-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaParasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
How vivax parasites cause severe malaria is not known. In contrast to falciparum parasites, the number of vivax parasites circulating in peripheral blood is low, and there is thought to be little sequestration of parasitized red cells within endothelium-lined small blood vessels in vital organs. Total parasite burden (circulating plus hidden) and activation and dysfunction of the endothelial cells lining blood vessels all contribute to severe disease in falciparum malaria, but have not been evaluated in severe vivax malaria. We measured parasite lactate dehydrogenase (pLDH) and P. vivax-pLDH (PvLDH) as proxies of total parasite biomass and found that, as in falciparum malaria, the total biomass of vivax parasites is underestimated by counting parasites circulating in peripheral blood, suggesting a hidden burden of vivax parasites. Markers of total vivax biomass were strongly associated with illness-severity and inflammatory cytokines, suggesting that this hidden burden is capable of contributing to generalised inflammation and hence severe disease. Number of peripheral vivax parasites, but not total biomass, correlated with activation of endothelial cells, suggesting that the hidden vivax-infected red cells may accumulate in parts of organs without endothelium, such as the slow-circulation of the spleen or non-blood-vessel parts of the bone marrow. Severe vivax malaria was associated with increased endothelial activation and impaired microvascular function, suggesting that these processes also contribute to impaired blood flow and disease.
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004558
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004558Summary
How vivax parasites cause severe malaria is not known. In contrast to falciparum parasites, the number of vivax parasites circulating in peripheral blood is low, and there is thought to be little sequestration of parasitized red cells within endothelium-lined small blood vessels in vital organs. Total parasite burden (circulating plus hidden) and activation and dysfunction of the endothelial cells lining blood vessels all contribute to severe disease in falciparum malaria, but have not been evaluated in severe vivax malaria. We measured parasite lactate dehydrogenase (pLDH) and P. vivax-pLDH (PvLDH) as proxies of total parasite biomass and found that, as in falciparum malaria, the total biomass of vivax parasites is underestimated by counting parasites circulating in peripheral blood, suggesting a hidden burden of vivax parasites. Markers of total vivax biomass were strongly associated with illness-severity and inflammatory cytokines, suggesting that this hidden burden is capable of contributing to generalised inflammation and hence severe disease. Number of peripheral vivax parasites, but not total biomass, correlated with activation of endothelial cells, suggesting that the hidden vivax-infected red cells may accumulate in parts of organs without endothelium, such as the slow-circulation of the spleen or non-blood-vessel parts of the bone marrow. Severe vivax malaria was associated with increased endothelial activation and impaired microvascular function, suggesting that these processes also contribute to impaired blood flow and disease.
Introduction
While P. falciparum accounts for a majority of severe and fatal malaria cases worldwide, P. vivax is a major cause of morbidity outside of Africa, causing an estimated 70–390 million malaria cases per year [1]. Although previously considered benign, P. vivax is now recognized as capable of causing severe and fatal disease [2], [3], [4], [5], [6], [7], [8], [9], [10]. Despite this, little is known about the pathogenesis of severe disease in vivax malaria.
In falciparum malaria, severe and fatal disease is characterised by cytoadherence of parasitized red blood cells (RBCs) to activated and dysfunctional endothelium, leading to parasite sequestration with microvascular obstruction [11], [12], [13]. As a result of sequestration the mature schizont stages of P. falciparum are rarely seen in peripheral blood [14]. Total parasite biomass is underestimated by circulating parasitemia quantitated by microscopy of peripheral blood, and is more accurately quantitated by plasma P. falciparum histidine rich protein-2 (HRP2). Pathogenesis and disease severity in falciparum malaria are both biomass-related: in contrast to peripheral parasitemia [15], [16], [17], plasma HRP2 is strongly and independently correlated with disease severity and mortality among both children [17], [18], [19], [20] and adults [21], [22].
Cytoadherence of infected RBCs to endothelial cells is 10-fold less in P. vivax infection than in falciparum malaria [23], and autopsy evidence for sequestration within the endothelium-lined microvasculature of vital-organs in vivax malaria is minimal [2], [4], [24]. This, together with the lower parasitemias resulting from preferential invasion of reticulocytes, is thought to account for the lower lethality of P. vivax [2]. The paucity of apparent endothelial microvascular sequestration in vivax malaria has led to the assumption that peripheral parasitemia reflects total parasite biomass. However, this assumption has been questioned [3], [25], [26], with more adhesive schizont-stages of P. vivax known to be under-represented in peripheral blood [26], [27]. Accumulation of P. vivax-infected RBCs in the spleen or bone marrow has been hypothesised [3], [25], [26] but not yet systematically investigated. In contrast to falciparum malaria, no study has evaluated the relationships between total parasite biomass, disease severity and systemic inflammation in vivax malaria. We propose that in vivax malaria, parasite lactate dehydrogenase (pLDH) and P. vivax-pLDH (PvLDH), produced by viable or recently killed parasites, may be used to estimate total parasite biomass. While plasma pLDH has been shown to demonstrate only moderate correlation with peripheral parasitemia in vivax malaria [28], a pLDH antigen capture enzyme-linked immunosorbent assay demonstrated a direct relationship between parasite production of pLDH and total P. vivax parasite concentration ex vivo, including all parasite stages, suggesting that pLDH reflects total P. vivax parasite biomass [29]. As with HRP2 in falciparum malaria [30], pLDH is produced to a greater extent by P. vivax schizonts and trophozoites than by ring-form parasites [31], and given the under-representation of mature P. vivax stages in peripheral blood [26], [27], pLDH may be a better marker of total parasite biomass and a better prognostic indicator than peripheral parasitemia.
In falciparum malaria, clinical severity and mortality are also independently associated with impaired microvascular function [13] and with increased angiopoietin-2 (Ang-2) [32], a key product of endothelial Weibel-Palade Bodies (WPB) and an autocrine mediator of endothelial activation [33]. Despite the apparent paucity of endothelial microvascular sequestration in vital organs, P. vivax has been associated with greater endothelial activation than P. falciparum, with Ang-2 concentrations higher in non-severe vivax compared to non-severe falciparum malaria [34]. However the relationships between clinical severity, microvascular dysfunction and endothelial WPB exocytosis have not been assessed in vivax malaria.
We tested the hypotheses that in vivax malaria, peripheral parasitemia would underestimate total parasite biomass, and that markers of total parasite biomass would be related to systemic inflammation and clinical severity. We also determined the relationship between vivax disease severity and endothelial activation and microvascular function, with patients with non-severe and severe falciparum malaria included for comparison. We found that total vivax biomass was underestimated by peripheral parasitemia, and was associated with both systemic inflammation and disease severity in vivax malaria. Severe vivax malaria was associated with increased endothelial activation and WPB exocytosis and with impaired microvascular function, comparable to that seen in severe falciparum malaria.
Results
Patients
A total of 192 malaria patients and 74 healthy controls were enrolled. Malaria patients included 62 with vivax malaria (53 non-severe, 9 severe) and 130 with falciparum malaria (109 non-severe, 21 severe). The clinical and epidemiological features of 43 patients with vivax malaria (including 7 with severe disease) and 122 patients with falciparum malaria (including 13 with severe disease) have been previously reported [35]. Baseline characteristics are shown in Table 1. Among the 9 patients with severe vivax malaria, severity criteria included hypotension (n = 6), respiratory distress (n = 1), jaundice (n = 2), metabolic acidosis (n = 1), abnormal bleeding (n = 1) and multiple convulsions (n = 1). Three patients had 2 severity criteria and 6 had one. Pre-antibiotic blood cultures were negative in 4 patients, positive for Streptococcus pneumoniae in one [35], and not done in 4. No deaths occurred from either species.
Tab. 1. Baseline characteristics of vivax and falciparum malaria patients and healthy controls. 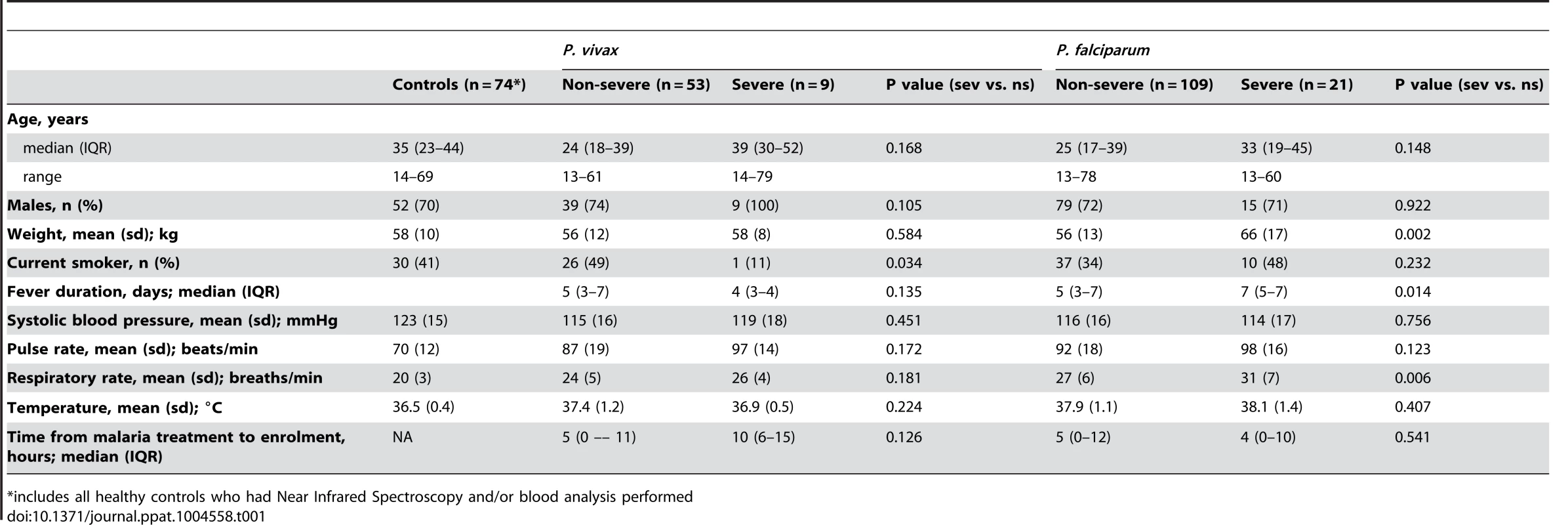
*includes all healthy controls who had Near Infrared Spectroscopy and/or blood analysis performed Peripheral parasitemia, schizontemia, markers of total parasite biomass and disease severity
In patients with vivax malaria, the median peripheral parasitemia was 2.4-fold higher in severe compared to non-severe disease (10,243 parasites/µL vs. 4,209 parasites/µL; P = 0.021), while the median PvLDH was 3.7-fold higher (96.6 ng/ml vs. 26.2 ng/ml; P = 0.021) and pLDH 6.9-fold higher (308 ng/ml vs. 44.6 ng/ml; P = 0.015) (Table 2 and Fig. 1). After removing the patient with severe vivax malaria and concurrent bacteremia from the analysis, the median peripheral parasitemia was 1.8-fold higher in severe compared to non-severe disease (7,865 parasites/µL vs. 4,209 parasites/µL; P = 0.047), while the median PvLDH was 4.8-fold higher (125 ng/ml vs. 26.2 ng/ml; P = 0.002) and pLDH 7.3-fold higher (326 ng/ml vs. 44.6 ng/ml; P = 0.002). In falciparum malaria, median peripheral parasitemia and plasma HRP2 were 4.2-fold and 7.4-fold higher, respectively, in severe compared to non-severe disease (Table 2).
Fig. 1. Parasitemia (A) and total parasite biomass [pLDH (B), PvLDH (C), and HRP2 (D)] among patients with severe and non-severe vivax and falciparum malaria. ![Parasitemia (A) and total parasite biomass [pLDH (B), PvLDH (C), and HRP2 (D)] among patients with severe and non-severe vivax and falciparum malaria.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/e3f1cb88440620cd12bdf09dd44f816b.png)
Tab. 2. Parasite count and parasite biomass among patients with P. vivax and P. falciparum malaria. 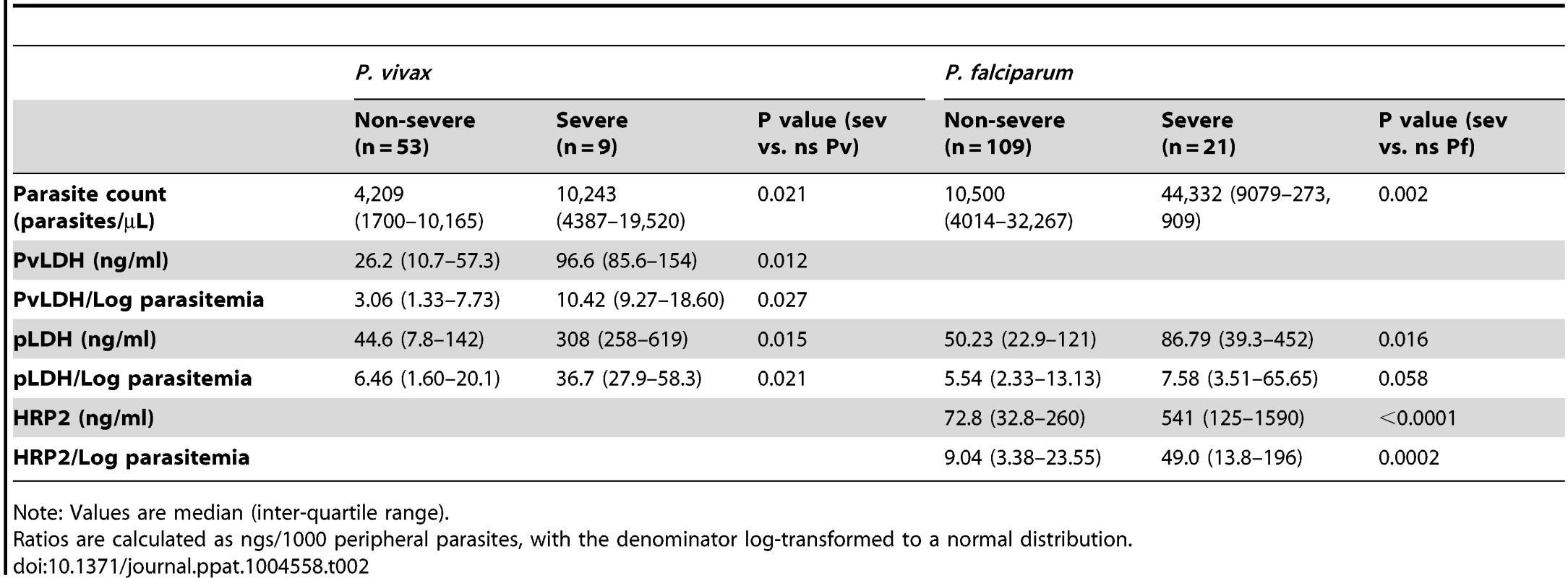
Note: Values are median (inter-quartile range). To estimate whether the “hidden parasite biomass” was larger in severe compared to non-severe malaria, we calculated the ratio of plasma pLDH and plasma PvLDH concentration to peripheral parasite density in vivax malaria, and the ratio of plasma HRP2 concentration to peripheral parasite density in falciparum malaria. Among patients with vivax malaria the ratios of PvLDH and pLDH to parasite density (log) were 3.7-fold and 5.7-fold higher, respectively, in severe compared to non-severe disease. The corresponding fold-increase in the ratio of plasma HRP2 to parasite count (log) in patients with severe compared to non-severe falciparum malaria was 5.4.
Among patients with vivax malaria pLDH and PvLDH were strongly correlated (ρ = 0.90, P<0.0001). However there was only a modest correlation between PvLDH and parasitemia (ρ = 0.27, P = 0.033), and between pLDH and parasitemia (ρ = 0.28, P = 0.028), comparable to the correlation between parasitemia and plasma HRP2 among patients with falciparum malaria (ρ = 0.41, P<0.0001) (Fig. 2).
Fig. 2. Parasitemia and markers of total parasite biomass among patients with vivax and falciparum malaria. 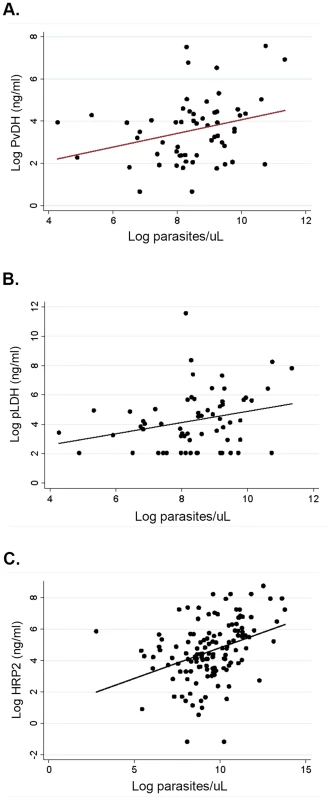
(A) Correlation between parasitemia and PvLDH among patients with vivax malaria, Spearman's ρ = 0.27, P = 0.033, (B) Correlation between parasitemia and pLDH among patients with vivax malaria, Spearman's ρ = 0.27, P = 0.028, and (C) correlation between parasitemia and HRP2 among patients with falciparum malaria, Spearman's ρ = 0.41, P = <0.0001. Among patients with vivax malaria who had detectable pLDH and PvLDH levels on enrolment and in whom longitudinal measurements could be performed (until day 3 or until undetectable), levels were undetectable by day 3 in 16/25 (64%) and 22/31 (71%) patients respectively.
In ex vivo assay conditions, P. vivax parasites exist as mature schizonts for an estimated 5% of their 48-hour life-cycle [36]. Despite this, only 14/59 (24%) patients with vivax malaria had any circulating schizonts detectable on peripheral blood film, including 2/9 (22%) with severe and 12/50 (24%) with non-severe disease. Of those with schizonts detectable, schizonts comprised <5% of circulating parasites in 11/14 (78%) patients, including one with severe and 10 with non-severe disease. There was a wide variation in the proportion of peripheral P. vivax parasites at ring (median 33%, range 0–100%, IQR 9–77%) and trophozoite stage (median 67%, range 0–100%, IQR 20–91%). Among patients with severe and non-severe vivax malaria, there were no differences between the median percentage of rings or trophozoites in peripheral blood.
Markers of endothelial activation and thrombosis, cytokines, and clinical disease
Median concentrations of Ang-2 and von Willebrand Factor (vWF), the two major products of endothelial WPB release, were both increased in patients with severe and non-severe vivax malaria compared to controls (P<0.01 for all comparisons), with Ang-2 nearly twice as high in severe compared to non-severe disease (P<0.0001) (Table 3). The median concentrations of the endothelial adhesion receptors E-selectin and ICAM1 were also increased in severe and non-severe vivax malaria compared to controls (P<0.001 for all comparisons). In patients with vivax malaria there was no association between age and any of the endothelial activation markers measured. Median concentrations of Ang-2, E-selectin and ICAM1 were at least as high in severe and non-severe vivax malaria as they were in severe and non-severe falciparum malaria (Table 3).
Tab. 3. Laboratory and physiological measurements among patients with vivax and falciparum malaria and healthy controls. 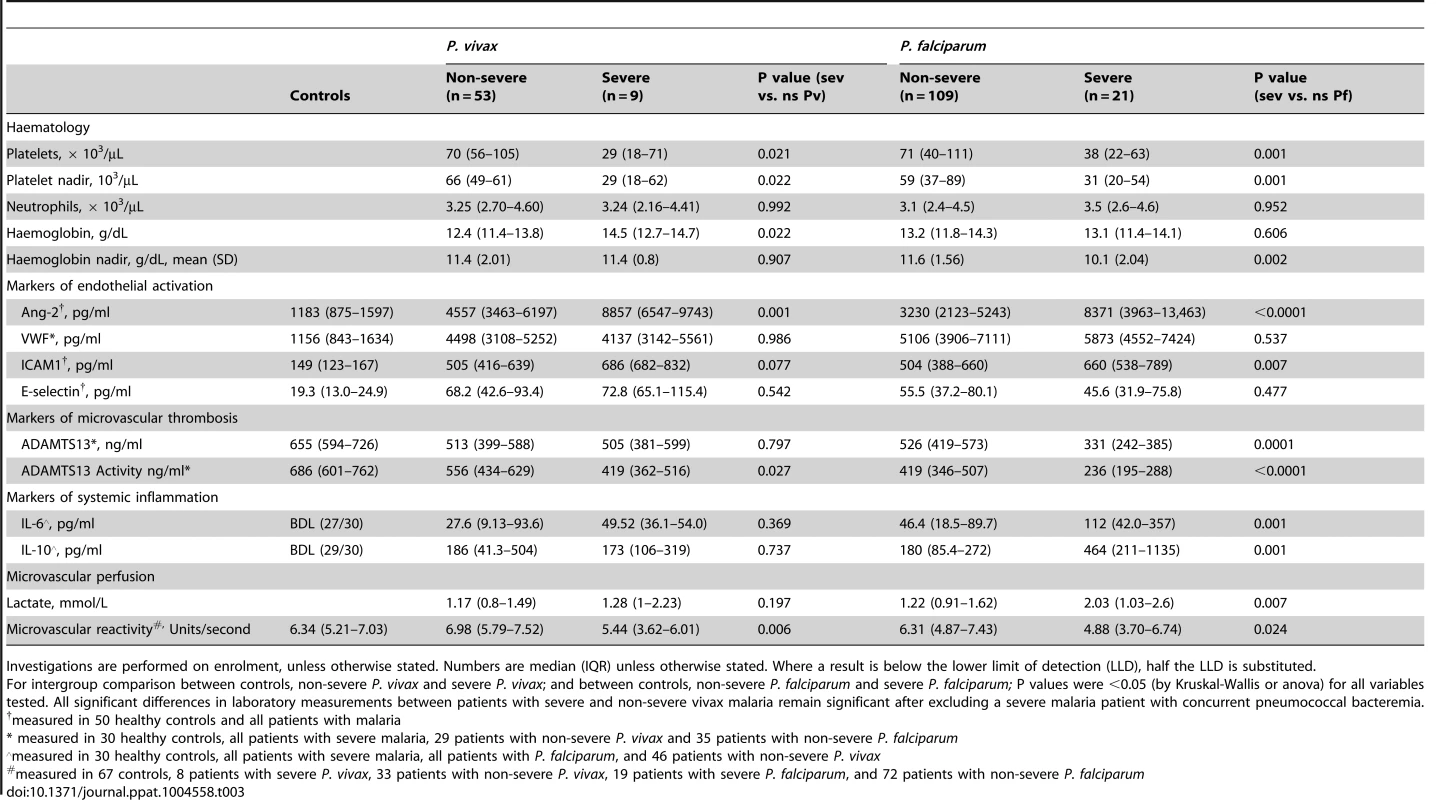
Investigations are performed on enrolment, unless otherwise stated. Numbers are median (IQR) unless otherwise stated. Where a result is below the lower limit of detection (LLD), half the LLD is substituted. The median concentrations of the vWF-cleaving protease, ADAMTS13, and its activity, were lower among patients with severe and non-severe vivax malaria compared to controls (P<0.001 for all comparisons), with median ADAMTS13 activity also lower in severe compared to non-severe vivax malaria (P = 0.027).
Median concentrations of IL-6 and IL-10 were higher in severe and non-severe vivax malaria compared to controls (Table 3).
Parasitemia, markers of total parasite biomass, and biomarkers of severity in vivax and falciparum malaria
In patients with vivax malaria, peripheral parasitemia correlated with the endothelial WPB products, VWF (ρ = 0.53, P<0.0001) and Ang-2 (ρ = 0.36, P = 0.004), as well as the endothelial adhesion receptor E-selectin (ρ = 0.33, P = 0.009), and was inversely correlated with activity of the VWF-cleaving protease ADAMTS13 (ρ = −0.31, P = 0.055) (Table 4). Importantly, and in contrast to peripheral parasitemia, no correlation was seen between either of the total parasite biomass markers PvLDH or pLDH, and the endothelial products VWF, Ang-2, or E-selectin (Table 4).
Tab. 4. Correlation coefficients of baseline parasitemia and parasite biomass markers among patients with vivax and falciparum malaria. 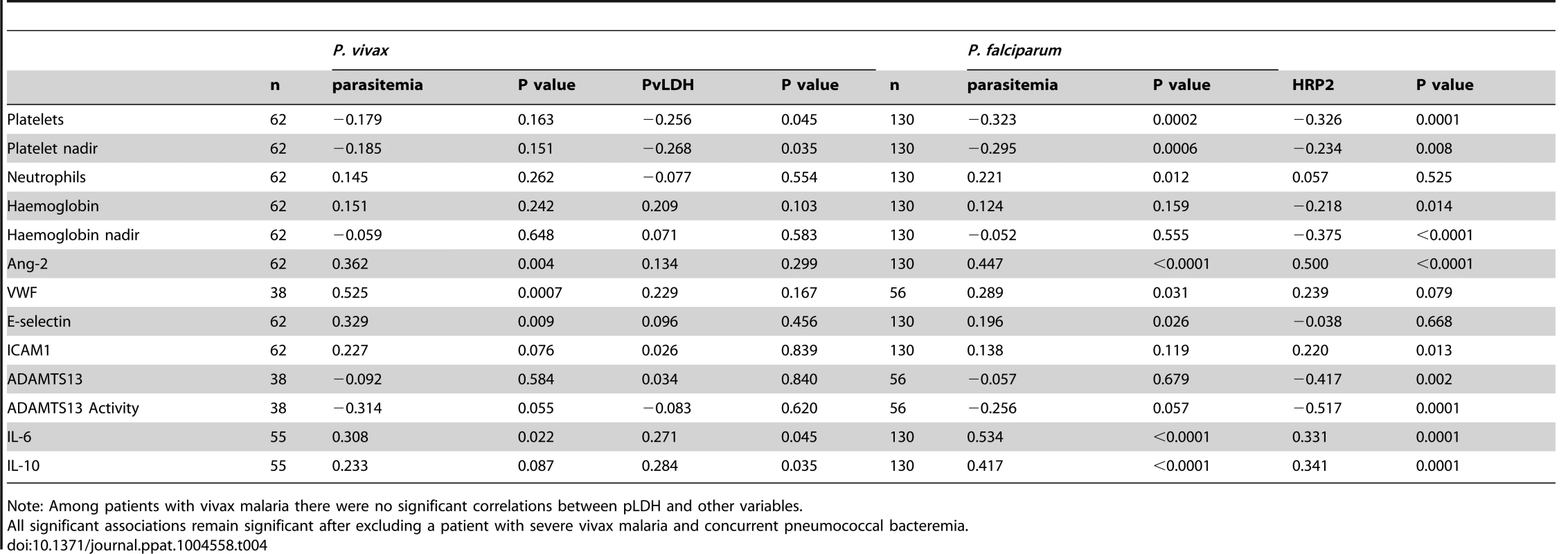
Note: Among patients with vivax malaria there were no significant correlations between pLDH and other variables. While not associated with markers of endothelial activation, PvLDH was correlated with the systemic inflammatory markers IL-6 (ρ = 0.27, P = 0.045) and IL-10 (ρ = 0.28, P = 0.035), and, in contrast to peripheral parasitemia, was inversely correlated with baseline platelet count (ρ = −0.26, P = 0.045) and with platelet nadir (ρ = −0.27, P = 0.035).
Among patients with falciparum malaria, biomarkers of systemic inflammation correlated with both peripheral parasitemia and the total parasite biomass marker, plasma HRP2 (Table 4). In addition, and in contrast to vivax malaria, endothelial activation correlated not only with peripheral parasitemia but also with total parasite biomass (Table 4). Baseline platelet count and platelet nadir also correlated with both peripheral parasitemia and HRP2 (Table 4).
Ang-2, ADAMTS13, and biomarkers of severity among patients with vivax malaria
In patients with vivax malaria, Ang-2, in addition to correlating with parasitemia, correlated with the adhesion receptors E-selectin (ρ = 0.40, P = 0.001) and ICAM-1 (ρ = 0.54, P<0.0001), and inversely with
ADAMTS13 antigen (ρ = −0.41, P = 0.010) and activity levels (ρ = −0.60, P = 0.0001), with all associations remaining significant after adjusting for parasitemia.
ADAMTS13 antigen and activity levels correlated with platelet count (ρ = 0.36, P = 0.025; and ρ = 0.56, P = 0.0003, respectively), with this association remaining significant after adjusting for parasitemia. IL-6, elevated in vivax malaria, is known to be a major inhibitor of ADAMTS13 activity [37]. In vivax malaria, IL-6 was inversely correlated with ADAMTS13 activity (ρ = −0.40, P = 0.013) and platelet nadir (r = −0.44, p = 0.0009). No association occurred between lactate and ADMATS13 antigen and activity levels, or between lactate and Ang-2.
Microvascular function
Microvascular reactivity (assessed using Near Infrared Resonance Spectroscopy [13]) was decreased in patients with severe vivax malaria (median 5.44 units/sec) compared to those with non-severe vivax malaria (median 6.98 units/sec; P = 0.006) and to controls (median 6.34, P = 0.027) (Table 3 and Fig. 3). Microvascular reactivity was also impaired among patients with severe falciparum malaria (median 4.88 units/sec) compared to those with non-severe falciparum malaria (6.31 units/sec; P = 0.024) and controls (P = 0.016), with the degree of impairment similar between severe vivax and severe falciparum malaria. In both species, there was no significant difference between controls and patients with non-severe malaria.
Fig. 3. Microvascular function (skeletal muscle reoxygenation) among patients with vivax (A) and falciparum (B) malaria. 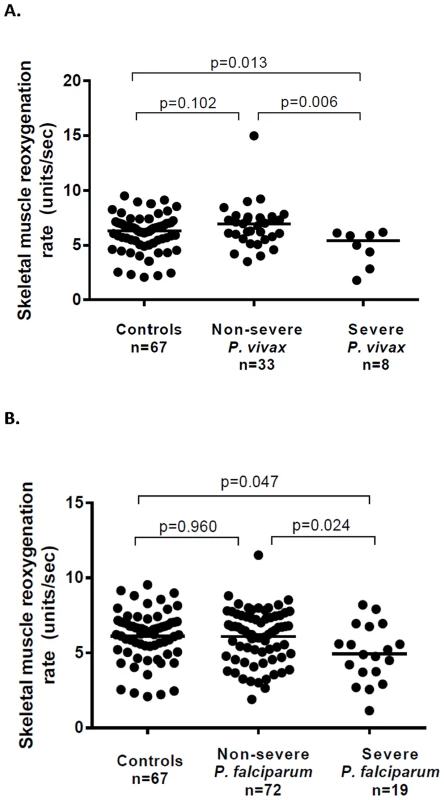
Among patients with vivax malaria, microvascular reactivity was inversely associated with the perfusion marker, lactate, in severe (ρ = −0.74, P = 0.04), but not non-severe disease (ρ = 0.09, P = 0.62). In severe disease ADAMTS13 antigen and activity levels correlated with microvascular reactivity (ρ = 0.64 and P = 0.09, for both associations), although no association occurred in non-severe disease (ρ = 0.07, P = 0.78; and ρ = −0.02, P = 0.95; respectively). No association was found between microvascular reactivity and other variables in vivax malaria, including parasitemia or biomass markers.
In patients with falciparum malaria, microvascular reactivity was inversely correlated with parasitemia (ρ = −0.22, P = 0.035), and positively correlated with platelet count (ρ = 0.017, P = 0.017). Among patients with severe and non-severe falciparum malaria, no association occurred between microvascular reactivity and lactate, or between microvascular reactivity and ADAMTS13 antigen or activity. In both species there was no association between microvascular reactivity and age.
Among patients with vivax malaria, 6 patients with severe and 16 with non-severe disease had NIRS repeated on day 3, with no change from baseline noted in either group (median microvascular reactivity on day 3 = 5.59 units/sec and 7.39 units/sec respectively). In severe falciparum, microvascular reactivity improved from 4.88 units/sec at enrolment to 6.51 units/sec on day 3 (P = 0.039), although no difference was noted among 37 patients with non-severe falciparum malaria (median microvascular reactivity on day 3 = 6.56 units/sec, P = 0.437)
Discussion
Peripheral parasitemia underestimates measures of total parasite biomass in vivax malaria and circulating schizonts are under-represented in peripheral blood, suggesting tissue accumulation of a hidden biomass of P. vivax-infected RBCs. The proportions of total parasite biomass detectable in peripheral blood in uncomplicated and severe disease were of comparable magnitude in vivax and in falciparum malaria. The association between P. vivax peripheral parasitemia and total parasite biomass is modest, with total parasite biomass more strongly associated with disease severity compared to peripheral parasitemia. The smaller proportion of total P. vivax biomass detectable in peripheral blood in severe compared to non-severe disease suggests that hidden biomass is greater in severe vivax malaria and contributes to pathogenesis of disease. The association of total vivax parasite biomass with systemic inflammation suggests that the hidden biomass is capable of mediating systemic inflammatory pathology. In contrast to peripheral P. vivax parasitemia, total parasite biomass did not correlate with endothelial activation, and we therefore speculate that accumulation of P. vivax-infected RBCs may occur in parts of the circulation devoid of endothelium.
In falciparum malaria, peripheral parasitemia underestimates the total parasite biomass due to sequestration of parasitized RBCs within endothelium-lined microvasculature. While P. vivax is not thought to sequester in the endothelium-lined microvasculature to the same degree as P. falciparum, limited histopathological reports show intact P. vivax-infected RBCs in the bone marrow [38], [39], [40], [41], [42], [43] and spleen [44], [45], organs containing circulatory compartments that are not endothelium-lined. The spleen is a lymphoid organ whose primary role is to clear abnormal erythrocytes from the circulation, and hence plays a fundamental role in removing parasitized RBCs, especially in falciparum malaria where RBC deformability is markedly decreased. In falciparum malaria, endothelial cytoadherence of infected RBCs enable the parasite to sequester in non-splenic microvasculature and avoid passage through the spleen. In contrast, P. vivax-infected RBCs cytoadhere with less avidity to endothelial cells [23], and demonstrate increased deformability [46], [47], allowing circulation through the spleen. While 80-90% of splenic blood flow occurs through endothelial-lined sinuses, the remainder circulates through a parallel slow, open circulation in the splenic cords, devoid of endothelial cells [48]. In a recent report involving a splenectomised patient with vivax malaria, large numbers of intact non-phagocytosed P. vivax-infected reticulocytes were found in the splenic cords [44]. Accumulation of P. vivax-infected reticulocytes in the slow open microcirculation of the spleen, possibly through reticulocyte adherence to non-endothelial resident cells [49], [50], [51], [52], has been proposed as a mechanism by which P. vivax may avoid macrophage clearance [53] yet readily invade new reticulocytes.
The findings of our study suggest that tissue accumulation of P. vivax does occur and is greatest in severe vivax malaria, and we speculate it may occur in a location devoid of endothelium, such as the slow circulation of the spleen. We found no correlation between the P. vivax biomarkers of total biomass, pLDH and PvLDH, and either of the two products of endothelial WPB release, Ang-2 or VWF, or the endothelial adhesion receptor E-selectin. In contrast, peripheral parasitemia correlated with endothelial activation (Ang-2, VWF and E-selectin) in both vivax and falciparum malaria, and with total biomass (HRP2) in falciparum malaria. These findings suggest that endothelial cells may be activated by P. vivax-infected RBCs circulating through the peripheral microvasculature, but not by the hidden P. vivax biomass. Accumulation of P. vivax in the endothelium-free open circulation of the spleen may account for these findings. The finding that P. vivax peripheral parasitemia and markers of total parasite biomass both correlated with the leukocyte-derived cytokines may reflect the ability of circulating and tissue-accumulated P. vivax to stimulate leukocytes in both peripheral blood and leukocyte-containing organs such as the spleen or bone marrow, and thereby mediating organ dysfunction secondary to systemic inflammation.
Although underestimating total biomass, peripheral parasitemia was still higher in severe compared to non-severe malaria in both P. vivax and P. falciparum, and endothelial activation was higher in patients with severe disease. Ang-2 concentrations were twice as high in severe compared to non-severe vivax malaria, and comparable to levels seen in severe falciparum malaria. Ang-2, which causes autocrine endothelial activation, has previously been shown to be markedly elevated in severe falciparum malaria and a consistent predictor of death in both adults [32], [54], [55] and children [56], [57]. In severe falciparum malaria Ang-2 correlates with impaired endothelial function, lactate, plasma HRP2, ICAM-1, and E-selectin [32]. In our study, Ang-2 was also associated with ICAM-1 and E-selectin among patients with vivax malaria, consistent with the role of Ang-2 in activating endothelium in vivax as well as falciparum malaria [32]. Our findings of increased endothelial activation in severe vivax malaria are consistent with a recent study reporting higher concentrations of ICAM-1 and VCAM-1 in severe compared to uncomplicated vivax malaria [58].
Microvascular function was significantly impaired in severe vivax malaria, comparable to the impairment seen in severe falciparum malaria. As previously shown in severe falciparum malaria [13], the impairment of microvascular reactivity in severe vivax malaria was strongly associated with blood lactate, suggesting that impaired tissue perfusion is contributing to organ dysfunction in severe vivax malaria.
Plasma ADAMTS13 activity was decreased in vivax malaria, with deficiency associated with both severe disease and with impaired microvascular function. ADAMTS13 is a protease that cleaves ultra-large and prothrombogenic VWF multimers (UL-VWF), and deficiency leads to accumulation of UL-VWF with resultant increase in platelet aggregation and adhesion, and microvascular thrombosis. Accumulation of UL-VWF resulting from ADAMTS13 deficiency is characteristic of the microangiopathic disease thrombotic thrombocytopenic purpura (TTP), and has been reported in patients with both falciparum and vivax malaria [59]. While renal failure was not a feature of patients with severe vivax malaria in our study, thrombotic microangiopathy has been reported in severe vivax disease elsewhere [60], [61]. In our study, lower ADAMTS13 antigen and activity levels were associated with lower platelet counts, and with increased concentrations of IL-6, a known specific inhibitor of ADAMTS13 activity [37]. We speculate that biomass-related IL-6 may contribute to impaired ADAMTS13 activity and accumulation of UL-VWF multimers, and may thereby contribute to microvascular dysfunction, thrombocytopenia and disease severity in vivax malaria. Further studies however are required to confirm the mechanisms underlying ADAMTS13 deficiency in vivax malaria, and to investigate the role of thrombotic microangiopathy.
Our study had several limitations. Firstly, pLDH and PvLDH have not been previously validated as biomass markers in vivax malaria. Other factors may contribute to the plasma concentrations of these markers, such as host metabolic clearance rate, distribution within the host and diffusion rates from host tissue, and natural variation in parasite expression. Release of pLDH solely at the time of schizont rupture may provide a possible alternative explanation for the association between concentrations of pLDH/PvLDH, systemic inflammation and disease severity, with schizogony also associated with an inflammatory response [62]. However, we have demonstrated that ex-vivo concentrations of PvLDH increase progressively during short term culture of P. vivax, indicating that PvLDH is secreted throughout the parasite life-cycle and supporting the use of pLDHand PvLDH as surrogate markers of parasite biomass. Host inflammatory response may contribute in part to the marked under-representation of P. vivax schizonts in peripheral blood, however this would apply similarly to hosts infected with other Plasmodium species with under-representation of schizonts in peripheral blood, such as P. falciparum or P. coatneyi, whose schizonts have been clearly shown to sequester in tissues [63].
The small number of patients with severe vivax malaria (n = 9) limited our ability to evaluate the association between disease severity and other variables, and to assess for associations of parasite biomass and/or parasitemia that may have occurred only among patients with severe disease. In addition, with multiple comparisons we are unable to exclude the possibility that associations may have occurred by chance. However, the magnitude and direction of the associations with disease severity and with different measures of biomass are consistent, plausible, and are comparable to those in patients with falciparum malaria. Pre-antibiotic blood cultures were not performed in all our patients, and hence it is possible that as in falciparum malaria [64], [65], concurrent bacteremia may have contributed to the clinical features of some patients with severe vivax malaria. However, the much higher biomass in patients with severe compared to non-severe vivax cannot have been accounted for by concurrent bacteremia, or the parasitemia - or biomass-related associations we found. Finally, while we demonstrated increased endothelial activation among patients with vivax malaria in proportion to disease severity [34], the causes of WPB exocytosis in vivax malaria were not evaluated and require further study.
In summary, peripheral parasitemia underestimates total parasite biomass in vivax malaria, particularly in severe disease, suggesting tissue accumulation of a hidden biomass of P. vivax-infected RBCs. While histological studies are needed to confirm this, we propose that a hidden P. vivax biomass is capable of mediating systemic inflammation which may contribute to organ dysfunction. The correlation of markers of total P. vivax biomass with systemic inflammation but not with markers of endothelial activation is consistent with the hypothesis that accumulation of P. vivax-infected red cells may occur in parts of the circulation devoid of endothelium, such as the open circulation of the spleen or extravascular bone marrow. In contrast only peripheral parasitemia was associated with endothelial activation and the Weibel Palade Body products Ang-2 and VWF. The association between clinical severity and endothelial activation, ADAMTS13 deficiency, thrombocytopenia, and impaired microvascular function suggests that thrombotic microangiopathy, systemic inflammation and microvascular dysfunction may contribute to pathogenesis of disease in vivax malaria.
Methods
Study site and patients
This study was conducted in Sabah, Malaysia, a region currently in the pre-elimination phase of malaria control and where P. vivax endemicity is low [66], [67]. Patients were enrolled as part of a prospective clinical and epidemiological study of all malaria patients admitted to Queen Elizabeth Hospital, an adult tertiary referral hospital [35]. Consecutive patients with PCR-confirmed vivax or falciparum monoinfection were enrolled from September 2010 – October 2012 (with non-severe falciparum malaria patients included up until October 2011) if they were non-pregnant, ≥12 years old, had no major comorbidities or concurrent illness, were within 18 hours of commencing antimalarial treatment, had haemoglobin >7.0 g/dL, and had not been previously enrolled in the study. Clinical details of patients enrolled from September 2010 – October 2011, in addition to details regarding excluded patients, have been previously reported [35]. Severe malaria was defined as the presence of ≥1 of: unrousable coma (Glasgow Coma Scale score <11); multiple ( >2) convulsions; respiratory distress (respiratory rate >30 breaths per minute and oxygen saturation <94%); hypotension (systolic blood pressure ≤80 mm Hg); jaundice (bilirubin >43 µmol/L plus parasitemia >20 000/µL [P. vivax] or >100,000 [P. falciparum] and/or creatinine >132 µmol/L); significant abnormal bleeding; hypoglycaemia (blood glucose <2.2 mmol/L); metabolic acidosis (bicarbonate <15 mmol/L or lactate >4 mmol/L); acute kidney injury (AKI; creatinine >265 µmol/L); hyperparasitemia (P. falciparum parasitemia >10%). Healthy controls were visitors or relatives of malaria patients, with no history of fever in the past 48 hours and with blood film negative for malaria parasites.
Standardized history and physical examination were documented. Haematology, biochemistry, acid-base parameters, and lactate (by bedside blood analysis; iSTAT system) were obtained on admission. Parasite counts were determined by microscopy, and parasite species were identified by PCR [68], [69]. Patients were treated according to hospital guidelines, as previously described [35].
Endothelial activation, WPB release, ADAMTS13 and cytokines
Venous blood collection in lithium heparin was centrifuged within 30 minutes of collection and plasma was stored at −70°C. Plasma concentrations of the endothelial activation markers ICAM-1, E-selectin, and Ang-2 were measured using ELISA (R&D Systems), and IL-6 and IL-10 were measured by flow cytometry (BD Cytometric Bead Array). Antigen concentrations of vWF and the vWF-cleaving enzyme, ADAMTS13, were measured in platelet poor plasma using ELISA (America Diagnostica), and ADAMTS 13 activity by fluorescence resonance energy transfer (FRET), as previously described [70].
Parasitemia and markers of total parasite biomass
Peripheral blood parasitemia was quantitated per 200 white blood cells by microscopy and expressed as parasites/µL based on automated white cell count. Parasite stage distribution was quantitated on microscopy of peripheral blood. Plasma HRP2 (for P. falciparum) [32], genus-specific pLDH (for P. falciparum and P. vivax) and PvLDH (for P. vivax) were measured by ELISA [28] as proxies for total parasite biomass. Ratios of plasma pLDH and PvLDH to peripheral parasite density were expressed as ngs/1000 peripheral parasites, with the denominator log-transformed.
PvLDH production in vitro
To confirm evidence of PvLDH secretion across the parasite life-cycle, four cryopreserved P. vivax isolates with a high proportion of ring-stages were thawed and cultured ex-vivo as previously described [71], over 48–54 hours. Starting parasitemias were 3623/µL (98% rings), 10,605/µL (98% rings), 19,342/µL (65% rings), and 24,680/µL (98% rings). Thick and thin films were prepared at serial time points for stage differential and culture supernatant sampled for concentration of PvLDH. A progressive increase in concentration of PvLDH in culture supernatant across the parasite life-cycle was demonstrated (Text S1), indicating secretion of PvLDH by all parasite stages. In vitro parasitemia remained stable throughout the culture duration.
Microvascular function
Microvascular function was assessed using Near Infrared Spectroscopy (InSpectra 650, Hutchinson Technology, Hutchinson, MN) which uses a probe applied to the thenar eminence to noninvasively measure microcirculatory oxygenation (tissue oxygen saturation; StO2) before and after an ischaemic stress, as previously described [13]. To induce an ischaemic stress, a vascular cuff was inflated to 200 mm Hg for 5 minutes and then rapidly deflated. Microvascular function was defined as the gradient of the StO2 recovery slope from release of the vascular cuff until StO2 had reached 85% of the baseline value.
Statistical methods
Statistical analysis was performed with STATA software (version 10.1; Statacorp, College Station, TX, USA). For continuous variables intergroup differences were initially compared using analysis of variance or Kruskal-Wallis tests depending on distribution. Student's T-test or Mann-Whitney tests were used for post-hoc pair-wise comparisons. Categorical variables were compared using χ2 or Fisher's exact test. Associations between continuous variables were assessed using Spearman's (ρ) or Peason's (r) correlation coefficients, depending on distribution.
Ethics statement
The study was approved by the Ethics Committees of the Malaysian Ministry of Health and Menzies School of Health Research. Informed written consent was provided by all participating adults, and by the parent or guardian of any participant aged <18 years.
Supporting Information
Zdroje
1. PriceRN, DouglasNM, AnsteyNM (2009) New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis 22 : 430.
2. AnsteyNM, DouglasNM, PoespoprodjoJR, PriceR (2012) Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol 80 : 151–201.
3. BairdJK (2013) Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin Microbiol Rev 26 : 36–57.
4. LacerdaMVG, FragosoSCP, AlecrimMGC, AlexandreMAA, MagalhãesBML, et al. (2012) Postmortem characterization of patients with clinical diagnosis of Plasmodium vivax malaria: to what extent does this parasite kill? Clin Infect Dis. 55: e67–74.
5. TjitraE, AnsteyNM, SugiartoP, WarikarN, KenangalemE, et al. (2008) Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5: e128.
6. KocharDK, DasA, KocharSK, SaxenaV, SirohiP, et al. (2009) Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg 80 : 194–198.
7. BarcusMJ, BasriH, PicarimaH, ManyakoriC, ElyazarI, et al. (2007) Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg 77 : 984–991.
8. LançaEFC, MagalhãesBML, Vitor-SilvaS, SiqueiraAM, BenzecrySG, et al. (2012) Risk factors and characterization of Plasmodium vivax-associated admissions to pediatric intensive care units in the Brazilian Amazon. PLoS ONE 7: e35406.
9. AndradeBB, Reis-FilhoA, Souza-NetoSM, ClarêncioJ, CamargoL, et al. (2010) Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J 9 : 13.
10. Douglas NM, Pontororing G, Lampah D, Yeo T, Kenangalem E, et al.. (2014) Mortality attributable to Plasmodium vivax malaria: a clinical audit from Papua, Indonesia. BMC Med In Press.
11. TurnerGDH, MorrisonH, JonesM, DavisTME, LooareesuwanS, et al. (1994) An immunohistochemical study of the pathology of fatal malaria: evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 145 : 1057.
12. DondorpA, InceC, CharunwatthanaP, HansonJ, Van KuijenA, et al. (2008) Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis 197 : 79–84.
13. YeoTW, LampahDA, KenangalemE, TjitraE, PriceRN, et al. (2013) Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis 207 : 528–536.
14. MacPhersonG, WarrellM, WhiteN, LooareesuwanS, WarrellD (1985) Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 119 : 385.
15. MarshK, ForsterD, WaruiruC, MwangiI, WinstanleyM, et al. (1995) Indicators of life-threatening malaria in African children. N Engl J Med 332 : 1399–1404.
16. JaffarS, Van HensbroekMB, PalmerA, SchneiderG, GreenwoodB (1997) Predictors of a fatal outcome following childhood cerebral malaria. Am J Trop Med Hyg 57 : 20–24.
17. RubachMP, MukembaJ, FlorenceS, JohnB, CrookstonB, et al. (2012) Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS ONE 7: e35985.
18. FoxLL, TaylorTE, PensuloP, LiombaA, MpakizaA, et al. (2013) HRP2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J Infect Dis 208 : 500–503.
19. HendriksenIC, Mwanga-AmumpaireJ, von SeidleinL, MtoveG, WhiteLJ, et al. (2012) Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 measurement. PLoS Med 9: e1001297.
20. CunningtonA, BretscherM, NogaroS, RileyE, WaltherM (2013) Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. J Infect 67 : 220–230.
21. DondorpAM, DesakornV, PongtavornpinyoW, SahassanandaD, SilamutK, et al. (2005) Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2: e204.
22. YeoT, LampahD, TjitraE, GitawatiR, DarcyC, et al. (2010) Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog 6 : 214–217.
23. CarvalhoBO, LopesSC, NogueiraPA, OrlandiPP, BargieriDY, et al. (2010) On the cytoadhesion of Plasmodium vivax–infected erythrocytes. J Infect Dis 202 : 638–647.
24. ValechaN, PintoRGW, TurnerGDH, KumarA, RodriguesS, et al. (2009) Histopathology of fatal respiratory distress caused by Plasmodium vivax malaria. Am J Trop Med Hyg 81 : 758–762.
25. World Health Organization (2013) World Malaria Report 2013. Geneva: World Health Organization.
26. Lopes SC, Albrecht L, Carvalho BO, Siqueira AM, Thomson-Luque R, et al.. (2014) Paucity of Plasmodium vivax mature schizonts in peripheral blood is associated with their increased cytoadhesive potential. J Infect Dis: 1403–1407.
27. Field JW, Shute PG (1956) The Microscopic Diagnosis of Human Malaria. Vol. 2: A Morphological Study of the Erythrocytic Parasites. Kuala Lumpur: Institute for Medical Research.
28. JangJW, ChoCH, HanET, AnSS, LimCS (2013) pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test. Malar J 12 : 181.
29. DruilheP, BrasseurP, BlancC, MaklerM (2007) Improved assessment of Plasmodium vivax response to antimalarial drugs by a colorimetric double-site plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob Agents Chemother 51 : 2112–2116.
30. DesakornV, DondorpAM, SilamutK, PongtavornpinyoW, SahassanandaD, et al. (2005) Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg 99 : 517–524.
31. BascoLK, MarquetF, MaklerMM, LebrasJ (1995) Plasmodium falciparum and Plasmodium vivax: Lactate-Dehydrogenase Activity and Its Application for in Vitro Drug Susceptibility Assay. Exp Parasitol 80 : 260–271.
32. YeoTW, LampahDA, GitawatiR, TjitraE, KenangalemE, et al. (2008) Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proceedings of the National Academy of Sciences 105 : 17097–17102.
33. FiedlerU, ReissY, ScharpfeneckerM, GrunowV, KoidlS, et al. (2006) Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med 12 : 235–239.
34. YeoTW, LampahDA, TjitraE, PieraK, GitawatiR, et al. (2010) Greater endothelial activation, Weibel-Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: a prospective study in Papua, Indonesia. J Infect Dis 202 : 109–112.
35. BarberBE, WilliamT, GriggMJ, MenonJ, AuburnS, et al. (2013) A prospective comparative study of knowlesi, falciparum and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and P. vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis 56 : 383–397.
36. KerlinDH, BoyceK, MarfurtJ, SimpsonJA, KenangalemE, et al. (2012) An analytical method for assessing stage-specific drug activity in Plasmodium vivax malaria: implications for ex vivo drug susceptibility testing. PLoS Negl Trop Dis 6: e1772.
37. BernardoA, BallC, NolascoL, MoakeJF, DongJ-f (2004) Effects of inflammatory cytokines on the release and cleavage of the endothelial cell–derived ultralarge von Willebrand factor multimers under flow. Blood 104 : 100–106.
38. ImirzaliogluC, SoydanN, SchallerM, BretzelRG, ChakrabortyT, et al. (2006) Diagnosis of mixed Plasmodium malariae and P. vivax infection in a development aid volunteer by examination of bone-marrow specimens by real-time PCR. J Clin Microbiol 44 : 2307–2310.
39. O'DonnellJ, GoldmanJ, WagnerK, EhingerG, MartinN, et al. (1998) Donor-derived Plasmodium vivax infection following volunteer unrelated bone marrow transplantation. Bone Marrow Transplant 21 : 313.
40. PanichakulT, SattabongkotJ, ChotivanichK, SirichaisinthopJ, CuiL, et al. (2007) Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol 37 : 1551–1557.
41. RuY-X, MaoB-Y, ZhangF-k, PangT-x, ZhaoS-x, et al. (2009) Invasion of erythroblasts by Plasmodium vivax: a new mechanism contributing to malarial anemia. Ultrastruct Pathol 33 : 236–242.
42. YoeliM (1948) Non-pigmented malaria parasites in the bone marrow from a mixed infection of Leishmania and Plasmodium vivax. Trans R Soc Trop Med Hyg 42 : 99–IN95.
43. RaghunandanJ, RajeshwariK, DubeyA, SinghT (2012) Peripheral gangrene in an 18-month-old boy with Plasmodium vivax malaria. Paediatrics and International Child Health 32 : 164–166.
44. SiqueiraAM, MagalhaesBML, MeloGC, FerrerM, CastilloP, et al. (2012) Spleen rupture in a case of untreated Plasmodium vivax infection. PLoS Negl Trop Dis 6: e1934.
45. Hehir P (1927) Malaria in India. London: Oxford University Press.
46. SuwanaruskR, CookeBM, DondorpAM, SilamutK, SattabongkotJ, et al. (2004) The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J Infect Dis 189 : 190–194.
47. HandayaniS, ChiuDT, TjitraE, KuoJS, LampahD, et al. (2009) High deformability of Plasmodium vivax-infected red blood cells under microfluidic conditions. J Infect Dis 199 : 445–450.
48. BuffetPA, SafeukuiI, DeplaineG, BrousseV, PrendkiV, et al. (2011) The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117 : 381–392.
49. Tablin F, Chamberlain JK, Weiss L (2002) The microanatomy of the mammalian spleen. In: Bowdler AJ, editor. The Complete Spleen. New Jersey: Humana Press. pp.11–22.
50. WeissL (1991) Barrier cells in the spleen. Immunol Today 12 : 24.
51. GroomA, SchmidtE, MacDonaldI (1991) Microcirculatory pathways and blood flow in spleen: new insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc 5 : 159.
52. SongS, GroomA (1971) Storage of blood cells in spleen of the cat. American Journal of Physiology—Legacy Content 220 : 779–784.
53. del PortilloHA, LanzerM, Rodriguez-MalagaS, ZavalaF, Fernandez-BecerraC (2004) Variant genes and the spleen in Plasmodium vivax malaria. Int J Parasitol 34 : 1547–1554.
54. JainV, LucchiNW, WilsonNO, BlackstockAJ, NagpalAC, et al. (2011) Plasma levels of angiopoietin-1 and-2 predict cerebral malaria outcome in Central India. Malar J 10 : 383.
55. PrapansilpP, MedanaI, MaiNTH, DayNP, PhuNH, et al. (2013) A clinicopathological correlation of the expression of the angiopoietin-Tie-2 receptor pathway in the brain of adults with Plasmodium falciparum malaria. Malar J 12 : 1–15.
56. ConroyAL, GloverSJ, HawkesM, ErdmanLK, SeydelKB, et al. (2012) Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study. Crit Care Med 40 : 952.
57. ErdmanLK, DhabangiA, MusokeC, ConroyAL, HawkesM, et al. (2011) Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS ONE 6: e17440.
58. RazaA, GhanchiNK, Sarwar ZubairiA, RaheemA, NizamiS, et al. (2013) Tumour necrosis factor alpha, interleukin-10, intercellular and vascular adhesion molecules are possible biomarkers of disease severity in complicated Plasmodium vivax isolates from Pakistan. PLoS ONE 8: e81363.
59. de MastQ, GrootE, AsihPB, SyafruddinD, OostingM, et al. (2009) ADAMTS13 deficiency with elevated levels of ultra-large and active von Willebrand factor in P. falciparum and P. vivax malaria. Am J Trop Med Hyg 80 : 492.
60. Sinha A, Singh G, Bhat AS, Mohapatra S, Gulati A, et al.. (2013) Thrombotic microangiopathy and acute kidney injury following vivax malaria. Clinical and Experimental Nephrology: 1–7.
61. SaharanS, KohliU, LodhaR, SharmaA, BaggaA (2009) Thrombotic microangiopathy associated with Plasmodium vivax malaria. Pediatr Nephrol 24 : 623–624.
62. KarunaweeraND, GrauG, GamageP, CarterR, MendisKN (1992) Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proceedings of the National Academy of Sciences 89 : 3200–3203.
63. Coatney GR, Collins WE, Warren MW, Contacos PG (1971) The primate malarias. Washington: US Government Printing Office.
64. WereT, DavenportGC, HittnerJB, OumaC, VululeJM, et al. (2011) Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol 49 : 671.
65. BerkleyJ, MwarumbaS, BramhamK, LoweB, MarshK (1999) Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg 93 : 283–286.
66. AbdullahNR, BarberBE, WilliamT, NorahmadNA, SatsuUR, et al. (2013) Plasmodium vivax population structure and transmission dynamics in Sabah, Malaysia. PLoS ONE 8: e82553.
67. WilliamT, JelipJ, MenonJ, AnderiosF, MohammadR, et al. (2014) Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J 13 : 390.
68. PadleyD, MoodyA, ChiodiniP, SaldanhaJ (2003) Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol 97 : 131–137.
69. ImwongM, TanomsingN, PukrittayakameeS, DayNPJ, WhiteNJ, et al. (2009) Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol 47 : 4173.
70. KokameK, NobeY, KokuboY, OkayamaA, MiyataT (2005) FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol 129 : 93–100.
71. RussellB, ChalfeinF, PrasetyoriniB, KenangalemE, PieraK, et al. (2008) Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother 52 : 1040–1045.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



