-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
article has not abstract
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004568
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004568Summary
article has not abstract
Introduction
Despite recent advancements in the diagnosis and management of fungal infections [1], invasive fungal diseases remain a major cause of morbidity and mortality in immunocompromised patients and are major drivers of elevated healthcare costs [2]. In this context, early diagnosis is a key factor. However, current diagnostic approaches, including laboratory tests and computer tomography, have limitations, especially in terms of sensitivity and specificity [3]. Therefore, empirical therapy has often evolved as the standard of care, irrespective of the immediate and long-term consequences in terms of cost, development of drug resistance, or toxicity [4].
An exceptional challenge is the development of imaging modalities providing not only high specificity and sensitivity but also localization of the infection site. In particular, nuclear medicine imaging techniques using radiolabelled probes (radiotracers) have the potential to specifically target the underlying pathophysiological mechanisms of the pathogen leading to molecular localization of the infection site in patients.
Traditionally applied in the context of planar scintigraphy and single photon emission tomography (SPECT) in the past decade, Positron-Emission Tomography (PET) has evolved as a major clinical imaging technique, particularly in oncology [5]. This technology provides improved sensitivity and resolution based on the coincidence detection of photons emitted from radionuclei resulting from annihilation of positrons. Its tremendous success in oncology is mainly based on 2-[18F] fluorodeoxyglucose specifically accumulating in cells in dependence of their glucose consumption [6]. In this process, termed “molecular trapping,” the radiolabelled glucose molecule is actively transported into the cell, followed by its phosphorylation. The incorporated Fluor blocks further metabolic processing and traps the radionuclide 18F inside the cell, leading to an intense radioactive signal in affected cells. Other clinically used PET probes, such as [18F]-3′-fluoro-3′-deoxy-L-thymidine, [18F]-choline derivatives, or radiolabelled peptides, such as 68Ga-DOTATOC, show a similar trapping mechanism based on initial active transport in, or receptor-specific recognition by, diseased cells with promising clinical applications in oncology [7]. Besides accumulation in the target, favorable pharmacokinetics of radiotracers, such as rapid transport to and low retention in non-target sites/cells as well as efficient elimination from the body, ideally via renal excretion, are required. These features allow early imaging with short-lived radionuclides such as 18F with a half-life of 110 minutes, resulting in a low radiation burden for the patient.
Therefore, a radiotracer for specific imaging of fungal infections should ideally fulfill similar criteria: specific accumulation in the pathogen combined with favorable pharmacokinetics, including rapid elimination from healthy tissue. Ideally, the pathogen should recognize the radiotracer as an apparent molecule of interest, boosting its active uptake and accumulation.
Attempts to Image Fungal Infections with Radiolabeled Probes
A number of non-specific radiotracers have been applied for imaging of infections. These include 111In - or 99mTc-labelled leucocytes, 99mTc-anti-granulocyte antibody, 99mTc-diphosphonates in the context of bone scanning, 67Ga-citrate, and even 2-[18F]-fluorodeoxyglucose [8]. These probes target predominantly secondary effects of infection, such as increased blood flow and vascular permeability, activated endothelial cells, or polymorphonuclear cell migration. Therefore, several attempts have been made to develop more specific radiotracers for fungal infections [9]. A widely studied group of compounds are antimicrobial peptides, which typically interact through their cationic domains with the anionic surface of microorganisms, leading to antimicrobial activity. Particularly, 99mTc-labelled ubiquicidin and human lactoferrin derivatives for SPECT have been developed with promising results in patients with bacterial infections, but no specificity for fungal infections preclinically. Another approach was the use of 99mTc-labelled fluconazole, a widely used triazole antifungal drug that inhibits ergosterol biosynthesis, which, however, accumulated poorly in A. fumigatus in a murine infections model [9]. 123I-labelling of chitinase and 99mTc-labelling of the chitin-binding protein CBP21 have been described as specific imaging agents for fungal infections, but were never translated to human studies [9]. Moreover, 99mTc-labelled polyethylene glycol (PEG)-liposomes and 99mTc-interleukin 8 showed interesting preclinical results, but these compounds show unfavorable pharmacokinetics [9]. All these agents lack specificity for fungal pathogens and, therefore, have not found their way into clinical fungal infection imaging.
Attempts to Specifically Image Aspergillosis
Among fungal pathogens, Aspergillus fumigatus is still the most common opportunistic mold pathogen infecting humans with a fourfold increase of invasive pulmonary aspergillosis over the last 30 years [10]. Recently, interesting approaches to develop a radiotracer specific for A. fumigatus have been reported. 99mTc-labeled phosphorodiamidate morpholino (MORF) oligomers that hybridize to the fungal ribosomal RNA were found to accumulate in infected lungs as compared to a control construct [11]. The cyclic peptide c(CGGRLGPFC)-NH2, identified by phage display technology to bind in vitro to the surface of conidia and hyphae of A. fumigatus, is able to image A. fumigatus lung infection in a mouse model using SPECT, when labeled with 111In [12]. Moreover, an Aspergillus-specific monoclonal antibody used for serodiagnosis of aspergillosis is currently being examined for its potential in PET imaging [13]. These promising initial results indicate the potential to specifically target A. fumigatus. However, all of these methods appear to have one major drawback: namely, a lack of active uptake by the pathogen leading to signal intensification at the infection site. This has led us to investigate the potential of the siderophore system as a target for a radiotracer with high specificity and sensitivity for fungal infections.
Siderophore Metabolism of A. Fumigatus as the Basis for Molecular Imaging
During infection, pathogens encounter an essentially iron-free environment as the available iron is tightly sequestered by host proteins, e.g., hemoglobin, transferrin, lactoferrin, and ferritin [14]. Consequently, pathogens had to evolve mechanisms for “stealing” host iron. A. fumigatus possesses two high-affinity iron uptake systems, reductive iron assimilation and siderophore-mediated iron acquisition [15]. Both systems are transcriptionally upregulated during iron starvation in vitro as well as in vivo in a murine model for pulmonary aspergillosis confirming that A. fumigatus faces iron starvation conditions during infection [16, 17]. In the murine infection model, genetic inactivation of siderophore biosynthesis, but not of reductive iron assimilation, attenuated virulence of A. fumigatus, demonstrating that siderophore-mediated iron assimilation plays the major role for virulence [15]. Siderophores are ferric iron-specific chelators secreted by a diverse range of bacteria and fungi, displaying a variety of structures depending on the producer [18]. With iron binding constants of 1020–1050, siderophores are able to sequester iron from iron-binding host proteins such as transferrin [19]. A. fumigatus secretes two hydroxamate-type siderophores (Fig. 1A), fusarinine C (FSC), which consists of three N5-anhydromevalonyl-N5-hydroxyornithine residues cyclically linked by ester bonds, and its N2-acetylated derivative triacetylfusarinine C (TAFC). FSC/TAFC synthesis involves six enzymes dedicated solely to siderophore biosynthesis [20–22]. The cellular export mechanism for siderophores remains to be characterized. After chelation of iron, the uptake of ferri-siderophores is mediated by specific transporters, which belong to the Siderophore Iron Transporter (SIT) subfamily of the major facilitator protein superfamily [23]. A. fumigatus possesses seven SIT proteins, five of which are transcriptionally upregulated by iron starvation. Among these, MirB was identified as the transporter for TAFC while the FSC transporter remains to be identified [24, 25]. In the cell, the trilactone rings of TAFC and FSC are hydrolyzed by specific esterases [26, 27]. The released iron is transferred to the metabolism or stored either in the intracellular siderophore ferricrocin or within the vacuole [28]. A scheme of TAFC-mediated iron uptake is shown in Fig. 1B.
Fig. 1. Siderophore mediated-iron uptake in A. fumigatus. 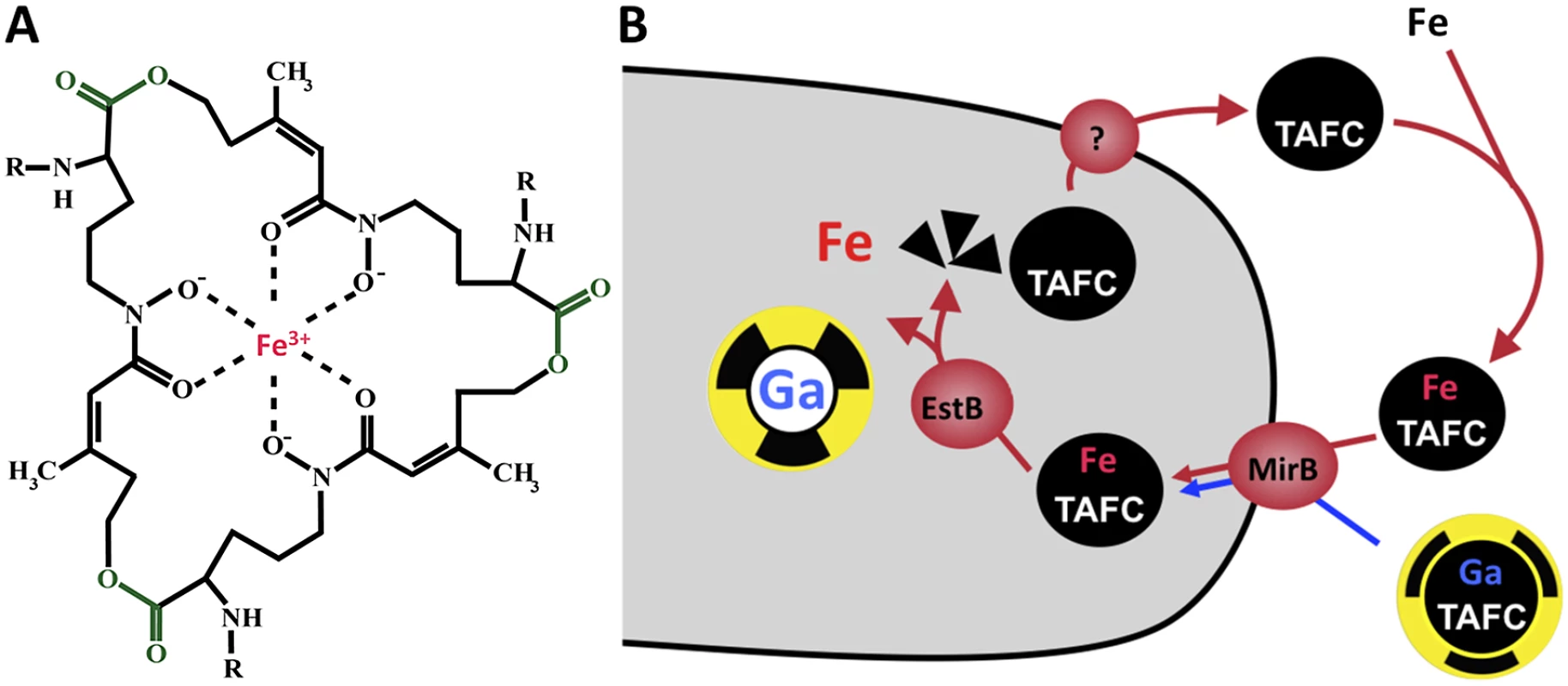
(A) FSC/TAFC is shown in the ferri-form; the ester bonds separating the three N5-acetyl-/N5-anhydromevalonyl-N5-hydroxyornithine residues are shown in green; for TAFC-based nuclear imaging, the iron (shown in red) is replaced by 68Ga. FSC, R = H; TAFC, R = acetyl. (B) TAFC-mediated uptake of iron and gallium into fungal hypha. Most fungal species produce siderophores, but there are notable exceptions, such as the fungal model species Saccharomyces cerevisiae, Cryptococcus neoformans, and Candida spp. [23]. SIT members are encoded by all fungal species. Consistently, non-siderophore-producing species were also shown to use such transporters for uptake of xenosiderophores, siderophores produced by other microorganisms [29]. Moreover, siderophore-producing fungal species are also able to take up xenosiderophores [30]. The siderophore system is confined to the fungal and bacterial kingdoms, i.e., the enzymes and transporters involved in biosynthesis and uptake of siderophores are not present in vertebrate cells.
A Trojan Horse for the Iron-Searching Pathogen
Siderophore transporters represent a promising target for molecular imaging approaches in fungal infections due to the following reasons: (i) they are highly upregulated during infection, which is underlined by their crucial role in virulence of A. fumigatus; (ii) these transporters are not present in human cells and therefore their specific substrates do not interact with the human physiology; (iii) the energy-dependent active uptake leads to accumulation of a (radio)labeled substrate in the pathogen; (iv) the low molecular mass of siderophores (e.g., the Mrof desferri-TAFC is 853 Da) has the advantage of rapid diffusion from the circulation into infected tissues; (v) the chemical features of TAFC, but not all siderophores, result in low binding to serum components, which is crucial for a high signal-to-noise ratio and rapid clearance from non-target tissue and elimination from the body via renal excretion; (vi) radiolabelling of siderophores can be achieved easily by replacing Fe(III) in the siderophore with an iron-mimicking radionuclide. There is no iron isotope with suitable properties for imaging in terms of half-life and photon emission. However, it has been known for a long time that Ga(III) is an isosteric diamagnetic substitute for Fe(III), which has been used to characterize siderophore complexes and siderophore uptake, revealing indistinguishable uptake compared to the Fe(III) counterpart in Ustilago sphaerogena [31, 32]. Recently, the interest in 68Ga has increased tremendously with the establishment of PET as a clinical imaging modality [33]. 68Ga with a physical half-life of 68 minutes can be obtained from a 68Ge/68Ga generator without the requirement of cyclotron installations and exhibits a very low radiation burden to the patient, comparable or even lower to that of 18F, the most widely used radionuclide for PET.
In a proof of concept study [34], we demonstrated that desferri-siderophores, particularly TAFC, can be easily radiolabelled with 68Ga exhibiting favorable hydrophilic properties and high chemical stability. Uptake of 68Ga-TAFC in A. fumigatus was upregulated under iron starvation conditions and could be blocked with an excess of siderophore or NaN3, indicating specific and energy-dependent uptake (Fig. 2A). In vivo, in an aspergillosis rat model, 68Ga-TAFC was rapidly excreted, exclusively renally as intact complex, indicating excellent metabolic stability. Compared to healthy lungs, 68Ga-TAFC was taken up in a more than 20-fold higher concentration in infected lung tissue. A variety of different siderophores, including FSC, TAFC, coprogen, as well as various ferrichrome-type and ferroxamine-type-siderophores, displayed excellent 68Ga-radiolabeling [30]. However, only 68Ga-TAFC and 68Ga-ferrioxamine E (FOXE), a siderophore produced by various Streptomycetes species [35], displayed a good combination of fungal uptake in culture combined with high chemical and metabolic stability as well as suitable pharmacokinetics for imaging, i.e., rapid clearance from organs and circulation with predominant renal excretion. High contrast imaging of A. fumigatus pulmonary infection in a rat model could be achieved using Micro-PET/computed tomography (CT) technology, exhibiting pronounced accumulation of 68Ga-TAFC in infected areas (Fig. 2B). This was achieved already early after the onset of infection and increased with its severity and correlated with abnormal CT images [36]. Most vertebrates produce siderophore-scavenging proteins, termed siderocalins, to limit the growth of pathogens. The major siderocalin found in the murine and human blood, NGAL/Lcn2, does not recognize either TAFC nor FOXE [37] and, consequently, does not impede these tracers.
Fig. 2. In vitro and in vivo uptake of 68Ga-TAFC by A. fumigatus. 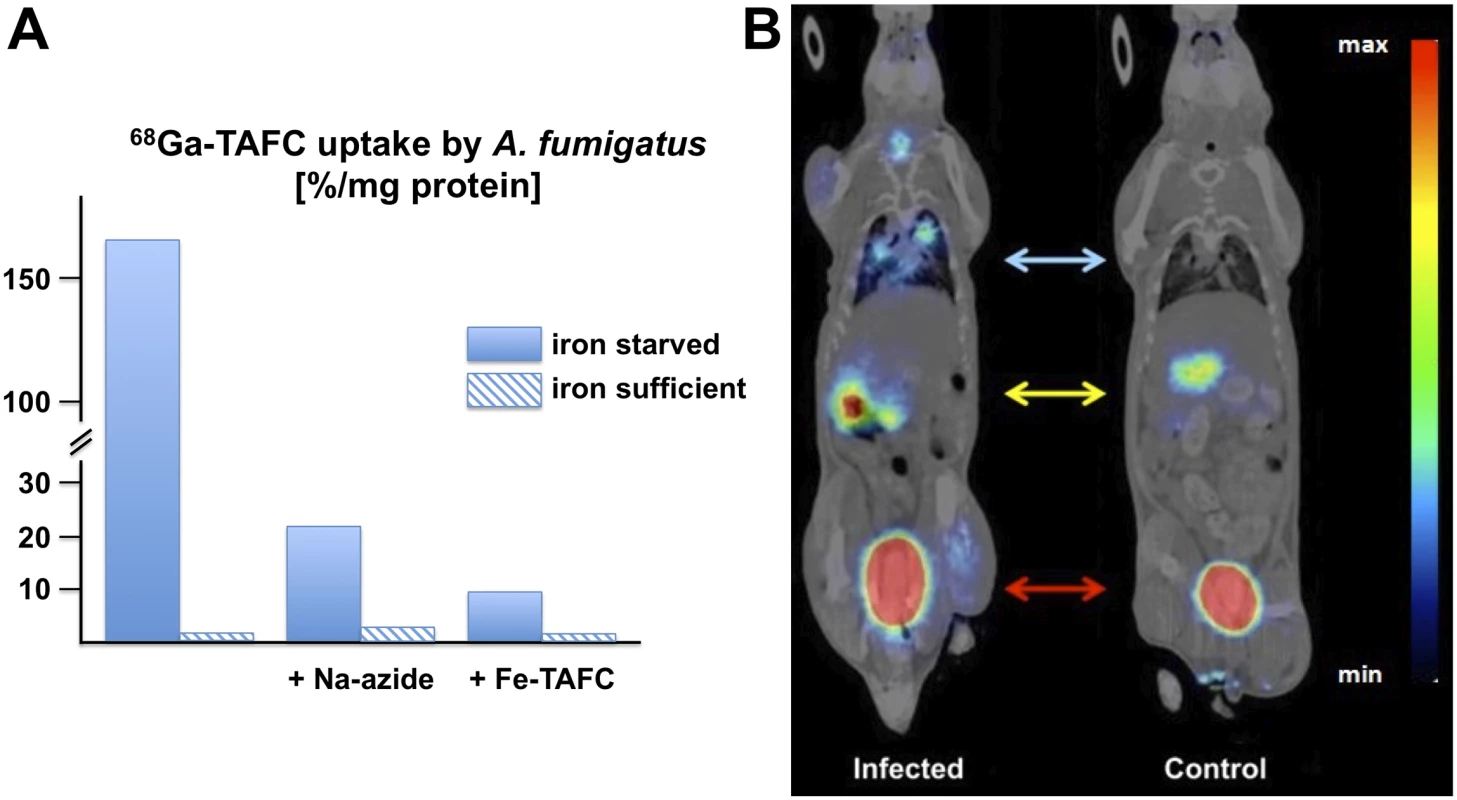
(A) In-vitro uptake of 68Ga-TAFC in A. fumigatus cultures, showing induction of uptake during iron starvation, energy-dependence, and saturation by excess of ferric TAFC. (B) Micro-PET/CT (Albira PET/SPECT/CT small animal imaging system, Bruker Biospin Corporation, Woodbridge, CT, USA) imaging of A. fumigatus (coronal slices) in a rat infection model 45 minutes post intravenous injection of 68Ga-TAFC showing clear accumulation (blue arrow) in infected lung tissue (M. Petrik, unpublished). Accumulation of 68Ga-TAFC in kidney (yellow arrow) and bladder (red arrow) is caused by rapid renal excretion of the tracer. The colors reflect the signal intensity increasing from blue to green, yellow and red. Is the Trojan Horse Only Finding the Right Target?
Before using such a potentially powerful imaging weapon, potential limitations have to be addressed, such as the sensitivity and specificity of such a method. In a clinical setting, the iron status or antifungal medication of the patient may influence the activity of the siderophore system and the rate of fungal iron acquisition, thereby influencing the radiotracer uptake und consequently the sensitivity of this method. Patients who acquire fungal infections often suffer from iron overload (e.g., from blood transfusions), which is a major risk factor in the onset of the infection. In a preliminary study, no significant influence of iron overload of A. fumigatus infected rats on lung uptake was observed. The influence of pre - or concomitant medication still has to be addressed in a preclinical model.
Regarding specificity, the discrimination between sterile inflammation, tumors, and real infection is crucial. In this respect, significant accumulation of 68Ga TAFC was neither found in a sterile inflammation model nor in tumor cells [38]. As TAFC is produced by different fungal species, e.g., A. nidulans and A. fumigatus, and might be used as a xenosiderophore by non-produces, the specificity among microbial pathogens is another important issue. 68Ga-TAFC uptake studies revealed in vitro high uptake by A. fumigatus, considerable uptake by Rhizopus oryzae and Fusarium solani, but no significant uptake by Aspergillus terreus, Aspergillus flavus, Candida albicans, as well as the bacterial species Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, or Mycobacterium smegmatis. In comparison, FOXE displayed the highest uptake by A. fumigatus, and considerable uptake by A. terreus, A. flavus, Rhizopus oryzae, Fusarium solani, as well as the bacterial species Staphylococcus aureus. In vivo, however, neither TAFC nor FOXE were found to accumulate in a rat S. aureus abscess model [38]. Taken together, TAFC and FOXE have overlapping species specificity with the best uptake observed for A. fumigatus, among all tested species. Nevertheless, further in vivo studies are required to clarify the species specificity. Moreover, the sensitivity of this approach compared to current diagnostic methods has to be addressed, ideally in a clinical setting.
Conclusion
From these studies, we conclude that 68Ga-labeled siderophores, particularly 68Ga-TAFC, have a high potential to be used as fungal radiotracers: easily generated by direct radiolabeling of the desferri-form by incubation with a 68Ga-solution in a pharmaceutically compatible acetate buffer, 68Ga-TAFC displays high stability both in solution and in vivo, and rapidly reaches the infection site where the pathogen misinterprets the radiolabeled tracer as an iron source and accumulates this “Trojan horse.” The next major step forward in this attempt to provide a tool for molecular imaging of fungal infections will be the proof of concept in a clinical study that is currently in its planning phase. It will show whether targeting of a molecular process related to the pathophysiology of infection indeed will be successful to provide the clinical information required to establish Odysseus’ concept of the Trojan horse at the molecular level.
Zdroje
1. Hayes GE, Denning DW (2013) Frequency, diagnosis and management of fungal respiratory infections. Curr Opin Pulm Med 19 : 259–265. doi: 10.1097/MCP.0b013e32835f1ad1 23411576
2. Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36 : 1–53. doi: 10.3109/10408410903241444 20088682
3. Steinbach WJ (2013) Are we there yet? Recent progress in the molecular diagnosis and novel antifungal targeting of Aspergillus fumigatus and invasive aspergillosis. PLoS Pathog 9: e1003642. doi: 10.1371/journal.ppat.1003642 24204250
4. Maertens JA, Nucci M, Donnelly JP (2012) The role of antifungal treatment in hematology. Haematologica 97 : 325–327. doi: 10.3324/haematol.2012.061952 22383742
5. Lin M, Shon IH, Lin P (2010) Positron emission tomography: current status and future challenges. Intern Med J 40 : 19–29. doi: 10.1111/j.1445-5994.2009.02072.x 20561362
6. Haubner R (2010) PET radiopharmaceuticals in radiation treatment planning—synthesis and biological characteristics. Radiother Oncol 96 : 280–287. doi: 10.1016/j.radonc.2010.07.022 20724013
7. Wadsak W, Mitterhauser M (2010) Basics and principles of radiopharmaceuticals for PET/CT. Eur J Radiol 73 : 461–469. doi: 10.1016/j.ejrad.2009.12.022 20181453
8. Signore A, Glaudemans AW (2011) The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann Nucl Med 25 : 681–700. doi: 10.1007/s12149-011-0521-z 21837469
9. Lupetti A, de Boer MG, Erba P, Campa M, Nibbering PH (2011) Radiotracers for fungal infection imaging. Med Mycol 49 Suppl 1: S62–69. doi: 10.3109/13693786.2010.508188 20795767
10. Kwon-Chung KJ, Sugui JA (2013) Aspergillus fumigatus--what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 9: e1003743. doi: 10.1371/journal.ppat.1003743 24348239
11. Wang Y, Chen L, Liu X, Cheng D, Liu G, et al. (2013) Detection of Aspergillus fumigatus pulmonary fungal infections in mice with (99m)Tc-labeled MORF oligomers targeting ribosomal RNA. Nucl Med Biol 40 : 89–96. doi: 10.1016/j.nucmedbio.2012.10.001 23142409
12. Yang Z, Kontoyiannis DP, Wen X, Xiong C, Zhang R, et al. (2009) Gamma scintigraphy imaging of murine invasive pulmonary aspergillosis with a (111)In-labeled cyclic peptide. Nucl Med Biol 36 : 259–266. doi: 10.1016/j.nucmedbio.2008.12.004 19324271
13. Thornton CR (2014) Breaking the mould—novel diagnostic and therapeutic strategies for invasive pulmonary aspergillosis in the immune deficient patient. Expert Rev Clin Immunol 10 : 771–780. doi: 10.1586/1744666X.2014.904747 24689528
14. Cassat JE, Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13 : 509–519. doi: 10.1016/j.chom.2013.04.010 23684303
15. Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, et al. (2004) Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med 200 : 1213–1219. doi: 10.1084/jem.20041242 15504822
16. McDonagh A, Fedorova ND, Crabtree J, Yu Y, Kim S, et al. (2008) Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog 4: e1000154. doi: 10.1371/journal.ppat.1000154 18787699
17. Schrettl M, Kim HS, Eisendle M, Kragl C, Nierman WC, et al. (2008) SreA-mediated iron regulation in Aspergillus fumigatus. Mol Microbiol 70 : 27–43. doi: 10.1111/j.1365-2958.2008.06376.x 18721228
18. Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27 : 637–657. doi: 10.1039/b906679a 20376388
19. Hissen AH, Moore MM (2005) Site-specific rate constants for iron acquisition from transferrin by the Aspergillus fumigatus siderophores N′,N′′,N′′′-triacetylfusarinine C and ferricrocin. J Biol Inorg Chem 10 : 211–220. doi: 10.1007/s00775-005-0630-z 15770504
20. Haas H (2012) Iron—A Key Nexus in the Virulence of Aspergillus fumigatus. Front Microbiol 3 : 28. doi: 10.3389/fmicb.2012.00028 22347220
21. Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, et al. (2007) Distinct Roles for Intra - and Extracellular Siderophores during Aspergillus fumigatus Infection. PLoS Pathog 3: e128. doi: 10.1371/journal.ppat.0030128
22. Yasmin S, Alcazar-Fuoli L, Grundlinger M, Puempel T, Cairns T, et al. (2012) Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc Natl Acad Sci U S A 109: E497–504. doi: 10.1073/pnas.1106399108 22106303
23. Haas H, Eisendle M, Turgeon BG (2008) Siderophores in fungal physiology and virulence. Annu Rev Phytopathol 46 : 149–187. doi: 10.1146/annurev.phyto.45.062806.094338 18680426
24. Raymond-Bouchard I, Carroll CS, Nesbitt JR, Henry KA, Pinto LJ, et al. (2012) Structural requirements for the activity of the MirB ferrisiderophore transporter of Aspergillus fumigatus. Eukaryot Cell 11 : 1333–1344. doi: 10.1128/EC.00159-12 22903978
25. Haas H, Schoeser M, Lesuisse E, Ernst JF, Parson W, et al. (2003) Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C. Biochem J 371 : 505–513. doi: 10.1042/BJ20021685 12487628
26. Kragl C, Schrettl M, Abt B, Sarg B, Lindner HH, et al. (2007) EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus. Eukaryot Cell 6 : 1278–1285. doi: 10.1128/EC.00066-07 17586718
27. Grundlinger M, Gsaller F, Schrettl M, Lindner H, Haas H (2013) Aspergillus fumigatus SidJ mediates intracellular siderophore hydrolysis. Appl Environ Microbiol 79 : 7534–7536. doi: 10.1128/AEM.01285-13 24038704
28. Gsaller F, Eisendle M, Lechner BE, Schrettl M, Lindner H, et al. (2012) The interplay between vacuolar and siderophore-mediated iron storage in Aspergillus fumigatus. Metallomics 4 : 1262–1270. doi: 10.1039/c2mt20179h 23151814
29. Philpott CC (2006) Iron uptake in fungi: a system for every source. Biochim Biophys Acta 1763 : 636–645. doi: 10.1016/j.bbamcr.2006.05.008 16806534
30. Petrik M, Haas H, Schrettl M, Helbok A, Blatzer M, et al. (2012) In vitro and in vivo evaluation of selected 68Ga-siderophores for infection imaging. Nucl Med Biol 39 : 361–369. doi: 10.1016/j.nucmedbio.2011.09.012 22172389
31. Llinas M, Klein MP, Neilands JB (1970) Solution conformation of ferrichrome, a microbial iron transport cyclohexapeptide, as deduced by high resolution proton magnetic resonance. J Mol Biol 52 : 399–414. doi: 10.1016/0022-2836(70)90409-2 5492288
32. Emery T, Hoffer PB (1980) Siderophore-mediated mechanism of gallium uptake demonstrated in the microorganism Ustilago sphaerogena. J Nucl Med 21 : 935–939. 7420194
33. Velikyan I (2013) Prospective of (6)(8)Ga-radiopharmaceutical development. Theranostics 4 : 47–80. doi: 10.7150/thno.7447 24396515
34. Petrik M, Haas H, Dobrozemsky G, Lass-Florl C, Helbok A, et al. (2010) 68Ga-siderophores for PET imaging of invasive pulmonary aspergillosis: proof of principle. J Nucl Med 51 : 639–645. doi: 10.2967/jnumed.109.072462 20351354
35. Barona-Gomez F, Wong U, Giannakopulos AE, Derrick PJ, Challis GL (2004) Identification of a cluster of genes that directs desferrioxamine biosynthesis in Streptomyces coelicolor M145. J Am Chem Soc 126 : 16282–16283. doi: 10.1021/ja045774k 15600304
36. Petrik M, Franssen GM, Haas H, Laverman P, Hortnagl C, et al. (2012) Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur J Nucl Med Mol Imaging 39 : 1175–1183. doi: 10.1007/s00259-012-2110-3 22526953
37. Sia AK, Allred BE, Raymond KN (2013) Siderocalins: Siderophore binding proteins evolved for primary pathogen host defense. Curr Opin Chem Biol 17 : 150–157. doi: 10.1016/j.cbpa.2012.11.014 23265976
38. Petrik M, Haas H, Laverman P, Schrettl M, Franssen GM, et al. (2014) 68Ga-triacetylfusarinine C and 68Ga-ferrioxamine E for Aspergillus infection imaging: uptake specificity in various microorganisms. Mol Imaging Biol 16 : 102–108. doi: 10.1007/s11307-013-0654-7 23818006
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



