-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
Caenorhabditis elegans CEP-1 and its mammalian homolog p53 are critical for responding to diverse stress signals. In this study, we found that cep-1 inactivation suppressed the prolonged lifespan of electron transport chain (ETC) mutants, such as isp-1 and nuo-6, but rescued the shortened lifespan of other ETC mutants, such as mev-1 and gas-1. We compared the CEP-1-regulated transcriptional profiles of the long-lived isp-1 and the short-lived mev-1 mutants and, to our surprise, found that CEP-1 regulated largely similar sets of target genes in the two mutants despite exerting opposing effects on their longevity. Further analyses identified a small subset of CEP-1-regulated genes that displayed distinct expression changes between the isp-1 and mev-1 mutants. Interestingly, this small group of differentially regulated genes are enriched for the “aging” Gene Ontology term, consistent with the hypothesis that they might be particularly important for mediating the distinct longevity effects of CEP-1 in isp-1 and mev-1 mutants. We further focused on one of these differentially regulated genes, ftn-1, which encodes ferritin in C. elegans, and demonstrated that it specifically contributed to the extended lifespan of isp-1 mutant worms but did not affect the mev-1 mutant lifespan. We propose that CEP-1 responds to different mitochondrial ETC stress by mounting distinct compensatory responses accordingly to modulate animal physiology and longevity. Our findings provide insights into how mammalian p53 might respond to distinct mitochondrial stressors to influence cellular and organismal responses.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004097
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004097Summary
Caenorhabditis elegans CEP-1 and its mammalian homolog p53 are critical for responding to diverse stress signals. In this study, we found that cep-1 inactivation suppressed the prolonged lifespan of electron transport chain (ETC) mutants, such as isp-1 and nuo-6, but rescued the shortened lifespan of other ETC mutants, such as mev-1 and gas-1. We compared the CEP-1-regulated transcriptional profiles of the long-lived isp-1 and the short-lived mev-1 mutants and, to our surprise, found that CEP-1 regulated largely similar sets of target genes in the two mutants despite exerting opposing effects on their longevity. Further analyses identified a small subset of CEP-1-regulated genes that displayed distinct expression changes between the isp-1 and mev-1 mutants. Interestingly, this small group of differentially regulated genes are enriched for the “aging” Gene Ontology term, consistent with the hypothesis that they might be particularly important for mediating the distinct longevity effects of CEP-1 in isp-1 and mev-1 mutants. We further focused on one of these differentially regulated genes, ftn-1, which encodes ferritin in C. elegans, and demonstrated that it specifically contributed to the extended lifespan of isp-1 mutant worms but did not affect the mev-1 mutant lifespan. We propose that CEP-1 responds to different mitochondrial ETC stress by mounting distinct compensatory responses accordingly to modulate animal physiology and longevity. Our findings provide insights into how mammalian p53 might respond to distinct mitochondrial stressors to influence cellular and organismal responses.
Introduction
Mitochondria are major sites of numerous metabolic processes, in particular electron transport and ATP production, and are essential for life. Not surprisingly, mitochondria also play central roles in aging and disease [1]. In model organisms such as worms, flies, and mice, specific point mutations or RNAi knockdowns directly affecting the electron transport chain (ETC) result in varying effects on development and longevity, ranging from developmental arrest and shortened survival to extended lifespan. The extended lifespan associated with moderate mitochondrial ETC dysfunction was surprising and further highlights the complex relationship between mitochondrial function and aging. An emerging model posits that a moderate reduction in mitochondrial ETC function can lead to compensatory responses that lengthen lifespan [2]–[5], whereas a more severe reduction in mitochondrial ETC function, beyond an innate threshold, will lead to developmental arrest and/or early death [6]–[7]. How different degrees of mitochondrial dysfunction result in opposing effects on longevity remains largely unknown.
Caenorhabditis elegans represents a powerful model to study the genetic basis of cellular and organismal changes in response to mitochondrial dysfunction. Previous findings in C. elegans have revealed a number of long-lived and short-lived ETC mutants. The nuo-6(qm200) mutant, which harbors a point mutation in the NADH-ubiquinone oxidoreductase of complex I, the isp-1(qm150) mutant, which harbors a point mutation in the rieske iron sulphur subunit of complex III, and the clk-1(e2519) mutant, with a point mutation in a coenzyme Q biosynthesis enzyme, exhibit substantial lifespan extension [8]–[9]. In contrast, the mev-1(kn-1) mutant, with a point mutation in the succinate dehydrogenase subunit c of complex II, and the gas-1(fc21) mutant, with a point mutation in the NADH:ubiquinone oxidoreductase NDUFS2 subunit of complex I, live significantly shorter than wild-type worms [10]. Furthermore, large-scale RNAi screens have revealed that RNAi-mediated inactivation of many of the ETC subunits result in prolonged or shortened lifespan [11].
Studies using genetic mutants and RNAi-mediated knockdown of ETC components in worms have begun to reveal the mechanistic basis of the longevity outcomes associated with mitochondrial dysfunction. Reactive oxygen species (ROS) have emerged as an important signaling intermediate in the ETC mutants. Specifically, nuo-6, isp-1, and mev-1 mutants have been shown to exhibit elevated levels of mitochondrial superoxide, and antioxidant treatment of these worms was able to revert their longevity phenotype [12]–[13]. Interestingly, the long-lived clk-1 mutant was not found to exhibit a higher level of mitochondrial superoxide, and antioxidant treatment had no impact on its lifespan, suggesting that the clk-1 mutation influences lifespan independent of ROS. In addition to increased ROS levels, the ETC mutants also exhibit an altered metabolism. The long-lived nuo-6, isp-1, and clk-1 mutants share similar metabolic profiles that are distinct from that of the mev-1 short-lived mutant [14]–[15]. An elevated production of metabolites, such as α-ketoacids and α-hydroxyacids, has been proposed to act as a pro-longevity signal in the long-lived ETC mutants. Furthermore, studies that have largely employed RNAi-mediated knockdown of various ETC subunits demonstrated that an imbalanced stoichiometry of the ETC protein subunits triggered a strong mitochondrial unfolded protein response (mtUPR). In this scenario, processed peptides in the mitochondria are thought to serve as the signal that activates several transcriptional regulators, including UBL-5 and ATFS-1, to induce transcriptional responses necessary to restore proteostasis in the mitochondria, which contributes to longevity determination [16]. Lastly, several RNAi and candidate screens have identified additional transcription factors, such as CEP-1 [17], CEH-23 [18], and TAF-4 [19] that mediate the lifespan of various ETC mutants.
The transcription factor p53 has recently emerged as a key regulator of metabolic balance [20]–[22]. Despite its importance, how p53 senses metabolic stress and accordingly regulates molecular changes that determine the physiological outcomes of an organism remains poorly understood. C. elegans CEP-1, the sole homolog of the mammalian p53 family [23] (p53, p63 and p73), is known to mediate the lifespan changes in worms with mitochondrial dysfunction. Inactivation of cep-1 has been shown to partially suppress the extended longevity of isp-1 mutant worms [24]. Furthermore, using different concentrations of RNAi to cause different degrees of knockdown of several ETC components demonstrated that CEP-1 is required for the increased longevity under mild mitochondrial disruption as well as the shortened lifespan when mitochondrial damage is more severe [25]. Therefore, CEP-1 exerts opposite effects on lifespan that likely depend on the levels of mitochondrial stress experienced. The underlying mechanism governing this intriguing duality of CEP-1 function is not known.
In this study, we sought to further characterize the role of CEP-1 in the longevity of several mitochondrial ETC mutants. Our results indicate that CEP-1 is a critical mediator of the lifespan of several mitochondrial mutants, suggesting that CEP-1 plays a central role in sensing mitochondrial distress and coordinating physiological outcomes accordingly. We also evaluated the CEP-1-regulated transcriptomes in the long-lived isp-1 and the short-lived mev-1 mitochondrial ETC mutants. Despite the opposing roles that CEP-1 appears to play in determining the lifespan of these mutants, the CEP-1-regulated transcriptional profiles were largely similar in these mutants. Nevertheless, the expression of a small group of genes was differentially regulated by CEP-1 between the long-lived isp-1 and the short-lived mev-1 mutants. Interestingly, this small group of genes is enriched for the Gene Ontology functional group “aging”, indicating they are over-represented by genes previously known to have a role in aging in worms. We functionally validated one of these differentially regulated genes, ftn-1, which encodes ferritin in C. elegans, by demonstrating that its RNAi-mediated depletion significantly impacted the lifespan of the isp-1 but not mev-1 mutant. This result supports our hypothesis that CEP-1 can differentially regulate a small subset of target genes to achieve distinct longevity outcomes in response to mutations in different ETC components.
Results
CEP-1 exerts opposing effects on longevity and development in the long-lived isp-1 and short-lived mev-1 mutants
Previous results suggested that cep-1 is required for the lifespan extension associated with mild mitochondrial dysfunction and the shortened lifespan associated with severe mitochondrial dysfunction [25]. We confirmed these results and demonstrated that inactivation of cep-1 partially but consistently suppressed the extended lifespan of isp-1 mutant animals (Fig. 1A), (Fig. S1A, Table S1, S2). We further showed that inactivation of cep-1 largely restored the lifespan of mev-1 mutants to wild-type (Fig. 1B), (Fig. S1A, Table S1, S2). Our data are therefore consistent with previous findings suggesting that CEP-1 can respond to different degrees of mitochondrial dysfunction and modulate lifespan accordingly.
Fig. 1. CEP-1 mediates the longevity and development of two mitochondrial mutants in C. elegans. 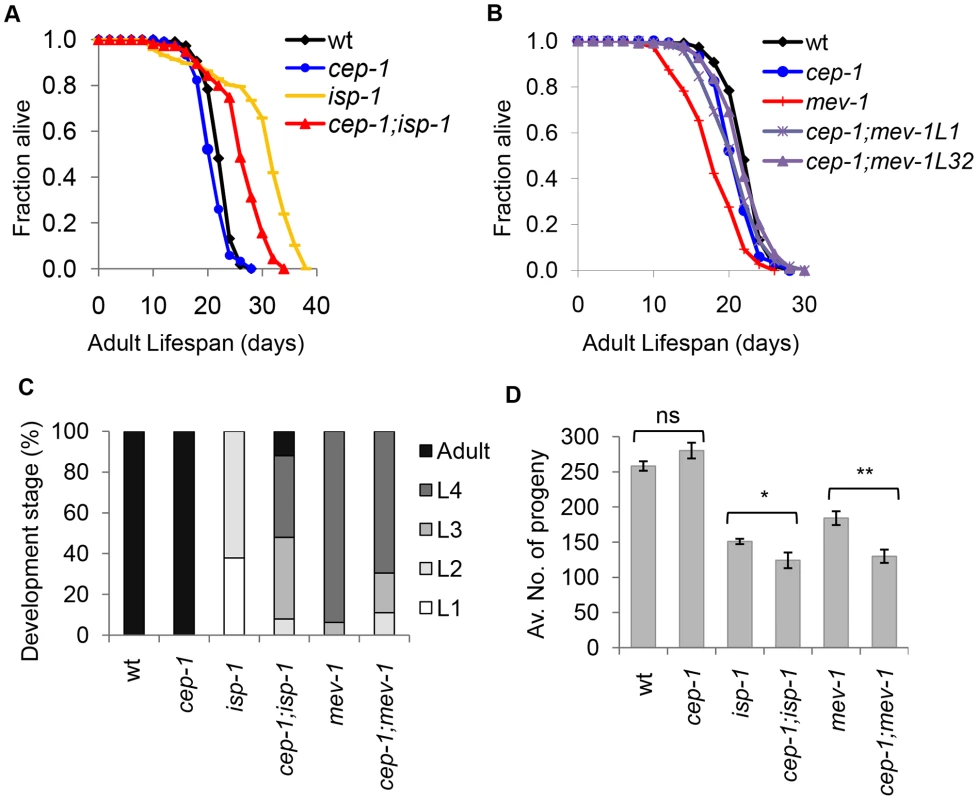
(A) cep-1 mutation partially suppresses isp-1 mutant longevity as the cep-1;isp-1 double mutant lifespan is shorter than that of the isp-1 single mutant. (B) cep-1 mutation restores the mev-1 mutant lifespan as the lifespans of two cep-1;mev-1 isolates (L1, L32) are similar to that of wt. (C) The percentage of worms at each developmental stage was quantified for wt, cep-1, isp-1, cep-1;isp-1, mev-1, and cep-1;mev-1 mutant worms after 60 hr of growth from the embryonic stage at 20°C. (D) The average number of progeny production for each line was calculated from 5 to 10 worms. The isp-1 and mev-1 mutants produce significantly less progeny than wt. The cep-1;isp-1 and cep-1;mev-1 double mutants display significantly lower brood sizes than their respective single mutant controls (*p<0.05, **p<0.0005). The error bars represent standard errors. Statistical analysis was performed using a two-tailed t-test. In addition to lifespan changes, mitochondrial ETC mutants also develop slowly and display reduced brood sizes. To assess whether CEP-1 participates in the development of mitochondrial ETC mutants, the development time of isp-1 and mev-1 mutants with or without cep-1 was compared to that of wild-type (wt) worms. Synchronized embryos of the various strains were allowed to develop at 20°C for 60 hours, and the number of adults and larvae were counted (Fig. 1C, Table S3). The data showed that wt and cep-1 mutant worms developed at similar rates, and 100% of the populations had reached adulthood by 60 hr. As expected, the isp-1 mutant worms grew slowly, and the majority were in the L1 (37%) and L2 (62%) stages after 60 hr. However, cep-1;isp-1 double mutants developed noticeably faster, and the majority were L3s (40%), L4s (40%), and adults (12%) after 60 hr. These data suggest that cep-1 inactivation partially recues the slow development of the isp-1 mutant. Interestingly, cep-1 inactivation exerted an opposite effect on mev-1 mutant development. The mev-1 mutants were slightly developmentally delayed, where the majority of mev-1 mutant worms were in the L3 (6%) and L4 (93%) stages at 60 hr. The development rate of the cep-1;mev-1 double mutant worms was heterogeneous and further delayed (L2 (11%), L3 (19%) and L4 (69%) at 60 hr) compared to mev-1 single mutant worms.
To examine a possible role for CEP-1 in the reproduction of mitochondrial ETC mutants, the average brood size of isp-1 and mev-1 mutants with or without cep-1 was compared to that of wt animals. The brood size of the cep-1 mutant did not significantly differ from wt (p = 0.1), and, as expected, the mitochondrial mutants (isp-1, mev-1) displayed significantly lower brood sizes compared to wt (p<0.0001). The double mutants cep-1;isp-1 and cep-1;mev-1 exhibited a further brood size reduction compared to isp-1 and mev-1 single mutants, respectively (p≤0.05) (Fig. 1D, ). These results suggest that cep-1 inactivation further exacerbates the reproductive defect associated with mitochondrial dysfunction.
Taken together, cep-1 appears to participate in multiple physiological outcomes of ETC mutants. cep-1 activity promotes, at least partially, both the slower development and the longer lifespan of the isp-1 mutant but is required to prevent further reproductive deterioration. In contrast, cep-1 activity promotes a shortened mev-1 mutant lifespan but is required for a relatively normal developmental rate. Similar to its role in isp-1 reproduction, cep-1 prevents reproductive decline in mev-1 mutants. Since cep-1 loss similarly impacts the reproductive success of isp-1 and mev-1 mutants, but its inactivation has opposing effects on the lifespan and developmental rate of these mutants, the role of cep-1 in reproduction might be independent of its role in lifespan and development in ETC mutants.
CEP-1 mediates reduced physiological germline apoptosis in the long-lived isp-1 mutant
CEP-1 is a well-established key regulator of stress-induced apoptosis [24]. Since CEP-1 is a crucial mediator of the longevity outcomes of isp-1 and mev-1 mutants, we asked whether CEP-1 does so by modulating apoptosis in these mutants. While apoptosis occurs throughout embryonic and larval development in C. elegans, we reasoned that monitoring apoptosis in adults would be more relevant in investigating adult lifespan. In C. elegans adults, physiological and stress-induced apoptosis occurs in the germline. We monitored physiological apoptosis in the germline of wild-type, isp-1 and mev-1 mutants with or without cep-1 inactivation. CEP-1 is best characterized for its role in stress-induced apoptosis, and our data indicated that loss of cep-1 alone only mildly affected physiological apoptosis in the germline. The short-lived mev-1 mutant and mev-1;cep-1 double mutant exhibited wt levels of physiological germline apoptosis (Fig. 2). Interestingly, the long-lived isp-1 mutant displayed a significantly lower level of physiological germline apoptosis, which was completely rescued in the cep-1;isp-1 double mutant (Fig. 2). These data suggest that CEP-1 protects against physiological germline apoptosis in the isp-1 mutant. As CEP-1 has largely been demonstrated to promote apoptosis in C. elegans, this new role of CEP-1 in protecting against apoptosis merits further investigation.
Fig. 2. CEP-1 mediates reduced physiological germline apoptosis in the isp-1 mutant. 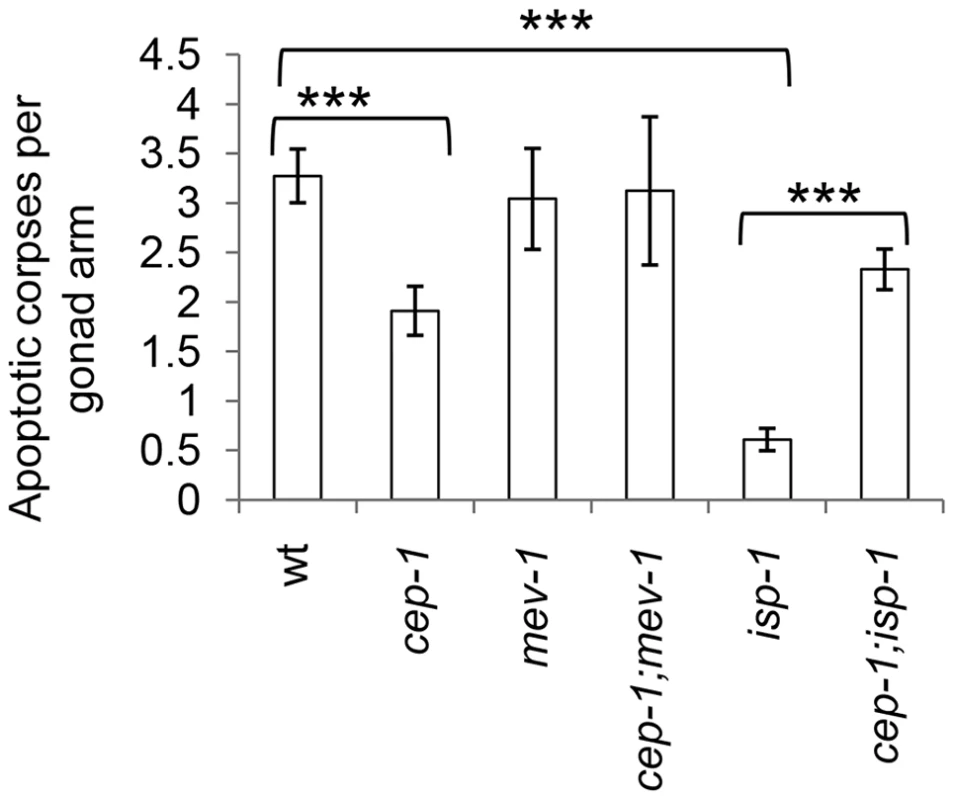
Physiological levels of apoptosis were quantified by counting the number of apoptotic corpses per gonad arm in various C. elegans strains. The corpses were counted using DIC microscopy at 63× magnification 48 hr post L4. The data represent the average of at least 3 independent experimental replicates (n≥15 gonad arms for each) ± standard error. Statistical analysis was done using the Mann-Whitney U-test. ***p<0.001. CEP-1-mediated transcriptional profiles in long-lived isp-1 and short-lived mev-1 mutants
CEP-1 appears to dually mediate the lifespan of mitochondrial mutants. We hypothesized that moderate mitochondrial dysfunction initiates a CEP-1-dependent defensive response that promotes longevity in long-lived mitochondrial mutants. Conversely, decreasing mitochondrial function beyond a threshold in short-lived mitochondrial mutants likely engages CEP-1 in a different way that results in a shortened lifespan. Since mammalian p53 is a well-established transcription factor, we sought to investigate the transcriptional response induced by CEP-1 in both a long-lived, isp-1, and a short-lived, mev-1, mitochondrial mutant. We hypothesized that the genes differentially regulated by CEP-1 between isp-1 and mev-1 mutants may mediate the distinct CEP-1 lifespans of the isp-1 and mev-1 mutants (see below). We compared the transcriptional profiles of synchronized isp-1(qm150) and cep-1(gk138);isp-1(qm150) young adults, as well as mev-1(kn1) and cep-1(gk138);mev-1(kn1), using the Agilent 4×44K oligonucleotide microarray (Table S4).
Although CEP-1 exerts opposing effects on the lifespans of isp-1 and mev-1 mutants, hierarchical gene cluster analysis revealed that the CEP-1-regulated transcriptional profiles in long-lived isp-1 and short-lived mev-1 mutants were largely similar (correlation coefficient of 0.58) (Fig. 3A). Only a small number of genes exhibited cep-1-dependent differential regulation. Data analysis using the statistical tool SAM (Significance Analysis of Microarray) with a FDR (false discovery rate) of 0.5% revealed that CEP-1 regulated the expression of 3,404 genes (Table S5) in a similar manner in the long-lived isp-1(qm150) and short-lived mev-1(kn1) mutants (Fig. 3B, Fig. S2A). SAM analysis with a FDR of 1% followed by a gene list comparison also revealed 71 genes (Table S6, see Materials & Methods for details on gene list filtering) that were differentially regulated by CEP-1 in the isp-1(qm150) and mev-1(kn1) mutants (Fig. 3B, Fig. S2B). The expression of these genes largely differed quantitatively rather than qualitatively, i.e., they generally showed a greater CEP-1-mediated regulation in the isp-1 or mev-1 mutant backgrounds. To validate our microarray findings, we selected six genes (F15E6.8/dct-7, C37A5.2/fipr-22, F28C6.1/AP-2 like TF, C52D10.6/skr-12, C08A9.1/sod-3 and F57B9.9/abu-13) based on their classification by SAM analysis and performed quantitative reverse transcription PCR (qRT-PCR) using an independent biological sample. The expression changes of all six genes echoed the microarray results (Fig. S3).
Fig. 3. CEP-1-regulated transcriptomes in isp-1 and mev-1 mutants. 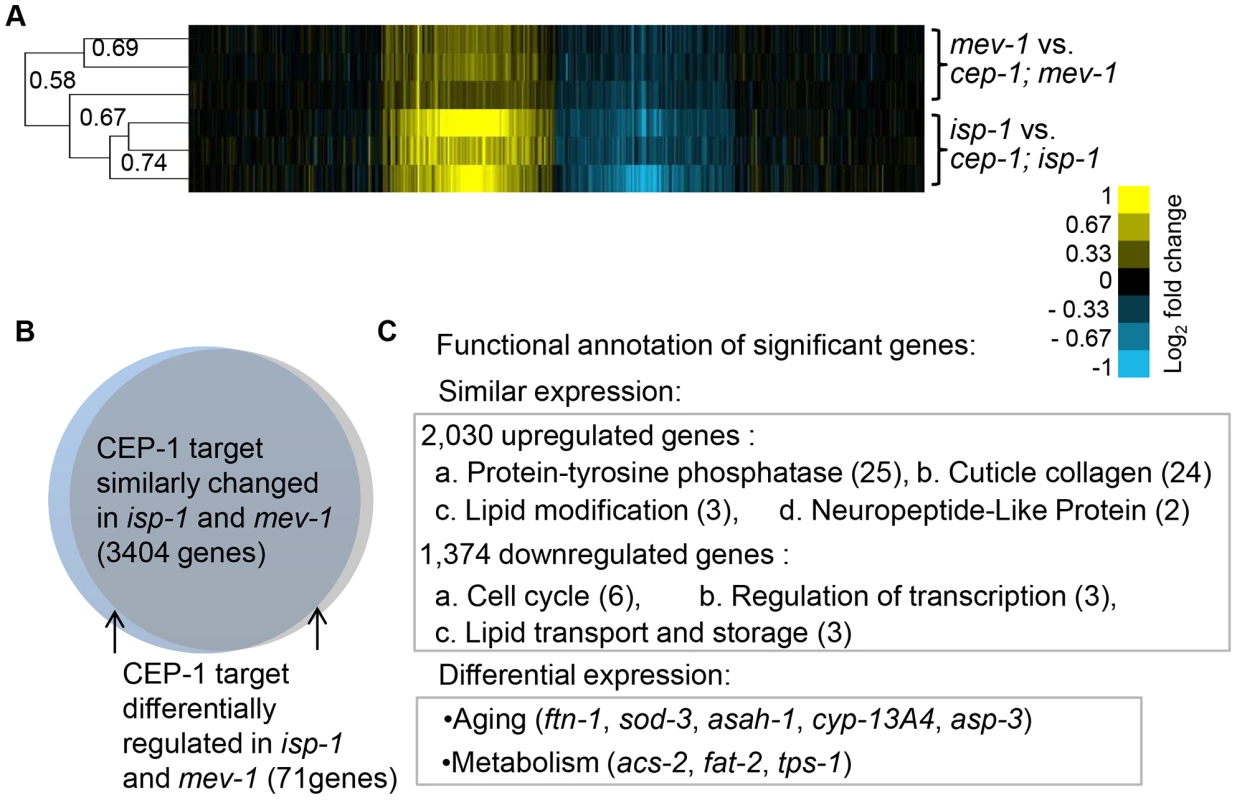
(A) CEP-1-regulated genes in isp-1 and mev-1 mutants are largely similar. Hierarchical single linkage gene clustering was performed, and the dendrogram shows the clustered relationship of individual arrays. The numbers on the dendrogram represent the correlation coefficients between arrays. Yellow: upregulated, Blue: downregulated, Black: no change. (B) The expression of CEP-1-regulated genes that were significantly changed in isp-1 and mev-1 mutants, identified by SAM analysis, are represented in the Venn diagram. (C) DAVID functional annotation of similarly and differentially expressed CEP-1-regulated genes in isp-1 and mev-1 mutants. The numbers represent the enrichment scores for each group (score>1.3 is considered as significant). Several examples of aging and metabolic genes are listed. To examine the biological processes of the CEP-1-regulated genes identified by SAM, we performed Gene Ontology (GO) analysis using DAVID (Database for Annotation, Visualization, and Integrated Discovery). We focused on the GO term categories that were most significantly enriched in our dataset compared to the distribution in the C. elegans genome. Interestingly, the small group of CEP-1-regulated genes that differentially changed in isp-1 and mev-1 mutants were enriched for genes with known roles in aging and metabolism (Fig. 3C, Table S7C). This finding supports our hypothesis that the genes differentially regulated by CEP-1 in isp-1 and mev-1 mutants are likely particularly important for the opposing effects on lifespan that CEP-1 exerts in these mutants. The genes that were similarly regulated by CEP-1 in isp-1 and mev-1 mutants likely account for the effects CEP-1 has on development, reproduction, and other physiological changes in these mutants and might also contribute to the final longevity (Fig. 3C, Tables S7A, S7B).
The CEP-1-regulated gene ftn-1 contributes to the longer lifespan of the isp-1 mutant
The microarray results helped narrow our analysis to a small number of CEP-1-regulated genes (Fig. 3C) that might contribute to the dual effects CEP-1 exerts on the longevity of mitochondrial mutants with varying degrees of dysfunction. One of these genes, ftn-1, encodes the C. elegans homolog of the ferritin heavy chain. Using qRT-PCR, we observed that ftn-1 expression was repressed ∼2 fold in the cep-1 mutant and induced ∼1.5 fold in the isp-1 mutant compared to wt. The cep-1;isp-1 double mutant displayed a similar repressed level of ftn-1 expression as observed for the cep-1 single mutant (Fig. S4A). While ftn-1 expression was induced in the mev-1 mutant, similar to the isp-1 mutant, this induction was not changed in the cep-1;mev-1 double mutant (), consistent with the microarray results. Inspection of the ftn-1 upstream sequence did not reveal a known p53 binding motif, suggesting that CEP-1 might not directly regulate ftn-1 transcription. C. elegans harbors another ftn-1 homolog, ftn-2, but its expression was not changed in the ETC mutants compared to wt (Fig. S4B). Lastly, we used the Pftn-1::gfp strain, where a GFP reporter is fused to the ftn-1 promoter [26], to assess whether the microarray and qRT-PCR results translated into observably meaningful ftn-1 expression changes. Pftn-1::gfp was substantially induced in isp-1 mutants and was completely suppressed in cep-1;isp-1 double mutants (Fig. 4A), consistent with our microarray and qRT-PCR results. Additionally, Pftn-1::gfp expression was slightly induced in mev-1 mutants and unchanged in cep-1;mev-1 double mutants. Taken together, our data indicate that CEP-1 is important for ferritin regulation in wt and isp-1 mutants but is dispensable in the mev-1 mutant.
Fig. 4. CEP-1-regulated ferritin induction partially mediates the extended lifespan of isp-1 mutants. 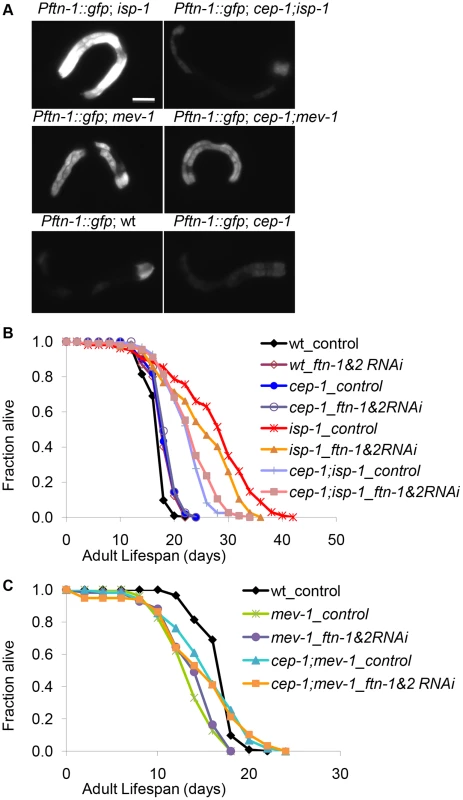
(A) Pftn-1::gfp expression in wt, cep-1, isp-1, cep-1;isp-1, mev-1, and cep-1;mev-1 mutant worms. Scale bar = 100 µm. (B, C) The lifespans of wt, cep-1, isp-1, cep-1;isp-1, mev-1 and cep-1;mev-1 mutant worms treated with ftn-1 and ftn-2 double RNAi. L4440 is a treatment control. Ferritin regulates the storage and release of iron. As iron homeostasis is known to play an important role during mitochondrial dysfunction, we wanted to investigate whether ferritin regulation was responsible for the dual roles of CEP-1 in mitochondrial ETC mutant longevity. To assess whether ferritin upregulation promoted isp-1 mutant longevity, we examined the lifespan of isp-1 and cep-1;isp-1 mutants after ftn-1 and ftn-2 knockdown by RNAi. We knocked down ftn-1 and ftn-2 simultaneously to prevent possible functional redundancy, which may preclude observable phenotypes when either gene is knocked down alone. Double RNAi-mediated knockdown of ftn-1 and ftn-2 significantly suppressed the extended lifespan of isp-1 mutants, although not to the same degree as cep-1 inactivation. Importantly, ftn-1/2 RNAi did not further suppress the lifespan of the cep-1;isp-1 double mutant (Fig. 4B, Fig. S5), suggesting that cep-1 and ftn-1/2 act in the same genetic pathway to mediate isp-1 longevity, corroborating our model that ftn-1/2 are downstream targets of CEP-1. Consistent with our expression results suggesting that ftn-1/2 are not regulated by CEP-1 in the short-lived mev-1 mutant, the mev-1 mutant lifespan was not affected by ftn-1/2 RNAi (Fig. 4C, Fig. S5).
CEP-1 is key to physiological changes in multiple ETC mutants
Given the important role CEP-1 plays in determining the lifespan of isp-1 and mev-1 mutants, we next tested whether cep-1 is generally required for the longevity of additional ETC mutants that have been well characterized in C. elegans. We explored the long-lived nuo-6(qm200) mutant, which harbors a point mutation in the NADH-ubiquinone oxidoreductase of complex I, the long-lived clk-1(e2519) mutant, which harbors a point mutation in a coenzyme Q biosynthesis enzyme, and the short-lived gas-1(fc21) mutant, which has a point mutation in the NADH:ubiquinone oxidoreductase NDUFS2 subunit of complex I. The cep-1 null mutation largely suppressed the long-lived phenotype of the nuo-6 mutant but did not affect the longevity of the clk-1 mutant, as the cep-1;clk-1 double mutant lived as long as the clk-1 single mutant (Fig. 5A, 5B, Fig. S1A). Furthermore, cep-1 deletion rescued the short-lived phenotype of the gas-1 mutant, similar to its effect on the mev-1 mutant lifespan (Fig. 5C, Fig. S1A). Taken together, the data suggest that CEP-1 crucially participates in the longevity outcome of multiple ETC mutants.
Fig. 5. CEP-1 mediates the longevity and development of several mitochondrial mutants in C. elegans. 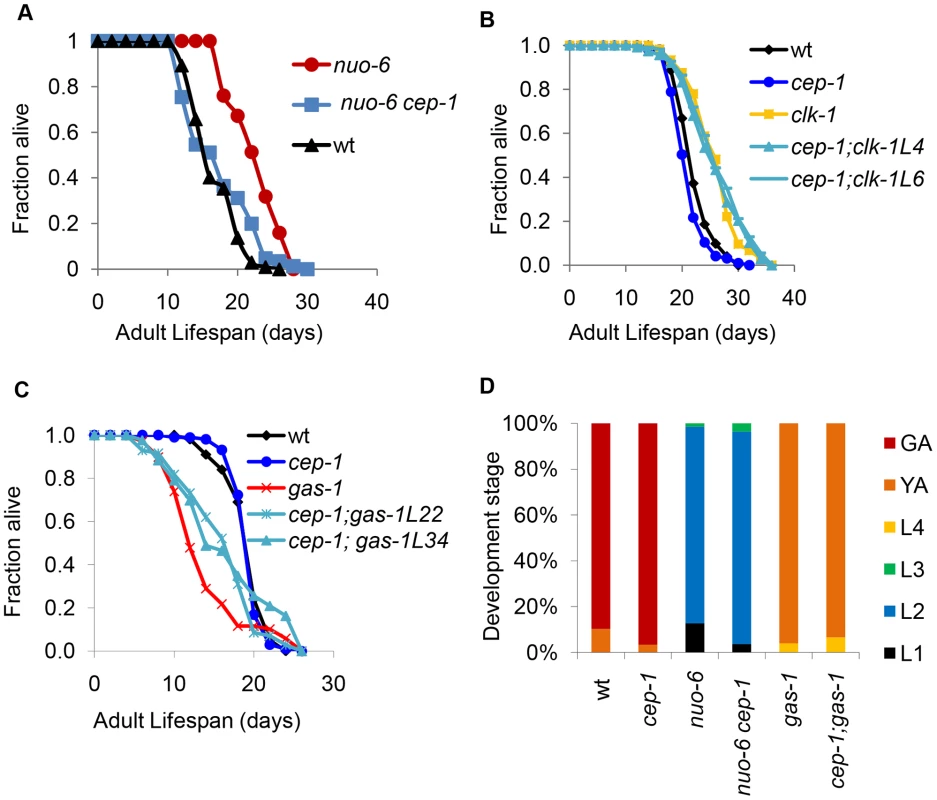
(A) cep-1 mutation fully suppresses the long lifespan of the nuo-6 mutant. (B) cep-1 mutation does not suppress clk-1 mutant longevity as the lifespans of two cep-1;clk-1 double mutant isolates (L4, L6) are similar to that of the clk-1 single mutant. (C) cep-1 mutation partially restores gas-1 mutant lifespan as two isolates of cep-1;gas-1 (L22, L34) live longer than the gas-1 single mutant. (D) The percentage of worms at each developmental stage was quantified as described in Fig. 1C. cep-1 deletion has little impact on nuo-6 and gas-1 mutant development. The fact that cep-1 abrogation did not affect the clk-1 mutant lifespan was intriguing. All of the well-characterized mitochondrial ETC mutants in C. elegans (isp-1, nuo-6, mev-1, and gas-1), with the exception of clk-1, have been shown to harbor elevated levels of mitochondrial superoxide. In fact, antioxidant treatment of all of these mutants, again with the exception of clk-1, has been shown to suppress (the long-lived) isp-1 and nuo-6 or rescue (the short-lived) mev-1 and gas-1 mutant lifespans. Therefore, the cep-1-mediated lifespans of these mitochondrial ETC mutants parallel the proposed roles that elevated ROS is exerting in these mutants, i.e., CEP-1 mediates the lifespans of mutants with elevated ROS but not of mutants without. This observation suggests the interesting possibility that CEP-1 might somehow be linked to the ROS-mediated longevity increases observed in the long-lived mitochondrial ETC mutants. Future studies aimed at thoroughly investigating the relationship between CEP-1 and ROS-mediated longevity will likely yield fruitful insights.
As cep-1 impacted the development rates of isp-1 and mev-1 mutants, we tested whether cep-1 similarly affected the development of nuo-6 and gas-1 mutants. Our data indicated that cep-1 deletion had little impact on nuo-6 and gas-1 mutant development (Fig. 5D), unlike what we found for isp-1 and mev-1 mutants (Fig. 1C). Therefore, whereas cep-1 mediates the lifespans of all four ETC mutants tested here (isp-1, nuo-6, mev-1, and gas-1), its involvement in the development of each of the mutants is mixed, suggesting that the role of CEP-1 in development may be distinct from its role in longevity in these various mutants.
Given that we observed an important role for CEP-1 in ftn-1 regulation and iron homeostasis in ETC mutant longevity, we further tested whether ftn-1 & ftn-2 are regulated by CEP-1 in nuo-6 and gas-1 mutants and whether ftn-1/2 are required for their longevity. Strikingly, ftn-1 was greatly induced in the nuo-6 mutant, even more so than in the isp-1 mutant (Fig. 6C). Somewhat surprisingly, cep-1 was not required for this induction, unlike in the isp-1 mutant. We also demonstrated that RNAi-mediated depletion of ftn-1/2 substantially suppressed the extended lifespan of the nuo-6 mutant, similar to their depletion in the isp-1 mutant (Fig. 6A). Although CEP-1 is essential for ftn-1 induction in the isp-1 mutant, it is dispensable in the nuo-6 mutant; however, both cep-1 and ftn-1/2 are important for the longevity of nuo-6 and isp-1 mutants. Therefore, while iron homeostasis and cep-1 are both important for longevity determination in the long-lived isp-1 and nuo-6 mutants, CEP-1 regulates ftn-1 only in the isp-1 mutant. We hypothesize that another transcription factor likely regulates ftn-1 expression in the nuo-6 mutant. Interestingly, ftn-1 was induced in the short-lived gas-1 mutant independently of cep-1, similar to that in the short-lived mev-1 mutant (Fig. 6C). Additionally, depleting ftn-1/2 did not rescue the shortened lifespan of the gas-1 mutant, similar to that observed in the mev-1 mutant (Fig. 6B). Therefore, iron homeostasis might be a determinant of isp-1 and nuo-6 longevity, but it does not appear to be important for mediating the lifespans of gas-1 and mev-1 mutants. Lastly, ftn-1 was not induced in the clk-1 mutant, another indication that clk-1 mutant might engage a different mechanism to extend lifespan compared to the other mitochondrial ETC mutants studied here.
Fig. 6. ftn-1 is differentially expressed in various ETC mutants and mediates their lifespan outcomes. 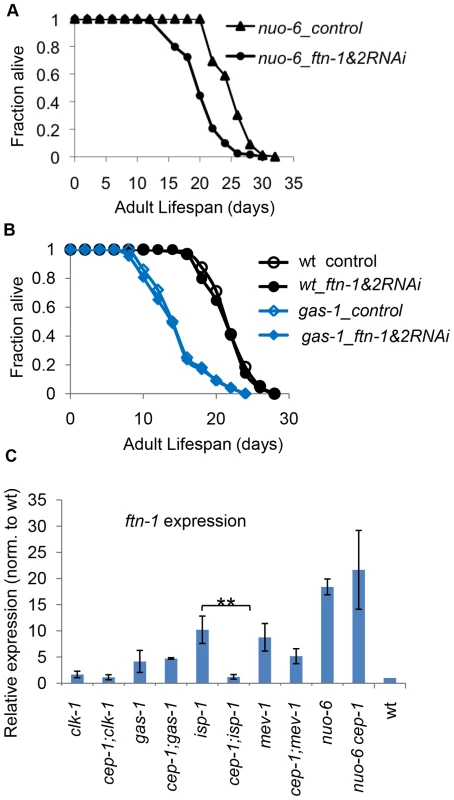
(A, B) RNAi-mediated knockdown of ftn-1 attenuates the long life of the nuo-6 mutant but does not impact the lifespan of the short-lived gas-1 mutant. (C) qRT-PCR results of ftn-1 expression levels in various ETC mutants. The two-tailed student t-tests were performed to determine significant difference in ftn-1 expression levels with and without CEP-1 in each ETC mutant background. The distinct ftn-1 results led us to consider more broadly whether isp-1 & nuo-6 and gas-1 & mev-1 share some common molecular signatures, which could account for their similar longevity (extended or shortened, respectively). We examined the expression of a handful of CEP-1-regulated genes that we identified earlier in isp-1/mev-1 mutants. Using qRT-PCR, we first analyzed the expression of nine genes that were differentially regulated by CEP-1 in isp-1 and mev-1 mutants (Fig. 7). Overall, we observed very different patterns for how these genes responded to the absence of cep-1 in the two long-lived mutants (isp-1 and nuo-6). The majority of genes that showed substantial cep-1-dependent induction in isp-1 mutants barely changed when cep-1 was depleted in nuo-6 mutants; only two genes (cyp-35A5, F09C8.1) showed consistent, but moderate, cep-1-dependent expression changes between isp-1 and nuo-6. Interestingly, the response of these target genes to cep-1 depletion was more similar between the two short-lived mutants (mev-1 and gas-1). However, it is worth noting that the expression of the majority of these genes barely changed in mev-1 and gas-1 mutants in response to cep-1 deletion (Fig. 7).
Fig. 7. Expression of differentially regulated CEP-1 targets in other ETC mutants. 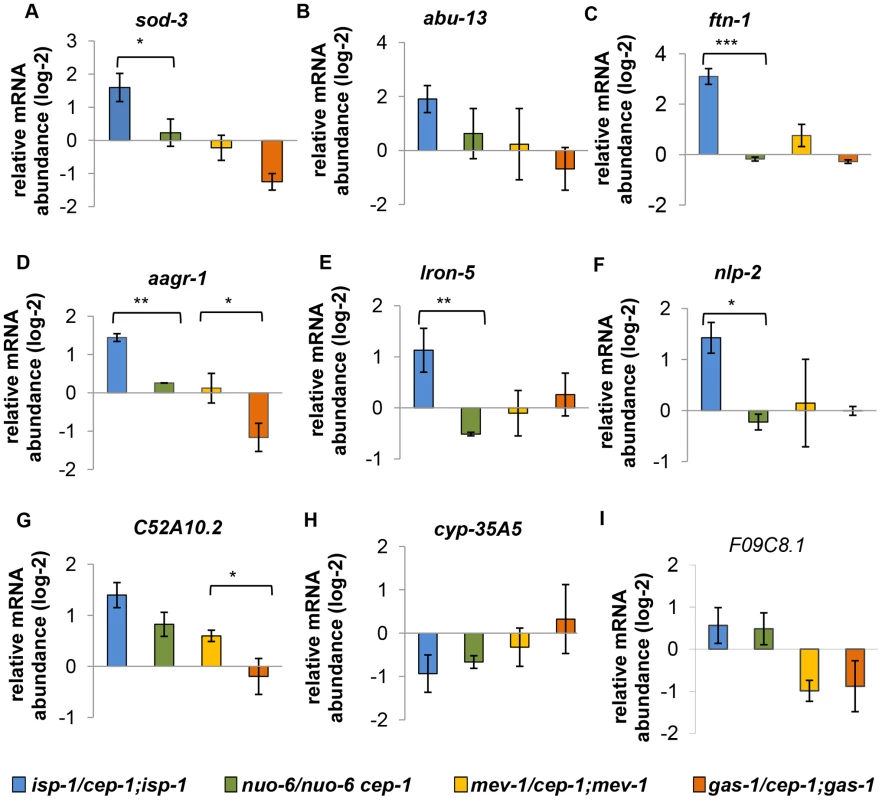
(A–I) These genes are differentially regulated by CEP-1 between isp-1 and mev-1 mutants. The relative expression of each gene was normalized to act-1 and wt. The average log2 ratio between ETC mutants with and without cep-1 from three independent experiments are plotted. The error bars represent standard errors. Two-tailed t-tests were performed to determine significant differences of the expression of CEP-1 gene targets between the long-lived isp-1(qm150) and nuo-6(qm200) mutants and the short-lived mev-1(kn1) and gas-1(fc21) mutants. *p<0.05, ** p<0.01, *** p<0.001. We also assayed the expression of genes that were similarly regulated by CEP-1 in isp-1 and mev-1 mutants and observed a similarly discordant pattern (Fig. 8). The regulation of these genes by CEP-1 was strikingly different between isp-1 and nuo-6 mutants but was much more similar between mev-1 and gas-1 mutants. In the absence of further genome-wide analysis, it is difficult to estimate the extent of common targets that are shared between (the long-lived) isp-1 and nuo-6 and between (the short-lived) mev-1 and gas-1 mutants. However, based on the small number of genes tested here using qRT-PCR, it appears that CEP-1 regulates some common genes between mev-1 and gas-1 mutants, which might account for its ability to restore a normal lifespan in these short-lived mutants. For the long-lived mutants, the situation is more complex; CEP-1 appears to regulate largely distinct genes between isp-1 and nuo-6 even though it is required for the extended lifespan of both mutants.
Fig. 8. Expression of similarly regulated CEP-1 targets in other ETC mutants. 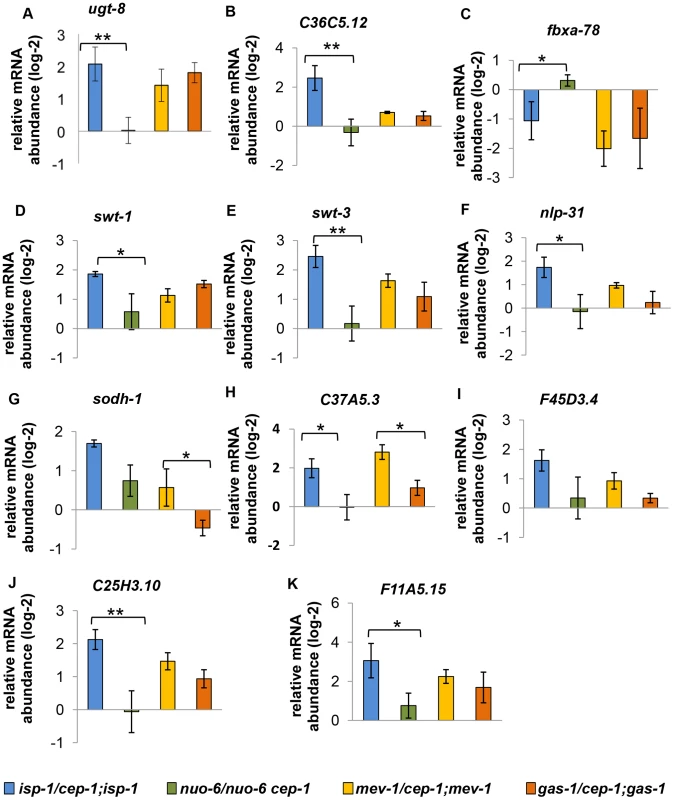
(A–K) These genes are similarly regulated by CEP-1 between isp-1 and mev-1 mutants. The relative expression of each gene was normalized to act-1 and wt. The average log2 ratio between ETC mutants with and without cep-1 from three independent experiments are plotted. The error bars represent standard errors. Two-tailed t-tests were performed to determine significant differences of the expression of CEP-1 target genes between the long-lived isp-1(qm150) and nuo-6(qm200) mutants and the short-lived mev-1(kn1) and gas-1(fc21) mutants. * p<0.05, ** p<0.01, *** p<0.001. The CEP-1-regulated transcriptional profiles of mitochondrial dysfunctional mutants are similar to those in worms exposed to UV irradiation
The best-characterized role of mammalian p53 is its ability to respond to DNA damage by inducing cell cycle arrest and repair or apoptosis. We wondered whether the CEP-1-regulated transcriptome changes in mitochondrial mutants would resemble changes observed in response to DNA damage. The global transcriptional profiles of wt or cep-1 mutant worms treated with UV, gamma, or x-ray irradiation have previously been published [27]–[28]. Using clustering analysis, we compared the CEP-1-dependent global expression profiles in response to mitochondrial dysfunction (in isp-1 or mev-1 mutants) to profiles 4 hr after exposure to UV irradiation, 6 hr after gamma-ray, or 2 hr after X-ray (Fig. S6A, Table S8). We observed a higher similarity between the CEP-1-regulated gene expression profiles in response to mitochondrial dysfunction and after UV treatment (correlation coefficient of 0.36) but less after gamma-ray treatment (correlation coefficient of 0.20). The published gene expression profiles after 2 hr of X-ray treatment did not display significant differences from the no treatment control and thus were not included in the downstream analysis.
We analyzed the CEP-1-regulated genes in response to mitochondrial dysfunction and UV and gamma irradiation. We focused on the gene sets that exhibited ≥1.4-fold changes under at least one of the stressed conditions (i.e., isp-1 mutant, mev-1 mutant, UV treatment, or gamma irradiation) and performed K-mean clustering to identify the gene sets that were either similarly regulated across all of the stressed conditions or differentially regulated under one or more conditions. The K-mean analysis revealed six distinct clusters (Fig. S6B, Table S9). We employed DAVID to examine the GO terms generated from genes representing each cluster (Fig. S6C, Table S10). Cluster-‘a’ (579 genes) represents genes that were upregulated by CEP-1 in response to all of the stressors. This group is particularly enriched for genes that function in phosphate metabolism, including kinases and phosphatases. Cluster-‘f’ (1,357 genes) represents genes that are also upregulated across all of the stressors but to a lesser extent than in cluster-‘a’. Cluster-‘f’ is enriched for ribosomal and ion transport proteins. Cluster-‘e’ (901 genes) is enriched for growth regulation and represents genes that are repressed by CEP-1 in response to mitochondrial dysfunction and to UV and gamma irradiation. Clusters-‘a’, ‘e’ and ‘f’ together suggest that CEP-1 regulates common transcriptional programs in response to stress, which might in turn induce key signaling pathways to counter the stress and simultaneous suppression of growth. Clusters-‘b’, ‘c’, ‘d’ represent genes that are similarly regulated by CEP-1 in response to mitochondrial dysfunction and UV but are different after gamma irradiation. These gene groups are significantly enriched for nematode cuticle and collagen proteins, cell cycle regulators, glycoproteins and signaling proteins. In summary, both mitochondrial dysfunction and UV irradiation are known to induce ROS, which might reflect the common CEP-1-regulated transcriptional changes observed here.
Discussion
The major function of p53 is to integrate stress signals and to orchestrate appropriate cellular responses. Under normal, unstressed conditions, p53 is maintained at low levels via proteosome-mediated degradation. Upon stress, p53 levels stabilize and activate stress response programs that range from cell repair to cell death [29]. Our genetic data indicate that mutations in the C. elegans ETC subunits isp-1 and nuo-6 engage CEP-1 in initiating a stress response that results in a longer lifespan. Conversely, mitochondrial defects, due to mutations in the ETC subunits mev-1 and gas-1, act through CEP-1 to confer a shorter lifespan. Taken together, our data suggest the intriguing possibility that CEP-1 can sense distinct dysfunctional mitochondrial processes and modulate overall longevity accordingly.
How CEP-1 is able to sense mitochondrial dysfunction caused by different ETC mutations remains unclear. ROS have been proposed to be important regulators of p53 [30]. ROS can induce DNA damage, which leads to p53 activation via DNA damage checkpoint pathways. ROS are also known to engage p53 directly through modifying the redox-sensitive Cystein (Cys) residues on p53 [31]. p53 contains several critical Cys residues located within the DNA-binding domain. Importantly, the Cys residues that are required to coordinate zinc and maintain the protein structure that enables interaction with the minor groove of target DNA are conserved between CEP-1 and human p53 [32]. As discussed earlier, many C. elegans ETC mutants have been shown to produce elevated levels of mitochondrial ROS. Altered ROS production in the mitochondrial ETC mutants might be coupled to CEP-1 via regulation of the conserved Cys residues. Upon activation, p53 is well-known to regulate the transcription of genes involved in ROS metabolism, including both antioxidant and pro-oxidant genes [33]. Therefore, depending on upstream signaling, CEP-1/p53 can either alleviate ROS stress or promote further ROS accumulation.
Deficiencies in the ETC can also affect cellular energy homeostasis. The isp-1 mutant has been shown to exhibit higher AMP:ATP ratios compared to wt worms, and aak-2, the AMPK α subunit of C. elegans, is partially necessary for the extended lifespan of isp-1 mutants [34]. In mammals, the cellular energy sensor AMP kinase (AMPK) is known to directly phosphorylate p53 at Ser15, leading to stabilization and transcriptional activation of p53 [35]. Intriguingly, this Serine residue is conserved between CEP-1 and human p53, and thus, it is possible that an altered AMP:ATP ratio in ETC mutants engages AMPK to regulate CEP-1 function. Further experiments are necessary to definitively identify the signals generated by C. elegans ETC mutants that lead to CEP-1 activation.
Although CEP-1 plays opposite roles in isp-1 and mev-1 longevity, CEP-1-regulated genes in these two mutants are strikingly similar. This observation suggests that CEP-1 induces similar compensatory responses to restore cellular homeostasis regardless of the specific mitochondrial ETC defect. Our GO analyses suggested, upon mitochondrial dysfunction, that CEP-1 likely promotes a kinases/phosphatase - and/or neuropeptide-mediated signaling cascade, activates metabolic processes poised for defense and detoxification and represses the energy demanding cell cycle program. Although mtUPR has emerged as a key pathway that mediates the physiological outcomes of ETC mutants, we did not observe changes in major mtUPR response genes, such as atfs-1, hsp-6, or hsp-60. Our data suggest that CEP-1 likely does not act through mtUPR to affect the lifespan of ETC mutants.
We were excited to identify a small number of genes that were differentially regulated by CEP-1 in the isp-1 and mev-1 mutants, and this gene set was over-represented for genes previously known to modulate aging. We demonstrated that one of these genes, ftn-1, was indeed functionally important for the cep-1-mediated alterations to longevity in the isp-1 mutant but not in the mev-1 mutant, further underscoring CEP-1's functional duality. Ferritin functions to store iron in a non-toxic form, to deposit it in a safe form, and to transport it to areas where it is required. Mitochondria are the sites of iron-sulfur cluster synthesis, which are critical catalytic and structural components of many cellular proteins [36]. Conversely, the presence of iron in mitochondria must be tightly regulated, as free iron can react with ROS and further produce hydroxyl radicals through the Fenton reaction. As isp-1 encodes an iron-sulphur cluster protein and can bind to iron, iron levels may accumulate in the isp-1 mutant and thus upregulate ftn-1 as a compensatory response to restore iron homeostasis. However, this hypothesis requires a thorough investigation of iron homeostasis in isp-1 and the other ETC mutants to be validated. Additionally, further functional analysis of the small group of genes differentially regulated by CEP-1 in isp-1 and mev-1 mutants will likely reveal new genes important for longevity and illuminate how CEP-1 modulates the lifespans of animals with different ETC mutations.
Whereas CEP-1 is essential in mediating the extended lifespan of both nuo-6 and isp-1 mutants, our results, albeit based on only a handful of target genes, suggest that CEP-1 regulates distinct genes in each case. This is quite surprising, especially considering that CEP-1 appears to regulate largely similar genes in gas-1 and mev-1 mutants. These differences might be due to the specific complex that is compromised and the precise point of electron transport that is defective in the various ETC mutants. During normal electron transport, electrons from complex I or complex II can be passed onto complex III. In the nuo-6 mutant, where complex I is compromised, fuels can still enter the ETC via complex II, allowing for some degree of electron transport. The situation might be quite different in the isp-1 mutant, which impairs complex III and would be expected to block electrons transferred from either complex I or complex II. In contrast, a defect in complex I, such as in gas-1, or in complex II, such as in mev-1, could have a similar consequence on electron transport if fuels are not limiting, as either likely partially compromises electron flow to complex III. Further experiments are necessary to elucidate whether and how different ETC mutations influence CEP-1 activity and gene regulation.
The best known function of CEP-1 in C. elegans is its ability to induce apoptosis upon stress. We, however, did not observe expression changes in egl-1, the key CEP-1 target for initiating apoptosis [37]. Intriguingly, our results suggest that CEP-1 may protect against physiological apoptosis specifically in the long-lived isp-1 mutant. This is an aspect of CEP-1 function that remains poorly characterized. Interestingly, analysis of our microarray results revealed that ced-8, ced-9, egl-38, genes known to regulate apoptosis [37], were specifically regulated in the isp-1 mutant in a cep-1-dependent manner, but their expression was not changed in the mev-1 mutant. Whether these genes might be central to the ability of CEP-1 to confer protection from physiological apoptosis will warrant further investigation. Future experiments to delineate whether repressed physiological apoptosis is required for the isp-1 lifespan extension and which CEP-1 target genes might contribute to protection from physiological apoptosis will likely provide important new insights into the function and physiological role of CEP-1/p53.
DNA damage is a major p53-activating stressor, so we compared the CEP-1-mediated transcriptional profiles in mitochondrial mutants and worms treated with UV or gamma irradiation. Our results demonstrated a considerably greater overlap between the CEP-1-regulated transcriptional response induced by ETC disruption and UV irradiation than by gamma irradiation. This might seem surprising given that UV irradiation and gamma irradiation are both genotoxic stressors that cause DNA damage, whereas mitochondrial ETC dysfunction might be considered a metabolic stress. However, UV irradiation is known to produce ROS, which might induce CEP-1 activation in a manner similar to mitochondrial ETC dysfunction, which is also known to induce ROS. Interestingly, the common set of genes that are upregulated by CEP-1 in mitochondrial mutants and in worms exposed to UV and gamma irradiation are highly enriched for kinases and phosphatases but not for DNA damage response genes. Therefore, it is likely that in response to mitochondrial dysfunction and different genotoxic stresses, CEP-1 can induce a core group of signal transduction molecules that initiate a downstream signaling cascade to mount a general stress response. In addition, CEP-1 appears to be able to sense specific damage and induce distinct responses associated with gamma irradiation and UV irradiation or mitochondrial ETC inhibition. Future dissection of the core and damage-specific responses of CEP-1/p53 will enhance our knowledge of the mechanisms that govern the function and regulation of p53, arguably one of the most important and ubiquitous tumor suppressors.
Materials and Methods
C. elegans strains
All strain stocks were kept at 16°C and grown under standard growth conditions. The following strains were used: Wild-type N2, isp-1(qm150), nuo-6(qm200), mev-1(kn-1), gas-1(fc21), clk-1(e2519), cep-1(gk138), and Pftn-1::gfp (GA641). Standard genetic methods were utilized to construct the following strains: cep-1(gk138);isp-1(qm150), nuo-6(qm200) cep-1(gk138), cep-1(gk138);clk-1(e2519), cep-1(gk138);mev-1(kn1), cep-1(gk138);gas-1(fc21), Pftn-1::gfp;isp-1(qm150), and Pftn-1::gfp;mev-1(kn-1).
Lifespan analysis
All lifespan assays were performed at 20°C on Nematode Growth Media (NGM) plates seeded with E. coli OP50 or RNAi bacteria. A detailed experimental procedure is described in the Supplementary Materials and Methods (Text S1). The survival function of each worm population was estimated using the Kaplan-Meier method, and statistical analysis was performed using a log-rank test (SPSS software). P≤0.001 was considered as significantly different from the control population. The independent trials were analyzed separately, and representative experiments are shown in the figures. All of the data from all the trials are shown in Supplementary Table S1. Percent mean lifespan differences from controls were plotted from multiple experiments in Fig. S1, and the data are shown in Supplementary Table S2.
Apoptosis assay
Worms of each strain were synchronized by picking, and the numbers of apoptotic corpses were counted 48 hours post L4. The corpses were assessed using Differential Interference Contrast (DIC) microscopy under 63× magnification as described in Lant and Derry (2013) [38]. For each strain, at least 3 independent experimental replicates were performed with n≥15, where n = number of gonad arms, for each replicate.
RNA isolation and microarray preparation
Total RNA was purified from synchronized young adult worms grown at 20°C on OP50 bacteria. Total RNA was isolated using Tri-reagent (Molecular Research Center, Inc.) and purified with the RNeasy kit (Qiagen). cRNA synthesis/amplification, Cy3/Cy5 dye labeling, and hybridization onto Agilent 4×44K C. elegans oligonucleotide microarrays were performed as previously described [39]. One of three replicate arrays was dye-flipped.
Microarray analysis
The normalized expression data were uploaded onto the Princeton University MicroArray database (PUMA [http://puma.princeton.edu]). The raw data were retrieved by SUID (Sequence Unique IDentifier) then averaged by SEQ_NAME with any remaining SUIDs removed. Log2-transformed fold-change data were acquired after setting spot filter criteria, where genes with >80% good data were used. The data were analyzed and visualized using Cluster 3 and TreeView [40]–[41].
The log2 ratios of wt vs. cep-1(gk138) with or without UV treatment were obtained from Derry et al. (2007). The log2 ratios of wt vs. cep-1 after gamma and X-ray irradiation data were obtained from Greiss et al. (2008). For the UV, gamma, and X-ray datasets, we averaged the intensity values of all three wt arrays for each treatment and used it as a reference. Then, we compared the results for each cep-1 array for the same treatment to the reference to obtain the log2 ratio.
SAM analysis
SAM analysis [42] was used to identify gene sets that were similarly and differentially regulated in isp-1 and mev-1 mutants in a cep-1-dependent manner from our microarray data. Log2-transformed fold-change data with no cutoff were submitted to SAM. One class analysis was used to identify genes that similarly changed significantly and consistently in isp-1 vs. cep-1;isp-1 and mev-1 vs. cep-1;mev-1 datasets. To identify genes differently changed between isp-1 vs. cep-1;isp-1 and mev-1 vs. cep-1;mev-1 datasets, SAM two-class unpaired analysis with a FDR = 1% was performed. The resulting gene list was compared with the gene list obtained from SAM one-class to exclude any duplicate genes. The unique 71 genes (Table S6) that were present only in SAM two-class analyses were considered differentially regulated between isp-1 and mev-1 mutants in a cep-1-dependent manner.
Gene Ontology classification
Worm Base IDs (WBID) of genes identified in SAM and K-mean clusters were input into the Functional annotation-clustering tool in DAVID (http://david.abcc.ncifcrf.gov/) [43] for gene annotation enrichment analysis. Functional annotation clustering was performed with the default criteria, and the enrichment score for each annotation cluster was determined.
Quantitative Reverse Transcription PCR (qRT-PCR):
Total RNA was isolated from synchronized young adult worms using Tri-reagent (Molecular Research Center, Inc.). cDNAs were synthesized with oligo-dT using the SuperScript III First-Strand Kit (Invitrogen). qRT-PCR reactions were performed using iQ SYBR Green Supermix (BIO-RAD) and the MyiQ Single Color Real-Time PCR Detection System (BIO-RAD). act-1 was used as the internal control. The qRT-PCR experiments were performed at least in triplicate using independent RNA/cDNA preparations.
GFP microscopy
For GFP fluorescence images, worms at the L1-L2 stage were paralyzed with levamisole on an agar pad. The GFP signal was visualized at 60× magnification using a Leica DM 5000B microscope. All images were captured with the same intensity and exposure time using Open Lab software.
Supporting Information
Zdroje
1. WallaceDC (2005) A mitochondrial paradigm of metabolic and degenerative disease, aging and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39 : 359–407.
2. CopelandJM, ChoJ, LoTJr, HurJH, BahadoraniS, et al. (2009) Extension of Drosophilla lifespan by RNAi of the mitochondrial respiratory chin. Curr Biol 19 (19) 1591–8.
3. LapointeJ, HekimiS (2008) Early mitochondrial dysfunction in long-lived Mclk1+/ − mice. J Biol Chem 283 (38) 26217–27.
4. ReaSL, VenturaN, JohnsonTE (2007) Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol Oct 2;5 (10) e259.
5. Van RaamsdonkJM, HekimiS (2009) Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 5 (2) e1000361.
6. IshiiN, FujiiM, HartmanPS, TsudaM, YasudaK, et al. (1998) A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 394 (6694) 694–7.
7. WalkerDW, HájekP, MuffatJ, KnoepfleD, CornelisonS, et al. (2006) Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc Natl Acad Sci U S A 103 (44) 16382–7.
8. FengJ, BussièreF, HekimiS (2001) Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell 1 (5) 633–44.
9. YangW, HekimiS (2010) Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell 9 (3) 433–47.
10. KondoM, Senoo-MatsudaN, YanaseS, IshiiT, HartmanPS, et al. (2005) Effect of oxidative stress on translocation of DAF-16 in oxygen-sensitive mutants, mev-1 and gas-1 of Caenorhabditis elegans. Mech Ageing Dev 126 (6–7) 637–41.
11. LeeSS, LeeRY, FraserAG, KamathRS, AhringerJ, et al. (2003) A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet 33 (1) 40–8.
12. YangW, HekimiS (2010) A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 8 (12) e1000556.
13. IshiiN, Senoo-MatsudaN, MiyakeK, YasudaK, IshiiT, et al. (2004) Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech Ageing Dev 125 (1) 41–6.
14. ButlerJA, VenturaN, JohnsonTE, ReaSL (2010) Long-lived mitochondrial (Mit) mutants of Caenorhabditis elegans utilize a novel metabolism. FASEB J 24 (12) 4977–88.
15. ButlerJA, MishurRJ, BhaskaranS, ReaSL (2013) A metabolic signature for long life in the Caenorhabditis elegans Mit mutants. Aging Cell 12 (1) 130–8.
16. NargundAM, PellegrinoMW, FioreseCJ, BakerBM, HaynesCM (2012) Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337 (6094) 587–90.
17. TorgovnickA, SchiaviA, TestiR, VenturaN (2010) A role for p53 in mitochondrial stress response control of longevity in C. elegans. Exp Gerontol 45 (7–8) 550–7.
18. WalterL, BaruahA, ChangHW, PaceHM, LeeSS (2011) The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biol 9 (6) e1001084.
19. KhanMH, LigonM, HusseyLR, HufnalB, FarberR2nd, et al. (2013) TAF-4 is required for the life extension of isp-1, clk-1 and tpk-1 Mit mutants. Aging 5 (10) 741–758.
20. MatobaS, KangJG, PatinoWD, WraggA, BoehmM, et al. (2006) p53 regulates mitochondrial respiration. Science 312 (5780) 1650–3.
21. JonesRG, PlasDR, KubekS, BuzzaiM, MuJ, et al. (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18 (3) 283–93.
22. MandalS, GuptanP, Owusu-AnsahE, BanerjeeU (2005) Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell 9 (6) 843–54.
23. BensaadK, VousdenKH (2007) p53: new roles in metabolism. Trends Cell Biol 17 (6) 286–91.
24. DerryWB, PutzkeAP, RothmanJH (2001) Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294 (5542) 591–5.
25. VenturaN, ReaSL, SchiaviA, TorgovnickA, TestiR, et al. (2009) p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell 8 (4) 380–93.
26. AckermanD, GemsD (2012) Insulin/IGF-1 and hypoxia signaling act in concert to regulate iron homeostasis in Caenorhabditis elegans. PLoS Genet 8 (3) e1002498.
27. DerryWB, BieringsR, van IerselM, SatkunendranT, ReinkeV, et al. (2007) Regulation of developmental rate and germ cell proliferation in Caenorhabditis elegans by the p53 gene network. Cell Death Differ 14 (4) 662–70.
28. GreissS, SchumacherB, GrandienK, RothblattJ, GartnerA (2008) Transcriptional profiling in C. elegans suggests DNA damage dependent apoptosis as an ancient function of the p53 family. BMC Genomics 15;9 : 334.
29. KruseJP, GuW (2009) Modes of p53 regulation. Cell 137 (4) 609–22.
30. LiuB, ChenY, St ClairDK (2008) ROS and p53: a versatile partnership. Free Radic Biol Med 44 (8) 1529–35.
31. HainautP, MannK (2001) Zinc binding and redox control of p53 structure and function. Antioxid Redox Signal 3 (4) 611–23.
32. HuyenY, JeffreyPD, DerryWB, RothmanJH, PavletichNP, et al. (2004) Structural differences in the DNA binding domains of human p53 and its C. elegans ortholog Cep-1. Structure 12 (7) 1237–43.
33. SablinaAA, BudanovAV, IlyinskayaGV, AgapovaLS, KravchenkoJE, et al. (2005) The antioxidant function of the p53 tumor suppressor. Nat Med 11 (12) 1306–13.
34. CurtisR, O'ConnorG, DiStefanoPS (2006) Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 5 (2) 119–26.
35. ImamuraK, OguraT, KishimotoA, KaminishiM, EsumiH (2001) Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 287 (2) 562–7.
36. LillR, MühlenhoffU (2008) Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu Rev Biochem 77 : 669–700.
37. SchumacherB, SchertelC, WittenburgN, TuckS, MitaniS, et al. (2005) C. elegans ced-13 can promote apoptosis and is induced in response to DNA damage. Cell Death Differ 12 (2) 153–61.
38. LantB, DerryWB (2013) Methods for detection and analysis of apoptosis signaling in the C. elegans germline. Methods 61 (2) 174–82.
39. ShawWM, LuoS, LandisJ, AshrafJ, MurphyCT (2007) The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol 17 (19) 1635–45.
40. de HoonMJ, ImotoS, NolanJ, MiyanoS (2004) Open source clustering software. Bioinformatics 20 (9) 1453–4.
41. SaldanhaAJ (2004) Java Treeview–extensible visualization of microarray data. Bioinformatics 20 (17) 3246–8.
42. TusherVG, TibshiraniR, ChuG (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98 (9) 5116–21.
43. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 (1) 44–57.
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



