-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
The flagellum organelle is an intricate multiprotein assembly best known for its rotational propulsion of bacteria. However, recent studies have expanded our knowledge of other functions in pathogenic contexts, particularly adherence and immune modulation, e.g., for Salmonella enterica, Campylobacter jejuni, Pseudomonas aeruginosa, and Escherichia coli. Flagella-mediated adherence is important in host colonisation for several plant and animal pathogens, but the specific interactions that promote flagella binding to such diverse host tissues has remained elusive. Recent work has shown that the organelles act like probes that find favourable surface topologies to initiate binding. An emerging theme is that more general properties, such as ionic charge of repetitive binding epitopes and rotational force, allow interactions with plasma membrane components. At the same time, flagellin monomers are important inducers of plant and animal innate immunity: variation in their recognition impacts the course and outcome of infections in hosts from both kingdoms. Bacteria have evolved different strategies to evade or even promote this specific recognition, with some important differences shown for phytopathogens. These studies have provided a wider appreciation of the functions of bacterial flagella in the context of both plant and animal reservoirs.
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004483
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1004483Summary
The flagellum organelle is an intricate multiprotein assembly best known for its rotational propulsion of bacteria. However, recent studies have expanded our knowledge of other functions in pathogenic contexts, particularly adherence and immune modulation, e.g., for Salmonella enterica, Campylobacter jejuni, Pseudomonas aeruginosa, and Escherichia coli. Flagella-mediated adherence is important in host colonisation for several plant and animal pathogens, but the specific interactions that promote flagella binding to such diverse host tissues has remained elusive. Recent work has shown that the organelles act like probes that find favourable surface topologies to initiate binding. An emerging theme is that more general properties, such as ionic charge of repetitive binding epitopes and rotational force, allow interactions with plasma membrane components. At the same time, flagellin monomers are important inducers of plant and animal innate immunity: variation in their recognition impacts the course and outcome of infections in hosts from both kingdoms. Bacteria have evolved different strategies to evade or even promote this specific recognition, with some important differences shown for phytopathogens. These studies have provided a wider appreciation of the functions of bacterial flagella in the context of both plant and animal reservoirs.
Introduction
The prokaryotic flagellum is best known as a motility organelle responsible for bacterial movement and necessary for chemotaxis [1]. An extraordinary multisubunit organelle, complex in its regulation and assembly, the flagellum has been the subject of extensive research over the past four decades and a central topic of evolutionary debate [2]. Ongoing research is still revealing surprises in various aspects, from assembly to function [3,4].
A switch in lifecycle from sessile to motile, e.g., exiting biofilms or established microcolonies in response to depletion in nutrients, effectively deems individual flagellate cells as pioneers, in search of more favourable environments. Often this means exploration of new hosts, habitats or niches. In this respect, flagella can be considered an early stage colonisation factor. Flagella from a variety of bacteria have been shown to bind to a diverse array of animal and plant substrates, and this review focuses on recent advances in our understanding of how flagella impact host-microbe interactions. The flagellum filament, attached to a transmembrane motor complex, is a long helical structure made up of hundreds of subunits of the flagellin protein, encoded by fliC (or homologues). The copy number and location on the bacterial cell surface varies between species. Here, adherence is described for intact flagella, while aspects of recognition and evasion relate to the flagellin protein. Polymorphisms in flagellin has provided a mechanism of sub-species differentiation, based on the “H” (Hauch) antigen type.
Twist and Stick: Interactions with Host Tissues
A role for flagella-mediated adherence has been demonstrated in many different plant species and animal infection models, for both pathogenic and opportunistic bacteria [4,5]. These results reveal a significant role for flagella during colonisation and, consequently, environmental transmission. Other factors also facilitate adherence, including electrostatic charge, or specific fimbrial-mediated interactions that may occur at subsequent stages and confer tissue tropism.
Initial flagella interactions drive formation of bacterial biofilms or microcolony communities and maintain their structure along colony surfaces through physical interactions [6]. Additionally, and perhaps surprisingly, these organelles can act as probes of uneven surfaces during biofilm formation, attaching within crevices [7]. Recent work demonstrated that individual cells, and consequently the growing colony, effectively become tethered to artificial surfaces that resemble microvilli; a scenario that is very likely to hold true for interactions with true biotic surfaces [7].
There is a large body of published work for flagella-mediated adherence, yet there are very few examples of specific interactions where both flagella and host determinants have been formally dissected. Instead, there is an emerging theme of nonspecific interactions, which has been challenging to investigate and likely relates to the biophysical properties of flagella (Fig. 1). Firstly, flagella organelles are long filaments that can reach up to 20 μm from the bacterial cell surface (Fig. 1A). It is therefore logical that flagella can be exploited as adhesive scaffolds and are involved in initial probing of surfaces as an early colonisation factor. Secondly, its motor can spin flagella filaments at speeds in excess of 15,000 rpm (Fig. 1B), which not only increases the chances of the filament coming into contact with surfaces, but also ensures it does so with force [8]. This is consistent with evidence that some flagella aren’t adhesive, but are involved in cellular binding and invasion in processes distinct from providing niche proximity through propulsion. In the absence of specific protein receptors, observation of intercalation and penetration into plant and animal membrane lipid layers by flagella could also be explained by this phenomenon. Thirdly, the flagellum filament is a polymeric structure, comprised of repeating epitopes of one or more flagellin types (Fig. 1C). Repeating epitopes are high avidity by definition: low affinity ionic interactions can be consolidated, amplified, and relevant if the binding substrate is also repetitive. With very few exceptions (innate immune receptors being the most notable), published examples of “specific” flagella binding interactions are with factors that are repetitive, such as polymeric proteins, proteoglycans, glycolipids, and phospholipids. Flagella therefore appear to be a tool with general properties that can be adapted to pathogenic colonisation of a diverse range of niches across plant and animal kingdoms.
Fig. 1. The biophysical properties of flagella, to “twist and stick,” lend themselves towards nonspecific adhesion. 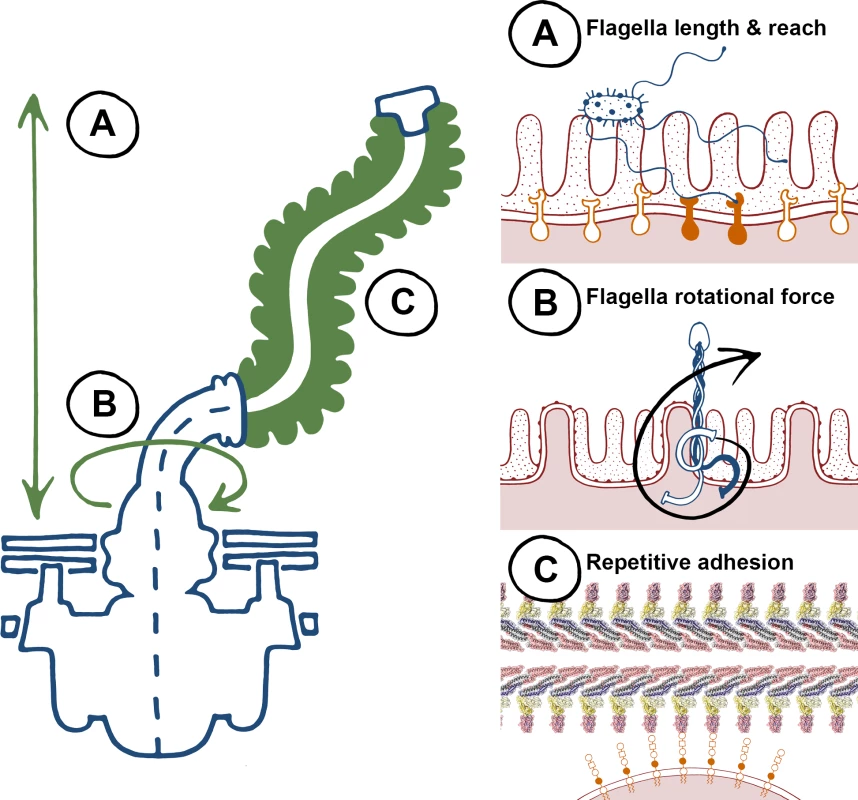
Left, a summary of key characteristics of the flagella apparatus that are advantageous for adherence, and right, specific properties highlighted on the left. (A) Flagellum length results in a long reach towards colonization surfaces as an early-stage anchor—higher affinity binding can occur at closer proximity with specific adhesins and receptors. (B) Flagella rotation generates force that promotes membrane interactions during initial adherence. (C) Flagella are highly repetitive structures, non-specific low affinity binding can result in adhesion at high avidities. Adhesion to plant tissues
Flagella-mediated adherence to plant tissues has previously been described for phytobacteria, such as Pseudomonas syringae to bean seedlings [9] and nitrogen-fixing bacteria Azospirillum brasilense to wheat roots [10]. Fresh vegetables or fruits are now recognised as secondary hosts involved in the transmission of human pathogenic bacteria through the food chain [11,12]. Flagella play a role in adherence to plants for multiple human pathogens, including Salmonella enterica, pathogenic Escherichia coli and Listeria monocytogenes. Attachment of S. enterica to basil leaf epidermis appears to be serovar specific, such that flagella mutants of serovar Senftenberg were significantly reduced in attachment compared to an isogenic parent, whereas S. enterica serovar Typhimurium (S. Typhimurium) mutants were not, presumably because of other adherence factors [13]. L. monocytogenes flagella adhere to sprouted seeds of alfalfa, broccoli, and radish [14]. Interestingly, a motility deficient mutant (motAB) was not deleteriously affected in adherence compared to the parental strain, demonstrating that flagella-mediated binding did not require chemotaxis or flagella rotation. Chemotaxis towards and tropism for stomatal guard cells has been reported for S. Typhimurium on lettuce leaves [15] and enteroaggregative E. coli (EAEC) on rocket leaves, respectively, although mutation of EAEC fliC did not reduced adherence to epidermal leaf cells [13]. Flagella expressed by enterohaemorrhagic E. coli (EHEC) O157:H7 and enterotoxigenic E. coli (ETEC) mediate attachment to rocket, spinach, lettuce, and basil leaves [16,17]. The requirement of flagella for systemic spread of S. enterica or EHEC from root to shoot of Arabidopsis thaliana plants is likely to relate more to motility and dissemination rather than to adhesion [18].
Adhesion to mammalian tissues
The role of flagella in bacterial adherence to mammalian hosts has been demonstrated for various bacterial species in a number of hosts [5]. Dissection of the different contributions of flagella for motility, adherence, and invasion is complex. This is exemplified by studies with Pseudomonas aeruginosa, where flagella-dependent uptake by macrophages occurs, despite only a marginal role for flagella-mediated binding to the cells [19]. In motile L. monocytogenes, flagella were required for optimal adherence and invasion of CaCo-2 cells, even after centrifugation onto monolayers, although the flagella alone were not adhesive [20]. This suggests that in this case rotational force was necessary for adherence and invasion, distinct from bacterial propulsion.
Flagella binding can be host tissue-specific for particular flagellar serotypes. Flagella from attaching and effacing bacteria EHEC O157:H7 and enteropathogenic E. coli (EPEC) O127:H6 have both been shown to be involved in the adherence to porcine gastric mucins and bovine primary intestinal epithelial cells and explants [21,22]. However, H7 serotype flagella appear to have a particular tropism for rectal epithelial cells. Complementation of an E. coli O157:H7 fliC mutant with fliCH7 restored adherence in this tissue to wild-type levels, whereas no significant effect was seen for the mutant complemented with an alternative fliC type (H6). An earlier study reported that while purified H2 and H6 flagella were adhesive to HeLa cells, H7 flagella was not [23], illustrating the importance of using relevant cell lines for infection models.
For some species, secretion of nonflagellin proteins occurs via the flagella apparatus, similar to that of the nonflagellar T3SS. For example, flagella-mediated secretion has been demonstrated for Campylobacter jejuni FlaC, an adhesin that facilitates binding to Hep-2 cells [24].
Interaction with mucus
Epithelial mucosal tissues act as a barrier between the animal host and its environment. Epithelial barrier cells are often coated by a layer of high viscosity mucus under a low viscosity mucus layer, both composed of water and a plethora of defensive compounds such as mucins. Mucins are highly glycosylated glycoproteins mainly composed of O-linked oligosaccharides, acidic monosaccharides with sialic acid, or modifications of other components, such as sulphate groups.
Flagellar-mediated motility through (animal) mucus can be a prerequisite for successful mucosal colonisation [25]. Helicobacter pylori swims through the mucus barrier, burrowing deep in the gastric mucus layer to escape the acidity of the stomach [26–28]. Yet, although Helicobacter flagella may be required for colonisation, there is no evidence of specific attachment of flagella to epithelial cells [29]. Indeed, mucus can repress motility, as with EHEC, ETEC, and S. Typhimurium, where incubation with mucus results in a down-regulation of flagella-associated genes or reduced swarming motility [30–32]. For Vibrio cholerae, passage through mucus can trap flagella, causing them to break and release the anti σ28-factor FlgM. This derepresses fliA, which encodes σ28, allowing optimal expression of late-class flagella genes, perhaps to replace the broken flagellum. Additionally, σ28 represses HapR, part of a quorum sensing network in V. cholera, allowing expression of genes required for toxin production [33]. This is an elegant mechanism of sequential and appropriate expression that occurs where expression of a single flagellum is needed and linked to discrete points of the infection cycle.
There are various reports of interactions between flagella and mucus. The opportunistic pathogen Stenotrophomonas maltophilia has been described to bind mouse tracheal mucus via its flagella [34]. Similarly, H6 and H7 flagellins from EPEC and EHEC, respectively, adhere to bovine mucus [35]. A certain FliD cap protein type (B-type) of P. aeruginosa strains binds to respiratory-derived mucins via the Lewis X determinants [36,37]. Probiotic E. coli strain Nissle flagella adhere to human intestinal mucus through gluconate [38], although gluconate is not directly associated with gastric nor intestinal human mucins [39,40]. It is possible that resident microbial communities degrade complex host mucus glycans and release gluconate into the mucus layer, providing a gluconate “decoy” as a means of competition [41,42]. In contrast, there are relatively few reports showing direct interactions of bacterial components with plant mucilage [43,44] and none as yet for flagella, although one could conceive the potential for such interactions. Mucilage secreted by plant root tip cells, to facilitate expansion of the growing root and provide protection against many microbes, is functionally but not structurally analogous to mucus [45].
Molecular targeting
While there are many studies demonstrating a role for flagella in adherence and colonisation of tissues, few have identified or characterised specific targets used by the flagella for binding. In animal cell models, P. aeruginosa flagella can recognise the basolateral surface of polarized lung epithelial cells through heparan sulphate, a highly sulphated proteoglycan [46]. A recent study supports a new role for surfactant protein A in binding and enhancing the clearance of P. aeruginosa flagellin, mediated in part by enhanced IL-1β production [47]. Furthermore, the P. aeruginosa flagellum has been described to bind glycolipids monosialoganglioside (GM) or disialoganglioside (GD), playing a role in the pulmonary infection where flagellin binding to GM1 was greater than to asialoGM1 [48]. In contrast, flagella from E. coli O113:H21 did not contribute to adherence, but did contribute to invasion via asialoGM1 [49].
Flagella-mediated adherence does not have to be direct; flagella can act as extended molecular scaffolds for other secreted adhesins. EtpA, a two-partner secretion exoprotein adhesin from ETEC, interacts with the conserved region of flagellin, by accessing the uncapped flagellum tips. This “bridge” allows the indirect adhesion of the flagella to intestinal mucosal tissues [50].
Recent work has highlighted the importance of membrane interactions in flagella-mediated adherence to plant cells. H6, H7, and H48 flagellins from E. coli were shown to bind algae sulphated polysaccharides and membrane phospholipids in an ionic charge-dependent manner. Specificity for phospholipid recognition allows flagella to intercalate into plant plasma membranes [51], in a manner that may be similar to the flexible, elongated type-III secretion system of phytopathogenic bacteria. The underlying mechanism is likely to be conserved with other eukaryotic hosts and thus a generic method of host–microbe interaction. As demonstrated with L. monocytogenes [20], the rotational force of the flagella may promote interactions and aid penetration of plant cell walls. Flagella rotation is also important for S. Typhimurium interactions with HeLa cells in a process called “near-surface swimming” [52].
Dodge: Immune Recognition
Flagellar-mediated host interactions incur a cost, as conserved regions in flagellin monomers are potent inducers of innate immune responses in vivo, across kingdoms. Consequently, recognition of flagellin leads to the greater clearance of flagellated versus nonflagellated strains [20,53–55]. Therefore, there are many immunomodulatory strategies, the simplest being alteration of flagella production and selection of bacteria that are less flagellate upon host-cell contact [56].
Structural recognition
The flagellin monomer is organised into four connected domains designated D0, D1, D2, and D3 (Fig. 2) [57]. To form these domains, flagellin peptides fold back on themselves, like an elaborate hairpin, with the termini associated with one another. The D2–D3 domains are highly variable and generate the antigenic diversity described as H-serotypes [58–60]. The termini that form the D0–D1 domains are well conserved across all bacterial flagellins. Both N - and C-termini are rich in hydrophobic residues, which form coiled–coil interfaces that allow them to associate with one another; these interfaces are also required for filament polymerisation [3,61,62]. Importantly, the flagellin needs to be disassociated to make D0–D1 accessible to innate immune receptors, in contrast to the polymeric structure involved in binding interactions. Flagellin recognition occurs in plant cells [63] and animal cells of both invertebrates [64] and vertebrates [65]. The receptors for these conserved regions are Toll-like receptor 5 (TLR5) for extracellular flagellin [66–68] and NAIP5-NLRC4 for intracellular flagellin [69] in vivo, and Flagellin sensitive 2 (FLS2) receptor for flagellin in planta (Fig. 2) [70].
Fig. 2. Cross-kingdom immune recognition of flagellin structures. 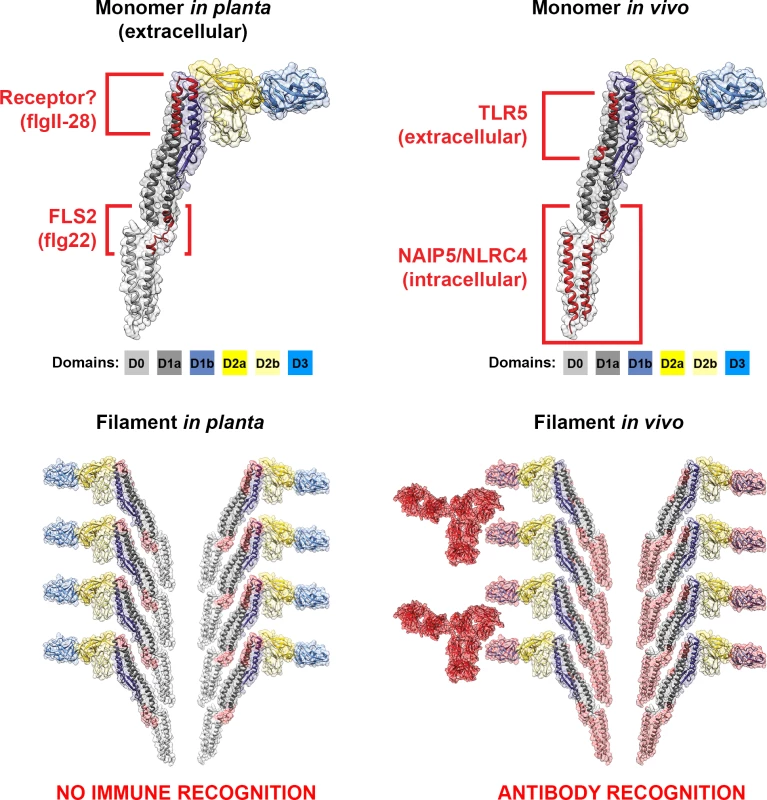
Top: backbone of the key residues of flagellin recognized by plant (left) and animal (right) innate immune receptors are highlighted in red. FliC from S. enterica is presented as a “model” flagellin, with reports for recognition by both TLR5 and FLS2 receptors. These residues are superimposed on the solved flagellin structure (PDB# 1UCU) in UCSF Chimera [113]. Surfaces and backbone are coloured according to previously assigned structural domains as indicated below each monomer [57]. Bottom: recognition of flagella filaments by plant (left) and animal (right) innate immune receptors does not occur as key residues (surfaces highlighted in red) are hidden within the filament structure. However, immune recognition still occurs in animals via antibody recognition of the D3 domain. Plant and mammal cell membrane receptors share the same architecture of extracellular leucine-rich repeats (LRR) and cytoplasmic association with a conserved family of serine-threonine kinases, although the LRR domains of those two receptors are very divergent [71]. Immune recognition of flagellin is based on highly conserved motifs that span diverse bacterial species (Fig. 2). TLR5 interacts with two short flagellin peptides from both termini (LQRIRELAVQ and LGAIQN) [67,72]. The D1 domain makes a substantial contribution to both high-affinity binding and TLR5 signalling, whereas D0 contributes to TLR5 signalling, but has little or no effect on binding [73]. In plants, FLS2 recognises a conserved N-terminal 22 amino acid peptide in the D0 domain (QRLSTGSRINSAKDDAAGLQIA), termed flg22, derived from P. syringae pv. tabaci [74,75]. A newly described flagellin-derived MAMP, flgII-28 (located in the D1 domain, Fig. 2), has been shown to be recognized independently of FLS2 by tomato and other solanaceous plants, but not by Arabidopsis [76,77].
Flagellin can also be recognised intracellularly in animal cells through another pathway involving receptors from the NOD-like receptor (NLR) family of intracellular pattern recognition or signalling molecules. The NLR apoptosis-inhibitory protein-5 (NAIP5), binds to 35 amino acids from the C-terminus and 52 amino acids from the N-terminus conserved regions of flagellin [78,79]. Unlike mammalian cells, plant cells do not have a cytoplasmic receptor to detect flagellin, as demonstrated by artificial delivery of phytopathogenic bacterial flagellin into the cytosol [80]. This feature may have evolved to prevent exploitation by necrotrophic pathogens that gain from death-associated immune responses.
Immune evasion by flagella
Since flagellin is such an important immunogen, bacteria have evolved multiple strategies to avoid or evade recognition (Fig. 3). Some pathogens enocode multiple flagellin types, which may relate to evasion or even niche versatility [81]. The majority of S. enterica encode two flagellin types (phase 1 & 2), under phase variable control of expression [81–84]. A third flagellin gene, flpA, has also been described for a particular S. enterica isolate [84]. However, control of expression may be of greater importance in evasion. Nonflagellate mutants out-competed flagellated EHEC O157:H7 colonisation in cattle [85], and nonflagellated strains of plant pathogens Xanthomonas fuscans subsp. fuscans are isolated from natural epidemics of plant disease [86]. Furthermore, attenuation of infection occurred when phase 2 flagella were constitutively expressed in S. Tyhimurium, following oral or intravenous inoculation of mice were in the mouse model of infection [87].
Fig. 3. A variety of mechanisms employed to “dodge” the flagellin innate immune response. 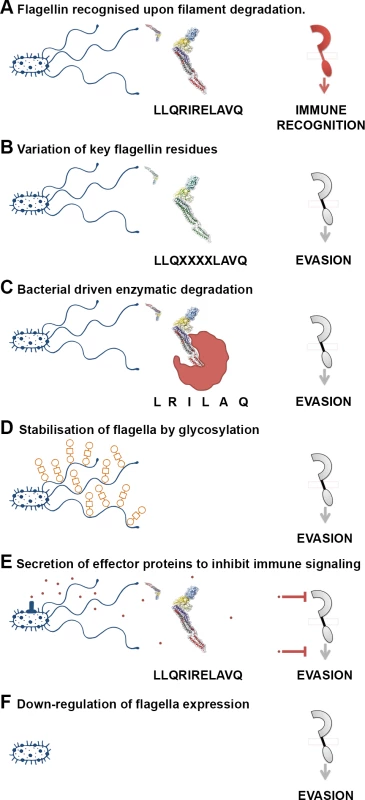
(A) Flagella filaments degrade, releasing monomeric flagellin, the residues of which are recognised by receptor TLR5, NLRC4, or FLS2, resulting in cytokine release or PTI. The example residues and receptor (right) shown are involved in TLR5 recognition. (B) Flagellin recognition by TLR5, NLRC4, or FLS2 is evaded by variation in key residues involved in flagellin detection, which can necessitate compensatory mutations. (C) Bacteria secrete enzymes that specifically target and degrade monomeric flagellin, preventing its recognition by TLR5, NLRC4, or FLS2. (D) Post-translational glyosylation of flagellin is thought to enhance flagella stability; reduced release of flagellin from flagella filaments will result in reduced recognition by TLR5 or FLS2. (E) Bacteria secrete effector proteins that interfere with TLR5, NLRC4, or FLS2 recognition either by direct inhibition of receptor expression or binding, or by inhibition of downstream signalling pathways. (F) Bacteria down-regulate or switch off flagella expression when motility and/or binding are no longer required. Amino acid substitution enables several animal bacterial pathogens and commensals to reduce activation of TLR5 signalling [88]. Differences in recognition and subsequent signalling elicited by different flagellins suggests host discrimination between pathogenic and commensal bacteria, as a nonpathogenic strain of E. coli (K-12) elicited a less pronounced flagellin response than did a pathogenic strain of S. Typhimurium [89]. Divergence in the protofilament number in C. jejuni compared to S. Typhimurium serves to evade the TLR5 recognition, due to substantially different packing of the D1 domains [90].
Immune activation in plant hosts varies depending on variation in the flg22 sequence of not only phytopathogenic bacteria [76,77,88,91] but also human pathogenic bacteria. Variation in five amino acids of the flg22 peptide derived from certain S. Senftenberg isolates induces a reduced pattern-triggered immunity (PTI) in plants in comparison to that from S. Typhimurium [92]. Likewise, plant growth-promoting rhizobacteria (PGPRs), such as Sinorhizobium meliloti and Agrobacterium tumefaciens have divergent flg22 epitopes that do not elicit any responses [75]. There is also evidence to suggest that the PGPR Burkholderia phytofirmans has evolved to evade the grapevine immune recognition system via FLS2 altogether [93].
As an alternative strategy, some bacteria evade immune recognition of flagellin independently of the flg22 peptide (Fig. 3). Phytopathogenic bacteria secrete effector proteins that specifically target FLS2 counteracting detection in plants. While some effectors rapidly act on FLS2 to shut down the PTI response [94,95], others suppress FLS2 accumulation and subsequent signalling cascades [96]. FLS2-induced stomatal closure is a characterised response to prevent pathogens from entering internal plant tissue [97], dependent on the action of oxylipins, rather than the phytohormone abscisic acid (ABA) [98]. Some isolates of P. syringae can prevent stomatal closure via secretion of a phytotoxin (coronatine) [99]. Intriguingly, S. Typhimurium can also delay stomatal closure, although to a lesser extent, via potential effector proteins other than coronatine, which may target the oxylipin pathway. EHEC O157:H7, which shares the same flg22 sequence as S. Typhimurium, cannot prevent stomatal closure, suggesting active manipulation of PTI by Salmonella [100]. P. aeruginosa, an opportunistic pathogen of plants and animals, produces an alkaline protease (AprA) to specifically degrade flagellin monomers. This strategy effectively evades the immune recognition in both kingdoms by degrading the natural ligand of TLR5 and FLS2 [101].
Post-translational modification
An alternative mechanism of evading recognition may come from modification of flagellin, reported for a number of animal and phytopathogenic bacteria [102,103]. Glycosylation enhances the structural stability of flagellin, preventing exposure of the flg22 region to FLS2 recognition, thereby evading the plant immune response [104]. There are multiple examples of O-glycosylation, well characterised for the flagellins of C. jejuni and Campylobacter coli [105]. Other examples include P. aeruginosa [106] and Shewanella oneidensis [107]. However, C. jejuni flagellar glycosylation was not involved in evasion of TLR5 recognition [108]. Perhaps it relates more to virulence and adherence, rather than filament stability [109–111]. Interestingly, S. Typhimurium flagellin is methylated at multiple lysine residues, yet this has no known impact on flagellar function [112]. The role of flagella post-translational modification in immune recognition clearly needs further investigation.
Concluding Remarks
Flagella enable pathogens to exploit or capitalise on various niches associated with the host. Although they display a range of functions, these are intrinsically linked to host colonisation and their own biophysical properties. Flagella are therefore not a virulence factor per se, but rather an early stage colonisation factor. They facilitate individual, pioneering cells to access, bind and invade new plant and animal tissues, and if successful in avoiding host recognition and clearance, to establish new colonies. More work is needed to understand how bacteria progress from flagella expression to flagella disassembly, both in the context of expression of more specialised colonisation factors that target specific ligands, and in immune recognition. The location of known ligands and the differences between decoys and membrane bound receptors also need to be addressed. Undoubtedly, future research will uncover further surprises for “twist and stick” or “dodge” flagella.
Zdroje
1. Sourjik V, Wingreen NS (2012) Responding to chemical gradients: bacterial chemotaxis. Curr Opin Cell Biol 24 : 262–268. doi: 10.1016/j.ceb.2011.11.008 22169400
2. Egelman EH (2010) Reducing irreducible complexity: divergence of quaternary structure and function in macromolecular assemblies. Curr Opin Cell Biol 22 : 68–74. doi: 10.1016/j.ceb.2009.11.007 20006482
3. Evans LD, Poulter S, Terentjev EM, Hughes C, Fraser GM (2013) A chain mechanism for flagellum growth. Nature 504 : 287–290. doi: 10.1038/nature12682 24213633
4. Rossez Y, Holmes A, Wolfson EB, Gally DL, Mahajan A, et al. (2014) Flagella interact with ionic plant lipids to mediate adherence of pathogenic Escherichia coli to fresh produce plants. Environ Microbiol 16 : 2181–2195. doi: 10.1111/1462-2920.12315 24148193
5. Haiko J, Westerlund-Wikström B (2013) The role of the bacterial flagellum in adhesion and virulence. Biology 2 : 1242–1267. doi: 10.3390/biology2041242 24833223
6. Pratt LA, Kolter R (1998) Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30 : 285–293. 9791174
7. Friedlander RS, Vlamakis H, Kim P, Khan M, Kolter R, et al. (2013) Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc Natl Acad Sci USA 110 : 5624–5629. doi: 10.1073/pnas.1219662110 23509269
8. Magariyama Y, Sugiyama S, Muramoto K, Maekawa Y, Kawagishi I, et al. (1994) Very fast flagellar rotation. Nature 371 : 752. 7935835
9. Haefele DM, Lindow SE (1987) Flagellar motility confers epiphytic fitness advantages upon Pseudomonas syringae. Appl Environ Microbiol 53 : 2528–2533. 16347469
10. Michiels KW, Croes CL, Vanderleyden J (1991) Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. J Gen Microbiol 137 : 2241–2246.
11. Holden N, Pritchard L, Toth I (2009) Colonization outwith the colon: plants as an alternative environmental reservoir for human pathogenic enterobacteria. FEMS Microbiol Rev 33 : 689–703. doi: 10.1111/j.1574-6976.2008.00153.x 19076238
12. Grad YH, Lipsitch M, Feldgarden M, Arachchi HM, Cerqueira GC, et al. (2012) Genomic epidemiology of the Escherichia coli O104:H4 outbreaks in Europe, 2011. Proc Natl Acad Sci USA 109 : 3065–3070. doi: 10.1073/pnas.1121491109 22315421
13. Berger CN, Shaw RK, Ruiz-Perez F, Nataro JP, Henderson IR, et al. (2009) Interaction of enteroaggregative Escherichia coli with salad leaves. Environ Microbiol Rep 1 : 234–239. doi: 10.1111/j.1758-2229.2009.00037.x 23765852
14. Gorski L, Duhé JM, Flaherty D (2009) The use of flagella and motility for plant colonization and fitness by different strains of the foodborne pathogen Listeria monocytogenes. PLoS ONE 4: e5142. doi: 10.1371/journal.pone.0005142 19357783
15. Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, et al. (2009) Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol 75 : 6076–6086. doi: 10.1128/AEM.01084-09 19648358
16. Saldana Z, Sanchez E, Xicohtencatl-Cortes J, Puente JL, Giron JA (2011) Surface structures involved in plant stomata and leaf colonization by Shiga-toxigenic Escherichia coli O157:H7. Front Microbiol 2 : 119. doi: 10.3389/fmicb.2011.00119 21887151
17. Shaw RK, Berger CN, Pallen MJ, Sjoeling A, Frankel G (2011) Flagella mediate attachment of enterotoxigenic Escherichia coli to fresh salad leaves. Environ Microbiol Rep 3 : 112–117. doi: 10.1111/j.1758-2229.2010.00195.x 23761239
18. Cooley MB, Miller WG, Mandrell RE (2003) Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae. Appl Environ Microbiol 69 : 4915–4926. 12902287
19. Mahenthiralingam E, Speert DP (1995) Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun 63 : 4519–4523. 7591095
20. Dons L, Eriksson E, Jin Y, Rottenberg ME, Kristensson K, et al. (2004) Role of flagellin and the two-component CheA/CheY system of Listeria monocytogenes in host cell invasion and virulence. Infect Immun 72 : 3237–3244. 15155625
21. Erdem AL, Avelino F, Xicohtencatl-Cortes J, Giron JA (2007) Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J Bacteriol 189 : 7426–7435. 17693516
22. Mahajan A, Currie CG, Mackie S, Tree J, McAteer S, et al. (2009) An investigation of the expression and adhesin function of H7 flagella in the interaction of Escherichia coli O157: H7 with bovine intestinal epithelium. Cell Microbiol 11 : 121–137. doi: 10.1111/j.1462-5822.2008.01244.x 19016776
23. Giron JA, Torres AG, Freer E, Kaper JB (2002) The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol Microbiol 44 : 361–379. 11972776
24. Song YC, Jin S, Louie H, Ng D, Lau R, et al. (2004) FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol Microbiol 53 : 541–553. 15228533
25. McGuckin MA, Linden SK, Sutton P, Florin TH (2011) Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9 : 265–278. doi: 10.1038/nrmicro2538 21407243
26. Schreiber S, Konradt M, Groll C, Scheid P, Hanauer G, et al. (2004) The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci USA 101 : 5024–5029. 15044704
27. Eaton K, Morgan D, Krakowka S (1992) Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J Med Microbiol 37 : 123–127. 1629897
28. Ottemann KM, Lowenthal AC (2002) Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect Immun 70 : 1984–1990. 11895962
29. Clyne M, Ocroinin T, Suerbaum S, Josenhans C, Drumm B (2000) Adherence of isogenic flagellum-negative mutants of Helicobacter pylori and Helicobacter mustelae to human and ferret gastric epithelial cells. Infect Immun 68 : 4335–4339. 10858255
30. Kim JC, Yoon JW, Kim C-H, Park M-S, Cho S-H (2012) Repression of flagella motility in enterohemorrhagic Escherichia coli O157: H7 by mucin components. Biochem Biophys Res Comm 423 : 789–792. doi: 10.1016/j.bbrc.2012.06.041 22713459
31. McCormick B, Stocker B, Laux D, Cohen P (1988) Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestine of streptomycin-treated mice. Infect Immun 56 : 2209–2217. 3044995
32. McCormick BA, Laux DC, Cohen PS (1990) Neither motility nor chemotaxis plays a role in the ability of Escherichia coli F-18 to colonize the streptomycin-treated mouse large intestine. Infect Immun 58 : 2957–2961. 2201640
33. Liu Z, Miyashiro T, Tsou A, Hsiao A, Goulian M, et al. (2008) Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl Acad Sci USA 105 : 9769–9774. doi: 10.1073/pnas.0802241105 18606988
34. Zgair AK, Chhibber S (2011) Adhesion of Stenotrophomonas maltophilia to mouse tracheal mucus is mediated through flagella. J Med Microbiol 60 : 1032–1037. doi: 10.1099/jmm.0.026377-0 21415208
35. Erdem AL, Avelino F, Xicohtencatl-Cortes J, Girón JA (2007) Host protein binding and adhesive properties of H6 and H7 flagella of attaching and effacing Escherichia coli. J Bacteriol 189 : 7426–7435. 17693516
36. Scharfman A, Arora SK, Delmotte P, Van Brussel E, Mazurier J, et al. (2001) Recognition of Lewis x derivatives present on mucins by flagellar components of Pseudomonas aeruginosa. Infect Immun 69 : 5243–5248. 11500392
37. Arora SK, Ritchings BW, Almira EC, Lory S, Ramphal R (1998) The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun 66 : 1000–1007. 9488388
38. Troge A, Scheppach W, Schroeder BO, Rund SA, Heuner K, et al. (2012) More than a marine propeller – the flagellum of the probiotic Escherichia coli strain Nissle 1917 is the major adhesin mediating binding to human mucus. Int J Med Microbiol 302 : 304–314. doi: 10.1016/j.ijmm.2012.09.004 23131416
39. Rossez Y, Maes E, Darroman TL, Gosset P, Ecobichon C, et al. (2012) Almost all human gastric mucin O-glycans harbor blood group A, B or H antigens and are potential binding sites for Helicobacter pylori. Glycobiol 22 : 1193–1206. doi: 10.1093/glycob/cws072 22522599
40. Robbe C, Capon C, Coddeville B, Michalski J (2004) Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J 384 : 307–316. 15361072
41. Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, et al. (2005) Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307 : 1955–1959. 15790854
42. Peekhaus N, Conway T (1998) What’s for dinner?: Entner-Doudoroff metabolism in Escherichia coli. J Bacteriol 180 : 3495–3502. 9657988
43. RdO Pinheiro, Boddey LH, James EK, Sprent JI, Boddey RM (2002) Adsorption and anchoring of Azospirillum strains to roots of wheat seedlings. Plant Soil 246 : 151–166.
44. Watt M, Hugenholtz P, White R, Vinall K (2006) Numbers and locations of native bacteria on field-grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environ Microbiol 8 : 871–884. 16623744
45. Driouich A, Follet-Gueye M-L, Vicré-Gibouin M, Hawes M (2013) Root border cells and secretions as critical elements in plant host defense. Curr Opin Plant Biol 16 : 489–495. doi: 10.1016/j.pbi.2013.06.010 23856080
46. Bucior I, Pielage JF, Engel JN (2012) Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Path 8: e1002616. doi: 10.1371/journal.ppat.1002616 22496644
47. Ketko AK, Lin C, Moore BB, LeVine AM (2013) Surfactant protein A binds flagellin enhancing phagocytosis and IL-1β production. PLoS ONE 8: e82680. doi: 10.1371/journal.pone.0082680 24312669
48. Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, et al. (1998) Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun 66 : 43–51. 9423837
49. Rogers TJ, Thorpe CM, Paton AW, Paton JC (2012) Role of lipid rafts and flagellin in invasion of colonic epithelial cells by Shiga-toxigenic Escherichia coli O113: H21. Infect Immun 80 : 2858–2867. doi: 10.1128/IAI.00336-12 22689816
50. Roy K, Hilliard GM, Hamilton DJ, Luo J, Ostmann MM, et al. (2008) Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 457 : 594–598. doi: 10.1038/nature07568 19060885
51. Rossez Y, Holmes A, Wolfson EB, Gally DL, Mahajan A, et al. (2013) Flagella interact with ionic plant lipids to mediate adherence of pathogenic Escherichia coli to fresh produce plants. Environ Microbiol 16 : 2181–2195. doi: 10.1111/1462-2920.12315 24148193
52. Misselwitz B, Barrett N, Kreibich S, Vonaesch P, Andritschke D, et al. (2012) Near surface swimming of Salmonella Typhimurium explains target-site selection and cooperative invasion. PLoS Pathog 8: e1002810. doi: 10.1371/journal.ppat.1002810 22911370
53. Lai MA, Quarles EK, López-Yglesias AH, Zhao X, Hajjar AM, et al. (2013) Innate immune detection of flagellin positively and negatively regulates Salmonella infection. PLoS ONE 8: e72047. doi: 10.1371/journal.pone.0072047 23977202
54. Lockman HA, Curtiss R (1990) Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun 58 : 137–143. 2152887
55. Olsen JE, Hoegh-Andersen KH, Casadesús J, Rosenkranzt J, Chadfield MS, et al. (2013) The role of flagella and chemotaxis genes in host pathogen interaction of the host adapted Salmonella enterica serovar Dublin compared to the broad host range serovar S. Typhimurium. BMC Microbiol 13 : 67. doi: 10.1186/1471-2180-13-67 23530934
56. Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, et al. (2013) Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe 14 : 571–581. doi: 10.1016/j.chom.2013.10.009 24237702
57. Yonekura K, Maki-Yonekura S, Namba K (2003) Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424 : 643–650. 12904785
58. Reid SD, Selander RK, Whittam TS (1999) Sequence diversity of flagellin (fliC) alleles in pathogenic Escherichia coli. J Bacteriol 181 : 153–160. 9864325
59. Brenner F, Villar R, Angulo F, Tauxe R, Swaminathan B (2000) Salmonella nomenclature. J Clin Microbiol 38 : 2465–2467. 10878026
60. Popoff MY, Bockemühl J, Gheesling LL (2003) Supplement 2001 (no. 45) to the Kauffmann–White scheme. Res Microbiol 154 : 173–174. 12706505
61. Beatson SA, Minamino T, Pallen MJ (2006) Variation in bacterial flagellins: from sequence to structure. Trend Microbiol 14 : 151–155. 16540320
62. Yonekura K, Maki-Yonekura S, Namba K (2003) Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424 : 643–650. 12904785
63. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 : 265–276. 10377992
64. Lemaitre B, Reichhart JM, Hoffmann JA (1997) Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA 94 : 14614–14619. 9405661
65. McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB (2000) High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun 68 : 5525–5529. 10992449
66. Eaves-Pyles TD, Wong HR, Odoms K, Pyles RB (2001) Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. J Immunol 167 : 7009–7016. 11739521
67. Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, et al. (2003) Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature Immunol 4 : 1247–1253. 14625549
68. Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, et al. (2001) The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410 : 1099–1103. 11323673
69. Zhao Y, Yang J, Shi J, Gong Y-N, Lu Q, et al. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477 : 596–600. doi: 10.1038/nature10510 21918512
70. Gomez-Gomez L, Boller T (2000) FLS2: an LRR receptor–like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5 : 1003–1011. 10911994
71. Ausubel FM (2005) Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6 : 973–979. 16177805
72. Jacchieri SG, Torquato R, Brentani RR (2003) Structural study of binding of flagellin by Toll-like receptor 5. J Bacteriol 185 : 4243–4247. 12837800
73. S-i Yoon, Kurnasov O, Natarajan V, Hong M, Gudkov AV, et al. (2012) Structural basis of TLR5-flagellin recognition and signaling. Science 335 : 859–864. doi: 10.1126/science.1215584 22344444
74. Gómez-Gómez L, Boller T (2000) FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5 : 1003–1011. 10911994
75. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 : 265–276. 10377992
76. Cai R, Lewis J, Yan S, Liu H, Clarke CR, et al. (2011) The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Path 7: e1002130. doi: 10.1371/journal.ppat.1002130 21901088
77. Clarke CR, Chinchilla D, Hind SR, Taguchi F, Miki R, et al. (2013) Allelic variation in two distinct Pseudomonas syringae flagellin epitopes modulates the strength of plant immune responses but not bacterial motility. New Phytol 200 : 847–860. doi: 10.1111/nph.12408 23865782
78. Halff EF, Diebolder CA, Versteeg M, Schouten A, Brondijk TH, et al. (2012) Formation and structure of a NAIP5-NLRC4 inflammasome induced by direct interactions with conserved N - and C-terminal regions of flagellin. J Biol Chem 287 : 38460–38472. doi: 10.1074/jbc.M112.393512 23012363
79. Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9 : 1171–1178. doi: 10.1038/ni.1646 18724372
80. Wei HL, Chakravarthy S, Worley JN, Collmer A (2013) Consequences of flagellin export through the type III secretion system of Pseudomonas syringae reveal a major difference in the innate immune systems of mammals and the model plant Nicotiana benthamiana. Cell Microbiol 15 : 601–618. doi: 10.1111/cmi.12059 23107228
81. McQuiston JR, Fields PI, Tauxe RV, Logsdon JM, Jr. (2008) Do Salmonella carry spare tyres? Trends Microbiol 16 : 142–148. doi: 10.1016/j.tim.2008.01.009 18375124
82. Alm R, Guerry P, Trust T (1993) The Campylobacter sigma 54 flaB flagellin promoter is subject to environmental regulation. J Bacteriol 175 : 4448–4455. 8331072
83. Silverman M, Zieg J, Hilmen M, Simon M (1979) Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc Natl Acad Sci USA 76 : 391–395. 370828
84. Smith NH, Selander RK (1991) Molecular genetic basis for complex flagellar antigen expression in a triphasic serovar of Salmonella. Proc Natl Acad Sci USA 88 : 956–960. 1992487
85. Dobbin HS, Hovde CJ, Williams CJ, Minnich SA (2006) The Escherichia coli O157 flagellar regulatory gene flhC and not the flagellin gene fliC impacts colonization of cattle. Infect Immun 74 : 2894–2905. 16622228
86. Darrasse A, Carrere S, Barbe V, Boureau T, Arrieta-Ortiz ML, et al. (2013) Genome sequence of Xanthomonas fuscans subsp. fuscans strain 4834-R reveals that flagellar motility is not a general feature of xanthomonads. BMC Genomics 14 : 761. doi: 10.1186/1471-2164-14-761 24195767
87. Ikeda JS, Schmitt CK, Darnell SC, Watson PR, Bispham J, et al. (2001) Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect Immun 69 : 3021–3030. 11292720
88. Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, et al. (2005) Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA 102 : 9247–9252. 15956202
89. Yang J, Zhang E, Liu F, Zhang Y, Zhong M, et al. (2014) Flagellins of Salmonella Typhi and nonpathogenic Escherichia coli are differentially recognized through the NLRC4 pathway in macrophages. J Innate Immun 6 : 47–57. doi: 10.1159/000351476 23816851
90. Galkin VE, Yu X, Bielnicki J, Heuser J, Ewing CP, et al. (2008) Divergence of quaternary structures among bacterial flagellar filaments. Science 320 : 382–385. doi: 10.1126/science.1155307 18420936
91. Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF (2006) Within-species flagellin polymorphism in Xanthomonas campestris pv campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell 18 : 764–779. 16461584
92. Garcia AV, Charrier A, Schikora A, Bigeard J, Pateyron S, et al. (2014) Salmonella enterica flagellin is recognized via FLS2 and activates PAMP-triggered immunity in Arabidopsis thaliana. Mol Plant 7 : 657–674. doi: 10.1093/mp/sst145 24198231
93. Trdá L, Fernandez O, Boutrot F, Héloir M-C, Kelloniemi J, et al. (2014) The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol 201 : 1371–1384. doi: 10.1111/nph.12592 24491115
94. Shan L, He P, Li J, Heese A, Peck SC, et al. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microb 4 : 17–27. doi: 10.1016/j.chom.2008.05.017 18621007
95. Xiang T, Zong N, Zou Y, Wu Y, Zhang J, et al. (2008) Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol 18 : 74–80. 18158241
96. Hann DR, Domínguez – Ferreras A, Motyka V, Dobrev PI, Schornack S, et al. (2014) The Pseudomonas type III effector HopQ1 activates cytokinin signaling and interferes with plant innate immunity. New Phytol 201 : 585–598. doi: 10.1111/nph.12544 24124900
97. Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126 : 969–980. 16959575
98. Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513 doi: 10.1371/journal.pbio.1001513 23526882
99. Underwood W, Melotto M, He SY (2007) Role of plant stomata in bacterial invasion. Cell Microbiol 9 : 1621–1629. 17419713
100. Roy D, Panchal S, Rosa BA, Melotto M (2013) Escherichia coli O157:H7 induces stronger plant immunity than Salmonella enterica Typhimurium SL1344. Phytopathol 103 : 326–332. doi: 10.1094/PHYTO-09-12-0230-FI 23301812
101. Bardoel BW, van der Ent S, Pel MJ, Tommassen J, Pieterse CM, et al. (2011) Pseudomonas evades immune recognition of flagellin in both mammals and plants. PLoS Path 7: e1002206. doi: 10.1371/journal.ppat.1002206 21901099
102. Ichinose Y, Taguchi F, Yamamoto M, Ohnishi-Kameyama M, Atsumi T, et al. (2013) Flagellin glycosylation is ubiquitous in a broad range of phytopathogenic bacteria. J Gen Plant Pathol 79 : 359–365.
103. Ewing CP, Andreishcheva E, Guerry P (2009) Functional characterization of flagellin glycosylation in Campylobacter jejuni 81–176. J Bacteriol 191 : 7086–7093. doi: 10.1128/JB.00378-09 19749047
104. Hirai H, Takai R, Iwano M, Nakai M, Kondo M, et al. (2011) Glycosylation regulates specific induction of rice immune responses by Acidovorax avenae flagellin. J Biol Chem 286 : 25519–25530. doi: 10.1074/jbc.M111.254029 21628471
105. Logan SM, Trust TJ, Guerry P (1989) Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol 171 : 3031–3038. 2722741
106. Brimer CD, Montie T (1998) Cloning and comparison of fliC genes and identification of glycosylation in the flagellin of Pseudomonas aeruginosa a-type strains. J Bacteriol 180 : 3209–3217. 9620973
107. Sun L, Jin M, Ding W, Yuan J, Kelly J, et al. (2013) Post-translational modification of flagellin FlaB in Shewanella oneidensis. J Bacteriol 195 : 2550–2561. doi: 10.1128/JB.00015-13 23543712
108. de Zoete MR, Keestra AM, Wagenaar JA, van Putten JP (2010) Reconstitution of a functional Toll-like receptor 5 binding site in Campylobacter jejuni flagellin. J Biol Chem 285 : 12149–12158. doi: 10.1074/jbc.M109.070227 20164175
109. Arora SK, Neely AN, Blair B, Lory S, Ramphal R (2005) Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect Immun 73 : 4395–4398. 15972536
110. Szymanski CM, Burr DH, Guerry P (2002) Campylobacter protein glycosylation affects host cell interactions. Infect Immun 70 : 2242. 11895996
111. Taguchi F, Suzuki T, Takeuchi K, Inagaki Y, Toyoda K, et al. (2009) Glycosylation of flagellin from Pseudomonas syringae pv. tabaci 6605 contributes to evasion of host tobacco plant surveillance system. Physiol Mol Plant Pathol 74 : 11–17.
112. Tronick SR, Martinez RJ (1971) Methylation of the flagellin of Salmonella typhimurium. J Bacteriol 105 : 211–219. 5541007
113. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem 25 : 1605–1612. 15264254
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



