-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAsymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
At key moments in development, asymmetric cell divisions give rise to daughter cells of differing characteristics, a process that promotes cell-type diversity in complex organisms. The first cell division of the C. elegans early embryo is a powerful model for understanding asymmetric cell division because the timing of divisions and the placement of their division planes are precise and reproducible. We surveyed the mRNA content of each daughter cell in the C. elegans 2-cell embryo using low-input RNA sequencing. We identified several hundred asymmetric transcripts and tested them for functions in development. We found that the gene neg-1 produced mRNA and protein preferentially on the anterior (head-side) of 2-cell and 4-cell stage embryos and that loss of neg-1 led to consequences in anterior morphogenesis later in development. We also analyzed the asymmetric transcripts using quantitative microscopy, bioinformatics comparisons with previously existing datasets, and RNA sequence motif discovery to gain insight to the mechanisms by which asymmetric abundance patterns arise.
Published in the journal: . PLoS Genet 11(4): e32767. doi:10.1371/journal.pgen.1005117
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005117Summary
At key moments in development, asymmetric cell divisions give rise to daughter cells of differing characteristics, a process that promotes cell-type diversity in complex organisms. The first cell division of the C. elegans early embryo is a powerful model for understanding asymmetric cell division because the timing of divisions and the placement of their division planes are precise and reproducible. We surveyed the mRNA content of each daughter cell in the C. elegans 2-cell embryo using low-input RNA sequencing. We identified several hundred asymmetric transcripts and tested them for functions in development. We found that the gene neg-1 produced mRNA and protein preferentially on the anterior (head-side) of 2-cell and 4-cell stage embryos and that loss of neg-1 led to consequences in anterior morphogenesis later in development. We also analyzed the asymmetric transcripts using quantitative microscopy, bioinformatics comparisons with previously existing datasets, and RNA sequence motif discovery to gain insight to the mechanisms by which asymmetric abundance patterns arise.
Introduction
Asymmetric cell divisions produce daughter cells of different size, molecular content, or developmental potential. These events promote tissue-type diversity in developing embryos, specify terminal differentiation, and allow for the maintenance of adult tissues [1, 2]. Asymmetric cell divisions trigger divergent cell fates through the unequal distribution of cell fate determinants or by moving daughter cells into different morphogen fields [3, 4]. Searches to identify intrinsic cell fate determinants and the mechanisms that guide their asymmetric distribution have been difficult to adapt to high-throughput strategies. A key challenge is separating daughter cells with sufficient purity and yield for genome-wide and proteome-wide assays. We sought to overcome this challenge in Caenorhabditis elegans by coupling a low-input RNA-seq protocol [5, 6] with hand-dissection of blastomeres, which ensures absolute purity in each pool of isolated cells (Fig 1A).
Fig. 1. Differential transcript abundance in AB and P1 blastomeres following the first embryonic division. 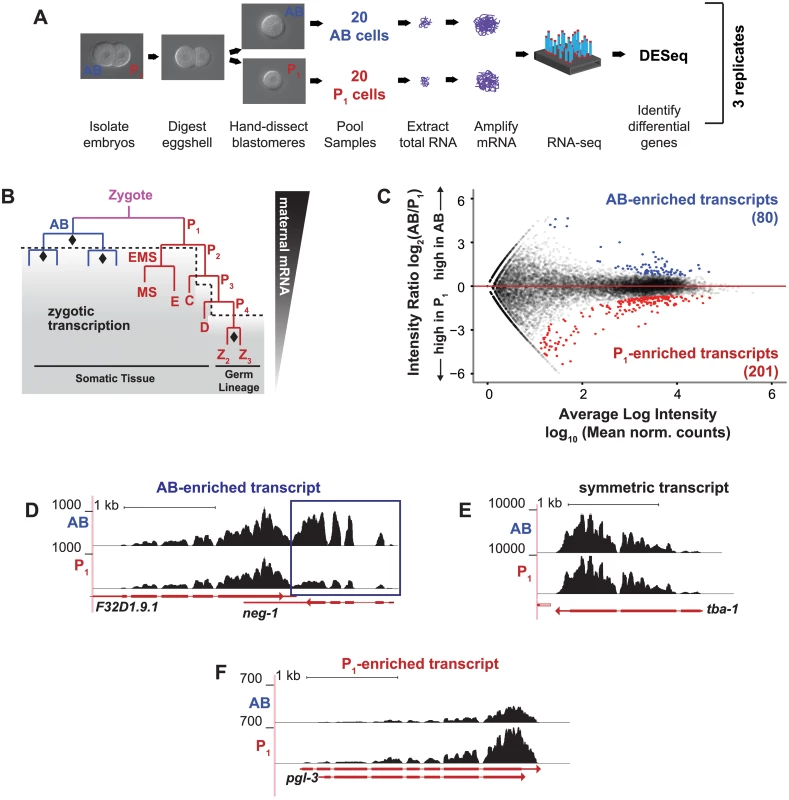
(A) C. elegans wild type embryos were harvested from gravid adults. AB and P1 blastomeres were separated by microdissection, and mRNA was extracted, amplified and sequenced. (B) The first cell division is asymmetric and creates the anterior AB cell and posterior P1 cell. RNA in the AB and P1 cells is exclusively maternal prior to the onset of zygotic transcription (dashed line, shading). Symmetric cell divisions are marked by diamonds. (C) For each gene, the normalized ratio of RNA-seq read counts between P1 and AB is plotted against the mean normalized read count for each transcript (Materials and Methods). Asymmetrically abundant transcripts are shown in blue (AB-enriched) and red (P1-enriched). (D) RNA-amp-seq traces for an AB-enriched transcript (neg-1, boxed in blue) (E) Same as D for a symmetric transcript (tba-1) (F) Same as D for a P1-enriched transcript (pgl-3). In C. elegans, an asymmetric cell division cleaves the recently-fertilized zygote into a larger anterior AB cell and a smaller posterior P1 cell, each of which has distinctive characteristics and fates (Fig 1B) [7]. The AB cell undergoes a series of rapid, symmetric cell divisions to eventually produce hypodermal, muscle, and neuronal tissue [8–10]. The smaller daughter cell, P1, is rich in perinuclear bodies of ribonucleoparticles called P granules. Unlike the AB cell that undergoes symmetric cell division, the P1 cell initiates a series of successive asymmetric cell divisions in which the smaller cell progressively inherits P granules and primordial germ cell fate and the larger cell becomes somatic tissues such as hypodermal, intestine, neuronal, and muscle tissues [8, 11, 12]. The somatic branches of the AB - and P1-lineages are transcriptionally active but the germ cell branch of the P1 lineage remains largely transcriptionally quiescent with some notable exceptions [13–16] (Fig 1B).
Four features make the first division of C. elegans embryogenesis an excellent model for studying the apportionment of mRNA through asymmetric cell division. First, the cell divisions of the early embryo are invariant and cell fates are precisely mapped [8, 13, 15–19], allowing one to connect any mRNA asymmetries to functional consequences later in embryogenesis. Second, many of the mechanisms and proteins responsible for polarity and asymmetry have been identified, ultimately allowing the mechanisms that drive mRNA partitioning to be placed into an established framework [20]. Third, zygotic transcription does not initiate until the 4-cell stage and becomes widespread at the 16-cell stage [13, 15, 21], meaning that RNA segregation, stabilization, and degradation can be observed independent of de novo transcription. Fourth, a few transcripts have been previously identified as asymmetrically abundant at this stage using in situ hybridization (mex-3, pos-1) allowing for verification by independent methods [22–24].
A previous study [16] identified 14 AB-enriched mRNA transcripts and 4 P1-enriched transcripts using a single-cell RNA-seq approach, in which only one cell is measured in each sample. Because our goal was to obtain as comprehensive a set of asymmetrically patterned genes as possible and to guard against cell-to-cell variability and noise, each of our samples consisted of a pool of 20 individual hand-dissected cells. By pooling AB and P1 cells, we were able to achieve lower variance in our samples (S2 Fig), allowing us to identify 80 AB-enriched and 201 P1 enriched transcripts. This is consistent with other studies showing that pooling cells to quantities of 20-cells or more buffers against cell-to-cell variation and yields more reliable and reproducible quantification [25]. The larger number of asymmetric transcripts we discovered allowed us to derive common properties of mRNAs that are asymmetrically partitioned in the early embryo. We complemented our transcriptome profiling approach with quantitative microscopy to verify our findings and further resolve patterns of mRNA distribution. We also used our dataset to identify neg-1 as a gene newly discovered to be important for anterior morphology.
Results
Determination of RNA abundance in AB and P1 blastomeres by RNA-amp-seq
We developed a low input RNA-seq protocol (RNA-amp-seq) that maintains relative transcript abundance and minimizes the potential for contamination. Total RNA was amplified by in vitro transcription (Eberwine amplification) of a low input cDNA pool [26], and amplified RNA was subjected to RNA-seq. Transcript abundance determined by RNA-amp-seq had a high correlation to standard RNA-seq (r2 = 0.84; S1A Fig) and preserved calls of enriched and depleted transcripts (S1B Fig). Although there is high concordance between amplified and unamplified samples, some transcripts may not be captured by the amplification procedure, resulting in false negatives. These results agree with previous studies demonstrating that in vitro transcription amplifies whole transcriptomes linearly, preserving relative transcript abundances [6, 16, 27].
To obtain AB and P1 specific transcriptomes, we isolated AB and P1 from 2-cell stage embryos. Two-cell stage embryos were removed from their eggshells with chitinase and chymotrypsin treatment and were extracted from the remaining envelope through mechanical sheering. AB and P1 cells were separated using a hollow-tipped glass needle attached to a mouth aspirator [28, 29]. Once separated, the two blastomeres were clearly distinguishable by their relative sizes. Twenty matched AB and P1 cells were pooled for each of three replicates (Fig 1A). We performed RNA-amp-seq and identified transcripts with statistically significant differential abundances [30–33]. Eighty of these RNAs were enriched in the anterior AB cell and 201 in the posterior P1 cell (Fig 1C–1G) with a Benjamani-Hochberg-adjusted P value of less than 0.10. For some analyses, these were ranked by adjusted P value (S1 Dataset, S1 Table).
Complementary techniques confirm asymmetrically abundant transcripts
We used several independent methods to confirm subsets of our identified asymmetrically abundant mRNA transcripts.
First, we compared asymmetric transcripts to an existing database of C. elegans RNA in situ hybridization images [34]. Note that the in situ database was created systematically and was not designed or optimized to detect transcript asymmetries at the two-cell stage. Therefore, a lack of a discernable positive signal was not evidence of a false positive in our dataset. We queried in situ hybridization entries for our 80 AB-enriched, 201 P1-enriched, and the 80 symmetric transcripts with the most uniform distribution (of 7664) (Fig 2A–2D, S2 Dataset). Many transcripts were absent from the online database, showed no staining, or were uninterpretable. Entries that had successful staining were scored in a blind survey that was used to generate a symmetry score. We found a strong association between entries that were identified as AB-enriched or P1-enriched by our RNA-seq analysis and those that yielded either high or low symmetry scores, respectively (Fig 2A–2D, S2 Dataset). Rates among all in situ images with positive staining were less than 3% AB-enriched and less than 1% P1-enriched (97% symmetric) when we queried a random set of 100 genes present at the 2-cell stage. Given that the hybridizations were not optimized for detecting cell-to-cell variation at the 2-cell stage of development, it is remarkable that so many of our transcripts were validated by this resource.
Fig. 2. Complementary methods, in situ hybridization and qRT-PCR, support RNA-seq measurements. 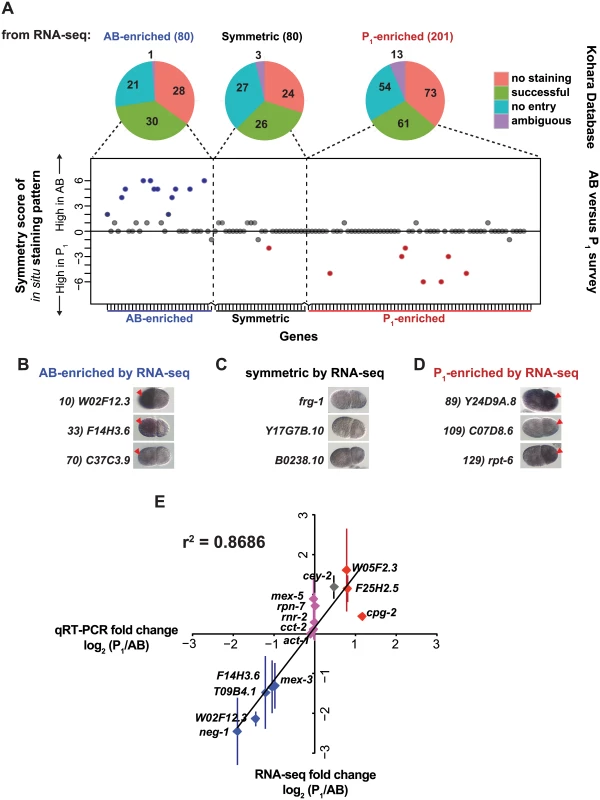
(A) The 80 AB-enriched, the 201 P1-enriched, and a subset of the middle 80 symmetric transcripts (S3 Dataset) were queried in a publically available collection of in situ hybridization images [34]. Pie charts show the proportion of transcripts with no entry (blue), successful staining (green), those that were in the database but showed no signal in any of the samples (No staining, red), and those that were otherwise interpretable due to overexposure, focus, or other problems (ambiguous, purple). Successfully stained entries were scored in a blind survey, and their results are plotted for each entry queried. Transcripts with scores higher than 2 are marked in blue and lower than 2 are marked in red. (B) Representative in situ hybridization images of AB-enriched transcripts [34]. The anterior AB cell is always oriented to the left of the posterior P1 cell. Red arrowheads indicate the cell with higher observed signal. All images in B, C, and D were taken from the Nematode Expression Data Base (http://nematode.lab.nig.ac.jp/db2/index.php). (C) As in B for symmetric transcripts (D) As in B for P1-enriched transcripts. (E) qRT-PCR was performed on pools of 5 AB and matched P1 cells for a subset of genes classified as AB-enriched (purple), P1-enriched (red), and symmetric (pink) by RNA-seq. Fold-change (P1/AB) as measured by qRT-PCR (Y-axis) and by RNA-seq (X-axis) is plotted. Each data point represents at least 2 independent biological samples, each of which was measured in at least 2 technical replicates; standard error of the mean is shown for biological replicates. Second, we performed qRT-PCR on pools of five blastomeres to measure the abundance of individual RNA transcripts. We selected transcripts to test from each set of genes: AB-enriched, P1-enriched, and symmetric. The ratio of transcript abundance (P1/AB) fold change determined by qRT-PCR was highly correlated with that determined by RNA-seq (r2 = 0.86) (Fig 2D). One caveat to this technique is that reliable quantification requires a linear standard dilution series for each primer set. Only transcripts with mean RNA-seq abundance values over 15,000 passed this requirement and were quantifiable by this method.
We also compared our data to the few transcripts whose mRNA localization had been previously characterized at the 2-cell stage of development. The mex-3 mRNA transcript is preferentially abundant in the AB cell [23], pos-1 is P1-enriched [24], and cey-2 appears initially uniform in early 2-cell stage but becomes P1-enriched in late 2-cell stage [22]. Of these transcripts, mex-3 appeared in our set of statistically significant AB-enriched transcripts. However, neither cey-2 nor pos-1 appeared on our list of P1-enriched transcripts. Though cey-2 and pos-1 were enriched 1.4 fold and 1.2 fold in the P1 cell in our dataset, they did not meet the conservative FDR-adjusted P value threshold we used. The transcripts for ama-1, eft-4, dpy-3, act-1, and tba-1 were previously characterized as having uniform distribution at the 2-cell stage [22] and appear among our symmetrically patterned transcripts. Further, a recent study reported that a transcript we identified as AB-enriched, W02F12.3, was quantifiable as AB-enriched by microscopy [35].
There was high concordance between the AB and P1-enriched transcripts reported in Hashimshony et al. [16] and those identified by our study (S2B and S2C, Fig). Five of the 14 genes previously identified as AB-enriched were also classified as AB-enriched in our study, and 3 of 4 P1-enriched genes previously identified were also in our set of 201 P1-enriched genes (S2B and S2C, Fig). Both Hashimshony et al. and our study failed to identify cey-2 and pos-1 transcripts as P1-enriched, which had been previously shown by in situ hybridization [22, 24].
Single-molecule RNA FISH revealed subcellular localization of asymmetric transcripts
The limitations of qRT-PCR and the publicly available in situ hybridization datasets motivated us to employ an alternative method of measuring mRNA transcript abundance. We performed quantitative in situ hybridization, also known as single-molecule FISH (smFISH). In separate experiments, we used probes designed to hybridize to three AB-enriched transcripts (mex-3, rank #4; neg-1, rank #42; tes-1, rank #77 of 80 total), three P1-enriched transcripts (chs-1, rank #1; pgl-3, rank #32; bpl-1, rank #170 of 201 total), and three symmetric transcripts (gpd-2, set-3, and B0495.7). We chose mex-3 because it has been previously reported to be AB-enriched and neg-1 because of our interest in this gene specifically. Other candidates were chosen to span a range of expression levels and to represent asymmetric transcripts at the top and bottom of our RNA-seq-based P value ranked lists. For each probe set, in each cell of the 2-cell embryo, both the number of fluorescent particles and the total fluorescence generated by the particles were quantified (Fig 3A–3F, S4A and S4B Fig). We report the ratio of “particle density”, where “particle density” is the volume-normalized count of fluorescent particles in the P1 cell as compared to the AB cell (Fig 3A–3F). In the case of mex-3, which is known to be enriched in AB, and neg-1, which was discovered to be AB-enriched by RNA-seq in this study, quantitative analysis confirmed AB enrichment (Fig 3A and 3B).
Fig. 3. Quantitative in situ hybridization using smFISH probes. 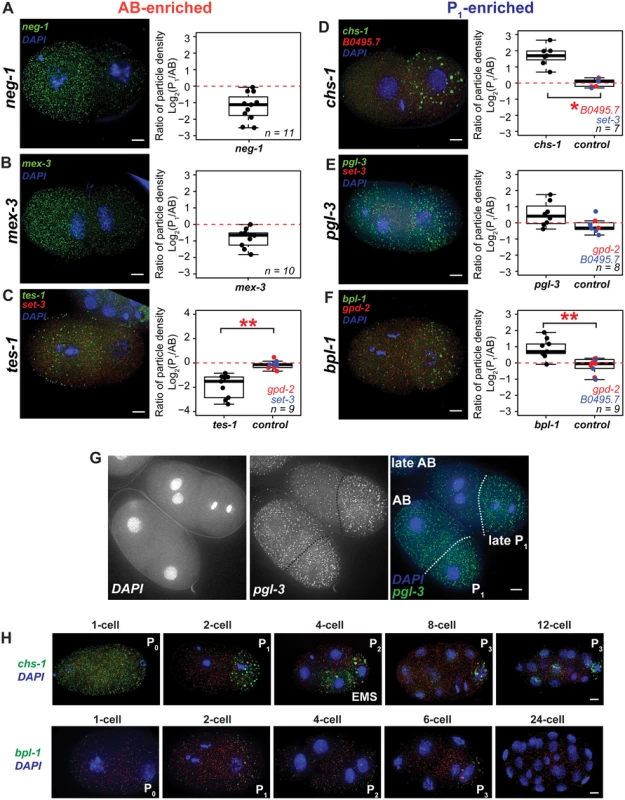
(A-F) Three AB-enriched, three P1-enriched, and three symmetric transcripts were imaged using sets of smFISH probes. (A-B) Images of wild type (N2) embryos grown at 20°C, fixed, and hybridized with mex-3 or neg-1 probes are shown. Ratios of particle density are reported (see Materials and Methods). Positive values indicate greater P1 cell particle densities; negative values indicate greater AB cell densities. neg-1 ranked #4 and mex-3 ranked #42 of 80 AB-enriched transcripts when ordered by P value. neg-1 (n = 11); mex-3 (n = 10). (C-F) tes-1, chs-1, pgl-3, and bpl-1 were multiplexed with control probe sets labeled with a different fluorophore (hybridizing to either gpd-2, set-3, or B0495.7) to image both an asymmetric and symmetric transcript within the same embryo. tes-1 was ranked #77 of 80 AB-enriched transcripts. chs-1 (rank #1), pgl-3 (rank #32), and bpl1 (rank #170) were among 201 P1-enriched transcripts. *P < 0.05; **P < 0.005, calculated using the Wilcoxon signed-rank test for paired samples. tes-1 (n = 9); chs-1 (n = 8); pgl-3 (n = 7); bpl-1 (n = 9). (G) P1-biased density of pgl-3 particles was more dramatic in late 2-cell stage embryos undergoing mitosis. (H) chs-1 and bpl-1 transcripts in fixed embryos over several stages of early embryonic development. All embryos are shown counterstained with DAPI. Maximal projections of optical sections are shown. Scale bars 5 μm. We next performed multiplex FISH with dual probe sets, which allowed us to compare an asymmetric transcript to a symmetric transcript within the same embryo. This approach provided an internal control and buffered against variations in hybridization efficiency. We tested for significant differences in the ratios of particle densities (P1/AB) of tes-1 (AB-enriched) and chs-1, pgl-3, and bpl-1 (P1-enriched) relative to gpd-2, set-3, or B0495.7 (symmetric transcripts). The three symmetric transcripts were nearly always within a 2-fold range of abundance in the AB and P1 cells (Fig 3C–3F). In contrast, tes-1 was up to 8-fold enriched in the AB cell relative to P1 (Fig 3C), confirming AB enrichment. This is important because tes-1 is a low-abundance transcript that is not reliably detectable by qRT-PCR. It is the 77th of 80 AB-enriched asymmetric transcripts ranked by P value. Thus, we have observed that even transcripts near the bottom of our asymmetrically abundant lists can exhibit reproducible, quantitative, and microscopically verifiable asymmetric patterns.
Three transcripts that were P1-enriched by RNA-seq were tested by smFISH (chs-1, pgl-3, and bpl-1). Of these, chs-1 and bpl-1 transcripts were P1-biased relative to internal controls (Fig 3D–3F). chs-1 and bpl-1 are ranked at the top and the bottom of the RNA-seq P1-enriched list of transcripts respectively. pgl-3 was also tested but did not show statistically significant differences between an internal control (Fig 3E). Instead, pgl-3 showed high variability in P1-enrichment ranging from marginal AB-enrichment to 4-fold higher P1–enriched particle density. We noticed that cells with higher ratios of P1 particle density were often later 2-cell stage embryos (Fig 3H) indicating that variability may be due to a greater degree of asymmetry in older 2-cell embryos, and a lesser degree in 1-cell embryos.
We also noted a qualitative difference in the chs-1 hybridization signal. The fluorescence particles appeared much larger than signal produced from other transcripts. This granular pattern was suggestive of P granule localization (Fig 3D–3I). Because of their granular nature, we were not able to quantitate these transcripts with reliable single molecule resolution. For this reason we also measured total fluorescence for all our experiments. However, even in the case of chs-1, which had the most notable granular patterning, the ratios and statistics calculated from total fluorescence density measurements closely paralleled measurements for particle density (S4 Fig).
chs-1, pgl-3, and, bpl-1 continue to associate with posterior cells in subsequent embryonic cell divisions
smFISH allowed us to observe RNA abundance patterns in embryos throughout development. The chs-1, pgl-1, and bpl-1 transcripts exhibited uniform transcript abundance in 1-cell stage embryos whereas transcripts in 2-cell stage embryos were localized asymmetrically (Fig 3G and 3H, S5 Fig). This suggests that differential transcript degradation or stabilization may contribute to pattern these particular mRNAs. Cell-specific distributions continued for chs-1 and bpl-1 transcripts, with particles concentrated in one or two posterior cells over the course of several cell divisions (Fig 3H).
Time-lapse microscopy following dsRNA treatment reveals a function for neg-1 in development
We hypothesized that genes encoding asymmetrically abundant transcripts might have lineage-specific roles in the development of the early embryo. At least one known example supports this idea. MEX-3 mRNA and protein are both distributed preferentially to the AB cell and mex-3 is required for proper AB lineage specification. We used RNAi to survey the knockdown phenotypes of 33 of our asymmetrically abundant transcripts (Fig 4A). Though RNAi assessments have been performed in the early embryo for some of these genes as part of systematic studies [36–42], we re-tested these 33 genes and scored and ranked relative embryonic lethality under uniform and controlled conditions. RNAi corresponding to 9 of the 33 genes tested yielded greater than 5% embryonic lethality in either wild type (N2) or sensitized (rrf-3) worms [40] (Fig 4A).
Fig. 4. neg-1 is required for morphogenesis. 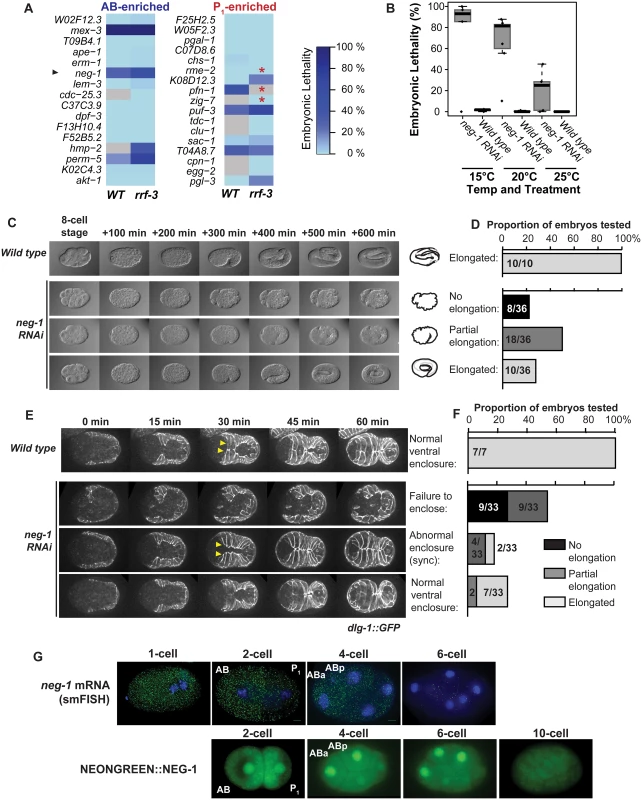
(A) The embryonic lethality resulting from RNAi depletion of asymmetrically abundant transcripts in wild type (N2) and RNAi sensitive (rrf-3) strains is shown. Embryonic lethality is depicted using the illustrated colorscale. Samples not tested are colored in gray. Worms that showed reduced brood size in response to RNAi feeding are marked with a red asterisk. (B) neg-1 dsRNA injection is lethal and cold sensitive. Worms were grown at 20°C, injected with dsRNA or left uninjected at L4, and then moved to 15°C, 20°C, or 25°C. Embryonic lethality for the resulting embryos was scored for 3–4 replicates of 6 worms each. (C) Time-lapse microscopy of embryonic development from neg-1 dsRNA-injected mothers at 20°C. Wild type embryos hatched normally under these conditions, but neg-1 depleted embryos exhibited a range of elongation phenotypes from showing no signs of elongation, to partial elongation, to complete elongation. (D) Tabulation of embryo developmental phenotypes shown in (C). (E) dlg-1::GFP embryos depleted of neg-1 were imaged and compared by time lapse microscopy at 20°C to wild type embryos. dlg-1::GFP is a fluorescent marker for apical adherens junctions of the hypodermal and gut epithelial cells. neg-1 depletion disrupted ventral enclosure. Leader cells are noted by yellow arrowheads. (F) Tabulation of the dlg-1::GFP phenotypes shown in (E). Hypodermal cell defects were predictive of a failure to hatch. (G) Visualization of neg-1 mRNA transcripts in fixed, wild type (N2) embryos using smFISH probes hybridized to neg-1 and DAPI as a nuclear stain. Scale bar 5 μm. Below, live embryos expressing NEONGREEN::NEG-1 driven from a construct that included the full length NEG-1 3’UTR and 100 bp of downstream sequence. We next tested whether any of these 9 genes with embryonic lethality phenotypes were involved in lineage-specific functions by performing timecourse microscopy after dsRNA injection. RNAi to neg-1 yielded the most dramatic anatomical defects, so we selected this gene for further study (Fig 4B–4D). neg-1 encodes a small protein of unknown function predicted to contain an unstructured N-terminus and a potentially structured and positively charged C-terminus [43]. The only known homologs to this protein occur in C. elegans and C. brenneri. F32D1.6 has recently been named neg-1 (Negative Effect on Gut development 1).
neg-1 is required for morphogenesis
We found that neg-1 RNA was enriched and highly abundant in the anterior AB cell (Fig 1D, Fig 2E, Fig 3A). Disruption of neg-1 by RNAi led to partially-penetrant embryonic lethality, with a median lethality rate of 96% by injection at 15°C, 81% at 20°C and 30% at 25°C (Fig 4B), and thus was cold-sensitive. Lethality was lower by RNAi feeding (30% at 20°C), which is consistent with previously published studies of neg-1 RNAi feeding, which reported 40% lethality [44] (Fig 4A).
To achieve a more detailed characterization of the cause of embryonic lethality, we performed time-lapse microscopy on embryos from mothers that either were or were not injected with neg-1 dsRNA (Materials and Methods) (Fig 4C and 4D). These experiments were conducted at 20°C. Among neg-1 depleted embryos, 22.2% arrested without apparent signs of elongation, 50% arrested with partial elongation and 27.7% elongated. In embryos undergoing partial elongation, we noticed a failure of the cells in the anterior of the embryo to enclose, which likely caused the elongation defect. During this time, development of the posterior continued normally (Fig 4C and 4D). This is reminiscent of hammerhead (hmr-1) mutation in that the hypodermis failed to enclose the anteroventral regions of the embryo leading to a failure of elongation [45]. It is also similar to humpback (hmp-1, hmp-2) phenotypes although we did not observe the classic dorsal rippling or bulging that is typical of those mutants [8, 45, 46].
We suspected that the primary defect in neg-1 depleted embryos was a failure of the hypodermis to fully enclose. During elongation, hypodermal cells encircle the embryo by extending from the dorsal half of the embryo and expanding to the ventral side, meeting and forming junctions at the ventral midline [47]. Once the ventral enclosure is complete, circumferential constriction of seam cells (a file of mid-body cells) and hypodermal cells powers the extension of the body into an elongated shape [48]. To more precisely monitor hypodermal cell behavior in neg-1 knock-downs, we performed time-lapse microscopy in a dlg-1::GFP strain that marks apical adherens junctions of epithelial cells of the hypodermis and gut [49]. We found that in 55% of neg-1 RNAi-treated embryos (18/33), hypodermal cells were misshapen prior to ventral enclosure (Fig 4E and 4F). Within this group, 9 embryos failed to complete ventral enclosure and did not undergo elongation (Fig 4E and 4F). The other 9 did not complete ventral enclosure on the anterior end and only partially underwent elongation. In these embryos, elongation led to an organized posterior region of the worm but forced internal cells out through the unenclosed anterior leading to the hammerhead-like phenotype. Interestingly, 18% (6/33) of embryos exhibited hypodermal cell enclosure that was largely normal, but mistimed. Typically, two actin-rich “leader” cells on flanking sides of the body are the first to meet at the ventral midline and are then followed by the more-posterior hypodermal cells. In the subset of embryos with defective timing, the leader cells and posterior cells met synchronously at the ventral midline. Of these 6 mistimed-enclosure embryos, 4 failed to complete elongation, whereas the other two hatched. The remainder of embryos (9/33, 27%) exhibited normal hypodermal morphogenesis, and of these, 7 of 9 hatched (Fig 4E and 4F). While there was a strong relation between abnormal behavior of hypodermal cells in neg-1 compromised embryos and the likelihood of successful hatching (Fig 4F), the correlation was not perfect, suggesting that processes other than hypodermal cell behavior are also defective when neg-1 is lacking.
mRNA abundance usually, but not always, correlates with protein production. To test whether anterior neg-1 mRNA enrichment correlated with NEG-1 protein enrichment, we compared smFISH staining for neg-1 mRNA to fluorescence in worms harboring a NeonGreen::neg-1 protein fusion. As visualized by smFISH, neg-1 mRNA appeared uniform in the 1-cell fertilized embryo, became localized to the anterior in the AB cell and its daughters, and then declined dramatically in signal during the 6–10-cell stages (Fig 4G). We observed NeonGreen fluorescence in the ABa and ABp nuclei at the 4-cell stage. NeonGreen persisted in the anterior nuclei of the 6-cell and 8-cell stage embryos, but then decreased by the 10–32-cell stages. Therefore, anterior enrichment of neg-1 mRNA preceded fluorescent protein accumulation by one cell cycle, and the pattern of anterior-localization between mRNA and protein was correlated.
Gene categories associated with AB-enriched transcripts are different from those associated with P1-enriched transcripts
Having identified an anterior transcript required for embryogenesis and required for full function in many AB-derived hypodermal cells, we asked whether asymmetric transcripts had functions associated with the lineage in which they were enriched. Transcripts that accumulated in AB were enriched in GO biological process categories [50] that were distinct from the categories enriched in P1 transcripts (Fig 5A). Categories associated with the AB transcripts included organelle organization, cellular organization, chromosome organization, and cell-cycle processes, locomotion, and morphogenesis (Fig 5A, S5 Dataset). The AB cell lineage undergoes more rapid cell cycle progression than the P1 lineage [8,9,49,50], which explains the enrichment for genes involved in cell cycle processes.
Fig. 5. Functions associated with AB and P1-enriched transcripts. 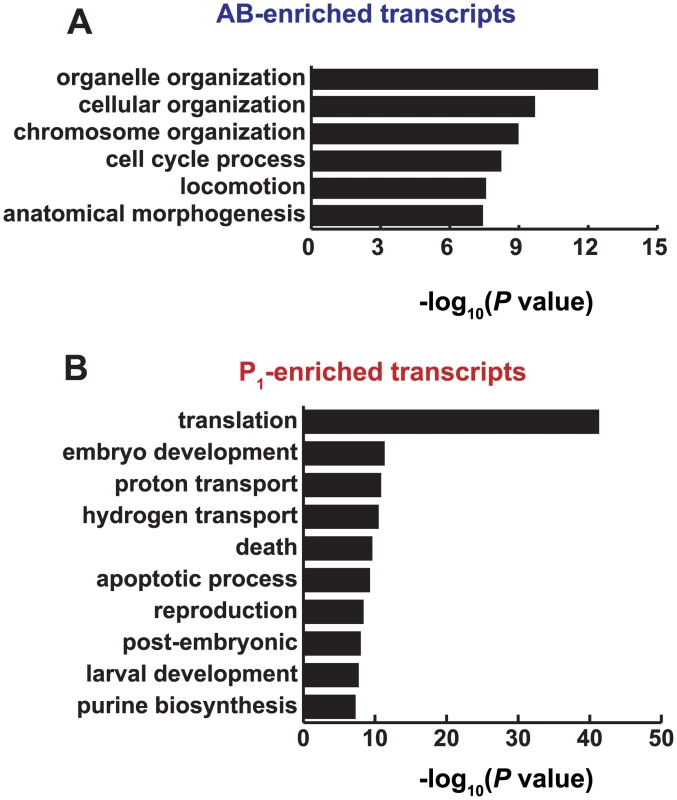
(A) GO ontology terms over-represented in genes whose transcripts were AB-enriched at the 2-cell stage of development. Categories were identified using the GOrilla algorithm for identifying categorical significance from ranked lists [50] and were summarized using the REViGO algorithm [51]. (B) Same as A for P1-enriched. Transcripts preferentially abundant in the posterior P1 cell were associated with translational control, embryo development, proton transport, and cell death (Fig 5A, S5 Dataset). Although translational control is prevalent throughout C. elegans embryogenesis, germ line development is especially dependent on translational control due to the transcriptional quiescence of this lineage until later stages of embryogenesis [12, 52, 53]. Competition and cooperation among RNA-binding proteins, some of which are asymmetrically distributed in the AB and P1 lineages, control translational repression and activation of maternally inherited mRNAs [54]. For example, MEX-3 is required for full translational repression of PAL-1, NOS-2, GLP-1, and ZIF-1 in anterior blastomeres [23, 55–57], and POS-1 and GLD-1 account for translational repression of key transcripts in posterior blastomeres [24, 55, 58].
Maternal degraded transcripts are over-represented among asymmetric transcripts
Maternally loaded mRNA transcripts of the C. elegans early embryo have been previously categorized into classes according to the dynamics of their distribution. Whereas Class I mRNAs are maintained in the embryo over time, Class II mRNAs are progressively degraded in somatic blastomeres. Known Class II mRNAs are limited to a few examples such as nos-1 and nos-2, which degrade in somatic cells beginning at the 4-cell stage but co-localize with the P granules in the P lineage [59]. Baugh et al. identified 1749 genes that produced “Maternal Degradation” [MD] transcripts by microarray time-course assays on whole embryos. MD transcripts are hypothesized to contain Class II mRNAs as well mRNAs that are degraded in other spatial patterns [60].
AB-enriched and P1-enriched transcripts were almost twice as likely to be categorized as MD maternal mRNAs when compared to symmetrically distributed transcripts (43.9% [26/66] of AB-enriched, 51.0% of P1 enriched [25/147], and 26.1% [737/2815] of symmetric transcripts were MD; Fig 6A–6C). This suggests that asymmetrically distributed transcripts were more likely to decrease in relative abundance as embryogenesis progressed compared to symmetrically distributed transcripts. This decline in abundance could be due to a uniform degradation of the transcript across the whole embryo or a relative reduction in the amount of transcript as it becomes restricted to a specific cell lineage. Indeed, by in situ hybridization (Fig 3H, S4 Fig) transcripts asymmetrically abundant in the P1 cell often exhibited P2-specific localization at the 4-cell stage.
Fig. 6. Characteristics of asymmetrically abundant genes. 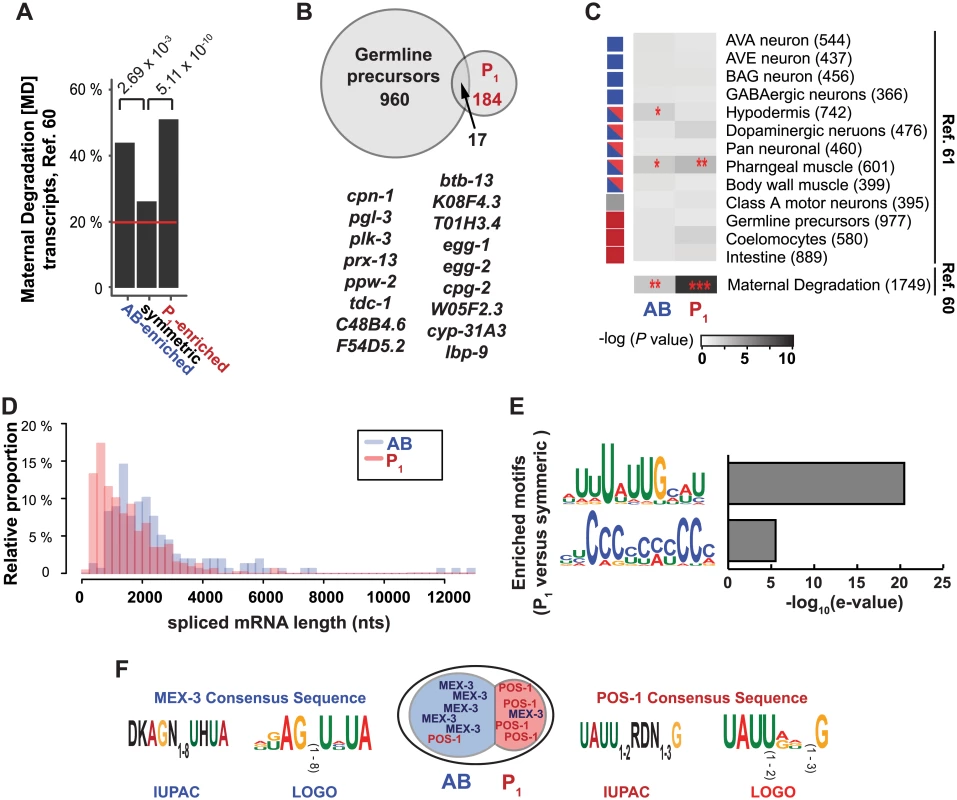
(A) The prevalence of Maternal Degradation [MD] transcripts (n = 1812) [60] among AB-enriched (n = 80), P1-enriched (n = 201), and symmetric transcripts (n = 2815). MD transcripts make up 19.8% of genes expressed at the 2-cell stage (horizontal red line). (B) Overlap between the P1-enriched gene set and transcripts enriched in Z2 and Z3 cells [61]. All individual genes that overlap are listed. (C) Association of cell-type specific transcripts [61] with the AB-enriched and P1-enriched gene sets. (*P < 5x10-2; **P < 5x10-3; ***P < 5x10-9, by Fisher’s exact test). Squares on the left indicate the lineage of origin for each tissue or cell type (blue = AB-lineage, blue and red = AB and P1 lineages, red = P1-lineage). For comparison, overlap between the MD transcripts (Fig 6A) is also plotted. (D) Lengths of spliced gene models for AB-enriched genes and P1-enriched genes plotted as a histogram. The P value for the likelihood that the two distributions were drawn from the same population was calculated using Wilcoxon rank-sum (Mann-Whitney U) test with continuity correction (P < 1.0x10-16 for all genes and P = 1.22x10-15 when the three outlying AB-enriched gene models were excluded). (E) Identification of de novo RNA sequence motifs specific to the 3′ UTRs of P1-enriched transcripts as compared to symmetric transcripts [62]. No significant motifs were identified between AB-enriched transcripts compared to symmetric transcripts. (F) POS-1 and MEX-3 proteins are known to be asymmetrically localized at the 2-cell stage of development. The consensus sequences required for POS-1 [63] and MEX-3 [64] recognition are shown as IUPAC codes and transformed into WEBLOGOs for ease of comparison with de novo logos (Fig 6E). Transcription is largely quiescent in the P1 cell and its descendants, so it is possible that maternal mRNAs are specifically sequestered in that lineage for later use in germ line development. The transcriptomes of the Z2/Z3 progenitor germ cells isolated from early embryos have been previously characterized by sorting them using Ppie-1::GFP::PGL-1 fluorescent markers [61]. We did not find a statistically significant over-representation of P1-enriched transcripts among Z2/Z3-enriched transcripts (Fig 6B and 6C). This indicates that P1-enriched transcripts are not set aside for retention in the Z2/Z3 germ line precursors at a higher rate than those found in the AB-enriched or symmetric sets at the 2-cell stage. However, individual P1-enriched transcripts may be of particular interest in germ line precursor biology. Indeed, transcripts that are both enriched in P1 and in Z2/Z3 include pgl-3, which encodes one of the two key scaffolding proteins of the P granules [65] (Fig 6B), and plk-3, a component whose presence is correlated with P granule development [66].
To determine whether asymmetric transcripts were associated later in development with a specific cell or tissue type, we asked whether AB - or P1-enriched transcripts were over-represented in any specific embryonic cell type [61]. We found significant associations between AB transcripts and hypodermal transcripts, AB transcripts and pharyngeal transcripts, and between P1 transcripts and pharyngeal transcripts (Fig 6C, S6 Dataset). However, we saw no clear association between AB-enriched transcripts and cell types that were exclusive to the AB-lineage or between P1-enriched transcripts and cell types that were exclusive to the P1-lineage. This suggests that the primary purpose of asymmetric transcript abundance following the first embryonic division is not to retain transcripts for cells that arise later in each lineage.
Together these results suggest that mRNAs asymmetrically abundant at the 2-cell stage tend to decline rapidly in relative abundance as embryogenesis progresses, but that only a small fraction of the corresponding transcripts are tissue-specific or lineage-specific later in development. These findings are consistent with the observation that the transcriptome undergoes waves of change throughout development [60]. These waves are shaped through RNA degradation and zygotic transcription [67, 68].
Distinct features and sequence content of asymmetric transcripts provide clues to potential mechanisms of asymmetric distribution
RNA-binding proteins recognize sequences and topologies in their RNA targets to affect localization, stabilization, polyadenylation, sequestration or degradation. We asked whether transcripts enriched in the AB or P1 cells shared RNA sequence features that could account for their patterning. Though we found no associations between 5′ splice leader usage, 5′ UTR length, 5′ UTR nucleotide composition, 3′ UTR length, or 3′ UTR sequence composition and cell-specific categories (S6A and S6B Fig), we found that short gene models (both spliced and unspliced) were associated with P1 enrichment, and longer gene models with AB-enrichment. The median AB-enriched transcript length was longer than the median length of symmetric transcripts, which in turn was longer than the median of P1-enriched transcripts (Fig 6D, S6C Fig). A large number of P1-enriched transcripts were very short (250–750 bp), a length class that was virtually non-existent in the AB set. Of the 73 genes whose models yielded spliced lengths of less than 750 bp and were P1-enriched, 13 were neuropeptide-like proteins (nlp and flp) and 14 were ribosomal protein components (rpl or rps).
We speculated that asymmetrically abundant transcripts might contain different RNA-binding protein associated sequence motifs or miRNA target sites. We searched de novo for RNA sequences that distinguished the AB-enriched or P1-enriched 3′ UTRs from those of the symmetric set. We identified two motifs over-represented in the 3′ UTRs of P1-enriched genes: UUUAUUGCAU and polyC (10-mer C) but none that were over-represented in the AB-enriched set of genes (Fig 6E) [62, 69, 70]. The UUUAUUGCAU motif is an Adenine and Uracil rich element (ARE) that contains features similar to known POS-1 and MEX-3 recognition sequences. The UAUU sequence is similar to the zinc-finger coordinated UAUU sequence found in the recognition sequence of the CCCH-type zinc finger protein POS-1 (UA(U2-3)RD(N1-3)G) and its human homolog, tristetraprolin (TPP-1, UAUUUAUU). The UUUAUUGCAU motif also resembles two stretches of UUUAUUGA within the nos-2 3′ UTR that are perfectly conserved in three Caenorhabditis species, are required for MEX-3 binding in vitro, and are required for preventing NOS-2 protein accumulation in anterior blastomeres [52, 56]. POS-1 and MEX-3 are two RNA binding proteins that are asymmetrically distributed in the 2-cell stage embryo and whose target sequence motifs have been described (Fig 6F). We noticed a striking similarity between the POS-1 target sequence and the P1-enriched sequence we identified (Fig 6E) suggesting that POS-1 or another CCCH-type zinc finger may function in the retention of P1-enriched transcripts.
Discussion
An expanded catalog of asymmetrically patterned mRNAs
We have expanded the knowledge of which mRNA molecules are asymmetrically distributed during the first cell division in C. elegans, a powerful model for asymmetric cell division and early development. Our approach led to the identification of neg-1. a gene that produces anterior mRNA and protein and is important for ventral enclosure and elongation, processes that involve hypodermal cells derived from the AB-lineage.
neg-1 is an anterior-enriched transcript that is important for anterior morphogenesis
We identified a transcript, neg-1, that is more abundant in the anterior cell following the first cell division of the C. elegans zygote. The protein product of this transcript is also preferentially localized to anterior nuclei, starting in the following cell division. Disruption of neg-1 by RNAi led to defective anterior morphogenesis due to failures in hypodermal cell ventral enclosure and elongation. The hypodermal leader cells (ABpraappap, ABpraapppa, ABplaappap, and ABplaapppa), the ventral cells posterior to the leader cells (ABpraapppp, ABplaapppp, and the P cells), and the seam cells (H0, H1, V1–V6, and T) are all derived from the AB lineage and are all actively involved in hypodermal cell enclosure along the ventral midline. The C founder cell yields other hypodermal cells but these are posterior and dorsally located and do not play as large a role in ventral enclosure [8, 71]. Thus, the data presented here show that the asymmetrically abundant neg-1 mRNA is associated with subsequent asymmetric protein abundance, and that loss of neg-1 function has consequences on anterior morphogenesis. It remains an open question as to how a protein whose anterior patterning is most striking at the 4-cell stage yields an RNAi phenotype 300 minutes later. NEG-1 may alter or regulate gene expression in the AB lineage, which would be consistent with its positive charge and concentration in the nucleus.
Asymmetric transcripts are associated with biological processes that differentiate AB and P1 lineages
The functional characterization of neg-1 in this manuscript indicates that cell-specific RNA profiling may be a fruitful way to identify transcripts and proteins that contribute to lineage-specific processes. We identified neg-1 based on its preferential abundance in the AB cell. With this in mind, other transcripts identified in our study warrant special attention with respect to their potential for lineage-specific functions. For example, cdc-25.3 was AB-enriched. Its homolog cdc-25.1 regulates relative cell-cycle rate in other lineages [9]. cdc-25.1 and cdc-25.3 may work together to regulate lineage-dependent cell-cycle rates. Many of the AB-enriched transcripts, such as erm-1 and hmp-2, are associated with epithelial adherens junctions, possibly foreshadowing the important role of hypodermal cell function among the progenitors of the AB lineage. Cytoskeletal regulatory genes enriched in the AB lineage such as cyk-1 and dnc-1 may indicate the requirement for greater cytoskeletal control in the AB lineage.
RNAs enriched in the P1 cell also function in processes known to be important to the P lineage. One such transcript encodes PGL-3, the protein that comprises the scaffold for P granules. Further, several RNAs encoding ribosomal protein genes and RBPs important for translation were over-represented in the P1 cell. This is consistent with the differential translation of specific mRNAs (glp-1, pal-1, and nos-2) in the anterior and posterior blastomeres that generates proteomic diversity prior to the onset of zygotic transcription [52, 57, 72]. Because key RBPs that regulate those mRNAs also bind other targets, cohorts of mRNAs may be coordinately regulated [73, 74]. The process of translation in general may be distinct between the P lineage and somatic lineages because P granules and germ granules contain mRNAs and proteins, such as IFE-1, that regulate general aspects of translational biology [75]. Future studies may address how P1-enriched transcripts associated with translation impact protein production in that lineage.
Asymmetric distribution of RNA molecules as a mechanism for cell fate determination
The search for asymmetrically partitioned cell-fate determinants has traditionally concentrated on differential gene expression patterns, which can arise through both transcriptional differences and differential post-transcriptional regulation of mRNAs. For example, ASH1 mRNA localizes to Saccharomyces cerevisiae bud cells and marks bud-versus-mother cell identity [76, 77], and the mRNA transcripts of gurken, bicoid, oskar, and nanos distribute asymmetrically in the Drosophila melanogaster embryo and are required for embryonic patterning (recently reviewed in [78] and [79]). The concentration of a particular mRNA transcript in a specific lineage can also be important for lineage-related functions even if it is not acting to determine the identity of that lineage directly.
Our study identified an asymmetrically distributed mRNA transcript, neg-1, which was important for morphogenesis. However, we have yet to establish whether neg-1 is a cell fate determinant or whether AB-enrichment of neg-1 mRNA is required for its function for embryogenesis. The AB-enrichment of neg-1 mRNA preceded an anterior enrichment of NEG-1 protein by one cell division, but it remains possible that the asymmetric RNA localization is not required for asymmetric NEG-1 protein localization. For example, translational control could be the primary mechanism shaping the proteomes of daughter cells. Translation of specific transcripts is regulated during oogenesis, early embryonic development, and in the germ cell lineage. It could be that the asymmetric mRNAs we observe arise as a downstream effect of translational control. Even if so, cataloging asymmetrically abundant mRNAs is likely to be an efficient way to identify genes that are spatially regulated and important for development.
How do mRNAs become asymmetrically abundant?
The initial symmetry of the round oocyte is broken by the ellipsoid shape of the fertilized egg and by the entry point of the sperm centrosome [7, 80, 81]. The PAR proteins transduce those polarity signals to create a polarized actinomyosin network, directional cytoplasmic flow, and a skewed arrangement of the mitotic spindle [82–86]. As a result, the division plane of first mitosis in the C. elegans zygote is situated closer to the posterior. This process involves progressive stages of polarity initiation, maintenance, and amplification. How is mRNA location and abundance regulated during this process? While a variety of mechanisms, including differential diffusion, stability, degradation, localization, sequestration, or polyadenylation may be regulating the RNA abundance patterns we identified in our study, our data provided two clues regarding where to look next.
First, we observed that AB-enriched transcripts were significantly longer than symmetric transcripts, and that these were, in turn, longer than P1-enriched transcripts. It is possible the shorter length of the P1-transcripts decreases the likelihood that they will contain miRNA binding sites or RNA-Binding Protein (RBP) target sequences, allowing for preservation of the shorter mRNAs in the P1 cell. Alternatively, the shorter 3′ UTRs may lack motifs that direct or stabilize transcripts in the AB cell. Finally, it is possible that bulk cytoplasmic flow within the zygote passively accumulates smaller transcripts in the posterior end of the cell, whereas longer transcripts are retained in the anterior side. Given that the actinomyosin network is known to adopt asymmetric qualities after fertilization [84] and given that poroelastic diffusion properties of the cytoplasm are highly dependent on actiomyosin [87], passive diffusion may contribute to differential distribution of molecules in the early embryo.
Second, we identified an AU-riche element (UUUAUUGCAU) that was over-represented among the P1-enriched transcripts. This motif bears striking resemblance to the recognition motif of POS-1 (UA(U2-3)RD(N1-3)G) but also bears similarity to the direct repeats (called DR1 and DR2) recognized by MEX-3 within the nos-2 3′ UTR (UUUAUUGA) [63, 74]. In general, AU-rich elements are associated with mRNA stability and decay via RBPs. Our identified sequence motif could be a MEX-3 recognition motif, a degenerate POS-1 motif, the binding motif for another RBP such as MEX5/6, or a motif that is bound combinatorially by multiple RBPs. nos-2 is translationally repressed in anterior blastomeres in a MEX-3-dependent manner [56], and nos-2 mRNA is degraded in anterior blastomeres in a MEX-5/MEX-6-dependent manner [52]. It is possible that multiple P1-enriched transcripts and their protein products are similarly restricted from anterior blastomeres. Alternatively, POS-1 or some other RBP may recognize this degenerate motif to sequester transcripts in the P1 cell.
In addition to these two observations derived from the bulk behavior of asymmetric transcripts in our RNA-seq approach, our smFISH observations also provide some guidance regarding mechanism. The transcripts chs-1 and bpl-1 were uniformly distributed in 1-cell embryos just before division and apparently became asymmetrically abundant after division occurred. In addition, the asymmetry of the pgl-3 transcript seemed more dramatic in late 2-cell stage embryos compared to early 2-cell embryos. This suggests that mechanisms responsible for asymmetric abundance of some mRNA transcripts are not wholly dependent on differential trafficking prior to cell division. Differential transcript stabilization or degradation may be responsible for generating cell-specific mRNA patterns throughout the cell cycle. We identified a PolyC sequence over-represented in the 3′ UTRs of P1-enriched transcripts. Recently, PolyC motifs were shown to be required for a wave of post-fertilization RNA degradation during the oocyte-to-embryo transition [88]. It is possible that the P1-lineage may be more resistant to this degradation than the AB-lineage.
The smFISH experiments also revealed that chs-1 and bpl-1 transcripts were present in larger granular particles. Despite the superficial resemblance to P granules, we were unable to determine whether these structures were P granules. Unlike P granules, the chs-1 and bpl-1 transcript granules did not appear to coalesce or posteriorly localize during prophase of the 1-cell stage embryo. It is possible that granular association promotes P1-enrichment of transcripts through sequestration or stabilization.
Asymmetric cell divisions are commonplace in development and influence fate decisions in systems ranging from plant stomata to cancer stem cells. The C. elegans early embryo is unique in the reproducibility of division timings and planes, the highly deterministic nature of the resulting lineages, and the lack of zygotic transcription in the earliest divisions. This study determined the mRNA abundance in each of the two cells created after the first zygotic cell division. This led to the identification of a new gene important for lineage-specific functions and clues regarding the post-transcriptional mechanisms that control cellular mRNA abundance in early embryogenesis.
Materials and Methods
Worm strains and isolations
N2 (wild type) worm strains were obtained from the Caenorhabditis Genome Center and maintained at 20°C unless otherwise indicated using standard protocols [89]. dlg-1::GFP (FT64) was obtained from the CGC.
Blastomere dissection
Blastomere isolations were performed by embryo isolation, eggshell degradation and micromanipulated separation as previously described [90]. We used chitinase from Streptomyces griseus (sigma, C6137) at 10 U/ml due to the recent unavailability of Serratia marcascens chitinase. Isolated AB and P1 cells were dropped directly into Trizol solution and stored at -80°C if not immediately processed.
RNA isolation
Blastomere preparations were thawed and re-frozen up to three cycles to lyse them. RNA extraction was performed using a Trizol/chloroform extraction followed by RNeasy Micro (Qiagen) preparation using On Column DNaseI Digestion (Qiagen).
mRNA amplification and sequencing
RNA amplification was performed by in vitro transcription of an oligo dT-primer fused T7 promoter producing up to a 10,000-fold increase in mRNA using one round of a TargetAmp aRNA Amplification Kit (Epicentre). The resulting in vitro amplified RNA was used for a standard RNA-seq protocol (Illumina mRNA Sequencing Sample Preparation Guide, September 2009). Magna beads were used for purification steps and for the final purification. Gel extraction was not necessary due to the size of in vitro amplified RNA fragments. Samples were sequenced on either GA-2 or Hi-Seq Illumina sequencers with either 36 or 50 rounds of sequencing. Data from this study are available on NCBI GEO (GSE59943)
Reviewer access: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE59943
Comparison between mRNA amplification and sequencing to traditional mRNA-seq
We sequenced 5 ug of unamplified RNA by the standard Illumina RNA-seq methodology. The same RNA sample was diluted down to 500 pg and sequenced using RNAamplification and sequencing.
RNA-seq analysis
Sequencing reads were filtered for primer and adapter sequences [91] and a minimal quality score of 20 using Tagdust (Version 1.12) [91], aligned to the C. elegans ce10 genome using Tophat (1.4.0) [30]. The resulting alignment files were quantified using HT-Seq (0.5.3) [33] and the RefSeq gene annotation for WS220 downloaded March 23, 2012 and reads were assigned per gene model. The significance of differentially represented transcripts were quantified by DESeq (1.2.1) [32] in R 2.12 [92] using size estimation normalization.
Differential expression analysis
Reads were called as present if the number of mapped reads met a minimum 50 reads in the mean of all samples and at least 1 read in the mean of either AB or P1 samples. We identified 7945 present genes of 20,240 of the predicted gene models. Present genes were called as giving rise to significantly asymmetric transcripts if they met a P value < 0.1 after FDR adjustment using the Benjamani-Hochberg method within the DEseq package (1.2.1) [32] in R 2.12 [92] (All other R analyses were performed in 3.0). When necessary, genes were ranked by negative log P value.
Comparison to publicly available in situ hybridization images
The 80 AB-enriched transcripts and 201 P1-enriched transcripts identified by RNA-seq were surveyed for entries in a publicly available database of RNA in situ hybridization images [34]. A subset of 80 (of 7664) symmetric transcripts were selected as a comparison. These 80 were the middle-most symmetric transcripts as ranked by log2 P1/AB fold change. Many transcripts were absent from the online database, showed no staining, or were uninterpretable for other reasons. Entries that had successful staining were scored in a blind survey we constructed. Multiple reviewers categorized sets of images from each entry as either “AB”, “Marginal AB”, “Symmetric”, “Marginal P1-biased”, P1”, “No expression”, or “Ambiguous”. The rubric for these categories is included as S1 Text. The full dataset including each reviewer’s response, the combined overall score, and links to publicly available images [34, 93] is included as S3 Dataset.
qRT-PCR
Pools of five P0, AB, and P1 blastomeres were isolated using dissection methods above and immediately processed using the CellsDirect cDNA kit (Invitrogen). The abundance of mRNA transcripts was quantified using the SYBR Maxima qPCR system (Thermo Scientific) on an ABI 7900HT Fast Real Time PCR System using a dilution series to calibrate relative abundance of each transcript. Only transcripts with an abundance over 15,000 mean reads passed the requirement of generating a linear standard dilution curve over a 1–1/1000 fold dilution series. Primers were designed to lie within the 3′ most exon and within 200 bp of the terminal codon. Each gene reported represents at least 2 independent biological samples, each of which was measured in 3 technical replicates; means of sample means and standard error of the mean were calculated.
smFISH
Stellaris smFISH fluorescent probe sets (Biosearch) were generated to hybridize to mex-3 neg-1, tes-1, chs-1, pgl-3, bpl-1, B0495.7, set-3, and gpd-2 (S2 Text). N2 worms were grown at 20°C to gravidity on NGM plates, bleached for embryos, resuspended in -20°C methanol, freeze cracked in liquid nitrogen, and fixed at -20°C for 2–48 hours. A protocol that combined Shaffer et al. [94] and Ji et al. [95] was performed. Embryos were equilibrated in WB1 (100 mg/ml dextran sulfate, 10% formamide, 2 x SSC), hybridized in hybridization buffer (100 mg/ml dextran sulfate, 1 mg/ml E. Coli tRNA, 2 mM vanadyl ribonucleoside complex, 0.2 mg/ml BSA) containing 50 pmoles of each primer set. Hybridization at 30°C overnight was followed by two WB1 washes, DAPI staining, and three 2 x SSC washes. Embryos were mounted as described in Ji et al. [95] using SlowFade (Life Technologies) to prevent photobleaching.
All smFISH images were acquired using a Cool Snap HQ2 camera on a DeltaVision-modified inverted microscope (IX71; Olympus), with a UPlanSApo 100 x (1.40 NA) objective and SoftWorx software (Applied Precision) using fixed exposure and acquisition conditions (0.3 μm z-stacks, up to three wavelengths). Images were deconvoluted. Quantification was performed using specialized scripts in ImageJ and R to identify thresholded particles, their pixel intensities, and their cell boundaries. The number of discrete particles was quantified and normalized to cell area (proportional to volume because cells are flattened). The total internal intensity of each particle was also measured, and the background-subtracted fluorescence was normalized to cell area. For statistical tests, two probes were multiplexed within the same embryo and Wilcoxon signed-rank tests were performed on the paired ratios.
RNAi feeding and embryonic lethality phenotyping
dsRNA feeding was carried out as previously described [96] by moving worms to RNAi feeding plates at L4 stages of development. 24 hours and 48 hours after feeding, worms were assessed for their ability to lay viable embryos by capturing all eggs laid within the next 24-hours and scoring for hatching and viability 48–72 hours later. For each strain queried, we performed 2–3 replicates in which 4–6 mothers were assayed at both the 24 - and 48-hour time points.
RNAi injection and 3D microscopy
dsRNA was produced using the T7 RiboMAX Expression System (Promega, P1700). RNA was injected into L4 stage worms as previously described [96, 97]. Images were acquired 24 hours post-injection for injected, uninjected, and mock-injected worms. Experiments were carried out at 20°C unless otherwise noted. RNAi knockdown and control worms were imaged on the same slide. Time-lapse images collected in Fig 5C, were acquired using Nomarski DIC optics on a Nikon Eclipse E800 using a Nikon 1.40 NA 60x objective as previously described in Dickinson et al. [98]. Images were collected every 2 minutes over 9 hours with z-stacks at 1 μm intervals for a total depth of 30 μm. For Fig 5D, images were captured on a spinning disc CSU-XI Yokogawa confocal system mounted on an inverted microscope (Eclipse Ti, Nikon), 16-bit cooled CCD camera (Image E, Hamamatsu), 50 mW air-cooled Arbon laser (Laser Physics), Nikon 1.4 NA 60x objective, and Metamorph Software. Images were captured every 15 min over 9 hours with z-stacks at 3 μm intervals for a total depth of 22 μm. Embryos were illuminated at 488 nm at 25% power, 500 ms, 1x1 bin for GFP settings and 100 ms, 1 x 1 bin for DIC settings.
mNEONGREEN::NEG-1::NEG-1-3′UTR transgenic worms
We fused the mex-5 promoter driving mneongreen (Allele Biotechnology) in frame, to the neg-1 gene beginning at its start site and containing its full 3’ UTR plus 100 base pairs downstream. This was cloned into a hygromyocin selectable MOSSCI plasmid using a pCFJ150 backbone [99] constructed using Gibson Assembly (New England BioLabs). The resulting mex-5promoter::neongreen::neg-1::neg-1-3’UTR plasmid was transformed into N2 worms, selected with hygromycin, and counter selected for single copy insertion using the peel-1 system. NEONGREEN::NEG-1 fluorescence was imaged on an inverted microscope (IX71; Olympus), with an 100 x objective, and using 0.3 μm sections. Max intensity projections are shown.
Gene ontology analysis
The list of present genes were separated by fold change and ranked by FDR adjusted P value as calculated in DESeq to produce a ranked list of AB-enriched genes and P1-enriched genes. Ranked lists were processed as input by the GOrilla algorithm and a false discovery rate-adjusted P value of 10-5 was set as a minimum cutoff [50]. GO terms and scores were summarized in REViGO to merge excessively overlapping terms [51]. Categories were filtered for those with frequencies of less that 5% of the genome and the REViGO adjusted log10 P values greater than 7 were plotted.
Comparisons with publically available transcriptome datasets
The intersection between Maternal Degraded (MD) mRNAs [60] and AB-enriched, P1-enriched, and symmetric genes was calculated. Symmetric genes were defined as the 50% of present genes with the largest P values, as calculated in DEseq. The association between maternal mRNA category and differential abundance category was calculated using Fisher’s exact test. The intersection between tissue-specific transcriptomes [61] and AB-enriched, P1-enriched, and symmetric sets were also calculated. The association between representation and category was calculated using Fisher’s exact test between symmetric and AB-enriched or symmetric and P1-enriched categories.
RNA feature characterization and analysis
Splice leader (SL) sequences, 5′ UTR sequences, spliced gene models, unspliced gene models, and 3′ UTR sequences were obtained from Mangone et al. [100] and Wormbase (November 27, 2013). To avoid redundancy in pools of sequences, only the longest 3′ UTR from the union set of multiple 3′ UTR models identified in both Mangone et. al. and Wormbase annotations were used. Association between cell-type category (AB-enriched, P1-enriched, symmetric) and gene feature were calculated using Fisher’s exact test for discrete data and Wilcoxon rank-sum (Mann-Whitney U) test for continuous data.
De novo RNA motif searching
De novo motif searching was carried out using MEME (4.9.0). We sought discriminating motifs 5–12 nucleotides in length that distinguished 3′ UTR sequences in the AB-enriched or the P1-enriched set from the symmetric set focusing on the sense strand. 3′ UTR gene models were identified as the overlap between Mangone et al. [100] and Wormbase (November 27, 2013). The search model [–mod zoops] was used.
Accession numbers
High throughput sequencing data was submitted to NCBI Geo as GSE59943 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE59943). Raw sequences are available as SRP/SRP045/SRP045110. GSE59943_ABP1_Nishimura_raw_matrix.txt.gz reports the number of raw read counts for each gene model, tabulated for 20,240 genes. GSE59943_ABP1_Nishimura_normalized_matrix.txt.gz reports the number of size-normalized read counts for each gene model, tabulated for 20,240 genes. GSE59943_AB_average.wig.gz is a wig file that averages three scaled AB samples. This file is suitable for upload to UCSC genome browser. GSE59943_P1_average.wig.gz is a wig file that averages three scaled P1 samples. This file is suitable for upload to UCSC genome browser.
Supporting Information
Zdroje
1. Hawkins N, Garriga G. Asymmetric cell division: from A to Z. Genes Dev. 1998;12(23):3625–38. Epub 1998/12/16. 9851969
2. Knoblich JA. Asymmetric cell division during animal development. Nat Rev Mol Cell Biol. 2001;2(1):11–20. Epub 2001/06/20. 11413461
3. Tajbakhsh S, Rocheteau P, Le Roux I. Asymmetric cell divisions and asymmetric cell fates. Annu Rev Cell Dev Biol. 2009;25 : 671–99. doi: 10.1146/annurev.cellbio.24.110707.175415 19575640
4. Li R. The art of choreographing asymmetric cell division. Dev Cell. 2013;25(5):439–50. doi: 10.1016/j.devcel.2013.05.003 23763946
5. Nagalakshmi U, Waern K, Snyder M. RNA-Seq: a method for comprehensive transcriptome analysis. Curr Protoc Mol Biol. 2010;Chapter 4:Unit 4 11 1–3. Epub 2010/01/14. doi: 10.1002/0471142727.mb0411s89 20069539
6. Tang F, Barbacioru C, Nordman E, Li B, Xu N, Bashkirov VI, et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc. 2010;5(3):516–35. Epub 2010/03/06. doi: 10.1038/nprot.2009.236 20203668
7. Gönczy P, Rose LS. Asymmetric cell division and axis formation in the embryo. WormBook. 2005 : 1–20.
8. Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Developmental biology. 1983;100 : 64–119. 6684600
9. Bao Z, Zhao Z, Boyle TJ, Murray JI, Waterston RH. Control of cell cycle timing during C. elegans embryogenesis. Dev Biol. 2008;318(1):65–72. doi: 10.1016/j.ydbio.2008.02.054 18430415
10. Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324(5935):1729–32. Epub 2009/05/23. doi: 10.1126/science.1172046 19460965
11. Hird SN, Paulsen JE, Strome S. Segregation of germ granules in living Caenorhabditis elegans embryos: cell-type-specific mechanisms for cytoplasmic localisation. Development. 1996;122(4):1303–12. Epub 1996/04/01. 8620857
12. Wang JT, Seydoux G. Germ cell specification. Adv Exp Med Biol. 2013;757 : 17–39. doi: 10.1007/978-1-4614-4015-4_2 22872473
13. Seydoux G, Mello CC, Pettitt J, Wood WB, Priess JR, Fire A. Repression of gene expression in the embryonic germ lineage of C. elegans. Nature. 1996;382(6593):713–6. Epub 1996/08/22. 8751441
14. Seydoux G, Dunn MA. Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development. 1997;124(11):2191–201. Epub 1997/06/01. 9187145
15. Guven-Ozkan T, Nishi Y, Robertson SM, Lin R. Global transcriptional repression in C. elegans germline precursors by regulated sequestration of TAF-4. Cell. 2008;135(1):149–60. Epub 2008/10/16. doi: 10.1016/j.cell.2008.07.040 18854162
16. Hashimshony T, Wagner F, Sher N, Yanai I. CEL-Seq: Single-Cell RNA-Seq by Multiplexed Linear Amplification. Cell Rep. 2012;2(3):666–73. Epub 2012/09/04. doi: 10.1016/j.celrep.2012.08.003 22939981
17. Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56(1):110–56. 838129.
18. Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol. 1979;70(2):396–417. 478167
19. Batchelder C, Dunn MA, Choy B, Suh Y, Cassie C, Shim EY, et al. Transcriptional repression by the Caenorhabditis elegans germ-line protein PIE-1. Genes Dev. 1999;13(2):202–12. Epub 1999/01/30. 9925644
20. Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol. 2008;9(5):355–66. Epub 2008/04/24. doi: 10.1038/nrm2388 18431399
21. Ghosh D, Seydoux G. Inhibition of transcription by the Caenorhabditis elegans germline protein PIE-1: genetic evidence for distinct mechanisms targeting initiation and elongation. Genetics. 2008;178(1):235–43. Epub 2008/01/19. doi: 10.1534/genetics.107.083212 18202370
22. Seydoux G, Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development (Cambridge, England). 1994;120 : 2823–34. 7607073
23. Draper BW, Mello CC, Bowerman B, Hardin J, Priess JR. MEX-3 is a KH domain protein that regulates blastomere identity in early C. elegans embryos. Cell. 1996;87 : 205–16. 8861905
24. Tabara H, Hill RJ, Mello CC, Priess JR, Kohara Y. pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development (Cambridge, England). 1999;126 : 1–11.
25. Marinov GK, Williams BA, McCue K, Schroth GP, Gertz J, Myers RM, et al. From single-cell to cell-pool transcriptomes: Stochasticity in gene expression and RNA splicing. Genome Res. 2014;24(3):496–510. doi: 10.1101/gr.161034.113 24299736
26. Eberwine J, Yeh H, Miyashiro K, Cao Y, Nair S, Finnell R, et al. Analysis of gene expression in single live neurons. Proc Natl Acad Sci U S A. 1992;89(7):3010–4. Epub 1992/04/01. 1557406
27. Islam S, Kjallquist U, Moliner A, Zajac P, Fan JB, Lonnerberg P, et al. Characterization of the single-cell transcriptional landscape by highly multiplex RNA-seq. Genome Res. 2011;21(7):1160–7. Epub 2011/05/06. doi: 10.1101/gr.110882.110 21543516
28. Goldstein B. Induction of gut in Caenorhabditis elegans embryos. Nature. 1992;357 : 255–7. 1589023
29. Werts AD, Roh-Johnson M, Goldstein B. Dynamic localization of C. elegans TPR-GoLoco proteins mediates mitotic spindle orientation by extrinsic signaling. Development. 2011. Epub 2011/09/10.
30. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25(9):1105–11. Epub 2009/03/18. doi: 10.1093/bioinformatics/btp120 19289445
31. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. Epub 2012/03/03. doi: 10.1038/nprot.2012.016 22383036
32. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. Epub 2010/10/29. doi: 10.1186/gb-2010-11-10-r106 20979621
33. Anders S. HTSeq: Analysing high-throughput sequencing data with Python. 2013.
34. Tabara H, Motohashi T, Kohara Y. A multi-well version of in situ hybridization on whole mount embryos of Caenorhabditis elegans. Nucleic Acids Res. 1996;24(11):2119–24. Epub 1996/06/01. 8668544
35. Spiró Z, Gönczy P. Polarity-dependent asymmetric distribution and MEX-5/6-mediated translational activation of the era-1 mRNA in C. elegans embryos PLoS ONE. 2015.
36. Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408(6810):325–30. Epub 2000/12/01. 11099033
37. Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2(1):RESEARCH0002. Epub 2001/02/24. 11178279
38. Zipperlen P, Fraser AG, Kamath RS, Martinez-Campos M, Ahringer J. Roles for 147 embryonic lethal genes on C. elegans chromosome I identified by RNA interference and video microscopy. EMBO J. 2001;20(15):3984–92. Epub 2001/08/03. 11483502
39. Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30(4):313–21. Epub 2003/06/28. 12828945
40. Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PV, Kamath RS, et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1(1):E12. Epub 2003/10/14. 14551910
41. Maeda I, Kohara Y, Yamamoto M, Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr Biol. 2001;11(3):171–6. Epub 2001/03/07. 11231151
42. Sonnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434(7032):462–9. Epub 2005/03/26. 15791247
43. Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4(3):363–71. doi: 10.1038/nprot.2009.2 19247286
44. Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421(6920):231–7. Epub 2003/01/17. 12529635
45. Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141(1):297–308. 9531567
46. Armenti ST, Nance J. Adherens junctions in C. elegans embryonic morphogenesis. Subcell Biochem. 2012;60 : 279–99. doi: 10.1007/978-94-007-4186-7_12 22674076
47. Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124(15):2889–901. Epub 1997/08/01. 9247332
48. Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol. 1986;117(1):156–73. 3743895
49. McMahon L, Legouis R, Vonesch JL, Labouesse M. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J Cell Sci. 2001;114(Pt 12):2265–77. 11493666
50. Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10 : 48. doi: 10.1186/1471-2105-10-48 19192299
51. Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6(7):e21800. Epub 2011/07/27. doi: 10.1371/journal.pone.0021800 21789182
52. D'Agostino I, Merritt C, Chen PL, Seydoux G, Subramaniam K. Translational repression restricts expression of the C. elegans Nanos homolog NOS-2 to the embryonic germline. Dev Biol. 2006;292(1):244–52. Epub 2006/02/28. 16499902
53. Merritt C, Rasoloson D, Ko D, Seydoux G. 3' UTRs are the primary regulators of gene expression in the C. elegans germline. Curr Biol. 2008;18(19):1476–82. Epub 2008/09/27. doi: 10.1016/j.cub.2008.08.013 18818082
54. Evans TC, Hunter CP. Translational control of maternal RNAs. WormBook. 2005 : 1–11. Epub 2007/12/01.
55. Oldenbroek M, Robertson SM, Guven-Ozkan T, Gore S, Nishi Y, Lin R. Multiple RNA-binding proteins function combinatorially to control the soma-restricted expression pattern of the E3 ligase subunit ZIF-1. Dev Biol. 2012;363(2):388–98. doi: 10.1016/j.ydbio.2012.01.002 22265679
56. Jadhav S, Rana M, Subramaniam K. Multiple maternal proteins coordinate to restrict the translation of C. elegans nanos-2 to primordial germ cells. Development. 2008;135(10):1803–12. Epub 2008/04/18. doi: 10.1242/dev.013656 18417623
57. Hunter CP, Kenyon C. Spatial and temporal controls target pal-1 blastomere-specification activity to a single blastomere lineage in C. elegans embryos. Cell. 1996;87(2):217–26. Epub 1996/10/18. 8861906
58. Ogura K, Kishimoto N, Mitani S, Gengyo-Ando K, Kohara Y. Translational control of maternal glp-1 mRNA by POS-1 and its interacting protein SPN-4 in Caenorhabditis elegans. Development. 2003;130(11):2495–503. Epub 2003/04/19. 12702662
59. Subramaniam K, Seydoux G. nos-1 and nos-2, two genes related to Drosophila nanos, regulate primordial germ cell development and survival in Caenorhabditis elegans. Development. 1999;126(21):4861–71. Epub 1999/10/16. 10518502
60. Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130(5):889–900. Epub 2003/01/23. 12538516
61. Spencer WC, Zeller G, Watson JD, Henz SR, Watkins KL, McWhirter RD, et al. A spatial and temporal map of C. elegans gene expression. Genome Res. 2011;21(2):325–41. Epub 2010/12/24. doi: 10.1101/gr.114595.110 21177967
62. Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, et al. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–8. doi: 10.1093/nar/gkp335 19458158
63. Farley BM, Pagano JM, Ryder SP. RNA target specificity of the embryonic cell fate determinant POS-1. RNA (New York, NY). 2008;14 : 2685–97. doi: 10.1261/rna.1256708 18952820
64. Pagano JM, Farley BM, Essien KI, Ryder SP. RNA recognition by the embryonic cell fate determinant and germline totipotency factor MEX-3. Proc Natl Acad Sci U S A. 2009;106(48):20252–7. Epub 2009/11/17. doi: 10.1073/pnas.0907916106 19915141
65. Kawasaki I, Amiri A, Fan Y, Meyer N, Dunkelbarger S, Motohashi T, et al. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics. 2004;167(2):645–61. 15238518
66. Spike CA, Bader J, Reinke V, Strome S. DEPS-1 promotes P-granule assembly and RNA interference in C. elegans germ cells. Development. 2008;135(5):983–93. Epub 2008/02/01. doi: 10.1242/dev.015552 18234720
67. Schauer IE, Wood WB. Early C. elegans embryos are transcriptionally active. Development. 1990;110(4):1303–17. Epub 1990/12/01. 2100265
68. Edgar LG, Wolf N, Wood WB. Early transcription in Caenorhabditis elegans embryos. Development. 1994;120(2):443–51. Epub 1994/02/01. 7512022
69. Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2 : 28–36. Epub 1994/01/01. 7584402
70. Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–89. doi: 10.1016/j.molcel.2010.05.004 20513432
71. Altun ZFaH D.H. Epithelial system, hypodermis. In WormAtlas2009.
72. Evans TC, Crittenden SL, Kodoyianni V, Kimble J. Translational control of maternal glp-1 mRNA establishes an asymmetry in the C. elegans embryo. Cell. 1994;77(2):183–94. Epub 1994/04/22. 8168128
73. Wright JE, Gaidatzis D, Senften M, Farley BM, Westhof E, Ryder SP, et al. A quantitative RNA code for mRNA target selection by the germline fate determinant GLD-1. EMBO J. 2011;30(3):533–45. Epub 2010/12/21. doi: 10.1038/emboj.2010.334 21169991
74. Farley BM, Ryder SP. POS-1 and GLD-1 repress glp-1 translation through a conserved binding-site cluster. Mol Biol Cell. 2012;23(23):4473–83. doi: 10.1091/mbc.E12-03-0216 23034181
75. Amiri A, Keiper BD, Kawasaki I, Fan Y, Kohara Y, Rhoads RE, et al. An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans. Development. 2001;128(20):3899–912. 11641215
76. Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science. 1997;277(5324):383–7. 9219698
77. Takizawa PA, Sil A, Swedlow JR, Herskowitz I, Vale RD. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature. 1997;389(6646):90–3. 9288973
78. Becalska AN, Gavis ER. Lighting up mRNA localization in Drosophila oogenesis. Development. 2009;136(15):2493–503. doi: 10.1242/dev.032391 19592573
79. Lasko P. mRNA localization and translational control in Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2012;4(10). Epub 2012/08/07. doi: 10.1101/cshperspect.a013300 23028121
80. Marston DJ, Goldstein B. Symmetry breaking in C. elegans: another gift from the sperm. Dev Cell. 2006;11(3):273–4. Epub 2006/09/05. 16950117
81. Jenkins N, Saam JR, Mango SE. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science. 2006;313(5791):1298–301. 16873611
82. Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52(3):311–20. Epub 1988/02/12. 3345562.
83. Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol. 2004;20 : 427–53. Epub 2004/10/12. 15473847
84. Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7(3):413–24. Epub 2004/09/15. 15363415
85. Cowan CR, Hyman AA. Acto-myosin reorganization and PAR polarity in C. elegans. Development. 2007;134(6):1035–43. Epub 2007/02/09. 17287245
86. Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138(5):799–809. Epub 2011/02/10. doi: 10.1242/dev.053538 21303844
87. Moeendarbary E, Valon L, Fritzsche M, Harris AR, Moulding DA, Thrasher AJ, et al. The cytoplasm of living cells behaves as a poroelastic material. Nat Mater. 2013;12(3):253–61. doi: 10.1038/nmat3517 23291707
88. Stoeckius M, Grün D, Kirchner M, Ayoub S, Torti F, Piano F, et al. Global characterization of the oocyte-to-embryo transition in Caenorhabditis elegans uncovers a novel mRNA clearance mechanism. EMBO J. 2014.
89. Stiernagle T. Maintenance of C. elegans. WormBook. 2006 : 1–11.
90. Edgar LG, Goldstein B. Culture and manipulation of embryonic cells. Methods Cell Biol. 2012;107 : 151–75. doi: 10.1016/B978-0-12-394620-1.00005-9 22226523
91. Lassmann T, Hayashizaki Y, Daub CO. TagDust—a program to eliminate artifacts from next generation sequencing data. Bioinformatics. 2009;25(21):2839–40. doi: 10.1093/bioinformatics/btp527 19737799
92. Team RC. R: A language and environment for statistical computing. In: Computing RFfS, editor. 2013. doi: 10.3758/s13428-013-0330-5 23519455
93. Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7 Suppl 1:S12.1–4.
94. Shaffer SM, Wu MT, Levesque MJ, Raj A. Turbo FISH: a method for rapid single molecule RNA FISH. PLoS One. 2013;8(9):e75120. doi: 10.1371/journal.pone.0075120 24066168
95. Ji N, van Oudenaarden A. Single molecule fluorescent in situ hybridization (smFISH) of C. elegans worms and embryos. WormBook. 2012 : 1–16. doi: 10.1895/wormbook.1.154.1 23255345
96. Sawyer JM, Glass S, Li T, Shemer G, White ND, Starostina NG, et al. Overcoming redundancy: an RNAi enhancer screen for morphogenesis genes in Caenorhabditis elegans. Genetics. 2011;188(3):549–64. Epub 2011/04/30. doi: 10.1534/genetics.111.129486 21527776
97. Dudley NR, Labbé JC, Goldstein B. Using RNA interference to identify genes required for RNA interference. Proc Natl Acad Sci U S A. 2002;99(7):4191–6. 11904378
98. Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat Methods. 2013;10(10):1028–34. doi: 10.1038/nmeth.2641 23995389
99. Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40(11):1375–83. doi: 10.1038/ng.248 18953339
100. Mangone M, Manoharan AP, Thierry-Mieg D, Thierry-Mieg J, Han T, Mackowiak SD, et al. The landscape of C. elegans 3'UTRs. Science. 2010;329(5990):432–5. doi: 10.1126/science.1191244 20522740
Štítky
Genetika Reprodukční medicína
Článek Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in CrustaceansČlánek Adventures in WonderlandČlánek Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and InfectivityČlánek Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in FilamentsČlánek Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in ArabidopsisČlánek Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression ControlČlánek The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent EnhancersČlánek Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of MitochondriaČlánek The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma FormationČlánek Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 4
-
Všechny články tohoto čísla
- Retraction: Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- Adventures in Wonderland
- Experimental Swap of 's Assortative Mating Preferences Demonstrates Key Role of X-Chromosome Divergence Island in Incipient Sympatric Speciation
- Chromosome Replacement and Deletion Lead to Clonal Polymorphism of Berry Color in Grapevine
- The Protein Quality Control Machinery Regulates Its Misassembled Proteasome Subunits
- Genome-Wide Association Study Identifies as a Critical Gene for Susceptibility to Noise-Induced Hearing Loss
- Genomic Location of the Major Ribosomal Protein Gene Locus Determines Global Growth and Infectivity
- Viable Neuronopathic Gaucher Disease Model in Medaka () Displays Axonal Accumulation of Alpha-Synuclein
- Multi-locus Analysis of Genomic Time Series Data from Experimental Evolution
- The Genetic Legacy of the Expansion of Turkic-Speaking Nomads across Eurasia
- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- The Pif1 Helicase, a Negative Regulator of Telomerase, Acts Preferentially at Long Telomeres
- Inhibiting K63 Polyubiquitination Abolishes No-Go Type Stalled Translation Surveillance in
- SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) Function Interdependently to Promote Axon Guidance by Regulating the MIG-2 GTPase
- Spatial Fluctuations in Expression of the Heterocyst Differentiation Regulatory Gene in Filaments
- Synergistic and Independent Actions of Multiple Terminal Nucleotidyl Transferases in the 3’ Tailing of Small RNAs in Arabidopsis
- Host Genetic Variation Influences Gene Expression Response to Rhinovirus Infection
- Contribution of Large Region Joint Associations to Complex Traits Genetics
- Volatility of Mutator Phenotypes at Single Cell Resolution
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Genome-Wide Negative Feedback Drives Transgenerational DNA Methylation Dynamics in Arabidopsis
- A Multi-layered Protein Network Stabilizes the FtsZ-ring and Modulates Constriction Dynamics
- Systematic Dissection of the Sequence Determinants of Gene 3’ End Mediated Expression Control
- Genome Sequencing of the Perciform Fish Provides Insights into Molecular and Genetic Mechanisms of Stress Adaptation
- Natural Variant E610G Is a Semi-dominant Suppressor of IAP-Induced RNA Processing Defects
- The Alkaline Response Pathway: Identification of a Novel Rim Pathway Activator
- Transgenerational Inheritance of Diet-Induced Genome Rearrangements in Drosophila
- A Single Nucleotide Polymorphism Uncovers a Novel Function for the Transcription Factor Ace2 during Hyphal Development
- DNA Damage Response and Spindle Assembly Checkpoint Function throughout the Cell Cycle to Ensure Genomic Integrity
- The Functional Interplay Between the t(9;22)-Associated Fusion Proteins BCR/ABL and ABL/BCR in Philadelphia Chromosome-Positive Acute Lymphatic Leukemia
- Extreme Recombination Frequencies Shape Genome Variation and Evolution in the Honeybee,
- Beyond Glycolysis: GAPDHs Are Multi-functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses
- Comprehensive Profiling of Amino Acid Response Uncovers Unique Methionine-Deprived Response Dependent on Intact Creatine Biosynthesis
- Windpipe Controls Intestinal Homeostasis by Regulating JAK/STAT Pathway via Promoting Receptor Endocytosis and Lysosomal Degradation
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
- Cross-Population Joint Analysis of eQTLs: Fine Mapping and Functional Annotation
- The Power of Gene-Based Rare Variant Methods to Detect Disease-Associated Variation and Test Hypotheses About Complex Disease
- The Chromatin Remodeler CHD8 Is Required for Activation of Progesterone Receptor-Dependent Enhancers
- Competition between VanU Repressor and VanR Activator Leads to Rheostatic Control of Vancomycin Resistance Operon Expression
- A Missense Change in the Gene Links Aberrant Autophagy to a Neurodegenerative Vacuolar Storage Disease
- Simultaneous Discovery, Estimation and Prediction Analysis of Complex Traits Using a Bayesian Mixture Model
- Selection against Heteroplasmy Explains the Evolution of Uniparental Inheritance of Mitochondria
- Genome-Destabilizing Effects Associated with Top1 Loss or Accumulation of Top1 Cleavage Complexes in Yeast
- Imputation-Based Population Genetics Analysis of Malaria Parasites
- Heterozygosity for a Hypomorphic Polβ Mutation Reduces the Expansion Frequency in a Mouse Model of the Fragile X-Related Disorders
- Neto-Mediated Intracellular Interactions Shape Postsynaptic Composition at the Neuromuscular Junction
- Ndd1 Turnover by SCF Is Inhibited by the DNA Damage Checkpoint in
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- The DNA Helicase Recql4 Is Required for Normal Osteoblast Expansion and Osteosarcoma Formation
- Spastin Binds to Lipid Droplets and Affects Lipid Metabolism
- Maintenance of Glia in the Optic Lamina Is Mediated by EGFR Signaling by Photoreceptors in Adult Drosophila
- Auxin Influx Carriers Control Vascular Patterning and Xylem Differentiation in
- Dual-Specificity Anti-sigma Factor Reinforces Control of Cell-Type Specific Gene Expression in
- The Lowe Syndrome Protein OCRL1 Is Required for Endocytosis in the Zebrafish Pronephric Tubule
- Postnatal Loss of Hap1 Reduces Hippocampal Neurogenesis and Causes Adult Depressive-Like Behavior in Mice
- CAPER Is Vital for Energy and Redox Homeostasis by Integrating Glucose-Induced Mitochondrial Functions via ERR-α-Gabpa and Stress-Induced Adaptive Responses via NF-κB-cMYC
- Distinct and Cooperative Activities of HESO1 and URT1 Nucleotidyl Transferases in MicroRNA Turnover in
- The Evolutionary Origination and Diversification of a Dimorphic Gene Regulatory Network through Parallel Innovations in and
- MAPK Signaling Pathway Alters Expression of Midgut ALP and ABCC Genes and Causes Resistance to Cry1Ac Toxin in Diamondback Moth
- Spatio-temporal Remodeling of Functional Membrane Microdomains Organizes the Signaling Networks of a Bacterium
- Asymmetric Transcript Discovery by RNA-seq in . Blastomeres Identifies , a Gene Important for Anterior Morphogenesis
- A Stress-Induced Small RNA Modulates Alpha-Rhizobial Cell Cycle Progression
- Systematic Profiling of Poly(A)+ Transcripts Modulated by Core 3’ End Processing and Splicing Factors Reveals Regulatory Rules of Alternative Cleavage and Polyadenylation
- The UPR Branch IRE1- in Plants Plays an Essential Role in Viral Infection and Is Complementary to the Only UPR Pathway in Yeast
- A Non-canonical RNA Silencing Pathway Promotes mRNA Degradation in Basal Fungi
- Co-chaperone p23 Regulates . Lifespan in Response to Temperature
- Re-replication of a Centromere Induces Chromosomal Instability and Aneuploidy
- Shade Avoidance Components and Pathways in Adult Plants Revealed by Phenotypic Profiling
- Lipid-Induced Epigenomic Changes in Human Macrophages Identify a Coronary Artery Disease-Associated Variant that Regulates Expression through Altered C/EBP-Beta Binding
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Lack of GDAP1 Induces Neuronal Calcium and Mitochondrial Defects in a Knockout Mouse Model of Charcot-Marie-Tooth Neuropathy
- Proteolysis of Virulence Regulator ToxR Is Associated with Entry of into a Dormant State
- Frameshift Variant Associated with Novel Hoof Specific Phenotype in Connemara Ponies
- Ataxin-2 Regulates Translation in a New BAC-SCA2 Transgenic Mouse Model
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



