-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMultiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
Arabidopsis thaliana cryptochrome 2 (CRY2) mediates light control of flowering time. CIB1 (CRY2-interacting bHLH 1) specifically interacts with CRY2 in response to blue light to activate the transcription of FT (Flowering Locus T). In vitro, CIB1 binds to the canonical E-box (CACGTG, also referred to as G-box) with much higher affinity than its interaction with non-canonical E-box (CANNTG) DNA sequences. However, in vivo, CIB1 binds to the chromatin region of the FT promoter, which only contains the non-canonical E-box sequences. Here, we show that CRY2 also interacts with at least CIB5, in response to blue light, but not in darkness or in response to other wavelengths of light. Our genetic analysis demonstrates that CIB1, CIB2, CIB4, and CIB5 act redundantly to activate the transcription of FT and that they are positive regulators of CRY2 mediated flowering. More importantly, CIB1 and other CIBs proteins form heterodimers, and some of the heterodimers have a higher binding affinity than the CIB homodimers to the non-canonical E-box in the in vitro DNA-binding assays. This result explains why in vitro CIB1 and other CIBs bind to the canonical E-box (G-box) with a higher affinity, whereas they are all associated with the non-canonical E-boxes at the FT promoter in vivo. Consistent with the hypothesis that different CIB proteins play similar roles in the CRY2-midiated blue light signaling, the expression of CIB proteins is regulated specifically by blue light. Our study demonstrates that CIBs function redundantly in regulating CRY2-dependent flowering, and that different CIBs form heterodimers to interact with the non-canonical E-box DNA in vivo.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003861
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003861Summary
Arabidopsis thaliana cryptochrome 2 (CRY2) mediates light control of flowering time. CIB1 (CRY2-interacting bHLH 1) specifically interacts with CRY2 in response to blue light to activate the transcription of FT (Flowering Locus T). In vitro, CIB1 binds to the canonical E-box (CACGTG, also referred to as G-box) with much higher affinity than its interaction with non-canonical E-box (CANNTG) DNA sequences. However, in vivo, CIB1 binds to the chromatin region of the FT promoter, which only contains the non-canonical E-box sequences. Here, we show that CRY2 also interacts with at least CIB5, in response to blue light, but not in darkness or in response to other wavelengths of light. Our genetic analysis demonstrates that CIB1, CIB2, CIB4, and CIB5 act redundantly to activate the transcription of FT and that they are positive regulators of CRY2 mediated flowering. More importantly, CIB1 and other CIBs proteins form heterodimers, and some of the heterodimers have a higher binding affinity than the CIB homodimers to the non-canonical E-box in the in vitro DNA-binding assays. This result explains why in vitro CIB1 and other CIBs bind to the canonical E-box (G-box) with a higher affinity, whereas they are all associated with the non-canonical E-boxes at the FT promoter in vivo. Consistent with the hypothesis that different CIB proteins play similar roles in the CRY2-midiated blue light signaling, the expression of CIB proteins is regulated specifically by blue light. Our study demonstrates that CIBs function redundantly in regulating CRY2-dependent flowering, and that different CIBs form heterodimers to interact with the non-canonical E-box DNA in vivo.
Introduction
Cryptochromes are photolyase-like photoreceptors regulating photomorphogenesis in plants and the circadian clock in plants and animals [1]–[4]. The Arabidopsis thaliana genome encodes at least two cryptochromes, cryptochrome 1 (CRY1) and cryptochrome 2 (CRY2). The major function of CRY1 is to mediate blue light-dependent de-etiolation responses [5], whereas CRY2 mediates primarily photoperiodic regulation of floral initiation [6]. Cryptochromes may mediate photoperiodic control of floral initiation by at least three different mechanisms: 1. Cryptochromes mediate light suppression of the COP1-dependent degradation of CONSTANS (CO) [7]–[9], which is a major transcription regulator of floral initiation. CO is a critical positive regulator of flowering in long day condition, CO promotes the flowering initiation by activating transcription of the florigen gene FT [10], which encodes a mobile transcriptional regulator that migrates from leaves to the apical meristem to activate transcription of floral meristem identity genes [11], [12]. 2. Cryptochromes regulate the light entrainment of the circadian clock [13], and then affect the expression of CO. 3. Cryptochromes directly modulate the transcription of FT through interaction with CIB1, a basic-helix-loop-helix (bHLH) transcription factor, which was isolated in a blue light differentiated yeast-two-hybrid screen [14].
In Arabidopsis, at least three types of photoreceptors, crypto!chromes, the LOV-domain/F-box proteins FKF/ZTL, and phytochromes, are involved in the control of overlapping physiological functions essential to plant development, such as de-etiolation and photoperiodic flowering. Direct interaction between photoreceptors and their respective target proteins have been recognized as a fundamental mechanism underlying the signal transduction of those photoreceptors. Light-dependent protein-protein interaction has been demonstrated for phytochromes, FKF/ZTL and cryptochromes. For example, phytochromes interact with several target proteins with a wavelength preference, including a nucleoside diphosphate kinase (NDPK2), a protein phosphotase (PAPPs), a response regulator (ARR4), and several bHLH transcription factors (PIF proteins), to modulate phytochrome function and regulation [15]–[21]. The FMN-containing blue light receptors, FKF1 and ZTL, interact with a clock protein, GI, in a blue light-dependent manner to control the stability of their targets, CDF and TOC1, as well as the circadian rhythmic transcription and photoperiodic flowering [22]–[26]. Similarly, Arabidopsis CRY2 undergoes blue light-specific interaction with CIB1 and also SPA1 [9], [14].
Arabidopsis CIB1 is the first blue light-dependent CRY2-interacting protein identified in plants [14], [27], [28]. CIB1 positively regulates floral initiation in a CRY2-dependent manner. CIB1 binds to the canonical E-box (CACGTG, G-box) in vitro with a much higher affinity than to non-canonical E-box elements (CANNTG), but it appears to affect transcription, with similar activities, of promoters containing canonical or non-canonical E-box in vivo. It was shown in a transient Arabidopsis transcription assay that CIB1 acted as a CRY - and blue light-dependent transcription regulator, and the in vivo transcriptional regulation activity of CIB1 seems indiscriminatory toward canonical and non-canonical E-boxes. CIB1 stimulates FLOWERING LOCUS T (FT) messenger RNA expression. It interacts with the chromatin DNA of the FT gene that lacks a canonical E-box but contains various non-canonical E-box elements. These results suggest a significant difference between the CIB1 DNA-binding activity in vitro and its transcription regulatory activity in vivo. One possible interpretation of this dilemma would be that CIB1 heterodimerizes with other bHLH proteins to alter their preference or affinity to different DNA sequences in vivo.
In this study, we performed a systematic biochemical and genetic analysis to isolate additional members of the bHLH family related to CIB1, and found that at least three additional CIB1-related bHLH proteins, referred to as CIB2, CIB4, and CIB5, can interact with CRY2 and/or CIB1. CIBs function redundantly to activate the transcription of FT and flowering initiation. More importantly, when added individually in vitro they all exhibit higher binding affinity for the canonical E-box (G-box), but they undergo a switch in preference for the non-canonical E-box of the FT promoter when combined. This is presumably due to a switch from homodimerization to heterodimerization. These results suggest that multiple CIB proteins act redundantly in the CRY2-CIB signal transduction pathway to mediate promotion of floral initiation. Consistent with our hypothesis, CIBs are specifically involved in CRY2 signaling, the expression of CIBs proteins is regulated specifically by blue light.
Results
Multiple bHLH proteins demonstrate CRY2-dependent activity of promoting floral initiation
Overexpression of CIB1 results in accelerated flowering in the wild-type background but not in the cry1cry2 mutant background, demonstrating that the floral promotion activity of CIB1 is dependent on cryptochromes. However, the monogenic cib1 mutant shows no phenotypic alterations, whereas the cib1cib5 double mutant flowers slightly later than the wild type plants in a specific condition [14], suggesting that CIB1 acts together with additional CIB1-related proteins to promote CRY2-dependent floral initiation. In order to isolate additional CIB1-related proteins that are involved in floral initiation, we first peformed a phylogenetic analysis (Figure S1A), and found out that 6 out of 17 members of the bHLH subfamily 18, are more closely related to CIB1. We then examined their ability to interact with CRY2. 4 of the 6 CIB1-related bHLH proteins examined (CIB2-At5g48560, CIB3-At3g07340, CIB4-At1g10120, CIB5-At1g26260) interacted with CRY2 in vitro in a pull-down assay (Figure S1B). The two that do not interact with CRY2 were named CIL1 (At1g68920) and CIL2 (At3g23690) (CIB1 Like protein). The T-DNA insertion mutations are available for 4 of the 6 CIB1-related genes (cib2, cib3, cib5 and cil1), but none of these monogenic mutants showed apparent phenotypic alterations (data not shown). Transgenic plants expressing 35S::MycCIB2, 35S::MycCIB4, 35S::MycCIB5, and 35S::MycCIL1 flowered significantly earlier than the wild type parents in long day condition, while transgenic plants expressing 35S::MycCIB3 and 35S::MycCIL2 showed no obvious flowering phenotype (Figure 1A–H, Figure S2A–D). Furthermore, in contrast to transgenic plants overexpressing CIBs in the wild-type background that flowered significantly earlier than the parent, transgenic plants overexpressing CIB4 and CIB5 in the cry1cry2 mutant background flowered at the same time as the cry1cry2 parent in the long day condition (Figure 1 I–J), suggesting that the activity of these CIBs on floral initiation depend on CRY2 and that they also act as CRY2-signaling proteins.
Fig. 1. Multiple bHLH promote flower initiation in long day condition. 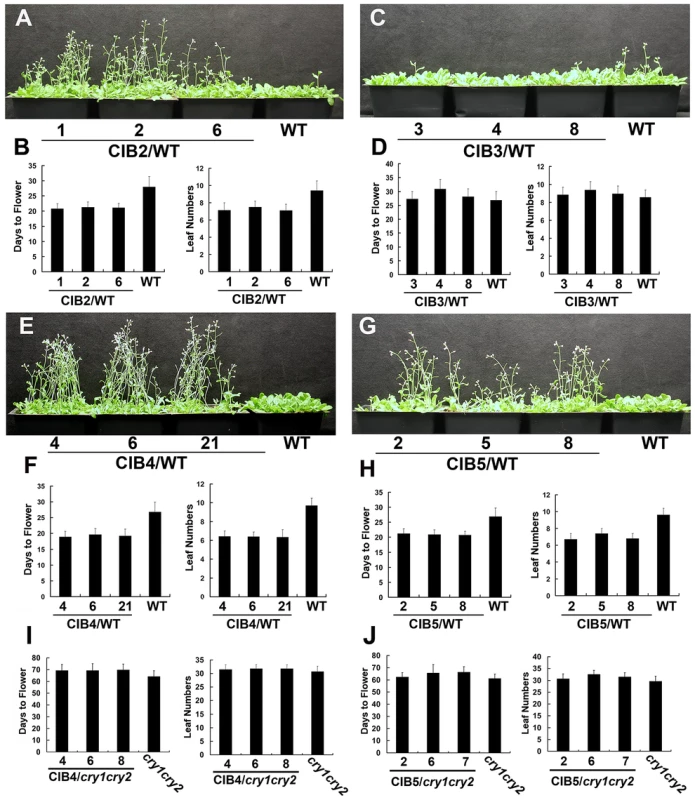
(A–H) Flowering phenotype of different transgenic lines in long day. Three independent overexpress lines expressing 35S::Myc-CIB2 (A–B), 35S::Myc-CIB3 (C–D), 35S::Myc-CIB4 (E–F), 35S::Myc-CIB5 (G–H) and the WT control were grown in LD (16-h light/8-h dark) for 23 days when the pictures were taken. The quantitative flowering times measured as days to flower and the number of rosette leaves at the day floral buds became visible, and the standard deviations (n≥20) are shown. (I–J) Three independent overexpress lines expressing 35S::Myc-CIB4 (I) or 35S::Myc-CIB5 (J) in cry1cry2 background and the cry1cry2 control were grown in LD (16-h light/8-h dark). The quantitative flowering times measured as days to flower and the number of rosette leaves at the day floral buds became visible, and the standard deviations (n≥20) are shown. CIB proteins promote flowering redundantly by activating FT mRNA expression
Considering the functional redundancy of these CIBs, we generated dominant repressor versions of CIB1, CIB4 and CIB5 using chimeric repressor silencing technology, in which CIB1, CIB4, CIB5 were fused to a 12-amino acid EAR motif, which serves as a very strong repressor domain [29]. In contrast to the Myc-CIB1 overexpression plants [14], expression of Myc-CIB1-EAR under the drive of 35S promoter resulted in a marked delayed flowering phenotype (Figure 2C–D), which suggests that CIB1 functions as a transcription activator for flowering and FT expression. The fusion of the EAR motif does not affect the interaction between CRY2 and CIB1, as they still interact with each other in a blue light dependent manner (Figure S3). For the yeast two-hybrid assay, cells expressing both CRY2 and CIB1-EAR showed fluence rate-dependent increase of the β-gal activity after normalization for cell number. Yeast cells exposed to higher fluence rate of blue light for the same duration of irradiation exhibited higher β-gal activity (Figure S3A), suggesting a more robust interaction of CRY2 and CIB1-EAR under stronger light. As expected, cells irradiated with blue light of the same fluence rate but for a longer duration of irradiation also exhibited higher β-gal activity (Figure S3A). Then we examined the CRY2 and CIB1-EAR complex formation in plants expressing MycCIB1-EAR, with a coimmunoprecipitation (co-IP) assay. Seedlings were pre-treated with the proteasome inhibitor MG132 to block blue light dependent CRY2 degradation [30]. Samples were then exposed to red light, or blue light (20 µmol m−2 s−1), and subjected to co-IP analyses. CIB1-EAR was co-precipitated with CRY2 in samples irradiated with blue light but not red light (Figure S3B). Blue light stimulates the accumulation of the CRY2-CIB1-EAR complex in plant cells, like it does with the CRY2-CIB1 complex. Transgenic plants expressing 35S::Myc-VP16-CIB1, in which CIB1 was fused to the VP16 activation motif, show an early flowering phenotype (Figure 2A–B), as observed by overexpression of CIB1, while expression of 35S::Myc-CIB4-EAR or 35S::Myc-CIB5-EAR also leads to a dramatic late flowering phenotype (Figure S2E–H), as observed with Myc-CIB1-EAR, which confirms that these CIBs function as transcription activators in regulating flowering.
Fig. 2. CIB proteins promote flowering redundantly by activating FT mRNA expression. 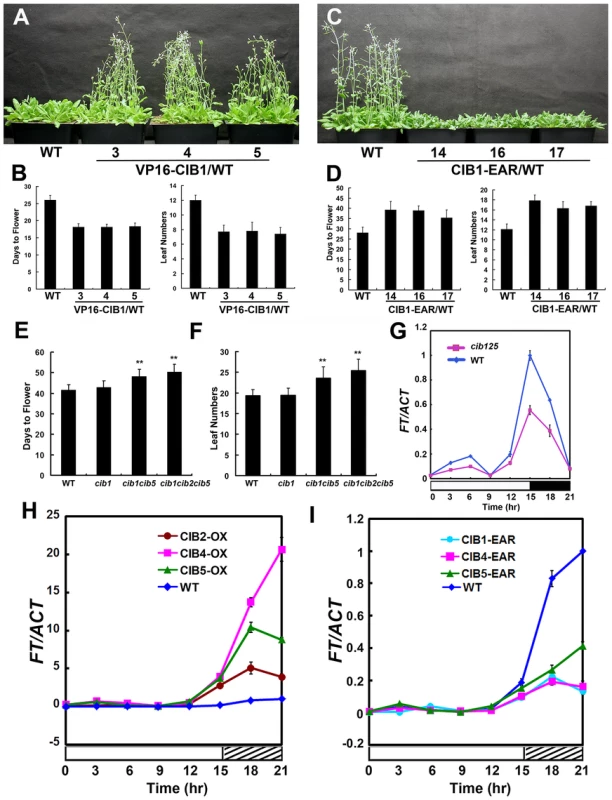
(A–D) Flowering phenotype in long day. Three independent 35S::VP16-Myc-CIB1 (A–B) transgenic lines and the WT control were grown in long day for 23 days when the picture was taken. 35S::Myc-CIB1-EAR (C–D) plants and the WT control were grown in long day for 33 days when the picture was taken. The quantitative flowering times measured as days to flower and the number of rosette leaves at the day floral buds became visible, and the standard deviations (n≥20) are shown. (E–F) The cib15 double and the cib125 triple mutant showed a mild but statistically significant delay of flowering under a photoperiodic inductive condition. Plants were grown in short-day photoperiod (9 hL/15 hD) for 20 days, transferred to long-day photoperiod (16 hL/8 hD) for 4 days, and moved back to short-day to continue grow until flowering. Days from sawing to flowering and number of rosette leaves at the time of flowering are shown with the standard deviations (n>20). (G) A comparison of the FT mRNA expression in the cib1cib2cib5 triple mutant and the wild type. Plants were grown in short-day photoperiod (9 hL/15 hD) for 20 days and transferred to long-day photoperiod (16 hL/8 hD) for 4 days, samples were collected every 3 hr for 24 hr in the fourth day of long day at the time indicated for the qPCR analysis. (H–I) Quantitative PCR results showing mRNA expression of FT in the wild type (WT), transgenic lines expressing the 35S::Myc-CIB2, 35S::Myc-CIB4, 35S::Myc-CIB5 or 35S::Myc-CIB1-EAR, 35S::Myc-CIB4-EAR, 35S::Myc-CIB5-EAR transgene in the wild-type background grown in long-day (16 hL/8 hD) for 6 days then moved to continue white light for one day. Samples were collected every 3 hr for 24 hr in the continuous white light. Each experiment was performed at least three times with similar results. To further test the genetic redundancy among these bHLH genes, cib2, cib5, cil1 mutant alleles were isolated (Material Method and Figure S4). We got cib4 (SALK_027284) seeds from ABRC, but none of the seeds have a T-DNA insertion even though the seeds were ordered twice. Plants carrying different combinations of mutations were constructed. The cib125 triple mutant showed a statistically significant delay of flowering under the photoperiodic induction condition [8], [14] (Figure 2E–F).
Transgenic plants overexpressing CIB2, CIB4 or CIB5 exhibited elevated mRNA expression of the flowering-time gene FT (Figure 2H), while cib125 triple mutant (Figure 2G) or transgenic plants overexpressing CIB1-EAR, CIB4-EAR, CIB5-EAR all exhibited decreased expression of FT (Figure 2I). We conclude that CIBs promote flowering redundantly by activating FT mRNA expression.
Blue light-dependent CRY2-CIB5 interaction in plant cells
CIB2, CIB4, and CIB5 can all interact with CRY2 in vitro (Figure S1) and they are nuclear proteins. CIB2-YFP, CIB4-YFP and CIB5-YFP can all be detected in the nucleus in tobacco, and the green fluorescence of CIB2-YFP, CIB4-YFP, CIB5-YFP co-localizes with the red fluorescence of CRY2-mCherry, especially in the photobodies (Figure 3A). We examined CRY2-CIBs interaction in plant cells using the BiFC (Bimolecular fluorescence complementation) assay [31], [32], [33]. In tobacco leaf epidermal cells coexpressing the C-terminal half of CFP fused to CRY2 (cCFP–CRY2) and the N-terminal half of YFP fused to CIB1 (nYFP–CIB1), or N-terminal half of YFP fused to CIB2 (nYFP-CIB2), or nYFP-CIB5, strong YFP fluorescence was observed (Figure 3B). In contrast, no YFP signal was observed when cCFP–CRY2 and nYFP-CIB4 or no-fusion nYFP (Figure 3B), or nYFP-CIB1/2/4/5 and no-fusion cCFP, were cotransformed (data not shown). CIB2 and CIB5 but not CIB4 interact with CRY2 in planta even though all of the three interact with CRY2 in vitro. To further examine the in vivo interaction of CIB5 and CRY2, co-IP was applied. Seedlings were pre-treated with the proteasome inhibitor MG132 to block blue light dependent CRY2 degradation [30]. Samples were then exposed to red light, white light, or blue light (20 µmol m−2 s−1), and subjected to co-IP analyses. CIB5 was co-precipitated with CRY2 in samples irradiated with white light or blue light but not red light (Figure 3C). We conclude that CIB2 and CIB5 interact with CRY2 in vivo and at least CIB5 can undergo blue light-dependent physical interaction with CRY2 like CIB1. We did not detect the in vivo interaction of CRY2 and CIB4, even that CIB4 exhibited CRY2 dependent activity on floral initiation, one possibility is that CIB4 interact with CIB1 or CIB5 or other CRY2 interacting bHLH proteins to form heterodimer to regulate flowering downstream of CRY2.
Fig. 3. Blue light-dependent CRY2-CIB5 interaction in plant cells. 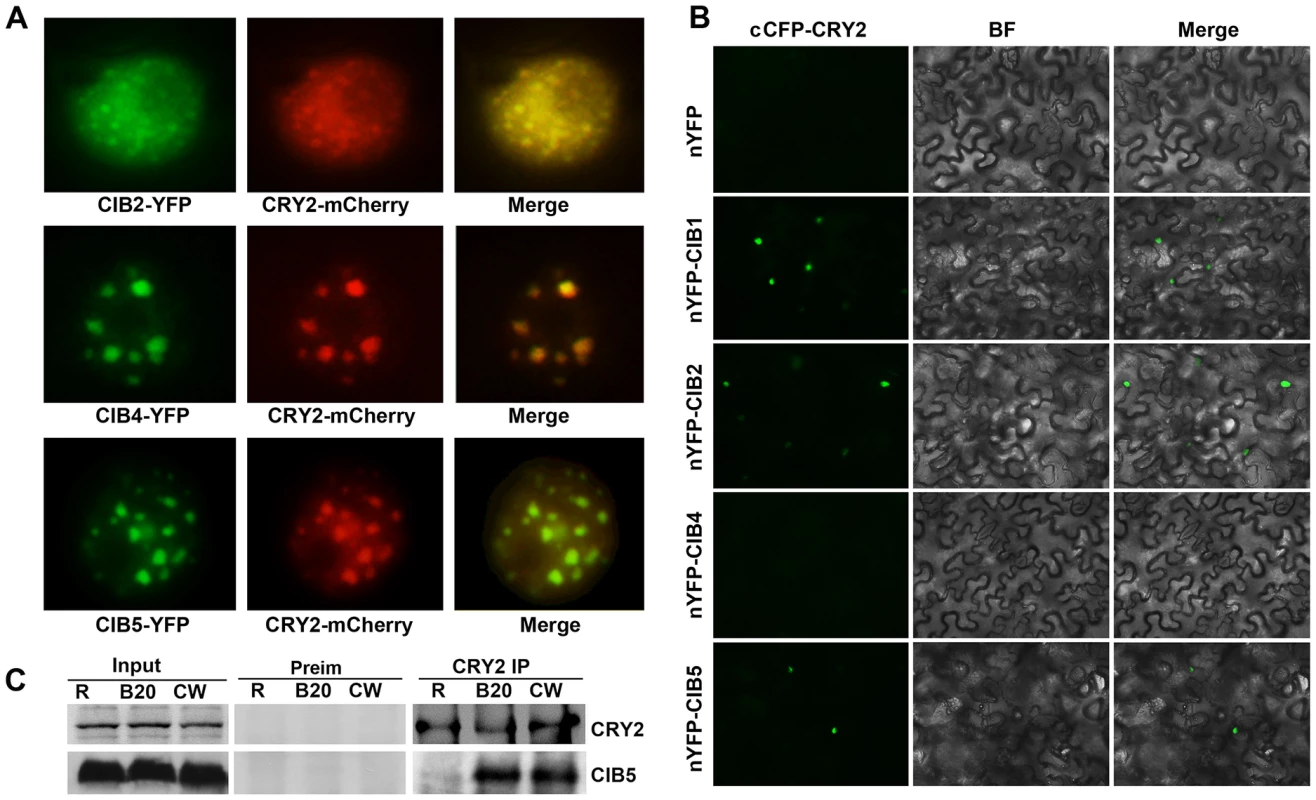
(A) Fluorescent microscopy images showing that CIB3, CIB4 and CIB5 (Green) all co-localize with CRY2 (Red) in the nucleus. (B) Bimolecular fluorescence complementation assays of the in vivo protein interaction. Leaf epidermal cells of N. benthamiana were cotransformated with cCFP–CRY2 and nYFP, or nYFP-CIB1, or nYFP-CIB2, or nYFP-CIB4, or nYFP-CIB5. BF, Bright Field; Merge, overlay of the YFP and bright field images. (C) The co-immunoprecipitation assay showing the blue light dependent CRY2-CIB5 interaction in planta. Co-IP assays of samples prepared from 12-day-old 35S::MycCIB5 seedlings grown in continuous red light, pre-treated in MG132, then exposed to white light (W), or red light (R), or blue light (B, 20 µmol m−2 s−1, 20 min). Total proteins (Input) or IP product of anti-CRY2 antibody (CRY2-IP) or preimmune serum (Preim) were probed, in immunoblots, by the anti-CRY2 antibody (CRY2), stripped and reprobed by the anti-Myc (MycCIB1) antibody. CIB4 and CIB5 associate with the chromatin regions of the FT gene
CIB1 interacts with the chromatin DNA of the FT gene that possesses various non-canonical E-box elements but no canonical E-boxes. CIB2, CIB4, and CIB5 work redundantly with CIB1 to promote flowering by activating FT expression. We therefore examined whether CIB4 and CIB5 might interact with the FT gene as CIB1 does, using the ChIP-qPCR and Chip-PCR assay. Both the Chip-qPCR (Figure 4B–C) and Chip-PCR (Fig. S5A–C) show that in vivo, CIB4 and CIB5 are associated with the same chromatin region of the FT promoter (region c) as CIB1, which contains non-canonical E-box sequences (CAAGTG, CACCTG). Given that CRY2 control of FT transcription took place primarily in the vascular bundle cells [34], we also tested whether CIB2, CIB4 and CIB5 were expressed in the vascular bundle cells. Analyses of the GUS (β-glucuronidase) reporter expression in transgenic plants expressing GUS under control of the CIB2, CIB4 or CIB5 promoter demonstrated that these promoters were active in the vascular bundle cells (Figure 4A). Finally, we analyzed the transcription activity of CIBs on the FT promoter. A transient transcription assay in tobacco leaves was used. We used a dual-LUC reporter plasmid that encodes a firefly luciferase (LUC) driven by the FT promoter (−2000 bp to 0 bp) and a Renilla luciferase (REN) driven by the constitutive 35S promoter (Figure 4D) [14], [35]. Our result indicates that CIB1, CIB2, CIB4 or CIB5 all can promote the transcription of the FT promoter-LUC gene (Figure 4E). These results support our hypothesis that CIBs interact with the non-canonical E-box regulatory elements of the FT gene, whereas CRY2 interacts with at least CIB1 and CIB5 in response to blue light to affect FT transcription and floral initiation.
Fig. 4. ChIP-qPCR showing interaction of CIB4 and CIB5 with chromatin regions of the FT gene. 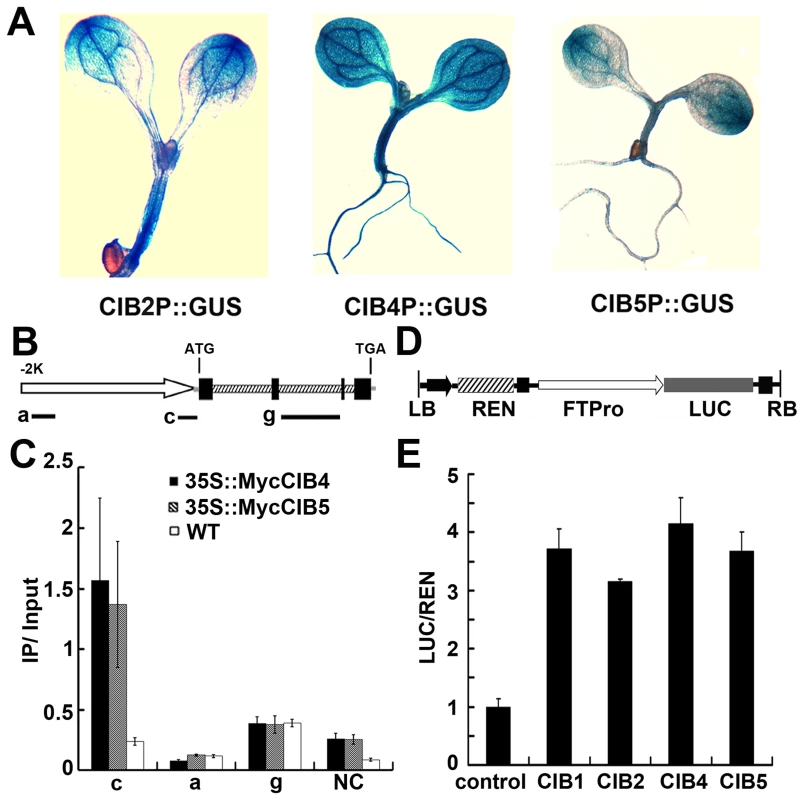
(A) GUS staining of seedlings expressing CIB2::GUS, CIB4::GUS, CIB5::GUS transgene. (B) A diagram depicting the putative promoter (arrow), 5′ UTR (grey line), exons (black boxes), introns (dashed boxes), 3′ UTR (grey line) of the FT gene. Black solid lines depict the DNA regions that were amplified by ChIP-PCR using the indicated primer sets. (C) Representative results of the ChIP-qPCR assays. Chromatin fragments (∼500 bp) were prepared from 7-day-old transgenic seedlings expressing 35S::Myc-CIB4 or 35S::Myc-CIB5, immunoprecipitated by the anti-Myc antibody, and the precipitated DNA were qPCR-analysised using the primer pairs indicated. The IP/input ratios are shown with the standard deviations (n≥3). (D) Structure of the FT promoter–driven dual-Luc reporter gene. 35S promoter (black arrow), FT promoter (−2000 bp–0 bp) (white arrow head), REN luciferase (REN), firefly luciferase (LUC), and T-DNA (LB and RB) are indicated. (E) Relative reporter activity (LUC/REN) in planta with different effectors (CIB1/2/4/5) expression. Control: transiently expressed reporter only, CIB1: transiently expressed reporter and CIB1, CIB2: reporter and CIB2, CIB4: reporter and CIB4, CIB5: reporter and CIB5. Tobacco leaves were transfected with the reporter and the effector (CIB1 or CIB2 or CIB4 or CIB5); kept in white light for 3 days. The relative LUC activities normalized to the REN activity are shown (LUC/REN, n = 3). CIB1 can heterodimerize with other CIBs
The bHLH factors can form homo - or heterodimers to bind to specific DNA motifs, such as the canonical E-box (CACGTG) or the non-canonical E-box (CANNTG) [36]. CIB1 binds to the canonical E-box in vitro with a higher affinity than with other non-canonical E-box elements. However, CIB1 binds to the chromatin region of the FT promoter in vivo, which only contains the non-canonical E-box but not the canonical E-box [14]. We hypothesized that CIB1 works redundantly with other CIB1-related bHLH proteins, and different CIB proteins may heterodimerize to interact with the non-canonical E-box DNA in vivo. We already showed that CIB proteins promote flowering redundantly by activating FT mRNA expression, and CRY2 interacts with at least CIB1 and CIB5 in response to blue light to affect FT transcription and floral initiation. To further test whether CIB1 can form heterodimers with CIB2, CIB4 and CIB5, we first checked the co-localization of CIB1 and CIB2, CIB4, CIB5. The green fluorescence of CIB2-YFP, CIB4-YFP, CIB5-YFP co-localizes with the red fluorescence of CIB1-mCherry (Figure 5A). We used the bimolecular fluorescence complementation assay to check the interaction of CIB1 with CIBs [31], [32], [33]. In tobacco leaf epidermal cells coexpressing the C-terminal half of CFP fused to CIB1 (cCFP–CIB1) and the N-terminal half of YFP fused to CIB1 (nYFP–CIB1), or N-terminal half of YFP fused to CIB2 (nYFP-CIB2), or nYFP-CIB4 or nYFP-CIB5, strong YFP fluorescence was observed (Figure 5B). In contrast, no YFP signal was observed when cCFP–CIB1 and no-fusion nYFP (Figure 5B), or nYFP-CIB1/2/4/5 and no-fusion cCFP, were cotransformed (data not shown). These indicate that CIB1 can form heterodimer with CIB2, CIB4 and CIB5 in planta.
Fig. 5. CIB1 interacts with CIBs. 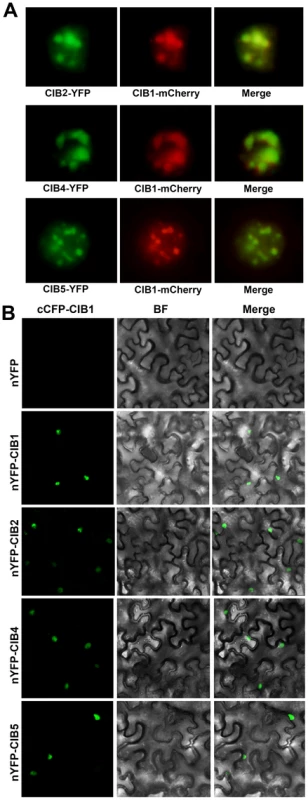
(A) Fluorescent microscopy images showing that CIB2, CIB4 and CIB5 (Green) all co-localize with CIB1 (Red) in the nucleus. (B) Bimolecular fluorescence complementation assays of the in vivo protein interaction. Leaf epidermal cells of N. benthamiana were cotransformated with cCFP–CIB1 and nYFP [31], or nYFP-CIB1, or nYFP-CIB2, or nYFP-CIB4, or nYFP-CIB5. BF, Bright Field; Merge, overlay of the YFP and bright field images. CIB heterodimers bind to the non-canonical E-box sequence of the FT promoter in vitro
CIB1 binds to the canonical E-box (G-box) with highest binding affinity in vitro, although it shows similar binding affinity to both canonical and non-canonical E-boxes in vivo. The specificity of the interaction between CIB2, CIB4, CIB5 and the canonical E-box were verified using electrophoretic mobility shift assays (EMSAs). The result confirmed that they all bind specifically to the canonical E-box in vitro (Fgiure 6A–C), and a single-nucleotide mutation within the canonical E-box sequence significantly reduced the ability of the mutant DNA to interact with CIB2, CIB4, and CIB5 (Figure S6A–C). CIB1, CIB2, CIB4, and CIB5 all bind to the canonical E-box in vitro with a higher affinity than their interaction with other non-canonical E-box DNA sequence. However, CIB1, CIB4, and CIB5 all associated with the FT promoter in vivo, which lacks a canonical E-box but contains several non-canonical E-boxes (CAAGTG, CACCTG). We hypothesized that different CIBs may form heterodimers to interact with the non-canonical E-box DNA in vivo. We already showed that CIB1 can heterodimerize with CIBs. To further test this hypothesis, we did an in vitro EMSA assay by using different combination of the CIB proteins. As we expected, the CIB1–CIB3, CIB1–CIB4, CIB2–CIB4, CIB2–CIB5, and CIB4–CIB5 heterodimers all bind to the non-canonical E-box sequence of the FT promoter (−334 to −311 bp, sequence:AGTGGCTACCAAGTGGGAGATATA), while CIB1–CIB5 does not (Figure 6D). The combination of CIB1 with CIB2 or CIB4 did not significantly change the binding affinity of CIB1 to canonical E-box since the binding affinity of CIB1 to canonical E-box is already very high (Figure S6D–E). To further test our hypothesis, the transient dual-LUC assay was employed again. The FTpro-LUC (Figure 4E) reporter was infiltrated into tobacco leaves together with Agrobacteria cells harboring one CIB (either CIB1, CIB2, CIB4 or CIB5) or with half the amount of CIB1 plus half the amount of CIB2, or CIB4 or CIB5. The expression level of FT promoter-LUC is about two times higher when CIB1 was combined with CIB2 or CIB4 than when only one CIB protein was infiltrated, even though the same amount of Agrobacteria cells were infiltrated (Figure 6 F–G). The transcription of the reporter is increased about 30% percent with the combination of CIB1 and CIB5 (Figure 6H). All these results indicate that although CIB proteins have the highest affinity for the canonical E-box in vitro, the heterodimers of different CIBs proteins bind the non-canonical E-box elements both in vivo and in vitro.
Fig. 6. CIB heterodimers bind to the non-canonical E-box sequence of the FT promoter. 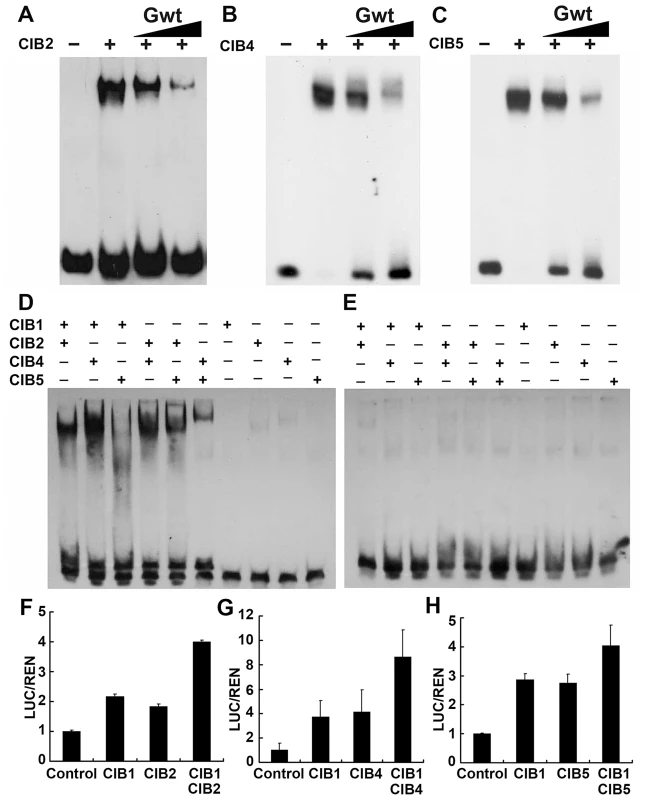
(A–C) Competitive electrophoretic mobility shift assay (EMSA) showing binding of CIB2 (A), CIB4 (B), and CIB5 (C) to the G-box DNA (canonical E-box) in vitro. Relative amounts of the un-labeled competitive oligonucleotide containing the G-box sequence used in the reactions are indicated on the top. (D–E) An EMSA experiment showing association of the CIB heterodimers, but not monomers, with the non-canonical E-box DNA of the FT promoter (region c in Figure 4B). The indicated CIB proteins were expressed and purified from E. coli, and incubated with the labeled oligonucleotide containing the E-box DNA of the FT promoter (D) or the same sequence except that the E box was replaced with AAAAAA sequence (E) (F–H) Transient assays show CIBs (CIB1/2/4/5) activation of the FTpro::LUC reporter gene. (F) Control: transiently expressed reporter only, CIB1: reporter and CIB1, CIB2: reporter and CIB2, CIB1 CIB2: reporter, CIB1 and CIB2 together. (G) CIB4: reporter and CIB4, CIB1 CIB4: reporter, CIB1 and CIB4. (H) CIB5: reporter and CIB5, CIB1CIB5: reporter, CIB1 and CIB5. Tobacco leaves were transfected with the reporter and the effectors; kept in white light for 3 days. The relative LUC activities normalized to the REN activity are shown (LUC/REN, n = 3). Error bars indicate SD of three biological repeats. CIBs proteins are regulated exclusively by blue light
As we know, most of the proteins involved in light signal transduction are light regulated, such as CRY2 protein which gets degraded under blue light [4], [37], PHYA protein undergoes rapid degradation in red light [38], and PIFs get degraded in the presence of red light [39]. Consistent with the hypothesis that CIBs act as CRY2-signaling proteins, they are also blue light regulated. Similar to CIB1 [40], the expression of CIB2, CIB4, and CIB5 proteins are regulated by blue light in a wavelength-specific manner. To study the regulation of these proteins, we used transgenic plants expressing either the Myc-tagged CIB4 or Myc-tagged CIB5 fusion protein, which are controlled by the constitutive 35S promoter (35S:Myc-CIB4, 35S:Myc-CIB5), and the luciferase tagged CIB1, CIB2, CIB4, or CIB5 fusion proteins which are under the control of the 35S constitutive promoter (35S:LUC-CIB1, 35S:LUC-CIB2, 35S:LUC-CIB4, 35S:LUC-CIB5). For unknown reasons, neither of the Myc-CIB2 or Flag-CIB2 fusion proteins was detected by the immunoblots, although they all showed early flowering phenotype and overexpressed mRNA. The immunoblot experiments showed that CIB4 and CIB5 proteins were barely detectable in plants grown in the dark or red light, but they started to accumulate soon after plants were exposed to blue light (Figure 7A, Figure S7). While abundant CIB4 and CIB5 proteins were detected in plants exposed to blue light, the CIB4, CIB5 protein level decreased after plants were transferred from blue light to dark, red light, or far-red light (Figure 7A, Figure S7). Results of the luciferase activity analysis corroborated with the immunoblot analysis. LUC-CIB1, LUC-CIB2, LUC-CIB4, LUC-CIB5 fusion proteins all get degraded after the plants were moved from blue light to dark condition in two different luciferase assays, the planta bioluminescence assay (Figure 7C) and the luciferase assay of plant extracts (Figure 7D). Treatment of Myc-CIB4 and Myc-CIB5 OX seedlings with the 26S proteasome inhibitor MG132 prevented the decline of CIB4 and CIB5 proteins abundance in the absence of blue light (Figure 7B). EAR fusion does not affect protein degradation since Myc-CIB1-EAR fusion protein also gets degraded without blue light but is accumulated in the blue light condition (Figure S7). These results demonstrate that like CIB1, in the absence of blue light, CIB2, CIB4 and CIB5 are also degraded by the 26S proteasome, and that blue light suppresses their degradation.
Fig. 7. Immunoblots and luciferase assays showing light regulation of CIBs protein expression. 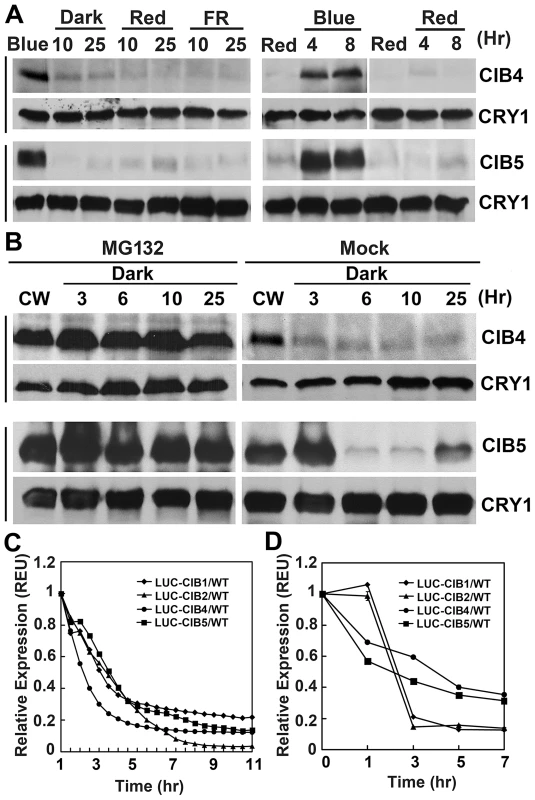
(A) Transgenic plants expressing the 35S::Myc-CIB4 and 35S::Myc-CIB5 transgenes were grown in long day for 3 weeks, treated with blue light (Blue) for 16 hr, and transferred to dark (Dark), red light (Red, 20 µmol m−2 s−1), or far red light (FR, 5 µmol m−2 s−1) for the indicated time (Left). Alternatively, the 3-week-old plants were first treated with red light for 16 hr (Red), and transferred to blue light (Blue, 35 µmol m−2 s−1) or kept in red light (Red, 20 µmol m−2 s−1) for the indicated time. (B) Immunoblot showing the inhibition of CIB4 and CIB5 degradation in darkness by the proteasome inhibitor MG132. Plants expressing the 35S::Myc-CIB4 or 35S::Myc-CIB5 transgenes were grown in continuous white light (CW) for 3 weeks, leaves were excised and incubated with MG132 (50 µmol/L) or mock solution (0.1% DMSO) in darkness for the indicated time. (C–D) A luciferase assay showing decreased levels of LUC-CIB1, LUC-CIB2, LUC-CIB4, and LUC-CIB5 fusion proteins in the absence of blue light. Transgenic Arabidopsis seedlings expressing the indicated LUC-fusion CIB proteins were grown in continuous blue light for 7 days, and transferred to dark (C) or red light (D) for the indicated time. The luciferase activity was measured by a CCD camera (C) or by a luminometer (D). For (C)), the bioluminescence/20 seedlings were measured by a CCD camera and shown after background subtraction. For (D), the relative levels of LUC activity (REU) was calculated by the formula [LUCRed/mgRed]/[LUCBlue/mgBlue]. LUCBlue and LUCRed: luciferase activity of dark- or blue light-treated samples, mgRed and mgBlue: total proteins (mg) of dark- or blue light-treated samples. Discussion
We investigated the function and biochemical mechanisms of 3 CIB1-related proteins, CIB2, CIB4, and CIB5 in this study. We showed that CIB1, CIB2, CIB4, and CIB5 function redundantly to activate the transcription of FT and that they are positive regulators of CRY2 mediated flowering. CIB1 and the CIB1 related bHLHs can form heterodimers and some of those heterodimers have higher binding affinity to the non-canonical E-box, which explains why CIB1 and other CIBs binds to the canonical E-box (CACGTG, G-box) in vitro with a higher affinity than to the non-canonical E-box elements (CANNTG), while they all associate with the FT chromatin which only contains non-canonical E-boxes. To our knowledge, this is the first evidence in plants that heterodimerization of distinct bHLH proteins can affect the specificity of the elements bound by bHLH proteins. Consistent with our hypothesis that CIBs are specifically involved in CRY2 signaling, the expression of CIBs proteins is regulated specifically by blue light. Our study demonstrates that CIBs function redundantly in regulating CRY2-mediated flowering, and more importantly, different CIBs genes form heterodimers to interact with the non-canonical E-box DNA in vivo.
CIBs act redundantly to promote flowering
The bHLH proteins are one of the largest transcription factor families in eukaryotes, and there are about 170 bHLH proteins in Arabidopsis. bHLH transcription factors can form homo - or heterodimers through their HLH domain, and they bind to canonical E-box (G-box) or non-canonical E-box through the basic domain [36]. In Arabidopsis, phytochromes interact with several bHLH transcription factors, known as PIF proteins, in a light dependent manner to modulate phytochrome function and regulation [15]–[21]. Cryptochromes regulate gene expression by modulating activities of the circadian clock [41], and interact directly with transcription factors, such as CIB1 to regulate gene transcription [14]. We showed that several CIB1 related bHLH genes, CIB2, CIB4, CIB5, and CIL1 all can promote flowering initiation in the long day condition. Expression of the dominant repressor version of CIB1, CIB4 or CIB5 resulted in a marked delayed flowering phenotype. Impairment of three of these bHLH genes in the cib125 triple mutant caused a statistically significant delay of flowering under the photoperiodic induction condition [8], [14], demonstrating that these genes are essential for the CRY2 mediated flowering in the wild type plants. Nevertheless, there may still be other CIB related proteins involved, such as CIL1, since overexpression of CIL1 also results in an early flowering phenotype. There are also other bHLH members in the same clade with CIB1, such as BEEs [42].
CIBs belong to the subfamily 18 of bHLH proteins. The subfamily 18 contains 17 members, including BEE1 (BR enhanced expression), BEE2, and BEE3 [36], [42]. The mRNA expression of the three BEE genes and some other members of this subfamily are regulated by brassinosteroids [42]. Genetic analysis demonstrates that the three BEE proteins are functionally redundant positive regulators of brassinosteroids signaling [42]. Among other members of this subfamily, CESTA, is a positive regulator of brassinosteroids biosynthesis [43] while BIGPETALp (BPEp) affects Arabidopsis thaliana petal growth by influencing cell expansion [44]. Very recently, ACEs/CIB5 was reported to be involved in regulating cell elongation, where it was shown that PRE1 (a HLH protein that regulate growth downstream of a wild range of signals) [45], IBH1 (HLH factor that inhibit cell elongation) [45], [46], and the ACEs constitute a triantagonistic bHLH system, that competitively regulates cell elongation. ACEs/CIB5 activates the enzyme genes for cell elongation directly, while IBH1 negatively regulates cell elongation by interacting with ACEs to interfere with DNA binding. PER1 interacts with IBH1 so that IBH1 can not affect ACEs [45]. ACE1 is actually our CIL1, ACE2 is our CIB4, while ACE3 is our CIL2, and we also observed a mild hypocotyle phenotype of these overexpression lines (data not shown). Zhiyong Wang's group also showed that PRE1, IBH1 and HBI1 work together and formed an antagonistic switch to regulate cell elongation under the control of multiple external and endogenous signals [47]. HBI1 is also a member of subfamily 18. CIB4, CIB5 and CIL1 promote flowering initiation together with CIB1 and CIB2, while they are also involved in regulating cell elongation, so these bHLH transcription factors may play different roles in different signal transduction pathways, and regulate different target genes.
CIB heterodimers bind to the non-canonical E-box sequence of the FT promoter in vivo
bHLH proteins are well known to dimerize, they can form both homodimers and heterodimers [36]. bHLH transcription factors can form a heterodimer with HLH proteins, HLH proteins interact with bHLH proteins to interfere with the DNA binding of the bHLH protein, for example, PRE1 and IBH1 can dimerize with ACEs or HBI1 to regulate the cell elongation [45], [47]. It was reported previously that mouse cryptochromes physically interact with two bHLH proteins, CLOCK and BMAL, to suppress their activity of the E-box–dependent transcription. CLOCK and BMAL form heterodimers to regulate transcription [48], [49]. In plants, the bHLH transcription factor INDEHISCENT (IND) and SPATULA (SPT) can interact with each other to regulate tissue patterning in Arabidopsis [50]. The bHLH protein LONG HYPOCOTYL IN FAR-RED 1 (HFR1), plays a role in photomorphogenesis by forming non-DNA binding heterodimers with PIFs [51], [52]. Recently, it was reported that the HLH protein KIDARI (KDR) can dimerize with HFR, so that HFR cannot interact with PIF4 [53]. The HLH protein PAR1 can also interact with PIF4 to inhibits PIF4 mediated gene activation, while the HLH protein PRE1 interact with PAR1 to activate PIF4 [54]. These revealed that the PIF4 activity is regulated through a double layer of competitive inhibition of HFR1 and KDR [53] or PAR1 and PRE1 [54]. PIF3 and PIF4 can also form heterodimers, and the heterodimers are still capable of recognizing the G-box motif in a sequence-specific manner, the same as the PIF3 or PIF4 homodimers [36]. In C. elegans, some bHLH proteins can form heterodimers, and none of those participate in herodimeric interactions exhibit significant sequence-specific DNA binding on their own, they exhibit sequence-specific DNA-binding only when they form heterodimers [55]. Here we reported that CIB1 can dimerize with CIB2, CIB4 and CIB5 in vivo (Figure 5). CIB1, CIB2, CIB4, CIB5 all bind to the canonical E-box (G-box) with a much higher affinity than with other non-canonical E-box DNA sequence in vitro [14] (Figure 6A, Figure S5). However, CIB1, CIB4, CIB5 all associated with the FT promoter in vivo, which contains only non-canonical E-boxes but not canonical E-boxes [14] (Figure 4). The CIB1–CIB2, CIB1–CIB4, CIB2–CIB4, CIB2–CIB5, and CIB4–CIB5 heterodimers all bind to the non-canonical E-box sequence of the FT promoter in vitro (Fgiure 6D). Furthermore, expression of CIB1 and CIB2 or CIB1 and CIB4 together promotes the expression of the FT promoter-LUC to a much higher level compared with expression only one of them (Figure 6 F, G). Although CIB proteins have the highest affinity to the canonical E-box (G-box) in vitro, the heterodimers of different CIB proteins bind non-canonical E-box elements both in vivo and in vitro. We show direct evidence here that a bHLH protein can dimerize with more than one partner and to form heterodimers, and furthermore, that heterodimerization can modulate the DNA binding affinity of those bHLH transcription factors. Heterodimerization may be very important for the specificity of bHLH proteins.
CIBs proteins are regulated specifically by blue light
Most of the proteins that are involved in light signaling are light regulated. For example, the photoreceptor CRY2 protein gets degraded under blue light [37], [56], [57] while phyA undergoes rapid degradation in red light [38], ZTL is stabilized under blue light [26]. Phytochromes interact with PIFs in response to light and induce rapid phosphorylation, poly-ubiquitylation and degradation of PIFs through the ubiquitin/26S proteasome pathway to promote photomorphogenesis [39]. Consistent with our hypothesis that CIBs are specifically involved in blue light signaling, we discovered that the protein expression of CIBs is regulated specifically by blue light. CIB1, CIB2, CIB4, and CIB5 proteins are degraded in the absence of blue light, via the ubiquitin/26S proteasome pathway, in the dark, red, and FR light (Figure 7, Figure S7) [40]. The degradation of CIBs are suppressed in blue light, resulting in the accumulation of CIBs in blue light, CIBs are unique compared to other light-signaling proteins that showed light-regulated protein turnover.
Materials and Methods
Plant materials
Except where indicated, the Columbia ecotype of Arabidopsis was used. The cry1cry2, cib1, cib5, cib1cib5 mutants have been previously described. The cib2 T-DNA insertion mutant (SALK_055827) was obtained from ABRC (http://www.arabidopsis.org/index.jsp). Transgenic Arabidopsis lines were prepared by floral dip transformation method [58], [59]. Phenotypes of transgenic plants were verified in at least 3 independent transgenic lines. The binary plasmids encoding the 35S:Myc-CIB2, 35S:Myc-CIB3, 35S:Myc-CIB4, 35S:Myc-CIB5, 35S:Myc-CIL1, 35S:Myc-CIL2, 35S:Myc-CIB1EAR, 35S:Myc-CIB4EAR, 35S:Myc-CIB5EAR, 35S:Myc-VP16CIB1, 35S:LUC-CIB1, 35S:LUC-CIB2, 35S:LUC-CIB4, 35S:LUC-CIB5, CIB2P:GUS, CIB4P:GUS, CIB5P:GUS, 35S:CIB2-YFP, 35S:CIB4-YFP, 35S:CIB5-YFP, 35S:CRY2-mCherry, 35S:CIB1-mCherry were prepared by conventional and/or GATEWAY methods. CIB2P, CIB4P and CIB5P represent the CIB2 promoter (−2150 nt to −1 nt), CIB4 promoter (−2592 nt to −1 nt) or CIB5 promoter (−1752 nt to −1 nt), respectively. The cib1cib3cib5 triple mutant was prepared by genetic crosses.
The in vitro pull-down
The in vitro pull-down protein-protein interaction assay was modified from that described previously [14]. CRY2 protein expressed and purified from insect cells was incubated with the S35 labled CIB proteins prepared by the in vitro transcription/translation reactions (TnT, Promega). Ni-affinity beads were used to pull down the protein complexes.
Co-localization and BiFC assay
The BiFC assay was based on that described previously with slight modifications [14], [33], CRY2 or CIB1 and CIBs were fused to N-terminus of YFP or C-terminus of CFP, transformed to Agrobacterium strain GV3101 containing pSoup-P19 plasmid that encodes the suppressor of gene silencing [35]. Overnight cultures of Agrobacteria were collected by centrifugation, re-suspended in MES buffer to 0.8 OD600, mixed, and incubated at room temperature for 2 hr before infiltration. Agrobacteria suspension in a 2 ml syringe (without the metal needle) was carefully press-infiltrated manually onto healthy leaves of 3-week-old Nicotiana benthamiana. Plants were left under continuous white light for 3 day after infiltration.
Luciferase assays
In planta bioluminescence was analyzed by a cool CCD camera as previously described [60]. To compare the level of expression of LUC-fusion proteins in planta, plants were sprayed with luciferin solution (1 mM luciferin and 0.01% Triton X-100), image captured 5 min later by a CCD camera [61], and analyzed using the Image J software (http://rsb.info.nih.gov/ij/). The luciferase activity of plant extract was analyzed by a luminometer (Promega 20/20), using commercial LUC reaction reagents according to the manufacturer's instruction (Promega).
Immunoblot
Immunoblot is as described previously [14], [30], [62]. Our attempts to prepare the anti-CIB1, anti-CIB2, anti-CIB4 and anti-CIB5 antibodies resulted in antisera that recognized CIB1, CIB2, CIB4, CIB5 proteins expressed in E.coli, but not plant proteins. For immunoblots, a mouse monoclonal anti-Myc antibody 4A6 (Millipore, #05-724, 1∶4000 dilution for immunoblot and 1∶100 for immunostain) was used to detect Myc-CIB1, Myc-CIB1-EAR, Myc-CIB4, and Myc-CIB5 fusion proteins.
co-immunoprecipitation
co-immunoprecipitation (co-IP) is as described previously [14]. For co-IP, 12-day-old 35S::Myc-CIB5 seedlings grown in continuous red light were used, tissues were excised and incubated in MG132 for 3 hour before exposed to white light (W), blue light (B) or red light (R) for 20 minute, grounded in liquid nitrogen, homogenized in Binding Buffer (20 mM HEPES [pH 7.5], 40 mM KCl, 1 mM EDTA, 1% Triton X-100, 1 mM PMSF), and centrifuged at 16,000 g for 15 min. 1 ml supernatant was mixed with 25 µl anti-CRY2-IgG-coupled protein-A Sepharose, incubated at 4°C for 30 min. 5 µl anti-CRY2 antiserum was incubated with 20 µl protein-A Sepharose beads in a 100 µl binding, at 4°C for 2 hour, and used soon after. The mixture was transferred to a spin cup (Pierce), washed (ca. 20 sec each) 5 times with Washing Buffer (20 mM HEPES [pH 7.5], 40 mM KCl, 1 mM EDTA, 0.1% Triton X-100). The bound proteins were eluted from the affinity beads with 4× SDS-PAGE sample buffer, and analyzed by immunoblot.
EMSA assay
The EMSA assay was as described [14]. CIB1, CIB3,CIB4 and CIB5 were expressed in E. coli using the pCOLD-TF expression system according to the manufacturer's instructions (Takara Bio Inc. Cat#3365). His-TF-CIB1, His-TF-CIB3, His-TF-CIB4 and His-TF-CIB5 fusion proteins were purified using Ni-affinity chromatography. The synthetic oligonucleotides (Table S1) were PCR amplified, and labeled with DIG (digoxigenin) by terminal transferase according to the manufacturer's instruction (DIG Gel Shift Kit, Roche). Total 100 ng protein was added in each binding reaction, when two CIBs protein were added, each was 50 ng.
qPCR and GUS assays
Total RNAs were isolated using the Illustra RNAspin Mini kit (GE healthcare). cDNA was synthesized from 1 µg total RNA using SuperScript first-strand cDNA synthesis system (Invitrogen). Platinum SYBR Green qPCR Supermix-UDG (Invitrogen) or SYBR Premix Ex Tag (Takara) was used for qPCR reaction, using the MX3000 System (Stratagene). The level of ACTIN mRNA expression (At3g18780, Table S1) was used as the internal control. The expression of GUS (beta-glucuronidase) was analyzed as described [63].
Supporting Information
Zdroje
1. CashmoreAR (2003) Cryptochromes: enabling plants and animals to determine circadian time. Cell 114 : 537–543.
2. SancarA (2003) Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev 103 : 2203–2237.
3. LinC, ShalitinD (2003) Cryptochrome Structure and Signal transduction. Annu Rev Plant Biol 54 : 469–496.
4. LiuH, LiuB, ZhaoC, PepperM, LinC (2011) The action mechanisms of plant cryptochromes. Trends Plant Sci 16 : 684–691.
5. AhmadM, CashmoreAR (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 : 162–166.
6. GuoH, YangH, MocklerTC, LinC (1998) Regulation of Flowering Time by Arabidopsis Photoreceptors. Science 279 : 1360–1363.
7. ValverdeF, MouradovA, SoppeW, RavenscroftD, SamachA, et al. (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303 : 1003–1006.
8. YanovskyMJ, KaySA (2002) Molecular basis of seasonal time measurement in Arabidopsis. Nature 419 : 308–312.
9. ZuoZ, LiuH, LiuB, LiuX, LinC (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21 : 841–847.
10. SearleI, CouplandG (2004) Induction of flowering by seasonal changes in photoperiod. Embo J 23 : 1217–1222.
11. CorbesierL, VincentC, JangS, FornaraF, FanQ, et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316 : 1030–1033.
12. LifschitzE, EviatarT, RozmanA, ShalitA, GoldshmidtA, et al. (2006) The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci U S A 103 : 6398–6403.
13. JangS, MarchalV, PanigrahiKC, WenkelS, SoppeW, et al. (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. Embo J 27 : 1277–1288.
14. LiuH, YuX, LiK, KlejnotJ, YangH, et al. (2008) Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322 : 1535–1539.
15. SweereU, EichenbergK, LohrmannJ, Mira-RodadoV, BaurleI, et al. (2001) Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294 : 1108–1111.
16. NiM, TeppermanJM, QuailPH (1998) PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95 : 657–667.
17. HuqE, Al-SadyB, HudsonM, KimC, ApelK, et al. (2004) PHYTOCHROME-INTERACTING FACTOR 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305 : 1937–1941.
18. QuailPH (2002) Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 3 : 85–93.
19. Al-SadyB, KikisEA, MonteE, QuailPH (2008) Mechanistic duality of transcription factor function in phytochrome signaling. Proc Natl Acad Sci U S A 105 : 2232–2237.
20. ChoiG, YiH, LeeJ, KwonY-K, SohMS, et al. (1999) Phytochrome signalling is mediated through nucleosidediphosphate kinase 2. Nature 401 : 610–613.
21. RyuJS, KimJI, KunkelT, KimBC, ChoDS, et al. (2005) Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing phytochrome stability and affinity for a signal transducer. Cell 120 : 395–406.
22. NelsonDC, LasswellJ, RoggLE, CohenMA, BartelB (2000) FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101 : 331–340.
23. SomersDE, SchultzTF, MilnamowM, KaySA (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101 : 319–329.
24. ImaizumiT, TranHG, SwartzTE, BriggsWR, KaySA (2003) FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426 : 302–306.
25. SawaM, NusinowDA, KaySA, ImaizumiT (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318 : 261–265.
26. KimWY, FujiwaraS, SuhSS, KimJ, KimY, et al. (2007) ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449 : 356–360.
27. Idevall-HagrenO, DicksonEJ, HilleB, ToomreDK, De CamilliP (2012) Optogenetic control of phosphoinositide metabolism. Proc Natl Acad Sci U S A 109: E2316–2323.
28. KennedyMJ, HughesRM, PeteyaLA, SchwartzJW, EhlersMD, et al. (2010) Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods 7 : 973–975.
29. OhtaM, MatsuiK, HiratsuK, ShinshiH, Ohme-TakagiM (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13 : 1959–1968.
30. YuX, KlejnotJ, ZhaoX, ShalitinD, MaymonM, et al. (2007) Arabidopsis Cryptochrome 2 Completes Its Posttranslational Life Cycle in the Nucleus. Plant Cell 19 : 3146–3156.
31. Bracha-DroriK, ShichrurK, KatzA, OlivaM, AngeloviciR, et al. (2004) Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J 40 : 419–427.
32. WalterM, ChabanC, SchutzeK, BatisticO, WeckermannK, et al. (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40 : 428–438.
33. BaiMY, ZhangLY, GampalaSS, ZhuSW, SongWY, et al. (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci U S A 104 : 13839–13844.
34. MocklerTC, YuX, ShalitinD, ParikhD, MichaelTP, et al. (2004) Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc Natl Acad Sci U S A 101 : 12759–12764.
35. HellensRP, AllanAC, FrielEN, BolithoK, GraftonK, et al. (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1 : 13.
36. Toledo-OrtizG, HuqE, QuailPH (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15 : 1749–1770.
37. YuX, KlejnotJ, ZhaoX, ShalitinD, MaymonM, et al. (2007) Arabidopsis cryptochrome 2 completes its posttranslational life cycle in the nucleus. Plant Cell 19 : 3146–3156.
38. CloughRC, Jordan-BeebeET, LohmanKN, maritaJM, WalkerJM, et al. (1999) Sequences within the N - and C-terminal domains of phytochrome A are required for PFR ubiquitination and degradation. Plant J 17 : 155–167.
39. Al-SadyB, NiW, KircherS, SchaferE, QuailPH (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell 23 : 439–446.
40. LiuH, WangQ, LiuY, ImaizumiT, et al. (2013) The Arabidopsis photoreceptors CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms. Proc Natl Acad Sci U S A (in press).
41. SomersDE, DevlinPF, KaySA (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282 : 1488–1490.
42. FriedrichsenDM, NemhauserJ, MuramitsuT, MaloofJN, AlonsoJ, et al. (2002) Three redundant brassinosteroid early response genes encode putative bHLH transcription factors required for normal growth. Genetics 162 : 1445–1456.
43. PoppenbergerB, RozhonW, KhanM, HusarS, AdamG, et al. (2011) CESTA, a positive regulator of brassinosteroid biosynthesis. EMBO J 30 : 1149–1161.
44. VaraudE, BrioudesF, SzecsiJ, LerouxJ, BrownS, et al. (2011) AUXIN RESPONSE FACTOR8 regulates Arabidopsis petal growth by interacting with the bHLH transcription factor BIGPETALp. Plant Cell 23 : 973–983.
45. IkedaM, FujiwaraS, MitsudaN, Ohme-TakagiM (2012) A triantagonistic basic helix-loop-helix system regulates cell elongation in Arabidopsis. Plant Cell 24 : 4483–4497.
46. ZhangLY, BaiMY, WuJ, ZhuJY, WangH, et al. (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21 : 3767–3780.
47. BaiMY, FanM, OhE, WangZY (2013) A triple helix-loop-helix/basic helix-loop-helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis. Plant Cell 24 : 4917–4929.
48. YoungMW, KaySA (2001) Time zones: a comparative genetics of circadian clocks. Nat Rev Genet 2 : 702–715.
49. SancarA (2004) Regulation of mammalian circadian clock by cryptochrome. J Biol Chem 279 : 34079–34082.
50. GirinT, PaicuT, StephensonP, FuentesS, KornerE, et al. (2011) INDEHISCENT and SPATULA interact to specify carpel and valve margin tissue and thus promote seed dispersal in Arabidopsis. Plant Cell 23 : 3641–3653.
51. FairchildCD, SchumakerMA, QuailPH (2000) HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev 14 : 2377–2391.
52. HornitschekP, LorrainS, ZoeteV, MichielinO, FankhauserC (2009) Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J 28 : 3893–3902.
53. HongSY, SeoPJ, RyuJY, ChoSH, WooJC, et al. (2012) A competitive peptide inhibitor KIDARI negatively regulates HFR1 by forming nonfunctional heterodimers in Arabidopsis photomorphogenesis. Mol Cells 35 : 25–31.
54. HaoY, OhE, ChoiG, LiangZ, WangZY (2012) Interactions between HLH and bHLH factors modulate light-regulated plant development. Mol Plant 5 : 688–697.
55. GroveCA, De MasiF, BarrasaMI, NewburgerDE, AlkemaMJ, et al. (2009) A multiparameter network reveals extensive divergence between C. elegans bHLH transcription factors. Cell 138 : 314–327.
56. YuX, SayeghR, MaymonM, WarpehaK, KlejnotJ, et al. (2009) Formation of nuclear bodies of Arabidopsis CRY2 in response to blue light is associated with its blue light-dependent degradation. Plant Cell 21 : 118–130.
57. LiX, WangQ, YuX, LiuH, YangH, et al. (2011) Arabidopsis cryptochrome 2 (CRY2) functions by the photoactivation mechanism distinct from the tryptophan (trp) triad-dependent photoreduction. Proc Natl Acad Sci U S A 108 : 20844–20849.
58. CloughS, BentAF (1998) Floral dip: a simple method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 : 735–743.
59. WeigelD, AhnJH, BlazquezMA, BorevitzJO, ChristensenSK, et al. (2000) Activation tagging in arabidopsis. Plant Physiol 122 : 1003–1014.
60. KnowlesSM, LuS, TobinE (2008) Testing Time: can ethanol-induced pulses of proposed oscillator components phase shift rhythms in Arabidopsis? J Biol Rhythms In press.
61. KnowlesSM, LuSX, TobinEM (2008) Testing time: can ethanol-induced pulses of proposed oscillator components phase shift rhythms in Arabidopsis? J Biol Rhythms 23 : 463–471.
62. YuX, ShalitinD, LiuX, MaymonM, KlejnotJ, et al. (2007) Derepression of the NC80 motif is critical for the photoactivation of Arabidopsis CRY2. Proc Natl Acad Sci U S A 104 : 7289–7294.
63. JeffersonRA, KavanaghTA, bevanMW (1987) GUS fusions: β-glucoronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO 6 : 3901–3907.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



