-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDistinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
The cranial bones and dermis differentiate from mesenchyme beneath the surface ectoderm. Fate selection in cranial mesenchyme requires the canonical Wnt effector molecule β-catenin, but the relative contribution of Wnt ligand sources in this process remains unknown. Here we show Wnt ligands are expressed in cranial surface ectoderm and underlying supraorbital mesenchyme during dermal and osteoblast fate selection. Using conditional genetics, we eliminate secretion of all Wnt ligands from cranial surface ectoderm or undifferentiated mesenchyme, to uncover distinct roles for ectoderm - and mesenchyme-derived Wnts. Ectoderm Wnt ligands induce osteoblast and dermal fibroblast progenitor specification while initiating expression of a subset of mesenchymal Wnts. Mesenchyme Wnt ligands are subsequently essential during differentiation of dermal and osteoblast progenitors. Finally, ectoderm-derived Wnt ligands provide an inductive cue to the cranial mesenchyme for the fate selection of dermal fibroblast and osteoblast lineages. Thus two sources of Wnt ligands perform distinct functions during osteoblast and dermal fibroblast formation.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004152
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004152Summary
The cranial bones and dermis differentiate from mesenchyme beneath the surface ectoderm. Fate selection in cranial mesenchyme requires the canonical Wnt effector molecule β-catenin, but the relative contribution of Wnt ligand sources in this process remains unknown. Here we show Wnt ligands are expressed in cranial surface ectoderm and underlying supraorbital mesenchyme during dermal and osteoblast fate selection. Using conditional genetics, we eliminate secretion of all Wnt ligands from cranial surface ectoderm or undifferentiated mesenchyme, to uncover distinct roles for ectoderm - and mesenchyme-derived Wnts. Ectoderm Wnt ligands induce osteoblast and dermal fibroblast progenitor specification while initiating expression of a subset of mesenchymal Wnts. Mesenchyme Wnt ligands are subsequently essential during differentiation of dermal and osteoblast progenitors. Finally, ectoderm-derived Wnt ligands provide an inductive cue to the cranial mesenchyme for the fate selection of dermal fibroblast and osteoblast lineages. Thus two sources of Wnt ligands perform distinct functions during osteoblast and dermal fibroblast formation.
Introduction
The bones of the skull vault develop in close contact with the embryonic skin to enclose the brain. In the mouse embryo, both bone-forming osteoblasts and skin-forming dermal fibroblasts are derived from cranial neural crest and paraxial mesoderm [1]. At E11.5, cranial dermal fibroblast progenitors undergo specification beneath the surface ectoderm while osteoblast progenitors are specified in a deeper layer of cranial mesenchyme above the eye [2]–[4]. Subsequently, osteoblast progenitors proliferate and migrate apically beneath the dermal progenitors [1], [4]. Both cell types secrete collagen as extracellular matrix, but skull bones provide physical protection for the brain, while the overlying dermis lends integrity to the skin and houses the epidermal appendages [5].
Both paracrine and autocrine intercellular signals function in early bone and skin development. In craniofacial bone formation the mesenchyme sets the timing of ossification [6], [7], while the surface ectoderm functions in a permissive manner [8]. Likewise, during skin formation ectodermal signals are essential for formation of the trunk hair-follicle forming dermis [9], [10], but the cranial dermal mesenchyme determines epidermal appendage identity such as hair or feather [11]. Further delineation of specific ectoderm-mesenchyme signaling during early development of the bone and dermis is required to overcome challenges in the engineering of replacement connective tissues.
Mesenchymal canonical Wnt/β-catenin signal transduction is essential in the specification and morphogenesis of both craniofacial dermis and bone [2], [3], [12]–[15], and dysregulation in components of such signaling pathways is associated with diseases of bone and skin [1], [2], [16]–[18]. Wntless (Wls) functions specifically in trafficking of Wnt ligands and is required for the efficient secretion of Wnt ligands. [2]–[4], [19]–[28]. Genetic deletion of Wls in mice is likely to dramatically reduce the levels of active Wnt ligands and can recapitulate phenotypes obtained by genetic ablation of Wnt ligands in mice [1], [4], [29]. Wnt ligand binding to target cell surface receptors (Fzd and LRP5/6) results in nuclear translocation of β-catenin, which binds to TCF/LEF transcription factors and activates expression of downstream targets. Certain Wnt ligands also activate the non-canonical Wnt/Planar Cell Polarity (PCP) pathway, which influences cellular movements [5], [30], [31]. β-catenin is essential in osteoblast differentiation and inhibition of chondrogenesis [6], [7], [12]–[14]; however, deletion of individual Wnt ligands resulted only in mild effects on bone differentiation [8], [32], [33]. β-catenin is also a central regulator of early dermal specification [3], [9], [10], [34], [35], and roles for Wnt ligands so far have only been directly shown later during hair follicle initiation [9], [11], [36]. In bone and skin development, redundant functions of multiple Wnts may compensate for deletion of individual ligands. Conventional knockouts of individual ligands removed Wnt expression from all cells in the embryo, and have confounded the identification of tissue sources of Wnt ligands in bone and skin development. Thus, the relative contributions from different sources of Wnt ligands for fate selection in cranial mesenchyme remain unknown.
Previous limitations were the lack of genetic tools to spatiotemporally manipulate early surface ectoderm and mesenchyme, and an inability to circumvent the intrinsic redundancy of Wnt ligands. We took a conditional approach to ablate the efficient secretion of Wnt ligands from either surface ectoderm or cranial mesenchyme prior to fate selection of the cranial bone and dermal lineages. Our findings provide key insights into how local developmental signals are utilized during morphogenesis to generate the cranial bone and dermal lineages.
Results
We found that the genes for most Wnt ligands were expressed in the cranial mesenchyme (Figure 1A) and surface ectoderm (Figure 1B) during the specification of two separate lineages such as cranial osteoblast and dermal fibroblasts in E12.5 mouse embryos (Figure S1, S7, Table 1). To identify the cells with the potential to secrete Wnt ligands, we examined the spatiotemporal expression of Wls, the Wnt ligand trafficking regulator. We detected Wls protein expression from E11.5-E12.5 in the cranial surface ectoderm and in the underlying mesenchyme (Figure 1C, G). Both the Runx2-expressing cranial bone progenitor domain and the Dermo1/Twist2-expressing dermal progenitor domain expressed Wls [3], [37] (Figure 1C, D, E, G). Wnt signaling activation was also visualized in the cranial ectoderm, bone and dermal progenitors by expression of target gene, Lef1 and nuclear localized β-catenin (Figure 1D, F, H, I). During specification of cranial bone and dermis, ectodermal and mesenchymal tissues secreted Wnt ligands, and the dermal and bone progenitors actively transduced Wnt signaling via β-catenin (Figure 1J).
Fig. 1. Expression of Wnt ligands, Wntless, and Wnt signaling response in cranial ectoderm and mesenchyme. 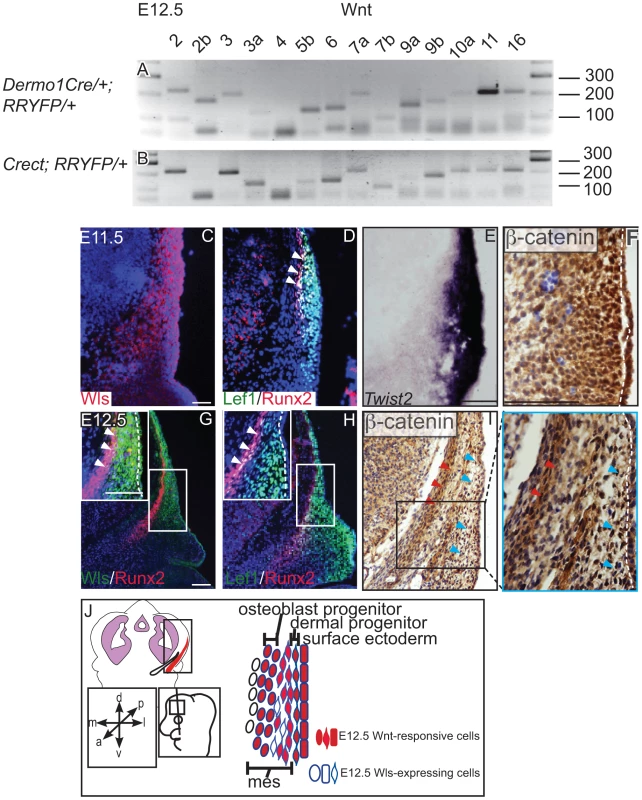
(A, B) RT-PCR for individual Wnt ligands was performed on cDNA from purified mouse embryonic cranial mesenchyme and surface ectoderm. (C, D G, H) Indirect immunofluorescence with DAPI counterstained nuclei (blue), (E) in situ hybridization, or immunohistochemistry (F, I) was performed on coronal mouse embryonic head sections. (G, H, I) Boxes indicate region in insets at higher magnification. White arrowheads indicate co-expression of (G) Wls/Runx2 or (D,H) Lef1/Runx2, (I) red arrowheads indicate osteoblast progenitors, and blue arrowheads indicate dermal progenitors. (F–I) White hatched lines demarcate ectoderm from mesenchyme. (J) Summary scheme of E12.5 supraorbital cranial mesenchyme. (J) Embryonic axes, figure depicts lateral view of embryonic head, region of interest in sections used in figures are shown. Scale bars represent 100 µm. Tab. 1. Primer sequences for RT-PCR of mouse Wnt genes. 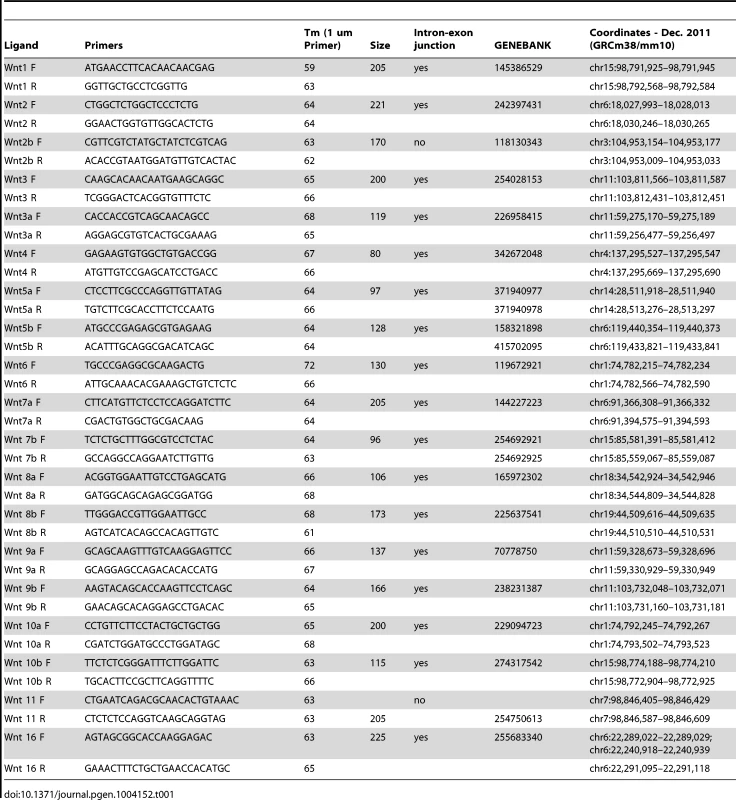
To dissect the requirements of ectodermal and mesenchymal Wnt signals, we generated mutant mice with conditional deletion of Wls [38] in the early surface ectoderm using Crect [39] and in the whole cranial mesenchyme using Dermo1Cre [40]. Crect efficiently recombined the Rosa26 LacZ Reporter (RR) in the cranial ectoderm by E11.5 (Figure S4K), but left Wls protein expression intact in the mesenchyme (Figure 2A, E, B, F) [41]. Dermo1Cre recombination showed β-galactosidase activity and Wls deletion restricted to the cranial mesenchyme and meningeal progenitors at E12.5, and Wls protein was still expressed in the ectoderm in mutants (Figure 2C, D, G, H).
Fig. 2. Deletion of Wntless in the cranial ectoderm and mesenchyme. 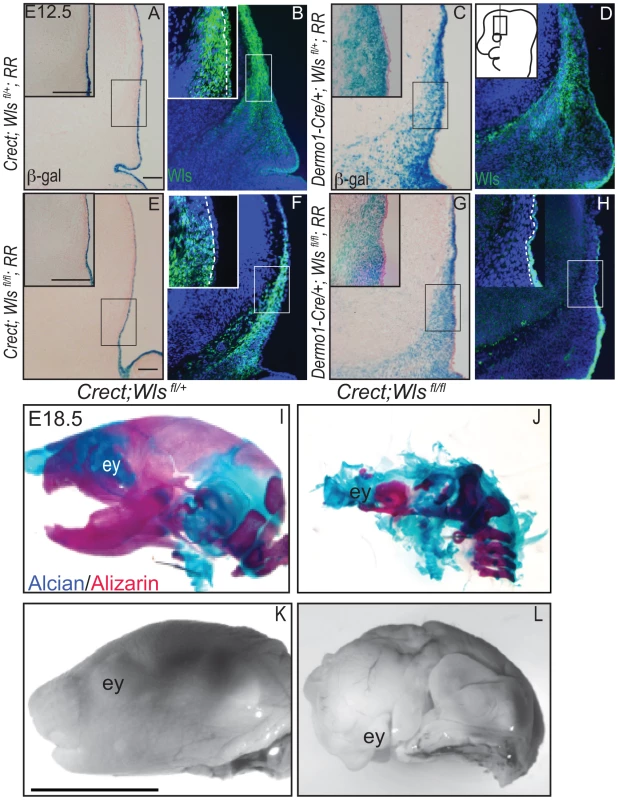
(A, C, E, G) β-galactosidase staining with eosin counterstain or (B, D, F, H) indirect immunofluorescence with DAPI-stained nuclei (blue) was performed on coronal mouse E12.5 head sections. (A–H) Box outlines indicate region in inset and white hatched line in insets demarcates cranial ectoderm from mesenchyme. (I–L) Lateral view of whole-mount skeletal preps or gray-scaled bright field images of embryonic mouse heads. Ey, eye. Scale bars for sections represent 100 µm. Scale bar (K) for whole mount pictures (I–L) represents 5 mm. Diagram inset in (D) depicts lateral view of embryonic head with box outlining region of interest. First, we compared the extent to which Wls deletion from ectoderm or mesenchyme affected formation of the craniofacial skeleton. E18.5 Crect; RR; Wls fl/fl mutant embryos, which experienced perinatal lethality, demonstrated a hypoplastic face with no recognizable upper or lower jaw most likely due to decrease in cell survival of branchial arch mesenchyme (Figure S5). In the remaining tissue, facial mesenchyme patterning was grossly comparable to controls for most of the markers examined (Figure S5). Notably, the mutants showed no sign of mineralization in the skull vault (Figure 2I–L). The later deletion of Wls from the ectoderm using the Keratin14Cre line resulted in comparable skull bone ossification as controls (Figure S2). Dermo1Cre; RR; Wls fl/fl mutant embryos exhibited lethality after E15.5, which precluded assessment of skeletogenesis by whole-mount. We generated En1Cre/+; RR; Wls fl/fl mutants, using a Cre that recombines in early cranial mesenchyme but lacks activity in meningeal progenitors (Figure S3 E′, F′) [3]. En1Cre/+; RR; Wls fl/fl mutants survived until birth, and demonstrated reduced bone differentiation and mineralization (Figure S3) as well as intact dermis in the supraorbital region with hair follicles (Figure S3). The more severe arrest in Crect; RR; Wls fl/fl mutants (Figure 2) suggested ectoderm Wls appears to play an earlier role than mesenchymal Wls in cranial development.
We next examined the effects of ectoderm or mesenchyme Wls deletion on cranial bone and dermal development by histology. We found Von Kossa staining for bone mineral was absent in Crect; RR; Wls fl/fl mutants (Figure 3A, B). The thin domain of mesenchyme above the eye in mutants appeared undifferentiated and showed no condensing dermal cells or early stage hair follicles. Additionally, the baso-apical expansion of both dermis and bone was evident by E15.5 in controls, but not in the thin cranial mesenchyme of mutants (Figure 3A–B red arrowhead). Although ossification was absent, we observed the presence of thin nodules of ectopic, alcian blue-stained cartilage (Figure 3E–F). Therefore the result of Wls deletion in the ectoderm was an absence of skull ossification and hair-inducing dermis, a failure of baso-apical expansion of mesenchyme, and the presence of ectopic chondrocyte differentiation. By comparison, Dermo1Cre; RR; Wls fl/fl mutants showed a reduction in mineralized bone (Figure 3C–D) without ectopic cartilage formation (Figure 3 G–H). The mutant mesenchyme nonetheless condensed and formed sufficient hair-follicle generating dermis in the supraorbital region to support the supraorbital vibrissae hair follicle and fewer primary guard hair follicles (Figure 3 C, D, C′, D′, black arrowheads). Compared to the control apical region of the head, the mutant lacked sufficient condensed dermal layer to support normal number and differentiation of hair follicles (Fig. 3 C″, D″). Reduced mineralization without ectopic chondrogenesis as well as hair-follicle formation were also present in En1Cre/+; Wls fl/fl mutants (Figure S3). Our data suggest that Wls deletion using the Dermo1Cre resulted in diminished bone mineralization with thinner dermis and fewer hair follicles.
Fig. 3. Distinct requirements for Wntless in the cranial ectoderm and mesenchyme. 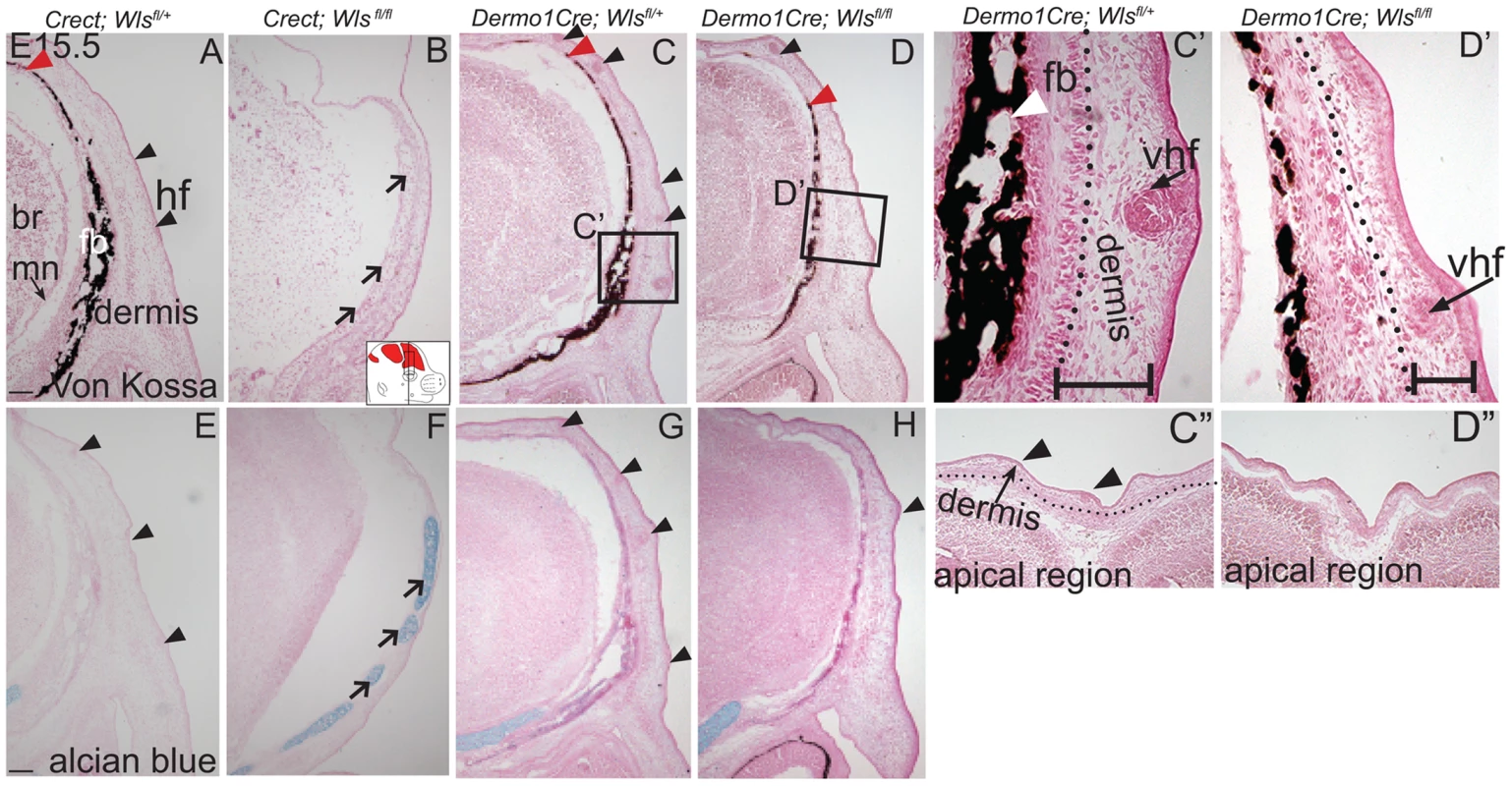
(A, B, C, D, C′, D′) Von Kossa staining, or (E–H) alcian blue staining was performed on coronal mouse embryonic head sections and counterstained with eosin. Br, brain, fb, frontal bone, vhf, supraorbital vibrissae hair follicle, mn, meningeal progenitors. Black arrowheads indicate guard hair follicles (hf), red arrowheads indicate dorsal extent of ossified frontal bone, and open black arrows indicate ectopic cartilage. (C′, D′ C″, D″) Black dotted line demarcates the lower limit of the dermal layer and the black bracket shows dermal thickness. Diagrams inset (B) figure depicts lateral view of E15.5 embryonic head with plane of section and region of interest. Red regions in diagram represent bone primordia. Scale bars (A,E) represent 100 µm. Deletion of Wls from the ectoderm resulted in complete absence of skull vault mineralization with failure of dermis formation, pointing to early defects in formation of the two lineages. Therefore we tested if cranial mesenchyme undergoes proper patterning, fate selection, and differentiation in the absence of Wls. Msx2 and Dlx5 that are early markers of skeletogenic patterning in cranial mesenchyme were expressed in Crect; Wls fl/fl mutants (Figures 4A, H, S4). The number of Msx2+ progenitor cells was not significantly different in controls and mutants (191±9.4 in controls and 206±24 in mutants, P-value = 0.23). However, few Runx2+ osteoblast progenitors formed in Crect; RR; Wls fl/fl mutant embryos, and expression shifted directly beneath the surface ectoderm (Figure 4B, I). During subsequent differentiation, condensing osteoblast progenitors express alkaline phosphatase (AP; Figure 4C, S4), but ectoderm Wnt-secretion deficient embryos lacked AP activity entirely (Figure 4J, S4). Markers of early osteoblast progenitors from other signaling pathways, Bmp4 and PTHrP (Figure 4D–E, K–L) were also absent in mutants, suggesting an arrest in osteoblast progenitor differentiation. The block was persistent as committed osteoblast progenitors expressing Osx were present in controls but not mutants (Figure 4F, M). Cell survival was not affected in the cranial mesenchyme prior to changes in marker expression (Figure S4A–D). We did not find significant difference in cell proliferation of the underlying mesenchyme (47%±4 in controls and 51%±2; P-value = 0.12). Whereas chondrocytes expressed Sox9 only at the skull base in controls, in mutants, ectopic Sox9-expressing chondrocyte progenitors and cartilage formed within the frontal bone domain (Figure 4G, N, Q, U). In spite of the effect of ectoderm-Wls deletion on mesenchyme, surface ectoderm expression of the differentiation marker, Keratin 14 (K14) was unaffected (Figure S4E,F). Next, we examined formation of dermal fibroblast progenitors in Crect; RR; Wls fl/fl mutant embryos. Cranial dermal fibroblast progenitors expressed the markers, Twist2 [3], [37] and Insulin Growth factor 2 (IGF2) by E12.5 in supraorbital mesenchyme (Figure 4O, P), but mutant embryos lacked Twist2 and IGF2 expression (Figure 4S, T). Twist2 expression became more progressively restricted to upper dermal fibroblasts during differentiation in controls, but was completely absent from cranial supraorbital mesenchyme of mutants (Figure 4 R, V). The altered cell fate marker expression at E12.5 (Figure 4, S4 I, J) immediately after deletion of ectoderm Wls (Figure S4K) was indicative of primary defects in mesenchymal cell fate selection. Together, our data suggest ectoderm Wnts form a non-cell autonomous inductive signal to the underlying mesenchyme for specification of osteoblast and dermal fibroblast progenitors, and for repression of chondrogenesis.
Fig. 4. Ectoderm deletion of Wntless leads to loss of cranial bone and dermal lineage markers in the mesenchyme. 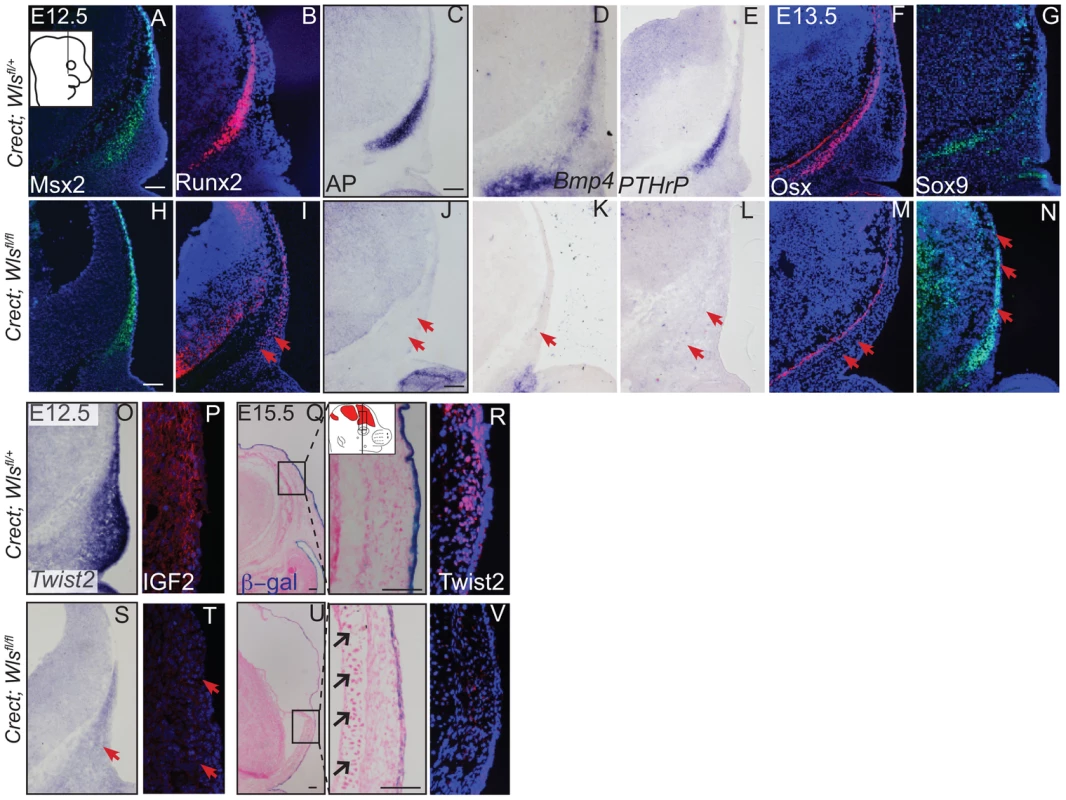
Indirect immunofluorescence with DAPI-stained (blue) nuclei was performed on coronal mouse embryonic head sections at E12.5 or as indicated (A,B, F, G, H, I, M, N, P, R, T, V). Alkaline Phosphatase staining (C, J), in situ hybridization (D, E, K, L, O, S), or β-galactosidase staining with eosin counterstain (Q, U) was performed on coronal tissue sections. Diagram in (A) demonstrates plane of section and region of interest for E12.5-E13.5 (A–T). Box and dashed lines in (Q, U) demonstrate the region of high magnification, and β-galactosidase stained sections were included for perspective for (R, V). Diagram inset in high magnification photograph from (Q) shows plane of section and region of interest for E15.5. Red arrows indicate changes in marker expression and black arrows in (U) high magnification indicate ectopic cartilage. Scale bars represent 100 µm. Next, we determined if mesenchyme Wls deletion resulted in a later defect in differentiation of cranial bone and dermal fibroblast progenitors. In En1Cre; RR; Wls fl/fl mutants, Runx2 expression in osteoblast progenitors was intact without ectopic Sox9 expression, but showed diminished expression of the skeletal differentiation marker, Osx and ossification (Figure S3). Wnt responsiveness by Axin2 expression was comparable in control and mutant cranial mesenchyme at E14.5 (Figure S3). In Dermo1Cre; RR; Wls fl/fl mutants, Runx2 expression was also unaffected during fate selection stages (Figure 5A, G, B, H). However, during later osteoblast progenitor differentiation (E15.5), Osx was diminished in mutants at E15.5 (Figure 5C, I). In dermal progenitors undergoing specification, Twist2 expression was unaffected (Figure 5D,J), and surface ectoderm differentiation marker, K14, was appropriately expressed (Figure S6C, D). Additionally at later stages in the mutant, we observed thinner dermis, which was sufficient to support initiation of fewer guard hair follicles (data not shown) and supraorbital vibrissae hair follicle formation (Figs. 3C, D; 5E, K). Furthermore, no ectopic expression of Sox9 occurred in mesenchyme Wls-deficient mutants (Figs. 5F, L). Deletion of mesenchyme-Wls did not lead to decrease in cell survival as monitored by expression of activated-Caspase3 (Figure S6A–B). Prior to E15.5, cell proliferation of osteoblast, dermal, and surface ectoderm progenitors was not significantly different from controls (Figure S6). Based on Dermo1Cre- and En1Cre- deletion of Wls, mesenchyme-derived Wnt ligands are not required for differentiation of dermal progenitors but are indispensable for later differentiation of osteoblast progenitors. Next, we tested the spatiotemporal requirement for mesenchyme Wls in Wnt signaling transduction. Nuclear β-catenin and Axin2 expression were comparable in the mesenchyme of mutants during fate selection stages at E12.5 (Figure 5M, N, Q, R). As differentiation occurs, expression of Axin2 and Lef1 was selectively diminished in the osteoblast progenitor domain of mesenchyme-Wls mutants compared to the controls (Figure 5O, P, S, T). Thus, mesenchyme Wnt ligands appeared to be important in mesenchyme Wnt signal transduction during osteoblast differentiation and ossification as opposed to earlier lineage specification events.
Fig. 5. Mesenchyme deletion of Wntless leads to diminished differentiation and Wnt responsiveness in the bone lineage. 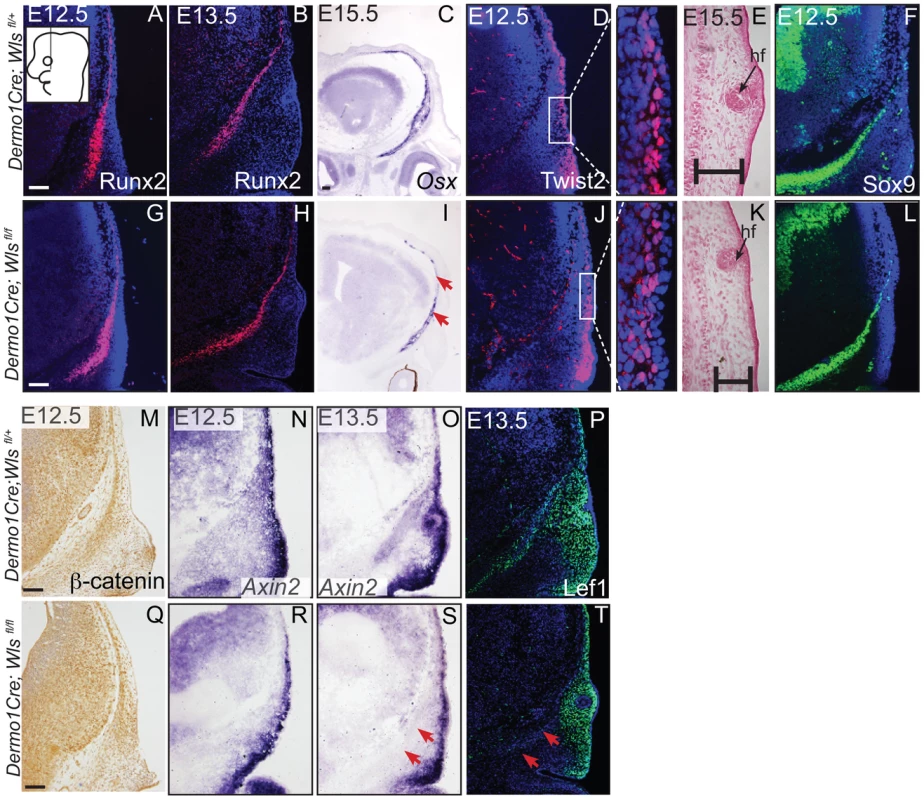
Indirect immunofluorescence with DAPI-stained (blue) nuclei (A, B, D, F, G, H, J, L, P, T) and immunohistochemistry (M,Q) was performed on coronal mouse embryonic head sections In situ hybridization (C, I, N, O, R, S) or eosin counterstain (E, K), was performed on coronal tissue sections of embryonic murine heads at the indicated stages. Diagram in (A) demonstrates plane of section and region of interest for E11.5-E12.5. Box in (D, J) demonstrate the region of high magnification. (I, S, T) Red arrows highlight changes in marker expression in osteoprogenitor domain. (E,K) vhf: subraorbital vibrissae hair follicle and black bracket indicates the dermal layer. (A,G) Scale bars represent 100 µm. Next, we examined the source of Wnts for the onset of Wnt responsiveness in the mesenchyme. During dermal and osteoblast progenitor cell fate selection, Wnt ligands, inhibitors, and target genes are expressed in spatially segregated patterns. Wnt10a and Wnt7b were expressed in surface ectoderm (Figure 6A–B), Wnt11 was expressed in sub-ectodermal mesenchyme (Figure 6C), and Wnt16 mRNA was expressed in medial mesenchyme (Figure 6D). Notably, the soluble Wnt inhibitor, Dickkopf2 (Dkk2) mRNA was localized to the deepest mesenchyme overlapping with cranial bone progenitors (Figure 6E). Wnt ligands can induce nuclear translocation of β-catenin in a dose-dependent manner leading to the expression of early target genes [42], [43]. At E11.5, expression of nuclear β-catenin was present in both dermal and osteoblast progenitors, and the highest intensity of nuclear localization was found in the surface ectoderm and dermal mesenchyme (Figure 1F). Wnt target genes Lef1, Axin2, and TCF4 were patterned in partially complementary domains. Expression of Tcf4 protein was visible in the skeletogenic mesenchyme (Figure 6F). Tcf4 expression expanded into the mesenchyme under the ectoderm in ectoderm Wls-deficient mutants (Figure 6I–J) and was diminished in mesenchyme Wls-deficient mutants compared to controls (Figure 6K–L). Lef1 and Axin2 were expressed at the highest intensity in the dermal progenitors beneath the ectoderm (Figure 6 G, H). At E12.5, Lef1 expression was completely abolished in the mesenchyme of ectoderm-Wls mutants, but was comparable to controls in the absence of mesenchyme-Wls (Figure 6M–P). The onset of Wnt signaling response in the mesenchyme as measured by Lef1, Axin2, and nuclear β-catenin expression (Figure 6O–T) required ectoderm Wls. By contrast, no single tissue source of Wnt ligands was required to maintain TCF4 expression.
Fig. 6. Generation of Wnt responsiveness in the cranial mesenchyme. 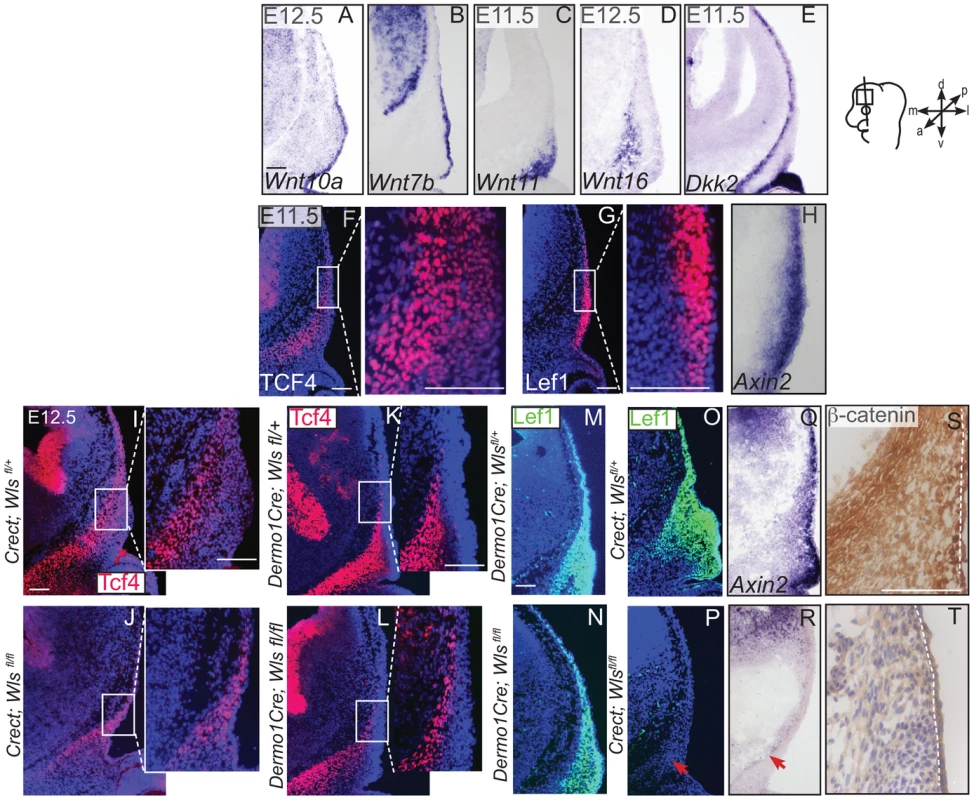
In situ hybridization (A–E, H, Q, R), immunohistochemistry (S, T) or indirect immunofluorescence with DAPI-stained nuclei (blue) was performed on coronal mouse embryonic head sections (F, G, I–P). (S, T) White dotted line demarcates ectoderm from mesenchyme. Embryonic head diagram depicts region of interest and plane of section. Embryonic axes for the sections are presented. Scale bars represent 100 µm. Finally, we tested whether cranial surface ectoderm Wnt ligands regulate the onset of Wnt ligand mRNA expression in the underlying mesenchyme (Figure 7). The non-canonical ligands Wnt5a and Wnt11 were expressed in cranial mesenchyme, with the highest expression corresponding to dermal progenitors. Wnt4, which signals in canonical or non-canonical pathways [44], was expressed strongly in dermal progenitors, as well as in osteoblast progenitors and in the skull base (Figure 7A–C). Wnt3a and 16, which signal in the canonical pathway via β-catenin and have roles in intramembranous bone formation, were expressed medially in the cranial mesenchyme containing cranial bone progenitors (Figure 7D, E) [12]–[14], [45]. Expression of Wnt5a Wnt11, Wnt3a, Wnt16 mRNAs was absent from the mesenchyme of Crect; RR; Wls fl/fl mutants whereas some Wnt4 expression was maintained (Fig. 7F–J). En1Cre deletion of β-catenin in the cranial mesenchyme [12] also resulted in an absence of Wnt5a and Wnt11 expression, except in a small portion of supraorbital lineage-labeled mesenchyme, suggesting a phenocopy of Crect;Wls mutants (Figure 7K, L, M). In contrast, Wnt5a, Wnt11, and Wnt4 expression were present in the Dermo1Cre; RR; Wlsfl/fl mutants (Figure 7N–S). However, the Wnt-expressing domains were smaller and only located close to the surface ectoderm, but nonetheless were lineage-labeled (Figure 7E–G, L–N; not shown). Thus, consistent with a role as initiating factors, ectoderm Wnt ligands and mesenchyme β-catenin were required for expression of certain Wnt ligands in the cranial mesenchyme during lineage selection. Mesenchymal Wnt ligands may in turn be required later for osteoblast differentiation (Figure 7T).
Fig. 7. Mesenchyme Wnt ligand expression is dependent on ectoderm Wls and mesenchymal β-catenin. 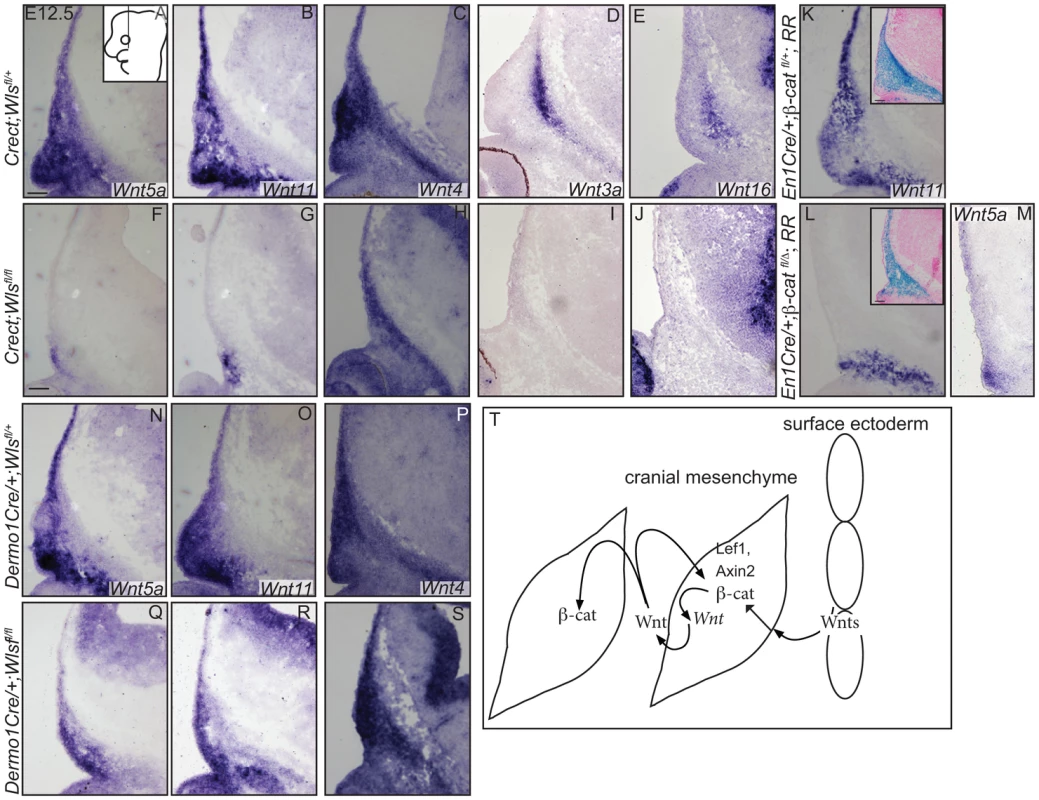
(A–S) In situ hybridization was performed on coronal mouse embryonic head sections. Diagram of embryonic head in (A) inset depicts region of interest and plane of section. Insets in (K, L) show β-galactosidase staining and eosin counterstaining on serial sections. (T) A working model for role of tissue sources of Wnt ligands during cranial mesenchymal lineage fate selection. Scale bars represent 100 µm. Discussion
Here we obtained data suggesting that ectodermal and mesenchymal Wnts function distinctly in early dermal and osteoblast progenitor specification and differentiation. Wnt ligands are expressed in the cranial surface ectoderm and mesenchyme, and ectoderm Wnts are required to generate an inductive cue for the specification of multiple lineages in the cranial mesenchyme. The dermal progenitors and osteoblast progenitors closest to the ectoderm experience the highest concentrations of nuclear β-catenin, in response to Wnt ligands from overlying ectoderm. Subsequent differentiation of osteoblast and dermal fibroblast progenitors requires Wls from the mesenchyme. Thus our study demonstrates that two different sources of Wnt signals coordinate to form two separate lineages, bone and dermis.
We present evidence to demonstrate that ectoderm Wnts generate an inductive cue of Wnt signaling in the mesenchyme to specify cranial bone and dermal lineages. The mechanism remains elusive; however, there are at least three possible models. First, the spatial segregation of Wnt pathway transcription cofactors such as Lef1 and TCF4, partially by lineage, provides a mechanism to generate different lineage programs. Second, a threshold-dependent model may also exist to generate multiple lineages from the same signal. At E11.5–12.5, dermal progenitors are closest to the ectoderm Wnt source and exhibit the highest Wnt signaling reporter activity and markers induced by constitutive activation of β-catenin in mesenchyme (Figure 1) [3], [9], [46]. High levels of Wnt pathway activity preclude osteoblast marker expression in the mesenchyme [12]. Consistently, osteoblast progenitors are present farther away from the ectoderm in an overlapping domain to at least one Wnt inhibitor, Dkk2 [47] (Figure 6E). Finally, the osteoblast response to ectodermal Wnts may be indirect; osteoblast progenitors may require a separate signal relayed from dermal progenitors. Future genetic experiments with new reagents will be required to distinguish between these models and test direct or indirect requirements of Wnt sources in osteoblast and dermis formation.
During fate selection of cranial dermal and osteoblast progenitors, upstream ectodermal Wnt ligands initiate expression of a subset of mesenchymal Wnt ligands via β-catenin. Ectoderm Wnts also act upstream of mesenchyme Wnts in mouse limb development [48]. Here, ectoderm Wnts act in a temporally earlier role than mesenchyme Wnts, and other studies support a direct relationship. In at least one instance, mesenchyme Wnt ligands are direct targets of canonical Wnt signaling [49]. Alternatively, ectoderm and mesenchyme Wnts may signal in parallel pathways to the mesenchyme. The signal that acts upstream to initiate Wnt ligand expression in the cranial ectoderm remains unknown.
We report here that osteoblast differentiation requires distinct Wnt signals from surface ectoderm and mesenchyme. β-catenin deletion in the ectoderm did not inhibit skull bone mineralization [39], so autocrine effects of Wls deletion on the ectoderm were unlikely to contribute to the skull phenotype. However, removal of surface ectoderm Wls resulted in ectopic chondrogenesis (Figure 3), which phenocopied mesenchymal β-catenin deletion [12]. In contrast, mesenchymal Wls deletion did not result in ectopic cartilage formation, suggesting repression of chondrogenesis in cranial mesenchyme requires an early, ectoderm Wnt signal. Our results thus implicate β-catenin here as a Wnt pathway factor that acts in the nucleus to repress chondrogenesis and functions downstream of ectoderm ligands. Ectoderm Wnt ligands thus provide an inductive cue acting on osteoblast progenitors while the cells are closest to the ectoderm. Indeed, later deletion of Wls from the ectoderm using the K14Cre line did not give rise to a skull bone ossification phenotype (Figure S2). During osteoblast progenitor differentiation, Wls deletion with Dermo1Cre resulted in a similar but more severe differentiation arrest than the more restricted En1Cre. Consistently, using a different Wls mutant allele, deletion of mesenchymal Wnts led to absence of osteoblast differentiation expression and reduced cell proliferation [50]. We show that the mesenchyme Wnts maintain the differentiation process but require an inductive ectoderm Wnt signal.
We demonstrate that dermal progenitors require ectodermal Wls for specification and mesenchymal Wls for normal differentiation (Figs. 4–5). Cranial dermal progenitors located beneath the ectoderm require β-catenin for specification [3], but the tissue contribution of Wnt sources remained previously undetermined. Here, a mesenchymal Wls source is indispensable in the dermal lineage for normal differentiation, thickness, and hair follicle patterning. Previous reports in murine trunk skin development suggested that ectoderm Wnts alone are essential in hair follicle induction [9], [10]. Differential requirements may exist for mesoderm-derived trunk dermal progenitors and cranial neural crest-derived dermal progenitors. Future studies will be needed to uncover the requirements for a mesenchymal Wnt signal in dermal fibroblast differentiation in different parts of the embryo.
Conditional Wls deletion resulted in a failure of cranial dermal and osteoblast progenitors to undergo baso-apical extension (Figure 3), a process that occurs independently of β-catenin [12]. Since Wls deletion blocked secretion of canonical and non-canonical Wnt ligands, extension defects in the mesenchyme are consistent with known roles for non-canonical Wnt ligands in orienting cell movements [51]. Homozygous null mutants of core planar cell polarity (PCP) components lacked proper skull tissue development and neural tube closure [52]. However, mutants for individual non-canonical Wnt ligands lack a cranial PCP phenotype. In the cranial mesenchyme, non-canonical Wnt5a or Wnt11 ligands were expressed in overlapping expression domains, suggesting the ligands function redundantly [53] (Figure 7). Therefore, the role of PCP signaling remains to be rigorously tested in conditional mutant mice. The non-canonical and canonical Wnt signaling pathways interact extensively. In our study, canonical β-catenin transduction, in response to ectodermal Wnts, initiates non-canonical Wnt ligand expression (Figure 7), consistent with reports from other systems [30], [49], [51]. Our results reinforce the role of non-canonical Wnt ligands in the pathogenesis of craniofacial anomalies [54], [55]. The ability of exogenous non-canonical Wnts to compensate for Wls deletion in the baso-apical extension of dermal and osteoblast progenitors remains to be tested.
Our results from tissue-specific deletion of Wls have implications in diseases with dysregulation of dermal fibroblasts or osteoblasts, and in understanding the pathogenesis of craniofacial birth defects. Removal of Wls from the ectoderm by E12.5 of mouse development reveals a default state for formation of cartilage in the cranial skeleton and dermis if all Wnt secretion were absent from the ectoderm. This forms an important baseline state that can be used to interpret less severe genetic conditions resulting from loss or mutation of individual Wnt ligands. In this respect, we hypothesize that mutations in the Wnt secretory pathway may underlie diseases of osteoblasts, and dermal fibroblasts, warranting continued investigation into the role of Wnt production in bone and skin formation and homeostasis [15], [17], [18], [45], [56]–[58]. Understanding the signals surrounding osteoblast and dermal fibroblast formation is crucial to meet the demands of engineering appropriate connective tissues.
Materials and Methods
Mice and genotyping
Conditional functional studies were conducted using Crect, Keratin 14Cre; Dermo1Cre, En1Cre, β-catenin deleted, conditional β-catenin floxed mice [39], [40], [59]–[62]. Mice and embryos were genotyped as described previously. The conditional loss-of-function floxed allele for Wls (Wlsfl/fl) was described previously [38]. RR/RR mice harboring a LacZ transgene downstream of a floxed stop transcription signal in the ubiquitous Rosa26 locus were obtained for lineage tracing [41]. For timed matings the vaginal plug day was assigned as E0.5. At desired time points, embryos were harvested and processed for frozen sections as previously described [34]. For each experiment, at least three to five different mutants with littermate controls from 2–3 litters were analyzed. At least three to five litters were used for all analyses. Case Western Reserve Institutional Animal Care and Use Committee approved all animal procedures.
In situ hybridization, immunohistochemistry, and histology
Embryos were fixed in 4%PFA, cryopreserved, and sectioned at 8–12 µm. In situ hybridization, β-galactosidase with eosin counter-staining, and immunohistochemistry were performed essentially as described [34], [35]. Alcian blue staining of sections was performed as described. For Von Kossa staining of frozen sections, slides were fixed with 4% PFA, incubated in the dark with 2% silver nitrate, rinsed, exposed to light, and counterstained with eosin. In situ probes for Twist2 (Eric Olson, Dallas, TX), Pthrp, Wnt4 (V. Lefebvre, Cleveland, OH), Wnt5a (Andrew McMahon, Boston, MA), Wnt11 (Steve Potter, Cincinnati, OH), Axin2 (Brian Bai), BMP4, Wnt7b, Dlx5 (Gail Martin, San Francisco, CA), Wnt16 (Yingzi Yang, Bethesda, MD) and Osx (Matthew Warmann, Boston, MA) were gifts. For Wnt10a, cDNA was amplified from E12.5 RNA using primer F: GCTATTTAGGTGACACTATAGGCGCTCTGGGTAAACTGAAG, primer R: TTGTAATACGACTCACTATAGGGAGAGCCAACCACCTCTCTCA, and in vitro transcription of antisense mRNA with T7 polymerase. For Dkk2, PCR primers DKK2-F(5′-GACATGAAGGAGACCCATGCCTACG-3′ and DKK2-T7R 5′-TGTAATACGACTCACTATAGGGCATAGATGAGGCACATAACGGAAG-3′ were used.
Primary antibodies for Runx2, Sox9, Twist2, Lef1, Osx, Msx2, Ki67, IGF2, Wls, and β-catenin (goat anti-Runx2; 1∶20, R&D Biosystems; rabbit anti-Sox9; 1∶100; Millipore; mouse anti-Twist2, 1∶500, Santa Cruz; rabbit anti-Lef1, 1∶100, Abcam; rabbit anti-Osx, 1∶400, Abcam; mouse Msx1/2, 1∶50, DSHB; activated Caspase3, 1∶250, Abcam; rabbit Ki67; 1∶500 Abcam; rabbit IGF2 1∶400, Cell Signaling); rabbit anti-Wls, 1∶2000, gift from Richard Lang; mouse β-catenin 1∶100 BD Biosciences) were used for indirect immunofluorescence and immunohistochemistry. All control/mutant pairs were photographed at the same magnification. Number of Msx2+ cells was counted from a fixed field in 10 different sections from 4 embryos.
Proliferation index was assessed by percent of cells with Ki67 expression in the Runx2 expression domain, in the dermal mesenchyme in the Twist2 domain, and surface ectoderm in the Keratin14 expressing cells. Similar numbers of cells in each domain were analyzed between four controls and mutants. Statistical significance for all quantifications was calculated using two-tailed Student t-test.
Alcian blue and Alizarin red and AP staining
Embryos were sacrificed, skinned and eviscerated, fixed in 95% ethanol, then stained for 24 hours each in 0.03% Alcian blue and 0.005% Alizarin red. Stained embryos were subsequently cleared in graded series of potassium hydroxide and glycerol until photography, after which they were stored in 0.02% Sodium Azide in glycerol. Whole mount Alkaline phosphatase staining was performed as previously described [63] with the addition of a 70% ethanol overnight incubation step after fixation in 4% PFA.
RT-PCR
Cranial mesenchyme and surface ectoderm were micro-dissected from E12.5 embryos and flash frozen in liquid nitrogen. Total RNA was isolated using the Qiagen RNEasy micro kit, and cDNA was reverse transcribed using the ABI kit. RT-PCR for most of the Wnt ligands was amplified for 35 cycles of 94°C for 15 seconds, 66°C for 30 seconds, and 72°C for 60 seconds and the products were resolved on a 3% agarose gel. For Wnt1, 5b, 8a, 8b, 10b the annealing temperature was 55°C for 30 seconds. Primer sequences for RT-PCR are listed in Table 1.
Supporting Information
Zdroje
1. JiangX (2002) Tissue Origins and Interactions in the Mammalian Skull Vault. Developmental Biology 241 : 106–116.
2. HsuY-H, ZillikensMC, WilsonSG, FarberCR, DemissieS, et al. (2010) An integration of genome-wide association study and gene expression profiling to prioritize the discovery of novel susceptibility Loci for osteoporosis-related traits. PLoS Genet 6: e1000977.
3. TranTH, JarrellA, ZentnerGE, WelshA, BrownellI, et al. (2010) Role of canonical Wnt signaling/ß-catenin via Dermo1 in cranial dermal cell development. Development 137 : 3973–3984.
4. YoshidaT, VivatbutsiriP, Morriss-KayG, SagaY, IsekiS (2008) Cell lineage in mammalian craniofacial mesenchyme. Mechanisms of Development 125 : 797–808.
5. HardyMH (1992) The secret life of the hair follicle. Trends Genet 8 : 55–61.
6. SchneiderRA, HelmsJA (2003) The cellular and molecular origins of beak morphology. Science 299 : 565–568.
7. MerrillAE, EamesBF, WestonSJ, HeathT, SchneiderRA (2008) Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development 135 : 1223–1234.
8. HallBK (1981) The induction of neural crest-derived cartilage and bone by embryonic epithelia: an analysis of the mode of action of an epithelial-mesenchymal interaction. J Embryol Exp Morphol 64 : 305–320.
9. ChenD, JarrellA, GuoC, LangR, AtitR (2012) Dermal -catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 139 : 1522–1533.
10. FuJ, HsuW (2012) Epidermal Wnt Controls Hair Follicle Induction by Orchestrating Dynamic Signaling Crosstalk between the Epidermis and Dermis. Journal of Investigative Dermatology 133 : 890–898.
11. EamesBF, SchneiderRA (2005) Quail-duck chimeras reveal spatiotemporal plasticity in molecular and histogenic programs of cranial feather development. Development 132 : 1499–1509.
12. GoodnoughLH, ChangAT, TreloarC, YangJ, ScacheriPC, et al. (2012) Twist1 mediates repression of chondrogenesis by beta-catenin to promote cranial bone progenitor specification. Development 139 : 4428–4438.
13. DayT, GuoX, GarrettbealL, YangY (2005) Wnt/β-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Dev Cell 8 : 739–750.
14. HillT, SpaterD, TaketoM, BirchmeierW, HartmannC (2005) Canonical Wnt/β-Catenin Signaling Prevents Osteoblasts from Differentiating into Chondrocytes. Dev Cell 8 : 727–738.
15. ZhongZ, Zylstra-DiegelCR, SchumacherCA, BakerJJ, CarpenterAC, et al. (2012) Wntless functions in mature osteoblasts to regulate bone mass. Proceedings of the National Academy of Sciences 109: E2197–E2204.
16. RichardsJB, RivadeneiraF, InouyeM, PastinenTM, SoranzoN, et al. (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371 : 1505–1512.
17. WangX, Reid SuttonV, Omar Peraza-LlanesJ, YuZ, RosettaR, et al. (2007) Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nat Genet 39 : 836–838.
18. RivadeneiraF, StyrkársdottirU, EstradaK, HalldórssonBV, HsuY-H, et al. (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41 : 1199–1206.
19. BänzigerC, SoldiniD, SchüttC, ZipperlenP, HausmannG, et al. (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125 : 509–522.
20. BartschererK, PelteN, IngelfingerD, BoutrosM (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125 : 523–533.
21. FuJ, JiangM, MirandoAJ, YuHM, HsuW (2009) Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proceedings of the National Academy of Sciences 106 : 18598–18603.
22. GoodmanRM, ThombreS, FirtinaZ, GrayD, BettsD, et al. (2006) Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 133 : 4901–4911.
23. BelenkayaTY, WuY, TangX, ZhouB, ChengL, et al. (2008) The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 14 : 120–131.
24. Franch-MarroX, WendlerF, GuidatoS, GriffithJ, Baena-LopezA, et al. (2008) Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 10 : 170–177.
25. PortF, KusterM, HerrP, FurgerE, BänzigerC, et al. (2008) Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 10 : 178–185.
26. PortF, BaslerK (2010) Wnt trafficking: new insights into Wnt maturation, secretion and spreading. Traffic 11 : 1265–1271.
27. YangP-T, LorenowiczMJ, SilhankovaM, CoudreuseDYM, BetistMC, et al. (2008) Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 14 : 140–147.
28. DasS, YuS, SakamoriR, StypulkowskiE, GaoN (2012) Wntless in Wnt secretion: molecular, cellular and genetic aspects. Front Biol (Beijing) 7 : 587–593.
29. FuJ, Ivy YuH-M, MaruyamaT, MirandoAJ, HsuW (2011) Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn 240 : 365–371.
30. van AmerongenR, NusseR (2009) Towards an integrated view of Wnt signaling in development. Development 136 : 3205–3214.
31. CleversH, NusseR (2012) Wnt/beta-catenin signaling and disease. Cell 149 : 1192–1205.
32. SpaterD, HillTP, O'Sullivan RJ, GruberM, ConnerDA, et al. (2006) Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development 133 : 3039–3049.
33. BennettCN, LongoKA, WrightWS, SuvaLJ, LaneTF, et al. (2005) Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 102 : 3324–3329.
34. AtitR, SgaierSK, MohamedOA, TaketoMM, DufortD, et al. (2006) Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Developmental Biology 296 : 164–176.
35. OhtolaJ, MyersJ, Akhtar-ZaidiB, ZuzindlakD, SandesaraP, et al. (2008) beta-Catenin has sequential roles in the survival and specification of ventral dermis. Development 135 : 2321–2329.
36. HuB, LefortK, QiuW, NguyenB-C, RajaramRD, et al. (2010) Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes & Development 24 : 1519–1532.
37. LiL, CserjesiP, OlsonEN (1995) Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Developmental Biology 172 : 280–292.
38. CarpenterAC, RaoS, WellsJM, CampbellK, LangRA (2010) Generation of mice with a conditional null allele for Wntless. genesis 48 : 554–558.
39. ReidBS, YangH, MelvinVS, TaketoMM, WilliamsT (2011) Ectodermal Wnt/beta-catenin signaling shapes the mouse face. Developmental Biology 349 : 261–269.
40. YuK (2003) Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130 : 3063–3074.
41. SorianoP (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21 : 70–71.
42. WillertK, BrownJD, DanenbergE, DuncanAW, WeissmanIL, et al. (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423 : 448–452.
43. RudloffS, KemlerR (2012) Differential requirements for beta-catenin during mouse development. Development 139 : 3711–3721.
44. LyonsJP, MuellerUW, JiH, EverettC, FangX, et al. (2004) Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res 298 : 369–387.
45. ZhengHF, TobiasJH, DuncanE, EvansDM, ErikssonJ, et al. (2012) WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet 8: e1002745.
46. ManiP, JarrellA, MyersJ, AtitR (2010) Visualizing canonical Wnt signaling during mouse craniofacial development. Dev Dyn 239 : 354–363.
47. FjeldK, KettunenPI, FurmanekT, KvinnslandIH, LuukkoK (2005) Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn 233 : 161–166.
48. ZhuX, ZhuH, ZhangL, HuangS, CaoJ, et al. (2012) Wls-mediated Wnts differentially regulate distal limb patterning and tissue morphogenesis. Developmental Biology 1–11.
49. ZhouW, LinL, MajumdarA, LiX, ZhangX, et al. (2007) Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet 39 : 1225–1234.
50. MaruyamaT, JiangM, HsuW (2012) Gpr177, a novel locus for bone-mineral-density and osteoporosis, regulates osteogenesis and chondrogenesis in skeletal development. J Bone Miner Res 28 : 1150–1159.
51. GrosJ, SerralboO, MarcelleC (2009) WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature 457 : 589–593.
52. GaoB, SongH, BishopK, ElliotG, GarrettL, et al. (2011) Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev Cell 20 : 163–176.
53. YamaguchiTP, BradleyA, McmahonAP, JonesS (1999) A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126 : 1211–1223.
54. KibarZ, TorbanE, McDearmidJR, ReynoldsA, BerghoutJ, et al. (2007) Mutations in VANGL1 associated with neural-tube defects. N Engl J Med 356 : 1432–1437.
55. LeiYP, ZhangT, LiH, WuBL, JinL, et al. (2010) VANGL2 mutations in human cranial neural-tube defects. N Engl J Med 362 : 2232–2235.
56. GrzeschikK-H, BornholdtD, OeffnerF, KönigA, Del Carmen BoenteM, et al. (2007) Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nat Genet 39 : 833–835.
57. BarrottJJ, CashGM, SmithAP, BarrowJR, MurtaughLC (2011) Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proceedings of the National Academy of Sciences 108 : 12752–12757.
58. PettiM, SamanichJ, PanQ, HuangCK, ReinmundJ, et al. (2011) Molecular characterization of an interstitial deletion of 1p31.3 in a patient with obesity and psychiatric illness and a review of the literature. Am J Med Genet A 155A: 825–832.
59. KimmelRA, TurnbullDH, BlanquetV, WurstW, LoomisCA, et al. (2000) Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes & Development 14 : 1377–1389.
60. DassuleHR, LewisP, BeiM, MaasR, McmahonAP (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127 : 4775–4785.
61. BraultV, MooreR, KutschS, IshibashiM, RowitchDH, et al. (2001) Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128 : 1253–1264.
62. HaegelH, LarueL, OhsugiM, FedorovL, HerrenknechtK, et al. (1995) Lack of beta-catenin affects mouse development at gastrulation. Development 121 : 3529–3537.
63. IshiiM, MerrillAE, ChanY-S, GitelmanI, RiceDPC, et al. (2003) Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development 130 : 6131–6142.
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



