-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFunction and Evolution of DNA Methylation in
The parasitoid wasp Nasonia vitripennis is an emerging genetic model for functional analysis of DNA methylation. Here, we characterize genome-wide methylation at a base-pair resolution, and compare these results to gene expression across five developmental stages and to methylation patterns reported in other insects. An accurate assessment of DNA methylation across the genome is accomplished using bisulfite sequencing of adult females from a highly inbred line. One-third of genes show extensive methylation over the gene body, yet methylated DNA is not found in non-coding regions and rarely in transposons. Methylated genes occur in small clusters across the genome. Methylation demarcates exon-intron boundaries, with elevated levels over exons, primarily in the 5′ regions of genes. It is also elevated near the sites of translational initiation and termination, with reduced levels in 5′ and 3′ UTRs. Methylated genes have higher median expression levels and lower expression variation across development stages than non-methylated genes. There is no difference in frequency of differential splicing between methylated and non-methylated genes, and as yet no established role for methylation in regulating alternative splicing in Nasonia. Phylogenetic comparisons indicate that many genes maintain methylation status across long evolutionary time scales. Nasonia methylated genes are more likely to be conserved in insects, but even those that are not conserved show broader expression across development than comparable non-methylated genes. Finally, examination of duplicated genes shows that those paralogs that have lost methylation in the Nasonia lineage following gene duplication evolve more rapidly, show decreased median expression levels, and increased specialization in expression across development. Methylation of Nasonia genes signals constitutive transcription across developmental stages, whereas non-methylated genes show more dynamic developmental expression patterns. We speculate that loss of methylation may result in increased developmental specialization in evolution and acquisition of methylation may lead to broader constitutive expression.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003872
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003872Summary
The parasitoid wasp Nasonia vitripennis is an emerging genetic model for functional analysis of DNA methylation. Here, we characterize genome-wide methylation at a base-pair resolution, and compare these results to gene expression across five developmental stages and to methylation patterns reported in other insects. An accurate assessment of DNA methylation across the genome is accomplished using bisulfite sequencing of adult females from a highly inbred line. One-third of genes show extensive methylation over the gene body, yet methylated DNA is not found in non-coding regions and rarely in transposons. Methylated genes occur in small clusters across the genome. Methylation demarcates exon-intron boundaries, with elevated levels over exons, primarily in the 5′ regions of genes. It is also elevated near the sites of translational initiation and termination, with reduced levels in 5′ and 3′ UTRs. Methylated genes have higher median expression levels and lower expression variation across development stages than non-methylated genes. There is no difference in frequency of differential splicing between methylated and non-methylated genes, and as yet no established role for methylation in regulating alternative splicing in Nasonia. Phylogenetic comparisons indicate that many genes maintain methylation status across long evolutionary time scales. Nasonia methylated genes are more likely to be conserved in insects, but even those that are not conserved show broader expression across development than comparable non-methylated genes. Finally, examination of duplicated genes shows that those paralogs that have lost methylation in the Nasonia lineage following gene duplication evolve more rapidly, show decreased median expression levels, and increased specialization in expression across development. Methylation of Nasonia genes signals constitutive transcription across developmental stages, whereas non-methylated genes show more dynamic developmental expression patterns. We speculate that loss of methylation may result in increased developmental specialization in evolution and acquisition of methylation may lead to broader constitutive expression.
Introduction
DNA methylation is an important epigenetic modification found in many plants and animals [1]–[5]. In mammals, DNA methylation is associated with important epigenetic processes such as genomic imprinting [6], histone modifications and X chromosome inactivation [7], [8], and plays an important role in brain development [9]. Clusters of CpG sites (CpG islands or CGIs) are often found in the 5′ regulatory regions including the promoter regions in mammals [10], [11]. Methylation at the promoter will typically result in silencing of the gene [12]. The promoters of transposable elements (TEs) are also often repressed by DNA methylation [13]. Non-CpG methylation has been observed in mammals, with high percentages in embryonic stem cells [14].
DNA methylation is also widespread in invertebrates [4], [15]–[26]. In contrast with mammals, methylation typically occurs over gene bodies, and is correlated with elevated gene expression [4], [15], [16], [18], [19], [22], [27], rather than gene inactivation. Consistent with gene activation, several studies of invertebrate methylation have reported that methylated genes tend to have “house-keeping functions”, whereas non-methylated genes are more tissue-specific [18], [28], [29].
DNA methylation is not universal among invertebrates [30], [31]. For example, the fruit fly Drosophila melanogaster lacks DNA methylation in adults due to the loss of two of three DNA methyltranferases (Dnmt1 and Dnmt3), and the reported DNA methylation found in early embryonic stages [32], [33] may be due to bisulfite conversion artifacts [31]. Nevertheless, in insects a combination of insect genome sequencing, identification of a full complement of DNMTs, and indirect or direct quantification of methylation, has uncovered genome-wide methylation in many species. A common indirect computational method for identifying genome-wide methylation is gene specific depletion of expected frequencies of CpG relative to observed (CpG O/E), which occurs in methylated genes due to mutational biases of methylated C to T [16]. This approach yielded evidence of genome-wide methylation in a number of insects, including the honeybee Apis mellifera, parasitoid wasp Nasonia vitripennis, pea aphid Acrythiosiphon pisum, and others [16], [26], [34]–[36]. Direct methods that have been used to quantify genome-wide methylation in insects include methylation sensitive restriction enzymes [37] and methylated DNA immunoprecipitation (MeDIP) [21]. However, to achieve single-base resolution of methylation in the genome requires bisulfite conversion coupled with high throughput sequencing, which has so far only been reported for honeybee (Apis mellifera) [15], [19], silkworm (Bombyx mori) [15], [22] and ants Camponotus floridanus, Harpegnathos saltator [18] and Solenopsis invicta [38].
Most of the work on arthropod DNA methylation has focused on the social insects (honeybees and ants) where alternative castes drive an interest in developmental processes that modulate caste determination [18], [28], [29]. Investigations in honeybee and ants have suggested an association between alternate splicing and methylation [18], [39]. N. vitripennis is a non-social haplodiploid parasitoid wasp with a well annotated reference genome [34], [40], [41]. Prior studies have revealed DNA methylation in Nasonia [20] and the presence of requisite DNA methyltransferases, including three members of Dnmt1 [34]. Here, we report findings of a whole-genome bisulfite sequencing (WGBS-seq) study that provides base-pair resolution of the genome of Nasonia vitripennis, a non-social Hymenopteran species [34], [40], [41]. The highly inbred strain of Nasonia used here allows for precisely mapping of WGBS-seq reads and CpG methylation calls to the genome without the complications caused by SNP variation found within heterologous DNA samples from variable strains or populations. We analyze whole genome patterns of DNA methylation in N. vitripennis, including the relationship between methylation, gene expression, expression breadth, and gene length, clustering of methylated CpG sites and methylated genes in the genome, patterns of methylation among transposons, non-CpG methylation, methylome comparisons with Apis, and changes in gene expression correlated with changes in methylation among paralogs in the Nasonia lineage. The Nasonia methylome helps to shed light on the function(s) and evolution of DNA methylation in insects.
Results
A. Base-pair resolution profile of CpG DNA methylation in Nasonia vitripennis
To profile the Nasonia methylome, we performed Illumina whole-genome bisulfite sequencing (WGBS-seq) in adult female samples with 25× haploid genome coverage (Figure S1) and 16.2× average CpG coverage (Figure S2). From the control lambda DNA alignments, the bisulfite conversion efficiency was 99.7% (Table S1), indicating highly efficient conversion. Additional quality control metrics and procedures to assure the high quality of this methylome are described in Materials and Methods.
Across the 8 million CpG sites in the Nasonia genome covered by our data, the average percentage methylation is 1.45%, and 1.6% of sites are defined as methylated CpG sites (mCpG) based on our criteria of the site having at least 10× coverage and >10% methylation (see Materials and Methods, Table 1 and Table S2). The percentage of methylation is not uniform across mCpG sites – those with 100% methylated sites are highly enriched, and the distribution is biased toward highly methylated sites with >75% methylation (Figure S3). In other words, CpG sites tend to either be largely non-methylated or highly methylated. We established that genome-wide bisulfite sequencing correctly identifies methylated and non-methylated CpGs by sequencing multiple clones from bisulfite converted DNA from three randomly chosen methylated genes and three non-methylated genes (Figures S4 S5, S6, S7, S8, S9 and Text S1).
Tab. 1. Summary of DNA methylation status for CpG islands and methylated CpG clusters. 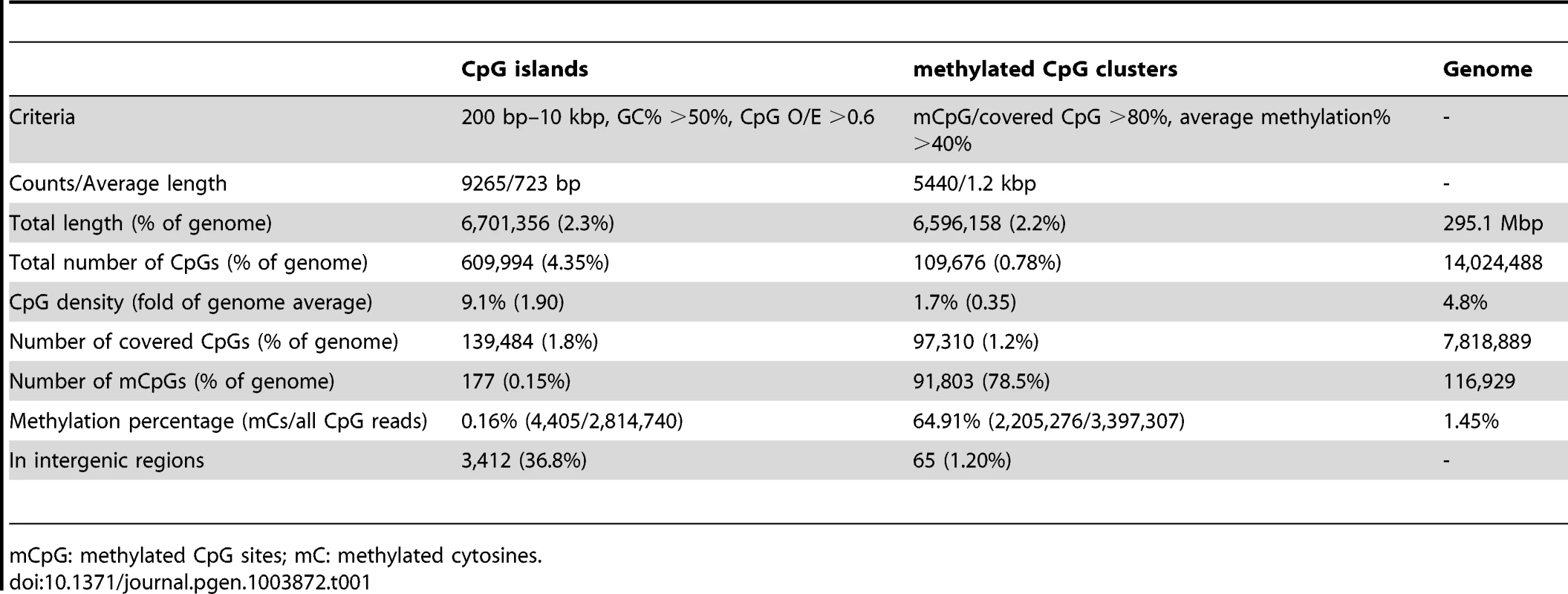
mCpG: methylated CpG sites; mC: methylated cytosines. Below we describe some of the striking patterns observed in the methylome of Nasonia.
A.1. CpG methylation occurs on gene bodies and is enriched in the 5′ coding region
DNA methylation in Nasonia predominantly occurs over gene bodies, and in particular over exons (Figure 1). While only containing 10% of 14 million CpGs, the annotated coding regions in Nasonia OGS2 (Official Gene Set v2; see Evidential Genes for Nasonia vitripennis at http://arthropods.eugenes.org/genes2/nasonia/) [42] are significantly enriched for mCpGs (61.4%, P-value<2.2×10−16, Chi-squared test; Figure 1A). Overall, 11.9% of CpGs located in exons are methylated. By contrast, the intergenic (0.2%), intronic (0.7%) and 1 kbp flanking regions of genes (1%) are depleted of methylated CpGs (Figure 1A). mCpGs are also clustered in the Nasonia genome, 78.5% of which are found in 5,440 clusters (Table 1 and Text S2). 98.8% of mCpG clusters are in gene regions (Table 1), which is consistent with gene body methylation. Furthermore, among the 65 mCpG clusters in “intergenic” regions, we found detectable expression in adult female RNA-seq data for 42 (Table S3). We therefore conclude that methylated CpG islands in Nasonia occur almost exclusively within transcribed genes.
Fig. 1. Distribution of CpG DNA methylation in the Nasonia genome across protein-coding genes. 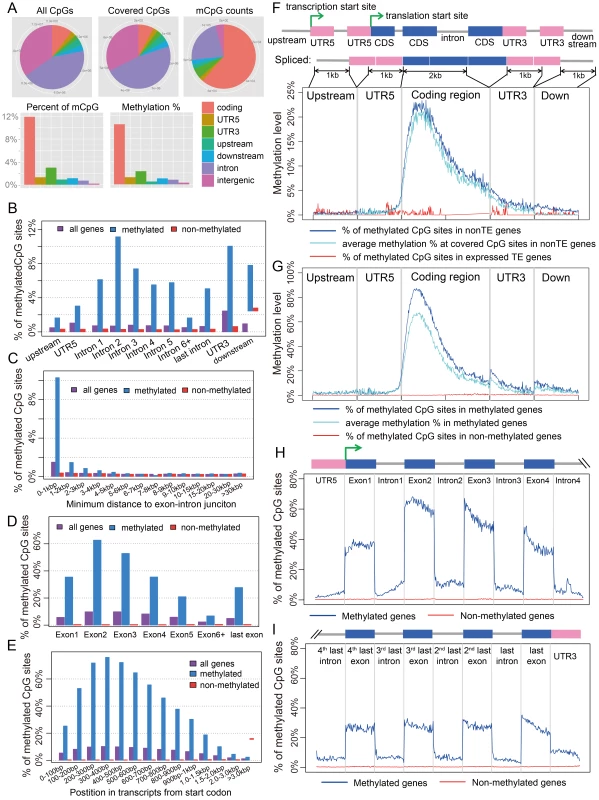
(A) Distributions across genomic features for all 14 million CpG sites (Top left), 8 million covered CpG sites (Top middle) and methylated CpG sites (mCpGs, Top right). Plotted in the bottom panel are the distributions for percentage of mCpGs and methylation percentage at covered CpG sites. (B) Percentage of mCpGs in the 1 kbp upstream, 1 kbp downstream, UTR and intronic regions for methylated (blue), non-methylated (red) and all genes (purple). (C) Percentage of mCpGs in introns for methylated (blue), non-methylated (red) and all genes (purple), binned by the nearest distance to the exon-intron junctions. (D) Percentage of mCpGs across exons for methylated (blue), non-methylated (red) and all genes (purple). (E) Percentage of mCpGs in the coding region starting from first codon for methylated (blue), non-methylated (red) and all genes (purple). (F) Methylation level in 1 kbp upstream, 1 kbp 5′-UTR, first 2 kbp coding, 1 kbp 3′-UTR and 1 kbp downstream regions for 1,540 expressed transposable element genes (TE genes) and 16,186 non-TE genes. Dark blue line: percentage of mCpGs for non-TE genes; light blue line: average methylation percentage across covered CpGs for non-TE genes; red line: percentage of mCpGs for TE genes. (G) Methylation level in 1 kbp upstream, 1 kbp 5′-UTR, first 2 kbp coding, 1 kbp 3′-UTR and 1 kbp downstream regions for 4,751 methylated non-TE genes and 12,975 non-methylated non-TE genes. Dark blue line: percentage of mCpGs for methylated genes; light blue line: average methylation percentage across covered CpGs for methylated genes; red line: percentage of mCpGs for non-methylated genes. (H–I) Plot of Percentage of methylated CpG sites in the 5′UTR, the first four exons and introns (H) and 3′UTR, the last four exons and introns (I) for methylated (blue) and non-methylated genes (red). All exons, introns and UTRs were rescaled to the same length. To compare Nasonia mCpG clusters to mammalian-type CpG islands, we ran predictions of CpG islands in the Nasonia genome using the same criteria as in mammals [20] (see Materials and Methods). Of 9,265 CpG islands, 36.8% occurred outside of gene bodies and these were nearly universally not methylated (0.15% mCpGs). Methylation also shows a clear pattern of being enriched at the beginning of genes (Figure 1C,F,G). Based on this pattern, we define genes with >10% methylated CpGs in the first 1 kbp coding region as methylated genes, and genes with ≤10% methylated CpGs in the first 1 kpb as non-methylated genes (see Materials and Methods). Methylation is largely absent in genes defined as non-methylated (0.31% mCpGs) (Figure 1B–I). In methylated genes, the highest levels of methylation occur in the 5′ exons of genes classified as methylated, and decline toward the 3′ region of the gene (Figure 1G–I). Exon methylation in methylated genes peaks at exon 2 or 400–500 bp into the coding region (Figure 1D–H); intron methylation was observed around the exon-intron junctions and also peaks at intron 2 (Figure 1B,C,H). In N. vitripennis, 26.7% of protein-coding genes are methylated among the 17,726 genes for which we have sufficient coverage to score methylation status. Excluding 1,540 expressed transposon genes, 4,739 (29.3%) of protein-coding gene are methylated. Our genome-wide investigation also confirms an association between the ratio of observed to expected CpG (CpG O/E) and DNA methylation status, a pattern that was predicted earlier based on bisulfite sequencing of 18 individual genes [20] (Figure S10 and Text S3).
A.2. Transposons are rarely methylated
Among 17,726 annotated genes in OGS2 with adequate uniquely mapped read coverage, 1,540 are expressed transposable element genes (expressed TE genes). The TE genes were characterized in OGS2 with detectable expression level in at least one developmental stage [42]. In adult females, 99.8% of these TE genes are non-methylated (Figure 1F). However, because many TEs occur in multiple copies in the genome with insufficient divergence to be uniquely mapped, we also quantified the DNA methylation percentages in 839 repetitive TEs annotated in the Nasonia genome paper [34] that were not covered by uniquely mapped reads (see Materials and Methods). Among the 803 elements with adequate WGBS-seq coverage, only five (GYPSY, SPRINGER, SNAKEHEAD, IFAC and BLASTOPIA) have >5% methylation averaging across CpG positions, and the top three are highly expressed in adult female RNA-seq data (Table S4). Therefore, we can conclude that TEs are rarely methylated and when they are, it can be associated with activation rather than inactivation. This finding contrasts sharply with methylation patterns in plants and mammals, in which methylation of TEs is involved in transcription suppression [13], [43].
A.3. CpG methylation shows a strong exon/intron pattern, and “marks” the beginning and end of protein-coding regions
There is a strong exon/intron patterning to methylation, with significantly heavier methylation levels occurring over exons, and declining in adjacent introns (Figure 1H, I). For example, there is significantly higher methylation in both the leading (P-value<2.2×10−16, Wilcoxon Matched-Pairs Signed Ranks Test - WMSRT) and trailing coding exons (P-value<2.2×10−16, WMSRT) relative to the intervening intron between the first two 5′ coding exons. The pattern persists even as overall methylation level decreases toward the 3′ regions of genes (Figure 1H, I).
In addition, the protein-coding regions of methylated genes are enriched for methylation relative to flanking untranslated regions (UTRs) of the same genes. For methylated genes, only 3.0% of the covered CpGs are methylated in the 5′ UTRs (Figure 1B) compared to 35.5% in the first coding exons (Figure 1D). Levels of methylation increase following the start codon for protein-coding genes, with significantly lower levels of mCpGs within 500 bp 5′ of the start codon (1336/26350 or 5.1% mCpGs) relative to 500 bp 3′ of the start codon (30544/46513 or 65.7% mCpGs; (Figure 1G; P-value<2.2×10−16, Chi-squared test). We are confident in the UTR identifications for Nasonia OGS2 because they are based on extensive RNA sequencing and tiling array data (see http://arthropods.eugenes.org/genes2/nasonia/), and consistent with our own adult RNA-seq data.
For smaller genes (e.g. with coding region <1 kbp), the end of the protein-coding region after the stop codon is also “marked” by reduced methylation level (Figure 1H). Comparison of methylation levels 100 bp before and 100 bp after the stop codon (with ≥4 covered CpGs) shows a significant decline in methylation level of the 3′UTR in genes with protein coding regions <1 kbp (11.4% mean before, 5.7% mean after, P-value = 0.003, WMSRT). The same does not hold, however, for genes of greater length (1.6% mean before, 1.6% mean after, P-value = 0.97, WMSRT). For genes shorter than 1 kbp, the relative number of genes with higher mCpG percentage before the stop codon is also significantly greater than those with lower mCpG percentage (P-value = 4.2×10−7, Chi-squared test), but this is not the case for larger genes (P-value = 0.21, Chi-squared test). Implications of the apparent tagging of the protein-coding exons and start codon from methylated genes are explored in the Discussion.
A.4. Correlation of transcript length and methylation is driven by 5′ bias in methylation
In Nasonia, we initially observed a significant negative correlation (Spearman's rank correlation coefficient ρ = −0.52, P-value<2×10−16) between transcript length and the percentage of mCpGs in methylated genes, when the entire transcribed region is used (Figure 2A). However, the majority of DNA methylation is located in the first 1 kbp of the coding region (Figure 1E,G). When we examined the relationship using one kbp 5′ of coding regions (Figure 2A), the correlation disappeared (Spearman's ρ = −0.03 P-value>0.05). Therefore, the correlation with gene length is a byproduct of the 5′ bias to the distribution of methylation within genes.
Fig. 2. DNA methylation and gene length, exon number and gene locations. 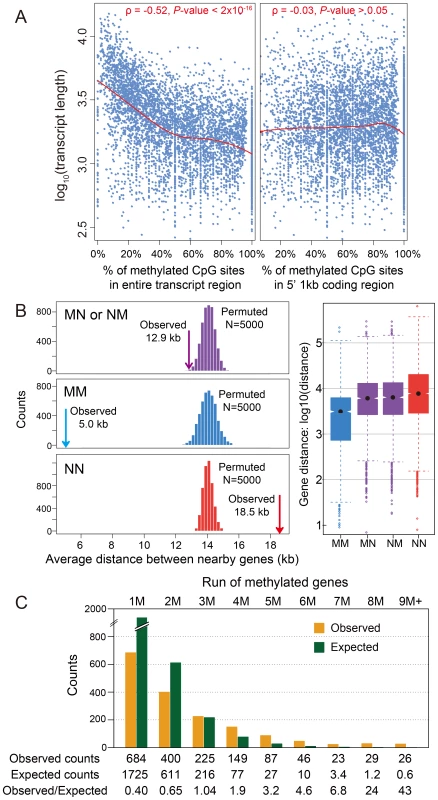
(A) Scatterplot for gene length (log10) and percentage of methylated CpG sites for methylation genes in the entire transcript region (left) and in 5′ 1 kbp coding region (right). The fitted lines using non-parametric local regression are shown in red. (B) Left: Distance between neighboring methylated genes (MM), non-methylated genes (NN) and methylated-non-methylated genes (MN or NM). The expected distributions for the three classes calculated by permuting the methylation status (N = 5,000) were plotted (MM: blue; NN: red; MN or NM: purple). The observed mean distance for each group was shown using arrows. Right: Distribution of the distance for the four classes (MM, NN, MN and NM). (C) Distribution of observed (orange) and expected (blue) counts for consecutive run of methylated genes. The expected counts were computed assuming the methylation status is randomly distributed. A.5. Methylated genes are clustered in the Nasonia genome
Tandem methylated genes (MM) and non-methylated gene pairs (NN) are significantly over-represented compared to MN and NM pairs (Figure S11A; P-value<2.2×10−16, Chi-squared test), suggesting that methylated genes are clustered in the genome. The average distance between MM gene pairs (4.8 kbp) is much shorter than the expected distance under random distribution of methylated genes, and the distance for NN gene pairs is significantly longer (18.5 kbp) (Figure 2B; P-value<2.2×10−16, Mann-Whitney U Test). Moreover, consecutive runs of methylated genes (1M, 2M, 3M, etc.), are longer than expected by chance (Figure 2C; P-value<2.2×10−16, Chi-squared test), with a mean cluster size of 2.48. Neighboring genes within distances <1 kbp and coding on opposite strands (i.e., in head-head and tail-tail formations) are enriched among methylated genes, and head-head formations comprise the highest fraction of methylation gene pairs (Figure S11B). This observed pattern of neighboring genes significantly sharing their methylation status (MM or NN) suggests potential co-regulation of methylation (Figure S11B). In conclusion, methylated genes tend to occur in small clusters within the genome.
A.6. The Nasonia genome lacks non-CpG DNA methylation
Non-CpG DNA methylation is rarely observed in the Nasonia genome; only 0.18% of Cs among the 60 million non-CpG positions with adequate read-depth are methylated (Table S5, Figure S12 and Text S4), which is less than the unconverted Cs in the lambda DNA used as a bisulfite control (Table S1). Therefore, many of these counts are likely experimental artifacts of bisulfite conversion or nucleotide mismatches in the reference genome (Table S6 and Figure S13). For example, of 28 top candidate non-CpG methylation sites with >30% unconverted Cs, eight (4 in top 10) are actually methylated at CpG sites, but were misidentified as non-CpG methylation due to sequence errors in the reference genome sequence (Table S6, Figure S13 and Text S4). Only one candidate non-CpG methylation site out of four examined was verified within the coding region of a gene (Figure S14 and Text S4).
B. CpG methylation and gene expression
We next investigated associations between DNA methylation and gene expression, using a combination of RNA-seq data from adult females and genome-wide tiling microarray data from five different developmental stages: early embryo, late embryo, larva, pupa, and adult (Figure S15 and Dataset S1, See Materials and Methods). Here, we compare expression patterns across developmental stages, and also examine copies of duplicated genes that differ in their methylation status.
B.1. Methylated genes show higher median expression levels
The relationship between methylation status and gene expression level was investigated using two different data sets – RNA-seq data for adult females and tiling microarray data for 5 different developmental stages (early embryo, late embryo, larva, pupa, adult). The RNA-seq results displayed a bimodal distribution of gene expression level in adult females (Figure 3A, P-value<2.2×10−16, Hartigans' dip test for unimodality) [44], [45]. Methylated genes have significantly higher expression level than non-methylated genes (P-value<2.2×10−16, Mann-Whitney U Test) and they showed markedly different patterns. The distribution of gene expression levels for methylated genes was unimodal (P-value = 1) and is generally composed of the higher expressed genes (Figure 3A), whereas the expression of the non-methylated genes is bimodal in distribution, with the moderately expressed set of genes overlapping with the expression levels observed from the methylated genes (Figure 3A, P-value = 0.03). Examination of the expression level for all genes reveals that non-methylated genes constitute the vast majority of low expressed genes. Furthermore, the non-methylated genes account for 99% of the genes that were not found to be expressed in the adult female RNA-seq data (FPKM <1).
Fig. 3. DNA methylation, gene expression and expression breadth. 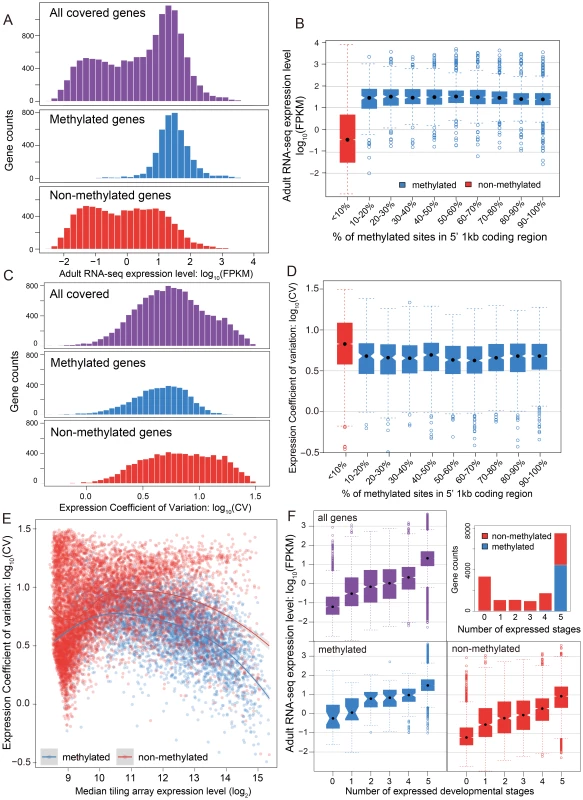
(A) Distribution of RNA-seq expression level (log10 FPKM) in adult female for methylated (blue), non-methylated (red) and all genes (purple). (B) Distribution of RNA-seq expression level (log10 FPKM) in adult female for groups of genes binned by percentage of methylated CpG sites in 5′ 1 kbp coding region. Red: non-methylation genes; blue: methylated genes. (C) Histograms for distribution of expression coefficient of variation (log10 expression CV) in five developmental stages (early embryo, late embryo, larvae, pupae and adult) for methylated (blue), non-methylated (red) and all genes (purple). (D) Distribution of expression breadth measurement (log10 expression CV) in six developmental stages for groups of genes binned by percentage of methylated CpG sites in 5′ 1 kbp coding region. Red: non-methylation genes; blue: methylated genes. (E) Scatterplot of expression breadth (log2 expression CV) on y-axis against median expression level (log2 signal intensity) in tiling array on x-axis, color-coded by adult female methylation status (blue: methylated genes; red: non-methylated genes). Fitted lines using non-parametric local regression are shown for methylated and non-methylated genes respectively. (F) Top right panel: Stacked barplot for expressed methylated and non-methylated genes with 0 to 6 expressed stages. Red: unmethylation genes; blue: methylated genes. Top left and bottom panel: boxplot for distribution of adult female RNA-seq expression level (log10 FPKM) for methylated (in blue), non-methylated (in red) and all genes (in purple) expressed in 0–5 developmental stages. In conclusion, DNA methylation in adult females is positively correlated with gene expression level in adult females, and most methylated genes are more highly expressed than typical for non-methylated genes (Figure S16). Nevertheless, methylation status is clearly not the only determinant for high gene expression, as many non-methylated genes also show high expression levels. The same general pattern was observed in tiling array data using median expression level across development (Figure S17). To examine whether there is a simple linear relationship between gene expression level and the percentage of mCpGs in methylated genes, we tested the difference of expression level for genes in different classes of mCpG percentage (Figure 3B). Among the methylated genes, we observed no positive correlation between methylation percentage and expression level (Spearman's ρ = −0.08). Therefore, gene expression is correlated with methylation status (methylated vs. non-methylated), but does not increase with increasing methylation level among methylated genes.
B.2. Methylated genes are constitutively expressed during development
Two metrics of gene expression change across development were calculated from the genome-wide tiling path microarray data: a coefficient of expression-level variation across the five developmental stages (expression CV), and the number of stages when gene expression is detected above baseline (see Methods & Materials).
While mean expression CV is lower in methylated (5.07) than non-methylated (6.07) genes, it is clear that both CV and median expression level across development covary (Figure 3C), which is confirmed in a logistic regression analysis (Text S5, Table S7 and S8). Because CV varies as a function of expression level, we examined the expression CV against the median expression level across development (Figure 3E). Excluding genes with very low expression (level<9) because there are too few methylated genes to make a proper comparison, we find that methylated genes have lower expression variation than non-methylated genes across a wide range of median expression levels. Dividing median expression level into three categories (9–11, 11–13, >13), methylated genes show significantly lower CV than do non-methylated genes for all categories (P-value<2.2×10−16 for all three categories, Mann-Whitney U Test; Table S9). The same trend is obtained when adult RNA-seq expression level is used in place of median expression across development (Figure S18, S19). Methylated genes have lower CVs across a broad range of median expression levels, indicating that they are expressed more evenly across development.
We next investigated the relationship between the methylation status of genes and the number of developmental stages with nonzero gene expression (see Materials and Methods). The majority of methylated genes (95%) are expressed broadly in all five developmental stages and less than 0.8% of the methylated genes have expression values below 9 in all five stages (Figure 3F). In contrast, only 28% of the non-methylated genes are expressed in all five stages, and 30% are absent (expression value <9) in all stages (Figure 3F). However, there is still a good proportion of non-methylated genes (3062 genes or 28%) that are expressed in all five stages, allowing us to compare expression breadth to median expression level across stages. Whereas it is not the case that all non-methylated genes are stage-specific, most methylated genes show broad expression across developmental stages, even when their median level of expression is relatively low.
The number of expressed stages is also correlated with the gene expression level. Genes present in more life stages tend to have a higher expression level (Figure S20). Taken together, these results strongly suggest that methylation is a general signal for constitutive expression of genes across development, and that this applies both to moderately expressed and highly expressed genes. Studies in Apis [28], [29], found that methylated genes are more broadly expressed across tissue/cell types. Here we show that methylated genes in Nasonia are more broadly expressed across developmental stages.
B.3. Methylated genes are enriched for basal cellular functions
We used blast2go (v2.6.0) to explore the enrichment of Gene Ontology (GO) term categories among methylated genes in Nasonia. This analysis reveals that methylated genes were generally enriched for basal cellular functions, such as translation, mRNA processing, and post-translational modifications (Table S10 and Figure S21). As the expression of methylated genes is distributed to the right of the median genome expression, we were concerned that the GO-term enrichment may be confounded by expression level differences between methylated and non-methylated genes. To adjust for this, we carried out a second analysis using gene lists restricted to low-, medium-, and high-expressed genes (See Materials and Methods). The GO-term enrichment among low-expressed methylated genes (Table S11) closely reflected those observed for all methylated genes (Table S10), however, for the medium - and high-expressed methylated genes (Table S12 and S13), cellular component terms became significantly enriched, specifically terms related to intracellular organelles. Both results are consistent with the conclusion that methylated genes in Nasonia are typically involved in cellular “house-keeping” functions, especially those involving translation, transcription and organelles.
B.4. Methylation is not required for differential splicing
We investigated patterns of DNA methylation in genes showing alternative splicing, to determine whether a signal of the alternative splice forms is apparent. We found no genome-wide correlation between methylation status and alternative splicing in adult females (Figure 4A–C and Figures S22 and S23, See Materials and Methods). Genes showing differential splicing are not more likely to be methylated than expected by chance (Figure 4A; P-value = 0.49, Chi-squared test), and there was no significant difference in the degree of alternative splicing between methylated and non-methylated genes, quantified by the fraction of major spliced forms (Figure 4B, P-value = 0.65, Kolmogorov-Smirnov test). In methylated genes with multiple methylated CpG clusters, we found in most cases that alternative exons within the first 1 kbp of the coding region do retain methylation (Text S6, Table S14 and Figure S24). However, as non-methylated genes also show extensive alternative splicing (Figure 4C), DNA methylation is clearly not required for differential splicing in Nasonia.
Fig. 4. DNA methylation and alternative splicing. 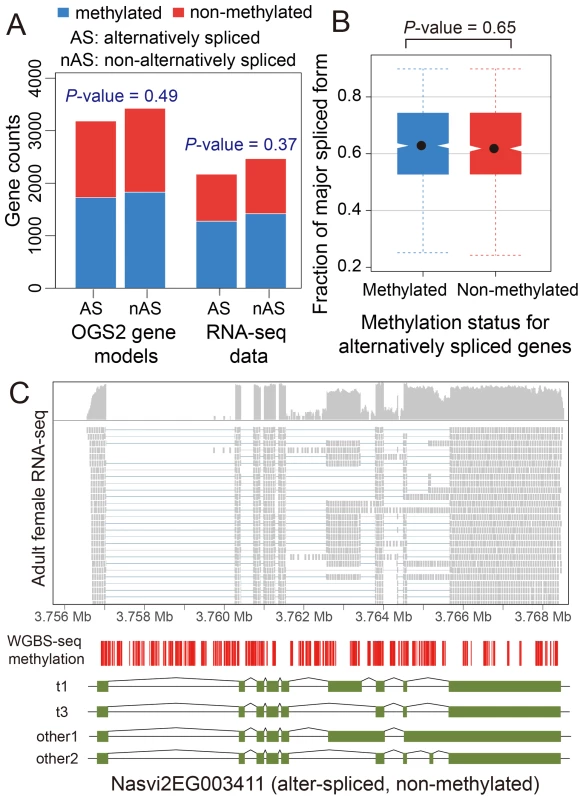
(A) Counts of alternatively spliced and non-alternatively spliced genes with different methylation status from OGS2 gene models (left) and RNA-seq data (right). AS: alternatively spliced; nAS: non-alternatively spliced. Methylated is shown in blue and non-methylated shown in red. (B) Distribution of fraction of major spliced forms for alternatively spliced methylated (blue) and non-methylated genes (red). (C) Gene expression, DNA methylation and alternative splicing profile for a non-methylated gene Nasvi2EG003411. Plotted at the top is the IGV browser screenshot showing adult female RNA-seq coverage (on log scale) and read alignments in the gene region. Plotted at the bottom are the CpG methylation profile at covered CpG sites from WGBS-seq data and the exon model of the alternatively spliced transcripts from OGS2 gene models. A vertical bar was drawn for each CpG at its position in the gene, color-coded by the methylation percentage in proportion to the bar length (blue: methylated Cs; red: non-methylated Cs). All 587 covered CpGs in the gene region were non-methylated. Two of the three OGS2 transcript variants, Nasvi2EG003411t1 (labelled as t1) and Nasvi2EG003411t3 (labelled as t3), were covered in the RNA-seq data with 47% and 41% of the transcript abundance, respectively. Two of the remaining minor transcript variants (other1 and other2) were also plotted. C. Comparative Genomics of Methylated Genes
C.1. Methylated genes are more conserved in evolution
To check the conservation status for methylated genes, we investigated 5,039 Nasonia single-copy genes covered in our WGBS-seq data that have either one or zero orthologs in each of seven other insect species (Apis mellifera, Tribolium castaneum, Bombyx mori, Anopheles gambiae, Drosophila melanogaster, Pediculus humanus and Acyrthosiphon pisum; see Materials and Methods). For these genes, we compared the methylation status in Nasonia to other factors among three gene conservation categories: genes present in single copy in all eight insect species (conserved genes), genes present in honeybee and Nasonia but not in other species (Hymenoptera-specific genes) and genes present only in Nasonia (Nasonia-specific genes) (Figure 5A). Nasonia methylated genes account for 71% of the genes present in all species, compared to 27% of Hymenoptera-specific genes and 14% of Nasonia-specific genes (Figure 5B). Therefore, Nasonia methylated genes were highly enriched in the conserved gene class (P-value<2×10−16, Chi-square test). The degree of methylation in methylated genes, measured by percentages of methylated CpG sites, was not significantly different among the three classes (P-value = 0.64, Kruskal-Wallis rank sum test) (Figure 5C).
Fig. 5. DNA methylation and gene conservation. 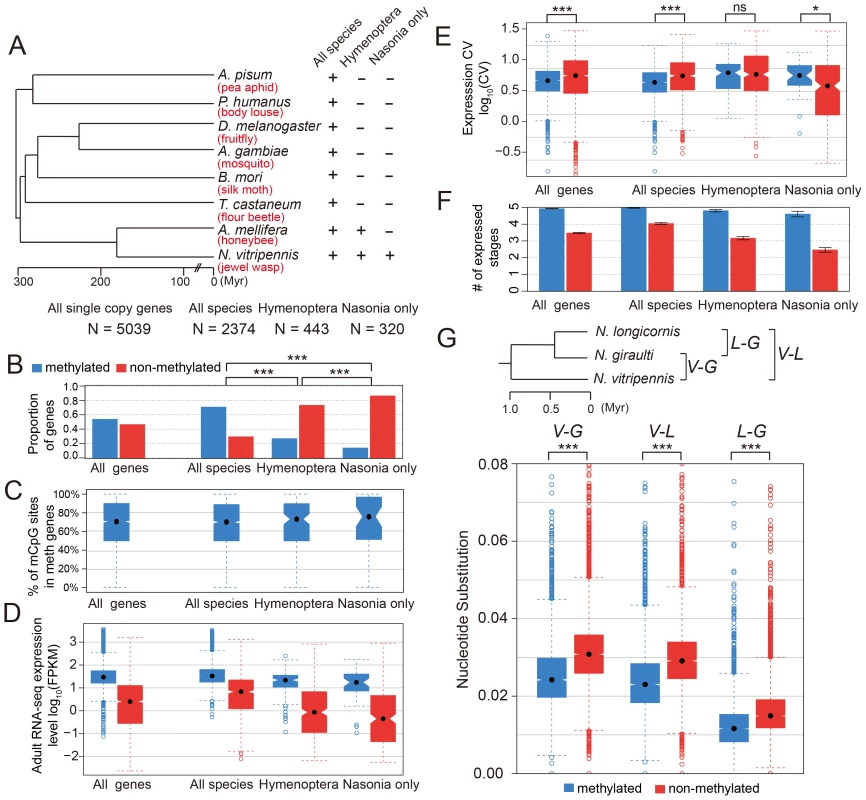
(A) Phylogenetic tree of eight insect species: Nasonia vitripennis, Apis mellifera, Tribolium castaneum, Bombyx mori, Anopheles gambiae, Drosophila melanogaster, Pediculus humanus and Acyrthosiphon pisum. The methylation status and correlating factors were plotted in (B–F) for four groups of genes: all 5,039 Nasonia single-copy genes with one or zero ortholog in seven other insect species, 2,374 genes with one orthologs in all eight insect species, 443 genes with one orthologs in Apis and Nasonia but missing in other six species, and 320 genes present only in Nasonia. The y-axes plotted in (B–F) are (B): proportion of methylated (blue) and non-methylated genes (red); (C): percentage of methylated CpG sites in methylated genes; (D): adult RNA-seq expression levels (log10FPKM); (E): coefficient of variation of expression level in tiling array across six developmental stages; (F): number of expressed tissues. (G) Top: Phylogenetic tree of three Nasonia species: N. longicornis (L), N. giraulti (G) and N. vitripennis (V). Bottom: boxplots of nucleotide substitution rates between V–L, V–G and L–G. Methylated genes have higher expression level (in Nasonia) in all three conservation classes (P-value<10−10, Mann-Whitney U Test), which is consistent with the methylation-expression correlation we observed (Figure 5D). In addition, Hymenoptera-specific and Nasonia-specific genes have lower expression levels compared to the conserved genes (P-value<2.2×10−16, Mann-Whitney U Test); however, the over-representation of non-methylated genes among them was not purely due to the expression difference. For all Nasonia single copy genes, non-methylated genes have higher expression variability across the five life stages (P-value<2.2×10−16, Mann-Whitney U Test, one-side). This is also true for the conserved genes (the “all species” category; P-value = 9.1×10−16, Mann-Whitney U Test, one-side). However, there is no significant difference in expression variability for hymenopteran-specific genes (P-value = 0.17, Mann-Whitney U Test, one-side), while Nasonia-specific genes showed the opposite pattern with (P-value = 0.018, Mann-Whitney U Test, one side) (Figure 5E). The reverse pattern found in Nasonia-specific methylated genes is relatively weak, although statistically significant.
Because expression level of non-methylated genes declines with decreasing conservation (Figure 5D) and CV co-varies with expression level (Figure 3E), CV is not the best index of expression breadth when comparing methylated and non-methylated genes of different conservation levels. We therefore examined how broadly genes are expressed across development for different conservation levels and methylation status (Figure 5F). Methylated genes are expressed more broadly than non-methylated genes for all three conservation categories. Conserved non-methylated genes (i.e. present in all species) are expressed in 4 stages on average, but the number dropped to 3.1 for hymenopteran-specific genes and further dropped to 2.5 for Nasonia-specific genes; methylated genes showed a much less dramatic decline, from 4.97 for all species to 4.81 for hymenoptera-specific genes and 4.60 for Nasonia-specific genes (Figure 5F). The median values were significantly different for all three categories (Table S15). These results show that methylated genes are more broadly expressed than non-methylated genes across conservation categories, and therefore indicate that even more recently evolved methylated genes acquire broader constitutive expression across development than comparable non-methylated genes.
C.2. There is significant conservation of gene methylation status between Nasonia and Apis
We next compared patterns between Nasonia and Apis, each being a representative of two major groups of Hymenoptera that have diverged approximately 180 MYA [34]. The honeybee (Apis) methylome data were available in the literature [15]. There were 3,206 Nasonia-Apis 1∶1 orthologous gene-pairs with methylation status called in both species. Of these, 71.9% are methylated in Nasonia compared to 47.7% in Apis. Note that the calling of methylation status is different between the Nasonia and Apis, as data on the distribution of methylated sites within genes (i.e. 5′ to 3′) was not available to us for Apis (see Materials and Methods). Despite these methodological limitations, there is a strong positive correlation in gene methylation status between Apis and Nasonia (P-value<2.2×10−16, Chi-squared test), with 42.2% of genes methylated in both species, compared to an expected 34.3%. Furthermore, when we calculated the % of methylated CpGs across the entire gene (the same as done for Apis), only 5% of the non-methylated genes changed status to methylated, and the finding of general conservation of methylation status was still found. These findings, based on genome-wide methylation criteria, are consistent with an earlier study showing conservation in gene methylation between Nasonia and Apis, based on inferred methylation from CpG O/E [20].
C.3. Methylated genes evolve more slowly within the Nasonia clade
We also examined methylation status and gene conservation at a shorter evolutionary time scale among Nasonia species. The nucleotide substitution rates in ∼7,000 genes were compared across three Nasonia species: N. longicornis, N. giraulti and N. vitripennis (see Materials and Methods). In all comparisons, methylated genes have lower nucleotide substitution rates (P-value<2.2×10−16, Mann-Whitney U Test) (Figure 5H).
C.4. Apis to Nasonia differences in methylation associate with gene ontology
To investigate whether GO-categories of methylated genes are conserved between Apis and Nasonia, we identified all 1-to-1 orthologs between Nasonia and Apis for which we had confident methylation status calls (3206 loci), and tested for enrichment of GO-terms where methylation status was either conserved or diverged between these two hymenopteran species. Once again, the most significantly enriched GO-terms for genes methylated in both Nasonia and Apis (1354 loci) are in categories associated with basal cellular processes such as metabolism and organelle function (Table S16). Next we restricted our lists to genes showing lineage-specific methylation within either Nasonia or Apis. For genes with methylation only in Nasonia (682 loci), ribonucleoprotein complex was enriched at the 5% FDR cutoff level (Table S17). No GO term enrichment was observed at this cutoff for genes methylated in Apis only (176 loci); however, processes related to sensory system were enriched at a more permissive FDR cutoff (results not shown).
C.5. When duplicated genes lose methylation, they evolve more quickly and become more developmentally specialized
Finally, we investigated the patterns of evolution of genes that have undergone gene duplications in the clade leading to Nasonia. A total of 145 orthologous gene sets were identified that are present in a single copy in all other Hymenoptera (OrthoDB, 13 taxa examined) [46], but which have undergone a gene duplication in the Nasonia clade. Methylation status in both Nasonia duplicates and the Apis paralog was available for 33 of these. In 9 (27%), the Apis ortholog and both Nasonia paralogs are methylated. In 8 cases, one of the Nasonia paralogs was non-methylated (N) whereas the other paralog and Apis gene was methylated (M) (Table S18). Those 8 gene pairs are present in a single copy across all Hymenoptera, with the exception of Nasonia. We therefore infer that they underwent a lineage-specific duplication, followed by loss of methylation in one of the paralogs. We examined gene expression and rates of divergence in each M to N conversion, using Apis as the outgroup. Despite the small sample size, several striking patterns are observed. First, in 7 of 8 cases, the N paralog has lower median expression across developmental stages than does the M (P-value = 0.016, WMSRT). In one case, the N paralog expression was close to the minimum detection level at all five developmental stages in the tiling array data, and we therefore excluded it as possible pseudogene. In the remaining 7 cases, the N paralogs showed significantly lower median expression levels (Figure 6A; P-value = 0.031, WMSRT). As is apparent in Figure 6, whereas the median expression is lower for the N genes, they show a greater variation of expression (P-value = 0.016, WMSRT in coefficient of variation) and greater maximum expression difference than do their M paralogs (P-value = 0.031, WMSRT), indicating that the N genes have maintained or evolved high expression within certain life stages. Finally, the N genes have significantly longer branch lengths (Figure 6B, P-value = 0.016, WMSRT) than their M paralogs, indicating more rapid evolution. These results suggest that loss of methylation status following gene duplication correlates with loss of constitutive expression across developmental stages, and possibly increased evolution and specialization of the duplicated gene.
Fig. 6. Paralog analysis. 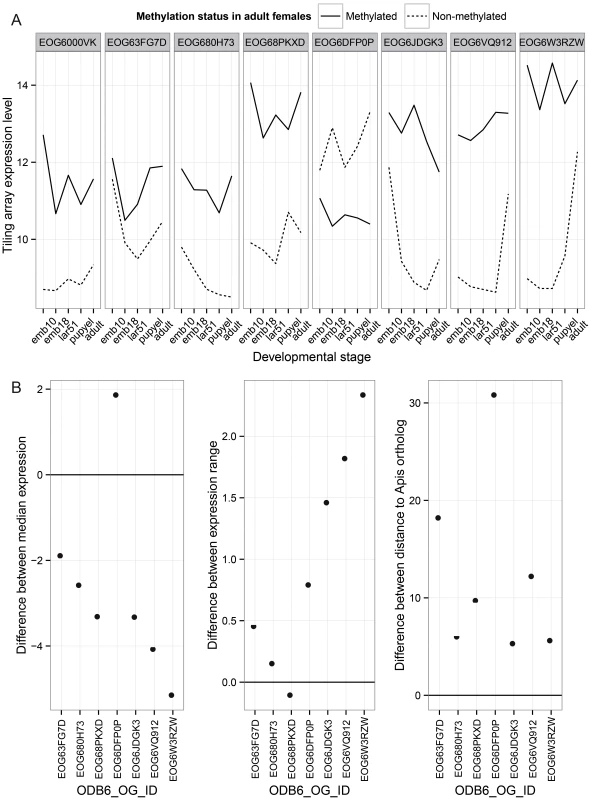
Differences between two paralogs that have changed in methylation status in the Nasonia lineage are shown. (A) Comparisons of expression pattern across developmental stages for duplicated genes in the Nasonia lineage where one gene is methylated (M) and the other lost methylation (N). These genes have 1∶1 orthologs in other hymenopteran species, and the ortholog is methylated in Apis. (B) Those paralogs that lost methylation show significant reductions in median expression level across development relative to the M paralog (N–M), significant increases in the range of expression level (N–M), and significantly greater divergence from the Apis ortholog (N–M). Discussion
In this study, we profiled the genome-wide methylation at base-pair resolution in Nasonia and found several striking features. First, 1.6% of covered CpG sites are methylated in the Nasonia genome, and the methylated CpGs are clustered along the genome. As found in several other invertebrates [15], [18], [19], DNA methylation is located mainly in the gene bodies in Nasonia, with coding genes falling into two distinct groups: around 30% of genes are methylated and show strong CpG methylation in 5′ exons, while DNA methylation is largely absent in the remaining genes. To compare the global methylation level across hymenopteran species, we calculated the percentage of methylated CpGs (mC/C) in Nasonia, Apis and ants (Text S7). Although it is difficult to compare genome-wide methylation levels due to differences in methodology, it appears that Nasonia (1.6%) has a higher overall methylation level than is found in honeybees (0.8%) or ants (1.05% in Camponotus and 0.68% in Harpegnathos).
Unlike mammals, where methylation is associated with suppression of transposon gene expression, with rare exceptions TEs are not methylated in Nasonia. The finding is in concordance with honeybee TE methylation profile [19], and suggests that DNA methylation is not required for TE repression in insects. In ants, TE methylation is at the genomic background level, but certain types of TE are hypermethylated and the pattern is species-specific [18]. In our data, we found five retrotransposon families with >5% methylation across CpG sites. The top three methylated TE types (SNAKEHEAD, GYPSY and SPRINGER) are highly expressed in the adult female RNA-seq data (Table S4), suggesting that DNA methylation may actually enhance expression of these elements. We do not know how this is accomplished, but it is possible that certain TEs may contain (or land near) sequence signals that promote DNA methylation. But globally the vast majority of TEs show no methylation in Nasonia.
Close examination of methylation in coding genes revealed a striking matching of methylation with the transcription unit. Methylation is low in 5′ UTR and increases rapidly near the transcription start site. Methylation is then consistently higher on exons and decreases significantly on introns, resulting in a clear delineation of exon-intron boundaries by methylation “tagging”. Finally, at least for methylated genes <1 kbp in length, methylation also declines significantly in the 3′UTR (after the stop codon). These patterns across the gene region suggest that DNA methylation provides “tags” that mark exons and targets introns for excision during transcription, but also that mark location of translational start and stop, even though translation occurs in the cytoplasm and is not directly associated with the DNA. If methylation affects the rate of transcription, then it is possible that methylation-induced transcriptional pausing at the exon-intron boundary could play a role in splicing [47]. However, how would the DNA methylation signal result in tagging of mature mRNA to demarcate translational initiation and termination? One possibility is through directing mRNA base modifications. For example, in mammals methylation of the N6 position of adenosine (m6A) has been shown to accumulate at stop codons and 3′UTR [48], suggesting a possible signal for translation termination.
It has been hypothesized that in insects DNA methylation regulates alternative splicing [19]; however, a direct causal relationship between methylation and differential splicing remains unsubstantiated. In Nasonia, we found no global correlation between methylation status and alternative splicing, although methylation changes across exon/intron boundaries suggested a potential link between DNA methylation and splicing. We should emphasize that DNA methylation is not required for either intron splicing or coding region demarcation, as non-methylated genes show both. Nevertheless, it is possible that methylation expedites these signals for a subset of methylated house-keeping genes, which we have shown to be expressed constitutively and at higher levels. Investigating these mechanisms is an interesting avenue for future research.
In Nasonia, the exon-intron pattern is augmented by a strong 5′ bias in level of methylation. The majority of DNA methylation was within the first 1 kbp coding exons and clearly drops beyond that in Nasonia, although an exon-intron distinction is still discernible in larger genes. A similar 5′-biased DNA methylation pattern has been observed in ants [18]. Studies in honeybee have reported a negative correlation between gene length and methylation status [49] and we observed the same pattern in Nasonia when the methylation percentage across the entire gene was used; however, this pattern disappears in Nasonia when the score of methylation level is restricted to the first 1 kbp of the coding region. We found little evidence for non-CpG methylation in Nasonia, but were able to confirm a single case. Therefore, non-CpG methylation is present, but it is extremely rare in Nasonia. Most candidate non-CpG methylation sites were located in genes nested in CpG methylation clusters. These findings suggest that non-CpG methylation may result from the inaccurate methylation at non-CpG sites by the CpG methylation machinery. It may strengthen the CpG methylation cluster, but the biological significance remains an open question.
In mammals, DNA methylation at promoter regions is often associated with suppression of gene expression [50], [51]. However, in insects, DNA methylation has been shown to be positively correlated with expression level in silkworm and ants [18], [22]. Here, we also observed a strong positive correlation between methylation and gene expression level; however, methylation is more strongly associated with constitutive expression across development independent of expression level. The distribution of expression levels for methylated genes is unimodal, matching the high expression class. Non-methylated genes show a bimodal distribution, with a mixture of both low and moderate expression, indicating DNA methylation is not the only factor affecting expression level. Other epigenetic marks such as histone modifications are likely to play a role in expression differences among non-methylated genes.
By comparing gene expression levels across five developmental stages, we found that methylated genes show more even expression across stages, and this pattern applies to both highly - and moderately-expressed methylated genes. The finding complements studies in honeybee, which found methylated genes to be expressed across multiple tissues, whereas non-methylated genes showed a more spatially restricted expression pattern [28], [29]. In both cases, methylation appears to be more prevalent in genes that are constitutively expressed across development and tissue types. GO-term analysis showed that methylated genes in Nasonia are enriched for genes with housekeeping functions, as observed in honeybee and ants [18], [19], [21]. Furthermore, genes methylated in Nasonia tend to be more evolutionarily conserved, as also found in recent studies in ants and other invertebrates [27], [52]. Housekeeping genes tend to be expressed in most tissue and cell types, which may explain the low expression variability for methylated genes across stages.
Further support for the role of methylation in constitutive expression of genes comes from the study of duplicated genes that have lost methylation relative to their paralog in the Nasonia lineage. Comparing non-methylated and methylated paralogs reveals both a marked median reduction in expression level, and evolution toward more developmental stage-specific expression patterns in the non-methylated genes. Functional category enrichment analysis showed that methylated genes are enriched for basic cellular functions, such as transcription and translation, as also found in honeybee and ants [18], [19], [21]. Our comparative genomic analysis also shows that many genes have maintained their methylation status across the long evolutionary time scale from Apis to Nasonia. This probably reflects the role of methylation in constitutive expression of basal housekeeping genes. We also find that methylated genes are enriched among the class of genes that are conserved among insects, while non-methylated genes are enriched among Hymenoptera-specific and Nasonia-specific genes. Nevertheless, methylated genes are expressed more broadly across development than are non-methylated genes for each of these conservation categories. Even the more recently evolved “Nasonia-specific” methylated genes show broad expression across developmental stages (median 4.60), considerably greater than for non-methylated genes (median 2.5). This suggests that broader constitutive expression is a hallmark of methylated genes whether they are conserved or recently evolved.
Bisulfite sequencing and expression profiling in our study were done on whole insects. Therefore, it could be argued that the correlation between methylation status and expression level occurs because genes that are methylated in more tissues show both higher levels of methylation and higher expression. In other words, tissue specific changes in methylation regulate tissue-specific gene expression, and this creates a correlation between methylation status and gene expression in whole animals. Although a possibility, we found that among methylated genes there is no correlation between level of methylation and level of expression (Figure 3B), which would be expected if the proportion of tissues in which the gene is methylated was driving the pattern. Future work will help resolve whether some genes are being differentially regulated by changes in methylation status. However, it appears that in general DNA methylation is a hallmark of genes that are constitutively “turned on”, at least across developmental stages.
In some eusocial organisms such as honeybee and ants, DNA methylation was shown to be related to caste determination [18], [19], [53]. In Nasonia, we have no evidence as yet that changes in methylation regulate specific developmental programs. In contrast, the general data reported above suggest that its primary role is in maintaining constitutive (and perhaps higher) expression of a subset of important cellular “house-keeping” genes, whereas non-methylated genes are more involved in stage-specific differences in expression.
Investigating the role of methylation in epigenetic processes (e.g. sexual differentiation, tissue-specific gene expression) will motivate the future study of establishment, maintenance, epigenetic reprogramming and interactors of DNA methylation in Nasonia and other insects. Comparison among closely related Nasonia also provides the opportunity to study the microevolution of DNA methylation. In addition, the ability to genetically dissect species differences in Nasonia through inter-fertile crosses [41], [54], [55] could provide tools for the genetic investigation of cis-regulatory mechanisms of DNA methylation.
Materials and Methods
Sample collection, genomic DNA and total RNA extraction
Genomic DNA samples were extracted from a pool of 50 24 h adult females from the standard N. vitripennis strain AsymCX using DNeasy Blood & Tissue Kit (Qiagen, CA). This is the same strain used for the Nasonia genome project [34] and is cured of the intracellular bacterium Wolbachia.
For RNA-seq, total RNA samples were extracted from adult females ∼24 h following eclosion from pupation, using RNeasy Plus mini kit (Qiagen, CA) following the manufacturer's protocol. The DNA, RNA concentration and the A260 nm/A280 nm absorption ratios were measured by NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, DE) to assess quality. RNA integrity was checked using the Agilent 2100 Bioanalyzer (Agilent Technologies, CA). All of the samples had a RIN (RNA integrity number) in the range 9.8–10.0 (RINmax = 10.0).
For tiling microarrays, total RNA was extracted from samples of 5 different life stages, 0–10 h embryos, 18–30 h embryos, 51–57 h larvae, day yellow pupae (little to no red eye pigment), and 1 day post eclosion adults. To generate the samples, mated females were first singly given two Sarcophaga bullata hosts for 48 h and then given one host for 6 hours, with access to the host restricted to one end for ease of embryo collection. Embryos or larvae were then collected from the hosts. Under this experimental design, females typically produce 85–95% female offspring, and these percentages were confirmed using control hosts where the offspring were permitted to complete development. Therefore, the wasps from these samples are predominantly female, although individual embryos or larvae were not sexed. For pupal collections, hosts were opened and female pupae from the “yellow pupal” stage were collected. Adult females were collected for RNA extraction ∼24 h after eclosion from the pupal stage. Six replicates per sample were used, averaging 400 individuals per replicate for embryos, 300 for larvae, 20 for pupae and 20 for adults. Samples were extracted in Trizol (Invitrogen, cat#15596-026) and then sent to the Indiana University Center for Genomics and Bioinformatics for sample preparation and tiling microarray analysis using previously published methods.
WGBS-seq and mRNA-seq library preparation and Illumina sequencing
20 µg of female Nasonia genomic DNA and 5 µg non-methylated control lambda DNA (catalog #: D1521, Promega, WI) were sheared by Covaris S2 system (Covaris, MA) for 480 second with 10% duty cycle, level 5 intensity and 200 cycles per burst. The DNA fragments were purified with Zymo DNA Clean & Concentrator-5 columns (Zymo Research, CA), size-selected for 130–180 bp with E-Gel system (Life technologies, CA) and QIAquick Gel Extraction Kit (Qiagen, CA), end-repaired with NEBnext end repair module and NEBnext dA tailing module (New England Biolabs Inc., MA), ligated with Illumina methylated PE adapter oligo (part #1005560, Illumina, CA) and then purified with Agencourt AMPure XP beads (Beckman Coulter, CA). We performed bisulfite conversion on purified Nasonia adult DNA and lambda control DNA using Qiagen EpiTect Bisulfite kit with 2× bisulfite conversion cycles to improve the conversion efficiency and then purified the elute by AMPure XP beads. The purified converted DNA was amplified with PfuTurbo Cx Hotstart DNA Polymerase (Agilent Technologies, CA) using 15 cycles. The final libraries were purified again using AMPure XP beads and the library concentration was measured by Qubit (Life technologies, CA). The library size distribution was checked by Agilent 2100 Bioanalyzer (Agilent Technologies, CA).
We mixed 0.5% of the lambda control DNA library in the Nasonia DNA WGBS-seq library, and performed Illumina short-read sequencing in one 84 bp lane on Genome Analyzer IIx (GAIIx) and one 101 bp paired-end lane on HiSeq2000 instrument. Image analysis and base calling were performed by the Illumina instrument software. In total, we obtained 27,766,713 reads from the GAIIx lane and 89,739,445 reads from the HiSeq2000 lane. Illumina WGBS-seq data have been deposited in GEO under accession no. GSE43423.
The mRNA-Seq library was made from 3.5 µg total RNA samples from 24 h adult females, using TruSeq RNA Sample Preparation Kits v2 (Illumina Inc., CA). The library was sequenced on an Illumina HiSeq2000 instrument and we obtained 65,334,896 reads. IIlumina RNA-seq data in this study have been deposited in GEO under accession no. GSE43422.
WGBS-seq and mRNA-seq read alignments and data analysis
The Illumina quality score and nucleotide distribution were checked by the FASTX toolkits (http://hannonlab.cshl.edu/fastx_toolkit/index.html). The adapter sequences were removed from the raw reads by custom scripts (0.7% in GAIIx lane and 0.9% in HiSeq lane). To include only high quality bases in our analysis, the sequence reads were trimmed to 75 bp. After trimming, the GAIIx and HiSeq (read 1 only) data gave us 8.75 Gbp of sequences or 25× coverage of the haploid genome, assuming 350 Mbp genome size.
We first aligned the reads to the plus and minus strands of non-methylated lambda genome (NCBI reference sequence NC_001416) with all Cs converted to Ts, using BWA with 4 mismatches [56]. A total of 746,736 (0.64%) reads were uniquely mapped to the lambda genome without indels, resulting 1155× coverage of lambda genome. We estimated the unconverted Cs to be 0.31% by subtracting the background T→C sequence error from the remaining unconverted Cs, therefore the final bisulfite conversion efficiency, at 99.69%, was ideal for downstream analysis. The Illumina sequencing error rates for each type of nucleotide in the GAIIx and HiSeq lane were also estimated from the lambda control alignments (Table S1).
From the N. vitripennis reference scaffolds [34], we built C→T converted reference genomes for both the Watson (+) and Crick (−) strand separately, with all Cs in CpGs context remains Cs (meth_genome) and all CpG Cs converted to Ts (unmeth_genome). The rest Illumina sequencing reads were aligned to the converted genomes with BWA [56] with a maximum of 4 mismatches, and summarized in a single BAM file (Figure S1). We tested 4, 6, 8 and 10 mismatches and found 4 mismatches will give the best mapping percentage without ambiguity due to reduced genome complexity after bisulfite conversion. ∼80% of the reads could be mapped to the converted Nasonia reference genome. To get accurate methylation estimation, we only used uniquely mapped reads without any indel (60% of total reads) for the methylation quantification. CpG methylation percentages were estimated from the proportion of remaining Cs in CpG context (Table S2). Non-CpG methylation was also quantified (Table S5).
We aligned adult female RNA-seq reads to the Nasonia reference scaffolds using TopHat v1.4.1 [57] with a maximum of three mismatches. 94% of the reads were uniquely mapped to the genome. Total expression level (FPKM: Fragments Per Kilobase-pair of exon Model) was calculated using Cufflinks v1.3.0 [58] based on all mapped reads from the TopHat alignments. The multiple mapped reads were weighted using the “-u” parameter in Cufflinks. The RNA-seq alignments were viewed in the IGV browser [59], [60].
CpG methylation quantification and gene methylation analysis
Among the 14,024,488 CpG sites in Nasonia haploid genome, we covered >90% with 2 or more uniquely aligned reads and >55% with 10 or more reads. The average coverage at CpG sites is 16.2× (Figure S2). To obtain accurate quantification of the methylation percentages, we only included ∼8 M CpGs sites with 10 or more coverage (covered CpGs). To quantify the CpG methylation levels, we used two metrics: percentage of methylated CpGs (percentage of mCpGs) and average methylation percentage in covered CpGs (methylation percentage). Methylated CpGs (mCpGs) are defined as CpG sites with >10% methylated Cs and ≥10 coverage. This definition requires at least two unconverted C containing reads to call a site methylated, therefore a single T→C Illumina sequence error will not results a spurious methylated site.
Methylation percentage is the average methylated percentage over all CpGs in a particular region, which is the total number of unconverted Cs divided by the total number of reads at CpG sites. The methylated CpG sites were annotated using both the Nasonia OGS1.2 (official gene set) and OGS2 gene models [42]. OGS2 gene models incorporated both whole genome tiling expression array and RNA-seq data from multiple tissues at multiple developmental time points, proving high quality support for 5′ - and 3′-UTR annotation. Among the 14,024,488 CpGs, 1,159,303 were located in overlapped gene models and were excluded from the analysis. To determine the gene methylation status, we calculated the percentage of mCpG among the covered CpG sites (depth ≥10) in both the first 1 kbp coding region and in the entire transcript region. Since the majority of the mCpGs are located in the first 1 kbp coding region and the methylation level is under the UTR level beyond 2 kbp (Figure 1G), long genes with heavy methylation at the beginning will be averaged out if the entire transcript length was used. Therefore, we inferred the gene methylation status using the percentage of mCpG in the first 1 kbp coding region. Because single or sparse mCpG could be spuriously generated by T→C sequencing error, local incomplete bisulfite conversion or alignment problems, we applied arbitrary cut-off and genes with at least four covered CpGs and >10% mCpG in the first 1 kbp coding region are classified as methylated genes; genes with ≤10% mCpG are defined as non-methylated genes.
To quantify the DNA methylation in repetitive elements and retrotransposons, we built a non-redundant repeat sequence database for the repeat library and retroid elements annotation from the Nasonia genome project [34]. From the 1195 sequences in the repeat library, 763 that are >100 bp in length and contain 4 or more CpGs were kept. Simple repeats and STRs were excluded from the analysis. The longest element in each of the 76 retroid families was included in repetitive elements database. We aligned the unmapped and non-uniquely mapped WGBS-seq reads to the database, and quantified the methylation percentage at CpG positions. Elements with average read depth four or more were included in the analysis.
Characterizing the CpG islands and methylated CpG clusters in the Nasonia genome
To search for mammalian type CpG islands (CGIs) in the Nasonia genome, we ran predictions of CGIs in the Nasonia genome using the same criteria as in mammals [10]: GC percent >50%, CpG O/E (observed/expected CpGs) ratio >0.6, and greater than 200 bp in length. 9,265 CGIs were found in the Nasonia genome. We define methylated CpG clusters (mCpGCLs) as regions with >80% methylated CpGs and >40% average methylation percentage, and we found 5,440 mCpGCLs in the Nasonia genome.
Analysis of clustering of methylated genes in the Nasonia genome
To determine whether the methylated genes are clustered or randomly distributed in the Nasonia genome, we analyzed the frequency and distance between neighboring gene pairs (MM: methylated-methylated; MN: methylated-nonmethylated; NM: nonmethylated-methylated; NN: nonmethylated-nonmethylated), as well as the consecutive runs of methylated genes. Scaffold rather than the chromosomal locations were used for the analysis because neighboring genes on two different scaffolds are not in proximity. To eliminate the effect of short scaffolds with few genes in them, only the top 100 largest scaffolds were included for the analysis, containing 11,683 genes with methylation status.
Validation of methylated and non-methylated genes using cloning and sequencing method
To confirm methylation status of individual genes, DNA from 20 pooled 24–27 h virgin Nasonia vitripennis (strain Asymcx) females was extracted using the Qiagen DNeasy Blood and Tissue Kit (Cat No. 69504). The bisulfite conversion was performed by the Qiagen EpiTect Bisulfite Kit (Cat No. 59104) with 1.5 µg of starting DNA. Bisulfite PCR primers for six selected genes were designed using Methyl Primer Express software v1.0 (Applied Biosystems by Life Technologies, CA). The amplified PCR product was gel purified and cloned using Promega pGEM-T Easy Vector System II (Cat No. A1380). Direct PCR from the E. coli “white” colonies with T7 and SP6 primers was used to select colonies with the right insert size, which were then inoculated in LB broth with ampicillin and the plasmid was extracted using the QIAprep Miniprep kit (Cat No. 27104). Prism BigDye Terminator v3.1 Cycle Sequencing Ready Run Kit (Applied Biosystems) was used to prepare the products for sequencing. BigDye clean-up was completed using ABgene Dye Terminator Removal Kit (Cat No. AB-0943). Sequencing was completed at the Function Genomic Center at the University of Rochester.
Tiling microarray sample preparation
We used NimbleGen high-density 2 (HD2) arrays for transcriptome investigations. The custom 4-array (chip) set consisted of 8.4 million isothermal long-oligonucleotide probes that are 50–60 nt in length and that span the Nasonia genome sequence at overlapping intervals of 33 bp, on average. Each slide contained 27,000 Markov model random probes that are not represented in the genome for setting background level thresholds. All probes were designed using NimbleGen's ArrayScribe software and the quality assurance tests of the probes were conducted using Indiana University's Centre for Genomics and Bioinformatics in-house algorithms. Signal to background ratios were determined by first calling probes that fluoresced at intensities greater than 99% of the random probes' signal intensities; therefore only 1% of fluorescing probes are likely to be false positives. The arrays reliably produced high signal to background ratios; log2 ratios of eight were observed for signal over background.
We conducted three replicates each using RNA from independent biological extractions of female early embryo (0–10 h), late embryo (18–30 h), 1st instar larvae, and pupae. Additional experiments were performed comparing transcription in testis and the female reproductive tract. Samples were prepared at 25°C as follows: Approximately 100 N. vitripennis (AsymCX) virgins were collected as black pupae. After eclosion, females were provided with males and allowed to mate overnight. Females were initially provisions 15–20 Sarchophaga bullata hosts in groups of 20 females for 24 h to induce production of eggs. The hosts were then removed and females were left overnight (∼18 h). Mated females produced 85% female progeny under the design used here, and therefore the embryo and larval collections are predominantly female offspring. To collect embryos, individual females were given access to a host at one end (to restrict the oviposition site) and allowed to lay eggs for 6–10 h before being removed. Embryos were then harvested immediately (early embryos), 18 h later (late embryos), or 51 h later (1st instar larvae). All embryos and larvae were collected in an RNase free environment. The host was cracked open and the “cap” removed to expose the embryo. Dissecting needles were used to gently scrape embryos from the surface of the host and transfer them into a 1.5 ml tube pre-chilled on dry ice. Samples were stored at −80°C. If at any time the host was punctured or embryos were exposed to host hemolymph, they were discarded. Estimates of the number of embryos per replicate (three per life stage/sex) were recorded; early embryos ranged from 300–900, late embryo 140–500, 1st instar larvae 245–520. Since sex cannot be determined at larval stage, some of the mated female hostings were allowed to mature to adulthood then males and females were counted to determine the sex ratio. Early larvae showed an average of 82.9% females and late larvae had an average of 84.2%. Pupae collections were made among the progeny of mated females provided with hosts for 48 hrs. They were sorted by sex and stage (early yellow, red-eye, half black, and black pupae). Equal numbers (S20) of pupae from each stage were then pooled prior to RNA extraction. Female reproductive tracts (30 per replicate) were removed from 1–3 days post eclosion virgin females and transferred to a tube on dry ice prior to RNA extraction.
Tissue was disrupted and homogenized using Trizol reagent (Invitrogen), and extracted RNA was purified using the Qiagen RNeasy protocol with optimal, on column DNase treatment from specific tissues. Beginning with at least 0.5 µg of total RNA (for early to late embryo) or at least 1.0 µg (for other tissue types), a single round of amplification using MessageAmp II aRNA kit (Ambion) produced between 30 and 45 µg of cRNA for embryo RNA and greater than 100 µg for all other tissue types. Starting with 10 µg of cRNA, double strand cDNA synthesis was carried out using the Invitrogen SuperScript Double-Stranded cDNA Synthesis kit using random hexamer primer followed by DNA labeling using 1 O.D. CY-labeled random nonomer primer and 100 U Klenow fragment (3>5 exo) per 1 µg double-stranded cDNA. The use of random primers ensured that all transcripts hybridize to the array, which contains probes designed solely from a single strand of the DNA sequence. Both sexes for each tissue type were alternatively labelled and a dye-swap was included among the replicate experiments. Dual-color hybridization, post-hybridization washing and scanning were done according to the manufacturer's instructions. Images were acquired using a GenePix 4200A scanner with GenePix 6.0 software. The data from these arrays were extracted using the software NimbleScan 2.4 (Roche NimbleGen). The normalized tiling array data can be found in Dataset S1.
Tiling array data analysis
The data analysis was performed using the statistical software package R (http://www.r-project.org/) and Bioconductor (http://www.bioconductor.org/) [61]. The signal distributions across chips, samples and replicates were adjusted to be equal according to the mean fluorescence of the random probes on each array. All probes including random probes were quantile normalized across replicates. Scores were assigned for each predicted OGS v2 gene, for each sample, based on the median log2 fluorescence over background intensity of probes falling within the boundaries of the largest gene transcript. The genes were deemed to be transcribed only when greater than ½ or their tiled length was expressed. On average, the 23,161 interrogated genes were tiled by 95.4±1.1 probes. Genes validated by tiling array or EST data are available online at http://www.hymenopteragenome.org/nasonia/?q=sequencing_and_analysis_consortium_datasets.
Analysis of alternative splicing
We used two methods to obtain the alternative splicing status for Nasonia transcripts. First, we used the alternative splicing status from the OGS2 gene models with good intron information support. Genes with more than one OGS2 transcripts per gene were considered as alternatively spliced genes, and genes with a single form in OGS2 were considered as non-spliced genes. We also inferred the alternative splicing status from the adult female RNA-seq data using Cufflinks software. Moderately and highly expressed genes with expression level FPKM>2 were included in the study because sufficient RNA-seq coverage is needed to detect the alter-spliced forms in the RNA-seq data. Genes with the percentage of second most abundant forms greater than 10% were considered as alternatively spliced genes.
Methylation conservation and GO-term enrichment analysis
For inference about conservation of methylation status of genes, loci were called Nasonia-specific if they did not have a homolog in OrthoDB BLASTp homolog (1e-5) to a database containing Human, Mouse, Xenopus, Apis mellifera, Drosophila melanogaster, and Anopheles gambiae. Arthropod-specific loci were those Nasonia sequences that had strong BLASTp hits (1e-5) to Apis mellifera, Drosophila melanogaster, Anopheles gambiae, but had no homology to proteins from Human, Mouse or Xenopus. GO term enrichment analysis was performed using blast2go [62] with the Nasonia OGS2 protein sequences and a BLASTp cut-off score of 1E-3 for assigning terms. Enrichment was determined using Fisher exact test as implemented by blast2go, with the cut-off for enrichment set to a 5% false discovery rate. The background gene set was restricted to the 17726 Nasonia genes with a known adult female methylation status as determined by bisulfite sequencing. For enrichment across different expression levels, genes were divided into low (9–11), medium (11–13) and high expression (13–15) based on median array expression (Table S11, S12, S13), with the background restricted to all genes with known methylation status that fell within that expression range. For GO-term analysis of genes with conserved methylation status between Apis and Nasonia, 1∶1 orthologs were selected based on their known methylation status for Apis (taken from [15]).
Comparative genomic analysis of methylated genes
The orthology status for thirteen Hymenoptera insect species (Acromyrmex echinatior, Apis florea, Apis mellifera, Atta cephalotes, Bombus impatiens, Bombus terrestris, Camponotus floridanus, Harpegnathos saltator, Linepithema humile, Megachile rotundata, Nasonia vitripennis, Pogonomyrmex barbatus, and Solenopsis invicta) was obtained from OrthoDB [46]. The updated Official Gene Set 2.0 (OGS2) for Nasonia vitripennis was used in this analysis (http://arthropods.eugenes.org/genes2/nasonia/). The honeybee methylation status was from Zemach et al. 2010 [15]. The nucleotide substitution rates between three Nasonia species (N. longicornis, N. giraulti and N. vitripennis) were from the Nasonia genome project [34]. Analysis of paralogs that had undergone changes in methylation status was accomplished by first identifying all genes that had 1∶1 orthologs in thirteen sequenced hymenopteran genomes, but are duplicated in N. vitripennis, using the OrthoDB database [46]. These were then divided into categories based on methylation status. Rates of evolution of the Nasonia genes relative to the Apis orthologs were measured by comparing pairwise distances of protein alignments scores obtained from the AllAll tool (available at http://www.cbrg.ethz.ch/services/AllAll). Median expression level, range in expression and largest difference in expression were calculated using tiling microarray data.
Statistical analyses
The logistic regression analysis of the effect of expression level and expression breadth on gene methylation status was performed using the LOGISTIC procedure in SAS 9.1 (Text S5). The statistical software R (version 2.13.0, www.r-project.org) was used for the rest of the statistical tests. Comparisons between matched gene samples were conducted using the Wilcoxon Matched-Pairs Signed Ranks Test (WMSRT) implemented in wilcox.test() function in the stats package. The test P-value of unimodality of gene expression distribution for methylated and non-methylated genes was calculated using the Hartigans' dip test for unimodality (dip package).
Supporting Information
Zdroje
1. HeXJ, ChenT, ZhuJK (2011) Regulation and function of DNA methylation in plants and animals. Cell Res 21 : 442–465.
2. JonesPA (2012) Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13 : 484–492.
3. KloseRJ, BirdAP (2006) Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31 : 89–97.
4. SuzukiMM, BirdA (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9 : 465–476.
5. BirdA (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16 : 6–21.
6. PaulsenM, Ferguson-SmithAC (2001) DNA methylation in genomic imprinting, development, and disease. J Pathol 195 : 97–110.
7. HellmanA, ChessA (2007) Gene body-specific methylation on the active X chromosome. Science 315 : 1141–1143.
8. NorrisDP, BrockdorffN, RastanS (1991) Methylation status of CpG-rich islands on active and inactive mouse X chromosomes. Mamm Genome 1 : 78–83.
9. ListerR, MukamelEA, NeryJR, UrichM, PuddifootCA, et al. (2013) Global Epigenomic Reconfiguration During Mammalian Brain Development. Science 341 (6146) 1237905.
10. Gardiner-GardenM, FrommerM (1987) CpG islands in vertebrate genomes. J Mol Biol 196 : 261–282.
11. AntequeraF, BirdA (1993) Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci U S A 90 : 11995–11999.
12. DeatonAM, BirdA (2011) CpG islands and the regulation of transcription. Genes Dev 25 : 1010–1022.
13. JonesPA, TakaiD (2001) The role of DNA methylation in mammalian epigenetics. Science 293 : 1068–1070.
14. ListerR, PelizzolaM, DowenRH, HawkinsRD, HonG, et al. (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462 : 315–322.
15. ZemachA, McDanielIE, SilvaP, ZilbermanD (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328 : 916–919.
16. GlastadKM, HuntBG, YiSV, GoodismanMA (2011) DNA methylation in insects: on the brink of the epigenomic era. Insect Mol Biol 20 : 553–565.
17. GaveryMR, RobertsSB (2010) DNA methylation patterns provide insight into epigenetic regulation in the Pacific oyster (Crassostrea gigas). BMC Genomics 11 : 483.
18. BonasioR, LiQ, LianJ, MuttiNS, JinL, et al. (2012) Genome-wide and Caste-Specific DNA Methylomes of the Ants Camponotus floridanus and Harpegnathos saltator. Curr Biol 22 : 1755–1764.
19. LykoF, ForetS, KucharskiR, WolfS, FalckenhaynC, et al. (2010) The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLoS Biol 8: e1000506.
20. ParkJ, PengZ, ZengJ, ElangoN, ParkT, et al. (2011) Comparative analyses of DNA methylation and sequence evolution using Nasonia genomes. Mol Biol Evol 28 : 3345–3354.
21. WurmY, WangJ, Riba-GrognuzO, CoronaM, NygaardS, et al. (2011) The genome of the fire ant Solenopsis invicta. Proc Natl Acad Sci U S A 108 : 5679–5684.
22. XiangH, ZhuJ, ChenQ, DaiF, LiX, et al. (2010) Single base-resolution methylome of the silkworm reveals a sparse epigenomic map. Nat Biotechnol 28 : 516–520.
23. GaoF, LiuX, WuXP, WangXL, GongD, et al. (2012) Differential DNA methylation in discrete developmental stages of the parasitic nematode Trichinella spiralis. Genome Biol 13: R100.
24. TweedieS, CharltonJ, ClarkV, BirdA (1997) Methylation of genomes and genes at the invertebrate-vertebrate boundary. Mol Cell Biol 17 : 1469–1475.
25. BrachtJR, PerlmanDH, LandweberLF (2012) Cytosine methylation and hydroxymethylation mark DNA for elimination in Oxytricha trifallax. Genome Biol 13: R99.
26. WalshTK, BrissonJA, RobertsonHM, GordonK, Jaubert-PossamaiS, et al. (2010) A functional DNA methylation system in the pea aphid, Acyrthosiphon pisum. Insect Mol Biol 19 Suppl 2 : 215–228.
27. SardaS, ZengJ, HuntBG, YiSV (2012) The Evolution of Invertebrate Gene Body Methylation. Mol Biol Evol 29 : 1907–16.
28. ForetS, KucharskiR, PittelkowY, LockettGA, MaleszkaR (2009) Epigenetic regulation of the honey bee transcriptome: unravelling the nature of methylated genes. BMC Genomics 10 : 472.
29. ElangoN, HuntBG, GoodismanMA, YiSV (2009) DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci U S A 106 : 11206–11211.
30. SimpsonVJ, JohnsonTE, HammenRF (1986) Caenorhabditis elegans DNA does not contain 5-methylcytosine at any time during development or aging. Nucleic Acids Res 14 : 6711–6719.
31. RaddatzG, GuzzardoPM, OlovaN, FantappieMR, RamppM, et al. (2013) Dnmt2-dependent methylomes lack defined DNA methylation patterns. Proc Natl Acad Sci U S A 110 : 8627–8631.
32. LykoF, RamsahoyeBH, JaenischR (2000) DNA methylation in Drosophila melanogaster. Nature 408 : 538–540.
33. GowherH, LeismannO, JeltschA (2000) DNA of Drosophila melanogaster contains 5-methylcytosine. EMBO J 19 : 6918–6923.
34. WerrenJH, RichardsS, DesjardinsCA, NiehuisO, GadauJ, et al. (2010) Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 327 : 343–348.
35. SrinivasanDG, BrissonJA (2012) Aphids: a model for polyphenism and epigenetics. Genet Res Int 2012 : 431531.
36. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443 : 931–949.
37. SmithCR, SmithCD, RobertsonHM, HelmkampfM, ZiminA, et al. (2011) Draft genome of the red harvester ant Pogonomyrmex barbatus. Proc Natl Acad Sci U S A 108 : 5667–5672.
38. HuntBG, GlastadKM, YiSV, GoodismanMA (2013) Patterning and regulatory associations of DNA methylation are mirrored by histone modifications in insects. Genome Biol Evol 5 : 591–598.
39. FloresKB, WolschinF, AllenAN, CorneveauxJJ, HuentelmanM, et al. (2012) Genome-wide association between DNA methylation and alternative splicing in an invertebrate. BMC Genomics 13 : 480.
40. BeukeboomL, DesplanC (2003) Nasonia. Curr Biol 13: R860.
41. WerrenJH, LoehlinDW (2009) The parasitoid wasp Nasonia: an emerging model system with haploid male genetics. Cold Spring Harb Protoc 2009: pdb emo134.
42. Munoz-TorresMC, ReeseJT, ChildersCP, BennettAK, SundaramJP, et al. (2011) Hymenoptera Genome Database: integrated community resources for insect species of the order Hymenoptera. Nucleic Acids Res 39: D658–662.
43. SazeH, TsuganeK, KannoT, NishimuraT (2012) DNA methylation in plants: relationship to small RNAs and histone modifications, and functions in transposon inactivation. Plant Cell Physiol 53 : 766–784.
44. HartiganJA, HartiganPM (1985) The Dip Test of Unimodality. Annals of Statistics 13 : 70–84.
45. HartiganPM (1985) Computation of the Dip Statistic to Test for Unimodality. Applied Statistics-Journal of the Royal Statistical Society Series C 34 : 320–325.
46. WaterhouseRM, TegenfeldtF, LiJ, ZdobnovEM, KriventsevaEV (2013) OrthoDB: a hierarchical catalog of animal, fungal and bacterial orthologs. Nucleic Acids Res 41: D358–365.
47. AndersenPK, JensenTH (2010) A pause to splice. Mol Cell 40 : 503–505.
48. MeyerKD, SaletoreY, ZumboP, ElementoO, MasonCE, et al. (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149 : 1635–1646.
49. ZengJ, YiSV (2010) DNA methylation and genome evolution in honeybee: gene length, expression, functional enrichment covary with the evolutionary signature of DNA methylation. Genome Biol Evol 2 : 770–780.
50. WolffeAP, MatzkeMA (1999) Epigenetics: regulation through repression. Science 286 : 481–486.
51. WeberM, HellmannI, StadlerMB, RamosL, PaaboS, et al. (2007) Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39 : 457–466.
52. SimolaDF, WisslerL, DonahueG, WaterhouseRM, HelmkampfM, et al. (2013) Social insect genomes exhibit dramatic evolution in gene composition and regulation while preserving regulatory features linked to sociality. Genome Res 23 : 1235–1247.
53. HerbBR, WolschinF, HansenKD, AryeeMJ, LangmeadB, et al. (2012) Reversible switching between epigenetic states in honeybee behavioral subcastes. Nat Neurosci 15 : 1371–1373.
54. LoehlinDW, WerrenJH (2012) Evolution of shape by multiple regulatory changes to a growth gene. Science 335 : 943–947.
55. NiehuisO, BuellesbachJ, GibsonJD, PothmannD, HannerC, et al. (2013) Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature 494 : 345–348.
56. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
57. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111.
58. TrapnellC, WilliamsBA, PerteaG, MortazaviA, KwanG, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28 : 511–515.
59. ThorvaldsdottirH, RobinsonJT, MesirovJP (2012) Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14 : 178–92.
60. RobinsonJT, ThorvaldsdottirH, WincklerW, GuttmanM, LanderES, et al. (2011) Integrative genomics viewer. Nat Biotechnol 29 : 24–26.
61. GentlemanRC, CareyVJ, BatesDM, BolstadB, DettlingM, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80.
62. ConesaA, GotzS, Garcia-GomezJM, TerolJ, TalonM, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21 : 3674–3676.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



