-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
Human cancer genomes are highly complex, making it challenging to identify specific drivers of cancer growth, progression, and tumor maintenance. To bypass this obstacle, we have applied array comparative genomic hybridization (array CGH) to zebrafish embryonal rhabdomyosaroma (ERMS) and utilized cross-species comparison to rapidly identify genomic copy number aberrations and novel candidate oncogenes in human disease. Zebrafish ERMS contain small, focal regions of low-copy amplification. These same regions were commonly amplified in human disease. For example, 16 of 19 chromosomal gains identified in zebrafish ERMS also exhibited focal, low-copy gains in human disease. Genes found in amplified genomic regions were assessed for functional roles in promoting continued tumor growth in human and zebrafish ERMS – identifying critical genes associated with tumor maintenance. Knockdown studies identified important roles for Cyclin D2 (CCND2), Homeobox Protein C6 (HOXC6) and PlexinA1 (PLXNA1) in human ERMS cell proliferation. PLXNA1 knockdown also enhanced differentiation, reduced migration, and altered anchorage-independent growth. By contrast, chemical inhibition of vascular endothelial growth factor (VEGF) signaling reduced angiogenesis and tumor size in ERMS-bearing zebrafish. Importantly, VEGFA expression correlated with poor clinical outcome in patients with ERMS, implicating inhibitors of the VEGF pathway as a promising therapy for improving patient survival. Our results demonstrate the utility of array CGH and cross-species comparisons to identify candidate oncogenes essential for the pathogenesis of human cancer.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003727
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003727Summary
Human cancer genomes are highly complex, making it challenging to identify specific drivers of cancer growth, progression, and tumor maintenance. To bypass this obstacle, we have applied array comparative genomic hybridization (array CGH) to zebrafish embryonal rhabdomyosaroma (ERMS) and utilized cross-species comparison to rapidly identify genomic copy number aberrations and novel candidate oncogenes in human disease. Zebrafish ERMS contain small, focal regions of low-copy amplification. These same regions were commonly amplified in human disease. For example, 16 of 19 chromosomal gains identified in zebrafish ERMS also exhibited focal, low-copy gains in human disease. Genes found in amplified genomic regions were assessed for functional roles in promoting continued tumor growth in human and zebrafish ERMS – identifying critical genes associated with tumor maintenance. Knockdown studies identified important roles for Cyclin D2 (CCND2), Homeobox Protein C6 (HOXC6) and PlexinA1 (PLXNA1) in human ERMS cell proliferation. PLXNA1 knockdown also enhanced differentiation, reduced migration, and altered anchorage-independent growth. By contrast, chemical inhibition of vascular endothelial growth factor (VEGF) signaling reduced angiogenesis and tumor size in ERMS-bearing zebrafish. Importantly, VEGFA expression correlated with poor clinical outcome in patients with ERMS, implicating inhibitors of the VEGF pathway as a promising therapy for improving patient survival. Our results demonstrate the utility of array CGH and cross-species comparisons to identify candidate oncogenes essential for the pathogenesis of human cancer.
Introduction
Rhabdomyosaroma (RMS) is the most common soft tissue sarcoma of childhood [1] and falls into two major histopathologic subtypes in children - embryonal and alveolar. Embryonal rhabdomyosaroma (ERMS) accounts for approximately 60% of childhood cases and is frequently associated with RAS pathway activation [2]–[5]. Treatment for either RMS subtype requires surgical resection, chemotherapy, and radiation with overall poor prognosis for patients with high-risk features, metastasis, or relapse disease. Thus, there is great interest in elucidating key molecular pathways and genetic factors that are involved in continued RMS growth and tumor maintenance. Cytogenetic studies, including array Comparative Genomic Hybridiation (array CGH), identify frequent but inconsistent gains and losses of whole or partial chromosome arms and rare focal high-level amplifications in both human ERMS and ARMS [5]–[9], largely precluding the identification of specific drivers of cancer in this disease. Moreover, array CGH and cross-species comparisons between mouse and human RMS have largely failed to identify functionally important genes contained within common copy number alterations (CNAs). In one report, RMS that arose in Ptch1+/− Blmtm3Brd/tm3Brd (a hypomorphic Blm allele) mice exhibited a gain of chromosome 10 in 80% of cases [10], but the oncogenes associated with this chromosomal gain remain undefined due to the large number of candidate genes found within this region. Moreover, extension of these findings to human RMS has not been reported. Rubin et al. recently showed that greater than 30% of ERMS arising in mice that harbor p53 homozygous deletion and/or Ptch1 heterozygous deletion lack a defined molecular signature or genetic lesion, suggesting undiscovered pathways likely contribute to ERMS transformation, growth, and tumor maintenance [11]. To date, there remains a need for novel gene discovery methods to identify genes and pathways essential for tumor growth, progression, and maintenance in human cancer – including ERMS.
Zebrafish cancer shares molecular and pathological similarities to human disease [4], [12]–[16]. For example, Lam et al. (2006) was the first to use comparative analysis of microarray data from zebrafish and human liver tumors to demonstrate a conserved molecular profile during tumor progression [13]. Building on this work, microarray gene expression studies of zebrafish ERMS and cross-species comparison to human disease identified RAS pathway activation as a common initiating event in zebrafish and human ERMS. Activating RAS mutations have also been identified in numerous studies of human ERMS [2]–[5], [17]. Most recently, Paulson et al reported that 11 of 26 (42%) human ERMS samples harbored activating RAS mutations along with acquisition of additional CNAs as detected array CGH [5], suggesting that additional genetic lesions are likely required to drive oncogenic transformation to ERMS. Not surprisingly, zebrafish cancers also exhibit recurrent chromosomal gains and losses similar to those found in human cancer. For example, transgenic models of zebrafish melanoma, T-cell acute lymphoblastic leukemia (T-ALL), and ERMS contain genomic imbalances including high-level gains and losses [18]. However, specific driver events could not be identified in these studies due to the low resolution of this platform. Using high-resolution array CGH, Zhang et al (2010) demonstrated the aneuploid nature of zebrafish malignant nerve sheath tumors (MPNST), a feature that also characterizes the human disease, and identified a subset of genes that are co-amplified as high-copy gains in human MPNST [19]. High-resolution array CGH has also been applied to zebrafish T-ALL and identified a subset of genes contained within CNAs that were also amplified or deleted in human disease [20]. These latter two studies have demonstrated the utility of array CGH technology in detecting copy number aberrations and candidate driver genes in zebrafish tumor models, yet functional relevance of identified genes in human disease has not been reported nor have these genes been assessed for roles in regulating tumor maintenance – providing novel targets for therapy in established tumors.
Capitalizing on a zebrafish model of kRASG12D-induced ERMS that shares common histopathological, genetic, and molecular characteristics of human ERMS [4], [21], [22], we have utilized high-resolution array CGH to identify novel CNAs in ERMS. Remarkably, our array CGH analysis revealed focal CNAs that span short genomic regions and contain only 1–3 genes. To validate the functional significance of amplified genes in human ERMS, we prioritized six genes for initial characterization in human ERMS cell lines. Of these six genes, gene knockdown of Cyclin D2 (CCND2), Homeobox C6 (HOXC6), PlexinA1 (PLXNA1) inhibited proliferation of human ERMS. PLXNA1 also exhibited important roles in blocking ERMS cells in early stages of muscle differentiation, enhancing migration, and altering anchorage-independent growth. CCND2, HOXC6, PLXNA1, and Vascular Endothelial Growth Factor A (VEGFA) were also highly expressed in a large fraction of human primary RMS, supporting prominent roles for these genes in rhabdomyosarcoma. Chemical inhibition of VEGF signaling reduced tumor growth in vivo with an associated decrease in angiogenesis, implicating VEGF inhibitors as promising therapeutic agents for ERMS. Taking advantage of tractable features of zebrafish cancer genomes such as smaller CNA intervals and regions of conserved homology with human disease, our study demonstrates the effective use of array CGH to identify oncogenes required for continued tumor growth in human rhabdomyosaroma, providing novel therapeutic targets for the treatment of ERMS.
Results
Zebrafish array CGH identifies novel and conserved CNAs in human ERMS
Array CGH was performed on genomic DNA isolated from twenty kRASG12D-induced zebrafish ERMS and compared to adjacent normal tissue. Array CGH revealed a complex CNA pattern with relative gains being observed more frequently than losses. For example, we identified 190 regions of amplification and 35 deletions recurrent in ≥3 zebrafish tumors analyzed (Table S1). Remarkably, only 2 of 20 zebrafish samples exhibit evidence of aneuploidy, contrasting starkly with human ERMS where nearly all human RMS harbored regions of extensive aneuploidy [5]. While 10 zebrafish ERMS showed evidence for CNAs in coding regions of the genome, only 3 exhibited a high frequency of multiple gains (Table 1 and S1). In total, we identified 19 gains and 2 losses in gene-containing amplicons that were recurrent in at least three zebrafish ERMS samples. Candidate genes in these regions were predominantly amplified as low-level gains, which averaged 1–3 genes and spanned only 48+/−27 kb (+/ − SD, Table 1; Fig. 1A). Copy number alterations were validated by qPCR of genomic DNA (Fig. S1).
Fig. 1. Array CGH reveals cancer-specific chromosomal abnormalities in zebrafish ERMS. 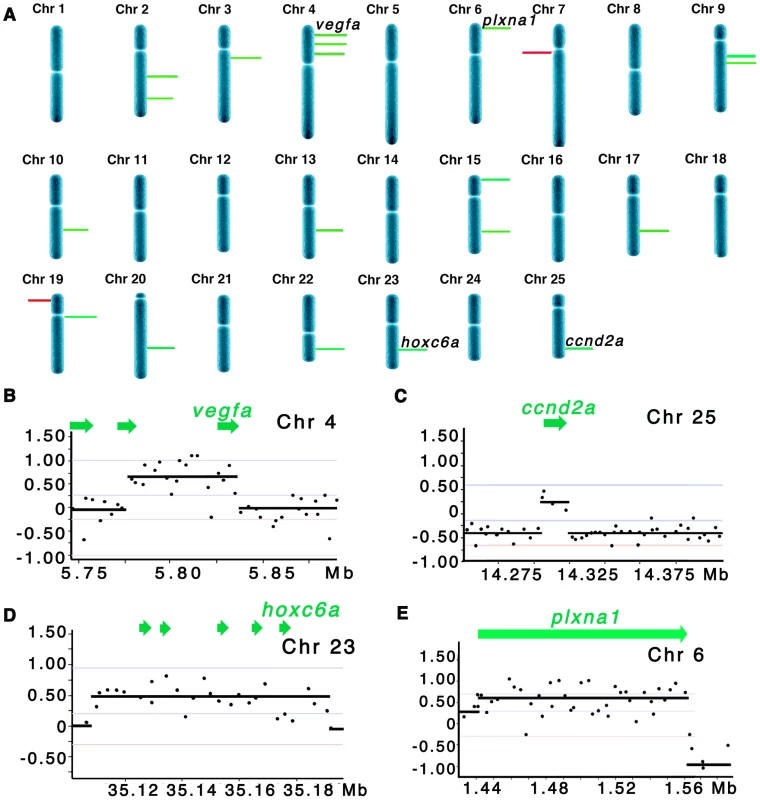
(A) Summary of common gene-containing CNA gains (green) and losses (red) in 20 animals examined. Only recurrent CNAs found in ≥3 samples are shown. The height of each bar correlates with the frequency of each aberration. Detailed view of regional gains for vegfa on chromosome 4 (B), ccnd2a on chromosome 25 (C), hoxc6a on chromosome 23 (D), and plxna1 on chromosome 6 (E). Y-axis denotes log2 ratio of the probes and X-axis denotes genomic coordinates. Tab. 1. Comparison of array CGH analyses in zebrafish and human ERMS. 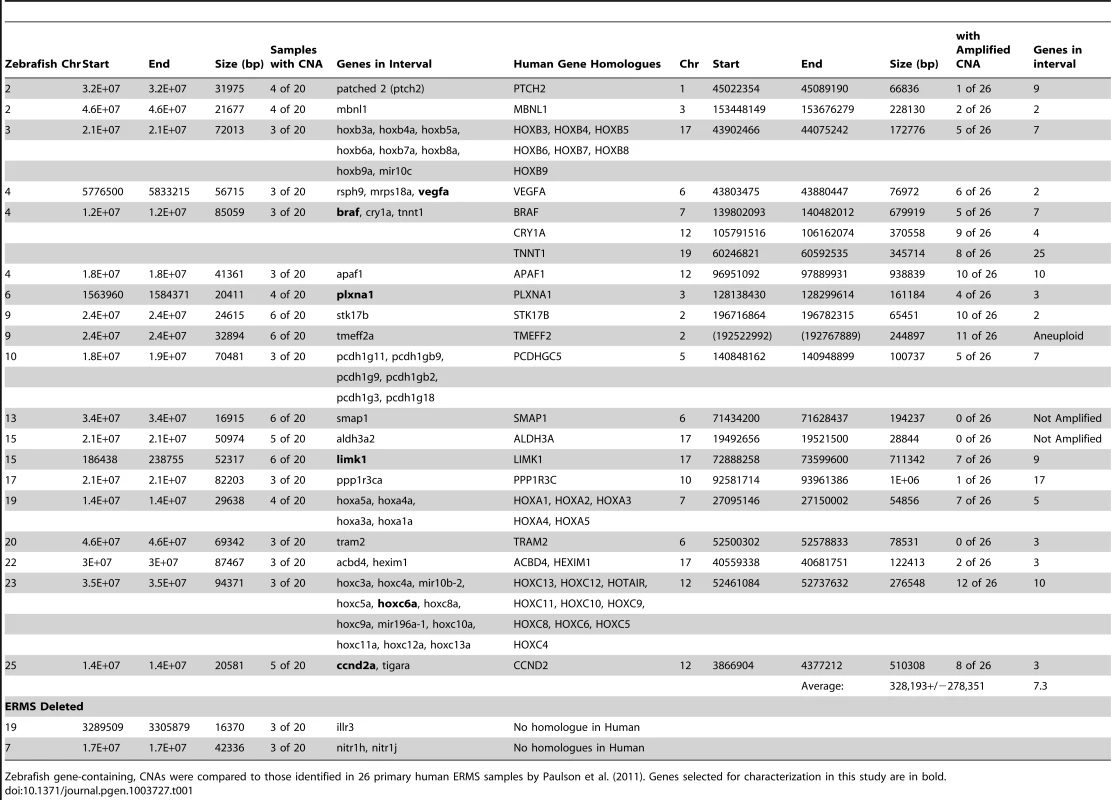
Zebrafish gene-containing, CNAs were compared to those identified in 26 primary human ERMS samples by Paulson et al. (2011). Genes selected for characterization in this study are in bold. To assess whether CNAs identified in zebrafish ERMS were conserved in human disease, zebrafish array CGH data was compared to the high-resolution array CGH data from 26 human ERMS samples [5]. Of the 26 samples, 11 carried activating RAS mutations as assessed by Sanger sequencing analysis [5]. The two regions of chromosomal loss in zebrafish ERMS contained zebrafish-specific genes that failed to have human homologues (Table 1). By contrast, genes contained within the 19 CNA gains mapped to 21 distinct homologous genomic regions in human. Utilizing the same statistical algorithms and threshold settings as outlined by Paulson et al [5], we discovered that 18 of 21 homologous regions were also amplified as low-copy gains in human ERMS samples (Table 1). Like zebrafish ERMS, these CNAs were focal, low-copy gains spanning 328 kB+/−278 kB and contained 7.3 genes on average (Table 1 and S1).
To demonstrate the efficacy of our array CGH approach to identify evolutionarily conserved oncogenes essential for driving tumor progression and maintenance, we prioritized CNAs that contained genes and were amplified in both zebrafish and human ERMS. In total, six candidate genes were prioritized for further study in human ERMS based on the following criteria. 1) Candidate oncogenes were differentially expressed in human ERMS compared to ARMS and/or normal muscle as assessed by microarray gene expression studies. 2) Genes that have known oncogenic activity in other cancer types, but yet ascribed functional roles in ERMS, were prioritized for additional study to serve as “proof of concept” genes in our cross-species comparative study. 3) A subset of genes was selected which have unknown function in ERMS and represent potential novel oncogenes. 4) Amplified CNA regions that harbored a single human homologue were also prioritized. Based on these criteria, CCND2, HOXC6, PLXNA1, VEGF, BRAF and LIMK1 were selected for further study (Table 1). CCND2, PLXNA1, VEGFA, and LIMK1 were the single genes contained within the amplified CNA intervals in human disease. BRAF was the only gene in the interval that was overexpressed in human ERMS, whereas CRY1 and TNNT1 identified within the same amplified interval were not differentially expressed when comparing human ERMS to normal muscle (Fig. S2). HOXC6 has been reported to be highly expressed in human ERMS compared with ARMS [23], suggesting an possible role in modulating tumor growth. While CCND2, a cell cycle regulator, VEGFA, an essential regulator of angiogenesis in a variety of cancer types and BRAF, an oncogene in a variety of cancers, most likely serve as our “proof of concept” genes for demonstrating functional significance in human ERMS. LIMK1, HOXC6 and PLXNA1 represent potential novel candidate genes for driving tumor growth of ERMS.
A subset of amplified genes play essential roles in regulating ERMS proliferation
The six candidate genes were first assessed for anti-proliferative effects in human RD and SMS-CTR ERMS cell lines by siRNA knockdown, establishing a role for these genes in continued tumor growth and maintenance. Importantly, each of these human ERMS cell lines contains mutationally-activated RAS alleles, mimicking the zebrafish model. Effective gene knockdown was validated by quantitative RT-PCR and/or Western analysis (Fig. 2 A and Fig. S3 B and S4 A). A quantitative VEGFA ELISA confirmed lower levels of secreted VEGFA in the growth medium from cells transfected with VEGFA siRNA (Fig. S3 A, p<0.05). Gene-specific siRNA knockdown of CCND2, HOXC6 and PLXNA1 resulted in reduced cell viability as assessed by a luminescent cell viability assay in both cell lines (Fig. 2 B and S4 B–H). By contrast, knockdown of BRAF, LIMK1, or VEGFA failed to alter viability and/or growth in both cell lines (Fig. 2 B and 2 C and data not shown). Following validation of growth effects using two additional siRNAs for CCND2, HOXC6 and PLXNA1 in RD and SMS-CTR cell lines (Fig. 2 C and S4 B–H), these genes were prioritized for additional functional studies. For example, knockdown of CCND2, HOXC6 and PLXNA1 resulted in reduced EDU incorporation when compared to cells transfected with control siRNA in both RD and SMS-CTR cell lines, suggesting that inhibition of cell growth resulted from a block in proliferation (Fig. 2 D). Apoptosis was not altered by gene knock down as assessed by Annexin V staining (Fig. S5). In total, our data uncovered important roles for CCND2, HOXC6 and PLXNA1 in regulating ERMS proliferation, validating the role of several novel genes in regulating continued tumor cell proliferation in human ERMS cells.
Fig. 2. CCND2, HOXC6 and PLXNA1 exert important roles in human ERMS cell proliferation. 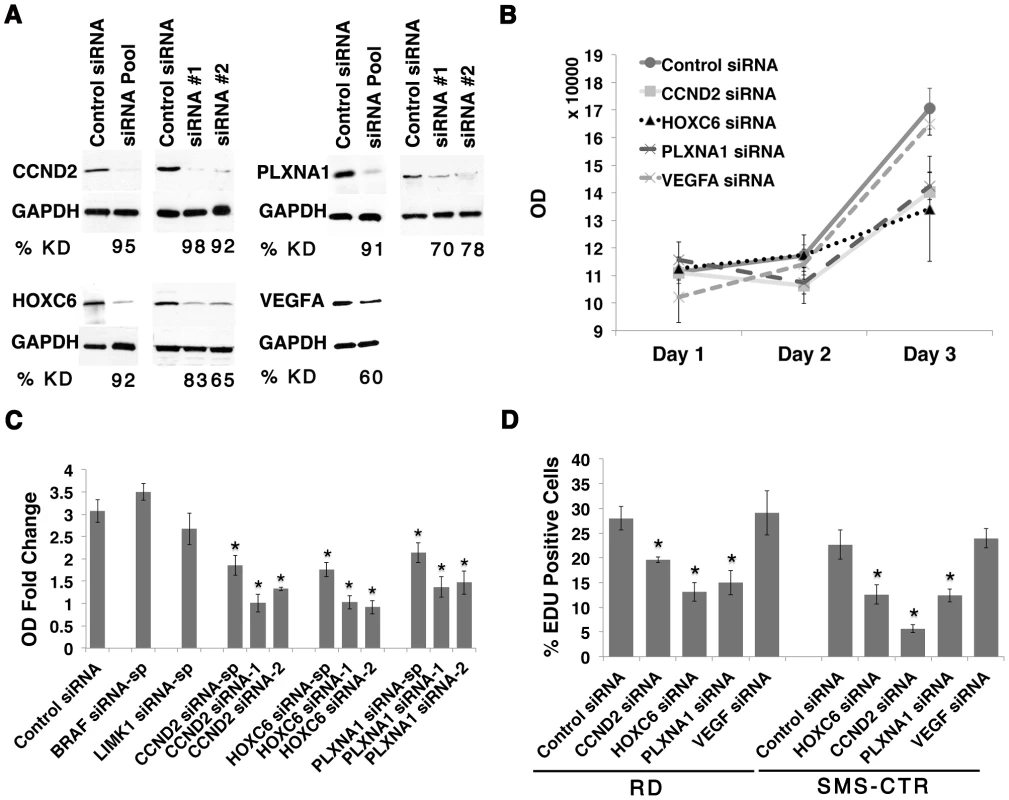
(A) Western analysis following siRNA knockdown in the RD cell line. The percentage of knockdown (% KD) is indicated below each lane (representative example shown for three independent replicates). (B) Viability following siRNA gene knockdown in RD cells was assessed by cell-titer glo assay. (C) Graph summarizing the results of the cell-titer glo assay. OD-fold change over 3 days in RD cell line from knockdown by smart-pool and two individual gene-specific siRNAs is indicated. (D) Summary of EDU proliferation analysis from RD and SMS-CTR ERMS cell lines,. (Asterisks denote significant differences between siRNA knockdown compared to control siRNA (p<0.05). Each error bar in B, C, and D denotes standard deviation of 3 independent experiments. Knockdown of PLXNA1 results in increased terminal differentiation and impaired anchorage-independent growth in human ERMS
ERMS expresses myogenic factors such as MYOD and MYF5 yet it fails to complete normal myogenesis secondary to differentiation arrest [24], [25]. As a result, ERMS is composed of heterogeneous subpopulations of proliferating tumor cells that vary in their differentiation status. Therefore, oncogenes that are essential for regulating proliferation of ERMS cells likely also play a role in modulating their differentiation status. Thus, we determined whether CCND2, HOXC6, and PLXNA1 also played a role in blocking differentiation of ERMS. Knockdown of PLXNA1 resulted in increased formation of multinucleated myocytes and induction of myosin heavy chain expression in RD cells (Fig. 3 B, p = 0.03). By contrast, siRNA knockdown of CCND2 and HOXC6 did not alter the differentiation status of human RD cell (Fig. 3 E). To validate the phenotype of PLXNA1 knockdown, two independent PLXNA1 shRNA knockdown stable lines were generated and cultured under differentiation condition. Both PLXNA1 shRNAs induced robust gene knockdown compared to control scrambled shRNA (Fig. 3 F), resulting in increased numbers of multinucleated-myocytes and myosin heavy chain expression (Fig. 3 D–E, p = 0.01).
Fig. 3. Knockdown of PLXNA1 induced differentiation and impaired anchorage-independent growth of human ERMS cells. 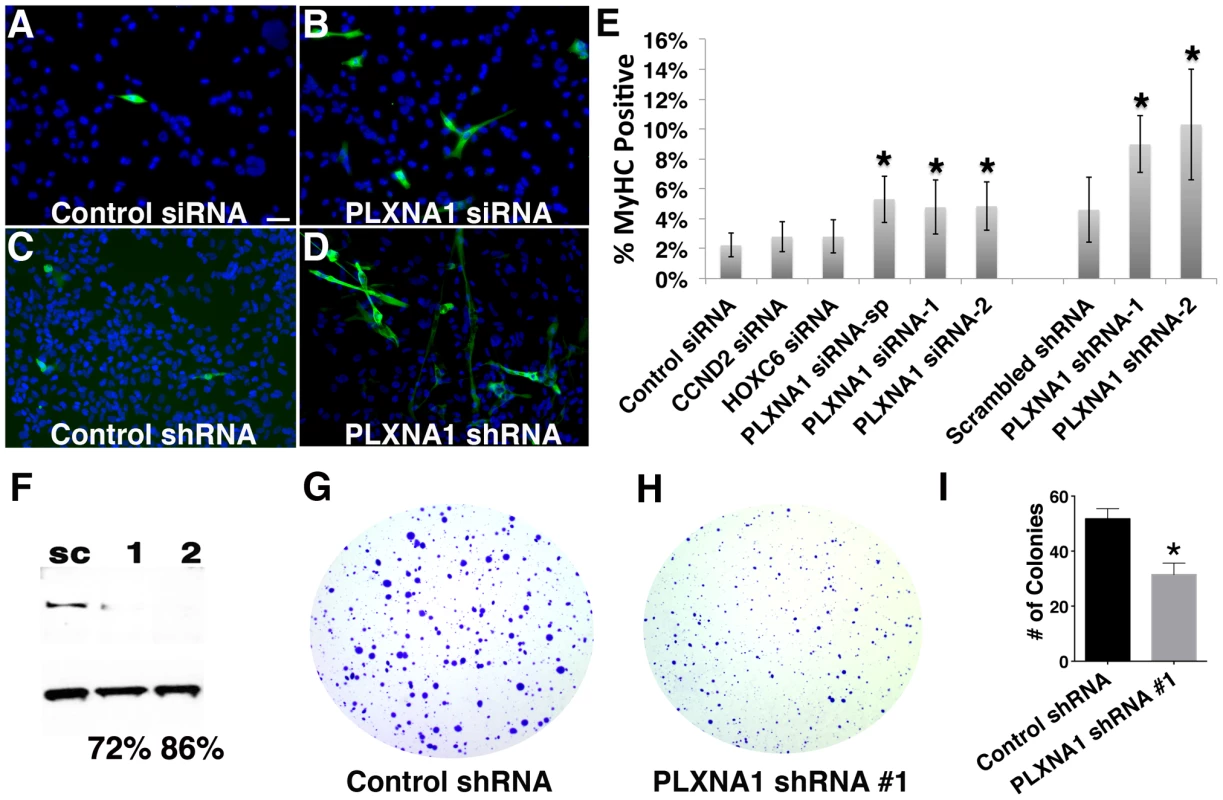
RD cells stained with myosin heavy chain (MF20) and DAPI following culture under differentiation conditions for 72 hrs. (A) Control siRNA. (B) PLXNA1 smart-pool siRNA. (C) Control scrambled shRNA. (D) PLXNA1 shRNA-1. DAPI, blue; MF20-positive cell, green. (E) Quantification of MF-20 immunofluorescence in siRNA and shRNA-knockdown RD cells. Asterisk indicates significant differences between gene knock- down and control cells (p<0.05). Error bars denote standard deviation. (F) Western analysis of PLXNA1 shRNA stable knockdown; sc, scrambled control shRNA; 1, PLXNA1 shRNA-1; 2, PLXNA1 shRNA-2. A soft agar colony formation assay to assess PLXNA1 knockdown effects on anchorage-independent growth (G–I). (G) Control scrambled shRNA. (H) PLXNA1 shRNA. (I) Quantification of colony formation assay results. Error bar indicates standard deviation from triplicate experiments. PLXNA1 also played a critical role in regulating anchorage-independent growth in colony formation assays. Stable knockdown of PLXNA1 resulted in impaired anchorage-independent growth with decreased colony formation two-fold over 15 days when compared to RD cells transduced with control shRNA (Fig. 3 G–I, p = 0.0003). Moreover, colonies were smaller in size, likely reflecting the prominent role of PLXNA1 in regulating cell growth. Together, our findings indicate that PLXNA1 plays an essential important role in regulating proliferation and differentiation in transformed ERMS.
Knockdown of PLXNA1 results in impaired migration of human ERMS cell lines
Migratory behavior of tumor cells in vitro can be a useful predictive index of cell invasion and metastasis in vivo. Genes and pathways that are essential for regulating the migratory behavior of tumors cells can likely serve important functions in mediating metastasis and therefore are potential targets for novel therapy. Wound healing and transwell migration assays were used to assess a role for CCND2, HOXC6, PLXNA1 and VEGFA in migration of human RD and SMS-CTR ERMS cell lines. Knockdown of PLXNA1 by siRNAs (smart-pool and individual siRNAs) and shRNAs resulted in impaired migration in both RD and SMS-CTR cells over 22 hours (p<0.02 for RD and p≤0.04 SMS-CTR, Fig. 4 G–I, Fig. S6). By contrast, knockdown of CCND2, HOXC6 and VEGFA did not affect migration of either RD or SMS-CTR cells (p>0.25, Fig. 4 A–F, I). As an independent assessment of ERMS cell migration, PLXNA1 stable shRNA knockdown cells were assessed for migration in a transwell assay. Knockdown of PLXNA1 in RD cells with two independent gene-specific shRNAs resulted in >50% reduction in transwell migration (p = 0.03 for shRNA-A and p = 0.0038 for shRNA-B, Fig. 4 J). Together, these results support an additional role for PLXNA1 in regulating migratory behavior of human ERMS cells.
Fig. 4. Knockdown of PLXNA1 impairs migration of human ERMS cells. 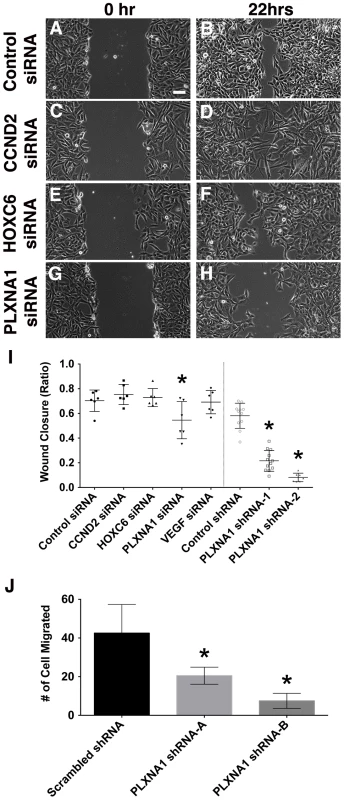
Representative images of ERMS cells transfected with gene-specific siRNAs at 0 hr (A, control siRNA; C, CCND2 siRNA; E, HOXC6 siRNA; G, PLXNA1 siRNA) and 22 hrs (B, control siRNA; D, CCND2 siRNA; F, HOXC6 siRNA; H, PLXNA1 siRNA) following gap creation. Scale bar indicates 100 µm. (I) Quantification of data from wound healing assay. Each error bar indicates standard deviation across 5–6 independent replicates. (J) A Transwell migration assay was performed in RD cells that stably express either a control shRNA or two independent PLXNA1 shRNAs. Migration was assessed after 24 hours. Each error bar indicates standard deviation across six fields at 200× magnification. Asterisks denote p<0. 05. Inhibition of VEGFA results in reduced angiogenesis and tumor growth
The VEGFA pathway often exerts powerful roles in regulating cancer-induced angiogenesis, which would have been missed in our human cell culture assays. To assess a role for VEGFA in modulating tumor growth in vivo, ERMS-bearing zebrafish were treated with the VEGF receptor tyrosine kinase inhibitor, cediranib, or DMSO vehicle for 7 days and assessed for effects on tumor growth. Relative tumor growth as determined by the ratio of tumor volume change between pre - and post-treatment was reduced by three-fold in cediranib-treated fish when compared to those treated with vehicle (Fig. 5A–M, p = 0.0017, Student's T-test). As VEGFA is known to promote angiogenesis during tumor progression in a variety of cancers, we next assessed whether inhibition of VEGFA also blocked angiogenesis in ERMS in vivo. In order to visualize angiogenesis in established tumors, ERMS co-expressing rag2-KRASG12D and rag2-dsRED were transplanted into irradiated fli1-GFP fish that exhibit vessel-specific GFP expression [26]. Fish with engrafted ERMS were treated with either cediranib or DMSO vehicle for 7 days. Animals were assessed for differences in both overall tumor growth and microvessel density as determined by cryosections of tumors. ERMS-affected animals treated with cediranib showed a significant reduction in tumor growth with an accompanied two-fold reduction in tumor microvessel density when compared to those treated with vehicle control (N = 5 for each group, p = 0.006, Fig. 5 N–P). Cediranib-treated ERMS did not exhibit a difference in proliferation when compared to vehicle control-treated tumors (Fig. 5 Q–S), consistent with our results for VEGFA gene knockdown in human ERMS cell lines. Together, these data suggest that activation of the VEGF pathway promotes ERMS tumor progression through enhanced angiogenesis.
Fig. 5. Chemical inhibition of VEGF signaling by cediranib reduces ERMS growth in vivo. 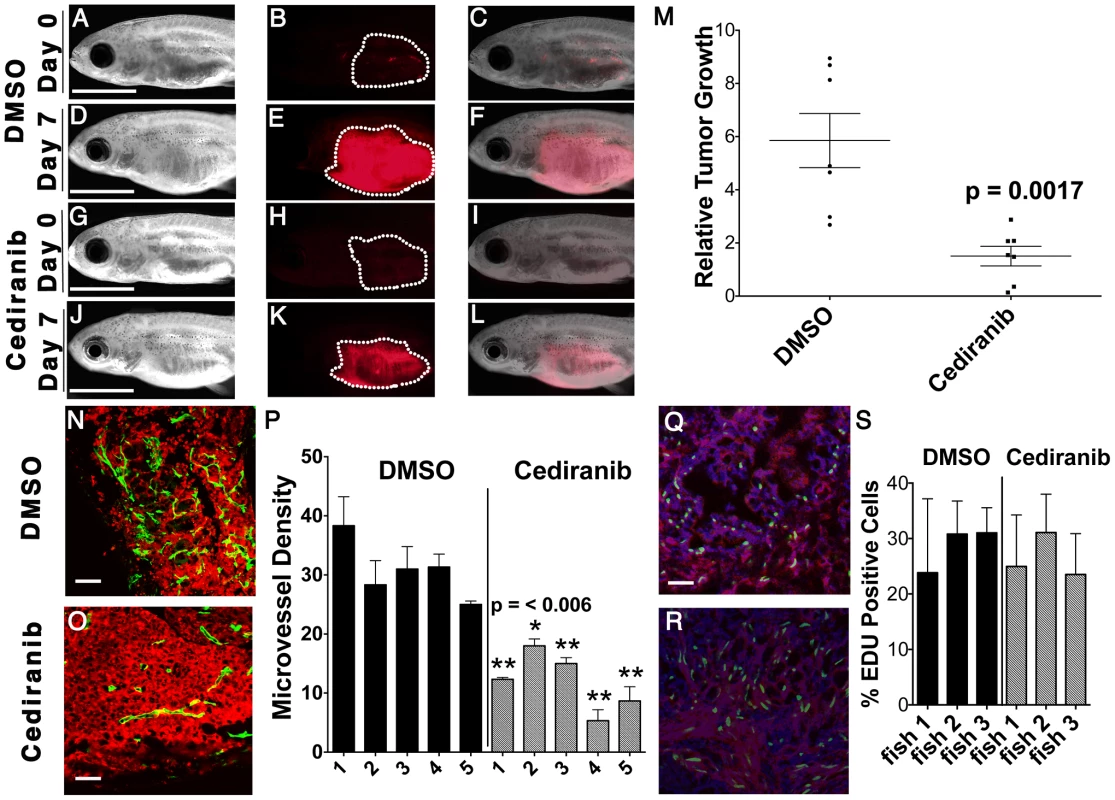
Syngeneic CG1 fish were transplanted with ERMS cells that co-expressed rag2-KRASG12D and rag2-dsRED. Fish with engrafted tumors were treated with DMSO vehicle (A–F) or 100 nM of cediranib for 7 days (G–L). Pre-treatment (A–C and G–I) and post-treatment images (D–F and J–L) of representative fish. Bright field (A,D,G,J), dsRED fluorescence (B,E,H,K) and merged image planes (C,F,I,L). Scale bar is 3 mm. (M) Quantification of relative volume change for individual animals. (N–O) fli1-GFP transgenic zebrafish were transplanted with dsRED-labeled ERMS and treated with DMSO (N) and cediranib (O). Scale bar equals 50 µm. (P) Microvessel density quantification. Asterisk indicates statistically significant difference between DMSO and cediranib-treated groups based on student t-test. Each error bar indicates standard deviation from 3 fields of microvessels for each animal. EDU incorporation analysis in DMSO (Q) or cediranib (R) treated fish. Scale bar is 50 µm. (S) Quantification of EDU analysis across each cohort of animals. Each error bar indicates standard deviation of percent EDU+ cells found within 3 fields for each animal. CCND2, HOXC6, PLXNA1 and VEGFA are commonly expressed in human rhabdomyosaroma
Having established roles for CCND2, HOXC6, PLXNA1 and VEGFA in ERMS growth, we next wanted to assess the extent to which these proteins are expressed in human primary RMS. Immunohistochemistry was performed using antibodies to CCND2, HOXC6, PLXNA1 and VEGFA in primary human tumors and fetal muscle (Supplemental Table S2). In all, 8 pediatric and 11 adult ERMS and 3 pediatric and 4 adult alveolar RMS (ARMS) were analyzed. Remarkably, CCND2, HOXC6, PLXNA1 and VEGFA protein expression were detected in a majority of RMS samples while antibody staining for each was largely negative in fetal muscle (Fig. 6). Specifically, HOXC6 protein expression was detected in 14 of 19 ERMS with strong, diffuse staining being found in 6 of the 14 cases (1 adult and 5 pediatric). In contrast, only 2 of 7 ARMS showed weak, positive staining for HOXC6, consistent with lower-level gene transcript levels being detected in pediatric ARMS compared to ERMS (Supplemental Fig. S7). CCND2, PLXNA and VEGFA were expressed at comparable frequency in both subtypes of RMS. For example, CCND2 was detected in 15 of 19 ERMS and 5 of 7 ARMS, while PLXNA1 expression was found in 17 of 19 ERMS and 6 of 7 ARMS. VEGFA antibody staining was detected in the tumor cells and the vasculature in 10 of 19 ERMS (strong staining in 1 adult and 1 pediatric case), while 6 of 7 ARMS exhibited weak staining in all cases analyzed. Additional immunohistochemical analysis of a tissue microarray from Children's Oncology Group revealed positive VEGF expression in 31 of 38 ERMS and 3 of 6 ARMS (Table S3). Of the 38 cases of ERMS, 29 cases showed strong and diffuse staining. Our analysis suggests that despite these oncogenes being infrequently amplified in human disease, their protein expression levels are elevated in a majority of human ERMS. These data imply important roles for these genes in regulating tumor growth in a large fraction of human ERMS and suggesting additional, as of yet undiscovered mechanisms that regulate expression of these genes.
Fig. 6. Genes contained within low copy CNAs are expressed in primary human rhabdomyosaroma but not normal fetal muscle. 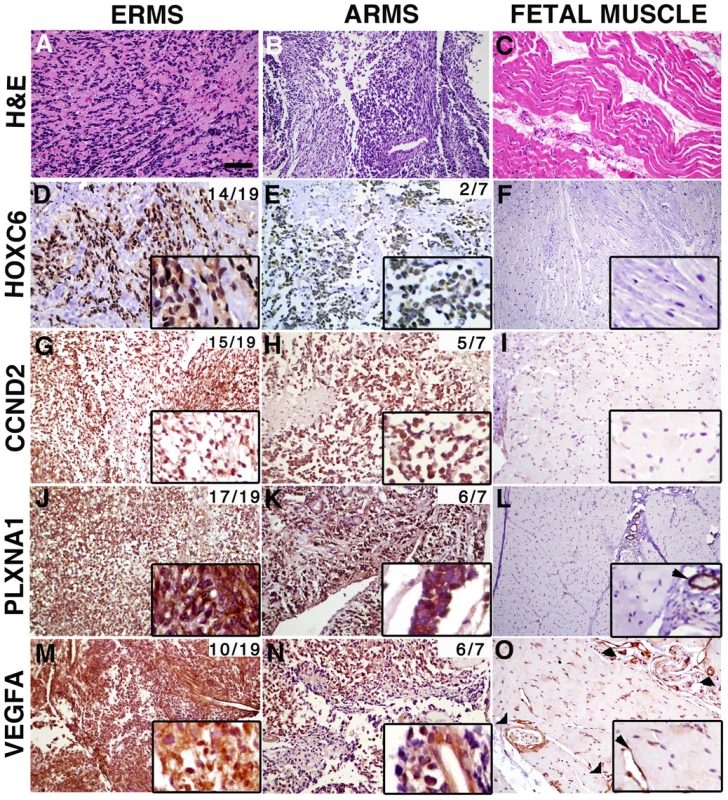
Immunohistochemistry of human primary RMS and fetal muscle tissue samples. Hematoxylin and Eosin stained sections (A–C). Expression of HOXC6, CCND2, PLXNA1 and VEGFA in embryonal rhabdomyosaroma (D, G, J, M), alveolar rhabdomyosaroma (E, H, K, N) and fetal muscle (F, I, L, O). Magnified views of staining are shown in insets. In fetal muscle, PLXNA1 and VEGFA are only expressed in the vasculature (examples indicated by arrowheads in L and O and corresponding insets). The cumulative frequency of positive staining within tumor subtype is shown in the top right corner of each panel (pediatric and adult samples combined). Scale bar (panel A) = 50 µm. High VEGFA expression correlates with clinical outcome
To assess whether dysregulated expression of CCND2, HOXC6, PLXNA1 and VEGFA correlates with clinical outcome, Kaplan Meier analyses were completed using microarray gene expression data from primary ERMS and ARMS [23]. Samples were stratified based on high and low median expression for each gene and each assessed as an independent predictor of survival. Based on this analysis, differential expression of CCND2 and PLXNA1 did not correlate with overall survival outcome in either ERMS or ARMS (Fig. S8). HOXC6 was differentially upregulated in ERMS compared to ARMS (Fig. S7); thus, high expression of HOXC6 correlated with better overall survival (Fig. 7 A), a finding consistent with previous studies demonstrating better clinical outcome for ERMS patients compared to those with ARMS [27]. Finally, samples with high mRNA expression of VEGFA correlated with low overall clinical survival in the ERMS cohort but did not predict survival outcome in ARMS (Fig. 7 B). In addition, VEGFA expression did not correlate with clinical stage, indicating that it is likely an independent prognostic indicator (Fig. S9). These data implicate important roles of VEGFA in promoting ERMS tumor progression and identify VEGFA as a biomarker with likely use in stratifying ERMS patients into high and low-risk groups.
Fig. 7. Elevated VEGFA expression correlates with poor clinical outcome in rhabdomyosarcoma. 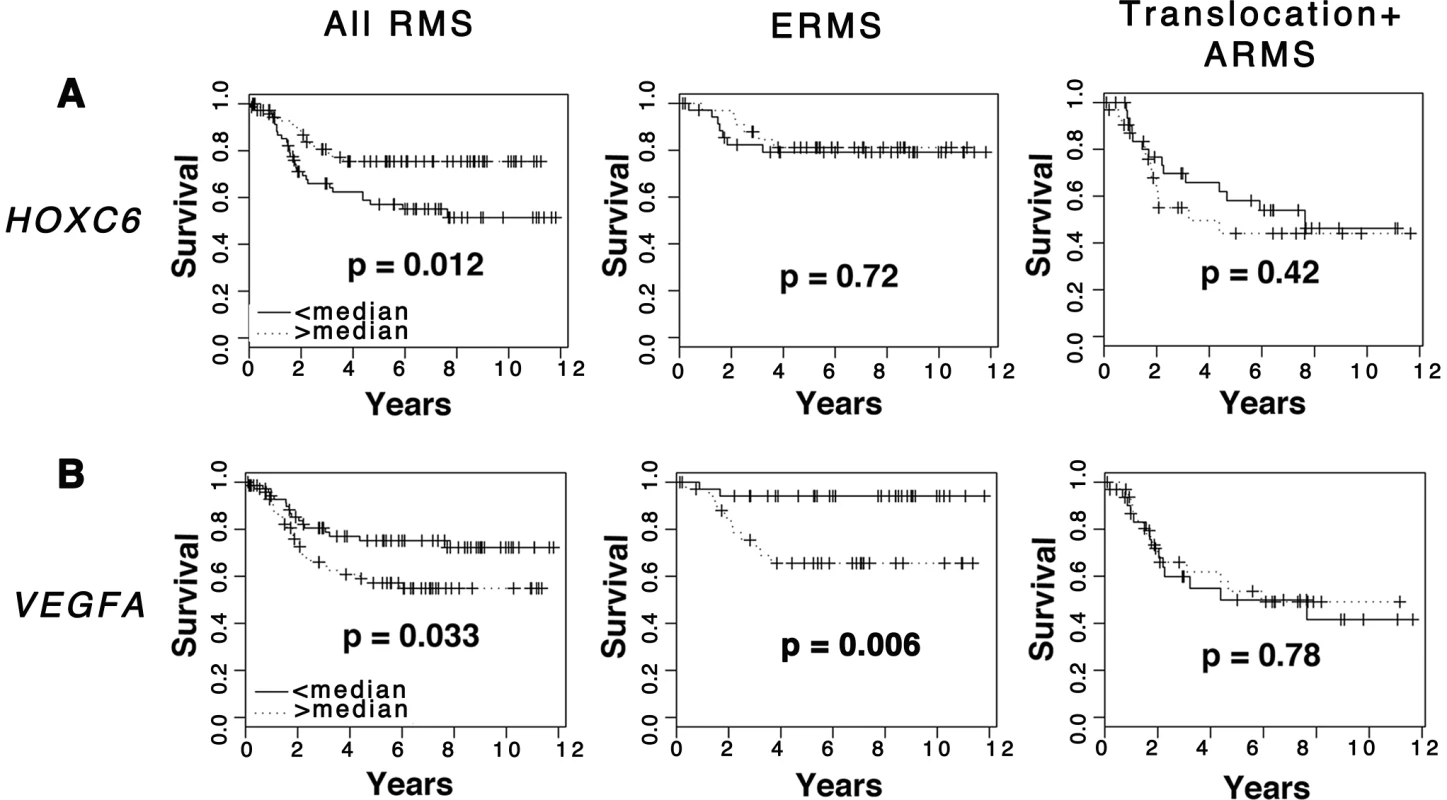
Kaplan-Meier analysis was completed using microarray gene expression data for which patient outcome is available. Comparison was made in all RMS patients, ERMS patients only, and translocation-positive ARMS patients only. (A) HOXC6. (B) VEGFA. Discussion
Prior cytogenetic and array CGH studies in human ERMS demonstrate inconsistent and non-specific partial to whole chromosomal aneuploidy across different primary tumors making it difficult to identify critical genes essential for driving tumor growth. Utilizing a zebrafish model of RAS-induced ERMS that mimics the human disease [4], [21] and subsequent array CGH analyses of genomic DNA from tumor vs. matched normal, we were able to rapidly identify candidate gene-containing regions that likely contribute to ERMS pathogenesis. The 19 CNA gains that were recurrently amplified in zebrafish ERMS mapped to 21 homologous regions within the human genome. Remarkably, 18 of these regions also demonstrated low-level genomic amplification in human ERMS. To validate that candidate genes contained within these intervals exert important roles in continued tumor growth and maintenance, we characterized the function of six amplified genes in human ERMS cell lines and conclusively demonstrated functional significance of CCND2, HOXC6, PLXNA1 in proliferation of human ERMS. PLXNA1 also has important roles in regulation differentiation and migration of ERMS cells. As the in vitro analyses performed in this study would not be able to assess other aspects of tumorigenesis such as neovascularization and tumor initiation, we utilized the zebrafish in vivo model to demonstrate the important role of VEGF-A pathway in mediating angiogenesis during tumor growth. In total, our work identified roles for 4 of 6 candidate genes identified in our cross-species array CGH studies for eliciting important roles in human ERMS. Importantly, this strategy is not limited to zebrafish ERMS, and will likely provide powerful new methods to identify novel tumor-suppressor and oncogenes in a wide range of zebrafish and human tumors.
Data from our array CGH study and previous studies of zebrafish cancer revealed low-level CNA gains as a frequent DNA alteration in cancer, yet this class of mutation has not commonly been studied due to the difficulty in identifying relevant and meaningful genes in these regions. Importantly, zebrafish allows for the easy identification of low-level gene amplifications. In total, our data is consistent with a model where zebrafish tumor cells undergo acquisition of low-amplitude gains, likely represented as single copy gains within CNA regions. For example, we have also observed that clonal-populations of purified T-ALL cells (90% enriched for blasts) also contain low-amplitude gains [Blackburn et al., unpublished]. Moreover, Rudner et al. (2011) recently showed that a majority of amplified, gene-containing CNAs found in zebrafish T-ALL were also amplified in human disease [20]. Upon re-analysis of this data, we find that 72% of the reported amplified regions were detected as low-level gains in zebrafish T-ALL, yet were not reported as such. Zhang et al. identified large regions of aneuploidy and high-level CNA gains in zebrafish malignant peripheral nerve sheath tumors when assessed by array CGH, but also identified numerous regions of low-level CNA gains, which were dismissed as potential causative lesions in cancer. Thus, despite these previous two reports observing low-level CNA gains in zebrafish malignancy, neither reported the functional importance of this class of genes to promote tumor progression and maintenance in zebrafish or human disease. Although it is formally possible that low level gains detected in zebrafish ERMS represent high-copy gains masked by a high degree of tumor cell heterogeneity and/or contamination of normal DNA from non-transformed blood, fibroblasts and stroma, our data strongly argue that low-copy amplification is a common attribute found in zebrafish and human cancer.
Interestingly, even though the functional relevance of low-level gains such as genomic duplication events have been infrequently reported in human cancer, this type of DNA alteration often predicts important clinical parameters such as disease susceptibility, therapy resistance and adverse prognosis. For example, duplication of a region on chromosome 6q27 is detected in individuals affected with familial chordoma, a rare bone cancer, but not among unaffected individuals within the same family [28]. MYB tandem duplication occurs in pediatric T-ALL and results from homologous recombination at ALU repetitive sequences flanking the MYB locus. Elevated MYB expression is associated with poor outcome in T-ALL [29]. Similarly, focal tandem duplication also contributes to chemotherapy resistance in patients with high-grade ovarian cancer [30]. These findings indicate that low-level CNA gains have important clinical prognostic relevance and likely play important functional roles in human cancer. Finally, we also found that genes within each CNA are highly expressed in a majority of human RMS despite being infrequently amplified as low-copy CNAs, suggesting the importance of these gene pathways in regulating a large fraction of human ERMS and that additional mechanisms underlying the dysregulation of this class of genes in cancer is likely.
Our work has identified essential roles for four genes in modulating ERMS growth, maintenance, migration, and neovascularization. Of these genes, CCND2, HOXC6 and PLXNA1 exhibited important roles in regulating proliferation in human ERMS cell lines. PLXNA1 also had additional roles in arresting ERMS cells in early stages of muscle differentiation, in enhancing tumor cell migration, and in altering anchorage-independent growth. Despite the fact that these genes and/or related family members have been ascribed functions in other cancer types, their contributions to the pathogenesis of ERMS have not been previously characterized. For example, HOXC6, a homeobox transcription factor, regulates the expression of genes including BMP7, FGFR2, IGFP3 and PDGFRA to influence oncogenic activities in prostate cancer [31]. HOXC6 is highly expressed in ERMS but not ARMS [23], suggesting a specific and independent role in regulating growth in the human ERMS subtype. A role for HOXC6 in regulating continued RMS growth had not been reported until this study. CCND2 belongs to the D-type G1 cyclins (D1, D2 and D3). While cyclin D1 is frequently dysregulated in cancer and is a marker for disease progression [32], the involvement of cyclin D2 in cancer is not as well characterized. CCND2 is amplified in 2% of gliomas and in zebrafish and human MPNSTs [19], [33]. Finally, PLXNA1 belongs to a highly conserved family of transmembrane receptors that bind semaphorins and have been shown to mediate neuronal cell migration, guidance, and patterning [34], [35]. In humans, nine plexins group into four subfamilies and several have been implicated as having roles in cancer progression and growth. In particular, plexin-B1 can function as an oncogene by promoting proliferation and survival of B-Cell Lymphoblastic Lymphoma cells and invasion of ovarian and breast tumor cells [36]–[38]. Plexin-A1, the gene identified in our study, has been shown to activate the VEGF receptor and NF-κB to promote survival of malignant mesothelioma cells [39], suggesting a complex interplay of PLXNA1 in cell survival and neovascularization. Taken together, our study has demonstrated prominent and novel roles for CCDN2, HOXC6, and PLXNA1 in modulating ERMS proliferation while PLXNA1 exerts important additional roles in regulating differentiation and migration. None of these genes have been previously implicated as important modulators of ERMS growth and maintenance, suggesting that our cross species array CGH studies will be valuable for uncovering genetic lesions across a wide range of zebrafish and human cancers.
VEGF pathway activation promotes tumor angiogenesis and progression in a variety of human cancers, and elevated VEGF expression correlates with poor prognosis in certain tumor types [40]–[42]. However, until our report, the prognostic impact of VEGFA expression in human ERMS had not been described. Here, we show that VEGFA is amplified as a low-copy gain in a small cohort of zebrafish and human RMS and yet highly expressed in a majority of human patient samples. High VEGFA mRNA expression correlated with poor clinical outcome in human ERMS, underscoring the importance of this pathway in driving continued tumor growth. As VEGFA expression level is not linked to clinical stage, it represents an important independent prognostic indicator and a potential biomarker for therapy stratification. Chemical inhibition of VEGF signaling in our pre-clinical in vivo model effectively suppressed tumor growth by reducing angiogenesis, consistent with the findings from a pre-clinical testing of VEGFR inhibitors on a small number of human RMS xenografts into mice [43]. Although clinical trials of VEGF inhibitors in other types of cancers have exhibited mixed results [44]–[46], our data suggest that targeting the VEGF pathway may be a promising therapeutic option to curb tumor growth in a subset of high-risk ERMS patients.
In summary, our array CGH studies of zebrafish cancer have identified conserved CNA gains with functional significance in human ERMS. As proof of principle, we have also demonstrated the utility of zebrafish array CGH studies to identify oncogenes that are essential for continued tumor growth in both zebrafish and human ERMS. Our work also provides 13 additional CNA gains that are conserved in zebrafish and human ERMS for which an essential genetic lesion has yet to be identified – providing potential genes to interrogate in the future. Our studies suggest that most amplified CNAs will contain genes that regulate important processes in cancer maintenance and growth. Moreover, our study reveals a number of tractable features of zebrafish cancer genomes such as small-size CNAs containing few genes within each region of chromosomal aberration, thereby positioning the zebrafish as an effective model system for discovering novel genes required for continued tumor growth and maintenance within a wide diversity of cancer types.
Methods
Animal and human protocol approval
Studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care under protocol #2011N000127 (zebrafish) and by the Partners Human Research Committee under IRB protocol #2009-P-002756 (human).
Array comparative hybridization
TuAB-strain zebrafish were co-injected at the one-cell stage with linearized rag2-KRASG12D and rag2-dsRED DNA constructs as previously described [4], [47]. dsRED-labeled ERMS and adjacent non-neoplastic tissues were dissected from tumor-bearing animals at 30–40 days of life. RNA and DNA were extracted by Trizol (Sigma). Tumor DNA was labeled with Cy5 (Bioprime system, Invitrogen, Carlsbad, CA) and hybridized against the matched normal samples labeled with Cy3 onto the custom SurePrint G3 400k CGH microarray (Agilent Technologies, Santa Clara, CA). Array image scans were extracted using Agilent Feature Extraction software (Agilent Technologies, Inc, Santa Clara, CA), normalized for signal intensity, and imported into the Nexus Copy Number software program version 5.1 (Biodiscovery, Inc., El Segundo, CA). CNA calls were generated based on log2 ratio output files using a rank segmentation algorithm. Settings were optimized using self-self hybridizations to reduce false positive calls. The parameters include significance threshold 1.0 E-5, maximum continuous probe spacing of 200 kb, minimum number of probes per sequence of 3, and log2 ratios of 1.0, 0.25, −0.25 and −1.0 for high-level amplifications, gains, losses and deletions, respectively. CNAs of interest were determined using the aggregate function in Nexus. Aggregates are represented as segmented regions of gain or loss shared by a set of samples with the number of samples sharing the event referred to as the aberration frequency. The minimum aberration frequency required for analysis in our study was set at ≥15% (n≥3 of 20 zebrafish ERMS contained a common region of gene amplification).
For the human ERMS sample analysis, normalized log2 intensity files (series number GSE27392) were downloaded from Gene Expression Omnibus (GEO) at the National Center for Biotechnology Information (NCBI) and imported into and analyzed using Nexus Copy Number software (version 5.1, BioDiscovery). This program analyzes log2 ratio output files using a rank segmentation algorithm similar to circular binary segmentation. Samples were segmented following the removal of the greatest 3% of outliers and a minimum five-probe requirement at a significance threshold of 1E-08. Gains and losses were defined as regions exhibiting log2 values of 0.2 and −0.18, respectively, with high-level amplifications and deletions defined as log2 values greater than 0.5 and less than −0.5. Following the identification of human ERMS aberrations, homologous human regions of zebrafish ERMS CNAs were analyzed to determine whether common low-level amplifications were present in both zebrafish and human ERMS samples.
Immunohistochemistry
Paraffinized human primary rhabdomyosaroma (5 ERMS and 3 ARMS), US Biomax tissue microarray (14 ERMS and 4 ARMS, Rockville, MD), and a Children's Oncology Group tissue microarray were analyzed by immunohistochemical staining as previously described [48]. HOXC6 (Sigma, 1∶200), CCND2 (Ab-Cam, 1∶100), PLXNA1 (Ab-Cam; 1∶200) and VEGFA (Ab-Cam; 1∶125). BGAR - biotinylated goat anti-rabbit (Vector #BA-1000) was used as the secondary antibody. Pathology review was completed independently by E.C. and G.P.N.
Cell lines, siRNA transfection, stable shRNA knockdown and Western analysis
The human RD cell line was obtained from ATCC cell biology collection (Manassas, Virginia) and the SMS-CTR cell line provided by Dr. Corrine Linardic (Duke University, North Carolina). Cells were seeded at a density of 5×102 cells in 6-well plates in 2 ml of antibiotic-free 10% FBS/DMEM. 50 pg of gene-specific smart-pool or control siRNA were transfected into cells using RNAiMax lipofectamine transfection reagent (Invitrogen). For stable knockdowns, scrambled and gene-specific shRNAs in pLKO.1-based lentiviral vectors were packaged in 293T cells. shRNAs were obtained from molecular profiling laboratory at the Cancer Center of Massachusetts General Hospital (Table S4). RD cells were infected with viral particles for 24 hours at 37 degrees with polybrene (Millipore) at 4 µg/mL and then selected with puromycin (In Vivo Gene) at 10 µg/mL in 10%FBS/DMEM for 15 days to obtain stable lines. Total cell lysates from knockdown experiments were immunoblotted using primary antibodies against HOXC6 (1∶500), CCND2 (1∶1000), PLXNA1 (1∶1000) and VEGFA (1∶1000). All Western analysis was completed three times per experiment and average percent knockdown is noted. Incubation with HRP-conjugated secondary antibody (1∶2000) was performed in 5% milk/TBST for 2 hours.
Cell proliferation and apoptosis assays
siRNA transfected cells were assessed by Cell Titer Glo assay as per the manufacturer's instructions (Promega). Cells were also pulsed with EDU for 2 hours, harvested at 48 hours post-transfection and processed using the EDU ClickIt Flow Cytometry Assay kit (Alexa Fluor 647 dye, Invitrogen). Unstained cells were used as the negative sample to facilitate gating in flow cytometry. To assess apoptosis, cells were harvested at 48 hours post-transfection and labeled with PE Annexin V and 7-AAD using the PE Annexin V Apoptosis Detection Kit (BD Pharmagin). Unstained cells, cells treated with PE Annexin V only and 7-AAD only were used to set up gates for flow cytometry. Each analysis was performed in triplicate. A student's T-test was performed to assess whether the difference in the percentage of Annexin V-positive cells between test samples and control siRNA-transfected cells was significant.
Cell migration assays
A wound-healing assay was performed in cells transiently transfected with siRNA and/or cells that stably express a gene-specific shRNA. Cell were seeded into 6-well plates and grown to nearly confluent density. A scrape was made in each well using a pipette tip, and cell migration across the gap was assessed after 22 hours. Images were taken at 0 and 22 hrs to calculate the percentage of gap closure. ERMS cells were also analyzed for altered migration in a transwell assay. Specifically, 2×104 cells were seeded in 6.5 mm-membrane inserts (Corning) in DMEM and were allowed to migrate through the permeable membranes (8.0 µM pore size) toward the bottom chamber containing medium with 10% FBS. Cells were then fixed with 4% paraformaldehyde after 24 hours and stained with hematoxylin for 30 minutes. Unmigrated cells from the inserts were removed. Six random fields of the migrated cells on the membranes were imaged using the Olympus light microscope (Model MVX10, 400× magnification) and manually counted. A Student's T-test was performed to assess differences between the control and experimental groups.
Soft-agar colony formation assay
A base layer of 1% agar in 10% FBS/DMEM was prepared in 6-well plates. Cells were resuspended in 0.5% low-melting point agarose/10%FBS/DMEM and overlaid on the base layer with 2.5×103 cells per well and subsequently kept in the humidified incubator with 5% CO2 with media change every 3 days for 15 days. Cells were fixed with 4% paraformaldehyde and stained with 0.05% crystal violet. Colony formation assay for each shRNA stable knockdown line was performed in triplicate. Image for each well containing soft agar colonies was taken at low magnification by light microscopy. Colony count was performed using the ImageJ software and differences assessed by Student T-test.
Chemical treatment of zebrafish with ERMS
Six-week old CG1 syngeneic fish were transplanted with 3×104 unsorted tumor cells arising from dsRED-positive ERMS from CG1 strain fish (Mizgireuv and Revskoy 2006; Smith et al., 2010). Engrafted animals were treated at 6-days post-transplantation with 100 nM of cediranib (Selleck) and vehicle control (DMSO) for 7 days (including 2 24-hr drug holidays). Tumor volume was assessed by imaging animals pre-treatment and post-treatment. Tumor volume was calculated by multiplying tumor area by fluorescent intensity using image J. A Student's T-test was performed to assess differences between tumor size in the control and experimental groups.
Estimating microvessel density
Six-week old fli1-GFP fish were irradiated at 25 Gy and transplanted with 3×104 unsorted ERMS cells from fish with dsRED-positive ERMS. Fish with engrafted tumors were treated with cediranib as described above. At the end of treatment period, tumor tissues were isolated, fixed in 4% paraformaldehyde for 30 minutes and snap frozen. 5 µM Frozen sections were mounted in DAPI-containing Vectashield (Invitrogen). GFP and dsRED images were obtained at 200× magnification using a Nikon confocal microscope. Microvessel density was quantified using Weidner et al. criteria [49] and differences assessed by Student T-test.
Kaplan-Meier analysis
Kaplan-Meier analysis was completed using R with the survival package. Median expression level for each gene was used to group samples into high and low expression. Chi-squared tests were used to assess overall survival differences between groups. Statistical significance was defined as a p-value less than 0.05.
Supporting Information
Zdroje
1. LinaberyAM, RossJA (2008) Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 112 : 416–432.
2. ChenY, TakitaJ, HiwatariM, IgarashiT, HanadaR, et al. (2006) Mutations of the PTPN11 and RAS genes in rhabdomyosarcoma and pediatric hematological malignancies. Genes Chromosomes Cancer 45 : 583–591.
3. HettmerS, LiuJ, MillerCM, LindsayMC, SparksCA, et al. (2011) Sarcomas induced in discrete subsets of prospectively isolated skeletal muscle cells. Proc Natl Acad Sci U S A 108 : 20002–20007.
4. LangenauDM, KeefeMD, StorerNY, GuyonJR, KutokJL, et al. (2007) Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev 21 : 1382–1395.
5. PaulsonV, ChandlerG, RakhejaD, GalindoRL, WilsonK, et al. (2011) High-resolution array CGH identifies common mechanisms that drive embryonal rhabdomyosarcoma pathogenesis. Genes Chromosomes Cancer 50 : 397–408.
6. BridgeJA, LiuJ, QualmanSJ, SuijkerbuijkR, WengerG, et al. (2002) Genomic gains and losses are similar in genetic and histologic subsets of rhabdomyosarcoma, whereas amplification predominates in embryonal with anaplasia and alveolar subtypes. Genes Chromosomes Cancer 33 : 310–321.
7. GoldsteinM, MellerI, IssakovJ, Orr-UrtregerA (2006) Novel genes implicated in embryonal, alveolar, and pleomorphic rhabdomyosarcoma: a cytogenetic and molecular analysis of primary tumors. Neoplasia 8 : 332–343.
8. MissiagliaE, SelfeJ, HamdiM, WilliamsonD, SchaafG, et al. (2009) Genomic imbalances in rhabdomyosarcoma cell lines affect expression of genes frequently altered in primary tumors: an approach to identify candidate genes involved in tumor development. Genes Chromosomes Cancer 48 : 455–467.
9. PanditaA, ZielenskaM, ThornerP, BayaniJ, GodboutR, et al. (1999) Application of comparative genomic hybridization, spectral karyotyping, and microarray analysis in the identification of subtype-specific patterns of genomic changes in rhabdomyosarcoma. Neoplasia 1 : 262–275.
10. DavariP, HebertJL, AlbertsonDG, HueyB, RoyR, et al. (2010) Loss of Blm enhances basal cell carcinoma and rhabdomyosarcoma tumorigenesis in Ptch1+/ − mice. Carcinogenesis 31 : 968–973.
11. RubinBP, NishijoK, ChenHI, YiX, SchuetzeDP, et al. (2011) Evidence for an unanticipated relationship between undifferentiated pleomorphic sarcoma and embryonal rhabdomyosarcoma. Cancer Cell 19 : 177–191.
12. GoesslingW, NorthTE, LoewerS, LordAM, LeeS, et al. (2009) Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136 : 1136–1147.
13. LamSH, WuYL, VegaVB, MillerLD, SpitsbergenJ, et al. (2006) Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol 24 : 73–75.
14. LiuS, LeachSD (2011) Zebrafish models for cancer. Annu Rev Pathol 6 : 71–93.
15. PattonEE, WidlundHR, KutokJL, KopaniKR, AmatrudaJF, et al. (2005) BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol 15 : 249–254.
16. WhiteRM, CechJ, RatanasirintrawootS, LinCY, RahlPB, et al. (2011) DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471 : 518–522.
17. StrattonMR, FisherC, GustersonBA, CooperCS (1989) Detection of point mutations in N-ras and K-ras genes of human embryonal rhabdomyosarcomas using oligonucleotide probes and the polymerase chain reaction. Cancer Res 49 : 6324–6327.
18. FreemanJL, CeolC, FengH, LangenauDM, BelairC, et al. (2009) Construction and application of a zebrafish array comparative genomic hybridization platform. Genes Chromosomes Cancer 48 : 155–170.
19. ZhangG, HoerschS, AmsterdamA, WhittakerCA, LeesJA, et al. (2010) Highly aneuploid zebrafish malignant peripheral nerve sheath tumors have genetic alterations similar to human cancers. Proc Natl Acad Sci U S A 107 : 16940–16945.
20. RudnerLA, BrownKH, DobrinskiKP, BradleyDF, GarciaMI, et al. (2011) Shared acquired genomic changes in zebrafish and human T-ALL. Oncogene 30 : 4289–4296.
21. IgnatiusMS, ChenE, ElpekNM, FullerAZ, TenenteIM, et al. (2012) In vivo imaging of tumor-propagating cells, regional tumor heterogeneity, and dynamic cell movements in embryonal rhabdomyosarcoma. Cancer Cell 21 : 680–693.
22. LeX, LangenauDM, KeefeMD, KutokJL, NeubergDS, et al. (2007) Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci U S A 104 : 9410–9415.
23. DavicioniE, AndersonJR, BuckleyJD, MeyerWH, TricheTJ (2010) Gene expression profiling for survival prediction in pediatric rhabdomyosarcomas: a report from the children's oncology group. J Clin Oncol 28 : 1240–1246.
24. MerlinoG, HelmanLJ (1999) Rhabdomyosarcoma–working out the pathways. Oncogene 18 : 5340–5348.
25. TapscottSJ, ThayerMJ, WeintraubH (1993) Deficiency in rhabdomyosarcomas of a factor required for MyoD activity and myogenesis. Science 259 : 1450–1453.
26. LawsonND, WeinsteinBM (2002) In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248 : 307–318.
27. RaneyRB, AndersonJR, BarrFG, DonaldsonSS, PappoAS, et al. (2001) Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol 23 : 215–220.
28. YangXR, NgD, AlcortaDA, LiebschNJ, SheridanE, et al. (2009) T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet 41 : 1176–1178.
29. LahortigaI, De KeersmaeckerK, Van VlierbergheP, GrauxC, CauwelierB, et al. (2007) Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet 39 : 593–595.
30. NgCK, CookeSL, HoweK, NewmanS, XianJ, et al. (2011) The role of tandem duplicator phenotype in tumour evolution in high-grade serous ovarian cancer. J Pathol 226 : 703–12.
31. McCabeCD, SpyropoulosDD, MartinD, MorenoCS (2008) Genome-wide analysis of the homeobox C6 transcriptional network in prostate cancer. Cancer Res 68 : 1988–1996.
32. MusgroveEA, CaldonCE, BarracloughJ, StoneA, SutherlandRL (2011) Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11 : 558–572.
33. BuschgesR, WeberRG, ActorB, LichterP, CollinsVP, et al. (1999) Amplification and expression of cyclin D genes (CCND1, CCND2 and CCND3) in human malignant gliomas. Brain Pathol 9 : 435–442; discussion 432–433.
34. KrugerRP, AurandtJ, GuanKL (2005) Semaphorins command cells to move. Nat Rev Mol Cell Biol 6 : 789–800.
35. NegishiM, OinumaI, KatohH (2005) Plexins: axon guidance and signal transduction. Cell Mol Life Sci 62 : 1363–1371.
36. GranzieroL, CircostaP, ScielzoC, FrisaldiE, StellaS, et al. (2003) CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood 101 : 1962–1969.
37. ValenteG, NicotraG, ArrondiniM, CastinoR, CapparucciaL, et al. (2009) Co-expression of plexin-B1 and Met in human breast and ovary tumours enhances the risk of progression. Cell Oncol 31 : 423–436.
38. YeS, HaoX, ZhouT, WuM, WeiJ, et al. (2010) Plexin-B1 silencing inhibits ovarian cancer cell migration and invasion. BMC Cancer 10 : 611.
39. CatalanoA, LazzariniR, Di NuzzoS, OrciariS, ProcopioA (2009) The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-kappaB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res 69 : 1485–1493.
40. KasebAO, MorrisJS, HassanMM, SiddiquiAM, LinE, et al. (2011) Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol 29 : 3892–3899.
41. MaaeE, OlsenDA, SteffensenKD, JakobsenEH, BrandslundI, et al. (2012) Prognostic impact of placenta growth factor and vascular endothelial growth factor A in patients with breast cancer. Breast Cancer Res Treat 133 : 257–65.
42. PrinsMJ, VerhageRJ, Ten KateFJ, van HillegersbergR (2012) Cyclooxygenase Isoenzyme-2 and Vascular Endothelial Growth Factor are Associated with Poor Prognosis in Esophageal Adenocarcinoma. J Gastrointest Surg 16 : 956–66.
43. MarisJM, CourtrightJ, HoughtonPJ, MortonCL, GorlickR, et al. (2008) Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer 50 : 581–587.
44. BoucheO, Maindrault-GoebelF, DucreuxM, LledoG, AndreT, et al. (2011) Phase II trial of weekly alternating sequential BIBF 1120 and afatinib for advanced colorectal cancer. Anticancer Res 31 : 2271–2281.
45. FountzilasG, FragkoulidiA, Kalogera-FountzilaA, NikolaidouM, BobosM, et al. (2010) A phase II study of sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother Pharmacol 65 : 649–660.
46. IwamotoFM, LambornKR, RobinsHI, MehtaMP, ChangSM, et al. (2010) Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol 12 : 855–861.
47. SmithAC, RaimondiAR, SalthouseCD, IgnatiusMS, BlackburnJS, et al. (2010) High-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell acute lymphoblastic leukemia. Blood 115 : 3296–3303.
48. GuyonJR, MosleyAN, ZhouY, O'BrienKF, ShengX, et al. (2003) The dystrophin associated protein complex in zebrafish. Hum Mol Genet 12 : 601–615.
49. WeidnerN, SempleJP, WelchWR, FolkmanJ (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324 : 1–8.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



