-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIncremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
Mutations causing replication stress can lead to genomic instability (GIN). In vitro studies have shown that drastic depletion of the MCM2-7 DNA replication licensing factors, which form the replicative helicase, can cause GIN and cell proliferation defects that are exacerbated under conditions of replication stress. To explore the effects of incrementally attenuated replication licensing in whole animals, we generated and analyzed the phenotypes of mice that were hemizygous for Mcm2, 3, 4, 6, and 7 null alleles, combinations thereof, and also in conjunction with the hypomorphic Mcm4Chaos3 cancer susceptibility allele. Mcm4Chaos3/Chaos3 embryonic fibroblasts have ∼40% reduction in all MCM proteins, coincident with reduced Mcm2-7 mRNA. Further genetic reductions of Mcm2, 6, or 7 in this background caused various phenotypes including synthetic lethality, growth retardation, decreased cellular proliferation, GIN, and early onset cancer. Remarkably, heterozygosity for Mcm3 rescued many of these defects. Consistent with a role in MCM nuclear export possessed by the yeast Mcm3 ortholog, the phenotypic rescues correlated with increased chromatin-bound MCMs, and also higher levels of nuclear MCM2 during S phase. The genetic, molecular and phenotypic data demonstrate that relatively minor quantitative alterations of MCM expression, homeostasis or subcellular distribution can have diverse and serious consequences upon development and confer cancer susceptibility. The results support the notion that the normally high levels of MCMs in cells are needed not only for activating the basal set of replication origins, but also “backup” origins that are recruited in times of replication stress to ensure complete replication of the genome.
Published in the journal: . PLoS Genet 6(9): e32767. doi:10.1371/journal.pgen.1001110
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001110Summary
Mutations causing replication stress can lead to genomic instability (GIN). In vitro studies have shown that drastic depletion of the MCM2-7 DNA replication licensing factors, which form the replicative helicase, can cause GIN and cell proliferation defects that are exacerbated under conditions of replication stress. To explore the effects of incrementally attenuated replication licensing in whole animals, we generated and analyzed the phenotypes of mice that were hemizygous for Mcm2, 3, 4, 6, and 7 null alleles, combinations thereof, and also in conjunction with the hypomorphic Mcm4Chaos3 cancer susceptibility allele. Mcm4Chaos3/Chaos3 embryonic fibroblasts have ∼40% reduction in all MCM proteins, coincident with reduced Mcm2-7 mRNA. Further genetic reductions of Mcm2, 6, or 7 in this background caused various phenotypes including synthetic lethality, growth retardation, decreased cellular proliferation, GIN, and early onset cancer. Remarkably, heterozygosity for Mcm3 rescued many of these defects. Consistent with a role in MCM nuclear export possessed by the yeast Mcm3 ortholog, the phenotypic rescues correlated with increased chromatin-bound MCMs, and also higher levels of nuclear MCM2 during S phase. The genetic, molecular and phenotypic data demonstrate that relatively minor quantitative alterations of MCM expression, homeostasis or subcellular distribution can have diverse and serious consequences upon development and confer cancer susceptibility. The results support the notion that the normally high levels of MCMs in cells are needed not only for activating the basal set of replication origins, but also “backup” origins that are recruited in times of replication stress to ensure complete replication of the genome.
Introduction
In late mitosis to early G1 phase of the cell cycle, DNA replication origins are selected and bound by the hexameric origin recognition complex (ORC; [1]). ORC then recruits the initiation factors CDC6 and CDT1, which are required for loading MCM2-7, thereby forming the “pre-replicative complex” (pre-RC). The formation of pre-RCs is termed origin “licensing” and this gives origins competency to initiate a single round of DNA synthesis before entering S phase. MCM2-7 is a hexamer of six distinct but structurally-related minichromosome maintenance (MCM) proteins (reviewed in [2]–[5]). In vivo and in vitro evidence indicates that the MCM2-7 complex is the replicative helicase [6]–[8].
MCM2-7 proteins are abundant in proliferating cells [9], and are bound to chromatin in amounts exceeding that which is present at active replication origins or required for complete DNA replication [10]–[14]. Although these and other studies showed that drastic decreases in MCMs are tolerated by dividing cells, there are certain deleterious consequences. In Xenopus extracts and mammalian cells, excess chromatin-bound MCM2-7 complexes occupy dormant or “backup” origins that are activated under conditions of replication stress, compensating for stalled or disrupted primary replication forks [11], [15]–[16]. The depletion of these backup licensed origins was associated with elevated chromosomal instability and susceptibility to replication stress, factors that might predispose to cancer.
In previous work, Shima et al found that a hypomorphic allele of mouse Mcm4 (Mcm4Chaos3) caused high levels of GIN and extreme mammary cancer susceptibility in the C3HeB/FeJ background [17]. This provided the first concrete evidence that endogenous mutations in replication licensing machinery may have a causative role in cancer development. The ethylnitrosourea (ENU)-induced Mcm4Chaos3 point mutation changed PHE to ILE at residue 345 (Phe345Ile). This amino acid is conserved across diverse eukaryotes and is important for interaction with other MCMs [18]. Budding yeast engineered to bear the orthologous mutation exhibit DNA replication defects and GIN [17], [19]. Surprisingly, MEFs from Mcm4Chaos3 mice not only had reduced levels of MCM4, but also MCM7 [17], suggesting that the point mutation might destabilize the MCM2-7 complex. Subsequently, it was reported that mice containing 1/3 the normal level of MCM2 succumbed to lymphomas at a very young age, and had diverse stem cell proliferation defects [20]. These mice also had 27% reduced levels of MCM7 protein, and their cells exhibited decreased replication origin usage when under replication stress (treatment with hydroxyurea) conditions [21]. These studies imply that relatively modest decreases in any of the MCMs may be sufficient to cause cancer susceptibility, developmental defects, and GIN [20].
Here, we report that genetically-induced reductions of MCM levels in mice, achieved by breeding combinations of MCM2-7 alleles, caused several health-related defects including increased embryonic lethality, GIN, cancer susceptibility, growth retardation, defective cell proliferation, and hematopoiesis defects. Remarkably, genetic reduction of MCM3, which mediates nuclear export of excess MCM2-7 complexes in yeast [22], rescued many of these defects, presumably attributable to observed increases in chromatin-bound MCM levels. These data suggest that relatively minor misregulation or destabilization of MCM homeostasis can have serious consequences for health, viability and cancer susceptibility of animals.
Results
Mcm4Chaos3/Chaos3 cells exhibit pan-reduction of total and chromatin-bound MCM2-7 due to decreased mRNA levels
To extend previous findings that Mcm4Chaos3Chaos3 cells exhibited decreases in MCM4 and MCM7 protein, and to determine if the decreased levels were differentially compartmentalized in the cell, we quantified soluble and chromatin-bound MCM2-7 levels in mouse embryonic fibroblasts (MEFs) by Western blot analysis. As shown in Figure 1A, all MCMs were decreased in both compartments by at least 40% compared to WT cells. Because Mcm4Chaos3/Chaos3 MEF cultures have slightly decreased proliferation and G2/M delay (Figure 1A and [17]), it is possible that the lower MCM levels in mutant MEFs are entirely attributable to growth defects. To test this, we assessed the levels of nuclear MCM2 in S-phase cells by flow cytometry (Figure 1B). Although MCM2 levels in WT and Mcm4Chaos3/Chaos3 G1 nuclei were essentially the same (P = .65; t-test), mutant cells transitioned from G1 to S with 40% less nuclear MCM2 content than in WT (P<.02; t-test). The levels of nuclear MCM2 in WT decreased through S phase more sharply than in mutants, which transitioned to G2 with only ∼23% less than controls (Figure 1B). This differential decline is apparent in the flow plots, where WT cells exhibit a greater downward slope in the S compartment (Figure 1B). The decreases in MCM2 from early to late S were 51% in WT and 38% in mutants. The MCM2 intra-S modulation phenomenon is also addressed in subsequent experiments. The marked differences in nuclear MCM2 concentration between actively proliferating (S-phase) WT and mutant cells indicates that a biochemical or regulatory basis, rather than a population skewing, underlies the differences in protein levels.
Fig. 1. MCM2-7 proteins and mRNA are reduced in Mcm4Chaos3/Chaos3 MEFs, particularly in early S phase. 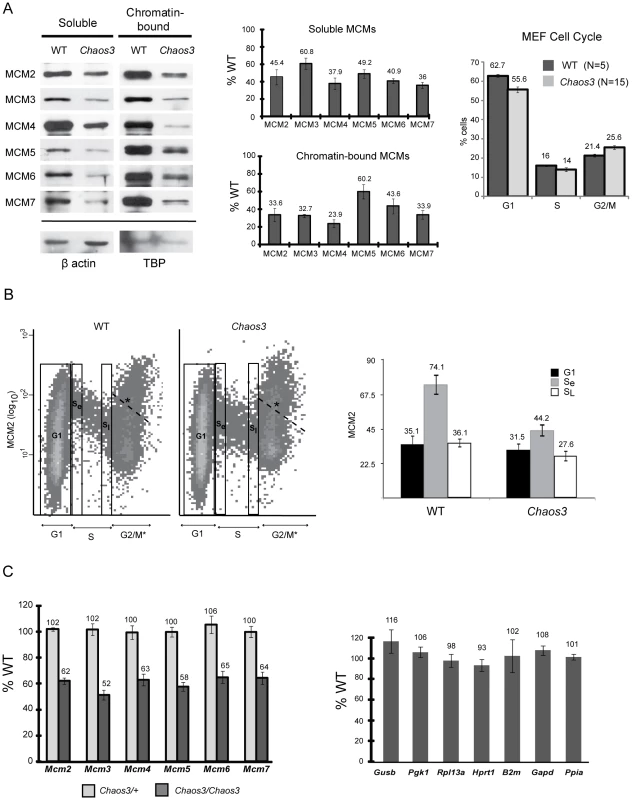
(A) Western blot analysis of MCM2-7 (left panel). Soluble or chromatin-bound protein was electrophoresced on PAGE gels, electrotransferred, and the blots were immunolabeled with the indicated antibodies. The bands correspond to the predicted molecular weights of these proteins, and for MCM2, the identity of the band was verifed by RNAi knockdown and transient overexpression in NIH 3T3 cells. TBP = TATA box binding protein. Quantification of Western blot data by densitometry is shown in the center panel. The amounts relative to WT cells (after normalization to the controls) are plotted. Error bars represent SEM, derived from 4 replicate experiments. The rightmost panel graphs the results of flow cytometric analysis of unsynchronized MEF culture cell cycle profiles, based on DNA content. (B) Flow cytometric quantification of MCM2 content (fluorescence intensity of antibody staining) is plotted on the Y axis, vs DNA content on the X axis. Plotted at the right is the mean fluorescent intensity of the 3 gated (boxed) regions from the flow data. Se = early S phase; SL = late S phase. The labeled cell cycle stages are based on DNA content. However, because light scatter was inadequate to distinguish individual nuclei from clumped nuclei or artifactual structures, the 4c category (denoted G2/M*) contains events other than G2/M nuclei. *We drew a dashed line representing an arbitrary cutoff above which contains such undefined events. (C) qRT-PCR analysis of Mcm mRNAs (left panel) and control genes (right panel), in the three indicated genotypes of MEFs. Relative transcript levels were normalized to β-actin. Charted are the percent levels of the indicated RNAs in mutant compared to WT (considered to be 100%). At least 3 replicate cultures were analyzed for each genotype. Error bars are SEM. In all panels, the raw data shown are from MEFs established from littermates. Furthermore, the replicates involved MEFs from pairs of littermates. Chaos3 = Mcm4Chaos3/Chaos3; WT = +/+. Another possible explanation for the coordinated decrease in MCMs is that the mutant MCM4Chaos3 protein destabilizes the MCM2-7 hexamer and causes subsequent degradation of uncomplexed MCMs. Other groups reported that knockdown of Mcm2, Mcm3, or Mcm5 in human cells decreased the amount of other chromatin-bound MCMs [15]–[16], leading to a similar proposition that the cause was MCM2-7 hexamer destabilization [16]. If true, then we would expect mRNA levels to be unchanged in mutant cells. To test this, we performed quantitative RT-PCR (qRT-PCR) analysis of Mcm2-7, and several control housekeeping genes in Mcm4Chaos3/Chaos3 MEFs. Analysis of 5 littermate pairs of primary MEF cultures revealed that transcript levels for each of these genes in mutant cells was 51–65% of WT, similar to the protein decreases (Figure 1C). Levels of mRNA in the 7 housekeeping genes analyzed were not altered significantly (Figure 1C, right panel). This data suggest that either reduced MCM4 levels per se, or defects resulting from the Mcm4Chaos3 allele, cause a decrease in the levels of all Mcm mRNAs. Interestingly, the mRNA reduction appears to occur post-transcriptionally, a phenomenon that is currently under investigation (Chuang and Schimenti, unpublished observations).
Decreased Mcm gene dosages cause elevated chromosomal instability and Mcm2-specific pan-decreases in Mcm mRNAs
The Mcm4Chaos3 allele was identified in a forward genetic screen for mutations causing elevated micronuclei (MN) in red blood cells, an indicator of GIN [17]. While the altered MCM4Chaos3 protein may cause DNA replication errors as does a yeast allele engineered to contain the same amino acid change [19], it is also possible that the decrease in overall MCM levels in Mcm4Chaos3 mutants contributes to, or is primarily responsible for, elevated S-phase DNA damage and GIN as is seen in various cell culture models (see Introduction). To test this possibility, we generated mice from ES cells bearing gene trap insertions in Mcm2, Mcm3, Mcm6, and Mcm7 (Figure 2A; alleles are designated as Mcm#Gt). These gene traps are designed to disrupt gene expression by fusing the 5′ end of the endogenous mRNA (via use of a splice acceptor) to a vector-encoded reporter, resulting in a fusion protein lacking the C-terminal portion of the endogenous (MCM) protein. As with a previously-reported Mcm4 gene trap [17], each of these alleles proved to be recessive embryonic lethal (Figure S1). Furthermore, each allele appeared to be a null, since mRNA levels in heterozygous MEF cultures were ∼50% lower than WT controls (Figure 2B). To determine if heterozygosity for various Mcms caused pan-decreases in Mcm mRNA levels as does homozygosity for Mcm4Chaos3, mRNA levels for each of the Mcm2-7 genes were also quantified. Whereas Mcm2Gt/+ cells did show ∼20% decreases in the other Mcms, the Mcm3, Mcm4, Mcm6 and Mcm7 gene trap alleles did not (Figure 2B). Thus, it appears that the marked Mcm pan-decreases in Mcm4Chaos3/Chaos3 cells are not due to decreased Mcm4 RNA per se, but rather a response to replication defects cause by the mutant protein. Notably, the pan Mcm2-7 downregulation in Mcm2Gt/+ cells is consistent with the observation that MCM7 is decreased in Mcm2IRES-CreERT2/IRES-CreERT2 mice, although mRNA levels were not evaluated in that study [20].
Fig. 2. Mcm gene trap alleles and associated mRNA levels, peripheral blood micronuclei, and cancer frequency. 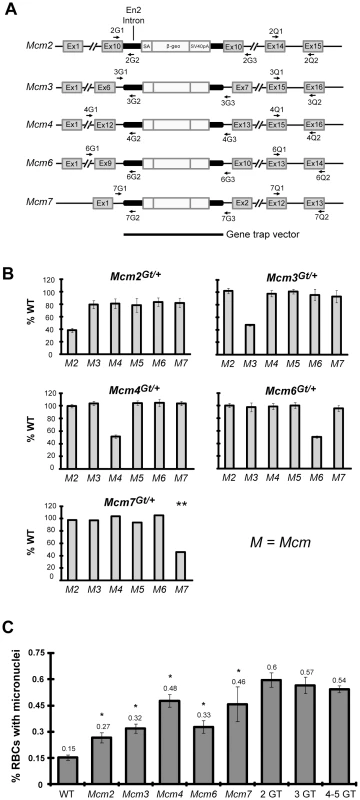
(A) Genomic structures of mutated Mcm genes. Indicated is the intron/exon structure of each gene (not to scale), the locations of the gene trap insertions, and qPCR primer locations. (B) qRT-PCR analyses of MEF mRNA from gene trap heterozygotes. Charted are the percent levels of the indicated RNAs in mutant compared to WT (considered to be 100%). For all but Mcm7, the data were obtained from at least 3 MEF cultures from different embryos. The Mcm7 data represents the average of three replicates from 1 MEF culture, hence there are no error bars. Otherwise, error bars show SEM. (C) Micronucleus levels in Mcm gene trap-bearing male mice. At least 5 animals were analyzed for each single gene trap mutant allele. The “2GT” (two gene trap) group contains: 4 mice doubly heterozygous for Mcm2Gt and Mcm3Gt (“Mcm2/3”), 4 Mcm2/4 mice, and 4 Mcm3/4 mice. The 3GT group contains: 4 Mcm2/3/4 mice, 1 Mcm2/3/6 mouse, 1 Mcm2/4/6 mouse, and 3 Mcm3/4/6 mice. The 4-5GT group contains: 3 Mcm2/3/4/6 mice, 1 Mcm2/3/6/7 mouse, and 2 Mcm2/3/4/6/7 mouse. SEM bars are shown. After breeding the gene trap alleles into the C3HeB/FeJ genetic background for at least 2 generations (Mcm4Chaos3/Chaos3 females get mammary tumors in this background), blood MN levels were measured. Heterozygosity for each allele caused an increase in the fraction of cells with MN (Figure 2C). Compound heterozygosity further increased MN on average, as did heterozygosity for 3 or more gene traps (Figure 2C), indicating that genetically-based decreases in any of the MCMs precipitate GIN.
Genetic reductions of Mcm2, Mcm6 or Mcm7 in an Mcm4Chaos3/Chaos3 background causes partial synthetic lethality, severe growth defects and (for Mcm2) dramatically accelerated cancer onset
As outlined above, previous studies showed that reductions of particular MCMs in cells or mice reduces the levels of other MCMs, causing GIN, cancer, and developmental defects. However, the reduction in MCM levels required to precipitate these consequences, and whether there is a threshold effect, is unclear. To explore the consequences of incremental MCM reductions on viability and cancer in mice, we crossed the Mcm4Chaos3 and gene trap alleles into the same genome. In the case of Mcm2, there was a striking and highly significant shortfall of Mcm4Chaos3/Chaos3 Mcm2Gt/+ offspring at birth (Figure 3A; Figure S2). Heterozygosity for Mcm2Gt itself was not haploinsufficient, as indicated by Mendelian transmission of Mcm2Gt in crosses of heterozygotes to WT (119/250; χ2 = 0.448). These results demonstrate that there is a synthetic lethal interaction between Mcm4Chaos3 and Mcm2Gt that is related to MCM2 levels. Additionally, the surviving Mcm4Chaos3/Chaos3 Mcm2Gt/+ offspring were severely growth retarded; males weighed ∼50% less than Mcm4Chaos3/Chaos3 siblings (Figure 3B; this genotype causes disproportionate female lethality). Another indication of a quantitative MCM threshold effect is that C3H-Mcm4Chaos3/Chaos3 mice are developmentally normal, but Mcm4Chaos3/Gt animals die in utero or neonatally (Figure 3A) [23].
Fig. 3. Synthetic lethality and growth retardation between Mcm4Chaos3 and Mcm2, Mcm6 and Mcm7. 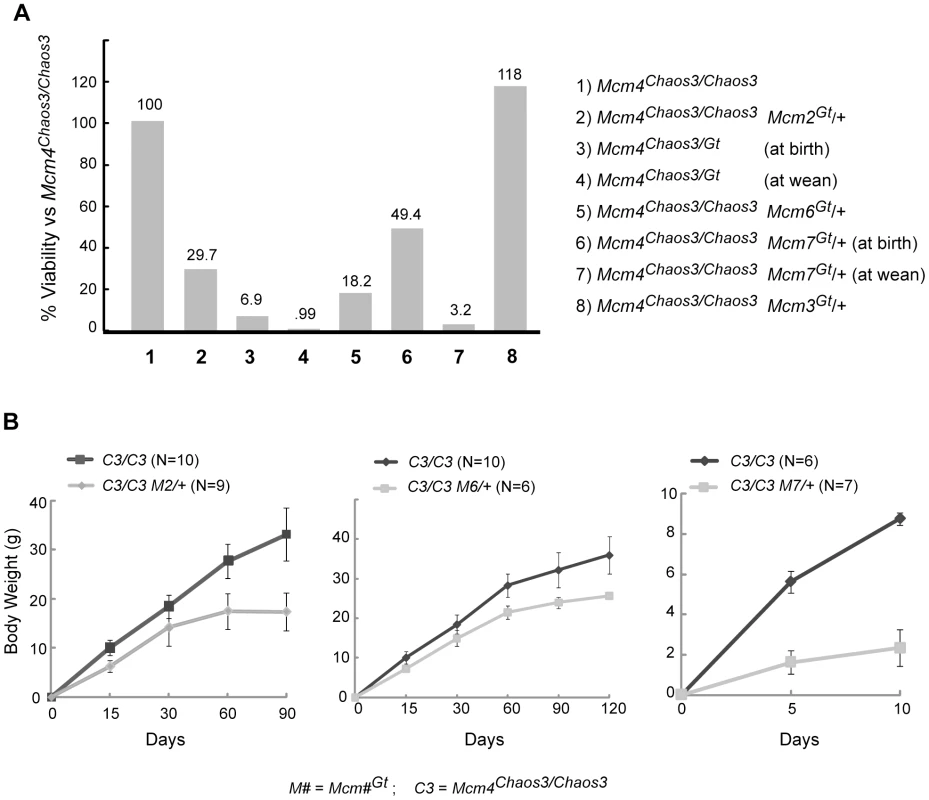
(A) Graphed are viability data from crosses presented in Figure S2, which includes statistics. Unless otherwise indicated, the values represent expected proportions of indicated genotypes that were present at wean. (B) Weights of surviving animals are graphed over time. SEM bars are shown. The synthetic interaction between Mcm4Chaos3 and Mcm2Gt might be specific, or it may reflect a general consequence of reduced replication licensing (and consequent elevated replication stress). We therefore tested whether hemizygosity for Mcm3, Mcm6 or Mcm7 would also cause synthetic phenotypes in the Mcm4Chaos3/Chaos3 background. The Mcm4Chaos3/Chaos3 Mcm6Gt/+ genotype caused highly penetrant embryonic lethality; only 10% of the expected number of such animals survived to birth (Figure 3A; Figure S2). The Mcm4Chaos3/Chaos3 Mcm7Gt/+ genotype caused both embryonic and postnatal lethality. The number of liveborns was ∼50% of the expected value, and only 8% of those (5/62) survived to weaning (Figure 3A; Figure S2). Additionally, as with Mcm2, hemizygosity for Mcm6Gt and Mcm7Gt in the Mcm4Chaos3/Chaos3 background caused growth retardation (Figure 3B). The decrease in male weight was ∼20% and ∼80% respectively, compared to Mcm4Chaos3/Chaos3 siblings at the oldest age measured (Mcm4Chaos3/Chaos3 Mcm7Gt/+ animals died before wean, so the oldest weights were taken at 10 dpp). In contrast to the synthetic phenotypes with Mcm2, 4, 6 and 7, there was no significant decrease in viability (Figure 3A) or weight (not shown) in Mcm4Chaos3/Chaos3 Mcm3Gt/+ mice. This seeming inconsistency is addressed in the following section.
As mentioned earlier, mice with ∼35% of WT MCM2 protein, but not 62%, showed early latency (10–12 week) lymphoma susceptibility [20]. To identify if there is a critical MCM threshold for cancer susceptibility, we aged a cohort of Mcm2Gt/+ mice, representing approximately intermediate MCM2 levels. As shown in Figure 4A, these animals did not show a dramatic cancer-related mortality in the first 12 months of life. However, we did find that ∼3/4 of these animals had tumors at death or necropsy by 18 months of age (data not shown). These combined data are suggestive of a potential gradient of susceptibility, but that there is a critical minimum threshold of MCM levels, between ∼35 and 50% in the case of MCM2, required to avoid early cancer and other developmental defects.
Fig. 4. Premature morbidity and cancer susceptibility in Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice. 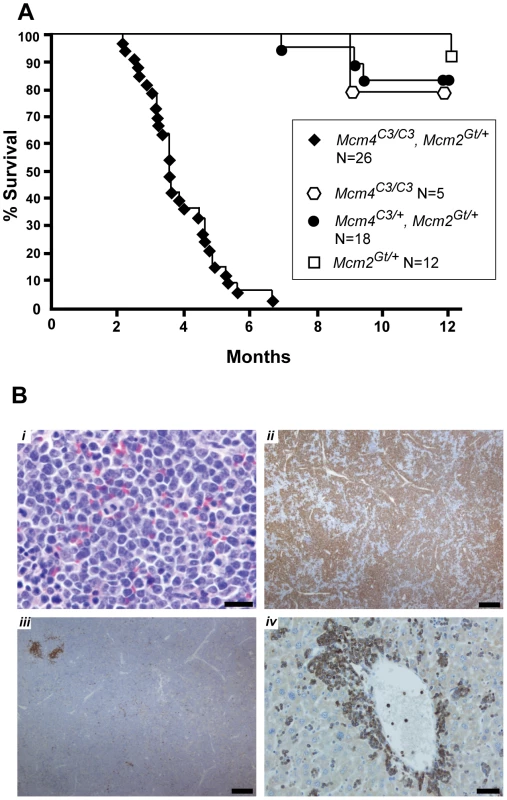
(A) Kaplan-Meier survival plot of the indicated genotypes. Animals of both sexes are combined. “C3” = Chaos3. (B) Spleen and liver histopathology of a Mcm4C3/C3 Mcm2Gt/+ male diagnosed with T cell leukemic lymphoma. i. H&E stained spleen. Neoplastic cells have abundant cytoplasm, 1–2 nucleoli and a high mitotic rate, consistent with lymphoblastic lymphoma. Bar = 20 µm. ii. Neoplastic cells in spleen demonstrate immunoreactivity with anti-CD3 (brown; immunoperoxidase staining with DAB chromogen & hematoxalin counterstain), indicating T lymphocytes. Bar = 200µm. iii. In spleen, immunoreactivity (brown) with anti-PAX-5 (a B cell marker) is limited to follicular remnants and scattered individual cells. Bar = 200 µm. iv. In liver, neoplastic cells surround central veins and expand sinusoids (see also Figure S4) and demonstrate immunoreactivity (brown) with the anti-CD3 T lymphocyte marker. Bar = 50 µm. To further resolve this phenomenon, surviving Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice were aged and monitored. They began dying at 2 months of age, and all were dead (or sacrificed when they appeared moribund) by 7 months (Figure 4A). Gross necropsy and histopathological analyses revealed or suggested lymphomas/leukemias in 20 of these animals (summarized in Table S1 with histological examples in Figure S3; detailed histopathology analysis of a T cell leukemic lymphoma is presented in Figure 4B). Six of these had chest tumors that were likely thymic lymphomas. The cause of death for the remaining 7 animals was undetermined. Consistent with previous studies [17], most Mcm4Chaos3/Chaos3 mice hadn't yet succumbed from tumors or other causes by 12 months of age. Additional animals of these genotypes are incorporated in Figure 6, but histopathological analyses weren't conducted. These data show clearly that removing a half dose of MCM2 from Mcm4Chaos3/Chaos3 cells is sufficient to produce greatly elevated cancer predisposition to the already-underrepresented survivors at wean. Mcm4Chaos3/Chaos3 Mcm2Gt/+ MEFs had 45% the amount of Mcm2 mRNA as Mcm4Chaos3/Chaos3 cells (Figure 7C), which already had a 38% reduction compared to WT (Figure 1). Thus, Mcm2 RNA was reduced to ∼17% of WT. To determine if elevated GIN might be responsible for the cancer susceptibility phenotype, we measured erythrocyte MN. Whereas the percentage of micronucleated RBCs in Mcm4Chaos3/Chaos3 mice was 4.18±0.26 (mean±SEM, N = 12), Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice averaged 5.85±0.47 (N = 16), indicating a synergistic increase (P<0.01). Overall, the data support the notion that in whole animals, reduction of MCMs to under 50% of WT causes severe developmental and physiological problems.
Rescue of phenotypic defects in Mcm4Chaos3/Chaos3 and Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice by reducing Mcm3 genetic dosage
The data reported here and elsewhere [17], [20] support a model where phenotypic severity is proportionally related to MCM concentrations. However, our genetic experiments uncovered one notable exception: hemizygosity for Mcm3 did not cause any severe haploinsufficiency phenotypes (increased lethality and decreased weight) as did Mcm2/6/7 in the Mcm4Chaos3/Chaos3 background, or Mcm4Gt in trans to Mcm4Chaos3 (Figure 3A; Figure S2). Since extreme reductions of MCM3 in cultured human cells caused GIN and cell cycle arrest [16], the absence of synthetic effects with McmChaos3 led us to hypothesize that either mice are more tolerant to lower levels of this particular MCM, or that MCM3 is present in a stoichiometric excess compared to the other MCMs, at least in a subset of cell types. To explore these issues we performed additional phenotype analyses, and also sought to uncover potential effects of MCM3 reduction by reducing other MCMs simultaneously.
Strikingly, rather than exacerbating the synthetic lethality in Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice, Mcm3Gt heterozygosity significantly rescued their viability to 72.5% from 29.7% (Figure 5A and Fig S3). Not only was viability rescued, but also growth (weight) of Mcm4Chaos3/Chaos3 Mcm2Gt/+ Mcm3Gt/+ survivors compared to Mcm4Chaos3/Chaos3 Mcm2Gt/+ animals produced from the same matings (Figure 5B). Mcm3 hemizygosity also significantly rescued the near 100% lethality of Mcm4Chaos3/Gt animals (nearly 6 fold increased viability), and doubled the viability of Mcm4Chaos3/Chaos3 Mcm6Gt/+ mice (Figure 5A; Figure S3). Rescue of Mcm4Chaos3/Chaos3 Mcm7Gt/+ was not observed (not shown).
Fig. 5. Rescue of phenotypes by Mcm3 hemizygosity. 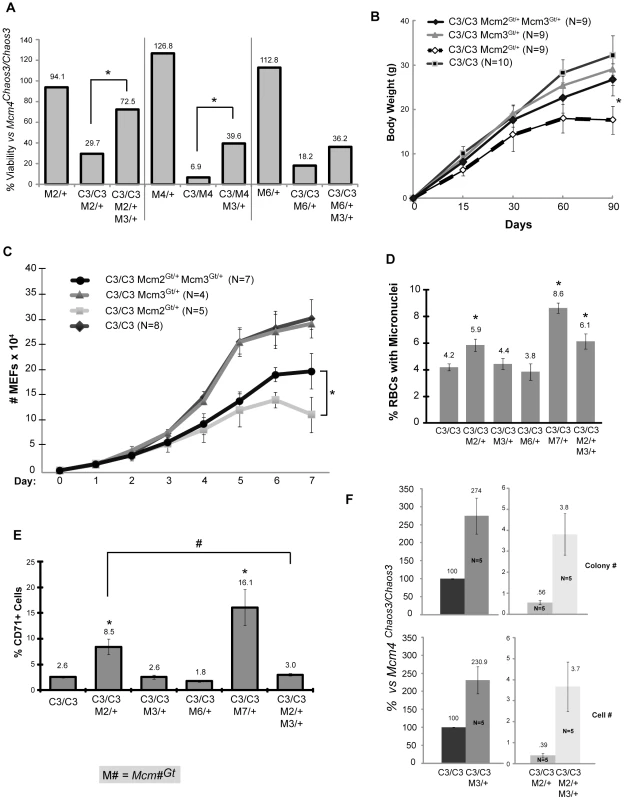
(A) Heterozygosity for Mcm3Gt rescues the low viability of various mutant genotypes (asterisk indicates significance at P<0.05 by FET). The raw data are presented in Figure S4. (B) Male body weights of combination mutant mice. The weights of Mcm4Chaos3/Chaos3 Mcm2Gt/+ Mcm3Gt/+ mice are significantly higher (asterisk; P<0.01, Student's t-test) at 90 days than Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice. Error bars represent SEM. (C) Mcm4Chaos3/Chaos3 Mcm2Gt/+ MEF proliferation defects are partially rescued by Mcm3 hemizygosity. The effect is significant after 6 days in culture (P<0.05, Student's t-test; Error bars represent SEM). (D) Micronucleus levels in Mcm4Chaos3/Chaos3 mice bearing additional gene trap alleles. At least 5 males were analyzed for each genotype. Error bars represent SEM. Asterisk indicates P<0.05 (student's t-test.) compared to Mcm4Chaos3/Chaos3 alone. (E) CD71+ reticulocyte ratios in mutant male mice. At least 5 animals were analyzed from each class. The samples are identical to those in “D”. All scored cells were anucleate peripheral blood cells Error bars represent SEM. Asterisks and “#” indicate P<0.05 (Student's t-test) when compared to Mcm4Chaos3/Chaos3 and Mcm4Chaos3/Chaos3 Mcm2Gt/+ cohorts, respectively. (F) Mcm2 hemizygosity decreases efficiency of reprogramming Mcm4Chaos3/Chaos3 MEFs into iPS cells, and Mcm3 hemizygosity significantly increases reprogramming efficiency. Two methods of quanitifying reprogramming were used as described in Materials and Methods. “Cell number” refers flow cytometric quantification of LIN28/SSEA1 double positive cells from primary cultures of reprogrammed MEFs. Relative reprogramming efficiencies were normalized to Mcm4Chaos3/Chaos3 MEFs (considered to be 100%). Error bars represent SEM. All samples within quantification class are significantly different from one another (P<0.05, Student's t-test). C3 = Mcm4Chaos3; M = Mcm. The rescue of the reduced growth phenotype by Mcm3 hemizygosity led us to evaluate the proliferation of compound mutant cells. Whereas Mcm4Chaos3/Chaos3 and Mcm4Chaos3/Chaos3 Mcm3Gt/+ primary MEFs proliferated at identical rates, Mcm4Chaos3/Chaos3 Mcm2Gt/+ MEFs showed a severe growth defect beginning ∼5 days in culture (Figure 5C). As with whole animals, MEF growth was partially but significantly rescued by Mcm3 hemizygosity.
Since the Mcm4Chaos3 and Mcm2Gt alleles causes elevated GIN (micronuclei in RBCs), we considered the possibility that the Mcm3 rescue effect might be related to an attentuation of GIN. Accordingly, we measured MN levels in Mcm4Chaos3/Chaos3 mice with different combinations of other Mcm mutations. As shown in Figure 5D, hemizygosity for Mcm2 and Mcm7 caused a significant elevation in MN levels, unlike Mcm3. However, the increased MN in Mcm4Chaos3/Chaos3 Mcm2Gt/+ was not rescued by Mcm3 hemizygosity. This suggests that the synthetic lethality and mouse/cell growth defects are not related to GIN per se. However, in the course of measuring MN in enucleated peripheral blood cells, we noticed that the ratio of CD71+ cells was significantly higher in both Mcm4Chaos3/Chaos3 Mcm2Gt/+ and Mcm4Chaos3/Chaos3 Mcm7Gt/+ mice (3.3 and 6.2 fold, respectively; Figure 5E). This increase in the ratio of reticulocytes (erythrocyte precursors; immature RBCs) to total RBCs is characteristic of anemia. Hemizygosity for Mcm3, which alone had no effect on CD71 ratios of Chaos3 mice, corrected completely this abnormal phenotype in Mcm4Chaos3/Chaos3 Mcm2Gt/+animals (Figure 5E).
Because MCM2-depleted mice were reported to have stem cell defects [20], and Mcm4Chaos3/Chaos3 Mcm#Gt/+ mice had clear developmental abnormalities, we examined the efficiency of reprogramming mutant MEFs into induced pluripotent stem cells (iPS). The efficiency was quantified using either : 1) iPS-like colony formation, or 2) cells counts of SSEA1 and LIN28 positive cells by flow cytometry. Both gave similar results. Mcm4Chaos3/Chaos3 Mcm2Gt/+ cells were severely compromised in the ability to form iPS cells compared to Mcm4Chaos3/Chaos3 (∼200 fold less efficient; Figure 5F). However, additionally reducing Mcm3 by 50% increased iPS formation from both Mcm4Chaos3/Chaos3and Mcm4Chaos3/Chaos3 Mcm2Gt/+ MEFs by ∼2.5 and 10 fold, respectively.
Finally, we found that reduced MCM3 levels could rescue the cancer susceptibility of two different Chaos3 models. As shown earlier (Figure 4), Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice were highly cancer-prone with an average latency of <4 months. When a dose of Mcm3 was removed from mice of this genotype, lifespan was extended dramatically in both sexes as a consequence of delayed cancer onset, and the cancer spectrum shifted from lymphoma/thymoma towards mammary tumors (Figure 6A). Additionally, hemizygosity of Mcm3 delayed (or eliminated) the onset of mammary tumorigenesis in Mcm4Chaos3/Chaos3 females by ∼4 or more months (Figure 6B). However, although Mcm3 hemizygosity rescued viability of Mcm4Chaos3/Gt mice (Figure 5A), these animals were cancer prone with a shorter latency (by ∼6 months) and different spectrum (primarily lymphomas) than Mcm4Chaos3 homozygotes.
Fig. 6. Inhibition of Chaos3 cancers by MCM3 reduction. 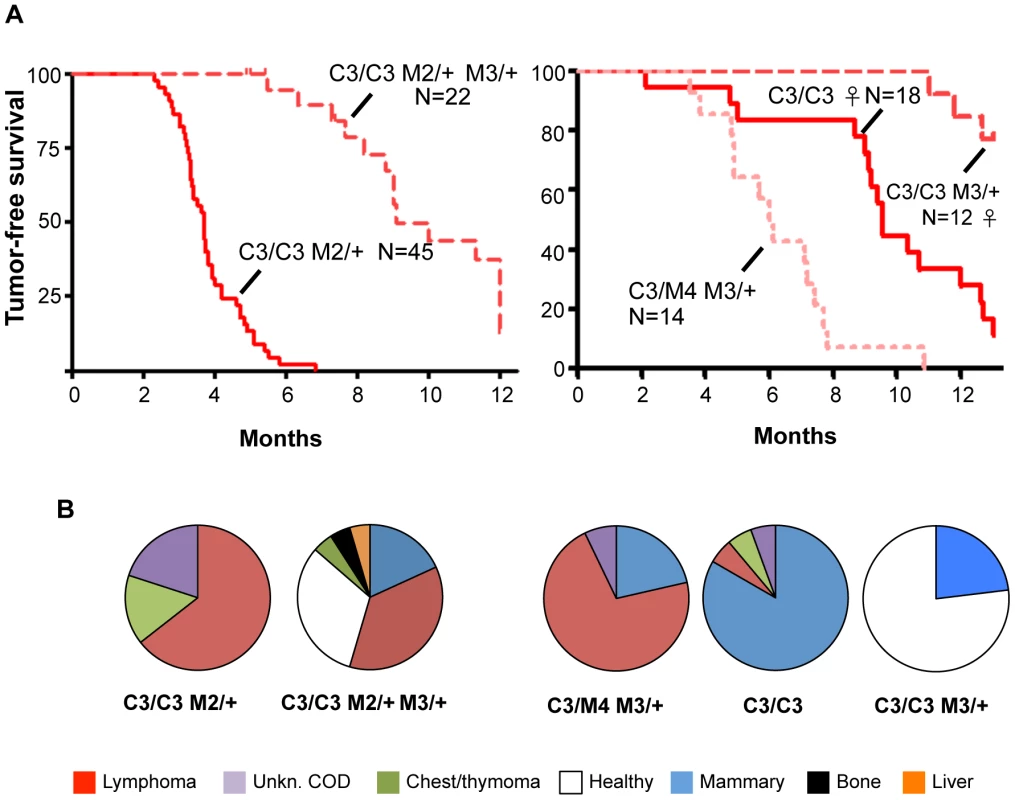
(A) Kaplan-Meier graphs of cohorts of the indicated genotypes. C3 = Mcm4Chaos3; M# = Mcm#Gt. In the left panel, the experiment was terminated at 12 months, with ∼1/3 animals tumor-free and healthy at the time (see “B”). Unless otherwise indicated, the cohorts contained both sexes. (B) Pie charts of cancer types in mice from “A.” COD = cause of death; Unkn = unknown. Classification of cancer types was assigned during necropsy, not from histological analysis. Decreased MCM3 increases chromatin-bound levels of other MCMs
We considered two possibilities to explain the surprising phenotypic rescues of reduced MCM genotypes (Mcm4Chaos3/Chaos3 ; Mcm4Chaos3/Chaos3 Mcm2/6Gt/+ ; Mcm4Chaos3/Gt) by additional MCM3 reduction (Mcm3Gt/+). One is that the phenotypes are related to altered stoichiometry of MCM monomers, and that disproportionally high amounts of MCM3 relative to MCM4 and MCM2/6/7 have a dominant negative effect. However, as demonstrated above, levels of MCM3 are proportionally reduced in Mcm4Chaos3/Chaos3 cells (Figure 1). The second possibility is that decreased levels of MCM3 leads to a favorable change in the amounts or subcellular localization of MCMs. Various experiments have indicated that MCM2-7 hexamers or subcomplexes must be assembled in the cytoplasm before nuclear import in yeast [4], and in mice, nuclear import appears to require MCM2 and MCM3 [24]. MCMs shuttle between the nucleus and cytoplasm during the cell cycle in S. cerevisiae. Although in most other organisms MCMs are reported to be predominantly and constitutively nuclear localized throughout the cell cycle, dynamic redistribution between the nucleus and cytoplasm has been observed in hormonally-treated mouse uterine cells [25]. In budding yeast, nuclear export is dependent upon Mcm3, which has a nuclear export signal (NES) that is recognized by Cdc28 to promote export of MCM2-7 [22]. Analysis of mouse and human MCM3 using NES prediction software (www.cbs.dtu.dk/services/NetNES/) [26] revealed the presence of homologously-positioned, leucine-rich potential NESs (Figure 7A). Therefore, we hypothesized that the rescue of phenotypes by Mcm3 hemizygosity is due to decreased MCM protein export from the nucleus, or alternatively, increased nuclear import or stabilization that allows greater access of all MCMs for licensing chromatin.
Fig. 7. MCM3 regulates nuclear and chromatin-bound MCM levels. 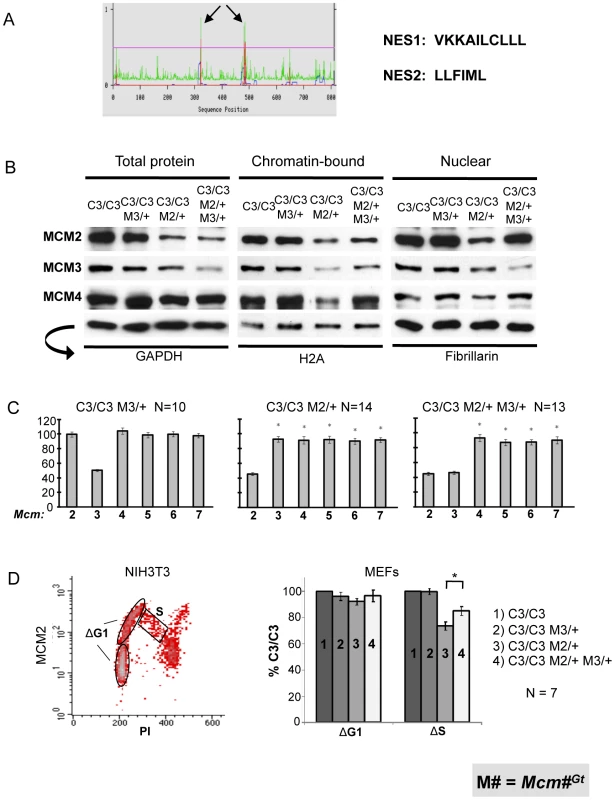
(A) Predicted nuclear export sequences (NES) in mouse MCM3 (see text). (B) Western blot analysis of MCM2/3/4 in the indicated genotypes of MEFs. Three different protein fractions were examined, with the indicated (arrow) loading controls at the bottom. (C) qRT-PCR analysis of Mcm2-7 mRNAs in MEFs of the indicated genotypes. (D) Nuclear MCM2 dynamics during the cell cycle. The flow plot is of isolated nuclei stained for DNA content (PI = propidium iodide) on the X-axis, and MCM2 on the Y-axis. NIH3T3 cells show dramatically the decrease in nuclear MCM2 through S phase. Flow cytometric data from the 4 MEF genotypes indicated in the right panel were used to calculate two values, ΔG1 and ΔS. The regions for the calculation of these values are indicated, and the values plotted in the right panel. The G1 (1N DNA content) phase nuclei were divided into two equal groups based on MCM2 signal intensity (Y-axis): the lower half, considered to be early-G1, and the upper half, considered to be late-G1. The ΔG1 value was calculated as the difference between the early and late MCM2 signal intensity averages. The ΔS value was calculated as : (average MCM2 intensity in the S population) – (early G1 average intensity). C3 = Mcm4Chaos3; M = Mcm. C3 is set at 100%. The asterisk indicates significance by Student's t-test (P<0.05). To explore this hypothesis, we performed Western blot analysis of MCM levels in Mcm4Chaos3/Chaos3 MEFs with or without the Mcm3Gt and/or Mcm2Gt alleles, and examined the effects of Mcm3 dosage on the levels of nuclear and chromatin-bound MCM2 and MCM4. The results are presented in Figure 7B. In all cases, the genetic reductions of Mcm2 and Mcm3 led to corresponding decreases in the cognate mRNA levels (Figure 7C), with only minor additional decreases of other MCM mRNAs (beyond that already caused by homozygosity for Mcm4Chaos3) occuring in the context of Mcm2 hemizygosity (similar to Mcm2Gt/+ MEFs in Figure 2B). The overall levels of total, nuclear, and chromatin-bound MCM2 and MCM4 were unaffected by hemizygosity of Mcm3 in Mcm4Chaos3/Chaos3 cells (Figure 7B). When Mcm2 levels were genetically reduced by half, a condition causing the severe phenotypic effects described earlier, this caused a marked decrease in the level of chromatin-bound MCM3 and MCM4 (in addition to MCM2 itself), although total and nuclear MCM3/4 levels were affected to a lower degree or not at all. Strikingly, the decreased levels of chromatin-bound MCM2/3/4 in Mcm4Chaos3/Chaos3 Mcm2Gt/+ MEFs were reversed by Mcm3 heterozygosity, but levels of total MCM2 and MCM4 were not restored. The increase of chromatin-bound MCMs occured despite the presence of less MCM3, suggesting that MCM3 is present at levels in excess of that needed to bind chromatin, presumably for pre-RC formation in the context of the MCM2-7 hexamer. In conclusion, a 50% reduction in total MCM3 increases MCM2/4 loading onto chromatin when MCM2 is otherwise limiting, and this rescue is associated with amelioration of several phenotypes.
We found that elevation of nuclear MCMs in the Mcm3Gt/+ MEFs was often (as shown in Figure 7B), but not consistently elevated across samples by Western analysis (not shown). Therefore, we quantified MCM2 during the cell cycle by flow cytometric analysis of nuclei from 7 replicate MEF cultures. Similar to WT MEFs (examples in Figure 1B), NIH3T3 cells showed a decrease of nuclear MCM2 during S phase progression (Figure 7D, left panel). However, all genotypes with in the Mcm4Chaos3/Chaos3 background had a reduced decline. Thus, for comparative quantitation across genotypes, we compared the levels of MCM2 levels at the beginning of G1 vs. that in S phase (regions used for these calculations are indicated in the left panel), using the calculation described in the Figure 7 legend. The data are graphed in the right panel. The data revealed that regardless of genotype, the difference in average amounts of nuclear MCM2 at the beginning and end of G1 (ΔG1) did not vary. Compared to Mcm4Chaos3/Chaos3, cells lacking 1 dose of Mcm2 had relatively lower levels of S phase MCM2 (ΔS) compared to early G1. Additional removal of an Mcm3 dose partially rescued the ΔS value, indicating that these cells had ∼16% more nuclear MCM2 in S phase compared to Mcm4Chaos3/Chaos3 cells hemizygous for Mcm2 alone, despite overall reduced MCM2 levels in the cell (Figure 7B, left panel).
Discussion
MCM2-7 proteins exist abundantly in proliferating cells and are bound to chromatin in amounts exceeding that required to license all replication origins that initiate DNA synthesis [9]–[12], [14]. The role of excess chromatin-bound MCM2-7 has been a mystery referred to as the “MCM paradox” [27], perpetuated by observations that drastic MCM reductions in certain systems can be compatible with normal DNA replication or cell proliferation [13], [28]–[30]. However, these circumstances are not universal, and reductions are not entirely without consequences. Early studies showed that a reduction in MCMs resulted in decreased usage of certain ARSs [12] and conferred genome instability [31] in yeasts. In cell culture systems, depletion of certain MCMs have been found to cause cell cycle defects, checkpoint abberations and GIN [13], [16]–[17], [29], [32].
Recent work has shed light on aspects of the MCM paradox. Using Xenopus egg extracts attenuated for licensing by addition of geminin (an inhibitor of CDT1, which is required for MCM loading onto origins), one study proposed that excess chromatin-bound MCM2-7 complexes license “dormant” origins that can be activated to rescue stalled or damaged replication forks, a situation that can become important under conditions of replication stress [11]. Similar results were subsequently reported for human cells depleted of MCMs by siRNA [15]–[16], and for replication stressed MCM2-deficient MEFs [21]. Our finding that nuclear MCM2 levels decrease as S-phase progresses, and moreso in WT than in Mcm4Chaos3/Chaos3 MEFs, is consistent with the dormant origin hypothesis. The decrease may reflect displacement of dormant hexamers by active replisomes, followed by subsequent degradation or nuclear export. If WT nuclei have more dormant licensed origins than Chaos3 mutants, then WT cells would be expected show a greater loss of MCMs.
The isolation of Mcm4Chaos3 provided the first demonstration that mutant alleles of essential replication licensing proteins can cause GIN and cancer [17]. Diploid budding yeast containing the same amino acid change in scMcm4 as the mouse Mcm4Chaos3 exhibited Rad9-dependent G2/M delay (Rad9 is a DNA damage checkpoint protein), elevated mitotic recombination, chromosome rearrangements, and intralocus mutations [19] (Li, X. and Tye, B., personal communication). One explanation for these outcomes is that the Chaos3 mutation impairs MCM4 biochemically in a manner leading to elevated replication fork defects, and that these defects lead to the GIN and cancer phenotypes. Alternatively, and/or in addition, the observed associated pan-reductions of MCMs in mouse cells [17] raised the possibility that decreased replication licensing might be the primary or ancillary cause for the mouse phenotypes.
The subsequent finding that mice (Mcm2IRES-CreERT) containing ∼1/3 the normal level of MCM2 had GIN and and cancer lent support for the idea that reductions in MCMs contribute to the Chaos3 phenotypes [20]. Although amounts of all MCMs were not investigated in Mcm2IRES-CreERT/IRES-CreERT mice, 65% reduction of MCM2 caused a reduction of dormant replication origins in MEFs that were replication stressed by hydroxyurea [21]. In Mcm4Chaos3/Chaos3 mice, we hypothesize that in the context of Mcm2, 6 or 7 heterozygosity, which further reduces overall and chromatin-bound MCM levels below that already caused by Mcm4Chaos3 (measured to be <20% of WT mRNA levels for Mcm2), MCMs are reduced to a degree that compromises cell proliferation. This then translates into the various developmental defects and increased cancer susceptibility we observed. Whatever the exact mechanistic cause of these phenotypes, it is clear that the phenotypes are related to reduction of one or more MCMs below a threshold level that is <50%. The severe developmental consequences of MCM depletion in mice suggests that certain cell types in the developing embryo are highly sensitive to the effects of replicative stress, and/or that relatively minor cell growth perturbations of such cells are not well-tolerated in the context of complex, rapidly-occuring developmental events. The molecular basis for these phenotypes does not appear to be directly related to GIN, because whereas Mcm3 hemizygosity rescued several phenotypes, and delayed cancer latency in Mcm4Chaos3/Chaos3 mice, it did not concommitantly decrease MN. This suggests that phenotypes such as decreased proliferation and embryonic death are caused by genetically-induced replication stress, moreso (or in addition to) than GIN alone.
Our genetic studies indicate that there is a quantitative MCM threshold required for embryonic viability, as demonstrated by the synthetic lethalities we observed when combining homozygosity of Mcm4Chaos3 with Mcm2Gt, Mcm6Gt or Mcm7Gt heterozygosity, but not in the heterozygous single mutants. Additionally, the Mcm4Chaos3/Gt genotype, which reduced MCM levels below 50%, caused embryonic and neonatal lethality [17]. Underscoring the exquisite sensitivity of whole animals to subtle perturbations in the DNA replication machinery were the remarkable phenotypic rescues (viability, growth, iPS efficiency, etc.) by Mcm3 hemizygosity. The decreased MCM dosage led to increases in S phase nuclear MCMs and chromatin-bound MCMs, presumably reflecting increased replication origin formation. The various single and compound mutants described here and elsewhere [20], which show that 50% reductions of any one MCM is well-tolerated but decreases of ∼2/3 are not, supports the idea of a threshold effect, and suggests that the threshold lies somewhere between 1/3 and 1/2 of normal MCM levels (at least in the cases of MCM2, MCM6 and MCM7).
These results also emphasize the importance of relevant physiological models, both in general and with respect to the MCMs. RNAi knockdown of MCM3 in human cells to ∼3% normal levels was still compatible with normal short-term proliferation, although the cells had GIN and high sensitivity to replication stress [16]. It is doubtful such a drastic situation would be recapitulated in vivo (it would likely result in embryonic lethality as in Mcm3Gt/Gt mice). Nevertheless, it is noteworthy in that study that MCM3 depletion was better tolerated than knockdowns of any other member of the replicative helicase.
The finding that reductions in MCM3 rescued MCM2/4/6 depletion phenotypes lends insight into dynamics and regulation of mammalian DNA replication. In budding yeast, MCMs shuttle between the nucleus and cytoplasm during the cell cycle. MCM2-7 multimers must be assembled in the cytoplasm before being imported into the nucleus during G1 phase [4]. The MCM2-7 importation is dependent upon synergistic nuclear localization signals (NLS) on Mcm2 and Mcm3 [22]. In order to prevent over-replication of the genome, MCMs are exported from the nucleus during S, G2 and M [4]. This export is dependent upon Mcm3, which has a nuclear export signal (NES) that is recognized by Cdc28 to promote MCM2-7 export in a Crm1-dependent manner [22].
In contrast to budding yeast, MCMs that have been studied (MCM2/3/7) are primarily nuclear-localized throughout the cell cycle in metazoans and in fission yeast [4]. Upon dissociation from chromatin during S phase, MCM2-7 complexes are reported to remain in the nucleus but are sequestered via attachement to the nuclear envelope or other nuclear structures [24], [33]–[35]. Interestingly, mcm mutations in fission yeast that disrupt intact MCM2-7 heterohexamers triggers active redistribution of MCMs to the cytoplasm [36]. Additionally, re-distribution of MCMs between the cytoplasmic and nuclear compartments has been observed in hormonally-treated mouse uterine cells [25].
Our observations support the idea that intracellular re-distribution of MCMs also occurs in mammals, and that it is an important regulatory process. Staining of MCM2 in intact nuclei of normal NIH 3T3 fibroblasts and MEFs show a steady decline (but not elimination) as S phase progresses. Furthermore, it appears that the process of nuclear MCM2 elimination during S phase is regulated, since in situations of decreased MCMs (as in the Mcm4Chaos3/Chaos3 mutant), there is decreased loss of nuclear MCM2 during S phase.
Three lines of experimentation implicate MCM3 as playing a key role in regulating intracellular MCM localization: 1) Rescue of reduced-MCM phenotypes by genetic reduction of MCM3; 2) Increased S-phase nuclear MCM2 by Mcm3 hemizygosity in MCM-depleted cells (Figure 7D); and increased chromatin-bound MCM2/4 by Mcm3 hemizygosity in MCM-depleted cells. Our data suggests that MCM3 acts as a negative regulator that prevents re-assembly or reloading of MCM complexes as they dissociate from DNA during replication. As described earlier, mouse and human MCM3 have predicted NESs in similar positions of their primary amino acid sequences as do the yeast genes. Thus, one explanation for these phenomena is that decreased MCM3 suppresses MCM2-7 nuclear export, which occurs normally and which may be accentuated by the Chaos3 mutation in a fashion analogous to mcm mutant fission yeast discussed above [36]. This would effectively increase the amounts of MCMs available for replication licensing. More work is required to determine if the rescue mechanism is indeed related to a decrease in MCMs export, as opposed to direct or indirect involvement in other events such as increased nuclear import or enhanced chromatin loading.
With respect to the early lymphoma susceptibility phenotype in Mcm4Chaos3/Chaos3 Mcm2Gt/+ mice, it is unclear whether the type of tumor is dictated primarily by the particular Mcm depletion (in this case MCM2, thus resembling Mcm2IRES-CreERT2/IRES-CreERT2 animals), the genetic background, or the age of particular cancer onset (if animals die of thymic lymphoma at an early age, they will be unable to manifest later-arising mammary tumors). The compound mutant mice used for the aging aspects of this study were bred to at least the N3 generation in strain C3H. Mcm4Chaos3/Chaos3 mice congenic in this background are predisposed exclusively to mammary tumors, whereas lymphomas were observed in mutants of mixed background [17]. Presently, we favor the idea that genetic background and age of tumor type onset are primary determinants of the cancers that arise in the mice we have studied thus far. Genetic background has also been reported to influence tumor latency in MCM2-deficient mice [21].
The MCM2-7 pan-reduction in Chaos3 cells is consistent with other studies involving mutation or knockdown of a single MCM in mammalian cells [16], [20], [29], [37]. In these examples of parallel MCM decreases, the general assumption is that there is hexamer destabilization or impaired MCM chromatin loading followed by degradation of monomers. However, we found that the protein decreases are related to decreased mRNA levels. These large (∼40%) decreases do not appear to be attributable to transcriptional alterations from cell cycle disruptions (these cells have a small elevation in the G2/M population), but rather occur at the post-transcriptional level (unpublished observations). Since we also found that MEFs carrying only 1 functional Mcm2 allele caused ∼20% decreases of Mcm3-7 mRNAs, it is possible that mRNA downregulation drove MCM reductions in these other model systems. However, the mechanism for coordinated mRNA regulation, and what triggers it, is a mystery that we are currently investigating.
Our data contribute to a growing body of data that replication stress, which can occur via perturbations of the DNA replication machinery, plays a significant role in driving cancer [38]–[41]. While the Mcm4Chaos3 mutation is an unique case, the deleterious consequences of MCM reductions suggest that genetically-based variability in DNA replication factors can have physiological consequences. Such variability in functions or levels may be caused by Mendelian mutations or multigenic allele interactions. Mutations affecting transcriptional activity of one or more Mcms, which might occur in non-coding cis-linked sequences or unlinked transcription factors, could have such effects. This has implications for cancer genome resequencing projects, whereby such mutations would not be obviously associated with MCM expression. The allelic collection we generated, when used alone or in combination with each other or Mcm4Chaos3/Chaos3 mice, allow the generation of mouse models with a graded range of MCM levels. These should be valuable for investigations into the impact of replication stress on animal development, cancer formation, and cellular homeostasis.
Materials and Methods
MEF culture and proliferation assays
MEFs from 12.5 - to 14.5-dpc embryos were cultured in DMEM+10% FBS, 2 mM GlutaMAX, and penicillin-streptomycin (100 units/ml). Assays were conducted on cells at early passages (up to P3). For cell proliferation assays, 5×104 cells were seeded per well of a 6 well plate. They were then cultured and harvested at the indicated time points to perform cell counts.
iPS induction from MEFs using lentiviral vectors
Doxycycline inducible lentiviral vectors [42] were prepared by co-transecting viral packaging plasmids psPAX2 and and pMD2.G along with vectors encoding rtTA, Oct4, Sox2, Klf4, or c-Myc (plasmids were obtained from Addgene.org, serial numbers 12259, 12260, 20323, 20322, 20324, and 20326) into 293T cells using TransIT-Lt1 transfection reagent (Mirus). Viral supernatants were collected at 48 and 72 hours, and concentrated using a 30kd NMWL centrifugal concentrator. MEFs from 13.5d embryos, up to P3, were seeded to gelatin coated tissue plates at a density of 6.75×103 cells/cm2 and allowed to attach in standard MEF media for 24 hours before infection with lentiviral vectors. After 24 hours incubation the culture media was changed to KO-DMEM supplemented with 15% KO serum replacement (Gibco), recombinant LIF, 2 µg/mL doxycycline (Sigma), 100 µm MEM non-essential amino acids solution, 2mM GlutaMax, 100 units/mL penicillin and 100 µg/mL streptomycin (Gibco). The induction media was refreshed daily for 13 days until the cells were passaged to 100 mm plates prepared with irradiated feeders. Cells were cultured for an additional 10 days in the induction media in the absence of doxycyline before iPS colony counting, cell counts, and flow cytometry.
For flow cytometric quantification of iPS cells derived from reprogramming of MEFs, ∼1×106 cells were trypsinized for 10 minutes, then washed twice with cold PBS. They were gently but completely resuspended in 1ml of 4% paraformaldehyde in PBS at room temperature for 30 minutes. The fixed cells were pelleted by centrifugation at 500×G for 2 minutes and washed twice with 10 ml TBS-TX (0.1% Triton X-100) buffer. For antibody staining, the cells were blocked with 1ml TBS-TX buffer with 1% BSA for 15 min at room temperature, then stained with primary “stemness” antibodies (monoclonal anti-SSEA1, Millipore; rabbit polyclonal anti-LIN28, Abcam) for 60 min, washed twice, then secondary antibody was applied for 60 minutes. Immunolabeled cells were analyzed by flow cytometry using a 488nm laser. Secondary antibodies were goat anti-mouse IgG-FITC (South Biotech) and goat anti-rabbit IgG-594 (Molecular Probes). Cells were considered to be iPS cells if they were LIN28/SSEA1 positive. Calibration of the flow cytometer and gates were set using untransfected MEFs as negative controls, and v6.4 ES cells as positive controls.
For quantification by colony formation, plates containing the passaged reprogrammed cells were examined microscopically at 20×, and 4 fields were scored and averaged. Colonies were considered as iPS clones based on morphological criteria: well defined border, three-dimensionality, and tight packing of cells.
Flow cytometric analyses of micronuclei and iPS cells
Micronucleus assays, which include CD71 staining, were performed essentially as described [43].
Nuclei isolation and immunofluorescence staining
MEFs were plated at 4×106 cells/150 mm culture dish for 60 hr, trypsinized, then resuspended in 1ml PBS. To the suspension was added TX-NE (320 mM sucrose, 7.5 mM MgCl2, 10 mM HEPES, 1% Triton X-100, and a protease inhibitor cocktail). The cells were gently vortexed for 10 seconds and incubated on ice for 30 min. Dounce homogenization was unnecessary. Nuclei were then pelleted by centrifugation at 500×G for 2 min and washed twice with 10 ml TX-NE, then resuspended in 1ml TX-NE. Nuclei yield and integrity was monitored microscopically with trypan blue staining. The nuclei were fixed by adding 15ml cold methanol for 60 min on ice. The fixed nuclei were pelleted by centrifugation at 500×G for 2 min, then washed twice with 10 ml TBS-TX (0.1% TX-100). 1×106 nuclei were placed into 1.5ml tubes in 1ml TBS-TX buffer+1% BSA for 15 min at room temperature. The primary antibody (Rabbit anti-mouse MCM2) was added for 60 min, then secondary antibody (FITC goat anti-rabbit) was added for 60 min. Finally, the nuclei were stained with propidium iodide (PI), and RNAse treated (batches optimized empirically) for 30 mins. Immunolabeled nuclei were analyzed by flow cytometry (using a BD FACSCalibur cytometer with CellQuest software), exciting the PI and FITC with a 488nm laser.
Generation and validation of mouse lines bearing mutant Mcm alleles
ES cell lines containing gene trap insertions in Mcm genes were obtained from Bay Genomics [Mcm3 (RRR002), Mcm6 (YHD248), Mcm7 (YTA285)] or the Sanger Institute [Mcm2 (ABO178)]. The Mcm4 line was previously reported [17]. Allele names are abbreviated as, for example, Mcm3Gt instead of the full name Mcm3Gt(RRR002)Byg. All of the original ES cells were of strain 129 origin, and the alleles were backcrossed into C3HeB/FeJ for ≥4 generations.
To identify the exact insertion sites of the gene trap vectors, a “primer walking” procedure was used. This involved priming PCR reactions with :1) a fixed vector primer, and 2) one of a series of primers series corresponding to the intron in which the vector presumably integrated. PCR products were then sequenced. Genotyping of gene-trap-bearing mice was performed either by PCR amplification of the neomycin resistance gene within the vector, or by using insertion-specific assays (Table S2).
Western blot analysis
Cytosolic and chromatin-bound protein was extracted as described [44]. Antibody binding was detected with a Pierce ECL kit. Band were quantified using NIH Image J software. Antibodies - aMCM2: ab31159 (Abcam); aMCM3 : 4012 (Cell Signaling); aMCM4: ab4459 (Abcam); aMCM5: NB100-78261 (Novus); aMCM6: NB100-78262 (Novus); aMCM7: ab2360 (Abcam); aBeta-actin: A1978 (Sigma); aTBP: NB500-700 (Novus).
Quantitative RT-PCR (qPCR)
Total RNA from P1 MEFs was DNAse I treated, then cDNA was synthesized from 1 µg of total RNA using the Invitrogen SuperScript III ReverseTranscriptase kit with the supplied Olige-dT or random-hexamer primers. qPCR reactions were performed in triplicate on 1 ng or 10 ng of cDNA by using the SYBR power green RT-PCR Master kit (Applied Biosystems; 40 cycles at 95°C for 10 s and at 60°C for 1 min), and real-time detection was performed on an ABI PRISM 7300 and analyzed with Geneamp 5700 software. The specificity of the PCR amplification procedures was checked with a heat-dissociation step (from 60°C to 95°C) at the end of the run and by gel electrophoresis. Results were standardized to β-actin. The PCR primers are listed in Table S1.
Supporting Information
Zdroje
1. GilbertDM
2001 Making sense of eukaryotic DNA replication origins. Science 294 96 100
2. BlowJJ
DuttaA
2005 Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6 476 486
3. LeiM
2005 The MCM complex: its role in DNA replication and implications for cancer therapy. Curr Cancer Drug Targets 5 365 380
4. ForsburgSL
2004 Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev 68 109 131
5. TyeBK
1999 MCM proteins in DNA replication. Annu Rev Biochem 68 649 686
6. MoyerSE
LewisPW
BotchanMR
2006 Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A 103 10236 10241
7. LabibK
TerceroJA
DiffleyJF
2000 Uninterrupted MCM2-7 function required for DNA replication fork progression. Science 288 1643 1647
8. BochmanML
SchwachaA
2008 The Mcm2-7 complex has in vitro helicase activity. Mol Cell 31 287 293
9. StoeberK
TlstyTD
HapperfieldL
ThomasGA
RomanovS
2001 DNA replication licensing and human cell proliferation. J Cell Sci 114 2027 2041
10. EdwardsMC
TutterAV
CveticC
GilbertCH
ProkhorovaTA
2002 MCM2-7 complexes bind chromatin in a distributed pattern surrounding the origin recognition complex in Xenopus egg extracts. J Biol Chem 277 33049 33057
11. WoodwardAM
GohlerT
LucianiMG
OehlmannM
GeX
2006 Excess Mcm2-7 license dormant origins of replication that can be used under conditions of replicative stress. J Cell Biol 173 673 683
12. LeiM
KawasakiY
TyeBK
1996 Physical interactions among Mcm proteins and effects of Mcm dosage on DNA replication in Saccharomyces cerevisiae. Mol Cell Biol 16 5081 5090
13. CrevelG
HashimotoR
VassS
SherkowJ
YamaguchiM
2007 Differential requirements for MCM proteins in DNA replication in Drosophila S2 cells. PLoS ONE 2 e833
14. YoungMR
TyeBK
1997 Mcm2 and Mcm3 are constitutive nuclear proteins that exhibit distinct isoforms and bind chromatin during specific cell cycle stages of Saccharomyces cerevisiae. Mol Biol Cell 8 1587 1601
15. GeX
JacksonDA
BlowJJ
2007 Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev 21 3331 3341
16. IbarraA
SchwobE
MendezJ
2008 Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc Natl Acad Sci U S A 105 8956 8961
17. ShimaN
AlcarazA
LiachkoI
BuskeTR
AndrewsCA
2007 A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet 39 93 98
18. FletcherRJ
BishopBE
LeonRP
SclafaniRA
OgataCM
2003 The structure and function of MCM from archaeal M. Thermoautotrophicum. Nat Struct Biol 10 160 167
19. LiX
SchimentiJ
TyeB
2009 Aneuploidy and improved growth are coincident but not causal in a yeast cancer model. PLoS Biology 7 e1000161
20. PruittSC
BaileyKJ
FreelandA
2007 Reduced Mcm2 expression results in severe stem/progenitor cell deficiency and cancer. Stem Cells 25 3121 3132
21. KunnevD
RusiniakME
KudlaA
FreelandA
CadyGK
DNA damage response and tumorigenesis in Mcm2-deficient mice. Oncogene
22. LikuME
NguyenVQ
RosalesAW
IrieK
LiJJ
2005 CDK phosphorylation of a novel NLS-NES module distributed between two subunits of the Mcm2-7 complex prevents chromosomal rereplication. Mol Biol Cell 16 5026 5039
23. ShimaN
BuskeTR
SchimentiJC
2007 Genetic screen for chromosome instability in mice: Mcm4 and breast cancer. Cell Cycle 6 1135 1140
24. KimuraH
OhtomoT
YamaguchiM
IshiiA
SugimotoK
1996 Mouse MCM proteins: complex formation and transportation to the nucleus. Genes Cells 1 977 993
25. PanH
DengY
PollardJW
2006 Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A 103 14021 14026
26. la CourT
KiemerL
MolgaardA
GuptaR
SkriverK
2004 Analysis and prediction of leucine-rich nuclear export signals. Protein Eng Des Sel 17 527 536
27. HyrienO
MarheinekeK
GoldarA
2003 Paradoxes of eukaryotic DNA replication: MCM proteins and the random completion problem. Bioessays 25 116 125
28. TsaoCC
GeisenC
AbrahamRT
2004 Interaction between human MCM7 and Rad17 proteins is required for replication checkpoint signaling. Embo J 23 4660 4669
29. CortezD
GlickG
ElledgeSJ
2004 Minichromosome maintenance proteins are direct targets of the ATM and ATR checkpoint kinases. Proc Natl Acad Sci U S A 101 10078 10083
30. OehlmannM
ScoreAJ
BlowJJ
2004 The role of Cdc6 in ensuring complete genome licensing and S phase checkpoint activation. J Cell Biol 165 181 190
31. LiangDT
HodsonJA
ForsburgSL
1999 Reduced dosage of a single fission yeast MCM protein causes genetic instability and S phase delay. J Cell Sci 112(Pt 4) 559 567
32. Ekholm-ReedS
MendezJ
TedescoD
ZetterbergA
StillmanB
2004 Deregulation of cyclin E in human cells interferes with prereplication complex assembly. J Cell Biol 165 789 800
33. MadineMA
KhooCY
MillsAD
MusahlC
LaskeyRA
1995 The nuclear envelope prevents reinitiation of replication by regulating the binding of MCM3 to chromatin in Xenopus egg extracts. Curr Biol 5 1270 1279
34. KimuraH
NozakiN
SugimotoK
1994 DNA polymerase alpha associated protein P1, a murine homolog of yeast MCM3, changes its intranuclear distribution during the DNA synthetic period. Embo J 13 4311 4320
35. FujitaM
KiyonoT
HayashiY
IshibashiM
1996 hCDC47, a human member of the MCM family. Dissociation of the nucleus-bound form during S phase. J Biol Chem 271 4349 4354
36. PasionSG
ForsburgSL
1999 Nuclear localization of Schizosaccharomyces pombe Mcm2/Cdc19p requires MCM complex assembly. Mol Biol Cell 10 4043 4057
37. LinDI
AggarwalP
DiehlJA
2008 Phosphorylation of MCM3 on Ser-112 regulates its incorporation into the MCM2-7 complex. Proc Natl Acad Sci U S A 105 8079 8084
38. BartkovaJ
HorejsiZ
KoedK
KramerA
TortF
2005 DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434 864 870
39. GorgoulisVG
VassiliouLV
KarakaidosP
ZacharatosP
KotsinasA
2005 Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434 907 913
40. Di MiccoR
FumagalliM
CicaleseA
PiccininS
GaspariniP
2006 Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444 638 642
41. BartkovaJ
RezaeiN
LiontosM
KarakaidosP
KletsasD
2006 Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444 633 637
42. BrambrinkT
ForemanR
WelsteadGG
LengnerCJ
WernigM
2008 Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2 151 159
43. ReinholdtL
AshleyT
SchimentiJ
ShimaN
2004 Forward genetic screens for meiotic and mitotic recombination-defective mutants in mice. Methods Mol Biol 262 87 107
44. FujitaM
KiyonoT
HayashiY
IshibashiM
1997 In vivo interaction of human MCM heterohexameric complexes with chromatin. Possible involvement of ATP. J Biol Chem 272 10928 10935
Štítky
Genetika Reprodukční medicína
Článek Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator ofČlánek Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline AnalysisČlánek Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoAČlánek Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.Článek Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 9
-
Všechny články tohoto čísla
- Optimal Strategy for Competence Differentiation in Bacteria
- Mutational Patterns Cannot Explain Genome Composition: Are There Any Neutral Sites in the Genomes of Bacteria?
- Frail Hypotheses in Evolutionary Biology
- Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits
- Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator of
- Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline Analysis
- Germline Variation Controls the Architecture of Somatic Alterations in Tumors
- Mice Doubly-Deficient in Lysosomal Hexosaminidase A and Neuraminidase 4 Show Epileptic Crises and Rapid Neuronal Loss
- Analysis of Population Structure: A Unifying Framework and Novel Methods Based on Sparse Factor Analysis
- FliO Regulation of FliP in the Formation of the Flagellum
- Cdc20 Is Critical for Meiosis I and Fertility of Female Mice
- dMyc Functions Downstream of Yorkie to Promote the Supercompetitive Behavior of Hippo Pathway Mutant Cells
- DCAF26, an Adaptor Protein of Cul4-Based E3, Is Essential for DNA Methylation in
- Genome-Wide Double-Stranded RNA Sequencing Reveals the Functional Significance of Base-Paired RNAs in
- An Immune Response Network Associated with Blood Lipid Levels
- Genetic Variants and Their Interactions in the Prediction of Increased Pre-Clinical Carotid Atherosclerosis: The Cardiovascular Risk in Young Finns Study
- The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoA
- Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
- Orphan CpG Islands Identify Numerous Conserved Promoters in the Mammalian Genome
- Analysis of the Basidiomycete Reveals Conservation of the Core Meiotic Expression Program over Half a Billion Years of Evolution
- ETS-4 Is a Transcriptional Regulator of Life Span in
- The SR Protein B52/SRp55 Is Required for DNA Topoisomerase I Recruitment to Chromatin, mRNA Release and Transcription Shutdown
- The Baker's Yeast Diploid Genome Is Remarkably Stable in Vegetative Growth and Meiosis
- Chromatin Landscape Dictates HSF Binding to Target DNA Elements
- The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice
- Accurately Assessing the Risk of Schizophrenia Conferred by Rare Copy-Number Variation Affecting Genes with Brain Function
- Widespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
- The Characterization of Twenty Sequenced Human Genomes
- The Genome of a Pathogenic : Cooptive Virulence Underpinned by Key Gene Acquisitions
- A Single Element Maintains Repression of the Key Developmental Regulator
- Identification of New Genetic Risk Variants for Type 2 Diabetes
- Effect of Correlated tRNA Abundances on Translation Errors and Evolution of Codon Usage Bias
- Evidence of Selection upon Genomic GC-Content in Bacteria
- Proteomic Changes Resulting from Gene Copy Number Variations in Cancer Cells
- Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.
- Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study
- Response to Mechanical Stress Is Mediated by the TRPA Channel Painless in the Heart
- DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
- Incremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
- Loss of Maternal ATRX Results in Centromere Instability and Aneuploidy in the Mammalian Oocyte and Pre-Implantation Embryo
- Comparative Genomic Hybridization (CGH) Reveals a Neo-X Chromosome and Biased Gene Movement in Stalk-Eyed Flies (Genus )
- Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
- Gene–Environment Interactions at Nucleotide Resolution
- Dementia Revealed: Novel Chromosome 6 Locus for Late-Onset Alzheimer Disease Provides Genetic Evidence for Folate-Pathway Abnormalities
- Critical Functions of Rpa3/Ssb3 in S-Phase DNA Damage Responses in Fission Yeast
- Preferential Re-Replication of Heterochromatin in the Absence of Geminin
- The Potential for Enhancing the Power of Genetic Association Studies in African Americans through the Reuse of Existing Genotype Data
- Evidence That Mutation Is Universally Biased towards AT in Bacteria
- Perturbation Analysis of Heterochromatin-Mediated Gene Silencing and Somatic Inheritance
- Diversity of Eukaryotic DNA Replication Origins Revealed by Genome-Wide Analysis of Chromatin Structure
- Genetic Deletion of the Desmosomal Component Promotes Tumor Microinvasion in a Mouse Model of Pancreatic Neuroendocrine Carcinogenesis
- The Metabolic Enzyme ManA Reveals a Link between Cell Wall Integrity and Chromosome Morphology
- SNPs Associated with Cerebrospinal Fluid Phospho-Tau Levels Influence Rate of Decline in Alzheimer's Disease
- Synthesizing and Salvaging NAD: Lessons Learned from
- A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis
- An Insect Herbivore Microbiome with High Plant Biomass-Degrading Capacity
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Synthesizing and Salvaging NAD: Lessons Learned from
- Optimal Strategy for Competence Differentiation in Bacteria
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



