-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaOxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
The accumulation of somatic mitochondrial DNA (mtDNA) mutations is implicated in aging and common diseases of the elderly, including cancer and neurodegenerative disease. However, the mechanisms that influence the frequency of somatic mtDNA mutations are poorly understood. To develop a simple invertebrate model system to address this matter, we used the Random Mutation Capture (RMC) assay to characterize the age-dependent frequency and distribution of mtDNA mutations in the fruit fly Drosophila melanogaster. Because oxidative stress is a major suspect in the age-dependent accumulation of somatic mtDNA mutations, we also used the RMC assay to explore the influence of oxidative stress on the somatic mtDNA mutation frequency. We found that many of the features associated with mtDNA mutations in vertebrates are conserved in Drosophila, including a comparable somatic mtDNA mutation frequency (∼10−5), an increased frequency of mtDNA mutations with age, and a prevalence of transition mutations. Only a small fraction of the mtDNA mutations detected in young or old animals were G∶C to T∶A transversions, a signature of oxidative damage, and loss-of-function mutations in the mitochondrial superoxide dismutase, Sod2, had no detectable influence on the somatic mtDNA mutation frequency. Moreover, a loss-of-function mutation in Ogg1, which encodes a DNA repair enzyme that removes oxidatively damaged deoxyguanosine residues (8-hydroxy-2′-deoxyguanosine), did not significantly influence the somatic mtDNA mutation frequency of Sod2 mutants. Together, these findings indicate that oxidative stress is not a major cause of somatic mtDNA mutations. Our data instead suggests that somatic mtDNA mutations arise primarily from errors that occur during mtDNA replication. Further studies using Drosophila should aid in the identification of factors that influence the frequency of somatic mtDNA mutations.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1003974
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003974Summary
The accumulation of somatic mitochondrial DNA (mtDNA) mutations is implicated in aging and common diseases of the elderly, including cancer and neurodegenerative disease. However, the mechanisms that influence the frequency of somatic mtDNA mutations are poorly understood. To develop a simple invertebrate model system to address this matter, we used the Random Mutation Capture (RMC) assay to characterize the age-dependent frequency and distribution of mtDNA mutations in the fruit fly Drosophila melanogaster. Because oxidative stress is a major suspect in the age-dependent accumulation of somatic mtDNA mutations, we also used the RMC assay to explore the influence of oxidative stress on the somatic mtDNA mutation frequency. We found that many of the features associated with mtDNA mutations in vertebrates are conserved in Drosophila, including a comparable somatic mtDNA mutation frequency (∼10−5), an increased frequency of mtDNA mutations with age, and a prevalence of transition mutations. Only a small fraction of the mtDNA mutations detected in young or old animals were G∶C to T∶A transversions, a signature of oxidative damage, and loss-of-function mutations in the mitochondrial superoxide dismutase, Sod2, had no detectable influence on the somatic mtDNA mutation frequency. Moreover, a loss-of-function mutation in Ogg1, which encodes a DNA repair enzyme that removes oxidatively damaged deoxyguanosine residues (8-hydroxy-2′-deoxyguanosine), did not significantly influence the somatic mtDNA mutation frequency of Sod2 mutants. Together, these findings indicate that oxidative stress is not a major cause of somatic mtDNA mutations. Our data instead suggests that somatic mtDNA mutations arise primarily from errors that occur during mtDNA replication. Further studies using Drosophila should aid in the identification of factors that influence the frequency of somatic mtDNA mutations.
Introduction
Mitochondria play crucial cellular roles in energy production, Ca2+ buffering, metabolite synthesis, and programmed cell death in metazoans [1]–[3]. While most mitochondrial proteins are encoded in the nuclear genome, mitochondria also contain a compact genome that generally encodes 37 genes [4], and germline mutations that disrupt the functions of mitochondrial DNA (mtDNA) encoded genes cause a number of devastating familial syndromes [5]. mtDNA mutations also occur in somatic tissues, and the accumulation of somatic mtDNA mutations is implicated in aging and common diseases of the elderly, including cancer, diabetes, and neurodegenerative disease [5]–[8]. Because there are multiple copies of mtDNA in any given cell, when mtDNA mutations occur, they frequently coexist with wild-type (WT) mtDNA, a condition known as heteroplasmy. For reasons that are not presently understood, many mtDNA mutations expand clonally within a cell, such that a single somatic mtDNA mutation can ultimately represent a large fraction of the mtDNA within a given cell or tissue [9]. Although the ratio of mutated to WT mtDNA is believed to play a critical pathological role in diseases associated with mtDNA mutations [10], the molecular mechanisms that influence this ratio are poorly understood.
The frequency of somatic mtDNA mutations can exceed the mutation frequency of the nuclear genome by several orders of magnitude [10], and various factors have been proposed to account for this high mutation frequency. Because mitochondria are the major cellular source of DNA-damaging reactive oxygen species (ROS), it is believed that ROS-mediated damage is an important contributor to somatic mtDNA mutations. Indeed, Harman proposed in the 1970s that ROS damage to mtDNA causes mtDNA mutations that result in production of dysfunctional respiratory chain components, which in turn produce increased amounts of ROS, thus leading to a vicious cycle responsible for aging [11]. Another possible source of the high mtDNA mutation frequency is mtDNA replication errors. Unlike the nuclear genome, which does not replicate in postmitotic tissues, mtDNA replicates throughout life in postmitotic tissues [10], and this ongoing replication can potentially lead to the accumulation of replication errors. Finally, the relative lack of particular DNA repair pathways and protective histones in mitochondria has also been suggested as a source of the high frequency of mtDNA mutations [12]. However, the relative contributions of these and other possible causes of the age-dependent accumulation of somatic mtDNA mutations remain unclear.
Recent work in Drosophila melanogaster has contributed greatly to our understanding of mitochondrial quality control [13], [14], so we sought to use Drosophila as a simple, genetically tractable model system to explore the factors that influence somatic mtDNA mutations. Although much is known about the frequency and distribution of somatic mtDNA mutations in vertebrates [10], these matters are largely unexplored in invertebrates. Only three previous studies have attempted to test whether somatic mtDNA mutations accumulate in Drosophila, and these studies have yielded contradictory findings [15]–[17]. While technical issues likely explain these discordant findings, a limitation of all three studies is the lack of quantitative estimates of the somatic mtDNA mutation frequency, including the somatic mtDNA point mutation frequency. Thus, we used an approach that would enable us to address these issues in Drosophila.
To explore the distribution, frequency, and causes of somatic mtDNA mutations in Drosophila, we used the Random Mutation Capture (RMC) assay [18], [19]. We found that the somatic mtDNA mutation frequencies in fly tissues are similar to those reported in vertebrates, and that their frequencies increase with age. However, the specific types of mtDNA mutation detected, and the results of gene perturbations targeting oxidative stress and repair pathways, indicate that oxidative stress is not a major contributor to the somatic mtDNA mutation frequency. Instead, our findings suggest that somatic mtDNA mutations arise primarily from errors that occur during DNA replication.
Results
mtDNA replication occurs in somatic tissues of Drosophila
Previous work has shown that mtDNA synthesis occurs in mitochondria isolated from adult flies [20] and that mtDNA replication intermediates can be detected in adult Drosophila tissues [21], suggesting that mtDNA replication occurs in the somatic tissues of adult Drosophila. Given that DNA replication is essential to the formation of point mutations we performed an additional experiment to verify that mtDNA replication occurs in somatic tissues of Drosophila adults. We fed adult flies 5-bromo-2′-deoxyuridine (BrdU) and used Southwestern blot analysis and immunofluorescence confocal microscopy to test whether this thymidine analog was incorporated into mtDNA. After 72 hours of exposure to BrdU, DNA was isolated from Drosophila heads, digested with BglII, then subjected to Southwestern blot analysis using antiserum against BrdU. Because the Drosophila mitochondrial genome is 19.5 kb in size and contains only one BglII site, BrdU labeling should yield a single 19.5 kb band. Consistent with this prediction, our analysis revealed a single 19.5 kb band, indicating BrdU incorporation into mtDNA (Figure S1A). To further validate this finding, we used immunohistochemistry and confocal microscopy to test whether BrdU immunofluorescence colocalizes with mitochondria in the Drosophila thoracic ganglion. After feeding adult flies BrdU for 72 hours, the thoracic ganglion was dissected and stained with MitoTracker Deep Red to detect mitochondria and then incubated with an antibody against BrdU. Confocal microscopy revealed anti-BrdU immunofluorescence that colocalized with MitoTracker Deep Red fluorescence in BrdU-labeled animals, but not in unlabeled controls (Figure S1B). The finding that only a subset of mitochondria stained positively for BrdU is consistent with previous work indicating that mtDNA replication occurs at a low rate in the nervous system [22]. While we cannot rule out the possibility that some mitochondrial BrdU labeling is due to repair of mtDNA, our data together with previous work supports the occurrence of mtDNA replication in the adult somatic tissues of Drosophila [20], [21].
Multiple TaqI sites are suitable for detecting mutations in Drosophila mtDNA
A major challenge in detecting somatic mtDNA mutations is that they arise independently in different cells, such that any single mtDNA mutation typically lacks sufficient abundance within a tissue to be reliably detected by direct sequencing [23], [24]. Thus, we chose to use the Random Mutation Capture (RMC) assay to quantify the mtDNA mutation frequency in Drosophila because this assay can detect sequence variants at a detection limit of 10−8 [9]. The RMC assay measures the frequency at which a particular TaqI restriction site in mtDNA is eliminated through mutation. The RMC assay is a qPCR-based assay that uses two PCR primer sets. One primer set is used to amplify a portion of the mtDNA that contains a single TaqI restriction site (the test primers); the other primer set is used to amplify a portion of mtDNA that lacks a TaqI restriction site (the control primers). These primer sets are then used in qPCR reactions with TaqI-digested mtDNA to estimate the total number of mtDNA molecules present in the sample (determined by the qPCR reaction containing the control primer set) and the number of mtDNA molecules bearing mutations that eliminate the TaqI site (determined by the qPCR reaction containing the test primer set). In addition to the exquisite sensitivity with which RMC can detect mtDNA mutations, another advantage of this method is that WT molecules are eliminated by TaqI digestion prior to PCR amplification, and thus PCR amplification errors do not confound the mtDNA mutation frequency estimate. Previous work indicates that the majority of mtDNA mutations detected with this method are single base pair substitutions; however, small deletions and insertions can also be detected [25].
The D. melanogaster mtDNA sequence contains 33 TaqI sites (Figure 1). Because the mutability of these sites could vary, we sought to measure the mtDNA mutation frequency at multiple TaqI sites (Figure 1, Table S1). Four TaqI sites were excluded from analysis because the extreme AT-richness of the flanking sequence precluded primer design. Another 13 TaqI sites were excluded because their proximity to one or more adjacent TaqI sites did not allow for the design of primer sets that amplify only a single TaqI site. We designed primer pairs to the flanking sequences of the remaining 16 TaqI sites and used them in experiments aimed at qPCR primer optimization (see Materials and Methods). Only 4 out of these 16 primer sets had optimum amplification profiles (Table S1). These 4 primer sets amplified TaqI sites in three different genes, and we used three of these test primer sets (corresponding to three different genes) for our studies. The three TaqI sites interrogated in our study are located in mt:Cyt-b (Complex III subunit), mt:CoI (Complex IV subunit), and mt:tRNA:Arg (Figure 1, Table S1). The control primer pair anneals to sequences in the mt:CoIII gene (Complex IV subunit) (Table S1).
Fig. 1. The locations of TaqI sites in Drosophila mtDNA. 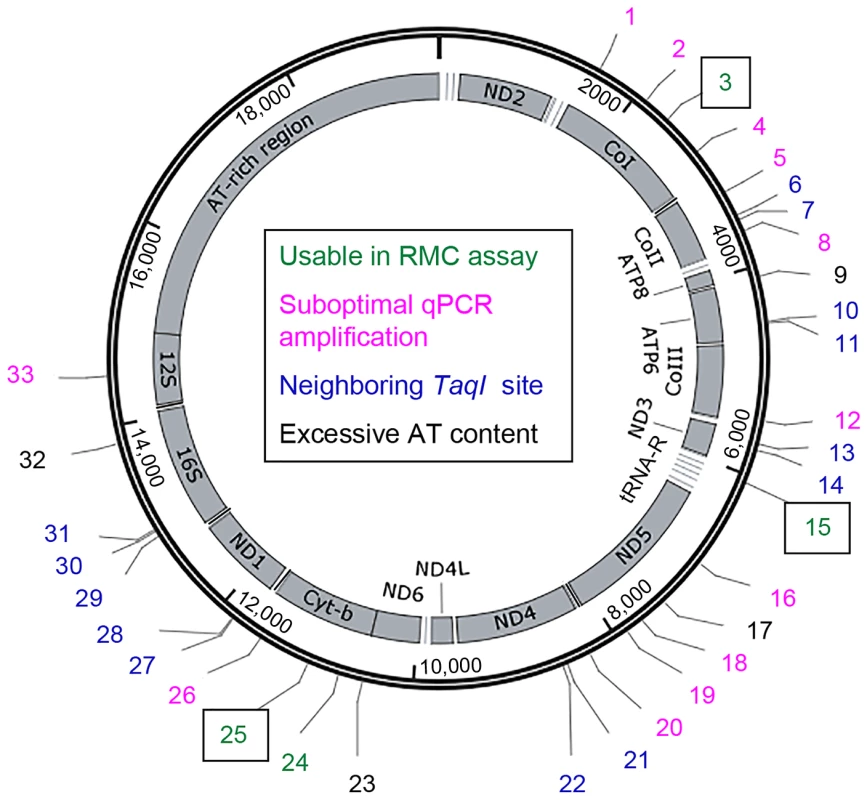
Schematic depiction of the Drosophila melanogaster mitochondrial genome. Numbers (1-33) indicate the different TaqI sites, color coded according to their utility for RMC analysis as indicated. Boxed numbers indicate the TaqI sites used for RMC analysis in our work. tRNA genes are represented by horizontal lines adjacent to other mitochondrial genes. Somatic mtDNA mutations accumulate with age in Drosophila
To explore the frequency and distribution of somatic mtDNA mutations in Drosophila, we used an isogenic fly line (w1118) to control for the possible influence of nuclear genetic background on the mtDNA mutation frequency in comparisons between cohorts. We isolated mtDNA separately from the heads and thoraces of adult flies, which contain primarily nervous tissue and muscle tissue, respectively so that the somatic mtDNA mutation frequency could be compared in these tissues. The abdomen was excluded from analysis because mtDNA replication in the female germline might confound our analysis of the somatic mtDNA mutation frequency.
RMC analysis of mtDNA isolated from the heads and thoraces of 1 - to 3-day-old flies (hereafter termed “young” animals; Figure S2B, Table S2, Table S3) revealed a similar mtDNA mutation frequency between the three TaqI sites within the same tissue for either young heads or thoraces (Figure S2). Therefore, we pooled the data from the three TaqI sites to achieve greater statistical power, and detected a small but significant increase in the mtDNA mutation frequency in young thoraces relative to heads (Figure 2A). The mtDNA mutation frequency in heads and thoraces (∼10−5) was similar to the mtDNA mutation frequency reported in vertebrates [25], [26], and variation in the mtDNA mutation frequency between tissues has also been reported in vertebrates [25], [26].
Fig. 2. Mitochondrial DNA mutation frequency increases with age and is greater in thoraces than in heads. 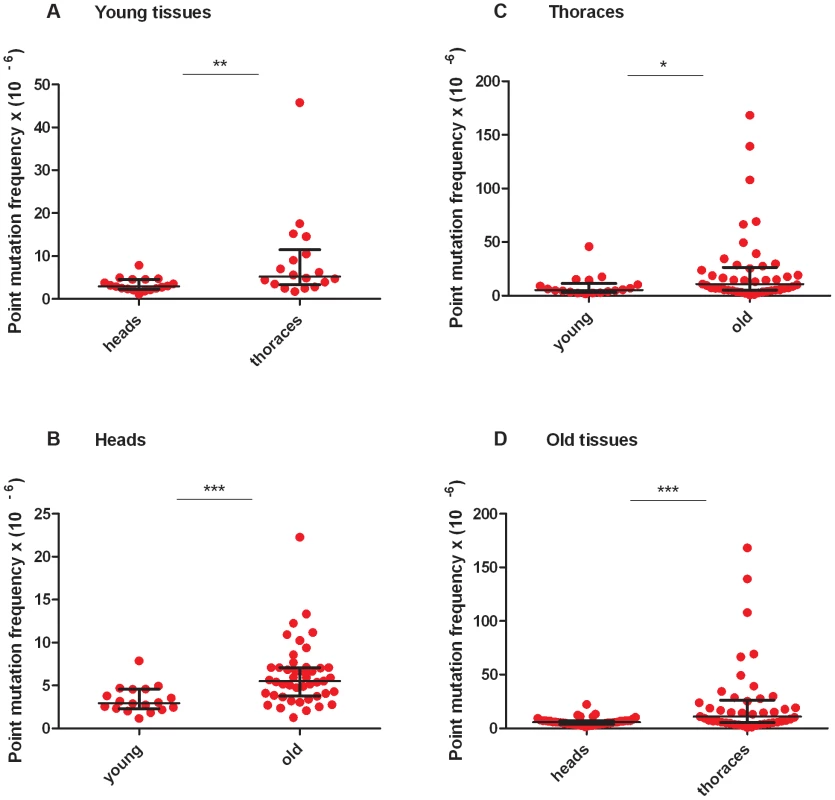
(A) The mtDNA mutation frequency is greater in young thoraces than in young heads (**p<0.01). (B) The mtDNA mutation frequency is greater in old heads than in young heads (***p<0.001) (C) and is greater in old thoraces than in young thoraces (*p<0.05). (D) The mtDNA mutation frequency is greater in old thoraces than in old heads (**p<0.001). Horizontal bars represent the median mutation frequency, and error bars indicate the interquartile range. Significance was tested using Mann-Whitney unpaired U tests. We next asked whether the frequency of somatic mtDNA mutations increases with age in Drosophila. Prior to measuring the mtDNA mutation frequency in older flies, we performed a lifespan analysis on the w1118 strain to identify a suitable age range for RMC experiments (Figure S2A). We used flies aged between 40 and 66 days (hereafter termed “old” animals) for our studies. As in young tissues, the mutation frequency was similar between TaqI sites within the same tissue for either old heads or thoraces (Figure S2C, S2D, Table S2, Table S3). Using pooled data from TaqI sites, RMC analysis revealed a significant increase in mtDNA mutation frequency with age in both heads and thoraces (Figure 2B, C). As in young animals, the mtDNA mutation frequency was higher in thoraces than in heads from old animals (Figure 2D). In contrast to previous work in mice showing an exponential increase in the frequency of mtDNA mutations with age [25], our data fit best to a model of linear increase in the mutation frequency with age (Figure S3). However, this discrepancy might simply reflect the dramatic increase in mtDNA mutation frequency that occurs in mice near the end of life when only 10% of the population is still alive [25], and the fact that the oldest flies examined in our studies involved an age where 25% of the population was still alive.
Upon closer inspection of our data, it appeared that much of the age-dependent increase in the mtDNA mutation frequency was caused by outlier samples with high mtDNA mutation frequencies (Figure 2). Because mtDNA mutations expand clonally in humans, we hypothesized that these outlier samples represent rare events in which a mutation within the TaqI site occurred early in development, and then clonally expanded in just one of the 100 flies in the sample. Assuming that a single fly fully accounts for the mtDNA mutation frequency in the outlier sample with the highest mtDNA mutation frequency, we estimate that the frequency of the clonally expanded mutation within that fly would approach 0.2%. An mtDNA mutation frequency of 0.2% is well below the threshold required to cause disease in humans for all known pathogenic mtDNA mutations [27], and thus would likely be well tolerated in flies even in the event of a deleterious mutation. A prediction of the hypothesis that outlier samples with high mtDNA mutation frequencies are a consequence of a single fly with an abundant clonally expanded mutation is that these samples should have a single predominating mutation type. By contrast, samples with an mtDNA mutation frequency nearer to the mean (non-outliers) would be more likely to contain a mixture of different mutation types that are contributed by multiple individuals in the population.
To test the hypothesis that outlier samples with high mtDNA mutation frequencies represent clonal expansion events, we compared the distribution of mutations in outlier and non-outlier samples by cloning and sequencing TaqI resistant DNA from these samples (Figure S4). We defined outlier samples as those that lie outside the 95% confidence interval of the mean for a given group. Our analysis revealed a trend towards an increased frequency of the predominating mutation in outlier samples, with the frequency greatest in the head and thorax samples with the highest mutation frequencies. However, this difference did not reach statistical significance (p = 0.09, Student's t-test). Thus, our data suggests that clonal expansion may be the explanation for the high mutation frequency in outlier samples; however, it does not allow us to rule out other explanations for outlier samples, including simple chance deviations from the mean.
Oxidative stress is only a minor contributor to somatic mtDNA mutations
Mitochondria are major producers of superoxide anion [28], which can damage DNA [29] and is implicated in the age-dependent accumulation of somatic mtDNA mutations [11], [30], [31]. To test whether superoxide and other ROS are a major cause of somatic mtDNA mutations in Drosophila, we performed several experiments. First, we analyzed the mtDNA mutation spectrum using data obtained from cloning and sequencing RMC processed samples. Superoxide anion reacts with deoxyguanosine to produce 8-hydroxy-2′-deoxyguanosine (8-oxo-dG) [32], which results in G∶C to T∶A transversions following DNA replication [29]. Thus, if oxidative stress is a major cause of somatic mtDNA mutations, there should be a preponderance of G∶C to T∶A transversion mutations in our samples. However, G∶C to T∶A transversions represented less than 10% of the mutations detected, regardless of the age of animals (Figure 3). Moreover, the frequency of G∶C to T∶A transversions did not increase appreciably with age (5% in young flies and 8% in old flies), and thus are not a major contributor to the increase in mtDNA mutation frequency with age. The most common mutation type detected in our samples was G∶C to A∶T transitions (80% of mutations in young flies and 86% in old flies). Further analysis revealed that the G∶C to A∶T transition mutations exhibited a strand bias, whereby G to A transitions occur more frequently on the major strand (the coding strand for the majority of mitochondrial genes) in both young and old tissues (Figure S5). Because strand asymmetric mutation accumulation has been postulated to reflect different mutation susceptibilities of the leading and lagging strands during DNA replication [33], our findings suggest that somatic mtDNA mutations occur primarily during mtDNA replication in Drosophila.
Fig. 3. GC∶TA transversions account for only a small percentage of mtDNA mutations. 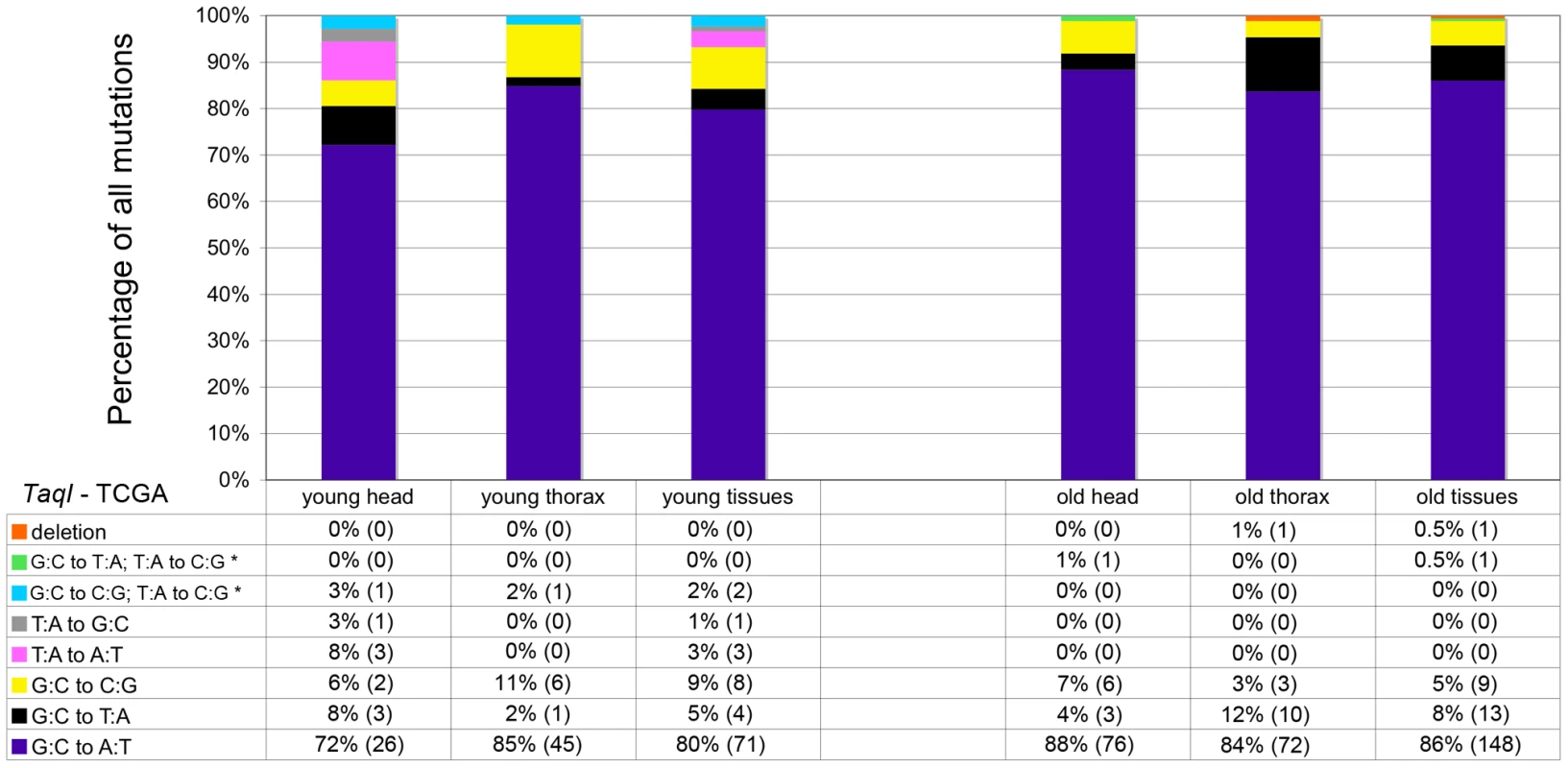
The percentage and total number (in parentheses) of each type of mutation identified at TaqI sites in young and old animals. Results depict data obtained separately from heads and thoraces and also combined. The wild-type TaqI sequence (TCGA) is shown for reference. * = Two mutations identified within a single cloned TaqI site. Biochemical studies indicate that 8-oxo-dG lesions block DNA replication, which may result in mtDNA depletion [34]. Depletion of mtDNA caused by 8-oxo-dG lesions could, therefore, possibly explain the finding that G∶C to T∶A transversion mutations did not increase appreciably with age. To address this hypothesis, we analyzed mtDNA copy number in fly heads using qPCR. We found no difference in mtDNA copy number relative to nuclear DNA between young and old flies (Figure S6). Therefore, mtDNA depletion does not account for the relative lack of an age-related increase in G∶C to T∶A mutation frequency.
To further explore the influence of oxidative stress on somatic mtDNA mutations in Drosophila, we tested whether flies deficient in the oxidative stress response pathway accumulate somatic mtDNA mutations at a higher rate. Superoxide anion is primarily detoxified in mitochondria by mitochondrially localized manganese superoxide dismutase 2 (Sod2). While null alleles of the Drosophila Sod2 gene exist, Sod2 null homozygotes survive for only a few hours as adults [35]. To circumvent this limitation, we generated transheterozygous flies that bear the Sod2n283 null mutation in combination with a hypomorphic mutation of the Sod2 gene (Sod2wk). Studies of Sod2n283/Sod2wk transheterozygotes (hereafter referred to as Sod2 mutants) have shown that these mutants exhibit increased oxidative stress, but are viable for nearly 50 days as adults [36].
Before conducting RMC analysis on Sod2 mutants, we verified that they would be suitable for our studies by performing a lifespan analysis (Figure 4A). We found that the median lifespan of Sod2 mutants was 41 days and that both the median and the maximum lifespan of Sod2 mutants were shortened by ∼30% relative to our isogenic w1118 strain. We measured the somatic mtDNA mutation frequency in 40 - to 42-day-old Sod2 mutants. Because our data indicate that the somatic mtDNA mutation pattern is similar in heads and thoraces and the Sod2 gene is ubiquitously expressed [37], we examined the mutation frequency in Sod2 mutants using only heads. Surprisingly, we detected no increase in the mtDNA mutation frequency at any of the individual TaqI sites analyzed in 40 - to 42-day-old Sod2 mutants relative to age-matched control animals (Figure S7). Pooled data from all three TaqI sites also failed to reveal a difference in the mutation frequency between Sod2 mutants and controls (Figure 4B, Table S2, Table S3). These findings provide further support for the conclusion that oxidative stress does not contribute significantly to the somatic mtDNA mutation frequency.
Fig. 4. Oxidative stress response pathway mutants do not have an increased mtDNA mutation frequency. 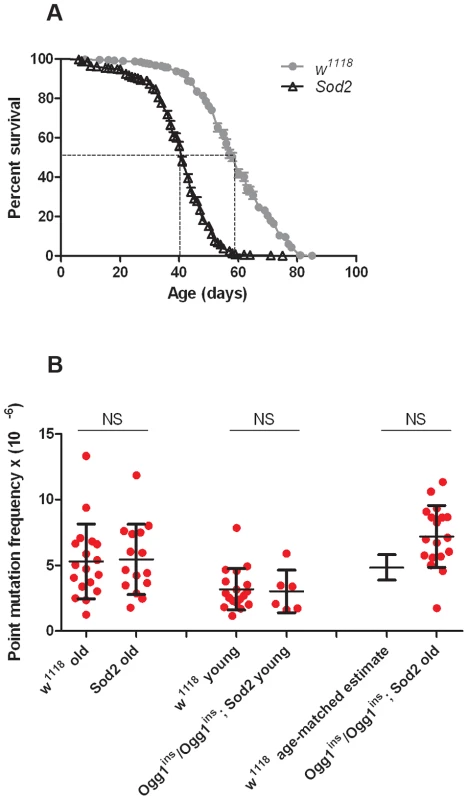
(A) Lifespan of w1118 and Sod2 mutant flies. The median lifespan (indicated by dashed lines) was 60 days for w1118 flies and 41 days for Sod2 mutants. (B) The mutation frequency using pooled data from all three TaqI sites in old Sod2 mutants (39- to 41-day-old), young Ogg1ins/Ogg1ins; Sod2 double mutants (1- to 3- day-old), old Ogg1ins/Ogg1ins; Sod2 double mutants (26- to 28- day-old), and age-matched w1118 controls. The 26- to 28- day-old control estimate was obtained from linear regression of the age-dependent w1118 mutation frequency. Horizontal bars represent the median mutation frequency, and error bars indicate the interquartile range. There was no significant difference in mutation frequency between mutants and age-matched controls (Mann-Whitney unpaired U tests). A caveat of our findings with Sod2 mutants is that efficient repair of oxidatively damaged bases might account for the lack of effect of Sod2 deficiency on the mtDNA mutation frequency. Previous work indicates that the 8-oxo-dG lesions formed by superoxide anion attack on mtDNA are primarily removed by a mitochondrially localized 8-oxoguanine glycosylase [32], [38]. Studies in Drosophila indicate that there are two genes, dOgg1 (referred to as Ogg1) and Ribosomal protein S3 (RpS3) that encode 8-oxoguanine glycosylase activity [39]. However, only the Ogg1 protein sequence bears significant homology to the human Ogg1 protein, and only Ogg1 appears to contain an N-terminal mitochondrial targeting sequence (Figure 5A). These findings suggest that Ogg1 is primarily responsible for the repair of mitochondrial 8-oxo-dG lesions in Drosophila.
Fig. 5. An Ogg1 mutation diminishes Ogg1 activity and interacts genetically with Sod2. 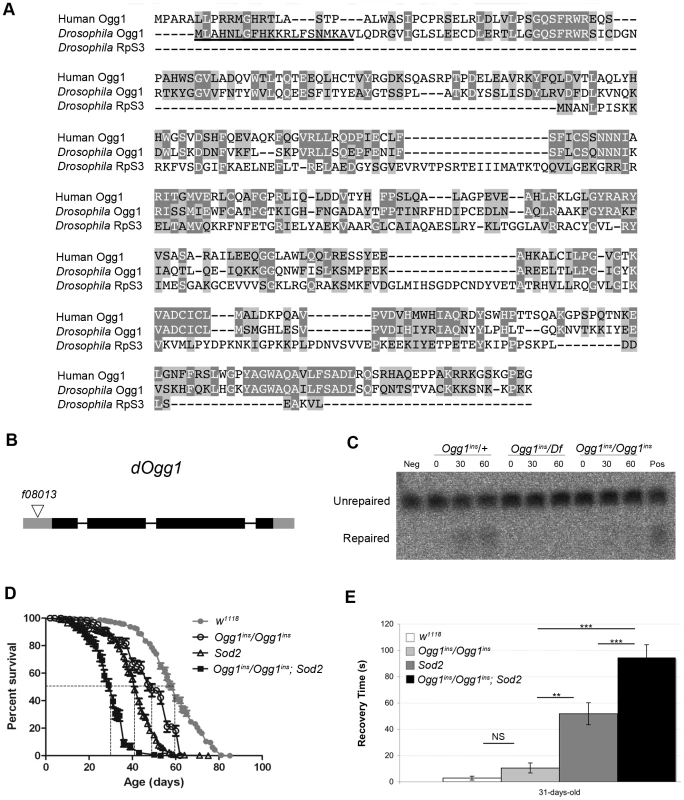
(A) Amino acid sequence alignment of human Ogg1 with Drosophila Ogg1 and RpS3. Dark shading denotes amino acid identities; light shading denotes amino acid similarities. The putative N-terminal mitochondrial targeting sequence of Drosophila Ogg1 is underlined. (B) The Ogg1 gene comprises four exons, designated by rectangles, and the coding sequence is indicated with dark gray shading. The Ogg1f08013 piggyBac insertion (designated by a triangle) is located 78 base pairs upstream of the Ogg1 translation start site. (C) 8-oxo-dG repair activity was assayed by monitoring cleavage of a 32P-labeled synthetic probe containing 8-oxo-dG, using protein extracts from flies of the indicated genotypes (Df = the Df(1)BSC627 deletion that removes Ogg1; ins = the Ogg1f08013 piggyBac insertion). Results of the assay are shown following 0, 30, and 60 minutes of incubation. Neg = No protein; Pos = Mouse brain protein extract. (D) Lifespan of w1118, Ogg1ins/Ogg1ins, Sod2, and Ogg1ins/Ogg1ins; Sod2 double mutant flies. The median lifespan (indicated by dashed lines) was 60 days for w1118, 49 days for Ogg1ins mutants, 41 days for Sod2 mutants, and 31 days for Ogg1ins; Sod2 double mutants. All lifespan curves are statistically different from one another using log rank tests (p<0.001). (E) w1118, Ogg1ins/Ogg1ins, Sod2, and Ogg1ins/Ogg1ins; Sod2 double mutant flies were subjected to a mechanical stress sensitivity test at 31 days. Values represent mean time (seconds) of recovery from mechanical stress, and error bars represent standard error of the mean. Significance was determined using one-way ANOVA followed by Tukey's post hoc tests (**p<0.01; ***p<0.001). To test the hypothesis that efficient repair of 8-oxo-dG lesions explains the lack of increased mutation frequency in Sod2 mutants, we inactivated Ogg1 in an Sod2 mutant background. A search of FlyBase revealed an Ogg1 allele (Ogg1f08013; hereafter referred to as Ogg1ins) caused by a piggyBac transposable element insertion located 78 bp upstream of the translation start site in the Ogg1 gene, as well as a large deletion (Df(1)BSC627) that removes the Ogg1 gene [40] (Figure 5B). We prepared protein lysates from Ogg1ins heterozygotes (Ogg1ins/+), Ogg1ins homozygotes (Ogg1ins/Ogg1ins), and Ogg1ins hemizygotes (Ogg1ins/Df(1)BSC627) and used them to assay 8-oxo-dG DNA repair activity. We found that Ogg1ins homozygotes and hemizygotes had reduced DNA repair activity relative to the heterozygous control (Figure 5C). The magnitude of this defect was greater in extracts from Ogg1ins hemizygotes relative to Ogg1ins homozygotes, indicating that the Ogg1ins mutation represents a hypomorphic loss-of-function allele of the Ogg1 gene.
To test whether Ogg1 activity masks a somatic mtDNA mutator phenotype of Sod2 mutants, we constructed and analyzed Ogg1ins/Ogg1ins; Sod2 double mutants. Behavioral studies of the double mutants revealed that the Ogg1ins mutation reduced lifespan and enhanced stress-induced seizure (bang-sensitive) phenotypes of Sod2 mutants (Figure 5D, E), suggesting that these genes act in a common pathway. To test whether these genetic interactions reflect an increased mtDNA mutation frequency in the double mutants, we measured the mtDNA mutation frequency in the heads of young (1 - to 3-day-old) and old (26 - to 28-day-old) Ogg1ins/Ogg1ins; Sod2 double mutants. We used 26 - to 28-day-old animals as our “old” sample, because this is near the median lifespan of the double mutants (Figure 5D). The mtDNA mutation frequency at the corresponding age in w1118 controls was obtained using linear regression (Figure S3A). To increase our ability to detect small differences, we pooled data from all three TaqI sites analyzed. Despite this measure, we were unable to detect a difference in somatic mtDNA mutation frequency between young double mutants and age-matched controls (Figure 4B). While there was a trend towards an increase in somatic mtDNA mutations in old double mutants, this difference did not reach significance (p = 0.08). Together, our findings indicate that oxidative stress is not a major contributor to the somatic mtDNA mutation frequency in Drosophila.
Discussion
The accumulation of somatic mtDNA mutations is thought to contribute to aging and common age-related diseases, including cancer and Parkinson's disease. The causes of somatic mtDNA mutations are poorly understood, as are the factors that influence their cellular frequency following their occurrence. Given the powerful genetic tools available in Drosophila for studying fundamental biological processes, we explored the utility of this model system to investigate the mechanisms that influence the frequency of somatic mtDNA mutations. We show that features associated with mtDNA mutations in vertebrates are conserved in Drosophila, including the somatic mtDNA mutation frequency, an increased frequency of somatic mtDNA mutations with age, a prevalence of G∶C to A∶T transition mutations, and possibly, clonal expansion of somatic mtDNA mutations. These findings indicate that Drosophila will serve as a valuable model system in which to examine the mechanisms influencing somatic mtDNA mutation frequency.
Although oxidative stress is commonly cited as a major contributor to somatic mtDNA mutations [31], our work does not support this idea. The fraction of G∶C to T∶A transversion mutations, a consequence of 8-oxo-dG lesions, is small in young flies and does not increase appreciably with age. These findings parallel recent results in mice and humans showing a similarly low occurrence of G∶C to T∶A transversion mutations in samples from both young and old individuals [25], [41], [42]. Furthermore, we found that mutations in genes that oppose superoxide-induced DNA damage do not detectably influence the mtDNA point mutation frequency. However, because the total number of somatic mtDNA mutations increases with age but the percentage of G∶C to T∶A transversions remained nearly constant, our data do leave open the possibility that oxidative stress is a minor contributor to the increased burden of somatic mtDNA mutations with age. Moreover, while our studies indicate that superoxide is not a major cause of mtDNA point mutations, further work will be required to determine whether superoxide contributes to the frequency of mtDNA deletions, and to assess the influence of other forms of ROS, such as hydroxyl radicals, on mtDNA mutation frequency.
How can we reconcile our current findings with the body of work supporting a role of ROS in mtDNA mutations? Such work includes direct sequencing of mtDNA [43], [44], and a study showing that catalase expression in mouse mitochondria reduced the frequency of mtDNA mutations [45]. One possible explanation for at least some of the conflicts involving sequencing is that PCR amplification of DNA that contains 8-oxo-dG lesions results in G∶C to T∶A transversion mutations during the amplification process. Sequencing of material containing amplification-induced mutations could thus result in an overestimation of the true G∶C to T∶A transversion frequency. Further compounding this potential problem is the finding that oxidative modification of mtDNA can occur during mtDNA isolation [46]. By contrast, the RMC assay is immune to this type of artifact because TaqI is capable of digesting oxidized DNA, and thereby removes oxidized molecules prior to mutation detection by qPCR [18], [25]. Given that the study involving catalase ovexpression also used RMC to monitor mtDNA mutation frequency, we cannot presently offer an explanation for this conflict. However, it is important to emphasize that the findings from studies of catalase overexpression do not necessarily represent a direct conflict with our current conclusion because our findings leave open the possibility that ROS makes a small contribution to the mtDNA mutation frequency. Nevertheless, further studies will be required to explain the sizeable magnitude of the effect of catalase overexpression on the mtDNA mutation frequency, given the small contribution of ROS to mtDNA mutations suggested by our work.
If oxidative stress accounts for only a small fraction of the somatic mtDNA mutation burden, what then is the major source of these mutations? Previous work has shown that expression of a proofreading-deficient form of mtDNA polymerase (Pol-γ) causes primarily G∶C to A∶T transition mutations [47]. This finding raises the possibility that Pol-γ misincorporation errors are responsible for the G∶C to A∶T mutations that account for the majority of the somatic mtDNA mutations detected in our work. However, while Pol-γ replication errors may account for some of the G∶C to A∶T transition mutations that occur in somatic tissues, this model is difficult to reconcile with the finding that most of these mutations accumulate in a strand asymmetric fashion. Instead, we propose that most of the G∶C to A∶T transition mutations that occur in somatic tissues arise because of differing sensitivities of the leading and lagging strands to mutagenesis during mtDNA replication. In Drosophila, the mtDNA minor strand is replicated in a continuous fashion (the leading strand) and the major strand is replicated in a discontinuous fashion (the lagging strand) [48]–[50]. During leading strand synthesis, the template for the lagging strand (the minor strand) is transiently single-stranded, and thus more susceptible to DNA damage than the template for the leading strand (the major strand). One type of DNA damage that is compatible with our findings is spontaneous deamination of cytosine [51]–[53], the rate of which increases several orders of magnitude in single-stranded DNA [33]. Selective cytosine deamination on the minor strand during leading strand synthesis would offer an explanation for the preponderance of C to T mutations that we detect on the minor strand (equivalent to G to A mutations on the major strand). While transcription can also lead to asymmetric accumulation of mutations on the non-transcribed strand [54]–[56], the pattern of mutational accumulation observed in our work is inconsistent with a transcriptional origin because most of the C to T mutations occur on the transcribed strand. The strand asymmetric mutational bias detected in our study is consistent with previous findings in aging human brain [42] and in the Drosophila germline [57], suggesting that spontaneous cytosine deamination on the lagging strand template during replication is a conserved mechanism of mtDNA mutation accumulation.
Another important question that is raised by our findings is why Ogg1 mutations enhance Sod2 mutant phenotypes if the double mutants do not have a significantly higher mtDNA mutation frequency than control flies. While we believe that ROS-mediated damage to proteins and/or lipids [35], [36] is the primary explanation for the shortened lifespan and bang sensitivity of Sod2 mutants, the genetic interaction between the Sod2 and Ogg1 mutations cannot readily be explained by an increase in protein or lipid damage in the double mutants because there is no evidence that Ogg1 is involved in the repair of damaged proteins or lipids. However, recent studies show that several other factors involved in base excision repair also function in apoptosis and transcriptional regulation [58], [59]. Thus, one possibility is that Ogg1 has a function other than DNA repair, and that the loss of this function is responsible for the genetic interaction with Sod2. Alternatively, Ogg1 may also play a role in nuclear DNA repair, and the genetic interaction between Ogg1 and Sod2 may reflect inefficient repair of oxidatively damaged nuclear DNA. Finally, the genetic interaction between Ogg1 and Sod2 may reflect an increase in the somatic mtDNA mutation frequency that was below the detection limit of our assay. Although we did not observe a statistically significant increase in the mtDNA mutation frequency in the Ogg1; Sod2 double mutants, there was a clear trend in this direction. Future studies will be required to distinguish between these possibilities.
In conclusion, our study sheds additional light on the genesis of mtDNA mutations, and also indicates that Drosophila is a tractable genetic model system with which to study the mechanisms that influence the frequency of mtDNA mutations. Little is known of the cellular factors that influence heteroplasmy, clonal expansion, and the inheritance of mtDNA mutations. The use of Drosophila should facilitate rapid advancement in our understanding of mtDNA mutations and their roles in aging and disease.
Materials and Methods
Fly strains and maintenance
Flies were maintained on standard cornmeal-molasses food (70% cornmeal, 10.5% molasses, 2.5% yeast, and 1% agar) at 25°C. The w1118 isogenic fly strain, Ogg1 PiggyBac element insertion (Ogg1f08013) strain, and Ogg1 deletion (Df(1)BSC627) strain were obtained from the Bloomington Drosophila Stock Center. The Sod2n283 and Sod2wk mutants have been described previously [35], [36].
Behavioral analysis
Lifespan analysis
One - to two-day-old flies were placed in vials in groups of up to 20 flies. Every 2–3 days flies were transferred to fresh vials and the number of deaths was recorded. Each lifespan trial was repeated at least three times, with a minimum of 100 flies per genotype analyzed. Survivorship was plotted as a function of time (in days) using GraphPad Prism software version 5.04 (GraphPad Software, Inc., La Jolla, CA). Survival curves were compared between genotypes using the log-rank test to determine significance.
Bang sensitivity analysis
Flies were tested for sensitivity to mechanical stress using a modified version of a published procedure [60]. Briefly, one - to two-day-old flies were placed into vials in groups of 2 and aged to 31 days. Fly vials were inverted and vortexed on the maximum setting for ten seconds, and the time required for flies to recover from paralysis and/or seizures was recorded. A minimum of 20 flies was tested per genotype.
5-bromo-2′-deoxyuridine (BrdU) labeling and analysis
One-day-old flies were collected, starved for 24 hours, and then transferred to vials lined with Whatman filter paper (GE Healthcare, Pittsburg, PA). Flies were fed BrdU by adding a solution of 1 mg/mL BrdU (Sigma-Aldrich, St. Louis, MO) in 5% sucrose and 5% yeast directly to the filter paper. Fresh BrdU containing solution was added to the filter paper twice a day for three days. Following BrdU labeling, tissues were collected for Southwestern blot analysis or confocal microscopy.
Southwestern analysis
Flies were flash frozen in liquid nitrogen and vortexed for 10 to 15 seconds to dissociate body parts. One hundred fly heads were collected and homogenized in 300 µL buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 20 mM EDTA, 1% SDS) in a 1.5-mL Eppendorf tube using a plastic pestle. RNase A (Applied Biosystems, Foster City, CA) was added to a final concentration of 10 µg/mL and incubated at 37°C for 30 minutes. Following RNase A treatment, proteinase K (Qiagen, Valencia, CA) was added to a final concentration of 200 µg/mL and incubated either overnight at 37°C or for three hours at 56°C. An equal volume of cold phenol/chloroform/isoamyl alcohol (25∶24∶1; Fisher, Pittsburgh, PA) was then added, and the samples were inverted ten times, mixed, and centrifuged at 15,800× g for 10 minutes. The aqueous phase containing the DNA was collected and the phenol/chloroform/isoamyl alcohol extraction was repeated once, followed by a single extraction with chloroform. The DNA was then precipitated with 3M sodium acetate pH 5.2 and isopropanol and centrifuged at 15,800× g for 25 minutes at 4°C. The DNA pellet was resuspended in 10 mM Tris-HCl and 0.1 mM EDTA (pH 8.0), and 3 µg of DNA was immediately digested with BglII (New England Biolabs, Beverly, MA) for at least 16 hours at 37°C. The digested DNA was then subjected to Southern blot analysis using nylon membranes (Millipore Corp, Billerica, MA). DNA crosslinking to membranes was performed using a UV Stratalinker 1800 on the auto crosslink setting (Agilent Technologies, Santa Clara, CA). The membrane was blocked for 1 hour in 5% milk in PBS at 25°C, then incubated with antiserum to BrdU (Abcam #92837, Cambridge, MA) at a 1∶1000 dilution overnight. Membranes were then washed in 0.1% Tween in PBS, followed by incubation with HRP-conjugated anti-chicken secondary antibody (Sigma-Aldrich #A9046, St. Louis, MO) at a 1∶80,000 dilution. Western Blotting Substrate (Fisher, Pittsburgh, PA) was used to detect peroxidase signal.
Immunohistochemistry and confocal microscopy
BrdU-labeled tissues were prepared for confocal microscopy by dissecting the thoracic ganglion in cold PBS using a dissecting microscope. The thoracic ganglion was stained with 25 nM MitoTracker Deep Red for 25 minutes (Invitrogen, Carlsbad, CA), rinsed with 0.1% Triton X-100 in PBS (wash buffer), and fixed for 30 minutes in 4% paraformaldehyde (Ted Pella Inc., Redding, CA) in PBS at 4°C. After rinsing in wash buffer, the thoracic ganglion was incubated in 1 M HCl for 30 minutes at 25°C, then blocked in 0.1% Triton X-100 with 10% heat-inactivated FBS in PBS (blocking buffer) for 1 hour at 25°C. Primary antibody to BrdU (Abcam #Ab6326, Cambridge, MA) was then added at 1∶100 dilution and incubated overnight at 4°C. After washing with wash buffer, Alexa Fluor 488 goat anti-rat IgG (Molecular Probes #A11006, Eugene, OR) was added to blocking buffer at 1∶10,000 dilution and incubated for 2 hours at 25°C. Stained thoracic ganglia were washed with wash buffer, mounted in Fluoromount (Sigma-Aldrich, St. Louis, MO), and imaged using an Olympus FV-1000 with a 100× lens and 2–4× digital zoom.
Random mutation capture assay
qPCR primer design and validation
TaqI test primers were designed to flank TaqI sites and amplify products sized between 95 and 250 bp using NCBI's Primer-BLAST program (Primer3, [61]) (Primer-Blast, National Center for Biotechnology Information, National Library of Medicine, Bethesda, MD). The Primer-BLAST algorithm was also used to optimize primer specificity by testing for mis-priming to related nuclear and mitochondrial sequences. There are four known Drosophila mitochondrial pseudogenes that reside in the nuclear DNA [62], and these sequences were excluded from primer design to ensure that our primers were specific for mitochondrial sequences. Control primers were designed in the same manner as test primers but do not flank a TaqI site. Because the Drosophila mtDNA has a high A∶T content, control primers were sought at mtDNA sequences with a relatively high G∶C content.
We validated the efficiency of control and test primer pairs by measuring the cycle threshold (Ct; the cycle number at which the signal rises above background) using a four-fold dilution series of undigested mtDNA, and plotting Ct vs. dilution (Table S1). Theory predicts that product abundance doubles at each cycle of PCR amplification, so the slope of Ct vs. dilution should be 2. Primer pairs that deviated by 15% or more from a slope value of 2 were discarded. Only test and control primers that yielded slope values within 5% of one another were used in our analyses (Table S1). Finally, we sequenced the PCR product produced by each primer pair that passed our quality control thresholds to ensure that only the correct target was amplified.
DNA isolation
One - to three-day-old flies were collected and transferred to fresh food every 2–3 days until they reached the ages chosen for RMC analysis. Flies were then flash frozen in liquid nitrogen in groups of 100 and vortexed for 10 to 15 seconds to dissociate body parts. Heads were collected, and the thorax was sectioned from the abdomen using a razor blade. Groups of 100 heads or thoraces were then processed to isolate DNA as described above (Southwestern analysis). The DNA pellet was resuspended in 10 mM Tris-HCl and 0.1 mM EDTA (pH 8.0), and was then used for TaqI digestion or frozen at −80°C until use.
TaqI digestion: The methods for the RMC assay are described in detail in reference [19]. Briefly, each sample of ∼10 µg of DNA was treated with 100 U of TaqI restriction enzyme (New England Biolabs, Beverly, MA) and incubated at 65°C. An additional 100 U were added every hour for a total of 10 hours to promote complete DNA digestion.
qPCR experiments
qPCR experiments were carried out on an MJ Research DNA Engine Opticon 2 System (Hercules, CA) using Power SYBR Green PCR Master Mix (Agilent, Wilmington, DE). The PCR cycle consisted of an initial denaturing step at 95°C for 10 minutes, followed by 45 cycles of denaturing at 95°C for 30 seconds, annealing at 60°C for 1 min, and extension at 72°C for 15 sec. After the 45 cycles of PCR, a final extension step was performed at 72°C for 1 min. Melting curves were performed on all PCR products and analyzed using the Bio-Rad Opticon Monitor II software to ensure that primers amplified a single product.
Mutation quantification
To quantify the mutation frequency, we first used the Bio-Rad Opticon Monitor II software to determine the Ct values of the control primers (Ctcontrol), representing total mtDNA molecules, and the Ct values of the test primers (Cttest), representing mutant mtDNA molecules. The ratio of mutant to WT mtDNA molecules was then calculated from the difference in Ct values using the following equation: Point mutation frequency = Mutant molecules/WT molecules = 1/2ΔCt×4, where ΔCt = Ctcontrol−Cttest. This value is multiplied by a factor of 4 because mutations at any of the four bases in the TaqI restriction site would prevent digestion. Following qPCR amplifcation, the amplification products were either sequenced or digested with TaqI and subjected to densitometry to estimate the percentage of contaminating WT molecules that remained. This percentage was then used to adjust the mtDNA mutation calculation to account for contaminating WT mtDNA.
Clonal expansion
To test for clonal expansion, we cloned DNA from eight individual samples, performed Sanger sequencing on at least 10 colonies per sample, and quantified the percentage of each type of mutation detected. Data was collected from the TaqI sites located in the mt:Cyt-b or mt:CoI genes. Additionally, we compared the total amount of mtDNA and total number of mtDNA copies in outlier and non-outliers samples. This analysis revealed that neither of these parameters differed between outliers and non-outliers (Table S4).
DNA copy number
Total DNA was isolated as described above (Random Mutation Capture Assay: DNA isolation) from the heads of young and old flies and used as template in qPCR. qPCR settings were as described above (Random Mutation Capture Assay: qPCR experiments). Relative copy number of mtDNA to nuclear DNA was determined using control mtDNA primers against mt:CoIII (see Table S1) and nuclear DNA primers against β-Tubulin [63].
Ogg1 repair of 8-oxo-dG
Ogg1 activity was assayed using fly head homogenates as previously described [64]. Briefly, the heads from 15–18 anesthetized flies, aged 1 to 3 days, were collected on ice using a razor blade and homogenized in 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, 1 mM DTT, 0.5 mM spermine, 0.5 mM spermidine, protease inhibitor, and 50% glycerol. A 1∶10 volume of 2.5 M KCl was added to this homogenate and mixed at 4°C for 30 minutes. Samples were then centrifuged at 15,800× g for 30 minutes and the supernatant was collected and frozen at −80°C until use in the 8-oxo-dG repair assay. Ogg1 activity was measured at 37°C using 30 µg of protein extract and a 32P-labeled synthetic probe containing 8-oxo-dG for the indicated times. The reaction was stopped by placing the solution on ice and adding 20 µl of loading buffer containing 90% formamide, 10 mM of NaOH, and Blue/Orange Loading Dye (Promega Corp., Madison, WI). The solution was then heated at 95°C for 3 min and placed on ice. The samples were loaded onto a polyacrylamide gel (20%) in 7 M urea and 1× TBE running buffer and run at 400 mV for 2 h. An FLA-3000 Series Fuji Film Fluorescent Image Analyzer was used to obtain images from the gel. All reagents used in this analysis were obtained from Sigma-Aldrich unless indicated otherwise (St. Louis, MO).
Sequence algorithms
Clustal Omega (Cambridge, UK) was used to align human Ogg1 and Drosophila Ogg1 and RpS3 sequences [65], [66], and MitoPROT (Munich, Germany) was used to identify N-terminal mitochondrial targeting sequences in Drosophila Ogg1 [67]. Snap Gene software was used to create the Drosophila mtDNA map with TaqI sites (GSL Biotech LLC, Chicago, IL)
Statistics
Significance was determined using Mann-Whitney unpaired U-tests to compare mtDNA mutation frequencies; log-rank test to compare survival curves for lifespan analyses; a one-way ANOVA followed by Tukey's post hoc test for bang-sensitive experiments; a two-tailed Student's t-test for mtDNA copy number comparison, and Pearson's correlation coefficient for linear correlation of mtDNA mutation frequency with age.
Supporting Information
Zdroje
1. McBrideHM, NeuspielM, WasiakS (2006) Mitochondria: more than just a powerhouse. Current Biology 16: R551–560.
2. WallaceDC (2012) Mitochondria and cancer. Nature reviews Cancer 12 : 685–698.
3. WangC, YouleRJ (2009) The role of mitochondria in apoptosis. Annual Review of Genetics 43 : 95–118.
4. BooreJL (1999) Animal mitochondrial genomes. Nucleic Acids Res 27 : 1767–1780.
5. DiMauroS, HiranoM (2005) Mitochondrial encephalomyopathies: an update. Neuromuscular disorders : NMD 15 : 276–286.
6. ParkCB, LarssonNG (2011) Mitochondrial DNA mutations in disease and aging. The Journal of cell biology 193 : 809–818.
7. BenderA, KrishnanKJ, MorrisCM, TaylorGA, ReeveAK, et al. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genetics 38 : 515–517.
8. KraytsbergY, KudryavtsevaE, McKeeAC, GeulaC, KowallNW, et al. (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nature Genetics 38 : 518–520.
9. de GreyAD (2009) How is mutant mitochondrial DNA clonally amplified? Much new evidence, still no answers. Rejuvenation Res 12 : 217–219.
10. LarssonNG (2010) Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem 79 : 683–706.
11. HarmanD (1972) The biologic clock: the mitochondria? Journal of the American Geriatrics Society 20 : 145–147.
12. BrierleyEJ, JohnsonMA, LightowlersRN, JamesOF, TurnbullDM (1998) Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann Neurol 43 : 217–223.
13. GuoM (2010) What have we learned from Drosophila models of Parkinson's disease? Prog Brain Res 184 : 3–16.
14. KohH, ChungJ (2012) PINK1 as a molecular checkpoint in the maintenance of mitochondrial function and integrity. Mol Cells 34 : 7–13.
15. CallejaM, PenaP, UgaldeC, FerreiroC, MarcoR, et al. (1993) Mitochondrial DNA remains intact during Drosophila aging, but the levels of mitochondrial transcripts are significantly reduced. The Journal of Biological Chemistry 268 : 18891–18897.
16. SchwarzeSR, WeindruchR, AikenJM (1998) Decreased mitochondrial RNA levels without accumulation of mitochondrial DNA deletions in aging Drosophila melanogaster. Mutat Res 382 : 99–107.
17. YuiR, MatsuuraET (2006) Detection of deletions flanked by short direct repeats in mitochondrial DNA of aging Drosophila. Mutat Res 594 : 155–161.
18. BielasJH, LoebLA (2005) Quantification of random genomic mutations. Nat Methods 2 : 285–290.
19. VermulstM, BielasJH, LoebLA (2008) Quantification of random mutations in the mitochondrial genome. Methods 46 : 263–268.
20. Martinez-AzorinF, CallejaM, Hernandez-SierraR, FarrCL, KaguniLS, et al. (2008) Over-expression of the catalytic core of mitochondrial DNA (mtDNA) polymerase in the nervous system of Drosophila melanogaster reduces median life span by inducing mtDNA depletion. J Neurochem 105 : 165–176.
21. JoersP, JacobsHT (2013) Analysis of replication intermediates indicates that Drosophila melanogaster mitochondrial DNA replicates by a strand-coupled theta mechanism. PLoS One 8: e53249.
22. GrossNJ, GetzGS, RabinowitzM (1969) Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem 244 : 1552–1562.
23. JonesS, ChenWD, ParmigianiG, DiehlF, BeerenwinkelN, et al. (2008) Comparative lesion sequencing provides insights into tumor evolution. Proceedings of the National Academy of Sciences of the United States of America 105 : 4283–4288.
24. ShendureJ, JiH (2008) Next-generation DNA sequencing. Nature Biotechnology 26 : 1135–1145.
25. VermulstM, BielasJH, KujothGC, LadigesWC, RabinovitchPS, et al. (2007) Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet 39 : 540–543.
26. KhrapkoK, VijgJ (2009) Mitochondrial DNA mutations and aging: devils in the details? Trends Genet 25 : 91–98.
27. GreavesLC, ReeveAK, TaylorRW, TurnbullDM (2012) Mitochondrial DNA and disease. J Pathol 226 : 274–286.
28. MurphyMP (2009) How mitochondria produce reactive oxygen species. Biochem J 417 : 1–13.
29. De BontR, van LarebekeN (2004) Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 19 : 169–185.
30. HarmanD (1956) Aging: a theory based on free radical and radiation chemistry. Journal of gerontology 11 : 298–300.
31. WallaceDC (2001) A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp 235 : 247–263 discussion 263-246.
32. MishraPC, SinghAK, SuhaiS (2005) Interaction of singlet oxygen and superoxide radical anion with guanine and formation of its mutagenic modification 8-oxoguanine. International Journal of Quantum Chemistry 102 : 282–301.
33. FredericoLA, KunkelTA, ShawBR (1990) A sensitive genetic assay for the detection of cytosine deamination: determination of rate constants and the activation energy. Biochemistry 29 : 2532–2537.
34. GraziewiczMA, BienstockRJ, CopelandWC (2007) The DNA polymerase gamma Y955C disease variant associated with PEO and parkinsonism mediates the incorporation and translesion synthesis opposite 7,8-dihydro-8-oxo-2′-deoxyguanosine. Hum Mol Genet 16 : 2729–2739.
35. DuttaroyA, PaulA, KunduM, BeltonA (2003) A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics 165 : 2295–2299.
36. PaulA, BeltonA, NagS, MartinI, GrotewielMS, et al. (2007) Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech Ageing Dev 128 : 706–716.
37. ChintapalliVR, WangJ, DowJA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 : 715–720.
38. de Souza-PintoNC, EideL, HogueBA, ThyboT, StevnsnerT, et al. (2001) Repair of 8-oxodeoxyguanosine lesions in mitochondrial dna depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer Res 61 : 5378–5381.
39. DherinC, DizdarogluM, DoerflingerH, BoiteuxS, RadicellaJP (2000) Repair of oxidative DNA damage in Drosophila melanogaster: identification and characterization of dOgg1, a second DNA glycosylase activity for 8-hydroxyguanine and formamidopyrimidines. Nucleic acids research 28 : 4583–4592.
40. McQuiltonP, St PierreSE, ThurmondJ, FlyBaseC (2012) FlyBase 101–the basics of navigating FlyBase. Nucleic Acids Res 40: D706–714.
41. AmeurA, StewartJB, FreyerC, HagstromE, IngmanM, et al. (2011) Ultra-deep sequencing of mouse mitochondrial DNA: mutational patterns and their origins. PLoS Genet 7: e1002028.
42. KennedySR, SalkJJ, SchmittMW, LoebLA (2013) Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet 9: e1003794.
43. WeiYH (1998) Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med 217 : 53–63.
44. Ruiz-PesiniE, LottMT, ProcaccioV, PooleJC, BrandonMC, et al. (2007) An enhanced MITOMAP with a global mtDNA mutational phylogeny. Nucleic Acids Res 35: D823–828.
45. DaiDF, SantanaLF, VermulstM, TomazelaDM, EmondMJ, et al. (2009) Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119 : 2789–2797.
46. BokovA, ChaudhuriA, RichardsonA (2004) The role of oxidative damage and stress in aging. Mech Ageing Dev 125 : 811–826.
47. SpelbrinkJN, ToivonenJM, HakkaartGA, KurkelaJM, CooperHM, et al. (2000) In vivo functional analysis of the human mitochondrial DNA polymerase POLG expressed in cultured human cells. J Biol Chem 275 : 24818–24828.
48. GoddardJM, WolstenholmeDR (1978) Origin and direction of replication in mitochondrial DNA molecules from Drosophila melanogaster. Proc Natl Acad Sci U S A 75 : 3886–3890.
49. GoddardJM, WolstenholmeDR (1980) Origin and direction of replication in mitochondrial DNA molecules from the genus Drosophila. Nucleic Acids Res 8 : 741–757.
50. SaitoS, TamuraK, AotsukaT (2005) Replication origin of mitochondrial DNA in insects. Genetics 171 : 1695–1705.
51. WagnerJR, CadetJ (2010) Oxidation reactions of cytosine DNA components by hydroxyl radical and one-electron oxidants in aerated aqueous solutions. Acc Chem Res 43 : 564–571.
52. KreutzerDA, EssigmannJM (1998) Oxidized, deaminated cytosines are a source of C→T transitions in vivo. Proc Natl Acad Sci U S A 95 : 3578–3582.
53. ZhengW, KhrapkoK, CollerHA, ThillyWG, CopelandWC (2006) Origins of human mitochondrial point mutations as DNA polymerase gamma-mediated errors. Mutat Res 599 : 11–20.
54. BeletskiiA, BhagwatAS (1998) Correlation between transcription and C to T mutations in the non-transcribed DNA strand. Biol Chem 379 : 549–551.
55. FrancinoMP, ChaoL, RileyMA, OchmanH (1996) Asymmetries generated by transcription-coupled repair in enterobacterial genes. Science 272 : 107–109.
56. FrancinoMP, OchmanH (2001) Deamination as the basis of strand-asymmetric evolution in transcribed Escherichia coli sequences. Mol Biol Evol 18 : 1147–1150.
57. Haag-LiautardC, CoffeyN, HouleD, LynchM, CharlesworthB, et al. (2008) Direct estimation of the mitochondrial DNA mutation rate in Drosophila melanogaster. PLoS Biol 6: e204.
58. BernsteinC, BernsteinH, PayneCM, GarewalH (2002) DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res 511 : 145–178.
59. de Souza-PintoNC, MasonPA, HashiguchiK, WeissmanL, TianJ, et al. (2009) Novel DNA mismatch-repair activity involving YB-1 in human mitochondria. DNA Repair (Amst) 8 : 704–719.
60. GanetzkyB, WuCF (1982) Indirect Suppression Involving Behavioral Mutants with Altered Nerve Excitability in DROSOPHILA MELANOGASTER. Genetics 100 : 597–614.
61. RozenS, SkaletskyH (2000) Primer3 on the WWW for general users and for biologist programmers. Methods in molecular biology 132 : 365–386.
62. RogersHH, Griffiths-JonesS (2012) Mitochondrial pseudogenes in the nuclear genomes of Drosophila. PLoS One 7: e32593.
63. ZhangK, LiZ, JaiswalM, BayatV, XiongB, et al. (2013) The C8ORF38 homologue Sicily is a cytosolic chaperone for a mitochondrial complex I subunit. J Cell Biol 200 : 807–820.
64. Cardozo-PelaezF, BrooksPJ, StedefordT, SongS, Sanchez-RamosJ (2000) DNA damage, repair, and antioxidant systems in brain regions: a correlative study. Free Radic Biol Med 28 : 779–785.
65. SieversF, WilmA, DineenD, GibsonTJ, KarplusK, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology 7 : 539.
66. GoujonM, McWilliamH, LiW, ValentinF, SquizzatoS, et al. (2010) A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic acids research 38: W695–699.
67. ClarosMG, VincensP (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241 : 779–786.
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



