-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRegulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
Analogously to chromosome cohesion in eukaryotes, newly replicated DNA in E. coli is held together by inter-sister linkages before partitioning into daughter nucleoids. In both cases, initial joining is apparently mediated by DNA catenation, in which replication-induced positive supercoils diffuse behind the fork, causing newly replicated duplexes to twist around each other. Type-II topoisomerase-catalyzed sister separation is delayed by the well-characterized cohesin complex in eukaryotes, but cohesion control in E. coli is not currently understood. We report that the abundant fork tracking protein SeqA is a strong positive regulator of cohesion, and is responsible for markedly prolonged cohesion observed at “snap” loci. Epistasis analysis suggests that SeqA stabilizes cohesion by antagonizing Topo IV-mediated sister resolution, and possibly also by a direct bridging mechanism. We show that variable cohesion observed along the E. coli chromosome is caused by differential SeqA binding, with oriC and snap loci binding disproportionally more SeqA. We propose that SeqA binding results in loose inter-duplex junctions that are resistant to Topo IV cleavage. Lastly, reducing cohesion by genetic manipulation of Topo IV or SeqA resulted in dramatically slowed sister locus separation and poor nucleoid partitioning, indicating that cohesion has a prominent role in chromosome segregation.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003673
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003673Summary
Analogously to chromosome cohesion in eukaryotes, newly replicated DNA in E. coli is held together by inter-sister linkages before partitioning into daughter nucleoids. In both cases, initial joining is apparently mediated by DNA catenation, in which replication-induced positive supercoils diffuse behind the fork, causing newly replicated duplexes to twist around each other. Type-II topoisomerase-catalyzed sister separation is delayed by the well-characterized cohesin complex in eukaryotes, but cohesion control in E. coli is not currently understood. We report that the abundant fork tracking protein SeqA is a strong positive regulator of cohesion, and is responsible for markedly prolonged cohesion observed at “snap” loci. Epistasis analysis suggests that SeqA stabilizes cohesion by antagonizing Topo IV-mediated sister resolution, and possibly also by a direct bridging mechanism. We show that variable cohesion observed along the E. coli chromosome is caused by differential SeqA binding, with oriC and snap loci binding disproportionally more SeqA. We propose that SeqA binding results in loose inter-duplex junctions that are resistant to Topo IV cleavage. Lastly, reducing cohesion by genetic manipulation of Topo IV or SeqA resulted in dramatically slowed sister locus separation and poor nucleoid partitioning, indicating that cohesion has a prominent role in chromosome segregation.
Introduction
Chromosome dynamics studies in E. coli using either fluorescent in situ hybridization (FISH) or fluorescent repressor proteins bound to arrays of operator sequences (FROS) have shown that there is a significant time delay between passage of the replication fork and separation of replicated sequences into two visible foci [1]–[6]. Comprehensive surveys across the E. coli chromosome indicate that this delay is ∼10 minutes at most sites [3], [6], suggesting that a several hundred kilobase sliding window of sister “non-separation” (i.e., cohesion) follows each replication fork. Superimposed on this brief and progressive cohesion program, three regions have been identified that exhibit much longer cohesion, including the replication origin, oriC and two broad domains on the right chromosome arm [3], [6]. The two late-splitting right arm regions, which we term “snaps”, are further unique in that their cohesion is lost simultaneously and is accompanied by a major global nucleoid reorganization event that gives rise to a bilobed nucleoid morphology [6]. This abrupt transition involves significant nucleoid expansion [7] and comprises a sister individualization step in which each nucleoid lobe contains one partially replicated daughter chromosome [6]. These data led us to propose that snap regions promote efficient chromosome segregation by resisting global sister chromosome separation until an appropriate time in the cell cycle. In this light, snaps may be analogous to eukaryotic centromere elements, which provide essential tension for microtubule-assisted chromosome segregation (Discussion).
Although there is no known bacterial equivalent of the eukaryotic cohesin complex that holds sisters together by a covalent ring structure [8], several lines of evidence suggest that colocalized sister regions in E. coli form a molecular complex. First, for the duration of the segregation delay, “cohered” regions remain within the resolution limit of fluorescence microscopy, ∼230 nm [6]. Subsequent separation is very rapid (1–2 µm in 1–3 min; [9]), implying that segregation tension is counteracted by covalent linkages during cohesion. Second, disruption of the oriC partitioning apparatus by eliminating MukB does not cause increased oriC cohesion [10], as would be expected if newly replicated regions merely passively colocalized until acted upon by segregation machinery. Third, a critical component of cohesion in E. coli appears to be the decatenating enzyme topoisomerase IV (Topo IV), suggesting that part or all of the basis for cohesion is entanglement of replicated DNA behind the fork [4]. Fourth, inter-sister recombination exchanges occur more frequently between cohered loci [11], indicating that homologous sequences physically interact during the colocalization period, and are not merely in the same subcellular vicinity.
Currently, the only known mediator of cohesion in E. coli is the well-conserved type-II topoisomerase, Topo IV. Inactivation of Topo IV via a temperature-sensitive mutation led to a reduction in sister separation near oriC [4], [11], and also within the terminus region [11], implying that Topo IV modulates cohesion across the E. coli chromosome. Topo IV, which relaxes positively supercoiled DNA molecules by a double-stranded cut/passage/ligation mechanism [12], was initially thought to act primarily in the terminus region, where converging replication forks generate maximal positive supercoiling. However, Topo IV is also present at the replisome continually during replication [13], [14], which suggests that positive supercoils frequently migrate behind the replication fork, causing nascent sister duplexes to wind around each other in a precatenane structure. Single molecule studies estimate that Topo IV, present at ∼1000 molecules per cell, has a total unlinking capacity of ∼6000 strand passages per second [15], several orders of magnitude faster than the rate at which precatenanes are predicted to form [16]. In contrast, cohesion lasts at minimum ∼7 minutes and up to 30 minutes along snap regions [6]. Thus, it appears that either cohesion involves another molecular linking component besides precatenanes, or, that Topo IV is negatively regulated by an unknown factor.
To investigate how sister cohesion is regulated in E. coli, we analyzed cohesion timing in a broad range of chromosome structure and segregation mutants. Candidate cohesion regulatory proteins included the SMC-like proteins MukB and RecN, the nucleoid associated proteins HU, IHF and Fis, the replication fork tracking protein SeqA and its binding regulator Dam, and Topo IV. MukB and RecN are the only E. coli proteins with structural similarity to eukaryotic cohesin [17], and could potentially promote cohesion by forming protein bridges across sister chromosomes [1]. The “histone-like” proteins HU, IHF and Fis, and the abundant DNA binding protein SeqA, are important for maintaining nucleoid structure and supercoiling [18], and could also modulate cohesion through bridging or by net effects on chromosome compaction [19]. SeqA in particular is well positioned to regulate cohesion because it binds strongly and specifically to newly replicated DNA [20]. As DNA exits the replication fork the newly synthesized strand is unmethylated for a period of 5–10 minutes, before remethylation by Dam methylase [21]. During this period of hemimethylation, GATC sequences are bound by SeqA, with potentially several hundred molecules bound behind each fork [19], and SeqA-GFP fusions forming large foci near or adjacent to sites of DNA replication [19], [22], [23]. It may not be coincidence that hemimethylation and cohesion periods (of typical non-snap loci) are very near equal. Importantly, in addition to a direct (bridging) mechanism, any of these proteins could regulate cohesion indirectly by affecting the processing of DNA catenanes. Supporting this idea, both SeqA and MukB interact with Topo IV in vivo and have been shown to strongly affect Topo IV decatenase activity in vitro [24]–[26].
Results
Chromosome cohesion is oppositely regulated by SeqA and Topo IV
To identify factors involved in the regulation of chromosome cohesion, we performed a candidate screen for mutants that have increased or decreased sister cohesion. Mutants were selected that displayed both a moderate to severe chromosome segregation phenotype and abnormal nucleoid shape or compaction (Introduction). Cohesion in these strains was determined in exponential cultures at the well-characterized gln locus using our standard non-synchronized cell assay (Figure 1A). gln copy number is determined by first measuring oriC copies per cell by rifampicin runoff flow cytometry, then measuring the relative ratio of gln copies to oriC copies by quantitative real time PCR (qPCR). In parallel, gln foci per cell is determined by FROS in which a tandem array of tetO binding sequences is inserted into the chromosome and subsequently bound by a fluorescent TetR-YFP fusion protein. The duration of sister co-localization (cohesion) is then proportional to the difference in gln copy number and gln foci per cell. This assay requires high efficiency of fluorescence detection (below) and sufficient resolution of segregated loci. Because initial segregation velocities are rapid, about 0.4 µm/min in the current study with final positions 3–10 times greater than the resolution distance of light microscopy (shown below), we estimate that sister loci appear as two fluorescent foci <30 seconds after loss of sister cohesion. Although this cohesion assay is valid under any growth rate, cells were grown in minimal media supplemented with alanine or succinate as indicated to minimize overlapping replication cycles, which simplified microscopy analysis.
Fig. 1. Cohesion in E. coli chromosome structure mutants. 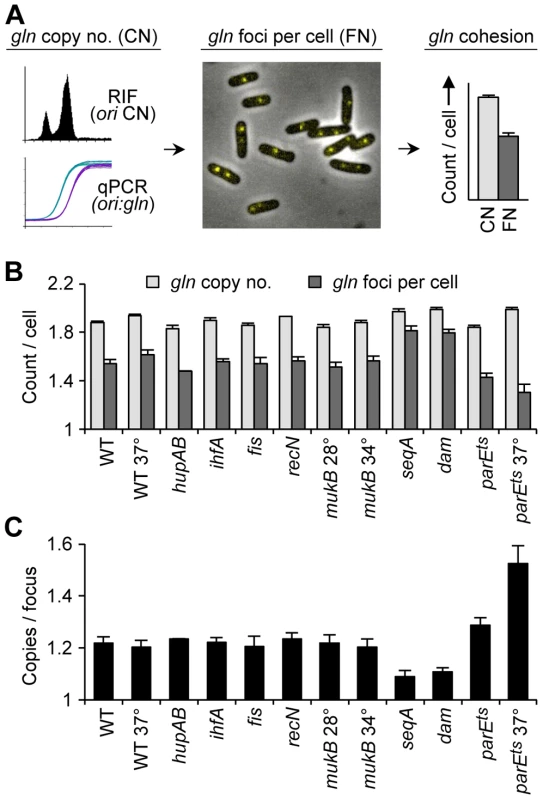
(A) Cohesion assay. Cohesion values (gln copies per focus) are determined by independently measuring gln copy number and resolvable gln foci per cell in asynchronous exponential cells bearing a tetO array at gln and expressing fluorescent TetR-YFP. See text for details. (B) gln copy number and TetR-YFP foci per cell in wild-type and mutant cells bearing a tetO array at the gln locus. Cells were grown exponentially in minimal alanine media, and gln copy number and foci per cell were determined (Materials and Methods). Cells were grown continually at 30°C or shifted to the indicated temperature 4 hours prior to analysis. Values are means of three independent experiments ±1 standard deviation (SD). (C) Cohesion values (average number of gln copies per gln focus) for candidate mutant strains. Focus values were adjusted for small inefficiencies of fluorescent detection (Materials and Methods). The gln locus, located on the right chromosome arm 130 kb from oriC, normally exhibits a 30 minute cohesion period under similar growth conditions [2], [6]. In the present study, gln was present at 1.9 copies per cell in wild-type cells, indicating that most cells had one or two chromosomes and that replication initiation occurred relatively early in the cell cycle (Figure 1B; Figure S1). Mutant strains had similar copy numbers, ranging between 1.8 and 2.0 gln loci per cell. Average gln TetR-YFP foci per cell for wildtype, hupAB, ihfA, fis, recN and mukB, was ∼20% lower than the respective gln copy number (Figure 1B). This suggests that a significant fraction of foci in these strains harbored two colocalized gln loci (separated by <0.2 µm) in a state of cohesion. Due to the inherent limitations of fluorescence labeling and imaging, the observed number of fluorescent tetO/TetR-YFP complexes per cell is slightly undervalued, leading to an overestimation of cohesion. To correct for this, efficiencies were determined for each FROS experiment (94%±4%), and raw focus counts were adjusted (≤+0.10 foci per cell; Materials and Methods). This method was verified by determining the number of gln foci in a population of non-replicating, and presumably cohesion-less, stationary phase cells (Figure S2). Resulting gln copies per focus values for wildtype and most mutants were 1.22±0.03 (Figure 1C), indicating that most strains, including ΔmukBEF, had normal gln cohesion (∼30 min; [6]).
In contrast to WT, ΔseqA cells contained only 1.09 gln copies per focus (Figure 1C), indicating that gln cohesion is reduced ∼60% in the absence of SeqA protein. A dam mutant, which is defective in GATC methylation and thus does not target SeqA to newly replicated DNA, had nearly identical gln cohesion as ΔseqA as expected. An opposite effect on cohesion was seen in cells with reduced levels of Topo IV. Cells bearing a parE10(Ts) mutation that produces a defective Topo IV protein at 42°C [27], [28] showed a sharp increase in cohesion when incubated at the semi-permissive temperature of 37°C (Figure 1C), indicating that Topo IV mediates cohesion at an arm locus in addition to its role at oriC and ter [4], [11].
Synchronized cell analysis
Although we infer from non-synchronized “batch” culture analysis that ΔseqA and parE10 cells have shorter and longer gln cohesion periods respectively (Figure 1), it is possible that these mutants produce mixed populations of cells (with altered replication timing) that could bias cohesion measurements. To address this, we examined the dynamics of gln cohesion during the cell cycle by synchronized cell analysis (Figure 2). Wild-type, ΔseqA, ΔmukBEF, and parE10 mutant cells were synchronized by the baby machine method, which results in 75–85% synchrony and cells that are unperturbed for rates of mass increase, DNA replication and cell division [29]. Synchronized cells were then assayed for gln replication and gln splitting for two hours after cell birth, the equivalent of one cell cycle for wild-type cells at 30°C. In wild-type cells at both 30°C and 37°C, gln copy numbers rose steeply after cell birth (Figure 2A, top panels, gray), followed by an increase in gln foci per cell ∼30 minutes later (black). Integrating the area under the raw data curves at each time point yields cumulative curves (Figure 2A, dashed lines), which describe the percentage of cells among the synchronous fraction that have replicated or segregated over time [2]. Cohesion periods are thus defined as the time interval between the replication and segregation cumulative curves. For wild-type cells, gln cohesion lasted 31 minutes at 30°C and 26 minutes at 37°C in agreement with previous studies [2], [6].
Fig. 2. Synchronized cell analysis of ΔseqA, ΔmukB and parE10 strains. 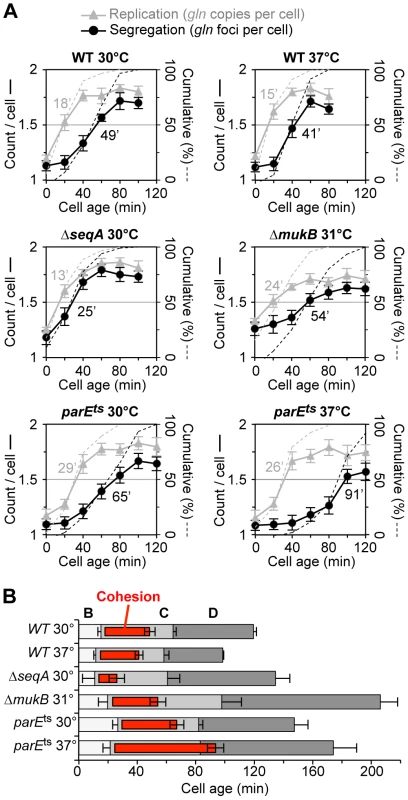
(A) Timing of replication and segregation of the gln locus. Cells were synchronized by baby machine in minimal alanine media at the indicated temperature and assayed for gln copy number (grey triangles) and number of TetR-YFP foci per cell (black circles) during the cell cycle. Values are means of two experiments ±1 SD. Dashed lines indicate the cumulative percentage of cells in the synchronized fraction that have replicated and segregated the gln locus (right ordinate), with times at 50% shown in minutes after birth. (B) Cell cycle diagrams are shown based on the timing of oriC, gln and ter duplication by qPCR (Materials and Methods). Doubling times in minimal alanine media were: WT/30°C, 119 min; WT/37°C, 98 min; ΔseqA/30°C, 134 min; ΔmukB/30°C, 211 min; parE10/30°C, 147 min; parE10/37°C, 174 min. As was seen with wildtype, ΔseqA cells exhibited steep increases in gln copies and gln foci during the cell cycle. However, gln foci split much sooner after replication in ΔseqA cells (Figure 2A, middle left panel). Cumulative curve analysis indicates that cohesion lasted about 12 minutes in ΔseqA cells, about 1/3 the normal duration of gln cohesion (Figure 2B). Confirming results from our initial screen, mukB mutant cells exhibited ∼30 minutes of gln cohesion at 31°C, the highest temperature that supported steady state growth (Figure 2A, middle right panel). Synchrony in mukB cells was relatively poor (note shallow curves for gln copy number and foci per cell), but gln splitting was clearly delayed after replication to a similar extent as wildtype. We conclude that like oriC [10], gln cohesion does not require MukB protein. In contrast, parE10 cells showed severely delayed gln splitting at the semi-permissive temperature of 37°C (Figure 2A, lower right panel). Under this condition, gln cohesion lasted about 65 minutes, 2-fold longer than when cells were grown at 30°C (left panel). Importantly, the segregation delay was not caused by indirect effects of temperature, as wild-type cells showed an even shorter cohesion period at 37°C compared to 30°C. Interestingly, both seqA and parE10 cells had significantly longer post-replication D periods than wild-type cells at the same temperature (Figure 2B), indicating that cell division was delayed. This delay may stem from late sister segregation caused by improper cohesion, although indirect effects on segregation cannot be ruled out (mukB cells also had extended D periods, Figure 2B).
SeqA binding determines the duration of cohesion
Synchronized cell analysis showed that gln cohesion, normally lasting ∼30 minutes, was reduced to ∼12 minutes in the absence of SeqA protein. To test whether SeqA mediates cohesion at sites other than gln, cohesion was measured in wild-type and ΔseqA cells at 5 chromosomal loci (see map, Figure 3C): two late-splitting snap loci (gln and psd), two fast-splitting non-snap loci (dnaB and arcA), and oriC, which exhibits late-splitting but with much different timing than snap loci [6]. At oriC and both snap sites (gln and psd), cohesion was ∼60% reduced in ΔseqA compared to wildtype (Figure 3A). ΔseqA cells were slightly elongated (3.1 µm compared to 2.4 µm for WT) with ∼2% anucleate cells (Figure 3B, arrow), suggesting that nucleoid segregation is partially defective. Although it is possible that aberrant replication initiation causes these effects, under the current slow growth conditions the ΔseqA initiation phenotype is greatly suppressed as indicated by only a 5-minute advanced initiation timing (Figure 2), suggesting that segregation problems are due to reduced cohesion (Discussion). Unlike snap cohesion, cohesion at the two non-snap loci dnaB and arcA was not measurably different in ΔseqA cells (Figure 3A), although any subtle (<20%) changes in cohesion at these sites might be below our current level of detection. Subsequent experiments showing that an overabundance of SeqA causes prolonged cohesion at dnaB suggest that SeqA is able to promote cohesion at non-snap loci under some conditions (below).
Fig. 3. SeqA is responsible for delayed separation at late-splitting loci. 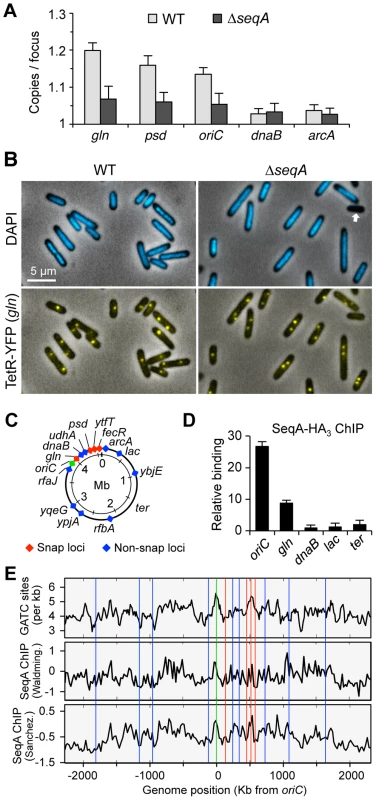
(A) Loss of SeqA reduces cohesion at snaps and oriC, but not at non-snap loci dnaB and arcA. WT and ΔseqA strains bearing tetO arrays at each of the five loci shown were grown in minimal alanine media and assayed for cohesion as described in Figure 1 (3 independent experiments ±1 SD). (B) Representative micrographs of wildtype and ΔseqA cells showing nucleoids (DAPI) and gln TetR-YFP foci. ΔseqA cells exhibit ∼2% anucleate cells (arrow). (C) E. coli chromosome map with cohesion-characterized loci [6]. Snap loci (red) and oriC (green) have prolonged cohesion periods (19–30 min); non-snap loci (blue) have short cohesion periods (7–10 min). (D) Sites with prolonged cohesion bind more SeqA. SeqA binding levels at 5 loci shown were determined by ChIP-qPCR. Relative binding (2−ΔΔCt) indicates SeqA binding relative to the poorest binding sequence, dnaB. (E) Genomic analysis of GATC frequency, SeqA binding, and cohesion. GATC per kb (top panel), and SeqA binding from two SeqA ChIP-chip studies; Waldminghaus et. al. [31] (middle panel) and Sanchez-Romero et. al. [32] (lower panel), 40-kb moving average of SeqA binding is shown (log2 ratio of IP to input fluorescence). Positions of cohesion-characterized loci are shown as colored vertical lines. To evaluate whether sites exhibiting higher cohesion are enriched in SeqA binding, we performed chromatin immunoprecipitation against HA-tagged SeqA protein, followed by site-specific analysis of immunoprecipitated DNA by qPCR (ChIP-qPCR). As expected, oriC DNA bound much more SeqA than the non-snap locus dnaB (25-fold enrichment, Figure 3D). The snap locus gln also showed elevated SeqA binding (10-fold over dnaB), whereas two other non-snap loci, lac and ter, exhibited similar low levels of SeqA binding. The relatively high abundance of oriC DNA on the SeqA complexes, probably reflects a small but very dense cluster of GATC sequences within the origin itself (known as the 13-mers, see [30]).
To examine whether the above correlation between cohesion and SeqA binding extend to other sites on the chromosome, we compared cohesion at 15 characterized loci from our earlier study [6] to genomic SeqA binding data from two E. coli microarray ChIP-chip studies [31], [32] as well as the frequency of GATC sequences (Figure 3E). Several insights emerge from this analysis. First, large-scale SeqA binding trends (40-kb moving average shown) from both ChIP-chip studies are quite similar, and generally reflect the density of GATC sequences, but not perfectly. This likely reflects the fact that cooperative SeqA binding is optimal when adjacent GATC spacing places them on the same helical face [19], thus some GATC sequences do not bind SeqA well. Second, several prominent peaks and valleys are present in the SeqA binding plots, and these fluctuations correspond generally to locations of snaps and non-snaps, respectively. Third, a higher resolution analysis of the ChIP-chip data near our sites of interest (5-kb moving average, Figure S3) resulted in an improved correlation between SeqA binding and cohesion, suggesting that cohesion levels may be regulated by local variations in SeqA binding (Discussion).
Cohesion at snap and non-snap loci after Topo IV inactivation
To further evaluate the role of Topo IV in regulating cohesion, we measured copy number and foci per cell at a snap locus (gln) and a non-snap locus (dnaB) after inactivation of Topo IV via a temperature sensitive mutation (Figure 4). WT or parE10 cells were grown at 30°C in minimal succinate media to early log phase (WT doubling time ∼90 min), shifted to the non-permissive temperature of 42°C, and assayed as described in Figure 1. Cohesion at the gln locus increased steadily in parE10 cells after temperature upshift, reaching a maximum of ∼2.2 copies per focus by 4 hours (Figure 4A, dark red symbols). Cohesion at the fast-splitting non-snap dnaB locus was also prolonged by depletion of Topo IV (Figure 4B), reaching a maximum of ∼1.4 copies per focus by 4 hours (dark blue symbols). Cohesion did not significantly change at either locus in par+ cells after temperature upshift (Figure 4AB, light shaded symbols). Interestingly, in all four cases cohesion decreased during the first 30 minutes after temperature upshift, suggesting that high temperature induced conformational changes to DNA that facilitated cohesion loss. Increased cohesion in parE10 cells was not due to replication fork stalling, as shown by complete replication runoff after rifampicin treatment (Figure S4A) and continued DNA synthesis by radioactive thymidine incorporation (Figure S4B).
Fig. 4. Topo IV reduces cohesion at both snap and non-snap loci. 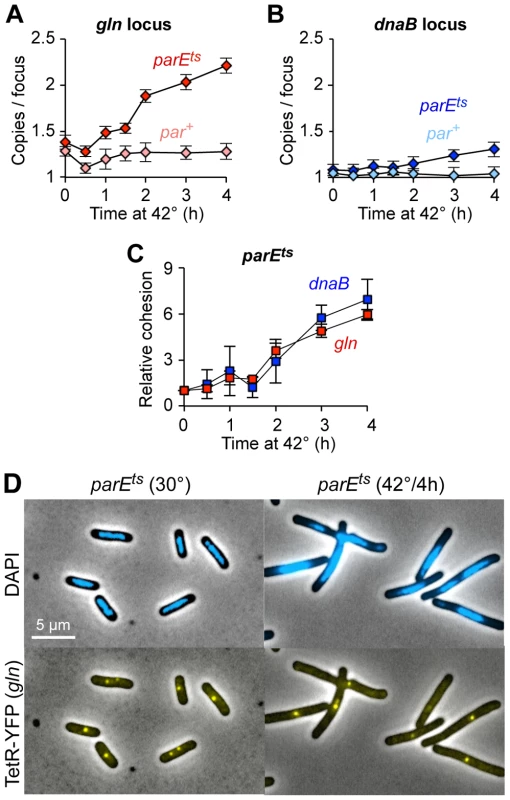
(A–B) Raw cohesion values at gln (A) and dnaB (B) after Topo IV inactivation. Copy number per TetR-YFP focus was determined in parE10 (dark shaded symbols) and par+ control (light shaded symbols) cells after shift to restrictive temperature. Values are means of 3–4 experiments ±1 SD. Cells were grown to early log phase at 30°C in minimal succinate medium, shifted to 42°C, and assayed as described in Figure 1. (C) Relative gln and dnaB cohesion after Topo IV inactivation, normalized to 30°C. The difference in cohesion (copies/focus) between parE10 and WT at each time point relative to the difference at t = 0 is shown for gln and dnaB. (D) Representative micrographs of wild-type and parE10 cells at 30°C and 42°C. Although absolute cohesion levels were higher at gln than at dnaB under all conditions, the relative rate of increase in cohesion after Topo IV inactivation was similar for both loci (Figure 4C). Thus, gln and dnaB were equally sensitive to a lack of Topo IV, further implying that all sequences experience similar levels of catenation. We postulate that higher observed cohesion at gln and other snap loci is caused by another mechanism at these sites, presumably mediated by SeqA, which either inhibits Topo IV and/or directly facilitates sister cohesion (Discussion). Additionally, the present data provide insight into how sister chromosomes are arranged during development of the par phenotype. By 4 hours after Topo IV inactivation, cells appear elongated with large unsegregated nucleoids, usually with one or two closely spaced gln foci at midcell (Figure 4D). This phenotype is maintained for longer 42°C incubations (data not shown), and cells eventually arrest growth with multiple half-segregated chromosomes (see two-color FISH labeling, Figure S5).
Genetic interactions between Topo IV and SeqA
To determine the epistatic relationship between Topo IV and SeqA, we tested a parE10 ΔseqA double mutant for temperature sensitivity and cohesion. Single mutant parE10 cells exhibited partial growth at 38°C, with ∼25% reduction in colony forming units (CFU) compared to 30°C, and no growth at 42°C (Figure 5A; 5B, green), while ΔseqA single mutants (orange) grew well at all temperatures. Double mutant parE10 ΔseqA cells (purple) showed intermediate growth at both 38°C and 42°C, indicating partial suppression of the parE10 Ts phenotype. Double mutant parE10 ΔseqA cells showed ∼40% decreased cohesion compared to parE10 alone, but suppression by ΔseqA was specific to gln (Figure 5C right, red bars); elevated dnaB cohesion in parE10 at 42°C was not significantly reduced by addition of ΔseqA (blue bars). The relationship between SeqA and Topo IV was further examined by mildly overexpressing each protein from a low copy inducible expression vector and testing gln and dnaB cohesion (Figure 5C left). Cells induced for Topo IV expression (Topo IV-OE) for one hour prior to observation had significantly reduced gln cohesion, to a level similar to that seen in ΔseqA (red bars). Conversely, cells overexpressing SeqA protein (SeqA-OE) had the opposite phenotype, with >2-fold increase in gln cohesion and dnaB cohesion, similar to parE10 cells at 42°C. This phenotypic similarity also extended to cell morphology; ΔseqA and Topo IV-OE cells had poorly separated nucleoids and closely spaced gln foci, while SeqA-OE and parE10 cells were very elongated often with one mid-cell gln focus (example, Figure S6). Topo IV expression was normal in ΔseqA cells (Figure S7) as shown previously [33].
Fig. 5. Genetic interactions between Topo IV and SeqA. 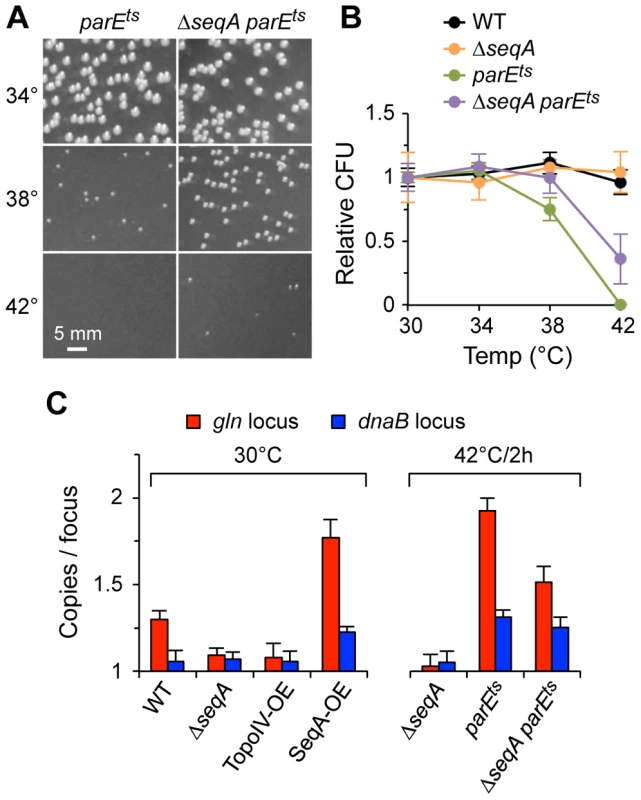
(A) ΔseqA partially suppresses the temperature sensitivity of parE10. parE10 single and ΔseqA parE10 double mutant cells were grown to exponential phase at 30°C, equal numbers of cells were plated and incubated at the indicated temperature for 24 h and colonies were photographed. (B) Quantification of colony formation data. Wild-type, ΔseqA and parE10 single and double mutants were plated and grown as described above, and the number of colonies relative to WT grown at 30°C were plotted (2 independent experiments ±1 SD). (C) Cohesion relationship between SeqA and Topo IV. Cohesion values were determined at gln and dnaB in cells carrying loss-of-function alleles or overexpression constructs of seqA and parE. Overexpression (OE) was achieved by transformation with low-level expression plasmids (Materials and Methods). Vector plasmids showed wild-type cohesion levels at gln and dnaB (1.31±0.07 and 1.05±0.04 copies/focus, respectively). Because the cohesion phenotype of a parE10 ΔseqA double mutant most closely resembled that of a parE10 single mutant, the simplest interpretation of the above results is that parE is epistatic to seqA (SeqA acts upstream of Topo IV in a single pathway). Although this conclusion assumes complete penetrance of the parE10 mutation (no partial or compensating activity at 42°C), it is supported by the fact that Topo IV overexpression was able to reduce cohesion levels well below wild-type, even in the presence of SeqA protein, indicating that all cohesion probably occurs via a precatenane mechanism (Discussion). Cohesion along snap regions is apparently more complicated, where it is clear that SeqA has some Topo IV-independent function (ΔseqA reduced gln cohesion ∼40% in a parE10 background). Such function could be direct bridging of sister chromosomes or negative regulation of compensating topoisomerases (Gyrase or Topo III).
Cohesion is required for efficient chromosome segregation
Coordinated separation of gln and four other late-splitting snap loci on the right chromosome arm is accompanied by a 35% increase in nucleoid volume and deformation of the nucleoid into a bi-lobed mass with one copy of each replicated sequence positioned within each lobe [6]. This suggests that cohesion loss along these tightly cohered regions initiate and/or drive a key sister individualization step in E. coli chromosome segregation. We tested this hypothesis by measuring the rate of separation of segregating sister loci in cells with reduced cohesion after genetic manipulation of SeqA or Topo IV. Time-lapse analysis of gln segregation was performed by growing and imaging cells directly on agarose-coated slides. Cells bearing a tetO array at gln and a photostable TetR-mCherry fusion that allowed multiple (10–15) exposures were imaged every 10 minutes through one complete doubling time (2 h). Under these conditions, the majority of cells underwent a single round of replication per cell cycle and contained either one or two gln foci (data not shown). Control cells exhibited abrupt gln separation with an average inter-gln distance of ∼1 µm immediately after appearance of two gln foci (Figure 6A, left panel, t = 0). Inter-gln distance continued to increase to ∼1.5 µm by 20 minutes after splitting, then gradually increased to a maximum of ∼2 µm before cell division. This corresponds to an initial separation speed of ∼0.15 µm/min, slowing to the rate of cell elongation (∼0.02 µm/min) by 20 minutes after focus duplication (Figure S8A). When images were acquired every 3 minutes, split gln foci still initially appeared ∼1 µm apart, indicating that the actual speed of focus separation likely exceeded 0.4 µm/min (Figure S8B; Movie S1). This estimation is in line [9] or slightly higher [34] than previous measurements.
Fig. 6. Cells with reduced cohesion have impaired chromosome segregation. 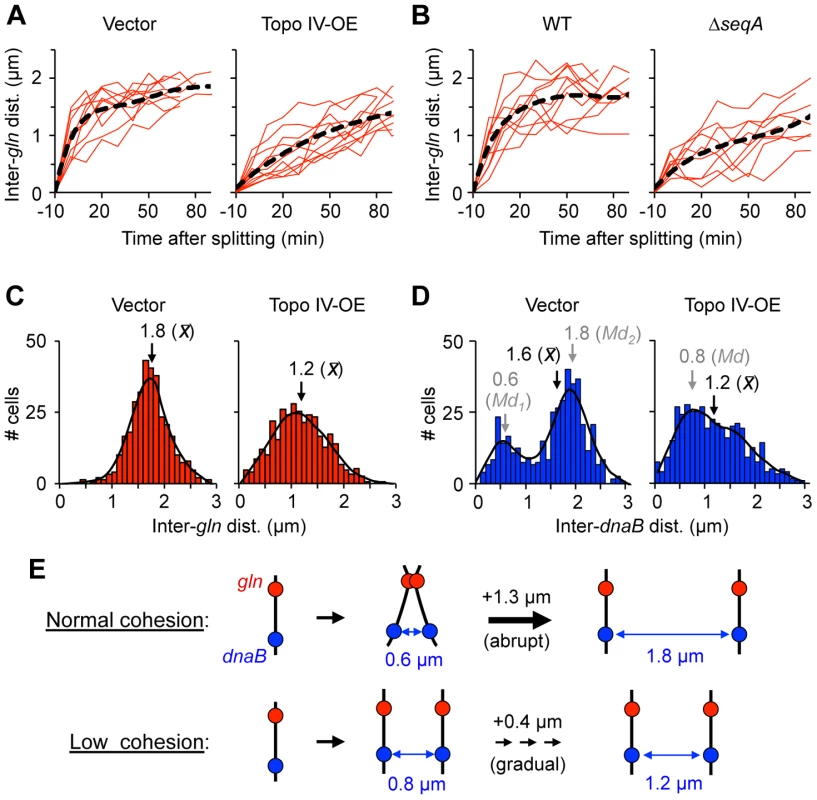
(A) Separation velocity is reduced in cells overexpressing Topo IV. Cells bearing a tetO array at gln and expressing TetR-mCherry were transformed with pDB332, a weak Topo IV expression plasmid (Topo IV-OE), or the empty vector pBAD322-kan (Vector), grown to log phase in AB succinate +CAA, induced for two hours, placed onto agarose pads and imaged by time-lapse microscopy. Inter-focus distance was measured every 10 minutes through one cell cycle. Time zero represents the first time point at which two foci appeared. Regression line (black) is shown for 10 independent cells (red). (B) Separation velocity is reduced in ΔseqA cells. Cells were grown and analyzed as above. (C–D) Topo IV overexpressing cells exhibit abnormal gln (C) and dnaB (D) focus positions. Inter-focus distances were determined in exponentially growing cells by FROS. Regression line (black), and mean (x) and mode (Md) inter-focus distances are shown for each histogram (n = 500). (E) Graphical summary of gln and dnaB segregation data in cells with normal or reduced cohesion (see Text for details). In cells overexpressing Topo IV for one hour before imaging, gln separation was much slower, with split gln foci initially appearing ∼0.4 µm apart (Figure 6A, right panel) and separation speeds ∼1/3 of that seen in non-overproducing cells (Figure S8). Supporting the time-lapse data, inter-gln distance after Topo VI overexpression in exponentially growing batch culture cells (n = 500) was significantly reduced, with a wider distribution compared to vector control cells (Figure 6C). Similarly to Topo IV overexpression, ΔseqA cells showed protracted gln segregation with ∼70% decrease in initial gln separation velocity compared to WT (Figure 6B, right panel; Movie S2). This finding implies that cohesion specifically along late-splitting snap regions is required for efficient chromosome segregation.
An equally pronounced effect of Topo IV overexpression was seen on the distribution of inter-dnaB foci (Figure 6D). The non-snap dnaB locus normally exhibits a bimodal distribution of inter-sister distances corresponding to times before and after snap separation [6]. This pattern, which was seen in vector control cells (Figure 6D, left panel), implies that dnaB segregation occurs in two discrete steps: an initial separation to 0.6 µm apart, followed by a second larger separation event (to 1.9 µm) later in the cell cycle (Illustrated in Figure 6E). Other non-snap loci behave similarly, and we have proposed that early separation of these loci is restrained by long-lived connections along snap regions [6]. After cohesion reduction by overexpression of Topo IV, this bi-modal positioning was lost, and sister dnaB loci had a ∼25% lower average inter-focus distance than vector control cells (Figure 6D, right panel). We conclude that reduced cohesion causes inefficient segregation of both snap and non-snap loci.
Discussion
An examination of cohesion in 8 chromosome structure and segregation mutants identified Topo IV and SeqA as strong and opposite mediators of sister chromosome cohesion in E. coli. Mutants deleted for the nucleoid associated proteins HU, IHF or Fis, all of which can condense DNA by bridging adjacent chromosomal segments [18], exhibited no detectable loss or gain of cohesion. Similarly, mutants of the SMC-like proteins RecN and MukB, structurally related to the cohesin proteins responsible for linking homologous chromosomes in eukaryotes, also had no effect on cohesion in our study. Depletion of Topo IV via a temperature-sensitive parE10 mutation resulted in a rapid increase in sister cohesion at all loci, development of a large (multi-chromosome) unsegregated nucleoid, and eventual cell cycle arrest. Conversely, deleting seqA caused up to a 60% decrease in sister cohesion, with the largest decreases seen at loci that normally bind high levels of SeqA. Reductions in cohesion had adverse effects on chromosome segregation, including sluggish separation speeds and incomplete nucleoid division.
Precatenanes occur universally along the E. coli chromosome
We and others have previously showed that most chromosomal loci experience a 7–10 minute delay between passage of the replication fork and separation beyond a resolvable (∼230 nm) distance [3], [6]. During this period of colocalization, homologous sequences physically interact [11], suggesting that similar to eukaryotic chromosomes, sisters are tightly juxtaposed during cohesion. At a fork speed of 700 nt/sec [6], this means that a 300–400 kb sliding window of tight sister cohesion occur behind each replication fork. Superimposed on this progressive cohesion program, oriC and two broad >100 kb segments on the right chromosome arm remain cohered for 20–30 minutes [3], [5], [6]. Late-splitting right arm loci, or snaps, are further distinct from oriC and the rest of the chromosome in that they separate in unison and concomitantly with appearance of bi-lobed nucleoids [2], [6].
Prior work by the Sherratt and Espeli labs indicated that segregation of oriC and ter sequences is modulated by Topo IV [4], [11]. Theoretically, duplex tension generated by the replicative helicases can migrate back behind the fork twisting nascent sister chromatids around each other as originally proposed by Cozzarelli and colleagues [35] (Figure 7A). Resolution of inter-sister twists, or precatenanes, requires a highly specific double strand cleavage, strand passage and ligation reaction that is mediated by the essential and highly conserved Topo IV protein [12]. Our current results extend the role of Topo IV to mediating cohesion of arm loci, including the late-splitting snap regions. Depleting Topo IV by shifting a parE10 mutant to non-permissive temperature caused an immediate block of sister separation at all loci tested, resulting in the classic par phenotype of large undivided nucleoids in elongated cells (Figure 4). Conversely, overexpression of Topo IV resulted in dramatically reduced cohesion at all loci (Figure 5). From these data, it can be argued that precatenanes are the fundamental basis of all cohesive linkages in E. coli. Importantly however, precatenanes do not readily explain the phenomenon of late-splitting snaps. Although snaps are cohered 2–3 times longer than non-snap loci, both loci responded identically to loss of Topo IV (Figure 4C), indicating that high cohesion at snap loci is likely caused by another mechanism than Topo IV (below).
Fig. 7. Models for cohesion and cohesion-mediated chromosome segregation. 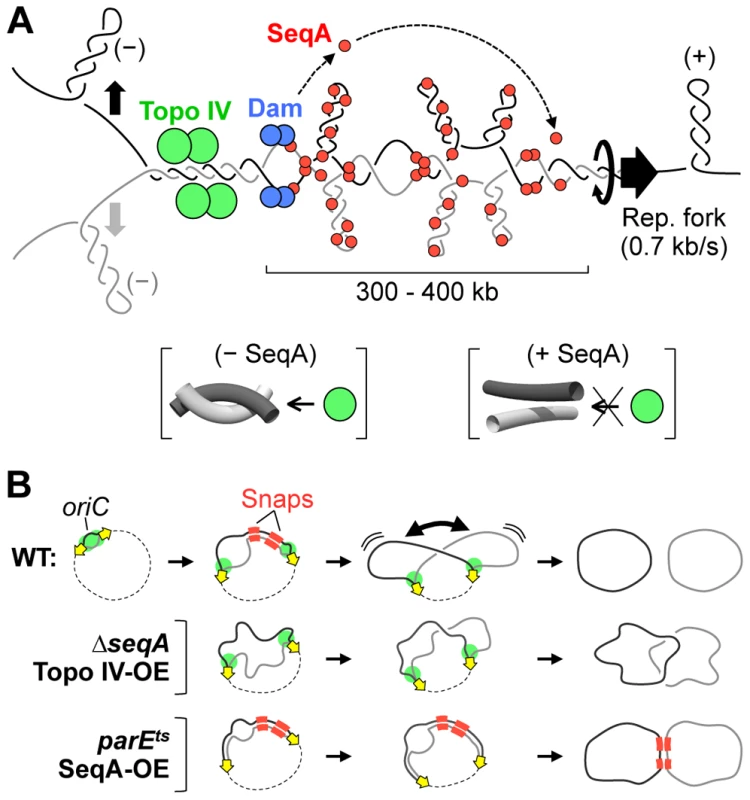
(A) SeqA-dependent precatenane removal. Positive supercoils migrate behind the replisome, entwining newly replicated sister regions. Resolution of precatenanes by Topo IV (green) is delayed by SeqA (red), which binds to hemimethylated DNA tracts behind the fork. Five to ten minutes after fork passage, DNA is remethylated by Dam (blue), releasing SeqA, and allowing Topo IV to resolve inter-sister links. SeqA may inhibit Topo IV by restraining supercoils, which transforms Topo IV-reactive hooked juxtaposition crossings (lower left brackets) to Topo IV-unreactive unhooked crossings (lower right brackets). (B) Simultaneous release of cohesion along right-arm snap regions (red) promotes abrupt sister separation and results in individualized daughter nucleoids (middle). Premature cohesion loss results in poor sister individualization (top). Deficient removal of cohesion results in late/unfinished sister separation (bottom). SeqA delays separation of newly replicated DNA
Plasmid studies and in vivo estimates of decatenation kinetics indicate that the abundant Topo IV protein likely has a cellular precatenane unlinking capacity equal to or even faster than the rate that they are formed [16], [28], suggesting that additional factor(s) exist in E. coli to impede sister separation. We propose that SeqA protein fulfills this role by binding to the same newly replicated DNA stretches acted on by Topo IV. Null mutants of seqA exhibited significantly reduced cohesion, and this effect was strongest at sites containing a high local concentration of GATC sites. As predicted by this model, late-splitting snap loci and oriC have higher than average local GATC frequency and bind ≥10-fold more SeqA than non-snap loci by ChIP-qPCR. When SeqA is overexpressed, cohesion time increases >2-fold at both snap and non-snap loci, with a cohesion and nucleoid phenotype indistinguishable from parE10 at 42°C (Figures 5,S6). We conclude that SeqA is the primary timekeeper for sister cohesion, and is solely responsible for extended cohesion observed along snap regions.
SeqA is uniquely suited to mediate sister cohesion due to its high specificity for hemimethylated DNA. Newly replicated strands are unmethylated for 5–10 minutes after passage of the replication fork before methylation by Dam [21], and SeqA-GFP fusions form large, relatively immobile foci adjacent to the replisomes [19], [22], [36]. In the absence of Dam, SeqA does not form these complexes [22], and in our study dam - cells exhibited a low cohesion phenotype identical to seqA− (Figure 1). Based on an average spacing of one favorable SeqA binding site per kb [19], an estimated 100–200 SeqA dimers are continually bound behind each fork (Figure 7A). Our data indicate that oriC and snaps bind several fold more SeqA than the more typical non-snap DNA, which may induce a higher order SeqA complex with increased stability [23], [37]. Given that overexpression of SeqA delays remethylation of origin DNA [38], and SeqA dimers can oligomerize into Dam-resistant RecA-like filaments along GATC-dense DNA fragments in vitro [23], [37], it is possible that SeqA binds as individual dimers along most of the chromosome and as a continuous or semi-continuous filament along snap regions.
A two pathway model
Two models can explain how SeqA modulates sister cohesion. SeqA could stimulate cohesion directly by forming protein-protein linkages across sister chromosomes, or it could promote cohesion indirectly by inhibiting the activity of Topo IV along precatenated junctions. The best evidence for a direct mechanism is that double mutant ΔseqA parE10 cells exhibited an intermediate cohesion phenotype to each of the single mutants. Although the parE10 mutation was partially epistatic to ΔseqA (double mutants more closely resembled parE10), it is clear that at least part of SeqA's ability to promote cohesion was independent of Topo IV, suggesting that these proteins reside in different pathways. In support, purified SeqA has been shown to physically tether hemimethylated oriC-containing molecules in an in vitro replication system [36]. Additionally, the binding characteristics of SeqA dimers suggest that oligomerization may be facilitated by individual subunits binding across opposing homologous GATC sites as they exit the fork [37]. It is unclear how SeqA nucleoprotein complexes are eventually disassembled, but SeqA molecules have an on/off rate that exceeds the hemimethylation period [39], suggesting that another factor controls the lifespan of SeqA complexes, possibly Dam.
A one pathway model
If increased cohesion at snap loci is indeed due to inter-sister bridging by SeqA, then overexpression of Topo IV would be expected to have little effect on snap cohesion in a seqA+ strain. This was clearly not the case in our study: Topo IV overexpression resulted in a 3-fold reduction in cohesion at the gln snap locus, with cohesion and nucleoid phenotypes identical to ΔseqA. Similarly, SeqA overexpression phenocopied parE10 at 42°C. The simplest interpretation of these results is that SeqA and Topo IV reside in the same pathway, with SeqA inhibiting Topo IV decatenation. Observed partial synergism between ΔseqA and parE10 (two pathway, above) could result from SeqA inhibiting DNA gyrase or Topo III, which are known to partially compensate for Topo IV function at the replication fork [15].
How could SeqA inhibit precatenane removal? If present in sufficient quantities, SeqA could conceivably physically block access of Topo IV to catenated structure. However this may be unlikely given that Topo IV binding is not sequence-specific, and typical DNA exhibiting ∼10 minutes of cohesion contain only sparse (∼one per kb) SeqA binding sites [19]. Instead, we favor a topological-based mechanism in which SeqA binding temporarily sequesters positive supercoils behind the fork, preventing Topo IV from recognizing catenated DNA crossings. Normally in positively supercoiled DNA, duplex crossings (inter or intra-molecular) adopt a tight geometry with signature “hooked juxtapositions” (Figure 7A) that are recognized and cleaved by Topo IV and gyrase [40]. This mechanism may explain how Type-II cleavage, strongly cytotoxic if unregulated and used as a chemotherapeutic, is limited to only positively supercoiled regions [16], [40]. SeqA binding, which is known to alter DNA twist or writhe by restraining supercoils [23], might relax inter-sister junctions, preventing Topo IV-mediated decatenation (Figure 7A). Further, SeqA has been shown to directly modulate Topo IV-mediated cleavage in vitro, inhibiting decatenation at high SeqA concentrations and favoring decatenation at lower concentrations [24]. We speculate that variable binding of SeqA along the E. coli chromosome results in a wide dynamic range of Topo IV regulation, and may explain the highly variable and “patchy” behavior of sister cohesion. An analogous mechanism may operate in eukaryotes, in which the ring-like cohesin complex retards decatenation of sister chromosomes by inhibiting topoisomerase II, the eukaryotic homolog of bacterial Topo IV [41].
Chromosome snaps mediate efficient chromosome segregation
In wild-type cells, sister snap segregation is very rapid, with foci appearing 1.5 µm apart within 20 minutes after splitting, and separating with an initial velocity of ≥0.4 µm/min. Repressing cohesion via a seqA deletion or overexpression of Topo IV, resulted in 30% reduced final inter-sister distances and 70% slower initial separation velocities (Figures 6,S8). Although we hypothesize that the observed segregation defects in these strains were a direct consequence of reduced cohesion, it is possible that they were caused instead by effects on cell cycle timing or nucleoid compaction. For example, ΔseqA cells initiate prematurely [20], which could potentially advance segregation timing beyond its normal cell cycle window. They also exhibit over-condensed nucleoids, which might reflect some inability to separate newly replicated regions from the replisome [19]. However, replication timing defects in ΔseqA cells are suppressed under slow growth conditions [20], and replication initiation was only five minutes earlier than WT in our experiments (Figure 2A). Moreover, Topo IV overexpression, which has no known effect on the timing of DNA replication, resulted in slowed sister segregation that was indistinguishable from ΔseqA. In sum, we conclude that poor segregation in these strains was a direct result of reduced sister cohesion.
These findings provide direct supporting evidence for a previously proposed model in which snaps mediate a key mid-replication chromosome reorganization event (Figure 7B; [6]). This event involves the following coordinate chromosome transformations: 1) simultaneous release of inter-sister linkages along both snap regions, 2) conversion of the nucleoid from unilobed to bilobed morphology, 3) 35% increase in total nucleoid volume, 4) further dramatic separation of replicated non-snap loci, and 5) placement of one copy of each thus-far replicated sequence in each daughter nucleoid lobe. The net effect of these changes are conversion of the nucleoid from a highly condensed mixed state to a relaxed pre-divisional state with spatially individualized sister chromosomes (Figure 7B). Recent work from the Kleckner lab has shown that E. coli progresses through four chromosome expansion stages (T1–T4), with the above described T2 transition being the most prominent in terms of sister separation [7].
How could holding sisters together promote their separation? Cohesion at eukaryotic centromeres directs sister chromatid segregation by providing counter tension between opposing microtubule assemblies. Similarly, it is possible that snaps resist global separation of replicated E. coli chromosomes until they are acted on by an ‘external’ segregation mechanism such as MukB [42], FtsK or MreB. Or, in theory, pushing forces generated between highly confined snap segments during cohesion, and their simultaneous release, could drive sister separation without outside influence [7]. Such cycles of restraint and programmed release of DNA confinements are proposed to be a general basis for chromosome movements observed in eukaryotes [43]. In fact, release of cohesion along chromosome arms in prometaphase is required for the generation of compact side-by-side sister chromatids long before microtubule involvement ([43] and references therein). Given that identified snap regions comprise only a small fraction of the total genome, we speculate that snap splitting is a triggering mechanism for a global nucleoid reorganization event that relies on a combination of internally and externally derived forces. Despite dramatically slowed sister separation velocities in the absence of cohesion, most cells were eventually able to complete chromosome segregation, with moderate cell elongation and production of anucleate cells (e.g., Figure 3B). Thus, the significance of cohesion in the greater E. coli chromosome segregation program remains somewhat clouded. It is logical to assume that segregation defects in cohesion-less cells observed under the current slow growth conditions are compounded during multi-forked replication, which is in agreement with the rich media sensitivity of ΔseqA strains [20], [30].
SeqA, the multi-faceted genome stability factor
SeqA plays a prominent role in nearly every phase of genome duplication and inheritance. First discovered in a screen for mutations that allowed replication of a hemimethylated oriC plasmid [44], SeqA binds and sequesters oriC immediately after replication starts for about one third of the replication period, during which oriC is refractory to further initiations [21], [44]. There is also evidence that SeqA stabilizes replication fork progression: seqA mutants grown in rich medium exhibit stalled replication forks after rifampicin runoff [20], and they are hypersensitive to the replication elongation inhibitors hydroxyurea (HU) or azidothymidine (AZT) [45]. SeqA's ability to organize replication forks (or at least the DNA created by forks) into so-called “hyperstructures” is well documented [19], [22], [36]. This activity has been hypothesized to improve fork progression by concentrating replication proteins to a central location [36], [46] and even to drive chromosome segregation by continually condensing daughter nucleoids on either side of the replisome [19]. Our current work shows that SeqA promotes sister cohesion, and that extended cohesion along snap regions is involved in a global chromosome reorganization event that is important for efficient chromosome segregation. Through its capacity to indefinitely cohere DNA, SeqA may also mediate cell cycle blockage during the stringent response, as indicated by a requirement of SeqA for nutritional deprivation-induced chromosome segregation blockage, independently of its function at oriC [45]. Logically, cohesion in E. coli may also drive homologous recombination dependent DNA repair by co-localizing sister molecules immediately after replication, presumably when double strand breaks are created. Supporting this model, seqA mutations result in mild SOS induction [33], [47], and are synthetically lethal with recA mutations in rich medium [47].
Materials and Methods
Bacterial strains and growth conditions
The genetic background for all strains is DB81, a derivative of CM735 (metE46 trp-3 his-4 thi-1 GalK2 lacY1, lacZ4 mtl-1 ara-9 tsx-3 ton-1 rps-8, or rps-9 supE44 lambda) [48] containing the Ptac-fliCst synchronization allele [29]. Gentamycin-marked tetO array insertion strains were previously described [6]. Gene deletion or disruption alleles were obtained from the following sources: ΔseqA in-frame deletion [44]; dam13::Tn9 [49]; parE10 and parE1215 [27]; hupA::cat and hupB::kan [50]; mukBEF::kan [51]; fis767::kan [52]; and ihfA::cat [53]. Marked alleles were introduced into DB81 by P1 transduction selecting for antibiotic resistance or in the case of parE10 and parC1215 reversion of methionine auxotrophy, ΔseqA was introduced by the gene replacement vector pBIP [44]. Cells were grown in AB minimal media supplemented with 0.2% alanine and 20 µg/ml each of tryptophan, histidine, methionine and thiamine or 0.2% succinate and 0.1% casamino acids, as indicated. These media resulted in doubling times for DB81 at 30°C of 126 minutes and 83 minutes, respectively. Cell synchronization was carried out as previously described [6].
Fluorescence microscopy and analysis
All images were acquired with a Zeiss AxioImager Z1 microscope equipped with a Hamamatsu EM-CCD camera, and FROS and FISH data was analyzed using a custom Matlab image analysis program, FocusCounter (http://www.bcm.edu/genetics/bateslab). Raw foci/cell values were adjusted for focus detection inefficiency, determined empirically for each experiment based on the frequency of cells with zero foci (Figure S2). Detection inefficiencies ranged between 0.9% and 3.6% (avg. 1.4%, ±0.8%), resulting in final corrections of only +0.06 to +0.15 foci/cell. This method was validated by accurately calculating foci/cell in a control experiment with cohesion-less stationary phase cells (Figure S2).
FROS was performed as previously described [6]. Cells carrying the TetR-YFP expression plasmid pDB316 were grown to OD 0.2 with 50 ng/ml ampicillin, induced with 0.02% arabinose for 1 hour, then imaged directly without fixation. pDB316 is a derivative of pWX6 [54] that carries a deletion of the LacI-CFP gene and a spontaneous mutation that weakens expression. For time-lapse experiments, TetR-mCherry was expressed from pDB317, a derivative of the salicylate-inducible nahG promoter vector pKG110 that provides highly tunable expression at sub-micromolar concentrations of inducer. Cells were grown to OD 0.2 with 25 µg/ml chloramphenicol and 50 µg/ml anhydrotetracycline (to reduce TetR binding), induced with 0.5 µM sodium salicylate for one hour, placed onto agarose-coated slides (liquid media with 2% SeaKem ME low melting agarose) and imaged in a controlled temperature 37°C environment. Unless otherwise noted, ∼1000 cells are analyzed per sample for all experiments. An absence of replication pausing or blockage at the array site was confirmed by qPCR analysis for all FROS experiments (Figure S9AB). Such blocks can occur under high TetR expression and was observed with the original TetR expression plasmids pLAU53 or pWX6 ([54]; Figure S9). Additionally, when cohesion was analyzed in cells without a tetO array by FISH, copy number and foci per cell in both WT and parE10 strains were very similar to values obtained by FROS (Figure S10).
For FISH, DB81 cells (parE10 and parE+ derivatives) without tetO array were grown to exponential phase in minimal succinate medium and fixed with 2.5% paraformaldehyde. Three kilobase gln and dnaB probes were amplified by PCR (Table S1) and labeled with PromoFluor-500 or -594, respectively by nick translation (Promokine, Germany). In situ hybridization was performed as previously described [6].
Copy number and cell cycle determinations
The number of gln or dnaB loci per cell was determined by measuring the relative ratio of gln or dnaB loci to oriC loci by qPCR as previously described [6]. These ratios were then multiplied by the total number of oriC loci per cell determined by Rifampicin runoff analysis of duplicate cell samples (Figure S1). To exclude any error caused by possible rifampicin-resistant initiations in mutant cells (e.g., [53]), gln and dnaB copy numbers were verified by absolute quantification qPCR in which cell samples were spiked (1∶1) with a calibrator strain containing a unique sequence that was used to generate a standard curve of DNA copies per cell (values were ±0.08 of those shown in Figure 1). Real time qPCR was performed in 384-well plate in ABI Prism 7900HT Thermal Cycler using KAPA SYBR Fast qPCR reagent (Kapa Biosystems, USA) and analyzed with ABI-prism software (primers in Table S1).
Cohesion timing at a given locus in exponential cultures is measured as the ratio of locus copy number to foci per cell (Figure 1A), thus cell cycle determinations are not required. In synchronized cell experiments (Figure 2), cohesion duration is determined directly by measuring the timing of locus replication and segregation [6]. Locus replication time is equal to the point at which 50% of cells have duplicated locus copy number by qPCR (the replication cumulative curve), and locus segregation time is the point at which 50% of cells have duplicated the number of foci per cell (the segregation cumulative curve). Similarly, the timing of replication initiation and termination are equal to the point at which 50% of cells duplicate the oriC and ter loci, respectively. Resulting B, C and D periods (Figure 2B) are generated from the above replication timing and generation time.
Overexpression studies
For Topo IV overexpression, the parC and parE open reading frames were amplified from the chromosome by PCR with EcoRI and HindIII restriction tails at 5′ and 3′ ends (Table S1) and cloned into pBAD322-kan [55], a low copy arabinose-inducible vector designed to express genes that are toxic at high levels. The resulting plasmid, pDB332, modestly overexpressed Topo IV after two hours induction with 0.02% arabinose (6-fold over WT; Figure S7), did not impede growth or cause cell filamentation after many generations of growth, and completely suppressed temperature sensitivity of both parE10 and parE1215 alleles (data not shown). This suggests that Topo IV overexpression did not block chromosome segregation or create DSBs, which could bias chromosome segregation analyses. For SeqA overexpression, the seqA ORF-containing BssHII fragment (excluding the downstream pgm gene) was cloned into the expression vector pGC2 under Plac promoter control and containing the lacIQ fragment to reduce leaky expression, resulting in pDB338. Induction of pDB338 containing cells with 25 nM IPTG for 2 hours did not exhibit decreased DNA synthesis by flow cytometry (data not shown), as can occur under high SeqA expression [38].
ChIP-qPCR
Three copies of the haemagglutinin (HA) epitope (TACCCATACGACGTCCCAGACTACGCT) were cloned onto the 3′ end of seqA, and integrated into the endogenous DB81 seqA locus via pBIP gene replacement. The resulting SeqA-HA3 protein exhibits a seqA+ phenotype as shown by normal growth rate and synchronous replication initiations (Figure S11). Chromatin immunoprecipitation was performed essentially as in [32]. Briefly, DB81 seqA-HA3 cells were grown in AB alanine media to early log phase, formaldehyde cross-linked, lysed, and sonicated to fragment DNA. Triplicate samples of cross-linked SeqA-HA3-DNA were immunoprecipitated with monoclonal 12CA5 anti-HA antibody (Roche). Samples were washed, cross-links were reversed, and DNA was purified. Total DNA was also prepared from identical control “input” samples not subjected to immunoprecipitation. The relative abundance of five different sequences of bound DNA was determined by qPCR using specific primer pairs previously described [6]. For each input and IP DNA sample, qPCR was performed in triplicate and amplification Ct values were averaged. Fold-enrichment of bound DNA at each site was determined by the ΔΔCt method, where ΔΔCt equals the difference in amplification of IP DNA and input DNA for each site relative to dnaB (the locus showing lowest abundance in IP samples). Thus, ΔΔCtsitex = (CtIP−Ctinput)sitex−(CtIP−Ctinput)dnaB, and fold-enrichment = 2−ΔΔCt.
Supporting Information
Zdroje
1. SunakoY, OnogiT, HiragaS (2001) Sister chromosome cohesion of Escherichia coli. Mol Microbiol 42 : 1233–1241.
2. BatesD, KlecknerN (2005) Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 121 : 899–911.
3. NielsenHJ, LiY, YoungrenB, HansenFG, AustinS (2006) Progressive segregation of the Escherichia coli chromosome. Mol Microbiol 61 : 383–393.
4. WangX, Reyes-LamotheR, SherrattDJ (2008) Modulation of Escherichia coli sister chromosome cohesion by topoisomerase IV. Genes Dev 22 : 2426–2433.
5. EspeliO, MercierR, BoccardF (2008) DNA dynamics vary according to macrodomain topography in the E. coli chromosome. Mol Microbiol 68 : 1418–1427.
6. JoshiMC, BourniquelA, FisherJ, HoBT, MagnanD, KlecknerN, BatesD (2011) Escherichia coli sister chromosome separation includes an abrupt global transition with concomitant release of late-splitting intersister snaps. Proc Natl Acad Sci U S A 108 : 2765–2770.
7. FisherJK, BourniquelA, WitzG, WeinerB, PrentissM, KlecknerN (2013) Four-Dimensional Imaging of E. coli Nucleoid Organization and Dynamics in Living Cells. Cell 153 : 882–895.
8. NasmythK, HaeringCH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43 : 525–558.
9. GordonGS, SitnikovD, WebbCD, TelemanA, StraightA, LosickR, MurrayAW, WrightA (1997) Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell 90 : 1113–1121.
10. DanilovaO, Reyes-LamotheR, PinskayaM, SherrattD, PossozC (2007) MukB colocalizes with the oriC region and is required for organization of the two Escherichia coli chromosome arms into separate cell halves. Mol Microbiol 65 : 1485–1492.
11. LesterlinC, GigantE, BoccardF, EspeliO (2012) Sister chromatid interactions in bacteria revealed by a site-specific recombination assay. EMBO J 31 : 3468–3479.
12. DeiblerRW, RahmatiS, ZechiedrichEL (2001) Topoisomerase IV, alone, unknots DNA in E. coli. Genes Dev 15 : 748–761.
13. EspeliO, LevineC, HassingH, MariansKJ (2003) Temporal regulation of topoisomerase IV activity in E. coli. Mol Cell 11 : 189–201.
14. KhodurskyAB, PeterBJ, SchmidMB, DeRisiJ, BotsteinD, BrownPO, CozzarelliNR (2000) Analysis of topoisomerase function in bacterial replication fork movement: use of DNA microarrays. Proc Natl Acad Sci U S A 97 : 9419–9424.
15. PostowL, CrisonaNJ, PeterBJ, HardyCD, CozzarelliNR (2001) Topological challenges to DNA replication: conformations at the fork. Proc Natl Acad Sci U S A 98 : 8219–8226.
16. LopezV, Martinez-RoblesML, HernandezP, KrimerDB, SchvartzmanJB (2012) Topo IV is the topoisomerase that knots and unknots sister duplexes during DNA replication. Nucleic Acids Res 40 : 3563–3573.
17. PetrushenkoZM, SheW, RybenkovVV (2011) A new family of bacterial condensins. Mol Microbiol 81 : 881–896.
18. Johnson RC, Johnson LM, Schmidt JW, Gardner JF (2005) Major nucleoid proteins in the structure and function of the Eschrichia coli chromosome. In: Higgins NP, editor. The Bacterial Chromosome. Washington, D.C.: ASM Press. pp. 65–132.
19. BrendlerT, SawitzkeJ, SergueevK, AustinS (2000) A case for sliding SeqA tracts at anchored replication forks during Escherichia coli chromosome replication and segregation. EMBO J 19 : 6249–6258.
20. LuM, CampbellJL, BoyeE, KlecknerN (1994) SeqA: a negative modulator of replication initiation in E. coli. Cell 77 : 413–426.
21. CampbellJL, KlecknerN (1990) E. coli oriC and the dnaA gene promoter are sequestered from Dam methyltransferase following the passage of the chromosomal replication fork. Cell 62 : 967–979.
22. OnogiT, NikiH, YamazoeM, HiragaS (1999) The assembly and migration of SeqA-Gfp fusion in living cells of Escherichia coli. Mol Microbiol 31 : 1775–1782.
23. OdsbuI, KlungsoyrHK, FossumS, SkarstadK (2005) Specific N-terminal interactions of the Escherichia coli SeqA protein are required to form multimers that restrain negative supercoils and form foci. Genes Cells 10 : 1039–1049.
24. KangS, HanJS, ParkJH, SkarstadK, HwangDS (2003) SeqA protein stimulates the relaxing and decatenating activities of topoisomerase IV. J Biol Chem 278 : 48779–48785.
25. HayamaR, MariansKJ (2010) Physical and functional interaction between the condensin MukB and the decatenase topoisomerase IV in Escherichia coli. Proc Natl Acad Sci U S A 107 : 18826–18831.
26. LiY, StewartNK, BergerAJ, VosS, SchoefflerAJ, BergerJM, ChaitBT, OakleyMG (2010) Escherichia coli condensin MukB stimulates topoisomerase IV activity by a direct physical interaction. Proc Natl Acad Sci U S A 107 : 18832–18837.
27. KatoJ, NishimuraY, ImamuraR, NikiH, HiragaS, SuzukiH (1990) New topoisomerase essential for chromosome segregation in E. coli. Cell 63 : 393–404.
28. ZechiedrichEL, CozzarelliNR (1995) Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev 9 : 2859–2869.
29. BatesD, EpsteinJ, BoyeE, FahrnerK, BergH, KlecknerN (2005) The Escherichia coli baby cell column: a novel cell synchronization method provides new insight into the bacterial cell cycle. Mol Microbiol 57 : 380–391.
30. StepankiwN, KaidowA, BoyeE, BatesD (2009) The right half of the Escherichia coli replication origin is not essential for viability, but facilitates multi-forked replication. Mol Microbiol 74 : 467–479.
31. WaldminghausT, SkarstadK (2010) ChIP on Chip: surprising results are often artifacts. BMC Genomics 11 : 414.
32. Sanchez-RomeroMA, BusbySJ, DyerNP, OttS, MillardAD, GraingerDC (2010) Dynamic Distribution of SeqA Protein across the Chromosome of Escherichia coli K-12. mBio 1: e00012–10.
33. Lobner-OlesenA, MarinusMG, HansenFG (2003) Role of SeqA and Dam in Escherichia coli gene expression: a global/microarray analysis. Proc Natl Acad Sci U S A 100 : 4672–4677.
34. ElmoreS, MullerM, VischerN, OdijkT, WoldringhCL (2005) Single-particle tracking of oriC-GFP fluorescent spots during chromosome segregation in Escherichia coli. J Struct Biol 151 : 275–287.
35. PeterBJ, UllspergerC, HiasaH, MariansKJ, CozzarelliNR (1998) The structure of supercoiled intermediates in DNA replication. Cell 94 : 819–827.
36. FossumS, CrookeE, SkarstadK (2007) Organization of sister origins and replisomes during multifork DNA replication in Escherichia coli. EMBO J 26 : 4514–4522.
37. GuarneA, BrendlerT, ZhaoQ, GhirlandoR, AustinS, YangW (2005) Crystal structure of a SeqA-N filament: implications for DNA replication and chromosome organization. EMBO J 24 : 1502–1511.
38. BachT, KreklingMA, SkarstadK (2003) Excess SeqA prolongs sequestration of oriC and delays nucleoid segregation and cell division. EMBO J 22 : 315–323.
39. WaldminghausT, WeigelC, SkarstadK (2012) Replication fork movement and methylation govern SeqA binding to the Escherichia coli chromosome. Nucleic Acids Res 40 : 5465–5476.
40. LiuZ, ZechiedrichL, ChanHS (2010) Action at hooked or twisted-hooked DNA juxtapositions rationalizes unlinking preference of type-2 topoisomerases. J Mol Biol 400 : 963–982.
41. FarcasAM, UluocakP, HelmhartW, NasmythK (2011) Cohesin's concatenation of sister DNAs maintains their intertwining. Mol Cell 44 : 97–107.
42. WeitaoT, NordstromK, DasguptaS (1999) Mutual suppression of mukB and seqA phenotypes might arise from their opposing influences on the Escherichia coli nucleoid structure. Mol Microbiol 34 : 157–168.
43. KlecknerN, ZicklerD, JonesGH, DekkerJ, PadmoreR, et al. (2004) A mechanical basis for chromosome function. Proc Natl Acad Sci U S A 101 : 12592–12597.
44. SlaterS, WoldS, LuM, BoyeE, SkarstadK, et al. (1995) E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell 82 : 927–936.
45. FerulloDJ, LovettST (2008) The stringent response and cell cycle arrest in Escherichia coli. PLoS Genet 4: e1000300.
46. NorrisV, FralickJ, DanchinA (2000) A SeqA hyperstructure and its interactions direct the replication and sequestration of DNA. Mol Microbiol 37 : 696–702.
47. KouzminovaEA, RotmanE, MacomberL, ZhangJ, KuzminovA (2004) RecA-dependent mutants in Escherichia coli reveal strategies to avoid chromosomal fragmentation. Proc Natl Acad Sci U S A 101 : 16262–16267.
48. HansenFG, von MeyenburgK (1979) Characterization of the dnaA, gyrB and other genes in the dnaA region of the Escherichia coli chromosome on specialized transducing phages lambda tna. Mol Gen Genet 175 : 135–144.
49. MarinusMG, CarrawayM, FreyAZ, BrownL, ArrajJA (1983) Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet 192 : 288–289.
50. HuismanO, FaelenM, GirardD, JaffeA, ToussaintA, et al. (1989) Multiple defects in Escherichia coli mutants lacking HU protein. J Bacteriol 171 : 3704–3712.
51. YamazoeM, OnogiT, SunakoY, NikiH, YamanakaK, et al. (1999) Complex formation of MukB, MukE and MukF proteins involved in chromosome partitioning in Escherichia coli. EMBO J 18 : 5873–5884.
52. JohnsonRC, BallCA, PfefferD, SimonMI (1988) Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc Natl Acad Sci U S A 85 : 3484–3488.
53. Von FreieslebenU, RasmussenKV, AtlungT, HansenFG (2000) Rifampicin-resistant initiation of chromosome replication from oriC in ihf mutants. Mol Microbiol 37 : 1087–1093.
54. LauIF, FilipeSR, SoballeB, OkstadOA, BarreFX, et al. (2003) Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol Microbiol 49 : 731–743.
55. CronanJE (2006) A family of arabinose-inducible Escherichia coli expression vectors having pBR322 copy control. Plasmid 55 : 152–157.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



