-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
Preplacodal ectoderm arises near the end of gastrulation as a narrow band of cells surrounding the anterior neural plate. This domain later resolves into discrete cranial placodes that, together with neural crest, produce paired sensory structures of the head. Unlike the better-characterized neural crest, little is known about early regulation of preplacodal development. Classical models of ectodermal patterning posit that preplacodal identity is specified by readout of a discrete level of Bmp signaling along a DV gradient. More recent studies indicate that Bmp-antagonists are critical for promoting preplacodal development. However, it is unclear whether Bmp-antagonists establish the proper level of Bmp signaling within a morphogen gradient or, alternatively, block Bmp altogether. To begin addressing these issues, we treated zebrafish embryos with a pharmacological inhibitor of Bmp, sometimes combined with heat shock-induction of Chordin and dominant-negative Bmp receptor, to fully block Bmp signaling at various developmental stages. We find that preplacodal development occurs in two phases with opposing Bmp requirements. Initially, Bmp is required before gastrulation to co-induce four transcription factors, Tfap2a, Tfap2c, Foxi1, and Gata3, which establish preplacodal competence throughout the nonneural ectoderm. Subsequently, Bmp must be fully blocked in late gastrulation by dorsally expressed Bmp-antagonists, together with dorsally expressed Fgf and Pdgf, to specify preplacodal identity within competent cells abutting the neural plate. Localized ventral misexpression of Fgf8 and Chordin can activate ectopic preplacodal development anywhere within the zone of competence, whereas dorsal misexpression of one or more competence factors can activate ectopic preplacodal development in the neural plate. Conversely, morpholino-knockdown of competence factors specifically ablates preplacodal development. Our work supports a relatively simple two-step model that traces regulation of preplacodal development to late blastula stage, resolves two distinct phases of Bmp dependence, and identifies the main factors required for preplacodal competence and specification.
Published in the journal: . PLoS Genet 6(9): e32767. doi:10.1371/journal.pgen.1001133
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001133Summary
Preplacodal ectoderm arises near the end of gastrulation as a narrow band of cells surrounding the anterior neural plate. This domain later resolves into discrete cranial placodes that, together with neural crest, produce paired sensory structures of the head. Unlike the better-characterized neural crest, little is known about early regulation of preplacodal development. Classical models of ectodermal patterning posit that preplacodal identity is specified by readout of a discrete level of Bmp signaling along a DV gradient. More recent studies indicate that Bmp-antagonists are critical for promoting preplacodal development. However, it is unclear whether Bmp-antagonists establish the proper level of Bmp signaling within a morphogen gradient or, alternatively, block Bmp altogether. To begin addressing these issues, we treated zebrafish embryos with a pharmacological inhibitor of Bmp, sometimes combined with heat shock-induction of Chordin and dominant-negative Bmp receptor, to fully block Bmp signaling at various developmental stages. We find that preplacodal development occurs in two phases with opposing Bmp requirements. Initially, Bmp is required before gastrulation to co-induce four transcription factors, Tfap2a, Tfap2c, Foxi1, and Gata3, which establish preplacodal competence throughout the nonneural ectoderm. Subsequently, Bmp must be fully blocked in late gastrulation by dorsally expressed Bmp-antagonists, together with dorsally expressed Fgf and Pdgf, to specify preplacodal identity within competent cells abutting the neural plate. Localized ventral misexpression of Fgf8 and Chordin can activate ectopic preplacodal development anywhere within the zone of competence, whereas dorsal misexpression of one or more competence factors can activate ectopic preplacodal development in the neural plate. Conversely, morpholino-knockdown of competence factors specifically ablates preplacodal development. Our work supports a relatively simple two-step model that traces regulation of preplacodal development to late blastula stage, resolves two distinct phases of Bmp dependence, and identifies the main factors required for preplacodal competence and specification.
Introduction
Cranial placodes provide major contributions to the paired sensory organs of the head. Examples include the anterior pituitary, the lens of the eye, the olfactory epithelium, the inner ear, and clusters of sensory neurons in the trigeminal and epibranchial ganglia [1]–[4]. Though diverse in fate, all placodes are thought to arise from a zone of pluripotent progenitors termed the preplacodal ectoderm. Preplacodal cells arise from the nonneural ectoderm immediately adjacent to neural crest. Neural crest cells originate in the lateral edges of the neural plate and later migrate to placodal regions to contribute to the corresponding sensory structures [1], [2]. However, while neural crest has been analyzed extensively, little is known about the early requirements for preplacodal development. Various preplacodal markers, including members of the eya, six and dlx gene families, are expressed at high levels along the neural-nonneural interface around the anterior neural plate near the end of gastrulation [1]–[7]. How these genes are regulated is still unclear, but modulation of Bmp signaling appears to be critical. In a classical model (Fig. 1A), ectoderm is patterned during gastrulation by readout of a Bmp morphogen gradient. Such a gradient could coordinate specification of preplacodal ectoderm and neural crest in juxtaposed domains, with preplacodal ectoderm requiring slightly higher levels of Bmp than neural crest [8]–[15].
Fig. 1. Models for the role of Bmp in preplacodal specification. 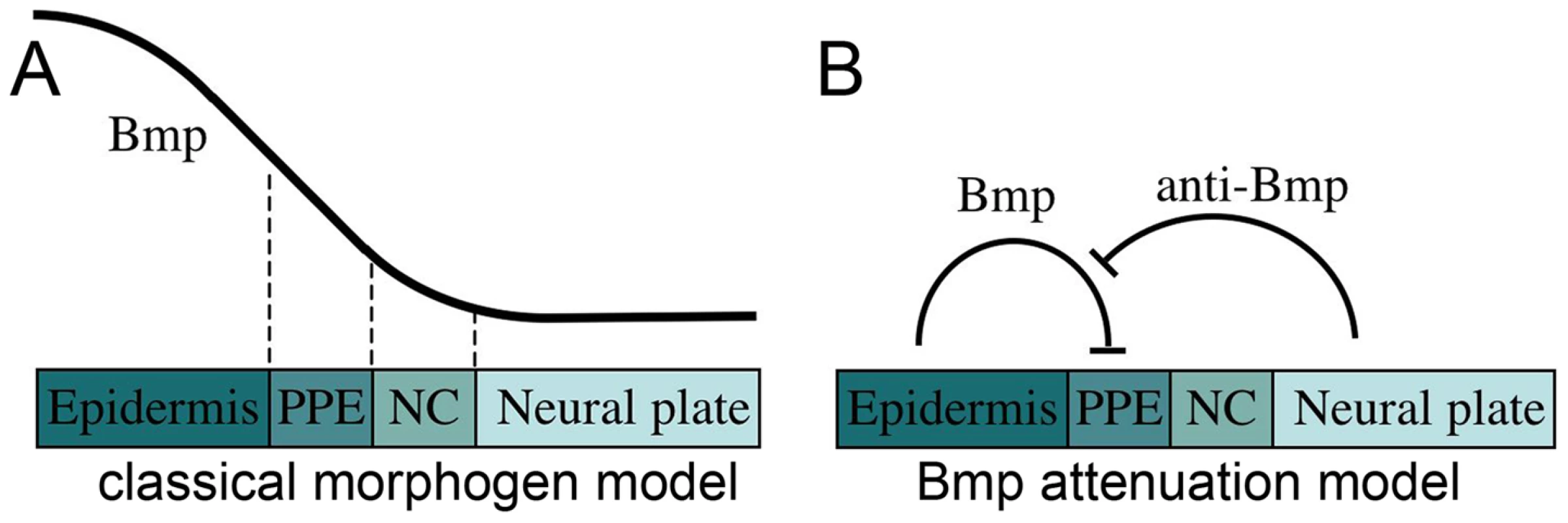
(A) Classical model in which a Bmp morphogen gradient directly specifies multiple fates, including epidermal ectoderm, preplacodal ectoderm (PPE), neural crest (NC) and neural plate, at discrete threshold concentrations. (B) Bmp-attenuation model in which Bmp-antagonists, secreted from the dorsal tissue of the embryo, promotes preplacodal fate in nonneural ectoderm abutting the anterior neural plate. In this model, Bmp must be fully blocked to permit preplacodal specification. Numerous studies provide strong support for the notion that neural crest requires a specific low threshold of Bmp signaling. In zebrafish mutations or inducible transgenes that weaken overall Bmp signaling can expand neural crest throughout the ventral domain [12], [13], [15]. Similarly, development of neural crest in Xenopus is stimulated by misexpression of moderate but not high levels of Bmp-antagonists [11].
In contrast, available data are ambiguous with regard to Bmp's role in preplacodal specification. A number of Bmp-antagonists expressed near the neural-nonneural interface late in gastrulation are required for normal preplacodal development [16], [17]. Similarly, high-level misexpression of Bmp antagonists expands preplacodal gene expression partway into the nonneural ectoderm [18]–[21]. These findings have been alternately interpreted as support for either of two competing models: Some investigators have argued that Bmp-antagonists titrate Bmp signaling to a specific level appropriate for preplacodal specification, consistent with the Bmp morphogen model [18], [19] (Fig. 1A). Others counter that these misexpression conditions are likely to fully block Bmp signaling [20], [21], leading to an alternative model in which preplacodal specification requires attenuation of Bmp (Fig. 1B). These opposing models invoke fundamentally different mechanisms: In the morphogen model Bmp is a positive requirement whereas in the attenuation model Bmp is an inhibitor that must be fully blocked to permit preplacodal development. Notably, none of these studies has measured changes in the level of Bmp signaling associated with their experimental manipulations, making it impossible to distinguish between the opposing models. A similar uncertainty applies to genetic studies in zebrafish, which suggest that neither of the models in Fig. 1 is fully adequate. Mutations that strongly impair Bmp signaling eliminate preplacodal development [12], [13], revealing a definite requirement for Bmp. However, none of the mutations that impair Bmp to a lesser degree expand preplacodal fate throughout the ventral ectoderm, in sharp contrast to neural crest [12], [13]. Although these data fail to support predictions of the Bmp morphogen model for preplacodal specification, it is possible that available mutations do not expand the appropriate range of Bmp signaling required for preplacodal ectoderm, if one exists. Thus the status of Bmp signaling during preplacodal specification remains an important unresolved question.
In addition to differing requirements for Bmp, preplacodal ectoderm and neural crest appear to be specified at different times. Recent studies in chick and zebrafish suggest that neural crest is specified by the beginning of gastrulation [15], [22]. In contrast, preplacodal ectoderm appears to be specified during late gastrula or early neurula stages, as suggested by studies in chick and Xenopus [20], [21]. This difference in timing is especially relevant for the Bmp-attenuation model (Fig. 1B). Specifically, the lag in preplacodal specification allows time to reshape the Bmp gradient without jeopardizing the earlier requirement of neural crest for Bmp. There are currently no data to show when preplacodal specification occurs in zebrafish.
Other signals from dorsal tissues also appear critical for preplacodal development. In chick and Xenopus, grafting neurectoderm into more ventral regions induces expression of preplacodal markers in surrounding host tissue [20], [21], [23]. Moreover, combining misexpression of Bmp antagonists with Fgf8, a relevant dorsal signal, is sufficient to induce at least some preplacodal markers; neither Fgf8 nor Bmp-antagonism is sufficient [20], [21]. Various transcription factors have also been implicated in preplacodal development, but most appear to act after preplacodal specification to influence fates of cells in different regions of this domain [2], [3].
Here we provide the first direct evidence for a 2-step model in which Bmp is required only transiently during blastula/early gastrula stage to directly or indirectly induce ventral expression of four transcription factors, Tfap2a, Tfap2c, Gata3 and Foxi1, which establish preplacodal competence throughout the nonneural ectoderm. In this context, Bmp does not act as a morphogen because it does not distinguish between preplacodal and epidermal ectoderm within the nonneural domain. We initially focused on foxi1, gata3, tfap2a and tfap2c as potential competence factors because they show similar early expression patterns throughout the nonneural ectoderm and all have been implicated in later development of various subsets of cranial placodes [2], [3], [24]–[29]. Once expressed, preplacodal competence factors no longer require Bmp for their maintenance. Near the end of gastrulation, Bmp must be fully blocked by dorsally expressed Bmp-antagonists, which combined with Fgf, are necessary and sufficient to induce preplacodal development within the zone of competence.
Results
Requirements for Bmp
To monitor early preplacodal development, we followed expression of dlx3b, eya1 and six4.1. dlx3b is the earliest marker, initially showing a low level of expression throughout the nonneural ectoderm at 8 hpf, with strong upregulation in preplacodal ectoderm and downregulation in ventral ectoderm by 9 hpf (late gastrulation) [5]. Expression of six4.1 and eya1 first appear in preplacodal ectoderm by 10 hpf (the close of gastrulation), and a low level of six4.1 is also seen in scattered mesendodermal cells in the head [6], [7]. For comparison, we also monitored the neural crest marker foxd3, which is expressed specifically in premigratory neural crest by 10 hpf [30], [31].
To assess the role of Bmp in preplacodal specification, we treated embryos at various times with dorsomorphin (DM), a pharmacological inhibitor of Bmp signaling [32]. Although we used DM at higher concentrations than previously reported [32], it did not appear to cause defects beyond the phenotypes associated with Bmp pathway mutants (see below). Thus, unintended non-specific effects of the drug, if present, are apparently mild and do not interfere with the ability to block Bmp signaling.
We initially performed a dose-response to assess the effects of DM when added at 5, 6 or 7 hpf (Table 1). As expected, embryos were increasingly dorsalized after exposure to increasing concentrations of DM, and earlier exposure caused greater dorsalization than later exposure. Exposing embryos to 50 or 100 µM DM beginning at 5 hpf mimicked strong loss of function mutations in the Bmp pathway [8], [12], [13], [33] and resulted in complete dorsalization (Table 1). In confirmation, exposure to 100 µM DM at 5 hpf eliminated phospho-Smad1/5/8 staining within 15 minutes (Fig. S1A), indicating rapid and complete cessation of Bmp signaling. Additionally, mRNA for sizzled, a feedback inhibitor of Bmp [34], decayed rapidly under these conditions, with only weak staining after 30 minutes and none after 1 hour (Fig. S1B).
Tab. 1. Stage- and dose-dependent dorsalization caused by dorsomorphin (DM). 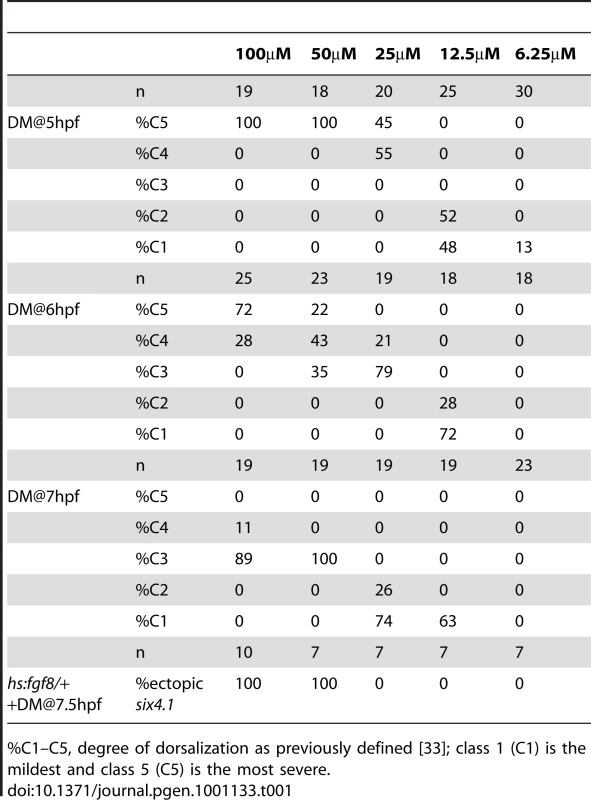
%C1–C5, degree of dorsalization as previously defined [33]; class 1 (C1) is the mildest and class 5 (C5) is the most severe. Because the role of Bmp in neural crest specification has been well characterized [11]–[13], [15], we tested whether DM could affect this tissue as predicted by these previous studies. Adding 100 or 200 µM DM beginning at 4 hpf totally ablated neural crest formation (Fig. 2A and data not shown). However, adding 50 µM DM at 4 hpf led to ventral expansion of cranial neural crest to fully displace the nonneural ectoderm, similar to the effects of mutations that weaken overall Bmp signaling in zebrafish [12], [13]. These conditions are thought to create a broad plateau of low Bmp signaling appropriate for neural crest specification, providing strong support for the role of Bmp as a morphogen in specifying neural crest. Interestingly, after initially treating embryos with 50 µM DM at 4 hpf, fully blocking Bmp with a super-saturating dose of DM at 5, 6, or 7 hpf does not prevent formation of cranial neural crest, though the domain is somewhat reduced when Bmp is blocked earlier. These data are consistent with the effects of timed misexpression of Chordin [15], showing that Bmp acts very early in cranial neural crest specification and is no longer needed after late blastula/early gastrula stage.
Fig. 2. Distinct responses of neural crest and preplacodal ectoderm to graded impairment of Bmp. 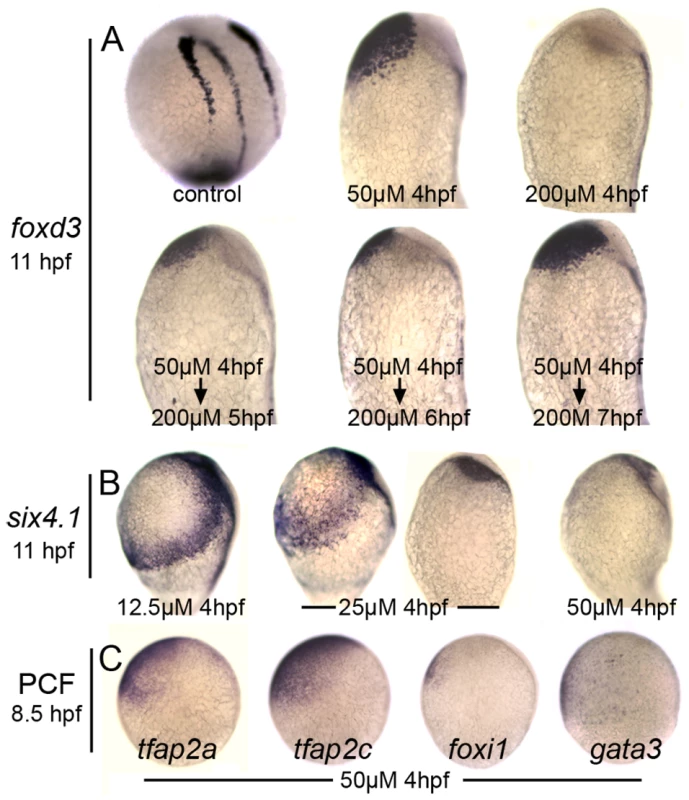
(A) Lateral views of foxd3 expression at 11 hpf with anterior up and dorsal to the right. Embryos were treated with indicated concentrations of DM added at 4 hpf. Where indicated the DM concentration was increased to 200 µM (complete Bmp-inhibition) at 5 hpf, 6 hpf or 7 hpf. (B) Lateral views of six4.1 expression at 11 hpf in embryos treated with indicated concentrations of DM beginning at 4 hpf. Treatment with 25 µM DM yields two discrete responses, one in which six4.1 remains confined to two bilateral stripes flanking the neural plate and the other in which six4.1 expression is lost. (C) Lateral views showing expression of preplacodal competence factors tfap2a, tfap2c, foxi1 and gata3 in embryos were treated with 50 µM DM beginning at 4 hpf. Note that tfap2a/c remain broadly expressed in ventral ectoderm whereas foxi1 and gata3 are nearly eliminated. Analysis of preplacodal markers revealed a different pattern of Bmp-dependence. First, preplacodal ectoderm (Fig. 2B) and epidermal ectoderm (not shown) are totally ablated by exposure to 50 µM DM, reflecting loss of all nonneural ectoderm. Accordingly, this treatment eliminated expression of putative preplacodal competence factors foxi1 and gata3, though tfap2a and tfap2c continue to be expressed (Fig. 2C). The latter two genes are also required in the lateral edges of the neural plate for neural crest development [29], [35]. Second, we found no dose of DM that caused expansion of preplacodal markers throughout the ventral ectoderm. Instead, exposure to 25 µM at 4 hpf yielded two distinct responses; either preplacodal markers were lost entirely or preplacodal ectoderm was shifted ventrally but was still confined to two bilateral stripes bordering the neural plate (Fig. 2B and data not shown). Thus, there does not appear to be a specific level of Bmp that can expand the preplacodal ectoderm at the expense of more ventral (epidermal) ectoderm.
To characterize the temporal requirements for Bmp, embryos were treated with 100 µM DM at different times during late blastula and early gastrula stages and subsequently analyzed for expression patterns of various ectodermal markers. As expected from the severe dorsalization caused by administering this dose at 5 hpf (Table 1), neural markers were expanded throughout the ectoderm and all nonneural markers were lost, including putative preplacodal competence factors (Fig. 3D, E, G–J). Additionally, definitive preplacodal markers dlx3b, eya1 and six4.1 were not expressed in these embryos (Fig. 3A–C). In contrast, exposure to 100 µM DM from 7 hpf resulted in only partial dorsalization (Table 1, Fig. 3D, F) and all embryos expressed nonneural markers, albeit in diminished ventral domains (Fig. 3E, G–J). Preplacodal markers dlx3b, eya1 and six4.1 were expressed on time by 10.5 hpf (Fig. 3A–C). Moreover, all placodal derivatives were produced on time in embryos treated with 100 µM DM from 7 hpf, including the anterior pituitary, olfactory, lens, trigeminal, epibranchial and otic placodes (Fig. 4B, E, H, K, N, Q, T, W) [36]–[46]. Adding 100 µM DM at 6 hpf yielded two classes of embryos, with roughly half being fully dorsalized and the rest resembling the partially dorsalized embryos obtained with 100 µM DM at 7 hpf (Fig. S2, Table 1). Adding 100 µM DM at 5.5 hpf eliminated eya1 and six4.1 expression in all embryos, though some embryos still expressed dlx3b in bilateral stripes (Fig. S2). These data indicate that embryos make a transition around 5.5–6 hpf after which Bmp is no longer required for preplacodal development. As with treatment during blastula stage, treatment with 100 µM DM during gastrulation eliminated phospho-Smad1/5/8 accumulation and sizzled expression, confirming loss of Bmp signaling [15], [34] (Fig. 3K, L). Additionally, the effects of adding 100 µM DM at 7 hpf were identical to the effects of 500 µM DM, the highest dose tested (data not shown), arguing that the block to Bmp signaling was saturated at these doses. Nevertheless, to ensure that Bmp was fully blocked, we combined addition of 100 µM DM at 7 hpf with activation of heat shock-inducible transgenes encoding Chordin and/or dominant-negative Bmp receptor [15], [45] (Fig. 3M, N). The effects on preplacodal specification and morphological development were identical to treatment with 100 µM DM alone. These data show that Bmp is not directly required after the onset of gastrulation for preplacodal specification. The data further show that Bmp signaling is required to induce expression of putative competence factors foxi1, gata3, tfap2a and tfap2c during blastula stage, but is not required to maintain them thereafter (Fig. 3G–J).
Fig. 3. Stage-dependent requirements for Bmp. 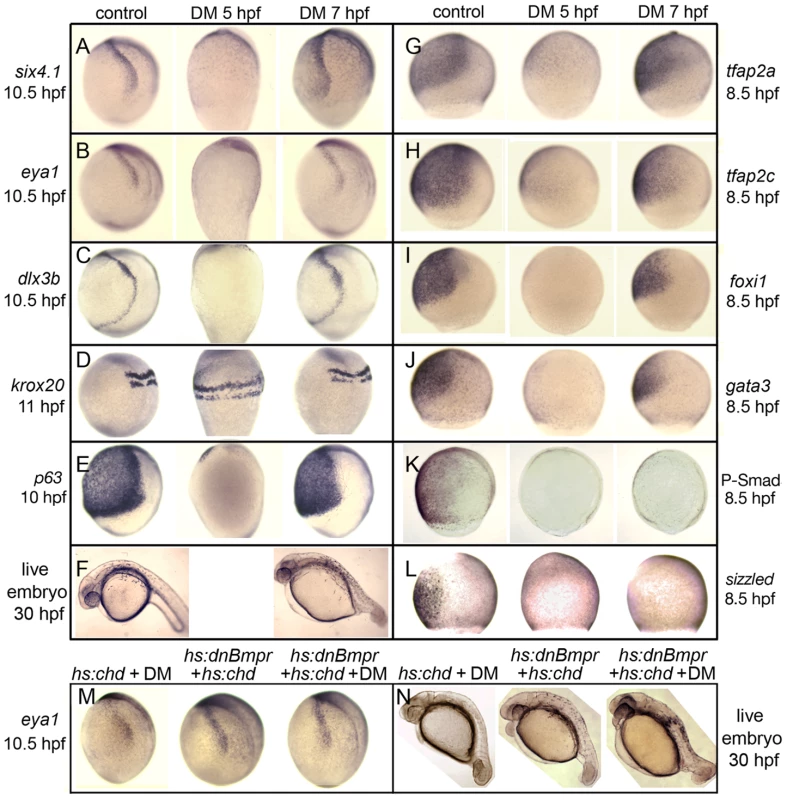
(A–E, G–L) Analysis of indicated gene expression patterns in control embryos and embryos treated with 100 µM dorsomorphin (DM) at 5 hpf or 7 hpf. Lateral views with dorsal to the right and anterior up. Expression of six4.1, eya1 and dlx3b (A–C) in PPE, krox20 in hindbrain(D) and p63 in epidermal ectoderm (E). Expression of competence factor genes tfap2a, tfap2c, foxi1 and gata3 (G–J). Reporters of Bmp-signaling, Phospho-Smad1/5/8 antibody staining (K) and sizzled in situ hybridization (L). Note the complete loss of Bmp signaling by 100 µM DM-treatment either at 5 hpf or 7 hpf. (F) Lateral views of live embryos at 30 hpf. Embryos treated with DM at 7 hpf show a partially dorsalized C3 phenotype [33]. (M, N) Tg(hs:chd) and/or Tg(hs:dnBmpr) embryos heat-shocked and treated with 100 µM DM at 7.5 hpf. eya1 expression (M) and C3 phenotypes (N) are comparable to embryos treated with 100 µM DM alone. Fig. 4. Formation of cranial placodes requires competence factors but not Bmp during gastrulation. 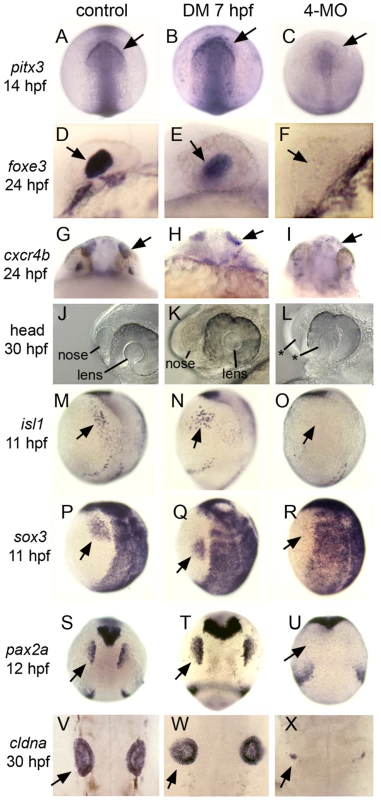
Analysis of various cranial placode markers in control embryos, embryos treated with 100 µM DM at 7 hpf, or foxi1/gata3/tfap2a/c quadruple morphants (4-MO). Arrows indicate relevant expression domains in placodal tissues. (A–C) Dorsal views (anterior up) of pitx3 expression in anterior pituitary and lens placode. (D–F) Lateral views (anterior to left) of foxe3 expression in the lens placode. (G–I) Frontal views of cxcr4b expression in olfactory placode. (J–L) Lateral views (anterior to left) showing the lens and nasal pits in live specimens at 30 hpf. Asterisks in (L) depict the absence of morphologically discernable structures. (M–O) Lateral views (anterior up) of isl1 expression in the trigeminal placode. (P–R) Lateral views (anterior up) of sox3 expression in the epibranchial placode. (S–U) Dorsal views (anterior up) of pax2a expression in the otic placode. (V–X) Dorsal views (anterior up) of cldna expression in the otic vesicle. All placodal markers are expressed normally in DM-treated embryos. Expression of cldna is severely deficient in quadruple morphants (X, n = 13/21) or ablated altogether (8/21, not shown). All other placodal markers are ablated in quadruple morphants (n≥10 for each marker). Requirement for ventrally expressed competence factors
We hypothesized that foxi1, gata3, tfap2a and tfap2c encode preplacodal competence factors because they are expressed early throughout the nonneural ectoderm yet are specifically required for later development of various subsets of placodes [24]–[29]. To test the functions of these genes, we injected morpholino oligomers (MOs) to knockdown their functions. Knockdown of any one gene had no discernable effect on preplacodal gene expression (data not shown), though loss of foxi1 specifically impairs development of the otic and epibranchial placodes [27], [28]. Knockdown of both foxi1 and gata3 enhanced the otic placode deficiency (data not shown), and caused a slight reduction in expression levels of dlx3b, eya1 and six4.1 (Fig. 5A). Knockdown of both tfap2a and tfap2c caused a stronger reduction in expression levels of preplacodal markers (Fig. 5B). Co-injecting either gata3-MO or foxi1-MO with tfap2a/c-MOs further reduced preplacodal gene expression (data not shown) whereas simultaneous knockdown of foxi1, gata3, tfap2a and tfap2c (quadruple morphants) resulted in complete loss of preplacodal gene expression (Fig. 5C). Moreover, development of all cranial placodes (pituitary, olfactory, lens, trigeminal, otic and epibranchial) was severely deficient or totally ablated in all quadruple morphants examined (Fig. 4C, F, I, L, O, R, U, X). Disruption of preplacodal development in quadruple morphants did not reflect general impairment of nonneural ectoderm, as the epidermal marker p63 [46], [47] was appropriately expressed in the ventral ectoderm (Fig. 5D). Additionally, quadruple morphants did not exhibit elevated cell death, as indicated by relatively normal levels of staining with the vital dye acridine orange [48] (data not shown). These data show that foxi1, gata3, tfap2a and tfap2c are specifically required for formation of preplacodal ectoderm and all placodal derivatives, and are partially redundant in this function.
Fig. 5. Knockdown of competence factors impairs preplacodal specification. 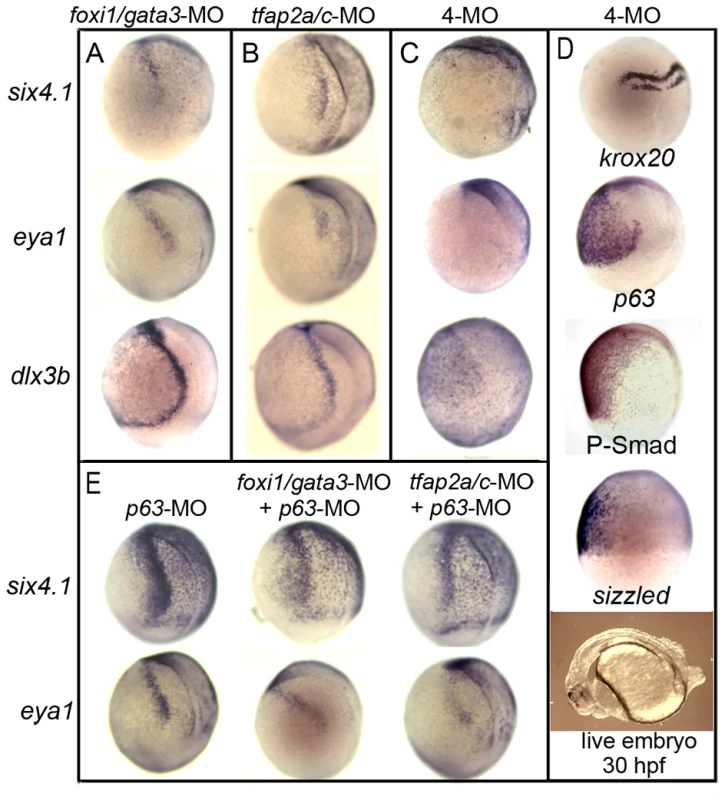
(A–C) Expression of preplacodal markers at 10.5 hpf in (A) foxi1/gata3 double morphants, (B) tfap2a/c double morphants, (C) foxi1/gata3/tfap2a/c quadruple morphants (4-MO). Note the complete loss of preplacodal markers in C. (D) Expression of krox20, p63, P-smad and sizzled during gastrulation in foxi1/gata3/tfap2a/c quadruple morphants. Morphology of a live quadruple morphant at 30 hpf is also shown. (E) Expression of six4.1 and eya1 in p63 morphants alone or in combination with tfap2a/c-MO or foxi1/gata3-MO. All images show lateral views with dorsal to the right and anterior up, except for the live specimen in (D), which shows a lateral view with anterior to the left. Importantly, quadruple morphants retained a neural-nonneural interface (Fig. 4R and Fig. 5D), the region normally associated with preplacodal specification. Moreover, Bmp signaling also persisted in quadruple morphants as shown by continued ventral accumulation of phospho-Smad1/5/8 and expression of sizzled (Fig. 5D). Expression of fgf3, fgf8 and the Fgf-target gene erm were also appropriately localized in quadruple morphants (data not shown). Thus, neither Bmp signaling, Fgf signaling, nor neural-nonneural interactions are sufficient for preplacodal specification in this background. These data support the hypothesis that foxi1, gata3, tfap2a and tfap2c are required for preplacodal competence or early differentiation.
Although p63 is normally co-expressed with preplacodal competence factors and is only known to regulate epidermal development [46], [47], we examined whether it is required for preplacodal development. Knockdown of p63 did not detectably alter preplacodal development, nor did it enhance the deficits in preplacodal gene expression or morphological development seen in foxi1-gata3 or tfap2a/c double morphants (Fig. 5E, and data not shown). This further shows that not all early Bmp-target genes are required for preplacodal development and that the requirement for foxi1, gata3, tfap2a and tfap2c is relatively specific.
We also investigated the requirements for foxi1, gata3, tfap2a and tfap2c in neural crest formation. Knockdown of both foxi1 and gata3 did not alter expression of foxd3 (data not shown), whereas knockdown of tfap2a/c completely eliminated expression of foxd3 as reported previously [29], [35]. Not surprisingly, foxd3 expression is also ablated in foxi1-gata3-tfap2a/c-quadruple morphants (data not shown). This likely reflects a cell-autonomous requirement for tfap2a/c in neural crest specification [29], [35].
Dorsal misexpression of preplacodal competence factors
To further test the functions of preplacodal competence factors, we generated constructs to misexpress foxi1, gata3 and tfap2a under the control of the hsp70 heat shock promoter [49]. We reasoned that if these genes provide preplacodal competence, then misexpressing them in dorsal ectoderm, where preplacodal inducing factors are normally expressed, should be sufficient to induce ectopic expression of preplacodal genes. We performed transient transfections to introduce hs:tfap2a and hs:gata3 whereas a stable transgenic line was used for hs:foxi1 (see Materials & Methods). Global heat shock-activation of any one of these genes at 4.5 hpf (late blastula) or 5.5 hpf (early gastrula) resulted in scattered ectopic expression of preplacodal markers within the neural plate by 11 hpf (Fig. 6A–C, and data not shown). In most experiments, over half of embryos showed ectopic expression of preplacodal genes. Co-activation of any two heat shock genes yielded more robust and widespread expression of preplacodal genes in the neural plate, with nearly complete penetrance in most experiments. For reasons that are unclear, misexpression of competence factors at these stages caused widening of the neural plate and narrowing of the ventral Bmp signaling domain (Fig. S3). Nevertheless, Bmp signaling and general DV patterning are still evident following activation of hs:foxi1, hs:gata3 and/or hs:tfap2a (Fig. S3). Importantly, we never observed ectopic expression of the epidermal marker p63 in the neural plate following misexpression of competence factors, indicating that preplacodal competence factors do not induce all nonneural fates in this domain. Co-activation of all three transgenes at 4.5 hpf led to widespread expression of preplacodal genes, but also caused severe axial patterning defects during gastrulation, making results difficult to interpret (data not shown). However, mosaic misexpression of all three competence factors at 4.5 hpf avoided defects in axial patterning yet still led to dorsal expression of dlx3b and six4.1 in a subset of misexpressing cells (Fig. 6D). These data are consistent with the hypothesis that foxi1, gata3 and tfap2a are sufficient to render dorsal ectoderm competent to express preplacodal genes in response to dorsally expressed inducing factors.
Fig. 6. Misexpression of competence factors induces ectopic expression of preplacodal markers. 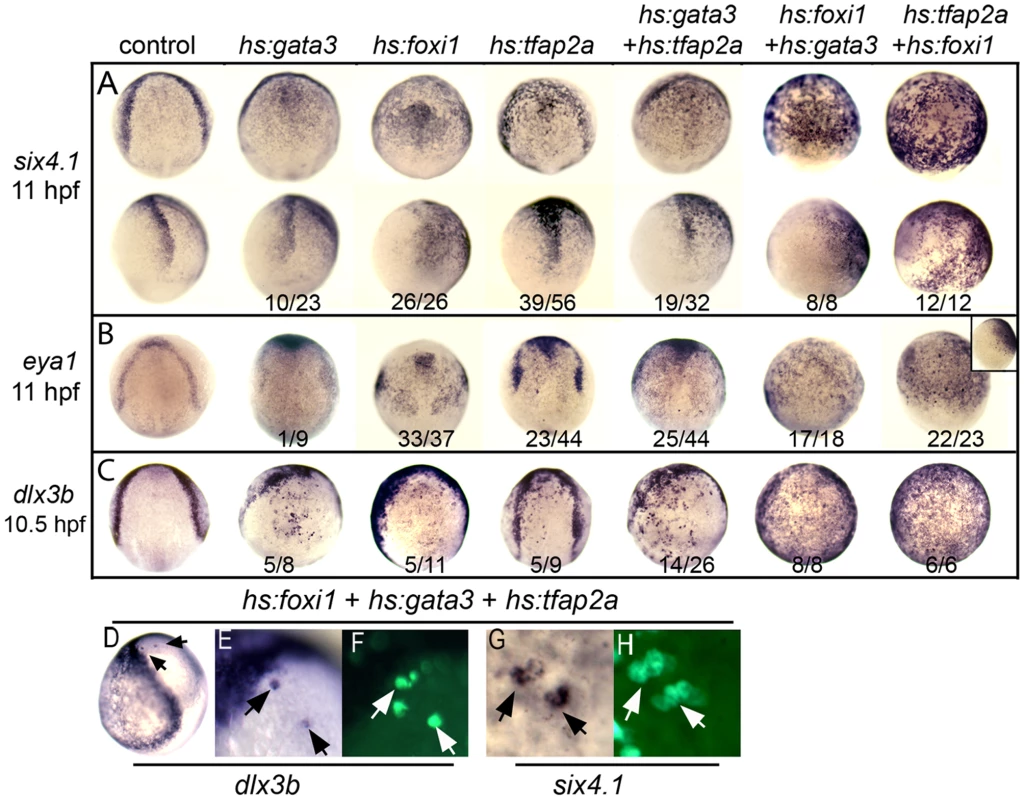
(A–C) Analysis of indicated gene expression patterns in control embryos and embryos carrying hs:gata3, hs:foxi1 and/or hs:tfap2a heat-shocked at 30% epiboly (4.5 hpf). Dorsal views with anterior up except bottom row in A, inset in B, which are lateral views with dorsal to the right. Note the ectopic expression of PPE markers, six4.1 (A), eya1 (B) and dlx3b (C) in neuroectoderm of embryos misexpressing one or more competence factors. (D–H) Dorsolateral views (anterior up) of mosaic embryos showing ectopic expression of dlx3b and six4.1 at 10.5 hpf. Donor cells obtained from Tg(hs:foxi1) injected with hs:gata3 and hs:tfap2a plasmid were transplanted into wild type hosts and heat shocked at 4.5 hpf at 39°C. Transplanted cells were identified with Strepavidin-FITC (arrows F, H). Mosaic embryos shows cell autonomous expression of dlx3b and six4.1 in the neural plate (compare E, F and G, H). In addition to their role in preplacodal development, Tfap2a and Tfap2c are required for neural crest [29], [35], whereas Foxi1 and Gata3 are required for preplacodal ectoderm but not neural crest. We asked whether these differing roles in neural crest could also be distinguished in misexpression experiments. Similar to the effects of injecting tfap2a mRNA [29], we found that misexpression of hs:tfap2a, either alone or in combination with other competence factors, resulted in ectopic foxd3 expression in the neural plate (Fig. S4). In contrast, activation of hs:foxi1 and/or hs:gata3 did not induce ectopic foxd3 expression (data not shown), but instead reduced expression of foxd3 in the endogenous neural crest domain (Fig. S4). Importantly, these findings show that formation of ectopic preplacodal tissue is not always associated with neural crest, further arguing that preplacodal competence can be regulated independently from other ectodermal fates.
Ventral misexpression of preplacodal-inducing factors
We next attempted to induce preplacodal development throughout the zone of competence in the nonneural ectoderm by providing appropriate inductive signals normally limited to dorsal tissue. Previous studies have implicated dorsally expressed Bmp-antagonists and Fgfs as preplacodal inducers [16]–[21]. To mimic such signals throughout the nonneural ectoderm, we used heat shock-inducible transgenic lines to misexpress Fgf3 or Fgf8 (hs:fgf3 and hs:fgf8) while blocking Bmp with DM. Using standard heat shock conditions (39°C for 30 minutes) to activate hs:fgf8 combined with DM treatment at 7.5 hpf fully dorsalized the embryo and was not informative. However, full dorsalization was avoided by prolonged incubation at more moderate temperatures, achieving a weaker level of transgene activation. Incubating hs:fgf8/+ transgenic embryos at 35°C with 100 µM DM from 7.5–10.5 hpf resulted in expression of eya1 and six4.1 throughout the nonneural ectoderm in all embryos (Fig. 7B, F). Diffuse ectopic expression of erm confirmed that this heat shock regimen elevated Fgf signaling within nonneural ectoderm (Fig. 7I–K). Similar results were obtained with hs:fgf3/+ transgenic embryos incubated at 36°C with 100 µM DM from 7–10.5 hpf (Fig. 7D, H). Activation of hs:fgf3 or hs:fgf8 alone was not sufficient to activate ectopic preplacodal gene expression (Fig. 7A, C, E, G). These data show that the entire nonneural ectoderm is competent to express preplacodal genes in response to Fgf plus inhibition of Bmp.
Fig. 7. The entire nonneural ectoderm is competent to form preplacodal tissue. 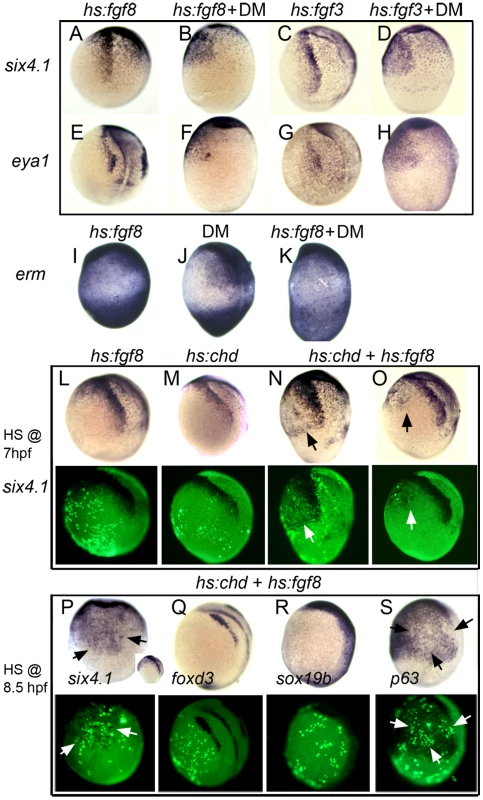
(A–H) Expression of preplacodal markers in (A, B, E, F) Tg(hs:fgf8) embryos incubated at 35°C from 7.5–10.5 hpf, or (C, D, G, H) Tg(hs:fgf3) embryos incubated at 36°C from 7–10.5 hpf. 100 µM DM was added as indicated. (I–K) Expression of erm in (I) Tg(hs:fgf8) embryo incubated at 35°C without DM, (J) a non-transgenic embryos incubated at 35°C with 100 µM DM, and (K) a Tg(hs:fgf8) embryo incubated at 35°C with 100 µM DM. (L–S) Mosaic misexpression of Fgf8 and/or Chordin. (L–O) Brightfield images (top row) and fluorescent images (bottom row) of host embryos with cells transplanted from Tg(hs:fgf8) (L), Tg(hs:chd) (M) or Tg(hs:fgf8); Tg(hs:chd) donor embryos (N, O). Donor embryos were injected with lineage tracer (biotin-dextran) and transplanted at mid-blastula (L, M, N) or early gastrula stage (O) into unlabeled host embryos. Embryos were heat-shocked at 39°C for 30 minutes at 7 hpf and examined for six4.1 expression at 10.5 hpf. Transplanted transgenic cells were identified by Strepavidin-FITC staining after in situ hybridization. All panels show lateral views of host embryos with anterior up. Mosaic embryos with Tg(hs:fgf8);Tg(hs:chd) double transgenic cells showed ectopic six4.1 expression in surrounding ventral ectoderm (N, O), whereas no ectopic six4.1 expression was detected following activation of hs:fgf8 alone (L) or hs:chd alone (M). (P–S) Brightfield images (top row) and fluorescent images (bottom row) of host embryos with cells transplanted during early gastrula stage from double heterozygous Tg(hs:fgf8); Tg(hs:chd) embryos. Embryos were heat shocked for 30 minutes at 39°C beginning at 8.5 hpf and examined for expression of six4.1 (preplacodal ectoderm), foxd3 (neural crest), sox19b (neural plate) or p63(epidermal ectoderm) at 10.5 hpf. All panels show lateral views except (P) which shows a ventral view (lateral view in inset) and (S) which shows ventro-lateral view. Heat shock activation at 8.5 hpf (P) leads to stronger ectopic expression of six4.1 than heat shock at 7 hpf (O). No ectopic expression of foxd3 or sox19b is detected (Q, R) whereas p63 expression appears downregulated in and around transgenic cells (arrows in S). We next titrated the dose of DM required for ectopic induction of preplacodal genes. Incubating hs:fgf8/+ embryos at 35°C with 50 µM DM at 7 hpf led to ventral expression of preplacodal genes, but lower concentrations of DM were not sufficient (Table 1). The finding that 25 µM DM is not sufficient indicates that even very low levels of Bmp signaling can block preplacodal gene activation.
To express inductive signals with greater spatial control, we generated mosaic embryos to locally co-misexpress Fgf8 and Chordin. Donor cells carrying both hs:fgf8 and hs:chd transgenes were transplanted into non-transgenic host embryos at the mid-blastula stage to obtain a random distribution of misexpressing cells. To achieve maximal transgene activation, mosaics were heat-shocked at 39°C for 30 minutes beginning at 7 hpf and then maintained at 33°C until tailbud stage (10 hpf). Of 4 mosaic embryos harboring transgenic donor cells on the ventral side, all showed significant ventral expression of six4.1 in surrounding host cells (Fig. 7N). In another experiment, transgenic donor cells were transplanted directly to the ventral side at the early gastrula stage (6 hpf). Following heat shock at 7 hpf, all mosaic embryos (n = 4) showed ectopic six4.1 expression in surrounding host cells (Fig. 7O). In contrast, no ectopic six4.1 expression was seen following mosaic misexpression of hs:fgf8 alone (n = 13) or hs:chd alone (n = 10) (Fig. 7L, 7M). This confirms that both Fgf and Bmp-antagonists are required to induce expression of preplacodal genes.
Because preplacodal specification has been reported to occur near the end of gastrulation in frog and chick embryos [20], [21], we tested whether activation of hs:fgf8; hs:chd cells at later stages could also stimulate ectopic preplacodal gene expression. Heat shock activation of ventrally transplanted transgenic cells at 8.5 hpf (yielding peak transgene expression at 9 hpf) led to robust ectopic expression of six4.1 in surrounding host ectoderm by 11 hpf (Fig. 7P). This suggests that in zebrafish, too, preplacodal specification occurs near the end of gastrulation.
Importantly, activation of hs:fgf8 and hs:chd did not lead to ectopic expression of the general neural plate marker sox19b nor the neural crest marker foxd3 (Fig. 7Q, R). Thus, induction of ectopic six4.1 expression did not result indirectly from ectopic formation of neural plate. On the other hand, activating transgenic cells at 8.5 hpf caused downregulation of p63, suggesting that nearby host cells lose epidermal identity in response to preplacodal specifying signals.
Finally, we reassessed the requirement for Fgf during normal preplacodal specification. Previous studies have reported that expression of preplacodal markers does not require Fgf in zebrafish [50]–[53]. We find that blocking Fgf by adding the pharmacological inhibitor SU5402 at 8.5 hpf did not block expression of preplacodal markers, but levels of expression were reduced (Fig. S5). We speculated that Pdgf, which is also dorsally expressed near the end of gastrulation [54] and activates a similar signal transduction pathway, might provide redundancy with Fgf. We tested this by applying another inhibitor, AG1295, which blocks Pdgf activity in zebrafish [55]. Treatment with AG1295 alone had little effect on preplacodal gene expression, but co-incubation with AG1295 and SU5402 from 8.5 hpf led to further reduction of preplacodal gene expression (Fig. S5). Indeed, expression of eya1 was almost totally eliminated in the preplacodal domain, though robust expression continues in the cranial mesoderm. These data support the hypothesis that Fgf and Pdgf are partially redundant dorsal factors required for preplacodal specification.
Discussion
We have presented data supporting a relatively simple two-step model of preplacodal development (Fig. 8). First, during late blastula/early gastrula stage Bmp establishes a broad zone of preplacodal competence throughout the nonneural ectoderm. Second, near the end of gastrulation signals from dorsal tissue locally specify preplacodal ectoderm bordering the anterior neural plate. Interestingly, Nguyen et al. proposed a broadly similar two-step model based on analysis of Bmp-pathway mutants in zebrafish [12]. However, at that time neither the molecular basis of preplacodal competence nor the signals required for preplacodal specification were known. Additionally, more recent studies have led to disagreement as to whether Bmp is required at a specific low level or must be blocked entirely for preplacodal specification [18]–[21]. Our model resolves the role of Bmp, confirms that Fgf plus Bmp-antagonists are sufficient for preplacodal specification, shows for the first time that Fgf and Pdgf cooperate as redundant preplacodal inducing factors, and highlights the importance of Foxi1, Gata3, Tfap2a and Tfap2c as preplacodal competence factors. We also readdress mechanisms of neural crest specification, which show a number of crucial differences from preplacodal ectoderm.
Fig. 8. A model for sequential phases of preplacodal development. 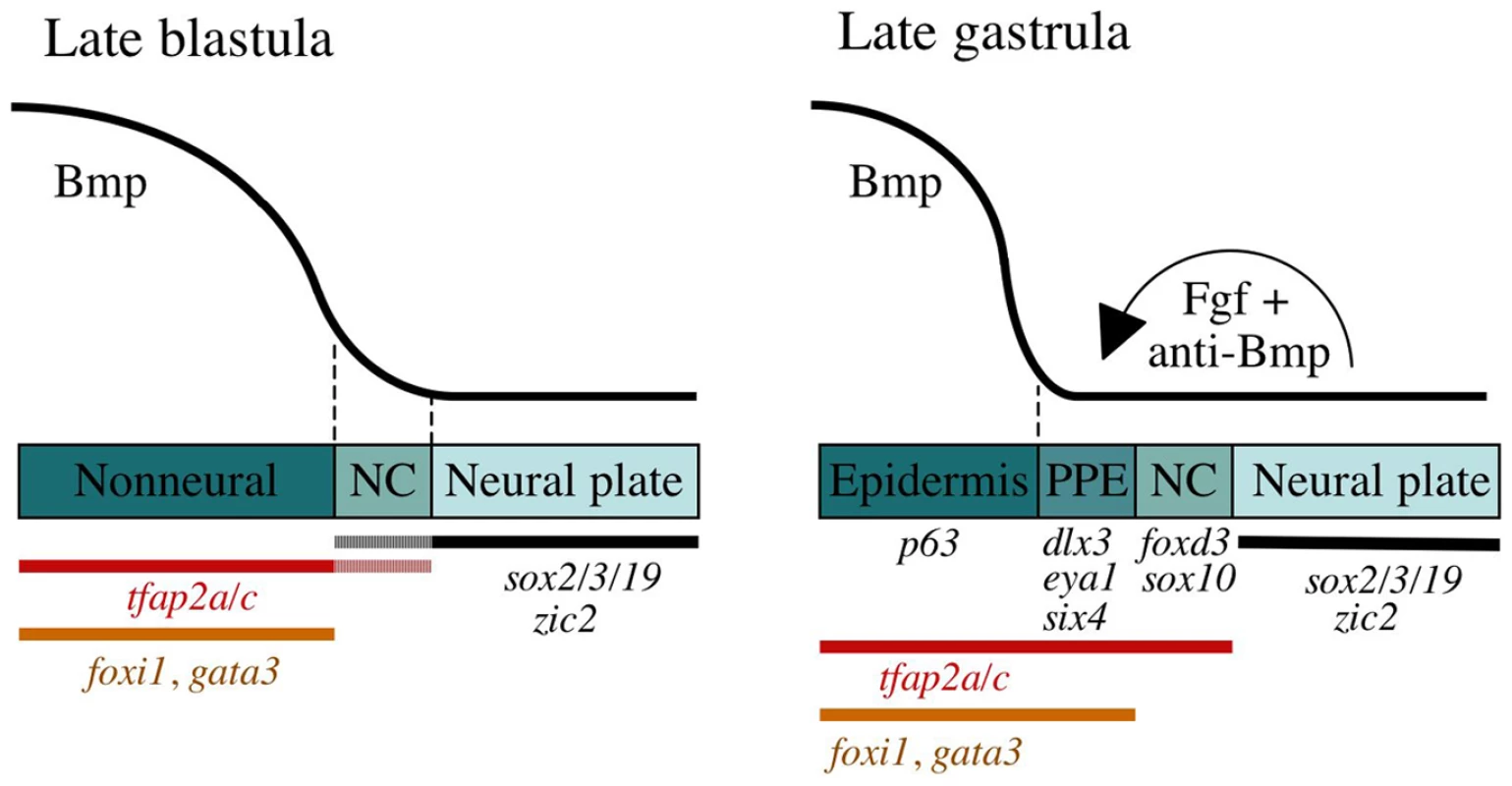
During late blastula stage, Bmp acts as a morphogen that specifies neural crest (NC) within a narrow but low range of signaling, whereas higher levels of Bmp signaling establish the nonneural ectoderm as a broad zone of uncommitted cells with potential to form epidermal or preplacodal ectoderm (PPE). Within the nonneural ectoderm, changing levels of Bmp do not distinguish preplacodal from epidermal potential, and preplacodal competence factors are uniformly induced throughout this domain. However, expression of tfap2a/c overlaps with the lateral edges of the neural plate where, perhaps in combination with neural markers, they cell-autonomously specify NC fate. During late gastrula stage (9–10 hpf), PPE fate is specified in competent cells near the neural-nonneural border by dorsally expressed Bmp antagonists, Fgf and Pdgf. Complete attenuation of Bmp is required for PPE specification. Relevant markers for each ectodermal domain are shown. Distinct roles for Bmp in specification of neural crest and preplacodal ectoderm
Using DM to finely control Bmp signaling, we show that Bmp regulates neural crest and preplacodal ectoderm by markedly different mechanisms. In agreement with earlier genetic studies in zebrafish [12], [13], [15], our data indicate that neural crest is specified by a discrete low level of Bmp signaling as predicted by the classical morphogen model (Fig. 1A). Adding DM at 4 hpf at a dose sufficient to fully block Bmp signaling ablates neural crest formation, whereas a slightly lower dose causes a dramatic ventrolateral expansion of neural crest to fully displace nonneural ectoderm (Fig. 2A). Fully blocking Bmp after the onset of gastrulation does not block neural crest, in agreement with studies involving timed misexpression of Chordin [15]. These data suggest that cranial neural crest is already specified by early gastrula stage, after which it no longer requires Bmp. In chick, too, neural crest is specified by early gastrula stage [22].
Preplacodal ectoderm, marked by expression of dlx3b, eya1 and six4.1, develops in two distinct phases with distinct signaling requirements, neither of which resemble the pattern shown by neural crest. Preplacodal ectoderm requires a robust Bmp signal during late blastula/early gastrula, but unlike neural crest, there does not appear to be a specific range of Bmp signaling that uniquely specifies preplacodal fate. We found no dose of DM that could expand the preplacodal ectoderm in a manner similar to neural crest. Instead, increasing the concentration of DM (lowering Bmp signaling) either shifted discrete bilateral stripes of preplacodal ectoderm to a more ventral position or eliminated them altogether, depending on the degree of neural plate expansion. Indeed, treatment with a single dose (25µM DM beginning at 4 hpf) yielded both classes of embryo, with nothing in between. Thus, DM cannot expand preplacodal ectoderm at the expense of epidermal ectoderm, indicating that changing Bmp levels do not distinguish between these fates.
The requirement for Bmp changes during the second phase of preplacodal development beginning soon after the onset of gastrulation. Adding a full blocking dose of DM at 7 hpf does not block preplacodal specification, even if transgenic Chordin and dominant-negative Bmp receptor are also activated during this period. Thus, Bmp is not required during gastrulation for preplacodal specification. By extension, the requirement of preplacodal ectoderm for locally secreted Bmp-antagonists [16]–[21] cannot reflect a requirement for a specific low threshold of Bmp; instead Bmp-antagonists are presumably needed to fully attenuate Bmp. This conclusion is further supported by our experiments showing that a full blocking dose of DM is required to induce ectopic preplacodal markers throughout the ventral ectoderm (Fig. 7, Table 1, and see below).
Other essential signals
We have found that Fgf combined with Bmp attenuation is sufficient to induce preplacodal markers in ventral ectoderm, as has been shown in chick and frog [20], [21], suggesting that this mechanism is broadly conserved. Thus, using heat shock-inducible transgenes, we show that misexpression of Fgf combined with DM treatment is sufficient to induce ectopic preplacodal markers anywhere within the nonneural ectoderm. This supports two important conclusions. First, it demonstrates that the entire nonneural ectoderm is competent to form preplacodal ectoderm, even at the ventral midline far from the neural plate. This is consistent with the expression domains of preplacodal competence factors (see below). Second, although Fgf and Bmp-antagonists likely constitute a small subset of signals associated with the neural-nonneural border, no other signals are needed to trigger preplacodal development. Fgf and Bmp-attenuation induces ectopic expression of preplacodal markers in chick and Xenopus [20], [21], though this combination of signals also induces expression of general neural plate markers in those species. By contrast, our experimental conditions do not induce formation of ectopic neural plate or neural crest, tissues that could themselves have induced ectopic preplacodal markers [20], [21], [23]. Thus induction of ectopic preplacodal ectoderm appears to be a direct and specific response to Fgf combined with Bmp attenuation, at least in zebrafish.
In addition to being able to induce ectopic preplacodal markers, we have found that Fgf is required in zebrafish for normal preplacodal development, and furthermore that Pdgf acts partially redundantly in this process. Fgf and Pdgf have been shown to regulate distinct aspects of gastrulation, with Fgf promoting dorsal fate specification and Pdgf promoting convergence towards the dorsal midline [55], [56]. Although Fgf is not absolutely required for expression of general preplacodal markers [50]–[53], we find that treating embryos with the Fgf inhibitor SU5402 during the latter half of gastrulation reduces the level of expression of preplacodal markers. Treating embryos with the Pdgf inhibitor AG1295 alone has no effect on preplacodal specification, but blocking both Fgf and Pdgf further reduces preplacodal gene expression, nearly eliminating eya1 expression. Homologs of Fgf and Pdgf are preferentially expressed in dorsal tissues near the end of gastrulation [54], [56], [57] and likely activate the same signal transduction pathways required for preplacodal specification. It is not known whether Pdgf regulates preplacodal development in other species, but Pdgf and Fgf are specifically required for induction of the trigeminal placode in chick [58].
In this study we have not addressed the role of Wnt inhibitors, which are also required for preplacodal development [18], [21]. Numerous Wnt inhibitors are abundantly expressed in the head and are vital for cranial development in general, including preplacodal ectoderm. Otherwise, preplacodal fate is restricted from the trunk and tail by posteriorizing Wnt signals [59], [60].
The role of competence factors
We show that Tfap2a, Tfap2c, Foxi1 and Gata3 act as partially redundant competence factors required specifically for preplacodal development. These genes are expressed uniformly within the nonneural ectoderm beginning in late blastula stage. Knockdown of individual competence factors can impair development of discrete subsets of cranial placodes but formation of preplacodal ectoderm is not detectably altered [24]–[29]. In contrast, knockdown all four competence factors specifically blocks formation of preplacodal ectoderm and all placodal derivatives (Fig. 4, Fig. 5). Importantly, formation of a ventral Bmp gradient and the neural-nonneural interface still occurs. Formation of this region reflects a signaling environment that normally promotes preplacodal development yet, without the four competence factors, cells in the nonneural ectoderm cannot respond to such signals. Conversely, misexpression of one or more competence factors in the neural plate, where preplacodal inducing signals are expressed, leads to ectopic expression of preplacodal markers (Fig. 6). Although global misexpression of competence factors causes various developmental defects, localized mosaic misexpression avoids global perturbation yet still results in cell-autonomous expression of preplacodal markers in the neural plate. Thus, these genes are necessary and sufficient to render cells competent to form preplacodal ectoderm, while additional dorsal signals are required for overt specification of preplacodal fate.
Though tfap2a/c, foxi1 and gata3 are required for preplacodal ectoderm, they are neither necessary nor sufficient for epidermal fate: Expression of the epidermal marker p63 remains appropriately localized following either knockdown or misexpression of preplacodal competence factors (Fig. 5, Fig. 6). Conversely, knockdown of p63 does not detectably impair preplacodal development nor enhance the effects of knocking down subsets of preplacodal competence factors (Fig. 5). The simplest interpretation is that Bmp initially co-induces epidermal and preplacodal potential throughout the nonneural ectoderm, with fate specification occurring later according to differences in local signaling.
Differential regulation of preplacodal competence factors by Bmp explains the differing Bmp-requirements of preplacodal ectoderm vs. neural crest. tfap2a, tfap2c, foxi1 and gata3 all require Bmp for ventral expression during blastula stage. Because these genes are expressed uniformly throughout the nonneural ectoderm, it is now clear why no dose of DM is capable of expanding preplacodal ectoderm at the expense of epidermal ectoderm, though both fates can be eliminated together at sufficiently high concentrations. However, tfap2a and tfap2c are expressed in a broader domain that includes the lateral edges of the neural plate where they are required for neural crest specification [29], [35]. The broader domain of expression suggests that tfap2a and tfap2c can be induced by a lower level of Bmp than foxi1 and gata3. Indeed, we identified a dose of DM that permits continued broad expression of tfap2a/c but eliminates expression of foxi1 and gata3 (Fig. 2). Thus the greater sensitivity of tfap2a/c to Bmp explains the ability of a low threshold of Bmp to expand neural crest at the expense of nonneural ectoderm. After the onset of gastrulation, expression of all four genes becomes independent of Bmp. This is an important regulatory feature because it allows maintenance of preplacodal competence as Bmp signaling is attenuated along the neural-nonneural border during preplacodal specification. Likewise, stability of tfap2a/c in the neural plate safeguards neural crest fate after Bmp signaling abates.
It is still unclear how tfap2a/c can alternately promote either neural crest or preplacodal development. We speculate that the overlap of tfap2a/c with early markers of neural plate such as sox2/3/19 favors neural crest, whereas overlap with foxi1 and gata3 in the nonneural ectoderm favors preplacodal development (Fig. 8). However, misexpression of tfap2a in the neural plate can induce both neural crest and preplacodal markers, albeit in non-overlapping clusters of cells (Fig. S4). It is possible that the level of tfap2a and tfpa2c also influences its developmental function. Both genes show diminishing expression near the edges of the neural plate, which might facilitate their neural crest functions. Similarly, cell-to-cell variation in the level of hs:tfap2a transgene expression might explain the ability to activate ectopic preplacodal and neural crest markers in dorsal ectoderm.
The long lag between expression of competence factors and expression of preplacodal markers remains unexplained. That is, why are preplacodal competence factors expressed prior to gastrulation yet preplacodal markers are not induced until the end of gastrulation? We cannot accelerate expression of preplacodal markers by changing the time of activation of hs:fgf8 and hs:chd. Regardless of whether we activated these transgenes at 7 hpf or 8.5 hpf, we only detected ectopic expression of preplacodal markers at 10.5–11 hpf, the same time these genes are induced within the endogenous preplacodal domain. It is possible that competence factors require sufficient time to “condition” ectoderm, for example through chromatin remodeling [61], or by activating other essential co-factors. These are important issues that require further investigation.
Materials and Methods
Standard development, staging, and pharmacological inhibitor treatment
Embryos were developed under standard conditions at 28.5°C except where noted and staged according to standard protocols [62]. To block Bmp, dorsomorphin (DM) (Calbiochem, 171260) was added to the fish water from a 10mM stock in DMSO. Embryos were treated without removing their chorions. Treatment was carried out in 24-well plates, with 40 embryos in 0.5 ml of solution per well. Relevant controls were incubated in fish water containing an equal concentration of DMSO to that of treated embryos. DM solutions should be exposed to as little light as possible as the drug is photo-unstable. Stock solution of DM may be stored in small aliquots at −80°C for several months, but storage at warmer temperatures and repeated freeze-thaw significantly reduces activity. To Block Fgf, SU5402 (Calbiochem) was diluted from a 10 mM stock in DMSO. To block Pdgf, AG1295 (Calbiochem) was diluted from a 20mM stock in DMSO.
In situ hybridization and immunostaining
Fixation and in situ hybridization were performed as previously described [48], [57]. Immunostaining for phosphorylated Smads was carried out as described [15] with minor modifications. The primary antibody was used at a concentration 1∶150 (anti-pSmad1/5/8 antibody; Cell Signaling Technology). Secondary antibody was HRP-conjugated anti-rabbit IgG at 1∶200 (Santa Cruz Biotechnology).
Morpholino injection
For gene knockdown experiments, embryos were injected with 5ng per morpholino as indicated. Morpholino sequences for foxi1, tfap2a, tfap2c and p63 have been previously published [27], [29], [63]. To knockdown gata3, either of two morpholinos was used: For blocking translation, gata3-MO1 TCCGGACTTACTTCCATCGTTTATT; for blocking mRNA splicing at the exon1-intron1 junction, gata3-MO2 AGAACTGGTTTACTTACTGTGAGGT. Neither gata3-MO1 nor gata3-MO2 produced discernable phenotypes on their own, but both showed identical interactions with morpholinos for other competence factors. The ability of gata3-MO2 to diminish production of mature gata3 mRNA was confirmed with RT-PCR (Fig. S6). The MO-generated phenotypes described in this study were 100% penetrant, except where noted in the text. At least 10 specimens were examined or each experimental time point, unless stated otherwise.
Gene misexpression
Full length cDNAs of foxi1, gata3, tfap2a, fgf3 and fgf8 were ligated to hsp70 heat shock promoter [49] with flanking I-SceI meganuclease sites [64], [65]. Recombinant plasmid (10–40 pg/nl) was coinjected with I-SceI meganuclease (NEB, 0.5 U/µl) into 1-cell stage embryos. For transient ectopic expression, injected embryos were heat-shocked in a recirculating water bath. Stable transgenic lines Tg(hsp70:fgf8a)x17, Tg(hsp70:fgf3)x18 and Tg(hsp70:foxi1)x19 were generated by raising injected embryos to adult and screening by PCR for germline transmission. Heterozygous transgene-carriers were easily distinguished based on the phenotype following heat shock at 30% epiboly: Activation of Tg(hsp70:fgf8a)x17 or Tg(hsp70:fgf3)x18 caused dorsalization of the embryo, whereas activation of Tg(hsp70:foxi1)x19 caused anterior truncations with defects in forebrain and eyes (Fig. S3H). The Tg(hsp70l:dnBmpr-GFP) transgenic line [45] was provided by ZIRC. Tg(hsp70:chordin) [15] was generously provided by Mary Mullins.
In most experiments, transgenic embryos were heterozygous for the transgenes in question, with the exception that homozygous Tg(hsp70:chordin)/Tg(hsp70:chordin) embryos were used to misexpress chd. To misexpress foxi1, tfap2a and gata3, embryos were heat shocked at 39°C for 30 min at various times as indicated in the text. Tg(hsp70l:dnBmpr-GFP) and Tg(hsp70:chordin) embryos were heat shocked at 39°C for 30 min at 7.5 hpf; Tg(hsp70:fgf8a)and Tg(hsp70:fgf3) embryos at 35°C for 3 hr from 7.5 hpf. After heat shock, the plate containing the embryos was transferred into a 28.5°C incubator until fixation or observation.
Cell transplantation
Donor embryos were injected with lineage tracer (mix of lysine fixable rhodamine dextran, 10000 MW, and 5% biotin dextran, 10000 MW, in the ratio of 1∶9 in 0.2 M KCl) at the one-cell stage. Cells were transplanted either from blastula stage donors into blastula stage hosts or from blastula stage donors into gastrula stage (∼6 hpf) hosts. Mosaic embryos were then heat-shocked at 39°C for 30 min at 7 hpf and subsequently maintained at 33°C until fixed. Transplanted cells were identified in the hosts by streptavidin-FITC antibody staining.
Cell death assays
Embryos were dechorinated and incubated for 1 hour on agarose-coated plates containing fish water with acridine orange (AO) (1µg/ml), as modified from [48]. The embryos were then briefly washed and immediately examined under a fluorescence microscope.
Supporting Information
Zdroje
1. BakerCVH
Bronner-FraserM
2000 Vertebrate cranial placodes I. Embryonic induction. Dev Biol 232 1 61
2. SchlosserG
2006 Induction and specification of cranial placodes. Dev Biol 294 303 351
3. StreitA
2007 The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol 51 447 461
4. BrugmannSA
MoodySA
2005 Induction and specification of the vertebrate ectodermal placodes: precursors of the cranial sensory organs. Biol Cell 97 303 319
5. AkimenkoMA
EkkerM
WegnerJ
LinW
WesterfieldM
1994 Combinatorial expression of three zebrafish genes related to Distal-less: part of a homeobox gene code for the head. J Neurosci 14 3475 3486
6. SahlyI
AndermannP
PetitC
1999 The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol 209 399 410
7. KobayashiM
OsanaiH
KawakamiK
YamamotoM
2000 Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech Dev 9 151 155
8. KishimotoY
LeeKH
ZonL
HammerschmidtM
Schulte-MerkerS
1997 The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124 4457 4466
9. NeaveB
HolderN
PatientR
1997 A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech Dev 62 183 195
10. WilsonPA
LagnaG
SuzukiA
Hemmati-BrivanlouA
1997 Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development 124 3177 3184
11. MarchantL
LinkerC
RuizP
GuerreroN
MayorR
1998 The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol 198 319 329
12. NguyenVH
SchmidB
TroutJ
ConnorsSA
EkkerM
1998 Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol 199 93 110
13. BarthKA
KishimotoY
RohrKB
SeydlerC
Schulte-MerkerS
1999 Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development 126 4977 4987
14. ReversadeB
De RobertisEM
2005 Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123 1147 1160
15. TuckerJA
MintzerKA
MullinsMC
2008 The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell 14 108 119
16. EsterbergR
FritzA
2008 dlx3b/4b are required for the formation of preplacodal region and otic placode through modulation of Bmp activity. Dev Biol 325 189 199
17. KwonHJ
RileyBB
2009 Mesendodermal signals required for otic induction: Bmp-antagonists cooperate with Fgf and can facilitate formation of ectopic otic tissue. Dev Dyn 238 1582 1594
18. BrugmannSA
PandurPD
KenyonKL
PignoniF
MoodySA
2004 Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development 131 5871 5881
19. GlavicA
HonoréMS
FeijóoCG
BastidasF
AllendeML
2004 Role of BMP signaling and the homeoprotein Iroquois in the specification of the cranial placodal field. Dev Biol 272 89 103
20. AhrensK
SchlosserG
2005 Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol 288 40 59
21. LitsiouA
HansonS
StreitA
2005 A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132 4051 4062
22. BaschML
Bronner-FraserM
Barcía-CastroMI
2006 Specification of the neural crest occurs during gastrulation and requires Pax7. Nature 441 218 222
23. WodaJM
PastagiaJ
MercolaM
ArtingerKB
2003 Dlx proteins position the neural plate border and determine adjacent cell fates. Development 130 331 342
24. NeaveB
RodawayA
WilsonSW
PatientR
HolderN
1995 Expression of zebrafish GATA 3 (gta3) during gastrulation and neurulation suggests a role in the specification of cell fate. Mech Dev 51 169 182
25. ShengG
SternCD
1999 Gata2 and Gata3: novel markers for early embryonic polarity and for non-neural ectoderm in the chick embryo. Mech Dev 87 213 219
26. KarisA
PataI
van DoorninckJH
GrosveldF
de ZeeuwCI
2001 Transcription factor GATA-3 alters pathway selection of olivochochlear neurons and affects morphogenesis of the ear. J Comp Neurol 429 615 630
27. SolomonKS
KudohT
DawidIB
FritzA
2003 Zebrafish foxi1 mediates otic placode formation and jaw development. Development 130 929 940
28. LeeSA
ShenEL
FiserA
SaliA
GuoS
2003 The zebrafish forkhead transcription factor Foxi1 specified epibranchial placode-derived sensory neurons. Development 130 2669 2679
29. LiW
CornellRA
2007 Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Dev Biol 304 338 354
30. KelshRN
DuttonK
MedlinJ
WisenJS
2000 Expression of zebrafish fkd6 in neural crest-derived glia. Mech Dev 93 161 164
31. Montero-BalaguerM
LangMR
Weiss SachdevS
KnappmeyerC
StewartRA
2006 The mother superior mutation ablates foxd3 activity in neural crest progenitor cells and depletes neural crest derivatives in zebrafish. Dev Dyn 235 3199 3212
32. YuPB
HongCC
SachidanandanC
BabittJL
DengDY
2008 Dorsomorphin inhibits Bmp signals required for embryogenesis and iron metabolism. Nature Chem Biol 4 33 41
33. MullinsMC
HammerschmidtM
KaneDA
OdenthalJ
BrandM
1996 Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123 81 93
34. YabeT
ShimizuT
MuraokaO
BaeYK
HirataT
2003 Ogon/Secreted Frizzled functions as a negative feedback regulator of Bmp signaling. Development 130 2705 2716
35. HoffmanTL
JavierAL
CampeauSA
KnightRD
SchillingTF
2007 Tfap2 transcription factors in zebrafish neural crest development and ectodermal evolution. J Exp Zool 308B 679 691
36. KraussS
JohansenT
KorzhV
FjoseA
1991 Expression of the zebrafish paired box genes pax[zf-b] during early neurogenesis. Development 113 1193 1206
37. KollmarR
NakamuraSK
KapplerJA
HudspethAJ
2001 Expression and phylogeny of claudins in vertebrate primordia. Proc Natl Acad Sci 98 10196 10201
38. KajiT
ArtingerKB
2004 dlx3b and dlx4b function in development of Rohon-Beard sensory neurons and trigeminal placode in the zebrafish neurula. Dev Biol 276 523 540
39. DuttaS
DietrichJE
AspöckG
BurdineRD
SchierA
2005 pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development 132 1579 1590
40. KnautH
BladerP
SträhleU
SchierAF
2005 Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron 47 653 666
41. SwindellEC
ZilinskiCA
HashimotoR
ShahR
LaneME
2008 Regulation and function of foxe3 during early zebrafish development. Genesis 46 177 183
42. MiyasakaN
KnautH
YoshiharaY
2007 Cxcl12/Cxcr4b chemokine signaling is required for placode assembly and sensory axon pathfinding in the zebrafish olfactory system. Development 134 2459 2468
43. NikaidoM
DoiK
ShimizuT
HibiM
KikuchiY
2007 Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn 236 564 571
44. SunSK
DeeCT
TripathiVB
RengifoA
HirstCS
2007 Epibranchial and otic placodes are induced by a common Fgf signal, but their subsequent development is independent. Dev Biol 303 675 686
45. PyatiUJ
WebbAE
KimelmanD
2005 transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development 132 2333 2343
46. BakkersJ
HildM
KramerC
Furutani-SeikiM
HammerschmidtM
2002 Zebrafish ΔMp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell 2 617 627
47. LeeH
KimelmanD
2002 A dominant-negative form of p63 is required for epidermal proliferation in zebrafish. Dev Cell 2 607 616
48. PhillipsBT
KwonHY
MeltonC
HoughtlingP
FritzA
2006 Zebrafish msxB, msxC and msxE function together to refine the neural-nonneural border and regulate cranial placodes and neural crest development. Dev Biol 294 376 390
49. ShojiW
YeeCS
KuwadaJY
1998 Zebrafish Semaphorin Z1a collapses specific growth cones and alters their pathway in vivo. Development 125 1275 1283
50. HansS
ChristisonJ
LiuD
WesterfieldM
2007 Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol 7 5
51. SolomonKS
KwakSJ
FritzA
2004 Genetic interactions underlying otic placode induction and formation. Dev Dyn 230 419 433
52. LégerS
BrandM
2002 Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev 119 91 108
53. LiuD
ChuH
MavesL
YanYL
MorcosPA
2003 Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development 130 2213 2224
54. LiuL
KorzhV
BablsubramaniyanNV
EkkerM
GeR
2002 Platelet-derived growth factor A (pdgf-a) expression during zebrafish embryonic development. Dev Genes Evol 212 298 301
55. MonteraJA
KilianB
ChanJ
BaylissPE
HeisenbergCP
2003 Phosphoinositide 3-kinase is required for process outgrowth and cell polarization of gastrulating mesendodermal cells. Curr Biol 13 1279 1289
56. KudohT
ConchaML
HouartC
DawidIB
WilsonSW
2004 Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development 131 3581 3592
57. PhillipsBT
BoldingK
RileyBB
2001 Zebrafish fgf3 and fgf8 encode redundant functions required for otic placode induction. Dev Biol 235 351 365
58. McCabeKL
Bronner-FraserM
2008 Essential role for PDGF signaling in ophthalmic trigeminal placode induction. Development 135 186301874
59. PattheyC
GunhagaL
EdlundT
2008 Early development of the central and peripheral nervous systems is coordinated by Wnt and Bmp signals. PLoS ONE 3 31625 doi:10.1371/journal.pone.0001625
60. PattheyC
GunhagaL
EdlundT
2009 Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development 136 73 83
61. YamJ
XuL
CrawfordG
WangZ
BurgessSM
2006 The forkhead transcription factor Foxi1 remains bound to condensed mitotic chromosomes and stably remodels chromatin structure. Mol Cell Biol 26 155 168
62. KimmelCB
BallardWW
KimmelSR
UlmannB
SchillingTF
1995 Stages of embryonic development of the zebrafish. Dev Dyn 203 253 310
63. SidiS
SandaT
KennedyRD
HagenAT
JetteCA
2008 Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl2, and Caspase-3. Cell 133 864 877
64. ThermesV
GrabherC
RistoratoreF
BourratF
ChoulikeA
2002 I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118 91 98
65. RemboldM
LahiriK
FoulkesNS
WittbrodtJ
2006 Transgenesis in fish: efficient selection of transgenic fish by co-injection with a fluorescent reporter construct. Nature Protocols 1 1133 1139
Štítky
Genetika Reprodukční medicína
Článek Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator ofČlánek Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline AnalysisČlánek Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoAČlánek Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.Článek Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 9
-
Všechny články tohoto čísla
- Optimal Strategy for Competence Differentiation in Bacteria
- Mutational Patterns Cannot Explain Genome Composition: Are There Any Neutral Sites in the Genomes of Bacteria?
- Frail Hypotheses in Evolutionary Biology
- Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits
- Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator of
- Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline Analysis
- Germline Variation Controls the Architecture of Somatic Alterations in Tumors
- Mice Doubly-Deficient in Lysosomal Hexosaminidase A and Neuraminidase 4 Show Epileptic Crises and Rapid Neuronal Loss
- Analysis of Population Structure: A Unifying Framework and Novel Methods Based on Sparse Factor Analysis
- FliO Regulation of FliP in the Formation of the Flagellum
- Cdc20 Is Critical for Meiosis I and Fertility of Female Mice
- dMyc Functions Downstream of Yorkie to Promote the Supercompetitive Behavior of Hippo Pathway Mutant Cells
- DCAF26, an Adaptor Protein of Cul4-Based E3, Is Essential for DNA Methylation in
- Genome-Wide Double-Stranded RNA Sequencing Reveals the Functional Significance of Base-Paired RNAs in
- An Immune Response Network Associated with Blood Lipid Levels
- Genetic Variants and Their Interactions in the Prediction of Increased Pre-Clinical Carotid Atherosclerosis: The Cardiovascular Risk in Young Finns Study
- The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoA
- Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
- Orphan CpG Islands Identify Numerous Conserved Promoters in the Mammalian Genome
- Analysis of the Basidiomycete Reveals Conservation of the Core Meiotic Expression Program over Half a Billion Years of Evolution
- ETS-4 Is a Transcriptional Regulator of Life Span in
- The SR Protein B52/SRp55 Is Required for DNA Topoisomerase I Recruitment to Chromatin, mRNA Release and Transcription Shutdown
- The Baker's Yeast Diploid Genome Is Remarkably Stable in Vegetative Growth and Meiosis
- Chromatin Landscape Dictates HSF Binding to Target DNA Elements
- The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice
- Accurately Assessing the Risk of Schizophrenia Conferred by Rare Copy-Number Variation Affecting Genes with Brain Function
- Widespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
- The Characterization of Twenty Sequenced Human Genomes
- The Genome of a Pathogenic : Cooptive Virulence Underpinned by Key Gene Acquisitions
- A Single Element Maintains Repression of the Key Developmental Regulator
- Identification of New Genetic Risk Variants for Type 2 Diabetes
- Effect of Correlated tRNA Abundances on Translation Errors and Evolution of Codon Usage Bias
- Evidence of Selection upon Genomic GC-Content in Bacteria
- Proteomic Changes Resulting from Gene Copy Number Variations in Cancer Cells
- Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.
- Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study
- Response to Mechanical Stress Is Mediated by the TRPA Channel Painless in the Heart
- DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
- Incremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
- Loss of Maternal ATRX Results in Centromere Instability and Aneuploidy in the Mammalian Oocyte and Pre-Implantation Embryo
- Comparative Genomic Hybridization (CGH) Reveals a Neo-X Chromosome and Biased Gene Movement in Stalk-Eyed Flies (Genus )
- Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
- Gene–Environment Interactions at Nucleotide Resolution
- Dementia Revealed: Novel Chromosome 6 Locus for Late-Onset Alzheimer Disease Provides Genetic Evidence for Folate-Pathway Abnormalities
- Critical Functions of Rpa3/Ssb3 in S-Phase DNA Damage Responses in Fission Yeast
- Preferential Re-Replication of Heterochromatin in the Absence of Geminin
- The Potential for Enhancing the Power of Genetic Association Studies in African Americans through the Reuse of Existing Genotype Data
- Evidence That Mutation Is Universally Biased towards AT in Bacteria
- Perturbation Analysis of Heterochromatin-Mediated Gene Silencing and Somatic Inheritance
- Diversity of Eukaryotic DNA Replication Origins Revealed by Genome-Wide Analysis of Chromatin Structure
- Genetic Deletion of the Desmosomal Component Promotes Tumor Microinvasion in a Mouse Model of Pancreatic Neuroendocrine Carcinogenesis
- The Metabolic Enzyme ManA Reveals a Link between Cell Wall Integrity and Chromosome Morphology
- SNPs Associated with Cerebrospinal Fluid Phospho-Tau Levels Influence Rate of Decline in Alzheimer's Disease
- Synthesizing and Salvaging NAD: Lessons Learned from
- A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis
- An Insect Herbivore Microbiome with High Plant Biomass-Degrading Capacity
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Synthesizing and Salvaging NAD: Lessons Learned from
- Optimal Strategy for Competence Differentiation in Bacteria
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



