-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
Visceral leishmaniasis (VL), a globally important parasitic disease responsible for over 40,000 deaths p.a., results in pronounced changes in splenic organisation associated with impaired immune function and persistent parasite infection. We have previously shown that receptor tyrosine kinase (RTKi) inhibitors can restore splenic architecture and improve immunocompetence, and that mononuclear phagocytes (MPs) are involved in this process. Here, we provide evidence that neurotrophin receptor Ntrk2 (also known as TrkB) plays a role in the pathologic remodeling of the spleen that accompanies experimental Leishmania donovani-infection. We show that following infection of mice with L.donovani, Ntrk2 is expressed on splenic endothelial cells that are closely associated with F4/80hiCD11bloCD11c+ macrophages expressing Ntrk2 ligands. Administration of the Ntrk2 antagonist ANA-12 to infected mice significantly inhibited compartment-specific vascular remodeling of the spleen. This study expands our understanding of the pathogenesis of experimental VL and also demonstrates the potential of Ntrk2/Bdnf as targets for treatment of infection-induced vascular remodeling.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004681
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004681Summary
Visceral leishmaniasis (VL), a globally important parasitic disease responsible for over 40,000 deaths p.a., results in pronounced changes in splenic organisation associated with impaired immune function and persistent parasite infection. We have previously shown that receptor tyrosine kinase (RTKi) inhibitors can restore splenic architecture and improve immunocompetence, and that mononuclear phagocytes (MPs) are involved in this process. Here, we provide evidence that neurotrophin receptor Ntrk2 (also known as TrkB) plays a role in the pathologic remodeling of the spleen that accompanies experimental Leishmania donovani-infection. We show that following infection of mice with L.donovani, Ntrk2 is expressed on splenic endothelial cells that are closely associated with F4/80hiCD11bloCD11c+ macrophages expressing Ntrk2 ligands. Administration of the Ntrk2 antagonist ANA-12 to infected mice significantly inhibited compartment-specific vascular remodeling of the spleen. This study expands our understanding of the pathogenesis of experimental VL and also demonstrates the potential of Ntrk2/Bdnf as targets for treatment of infection-induced vascular remodeling.
Introduction
Disruption in lymphoid tissue organisation is evident in a wide array of chronic inflammatory disorders, including cancer, infectious disease, psoriasis, liver disorders, autoimmune and metabolic diseases, and the persistence of vascular remodelling has been shown to impair immune responses and promote the chronicity of inflammation [1–4]. It is perhaps no surprise, therefore, that anti-angiogenic drugs are becoming an important therapeutic option for an array of chronic diseases and disorders, including those of infectious origin. HIV, malaria, schistosomiasis and leishmaniasis all promote chronic inflammation and remodelling of lymphoid organs [5–8], and murine models of these diseases have been used extensively to study the mechanistic basis of stromal cell and vascular remodelling and their consequences in terms of immune dysfunction and disease progression [7,9–11].
Splenomegaly is a defining characteristic of the parasitic disease visceral leishmaniasis (VL) and like many examples of splenomegaly, VL is associated with a remodeling of splenic architecture [4,12]. In murine experimental VL, enlargement of red pulp vessels and neovascularization of the white pulp are prominent. Previously, we demonstrated that administration of the broad spectrum receptor tyrosine kinase inhibitor (RTKi) sunitinib maleate to Leishmania donovani-infected mice halted progressive vascular remodeling, reduced mononuclear phagocyte (MP) number and improved the efficacy of immune-dependent drugs [4,13,14]. Compartment-specific remodeling of the red pulp vasculature was subsequently identified as a function of Ly6C+ inflammatory monocytes [14], whereas these cells played no apparent role in regulating white pulp neovascularisation or the loss of follicular dendritic cells (FDC) and fibroblastic reticular cells (FRC), suggesting alternate and independent mechanisms exist to regulate these processes.
Neurotrophins and their receptors are novel targets for angiogenic therapies [15,16]. For example, Bdnf is recognised as an angiogenic factor, being critical for the establishment of cardiac vasculature during development [17,18], and for promoting Ntrk2-dependent skeletal muscle neoangiogenesis in adult ischemic hind limbs [19]. However, it is the limited tissue expression of the neutrophin receptors, such as Ntrk2, which makes them so desirable as targets for therapy, with the potential for aberrant expression in pathological conditions in other tissues being largely overlooked.
MPs are recognized as playing a significant role in inflammation-induced angiogenesis [2,20] and MP are known to be a source of Bdnf and NT-4/5 with their production being increased upon inflammatory stimulation [21,22]. Chronic parasitic and bacterial infections have also been associated with elevated levels of Bdnf/BDNF [23–25]. For example, serum levels of BDNF were significantly higher in patients suffering from chronic Chagas disease compared to healthy controls [25]. However, the cellular source(s) of neurotrophin were not determined in these studies. A causal link, therefore, between neurotrophin expression and infection-induced, macrophage-directed angiogenesis has not been previously reported.
Here, we report that Ntrk2 is aberrantly expressed on endothelial cells that form newly emerging vessels within the white pulp during L. donovani infection and that expression of Bdnf is up regulated in a sub-population of MPs with phenotypic similarity to resident tissue macrophages. Together, these observations have important implications for understanding the pathogenesis of VL and illustrate the potential for aberrant expression of angiogenic receptors and their ligands during inflammation.
Results
Characterisation of MPs in Leishmania infected mice
To determine whether MPs were involved in the process of white pulp remodeling, we first characterized MPs in the spleen of sunitinib-treated and control mice after the onset of splenomegaly (d28 post infection;[4]). Three distinct populations of CD11c+MHCII+ MPs were identified in infected mice based on CD11b, F4/80 and forward/side scatter profile and morphology: F4/80hiCD11blo cells (large, macrophage-like morphology; ∼80% containing parasites; Fig. 1A); F4/80loCD11bhi cells (smaller, classic macrophage morphology, <5% infected; Fig. 1B); and F4/80loCD11blo cells (small, dendritic cell morphology, no parasites; Fig. 1C). These MP populations increased in number 9–14 fold in infected compared to naïve mice. Although administration of sunitinib reduced the number of all populations, this was most marked for the F4/80hiCD11blo cells (Fig. 1A-C, right panels).
Fig. 1. Sensitivity of mononuclear phagocytes in L.donovani infected mice to RTKi treatment. 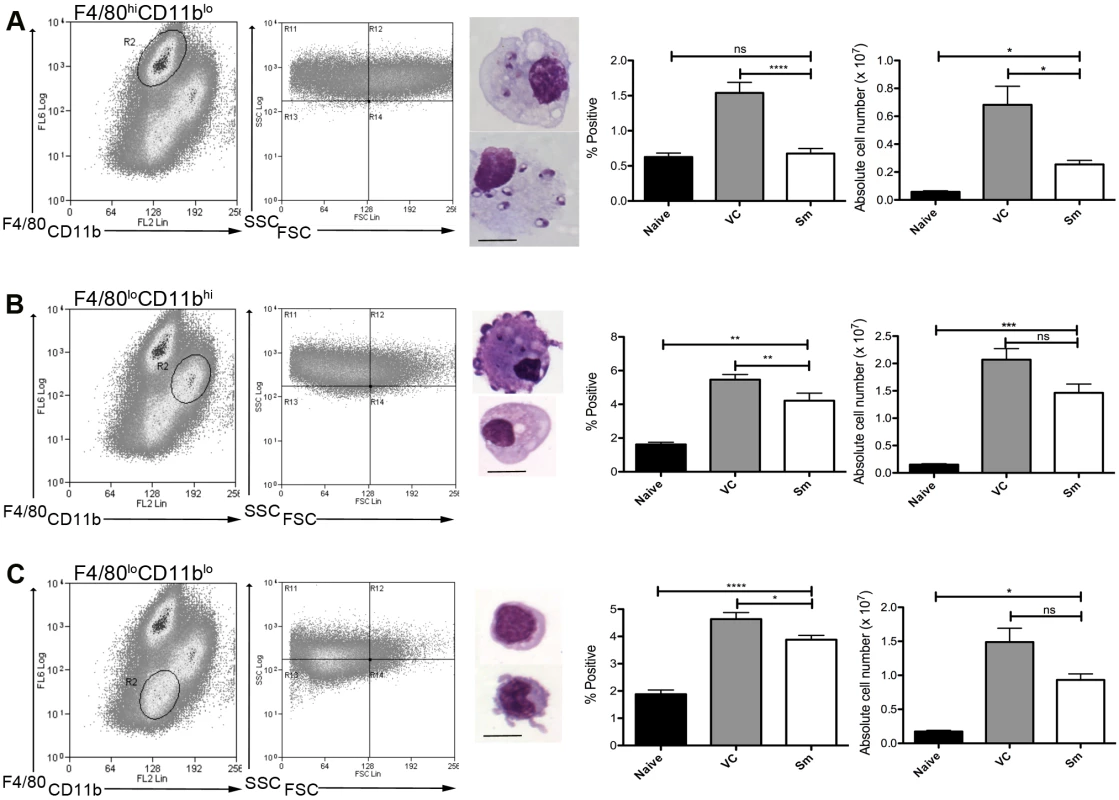
CD11c+MHCII+ MPs in d28 L. donovani infected mice were distinguished on the basis of F4/80 and CD11b expression, forward/side scatter profile and morphology into: (A) large CD11c+MHCII+F4/80hiCD11blo (F4/80hiCD11blo) cells with macrophage-like morphology, of which ∼80% harbored intracellular parasites; (B) slightly smaller CD11c+MHCII+F4/80loCD11bhi (F4/80loCD11bhi) cells with classic macrophage morphology, of which <5% harbored parasites; (C) much smaller CD11c+MHCII+F4/80loCD11blo (F4/80loCD11blo) cells with dendritic cell-like morphology and no observable parasites. Representative dot plots show pre-sorted populations with ellipsoid sort gates based on F4/80 and CD11b expression. Scale bar in micrographs = 10microns. The frequency and absolute numbers of each population is given in the right hand panels in naïve mice, infected mice and infected mice treated orally with sunitinib (Sm) for 7 days. P values = * <0.05, ** <0.008, ***<0.001, ns = not significant. As the sensitivity of F4/80hiCD11blo MPs to sunitinib treatment correlated with inhibition of white pulp neovascularisation, we further characterized these cells in both untreated infected and sunitinib-treated infected mice. Phenotypically, F4/80hiCD11blo MPs from both groups of mice were CD68+Ly6G/C- CD80+ SIGNR1loCD115+/- (Fig. 2A-C), suggesting that these MPs might be resident rather than inflammatory monocytes / macrophages. To further characterize these cells, we used an in-house MP-targeted oligoarray (consisting of >500 genes representing multiple GO pathways; S1 Table) to identify genes differentially expressed (DE) in F4/80hiCD11blo MPs vs. a reference population of F4/80hiCD11blo peritoneal MPs. The top DE gene was Slc40a1, an iron export protein expressed at high levels by tissue resident red pulp macrophages (Table 1), with many other DE genes being associated with pro-inflammatory and IFNγ-regulated responses, e.g. Cxcl9, Il18, Fpr2 and Ccl4 (Table 2 and S2 Table).
Fig. 2. Phenotypic analysis of F4/80hiCD11blocells. 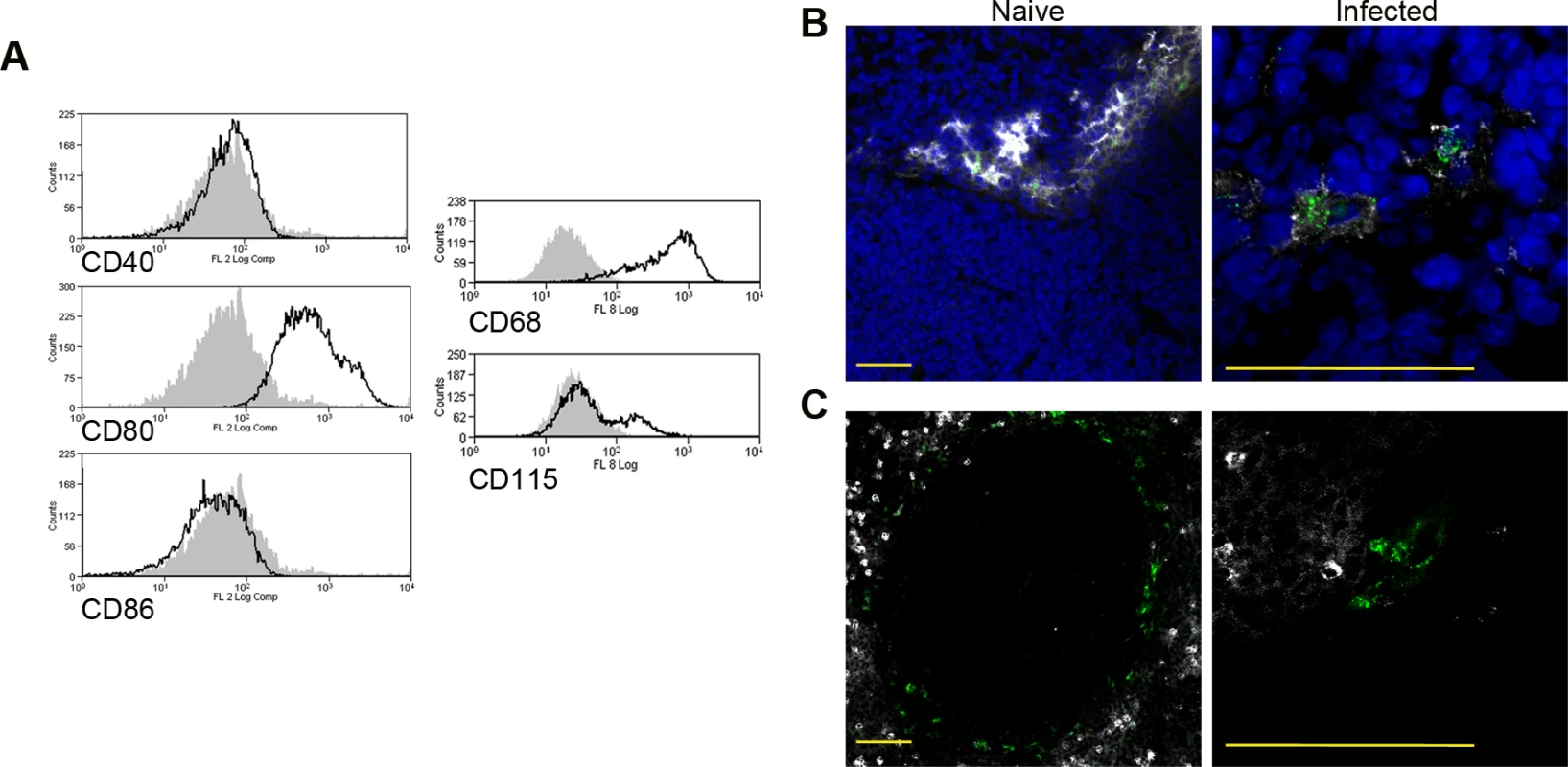
Splenocytes isolated from L.donovani infected mice at 28 days post infection were stained with a panel of myeloid cell markers. CD11c+MHCII+F4/80hiCD11blo MPs were positive for CD80, CD68 and a small proportion (15%) expressed CD115 (A: isotype control, filled grey histogram). Strong SIGNR1 (white) and FITC-dextran (green) labeling co-localise in the marginal zone of naïve mice (B), whereas in infected mice FITC-dextran+ (green) cells had low expression of SIGNR1 (white). FITC-dextran+ (green) cells were negative for GR1 (white) in both naïve and infected mice (C). Scale bars = 100 microns. Tab. 1. Altered gene expression in F4/80<sup>hi</sup>CD11b<sup>lo</sup> cells compared to control macrophages. 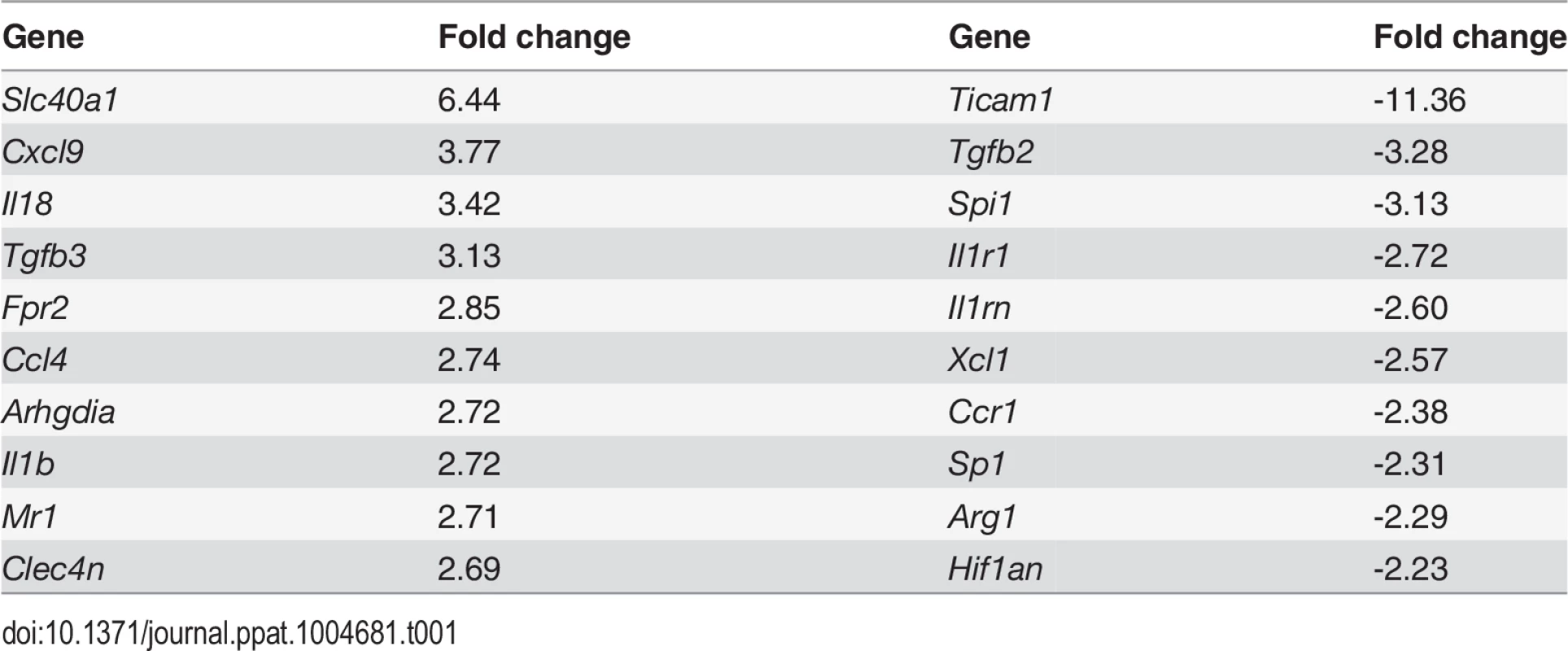
Tab. 2. Comparison of growth factor mRNA abundance in F4/80<sup>hi</sup>CD11b<sup>lo</sup> cells compared to other MP populations isolated from <i>L. donovani</i>-infected spleens. 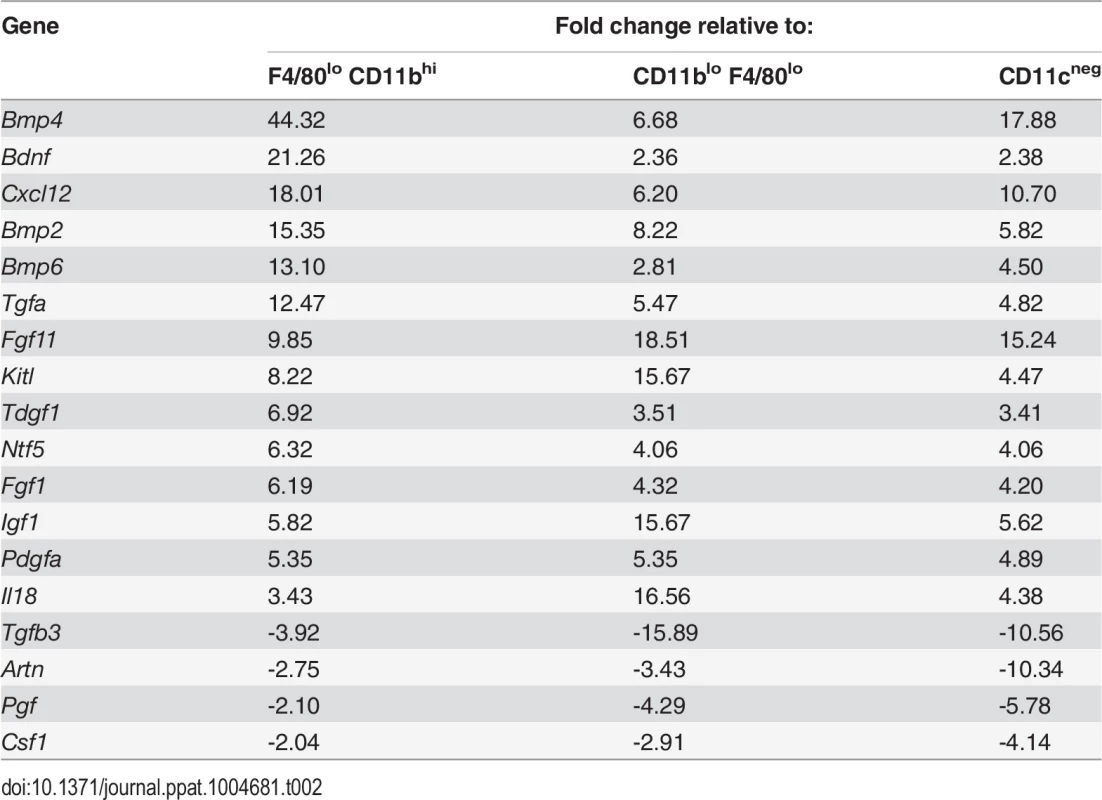
F4/80hi CD11blo MPs are associated with blood endothelial cells in the splenic white pulp
F4/80hi macrophages were described 30 years ago immediately adjacent to arterioles in the periarteriolar lymphoid sheath [26,27]. We localized F4/80hiCD11blo MPs in situ, taking advantage of their expression of SIGNR1, a receptor for the uptake of FITC-dextran [28], and the absence of SIGNR1hi marginal zone macrophages in L. donovani infected mice [11]. 74% of cells labeled intra-vitally with FITC-dextran were CD11c+F4/80hiCD11blo (gating strategy in S1 Fig.). In situ, F4/80hiCD11blo FITC-dextran+ MPs were located in either the white pulp region of the spleen or adjacent to the MZ (Fig. 3A, B). F4/80hiCD11blo MPs were most commonly (87.5%±7.2) found in association with Meca32+ vessels adjacent to the MZ or no more than a distance of two cell nuclei away (8.3% ± 8), and located predominantly at vessel junctions (Fig. 3C, D). x-y-z - reconstructions confirmed that they were closely associated with smooth muscle actin (SMA)-positive cells (S1 Video). Finally, 3D-rendering of z-plane images confirmed the presence of F4/80hiCD11blo MPs tightly associated with vessel junctions and vasculature that protruded into the white pulp from the marginal sinus (Fig. 3E-F and S2 Video).
Fig. 3. F4/80hiCD11blo MPs are located in close proximity to white pulp vasculature and possess angiogenic properties. 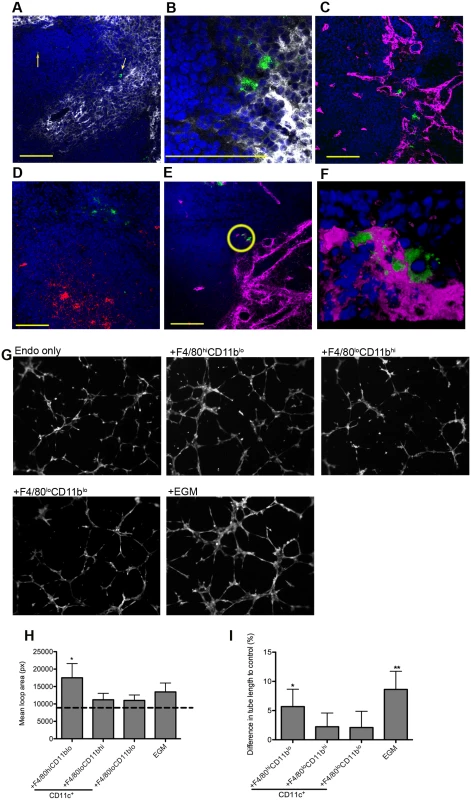
F4/80hiCD11blo cells (FITC-dextran, green; yellow arrows) identified in fresh frozen sections as located either in or bordering the white pulp (A,B). Red pulp F4/80+ macrophages are also shown (white). F4/80hiCD11blo cells (FITC-dextran, green) were found in close association with endothelial cells (C, E; Meca-32, magenta) but not follicular dendritic cells (D; FDCM1, red). High magnification image of area depicted by yellow circle in e (F). All sections were counterstained with DAPI (blue). Scale bars = 100 microns. F4/80hiCD11blo cells, but not other splenic MPs tested, drive SVEC4–10 endothelial cell tube formation on a gelled basement membrane extract (G). Representative images are shown. An optimised cocktail of growth factors (EGM) was used as a positive control. Quantitative analysis of SVEC4–10 mean loop area (H) and difference in tube length (I), in the presence of each MP population or control growth factors. Mean loop area in the absence of growth factors or MPs is shown as a dotted line in h. *p = 0.05, **p = 0.02. Images were analysed using WimTube software and data are expressed as mean ± SEM from at least three independent experiments. F4/80hi CD11blo MPs are pro-angiogenic
The close association of F4/80hiCD11blo MPs with vessels penetrating the white pulp suggested that there maybe a causal relationship with vascular remodeling. To determine the angiogenic potential of F4/80hiCD11blo MPs, we used SVEC4–10 mouse endothelial cells in an in vitro tube formation assay. SVEC4–10 cells cultured in the presence of an optimized cocktail of growth factors (EGM) migrated, directionally align and formed tube networks to a greater extent than cells cultured in in basal media (EBM) (Fig. 3G). Addition of F4/80loCD11bhi and F4/80loCD11blo cells had negligible tube promoting activity (<20% of maximal activity; difference in tube length of 2.2±2.3% and 2.1±2.7%, respectively vs. EBM control), whereas F4/80hiCD11blo MPs significantly enhanced mean loop area and tube length (5.7±3.0% and 8.6±3.1% for F4/80hi CD11blo MPs and EGM respectively, compared to EBM control; Fig. 3G-I). Hence, of the splenic MPs tested, only F4/80hiCD11blo MPs displayed angiogenic activity in vitro.
To identify factors associated with the angiogenic activity of these MPs, we studied the expression of 84 angiogenesis-related genes using PCR array. 69% (58/84) of genes were DE (2-fold cut-off) in F4/80hiCD11blo MPs relative to F4/80loCD11bhi MPs (46 up, 12 down), 50% (42/84) were DE relative to F4/80loCD11blo MPs (28 up, 14 down) and 55% (46/84) were DE relative to non-adherent CD11c- splenocytes (28 up, 18 down). 21% (18/84; 14 up, 4 down) were DE relative to all groups (Table 2). These included several members of the Tgf family and known ligands for sunitinib, including Pdgf, Kitl Artn, Pgf and Csf1. Neurotrophic factors Fgf11, Bdnf and Ntf5 (neurotrophin 4/5) were all up regulated in F4/80hiCD11blo MPs (Table 1). F4/80hiCD11blo MPs also expressed higher levels of Bdnf protein compared to other MP populations studied (ΔMFI = 811 vs ΔMFI = 247 and ΔMFI = 139; Fig. 4A). Although we cannot rule out a causal link between intracellular infection of F4/80hiCD11blo MPs by L. donovani amastigotes and their Bdnf expression, Bdnf gene expression was not enhanced following L. donovani infection of a variety of other MPs in vitro [29] and in vivo [30]. In summary, these data demonstrate that F4/80hiCD11blo MPs in infected mice: i) are appropriately positioned in the tissue; ii) express neurotrophins with known angiogenic activity; and iii) are pro-angiogenic in vitro.
Fig. 4. Infection-induced expression of Bdnf and Ntrk2 in spleen. 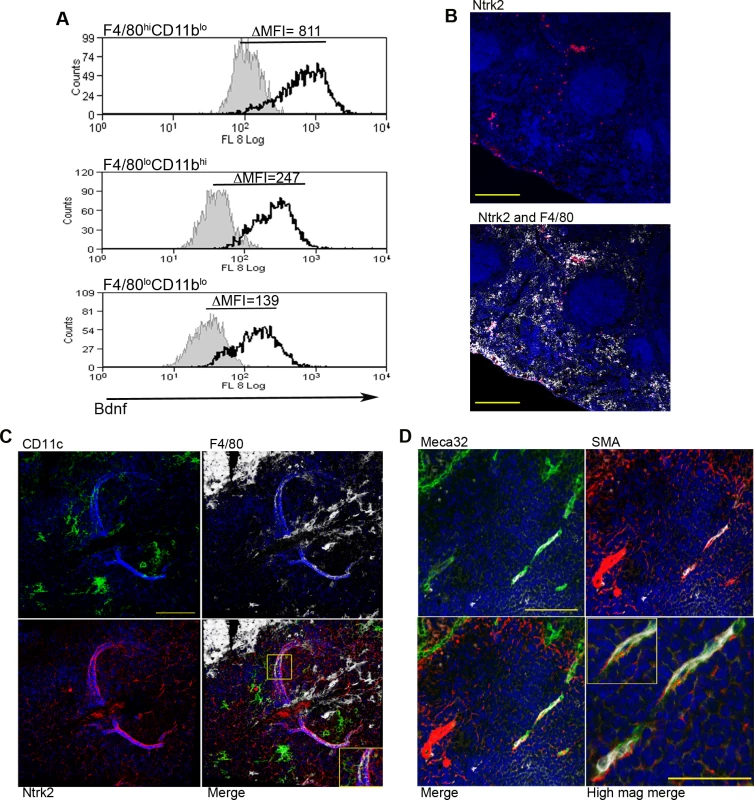
Bdnf (black line) was expressed at significantly higher levels by F4/80hiCD11blo cells compared to the other MP populations tested, as revealed by delta median fluorescence intensity levels (ΔMFI) using intracellular flow cytometry (A). Isotype control staining is highlighted by the solid grey histogram. Ntrk2 (red) expression in naïve mouse spleen is found in a subset of splenic red pulp macrophages (F4/80+, white) in naïve mouse spleen (B). Scale bar = 200microns. In L. donovani infected mice splenic white pulp vessels expressed Ntrk2 (C: red). F4/80+ (white) CD11c+ (green) MPs are found in close association with Nrtk2+ vessels (C: merge and inset). Endothelial cells (Meca-32, green) but not smooth muscle actin-positive cells (SMA, red) in white pulp express Nrtk2 (white, D and inset). All sections were stained with DAPI (blue). Scale bars = 100 microns and 50 microns in high magnification merge image. Nrtk2 expression on vasculature following infection in vivo
Spleen tissue sections were analyzed for the expression of Ntrk2, the receptor for Bdnf to investigate a possible role for this pathway in vascular remodeling in vivo. In the naïve spleen, Ntrk2 expression was restricted to a population of F4/80+ red pulp MPs, corresponding to previous reports [31] (Fig. 4B). Expression of Ntrk2 was not altered at d1 post infection, suggesting that induction of Ntrk2 was not the result of inflammatory responses that immediately follow infection. Induced expression was seen in the marginal zone area at day 7 post infection and staining within the white pulp area was beginning to become apparent at day 14 (S2 Fig.). In spleens from chronically infected mice, Nrtk2 was aberrantly expressed on stromal cells, with white pulp vessels showing the highest intensity of staining for Ntrk2 (Fig. 4C). Ntrk2+ vessels were found to be in extremely close association with CD11c+F4/80+ MPs (Fig. 4C). Ntrk2 expression has been reported on endothelial cells and smooth muscle cells in developing and adult cardiac tissue [18,32]. Co-staining with Meca32 indicated that Ntrk2 was expressed on splenic endothelial cells, but vessels associated with high levels of SMA staining did not express detectable levels of Ntrk2 (Fig. 4D). As SMA acquisition is a marker of vessel maturity [33], these data suggest that Ntrk2 is up regulated only on developing or immature blood vessels.
Pharmacologic blockade of Ntrk2 inhibits neovascularisation
Finally, to determine whether this pathway played a functional role in vascular remodeling, we inhibited Ntrk2 signaling in vivo using the small molecule antagonist ANA-12 [34]. Mice were treated for 7 days i.p. with sunitinib or ANA-12 commencing on day 21 p.i. [4] (Fig. 5A). Quantitative image analysis indicated that sunitinib [4] but not ANA-12 inhibited red pulp vascular remodeling (Fig. 5B and C). In contrast, both sunitinib and ANA-12 significantly inhibited white pulp neovascularization (50%±10 and 34%±8 reduction vs. vehicle control, respectively; Fig. 4B and D). ANA-12 did not alter splenic parasite burden, as was also the case for sunitinib (Fig. 5E). Unlike sunitinib, however, ANA-12 treatment had no significant effect on spleen size (Fig. 5F) suggesting that splenomegaly and red pulp vascular remodeling are linked. Similarly, MP frequency was unaltered following ANA-12 treatment (1.15±0.1% vs 1.0±0.1% for F4/80hiCD11blo MPs, 5.83±0.5% vs 5.82±0.3% for F4/80loCD11bhi MPs and 5.25±0.5% vs 5.3±1.5% for F4/80loCD11blo MPs, comparing ANA-12 to vehicle). Collectively, these data demonstrate that targeting Ntrk2 signaling in vivo selectively inhibits ongoing white pulp neovascularization, without affecting MP infiltration and/or proliferation or splenomegaly and red pulp vascular remodeling (Fig. 6).
Fig. 5. White pulp neovascularization during chronic infection is dependent on Nrtk2. 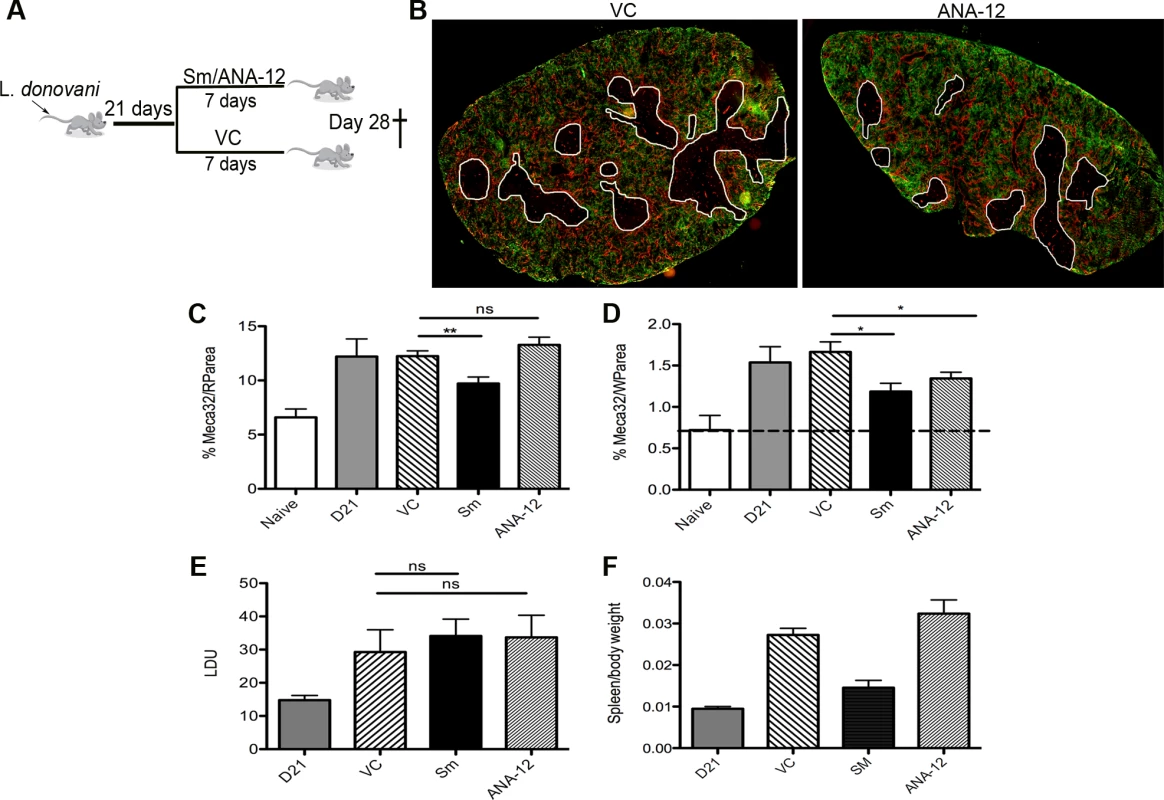
Drug treatment schedule: 21 days post infection with L. donovani, mice were treated with ANA-12, sunitinib maleate (Sm) or vehicle control (VC), daily for 7 days (A). Spleen sections from control and drug-treated mice were stained for endothelial cells (Meca-32, red) and F4/80 (green) to identify boundary of the red and white pulp (white lines). Representative whole slide scanned images are shown (B). The proportion of red pulp area (C) occupied by endothelial cells (Meca-32) and expression of Meca32 relative to total white pulp area (D) was determined by computer-assisted morphometry. Data are expressed as mean ± SEM of at least two independent experiments where n = 5 for each treatment. ** p<0.02 *p<0.05. Spleens were examined after drug treatment for parasite burdens (E) and size/body weight ratio (F). Fig. 6. Schematic diagram of the effects of treatment with RTK inhibitors during L.donovani infection. 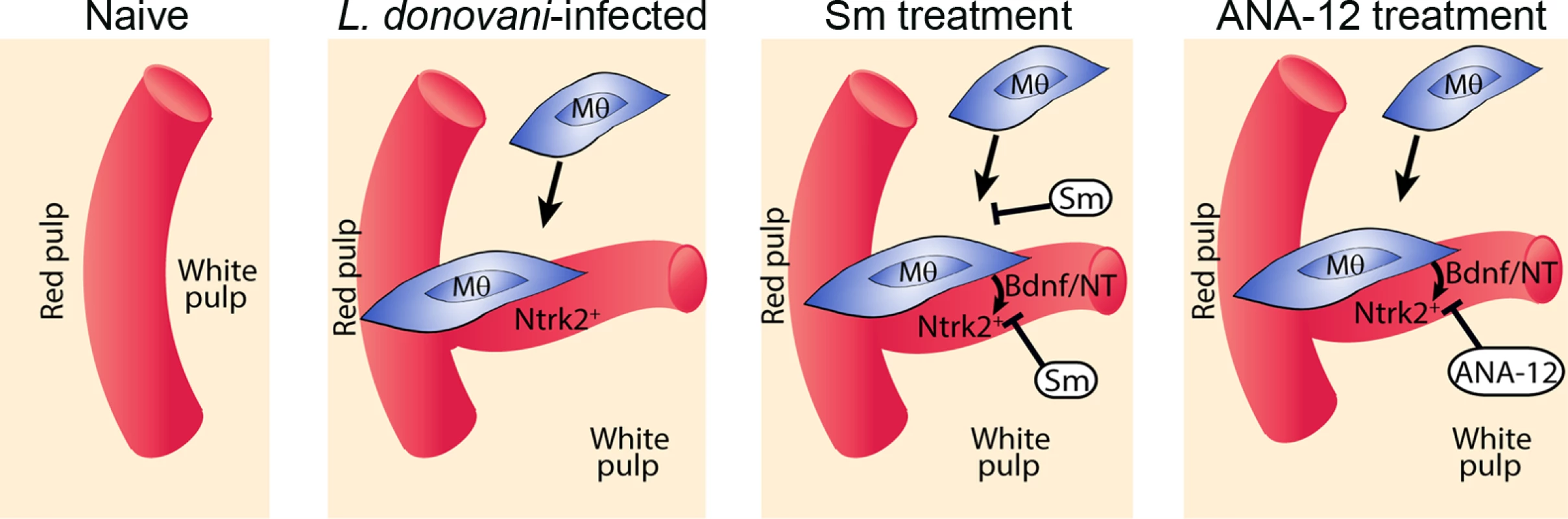
Cartoon to depict processes inhibited by the broad spectrum RTKi (Sm) and the selective Nrtk2 inhibitor (ANA-12) during infection-associated neovascularization of white pulp. Discussion
Chronic inflammation results from sustained immune mediated inflammatory responses leading to significant tissue destruction and / or remodeling, but the cells and mechanisms involved in these processes, especially in the context of infectious disease, are not fully understood. Our data provides the first evidence that the neurotrophic receptor Ntrk2 and its ligands play a role in mediating pathological vascular remodeling. First, we show that Ntrk2 is aberrantly expressed on lymphoid tissue (white pulp) vasculature in the spleen of mice with chronic infection-associated inflammation. This contrasts with the largely CNS-restricted expression of Ntrk2 expression observed in healthy adult tissues. Second, we have identified a population of splenic F4/80hi CD11blo MPs that express Ntrk2 ligands and possess all the characteristics needed to drive white pulp vascular remodeling. Third, we demonstrate that a selective antagonist of Ntrk2 inhibits ongoing white pulp neovascularization.
Angiogenesis and inflammation have long been thought of as co-dependent processes and there is increasing evidence that disruption to vasculature prolongs and intensifies the inflammatory response [35]. It has been shown that MPs are recruited to sites of neoangiogenesis and support neovascularization by releasing angiogenic factors, including VEGF, PDGF, FGF and metalloproteinases [36–38]. Recently, evidence has suggested that neuronal factors such as neurotrophic factors, ephrins, bone morphogenetic proteins (BMPs) and their receptors [19,39,40], can also play critical roles as angiogenic regulators. A study by Kermani et al highlighted that Bdnf promoted neovascularization in ischemic adult limbs and this correlated with the expression of Ntrk2 on endothelial cells. In agreement with that study, we found enrichment of Bdnf in F4/80hi CD11blo MPs and could directly show that these cells were closely associated with endothelial cells expressing Ntrk2. The expression of Ntrk2 by splenic endothelial cells was only observed in chronic infection and was not evident in the steady-state.
ANA-12 is a low molecular weight heterocyclic compound that was identified using a novel structure-based in silico screening technique as a selective Ntrk2 inhibitor [34]. In mice, ANA-12 has been shown to have anti-depressant and anti-anxiolytic activity [34] and it alters cocaine-mediated behaviour in rats [41]. Ntrk2 is also targeted, less selectively by other RTKi’s in the clinic or in early stage clinical development e.g. Lestaurtinib (for neuroblastoma;[42]) and PLX7486 (pancreatic adenocarcinoma; ClinicalTrials.gov identifier: NCT01804530). If aberrant expression of Ntrk2 is confirmed in other settings of chronic inflammation associated with pathogenic angiogenesis, Ntrk2-selective drugs may provide a new therapeutic option for treatment of these conditions. Importantly, the potential for aberrant expression and function of this neurotrophic receptor, as shown here, also highlights the need to consider inflammation when designing organ-specific therapeutics.
Developmentally, tissue macrophages are known to have functions in matrix remodeling [43], epithelial proliferation and outgrowth [44], angiogenesis and tissue organization [45,46]. It has been suggested that in chronic disease, the developmental activities of macrophages are dysregulated and that these activities contribute to disease pathology [47]. This is evident in some models of cancer where depletion of macrophages or inhibition of macrophage access to the tumor site inhibits tumor growth and angiogenesis[48–51]. Gene expression signatures of tumour-associated macrophages revealed enrichment in genes associated with developmental functions including matrix remodeling, and angiogenesis [52]. Consistent with these findings, F4/80hi CD11blo MPs isolated from the spleen of L. donovani-infected mice revealed enrichment in expression of genes functionally related to cancer and cell survival. These cells displayed angiogenic properties in vitro and given their spatial relationship with emerging blood vessels, it is highly likely that these cells are playing a role in neovascularisation in vivo.
Splenic macrophages are themselves a heterogeneous population, of which the immunological phenotype and immunological function of all populations has still not been extensively explored, particularly during chronic inflammation. The CD11c+MHCII+F4/80hi CD11blo MP population identified here that is expanded during L. donovani infection is rare in the spleen of uninfected mice, making it hard to draw direct comparisons with any function of these cells in the steady-state. F4/80+ macrophages found in the white pulp of the spleen were originally described 30 years ago in adult mice to be immediately adjacent to arterioles in the periarteriolar lymphoid sheath [26], and later studies defined these MPs as having high expression of the F4/80 antigen [27]. Although the low expression of SIGNR1 suggests that these MPs may have some relationship to marginal zone macrophages, cell-tracking studies of MZM in the spleens of infected mice suggest that most bone fide MZM are lost from the spleen during infection [11]. A formal identification of the relationship of the CD11c+MHCII+F4/80hi CD11blo MP population described here to different splenic MP populations present in the steady state would require lineage tracking studies that are beyond the scope of the current manuscript.
Finally, the impact of ANA-12 on tissue remodeling was highly selective compared to the impact we have previously observed using a broader spectrum RTKi, sunitinib maleate. Following sunitinib administration, the ongoing remodeling of red and white pulp vasculature is halted and follicular dendritic cell and fibroblastic reticular cell networks are largely restored [4]. In contrast, the impact of ANA-12 administration was selective for white pulp neovascularization. Likewise, whereas red pulp vasculature is remodeled largely by Ly6C+ inflammatory monocytes, white pulp neovascularization appears to reflect the more local behavior of MPs with a more resident-like phenotype. Further studies will be required to determine whether selective blockade of white pulp neovascularization using ANA-12 has any selective effects on immune function than might support its use in combination therapy for disease such as leishmaniasis, where lymphoid tissue remodeling is so prominent. However, in common with broader spectrum RTKi, placental expression of Ntrk2 and the influence of maternal energetic status on placental Ntrk2 expression[53] would likely be an impediment to development of this and similar drugs for the treatment of women of childbearing age.
In conclusion, we have shown that selected features of pathogenic angiogenesis induced by chronic infection are mediated through the interaction of a neurotrophic receptor with ligands produced by local MPs. Our data provide new insights into the role of the neurotrophins and their receptors in inflammation, and further characterize the exquisite compartment specific nature of tissue remodeling processes. The role of macrophage expression of Bdnf in other chronic infectious and inflammatory disease settings clearly warrants further study.
Materials and Methods
Ethics statement
All animal care and experimental procedures were regulated under the Animals (Scientific Procedures) Act 1986 (revised under European Directive 2010/63/EU) and were performed under UK Home Office License (Ref # PPL 60/4377) and with approval from the Animal Procedures and Ethics Committee of the Department of Biology, University of York.
Experimental infection and treatment
Female C57BL6 CD45.1 and CD45.2 mice were obtained from Charles River UK, housed under specific pathogen-free conditions and used at 6–10 weeks of age. The Ethiopian strain of L. donovani (LV9) was maintained by passage in RAG-2-/- mice. Mice were infected by injecting 3x107 amastigotes i.v. via the lateral tail vein (total n = 20 for each treatment experiment). Animals were then allocated to treatment groups. Sunitinib maleate (35mg/kg; Sequoia Research Products Ltd) (n = 5) or ANA-12 (1mg/kg; Sigma) (n = 5) was administered daily via oral gavage or intraperitoneally for 7d. Vehicle-treated mice received citrate-buffered saline, pH 3.5 (Sm) or PBS/1% DMSO (ANA-12) (n = 5). A sample size of 5 for each experiment was required to detect a 30% change in vascularization at 80% power based on previous data [4]. For FITC-dextran uptake, 70,000 MW FITC-dextran was injected i.v. at 10mg/ml one hour before animals were sacrificed.
Cell culture of SVEC4–10 cells
Murine endothelial cells (ATCC) were grown in DMEM media supplemented with 10% heat inactivated FCS in an atmosphere of 95% air and 5% CO2 at 37°C in plastic flasks. At confluence, the cells were subcultured at a 1 : 3 ratio and used at passage numbers three through to ten. Cells were tested for mycoplasma contamination using MycolAlert mycoplasma detection kit (Lonza, Cat No: LT07–418)
Microarray and pathway analysis
Custom microarrays with 568 gene probes, including 32 control probes were printed in-house. The 536 test probes were chosen to provide insight into the various functions and signalling pathways of macrophages.
Total RNA was isolated from splenic macrophages from L.donovani-infected mice (experimental samples) and from naive pooled peritoneal macrophages (reference sample). Amplified reference and experimental RNA fluorescently labelled with Cy3 or Cy5 were combined in equal amounts on custom printed microarray slides and hybridised overnight at 42°C. The average experimental: reference ratios were compared by t-tests. Microarray data was analysed using Ingenuity Pathways Analysis. The web-based pathways analysis tool IPA (Ingenuity Systems, www.ingenuity.com) was used to identify biological and molecular networks. Knowledge coming from published, peer-reviewed scientific publications is stored in the Ingenuity Pathways Knowledge Base (IPKB) and is continuously updated, this web-based tool then allows for the mapping of gene expression data into relevant pathways based on their functional annotation and known molecular interactions. The genes considered to have been differentially regulated to a significant extent when comparing F4/80hiCD11blo cells with conventional peritoneal macrophages were uploaded into IPA along with the gene identifiers and corresponding fold change values. In the network analysis, networks of these genes are then algorithmically generated based on their connectivity. The functional analysis of a network identified the biological functions and/or diseases that were most significant to the genes in the network, and the functional analysis of the entire data set identified the biological functions and/or diseases that were most significant to the data set.
Flow cytometry and cell sorting
Mononuclear cells were prepared from the spleens of C57BL6 L. donovani infected mice. Isolated cells were labelled with F4/80 (clone: BM8), CD11c (clone: N418), CD80 (clone: 16–10A1), CD86 (clone: GL1), CD40 (clone: IC10), CD115 (clone: AFS98), MHCII (clone: M5/114), CD11b (clone: M1/70), Gr-1 (Ly6C/G clone: RB6–8C5) all purchased from eBioscience and CD68 (clone FA-11) and CD169 (clone MOMA-1) were purchased from Serotec. Intracellular cytokine staining was performed following surface staining on fixed cell (2% paraformaldehyde) and then permeabilised with 0.5% saponin. Cells were analyzed using a cyAn flow cytometer and analyzed using Summit software (Beckman Coulter). Cells were sorted based on forward and side scatter and expression of PE or PE-Cy7 conjugated anti-CD11c, Alexa Fluor 450 anti-MHCII, Alexa Fluor 488 conjugated anti-CD11b and Alexa Fluor 647 anti-F480 on a MoFlo cell sorter (Beckman Coulter). Sorted cells were then used for tube formation assays, analysed for mRNA expression or morphological analysis was carried out with approximately 3000 sorted cells spun onto glass slides, fixed in methanol and stained with Giemsa. Light microscopy was conducted on a Zeiss Axioplan and imaged with an Optronics CCD camera using MagnaFire software (Optronics).
Confocal microscopy
Phenotypic analysis of MPs was also carried out on splenic frozen sections. Sections (6–10μm) from FITC-dextran injected, infected or uninfected mice were acetone fixed and labeled with biotin conjugated Gr-1 or biotin conjugated F4/80 and Alexa fluor 647 SIGNR1 (eBioscience).
Thicker sections (10–30μm) were used to assess the location of MPs within the spleen. Sections were labeled with a purified rat anti mouse pan endothelial cell antigen antibody (Biolegend, clone Meca-32) and/or a Cy3 labelled monoclonal antibody against α-Smooth Muscle Actin (α-SMA) (Sigma, Clone 1A4) were used to identify endothelial cells or vessels. Rat anti-mouse FDC antibody (FDCMI) (BD Pharmingen) was used to detect follicular dendritic cells. Fluorochrome conjugated goat anti-rat antibody (Invitrogen) were used for detection of purified antibodies. Sections were counterstained with DAPI and mounted in Pro-long Gold anti-fade (Invitrogen) and visualized using a Carl Zeiss upright LSM META 510, inverted LSM META 710 confocal microscope or a Carl Zeiss Axio Scan.Z1 digital slide scanner with brightfield and 4-colour fluorescence.
Tube formation assay
Angiogenesis assays were carried out as described previously [54]. Isolated splenocytes from chronically infected mice were first enriched for MPs by plastic adherence for 1hr at 37oC in RPMI (10% FCS) before cell sorting. Proliferating SVEC4–10 cells (1.5 x104 cells) were added to sorted populations of MPs or non-adherent cells (2 x 104) in endothelial basal media-2, with no FCS, (EBM-2; Lonza) and then plated onto growth factor-reduced Cultrex basement membrane (BME; Trevigen) coated 96 well plates. Negative controls of SVEC4–10 cells in EBM-2 and positive controls of SVEC4–10 cells in endothelial cell growth media-2 (EGM-2; Lonza) containing FCS and growth supplements (SingleQuot kit; Lonza) were included. Tube formation was assayed 4 hours after plating. Images were taken using a Zeiss inverted fluorescent microscope with x5 objective. All conditions were set up in each experiment in triplicate and imaged. Images were then uploaded onto http://www.wimasis.com/ for independent blind analysis using WimTube, web-based image analysis software, which quantitatively evaluated the generation of new vessels. Assays were analyzed by WimTube Quantitative Image Analysis.
Growth factor PCR array
Total RNA was extracted from the sorted cells using the RNeasy RNA isolation kit (Qiagen), and then quantified using a Nanodrop ND-100. For the PCR array, 200ng of total RNA was reverse transcribed using RT2 First Stand Kit (Qiagen), and cDNA was directly added to PCR Master mix containing SYBR green. The mixtures were then aliquoted into 96-well PCR array plates, to profile the expression of 84 growth factor pathway-related genes using a mouse growth factor signalling pathway RT2 Profiler PCR array (PAMM-041Z, Qiagen) according to the manufacturer’s instructions. The array also included 6 housekeeping genes and 3 RNA as internal controls. Arrays were run on an ABI 7300HT qPCR instrument equipped with SDS 2.3 software, using RT2 SYBR Green/ROX qPCR master mix (Qiagen). Data analysis was done by the 2-ΔΔCt method on the manufacturer’s Web portal http://www.SABiosciences.com/pcrarraydataanalysis.php
Statistics
Data are expressed values as means ± SEM. Comparison was performed using the unpaired Student’s t-test (for data following a Gaussian distribution) and the Mann-Whitney test (for data that did not assume Gaussian distribution). D’Agostino and Pearson omnibus normality test was used to test for Gaussian distribution. A probability of less than 5% (P < 0.05) was considered to be statistically significant. All statistical analyses were performed with Prism v5.01 (GraphPad, Inc.) software.
Supporting Information
Zdroje
1. Mueller SN, Germain RN (2009) Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol 9 : 618–629. doi: 10.1038/nri2588 19644499
2. Carmeliet P (2003) Angiogenesis in health and disease. NatMed 9 : 653–660. 12778163
3. Heidenreich R, Rocken M, Ghoreschi K (2009) Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol 90 : 232–248. doi: 10.1111/j.1365-2613.2009.00669.x 19563608
4. Dalton JE, Maroof A, Owens BM, Narang P, Johnson K, et al. (2010) Inhibition of receptor tyrosine kinases restores immunocompetence and improves immune-dependent chemotherapy against experimental leishmaniasis in mice. J Clin Invest 120 : 1204–1216. doi: 10.1172/JCI41281 20234089
5. Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, et al. (2011) Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 121 : 998–1008. doi: 10.1172/JCI45157 21393864
6. Zeng M, Southern PJ, Reilly CS, Beilman GJ, Chipman JG, et al. (2012) Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog 8: e1002437. doi: 10.1371/journal.ppat.1002437 22241988
7. Cadman ET, Abdallah AY, Voisine C, Sponaas AM, Corran P, et al. (2008) Alterations of splenic architecture in malaria are induced independently of Toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun 76 : 3924–3931. doi: 10.1128/IAI.00372-08 18559428
8. Brand C, Oliveira FL, Takiya CM, Palumbo A Jr., Hsu DK, et al. (2012) The involvement of the spleen during chronic phase of Schistosoma mansoni infection in galectin-3-/ - mice. Histol Histopathol 27 : 1109–1120. 22763883
9. Scandella E, Bolinger B, Lattmann E, Miller S, Favre S, et al. (2008) Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol 9 : 667–675. doi: 10.1038/ni.1605 18425132
10. Benedict CA, De Trez C, Schneider K, Ha S, Patterson G, et al. (2006) Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog 2: e16. 16518465
11. Engwerda CR, Ato M, Cotterell SE, Mynott TL, Tschannerl A, et al. (2002) A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. AmJPathol 161 : 429–437.
12. Zijlstra EE, el-Hassan AM (2001) Leishmaniasis in Sudan. Visceral leishmaniasis. Trans R Soc Trop Med Hyg 95 Suppl 1: S27–58. 11370250
13. Kaye PM, Svensson M, Ato M, Maroof A, Polley R, et al. (2004) The immunopathology of experimental visceral leishmaniasis. ImmunolRev 201 : 239–253. 15361245
14. Yurdakul P, Dalton J, Beattie L, Brown N, Erguven S, et al. (2011) Compartment-specific remodeling of splenic micro-architecture during experimental visceral leishmaniasis. Am J Pathol 179 : 23–29. doi: 10.1016/j.ajpath.2011.03.009 21703391
15. Neri D, Bicknell R (2005) Tumour vascular targeting. Nat Rev Cancer 5 : 436–446. 15928674
16. Cristofaro B, Emanueli C (2009) Possible novel targets for therapeutic angiogenesis. Curr Opin Pharmacol 9 : 102–108. doi: 10.1016/j.coph.2008.11.006 19071062
17. Kermani P, Hempstead B (2007) Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med 17 : 140–143. 17482097
18. Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, et al. (2000) Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 127 : 4531–4540. 11023857
19. Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, et al. (2005) Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest 115 : 653–663. 15765148
20. Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C (1994) Macrophages and angiogenesis. J Leukoc Biol 55 : 410–422. 7509844
21. Vega JA, Garcia-Suarez O, Hannestad J, Perez-Perez M, Germana A (2003) Neurotrophins and the immune system. J Anat 203 : 1–19. 12892403
22. Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, et al. (1999) Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189 : 865–870. 10049950
23. Barichello T, Dagostim VS, Generoso JS, Simoes LR, Dominguini D, et al. (2014) Neonatal Escherichia coli K1 meningitis causes learning and memory impairments in adulthood. J Neuroimmunol 272 : 35–41. doi: 10.1016/j.jneuroim.2014.05.003 24857717
24. Serghides L, McDonald CR, Lu Z, Friedel M, Cui C, et al. (2014) PPARgamma agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathog 10: e1003980. doi: 10.1371/journal.ppat.1003980 24603727
25. Martinelli PM, da Costa Rocha MO, Teixeira AL, do Carmo Pereira Nunes M, da Silva Camargos ER (2011) Brain-derived neurotrophic factor is up regulated in chronic Chagas disease. Int J Cardiol 149 : 277–278. doi: 10.1016/j.ijcard.2011.02.058 21420187
26. Hume DA, Robinson AP, MacPherson GG, Gordon S (1983) The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med 158 : 1522–1536. 6355361
27. Hume DA, Perry VH, Gordon S (1984) The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec 210 : 503–512. 6524692
28. Geijtenbeek TB, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, et al. (2002) Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood 100 : 2908–2916. 12351402
29. Phillips R, Svensson M, Aziz N, Maroof A, Brown N, et al. (2010) Innate killing of Leishmania donovani by macrophages of the splenic marginal zone requires IRF-7. PLoS Pathog 6: e1000813. doi: 10.1371/journal.ppat.1000813 20300600
30. Beattie L, d'El-Rei Hermida M, Moore JW, Maroof A, Brown N, et al. (2013) A transcriptomic network identified in uninfected macrophages responding to inflammation controls intracellular pathogen survival. Cell Host Microbe 14 : 357–368. doi: 10.1016/j.chom.2013.08.004 24034621
31. Perez-Perez M, Garcia-Suarez O, Blanco-Gelaz MA, Esteban I, Ciriaco E, et al. (2004) TrkB mRNA and protein in mouse spleen: structure of the spleen of functionally deficient TrkB mice. Cell Tissue Res 316 : 179–187. 15045579
32. Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, et al. (1995) Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol 147 : 309–324. 7639328
33. Benjamin LE, Hemo I, Keshet E (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125 : 1591–1598. 9521897
34. Cazorla M, Premont J, Mann A, Girard N, Kellendonk C, et al. (2011) Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest 121 : 1846–1857. doi: 10.1172/JCI43992 21505263
35. Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD (1997) The codependence of angiogenesis and chronic inflammation. FASEB J 11 : 457–465. 9194526
36. Giraudo E, Inoue M, Hanahan D (2004) An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 114 : 623–633. 15343380
37. Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, et al. (2003) VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res 92 : 378–385. 12600898
38. Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, et al. (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 113 : 1040–1050. 15057311
39. Mansson-Broberg A, Siddiqui AJ, Genander M, Grinnemo KH, Hao X, et al. (2008) Modulation of ephrinB2 leads to increased angiogenesis in ischemic myocardium and endothelial cell proliferation. Biochem Biophys Res Commun 373 : 355–359. doi: 10.1016/j.bbrc.2008.06.036 18571496
40. Smadja DM, Bieche I, Silvestre JS, Germain S, Cornet A, et al. (2008) Bone morphogenetic proteins 2 and 4 are selectively expressed by late outgrowth endothelial progenitor cells and promote neoangiogenesis. Arterioscler Thromb Vasc Biol 28 : 2137–2143. doi: 10.1161/ATVBAHA.108.168815 18818419
41. Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013) Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16 : 42–47. doi: 10.1038/nn.3280 23242310
42. Iyer R, Evans AE, Qi X, Ho R, Minturn JE, et al. (2010) Lestaurtinib enhances the antitumor efficacy of chemotherapy in murine xenograft models of neuroblastoma. Clin Cancer Res 16 : 1478–1485. doi: 10.1158/1078-0432.CCR-09-1531 20179224
43. Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW (2006) Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn 235 : 3222–3229. 17029292
44. Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA (1999) Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development 126 : 2141–2147. 10207139
45. Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, et al. (2005) WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437 : 417–421. 16163358
46. Rao S, Lobov IB, Vallance JE, Tsujikawa K, Shiojima I, et al. (2007) Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch. Development 134 : 4449–4458. 18039971
47. Pollard JW (2009) Trophic macrophages in development and disease. Nat Rev Immunol 9 : 259–270. doi: 10.1038/nri2528 19282852
48. Oguma K, Oshima H, Aoki M, Uchio R, Naka K, et al. (2008) Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J 27 : 1671–1681. doi: 10.1038/emboj.2008.105 18511911
49. Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, et al. (2007) Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol 127 : 2031–2041. 17460736
50. Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, et al. (2004) Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res 64 : 5378–5384. 15289345
51. Paulus P, Stanley ER, Schafer R, Abraham D, Aharinejad S (2006) Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res 66 : 4349–4356. 16618760
52. Ojalvo LS, King W, Cox D, Pollard JW (2009) High-density gene expression analysis of tumor-associated macrophages from mouse mammary tumors. Am J Pathol 174 : 1048–1064. doi: 10.2353/ajpath.2009.080676 19218341
53. Mayeur S, Silhol M, Moitrot E, Barbaux S, Breton C, et al. (2010) Placental BDNF/TrkB signaling system is modulated by fetal growth disturbances in rat and human. Placenta 31 : 785–791. doi: 10.1016/j.placenta.2010.06.008 20615547
54. Arnaoutova I, Kleinman HK (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5 : 628–635. doi: 10.1038/nprot.2010.6 20224563
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



