-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
Mitochondrial dysfunction has been implicated in the pathogenesis of biliary atresia (BA). This study aimed to determine whether a specific mitochondrial DNA haplogroup is implicated in the pathogenesis and prognosis of BA. We determined 40 mitochondrial single nucleotide polymorphisms in 15 major mitochondrial haplogroups by the use of 24-plex PCR and fluorescent beads combined with sequence-specific oligonucleotide probes in 71 patients with BA and in 200 controls in the Taiwanese population of ethnic Chinese background. The haplogroup B4 and E prevalence were significantly lower and higher respectively, in the patients with BA than in the controls (odds ratios, 0.82 [p = 0.007] and 7.36 [p = 0.032] respectively) in multivariate logistic-regression analysis. The 3-year survival rate with native liver was significantly lower in haplogroup E than the other haplogroups (P = 0.037). A cytoplasmic hybrid (cybrid) was obtained from human 143B osteosarcoma cells devoid of mtDNA (ρ0 cell) and was fused with specific mtDNA bearing E and B4 haplogroups donated by healthy Taiwanese subjects. Chenodeoxycholic acid treatment resulted in significantly lower free radical production, higher mitochondrial membrane potential, more viable cells, and fewer apoptotic cybrid B4 cells than parental 143B and cybrid E cells. Bile acid treatment resulted in a significantly greater protective mitochondrial reaction with significantly higher mitochondrial DNA copy number and mitofusin 1 and 2 concentrations in cybrid B4 and parental cells than in cybrid E cells. The results of the study suggested that the specific mitochondrial DNA haplogroups B4 and E were not only associated with lower and higher prevalence of BA respectively, in the study population, but also with differential susceptibility to hydrophobic bile acid in the cybrid harboring different haplogroups.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003696
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003696Summary
Mitochondrial dysfunction has been implicated in the pathogenesis of biliary atresia (BA). This study aimed to determine whether a specific mitochondrial DNA haplogroup is implicated in the pathogenesis and prognosis of BA. We determined 40 mitochondrial single nucleotide polymorphisms in 15 major mitochondrial haplogroups by the use of 24-plex PCR and fluorescent beads combined with sequence-specific oligonucleotide probes in 71 patients with BA and in 200 controls in the Taiwanese population of ethnic Chinese background. The haplogroup B4 and E prevalence were significantly lower and higher respectively, in the patients with BA than in the controls (odds ratios, 0.82 [p = 0.007] and 7.36 [p = 0.032] respectively) in multivariate logistic-regression analysis. The 3-year survival rate with native liver was significantly lower in haplogroup E than the other haplogroups (P = 0.037). A cytoplasmic hybrid (cybrid) was obtained from human 143B osteosarcoma cells devoid of mtDNA (ρ0 cell) and was fused with specific mtDNA bearing E and B4 haplogroups donated by healthy Taiwanese subjects. Chenodeoxycholic acid treatment resulted in significantly lower free radical production, higher mitochondrial membrane potential, more viable cells, and fewer apoptotic cybrid B4 cells than parental 143B and cybrid E cells. Bile acid treatment resulted in a significantly greater protective mitochondrial reaction with significantly higher mitochondrial DNA copy number and mitofusin 1 and 2 concentrations in cybrid B4 and parental cells than in cybrid E cells. The results of the study suggested that the specific mitochondrial DNA haplogroups B4 and E were not only associated with lower and higher prevalence of BA respectively, in the study population, but also with differential susceptibility to hydrophobic bile acid in the cybrid harboring different haplogroups.
Introduction
Biliary atresia (BA) is characterized by progressive inflammatory cholangiopathy in infancy. The pathogenesis of BA may involve free radical products derived from mitochondria, resulting in significantly increased superoxide dismutase activity [1]. In the early stage of BA, augmented oxidative DNA and mitochondrial DNA (mtDNA) damage and apoptotic activities were also observed, which was associated with a decreased mitochondrial copy number [2].
The incidence of BA has been reported to be high in some geographic areas, including French Polynesia and Taiwan [3]–[5]. An interesting finding from a mitochondrial lineage analysis revealed that a specific mitochondrial haplogroup was associated with particular aboriginal races in Polynesia and Taiwan [6], with a rapid dispersal of maternal lineages from Taiwan approximately 4000 years ago [7]–[9]. Mitochondria are maternally inherited and accumulate mutations faster than does nuclear DNA [6]. An analysis of normal human mitochondrial DNA (mtDNA) identified many haplogroup-specific patterns of polymorphisms that have arisen over the last 150,000–200,000 years [10]–[12]. Studies in the past 10 years have identified an association of mtDNA single-nucleotide polymorphisms with many diseases [13]–[15]. The mtDNA mutation and mtDNA haplogroups are important in understanding the etiology and pathogenesis of human disease [16]–[18], as mutations of the mitochondrial D-Loop sequence have been demonstrated to be a risk factor for hepatocellular carcinoma (HCC) development [18].
In the present study, we identified the importance of the association of haplogroups B4 and E with BA, which confer resistance or susceptibility to hydrophobic bile acid and possibly to the different incidence of BA in different populations.
Results
Association of BA with specific mitochondrial haplogroups
The demographic data of 71 patients with BA, 52 children and 148 adults as controls, are shown in Table 1. Except for age, both control groups were devoid of liver diseases and their genetic distributions were almost identical except for one case of haplogroup E in the adult controls. It was therefore put together in the following statistical comparison of haplogroup prevalence with the patients with BA.
Tab. 1. Descriptive data of studied population. 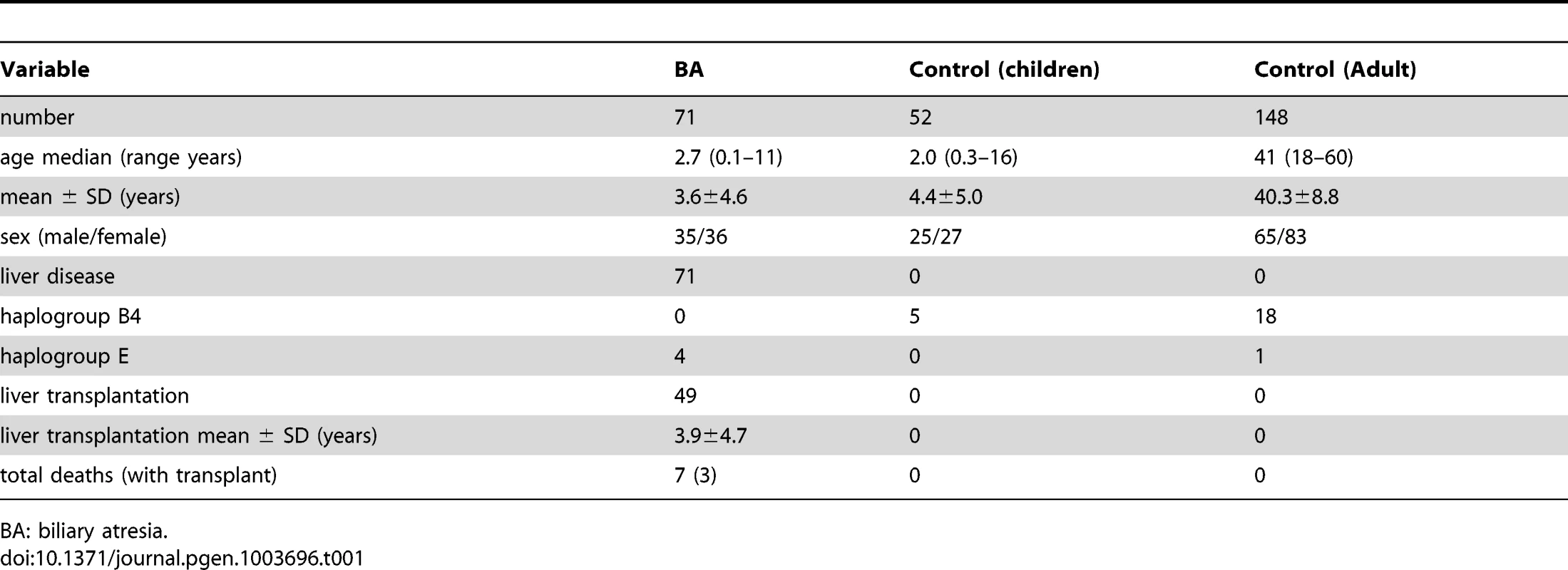
BA: biliary atresia. There were 4 cases of haplogroup E, all received kasai portoenterostomy. However, none survived with native liver. Among them, 3 received liver transplantation at ages 8 months, 1 year 5 months, 1 year 10 months, and the other one died at 3 years 1 month of age without receiving liver transplantation. The 3-year survival rate with native liver was significantly lower in haplogroup E than the other haplogroups (25.0% vs 48.6%, P = 0.037).
Evaluation of haplogroup distributions revealed that the haplogroup B4 was less prevalent in patients with BA than in the control group (odds ratio [OR], 0.71; 95% confidence interval [CI], 0.66–0.77; p = 0.001). In contrast, haplogroup E was more prevalent in BA than in the control group (OR, 11.88; 95% CI, 1.31–108.16; p = 0.018; Table 2). Multivariate logistic-regression analysis of haplogroups associated with BA with adjustment for age and sex, showing the risk for haplogroup B4 was p = 0.007, OR 0.82, 95%CI 0.71–0.85 and for haplogroup E was p = 0.032, OR 7.36, 95%CI 1.22–94.6.
Tab. 2. Association between biliary atresia (BA) and the mitochondrial haplogroups. 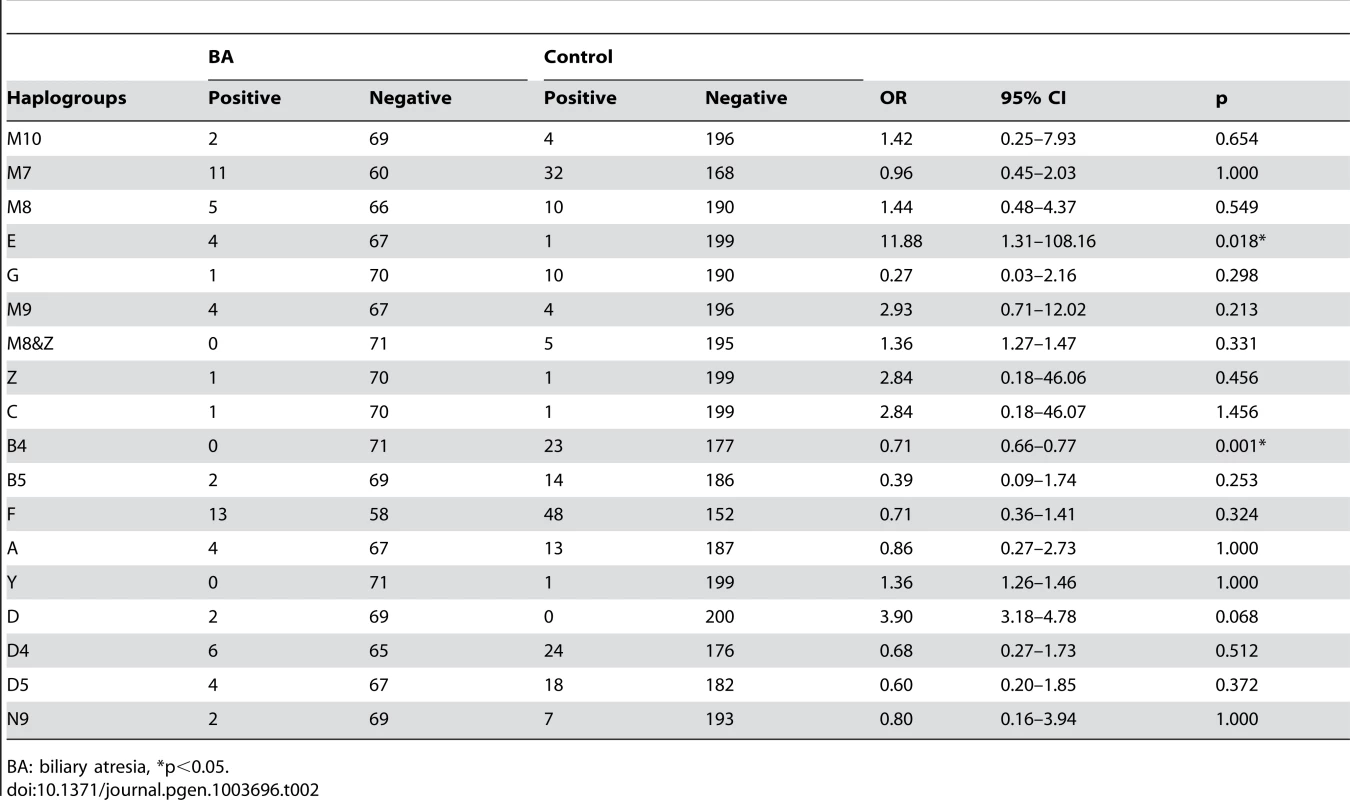
BA: biliary atresia, *p<0.05. The main DNA sequence difference(s) between mtDNA of B4 and E
The main differences between our mtDNA B4 and E are: T3027C (16SrRNA), G4491A (ND2, V 8 I), G7266T (COI, S 455 A), G7598A (COII, A 5 T), A7934G (COII, I 117 V), A8701G (ATPase6, T 59 A), A10398G (ND3, T 114 A), C10400T (ND3, T 114 A), T14577C (ND6, I 33 V), T16217C (D-loop), detected following a full-length sequencing of haplogroups B4 and E, shown in Table S1.
Differential mitochondrial membrane potential response to bile acids in cybrid B4 and E cells
Before conducting the experiments on different cybrids, we confirmed the absence of Cox-II in 143B ρ0 cells depleted of mtDNA but not in the wild-type 143B and cybrid B4 and E cells (Figure S1A). Organic anion-transporting polypeptides (OATPs) are sodium-independent organic anion transporters found in a variety of tissues, including those of the liver, and in the studied 143B cells. OATPs contribute to the transport of bile acids. To assess the responsiveness of the cybrids to bile acid treatment, we identified the bile acid receptor OATP mRNA (Figure S1B) and protein expression in the cybrids (Figures S1C and D).
In comparison with the wild-type 143B cells without bile acid treatment, the 143B cells and cybrid B4 and E cells treated with bile acid had a significantly decreased mitochondrial membrane potential (MMP). The change was time - and dose-dependent in all the 3 cell lines (Figures 1A and B). MMP was significantly higher in the cybrid B4 cells than in the cybrid E and parental 143B cells (Figure 1C).
Fig. 1. Time- and dose-dependent mitochondrial membrane potential in the parental 143B cells, and cybrid B4 and E cells in response to the bile acid treatment. 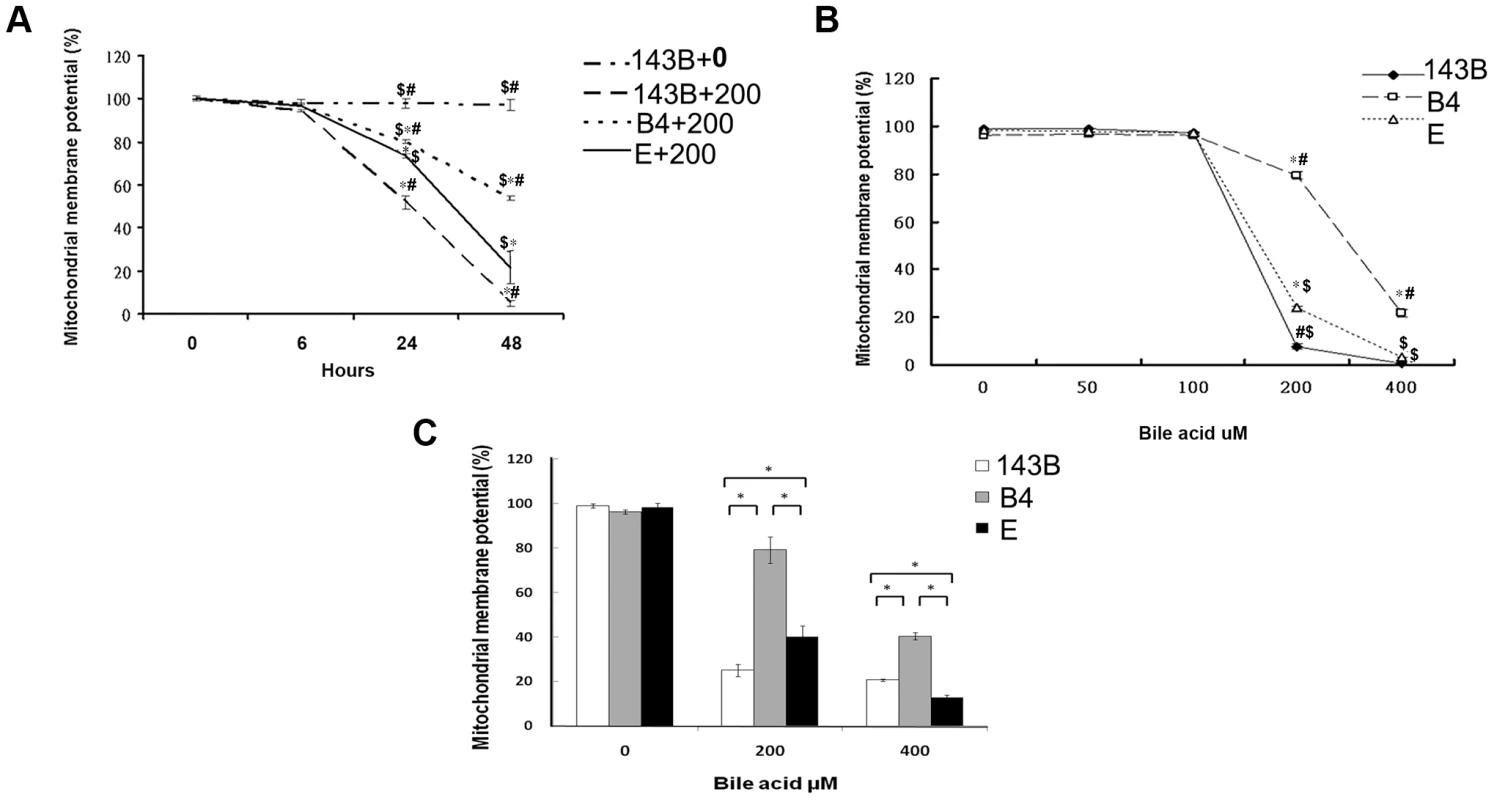
(A) Time-dependency of mitochondrial membrane potential in parental 143B cells, B4 and E cybrids in response to 200 uM of bile acid. * P<0.001 compared to parental 143B cell+0 uM of bile acid at the same times. # P<0.001 compared to cybrid E+200 uM of bile acid, $ P<0.001 compared to cybrid 143B+200 uM of bile acid at the same times. (B) dose-dependency of mitochondrial membrane potential in parental 143B cells, B4 and E cybrids in response to different concentrations of bile acid, ranging from 0 uM to 400 uM. * P<0.001 compared to parental 143B cell at the same doses of bile acid. # P<0.001 compared to cybrid E at the same doses of bile acid. $ P<0.001 compared to cybrid B4 at the same doses of bile acid. (C) Mitochondrial membrane potential in parental 143B cells, B4 and E cybrids. The results represented were mean ± SE in six times tests. * P<0.05 compared at the same doses of bile acid. Difference in reactive oxygen species production and mitochondrial copy number between the cybrid B4 and E cells in response to bile acid treatment
A flow cytometric measurement showed that the cybrid B4 cells had lower reactive oxygen species (ROS) production than the cybrid E and parental cells (Figure 2A).
Fig. 2. Measurement of reactive oxygen species (ROS) production and mitochondrial DNA copy number. 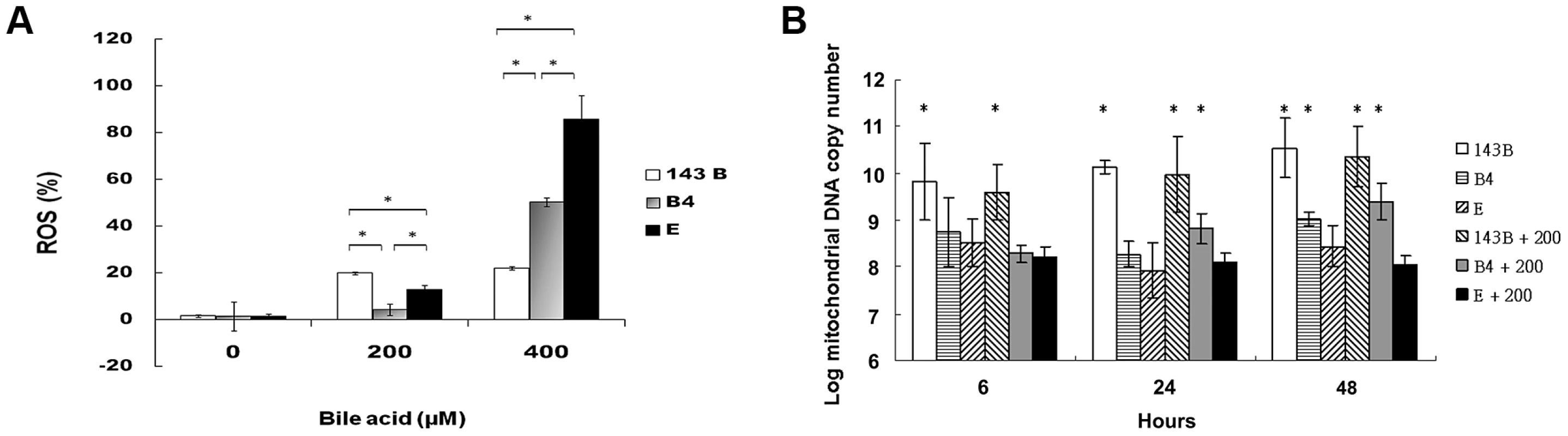
(A) Flow cytometric measurement of reactive oxygen species in parental 143B cells, B4 and E cybrids after treatment with 0, 200 and 400 uM of bile acid for 24 h. * P<0.05 compared at the same doses of bile acid. (B) Changes of mitochondrial DNA copy number in parental 143B cells, B4 and E cybrids after treatment with 0 or 200 uM bile acid for 6, 24 and 48 h. The results represented were mean ± SE in six times tests * P<0.05 compared to cybrid E+200 uM of bile acid. mtDNA copy number usually measures the responsiveness of mitochondria to stress. In this respect, cybrid B4 was unique in that its mtDNA copy number increased significantly compared with those of the parental 143B and cybrid E cells at 24 and 48 h after the bile acid treatment. On the contrary, mtDNA copy number significantly decreased as early as 6 h after the bile acid treatment in the E cybrid cells compared with the parental 143B and B4 cybrid cells (Figure 2B).
Less apoptosis in cells harboring haplogroup B4 than in parental or cybrid E cells treated with bile acid
Hydrophobic bile acids such as chenodeoxycholic acid are generally toxic to the cells with bile acid transporters. To determine apoptosis, 143B cells and cybrid B4 and E cells were treated with different doses of chenodeoxycholic acid for 24 and 48 h. After treatment, annexin V and PI double staining was performed, revealing significantly less apoptosis in the cybrid B4 cells than in the cybrid E and parental cells (Figure 3A, after 24 h of treatment).
Fig. 3. Cell apoptosis and viability. 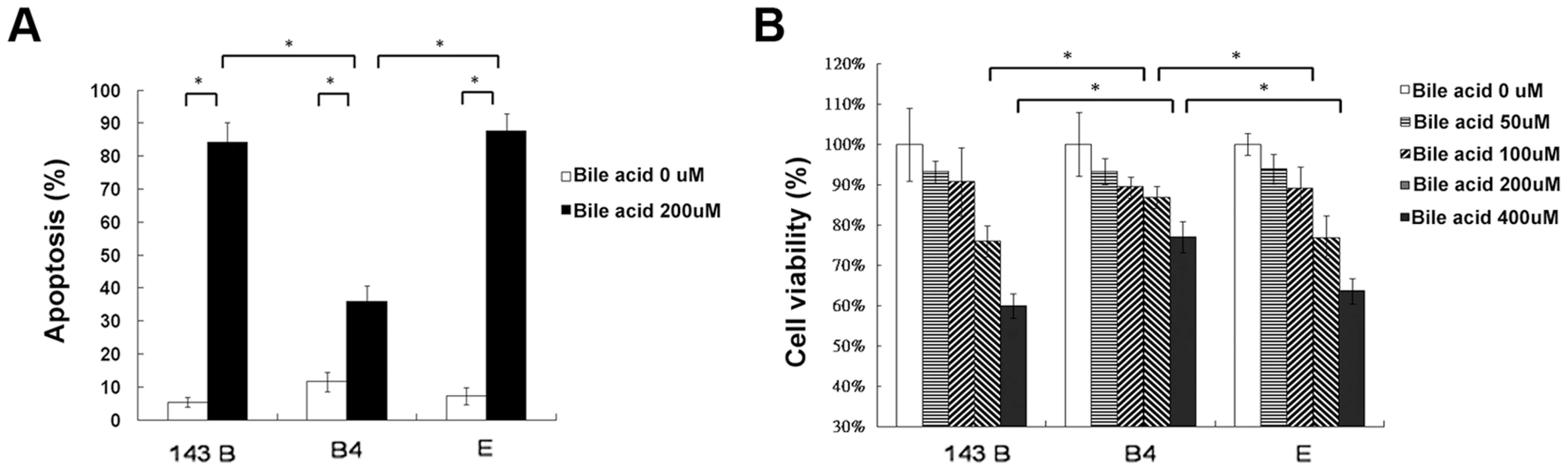
(A) Quantitation of flow cytometric measurement of apoptosis in parental 143B cells, B4 and E cybrids after treatment with 200 uM bile acid for 24 h and doubly stained with annexin V and PI. (B) The cell viability, measured by MTT assay, in parental 143B cells, B4 and E cybrids after treatment with 200 uM bile acid for 24 h. The results represented were mean ± SE in six times tests * P<0.05. Cell viability, as measured by MTT
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), indicated a dose-dependent decrease in viable cells among the 143Bcells, and cybrid B4 and E cells treated with increasing amount of bile acid. However, the cybrid E cells were most vulnerable to the bile acid treatment, having significantly fewer viable cells than the B4 and parental 143B cells at a dose higher than 200 µM for 24 and 48 h after the treatment (Figure 3B, after 24 h of treatment).
Significantly higher mitochondrial fusion protein expression in cybrid B4 cells than in cybrid E and parental cells treated with bile acid
Mitochondrial fusion is essential for organelle function and cellular homeostasis [19]. To assess mitochondrial fusion, the 143B cells, and cybrid B4 and E cells were treated with 200-µM chenodeoxycholic acid for 6, 24, and 48 h and were harvested to determine the mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) expression levels. As expected, significantly higher Mfn1 and Mfn2 expression levels were found in the cybrid B4 cells than in the cybrid E and parental 143B cells at 24 and 48 h after treatment (Figures 4A and 4B).
Fig. 4. Analysis of the mitochondrial fusion molecules expression in the parental 143B cells, and cybrid B4 and E cells after the bile acid treatment. 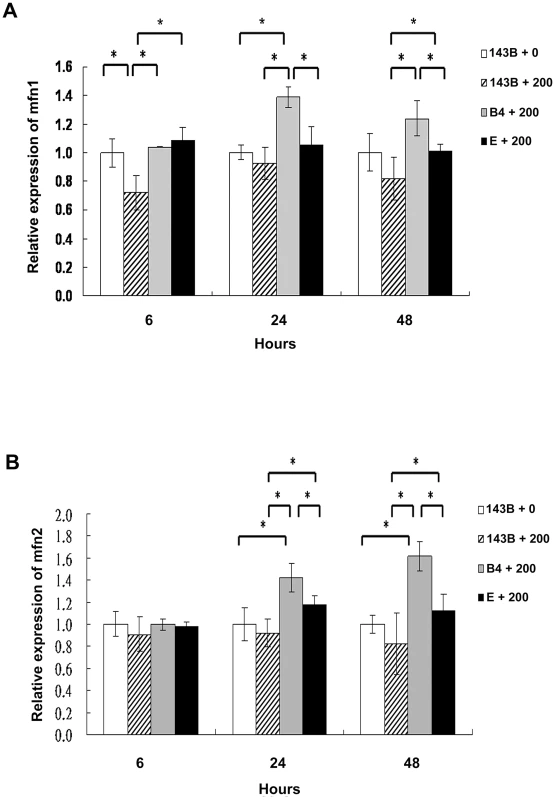
Western blot analysis of mitofusin 1 (mfn1) and mitofusin 2 (mfn2) expression in parental 143B cells, B4 and E cybrids after treatment with bile acid. (A) Histogram showed statistical difference between groups in mfn1 expression. Western blot of mfn 1 expression in parental 143B cells, B4 and E cybrids after treatment with 200 uM bile acid for 24 h and 48 h. (B) Histogram showed statistical difference between groups in mfn2 expression. Western blot of mfn 2 expression in parental 143B cells, B4 and E cybrids after treatment with 200 uM bile acid for 24 h and 48 h. The results represented were mean ± SE in six times tests * P<0.05. Cybrid B4 exhibited better tolerance and respiratory function, and less glycolytic reaction following bile acid exposure than did cybrid E
Our group has reported that cybrids with various mtDNA haplogroups demonstrated differences in oxidative phosphorylation (OXPHOS) capacity and viability under oxidative stress, suggesting mtDNA polymorphism contributes to differential tolerance against exogenous insult [20]. As shown in Figure 3B, cybrid B4 had better cell viability than cybrid E after exposure to bile acid. To assess whether the difference was due to different OXPHOS capacity and glycolysis, oxygen consumption rate (OCR) and lactate production were examined. Cybrid B4 had higher OCR in which ADP-limited (state 4), ADP-stimulated (state 3), and FCCP-induced (uncoupled) respiration than did cybrid E, 143B and ρ0 in either absence or presence of bile acid exposure (Figures 5A and 5B).In contrast, following bile acid exposure, cybrid E and parental 143B, but not cybrid B4 and ρ0, demonstrated a surge of lactate production (Figure 5C). These results indicated that the variations in tolerance against bile acid are correlated with mtDNA polymorphism-associated mitochondrial OXPHOS capacity. The differential viability and respiratory function in the presence of bile acid couple with metabolic shift to glycolysis.
Fig. 5. Effects of bile acid on OXPHOS and glycolysis in cybrid B4, cybrid E, parental 143B and ρ0 cell. 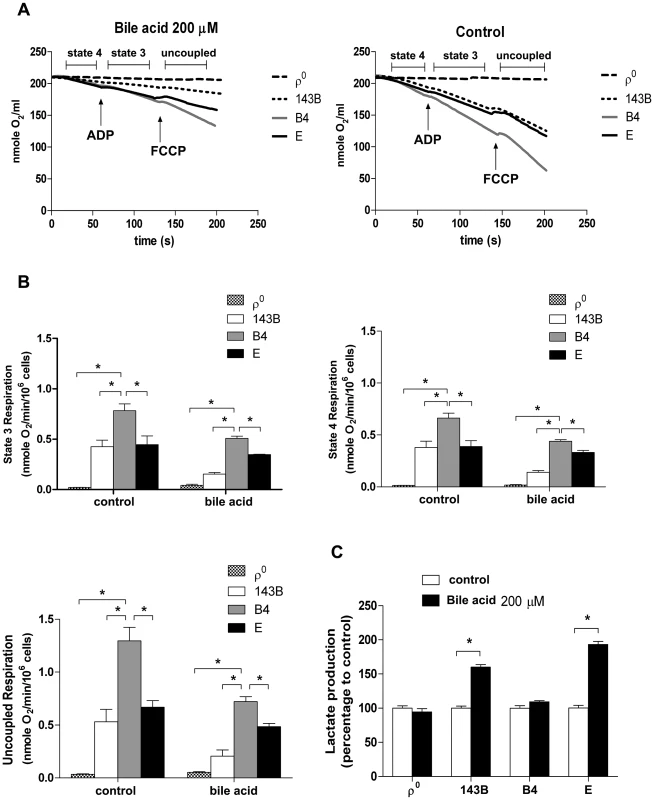
Cybrid B4, cybrid E, parental 143B and ρ0 were treated with PBS or 200 µM bile acid (BA) for 24 h. (A) Measuring respiratory function, oxygen content in medium is illustrated by polarographic curves. Arrows indicated the point of adding drugs. Mitochondrial state 4 respiration was determined using 10 mM glutamate/malate as substrate. State 3 and uncoupled respiration were determined in the presence of 0.2 mM ADP and 1 µM FCCP respectively. 5×106 cells/mL were resuspended in 100 µl respiratory medium. (B) Histogram showing the resulting oxygen consumption rate. (C) Culture medium was collected to detect the production of lactate, which was normalized to cell number. The results represented were mean ± SE in six times tests * P<0.05. Discussion
In this study, we found that the mtDNA haplogroups B4 and E were associated with BA, although with entirely different implications. We found that whereas the haplogroup B4 was significantly less likely to occur in the patients with BA, the haplogroup E was more likely to occur in the patients with BA than in the healthy controls. Given that deregulation of mitochondrial function has been implicated in the pathogenesis of BA [1], [2], we studied the differential responses of cybrids B4 and E to a hydrophobic bile acid (chenodeoxycholic acid)-induced oxidative injury. As expected, we found that cybrid B4 maintained a significantly higher MMP and produced significantly less oxygen free radicals than the cybrid E and parental cells in response to the bile acid treatment. Furthermore, mitochondrial copy number and mitochondrial fusion protein expression, two protective responses of mitochondria to stress, were significantly higher in the cybrid B4 cells than in the cybrid E and parental cells, which was associated with significantly fewer apoptotic cybrid B4 cells than apoptotic cybrid E and parental cells. Overall, these findings suggested a protective effect of the haplogroup B4 from oxidative liver injury, which is at least in part due to inherently better mitochondrial function.
Consistent with our findings is a significantly higher evolutionary rate of mtDNA than that of nuclear DNA [21] because mtDNA is more vulnerable to high levels of ROS, lack of protective histones, and limited capacity for DNA repair than nuclear DNA [22]. Mitochondrial dysfunction has been linked to organ failures [23], [24], and polymorphisms in the mtDNA are expected to contribute more extensively to functional differences between individuals compared with polymorphisms in nuclear DNA [15]. It is not surprising that many human diseases have been associated with pathogenic mutations in the mtDNA or haplogroup-associated polymorphisms [16], [25], [26]. In particular, the sequence polymorphism in the D-loop region of the mtDNA was a risk factor for HCC [18]. The single nucleotide polymorphisms in the D-loop region of mtDNA 16266C/T, 16293A/G, 16299A/G, 16303G/A, 242C/T, 368A/G, and 462C/T minor alleles, were associated with increased risk for alcohol - HCC, and the 523A/del was associated with increased risks of alcohol - HCC and HBV-HCC [18].
Each mammalian cell contains several hundreds to more than a thousand mitochondria, and each organelle harbors 2–10 copies of mtDNA [27]. The mtDNA copy number in a specific cell type is usually precisely maintained but might be subjected to change during cell growth and differentiation [28]–[30]. In our study, the increased mtDNA copy number in the haplogroup B4 was a protective response, which is similar to the protective response of human beings against sepsis either through increased heat generation because of higher electron transport rates or looser coupling [31], or through ROS production to reduce infection [12]. About 96% of our population group, including the B4 cybrid line used in this study, carry T16217C but only 12% carry the additional A16247G, C16261T control region variants [32], [33]. The combinations of these variants are specifically identified as a Polynesian motif, which has also been reported in some of the Chinese population [32]. In our study (Table S1), there are eight mtDNA sequence alterations between B4 and E which cause amino acid changes. Four alterations occurred at subunits of respiratory complex I, three at complex IV and one change at complex V. Previously, mitochondrial complexes I and IV were suggested as being the main site of mitochondrial electron leakage [34]. Thus, it is feasible to hypothesize that amino acid alteration in the subunits of these enzyme complexes could lead to mitochondrial functional change, including a higher oxidative stress in haplogroup E, which is associated with significantly lower MMP, higher ROS production, and thus higher cellular apoptosis. The results also imply less likely occurrence of the haplogroup B4 in the patients with BA than in the haplogroup E.
Initial decreases in MMP and subsequent ROS production are a characteristic feature of pre-apoptosis [35]–[38]. In the present study, the bile acid treatment resulted in an initial significant decrease in MMP, which was associated with an excessive intracellular ROS production in the cybrid and parental cells in a time - and dose-dependent manner. The mtDNA-based differences, including different mtDNA and mtRNA levels, mitochondrial protein synthesis, cytochrome oxidase activity and level, normalized oxygen consumption, and mitochondrial inner membrane potential and growth capacity, were also found between cybrids [39]. Such inherited differences in mitochondrial function, including oxidative phosphorylation capacity [40], can help to explain the differential bioenergetic threshold toward oxidative liver injury between populations with different haplogroups that confer their different susceptibility to BA.
Occasional dysfunctional mitochondria can be complemented functionally through mitochondrial fusion. In cells lacking mitochondrial fusion, dysfunctional mitochondria may accumulate, leading to injury-induced cell death [39]. Mfn1 is the main mediator of mitochondrial fusion and homeostasis [41]. High Mfn1 expression levels significantly improved embryo development by increasing ATP levels and MMP while reducing H2O2 generation [41]. Mfn2 is also important in mtDNA transmission to daughter cells by tethering the mitochondrial membrane [42]. Downregulation of Mfn2 was found to induce cellular apoptosis, whereas overexpression of Mfn2 diminished cellular apoptosis [39]. In our study, we found that the cybrid B4 cells had higher Mfn1 and Mfn 2 expression levels than the cybrid E and parental cells in response to the bile acid treatment. The latter may also help to explain why apoptosis was significantly less in the cybrid B4 cells than in cybrid E and parental cells after the bile acid treatment.
Kim reported that synthetic bile acids induce apoptosis of osteosarcoma cells through a caspase and mitochondrial pathway [43]. Bile acid increases osteoblast differentiation, and neutralizes the detrimental effects of lithocholic acid in jaundiced patients [44]. In this study, the parental osteosarcoma cells harboring haplogroup X, after bile acid treatment, had different MMP, higher mtDNA copy number, different ROS production, higher cellular apoptosis and lower Mfn1 and Mfn 2 expression levels. We cannot exclude the differential effects of the hapogroup B4 and E from “wild type” as related to their mtDNA haplogroup, as well as to the change of the characteristics of osteosarcoma cells during the process of constructing cybrids.
From the findings that the patients with BA harboring haplogroup E had worse prognosis than other haplogroups and had to receive liver transplant earlier or else died, it is reasonable to check haplogroups in every BA case in the future to predict the outcomes as early as possible.
In conclusion, the study provides a novel insight into the etiopathogenesis of BA, based on the genetic background of mitochondria. The use of the cybrid models helped to establish the association of haplogroups B4 and E with BA and to clarify the population-based differences in the incidence of BA.
Materials and Methods
Patients and ethics statement
The study was approved by the Ethics and Clinical Research Committee of the Chang Gung Memorial Hospital (No. 97-2268A3). Seventy-one patients with BA and 200 healthy controls in the Taiwanese population of ethnic Chinese background were recruited from the Kaohsiung Chang Gung Memorial Hospital.
BA was confirmed on the basis of results of liver histological examination and intraoperative findings, which included 35 males and 36 females ranging in age from 0.1–11 years (median 2.7). All received screening for cytomegalovirus, rubella, herpes infection, but no specific causative factors were identified. As our hospital is the biggest referring center for liver transplantation in Taiwan, there are more patients referred for liver transplant after failed Kasai procedure in the other hospitals than receiving the operation primarily in this hospital. Accordingly, only eighteen patients survived with their native liver, while the remaining 53 patients either survived with liver transplant (46) or died (7).
The control group included 52 children and 148 adults. The 52 children were originally recruited for test of reaction to allergens and were proven negative for allergy. There were 25 males and 27 females, ranging in age from 0.3 to 16 years (medium 2.0). The adult population comprised 65 males and 83 females, ranging in age from 18 to 60 (medium 41). They were healthy Taiwanese subjects randomly selected from the health screening center. None of the subjects had a history of BA, cholestatic or autoimmune liver diseases (Table 1). The genetic background was similar in both control groups and was put together for statistical calculation. Informed written consent was obtained from their legal guardians or the participants for use of their blood in the study.
Methods for determination of mitochondrial haplogroup
We performed haplogroup analysis, which included 40 mitochondrial single nucleotide polymorphisms in 15 major mitochondrial haplogroups. Genomic DNA was extracted from the whole blood. We used 24 pair primers to perform the gene amplification by multiplex polymerase chain reaction (PCR), which has been described as in Liou et al [32], [45]. The range of amplicon size was 190–300 base pairs (bp). We used 94 probes for mitochondrial haplogroup determination [32], [45]. The reactions were then measured by the Luminex100 flow cytometer. The detailed method for genotyping has been described in the protocol by Itoh Y et al [32], [45], [46].
Genotyping of mtDNA
Venous blood (3 mL) was collected from each subject into tubes containing 50 mmol/L EDTA (disodium salt), and genomic DNA was isolated by use of a commercial kit (Genomix, Talent, Trieste, Italy) [32]. Mitochondrial polymorphisms were determined with sequence-specific oligonucleotide probes by the use of suspension array technology (Luminex 100; Luminex, Austin, TX). The details have been described as in Liou et al [32].
Selection of mitochondrial polymorphism for haplogroup classification
The human mitochondrial single nucleotide polymorphism (mtSNP) database provided in the Mitomap website (http://www.mitomap.org/MITOMAP) was used for reference. We selected 40 mtSNPs that defined 15 major haplogroups (A, B, C, D, E, F, G, M7, M8, M9, M10, M11, M12, M13, N9) and their constitutive 13 sub-haplogroups (B4, B5, D4, D5, F1, F2, F3, F4, M7a, M7b, M7c, M8a, N9a) in this study. The selection was based on the previously constructed phylogenetic trees for Chinese and Japanese populations [32], [47], [48].
Generation of ρ0 cells
Human osteosarcoma 143B cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/liter of glucose, supplemented with 10% (v/v) FBS, 50 units of penicillin/ml, and 50 µg/ml of streptomycin at 37°C in 5% CO2. For ρ0 cells generation, cells were grown in the presence of 50 ng/ml ethidium bromide in DMEM containing 4.5 g/liter of glucose, supplemented with 10% (v/v) FBS, 50 units of penicillin/ml, 50 µg/ml of streptomycin, 50 µg/ml of uridine, and 110 µg/ml of sodium pyruvate (ρ0 medium) at 37°C in 5% CO2 for three months. The medium was changed every 2–3 days and the cells were re-plated once a week. The absence of mitochondrial DNA was determined by real-time PCR or by Western blot confirmation of low or absent cytochrome c oxidase-II (Cox-II) expression [49].
Construction of cybrids containing haplogroup B4 and E
Platelets were used as mtDNA donor. Fusion of platelets from the healthy Taiwanese volunteers with haplogroup B4 and E with 143B ρ0 cells were carried out in the presence of 50% (wt/vol) polyethylene glycol 1500 (Boehringer Mannheim). The fusion mixture was recovered for 1 week in ρ0 medium and then cultivated in selection DMEM medium, in which even cybrids with very low Cox-II expression were shown to grow without pyruvate and uridine. Negative control plates consisted of 143B ρ0 cells, which had undergone trans-mitochondrial fusion in the absence of platelets. On days 14–30 after fusion, successful cybrid clones growing in the medium were isolated clonally by limited dilution, while negative control cells died out. The presence of exogenously imported mtDNA, or specific mtDNA haplogroup in the cybrid was confirmed by Luminex100™ system.
MTT assay
Human osteosarcoma 143B cells, 143Bρ0 cells and cybrids carrying B4 and E haplogroups were washed once with phosphate-buffered saline (PBS), followed by the addition of 1 mL DMEM containing 0.05 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2 and 5-diphenyltetrazolium bromide (MTT; Sigma). After incubation at 37°C for 1 h, the media were removed and the formazan crystals in the cells were dissolved in 1 mL DMSO. Cell viability was detected at an OD570 nm using a spectrophotometer.
Measurement of reactive oxygen species (ROS) production
To evaluate ROS levels, cybrid B4 and E cells (6×105 cells/flask) were incubated with different concentrations of chenodeoxycholic acid for different time periods. Cells were then stained with 10 µM of Dichlorofluorescein diacetate for 30 min at 37°C, and then detached with trypsin/EDTA. Cells were collected in 1× PBS, washed twice by centrifugation (1500 rpm; 5 min), and resuspended in 0.5 mL 1× PBS for ROS production. It was measured by flow cytometry utilizing a BD Biosciences FACScan system. ROS production was expressed as mean fluorescence intensity (MFI), which was calculated by CellQuest software.
Mitochondrial membrane potential (Δψ)
6×105 cells were plated in six-well plates and allowed to attach for 16–18 h. After being treated with bile acids at different concentration for 24 h or 48 h, the cells were harvested by treatment with trypsin, washed in PBS, and resuspended in 200 ng/mL of Rhodamine 123 (Invitrogen). After incubation for 30 min at 37°C, the cells were washed three times and resuspended in 500 µL of PBS. Cytofluorimetric analysis was performed using a fluorescence-activated cell scanner machine (BD Biosciences FACScan system) [2], [49].
Determination of mtDNA copy number
DNA samples were extracted from cybrid cells. The mtDNA copy numbers were measured by a real-time PCR and corrected by simultaneous measurement of the nuclear DNA (β-actin gene). The forward and reverse primers complementary to the nuclear β-actin gene and ND1 gene were as presented before [2]. The threshold cycle number (Ct) values of the β-actin gene and the ND1 gene were determined for each individual quantitative PCR run. Relative copy number (Rc) = 2ΔCT, where ΔCT was the Ct ß-actin - Ct ND1 [50]. Each measurement was carried out at least three times and normalized in each experiment against a serial dilution of a control DNA sample.
Flow cytometric measurement of cell apoptosis
The cybrid B4 and, E cells (6×105 cells/flask) were incubated with various concentrations of bile and H2O2 and harvested at 6, 24 and 48 h respectively. The percentage of cells in different phases of cell cycle was analyzed by flow cytometry after staining with propidium iodide (PI). Methodology is described briefly as follows; cells were fixed overnight with 70% ethanol at 4°C and washed twice with PBS. Then cells were centrifuged 1,500 RPM for 5 min and supernatant discarded. The cell pellet was resuspended in 1 ml PI (50 µg/ml)/0.05%Triton X-100 staining solution with RNase A (0.1 mg/ml) and incubated for 30 min at 37°C. Additionally, an apoptosis assay was performed using an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences). The samples were analyzed on a Becton Dickinson FACScan flow cytometer using the CellQuest software. Annexin V positive/PI negative and Annexin V positive/PI positive cells were defined as necrotic and apoptotic cells respectively.
Western blotting analysis
The cells were homogenized in a buffer then centrifuged at 14,000 g. Protein (40 µg) from the supernatant of each sample was separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes for electrophoresis. The membranes were blocked in TBST buffer for 1 h at room temperature after which the blots were incubated with a primary cytochrome c oxidase-II (Cox-II), OATP (organic anion-transporting polypeptide), Mfn1, Mfn2 antibody (Santa Cruz Biotech.), followed by a secondary alkaline phosphatase-conjugated anti-IgG antibody (1∶5000; Promega). The Western blots were visualized with the Blot AP System (Promega). Alpha-tubulin or b-actin was used as internal control.
Oxygen consumption analysis
Detection of oxygen consumption in permeabilized cells was described previously with some modifications [51], [52]. Oxygen consumption was monitored by a Clark electrode (Mitocell S200 micro respirometry system; Strathkelvin Instruments, Motherwell, UK). Cells (100 µL at 5×106 cells/mL) in a respiratory medium (100 mM KCl, 3 mM MgCl2, 20 mM HEPES, 1 mM EDTA, and 5 mM KH2PO4; pH 7.4) were permeabilized by digitonin (optimal concentration 32.5 µg/mL as determined by trypan blue staining) and loaded into a 200 µL MT200 Respirometer chamber, suspended by a fixed-speed solid-state magnetic stirrer inside the chamber and maintained at 37°C by a circulating water bath. Mitochondrial state 4 respiration was determined using 10 mM glutamate/malate as substrates of respiratory complex I in the absence of ADP. Subsequently, mitochondrial state 3 was measured in the presence of 0.2 mM ADP. The following uncoupled respiration was determined by the addition of 1 µM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) to the respiratory medium. Oxygen level in medium was expressed in nanomoles of O2 per mL. Oxygen consumption rate was expressed in nanomoles of O2 per minute per 106 cells. Lactate was measured in collected culture medium using the Lactate Colorimetric Assay Kit (Abcam).
Statistical analysis
Frequencies of all mitochondrial haplogroups in the BA patients and controls were tested for independency using Pearson chi-square statistics and Fisher's exact test as appropriate. Cybrid data were expressed as mean ± SE. Comparisons between different cybrids and parental cells were analyzed by the ANOVA with Bonferroni's correction when multiple comparisons were evaluated. We performed multivariate logistic-regression analysis, with BA as a dependent variable and independent variables including age and sex. Patient survival was assessed using the Kaplan–Meier method and compared between groups using the log–rank test. Survival with native liver was analyzed using time from birth until the time of follow-up excluding those who died or received liver transplantation. A p-value <0.05 was considered statistically significant. The statistical analyses were performed using the Statistical Package for Social Science (SPSS, version 12) software package.
Supporting Information
Zdroje
1. BroideE, KlinowskiE, KoukoulisG, HadzicN, PortmannB, et al. (2000) Superoxide dismutase activity in children with chronic liver diseases. J Hepatol 32 : 188–192.
2. TiaoMM, LinTK, KuoFY, HuangCC, DuYY, et al. (2007) Early stage of biliary atresia is associated with significant changes in 8-hydroxydeoxyguanosine and mitochondrial copy number. J Pediatr Gastroenterol Nutr 45 : 329–334.
3. VicP, GestasP, MalletEC, ArnaudJP (1994) [Biliary atresia in French Polynesia. Retrospective study of 10 years]. Arch Pediatr 1 : 646–651.
4. LinYC, ChangMH, LiaoSF, WuJF, NiYH, et al. (2011) Decreasing rate of biliary atresia in Taiwan: a survey, 2004–2009. Pediatrics 128: e530–536.
5. GrizeljR, VukovicJ, NovakM, BatinicaS (2010) Biliary atresia: the Croatian experience 1992–2006. Eur J Pediatr 169 : 1529–1534.
6. SykesB, LeiboffA, Low-BeerJ, TetznerS, RichardsM (1995) The origins of the Polynesians: an interpretation from mitochondrial lineage analysis. Am J Hum Genet 57 : 1463–1475.
7. SoaresP, RitoT, TrejautJ, MorminaM, HillC, et al. (2011) Ancient voyaging and Polynesian origins. Am J Hum Genet 88 : 239–247.
8. TrejautJA, KivisildT, LooJH, LeeCL, HeCL, et al. (2005) Traces of archaic mitochondrial lineages persist in Austronesian-speaking Formosan populations. PLoS Biol 3: e247.
9. TabbadaKA, TrejautJ, LooJH, ChenYM, LinM, et al. (2010) Philippine mitochondrial DNA diversity: a populated viaduct between Taiwan and Indonesia? Mol Biol Evol 27 : 21–31.
10. CannRL, StonekingM, WilsonAC (1987) Mitochondrial DNA and human evolution. Nature 325 : 31–36.
11. WallaceDC (1999) Mitochondrial diseases in man and mouse. Science 283 : 1482–1488.
12. WallaceDC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39 : 359–407.
13. FukuN, OshidaY, TakeyasuT, GuoLJ, KurataM, et al. (2002) Mitochondrial ATPase subunit 6 and cytochrome B gene polymorphisms in young obese adults. Biochem Biophys Res Commun 290 : 1199–1205.
14. GuoLJ, OshidaY, FukuN, TakeyasuT, FujitaY, et al. (2005) Mitochondrial genome polymorphisms associated with type-2 diabetes or obesity. Mitochondrion 5 : 15–33.
15. NishigakiY, YamadaY, FukuN, MatsuoH, SegawaT, et al. (2007) Mitochondrial haplogroup A is a genetic risk factor for atherothrombotic cerebral infarction in Japanese females. Mitochondrion 7 : 72–79.
16. DimauroS, DavidzonG (2005) Mitochondrial DNA and disease. Ann Med 37 : 222–232.
17. TaylorRW, TurnbullDM (2005) Mitochondrial DNA mutations in human disease. Nat Rev Genet 6 : 389–402.
18. ZhangR, ZhangF, WangC, WangS, ShiaoYH, et al. (2010) Identification of sequence polymorphism in the D-Loop region of mitochondrial DNA as a risk factor for hepatocellular carcinoma with distinct etiology. J Exp Clin Cancer Res 29 : 130.
19. ChenY, LiuY, DornGW2nd (2011) Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109 : 1327–1331.
20. LinTK, LinHY, ChenSD, ChuangYC, ChuangJH, et al. (2012) The creation of cybrids harboring mitochondrial haplogroups in the Taiwanese population of ethnic Chinese background: an extensive in vitro tool for the study of mitochondrial genomic variations. Oxid Med Cell Longev 2012 : 824275.
21. PakendorfB, StonekingM (2005) Mitochondrial DNA and human evolution. Annu Rev Genomics Hum Genet 6 : 165–183.
22. LightowlersRN, ChinneryPF, TurnbullDM, HowellN (1997) Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends Genet 13 : 450–455.
23. ProttiA, SingerM (2007) Strategies to modulate cellular energetic metabolism during sepsis. Novartis Found Symp 280 : 7–16 discussion 16–20, 160–164.
24. AmoT, YadavaN, OhR, NichollsDG, BrandMD (2008) Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene 411 : 69–76.
25. HerrnstadtC, HowellN (2004) An evolutionary perspective on pathogenic mtDNA mutations: haplogroup associations of clinical disorders. Mitochondrion 4 : 791–798.
26. DiMauroS, SchonEA (2001) Mitochondrial DNA mutations in human disease. Am J Med Genet 106 : 18–26.
27. RobinED, WongR (1988) Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol 136 : 507–513.
28. WiesnerRJ, KurowskiTT, ZakR (1992) Regulation by thyroid hormone of nuclear and mitochondrial genes encoding subunits of cytochrome-c oxidase in rat liver and skeletal muscle. Mol Endocrinol 6 : 1458–1467.
29. RenisM, CantatoreP, Loguercio PolosaP, FracassoF, GadaletaMN (1989) Content of mitochondrial DNA and of three mitochondrial RNAs in developing and adult rat cerebellum. J Neurochem 52 : 750–754.
30. ShayJW, PierceDJ, WerbinH (1990) Mitochondrial DNA copy number is proportional to total cell DNA under a variety of growth conditions. J Biol Chem 265 : 14802–14807.
31. BaudouinSV, SaundersD, TiangyouW, ElsonJL, PoynterJ, et al. (2005) Mitochondrial DNA and survival after sepsis: a prospective study. Lancet 366 : 2118–2121.
32. LiouCW, ChenJB, TiaoMM, WengSW, HuangTL, et al. (2012) Mitochondrial DNA coding and control region variants as genetic risk factors for type 2 diabetes. Diabetes 61 : 2642–2651.
33. ParkKS, ChanJC, ChuangLM, SuzukiS, ArakiE, et al. (2008) A mitochondrial DNA variant at position 16189 is associated with type 2 diabetes mellitus in Asians. Diabetologia 51 : 602–608.
34. LiuY, FiskumG, SchubertD (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80 : 780–787.
35. LingX, ZhouY, LiSW, YanB, WenL (2010) Modulation of mitochondrial permeability transition pore affects multidrug resistance in human hepatocellular carcinoma cells. Int J Biol Sci 6 : 773–783.
36. JiYB, QuZY, ZouX (2011) Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp Toxicol Pathol 63 : 69–78.
37. LyJD, GrubbDR, LawenA (2003) The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 8 : 115–128.
38. GottliebE, Vander HeidenMG, ThompsonCB (2000) Bcl-x(L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 20 : 5680–5689.
39. Jahani-AslA, CheungEC, NeuspielM, MacLaurinJG, FortinA, et al. (2007) Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. J Biol Chem 282 : 23788–23798.
40. Gomez-DuranA, Pacheu-GrauD, Lopez-GallardoE, Diez-SanchezC, MontoyaJ, et al. (2010) Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet 19 : 3343–3353.
41. HuaS, ZhangH, SongY, LiR, LiuJ, et al. (2012) High expression of Mfn1 promotes early development of bovine SCNT embryos: improvement of mitochondrial membrane potential and oxidative metabolism. Mitochondrion 12 : 320–327.
42. ScottSV, Cassidy-StoneA, MeeusenSL, NunnariJ (2003) Staying in aerobic shape: how the structural integrity of mitochondria and mitochondrial DNA is maintained. Curr Opin Cell Biol 15 : 482–488.
43. KimG, HerY, ParkJ, MoonY, YooY, et al. (2004) Synthetic Bile Acid Derivative HS-1200-induced Apoptosis of Human Osteosarcoma Cells. The Korean J Anat 37 : 449–457.
44. DubreuilM, Ruiz-GaspaS, GuanabensN, PerisP, AlvarezL, et al. (2013) Ursodeoxycholic acid increases differentiation and mineralization and neutralizes the damaging effects of bilirubin on osteoblastic cells. Liver Int 33 : 1029–1038.
45. LiouCW, LinTK, ChenJB, TiaoMM, WengSW, et al. (2010) Association between a common mitochondrial DNA D-loop polycytosine variant and alteration of mitochondrial copy number in human peripheral blood cells. J Med Genet 47 : 723–728.
46. ItohY, MizukiN, ShimadaT, AzumaF, ItakuraM, et al. (2005) High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a PCR-SSOP-Luminex method in the Japanese population. Immunogenetics 57 : 717–729.
47. TanakaM, CabreraVM, GonzalezAM, LarrugaJM, TakeyasuT, et al. (2004) Mitochondrial genome variation in eastern Asia and the peopling of Japan. Genome Res 14 : 1832–1850.
48. YaoYG, KongQP, BandeltHJ, KivisildT, ZhangYP (2002) Phylogeographic differentiation of mitochondrial DNA in Han Chinese. Am J Hum Genet 70 : 635–651.
49. ChangYC, LiaoCB, HsiehPY, LiouML, LiuYC (2008) Expression of tumor suppressor p53 facilitates DNA repair but not UV-induced G2/M arrest or apoptosis in Chinese hamster ovary CHO-K1 cells. J Cell Biochem 103 : 528–537.
50. TiaoMM, LinTK, LiouCW, WangPW, ChenJB, et al. (2009) Early transcriptional deregulation of hepatic mitochondrial biogenesis and its consequent effects on murine cholestatic liver injury. Apoptosis 14 : 890–899.
51. GrangerDL, LehningerAL (1982) Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol 95 : 527–535.
52. VrbackyM, KrijtJ, DrahotaZ, MelkovaZ (2003) Inhibitory effects of Bcl-2 on mitochondrial respiration. Physiol Res 52 : 545–554.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



