-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaReversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
Stress-induced changes of gene expression are crucial for survival of eukaryotic cells. Regulation at the level of translation provides the necessary plasticity for immediate changes of cellular activities and protein levels. In this study, we demonstrate that exposure to oxidative stress results in a quick repression of translation by deactivation of the aminoacyl-ends of all transfer-RNA (tRNA). An oxidative-stress activated nuclease, angiogenin, cleaves first within the conserved single-stranded 3′-CCA termini of all tRNAs, thereby blocking their use in translation. This CCA deactivation is reversible and quickly repairable by the CCA-adding enzyme [ATP(CTP):tRNA nucleotidyltransferase]. Through this mechanism the eukaryotic cell dynamically represses and reactivates translation at low metabolic costs.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003767
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003767Summary
Stress-induced changes of gene expression are crucial for survival of eukaryotic cells. Regulation at the level of translation provides the necessary plasticity for immediate changes of cellular activities and protein levels. In this study, we demonstrate that exposure to oxidative stress results in a quick repression of translation by deactivation of the aminoacyl-ends of all transfer-RNA (tRNA). An oxidative-stress activated nuclease, angiogenin, cleaves first within the conserved single-stranded 3′-CCA termini of all tRNAs, thereby blocking their use in translation. This CCA deactivation is reversible and quickly repairable by the CCA-adding enzyme [ATP(CTP):tRNA nucleotidyltransferase]. Through this mechanism the eukaryotic cell dynamically represses and reactivates translation at low metabolic costs.
Introduction
Environmental stress or suboptimal growth conditions reduce cell viability and put cells at risk. Cells maintain their internal homeostasis by adequate reprogramming of metabolic activities at all levels of gene expression, including chromatin remodeling, mRNA expression and degradation, translation and protein degradation. Given the considerable time needed to activate new genes and/or de novo synthesize mRNA, the translation of existing mRNAs provides the necessary plasticity for the cell to selectively and rapidly respond to stress [1], [2]. Translation is divided into three distinct phases: initiation, elongation and termination. Translation initiation, as a rate-limiting process, is a major point to reprogram translation in response to stress [3], [4]. A key mechanism to repress translation initiation is the phoshorylation of the alpha-subunit of translation initiation factor 2 (eIF2) by stress-activated kinases [5], [6]. However, a sizeable set of cellular mRNAs are initiated in an eIF2-independent manner, which allows for escaping the global kinase-dependent inhibition of translation initiation [3], [4]. It remains elusive, which alternative mechanisms the cell employs to regulate translation during adverse environmental stress.
Transfer RNAs (tRNAs) enter ribosome-mediated protein biosynthesis in a translationally competent state, which includes post-transcriptional modifications at various positions, including the anticodon loop, and the presence of an intact single-stranded CCA-sequence at the 3′-terminus that is required for amino acid attachment by the corresponding aminoacyl-tRNA-synthetase [7]. The CCA ends are generated and maintained by the CCA-adding enzyme [8]. Some bacteria carry tRNA genes encoding CCA termini, thus the CCA-adding enzyme is primarily involved in repairing damaged CCA ends in these organisms [9]. In contrast, all eukaryotic tRNA genes lack the CCA ends and the role of the CCA-adding enzyme is to attach post-transcriptionally the CCA overhang to the 3′-termini of all tRNAs [8], [10], [11]. The functional repertoire of the CCA-adding enzyme has been expanded by its recently discovered role in the quality control of hypo-modified tRNAs [12].
Mature, translationally competent tRNAs are very stable under normal growth conditions, with a half-life of approximately one to several hours [13]. However, environmental changes dynamically modulate the concentration of the tRNA pool. Some tRNAs are cleaved in the anticodon loop in response to various environmental stress factors (e.g., oxidative stress, heat shock or ultraviolet irradiation) [14], [15], [16], [17]. The endonucleolytic tRNA cleavage is a conserved feature in higher eukaryotes. Thereby, two tRNA-halves (designated 5′ - and 3′-tiRNAs) are generated by a ubiquitously expressed enzyme, angiogenin [18]. This cleavage, however, does not significantly reduce the level of mature tRNAs, which implies that tiRNAs may rather act as a signal transducer to modulate translation of specific mRNAs, than to globally repress translation [18]. Furthermore, in response to external stimuli, retrograde translocation of mature tRNAs to the nucleus[19] or selective charging of different tRNA isoacceptors [20] transiently alter the pool of translationally active tRNAs in the cytoplasm. Consequently, these stress-induced alterations in the tRNA concentration will decrease the amount of ternary complex (that is, the complex of charged tRNA with the GTP-loaded elongation factor). However, the primary mechanism, that triggers a general inhibition of translation elongation during stress, remains surprisingly elusive.
Here, using high-sensitive approaches to probe the structural integrity of cellular tRNAs, we show that upon exposure to oxidative stress all tRNAs are rapidly deactivated by a cleavage within their 3′-CCA termini by oxidative stress-activated nuclease, angiogenin. Since 3′-CCA ends are ubiquitous for all tRNAs, angiogenin-induced deactivation of tRNAs provides a means for global repression of translation at the level of elongation. On a much slower scale, at longer times of exposure to stress, some tRNAs are also cut in their anticodon. The CCA ends deactivation is reversible and quickly repairable by the CCA-adding enzyme. We propose that this is a mechanism to dynamically repress and reset translation at low metabolic costs.
Results
3′-CCA ends of tRNAs are first cleaved by oxidative stress
Angiogenin, the nuclease that endonucleolytically cleaves tRNAs during oxidative stress, is constitutively expressed, but kept inactive through an inhibitor RNH1 [18]. Oxidative stress dissociates the inhibitor and activates angiogenin [21]. To investigate the susceptibility of the cellular tRNAs to angiogenin-mediated cleavage, we exposed confluent HeLa cells to arsenite which elicits oxidative stress and activates angiogenin. A small amount of tiRNAs was generated, but only at prolonged exposure to arsenite (>30 min) (Figure 1A). Next, we used tRNA microarrays [22] with immobilized oligonucleotide probes complementary to the full-length tRNA sequences to determine the susceptibility of each tRNA species to angiogenin. Only a subset of all tRNAs bearing a CA sequence in the anticodon loop was predominantly cleaved into tiRNAs; a minor fraction with UA or GC motifs in the anticodon loop was also cleaved (Figure S1A). This cleavage pattern mirrors the substrate specificity of angiogenin: it targets single-stranded ribonucleic acid sequences with 10–30-fold higher preference for CA over UA [23] and 3-fold higher for CA over CG [24]. While there is a large variability in the composition of the anticodon loops of all tRNAs and only a fraction of tRNAs possesses a CA-motif in the anticodon loop (Figure S1B), we realized that the ubiquitous, single-stranded 3′-CCA sequence post-transcriptionally attached to all eukaryotic tRNAs [8], bears the strongest angiogenin recognition motif, the CA motif. To investigate whether the 3′-CCA ends can be targeted by angiogenin upon exposure to oxidative agents, we used enzymatic ligation of a fluorescent stem-loop oligonucleotide that complementary pairs only to the intact 3′-CCA end of tRNA (Figure 1B, schematic inset). The fluorescent signal, which is proportional to the amount of tRNAs with intact 3′-CCA ends, decreased noticeably in conditions of severe oxidative stress while the total tRNA amount remained relatively constant (500 µM arsenite; Figure 1B), consistent with the idea that the 3′-CCA ends of tRNAs are primary substrates of angiogenin. Oxidative stress altered the structural integrity of the 3′-CCA termini of tRNAs in a dose-sensitive manner; at lower dose of stress (100 µM arsenite) much smaller fraction of tRNAs than at high stress dose (500 µM arsenite) was unable to ligate the fluorescent oligonucelotide (Figure 1B). To our surprise, the removal of the 3′-CCA ends of the tRNAs occurred on a much faster time scale (Figure 1B) compared to the appearance of the tiRNA fragments (Figure 1A).
Fig. 1. Oxidative stress-mediated tRNA cleavage in HeLa cells. 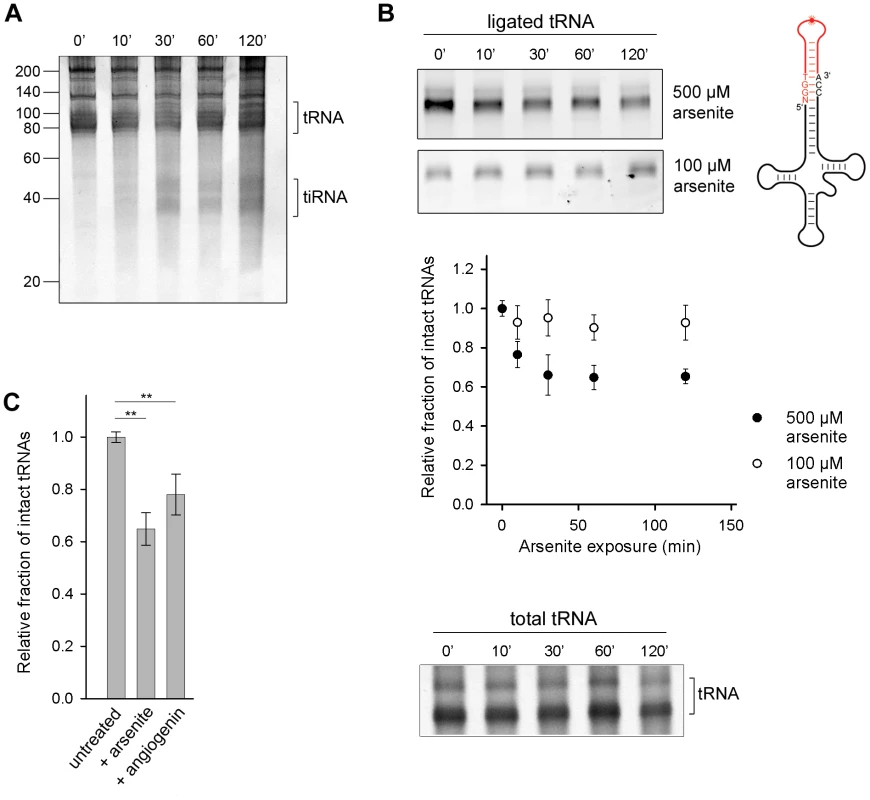
(A) Minor fraction tiRNAs are generated by longer exposure to 500 µM arsenite (>30 min). The numbers on the left denote the DNA ladder in nt. (B) Arsenite alters the integrity of the 3′-CCA end of full-length tRNAs in a dose-dependent manner. The amount of tRNAs with intact CCA ends was analyzed by their ability to ligate to a fluorescently labeled oligonucleotide (schematic inset) which forms a loop and binds only intact 3′-CCA ends (upper two gels). The intensity of tRNAs with intact CCA termini was quantified from the gels, normalized to the total tRNA amount at each time point and presented as relative values ± SEM (from three independent experiments) to the amount of initial, untreated sample which was set as 1.0. The amount of the total tRNA remained almost unchanged when cells were exposed to arsenite (500 µM) and visualized by SYBR Green (bottom gel marked as total tRNA). (C) Increase of the cellular concentration of angiogenin alters the 3′-CCA integrity of tRNAs. Angiogenin was upregulated by ectopic expression under the control of a CMV promoter for 8 h (+angiogenin). The sample representing arsenite stress (+arsenite) corresponds to the 60-min data point at 500 µM arsenite in panel (B) and is used for comparison. The intensity of tRNAs with intact CCA termini was the quantified as described for panel (B). ** for p<0.01. To confirm that angiogenin cleaves the 3′-CCA termini of tRNAs upon exposure to arsenite, we upregulated the level of aniogenin and analyzed the integrity of the 3′-CCA ends using the fluorescent oligonucleotide-ligation approach. A small increase in the cellular level of angiogenin led to a noticeable enrichment of tRNAs with cleaved 3′-CCA termini, confirming its role in the oxidative stress-mediated cleavage of the 3′-CCA ends (Figure 1C). Note that angiogenin can be only moderately upregulated for short expression times (Figure S2A); longer expression perturbs the vitality of the cell. The ectopically enhanced levels of angiogenin, a fraction of it might be additionally deactivated by the excess of the RNH1 inhibitor in the cell, is most likely far below the concentration of stress-activated angiogenin, thus the effect of arsenite-induced 3′-CCA end cleavage (+arsenite) was much stronger (Figure 1C).
Intrigued by the different time scales of the stress-induced alterations of cellular tRNAs, we next analyzed the kinetics of 3′-CCA end cleavage and tiRNA generation in vitro. Total tRNA was isolated from confluent, non-stressed HeLa cells, radioactively labeled at their 5′ - or the 3′-end and subsequently subjected to angiogenin treatment. Strikingly, while 5′-labeled tRNAs are still visible at 120 min of incubation with angiogenin, the 3′-labeled tRNAs completely disappeared after 30 min (Figure 2A). The fast decay of the signal of the 3′-labeled full length tRNAs than the 5′-labeled tRNAs (Figure 2A) is consistent with a preferred and much faster cleavage in the 3′-CCA termini of the tRNAs. All tRNAs were equally sensitive to angiogenin cleavage at the 3′-CCA termini; the signal for all 3′-radioactively labeled tRNAs decayed almost simultaneously during the angiogenin treatment (Figure 2B and S3). In the fluorescent oligonucleotide-ligation approach (Figure 1B, schematic inset), we observed a clear progressive decrease of the yield of the oligonucleotide ligated to the 3′-CCA end of the tRNAs upon angiogenin treatment (Figure 2C). Notably, fragments migrating at the height of the tiRNAs appeared much later (Figure 2C), suggesting that angiogenin degraded the 3′-CCA ends more rapidly than it cleaved tRNAs in the anticodon loop, thus recapitulating the observations in HeLa cells (Figure 1). To define the exact cleavage site in the 3′-CCA end, we used internally radioactively labeled variants of tRNAPhe(GAA) with intact 3′-CCA and truncated 3′-CC end. tRNAPhe(GAA) lacks the CA motif in the anticodon loop and hence, only the 3′-CCA terminus is susceptible to angiogenin cleavage. Angiogenin cleaved endonucleolytically within the 3′-CCA motif between the C and A nucleotide and removed exclusively the adenosine residue (Figure S4), implying a high CA-dependent endonucleolytic activity of angiogenin.
Fig. 2. The CCA sequence at the 3′-termini of all tRNAs is first cleaved by angiogenin in vitro. 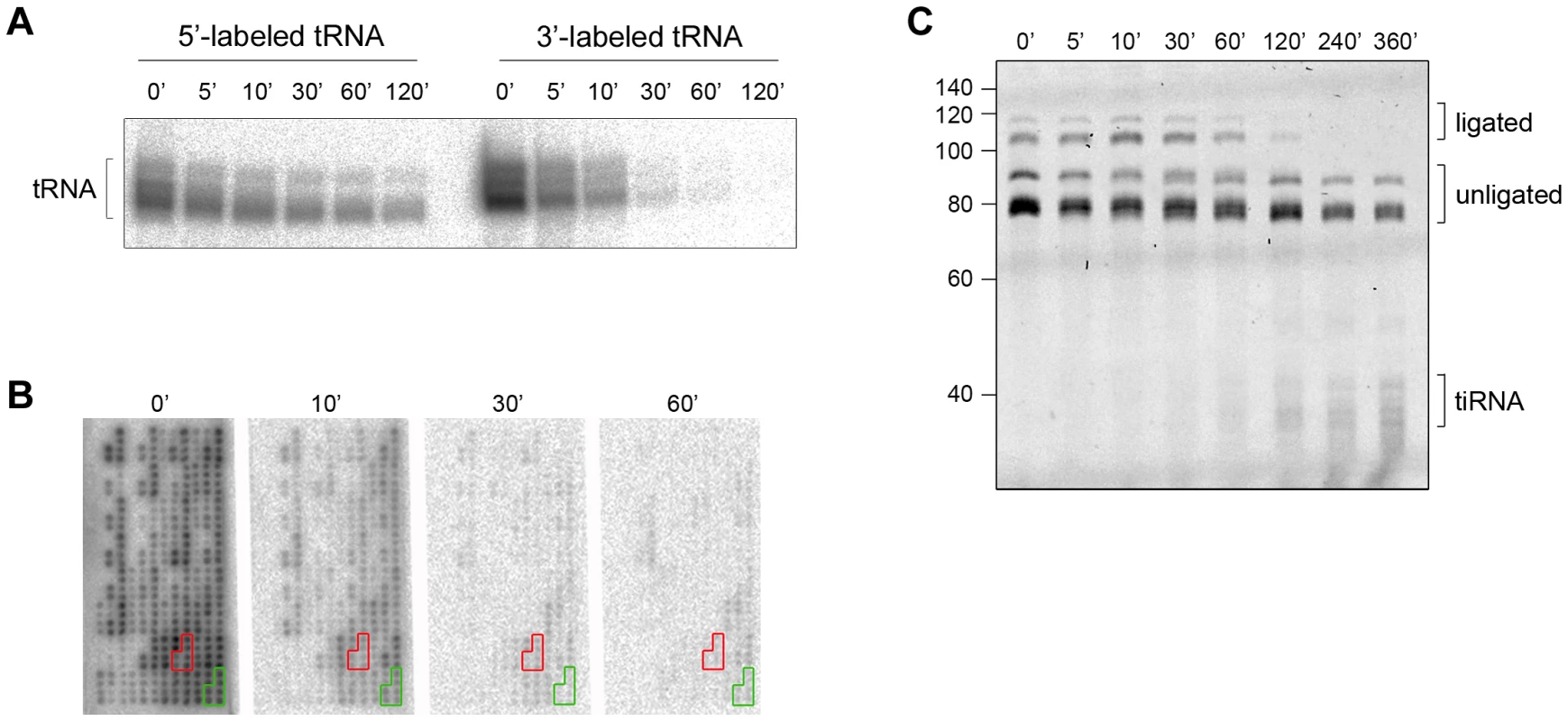
(A) Angiogenin (1 µM) digestion of total HeLa tRNA radioactively labeled at either 5′- or 3′-end. (B) 3′-radioactively labeled HeLa tRNAs treated with 1 µM angiogenin for different times and their intact 3′-termini were visualized with tRNA macroarrays. Only tRNAs (or fragments of them) with intact 3′-ends are visible on the microarrays. Two exemplary tRNAs (Ala-IGC, green and Gln-yTG, red) are marked. Probes for each tRNA are arranged in clusters of six replicates. (C) Analysis of the integrity of the 3′-CCA end of full-length tRNAs with the specific oligonucleotide-ligation approach after angiogenin (0.2 µM) treatment for various times. The gel was visualized with SYBR Green. Note, to better resolve the kinetics of cleavage we decreased the concentration of angiogenin to 0.2 µM; thus the time points here are not directly comparable with the time points in panels A, B. The numbers on the left denote the DNA ladder in nt. Multiple bands for tiRNAs, full-length tRNAs, ligated and unligated tRNAs are detected (panels A and C) due to the natural variations in tRNAs length. Cleaved 3′-CCA ends are repaired by the CCA-adding enzyme
While in vitro all tRNAs lost their 3′-ends after 10 min, as evidenced by almost complete signal extinction of the 3′-labeled full-length tRNAs (Figure 2A), in vivo the signal plateaued at about 60% of the initial signal intensity (Figure 1B). Translationally competent tRNAs are aminoacylated and complexed with elongation factor EF1α, which may protect tRNAs from angiogenin cleavage. Aminoacylation per se did not influence angiogenin cleavage (Figure S5). The crystal structure of the ternary complex from Thermus aquaticus indicates that EF1α contacts only the phosphate groups of tRNA bases 73–75 [25]. [Note, C75A76 is endonucleolytically targeted by angiogenin]. Thus, the elongation factor EF1α may marginally interfere with the angiogenin binding and partly protect the aminoacyl-tRNA.
In cells, the CCA-adding enzyme repairs the partially degraded 3′-CCA ends of tRNAs without a nucleic acid template and highly discriminates between adding cytidine at position 75 and adenosine at position 76 [8], [10], [11]. Thus, we hypothesized that the lower amount of tRNAs with deactivated CCA termini in HeLa cells (Figure 1B), compared to the in vitro angiogenin treatment (Figure 2A), might represent a steady-state equilibrium between the angiogenin-mediated cleavage and simultaneous repair by the CCA-adding enzyme whose activity remained unchanged upon the arsenite treatment (Figure S4C). To determine the effect of these two opposing processes, total HeLa tRNAs were successively treated with angiogenin and human CCA-adding enzyme. Indeed, the CCA-adding enzyme repaired the CCA termini (Figure 3A) by adding the cleaved adenosine (Figure 3B), implying that stress-damaged 3′-CCA ends of tRNAs can be easily repaired and tRNAs are converted back to translationally-competent species. The angiogenin-catalyzed endonucleolytic cleavage of the CA motif results in a 3′-terminal 2′,3′-cyclic phosphate at the cytosine residue. Thus, prior to treatment with CCA-adding enzyme HeLa tRNAs (Figure 3A) or single tRNAPheCC were treated with T4 polynukleotide kinase (PNK). In the cell, the 3′-end cyclization products are quickly hydrolyzed to 3′OH by 2′,3′-cyclic 3′-phosphodiesterases [26], [27].
Fig. 3. Human CCA-adding enzyme is able to repair damaged CCA ends of tRNAs. 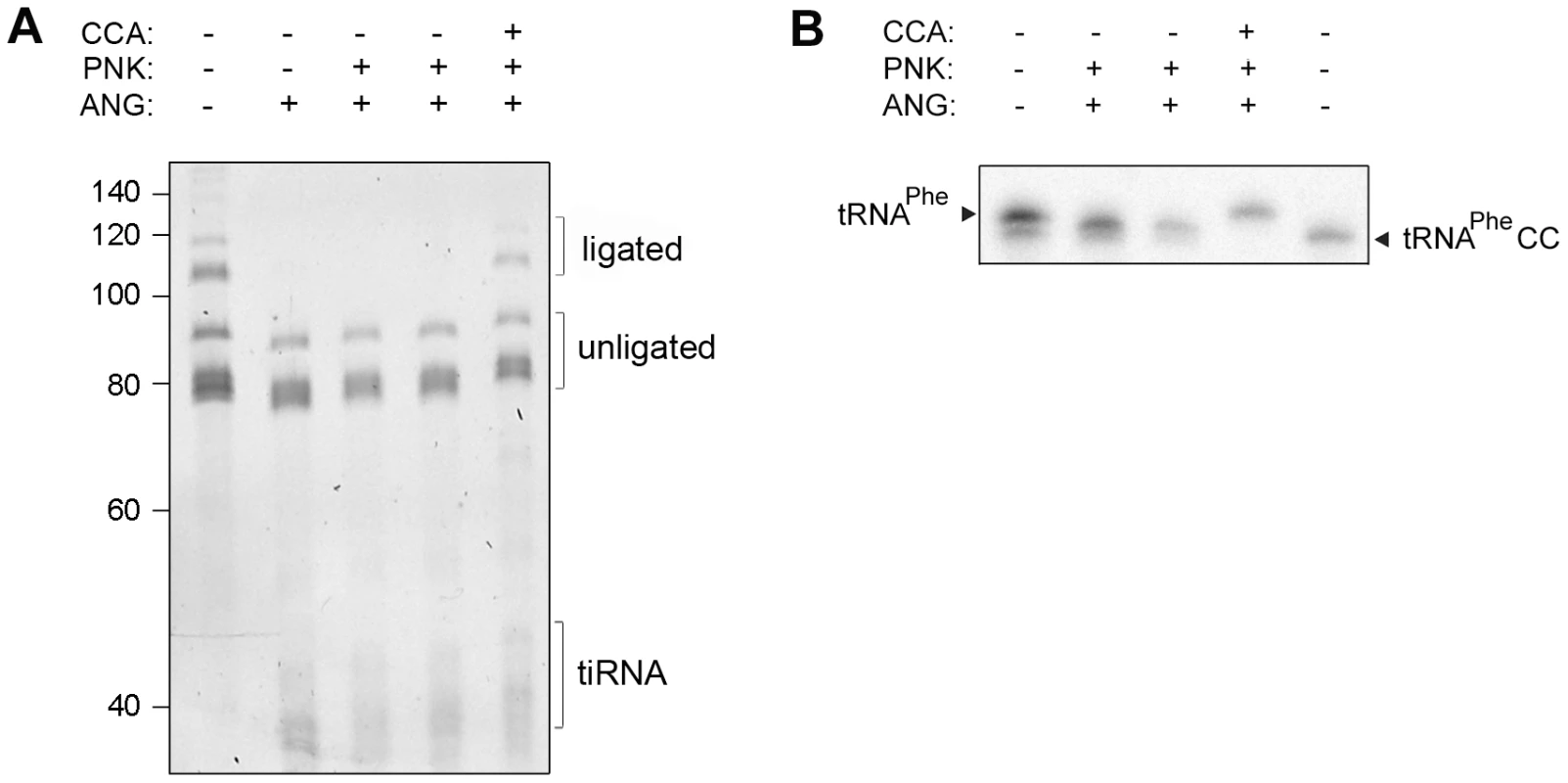
(A) Total HeLa tRNAs and (B) internally radioactively labeled yeast tRNAPhe were incubated successively with angiogenin and human CCA-adding enzyme. Subsequently to the angiogenin treatment (4 h), T4 polynucleotide kinase (PNK) (45 min) was added which converts the 2′,3′-cyclophosphate ends [42] generated by the angiogenin cleavage to free 3′OH. After purification tRNAs were subjected to treatment with the CCA-adding enzyme (30 min). The 3′-CCA end integrity of the HeLa tRNAs was determined with the fluorescent oligonucleotide (Figure 1A, schematic inset). tRNAPhe lacking the terminal 3′-adenosine (tRNAPheCC) served as a control. The numbers on the left denote the DNA ladder in nt. An attempt to reduce the cellular concentration of the CCA-adding enzyme was unsuccessful: even though de novo synthesis of the enzyme was significantly inhibited by targeting its mRNA with specific siRNA probe (Figure S2B), the concentration of the mature CCA-adding enzyme remained unchanged (Figure S2C). As the CCA-adding activity is essential for cell viability, an intrinsic robustness of this enzyme has the advantage of maintaining a constant function and permitting a prompt stress response.
3′-CCA ends deactivation represses translation elongation
The 3′-CCA ends are indispensable for tRNA aminoacylation and subsequently for translation. What is the effect of angiogenin-induced deactivation of the 3′-CCA termini of cellular tRNAs on protein translation? Exposure of HeLa cells to acute oxidative stress (500 µM arsenite) altered the polysomal profile and shut down translation (Figure 4A). Importantly, at low arsenite concentration (100 µM) the cells retained some translation activity, detectable as a considerable polysomal fraction (Figure 4A). The most potent inhibition of translation is mediated by eIF2α phosphorylation upon oxidative stress via haem-regulated inhibitor kinase (HRI), which represses translation of mRNAs with scanning - or cap-dependent translation initiation [3]. By contrast, a sizeable subset of genes are translated through a cap-independent mechanism: internal ribosome-entry sites (IRES) direct translation initiation without the aid of canonical initiation factors and initiator Met-tRNA [28]. We hypothesized that cap-dependent translation will be influenced at much lower arsenite concentrations compared to mRNAs with scanning-independent initiation; the combined effect of oxidative stress on eIF2α phosphorylation and the deactivation of the 3′-CCA ends of all tRNAs will have much higher impact on mRNAs initiated in a scanning-dependent manner. In contrast, in the IRES-initiated translation, as only the 3′-CCA-end inactivation should play a role the effect should be less pronounced. We therefore tested the effect of two arsenite concentrations, representing severe (500 µM) and moderate (100 µM) oxidative stress using bicistronic mRNA encoding renilla luciferase (Rluc), initiated in a cap-controlled manner, and firefly luciferase (Fluc), initiated via cricket paralysis virus IRES (CrPV-IRES) (Figure 4B), an IRES sequence described to confer translation independent of any initiation factor [29]. At a low arsenite concentration (100 µM), the Fluc activity remained at >80%, while Rluc activity progressively decreased, indicating much potent inhibition of cap-dependent initiation compared to IRES-dependent initiation (Figure 4C,D). At a high arsenite concentration (500 µM), however, a similar decrease for both Rluc and Fluc activity was observed, implying that both IRES-dependent and scanning-controlled initiation were equally inhibited (Figure 4C,D). This cannot be attributed to the decrease of mRNA levels, since the mRNA expression levels of the bicistronic construct remained similar upon stress exposure (Figure S6). Variations in the transfection efficiency are not likely; transfection efficiency was equal in all experiments as assessed with fluorescent reporter. This suggests that under acute oxidative stress translation of all mRNAs is globally repressed, while moderate oxidative stress affects more strongly the cap-dependent than the IRES-controlled initiation due to the combined effect on eIF2α phosphorylation and the tRNAs deactivation.
Fig. 4. Scanning-independent translation initiation is less influenced by low dose oxidative stress. 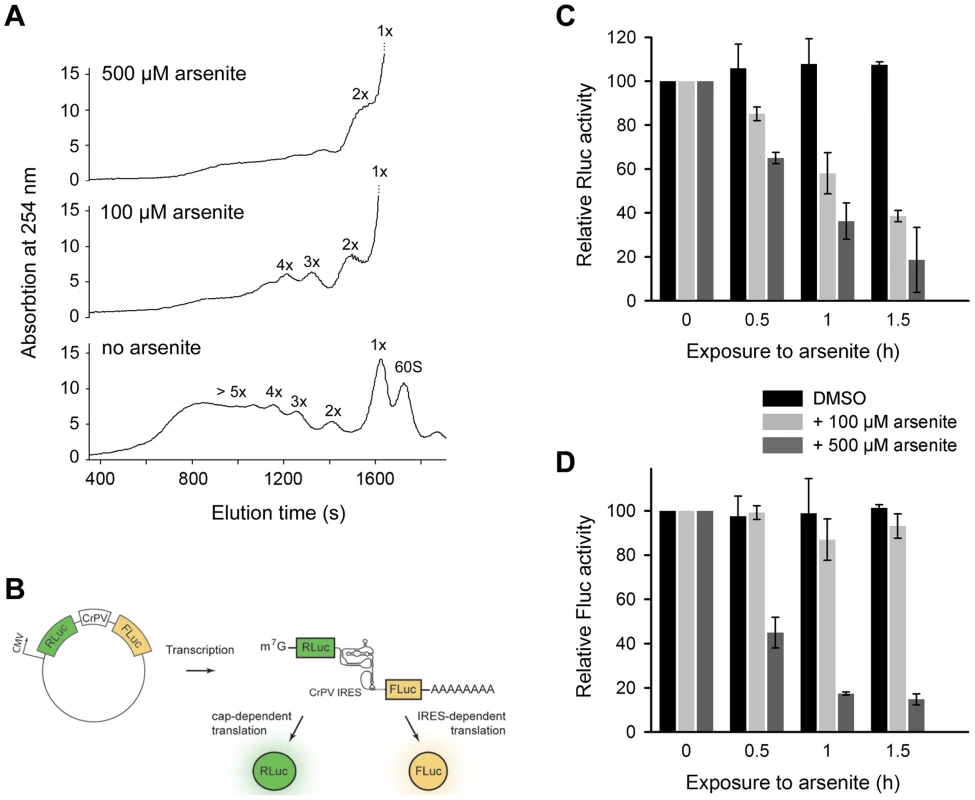
(A) Polysomal profiles of untreated or arsenite treated HeLa cells. (B) Schematic of the plasmid used to monitor cap-dependent and IRES-dependent translation initiation. Inhibition of cap-dependent, Rluc (C) or IRES-mediated, Fluc (D) translation upon exposure to oxidative stress. HeLa cells expressing the bicistronic construct encoding Rluc under the CMV-promoter (scanning-dependent translation) and Fluc under the CrPV-IRES (non-scanning controlled translation) were exposed to different arsenite concentrations for various times. Addition of DMSO to the cells served as a control. Data in (C) and (D) ± SEM are normalized to the first data point for which the activity was set as 100. Discussion
Here, we analyze the effect of oxidative stress on the structural integrity of the cellular tRNAs and define the mechanisms of oxidative stress-induced global repression of translation at the level of elongation. Our observations clearly suggest a sequential order of tRNA deactivation upon exposure to oxidative stress: the 3′-terminal CCA sequence is first targeted, while the deactivation into tRNA halves occurs much later. The first event, the CCA cleavage, is not restricted to specific tRNAs; the CCA ends of all tRNAs can be targeted by angiogenin. At severe oxidative stress (500 µM) all tRNAs are rapidly deactivated which leads to a global repression of translational elongation of both mRNAs with scanning and non-scanning (IRES) controlled initiation. The deactivation of the 3′-CCA ends is a mechanism to reversibly repress translation at very low metabolic costs; the 3′-CCA tRNA ends are quickly repaired by the CCA-adding enzyme [8], [10], [30] and translation is reset. In contrast, cleavage in the anticodon loop proceeds on a much slower timescale and is specific for only a subset of tRNAs, so that tiRNAs with specific primary sequences can be generated. This mirrors the reported specificity of the tiRNAs to either selectively target translation of a defined fraction of mRNAs [14] or trigger formation of stress granules [31]. The role of some tiRNAs to silence specific functions [14] indirectly suggests a separation of the tRNA halves upon a cleavage in the anticodon loop. Regeneration of such tRNAs is more metabolically demanding for the cell, as the cleaved tRNAs can be replaced only through a new transcription cycle. A sizeable fraction of tRNAs with cleaved anticodons may not dissociate into tiRNA halves and undergo a repair by tRNA ligases [32]. In vertebrates, the tRNA-ligase activity is coupled to tRNA splicing and is mainly localized in the nucleus [33], [34]; a cytoplasmic localization, although conceivable given the observation for cytoplasmic mitochondrial surface in yeast and plants [35], [36], has not yet been described. This, in turn, would require a translocation of the cleaved tRNAs into the nucleus. Stress-induced retrograde translocation to the nucleus has been shown for mature tRNAs [19]. If upon cleavage within the anticodon the tRNA structure is maintained nearly to the native one, the retrograde transport into the nucleus would be possible and tRNAs can be repaired by the tRNA ligases.
Arsenite derived oxidative stress has also been shown to induce elevated tRNA misacylation with methionine [37]. However, Met-misacylation was obtained upon 1 µM arsenite which is much lower than the concentrations in the experiments presented here (100 or 500 µM). Furthermore, the duration of the treatment is much longer (4 hours) [37], which exceeds the time of the first response towards oxidative stress – the 5′-CCA-ends cleavage. Met-misacylation has been proposed to potentially serve as a protective mechanism for cell's own proteins against oxidative inactivation [37]. This mechanism is distinct from the 3′-CCA cleavage which is useful to regulate global translation activity.
Our observation for selective translation of transcripts with scanning-independent initiation under moderate oxidative stress (100 µM arsenite) adds another layer to selectively reprogram protein translation under stress at the level of elongation. The inhibition of translation initiation through eIF2α phosphorylation upon oxidative stress is a potent mechanism to repress translation of mRNAs with scanning-controlled initiation [3], [16]. At low doses of arsenite (100 µM arsenite) translation of mRNAs with cap-dependent translation initiation is compromised, while the non-scanning, IRES-dependent translation continues to function to a certain level (Figure 4) as in the cell only a small fraction of the total tRNA pool is with deactivated 3′-CCA ends (Figure 1B). Finally, as many transcripts involved in proliferation, differentiation and apoptosis [16] are initiated in a cap-independent manner, the observed differential inactivation of protein synthesis which allows these mRNAs to bypass the global translational repression and activate the selective stress response [3], [16], [38].
Materials and Methods
In vivo experiments and total tRNA isolation
HeLa cells were usually cultured in DMEM with 10% fetal bovine serum and L-Glu (2 mM) to 80–90% confluency. Oxidative stress was exerted by adding 100 or 500 µM sodium arsenite (Fluka) for indicated times. Human angiogenin was cloned in pCDNA3 plasmid (Invitrogen) and transfected in sub-confluent HeLa cells using polyethylenimine (PEI, Polysciences Europe GmbH). After 8 h angiogenin expression was detected with polyclonal antibodies (1∶1000, Santa Cruz Biotechnology). To decrease the expression of CCA-adding enzyme, pSuper plasmid (Oligoengine) bearing shRNA (5′-CCGGCGCAGAGATCTCACTATAAATCTCGAGATTTATAGTGAGATCTCTGCGTTTTTG-3′) that targeted the CCA-adding enzyme mRNA was transfected using polyethylenimine and expressed for 12 h. shRNA with the same, but randomly scrambled sequence was used as a control. Prior to harvesting, an aliquot of cells was additionally exposed to 500 µM sodium arsenite for 1 h. mRNA was quantified by real-time qRT-PCR and the protein level with polyclonal antibodies (1∶200, Santa Cruz Biotechnology) against human CCA-adding enzyme.
Statistical analyses were performed with Fisher's exact test. Differences were considered statistically significant when p<0.05.
A bicistronic construct was created by cloning renilla luciferase (Rluc) gene under the CMV promoter and downstream of it a firefly luciferase (Fluc) gene under the cricket paralysis virus IRES (CrPV-IRES) into pECFP-C1 (Clontech); note CFP was deleted from pECFP-C1 prior to cloning. HeLa cells were transfected with this bicistronic reporter construct using polyethylenimine (PEI, Polysciences Europe GmbH) and expressed for 8 h in total. Prior to harvesting cells were exposed to 100 and 500 µM sodium arsenite for various times and harvested. Luciferase activities were measured using Dual-Luciferase® Reporter Assay System (Promega).
For isolation of non-charged tRNAs, HeLa cells were harvested by mechanical scrapping and total RNA was isolated with TriReagent (Sigma-Aldrich) according to the manufacturer's protocol. For isolation of charged tRNAs, total RNA was isolated under acidic conditions. Briefly, HeLa cells were re-suspended in 0.3 M NaOAc 10 mM EDTA pH 4.5 and extracted two times with acidic phenol. The aqueous phase, containing RNA, was precipitated with one volume isopropanol and washed with 80% ethanol. The total uncharged or charged tRNAs were separated on 10% PAGE gels at 4°C. Bands corresponding to the tRNAs were visualized by UV-shadowing, cut and eluted from the gel overnight at 4°C, for uncharged tRNAs in elution buffer (50 mM potassium acetate, 200 mM potassium chloride, pH 7.0) or for charged tRNAs in acidic elution buffer (0.3 M NaOAc, 10 mM EDTA pH 4.5).
Polysome profiling
1.5 Mio. HeLa cells were treated for 30 min with 100 or 500 µM arsenite. Ten minutes prior to harvesting, cycloheximide (CHX) to a final concentration of 100 µg/ml was added to the medium. Cells were trypsinized (trypsin solution was also supplemented with CHX) and collected by centrifugation at 232×g for 5 min. The cell pellet was resuspended in 320 µl of ice-cold lysis buffer (10 mM Tris-HCl pH 7.4, 5 mM MgCl2, 100 mM KCl, 1% Triton-X, 100 µg/ml, 2 mM DTT) and cells were sheared with a 26-gauge syringe. After pelleting of the debris at 5000×g for 8 min at 4°C, the supernatant was layered onto 15 to 50% (w/v) sucrose gradient (20 mM HEPES-KOH pH 7.4, 5 mM MgCl2, 100 mM KCl, 100 µg/ml CHX, 2 mM DTT) and centrifuged for 1.5 h at 35,000 rpm in SW 55Ti rotor (Beckman) at 4°C. The gradient was slowly pumped out from the bottom of the tubes and A254 nm was recorded via a flow-through UV spectrophotometer cell (Pharmacia LKB-UV-M II).
Total mRNA extraction and quantitative RT-PCR
One µg of the total mRNA from HeLa cells was treated with DNase I (Fermentas), the cDNA was synthesized with reverse transcriptase using oligo-dT primer (both Fermentas) and quantified using the 2× Fast SYBR® Green Master Mix (Applied Biosystems) and the 7500 Fast Real-Time PCR system (Applied Biosystems). The following primers were used for amplification of the bicistronic Fluc-Rluc construct: forward (5′-GCTGTTTCTGAGGAGCCTTC-3′) and reverse (5′-GCACTCTGATTGACAAATACGATT-3′), and for CCA-adding enzyme: forward (5′-GATCGCAAAAGAGGAGAAAAAC-3′) and reverse (5′-GCATCAGGTTCCCTAGAATC-3′). mRNA expression was normalized to β-actin.
Charging assay
The degree of aminoacylation of isolated HeLa tRNAs was tested using the periodate protection assay described previously [39]. Total tRNA sample was treated with 50 mM sodium periodate, which oxidizes the 3′-ends of uncharged tRNAs, which prevents the ligation of the fluorescent stem-loop DNA/RNA oligonucleotide. tRNAs are resolved on denaturing 10% PAGE and the ligation efficiency serves as a measure for the levels of charged tRNAs.
tRNA microarrays
tRNA probes covering the full-length sequence of 42 cytosplasmic tRNA species with sequences described previously [22] were spotted onto amino-coated slides. The probes for each tRNA are arranged in clusters of six replicates. Radioactively labeled tRNA samples were mixed with 0.17 mg/ml salmon sperm DNA (Invitrogen), 0.17 mg/ml polyA (Sigma-Aldrich) in hybridization buffer (Sigma-Aldrich) and hybridized on the microarrays for 16 h at 60°C. Subsequently, the microarrays were washed three times in 6×SSC at 35°C and once in 2×SSC and 0.2×SSC at 30°C. The composition of the 20×SSC buffer was as follow: 3 M sodium chloride, 300 mM sodium citrate, 0.1% SDS. Radioactivity was detected on a FUJI BAS scanner.
In vitro tRNA transcription
Yeast tRNAPhe with intact CCA ends or tRNAPheCC were generated according to the procedure described in [40]. Radioactive, internally labeled transcripts were synthesized in the presence of 3 µCi 32P-α-ATP.
Radioactive 5′-tRNA labeling
Total HeLa tRNA was dephosphorylated with calf intestine alkaline phosphatase (Roche) for 30 min at 37°C. The enzyme was removed by phenol/chloroform extraction and the tRNA was precipitated. Radioactive phosphate was incorporated by T4 polynukleotide kinase (USB) and 32P-γ-ATP for 30 min at 37°C. Radioactively labeled RNA was separated on a denaturing 10% PAGE gel, tRNA bands were cut and eluted in the elution buffer (4 h, 25°C).
Radioactive 3′-tRNA labeling
Total HeLa tRNAs were deacylated for 45 min at 37°C in 0.1 M TrisHCl, pH 9.0 and dephosphorylated with T4 polynucleotide kinase (USB) in the absence of ATP. 3′-CMP was phosphorylated with 32P-γ-ATP (30 min, 37°C) using PNK (Fermentas) and ligated to the RNA with T4 RNA ligase (NEB) by incubation over night at 16°C. Radioactively labeled RNAs were separated on a denaturing 10% PAGE gel, tRNA bands were cut and eluted in the elution buffer (4 h, 25°C).
In vitro angiogenin digestion
To prepare the tRNA for subsequent digestions, isolated tRNA was heated at 90°C for 2 min and cooled down at room temperature in 30 mM HEPES 30 mM sodium chloride, pH 7.0 for 3 min. MgCl2 and BSA were added to final concentrations of 2 mM and 0.01%, respectively, and further incubated for 5 min at 37°C. Recombinant human angiogenin (R&D systems) was added to a final concentration of 0.2 or 1 µM to the total HeLa or yeast tRNAPhe and incubated at 37°C for the indicated times. In the radioactive experiments, total non-labeled HeLa tRNA was spiked with radioactive 5′ - or 3′-labeled tRNA. The reactions were stopped by extraction with phenol/chloroform or adding gel loading buffer (95% formamide, 0.025% SDS, 0.5 mM EDTA, 0.25% (w/v) bromophenolblue, 0.25% (w/v) xylene cyanol) and shock freezing in liquid nitrogen. To test the integrity of the 3′-CCA ends, a fluorescent stem-loop RNA/DNA oligonucleotide, with a sequence described previously [22], was ligated over night at 16°C with T4 DNA ligase (NEB). Full-length tRNA and tiRNAs were separated on a denaturing 10% PAGE gel.
In vitro repair of the CCA termini with CCA-adding enzyme
Human CCA-adding enzyme was purified as described [41]. Total HeLa tRNA and 3′-radioactive labeled yeast tRNAPhe (0.5 µM) were treated with 0.2 µM angiogenin at 37°C for 4 h, dephosphorylated with PNK to convert the 2′,3′-cyclophosphate generated by angiogenin to 3′-OH [40] and subsequently treated with 50 nM human CCA-adding enzyme at 30°C for 30 min in 20 mM HEPES pH 7.6, containing 20 mM KCl, 6 mM MgCl2, 2 mM DTT and 1 mM NTPs.
Determination of the integrity of the CCA termini of tRNAs
Oxidative stress was exerted by adding 500 µM sodium arsenite (Fluka) to confluent HeLa cells for indicated times. RNA was isolated using mirVana miRNA Isolation kit (Ambion) and subsequently deacylated in 0.1 M Tris.HCl buffer, pH 9.0 at 37°C for 30 min. Fluorescent stem-loop RNA/DNA oligonucleotide [22] (Figure 1B, schematic inset) was ligated over night at 16°C with T4 DNA ligase (NEB). Ligation efficiency was analyzed by resolving the samples on denaturing 10% PAGE and detected by fluorescence (Fujifilm LAS-4000) or SYBR Green (Invitrogen) staining.
Supporting Information
Zdroje
1. TurnerM (2011) Is transcription the dominant force during dynamic changes in gene expression? Adv Exp Med Biol 780 : 1–13.
2. AndersonP, KedershaN (2009) RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol 10 : 430–436.
3. HolcikM, SonenbergN (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6 : 318–327.
4. SonenbergN, HinnebuschAG (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136 : 731–745.
5. DonnellyN, GormanAM, GuptaS, SamaliA (2013) The eIF2alpha kinases: their structures and functions. Cell Mol Life Sci doi: 10.1007/s00018-012-1252-6
6. HardingHP, ZhangY, ZengH, NovoaI, LuPD, et al. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11 : 619–633.
7. IbbaM, SollD (2000) Aminoacyl-tRNA synthesis. Annu Rev Biochem 69 : 617–650.
8. XiongY, SteitzTA (2006) A story with a good ending: tRNA 3′-end maturation by CCA-adding enzymes. Curr Opin Struct Biol 16 : 12–17.
9. SchurerH, SchifferS, MarchfelderA, MorlM (2001) This is the end: processing, editing and repair at the tRNA 3′-terminus. Biol Chem 382 : 1147–1156.
10. LizanoE, ScheibeM, RammeltC, BetatH, MorlM (2008) A comparative analysis of CCA-adding enzymes from human and E. coli: differences in CCA addition and tRNA 3′-end repair. Biochimie 90 : 762–772.
11. XiongY, SteitzTA (2004) Mechanism of transfer RNA maturation by CCA-adding enzyme without using an oligonucleotide template. Nature 430 : 640–645.
12. WiluszJE, WhippleJM, PhizickyEM, SharpPA (2011) tRNAs marked with CCACCA are targeted for degradation. Science 334 : 817–821.
13. DittmarKA, MobleyEM, RadekAJ, PanT (2004) Exploring the regulation of tRNA distribution on the genomic scale. J Mol Biol 337 : 31–47.
14. IvanovP, EmaraMM, VillenJ, GygiSP, AndersonP (2011) Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43 : 613–623.
15. ThompsonDM, LuC, GreenPJ, ParkerR (2008) tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14 : 2095–2103.
16. YamasakiS, AndersonP (2008) Reprogramming mRNA translation during stress. Curr Opin Cell Biol 20 : 222–226.
17. SaikiaM, KrokowskiD, GuanBJ, IvanovP, ParisienM, et al. (2012) Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem 287 : 42708–42725.
18. YamasakiS, IvanovP, HuGF, AndersonP (2009) Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185 : 35–42.
19. ShaheenHH, HoretskyRL, KimballSR, MurthiA, JeffersonLS, et al. (2007) Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc Natl Acad Sci U S A 104 : 8845–8850.
20. ElfJ, NilssonD, TensonT, EhrenbergM (2003) Selective charging of tRNA isoacceptors explains patterns of codon usage. Science 300 : 1718–1722.
21. BlazquezM, FominayaJM, HofsteengeJ (1996) Oxidation of sulfhydryl groups of ribonuclease inhibitor in epithelial cells is sufficient for its intracellular degradation. J Biol Chem 271 : 18638–18642.
22. DittmarKA, GoodenbourJM, PanT (2006) Tissue-specific differences in human transfer RNA expression. PLoS Genet 2: e221.
23. RussoN, AcharyaKR, ValleeBL, ShapiroR (1996) A combined kinetic and modeling study of the catalytic center subsites of human angiogenin. Proc Natl Acad Sci U S A 93 : 804–808.
24. HarperJW, ValleeBL (1989) A covalent angiogenin/ribonuclease hybrid with a fourth disulfide bond generated by regional mutagenesis. Biochemistry 28 : 1875–1884.
25. NissenP, KjeldgaardM, ThirupS, PolekhinaG, ReshetnikovaL, et al. (1995) Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 270 : 1464–1472.
26. GravelM, RobertF, KottisV, GallouziIE, PelletierJ, et al. (2009) 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: a novel RNA-binding protein that inhibits protein synthesis. J Neurosci Res 87 : 1069–1079.
27. RemusBS, ShumanS (2013) A kinetic framework for tRNA ligase and enforcement of a 2′-phosphate requirement for ligation highlights the design logic of an RNA repair machine. RNA 19 : 659–69.
28. HellenCU, SarnowP (2001) Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 15 : 1593–1612.
29. WilsonJE, PestovaTV, HellenCU, SarnowP (2000) Initiation of protein synthesis from the A site of the ribosome. Cell 102 : 511–520.
30. HouYM (2010) CCA addition to tRNA: implications for tRNA quality control. IUBMB Life 62 : 251–260.
31. EmaraMM, IvanovP, HickmanT, DawraN, TisdaleS, et al. (2010) Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem 285 : 10959–10968.
32. WangLK, ShumanS (2005) Structure-function analysis of yeast tRNA ligase. RNA 11 : 966–975.
33. HopperAK, ShaheenHH (2008) A decade of surprises for tRNA nuclear-cytoplasmic dynamics. Trends Cell Biol 18 : 98–104.
34. PopowJ, SchleifferA, MartinezJ (2012) Diversity and roles of (t)RNA ligases. Cell Mol Life Sci 69 : 2657–2670.
35. ParkMY, WuG, Gonzalez-SulserA, VaucheretH, PoethigRS (2005) Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A 102 : 3691–3696.
36. YoshihisaT, Yunoki-EsakiK, OhshimaC, TanakaN, EndoT (2003) Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol Biol Cell 14 : 3266–3279.
37. NetzerN, GoodenbourJM, DavidA, DittmarKA, JonesRB, et al. (2009) Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462 : 522–526.
38. BairdSD, TurcotteM, KornelukRG, HolcikM (2006) Searching for IRES. RNA 12 : 1755–1785.
39. ZaborskeJM, WuX, WekRC, PanT (2010) Selective control of amino acid metabolism by the GCN2 eIF2 kinase pathway in Saccharomyces cerevisiae. BMC Biochem 11 : 29.
40. SchurerH, LangK, SchusterJ, MorlM (2002) A universal method to produce in vitro transcripts with homogeneous 3′ ends. Nucleic Acids Res 30: e56.
41. ReichertAS, ThurlowDL, MorlM (2001) A eubacterial origin for the human tRNA nucleotidyltransferase? Biol Chem 382 : 1431–1438.
42. RybakSM, ValleeBL (1988) Base cleavage specificity of angiogenin with Saccharomyces cerevisiae and Escherichia coli 5S RNAs. Biochemistry 27 : 2288–2294.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



