-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaViable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
Parkinson’s disease (PD) is a neurodegenerative disease characterized by intraneuronal accumulation of alpha-synuclein (α-syn) called Lewy bodies and Lewy neurites. Recent genetic studies have revealed that mutations in glucocerebrosidase (GBA), a causative gene of Gaucher disease (GD), are a strong risk for PD. However, its pathological mechanisms leading to PD remain largely unknown. Here, we generated GBA nonsense mutant (GBA-/-) medaka which survive long enough for pathological analysis of disease progression. These mutant medaka display not only the phenotypes resembling human neuronopathic GD but also axonal accumulation of α-syn accompanied by impairment of the autophagy-lysosome pathway. Furthermore, the present study demonstrates this α-syn accumulation has negligible contribution to the pathogenesis of neuronopathic GD in medaka. GBA-/ - medaka represent a valuable model for exploring the pathological mechanisms of PD with GBA mutations as well as neuronopathic GD, and our findings have important implications for the association of GBA mutations with PD.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005065
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005065Summary
Parkinson’s disease (PD) is a neurodegenerative disease characterized by intraneuronal accumulation of alpha-synuclein (α-syn) called Lewy bodies and Lewy neurites. Recent genetic studies have revealed that mutations in glucocerebrosidase (GBA), a causative gene of Gaucher disease (GD), are a strong risk for PD. However, its pathological mechanisms leading to PD remain largely unknown. Here, we generated GBA nonsense mutant (GBA-/-) medaka which survive long enough for pathological analysis of disease progression. These mutant medaka display not only the phenotypes resembling human neuronopathic GD but also axonal accumulation of α-syn accompanied by impairment of the autophagy-lysosome pathway. Furthermore, the present study demonstrates this α-syn accumulation has negligible contribution to the pathogenesis of neuronopathic GD in medaka. GBA-/ - medaka represent a valuable model for exploring the pathological mechanisms of PD with GBA mutations as well as neuronopathic GD, and our findings have important implications for the association of GBA mutations with PD.
Introduction
Gaucher disease (GD) is the most common lysosomal storage disease and is caused by homozygous mutations in glucocerebrosidase (GBA). Mutations in GBA lead to decreased enzymatic activity of glucocerebrosidase (GCase) and result in the accumulation of its substrates, glucocerebroside and glucosylsphingosine[1,2]. GD is classically divided into three subtypes: a non-neuronopathic form (type 1), an acute neuronopathic form (type 2), and a subacute neuronopathic form (type 3). Visceral manifestations of all forms are characterized by hepatosplenomegaly, cytopenia, and skeletal disease. Pathologically, the accumulation of lipid-laden macrophages, called Gaucher cells, are observed in the affected organs. Neurological manifestations of neuronopathic forms include brainstem dysfunction, intellectual disability, seizures, and myoclonic movement. Pathological features of neuronopathic forms are neuronal loss, astrogliosis, microgliosis, and perivascular accumulation of Gaucher cells[3]. The most severe neuronopathic form, called the perinatal lethal type, has also been reported[4]. Common presentations of patients with the perinatal lethal type are hydrops fetalis and congenital ichthyosis. Almost no residual GCase enzymatic activity is found in these cases. Because currently available therapies are ineffective for neurological manifestations, a strong demand exists for elucidation of the pathological mechanisms and the development of novel therapies.
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder. GBA has recently drawn considerable attention because heterozygous mutations in this gene confer a high risk for sporadic PD[5,6]. In addition, patients with type 1 GD also have an increased life-time risk of developing PD[7]. PD patients carrying GBA mutations show intraneuronal accumulation of alpha-synuclein (α-syn) called Lewy bodies and Lewy neurites, which are the pathological hallmarks of sporadic PD[3]. Several cellular, animal, and postmortem studies have indicated an association between GBA mutations and α-syn accumulation. For example, deficiency in GCase enzymatic activity causes lysosomal dysfunction and α-syn accumulation[8,9,10,11,12]. Increased α-syn in turn creates a vicious cycle by inhibiting the trafficking of GCase to lysosomes, thus leading to decreased GCase activity in lysosomes[9]. Consistent with this notion, mouse models overexpressing α-syn and postmortem tissue from patients with PD show reduced GCase activity in the brains[13,14,15]. Although several hypotheses have been proposed, further mechanisms of how GBA mutations contribute to the development of PD remain elusive.
Medaka (Oryzias latipes) are a versatile vertebrate animal model for disease research. These fish are easy to handle, have a relatively short generation time (2–3 months), produce a large number of progeny per generation, and have several inbred strains[16]. Importantly, medaka have an advantage as an animal model of PD due to endogenous α-syn in contrast to invertebrate models that lack α-syn. Moreover, several genetic manipulations can be performed in medaka in addition to established transgenic techniques[17,18,19,20,21]. So far, we have reported genetic PD models of medaka that develop locomotor dysfunction accompanied by the selective loss of dopaminergic and noradrenergic neurons[22,23]. Considering these lines of evidence, medaka have the potential to be a new animal model of PD.
Here, we generated GBA nonsense mutant medaka and found that homozygous GBA nonsense mutant (GBA-/-) medaka are a viable neuronopathic GD model. GBA-/- medaka developed remarkable α-syn accumulation in the brains and thus provide novel insights into the association of GBA mutations with α-syn accumulation. Furthermore, we revealed minimal contribution of endogenous α-syn to the pathogenesis of neuronopathic GD in medaka.
Results
Generation of GBA nonsense mutant medaka
We generated GBA nonsense mutant medaka to investigate the mechanisms by which GBA mutation leads to PD. To identify medaka GBA orthologs, we searched the medaka genome database (http://www.ensembl.org/Oryzias_latipes/Info/Index) with the basic local alignment search tool and found only one ortholog of human GBA. We cloned the medaka GBA with reverse transcription-polymerase chain reaction (RT-PCR) and rapid amplification of cDNA ends and found that this gene has 11 exons encoding a protein of 522 amino acids. The amino acid sequence of medaka GBA showed 53% homology to that of human GBA (S1 Fig). Next, we screened a targeting-induced local lesions in genome (TILLING) library for medaka GBA using a high-resolution melting assay[17,24]. We identified a nonsense mutant (W337X) and generated the nonsense mutant medaka by in vitro fertilization (Fig. 1A). We examined GCase activity in the brains of GBA mutants after crossing with heterozygous mutants. GBAW337X/W337X (GBA-/-) medaka showed complete deficiency in GCase activity, and GBAWT/W337X (GBA+/-) medaka showed a decrease in GCase activity of about 50% compared to wild-type (GBA+/+) medaka (Fig. 1B). Although humans and mice lacking GCase activity die soon after birth[4,25,26], GBA-/- medaka survived for more than 3 months, enabling us to analyze the pathological progression (Fig. 1D). GBA-/- medaka showed abnormal rotating swimming movement at 2 months (S1, S2 Movie) and the abnormal appearance of a bent spine at 3 months (Fig. 1C). High levels of glucocerebroside accumulated in the brains of GBA-/- medaka (Fig. 1E), whereas the amount of galactocerebroside, an isomer of glucocerebroside, was not changed. Glucocerebroside with C18 fatty acids was the most dominant type in the brains of GBA-/- medaka (S2 Fig), an observation that is consistent with the neuronopathic GD mouse model[27].
Fig. 1. Generation of GBA nonsense mutant medaka. 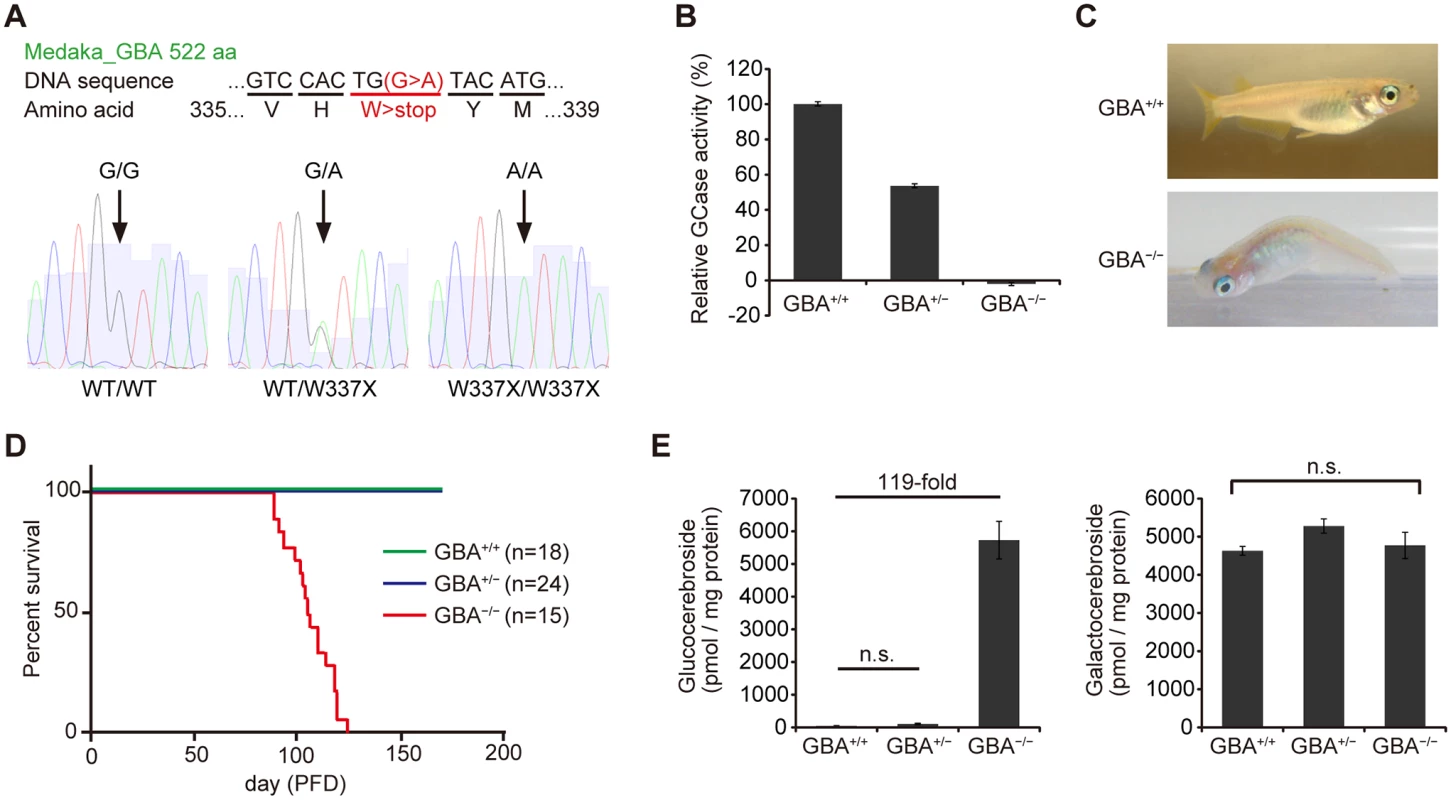
(A) Upper panel: DNA and amino acid sequences of GBA nonsense mutant medaka. Lower panels: Sequence data for each genotype. A = green, T = red, G = black, and C = blue. (B) Relative GCase activity in medaka brains (n = 10). (C) Abnormal posture (‘bent spine’) in GBA-/- medaka at 3 months. (D) Survival curves for each genotype. PFD, post-fertilization day. (E) Quantification of glucocerebroside and galactocerebroside in medaka brains at 3 months with SFC/MS/MS (n = 3–4). For all analyses, data are the mean ± standard error of the mean (SEM). n.s. means not significant. GBA-/- medaka showed neuronopathic GD-like pathology
We performed pathological analyses of GBA-/- medaka. Patients with GD show Periodic acid-Schiff-positive Gaucher cells in affected visceral organs such as the liver, spleen, and bone marrow, whereas GBA-/- medaka showed abnormal Periodic acid-Schiff-positive cells in the spleen and kidney, but not in the liver, at 3 months (S3 Fig). Next, we examined the brains of GBA-/- medaka and found abnormal cells with large vacuoles mainly in the periventricular gray zone of the optic tectum (Fig. 2A, B). Transmission electron microscopy revealed that these cells were macrophage-like and had large vacuoles containing filamentous structures (Fig. 2C). Similar filamentous structures are observed in Gaucher cells of patients with GD and mouse models of GD[26,28,29]. The staining intensity with Luxol fast blue was decreased, and single-stranded DNA (ssDNA)-positive cells were observed in GBA-/- medaka (Fig. 2D), indicating myelin loss and cell death, respectively. In situ hybridization for apolipoprotein E (APOE), a microglial marker in teleost fish[30], revealed proliferating activated microglia in GBA-/- medaka (Fig. 2D). Teleost fish have glial fibrillary acidic protein (GFAP)-expressing radial glial cells (or ependymoglial cells) instead of astrocytes as in mammals[31]. Humans and mice with neuronopathic GD show astrogliosis in their brains[3,32], whereas neither proliferation of GFAP-positive radial glial cells nor elevated levels of GFAP were observed in GBA-/- medaka (Fig. 2D, E). To investigate the type of neuronal cells that die in GBA-/- medaka, we counted the numbers of tyrosine hydroxylase (TH)-positive dopaminergic neurons in the middle diencephalon, which corresponds to the human substantia nigra[33], TH-positive noradrenergic neurons in the locus coeruleus, and tryptophan hydroxylase (TPH)-positive serotonergic neurons in the superior raphe at 2 and 3 months. GBA-/- medaka showed progressive cell loss of all these neurons (Fig. 2F). Consistent with these findings, the amount of TH was decreased in GBA-/- medaka (Fig. 2G). Collectively, GBA-/- medaka exhibited neuronopathic GD-like pathology including progressive and non-selective neuronal loss.
Fig. 2. Pathological analyses of GBA-/- medaka. 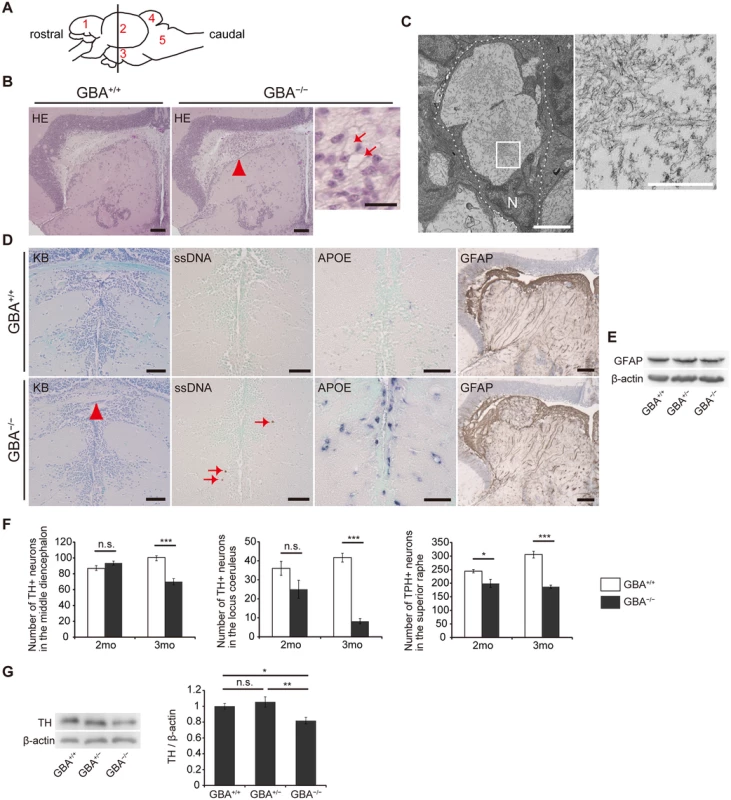
(A) Schematic of a lateral view of the medaka brain. Each number signifies a part of the brain. 1: telencephalon, 2: optic tectum, 3: diencephalon, 4: cerebellum, 5: medulla oblongata. The brain sections used for pathological analyses in the present study are illustrated by the vertical line. (B) Hematoxylin and eosin staining. Abnormal cells observed in the periventricular gray zone of the optic tectum (arrowhead) in GBA-/- medaka at 3 months. Scale bars, 50 μm. Right panel: High-magnification image showing abnormal cells with large vacuoles (arrows). Scale bar, 5 μm. (C) Transmission electron micrographs showing abnormal macrophage-like cells. Left panel: A whole-cell image of an abnormal macrophage-like cell. Dashed lines outline a whole abnormal cell. N, Nucleus. Scale bar, 2 μm. Right panel: High-magnification image of filamentous structures in vacuoles. Scale bar, 500 nm. (D) Klüver-Barrera (KB) staining, ssDNA immunohistochemistry, APOE in situ hybridization, and GFAP immunohistochemistry in the diencephalon. Commissura posterior with decreased Luxol fast blue staining intensity (arrowhead) and ssDNA-positive dead cells (arrows) in GBA-/- medaka. APOE in situ hybridization revealed proliferating activated microglia in GBA-/- medaka. The staining intensity and area of GFAP were not changed in GBA-/- medaka. Scale bars, 50 μm. (E) Western blot analysis of GFAP and β-actin. The expression level of GFAP was not changed among genotypes. (F) Number of TH-positive neurons in the middle diencephalon, number of TH-positive neurons in the locus coeruleus, and number of TPH-positive neurons in the superior raphe at 2 and 3 months. In GBA-/- medaka, progressive neuronal loss was observed in all types of neurons (n = 4–6, *p < 0.05, ***p < 0.001). (G) Western blot analysis of TH and β-actin (n = 7, *p < 0.05, **p < 0.01). For all analyses, data are the mean ± SEM. GBA-/- medaka developed α-syn accumulation in axonal swellings containing autophagosomes
Medaka express α-syn, which is a protein consisting of 127 amino acids. To investigate α-syn pathology in GBA-/- medaka, we created a medaka α-syn antibody against the epitope of amino acids 90 to 104 (S4A Fig). Medaka also express β-synuclein, γ-synuclein-a, and γ-synuclein-b in addition to α-syn, but these other synucleins do not have amino acid sequences homologous to the epitope of the medaka α-syn antibody (S4B Fig). Next, we generated α-syn deletion mutant medaka using TALENs. Deletion of 11 bases near the start codon in α-syn resulted in a frame shift mutation (S4C Fig). Wild-type, heterozygous, and homozygous α-syn deletion mutant (α-syn+/+, α-syn+/-, and α-syn-/-, respectively) medaka could be distinguished with PCR analysis of α-syn (S4D Fig). RT-PCR analysis of α-syn mRNA revealed that α-syn-/- medaka did not express intact α-syn mRNA (S4E Fig). Western blot analysis with the medaka α-syn antibody revealed a 14-kDa band, which was specifically found in α-syn+/+ medaka (S4F Fig). The authenticity of the antibody was confirmed by the lack of immunostaining with the medaka α-syn antibody in the brains of α-syn-/- medaka (S4G Fig). Consistent with the findings in mammals, medaka α-syn was mainly found presynaptically with immunoelectron microscopy (S4H Fig).
Then, we performed immunohistochemical analysis and found abundant α-syn accumulation in the brains of GBA-/- medaka at 3 months (Fig. 3A). α-syn accumulation was also observed at 2 months, but not at 1 month (S5 Fig). Abnormal structures observed with toluidine blue staining were similar to α-syn accumulations in size and distribution (Fig. 3A). Transmission electron microscopy revealed numerous axonal swellings containing vacuoles and other various materials in the brains of GBA-/- medaka (Figs. 3B, S6A). A considerable number of these vacuoles had double membranes and sometimes contained mitochondria, suggesting that these vacuoles were autophagosomes. Consistent with this, LC3, an autophagosomal marker, accumulated in the brains of GBA-/- medaka (Fig. 3A). Moreover, immunogold-labeled α-syn was detected in axonal swellings with immunoelectron microscopy (Fig. 3C). Conventional and confocal double immunofluorescence microscopy revealed that accumulated α-syn colocalized with LC3 accumulations (Fig. 3D), and a considerable portion of the α-syn signals in axonal swellings colocalized with LC3 signals (Figs. 3E, S6B). In addition to these findings, ubiquitin also accumulated in α-syn-positive axonal swellings (Figs. 3E, S6B). To examine the α-syn expression level and solubility, we performed sequential biochemical fractionation assays. The total amount of α-syn in the Triton-soluble fraction was not increased in GBA-/- medaka. However, when adjusted for the amount of neuron-specific enolase (NSE) and considering the robust neuronal loss in GBA-/- medaka, α-syn was significantly increased in GBA-/- medaka (Fig. 3F). The decreased amount of neurofilament also reflected the neuronal loss in GBA-/- medaka (Fig. 3F). No apparent α-syn band was detected in the Triton-insoluble, SDS-soluble fraction in all genotypes. In agreement with the accumulation of autophagosomes in axonal swellings, the ratio of LC3-II to LC3-I was increased in GBA-/- medaka (Fig. 3F).
Fig. 3. Axonal swellings with α-syn accumulation in GBA-/- medaka. 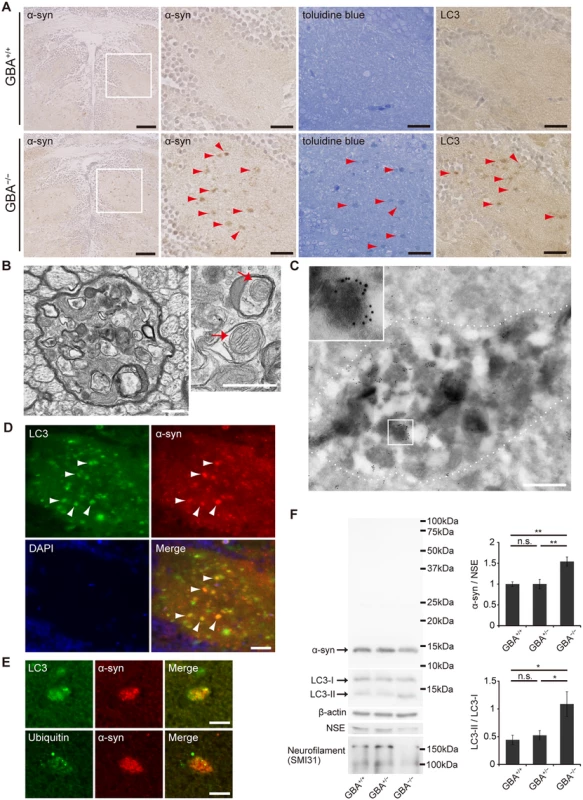
(A) α-syn and LC3 immunohistochemistry, and toluidine blue staining in the diencephalon at 3 months. Left panels: α-syn accumulations were observed in GBA-/- medaka. Scale bars, 50 μm. Other panels: outlined area of left panels. The distribution of α-syn accumulations was similar to that of abnormal structures observed with toluidine blue staining and LC3 accumulations (arrowheads). Scale bars, 20 μm. (B) Transmission electron micrographs of axonal swellings in GBA-/- medaka. Left panel: a swelling of a myelinated axon containing vacuoles and electron-dense bodies. Right panel: vacuoles in an axonal swelling containing mitochondria (arrows). Scale bars, 500 nm. (C) Immunoelectron micrograph of an axonal swelling in a GBA-/- medaka with immunogold-labeled α-syn. Dashed lines outline an axonal swelling containing vacuoles and electron-dense bodies. The boxed area is enlarged in the inset. Scale bar, 500 nm. (D) Double immunostaining for LC3 (green) and α-syn (red) in GBA-/- medaka. Nuclei were visualized with DAPI (blue). α-syn accumulations colocalized with LC3 accumulations (arrowheads). Scale bar, 20 μm. (E) Conforcal microscope images of α-syn-positive axonal swellings. Upper panels: Double immunostaining for LC3 (green) and α-syn (red). A considerable portion of the α-syn signals colocalized with LC3 signals. Lower panels: Double immunostaining for ubiquitin (green) and α-syn (red). Ubiquitin colocalized with an α-syn accumulation. Scale bars, 5 μm. (F) Western blot analysis of α-syn, LC3, β-actin, NSE, and neurofilament at 3 months (n = 6–7, *p < 0.05, **p < 0.01). For all analyses, data are the mean ± SEM. Meanwhile, we also investigated the phenotypes of GBA+/- medaka because heterozygous mutations in GBA are a strong risk for PD[5,6]. However, GBA+/- medaka even at 12 months did not show any apparent abnormal phenotypes including α-syn pathology, the numbers of TH-positive neurons, swimming movement, or the amounts of several neurotransmitters (S7A–S7E Fig).
Impairment of the autophagy-lysosome pathway in neurons of GBA-/- medaka
Previous studies of GD mouse models reported that p62/SQSTM1 (an autophagic substrate) accumulates in neurons and astrocytes[34], and the number of Cathepsin D-positive puncta is decreased in neurons[35]. These observations prompted us to examine the autophagy-lysosome pathway in GBA-/- medaka. Immunohistochemical analysis revealed that ubiquitin - and p62-positive aggregates were observed mainly in the perikarya of neurons (Figs. 4A, D, S8B). These aggregates were observed only in neurons and not in GFAP-positive radial glial cells or Lycopersicon Esculentum (Tomato) Lectin (LEL)-positive microglia (S8A Fig). LEL is an excellent teleost microglial marker[36]. Western blot analysis showed that the amounts of ubiquitin and p62 were increased in the brains of GBA-/- medaka (Fig. 4C). In contrast, these aggregates did not colocalize with LC3 accumulations or Cathepsin D-positive organelles (Figs. 4D, S8B). These organelles, which are putative lysosomes, showed decreased Cathepsin D staining intensity and abnormal morphology (Fig. 4A). Consistent with this, neurons that contained lysosome-like organelles filled with filamentous structures were observed with transmission electron microscopy (Fig. 4B).
Fig. 4. Impairment of the autophagy-lysosome pathway in GBA-/- medaka. 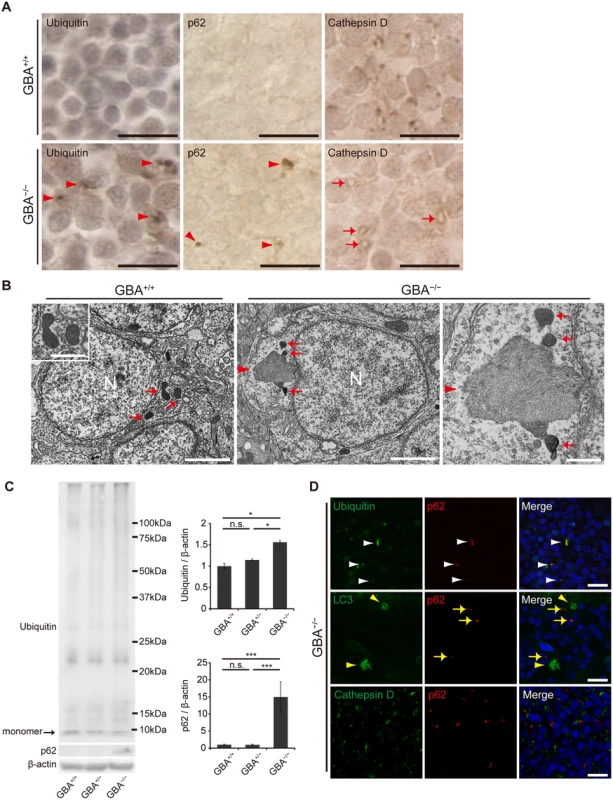
(A) Ubiquitin, p62, and Cathepsin D immunohistochemistry in the neuronal layer of the optic tectum at 3 months. Almost all of the cells in these figures are neurons. Ubiquitin-positive and p62-positive aggregates were observed in the perikarya of neurons in GBA-/- medaka at 3 months (arrowheads). Morphologically abnormal organelles with decreased Cathepsin D staining intensity were observed in GBA-/- medaka (arrows). Scale bars, 10 μm. (B) Transmission electron micrographs of neurons. Left panel: Neuron of a GBA+/+ medaka. Electron-dense organelles (arrows) are likely lysosomes. N, Nucleus. Scale bar, 2 μm. The inset shows a high-magnification image of electron-dense organelles. Scale bar, 500 nm. Middle panel: Neuron of a GBA-/- medaka. An aggregate containing filamentous structures (arrowhead) is continuous with an electron-dense organelle. N, Nucleus. Scale bar, 2 μm. Right panel: High-magnification image of an aggregate of filamentous structures (arrowhead) and electron-dense organelles (arrows) in a GBA-/- medaka. Scale bar, 500 nm. (C) Western blot analysis of ubiquitin, p62, and β-actin (n = 4–7, *p < 0.05, ***p < 0.001). (D) Conforcal microscope images of GBA-/- medaka. Upper panels: ubiquitin (green) and p62 (red). p62-positive aggregates colocalized with ubiquitin-positive aggregates (white arrowheads). Middle panels: LC3 (green) and p62 (red). p62-positive aggregates (yellow arrows) did not colocalize with LC3 accumulations (yellow arrowheads). Lower panels: Cathepsin D (green) and p62 (red). p62-positive aggregates did not colocalize with Cathepsin D-positive organelles. Nuclei were visualized with DAPI (blue). Scale bars, 10 μm. For all analyses, data are the mean ± SEM. The phenotypes of GBA-/- medaka were rescued by transgenic expression of GBA
GBA nonsense mutant medaka have random point mutations in the genome at loci other than GBA. Therefore, we performed a rescue experiment to determine whether the abnormal phenotypes observed in GBA-/- medaka were really caused by GBA mutation. We created medaka GBA-expressing vectors driven by a medaka growth-associated protein 43 (GAP-43) promoter (S9A Fig)[37]. GAP-43 mRNA is expressed mainly in nervous system in medaka[37]. We established six medaka GBA transgenic lines (Tg(GAP-43:GBA)) using these vectors and crossed them with GBA nonsense mutant medaka. Each line of GBA-/- medaka with GBA transgene (Tg(GAP-43:GBA);GBA-/-) showed GCase activity of various levels in the brains at 3 months (S9B Fig). All lines of Tg(GAP-43:GBA);GBA-/- medaka showed normal swimming movement at 2 months (S3 Movie). Also, the swimming distance was increased in Tg(GAP-43:GBA)line3;GBA-/- medaka (S9C Fig). Pathological analysis of the brains of Tg(GAP-43:GBA)line3;GBA-/- medaka including hematoxylin and eosin staining, in situ hybridization for APOE, and immunohistochemistry for p62 and α-syn revealed no apparent abnormality (S9D Fig). We concluded that the abnormal phenotypes observed in the brains of GBA-/- medaka were caused by the GBA mutation.
Disruption of α-syn in GBA-/- medaka did not improve the phenotypes
Many studies have reported that accumulated α-syn caused by α-syn overexpression results in neurotoxicity[38]. Neuroinflammation is observed in the brains of patients with PD, and much evidence shows that neuroinflammation promotes disease progression[39]. Moreover, a recent study reported that oligomeric α-syn released from neurons activates inflammatory responses in microglia[40].
We examined the toxicity of α-syn in GBA-/- medaka by crossing GBA nonsense mutant medaka with α-syn deletion mutant medaka. α-syn-/- medaka showed no apparent abnormal phenotypes in their outer appearance, swimming movement, or life span like α-syn-disrupted mice[41]. The life spans of GBA-/-α-syn+/- and GBA-/-α-syn-/- medaka were not prolonged compared to that of GBA-/-α-syn+/+ medaka (Fig. 5A). Moreover, the number of LC3-positive puncta was not changed in GBA-/-α-syn-/- medaka, indicating that α-syn was not primarily involved in formation of axonal swellings (Fig. 5B). The numbers of dopaminergic neurons in the middle diencephalon and noradrenergic neurons in the locus coeruleus were not changed in either GBA-/- α-syn+/- or GBA-/- α-syn-/- medaka (Fig. 5C). We also examined the extent of neuroinflammation with in situ hybridization for APOE and quantitative RT-PCR for tumor necrosis factor (TNF)α mRNA. The number of APOE-positive cells and the expression level of TNFα were not changed (Fig. 5D, E). Collectively, we found no evidence for α-syn involvement in the short life span, formation of axonal swellings, dopaminergic and noradrenergic neuronal loss, and neuroinflammation that are observed in GBA -/- medaka.
Fig. 5. Disruption of α-syn did not change the pathological phenotypes in GBA-/- medaka. 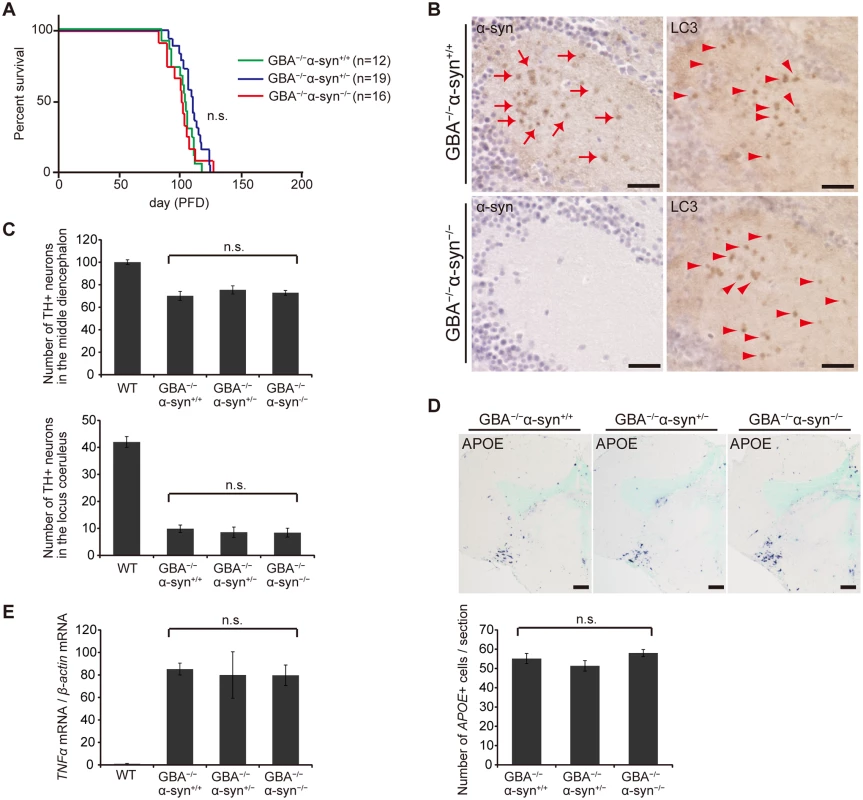
(A) Survival curves for each genotype. Life spans were not changed among genotypes. (B) α-syn and LC3 immunohistochemistry in the diencephalon. α-syn accumulations were observed only in GBA-/-α-syn+/+ medaka (arrows). The number of LC3-positive puncta was not different between GBA-/-α-syn+/+ and GBA-/-α-syn-/- medaka (arrowheads). Scale bars, 20 μm. (C) Number of TH-positive neurons in the middle diencephalon and the locus coeruleus at 3 months (n = 6). (D) Upper panel: APOE in situ hybridization in the optic tectum and the diencephalon. Lower panel: The number of APOE-positive cells per section (n = 9). Scale bars, 50 μm. (E) Quantification of TNFα mRNA levels normalized to β-actin mRNA in medaka brains with quantitative RT-PCR (n = 8). For all analyses, data are the mean ± SEM. Discussion
To date, several genetic animal models of neuronopathic GD including mouse, dog, and sheep have been reported[42]. Gba null mice die within 24 hours of birth due to permeability barrier defects in the skin[26,43]. Thus, conditional knockout K14-lnl/lnl mice with normal GCase activity in their skin were generated[27]. However, use of these mice is limited because they survive only 2 weeks after birth. Mice harboring a Gba missense mutation combined with prosaposin - or saposin C-deficient mice, another neuronopathic GD model, also show neuronopathic abnormalities[29,34] and have the advantage of longer survival than K14-lnl/lnl mice. However, the relevance of these mice to neuronopathic GD is controversial. The present study revealed that GBA-/- medaka survive long enough for pathological analysis of disease progression. As an example, GBA-/- medaka showed α-syn accumulation at 2 months, not at 1 month after fertilization. Considering these observations, GBA-/- medaka are useful as a viable neuronopathic GD model with endogenous α-syn accumulation.
GBA-/- medaka showed several phenotypes different from those of mammalian neuronopathic GD model. Firstly, the skin of GBA-/- medaka looks intact whereas severe skin lesion is observed in patients with perinatal lethal type GD and Gba null mice[4,43]. Secondly, GBA-/- medaka exhibited PAS-positive abnormal cells in spleen and kidney which presumably correspond to human Gaucher cells. In patients with GD, Gaucher cells are not found in kidney, but in liver, spleen and bone marrow. These differences could be explained by the fact that in adult teleost fish kidney has hematopoietic function instead of mammalian bone marrow[44]. Lastly, the proliferation of GFAP-positive radial glial cells was not observed in GBA-/- medaka whereas astrogliosis is observed in humans and mice with neuronopathic GD. It was reported that the reaction of GFAP-positive radial glial cells to inflammation is different from that of mammalian astrocytes[31].
Lysosomes play a fundamental role in the autophagic pathway by fusing with autophagosomes and digesting their contents[45]. In general, lysosomal dysfunction results in defective digestion and accumulation of autophagic substrates such as polyubiquitinated proteins, p62, and dysfunctional mitochondria, accompanied by accumulation of autophagosomes. Axonal swellings containing autophagosome-like structures are observed in mouse models of lysosomal storage diseases such as neuronopathic GD mice, Niemann-pick type C1 mice, Cathepsin D-deficient mice, and Cathepsin B/L double-deficient mice[34,46,47]. A previous study reported that autophagosomes are formed in the distal part of the axon and undergo retrograde transport toward the cell body[48]. Another previous study demonstrated that inhibition of lysosomal function in primary neurons from mouse embryos disrupts the axonal transport of autophagosomes and causes their accumulation in axons[49]. Considering these lines of evidence, we propose that GCase deficiency primarily causes lysosomal dysfunction, leading to disrupted retrograde transport of autophagosomes in axons and formation of axonal swellings with α-syn accumulation (Fig. 6). Contrary to our expectations, p62-positive aggregates did not colocalize with LC3 - and α-syn-positive axonal swellings, but were mainly located in the perikarya of neurons. These results suggest the possibility that presynaptic α-syn is transported proximally and degraded in a p62-independent autophagy-lysosome pathway. A previous study supports this hypothesis. Conditional knockout mice lacking ATG7 in TH-positive neurons show α-syn aggregates in swollen axons in the striatum, but not in cell bodies in the midbrain, indicating that autophagic dysfunction initially causes α-syn aggregates in the distal part of axons[50]. In addition, another previous study demonstrated that α-syn aggregation occurs earlier in axons than in neuronal cell bodies in the cardiac sympathetic nervous system in PD patients[51]. Because axonal retrograde transport may be involved in the degradative pathway of α-syn and may be a therapeutic target in PD, further studies are required to elucidate the precise mechanisms.
Fig. 6. Pathological findings and proposed pathological mechanisms in neurons of GBA-/- medaka. 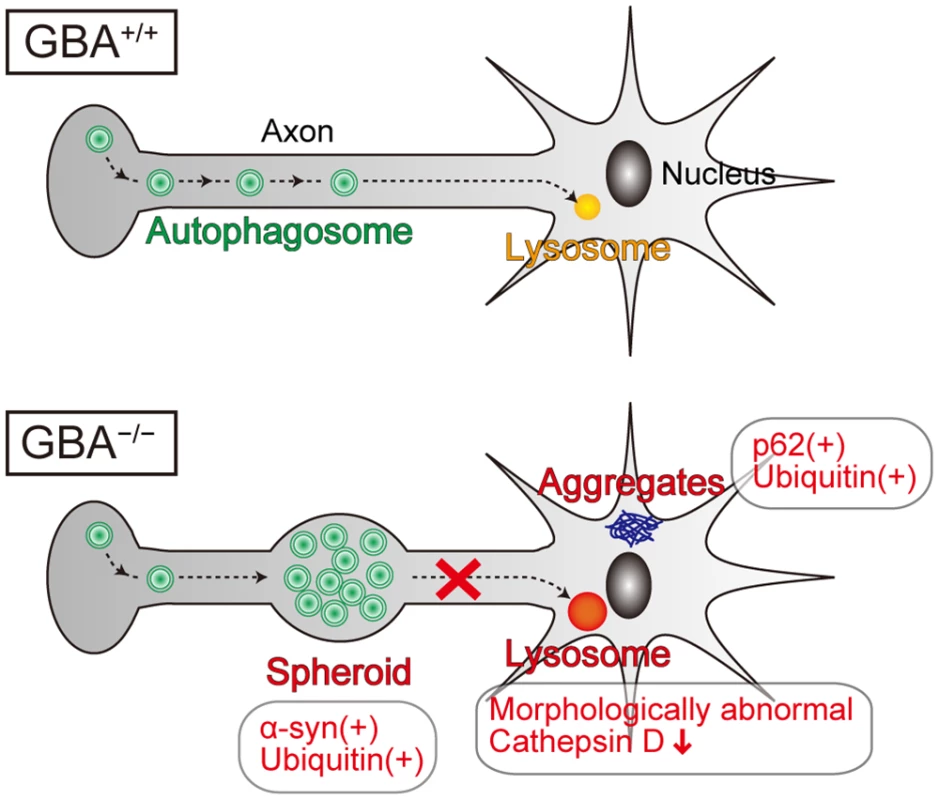
Neurons of GBA-/- medaka showed lysosomal dysfunction, which is reflected in morphologically abnormal structures with decreased Cathepsin D staining intensity, and axonal swellings containing autophagosomes where α-syn and ubiquitin accumulate. p62-positive aggregates, which colocalize with ubiquitin, are not located in axonal swellings, but presumably in soma or dendrites. Considering the previous studies about the axonal transport of autophagosomes, we propose that GCase deficiency primarily causes lysosomal dysfunction, which leads to disrupted retrograde transport of autophagosomes in axons and formation of axonal swellings with α-syn accumulation. Because GBA+/- medaka as old as 12 months did not show any apparent abnormal phenotypes, we could not directly investigate how heterozygous GBA mutations cause PD. Meanwhile, according to a recent report, induced pluripotent stem cell-derived neurons from PD patients carrying heterozygous GBA mutations show α-syn accumulation, an impaired autophagy-lysosome pathway, and dysregulation of calcium homeostasis[12]. The reason for differences in phenotypes between in vivo and in vitro models is unclear. However, our findings from GBA+/- medaka seem to be reasonable because the penetrance of PD in GBA mutation carriers is estimated to be at most 30% by the age of 80 years[52]. Thus, second hits such as environmental factors and other genetic factors are probably required for the development of PD pathology in vivo.
Cellular and animal PD models overexpressing α-syn have provided evidence for the various potential toxic mechanisms of α-syn. However, few studies have demonstrated the pathological role of endogenous α-syn in vivo, which may reflect the authentic role for α-syn in PD. A previous study showed that α-syn null mice are resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity compared with wild-type mice[53]. However, it is unclear whether this improvement is due to attenuation of α-syn toxicity or α-syn-mediated changes in the presynaptic machinery. Another previous study showed that the formation of intraneuronal inclusions and neurodegeneration in 26S proteasome-depleted mice is independent of α-syn[54]. In the present study, disruption of α-syn did not improve the life span, neuronal loss, or neuroinflammation in GBA-/- medaka. Moreover, α-syn was not involved in the accumulation of autophagosomes in axons. Our data indicate that α-syn accumulation is a downstream event, and other severe pathological factors may obscure the involvement of α-syn in the pathogenesis of neuronopathic GD in medaka.
In summary, the present study showed that GBA-/- medaka are useful as a viable neuronopathic GD model with endogenous α-syn accumulation. Long-term survival of these fish allows us to observe the pathological progression. Our data revealed that GCase deficiency causes lysosomal dysfunction in neurons and α-syn accumulation in axonal swellings containing autophagosomes. Axonal transport of α-syn may play an important role in the mechanisms of GBA mutations leading to PD and may also be a therapeutic target in PD. Furthermore, we demonstrate the minimal contribution of α-syn to the pathogenesis of neuronopathic GD in medaka. GBA-/- medaka represent a valuable model for exploring the pathological mechanisms and also provide a new platform for developing treatments in PD with GBA mutations as well as neuronopathic GD.
Materials and Methods
Ethics statement
Medaka were anesthetized in 0.02% tricaine in fish water and then sacrificed. All experimental procedures used in this study followed national guidelines. The Animal Research Committee of Kyoto University granted a formal waiver of ethical approval and also granted permission.
Maintenance of medaka
Medaka of the Kyoto-cab strain, a substrain of Cab, were maintained at 27°C in a recirculating aquaculture system. Adult fish were kept in a reproduction regimen (14 hr light/10 hr dark). Eggs were kept in a dark box at 28°C.
Cloning of medaka genes
RNA was extracted from wild-type medaka brains with Qiazol (QIAGEN) according to the manufacturer’s instructions. cDNA was synthesized using the PrimeScript RT reagent kit (Perfect Real Time) (TaKaRa, #RR037A). To identify medaka GBA, SNCA, SNCGb, p62/SQSTM1, and MAP1LC3B orthologs, we referred to the medaka genome database (http://www.ensembl.org/Oryzias_latipes/Info/Index). Because their cDNA sequences and amino acid sequences were not completely known, we determined their cDNA sequences using a combination of RT-PCR and rapid amplification of cDNA ends, the products of which were generated using Seegene’s Capfishing kit (Seegene). The cDNA sequences can be found in the European Nucleotide Archive (ENA, accession numbers LM644999–645003).
Generation of GBA nonsense mutant medaka
GBA mutant medaka were generated as described[17]. To find GBA mutations in the TILLING library, we narrowed down mutated candidates from 5,771 samples using a high-resolution melting assay, followed by determination of the DNA sequences[24]. We screened the TILLING library for exons 1–2, exons 5–7, exon 8, and exons 9–11 of GBA. In vitro fertilization was carried out using sperm from a sample with the favorable mutation. To genotype the progeny of GBAWT/W337X mutants, PCR was performed with the following primer set (5′-AGGGTTGAAGGGGTTAAGCA-3′, 5′-TTGTAACCAGTACCGCAGCA-3′), designed HybProbes (5′-LC Red 640-CATGTACCAGTGGACG-Phosphate-3′, 5′-CCTAAGCTTATATCTGCAGGGACTAAACTGT-Fluorescein-3′), and LightCycler 480 Probes Master in LightCycler 480 (Roche) according to the manufacturer's instructions. The genotypes can be distinguished with high-resolution melting curve analysis. GBAWT/W337X mutants were back-crossed to Kyoto-Cab medaka at least seven times and then crossed to obtain GBAW337X/W337X mutants.
Rescue experiment
For the rescue experiment, we established GBA transgenic medaka in which medaka GBA expression was driven by the medaka GAP-43 promoter (Tg(GAP-43:GBA)). We used an insulator located in the upstream region of sea urchin (Hemicentrotus pulcherrimus) arylsulfatase[55,56]. The transgenic construct was flanked by two insulators and included the medaka GAP-43 promoter followed by an internal ribosome entry site (IRES), enhanced GFP (EGFP), and the Simian virus 40 polyadenylation site or the medaka β-actin 3′-untranslated region (3′UTR) (S8A Fig). The medaka GAP-43 promoter contained a 1.0-kb fragment of the 5′-flanking region of the gene. A DNA fragment of the transgene was inserted into EcoR I/Sal I restriction enzyme sites of the pDs-actb2k-EGFP plasmid. pDs-actb2k-EGFP was constructed by inserting an Xho I/Spe I fragment from pactb2k-EGFP into the Xho I/Spe I site of the pDs-GTDEL4 plasmid, which contains 5′ - and 3′-Ds sequences[21,57]. The resultant vector was injected into the cytoplasm of fertilized Kyoto-Cab eggs before the first cleavage as described[20].
Generation of α-syn deletion mutant medaka
α-syn deletion mutant medaka were generated with TALENs as described[58]. To genotype the progeny of α-syn deletion mutants, PCR was performed with the following primer set (5′-GATCCCGAGCCATCCAC-3′, 5′-TGCAACTGTGGAAACACCAT-3′), followed by electrophoresis in a 10% (w/v) polyacrylamide gel (S4D Fig). RT-PCR for α-syn was performed with the following primer sets (α-syn: 5′-GATCCCGAGCCATCCAC-3′, 5′-TTTGGAGAAACCCTTCATTAAC-3′; β-actin: 5′-TCCACCTTCCAGCAGATGTG-3′, 5′-AGCATTTGCGGTGGACGAT-3′) (S4E Fig).
Behavioral analyses
The spontaneous swimming movement of medaka was traced using a video camera positioned above the water tank and analyzed with ethovision XT 5 (Noldus). The water tank was a transparent circular container (20 cm diameter, 2 cm water depth, room temperature). Image acquisition began 5 min after medaka were placed in a new water tank. Data were collected continuously for the subsequent 3 min.
GCase enzymatic activity assay
The assay for GCase enzymatic activity was performed as described[11]. Medaka brains were homogenized in 40 μl sample buffer (10 mM Tris-HCl, 150 mM NaCl, 1% (v/v) Triton X-100, pH 7.4), sonicated, and centrifuged at 10,000 ×g at 4°C for 5 min. Aliquots containing 50 μg protein were incubated in assay buffer (5 mM 4-Methylumbelliferyl β-d-glucopyranoside (Wako, #324–37441), 1% (w/v) sodium taurocholate (Wako, #197–10033), 50 mM sodium citrate, 50 mM sodium phosphate, pH 5.0) in the presence or absence of 2 mM Conduritol B epoxide (Toronto Research Chemicals, #C666000) in a total volume of 100 μl at 37°C for 4 hr. The reaction was stopped by adding 100 μl of 0.4 M glycine, pH 10.8, and the fluorescence at 460 nm (emission 355 nm) was measured with Fluoroskan Ascent FL (Thermo Fisher).
Lipid analyses with supercritical fluid chromatography (SFC)/mass spectrometry (MS)/MS
Medaka brains were stored at—80°C until analyses and homogenized in 1 ml tissue homogenization buffer (250 mM sucrose, 25 mM KCl, 50 mM Tris-HCl, 0.5 mM EDTA, pH 7.4). Aliquots containing 1 mg protein were used for the analyses. Levels of glucocerebroside and galactocerebroside were measured with high-performance liquid chromatography-tandem MS as described with modification using SFC separation[59].
Generation of the medaka α-syn antibody
A synthetic peptide corresponding to amino acids 90–104 of medaka α-syn conjugated to Keyhole Limpet Hemocyanin was used for immunization of rabbits. Serum was obtained 49 days after immunization and purified with affinity chromatography.
Immunohistochemical analyses
Frozen and paraffin sections were used for immunohistochemical analyses. For frozen sections, medaka brains were fixed with 4% (w/v) paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C for 4 hr and then placed in 30% (w/v) sucrose at 4°C for more than 16 hr. Samples were embedded in Surgipath FSC 22 (Leica), and 14-μm-thick sections were obtained with a LEICA CM 1900. For paraffin sections, medaka brains were fixed in 4% (w/v) PFA at 4°C for 16 hr, dehydrated, and embedded in paraffin. Sections with a thickness of 6 to 20 μm were obtained with a Microm HM 325. For immunohistochemical analyses, the following primary antibodies and a lectin were used: anti-Cathepsin D (Calbiochem, #IM03, 1 : 200), anti-GFAP (Sigma-Aldrich, #G3893, 1 : 1000), anti-LC3 (Santa Cruz, #sc-16755, 1 : 100), anti-medaka α-syn (1 : 2000), anti-p62 (MBL, #PM045, 1 : 2000), anti-ssDNA (DAKO, #A4506, 1 : 2000), anti-TH (Millipore, #MAB318, 1 : 1000), anti-TPH (Abcam, #ab3907–50, 1 : 1000), anti-ubiquitin (Santa Cruz, #sc-8017, 1 : 50), anti-ubiquitin (DAKO, #Z0458, 1 : 1000), and biotinylated Lycopersicon Esculentum (Tomato) Lectin (VECTOR, #B-1175, 5 <g/ml). The sections were incubated at 4°C with primary antibodies or lectin for 1 to 3 days and then processed for visualization. As secondary antibodies, ImmPRESS (VECTOR) was used for diaminobenzidine staining, and Alexa Fluor-conjugated antibodies (Molecular Probes) were used for immunofluorescence. Sections were observed with an Olympus CX41 microscope, a BZ-9000 fluorescence microscope (KEYENCE), and an Olympus FV-1000 confocal laser scanning microscope. For confocal microscope images, Pearson's coefficient correlation (r) was calculated using Olympus software.
Measurement of mRNA in medaka brains
RNA was extracted from medaka brains with Qiazol (QIAGEN) according to the manufacturer’s instructions. cDNA was generated with reverse transcription using the PrimeScript RT reagent kit (Perfect Real Time) (TaKaRa, #RR037A). The amount of cDNA was quantified with real-time PCR using LightCycler 480 SYBR Green I Master (Roche, #04887352001) and Roche LightCycler 480. The following primer sets were used (TNFα: 5′-ATTGGAGTGAAAGGCCAGAA-3′, 5′-ACTAATTTGAGACCGCCACG-3′; β-actin: 5′-TCCACCTTCCAGCAGATGTG-3′, 5′-AGCATTTGCGGTGGACGAT-3′).
In situ hybridization
The vector including a portion of the medaka APOE cDNA sequence was generously provided by Dr. H. Mitani (Tokyo University, Tokyo, Japan) (in submission). In situ hybridization was performed as described[60]. Counterstaining was performed with methyl green (Sigma-Aldrich, #M8884).
Western blot analyses
For Triton-soluble fractions, samples were homogenized in high-salt buffer containing 1% Triton X-100 (750 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl, 1% (v/v) Triton X-100, pH 7.5) and centrifuged at 20,400 ×g at 4°C for 5 min. For SDS-soluble fractions, the pellet was subsequently sonicated in SDS buffer (50 mM Tris-HCl, 2% SDS, pH 7.4) followed by centrifugation at 20,400 ×g at 4°C for 5 min. The supernatant was boiled in sample buffer (1% (w/v) SDS, 12.5% (w/v) glycerol, 0.005% (w/v) bromophenol blue, 2.5% (v/v) 2-mercaptoethanol, 25 mM Tris-HCl, pH 6.8). Samples were separated on 10%, 12%, or 14% (w/v) gels for SDS-PAGE. Samples containing 20 μg protein were loaded in each lane for Triton-soluble fractions, and SDS-soluble fractions were loaded according to the amount of protein in Triton-soluble fractions. Proteins were transferred to polyvinylidene difluoride membranes with a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad). For detection of medaka α-syn and mouse α-syn, the membranes were treated with 0.4% (w/v) PFA in PBS for 30 min at room temperature before blocking to prevent detachment of α-syn from the blotted membranes[61]. For western blot analyses, the following primary antibodies were used: anti-β-actin (Sigma-Aldrich, #A1978, 1 : 5000), anti-GFAP (Sigma-Aldrich, #G3893, 1 : 1000), anti-LC3 (MBL, #PM036, 1 : 2000), anti-NSE (DAKO, #M0873, 1 : 500), anti-α-syn (BD Transduction, #610787, 1 : 2000), anti-medaka α-syn (1 : 10,000), anti-phosphorylated neurofilament (COVANCE, #smi-31r, 1 : 1000), anti-p62 (MBL, #PM045, 1 : 500), anti-TH (Millipore, #MAB318, 1 : 1000), and anti-ubiquitin (Santa Cruz, #sc-8017, 1 : 50). The membranes were incubated with primary antibodies for 1 to 3 days at 4°C, followed by reaction with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) for 1 hr at room temperature. Immunoreactive bands were detected with Chemi-Lumi One Super (Nacalai tesque), and the chemiluminescent signal was detected with Fujifilm LAS-3000. Densitometric analyses were performed using ImageJ software (National Institutes of Health).
High performance liquid chromatography
To measure the amounts of dopamine, noradrenaline and serotonin in medaka brains, high performance liquid chromatography was performed as described[60].
Transmission electron microscopy and toluidine blue staining
Medaka brains were fixed with 4% (w/v) PFA and 2% (v/v) glutaraldehyde (Wako, #072–01961) in 0.1 M phosphate buffer (PB) at 4°C for 16 hr. After rinsing in 0.1 M PB, samples were postfixed with 1% (w/v) OsO4 in 0.1 M PB for 2 hr. Then, samples were dehydrated, penetrated with ethanol and a propylene oxide series, and embedded in Epon. Sections were obtained with an EM UC6 ultramicrotome (Leica). Sections with a thickness of 1 μm were used for toluidine blue staining. Sections with a thickness of 60 to 80 nm were stained with uranyl acetate and lead citrate and observed with a Hitachi H-7650 transmission electron microscope.
Immunoelectron microscopy
Immunoelectron microscopy using ultrathin cryosections was performed as described[62]. Briefly, brains were quickly removed from medaka and immersed in 4% PFA buffered with 0.1 M PB (pH 7.2) at 4°C for 2 hr, washed thoroughly with 7.5% sucrose in 0.1 M PB (pH 7.2), and embedded in 12% gelatin. The samples were rotated in 2.3 M sucrose in 0.1 M PB overnight at 4°C, placed on a specimen holder (Leica Microsystems), and quickly plunged into liquid nitrogen. Ultrathin cryosections were cut with a Leica UC6/FC6 and UC7/FC7 (Leica Microsystems) at about—120°C. Sections about 60 nm thick were picked up with a 1 : 1 mixture of 2% methylcellulose and 2.3 M sucrose and transferred to a nickel grid with a carbon-coated Formvar supporting film. The sections were rinsed with PBS containing 0.02 M glycine, treated with 1% bovine serum albumin in PBS, and incubated overnight at 4°C with rabbit anti-medaka α-syn antibody (1 : 30). They were then incubated with goat anti-rabbit IgG conjugated to 10 nm colloidal gold particles (1 : 40) (British Biocell International) for 1 hr at room temperature. Immunostained sections were fixed with 1% glutaraldehyde in PBS. After completion of the labeling, the sections were embedded in a thin layer of 2% methylcellulose with 0.4% uranyl acetate (pH 4.0), air-dried, and observed with a Hitachi H-7100 electron microscope. For control experiments, ultrathin sections were reacted only with the gold particle-conjugated secondary antibody.
Statistical analyses
A two-tailed paired Student’s t-test or one-way ANOVA with Tukey’s post-hoc test was used for analyses. Statistical calculations were performed with Microsoft Excel or GraphPad Prism Software, Version 5.0.
Supporting Information
Zdroje
1. Futerman AH, Zimran A (2006) Gaucher disease: CRC Press.
2. Grabowski GA (2008) Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet 372 : 1263–1271. doi: 10.1016/S0140-6736(08)61522-6 19094956
3. Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, et al. (2004) Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 82 : 192–207. 15234332
4. Eblan MJ, Goker-Alpan O, Sidransky E (2005) Perinatal lethal Gaucher disease: a distinct phenotype along the neuronopathic continuum. Fetal Pediatr Pathol 24 : 205–222. 16396828
5. Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, et al. (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 132 : 1783–1794. doi: 10.1093/brain/awp044 19286695
6. Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med 361 : 1651–1661. doi: 10.1056/NEJMoa0901281 19846850
7. Bultron G, Kacena K, Pearson D, Boxer M, Yang R, et al. (2010) The risk of Parkinson's disease in type 1 Gaucher disease. J Inherit Metab Dis 33 : 167–173. doi: 10.1007/s10545-010-9055-0 20177787
8. Manning-Bog AB, Schule B, Langston JW (2009) Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology 30 : 1127–1132. doi: 10.1016/j.neuro.2009.06.009 19576930
9. Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, et al. (2011) Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146 : 37–52. doi: 10.1016/j.cell.2011.06.001 21700325
10. Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, et al. (2011) CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci U S A 108 : 12101–12106. doi: 10.1073/pnas.1108197108 21730160
11. Xu YH, Sun Y, Ran H, Quinn B, Witte D, et al. (2011) Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol Genet Metab 102 : 436–447. doi: 10.1016/j.ymgme.2010.12.014 21257328
12. Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, et al. (2014) iPSC-derived neurons from GBA1-associated Parkinson's disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5 : 4028. doi: 10.1038/ncomms5028 24905578
13. Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, et al. (2012) Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol 72 : 455–463. doi: 10.1002/ana.23614 23034917
14. Sardi SP, Singh P, Cheng SH, Shihabuddin LS, Schlossmacher MG (2012) Mutant GBA1 expression and synucleinopathy risk: first insights from cellular and mouse models. Neurodegener Dis 10 : 195–202. doi: 10.1159/000335038 22327140
15. Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, et al. (2014) Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson's disease. Brain 137 : 834–848. doi: 10.1093/brain/awt367 24477431
16. Wittbrodt J, Shima A, Schartl M (2002) Medaka—a model organism from the far East. Nat Rev Genet 3 : 53–64. 11823791
17. Taniguchi Y, Takeda S, Furutani-Seiki M, Kamei Y, Todo T, et al. (2006) Generation of medaka gene knockout models by target-selected mutagenesis. Genome Biol 7: R116. 17156454
18. Ansai S, Kinoshita M (2014) Targeted mutagenesis using CRISPR/Cas system in medaka. Biol Open 3 : 362–371. doi: 10.1242/bio.20148177 24728957
19. Ansai S, Sakuma T, Yamamoto T, Ariga H, Uemura N, et al. (2013) Efficient targeted mutagenesis in medaka using custom-designed transcription activator-like effector nucleases. Genetics 193 : 739–749. doi: 10.1534/genetics.112.147645 23288935
20. Kinoshita M, Kani S, Ozato K, Wakamatsu Y (2000) Activity of the medaka translation elongation factor 1alpha-A promoter examined using the GFP gene as a reporter. Dev Growth Differ 42 : 469–478. 11041488
21. Ansai S, Ochiai H, Kanie Y, Kamei Y, Gou Y, et al. (2012) Targeted disruption of exogenous EGFP gene in medaka using zinc-finger nucleases. Dev Growth Differ 54 : 546–556. doi: 10.1111/j.1440-169X.2012.01357.x 22642582
22. Matsui H, Gavinio R, Asano T, Uemura N, Ito H, et al. (2013) PINK1 and Parkin complementarily protect dopaminergic neurons in vertebrates. Hum Mol Genet 22 : 2423–2434. doi: 10.1093/hmg/ddt095 23449626
23. Matsui H, Sato F, Sato S, Koike M, Taruno Y, et al. (2013) ATP13A2 deficiency induces a decrease in cathepsin D activity, fingerprint-like inclusion body formation, and selective degeneration of dopaminergic neurons. FEBS Lett 587 : 1316–1325. doi: 10.1016/j.febslet.2013.02.046 23499937
24. Ishikawa T, Kamei Y, Otozai S, Kim J, Sato A, et al. (2010) High-resolution melting curve analysis for rapid detection of mutations in a Medaka TILLING library. BMC Mol Biol 11 : 70. doi: 10.1186/1471-2199-11-70 20840787
25. Tayebi N, Cushner SR, Kleijer W, Lau EK, Damschroder-Williams PJ, et al. (1997) Prenatal lethality of a homozygous null mutation in the human glucocerebrosidase gene. Am J Med Genet 73 : 41–47. 9375921
26. Tybulewicz VL, Tremblay ML, LaMarca ME, Willemsen R, Stubblefield BK, et al. (1992) Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature 357 : 407–410. 1594045
27. Enquist IB, Lo Bianco C, Ooka A, Nilsson E, Mansson JE, et al. (2007) Murine models of acute neuronopathic Gaucher disease. Proc Natl Acad Sci U S A 104 : 17483–17488. 17954912
28. Pennelli N, Scaravilli F, Zacchello F (1969) The morphogenesis of Gaucher cells investigated by electron microscopy. Blood 34 : 331–347. 5804023
29. Sun Y, Quinn B, Witte DP, Grabowski GA (2005) Gaucher disease mouse models: point mutations at the acid beta-glucosidase locus combined with low-level prosaposin expression lead to disease variants. J Lipid Res 46 : 2102–2113. 16061944
30. Peri F, Nusslein-Volhard C (2008) Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133 : 916–927. doi: 10.1016/j.cell.2008.04.037 18510934
31. Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J (2012) Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60 : 343–357. doi: 10.1002/glia.22269 22105794
32. Farfel-Becker T, Vitner EB, Pressey SN, Eilam R, Cooper JD, et al. (2011) Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum Mol Genet 20 : 1375–1386. doi: 10.1093/hmg/ddr019 21252206
33. Rink E, Wullimann MF (2001) The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res 889 : 316–330. 11166725
34. Sun Y, Liou B, Ran H, Skelton MR, Williams MT, et al. (2010) Neuronopathic Gaucher disease in the mouse: viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum Mol Genet 19 : 1088–1097. doi: 10.1093/hmg/ddp580 20047948
35. Vitner EB, Dekel H, Zigdon H, Shachar T, Farfel-Becker T, et al. (2010) Altered expression and distribution of cathepsins in neuronopathic forms of Gaucher disease and in other sphingolipidoses. Hum Mol Genet 19 : 3583–3590. doi: 10.1093/hmg/ddq273 20616152
36. Cuoghi B, Mola L (2007) Microglia of teleosts: facing a challenge in neurobiology. Eur J Histochem 51 : 231–240. 18162452
37. Fujimori KE, Kawasaki T, Deguchi T, Yuba S (2008) Characterization of a nervous system-specific promoter for growth-associated protein 43 gene in Medaka (Oryzias latipes). Brain Res 1245 : 1–15. doi: 10.1016/j.brainres.2008.09.071 18951884
38. Dawson TM, Ko HS, Dawson VL (2010) Genetic animal models of Parkinson's disease. Neuron 66 : 646–661. doi: 10.1016/j.neuron.2010.04.034 20547124
39. Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 8 : 382–397. doi: 10.1016/S1474-4422(09)70062-6 19296921
40. Kim C, Ho DH, Suk JE, You S, Michael S, et al. (2013) Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4 : 1562. doi: 10.1038/ncomms2534 23463005
41. Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, et al. (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25 : 239–252. 10707987
42. Farfel-Becker T, Vitner EB, Futerman AH (2011) Animal models for Gaucher disease research. Dis Model Mech 4 : 746–752. doi: 10.1242/dmm.008185 21954067
43. Holleran WM, Ginns EI, Menon GK, Grundmann JU, Fartasch M, et al. (1994) Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest 93 : 1756–1764. 8163674
44. Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, et al. (2006) Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25 : 963–975. 17157041
45. Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, et al. (2012) Autophagy in lysosomal storage disorders. Autophagy 8 : 719–730. doi: 10.4161/auto.19469 22647656
46. Liao G, Yao Y, Liu J, Yu Z, Cheung S, et al. (2007) Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1 -/ - mouse brain. Am J Pathol 171 : 962–975. 17631520
47. Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, et al. (2005) Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am J Pathol 167 : 1713–1728. 16314482
48. Maday S, Wallace KE, Holzbaur EL (2012) Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 196 : 407–417. doi: 10.1083/jcb.201106120 22331844
49. Lee S, Sato Y, Nixon RA (2011) Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J Neurosci 31 : 7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011 21613495
50. Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, et al. (2012) Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci 32 : 7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012 22649237
51. Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, et al. (2008) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain 131 : 642–650. 18079166
52. Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, et al. (2012) Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology 78 : 417–420. doi: 10.1212/WNL.0b013e318245f476 22282650
53. Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, et al. (2002) Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99 : 14524–14529. 12376616
54. Paine SM, Anderson G, Bedford K, Lawler K, Mayer RJ, et al. (2013) Pale body-like inclusion formation and neurodegeneration following depletion of 26S proteasomes in mouse brain neurones are independent of alpha-synuclein. PLoS One 8: e54711. doi: 10.1371/journal.pone.0054711 23382946
55. Ochiai H, Sakamoto N, Suzuki K, Akasaka K, Yamamoto T (2008) The Ars insulator facilitates I-SceI meganuclease-mediated transgenesis in the sea urchin embryo. Dev Dyn 237 : 2475–2482. doi: 10.1002/dvdy.21690 18729225
56. Takagi H, Inai Y, Watanabe S, Tatemoto S, Yajima M, et al. (2012) Nucleosome exclusion from the interspecies-conserved central AT-rich region of the Ars insulator. J Biochem 151 : 75–87. doi: 10.1093/jb/mvr118 21930654
57. Emelyanov A, Gao Y, Naqvi NI, Parinov S (2006) Trans-kingdom transposition of the maize dissociation element. Genetics 174 : 1095–1104. 16951067
58. Ansai S, Inohaya K, Yoshiura Y, Schartl M, Uemura N, et al. (2014) Design, evaluation, and screening methods for efficient targeted mutagenesis with transcription activator-like effector nucleases in medaka. Dev Growth Differ 56 : 98–107. doi: 10.1111/dgd.12104 24286287
59. Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, et al. (2010) Sphingolipid analysis by high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Adv Exp Med Biol 688 : 46–59. 20919645
60. Matsui H, Taniguchi Y, Inoue H, Kobayashi Y, Sakaki Y, et al. (2010) Loss of PINK1 in medaka fish (Oryzias latipes) causes late-onset decrease in spontaneous movement. Neurosci Res 66 : 151–161. doi: 10.1016/j.neures.2009.10.010 19895857
61. Lee BR, Kamitani T (2011) Improved immunodetection of endogenous alpha-synuclein. PLoS One 6: e23939. doi: 10.1371/journal.pone.0023939 21886844
62. Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, et al. (2000) Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J Neurosci 20 : 6898–6906. 10995834
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



