-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlaying the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
article has not abstract
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003918
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003918Summary
article has not abstract
Within the vertebrate nervous system, specialized glial cell types ensheath the axons of neurons with multiple wraps of membrane (myelin) in order to increase the speed and efficiency of nerve conduction. In the central nervous system, this role is fulfilled by oligodendrocytes; Schwann cells carry out the equivalent function within the peripheral nervous system. In spite of their common function, there are some substantial differences between oligodendrocytes and Schwann cells. For starters, they have different embryonic origins, arising from the neural tube and neural crest respectively. Each oligodendrocyte may myelinate anywhere from 1 to 50 axons, whereas a myelinating Schwann cell will devote its energy to a single axon. Even the major protein components of myelin in the peripheral and central nervous systems are a somewhat inexplicable mix; both incorporate the Myelin Basic Protein (MBP), but the major peripheral myelin protein, Protein Zero (P0), is replaced in the central nervous system with Proteolipid Protein (PLP). The transcription networks underlying differentiation and myelination in each cell type are also largely distinct, although one consistency is that the HMG-domain transcription factor Sox10 is required for successful myelination by both cell types [1], [2].
In this issue of PLOS Genetics, Hornig and colleagues [3] give another striking example of two very different genes being co-opted by Sox10 to drive the myelination process in each cell type.
Within the Schwann cells, Sox10 is known to directly induce the transcription of another transcription factor, Krox20 (also known as Egr2) [4]. Sox10 and Krox20 subsequently act in concert at myelin gene enhancers [5], [6] (Figure 1). Unlike Schwann cells, oligodendrocytes do not express Krox20, however recent work has identified a putative functional replacement, Myelin Regulatory Factor (Myrf, previously known as C11Orf9, MRF, and GM98). Just as Krox20 is upregulated in myelinating Schwann cells, Myrf is upregulated during oligodendrocyte differentiation and is required for them to myelinate [7].
Fig. 1. Feed-forward transcriptional networks regulating myelination in Schwann cells and oligodendrocytes. 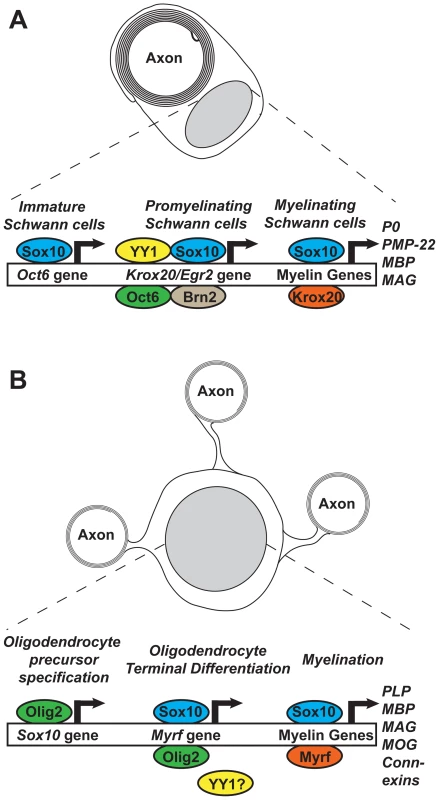
(A) Sox10 is present within immature Schwann cells and acts to induce expression from the Oct6 gene via a downstream enhancer [13]. Upon signaling from axonal neuregulin, Sox10, Oct6, Brn2, and YY1 act to drive Krox20 expression via a +35 kb upstream enhancer [4], [14]. Krox20 and Sox10 then synergistically activate myelin genes. (B) Within the developing central nervous system, Olig2 acts during oligodendrocyte precursor specification to induce the expression of Sox10 [15]. During terminal differentiation, Sox10 and Olig2 induce the expression of Myrf, with Sox10 binding the intron 1 enhancer/ECR9 [3]. Sox10 and Myrf then act at myelin gene promoters and enhancers to drive myelin gene expression. See [16], [17] for a more comprehensive review of the mechanisms controlling Schwann cell and oligodendrocyte development and myelination, respectively. Hornig and colleagues now demonstrate that Krox20 and Myrf not only have an analogous role in driving myelination in their respective cell types, they also share a remarkably similar relationship with Sox10. Just as Sox10 directly regulates Krox20 in Schwann cells, they find it is also required for the induction of Myrf during terminal oligodendrocyte differentiation in vivo. This regulation by Sox10 was mapped to an enhancer in the first intron of the Myrf gene containing several Sox consensus motifs. They show this enhancer is bound by Sox10 and is sufficient to drive gene expression in developing oligodendrocytes.
Perhaps equally strikingly, the Sox10 and Myrf proteins were found to subsequently physically interact and act synergistically at key myelin gene enhancers, including upstream of the MBP gene. This corroborates recent ChIP-Seq data indicating that the two bind to partially overlapping genomic regions within oligodendrocytes [8]. This, once again, closely mirrors the functional relationship between Sox10 and Krox20 in the PNS, where they act synergistically in the myelinating Schwann cells to regulate myelin gene expression [5], [6]. Intriguingly, both Hornig et al. and Bujalka et al. find that although there is some clear overlap and synergy between the myelin gene regulatory regions bound by Sox10 and Myrf, there are also some distinct differences, with many regulatory elements being targeted by just one factor [3], [8]. This suggests that the two do not necessarily act as part of an obligatory protein complex, instead sharing overlapping but subtly distinct roles.
These findings further cement the central role of Sox10 in the regulation of the differentiation of both Schwann cells and oligodendrocytes and their subsequent myelination. Indeed, its role is remarkably well-conserved in peripheral and central glia given how few other key transcription factors are common between the two. As Hornig et al. point out, on the surface Myrf appears to be an unlikely functional replacement for Krox20. Krox20 is a fairly well characterized zinc finger transcription factor, with a variety of roles in development. In contrast, Myrf is something of the eccentric elderly uncle in the transcription factor family. It has few close homologs but shows homology to the yeast transcription factor Ndt80 [9], also incorporating structural domains from bacteriophage proteins [8], [10]. Within vertebrates, its roles outside central nervous system myelination remain undefined. Nevertheless, the parallels between Krox20 and Myrf, as well as the relationship they share with Sox10, are clear.
A number of questions are raised by these findings.
As Hornig et al. note, Sox10 is present within the oligodendrocyte precursors for some time before it acts to promote the expression of Myrf. This indicates the presence of additional regulatory mechanisms. Recent work suggests that chromatin remodeling by Brg1 and Olig2 alters the accessibility of key genes, including Myrf, at the onset of terminal differentiation [11]. It is highly feasible that other factors such as Nkx2.2 and YY1 will also have a direct role in this regulation. The broader cellular events and molecular partners that direct Sox10 to the Myrf intronic enhancer at the critical point of oligodendrocyte differentiation will be important to determine.
Secondly, Hornig and colleagues found that Sox10 physically interacts with the C-terminal region of Myrf. Several groups have recently reported that the Myrf protein is cleaved as a prerequisite for its transcription factor function and that the C-terminal domain appears to be excluded from the nucleus [8], [10], [12]. The functional role of this physical interaction between Sox10 and the C-terminal of Myrf (and indeed the role of the C-terminal domain of Myrf more generally) will therefore be important to clarify.
Finally, and perhaps most intriguingly, the question of how Schwann cells and oligodendrocytes have come to perform essentially the same function (ensheathing nerve cells) during vertebrate evolution while using overlapping, but clearly discrete, sets of transcription regulators remains to be resolved. Do they share a common cellular ancestor, or (as Hornig and colleagues speculate), has the myelination process evolved independently in the central and peripheral nervous systems with Sox10, the common element in an otherwise largely mixed bag of genes? It seems possible that careful analysis of the neural cells that co-express Sox10, Krox20, and Myrf in our evolutionary distant relatives may hold the key to the origins of these cells and their relationship to each other.
Zdroje
1. BritschS, GoerichDE, RiethmacherD, PeiranoRI, RossnerM, et al. (2001) The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15 : 66–78.
2. StoltCC, RehbergS, AderM, LommesP, RiethmacherD, et al. (2002) Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 16 : 165–170.
3. HorningJ, FröbF, VoglM, Hermans-BorgmeyerI, TammE, et al. (2013) The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet 9 e1003907 doi: 10.1371/journal.pgen.1003907
4. GhislainJ, CharnayP (2006) Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep 7 : 52–58.
5. SrinivasanR, SunG, KelesS, JonesEA, JangSW, et al. (2012) Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve. Nucleic Acids Res 40 : 6449–6460.
6. LeBlancSE, JangSW, WardRM, WrabetzL, SvarenJ (2006) Direct regulation of myelin protein zero expression by the Egr2 transactivator. J Biol Chem 281 : 5453–5460.
7. EmeryB, AgalliuD, CahoyJD, WatkinsTA, DugasJC, et al. (2009) Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138 : 172–185.
8. BujalkaH, KoenningM, JacksonS, PerreauVM, PopeB, et al. (2013) MYRF Is a Membrane-Associated Transcription Factor That Autoproteolytically Cleaves to Directly Activate Myelin Genes. PLoS Biol 11: e1001625 doi: 10.1371/journal.pbio.1001625
9. FingermanIM, SutphenK, MontanoSP, GeorgiadisMM, VershonAK (2004) Characterization of critical interactions between Ndt80 and MSE DNA defining a novel family of Ig-fold transcription factors. Nucleic Acids Res 32 : 2947–2956.
10. LiZ, ParkY, MarcotteEM (2013) A Bacteriophage Tailspike Domain Promotes Self-Cleavage of a Human Membrane-Bound Transcription Factor, the Myelin Regulatory Factor MYRF. PLoS Biol 11: e1001624 doi: 10.1371/journal.pbio.1001624
11. YuY, ChenY, KimB, WangH, ZhaoC, et al. (2013) Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 152 : 248–261.
12. SenooH, ArakiT, FukuzawaM, WilliamsJG (in press) A new kind of membrane-tethered eukaryotic transcription factor that shares an auto-proteolytic processing mechanism with bacteriophage tail-spike proteins. Journal of Cell Sci Advance Online Article September 17, 2013.
13. JagalurNB, GhazviniM, MandemakersW, DriegenS, MaasA, et al. (2011) Functional dissection of the Oct6 Schwann cell enhancer reveals an essential role for dimeric Sox10 binding. J Neurosci 31 : 8585–8594.
14. HeY, KimJY, DupreeJ, TewariA, Melendez-VasquezC, et al. (2010) Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci 13 : 1472–1480.
15. KuspertM, HammerA, BoslMR, WegnerM (2011) Olig2 regulates Sox10 expression in oligodendrocyte precursors through an evolutionary conserved distal enhancer. Nucleic Acids Res 39 : 1280–1293.
16. PereiraJA, Lebrun-JulienF, SuterU (2012) Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci 35 : 123–134.
17. HeL, LuQR (2013) Coordinated control of oligodendrocyte development by extrinsic and intrinsic signaling cues. Neurosci Bull 29 : 129–143.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



