-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNew Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
The apple is the most common and culturally important fruit crop of temperate areas. The elucidation of its origin and domestication history is therefore of great interest. The wild Central Asian species Malus sieversii has previously been identified as the main contributor to the genome of the cultivated apple (Malus domestica), on the basis of morphological, molecular, and historical evidence. The possible contribution of other wild species present along the Silk Route running from Asia to Western Europe remains a matter of debate, particularly with respect to the contribution of the European wild apple. We used microsatellite markers and an unprecedented large sampling of five Malus species throughout Eurasia (839 accessions from China to Spain) to show that multiple species have contributed to the genetic makeup of domesticated apples. The wild European crabapple M. sylvestris, in particular, was a major secondary contributor. Bidirectional gene flow between the domesticated apple and the European crabapple resulted in the current M. domestica being genetically more closely related to this species than to its Central Asian progenitor, M. sieversii. We found no evidence of a domestication bottleneck or clonal population structure in apples, despite the use of vegetative propagation by grafting. We show that the evolution of domesticated apples occurred over a long time period and involved more than one wild species. Our results support the view that self-incompatibility, a long lifespan, and cultural practices such as selection from open-pollinated seeds have facilitated introgression from wild relatives and the maintenance of genetic variation during domestication. This combination of processes may account for the diversification of several long-lived perennial crops, yielding domestication patterns different from those observed for annual species.
Published in the journal: . PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002703
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002703Summary
The apple is the most common and culturally important fruit crop of temperate areas. The elucidation of its origin and domestication history is therefore of great interest. The wild Central Asian species Malus sieversii has previously been identified as the main contributor to the genome of the cultivated apple (Malus domestica), on the basis of morphological, molecular, and historical evidence. The possible contribution of other wild species present along the Silk Route running from Asia to Western Europe remains a matter of debate, particularly with respect to the contribution of the European wild apple. We used microsatellite markers and an unprecedented large sampling of five Malus species throughout Eurasia (839 accessions from China to Spain) to show that multiple species have contributed to the genetic makeup of domesticated apples. The wild European crabapple M. sylvestris, in particular, was a major secondary contributor. Bidirectional gene flow between the domesticated apple and the European crabapple resulted in the current M. domestica being genetically more closely related to this species than to its Central Asian progenitor, M. sieversii. We found no evidence of a domestication bottleneck or clonal population structure in apples, despite the use of vegetative propagation by grafting. We show that the evolution of domesticated apples occurred over a long time period and involved more than one wild species. Our results support the view that self-incompatibility, a long lifespan, and cultural practices such as selection from open-pollinated seeds have facilitated introgression from wild relatives and the maintenance of genetic variation during domestication. This combination of processes may account for the diversification of several long-lived perennial crops, yielding domestication patterns different from those observed for annual species.
Introduction
Domestication is a process of increasing codependence between plants and animals on the one hand, and human societies on the other [1], [2]. The key questions relating to the evolutionary processes underlying domestication concern the identity and geographic origin of the wild progenitors of domesticated species [3], the nature of the genetic changes underlying domestication [4], [5], the tempo and mode of domestication (e.g., rapid transition versus protracted domestication) [6] and the consequences of domestication for the genetic diversity of the domesticated species [7], [8], [9], [10]. An understanding of the domestication process provides insight into the general mechanisms of adaptation and the history of human civilization, but can also guide modern breeding programs aiming to improve crops or livestock species further [11], [12].
Plant domestication has mostly been studied in seed-propagated annual crops, in which strong domestication bottlenecks have often been inferred, especially in selfing annuals, such as foxtail millet, wheat and barley [11], [13], [14], [15], [16], [17]. Genetic data have suggested that domestication or the spread of domesticated traits has been fairly rapid in some annual species (e.g, maize or sunflower), with limited numbers of populations or species contributing to current diversity [10], [18], [19], [20], [21], [22]. In contrast, a combination of genetics and archaeology suggested a protracted model of domestication for other annual crops, and in particular for the origin of wheat or barley in the Fertile Crescent [11], [23]. However, the genetic consequences of domestication have been little investigated in long-lived perennials, such as fruit trees [24], [25], [26]. Trees have several biological features that make them fascinating and original models for investigating domestication: they are outcrossers with a long lifespan and a long juvenile phase, and tree populations are often large and connected by high levels of gene flow [27], [28].
Differences in life-history traits probably result in marked differences in the mode and speed of evolution between trees and seed-propagated selfing annuals [27], [28], [29]. For example, outcrossing may tend to make domestication more difficult, in part because the probability of fixing selected alleles is lower than in selfing crops [6], [13]. The combination of self-incompatibility and a long juvenile phase also results in highly variable progenies, making breeding a slow and expensive process, and rendering crop improvement difficult. The development of vegetative propagation based on cuttings or grafting has been a key element in the domestication of long-lived perennials, allowing the maintenance and spread of superior individuals despite self-incompatibility [30]. However, the use of such techniques has further decreased the number of sexual cycles in tree crops since the initial domestication event, adding to the effect of long juvenile phases in limiting the genetic divergence between cultivated trees and their wild progenitors [30], [31], [32], [33]. Thus, domestication can generally be considered more recent, at least in terms of the number of generations, in fruit tree crops than in seed-propagated selfing annuals.
Given the slow process of selection and the limited number of generations in which humans could exert selection, the protracted nature of the domestication process in trees has probably resulted in limited bottlenecks [25], [31] and in a weaker domestication syndrome [34] than in seed-propagated annuals. Nevertheless, many cultivated fruit trees clearly display morphological, phenotypic and physiological features typical of a domestication syndrome, such as large fruits and high sugar or oil content [32], [35]. Many aspects of fruit tree domestication have been little studied [25]. Consequently, most of the hypotheses concerning the consequences of particular features of trees for their domestication/diversification remain to be tested. Recent studies on grapevines, almond and olive trees have provided illuminating insights, such as the importance of outcrossing and interspecific hybridization [36], [37], [38], but additional studies of other species are required to draw more general conclusions.
Here, we investigated the origins of the domesticated apple Malus domestica Borkh., one of the most emblematic and widespread fruit crops in temperate regions [35]. A form of apple corresponding to extant domestic apples appeared in the Near East around 4,000 years ago [39], at a time corresponding to the first recorded uses of grafting. The domesticated apple was then introduced into Europe and North Africa by the Greeks and Romans and subsequently spread worldwide [35]. While the ancestral progenitor has been clearly identified as being M. sieversii, the identity and relative contributions of other wild species present along the Silk route that have contributed to the genetic makeup of apple cultivars remain largely unknown. This is surprising given the potential importance of this knowledge for plant breeding and for our understanding of the process of domestication in fruit trees.
The wild Central Asian species M. sieversii (Ldb.) M. Roem has been identified as the main contributor to the M. domestica genepool based on similarities in fruit and tree morphology, and genetic data [40], [41], [42], [43]. The Tian Shan forests were identified as the geographic area in which the apple was first domesticated, on the basis of the considerable intraspecific morphological variability of wild apple populations in this region [44], [45]. Nucleotide variation for 23 DNA fragments even suggested that M. sieversii and M. domestica belonged to a single genepool (which would be called M. pumila Mill.), with phylogenetic networks showing an intermingling of individuals from the two taxa [43]. Some authors have also suggested possible contributions of additional wild species present along the Silk Route: M. baccata (L.) Borkh, which is native to Siberia, M. orientalis Uglitz., a Caucasian species present along western sections of the ancient trade routes, and M. sylvestris Mill. (European crabapple), a species native to Europe [46], [47], [48], [49]. These hypotheses were based on the history of human migration and trade, the lack of phylogenetic resolution between M. domestica and these four wild species [41], [42], genetic evidence of hybridization at a local scale between domesticated apple and M. sylvestris [40], and the recent finding of sequence haplotype sharing between M. sylvestris and M. domestica [50]. However, such secondary contributions remain a matter of debate, mostly due to the difficulty of distinguishing introgression from incomplete lineage sorting [43], [50], [51]. The three wild species occurring along the Silk Route all bear small, astringent, tart fruits. None of these species has the fruit quality of M. sieversii, but they may have contributed other valuable horticultural traits, such as later flowering, resistance to pests and diseases, capacity for longer storage or climate adaptation. The organoleptic properties of the fruits of these wild species may also have been selected during domestication, for the preparation of apple-based beverages, such as ciders [46], [52]. Cider apples are indeed smaller, bitter and more astringent than dessert apples and bear some similarity to M. sylvestris apples. There is also evidence to suggest that Neolithic and Bronze Age Europeans were already making use of M. sylvestris [39].
In this study, we used a comprehensive set of apple accessions sampled across Eurasia (839 accessions from China to Spain; Figure 1 and Figure S1; Table S1) and 26 microsatellite markers distributed evenly across the genome to investigate the following questions: 1) Is there evidence for population subdivision within and between the five taxa M. domestica, M. baccata, M. orientalis, M. sieversii and M. sylvestris? 2) How large is the contribution of wild species other than the main progenitor, M. sieversii, to the genome of M. domestica? 3) Does M. domestica have a genetic structure associated with its different possible uses (i.e., differences between cider and dessert apples)? 4) What consequences have domestication, subsequent crop improvement and vegetative propagation by grafting had for genetic variation in cultivated apples? Most of our samples of M. domestica corresponded to cultivars from Western Europe (Figure 1 and Figure S1), as almost all the cultivars available in modern collections (including American, Australasian cultivars) are of European ancestry and this region is therefore the most relevant area for the detection of possible secondary introgression from the European crabapple.
Fig. 1. Geographic origins of the samples of the four wild Malus species used: M. sylvestris (blue), M. orientalis (yellow), M. baccata (purple), and M. sieversii (red). 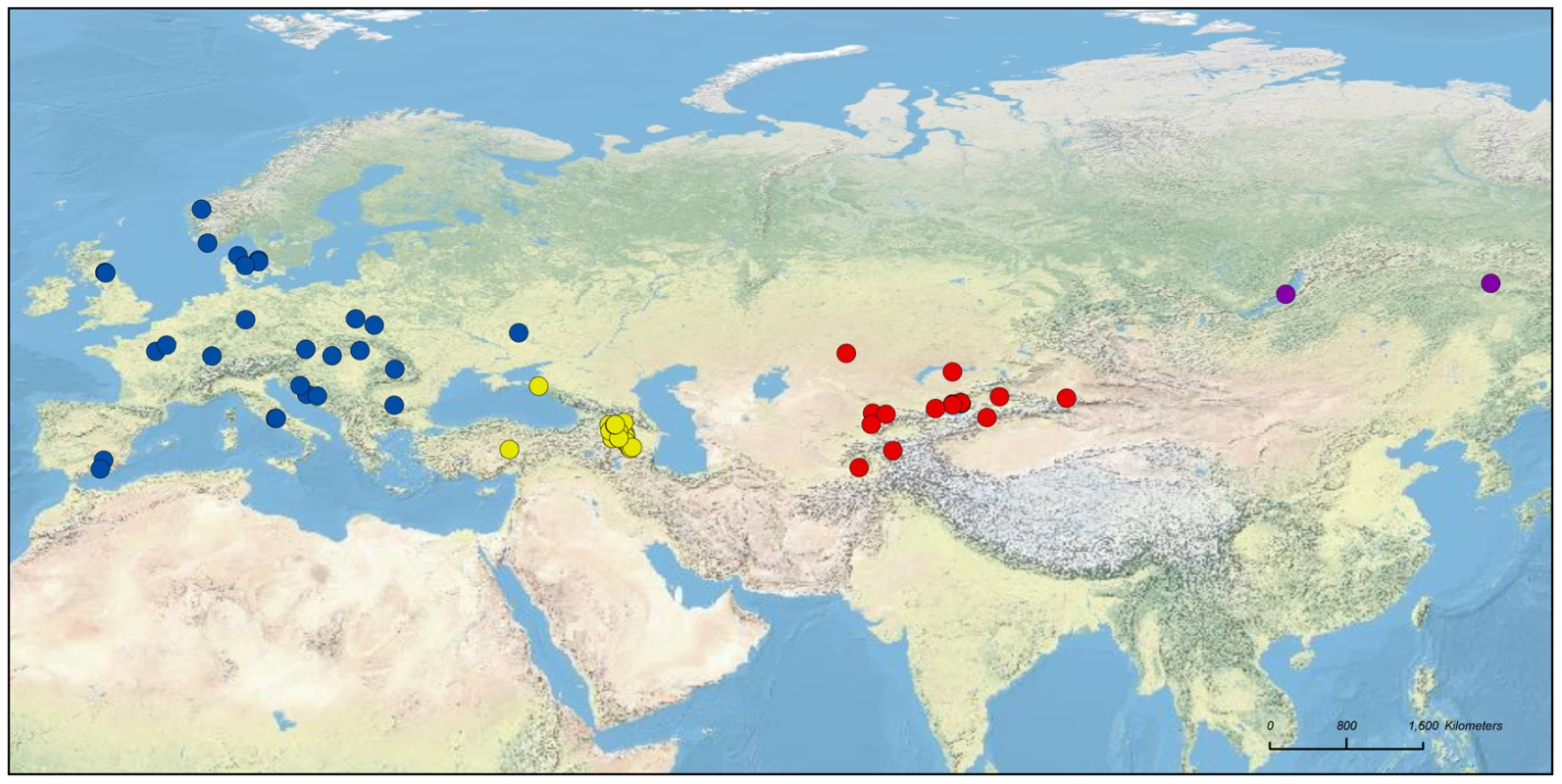
Samples of unknown origin (N = 28) were not projected onto the map. Results
High diversity and low deviations from random mating expectations within species
Our sampling scheme (Figure 1 and Figure S1), based on the collection of a single tree for each apple variety, was designed to avoid the sampling of clones. However, there may still be some clonality if some varieties differing by only a few mutations were propagated by grafting. We corrected for this potential clonality, using the clonal assignment procedures implemented in GENODIVE [53]. We found no pair of samples assigned to the same clonal lineage unless using a threshold of 22 pairwise differences between multilocus genotypes, indicating that our samples did not include any clonal genotypes (the threshold corresponds to the maximum genetic distance allowed between genotypes deemed to belong to the same clonal lineage).
Many apple cultivars, including modern cultivars in particular, share recent common ancestors, and siblings or clones of wild species can also be collected unintentionally in the field. Because these features could result in a spurious genetic structure due to the presence of closely related individuals in the dataset, we checked for the presence of groups of related individuals in our dataset between M. domestica cultivars and between the individuals of each wild species. The percentage of pairs with a pairwise relatedness (rxy) greater than 0.5 (i.e., full sibs) was: 0.4% in M. domestica (N = 168 pairs), 0.3% in M. sieversii (N = 79), 0.004% in M. orientalis (N = 20), and 0.7% in M. baccata (N = 40). For M. sylvestris, no individual pair with rxy>0.5 was identified. However, the distribution of pairwise relatedness rxy among M. domestica cultivars did not deviate significantly from a Gaussian distribution centred on 0 and with a low variance (Fisher's exact test, P≈1, standard deviation = 0.11, Figure S2). This suggests that closely related cultivars are unlikely to have biased subsequent analyses of population structure. We also checked for the limited effect of relatedness on our conclusions by performing all analyses of population subdivision on both the full dataset and a pruned dataset excluding related individuals (see below).
We tested the null hypothesis of random mating within each species by calculating FIS, which measures inbreeding. All five Malus species had relatively low values of FIS, although all were significantly different from zero (Table 1), suggesting that each species corresponded to an almost random mating unit. This is consistent with the self-incompatibility system of these species and indicates a lack of widespread groups of related individuals in M. domestica. Low FIS values at species level also indicate a lack of population structure within species. The higher values of FIS observed in M. baccata probably resulted from the occurrence of null alleles, as the microsatellite markers were developed in M. domestica, to which M. baccata is the most distantly related (Table 2). The lowest FIS value was that obtained for M. domestica, reflecting outcrossing between dissimilar parents in breeding programs, or that selection targeted higher levels of heterozygosity [54].
Tab. 1. Summary of genetic variation in the five Malus species. 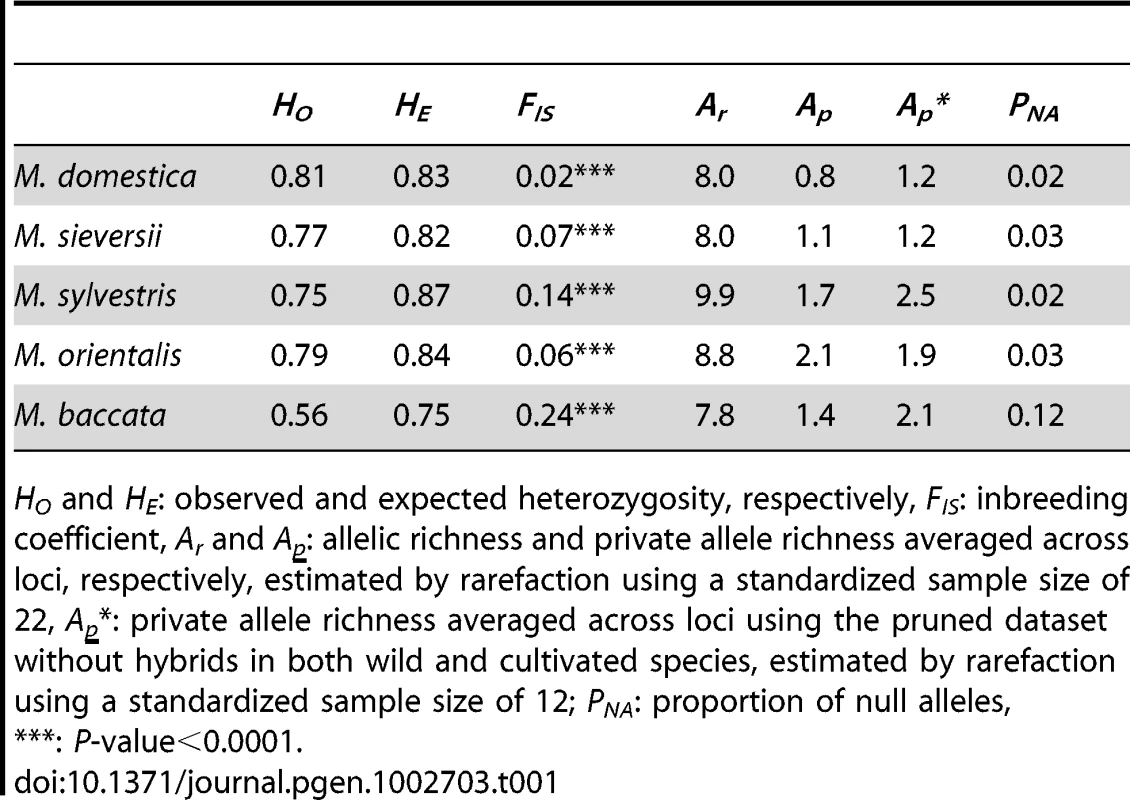
HO and HE: observed and expected heterozygosity, respectively, FIS: inbreeding coefficient, Ar and Ap: allelic richness and private allele richness averaged across loci, respectively, estimated by rarefaction using a standardized sample size of 22, Ap*: private allele richness averaged across loci using the pruned dataset without hybrids in both wild and cultivated species, estimated by rarefaction using a standardized sample size of 12; PNA: proportion of null alleles, Tab. 2. Pairwise differentiation (FST) between the five Malus species. 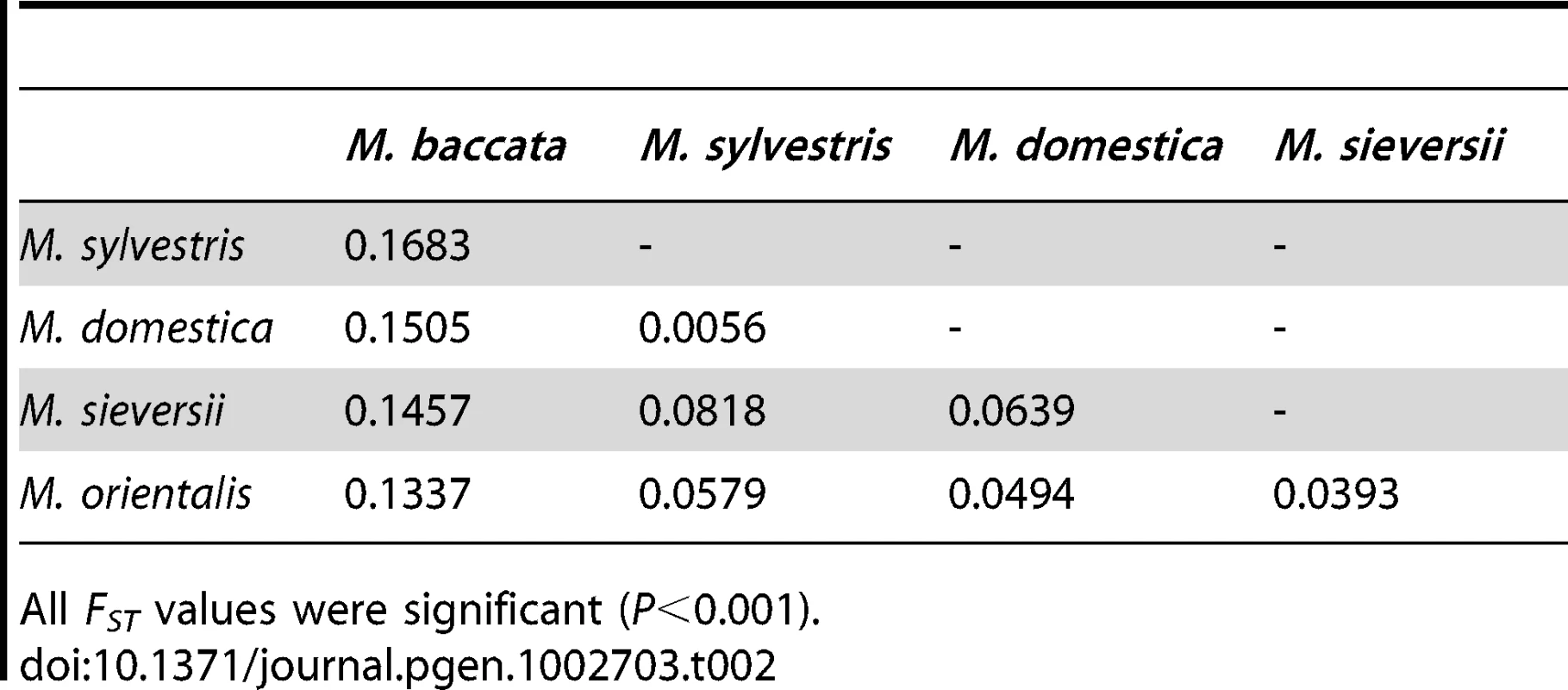
All FST values were significant (P<0.001). The five Malus species form well separated genetic clusters
We used the ‘admixture model’ implemented in STRUCTURE 2.3 [55] to infer population structure and introgression. Analyses were run for population structure models assuming K = 1 to K = 8 distinct clusters (Figure 2). The ΔK statistic, designed to identify the most relevant number of clusters by determining the number of clusters beyond which there is no further increase in likelihood [56], was greatest for K = 3 (ΔK = 6249, Pr|ln L = −78590). However, the clusters identified at higher K values may also reveal a genuine and biologically relevant genetic structure, provided that they are well delimited [57]. The five Malus species were clearly assigned to different clusters for models assuming K≥6 clusters and for a minor clustering solution (“mode”) at K = 5 (Figure 2). The major mode (i.e., the clustering solution found in more than 60% of the simulation replicates) observed at K = 5 grouped together M. sylvestris and M. domestica genotypes. Increasing the number of clusters above K = 6 identified no additional well-delimited clusters corresponding to a subdivision of a previous cluster. Instead, it simply introduced heterogeneity into membership coefficients, indicating that the clustering of the five Malus species into separate genepools was the most relevant clustering solution. We checked that the presence of related pairs of cultivars in our dataset did not bias clustering results, by repeating the analysis on a pruned dataset (N = 489) excluding all related individuals in wild and cultivated species (i.e., excluding all pairs with rxy≥0.5). Similar results were obtained, with the same five distinct clusters identified as for the full dataset.
Fig. 2. Proportions of ancestry of Malus genotypes from five species (N = 770) from K = 2 to K = 8 ancestral genepools (“clusters”) inferred with the STRUCTURE program. 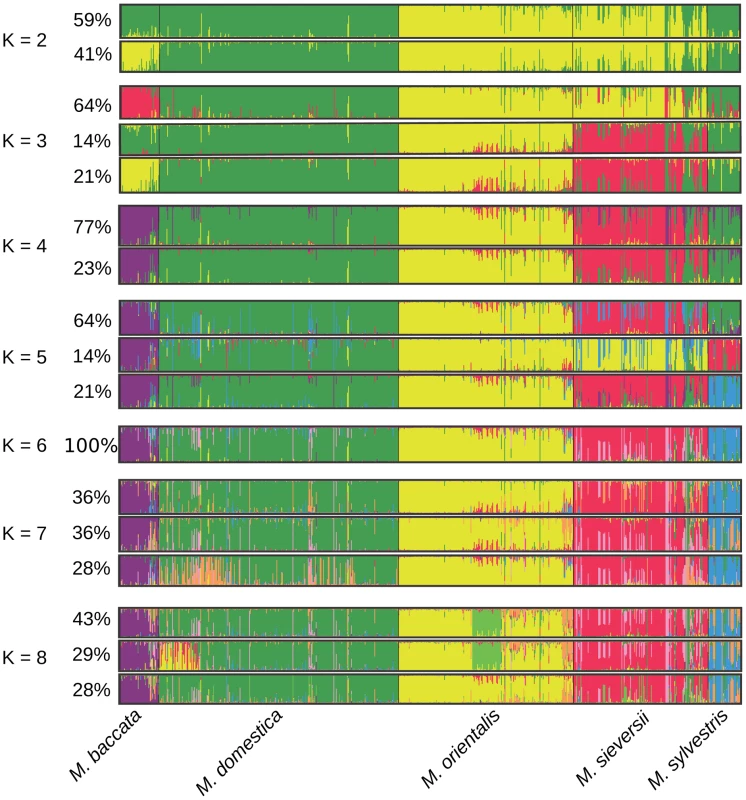
Each individual is represented by a vertical bar, partitioned into K segments representing the amount of ancestry of its genome in K clusters. When several clustering solutions (“modes”) were represented within replicate runs, the proportion of simulations represented by each mode is given. We estimated the genetic differentiation between the five Malus species by calculating pairwise FST (Table 2). All FST values were highly significant (P<0.001) and seemed to indicate a West to East differentiation gradient of M. domestica with the wild species. The highest level of differentiation was that between M. baccata and the other Malus species, and the lowest level of differentiation was that between M. domestica and the westernmost species, M. sylvestris (Table 2). Malus domestica was markedly more differentiated from its main progenitor M. sieversii (FST = 0.0639) than from the European M. sylvestris (FST = 0.006) and it was only slightly less differentiated from the Caucasian M. orientalis (FST = 0.049).
No bottleneck during apple domestication
We first searched for footprints of a domestication bottleneck by comparing levels of microsatellite variation in M. domestica and wild species. There was no significant difference in genetic diversity (as measured by expected heterozygosity, HE) between M. domestica and M. baccata, M. orientalis or M. sieversii, but HE was significantly higher in M. sylvestris than in M. domestica (Table 1). Significant differences in allelic richness (Ar) were found between M. domestica and M. orientalis (Wilcoxon signed rank test, P = 0.03) or M. sylvestris (P<10−8), but not between M. domestica and either M. baccata (P = 0.9) or M. sieversii (P = 0.9) (Table 1).
We used the method implemented in the BOTTLENECK program [58], comparing the expected heterozygosity estimated from allele frequencies with that estimated from the number of alleles and the sample size, which should be identical for a neutral locus in a population at mutation-drift equilibrium. Inferences about historical changes in population size are based on the prediction that the expected heterozygosity estimated from allele frequencies decreases faster than that estimated under a given mutation model at mutation-drift equilibrium in populations that have experienced a recent reduction in size. BOTTLENECK analysis showed no significant deviation from mutation-drift equilibrium in any of the five species, under either stepwise or two-phase models of microsatellite evolution (one-tailed Wilcoxon signed rank test, P>0.95). We therefore detected no signal of a demographic bottleneck associated with the domestication of apples.
Variable recent contributions of wild relative species to the M. domestica genepool, with the strongest introgression from M. sylvestris
We used the admixture coefficients estimated by STRUCTURE to assess the recent contribution of the various wild species to the M. domestica genepool. STRUCTURE analyses of the full dataset showed some admixture among Malus species for the minor mode separating the five species at K = 5. Admixture coefficients were higher between M. domestica and M. sylvestris (α = 0.23) than between M. domestica and respectively M. sieversii (α = 0.06), M. orientalis (α = 0.034) and M. baccata (α = 0.032).
We further analysed the contribution of each wild species to the genome of M. domestica by running STRUCTURE separately on each pair of species including M. domestica (Figure 3; Table 3 and Table S2). Malus domestica genotypes with membership coefficients ≥0.20 in a wild species genepool were considered to display introgression. Using this somehow arbitrary cut-off value, STRUCTURE analyses revealed that 26% of M. domestica cultivars displayed introgression from the European crabapple, M. sylvestris (Table 3 and Table S2). By contrast, only 2%, 3% and 0.02% of the M. domestica genotypes displayed introgression from M. sieversii, M. orientalis and M. baccata, respectively (Table 3 and Table S2). The M. domestica cultivars displaying admixture with the M. sylvestris genepool were mostly Russian (e.g., “Antonovka”, “Antonovka kamenicka”, “Novosibirski Sweet”, “Yellow transparent”), French (e.g., “Blanche de St Anne”, “St Jean”, “Api” and “Michelin”) and English (e.g., “Worcester Pearmain” and “Fiesta”). The M9 dwarf apple cultivar (“Paradis jaune de Metz”, [59]) commonly used as a rootstock also appeared to display introgression from the European crabapple (proportion of ancestry in the M. domestica genepool: 0.28; Table S2). When French cultivars were removed from the dataset (N = 89) and pairwise STRUCTURE analyses were repeated for all species pairs including M. domestica, 18% of cultivars displayed introgression from M. sylvestris, including commercial cultivars such as Granny Smith, Michelin, Antonovka and Ajmi (Figure S3) with a mean membership coefficient of M. sylvestris into M. domestica genepool of 47%. Malus sylvestris thus appears to have made a significant contribution to the M. domestica genepool through recent introgression, building on the more ancient contribution (see below) of the Asian wild species M. sieversii. We also note that a few M. domestica individuals appeared to display introgression from several wild species (Table S2), and that M. baccata ornamental cultivars, such as M. baccata flexilis, M. baccata Hansen's and M. baccata gracilis, were partially or even mostly assigned (from 32% to >80%) to the M. domestica genepool (Table S3).
Fig. 3. Proportions of ancestry in two ancestral genepools inferred with the STRUCTURE program, based on datasets including M. domestica (green, N = 299) and each of the four wild Malus species (red). 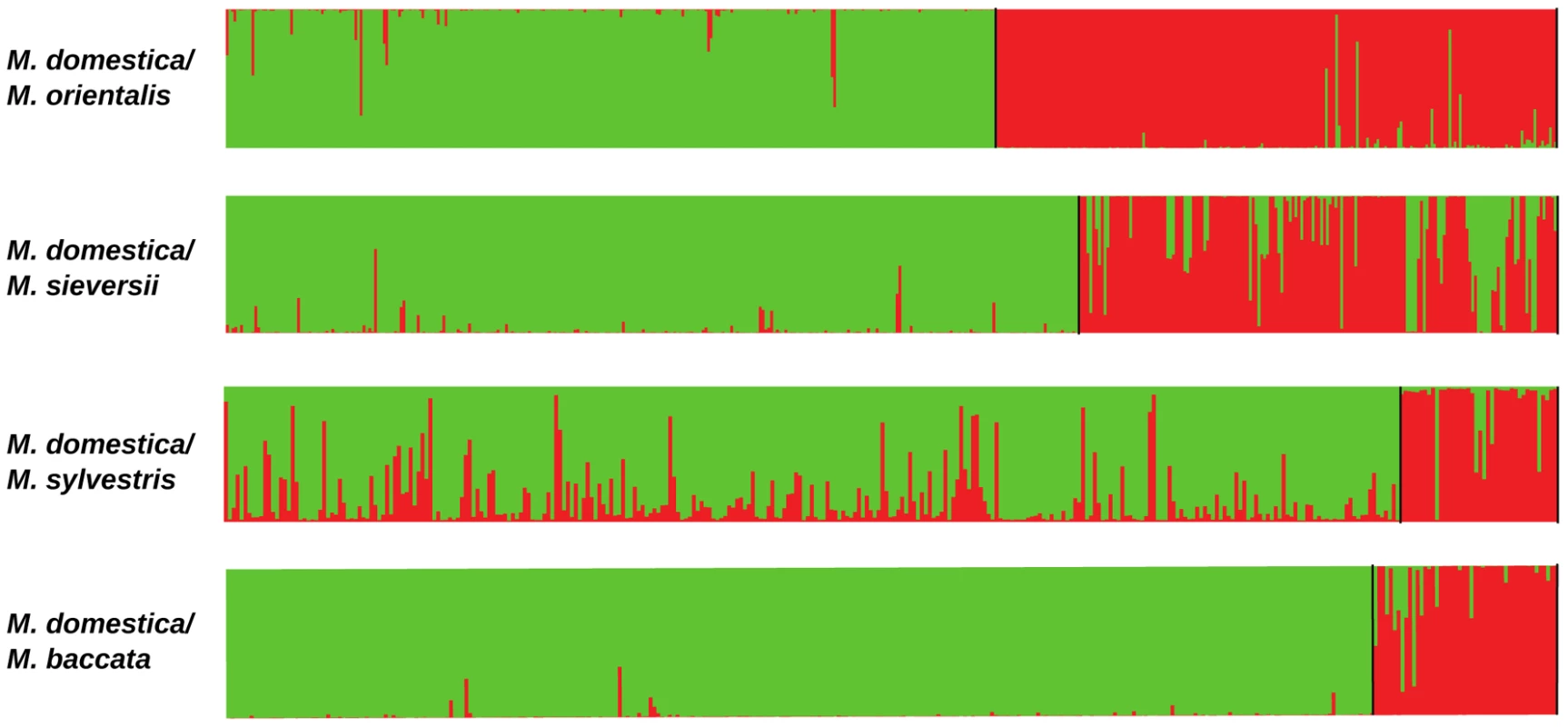
The x-axis is not to scale (details in Table S2). Tab. 3. Mean proportions of assignment to each of the two species in species pair comparisons (K = 2) including <i>M. domestica</i> (Genepool 1) and each of the four wild <i>Malus</i> species (Genepool 2). 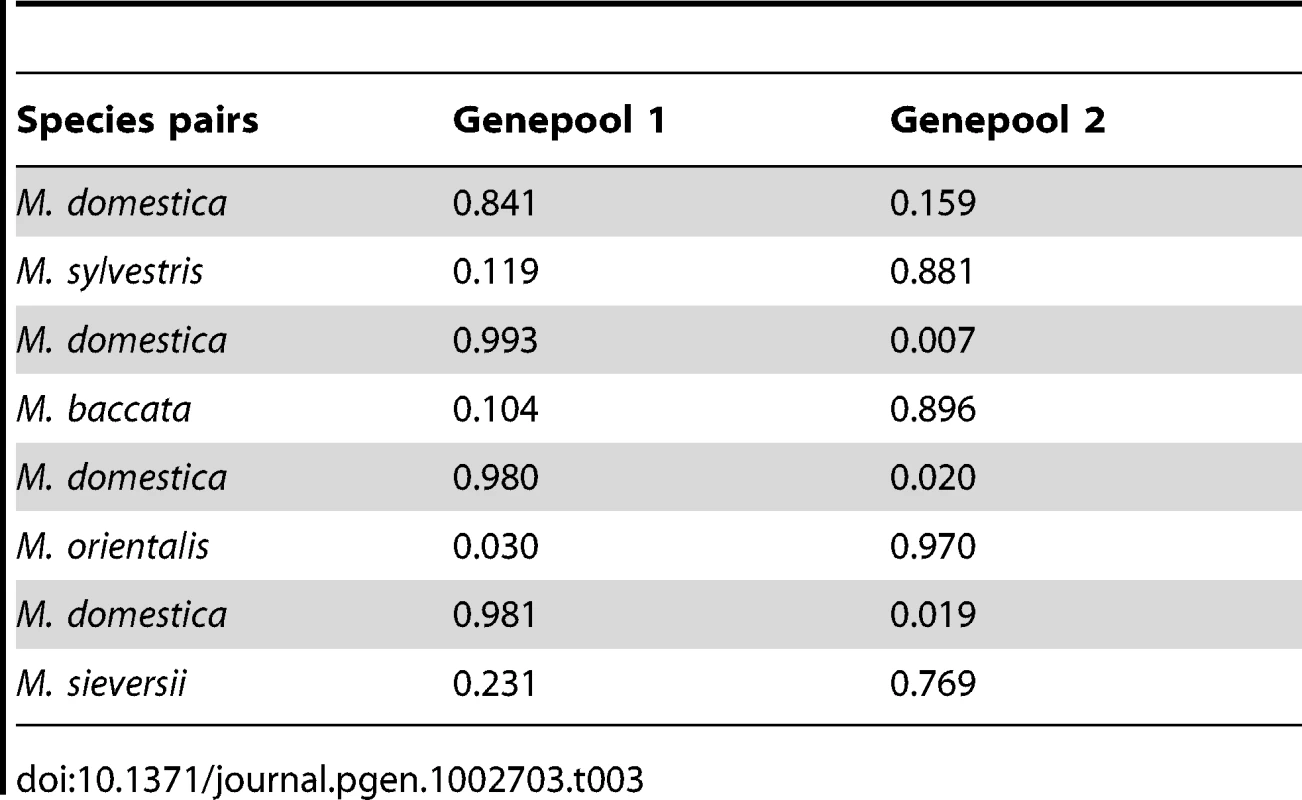
Wild Central Asian apple origin of the M. domestica genepool
Previous studies [43], [50], [60] identified the Central Asian wild apple M. sieversii as the main progenitor of M. domestica on the basis of DNA sequences. Due to the large contribution by M. sylvestris detected in our dataset, corresponding mostly to Western European cultivars, M. domestica and M. sylvestris appeared to be the most closely related pair of species in our analyses of microsatellite markers. We investigated the more ancient contribution of M. sieversii to the M. domestica genepool, by reassessing the genetic differentiation between species in analyses restricted to “pure” individuals (i.e., assigned at ≥0.9 to their respective genepools) from both wild and cultivated species. All FST values were highly significant (P<0.001), but the ranking of FST values between M. domestica and the various wild species was affected: the highest differentiation was still observed between M. domestica and M. baccata (FST = 0.22), but the lowest differentiation was observed between M. domestica and M. sieversii (FST = 0.11). Regarding the differentiation between M. sylvestris and M. domestica, we observed the opposite of what was found with the full dataset: M. sylvestris appeared to be more strongly differentiated (FST = 0.14) from M. domestica than M. sieversii. Thus, by removing signals of recent introgression between cultivated and wild species we were able to confirm that M. sieversii was the initial progenitor of M. domestica.
Recent introgression from M. domestica into wild species
The finding of a significant level of introgression from wild species into cultivated apple suggested that gene flow might also have occurred in the opposite direction. STRUCTURE analyses of pairs of species confirmed this hypothesis (Figure 3), revealing possible introgression of genetic material into M. sylvestris, M. baccata, M. orientalis and M. sieversii from M. domestica (mean proportions of ancestry in the M. domestica genepool of 0.12, 0.10, 0.03 and 0.23, respectively; Table 3). Considering genotypes with membership coefficients ≥0.9 in the M. domestica genepool as misclassified, we found a total of N = 31 misclassified wild Malus individuals. These results suggest gene flow from the domesticated apple genepool could significantly affect the genetic integrity of wild apple relatives, their future evolution and, possibly, their use as resources for crop improvement.
Inference of demographic history
Model-based Bayesian clustering algorithms, such as that implemented in STRUCTURE, have a high level of power only for the detection of recent introgression events [55], [61], [62]. We therefore investigated the contributions of M. sylvestris and M. orientalis to the M. domestica genepool using approximate Bayesian computation (ABC) methods that offer a more historical perspective on gene flow [63]. We used a demographic model implementing admixture events [64].
We compared several admixture models to infer what species pairs underwent introgression events and to estimate introgression rates [64]. Malus baccata was not included in these analyses because of its high level of divergence from M. domestica. We assumed, as suggested by previous studies, that M. domestica derived originally from M. sieversii. The most complex model simulated sequential admixtures between M. domestica and all wild species. Other models sequentially removed introgression with each wild species, the order being based on FST values and admixture rates inferred by STRUCTURE. The compared models were the following: (i) the model a assumed that M. domestica was derived from M. sieversii and that the ancestral M. domestica population was involved in reciprocal introgression events with M. orientalis and M. sylvestris, and subsequently introgressed back into M. sieversii (Figure 4a), (ii) model b was similar to the model a, but without introgression events from M. domestica into wild species (Figure 4b), (iii) the model c included a single introgression event, from M. sylvestris into M. domestica (Figure 4c), and (iv) the model d simulated no admixture (Figure 4d). The number of parameters estimated in the model was limited by fixing the times of admixture with M. orientalis, M. sylvestris and M. sieversii at 600, 200 and 13 generations before the present, respectively. We used the following underlying hypotheses: (i) as the juvenile period of Malus lasts five to 10 years, we assumed a generation time of 7.5 years, (ii) admixture between ancestral M. domestica and M. orientalis in the Caucasus occurred approximately 4,500 years ago, shortly before the appearance of sweet apples in the Middle East (4,000 years ago), (iii) admixture between ancestral M. domestica and M. sylvestris in Europe occurred approximately 1,500 years ago, soon after the introduction of domesticated apples into Europe by the Greeks and Romans (iv) back-introgression into M. sieversii from M. domestica occurred approximately 100 years ago, when the cultivation of modern varieties reached Central Asia.
Fig. 4. Admixture models compared in approximate Bayesian computations. 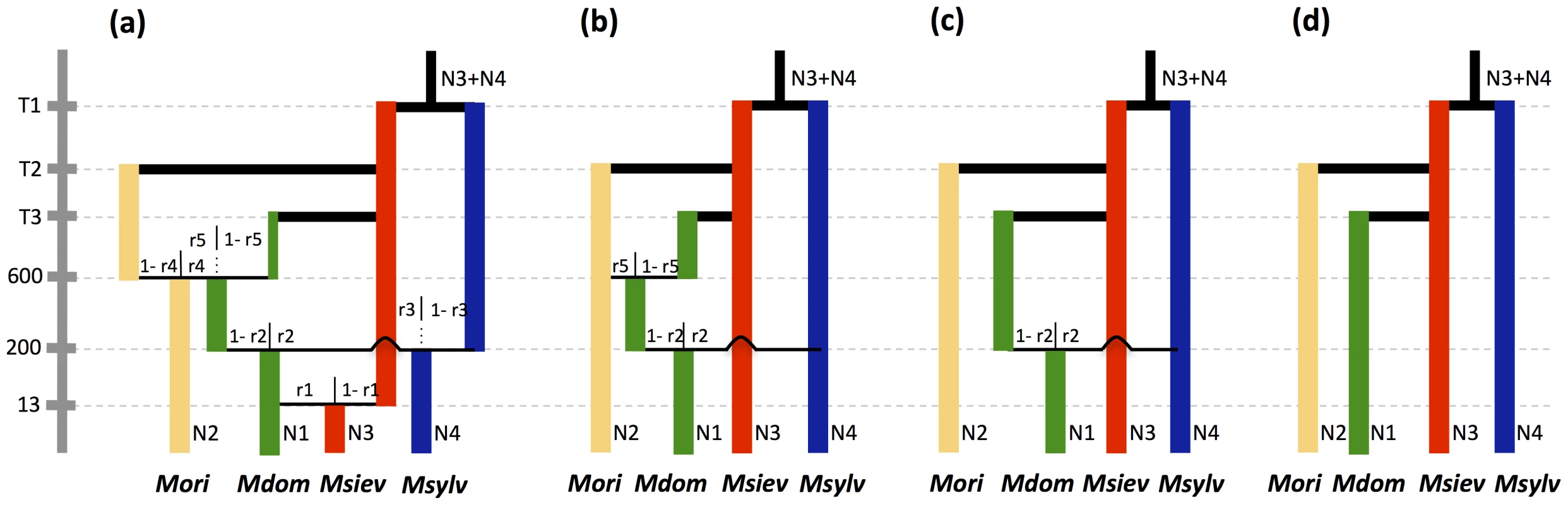
Model a assumes that M. domestica is derived from M. sieversii and that the ancestral M. domestica population was involved in reciprocal introgression events with M. orientalis and M. sylvestris, and subsequently introgressed back into M. sieversii. Model b assumes no introgression from M. domestica into wild species, model c assumes the only admixture event is from M. sylvestris into M. domestica, and model d assumes no admixture. Admixture times between M. domestica and the three wild species were fixed (see text). Abbreviations: Nk, effective population sizes; Tk, divergence times; r1, r3, r4 introgression from M. domestica into M. sieversii, M. sylvestris, and M. orientalis respectively; r2, r5 introgression from M. sylvestris and M. orientalis, respectively, into M. domestica. The relative posterior probabilities computed for each model provided strongest statistical support for model c, which assumed a single introgression event, from M. sylvestris into M. domestica (Table 4; posterior probability [p] = 0.67, 95% confidence interval: 0.63–0.72). Note that the model without admixture (model d) had the lowest relative posterior probability (Table 4). In analyses under alternative admixture models (models a and b), the posterior distributions were flat for introgression between M. domestica and M. orientalis and highly skewed towards low values for introgression into M. sylvestris and M. sieversii (not shown), which is consistent with statistical support being highest for model c.
Tab. 4. Relative posterior probabilities (p) for the four historical models compared using approximate Bayesian computations. 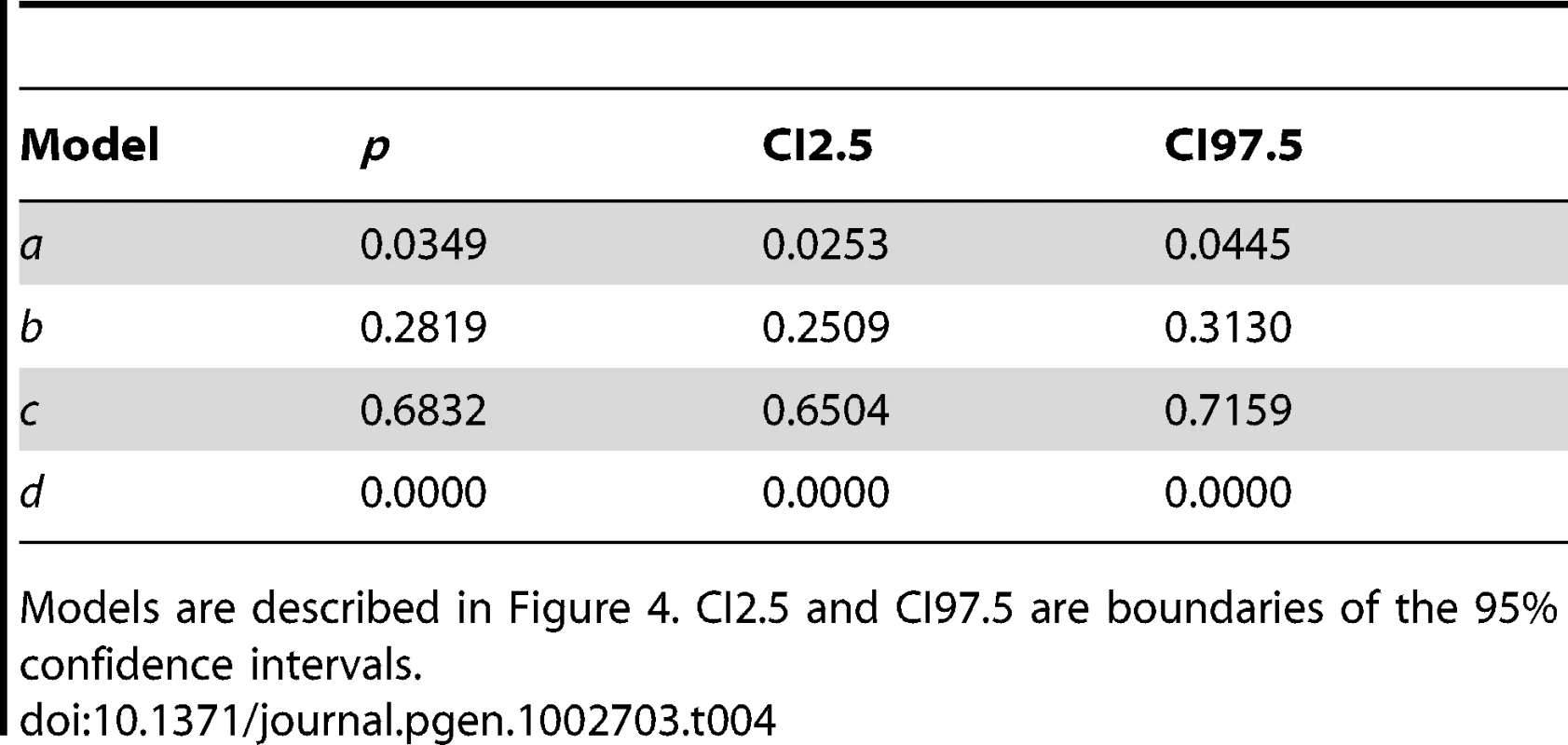
Models are described in Figure 4. CI2.5 and CI97.5 are boundaries of the 95% confidence intervals. Given that the model c was clearly favoured, parameter estimates are shown below only for this model (Table 5; prior distributions in Table S4). The contribution of M. sylvestris to the M. domestica genepool was estimated at about 61% (95% credibility interval [95% CI]: 50–68%). We obtained estimates of effective population sizes of 3,520 (95% CI: 2,090–5,680) for M. domestica, 13,200 (95% CI: 6,920–19,300) for M. sieversii, 34,600 (95% CI: 15,100–48,000) for M. sylvestris, and 28,300 (95% CI: 11,700–64,000) for M. orientalis. Using a generation time of 7.5 years, the divergence between M. domestica and M. sieversii (T3) was estimated to have occurred 17,700 years ago (95% CI: 6,225–25,200), which is earlier than previously thought, but we note that the credibility interval is quite large. We estimated that M. sylvestris and M. sieversii diverged about 83,250 years ago (T1, 95% CI: 40,575–334,500), with M. orientalis and M. sieversii diverging about 20,775 years ago (T2, 95% CI: 9,900–47,775).
Tab. 5. Demographic and mutation parameters estimated using approximate Bayesian computation for model c. 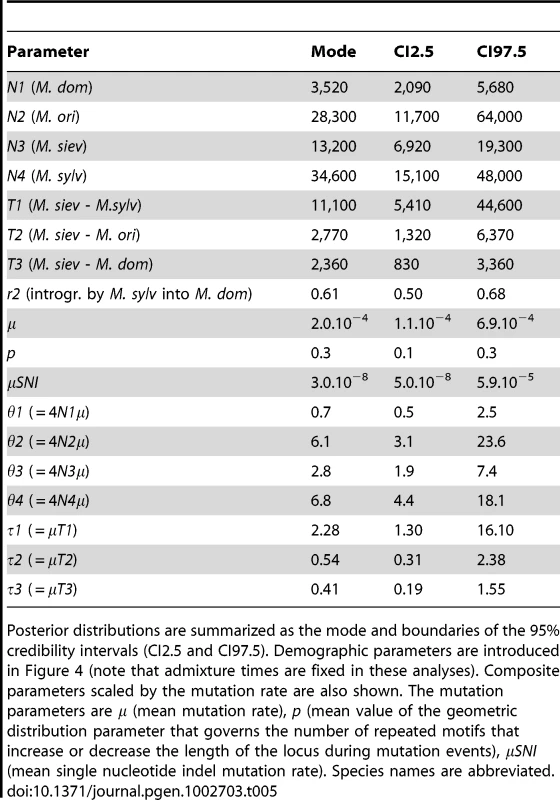
Posterior distributions are summarized as the mode and boundaries of the 95% credibility intervals (CI2.5 and CI97.5). Demographic parameters are introduced in Figure 4 (note that admixture times are fixed in these analyses). Composite parameters scaled by the mutation rate are also shown. The mutation parameters are μ (mean mutation rate), p (mean value of the geometric distribution parameter that governs the number of repeated motifs that increase or decrease the length of the locus during mutation events), μSNI (mean single nucleotide indel mutation rate). Species names are abbreviated. The results above were obtained using the full dataset. We checked the validity of our inferences by conducting analyses on the dataset without admixed and misclassified individuals and using different times of admixture, by assessing the goodness-of-fit of models to data, and by checking that sufficient power was achieved to discriminate among competing models (Text S1; Tables S5, S6, S7). Overall, ABC analyses all provided clear support for a model with contribution of the European crabapple into the domesticates, although the estimated value of the actual contribution of M. sylvestris is probably overestimated here, and should therefore be treated with caution. Indeed, the simulation of a single introgression event hundreds of years ago most likely demanded higher rates of introgression to account for the actual genetic contribution of M. sylvestris into M. domestica than would be needed under continuous gene flow over a long period.
Weak genetic structure within M. domestica: linked to cultivar use or geography?
As cider cultivars produce apples that are smaller, more bitter and astringent than dessert cultivars, we expected to observe genetic differentiation between these two groups of cultivars and a closer genetic proximity of cider cultivars to M. sylvestris [35], [65]. Neither hypothesis was supported by our data. The classification of apples into “dessert” and “cider” varieties as prior information for STRUCTURE (Locprior model) revealed a very weak tendency of cider and dessert cultivars to be assigned to different clusters at K = 2 (Figure 5), but increasing K did not further result in clearer differentiation between the two types of cultivars. At K = 2, M. domestica cider genotypes had a mean membership of 94.7%, and M. domestica dessert genotypes had a mean membership of 52.5%. However, STRUCTURE analyses without this prior information gave essentially the same clustering patterns at K = 2 (G′ = 0.95 similarity to analyses using classification to assist clustering). The weak differentiation between cider and dessert cultivars (FST = 0.02) and their high level of admixture in STRUCTURE analyses (Figure 5) indicated a shallow subdivision of the M. domestica genepool. Analyses on a pruned dataset from which closely related individuals had been removed (i.e., pairs of genotypes with rxy≥0.5; N = 172) revealed the same pattern, confirming that the presence of related cultivars in the dataset did not bias clustering analyses. STRUCTURE was also run on a dataset including all M. sylvestris genotypes, to test the hypothesis that cider cultivars would display a higher level of introgression from the European crabapple. However, the opposite pattern was observed: the proportion of genotypes displaying introgression from M. sylvestris was actually significantly higher in dessert than in cider cultivars (36.4% and 15.5% respectively, χ2 = 16.9, P = 4×10−5). Finally, little genetic differentiation was observed between groups of cultivars of different geographic origins (95% CI: −0.8–0.6, Table S8).
Fig. 5. Proportions of ancestry of <i>M. domestica</i> genotypes (cider and dessert apples) in two ancestral genepools inferred with the STRUCTURE program. 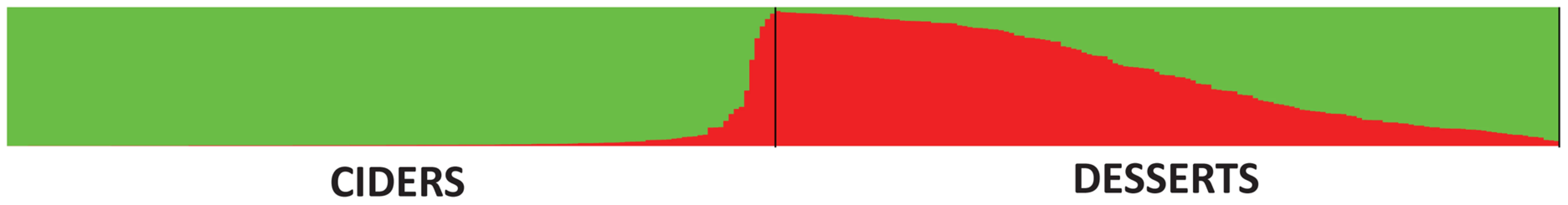
Discussion
The apple is so deeply rooted in the culture of human populations from temperate regions that it is often not recognized as an exotic plant of unclear origin. We show here that the evolution of the domesticated apple involved more than one geographically restricted wild species. The domesticated apple did not arise from a single event over a short period of time, but from evolution extending over thousands of years. The genepool of the current domesticated apple varieties has been enriched by the contribution of at least two wild species. Malus species have a self-incompatibility system; apple domestication and traditional variety improvement have therefore been based mostly on the selection of the best phenotypes grown from open-pollinated seeds. This breeding strategy has probably favoured the incorporation of genetic material from multiple wild sources and the maintenance of high levels of genetic variation in domesticated apples, despite the extensive use of large-scale vegetative propagation of superior individuals by grafting. Our results are consistent with those reported for the few other woody perennials studied to date, such as grape [37], red mombin [26] and olive trees [36], and support the view that domestication in long-lived plants differs in many respects from the scenarios described for seed-propagated annuals.
Weak differentiation from wild progenitors and the Central Asian origin of M. domestica
Malus sieversii was previously identified as the main contributor to the M. domestica genome on the basis of morphological and sequence data [41], [43]. The flanks of the Tian Shan mountains have been identified as a likely initial site of domestication, based on the high morphological variability of the wild apples growing in this region, and their similarity to sweet dessert apples [44], [45]. We show here, using a set of rapidly evolving genetic markers distributed throughout the genome and a large sampling, that M. domestica now forms a distinct, random mating group, surprisingly well separated from M. sieversii, with no difference in levels of genetic variation between the domesticate and its wild progenitor. This contrasts with the pattern previously reported, based on a twenty three-gene phylogenetic network [43], where domesticated varieties of apple appeared nested within M. sieversii. After the removal of individuals showing signs of recent admixture, M. sieversii and M. domestica nevertheless appeared to be the pair of species most closely related genetically, confirming their progenitor-descendant relationship.
Lack of a domestication bottleneck
Apple breeding methods (grafting and “chance seedling” selection), life-history traits specific to trees and/or the genetic architecture of selected traits have likely played a role in the conservation of levels of genetic diversity in cultivated apples similar to those in wild apples. Some factors, such as “chance seedling” selection [66], may even have increased genetic diversity, by favouring outcrossing events among domesticates and introgression from wild species [39]. The low inbreeding coefficients inferred in domesticated apples and the low level of differentiation between cultivated and wild apple populations [40], [54], [67], [68] indicate a high frequency of crosses between individuals of M. domestica, M. sieversii and other wild relatives hailing from diverse geographic origins. Such a high level of gene flow has likely contributed to maintenance of a high level of genetic diversity in domesticated apples.
The grafting technique, which was probably developed around 3,000 years ago, has made it possible to propagate superior individuals clonally. The spread of grafting, together with the lengthy juvenile phase (5–10 years) and the long lifespan of apples, may have imposed strong limits on the intensity of the domestication bottleneck thereby limiting the loss of genetic diversity [27], [28], [31]. By decreasing the number of generations since domestication, these factors have probably also helped to restrict the differentiation between domesticates and wild relatives. In theory, grafting may have limited the size of the apple germplasm dispersed early on to a few very popular genotypes, thereby provoking a sudden shrink in effective population size and a loss of diversity. However, we found no evidence that the clonal propagation of apples resulted in a long-lasting decrease in population size or clonal population structure. We can speculate that this may be due to a combination of various factors such as: gene flow with wild species, small-scale propagation (many farmers producing a few grafts each), a large variation in preferences for taste and other quality characteristics between farmers and cultures, large differences in growth conditions leading to the adoption of different sets of genotypes in different regions or the typical behaviour of hobby breeders, who tend to spot particular differences and multiply them. Similarly, for grape, there are huge numbers of old varieties and as much genetic variation in cultivated varieties as in wild-relative progenitors [37].
A major secondary contribution from the European crabapple
There has been a long-running debate concerning the possible contribution of other wild species present along the Silk Route to the genetic makeup of M. domestica [40], [46], [47], [65], [69]. Our results clearly show that interspecific hybridization has been a potent force in the evolution of domesticated apple varieties. Apple thus provides a rare example of the evolution of a domesticated crop over a long period of time and involving at least two wild species (see also the cases of olive tree and avocado [24], [26], [37], [70]). A recent study argued that introgression from M. sylvestris into the M. domestica genepool was the most parsimonious explanations for shared gene sequence polymorphisms between the two species [50]. Using an unprecedentedly large dataset, more numerous and more rapidly evolving markers and a combination of inferential methods, we provide a comprehensive view of the history of domestication in apple. We confirm that M. sieversii was the initial progenitor and show that the wild European crabapple M. sylvestris has been a major secondary contributor to the diversity of apples, resulting in current varieties of M. domestica being more closely related to M. sylvestris than to their central Asian progenitor. This situation is reminiscent of that for maize, in which the cultivated crop Zea mays is genetically more closely related to current-day highland landraces than to lowland Z. mays ssp. parviglumis from which the crop was domesticated [71]. This pattern has been attributed to large-scale gene flow from a secondary source, a second subspecies of teosinte, Z. mays ssp. mexicana, into highland maize populations [71].
The usefulness of wild relatives for improving elite cultivated crop genepools has long been recognised and the exploitation of wild resources is now considered a strategic priority in breeding and conservation programs for most crops [11], [12], [44]. Domesticated apples are unusual in that the contribution of wild relatives probably occurred early and unintentionally in the domestication process, preceding even the use of controlled crosses. The use of genetic markers with lower mutation rates than our set of microsatellites might also make it possible to investigate the contribution of more phylogenetically distant apple species growing in areas away from the Silk Route to the diversification of modern apple cultivars.
The Romans introduced sweet apples into Europe at a time at which the Europeans were undoubtedly already making cider from the tannin-rich fruits of the native M. sylvestris [35], [72]. Cider is not typical of Asia [35], but it was widespread in Europe by the time of Charlemagne (9th century, [73]). Large numbers of apple trees were planted for cider production in France and Spain from the 10th century onwards [48], [52]. The very high degree of stringency of cider apples (often to the extent that they are inedible) led to the suggestion that cider cultivars arose from hybridization between M. sylvestris and sweet apples [35], [46], [65]. We show here that the genetic structure within the cultivated apple genepool is very weak, with poor differentiation between cider and dessert apples. Cider cultivars thus appear to be no more closely genetically related to M. sylvestris than dessert cultivars. As wild Asian apples are known to cover the full range of tastes [44], [46], it is possible that fruits with the specific characteristics required for cider production were in fact initially selected in Central Asia and subsequently brought into Europe. There is a long-standing tradition of cider production in some parts of Turkey [35], for instance, which is potentially consistent with an Eastern origin of cider cultivars. However, the low level of genetic differentiation between dessert and cider apples indicates that, even if different types of apples were domesticated in Asia and brought to Europe, they have not diverged into independent genepools.
Concluding remarks
This study settles a long-running debate by confirming that 1) M. domestica was initially domesticated from M. sieversii, and 2) M. domestica subsequently received a significant genetic contribution from M. sylvestris, much larger than previously suspected [35], at least in Western Europe, where originated most of our samples and most cultivar diversity. The higher level of introgression of the European crabapple into the domesticated apple in this study than in previous studies [43], [50], [51] may be attributed to the use of a larger and more representative set of M. domestica genotypes coupled with the genotyping of numerous and rapidly evolving markers known to trace back more recent events.
Our inferences also have important implications for breeding programs and for the conservation of wild species of apple. The major contribution of the various wild species to the M. domestica genepool highlights the need to invest efforts into the conservation of these species, which may contain unused genetic resources that could further improve the domesticated apple germplasm [74], such as disease resistance genes or genes encoding specific organoleptic features.
Materials and Methods
Sample collection and DNA extraction
Leaf material was retrieved from the collections of various institutes (INRA Angers, France; USDA - ARS, Plant Genetic Resources Unit, Geneva, NY; ILVO Melle, Belgium) and from a private apple germplasm repository in Brittany for M. domestica (N = 368, Figure S1 including only diploid cultivars N = 299) and from forests for the four wild species (Figure 1; Table S1). Malus sieversii (N = 168) material was collected from 2007 to 2010 in the Chinese Xinjiang province (N = 26), Kyrgyzstan (N = 5), Uzbekistan (N = 1), Tajikistan (N = 1) and Kazakhstan (N = 114). Malus orientalis (N = 215) was sampled in 2009 in Armenia (N = 203), Turkey (N = 5) and Russia (N = 5). Malus sylvestris (N = 40) samples were obtained from 15 European countries. Malus baccata (N = 48) was sampled in 2010 in Russia. The origins of M. domestica cultivars were: France (N = 266), Great Britain (N = 12), USA (N = 12), Russia (N = 7), the Netherlands (N = 6), Australia (N = 4), Belgium (N = 4), Germany (N = 4), Japan (N = 3), Ukraine (N = 3), Tunisia (N = 2), Switzerland (N = 2), Spain (N = 2), New Zealand (N = 2), Israel (N = 1), Ireland (N = 1), Canada (N = 1), Armenia (N = 2) and unknown/debated (N = 34). Genomic DNA was extracted with the Nucleo Spin plant DNA extraction kit II (Macherey & Nagel, Düren, Germany) according to the manufacturer's instructions.
Microsatellite markers and polymerase chain reaction (PCR) amplification
Microsatellites were amplified by multiplex PCR, with the Multiplex PCR Kit (QIAGEN, Inc.). We used 26 microsatellites spread across the 17 chromosomes (one to three microsatellites per chromosome), in 10 different multiplexes previously optimised on a large set of genetically related progenies of M. domestica [75]. The four multiplexes (MP01, MP02, MP03, MP04; Table S9; Lasserre P. unpublished data) were performed in a final reaction volume of 15 µl (7.5 µl of QIAGEN Multiplex Master Mix, 10–20 µM of each primer, with the forward primer labelled with a fluorescent dye and 10 ng of template DNA). We used a touch-down PCR program (initial annealing temperature of 60°C, decreasing by 1°C per cycle down to 55°C). Six other multiplex reactions (Hi6, Hi4ab, Hi5-10, Hi13a, Hi13b, Hi4b) were performed using previously described protocols [75]. Genotyping was performed on an ABI PRISM X3730XL, with 2 µl of GS500LIZ size standard (Applied Biosystems). Alleles were scored with GENEMAPPER 4.0 software (Applied Biosystems). We retained only multilocus genotypes presenting less than 30% missing data.
Suitability of microsatellites for population genetic analyses
We checked the suitability of the markers for population genetic analyses. None of the 26 microsatellite markers deviated significantly from a neutral equilibrium model, as shown by the non significant P-values obtained in Ewen-Watterson tests [76], and no pair of markers was found to be in significant linkage disequilibrium in any of the species [77], [78]. The markers could therefore be considered unlinked and neutral.
Analyses of genetic variation and differentiation between the five species
Apple cultivars may be polyploid [79]. We therefore first checked for the presence of polyploidy individuals of M. domestica within our dataset. Individuals presenting multiple peaks on electrophoregrams were first re-extracted to eliminate contamination as a possible source of apparent polyploidy. We then checked whether they had been reported to be polyploidy in previous studies [79]. After completion of this checking procedure, we removed 69 polyploids (of the 368 samples) from subsequent analyses. We tested for the occurrence of null alleles at each locus with MICROCHECKER 2.2.3 software [80]. Allelic richness and private allele frequencies were calculated with ADZE software [81], for a sample size of 22. Heterozygosity (expected (HE) and observed (HO)), Weir & Cockerham F-statistics, deviation from Hardy-Weinberg equilibrium and genotypic linkage disequilibrium were estimated with GENEPOP 4.0 [77], [78]. The significance of differences between FST values was assessed in exact tests carried out with GENEPOP 4.0 [77], [78]. Individuals were assigned to clonal lineages with GENODIVE [53]. We estimated relatedness between pairs of cultivars and between pairs of individuals within each species, by calculating the rxy of Ritland and Lynch [82] with RE-RAT online software [83]. We tested whether the distributions of rxy deviated significantly from a Gaussian distribution with a mean of zero and a standard deviation equal to the observed standard deviation, by comparing observed and simulated distributions in Fisher's exact test (R Development Core Team, URL http://www.R-project.org).
Assessing bottlenecks during apple domestication and diversification
We tested for the occurrence of a bottleneck during apple domestication with the method implemented in BOTTLENECK [58], [84]. The tests were performed under the stepwise-mutation model (SMM) and under a two-phase model (TPM) allowing for 30% multistep changes. We used Wilcoxon signed rank tests to determine whether a population had a significant number of loci with excess genetic diversity.
Analyses of population subdivision
We used the individual-based Bayesian clustering method implemented in STRUCTURE 2.3.3 [55], [85], [86] to investigate species delimitation, intraspecific population structure and admixture. This method is based on Markov Chain Monte Carlo (MCMC) simulations and is used to infer the proportion of ancestry of genotypes in K distinct predefined clusters. The algorithm attempts to minimize deviations from Hardy–Weinberg and linkage equilibrium within clusters. Analyses were carried out without the use of prior information, except for analyses of population subdivision within the M. domestica genepool for which the “cider”/“dessert” classification of cultivars was used as prior information to assist clustering. K ranged from 1 to 8 for analyses of the five-species dataset and the M. domestica dataset, and was fixed at K = 2 for analyses of pairs of species including M. domestica and each of the wild species. Ten independent runs were carried out for each K and we used 500,000 MCMC iterations after a burn-in of 50,000 steps. We used CLUMPP v1.1.2 (Greedy algorithm) [87] to look for distinct modes among the 10 replicated runs of each K.
STRUCTURE analyses were run for the full dataset (N = 839) and for two pruned datasets excluding non-pure individuals (i.e., genotypes with <0.9 membership of their species' genepool) and related individuals (rxy≥0.5).
Inference of demographic history
We used the DIYABC program [88] to compare different admixture models and infer historical parameters. We simulated microsatellite datasets for 14 loci (Ch01h01, Ch01h10, Ch02c06, Ch02d08, Ch05f06, Ch01f02, Hi02c07, Ch02c09, Ch03d07, Ch04c07, Ch02b03b, MS06g03, Ch04e03, Ch02g01) previously reported to be of the perfect repeat type [89], [90], [91]. In total, we generated 5×105 simulated datasets for each model.
A generalized stepwise model (GSM) was used as the mutational model. The model had two parameters: the mean mutation rate (μ) and the mean parameter (P) of the geometric distribution used to model the length of mutation events (in numbers of repeats). As no experimental estimate of microsatellite mutation rate is available for Malus, the mean mutation rate was drawn from a uniform distribution by extreme values of 10−4 and 10−3, and the mutation rate of each locus was drawn independently from a Gamma distribution (mean = μ; shape = 2). The parameter P ranged from 0.1 to 0.3. Each locus L had a possible range of 40 contiguous allelic states (44 for CH02C06, 42 for CH04E03) and was characterized by individual values for mutation rate (μL) and the parameter of the geometric distribution (PL); μL and PL were drawn from Gamma distributions with the following parameter sets: mean = μ, shape = 2, range = 5×10−5–5×10−2 for μL, and mean = P, shape = 2, range = 0.01–0.9 for PL. As not all allele lengths were multiples of motif length, we also included single-nucleotide insertion-deletion mutations in the model, with a mean mutation rate (μSNI) and locus-specific rates drawn from a Gamma distribution (mean = μSNI; shape = 2). The summary statistics used were: mean number of alleles per locus, mean genetic diversity [92], genetic differentiation between pairwise groups (FST; [93]), genetic distances (δμ)2 [94].
We used a polychotomous logistic regression procedure [95] to estimate the relative posterior probability of each model, based on the 1% of simulated data sets closest to the observed data. Confidence intervals for the posterior probabilities were computed using the limiting distribution of the maximum likelihood estimators [64]. Once the most likely model was identified, we used a local linear regression to estimate the posterior distributions of parameters under this model [96]. The 1% simulated datasets most closely resembling the observed data were used for the regression, after the application of a logit transformation to parameter values.
Supporting Information
Zdroje
1. DiamondJ 1997 Guns, Germs, and Steel: The Fates of Human Societies Norton, W. W. & Company, Inc. Sales
2. ZederMAEmshwillerESmithBDBradleyDG 2006 Documenting domestication: the intersection of genetics and archaeology. Trends Genet 22 139 155
3. DiamondJ 2002 Evolution, consequences and future of plant and animal domestication. Nature 418 700 707
4. PuruggananMDFullerDQ 2009 The nature of selection during plant domestication. Nature 457 843 848
5. WrightSIGautBS 2005 Molecular population genetics and the search for adaptive evolution in plants. Mol Biol Evol 22 506 519
6. TenaillonMIManicacciD 2011 Maize origins: an old question under the spotlights. PrioulJ-LThévenotCMolnarT Advances in Maize (Essential Reviews in Experimental Biology): The Society for Experimental Biology 89 110
7. AllabyRGFullerDQBrownTA 2008 The genetic expectations of a protracted model for the origins of domesticated crops. PNAS 105 13982 13986
8. CaicedoALWilliamsonSHHernandezRDBoykoAFledel-AlonA 2007 Genome-wide patterns of nucleotide polymorphism in domesticated rice. PLoS Genet 3 e163.
9. DoebleyJFGautBSSmithBD 2006 The molecular genetics of crop domestication. Cell 127 1309 1321
10. GrossBLOlsenKM 2009 Genetic perspectives on crop domestication. Trends Plant Sci 15 529 537
11. BrownTAJonesMKPowellWAllabyRG 2009 The complex origins of domesticated crops in the Fertile Crescent. Trends Ecol Evol 24 103 109
12. FeuilletCLangridgePWaughR 2008 Cereal breeding takes a walk on the wild side. Trends Genet 24 24 32
13. GléminSBataillonT 2009 A comparative view of the evolution of grasses under domestication. New Phytol 183 273 290
14. KilianBÖzkanHWaltherAKohlJDaganT 2007 Molecular diversity at 18 loci in 321 wild and 92 domesticate lines reveal no reduction of nucleotide diversity during Triticum monococcum (Einkorn) domestication: implications for the origin of agriculture. Mol Biol Evol 24 2657 2668
15. KovachMJSweeneyMTMcCouchSR 2007 New insights into the history of rice domestication. Trends Genet 23 578 587
16. RussellJDawsonIKFlavellAJSteffensonBWeltzienE 2011 Analysis of >1000 single nucleotide polymorphisms in geographically matched samples of landrace and wild barley indicates secondary contact and chromosome-level differences in diversity around domestication genes. New Phytol 191 564 578
17. WangCChenJZhiHYangLLiW 2010 Population genetics of foxtail millet and its wild ancestor. BMC Genet 11 90
18. MatsuokaYVigourouxYGoodmanMMSanchezGJBucklerE 2002 A single domestication for maize shown by multilocus microsatellite genotyping. PNAS 99 6080 6084
19. OumarIMariacCPhamJ-LVigourouxY 2008 Phylogeny and origin of pearl millet (Pennisetum glaucum [L.] R. Br) as revealed by microsatellite loci. Theor Appl Genet 117 489 497
20. BlackmanBKScascitelliMKaneNCLutonHHRasmussenDA 2011 Sunflower domestication alleles support single domestication center in eastern North America. PNAS 108 14360 14365
21. HarterAVGardnerKAFalushDLentzDLByeRA 2004 Origin of extant domesticated sunflowers in eastern North America. Nature 430 201 205
22. TenaillonMIU'RenJTenaillonOGautBS 2004 Selection versus demography: A multilocus investigation of the domestication process in maize. Mol Biol Evol 21 1214 1225
23. TannoK-iWillcoxG 2006 How Fast Was Wild Wheat Domesticated? Science 311 1886
24. ChenHMorrellPLAshworthVETMde la CruzMCleggMT 2009 Tracing the geographic origins of major avocado cultivars. J Hered 100 56 65
25. MillerAGrossBL 2011 From forest to field: perennial fruit crops domestication. Am J Bot 98 1389 1414
26. MillerASchaalB 2005 Domestication of a Mesoamerican cultivated fruit tree, Spondias purpurea. PNAS 102 12801 12806
27. PetitRJHampeA 2006 Some evolutionary consequences of being a tree. Annu Rev Ecol Evol Syst 37 187 214
28. SavolainenOPyhäjärviT 2007 Genomic diversity in forest trees. Curr Opin Plant Biol 10 162 167
29. AusterlitzFMarietteSMachonNGouyonPHGodelleB 2000 Effects of colonization processes on genetic diversity: differences between annual plants and tree species. Genetics 154 1309 1321
30. JanickJ 2005 The origins of fruits, fruit growing, and fruit breeding Plant Breeding Reviews: John Wiley & Sons, Inc 255 321
31. MillerAJSchaalBA 2006 Domestication and the distribution of genetic variation in wild and cultivated populations of the Mesoamerican fruit tree Spondias purpurea L. (Anacardiaceae). Mol Ecol 15 1467 1480
32. ZoharyDSpiegel-RoyP 1975 Beginnings of Fruit Growing in the Old World. Science 319 327
33. ZoharyD 2004 Unconscious selection and the evolution of domesticated plants. Econ Bot 58 5 10
34. PickersgillB 2007 Domestication of plants in the Americas: insights from mendelian and molecular genetics. Ann Bot 100 925 940
35. JuniperBEMabberleyDJ 2006 The story of the apple Imber Press, Inc 240
36. BesnardGRubio de CasasRVargasP 2007 Plastid and nuclear DNA polymorphism reveals historical processes of isolation and reticulation in the olive tree complex (Olea europaea). J Biogeogr 34 736 752
37. MylesSBoykoAROwensCLBrownPJGrassiF 2011 Genetic structure and domestication history of the grape. PNAS 108 3530 3535
38. DelplanckeMAlvarezNEspíndolaAJolyHBenoitL 2011 Gene flow among wild and domesticated almond species: insights from chloroplast and nuclear markers. Evolutionary Applications in press
39. ZoharyDHopfM 2000 Domestication of plants in the Old World New York Oxford University Press 316
40. CoartEVan GlabekeSDe LooseMLarsenASRoldán-RuizI 2006 Chloroplast diversity in the genus Malus: new insights into the relationship between the European wild apple (Malus sylvestris (L.) Mill.) and the domesticated apple (Malus domestica Borkh.). Mol Ecol 15 2171 2182
41. HarrisSARobinsonJPJuniperBE 2002 Genetic clues to the origin of the apple. Trends Genet 18 426 430
42. RobinsonJPHarrisSAJuniperBE 2001 Taxonomy of the genus Malus Mill. (Rosaceae) with emphasis on the cultivated apple, Malus domestica Borkh. Plant Syst Evol 226 35 58
43. VelascoRZharkikhAAffourtitJDhingraACestaroA 2010 The genome of the domesticated apple (Malus×domestica Borkh.). Nature Genetics 42 833 839
44. DzhangalievAD 2003 The wild apple tree of Kazakhstan Hortic Rev John Wiley & Sons, Inc 63 303
45. VavilovNI 1926 Studies on the origin of cultivated plants. Trudy Byuro Prikl Bot 16 139 245
46. ForslinePLAldwinckleHSDicksonEELubyJJHokansonSC 2002 Collection, maintenance, characterization and utilization of wild apples of central Asia Hortic Rev John Wiley & Sons, Inc 1 61
47. RehderA 1940 Manual of cultivated trees and shrubs Macmillan, New York
48. BoréJMFleckingerJ 1997 Pommiers à cidre: variétés de France: INRA éditions, Paris, FRANCE (1997) (Monographie) 771
49. LubyJJAlspachPABusVGMOraguzieNC 2001 Field resistance to fire blight in a diverse apple (Malus sp.) germplasm collection. J Am Soc Hortic Sci 127 245 253
50. HarrisonNHarrisonRJ 2011 On the evolutionary history of the domesticated apple. Nat Genet 43 1043 1044
51. MichelettiDTroggioMSalaminiFViolaRVelascoR 2011 On the evolutionary history of the domesticated apple. Nat Genet 43 1044 1045
52. Pereira-LorenzoSRamos-CabrerAMFischerM 2009 Breeding Apple (Malus×Domestica Borkh). Breeding Plantation Tree Crops: Temperate Species Springer New York 33 81
53. MeirmansPGVan TienderenPH 2004 Genotype and genodive: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4 792 794
54. KoopmanWJMLiYCoartEVan De WegWEVosmanB 2007 Linked vs. unlinked markers: multilocus microsatellite haplotype-sharing as a tool to estimate gene flow and introgression. Mol Ecol 16 243 256
55. PritchardJKStephensMDonnellyP 2000 Inference of population structure using multilocus genotype data. Genetics 155 945 959
56. EvannoGRegnautSGoudetJ 2005 Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14 2611 2620
57. VerckenEFontaineMCGladieuxPHoodMEJonotO 2010 Glacial refugia in pathogens: European genetic structure of anther smut pathogens on Silene latifolia and Silene dioica. PLoS Pathog 6 e1001229.
58. CornuetJMLuikartG 1996 Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144 2001 2014
59. MabberleyDJJarvisCEJuniperBE 2001 The name of the apple. Telopea 9 2001
60. DiegoMMichelaTFrancescoSRobertoVRiccardoV 2011 On the evolutionary history of the domesticated apple. Nat Genet 43 1044 1045
61. AndersonECThompsonEA 2002 A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160 1217 1229
62. ExcoffierLEstoupACornuetJ-M 2005 Bayesian analysis of an admixture model with mutations and arbitrarily linked markers. Genetics 169 1727 1738
63. Ross-IbarraJTenaillonMGautBS 2009 Historical divergence and gene flow in the Genus Zea. Genetics 181 1399 1413
64. CornuetJ-MSantosFBeaumontMARobertCPMarinJ-M 2008 Inferring population history with DIY ABC: a user-friendly approach to approximate Bayesian computation. Bioinformatics 24 2713 2719
65. WagnerIWeedenNF 2000 Isozyme in Malus sylvestris, Malus×domestica and in related Malus species. Acta Horticulturae 538 51 56
66. GardinerSEBusVGMRusholmeRLChagnéDRikkerinkEHA 2007 Fruits and Nuts: Apple KoleC Springer Berlin Heidelberg 1 62
67. CoartEVekemansXSmuldersMJMWagnerIVan HuylenbroeckJ 2003 Genetic variation in the endangered wild apple (Malus sylvestris (L.) Mill.) in Belgium as revealed by amplified fragment length polymorphism and microsatellite markers. Mol Ecol 12 845 857
68. GharghaniAZamaniZTalaieAOraguzieNFatahiR 2009 Genetic identity and relationships of Iranian apple (Malus domestica Borkh.) cultivars and landraces, wild Malus species and representative old apple cultivars based on simple sequence repeat (SSR) marker analysis. Genet Resour Crop Ev 56 829 842
69. PonomarenkoV 1991 On a little known species Malus×asiatica (Rosaceae). Bot Zhurn 76 715 720
70. OlsenKMGrossBL 2008 Detecting multiple origins of domesticated crops. PNAS 105 13701 13702
71. van HeerwaardenJDoebleyJBriggsWHGlaubitzJCGoodmanMM 2011 Genetic signals of origin, spread, and introgression in a large sample of maize landraces. PNAS 108 1088 1092
72. OrtonV 1973 The American cider Book The story of America's natural beverage North Point Press
73. LeaAGHPiggottJR 2003 Fermented Beverage Production; 2, editor Kluwer Academic/Plenum
74. HajjarRHodgkinT 2007 The use of wild relatives in crop improvement: a survey of developments over the last 20 years. Euphytica 156 1 13
75. PatocchiAFernàndez-FernàndezFEvansKGobbinDRezzonicoF 2009 Development and test of 21 multiplex PCRs composed of SSRs spanning most of the apple genome. Tree Genet Genomes 5 211 223
76. ExcoffierLLischerHEL 2010 Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10 564 567
77. RaymondMRoussetF 1995 GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86 248 249
78. RoussetF 2008 Genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8 103 106
79. SchusterMBüttnerR 1995 Chromosome numbers in the Malus wild species collection of the genebank Dresden-Pillnitz. Genet Resour Crop Ev 42 353 361
80. Van OosterhoutCHutchinsonWFWillsDPMShipleyP 2004 Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4 535 538
81. SzpiechZAJakobssonMRosenbergNA 2008 ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 24 2498 2504
82. LynchMRitlandK 1999 Estimation of pairwise relatedness with molecular markers. Genetics 152 1753 1766
83. SchwackeLSchwackeJRoselP 2005 RE-RAT: relatedness estimation and rarefaction analysis tool. http://people.musc.edu/~schwaclh/
84. PirySLuikartGCornuetJM 1999 Computer note. BOTTLENECK: a computer program for detecting recent reductions in the effective size using allele frequency data. Journal of Heredity 90 502 503
85. FalushDStephensMPritchardJK 2003 Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164 1567 1587
86. HubiszMJFalushDStephensMPritchardJK 2009 Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9 1322 1332
87. JakobssonMRosenbergNA 2007 CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23 1801 1806
88. CornuetJ-MRavigneVEstoupA 2010 Inference on population history and model checking using DNA sequence and microsatellite data with the software DIYABC (v1.0). BMC Bioinformatics 11 401
89. LiebhardRGianfranceschiLKollerBRyderCDTarchiniR 2002 Development and characterisation of 140 new microsatellites in apple (Malus×domestica Borkh.). Mol Breeding 10 217 241
90. Silfverberg-DilworthEMatasciCVan de WegWVan KaauwenMWalserM 2006 Microsatellite markers spanning the apple (Malus×domestica Borkh.) genome. Tree Genet Genomes 2 202 224
91. GianfranceschiLSegliasNTarchiniRKomjancMGesslerC 1998 Simple sequence repeats for the genetic analysis of apple. Theor Appl Genet 96 1069 1076
92. NeiM 1978 Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89 583 590
93. WeirBSCockerhamCC 1984 Estimating F-Statistics for the analysis of population structure. Evolution 38 1358 1370
94. GoldsteinDBRuiz LinaresACavalli-SforzaLLFeldmanMW 1995 Genetic absolute dating based on microsatellites and the origin of modern humans. PNAS 92 6723 6727
95. FagundesNJRRayNBeaumontMNeuenschwanderSSalzanoFM 2007 Statistical evaluation of alternative models of human evolution. PNAS 104 17614 17619
96. BeaumontMAZhangWBaldingDJ 2002 Approximate Bayesian Computation in population genetics. Genetics 162 2025 2035
Štítky
Genetika Reprodukční medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 5
-
Všechny články tohoto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



