-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHelminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
article has not abstract
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004582
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004582Summary
article has not abstract
What Is the Immunoepidemiologic Model of Helminth and Tuberculosis Coinfection?
Tuberculosis (TB) caused by the bacteria Mycobacterium tuberculosis (Mtb) remains a global cause of considerable morbidity and mortality [1]. One of the biggest current challenges of TB control is our incomplete understanding of what constitutes protective immunity in TB-endemic areas of the world. Although close to 2,200 million people maintain the infection in a state of latency and act as a reservoir of infection, the risk factors for reactivation to active disease and subsequent transmission in these populations are poorly understood. These areas also report some of the lowest rates of efficacy of the Bacillus Calmette–Guérin (BCG) vaccine [2].
Helminth infections (soil transmitted and vector-borne) exhibit broad geographic overlap with areas of TB endemicity [3]. These complex eukaryotes have the ability to establish chronic, often asymptomatic infections and have evolved highly effective methods for subverting the immune system for their survival. Their immunomodulatory effects (as we discuss below) have been shown to extend to nonparasitic infections and vaccine responses [4, 5]. Our knowledge of how helminth-coinfection-induced immune regulation can affect TB-specific immunity and disease in an endemic area comes from three broad areas of study, with specific animal models providing supplemental evidence for particular immunologic phenomena at various stages of helminth infection. First, the effects of chronic maternal helminth infection can lead to in utero sensitization to helminth antigens [6, 7] and have the potential to affect neonatal responses to BCG vaccination [8] as well as TB-specific immunity. This has important implications, as children less than 3 years of age represent the major pediatric disease burden in endemic areas [9]. Secondly, repeated exposure to vector - and soil-transmitted helminths occurs with increasing age, leading to an age-related increase both in helminth prevalence and in the rate of acquiring TB [10, 11]. Lastly, adult subjects in endemic areas with chronic helminth infection show impaired cellular responses that may alter the responses to Mtb antigens and possibly contribute to a higher incidence of active TB disease [12]. In this context, it is important to keep in mind some of the challenges to addressing these questions in a clinical setting. The proper diagnosis of active helminth infection can be challenging and is dependent on various factors such as the species being tested, intensity of infection [13] in a given area, and type of diagnostic test used. Also, polyparasitism is not uncommon, especially for intestinal helminth infections, and newer molecular diagnostic tests might provide higher sensitivity and specificity [14] compared to traditional stool-based techniques. Coinfection with HIV can also be an important confounder, especially for immunologic assessments in these populations. Finally, immunomodulation caused by chronic helminth infection may take a variable amount of time to resolve after treatment (depending on type of species and whether chronic sequelae are present), making prospective studies difficult to perform.
How Does Helminth-Induced Immunomodulation Affect the Repertoire of T Cell Responses to Mycobacteria?
The question of what constitutes protective immunity in human TB is an evolving issue. A few well-defined risk factors such as advanced HIV disease and older age have been established; in addition, the pivotal protective role of a CD4+ response involving primarily interleukin 12 (IL-12), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) (Th1-like) has been established from human genetic and animal model studies [15]. There is experimental evidence that the earliest responses to the infective forms of helminth infections might actually be proinflammatory [16, 17] or of a mixed Th1/Th2 nature [18]. As patency and chronicity is established, however, there is an induction of Th2 populations as well as immunoregulatory T cell populations (both naturally occurring regulatory T cells [nTregs] and adaptive regulatory T cells [iTregs] [19, 20]). The potent immune skewing that occurs as a result of this also affects responses to bystander antigens [21]. In subjects with chronic helminth infections and evidence of mycobacterial infection, in vitro studies have revealed diminished Th1 and Th17 responses to mycobacterial antigens [22–24]; these diminished responses are related to overexpression of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and transforming growth factor beta (TGF-β) and to exaggerated Th2 responses [25]. Restoration of these responses has been documented after treatment of these infections [26].
How Does the Adaptive Skewing of the Immune Response in Helminth Infections Affect Antigen-Presenting Cells (APCs)?
Studies have shown direct and indirect effects of helminths on APCs. Decreased viability and function of dendritic cells (DCs) [27] as well as down-regulation of dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN, CD209), one of the receptors required for Mtb entry into DCs, was seen on exposure to live microfilariae [28]. In addition, impaired resistance to primary infection to Mtb was noted in a mouse model of infection with the intestinal helminth Nippostrongylus brasiliensis mediated through IL-4 receptor–mediated alternative macrophage activation [29]. Finally, subjects with latent TB and filarial coinfection have been shown to exhibit decreased toll-like receptor 2 (TLR2) and toll-like receptor 9 (TLR9) expression, which was reversed after successful antifilarial chemotherapy [30].
Does Maternal Helminth Infection Affect Neonatal Immunity to TB?
It is well established from in vitro and neonatal priming studies in animals that the cytokine/chemokine milieu in which a T cell has its primary encounter with antigen determines the response (Th1/Th2) and the eventual outcome of infection [31]. It is also known that the lack of an optimal Th1 response leads to impaired immunity to mycobacterial infection [15]. Not unexpectedly, therefore, it has been demonstrated that cord blood exposure to parasite antigens from the helminth-infected mother induces both a Th2-predominant response [32] and an expansion of Tregs or IL-10-producing Type 1 regulatory (Tr1) cells. Infants who were sensitized in utero to helminth antigens exhibited a diminished or lack of IFN-γ response to the mycobacterial antigen purified protein derivative (PPD). Additionally, it was shown in the same study that a diminished IFN-γ response to PPD was noted between 10–14 months of age if the pattern of helminth antigen-induced cytokine response at birth was predominantly Th2-like. Using the diagnostic tools available to these investigators, the rates of acquisition of parasitic infection by infants enrolled in this study were very low, suggesting that helminth-induced T cell priming at birth may have long-lasting consequences for immunologic memory. The concern that antenatal parasite infection might result in impaired vaccination response to BCG [33] led eventually to a randomized double blind placebo controlled trial [34] using albendazole and/or praziquantel that demonstrated no measurable effect of maternal deworming on BCG immunization in infants at 1 year of age. Sampling bias might have affected the results of this study, as recently reported by the authors [35].
What Is the Evidence of Helminth Infection Predisposing to TB Disease?
The association between active TB disease and coincident helminth infection has
been investigated primarily in cross-sectional studies [12], as prospective trials would need huge numbers of subjects to be followed over long periods of time. Animal models of mycobacterial challenge using different helminth species have not provided consistent results [36, 37], but it appears that helminth infection does not seem to have a significant impact on bacillary loads and clearance rates. In the only large prospective study conducted to date, baseline helminth infection status did not have any effect on incident rates of active pulmonary tuberculosis or on severity of disease [38].
Summary and Future Directions
Helminths have evolved complex mechanisms for immune subversion, with effects on both adaptive and innate immune responses that lead to their long-term persistence. Spillover effects on mycobacterial antigen responses have been seen in both in vitro and in vivo studies. A possible mechanism of interaction between the two infections is outlined in Fig. 1. No clear consensus has, however, emerged on whether this affects vaccine responses and enhances susceptibility to active TB disease. Although animal models provide important insights into specific pathways of immunomodulation, human studies have suffered from multiple constraints. Prospective studies following large cohorts with serial assessment for both helminth infection status as well as for development of active TB disease are logistically challenging to perform. Future studies will therefore need stringent definitions for inclusion criteria that incorporate the use of highly sensitive and specific diagnostic tools and clear enumeration of confounding variables like HIV, polyparasitism, and measures of intensity and stage of infection.
Fig. 1. Mechanism of immune modulation caused by helminth infections affecting immune responses and susceptibility to TB. 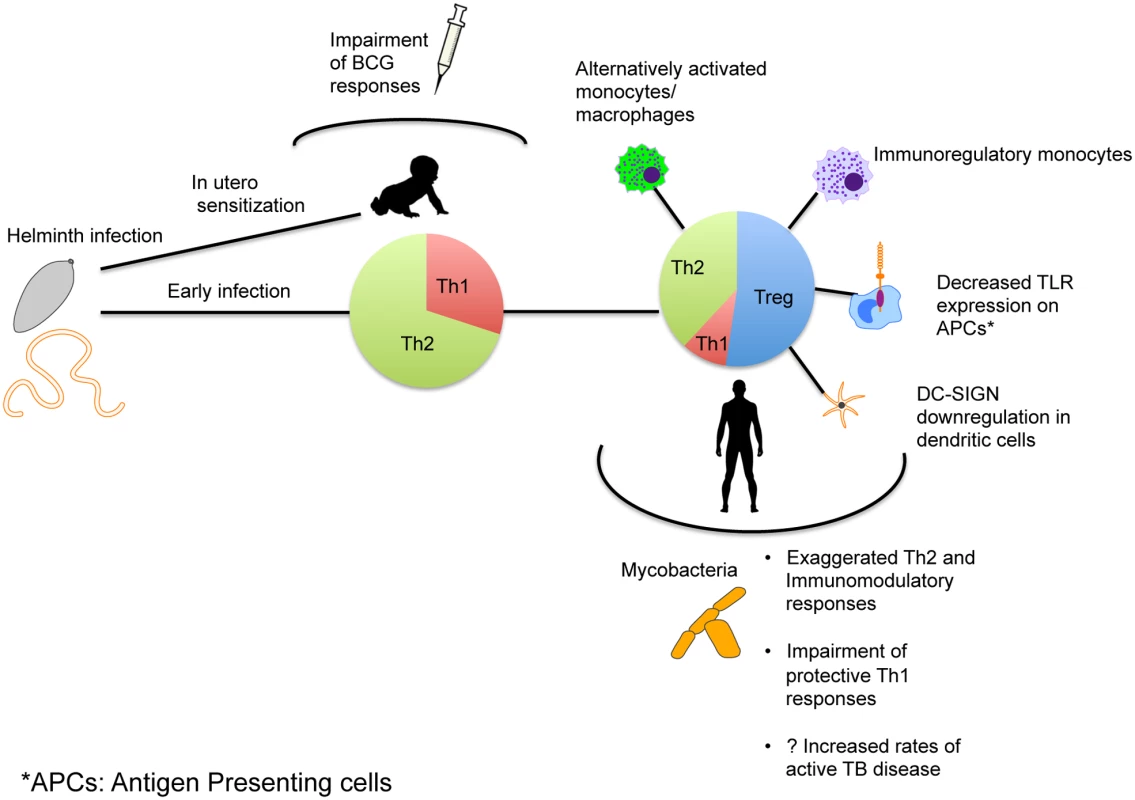
Exposure to helminth infection occurs early in areas of endemicity, with early mixed Th1/Th2 responses eventually leading to expansion of Th2 and Treg responses with establishment of chronic infection. This in turn can alter the phenotype and functionality of antigen-presenting cells as shown. The immune skewing that occurs as a result alters immune responses to Mtb and might affect susceptibility to TB. In utero sensitization to helminth antigens that leads to a similar skewing of the neonatal immune system can occur and thereby alter the immunogenicity of Mtb-specific vaccines.
Zdroje
1. WHO (2013) Global tuberculosis report 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed 22 December 2014. 25473701
2. Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, et al. (1994) Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama 271 : 698–702. doi: 10.1001/jama.271.9.698 8309034
3. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, et al. (2008) Helminth infections: the great neglected tropical diseases. J Clin Invest 118 : 1311–1321. doi: 10.1172/JCI34261 18382743
4. Cooper PJ, Chico ME, Losonsky G, Sandoval C, Espinel I, et al. (2000) Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis 182 : 1199–1206. doi: 10.1086/315837 10979918
5. Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB (2004) Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun 72 : 2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004 15102768
6. Guadalupe I, Mitre E, Benitez S, Chico ME, Nutman TB, et al. (2009) Evidence for in utero sensitization to Ascaris lumbricoides in newborns of mothers with ascariasis. J Infect Dis 199 : 1846–1850. doi: 10.1086/599214 19426111
7. Weil GJ, Hussain R, Kumaraswami V, Tripathy SP, Phillips KS, et al. (1983) Prenatal allergic sensitization to helminth antigens in offspring of parasite-infected mothers. J Clin Invest 71 : 1124–1129. doi: 10.1172/JCI110862 6343433
8. Malhotra I, Mungai P, Wamachi A, Kioko J, Ouma JH, et al. (1999) Helminth - and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol 162 : 6843–6848. 10352306
9. Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, et al. (2006) The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis 10 : 732–738. 16848333
10. Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, et al. (2006) Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis. In: Jamison DT, Breman JG, Measham AR, et al, editors. Disease Control Priorities in Developing Countries. Washington (D.C.): World Bank.
11. Wood R, Liang H, Wu H, Middelkoop K, Oni T, et al. (2010) Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 14 : 406–412. 20202297
12. Elias D, Mengistu G, Akuffo H, Britton S (2006) Are intestinal helminths risk factors for developing active tuberculosis? Tropical medicine & international health: TM & IH 11 : 551–558. doi: 10.1111/j.1365-3156.2006.01578.x 16553939
13. King CL, Connelly M, Alpers MP, Bockarie M, Kazura JW (2001) Transmission intensity determines lymphocyte responsiveness and cytokine bias in human lymphatic filariasis. J Immunol 166 : 7427–7436. doi: 10.4049/jimmunol.166.12.7427 11390495
14. Mejia R, Vicuna Y, Broncano N, Sandoval C, Vaca M, et al. (2013) A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg 88 : 1041–1047. doi: 10.4269/ajtmh.12-0726 23509117
15. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, et al. (1993) An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178 : 2249–2254. doi: 10.1084/jem.178.6.2249 7504064
16. Babu S, Nutman TB (2003) Proinflammatory cytokines dominate the early immune response to filarial parasites. J Immunol 171 : 6723–6732. doi: 10.4049/jimmunol.171.12.6723 14662876
17. Cooper PJ, Mancero T, Espinel M, Sandoval C, Lovato R, et al. (2001) Early human infection with Onchocerca volvulus is associated with an enhanced parasite-specific cellular immune response. J Infect Dis 183 : 1662–1668. doi: 10.1086/320709 11343216
18. Porthouse KH, Chirgwin SR, Coleman SU, Taylor HW, Klei TR (2006) Inflammatory responses to migrating Brugia pahangi third-stage larvae. Infect Immun 74 : 2366–2372. doi: 10.1128/IAI.74.4.2366-2372.2006 16552066
19. Babu S, Blauvelt CP, Kumaraswami V, Nutman TB (2006) Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. Journal of immunology 176 : 3248–3256. doi: 10.4049/jimmunol.176.5.3248 16493086
20. Metenou S, Nutman TB (2013) Regulatory T cell subsets in filarial infection and their function. Front Immunol 4 : 305. doi: 10.3389/fimmu.2013.00305 24137161
21. Kullberg MC, Pearce EJ, Hieny SE, Sher A, Berzofsky JA (1992) Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol 148 : 3264–3270. 1533656
22. Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, et al. (2009) Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis 200 : 288–298. doi: 10.1086/599797 19505258
23. Elias D, Britton S, Aseffa A, Engers H, Akuffo H (2008) Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine 26 : 3897–3902. doi: 10.1016/j.vaccine.2008.04.083 18554755
24. Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R (2007) Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol 147 : 45–52. doi: 10.1111/j.1365-2249.2006.03247.x 17177962
25. Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, et al. (1999) Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol 117 : 517–523. doi: 10.1046/j.1365-2249.1999.01015.x 10469056
26. Elias D, Wolday D, Akuffo H, Petros B, Bronner U, et al. (2001) Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol 123 : 219–225. doi: 10.1046/j.1365-2249.2001.01446.x 11207651
27. Semnani RT, Liu AY, Sabzevari H, Kubofcik J, Zhou J, et al. (2003) Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J Immunol 171 : 1950–1960. doi: 10.4049/jimmunol.171.4.1950 12902498
28. Talaat KR, Bonawitz RE, Domenech P, Nutman TB (2006) Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J Infect Dis 193 : 196–204. doi: 10.1086/498912 16362883
29. Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, et al. (2011) Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. The Journal of experimental medicine 208 : 1863–1874. doi: 10.1084/jem.20091473 21825018
30. Babu S, Bhat SQ, Kumar NP, Anuradha R, Kumaran P, et al. (2009) Attenuation of toll-like receptor expression and function in latent tuberculosis by coexistent filarial infection with restoration following antifilarial chemotherapy. PLoS neglected tropical diseases 3: e489. doi: 10.1371/journal.pntd.0000489 19636364
31. Seder RA, Paul WE (1994) Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol 12 : 635–673. doi: 10.1146/annurev.iy.12.040194.003223 7912089
32. Malhotra I, Ouma J, Wamachi A, Kioko J, Mungai P, et al. (1997) In utero exposure to helminth and mycobacterial antigens generates cytokine responses similar to that observed in adults. J Clin Invest 99 : 1759–1766. doi: 10.1172/JCI119340 9120021
33. Labeaud AD, Malhotra I, King MJ, King CL, King CH (2009) Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis 3: e442. doi: 10.1371/journal.pntd.0000442 19478847
34. Webb EL, Mawa PA, Ndibazza J, Kizito D, Namatovu A, et al. (2011) Effect of single-dose anthelmintic treatment during pregnancy on an infant′s response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet 377 : 52–62. doi: 10.1016/S0140-6736(10)61457-2 21176950
35. Millard JD, Muhangi L, Sewankambo M, Ndibazza J, Elliott AM, et al. (2014) Assessing the external validity of a randomized controlled trial of anthelminthics in mothers and their children in Entebbe, Uganda. Trials 15 : 310. doi: 10.1186/1745-6215-15-310 25100338
36. Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, et al. (2005) Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine 23 : 1326–1334. doi: 10.1016/j.vaccine.2004.09.038 15661380
37. Hubner MP, Killoran KE, Rajnik M, Wilson S, Yim KC, et al. (2012) Chronic Helminth Infection Does Not Exacerbate Mycobacterium tuberculosis Infection. PLoS neglected tropical diseases 6: e1970. doi: 10.1371/journal.pntd.0001970 23285308
38. Chatterjee S, Kolappan C, Subramani R, Gopi PG, Chandrasekaran V, et al. (2014) Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One 9: e94603. doi: 10.1371/journal.pone.0094603 24728010
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



