-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
Dorsoventral patterning of the embryonic axis relies upon the mutual antagonism of competing signaling pathways to establish a balance between ventralizing BMP signaling and dorsal cell fate specification mediated by the organizer. In zebrafish, the initial embryo-wide domain of BMP signaling is refined into a morphogenetic gradient following activation dorsally of a maternal Wnt pathway. The accumulation of β-catenin in nuclei on the dorsal side of the embryo then leads to repression of BMP signaling dorsally and the induction of dorsal cell fates mediated by Nodal and FGF signaling. A separate Wnt pathway operates zygotically via Wnt8a to limit dorsal cell fate specification and maintain the expression of ventralizing genes in ventrolateral domains. We have isolated a recessive dorsalizing maternal-effect mutation disrupting the gene encoding Integrator Complex Subunit 6 (Ints6). Due to widespread de-repression of dorsal organizer genes, embryos from mutant mothers fail to maintain expression of BMP ligands, fail to fully express vox and ved, two mediators of Wnt8a, display delayed cell movements during gastrulation, and severe dorsalization. Consistent with radial dorsalization, affected embryos display multiple independent axial domains along with ectopic dorsal forerunner cells. Limiting Nodal signaling or restoring BMP signaling restores wild-type patterning to affected embryos. Our results are consistent with a novel role for Ints6 in restricting the vertebrate organizer to a dorsal domain in embryonic patterning.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003822
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003822Summary
Dorsoventral patterning of the embryonic axis relies upon the mutual antagonism of competing signaling pathways to establish a balance between ventralizing BMP signaling and dorsal cell fate specification mediated by the organizer. In zebrafish, the initial embryo-wide domain of BMP signaling is refined into a morphogenetic gradient following activation dorsally of a maternal Wnt pathway. The accumulation of β-catenin in nuclei on the dorsal side of the embryo then leads to repression of BMP signaling dorsally and the induction of dorsal cell fates mediated by Nodal and FGF signaling. A separate Wnt pathway operates zygotically via Wnt8a to limit dorsal cell fate specification and maintain the expression of ventralizing genes in ventrolateral domains. We have isolated a recessive dorsalizing maternal-effect mutation disrupting the gene encoding Integrator Complex Subunit 6 (Ints6). Due to widespread de-repression of dorsal organizer genes, embryos from mutant mothers fail to maintain expression of BMP ligands, fail to fully express vox and ved, two mediators of Wnt8a, display delayed cell movements during gastrulation, and severe dorsalization. Consistent with radial dorsalization, affected embryos display multiple independent axial domains along with ectopic dorsal forerunner cells. Limiting Nodal signaling or restoring BMP signaling restores wild-type patterning to affected embryos. Our results are consistent with a novel role for Ints6 in restricting the vertebrate organizer to a dorsal domain in embryonic patterning.
Introduction
The vertebrate embryonic dorsal organizer, historically referred to as the Spemann organizer, breaks the symmetry of the blastula by defining its dorsal side and ultimately gives rise to axial mesoderm, which forms the notochord, the defining anatomical feature of the chordate lineage. In fish and frogs, induction of the organizer relies on a maternal Wnt signaling pathway that leads to the accumulation of β-catenin in nuclei on the prospective dorsal side of the embryo [1], [2]. A primary function of the organizer is to induce a region in the embryo that is competent to adopt dorsal fates, such as prechordal plate mesoderm and neural ectoderm, in the presence of widespread ventralizing BMP signaling.
Proper partitioning of axial versus non-axial cell fates during gastrulation is essential to ensure proper embryonic patterning. BMP signaling patterns tissues along the dorsoventral axis (DV), but does not act to partition axial versus non-axial fates. For example, in zebrafish bmp2b (swirl) ligand mutant embryos, loss of BMP signaling causes the expansion of dorsal neurectodermal and non-axial dorsal mesodermal cell fates at the expense of ventral cell fates without expanding the organizer itself [3], [4], [5], [6]. Thus, in the absence of ventral cell fate specification, other mechanisms ensure that the organizer is confined dorsally.
In zebrafish and Xenopus, several maternal and zygotic genes function to restrict the organizer to dorsal regions. Three related homeodomain-containing transcriptional repressors, Vox, Vent, and Ved play a key role in repressing dorsal organizer gene expression ventrolaterally in zebrafish [7], [8], [9], [10], [11]. These repressors are expressed ventrally and dorsolaterally, and their deficiency causes dorsal organizer gene expression to expand around the ventrolateral margin during late blastula stages at the expense of ventrolateral tissues. Xenopus Vox and Vent have been shown to directly repress the expression of the organizer genes chordin (chd) and goosecoid (gsc) [12], [13] and depletion of Gsc has been reported to lead to a 25-fold increase in vent expression [14]. Similarly in zebrafish, Vox and Vent have been shown to bind to the gsc promoter and to physically associate with Gsc protein [8], [9]. These and other data [15], [16] illustrate the cross-regulatory interactions between opposing ventralizing and dorsalizing transcriptional repressors that are essential for proper embryonic patterning.
Several additional genes are known to restrict organizer gene expression to dorsal regions and modulate the expression of vox, vent, and ved. Knockdown of Runx2bt2, a maternal isoform of Runx2b, delays induction of vox and vent, and eliminates ved expression [17]. Embryos deficient in maternal Runx2bt2 exhibit an expansion of dorsal organizer gene expression at late blastula stages with a reciprocal loss of ventrolateral tissues [17]. Expression of vox, vent, and ved is maintained during late blastula and early gastrula stages by zygotic Wnt8a signaling [10], [18]. By mid gastrulation, expression of these ventralizing transcriptional repressors is maintained by BMP signaling [10], [19]. Thus, a gene regulatory network involving Runx2bt2, Wnt8a and BMP signaling converges on vox, vent, and ved to maintain the specification of non-axial mesoderm.
The maternally supplied transcription factor pou5f3 (previously called pou5f1) also functions in restricting the organizer to the dorsal midline. Maternal-zygotic deficiency of pou5f3 (MZpou5f3) leads to severe dorsalization resulting from derepression of organizer genes ventrolaterally in the embryonic margin, and incomplete induction of the BMP pathway [20]. MZpou5f3 mutants also exhibit aberrant morphogenesis and fail to form endoderm [20], [21], [22]. Pou5f3 likely functions as a transcriptional activator of genes, including vox, that are required to repress dorsal organizer gene expression ventrolaterally [23], [24]. Thus, Pou5f3 is another mediator of organizer gene repression operating in parallel to the Wnt8a pathway and partially through the BMP pathway.
One of the earliest organizer genes induced downstream of the maternal Wnt pathway in zebrafish is bozozok (boz), a direct transcriptional repressor of bmp2b, vox, vent, and ved expression [8], [25], [26], [27], [28], [29], [30]. boz mutant embryos fail to form prechordal plate, notochord, forebrain, and ventral neural structures and display an increase of ventroposterior mesoderm [25]. boz mutant embryos can be rescued by suppressing Wnt8a signaling, indicating that Boz antagonizes zygotic wnt8a expression in the organizer to block non-axial fate development in the dorsal embryonic midline and allow axial development [26]. Boz stability is modulated by Lnx2b, a maternally supplied E3 ubiquitin ligase that can directly bind and ubiquitinate Boz [31], [32]. Loss of Lnx2b causes expression of boz and other organizer genes to expand into lateral regions of the late blastula, illustrating the importance of proper turnover of Boz.
The transcriptional repressors Vox, Vent, and Ved are essential for partitioning the mesoderm into axial versus non-axial domains in response to positive regulation from Runx2bt2, Pou5f3, the Wnt8, and BMP pathways, and negative inputs from dorsalizing transcriptional repressors such as Boz and Gsc. It is less clear how these pathways are molecularly integrated to regulate vox, vent, and ved expression and it is likely that additional maternally-provided factors function in this process. Accordingly, we performed a genetic screen for maternal-effect mutations to identify novel mediators of vertebrate embryonic patterning.
We isolated a novel recessive maternal-effect mutation p18ahub that causes a profound reduction in ventrolateral mesoderm with a reciprocal expansion in axial mesoderm, and frequently multiple independent axial-like domains. Consistent with radial expansion of the organizer, p18ahub mutant females produce embryos exhibiting ectopic dorsal forerunner cells, a unique population of non-involuting mesendodermal cells at the dorsal margin [33], [34], [35]. We can rescue p18ahub dorsalized mutant embryos either by limiting Nodal signaling or restoring BMP signaling. We determined through positional cloning that p18ahub is a mutation disrupting the integrator complex subunit 6 (ints6) gene, which encodes a highly conserved component of the Integrator Complex, a large multisubunit complex implicated in 3′ end processing of spliceosomal snRNAs [36]. Previously, ints6 was named deleted in cancer 1 (dice1) and investigated as a putative tumor suppressor gene in humans [37], [38], [39], [40]. Using a forward genetic approach, we have revealed a novel role for Ints6 in limiting the extent of dorsal organizer tissues during vertebrate embryogenesis.
Results
A vertebrate recessive maternal-effect dorsalizing mutation
We isolated p18ahub, a recessive maternal-effect dorsalizing mutation, in an ENU-induced mutagenesis screen designed to identify novel maternal factors required for early embryonic development and patterning in zebrafish (similar to that described in [41], [42]). Mutant females yielded embryos with similar phenotypes whether they were crossed to mutant or wild-type (WT) males, indicating a strictly maternal-effect defect with no zygotic contribution. The first defect evident in embryos from p18ahub mutant mothers (henceforth referred to as p18ahub mutant embryos) was a delay in the initiation of epiboly, the morphogenetic process by which the blastoderm cells move over and encompass the yolk cell [43]. As WT embryos reached the late blastula/50% epiboly stage (Figure 1A), mutant embryos typically had not initiated epiboly movements (Figure 1B). In early gastrulation stages, WT embryos displayed a single dorsal thickening corresponding to the embryonic axis (Figure 1C), whereas mutant embryos often developed a radial thickening possibly due to hyper convergence of cells around the entire embryonic margin (Figure 1D). Approximately 50% of mutant embryos (Figure 1G) lysed prior to 24 hpf.
Fig. 1. p18ahub mutants exhibit delayed progression of epiboly and severe dorsalization. 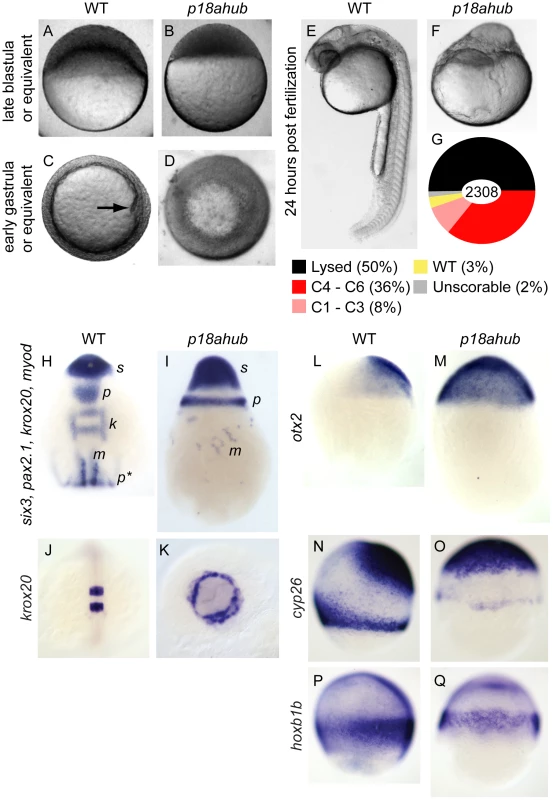
Late blastula stage WT embryo (A), and age-matched p18ahub embryo (B). WT embryo at an early gastrula stage (C) with the shield (organizer) on the dorsal side (arrow). A p18ahub embryo at an early gastrula stage (D) displaying apparent radial hyperconvergence with no definitive dorsal side. (A,B) lateral views; (C,D) animal pole views. WT (E) and p18ahub (F) embryos at 24 hours post fertilization (hpf) (lateral views). (G) Pie chart depicting proportion of embryos with indicated phenotypes at 24 hpf sampled over a total of 2308 embryos. (H and I), in situ hybridization on 3–5-somite stage WT (H, n = 15) and age-matched p18ahub (I, n = 13) embryos for six3 (s) expression in presumptive forebrain, pax2.1 expression in the midbrain-hindbrain boundary (p) and pronephros (p*), krox20 (k) in hindbrain rhombomeres 3 and 5, and myod (m) in paraxial mesoderm; dorsal view in (H), lateral view in (I), anterior to top. (J and K), 10-somite stage WT (J, n = 17, dorsal view), and age-matched p18ahub embryos (K, n = 18, anterior view) processed for krox20 in situ hybridization. (L–Q) in situ hybridization on mid gastrula stage WT and equivalent stage p18ahub embryos shown for: otx2, WT (L, n = 18) and p18ahub (M, n = 10); cyp26a, WT (N, n = 16) and p18ahub (O, n = 15); and hoxb1b, WT (P, n = 22) and p18ahub (Q, n = 21). Lateral views, anterior at top, dorsal to right. Thirty-five percent of mutant embryos surviving to 24 hours post fertilization (hpf) exhibited radial symmetry around the animal-vegetal axis and lacked any recognizable structures (Figure 1E,F). We categorized such embryos as class 6 dorsalized embryos. Class 5 embryos lacked recognizable structures but were not radially symmetric around the animal-vegetal axis and typically did not survive to 24 hpf. Class 4 through class 1 embryos (Figure 1G) exhibited progressively less severe dorsalization, as described [5]. To simplify the presentation of the data, we have combined phenotypic classes, C1–C3, and, C4–C6, in all figures, except that C5 embryos that did not survive to 24 hpf are included in a lysed category.
To better examine the epiboly and lysis defects of p18ahub embryos, we conducted time-lapse imaging (supplemental Movie S1 and Movie S2) of embryos from mid-blastula to mid-somitogenesis stages. p18ahub embryos were developmentally delayed and in some mutant embryos displayed prolonged epiboly (Movie S2). Some p18ahub embryos failed to undergo epiboly whatsoever and lysed by the equivalent of early gastrula stage in WT, with cells dispersing rapidly and the blastoderm disintegrating (Movie S1). In other p18ahub embryos, epiboly progressed to just prior to yolk plug closure (100% epiboly) when the hypoblast rapidly retracted and either the embryo lysed or the tissue dived down into the animal pole of the yolk cell (Movie S2), accounting for the morphology of embryos like the one shown in Figure 1F. Based on these studies it is likely that the lysed p18ahub embryos for a given clutch displayed either the early lysis phenotypes or were class 5 or 6 dorsalized.
To investigate if p18ahub embryos exhibit altered DV patterning, we examined the expression of genes of the dorsally-derived neurectoderm, dorsolaterally-derived somitic and paraxial mesoderm, and ventrally-derived pronephros, by in situ hybridization on 3 - to 5-somite stage embryos. The expression of both six3, which marks forebrain neurectoderm [44], and pax2.1, a marker of the midbrain-hindbrain boundary [45], was circumferentially expanded in p18ahub embryos (Figure 1H,I). krox20, which is expressed in rhombomeres 3 and 5 in the hindbrain [46], was often undetectable in severely dorsalized p18ahub embryos due to their severe delay (8/23 embryos displayed krox20 expression for the clutch represented in Figure 1I). In p18ahub embryos able to develop longer, krox20 expression was also expanded circumferentially (Figure 1J,K).
The expression of myod, a marker of paraxial and somitic mesoderm [47], was scattered in clusters of cells distributed circumferentially (Figure 1I, verified by examination of myod probe alone, not shown). Pronephric pax2.1 expression was often undetectable in mutant embryos (compare Figure 1I p* with H; verified by examination of pax2.1 probe alone, not shown), indicative of a severe reduction of ventroposterior mesoderm.
To investigate if patterning is also affected during gastrulation in p18ahub embryos, we examined the expression of the fore - and mid-brain marker otx2 [48] at mid gastrulation. We found that otx2 expression was expanded around the DV axis rather than restricted to a dorsoanterior region as in WT (Figure 1L and M). Importantly, however, otx2 was not expanded posteriorly as in wnt8a mutants [18], suggesting that the Wnt8a pathway is intact in p18ahub embryos. We also examined the expression of cyp26a and hoxb1b, markers of anterior neurectoderm and caudal hindbrain, respectively [49], [50]. Consistent with dorsalization, p18ahub embryos displayed expanded cyp26a expression around the DV axis (Figure 1N,O). Note also that the p18ahub embryos were delayed developmentally; the margin of the mutant embryo (the equivalent time of bud stage for WT) has not advanced as far as in WT. Compared to WT (Figure 1N), the p18ahub embryo displays reduced marginal cyp26a expression, which is consistent with the WT cyp26a expression pattern at earlier gastrula stages [50]. Expression of hoxb1b in the posterior neurectoderm is expanded in p18ahub embryos around the margin, although with reduced intensity compared to WT (Figure 1P,Q), likely also reflecting developmental delay [49]. It was necessary to age-match embryos for these experiments because we could not obtain sufficient numbers of p18ahub embryos at mid-gastrula stage due to their lysis. These data indicate that p18ahub embryos display an expansion of dorsal neurectodermal cell fates during gastrulation, but unlike zygotic wnt8a mutants, no significant expansion of anterior at the expense of posterior neurectoderm is observed in p18ahub embryos.
Although severely repressed, BMPs can signal in p18ahub embryos
To determine if the dorsalization of p18ahub embryos results from impaired BMP signaling, we examined BMP ligand gene expression, as well as expression of the BMP antagonist chordin (chd) in mutant embryos. bmp2b expression appeared normal in late blastula stage mutant embryos (data not shown). However, bmp2b and bmp4 expression were significantly reduced in mutant embryos by the early gastrula stage (Figure 2A,B,E,F) and severely reduced by mid gastrulation (Figure 2C, D, and data not shown), consistent with dorsalization. Since BMP gene expression is controlled by an autoregulatory feedback loop [3], , the loss of bmp2b and bmp4 expression in mutant embryos indicates severely reduced BMP signaling. chd expression, which is restricted to the dorsal side of the early zebrafish gastrula [53], is circumferentially expanded in mutant embryos at an early gastrula stage (Figure 2G,H), consistent with reduced BMP signaling and excessive dorsal fate specification in p18ahub embryos.
Fig. 2. Reduced bmp expression but intact BMP signal transduction in p18ahub mutants. 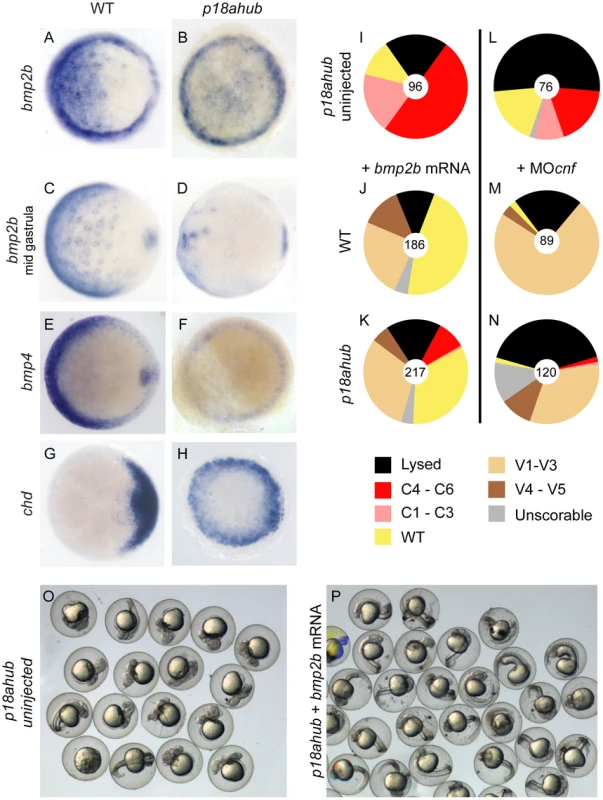
(A–H) In situ hybridization on early gastrula stage embryos or equivalent, except (C) and (D) are mid gastrula stage or equivalent. Embryos were stage-matched. Animal pole views, dorsal to the right for all. Expression of bmp ligand gene in WT embryos (A, n = 32, C, n = 43, and E, n = 21) and p18ahub embryos (B, n = 30, D, n = 18, and F, n = 16). Expression of the BMP antagonist chordin (chd) was confined to the dorsal side of WT embryos (G, n = 21) but was expanded around the entire margin of p18ahub embryos (H, n = 20) by early gastrulation. (I–N) Pie charts indicate fractions of embryos at 24 hpf that displayed the indicated phenotypes (categories described in the text). Number of embryos is shown at the center of each chart. p18ahub embryos (I, O, uninjected) that were injected with 20 pg bmp2b mRNA were rescued to WT or ventralized (K, P, +bmp2b mRNA) similarly to WT embryos (J, +bmp2b mRNA). p18ahub mutant embryos (L, uninjected) depleted of Chordin, Noggin1, and Follistatin-like 2b proteins by MO injection were similarly ventralized (N, +MOcnf) to WT embryos (M, +MOcnf). To determine if p18ahub embryos are defective mechanistically in BMP signal transduction, we injected mutant embryos with bmp2b mRNA, which moderately to severely ventralizes WT embryos [6] (Figure 2J). We found that forced expression of bmp2b restored ventral fates to WT in two-thirds of mutant embryos or moderately ventralized them (Figure 2I,K,O,P). Thus, the BMP signal transduction machinery in mutant embryos can function when BMP ligand is provided exogenously.
To further test whether the endogenous BMP signaling machinery is functional in p18ahub embryos, including the endogenous BMP ligands, mutant embryos were injected with translation-blocking morpholinos (MOs) targeting the secreted BMP antagonists Chordin, Noggin1, and Follistatin-like 2b [54]. Depletion of these BMP antagonists in mutant embryos caused mild to moderate ventralization, similar to that of WT embryos (Figure 2M,N). Thus, endogenous BMP ligands can signal in p18ahub embryos at a WT or greater level if BMP ligand function is permitted.
Organizer genes are derepressed in p18ahub embryos
Dorsalization can also be caused by a ventral expansion of dorsal organizer gene expression. To investigate the organizer in p18ahub mutant embryos, we examined expression of the organizer gene goosecoid (gsc) [55]. We found that, although gsc expression was induced normally at the mid blastula stage (data not shown), it was expanded in p18ahub embryos by early gastrulation (Figure 3A,B) and remained ectopically expressed through mid gastrula stages (Figure 3C,D) compared to WT embryos. Therefore, the dorsalization of p18ahub embryos involves a prominent expansion of gsc expression by early gastrulation, contrasting dorsalization resulting solely from defective BMP signaling [5].
Fig. 3. Dorsal organizer gene expression is expanded in p18ahub mutants. 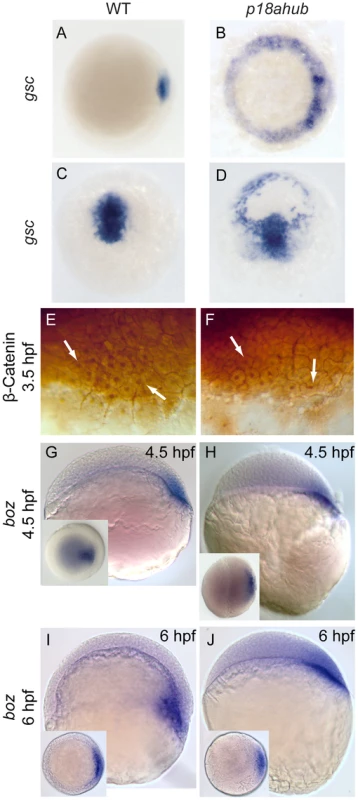
In situ hybridization using goosecoid (gsc) probe (A–D). Animal pole views, dorsal to right for (A) and (B); dorsal view, animal pole up in (C) and (D). WT embryos at early gastrula (A, n = 27) and mid gastrula stages (C, n = 41). p18ahub embryos (B, n = 33 and D, n = 10) displayed radially expanded gsc expression at the same stages. The embryo in (D) is tilted toward the viewer to show radial gsc expression. WT (E, n = 18) and p18ahub (F, n = 18) blastula stage embryos showed no obvious differences in the number or distribution of β-catenin immunopositive nuclei (arrows). (G–J) In situ hybridization for bozozok (boz), lateral views, dorsal to right; insets show animal pole views. WT and p18ahub embryos showed normal boz expression at late blastula (G, n = 19 and H, n = 18) and early gastrula stages (I, n = 14 and J, n = 16, respectively). As described in the text, epiboly progression was delayed in p18ahub embryos. At 6 hpf WT embryos form a morphologically apparent organizer, indicated by a thickening of the blastoderm on the dorsal side of the embryo and boz expression within the hypoblast. In contrast, at 6 hpf p18ahub embryos still appeared to be in a late blastula stage, although their boz expression was similar to WT. Wnt-mediated organizer induction is normal in p18ahub embryos
Since gsc expression was induced normally in p18ahub mutant embryos, it suggested that the organizer is induced normally by the maternal Wnt signaling pathway [1], [2]. To directly test this, we examined β-catenin nuclear localization as a readout of the maternal Wnt signaling pathway. We immunostained mid-blastula stage embryos (3.5 hpf) to visualize β-catenin in nuclei on the presumptive dorsal side of the embryo [1]. No differences were evident between WT and mutant embryos in β-catenin intensity or its localization in nuclei at the dorsal margin (Figure 3E, F, arrows). No nuclear localized β-catenin was observed ventrally in mutant embryos. Consistent with normal induction of the organizer, boz, a direct transcriptional target of the maternal Wnt pathway [56], was expressed normally in mutant embryos through 6 hpf, the equivalent of early gastrula stage (Figure 3G–J), unlike gsc and chd, which were expanded (Figure 2H and 3B). Thus, the organizer is induced normally in mutant embryos but the expression of some organizer genes becomes derepressed around the margin between late blastula and early gastrula stages. Furthermore, the dorsalization of p18ahub embryos does not rely upon ventrolateral expansion of boz.
The Wnt8a pathway is functionally intact but repressed in p18ahub embryos
Zygotic Wnt8a signaling in ventrolateral regions represses the expression of the organizer genes, gsc and chd, thus restricting the size of the organizer [7], [8], [9], [10], [11], [18], [19], [26]. Loss of Wnt8a or its mediators Vox, Vent, and Ved causes the expression of some organizer genes to expand and dorsalizes the embryo [7], [8], [9], [10], [11], [19]. Accordingly, we investigated the status of the Wnt8a pathway in mutant embryos. In WT late blastula and early gastrula stage embryos, wnt8a is expressed at the margin in a large domain extending from ventral to dorsolateral regions (Figure 4A) [18], [57]. However, in p18ahub late blastula stage embryos, wnt8a expression was restricted ventrally to a smaller domain (Figure 4B). Furthermore, wnt8a expression was undetectable in mutant embryos age-matched to their WT counterparts at early gastrula stage (not shown), perhaps reflecting a loss in the competence of marginal cells to express wnt8a. Since wnt8a is required for anteroposterior (AP) patterning of neural tissue [18] and a defect is not evident in AP patterning in p18ahub mutants, it suggests that the early, transient expression of wnt8a may be sufficient for AP patterning in p18ahub mutant embryos.
Fig. 4. The Wnt8a pathway is repressed but mechanistically intact in p18ahub embryos. 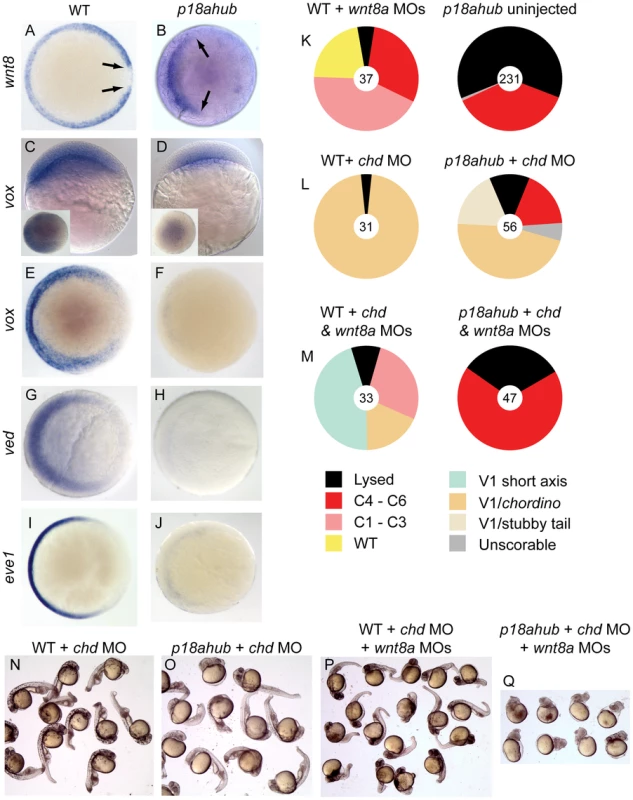
(A–J) In situ hybridization for Wnt8a pathway components or downstream genes. Animal pole views, dorsal at right, except (C, D) are lateral views, dorsal at right, insets show animal pole views. At an early gastrula stage in WT (A, n = 14) wnt8a was expressed around the margin but was absent from the axial mesoderm (arrows). In p18ahub embryos at a late blastula stage (B, n = 20) wnt8a expression was absent from the presumptive dorsal half of the embryo. The expression of vox was reduced in late blastula p18ahub embryos (D, 11/13 embryos) compared to WT embryos (C, n = 18). By early gastrulation, WT embryos (E, n = 20) showed prominent ventrolateral vox expression, while vox was nearly absent from equivalent stage p18ahub embryos (F, n = 12). ved was expressed similarly to vox in WT (G, n = 10) and was also nearly undetectable in p18ahub embryos (H, n = 10) at early gastrulation. eve1 in WT (I, n = 17) marks developing ventroposterior mesoderm and was nearly absent from p18ahub embryos by early gastrulation (J, n = 12). Splice blocking wnt8a MOs variably dorsalized WT embryos (K). (L) Injection of MO against chd ventralized both WT and p18ahub embryos (compare to p18ahub uninjected, K). (M) Simultaneous reduction of Chd and Wnt8a causes dorsalization in p18ahub embryos, indicating that endogenous Wnt8a signal transduction is intact and functional in p18ahub embryos that are depleted of Chd. Representative embryos at 1 dpf are shown in N–Q. We also examined the expression of vox and ved, two genes encoding transcriptional repressors acting downstream of Wnt8a to restrict organizer gene expression to the dorsal midline [10], [11], [19]. We found that vox expression was reduced in mutant embryos compared to age-matched WT embryos at a late blastula stage (Figure 4C,D). By early gastrulation, vox expression was greatly reduced or absent in mutant embryos (Figure 4E,F). We also conducted a time course experiment where embryos were collected from WT and p18ahub females over half hour intervals beginning at a mid-blastula stage (3 hpf) and subsequently examined for wnt8a and vox expression. In this experiment induction of neither gene was observed in p18ahub embryos by the equivalent of the early to mid gastrula stage (7 hpf) in WT (not shown). Maternal ved expression was evident in p18ahub embryos (data not shown); however, by an early gastrula stage ved expression was prominently reduced (Figure 4G,H). Early gastrula stage embryos also displayed reduced or nearly absent expression of eve1, a marker of ventroposterior mesoderm that requires BMP and Wnt8a signaling for its expression (Figure 4I,J) [58], [59], [60]. Thus, key mediators of the Wnt8a pathway are repressed in p18ahub embryos beginning as early as the late blastula stage, which can account for the loss of ventroposterior mesoderm.
We could not restore Wnt8a signaling in mutant embryos via injection of wnt8a mRNA, because early overexpression of wnt8a mimics the maternal Wnt signal for organizer induction and severely dorsalizes embryos [57]. To determine if the zygotic Wnt8a signaling pathway is functional in p18ahub embryos, we tested for Wnt8a function in p18ahub embryos rescued by depletion of BMP antagonists. If Wnt8a signaling is required, then it would indicate that the pathway is functional but likely fails to function in p18ahub embryos due to lack of full induction or maintenance of expression. To moderately increase BMP signaling in p18ahub embryos, we depleted one BMP antagonist, Chd, via MO injection. Loss of Chd alone rescued the majority of p18ahub embryos to a mild V1 ventralized phenotype, similar to loss of Chd in WT embryos (Figure 4K,L,N,O). Loss of wnt8a dorsalized WT embryos [18] (Figure 4K) and also dorsalized chd deficient embryos or caused posterior truncations (Figure 4M,P). Importantly, p18ahub embryos that were enabled to specify ventral tissues by Chd depletion became dorsalized again when Wnt8a was also depleted (Figure 4K–M,O,Q). These results indicate that the Wnt8a pathway is mechanistically intact in p18ahub embryos and that their ventralization or rescue to a WT phenotype by enhancement of BMP signaling depends on endogenous Wnt8a signaling.
Axial mesoderm is excessively specified at the expense of ventrolateral mesoderm in p18ahub embryos
Along with the maternal Wnt pathway, the dorsal organizer also depends on Nodal signaling for its induction (reviewed in [61]). Thus, we investigated the status of the Nodal pathway in p18ahub embryos. We examined expression of the Nodal ligand nodal-related 1 (ndr1, squint) in mutant and WT embryos [62]. ndr1 induction is initiated on the dorsal side of the embryo (Figure 5A) and requires Wnt signaling similarly to boz [2]. At a mid blastula stage, we observed no significant differences in the expression of ndr1 between WT and p18ahub embryos (Figure 5B) and ndr1 expression was never observed more animally outside of its normal marginal domain through an early gastrula stage equivalent (not shown).
Fig. 5. Excessive axial mesoderm at the expense of ventrolateral mesoderm in p18ahub embryos. 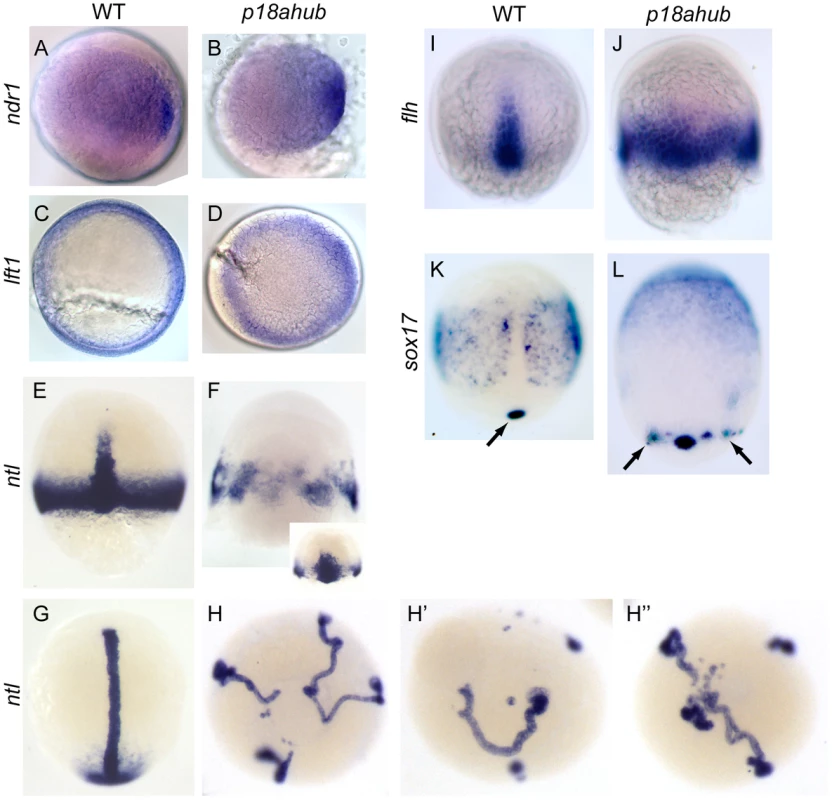
WT (A, n = 19) and stage-matched p18ahub (B, n = 20) embryos at a mid blastula stage, exhibited similar nodal related 1 (ndr1) expression. WT (C, n = 14) and age-matched p18ahub (D, n = 13) embryos at 6 hpf after synchronized matings displayed similar lefty1 (lft1) expression, although blastoderm involution was not evident in the mutants. (A–D) are animal pole views, dorsal to right. (E–L) are mid gastrula stage, except (G and H) are 3–5 somite stage or equivalent. Dorsal views, animal to top, except (H–H″) are animal pole views. WT embryos (E, n = 6) displayed marginal and axial ntl expression. In contrast, some p18ahub embryos displayed only axial ntl expression distributed circumferentially (F, n = 16), while others displayed broadened axial and reduced ventrolateral ntl expression (F inset, n = 8). In WT embryos (G, n = 22) ntl was expressed in the notochord and tail bud mesenchyme. In equivalent stage p18ahub embryos, (H–H″, n = 21), ntl expression was observed in multiple notochords terminating in smaller tail bud-like domains. Consistent with ectopic axial ntl expression, the expression of floating head (flh), a marker of notochord precursors, confined dorsally in WT embryos (I, n = 20), was expressed around the entire embryonic margin in p18ahub embryos (J, n = 16). In WT (K, n = 18) sox17 is expressed in endodermal precursor cells and in a single cluster of dorsal forerunner cells (arrow). In p18ahub embryos (L, n = 16) sox17 expression indicates the presence of ectopic clusters of dorsal forerunner cells (arrows). To further test if Nodal signaling is induced normally in p18ahub embryos, we examined expression of the Nodal feedback antagonist lefty1 (lft1, antivin1). lft1 is initially expressed dorsally but is subsequently expressed around the margin of the late blastula [63], [64], [65]. At an early gastrula stage (6 hpf), we observed no significant differences in the expression level of lft1 in mutant versus WT embryos (Figure 5C and D). From these data we conclude that Nodal signaling in p18ahub embryos is induced normally and likely operates normally at an early gastrula stage.
By mid gastrula stages the pan-mesodermal gene no tail (ntl) is expressed in two distinct domains, one corresponding to axial mesoderm, the developing notochord, and another corresponding to ventrolateral non-axial mesoderm [66]. At the equivalent of mid gastrula and early somite stages, ntl expression in presumptive non-axial mesoderm was reduced or absent, while axial mesoderm appeared to be expanded leading to multiple independent axes in some mutant embryos (9/9, 6/10, and 4/10 in three independent clutches) (Figure 5E–H″). Some mutant embryos displayed a significantly broadened single axial ntl domain with a prominent reduction in marginal ntl expression (Figure 5F inset). By the equivalent of early somite stages of development, mutant embryos clearly possessed multiple independent presumptive notochords marked by ntl expression (Figure 5H).
floating head (flh), a homeobox gene required for notochord specification, is induced at a late blastula stage independently of ntl but is a direct transcriptional target of ntl by mid gastrula stages [67], [68], [69]. Consistent with excessive axial ntl expression, flh was also circumferentially expanded in p18ahub embryos by mid gastrulation (Figure 5I,J). Therefore, genes marking axial mesoderm (gsc and flh) are ectopically expressed in p18ahub embryos by early gastrula stage, despite normal Wnt-mediated organizer induction and normal induction of the Nodal pathway.
p18ahub embryos display ectopic dorsal forerunner cells
Nodal signaling is required for the expression of the HMG - type transcription factor sox32 (casanova (cas)) in endodermal precursors by late blastula stages [35], [70]. cas is required along with pou5f3 (formerly spiel-ohne-grenzen, pou5f1) to induce sox17 and maintain endodermal precursor cells [21]. Both cas and pou5f3 are also required for the maintenance of dorsal forerunner cells [21], [33], a distinct population of non-involuting cells that also express sox17 at the leading edge of the dorsal margin of the blastoderm as it migrates over the yolk [34], [71]. We observed endodermal sox17 expression in p18ahub embryos at mid gastrulation (Figure 5L). However, endodermal precursor cells were located more animally than in WT (Figure 5K), perhaps due to altered cell movements resulting from loss of BMP signaling and dorsalization [72], [73]. Ectopic marginal expression of sox17 was observed in p18ahub mid gastrula stage embryos indicating that ectopic dorsal forerunner cells form in mutant embryos (Figure 5K,L, arrows).
In p18ahub embryos axial mesoderm and organizer gene expression are expanded ventrolaterally, but remain confined within a normal mesodermal domain. Importantly, we did not observe the animal-ward expansion of Nodal-dependent genes such as ntl or lft1 or excessive specification of mesendodermal precursor cells, which has been reported upon excessive Nodal signaling due to loss of Lefty [74], [75], [76] or ectopic expression experiments [77], [78]. Thus, Nodal signaling is likely not excessive in p18ahub embryos.
Suppression of Nodal signaling rescues patterning of p18ahub embryos
The secreted feedback inhibitor Lefty/Antivin (Lft1) regulates Nodal signaling [63], [64], [65], [79]. Misexpression of Lft1 in WT embryos severely limits mesendoderm induction with embryos closely resembling ndr1;ndr2 double mutants [62]. We found that injection of as little as 0.7 picograms (pg) of lft1 mRNA (Figure 6A, +0.7× middle row), a dose that only weakly perturbs WT embryos (Figure 6A, Minor head defects), could restore WT or nearly WT patterning in 33% of mutant embryos. Injection of 1 pg of lft1 mRNA (Figure 6A, +1× middle row) restored WT patterning in a larger fraction of mutant embryos and suppressed mesendoderm formation (oep-like) in 20% of mutant embryos. The same dose of lft1 mRNA injected into WT embryos blocked mesendoderm development in more than 50% of the embryos (Figure 6A, +1× bottom). Injection of 3 pg of lft1 mRNA into mutant and WT embryos inhibited mesendoderm induction in a similar fraction of embryos (Figure 6A, +3×). Thus, suppression of Nodal signaling can restore the balance between axial and non-axial fate specification in mutant embryos, similarly to restoring BMP signaling.
Fig. 6. Lefty misexpression suppresses patterning defects of p18ahub embryos. 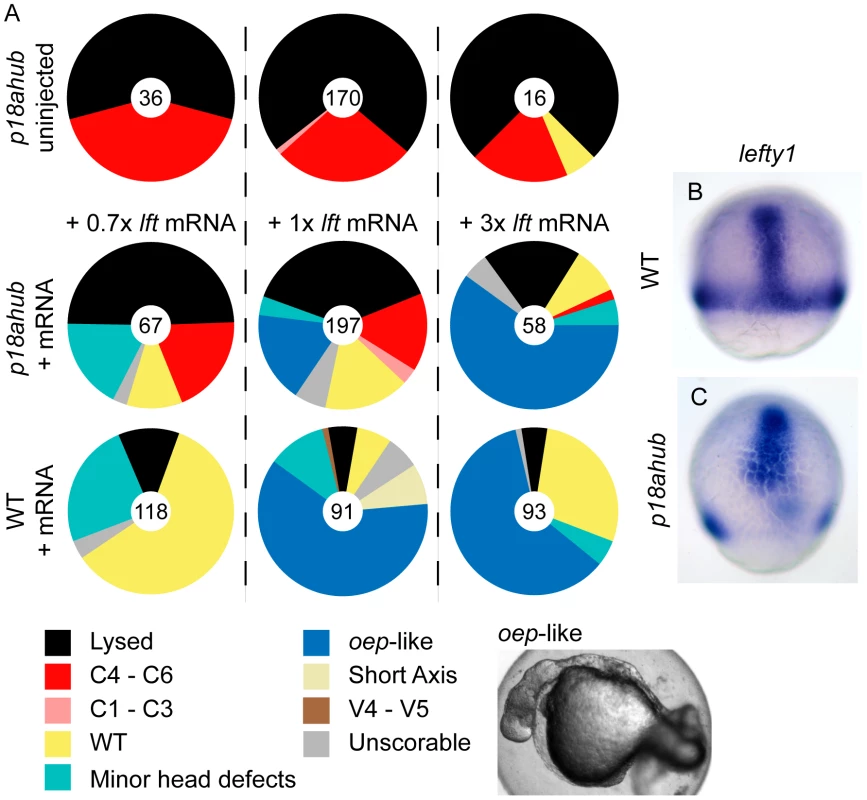
(A) Pie charts indicate fractions of embryos at 24 hpf with the indicated phenotypes. Number of embryos is at the center of each chart. Labels across indicate the amount of lefty1 (lft1) mRNA injected. Top row shows uninjected p18ahub control clutch for each experiment, middle row indicates p18ahub embryos injected with mRNA, and bottom row is WT embryos injected with mRNA. +1× lft mRNA corresponds to 1 pg mRNA. (B and C) In situ hybridization for lft1 in a mid gastrula stage WT (B, n = 12) and p18ahub (C, n = 13) embryos; lateral views, dorsal facing. We examined lft1 expression to determine if its reduction contributed to excess dorsal mesodermal gene expression at mid gastrulation in p18ahub embryos. In WT embryos lft1 was expressed around the embryonic margin as well as in the developing axis and dorsal forerunner cells (not shown) at mid gastrula stages (Figure 6B). In p18ahub embryos lft1 was expressed within the presumptive prechordal plate as well as in clusters of cells scattered around the margin at a mid gastrula stage (Figure 6C). These latter cells may represent remaining marginal cells having non-axial fates. Hence, an absence of lft1 expression cannot account for the severe dorsalization of p18ahub embryos.
p18ahub affects a highly conserved component of the Integrator Complex
To identify the molecular nature of p18ahub, we mapped the mutation to a chromosomal position by examining linkage to simple sequence length polymorphic (SSLP) markers. We first found linkage of p18ahub to z1660 on chromosome 9. Further fine mapping examining over 1100 meioses placed p18ahub within a 1.35 Mb interval between the SSLP marker z7120 and an SSLP marker that we generated for BAC CR545476.14 (Zv9). (Figure 7A). The interval displays synteny with human chromosome 13. It contains just over one dozen genes, none of which were known to function in DV patterning. We proceeded to sequence the open reading frames of cDNAs of genes within the interval.
Fig. 7. p18ahub affects the Integrator Complex Subunit 6 (Ints6). 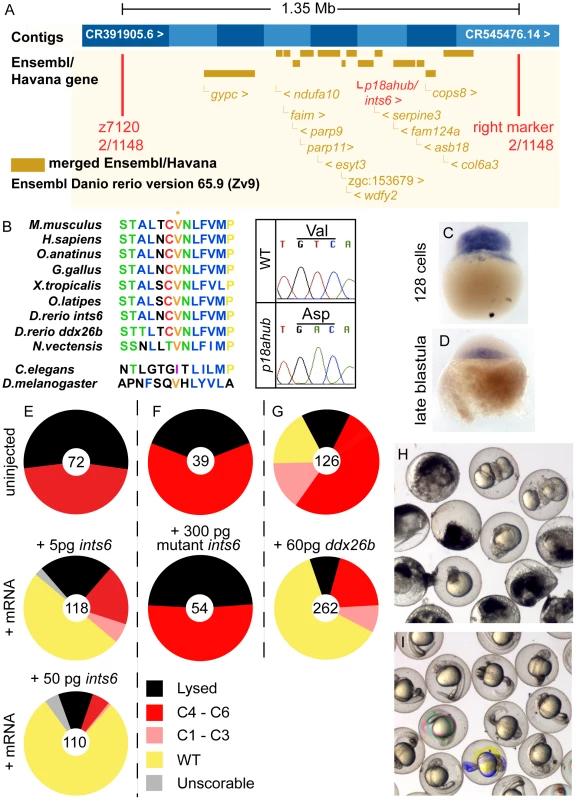
(A) p18ahub was narrowed to an approximately 1.35 Mb interval between SSLP marker z7120 and an SSLP marker that we generated in BAC CR545476.14 (Zv9) (right marker) by examining meiotic recombination between markers flanking the mutation. The recombinants found in the number of meioses examined at each marker is shown and the positions of markers are approximate. (B) p18ahub is a T to A transition in an exon of ints6 that converts valine 375 to an aspartate. An alignment of several Ints6 proteins was generated using ClustalW and reveals that V375 is nearly invariant among widely divergent species. (C and D) In situ hybridization for ints6 transcripts on WT embryos at (C) 128-cell (n = 8) and (D) late blastula stage (n = 19); lateral views, animal to top. (E–G) Rescue experiments: pie charts show fractions of embryos evaluated at 1 dpf with indicated phenotypes. Numbers of embryos are shown in the center of each chart. (H) Uninjected p18ahub mutant embryos and (I) mutant embryos rescued by injection of 50 pg ints6 mRNA, shown at 1 dpf. We found a T to A transition in an exon of the integrator complex subunit 6 (ints6) gene (GenBank Accession number KF700696, OMIM 604331), converting a nearly invariant valine to an aspartate at position 375 of the 854 amino acid predicted protein (Figure 7B). Human and zebrafish Ints6 orthologs are 66% identical indicating an overall high degree of evolutionary conservation. The only recognizable domain in Ints6 is an N-terminal von Willebrand factor type A motif (InterPro IPR002035), a broadly employed motif mediating interactions between diverse proteins. There is a second gene, ddx26b, on the X chromosome in humans and on chromosome 14 in zebrafish that is highly related to ints6. The zebrafish Ints6 and Ddx26b proteins are 61% identical also implying significant conservation of function between these homologs.
We examined the expression of ints6 by in situ hybridization. ints6 transcripts were present in eggs and embryos through the late blastula stage (Figure 7C,D, data not shown). ints6 transcripts declined during late blastula stages and were undetectable by early gastrulation (Figure 7, data not shown). We observed no alteration in the expression of ints6 in mutant embryos by in situ hybridization (not shown). These expression data are consistent with the recessive, maternal-effect inheritance of p18ahub and the maternal requirement for ints6.
Ints6 is the gene affected by p18ahub
To determine if dorsalization of p18ahub embryos was caused by the mutation in ints6, we injected mutant embryos with mRNA encoding WT Ints6. We found that as little as 5 pg of WT ints6 mRNA rescued 50% of mutant embryos completely (Figure 7E) and 50 pg rescued nearly 80% of mutant embryos to WT (Figure 7E,H,I). Thus, ints6 is the defective gene in p18ahub mutant embryos. We also injected mutant embryos with up to 300 pg of different preparations of p18ahub mutant ints6 mRNA and never detected rescue (Figure 7F). Thus, p18ahub is likely a strong loss-of-function or null allele. Overexpression of inst6 in WT embryos produced no phenotype (data not shown). The related zebrafish ddx26b mRNA also rescued p18ahub embryos (Figure 7G), although it is not provided maternally to the embryo (data not shown). We did not detect ddx26b expression via in situ hybridization on embryos through 24 hpf, although we were able to amplify cDNA corresponding to ddx26b from ovary RNA (not shown).
Discussion
Ints6 is a negative regulator of vertebrate organizer gene expression
We have identified a recessive maternal-effect mutation in integrator complex subunit 6 (ints6), a highly conserved member of an RNA processing machine for which no specific role in vertebrate development was known. Importantly, to our knowledge the p18ahub mutation represents the first mutation of the ints6 gene in any organism to be reported. Interestingly, the loss of ints6 causes a recessive, maternal-effect dorsalization whereby dorsal midline axial fates are expanded, generating multiple dorsal axes at the expense of ventrolateral fates, suggesting an expansion of the dorsal organizer itself or of organizer gene expression mediating dorsal fate specification. The dramatic radial dorsalization of affected embryos is not caused by an expansion of the maternal Wnt-mediated induction of the organizer or the induction of Nodal signaling. In ints6 mutant embryos axial mesoderm is expanded along the DV axis at late blastula stages but remains confined near the margin where mesendoderm is normally induced, indicating a DV patterning defect rather than a general expansion of mesoderm.
The Integrator Complex was identified as a complex of 12 subunits that co-purifies with Deleted in split hand/split foot 1 (DSS1) in human HEK293 cells [36]. The Integrator Complex associates with the C-terminal domain (CTD) of the large subunit of RNA polymerase II (RNAP) and has been implicated in 3′ end processing of the U1 and U2 snRNAs of the splicesosome [36]. Phosphorylation of serine 7 in the heptapeptide repeats present in the CTD directs the Integrator Complex-bound RNAP to snRNA genes rather than to the promoters of protein coding genes [36]. Prior to those studies, ints6 was referred to as deleted in cancer 1 and investigated as a putative human tumor suppressor gene, given the loss of its expression in several tumor-derived cell lines and tumor specimens from patients [38], [39]. It was shown that the promoter of ints6 is subject to hypermethylation in transformed cells and overexpression of ints6 can suppress the anchorage-independent growth of prostate tumor-derived and non-small cell lung carcinoma-derived cell lines [37], [40].
Although Ints6 is a member of a complex required to produce functional spliceosomal snRNAs [36], [80], the dorsalized phenotype of the ints6 mutant in the zebrafish is not suggestive of a broad maternal or zygotic function in snRNA 3′ end formation. We can rescue the dorsalized mutant phenotype by altering a number of pathways required for embryonic patterning. Such success in our rescue experiments is difficult to reconcile with a widespread splicing defect. Since we can rescue dorsalization by injecting WT ints6 mRNA, Ints6 functions in the embryo rather than during oogenesis, when maternal mRNAs are transcribed and spliced. Tao et al. have reported that MO-mediated knockdown of Integrator Complex subunits 5, 9, and 11 in the zebrafish results in defective hematopoiesis, possibly due to alteration of zygotic snRNA and mRNA processing [81]. The lysis defect that we observe in some early gastrula stage p18ahub embryos may reflect a more general function for Ints6, possibly in snRNA 3′ end formation or another unidentified role. In this regard p18ahub may be a hypomorphic allele that perturbs Ints6 function as part of the Integrator Complex.
The specific role of Ints6 within the Integrator Complex is unknown. RNAi-mediated knockdown of Ints6 in Drosophila S2 cultured cells causes low levels of splicing defects of snRNAs and of a U7-GFP reporter. These effects are orders of magnitude less than the alteration of snRNA processing reported in Drosophila S2 cells for RNAi-mediated knockdown of Ints9 or Ints11, putative catalytic subunits of the Integrator Complex [80]. It is also possible that the p18ahub missense mutation in ints6 affects some but not all roles of Ints6 equivalently.
Prior to our work C.elegans deleted in cancer 1 (dic-1) was the only ints6 homolog to be investigated in any developmental context [82]. However, C.elegans DIC-1 is highly divergent from zebrafish Ints6 displaying only 26% identity and may not represent a true ortholog. C.elegans DIC-1 is required to maintain the viability of oocytes through oogenesis and embryonic viability in general, since RNAi depletion in embryos leads to arrested development and widespread cell death. No defects in embryonic patterning were reported [82].
Early gastrula stage expansion of axial mesoderm in ints6 mutant embryos
Nuclear β-catenin was normally localized dorsally in mid-blastula stage ints6 mutant embryos indicating that maternal Wnt-mediated organizer induction is normal. Furthermore, boz expression was also normal in mutant embryos through the equivalent of an early gastrula stage [7], [11]. The expression of ndr1, lft1, and ntl in both WT and ints6 mutant embryos was initially confined to the dorsal side of the embryo and expanded normally ventrolaterally around the blastoderm margin by late blastula stages [62], [63], [64], [83]. The earliest alteration of organizer gene expression that we observed in p18ahub embryos was the derepression of marginal gsc expression at an early gastrula stage. By mid gastrulation, ntl and flh expression in the axial mesoderm was also extensively expanded in mutant embryos.
Several lines of evidence distinguish the induction of the Nodal pathway in the axial mesoderm from its induction throughout the margin during late blastula and early gastrula stages, but the molecular basis for this distinction is not fully understood [2], [62], [84], [85], [86]. Genes downstream of ndr1, like ntl, are also induced differentially in the axis versus the ventrolateral margin [62], [85]. Axial ntl is required for notochord formation, whereas non-axial ntl expression is required to establish ventroposterior mesoderm [87], [88]. Analyses of reporter gene constructs driven by different ntl enhancers indicate that, aside from Nodal signaling, both Wnt8a and BMP signaling contribute significantly to the ventrolateral expression of ntl [89]. In ints6 mutant embryos, loss of the non-axial expression domain of ntl is likely due to reduced ventrolateral BMP and Wnt8a signaling, thus leading to loss of non-axial mesoderm with a concomitant expansion of axial mesoderm.
ints6 mutants are deficient in ventrolateral Wnt8a signaling
Vox, Vent, and Ved are three critical repressors of organizer gene expression that are required to maintain the integrity of non-axial mesoderm. Initial induction of vox and zygotic ved expression is mediated through runx2bt2, a maternally provided splice isoform of runx2. Depletion of Runx2bt2 leads to a notable absence of ved expression at a late blastula stage, while vox and vent are expressed [17]. These defects are distinct from that of ints6 mutants, which display nearly absent vox and ved expression by early gastrulation.
The Wnt8a pathway is a critical negative regulator of organizer gene expression in ventrolateral non-axial mesoderm operating through Vox, Vent, and Ved [7], [10], [18]. wnt8a is induced in a reduced domain in ints6 mutant embryos but is not maintained by an early gastrula stage (Figure 4B). ints6 mutants exhibit reduced expression of vox and ved by the early gastrula stage, a time when these transcriptional repressors rely on Wnt8a for their expression. By the early gastrula stage, organizer gene derepression is evident in ints6 mutants (Figure 3B). Loss of Wnt8a signaling may cause organizer gene expression to expand, or alternatively the expanded expression of organizer genes in ints6 mutants may cause the loss of Wnt8a signaling.
Simultaneous loss of both zebrafish β-catenin proteins causes gsc and chd expression to expand around the embryonic margin, similarly to ints6 mutants [90]. β-catenin 1 and 2 function redundantly to transduce zygotic Wnt8a signaling, which has been proposed to suppress the ndr1-mediated induction of chd and gsc in the ventrolateral margin [91]. Loss of wnt8a expression in ints6 mutant embryos could similarly cause chd and gsc to expand around the ventrolateral margin. Our results suggest that Wnt8a signal transduction mechanisms are intact in ints6 mutants, given that chd MO-mediated rescue of p18ahub depends on Wnt8a signaling.
The dorsalization of ints6 mutants
Our results indicate that ints6 mutants are not mechanistically defective in BMP signaling. DV patterning of ints6 mutants can be restored to nearly WT by depleting the BMP antagonist Chd alone, or along with Noggin1 and Follistatin-like 2b. Induction of bmp2b expression is normal in ints6 mutants and loss of bmp2b and bmp4 expression by early gastrulation likely is the result of expanded organizer gene expression, including chd expression, or loss of Wnt8 signaling.
Maternal-zygotic pou5f3 (MZpou5f3) mutants, like ints6 mutants, display expanded organizer gene expression at late blastula stages and are severely dorsalized. In contrast to ints6 deficient embryos, MZpou5f3 mutants fail to initiate bmp2b and bmp4 expression, and show greatly reduced bmp7 expression [20]. In MZpou5f3 mutants, expansion of organizer gene expression is likely due to loss of repressors that rely on Pou5f3 for their expression [24]. Similarly to ints6 mutants, pou5f3 mutants exhibit aberrant morphogenesis and also display a delay in the completion of epiboly, [22], [92].
However, Pou5f3 has additional functions during gastrulation compared to Ints6. MZpou5f3 mutants fail to form endoderm and exhibit reduced sox17 expression in dorsal forerunner cells [20], [24], in contrast to the expanded populations of sox17-expressing forerunner cells observed in p18ahub embryos. Endodermal sox17 expression was observed in ints6 mutant embryos, although it was restricted more animally than in WT (Figure 5L). We examined the expression of pou5f3 in p18ahub embryos at a late blastula stage and observed no differences from WT (not shown). Thus, pou5f3 expression does not rely on ints6. It is possible that ints6 and pou5f3 cooperate in DV patterning and in morphogenesis of the early embryo.
The definitive placement of ints6 within a genetic pathway as well as the determination of its precise molecular functions will require biochemical characterization of the Ints6 protein. Ints6 may mediate DV patterning by supporting the expression or function of the Wnt8a pathway or specifically modulating the axial versus non-axial response to Nodal. Importantly, our molecular genetic approach has revealed a novel function for Ints6 in vertebrate embryonic patterning. Future studies will reveal its function within the complex gene regulatory network that restricts the organizer to dorsal regions and modulates axial versus non-axial tissues of the vertebrate body plan.
Materials and Methods
Ethics statement
The animal work performed in this study was approved by the Institutional Review Board of the University of Pennsylvania.
Zebrafish strains
p18ahub was isolated in an ENU-induced mutagenesis genetic screen for maternal-effect mutations utilizing a hybrid AB/TU genetic background for mapping purposes, similar to that described previously [41], [42]. The p18ahub mutation was found to be on the TU chromosome. Thus the line was outcrossed to AB to maintain it. Mutant females from both the original hybrid line as well as outcrossed lines were used. Embryos for in situ hybridization and injection experiments were derived from crosses of p18ahub mutant females to TL males. Due to delay in morphogenesis, the stage of p18ahub embryos is often based on the stage of corresponding age-matched WT reference embryos. Other criteria for embryo staging are described in the text.
In situ hybridization
Antisense RNA probes were synthesized from linearized plasmid templates and transcribed using either SP6 or T7 polymerases (Promega) and Roche digoxygenenin-labeled NTP mix (11277073910). To examine the expression of ints6 and ddx26b, full-length open reading frames were amplified using the following primers and cloned into pDONR221 and ultimately pCSDest using the Gateway system (Invitrogen). Note: coding sequences or complements are shown; attB1 and attB2 sites are omitted.
ints6 gateway For GTCCATGTAGAAGGGGCGAATATCAAC
ints6 gateway Rev GTGCTCGAGTCCTTCAAGTAGGGCAG
ddx26b gateway For GAGATAGGTCATATGCCGATTGTAGC
ddx26b gateway Rev TGAGATGTGACTTGCCACTATCTGC
The hybridization procedure was essentially as described at the Zebrafish Information Network (see http://zfin.org/zf_info/zfbook/chapt9/9.82.html), except Roche BM Purple alkaline phosphatase substrate (11442074001) was used and terminated by washing embryos briefly in PBS followed by overnight fixation in 4% paraformaldehyde/PBS at 4°C. Stained embryos were stored in methanol at −20°C and cleared in a 2∶1 mixture of benzyl benzoate∶benzyl alcohol for photography. Embryos were mounted in Canada Balsam and oriented under glass coverslips for imaging on either a Zeiss Axioscope microscope fitted with a ProgRes CF CCD camera (Jenoptik) or Leica MZ12-5 dissecting microscope fitted with a Photometrics CoolSnap CF CCD camera (Roper Scientific). In situ hybridization was performed 2 to 4 times for each probe independently on groups of mutant and age - or stage-matched WT control embryos, as indicated in the text, typically obtained from crosses on the same day.
Immunostaining for β-catenin
Embryos were fixed overnight at 4°C in 4% paraformaldehye/PBS and washed several times in PBS. Embryos were then dechorionated and placed in 100% methanol overnight or for long-term storage at −20°C. Embryos were rehydrated first in 50% MeOH for 10 minutes and then 3 times for 10 minutes in100% PBST (PBS with 0.5% Triton X-100). Embryos were blocked in Blocking Solution (10% fetal bovine serum/PBST) for one hour before receiving a 1∶500 dilution of rabbit anti-β-catenin antibody (Sigma C2206) in Blocking Solution and incubating at 4°C overnight. The next day embryos were washed 3 times for 10 minutes with 1 ml of Blocking Solution at room temperature before incubation in a 1∶500 dilution of HRP-conjugated goat-anti-rabbit antibody (Sigma A9169) in Blocking Solution for at least two hours at room temperature. Embryos were then washed 4 times in 1 ml PBST and developed in 1 ml of a 1∶3 dilution of a 15 ml solution of 50 mM Tris-Cl pH 7.5, 100 mM NaCl, 1 DAB (3,3′ diaminobenzidine) tablet (Sigma D5905), and 15 µl freshly added 30% H2O2. Staining was monitored visually under a dissecting microscope and terminated via washing in 50 mM Tris-Cl pH 7.5, 100 mM NaCl. Stained embryos were placed in 100% methanol for storage at −20°C and photographed as described above for in situ hybridized embryos.
Microinjection of embryos
mRNA or morpholinos (MOs) were injected into the yolk cell of 1 - to 2-cell stage embryos that were subsequently scored at 24 hours post fertilization (hpf) for phenotypes. In vitro transcribed mRNAs were generated from linearized plasmid DNA templates using the mMessage mMachine kit (Ambion). mRNAs were diluted to appropriate working concentrations in 100 mM KCl/10% Phenol Red just prior to injection. Templates for ints6 (WT or mutant) and ddx26b mRNAs were the same as those used to make in situ riboprobes described above. Other templates included: lft1-pCS2 [64], sqt-pCS2 [93], and FLAG-bmp2b [94]. MOs were obtained from GeneTools and prepared as stocks in water according to the manufacturers recommendations. Prior to injection, MOs were diluted to working concentrations in 1× Danieau's Solution (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5 mM Hepes pH 7.1–7.3). The sequences of the chordin, noggin1, and follistatin-like2b translation-blocking MOs are reported elsewhere [54]. We employed anti-chd MO at 0.82 ng/µl, anti-nog MO at 4.22 ng/µl, and anti-fstl2b MO at 8.45 ng/µl and typically injected 1 nl. To target processing of wnt8a transcripts we employed the splice-blocking MOs orf1 E1i1 and orf2 E4i4, as described [60].
Positional cloning of p18ahub
Our fine mapping strategy has been described elsewhere [41], [95]. Heterozygous p18ahub females and homozygous p18ahub males from the original AB/TU hybrid strain were crossed to obtain females recombinant between SSLP markers flanking the mutation. The initial interval placed p18ahub between z34824 and z6845. In total we examined 1148 meioses, narrowing the interval to approximately 1.35 Mb between z7120 and an SSLP marker that we generated against BAC CR545476 (forward primer – AGATGTAACTCATCCACTGTCATACACC, reverse primer – AACCGTTGAGAGGTTTCTAGCTAGTAC). We then proceeded to sequence all genes within the interval for which a cDNA was reported. We made oligo-dT-primed cDNA from the TU wild-type and the p18ahub mutant strains from total ovary RNA extracted using Trizol Reagent (Invitrogen 15596-018) according to the manufacturer's instructions, and PCR amplified the coding regions of candidate genes. PCR products were purified (Qiagen PCR clean-up kit) and both strands were sequenced. The ints6 ORF was sequenced using the primers described above (without att sites) (GenBank Accession number KF700696) and a T to A transition at nucleotide 1124 converting valine 375 to an aspartate was discovered in p18ahub-derived ovary cDNA. No mutation was found in genomic DNA derived from the G0 mutagenized male, consistent with the point mutation resulting from ENU-induced mutagenesis.
Time-lapse imaging
Embryos were mounted in 0.3% low-melt agarose in E3 media and photographed with a Leica MZ12.5 microscope and Micropublisher 5.0 RTV Non-Cooled camera at 5 minute intervals from mid blastula through early somitogenesis stages at room temperature under constant illumination. Images were combined into movies using ImageJ.
Supporting Information
Zdroje
1. SchneiderS, SteinbeisserH, WargaRM, HausenP (1996) Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57 : 191–198.
2. KellyC, ChinAJ, LeathermanJL, KozlowskiDJ, WeinbergES (2000) Maternally controlled (beta)-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127 : 3899–3911.
3. KishimotoY, LeeKH, ZonL, HammerschmidtM, Schulte-MerkerS (1997) The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124 : 4457–4466.
4. KramerC, MayrT, NowakM, SchumacherJ, RunkeG, et al. (2002) Maternally supplied Smad5 is required for ventral specification in zebrafish embryos prior to zygotic Bmp signaling. Dev Biol 250 : 263–279.
5. MullinsMC, HammerschmidtM, KaneDA, OdenthalJ, BrandM, et al. (1996) Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123 : 81–93.
6. NguyenVH, SchmidB, TroutJ, ConnorsSA, EkkerM, et al. (1998) Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 199 : 93–110.
7. ImaiY, GatesMA, MelbyAE, KimelmanD, SchierAF, et al. (2001) The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development 128 : 2407–2420.
8. KawaharaA, WilmT, Solnica-KrezelL, DawidIB (2000) Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc Natl Acad Sci U S A 97 : 12121–12126.
9. KawaharaA, WilmT, Solnica-KrezelL, DawidIB (2000) Functional interaction of vega2 and goosecoid homeobox genes in zebrafish. Genesis 28 : 58–67.
10. RamelMC, LekvenAC (2004) Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 131 : 3991–4000.
11. ShimizuT, YamanakaY, NojimaH, YabeT, HibiM, et al. (2002) A novel repressor-type homeobox gene, ved, is involved in dharma/bozozok-mediated dorsal organizer formation in zebrafish. Mech Dev 118 : 125–138.
12. MelbyAE, ClementsWK, KimelmanD (1999) Regulation of dorsal gene expression in Xenopus by the ventralizing homeodomain gene Vox. Dev Biol 211 : 293–305.
13. TrindadeM, TadaM, SmithJC (1999) DNA-binding specificity and embryological function of Xom (Xvent-2). Dev Biol 216 : 442–456.
14. SanderV, ReversadeB, De RobertisEM (2007) The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. The EMBO J 26 : 2955–2965.
15. GawantkaV, DeliusH, HirschfeldK, BlumenstockC, NiehrsC (1995) Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J 14 : 6268–6279.
16. OnichtchoukD, GlinkaA, NiehrsC (1998) Requirement for Xvent-1 and Xvent-2 gene function in dorsoventral patterning of Xenopus mesoderm. Development 125 : 1447–1456.
17. FloresMV, LamEY, CrosierKE, CrosierPS (2008) Osteogenic transcription factor Runx2 is a maternal determinant of dorsoventral patterning in zebrafish. Nat Cell Biol 10 : 346–352.
18. LekvenAC, ThorpeCJ, WaxmanJS, MoonRT (2001) Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1 : 103–114.
19. MelbyAE, BeachC, MullinsM, KimelmanD (2000) Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol 224 : 275–285.
20. ReimG, BrandM (2006) Maternal control of vertebrate dorsoventral axis formation and epiboly by the POU domain protein Spg/Pou2/Oct4. Development 133 : 2757–2770.
21. ReimG, MizoguchiT, StainierDY, KikuchiY, BrandM (2004) The POU domain protein spg (pou2/Oct4) is essential for endoderm formation in cooperation with the HMG domain protein casanova. Dev Cell 6 : 91–101.
22. LundeK, BeltingHG, DrieverW (2004) Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Current biology : CB 14 : 48–55.
23. BeltingHG, WendikB, LundeK, LeichsenringM, MossnerR, et al. (2011) Pou5f1 contributes to dorsoventral patterning by positive regulation of vox and modulation of fgf8a expression. Dev Biol 356 : 323–336.
24. OnichtchoukD, GeierF, PolokB, MesserschmidtDM, MossnerR, et al. (2010) Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol 6 : 354.
25. FekanyK, YamanakaY, LeungT, SirotkinHI, TopczewskiJ, et al. (1999) The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development 126 : 1427–1438.
26. Fekany-LeeK, GonzalezE, Miller-BertoglioV, Solnica-KrezelL (2000) The homeobox gene bozozok promotes anterior neuroectoderm formation in zebrafish through negative regulation of BMP2/4 and Wnt pathways. Development 127 : 2333–2345.
27. KoosDS, HoRK (1999) The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula. Dev Biol 215 : 190–207.
28. LeungT, BischofJ, SollI, NiessingD, ZhangD, et al. (2003) bozozok directly represses bmp2b transcription and mediates the earliest dorsoventral asymmetry of bmp2b expression in zebrafish. Development 130 : 3639–3649.
29. LeungT, SollI, ArnoldSJ, KemlerR, DrieverW (2003) Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastula-transition activation of boz expression. Dev Dyn 228 : 424–432.
30. YamanakaY, MizunoT, SasaiY, KishiM, TakedaH, et al. (1998) A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev 12 : 2345–2353.
31. RoH, DawidIB (2009) Organizer restriction through modulation of Bozozok stability by the E3 ubiquitin ligase Lnx-like. Nat Cell Biol 11 : 1121–1127.
32. RoH, DawidIB (2010) Lnx-2b restricts gsc expression to the dorsal mesoderm by limiting Nodal and Bozozok activity. Biochem Biophys Res Commun 402 : 626–630.
33. AlexanderJ, RothenbergM, HenryGL, StainierDY (1999) casanova plays an early and essential role in endoderm formation in zebrafish. Dev Biol 215 : 343–357.
34. CooperMS, D'AmicoLA (1996) A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol 180 : 184–198.
35. KikuchiY, AgathonA, AlexanderJ, ThisseC, WaldronS, et al. (2001) casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev 15 : 1493–1505.
36. BaillatD, HakimiMA, NäärAM, ShilatifardA, CoochN, et al. (2005) Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123 : 265–276.
37. RöpkeA, BuhtzP, BohmM, SegerJ, WielandI, et al. (2005) Promoter CpG hypermethylation and downregulation of DICE1 expression in prostate cancer. Oncogene 24 : 6667–6675.
38. WielandI, ArdenKC, MichelsD, Klein-HitpassL, BohmM, et al. (1999) Isolation of DICE1: a gene frequently affected by LOH and downregulated in lung carcinomas. Oncogene 18 : 4530–4537.
39. WielandI, RöpkeA, StummM, SellC, WeidleUH, et al. (2001) Molecular characterization of the DICE1 (DDX26) tumor suppressor gene in lung carcinoma cells. Oncology Research 12 : 491–500.
40. WielandI, SellC, WeidleUH, WieackerP (2004) Ectopic expression of DICE1 suppresses tumor cell growth. Oncology Reports 12 : 207–211.
41. DoschR, WagnerDS, MintzerKA, RunkeG, WiemeltAP, et al. (2004) Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell 6 : 771–780.
42. WagnerDS, DoschR, MintzerKA, WiemeltAP, MullinsMC (2004) Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell 6 : 781–790.
43. KimmelCB, BallardWW, KimmelS, UllmanB, SchillingTF (1995) Stages of Embryonic Development of the Zebrafish. Dev Dyn 203 : 253–310.
44. KobayashiM, ToyamaR, TakedaH, DawidIB, KawakamiK (1998) Overexpression of the forebrain-specific homeobox gene six3 induces rostral forebrain enlargement in zebrafish. Development 125 : 2973–2982.
45. KraussS, JohansenT, KorzhV, FjoseA (1991) Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development 113 : 1193–1206.
46. OxtobyE, JowettT (1993) Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res 21 : 1087–1095.
47. WeinbergES, AllendeML, KellyCS, AbdelhamidA, MurakamiT, et al. (1996) Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122 : 271–280.
48. MoriH, MiyazakiY, MoritaT, NittaH, MishinaM (1994) Different spatio-temporal expressions of three otx homeoprotein transcripts during zebrafish embryogenesis. Brain Res Mol Brain Res 27 : 221–231.
49. AlexandreD, ClarkeJD, OxtobyE, YanYL, JowettT, et al. (1996) Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acid-induced phenotype. Development 122 : 735–746.
50. KudohT, WilsonSW, DawidIB (2002) Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 129 : 4335–4346.
51. HildM, DickA, RauchGJ, MeierA, BouwmeesterT, et al. (1999) The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development 126 : 2149–2159.
52. SchmidB, FurthauerM, ConnorsSA, TroutJ, ThisseB, et al. (2000) Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127 : 957–967.
53. Schulte-MerkerS, LeeKJ, McMahonAP, HammerschmidtM (1997) The zebrafish organizer requires chordino. Nature. 387 : 862–863.
54. Dal-PraS, FurthauerM, Van-CelstJ, ThisseB, ThisseC (2006) Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol 298 : 514–526.
55. StachelSE, GrunwaldDJ, MyersPZ (1993) Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development 117 : 1261–1274.
56. RyuSL, FujiiR, YamanakaY, ShimizuT, YabeT, et al. (2001) Regulation of dharma/bozozok by the Wnt pathway. Dev Biol 231 : 397–409.
57. KellyGM, GreensteinP, ErezyilmazDF, MoonRT (1995) Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development 121 : 1787–1799.
58. JolyJS, JolyC, Schulte-MerkerS, BoulekbacheH, CondamineH (1993) The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development 119 : 1261–1275.
59. PyatiUJ, WebbAE, KimelmanD (2005) Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development 132 : 2333–2343.
60. RamelMC, BucklesGR, BakerKD, LekvenAC (2005) WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev Biol 287 : 237–248.
61. WengW, StempleDL (2003) Nodal signaling and vertebrate germ layer formation. Birth Defects Res C Embryo Today 69 : 325–332.
62. FeldmanB, GatesMA, EganES, DouganST, RennebeckG, et al. (1998) Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395 : 181–185.
63. BisgroveBW, EssnerJJ, YostHJ (1999) Regulation of midline development by antagonism of lefty and nodal signaling. Development 126 : 3253–3262.
64. ThisseC, ThisseB (1999) Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development 126 : 229–240.
65. MenoC, GritsmanK, OhishiS, OhfujiY, HeckscherE, et al. (1999) Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell 4 : 287–298.
66. Schulte-MerkerS, van EedenFJ, HalpernME, KimmelCB, Nusslein-VolhardC (1994) no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120 : 1009–1015.
67. HalpernME, ThisseC, HoRK, ThisseB, RigglemanB, et al. (1995) Cell-autonomous shift from axial to paraxial mesodermal development in zebrafish floating head mutants. Development 121 : 4257–4264.
68. TalbotWS, TrevarrowB, HalpernME, MelbyAE, FarrG, et al. (1995) A homeobox gene essential for zebrafish notochord development. Nature 378 : 150–157.
69. MorleyRH, LachaniK, KeefeD, GilchristMJ, FlicekP, et al. (2009) A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc Natl Acad Sci U S A 106 : 3829–3834.
70. DickmeisT, MourrainP, Saint-EtienneL, FischerN, AanstadP, et al. (2001) A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev 15 : 1487–1492.
71. MelbyAE, WargaRM, KimmelCB (1996) Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development 122 : 2225–2237.
72. von der HardtS, BakkersJ, InbalA, CarvalhoL, Solnica-KrezelL, et al. (2007) The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol 17 : 475–487.
73. MyersDC, SepichDS, Solnica-KrezelL (2002) Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol 243 : 81–98.
74. AgathonA, ThisseB, ThisseC (2001) Morpholino knock-down of antivin1 and antivin2 upregulates nodal signaling. Genesis 30 : 178–182.
75. ChenY, SchierAF (2002) Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol 12 : 2124–2128.
76. FeldmanB, ConchaML, SaudeL, ParsonsMJ, AdamsRJ, et al. (2002) Lefty antagonism of Squint is essential for normal gastrulation. Curr Biol 12 : 2129–2135.
77. AokiTO, MathieuJ, Saint-EtienneL, RebagliatiMR, PeyrierasN, et al. (2002) Regulation of nodal signalling and mesendoderm formation by TARAM-A, a TGFbeta-related type I receptor. Dev Biol 241 : 273–288.
78. PoulainM, LepageT (2002) Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish. Development 129 : 4901–4914.
79. ChenC, ShenMM (2004) Two modes by which Lefty proteins inhibit nodal signaling. Curr Biol 14 : 618–624.
80. EzzeddineN, ChenJ, WaltenspielB, BurchB, AlbrechtT, et al. (2011) A subset of Drosophila integrator proteins is essential for efficient U7 snRNA and spliceosomal snRNA 3′-end formation. Mol Cell Biol 31 : 328–341.
81. TaoS, CaiY, SampathK (2009) The Integrator subunits function in hematopoiesis by modulating Smad/BMP signaling. Development 136 : 2757–2765.
82. HanSM, LeeTH, MunJY, KimMJ, KritikouEA, et al. (2006) Deleted in cancer 1 (DICE1) is an essential protein controlling the topology of the inner mitochondrial membrane in C. elegans. Development 133 : 3597–3606.
83. HarveySA, SmithJC (2009) Visualisation and quantification of morphogen gradient formation in the zebrafish. PLoS Biol 7: e1000101.
84. ChenS, KimelmanD (2000) The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development 127 : 4681–4689.
85. GritsmanK, ZhangJ, ChengS, HeckscherE, TalbotWS, et al. (1999) The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell 97 : 121–132.
86. ShimizuT, YamanakaY, RyuSL, HashimotoH, YabeT, et al. (2000) Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech Dev 91 : 293–303.
87. GriffinK, PatientR, HolderN (1995) Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development 121 : 2983–2994.
88. Schulte-MerkerS, HammerschmidtM, BeuchleD, ChoKW, De RobertisEM, et al. (1994) Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development 120 : 843–852.
89. HarveySA, TumpelS, DubrulleJ, SchierAF, SmithJC (2010) no tail integrates two modes of mesoderm induction. Development 137 : 1127–1135.
90. BellipanniG, VargaM, MaegawaS, ImaiY, KellyC, et al. (2006) Essential and opposing roles of zebrafish beta-catenins in the formation of dorsal axial structures and neurectoderm. Development 133 : 1299–1309.
91. VargaM, MaegawaS, BellipanniG, WeinbergES (2007) Chordin expression, mediated by Nodal and FGF signaling, is restricted by redundant function of two beta-catenins in the zebrafish embryo. Mech Dev 124 : 775–791.
92. LachnitM, KurE, DrieverW (2008) Alterations of the cytoskeleton in all three embryonic lineages contribute to the epiboly defect of Pou5f1/Oct4 deficient MZspg zebrafish embryos. Dev Biol 315 : 1–17.
93. RebagliatiMR, ToyamaR, FrickeC, HaffterP, DawidIB (1998) Zebrafish nodal-related genes are implicated in axial patterning and establishing left-right asymmetry. Dev Biol 199 : 261–272.
94. LittleSC, MullinsMC (2009) Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11 : 637–643.
95. LiaoEC, ZonLI (1999) Simple sequence-length polymorphism analysis. Methods Cell Biol 60 : 181–183.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



