-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
Most people who are infected with Mycobacterium tuberculosis (M. tb) have latent tuberculosis infection (LTBI) with protective immunity. Patients with active tuberculosis have severe disease and ineffective immunity. Understanding how LTBI individuals control infection without developing disease provides important insight into the mechanisms of protective immunity against tuberculosis, and this information is essential for development of an effective vaccine. It is known that a lymphocyte population called T-cells contributes significantly to protective immunity against tuberculosis infection. In the current study, using human and murine models of M. tb infection, we found that a soluble factor, Rho GDP dissociation inhibitor (D4GDI), produced by a subpopulation of T-cells (CD4+CD25+Foxp3+) inhibits M. tb growth. We also found that D4GDI induces M. tb genes that are expressed during the non-replicative state. Our results suggest that D4GDI has a previously undescribed positive effect on immunity by enhancing host antimicrobial activity. These findings also may aid in understanding the factors that induce LTBI. Further, this information will facilitate development of improved vaccines and immunotherapeutic strategies to prevent and treat tuberculosis, respectively.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004617
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004617Summary
Most people who are infected with Mycobacterium tuberculosis (M. tb) have latent tuberculosis infection (LTBI) with protective immunity. Patients with active tuberculosis have severe disease and ineffective immunity. Understanding how LTBI individuals control infection without developing disease provides important insight into the mechanisms of protective immunity against tuberculosis, and this information is essential for development of an effective vaccine. It is known that a lymphocyte population called T-cells contributes significantly to protective immunity against tuberculosis infection. In the current study, using human and murine models of M. tb infection, we found that a soluble factor, Rho GDP dissociation inhibitor (D4GDI), produced by a subpopulation of T-cells (CD4+CD25+Foxp3+) inhibits M. tb growth. We also found that D4GDI induces M. tb genes that are expressed during the non-replicative state. Our results suggest that D4GDI has a previously undescribed positive effect on immunity by enhancing host antimicrobial activity. These findings also may aid in understanding the factors that induce LTBI. Further, this information will facilitate development of improved vaccines and immunotherapeutic strategies to prevent and treat tuberculosis, respectively.
Introduction
Tuberculosis (TB) causes an estimated 1.7 million deaths world-wide annually. Reducing morbidity and mortality from TB hinges on developing an improved vaccine, which in turn depends on understanding the immune response. T cells play a crucial role in protective immunity against Mycobacterium tuberculosis (M. tb) and other intracellular pathogens [1] in part through production of IFN-γ, which is required for resistance to infection [2]. However, uncontrolled T-cell responses can cause tissue damage, which can be reduced by regulatory CD4+ T-cells (Tregs) that express CD25 and Foxp3 [3].
It is generally believed that CD4+CD25+Foxp3+ T-cells inhibit effective immunity to microbial pathogens. CD4+Foxp3+ T-cells accumulate at sites of infection [4] and prevent efficient clearance of infection in mice infected with M. tb [5]. Recently we found that Tregs expand in response to M. tb in healthy tuberculin reactors and that M. tb mannose-capped lipoarabinomannan converts some CD4+CD25-Foxp3- cells to CD4+CD25+Foxp3+ cells [6]. We also found that the programmed death-1 receptor (PD-1) and cytokine inducible SH2-containing protein (CISH) control expansion of M. tb-induced Tregs [7]. Furthermore, human CD4+CD25+Foxp3+ cells produce TGF-β and IL-10, and inhibit IFN-γ production by CD4+ and CD8+ cells [6], suggesting that they may limit tissue inflammation and destruction. Other studies have found increased number of CD4+Foxp3+ T-cells in TB patients, which inhibit immune responses [6], [8], [9].
However, in humans, some activated T-cells express Foxp3 transiently and these cells lack classical regulatory function [10]–[12]. In the current report, we found that M. tb-activated CD4+CD25+ (85% Foxp3+) T-cells from individuals with latent tuberculosis infection (LTBI) can inhibit growth of M. tb in human monocyte-derived macrophages (MDMs) through production of Rho GDP dissociation inhibitor (D4GDI), a small GTP-binding protein. In addition, D4GDI reduced the bacillary burden in mice infected with M. tb. These findings demonstrate that human CD4+CD25+ (85% Foxp3+) T-cells can contribute to immune defenses by enhancing antimicrobial activity.
Results
CD4+CD25+ (85% Foxp3+) cells inhibit growth of M. tb in MDMs
To determine the effect of M. tb-activated CD4+CD25+Foxp3+ cells on intracellular mycobacterial growth, freshly isolated CD4+ cells and CD14+ monocytes from 6 LTBI were cultured with γ-irradiated M. tb H37Rv. After 4 days, CD4+CD25+ (85–90% Foxp3+) and CD4+CD25- (<5% Foxp3+) cells were isolated, as outlined in the methods. MDMs from the same donors were infected with M. tb H37Rv at a MOI of 1 : 2.5, as detailed in the methods, and M. tb-expanded CD4+CD25+ (85% Foxp3+) or CD4+CD25-Foxp3- cells were cultured in Transwells, or directly in the same wells containing infected macrophages. On day “0” (2 hr after infection) the number of CFU per well were 1.2 ± 0.38× 106. After 7 days, 16.5 ± 1.2 × 106 CFU per well were present in MDMs cultured without T-cells. Addition of CD4+CD25+ (85% Foxp3+) cells to the same well or in Transwells reduced CFU to 0.4 ± 0.2 × 106 (>95% inhibition, p = 0.001 for both comparisons, Fig. 1A). Addition of CD4+CD25-Foxp3- cells had no effect (Fig. 1A). This surprising result indicates that soluble factors produced by CD4+CD25+ (85% Foxp3+) cells strongly inhibit M. tb H37Rv growth in macrophages. This finding was unlikely to be due to contamination of CD4+CD25+ cells with activated CD4+ cells because very few cells expressed IFN-γ (Fig. 2D). Furthermore, we added a very low ratio of T-cells to macrophages (1 : 9), whereas published data indicate that much higher ratios of activated CD4+ cells are needed to reduce M. tb growth by 50–80% [13], [14]. Even though we used low numbers of CD4+CD25-Foxp3+cells for the above study, it is possible that the effects seen in the above experiment may be due to small numbers of contaminating IFN-γ - or IL-22-producing activated T-cells or NK cells. However, in 5 LTBI+ individuals, when CD4+CD25+Foxp3+ cells were cultured in Transwells with M. tb-infected MDMs, addition of anti-IFN-γ or anti-IL-22, alone or together, did not reduce inhibition of M. tb growth in MDMs by CD4+CD25+ (85% Foxp3+) cells (Fig. 1B).
Fig. 1. Effect of CD4+CD25+Foxp3+cells on M. tb growth. 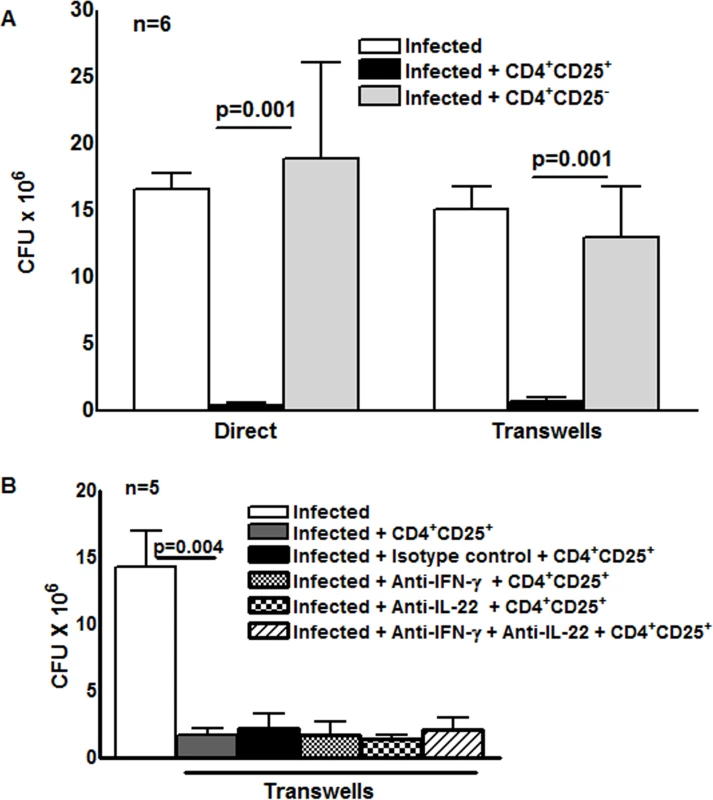
(A) Freshly isolated CD4+ cells and CD14+ monocytes from 6 individuals with LTBI were cultured with γ-irradiated M. tb H37Rv. After 4 days, CD4+CD25+ (85–90% Foxp3+) and CD4+CD25- (<5% Foxp3+) cells were isolated, as outlined in the methods. MDMs from the same donors were infected with H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM). After 2 hr MDMs were washed to remove extracellular bacteria and M. tb-expanded CD4+CD25+ (85–90% Foxp3+) or CD4+CD25- (<5% Foxp3+) cells were cultured in Transwells, or directly in the same wells, at a T-cell:macrophage ratio of 1:9. After 2 hr and 7 days, CFU were measured and mean values, p values and SEs are shown. In 6 individuals with LTBI the number of CFU after 2 hr was 1.2 ± 0.3 × 106. (B) H37Rv infected MDMs and M. tb-expanded CD4+CD25+ (85–90% Foxp3+) cells in Transwells were cultured as in panel A. To some wells, anti-IFN-γ (10 μg/ml), anti-IL-22 (10 μg/ml), alone or together (5 + 5 μg/ml), or isotype control antibodies (10 μg/ml) were added on days 0 and 3. After 7 days, CFU were measured and data for 5 individual LTBI+ donors was shown. Mean values, p values and SEs are shown. Fig. 2. A subset of Foxp3+ cells express D4GDI. 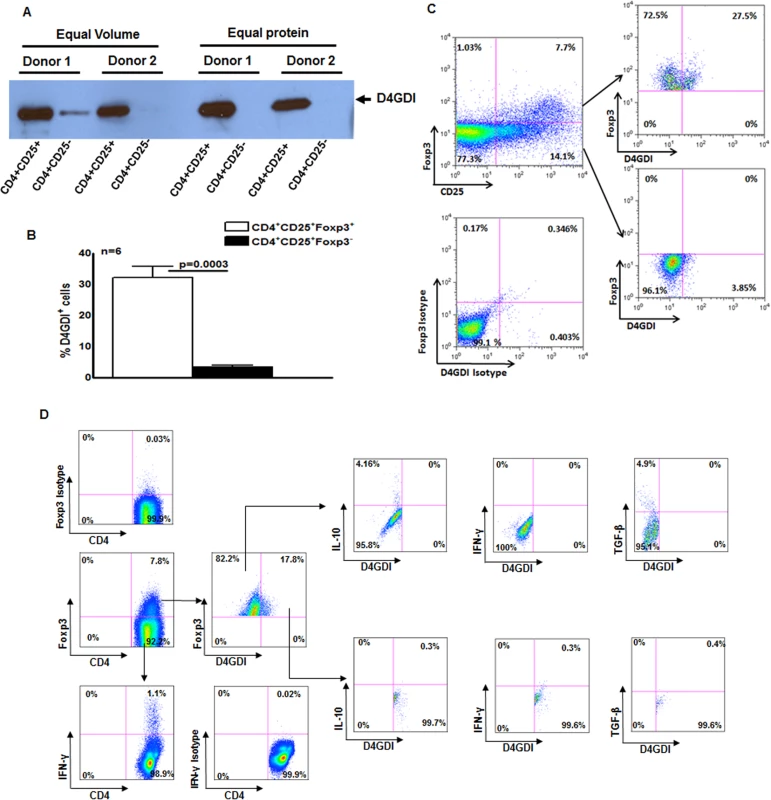
(A) CD4+ cells from PBMC of 6 donors with LTBI were cultured with autologous monocytes and γ-irradiated M. tb (10 μg/ml). After 4 days, CD4+CD25+ (85–90% Foxp3+) and CD4+CD25‑ (<5% Foxp3+) cells were isolated, and cultured overnight in serum-free medium. Culture supernatants were subjected to Western blotting with anti-D4GDI. Data from 2 individuals with LTBI are shown. (B) CD4+ cells and autologous monocytes from 6 donors were cultured as in panel A. After 4 days, the percentages of D4GDI+ cells in CD4+Foxp3+ and CD4+Foxp3‑ cells were determined by flow cytometry. Mean values, p values and SEs are shown. (C) A representative flow cytometry plot is shown. (D) PBMC from 5 individuals with LTBI were cultured with γ-irradiated M. tb H37Rv. After 4 days, the percentages ofCD4+Foxp3+D4GDI+IFN-γ+, CD4+Foxp3+D4GDI+IL-10+ and CD4+ Foxp3+D4GDI+TGF-β+ cells were determined by flow cytometry. A representative flow cytometry plot is shown. M. tb-expanded CD4+CD25+ (85%Foxp3+) cells produce D4GDI
To identify the soluble factor/s produced by M. tb-expanded CD4+CD25+Foxp3+ cells that inhibit M. tb H37Rv growth in macrophages, CD4+ cells and autologous monocytes from 6 persons with LTBI were cultured with γ-irradiated M. tb H37Rv. After 4 days, CD4+CD25+Foxp3+ and CD4+CD25-Foxp3- cells were isolated by immunomagnetic sorting, and cultured overnight in serum-free medium. The supernatants were concentrated and proteins in the supernatants were resolved by 2D gel electrophoresis. Only supernatants from CD4+CD25+Foxp3+ cells showed strong expression of a protein that was identified as D4GDI (100% match by LC MS/MS analysis, S1 Fig.).
We next found that D4GD1 was abundant in supernatants from M. tb-expanded CD4+CD25+ (85% Foxp3+) cells but not those from CD4+CD25-Foxp3- cells, using Western blotting (Fig. 2A). As additional confirmation, we isolated CD4+ cells from 6 individuals with LTBI, and cultured them with autologous monocytes and γ-irradiated M. tb for 4 days. We found that 33 ± 4% of CD4+Foxp3+ cells were D4GDI+, compared to only 3.2 ± 0.7% of CD4+CD25+Foxp3- cells (p = 0.0003, Figs. 2B and C). This suggests that a subpopulation of CD4+Foxp3+ cells produce D4GDI. In 5 individuals with LTBI, we measured IL-10, TGF-β and IFN-γ-positive CD4+CD25+Foxp3+D4GDI+ cells in γ-irradiated M. tb-stimulated PBMC. As shown in Fig. 2D, CD4+CD25+Foxp3+D4GDI- but not CD4+CD25+Foxp3+D4GDI+ cells are the source for IL-10 (5 ± 1.2% vs. 0.7 ± 0.3%, p = 0.02) and TGF-β (3.6 ± 2.1% vs. 0.5 ± 0.5%, p = 0.01). CD4+CD25+Foxp3- cells but not CD4+CD25+Foxp3+D4GDI- cells or CD4+CD25+ Foxp3+D4GDI+ cells are the major source or IFN-γ (1.2 ± 0.2% vs. 0.4 ± 0.2%, and 0.64 ± 0.1%, p = 0.06).
D4GDI inhibits growth of M. tb in human macrophages
We next wished to determine if D4GDI inhibits M. tb H37Rv growth in human MDMs. Recombinant D4GDI had no effect on uninfected MDM viability (S2 Fig.). Recombinant D4GDI reduced CFU in MDMs by 75–84% (p = 0.04, Fig. 3A). Next, we treated M. tb-expanded CD4+CD25+ (85% Foxp3+) cells with D4GDI siRNA, which inhibited D4GDI mRNA expression by 70% to 80%, as quantified by real-time PCR. In contrast, D4GDI siRNA did not affect Foxp3 expression. In 5 donors with LTBI, CD4+CD25+ (85% Foxp3+) cells treated with scrambled siRNA reduced M. tb growth in MDMs by 84% (p = 0.002, Fig. 3B). In contrast, CFU were 3-fold higher in MDMs exposed to D4GDI siRNA-transfected CD4+CD25+Foxp3+ cells (p = 0.01, Fig. 3B). CD4+CD25- cells treated with D4GDI or scrambled siRNA did not reduce CFU in MDMs. These findings indicate that D4GDI produced by M. tb-expanded CD4+CD25+ (85% Foxp3+) T-cells inhibits intracellular growth of M. tb.
Fig. 3. D4GDI inhibits growth of M. tb in macrophages. 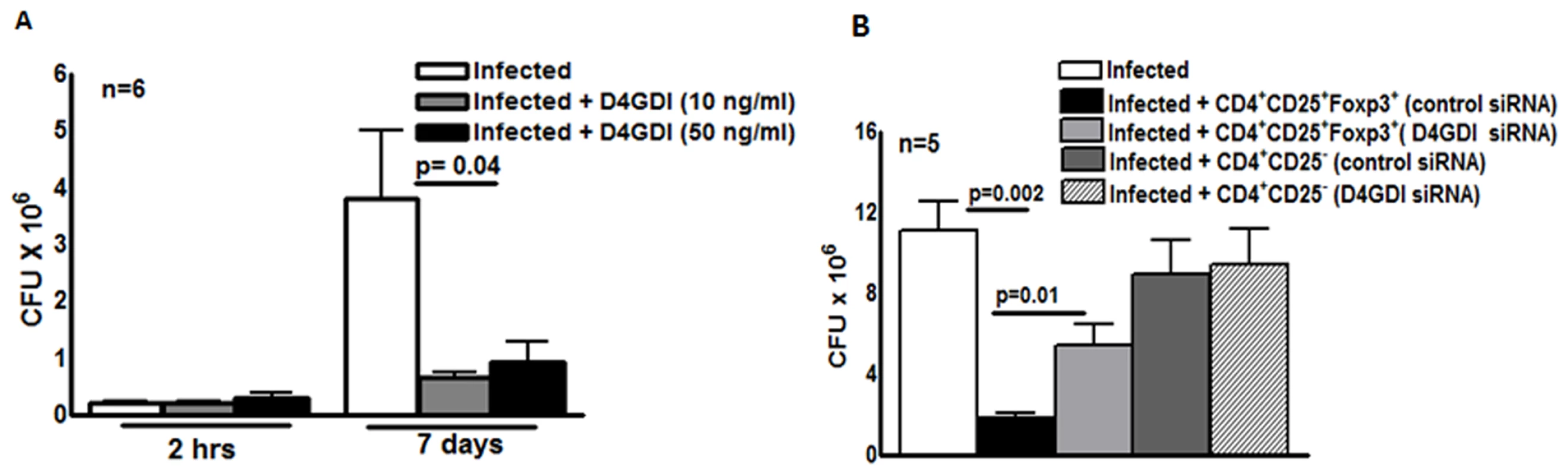
(A) Recombinant D4GDI inhibits growth of M. tb. MDMs from 6 LTBI+ individuals were infected with H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM), with or without recombinant D4GDI. After 7 days, CFU were measured. Mean values, p values and SEs are shown for CFU per well. (B) D4GDI siRNA reverses Treg-dependent M. tb growth inhibition in macrophages. CD4+ cells from PBMC of 5 donors with LTBI+ were transfected with siRNA for D4GDI or scrambled siRNA, then cultured with autologous monocytes and γ-irradiated M. tb (10 μg/ml). After 4 days, CD4+CD25+ (85–90% Foxp3+) and CD4+CD25- (<5% Foxp3+) cells were isolated, and cultured in Transwells with MDMs infected with H37Rv at a MOI of 1:2.5. After 7 days, CFU were measured. Mean values, p values and SEs are shown. D4GDI inhibits growth of M. tb in mice
Our data in humans (Fig. 3) suggest that CD4+CD25+ (85% Foxp3+) cells inhibit growth of M. tb through production of D4GDI. To determine if these cells express D4GDI during M. tb infection in vivo, we infected C57BL/6 mice with M. tb H37Rv. After 30 days, we quantified CD4+CD25+Foxp3+D4GDI+ cells in the lungs of uninfected and M. tb H37Rv-infected mice. Immunolabeling and flow cytometry showed that the total number of CD4+ cells increased 4.5 fold (p = 0.04, Fig. 4A), CD4+CD25+Foxp3+ cells increased around 9 fold (p = 0.009, Fig. 4B) and CD4+CD25+Foxp3+D4GDI+ cells increased more than 6-fold, compared to uninfected mice (p = 0.04, Fig. 4C).
Fig. 4. D4GDI inhibits growth of M. tb in mice. 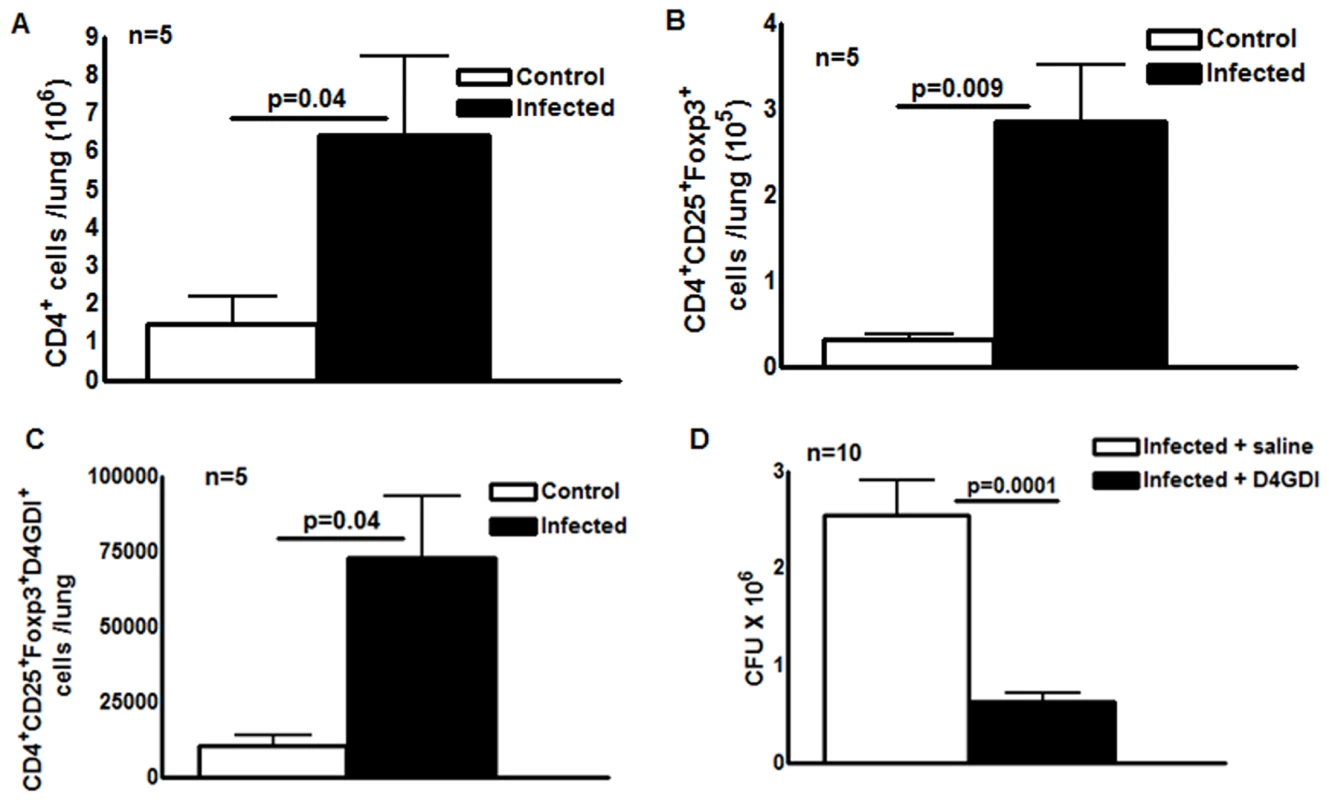
Ten wild type C57BL/6 mice were infected with 50–100 CFU of H37Rv by aerosol. Five mice were sacrificed after 24 hr and the bacterial burden in lungs was confirmed to be 50–100 CFU. The remaining five mice were sacrificed after 30 days. Total number of (A) CD4+ cells (B) CD4+CD25+Foxp3+ cells (C) CD4+CD25+Foxp3+D4GDI+ cells in the lungs of uninfected and M. tb-infected mice were measured by flow cytometry. (D) Twenty C57BL/6 wild type mice were infected with 50–100 CFU of M. tb H37Rv by aerosol. Ten infected mice were given recombinant D4GDI (10ng/ml) through tail vein injection on day 0, 4, 8, 12, 16, 20, 24 and 28 after infection. Thirty days later, bacterial burden in the lungs was measured by plating lung homogenates and counting CFU. Mean values, p values and SEs are shown. Further, we asked whether recombinant D4GDI can reduce bacterial burden in M. tb-infected mice. We infected C57BL/6 wild type mice with M. tb H37Rv by aerosol. Some infected mice were given recombinant D4GDI (10 ng/ml) through tail vein injection 0, 4, 8, 12, 16, 20, 24 and 28 days after infection. Thirty days later, we measured CFU in the lungs. Recombinant D4GDI reduced CFU by 4 fold (p = 0.0001, Fig. 4D). These findings suggest that D4GDI produced by M. tb-expanded CD4+CD25+Foxp3+ T-cells inhibits M. tb growth in mice.
Apoptotic Foxp3+ cells are a source of D4GDI
Our findings above suggest that T-cell supernatants contain D4GDI, which inhibits growth of M. tb in MDMs (Fig. 1 and S1 Fig.). However, in humans, D4GDI is expressed primarily by T and B cells [15] and is not a secreted protein. We asked whether D4GDI is released when terminally differentiated Foxp3+ cells undergo apoptosis. CD4+ cells and autologous monocytes from 6 persons with LTBI were cultured with γ-irradiated M. tb H37Rv. After 4 days, annexinV expression by CD4+CD25+Foxp3+, CD4+CD25+Foxp3- and CD4+CD25-Foxp3- cells was determined by flow cytometry. Among CD4+ cells exposed to γ-irradiated M. tb, 7.2 ± 1.9% of CD25+FoxP3+D4GDI+, 4.7 ± 0.3% of CD25+FoxP3+D4GDI- and 1.5 ± 0.6% of CD4+CD25+ FoxP3-D4GDI- cells are annexinV+ (Figs. 5A and C). As an alternative measure of apoptosis, we determined caspase 3 expression in cultured cells. Similar to the above findings, among CD4+ cells exposed to γ-irradiated M. tb, 15.8 ± 3.3% of CD25+Foxp3+D4GDI+ and 0.82 ± 0.1% of CD25+Foxp3+D4GDI-cells were caspase 3+ (S3 Fig.). Among CD4+CD25+Foxp3- cells 3.5 ± 2.1% of them were caspase 3+ (S3 Fig.) and none were D4GDI+, suggesting that only a subpopulation of CD4+Foxp3+D4GDI+ cells that undergo apoptosis release D4GDI. To further determine if D4GDI is specifically released by apoptotic CD4+Foxp3+ cells but not by CD4+Foxp3- cells, we cultured the latter cells in the presence of cyclosporin A (10 μg/ml), a known inducer of CD4+ T-cell apoptosis. As shown in S4 Fig., apoptotic CD4+Foxp3- cells did not express D4GDI despite induction of apoptosis.
Fig. 5. Apoptotic Foxp3+ cells are the source for D4GDI. 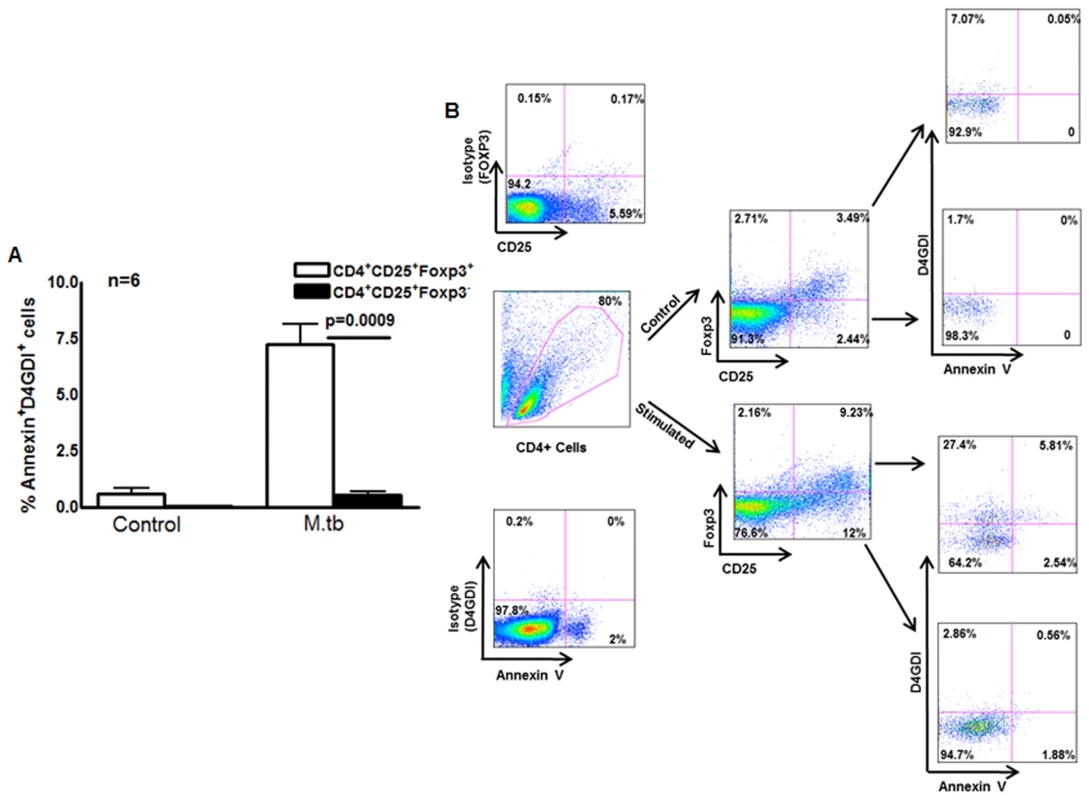
(A) Expression of D4GDI by apoptotic Foxp3+ cells. CD4+ cells and autologous monocytes from 6 persons with LTBI were cultured with γ-irradiated H37Rv. After 4 days, CD4+CD25+Foxp3+, CD4+CD25+Foxp3- and CD4+CD25-Foxp3- cells that expressed annexinV and/or D4GDI were identified by flow cytometry. Mean values, p values and SEs are shown. (B) A representative flow cytometry plot is shown. Macrophages internalize D4GDI
We next determined whether MDMs can take up D4GDI. MDMs from 6 healthy donors were infected with GFP - M. tb H37Rv as described in the methods, and some of the uninfected and infected MDMs were cultured with N-terminal-tagged GST-D4GDI fusion protein. Using confocal microscopy, as described in the methods, we found significant amounts of internalized D4GDI fusion protein in M. tb H37Rv-infected MDMs (S5 Fig.).
Interleukin-1 beta (IL-1β), Tumor Necrosis Factor-alpha (TNF-α) and Granulocyte Colony-Stimulating Factor (G-CSF) contribute to D4GDI-dependent inhibition of M. tb growth
To identify molecules that control D4GDI-mediated inhibition of M. tb growth in MDMs, we cultured M. tb H37Rv-infected MDMs from 5 to 6 healthy donors, with or without recombinant D4GDI. After 6 hr, culture supernatants were collected, and levels of 27 different cytokines and chemokines were measured, using a multiplex ELISA system. Recombinant D4GDI increased levels of interleukin-1 beta (IL-1β) from 43 ± 33 pg/ml to 193 ± 104 pg/ml (p = 0.02, Fig. 6A), tumor necrosis factor-alpha (TNF - α) from 88 ± 126 pg/ml to 129 ± 111 pg/ml (p = 0.03, Fig. 6B), and granulocyte colony-stimulating factor (G-CSF) from 122 ± 67 pg/ml to 223 ± 135 pg/ml (p = 0.02, Fig. 6C). Recombinant D4GDI had no effect on cytokine production by uninfected MDMs (Fig. 6). To identify additional molecules that are involved in D4GDI-mediated inhibition of M. tb growth, we compared gene expression profiles of M. tb-infected MDMs, cultured with or without recombinant D4GDI, using microarray analysis for 40,000 genes. MDMs from 4 healthy donors were infected with M. tb H37Rv and some of the infected MDMs were cultured with recombinant D4GDI. After 6 hr, total RNA was extracted from control, infected and D4GDI-treated infected MDMs. In all 4 donors, mRNA expression was >1.25-fold higher for 42 genes <1.25-fold lower for 4 genes in D4GDI-treated cells (S1 Table). Because the focus of our study is to identify the molecules that are involved in D4GDI-dependent inhibition of M. tb H37Rv growth, we selected four upregulated genes, IL-1β, TNF-α, MMP-12 and IL-27, for further study, because these molecules have been shown to play important roles in M. tb infection. D4GDI induced G-CSF production (Fig. 6C) but we did not find differences in mRNA expression. We also included G-CSF and a downregulated gene called SIVA (proapoptotic molecule) for further study. Transfection of M. tb-infected MDMs with IL-1β and TNF-α siRNAs reduced mycobacterial growth inhibition by D4GDI by 80% (p<0.05, Fig. 6H) and G-CSF siRNA decreased D4GDI-induced growth inhibition by 30% (p<0.05, Fig. 6H). In contrast, MMP-12, SIVA and IL-27 siRNAs had no effect on D4GDI-dependent M. tb H37Rv growth inhibition (Fig. 6H).
Fig. 6. IL-1β and TNF-α contribute to D4GDI-dependent inhibition of M. tb growth. 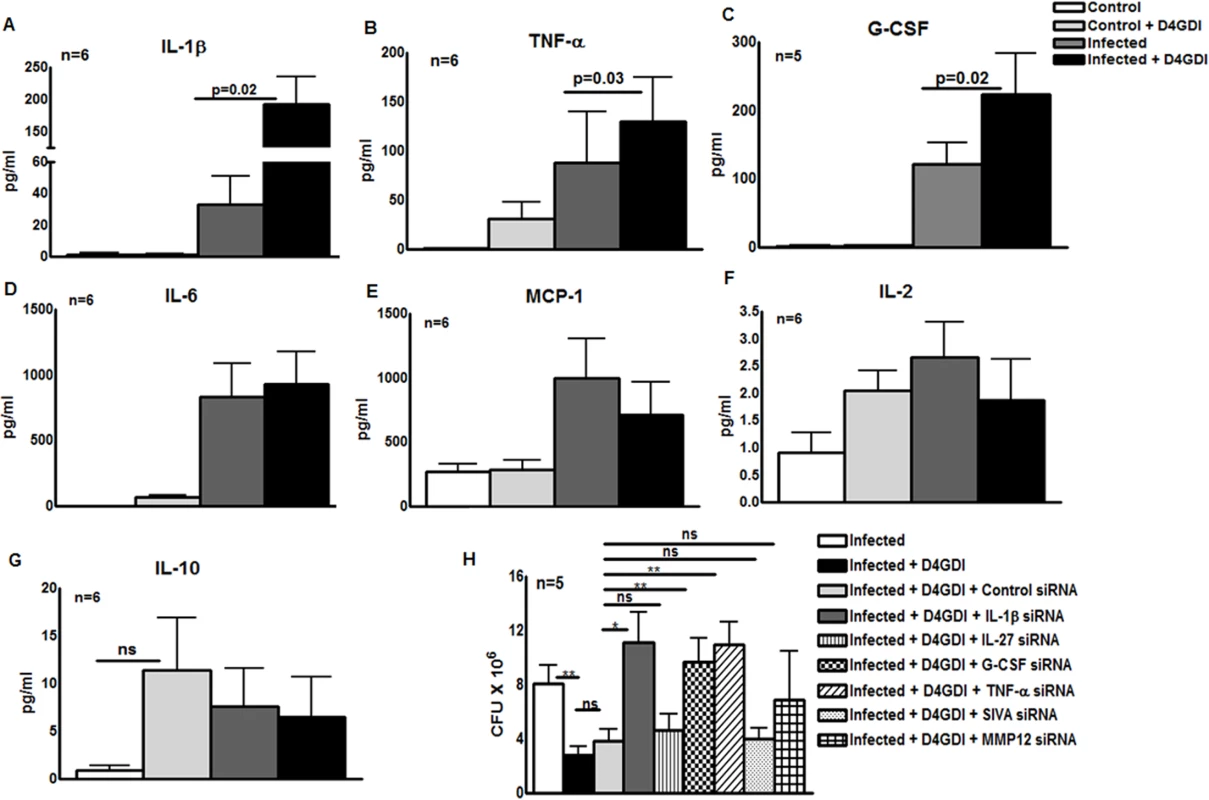
MDMs from 5 to 6 healthy donors with LTBI were infected with H37Rv, and control and infected MDMs were cultured with or without recombinant D4GDI. After 6 hr, culture supernatants were collected and levels of 27 different cytokines and chemokines were measured by multiplex ELISA. (A) IL-1β production. (B) TNF-α production. (C) G-CSF production. (D) IL-6 production. (E) MCP-1 production. (F) IL-2production. (G) IL-10 production. (H) Effect of IL-1β, IL-27, G-CSF, TNF-α, SIVA and MMP12 siRNAs on D4GDI-dependent M. tb growth inhibition. MDMs from 5 donors were transfected with siRNA for IL-1β, IL-27, G-CSF, TNF-α, SIVA, MMP-12 or scrambled siRNA (control siRNA), and infected with H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM). Some infected MDMs were cultured with recombinant D4GDI (10 ng/ml). After 7 days, CFU were measured. Mean values, p values and SEs are shown. *p<0.05, **p<0.005. Reactive oxygen species (ROS) contribute to D4GDI-induced IL-1β, TNF-α and G-CSF dependent growth inhibition
Because D4GDI increases production of ROS by mononuclear phagocytes in other experimental systems [15], [16], we wished to determine if D4GDI-induced IL-1β-, TNF-α - and G-CSF-dependent growth inhibition depended on ROS production. ROS production by MDMs was enhanced by infection with M. tb H37Rv, and this was further increased by addition of D4GDI (MFI of 1286.8 ± 501.9 vs. 598.4 ± 158.3, p = 0.01, Fig. 7A). ROS levels were similar with addition of D4GDI and hydrogen peroxide (Fig. 7A). To determine if D4GDI-dependent ROS production inhibits growth of M. tb H37Rv, we used N-acetylcysteine (NAC), a scavenger of ROS. D4GDI reduced CFU in infected MDMs by 84% and NAC completely abrogated this effect (p = 0.02, Fig. 7B), suggesting that D4GDI inhibits M. tb H37Rv growth by inducing M. tb-infected MDMs to produce ROS. We also found that transfection of M. tb-infected MDMs with IL-1β, TNF-α and G-CSF siRNAs reduced D4GDI-induced ROS production (p<0.05 Fig. 7C).
Fig. 7. Reactive oxygen species (ROS) contribute to D4GDI-induced IL-1β, TNF-α and G-CSF dependent growth inhibition. 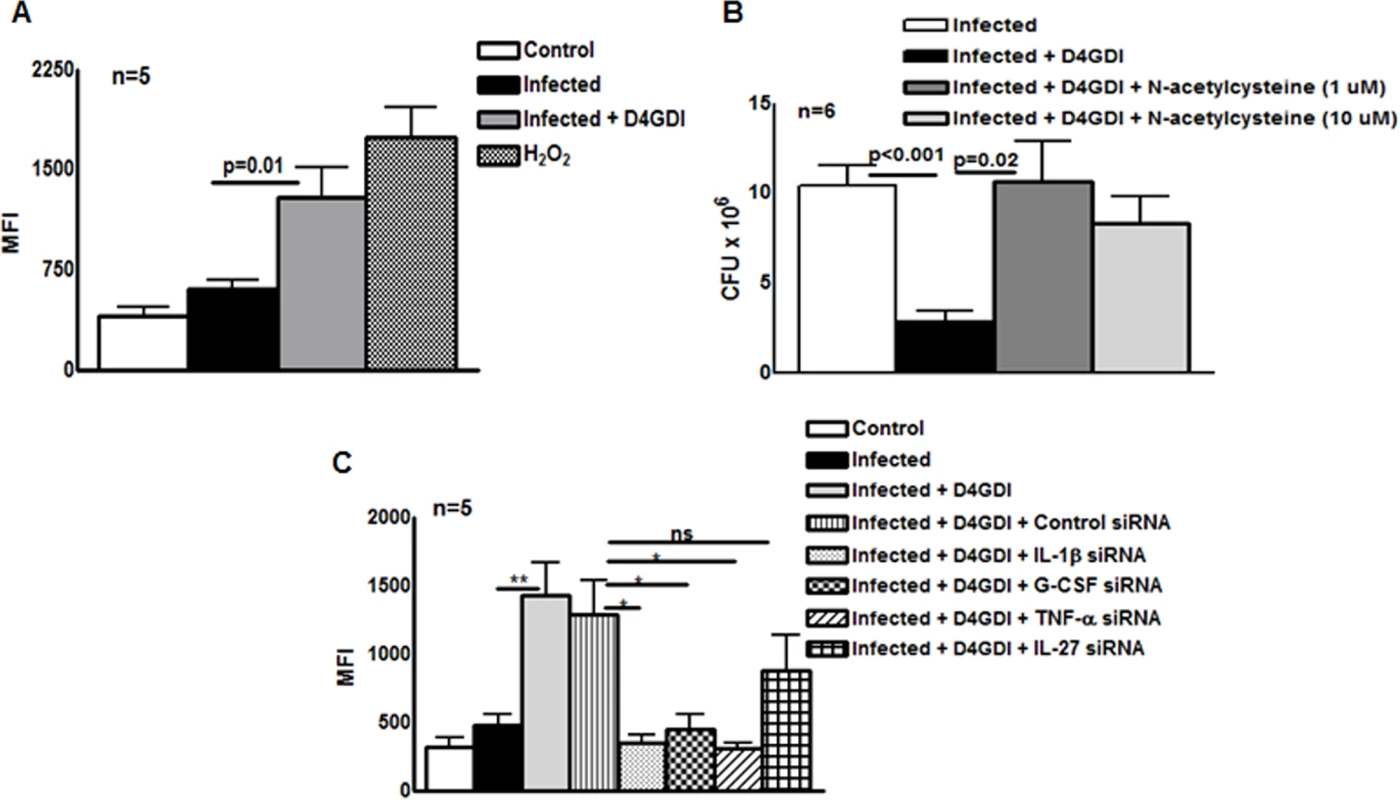
MDMs from 5 donors were infected with H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM). To some wells, recombinant D4GDI (10 ng/ml) or hydrogen peroxide (10 μM) were added. (A) After 24 hr, cells were labeled with 2′,7′-dichlorofluorescein, and ROS expression was measured by flow cytometry. (B) MDMs from 6 donors were cultured. N-acetylacysteine was added to some wells and CFU were measured after 7 days. (C) IL-1β or TNF-α regulate D4GDI-dependent ROS production. MDMs from 5 donors were transfected with siRNA for IL-1β, TNF-α, G-CSF, IL-27or scrambled siRNA (control siRNA), and infected with H37Rv at a MOI of 1:2.5. Some infected MDMs were cultured with recombinant D4GDI (10 ng/ml). After 24 hr, cells were labeled with 2′,7′-dichlorofluorescein, and ROS expression was measured by flow cytometry. Mean values and SEs are shown. *p<0.05, **p<0.005. D4GDI enhances the apoptosis of M. tb-infected macrophages
D4GDI increased the production of IL-1β, TNF-α, G-CSF and ROS by M. tb H37Rv-infected MDMs to inhibit M. tb growth. We next asked whether D4GDI-dependent growth inhibition is due to enhanced ROS-induced apoptosis of infected MDMs. M. tb H37Rv infection increased the percentage of apoptotic MDMs and this was further increased by addition of D4GDI (5.6 ± 0.9% vs. 1.7 ± 0.9%, Fig. 8). Addition of NAC, a scavenger of ROS, inhibited D4GDI-mediated apoptosis. We also found that D4GDI-induced apoptosis was reduced by transfection of M. tb-infected MDMs with siRNAs for IL-1β or TNF-α (p<0.05) but not by G-CSF siRNA (Fig. 8).
Fig. 8. D4GDI enhances apoptosis of M. tb-infected MDMs. 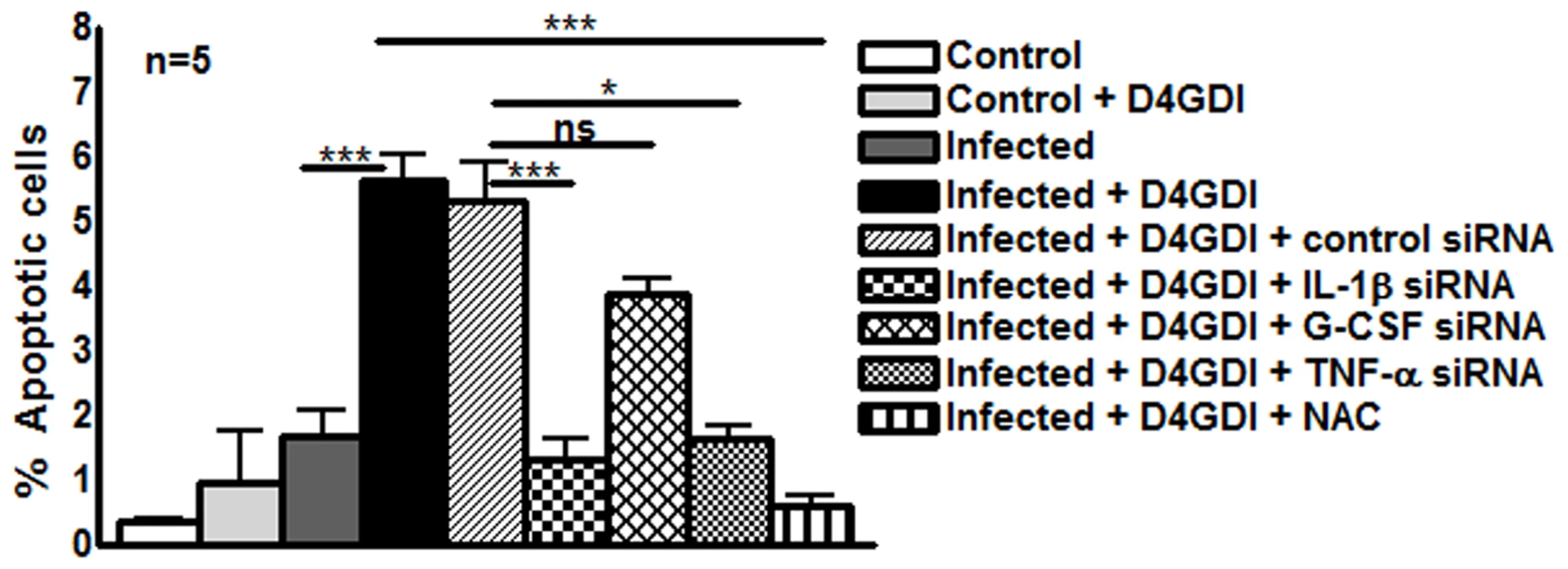
(A) Apoptosis of control and M. tb-infected MDMs in the presence of D4GDI. MDMs from 5 donors were infected with H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM). To some control and M. tb-infected MDM wells, recombinant D4GDI (10 ng/ml) was added. After 3 days, a TUNEL assay was performed to identify apoptotic cells by flow cytometry. MDMs were transfected with siRNA for IL-1β, TNF-α, G-CSF, or scrambled siRNA, and infected with H37Rv at a MOI of 1:2.5 (2.5 M. tb to one MDM). Some infected MDMs were cultured with recombinant D4GDI (10 ng/ml). After 3 days, a TUNEL assay was performed to determine the apoptotic cells by flow cytometry. Mean values and SEs are shown. *p<0.05, ***p<0.0005. M. tb-expanded CD4+CD25+ (85% Foxp3+) cells and D4GDI induce dosR expression by M. tb
To characterize the physiologic state of M. tb in MDMs during exposure to M. tb-expanded CD4+CD25+Foxp3+ T-cells, we used real-time PCR to measure expression of dosR, which encodes a transcription factor that is upregulated in response to hypoxia and nitric oxide [17]-[19]. DosR activates expression of multiple genes when M. tb enters a nonreplicative state, including dormancy induced by anaerobic conditions [20]. MDMs from 7 healthy tuberculin reactors were infected with M. tb H37Rv and cultured with autologous M. tb-expanded CD4+CD25+ (85% Foxp3+) or CD4+CD25- cells in Transwells. After 7 days, dosR expression was increased 4-fold in MDMs exposed to CD4+CD25+ (85% Foxp3+) cells (p = 0.04, Fig. 9A), but not in those exposed to CD4+CD25-Foxp3- cells.
Fig. 9. Effect of CD4+CD25+Foxp3+cells and D4GDI on M. tb expression of dosR. 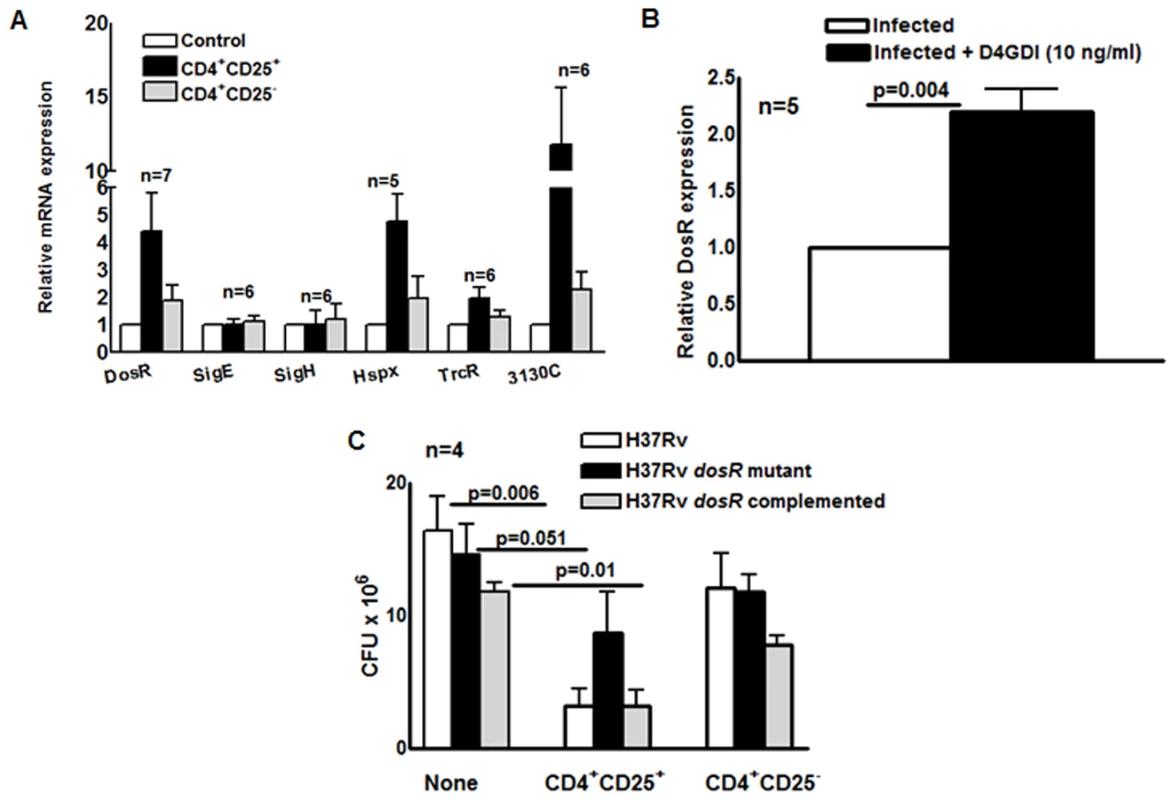
(A) MDMs from 7 donors with LTBI were infected with M. tb and cultured with autologous M. tb-expanded CD4+CD25+ (85–90% Foxp3+) or CD4+CD25- (<5% Foxp3+) cells in Transwells. After 7 days, mycobacterial mRNA was quantified by real-time PCR. Mean values and SEs are shown. (B) Effect of D4GDI on DosR expression. MDMs from 5 healthy donors were infected with H37Rv, with or without D4GDI (10 ng/ml). After 7 days, DosR expression was quantified by real time PCR. ** P = 0.004. (C) Effect of CD4+CD25+FoxP3+ cells on the growth of a M. tb DosR deletion mutant. MDMs from four donors with LTBI were infected with the H37Rv strains shown, and M. tb-expanded CD4+CD25+ (85–90% Foxp3+) cells were added in Transwells, at a T-cell:macrophage ratio of 1:9. After 7 days, CFU were measured. Mean values and SEs are shown. Mean values, p values and SEs are shown. Expression of Rv3130c and hspX, which are part of the DosR regulon, was also markedly increased in MDMs exposed to CD4+CD25+ (85% Foxp3+) cells (p<0.07, Fig. 9A). Addition of D4GDI to M. tb-infected MDMs also induced dosR expression two-fold (p = 0.004, Fig. 9B). Because DosR can be activated by multiple stresses [21], we evaluated other stress-associated regulators to determine whether D4GDI induced a generalized mycobacterial stress response that was not associated with non-replicating persistence. Expression of these genes (trcR, sigE and sigH) was not increased after exposure to CD4+CD25+ (85% Foxp3+) cells (Fig. 9A).
A M. tb dosR deletion mutant showed no growth defect in MDMs, compared to wild type M. tb H37Rv, but addition of CD4+CD25+ (85% Foxp3+) cells reduced CFU of wild type M. tb H37Rv by 80%, compared to only 40% for the dosR mutant (Fig. 9C). The dosR complemented strain behaved similarly to wild type M. tb H37Rv, confirming that this difference was due to dosR and not to effects on adjacent M. tb genes. These findings demonstrate that M. tb-expanded CD4+CD25+Foxp3+ T-cells and D4GDI inhibit intracellular mycobacterial growth through mechanisms that upregulate expression of dosR, and may induce M. tb to enter a state of non-replicating persistence.
D4GDI expression by TB patients
To investigate the potential contribution of D4GDI in vivo at the site of disease, we studied patients with tuberculous pleuritis, as the pleural immune response usually leads to clearing of local infection [22], [23]. Western blotting showed that D4GDI expression was significantly higher in pleural fluid than in serum of 10 patients with tuberculous pleuritis (Fig. 10A), suggesting that D4GDI is part of an effective local human immune response to M. tb infection.
Fig. 10. D4GDI expression by TB patients. 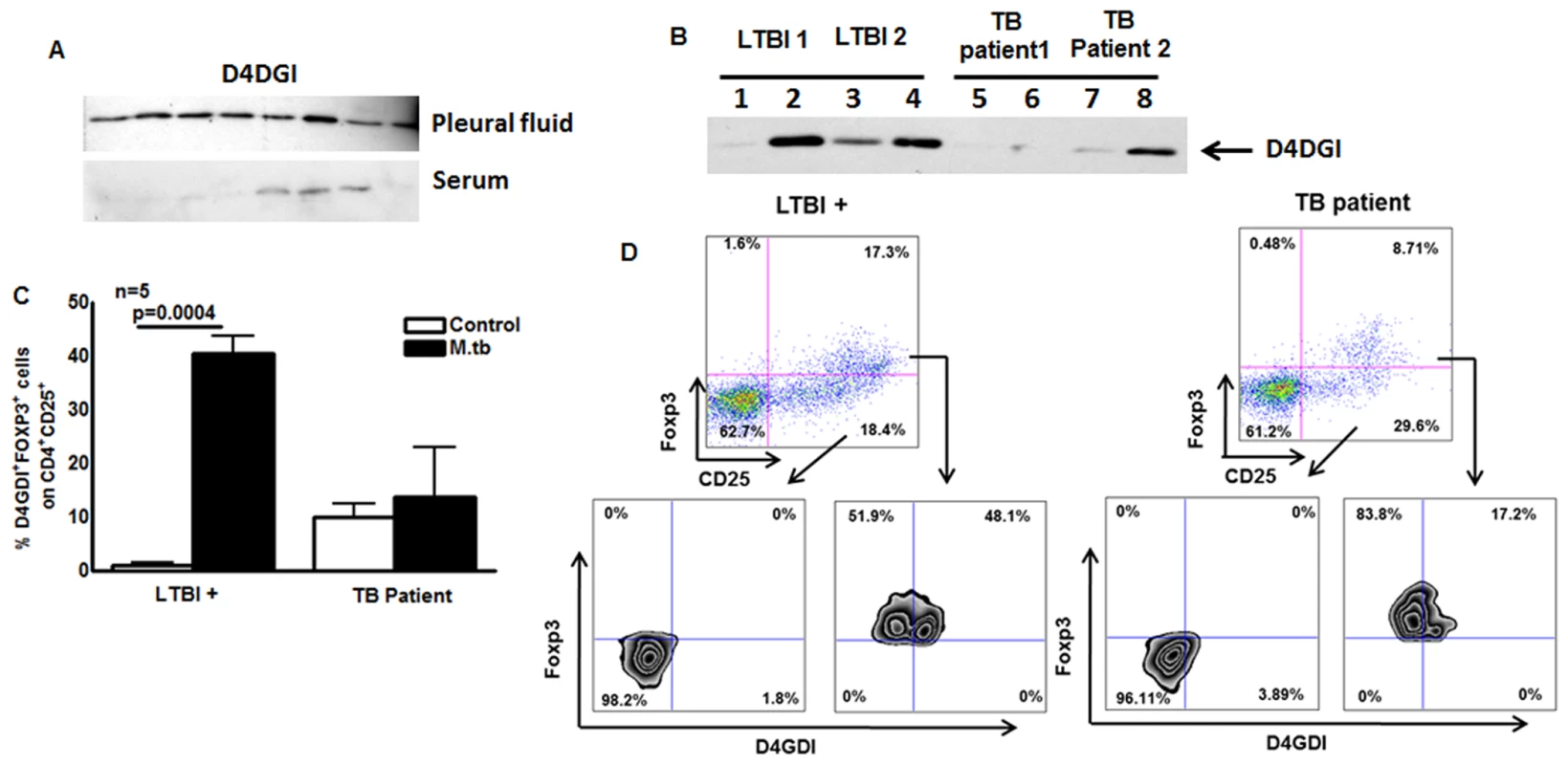
(A) D4GDI is highly expressed in pleural fluid of TB patients. Pleural fluid and serum from 10 patients were collected, and equal amounts of protein were subjected to Western blotting with a monoclonal antibody to D4GDI. Data from 8 patients are shown. (B) PBMC from TB patients express reduced amounts of D4GDI. PBMC from 5 donors with LTBI and 5 TB patients were cultured with γ-irradiated H37Rv (10 μg/ml). After 5 days, culture supernatants were subjected to Western blotting with monoclonal antibody to D4GDI. Data from 2 patients and 2 individuals with LTBI+ are shown. (C) Intracellular staining for FOXP3+D4GDI+ cells. CD4+ cells from PBMC of 5 donors with LTBI+ and 5TB patients were cultured with autologous monocytes in medium alone, or with γ-irradiated H37Rv (10 μg/ml). After 4 days, immunolabeling and flow cytometry were performed. (D) A representative flow cytometry plot is shown. Mean values, p values and SEs are shown. In contrast to tuberculous pleurtis, systemic immunity is reduced in patients with pulmonary TB, whose PBMC show decreased production of IFN-γ in response to mycobacterial antigens, compared to healthy persons with LTBI, who have protective immunity to M. tb [8]. We stimulated PBMC from TB patients and donors with LTBI with γ-irradiated M. tb, and measured D4GDI levels in culture supernatants by Western blotting. Expression of D4GDI was significantly reduced in TB patients, compared to findings in donors with LTBI+ (Fig. 10B). When CD4+ cells from 5 TB patients and 5 donors with LTBI+ were cultured with autologous monocytes and γ-irradiated M. tb for 4 days, the percentages of CD4+CD25+Foxp3+D4GDI+ cells were higher in persons with LTBI+ (p = 0.0004, Fig. 10C) compared to TB patients, suggesting reduced expansion of D4GDI+ cells in TB patients.
Discussion
In the current study we found that human CD4+CD25+ (85% Foxp3+) cells inhibit growth of M. tb in macrophages, providing the first evidence that CD4+CD25+ (85% Foxp3+) cells contribute to effective immunity against an intracellular pathogen. These salutary effects were mediated through production of D4GDI, which enhanced apoptosis of MDMs to inhibit M. tb growth through the production of IL-1β, TNF-α and reactive oxygen species. D4GDI was concentrated at the site of disease in tuberculosis patients, D4GDI+ cells were expanded in mice infected with M. tb, and recombinant D4GDI inhibited M. tb growth in mice, suggesting that this molecule contributes to immune defenses in vivo. Furthermore, CD4+CD25+ (85% Foxp3+) cells and D4GDI upregulated the mycobacterial gene DosR which activates expression of multiple genes when M. tb enters a nonreplicative state. The sum of these data suggest a novel role for human CD4+CD25+Foxp3+ cells in protection against infection and identify a new molecule, D4GDI, through which such protection is mediated.
Tregs influence the immune response to parasitic, bacterial, viral and fungal pathogens. The effects may be favorable or harmful to the host, depending on the pathogen and stage of infection. In Leishmania infection, Tregs persist at the site of infection, are essential for parasite persistence but favor host immunity to exogenous reinfection [24], [25]. In human Plasmodium falciparum infection, the percentage of Tregs correlates directly with parasitic growth [26] and depletion of Tregs enhances survival of mice infected with P. yoelii [27], indicating the negative effects of Tregs on the host in malaria. In contrast, Tregs suppress Helicobacter pylori-specific T cell responses [28], reducing gastric inflammation and ulceration [29]. Tregs negatively affect host immune responses to viral infections, such as HIV [30], cytomegalovirus [31], Epstein-Barr virus and hepatitis B and C [32]–[35]. We found that a subpopulation of CD4+CD25+Foxp3+ T-cells enhance immunity by producing D4GDI, which inhibits M. tb growth in human macrophages. Our studies are further supported by recent findings that in Candida albicans infected mice, Tregs enhance Th17 cytokine production to clear the fungal infection [36]. Recent studies have shown that human CD4+CD25+ cells express Foxp3 transiently and do not have classical regulatory function. Previously we found that M. tb-expanded CD4+CD25+Foxp3+ cells produce IL-10 and TGF-β to inhibit IFN-γ production by T-cells [6], [7]. Our findings in Fig. 2D indicate that the subpopulation of CD4+CD25+Foxp3+ cells that express D4GDI do not express IL-10 and TGF-β, suggesting that they are not immunosuppressive. Our current findings suggest that subpopulations of M. tb-expanded human Tregs differ in their function.
D4GDI is one of the Rho GDP dissociation inhibitors, small GTP-binding proteins that stabilize the GDP dissociation inhibitor-bound form of Rho GTPases, sequestering Rho GTPases at the plasma membrane [15], [37]. In humans, D4GDI is expressed primarily by T and B cells [15] and is involved in diverse cellular functions, including proliferation, cell signaling, cytoskeletal organization and apoptosis [15], [38], but there is limited information available on the role of D4GDI in microbial infection. Overexpression of D4GDI in T-cells reduces HIV replication through inactivation of RhoA, which reduces viral receptor clustering and lowers the efficiency of virus-cell membrane fusion [39]. However, given the differences in intracellular replication strategies of HIV and M. tb, this mechanism would not explain our current results.
We found that silencing of IL-1β and TNF-α gene expression reduces D4GDI-dependent inhibition of M. tb growth (Fig. 6). IL-1αβ-/- and IL-1R-/- mice are highly susceptible to M. tb infection [40], [41] and IL-1β inhibits M. tb growth in macrophages through caspase-3 activation [42], suggesting an important role for IL-1β in innate immune responses against M. tb infection. In humans, M. tb induces IL-1β production through its interaction with TLR2, TLR6 and NOD2 receptors [43]. In addition, TLR2/1-induced antimicrobial responses against M. tb depend on convergence of the IL-1β and vitamin D receptor activation pathways [44], [45]. In mice, caspase-1-independent IL-1β production is critical for host resistance to M. tb but does not require TLR signaling [41]. Similarly, TNF-α is essential for control of infection with M. tb in animals [46] and inhibits growth of M. tb in human monocytes and alveolar macrophages [11], [47], [48]. IL-1β inhibits M. tb growth in macrophages by enhancing TNF-α production and through caspase-3 activation [42], suggesting the vital role of these two molecules in M. tb infection. In the current study we found that a subpopulation of Tregs produce a novel molecule, D4GDI, which induces IL-1β and TNF-α production by human macrophages to inhibit M. tb growth.
We found that IL-1β and TNF-α produced by MDMs in response to D4GDI enhance ROS production, and inhibition of ROS reverses D4GDI-dependent inhibition of M. tb growth in macrophages (Fig. 7). ROS are vital in eliminating many pathogens, including M. tb [49], [50] and patients with chronic granulomatous disease, whose phagocytic cells fail to generate superoxide, are highly susceptible to infections with fungi and bacteria, including mycobacteria [51], [52]. D4GDI is recruited to the phagosome of RAW macrophages after infection with Listeria monocytogenes [53], but its role in ROS production is controversial. Some investigators concluded that D4DGI inhibited ROS production in response to Listeria [53], whereas others showed that phagocytic cells from D4GDI-deficient mice produce less superoxide [15], [16]. Our current findings suggest that D4GDI produced by CD4+CD25+Foxp3+ T-cells increases IL-1β and TNF-α production which enhance ROS production by human macrophages in response to M. tb infection, reducing bacillary replication. ROS can induce apoptosis, enhance autophagy and elicit production of antimicrobial peptides in M. tb-infected macrophages [54], [55], all of which can inhibit intracellular mycobacterial growth [56]–[58].
We found that ROS generated by D4GDI increase M. tb-infected macrophage apoptosis and mycobacterial killing (Fig. 8). Apoptosis is a potent mechanism for elimination of mycobacteria. More virulent strains of the M. tb complex inhibit apoptosis and grow more rapidly in macrophages, whereas attenuated strains such as H37Ra and BCG elicit greater apoptosis and show reduced intracellular growth [59], [60]. The M. tb nuoG gene inhibits macrophage apoptosis and a nuoG deletion mutant has reduced virulence in SCID mice [61]. ROS generated during M. tb infection induce signaling molecules, such as signal-regulating kinase, which induce apoptosis [62]. We found that D4GDI enhances apoptosis of M. tb infected macrophages through the production of IL-1β, TNF-α and ROS to inhibit M. tb growth.
Disease manifestations in human TB result not only from uncontrolled bacillary proliferation, but also from tissue inflammation due to a vigorous immune response. In this setting, the role of Tregs in the pathogenesis of TB remains controversial. Elegant experiments in mice suggest that Tregs inhibit immunity to M. tb by delaying trafficking of effector T cells to the site of infection [5], [63], but studies in macaques show that the frequency of Tregs is higher in animals that developed LTBI than in those that developed active disease, suggesting that Tregs do not cause progression to active disease [64]. Tregs are well known to downregulate immune responses in many experimental models, and CD4+Foxp3+ cells expand in response to inflammation during early TB in macaques [64], suggesting that they limit tissue destruction. We found that CD4+CD25+Foxp3+ cells that expand upon exposure to M. tb produce D4GDI, which inhibits mycobacterial growth in macrophages. D4GDI was selectively concentrated in pleural fluid of patients with tuberculous pleuritis (Fig. 10A), who have an effective immune response that clears local infection. In addition, PBMC from TB patients with ineffective immunity have reduced production of D4GDI (Fig. 10B). Furthermore, recombinant D4GDI reduced the bacillary burden in M. tb infected mice (Fig. 4B). Because CD4+Foxp3+ cells are enriched in pleural fluid of TB patients [65] and in mycobacterial granulomas [66], we speculate that CD4+CD25+ cells that express Foxp3 transiently at the site of disease release D4GDI, which inhibits M. tb growth. The sum of our studies in humans and murine models of infection suggest that D4GDI produced by CD4+CD25+Foxp3+ T-cells has a previously undescribed positive effect on immunity by enhancing host antimicrobial activity. Our results suggest that D4GDI is part of an effective local human immune response to M. tb infection in LTBI individuals. Previous studies have found increased Foxp3+cells at the site of TB disease [65], [67]. We found that apoptotic CD4+CD25+Foxp3+ cells express D4GDI and that high D4GDI levels are present at the site of disease, suggesting high turnover of CD4+CD25+FoxP3+ cells at the site of active disease. Further studies to understand the mechanisms that induce D4GDI+ cell expansion, production and turnover will provide new insight into the pathogenesis of TB, and identify strategies to enhance immune responses in TB patients.
We found that D4GDI causes intracellular M. tb to increase expression of DosR and DosR-regulated genes (Fig. 9). DosR controls expression of a suite of genes required during dormancy induced by anaerobic conditions, when M. tb enters a hypometabolic state with minimal replication [20], [68], [69]. DosR is activated by two sensor kinases, DosT and DosS, that fine-tune the response to changes in oxygen, nitrous oxide and carbon monoxide levels, and that appear to modulate the entry of M. tb into dormancy in a step-wise fashion [19], [20], [70]. Further work is required to determine the conditions that induce the DosR regulon following macrophage exposure to D4DGI. Mycobacterial growth inhibition by CD4+CD25+Foxp3+ cells was reduced in the M. tb dosR deletion mutant (Fig. 9B), suggesting that the growth inhibition depends in part on activation of the DosR regulon. Our results suggest that macrophage exposure to D4GDI triggers conditions that induce DosR, followed by entry of the bacteria into a nonreplicative state. These findings may aid in understanding the factors that induce LTBI.
In summary, our study demonstrates that D4GDI inhibit growth of M. tb in macrophages, providing the first evidence that D4GDI contribute to effective immunity against an intracellular pathogen. We are currently determining whether D4GDI can be used as an immunologic marker to detect persons at greatest risk of progression of LTBI to TB, so that this subpopulation can be targeted to receive isoniazid, reduce TB morbidity and improve public health. Additional studies are needed to determine if D4GDI can be used as an immunostimulatory treatment for TB and other infectious diseases.
Materials and Methods
Patient population
After obtaining informed consent, blood was obtained from 20 healthy persons with positive QuantiFERON-TB Gold tests, indicative of LTBI, 12 healthy persons with negative QuantiFERON-TB Gold tests and 15 HIV-seronegative patients with culture-proven TB who had received antituberculosis therapy for <4 weeks. All donors were between the ages of 18 and 65. Individuals with LTBI did not have a history of TB or HIV infection, and were not receiving therapy with immunosuppressive drugs.
Pleural fluid was obtained from 10 patients with tuberculous pleuritis who had received antituberculosis therapy for <5 days. All patients had unilateral exudative effusions, and the diagnosis was confirmed by culture of M. tb from pleural fluid or tissue, or by histologic evidence of granulomatous pleuritis, combined with a response to antituberculosis therapy.
Animals
All animal studies were performed on specific-pathogen-free 4–6-week-old female C57BL/6 mice and approved by The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler.
Ethics statement
All studies were approved by the Institutional Review Boards of the University of Texas Health Science Center at Tyler (Protocol #640) and the University of Southern California School of Medicine. All the human subjects were in between 18 to 65 years and written informed consent was obtained before enrolling in the study. The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler approved the animal work (Protocol #488). All animal procedures involving the care and use of mice were in accordance with the guidelines of NIH / OLAW (Office of Laboratory Animal Welfare).
M. tb strains
H37Rv, a virulent laboratory strain of M. tb, was used in most experiments. A dosR mutant, its complemented strain, and its parental strain of H37Rv, were used in some experiments [68].
Abs and other reagents
For flow cytometry, we used FITC anti-CD4, allophycocyanin (APC) anti-CD4, APC anti-CD25, PE anti-Foxp3, PE-Cy5 anti-Foxp3 (all from eBioscience), FITC anti-CD14, FITC anti-CD8, PE anti-PD1, FITC anti-D4GDI (Pierce), PE anti-IL-10, PE anti-TGF-β and PE anti-IFN-γ and PE anti-CD127 (e-Bioscience), APO-Direct TUNEL kit (e-Bioscience), Caspase-3 staining kit (BD Pharmingen) and DCFDA—Cellular Reactive Oxygen Species Detection Assay Kit (Abcam). γ-irradiated M. tb H37Rv was obtained from BEI Resources. In some experiments recombinant D4GDI (Cell Sciences) and cyclosporin A (Sigma) were used. For neutralization experiments, anti-IFN-γ (BD Pharmingen) and anti IL-22 (e-Biosciences) antibodies were used.
Isolation of M. tb-expanded CD4+CD25+Foxp3+ and CD4+CD25-Foxp3- cells
PBMC were isolated by differential centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech). CD4+ and CD14+ cells were isolated by positive immunomagnetic selection (MiltenyiBiotec), and were >95% CD4+ and CD14+, respectively, as measured by flow cytometry. CD4+ cells were cultured in 12-well plates at 2 ×106 cells/well in RPMI 1640 containing 10% heat-inactivated human serum, with 2 × 105 autologous CD14+ monocytes/well and γ-irradiated M. tb (10 µg/ml) at 37°C. After 4 days, CD4+CD25+ and CD4+CD25- cells were isolated, using the Treg isolation kit (MiltenyiBiotec), as described [11]. Eighty five-90% of isolated CD4+CD25+ cells expressed Foxp3, and <1% of CD4+CD25- cells were Foxp3+, as determined by intracellular staining.
Immunolabeling of intracellular Foxp3, IL-10, TGF-β, IFN-γ and D4GDI
For surface staining, 106 cells were resuspended in 100 μl of staining buffer (PBS containing 2% heat-inactivated FBS) and Abs. Cells were then incubated at 4°C for 30 min, washed twice and fixed in 1% paraformaldehyde, before acquisition using a FACS Calibur (BD Biosciences). In some experiments, intracellular staining for Foxp3, IL-10, TGF-β, IFN-γ and D4GDI was performed, according to the manufacturer’s instructions. Controls for each experiment included cells that were unstained, cells to which APC-conjugated goat or rat IgG or PE-conjugated rat IgG had been added and cells that were single stained, either for the surface marker or for intracellular molecules. For Foxp3, IL-10, TGF-β and IFN-γ analysis, we gated on CD4+ lymphocytes, and determined the percentages of CD25+ and Foxp3+ cells. For D4GDI, IL-10, TGF-β and IFN-γ analysis, we gated on CD4+Foxp3+ cells to identify D4GDI+ cells.
Infection of macrophages with M. tb, and coculture with CD4+CD25+Foxp3+ T-cells
CD14+ monocytes (106/well) were plated in 12-well plates in 1 ml of antibiotic-free RPMI 1640 containing 10% heat-inactivated human serum, and incubated at 37°C in a humidified 5% CO2 atmosphere for 4 days to differentiate into macrophages. At the same time, CD4+ cells and CD14+ monocytes were cultured with γ-irradiated M. tb for 4 days, and CD4+CD25+ and CD4+CD25- cells were isolated, as outlined above. In some experiments cells were cultured with Cyclosporin A (20μg/ml). MDMs were infected with M. tb H37Rv at a MOI of 1 : 2.5 (2.5 M. tb to one MDM), incubated for 2 hr at 37°C, washed to remove extracellular bacilli, and cultured in RPMI 1640 containing10% heat-inactivated human serum. To some wells, CD4+CD25+ or CD4+CD25- cells were added in Transwells or in the same well, at a ratio of 1 CD4+ cell:9 MDMs. To some wells anti-IFN-γ or anti-IL-22 or both, or isotype control antibodies were added on days 0 and 3.
Infected macrophages were cultured for 7 days, at which point macrophage viability was >90%. The supernatant was aspirated, and macrophages were lysed. The supernatant was centrifuged to pellet bacteria, and the pellets were added to the cell lysates. Bacterial suspensions were ultrasonically dispersed, serially diluted, and plated in triplicate on 7H10 agar. The number of colonies was counted after 3 weeks.
Analysis of proteins in T-cell supernatants
CD4+ cells and autologous monocytes were cultured with γ-irradiated H37Rv, as outlined above. After 4 days, CD4+ CD25+ cells (85–90% Foxp3+) and CD4+CD25- cells (<5% Foxp3+) were isolated by immunomagnetic sorting, and cultured in medium overnight. In some experiments, the supernatants were concentrated and 2D gel electrophoresis was performed, followed by LC-MS/MS analysis of differentially expressed proteins (Kendrick Laboratories, Madison, WI). In other experiments, supernatants were collected and 10μg from each sample was separated by 10% SDS-PAGE, transferred to nitrocellulose and probed with Abs to D4GDI or β-actin (Santa Cruz Biotechnology). After washing, the membranes were incubated with HRP-conjugated secondary Ab (Santa Cruz Biotechnology) and binding was detected by ECL (GE Healthcare).
MTT assay
The effect of recombinant D4GDI on the viability of MDMs was studied in 96-well plates using the MTT cell proliferation assay kit (ATCC). Briefly, after 7 days of D4GDI treatment, 10 µl of MTT reagent was added in the wells and incubated for 3 hr for the development of purple precipitates. Cells were then lysed in 100 μl of detection reagent and the plate was read at 570 nm after 2 hr of incubation. For blank wells, cells were first lysed and then MTT reagent was added.
Microarray analysis and Multiplex ELISA
H37Rv-infected MDMs were cultured with or without recombinant D4GDI. After 6 hr, culture supernatants were collected and RNA was isolated from MDMs, using TRIzol reagent (Life Technologies), and pooled RNA was converted to cDNA, followed by microarray analysis (UT, Southwestern, Dallas, TX, USA). In the culture supernatants, the following 27 cytokines and chemokines were measured using multiplex ELISA kit (Bio-Rad). The cytokines and chemokines analyzed were IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, Eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 (MCAF), MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF.
RNA extraction and real-time PCR analysis
MDMs were infected with H37Rv at a MOI of 1 : 2.5 (2.5 M. tb to one MDM), as outlined above, and treated with medium alone or recombinant D4GDI. In some experiments, CD4+CD25+ or CD4+CD25‑ cells were added in Transwells. After 3 days, RNA was isolated from 106 MDMs, and reverse transcribed, using the Clone AMV First-Strand cDNA synthesis kit (Life Technologies). For analysis of M. tb mRNA, host cells were lysed, bacteria were recovered by centrifugation, and then RNA was extracted by bead-beating in Trizol, followed by further purification.
Mycobacterial primer and probe sets were designed with primer express software (Applied Biosystems); probes were labeled with 5’-fluorescein phosphoramidite and 3’-TAMRA. The primers used to amplify human and M. tb cDNA are shown in S2 Table. Real-time PCR was performed using the Quantitect SYBR Green PCR kit (Qiagen) in a sealed 96-well microtiter plate (PE Applied Biosystems) on a spectrofluorometric thermal cycler (7700 PRISM, Applied Biosystems). PCR reactions were performed in triplicate as follows: 95°C for 10 min, and 45 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Expression of human and M. tb genes were normalized to the amount of GAPDH and 16S rRNA transcripts in each sample, respectively.
siRNA
Freshly isolated CD4+ cells were transfected with siRNA for D4GDI or scrambled siRNA. The next day, CD4+ cells were washed, and cultured with autologous monocytes and γ-irradiated M. tb H37Rv. CD4+CD25+ and CD4+CD25- cells were isolated after 5 days. The efficiency of siRNA knockdown was measured by real-time PCR of D4GDI expression.
MDMs were transfected with siRNA for IL-1β, TNF-α, IL-27, G-CSF, SIVA or MMP12, or control siRNA (all from Santa Cruz Biotechnology). The efficiency of siRNA knockdown was measured by real-time PCR to detect the relevant mRNA. Briefly, 106 MDMs were resuspended in 500 µl of transfection medium, and transfected with siRNA (6 pmoles). After 6 hr, an additional 250 µl of 2X RPMI complete medium was added, and cells were cultured overnight in a 24-well plate. The next day, MDMs were washed and infected with H37Rv, as outlined above, and CFU were measured after 3 days.
Confocal microscopy to determine internalization of D4GDI
Confocal microscopy was performed to detect intracellular D4GDI, as described previously [71]. MDMs on chamber slides (Lab Tek) were uninfected or infected with GFP expressing M. tb H37Rv at a multiplicity of infection of 1 : 2.5 (2.5 M. tb to one MDM) for 2 hr, washed thoroughly, and cultured overnight with GST-D4GDI. After overnight incubation, for intracellular staining, cells were first fixed in 2% paraformaldehyde in PBS (pH 7.2), permeabilized and nonspecific binding was blocked by incubating in blocking buffer. Cells were then incubated overnight with goat polyclonal anti-GST (5 mg/ml) in blocking buffer. As a control, monocytes were incubated with either blocking buffer alone or isotype control antibody (5 mg/ ml). Then, cells were stained with secondary antibody and DAPI (Molecular Probes) to identify the primary signal and nuclei. The cells were washed and mounted with aqueous gel mounting media (Biomedia) containing antifading agent. Confocal images were obtained, using an LSM 510 Meta confocal system (Carl Zeiss) equipped with an inverted microscope (Axio Observer Z1; Carl Zeiss). Immunostained cells were viewed through a Plan-APOCHROMAT 633/1.4 NA oil objective lens, with 2.53 digital magnification, to detect green fluorescence (GFP-expressing M. tb H37Rv), blue (DAPI) and red fluorescence (GST-D4GDI). Images were acquired with Zen 2007 software (Carl Zeiss), and scanned images were exported and processed using Adobe Photoshop version 7.0 software (Adobe Systems).
Measurement of apoptosis of M. tb-infected macrophages
The effect of recombinant D4GDI on apoptosis of M. tb-infected MDMs was measured by using the APO-Direct TUNEL kit. Briefly, MDMs were infected with M. tb H37Rv at a MOI of 1 : 2.5 (2.5 M. tb to one MDM). Some MDMs were cultured with D4GDI (10ng/ml). After 72 hr, cells were isolated using trypsin-EDTA and fixed in 1% paraformaldehyde and 70% ethanol in PBS. After overnight incubation at -20°C, cells were washed in washing buffer, resuspended in 50 μl of DNA labeling solution (provided in the kit) and incubated at 37°C. After 60 min, cells were washed twice in rinsing buffer and 500 μl of propidium iodide/RNAse A solution was added and incubated in the dark. After 30 min, cells were analyzed by FACS.
Infection of mice with M. tb
Wild type C57Bl/6 mice were infected with H37Rv, using an aerosol exposure chamber, as described previously [72], [73] to deposit ~50–100 bacteria in the lungs. To measure lung CFU, serially diluted lung homogenates were plated on 7H11 agar, supplemented with OADC. CFU were enumerated after 14–22 days incubation at 37°C.
Statistical analysis
Results are shown as the mean ± SE. Comparisons between groups were performed by a paired or unpaired t test, as appropriate.
Supporting Information
Zdroje
1. Flynn JL, Chan J (2001) Immunology of tuberculosis. Annu Rev Immunol 19 : 93–129. doi: 10.1146/annurev.immunol.19.1.93 11244032
2. Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, et al. (1993) Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178 : 2243–2247. doi: 10.1084/jem.178.6.2243 8245795
3. Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299 : 1057–1061. doi: 10.1126/science.1079490 12522256
4. Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, et al. (2007) Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med 204 : 2159–2169. doi: 10.1084/jem.20062105 17709423
5. Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, et al. (2007) Cutting Edge: Regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol 178 : 2661–2665. doi: 10.4049/jimmunol.178.5.2661 17312107
6. Garg A, Barnes PF, Roy S, Quiroga MF, Wu S, et al. (2008) Mannose-capped lipoarabinomannan - and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol 38 : 459–469. doi: 10.1002/eji.200737268 18203140
7. Periasamy S, Dhiman R, Barnes PF, Paidipally P, Tvinnereim A, et al. (2011) Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation with Mycobacterium tuberculosis. J Infect Dis 203 : 1256–1263. doi: 10.1093/infdis/jir011 21383382
8. Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, et al. (1999) Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis 180 : 2069–2073. doi: 10.1086/315114 10558973
9. Mahan CS, Thomas JJ, Boom WH, Rojas RE (2009) CD4+CD25(high) Foxp3+ regulatory T cells downregulate human Vdelta2+ T-lymphocyte function triggered by anti-CD3 or phosphoantigen. Immunology 127 : 398–407. doi: 10.1111/j.1365-2567.2008.02982.x 19019089
10. Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, et al. (2005) Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol 66 : 13–20. doi: 10.1016/j.humimm.2004.05.016 15620457
11. Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE (2007) Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 37 : 129–138. doi: 10.1002/eji.200636435 17154262
12. Roncarolo MG, Gregori S (2008) Is FOXP3 a bona fide marker for human regulatory T cells?. Eur J Immunol 38 : 925–927. doi: 10.1002/eji.200838168 18395862
13. Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, et al. (2001) CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol 167 : 2734–2742. doi: 10.4049/jimmunol.167.5.2734 11509617
14. Silver RF, Li Q, Boom WH, Ellner JJ (1998) Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol 160 : 2408–2417. 9498784
15. Dovas A, Couchman JR (2005) RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J 390 : 1–9. doi: 10.1042/BJ20050104 16083425
16. Guillemot JC, Kruskal BA, Adra CN, Zhu S, Ko JL, et al. (1996) Targeted disruption of guanosine diphosphate-dissociation inhibitor for Rho-related proteins, GDID4: normal hematopoietic differentiation but subtle defect in superoxide production by macrophages derived from in vitro embryonal stem cell differentiation. Blood 88 : 2722–2731. 8839868
17. Honaker RW, Leistikow RL, Bartek IL, Voskuil MI (2009) Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy. Infect Immun 77 : 3258–3263. doi: 10.1128/IAI.01449-08 19487478
18. Rustad TR, Sherrid AM, Minch KJ, Sherman DR (2009) Hypoxia: a window into Mycobacterium tuberculosis latency. Cell Microbiol 11 : 1151–1159. doi: 10.1111/j.1462-5822.2009.01325.x 19388905
19. Converse PJ, Karakousis PC, Klinkenberg LG, Kesavan AK, Ly LH, et al. (2009) Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun 77 : 1230–1237. doi: 10.1128/IAI.01117-08 19103767
20. Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, et al. (2010) The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol 192 : 1662–1670. doi: 10.1128/JB.00926-09 20023019
21. Kendall SL, Movahedzadeh F, Rison SC, Wernisch L, Parish T, et al. (2004) The Mycobacterium tuberculosis dosRS two-component system is induced by multiple stresses. Tuberculosis (Edinb) 84 : 247–255. doi: 10.1016/j.tube.2003.12.007
22. Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, et al. (1989) Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol 142 : 1114–1119. 2464638
23. Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, et al. (1990) Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol 145 : 149–154. 2113553
24. Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 : 502–507. doi: 10.1038/nature01152 12466842
25. Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, et al. (2002) The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol 169 : 3232–3241. doi: 10.4049/jimmunol.169.6.3232 12218142
26. Walther M, Tongren JE, Andrews L, Korbel D, King E, et al. (2005) Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23 : 287–296. doi: 10.1016/j.immuni.2005.08.006 16169501
27. Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, et al. (2004) Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat Med 10 : 29–30. doi: 10.1038/nm975 14702631
28. Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS (2003) Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect Immun 71 : 1755–1762. doi: 10.1128/IAI.71.4.1755-1762.2003
29. Harris PR, Wright SW, Serrano C, Riera F, Duarte I, et al. (2008) Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134 : 491–499. doi: 10.1053/j.gastro.2007.11.006 18242215
30. Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, et al. (2004) CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med 200 : 331–343. doi: 10.1084/jem.20032069 15280419
31. Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF (2004) Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol 78 : 2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004 14963140
32. Baumforth KR, Birgersdotter A, Reynolds GM, Wei W, Kapatai G, et al. (2008) Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates Up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol 173 : 195–204. doi: 10.2353/ajpath.2008.070845 18502823
33. Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, et al. (2004) An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology 40 : 1062–1071. doi: 10.1002/hep.20454 15486925
34. Stoop JN, van der Molen RG, Baan CC, van der Laan LJ, Kuipers EJ, et al. (2005) Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 41 : 771–778. doi: 10.1002/hep.20649 15791617
35. Peng G, Li S, Wu W, Sun Z, Chen Y, et al. (2008) Circulating CD4+ CD25+ regulatory T cells correlate with chronic hepatitis B infection. Immunology 123 : 57–65. doi: 10.1111/j.1365-2567.2007.02691.x 17764450
36. Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, et al. (2011) CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity 34 : 422–434. doi: 10.1016/j.immuni.2011.03.002 21435589
37. Garcia-Mata R, Boulter E, Burridge K (2011) The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 12 : 493–504. doi: 10.1038/nrm3153 21779026
38. Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420 : 629–635. doi: 10.1038/nature01148 12478284
39. Watanabe T, Urano E, Miyauchi K, Ichikawa R, Hamatake M, et al. (2012) The Hematopoietic Cell-Specific Rho GTPase Inhibitor ARHGDIB/D4GDI Limits HIV Type 1 Replication. AIDS Res Hum Retroviruses 28 : 912–922. doi: 10.1089/aid.2011.0180
40. Juffermans NP, Florquin S, Camoglio L, Verbon A, Kolk AH, et al. (2000) Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J Infect Dis 182 : 902–908. doi: 10.1086/315771 10950787
41. Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, et al. (2010) Caspase-1 independent IL-1beta production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol 184 : 3326–3330. doi: 10.4049/jimmunol.0904189 20200276
42. Jayaraman P, Sada-Ovalle I, Nishimura T, Anderson AC, Kuchroo VK, et al. (2013) IL-1beta promotes antimicrobial immunity in macrophages by regulating TNFR signaling and caspase-3 activation. J Immunol 190 : 4196–4204. doi: 10.4049/jimmunol.1202688 23487424
43. Kleinnijenhuis J, Joosten LA, van de Veerdonk FL, Savage N, van Crevel R, et al. (2009) Transcriptional and inflammasome-mediated pathways for the induction of IL-1beta production by Mycobacterium tuberculosis. Eur J Immunol 39 : 1914–1922. doi: 10.1002/eji.200839115 19544485
44. Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, et al. (2009) Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE 4: e5810. doi: 10.1371/journal.pone.0005810 19503839
45. Verway M, Bouttier M, Wang TT, Carrier M, Calderon M, et al. (2013) Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog 9: e1003407. doi: 10.1371/journal.ppat.1003407
46. Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, et al. (1995) Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2 : 561–572. doi: 10.1016/1074-7613(95)90001-2 7540941
47. Rook GA, Taverne J, Leveton C, Steele J (1987) The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology 62 : 229–234. 3119471
48. Hirsch CS, Ellner JJ, Russell DG, Rich EA (1994) Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol 152 : 743–753. 8283049
49. Fang FC (2004) Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2 : 820–832. doi: 10.1038/nrmicro1004 15378046
50. Freeman AF, Holland SM (2007) Persistent bacterial infections and primary immune disorders. Curr Opin Microbiol 10 : 70–75. doi: 10.1016/j.mib.2006.11.005 17208513
51. Babior BM (1999) NADPH oxidase: an update. Blood 93 : 1464–1476. 10029572
52. Casanova JL, Abel L (2002) Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 20 : 581–620. doi: 10.1146/annurev.immunol.20.081501.125851 11861613
53. Wu W, Hsu YM, Bi L, Songyang Z, Lin X (2009) CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat Immunol 10 : 1208–1214. doi: 10.1038/ni.1788 19767757
54. Miller JL, Velmurugan K, Cowan MJ, Briken V (2010) The type I NADH dehydrogenase of Mycobacterium tuberculosis counters phagosomal NOX2 activity to inhibit TNF-alpha-mediated host cell apoptosis. PLoS Pathog 6: e1000864. doi: 10.1371/journal.ppat.1000864 20421951
55. Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, et al. (2009) NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol 182 : 3696–3705. doi: 10.4049/jimmunol.0802217 19265148
56. Deretic V (2012) Autophagy as an innate immunity paradigm: expanding the scope and repertoire of pattern recognition receptors. Curr Opin Immunol 24 : 21–31. doi: 10.1016/j.coi.2011.10.006 22118953
57. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, et al. (1999) Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285 : 732–736. doi: 10.1126/science.285.5428.732 10426995
58. Behar SM, Martin CJ, Nunes-Alves C, Divangahi M, Remold HG (2011) Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect 13 : 749–756. doi: 10.1016/j.micinf.2011.03.002 21458584
59. Keane J, Remold HG, Kornfeld H (2000) Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol 164 : 2016–2020. doi: 10.4049/jimmunol.164.4.2016 10657653
60. Sly LM, Hingley-Wilson SM, Reiner NE, McMaster WR (2003) Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J Immunol 170 : 430–437. doi: 10.4049/jimmunol.170.1.430 12496428
61. Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, et al. (2007) Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog 3: e110. doi: 10.1371/journal.ppat.0030110 17658950
62. Kundu M, Pathak SK, Kumawat K, Basu S, Chatterjee G, et al. (2009) A TNF - and c-Cbl-dependent FLIP(S)-degradation pathway and its function in Mycobacterium tuberculosis-induced macrophage apoptosis. Nat Immunol 10 : 918–926. doi: 10.1038/ni.1754 19597496
63. Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB (2010) Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med 207 : 1409–1420. doi: 10.1084/jem.20091885 20547826
64. Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, et al. (2010) CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis 202 : 533–541. doi: 10.1086/654896 20617900
65. Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A (2006) Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 173 : 803–810. doi: 10.1164/rccm.200508-1294OC 16339919
66. Rahman S, Gudetta B, Fink J, Granath A, Ashenafi S, et al. (2009) Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions. Am J Pathol 174 : 2211–2224. doi: 10.2353/ajpath.2009.080941 19435796
67. Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, et al. (2009) FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med 179 : 1061–1070. doi: 10.1164/rccm.200804-529OC 19246720
68. Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, et al. (2003) Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48 : 833–843. doi: 10.1046/j.1365-2958.2003.03474.x 12694625
69. Karakousis PC, Yoshimatsu T, Lamichhane G, Woolwine SC, Nuermberger EL, et al. (2004) Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J Exp Med 200 : 647–657. doi: 10.1084/jem.20040646 15353557
70. Shiloh MU, Manzanillo P, Cox JS (2008) Mycobacterium tuberculosis senses host-derived carbon monoxide during macrophage infection. Cell Host Microbe 3 : 323–330. doi: 10.1016/j.chom.2008.03.007 18474359
71. Tan T, Lee WL, Alexander DC, Grinstein S, Liu J (2006) The ESAT-6/CFP-10 secretion system of Mycobacterium tuberculosis modulates phagosome maturation. Cell Microbiol 8 : 1417–1429. doi: 10.1111/j.1462-5822.2006.00721.x 16922861
72. Dhiman R, Periasamy S, Barnes PF, Jaiswal AG, Paidipally P, et al. (2012) NK1.1+ Cells and IL-22 Regulate Vaccine-Induced Protective Immunity against Challenge with Mycobacterium tuberculosis. J Immunol 189 : 897–905. doi: 10.4049/jimmunol.1102833 22711885
73. Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, et al. (2003) NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol 171 : 6039–6045. doi: 10.4049/jimmunol.171.11.6039 14634116
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



