-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
Vertically-transmitted facultative bacterial endosymbionts are common in invertebrates, and affect traits as diverse as the mode of sexual reproduction, speciation, and susceptibility to pathogens. Horizontal transmission of endosymbionts is thought to be infrequent in most species, and not to contribute to their spread through populations. Here we demonstrate that parasitoid wasps can act as vectors, transmitting the endosymbiont Wolbachia between whitefly hosts at a high rate. The ovipositors and mandibles of parasitoids can be contaminated with Wolbachia when probing infected whitefly. If these parasitoids then probe Wolbachia-free hosts and the whitefly survive, it will result in a stably infected line with increased fitness. Such vector-borne transmission may explain why endosymbionts are so widely distributed, and why genetically similar symbionts are often found in phylogenetically distant organisms.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004672
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004672Summary
Vertically-transmitted facultative bacterial endosymbionts are common in invertebrates, and affect traits as diverse as the mode of sexual reproduction, speciation, and susceptibility to pathogens. Horizontal transmission of endosymbionts is thought to be infrequent in most species, and not to contribute to their spread through populations. Here we demonstrate that parasitoid wasps can act as vectors, transmitting the endosymbiont Wolbachia between whitefly hosts at a high rate. The ovipositors and mandibles of parasitoids can be contaminated with Wolbachia when probing infected whitefly. If these parasitoids then probe Wolbachia-free hosts and the whitefly survive, it will result in a stably infected line with increased fitness. Such vector-borne transmission may explain why endosymbionts are so widely distributed, and why genetically similar symbionts are often found in phylogenetically distant organisms.
Introduction
Vertically transmitted intracellular bacteria often live in symbioses with their arthropod hosts [1]. These endosymbionts may be obligate (essential for host survival) or facultative, in which case they can increase or decrease host fitness [1–3]. When endosymbionts are obligate they typically share a long evolutionary history with their hosts and are found within specialized cells [4]. The facultative endosymbionts tend to have a more recent association with arthropods, but are nonetheless very common among arthropods. For instance, 40% of insect species are estimated to be infected with Wolbachia [5]. Similar to obligate endosymbionts, facultative endosymbionts are also transmitted vertically from mother to offspring with high fidelity and this is considered the primary transmission pathway [4]. However, through their evolution these endosymbionts have also been transmitted horizontally between different species [1,6,7], with closely related endosymbionts occurring in phylogenetically distant insects [1,7–12]. In the last two decades there have been multiple studies reporting evidence of Wolbachia transmission within and between both phylogenetically close and more distant species either through phylogenetic or transinfection studies [8,13–18].
The spread of endosymbionts in field populations by horizontal transmission, however, has received comparatively little attention, and the mechanisms driving horizontal transmission are only recently becoming apparent [1,3]. Horizontal transmission of symbionts has been documented when infected and uninfected parasitoid wasps develop within the same host insect [19–21], when infected males mate with uninfected females [22], when infected and uninfected whiteflies feed on the same host plant and the symbiont moves through the phloem sap [23], and when symbionts are acquired from the environment [24]. It is also possible for endosymbionts to be transmitted by vectors. Hamiltonella defensa and Regiella insecticola symbionts can be efficiently transmitted when a parasitoid wasp sequentially stabs an infected then an uninfected aphid [25]. Similarly, Spiroplasma can be transmitted between Drosophila species by ectoparasitic mites [26]. Here we report the efficient phoretic transfer of Wolbachia by the parastoid Eretmocerus sp. nr. furuhashii from infected whitefly Bemisia tabaci AsiaII7 to uninfected individuals..
The whitefly Bemisia tabaci is a small hemipterous insect that feeds on phloem sap of numerous host plants. It is currently considered as a complex of at least 24 distinct cryptic species that are morphologically indistinguishable but markedly differ in host range, ability to transmit viruses, insecticide resistance and the endosymbionts they are infected with [27–30]. The B. tabaci species complex harbours various bacterial symbionts, including Wolbachia. Wolbachia has been found in the ovarian cells of the host and at the circumference of and inside the bacteriocytes [31]. The AsiaII7 B. tabaci is indigenous to China and is one of the common cryptic species in South China (formerly “Cv” biotype) [32]. It was first discovered on variegated laurel Codiaeum variegatum in Guangzhou in 2006, and further studies indicated that this whitefly can damage various economically important ornamental plants [33]. Eretmocerus sp. nr. furuhashii is one of the dominant parasitoids of whitefly B. tabaci in South China [34,35]. It is a primary, solitary parasitoid which oviposits externally between the nymphal host and the leaf surface, but nonetheless penetrates whitefly nymphs with its mouth parts and ovipositor to examine them before laying and to feed.
The current study was motivated by a six-year long observation from 2007–2012 of two subcolonies, one for rearing whitefly B. tabaci AsiaII7 (hereafter “WR”, 5 cages) and the other for rearing the parasitoid E. sp. nr. furuhashii using AsiaII7 as hosts (hereafter “PR”, 6 cages). We found that the Wolbachia infection rate of whitefly housed with parasitoids was higher than that without parasitoids, gradually increasing during the surveillance period (S1 Fig.). Meanwhile, the prevalence of Wolbachia in E. sp. nr. furuhashii wasp was lower than that of the whitefly, but also gradually increased (S1 Fig.). Although this is not a replicated experiment, it led us to hypothesise that the coexistence of parasitoid and whitefly may promote the transmission of Wolbachia between different individuals of whitefly. Here we show that non-lethal host inspection of the whitefly nymphs by the wasp can transfer Wolbachia. The acquisition of Wolbachia benefited its host’s fitness in terms of faster immature development and increased adult survival.
Results
Behavioral observation of parasitoids visiting whitefly
To test whether parasitoids can vector Wolbachia, we allowed them to oviposit and feed on Wolbachia-infected whitefly and then transferred them to a cage of uninfected whitefly. By observing the behavior of E. sp. nr. furuhashii in the laboratory, we found that among the uninfected AsiaII7 nymphs which were visited by parasitoids for feeding or oviposition checking, 35.8% (38 of 106) died, adult parasitoids emerged from 34.0% (36 of 106), and 30.2% (32 of 106) resulted in adult whitefly. Wolbachia screening by PCR revealed that 93.8% (30 of 32) of the newly emerged whitefly individuals became infected after surviving the parasitoid penetration.
FISH detection and MLST of Wolbachia in whitefly and parasitoids
In order to confirm infection by Wolbachia, samples of both AsiaII7 nymphs (including the donor whitefly from PR cage, the newly infected nymphs and their offspring) and E. sp. nr. furuhashii were selected randomly for fluorescence in situ hybridization. We found different distributions of Wolbachia in the two-level trophic system. For the parasitoid E. sp. nr. furuhashii, Wolbachia was found both in their mouthparts and ovipositor after they fed on or penetrated whitefly hosts, but not in the ovaries (Fig. 1). Among the donor AsiaII7 nymphs there were two distinct patterns of infection. In some individuals Wolbachia was “scattered” in both the ovaries and somatic tissues (Fig. 2 A, B; 6 of 30 individuals), while in others it was “confined” to the bacteriocytes and ovaries (Fig. 2 C, D; 24 of 30 individuals).
Fig. 1. FISH detection of Wolbachia in adult Eretmocerus parasitoids. 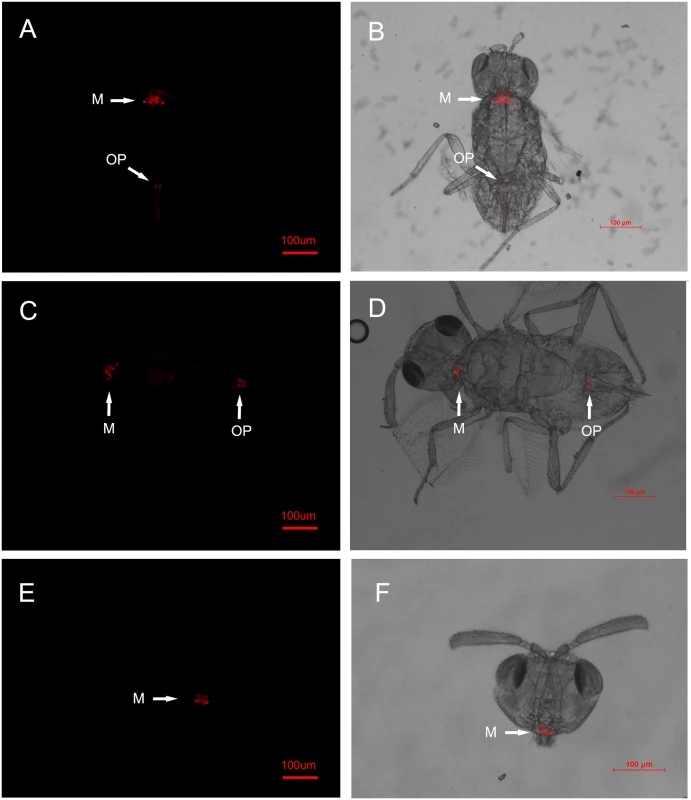
The E. sp. nr. furuhashii wasps were selected randomly 24–48 h after visiting Wolbachia-positive AsiaII7 whitefly nymphs. Panels (A-D): Wolbachia in parasitoid with different body poses, it was found both in the mouthparts (M) and ovipositors (OP); panels (E, F): Wolbachia in the parasitoid mouthparts (front view). Left panels (A, C, E): fluorescence in dark field; right panels (B, D, F): fluorescence in bright field. Fig. 2. FISH detection of Wolbachia in AsiaII7 whitefly. 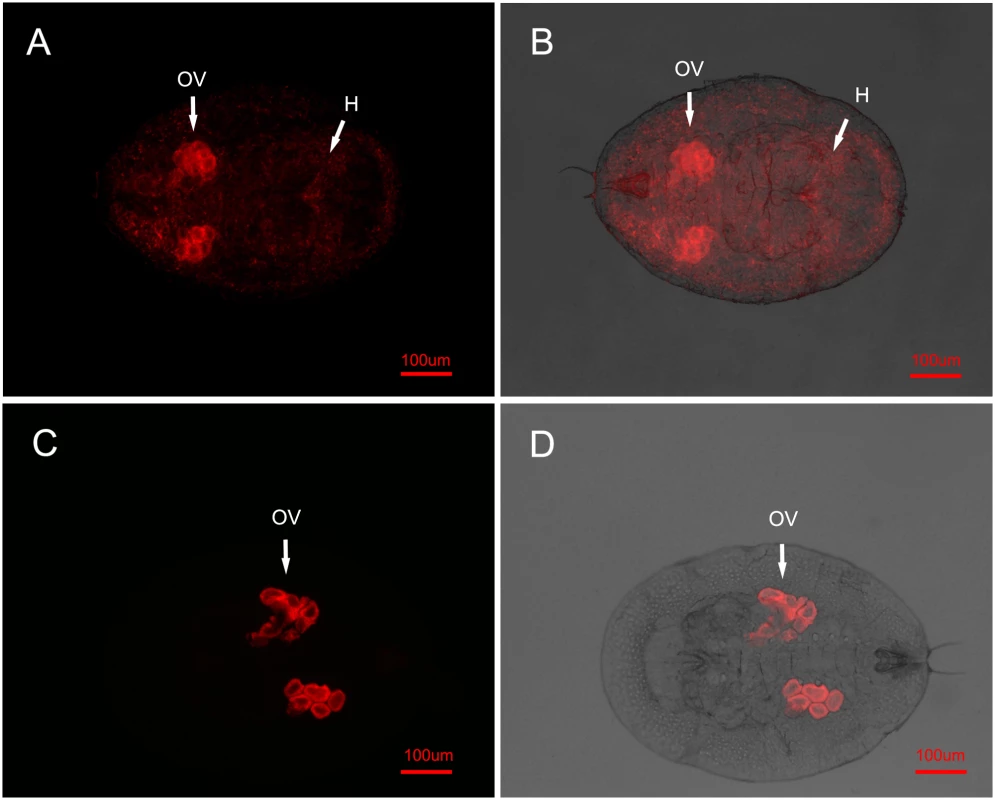
Wolbachia has two distributions in 3rd instar AsiaII7 nymphs randomly selected from the Eretmocerus rearing cages. Top panels (A, B): in the scattered pattern, Wolbachia was found both in the ovary (OV) and haemolymph (H) of whitefly; bottom panels (C, D): in the confined pattern, Wolbachia was only found in the ovary (OV) of whitefly. Left panels (A, C): fluorescence in dark field; right panels (B, D): fluorescence in bright field. In uninfected whitefly that had been visited by a contaminated parasitoid, Wolbachia was not visible during the first 72 h. This is presumably due to its low density, as qRT-PCR showed a continuous increase of Wolbachia during this time, indicating that the insect had been infected and the bacterium could replicate (S2 Fig.). In the offspring of whitefly visited by contaminated wasps, Wolbachia is clearly visible in 3rd instar nymphs. In all cases the symbiont had the scattered pattern (15 of the 16 AsiaII7 offspring harboured scattered Wolbachia, while Wolbachia was not detected in the other one).
To identify the Wolbachia strain in AsiaII7 whiteflies and Eretmocerus parasitoids, we sequenced five MLST genes and the wsp gene. This revealed that the Wolbachia strain from E. sp. nr. furuhashii was the same as in AsiaII7 (sequence type 388). By comparing the sequences those in the Wolbachia MLST database (http://pubmlst.org/wolbachia/) and by constructing phylogeny, this was identified as a new sequence type “ST388” (S1 Table, S3 Fig.).
Persistence of Wolbachia in whitefly and parasitoids
To be stably transmitted between generations Wolbachia must infect the female germ line. We established populations of whitefly from individuals that had been probed by contaminated parasitoids and measured the prevalence of Wolbachia for five generations. We found that 85.0 to 90.0% of the F1 to F5 progeny of the newly-infected whiteflies were Wolbachia-positive (Fig. 3), indicating the vertical transmission and long term persistence of Wolbachia in the newly-infected AsiaII7 populations. While we did not directly measure vertical transmission rates (uninfected whitefly may have been among the parents of each generation), these results indicate efficient transmission from parent to offspring. The negative control was uninfected in all 5 generations.
Fig. 3. The prevalence of newly-acquired Wolbachia in AsiaII7 whitefly over five generations. 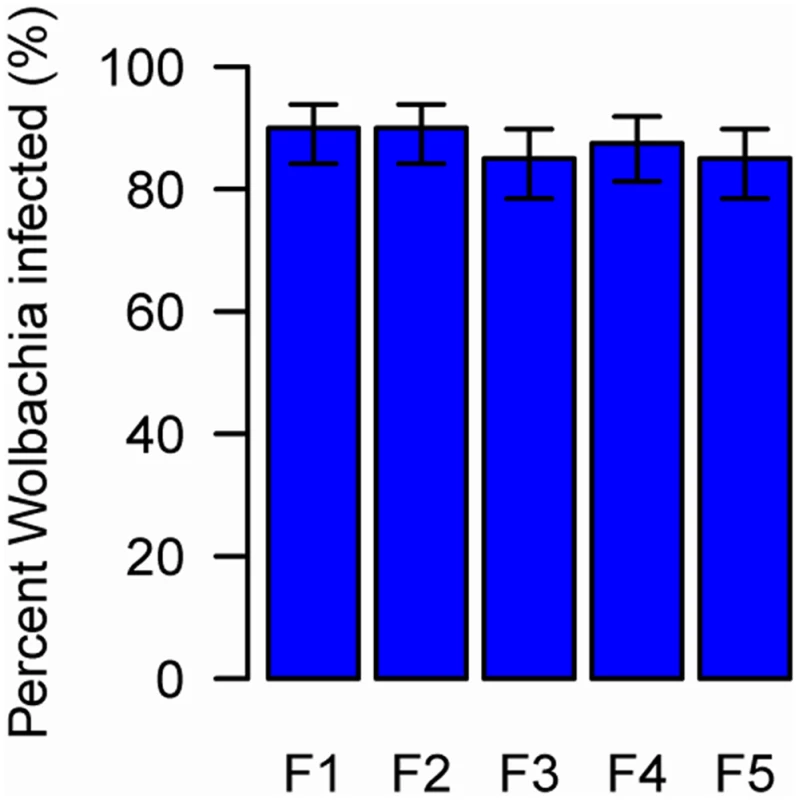
The proportion on infected whitefly was measured in a population over 5 generations. The population was founded by four pairs of new emerged AsiaII7 whitefly that had been infected with Wolbachia acquired from a contaminated wasp. The proportion of Wolbachia-infected offspring was monitored with PCR. The bars are standard errors. The means and standard errors were estimated using a generalized linear model and are back-transformed from the logit scale. The is no significant heterogeneity in the prevalence among generations (GLMM likelihood ratio test: χ2 = 0.92, df = 4, P = 0.92). Wolbachia DNA can be detected by PCR for at least 5 days in E. sp. nr. furuhashii after they fed or oviposited on Wolbachia-infected AsiaII7 nymphs (Fig. 4A). However, parasitoids contaminated with Wolbachia were only able to transmit it for the first 48 h, indicating that the bacteria are gradually losing their infectivity with time (Fig. 4B).
Fig. 4. The persistence of newly-acquired Wolbachia in parasitoids. 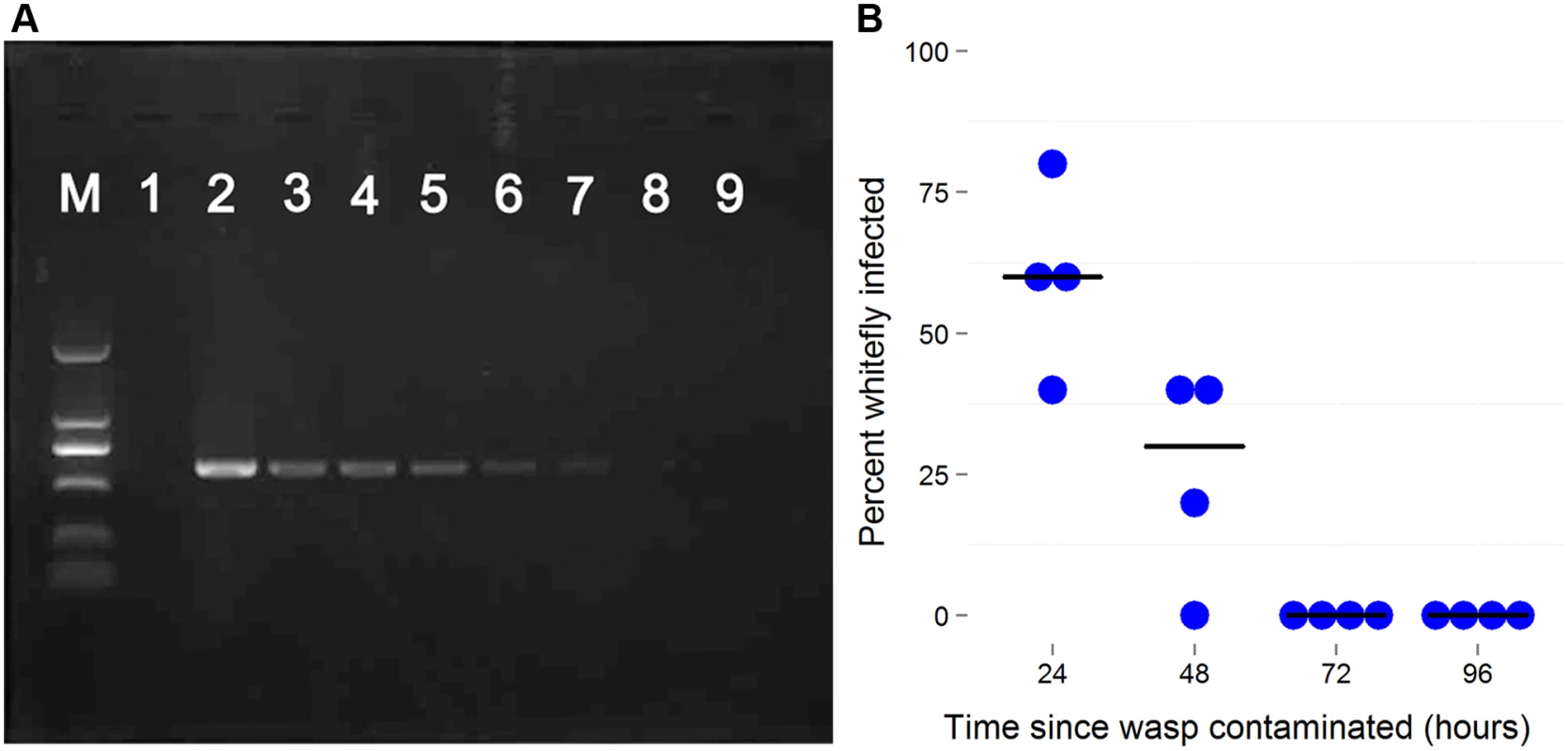
A: Wolbachia detection by PCR using wsp gene in the seven days after the parasitoid visited an infected host. In each PCR reaction Wolbachia DNA was extracted from 10 individuals. B: The decline in Wolbachia transmission rates with time after the wasp is contaminated. Female Eretmocerus wasps were released to parasitize Wolbachia negative AsiaII7 2nd-3rd instar nymphs 24, 48, 72 and 96 h after contamination with Wolbachia. The Wolbachia infection rate of whitefly that were visited by wasps but survived to adulthood was detected with PCR. Each point is the transmission rate to ten whiteflies in a single replicate of the experiment. The horizontal bars are the median. There is a significant decline in transmission rates (logistic generalized linear model: z = 3.50, P = 0.0005). Effects of Wolbachia on whitefly fitness
We established populations of whitefly with and without Wolbachia, and found that they had significant biological differences. Compared to uninfected AsiaII7, infected whiteflies developed significantly faster (Fig. 5, General Linear Mixed Model: t = 3.38, df = 6, P = 0.01) and had significantly increased longevity (Fig. 5, Cox proportional hazards mixed model: z = 3.86, P = 0.0001). There was no significant effect of Wolbachia on either juvenile survival (Fig. 5, Generalised linear mixed model: z = 1.6, P = 0.11) or fecundity (Fig. 5, General Linear Mixed Model: t = 0.64, df = 6, P = 0.55). Wolbachia was associated with a slightly higher proportion of female offspring, but this was not significant (Fig. 5, Generalised linear mixed model: z = 1.7, P = 0.09).
Fig. 5. Effects of Wolbachia on whitefly fitness. 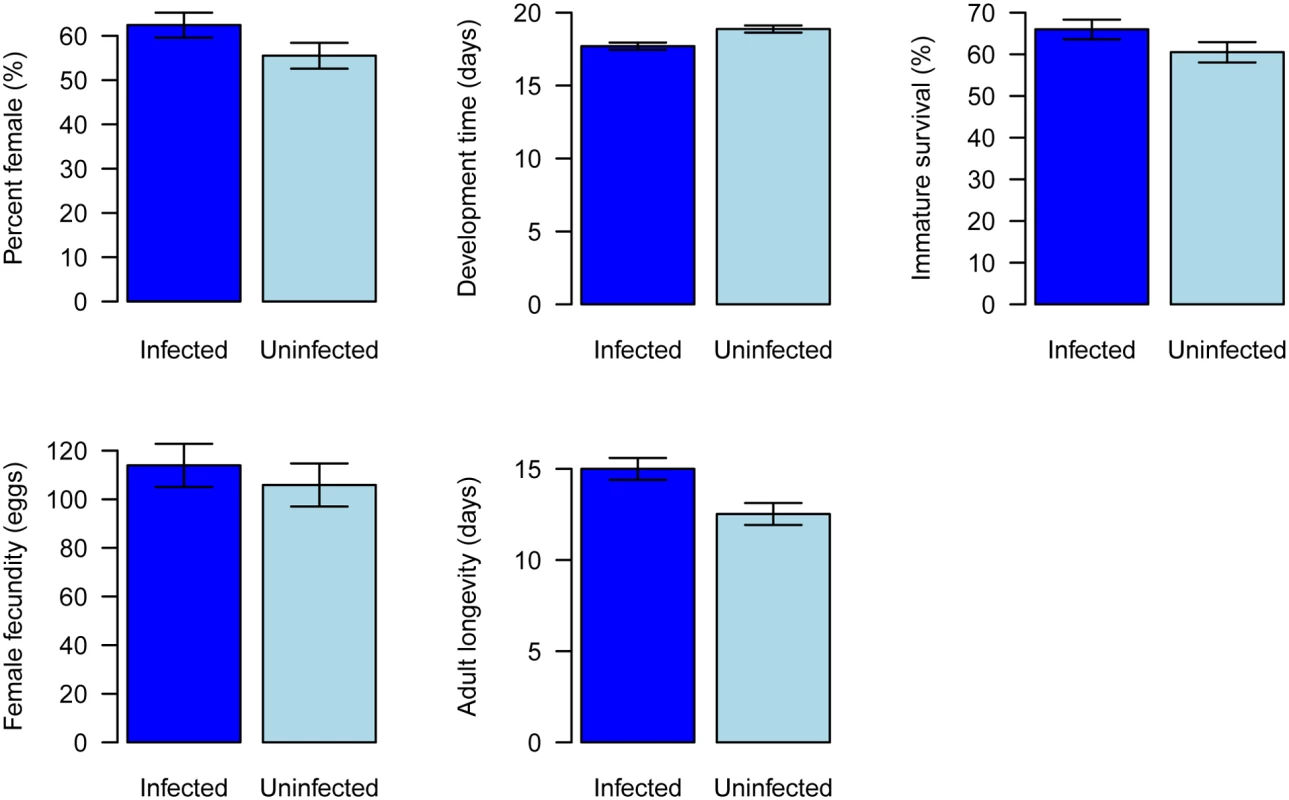
Traits affecting the fitness of newly Wolbachia-infected offspring were compared with those Wolbachia uninfected populations under laboratory conditions. A: percent female, B: development time, C: immature survival, D: female adult fecundity and E: female adult longevity. The data are means ± SE. Effects of horizontal transmission on the population dynamics of Wolbachia
To understand how horizontal transmission will affect the prevalence of a symbiont, we simulated the spread of Wolbachia through a population. To do this we modified existing models of Wolbachia dynamics to include an additional parameter w, which is the product of the number of new cases generated by horizontal transmission from a single infected host in an otherwise uninfected population and any reduction in vertical transmission efficiency of Wolbachia in the newly infected hosts relative to those infected from their mother. The value of w will be determined by factors including the frequency with which a vector visit hosts, the time that contaminated vectors remain infectious, and the probability that a contaminated vector infects an uninfected whitefly. Field data shows that the majority of wasps are contaminated by Wolbachia DNA (S4 Fig.), and our lab data suggests many of these will be infectious (DNA is detectable for 5 days, the infectious period is 2 days). We also find that about a third of nymphs survive parasitism. Therefore, w will be below the parasitism rate, but not dramatically so.
We first investigated the effect of horizontal transmission in the absence of any reproductive manipulation or effect on host fitness (Fig. 6A). The outcome depends on the rate of horizontal transmission relative to the rate at which the infection is lost due to imperfect vertical transmission. When horizontal transmission is low, the infection will tend to be lost, but moderate rates of parasitism (w = 0.06) can result in Wolbachia invading and reaching a stable equilibrium frequency. Fixation is prevented by imperfect vertical transmission.
Fig. 6. The effect of horizontal transmission on the spread of Wolbachia through populations. 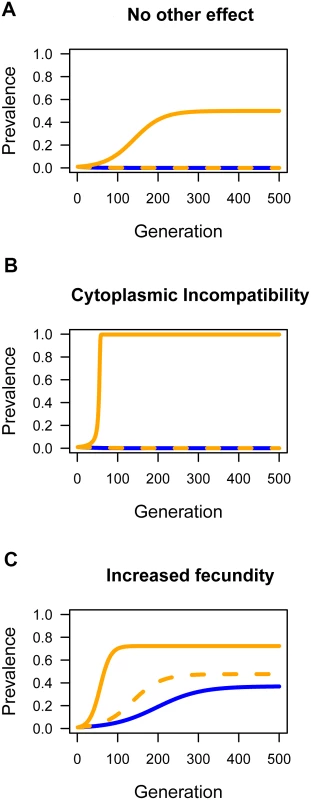
Simulations were performed to explore the effect of horizontal transmission on the prevalence of Wolbachia. The blue lines are where there was no horizontal transmission (w = 0), the dashed orange line was a low rate (w = 0.01), and the solid orange line a moderate rate of horizontal transmission (w = 0.06). A: The effect of horizontal transmission when there is not effect of Wolbachia on host fitness and no reproductive manipulation (H = 1, F = 1). B: Wolbachia induces cytoplasmic incompatibility (H = 0.1, F = 1). C: Wolbachia carries a fitness benefit (H = 1, F = 1.05). In all cases the starting prevalence was p = 0.01 and the rate of imperfect maternal transmission was μ = 0.03. Next, we examined how horizontal transmission will affect a strain of Wolbachia that causes the reproductive manipulation cytoplasmic incompatibility (CI; Fig. 6B). Combining CI and horizontal transmission can dramatically alter the outcome, with horizontal transmission allowing the near fixation of a strain that would otherwise be lost. The reason for this is that there is a threshold prevalence that CI strains must exceed in order to invade populations. Horizontal transmission can lower or eliminate this threshold. For the parameters used in Fig. 6B, if the starting prevalence is high, then Wolbachia can invade and reach a high prevalence without horizontal transmission.
Finally, we examined how horizontal transmission affects a strain that provides a fitness benefit to the host (Fig. 6C). Fitness benefits alone can allow the bacterium to invade the population as a mutualist and reach an intermediate equilibrium frequency maintained due to imperfect vertical transmission. However, horizontal transmission can accelerate the invasion and increase the equilibrium prevalence.
Discussion
Wolbachia has well-described effects on host physiology and reproduction that increase its prevalence in populations [1,5,36–38]. For instance, cytoplasmic incompatibility, female-biased offspring sex ratios and providing protection against viral infection are potent methods that may increase the frequency of infected relative to uninfected matrilines. These mechanisms all rely on bolstering the vertical transmission of the bacterium. On the other hand our study shows that within populations Wolbachia may spread horizontally as well. If horizontal transmission proves to be important in other insects, it is tempting to speculate that it may sometimes explain the observation of Wolbachia infections with no known phenotypic effect [39]. Furthermore, parasitoids could also potentially vector Wolbachia to novel species, such as the other cryptic species of B. tabaci which share parasitoids with AsiaII7. This could partly explain the plethora of phylogenetic evidence of discordance between host and symbiont phylogenies [8,11,12,40,41].
Parasitoid-vectored horizontal transmission of Wolbachia may be important in natural populations. AsiaII7 is an indigenous cryptic species of B. tabaci in south China, and we have surveyed field populations of this whitefly species and its parasitoids from 2006–2012. Wolbachia was detectable in about 30–40% of wild E. sp. nr. furuhashii throughout this time (S4 Fig.). Furthermore, most AsiaII7 individuals were Wolbachia-infected, and the prevalence increased during this time (S4 Fig.). Numerous factors may have contributed to this increase, including fitness benefits and horizontal transmission (see results).
It has been suggested that horizontal transmission from parasitoids to their hosts would be unlikely as parasitized hosts die [8]. The mode of transmission we have described here relies on the fact that parasitoids do not always kill hosts with which they interact. Given mixed-instar nymphs, Eretmocerus parasitoids of whitefly B. tabaci usually exhibit a clear preference for feeding on 1st and 4th instar nymphs, leaving 2nd and 3rd instars for oviposition, while Encarsia parasitoids prefer to feed on 1st and 2nd instars, leaving 3rd and 4th instars for oviposition [42,43]. Our FISH and PCR screening revealed that the ovipositors and mouthparts of parasitoids get contaminated with Wolbachia when they probe or feed on infected whitefly nymphs. When these parasitoids probe uninfected whitefly nymphs, about one third survived, and of those 93.8% became infected with Wolbachia. Similar to Eretmocerus emiratus parasitoids developing in Rickettsia-infected B. tabaci hosts [7], Wolbachia failed to penetrate the oocytes of the parasitoid, but this is not required for parasitoids to transmit the bacterium effectively.
Subsequent to horizontal transmission, the bacteria have to be transmitted efficiently from mother to daughter in order to persist in the population. In many such cases infections fail to persist [1,7] or are transmitted with poor fidelity [16,17,23,44,45]. For example, Wolbachia was transmitted at a low rate (3.2%) from an infected Drosophila simulans host to the parasitoid Leptopilina boulardi, and subsequently was lost within four generations [15]. In our experiment, Wolbachia persisted at a stable prevalence (85.0–90.0%) for at least five generations in AsiaII7 whitefly, suggesting that the vertical transmission rates were high. Our result support the recent study of Gehrer and Vorburger [25] in which they demonstrated that parasitoids can transfer the bacterial endosymbionts Hamiltonella defensa and Regiella insecticola by sequentially stabbing infected and uninfected individuals of the black bean aphid Aphis fabae. Similar to our results, this established new, heritable infections.
Vectors that transmit infection can either be biological vectors, where the infective agent replicates or develops in the vector, or phoretic (mechanical) vectors where it does not. Three lines of evidence indicated that the parasitoids are acting as phoretic vectors. First, FISH indicates that the Wolbachia is only found on the surface of the wasp and does not infect its tissues or ovaries. Second, the infectivity of the wasps rapidly declines after they have become contaminated with Wolbachia. Third, the Wolbachia infection was routinely monitored in population cages, and wasps were always uninfected unless they were recently exposed to infected AsiaII7 whiteflies. Therefore, it seems most likely that the wasp is acting as little more than a dirty needle. Our FISH experiments revealed that in all the newly infected AsiaII7 individuals, Wolbachia had a scattered distribution across tissues, while vertically infected insects tended to have Wolbachia largely confined to the ovaries. Therefore we cannot be sure that the confined distribution of Wolbachia in whitefly ovaries can also be horizontal transmitted from one individual to another by parasitoids.
Facultative endosymbionts, including Wolbachia, can change the fitness or biology of their hosts. For example, facultative symbionts in the pea aphid (Acyrthosiphon pisum) can protect their hosts against entomopathogenic fungi and parasitoid wasps, ameliorate the detrimental effects of heat, and influence host plant suitability [1,46–48]; B. tabaci Mediterranean cryptic species infected with Wolbachia showed decreased juvenile development time, increased juvenile survival, increased adult life span and an increased percentage of female progeny [49]. Similarly, our study also showed that Wolbachia-infected AsiaII7 survived for longer and developed faster. There was also an increase in the proportion of daughters produced, but our sample size was small and the effect was not statistically significant. Wolbachia always had imperfect vertical transmission, with infected females producing uninfected daughters, which may explain why natural populations are a mixture of infected and uninfected individuals.
Our study revealed changes in fitness caused by Wolbachia that can potentially increase the rate of population growth of whitefly, leading to more severe damage to crops. Fitness measurements can often prove context-dependent and hard to replicate, so it is important to reproduce our results in the field. Furthermore, our laboratory results should be replicated in case there are unaccounted for differences between our Wolbachia-infected and Wolbachia-free populations. However, assuming similar effects are found in nature, our results suggest that parasitoids used for biological control could result in unintended negative consequences when the control agent also allows the horizontal transmission of Wolbachia between matrilines or even species of pests. With increased chemical resistance of whitefly in many countries, parasitoids have become an important biocontrol agent to manage whitefly infestations, especially in greenhouses. The B. tabaci species complex is known to be host to at least 56 species of parasitoids, mostly from the genera Eretmocerus and Encarsia [35], and some of these have been commercially produced and applied. Usually one B. tabaci cryptic species can be parasitized by dozens of parasitoid species, and one parasitoid species can attack several B. tabaci cryptic species, or even more distantly related species of whitefly such as the greenhouse whitefly Trialeurodes vaporariorum. This may provide many opportunities for endosymbionts to horizontally transmit between different whitefly species, potentially causing the detrimental fitness changes. We propose that this unintended negative impact of parasitoids in pest biological control cannot be ignored.
Methods
Survey of Wolbachia infection in whitefly and parasitoid
Adult AsiaII7 whiteflies were first collected from variegated laurel plants, Codiaeum variegatum (L.), in Guangzhou in 2006 [50]. They were then reared on hibiscus in two separate greenhouses (15×7m) in the South China Agricultural University (SCAU). These were covered with PVC film on the top and nylon net (70 mesh) on the sides. Two subcolonies were set up using 5–6 cages in the laboratory, one of which was used for AsiaII7 whitefly rearing only and the other was used for parasitoid E. sp. nr. furuhashii rearing. In the parasitoid rearing cages, new and clean hibiscus plants were added to replace the old plants when needed. This allows both the B. tabaci hosts and Eretmocerus parasitoids to stably coexist because removing old plants takes the parasitoid pupae away. Both the AsiaII7 whitefly and Eretmocerus parasitoids were reared in an insect growth chamber at 26.0±0.5°C, 70–80% relative humidity, 14 : 10 h (L:D) photoperiod.
AsiaII7 samples were collected three times per year (Apr-May, Aug-Sep, Dec-Jan) from both the two subcolonies between 2007–2012. In each survey, 30–50 samples per plant and 3 plants were collected in each subcolony using an aspirator. All samples were preserved immediately in 95% ethanol. Whitefly species identity was confirmed using mtCOI sequences [51,52]. From each survey, 20 whitefly adults from each subcolony were selected randomly for PCR-based detection of Wolbachia following the methods of Ahmed et al. [53]. Briefly, Wolbachia was detected using primers for the wsp, ftsZ and 16S rRNA genes. Both negative controls (ddH2O) and a template DNA quality control (primary endosymbiont Portiera 16S rRNA gene to indicate the DNA quality of extraction) were included. Results were further confirmed using FISH. Alongside the whitefly collection, 20 individuals of E. sp. nr. furuhashii adults were also sampled for Wolbachia detection with PCR and FISH. To identify the Wolbachia strain in parasitoids and whiteflies, we used multi locus strain typing methods described in Baldo et al. [54].
Establishment of Wolbachia infected and uninfected AsiaII7 cultures
To establish Wolbachia-positive lines of AsiaII7, male/female pairs from the AsiaII7 rearing cages were allowed to reproduce on hibiscus, one pair per plant. Once the F1 progeny emerged, ten pairs of newly emerged adults from one parent were selected at random. Five pairs were tested for the presence of Wolbachia using PCR; the remaining five pairs were caged individually on hibiscus leaves. Any line that contained uninfected individuals was discarded until a line in which all five of the tested pairs were positive for Wolbachia was identified. This screening process, based on five pairs being selected from each generation, was continued for 10 generations prior to use in experiments. After the initial selection of the line, all individuals screened were positive for Wolbachia.
To establish an outbred population, which we used for measuring fitness and in other experiments, we mixed together a large number of these lines. In total 50 to 80 pairs of parents were randomly selected, and all the validated Wolbachia positive couples were released into a rearing cage to reproduce. The progeny that emerged were used for the fitness measurements below.
To establish a pure culture of Wolbachia-free whitefly, the process followed that used to establish the infected line except that here the initial line was the one where all five pairs tested negative. The infected and uninfected populations were sampled from the same original greenhouse population. Both the infected and uninfected lines of AsiaII7 were then kept in rearing cages (60×60×60 cm) in separated air-conditioned insect growth chamber at 26.0±0.5°C, 70–80% relative humidity, 14 : 10 (L:D) photoperiod and light intensity of approximately 3000 Lux. Wolbachia-infection status in the cages was monitored on a monthly basis by PCR.
Wolbachia horizontal transmission between AsiaII7 nymphs
In the laboratory, we examined horizontal transmission of Wolbachia from the infected AsiaII7 nymphs to the uninfected nymphs via parasitoids. About 10 pairs of uninfected AsiaII7 adults were released into a leaf cage to reproduce on hibiscus for 48 h. Progeny from these adults developed to 2nd-3rd instar, at which point all but 60 nymphs were removed. Before the experiment, three mated parasitoid females of E. sp. nr. furuhashii (2-day age) were first introduced into a leaf cage in which there are 2nd-3rd instar Wolbachia-positive AsiaII7 nymphs for 2h of feeding and oviposition. After that, the parasitoids were transferred into the leaf cages with the 60 Wolbachia-free 2nd-3rd instar nymphs. The parasitoids were allowed to feed and oviposit for another 2 h, oviposition behaviors were observed using a binocular dissecting microscope, and nymphs visited by the wasp were marked with indelible ink. We used 240 nymphs of AsiaII7 in total (60 in each repeat), of which 106 were visited by parasitoids. Seven days later, the number of nymphs that were parasitized (larval parasitoids are visible in whitefly hosts), the number of nymphs that were subjected to the insertion of an ovipositor but survived, and nymphs that were parasitized but died were recorded. Meanwhile, the emerged whiteflies and parasitoids were both collected for Wolbachia detection by PCR following the methods of Ahmed et al. [53]. All the transmission experiments were done at 26.0±0.5°C, 70–80% relative humidity, 14 : 10 h of L:D photoperiod, and the four repeats were replicated contemporaneously.
FISH detection of Wolbachia in whitefly and parasitoid
The FISH procedure to detect Wolbachia in whitefly AsiaII7 and parasitoid E. sp. nr. furuhashii followed the method of Xue et al. [49] and Sakurai et al. [55]. About twenty Eretmocerus female adults (2-day old) were recaptured 24–48 h after they had been released to attack abundant Wolbachia positive AsiaII7 nymphs in a leaf cage. For the whitefly samples, we investigated forty 3rd instar AsiaII7 nymphs from the Wolbachia-infected donor PR cages (thirty were finally FISH photographed successfully), a similar number of the Wolbachia newly infected AsiaII7 nymphs via parasitoid and their F1 generation offspring were randomly selected for FISH detection respectively.
Two 5’ rhodamine labeled Wolbachia probes (targeted 16S rRNA of Wolbachia) were used to increase the signal: W2 : 5’-CTTCTGTGAGTACCGTCATTATC-3’ and W3 : 5’-TCCTCTATCCTCTTTCAATC-3’. Stained samples were washed thoroughly twice in the same buffer at 48°C for 20 min. All the samples were observed using an inverted fluorescence microscope (Nikon Eclipse Ti-U). Specificity of the detection was confirmed using the uninfected whiteflies as controls.
In order to quantify the increase of Wolbachia after it was transmitted into Wolbachia negative AsiaII7 whiteflies, 60 early 3rd instar AsiaII7 nymphs that have been fed or probed by those Wolbachia-carrying Eretmocerus parasitoids were screened out and divided into three groups. Then the three groups were used for Wolbachia qRT-PCR detection 24–72 h after they were fed or probed by parasitoids using the primers and protocols described in Xue et al. [49].
Stable infection in whitefly
In order to know the stability of Wolbachia infection in the newly infected AsiaII7 B. tabaci, a pair of newly infected adults of whitefly were randomly selected and introduced into a leaf cage for 48 ho. Eggs were allowed to develop to F1 adults, at which point 10 of them were randomly selected and tested for Wolbachia. Then another pair of F1 generation adults were randomly selected again and released into a new leaf cage to reproduce F2 generation, among which 10 individuals of F2 adults were selected randomly for PCR detection. This procedure was repeated through to the F5 and only female adults were selected for PCR detection in each generation. A control experiment was conducted in which uninfected AsiaII7 whitefly was reared and checked for Wolbachia infection from F1 to F5 generations in separated cages. Both the Wolbachia infected and uninfected whitefly lines were reared in the insect growth chamber and four replicates were conducted in each generation contemporaneously.
Temporary infection in parasitoids
After E. sp. nr. furuhashii fed or penetrated whitefly nymphs, FISH showed that Wolbachia was found in their mouthparts and ovipositors, but not in their ovaries. We therefore hypothesized that these wasps were contaminated temporarily by Wolbachia and can transfer this endosymbiont to other hosts by feeding or oviposition. To confirm this hypothesis, 70 to 80 mated females of E. sp. nr. furuhashii were divided into seven groups and released into seven leaf cages with abundant 2nd-3rd instar Wolbachia-positive AsiaII7 nymphs. Parasitoids were allowed to oviposit or feed on the whitefly hosts for 48 h, then all of them were recaptured, introduced into seven Petri dishes (9 cm diameter), and fed with 10% honey water. Hereafter one group of the female parasitoids per day was used to detect the presence of Wolbachia DNA by PCR according to the method of Ahmed et al. [43]. To extract DNA, 10 parasitoids were homogenized in one tube due to the tiny titer of Wolbachia.
Changes in transmissibility of Wolbachia
In order to confirm the time that Wolbachia on the mouthparts and ovipositors of Eretmocerus wasps is alive and transmittable, five two-day-old female wasps were collected after they parasitized several Wolbachia-positive AsiaII7 nymphs. They were then released into a 5 cm diameter Petri dish, and fed with 10% honey solution but not given hosts to parasitize. After 24, 48, 72 and 96 h, these parasitoids were released into leaf cages to parasitize 2nd-3rd instar nymphs of Wolbachia-negative AisaII7. After 4–6 days, DNA was extracted from individual whitefly adults that emerged, and these samples were tested for the presence of Wolbachia by PCR. The experiment at each time point (24–96 h) was repeated four times contemporaneously.
Fitness effects of Wolbachia on AsiaII7 whitefly
To compare the biology of Wolbachia uninfected and infected AsiaII7 populations, their development time, immature survivorship, sex ratio, fecundity and adult longevity were investigated according to Qiu et al. [34]. Briefly, 10 pairs of newly emerged AsiaII7 adults were selected at random from the uninfected or infected populations (see above for how these were established). Each pair was introduced into a leaf cage on a hibiscus plant to lay eggs for 48 h (10 leaf cages in each replicate of the experiment), after which all but 20 eggs per leaf cage were removed. The twenty F1 eggs were allowed to develop to adults in the leaf cage, and their emergence were checked daily until all nymphs completed their development. The developmental time of one randomly-selected individual in each leaf cage was recorded (in total 10 individuals were selected in each replicate).
Meanwhile, a pair of Wolbachia positive and negative F1 emerged adults (0–12 h age, which is before whitefly begin laying eggs) were randomly selected from each of the 10 leaf cages, and each pair was introduced into a new leaf cage on a hibiscus plant for reproduction. Their eggs were counted daily until the female died, and newly emerged males were supplemented if the original male died before the experiment ended. The fecundity and longevity of F1 generation adults was recorded. In addition, about 100 F1 adults were randomly selected and their sex ratio recorded. All the experiments were performed in the insect growth chamber (26.0±0.5°C, 70–80% relative humidity, 14 : 10 h of L:D photoperiod). Care was taken to select uniform hibiscus plants. We replicated the experiments measuring fitness trait four times contemporaneously (i.e. there were four replicates of the Wolbachia-infected and four replicates of the Wolbachia-free treatments).
Data analysis
The data on the fitness effects of Wolbachia was analysed using a series of statistical models. In all the experiments measuring fitness components, both the Wolbachia-infected and Wolbachia-free treatments were replicated four times, with 10 pairs per replicate (80 pairs in total). The 8 replicates (4 Wolbachia-infected and 4 Wolbachia-free) were performed contemporaneously. In all cases the model included the full replicate structure of the experiment and no model simplification was performed.
The data on adult longevity consisted of observations on the lifespan of a single adult offspring from each pair (10 observations per replicate). Each adult was observed until it died, so there is no right censoring of the data. We analyzed this data using a Cox proportional hazard model, which included a fixed effect of Wolbachia infection status and a random effect of replicate (also see S2 Table). The model was fitted using the function coxme in R.
The data on fecundity consisted of a count of the number of eggs laid by each pair (10 egg counts per replicate). The data on immature development consisted of the development time of a single nymph from each pair (10 nymphs per replicate). We treated both of these datasets as Gaussian and analyzed them using a general linear model. The model included a fixed effect of Wolbachia infection status, a random effect of replicate and a residual that accounts for variation between pairs within each replicate.
The data on immature survival consisted of counts of nymphs from each pair that died or survived (10 ratios of dead: alive nymphs per replicate). The data on sex ratio consisted of counts of male and female offspring that were produced by each replicate. Both immature survival and sex ratio were treated as ratios and analysed using generalized linear mixed models with a binomial error structure. The model included a fixed effect of Wolbachia infection status, a random effect of replicate for the survival data, and a residual variance to allow for over-dispersion (i.e. variation between pairs within each replicate that was greater than that expected under binomial sampling).
The figures are plotted using parameters estimated from these models, with the exception of adult longevity where mean survival times were estimated for the figure using a model similar to that described above for the fecundity data. For the binomial data, the means and standard errors were back-transformed from the logit scale into proportions for plotting. The model parameters were estimated by maximum likelihood using the R functions lme and glmer in the package lme4 [56].
Multi locus sequence typing of Wolbachia and phylogenetic analysis
Multi locus sequence typing (MLST) was used to identify Wolbachia in both AsiaII7 whitefly and E. sp. nr. furuhashii parasitoids. Five MLST genes, gatB (wMel locus WD_0146), coxA (WD_0301), hcpA (WD_0484), ftsZ and fbpA (WD_1238), together with the Wolbachia surface protein gene (wsp) were used for Wolbachia strain identification. The process is described in Baldo et al. [54].
Concatenated sequences of the seven most closely related Wolbachia sequence types (STs) associated with different host species were selected from the MLST database and used for comparisons with the STs isolated from other whitefly cryptic species, AsiaII7 and the parasitoid, E. sp. nr. furuhashii. Three STs from supergroup A, D and F Wolbachia were used as outgroups. The best model was chosen using the Bayesian Information Criterion (BIC) in MEGA5 [57]. Phylogenetic analysis based on the concatenated data of the five Wolbachia MLST loci (2079 bp) was undertaken using maximum parsimony (MP) and maximum likelihood in MEGA5 [57] and PAUP version 4.0b [58]. T92+G model was used during ML tree constructions.
Simulations of Wolbachia dynamics
To understand how horizontal transmission can affect the dynamics of Wolbachia within insect populations we adapted the model of Hoffman et al. [59], who provide a full explanation of the model, the parameters and its assumptions. This model assumes discrete generations. Infected females produce a fraction μ of uninfected ova. The fecundity of Wolbachia-infected females relative to Wolbachia-free females is denoted by F. When the strain induced cytoplasmic incompatibility, the relative hatch rate of incompatible crosses is H. Let sh = 1-H and sf = 1-F. The prevalence (proportion of infected adults) at generation t is pt. In the absence of horizontal transmission, the prevalence at generation t+1 is denoted k. Following Hoffmann et al. [59]:
We then added an extra step in which vectors can transmit Wolbachia horizontally. We assume that Wolbachia in newly infected whitefly has the same effect on female fecundity and cytoplasmic incompatibility as in stably infected lines. We also assume that the rate of hosts being visited by vectors is constant across generations, that wasps visit whitefly randomly with respect to their Wolbachia infection status, and that a contaminated wasp can only infect the next whitefly that it visits (i.e. Wolbachia is lost by probing an uninfected host). Male and female whiteflies are assumed to be equally likely to be visited by wasps and to transmit or be infected by Wolbachia. For simplicity, we also modelled the effects of a vector that never kills its host, although unpublished simulations suggest this produces similar results to a parasitoid that can kill its hosts. We define a new parameter w as the number of new cases generated by horizontal transmission from a single infected host in an otherwise uninfected population. It is possible that females that are infected by horizontal transmission may transmit Wolbachia at a reduced rate. If this is the case, then w can be considered as the number of product of the number of new cases generated by horizontal transmission and the proportionate reduction in the transmission rate in these females. Therefore, the prevalence at generation t+1 is given by: The value of w will be determined by factors including the frequency with which vectors visit hosts relative to the time that the contaminated vectors remain infectious and the probability that a contaminated wasp infects an uninfected whitefly.Supporting Information
Zdroje
1. Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55 : 247–266. doi: 10.1146/annurev-ento-112408-085305 19728837
2. Jiggins FM, Hurst GDD (2011) Rapid insect evolution by symbiont transfer. Science 332 : 185–186. doi: 10.1126/science.1205386 21474745
3. Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, et al. (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332 : 254–256. doi: 10.1126/science.1199410 21474763
4. Buchner P (1965) Endosymbiosis of animals with plant microorganisms. New York: John Wiley and Sons Interscience Press. pp. 332–338.
5. Zug R, Hammerstein P (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One 7: e38544. doi: 10.1371/journal.pone.0038544 22685581
6. Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA (2003) Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol 12 : 1061–1075. 12753224
7. Chiel E, Zchori-Fein E, Inbar M, Gottlieb Y, Adachi-Hagimori T, et al. (2009) Almost there: transmission routes of bacterial symbionts between trophic levels. PLoS One 4: e4767. doi: 10.1371/journal.pone.0004767 19274091
8. Vavre F, Fleury F, Lepetit D, Fouillet P, Bouletreau M (1999) Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol 16 : 1711–1723. 10605113
9. Noda H, Miyoshi T, Zhang Q, Watanbe K, Deng K, Hoshizaki S (2001) Wolbachia infection shared among planthoppers (Homoptera: Delphacidae) and their endoparasite (Strepsiptera: Elenchidae): a probable case of interspecies transmission. Mol Ecol 10 : 2101–2106. 11555254
10. Shoemaker DD, Machado CA, Molbo D, Werren JH, Windsor DM, et al. (2002) The distribution of Wolbachia in fig wasps: correlations with host phylogeny, ecology and population structure. Proc R Soc Lond B: Biol Sci 269 : 2257–2267.
11. Baldo L, Ayoub NA, Hayashi CY, Russell JA, Stahlhut JK, et al. (2008) Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol Ecol 17 : 557–569. doi: 10.1111/j.1365-294X.2007.03608.x 18179432
12. Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 : 741–75. doi: 10.1038/nrmicro1969 18794912
13. Ahmed MZ, De Barro PJ, Ren SX, Greeff JM, Qiu BL (2013a) Evidence for horizontal transmission of secondary endosymbionts in the Bemisia tabaci cryptic species complex. PLoS One 8: e53084 doi: 10.1371/journal.pone.0053084 23308142
14. Boyle L, O’Neill SL, Robertson HM, Karr TL (1993). Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260 : 1796–1799. 8511587
15. Heath BD, Butcher RDJ, Whitfield WGF, Hubbard SF (1999) Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biol 9 : 313–316. 10209097
16. Kang L, Ma X, Cai L, Liao S, Sun L, et al. (2003) Superinfection of Laodelphax striatellus with Wolbachia from Drosophila simulans. Heredity 90 : 71–76. 12522428
17. Riegler M, Charlat S, Stauffer C, Mercot H (2004) Wolbachia transfer from Rhagoletis cerasi to Drosophila simulans: investigating the outcomes of host-symbiont coevolution. Appl Environ Microbiol 70 : 273–279. 14711652
18. Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C, et al. (2004) Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc Natl Acad Sci USA 101 : 15042–15045. 15469918
19. Huigens ME, de Almeida RP, Boons PA, Luck RF, Stouthamer R (2004) Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc Biol Sci 1538 : 509–15. 15129961
20. Huigens ME, Luck RF, Klaassen RH, Maas MF, Timmermans MJ, et al. (2000) Infectious parthenogenesis. Nature 405 : 178–179. 10821272
21. Duron O, Wilkes TE, Hurst GDD (2010) Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecol Lett1 3 : 1139–1148.
22. Moran NA, Dunbar HE (2006) Sexual acquisition of beneficial symbionts in aphids. Proc Natl Acad Sci U S A 103 : 12803–12806. 16908834
23. Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, et al. (2012) Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc R Soc B 279 : 1791–1796. doi: 10.1098/rspb.2011.2095 22113034
24. Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73 : 4308–4316. 17483286
25. Gehrer L, Vorburger C (2012) Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol Lett 8 : 613–615. doi: 10.1098/rsbl.2012.0144 22417790
26. Jaenike J, Polak M, Fiskin A, Helou M, Minhas M (2007) Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3 : 23–25. 17443956
27. De Barro PJ, Liu SS, Boykin LM, Dinsdale AB (2011) Bemisia tabaci: a statement of species status. Ann Rev Entomol 56 : 1–19. doi: 10.1146/annurev-ento-112408-085504 20690829
28. Horowitz AR, Kontsedalov S, Khasdan V, Ishaaya I. (2005) Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch Insect Biochem Physiol 58(4): 216–225. 15756703
29. Jiao XG, Xie W, Wang SL, Wu QJ, Pan HP, et al. (2013) Differences in host selection and performance between B and Q putative species of Bemisia tabaci on three host plants. Entomol Exp Appl 147(1): 1–8.
30. Su Q, Oliver KM, Pan HP, Jiao X, Liu B, et al. (2013) Facultative symbiont Hamiltonella confers benefits to Bemisia tabaci (Hemiptera: Aleyrodidae), an invasive agricultural pest worldwide. Environ Entomol 42(6): 1265–1271. doi: 10.1603/EN13182 24280594
31. Gottlieb Y, Ghanim M, Gueguen G, Kontsedalov S, Vavre F. et al. (2008) Inherited intracellular ecosystem: Symbiotic bacteria share bacteriocytes in whiteflies. FASEB J. 22 : 2591–99. doi: 10.1096/fj.07-101162 18285399
32. Qiu BL, Chen YP, Liu L, Peng WL, Li XX, et al. (2009) Identification of three major Bemisia tabaci biotypes in China based on morphological and DNA polymorphisms. Prog Nat Sci 19 : 713–718.
33. Qiu BL, Dang F, Li SJ, Ahmed MZ, Jin FL, et al. (2011) Comparison of biological parameters between the invasive B biotype and a new defined Cv biotype of Bemisia tabaci (Hemiptera: Aleyradidae) in China. J Pest Sci 84 : 419–427.
34. Qiu BL, De Barro PJ, Ren SX (2005) Development, survivorship and reproduction of Eretmocerus sp. nr. furuhashii (Hymenoptera: Aphelinidae) parasitizing Bemisia tabaci (Hemiptera: Aleyrodidae) on glabrous and non-glabrous host plants. Bull Entomol Res 95 : 313–319. 16048679
35. Li SJ, Xue X, Ahmed MZ, Ren SX, Du YZ, et al. (2011) Host plants and natural enemies of Bemisia tabaci (Homoptera: Aleyrodidae) in China. Insect Sci 18 : 101–120.
36. Teixeira L, Ferreira Á, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e1000002.
37. Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol Lett 281 : 215–220. doi: 10.1111/j.1574-6968.2008.01110.x 18312577
38. Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA 107 : 769–774. doi: 10.1073/pnas.0911476107 20080750
39. Ahmed MZ, Greyvenstein OFC, Erasmus C, Welch JJ, Greeff JM (2013b) Consistently high incidence of Wolbachia in global fig wasp communities. Ecol Entomol 38 : 147–154.
40. Kittayapong P, Jamnongluk W, Thipaksorn A, Milne JR, Sindhusake C (2003) Wolbachia infection complexity among insects in the tropical rice-field community. Mol Ecol 12 : 1049–60. 12753223
41. Verne S, Johnson M, Bouchon D, Grandjean F (2012) Effects of parasitic sex-ratio distorters on host genetic structure in the Armadillidium vulgare Wolbachia association. J Evol Biol 25 : 264–276. doi: 10.1111/j.1420-9101.2011.02413.x 22188300
42. Qiu BL, De Barro PJ, He YR, Ren SX (2007) Suitability of Bemisia tabaci (Hemiptera: Aleyrodidae) instars for the parasitization by Encarsia bimaculata and Eretmocerus sp. nr. furuhashii (Hymenoptera: Aphelinidae) on glabrous and hirsute host plants. Biocontrol Sci Tech 17 : 823–839.
43. Greenberg SM, Jones WA, Liu TX (2008) Bemisia tabaci (Homoptera: Aleyrodidae) instar effects on rate of parasitism by Eretmocerus mundus and Encarsia pergandiella (Hymenoptera: Aphelinidae). Entomol Sci 11 : 97–103. 20401334
44. McGraw EA, Merrit DJ, Droller JN, O’Neill SL (2002) Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A 99 : 2918–2923. 11880639
45. Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, et al. (2007) Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res 97 : 407–413. 17645822
46. Oliver KM, Moran NA, Hunter MS (2005) Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102 : 12796–12800.
47. Scarborough CL, Ferrari J, Godfray HCJ (2005) Aphid protected from pathogen by endosymbiont. Science 310 : 1781. 16357252
48. Teixeira L, Ferreira Á, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6(12): e1000002. doi: 10.1371/journal.pbio.1000002 19222304
49. Xue X, Li SJ, Ahmed MZ, De Barro PJ, Ren SX, et al. (2012) Inactivation of Wolbachia reveals its biological roles in whitefly host. PLoS One 7: e48148. doi: 10.1371/journal.pone.0048148 23144739
50. Qiu BL, Ren SX, Wen SY, Mandour NS (2006) Population differentiation of three biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae) in China by DNA polymorphism. J South China Agri Univ 27 : 29–33.
51. Dinsdale A, Cook L, Riginos C, Buckley YM, De Barro P (2010). Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann Entomol Soc Am 103 : 196–208.
52. Ahmed MZ, De Barro PJ, Olleka A, Ren SX, Mandour NS, et al. (2012) Use of consensus sequences to identify members of the Bemisia tabaci (Hemiptera: Aleyrodidae) cryptic species complex in Egypt and Syria. J Appl Entomol 136 : 510–519.
53. Ahmed MZ, Ren SX, Xue X, Li XX, Jin GH, et al. (2010) Prevalence of endosymbionts in Bemisia tabaci populations and their in vivo sensitivity to antibiotics. Curr Microbiol 61 : 322–328. doi: 10.1007/s00284-010-9614-5 20217091
54. Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, et al. (2006) Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72 : 7098–7110. 16936055
55. Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T (2005) Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropis, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl Environ Microbiol 71 : 4069–4075. 16000822
56. Bates DM, Maechler M, Bolker B, Walker S (2013) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0–5.
57. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739. doi: 10.1093/molbev/msr121 21546353
58. Swofford DL (2002) PAUP.* Phylogenetic Analysis Using Parsimony (*And Other Methods), version 4, Sinauer Associates, Sunderland, MA.
59. Hoffmann AA, Turelli M, Harshman LG (1990) Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetic 126(4): 933–948. 2076821
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





