-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWidespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
The X chromosome often plays a central role in hybrid male sterility between species, but it is unclear if this reflects underlying regulatory incompatibilities. Here we combine phenotypic data with genome-wide expression data to directly associate aberrant expression patterns with hybrid male sterility between two species of mice. We used a reciprocal cross in which F1 males are sterile in one direction and fertile in the other direction, allowing us to associate expression differences with sterility rather than with other hybrid phenotypes. We found evidence of extensive over-expression of the X chromosome during spermatogenesis in sterile but not in fertile F1 hybrid males. Over-expression was most pronounced in genes that are normally expressed after meiosis, consistent with an X chromosome-wide disruption of expression during the later stages of spermatogenesis. This pattern was not a simple consequence of faster evolutionary divergence on the X chromosome, because X-linked expression was highly conserved between the two species. Thus, transcriptional regulation of the X chromosome during spermatogenesis appears particularly sensitive to evolutionary divergence between species. Overall, these data provide evidence for an underlying regulatory basis to reproductive isolation in house mice and underscore the importance of transcriptional regulation of the X chromosome to the evolution of hybrid male sterility.
Published in the journal: . PLoS Genet 6(9): e32767. doi:10.1371/journal.pgen.1001148
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001148Summary
The X chromosome often plays a central role in hybrid male sterility between species, but it is unclear if this reflects underlying regulatory incompatibilities. Here we combine phenotypic data with genome-wide expression data to directly associate aberrant expression patterns with hybrid male sterility between two species of mice. We used a reciprocal cross in which F1 males are sterile in one direction and fertile in the other direction, allowing us to associate expression differences with sterility rather than with other hybrid phenotypes. We found evidence of extensive over-expression of the X chromosome during spermatogenesis in sterile but not in fertile F1 hybrid males. Over-expression was most pronounced in genes that are normally expressed after meiosis, consistent with an X chromosome-wide disruption of expression during the later stages of spermatogenesis. This pattern was not a simple consequence of faster evolutionary divergence on the X chromosome, because X-linked expression was highly conserved between the two species. Thus, transcriptional regulation of the X chromosome during spermatogenesis appears particularly sensitive to evolutionary divergence between species. Overall, these data provide evidence for an underlying regulatory basis to reproductive isolation in house mice and underscore the importance of transcriptional regulation of the X chromosome to the evolution of hybrid male sterility.
Introduction
The importance of proper gene regulation to the evolution of reproductive isolation between species is not well understood. Several studies have documented abnormal genome-wide patterns of expression in F1 hybrid offspring relative to their parental species [1]–[5]. However, two confounding factors make it difficult to determine the extent to which these data are directly relevant to the genetic basis of speciation. First, expression data from whole tissues reflect proportional transcript abundances across different cell types. Thus, genome-wide differences in hybrid expression could simply reflect quantitative differences in the cellular composition of tissues that manifest abnormal hybrid phenotypes [6], rather than true expression differences between cells. Second, many studies have focused on divergent crosses that produce severe F1 hybrid incompatibility phenotypes that uniformly affect a given sex. Without variability in F1 sterility, it is difficult to establish a causal relationship between reproductively isolating phenotypes and general expression patterns on a hybrid genomic background.
The X chromosome often plays a central role in the genetic underpinnings of reproductive isolation [7]. Hybrid inviability and sterility typically arise due to incompatible epistatic interactions between divergent genes [7]–[9]. Deleterious recessive incompatibilities are exposed on the X chromosome, but not the autosomes, of F1 hybrid males. This dominance-based model [10] provides a simple genetic explanation for the ubiquitous evolutionary pattern that hybrid inviability or sterility overwhelmingly afflicts the heterogametic sex first (i.e., Haldane's rule [11]). However, it does not explain why hybrid male sterility evolves much faster than male inviability [12]–[16]. Male sterility evolves particularly quickly on the X chromosome (i.e., the large X-effect [7], [17] for male sterility), which has been shown in Drosophila to accumulate a higher density of recessive mutations causing hybrid male sterility relative to the autosomes [14]–[16]. There are several evolutionary hypotheses to explain the rapid development of X-linked sterility, including more frequent positive selection on the X chromosome because of the immediate exposure of beneficial recessive mutations [18]–[20], recurrent genetic conflict over the meiotic transmission of the sex chromosomes [21]–[25], and rampant gene movement onto and off of the X chromosome [26]. None of these hypotheses are mutually exclusive and all plausibly contribute to the rapid evolution of hybrid male sterility.
Rapid X-linked evolution notwithstanding, the importance of the X chromosome for hybrid male sterility is surprising given that spermatogenic genes tend to be underrepresented on the X chromosome [27]–[29]. A possible mechanistic explanation for this discrepancy is that spermatogenesis may be particularly sensitive to disruption of gene expression on the X chromosome [16], [30]–[32]. In mammals [33], flies [34], and nematodes [35], transcription on the X chromosome is silenced during part of spermatogenesis, resulting in an under-representation of X-linked spermatogenic genes [27], [29], [36]. In mice, the X chromosome is inactivated at the pachytene stage of meiosis (i.e., meiotic sex chromosome inactivation or MSCI) when homologous autosomes synapse [37], [38]. Most of the X chromosome remains transcriptionally inactive for the duration of spermatogenesis (postmeiotic sex chromosome repression or PMSR) save a relatively small subset of postmeiotically expressed genes [39]. Mutations that disrupt synaptic pairing of autosomes can disrupt MSCI and PMSR, often resulting in male-limited sterility [40]–[42]. If MSCI and/or PMSR are also sensitive to evolutionary divergence between closely related species then disruption of X-inactivation during spermatogenesis may provide a general molecular basis for the large X-effect and the rapid evolution of hybrid male sterility [30]–[32].
Two closely related lineages of house mice, Mus musculus and M. domesticus, provide a powerful system for studying the role of gene regulatory divergence in speciation. The two species are recently diverged (∼500 KYA; [43]) and form a narrow hybrid zone across Europe. Laboratory crosses between M. domesticus and M. musculus often yield fertile females and sterile males [44]. The spermatogenic status of F1 males ranges from normal to complete meiotic arrest [31] or dramatic reductions in postmeiotic cells [44]. Two factors contribute to variation in F1 hybrid male sterility. First, multiple sets of epistatic incompatibilities are involved in spermatogenic failure [31], [44], including one or more X-autosome interactions that result in asymmetric sterility in some reciprocal crosses [44], [45]. All asymmetric crosses described so far yield sterile hybrid males when the maternal line is M. musculus, and introgression of the M. musculus X chromosome causes male sterility on a M. domesticus genetic background [46], [47]. Second, multiple autosomal incompatibilities are polymorphic within M. musculus and M. domesticus [45], [47]–[49]. Thus, F1 hybrid male fertility depends critically on both the direction of the cross and the genotype of the parental species.
One of the polymorphic incompatibilities, Hst1, has recently been localized to a single autosomal gene, PR-domain 9 or Prdm9 [50]. Prdm9 is involved in histone methylation [51] and causes aberrant expression of several interacting genes in sterile hybrid males [50]. Two previous studies [5], [52] have interrogated the evolution of gene expression between M. musculus, M. domesticus, and M. castaneus (another closely related species). The results of these studies were somewhat conflicting, with testis showing a clear excess of expression divergence between M. musculus and M. domesticus relative to brain or liver in only one of the experiments (i.e., [5]). This experiment also evaluated F1 hybrid male expression for two reciprocal crosses (M. domesticus and M. musculus; M. castaneus and M. musculus) [5]. F1 expression patterns were largely additive in most tissues and crosses; however, males from one cross (female M. musculus x male M. castaneus) showed an excess of mis-expressed transcripts in testis. The relevance of these data to mouse speciation remains unclear because sterility factors are polymorphic within house mice [45], [47]–[49] and male fertility phenotypes were not measured in this experiment.
Here we evaluate the role of gene expression in mouse speciation by using a reciprocal cross between M. domesticus and M. musculus that results in asymmetric hybrid male sterility. We directly associate aberrant expression patterns with hybrid male sterility by contrasting genome-wide expression data for both species with data from fertile and sterile F1 hybrids. Previously published phenotypic data from sterile hybrid males [44] were used to generate simple qualitative predictions for expected expression differences due to changes in the cellular composition of sterile hybrid testis. We then considered these predictions in the context of detailed information on the developmental timing of gene expression during spermatogenesis [39], [53].
Results/Discussion
Experimental design
The current work builds upon a previous study examining hybrid male sterility [44]. Previously, all eight pairwise interspecific crosses were performed between two wild-derived strains of M. domesticus (LEWES/EiJ, WSB/EiJ) and two wild-derived strains of M. musculus (PWK/PhJ, CZECHII/EiJ). F1 hybrid males from reciprocal interspecific crosses between CZECHII/EiJ and either strain of M. domesticus had small testis that produced few or no mature sperm. In contrast, males from crosses involving PWK/PhJ were only sterile when PWK/PhJ was the maternal strain (Figure 1).
Fig. 1. Experimental design and male reproductive phenotypes. 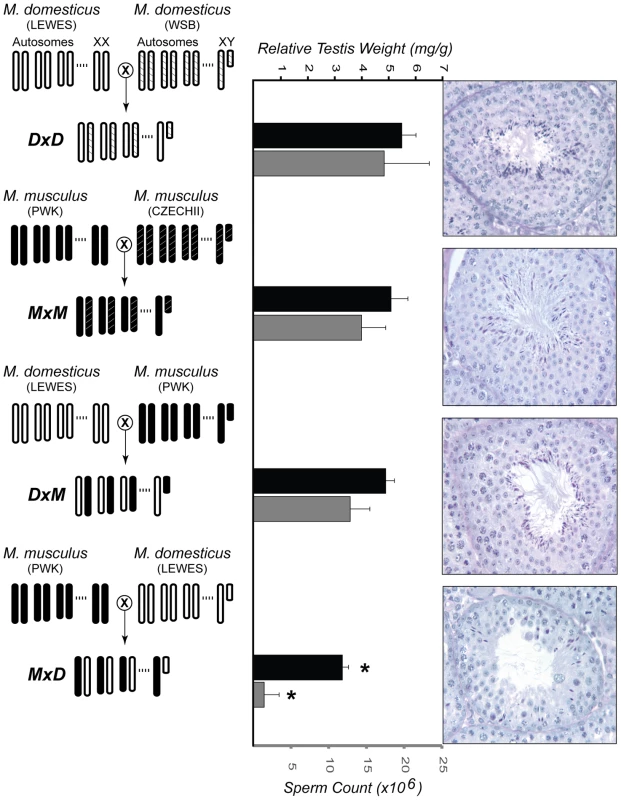
The crossing designs used to generate F1 males from two intraspecific crosses (DxD and MxM) and two interspecific crosses (DxM and MxD) are shown on the left. For each cross, the genotype for three autosomes and both sex chromosomes are given. Light-colored chromosomes (white and white-hatched) are from wild-derived strains of M. domesticus (LEWES/EiJ, WSB/EiJ) and dark-colored chromosomes (black and black-hatched) are from wild-derived strains of M. musculus (PWK/PhJ, CZECHII/EiJ). Previously published estimates [44] of relative testis weights (standardized for body weight) and sperm counts are given for males from each cross, in black and gray bars, respectively. Error bars indicate one standard deviation. (*) Hybrid MxD males were significantly reduced for both characters when compared to MxM and DxD males (pooled, Wilcoxon rank sum, P<0.01). For each genotype, representative histological cross-sections of a single seminiferous tubule are shown on the right. The first three genotypes showed normal progression of spermatogenesis, while the MxD males showed diminished numbers of germ cells overall, poor organization of the seminiferous epithelium, and a large reduction in the number of postmeiotic cells. Focusing on this latter asymmetric cross involving PWK/PhJ, we interrogated expression levels of ∼39,000 transcripts using Affymetrix Mouse Genome 430 2.0 GeneChips. For each of four genotypes (Figure 1), we examined expression levels of RNA isolated from whole testis in three 60-day old males resulting in 12 microarray experiments. This cross design was chosen specifically to evaluate the expression of a single M. musculus and M. domesticus X chromosome on both con - and heterospecific F1 backgrounds. Crosses within each species were performed to avoid confounding expression and phenotypic differences within and between species with differences between inbred and F1 genotypes. This design provides two important contrasts for evaluating the contribution of expression differences to reproductive isolation. First, comparison of testis expression levels within M. musculus (hereafter MxM) and within M. domesticus (hereafter DxD) males allows for the identification of genes with divergent expression levels between the species. Second, comparison between sterile hybrid males (hereafter MxD, maternal strain first) and all other males, including the fertile reciprocal hybrid (hereafter DxM), provides a direct contrast between normal and sterile males.
Strong conservation of testis expression between species on the X chromosome
Affymetrix 430 2.0 GeneChips were designed from the genome of the laboratory mouse C57BL/B6, which is largely of M. domesticus origin [54]. To help reduce the influence of probe mismatch, we incorporated probe performance into our analysis by down-weighting probes with high technical variance and only including genes that were detected in all samples based on Wilcoxon signed rank tests (P<0.01) between perfect versus mismatch signals. Of the 6,998 genes detected in all 12 samples (Table S1), 2,065 were significantly different between M. musculus and M. domesticus (P<0.05, pairwise t-tests). 1,435 of these genes remained significant at an estimated false discovery rate (FDR) of 5% (P<0.02364, pairwise t-tests).
The cellular composition of testis is highly heterogeneous, including populations of both somatic and germ line cells. Therefore, expression data collected from whole testis can be strongly influenced by the underlying cellular composition. We used published expression data [53] to identify groups of genes that show the greatest level of induction in somatic (Sertoli cells), mitotic (spermatogonia), meiotic (spermatocytes), or postmeiotic (round spermatids) cells. Of the 1,435 genes with significantly different expression between the species (FDR<0.05), 712 genes could be associated with a particular cell type. For these genes, loci with significantly higher expression in M. domesticus were enriched for meiotic genes but under-represented among mitotic genes (Table 1). Expression differences were not biased with respect to postmeiotic genes, suggesting that this difference does not reflect a simple shift in the onset of spermatogenesis (i.e., later development in M. musculus). Rather, it appears that evolutionary differences between the species are enriched to particular developmental time-points (i.e., meiosis). However, it is also possible that a subtle shift in the overall cellular composition of the testis has evolved between the species. Note that a slight majority of these differences resulted from transcripts that were more highly expressed in M. musculus (55%). These data suggest that probe effects due to evolutionary divergence are not a major factor in our analysis because probe mismatches to M. musculus should bias our results towards transcripts appearing more highly expressed in M. domesticus.
Tab. 1. Expression differences between M. domesticus and M. musculus across spermatogenic cell types. 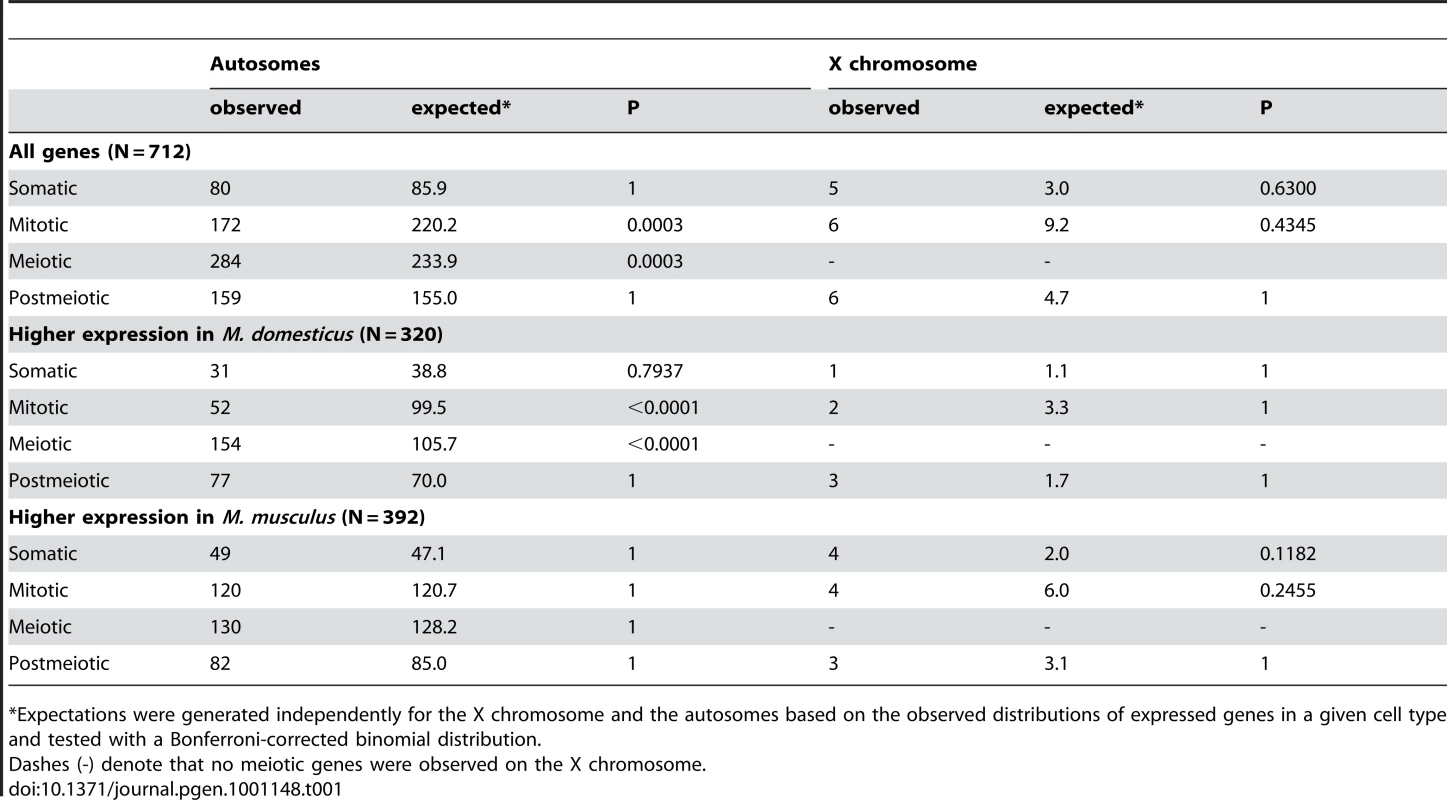
*Expectations were generated independently for the X chromosome and the autosomes based on the observed distributions of expressed genes in a given cell type and tested with a Bonferroni-corrected binomial distribution. Autosomal genes with divergent expression between species were distributed as expected given the genomic location of probes on the array (Figure 2). In contrast, we found half as many significant differences as expected on the X chromosome (22 observed versus 43.5 expected; Bonferroni-corrected P<0.003). The same under-representation of differences on the X chromosome was also obtained for the larger set of 2,065 genes (35 X-linked observed versus 63 expected; Bonferroni-corrected P<0.001) identified using a non-FDR corrected cutoff for the pairwise t-tests (P<0.05). These results are seemingly at odds with the prediction that the X chromosome may be disproportionately involved in adaptive evolution [18]. Although empirical evidence for faster X-linked evolution has been mixed [32], numerous studies have reported higher levels of protein divergence [55]–[57], a higher incidence of positive selection [56], [58], and an over-representation of certain classes of male reproductive genes [29], [59] on the X chromosome. We found that X-linked testis-expressed genes also show a significantly higher rate of protein evolution than autosomal testis-expressed genes (Wilcoxon signed rank P<0.0001 for dN/dS pairwise comparison versus orthologous rat genes; X chromosome, N = 152 genes, mean = 0.247, median = 0.160; autosomal dN/dS, N = 5,611 genes, mean = 0.165, median = 0.112). Thus, contrary to considerable evidence for rapid protein evolution on the X chromosome, our results demonstrate that testis gene expression on the X chromosome is actually more highly conserved between species of mice. Testis expression is significantly enriched for mitotic expression on the X chromosome (Table S2) and MSCI selects against expression during meiosis [29]. These developmental constraints also appear to limit X-linked expression divergence between species.
Fig. 2. Strong conservation of testis expression on the X chromosome between species. 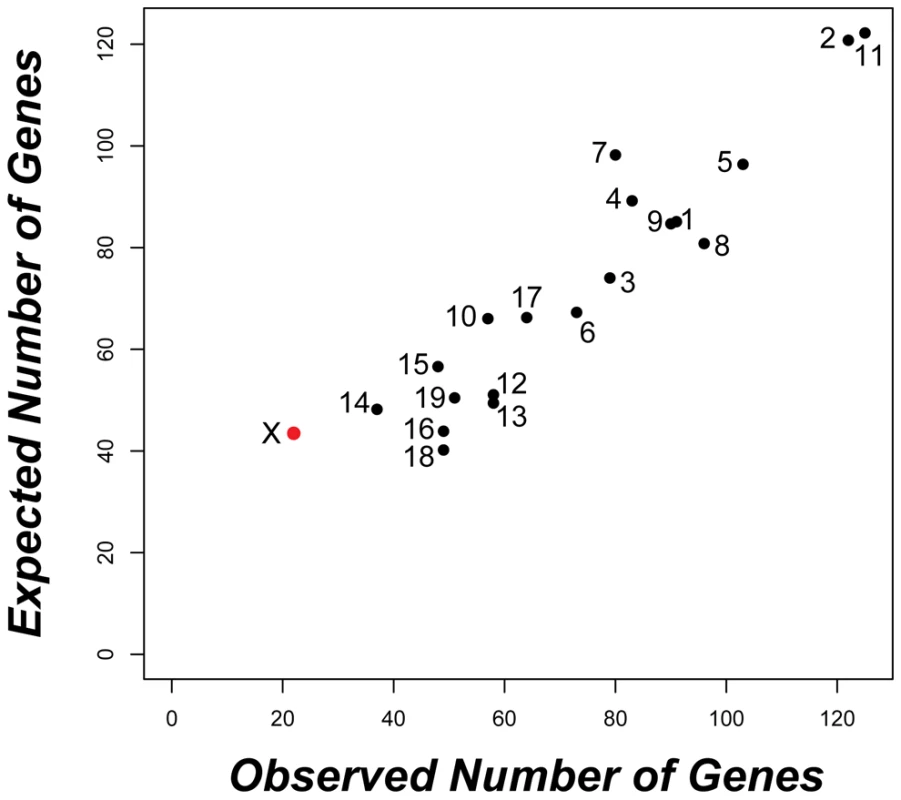
The observed versus expected distribution of the 1,435 genes with significantly different expression between M. musculus and M. domesticus (P<0.02364; FDR<0.05) is given for each chromosome. Only the X chromosome (red) showed a significant deviation (22 observed versus 43.5 expected; Bonferroni-corrected P<0.003) based on chromosome-wise hypergeometric tests. Widespread mis-expression of the X chromosome in sterile hybrid males
To associate expression divergence with reproductive isolation, we employed a hierarchical approach to define a conservative set of sterility-correlated genes. First, we contrasted each of the three fertile genotypes (MxM, DxD, DxM) with the MxD sterile F1 hybrid mice and identified all genes with significantly different expression between groups based on gene-by-gene t-tests (P<0.05; Figure 3). The estimated FDR among significant differences identified in these three pairwise contrasts ranged from 3.2% (MxD vs. DxD) to 19.3% (MxD vs. DxM). To help reduce the global FDR while enriching for expression differences directly correlated with the sterility phenotype, we focused only on the 902 genes that were significantly different between the reciprocal hybrids and at least one of the parental lines (Figure 3). We refer to these 902 genes as “sterility-correlated genes”. The autosomal distribution of sterility-correlated genes did not deviate from random expectations (Figure S1). However, opposite to what was observed between species, we detected a ∼three-fold enrichment of sterility-correlated genes on the X chromosome (81 observed versus 27.3 expected, Bonferroni-corrected P<0.0001). Importantly, an approximately three-fold enrichment of sterility-correlated genes on the X chromosome was consistently observed across different operational definitions of “sterility-correlated”: the 1,049 genes differing between sterile MxD males and fertile DxM males (94 observed versus 31.8 expected, Bonferroni-corrected P<0.0001), the 397 genes different in all three fertile vs. sterile pairwise contrasts (43 observed versus 12 expected, Bonferroni-corrected P<0.0001), and the 181 genes different between all three fertile vs. sterile pairwise contrasts and not different between all three fertile genotypes (21 observed versus 5.5 expected, Bonferroni-corrected P<0.0001). Likewise, we also observed a strong global enrichment of sterility-correlated genes on the X chromosome (46 observed versus 6.8 expected, Bonferroni-corrected P<0.0001) when using a more conservative cutoff (P<0.01; estimated FDR 0.5–7.5%) in our pairwise t-tests (Figure S2).
Fig. 3. Overlap of pairwise expression differences between sterile and fertile mice. 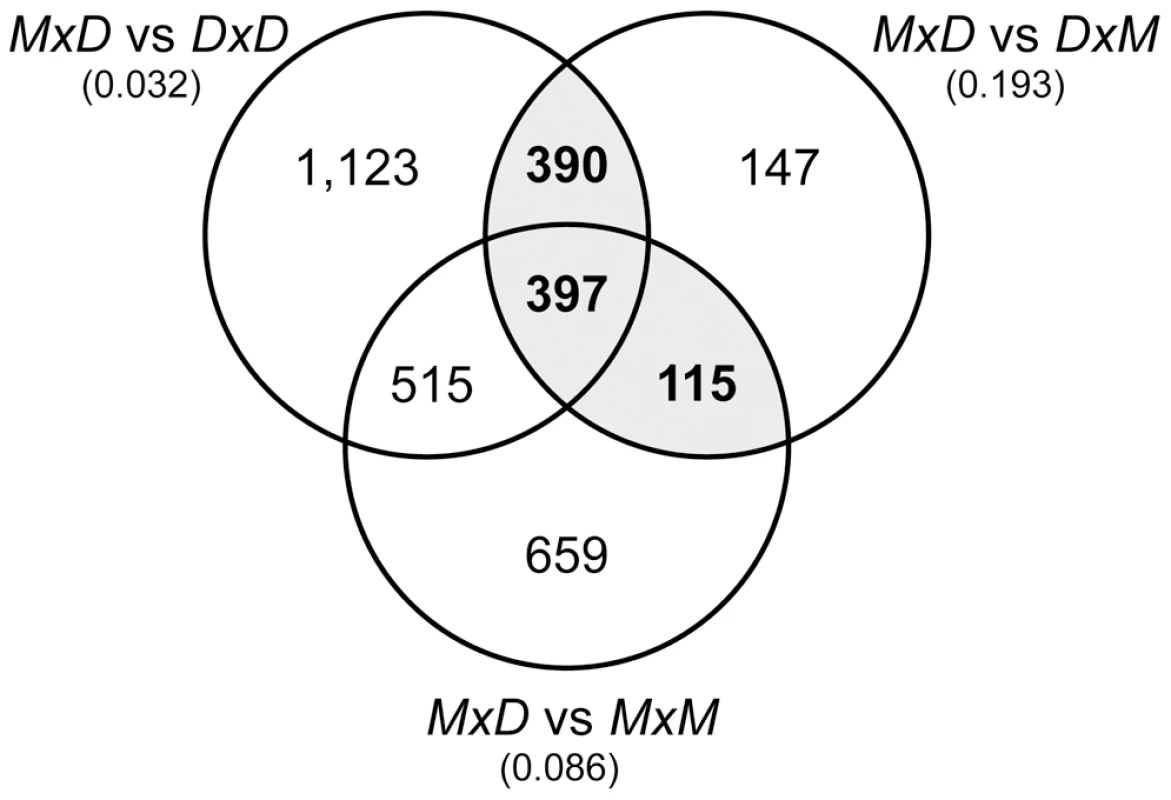
The Venn diagram gives the numbers of genes with significantly different expression (P<0.05) for the three pairwise contrasts between sterile MxD mice and the three fertile mouse genotypes (DxM, MxM, DxD). The estimated FDR for each comparison is given in parentheses. There were 902 genes that were significantly different between the reciprocal hybrids and at least one of the parental lines (gray shading). Histological analyses show that sterile MxD males have a dramatic reduction in the number of postmeiotic cells (Figure 1, [44]). Thus, postmeiotic cells comprise a smaller proportion of the overall cellular composition of testis in sterile MxD testis when compared to fertile males with normal spermatogenesis. Therefore, genes expressed late in spermatogenesis would be expected to show lower expression levels in these sterile males, even if transcript abundances per cell were equivalent. Because we are measuring transcript levels from a fixed amount of RNA extracted from whole testis, it follows that transcripts from mitotic cells would be proportionally more common in sterile males. This simple qualitative model, hereafter referred to as the “cellular composition hypothesis”, predicts that mitotic genes should appear to be over-expressed while postmeiotic genes should show lower expression in sterile hybrid males. For example, postmeiotic cells comprise ∼85% of the total cellular content of adult testis [60]. If postmeiotic cells only comprised 55% of the cells in the testis of sterile F1 males then we would expect an apparent reduction in postmeiotic expression to be accompanied by a three-fold proportional increase in the relative abundance of non-postmeiotic transcripts (i.e., an increase from 15% to 45% of total testis cellular composition). Only genes with higher postmeiotic expression and lower mitotic expression in sterile males are not confounded by cellular composition and potentially reflect true expression differences. Moreover, such differences should be highly conservative because the skew in cellular composition should reduce our power to detect true expression differences. In particular, the skew in cellular composition will lead to an underestimate of the magnitude of differences that is proportional to the underlying difference in relative abundance of the relevant cell type.
To evaluate our data in the context of the cellular composition hypothesis, we first binned the 902 sterility-correlated genes into three groups: genes with higher expression in sterile MxD mice, genes with lower expression in sterile MxD mice, and genes with intermediate expression in sterile MxD mice. We then identified 607 sterility-correlated genes that could be associated with one of four spermatogenic cell types [53]. Overall, postmeiotic genes were highly over-represented among sterility-correlated genes and there were many fewer meiotic genes than expected by chance (Table 2). However, this global pattern masks key differences in gene expression between the X chromosome and the autosomes. Autosomal sterility-correlated genes closely followed the predictions of the cellular composition hypothesis with most postmeiotic genes showing lower expression (180 of 211, ∼85%) and most mitotic genes showing higher expression (151 of 184, ∼82%) in sterile MxD males. These differences are confounded by the skewed cellular composition of the sterile versus fertile males and thus may not reflect true differences in expression. In stark contrast, most of the 902 sterility-correlated genes on the X chromosome were over-expressed in sterile MxD males (∼93% or 75 of 81). Thus, simple differences in the cellular composition of sterile and fertile hybrid males do not explain a majority of mis-expressed genes on the X chromosome. We also observed an almost 2-fold increase in over-expressed postmeiotic genes on the X chromosome (32 observed, 18 expected; Table 2). Strikingly, the X chromosome harbors only ∼6% of the postmeiotic genes in our dataset (48 of 806) yet 89% of the postmeiotic genes (32 of 36) that were over-expressed in sterile MxD males were X-linked. Overall, there were 45 sterility-correlated genes that could not be explained by simple differences in the cellular composition of testis from sterile and fertile males (higher postmeiotic, lower mitotic expression in sterile males, Table S3); thirty-two of these were over-expressed postmeiotic genes on the X chromosome (Figure 4).
Fig. 4. Forty-five sterility-correlated genes with patterns of expression robust to differences in testis cellular composition between fertile and sterile males. 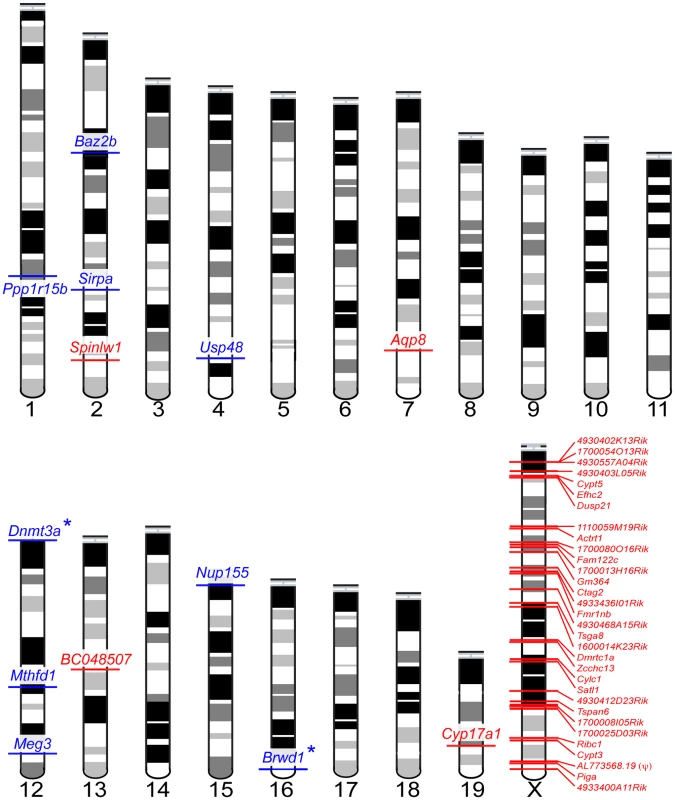
Postmeiotic genes over-expressed in sterile males are shown in red, mitotic genes under-expressed in sterile males are shown in blue. Autosomal loci with known male sterility knockout phenotypes are indicated with an (*). One locus, AL773568.19(ψ), is a transcribed pseudogene. Tab. 2. Expression of sterility-correlated genes across spermatogenic cell types. 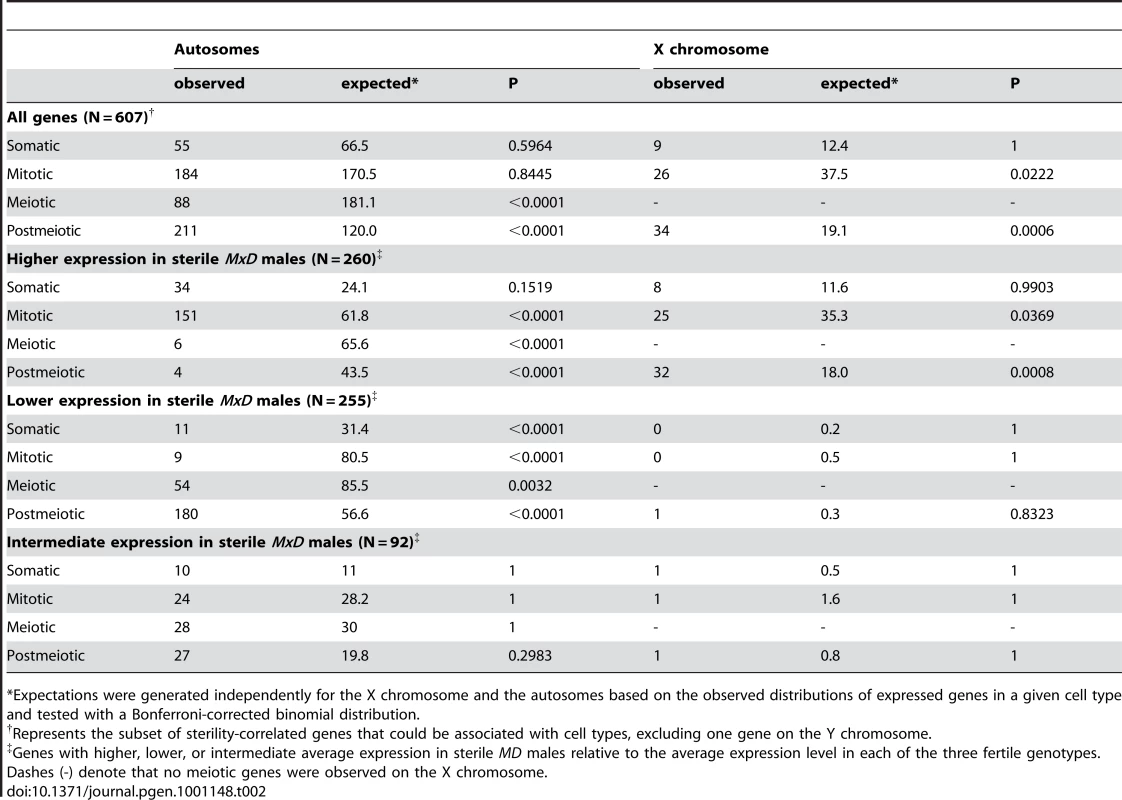
*Expectations were generated independently for the X chromosome and the autosomes based on the observed distributions of expressed genes in a given cell type and tested with a Bonferroni-corrected binomial distribution. Two patterns indicate that the signature of higher X-linked expression in sterile MxD males is a chromosome-wide phenomenon. First, the 32 postmeiotic genes over-expressed in sterile MxD males were distributed across the majority of the X chromosome (8.7–166.2 Mb, Table S3, Figure 4). Second, over-expression of the MxD X chromosome was also apparent when considering the per chromosome deviation of all 6,998 expressed genes (Figure 5). Genes on the X chromosome of the sterile MxD mice showed a mean increase of 17% compared to the per gene median expression level across all males (Figure 5A). In each of the six pairwise comparisons, the distribution of expression differences was significantly different between the X chromosome and the pooled autosomes (Figure 5B). However, the two chromosomal groups were similar and centered near zero for the three comparisons between fertile genotypes (Figure 5B, top panel), while the three contrasts involving sterile males all showed a dramatic shift towards higher X-linked expression in sterile MxD males (Figure 5B, bottom panel).
Fig. 5. Over-expression of the X chromosome in sterile MxD hybrid mice. 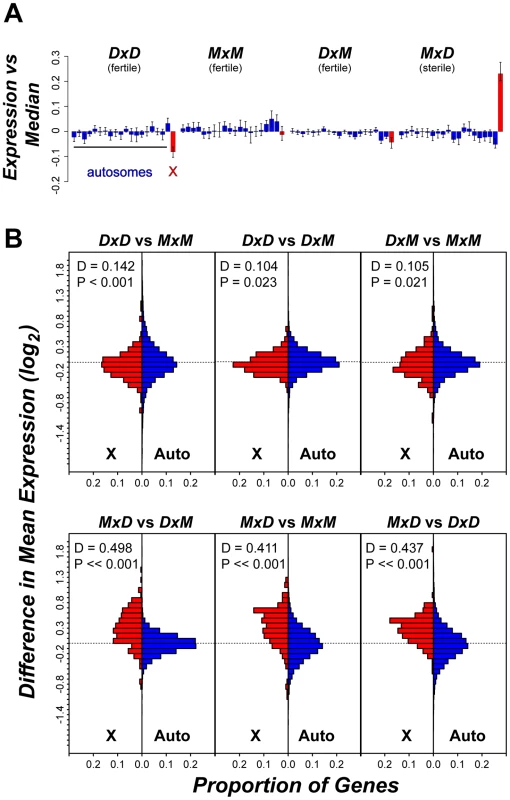
(A) Average per chromosome deviation (log2 scale) for each genotype versus the median per gene expression across all 12 males. The 19 autosomes (blue) are presented sequentially with the X chromosome (red). (B) Distribution of pairwise expression differences for the X chromosome versus the autosomes. For each pairwise comparison, the proportional distribution of gene-by-gene differences in mean expression (log2) is shown for the X chromosome (red) and the autosomes (blue) with results from a Kolmogorov-Smirnoff test. The observed over-expression of the X chromosome was also not merely a consequence of probe-induced artifacts. Higher expression of the X chromosome in sterile MxD mice is opposite of what would be expected if our results were strongly influenced by reduced probe affinity due to divergence from the M. domesticus-derived microarray, because the X chromosome in the sterile males is of M. musculus origin. More specifically, the fertile MxM mice and the sterile MxD mice share the same hemizygous M. musculus X chromosome and thus can be used to further evaluate X-linked expression on con - and heterospecific F1 backgrounds, independent of X-linked probe effects. A recent study [39] provided a detailed account of cell type specific expression patterns on the X chromosome, which is independent of the testis cell type associations we used above [53]. This work identified five general patterns of X-linked spermatogenic expression in mice: (1) expressed primarily in mitotic cells and repressed in meiotic and postmeiotic cells; (2) expressed in mitotic cells, repressed in meiotic cells, and expressed in postmeiotic cells; (3) expressed primarily in postmeiotic cells; (4) variable expression; (5) repressed in all cells. Associating these X-specific expression groups with our testis expression data from MxM and MxD males, we again found that most genes (154 of 180) showed higher average expression on the M. musculus X chromosome of MxD males, including 27 of 31 genes expressed primarily in postmeiotic cells (Figure S3). The strong tendency for X-linked genes to show higher expression in MxD males is in contrast to the slight bias (52% of genes) in the opposite direction for autosomal genes in this pairwise comparison.
By incorporating a detailed understanding of the sterility phenotype with information on the progression of gene expression during spermatogenesis, we were able to establish a striking association between F1 sterility and mis-regulation of the X chromosome that appears to be independent of differences in the cellular composition of the testis of sterile and fertile mice. The large role of the X chromosome also does not appear to be a direct consequence of greater evolutionary divergence for X-linked gene expression because X chromosome expression appears exceptionally conserved between species (Figure 2). Finally, the large role of the X chromosome inferred from this study is consistent with previous mapping results using these same strains, which showed that many loci distributed along the entire M. musculus X chromosome play an important role in hybrid male sterility [47]. Therefore, these data support the hypothesis that spermatogenic gene regulation on the X chromosome is particularly sensitive to incompatible interactions between the divergent genomes of M. musculus and M. domesticus. With the current data, we cannot determine whether higher expression of the X chromosome results from a failure of MSCI or abnormally high postmeiotic transcription. However, we did not observe a pachytene arrest during meiosis I [44] as might be expected given a complete failure of MSCI. Additional fine-scale examination of transcription on the X chromosome during the developmental progression of spermatogenesis should help resolve the exact timing and mechanism underlying this pattern.
X-inactivation, mis-expressed autosomal genes, and epigenetic regulation
Intrinsic hybrid incompatibilities typically arise due to epistatic interactions among divergent genes [7]–[9]. In our experiment, over-expression of the X chromosome only occurred on the MxD hybrid genomic background and thus likely involves one or more epistatic interactions between the M. musculus X chromosome and loci on the M. domesticus Y and/or the autosomes. Differences in the cellular composition of the testes of sterile and fertile mice may mask many differentially expressed autosomal transcripts between the sterile and fertile mice. Nevertheless, inspection of the 13 autosomal sterility-correlated genes with expression patterns that should be robust to differences in cellular composition (Figure 4; Table S3) revealed two compelling candidates for genes contributing to regulatory incompatibilities. First, DNA methyltransferase 3A (Dnmt3a) showed significantly reduced expression in sterile MxD males versus all three fertile genotypes (all P<0.021). Dnmt3a is essential for de novo DNA methylation [61] and conditional knockouts of Dnmt3a cause complete spermatogenic arrest characterized by a failure of germ cells to develop past round spermatids [62]. Under-expression of Dnmt3a, given its general role as a repressor of transcription via DNA methylation, and the global over-expression of X chromosome in our sterile mice (Figure 5), together raise the possibility of a direct connection between Dnmt3a and the failure of MSCI or PMSR. Dnmt3a is a direct negative regulator of Xist [63], which is required for X-inactivation in females [64]. In males, Xist is exclusively expressed in testis coincident with the onset of MSCI [65]. However, MSCI proceeds normally in males with a disrupted copy of Xist [66], suggesting X-inactivation proceeds through sex-specific mechanisms [41]. Thus, while Dnmt3a is essential for spermatogenesis, its underlying role in MSCI and PMSR remains unresolved.
Second, the transcription factor Brwd1 (Bromodomain and WD repeat domain containing 1) also showed significantly reduced expression in sterile hybrid MxD males versus the three other fertile genotypes (all P<0.005). Brwd1 is thought to influence transcriptional regulation and chromatin remodeling during spermatogenesis and oogenesis [67]. Mice homozygous for a null mutant of Brwd1 show both male and female sterility [67]. In testis, Brwd1 is most highly expressed in spermatogonia [53], yet disruption of Brwd1 in males results primarily in postmeiotic disruption of spermatogenesis, including dramatic reduction in postmeiotic spermatocytes, low epididymal sperm counts, abnormal sperm head morphology, and poor motility [67]. These phenotypes are qualitatively similar to those found in both sterile MxD males (Figure 1; [44]) and male-sterile strains of M. domesticus (LEWES) consomic for portions of the M. musculus (PWK) X chromosome [47].
In addition to Dnmt3a and Brwd1, a histone methyltransferase gene on chromosome 17, Prdm9, was recently determined to be involved in hybrid male sterility between M. musculus and M. domesticus [50], marking the first discovery of a hybrid sterility locus in a vertebrate. Prdm9 expression was not detected in any of the males in our experiment, suggesting expression levels were beyond the limits of microarray detection. Nevertheless, Prdm9 warrants further consideration in the context of abnormal F1 gene expression. Null mutants of Prdm9 disrupt homologous chromosome pairing and sex body formation during meiosis, resulting in male and female sterility [51]. Crosses between female M. musculus (PWD) and male C57BL/B6 (a laboratory strain predominantly of M. domesticus origin [54]) result in complete meiotic arrest of hybrid males due to an epistatic interaction between Prdm9 and multiple unidentified autosomal and X-linked factors [46], [68]. However, genotypic data suggest that Prdm9 is not involved in hybrid sterility in our experiment. Prdm9 is polymorphic for fertile and sterile alleles in laboratory strains of mice (i.e., ∼M. domesticus), and hybrid sterility only ensues when both sterility alleles are present [31]. The only protein-coding difference between sterile and fertile M. domesticus Prdm9 alleles is variation in the number of C-terminal C2H2 zinc-finger repeats [50]. We found that fertility-associated Prdm9 length variants also segregate between wild-derived strains of M. domesticus (LEWES, fertile allele; WSB, sterile allele) yet both of these strains produce sterile hybrid males with largely postmeiotic abnormalities when crossed with female M. musculus (PWK) [44]. Moreover, the sterile MxD males carry the Prdm9 length variant associated with fertility. Thus, if Prdm9 was involved in sterility of MxD males, it would require different allelic combinations than previously described [50].
The role of X-inactivation and epigenetic gene regulation in the evolution of hybrid male sterility
The global patterns we have described argue that disruption of gene regulation plays an important role in house mouse speciation. Several lines of evidence suggest that the X chromosome plays a large role in reproductive isolation in house mice, and that the genetic basis of this isolation is reasonably complex [47]. Three studies have attempted to dissect the genetic basis of hybrid sterility through introgression of the M. musculus X chromosome on to largely M. domesticus genomic backgrounds [46], [47], [69]. All three studies identified multiple QTL of large effect associated with male sterility spanning the X chromosome, but finer-scale localization of individual loci has thus far proven elusive. Interestingly, previous crosses with these same strains revealed a near additive effect of many loci along the entire X chromosome contributing to hybrid male sterility [47]. That observation, together with the genomic distribution of expression differences presented here, raises the possibility that sterility in these mice largely reflects the effects of disrupted transcriptional regulation of the X chromosome on a hybrid genomic background. This hypothesis predicts that the M. musculus X chromosome contains regulatory sequences along much of its length that do not interact properly with one or more M. domesticus autosomal loci. Chromosome-wide disruption of epigenetic silencing could also help explain an overall reduction in X-linked relative to autosomal gene flow between M. musculus X and M. domesticus observed in the European hybrid zone [70]–[73].
The X chromosome often plays a central role in speciation but the evolutionary basis for this has remained unclear. Several hypotheses, including faster evolution of the X chromosome [18] and an inherent sensitivity of spermatogenesis to disruption of X-linked gene regulation [30], [31], have been proposed to explain this phenomenon [32]. Our data provide empirical support for a regulatory basis to speciation in house mice and establish the importance of transcriptional regulation of the X chromosome in the evolution of hybrid male sterility, as originally proposed over 35 years ago [30]. Failure of MSCI may also play an important role in Drosophila speciation [32], where the X chromosome is enriched for over-expressed transcripts in testis of some sterile males [4]. In mammals, MSCI has long been argued as a critical check-point in male meiosis [74], [75] and failure of X-inactivation has been suggested to be an important cause of male sterility in humans and mice [41], [75]. In turn, disruption of X-inactivation may also prove to be an important mechanism contributing to two of the most general patterns in speciation genetics: Haldane's rule [11] and the disproportionately large effect of the X chromosome in hybrid male sterility [7], [17].
Methods
Ethics statement
Mice were maintained at the University of Arizona Central Animal Facility following Institutional Animal Care and Use Committee (IACUC) regulations.
Strains, animal husbandry, and male reproductive phenotypes
All breeding colonies were established using individuals purchased from the Jackson Laboratory (Bar Harbor, ME). LEWES/EiJ and WSB/EiJ were originally derived from natural populations of M. domesticus in eastern North America and the M. musculus strains CZECHII/EiJ and PWK/PhJ were isolated from different localities within the Czech Republic. After weaning, male offspring were housed in sibling groups until 40 days postpartum, and then caged singly until being sacrificed at 60 days old. We collected data for several male reproductive phenotypes including testis weight, sperm count, sperm motility, seminal vesicle weight, testis histology, and fecundity. A detailed description of these data, including experimental protocols, has been published previously [44].
Sample preparation and microarray processing
Immediately after males were euthanized, testes were dissected and cross-sectioned, placed in RNAlater (Ambion, Inc., Austin, TX), and archived at −80C. We extracted total RNA from whole testis using an RNeasy Midi kit (QIAGEN Inc., Valencia, CA). RNA sample quality and quantification was determined with an RNA Nano LabChip on an Agilent Bioanalyzer 2100 (Santa Clara, CA). Only samples with an RNA integrity number of 10 were used. Biotinylated complementary DNA was generated from 5 µg of total RNA and hybridized to the Affymetrix Mouse Genome 430 2.0 array (Santa Clara, CA). Sample quality control and microarray processing was performed following the manufacturer's instructions by the Genomics Shared Service at the University of Arizona. In order to estimate the between chip experimental variability, we followed the standard protocol of spiking in transcripts for three genes from the biotin synthesis pathway in E. coli (BioB, BioC, BioD) and one transcript from the recombinase gene from bacteriophage P1 (cre) as hybridization controls. For all of these transcripts there were two probe sets present on the array platform that we used - except for the BioB gene, which was targeted with three probe sets. This results in nine probesets for which we could evaluate the effect of hybridization onto different slides. The average Pearson correlation between the 12 microarray experiments of the signals from these nine probesets was observed to be very high (97.09%) and ranged between 93.76% and 99.99%.
Analysis of expression data
Updated transcript definitions can improve both the precision and accuracy of microarray data [76]. We used chip description files [77] downloaded from BRAINARRAY (version 11; http://brainarray.mbni.med.umich.edu). All data processing and analysis was conducted using R [78]. The 430 2.0 array was designed from the laboratory mouse genome, which is primarily derived from M. domesticus [54]. We used two approaches to help avoid systematic errors associated with this bias. First, data analysis was performed using probe logarithmic intensity error estimation (PLIER) on the signal intensity measurements as implemented with the justPlier function in BioConductor [79]. The PLIER algorithm is a model-based signal estimator that dynamically weights the probe signal intensity data using empirical probe performance. Each of the 6,998 genes was targeted with an average of 17 probes (range: 7–108). We used the PLIER algorithm to summarize the signals of these probes in order to obtain a robust gene level expression measurement. Second, we only considered genes with significantly detectable expression in all 12 individuals. A gene was considered expressed in an individual if the perfect match signal was significantly higher than the mismatch signal (Wilcoxon signed rank tests; P<0.01). Expression values were then quantile normalized to facilitate comparison across chips.
The primary goals of our experiment were to identify global patterns of testis gene expression with respect to (1) evolutionary divergence between M. musculus and M. domesticus and (2) divergence between sterile and fertile mice. To identify expression differences between species (DxD versus MxM), we first identified all genes with significantly different expression between groups based on gene-by-gene Student's t-tests (P<0.05), excluding genes with no variation between individuals. We then estimated the t-test p-value corresponding to an FDR of 5%, as implemented with fdrtool [80], to evaluate the robustness of all global patterns inferred from this pairwise contrast to multiple comparisons. Next we employed a hierarchical approach to define a conservative set of sterility-correlated genes. We first identified all genes with significantly different expression between groups based on gene-by-gene Student's t-tests (P<0.05) in each of the three possible pairwise comparisons between fertile genotypes (MxM, DxD, DxM) and the MxD sterile F1 hybrid mice. To estimate the FDR of these individual pairwise contrasts, we performed all ten possible sample-label permutations of each pairwise comparison to derive an empirical distribution of significant outcomes under the null hypothesis of no differences between the groups [81]. The FDR was then calculated as the ratio of the median number of significant outcomes in our permutations to the observed number of significant outcomes at a 5% cutoff. The estimated FDR's for individual pairwise comparisons in this study (3.2%–19.3%; see Results) are comparable to those in other studies [82], [83] and indicate that the results of the individual pairwise contrasts are not dominated by type I error. Nonetheless, because of the potential for false discovery of individual genes, we emphasize global patterns of expression difference with respect to genomic location rather than focusing on individual genes. To further reduce the FDR, we also restricted our focus to genes that were significantly different between the reciprocal hybrids and at least one of the parental lines. While direct estimation of the FDR for this hierarchically-defined set is complicated by non-independence of partially overlapping comparisons, this set of genes should be much more conservative than the three individual pairwise comparisons with respect to false positives associated with male sterility. Finally, we repeated all analyses using more stringent definitions of sterility-correlated and a more conservative threshold for our gene-by-gene t-tests (P<0.01, estimated FDR 0.5–7.5%) and observed the same global patterns with respect to expression divergence on the X chromosome.
To evaluate our data in the context of up - versus down-regulation of genes in sterile males we binned sterility-correlated genes into three groups: genes with higher mean expression in sterile MxD mice versus the mean expression of each of the three fertile genotypes, genes with lower expression in sterile mice, or genes where sterile mice showed intermediate levels of expression.
Gene set enrichment analysis
To determine if differentially expressed genes were randomly distributed across the genome we performed chromosome-wise hypergeometric tests with Bonferroni correction for multiple hypothesis testing. Gene annotation was based on Ensembl version 52 of NCBI build 37 of the mouse genome. Of the 6,998 expressed genes in our analysis, 6,882 were annotated as protein-coding genes, 13 as pseudogenes, two as retrotransposed genes, and one small nucleolar RNA.
We used the GermOnline Systems database [84] to associate genes with particular testis cell types. These cell type associations derive from a series of microarray experiments on enriched cell populations [53] and denote in which testis cell population [somatic (Sertoli cells), mitotic (spermatogonia), meiotic (spermatocytes), or postmeiotic (round spermatids) cells] a given gene showed the greatest level of induction in and is not necessarily indicative of cell type specific expression. We also used additional expression data [39] to provide a second, more detailed account of cell type specific expression patterns on the X chromosome. Using microarray analysis of enriched cell populations, X-linked genes were classified into five expression groups: group A - expressed in mitotic cells (A and B spermatogonia) and repressed in meiotic (pachytene spermatocytes) and postmeiotic cells (round spermatids); group B - expressed in mitotic cells, repressed in meiotic cells, and expressed in postmeiotic cells; group C - expressed in postmeiotic cells; group D - variable expression; group E - repressed in all cells. Genes with variable expression (group D) comprise a very small subset of X-linked genes [39] and were not included in our analysis. Bonferroni-corrected binomial tests were used to determine if subsets of genes were randomly distributed with respect to cell types. Because gene expression on X chromosome is non-random with respect to cell type (Table S2), expectations were generated independently for the X chromosome and the autosomes and were based on the observed distributions of the total number of expressed genes in a given cell type.
Molecular evolutionary analysis
We analyzed all one-to-one orthologs between mouse and rat using Ensembl annotation version 48 (www.ensembl.org; NCBI mouse build 37). Rates of protein evolution were calculated based on the number of nonsynonymous substitutions per nonsynonymous site (dN) normalized by the number of synonymous substitutions per synonymous site (dS), as previously reported [85].
Genotyping of Prdm9
The critical region of Prdm9 occurs in the C terminus, and the sterility phenotype correlates with alternative numbers of C2H2 repeats [50]. This region was targeted using published primers [50] centered around chromosome 17 position 15,249,000 (NCBI m36 mouse genome assembly). Ten pmol of each primer was combined with 5 nmol dNTP, 50 nmol MgCl2, BioRad Platinum taq polymerase, buffer, and water to 25 µL, and run for 35 cycles of: 94 C 20 sec, 57.5 C 20 sec, 68 C 90 sec. The classical inbred strains C57BL/6J (sterile allele, 12 C2H2 repeats) and C3H (fertile allele, 13 C2H2 repeats) were included as controls. PCR products were scored on a 2% agarose gel.
Data deposition
The expression data reported in this paper have been deposited in the NCBI Gene Expression Omnibus (GSE17684).
Supporting Information
Zdroje
1. MichalakP
NoorMAF
2003
Genome-wide patterns of expression in Drosophila pure species and hybrid males.
Mol Biol Evol
20
1070
1076
2. RanzJM
NamgyalK
GibsonG
HartlDL
2004
Anomalies in the expression profile of interspecific hybrids of Drosophila melanogaster and Drosophila simulans.
Genome Res
14
373
379
3. MaloneJH
ChrzanowskiTH
MichalakP
2007
Sterility and gene expression in hybrid males of Xenopus laevis and X. muelleri.
PLoS ONE
2
e781
doi:10.1371/journal.pone.0000781
4. MoehringAJ
TeeterKC
NoorMAF
2007
Genome-wide patterns of expression in Drosophila pure species and hybrid males. II. Examination of multiple-species hybridizations, platforms, and life cycle stages.
Mol Biol Evol
24
137
145
5. RottscheidtR
HarrB
2007
Extensive additivity of gene expression differentiates subspecies of the house mouse.
Genetics
177
1553
1567
6. Ortiz-BarrientosD
CountermanBA
NoorMAF
2007
Gene expression divergence and the origin of hybrid dysfunctions.
Genetica
129
71
81
7. CoyneJA
OrrHA
2004
Speciation.
Sunderland, MA
Sinauer Associates, Inc
545
8. DobzhanskyT
1937
Genetics and the origin of species.
New York
Columbia University Press
364
9. MullerHJ
1942
Isolating mechanisms, evolution, and temperature.
Biol Symp
6
71
125
10. TurelliM
OrrHA
2000
Dominance, epistasis and the genetics of postzygotic isolation.
Genetics
154
1663
1679
11. HaldaneJBS
1922
Sex ratio and unisexual sterility in animal hybrids.
J Genet
12
101
109
12. WuC-I
DavisAW
1993
Evolution of postmating reproductive isolation: the composite nature of Haldane's rule and its genetic bases.
Am Nat
142
187
212
13. HollocherH
WuCI
1996
The genetics of reproductive isolation in the Drosophila simulans clade: X vs autosomal effects and male vs female effects.
Genetics
143
1243
1255
14. TrueJR
WeirBS
LaurieCC
1996
A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans.
Genetics
142
819
837
15. TaoY
ChenS
HartlDL
LaurieCC
2003
Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. I. Differential accumulation of hybrid male sterility effects on the X and autosomes.
Genetics
164
1383
1397
16. MaslyJP
PresgravesDC
2007
High-resolution genome-wide dissection of two rules of speciation in Drosophila.
PLoS Biol
5
e243
doi:10.1371/journal.pbio.0050243
17. CoyneJA
OrrHA
1989
Two rules of speciation.
OtteD
EndlerJ
Speciation and Its Consequences
Sunderland, MA
Sinauer Associates
180
207
18. CharlesworthB
CoyneJA
BartonNH
1987
The relative rates of evolution of sex chromosomes and autosomes.
Am Nat
130
113
146
19. VicosoB
CharlesworthB
2006
Evolution of the X chromosome: unusual patterns and processes.
Nat Rev Genet
7
645
653
20. VicosoB
CharlesworthB
2009
Effective population size and the faster-X effect: an extended model.
Evolution
63
2413
2426
21. FrankSA
1991
Divergence of meiotic drive suppression systems as an explanation for sex-biased hybrid sterility and inviability.
Evolution
45
262
267
22. HurstLD
PomiankowskiA
1991
Causes of sex-ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's Rule and related phenomena.
Genetics
128
841
858
23. TaoY
HartlDL
2003
Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. III. Heterogeneous accumulation of hybrid incompatibilities, degree of dominance, and implications for Haldane's rule.
Evolution
57
2580
2598
24. McDermottSR
NoorMAF
2010
The role of meiotic drive in hybrid male sterility.
Philos Trans Roy Soc London B
365
1265
1272
25. MeiklejohnCD
TaoY
2010
Genetic conflict and sex chromosome evolution.
Trends Ecol Evol
25
215
223
26. MoyleLC
MuirCD
HanMV
HahnMW
2010
The contribution of gene movement to the "Two Rules of Speciation".
Evolution
64
1541
1557
27. ParisiM
NuttallR
NaimanD
BouffardG
MalleyJ
2003
Paucity of genes on the Drosophila X chromosome showing male-biased expression.
Science
299
697
700
28. RanzJM
Castillo-DavisCI
MeiklejohnCD
HartlDL
2003
Sex-dependent gene expression and evolution of the Drosophila transcriptome.
Science
300
1742
1745
29. KhilPP
SmirnovaNA
RomanienkoPJ
Camerini-OteroRD
2004
The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation.
Nat Genet
36
642
646
30. LifschytzE
LindsleyDL
1972
The role of X-chromosome inactivation during spermatogenesis.
Proc Natl Acad Sci U S A
69
182
186
31. ForejtJ
1996
Hybrid sterility in the mouse.
Trends Genet
12
412
417
32. PresgravesDC
2008
Sex chromosomes and speciation in Drosophila.
Trends Genet
24
336
343
33. RichlerC
SoreqH
WahrmanJ
1992
X-inactivation in mammalian testis is correlated with inactive X-specific transcription.
Nat Genet
2
192
195
34. HenseW
BainesJF
ParschJ
2007
X chromosome inactivation during Drosophila spermatogenesis.
PLoS Biol
5
e273
doi:10.1371/journal.pbio.0050273
35. KellyWG
SchanerCE
DernburgAF
LeeMH
KimSK
2002
X-chromosome silencing in the germline of C. elegans.
Development
129
479
492
36. ReinkeV
SmithHE
NanceJ
WangJ
Van DorenC
2000
A global profile of germline gene expression in C. elegans.
Mol Cell
6
605
616
37. HandelMA
2004
The XY body: a specialized meiotic chromatin domain.
Exp Cell Res
296
57
63
38. TurnerJMA
MahadevaiahSK
Fernandez-CapetilloO
NussenzweigA
XuXL
2005
Silencing of unsynapsed meiotic chromosomes in the mouse.
Nat Genet
37
41
47
39. NamekawaSH
ParkPJ
ZhangL-F
ShimaJE
McCarreyJR
2006
Postmeiotic sex chromatin in the male germline of mice.
Curr Biol
16
660
667
40. HomolkaD
IvanekR
CapkovaJ
JansaP
ForejtJ
2007
Chromosomal rearrangement interferes with meiotic X chromosome inactivation.
Genome Res
17
1431
1437
41. TurnerJMA
2007
Meiotic sex chromosome inactivation.
Development
134
1823
1831
42. ZamudioNM
ChongSY
O'BryanMK
2008
Epigenetic regulation in male germ cells.
Reproduction
136
131
146
43. GeraldesA
BassetP
GibsonB
SmithKL
HarrB
2008
Inferring the history of speciation in house mice from autosomal, X-linked, Y-linked and mitochondrial genes.
Mol Ecol
17
5349
5363
44. GoodJM
HandelMA
NachmanMW
2008
Asymmetry and polymorphism of hybrid male sterility during the early stages of speciation in house mice.
Evolution
62
50
65
45. Britton-DavidianJ
Fel-ClairF
LopezJ
AlibertP
BoursotP
2005
Postzygotic isolation between two European subspecies of the house mouse: estimates from fertility patterns in wild and laboratory-bred hybrids.
Biol J Linn Soc
84
379
393
46. StorchováR
GregorováS
BuckiováD
KyselováV
DivinaP
2004
Genetic analysis of X-linked hybrid sterility in the house mouse.
Mamm Genome
15
515
524
47. GoodJM
DeanMD
NachmanMW
2008
A complex genetic basis to X-linked hybrid male sterility between two species of house mice.
Genetics
179
2213
2228
48. ForejtJ
IványiP
1975
Genetic studies on male sterility of hybrids between laboratory and wild mice (Mus musculus L.).
Genet Res
24
189
206
49. VyskocilováM
PrazanovaG
PialekJ
2009
Polymorphism in hybrid male sterility in wild-derived Mus musculus musculus strains on proximal chromosome 17.
Mamm Genome
20
83
91
50. MiholaO
TrachtulecZ
VlcekC
SchimentiJC
ForejtJ
2009
A mouse speciation gene encodes a meiotic histone H3 methyltransferase.
Science
323
373
375
51. HayashiK
YoshidaK
MatsuiY
2005
A histone H3 methyltransferase controls epigenetic events required for meiotic prophase.
Nature
438
374
378
52. VoolstraC
TautzD
FarbrotherP
EichingerL
HarrB
2007
Contrasting evolution of expression differences in the testis between species and subspecies of the house mouse.
Genome Res
17
42
49
53. ChalmelF
RollandAD
Niederhauser-WiederkehrC
ChungSSW
DemouginP
2007
The conserved transcriptome in human and rodent male gametogenesis.
Proc Natl Acad Sci U S A
104
8346
8351
54. YangH
BellTA
ChurchillGA
Pardo-Manuel de VillenaF
2007
On the subspecific origin of the laboratory mouse.
Nat Genet
39
1100
1107
55. BainesJF
HarrB
2007
Reduced X-linked diversity in derived populations of house mice.
Genetics
175
1911
1921
56. BegunDJ
HollowayAJ
StevensK
HillierLW
PohY-P
2007
Population genomics: whole-genome analysis of polymorphism and divergence in Drosophila simulans.
PLoS Biol
5
e310
doi:10.1371/journal.pbio.0050310
57. BainesJF
SawyerSA
HartlD
ParschJ
2008
Effects of X-linkage and sex-biased gene expression on the rate of adaptive protein evolution in Drosophila.
Mol Biol Evol
25
1639
1650
58. BustamanteCD
Fledel-AlonA
WilliamsonS
NielsenR
HubiszMT
2005
Natural selection on protein-coding genes in the human genome.
Nature
437
1153
1157
59. DeanMD
GoodJM
NachmanMW
2008
Adaptive evolution of proteins secreted during sperm maturation: An analysis of the mouse epididymal transcriptome.
Mol Biol Evol
25
383
392
60. JancaFC
JostLK
EvensonDP
1986
Mouse testicular and sperm cell development characterized from birth to adulthood by dual parameter flow cytometry.
Biol Reprod
34
613
623
61. OkanoM
BellDW
HaberDA
LiE
1999
DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development.
Cell
99
247
257
62. TakashimaS
TakehashiM
LeeJ
ChumaS
OkanoM
2009
Abnormal DNA methyltransferase expression in mouse germline stem cells results in spermatogenic defects.
Biol Reprod
81
155
164
63. DoJT
HanDW
GentileL
Sobek-KlockeI
StehlingM
2008
Enhanced reprogramming of Xist by induced upregulation of Tsix and Dnmt3a.
Stem Cells
26
2821
2831
64. PennyGD
KayGF
SheardownSA
RastanS
BrockdorffN
1996
Requirement for Xist in X chromosome inactivation.
Nature
379
131
137
65. McCarreyJR
DilworthDD
1992
Expression of Xist in mouse germ cells correlates with X-chromosome inactivation.
Nat Genet
2
200
203
66. TurnerJMA
MahadevaiahSK
EillottDJ
GarchonHJ
PehrsonJR
2002
Meiotic sex chromosome inactivation in male mice with targeted disruptions of Xist.
J Cell Sci
115
4097
4105
67. PhilippsDL
WigglesworthK
HartfordSA
SunFY
PattabiramanS
2008
The dual bromodomain and WD repeat-containing mouse protein BRWD1 is required for normal spermiogenesis and the oocyte-embryo transition.
Dev Biol
317
72
82
68. ForejtJ
1981
Hybrid sterility gene located in T/t-H-2 supergene on chromosome 17.
ReisfeldR
FerroneS
Current trends in histocompatibility Immunogenetic and molecular profiles
London
Plenum Press
103
131
69. OkaA
MitaA
Sakurai-YamataniN
YamamotoA
TakagiN
2004
Hybrid breakdown caused by substitution of the X chromosome between two mouse subspecies.
Genetics
166
913
924
70. TuckerPK
SageRD
WarnerJ
WilsonAC
EicherEM
1992
Abrupt cline for sex chromosomes in a hybrid zone between two species of mice.
Evolution
46
1146
1163
71. DodB
JermiinLS
BoursotP
ChapmanVH
NielsenJT
1993
Counterselection on sex chromosomes in the Mus musculus European hybrid zone.
J Evol Biol
6
529
546
72. PayseurBA
KrenzJG
NachmanMW
2004
Differential patterns of introgression across the X chromosome in a hybrid zone between two species of house mice.
Evolution
58
2064
2078
73. MacholánM
MunclingerP
SugerkováM
DufkováP
BímováB
2007
Genetic analysis of autosomal and X-linked markers across a mouse hybrid zone.
Evolution
61
746
771
74. ForejtJ
GregorovaS
1977
Meiotic studies of translocations causing male sterility in mouse. I. Autosomal reciprocal translocations.
Cytogenet Cell Genet
19
159
179
75. ForejtJ
1985
Chromosomal and genic sterility of hybrid type in mice and men.
Exp Clin Immunogenetics
2
106
119
76. SandbergR
LarssonO
2007
Improved precision and accuracy for microarrays using updated probe set definitions.
BMC Bioinformatics
8
48
77. DaiM
WangP
BoydAD
KostovG
AtheyB
2005
Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data.
Nucleic Acids Res
33
e175
78. R Development Core Team
2009
R: A Language and Environment for Statistical Computing.
Vienna, Austria
R Foundation for Statistical Computing
79. GentlemanRC
CareyVJ
BatesDM
BolstadB
DettlingM
2004
Bioconductor: open software development for computational biology and bioinformatics.
Genome Biol
5
R80
80. StrimmerK
2008
fdrtool: a versatile R package for estimating local and tail area-based false discovery rates.
Bioinformatics
24
1461
1462
81. TusherVG
TibshiraniR
ChuG
2001
Significance analysis of microarrays applied to the ionizing radiation response.
Proc Natl Acad Sci U S A
98
5116
5121
82. SubramanianA
TamayoP
MoothaVK
MukherjeeS
EbertBL
2005
Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles.
Proc Natl Acad Sci U S A
102
15545
15550
83. EfronB
2007
Size, power, and false discovery rates.
Ann Stat
35
1351
1377
84. GattikerA
Niederhauser-WiederkehrC
MooreJ
HermidaL
PrimigM
2007
The GermOnline cross-species systems browser provides comprehensive information on genes and gene products relevant for sexual reproduction.
Nucleic Acids Res
35
D457
462
85. DeanMD
ClarkNL
FindlayGD
KarnRC
YiX
2009
Proteomics and comparative genomic investigations reveal heterogeneity in evolutionary rate of male reproductive proteins in mice (Mus domesticus).
Mol Biol Evol
26
1733
1743
Štítky
Genetika Reprodukční medicína
Článek Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator ofČlánek Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline AnalysisČlánek Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoAČlánek Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.Článek Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 9
-
Všechny články tohoto čísla
- Optimal Strategy for Competence Differentiation in Bacteria
- Mutational Patterns Cannot Explain Genome Composition: Are There Any Neutral Sites in the Genomes of Bacteria?
- Frail Hypotheses in Evolutionary Biology
- Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits
- Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator of
- Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline Analysis
- Germline Variation Controls the Architecture of Somatic Alterations in Tumors
- Mice Doubly-Deficient in Lysosomal Hexosaminidase A and Neuraminidase 4 Show Epileptic Crises and Rapid Neuronal Loss
- Analysis of Population Structure: A Unifying Framework and Novel Methods Based on Sparse Factor Analysis
- FliO Regulation of FliP in the Formation of the Flagellum
- Cdc20 Is Critical for Meiosis I and Fertility of Female Mice
- dMyc Functions Downstream of Yorkie to Promote the Supercompetitive Behavior of Hippo Pathway Mutant Cells
- DCAF26, an Adaptor Protein of Cul4-Based E3, Is Essential for DNA Methylation in
- Genome-Wide Double-Stranded RNA Sequencing Reveals the Functional Significance of Base-Paired RNAs in
- An Immune Response Network Associated with Blood Lipid Levels
- Genetic Variants and Their Interactions in the Prediction of Increased Pre-Clinical Carotid Atherosclerosis: The Cardiovascular Risk in Young Finns Study
- The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoA
- Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
- Orphan CpG Islands Identify Numerous Conserved Promoters in the Mammalian Genome
- Analysis of the Basidiomycete Reveals Conservation of the Core Meiotic Expression Program over Half a Billion Years of Evolution
- ETS-4 Is a Transcriptional Regulator of Life Span in
- The SR Protein B52/SRp55 Is Required for DNA Topoisomerase I Recruitment to Chromatin, mRNA Release and Transcription Shutdown
- The Baker's Yeast Diploid Genome Is Remarkably Stable in Vegetative Growth and Meiosis
- Chromatin Landscape Dictates HSF Binding to Target DNA Elements
- The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice
- Accurately Assessing the Risk of Schizophrenia Conferred by Rare Copy-Number Variation Affecting Genes with Brain Function
- Widespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
- The Characterization of Twenty Sequenced Human Genomes
- The Genome of a Pathogenic : Cooptive Virulence Underpinned by Key Gene Acquisitions
- A Single Element Maintains Repression of the Key Developmental Regulator
- Identification of New Genetic Risk Variants for Type 2 Diabetes
- Effect of Correlated tRNA Abundances on Translation Errors and Evolution of Codon Usage Bias
- Evidence of Selection upon Genomic GC-Content in Bacteria
- Proteomic Changes Resulting from Gene Copy Number Variations in Cancer Cells
- Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.
- Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study
- Response to Mechanical Stress Is Mediated by the TRPA Channel Painless in the Heart
- DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
- Incremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
- Loss of Maternal ATRX Results in Centromere Instability and Aneuploidy in the Mammalian Oocyte and Pre-Implantation Embryo
- Comparative Genomic Hybridization (CGH) Reveals a Neo-X Chromosome and Biased Gene Movement in Stalk-Eyed Flies (Genus )
- Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
- Gene–Environment Interactions at Nucleotide Resolution
- Dementia Revealed: Novel Chromosome 6 Locus for Late-Onset Alzheimer Disease Provides Genetic Evidence for Folate-Pathway Abnormalities
- Critical Functions of Rpa3/Ssb3 in S-Phase DNA Damage Responses in Fission Yeast
- Preferential Re-Replication of Heterochromatin in the Absence of Geminin
- The Potential for Enhancing the Power of Genetic Association Studies in African Americans through the Reuse of Existing Genotype Data
- Evidence That Mutation Is Universally Biased towards AT in Bacteria
- Perturbation Analysis of Heterochromatin-Mediated Gene Silencing and Somatic Inheritance
- Diversity of Eukaryotic DNA Replication Origins Revealed by Genome-Wide Analysis of Chromatin Structure
- Genetic Deletion of the Desmosomal Component Promotes Tumor Microinvasion in a Mouse Model of Pancreatic Neuroendocrine Carcinogenesis
- The Metabolic Enzyme ManA Reveals a Link between Cell Wall Integrity and Chromosome Morphology
- SNPs Associated with Cerebrospinal Fluid Phospho-Tau Levels Influence Rate of Decline in Alzheimer's Disease
- Synthesizing and Salvaging NAD: Lessons Learned from
- A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis
- An Insect Herbivore Microbiome with High Plant Biomass-Degrading Capacity
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Synthesizing and Salvaging NAD: Lessons Learned from
- Optimal Strategy for Competence Differentiation in Bacteria
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



