-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTelomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
Human chromosomes are capped by protective ends called telomeres. These ends are shortened during renewal of tissue and eventually become critically short, causing cells to become senescent or die. It is widely believed that lifestyle features such as smoking, obesity, physical inactivity, and possibly alcohol intake enhance shortening of telomeres. However, strong evidence to support such an interpretation is hard to find. We therefore tested whether these lifestyle factors are associated with telomere length change in 4,576 healthy individuals from the general population. Individuals had relative telomere length measured twice with a 10-year interval, and were then followed for mortality and morbidity for a further 10 years after the second measurement. We found change in telomere length to be more dynamic than previously believed, as we observed both shortening (in 56%) and lengthening (in 44%) among participants. Contrary to previous beliefs, we found telomere length change to be unaffected by lifestyle factors. Instead, we found the strongest association between past telomere length and age with change in telomere length over 10 years. Also, we found no association between change in telomere length and risk of all-cause mortality, cancer, chronic obstructive lung disease, diabetes mellitus, ischemic cerebrovascular disease, or ischemic heart disease.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004191
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004191Summary
Human chromosomes are capped by protective ends called telomeres. These ends are shortened during renewal of tissue and eventually become critically short, causing cells to become senescent or die. It is widely believed that lifestyle features such as smoking, obesity, physical inactivity, and possibly alcohol intake enhance shortening of telomeres. However, strong evidence to support such an interpretation is hard to find. We therefore tested whether these lifestyle factors are associated with telomere length change in 4,576 healthy individuals from the general population. Individuals had relative telomere length measured twice with a 10-year interval, and were then followed for mortality and morbidity for a further 10 years after the second measurement. We found change in telomere length to be more dynamic than previously believed, as we observed both shortening (in 56%) and lengthening (in 44%) among participants. Contrary to previous beliefs, we found telomere length change to be unaffected by lifestyle factors. Instead, we found the strongest association between past telomere length and age with change in telomere length over 10 years. Also, we found no association between change in telomere length and risk of all-cause mortality, cancer, chronic obstructive lung disease, diabetes mellitus, ischemic cerebrovascular disease, or ischemic heart disease.
Introduction
Telomeres are 1,500 to 15,000 basepairs long tandem repeat DNA sequences (TTAGGG)x which cap at the ends of linear chromosomes [1], [2]. During mitosis, telomere length is shortened due to the inability of the DNA polymerase to complete duplication of the lacking strand [3]. Eventually, telomeres become critical short causing cells to become senescent or die [1], [3].
Several cross-sectional and prospective studies have associated short telomere length with increased risk of any early death [2], [4], [5], cardiovascular disease [2], [6], cancer [7], [8], and early death after a cancer diagnosis [9]. At the same time cross-sectional studies have reported short telomere length to be associated with lifestyle factors such as smoking, obesity, physical inactivity, and possible alcohol intake [2], [10]–[12]. These observations have led to a general belief that telomere length is shortened by these factors, and that telomere length possibly could be a marker of biological age of tissues [12]–[14]. The alleged associations are quoted repeatedly, but strong evidence to support these hypotheses is difficult to find. Therefore, the association between lifestyle factors and short telomere length could simply be due to confounding. Moreover, it is uncertain whether change in lifestyle can change telomere length, and whether telomere length change is associated with risk of mortality and morbidity.
We tested the hypothesis that smoking, body weight, physical activity, and alcohol intake are associated with telomere length cross-sectionally and with telomere length change during 10 years observation in the general population. Furthermore, we tested whether telomere length change is associated with mortality and morbidity.
Results
Cross-sectional: Telomere length
Characteristics of participants as a function of examination specific telomere length quartiles are shown in Table 1. Cross-sectionally at the 1991–1994 examination, short telomere length was associated with increased age (P for trend across quartiles = 3×10−77), male gender (P = 0.02), current smoking (P = 8×10−3), increased body mass index (P = 7×10−14), physical inactivity (P = 4×10−17), but not with increased alcohol intake (P = 0.10). Correspondingly at the 2001–2003 examination, short telomere length was associated with increased age (P for trend across quartiles = 2×10−74), male gender (P = 1×10−5), increased body mass index (P = 4×10−4), and physical inactivity (P = 2×10−33), but not with current smoking (P = 0.32) or with increased alcohol intake (P = 0.01 for reduced intake). At study entry, we observed a 20 basepair decrease in telomere length per year of age (P<2×10−81; Figure 1). Variation in age explained 8% of the variation in telomere length (R2 = 0.08).
Fig. 1. Telomere length in basepairs as a function of age in years at the 1991–1994 examination. 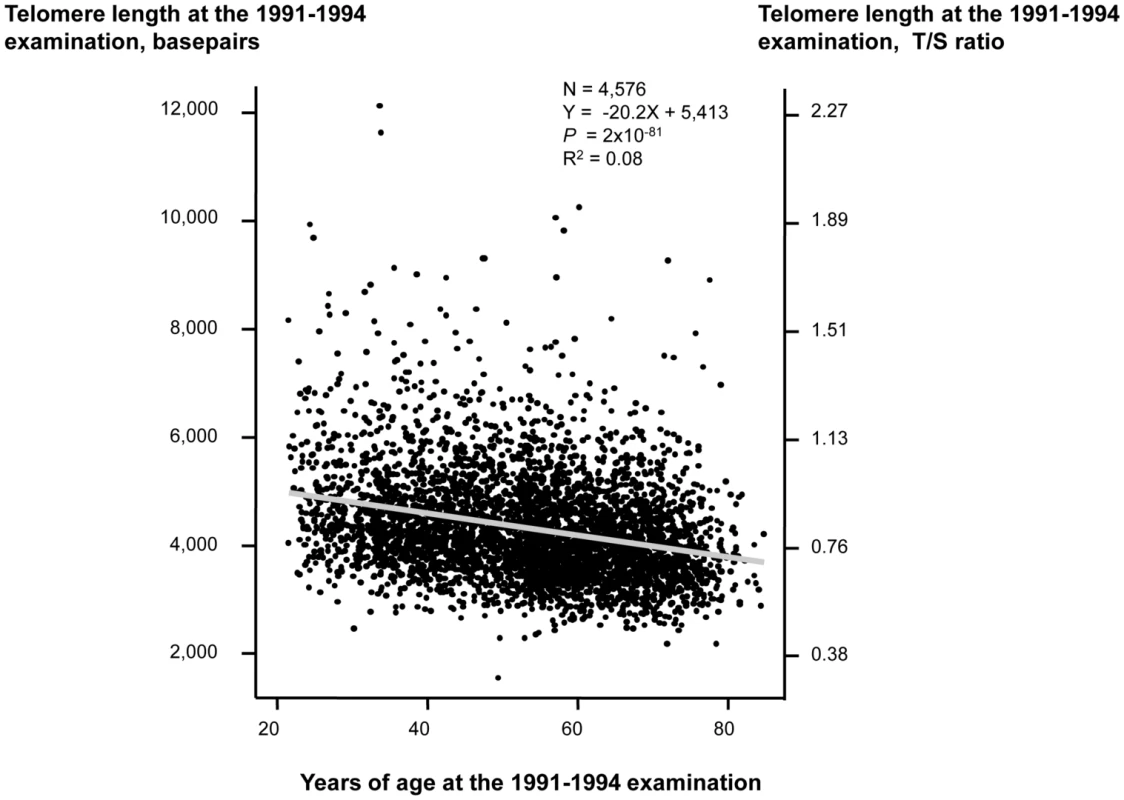
Linear regression is shown in equation and as a grey line. N = number of participants. P-value and R2 are for the correlation from the linear regression. Statistical tests were two-sided. Tab. 1. Characteristics of participants in the general population, the Copenhagen City Heart Study. 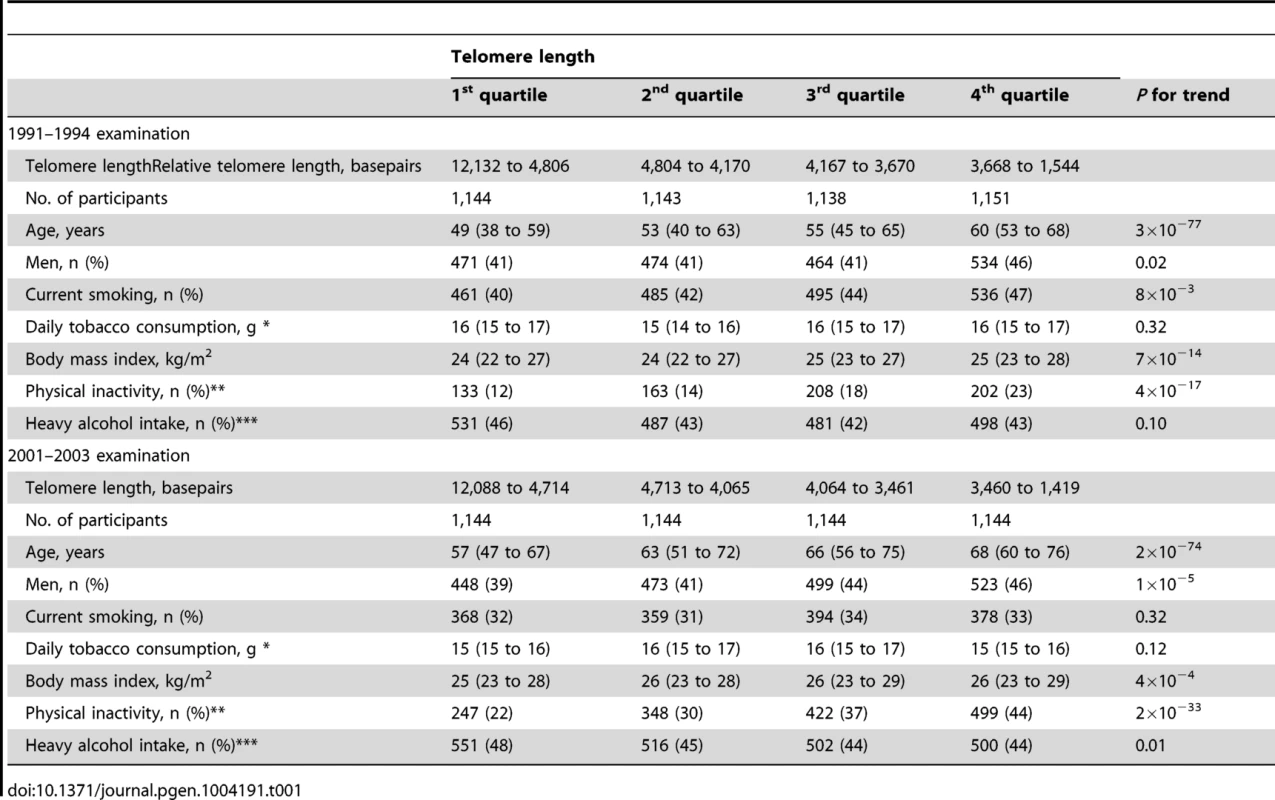
10 years observation: Telomere length change
During 10 years observation, 56% of participants lost and 44% gained telomere length (Figure 2). Mean change in telomere length was −19.3 basepairs per year. Ten year maximum loss and gain were −8,406 and +7,278 basepairs. Characteristics of participants stratified for 10 year loss or gain of telomere length were similar (Table S1). The telomere length distribution at each examination is shown in Figure S1.
Fig. 2. Absolute change in basepairs during 10 years observation. N = number of participants. 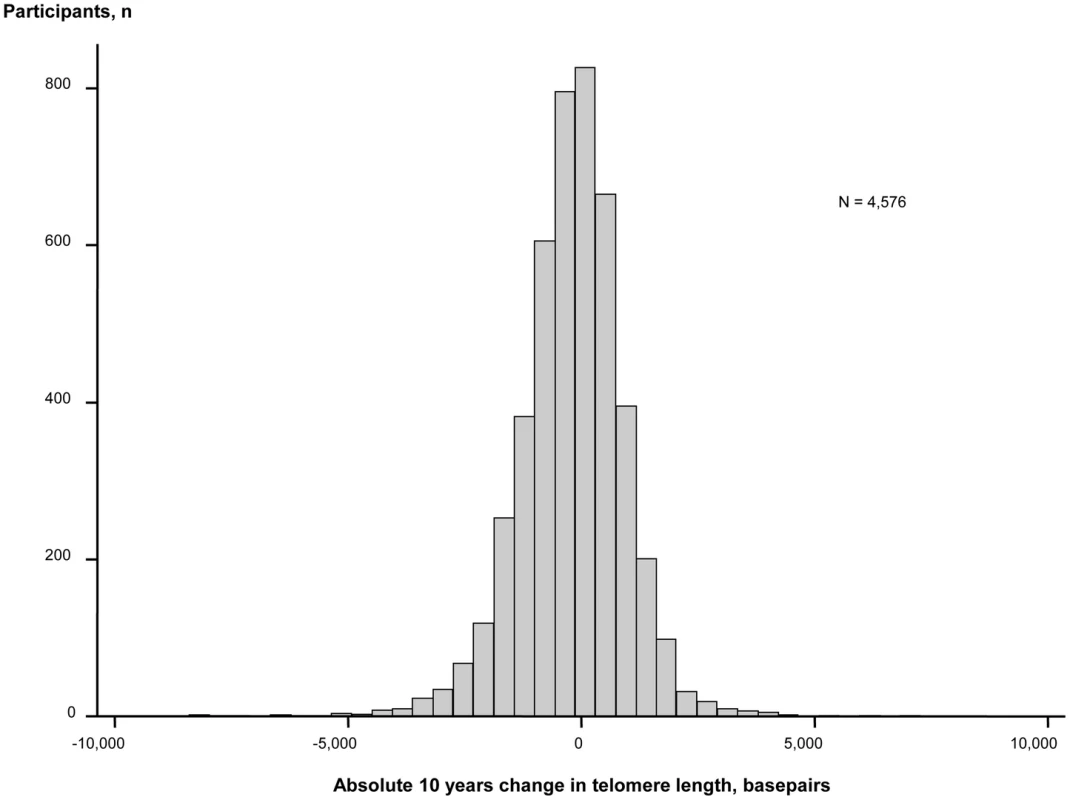
An inverse association of change in telomere length in either basepairs or in percent with baseline telomere length in basepairs are illustrated in Figure 3. Baseline telomere length was associated inversely with 10 years change in basepairs of telomere length, and variation in baseline telomere length explained 29% of variation in 10 years change in basepairs in telomere length (P<1×10−300, R2 = 0.29; Figure 3, left). Baseline telomere length was also associated inversely with 10 years percent change in telomere length and variation in baseline telomere length explained 18% of variation in 10 years percent change in telomere length (P = 3×10−200, R2 = 0.18; Figure 3, right).
Fig. 3. 10 year change in telomere length in basepairs or percent as functions of telomere length at the 1991–1994 examination. 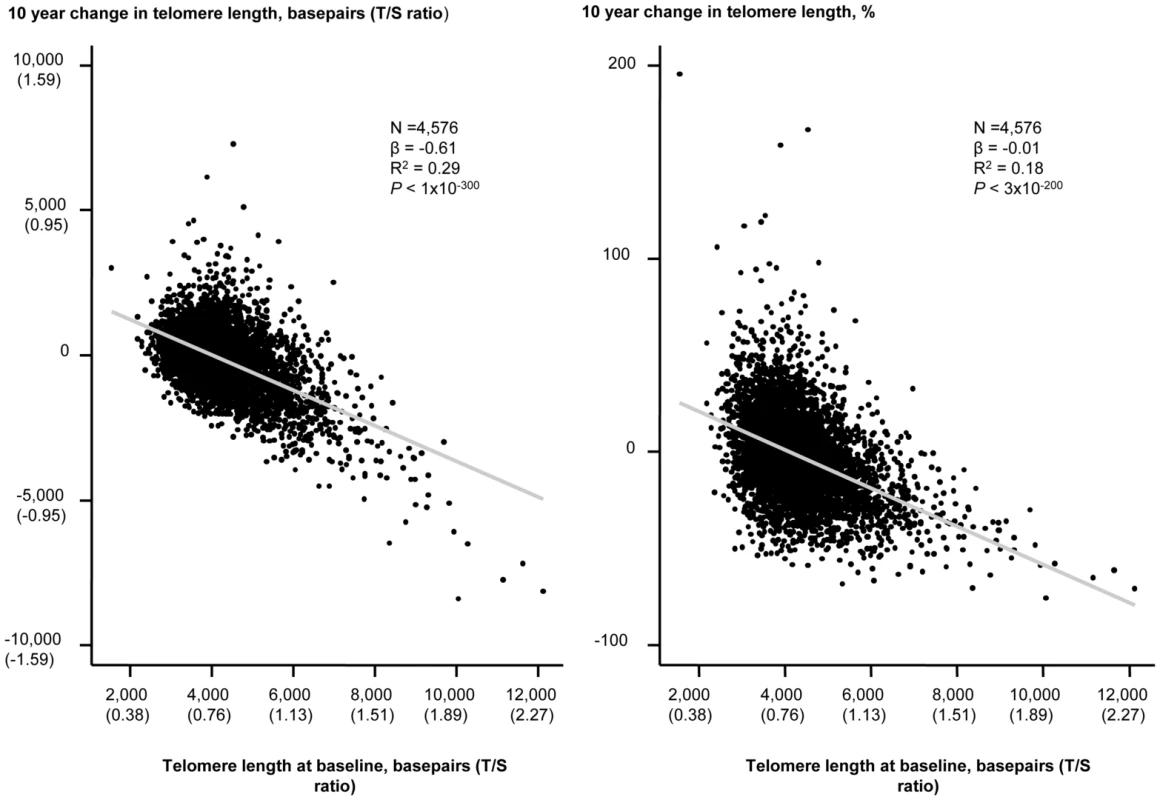
Linear regressions are shown in equations and as grey lines. N = number of participants. P-value and R2 are for the correlation from the linear regression. Statistical tests were two-sided. The independent predictors of change in leukocyte telomere length over 10 years were in multivariable models baseline telomere length and age at baseline (Table 2). Stratification on 10 year telomere length gain or loss showed similar results (Table S2). In overall analysis and in sub-analyses, baseline and 10 year inter-observational tobacco consumption, body weight, physical inactivity, and alcohol intake were not associated with change in telomere length.
Tab. 2. Independent predictors of change in telomere length from the 1991–1994 to the 2001–20013 examinations. 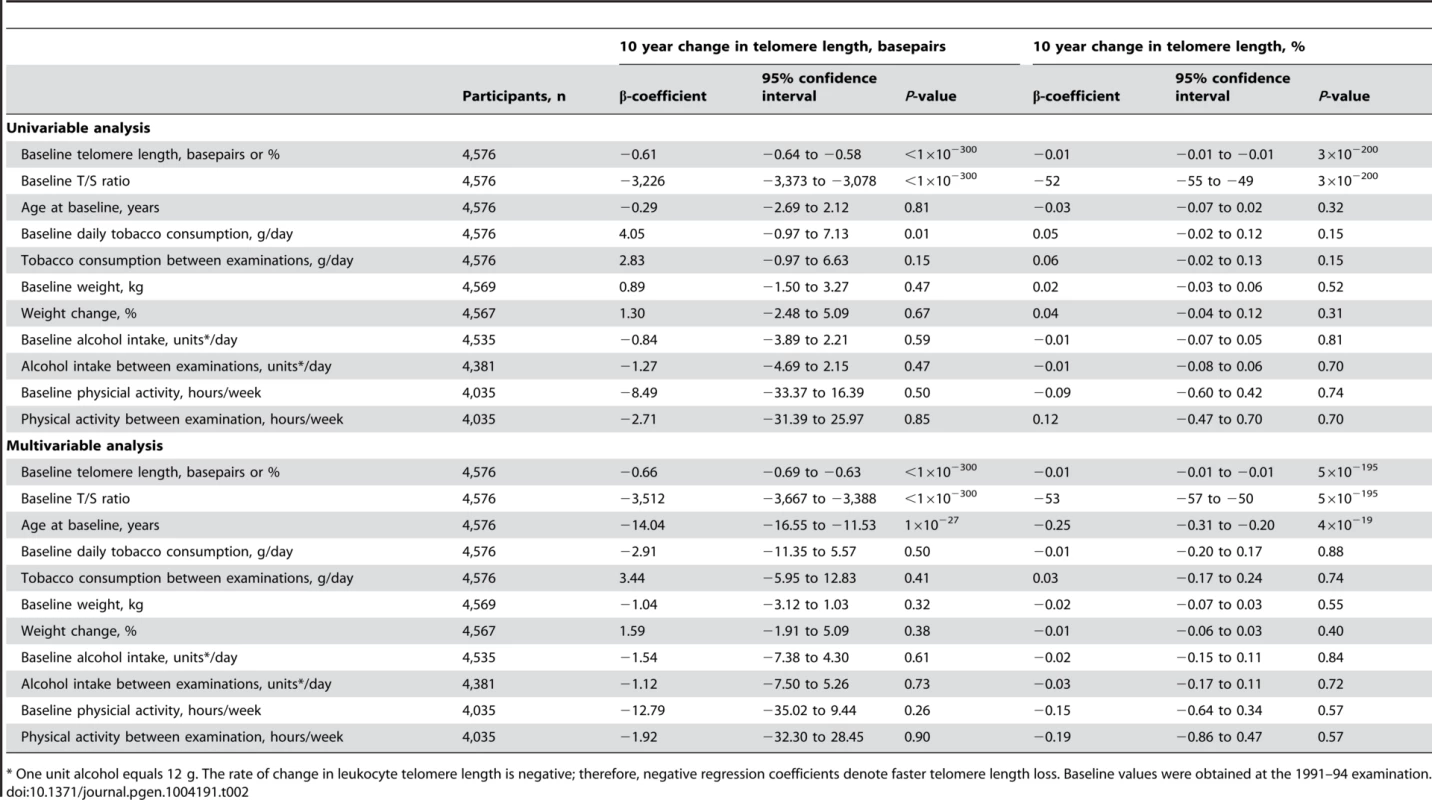
* One unit alcohol equals 12 g. The rate of change in leukocyte telomere length is negative; therefore, negative regression coefficients denote faster telomere length loss. Baseline values were obtained at the 1991–94 examination. The fact that telomere length at baseline was the only factor strongly associated with change in telomere length prompted us to investigate whether the telomere length change could be partly explained by regression towards the mean, ie. that the combined effect of biological and analytical variability will push the highest levels to a lower level on retesting and vice versa for those with the lowest levels [15]. This was indeed the case with a regression dilution ratio of 0.50 for the quartilation used in this study (Figure S2).
Prospective studies: Mortality and morbidity
Participants were grouped in quartiles according to change in telomere length over two examinations spanning 10 years, and were then followed for an additional up to 10 years after the second examination (Table 3). Median follow-up after the second examination were 8.1 years for survival, 6.7 years for cancer, and 7.9 years for chronic obstructive pulmonary disease, diabetes mellitus type II, ischemic cerebrovascular disease, and ischemic heart disease. We observed no association between quartiles of telomere length change and risk of early death (P for trend across quartiles = 0.21), cancer (P = 0.62), chronic obstructive pulmonary disease (P = 0.60), diabetes mellitus type II (P = 0.37), ischemic cerebrovascular disease (P = 0.60), or ischemic heart disease (P = 0.56). Stratification on 10-year telomere length gain or loss showed similar results (Table S3).
Tab. 3. Mortality and morbidity by quartiles of telomere length change. 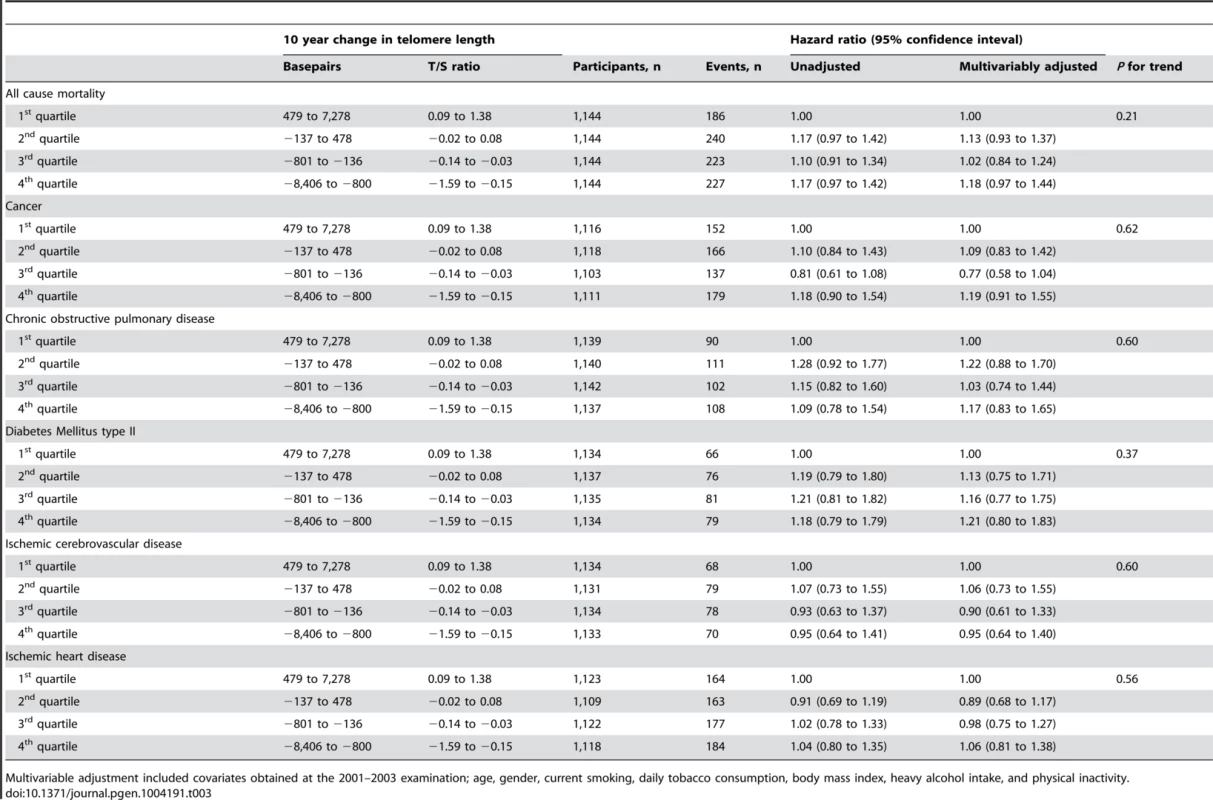
Multivariable adjustment included covariates obtained at the 2001–2003 examination; age, gender, current smoking, daily tobacco consumption, body mass index, heavy alcohol intake, and physical inactivity. In contrast, short telomere length was associated with all-cause mortality both at the 1991–94 examination (P = 0.00001) and at the 2001–03 examination (P = 0.007) (Figure 4), illustrating that the telomere length measurement included biological important information at both examinations.
Fig. 4. All-cause mortality by quartiles of telomere length at the 1991–94 examination (left) and at the 2001–03 examination (right). 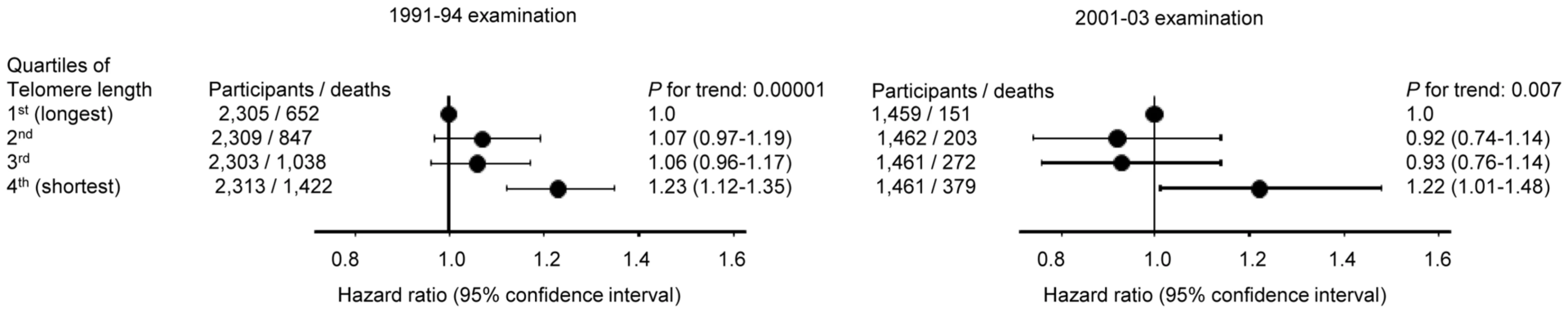
Hazard ratios were adjusted for covariates obtained at the date of examination; age, gender, current smoking, daily tobacco consumption, body mass index, heavy alcohol intake, and physical inactivity. Numbers of participants were all available participants with measured telomere lengths (1991–94; 9250, 2001–03; 5843). Follow-up began the day of the 1991–94 (for the 9250 participants examined in 1991–94) or the 2001–03 (for the 5843 participants examined in 2001–03) examination. Discussion
We found association of smoking, increased body weight, and physical inactivity with short telomere length cross-sectionally, but not with telomere length change during 10 years observation, while alcohol intake was associated with neither. Also, change in telomere length did not associate prospectively with mortality or morbidity in the general population, whereas both cross-sectional measurements did associate with mortality.
It it is somewhat surprising that we observed telomere elongation in nearly half of our participants. This suggests that telomere length is more dynamic than hitherto acknowledged, and that non-stem cells may have an underestimated regenerative potential. Although, telomere elongation is not widely discussed, other prospective studies have reported telomere elongation in 11% to 33% of adult individuals [16]–[19], not so different from the 44% with telomere elongation in the present study as we did not consider a middle group with unchanged telomere length. Interestingly, tropical python hatchlings are born with short telomere length and extend their telomeres as they grow. A slight gender bias, similar to what is observed cross-sectionally in humans, causes longer telomere in adult female pythons [20]. The observed telomere elongation may occur through telomerase activity or through other pathways. In support of the telomerase pathway, telomerase activity has been reported in some studies of non-malignant peripheral mononuclear blood cells [21], but not in all studies [22]–[24]. The last notion is supported by the observations that in tumors, where telomerase activity is present as opposed to their normal tissue counterparts, the telomeres are shorter [22]–[24]. On the other hand, we cannot completely exclude that our observed telomere elongation to some extent could be an artifact, either caused by a redistribution of leukocyte subpopulations between tissues, as telomere length vary among lymphocyte subpopulations [23], [25] or other factors [26]. But as more studies using different assays report telomere elongation [16]–[19], the possibility of telomere elongation being an artifact seems to diminish. Nevertheless, it is possible that telomere elongation in some individuals simply is explained by regression toward the mean, as seen for most measurements of biological parameters [15]. Biological and analytical variability combined will cause individuals with the highest levels to have a lower level on retesting and vice versa for those with the lowest levels (the latter will lead to apparent telomere elongation, when in fact it could simply represent regression toward the mean). In support of this possibility, telomere length at baseline was the only factor strongly associated with change in telomere length: according to regression toward the mean, those with the longest telomeres on the first examination will get the largest decrease in telomere length on retesting 10 years later, while those with the shortest telomeres on the first examination will get the largest increase in telomere length on retesting 10 years later. This was indeed the case, suggesting that future prospective studies of telomere length and risk of disease should probably be corrected for regression dilution bias as is common practice for many risk factor studies [15].
Most surprisingly, we were able to confirm strong associations between lifestyle and short telomere length cross-sectionally, but prospectively lifestyle did not associate with telomere length change. How can this be, when numerous reports have associated lifestyle and telomere length cross-sectionally, even the present study? We speculate that a part of the answer could be collider bias [27], a special kind of selection bias. In our study, all-cause mortality and morbidity is a collider since these outcomes are associated with short telomeres, and at the same time might reduce the likelihood for the participant of being (re)examined. Therefore, individuals with short telomeres were probably underrepresented already at the 1991–94 as well as at the 2001–03 examination, and the participants with short telomeres could theoretically be strongly selected survivors despite their short telomeres. Nevertheless, as short telomeres were associated with all-cause mortality after both examinations, this potential bias was not strong enough to eliminate the association between short telomeres and increased all-cause mortality. Therefore, we still consider that the lack of association between telomere length change and lifestyle to be plausible.
Prospectively, we found no association between change in telomere length and mortality and morbidity. This is in contrast to cross-sectional and prospective studies reporting increased risk of early death in this and other studies [4], [5], early death after a cancer diagnosis [9], cardiovascular disease [2], [6], chronic obstructive pulmonary disease [28], [29], diabetes mellitus type II [2], ischemic cerebrovascular disease [30], [31], and ischemic heart disease [2], [6] in individuals with the shortest telomeres at baseline.
Strengths of the present study include study size as we examined 4,576 individuals from the general population, and follow-up time as individuals were observed on average for 9.3 years for telomere length change and then followed for up to 10 years more for mortality and morbidity. Next, telomere length was measured with a very precise assay and the measurements were validated through inverse association with age, while the measurement of change in telomere length over 10 years was validated through association with baseline telomere length, as reported previously [2], [9]. And finally, due to the national Danish Civil Registration System, the national Danish Causes of Death Registry, and the national Danish Patient Registry, we had complete information on mortality and morbidity without losses to follow-up of even a single individual.
Limitations of the present study include that we examined whites only, and therefore our results may not necessarily be applicable to other ethnicities; however, we are not aware of data to suggest that our results should not be applicable to all races. Also, we measured telomere length in leukocytes from peripheral blood only, and not in all cell types in the body; however, leukocyte telomere length correlate highly with that in cells from other tissues [10], [32]–[34]. Finally, it could be argued that qPCR measures telomere content rather than telomere length, due to the nature of the method.
In conclusion, smoking, body weight, and physical inactivity were associated with short telomere length cross-sectionally, but not with telomere length change during 10 years observation, while alcohol intake was associated with neither. Also, change in telomere length did not associate prospectively with mortality or morbidity in the general population.
Methods and Materials
Ethics statement
The study was approved by Herlev Hospital and by Danish ethical committees (No. KF-V.100.2039/91, KF-01-144/01), and were conducted according to the Declaration of Helsinki. Participants gave written informed consent.
Study participants
Participants were individuals from the prospective Copenhagen City Heart Study. All participants were white and of Danish descent. They were randomly selected from the national Danish Civil Registration System to reflect the adult Danish population ranging from age 20 to 100 years. The 4,576 participants in the present study participated both in the 1991–94 and 2001–03 examinations, and gave blood samples for DNA extraction at both examinations. Participation rate for the Copenhagen City Heart Study was 55%.
Covariates
Prior to examinations, participants filled in self-administered questionnaires concerning present and past life-style and health status. This was completed together with examiners at the day of examination, followed by physical examination and blood sampling. The following covariates were obtained at both examinations: age in years, gender, current smoking (yes/no), daily tobacco consumption (g/day), body mass index (measured weight in kilograms divided by squared measured height in meters), heavy alcohol intake (no/yes) (yes if female and male participants reported a weekly alcohol intake above 87.5 g and 175 g), and physical inactivity (no/yes) (yes if participants reported less than 4 hours weekly exercise).
Ascertainment of endpoints
Information on vital status from day of the second blood sampling at the 2001–03 examination until June 7th 2011 was obtained from the national Danish Civil Registration System. Diagnosis of invasive cancer from January 1st 1943 through December 31st 2009 were obtained from the Danish Cancer Registry, which identifies 98% of all cancers diagnosed in Denmark [35], [36]. Diagnoses were classified according to the World Health Organization's International Classification of Diseases (ICD). Diagnoses of cancer were ICD-10 codes C00–D09. Diagnoses of chronic obstructive pulmonary disease, diabetes mellitus type II, ischemic cerebrovascular disease, and ischemic heart disease were obtained from January 1st 1977 through May 10th 2011 from the national Danish Patient Registry and the national Danish Causes of Death Registry, public registers to which all hospitalizations and deaths in Denmark are reported. Diagnoses of chronic obstructive pulmonary disease were ICD-8 codes 491–492 and ICD-10 codes J41–J44. Diagnoses of diabetes mellitus type II were ICD-8 codes 249–250 and ICD10 codes E10–E14. Diagnoses of ischemic cerebrovascular disease were ICD-8 codes 431–438 and ICD-10 codes I61-I66 and G45. Diagnoses of ischemic heart disease were ICD-8 codes 410–414 and ICD-10 codes I20-I25. For all registries follow-up was 100% complete, that is, we did not lose track of even a single individual.
Relative telomere length
We measured relative telomere length in DNA extracted from leukocytes in peripheral blood. Relative telomere length was measured on a CFX384 real-time PCR detection system (Bio-Rad Laboratories, Denmark), using a modified Monochrome Multiplex Quantitative PCR method [37]. In the duplex PCR, the single-copy gene ALB which encodes albumin was amplified simultaneously with the telomere template in the same well and was used as a reference to adjust for different amounts of DNA in different samples. Final concentrations of PCR reagents were 1X QuantiFast SYBR Green Master Mix (Qiagen), 20 ng DNA, 300 nmol/L forward telomere primer (5′-ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT-′3), 300 nmol/L reverse telomere primer (5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-′3), 350 nmol/L forward albumin primer (5′-CGGCGGCGGGCGGCGCGGGCTGGGCGGAAATGCTGCACAGAATCCTTG-′3), and 350 nmol/L reverse albumin primer (5′-GCCCGGCCCGCCGCGCCCGTCCCGCCGGAAAAGCATGGTCGCCTGTT-′3) in a volume of 10 µL. The present thermal cycling profile was elaborated from the original publication: Stage 1 : 15 min at 95°C; Stage 2 : 2 cycles of 15 s at 94°C, 15 s at 49°C; and Stage 3 : 32 cycles of 15 s at 94°C, 10 s at 62°C, 15 s at 73°C with signal acquisition, 10 s at 84°C, 15 s at 87°C with signal acquisition; Stage 4 : 1 cycle of 0.05 s at 65°C with signal acquisition. SYBR Green I, in QuantiFast, bind to double stranded DNA to produce a fluorescence signal. The 73°C reads provided the Ct values for the amplification of the telomere template (in early cycles when the albumin signal is still at baseline). The 87°C reads provided the Ct values for the amplification of the albumin template (at this temperature the telomere template is fully melted). The 65°C signal acquisition in Stage 4 was used to identify wells with primer-dimer formation for rerun. On each 384-well plate, DNA from participants was examined in quadruplicates together with triplicates of an internal control and a calibrator consisting of genomic DNA from the cell lines NTERA-2 and K562, respectively (purchased from DMSZ, Braunschweig, Germany). To determine the absolute telomere length (absTL) in basepairs of our calibrator, we measured its T/S ratio relative to the reference DNA sample used in Dr. Cawthon's 2009 methods [37], and then calculated the calibrator's absTL as [(T/S)×(absTL of the reference DNA)]. The absTL of this reference DNA is provided by the slope of the linear regression line in Figure 5 of ref. [38], since that slope equals the change in mean Terminal Restriction Fragment length (in basepairs) by Southern blot, per unit change in T/S, and the reference DNA, by definition, has T/S = 1.0 unit. The absTL of the calibrator derived in this way was 5,290 basepairs, which was comparable to previous reports for this cell line of 5,360 and 6,500 basepairs [39], [40]. Ct values are the raw results from a quantitative PCR analysis. Ct is short for cycle threshold, which is the number of PCR cycles needed to produce enough amplicons to raise the SYBR Green I fluorescence signal above a computer set threshold of the background fluorescence. T/S ratios were calculated as the cycle threshold value of the telomere amplicon divided by the cycle threshold value of the albumin amplicon [38].
Assay efficiency
DNA from each participant was measured in four different wells, resulting in four Ct values for the telomere signal and four Ct values for the albumin signal. A mean of the four telomere Ct values and the four albumin Ct values were calculated, and wells producing measurements outside mean Ct values ±2 SD were excluded, and new mean Ct values for telomere and albumin were calculated after exclusion of these outlier wells. Participants with less than two valid telomere and albumin Ct values were run again. For each participant we calculated a mean Ct value for the telomere assay (Cttel X) and for the albumin assay (Ctalb X). Similarly for the calibrator, we calculated means for Cttel and Ctalb across all plates (Cttel K562_All and Ctalb K562_All) and for each individual plate (Cttel K562_plate_X and Ctalb K562_plate_X). We calculated normalization factors for both assays on every plate. For the telomere assay, the normalization factor was: factortel_plate_X = (Cttel K562_All/Cttel K562_plate_X). Next, we calibrated Cttel and Ctalb of internal controls and participant samples. For the telomere assay, the calibration was: Cttel X _calibrated = Cttel X * factortel_plate_X. We then used the ΔΔCt calculation to derive the unknown relative telomere length of a participant sample (X): ΔCtX = Ctalb X_calibrated – Cttel X_calibrated. ΔCt K562 = Ctalb K562 – Cttel K562 and ΔΔCt = ΔCtX – ΔCt K562. The absolute telomere length of sample X was then calculated as = 2∧ΔΔCt * 5290 basepairs. Failed samples were measured a second round and a third if they failed again. Therefore, valid measurements of relative telomere lengths were available for more than 99.9% of participants.
Assay precision
The coefficient of variation was measured with the use of triplicates in each plate of DNA from the cell line NTERA-2, where ΔCt and the absolute telomere length were calculated exactly as for participant samples. Means and standard deviations were calculated for Cttel NTERA-2 and absolute telomere length values. The inter-assay coefficient of variation (CV) was determined using the calculation: CV = (standard deviation/mean)*100%. Coefficients of variation for the internal control were 1.8% for Cttel NTERA-2 at mean value of 18.0 and 9.3% for absolute telomere length in basepairs at the mean level of 2,577 basepairs. Intra-assay participant CV was 0 to 5.1% for Cttel and 0 to 3.6% for Ctalb.
Participants gave blood twice for DNA extraction with a roughly 10 years interval, and at both extractions we used the Qiagen blood kit. A median of 9.3 years passed between the first and second examination (SD = 0.43 years). We calculated 10 year change in telomere length using the formula: ((2001–03 telomere length – 1991–95 telomere length)/((2001–03 examination date – 1991–94 examination date)/365.25days)))*10 years. Thus, for each individual person we used the exact time difference between the two measurements and then converted it to a 10 year change.
Statistical analyses
We used the statistical software package STATA version 11.1 for analysis (StataCorp, College Station, Texas). All statistical tests were two-sided. Information on age, gender, and year of birth was complete, while information on other covariates was more than 99% complete. For multivariable analyses, missing continuous covariates were imputed based on age and gender, whereas missing categorical values were assigned to a missing category.
As quantitative PCR for relative telomere length measurement is sensitive to DNA quality, type of DNA extraction, time between sample collection and processing, and as there is some degree of variation among runs, we already made sure that differences in DNA storage and isolation would not produce a false overall change (reduction or elongation alike) in relative telomere length. We did this by performing a linear regression between the 1991–94 examination relative telomere length and age. The coefficients from this analysis were used to calibrate the association between the 2001–03 relative telomere length measurement and age, and thereby ensuring that the association between relative telomere length and age remained, while keeping the measured variance from the 2001–03 measurement. As this calibration reduced the likelihood that differences in DNA isolation and storage would produce a false overall change (reduction or elongation alike) in relative telomere length, it also means that we cannot examine the average change in relative telomere length in the whole population over 10 years, but we can accurately look at relative changes in telomere length within each individual.
In the cross-sectional analysis, participants were categorized according to examination specific quartiles of telomere length for trend tests. Individuals in the 1st quartile had the longest telomere length and individuals in the 4th quartile had the shortest telomere length.
Absolute 10 years change in telomere length in basepairs was calculated as ((telomere length in basepairs at the 2001–2003 examination – telomere length in basepairs at the 1991–1994 examination)/(years between examinations))*10. Percent 10 years change in telomere length was calculated as ((telomere length in basepairs at the 2001–2003 examination - telomere length in basepairs at the 1991–1994 examination)/(telomere length in basepairs at the 1991–1994 examination))*100%. We used linear regression to associate leukocyte change with age at baseline (years), baseline tobacco consumption (g/day), tobacco consumption between examinations (g/day), baseline body weight (kg), body weight change (%), baseline alcohol intake (units of 12 g alcohol/day), alcohol intake between examinations (units of 12 g alcohol/day), baseline physical activity (hours/week), and physical activity between examinations (hours/week). Baseline was the 1991–1994 examination.
For prospective analysis we tested the proportional hazards assumption visually by plotting -ln(-ln(survival)) versus ln(analysis time): no violations were observed. Study entry for prospective analysis was defined as the day of blood sampling at the 2001–2003 examination, except for the analysis of mortality as a function of telomere length after each examination separately (Figure 4), where follow-up began at the day of examination. Participants were categorized according to quartiles of telomere length change. Individuals in the 1st quartile gained the most and individuals in the 4th quartile lost the most telomere basepairs between examinations. Follow-up began at the day of blood sampling at the 2001–2003 examination, and ended at death, diagnosis of endpoint of interest, emigration (n = 39), or June 7th 2011 (for death endpoint), December 31st 2009 (for cancer endpoint), and May 10th 2011 (for chronic obstructive pulmonary disease, diabetes mellitus type II, ischemic cerebrovascular disease, and ischemic heart disease endpoints). Participants diagnosed with an endpoint of interest before the day of blood sampling at the 2001–2003 examination were excluded from analysis. For this reason numbers of participants among endpoints vary slightly. Multivariable adjustment included the following covariates obtained at the 2001–2003 examination: age (years), gender (male/female), current smoking (no/yes), daily tobacco consumption (g), body mass index (kg/m2), heavy alcohol intake (no/yes), and physical inactivity (no/yes).
Supporting Information
Zdroje
1. BlackburnEH (1991) Structure and function of telomeres. Nature 350 : 569–573 10.1038/350569a0 [doi]
2. WeischerM, BojesenSE, CawthonRM, FreibergJJ, Tybjaerg-HansenA, et al. (2012) Short Telomere Length, Myocardial Infarction, Ischemic Heart Disease, and Early Death. Arterioscler Thromb Vasc Biol 32 : 822–829.
3. WongJM, CollinsK (2003) Telomere maintenance and disease. Lancet 362 : 983–988 S0140-6736(03)14369-3 [pii];10.1016/S0140-6736(03)14369-3 [doi]
4. FitzpatrickAL, KronmalRA, KimuraM, GardnerJP, PsatyBM, et al. (2011) Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 66 : 421–429 glq224 [pii];10.1093/gerona/glq224 [doi]
5. WilleitP, WilleitJ, Kloss-BrandstatterA, KronenbergF, KiechlS (2011) Fifteen-year follow-up of association between telomere length and incident cancer and cancer mortality. JAMA 306 : 42–44 306/1/42-a [pii];10.1001/jama.2011.901 [doi]
6. WilleitP, WilleitJ, BrandstatterA, EhrlenbachS, MayrA, et al. (2010) Cellular Aging Reflected by Leukocyte Telomere Length Predicts Advanced Atherosclerosis and Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol 1649–1655.
7. MaH, ZhouZ, WeiS, LiuZ, PooleyKA, et al. (2011) Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One 6: e20466 10.1371/journal.pone.0020466 [doi];PONE-D-11-04747 [pii]
8. WentzensenIM, MirabelloL, PfeifferRM, SavageSA (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20 : 1238–1250 1055-9965.EPI-11-0005 [pii];10.1158/1055-9965.EPI-11-0005 [doi]
9. WeischerM, NordestgaardBG, CawthonRM, FreibergJJ, Tybjaerg-HansenA, et al. (2013) Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst 105 : 459–468 djt016 [pii];10.1093/jnci/djt016 [doi]
10. ButtHZ, AtturuG, LondonNJ, SayersRD, BownMJ (2010) Telomere length dynamics in vascular disease: a review. Eur J Vasc Endovasc Surg 40 : 17–26.
11. CherkasLF, HunkinJL, KatoBS, RichardsJB, GardnerJP, et al. (2008) The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 168 : 154–158.
12. von ZglinickiT, Martin-RuizCM (2005) Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 5 : 197–203.
13. Londono-VallejoJA (2008) Telomere instability and cancer. Biochimie 90 : 73–82.
14. MatherKA, JormAF, ParslowRA, ChristensenH (2011) Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci 66 : 202–213.
15. ClarkeR, ShipleyM, LewingtonS, YoungmanL, CollinsR, et al. (1999) Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 150 : 341–353.
16. AvivA, ChenW, GardnerJP, KimuraM, BrimacombeM, et al. (2009) Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol 169 : 323–329 kwn338 [pii];10.1093/aje/kwn338 [doi]
17. EhrlenbachS, WilleitP, KiechlS, WilleitJ, ReindlM, et al. (2009) Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol 38 : 1725–1734.
18. EpelES, MerkinSS, CawthonR, BlackburnEH, AdlerNE, et al. (2009) The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 1 : 81–88.
19. NordfjallK, SvensonU, NorrbackKF, AdolfssonR, LennerP, et al. (2009) The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet 5: e1000375 10.1371/journal.pgen.1000375 [doi]
20. UjvariB, MadsenT (2009) Short telomeres in hatchling snakes: erythrocyte telomere dynamics and longevity in tropical pythons. PLoS One 4: e7493 10.1371/journal.pone.0007493 [doi]
21. BroccoliD, YoungJW, deLT (1995) Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A 92 : 9082–9086.
22. HathcockKS, JeffreyCY, HodesRJ (2005) In vivo regulation of telomerase activity and telomere length. Immunol Rev 205 : 104–113 IMR267 [pii];10.1111/j.0105-2896.2005.00267.x [doi]
23. LinJ, EpelE, CheonJ, KroenkeC, SinclairE, et al. (2010) Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods 352 : 71–80 S0022-1759(09)00292-0 [pii];10.1016/j.jim.2009.09.012 [doi]
24. TrentinL, BallonG, OmettoL, PerinA, BassoU, et al. (1999) Telomerase activity in chronic lymphoproliferative disorders of B-cell lineage. Br J Haematol 106 : 662–668 bjh1620 [pii]
25. WengNP, GrangerL, HodesRJ (1997) Telomere lengthening and telomerase activation during human B cell differentiation. Proc Natl Acad Sci U S A 94 : 10827–10832.
26. SteenstrupT, HjelmborgJV, KarkJD, ChristensenK, AvivA (2013) The telomere lengthening conundrum–artifact or biology? Nucleic Acids Res 41: e131 gkt370 [pii];10.1093/nar/gkt370 [doi]
27. Rothman KJ, Greenland S, and Lash TL (2008) Modern Epidemiology. Lippencott-Raven Publishers; 2008 : 183–209. Lippencott-Raven Publishers. 186 p.
28. RodeL, BojesenSE, WeischerM, VestboJ, NordestgaardBG (2013) Short telomere length, lung function and chronic obstructive pulmonary disease in 46,396 individuals. Thorax 68 : 429–435 thoraxjnl-2012-202544 [pii];10.1136/thoraxjnl-2012-202544 [doi]
29. SavaleL, ChaouatA, Bastuji-GarinS, MarcosE, BoyerL, et al. (2009) Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179 : 566–571 200809-1398OC [pii];10.1164/rccm.200809-1398OC [doi]
30. DingH, ChenC, ShafferJR, LiuL, XuY, et al. (2012) Telomere length and risk of stroke in Chinese. Stroke 43 : 658–663 STROKEAHA.111.637207 [pii];10.1161/STROKEAHA.111.637207 [doi]
31. FitzpatrickAL, KronmalRA, GardnerJP, PsatyBM, JennyNS, et al. (2007) Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 165 : 14–21.
32. FriedrichU, GrieseE, SchwabM, FritzP, ThonK, et al. (2000) Telomere length in different tissues of elderly patients. Mech Ageing Dev 119 : 89–99.
33. OkudaK, BardeguezA, GardnerJP, RodriguezP, GaneshV, et al. (2002) Telomere length in the newborn. Pediatr Res 52 : 377–381.
34. WilsonWR, HerbertKE, MistryY, StevensSE, PatelHR, et al. (2008) Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J 29 : 2689–2694.
35. StormHH (1988) Completeness of cancer registration in Denmark 1943–1966 and efficacy of record linkage procedures. Int J Epidemiol 17 : 44–49.
36. StormHH, MichelsenEV, ClemmensenIH, PihlJ (1997) The Danish Cancer Registry–history, content, quality and use. Dan Med Bull 44 : 535–539.
37. CawthonRM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37: e21.
38. CawthonRM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30: e47.
39. AkiyamaM, YamadaO, KandaN, AkitaS, KawanoT, et al. (2002) Telomerase overexpression in K562 leukemia cells protects against apoptosis by serum deprivation and double-stranded DNA break inducing agents, but not against DNA synthesis inhibitors. Cancer Lett 178 : 187–197 S0304383501008382 [pii]
40. WangY, FangMY (2006) Effect of ginseng saponin, arsenic trioxide, beta-elemene combined with CTX on telomere-telomerase system in K562 cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi 14 : 1089–1095 1009-2137(2006)06-1089-07 [pii]
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



