-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaClassic Selective Sweeps Revealed by Massive Sequencing in Cattle
Human driven selection during domestication and subsequent breed formation has likely left detectable signatures within the genome of modern cattle. The elucidation of these signatures of selection is of interest from the perspective of evolutionary biology, and for identifying domestication-related genes that ultimately may help to further genetically improve this economically important animal. To this end, we employed a panel of more than 15 million autosomal SNPs identified from re-sequencing of 43 Fleckvieh animals. We mainly applied two somewhat complementary statistics, the integrated Haplotype Homozygosity Score (iHS) reflecting primarily ongoing selection, and the Composite of Likelihood Ratio (CLR) having the most power to detect completed selection after fixation of the advantageous allele. We find 106 candidate selection regions, many of which are harboring genes related to phenotypes relevant in domestication, such as coat coloring pattern, neurobehavioral functioning and sensory perception including KIT, MITF, MC1R, NRG4, Erbb4, TMEM132D and TAS2R16, among others. To further investigate the relationship between genes with signatures of selection and genes identified in QTL mapping studies, we use a sample of 3062 animals to perform four genome-wide association analyses using appearance traits, body size and somatic cell count. We show that regions associated with coat coloring significantly (P<0.0001) overlap with the candidate selection regions, suggesting that the selection signals we identify are associated with traits known to be affected by selection during domestication. Results also provide further evidence regarding the complexity of the genetics underlying coat coloring in cattle. This study illustrates the potential of population genetic approaches for identifying genomic regions affecting domestication-related phenotypes and further helps to identify specific regions targeted by selection during speciation, domestication and breed formation of cattle. We also show that Linkage Disequilibrium (LD) decays in cattle at a much faster rate than previously thought.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004148
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004148Summary
Human driven selection during domestication and subsequent breed formation has likely left detectable signatures within the genome of modern cattle. The elucidation of these signatures of selection is of interest from the perspective of evolutionary biology, and for identifying domestication-related genes that ultimately may help to further genetically improve this economically important animal. To this end, we employed a panel of more than 15 million autosomal SNPs identified from re-sequencing of 43 Fleckvieh animals. We mainly applied two somewhat complementary statistics, the integrated Haplotype Homozygosity Score (iHS) reflecting primarily ongoing selection, and the Composite of Likelihood Ratio (CLR) having the most power to detect completed selection after fixation of the advantageous allele. We find 106 candidate selection regions, many of which are harboring genes related to phenotypes relevant in domestication, such as coat coloring pattern, neurobehavioral functioning and sensory perception including KIT, MITF, MC1R, NRG4, Erbb4, TMEM132D and TAS2R16, among others. To further investigate the relationship between genes with signatures of selection and genes identified in QTL mapping studies, we use a sample of 3062 animals to perform four genome-wide association analyses using appearance traits, body size and somatic cell count. We show that regions associated with coat coloring significantly (P<0.0001) overlap with the candidate selection regions, suggesting that the selection signals we identify are associated with traits known to be affected by selection during domestication. Results also provide further evidence regarding the complexity of the genetics underlying coat coloring in cattle. This study illustrates the potential of population genetic approaches for identifying genomic regions affecting domestication-related phenotypes and further helps to identify specific regions targeted by selection during speciation, domestication and breed formation of cattle. We also show that Linkage Disequilibrium (LD) decays in cattle at a much faster rate than previously thought.
Introduction
The available genetic and archaeological evidences date cattle domestication back to the Neolithic period, around 10,000 BCE [1], [2]. Modern cattle are thought to have originated from multiple independent domestication events of aurochs (B. primigenius) primarily in southwest Asia and south Asia, resulting in the humpless taurine (B. taurus) and the humped zebu (B. indicus) groups respectively [3], [4]. Domestication of cattle had a major impact on human civilization as they provided physical power in agriculture and were a major source of milk, meat and leather products.
Domestication of cattle provides an excellent model of animal evolution. During the domestication process, cattle have adapted in morphology, physiology and behavior to captive life, and have been subject to artificial selection imposed by humans to increase yield, fertility and other processes. As a result, more than 900 breeds, each with distinct characteristics, have emerged throughout the world [5]. The phenotypes associated with domestication include milk and meat production, fertility, appearance including coat coloration, decreased fearfulness, social motivation, and mild temper [6]. The selection affecting these phenotypes has left detectable signatures of selection within the genome of modern cattle [7].
The signatures of selection in the genome, as a beneficial mutation arises and rapidly increases in frequency in the population, can be detected as (i) reduced local variability, (ii) deviations in the Site Frequency Spectrum (SFS) and (iii) increased linkage disequilibrium and extended haplotype structure. These signatures can be used to screen a genome for genes involved in recent adaptation. Numerous statistics have been developed aiming at detecting selection [8], [9], [10], [11], .
Previous genome-wide studies to detect positive selection in cattle have used SNP arrays, which suffer from ascertainment biases caused by the process used to discover SNPs [14], [15], [16] and limited resolution [17], [18], [19], [20], . In addition, these studies have focused on a single selection signature statistic that typically only detects selection during a certain time in the past. Many selection signals may, therefore, have remained un-detected by previous studies.
In this study, we use whole-genome re-sequencing data of 43 Fleckvieh animals [23], a German dual purpose cattle breed. We apply two different statistics, the integrated Haplotype Homozygosity Score (iHS) [12] and the Composite of Likelihood Ratio (CLR) [11] to detect past selection. iHS finds maximal power when a selected allele segregates at intermediate frequencies in the population, whereas the CLR statistics has most power right after the selected allele has gone to fixation. For this reason, the two statistics are complementary in the type of selection that they detect. Using whole genome sequence information rather than genotypes for pre-selected SNP panels avoids the problems caused by ascertainment. This design thus, provides additional power to detect selection missed by previous studies. In addition, we conduct a Genome-Wide Association mapping Study (GWAS) on appearance traits. We find evidence of strong signatures of selection in cattle during speciation, domestication and breed formation exemplified by several striking selective sweeps co-localized with major QTLs.
We also use the direct sequencing data to examine the pattern of Linkage Disequilibrium (LD) in the Fleckvieh breed. A detailed profile of LD over the entire genome is a quantity of interest, especially for the use in breeding programs implementing genomic selection. Previous studies of LD structure in cattle populations have used low resolution panels of ascertained SNPs mainly selected based on their minor allele frequency (MAF) and position on the genome [24], [25, among others].
Results and Discussion
Allele frequency distribution and LD
The data from Jansen et al [23] analyzed in this study includes roughly 20 times more SNPs than the 700K array previously used in cattle for examining LD. The increased SNP density provides a greater coverage of rare and low frequency SNPs than in any previous study based on SNP chip data.
The distribution of allele frequencies follow the same pattern as that observed for high-quality data in many other organisms including human populations [26], [27]. As predicted by population genetics theory, the frequency spectrum is a decreasing function, and among rare alleles there is a slight excess in the proportion of non-synonymous mutations relative to intergenic or synonymous mutations (Figure 1). The relative excess of non-synonymous mutations among rare alleles is presumed to be caused by selection acting on slightly deleterious mutations [26], [27].
Fig. 1. Comparison of the site frequency spectra from resequencing of 43 German Fleckvieh animals. 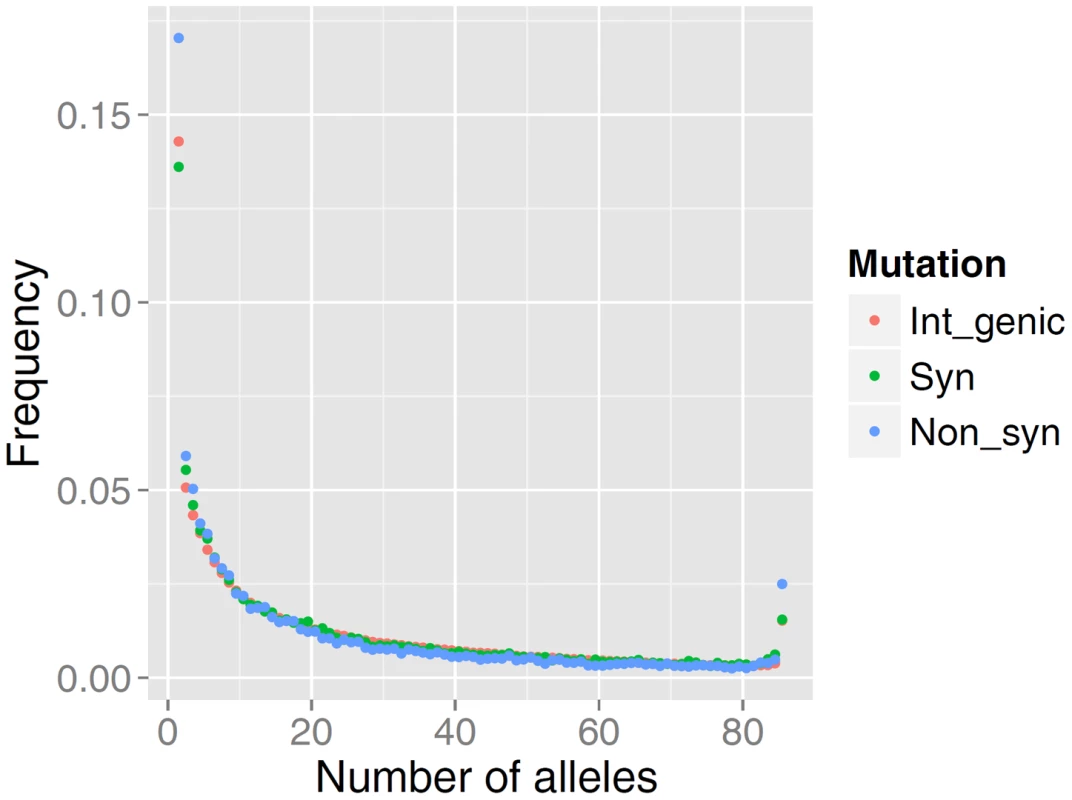
SFS is represented for non-synonymous (Non_syn), synonymous (Syn) and inter-genic polymorphic (Int_genic) variants. We found a mean value of r2 = 0.25 (sample SD = 0.29) for SNPs less than 20 kb apart (Figure 2). Table S1 summarizes more properties of LD as a function of physical distance. It is evident that average LD does not extend beyond the inter-marker space of 100 Kb across the genome. Previous studies in cattle however, found strong LD extending over several Megabasepairs [24], [25, among others]. However, LD as measured by r2 depends on allele frequencies [28], [25]; and the difference between this study and previous studies may partially be explained by the biased SNPs selection on the Illumina Bovine arrays, where SNPs mainly were ascertained based on allele frequency and a uniform distribution over the genome. Additionally, differences in the sample composition may explain the results, as LD is strongly affected by population structure. Characterizing LD using structured populations leads to an inflation of the LD statistics, which might have affected previous studies. Finally, genotyping error reduces apparent LD, and is a major concern for low - and intermediate-depth coverage re-sequencing data.
Fig. 2. A schematic representation of LD plotted as a function of distance. 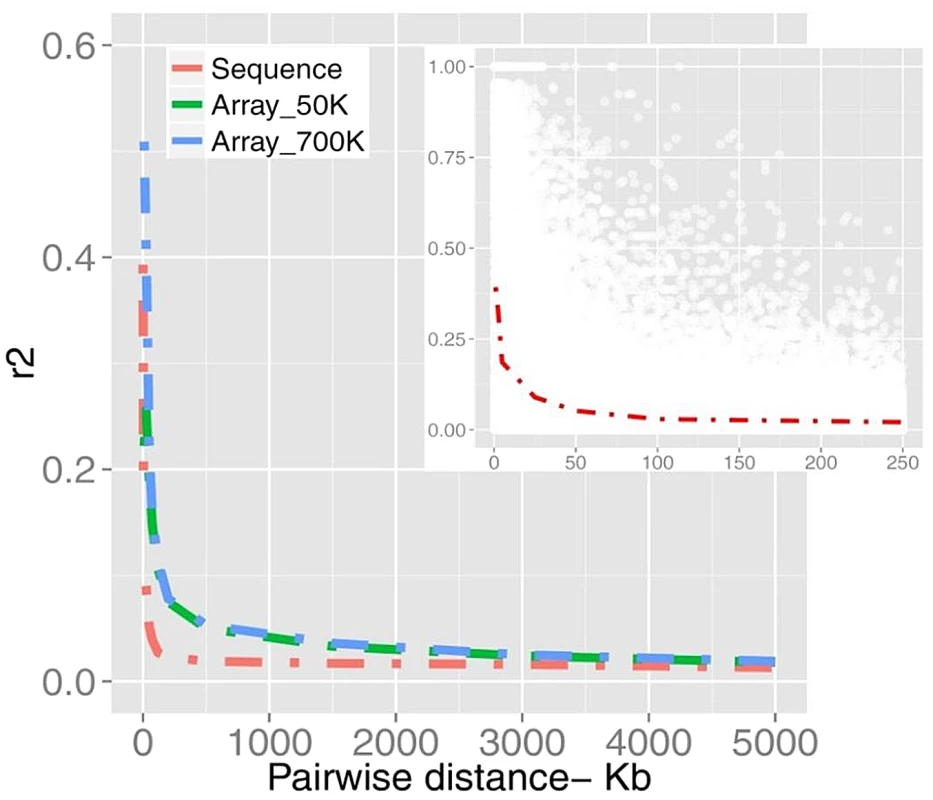
The decay of LD estimated from bovine SNP arrays of 50K and 700K (n = 1,293) are compared with the sequence data (n = 43). The inner plot displays a higher resolution of LD in pair-wise distances of <250 Kb from sequence data of which r2 values are down-sampled from all pairwise estimates (for more details see Material and Methods). To test the degree to which the differences in the LD curves are caused by these factors we examined LD in different scenarios. First we plotted the LD curve after excluding low-frequency variants from sequence data. As shown in Figure S1A, LD persists at a higher level when being estimated with frequent alleles. Second, we sub-selected the sequence data for those SNPs present in the 700k chip and compared LD from this sub-set with sequence and array based LD (Figure S1B). In the new dataset LD decayed faster than original array-based LD possibly due to the different sample composition. However, LD persisted at higher levels compared to the sequence LD due to the different allelic profile (p<0.001). Table S2 summarizes testing the strength of LD in two datasets estimated from SNP pairs in inter-marker distance bins up to 500 Kb. Further, to get insight into the quality of genotypes the concordance rate between sequences vs. array-derived genotypes was evaluated. We observed a significant concordance of 96.9% (±3.4%) based on 38,246 SNPs tested on chromosome 1. The LD curves before and after filtering out all but the highest quality SNPs were overlapped due to the fairly low discordance rate (data not shown). These results demonstrate that LD in cattle decays at a rate much faster than previously thought.
LD-based estimations of past effective population size [7], [25] should be revisited in light of the finding that the ‘true’ sequence-based LD profile is poorly estimated by SNP-chip based LD-estimates. Considering the relatively small effective population size in cattle, population level of LD is unexpectedly low, which suggests that effective population size was considerably larger in the very recent past.
Localizing selective sweeps
Evidence of positive selection was investigated through multiple statistics designed to detect signatures of selective sweeps. We calculated iHS per site and averaged them in non-overlapping 40 Kb windows across the genome, resulting in a total of 62,196 windows (Figure 3). The CLR was estimated using an identical grid size across the genome. We focused the analyses on windows for which the values of the statistics fell in the 99th percentile. Respectively, 68 and 73 candidate regions were identified from iHS and CLR analyses (Tables S3 and S4). There is a substantial overlap between the list of genes identified by CLR and iHS, reflecting the fact that the two tests take advantage of different but correlated patterns of a selective sweep. However, there are also regions solely identified by either metric, possibly because these statistics identify selection acting at different time scales. Two examples of candidate genes are shown in Figure 3: KIT and MITF, two pigmentation genes on Bos taurus (BTA) chromosomes 6 and 22.
Fig. 3. Genome-wide visualization of selection candidates (top 1% signals) localized by |iHS| (A) and CLR (B) metrics. 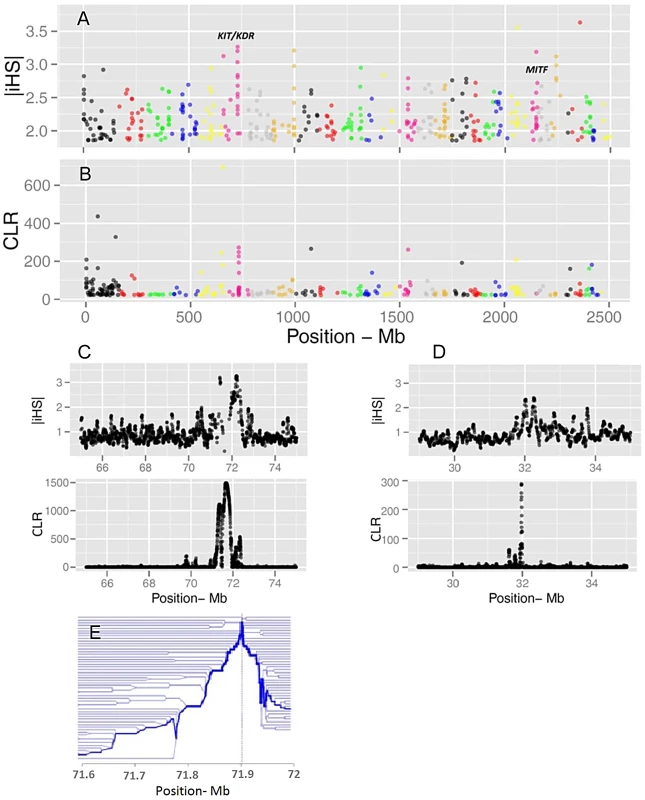
Each dot represents a non-overlapping window of 40 Kb along BTA1 to BTA29. Panels C and D show a high resolution illustration of the candidate regions for the KIT/KDR and MITF genes, respectively on BTA6 and BTA22. |iHS| is plotted in overlapping windows of 40 Kb in steps of 5 Kb, and a grid size of 5 Kb was chosen for the CLR statistic. Finally, Panel E is a haplotype bifurcation plot of the KIT/KDR genes. Putatively selected genes
Tables S3 and S4 summarize statistics for the genomic regions harboring the strongest selection signals. We used DAVID [29] to perform a functional analysis based on the list of all genes in the regions showing signatures of a selective sweep. We found no overall significant enrichment of any particular biological process after correction for multiple testing (data not shown). Nevertheless, we note that genes associated with a number of processes previously implicated in domestication-related changes are present within these regions. These include pigmentation, sensory perceptions, brain and neural system (for review see [6]) along with genes of immunity and blood clotting systems. For clarity and based on a priori interest, we divided genes into functional groups in line with domestication-related changes and discuss each group under separate heading. However, as most genes have pleiotropic effects, selection may possibly act on other functional effects of the genes than those highlighted here. In the following sections, we highlight some results from these analyses.
Patterned pigmentation
In mammals, coat color loci influence the development, differentiation, proliferation, and migration of melanocytes, the construction and transport of melanosomes, as well as the synthesis of melanin. In the genome-wide screen, the window with the strongest signal (PiHS = 0.00029, PCLR = 0.00011) coincides with a cluster of tyrosine kinase receptor genes (PDGFRA, KIT and KDR) on BTA6 (Figure 3). Among them, KIT is a widely studied gene with an important role in several critical pathways including melanogenesis. Genetic variation in the KIT gene has been shown to affect coat coloring pattern in a variety of mammals including cattle [30], horses [31], pigs [32] and mice [33]. Another strong selection candidate included microphthalmia-associated transcription factor (MITF, PiHS = 0.00172 and PCLR = 0.00709) a major candidate for patterned pigmentation in BTA22 [30]. KIT and MITF show complex interactions in that MITF is needed for the maintenance of KIT expression in melanoblasts and KIT signaling modulates MITF activity and stability in melanocyte cell lines. The mutual interaction between KIT and MITF is particularly interesting since mutations in any one of them lead to a strikingly overlapping phenotype of early loss of the melanocyte lineage [34].
Another particularly interesting selective sweep candidate in this group overlaps melanocortin 1 receptor (MC1R, PiHS = 0.00785 and PCLR = 0.00156) on BTA18 (also see Figure S2A), whose permanent activation results in black coat color, whereas loss of function mutations cause red coat color in different mammals including cattle [35]. MC1R gene expression is regulated by the MITF and has an autosomal recessive mode of inheritance (also see GWAS section).
Among the top selection candidates, we noticed three genes of the NRG – Erbb4 signaling pathway. This pathway is involved in the development and progression of melanocytes [36]. Our results revealed typical hitch-hiked patterns for NRG4 (PiHS = 0.00368, PCLR = 0.00221) and Erbb4 (PiHS = 0.00203, PCLR = 0.00855) genes along with an extremely deviated SFS for the pro-NRG2 like locus (PiHS = 0.66899, PCLR = 0.00589)(see also Figures S3 and S4). Interestingly, the same pathway appears to have been targeted by positive selection in humans [37]. Since precursors to pigment cells are also precursors to nerve cells, variants of genes in this pathway are also reported to be associated with various psychiatric phenotypes [38], [39]. Another strong signal that we speculate could be related to coat coloring is the ULBP3 gene (PiHS = 0.00691, PCLR = 0.00917) shown to be associated with the “sudden whitening of the hair” phenomenon [40].
Brain development and neurobehavioral functioning
Domesticated species differ from their wild ancestors, notably in behavioral traits such as reduced fear of humans and aggressiveness [6]. We noticed strong signals standing by some genes underlying extreme neurobehavioral phenotypes and psychiatric disorders (for instance see Figures S5, S6 and S7). Among the most pronounced candidates in our list are neuronal genes TMEM132D (PiHS = 0.00087, PCLR = 0.00021), CACNA1C (PiHS = 0.48366, PCLR = 0.00013), NRXN1 (PiHS = 0.00396, PCLR = 0.00018) and NPAS3 (PiHS = 0.34897, PCLR = 0.00137) reported as candidate QTLs for anxiety-related behavior, major depression and high risk of developing schizophrenia [41], [42], [43], [44]. One interesting observation was the presence of GRIK3 (PiHS = 0.00076, PCLR = 0.00761) among the genes with the strongest signal of selection. GRIK3 is a member of glutamine receptors suggested as QTL for reward-related learning [45]. Other putative selection candidate include OLIG1 (PiHS = 0.00031, PCLR = 0.00016), LAMC3 (PiHS = 0.00222, PCLR = 0.00325) and ATL1 (PiHS = 0.00227, PCLR = 0.00557) genes, which all have central roles in the development of brain cortex and formation of axons. Mutations in the LAMC3 are suggested to cause malformations of occipital cortical development in humans [46]. We speculate that these genes could have been affected by selection targeting at behavioral traits such as a modest temperament during domestication.
Sensory perception
We observed signals for selection targeting several sensory functions including olfaction and taste. The putative sweeps on BTA7 and BTA29 contain four clusters of olfactory receptor (OR) family genes (see Table 1). Olfactory receptors detect and identify a wide range of odors and chemosensory stimuli, a necessity to find food, detect mates and offspring, recognize territories and avoid danger (for review see [47]). OR genes are shown to have been under selection in humans [48] and domesticated animals including dog [49], swine [50] and cattle [7]. They are also reported to be duplicated within the bovine genome [51] suggesting that they may be under strong selection for newly evolving functions.
Tab. 1. A partial list of candidate regions revealed by both iHS and CLR analyses. 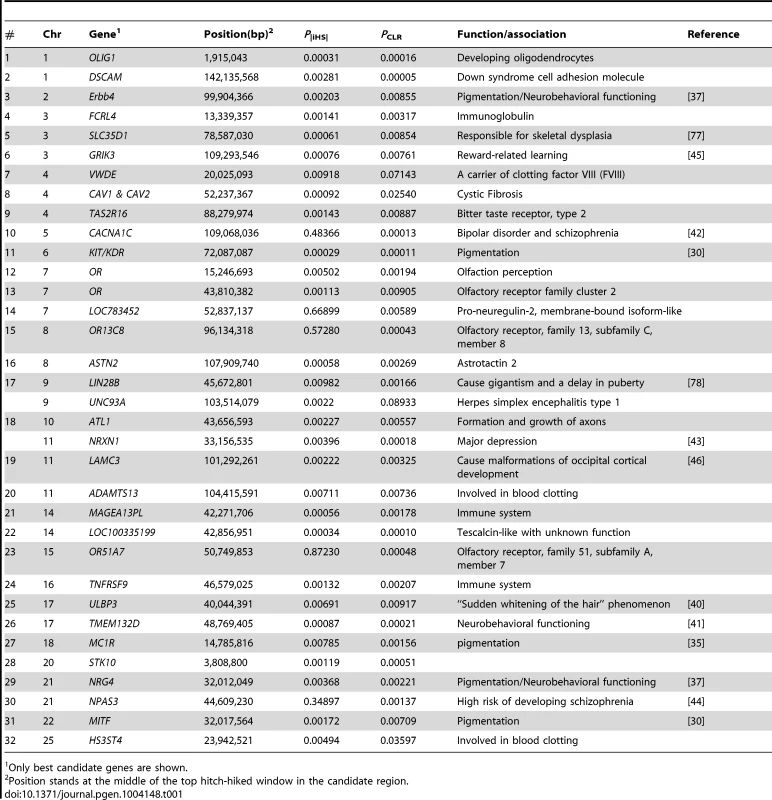
Only best candidate genes are shown. Another candidate selective sweep was localized within a bitter taste receptor gene (TAS2R16, PiHS = 0.00143, PCLR = 0.00887) that enables animals to properly distinguish food sources and prevent them from ingesting potentially harmful compounds such as noxious defense compounds produced by plants [52]. It has been argued that elimination of the need to search for food in wild animals after their domestication as well as adaptation to new dietary habits may relax the evolutionary constraint acting on these genes [53]. Selection has possibly targeted TAS2R16 as part of the new dietary habits emerging during cattle domestication.
Immune system and genetic disorders
Infectious diseases have been dominant threats to survival; therefore natural selection is expected to act strongly on innate immunity genes. Among the top selection candidates in our list are MAGEA13P-like (PiHS = 0.00056, PCLR = 0.00178) a member of melanoma-associated antigen family (see for instance Figure S7), FCRL4 (PiHS = 0.00141, PCLR = 0.00317) an immunoglobulin receptor gene, UNC93A (PiHS = 0.00220, PCLR = 0.08933), associated with Herpes simplex encephalitis type 1, and finally TNFRSF9 (PiHS = 0.00132, PCLR = 0.00207) induced by lymphocyte activation gene. Other noteworthy genes in our list are CAV1 and CAV2 (PiHS = 0.00092, PCLR = 0.02540) involved in Cystic Fibrosis, DSCAM (PiHS = 0.00281, PCLR = 0.00005) implicated in Down syndrome, SLC35D1 (PiHS = 0.00061, PCLR = 0.00854) responsible for skeletal dysplasia and a strong signal on Tescalin like gene (PiHS = 0.00034, PCLR = 0.00010) with unknown function (Figure S8).
Blood coagulation
We found three genes annotated with blood clotting functions in the region of selection signals. The von Willebrand factor D and EGF domains (VWDE, PiHS = 0.00918, PCLR = 0.07143) a carrier of clotting factor VIII (FVIII), ADAMTS13 (PiHS = 0.00711, PCLR = 0.00736) that cleaves VWDE, and heparan sulfate (glucosamine) 3-O-sulfotransferase 4 (HS3ST4, PiHS = 0.00494, PCLR = 0.03597) implicated in negative regulation of blood coagulation show signals of selection. This corresponds with some reports on primates along with human data suggesting potential signatures of positive selection for genes involved in blood clotting pathways [54], [55]. Further research on this group of genes would be required to address potential adaptation of blood coagulation genes in cattle.
There is a growing number of genome-wide scans for detecting historical positive selection in cattle and other farm animals. Previous studies in cattle however, have used low resolution panels of ascertained SNPs, mostly based on inter-population comparisons of site frequencies [18], [19], [20], [21], [22]. The notable candidate genes reported in these studies include GHR, PDGFRA, KIT and MC1R in association with body size and morphology traits in cattle. These signals generally differ from those reported by the Bovine HapMap consortium [7]. The most recent study based on 700k bovine array employed a composite framework that combines P-values from different tests across multiple breeds [56]. While the polymorphism content in both KIT and MC1R regions were under-represented for conducting an efficient selection scan, we found a poor overlap genome wide in comparison to our results. Besides different marker density and populations in both studies, the differences in the statistical approaches used could explain the discrepancy. The suggested statistical tests applied in this study recover selective events from different time periods and/or for different stages of the selective sweep (e.g., CLR vs. iHS). Furthermore, a selective sweep might be specific for one population and may not appear in other populations. Thus, combining results of multiple tests and across populations may mask real signals which could partly explain the low concordance with our single-breed single-test results.
Validating putative sweeps with GWAS
If our candidate regions are in fact enriched for genes affected by selection related to domestication traits, they should overlap with regions identified in QTL mapping studies on these traits. This hypothesis was verified in an exemplary fashion for the complex trait coat color. Coat color in cattle is usually regarded to be a trait controlled by few loci of large effect [30]. There is a high degree of variation in color apparent within Fleckvieh population. Fleckvieh animals are phenotypically characterized by being red, spotted or not, and having white legs and a white head, animals with red head occasionally occur but are considered as a deviation from the breed standard. We used the coat color traits recorded respectively as the proportion of daughters of bulls ‘without spotting’ and with ‘red head’ to validate putative sweeps for coloration phenotypes (Figure 4).
Fig. 4. Fleckvieh animals with different coat coloring phenotypes. 
(A) without spot, (B) spotted and (C) red head. The figures were kindly supplied by BAYERN-GENETIK GmbH (http://www.fleckvieh.de). We performed a Genome Wide Association Study (GWAS) on 3602 animals for which genotypes of 15,182,131 SNPs were imputed (see Material and Methods). The GWAS revealed eight SNPs with large effect on the ‘proportion of daughters without spotting’ (Figure 5B1). The SNP with the most significant effect is a non-genic variant in the vicinity of the MITF (P = 2.65e-58) gene on BTA22. Another highly significant but non-genic SNP coincides with a selection candidate region (e.g., Figure 3C) covering the KIT and KDR (P = 2.46e-44) loci. There are also two significant signals, respectively next to endothelin 3 (EDN3) (P = 2.42e-36) and an uncharacterized protein at the proximity of the membrane metallo-endopeptidase (MME) gene (P = 5.11e-14). EDN3 plays a significant role during the early development of melanocytes in their response to ultraviolet radiation, and in pathological conditions including melanoma. Severe pigmentation defects of mutations in EDN3 in mice, human and chicken are well-described (for review see [57]). In human models, MME is expressed at the surface of melanoma cells and are involved in the regulation of melanogenesis [58]. Although these SNPs were the top association signals for each of the identified QTL, distinguishing causal variants from nearby neutral loci may be the most difficult issue, as those variants possibly stay in LD with the actual selected locus that may produce similar signals due to genetic hitch-hiking.
Fig. 5. The visualization of the signals revealed by association analyses for coat coloring traits. 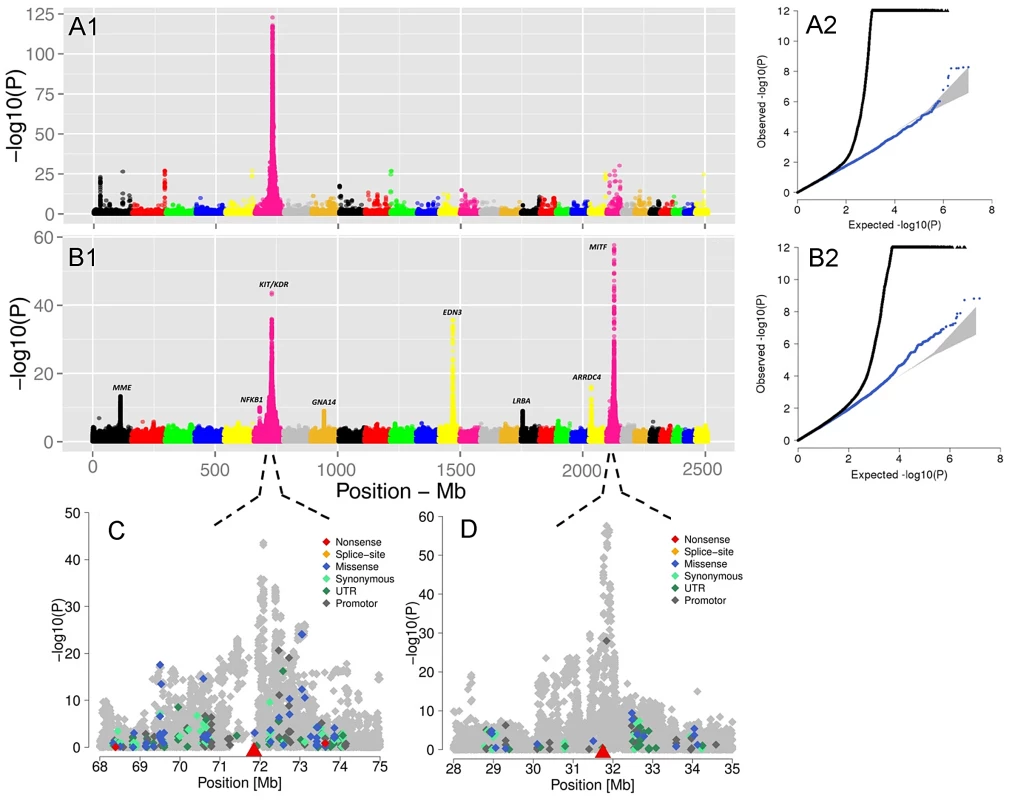
GWAS are presented for the proportion of daughters with red head (A1) and the proportion of daughters without spotting (B1) based on 15,182,131 imputed variants in 3062 Fleckvieh animals. In (B1) the largest effects emerge from eight SNPs summarized in Table 2 with MITF (P = 2.65e-58) and KIT/KDR (P = 2.46e-44) at the top. Together, KIT/KDR and MITF explained 36.25% of the residual variance of the trait in the studied population. A2 and B2 are the corresponding quantile-quantile plots. Shown in blue is the quantile-quantile plot resulting from removal of all SNPs in the region of significant genes listed in Table 2, for both traits. The shaded area is the 95% concentration band under the null hypothesis of no association. Panels C and D are detailed overviews of the associated regions on BTA6 and BTA22, respectively. Variants in the promoter (defined to encompass 1,000 bp upstream of the transcription start), in the untranslated regions (UTR) and in the amino-acid coding region are highlighted with different color. The red triangles indicate the genomic positions of KIT and MITF genes. Genomic relationship matrices were built separately for each chromosome and QTL using imputed variants. The phenotypic variation explained by each chromosome/QTL was then estimated with the effects of all chromosomes/QTL fitted simultaneously using GCTA [59]. All together, the imputed variants explained 82.37% of the phenotypic variation (i.e. 94% of the heritability) (Figure S9). Together, the eight identified QTL explained 49.76% of the phenotypic variation (i.e. 56.8% of the heritability).
The GWAS for the ‘proportion of daughters with red head’ was modeled in the same way. Visualizing of results shows a different genetic control for the red head phenotype when is compared to the spotting. The strongest GWAS revealed signal (P = 6.8e-125) was on the KIT locus on BTA6 (Figure 5A1), which alone explained 34.81% of the total variation of the trait (Table 2).
Tab. 2. A descriptive summary of GWA studies for coat color variation in Fleckvieh animals. 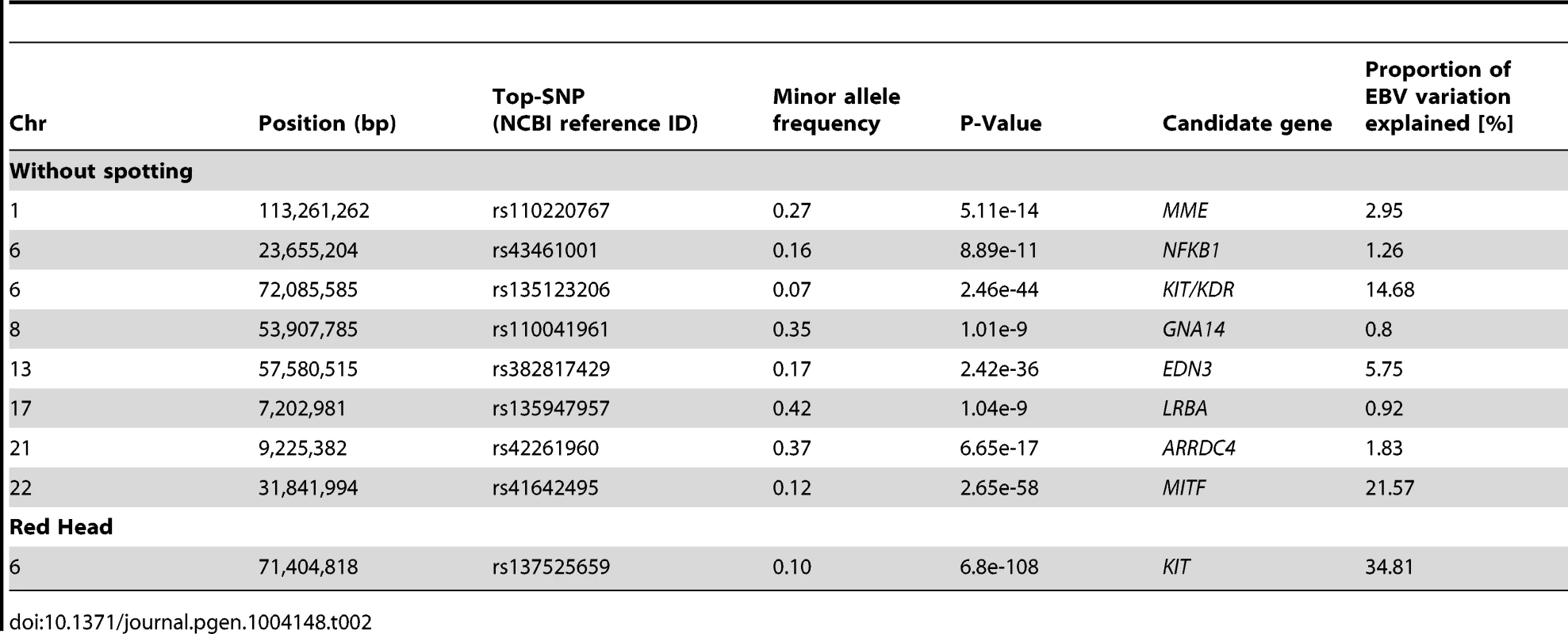
Previous GWAS on coat color in cattle have identified polymorphism in MC1R, KIT and MITF [35], . In both GWA analyses in this study, the KIT locus is associated, but MITF, EDN3 and MME are related only with spotting phenotype. This observation suggests that a different genetic mechanism regulates these phenotypes in the Fleckvieh breed. Recently, Pausch et al. [60] studied the genetics controlling the peculiar pigmentation surrounding the eyes in Fleckvieh animals using the 700K SNP panel. They found a strong association between MITF and KIT genes with the UV-protective eye area pigmentation in Fleckvieh cattle, but no association with EDN3 and MME genes. Comparing GWAS results from coat spotting, red head, and eye area pigmentation reveals a complex genetic background for the different pigmentation phenotypes in the Fleckvieh population.
To test for an overlap between the selection candidates and the QTL study, we performed two randomization experiments on coat spotting phenotype using either single SNPs or window-based estimates of |iHS| versus P-values from GWAS (see Methods). Both randomizations revealed no single randomized dataset more extreme than the real data.
While there is no association detected for MC1R gene in the GWAS, selection signature analyses suggests that there has been a selective sweep at or near the MC1R gene on BTA18 (Figure S2A). MC1R variants have been shown to alter pigment synthesis in a range of species, as well as coat color spotting in pigs [61]. At least three major alleles exist in cattle MC1R, the E+ wild type, ED dominant black locus, and e recessive red locus [35]. Since the red variant is fixed in Fleckvieh population, no [possible] association with coat spotting or red head can be traced through GWAS. This also explains why the locus is not very extreme when explored by the iHS statistic (see Figure S2A). We postulate that red hair has been under very recent selection (e.g., human driven selection during breed formation) resulting in fixation of the red variant in the Fleckvieh cattle, while simultaneously fixing alleles at nearby hitch-hiked loci. To validate this hypothesis, we calculated SNP-specific FST [62] for 1,173 SNPs on BTA18 which have been genotyped in 2,084 Holstein-Friesian (black and white coat color) and 2,539 Fleckvieh (red and white coat color) animals using 50K SNP arrays. This revealed a strong differentiation hit located at 14.42 Mb in immediate vicinity to MC1R, further supporting findings of selection analyses (Figure S2B). Therefore, our results exemplify how a historical selective sweep that underwent fixation can be localized by employing a population genetic approach.
We further conducted GWA studies for somatic cell count (SCC, Figure 6A) and body size (Figure S10) based on daughter-derived phenotypes (estimated breeding values, EBVs) for these traits. In cattle breeding schemes, somatic cell count measured as the log number of somatic cells per ml of milk is widely used as an indicator for incidence of mastitis. The GWAS for SCC revealed strong association on BTA22 nearby the LTF gene encoding lactotransferrin (Table S5). Due to its antimicrobial and anti-inflammatory activity [63], LTF is a strong candidate for mastitis resistance. The most significant SNP is located ∼8 Kb upstream of the translation start of LTF and might affect expression of LTF during mammary gland infections [64]. Another QTL is located on BTA3 and the most significant SNP (rs386094483, P = 7.95e-11) is a Lysine-to-Arginine substitution in the DC-STAMP domain containing 1-encoding gene (DCST1, p.K113R, Chr3 : 15613949) (Figure 6C). DCST1 plays an important role in the initiation of the immune system by antigen processing [65], [66], [67]. The affected amino acid is highly conserved among species and is a strong candidate causal mutation for the somatic cell count in cattle (Figure 6D).
Fig. 6. The visualization of the signals revealed by association analyses for SCC trait. 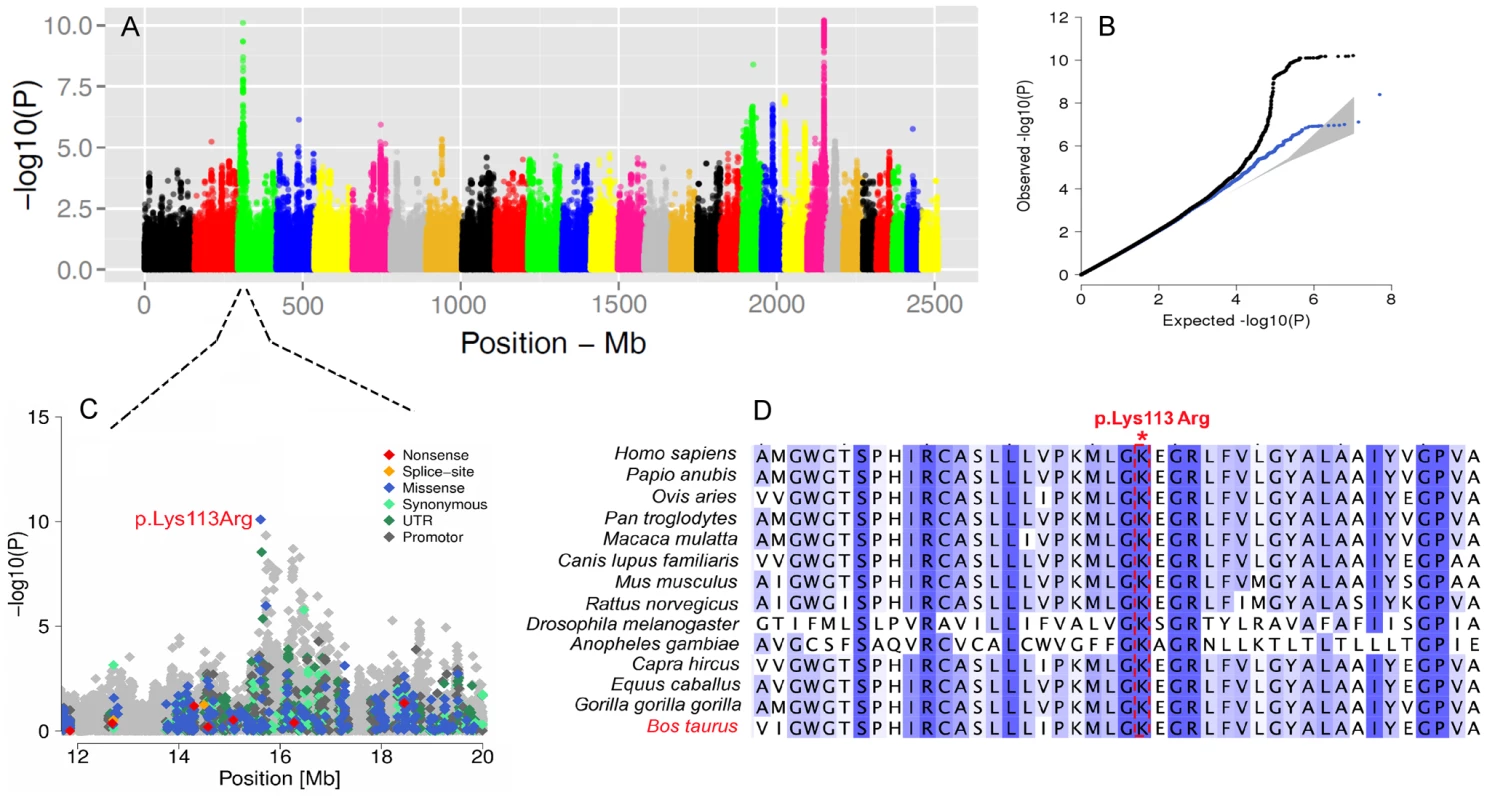
Manhattan plot presents the association of 15'182'131 imputed SNPs with the “somatic cell count” trait in 3602 Fleckvieh animals (A). Panel B represents corresponding quantile-quantile plot. Shown in blue is the quantile-quantile plot resulting from excluding SNPs in the region of significant genes. The shaded area represents 95% concentration band under the null hypothesis of no association. Panel C displays the associated region on BTA3 with a higher resolution. Variants in the promoter (defined to encompass 1,000 bp upstream of the transcription start), in the untranslated regions (UTR) and in the amino-acid coding region are highlighted with different color. Panel D provides a multi species alignment of DC-STAMP domain containing 1 encoded by DCST1 gene. The GWAS for body size revealed three QTLs. One example is the PLAG1 region on BTA14 which is significantly associated with body size (P = 1.5e-27). This region was already shown to affect growth-related traits in several species including cattle [68]. This QTL is also associated with the calving difficulties and an increased stillbirth rate in cattle, probably resulting from an enhanced fetal growth [69].
Table S5 presents a descriptive summary of the most significant SNPs and the proportion of EBV variance attributable to the five identified QTL regions. Altogether, the imputed variants explain 81.35% and 80.73% of the variation of EBV, respectively for the SCC and body size traits. A schematic illustration of chromosomal contributions for the total variance of these traits are represented respectively, in Figures S11 and S12.
In contrast to regions associated with color variation QTLs, these regions show no overlap with selection candidates. One possible explanation is that most selection affecting body size and somatic cell count has affected alleles that are no longer segregating in this breed. This raises the question as to why the currently segregating alleles affecting these traits do not show strong signals of selection. Possibly, the alleles that are still segregating, even after intense artificial selection during domestication, may have negative pleiotropic effects preventing them from increasing in frequency in the population. Additionally, selection is likely to have affected standing variation. If the selected mutations were segregating on multiple different haplotypes before selection started, both the iHs and the CLR statistic may have limited power to detect selection.
We present the first comprehensive study for localizing signatures of past selection in cattle based on full re-sequencing data. 106 candidate regions were identified containing genes with biological functions involved in blood clotting, immune-defense functions, pigmentation pattern, sensory perceptions and neurobehavioral functioning. The detection of genes related to pigmentation is not surprising since a specific coat color pattern, such as red (in various shadings) coat with white head, is constitutive for the breed definition of Fleckvieh and therefore fundamental for the breed formation process. Using the same samples, we also performed a coat color GWA study, and show that there is a strong overlap between genes identified in the GWAS and in the selection scan. As demonstrated for the gene MC1R, selection signatures can be detected in regions where anthropogenic selection has fixed the desired allele and, consequently, GWAS fails. This illustrates the potential for population genetic techniques to identify genomic regions relating to phenotypes of importance to breeders. Comparing GWAS results for different traits also provides further evidence regarding the complexity of genetics underlying coat coloring in cattle.
Materials and Methods
Ethics statement
DNA needed for the study was previously extracted from commercial AI bull semen straws. No ethics statement is thus required.
Sequenced-based imputation
For the purpose of this study we used data from Jansen et al. [23]. Briefly, it consists low to medium coverage (∼7.4-fold) sequence of the entire genomes of 43 key and contemporary animals representing ∼69% of the genetic diversity of the current German Fleckvieh population.
The sequence panel consisting of 15,182,131 SNPs with an average inter-marker space equal to 178±115 bp was used for a two-step imputation using default setting in Beagle [70] and Minimac [71], respectively. Imputation started from a medium density panel (50K SNPs) bridged by a high density panel (700K SNPs) to the full sequences using 43 reference animals. We evaluated the accuracy of sequence-based imputation for chromosomes 5, 15 and 25 within the high density panel (700K SNPs). Genotypes for randomly selected 66% of the SNPs were retained, while genotypes for the remaining SNPs were masked to mimic missing genotypes. Those were imputed using Beagle and Minimac (see above) based on sequence-derived genotypes of 43 re-sequenced animals. Imputation accuracy was assessed as the correlation between array-derived and imputed genotypes. This approach yielded high imputation accuracy for frequent alleles (e.g., MAF >5%) (Figure S13). However, the number of re-sequenced animals (n = 43) might not be sufficient for imputing low-frequency variants with a sophisticated accuracy, which agrees with a previous report in cattle [72].
The individual call-rate was >95% for all animals genotyped with SNP arrays. After quality control (call-rate per SNP >95%, minor allele frequency >0.5%, no significant deviation of the Hardy-Weinberg-Equilibrium (P>10-6), known chromosomal position), the medium and high density dataset comprised genotypes for 39,304 (n = 2,309) and 645,189 SNPs (n = 1,293), respectively.
Linkage disequilibrium
We quantified LD using the squared correlation coefficients (r2) between pairs of SNPs. Evaluations of SNP to SNP pairwise r2 were completed based on the panels of 15,000 SNPs randomly sampled across two segments of each 5 Mb on the chromosomes 5, 10, 15, 20 and 26. We then compared measures of r2 to those of 50K and 700K bovine arrays across the corresponding chromosomes.
Detecting positive selection
Evidence of positive selection was investigated through multiple statistics: We performed the Composite Likelihood Ratio test (CLR) using information from allele frequencies to detect a completed sweep [11]. Briefly, CLR relies on identifying skews in the allele frequency spectrum toward excess of rare and frequent alleles. To infer ongoing sweeps we employed the integrated Haplotype Homozygosity Score (iHS) that explores the structure of haplotype and essentially indicates unusually long haplotypes carrying the ancestral and derived allele [12]. Single site values for iHS were averaged in non-overlapping windows of 40 Kb across the genome resulting in a total of 62'196 windows. Window size was adapted based on the extent of LD as discussed above (Figure 2). The variance of SNP statistics within 40 kb windows (i.e., Var|iHS| = 0.27) was significantly smaller than that among randomly selected SNPs (i.e., Var|iHS| = 0.35), confirming that the windows effectively grouped SNPs with more similar statistic values. Figure S14 visualizes the number of SNPs distributed across sliding windows. To produce comparable results of the composite likelihood ratio (CLR) test, which is a multi-locus statistic, the grid size was taken as 40 kb. This resulted in 62,788 local CLR values across the genome. The empirical P-values were generated by genome wide ranking of |iHS| and CLR values. The list of |iHS| and CLR scores for all windows are available in supporting material as Dataset S1 and Dataset S2, respectively. The iHS metric was calculated using the R package ‘rehh’ [73]. For estimating CLRs we used ‘sweepfinder’ [11] with a background allele frequency spectrum calibrated genome-wide. We further used custom programs to calculate the ‘observed heterozygosity’ (Het), which should be reduced in regions affected by a sweep [74], Tajima's D [8], Fay and Wu H statistic [9], and the number of Segregating Loci (nSL), a recently developed statistic related to iHS in Rasmus Nielsen's Lab. The metrics were estimated using different window size to explore the sensitivity to the choice of window. We annotated candidate genomic regions by aligning the positions to the bovine genome sequence assembly build 6.1, to reveal genes and ESTs located in the respective region.
Randomization test
To test if the overlap between a selection scan and a GWAS is significantly different from that expected at random, we performed permutation tests. We generated 10,000 simulated data sets by permuting p-values among either single SNPs or windows. For example, we calculated empirical p-values for windows based on |iHS|, then counted the number of instances where a GWAS association (p-value<10−6, Bonferroni corrected threshold) overlaps a high-scoring (top 1%) selection window. The observed value of the overlap statistic was then compared to the distribution of overlap statistics in the permuted data sets.
Sequenced-based association study
After imputation, genotypes of 15,182,131 SNPs in 3602 individuals were used for a GWAS for four different traits: the square-root transformed proportion of daughters without spotting and with red head and estimated breeding values for somatic cell counts and body size. EMMAX [75] was used to fit the model , where is the vector of phenotypes, is the SNP effect, is a design matrix of allele dosages for the imputed SNPs, is the additive genetic effect , where is the additive genetic variance, is the realized genomic relationship matrix estimated using genotype information [76], and is the random residual term.
Supporting Information
Zdroje
1. Bradley DG, Cunningham EP (1999) Genetic aspects of domestication. In: Fries R, Ruvinsky A, Eds. The Genetics of Cattle. pp 15–31.
2. BollonginoR, BurgerJ, PowellA, MashkourM, VigneJ-D, et al. (2012) Modern Taurine Cattle Descended from Small Number of Near-Eastern Founders. Mol Biol Evol 29 : 2101–2104 doi:10.1093/molbev/mss092
3. LoftusRT, MacHughDE, BradleyDG, SharpPM, CunninghamP (1994) Evidence for two independent domestications of cattle. Proc Natl Acad Sci U S A 91 : 2757–2761.
4. TroyCS, MacHughDE, BaileyJF, MageeDA, LoftusRT, et al. (2001) Genetic evidence for Near-Eastern origins of European cattle. Nature 410 : 1088–1091 doi:10.1038/35074088
5. FAO (2007) The state of the world's animal genetics resources for food and agriculture. ftp://ftp.fao.org/docrep/fao/010/a1250e/a1250e02.pdf
6. Zeder MA.”Pathways to Animal Domestication (Melinda A. Zeder, 2012),” in BoneCommons, Item #11838, http://alexandriaarchive.org/bonecommons/items/show/1838 (accessed April 5, 2013).
7. The Bovine HapMap Consortium (2009) GibbsRA, TaylorJF, Van TassellCP, BarendseW, et al. (2009) Genome-Wide Survey of SNP Variation Uncovers the Genetic Structure of Cattle Breeds. Science 324 : 528–532 doi:10.1126/science.1167936
8. TajimaF (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 : 585–595.
9. FayJC, WuC-I (2000) Hitchhiking Under Positive Darwinian Selection. Genetics 155 : 1405–1413.
10. SabetiPC, ReichDE, HigginsJM, LevineHZP, RichterDJ, et al. (2002) Detecting recent positive selection in the human genome from haplotype structure. Nature 419 : 832–837 doi:10.1038/nature01140
11. NielsenR, WilliamsonS, KimY, HubiszMJ, ClarkAG, et al. (2005) Genomic scans for selective sweeps using SNP data. Genome Res 15 : 1566–1575 doi:10.1101/gr.4252305
12. VoightBF, KudaravalliS, WenX, PritchardJK (2006) A Map of Recent Positive Selection in the Human Genome. PLoS Biology 4: e72 doi:10.1371/journal.pbio.0040072
13. RubinC-J, ZodyMC, ErikssonJ, MeadowsJRS, SherwoodE, et al. (2010) Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464 : 587–591 doi:10.1038/nature08832
14. NielsenR (2000) Estimation of Population Parameters and Recombination Rates From Single Nucleotide Polymorphisms. Genetics 154 : 931–942.
15. KuhnerMK, BeerliP, YamatoJ, FelsensteinJ (2000) Usefulness of Single Nucleotide Polymorphism Data for Estimating Population Parameters. Genetics 156 : 439–447.
16. WakeleyJ, NielsenR, Liu-CorderoSN, ArdlieK (2001) The Discovery of Single-Nucleotide Polymorphisms–and Inferences about Human Demographic History. Am J Hum Genet 69 : 1332–1347 doi:10.1086/324521
17. LynnDJ, FreemanAR, MurrayC, BradleyDG (2005) A Genomics Approach to the Detection of Positive Selection in Cattle:. Genetics 170 : 1189–1196 doi:10.1534/genetics.104.039040
18. MacEachernS, HayesB, McEwanJ, GoddardM (2009) An examination of positive selection and changing effective population size in Angus and Holstein cattle populations (Bos taurus) using a high density SNP genotyping platform and the contribution of ancient polymorphism to genomic diversity in Domestic cattle. BMC Genomics 10 : 181 doi:10.1186/1471-2164-10-181
19. FloriL, FritzS, JaffrézicF, BoussahaM, GutI, et al. (2009) The Genome Response to Artificial Selection: A Case Study in Dairy Cattle. PLoS ONE 4: e6595 doi:10.1371/journal.pone.0006595
20. StellaA, Ajmone-MarsanP, LazzariB, BoettcherP (2010) Identification of Selection Signatures in Cattle Breeds Selected for Dairy Production. Genetics 185 : 1451–1461 doi:10.1534/genetics.110.116111
21. QanbariS, PimentelECG, TetensJ, ThallerG, LichtnerP, et al. (2010b) A genome-wide scan for signatures of recent selection in Holstein cattle. Anim Genet 41 : 377–389 doi:10.1111/j.1365-2052.2009.02016.x
22. QanbariS, GianolaD, HayesB, SchenkelF, MillerS, et al. (2011) Application of site and haplotype-frequency based approaches for detecting selection signatures in cattle. BMC Genomics 12 : 318 doi:10.1186/1471-2164-12-318
23. JansenS, AignerB, PauschH, WysockiM, EckS, et al. (2013) Assessment of the genomic variation in a cattle population by re-sequencing of key animals at low to medium coverage. BMC Genomics 14 : 446 doi:10.1186/1471-2164-14-446
24. SargolzaeiM, SchenkelFS, JansenGB, SchaefferLR (2008) Extent of linkage disequilibrium in Holstein cattle in North America. J Dairy Sci 91 : 2106–2117 doi:10.3168/jds.2007-0553
25. QanbariS, PimentelECG, TetensJ, ThallerG, LichtnerP, et al. (2010a) The pattern of linkage disequilibrium in German Holstein cattle. Anim Genet 41 : 346–356 doi:10.1111/j.1365-2052.2009.02011.x
26. BoykoAR, WilliamsonSH, IndapAR, DegenhardtJD, HernandezRD, et al. (2008) Assessing the Evolutionary Impact of Amino Acid Mutations in the Human Genome. PLoS Genet 4: e1000083 doi:10.1371/journal.pgen.1000083
27. LiY, VinckenboschN, TianG, Huerta-SanchezE, JiangT, et al. (2010) Resequencing of 200 human exomes identifies an excess of low-frequency non-synonymous coding variants. Nat Genet 42 : 969–972 doi:10.1038/ng.680
28. PritchardJK, PrzeworskiM (2001) Linkage Disequilibrium in Humans: Models and Data. Am J Hum Genet 69 : 1–14.
29. HuangDW, ShermanBT, LempickiRA (2008) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 4 : 44–57 doi:10.1038/nprot.2008.211
30. HayesBJ, PryceJ, ChamberlainAJ, BowmanPJ, GoddardME (2010) Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits. PLoS Genet 6: e1001139 doi:10.1371/journal.pgen.1001139
31. HaaseB, BrooksSA, TozakiT, BurgerD, PoncetP-A, et al. (2009) Seven novel KIT mutations in horses with white coat colour phenotypes. Animal Genetics 40 : 623–629 doi:10.1111/j.1365-2052.2009.01893.x
32. RubinC-J, MegensH-J, BarrioAM, MaqboolK, SayyabS, et al. (2012) Strong signatures of selection in the domestic pig genome. PNAS 109 : 19529–19536 doi:10.1073/pnas.1217149109
33. BaxterLL, HouL, LoftusSK, PavanWJ (2004) Spotlight on Spotted Mice: A Review of White Spotting Mouse Mutants and Associated Human Pigmentation Disorders. Pigment Cell Research 17 : 215–224 doi:10.1111/j.1600-0749.2004.00147.x
34. HouL, PanthierJJ, ArnheiterH (2000) Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development 127 : 5379–5389.
35. KlunglandH, VageDI, Gomez-RayaL, AdalsteinssonS, LienS (1995) The role of melanocyte-stimulating hormone (MSH) receptor in bovine coat color determination. Mammalian Genome 6 : 636–639 doi:10.1007/BF00352371
36. ChoiJ, YoungJAT, CallawayEM (2010) Selective viral vector transduction of ErbB4 expressing cortical interneurons in vivo with a viral receptor–ligand bridge protein. PNAS 107 : 16703–16708 doi:10.1073/pnas.1006233107
37. PickrellJK, CoopG, NovembreJ, KudaravalliS, LiJZ, et al. (2009) Signals of recent positive selection in a worldwide sample of human populations. Genome Res 19 : 826–837 doi:10.1101/gr.087577.108
38. StefanssonH, PeturssonH, SigurdssonE, SteinthorsdottirV, BjornsdottirS, et al. (2002) Neuregulin 1 and Susceptibility to Schizophrenia. The American Journal of Human Genetics 71 : 877–892 doi:10.1086/342734
39. MeiL, XiongW-C (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9 : 437–452 doi:10.1038/nrn2392
40. PetukhovaL, DuvicM, HordinskyM, NorrisD, PriceV, et al. (2010) Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 466 : 113–117 doi:10.1038/nature09114
41. ErhardtA, AkulaN, SchumacherJ, CzamaraD, KarbalaiN, et al. (2012) Replication and meta-analysis of TMEM132D gene variants in panic disorder. Transl Psychiatry 2: e156 doi:10.1038/tp.2012.85
42. NyegaardM, DemontisD, FoldagerL, HedemandA, FlintTJ, et al. (2010) CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry 15 : 119–121 doi:10.1038/mp.2009.69
43. BrownSM, ClapcoteSJ, MillarJK, TorranceHS, AndersonSM, et al. (2011) Synaptic modulators Nrxn1 and Nrxn3 are disregulated in a Disc1 mouse model of schizophrenia. Mol Psychiatry 16 : 585–587 doi:10.1038/mp.2010.134
44. YuL, ArbezN, NuciforaLG, SellGL, DelisiLE, et al. (2013) A mutation in NPAS3 segregates with mental illness in a small family. Mol Psychiatry doi:10.1038/mp.2012.192
45. MinelliA, ScassellatiC, BonviciniC, PerezJ, GennarelliM (2009) An association of GRIK3 Ser310Ala functional polymorphism with personality traits. Neuropsychobiology 59 : 28–33 doi:10.1159/000202827
46. BarakT, KwanKY, LouviA, DemirbilekV, SaygıS, et al. (2011) Recessive LAMC3 mutations cause malformations of occipital cortical development. Nat Genet 43 : 590–594 doi:10.1038/ng.836
47. AcheBW, YoungJM (2005) Olfaction: Diverse Species, Conserved Principles. Neuron 48 : 417–430 doi:10.1016/j.neuron.2005.10.022
48. Moreno-EstradaA, CasalsF, Ramírez-SorianoA, OlivaB, CalafellF, et al. (2008) Signatures of Selection in the Human Olfactory Receptor OR5I1 Gene. Mol Biol Evol 25 : 144–154 doi:10.1093/molbev/msm240
49. ChenR, IrwinDM, ZhangY-P (2012) Differences in Selection Drive Olfactory Receptor Genes in Different Directions in Dogs and Wolf. Mol Biol Evol 29 : 3475–3484 doi:10.1093/molbev/mss153
50. GroenenMAM, ArchibaldAL, UenishiH, TuggleCK, TakeuchiY, et al. (2012) Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491 : 393–398 doi:10.1038/nature11622
51. ElsikCG, TellamRL, WorleyKC (2009) The Genome Sequence of Taurine Cattle: A Window to Ruminant Biology and Evolution. Science 324 : 522–528 doi:10.1126/science.1169588
52. DongD, JonesG, ZhangS (2009) Dynamic evolution of bitter taste receptor genes in vertebrates. BMC Evolutionary Biology 9 : 12 doi:10.1186/1471-2148-9-12
53. LucaF, PerryGH, Di RienzoA (2010) Evolutionary Adaptations to Dietary Changes. Annual Review of Nutrition 30 : 291–314 doi:10.1146/annurev-nutr-080508-141048
54. KosiolC, VinařT, da FonsecaRR, HubiszMJ, BustamanteCD, et al. (2008) Patterns of Positive Selection in Six Mammalian Genomes. PLoS Genet 4: e1000144 doi:10.1371/journal.pgen.1000144
55. AthanasiadisG, EstebanE, Gayà -VidalM, DugoujonJ-M, MoschonasN, et al. (2010) Different Evolutionary Histories of the Coagulation Factor VII Gene in Human Populations?: Evolutionary Patterns of the F7 Gene. Annals of Human Genetics 74 : 34–45 doi:10.1111/j.1469-1809.2009.00557.x
56. UtsunomiyaYT, Pérez O'BrienAM, SonstegardTS, Van TassellCP, do CarmoAS, et al. (2013) Detecting Loci under Recent Positive Selection in Dairy and Beef Cattle by Combining Different Genome-Wide Scan Methods. PLoS ONE 8: e64280 doi:10.1371/journal.pone.0064280
57. Saldana-CaboverdeA, KosL (2010) Roles of endothelin signaling in melanocyte development and melanoma. Pigment Cell & Melanoma Research 23 : 160–170 doi:10.1111/j.1755-148X.2010.00678.x
58. AberdamE, AubergerP, OrtonneJ-P, BallottiR (2000) Neprilysin, a Novel Target for Ultraviolet B Regulation of Melanogenesis Via Melanocortins. Journal of Investigative Dermatology 115 : 381–387 doi:10.1046/j.1523-1747.2000.00075.x
59. YangJ, LeeSH, GoddardME, VisscherPM (2011) GCTA: A Tool for Genome-wide Complex Trait Analysis. The American Journal of Human Genetics 88 : 76–82 doi:10.1016/j.ajhg.2010.11.011
60. PauschH, WangX, JungS, KrogmeierD, EdelC, et al. (2012) Identification of QTL for UV-Protective Eye Area Pigmentation in Cattle by Progeny Phenotyping and Genome-Wide Association Analysis. PLoS ONE 7: e36346 doi:10.1371/journal.pone.0036346
61. KijasJM, MollerM, PlastowG, AnderssonL (2001) A frameshift mutation in MC1R and a high frequency of somatic reversions cause black spotting in pigs. Genetics 158 : 779–785.
62. AkeyJM, ZhangG, ZhangK, JinL, ShriverMD (2002) Interrogating a high-density SNP map for signatures of natural selection. Genome Res 12 : 1805–1814 doi:10.1101/gr.631202
63. NuijensJH, Berkel PHCvan, SchanbacherFL (1996) Structure and biological actions of lactoferrin. J Mammary Gland Biol Neoplasia 1 : 285–295 doi:10.1007/BF02018081
64. JensenK, GüntherJ, TalbotR, PetzlW, ZerbeH, et al. (2013) Escherichia coli - and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genomics 14 : 36 doi:10.1186/1471-2164-14-36
65. HartgersFC, VissersJL, LoomanMW, van ZoelenC, HuffineC, et al. (2000) DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur J Immunol 30 : 3585–3590 doi:#;#;;10.1002/1521-4141(200012)30 : 12<3585::AID-IMMU3585>3.0.CO;2-Y
66. SawataniY, MiyamotoT, NagaiS, MaruyaM, ImaiJ, et al. (2008) The role of DC-STAMP in maintenance of immune tolerance through regulation of dendritic cell function. Int Immunol 20 : 1259–1268 doi:10.1093/intimm/dxn082
67. SaneckaA, AnsemsM, ProsserAC, DanielskiK, WarnerK, et al. (2011) DC-STAMP knock-down deregulates cytokine production and T-cell stimulatory capacity of LPS-matured dendritic cells. BMC Immunol 12 : 57 doi:10.1186/1471-2172-12-57
68. KarimL, TakedaH, LinL, DruetT, AriasJAC, et al. (2011) Variants modulating the expression of a chromosome domain encompassing PLAG1 influence bovine stature. Nat Genet 43 : 405–413 doi:10.1038/ng.814
69. PauschH, FlisikowskiK, JungS, EmmerlingR, EdelC, et al. (2011) Genome-wide association study identifies two major loci affecting calving ease and growth-related traits in cattle. Genetics 187 : 289–297 doi: 10.1534/genetics.110.124057
70. BrowningBL, BrowningSR (2009) A Unified Approach to Genotype Imputation and Haplotype-Phase Inference for Large Data Sets of Trios and Unrelated Individuals. The American Journal of Human Genetics 84 : 210–223 doi:10.1016/j.ajhg.2009.01.005
71. HowieB, FuchsbergerC, StephensM, MarchiniJ, AbecasisGR (2012) Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet 44 : 955–959 doi:10.1038/ng.2354
72. PauschH, AignerB, EmmerlingR, EdelC, GötzK-U, et al. (2013) Imputation of high-density genotypes in the Fleckvieh cattle population. Genetics Selection Evolution 45 : 3 doi:10.1186/1297-9686-45-3
73. GautierM, VitalisR (2012) rehh: an R package to detect footprints of selection in genome-wide SNP data from haplotype structure. Bioinformatics 28 : 1176–1177 doi:10.1093/bioinformatics/bts115
74. SmithJM, HaighJ (1974) The hitch-hiking effect of a favourable gene. Genetics Research 23 : 23–35 doi:10.1017/S0016672300014634
75. KangHM, SulJH, ServiceSK, ZaitlenNA, KongS, et al. (2010) Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42 : 348–354 doi:10.1038/ng.548
76. HayesBJ, BowmanPJ, ChamberlainAC, VerbylaK, GoddardME (2009) Accuracy of genomic breeding values in multi-breed dairy cattle populations. Genetics Selection Evolution 41 : 51 doi:10.1186/1297-9686-41-51
77. HiraokaS, FuruichiT, NishimuraG, ShibataS, YanagishitaM, et al. (2007) Nucleotide-sugar transporter SLC35D1 is critical to chondroitin sulfate synthesis in cartilage and skeletal development in mouse and human. Nat Med 13 : 1363–1367 doi:10.1038/nm1655
78. ZhuH, ShahS, Shyh-ChangN, ShinodaG, EinhornWS, et al. (2010) Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat Genet 42 : 626–630 doi:10.1038/ng.593
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



