-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Case for Two-Component Signaling Systems As Antifungal Drug Targets
article has not abstract
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004632
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004632Summary
article has not abstract
The Impact of Fungal Diseases
The recent outbreak of fungal meningitis caused by Exserohilum rostratum in patients receiving contaminated steroid injections resulted in 64 deaths, receiving a lot of press and briefly bringing into the public eye the difficulty of treating systemic fungal infections [1]. What is generally less well appreciated, however, is that there are several other, much more common fungal pathogens that pose a serious health threat. Indeed, currently more people die from these fungal diseases worldwide than from tuberculosis or malaria [2]. The fungal pathogens most frequently responsible for human mortality are: Aspergillus fumigatus, Candida spp. (predominantly C. albicans), Cryptococcus neoformans, Pneumocystis carinii, and dimorphic fungi that cause endemic mycoses (Coccidioides immitis, Histoplasma capsulatum, Blastomyces dermatitides, and Paracoccidioides brasiliensis). Fungal pathogens pose an especially high risk to individuals with compromised immunity, and this population of susceptible hosts is growing [3,4]. There has been a steady increase in the incidence of fungal infections over recent decades, primarily due to the AIDS pandemic, an increase in patients receiving cancer chemotherapy and allogeneic bone marrow transplants, a higher incidence of seriously ill patients in intensive care units, and the aging of the human population [3–8].
Despite the extensive list of fungal pathogens and the increasing frequency of their occurrence, we have at our disposal only a very limited number of antifungal drugs. The past two decades have seen the emergence of two classes of antifungals: those that target ergosterol synthesis (the azoles) and those that target cell wall β-1,3 glucan synthase (echinocandins). Of the azoles, the triazoles have gained importance as alternatives to the more toxic amphotericin B. Triazoles are fungistatic and their continued use has resulted in an increase in triazole resistance among formerly sensitive species and a rising number of disease cases caused by intrinsically azole-resistant non-albicans Candida species [4]. Echinocandins are fungicidal and are the drug of choice for treating most fungal infections, but these drugs are not effective in treating infections caused by C. neoformans, and echinocandin resistance is increasing in some Candida species [9]. Clearly, there is an urgent need to discover new drug targets to meet the challenges posed by fungal infections.
Two-Component Signal Transduction Pathways in Fungal Pathogens as Potential Drug Targets
An ideal drug target is a fungal-specific protein that (1) functions as a virulence factor or is essential for fungal viability and (2) is absent from the host organism, such that its inhibition causes no toxicity in the host. The low number of drugs in our antifungal armamentarium is in part due to the relative evolutionary relatedness of fungi and mammals and the resulting paucity of fungal-specific proteins that meet these criteria. One class of molecules that do fit both of these criteria is comprised of proteins that function in the so-called two-component signal transduction pathways. These signaling pathways are based on the transfer of phosphoryl groups among their components (phosphorelays) and are one of the primary means by which bacteria and fungi sense and respond to environmental cues. Two-component signal transduction pathways present attractive targets for antifungal drug discovery because they exist in prokaryotes, plants, and lower eukaryotes but not in mammalian cells. Furthermore, while the genes encoding two-component system proteins are frequently not essential for viability, multiple studies have demonstrated the importance of two-component signal proteins for virulence in fungal pathogens, including A. fumigatus [10], C. albicans [11–13], C. neoformans [14], B. dermatitidis, and H. capsulatum [15] (Table 1).
Tab. 1. List of two-component signaling proteins and their functions in human fungal pathogens. 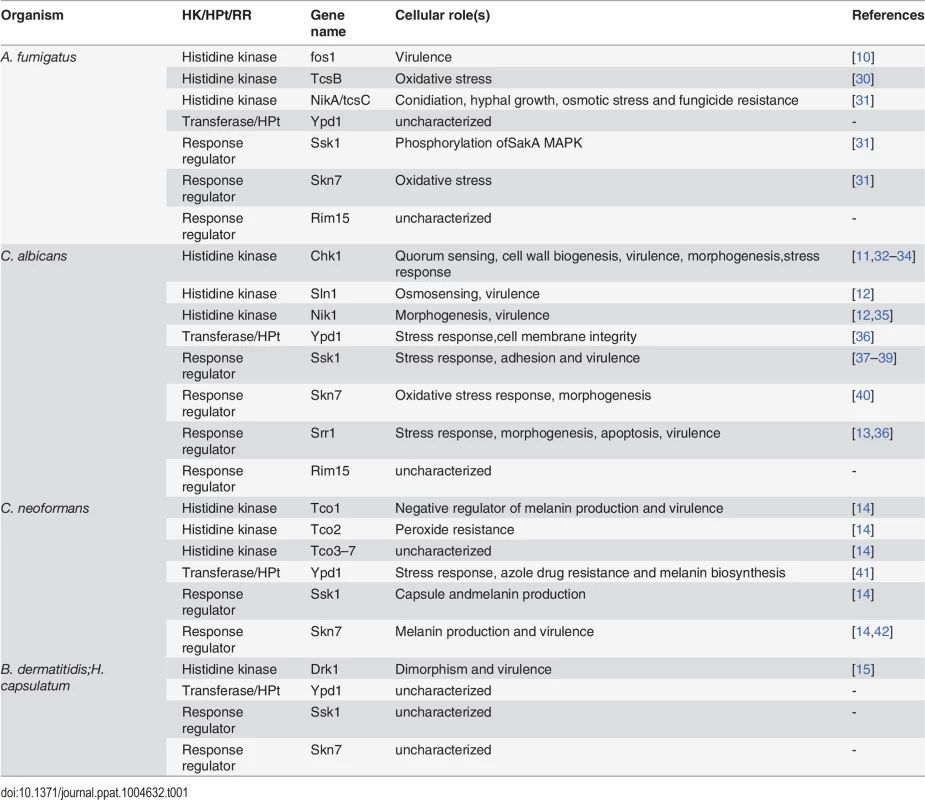
The term “two-component” is derived from bacterial systems where the phosphorelay generally involves two proteins: a histidine kinase (HK) and a response regulator (RR) protein (Fig. 1). In response to an environmental signal, the HK, which is frequently localized in the bacterial outer membrane, is autophosphorylated on a conserved histidine residue, followed by a transfer of the phosphoryl group to a cognate response regulator protein (RR) on a conserved aspartate residue. The phosphorylated RR then usually acts directly as a transcription factor to activate genes associated with chemotaxis, stress response, quorum sensing, sporulation, virulence factor expression, and antibiotic resistance [16]. Fungal two-component phosphorelays are more intricate in two respects (Fig. 1). First, the signaling cascade involves three proteins: HK, RR, and a histidine phosphotransferase (HPt) whose function is to shuttle the phosphate moiety from HK to RR [17]. Second, in fungi, the phosphorelay typically comprises four phosphorylation events: (1) the HK is autophosphorylated on a histidine residue within its histidine kinase domain; (2) the phosphate is transferred intramolecularly to an aspartate (His→Asp) in the HK receiver domain; (3) a third, intermolecular phosphotransfer occurs to the histidine residue present in the HPt domain on the transferase (His→Asp→His); and (4) the phosphoryl group is relayed to an aspartate on the RR protein (His→Asp→His→Asp). Thus, two-component–like phosphorelay systems are unusual in terms of mechanism: the amino acids that accept phosphoryl groups are either aspartate or histidine residues. These unique features may be exploited in designing specific inhibitors that would not affect the activity of conventional Ser/Thr/Tyr kinases more prevalent in mammalian systems.
Fig. 1. Schematic representation of two-component signal transduction pathways in bacteria and common human fungal pathogens. 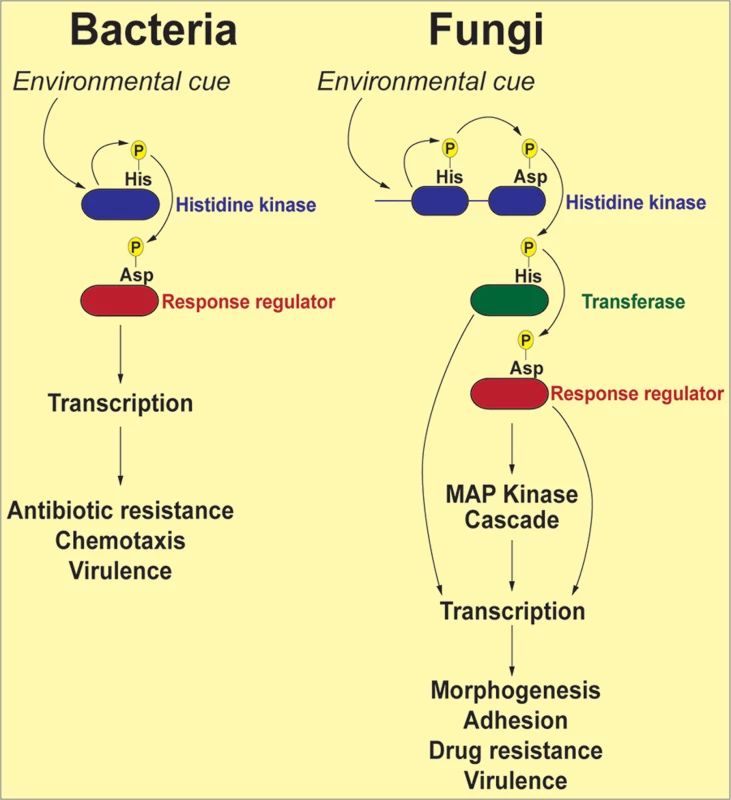
In response to environmental stimuli the histidine kinase (HK) is autophosphorylated on a histidine residue. In bacteria, the phosphate group from the histidine is transferred to an aspartate residue on a response regulator (RR), and the phosphorylated RR usually acts as a transcription factor to activate genes involved in response to the stimuli. In fungi, the phosphorelay involves three proteins: the phosphoryl group is first transferred intramolecularly from the histidine to an aspartate on the HK, then to a histidine on a transferase protein, and finally to an aspartate on a RR. The end result of these reactions is often activation of a downstream MAP kinase cascade, which, in turn, activates transcription factors whose target genes participate in the cellular response to environmental change. Depending on the system and the specific factors involved, the initial HK phosphorylation may occur either in response to stress or in response to removal of stress, with the ultimate outcome of either activating or down-regulating the transcription of stress response genes. A comprehensive list of all known fungal two-component signal transduction proteins and their roles in virulence-related processes can be found in Table 1. For a detailed discussion on the function of HKs and RRs in medically relevant fungi, the reader is directed to several excellent reviews [17–19].
High Throughput Screens for Fungal Two-Component System Inhibitors
Screening for inhibitors in vitro
Despite the acknowledged attractiveness of two-component systems as drug targets and their well-understood molecular mechanisms, thus far no two-component system inhibitor has reached the clinic, even though efforts to identify inhibitors of bacterial two-component systems stretch back approximately two decades. Most of the in vitro efforts to identify such inhibitors have focused on searching for compounds that could prevent HK phosphorylation activity [20]. However, many of the identified compounds did not exhibit competitive kinetics with ATP, inhibiting HK activity by other means, such as promoting HK aggregation [20]. These screens largely relied on detecting radioactively labeled phosphorylation substrates; however, phosphohistidines are highly unstable moieties [21], complicating these studies and making high-throughput screening difficult. Recently, a number of new tools have been developed to analyze HK activity, including an antibody that specifically recognizes phosphohistidine [22] and a fluorescent probe to detect histidine phosphorylation by HK in vitro [23]. The probe can label both the HK itself upon autophosphorylation and the downstream HK phosphorylation target [23], and can be thus used as a screening tool in identifying inhibitors of different steps of the phosphorelay. These tools, together with synthetic, natural, or peptide-based libraries, can facilitate rapid high-throughput screening of fungal HKs in vitro. The results of these screens can be combined with in vivo assays for two-component system activity described below.
Screening for inhibitors in vivo
While in vitro screens can identify compounds that act via a desired molecular mechanism, these compounds may not be active in cellular or organismal context. For example, several identified HK inhibitors with good activity in vitro failed to inhibit bacterial growth in vivo because they were sequestered in membranes and other lipid-rich compartments [20]. Another potential benefit of in vivo screens is that they can target any component of the two-component pathway, not just the HK. To identify compounds that inhibit two-component systems in vivo, several approaches have been used in both bacteria and fungi. One set of studies focused on fungal HK Nik1 because it belongs to one of six highly conserved HK families, suggesting that its inhibition may have broad spectrum antifungal activity. In particular, one study looked for compounds that specifically inhibited the growth of a Saccharomyces cerevisiae strain heterologously expressing C. albicans Nik1 [24]. However, while this work identified two new fungicidal compounds, it also showed that these compounds did not act via inhibition of Nik1 [24].
A conceptually different type of in vivo screening approach applied in both bacteria and fungi uses a strain where the function of a particular pathway is compromised by mutation; as a result, the screen strain shows exacerbated sensitivity relative to the wild-type strain to compounds that specifically inhibit this pathway [25,26]. Because two-component system genes are not essential, in this instance, growth rate or viability may not be informative screening end points. Rather, it may be worthwhile to screen for small molecules that significantly sensitize the screen strain to oxidative stress because two-component systems are necessary for normal oxidative stress resistance in various fungi (Table 1). In a diploid fungus, such as C. albicans, compromising the two-component system can be achieved by deleting one of the two copies of the gene encoding a HK or RR, creating a heterozygous mutant. In a haploid fungus, such as Candida glabrata, a temperature-sensitive allele of a two-component system gene can be utilized for the same purpose. This approach has been used to identify compounds that affect a variety of cellular pathways, including ergosterol biosynthesis, the actin cytoskeleton, and protein folding [26], and can be readily adapted to screen for two-component system inhibitors.
Another in vivo approach can take advantage of the fact that two-component signal transduction systems regulate gene expression, either via activating downstream MAP kinase cascades or by RR proteins acting as transcription factors [19]. Thus, it should be possible to screen for pathway-specific inhibitors using a reporter whose activation or repression depends on the functionality of the two-component system pathway. Recent studies examining the effects of two-component pathways on gene expression suggest that cell-wall–maintenance genes are strongly induced by a response to stress and that this induction requires two-component systems in several fungi, including C. albicans, S. cerevisiae, and C. neoformans [27–29]. Thus, promoters of individual cell-wall–maintenance genes may be fused to fluorescent markers and used as reporters in high-throughput screens for inhibitors. This approach would likely not be specific to the two-component pathway, but will also identify inhibitors of downstream signaling events, such as other steps in the corresponding MAP kinase cascade.
Concluding Remarks
The limited data available from studies of two-component proteins in fungal pathogens have revealed the critical functions of these proteins in adaptation to stress, regulation of virulence factors, and sensitivity to antifungal drugs, underscoring the importance of these signaling pathways in fungal pathogenesis. These features, together with the absence of two-component pathways in animals, make these proteins very attractive targets for antifungal drug discovery. Because two-component systems are found in all major fungal pathogens, drugs targeting these factors may have broad spectra. Recently developed tools for phosphohistidine analysis are likely to facilitate in vitro screening efforts, while complementary searches for pathway-based inhibitors may identify compounds that specifically inactivate two-component signal transduction in vivo.
Zdroje
1. Pappas PG (2013) Lessons learned in the multistate fungal infection outbreak in the United States. Curr Opin Infect Dis 26 : 545–550. doi: 10.1097/QCO.0000000000000013 24152763
2. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4 : 165rv113. doi: 10.1126/scitranslmed.3004404 23253612
3. Hof H (2010) Mycoses in the elderly. Eur J Clin Microbiol Infect Dis 29 : 5–13. doi: 10.1007/s10096-009-0822-5 19911208
4. Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20 : 133–163. 17223626
5. Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, et al. (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34 : 7–14. 11731939
6. Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN (2010) Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009). Diagn Microbiol Infect Dis 68 : 278–283. doi: 10.1016/j.diagmicrobio.2010.06.015 20846808
7. Wenzel RP (1995) Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis 20 : 1531–1534. 7548504
8. Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39 : 309–317. 15306996
9. Perlin DS (2014) Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drugs 74 : 1573–1585. doi: 10.1007/s40265-014-0286-5 25255923
10. Clemons KV, Miller TK, Selitrennikoff CP, Stevens DA (2002) fos-1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med Mycol 40 : 259–262. 12146755
11. Calera JA, Calderone R (1999) Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology 145 (Pt 6): 1431–1442.
12. Yamada-Okabe T, Mio T, Ono N, Kashima Y, Matsui M, et al. (1999) Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J Bacteriol 181 : 7243–7247. 10572127
13. Desai C, Mavrianos J, Chauhan N (2011) Candida albicans SRR1, a putative two-component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot Cell 10 : 1370–1374. doi: 10.1128/EC.05188-11 21841121
14. Bahn YS, Kojima K, Cox GM, Heitman J (2006) A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol Biol Cell 17 : 3122–3135. 16672377
15. Nemecek JC, Wuthrich M, Klein BS (2006) Global control of dimorphism and virulence in fungi. Science 312 : 583–588. 16645097
16. Mascher T, Helmann JD, Unden G (2006) Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev 70 : 910–938. 17158704
17. Fassler JS, West AH (2013) Histidine phosphotransfer proteins in fungal two-component signal transduction pathways. Eukaryot Cell 12 : 1052–1060. doi: 10.1128/EC.00083-13 23771905
18. Catlett NL, Yoder OC, Turgeon BG (2003) Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot Cell 2 : 1151–1161. 14665450
19. Chauhan N, Latge JP, Calderone R (2006) Signalling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat Rev Microbiol 4 : 435–444. 16710324
20. Kurosu M, Begari E (2010) Bacterial Protein Kinase Inhibitors. Drug Development Research 71 : 168–187.
21. Kee JM, Muir TW (2012) Chasing phosphohistidine, an elusive sibling in the phosphoamino acid family. ACS Chem Biol 7 : 44–51. doi: 10.1021/cb200445w 22148577
22. Kee JM, Oslund RC, Perlman DH, Muir TW (2013) A pan-specific antibody for direct detection of protein histidine phosphorylation. Nat Chem Biol 9 : 416–421. doi: 10.1038/nchembio.1259 23708076
23. Wilke KE, Francis S, Carlson EE (2012) Activity-based probe for histidine kinase signaling. J Am Chem Soc 134 : 9150–9153. doi: 10.1021/ja3041702 22606938
24. Tebbets B, Stewart D, Lawry S, Nett J, Nantel A, et al. (2012) Identification and characterization of antifungal compounds using a Saccharomyces cerevisiae reporter bioassay. PLoS One 7: e36021. doi: 10.1371/journal.pone.0036021 22574132
25. Okada A, Gotoh Y, Watanabe T, Furuta E, Yamamoto K, et al. (2007) Targeting two-component signal transduction: a novel drug discovery system. Methods Enzymol 422 : 386–395. 17628150
26. Xu D, Jiang B, Ketela T, Lemieux S, Veillette K, et al. (2007) Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog 3: e92. 17604452
27. Ko YJ, Yu YM, Kim GB, Lee GW, Maeng PJ, et al. (2009) Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot Cell 8 : 1197–1217. doi: 10.1128/EC.00120-09 19542307
28. Kruppa M, Goins T, Cutler JE, Lowman D, Williams D, et al. (2003) The role of the Candida albicans histidine kinase [CHK1) gene in the regulation of cell wall mannan and glucan biosynthesis. FEMS Yeast Res 3 : 289–299. 12689636
29. Li S, Dean S, Li Z, Horecka J, Deschenes RJ, et al. (2002) The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol Biol Cell 13 : 412–424. 11854400
30. Du C, Sarfati J, Latge JP, Calderone R (2006) The role of the sakA (Hog1) and tcsB (sln1) genes in the oxidant adaptation of Aspergillus fumigatus. Med Mycol 44 : 211–218. 16702099
31. Hagiwara D, Takahashi-Nakaguchi A, Toyotome T, Yoshimi A, Abe K, et al. (2013) NikA/TcsC histidine kinase is involved in conidiation, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS One 8: e80881. doi: 10.1371/journal.pone.0080881 24312504
32. Bernhardt J, Herman D, Sheridan M, Calderone R (2001) Adherence and invasion studies of Candida albicans strains, using in vitro models of esophageal candidiasis. J Infect Dis 184 : 1170–1175. 11598840
33. Kruppa M, Krom BP, Chauhan N, Bambach AV, Cihlar RL, et al. (2004) The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot Cell 3 : 1062–1065. 15302838
34. Torosantucci A, Chiani P, De Bernardis F, Cassone A, Calera JA, et al. (2002) Deletion of the two-component histidine kinase gene (CHK1) of Candida albicans contributes to enhanced growth inhibition and killing by human neutrophils in vitro. Infect Immun 70 : 985–987. 11796636
35. Srikantha T, Tsai L, Daniels K, Enger L, Highley K, et al. (1998) The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology 144 (Pt 10): 2715–2729. 9802013
36. Mavrianos J, Berkow EL, Desai C, Pandey A, Batish M, et al. (2013) Mitochondrial two-component signaling systems in Candida albicans. Eukaryot Cell 12 : 913–922. doi: 10.1128/EC.00048-13 23584995
37. Calera JA, Zhao XJ, Calderone R (2000) Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect Immun 68 : 518–525. 10639412
38. Chauhan N, Inglis D, Roman E, Pla J, Li D, et al. (2003) Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot Cell 2 : 1018–1024. 14555484
39. Li D, Bernhardt J, Calderone R (2002) Temporal expression of the Candida albicans genes CHK1 and CSSK1, adherence, and morphogenesis in a model of reconstituted human esophageal epithelial candidiasis. Infect Immun 70 : 1558–1565. 11854244
40. Singh P, Chauhan N, Ghosh A, Dixon F, Calderone R (2004) SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect Immun 72 : 2390–2394. 15039366
41. Lee JW, Ko YJ, Kim SY, Bahn YS (2011) Multiple roles of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformans. Eukaryot Cell 10 : 998–1002. doi: 10.1128/EC.05124-11 21642509
42. Wormley FL Jr., Heinrich G, Miller JL, Perfect JR, Cox GM (2005) Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect Immun 73 : 5022–5030. 16041017
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



