-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaChromosome Pairing: A Hidden Treasure No More
article has not abstract
Published in the journal: . PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002737
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002737Summary
article has not abstract
Many generations of biologists have been intrigued by the myriad structures that eukaryotic chromosomes can adopt and have questioned how their form relates to function [1], [2]. One organizational state that chromosomes adopt is pairing in a homology-dependent manner [3]–[8]. Although homolog pairing in meiosis has been extensively studied and is important for chromosome segregation, pairing of homologs in somatic cells is less well understood. In this issue of PLoS Genetics, Joyce et al. report the first comprehensive RNAi screen of genes regulating somatic chromosome pairing in Drosophila [9]. This study finally unlocks the treasure trove of Drosophila somatic chromosome pairing, sets the stage for much deeper mechanistic investigations, and most importantly, points to new avenues for understanding the functional significance of homologous chromosome pairing.
Drosophila presents a unique opportunity for identifying molecular regulators that establish, maintain, and antagonize homolog pairing because its homologous chromosomes are almost always paired in somatic cells. Metz described somatic cell homolog pairing in 1916 [10], while Painter first described polytene chromosomes in 1933 [11]—polytene chromosomes are found in some polyploid cells where many copies of homologous chromosomes and chromatids are paired along their lengths. Despite these early descriptions of chromosome pairing, many fundamental questions regarding homolog pairing still remain unanswered: Is meiotic homolog pairing mechanistically similar to pairing in somatic cells? Is pairing of homologous sequences in the context of polytene chromosomes similar to somatic or meiotic homolog pairing? In the absence of recombination - and meiosis-specific synaptonemal complex proteins, how do homologous sequences find each other in somatic cells? Are there negative regulators of pairing? Are there genomic regions or chromatin states that pair more efficiently than others? Most importantly, what is the biological relevance of homolog pairing in somatic cells? The answers to these questions have eluded us for almost a century because of limitations in cytological tools for measuring pairing and genetic tools for perturbing pairing dynamics.
Recent evidence has raised the exciting possibility that both pairing and anti-pairing forces may act on chromosomes to regulate the spatial juxtaposition of homologous sequences (Figure 1). Two previous studies in Drosophila identified Suppressor of Hairy Wing (Su(Hw)) and Topoisomerase II as pairing promoting factors [12], [13]. In a third study, the Kleisin subunit of condensin II, Cap-H2, was shown to be necessary and sufficient to antagonize pairing of homologs in the context of polytene chromosomes [14]. Cap-H2 mutant Drosophila males have chromosome unpairing defects in meiosis I, also providing evidence for a Cap-H2 anti-pairing activity [15]. Until now, the dearth of molecular models for somatic pairing has been mainly due to this paucity of “pairing” and “unpairing” factors.
Fig. 1. Dynamic chromosome pairing. 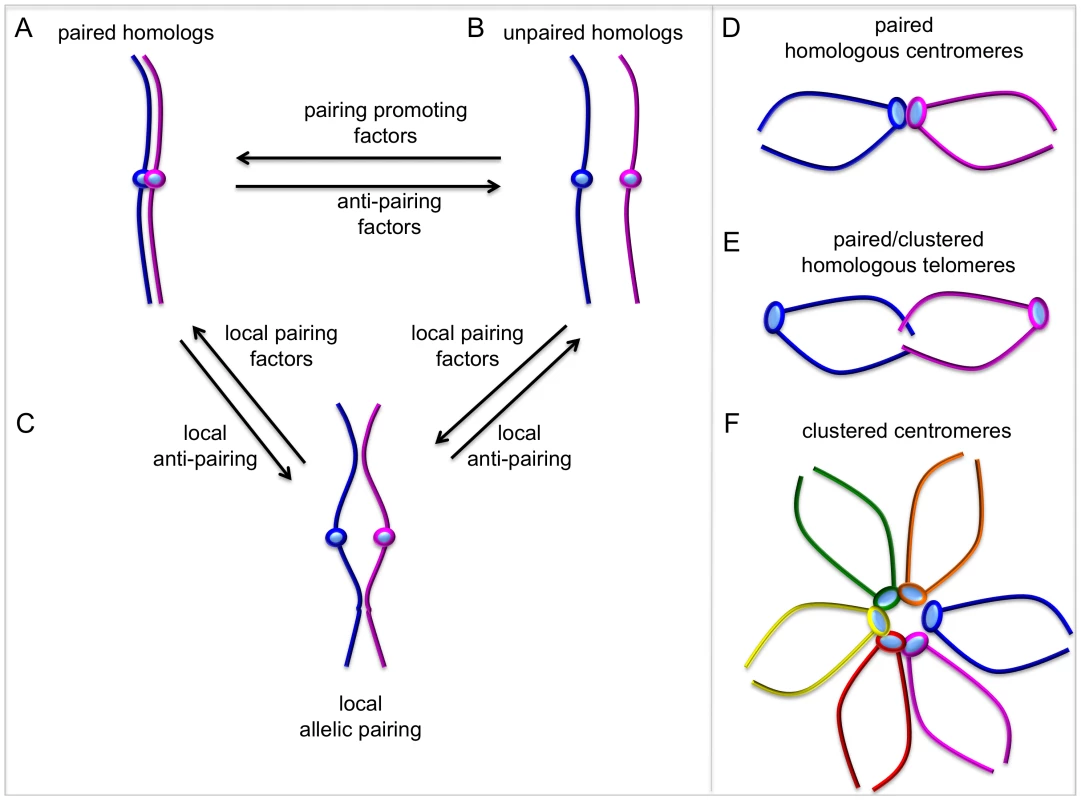
The term “pairing” describes the spatial juxtaposition of entire homologous chromosomes, allelic sequences, and/or homologous sequences at non-allelic locations. (A, B) Homologous chromosomes can exist as paired throughout their entire length and the relative activities of pairing and anti-pairing factors determine the degree of global chromosome pairing. (C) Local pairing and anti-pairing factors can affect pairing status of specific genes or chromosomal regions. Local “pairing centers” can nucleate global pairing, but it is unclear whether factors regulating local pairing are different from global pairing factors. (D–F) In some cases, centromeres or telomeres from homologs can pair or cluster with non-homologous chromosomes, while there is no pairing along chromosome arms. The ability to perturb homolog pairing by RNAi depletion [13], combined with FISH and high-throughput technology, has now made it possible to interrogate entire genomes and ask, nearly 100 years after Metz's initial description, “what genes regulate homologous chromosome pairing in somatic cells?” Joyce et al. [9] use a novel combination of whole genome RNAi, high-throughput imaging, and DNA FISH that represents a tremendous effort. This work is a significant advance because it provides an extensive “parts list” of mostly novel factors affecting pairing. In their elegant RNAi screen, Joyce et al. report 40 new pairing promoting genes (where previously we knew of two) and 65 new anti-pairing genes (where previously we knew of only one). Interestingly, identification of genes affecting pairing of heterochromatic or euchromatic regions, but not both, supports the idea that pairing of different chromatin domains may be regulated in different ways.
The pairing and anti-pairing genes code for cell cycle, protein turn-over machinery, and chromatin proteins, among others. Previous studies suggested that cell cycle regulation and chromosome pairing are related by showing that entry into S-phase and G2/M disrupt pairing [13], [16], [17]. It is also likely that some cell cycle genes directly regulate pairing or may even monitor pairing status. For example, if allelic or homolog pairing in G1 is important for specific gene expression states, then one might imagine that in cycling cells pairing may be preserved through multiple mitotic chromosome condensation/decondensation cycles. Alternatively, if DNA replication and chromosome compaction forces disrupt pairing, then G1-specific regulators may be required to re-establish pairing. Now that we know which cell cycle genes affect pairing, the next challenge is to understand how they function in pairing dynamics. Of the protein turn-over genes that promote pairing, the Slimb ubiquitin ligase is of particular interest. This is because the authors show that Slimb-RNAi disruption of pairing is rescued by RNAi depletion of condensin II genes. This again points to a condensin II anti-pairing activity. However, a direct link between condensation and pairing is yet to be determined. That Slimb may target one or more anti-pairing factors while components of the Anaphase-Promoting Complex (APC) promote pairing suggests a still more complex layer of pairing regulation that ties protein turn-over machinery back to cell cycle regulation. It will be of great interest to determine the direct targets of Slimb - and APC-mediated protein turn-over and how these targets function in pairing.
Perhaps the most exciting broad conclusions from this study are that chromosome pairing is much more complicated and dynamic than anyone had anticipated, and that an abundance of “pairing promoting” and “anti-pairing” factors provide opposing forces. The authors suggest that the degree of homolog pairing in somatic cells, at the gene level and at the whole chromosome level, is likely determined by the relative activities of pairing and anti-pairing factors (Figure 1). It is noteworthy that many of the genes revealed in this study also have orthologs in other species, including humans. With this new pairing parts list it will be possible now to ask how homolog pairing in different species is regulated, and more importantly, it will lead to new studies seeking to understand the biological relevance of somatic pairing in different species.
Zdroje
1. KosakSTGroudineM 2004 Form follows function: the genomic organization of cellular differentiation. Genes Dev 18 1371 1384
2. CremerTCremerM 2010 Chromosome territories. Cold Spring Harb Perspect Biol 2 a003889
3. WuCTMorrisJR 1999 Transvection and other homology effects. Curr Opin Genet Dev 9 237 246
4. DuncanIW 2002 Transvection effects in Drosophila. Annu Rev Genet 36 521 556
5. Grant-DowntonRTDickinsonHG 2004 Plants, pairing and phenotypes–two's company? Trends Genet 20 188 195
6. McKeeBD 2004 Homologous pairing and chromosome dynamics in meiosis and mitosis. Biochim Biophys Acta 1677 165 180
7. ZicklerD 2006 From early homologue recognition to synaptonemal complex formation. Chromosoma 115 158 174
8. TsaiJHMcKeeBD 2011 Homologous pairing and the role of pairing centers in meiosis. J Cell Sci 124 1955 1963
9. JoyceEFWilliamsBRXieTWuC-t 2012 Identification of genes that promote or antagonize somatic homolog pairing using a high-throughput FISH-based screen. PLoS Genet 8 e1002667 doi:10.1371/journal.pgen.1002667
10. MetzCW 1916 Chromosome studies on the Diptera: II. The paired association of chromosomes in the Diptera and its significance. J Exp Zool 21 213 279
11. PainterTS 1933 A New method for the study of chromosome rearrangements and the plotting of chromosome maps. Science 78 585 586
12. FritschCPloegerGArndt-JovinDJ 2006 Drosophila under the lens: imaging from chromosomes to whole embryos. Chromosome Res 14 451 464
13. WilliamsBRBatemanJRNovikovNDWuCT 2007 Disruption of topoisomerase II perturbs pairing in drosophila cell culture. Genetics 177 31 46
14. HartlTASmithHFBoscoG 2008 Chromosome alignment and transvection are antagonized by condensin II. Science 322 1384 1387
15. HartlTASweeneySJKneplerPJBoscoG 2008 Condensin II resolves chromosomal associations to enable anaphase I segregation in Drosophila male meiosis. PLoS Genet 4 e1000228 doi:10.1371/journal.pgen.1000228
16. CsinkAKHenikoffS 1996 Genetic modification of heterochromatic association and nuclear organization in Drosophila. Nature 381 529 531
17. FungJCMarshallWFDernburgAAgardDASedatJW 1998 Homologous chromosome pairing in Drosophila melanogaster proceeds through multiple independent initiations. J Cell Biol 141 5 20
Štítky
Genetika Reprodukční medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 5
-
Všechny články tohoto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



