-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaModulates the Unfolded Protein Response in during Infection
In the model organism Caenorhabditis elegans, the IRE1–XBP1 pathway, a major branch of the unfolded protein response (UPR), is required for host defense against pathogens. However, how innate immune responses activate the UPR is not fully understood. In this report, we find that Pseudomonas aeruginosa PA14 infection up-regulates the expression of the microRNA mir-233 in C. elegans. The response of mir-233 to P. aeruginosa PA14 infection is dependent on a major pathway of innate immunity, the p38 MAPK signaling cascade. The up-regulation of mir-233 is functionally important since a mutation in mir-233 leads to hypersensitivity of the nematode to the killing by P. aeruginosa PA14. Furthermore, we demonstrate that mir-233 contributes to the activation of the UPR by repressing the protein levels of its target SCA-1, a C. elegans homologue of the sarco/endoplasmic reticulum Ca2+-ATPase. Thus, mir-233 is an important regulator of the UPR during the innate immune response.
Published in the journal: . PLoS Pathog 11(1): e32767. doi:10.1371/journal.ppat.1004606
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004606Summary
In the model organism Caenorhabditis elegans, the IRE1–XBP1 pathway, a major branch of the unfolded protein response (UPR), is required for host defense against pathogens. However, how innate immune responses activate the UPR is not fully understood. In this report, we find that Pseudomonas aeruginosa PA14 infection up-regulates the expression of the microRNA mir-233 in C. elegans. The response of mir-233 to P. aeruginosa PA14 infection is dependent on a major pathway of innate immunity, the p38 MAPK signaling cascade. The up-regulation of mir-233 is functionally important since a mutation in mir-233 leads to hypersensitivity of the nematode to the killing by P. aeruginosa PA14. Furthermore, we demonstrate that mir-233 contributes to the activation of the UPR by repressing the protein levels of its target SCA-1, a C. elegans homologue of the sarco/endoplasmic reticulum Ca2+-ATPase. Thus, mir-233 is an important regulator of the UPR during the innate immune response.
Introduction
The innate immune system, the first line of defense against microbial infection, is evolutionarily conserved in both vertebrate and invertebrate animals. Activation of the innate immune system upon pathogen infection results in a definitive anti-microbial response to invading microbes. The genetically tractable model organism Caenorhabditis elegans has contributed greatly to advancing our understanding of innate immunity in animals [1], [2]. During the last decade, C. elegans-based studies have identified a variety of signaling pathways involved in innate immunity, including the p38 mitogen activated protein kinase (MAPK) PMK-1 signaling pathway [2], [3]. The p38 MAPK pathway plays a major role in the immune responses to pathogens, mainly through regulating the expression of secreted antimicrobials, including C-type lectins, ShK toxins, and CUB-like genes [4].
In eukaryotic cells, the endoplasmic reticulum (ER) is a principal site for the folding and maturation of most secreted and transmembrane proteins [5], [6]. Increased load of misfolded proteins that enter the ER can lead to ER stress. To cope with this stress, cells trigger a signal transduction system collectively termed the unfolded protein response (UPR). The UPR is conserved from yeast to human and is an integrated intracellular signaling pathway that links the ER lumen with the cytoplasm and nucleus. Accumulating evidence has revealed that innate immunity is a physiologically relevant source of ER stress in C. elegans [7], [8]. The PMK-1 signaling pathway is required for activation of the UPR induced by Pseudomonas aeruginosa infection [7] or pore-forming toxins produced by human pathogens, such as Staphylococcus aureus, Streptococcus pyogenes, and Aeromonas hydrophilia [8]. However, the molecular mechanism underlying activation of the UPR by PMK-1 in these processes remains unclear.
MicroRNAs (miRNAs), a class of RNA molecules of 22 nucleotides (nt) long, are pivotal regulators of gene expression in metazoa [9]. In animals, miRNAs mainly target specific mRNAs through imperfect base pairing with the 3′-untranslated region (3′ UTR) of these mRNAs [10]. miRNAs are involved in a wide variety of biological processes, including patterning of the nervous system, inflammation and immunity, cell death and proliferation, and development [11]. Since the first two miRNAs, lin-4 and let-7, were identified as regulators of developmental timing [12], [13], more than 175 of miRNA genes have been confirmed in C. elegans [14]. Previous studies have revealed that a reduction in total miRISC activity or mutations in dcr-1, drsh-1 and alg-1 genes enhances worm resistance to pathogenic bacteria P. aeruginosa PA14 or Bacillus thuringiensis DB27 [15], [16]. As these genes are required for miRNA processing, these results imply that miRNAs are probably involved in innate immune responses to pathogenic bacteria. Furthermore, Liu et al. [17] have demonstrated that the mir-84(n4037) and mir-241(n4316) mutants exhibit enhanced resistance, whereas the mir-48(n4097) mutant worms exhibit decreased resistance to P. aeruginosa infection. Thus, different let-7 miRNA homologs play distinct roles in innate immune responses to bacterial infection.
To better understand the role of miRNAs in innate immunity, we used RNA deep sequencing to carry out a comprehensive survey of miRNA expression in wild type (WT) animals grown on live P. aeruginosa PA14. We screened the up-regulated miRNAs and discovered that mir-233 was required for resistance to P. aeruginosa PA14 infection. Using a proteomic approach, we identified that sca-1, which encodes a C. elegans homologue of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) [18], was the target of mir-233. Down-regulation of SCA-1 protein levels by mir-233 resulted in activation of the UPR, which in turn conferred resistance to P. aeruginosa PA14 infection. Finally, our data demonstrate that the UPR pathway functions in the intestine, the major site of pathogen exposure.
Results
mir-233 is required for C. elegans innate immunity
To explore whether miRNAs are involved in innate immunity in C. elegans, we determined the miRNA expression profiles in worms exposed to P. aeruginosa PA14 using small RNA deep sequencing. We found that 40 miRNAs at 4 h, 68 miRNAs at 8 h, and 64 miRNAs at 12 h post-infection were up-regulated, respectively (S1 Table). We hypothesized that some of the miRNAs up-regulated in response to bacterial infection play a role in C. elegans innate immunity. Thus, we focused on the 88 miRNAs and miRNA families that were up-regulated after PA14 infection. To identify individual miRNAs that play prominent roles in innate immunity, we tested 47 available mutant strains of these 88 miRNAs. Whereas mutations in most of the tested miRNAs did not influence the immune phenotype, mir-232(ndf56);F13H10.5 and mir-233(n4761) mutants exhibited enhanced susceptibility to the killing by PA14 (Fig. 1A and S2 Table). Using quantitative RT-PCR (qRT-PCR), we confirmed that the expression of mir-232 was markedly elevated in worms at 4 h, 8 h, and 12 h after exposure to P. aeruginosa PA14, compared with worms grown in the standard laboratory food Escherichia coli OP50 (Fig. 1B). Meanwhile, up-regulation of mir-233 was observed in worms at 4 h post-infection. Furthermore, using transgenic animals that express mir-232p::gfp or mir-233p::gfp, we observed that PA14 infection significantly increased expression of mir-232p::gfp (Fig. 1C) and mir-233p::gfp (Fig. 1D).
Fig. 1. P. aeruginosa infection up-regulates the expression of mir-232 and mir-233, which are required for innate immunity. 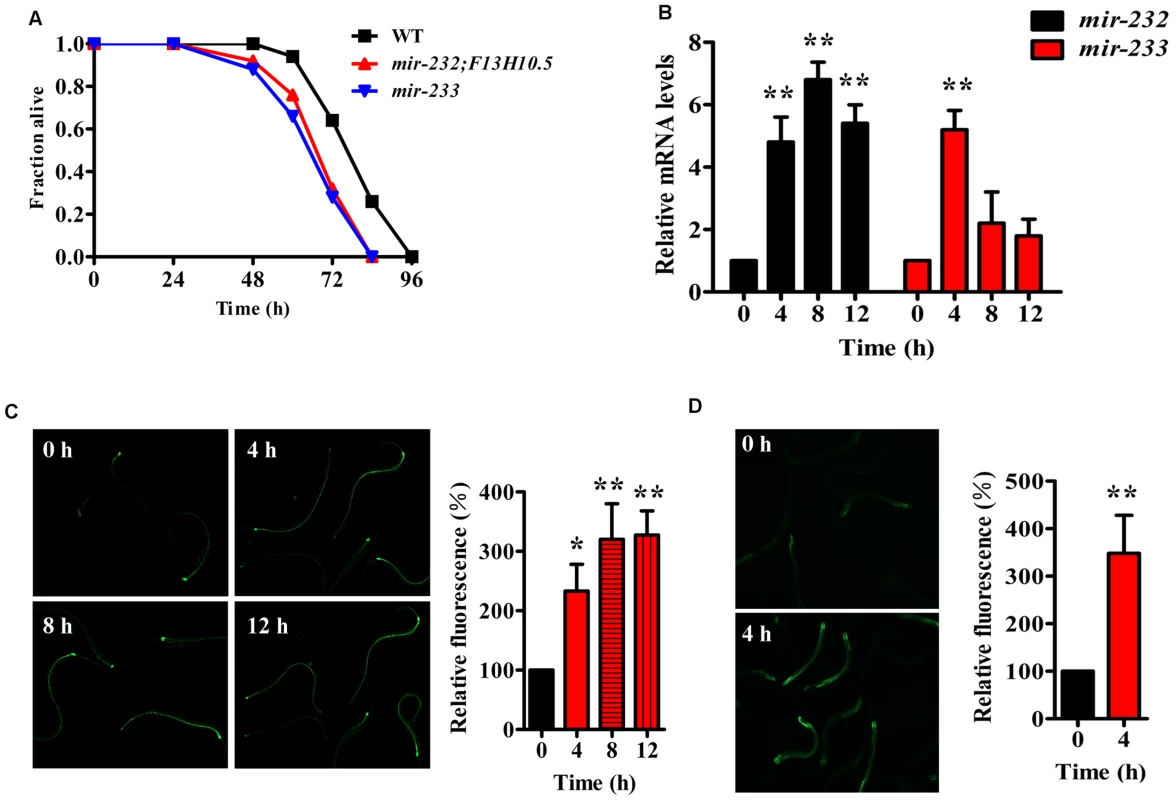
(A) Mutations in mir-232 and mir-233 reduced survival of worms exposed to P. aeruginosa PA14. The survival graph represents combined results of three independent experiments. P<0.01 versus wild type (WT). (B) qRT-PCR analysis of expression of mir-232 and mir-233 in WT worms after PA14 infection. The data are expressed as percent of control (the value of “0 hour” time point). **P<0.01 versus worms at 0 h. (C–D) The expression of mir-232p::gfp (C) and mir-233p:: gfp (D) in WT worms was significantly up-regulated by PA14 infection. The right parts show quantification of GFP levels. *P<0.05; **P<0.01 versus worms at 0 h. The mir-233(n4761) mutant is a deletion that removed not only mir-233, but also the gene W03G11.4. However, knockdown of W03G11.4 by RNAi had no impact on survival of WT worms after P. aeruginosa PA14 infection (S1A–S1C Fig.). Thus, the immune-deficient phenotype of the mir-233(n4761) mutant was not due to the removal of W03G11.4. Likewise, the mir-232(ndf56);F13H10.5 mutant animals also have two mutations. We found that knockdown of F13H10.5 by RNAi led to enhanced resistance to PA14 infection (S1D Fig.). These results suggest that the mutations in mir-232(ndf56) and F13H10.5 display a mixed effect on innate immunity. Thus, we focused on the role for mir-233 in innate immunity. In addition to its defect in a response to P. aeruginosa PA14 infection, the mir-233(n4761) worms were more sensitive to killings by the Gram-positive bacteria S. aureus ATCC 25923, Enterococcus faecalis ATCC 29212, and the Gram-negative bacterium Salmonella typhimurium 468 than WT animals (S2A–S2C Fig.). Furthermore, we found that the mir-233(n4761) mutant exhibited a comparable lifespan to WT worms (S3 Fig.), suggesting that the effect of mir-233 on the defense against P. aeruginosa is not due to changes in aging.
SCA-1 is a target of mir-233
Animal miRNAs usually bind to their target mRNAs, thus resulting in down-regulation of protein translation. It is reasoned that in response to bacterial infection, mir-233 functions to silence genes that have a negative impact on C. elegans innate immunity. Three different miRNA target prediction algorithms (MICRORNA, TargetScan, and DIANA-microT) suggested 38 putative target genes of mir-233 (S4 Fig.). To confirm the target genes of mir-233 that are involved in innate immunity, we used isobaric tags for relative and absolute quantitation (iTRAQ)-based quantitative proteomic approach to analyze the early changes in protein levels in worms exposed to P. aeruginosa PA14. As a commonly used quantitative proteomics method, iTRAQ allows for simultaneous comparative quantification of multiple samples within a single run, leading to a reduction of the experimental errors produced from individual experiments [19]. We found that the expression of 51, 45, and 85 proteins were up-regulated, while 19, 115, and 133 proteins were down-regulated at 4 h, 8 h, and 12 h post-infection, respectively (Fig. 2A and S3, S4, S5 Tables). However, when comparing our protein data set to two microarray data sets from the studies published by Shapira et al [20] and Troemel et al [4], we found very little overlap between our protein data set and the two microarray data sets. These results suggest that these gene expression changes are not reflected in our proteomic analysis. Many factors such as posttranscriptional regulation, variable rates of protein turnover, and post-translational modification, may account for the observed discordance between transcript and protein levels [21].
Fig. 2. The changes of protein levels in worms after P. aeruginosa infection. 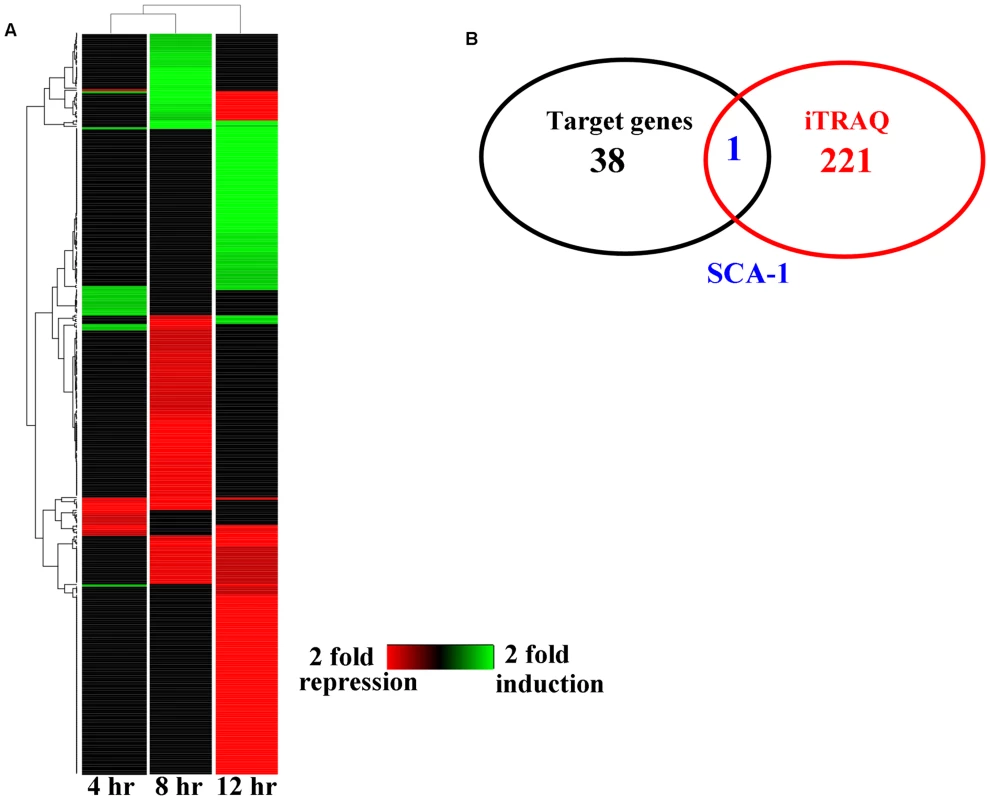
(A) Heat maps show significantly up- and down-regulated proteins after P. aeruginosa PA14 infection. Protein expression profiles were clustered hierarchically. (B) A Venn diagram comparing the overlap in target genes of mir-233 and proteins down-regulated by P. aeruginosa PA14 infection. We found only one gene (sca-1) overlapped between the down-regulated-protein encoding genes and the target genes of mir-233 (Fig. 2B). The protein levels of SCA-1 were markedly reduced at 8 h post-infection (Fig. 2A and S4 Table). miRNA target prediction algorithms revealed that the sca-1 gene contained one putative binding site for mir-233 in the 3′UTR region (Fig. 3A). SCA-1 is the C. elegans homologue of the mammalian SERCAs, which shows about 70% amino acid sequence identity and 80% similarity to the three human SERCA proteins [18]. Using Western blotting, we found that the protein levels of SCA-1 were decreased in WT worms at 8 h after P. aeruginosa PA14 infection (Fig. 3B). However, the protein levels of SCA-1 in the mir-233(n4761) mutant were significantly higher than those in WT worms after P. aeruginosa PA14 infection (Fig. 3B). In contrast, the mRNA levels of sca-1 in WT and the mir-233(n4761) animals were similar after PA14 infection (Fig. 3C), indicating that transcriptional regulation is unlikely to be involved in the reduced SCA-1 protein levels. To address whether mir-233 regulated the protein levels of SCA-1 through 3′UTR, we constructed a 4×NLS::GFP vector driven by the rpl-28 promoter, which contained the 3′UTR of sca-1 (Prpl-28::gfp:sca-1 3′UTR) [22]. Meanwhile, a sca-1-3′UTR mutant reporter construct was generated by replacing the putative mir-233 binding site with an oligonucleotide containing the exact identical sequence of mir-233. Specially, an Prpl-28::histone-24::mCherry:let-858 3′UTR construct that drives constitutive and mCherry expression was used as an internal control. We found that the GFP expression was markedly reduced in WT worms at 8 h after P. aeruginosa PA14 infection (Fig. 3D). However, mutagenesis of the putative binding site for mir-233 in the sca-1-3′UTR abolished the inhibition of the GFP expression in WT worms. Furthermore, we found that the GFP expression was much higher in the mir-233(n4761) mutant than that in WT worms after PA14 infection (Fig. 3D). These results suggest that mir-233 suppresses the protein levels of SCA-1 through binding to its 3′UTR and inhibiting its translation after PA14 infection.
Fig. 3. sca-1 is a target gene of mir-233. 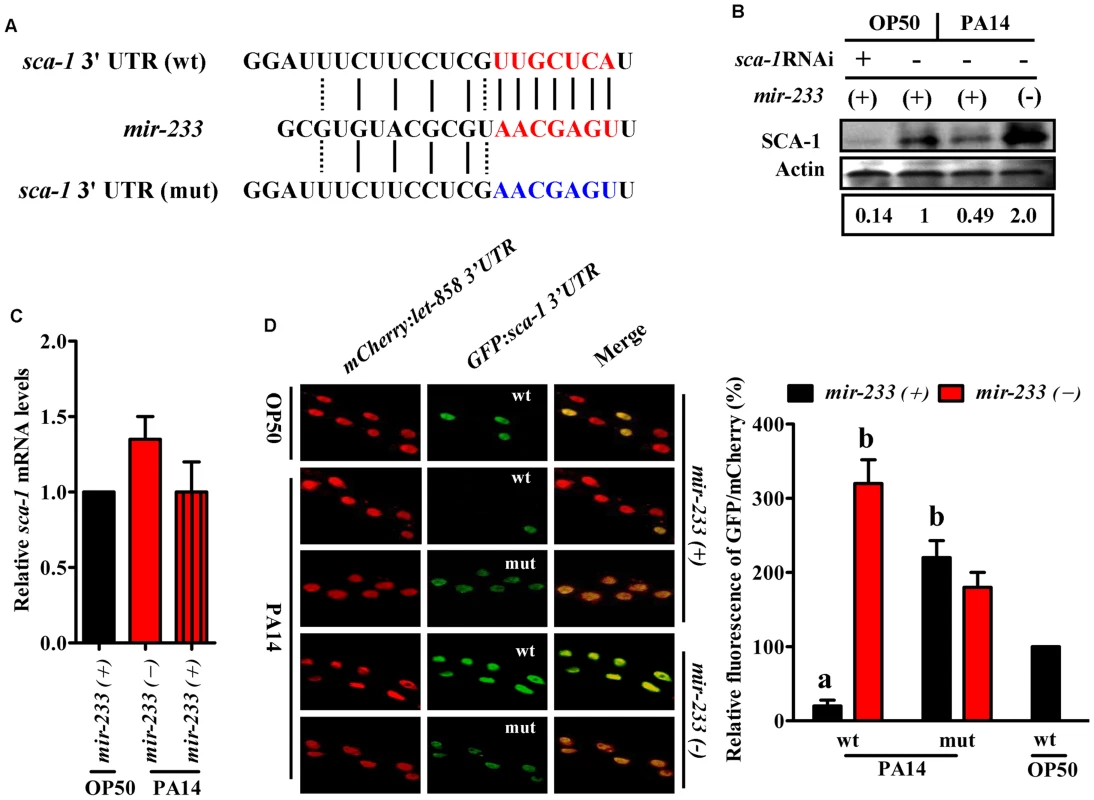
(A) Complementarity between the 3′ UTR of sca-1 gene and mir-233. mir-233 is presented in the form of DNA from 3′ to 5′ end. WT seeds are marked in red, mutated seeds in blue, Watson-Crick base pairing with a straight line and U-G wobbles with a dotted line. For constructing of sca-1-3′UTR (mut) reporter, the putative mir-233 binding site was replaced with an oligonucleotide containing the exact complementary sequence of mir-233. (B) The protein levels of SCA-1 were determined by Western blotting at 8 h after PA14 infection. The blot is typical of three independent experiments. P<0.05, mir-233(+) (wild type worms)+PA14 versus mir-233(+)+OP50; P<0.01, mir-233(-) (mir-233(n4761) mutant worms)+PA14 versus mir-233(+)+PA14; mir-233(+)+OP50+sca-1 RNAi versus mir-233(+)+OP50. (C) Deletion of mir-233 did not affect the mRNA levels of sca-1. (D) Fluorescence images of the sca-1-3′ UTR GFP reporter in worms grown on E. coli OP50 or P. aeruginosa PA14. Mutagenesis of the putative binding site for mir-233 in the sca-1-3′UTR led to derepression of the GFP expression in mir-233(+) worms after PA14 infection. Meanwhile, the GFP expression was significantly higher in mir-233(−) worms than that in mir-233(+) worms after PA14 infection. The lower panel shows quantification of GFP/mCherry ratio. The data are expressed as percent of control (the ‘wt’ construct on OP50). aP<0.01 versus mir-233(+)+wt on OP50; bP<0.01 versus mir-233(+)+wt on PA14. SCA-1 is a negative regulator of innate immunity in C. elegans
A mutation in sca-1(mca-4) has shown to result in embryonic and larval lethality [18], [23]. To test the role of sca-1 in pathogen susceptibility, we reduced the expression of the gene by RNAi. However, as demonstrated previously, the majority of sca-1(RNAi) worms died or were arrested at the L1 stage [18], [23], [24]. To overcome this problem, we diluted the sca-1 RNAi bacteria with an empty vector control at a ratio of 1 to 4. This dilution modified the severity of the phenotype enough to enable development. qRT-PCR analysis demonstrated that knockdown of sca-1 by RNAi in 1/4 dilution reduced approximately 50% of sca-1 mRNA levels (S5 Fig.). The 1/4 sca-1 RNAi exhibited enhanced resistance to P. aeruginosa PA14 infection in WT worms, compared to those subjected to the vector control RNAi (Fig. 4).
Fig. 4. sca-1 RNAi leads to enhanced resistance to P. aeruginosa infection. 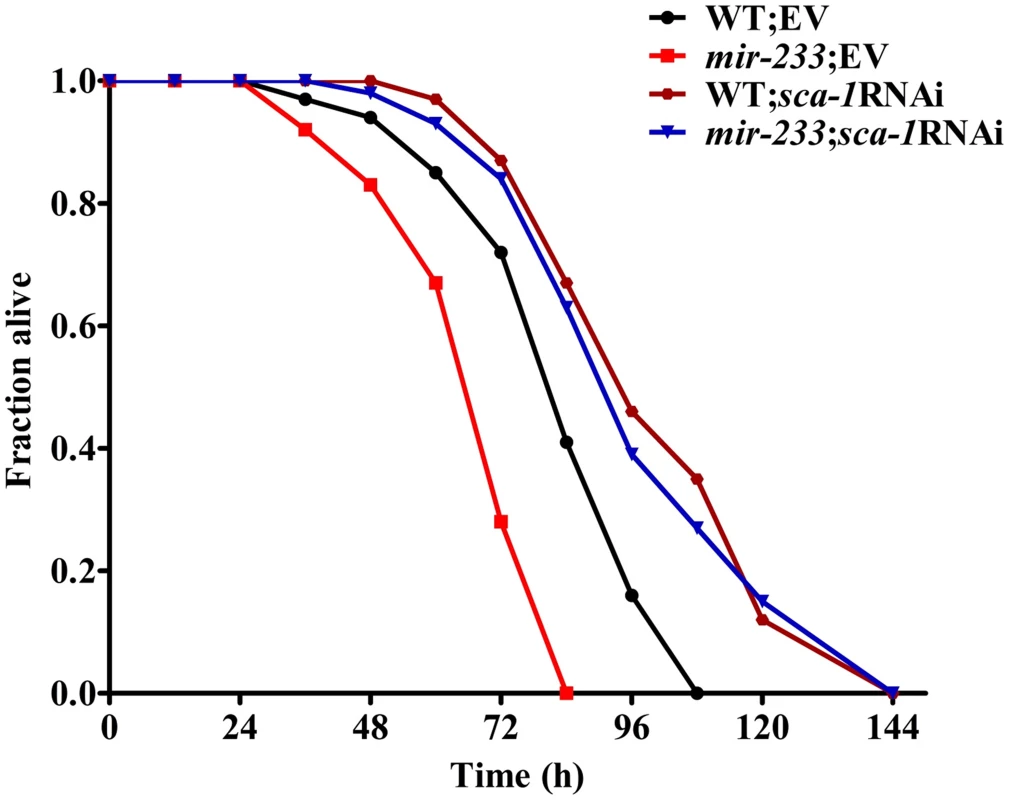
The survival graph represents combined results of three independent experiments. P<0.01 versus wild type worms (WT)+empty vector (EV). Next, we determined whether up-regulated expression of SCA-1 protein contributed to the immune-deficient phenotype of the mir-233(n4761) mutant. The mir-233(n4761) mutant subjected to sca-1 RNAi showed markedly increased survival relative to the mir-233(n4761) mutant exposed to the empty vector, to a degree that was comparable to the survival of WT worms subjected to sca-1 RNAi (Fig. 4). These results suggest that mir-233 is involved in innate immunity by suppressing SCA-1 expression.
mir-233 initiates the UPR by suppressing SCA-1
A previous study has demonstrated that P. aeruginosa PA14 infection activates the UPR [7]. The UPR is comprised of three branches, the ribonuclease inositol-requiring protein–1 (IRE-1), the PERK kinase homologue PEK-1, and the transcription factor ATF6 [5], [6]. In C. elegans, the IRE-1-XBP-1 pathway is required for resistance to P. aeruginosa infection or to the treatment of pore-forming toxins [7], [8]. IRE-1 splices an intron from XBP1 mRNA, producing the activated ‘spliced form’ of XBP-1 (XBP-1s) [5], [6]. As sca-1 RNAi leads to the UPR [25], we tested the role of mir-233 in the UPR. First, the levels of xbp-1s transcript were increased approximately 4-fold in WT worms, but not in the mir-233(n4761) mutant, at 8 h after P. aeruginosa PA14 infection (Fig. 5A). However, sca-1 RNAi restored the expression of xbp-1s in the mir-233(n4761) mutant exposed to PA14. The induction of BiP/GRP78, a molecular chaperone, reflects activation of the IRE-1-XBP-1 branch of the UPR [5], [6]. In C. elegans, hsp-4 gene encodes a homologue of mammalian BiP/GRP78, which is a target of XBP-1. Second, we detected the UPR activation using transgenic worms carrying Phsp-4::gfp [26], and found that P. aeruginosa PA14 infection induced the expression of Phsp-4::gfp in WT worms, but not in the mir-233(n4761) mutant (Fig. 5B). Furthermore, the expression of Phsp-4::gfp in the mir-233(n4761) mutant was significantly restored by sca-1 RNAi after PA14 infection (Fig. 5C). Similar results were obtained using qRT-PCR analysis for the mRNA levels of hsp-4 (Fig. 5D and 5E). Taken together, these results indicate that mir-233 is critically involved in silencing the sca-1 transcript to activate the IRE-1/XBP-1 pathway.
Fig. 5. mir-233 mediates the XBP-1-dependent UPR induced by P. aeruginosa infection. 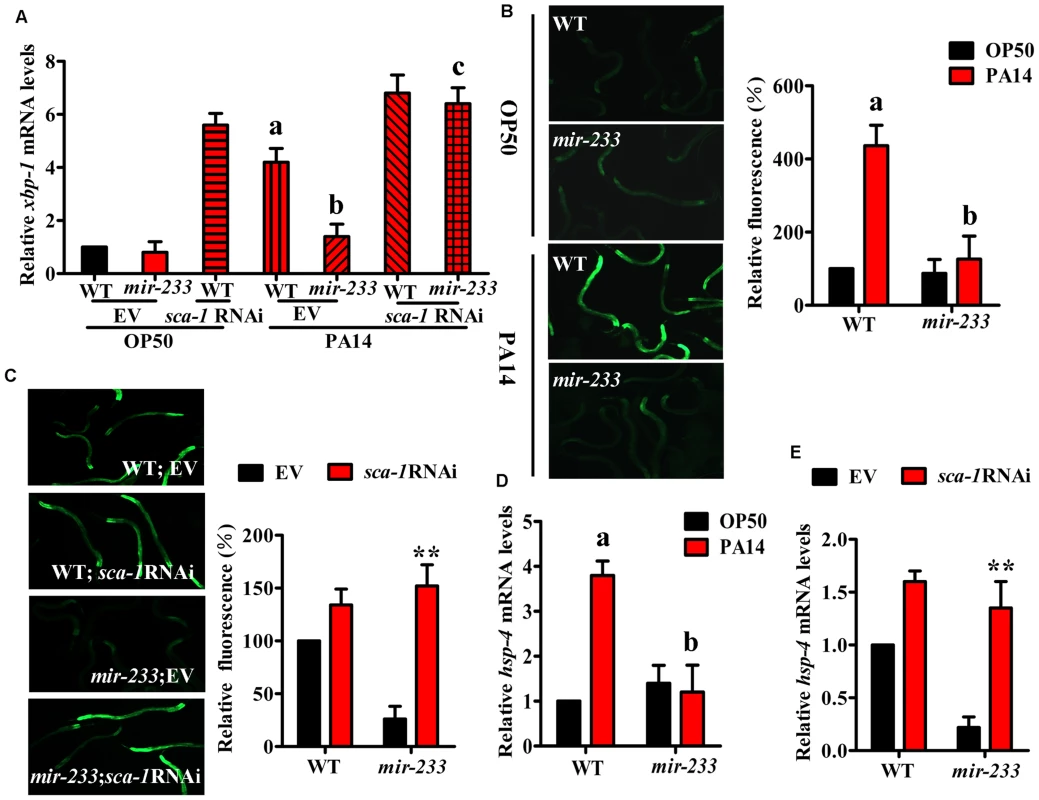
(A) qRT–PCR analysis of spliced xbp-1 mRNA levels at 8 h after P. aeruginosa PA14 infection. aP<0.01 versus wild type (WT) worms+EV on E. coli OP50; bP<0.05 versus WT+EV on PA14; cP<0.01 versus mir-233(n4761) on PA14. (B) Fluorescence images of WT and mir-233(n4761) animals carrying the Phsp-4::gfp transgene at 8 h after PA14 infection. The data are expressed as percent of control (WT on OP50). aP<0.01 versus WT on OP50; bP<0.05 versus WT on PA14. (C) sca-1 RNAi restored the expression of Phsp-4::gfp in the mir-233(n4761) worms grown on P. aeruginosa PA14. **P<0.01 versus mir-233+empty vector (EV). (D–E) qRT-PCR analysis of hsp4 mRNA levels after PA14 infection. (D) aP<0.01 versus WT on OP50; bP<0.05 versus WT on PA14. (E) **P<0.01 versus mir-233+EV. A previous study has suggested that the effect of the IRE-1/XBP-1 signaling on worm survival upon P. aeruginosa infection is due to increased tolerance and not increased resistance [7]. Here we tested P. aeruginosa accumulation in the intestine of the mir-233(n4761) mutant or worms subjected to sca-1 RNAi. We found that, like a mutation in xbp-1(zc12), the mutation in mir-233 or knockdown of sca-1 had no impact on the colony forming units (CFU) of PA14 in worms (S6A Fig.). Moreover, the accumulation of P. aeruginosa expressing GFP in the mir-233(n4761) or sca-1(RNAi) worms was comparable to that observed for WT worms with empty vector (S6B Fig.). In contrast, a mutation in pmk-1(km25), a conserved p38 MAPK pathway that plays a crucial role in innate immunity, resulted in a significant increase in accumulation of P. aeruginosa [7] (S6A–S6B Fig.). These results implicate that innate immune pathways may play distinct roles in the pathogenesis of infectious diseases.
PMK-1-mediated activation of the UPR through the mir-233/SCA-1 pathway
A previous study has shown that activation of the UPR in response to P. aeruginosa infection or to the pore-forming toxins produced by many human pathogens is mediated by the p38 MAPK orthologue PMK-1 [3], [8]. These results raised the possibility that PMK-1 could regulate the UPR through the mir-233/SCA-1 pathway. To test this hypothesis, we first examined the effect of PMK-1 on mir-233 expression. qRT-PCR analysis indicated that a mutation in pmk-1(km25) led to a significant decrease in both the levels of mir-233 and the expression of mir-233p::gfp at 4 h after PA14 infection (Fig. 6A and 6B). Second, the protein levels of SCA-1 in the pmk-1(km25) mutant were markedly higher than those in WT worms during PA14 infection (Fig. 6C). Finally, the expressions of xbp-1s and hsp-4 were reduced in the pmk-1(km25) mutant, compared with WT worms after PA14 infection (Fig. 6D and 6E), consistent with the observation reported by Richardson et al [7]. However, sca-1 RNAi restored the expressions of xbp-1s and hsp-4 in the pmk-1(km25) mutant to the same levels as WT worms (Fig. 6D and 6E). These results suggest that activation of the IRE-1/XBP-1 signaling by PMK-1 is through the mir-233/SCA-1 pathway.
Fig. 6. The mir-233/SCA-1 signaling functions as a downstream effector of p38 MAPK in the activation of the UPR. 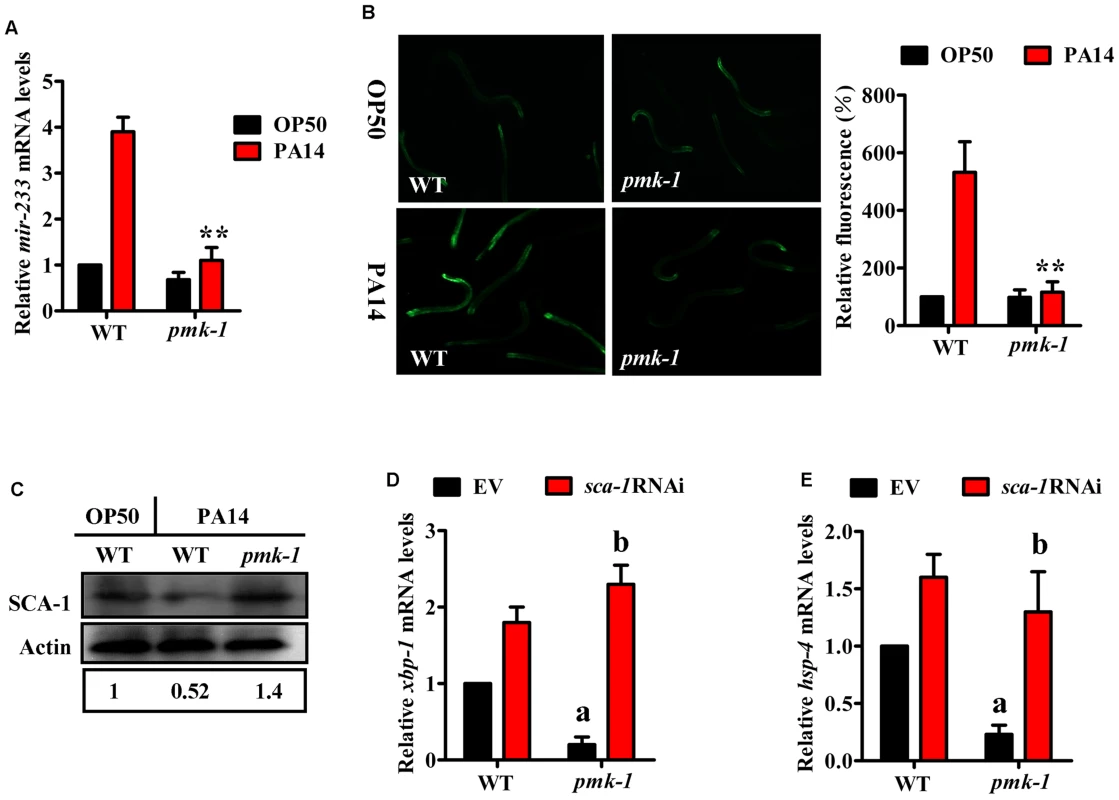
(A) qRT-PCR analysis of mir-233 expression in wild type (WT) and pmk-1(km25) worms at 4 h after P. aeruginosa PA14 infection. **P<0.01 versus WT on PA14. (B) The expression of mir-233p::gfp was significantly down-regulated by pmk-1 RNAi at 4 h after P. aeruginosa PA14 infection. The right parts show quantification of GFP levels. The data are expressed as percent of control (WT). **P<0.01 versus WT on PA14. (C) The protein levels of SCA-1 were determined by Western blotting after P. aeruginosa PA14 infection for 8 h. The blot is typical of three independent experiments. P<0.05, WT+PA14 versus WT+OP50; P<0.01, pmk-1(km25)+PA14 versus WT+PA14. (D–E) The expressions of xbp-1s (D) and hsp-4 (E) were determined by qRT-PCR at 8 h after PA14 infection. (D) aP<0.01 versus WT+empty vector (EV); bP<0.01 versus pmk-1(km25)+EV. (E) aP<0.01 versus WT+EV; bP<0.05 versus pmk-1(km25)+EV. It has been shown that the transcription factor ATF-7 functions as a regulator of PMK-1-mediated innate immunity via repression of immune gene expression [27], raising a possibility that the PMK-1-induced expression of mir-233 is mediated by ATF-7. To test this hypothesis, we tested the role of ATF-7 on mir-233 expression. However, we found that the expression of mir-233 in the atf-7(gk715) mutant was comparable to that in WT worms after PA14 infection (S7A Fig.). Meanwhile, atf-7 RNAi did not influence the expression of mir-233p::gfp in either WT or pmk-1(km25) worms after PA14 infection (S7B Fig.). Thus, ATF-7 is not involved in the induction of mir-233 mediated by PMK-1.
The UPR in the intestine is involved in resistance to P. aeruginosa infection
The IRE-1-XBP-1 signaling has shown to be required for resistance to P. aeruginosa infection during larval development [7]. We found that, like WT worms, all the sca-1(RNAi) worm eggs grown on plates with P. aeruginosa PA14 as the only food source could develop to the fourth larval stage (L4), comparable to their development on E. coli OP50 (S8 Fig.). In contrast, unlike WT or sca-1(RNAi) worms, the mir-233(n4761) mutant on P. aeruginosa PA14 plates exhibited severely attenuated larval development, and approximately 60% larva could not reach the L4 stage by 72 h (S8 Fig.).
Next, we asked if XBP-1 was required for resistance to P. aeruginosa PA14 in adult worms. We found that the xbp-1(zc12) mutant exhibited enhanced sensitivity of young adult worms to the killing by P. aeruginosa PA14 (Fig. 7A). It should be noted that the lifespan of the xbp-1(zc12) mutant was similar to that of WT worms (S9 Fig.), suggesting that the impact of XBP-1 on the defense against P. aeruginosa is not due to changes in aging. It has been reported that PEK-1, a branch of the UPR that is distinct from the IRE-1-XBP-1 pathway, is important for the worm resistance to P. aeruginosa infection under lower temperatures [28]. However, we found that the survival of the pek-1(ok275) mutant was comparable to that of WT animals after PA14 infection at standard temperature (25°C) (S10 Fig.).
Fig. 7. The SCA-1/XBP-1 pathway in the intestine functions as a regulator of innate immunity mediated by PMK-1. 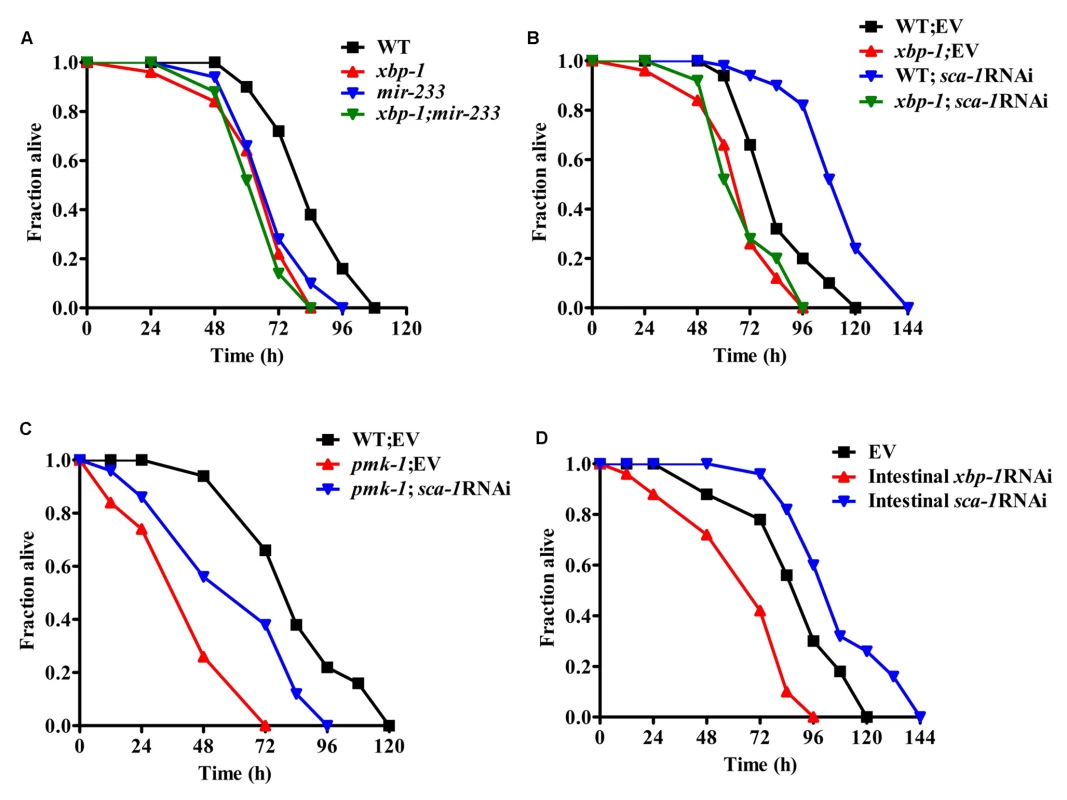
(A) Both xbp-1(zc12) and mir-233(n4761) mutants were more sensitive than wild type (WT) worms to the killing by P. aeruginosa PA14. P<0.01 versus WT. However, the mutation in mir-233(n4761) did not enhance susceptibility of the xbp-1(zc12) mutant to PA14 infection. (B) Although knockdown of sca-1 by RNAi promoted the survival of worms to PA14 infection, the survival of xbp-1(zc12); sca-1 RNAi worms was comparable to that of the xbp-1(zc12) worms after PA14 infection. P<0.01 versus WT+empty vector (EV). (C) The survival of the pmk-1(km25) mutant was partially restored by knockdown of sca-1. P<0.001, pmk-1(km25) versus WT; P<0.01, pmk-1(km25); sca-1 RNAi versus pmk-1(km25)+EV. (D) Intestinal-specific RNAi of sca-1 enhanced resistance, whereas intestinal-specific RNAi of xbp-1 led to enhanced sensitivity to PA14 infection. P<0.01 versus control (EV). All the survival graphs represent combined results of three independent experiments. The survival of the xbp-1(zc12);mir-233(n4761) double mutants was comparable to that of the xbp-1(zc12) mutant worms after PA14 infection (Fig. 7A). Although sca-1(RNAi) worms were more resistant to PA14 infection than WT worms, the survival of xbp-1(zc12); sca-1 RNAi was comparable to that of the xbp-1(zc12) mutant after P. aeruginosa PA14 infection, suggesting that the immune-resistant phenotype of sca-1(RNAi) worms is XBP-1-dependent (Fig. 7B). Unlike the mir-233(n4761) mutant, the immune-deficient phenotype of the pmk-1(km25) mutant was only partially rescued by knockdown of sca-1 (Fig. 7C). These results implicate that the UPR signaling is one of the downstream effectors in PMK-1-mediated innate immunity. Indeed, the survival of the xbp-1;pmk-1 double mutants was comparable to that of the pmk-1(km25) mutant (S11 Fig.).
We noticed that mir-233 was highly expressed in the intestine of C. elegans (S12 Fig.). We found that intestinal-specific sca-1 RNAi resulted in enhanced resistance to PA14 infection (Fig. 7D), whereas epidermal - and muscular-specific sca-1 RNAi did not affect the survival of worms (S13A–S13B Fig.). Likewise, intestinal-specific RNAi of xbp-1 enhanced sensitivity to PA14 infection (Fig. 7D), whereas epidermal - and muscular specific RNAi of xbp-1 failed to affect the survival of worms (S14A–S14B Fig.). These data suggest that the UPR in the intestine probably is required for innate immunity.
Discussion
The accumulation of unfolded proteins beyond the levels that ER can cope with leads to ER stress, which in turn activates ER stress signaling, the UPR [6]. Many of the molecular chaperones within the ER, such as GRP78, calnexin and calreticulin, are Ca2+ binding proteins [29]. Thus, the luminal Ca2+ is crucial for proper folding and maturation of proteins in the ER [30]. As a Ca2+-ATPase, the SERCA pump is responsible for transferring Ca2+ from the cytosol to the ER lumen via ATP hydrolysis [31]. In mammalian cells, inhibition of SERCA activity or down-regulation of SERCA expression is among the main mechanisms that evoke the UPR under a variety of pathophysiological conditions [30], [32]. For example, the oxidized LDL can induce oxidative stress in endothelial cells, which in turn inhibits SERCA by enhancing oxidation of the Cys674 residue of these ATPases [32]. Disturbed ER calcium stores leads to the UPR. In C. elegans, the UPR is induced in response to P. aeruginosa infection or to pore-forming toxins produced by a variety of human pathogens, suggesting that the innate immune responses represent a physiologically relevant inducer of the UPR [7], [8]. Our study reveals two novel regulatory components of the UPR: mir-233 and SCA-1. During P. aeruginosa infection, mir-233 negatively regulates the protein expression of SCA-1 through direct base pairing to the 3′ UTR of its mRNA. Suppression of SCA-1, in turn, promotes the activation of the UPR, which confers resistance to the pathogen.
In the current study, mir-233 was identified by screening 47 up-regulated miRNAs for susceptibility to P. aeruginosa infection. The mir-233(n4761) mutant animals were found to be susceptible to several other bacterial pathogens. These results suggest that mir-233 is required for immune responses to pathogenic bacteria in general. As demonstrated previously [33], [34], the mir-233(n4761) mutant animals showed normal phenotypes including locomotion, egg laying, pumping, and defecation. In addition, we found that a mutation in mir-233(n4761) did not influence the lifespan of worms. Thus, the pathogen killing assays reflect the effect of mir-233 on host defense against attacking pathogens, not mir-233-dependent changes in aging.
Previously, Horvitz and colleagues have revealed that a single mutation of the majority of miRNAs does not lead to obvious developmental or growth defects of worms [33], [34]. These observations lead them to hypothesize that there is significant functional redundancy among miRNAs. Our results reinforce the idea that most miRNAs act redundantly with other miRNAs or other pathways. Although 47 miRNA genes were up-regulated after P. aeruginosa PA14 infection, single mutations in most of these miRNAs did not influence innate immune responses to PA14 infection. However, we could not exclude the role of these miRNAs in innate immune responses to other microbes. It is possible that innate immunity is regulated by multiple miRNAs and a large number of target genes, which together consist of the miRNA-target network. Thus, a single mutation in these miRNAs does not de-repress the expression of immune-related genes as the other miRNAs that might regulate in parallel the target genes.
Our results demonstrate that mir-233 down-regulates the protein levels of its target gene SCA-1 during P. aeruginosa PA14 infection. SCA-1 is identified by iTRAQ analysis and miRNA target prediction algorithms. It should be noted that there was little overlap between the expressions of proteins at each of the time-points. The data from the proteomic analysis probably varied greatly, contributing to the statistically no significant difference between the expressions of proteins at each of the time-points. SCA-1 is the only C. elegans homolog of mammalian SERCA [18], which is required for the maintenance of intracellular Ca2+ homeostasis. In C. elegans, down-regulation of sca-1 by RNAi leads to Ca2+ depletion, thus inducing activation of the UPR [25]. Thus, these results imply a role for mir-233 in regulating the UPR during the immune response. Indeed, an increase in expression of xbp-1s and Phsp-4::gfp in response to PA14 infection are only observed in WT worms, but not in the mir-233(n4761) mutant. It has been shown that innate immunity constitutes a physiologically relevant source of ER stress in C. elegans [7], [8]. Although the molecular mechanism by which innate immunity-induced UPR is not clear, PMK-1, which plays a principal role in C. elegans defense against pathogens, is involved in the activation of the UPR. Our data demonstrate that PMK-1 mediates up-regulation of mir-233 expression after P. aeruginosa PA14 infection. In contrast, a mutation in pmk-1 leads to an increase in the protein expression of SCA-1. Meanwhile, knockdown of sca-1 by RNAi restores the induction of xbp-1s in the pmk-1(km25) mutant. Thus, PMK-1 promotes the activation of the UPR by regulating the mir-233/SCA-1 signaling during the immune response.
Of the three canonical braches of the UPR, the IRE-1-XBP-1 signaling is involved in resistance to P. aeruginosa and to pore-forming toxins in C. elegans [7], [8]. Our results demonstrate that knockdown of sca-1 by RNAi enhances immune resistance to P. aeruginosa infection in adult WT worms, but not in the xbp-1(zc12) mutant. Moreover, intestinal-specific knockdown of xbp-1 leads to enhanced susceptibility to PA14 infection. These results indicate that mir-233-mediated activation of the UPR in the intestine, which is in direct contact with pathogens, confers defense against P. aeruginosa infection.
The protective role of the UPR in innate immunity is not fully understood. During larval development, P. aeruginosa infection induces the PMK-1-dependent innate immune response, which in turn causes a disruption of ER homeostasis in the absence of the functional IRE-1–XBP-1 pathway [7]. Thus, Richardson et al [7] suggest that the principal mechanism by which XBP-1 promotes survival of worms during P. aeruginosa infection is to alleviate the detrimental effect induced by the immune response. These authors demonstrate that the UPR is also induced in response to collateral damage caused by activation of the p38 pathway, which is not associated with bacterial infection. These results, however, raises the possibility that the UPR regulated by mir-233 contributes to a more general stress resistance, but not innate immunity per se. Indeed, the UPR, which is activated by hypoxia, is required for protecting worms against hypoxic injury [35], [36]. As organisms are faced with a variety of stresses that results in protein misfolding and aggregation, the maintenance of the ER proteome may be a common mechanism to coordinate stress resistance.
In summary, we report that mir-233 is up-regulated by PMK-1 during P. aeruginosa PA14 infection. mir-233 down-regulates the protein expression of its target SCA-1, resulting in activation of the UPR. The UPR in turn confers C. elegans defense against P. aeruginosa PA14 infection. Thus, mir-233 is a key miRNA that modulates the UPR during the immune response.
Materials and Methods
Nematode strains
Mutated and transgenic strains used in this study include: xbp-1(zc12), pmk-1(km25), ZcIs[hsp-4::gfp], wwEx33[mir-232p::gfp+unc-119(+)], NR222 (rde-1(ne219); kzIs9[pKK1260(plin-12::nls::gfp), pKK1253(plin-26::rde-1), rol-6]); NR350 (rde-1(ne219); kzIs20[pDM#715(phlh-1::rde-1), pek-1(ok275), and atf-7(gk715) were kindly provided by the Caenorhabditis Genetics Center (CGC). The strain for intestinal-specific RNAi (sid-1(qt9); Is[vha-6pr::sid-1]; Is[sur-5pr:: gfp::nls]) were kindly provided by Dr. Gary Ruvkun (Massachusetts General Hospital, Harvard Medical School). miRNA mutants (from CGC) used in this study were listed in S6 Table. Mutants and transgenic strains were backcrossed three times into the N2 strain used in the laboratory. All strains were maintained on nematode growth media (NGM) and fed with E. coli OP50.
Infection with bacteria
Standard conditions were used for C. elegans growth at 20°C [37]. Synchronized populations of worms were cultivated at 20°C until the young adult stage. For all pathogen assays, 75 µg/ml of fivefluoro-2′-deoxyuridine (FUdR) was added to the assay plates to abolish the growth of progeny. P. aeruginosa PA14 (a gift from Dr. K Zhu, Institute of Microbiology, CAS) or E. faecalis ATCC 29212, or S. typhimurium 468, or S. aureus ATCC 25923 (gifts from Dr. WH Lee, Kunming Institute of Zoology, CAS) was grown in different medium [4], [38], [39], then seeded on slow-killing plates, which contained modified NGM (0.35% instead of 0.25% peptone) as described [4], [39]. Three plates of about 50–60 animals per plate were tested per assay and all experiments were performed three times independently at 25°C.
Small RNA deep sequencing
After infected with P. aeruginosa PA14 infection for 4 h, 8 h, and 12 h, worms were washed with M9 buffer for several times in order to remove bacteria. Then, worms were collected and RNAs were extracted using the mirVana miRNA Isolation Kit (Ambion), according to the manufacturer's instructions. Approximately 20 µg of RNAs per sample were submitted to Beijing Genomics Institute (BGI)-Shenzhen (Shenzhen, China) for sequencing. In brief, the sequencing was performed as follows: RNAs corresponding to 15–30 nt in size were purified by polyacrylamide gel electrophoresis (PAGE), and ligated with adapters to the 5′ and 3′ termini of the RNA. Then the RNAs were amplified by RT-PCR. cDNA libraries were sequenced using an Illumina Genome Analyzer. Illumina data can be found in the Gene Expression Omnibus (GEO) of NCBI under the accession number GSE17095742.
RNA interference
The clones of genes for RNAi were from the Ahringer library [40]. RNAi feeding experiments were performed on synchronized L1 larvae at 20°C for 40 h.
Development assays
The development assays were performed as described previously [7]. Strains (mir-233(n4761) mutant, sca-1(RNAi), and mir-233(n4761); sca-1(RNAi) worms) were egg laid on plates of PA14 (at least 80 eggs for each strain), and the fraction of worms growing to at least the L4 larval stage between the plates was averaged. Development was monitored daily for three days for experiments conducted at 25°C.
Construction of W03G11.4 RNAi
To generate a clone directed against W03G11.4, a 1420 bp fragment was amplified from genomic DNA by PCR using the primers 5′-GAC ATT ATG GTT GCT TCG-3′ (F) and 5′-GAG ATG CTG AGG TGA GAG-3′ (R). The fragment was TA-cloned into a PstI and KpnI-linearized L4440 feeding vector, as in the RNAi library [41].
Construction of transgenic strains
The vector mir-233p::gfp was generated by subcloning a 2.5 kb promoter fragment of mir-233 into an expression vector (pPD95.75). The construct was co-injected with the marker plasmid pRF4 containing rol-6(su1006) into gonads of WT worms by standard techniques [42], respectively. The transgenic worms were confirmed prior to assay. The expression of GFP was observed under a Zeiss Axioskop 2 plus fluorescence microscope (Carl Zeiss, Jena, Germany). Three plates of about 30 animals per plate were tested per assay and all experiments were performed three times independently.
Western blotting
After worms were homogenized in liquid nitrogen, the homogenate was lysed on ice for 30 min in lysis buffer (BioTeKe, Beijing, China). The proteins of lysates (50 µg per well) were separated on a 7% SDS-polyacrylamide gel. Proteins were then transferred to immobilon-PSQ transfer PVDF membrane (Millipore, Bedford, MA). Primary antibodies were anti-ATP2A2/SERCA2 antibodies (1∶1000 dilution; Cell Signaling, Beverly, MA), and anti-actin antibodies (1∶1000 dilution; Santa Cruz Biotech., Santa Cruz, CA). The secondary antibody was a peroxidase-coupled anti-rabbit IgG (1∶8000 dilution; Santa Cruz Biotech.). The membrane was exposed to Kodak X-OMAT film (Kodak, Xiamen, China), and the film was developed.
3′UTR reporters and microscopy
The ppD129.57 plasmid (a gift from Dr. Min Han, University of Colorado, Boulder, USA), which contains rpl-28 promoter:4NLS::gfp:let-858 3′UTR, was used as the vector for 3′UTR reporter constructs. The 3′UTRs of sca-1 were PCR amplified from genomic DNA and cloned into ppD129.57 to replace the let-858 3′UTR and make a sca-1-3′UTR (wt) reporter construct. A sca-1-3′UTR (mut) reporter construct was generated by replacing the putative mir-233 binding site with an oligonucleotide containing the exact complementary sequence of mir-233. The 3′UTR reporter constructs and the mCherry internal control plasmid (also a gift from Dr. M. Han) were coinjected into the gonad of WT and mir-233(n4761) worms following standard protocols. The transgenic worms were confirmed prior to assay. The expression of GFP and mCherry was monitored using a Zeiss Axioskop 2 plus fluorescence microscope.
Fluorescence analysis
ImageJ program (NIH) was used to quantify the fluorescence intensity of GFP or mCherry fluorescence. Equal regions of the worm were selected, and the intensities of fluorescence within the selected regions were measured with a standard size of 516×564 pixels. The mean pixelintensity (total pixel intensity/area) for each frame was then calculated. We expressed the fluorescence signal (F) as a ratio with the baseline fluorescence (F0). More than 30 worms per plate were observed to calculate the mean fluorescence intensity. Three plates were tested per assay and all experiments were performed three times independently.
Quantitative real-time PCR and detection of xbp-1 splicing
Total RNA was isolated from worms with the mirVana miRNA Isolation Kit (Ambion). Random-primed cDNAs were generated by reverse transcription of the total RNA samples with SuperScript II (Invitrogen). A real time-PCR analysis was conducted using SYBR Premix-Ex TagTM (Takara, Dalian, China) on an Applied Biosystems Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). act-1 was used for an internal control. The primers used for PCR were as follows: hsp-4: 5′ - TCA ATG ACG ACG ACA CGC -3′ (F), 5′ - CTC CAG AAC TTC GAG ACG G -3′ (R); xbp-1 splicing: 5′-ACC GTC TGC TCC TTC CTC AATG-3′ (F), 5′ - ACC GTC TGC TCC TTC CTC AAT G-3′ (R); act-1: 5′-GGG CGA AGA AGG AAA TGG TC-3′ (F), 5′ - CAG GTG GCG TAG GTG GAG AA -3′ (R).
iTRAQ-labeling proteomic analysis
After infected with P. aeruginosa PA14 infection for 4 h, 8 h, and 12 h, worms were washed with M9 buffer for several times. After worms were homogenized in liquid nitrogen, the homogenate was lysed on ice for 30 minutes in lysis buffer (BioTeKe, Beijing, China). Following centrifugation at 10,000× g for 10 min, the supernatant was collected. Approximately 100 µg of total proteins per sample were submitted to Beijing Genomics Institute (BGI)-Shenzhen for proteomic analysis. Briefly, after trypsin digestion of the samples, the peptides were labeled with 4-Plex iTRAQ reagents (Applied Biosystems). Then the mixtures of iTRAQ-labeled peptides were fractionated into 10 portions using SCX chromatography (Shimadzu, Japan), and were subjected to nanoelectrospray ionization followed by tandem mass spectrometry (MS/MS) using the TripleTOF 5600 System (AB SCIEX, USA). Candidate proteins were quantified using ProteinPilot Software 4.0.8085 (Applied Biosystems-MDS SCIEX Ins). We set a 1.5-fold change as the threshold and a two-tailed P-value <0.05 to identify significant changes.
Lifespan assays
Lifespan assays were conducted on NGM agar plates of E. coli OP50 at 20°C, starting with day 1 adults [43]. Animals were transferred to new plates during each day of their reproductive period and after that were transferred every third day. Survival of animals was monitored every day. Worms that did not move when gently prodded and displayed no pharyngeal pumping were marked as dead. Approximately 100 animals were tested on each plate with three replicates, and three independent assays were performed for each result.
Statistics
These results are mean ± SD of three independent experiments performed in triplicate. Differences in survival rates were analyzed using the log-rank test. The statistical significance of differences in gene expression and fluorescence intensity was assessed by performing a one-way ANOVA followed by a Student-Newman-Keuls test. Data were analyzed using SPSS11.0 software (SPSS Inc.).
Supporting Information
Zdroje
1. AballayA, AusubelFM (2002) Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr Opin Microbiol 5 : 97–101.
2. IrazoquiJE, UrbachJM, AusubelFM (2010) Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10 : 47–58.
3. KimDH, FeinbaumR, AlloingG, EmersonFE, GarsinDA, et al. (2002) A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 : 623–626.
4. TroemelER, ChuSW, ReinkeV, LeeSS, AusubelFM, et al. (2006) p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2: e183.
5. LiuCY, KaufmanRJ (2003) The unfolded protein response. J Cell Sci 116 : 1861–1862.
6. RonD, WalterP (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8 : 519–529.
7. RichardsonCE, KooistraT, KimDH (2010) An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 463 : 1092–1095.
8. BischofLJ, KaoCY, LosFC, GonzalezMR, ShenZ, et al. (2008) Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog 4: e1000176.
9. GuSG, PakJ, Barberan-SolerS, AliM, FireA, et al. (2007) Distinct ribonucleoprotein reservoirs for microRNA and siRNA populations in C. elegans. RNA 13 : 1492–1504.
10. BartelDP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136 : 215–233.
11. StefaniG, SlackFJ (2008) Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9 : 219–230.
12. LeeRC, FeinbaumRL, AmbrosV (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75 : 843–854.
13. ReinhartBJ, SlackFJ, BassonM, PasquinelliAE, BettingerJC, et al. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403 : 901–906.
14. HeikkinenL, KolehmainenM, WongG (2011) Prediction of microRNA targets in Caenorhabditis elegans using a self-organizing map. Bioinformatics 27 : 1247–1254.
15. IatsenkoI, SinhaA, RodelspergerC, SommerRJ (2013) New role for DCR-1/dicer in Caenorhabditis elegans innate immunity against the highly virulent bacterium Bacillus thuringiensis DB27. Infect Immun 81 : 3942–3957.
16. KudlowBA, ZhangL, HanM (2012) Systematic analysis of tissue-restricted miRISCs reveals a broad role for microRNAs in suppressing basal activity of the C. elegans pathogen response. Mol Cell 46 : 530–541.
17. LiuF, HeCX, LuoLJ, ZouQL, ZhaoYX, et al. (2013) Nuclear hormone receptor regulation of microRNAs controls innate immune responses in C. elegans. PLoS Pathog 9: e1003545.
18. ZwaalRR, Van BaelenK, GroenenJT, van GeelA, RottiersV, et al. (2001) The sarco-endoplasmic reticulum Ca2+ ATPase is required for development and muscle function in Caenorhabditis elegans. J Biol Chem 276 : 43557–43563.
19. ChenZ, WenB, WangQ, TongW, GuoJ, et al. (2013) Quantitative proteomics reveals the temperature-dependent proteins encoded by a series of cluster genes in thermoanaerobacter tengcongensis. Mol Cell Proteomics 12 : 2266–2277.
20. ShapiraM, HamlinBJ, RongJ, ChenK, RonenM, et al. (2006) A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci U S A 103 : 14086–14091.
21. ImielinskiM, ChaS, RejtarT, RichardsonEA, KargerBL, et al. (2012) Integrated proteomic, transcriptomic, and biological network analysis of breast carcinoma reveals molecular features of tumorigenesis and clinical relapse. Mol Cell Proteomics 11: M111 014910.
22. ZhangX, ZabinskyR, TengY, CuiM, HanM (2011) microRNAs play critical roles in the survival and recovery of Caenorhabditis elegans from starvation-induced L1 diapause. Proc Natl Acad Sci U S A 108 : 17997–18002.
23. ChoJH, BandyopadhyayJ, LeeJ, ParkCS, AhnnJ (2000) Two isoforms of sarco/endoplasmic reticulum calcium ATPase (SERCA) are essential in Caenorhabditis elegans. Gene 261 : 211–219.
24. NehrkeK, DentonJ, MowreyW (2008) Intestinal Ca2+ wave dynamics in freely moving C. elegans coordinate execution of a rhythmic motor program. Am J Physiol Cell Physiol 294: C333–344.
25. YanX, XingJ, Lorin-NebelC, EstevezAY, NehrkeK, et al. (2006) Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J Gen Physiol 128 : 443–459.
26. CalfonM, ZengH, UranoF, TillJH, HubbardSR, et al. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415 : 92–96.
27. ShiversRP, PaganoDJ, KooistraT, RichardsonCE, ReddyKC, et al. (2010) Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet 6: e1000892.
28. RichardsonCE, KinkelS, KimDH (2011) Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet 7: e1002391.
29. DickhoutJG, CarlisleRE, AustinRC (2011) Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res 108 : 629–642.
30. LiangCP, HanS, LiG, TabasI, TallAR (2012) Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment. Diabetes 61 : 2609–2620.
31. StutzmannGE, MattsonMP (2011) Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev 63 : 700–727.
32. DongY, ZhangM, WangS, LiangB, ZhaoZ, et al. (2010) Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 59 : 1386–1396.
33. MiskaEA, Alvarez-SaavedraE, AbbottAL, LauNC, HellmanAB, et al. (2007) Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet 3: e215.
34. Alvarez-SaavedraE, HorvitzHR (2010) Many families of C. elegans microRNAs are not essential for development or viability. Curr Biol 20 : 367–373.
35. AndersonLL, MaoX, ScottBA, CrowderCM (2009) Survival from hypoxia in C. elegans by inactivation of aminoacyl-tRNA synthetases. Science 323 : 630–633.
36. MaoXR, CrowderCM (2010) Protein misfolding induces hypoxic preconditioning via a subset of the unfolded protein response machinery. Mol Cell Biol 30 : 5033–5042.
37. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
38. AballayA, YorgeyP, AusubelFM (2000) Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol 10 : 1539–1542.
39. PowellJR, KimDH, AusubelFM (2009) The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response. Proc Natl Acad Sci U S A 106 : 2782–2787.
40. KamathRS, AhringerJ (2003) Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30 : 313–321.
41. TimmonsL, FireA (1998) Specific interference by ingested dsRNA. Nature 395 : 854.
42. MelloC, FireA (1995) DNA transformation. Methods Cell Biol 48 : 451–482.
43. SchulzTJ, ZarseK, VoigtA, UrbanN, BirringerM, et al. (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6 : 280–293.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and AdultsČlánek The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and HostČlánek Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for EntryČlánek Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Importance of Pathogen Load
- Implication of Gut Microbiota in Nonalcoholic Fatty Liver Disease
- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- Helminth-Induced Immune Regulation: Implications for Immune Responses to Tuberculosis
- The M3 Muscarinic Receptor Is Required for Optimal Adaptive Immunity to Helminth and Bacterial Infection
- An Iron-Mimicking, Trojan Horse-Entering Fungi—Has the Time Come for Molecular Imaging of Fungal Infections?
- Modulates the Unfolded Protein Response in during Infection
- Differential Reliance on Autophagy for Protection from HSV Encephalitis between Newborns and Adults
- Identification of HNRNPK as Regulator of Hepatitis C Virus Particle Production
- Parasite Biomass-Related Inflammation, Endothelial Activation, Microvascular Dysfunction and Disease Severity in Vivax Malaria
- : Trypanosomatids Adapted to Plant Environments
- Early Virus-Host Interactions Dictate the Course of a Persistent Infection
- TLR3 Signaling in Macrophages Is Indispensable for the Protective Immunity of Invariant Natural Killer T Cells against Enterovirus 71 Infection
- The Epstein-Barr Virus Encoded BART miRNAs Potentiate Tumor Growth
- Macrophage-Derived Human Resistin Is Induced in Multiple Helminth Infections and Promotes Inflammatory Monocytes and Increased Parasite Burden
- Dissemination of a Highly Virulent Pathogen: Tracking The Early Events That Define Infection
- Variability in Tuberculosis Granuloma T Cell Responses Exists, but a Balance of Pro- and Anti-inflammatory Cytokines Is Associated with Sterilization
- The Shear Stress of Host Cell Invasion: Exploring the Role of Biomolecular Complexes
- The Molecular Basis for Control of ETEC Enterotoxin Expression in Response to Environment and Host
- Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry
- Secreted Herpes Simplex Virus-2 Glycoprotein G Modifies NGF-TrkA Signaling to Attract Free Nerve Endings to the Site of Infection
- Preferential Use of Central Metabolism Reveals a Nutritional Basis for Polymicrobial Infection
- A New Family of Secreted Toxins in Pathogenic Neisseria Species
- A Human Type 5 Adenovirus-Based Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy
- Regulation of Oncogene Expression in T-DNA-Transformed Host Plant Cells
- GITR Intrinsically Sustains Early Type 1 and Late Follicular Helper CD4 T Cell Accumulation to Control a Chronic Viral Infection
- Cell Cycle-Independent Phospho-Regulation of Fkh2 during Hyphal Growth Regulates Pathogenesis
- Virus-Induced NETs – Critical Component of Host Defense or Pathogenic Mediator?
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Protective Efficacy of Centralized and Polyvalent Envelope Immunogens in an Attenuated Equine Lentivirus Vaccine
- Transmitted Virus Fitness and Host T Cell Responses Collectively Define Divergent Infection Outcomes in Two HIV-1 Recipients
- Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
- DNA Is an Antimicrobial Component of Neutrophil Extracellular Traps
- Uropathogenic Superinfection Enhances the Severity of Mouse Bladder Infection
- Well-Ordered Trimeric HIV-1 Subtype B and C Soluble Spike Mimetics Generated by Negative Selection Display Native-like Properties
- The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots
- Reprogramming of from Virulent to Persistent Mode Revealed by Complex RNA-seq Analysis
- Compartment-Specific and Sequential Role of MyD88 and CARD9 in Chemokine Induction and Innate Defense during Respiratory Fungal Infection
- Bacterial Flagella: Twist and Stick, or Dodge across the Kingdoms
- Elucidation of the RamA Regulon in Reveals a Role in LPS Regulation
- IL-1α Signaling Is Critical for Leukocyte Recruitment after Pulmonary Challenge
- Chronic Filarial Infection Provides Protection against Bacterial Sepsis by Functionally Reprogramming Macrophages
- Specificity and Dynamics of Effector and Memory CD8 T Cell Responses in Human Tick-Borne Encephalitis Virus Infection
- Promiscuous RNA Binding Ensures Effective Encapsidation of APOBEC3 Proteins by HIV-1
- Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Infections in Humans and Animals: Pathophysiology, Detection, and Treatment
- : Trypanosomatids Adapted to Plant Environments
- Environmental Drivers of the Spatiotemporal Dynamics of Respiratory Syncytial Virus in the United States
- Dengue Virus RNA Structure Specialization Facilitates Host Adaptation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



