-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaManipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
Adenoviruses are mostly considered self-limiting pathogens associated with respiratory, gastrointestinal and ocular infections; however, in immunocompromised subjects disseminated Ad infection can occur with life-threatening consequences. Many human Ads are capable of binding to coagulation factor X (FX). Following intravenous administration in animal models, FX binds directly to the major Ad capsid protein, the hexon, which subsequently results in virus accumulation in the liver. FX coating Ad5 also acts to shield against immune neutralisation via natural IgM antibodies and the classical complement system. Here we show that FX protection is not a conserved mechanism amongst Ads and identify the Ad5 hexon hypervariable regions (HVR) as the capsid proteins targeted by this host defense pathway. Furthermore, we show that genetic inclusion of Ad5 HVRs onto a native non-FX binder Ad26 to be sufficient to confer sensitivity to immune attack in vitro and in vivo. Using intravenous administration, we determine the significance of FX binding to the Ad5-derived HVRs with respect to defending the virus from neutralisation whilst mediating virus tropism. Our study gives new insight into the role of the viral HVRs and of FX at the interface between virus and host defense mechanisms.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004673
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004673Summary
Adenoviruses are mostly considered self-limiting pathogens associated with respiratory, gastrointestinal and ocular infections; however, in immunocompromised subjects disseminated Ad infection can occur with life-threatening consequences. Many human Ads are capable of binding to coagulation factor X (FX). Following intravenous administration in animal models, FX binds directly to the major Ad capsid protein, the hexon, which subsequently results in virus accumulation in the liver. FX coating Ad5 also acts to shield against immune neutralisation via natural IgM antibodies and the classical complement system. Here we show that FX protection is not a conserved mechanism amongst Ads and identify the Ad5 hexon hypervariable regions (HVR) as the capsid proteins targeted by this host defense pathway. Furthermore, we show that genetic inclusion of Ad5 HVRs onto a native non-FX binder Ad26 to be sufficient to confer sensitivity to immune attack in vitro and in vivo. Using intravenous administration, we determine the significance of FX binding to the Ad5-derived HVRs with respect to defending the virus from neutralisation whilst mediating virus tropism. Our study gives new insight into the role of the viral HVRs and of FX at the interface between virus and host defense mechanisms.
Introduction
For the immunocompromised host, human adenoviruses (Ad) have emerged as a significant pathogen capable of exploiting the impaired immunological response and becoming invasive, manifesting in prolonged, severe and life threatening conditions [1–5]. There are seven human species (A-G) of this common non-enveloped, double-stranded DNA virus. Whilst in healthy individuals infections are self-limiting, targeting defined tissues such as the lung, eye and gastrointestinal system over a short time frame, disseminated Ad infections occur when immunity is low (e.g. sufferers of hereditary immune deficiencies, patients undergoing immunosuppressive treatment [1–5]). Systemic infections can culminate in serious and diverse clinical syndromes, ranging from fulminant hepatic failure, coagulopathy, hemorrhagic cystitis, myocarditis, encephalopathy, nephritis to multi-organ failure [2,6]. The immunocompromised patient population is expanding due to increased use of immunosuppressive therapies (e.g. cytotoxic drugs) and consequently Ads are gaining increased recognition as a clinical problem. The presence of a high viral load in the blood is often strongly indicative of a severe outcome [5,6]. Incidence of infection is approximately 2.5–47% in stem cell transplant recipients, whilst paediatric transplantation patients are more prone to the disseminated disease, with mortality rates reaching up to 70% [5,7,8]. Despite the high risk, there is no FDA approved drug specific to treating Ad infection and therapeutic options can be limited. Advancing our knowledge of the complex mechanisms underlying Ad5 infection in vivo is of great importance.
Using species C Ad2/5 as the prototype, the in vitro infection pathway has been very well documented. Studies have finely detailed the individual steps from virus binding via the fiber knob protein to the primary cell surface coxsackie and adenovirus receptor (CAR) [9], engagement of the Ad penton base with αvβ3/5 integrins leading to internalization [10] and subsequent trafficking from endosomes to nuclear import [11,12]. However, the lack of suitable animal models, which allow viral replication and closely mimic the human immune system, has challenged the study of Ad infection in vivo. Nevertheless an abundance of valuable information has been gained about viral spread, immune responses and methods to combat such, especially from its popularity as a viral vector for gene therapy, vaccination and virotherapy protocols. Of the 60+ human Ads identified [13], Ad5 is the virus-based gene transfer vector most frequently employed. Concomitantly, Ad5 is also one of the most seroprevalent of the family. The use of Ad5 as a viral vector has deepened our understanding of virus:host binding events, involvement of innate and adaptive immunity and of the factors leading to the substantial accumulation of Ad5 particles in the liver following bolus injection into the bloodstream. When administered intravenously (I.V.) the virus rapidly encounters a multitude of interactions with circulating blood components. These include virion neutralisation by pre-existing antibodies [14], sequestration by Kupffer cells [15], MARCO+-expressing splenic macrophages [16], polymorphonuclear leukocytes [17], natural IgM and complement opsonisation [18,19]. Binding to platelets [20,21], erythrocytes [22,23], and blood coagulation factors [24–26] all contribute to the substantial interplay between the virus and host. Dissecting the precise interactions which occur in vivo is key to our understanding of the virus infection pathways partnered with an individual’s defence mechanisms.
Previous work suggests that Ads belonging to species C (Ad1, Ad2 and Ad5) are more commonly associated with disseminated disease than other types and have been implicated in severe hepatic failure [5,8]. Hepatitis is a frequent and serious consequence of systemic Ad infections [27–29]. Coagulation factor X (FX) plays a fundamental role in determining the characteristic hepatic tropism of Ad5 [24–26]. Selectively blocking FX prevents Ad liver transduction in rodent and non-human primates following I.V delivery of virus [30–32]. FX binds with nanomolar affinity to the Ad5 hexon hypervariable regions (HVR), and acts as a bridge to attach the virus to N and O-linked heparan sulphate proteoglycans (HSPG) on the surface of hepatocytes [24,33,34]. Crystallographic and cryogenic electron microscopy identified contact points within and around Ad5 HVR5 and HVR7 which are responsible for interacting with the FX Gla (γ-carboxylated glutamic acid) domain [32]. Genetically swapping regions or specific amino acids within the Ad5 HVR5 and HVR7 for those of a non-FX-binding Ad (e.g. species D Ad48 or Ad26) has proven an effective strategy to abrogate the FX interaction and diminish liver transduction [32,35]. In addition to binding the FX Gla domain at the HVR7 amino acid motif T423-E424-T425, Ad5 is also capable of interacting with coagulation factor VII (FVII) at these points [36]. However, FVII does not support Ad5 transduction as it binds to the virus in an alternate orientation to FX, with dimerization of the FVII serine protease domains disguising potential HSPG receptor binding sites [36]. Further to its influence on liver transduction, Doronin et al. showed that FX coating the virus triggers recognition by the innate immune system via nuclear factor-κB (NFκB) activation and subsequent TLR4/TRAF6/NFκB-mediated inflammation, an effect absent when Ad5 was genetically manipulated to be devoid of FX binding [37].
Recent work by Xu et al. has produced additional insight into the function of FX in adenovirus biology [19]. They indicated a role for FX in protecting Ad5 from attack by natural IgM antibodies and the classical complement system upon exposure to murine blood [19]. In co-operation with IgM, complement activation acts as an innate host defense mechanism and has previously been shown to result in neutralisation of invading pathogens including Ads [18,19,38]. In vitro data demonstrated that FX can prevent Ad5 from this neutralisation when incubated with murine serum [19]. In contrast to studies in wild-type mice, the Ad5:FX interaction was not essential for liver transduction in mice deficient in natural antibodies or the complement components C1q and C4 [19]. Instead FX binding to Ad5 acted as a protective "shield", decorating the viral capsid and preventing natural IgM and classical complement mediated inhibition of Ad gene transfer [19]. This study has led to some speculation surrounding the impact of FX in determining Ad liver tropism.
It is evident that blood components influence Ad tropism, whilst other interactions (e.g. Kupffer cell uptake) remain dominant barriers to widespread Ad dissemination. Here, we studied the binding events and mechanisms deciding the fate of the virus in circulation. We attempted to dissect the importance of these interactions in determining viral cellular uptake and tropism. In this study we used genetically mutated Ad vectors to identify key hexon regions responsible for IgM and complement-mediated attack. The Ad5 HVRs were identified as the critical viral capsid components. We then incorporated these regions and FX binding capability onto a non-FX-binding Ad26 background. We utilised this novel vector to investigate the significance of FX and the role of the Ad5 HVRs in the interplay between viral immune recognition and tropism in vivo.
Results
Ad5 hexon hypervariable regions are required for IgM and classical complement-mediated neutralisation
FX coating Ad5 shields the virus against IgM and classical complement-mediated immune attack [19]. Many other Ad types also bind to human FX (hFX) [24]. However it is unknown whether they are sensitive to neutralisation via the same pathway. Therefore, we compared the sensitivity of a selected panel of Ad vectors based on different species/types; the FX-binding Ad5, Ad35 and Ad50, and non-binding Ad48 and Ad26 [24] to neutralisation by murine serum in vitro. When Ad5 was incubated with C57BL/6 serum there was a significant increase in transduction compared to the media control, likely due to the presence of native FX in the murine serum (Fig. 1A). However when serum was pretreated with X-bp (binds the FX Gla domain blocking Ad:FX interaction [24]) Ad5 gene transfer was dramatically reduced, to levels significantly below control conditions, consistent with previous results [19] (Fig. 1A). This demonstrates that in the absence of FX Ad5 is sensitive to neutralisation by murine serum components [19]. In contrast, Ad35, Ad50, Ad48 and Ad26-mediated cell transduction was not affected by serum regardless of the presence of X-bp (Fig. 1A), indicating that these vectors are not sensitive to the same mechanism that mediates Ad5 neutralisation. It is noteworthy that the overall charge in the region of the Ad5 hexon hypervariable loops is more negative than that of Ad26/Ad35/Ad48/Ad50 (S1 Table). Protection from neutralisation by FX is also evident for Ad5 in rat serum (S1 Fig.).
Fig. 1. Ad5 HVRs mediate neutralisation by naïve murine serum. 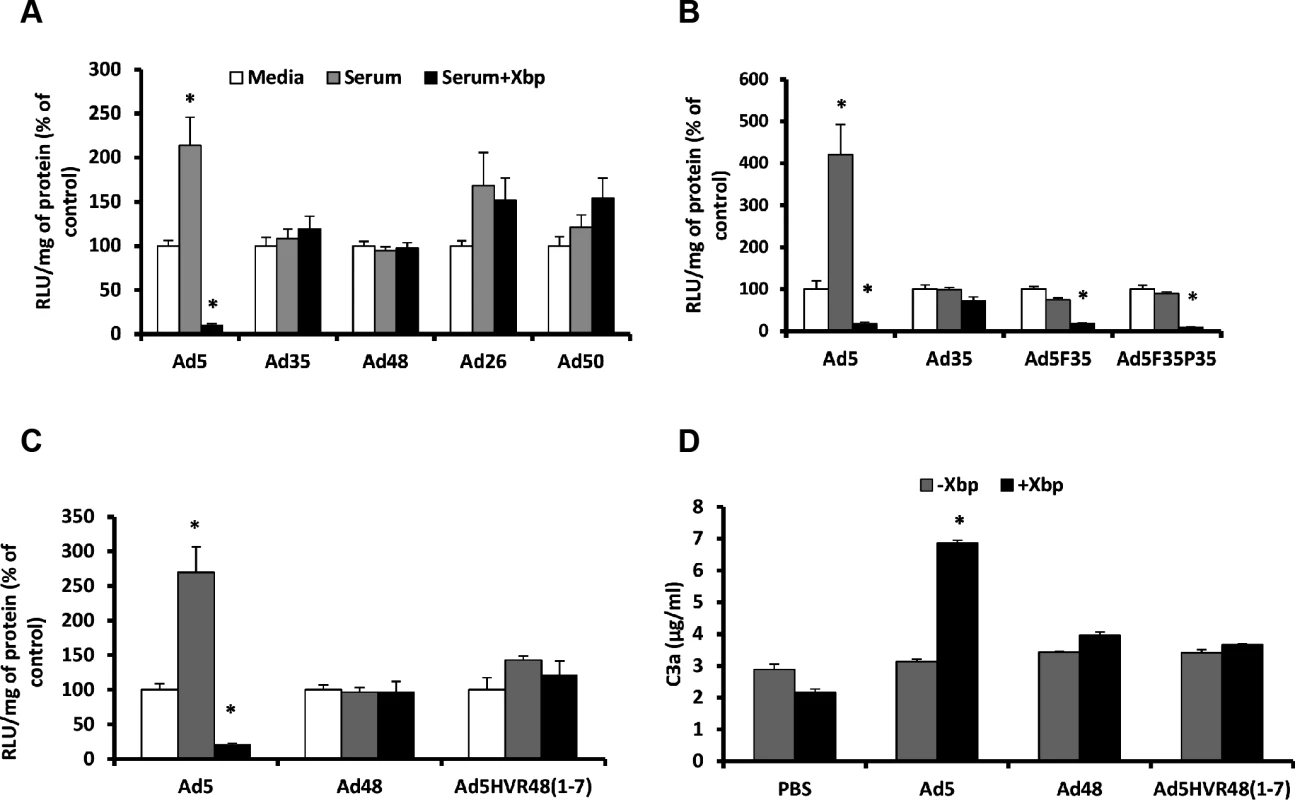
SKOV3 (A) or A549 cells (B-C): Vectors (2x1010 vp/mL) were incubated with RPMI-1640 media, 90% C57BL/6 mouse serum in RPMI-1640 media in the absence or presence of 40 μg/mL Xbp for 30 min at 37°C. Virus suspensions were diluted 200-fold in serum-free media and 100 μL added to cells for 2 h at 37°C before being replaced with RPMI-1640 media with 2% FCS. Transgene expression was quantified ∼16 h post-transduction and relative light units (RLU) normalized to mg total protein. Results for each vector are shown as percentage of the matched media control. White bars = media alone, grey bars = serum and black bars = serum + Xbp. (D) C57BL/6 mouse serum was incubated with Ad (5x1010 vp/mL) in the presence or absence of 40 μg/mL Xbp for 90 min at 37°C. C3a levels were quantified by ELISA. *p < 0.005 vs. matched controls. Next we investigated the role of different capsid proteins in mediating Ad5 neutralisation. For this we employed a range of Ad5-based chimeric vectors (S2 Table). We found no role for the fiber or penton base, as swapping those of Ad5 for those of Ad35 (i.e. the Ad5.F35 and Ad5.F35P35 vectors), still resulted in vector neutralisation in the absence of FX (Fig. 1B). Next we assessed the contribution of the Ad5 hexon in enabling neutralisation. Here we utilised Ad5.HVR48(1–7), which we previously showed to lack FX binding [24] (S2 Table). Ad5.HVR48(1–7) was not inhibited by serum components regardless of FX, thus illustrating an essential requirement for the Ad5 hexon HVRs in mediating neutralisation via this mechanism (Fig. 1C). In addition, unlike the parental Ad5, the Ad5.HVR48(1–7) chimeric vector caused no induction of C3 activation in serum preincubated with X-bp (Fig. 1D). Hence, Ad5.HVR48(1–7) cannot bind to FX [24] and is not sensitive to neutralisation. Therefore these data indicate a pivotal role for the Ad5 HVRs in enabling immune attack via the IgM/classical complement pathway.
Sensitivity of Ad5 HVR mutant/FX-binding deficient viruses to neutralisation
To investigate the contribution of different Ad5 HVRs in mediating neutralisation by mouse serum, we evaluated the sensitivity of Ad5 vectors containing a range of Ad26 HVR modifications. Ad26 was chosen for these studies as it does not bind FX [24] and is not susceptible to serum neutralisation in vitro (Fig. 1A). This series of Ad5/26 chimeric vectors (Ad5.HVR5(Ad26), Ad5.HVR7(Ad26) and Ad5.HVR5+7(Ad26)) (S2 Table) were previously shown to lack FX binding [32]. Unlike the Ad5 control, the Ad5 vectors with HVR(Ad26) swaps were all sensitive to inhibition by serum (Fig. 2A), suggesting the involvement of multiple HVRs in enabling neutralisation. Next, we employed a series of Ad5 vectors engineered with individual point mutations to alter specific amino acids to those found in Ad26 (S2 Table). These vectors were previously demonstrated to have reduced but not eliminated (e.g. Ad5.HVR5* (HVR5 mutations T270P and E271G) or abolished FX binding (e.g. Ad5.HVR7* (HVR7 mutations I421G, T423N, E424S, L426Y) and Ad5.HVR5*.HVR7*.E451Q (hereafter referred to as Ad5T*)) [32]. We evaluated vector sensitivity to neutralisation over a range of concentrations of C57BL/6 mouse serum (1–90% final volume) in vitro (Fig. 2B, S2 Fig.). In contrast to the media control, transduction of all FX-binding deficient Ad5 vectors was dramatically reduced in the presence of serum (Fig. 2B, S2 Fig.). This inhibitory effect was significantly lessened at lower serum concentrations (<25%) for the vectors engineered with point mutations, Ad5.E451Q, Ad5.HVR5*, Ad5.HVR7* and Ad5T* compared to vectors with entire HVR exchanges Ad5.HVR5+7(Ad26) (Fig. 2B). The latter vector remained highly sensitive to neutralisation even in the presence of 5% serum. This suggests that the sensitivity to serum is influenced by the location and extent of modifications to the Ad5 HVRs, and is, at least, partially independent of the complete loss of FX-binding capacity.
Fig. 2. Neutralisation of FX-binding deficient Ad5 vectors by naïve murine serum. 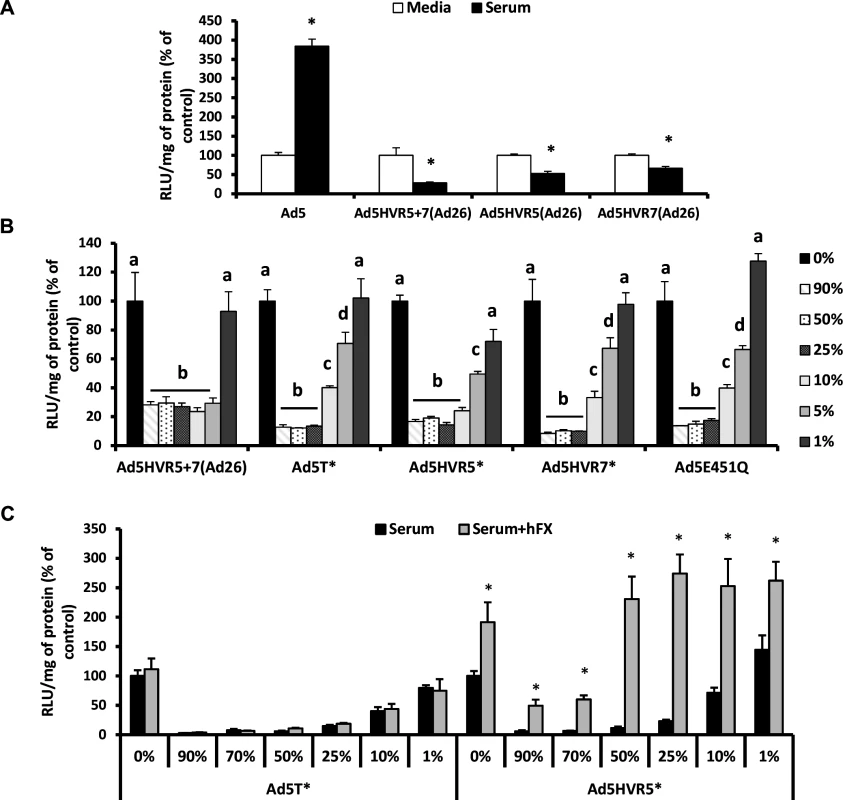
(A) A549 and (B) SKOV3 cells: Ad5 and its derivatives (2x1010 vp/mL) were incubated with RPMI-1640 media, 90% C57BL/6 mouse serum or 1–90% C57BL/6 mouse serum in RPMI-1640 media for 30 min at 37°C and (C) A549 cells: Ad5T* or Ad5.HVR5* (2x1010 vp/mL) were incubated with RPMI-1640 media, 1–90% C57BL/6 mouse serum in RPMI-1640 media in the absence or presence of 10 μg/mL hFX for 30 min at 37°C. Virus suspensions were diluted 200-fold in serum-free media and 100 μL added to cells for 2 h at 37°C before being replaced with RPMI-1640 media with 2% FCS. Transgene expression was quantified ∼16 h post-transduction and relative light units (RLU) normalized to mg total protein. Results for each vector are shown as percentage of the matched media control. Groups that do not share the same letter (a, b, c, d) are significantly different to samples within that group. *p < 0.05 vs. matched controls. It has previously been shown that Ad5.HVR5* is capable of direct interaction with FX, albeit at lower levels than Ad5, however detailed affinity kinetics are not available [32]. We hypothesized that immune components may compete with FX for binding sites within related exposed Ad5 HVR loops. Notably we found the concentration of hFX in human serum to be ∼50% lower than that of normal plasma (S3 Table). To test whether increasing concentrations of FX could protect Ad5.HVR5* from immune attack, we spiked C57BL/6 mouse serum with 10 μg/mL hFX and examined the susceptibility of the non-FX-binding Ad5T* control vector and Ad5.HVR5* to neutralisation (Fig. 2C). As expected the presence of hFX did not affect the neutralisation of Ad5T* by serum and mediated no increase in gene transfer (Fig. 2C). However, hFX prevented neutralisation of Ad5.HVR5*, particularly in lower concentrations of serum (Fig. 2C). The presence of hFX also increased gene transfer of Ad5.HVR5* indicating its ability to both protect and act as a bridge to HSPGs despite sub-optimal FX:hexon binding conditions [24]. These data indicate that both hFX and the murine serum components can compete with one another, likely through binding to similar HVRs, and this is dependent on their relative concentrations and/or affinities.
Generation and analysis of recombinant Ad26 virus containing modifications in hexon HVRs
To further dissect the role of the Ad5 HVRs and FX in protecting Ad from serum neutralisation, we engineered FX binding capacity into Ad26 by substituting the Ad5 HVRs into the Ad26 hexon. We attempted to generate a number of Ad26-based mutants however only three Ad26 chimeras were successfully packaged into mature viral vectors at high titer (Fig. 3A, S3 Fig., S4 Table). Specific hexon sequences were chosen for mutagenesis (S3 Fig.). These included the point mutant Ad26.Q461E (the corresponding Glu residue is conserved in all FX binding Ads) and the HVR exchanges Ad26.HVR5(Ad5).Q461E and Ad26.HVR5C [in which Ad26.HVR(1–3 and 5–7) were replaced by those of Ad5], respectively (Fig. 3A, S4 Table, S3 Fig.). To note, due to the genetic capsid modifications the vp:PFU ratio was ∼10 fold higher for both Ad26.HVR5C and Ad26.HVR5(Ad5).Q461E compared to the parental Ad26 vector (Fig. 3A). We next measured the ability of each virus to bind hFX by SPR. Ad26.HVR5C showed efficient binding when injected over a hFX biosensor chip, as did the positive control Ad5, while Ad26, Ad26.Q461E and Ad26.HVR5(Ad5).Q461E did not bind hFX (Fig. 3B). We then quantified affinity kinetics (Fig. 3C). The calculated association rate constant (ka) and dissociation rate constant (kd) values of the hFX for immobilized Ad26.HVR5C were 3.085x106 (1/Ms) and 1.068x10–2 (1/s), giving an overall equilibrium dissociation constant (KD) of 3.462x10–9 M. When Ad5 was immobilized and hFX was injected across the biosensor, ka and kd values were 1.308x106 (1/Ms) and 3.053x10–3 (1/s), giving a KD of 2.334x10–9 M (Fig. 3C), consistent with previously reported kinetics [24]. Therefore, incorporation of Ad5 HVR(1–3 and 5–7) into Ad26 generates de novo binding to FX by Ad26 at an affinity similar to Ad5.
Fig. 3. Generation of Ad26 hexon modified vectors and effects on FX binding by SPR. 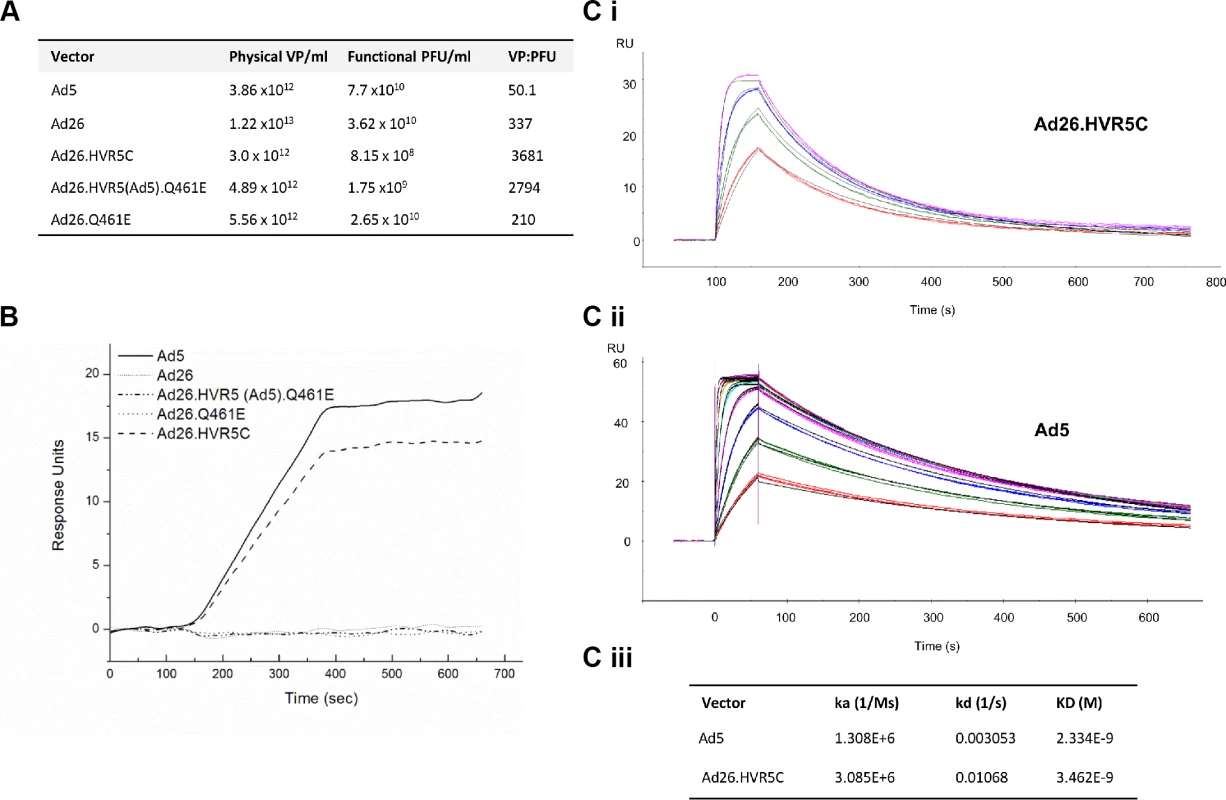
(A) Virus titers and vp/pfu ratios for each of the vectors produced. (B) SPR analysis of Ad5, Ad26 and mutant vector interaction with immobilized hFX (500 RU). Sensorgrams of 1011 vp/ml of vector injected at a flow rate of 30 μL/min. (C i) SPR analysis of hFX for immobilized Ad26.HVR5C. Samples analysed in triplicate at 4 concentrations of hFX. (C ii) SPR analysis of hFX for immobilized Ad5. Samples analysed in triplicate at 7 concentrations of hFX. The black lines represent the fit to the data. (C iii) Kinetic constants of hFX for immobilized Ad5 or Ad26.HVR5C as determined by SPR. Effect of de novo FX binding and Ad5 HVR inclusion on Ad26 sensitivity to mouse serum
Ad26.HVR5C provides a novel virus through which to ascertain whether inclusion of Ad5 HVR(1–3, 5–7) are sufficient for exposure to neutralisation and whether FX binding influenced the sensitivity of the parental virus to mouse serum. Therefore we assessed cellular transduction by the Ad26 mutants in the absence and presence of C57BL/6 or RAG2-/- murine sera +/ - X-bp. RAG2-/- mice are immune-deficient, lacking mature T and B lymphocytes, closely related to the RAG1-/- strain in which Ad5 liver tropism was previously demonstrated to be FX independent [19]. Parental Ad26 was resistant to neutralization by both strains of serum regardless of FX, again demonstrating Ad26 is not sensitive to the same mechanism that mediates Ad5 neutralisation (Fig. 4A). Interestingly, Ad26.HVR5C was resistant to neutralization in the presence of FX but sensitive to neutralization when immune-competent C57BL/6 serum was pre-incubated with X-bp, a similar profile to that seen with Ad5 (Fig. 4A). RAG2-/- serum did not neutralize Ad26.HVR5C or Ad5 (Fig. 4A), indicating a requirement for efficient T and/or B cell antibody function [19]. Ad26.HVR5(Ad5).Q461E and Ad26.Q461E were unaffected by either sera regardless of the presence of FX. Furthermore, whilst there were differences amongst the basal levels of C3a between vectors, both Ad5 and Ad26.HVR5C, but not Ad26 enhanced C3a in a FX-dependent manner. Notably, the FX protection mechanism is not dependent on the heparin binding exosite in the FX serine protease domain, as blocking these HSPG interacting sites did not alter Ad induced C3a levels compared to the matched serum only control (Fig. 4C). Hence, inclusion of the Ad5 HVRs in Ad26.HVR5C not only leads to FX binding, but also sensitises the virus to attack in immune-competent mouse serum indicating the importance of these hexon regions in both FX binding and immune recognition.
Fig. 4. FX protects Ad26.HVR5C against immune attack. 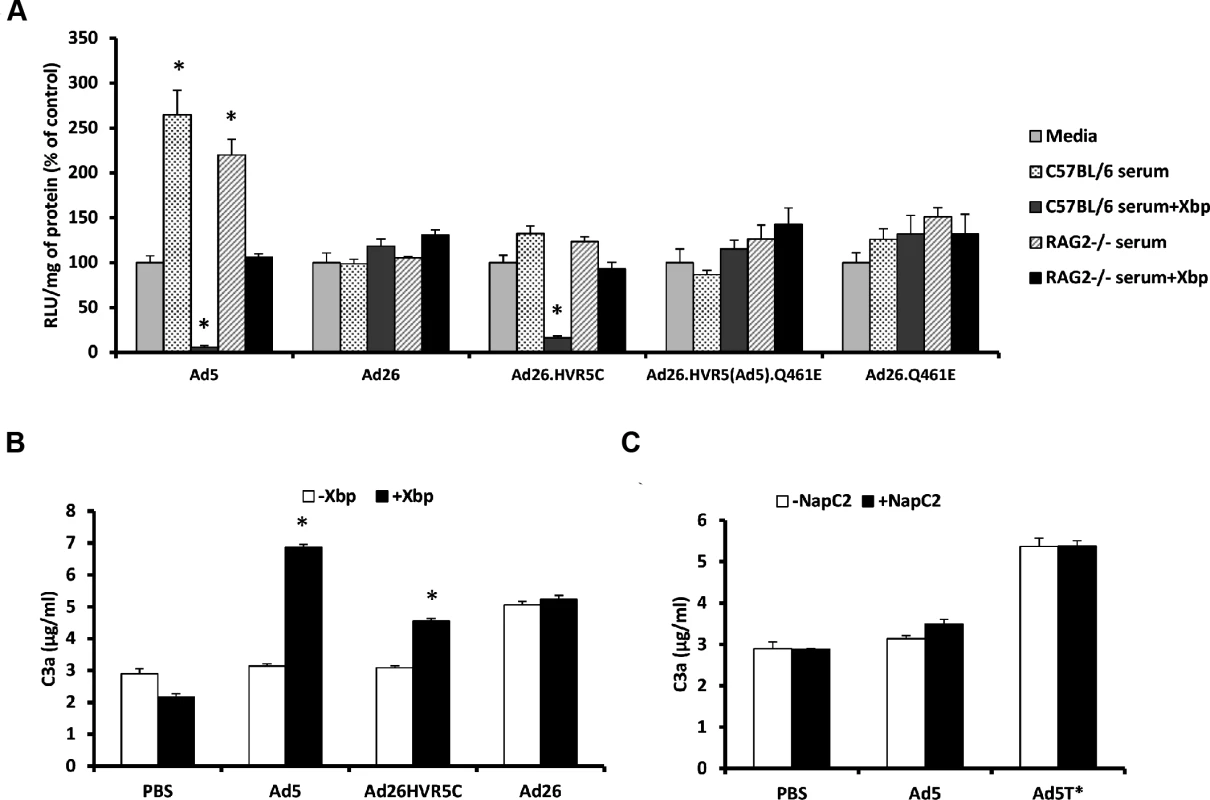
(A) 2x1010 vp/mL of Ad5, Ad26 or Ad26 hexon modified vectors were incubated with RPMI-1640 media, 90% C57BL/6 mouse serum, 90% C57BL/6 mouse serum with 40 μg/mL X-bp, 90% RAG2-/- mouse serum or 90% RAG2-/- mouse serum with X-bp, for 30 min at 37°C. Virus suspensions were diluted 200-fold in serum-free media and 100 μL added to SKOV3 cells for 2 h at 37°C before being replaced with RPMI-1640 media with 2% FCS. Transgene expression was quantified ∼16 h post-transduction and relative light units (RLU) normalized to mg total protein. Results for each vector are shown as percentage of the matched media control. *p < 0.001 vs. matched media controls. (B) C57BL/6 mouse serum was incubated with Ad (5x1010 vp/mL) in the presence or absence of 40 μg/mL Xbp for 90 min at 37°C. C3a levels were quantified by ELISA. (C) C57BL/6 mouse serum was incubated with Ad5 or Ad5T* (5x1010 vp/mL) in the presence or absence of 20 μg/mL NapC2 for 90 min at 37°C. C3a levels were quantified by ELISA. *p < 0.005 vs. matched controls. Effect of FX binding on recombinant Ad26 tropism
We next investigated whether creating Ads engineered to bind FX influenced their ability to interact with cells in vitro and in vivo. We performed cell binding and transduction assays with Ad26.HVR5C to assess vector:cell interaction profiles (Fig. 5). SKOV3 cells were employed for these assays as they express low levels of CAR [39] and allow focus on the FX-mediated pathway. Both cell binding and transduction for Ad26.HVR5C were significantly increased in the presence of FX compared to the parental Ad26 which was unaffected by FX (Fig. 5A-B). This suggests that Ad26.HVR5C functionally binds FX leading to Ad:FX engagement with cellular HSPGs and subsequent gene transfer. Next, we examined whether the Ad26.HVR5C hexon:FX interaction generates hepatic tropism via FX bridging to hepatocytes [24,33,34]. Immune competent MF1 control mice were first treated with warfarin to deplete vitamin K-dependent coagulation factors, and then administered I.V. with 1011 vp of Ad5, Ad26 or Ad26.HVR5C.luc+. Luciferase expression was visualised by whole-body bioluminescence imaging and quantified at 72 h. Ad26 produced low level, widely biodistributed gene transfer and this was unaltered by warfarin (Fig. 6A). However, both Ad5 and Ad26.HVR5C produced selective transduction of the liver in non-warfarin treated mice (Fig. 6A-C) albeit the levels mediated by Ad26.HVR5C were significantly lower than those of Ad5. Despite the vp:PFU ratio of Ad26.HVR5C being ∼10 fold lower than Ad26 (Fig. 3A), there was a significant increase in liver luciferase levels for the Ad26.HVR5C vector compared to Ad26, in non-warfarin treated animals (Fig. 6C). For both Ad5 and Ad26.HVR5C, liver transduction was reduced to less than 0.5% of control following warfarin treatment. Quantification of viral genome accumulation in the livers of Ad5 and Ad26.HVR5C injected animals revealed a similar pattern of virus-mediated gene transfer (Fig. 6D). Thus, inclusion of FX binding into Ad26.HVR5C leads to profound retargeting effects following I.V. injection into MF1 mice.
Fig. 5. FX-mediated Ad26.HVR5C cell surface binding and transduction. 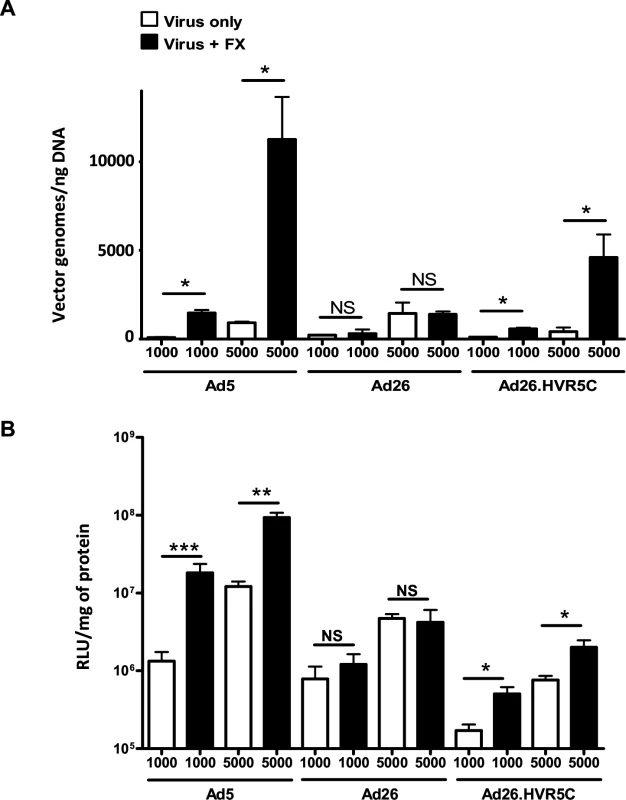
(A) Binding of 1000 or 5000 vp/cell of Ad5, Ad26 or Ad26.HVR5C was quantified after incubation with SKOV3 cells for 1 h at 4°C in the presence or absence of 10 μg/mL hFX. Vector genomes were detected by quantitative PCR. (B) SKOV3 cells were infected with 1000 or 5000 vp/cell of Ad5, Ad26 or Ad26.HVR5C in the absence or presence of 10 μg/mL hFX for 3 h at 37˚C, after which media was replaced by full RPMI-1640 media and cells incubated at 37˚C. Transgene expression was measured 48 h post-infection. *p < 0.001 vs. matched -/+FX controls. Fig. 6. In vivo analysis of Ad26.HVR5C mediated liver transduction in MF1 mice. 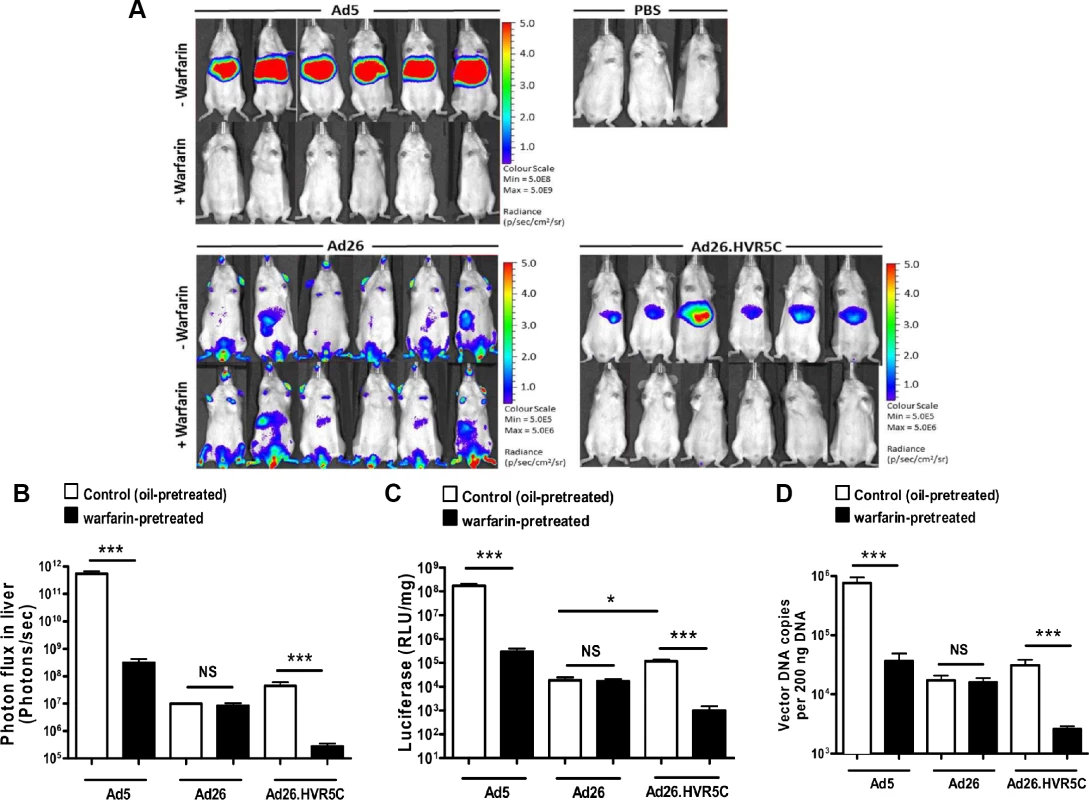
(A + B) Luciferase expression was visualised (A) and quantitatively assessed (B) by whole-body bioluminescence imaging 72 h after intravascular administration of 1x1011 vp/mouse Ad5, Ad26 or Ad26.HVR5C in the absence or presence of 133 μg/mouse warfarin (n = 6 animals/group) or PBS (n = 3 animals/group) in MF1 mice. (C) Livers were harvested 72 h post vector injection and processed for quantification of luciferase transgene expression and (D) quantification of viral genomes, using 200 ng of DNA for analysis. *p<0.05, ***p<0.001 and NS (not significant) vs the matched non-warfarin treated control. Ad5 HVRs mediate immune sensitivity in vivo and FX dictates Ad26.HVR5C hepatic tropism
We then performed the same experiment in immune-deficient RAG2-/- mice. Ad5 mediated FX-independent liver transduction in RAG2-/- mice, similar to that previously reported in RAG1-/- mice [19] (Fig. 7A-D). Ad26 demonstrated widespread gene expression in vivo which was equivalent in both non-warfarin and warfarin-treated mice (Fig. 7A). However, Ad26.HVR5C transduction was focused in the liver in non-warfarin treated RAG2-/- mice, whilst hepatic gene expression was dramatically decreased in the absence of FX (Fig. 7A-C). The transduction profile of Ad26.HVR5C in warfarin-treated mice was completely altered, no longer targeting the liver but instead exhibiting a more widespread biodistribution similar to the parental Ad26 (Fig. 7A). This demonstrates the ability of the hexon:FX interaction to determine Ad26.HVR5C hepatocyte uptake. Through this gain-of-function approach, incorporation of FX binding into Ad26, FX was shown to effectively alter vector tropism in vivo and dictate liver targeting of Ad26.HVR5C in control and immune-deficient mice.
Fig. 7. In vivo analysis of Ad26.HVR5C biodistribution in immune deficient mice. 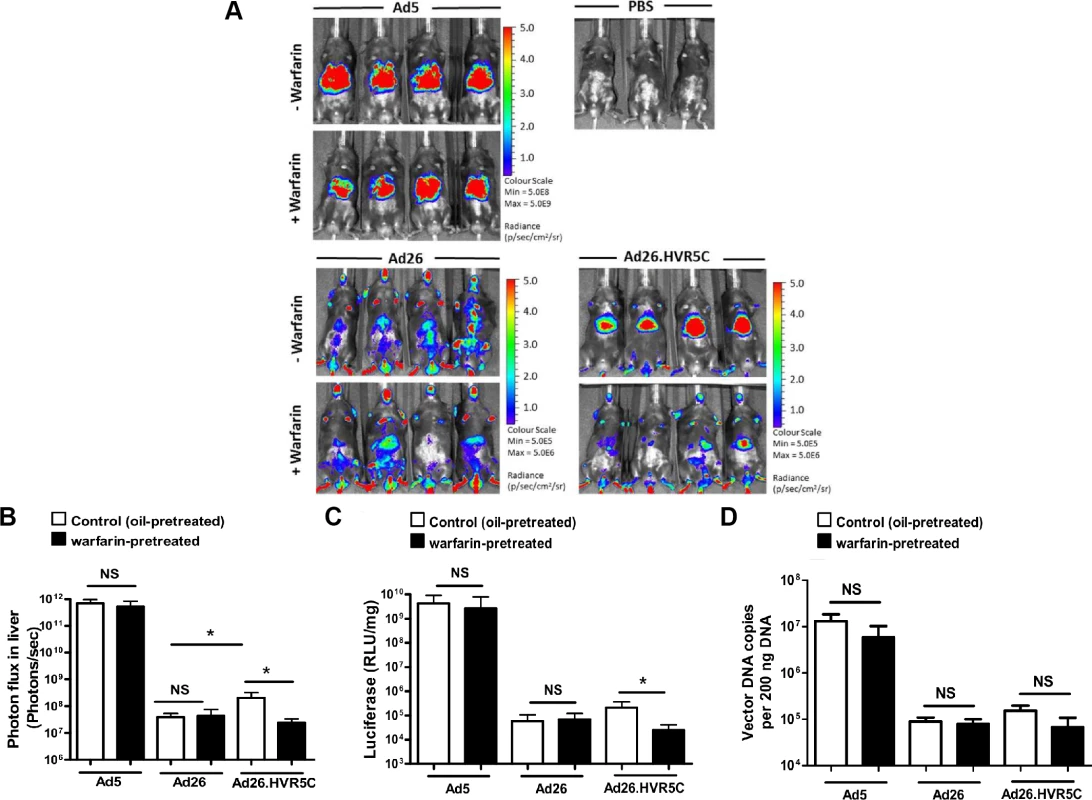
(A + B) Luciferase expression was visualised (A) and quantitatively assessed (B) by whole-body bioluminescence (IVIS) imaging 72 h after intravascular administration of 1x1011 vp/mouse Ad5, Ad26 or Ad26.HVR5C in the absence or presence of 133 μg/mouse warfarin (n = 4 animals/group) or PBS (n = 3 animals/group) in RAG2-/- mice. (C) Livers were harvested 72 h post-injection and processed for quantification of luciferase transgene expression and (D) quantification of viral genomes, using 200 ng of DNA for analysis. *p<0.05 and NS (not significant) vs the matched non-warfarin treated control. The high levels of gene expression by Ad5 in warfarin treated RAG2-/- mice (Fig. 7A) is in contrast to the diminished luciferase expression (Fig. 6A) observed in warfarin treated MF1 mice. This is due to IgM and classical complement-mediated vector neutralisation, similar to that demonstrated previously [19]. Unlike Ad26 but in parallel to Ad5, gene expression by Ad26.HVR5C was also inhibited in warfarin treated MF1 mice but not warfarin treated RAG2-/- mice (Fig. 6A, 7A). This suggests Ad26.HVR5C was neutralised in immune competent mice as a direct consequence of the engineered Ad5 HVRs. This indicates that in the immune competent setting, in the absence of a FX protective mechanism, engineering the Ad5 HVRs into Ad26 confers sensitivity to immune attack and vector neutralisation in vivo.
Discussion
Here, the role of the Ad5 HVRs and the significance of FX in defending the virus from host attack whilst determining liver targeting are described. We first identified the Ad5 HVRs as responsible for conferring sensitivity to serum neutralisation. We then genetically engineered the Ad5 hexon loops, onto a non-FX-binding Ad26 background to generate the novel vector Ad26.HVR5C. Employing this as an efficient tool, we deciphered the importance of Ad5 HVRs and FX in immune recognition, protection and biodistribution following systemic Ad administration.
Previous work reported that coagulation FX coats Ad5 to protect from IgM and classical complement-mediated immune neutralisation and that Ad5 liver uptake was not solely dependent on FX under immune-deficient conditions [19]. However the capsid proteins to which IgM and the classical complement components bind and initiate this cascade eventually leading to virus inactivation have not been identified. In addition to this work, Doronin et al., indicated that displacement of FX from the coagulation system through binding to Ad5 is sensed by the host and an inflammatory response is initiated [37]. Therefore, with both of these recent studies in mind, we attempted to identify the Ad capsid regions responsible for mediating immune neutralisation and further decipher the functional consequences of the Ad:FX interaction. We began by showing the FX protection mechanism was not conserved amongst human Ads. Ad5 was the only type tested susceptible to serum neutralisation in the absence of FX and using a series of genetic and pharmacological approaches this was shown to be reliant on the presence of the Ad5 HVRs. We then investigated the sensitivity of Ad5-based HVR mutants and FX-deficient vectors to neutralisation by serum from immune-competent mice and found that all viruses were inhibited, but interestingly there were differences amongst these inactivation levels. These variations indicate that it is more than simply the loss of FX binding or these contact points which likely dictates immune recognition. For our experiments we used fresh mouse serum. Unlike citrated or EDTA treated plasma there is no interference with calcium, which has previously been found to influence results [18]. During the coagulation process, a proportion of the coagulation factors will be activated and levels of intrinsic FX reduced. When we investigated the effects of spiking mouse serum with hFX and compared the null-binder Ad5T* to the FX-defective binder Ad5.HVR5*, we found hFX was capable of rescuing Ad5.HVR5* from neutralisation and further increasing gene transfer to levels above the media control. These data thereby suggests that hFX and the inhibitory murine serum components compete for binding within similar regions within the Ad5 hexon. Interestingly previous data has demonstrated using targeted PEGylation that similar Ad5 HVRs (1, 2, 5 and 7) are also responsible for interacting with scavenger receptors and subsequent Kupffer cell uptake, but in that case it has been suggested that FX and the scavenger receptors do not have overlapping binding sites [40].
Our work demonstrates a key role for the Ad5 HVRs in determining virus sensitivity to serum. We successfully engineered an Ad26 vector with Ad5-derived HVRs, Ad26.HVR5C, capable of FX-mediated cell binding and transduction in vitro. The species D Ad26 is a less seroprevalent virus than Ad5 [14]. It has been shown to utilise both CD46 [41] and CAR [42] as primary cellular receptors and is currently gaining a lot of attention as a vector-based vaccine against HIV [43]. Interestingly, the Ad5 HVR(1–3, 5–7) exchange in this Ad26-based vector suddenly made the virus susceptible to neutralisation by mouse serum, indicating the driving influence of the HVRs and the likelihood they possess a critical IgM recognising epitope. The smaller regions of exchange in Ad26.HVR5(Ad5).Q461E or the single point mutation in Ad26.Q461E were not sufficient to induce sensitivity to neutralisation. Conversely, incorporating the Ad26 HVR5+7 into Ad5 resulted in a vector (Ad5.HVR5+7(Ad26)) that remained susceptible to the inhibitory effects of serum. This indicates it is perhaps likely that multiple HVR loops working in conjunction which are responsible for mediating the crucial serum binding event. It will require further study to fine map the essential contact sites. IgM antibodies are broadly reactive, capable of binding to unrelated structures with low affinity [44] and mostly exist as a pentameric structure, containing ten potential antigen binding sites [45]. Therefore it is possible one IgM molecule may be capable of interacting across a wide hexon region. These natural antibodies are often directed against highly repetitive and charged motifs [46,47]. A recent study suggested the involvement of the electronegative potential of antigens in IgM recognition [47]. To note, the panel of recombinant Ad5-based FX-binding deficient viruses, with the point mutations were all modulated to have lower net negative charge, whilst the more sensitive entire HVR exchanges in Ad5.HVR5+7(Ad26) had more negative charge compared to the parental vector (S1 Table). In addition it is perhaps interesting that the net charge of the Ad5 hexon protein is more negative than the other vectors tested in this study, Ad26/Ad35/Ad48/Ad50. The HVRs of Ad types tested here differ significantly and charge-specific mutation analysis is required to further address this potential issue.
Engineering FX-binding into the Ad26 vector had profound effects on viral biodistribution in in vivo models of viral dissemination. In complete contrast to its parental virus, Ad26.HVR5C exhibited selective liver gene transfer in the presence of FX in immune-competent and deficient mice. In the absence of FX and immunity, Ad26.HVR5C reverted to its native tropism, with the same biodistribution pattern as Ad26. This demonstrates the tropism defining force instilled by the inclusion of FX binding. The interaction with FX instils new tropism in addition to its native Ad26 receptor usage (e.g. via CAR/CD46) [41,42]. It is evident from the work presented here, and from others [19], that Ad5 remains hepatotropic in warfarin treated immune-deficient mice. Therefore this suggests, that an alternative mechanism is utilised by Ad5 to transduce hepatocytes of immune deficient animals when FX binding is diminished. Further investigation is required to answer the important question of what is the precise mechanism determining Ad5 liver targeting in the absence of FX.
The relevance of the Ad:FX interactions and protection pathways in humans suffering from the disseminated disease is yet to be examined. Further study is therefore required to investigate whether human natural IgM can bind to Ads and if this initiates similar pathways as those in the mouse i.e. classical complement mediated neutralisation in the absence of FX [19]. It may also be beneficial to directly compare and interrogate the influences and interplay of complement, natural and acquired human immunoglobulins [48]. This may help to better predict the human immune defense to widespread Ad infection or when using Ads as gene therapy vectors. In the general population the majority of specific neutralising antibodies (e.g. IgG-based) are directed against the Ad5 hexon protein and particularly focused on the Ad5 HVRs [49,50]. Previous work has demonstrated that such neutralising human sera can prevent FX-mediated cellular binding and transduction in vitro [51]. In addition, in MF-1 mice preinjected with human sera, higher levels of pre-existing neutralising antibodies, correlated with decreased hepatocyte transduction in the presence of FX [51]. It would be interesting to determine whether different families of human immunoglobulins (IgG/IgM/IgA) can compete for binding sites within the Ad hexon HVRs, the relative affinities of these interactions and, further, whether FX coating of the hexon affects these processes. This would also be of clinical relevance in terms of gene therapy and for determining patient suitability or the type of Ad vector employed (FX-binding or non-FX-binding vectors) for intravascular Ad administration. Investigating the relevance of the FX protection mechanism from rodent to human models is an important next step for the field. Evidently, the complexity of Ad interactions with host factors is extensive and small animal models may only provide limited information. For instance, CAR-mediated Ad5 binding to human erythrocytes is an important clinical consideration however mouse erythrocytes are devoid of CAR expression, again highlighting the necessity for using human samples [22,52,53].
Despite the high morbidity and mortality rate associated with disseminated Ad infection there are currently no licensed specific anti-adenoviral drugs available for the management of the condition. Those that have been used in clinical settings, such as ribavirin, cidofovir, and vidarabiner, have yielded varied results and treatment options remain largely unsatisfactory [54,55]. A previous study identified small molecule pharmacological agents to block FX-mediated Ad5 infection following intravenous administration in a mouse model, and found it to be an effective strategy by which to prevent hepatic targeting [56]. However, in that case the pharmacological inhibitor was acting post the Ad5:FX binding event [56]. Doronin et al. used cryoEM and molecular dynamics flexible fitting simulations techniques to reveal an interaction between the FX Gla K10 residue and the Ad5 HVR7 residues E424 and T425 [37]. From our work it is evident that the host natural IgM and classical complement mediated defence mechanism is effectively governed via the Ad5 HVR loops, immune recognition is dependent on more than solely the loss of FX contact points, and FX functions in mediating liver uptake in both immune competent and deficient models. Identification of a small molecule inhibitor with favourable pharmacological properties capable of specifically blocking the Ad5:FX interaction at these contact points may enable a more robust host anti-viral immune response whilst limiting liver infection and thus be very valuable in treating systemic disease.
In summary, here we have identified the Ad5 HVRs as the key regions conferring sensitivity to the IgM and complement mediated host defence pathway. FX has both a pivotal role in protecting the virus from attack and has profound effects on Ad biodistribution. Through this work we gain a greater insight into the mechanism and significance of FX in protecting Ads against innate immunity and determining virus tropism.
Materials and Methods
Ethics statement
All animal procedures were approved by the University of Glasgow Animal Procedures and Ethics Committee and performed under UK Home Office license PPL 60/4429 in strict accordance with UK Home Office guidelines. All efforts were made to minimize suffering.
Cells
HEK293 (human embryonic kidney: ATCC CRL-1573) and HeLa (human cervical adenocarcinoma: ATCC CCL-2) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Paisley, UK) and SKOV3 (human ovarian carcinoma: ATCC HTB-77) and A549 (human lung carcinoma: ATCC CCL-185) cells in RPMI-1640 medium (Invitrogen), with 2 mM L-glutamine (Invitrogen), 10% fetal calf serum (FCS; PAA Laboratories) and 1 mM sodium pyruvate (Sigma-Aldrich, UK) at 37°C 5% CO2. PER.C6/55K cells [57] were cultured in DMEM with 2 mM L-glutamine, 10% FCS, 1 mM sodium pyruvate and 10 mM MgCl2, at 37°C 10% CO2.
Vector construction
E1/E3 deleted Ad5, Ad35, Ad5/35 chimeras (Ad5.F35, Ad5.F35P35), Ad26, Ad50, Ad48 and Ad5/48 chimeric (Ad5.HVR48(1–7)) vectors encoding CMV-luciferase reporter genes were generated as described previously [14,24,58]. S2 and S4 Tables provide detail on each of the mutated vectors used in this study. E1/E3 deleted Ad5 and FX-binding deficient Ad5/Ad26 chimeric vectors (Ad5.HVR5+7(Ad26), Ad5.HVR5*, Ad5HVR7*, Ad5E451Q and Ad5T*) encoding CMV-lacz reporter genes were generated as described previously [32]. Hexon gene-modified Ad26 vector genomes were constructed in the context of pAd26.luc, a plasmid that contains a PacI site-flanked, full-length Ad26 vector genome with E1, E3, and E4 deletions/modifications [14]. The vector genome is further equipped, at the site of E1 deletion, with a CMV promoter-driven expression cassette for firefly luciferase. To generate the desired hexon gene-modified pAd26.luc plasmids, the concerning Ad26/Ad5 hexon modifications (S4 Table) were first made in the context of a smaller ‘hexon shuttle’ plasmid (gene synthesis and subcloning by GeneArt/LifeTechnologies), and then shuttled into a hexon gene-deleted derivative of pAd26.luc by homologous recombination in E.coli BJ5183 (Stratagene/Agilent Technologies), as described previously [32]. S3 and S4 Figs. further describe construction and Ad26/Ad5 sequence alignment, highlighting the regions targeted for mutagenesis.
Vector amplification
Linearised Ad plasmids were transfected in PER.C6/55K using Lipofectamine 2000 (Invitrogen). Cells were harvested 10–14 days post-transfection. Viral particles (vp) were propagated and purified by CsCl gradients. Titers were determining by protein concentrations using micro-bicinchoninic acid (BCA) Protein Assay (Thermo Scientific). Titer calculations used the formula 1 μg protein = 4x109 vp and end-point dilution assays using PER.C6/55K for quantification of plaque forming units (pfu)/mL [59]. Purified Ads were analysed by SDS-PAGE followed by silver staining (Sigma-Aldrich) according to manufacturer’s instructions in order to verify the capsid composition and confirm the vector modifications did not interfere with the structural integrity of the particles.
Surface plasmon resonance (SPR)
Immobilized hFX: Performed using Biacore 2000 (GE Healthcare) as described [31]. Purified hFX was purchased from Cambridge Biosciences (Cambridge, UK). hFX was covalently immobilized onto the flowcell of a CM5 biosensor chip by amine coupling. Immobilized Ad: Performed using T200 (GE Healthcare). Virus was biotinylated using the EZ-link sulfo-NHS-LC biotinylation kit (ThermoFisher). The biotinylated products were coupled to streptavidin-coated sensorchips (SA; Biacore); Ad5 (482RU), Ad26.HVR5C (484RU). SPR was performed in 10 mM HEPES (pH7.4) 150 mM NaCl, 5 mM CaCl2, 0.005% Tween20 at a flow rate of 30 μL/min and sensorchips were regenerated by injection of 10 mM HEPES (pH 7.4) 150 mM NaCL, 3 mM EDTA, 0.005% Tween20. Kinetic analysis was performed using 2-fold serial dilutions (in duplicate, starting with 30 μg/mL) of hFX and fitted using a 1 : 1 binding model (Biacore Evaluation software, Biacore).
Cell binding assay
Cells were plated in 24-well formats (2x105 cells/well) and incubated overnight at 37˚C. Cells were cooled (4°C) for 30 min, washed with phosphate buffered saline (PBS) before adding 1000 or 5000 vp/cell Ad -/+ hFX. hFX was used at 10 μg/ml. Cells were incubated for 1 h at 4°C, washed with PBS, and harvested. DNA was extracted from cells using the QIAamp DNA mini kit (Qiagen) and quantified using Nanodrop (ThermoScientific). Viral genomes (200 ng DNA) were quantified by quantitative polymerase chain reaction (PCR) analysis (7900HT Sequence Detection System; Applied Biosystems) using Power SYBR Green PCR mastermix and CMV primers (Applied Biosystems).
Transduction assay
Cells were plated in 96-well formats (1x104 cells/well) and incubated overnight at 37˚C. Cells were infected with 1000 or 5000 vp/cell Ad -/+ hFX. Cells were incubated for 3 h at 37°C, washed with PBS, maintained with medium and harvested 48 h post-transfection. Luciferase activity was measured using the luciferase assay (Promega, Southhampton, UK). Protein concentrations were calculated by BCA. Values expressed as relative light units (RLU)/mg of protein.
In vivo transduction
8–9 week old male MF1 outbred mice (Harlan, UK) and Rag2 knockout mice (on a C57BL/6 genetic background) (kind gift from Dr Alison Michie, University of Glasgow) were used. Mice were warfarin-treated (133 μg/mouse) prior to virus administration as previously described [24]. 1011 vp of Ad in 100 μL PBS were administered via tail vein injection. 72 h post-injection IVIS imaging was performed and animals were sacrificed. Livers were harvested and luciferase activity measured. DNA was extracted from livers using the QIAamp DNA mini kit. Viral genomes in 200 ng DNA were quantified by qPCR as above.
Serum neutralization assay
Cells were plated in 96-well formats (1x104 cells/well) and incubated overnight at 37˚C. Fresh serum from C57BL/6 mice, Rag2-/- mice or Wistar rats was separated from whole blood, diluted in RPMI-1640 and incubated with 2x1010 vp/mL Ad in a final volume of 50 μL. 40 μg/mL X-bp was added to samples to block FX. Where specified serum was spiked with 10 μg/mL hFX prior to Ad addition. Controls were vectors alone in serum-free medium. Vectors were incubated with serum or medium for 30 min at 37°C. Mixtures were diluted 200-fold in serum-free medium. 1000 vp/well was added for 2 h at 37°C, then replaced with medium containing 2% FBS. After ∼16 h, cells were harvested for determination of transgene expression and protein content.
C3a ELISA
Serum was separated from fresh murine blood. Virus (5x1010 vp/mL) was incubated with 50 μL of serum-/+ 40 μg/mL Xbp or 20 μg/mL NapC2 (a kind gift from Dr G. Vlasuk (Corvas International, San Diego, CA, USA)) for 90 min at 37°C, then 10 mM EDTA was added. Samples were frozen at -80°C until evaluation in a mouse C3a ELISA as previously described [18]. The capture antibody for ELISA was rat anti-mouse C3a (BD Pharmingen #558250) and the detection antibody used was biotin rat anti-mouse C3a (BD Pharmingen #558251).
FX ELISA
The levels of hFX in human serum and plasma samples were measured using a Matched-pair antibody for ELISA of human Factor X FX-EIA (Quadratech Diagnostics, Surrey, UK) according to the manufacturer’s instructions. Purified hFX (Cambridge Bioscience) was used as the control.
Statistical analysis
Statistical significance was calculated using one-way ANOVA followed by Bonferroni post-hoc test with GraphPad Prism. In vitro results presented are representative data from three separate experiments with at least 3 experimental replicates per group. Each in vivo experiment was performed with a minimum of 3 animals per group (n = 4–6 for Ad treated groups, n = 3 for PBS groups). All error bars represent SEM.
Supporting Information
Zdroje
1. Chuang YY, Chiu CH, Wong KS, Huang JG, Huang YC, et al. (2003) Severe adenovirus infection in children. J Microbiol Immunol Infect 36 : 37–40. 12741731
2. Echavarría M (2008) Adenoviruses in Immunocompromised Hosts. Clinical Microbiology Reviews 21 : 704–715. doi: 10.1128/CMR.00052-07 18854488
3. Straussberg R, Harel L, Levy Y, Amir J (2001) A Syndrome of Transient Encephalopathy Associated With Adenovirus Infection. Pediatrics 107: e69. 11331719
4. Abzug MJ, Levin MJ (1991) Neonatal adenovirus infection: four patients and review of the literature. Pediatrics 87 : 890–896. 2034495
5. Lion T, Baumgartinger R, Watzinger F, Matthes-Martin S, Suda M, et al. (2003) Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood 102 : 1114–1120. 12702513
6. Echavarria M, Forman M, van Tol MJD, Vossen JM, Charache P, et al. (2001) Prediction of severe disseminated adenovirus infection by serum PCR. The Lancet 358 : 384–385. 11502321
7. Munoz RE, Piedra PA, Demmler GJ (1998) Disseminated Adenovirus Disease in Immunocompromised and Immunocompetent Children. Clinical Infectious Diseases 27 : 1194–1200. 9827268
8. Chakrabarti S, Mautner V, Osman H, Collingham KE, Fegan CD, et al. (2002) Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood 100 : 1619–1627. 12176880
9. Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, et al. (1997) Isolation of a Common Receptor for Coxsackie B Viruses and Adenoviruses 2 and 5. Science 275 : 1320–1323. 9036860
10. Wickham TJ, Mathias P, Cheresh DA, Nemerow GR (1993) Integrins ±v²3 and ±v²5 promote adenovirus internalization but not virus attachment. 73 : 309–319. 8477447
11. Greber UF, Suomalainen M, Stidwill RP, Boucke K, Ebersold MW, et al. (1997) The role of the nuclear pore complex in adenovirus DNA entry. 5998–6007 p.
12. Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, et al. (1999) Microtubule-dependent Plus - and Minus End–directed Motilities Are Competing Processes for Nuclear Targeting of Adenovirus. The Journal of Cell Biology 144 : 657–672. 10037788
13. Robinson CM, Singh G, Lee JY, Dehghan S, Rajaiya J, et al. (2013) Molecular evolution of human adenoviruses. Sci Rep 3. doi: 10.1038/srep03544 24384914
14. Abbink P, Lemckert AAC, Ewald BA, Lynch DM, Denholtz M, et al. (2007) Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. Journal of Virology 81 : 4654–4663. 17329340
15. Lieber A, He C-Y, Meuse L, Schowalter D, Kirillova I, et al. (1997) The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. Journal of virology 71 : 8798–8807. 9343240
16. Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, et al. (2009) Virus Binding to a Plasma Membrane Receptor Triggers Interleukin-1α-Mediated Proinflammatory Macrophage Response In Vivo. Immunity 31 : 110–121. doi: 10.1016/j.immuni.2009.04.015 19576795
17. Cotter MJ, Zaiss AK, Muruve DA (2005) Neutrophils Interact with Adenovirus Vectors via Fc Receptors and Complement Receptor 1. Journal of Virology 79 : 14622–14631. 16282462
18. Tian J, Xu Z, Smith JS, Hofherr SE, Barry MA, et al. (2009) Adenovirus Activates Complement by Distinctly Different Mechanisms In Vitro and In Vivo: Indirect Complement Activation by Virions In Vivo. Journal of Virology 83 : 5648–5658. doi: 10.1128/JVI.00082-09 19321608
19. Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, et al. (2013) Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nature medicine 19 : 452–457. doi: 10.1038/nm.3107 23524342
20. Othman M, Labelle A, Mazzetti I, Elbatarny HS, Lillicrap D (2007) Adenovirus-induced thrombocytopenia: the role of von Willebrand factor and P-selectin in mediating accelerated platelet clearance. Blood 109 : 2832–2839. 17148587
21. Stone D, Liu Y, Shayakhmetov D, Li Z-Y, Ni S, et al. (2007) Adenovirus-Platelet Interaction in Blood Causes Virus Sequestration to the Reticuloendothelial System of the Liver. Journal of Virology 81 : 4866–4871. 17301138
22. Carlisle RC, Di Y, Cerny AM, Sonnen AF-P, Sim RB, et al. (2009) Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. 1909–1918 p. doi: 10.1182/blood-2008-09-178459 19131551
23. Lyons M, Onion D, Green NK, Aslan K, Rajaratnam R, et al. (2006) Adenovirus Type 5 Interactions with Human Blood Cells May Compromise Systemic Delivery. Mol Ther 14 : 118–128. 16580883
24. Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, et al. (2008) Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132 : 397–409. doi: 10.1016/j.cell.2008.01.016 18267072
25. Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, et al. (2008) Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proceedings of the National Academy of Sciences 105 : 5483–5488. doi: 10.1073/pnas.0711757105 18391209
26. Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, et al. (2008) Substitution of Hexon Hypervariable Region 5 of Adenovirus Serotype 5 Abrogates Blood Factor Binding and Limits Gene Transfer to Liver. Mol Ther 16 : 1474–1480. doi: 10.1038/mt.2008.132 18560416
27. Bertheau P, Parquet N, Ferchal F, Gluckman E, Brocheriou C (1996) Fulminant adenovirus hepatitis after allogeneic bone marrow transplantation. Bone Marrow Transplant 17 : 295–298. 8640184
28. Chakrabarti S, Collingham KE, Fegan CD, Milligan DW (1999) Fulminant adenovirus hepatitis following unrelated bone marrow transplantation: failure of intravenous ribavirin therapy. Bone Marrow Transplant 23 : 1209–1211. 10382964
29. Johnson PR, Yin JA, Morris DJ, Desai M, Cinkotai KI, et al. (1990) Fulminant hepatic necrosis caused by adenovirus type 5 following bone marrow transplantation. Bone Marrow Transplant 5 : 345–347. 2161693
30. Alba R, Bradshaw AC, Mestre-Frances N, Verdier JM, Henaff D, et al. (2012) Coagulation factor X mediates adenovirus type 5 liver gene transfer in non-human primates (Microcebus murinus). Gene Ther 19 : 109–113. doi: 10.1038/gt.2011.87 21677690
31. Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, et al. (2006) Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108 : 2554–2561. 16788098
32. Alba R, Bradshaw AC, Parker AL, Bhella D, Waddington SN, et al. (2009) Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: effect of mutagenesis on FX interactions and gene transfer. Blood.
33. Bradshaw AC, Parker AL, Duffy MR, Coughlan L, van Rooijen N, et al. (2010) Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X. PLoS Pathog 6: e1001142. doi: 10.1371/journal.ppat.1001142 20949078
34. Duffy MR, Bradshaw AC, Parker AL, McVey JH, Baker AH (2011) A Cluster of Basic Amino Acids in the Factor X Serine Protease Mediates Surface Attachment of Adenovirus/FX Complexes. Journal of Virology 85 : 10914–10919. doi: 10.1128/JVI.05382-11 21849463
35. Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald RA, et al. (2010) Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. 2656–2664 p.
36. Irons EE, Flatt JW, Doronin K, Fox TL, Acchione M, et al. (2013) Coagulation Factor Binding Orientation and Dimerization May Influence Infectivity of Adenovirus-Coagulation Factor Complexes. Journal of Virology 87 : 9610–9619. doi: 10.1128/JVI.01070-13 23804638
37. Doronin K, Flatt JW, Di Paolo NC, Khare R, Kalyuzhniy O, et al. (2012) Coagulation factor X activates innate immunity to human species C adenovirus. Science 338 : 795–798. doi: 10.1126/science.1226625 23019612
38. Kojouharova M, Reid K, Gadjeva M (2010) New insights into the molecular mechanisms of classical complement activation. Molecular Immunology 47 : 2154–2160. doi: 10.1016/j.molimm.2010.05.011 20542571
39. Kim M, Zinn KR, Barnett BG, Sumerel LA, Krasnykh V, et al. (2002) The therapeutic efficacy of adenoviral vectors for cancer gene therapy is limited by a low level of primary adenovirus receptors on tumour cells. European Journal of Cancer 38 : 1917–1926. 12204675
40. Khare R, Reddy VS, Nemerow GR, Barry MA (2012) Identification of Adenovirus Serotype 5 Hexon Regions That Interact with Scavenger Receptors. Journal of Virology 86 : 2293–2301. doi: 10.1128/JVI.05760-11 22156515
41. Li H, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, et al. (2012) Adenovirus Serotype 26 Utilizes CD46 as a Primary Cellular Receptor and Only Transiently Activates T Lymphocytes following Vaccination of Rhesus Monkeys. Journal of Virology 86 : 10862–10865. doi: 10.1128/JVI.00928-12 22811531
42. Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, et al. (2010) Adenovirus-Based Vaccines: Comparison of Vectors from Three Species of Adenoviridae. Journal of Virology 84 : 10522–10532. doi: 10.1128/JVI.00450-10 20686035
43. Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, et al. (2012) First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Adenovirus Serotype 26 HIV-1 Env Vaccine (IPCAVD 001). Journal of Infectious Diseases.
44. Vollmers HP, Brändlein S (2006) Natural IgM antibodies: The orphaned molecules in immune surveillance. Advanced Drug Delivery Reviews 58 : 755–765. 16820243
45. Klimovich VB (2011) IgM and its receptors: Structural and functional aspects. Biochemistry (Moscow) 76 : 534–549. doi: 10.1134/S0006297911050038 21639833
46. Uchida K (2014) Natural antibodies as a sensor of electronegative damage-associated molecular patterns (DAMPs). Free Radical Biology and Medicine 72 : 156–161. doi: 10.1016/j.freeradbiomed.2014.03.016 24657731
47. Chikazawa M, Otaki N, Shibata T, Miyashita H, Kawai Y, et al. (2013) Multispecificity of Immunoglobulin M Antibodies Raised against Advanced Glycation End Products: INVOLVEMENT OF ELECTRONEGATIVE POTENTIAL OF ANTIGENS. Journal of Biological Chemistry 288 : 13204–13214. doi: 10.1074/jbc.M113.452177 23543734
48. Perreau M, Guerin M-C, Drouet C, Kremer EJ (2007) Interactions Between Human Plasma Components and A Xenogenic Adenovirus Vector: Reduced Immunogenicity During Gene Transfer. Mol Ther 15 : 1998–2007. 17712332
49. Sumida SM, Truitt DM, Lemckert AAC, Vogels R, Custers JHHV, et al. (2005) Neutralizing Antibodies to Adenovirus Serotype 5 Vaccine Vectors Are Directed Primarily against the Adenovirus Hexon Protein. The Journal of Immunology 174 : 7179–7185. 15905562
50. Roberts DM, Nanda A, Havenga MJE, Abbink P, Lynch DM, et al. (2006) Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441 : 239–243. 16625206
51. Parker AL, Waddington SN, Buckley SMK, Custers J, Havenga MJE, et al. (2009) Effect of Neutralizing Sera on Factor X-Mediated Adenovirus Serotype 5 Gene Transfer. Journal of Virology 83 : 479–483. doi: 10.1128/JVI.01878-08 18945780
52. Seiradake E, Henaff D, Wodrich H, Billet O, Perreau M, et al. (2009) The cell adhesion molecule "CAR" and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog 5: e1000277. doi: 10.1371/journal.ppat.1000277 19119424
53. Nicol CG, Graham D, Miller WH, White SJ, Smith TAG, et al. (2004) Effect of Adenovirus Serotype 5 Fiber and Penton Modifications on in vivo Tropism in Rats. Mol Ther 10 : 344–354. 15294181
54. Bordigoni P, Carret A-S, Venard V, Witz F, Le Faou A (2001) Treatment of Adenovirus Infections in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clinical Infectious Diseases 32 : 1290–1297. 11303263
55. Lenaerts L, Naesens L (2006) Antiviral therapy for adenovirus infections. Antiviral Research 71 : 172–180. 16698093
56. Duffy MR, Parker AL, Kalkman ER, White K, Kovalskyy D, et al. (2013) Identification of novel small molecule inhibitors of adenovirus gene transfer using a high throughput screening approach. Journal of Controlled Release 170 : 132–140. doi: 10.1016/j.jconrel.2013.05.007 23702233
57. Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, et al. (2003) Replication-Deficient Human Adenovirus Type 35 Vectors for Gene Transfer and Vaccination: Efficient Human Cell Infection and Bypass of Preexisting Adenovirus Immunity. Journal of Virology 77 : 8263–8271. 12857895
58. Greig JA, Buckley SMK, Waddington SN, Parker AL, Bhella D, et al. (2009) Influence of Coagulation Factor X on In Vitro and In Vivo Gene Delivery by Adenovirus (Ad) 5, Ad35, and Chimeric Ad5/Ad35 Vectors. Mol Ther 17 : 1683–1691. doi: 10.1038/mt.2009.152 19603000
59. Alba R, Baker A, Nicklin S (2012) Vector Systems for Prenatal Gene Therapy: Principles of Adenovirus Design and Production. In: Coutelle C, Waddington SN, editors. Prenatal Gene Therapy: Humana Press. pp. 55–84.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



