-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
The mechanisms that govern the formation, size and signaling output of in vivo niches remain poorly understood. Studies of Drosophila germline stem cells (GSCs) have suggested that chromatin programming greatly influences the behavior of these cells and their progeny. Previous work has shown that loss of the highly conserved histone demethylase Lsd1 results in ectopic niche signaling and an expanded GSC phenotype. To determine direct regulatory targets of Lsd1, we employed chromatin immunoprecipitation coupled with massive parallel sequencing (ChIP-seq) using specific cell populations inside and outside of the GSC niche. These experiments revealed that Lsd1 exhibits highly enriched binding to over one hundred genomic sites within a specific cell population. Furthermore, mis-regulation of some of these direct targets contributes to the expanded stem cell phenotype observed in Lsd1 mutants. These results provide insights into how Lsd1 directly restricts the size of the GSC microenvironment and establish a platform for understanding and exploring chromatin programming inside and outside an in vivo stem cell niche.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004200
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004200Summary
The mechanisms that govern the formation, size and signaling output of in vivo niches remain poorly understood. Studies of Drosophila germline stem cells (GSCs) have suggested that chromatin programming greatly influences the behavior of these cells and their progeny. Previous work has shown that loss of the highly conserved histone demethylase Lsd1 results in ectopic niche signaling and an expanded GSC phenotype. To determine direct regulatory targets of Lsd1, we employed chromatin immunoprecipitation coupled with massive parallel sequencing (ChIP-seq) using specific cell populations inside and outside of the GSC niche. These experiments revealed that Lsd1 exhibits highly enriched binding to over one hundred genomic sites within a specific cell population. Furthermore, mis-regulation of some of these direct targets contributes to the expanded stem cell phenotype observed in Lsd1 mutants. These results provide insights into how Lsd1 directly restricts the size of the GSC microenvironment and establish a platform for understanding and exploring chromatin programming inside and outside an in vivo stem cell niche.
Introduction
Stem cells undergo self-renewing divisions in which at least one daughter retains its stem cell identity, while the second daughter may or may not differentiate, depending on intrinsic and extrinsic cues. A balance between stem cell self-renewal and differentiation must be maintained for proper organ formation during development and tissue homeostasis in adulthood. Stem cells often reside in microenvironments called niches, and specific mechanisms tightly regulate the size and signaling output of these structures [1]. However, in vivo niches have often proven difficult to identify in mammalian tissues. As a result, much of the current understanding of niches stems from the study of invertebrate models such as the germline stem cells (GSCs) of the Drosophila ovary.
Drosophila female GSCs reside in a well-characterized niche at the tip of a structure called a germarium (Figure 1A). Within germaria, GSCs lie immediately next to a somatic cell niche comprised of cap cells and terminal filament cells [2]. Escort cells reside adjacent to the cap cells and line the anterior portion of the germarium. These cells act to shepherd the germ cells during the earliest stages of their differentiation [3], [4], after which developing germline cysts are enveloped by follicle cells derived from a second stem cell population within the germarium. Cap cells produce Decapentaplegic (Dpp), which in turn activates a canonical Bone Morphogenic Protein (BMP) signal transduction pathway in GSCs [5], [6]. BMP pathway activation results in the transcriptional repression of bag of marbles (bam) [7]–[9], a factor both necessary and sufficient for germ cell differentiation [10], [11]. Ectopic Dpp signaling outside the tip of the germarium results in an expanded GSC phenotype [5], [9].
Fig. 1. Further characterization of the Lsd1 mutant phenotype. 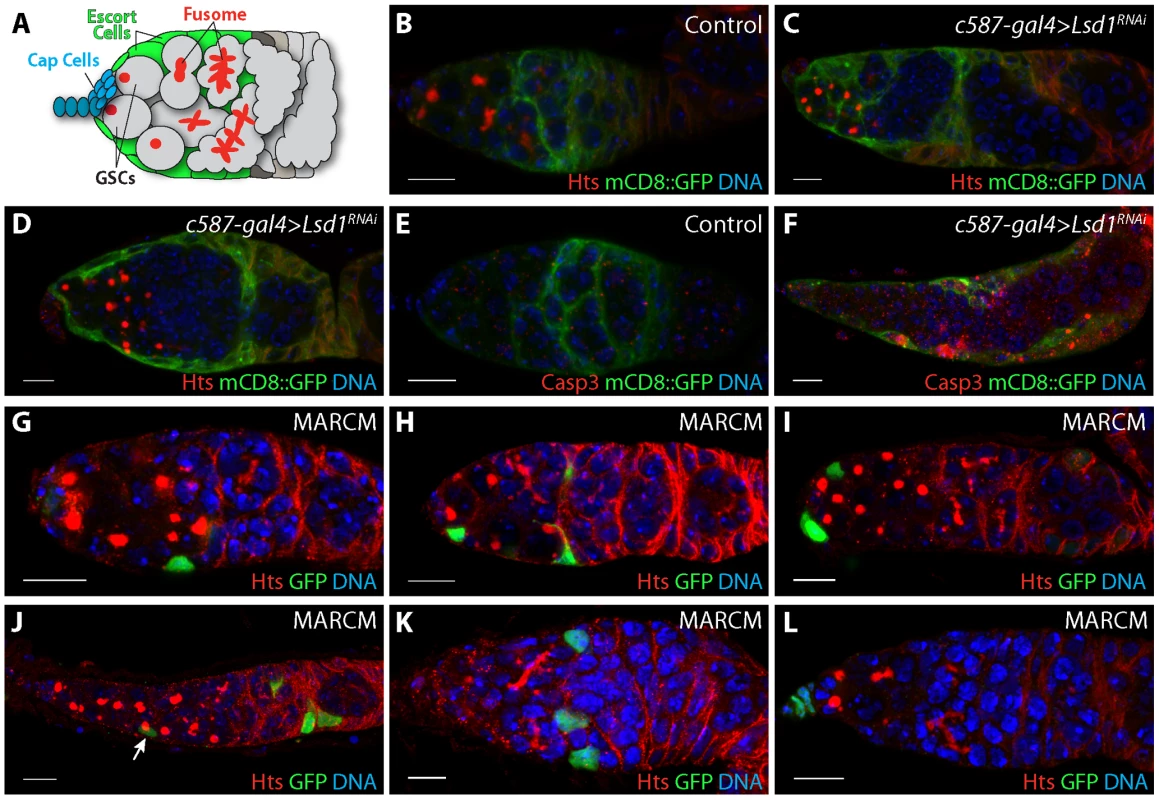
(A) Schematic of a Drosophila germarium. Two germline stem cells (GSCs) reside next to terminal filament and cap cells labeled in blue. GSCs carry a round endoplasmic reticulum-like organelle called a fusome or spectrosome. These fusomes become branched in appearance as GSC daughters form multicellular cysts. Lining the anterior end of the germarium next to the cap cells are the escort cells labeled in green. Germaria from (B) c587-gal4>mCD8::GFP and (C,D) c587-gal4>mCD8::GFP+Lsd1RNAi three day old females stained for Hts (red), GFP (green) and DNA (blue). RNAi knockdown of Lsd1 results in the accumulation single cells with round fusomes and the gradual retraction of escort cell extensions. (E) c587-gal4>mCD8::GFP and (F) c587-gal4>mCD8::GFP+Lsd1RNAi germaria from females shifted to 29°C for 3 days stained for activated Caspase 3 (Casp3), GFP (green) and DNA (blue). (G–L) MARCM Lsd1ΔN clones stained for Hts (red), GFP (green) and DNA (blue). Lsd1 mutant escort cell clones are associated with undifferentiated germ cells with round fusomes. Scale bars = 10 µM. Other pathways and neighboring cells likely regulate niche specific BMP signaling. For example, a recent study provides evidence that hedgehog (hh) produced by the cap cells stimulates the anterior escort cells to produce niche specific signals [12] Moreover, several additional intrinsic and extrinsic mechanisms help restrict Dpp ligand production and diffusion within the niche (reviewed in [13], [14]). One such mechanism involves the histone demethylase Lysine Specific Demethylase 1 (Lsd1). Lsd1 uses a flavin-dependent monoamine oxidative based mechanism to remove mono - and di-methyl groups from histone H3 on lysine 4 (H3K4me1 and H3K4me2) [15]. In mammals, Lsd1 has been shown to silence a number of distinct gene sets in different cellular contexts, including Notch targets, TGFβ-1 and various loci involved in the maintenance of embryonic stem cells [16]–[20]. Additional studies suggest that Lsd1 may also promote gene expression under certain circumstances [21]. Disruption of Drosophila Lsd1 results in a male and female sterile phenotype, marked by the expansion of GSC-like cells in the germarium [22], [23]. These cells exhibit ectopic BMP responsiveness and fail to initiate a normal differentiation program once they leave the cap cell niche [24].
To characterize the molecular mechanism by which Lsd1 restricts signaling outside the Drosophila female GSC niche, we used ChIP-seq to define direct binding sites of Lsd1 specifically in either escort cells or cap cells. These experiments revealed that Lsd1 binds to over one hundred sites in escort cells and provide further insights into how Lsd1 contributes to the chromatin programming of cells inside and outside of an in vivo niche.
Results
Further characterization of the Lsd1 loss-of-function phenotype
Escort cells send out extensions that closely contact germline cysts undergoing the early steps of differentiation [3], [4]. Escort cell death or genetically disrupting escort cell extensions can lead to an inappropriate expansion of GSC-like cells in the germarium [3]. Previous results showed that Lsd1 functions within escort cells to prevent expanded BMP signaling outside of the GSC niche [24]. This phenotype was accompanied by widespread cell death in both somatic cells and germ cells. Therefore we considered the possibility that the expansion of BMP signaling exhibited by Lsd1 mutants may depend on changes to escort cell morphology and number. To test this, we knocked down the expression of Lsd1 specifically in the escort cells and early follicle cells by crossing UAS-Lsd1RNAi into a c587-gal4; UAS-mCD8::GFP background and stained the resulting ovaries for GFP and the fusome marker Hts. Fusomes are highly vesiculated organelles that appear round in GSCs and cystoblasts, and become branched as these germ cells differentiate into multi-cellular cysts [25], [26]. Three days after eclosion, control samples appeared normal. These germaria typically contained two to four single cells (GSCs and cystoblasts) with round fusomes and escort cells that extended cytoplasmic processes between the developing cysts (Figure 1B). In contrast, the Lsd1 RNAi samples showed an expansion of GSC-like cells with round fusomes. Escort cell extensions were clearly present in some germaria, but were missing in others (Figure 1C,D). These observations suggested that while the knockdown of Lsd1 caused changes in escort cell morphology, the presence of extra single cells with round fusomes did not absolutely depend on a complete loss of escort cell extensions. However changes in escort cell morphology likely contributed to the phenotype over time. In addition, expression of Lsd1RNAi also led to an increase of death within escort cells, consistent with the widespread cell death previously noted in Lsd1 null mutant germaria (Figure 1E,F) [24].
Next we performed clonal analysis using the mosaic analysis with a repressible cell marker (MARCM) system to further analyze the Lsd1 mutant phenotype. Clonal germaria were stained for the positive clone marker GFP and for the fusome marker Hts. We categorized the relative position of escort cell clones along the anterior-posterior axis of the germarium. Cells were considered anterior escort cells if they were immediately next to the cap cells, posterior escort cells if they were immediately adjacent to the follicle stem cells and middle escort cells if they were located in any position in between. We induced control and Lsd1 mutant clones in parallel. Control escort cell clones were never associated with an obvious robust germline tumor phenotype, although we noted one exception in which a single germarium with control escort cell clones contained six single germline cells with round fusomes (1 out of 143 counted). By contrast, 17% (27/155) of the germaria that contained Lsd1 mutant escort cell clones displayed an expanded germline stem cell-like cell phenotype (Figure 1G–J). The relative position of Lsd1 mutant clones appeared to correlate with the appearance of a germline phenotype. The vast majority of germaria (96%; n = 27) that contained greater than 5 germline stem cell-like cells carried at least one Lsd1 mutant middle escort cell clone. We observed one example in which a germarium with a mild expansion of GSC-like cells contained an Lsd1 mutant anterior clone and posterior clone but no middle escort cell clones (Figure 1H). Of note, most germaria that carried middle escort cell clones did not exhibit a GSC expansion phenotype (98/125 germaria). While their appearance was rare, germaria with only anteriorly or posteriorly (Figure 1K) positioned escort cell clones did not display a robust GSC-like cell expansion phenotype. Similarly, loss of Lsd1 in the terminal filament did not result in an obvious phenotype (Figure 1L).
Germaria that contained Lsd1 mutant escort cell clones and exhibited an increased number of GSCs occasionally had an elongated and abnormal morphology (Figure 1J). Moreover, 22.1% of the germaria (n = 199) from Lsd1 mutant females lacked marked escort cell clones, compared to 13.9% of control germaria (n = 166), and the average number of Lsd1 mutant clones per germarium (4.72 escort cell clones/germarium) was lower compared to controls (8.77 escort cell clones/germarium), suggesting that either Lsd1 mutant escort cell progenitors exhibited reduced proliferation during development or died during the course of the experiment. If increased death within the escort cell population accounted for all the observed phenotypes, one might predict that complete loss of all Lsd1 mutant escort cell clones within a particular germarium would result in an increased number of GSC-like cells. However, we did not observe an expanded GSC phenotype in germaria that lacked clones from Lsd1 mutant females. Together all the phenotypic data suggest both that escort cells require Lsd1 function to limit GSC number and that loss of Lsd1 compromises the growth and survival of escort cells, consistent with previous observations [24] and those noted above, which in turn further exacerbates the observed germ cell phenotypes.
Mapping Lsd1 binding sites in specific cell populations within the germarium
To directly define the molecular mechanisms by which Lsd1 influences escort cell function, we elected to identify direct targets of Lsd1 regulation in these cells. Determining whether Lsd1 targeted potential candidate genes represented a significant challenge. For example, the size and complexity of the dpp promoter precluded our ability to assay whether Lsd1 directly targeted this gene using a PCR based chromatin immunoprecipitation (ChIP) approach. To systematically define Lsd1 binding sites, we conducted ChIP experiments coupled with massive parallel sequencing (ChIP-seq). We used a number of different Hemagglutinin (HA) tagged transgenes, including a N-terminally tagged UASt-HA::Lsd1 transgene that exhibits high expression in the somatic cells and a N-terminally tagged UASp-HA::Lsd1 transgene that displays relatively lower levels of expression in the somatic cells (Figure 2; S1). The UASt-HA::Lsd1 and UASp-HA::Lsd1 transgenes both fully rescued the Lsd1ΔN GSC tumor phenotype when driven by the c587-gal4 driver. Because Lsd1 is expressed ubiquitously throughout the ovary [24], we sought to determine whether this protein bound to distinct sites in different cell populations within the germarium. We expressed the UASt-HA::Lsd1 and UASp-HA::Lsd1 trangenes in cap cells and terminal filament cells using hh-gal4 (Figure 2A; S1) and in the escort cells and early follicle cells using the c587-gal4 driver (Figure 2B; S1). HA-directed ChIP assays were performed on dissected ovaries and the immunoprecipitated chromatin was compared to input chromatin as a control. Within escort cells and early follicle cells, products of the UASp-HA::Lsd1 and UASt-HA::Lsd1 trangenes bound to 207 and 191 sites respectively (Based on the FindPeaks algorithm using a p-value threshold of 1.00e-3 to maximize the number of potential peaks; Table S1, S2), with 100 common sites sharing some degree of overlap (Figure 2C). Within cap cells and terminal filament cells, the UASp-HA::Lsd1 and UASt-HA::Lsd1 transgenic products associated with 98 and 167 genomic loci respectively (Table S3, S4), with 37 overlapping loci in common between the two datasets (Figure 2C). Comparing all four datasets revealed 66 common peaks between terminal filament/cap cells and escort cells/early follicle cells (Figure 2C,D). 232 peaks appeared specific for escort cells and early follicle cells and 162 specific for cap cells and terminal filament cells (Figure 2C,D). MACs analysis [27] showed similar but broader peak calls (Table S5, S6, S7, S8). Lsd1 enrichment peaks were spread throughout the Drosophila genome (Figure S2) and showed a preference for the promoter and 5′UTR regions of genes (Figure S3). We were unable to isolate a sufficient number of cells to map H3K4 methylation across the escort cell genome. However, comparing our data with available data from the modENCODE project revealed that Lsd1 binding peaks correlate with valleys of H3K4 methylation in embryos (Figure S4), consistent with the established biochemical activity of Lsd1.
Fig. 2. Defining the targets of Lsd1 in the cap cell niche and the surrounding escort cells within Drosophila germaria. 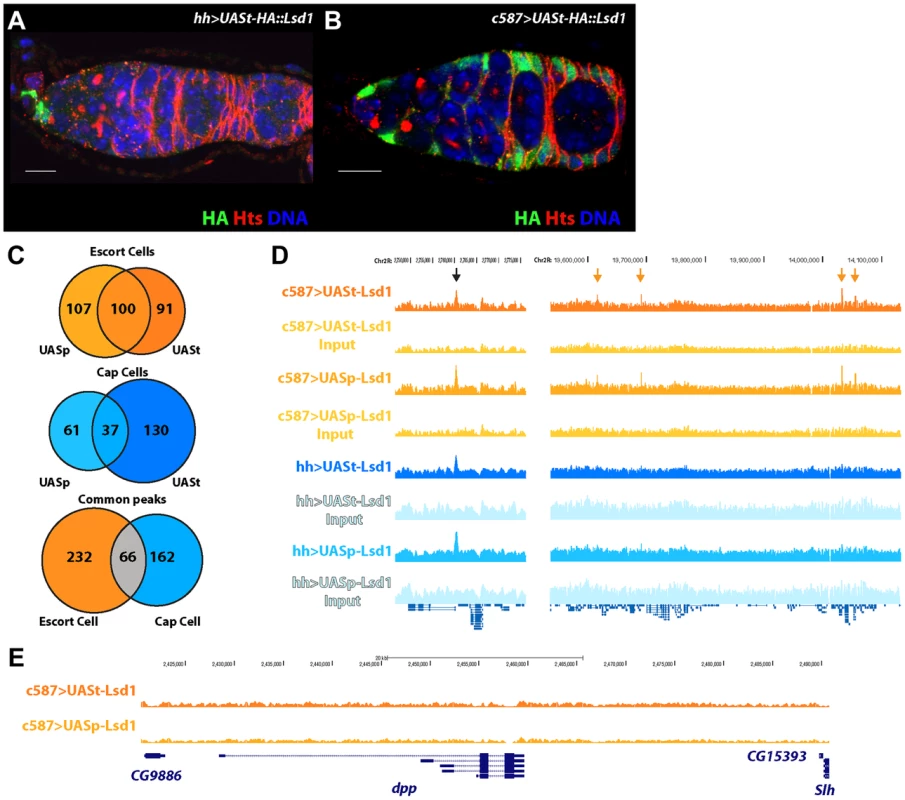
(A,B) Germaria stained for HA (green), to label the transgene, Hts (red), to label fusomes, and DNA (blue) (Scale bars, 10 µM). (A) hh-gal4>UASt-HA::Lsd1 germarium showing expression in cap cells and (B) c587-gal4>UASt-HA::Lsd1 germarium displaying expression in the escort cells and early follicle cells, but not in the cap cells. (C) Venn diagram showing the number of Lsd1 binding sites in escort cells and cap cells. (D) Lsd1 binding peaks on a segment of Chr2R. Black arrow points to a common peak in both the escort cell and cap cell populations. Orange arrows point to unique peaks seen only in the escort cell and early follicle cell population. (E) No Lsd1 binding sites are observed around the dpp locus in escort cells. Strikingly, we did not observe any enrichment for Lsd1 binding near the dpp locus in escort cells (Figure 2E), indicating that the repression of BMP signal transduction by Lsd1 must be through an indirect mechanism. We examined the annotation of genes near escort cell and early follicle cell peaks, cap cell and terminal filament peaks, shared peaks and from the UASt-HA::Lsd1 data sets (Table S9, S10, S11, S12). This analysis indicated that genes near escort cell specific Lsd1 binding peaks encode products with a diverse array of functions needed for development, basic cellular processes and reproduction (Figure S5A) [28], [29]. Further analysis of this gene set did not reveal significant enrichment for components of specific pathways. MEME analysis showed an enrichment of ACTGGAA elements within Lsd1 binding sites (Figure S5B). The significance of this enrichment remains unclear. These results suggested that the mis-expression of a functionally diverse set of genes likely contributes to the Lsd1 mutant phenotypes.
engrailed and hedgehog mis-regulation contribute to the Lsd1 mutant phenotype
The engrailed gene stood out as one potentially relevant target among the list of candidate genes. engrailed encodes a homeobox transcription factor that acts as a segment polarity gene during embryogenesis [30]–[32]. Previous results showed that engrailed regulates early ovarian development and that Engrailed protein expression is restricted to the terminal filament and cap cells in adult germaria [33]. Engrailed functions within these cells to help maintain GSCs [12]. Our ChIP-seq data revealed that Lsd1 exhibits enriched binding to a 2 kb region of the engrailed promoter in the escort cells (Figure 3A). We performed RNA RT-qPCR to look at the transcript levels of engrailed in Lsd1 mutants. We compared bam mutants to bam Lsd1 double mutants because these samples are comparable in size and have the same basic cellular makeup (Figure S6). This analysis revealed that engrailed transcript levels increased 6 fold in the absence of Lsd1 (Figure 3B).
Fig. 3. Lsd1 silences engrailed in the escort cells. 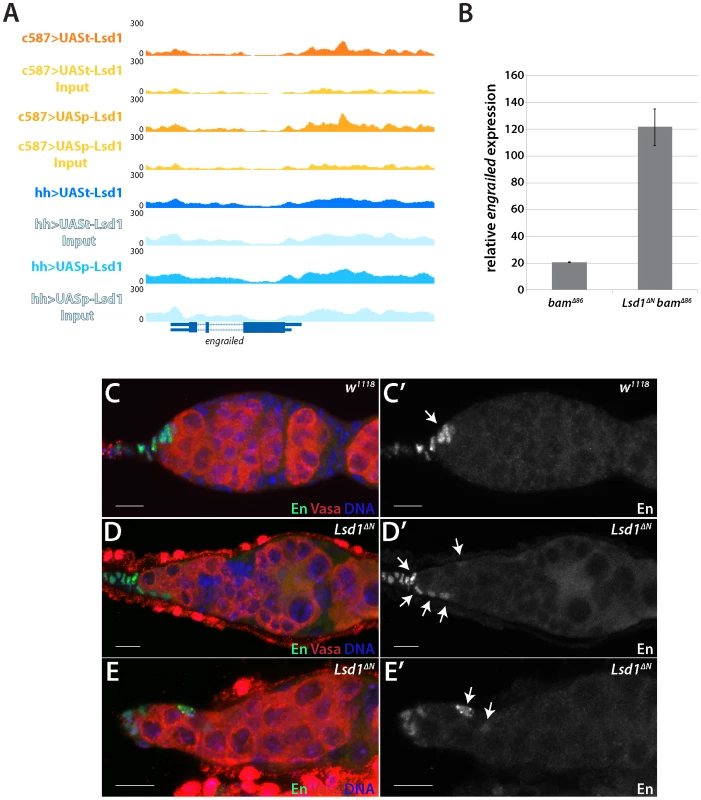
(A) A Lsd1 binding peak is observed in the engrailed promoter in escort cells. (B) RT-qPCR of whole ovary extracts from bamΔ86 and bamΔ86 Lsd1ΔN double mutants. The bamΔ86 Lsd1ΔN double mutant samples exhibit a relative 6-fold increase of engrailed transcripts when compared to bamΔ86 mutants. (C and C′) w1118 and (D–E′) Lsd1ΔN homozygous germaria stained for Engrailed (En) (green) and VASA (red) and DNA (blue). Arrows point to Engrailed positive cells. In w1118 control germaria, Engrailed is expressed in the terminal filament and cap cells, whereas Lsd1 mutants display engrailed expression in the anterior escort cells. Scale bars = 10 µM. Next, we tested whether Engrailed protein expression expanded in the absence of Lsd1. In wild type germaria, cap cells and terminal filament cells express readily detectable levels of Engrailed, whereas the escort cells do not (Figure 3C) [12], [33]. In Lsd1ΔN mutants, however, we observed ectopic Engrailed protein expression in a limited number of escort cells, in addition to the terminal filament and cap cells, in 85.7% (n = 91) of the germaria examined (Figure 3D). These Engrailed expressing escort cells tended to be positioned immediately adjacent to the normal niche, although occasionally we observed Engrailed expressing escort cells several cell positions away from the cap cells (Figure 3E). We cannot rule out the possibility that other cells also mis-expressed Engrailed, but at a level below our detection threshold. These data together suggest that Lsd1 serves to repress engrailed expression within a subpopulation of escort cells.
To test the functional relevance of ectopic Engrailed expression in escort cells, we assayed whether disruption of engrailed function, either through RNAi knockdown or loss-of-function mutations, modified the Lsd1 mutant phenotype. Knockdown of engrailed in an Lsd1 RNAi background (Figure 4A) suppressed the expanded GSC-like cell Lsd1 mutant phenotype (Figure 4B,E,F). Furthermore, three independent engrailed mutations also suppressed the Lsd1 RNAi-induced phenotype, so that the number of single cells with round fusomes decreased and cyst development within the germarium proceeded normally (Figure 4C–F). In all cases, engrailed suppression of the Lsd1 RNAi phenotype resulted in the formation of morphologically normal ovarioles with maturing egg chambers.
Fig. 4. engrailed mutations suppress the Lsd1 RNAi phenotype. 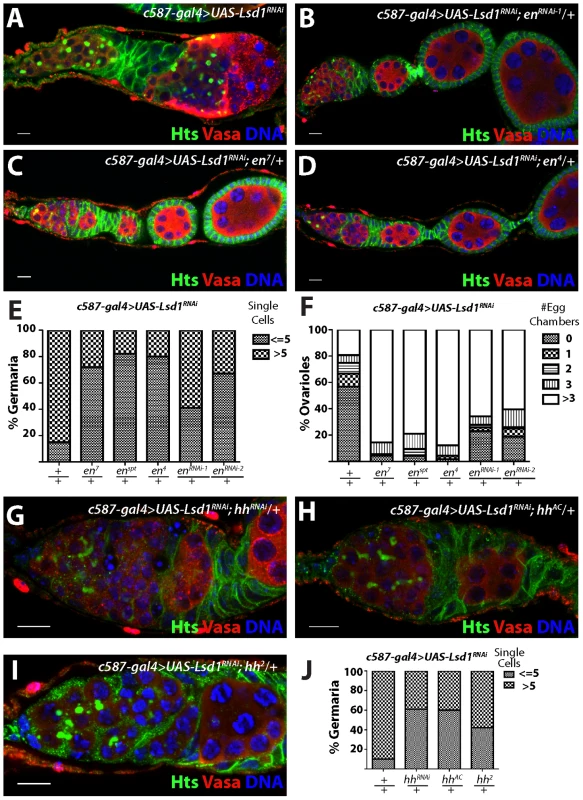
Ovarioles from (A) c587-gal4>UAS-Lsd1RNAi (B) c587-gal4>UAS-Lsd1RNAi; UAS enRNAi-1/+ (C) c587-gal4>UAS-Lsd1RNAi; en7/+ and (D) c587-gal4>UAS-Lsd1RNAi; en4/+ stained for Hts (green), VASA (red) and DNA (blue). (E) Graph showing the percentage of germaria that contain the indicated number of single germ cells with round fusomes for each genotype. (F) Graph showing the percentage of ovarioles that have a given number of developing egg chambers for each genotype. c587-gal4>UAS-Lsd1RNAi (n = 171); c587-gal4>UAS-Lsd1RNAi; en7/+ (n = 90); c587-gal4>UAS-Lsd1RNAi; enspt/+ (n = 95); c587-gal4>UAS-Lsd1RNAi; en4/+ (n = 115); c587-gal4>UAS-Lsd1RNAi; enRNAi-1/+ (n = 108); c587-gal4>UAS-Lsd1RNAi; enRNAi-2/+ (n = 96). (G) c587-gal4>UAS-Lsd1RNAi; UAS hhRNAi/+ (H) c587-gal4>UAS-Lsd1RNAi; hhAC/+ and (I) c587-gal4>UAS-Lsd1RNAi; hh2/+ stained for Hts (green), VASA (red) and DNA (blue). (J) Graph showing the percentage of germaria that contain the indicated number of single germ cells with round fusomes for each genotype. c587-gal4>UAS-Lsd1RNAi (n = 307); c587-gal4>UAS-Lsd1RNAi; UAS hhRNAi/+ (n = 222) c587-gal4>UAS-Lsd1RNAi; hhAC/+ (n = 88) and c587-gal4>UAS-Lsd1RNAi; hh2/+ (n = 184). Scale bars = 10 µM. In Drosophila, Engrailed drives the expression of hedgehog (hh), which in turn leads to the expression of dpp in adjacent cells [34]–[37]. Previous analysis showed an expansion of hh expression in Lsd1 mutant germaria [24]. To determine whether the mis-expression of hh in escort cells contributed to the Lsd1 mutant phenotype, we crossed both hh-specific UAS RNAi and hh mutant lines into a c587-gal4>UAS-Lsd1RNAi background. This analysis revealed that loss of hh function, similar to engrailed, suppressed the GSC-like expansion phenotype, resulting in the formation of germaria that exhibited normal germ cell differentiation (Figure 4G–J).
Mis-expression of engrailed in the escort cells results in a stem cell expansion phenotype
To assess whether mis-expression of engrailed and hh are sufficient to expand the number of stem cell-like cells in the germarium, we expressed transgenes corresponding to both genes within escort cells and early follicle cells using the c587-gal4 driver. Similar to the phenotype exhibited by Lsd1 mutants, ectopic expression of engrailed resulted in a stem cell-like cell expansion within 49.5% of germaria examined (n = 111). Many of these germline cells remained as single cells with round fusomes (Figure 5A). However, mis-expression of engrailed did not completely block cyst development and many ovarioles from c587-gal4>UAS-engrailed females contained maturing egg chambers.
Fig. 5. Ectopic expression of engrailed in the escort cells results in an expansion of stem cell-like cells in the germline. 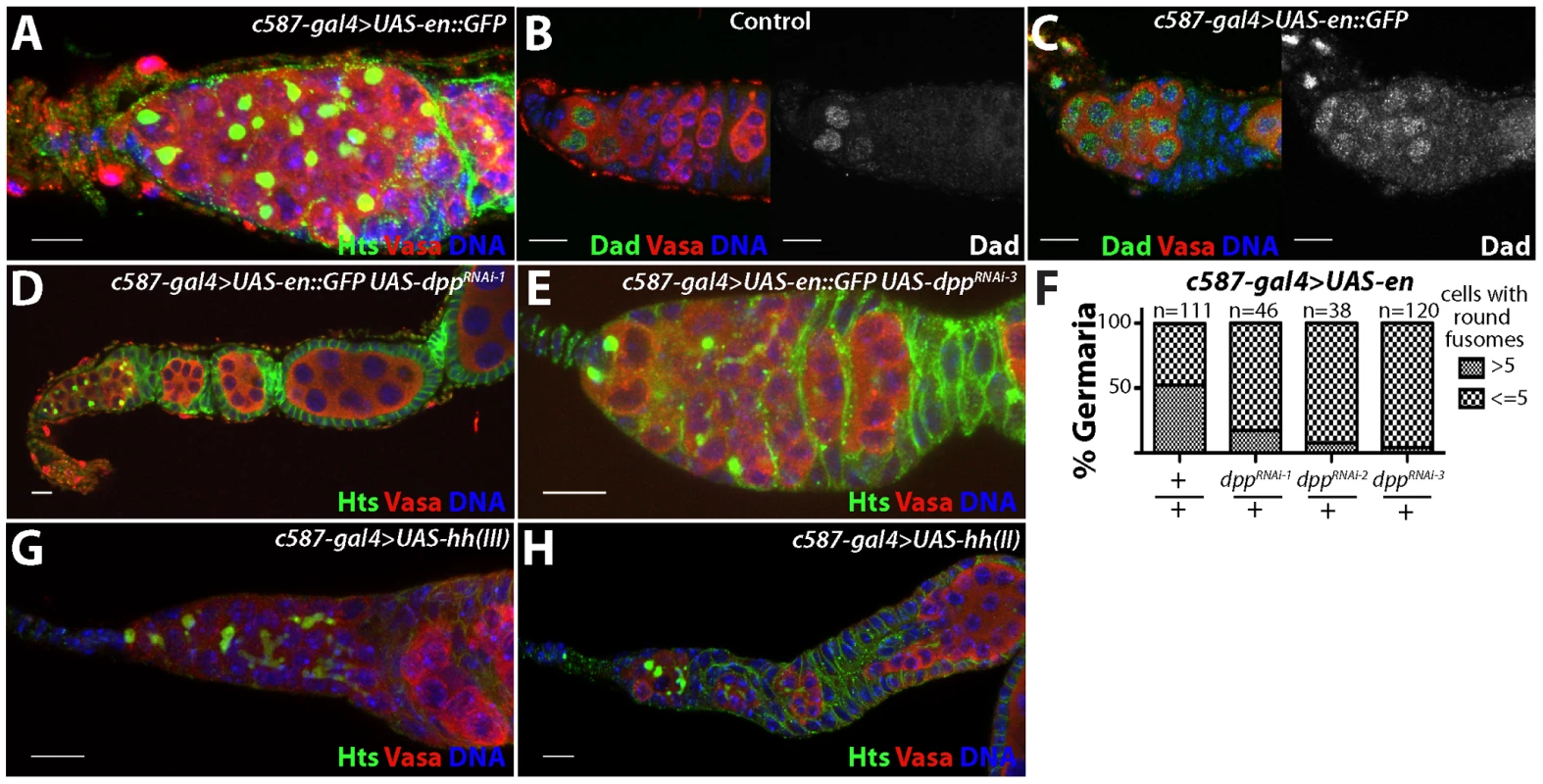
(A) c587-gal4>UAS-en::GFP germaria stained for Hts (green), VASA (red) and DNA (blue). (B) Control and (C) c587-gal4>UAS-en::GFP germaria stained for Dad-LacZ (Dad) (green), VASA (red) and DNA (blue). In control germaria, Dad-LacZ is expressed at high levels in the germline stem cells and its expression is reduced in the early differentiating cells. Germaria overexpressing engrailed display an expansion of Dad-LacZ positive cells. (D) Germaria from c587-gal4>UAS-en::GFP, UAS-dppRNAi-1 and (E) c587-gal4>UAS-en::GFP, UAS-dppRNAi-3 ovaries stained for Hts (green), VASA (red) and DNA (blue). Reducing the levels of dpp in the escort cells suppressed the engrailed overexpression phenotype. (F) Graph showing the percentage of germaria that contained a given number of cells with round fusomes for each genotype. (G) c587-gal4>UAS-hh(III) and (H) c587-gal4>UAS-hh(II) stained for Hts (green), VASA (red) and DNA (blue). Scale bars = 10 µM. In contrast to engrailed, over-expression of hh using two different transgenes did not obviously perturb early germ cell differentiation (Figure 5G,H). However, the mis-expression of these transgenes did result in follicle cell defects, consistent with previously published results [38]. These results indicated that the hh transgene is active in these cells but that hh over-expression in the escort cells and early follicle cells is not sufficient to induce an expansion of GSC-like cells in germaria.
Loss of Lsd1 results in expanded BMP signaling within the germline [24]. Based on the expansion of Engrailed expression in Lsd1 mutants and the similarities between the Lsd1 mutant and engrailed over-expression phenotypes, we reasoned that mis-expression of engrailed may also induce ectopic BMP signaling in the ovary. To test this, we used a Dad-lacZ enhancer trap as a positive transcriptional reporter of dpp signal transduction [9], [39]. In control ovarioles, stem cells express high levels of Dad-LacZ, whereas the expression of this reporter sharply decreases in differentiating cysts (Figure 5B). Upon engrailed mis-expression in the escort cells, the number of Dad-LacZ positive germline cells increases, likely reflecting expanded Dpp signaling (Figure 5C). Next, we knocked down the expression of dpp in the presence of the engrailed transgene and found that disruption of dpp suppressed the engrailed over-expression phenotype (Figure 5D–F). Together these results suggest that mis-expression of engrailed in Lsd1 mutants drives ectopic BMP signaling, resulting in the expanded GSC-like cell tumor phenotype.
To test whether ectopic engrailed expression can specifically affect adult escort cells, we performed a temporally controlled over-expression experiment. c587-gal4>UAS-engrailed larvae were kept at low temperature to prevent robust expression of the engrailed transgene. Ovaries from adult females maintained at a low temperature did not display ectopic Engrailed expression or an expanded undifferentiated cell phenotype (Figure 6A,A′). However, shifting c587-gal4>UAS-engrailed females to a higher temperature after eclosion resulted in ectopic engrailed expression in escort cells and early follicle cells, and a concomitant expansion of germline stem cell-like cells (Figure 6B–C). Thus, engrailed expression specifically in adults appears sufficient to induce ectopic BMP signaling in the anterior region of the germarium.
Fig. 6. engrailed mis-expression in adult escort cells results in delayed germ cell differentiation. 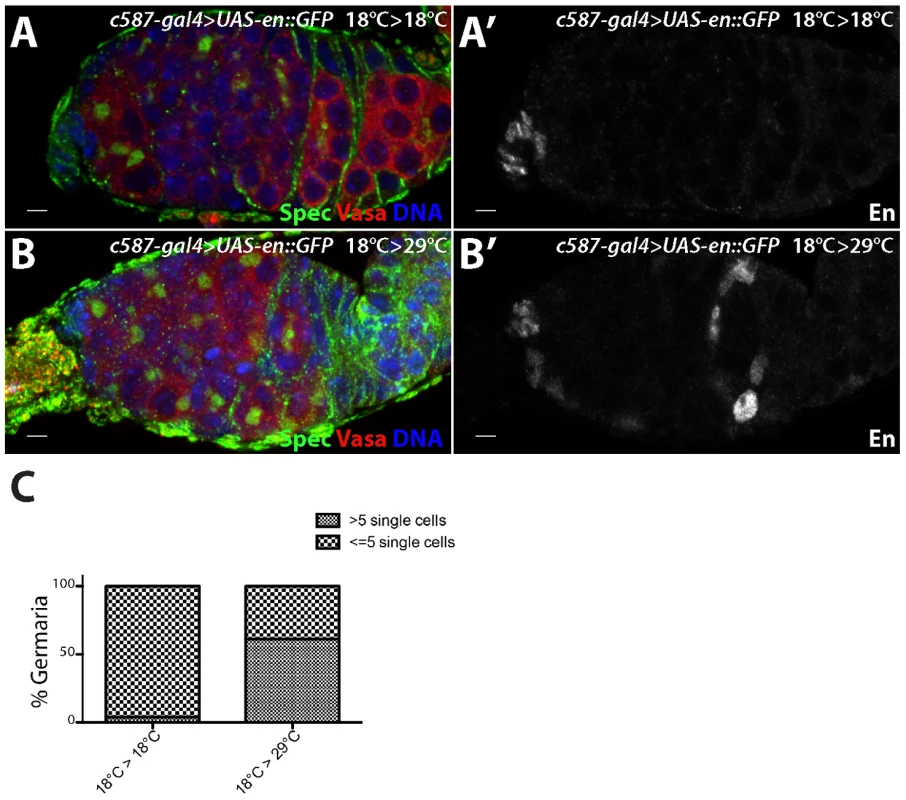
c587-gal4>UAS-en::GFP females were kept at (A) 18°C during both development and in adulthood or (B) moved to 29°C after eclosion. (A,B) The germaria were stained for alpha Spectrin (Spec) (green), VASA (red) and DNA (blue) and (A′,B′) Engrailed (En) (grayscale). (C) Graph showing the percentage of germaria with the indicated number of single germ cells with round fusomes when subjected to different temperatures. c587-gal4>UAS-en::GFP 18°C>18°C (n = 100), c587-gal4>UAS-en::GFP 18°C>29°C (n = 80). Scale bars = 10 µM. Other Lsd1 target genes
Lsd1 associates with the promoters of many genes besides engrailed, some of which could potentially play a role in regulating escort cell function. To begin to characterize whether Lsd1 modulates the expression of other potential target genes, we stained c587-gal4 control and c587-gal4>UAS-Lsd1RNAi ovaries using available antibodies. Cap cells and escort cells exhibit a shared peak of Lsd1 binding near the Rho1 gene (Table S11, 12). Previous results showed that loss of Rho1 results in escort cell defects and an expansion of GSC-like cells [3]. Knocking down Lsd1 levels did not appear to result in any dramatic change in Rho1 expression within the escort cells (compare Figure 7A and 7D). Likewise, Lsd1 also exhibits enriched binding near Apc1 (Tables S9, S12), a component of the Wnt signaling pathway. However antibody staining showed that Apc1 protein levels did not change to an appreciable degree upon knock-down of Lsd1 (Figure 7B,E). In contrast, the product of a third potential Lsd1 target gene, Broad, exhibited increased expression in c587-gal4>UAS-Lsd1RNAi samples relative to controls (Figure 7C,F). However, loss of broad did not appear to suppress the Lsd1 mutant phenotype (data not shown).
Fig. 7. raw represents another potential functional Lsd1 target gene. 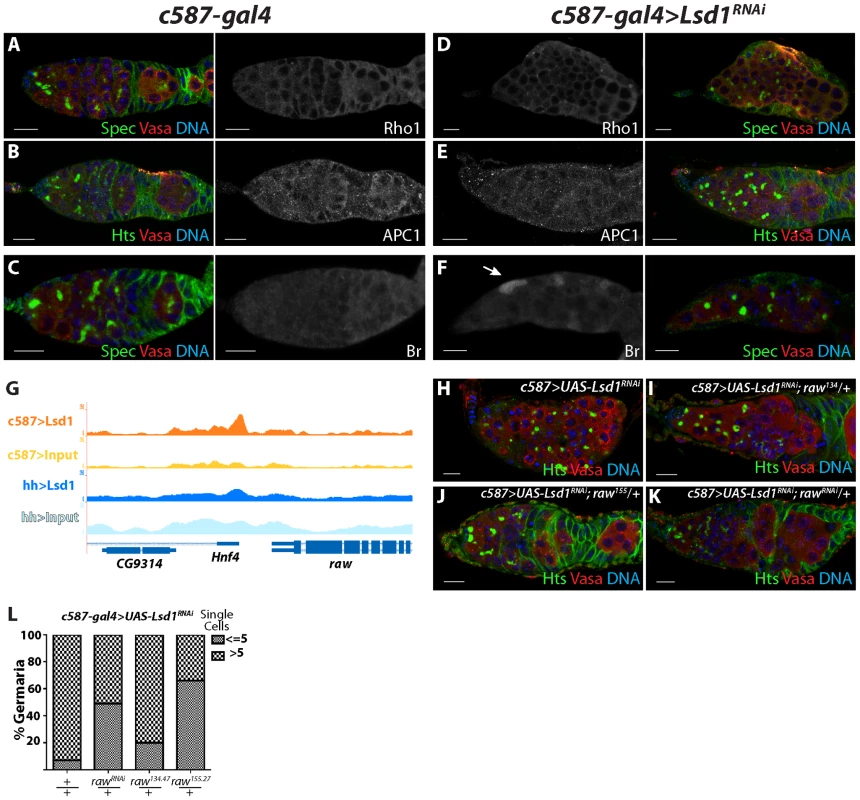
(A–C) c587-gal4 and (D–F) c587-gal4>UAS-Lsd1RNAi germaria stained for Rho1 (A,D), APC1 (B,E), and Broad (Br) (C,F). Alpha-Spectrin (Spec) or Hts (green), Vasa (red) and DNA (blue) staining of the same germaria are shown in color. (G) Screen shot of raw locus showing a peak of Lsd1 binding in the c587-gal4>UASt-HA-Lsd1 sample relative to the input DNA. (H) c587-gal4>UAS-Lsd1RNAi, (I) c587-gal4>UAS-Lsd1RNAi; UAS raw135/+ (J) c587-gal4>UAS-Lsd1RNAi; raw155/+ and (K) c587-gal4>UAS-Lsd1RNAi; rawRNAi/+ stained for Hts (green), VASA (red) and DNA (blue). (L) Graph showing the percentage of germaria that contain the indicated number of single germ cells with round fusomes for each genotype. c587-gal4>Lsd1RNAi (n = 351); c587-gal4>UAS-Lsd1RNAi; UAS rawRNAi/+ (n = 155); c587-gal4>UAS-Lsd1RNAi; raw135.47/+ (n = 224); and c587-gal4>UAS-Lsd1RNAi; raw155.27/+ (n = 229). Scale bars = 10 µM. The raw gene functions in the developing gonad to regulate the morphology of somatic cells as they interact with primordial germ cells [40], [41]. Lsd1 exhibits enriched binding just 3′ to the raw transcription termination site (Figure 7G). Antibodies were not available to assay whether loss of Lsd1 caused a change in Raw expression levels but raw mutant and RNAi lines partially suppressed the Lsd1 phenotype (Figure 7H–L). The raw134.47 allele weakly modified the GSC-like cell expansion phenotype exhibited by c587-gal4>UAS-Lsd1RNAi germaria, while both rawRNAi and a single copy of raw155.27 more strongly suppressed the c587-gal4>UAS-Lsd1RNAi phenotype, giving rise to a reduced number of germaria that carried more than 5 single cells with round fusomes. These genetic interactions suggest that mis-regulation of raw expression or activity also contributes to the Lsd1 mutant phenotype.
Encouraged by the finding that disruption of raw suppressed the Lsd1 mutant phenotype, we crossed a number of additional knockdown lines, corresponding to other putative Lsd1 target genes, into the c587-gal4>UAS-Lsd1RNAi background. We counted the total number of single germ cells with round fusomes within individual germaria from the resulting ovaries. This analysis showed that knockdown of 7 out of the 34 genes tested suppressed the c587-gal4>UAS-Lsd1RNAi phenotype to the point where fewer than 50% of the assayed germaria contained greater than 5 single cells with round fusomes (Figure 8A). This group included FK506-binding protein 1 (FK506-bp1), Glutamine:fructose-6-phosphate aminotransferase 1(Gfat1), CG11779, ken and barbie (ken), Anaphase Promoting Complex subunit 7 (APC7), barren (barr) and Hepatocyte nuclear factor 4 (Hnf4). These genes have varied functions and play roles in cell cycle regulation (APC7 and barr), juvenile hormone signaling (FK506-bp1), development of the genitalia (ken) and lipid metabolism (Hnf4). Lack of a clear functional link between these suppressors suggests that escort cells are particularly sensitive to perturbations in their gene expression programs. Together these data show that disruption of Lsd1 results in a complex phenotype, marked by increased BMP signaling in the germline and disruption of normal escort cell function, which likely involves the mis-expression of several direct and potentially indirect target genes (Figure 8B).
Fig. 8. Further genetic analysis shows that reduced levels of several genes suppress the Lsd1 mutant phenotype. 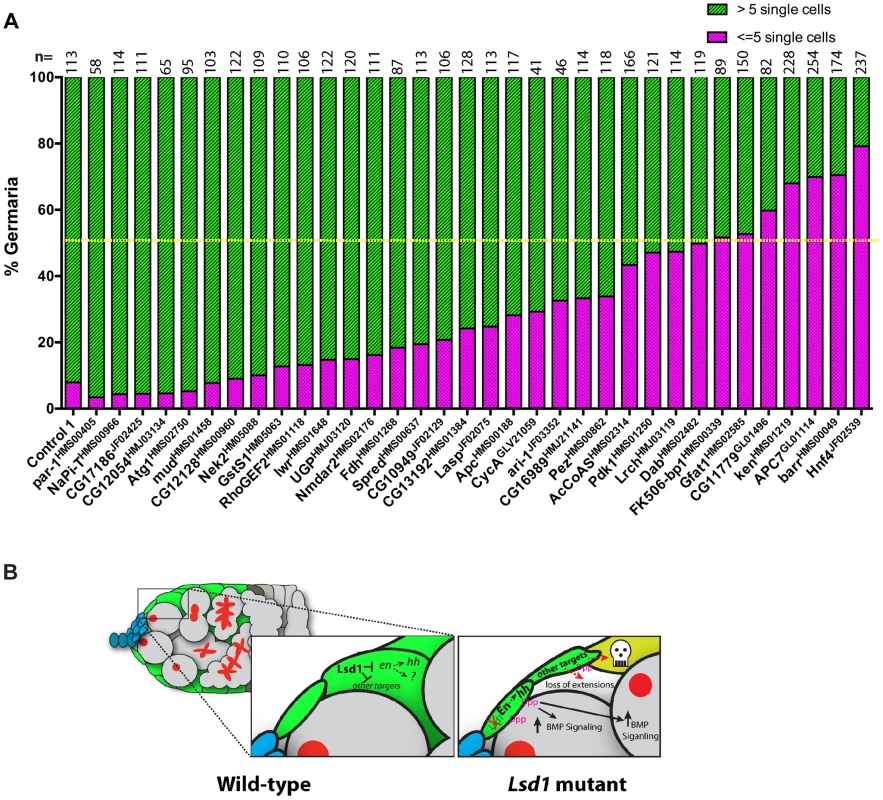
(A) The graph shows the percentage of germaria that contain the indicated number of single germ cells with round fusomes for each genotype. Control 1 represents c587-gal4>UAS-Lsd1RNAi; +/+ and the rest represent c587-gal4>UAS-Lsd1RNAi; gene specific RNAi/+. The resulting females were shifted to 29°C for seven days. The number (n) of germaria counted for each sample is indicated above the bar. The yellow dotted line marks the 50% threshold. All the indicated lines were crossed on their own to the c587-gal4 driver. None of the resulting ovaries displayed phenotypes except for CG13192HMS01384 (egg chamber defects) and par-1HMS00405 (follicle cell defects). (B) Model of Lsd1 function in escort cells. Discussion
Using ChIP-seq, we have systematically identified Lsd1 binding sites throughout the genomes of the cap cells/terminal filament cells and the escort cells/early follicle cells of the Drosophila ovary. The establishment of ChIP-based techniques using these specific cell populations and this comprehensive Lsd1 genome-wide dataset will facilitate further studies on the transcriptional hierarchies that regulate escort cell and cap cell function. Although Lsd1 mutants exhibit increased dpp expression [23], Lsd1 does not appear to directly target the dpp locus for silencing. Rather, Lsd1 represses the expression of the transcription factor engrailed. Mutations in the engrailed gene suppress the ovarian Lsd1 mutant phenotype and mis-expression of engrailed in escort cells results in increased BMP signaling and an expansion of undifferentiated germline cells within germaria. Thus engrailed may regulate dpp expression in germaria through either hh-dependent or independent mechanisms. Lsd1 also regulates additional genes adjacent to its other binding sites. Genetic analysis shows that the mis-regulation of these other target genes likely contributes to the Lsd1 mutant phenotype to varying degrees.
Lsd1 gene targets and function
We have found Lsd1 associates with a limited number of loci within two different cell populations. Lsd1 exhibits fairly broad peaks of binding, ranging in size from 166–262 bp based on the FindPeaks algorithm. MACs analysis calls even wider peaks (Supplementary Tables S5, S6, S7, S8). The significance of the width of these peaks remains unclear but suggests that Lsd1 either does not associate with single sequence specific elements at these sites or exhibits a certain degree of spreading upon recruitment to a particular locus. In Drosophila escort cells, Lsd1 binds to over 100 sites spread throughout the genome. Lsd1 binds to fewer sites in cap cells. While some Lsd1 binding sites overlap in cap cells and escort cells, the relatively large number of different sites suggests that Lsd1 recruitment depends on multiple and perhaps distinct cell-specific co-factors. MEME analysis [42], [43] reveals that many of the identified Lsd1 binding sites contain ACTGGAA elements. GGAA sequences are often present in the core binding sites of ETS transcription factors. The Drosophila genome encodes a number of ETS family members, none of which have been characterized in the somatic cells of the germarium. Determining the functional relevance of these specific GGAA sites within gene promoters and identifying the transcription factors that bind to them will require additional efforts.
For technical reasons and to enable comparisons of Lsd1 binding between escort cells/early follicle cells and cap cells/terminal filament cells, we elected to express the Lsd1 HA-tagged transgenes in an otherwise wild-type background. We acknowledge the possibility that endogenous Lsd1 may outcompete the HA-tagged transgenes for binding at specific sites in the escort cells and early follicle cells. Therefore sites identified in this study may be an underrepresentation of the total number of sites bound by endogenous Lsd1. Repeating the ChIP-seq analysis using material from rescued Lsd1ΔN females that express the HA-tagged Lsd1 transgene in escort cells and early follicle cells represents important work for the future.
We found that Lsd1 mutant samples exhibit a 6-fold increase in engrailed transcript levels relative to controls. Curiously, ectopic Engrailed protein expression was only observed in a small number of escort cells. Perhaps additional post-transcription mechanisms regulate the translation of Engrailed, and potentially other proteins, inside and outside of the cap cell niche. Such mechanisms would allow these cells to fine-tune their signaling output more than what could be achieved through transcriptional based mechanisms alone. Results presented here also suggest that escort cells are not uniform in nature and perhaps have specific functions or capabilities depending on their lineage and where they reside within the germarium. MARCM analysis shows that the loss of Lsd1 in some but not all escort cells results in a marked expansion of GSC-like cells within the germarium. Previous studies have also suggested that specific escort cells have distinct roles in supporting GSCs [12].
Technical considerations aside, the severity of the Lsd1 null phenotype compared to the engrailed over-expression phenotype, both in terms of the penetrance and extent of the GSC expansion phenotype and the accompanying germline and somatic cell death, suggests that engrailed is not the only biologically relevant target of Lsd1 regulation in the escort cells. Based on expression analysis (Figure 7), Lsd1 regulates the expression of some but not all genes adjacent to its binding sites. Our genetic analysis suggests that mis-regulation of the putative target raw (Figure 7) and several additional genes (Figure 8) also contribute to the Lsd1 mutant phenotype. Characterizing the transcriptional profile of escort cells from wild-type and Lsd1 mutant samples, which will have to await for improvements in current cell isolation and RNA profiling techniques, will help to further resolve which genes are direct and indirect targets of Lsd1 regulation. Such approaches may also reveal additional genes that participate in niche formation and function. Of note, the observation that functionally diverse genes can suppress the Lsd1 mutant phenotype suggests that escort cells are acutely sensitive to changes in their gene expression programs. While our data support a model that loss of Lsd1 initially results in mis-regulation of engrailed and other genes that, in turn, drive GSC expansion, it is clear that many of the escort cells that experience reduced Lsd1 function retract their cellular extensions and undergo cell death. This loss of escort cells further exacerbates the GSC expansion phenotype. Given the phenotypic complexity described here and elsewhere [3], care should be taken when analyzing gene function within the escort cell population.
Niche plasticity
How niches maintain stem cells and adjust their signaling output to ensure tissue homeostasis remains a fundamental question in stem cell biology. Elegant work has shown that terminal filament cells, cap cells and escort cells help to support the self-renewal of two to three germline stem cells at the tip of Drosophila germaria [5], [44]. The predominant signal emanating from the anterior tip of the germarium is Dpp, which acts locally to induce a canonical signal transduction cascade in GSCs, which in turn represses their differentiation [5], [9]. Several expression and genetic studies strongly suggest that terminal filament and cap cells, and perhaps the most anterior escort cells, are the primary source Dpp ligand [5], [9]. More recent work has suggested that Engrailed expression in cap cells non-autonomously promotes dpp expression in escort cells through a hedgehog dependent mechanism [12]. Loss of Lsd1 results in ectopic expansion of hh expression in escort cells [24] and data shown here (Figure 4) reveals that disruption of hh partially suppresses the Lsd1 mutant phenotype. However, consistent with previous results [38], over-expression of hh in escort cells does not result in an Lsd1-like mutant tumor phenotype (Figure 5G,H), demonstrating that hh is not sufficient to induce ectopic BMP signaling in the germarium. Given these observations, ectopic engrailed expression in escort cells likely targets additional genes besides hh to induce ectopic BMP signaling and promote the expansion of undifferentiated germ cells.
The finding that loss of Lsd1 or mis-expression of engrailed in adult escort cells leads to expanded Dpp signal transduction within germ cells throughout the anterior portion of the germarium indicates that subpopulations of escort cells are capable, and perhaps even poised, to express dpp under certain conditions. Such plasticity might allow the niche to expand and contract in response to various stimuli and environmental cues. Indeed, previous studies have shown that Jak/Stat and insulin signaling can influence the number of GSCs in the ovary [45]–[49]. Moreover, ongoing dynamic regulation of signaling may be a regular feature of niches under resting homeostatic conditions. The observation that long-term knock-down of dpp in escort cells results in a reduced number of GSCs at the tip of the germarium, but not their complete elimination, is consistent with the notion that escort cells contribute to the maintenance of GSCs in some manner [12]. Further work, with single-cell spatial and small-scale temporal resolution, will be needed to help clarify what cells express niche signals and when.
Inappropriate and extensive expansion of niches would be predicted to upset tissue homeostasis and perhaps even result in pathological conditions. Therefore robust but flexible mechanisms that depend on chromatin factors such as Lsd1 may be in place to precisely control the expansion and contraction of in vivo stem cell niches. The continued study of Drosophila cap cells and escort cells will provide further insights into how chromatin programming regulates niche plasticity.
Materials and Methods
Drosophila stocks
Drosophila stocks were maintained at room temperature on standard cornmeal-agar medium unless specified otherwise. The following fly strains were used in this study: w1118 was used as a control; Lsd1ΔN was provided by N. Dyson (Massachusetts General Hospital Cancer Center, Charlestown, MA); hh-gal4 and UAS-hh lines [50] were provided by J. Jiang (University of Texas Southwestern, Dallas, TX); c587-gal4 and Dad-LacZ were provided by A. Spradling (Carnegie Institution for Science, Baltimore, MD); the UAS-engrailed::GFP transgenic line was provided by Florence Maschat (Institute of Human Genetics, France); the raw134.47 and raw155.27 alleles were provided by Jennifer Mierisch (Loyola University of Chicago); en7, en4, enspt, UAS-mCD8::GFP, UAS-dppRNAi-1 (BL#-31172), UAS-dppRNAi-2 (BL#-31530), UAS-dppRNAi-3 (BL#-31531) and UAS-rawRNAi (BL#-31393), UAS-enRNAi-1 (BL#-33715), UAS-enRNAi-2 (BL#-26752), UAS-hhRNAi (BL#-31042), hhAC (BL#-1749), hh2 (BL#-3376), broadnpr-3 (BL#-29971), broad5 (BL#-29972), par-1HMS00405 (BL# - 32410), NaPi-THMS00966 (BL# - 34003), CG17186JF02425 (BL# - 27079), CG12054HMJ03134 (BL# - 50910), Atg1HMS02750 (BL# - 44034), mudHMS01458 (BL# - 35044), CG12128HMS00960 (BL# - 33997), Nek2HM05088 (BL# - 28600), GstS1HM05063 (BL# - 28885), RhoGEF2HMS01118 (BL# - 34643), lwrHMS01648 (BL# - 37506), UGPHMJ03120 (BL# - 50902), Nmdar2HMS02176 (BL# - 40928), FdhHMS01268 (BL# - 34937), SpredHMS00637 (BL# - 32852), CG10949JF02129 (BL# - 26231), CG13192HMS01384 (BL# - 34390), LaspJF02075 (BL# - 26305), ApcHMS00188 (BL# - 34869), CycAGLV21059 (BL# - 35694), ari-1JF03352 (BL# - 29416), CG16989HMJ21141 (BL# - 51017), PezHMS00862 (BL# - 33919), AcCoASHMS02314 (BL# - 41917), Pdk1HMS01250 (BL# - 34936), LrchHMJ03119 (BL# - 50901), DabHMS02482 (BL# - 42646), FK506-bp1HMS00339 (BL# - 32348), Gfat1HMS02585 (BL# - 42892), CG11779GL01496 (BL# - 43155), kenHMS01219 (BL# - 34739), APC7GL01114 (BL# - 38932), barrHMS00049 (BL# - 34068) and Hnf4JF02539 (BL# - 29375) lines were obtained from the Bloomington Stock Center. UAS-Lsd1RNAi was obtained from the National Institute of Genetics, Japan.
MARCM clones
Lsd1 mutant MARCM clones were generated by crossing Lsd1ΔN FRT 2A to yw122 tub-gal4 UAS-GFP;;tub-gal80 FRT 2A/TM6B (gift from Ben Ohlstein). The resulting larvae were heat-shocked twice a day at 37°C on days 5–7 after the cross was set. The resulting adult females were dissected and stained 7 days after they eclosed.
Cloning of tagged transgenes
The HA tagged transgenes of Lsd1 were created using Gateway Cloning (Invitrogen). The open reading frame (ORF) of Lsd1 was cloned into modified pTHW and pPHW destination vectors (http://emb.carnegiescience.edu/labs/murphy/Gateway%20vectors.html) that contained φC31 attB sites [51]–[53]. These constructs were injected into flies and transformed using φC31 integrase into the 51D landing site on the 2nd chromosome.
Immunostaining
Adult ovaries were dissected in Grace's medium and fixed in 4% (vol/vol) formaldehyde for 10 min. The ovaries were washed with PBT (1X PBS, 0.5% BSA, and 0.3% Triton-X 100) and stained with primary antibody overnight at 4°C. The ovaries were washed and incubated in secondary antibody at room temperature for 5 hrs. Ovaries were then washed again and mounted in Vectashield containing DAPI (Vector Laboratories). The following primary antibodies were used: mouse anti-Hts (1B1) (1∶20), mouse anti-Engrailed (4D9) (1∶2), mouse anti-Broad-core (25E9.D7) (1∶10), mouse anti-Rho1 (P1D9) (1∶50) and rat anti-VASA (1∶20) (Developmental Studies Hybridoma Bank), mouse anti-β-galactosidase (1∶200) (Promega), rabbit anti-APC1 [54] (1∶1000)(gift of E. Wieschaus), Rabbit anti-α-Spectrin [55](1∶1000)(gift from Ron Dubreuil), rabbit anti-GFP (1∶1000) (Molecular Probes), rat anti-HA 3F10 (Roche) and rabbit anti-cleaved Caspase-3 (1∶250) (Cell Signaling Technology). Fluorescence-conjugated secondary antibodies (Jackson Laboratories) were used at a dilution of 1∶200.
RNA isolation and RT-PCR
RNA was isolated from bamΔ86 and Lsd1ΔN bamΔ86 mutant ovaries using TRIzol (Invitrogen). The RNA was treated with DNase and subjected to RT-qPCR reaction using the Superscript III First-Strand Synthesis SuperMix (Invitrogen). The primers used to amplify engrailed mRNA are as follows:
engrailed forward: 5′ - GCCCGCCTGGGTGTACTG
engrailed reverse: 5′ - CGCTTCTCGTCGTTGGTCTTG
Chromatin immunoprecipitation and sequencing (ChIP-seq)
We used 1000 pairs of ovaries for each ChIP-seq reaction. Every 200 pairs of ovaries were dissected, fixed, washed and frozen immediately at −80°C. The entire protocol was done at 4°C unless otherwise indicated. The ovaries were dissected and fixed in 1 ml of 1% formaldehyde at room temperature for 10 mins. The crosslinking was stopped by the addition of 100 ml 1.25M glycine solution. The ovaries were washed three times with 1X cold PBS buffer and then sonicated in 500 µl ChIP Sonication Buffer (1%Triton X-100, 0.1% Deoxycholate, 50 mM Tris 8.1, 150 mM NaCl, 5 mM EDTA) on ice to achieve a final DNA length of 100 to 600 base pairs. The sample was centrifuged at maximum speed at 4°C to remove cell debris. The supernatant was transferred to a new tube and the sonicated sample was then blocked by adding Protein G agarose beads and incubating at 4°C for one hour. The beads were removed. 1% of the sample was kept aside as INPUT and to the remaining sample 3 ug rabbit-HA antibody (Abcam) was added and incubated overnight at 4°C.
The next day protein agarose G beads were added and incubated for 3 hours at 4°C. The beads was then washed well with ChIP Sonication Buffer (two times), High Salt Wash Buffer (1% Triton X-100, 0.1% Deoxycholate, 50 mM Tris 8.1, 500 mM NaCl, 5 mM EDTA) (three times), LiCl Immune Complex Wash Buffer (two times) and TE buffer. The protein bound to the beads was eluted using 500 µl Elution Buffer (1% SDS, 0.1M NaHCO3). The elution buffer was added to the INPUT samples and they were treated the same as the IP samples from this point. 20 µl of 5M NaCl was added to 500 µl of elution buffer and incubated at 65°C overnight.
The third day, the sample was treated with RNase A, Proteinase K and the DNA isolated using Qiagen PCR Purification kit. Subsequent library construction and sequencing of the input and immunoprecipitated DNA were conducted by the UT Southwestern McDermott Sequencing Center.
Bioinformatic analysis of ChIP-Seq data
The primary sequencing data was mapped to the fly reference genome dm3 using BioScope (1.2.1). During the alignment, three filter steps were applied to remove low quality, ambiguous and redundant reads. HA-Lsd1 binding regions were identified as genomic regions with a significant read enrichment and binding peak profile in the ChIP reads over the input reads using the FindPeaks module in the Homer software tool [56] with 10% false discovery rate (FDR). ChIP enrichment at important genome features such as specific chromosomes, promoters, downstream, exonic, intronic and distal intergenic regions was statistically analyzed with the Cis-regulatory Element Annotation System (CEAS) [57]. De novo motif discovery analysis for HA-Lsd1 binding regions was performed with the Multiple EM for motif elicitation (MEME) software tool [42], [43]. High quality motifs were aligned against transcription factor motifs retrieved from JASPAR [58] and TRANSFAC [59] using the TOMTOM software tool [42] to identify known transcription factor motifs that match the MEME predicted motifs. Potential protein-coding target genes associated with the identified HA-Lsd1 binding regions were identified based on the distance of their transcription start sites (TSSs) according to their RefSeq annotation in the dm3 assembly to binding peak summits. Genes with TSSs within 5 kb or nearest to an HA-Lsd peak summit were called as target genes. ChIP-Seq data has been deposited with NCBI GEO (http://www.ncbi.nlm.nih.gov/geo) under the accession code GSE54376.
Supporting Information
Zdroje
1. MorrisonSJ, SpradlingAC (2008) Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132 : 598–611.
2. KirillyD, XieT (2007) The Drosophila ovary: an active stem cell community. Cell Res 17 : 15–25.
3. KirillyD, WangS, XieT (2011) Self-maintained escort cells form a germline stem cell differentiation niche. Development 138 : 5087–5097.
4. MorrisLX, SpradlingAC (2011) Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138 : 2207–2215.
5. XieT, SpradlingAC (1998) decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94 : 251–260.
6. XieT, SpradlingAC (2000) A niche maintaining germ line stem cells in the Drosophila ovary. Science 290 : 328–330.
7. ChenD, McKearinD (2003) Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol 13 : 1786–1791.
8. ChenD, McKearinDM (2003) A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development 130 : 1159–1170.
9. SongX, WongMD, KawaseE, XiR, DingBC, et al. (2004) Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131 : 1353–1364.
10. McKearinD, OhlsteinB (1995) A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121 : 2937–2947.
11. McKearinDM, SpradlingAC (1990) bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev 4 : 2242–2251.
12. Rojas-RiosP, GuerreroI, Gonzalez-ReyesA (2012) Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS biology 10: e1001298.
13. EliazerS, BuszczakM (2011) Finding a niche: studies from the Drosophila ovary. Stem cell research & therapy 2 : 45.
14. HarrisRE, AsheHL (2011) Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO reports 12 : 519–526.
15. ShiY, LanF, MatsonC, MulliganP, WhetstineJR, et al. (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119 : 941–953.
16. ChenY, YangY, WangF, WanK, YamaneK, et al. (2006) Crystal structure of human histone lysine-specific demethylase 1 (LSD1). Proc Natl Acad Sci U S A 103 : 13956–13961.
17. WangY, ZhangH, ChenY, SunY, YangF, et al. (2009) LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 138 : 660–672.
18. AdamoA, SeseB, BoueS, CastanoJ, ParamonovI, et al. (2011) LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat Cell Biol 13 : 652–659.
19. Di StefanoL, WalkerJA, BurgioG, CoronaDF, MulliganP, et al. (2011) Functional antagonism between histone H3K4 demethylases in vivo. Genes & development 25 : 17–28.
20. MulliganP, YangF, Di StefanoL, JiJY, OuyangJ, et al. (2011) A SIRT1-LSD1 corepressor complex regulates Notch target gene expression and development. Molecular cell 42 : 689–699.
21. MetzgerE, WissmannM, YinN, MullerJM, SchneiderR, et al. (2005) LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437 : 436–439.
22. Di StefanoL, JiJY, MoonNS, HerrA, DysonN (2007) Mutation of Drosophila Lsd1 disrupts H3-K4 methylation, resulting in tissue-specific defects during development. Curr Biol 17 : 808–812.
23. RudolphT, YonezawaM, LeinS, HeidrichK, KubicekS, et al. (2007) Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell 26 : 103–115.
24. EliazerS, ShalabyNA, BuszczakM (2011) Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proceedings of the National Academy of Sciences of the United States of America 108 : 7064–7069.
25. de CuevasM, SpradlingAC (1998) Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development 125 : 2781–2789.
26. LinH, SpradlingAC (1995) Fusome asymmetry and oocyte determination in Drosophila. Dev Genet 16 : 6–12.
27. ZhangY, LiuT, MeyerCA, EeckhouteJ, JohnsonDS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome biology 9: R137.
28. MaereS, HeymansK, KuiperM (2005) BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21 : 3448–3449.
29. ShannonP, MarkielA, OzierO, BaligaNS, WangJT, et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research 13 : 2498–2504.
30. MorataG, LawrencePA (1975) Control of compartment development by the engrailed gene in Drosophila. Nature 255 : 614–617.
31. KornbergT (1981) Engrailed: a gene controlling compartment and segment formation in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 78 : 1095–1099.
32. DesplanC, TheisJ, O'FarrellPH (1985) The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature 318 : 630–635.
33. BolivarJ, PearsonJ, Lopez-OnievaL, Gonzalez-ReyesA (2006) Genetic dissection of a stem cell niche: the case of the Drosophila ovary. Developmental dynamics : an official publication of the American Association of Anatomists 235 : 2969–2979.
34. AlexandreC, VincentJP (2003) Requirements for transcriptional repression and activation by Engrailed in Drosophila embryos. Development 130 : 729–739.
35. LeeJJ, von KesslerDP, ParksS, BeachyPA (1992) Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell 71 : 33–50.
36. MorataG (2001) How Drosophila appendages develop. Nature reviews Molecular cell biology 2 : 89–97.
37. TabataT, EatonS, KornbergTB (1992) The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes & development 6 : 2635–2645.
38. ForbesAJ, LinH, InghamPW, SpradlingAC (1996) hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122 : 1125–1135.
39. TsuneizumiK, NakayamaT, KamoshidaY, KornbergTB, ChristianJL, et al. (1997) Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389 : 627–631.
40. JemcJC, MilutinovichAB, WeyersJJ, TakedaY, Van DorenM (2012) raw Functions through JNK signaling and cadherin-based adhesion to regulate Drosophila gonad morphogenesis. Developmental biology 367 : 114–125.
41. WeyersJJ, MilutinovichAB, TakedaY, JemcJC, Van DorenM (2011) A genetic screen for mutations affecting gonad formation in Drosophila reveals a role for the slit/robo pathway. Developmental biology 353 : 217–228.
42. BaileyTL, BodenM, BuskeFA, FrithM, GrantCE, et al. (2009) MEME SUITE: tools for motif discovery and searching. Nucleic acids research 37: W202–208.
43. BaileyTL, WilliamsN, MislehC, LiWW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic acids research 34: W369–373.
44. XieT, SongX, JinZ, PanL, WengC, et al. (2008) Interactions between stem cells and their niche in the Drosophila ovary. Cold Spring Harb Symp Quant Biol 73 : 39–47.
45. DecottoE, SpradlingAC (2005) The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell 9 : 501–510.
46. HsuHJ, Drummond-BarbosaD (2009) Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 106 : 1117–1121.
47. HsuHJ, Drummond-BarbosaD (2011) Insulin signals control the competence of the Drosophila female germline stem cell niche to respond to Notch ligands. Developmental biology 350 : 290–300.
48. Lopez-OnievaL, Fernandez-MinanA, Gonzalez-ReyesA (2008) Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development 135 : 533–540.
49. WangL, LiZ, CaiY (2008) The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol 180 : 721–728.
50. WangG, AmanaiK, WangB, JiangJ (2000) Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes & development 14 : 2893–2905.
51. BischofJ, MaedaRK, HedigerM, KarchF, BaslerK (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proceedings of the National Academy of Sciences of the United States of America 104 : 3312–3317.
52. BrandAH, PerrimonN (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 : 401–415.
53. RorthP (1998) Gal4 in the Drosophila female germline. Mechanisms of development 78 : 113–118.
54. AhmedY, NouriA, WieschausE (2002) Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development 129 : 1751–1762.
55. ByersTJ, DubreuilR, BrantonD, KiehartDP, GoldsteinLS (1987) Drosophila spectrin. II. Conserved features of the alpha-subunit are revealed by analysis of cDNA clones and fusion proteins. J Cell Biol 105 : 2103–2110.
56. HeinzS, BennerC, SpannN, BertolinoE, LinYC, et al. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Molecular cell 38 : 576–589.
57. ShinH, LiuT, ManraiAK, LiuXS (2009) CEAS: cis-regulatory element annotation system. Bioinformatics 25 : 2605–2606.
58. SandelinA, AlkemaW, EngstromP, WassermanWW, LenhardB (2004) JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic acids research 32: D91–94.
59. WingenderE, DietzeP, KarasH, KnuppelR (1996) TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic acids research 24 : 238–241.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



