-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaH2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
The histone H2A variant H2A.Z is essential for embryonic development and for proper control of developmental gene expression programs in embryonic stem cells (ESCs). Divergent regions of amino acid sequence of H2A.Z likely determine its functional specialization compared to core histone H2A. For example, H2A.Z contains three divergent residues in the essential C-terminal acidic patch that reside on the surface of the histone octamer as an uninterrupted acidic patch domain; however, we know little about how these residues contribute to chromatin structure and function. Here, we show that the divergent amino acids Gly92, Asp97, and Ser98 in the H2A.Z C-terminal acidic patch (H2A.ZAP3) are critical for lineage commitment during ESC differentiation. H2A.Z is enriched at most H3K4me3 promoters in ESCs including poised, bivalent promoters that harbor both activating and repressive marks, H3K4me3 and H3K27me3 respectively. We found that while H2A.ZAP3 interacted with its deposition complex and displayed a highly similar distribution pattern compared to wild-type H2A.Z, its enrichment levels were reduced at target promoters. Further analysis revealed that H2A.ZAP3 was less tightly associated with chromatin, suggesting that the mutant is more dynamic. Notably, bivalent genes in H2A.ZAP3 ESCs displayed significant changes in expression compared to active genes. Moreover, bivalent genes in H2A.ZAP3 ESCs gained H3.3, a variant associated with higher nucleosome turnover, compared to wild-type H2A.Z. We next performed single cell imaging to measure H2A.Z dynamics. We found that H2A.ZAP3 displayed higher mobility in chromatin compared to wild-type H2A.Z by fluorescent recovery after photobleaching (FRAP). Moreover, ESCs treated with the transcriptional inhibitor flavopiridol resulted in a decrease in the H2A.ZAP3 mobile fraction and an increase in its occupancy at target genes indicating that the mutant can be properly incorporated into chromatin. Collectively, our work suggests that the divergent residues in the H2A.Z acidic patch comprise a unique domain that couples control of chromatin dynamics to the regulation of developmental gene expression patterns during lineage commitment.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003725
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003725Summary
The histone H2A variant H2A.Z is essential for embryonic development and for proper control of developmental gene expression programs in embryonic stem cells (ESCs). Divergent regions of amino acid sequence of H2A.Z likely determine its functional specialization compared to core histone H2A. For example, H2A.Z contains three divergent residues in the essential C-terminal acidic patch that reside on the surface of the histone octamer as an uninterrupted acidic patch domain; however, we know little about how these residues contribute to chromatin structure and function. Here, we show that the divergent amino acids Gly92, Asp97, and Ser98 in the H2A.Z C-terminal acidic patch (H2A.ZAP3) are critical for lineage commitment during ESC differentiation. H2A.Z is enriched at most H3K4me3 promoters in ESCs including poised, bivalent promoters that harbor both activating and repressive marks, H3K4me3 and H3K27me3 respectively. We found that while H2A.ZAP3 interacted with its deposition complex and displayed a highly similar distribution pattern compared to wild-type H2A.Z, its enrichment levels were reduced at target promoters. Further analysis revealed that H2A.ZAP3 was less tightly associated with chromatin, suggesting that the mutant is more dynamic. Notably, bivalent genes in H2A.ZAP3 ESCs displayed significant changes in expression compared to active genes. Moreover, bivalent genes in H2A.ZAP3 ESCs gained H3.3, a variant associated with higher nucleosome turnover, compared to wild-type H2A.Z. We next performed single cell imaging to measure H2A.Z dynamics. We found that H2A.ZAP3 displayed higher mobility in chromatin compared to wild-type H2A.Z by fluorescent recovery after photobleaching (FRAP). Moreover, ESCs treated with the transcriptional inhibitor flavopiridol resulted in a decrease in the H2A.ZAP3 mobile fraction and an increase in its occupancy at target genes indicating that the mutant can be properly incorporated into chromatin. Collectively, our work suggests that the divergent residues in the H2A.Z acidic patch comprise a unique domain that couples control of chromatin dynamics to the regulation of developmental gene expression patterns during lineage commitment.
Introduction
Precise control of gene expression is critical for lineage commitment and proper development in all multicellular organisms. Regulation of chromatin structure has emerged as an important mechanism for modulating gene expression patterns in response to developmental cues. While post-translational histone modifications can influence chromatin structure and transcriptional activity, less is known about the role of histone variants. Histone variants are incorporated in a replication-independent manner and appear to mark structurally and functionally distinct chromatin domains [1]–[3]. The histone H2A variant H2A.Z is highly conserved among eukaryotes and is of particular interest because it plays an essential but poorly understood role in metazoan development including mammals [4]–[6]. H2A.Z has been implicated in a range of DNA-mediated processes such as gene expression, DNA repair, and genomic stability [7]–[9]. Notably, H2A.Z is required for proper execution of developmental gene expression programs during embryonic stem cell (ESC) differentiation [10], suggesting that H2A.Z has specialized functions to regulate lineage commitment.
A role for H2A.Z in gene regulation is supported by genome-wide localization studies showing that this variant flanks the nucleosome-free region at transcription start sites in a wide range of cell types [11], [12]. In particular, H2A.Z is incorporated at the majority of H3K4me3 modified promoter nucleosomes including bivalent promoters in ESCs that harbor both H3K4me3 and H3K27me3, marks of Trithorax and Polycomb, respectively [10], [11]. Bivalent promoters in ESCs are associated with lineage specific genes that are poised, but remain competent for activation [13], [14]. These studies suggest that H2A.Z contributes to formation of distinct chromatin states and that its incorporation at bivalent promoters may be necessary to allow for induction of lineage programs in response to developmental cues. Consistent with this idea, H2A.Z levels decreased at promoters upon gene activation [11], [15]–[17]. H2A.Z also occupied regulatory elements such as enhancers and boundary elements [11], [18], [19], indicating that H2A.Z is incorporated at regions that are subject to considerable chromatin regulation.
H2A.Z shares extensive homology with the major type histone H2A throughout the histone fold domain. However, divergent regions in the amino - and carboxy-terminal domains as well as the L1 loop region within the histone fold suggest that the two histones have different structural and functional properties. In vitro biophysical studies showed that H2A.Z incorporation stabilizes the dimer-tetramer interface and strongly favors formation of 30 nm fibers over formation of higher order chromatin folding that require fiber-fiber interactions when compared to canonical H2A, consistent with the idea that unique features of the variant contribute to specialized chromatin domains [20]–[22]. Moreover, H2A.Z and the histone H3 variant H3.3 can occupy the same nucleosome resulting in a double variant nucleosome that is enriched at active promoters as well as at highly regulated chromatin regions [23], [24]. These hybrid nucleosomes are characterized as unstable and highly salt labile, consistent with its presence in dynamic chromatin domains [25], [26]. Thus, dissecting the features of H2A.Z that distinguish it from core H2A is key to understanding its functional specialization and may provide new insights into the essential role of this variant during development.
The H2A.Z carboxy-terminal acidic patch comprises substitutions of H2A residues Asn89, Asn94, Lys95 for Gly92, Asp97, and Ser98. While the overall structure of the H2A.Z nucleosome appears similar to H2A-containing nucleosomes, these three divergent residues form an uninterrupted acidic patch that extends across the surface of the H2A.Z octamer resulting in a solvent-exposed cavity in the center of the nucleosome [27]. In Drosophila, domain-swap experiments demonstrated that the H2A.Z carboxy-terminus including the acidic patch is essential for development [28]. Later work in Xenopus involving site-specific mutagenesis of the divergent H2A.Z acidic patch residues resulted in embryos that exhibited significant developmental defects [6]. These studies suggest that the unique H2A.Z acidic patch plays an important role in establishing a novel chromatin state that is essential for embryonic development. In yeast, replacement of two residues in the H2A.Z acidic patch resulted in low nucleosome occupancy at the PHO5 promoter, suggesting that the acidic patch is necessary for interaction with its deposition complex and for proper incorporation into chromatin [29]. However, the amino acids mutated in this study are conserved between H2A.Z and canonical H2A, indicating that this phenotype is not likely specific to H2A.Z. Other loss-of-function studies in yeast have shown that the H2A.Z carboxy-terminal docking domain is critical for H2A.Z function; however, these studies examined truncations that retained an intact acidic patch domain [30], [31]. Thus, we currently lack a detailed mechanistic understanding of how this domain regulates chromatin conformation during development and whether it plays a similar role in mammals.
We investigated the role of the divergent H2A.Z acidic patch during ESC differentiation. ESCs are an ideal model for investigating how H2A.Z influences mammalian development because these cells maintain the potential to differentiate into all somatic cell types [32], [33]. We generated a mutant form of H2A.Z (denoted H2A.ZAP3), where the three divergent acidic patch residues in H2A.Z are replaced with the corresponding H2A amino acids. H2A.ZAP3 ESCs maintained the ability to self-renew, but these cells failed to differentiate properly. We found that while H2A.ZAP3 interacted with its deposition complex and displayed a highly similar distribution pattern compared to expression of the wild-type H2A.Z transgene (denoted H2A.ZWT), its enrichment levels were reduced at target promoters and were particularly diminished at the +1 nucleosome. Further analyses revealed that H2A.ZAP3 was less tightly associated with chromatin compared to H2A.ZWT suggesting that the mutant is more dynamic. Notably, bivalent genes that are poised for activation in ESCs displayed significant changes in expression compared to active genes suggesting that the poised state is more sensitive to H2A.Z regulation. Moreover, this group of genes showed reduced levels of the repressive chromatin mark H3K27me3 at H2A.ZAP3 target gene promoters compared to H2A.ZWT and displayed higher H3.3 enrichment, a variant associated with high chromatin flux. Consistent with this observation, we further showed that H2A.ZAP3 displayed higher mobility in chromatin compared to H2A.ZWT by fluorescence recovery after photobleaching (FRAP). Remarkably, ESCs treated with the transcriptional inhibitor flavopiridol partially restored the H2A.ZAP3 mobile fraction to wild-type levels and resulted in an increase in H2A.ZAP3 occupancy at target genes. Collectively, our results demonstrate that the divergent H2A.Z acidic patch mediates chromatin dynamics and indicate that control of H2A.Z dynamics is critical for the regulation of gene expression patterns during lineage commitment.
Results
H2A.Z acidic patch is necessary for ESC differentiation
The H2A acidic patch domain resides on the nucleosome surface, and in the case of H2A.Z contains three divergent residues that comprise an extended acidic patch [27] (Figure 1A). While disruption of the H2A.Z acidic patch results in early developmental defects in Drosophila and Xenopus, how it functions to regulate chromatin structure and whether this domain plays a similar role in mammals is unknown [6], [28]. In mouse, H2A.Z is encoded by two isoforms that differ by only 3 amino acid residues, denoted H2AFZ (H2A.Z) and H2AFV [34], [35] (Figure S1A). H2A.Z knockout mice die around the time of implantation [5], suggesting that H2AFV is unable to compensate for loss of H2A.Z and that the two isoforms are functionally distinct. We analyzed the relative abundance of H2A.Z and H2AFV in ESCs by mass spectrometry. While we detected both isoforms in ESCs, H2A.Z is ∼20-fold more abundant than H2AFV (Figure S1B). Thus, given the essential role of H2A.Z and its abundance in ESCs compared to H2AFV, we focused on dissecting the function of the divergent acidic patch in H2A.Z.
Fig. 1. H2A.Z acidic patch is necessary for ESC differentiation. 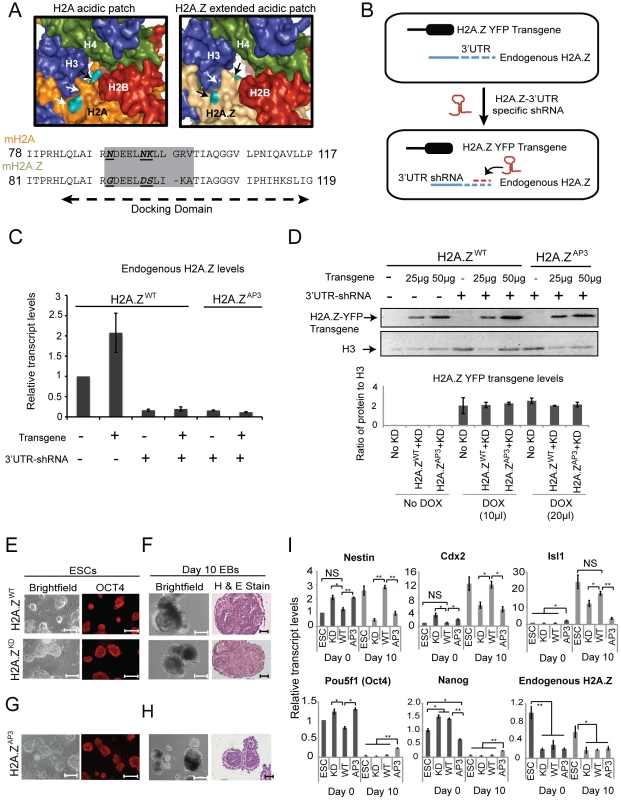
(A) Surface rendering of the H2A (left) and H2A.Z (right) nucleosome center. The H2A (orange) and H2A.Z (light brown) structures are shown with H2B (red), H3 (blue), and H4 (green) as indicated by the labels. The divergent residues (teal) are highlighted with arrows. The images were generated in Pymol using the following PDB files: 1AOI for canonical H2A-containing nucleosome structure and 1F66 for H2A.Z nucleosome structure. Below, sequence alignment of C terminal docking domain of H2A and H2A.Z. The acidic patch region is highlighted in a grey box. The bold, italicized and underlined residues indicate the divergent H2A.Z residues replaced in our study to the corresponding H2A residues. (B) Schematic diagram depicting the system used in this study to investigate the function of the H2A.Z acidic patch. (C) qRT-PCR representing the relative levels of endogenous H2A.Z transcript in H2A.ZWT and H2A.ZAP3 dox-inducible transgenic ESCs in the presence (+) and absence (−) of H2A.Z 3′UTR-specific shRNA. Transcript levels were normalized relative to Tubb5. Error bars represents standard deviation calculated from three independent biological replicates. (D) (Top) Western blot using H2A.Z antibodies on whole cell lysates isolated from dox-induced and uninduced (−/+) H2A.ZWT and H2A.ZAP3 transgenic ESC lines in the presence (+) and absence (−) of the H2A.Z 3′UTR-specific shRNA. Titrations of the dox-induced samples (25 µg and 50 µg of whole cell lysates) were performed to demonstrate comparable expression of H2A.Z-YFP transgene in H2A.ZWT and H2A.ZAP3 ESCs. H3 levels were used as a load control (lower exposure). Densitometric measurements were used to determine the ratio of transgene signal to H3 for the indicated samples (bottom) using ImageJ. Error bars represent standard deviations from a triplicate set of experiments. ESC colony morphology and OCT4 staining for H2A.ZWT, H2A.ZKD, (E) and H2A.ZAP3 (G) expressing ESC. Embryoid bodies (EBs) were generated from H2A.ZWT, H2A.ZKD (F), and H2A.ZAP3 (H) expressing ESCs. The left panels show bright field images of EBs cultured for 10 days in the absence of LIF. The right panels show hematoxylin and eosin stained sections of day 10 EBs for indicated cell lines. (I) qRT-PCR analyses showing mRNA levels of indicated pluripotency (Pou5f1/Oct4 and Nanog) and differentiation markers (Nestin, Cdx2, Isl1) in Day 0 and Day 10 EBs generated from unmodified ESCs, H2A.ZWT, H2A.ZKD, and H2A.ZAP3 ESCs. Student's t-test was performed to generate indicated p values. ***p<0.005, **p<0.01, *p<0.05. Error bars represent standard deviations from a triplicate set of experiments. Since H2A.Z and H2AFV share nearly identical amino acid sequences, available antibodies cannot distinguish between the two isoforms. To circumvent this limitation, we generated ESC lines that harbor a stably integrated Tet-inducible H2A.Z transgene fused to YFP (Figure 1B). Upon induction by doxycycline, we sorted for YFP expression and collected cells that displayed transgene expression comparable to endogenous H2A.Z levels for further analysis. To specifically test transgene function, we integrated short hairpins directed against the H2A.Z 3′ UTR into the inducible ESC lines (Figure 1B). This system allowed for targeted depletion of endogenous H2A.Z (denoted H2A.ZKD for knock-down) (Figure 1C, Figure S1D) without affecting transgene levels or H2AFV expression (Figure 1D, Figure S1C). Consistent with our previous work, H2A.ZKD ESCs displayed typical colony morphology, cell cycle kinetics, and normal expression of the pluripotency marker Pou5f1 (Oct4) suggesting that H2A.Z depletion does not affect self-renewal (Figure 1E, Figure S1E, F). To test differentiation potential, wild-type H2A.Z-YFP (denoted H2A.ZWT) and H2A.ZKD ESCs were induced to differentiate by allowing these cells to aggregate into embryoid bodies (EBs) in the absence of the pluripotency growth factor LIF. EBs are similar to egg cylinder stage embryos, albeit disorganized, and are capable of differentiation into tissues representing the three germ layers. H2A.ZKD ESCs failed to differentiate properly, and lacked the distinct differentiated structures observed in Day 10 EBs compared to H2A.ZWT (Figure 1F). Additionally, EBs from H2A.ZKD ESCs failed to activate developmental genes to levels observed in H2A.ZWT cells (Figure 1I), consistent with the idea that H2A.Z regulates lineage programs [11]. Importantly, expression of H2A.ZWT rescued the H2A.ZKD phenotype as measured by the restoration of ESC gene expression patterns as well as their capacity for multi-lineage differentiation (Figure 1F,I), whereas H2A-YFP did not compensate for loss of H2A.Z (data not shown). These data indicate that expression of the H2A.ZWT transgene recapitulates normal H2A.Z function. Thus, we used this system to dissect the role of the H2A.Z acidic patch during lineage commitment.
We replaced the divergent H2A.Z acidic patch residues by site-directed mutagenesis of Gly92, Asp97, and Ser98 to the equivalent residues in H2A - Asn89, Asn94, and Lys95 (Figure 1A). Similar to H2A.Z depletion, expression of the acidic patch mutant (denoted H2A.ZAP3) in H2A.ZKD ESCs did not affect self-renewal, colony morphology, or levels of the pluripotency marker OCT4 (Figure 1G). We observed, however, that H2A.ZAP3 EBs were smaller, morphologically distinct, and failed to differentiate properly compared to H2A.ZWT EBs as demonstrated by the lack of differentiated cell types at Day 10 and the inability to activate developmental gene expression programs during lineage commitment (Figure 1H,I). The smaller size of H2A.ZAP3 EBs was not a result of altered cell cycle kinetics, proliferation, or differences in levels of apoptotic cells relative to H2A.ZWT (Figure S1E–I). Moreover, the number of cells recovered from Day 10 H2A.ZAP3 EBs was comparable to that recovered from H2A.ZWT, and H2A.ZKD EBs (Figure S1J). Rather, we observed a larger number of small EBs in H2A.ZAP3 cultures compared to H2A.ZWT. Taken together, these data suggest that the divergent residues in the H2A.Z acidic patch are necessary for proper ESC differentiation.
H2A.Z acidic patch mutant is enriched at typical H2A.Z target genes
H2A.Z containing nucleosomes occupy majority of promoters from yeast to human as determined by genome-wide localization studies and its incorporation is critical for proper gene regulation [10], [11], [36]. Thus, we analyzed the localization pattern of the acidic patch mutant relative to H2A.Z. Given that current H2A.Z antibodies cannot distinguish between H2A.Z and H2AFV isoforms, we performed ChIP-Seq in ESCs using GFP antibodies that recognize the H2A.Z-YFP tag. This analysis revealed significant H2A.Z-specific enrichment at ∼11,000 promoters in ESCs (Table S1). We found by ChIP-Seq that H2A.Z enrichment overlapped with the majority of H3K4me3 promoters, a histone modification that is normally associated with transcriptional competence (Figure 2A) [11], [37]. Our H2A.Z data are similar to other genome wide reports using pan H2A.Z antibodies in ESCs [11], [36], indicating that the YFP tag does not affect H2A.Z incorporation.
Fig. 2. H2A.Z acidic patch is incorporated at lower levels at target genes. 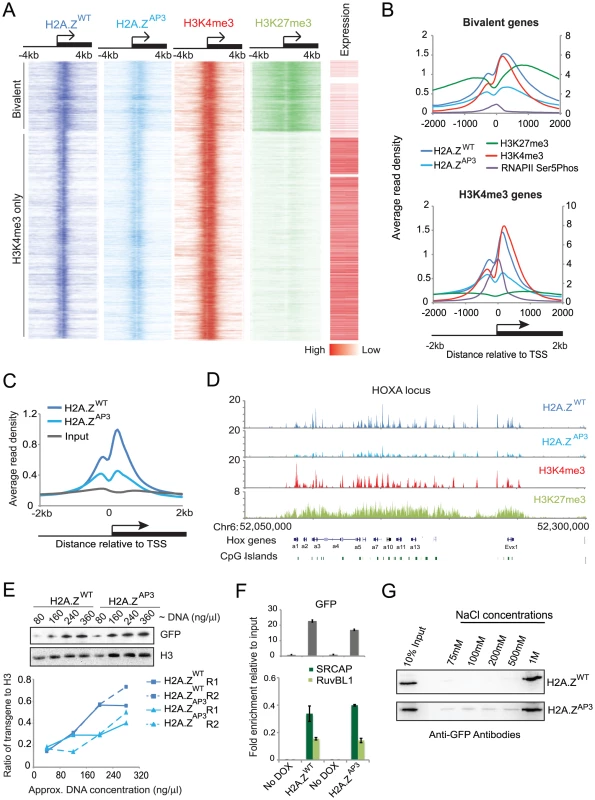
ChIP-Seq analysis of H2A.Z in ESCs shows that the divergent acidic patch residues are required for stable incorporation of H2A.Z (A) Density map of H2A.ZWT (dark blue), H2A.ZAP3 (light blue), H3K4me3 (red), and H3K27me3 (light green) enrichment at all H2A.Z target genes ordered from most H3K27me3 enriched genes to least H3K27me3 enriched genes in ESCs within the region −4 kb to +4 kb relative to the TSS. The right panel representing the expression levels of the corresponding genes in ESCs generated from RNA-Seq data. Red to white gradient represents genes with high to low expression levels respectively. (B) Average enrichment patterns of H2A.ZWT, H2A.ZAP3, H3K4me3, H3K27me3, and RNAP2-Ser5P +/−2 kb around the TSS at bivalent (top) and H3K4me3 (H3K27me3 negative) only promoters (bottom). H2A.ZWT, H2A.ZAP3, and H3K27me3 are plotted on the primary axis (right). H3K4me3 and RNAP2-Ser5P are plotted on the secondary axis (left). (C) Average read density plots comparing binding profiles of H2A.ZWT, H2A.ZAP3, and input at all H2A.Z target gene promoters in ESCs plotted +/−2 kb relative to TSS. The ChIP-Seq datasets for H2A.ZWT and H2A.ZAP3 were generated using GFP antibodies against the YFP transgene. (D) Genome profile of ChIP-Seq reads showing the distribution of H2A.ZWT (dark blue), H2A.ZAP3 (light blue), H3K4me3 (red), and H3K27me3 (light green) across the HoxA locus- a representative set of H2A.Z target genes. (E) Semi-quantitative western blot of H2A.ZWT and H2A.ZAP3 chromatin fractions probed with GFP and H3 (load control) using a range of DNA concentrations (top). Graph quantifying the ratio of transgene levels relative to H3 at the indicated DNA concentrations shows ∼1.85 fold more H2A.ZWT in chromatin fractions compared to H2A.ZAP3 (bottom). Fold change was calculated from the average ratio of each transgene to H3. Ratios for H2A.ZWT/H3 (0.439) and H2A.ZAP3/H3 (0.255) at the two intermediate DNA concentrations (160 µg/µl and 240 µg/µl) for replicate 1 (R1) were used to calculate the 1.72 (0.439/0.255) fold change between H2A.ZWT and H2A.ZAP3. Similar results were obtained for an independent replicate (R2). Ratios for H2A.ZWT/H3 (0.439) and H2A.ZAP3/H3 (0.219) at the two intermediate DNA concentrations (160 µg/µl and 240 µg/µl) were used to calculate the 2.0 (0.439/0.219) for R2. Thus, the levels of H2A.ZWT were on average 1.85-fold higher in chromatin-associated fractions relative to H2A.ZAP3. (F) Graph showing the ratio of SRCAP and RUVBL1 signal to their respective input signal, from co-immunoprecipitation analyses performed in H2A.ZWT and H2A.ZAP3 ESCs (in the endogenous H2A.Z knockdown background). Densitometric measurements of the western blots were performed in ImageJ. The standard deviations were generated from triplicates data points. (G) Nuclei isolated from H2A.ZWT and H2A.ZAP3 expressing ESCs were subjected to increasing salt concentrations as indicated. Histones were extracted at these salt concentrations and resolved by SDS-PAGE. Histones were detected by immunoblotting with GFP antibodies. In addition to its enrichment at active promoters, H2A.Z was enriched at bivalent promoters consistent with our prior ChIP-chip analysis [10]. Bivalent promoters are characterized by the enrichment of H3K4me3 and H3K27me3 marks, and these genes display low expression levels and reduced enrichment of RNA Polymerase II (RNAP2) (Figure 2A, B top) [13], [14]. In contrast, H2A.Z enrichment at active promoters (H3K4me3-only) was coincident with a strong RNAP2 peak at the TSS and higher expression of associated genes (Figure 2A, B bottom). Our analysis also revealed a bimodal H2A.Z distribution pattern around TSSs with a marked enrichment at the +1 nucleosome (Figure 2C), consistent with reports showing that H2A.Z flanks the nucleosome-depleted region [11]. Notably, we observed a broader distribution pattern of H2A.Z and H3K4me3 at bivalent genes compared to their enrichment at active genes suggesting that the chromatin structure differs at these two classes of promoters. In addition to promoters, we found that H2A.Z was enriched at a subset of distal enhancers identified in ESCs [38] (Table S1), similar to recent data using a pan-H2AZ antibody [36]. The enrichment at promoters as well as distal regulatory elements suggests that H2A.Z is incorporated at regions subject to considerable chromatin regulation.
We next analyzed H2A.ZAP3 localization across the ESC genome by ChIP-Seq. While H2A.ZAP3 enrichment was globally decreased, it occupied a highly similar set of promoter regions as well as distal enhancers (Figure 2C, Table S1). For example, H2A.ZAP3 displayed a similar overall spatial pattern of enrichment, albeit reduced compared to H2A.ZWT as shown across the large bivalent region encompassing the HOXA locus in ESCs (Figure 2D). Importantly, H2A.ZAP3 was expressed at similar levels as H2A.ZWT (Figure 1D, Figure S2A, B), indicating that reduced H2A.ZAP3 enrichment was not due to its lower abundance in ESCs. Interestingly, we observed a dramatic reduction in H2A.ZAP3 enrichment downstream of the TSS in ESCs, which is thought to mark the +1 nucleosome (Figure 2C). A number of studies have suggested that the +1 nucleosome possesses significant regulatory potential and that remodeling of this promoter nucleosome may be important for controlling gene expression by recruiting RNAP2 or by facilitating transcriptional elongation [39], [40]. Collectively, these data suggest that altered levels of H2A.ZAP3 as well as a particular reduction in the +1 nucleosome at promoters have consequences on the regulation of gene expression states.
One possibility for the observed lower levels of H2A.ZAP3 is that replacement of the divergent amino acids with those of H2A lead to its incorporation via a similar pathway as core histones. We expected that if H2A.ZAP3 is more broadly distributed along chromosomes, then its overall chromatin-associated fraction would be similar or higher relative to H2A.ZWT. To this end, we performed chromatin fractionation and probed for H2A.ZWT and H2A.ZAP3 using GFP antibodies in the respective transgenic ESC line (Figure S2B). Semi-quantitative immunoblots showed that the fraction of H2A.ZWT associated with chromatin is approximately 1.85 fold higher than H2A.ZAP3 suggesting that depletion of the mutant at TSSs does not lead to its random accumulation in chromatin (Figure 2E). Given recent evidence demonstrating that H2A.Z is redistributed during the cell cycle from promoters to heterochromatin regions in mouse trophoblast stem (TS) cells [41], next we examined the levels of H2A.ZAP3 at these regions. We first analyzed metaphase chromosomes (a time point when H2A.Z is enriched at heterochromatin in TS cells) and found that unlike the broad distribution of H2A across the chromosome H2A.ZAP3 ESCs showed a non-uniform distribution pattern and depletion at centromeric regions similar to H2A.ZWT (Figure S2C). Consistent with this observation, we found both H2A.ZAP3 and H2A.ZWT showed overall low enrichment at repetitive elements associated with heterochromatin including major satellite repeats in cycling cells (Figure S2D). Additionally, we found no change in the levels of H3K9me3, a modification highly enriched in heterochromatin, in H2A.ZWT and H2A.ZAP3 ESCs as well as in Day 5 RA differentiated cells by ChIP-qPCR (Figure S2E). The lower levels of H2A.Z at heterochromatin regions in ESCs compared to TS cells may reflect differences in cell cycle dynamics between the two cell types, as ESCs have a notably short G1. Together, these data suggest that mutations in the H2A.Z acidic patch do not result in the inappropriate incorporation of H2A.ZAP3 or disruption of heterochromatin.
H2A.Z acidic patch domain is necessary for stable chromatin association
The observed lower levels of H2A.ZAP3 at promoters suggested that the mutant is either more dynamically associated with chromatin or that it is not properly incorporated by its deposition complex. In yeast, deletion of either the carboxy-terminal docking domain or mutation of conserved residues within the acidic patch resulted in low H2A.Z occupancy at target genes [29]–[31]. This decrease in H2A.Z occupancy was attributed to the inability of the carboxy-terminal mutant to interact with its deposition complex SWR1. Site-specific incorporation of H2A.Z in mammalian cells is accomplished by the ATPase complex SRCAP (Snf2-related CREBBP activator protein), and in yeast H2A.Z removal appears to require the INO80 complex [42], [43]. Thus, we tested the ability of H2A.ZAP3 to interact with components of both of these complexes. We performed co-immunoprecipitation followed by immunoblot and found that H2A.ZAP3 interacted with SRCAP (catalytic component of SRCAP) and RUVBL1 (component of Tip60, SRCAP, and INO80 complexes) similar to H2A.ZWT (Figure S2F, Figure 2F). While it is possible that H2A.ZAP3 renders the deposition complex catalytically inactive, this scenario is unlikely because endogenous H2A.Z enrichment at promoters was unchanged over multiple passages in cells that also expressed the H2A.ZAP3 transgene (Figure S2G).
While H2A.Z specific chaperones such as CHZ1 have been identified in yeast [44], the CHZ1 homolog or chaperones that play similar roles in mammalian cells have not been studied in detail. Nevertheless, NAP1 is also a critical chaperone for histone incorporation in mammals including H2A.Z so we sought to test whether alterations in the acidic patch affected its interaction with this histone chaperone. Thus, we quantified the interaction between NAP1L1 and H2A.ZAP3, H2A.ZWT, as well as core H2A (Figure S2H, I). We found by co-immunoprecipitation using GFP antibodies that the amount of NAP1L1 bound to H2A.ZAP3 was modestly higher (<2-fold) compared to H2A.ZWT (Figure S2H, I). Surprisingly, we observed that lower levels of NAP1L1 co-immunoprecipitated with H2A compared to H2A.ZWT (Figure S2H, I). This result may be due to the much higher levels of stably associated H2A in chromosomes, of which only a fraction of H2A would interact with NAP1L1 unlike the continuous dynamic replacement of H2A.Z. Thus, these data are consistent with the idea that H2A.ZAP3 is more dynamic, resulting in a higher fraction of the mutant available to interact with histone chaperones.
We next tested the idea that H2A.ZAP3 is less tightly associated with chromatin compared to H2A.ZWT. To this end, we performed salt titrations on nuclei isolated from H2A.ZWT and H2A.ZAP3 ESCs. This analysis showed that while H2A.ZWT was stably associated with chromatin up to 500 mM NaCl and largely depleted at 1M NaCl (dimer loss typically occurs between 600–800 nM NaCl), a fraction of H2A.ZAP3 dissociates at the lower salt concentrations (75 mM–200 mM NaCl) (Figure 2G), suggesting that H2A.ZAP3 is less tightly associated with chromatin. The finding that H2A.ZAP3 is less tightly associated with chromatin by salt titration is consistent with the higher proportion of H2A.ZAP3 associated with NAP1L1 in ESCs. While we cannot rule out that H2A.ZAP3 incorporation is less efficient or that a small fraction is randomly distributed similar to incorporation of H2A, our data are consistent with the model that the unique H2A.Z extended acidic patch is critical for regulating its dynamic association with chromatin. These data also suggest that control of H2A.Z dynamics is important for regulation of gene expression programs during lineage commitment.
H2A.Z acidic patch is necessary for regulation of bivalent genes during ESC differentiation
H2A.Z knock-out mice die around the time of gastrulation when complex gene expression patterns are established during embryogenesis [5]. Moreover, ESCs depleted of H2A.Z fail to execute developmental gene expression programs when signaled to do so [10]. Furthermore, in vitro biophysical studies showed that an intact H2A.Z acidic patch is necessary for the ability of a nucleosomal template to fold into a 30-nm chromatin fiber and for the efficient repression of transcription [45], [46]. Thus, we analyzed global gene expression patterns in H2A.ZWT and H2A.ZAP3 cells in ESCs (Day 0 or D0) and at Day 3 (D3) of ESC differentiation (Table S2). We observed that a subset of genes showed higher expression levels in H2A.ZAP3 at D0 and that many of these genes remained expressed at higher levels in Day 3 H2A.ZAP3 EBs relative to H2A.ZWT (Figure 3A). These de-repressed genes have functions in vasculature development, pattern formation, and embryonic morphogenesis (e.g. Tbx20, Hoxb4, Foxc1) (Figure 3A). Genes that displayed higher expression levels in H2A.ZAP3 specifically at D3, function in steroid biosynthesis, signaling, and growth (e.g. Cyp51, Mvd, Wnt5a). In contrast, genes with lower expression levels at D3 in H2A.ZAP3 have roles in differentiation and transcription regulation (e.g. Notch4, Spag1, Neurod1, Wnt5a). These results indicate that H2A.ZAP3 incorporation leads to significant changes in the expression of genes with important developmental functions.
Fig. 3. H2A.Z acidic patch is necessary for regulation of bivalent genes during ESC differentiation. 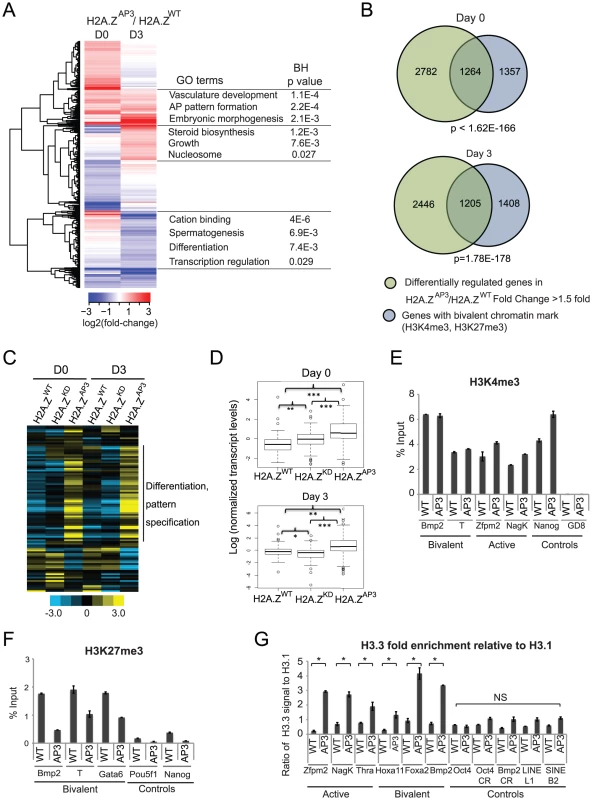
(A) Heat map displaying genes with absolute fold-changes of >1.5 fold at D0 or D3 in H2A.ZAP3 EBs relative to H2A.ZWT. Log2-fold changes in expression of these genes were hierarchically clustered using a Euclidean distance metric. Gene Ontology analysis of the clusters was performed using DAVID. (B) Venn diagrams representing overlap of differentially-regulated genes (>1.5 fold) in H2A.ZAP3 relative to H2A.ZWT (green) and genes with the bivalent chromatin mark within +/−2 kb of its TSS (blue) at D0 (top) and D3 (bottom). The p values were calculated by hypergeometric tests. (C) Heat map showing changes in transcript levels of a representative subset of genes (including developmental regulators, pluripotency factors, chromatin regulators and house keeping genes) using Nanostring in H2A.ZWT, H2A.ZKD, and H2A.ZAP3 ESCs at D0 and D3 of EB differentiation. The heat map was generated using log counts from each experiment, which were normalized to the geometric mean of the expression of housekeeping genes Gapdh, Tubb5, and Cltc (Table S3). Genes were then normalized across the 6 experiments. Yellow represents genes that are up-regulated 2 fold or over and blue represents genes down-regulated by 2 fold or more. (D) Boxplots representing the median expression levels of H2A.ZWT, H2A.ZKD, and H2A.ZAP3 D0 and D3 EBs were generated using log transformed, normalized gene expression values of all genes represented in the Nanostring probe set (Table S3). P values were calculated by unpaired student t-test. *p<0.008, **p<0.0008, and ***p<0.0002. The false discovery rate for a p value 0.0074 (<0.008) was generated using Monte Carlo simulation and found to be 3.9% from 10,000 random iterations, suggesting a small but significant change in median expression levels between Day 3 H2A.ZWT and H2A.ZKD EBs. qPCR was performed on ChIP DNA generated in H2A.ZWT and H2A.ZAP3 ESCs using H3K4me3 (E) and H3K27me3 (F) antibodies. (G) Quantitative qPCR was performed on ChIP-DNA enriched for H3.3 and H3.1 (Flag-HA tagged) using a mixture of Flag and HA antibodies in H2A.ZWT and H2A.ZAP3 containing HA-Flag tagged H3.3 and H3.1 transgenes independently. The ratio of H3.3 enrichment relative to H3.1 was plotted for active (Zfpm2, NagK, Thra) and bivalent (Hoxa11, Foxa2, Bmp2) H2A.Z target promoters. Relative enrichment ratios were also plotted for control regions- Pou5f1/Oct4 promoter, coding regions for Pou5f1 (Pou5f1-CR) and Bmp2 (Bmp2-CR) and heterochromatin elements (LINE L1, SINE B2). Student's t-test was performed to generate indicated p values. *p<0.05. Error bars represent standard deviations from a triplicate set of experiments. NS indicates p values >0.5. While H2A.Z is enriched at the majority of H3K4me3 marked promoters, we previously observed that bivalent genes exhibited significant changes upon H2A.Z depletion [10]. In ESCs, H2A.Z incorporation at bivalent promoters is required for precise regulation of developmental programs during the initial stages of lineage commitment. Notably, genes that showed changes in H2A.ZAP3 ESCs comprised a large cohort of developmental regulators (Figure 3A). Thus, we further tested the connection between H2A.Z and the regulation of bivalent genes. We compared the differentially regulated genes in H2A.ZAP3 cells (D0 and D3) with genes containing either bivalent (H3K4me3 and H3K27me3) or active (H3K4me3 only) histone marks. We found a significant overlap between genes that are differentially regulated and genes with bivalent marks (P<10−100, Figure 3B), whereas no significant overlap was observed with H3K4me3 only (active) genes (Figure S3A, top). Reciprocally, we grouped target genes as active or bivalent according to histone modification patterns and compared the expression levels of these two groups. Similarly, bivalent genes (H3K4me3, H3K27me3) in H2A.ZAP3 cells showed significant deviations in expression relative to H2A.ZWT, whereas active genes did not exhibit significant differences (Figure S3A, bottom, Table S2).
We focused further attention on the class of bivalent genes and compared their expression in H2A.ZAP3 relative to H2A.ZWT during ESC differentiation using the Nanostring mCounter assay as an independent measure of gene expression (Figure 3C, Table S4). The subset of genes in the Nanostring probe set included developmental regulators, lineage specific genes as well as pluripotency factors and housekeeping genes, comprising a subset of known H2A.Z target genes and negative controls (Table S4). Consistent with our RNA-Seq data, genes involved in differentiation and pattern specification were expressed at higher levels in H2A.ZAP3 ESCs (Figure 3C,D). Interestingly, bivalent genes are expressed at higher levels in H2A.ZAP3 relative to H2A.ZKD ESCs, suggesting that incorporation of the mutant results in a distinct chromatin state compared to loss of H2A.Z. Consistent with this idea, H2A.ZKD ESCs failed to activate lineage markers in D3 EBs [10], whereas many of these genes were expressed at higher levels in H2A.ZAP3 EBs (Figure 3C,D). The distinct transcriptional output of H2A.ZAP3 relative to H2A.ZKD may be due to the replacement of H2A.Z with H2A rather than its loss [36], whereas incorporation of H2A.ZAP3 may result in a constitutively dynamic nucleosome.
Given that H2A.Z is enriched at active genes and poised bivalent genes in ESCs (Figure 2A), next we analyzed H3K4me3 and H3K27me3 patterns by ChIP-qPCR in H2A.ZWT and H2A.ZAP3 ESCs (Figure 3E,F). While H3K4me3 enrichment patterns did not vary significantly between H2A.ZWT and H2A.ZAP3 at either class of H2A.Z target genes (Figure 3E), we found that H3K27me3 enrichment was reduced at bivalent genes in the acidic patch mutant ESCs. This observation is consistent with the de-repression of bivalent genes in H2A.ZAP3 ESCs. Together, these results suggest that the divergent H2A.Z residues play key roles in the formation of specialized chromatin domains that are necessary for maintenance of the poised state and for responding to developmental cues.
H2A.Z exists in hybrid nucleosomes along with the histone H3 variant H3.3 whose incorporation marks active promoters, enhancers, and insulator elements [23]–[25]. These double variant nucleosomes are characterized as highly unstable and salt labile, consistent with its enrichment in regions of highly dynamic chromatin. Given that H2A.ZAP3 appears to be loosely associated with chromatin relative to H2A.ZWT by salt titration, next we asked whether H3.3 was enriched at bivalent promoters in H2A.ZAP3 ESCs compared to H2A.ZWT. Since H3.3 differs from H3 by only three amino acids, antibodies against H3.3 cannot be used to effectively distinguish the variant from canonical H3. To circumvent this issue, we transfected an H3.3 C-terminal HA-Flag construct into H2A.ZWT and H2A.ZAP3 transgenic ESCs depleted of endogenous H2A.Z (Figure S3B). Similar lines were created with H3.1 C-terminal HA-Flag constructs (Figure S3B). ChIP-qPCR analyses using Flag and HA antibodies revealed that the ratio of H3.3 enrichment relative to H3.1 was higher at target promoters in H2A.ZAP3 ESCs relative to H2A.ZWT (Figure 3G). Given that H3.3 is associated with hyperdynamic chromatin, including active genes in ESCs [24]–[26], [47], the increase in H3.3 enrichment at TSSs is consistent with the dynamic nature of H2A.ZAP3 nucleosomes and de-repression of target genes in these cells. Taken together, our results demonstrate that the regulation of bivalent genes is highly sensitive to H2A.Z incorporation compared to active genes. In particular, the finding that a subset of genes remained highly expressed in H2A.ZAP3 cells during lineage commitment suggests that the acidic patch may be necessary to maintain the poised, silent chromatin state at bivalent genes.
Divergent acidic patch residues regulate H2A.Z dynamics
Nucleosome dynamics have important functional consequences on gene regulation [47]–[49]. Our data suggest that disruption of the divergent residues in the acidic patch results in a more dynamic association of H2A.ZAP3 in chromatin. Single cell analysis by fluorescence recovery after photobleaching (FRAP) has been used previously to probe the mobility and dynamics of chromatin-associated proteins in mammalian nuclei [48], [50]–[52]. FRAP studies showed that the mobility of core histones H2B-GFP and H3-YFP are significantly higher in ESCs compared to differentiated cell types [47]. These analyses led to the idea that ESC chromatin is in a hyperdynamic and transcriptionally-permissive state, whereas heterochromatin formation during differentiation leads to a decrease in core histone dynamics [47]. Furthermore, in vitro biophysical studies showed that H2A.Z-containing nucleosomal arrays impeded higher order chromatin folding compared to canonical nucleosomes, suggesting that H2A.Z incorporation may contribute to a unique chromatin environment [53]. Thus, we analyzed the recovery kinetics of H2A.ZWT-YFP relative to H2A-YFP in ESCs. As expected, H2A-YFP displayed recovery kinetics in ESCs similar to previous reports for H2B-GFP (∼20% mobile fraction) (Figure 4A,B, Table S5) [47]. Interestingly, the dynamics of H2A.ZWT was reduced compared to H2A in ESCs. For example, the mobile fraction of H2A was ∼20% compared to ∼13% for H2A.ZWT (P<0.02) (Figure 4B). Importantly, unbleached photo-imaging controls showed that imaging conditions did not incur inadvertent bleaching and loss of histone fluorescence signal during the experiment (Figure S4A, B). The higher mobility of H2A in ESCs is consistent with a global, transcriptionally permissive chromatin environment in ESC, while the slower recovery of H2A.ZWT is suggestive of a more specialized and distinct H2A.Z chromatin state. Our data are also consistent with prior work indicating that H2A.Z incorporation leads to a more stable nucleosome [21], [54] and that H2A.Z nucleosomes promote formation of 30 nm fibers [53].
Fig. 4. H2A.Z acidic patch influences chromatin stability and H2A.Z dynamics. 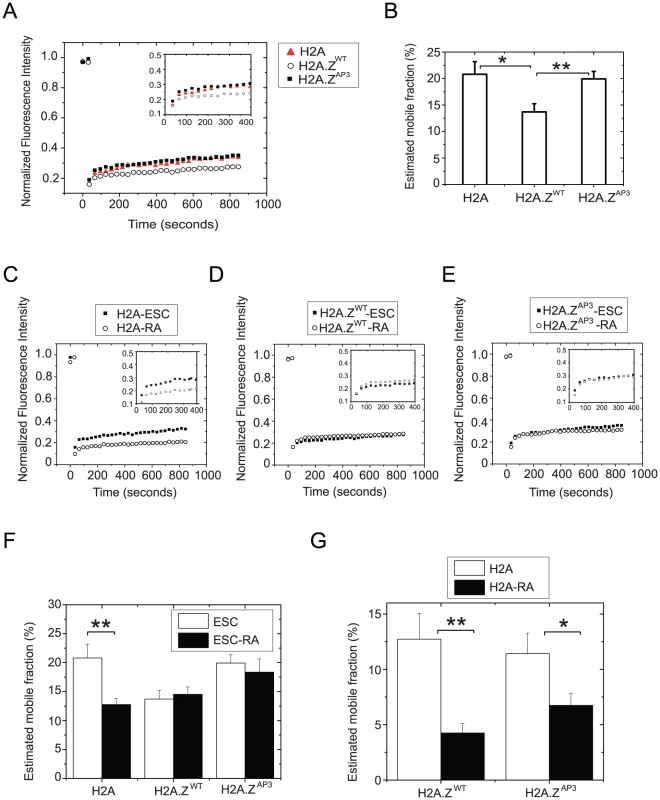
(A) Mean recovery curves generated from independent FRAP experiments performed on H2A, H2A.ZWT and H2A.ZAP3 expressing ESCs. Curves were generated by plotting mean fluorescence intensity of the bleached region measured every 30 secs for a duration of approximately 15 mins. (B) Recovery curves were used to determine percentage mobile fractions. (C–E) Similar recovery curves were generated from FRAP analyses performed on H2A.ZWT, H2A, and H2A.ZAP3 ESCs subjected to retinoic acid (RA)-induced differentiation for 5 days. (F) Graph representing the estimated mobile fractions of H2A, H2A.ZWT and H2A.ZAP3 in ESCs, and RA differentiated cells. *p<0.02, **p<0.004. NS indicates p values >0.6. P values were calculated using the standard unpaired Student t-test. Mean recovery curves were generated from individual curves from n>14 distinct cells for each cell type and condition. (G) Graph representing the estimated mobile fractions of H2A-mCherry in H2A.ZWT and H2A.ZAP3 (YFP transgenes) ESCs and day 5 RA differentiated cells. *p<0.04, **p<0.004. P values were calculated using the standard unpaired Student t-test. Mean recovery curves were generated from individual curves from n>12 distinct cells for each cell type and condition. Next we probed H2A.ZWT dynamics upon lineage commitment. ESCs were differentiated by addition of retinoic acid (RA) for five days and fluorescence recovery was measured on the final day. Consistent with an increase in chromatin condensation during lineage commitment due to heterochromatin formation, we found that H2A was less dynamic upon RA differentiation, as indicated by the reduction in the mobile fraction (∼20% in ESCs versus ∼12.5% in RA differentiated cells, P<0.02) (Figure 4C, F). In contrast, the recovery rate of H2A.ZWT was similar in ESCs and differentiated cells (Figure 4D, F, Table S5). The inability of H2A.Z to form more highly condensed chromatin structures typical of heterochromatin regions may explain why H2A.ZWT dynamics does not display a further reduction upon ESC differentiation. These data also suggest that H2A.Z dynamics is regulated via a different pathway compared to H2A.
Given that H2A.ZAP3 enrichment was lower in ESC chromatin by ChIP-Seq, we investigated its mobility in ESCs. We found that H2A.ZAP3 recovers significantly faster than H2A.ZWT and displays recovery kinetics similar to H2A (Figure 4A, B). For example, the H2A.ZAP3 mobile fraction was higher in ESCs compared to H2A.ZWT (20% versus 13%; P<0.006) and similar to H2A, suggesting that the divergent acidic patch residues play an important role in regulating H2A.Z dynamics. These observations are consistent with in vitro solution studies demonstrating that H2A.Z-containing nucleosome arrays harboring mutations in the acidic patch exhibited chromatin folding kinetics similar to H2A [55]. In contrast, the H2A.ZAP3 mobile fraction remained significantly higher upon differentiation compared with H2A, suggesting that H2A.ZAP3 nucleosomes are structurally distinct (P<0.044) (Figure 4E, F, Table S5). Taken together, these observations indicate that the reduced occupancy of H2A.ZAP3 in ESCs is likely due to its increased dynamics and suggest that the divergent H2A.Z acidic patch is important for formation of specialized chromatin states.
The difference in H2A.ZAP3 mobility upon RA differentiation relative to H2A may result from failure of these cells to properly differentiate. To determine if the mobile fraction was reflective of impaired differentiation capacity or purely a result attributable to H2A.ZAP3 dynamics, we introduced an H2A-mCherry transgene into H2A.ZWT and H2A.ZAP3 ESCs. To determine the mobile fraction of H2A-mCherry in both H2A.ZWT and H2A.ZAP3, we performed FRAP on Day 0 and Day 5 RA-differentiated ESCs. We found that the H2A dynamics was similar in H2A.ZWT and H2A.ZAP3 ESCs (Figure 4G, Figure S4C, D, Table S5) (12.7% and 11.4% respectively), suggesting that expression of the mutant variant does not significantly alter global H2A dynamics in the undifferentiated state. The relative differences in the percent mobile fraction for H2A-mCherry and H2A-YFP (22% versus 12.7%) is likely due to differences in their respective fluorophore properties [56]. Upon RA differentiation, we found H2A dynamics decreased in H2A.ZWT (4.3% in RA cells), consistent with formation of condensed heterochromatin during lineage commitment (Figure 4G, Figure S4C, Table S5). Similarly H2A dynamics was also reduced in H2A.ZAP3 retinoic acid induced cells (6.7% in RA cells), indicating that these cells form chromatin structures more similar to differentiated cell types (Figure 4G, Figure S4D, Table S5). Collectively, these data suggest that the acidic patch region is necessary for proper regulation of H2A.Z dynamics and that its incorporation directly controls gene expression during lineage commitment.
H2A.Z acidic patch is necessary for transcription-dependent chromatin dynamics
Active transcription is accompanied by rapid exchange of histone H2A/H2B dimers to accommodate the transiting RNA polymerase [48], [49], [51], [52]. Given that H2A.ZAP3 incorporation leads to altered chromatin dynamics and changes in gene expression, we hypothesized that the increase in H2A.ZAP3 mobility was linked to transcription. To test this idea, H2A.ZWT, H2A.ZAP3, and H2A ESCs were treated with flavopiridol, a reversible inhibitor of CDK9 that rapidly decreases RNAP2-dependent transcription. Consistent with previous observations, treatment of H2A.ZWT ESCs with 1 µM flavopiridol for 2 hrs resulted in a 30–44% decrease in RNAP2-dependent transcripts [57], [58] (Figure 5A). Notably, transcript levels were restored to control levels 2 hrs after the wash step. Prior FRAP studies in differentiated cells (HeLa) demonstrated a 3% reduction in the H2B mobile fraction upon treatment with the transcription inhibitor 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) [52]. While this overall fraction is low, it is expected that only a small subset of total H2B is associated with transcription given its broad distribution across the genome. Similarly, we found that H2A treated with flavopiridol exhibited an approximately 6% decrease in the mobile fraction in ESCs by FRAP relative to the DMSO control (P<0.02) (Figure 5B, Figure S5A). Importantly, H2A dynamics were restored to normal levels 2 hrs after removal of flavopiridol, suggesting that at least 6% of H2A in ESCs is linked to transcription (Figure 5B, Table S5). In contrast, we did not detect a measurable change in H2A.ZWT dynamics upon flavopiridol treatment as compared with untreated ESCs, suggesting H2A.ZWT dynamics is not specifically dependent on active transcription (Figure 5C, Figure S5B, Table S5). This result is consistent with the idea that H2A.Z incorporation results in an inherent steady-state dynamic at promoters [21], [54]. It is also possible that ESCs treated with flavopiridol show a moderate reduction in the H2A.ZWT mobile fraction that cannot be effectively resolved by FRAP [59]. In contrast, H2A.ZAP3 dynamics was decreased in ESCs upon flavopiridol treatment relative to H2A.ZWT (Figure 5D and Figure S5C). For example, we detected a ∼6% decrease in the H2A.ZAP3 mobile fraction upon flavopiridol treatment (P<0.01) similar to H2A, which was restored to near normal levels 2 hrs after flavopiridol removal. These observations were independently confirmed using DRB, an irreversible inhibitor of CDK9 and RNAP2-dependent transcription (Figure S5D–G, Table S5). Thus, our analysis suggests that the altered dynamics observed in H2A.ZAP3 ESCs are, in part, linked to transcription. These data are also consistent with disruption of a repressive chromatin state and higher expression of bivalent genes in H2A.ZAP3 cells.
Fig. 5. Disruption of H2A.Z acidic patch increases chromatin dynamics in a transcription-dependent manner. 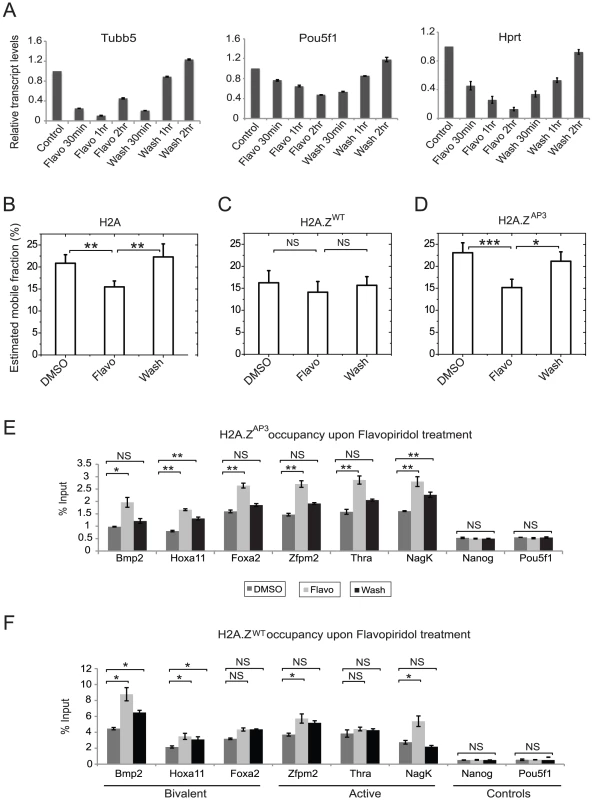
(A) qRT-PCR results showing a decrease in the transcript levels of active genes- Oct4, Tubb5, and Actin, upon treatment of 1 µM flavopiridol and restoration of transcript levels upon removal of the agent at the various time points in H2A.ZWT ESCs. (B–D) Graphs representing the estimated mobile fractions in H2A (B), H2A.ZWT (C), and H2A.ZAP3 (D) expressing ESCs upon treatment and removal of flavopiridol. ESCs treated with DMSO were used as a control. P values were generated by standard unpaired Student's t-test. *p<0.03, **p<0.015 and NS indicates p values >0.6. Quantitative PCR on ChIP DNA generated by using GFP antibodies in H2A.ZAP3 (E) and H2A.ZWT (F) ESCs treated with DMSO, 1 µM flavopiridol (Flavo) and 2 hrs after flavopiridol removal (Wash). Student's t-test was performed to test for statistical significance. * p<0.03, ** p<0.01, and NS represents not significant (>0.05). Error bars represent standard deviations from a triplicate set of experiments. To determine if the transcription-dependent decrease in H2A.ZAP3 dynamics is coincident with increase in H2A.ZAP3 occupancy at target genes, we performed ChIP on H2A.ZAP3 ESCs treated with DMSO, flavopiridol (Flavo) and 2 hours post flavopiridol removal (Wash). Consistent with our hypothesis, we found a significant increase in H2A.ZAP3 enrichment at target gene promoters with flavopiridol and partial reversal of this trend upon removal of flavopiridol (Figure 5E), suggesting that the decrease in H2A.ZAP3 dynamics upon flavopiridol treatment is coincident with higher H2A.Z chromatin occupancy. These data also support our findings that H2A.ZAP3 is properly incorporated at promoters. Similar ChIP analyses in H2A.ZWT ESCs revealed a more modest increase in H2A.ZWT occupancy at some but not all target genes upon flavopiridol treatment relative to H2A.ZAP3 (Figure 5F). This is also consistent with the FRAP data which indicates minimal change in H2A.ZWT mobile fraction upon flavopiridol treatment. These data are also consistent with in vitro biophysical studies showing that an intact H2A.Z acidic patch is necessary for the ability of a nucleosomal template to fold into a 30-nm chromatin fiber and for the efficient repression of transcription [45], [46]. Taken together, our work demonstrates that the divergent acidic patch is an important structural feature that mediates H2A.Z dynamics and maintenance of chromatin states necessary for regulation of inducible gene expression programs during lineage commitment.
Discussion
The essential histone variant H2A.Z has specialized functions that distinguish it from canonical H2A. The functional distinction between histone variants and core histones is mediated in large part by divergent regions in their respective amino acid sequences. These differences can impact how each histone is incorporated, the stability of the resulting nucleosome, higher order chromatin structure and its interactions with other factors. For example, H2A.Z harbors a unique extended acidic patch that contains three divergent residues compared to H2A. This region of H2A.Z is essential for development in Drosophila and Xenopus suggesting that it contributes specialized functions [6], [28], [60]. How the acidic patch domain influences H2A.Z function, however, is poorly understood. Here, we find that control of H2A.Z dynamics is mediated through the unique acidic patch region and that this regulation is critical for proper induction of developmental gene expression programs (Figure 6).
Fig. 6. H2A.Z acidic patch couples chromatin dynamics to gene expression regulation during ESC differentiation. 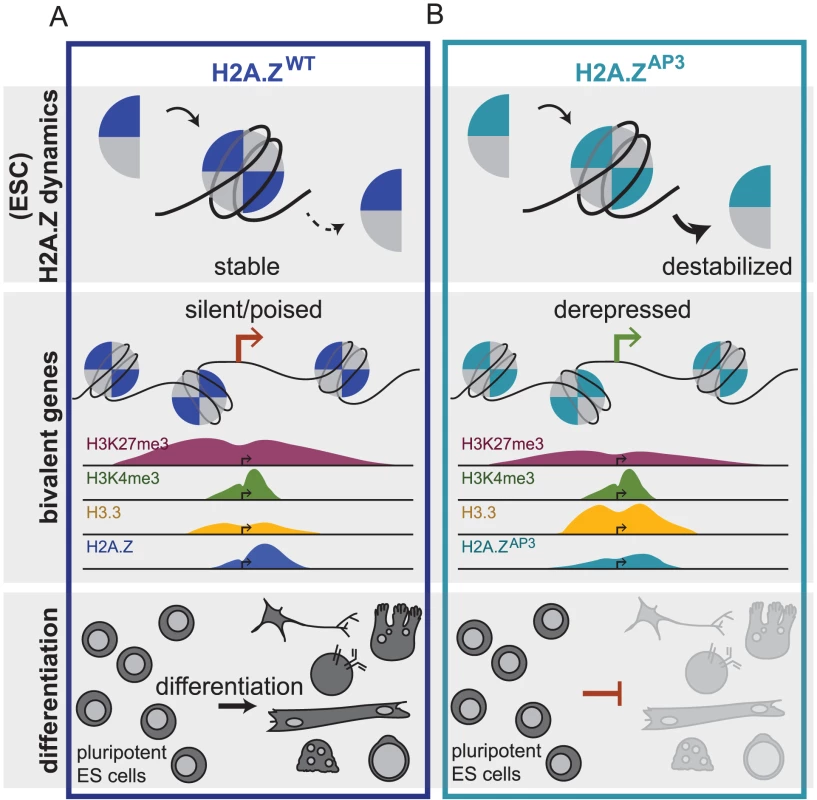
Schematic depicting H2A.Z dynamics in H2A.ZWT (A) and H2A.ZAP3 (B) ESCs and its consequence on gene expression of developmental genes and ultimately lineage commitment. The black arrow represents similar on rate for H2A.Z -containing dimers in H2A.ZWT and H2A.ZAP3 ESCs while a bold black arrow represents a greater off rate for H2A.ZAP3-H2B dimers whereas the hashed black arrow represents weaker off rate for H2A.Z-H2B dimers in H2A.ZWT. The model demonstrates how the divergent acidic patch domain functions to couple H2A.Z dynamics with the regulation of gene expression programs during ESC differentiation. H2A.Z acidic patch is necessary for proper regulation of bivalent genes and formation of specialized chromatin states during ESC differentiation
We analyzed the role of the three divergent H2A.Z acidic patch residues during ESC differentiation. While disruption of the acidic patch domain did not affect the global distribution of H2A.Z, its levels at target gene promoters were reduced, suggesting that the divergent acidic patch domain is an important determinant of H2A.Z incorporation or dynamics. We found that H2A.ZAP3 is less stably associated with chromatin relative to H2A.ZWT by salt extraction studies, suggesting that an intact acidic patch is necessary for stabilizing H2A.Z nucleosome structure. The dynamic association of H2A.ZAP3 with chromatin is also consistent with its lower enrichment at genomic sites. Prior in vitro studies show that H2A.Z forms stable 30 nm fibers at the expense of self-association [53], indicating that the unique acidic patch may be important for mediating distinct higher order chromatin structures.
H2A.Z is found at most H3K4me3 promoters of both active and poised genes and often flanks the nucleosome free region (NFR) around TSSs. We observed a particular reduction in H2A.ZAP3 levels in regions corresponding to the nucleosome downstream of the TSS, which likely represents the +1 nucleosome. The +1 nucleosome possesses significant regulatory potential and it is an important mediator of how a gene responds to environmental and developmental cues [39]. For example, a physical interaction between +1 nucleosome and stalled RNAP2 was demonstrated in Drosophila S2 cells and studies in C. elegans and Drosophila suggested that H2A.Z incorporation at the +1 nucleosome is important for maintaining the paused state of promoters [61]–[63]. Consistent with these findings, the accessibility of the TSS is determined in part by the displacement of the +1 nucleosome, which is important for recruitment of RNAP2. Additionally, the +1 nucleosome appears to be important for regulating transcriptional elongation through interactions with Mediator components [40]. Thus, H2A.Z incorporation at the +1 nucleosome may be critical for determining the specific transcriptional response to developmental cues. A similar phenomenon regarding the role of the H2A.Z +1 nucleosome has been observed in plants in response to changes in ambient temperature [15]. Notably, specific transcriptional responses cannot be achieved by replacement with H2A reinforcing the idea that H2A.Z incorporation leads to specialized chromatin states. Similarly, while H2A can substitute for H2A.Z upon loss of the variant in ESCs, target genes fail to activate properly during ESC differentiation [10], [36]. In contrast, incorporation of H2A.ZAP3 at TSSs, which is associated with a decrease in the +1 nucleosome, would allow for recruitment of RNAP2 and/or transcriptional elongation consistent with the persistent higher expression of bivalent genes during ESC differentiation (see below). Together, these data point to a critical role for regulating H2A.Z dynamics, particularly at the +1 nucleosome.
The decrease in H2A.ZAP3 enrichment led to changes in gene expression particularly at bivalent genes during ESC differentiation. H2A.Z is largely associated with active genes whose promoters are marked by H3K4me3 and it is enriched at a smaller subset of bivalent genes (comprising H3K4me3 and H3K27me3 modifications) in ESCs that are silent yet competent for activation. Why is the regulation of bivalent genes more sensitive to mutations in the acidic patch relative to active genes? We propose that the acidic patch is necessary for mediating H2A.Z dynamics or nucleosome stability upon incorporation at both poised and active target genes. Mutations in the acidic patch increase H2A.Z mobility and possibly alter the stability of the +1 nucleosome, which leads to expression of bivalent genes. Conversely, the increase in H2A.Z dynamics may have little impact on the expression of actively transcribed genes since elongation is occurring at these genes and perhaps other modes of regulation prevail to maintain genes in an active state [48]. This idea is also consistent with the observation that H2A.Z levels decrease upon gene activation [8].
In addition to H2A.Z, Polycomb Repressive Complexes (e.g. PRC1 and PRC2) are enriched at bivalent genes [10], [11]. PRCs are important for establishment and maintenance of repressed chromatin states and play key roles in dynamic regulation of gene expression during lineage commitment [64]. We suggest that disruption of the H2A.Z acidic patch alters the chromatin state and loss of PcG-mediated repression at bivalent genes. Consistent with this idea, we found that H3K27me3 levels were reduced at bivalent genes in H2A.ZAP3 ESCs, whereas H3K4me3 levels remained largely unchanged. These data further support our prior findings that functional coordination between PRCs and H2A.Z is important for regulating developmental gene expression patterns during ESC differentiation [10], [65]. Thus, the unique H2A.Z acidic patch may generally restrict the mobility of nucleosomes in ESCs. Changes in local chromatin compaction due to incorporation of the mutant may lead to changes in gene expression and subsequent loss of PRCs leading to a shift in the balance of chromatin associated factors that promote activation. In support of this model, we found that bivalent promoters gained H3.3 in H2A.ZAP3 ESCs, a histone variant typically incorporated at highly dynamic chromatin regions. Together, our data suggest that the divergent H2A.Z acidic patch is necessary for the formation of specialized chromatin states that allow for maintenance of the poised state and for proper induction of gene expression programs in response to developmental cues (Figure 6).
The H2A.Z acidic patch mediates histone dynamics in response to transcriptional cues
Given that H2A.Z displayed reduced chromatin association in ESCs compared to H2A.ZWT, we used single cell analysis to further analyze its dynamic association with chromatin. Interestingly, FRAP measurements revealed that H2A.Z was less dynamic than H2A as indicated by its reduced mobile fraction. These data are consistent with the idea that H2A.Z incorporation leads to the formation of a more stable nucleosome and the H2A.Z containing nucleosome arrays promote formation of 30 nm fibers [53]. While FRAP measurements represent overall steady-state chromatin dynamics, little is known regarding the on - and off-rates of H2A.Z. We suggest that this equilibrium is altered upon disruption of the divergent acidic patch residues resulting in a higher off rate and increased transcriptional output at genes that required H2A.Z for maintenance of the poised state (Figure 6). Moreover, our data suggest that the divergent acidic patch is a critical determinant of H2A.Z dynamics and that this region may be important for rapid activation of developmental programs. Remarkably, we show that while H2A.ZAP3 appears more dynamic, treatment with the transcriptional inhibitor flavopiridol led to a decrease in its mobile fraction as well as increased occupancy at target promoters. Thus, H2A.ZAP3 dynamics may also be regulated by the local chromatin environment as well as by interactions with other factors. Consistent with this idea, studies in Arabidopsis showed that H2A.Z is an important component of the thermosensory response. For example, H2A.Z was less sensitive to nuclease digestion at silent, inducible genes at normal temperature [15]. Upon activation of these genes by increasing temperature, H2A.Z-containing nucleosomes become more accessible to nuclease digestion suggesting that the variant is dynamically remodeled in response to inductive cues. Thus, it remains possible that H2A.Z modifications or interaction with other factors may mediate the function of the divergent acidic patch.
H2A.Z localization and dynamics is regulated from yeast to human in large part by two ATP-dependent remodelers, SRCAP and INO80, [66]–[69]. Thus, future studies will be important to determine how the divergent acidic patch cooperates with chromatin remodelers. For example, INO80 appears to be an important regulator of H2A.Z removal by catalyzing the exchange of H2A.Z for H2A [69]. Thus, one possibility is that H2A.ZAP3 is a better substrate for INO80 leading to a higher off rate and increased dynamics. Interestingly, Swc2 in yeast, a conserved subunit of SRCAP, binds directly to H2A.Z [70] and recent work indicates that this interaction may function as a “lock” that prevents rapid exchange of H2A.Z in chromatin [71]. Notably, domain swap experiments in yeast, where the H2A.Z C-terminal domain including the extended acidic patch is replaced with the analogous region of H2A blocks interaction with Swc2 and many other components of the SWR1 remodeling complex [70]. In contrast, deletion of the H2A.Z C-terminal region distal to the extended acidic patch maintained interactions with SWR1 [30]. Consistent with these observations, we found that H2A.ZAP3 interacted with SRCAP; however, it is possible that mutations in the extended acidic patch interfere with a specific interaction with YL1, the mammalian Swc2 homologue. Thus, an alternative scenario is that H2A.ZAP3 incorporation leads to SRCAP-mediated exchange of H2A.Z for H2A, which would likely influence dynamics.
The acidic patch has been proposed to mediate interactions with histone tails from neighboring nucleosomes, and this domain may also form a novel interaction surface for recruitment of other chromatin factors and downstream effectors [27], [53], [55], [60]. Thus, the unique H2A.Z acidic patch may interact with distinct subsets of factors that determine its specialized functions compared to H2A. In support of this idea, a recent proteomics analysis identified a number of chromatin-associated proteins enriched with H2A.Z [72]. These interactions may be important for regulating transcriptional output at target genes, ultimately allowing the cell to tune signals into specific responses that lead to changes in cell fate. In addition to its divergent structural features, H2A.Z is also subject to numerous posttranslational modifications (PTMs). For example, acetylation of the H2A.Z amino-terminus is a hallmark of active genes and has been implicated in gene regulation and chromosome stability [73]–[76], whereas the carboxy-terminal domain is modified by ubiquitylation and sumolyation, and these marks have been implicated in heterochromatin formation and DNA repair, respectively [77], [78]. As such, PTMs may regulate H2A.Z dynamics by promoting or inhibiting interactions with regulatory factors or by affecting its interactions with neighboring nucleosomes. Overall, our work provides critical insights into the role of the divergent H2A.Z acidic patch residues as a structural determinant that links chromatin dynamics, gene regulation, and ultimately cell fate. Moreover, these data provide a mechanistic explanation for the essential role of the divergent acidic patch during metazoan development.
Materials and Methods
Culture of mouse embryonic stem cells (mESCs)
V6.5 (129SvJae and C57BL/6) ESCs were plated with irradiated murine embryonic fibroblasts (MEFs) and grown under typical ESC conditions on gelatinized tissue culture plates. Briefly, cells were grown in Knockout DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone), leukemia inhibitory factor [16], non-essential amino acids (Invitrogen), L-glutamine (Invitrogen), and penicillin/streptomycin (Invitrogen) as previously described [79]. ESCs used for ChIP-Seq, RNA-Seq and qRT-PCR experiments were plated without MEFs for the final passage.
Transgenic ESC lines
H2A.ZWT-YFP, Mutant H2A.ZAP3-YFP and H2A-YFP constructs were generated from the pAd-cDNA (Addgene), which contains a dox-inducible CMV-promoter. The H2A.ZAP3 mutant was generated by replacing H2A.Z residues G92, D97, and S98 with the corresponding H2A amino acids (N, N and K respectively) using the instructions provided in the Quikchange Site Directed Mutagenesis Kit (Stratagene). We then replaced GFP in the vector with YFP (YFP exhibits lower background for imaging) to generate an in frame fusion C-terminal to H2A.Z. The resulting lentiviral constructs were transfected into 293 cells using the protocol outlined by the RNAi consortium (BROAD Institute, http://www.broadinstitute.org/rnai/public/). The viral supernatant generated 48 hrs after transfection was used to infect KH2 ESCs [80] to generate wild-type and mutant H2A.Z transgenic ESC lines. The YFP transgenic ESCs were induced with 1 µg/ml of doxycycline and FACS sorted for YFP positive cells.
shRNA-mediated depletion of endogenous H2A.Z
Lentiviral constructs expressing short hairpins specifically directed at the 3′ UTR of endogenous H2A.Z were introduced into the wild-type and mutant H2A.Z transgenic KH2 ESC lines. Sequences of the different H2A.Z 3′UTR-directed hairpin oligos are as follows: sh#1 5′ - AACAGCTGTCCAGTGTTGGTG-3′; sh#2 5′ - AATTAGCCTTCCAACCAACCA-3′. Hairpin oligos were annealed and cloned into pLKO.1 vector (Sigma) as detailed by the RNAi consortium, BROAD (http://www.broadinstitute.org/rnai/trc/lib). Since KH2 cells are puromycin resistant [80], blasticidin was used as a selection marker for the generation of endogenous H2A.Z-depleted transgenic KH2 ESCs. The puromycin marker in the pLKO.1 vector was removed by digestion with BamHI and KpnI and replaced with blasticidin. The blasticidin cDNA was PCR amplified from pLenti6.2/V5-DEST Gateway Vector (Invitrogen). V6.5 (129SvJae and C57BL/6) and the YFP transgenic ESCs were cultured as previously described [79]. The endogenous H2A.Z-depleted transgenic KH2 ESCs were cultured in the presence of blasticidin (5 µg/ml) on blasticidin-resistant feeder cells [81].
ESC differentiation
Retinoic acid-induced differentiation was performed by plating mESCs onto gelatinized tissue culture plates without MEFs and grown in the same mESC media as above but without LIF and supplemented with 1 µM all-trans retinoic acid (Sigma, R2625). Cells were collected after five days. mESCs were induced to form embryoid bodies [68] by plating on non-adherent plates from a starting density of 1–2 million ESCs per 10 ml of mESC media lacking LIF.
RNA isolation and quantitative real-time PCR
RNA was extracted using TRIzol (Invitrogen, 15596-018) or Izol (5PRIME, 2302700). Purified RNA was reverse transcribed using SuperScript III (Invitrogen, 18080-044) or M-MLV reverse transcriptase (Invitrogen, 28025-013) and random hexamers according to manufacturer protocols. Quantitative PCR reactions were performed with SYBR Green Master Mix (Roche). Primer sequences are listed in Table S4. Relative mRNA levels were determined in triplicate for each transcript using the manufacturer's software (Advanced Relative Quantification with Roche Lightcycler 480 Software Version 1.5) using control genes (Hprt, Gapdh, or Tubb5 levels) for normalization.
RNA-Seq library preparation and analysis methods
RNA was isolated using the protocol mentioned above. The purified RNA was the subjected to oligo (dT) selection, fragmentation and first and double strand synthesis with the Illumina Tru-Seq kit (RS-930-20 01) according to the manufacturer's instructions. DNA fragments above 30 bp was purified using SPRI-TE beads (Beckmann Coulter, Agencourt, A63880) according to manufacturer's instructions. The purified DNA was end-repaired and single A bases for adaptor ligations. The adaptor-ligated DNA was then subjected to double SPRI-TE purification to select for ∼200 bp fragments. These fragments were enriched and barcoded by PCR for multiplexing. A final SPRI-TE purification was performed to clean up the barcoded RNA-Seq libraries.
RNA-Seq data were aligned against the mouse reference genome (mm9) using Tophat 1.4.1 and Bowtie 0.12.7 in single-end mode, tolerating up to 2 mismatches and matches up to 20 locations transcriptome-wide. Gene expression was quantified with Cufflinks 1.3.0, and pairwise differential expression was analyzed using Cuffdiff 1.3.0. H3K4me3 and H3K27me3 enrichment peaks were obtained from our previous work [38]. Histone mark enrichment peaks whose boundaries fell within a region +/−2 kilobases (kbs) of a transcription start sites (TSS, based on RefSeq annotation of the mm9 mouse genome assembly) were identified and the number of H3K4me3 and H3K27me3 TSSs were recorded. RNA-Seq-based isoform expression data from Cufflinks (in RPKMs) were summed over each TSS and used as a metric for the transcriptional output originating from each TSS (Table S2). Median summed isoform expression levels were computed across all isoforms with H3K4me3 - or H3K4me3/H3K27me3-marked TSSs in the different ESCs (H2A.ZWT, H2A.ZKD, and H2A.ZAP3) and EB differentiation time points and compared by t-test. Similarly, the overlap between these subsets and the genes differentially regulated upon H2A.Z modulation was tested using the hypergeometric test. Finally, cumulative distribution of isoform expression differences upon H2A.Z modulation were computed by stratifying log2-tranformed isoform fold changes based on the presence of H3K4me3 and H3K27me3-bound regions within +/−2 kbs of the TSS. The significance of the differences between the cumulative distributions plots was assessed using the Kolmogorov-Smirnov test. All the sequencing results in the article have been deposited in GEO under the accession number GSE40065.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed as described [38] with the following modifications. Diagenode Bioruptor (UCD-200) was used to sonicate with 30 cycles of 30 sec on, 30 sec off. The samples were sonicated in 15 ml polystyrene tubes at 4°C while samples were immersed in ice cold water. Antibodies used for ChIP include: GFP (Abcam, ab290), H2A.Z (Abcam, ab4174), H3K27me3 (Cell Signaling Technology, #9733), H3K4me3 (Millipore, #07-473), Suz12 (Bethyl, A302-407A) and RNAP2 Ser5P (Abcam, ab5131).
ChIP and site-specific PCR
ChIP enriched DNA was quantified by quantitative PCR and the data analyses performed as described in [10]. qPCR reactions using SYBR Green (Roche) and gene-specific primers (Table S4) were performed on ChIP and whole cell extract (WCE) DNA. Reactions were performed in triplicate on the Roche LightCycler 480. % Input was calculated with the following formula: % Input = 2(Cp(WCE)-Cp(IP))×(% WCE).
ChIP-Seq library preparation and analysis methods
Approximately 200 ng of DNA was submitted to SPRI-works Fragment Library System I (Beckman Coulter) for each library prepared and sequenced on Illumina GAII. ChIP-seq reads were aligned to the mm9 genome assembly using Bowtie 0.12.3, allowing for two mismatches. To determine regions of the genome enriched for H2A.Z, mapped reads were extended to 200 bp (average fragment length) and allocated in 25-bp bins. A Poissonian model was used to determine statistically enriched bins with a P-value threshold set at 1E-9 as described previously [82]. Additionally, we required that genomic bins were at least 5 fold over input to be considered enriched peaks. All the sequencing results in the article have been deposited in GEO under the accession number GSE40065.
Fluorescence recovery after photobleaching (FRAP) analysis
ESCs were plated in the absence of MEFS on 0.2% gelatin in 2 mm Lab-Tek Chambered #1 borosilicate cover glass chambers containing a 1 mm glass slide 24 hours prior to imaging. Phenol-red free DMEM (Invitrogen 21063-045) was used to make ESC media for imaging purposes to minimize medium auto fluorescence. For differentiation experiments, ESCs were treated with 1 µM retinoic acid and plated on 0.2% gelatin in Lab-Tek Chambered cover glass 24 hours prior to imaging. YFP transgenic cells were additionally induced with 1 µg/ml of doxycycline 24 hours prior to imaging. Confocal fluorescence imaging was performed on a LSM 510 microscope (Carl Zeiss, Jena, Germany) with a 514 nm wavelength laser for YFP excitation, a 520–550 nm bandpass emission filter and a 100× 1.4 N. A. oil immersion objective. FRAP experiments were performed by photobleaching for a short 5.4 sec exposure to 100% laser intensity. To minimize error in the quantification of fluorescence recovery induced by the movement of unbleached chromatin into the bleached region due to the dynamic nature of ESC chromatin and changes in nuclear morphology, a 20 pixel sub-region within the 75 pixel bleached region was used was used to quantify fluorescence recovery using custom-written MATLAB routines (Mathworks, Natick, MA). In all cases the bleached region was sufficiently smaller than the size of the nucleus to minimize the effect of the original loss of fluorescent protein due to bleaching on maximal recovery. In any case, such loss would result in a uniform underestimation of mobile fractions in all tested instances, and not affect relative differences. Images were acquired using 30 sec acquisition intervals for approximately 14 mins with scan speed and imaging intervals optimized to minimize photobleaching during the recovery process. A minimum of 14 cells were analyzed for each FRAP experiment, with background correction performed by normalizing intensities to the maximum initial mean intensity in the bleach spot prior to photobleaching to generate individual FRAP curves. Mean data shown in results constitute the average of individual recovery curves. Mobile fractions (MF) were calculated from individual curves using MF = (Idip−Isat)/(1−Idip), where Idip is the value of the mean intensity immediately after the bleaching pulse, and Isat is the mean intensity at the end of the monitored recovery period. Student's t-test was used to calculate the statistical significance of the differences between estimated mobile fractions. For the flavopiridol experiments, cells were treated with 1 µM flavopiridol 2 hours before conducting photobleaching experiments. The post-wash FRAP experiments were performed on ESCs 2 hrs after washing away the inhibitor. For DRB experiments, ESCs were treated with 15 µM DRB for 2 hours and then imaged for FRAP as described above. All mobile fractions and the respective standard errors are listed in Table S5.
Histone extraction
Histone extracts were prepared by harvesting the cells first with ice-cold PBS supplemented with 5 mM sodium butyrate (106–107 cells). The cells were then washed in cold filter sterilized Lysis buffer (0.25M Sucrose, 3 mM CaCl2, 1 mM Tris pH 8.0, 0.5% NP-40). The nuclei were spun down at 3900 rpm for 5 min at 4°C. The supernatant was removed leaving the pellet of 50 µl of nuclei. This pellet was washed again with Wash solution (300 mM NaCl, 5 mM MgCl2, 5 mM DTT, 0.5% NP40) and spun under the same conditions as mentioned above. The supernatant was removed and the nuclei were resuspended in 50 µl of Extraction solution (0.5M HCl, 10% glycerol, 0.1M 2-mercaptoethylamine-HCl). The nuclei were then left in ice for 30 min after which they were spun at 13,000 rpm for 5 min at 4°C. The supernatant was transferred to siliconized tubes and 500 µl of acetone was added. The resulting solution was incubated at −20°C overnight. The resulting histone precipitate was spun at 13,000 rpm for 5 min for further analyses. Rabbit anti-H2A.Z antibody (Abcam, ab4174) and Rabbit anti-GFP antibody (Abcam, ab290) was used for western blot at concentrations recommended by the manufacturer.
Immunoblot analysis
Histone extracts, lysates and co-immunoprecipitated samples were resolved on SDS-PAGE gels and transferred on PVDF membrane (Bio-rad, 162-0177) using the Mini-Trans-Blot (Bio-rad, 170-3930). The transferred blot is then blocked with 5% milk in TBST (0.1% Tween-20 in 1× Tris-buffered saline-pH 7.4) for 1 hour at room temperature and incubated with the indicated antibodies prepared in TBST with 5% milk. The blots were then washed with TBST three times and incubated with species-specific HRP-conjugated secondary antibodies (Calbiochem). After incubation with secondary antibodies, the immunoblots was washed again with TBST and visualized using HRP substrate from Biorad (170-5070).
Immunostaining and histology
Cells were fixed with 4% paraformaldehyde for 20 minutes at room temperature, washed 3× with PBS, permeabilized in PBS with 0.2% TritonX, 0.1% Tween-20 for 30 minutes at room temperature, washed twice with PBS and blocked with PBS containing 0.1% Tween-20, 2% CCS (Cosmic Calf Serum, Invitrogen) for 1 hour at room temperature. The cells were then stained with anti-Oct4 antibody (mouse monoclonal, Santa Cruz, sc-5279, 1∶100) for 1 hour at room temperature, then washed twice more with block and stained with anti-mouse secondary (Alexafluor 594). After washing twice more, the cells were imaged using Nikon Eclipse Ti-S.
For histology, the EBs were fixed in 10% formalin for 20–30 mins and washed with PBS. Fixed EBs were then dehydrated by treating with a gradient of 70%, 80%, 90% and 100% ethanol for 20 mins each. The dehydrated EBs were finally cleared in xylene and embedded in paraffin overnight at 60°C. Sections of EBs at 0.4 microns were generated and placed on slides for staining. The sections are deparaffinized with xylene, rehydrated and stained with Harris Hematoxylin (Surgipath, 01560) and Eosin (Polyscientific, s176).
Qualitative and quantitative (SILAC) proteomics analyses
ESCs were cultured in 13C6/15N2-lysine(+8) 13C6/15N4-arginine(+10) (“SILAC heavy”) or naturally occurring lysine and arginine (“SILAC light”) medium according to [83]. Histones were purified from ES and differentiated cells essentially as described [84] except that a Zorbax C8 HPLC column was employed (Agilent). Each 1 min fraction collected from the HPLC separation of the histones was subjected to SDS-PAGE. Subsequent LC-MS/MS experiments were performed on an LTQ Velos-Orbitrap mass spectrometer (ThermoFisher Scientific) fed by an Agilent 1200 nano-HPLC system (Agilent) following procedures analogous to those described elsewhere [85]. First, the Coomassie-stained visible bands on PAGE separations of the HPLC fractions were interrogated by tryptic and chymotryptic digestion. Peptides unique to H2A.Z (and not derived from other H2A variants) were detected in bands of ∼14 kDa and ∼20 kDa. The ∼14 kDa variant of H2A.Z co-HPLC-separated with Histone H4 (∼12 kDa) while the ∼20 kDa variant co-HPLC-separated with Histone H2B (∼14 kDa). Next, these bands (from a parallel preparation) were subject to in-gel propionylation using propionic anhydride according to [86]. To study the C-terminal ubiquitination of H2A.Z, chymotryptic peptides were analyzed. To study the N-terminal acetylation of H2A.Z, tryptic peptides were analyzed. M/z values corresponding to the various acetylated and ubiquitin-residual peptides (The proteases will cleave ubiquitin as well as H2A.Z, leaving a branched peptide residual) were calculated. Separate acquisition methods were designed for the study of acetylation or ubiquitination. Selective-ion monitoring (SIM) windows were designed around these m/z as appropriate and data-independent MS/MS scans were acquired at these m/z ratios as dictated by each experiment. The sample was introduced to the mass spectrometer via liquid chromatography with conditions identical to those described [87].
Cell cycle and apoptosis analyses
Cell cycle kinetics using PI
A total of 5 million ESCs were resuspended in ice cold PBS to single-cell suspension and fixed using 95% ethanol at 4°C overnight. The fixed cells were then resuspended in PBS containing 1% BSA containing 50 µg/ml of propidium iodide (PI). The cells were then treated with RNAse A at a final concentration of 1 mg/ml at 37°C for 30 mins. The cells were then resuspended in PBS. DNA peak profiles were generated on a FACSCalibur (Becton Dickinson) flow cytometer at the Swanson Biotechnology Center Flow Cytometry Facility at the Koch Institute-MIT.
Cell cycle kinetics using BrdU incorporation
A total of 2 million cells were used for incorporation of BrdU using the BD Pharmingen APC BrdU Flow Kit (Cat.No: 552598) instructions. The ESCs were incubated with a final concentration of 10 µM BrdU for an hour. Day 5 RA-induced ESCs were incubated with the same concentration of BrdU for 3 hrs.
Annexin V staining
5 million cells were used for staining with Cy5 Annexin V using the BioVision Annexin V-Cy5 Apoptosis Kit (K103-25) according to manufacturer's instructions.
Cells counted from Day 10 embryoid bodies (EBs)
The Day 10 EBs are collected and washed with PBS. The washed EBs were resuspended in trypsin for 5 mins. The trysin was inactivated with ESC media containing serum. The cells were then counted using the Nexcelom Cell counter (Cellometer Auto T4).
Chromatin spreads
Chromosome spread analysis was performed as described in [88]. The cells were treated with a final concentration of 1 µg/ml of Nocodazole for 8 hours at 37°C. The treated cells were then washed with PBS. The mitosis-arrested cells were then collected and spread on slides using a Cytospin 4 (Thermo, Waltham, MA) [89]. The spread cells were then fixed with paraformaldehyde and permeabilized with detergent. The fixed slides were then stained for CENP-A at a dilution of 1∶800 (Cell Signaling Technology, #2048) and visualized with Alexa 594 secondary antibodies (1∶500). The DNA was visualized using DAPI and the GFP signal was from the YFP-fused transgene expression.
Supporting Information
Zdroje
1. BanaszynskiLA, AllisCD, LewisPW (2010) Histone variants in metazoan development. Dev Cell 19 : 662–674.
2. HenikoffS, AhmadK (2005) Assembly of variant histones into chromatin. Annu Rev Cell Dev Biol 21 : 133–153.
3. LugerK, DechassaML, TremethickDJ (2012) New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat Rev Mol Cell Biol 13 : 436–447.
4. GuillemetteB, GaudreauL (2006) Reuniting the contrasting functions of H2A.Z. Biochem Cell Biol 84 : 528–535.
5. FaastR, ThonglairoamV, SchulzTC, BeallJ, WellsJR, et al. (2001) Histone variant H2A.Z is required for early mammalian development. Curr Biol 11 : 1183–1187.
6. RidgwayP, BrownKD, RangasamyD, SvenssonU, TremethickDJ (2004) Unique residues on the H2A.Z containing nucleosome surface are important for Xenopus laevis development. J Biol Chem 279 : 43815–43820.
7. DrakerR, CheungP (2009) Transcriptional and epigenetic functions of histone variant H2A.Z. Biochem Cell Biol 87 : 19–25.
8. GuillemetteB, BatailleAR, GevryN, AdamM, BlanchetteM, et al. (2005) Variant histone H2A.Z is globally localized to the promoters of inactive yeast genes and regulates nucleosome positioning. PLoS Biol 3: e384.
9. SarmaK, ReinbergD (2005) Histone variants meet their match. Nat Rev Mol Cell Biol 6 : 139–149.
10. CreyghtonMP, MarkoulakiS, LevineSS, HannaJ, LodatoMA, et al. (2008) H2AZ is enriched at polycomb complex target genes in ES cells and is necessary for lineage commitment. Cell 135 : 649–661.
11. KuM, JaffeJD, KocheRP, RheinbayE, EndohM, et al. (2012) H2A.Z landscapes and dual modifications in pluripotent and multipotent stem cells underlie complex genome regulatory functions. Genome Biol 13: R85.
12. RaisnerRM, HartleyPD, MeneghiniMD, BaoMZ, LiuCL, et al. (2005) Histone variant H2A.Z marks the 5′ ends of both active and inactive genes in euchromatin. Cell 123 : 233–248.
13. AzuaraV, PerryP, SauerS, SpivakovM, JorgensenHF, et al. (2006) Chromatin signatures of pluripotent cell lines. Nat Cell Biol 8 : 532–538.
14. BernsteinBE, MikkelsenTS, XieX, KamalM, HuebertDJ, et al. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125 : 315–326.
15. KumarSV, WiggePA (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140 : 136–147.
16. SutcliffeEL, ParishIA, HeYQ, JuelichT, TierneyML, et al. (2009) Dynamic histone variant exchange accompanies gene induction in T cells. Mol Cell Biol 29 : 1972–1986.
17. HardyS, JacquesPE, GevryN, ForestA, FortinME, et al. (2009) The euchromatic and heterochromatic landscapes are shaped by antagonizing effects of transcription on H2A.Z deposition. PLoS Genet 5: e1000687.
18. CuiK, ZangC, RohTY, SchonesDE, ChildsRW, et al. (2009) Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 4 : 80–93.
19. BernsteinBE, KamalM, Lindblad-TohK, BekiranovS, BaileyDK, et al. (2005) Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120 : 169–181.
20. AbbottDW, IvanovaVS, WangX, BonnerWM, AusioJ (2001) Characterization of the stability and folding of H2A.Z chromatin particles: implications for transcriptional activation. J Biol Chem 276 : 41945–41949.
21. ParkYJ, DyerPN, TremethickDJ, LugerK (2004) A new fluorescence resonance energy transfer approach demonstrates that the histone variant H2AZ stabilizes the histone octamer within the nucleosome. J Biol Chem 279 : 24274–24282.
22. ParkYJ, LugerK (2008) Histone chaperones in nucleosome eviction and histone exchange. Curr Opin Struct Biol 18 : 282–289.
23. GoldbergAD, BanaszynskiLA, NohKM, LewisPW, ElsaesserSJ, et al. (2010) Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140 : 678–691.
24. JinC, ZangC, WeiG, CuiK, PengW, et al. (2009) H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet 41 : 941–945.
25. JinC, FelsenfeldG (2007) Nucleosome stability mediated by histone variants H3.3 and H2A.Z. Genes Dev 21 : 1519–1529.
26. ThakarA, GuptaP, IshibashiT, FinnR, Silva-MorenoB, et al. (2009) H2A.Z and H3.3 histone variants affect nucleosome structure: biochemical and biophysical studies. Biochemistry 48 : 10852–10857.
27. SutoRK, ClarksonMJ, TremethickDJ, LugerK (2000) Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat Struct Biol 7 : 1121–1124.
28. ClarksonMJ, WellsJR, GibsonF, SaintR, TremethickDJ (1999) Regions of variant histone His2AvD required for Drosophila development. Nature 399 : 694–697.
29. JensenK, SantistebanMS, UrekarC, SmithMM Histone H2A.Z acid patch residues required for deposition and function. Mol Genet Genomics 285 : 287–296.
30. WangAY, AristizabalMJ, RyanC, KroganNJ, KoborMS (2011) Key functional regions in the histone variant H2A.Z C-terminal docking domain. Mol Cell Biol 31 : 3871–3884.
31. WrattingD, ThistlethwaiteA, HarrisM, ZeefLA, MillarCB A conserved function for the H2A.Z C terminus. J Biol Chem 287 : 19148–19157.
32. JaenischR, YoungR (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132 : 567–582.
33. MurryCE, KellerG (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132 : 661–680.
34. DryhurstD, IshibashiT, RoseKL, Eirin-LopezJM, McDonaldD, et al. (2009) Characterization of the histone H2A.Z-1 and H2A.Z-2 isoforms in vertebrates. BMC Biol 7 : 86.
35. Eirin-LopezJM, Gonzalez-RomeroR, DryhurstD, IshibashiT, AusioJ (2009) The evolutionary differentiation of two histone H2A.Z variants in chordates (H2A.Z-1 and H2A.Z-2) is mediated by a stepwise mutation process that affects three amino acid residues. BMC Evol Biol 9 : 31.
36. HuG, CuiK, NorthrupD, LiuC, WangC, et al. (2013) H2A.Z facilitates access of active and repressive complexes to chromatin in embryonic stem cell self-renewal and differentiation. Cell Stem Cell 12 : 180–192.
37. BarskiA, CuddapahS, CuiK, RohTY, SchonesDE, et al. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129 : 823–837.
38. WamstadJA, AlexanderJM, TrutyRM, ShrikumarA, LiF, et al. (2012) Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell 151 : 206–220.
39. JiangC, PughBF (2009) Nucleosome positioning and gene regulation: advances through genomics. Nat Rev Genet 10 : 161–172.
40. NockA, AscanoJM, BarreroMJ, MalikS (2012) Mediator-regulated transcription through the +1 nucleosome. Mol Cell 48 : 837–848.
41. NekrasovM, AmrichovaJ, ParkerBJ, SobolevaTA, JackC, et al. (2012) Histone H2A.Z inheritance during the cell cycle and its impact on promoter organization and dynamics. Nat Struct Mol Biol 19 : 1076–1083.
42. RuhlDD, JinJ, CaiY, SwansonS, FlorensL, et al. (2006) Purification of a human SRCAP complex that remodels chromatin by incorporating the histone variant H2A.Z into nucleosomes. Biochemistry 45 : 5671–5677.
43. WongMM, CoxLK, ChriviaJC (2007) The chromatin remodeling protein, SRCAP, is critical for deposition of the histone variant H2A.Z at promoters. J Biol Chem 282 : 26132–26139.
44. LukE, VuND, PattesonK, MizuguchiG, WuWH, et al. (2007) Chz1, a nuclear chaperone for histone H2AZ. Mol Cell 25 : 357–368.
45. ThakarA, GuptaP, McAllisterWT, ZlatanovaJ (2010) Histone variant H2A.Z inhibits transcription in reconstituted nucleosomes. Biochemistry 49 : 4018–4026.
46. ZhouJ, FanJY, RangasamyD, TremethickDJ (2007) The nucleosome surface regulates chromatin compaction and couples it with transcriptional repression. Nat Struct Mol Biol 14 : 1070–1076.
47. MeshorerE, YellajoshulaD, GeorgeE, ScamblerPJ, BrownDT, et al. (2006) Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell 10 : 105–116.
48. HagerGL, McNallyJG, MisteliT (2009) Transcription dynamics. Mol Cell 35 : 741–753.
49. ThirietC, HayesJJ (2006) Histone dynamics during transcription: exchange of H2A/H2B dimers and H3/H4 tetramers during pol II elongation. Results Probl Cell Differ 41 : 77–90.
50. BhattacharyaD, TalwarS, MazumderA, ShivashankarGV (2009) Spatio-temporal plasticity in chromatin organization in mouse cell differentiation and during Drosophila embryogenesis. Biophys J 96 : 3832–3839.
51. KimuraH (2005) Histone dynamics in living cells revealed by photobleaching. DNA Repair (Amst) 4 : 939–950.
52. KimuraH, CookPR (2001) Kinetics of core histones in living human cells: little exchange of H3 and H4 and some rapid exchange of H2B. J Cell Biol 153 : 1341–1353.
53. FanJY, GordonF, LugerK, HansenJC, TremethickDJ (2002) The essential histone variant H2A.Z regulates the equilibrium between different chromatin conformational states. Nat Struct Biol 9 : 172–176.
54. ThambirajahAA, LiA, IshibashiT, AusioJ (2009) New developments in post-translational modifications and functions of histone H2A variants. Biochem Cell Biol 87 : 7–17.
55. FanJY, RangasamyD, LugerK, TremethickDJ (2004) H2A.Z alters the nucleosome surface to promote HP1alpha-mediated chromatin fiber folding. Mol Cell 16 : 655–661.
56. SeefeldtB, KasperR, SeidelT, TinnefeldP, DietzKJ, et al. (2008) Fluorescent proteins for single-molecule fluorescence applications. J Biophotonics 1 : 74–82.
57. FlynnRA, AlmadaAE, ZamudioJR, SharpPA Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc Natl Acad Sci U S A 108 : 10460–10465.
58. RahlPB, LinCY, SeilaAC, FlynnRA, McCuineS, et al. c-Myc regulates transcriptional pause release. Cell 141 : 432–445.
59. SpragueBL, PegoRL, StavrevaDA, McNallyJG (2004) Analysis of binding reactions by fluorescence recovery after photobleaching. Biophys J 86 : 3473–3495.
60. RangasamyD, GreavesI, TremethickDJ (2004) RNA interference demonstrates a novel role for H2A.Z in chromosome segregation. Nat Struct Mol Biol 11 : 650–655.
61. MavrichTN, JiangC, IoshikhesIP, LiX, VentersBJ, et al. (2008) Nucleosome organization in the Drosophila genome. Nature 453 : 358–362.
62. TevesSS, HenikoffS (2011) Heat shock reduces stalled RNA polymerase II and nucleosome turnover genome-wide. Genes Dev 25 : 2387–2397.
63. WhittleCM, McClinicKN, ErcanS, ZhangX, GreenRD, et al. (2008) The genomic distribution and function of histone variant HTZ-1 during C. elegans embryogenesis. PLoS Genet 4: e1000187.
64. SurfaceLE, ThorntonSR, BoyerLA Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell 7 : 288–298.
65. HuG, CuiK, NorthrupD, LiuC, WangC, TangQ, GeK, LevensD, Crane-RobinsonC, ZhaoK (2013) H2A.Z Facilitates Access of Active and Repressive Complexes to Chromatin in Embryonic Stem Cell Self-Renewal and Differentiation. Cell Stem Cell 12 : 1–13.
66. CuadradoA, CorradoN, PerdigueroE, LafargaV, Munoz-CanovesP, et al. (2010) Essential role of p18Hamlet/SRCAP-mediated histone H2A.Z chromatin incorporation in muscle differentiation. Embo J 29 : 2014–2025.
67. FazzioTG, HuffJT, PanningB (2008) An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell 134 : 162–174.
68. Papamichos-ChronakisM, KrebsJE, PetersonCL (2006) Interplay between Ino80 and Swr1 chromatin remodeling enzymes regulates cell cycle checkpoint adaptation in response to DNA damage. Genes Dev 20 : 2437–2449.
69. Papamichos-ChronakisM, WatanabeS, RandoOJ, PetersonCL (2011) Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144 : 200–213.
70. WuWH, AlamiS, LukE, WuCH, SenS, et al. (2005) Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat Struct Mol Biol 12 : 1064–1071.
71. WatanabeS, Radman-LivajaM, RandoOJ, PetersonCL (2013) A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science 340 : 195–199.
72. DrakerR, NgMK, SarcinellaE, IgnatchenkoV, KislingerT, et al. (2012) A combination of H2A.Z and H4 acetylation recruits Brd2 to chromatin during transcriptional activation. PLoS Genet 8: e1003047.
73. HalleyJE, KaplanT, WangAY, KoborMS, RineJ Roles for H2A.Z and its acetylation in GAL1 transcription and gene induction, but not GAL1-transcriptional memory. PLoS Biol 8: e1000401.
74. KimHS, VanoosthuyseV, FillinghamJ, RoguevA, WattS, et al. (2009) An acetylated form of histone H2A.Z regulates chromosome architecture in Schizosaccharomyces pombe. Nat Struct Mol Biol 16 : 1286–1293.
75. KroganNJ, BaetzK, KeoghMC, DattaN, SawaC, et al. (2004) Regulation of chromosome stability by the histone H2A variant Htz1, the Swr1 chromatin remodeling complex, and the histone acetyltransferase NuA4. Proc Natl Acad Sci U S A 101 : 13513–13518.
76. Valdes-MoraF, SongJZ, StathamAL, StrbenacD, RobinsonMD, et al. Acetylation of H2A.Z is a key epigenetic modification associated with gene deregulation and epigenetic remodeling in cancer. Genome Res 22 : 307–321.
77. KalocsayM, HillerNJ, JentschS (2009) Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell 33 : 335–343.
78. SarcinellaE, ZuzartePC, LauPN, DrakerR, CheungP (2007) Monoubiquitylation of H2A.Z distinguishes its association with euchromatin or facultative heterochromatin. Mol Cell Biol 27 : 6457–6468.
79. BoyerLA, PlathK, ZeitlingerJ, BrambrinkT, MedeirosLA, et al. (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441 : 349–353.
80. BeardC, HochedlingerK, PlathK, WutzA, JaenischR (2006) Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis 44 : 23–28.
81. IuchiS, DabelsteenS, EasleyK, RheinwaldJG, GreenH (2006) Immortalized keratinocyte lines derived from human embryonic stem cells. Proc Natl Acad Sci U S A 103 : 1792–1797.
82. MarsonA, LevineSS, ColeMF, FramptonGM, BrambrinkT, et al. (2008) Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134 : 521–533.
83. BendallSC, HughesC, StewartMH, DobleB, BhatiaM, et al. (2008) Prevention of amino acid conversion in SILAC experiments with embryonic stem cells. Mol Cell Proteomics 7 : 1587–1597.
84. ThomasCE, KelleherNL, MizzenCA (2006) Mass spectrometric characterization of human histone H3: a bird's eye view. J Proteome Res 5 : 240–247.
85. Sherman MKaNE (2000) Protein Sequencing and Identification Using Tandem Mass Spectrometry Sons JWa, editor: John Wiley and Sons.
86. GarciaBA, MollahS, UeberheideBM, BusbySA, MuratoreTL, et al. (2007) Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat Protoc 2 : 933–938.
87. JaffeJD, KeshishianH, ChangB, AddonaTA, GilletteMA, et al. (2008) Accurate inclusion mass screening: a bridge from unbiased discovery to targeted assay development for biomarker verification. Mol Cell Proteomics 7 : 1952–1962.
88. GoshimaG, KiyomitsuT, YodaK, YanagidaM (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J Cell Biol 160 : 25–39.
89. ToyodaY, YanagidaM (2006) Coordinated requirements of human topo II and cohesin for metaphase centromere alignment under Mad2-dependent spindle checkpoint surveillance. Mol Biol Cell 17 : 2287–2302.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



