-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInfection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
Malaria is transmitted by Plasmodium falciparum-infected Anopheles gambiae mosquitoes. Salivary gland contributes to the development of the parasite by creating a favorable environment for the infection and facilitating blood feeding and reproduction of the vector. However, the molecular mechanism by which the vector salivary gland modulates parasite/host interactions is not understood. We discovered that infection of the mosquito salivary gland upregulates several genes; among them, one codes for a protease inhibitor named Agaphelin. Notably, Agaphelin was found to exhibit multiple antihemostatic functions by targeting elastase. As a result, it inhibits platelet function which is required for blood to clot, and it prevents cleavage of TFPI, an anticoagulant that has recently been found to play a crucial role in thrombus formation in vivo. Agaphelin also attenuates neutrophils chemotaxis and the release of Neutrophil Extracellular Traps. These results provide evidence that neutrophils serve as a link between coagulation and the innate immune response. Agaphelin also exhibits anti-inflammatory and antithrombotic effects in vivo. Furthermore, Agaphelin did not promote bleeding, suggesting that targeting neutrophil exhibits potential therapeutic value. Altogether, these results highlight that the interplay between parasite, vector and host is a dynamic process that contributes and sustains the interface among Plasmodium, Anopheles and humans.
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004338
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004338Summary
Malaria is transmitted by Plasmodium falciparum-infected Anopheles gambiae mosquitoes. Salivary gland contributes to the development of the parasite by creating a favorable environment for the infection and facilitating blood feeding and reproduction of the vector. However, the molecular mechanism by which the vector salivary gland modulates parasite/host interactions is not understood. We discovered that infection of the mosquito salivary gland upregulates several genes; among them, one codes for a protease inhibitor named Agaphelin. Notably, Agaphelin was found to exhibit multiple antihemostatic functions by targeting elastase. As a result, it inhibits platelet function which is required for blood to clot, and it prevents cleavage of TFPI, an anticoagulant that has recently been found to play a crucial role in thrombus formation in vivo. Agaphelin also attenuates neutrophils chemotaxis and the release of Neutrophil Extracellular Traps. These results provide evidence that neutrophils serve as a link between coagulation and the innate immune response. Agaphelin also exhibits anti-inflammatory and antithrombotic effects in vivo. Furthermore, Agaphelin did not promote bleeding, suggesting that targeting neutrophil exhibits potential therapeutic value. Altogether, these results highlight that the interplay between parasite, vector and host is a dynamic process that contributes and sustains the interface among Plasmodium, Anopheles and humans.
Introduction
Hematophagous animals are strictly dependent on blood feeding for survival and reproduction. During feeding, the mouthparts of mosquitoes canulate or lacerate arterioles and venules, or penetrate hemorrhagic pools [1]–[3]. These events cause vascular injury, and the host response is accompanied by vasoconstriction, exposure of tissue factor (TF), endothelial cell injury, and activation of platelets, monocytes, and neutrophils [4]–[8]. Among these, neutrophils are particularly important in the early phase of inflammatory response and defense against infection. Accordingly, neutrophils phagocytose and kill intracellularly invading microorganisms in the phagosome by a mechanism involving reactive oxygen species, proteases, and antimicrobial peptides [4]–[8]. Evidences have also been provided that neutrophils can kill pathogens in an extracellular manner that does not require phagocytic uptake. This mechanism consists of web-like structures of DNA and proteins—known as neutrophil extracellular traps (NETs)—via a process called NETosis [4]–[8]. NETs released by activated neutrophils bind and kill pathogens. NETs are also important in inflammation and thrombus formation, as they operate as scaffolds to activate the extrinsic pathway via TF, the contact pathway via FXIIa, and platelet aggregation via histones [4]–[8].
Neutrophils also release granule contents containing enzymes such as elastase, proteinase-3, and cathepsin G. Elastase, a particularly inflammatory enzyme, interferes with thrombomodulin function [9], and cleaves TF pathway inhibitor (TFPI) [10], the physiological inhibitor of the extrinsic pathway [11]. More recently, it has been revealed that TFPI degradation by elastase plays a critical role in thrombosis in vivo [12], [13]. Elastase also induces proteinase-activated receptor activation, contributes to cytokine production and processing, inactivates metalloprotease inhibitors, degrades the extracellular matrix, promotes endothelial cell apoptosis and detachment, and affects leukocyte chemotaxis [14]–[16]. These events contribute to increased inflammatory and thrombotic tonus such as observed in ischemia reperfusion [17], acute myocardium infarction [6], venous thrombosis [18], [19], stroke [20] and cancer [21]. To regulate neutrophil function in general—and elastase activity in particular—a number of physiologic inhibitors of the enzyme have been described including serpins α-1 proteinase inhibitor and monocyte neutrophil elastase inhibitor (serpin B). In addition, elastase is inhibited by the chelonianin family of canonical inhibitors such as secretory leukocyte proteinase inhibitor (SLPI) and Elafin [22]. α2-Macroglobulin also inhibits elastase, among other enzymes. There is increasing evidence that these inhibitors, besides regulating inflammation by inhibiting proteolytic activity of proteases, also directly affect leukocyte chemotaxis and pro-inflammatory mediator release and may contribute to defense against invading pathogens. Therefore, elastase has been the target for several inhibitors (sivelestat, elafin, AZD9668) that have been tested under specific pathologic conditions associated with neutrophil dysfunction with mixed results according to different clinical trials [22].
In addition to physiologic inhibitors, bloodsucking arthropods exhibit an extensive repertoire of salivary molecules belonging to different family of proteins which target neutrophils, including protease inhibitors, chemokine-binding proteins and disintegrins which potentially affect inflammation [23]–[27]. However, direct blockade of neutrophil function by salivary proteins has not been shown to interfere with thrombosis in vivo, which is usually mediated by anticoagulants, platelet aggregation inhibitors, and vasodilators [2]. Here we demonstrate that the salivary gland (SG) of the malaria vector Anopheles gambiae presents several genes that are up - or down-regulated upon infection with Plasmodium falciparum, the main causative agent for severe malaria [28], [29]. Among the upregulated genes, we identified a putative antihemostatic peptide belonging to the family of Kazal type inhibitors. Agaphelin was found to inhibit elastase and proteinase-3, to modulate various neutrophil functions, and to prevent arterial thrombosis without impairing hemostasis. Agaphelin biological function emerges as a novel antihemostatic mechanism in hematophagy.
Results
Effects of P. falciparum on An. gambiae SG gene expression
We compared the gene expression of infected and uninfected SG tissues from An. gambiae mosquitoes. At least 43 genes were differentially expressed between infected and uninfected SGs; 5 (11.6%) were downregulated, while the remaining 38 (88.4%) were upregulated (Table 1). Among those upregulated were genes involved in chitin metabolism (AGAP002457), metal metabolisms (metallothioneins; AGAP001889 and AGAP001890), hemostasis (AGAP007907), antimicrobial humoral response (AGAP000999), regulation of saliva secretion and chemotaxis (AGAP002865), stress response (AGAP011970), lipid biosynthesis, transport (AGAP010973, AGAP002379, AGAP001185, AGAP002415), and signal transduction (AGAP001889), among others. The downregulated genes are related to housekeeping proteins and metabolism. To validate the microarray results, we performed nanostring RNA quantification for 19 An. gambiae genes. Overall, the microarray array and nanostring data presented a good degree of correlation (Pearson correlation coefficient of 0.95, with P<0.01).
Tab. 1. List of genes considered to be differentially expressed in salivary glands of A. gambiae, upon infection with Plasmodium falciparum. 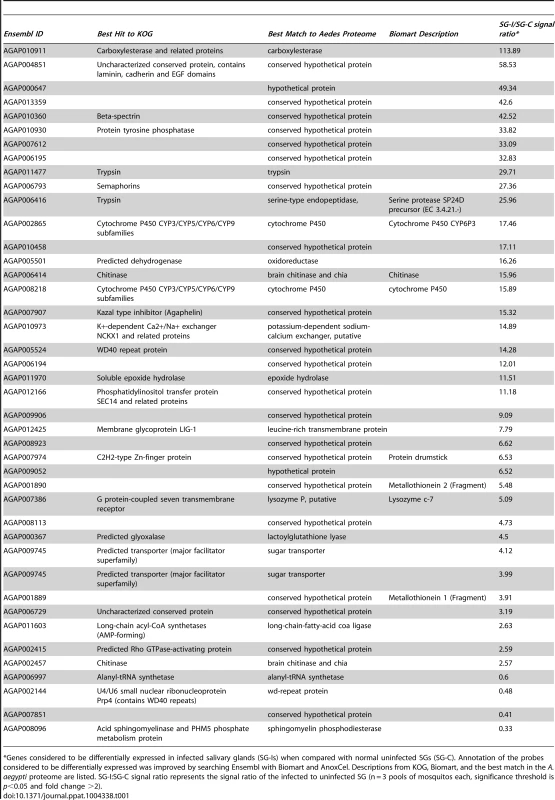
*Genes considered to be differentially expressed in infected salivary glands (SG-Is) when compared with normal uninfected SGs (SG-C). Annotation of the probes considered to be differentially expressed was improved by searching Ensembl with Biomart and AnoxCel. Descriptions from KOG, Biomart, and the best match in the A. aegypti proteome are listed. SG-I:SG-C signal ratio represents the signal ratio of the infected to uninfected SG (n = 3 pools of mosquitos each, significance threshold is p<0.05 and fold change >2). Characterization of Agaphelin
Among the genes that were upregulated in infected mosquito SG, we found one Kazal-type protease inhibitor, Agaphelin 15.32 time more expressed (gi118789673 GeneBank; AGAP007907, VectorBase database). Other anti-hemostatic proteins, such as the D7 family and apyrase did not reach the threshold indicative of up - or down-regulation. Agaphelin is also expressed in the midgut [30], but we did not find differential expression upon infection (microarray results and methods not shown). The Agaphelin gene is 255 bp long and encodes a 54 amino acid long mature protein containing a single Kazal inhibitory domain [31], with 6 cysteines and an estimated mol. wt. of 6.27 kDa and pI of 5.09. The protein contains a signal peptide predicted to be cleaved between residues AEA–DI, suggesting that it is secreted in the saliva. Figure 1A shows the alignment of Agaphelin with other Diptera family members.
Fig. 1. Characterization of Agaphelin. 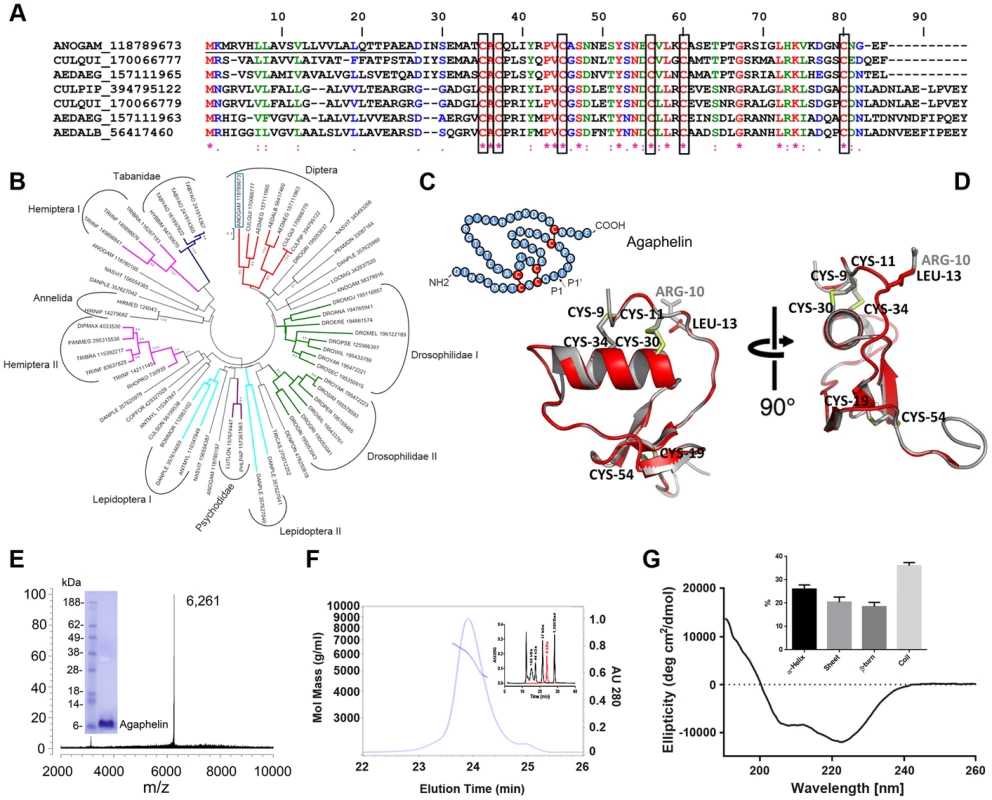
A) Clustal alignment of Agaphelin (gi| 118789673) with other proteins of the Kazal superfamily from Diptera. The boxes indicate the six conserved cysteines. Symbols below the alignment indicate: (*) identical sites; (:) conserved sites; (.) less-conserved sites. The underlined represent the signal peptide. B) Phylogram of Agaphelin (blue box) and other organisms obtained by the neighbor-joining algorithm using pairwise deletion and Poisson model. Sequences from the nonreduntant protein database of the National Center for Biotechnology Information (NCBI) are represented by the first three letters of their genus name, followed by the first three letters of the species name, followed by gi| accession number. Numbers in the phylogram nodes indicate percent bootstrap support for the phylogeny after 10,000 iterations. The bar indicates 10% amino acid divergence in the sequences. C) Secondary structure of Agaphelin [31], [34]. D) Structural model of Agaphelin (red) superimposed to Infestin 4 (gray). The six cysteines and the residue at position P1 from Agaphelin are labeled in black while the P1 residue of Infestin 4 is labeled in gray. Disulfide bonds are marked in yellow. E) Mass spectrometry analysis for Agaphelin. Inset: SDS/PAGE of Agaphelin, under reducing conditions. F) Analysis by SEC-MALS-HPLC provided the molar mass distribution of the main peak compared with absorbance at 280 nm. The continuous and interrupted lines represent absorbance 280 nm and MALS results, respectively. Inset: molecular weight markers (black lines) were loaded in the same column, and elution time was compared with Agaphelin (red line). G) Circular dichroism spectrum of Agaphelin showing a negative peak maxima at 222.6 nm and 208.0 nm and a positive peak maximum at 190 nm, indicating significant α-helix content. The ellipticity (degrees cm2/dmol) was plotted as a function of wavelength (nm) composition of Agaphelin. Data show the percentage of each type of secondary structure as determined by DichroWeb server (inset). The phylogenetic tree (Fig. 1B) indicates that Agaphelin and Kazal-type proteinase inhibitors belong to an expanded family of proteins from several Arthropoda organisms. Agaphelin has 41% identity with anticoagulant Infestin 4, an FXIIa inhibitor from T. infestans midguts (PDBID: 2ERW). It also shows a high level of identity with various other proteins with known crystal structures, including Anemonia elastase inhibitor (AEI) (35% identity, PDBID: 2LEO), 38% to Rhodniin, a specific thrombin inhibitor found in midguts of R. prolixus (PDBID 1TBQ), and to a thrombin inhibitor from A. aegypti. The Lepidoptera I clade clusters proteins are possibly related to defense against microorganisms and cocoon protection against predators, while a Kazal-type inhibitor from the black tiger shrimp Penaeus monodon showed inhibitory activity against subtilisin. The clade containing vasotab—a vasodilator from SGs of horse fly H. bimaculata [32]—and other sequences from Tabanidae has strong bootstrap support and clusters separately from Agaphelin, indicating different functions of proteins within the same family.
The predicted secondary structure of Agaphelin is presented in Fig. 1C. Within the Kazal domain, there are well conserved cysteine residues capable of forming three intradomain disulfide cysteine residues that can form disulfide bridges between cysteines 1–5, 2–4, and 3–6, resulting in a characteristic 3D structure [33]. Molecular homology modeling shows a high level of structural conservation of Agaphelin with other members of the Kazal family of inhibitors (Fig. 1D). It presents the characteristic structure of Kazal domains with a central α-helix inserted between two β-strands with a third one located toward the C-terminus. The reactive site P1 (located at position C2-X-P1) and the predicted conformation of the reactive site loop are structurally similar to those of other Kazal inhibitors, maintaining the canonical nature of the family. Agaphelin reactive site P1 contains a leucine (position 13) predicted to have activity against elastase, subtisilin, and chymotrypsin [34].
Synthesis of Agaphelin
Agaphelin was chemically synthesized and refolded yielding a peptide that was >95% pure. Mass-spectrometry analysis indicates a mass of 6,261 Da (Figure 1E), which is in agreement with a predicted mass of 6,267 Da for refolded Agaphelin; it migrates as a ∼6 kDa protein in SDS/PAGE (inset). In addition, Agaphelin elutes as ∼6 kDa protein in gel-filtration chromatography and the light-scattering plot determined that it exhibits a hydrodynamic radius of 1.4 nm, suggesting a globular conformation (Figure 1F). By SEC, 87% of the protein elutes as a monomer, 4% as aggregates, and 8% as break-down products. Consistent with the structure of folded Kazal inhibitors and its predicted secondary structure, the CDS of Agaphelin showed negative peak maxima at 222.6 nm and 208.0 nm and a positive peak maximum at 190 nm, indicating significant α-helix content (Fig. 1G). Analysis of the experimental circular dichroism data using the DichroWeb server gave a composition of 26%±5% α-helix, 20%±7% β-sheet, 18%±6% β-turn, and 36%±4% random coil. The composition of the predicted homology models using the DSSP program gave a predicted composition of 17.5%±1.8% α-helix, 16.3±1.44% β-sheet, 11.3±2.98 β-turn, and 54.9±2.77% not assigned to any category by DSSP (Figure 1G, inset).
Agaphelin is an inhibitor of elastase
Kazal-domain containing peptides are known to inactivate proteases [34]. Screening assays determined that Agaphelin inhibited elastase, proteinase-3, and chymotrypsin catalytic activity estimated with fluorogenic substrate (Fig. 2A). At large molar excess, it showed no or residual activity against other proteases involved with coagulation or inflammation (i.e., cathepsin G, trypsin, chymase, matryptase, β-tryptase, kallikrein, urokinase-type plasminogen activator, FXa, FXIa, FXIIa, plasmin, thrombin, and tissue-type plasminogen activator). As a control, another synthetic salivary kazal-type inhibitor from Triatoma infestans (gi149898841) which does not exhibit Leu in the P1 position, did not inhibit elastase or any other enzyme (data not shown) Agaphelin (1.5 µM) did not prolong PT or aPTT, while the heparin control prolonged the aPTT (10 µg/ml) and the PT (100 µg/ml) (not shown). Figure 2B shows that incubation of elastase with Agaphelin is accompanied by blockade of the enzyme catalytic activity, with an IC50 4.46±0.03 nM. BotDB server calculations using Cheng and Prusoff equation determined a Ki(app) 9.22±0.8 nM, assuming a competitive type of inhibition [35]. In addition, Agaphelin behaves as a slow-type inhibitor, since addition of enzyme to a mixture containing substrate and inhibitor is accompanied by progress curves with a downward concavity. SPR experiments were performed where Agaphelin was immobilized in sensor chips followed by injection of elastase as analyte. Analysis of the sensorgrams using Langmuir equation determined KD 17.4±3 nM (Figure 2C).
Fig. 2. Agaphelin inhibits elastase. 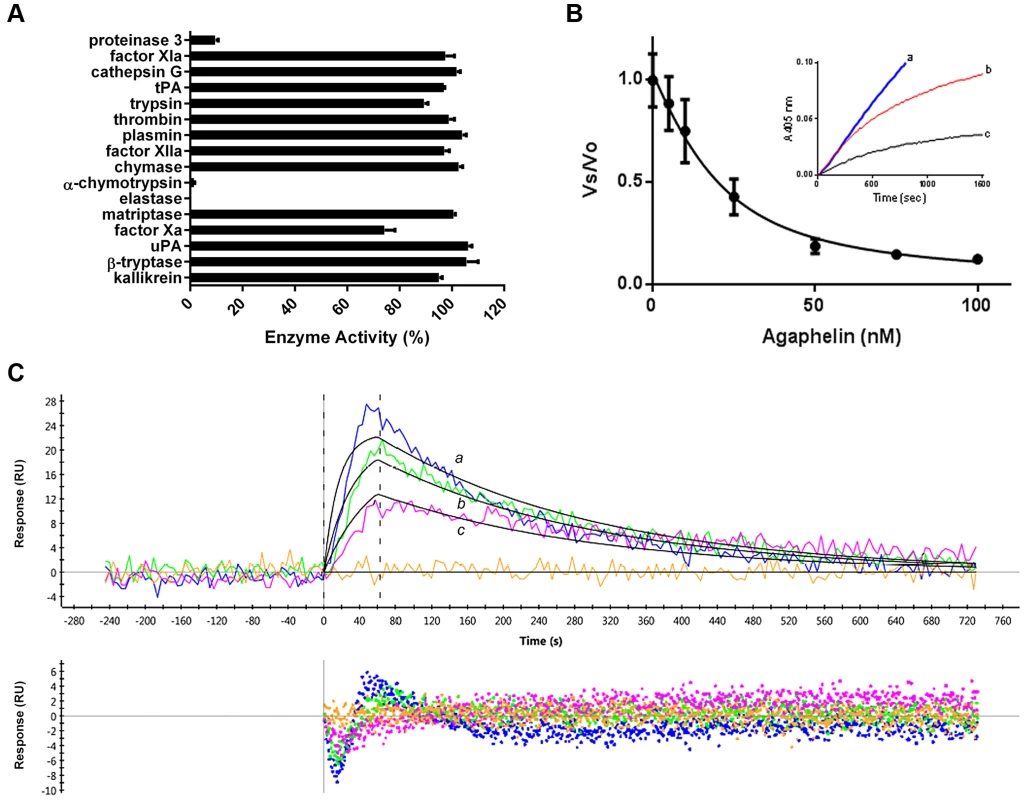
A) Agaphelin (1 µM) was tested against 16 different serine proteases in triplicates. Enzyme concentrations are provided in Material and Methods, (*, t-test; p≤0.05). B) Agaphelin inhibits elastase. Tight-type inhibitor: Elastase (18 nM) was incubated with Agaphelin (0–100 nM) for 30 min at RT followed by addition of chromogenic substrate MeOSuc-AAPV-pNA (600 µM). Residual activity was plotted as Vo (final velocity)/Vs (initial velocity). Inset: slow-type inhibitor: elastase (18 nM) was added to a mixture containing chromogenic substrate (600 µM) and Agaphelin (a, 0 nM; b, 150 nM, and c, 500 nM). C) Surface plasmon resonance experiments. Upper panel: Agaphelin was immobilized in a GLC chip and elastase was injected for 60 sec at 100 nM (a, blue), 50 nM (b, green), and 25 nM (c, pink). Dissociation of the Agaphelin-elastase complex was monitored for 600 sec. Representative sensograms (upper panel) are shown in black lines, and global fitting of the data points using the Langmuir equation is depicted. Lower panel: residual response. Kazal-type inhibitors may also promote vasodilation or exhibit antimicrobial activity [34]. Agaphelin (up to 512 µg/ml, 81 µM) was devoid of antimicrobial activity (calculated by the MCI) toward Staphylococcus epidermidis (ATCC12228), Enterococcus fecalis (ATCC29212), Escherichia coli (ATCC 25922), Klebsiella pneumonia (ATCC 10031), Proteus mirabilis (ATCC 29245), Serratia marcascens (ATCC13880), Acinetobacter baumannii (MDR1674627), Pseudomonas aeruginosa (ATCC27835), and Staphylococcus aureus (MRSA33591). Controls with ciprofloxacin indicated a MIC of <1 µg/ml (not shown). Agaphelin (512 µg/ml) was also tested for MBC and did not show activity for E. coli (ATCC29212), S. aureus MRSA (33591), or E. fecalis (ATCC29212) (not shown). In addition, Agaphelin (1 µM) did not promote vasodilation of the rat aorta, while nitrophorin (1 µM), a nitric-oxide releasing molecule, produced a robust response (not shown).
Agaphelin inhibits neutrophil chemotaxis
Inhibition of serine proteases may reduce neutrophil infiltration and neutrophil-mediated damage in various in vivo models of acute and chronic inflammation [15]. Agaphelin was tested on neutrophil chemotaxis toward fMLP using an EZ-TAXIScan chamber [36]. Figure 3A shows that Agaphelin is a potent inhibitor of neutrophil chemotaxis triggered by fMLP in vitro. Quantification of the results demonstrated that Agaphelin reduces several markers of chemotaxis including the total path length travelled by the cells (Fig. 3B), net path length (not shown), cell speed (Fig. 3C), and directionality (Fig. 3D) without affecting cell roundness (Fig. 3E).
Fig. 3. Agaphelin inhibits neutrophil chemotaxis in vitro. 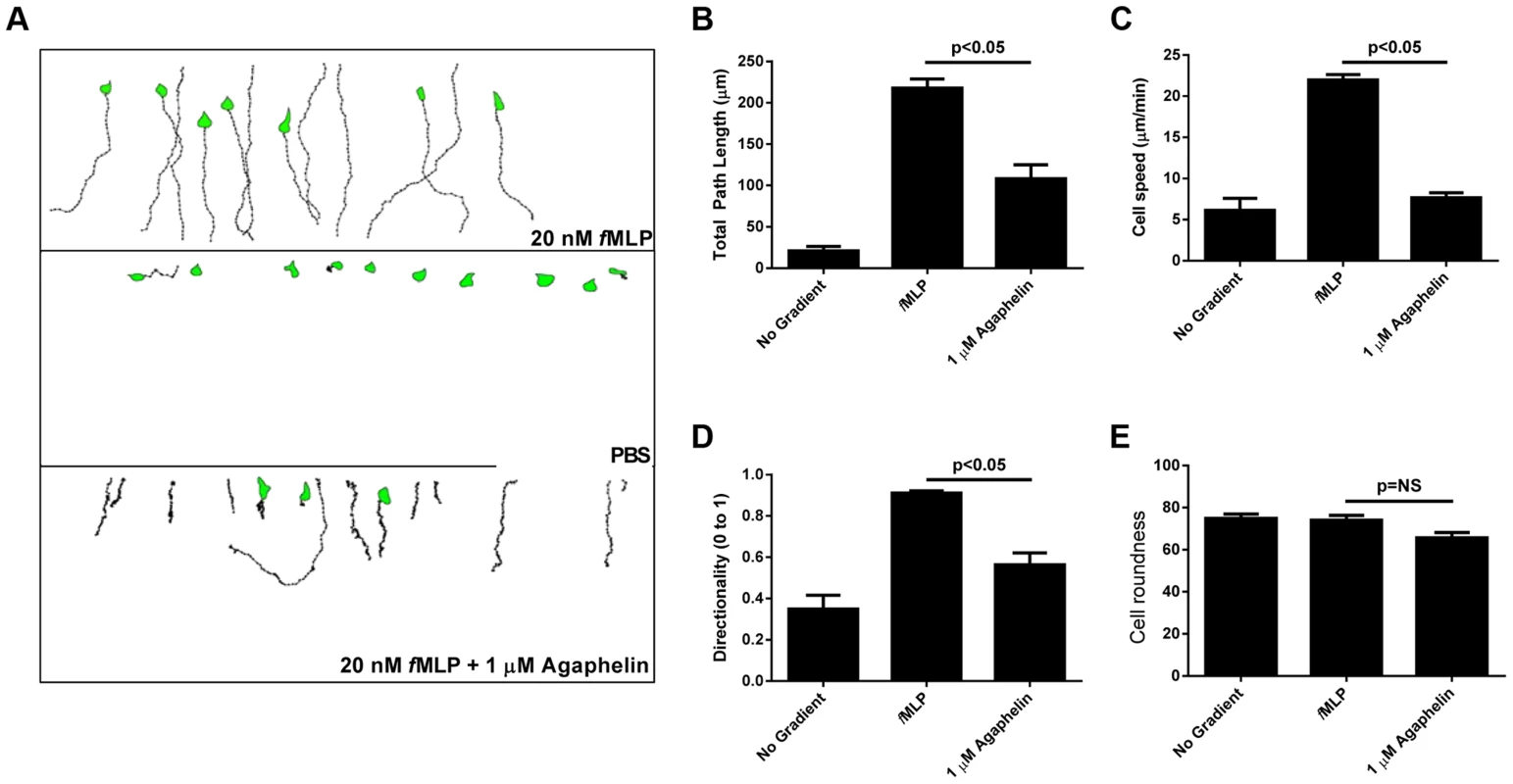
A) Chemotaxis of HL60 cells in EZ-TAXIScan chambers. Upper panel: HL60 cells incubated with PBS and then exposed to fMLP (20 nM) concentration gradient. Middle panel: HL60 cells incubated with buffer only and not exposed to fMLP. Lower panel: HL60 cells incubated with Agaphelin (1 µM) for 1 h and exposed to fMLP (20 nM). B–E) Quantification. Cell migration was analyzed based on the results presented in (A) and plotted for the following parameters: B) Total path length; C) Cell speed; D) Directionality, and E) Cell roundness. Data are expressed as the mean ± S.E (*, p≤0.05; t-test). Agaphelin inhibits platelet aggregation, cleavage of TFPI and neutrophil-induced coagulation
Neutrophils contribute to thrombosis through platelet aggregation, cleavage of TFPI, and release of NETs [4]. Elastase also plays a role in inflammation because it primes platelet aggregation induced by cathepsin [37]. Figure 4A shows that addition of elastase to platelets does not promote shape change or aggregation (left panel). On the other hand, low doses of cathepsin G activate platelets (middle panel). When elastase is added to platelets, followed by addition of cathepsin G, a robust aggregation is attained; however, when elastase was added to platelets previously incubated with Agaphelin, potentiation by cathepsin G no longer takes place (right panel). Control experiments demonstrated that Agaphelin did not affect cathepsin G-(Figure 4B) or collagen-induced platelet aggregation (Figure 4C). These results suggest that the effects on platelets were caused by blockade of elastase activity. Elastase has been reported to modulate coagulation tonus through cleavage of TFPI [10], [12], the physiologic inhibitor of TF [11]. Figure 4D confirms that elastase cleaves TFPI with the appearance of lower mol. wt. bands that are reportedly inactive as anticoagulants [10]; however, cleavage of TFPI was partially or completely blocked when elastase was pre-incubated with Agaphelin at 0.1 µM and 1 µM, respectively. Neutrophils induce coagulation of plasma through release of NETs and activation of the contact pathway [4]–[8]. In order to determine whether Agaphelin affect neutrophil-induced clot formation, inhibitor was incubated with neutrophil, followed by addition of phorbol myristate acetate (PMA) to induced release of procoagulant NETs. The mixture was added to citrated plasma and reactions started with CaCl2. Figure 4E shows that addition of neutrophils induces clot in 370 sec while in the presence of PMA clotting time is significantly shortened to 250 sec. However, Agaphelin-treated neutrophil completely loses its pro-coagulant activity. As a control, DNAse also reversed the procoagulant of PMA-activated neutrophils, suggesting that interfering with NETs formation resulted in inhibition of neutrophil-induced coagulation.
Fig. 4. Agaphelin inhibits elastase-mediated platelet aggregation, TFPI-cleavage by elastase, and neutrophil-induced coagulation. 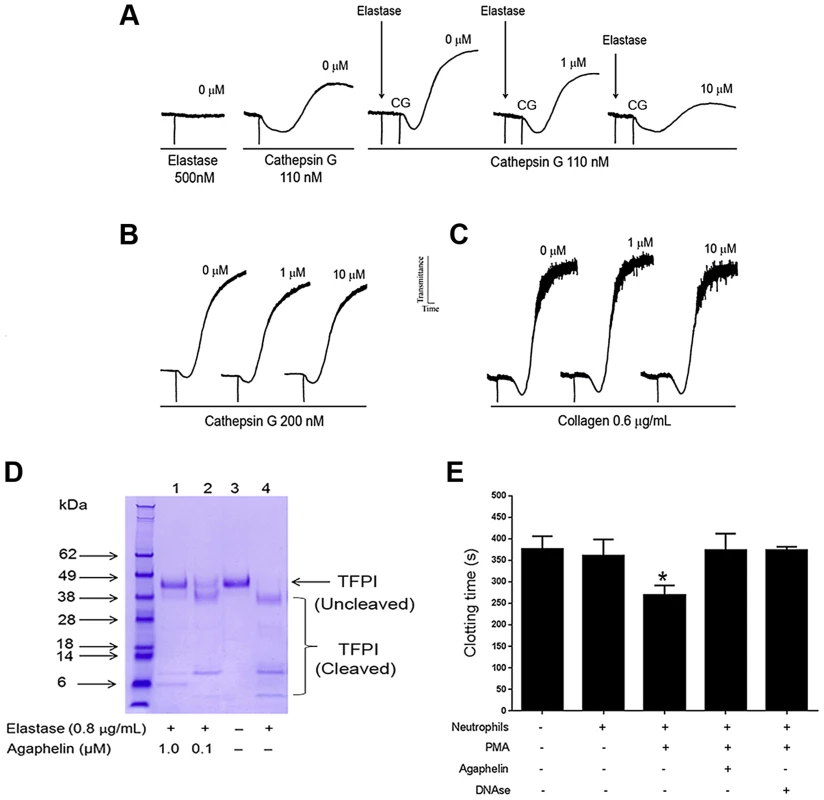
A) Platelet aggregation. Washed human platelets were stimulated with elastase only (500 nM, left panel), or cathepsin G only (110 nM, middle tracing), or elastase followed by cathepsin G (right tracing). In some experiments, Agaphelin (1 or 10 µM) was added to platelets followed by addition of elastase for 1 min, and cathepsin G. B) Agaphelin does not inhibit cathepsin G (200 nM) or C) collagen (0.6 µg/ml)-induced platelet aggregation. Aggregation response was monitored by turbidimetry using a Lumi-Aggregometer. D) TFPI cleavage. Agaphelin (0.1 µM and 1 µM) was incubated with 1 µg of TFPI in the presence of PBS or human neutrophil elastase (0.8 µg/ml). After 2 h, reactions were stopped by addition of LDS loading buffer (under reducing conditions, 10 mM DTT), boiled for 5 min, and loaded in 4–12% Nu-PAGE gel. Gels were Coomassie Blue-stained. E) Neutrophil-induced coagulation. Neutrophils (5×105 cells/well) were incubated with Agaphelin (1 µM), PBS (control) or DNAse (Dornase alfa, 4 µg/ml) for 1 hour, followed by addition of PMA (50 nM) for 3 hrs. Fifty µl of this suspension was added to 50 µl of plasma, and reactions were started by addition of CaCl2 (12.5 mM, final concentration)(*, p<0.05). Agaphelin inhibits NETs formation
Pharmacologic inhibition of elastase, or mice knockout (KO) for elastase have impaired formation of NETs [38]. To examine the effects of Agaphelin in this response, neutrophils were incubated with Agaphelin for 1 hour. Cells were then exposed to PMA for 3 hrs, and NETs were identified by confocal microscopy using antibodies against citrulinated histone, a marker of NETs formation [4]–[8]. Figure 5, left panel, shows that unstimulated neutrophils do not release NETs. In contrast, 5 nM PMA induces a strong response (central panel), which was dramatically inhibited by Agaphelin (1 µM, right panel). Quantification of results obtained with 4 different donors determined that Agaphelin promoted ∼65% inhibition (p = 0.03, t-test, n = 4) of NETs formation (Figure 5B). As a control, addition of DNAse (Dornase alfa, 4 µg/ml) completely abrogated the fluorescence signal associated with NETs (not shown).
Fig. 5. Agaphelin inhibits NETs formation. 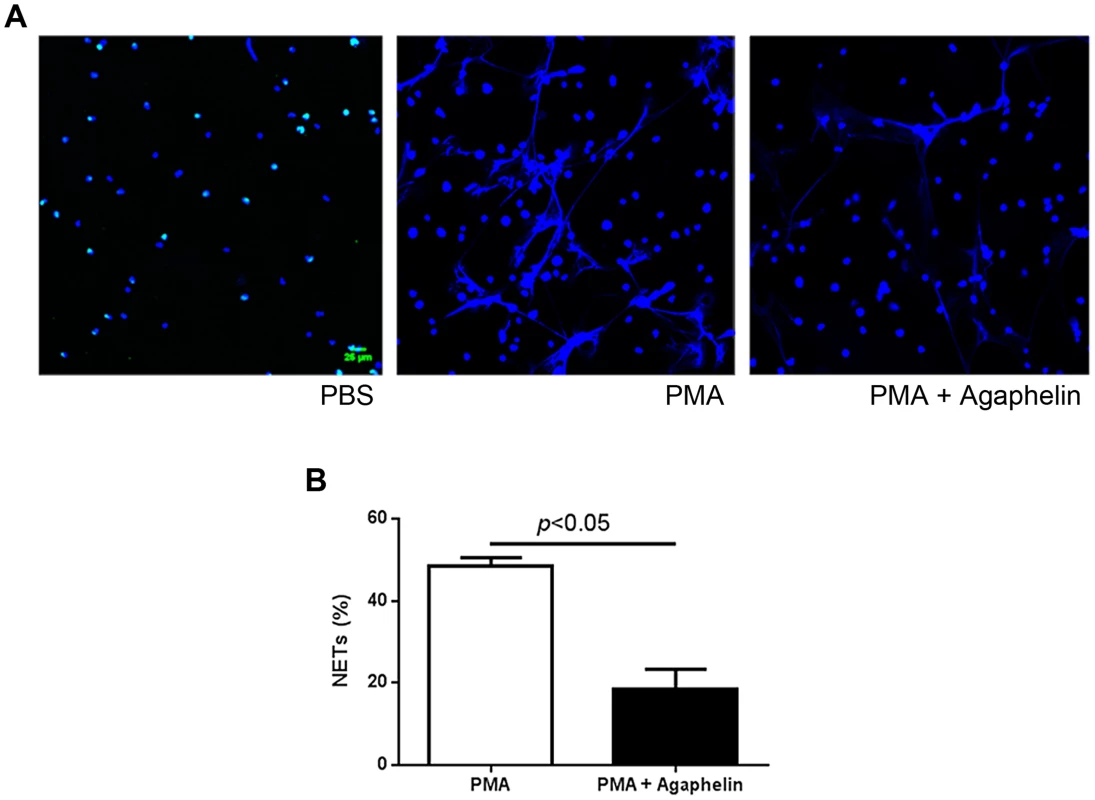
(A) Adherent neutrophils were incubated with Agaphelin or PBS (control) for 1 hour, and activated with PMA (5 nM, central panel), or PMA plus Agaphelin (right panel) for 3 h at 37°C. Left panel, non-activated neutrophil (no PMA). Formation of NETs was visualized under confocal microscopy, using antibody against citrullinated histone. Representative experiment is shown (4 different donors). B) Quantification. Performed as described in Methods. Sixty five per cent inhibition of NETs formation was attained with 1 µM Agaphelin (p = 0.03, t-test). Agaphelin displays anti-inflammatory and antithrombotic activities in vivo
We evaluated the effects of Agaphelin in a mouse model of acute inflammation induced by carrageenan. When inoculated into the mouse footpad, carrageenan induces an acute inflammatory process characterized by edema formation and intense neutrophil migration. This effect was confirmed here by an increase in paw thickness reaching a maximum 4 h post injection (Fig. 6A). Agaphelin at 1 mg/Kg promoted a non-significant reduction of edema formation. However, in the presence of 10 mg/kg of Agaphelin, paw edema induced by carrageenan was significantly inhibited (P<0.05). At 24 h, there were no differences in edema formation in any of the experimental groups. Next, we analyzed carrageenan-induced recruitment of neutrophils in the footpads by measuring tissue MPO activity at 4 h, the time point at which edema reaches a maximum (Fig. 6B). Statistically significant inhibition of MPO activity (30.9%) was observed when 1.0 mg/kg of Agaphelin was co-administered with carrageenan. The inhibition reached 43.2% when 10.0 mg/kg of Agaphelin was co-administered with carrageenan (P<0.01). Inoculation of Agaphelin (1 mg/Kg) only without carrageenan (control) did not induce edema or neutrophil accumulation (Fig. 6B).
Fig. 6. Agaphelin inhibits inflammation and neutrophil accumulation in vivo. 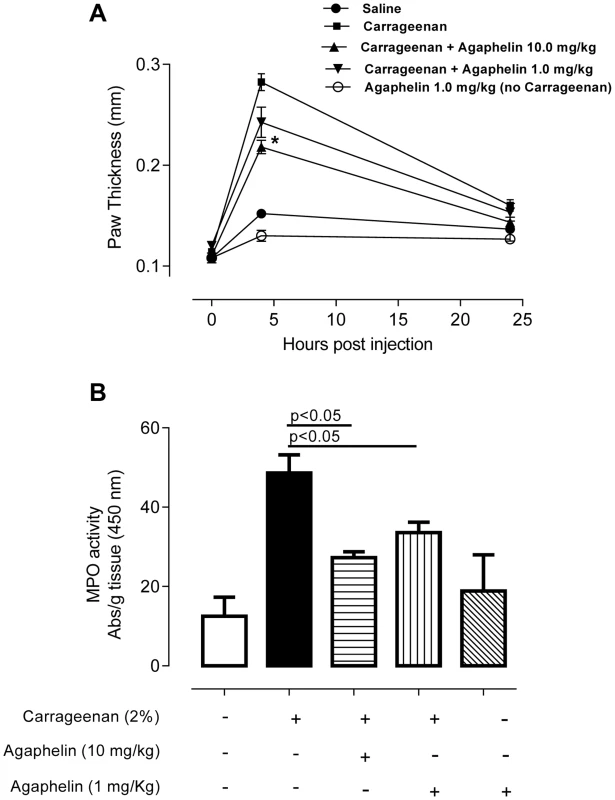
A) Paw edema in mice. Carrageenan (2%) was administered to mice, in the presence of saline or Agaphelin (1 or 10 mg/Kg). Agaphelin diluted in saline was injected as a control (without carrageenan). Edema formation was evaluated at 0, 4, and 24 h after as increase in paw thickness. B) Neutrophil recruitment in inflamed footpads was evaluated by measuring tissue myeloperoxidase activity, expressed as units of activity/g of tissue, after injection of carrageenan as above. Animals were euthanized 4 hours after. Statistical significance: *, p<0.05 or **, p<0.01 (one-way ANOVA followed by Tukey's post hoc test; n = 5 in each group), compared with carrageenan only. The effect of Agaphelin on thrombus formation in vivo was evaluated with FeCl3-induced carotid artery thrombosis model in mice. Figure 7A shows that after addition of FeCl3, occlusion of the artery occurs in approximately 18 min, as previously reported [39]. In contrast, Agaphelin (1 mg/Kg) promoted a complete inhibition of vessel occlusion after 60 min in 6/10 animals, demonstrating a consistent antithrombotic activity. Agaphelin did not prolong vessels occlusion in 4 animals, which is congruent with variations reported for this technique that involves the lesion size, temperature, concentration of FeCl3, and mice strains [40], [41]. To test the effects of Agaphelin in bleeding time, the tail transection method was used. Figure 7B shows that Agaphelin at antithrombotic concentration did not promote bleeding, indicating that it does not impair hemostasis.
Fig. 7. Agaphelin inhibits thrombosis in vivo, without impairing hemostasis. 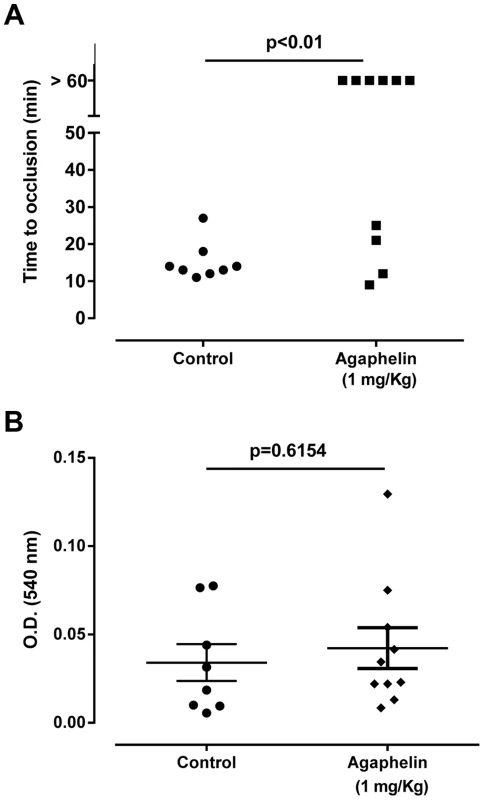
A) Arterial thrombosis. A paper filter infused with 7.5% FeCl3 was applied to the carotid artery, and blood flow was monitored with a perivascular flow probe for 60 min or until stable occlusion took place. Fifteen minutes before injury, Agaphelin was injected into the caudal veins of the mice. Each symbol represents one animal. *, p<0.05 (ANOVA with Dunnett post-test). B) Bleeding time. Bleeding was caused by a tail transection after intravenous injection of Agaphelin at the indicated concentrations. Absorbance at 540 nm (hemoglobin concentration) was used to estimate blood loss. *, p<0.05 (ANOVA with Dunnett post-test). Discussion
To understand how sporozoites affect gene expression in the SG, we employed Affymetrix chips containing approximately 14,900 A. gambiae transcripts. Microarray results demonstrate that several SGs genes are upregulated and a few others are downregulated upon infection with P. falciparum. Among those upregulated are genes involved in antimicrobial, humoral, and stress responses, chitin and metal metabolism, hemostasis, lipid biosynthesis, transport, signal transduction, regulation of saliva secretion, and chemotaxis, and also other genes described elsewhere [42]–[44]. Interestingly, we discovered that one upregulated gene codes for the protein Agaphelin, a novel monomeric member of the Kazal-family of inhibitors [45]. This family is evolutionarily expanded, since several orthologues were found in other Diptera, including Aedes sp and Culex sp.
Kazal inhibitors reportedly block several enzymes including subtilisin, granzyme A, elastase, proteinase K, thrombin, trypsin, α-chymotrypsin, and urokinase-type plasminogen activator [34]. Screening assays determined that chemically synthesized Agaphelin inhibits the catalytic activity of neutrophil elastase. This specificity is congruent with Leu in the P1 position of other Kazal inhibitors which exhibit the same inhibitory profile [23], [34], [45]. As a control, another synthetic salivary Kazal-type inhibitor from Triatoma infestans (gi 149898841) which does not exhibit Leu in the P1 position did not inhibit elastase or any other enzyme (data not shown). Kinetic experiments revealed that Agaphelin behaves as a slow and tight inhibitor of elastase with a Ki∼10 nM. This value is in good agreement with the KD of 17 nM calculated by SPR experiments. Our results also demonstrated that Agaphelin inhibits neutrophil-associated proteinase-3, which shares 57% homology with elastase. In contrast, it does not affect cathepsin G catalytic activity, which is only 37% homologous. Agaphelin also does not inhibit enzymes involved in coagulation or inflammation, nor does it prolong aPTT and PT. Agaphelin does not promote vasodilation or inhibit bacterial growth, two properties described before for members of the Kazal family of inhibitors [32], [46]. These experiments indicate that Agaphelin, due to its high specificity, is an ideal tool to understand the participation of neutrophil elastase in inflammatory events in vitro and in vivo.
Elastase exhibits a broad range of substrates and is released rapidly at high concentrations from neutrophil at sites of inflammation. Once released, serine protease may bind to the cell surface and remains relatively inaccessible to inhibitors, in contrast to soluble enzymes [47], [48]. Elastase and other serine proteases reportedly modulate several functions of neutrophils, including chemokine activation and degradation, cell recruitment, receptor activation, activation of lymphocytes, NET formation, and cleavage of adhesion molecules, apoptotic proteins, and anticoagulants [15]. Assays performed with TAXIScan chamber determined that Agaphelin interferes with chemotaxis toward fMLP and affected directionality, total path length, and speed of migrating cells. These results suggest that elastase plays a major regulatory role in chemotaxis in vitro. This contention is corroborated by previous reports showing that pharmacologic inhibition of elastase by L658758, Eglin C, or SLPI also blocks chemotactic neutrophil functions according to migration induced by PAF, fMLP or IL-8 [49]–[51]. While the mechanism is not precisely understood, blockade of cell surface proteinases inhibits intracellular signal for cytoskeletal change and polarization [49].
In agreement with in vitro results, Agaphelin reduces paw edema formation triggered by carrageenan and decreases neutrophil infiltration determined by tissue MPO activity, a sensitive marker of neutrophil accumulation. Others have shown that pharmacologic inhibition of elastase with L658758 or Eglin-C is accompanied by inhibition of neutrophil transmigration, based on intravital microscopy of inflamed microvessels [50]. KO mice for elastase also exhibit reduced zymozan-induced leukocyte migration through the cremasteric muscle venules and reduced production of IL-1β, CXCL1, and CCL3 [48]. Moreover, neutrophils from KO mice for both elastase and cathepsin G have impaired recruitment in a subcutaneous air-pouch model of inflammation, or arthritis induced by anti-collagen antibodies [52]. Mechanistically, it has been suggested that elastase inhibitors affect the function of other pro-inflammatory cells, therefore modulating chemokine release by neutrophils and leukocyte infiltration [15]. Elastase also cooperates with cell-surface integrins (α6β1) and PECAM-1, and cleaves E-cadherins, ICAM-1 and VCAM-1, which play an important role in cell-cell contact formation and in mediating neutrophil migration through the perivascular environment [15]. Altogether, inhibition of elastase is potentially associated with blockade of several relevant pathways that may result in diminished neutrophil-mediated inflammation observed here and reviewed elsewhere [15].
Elastase and proteinase-3 potentiate cathepsin G-induced platelet aggregation by a mechanism involving an increase in affinity of integrin αIIb for fibrinogen through limited proteolysis [37]. As expected for an elastase inhibitor, Agaphelin abrogated potentiation of cathepsin G-mediated aggregation by elastase, and this effect may decrease the contribution of platelets to the inflammatory tonus at the site of bite [53]. In addition, elastase cleaves TFPI [10], the physiological inhibitor of the extrinsic pathway [11]. This activity has been suggested to attenuate the anticoagulant effects of TFPI [10], [12]. Our experiments show that TFPI cleavage by elastase is completely abrogated by Agaphelin in vitro, and this effect may contribute to shifting the hemostatic balance toward a prothrombotic phenotype in vivo.
Neutrophils play a major role in thrombus formation through release of NETs by a complex mechanism named NETosis. While completion of this process may take hours, neutrophils may also rapidly expel NETs (within minutes) [8], [54]. Functionally, NETs contain pro-coagulant TF, promote contact pathway activation, contribute to platelet activation as a scaffold and through histones H3 and H4, and facilitates interaction of elastase with TFPI [4], [5], [7], [8], [12]. Our experiments demonstrate that Agaphelin reduces NETs formation in vitro, detected with antibodies against citrulinated histones, indicating that elastase is required for this process. In this context, elastase KO mice or pharmacologic inhibition of intracellular elastase with NEi exhibits impaired NETosis [38], [55]. It has been demonstrated that intracellular release of elastase, degradation of specific histones, and chromatin decondensation are critical for NETs formation by a mechanism synergized by myeloperoxidase and involving reactive oxygen species and the MAP-MEK-ERK pathway [4]–[8], [38]. Of relevance to Agaphelin's mechanism of action—which is a secreted protein—exogenously added elastase inhibitor serpin B1 is internalized, translocates into the nucleus, and inhibits NETs formation [56]. Furthermore, SLPI, another elastase inhibitor, is internalized by monocyte-like cells and found to bind to NF-κB binding sites and to inhibit p65 binding and promote inhibition of TNF-α and IL-8 generation [57].
Agaphelin inhibits arterial thrombus formation induced by FeCl3. While Agaphelin prevents vessel occlusion in most animals, 4 of them were not affected by the inhibitor. Conceivably, the mechanism of action of Agaphelin is more subtle than observed with other direct and potent salivary anticoagulants we have tested in this model [39], [58]–[60], besides variations inherent to this technique [40], [41]. Nevertheless, these results are congruent with inhibition of elastase activity, NETs formation, chemotaxis and platelet aggregation observed herein, in vitro. In fact, platelets participate in coagulation reactions and thrombus formation [61]–[63]. NETs contribute to thrombosis in baboons, which is prevented by DNase treatment [19]. Moreover, elastase KO mice exhibit less cleavage of TFPI and are resistant to thrombosis in vivo. Furthermore, degradation of TFPI by elastase is crucial for controlling the spread of intravascular bacteria [12]. These results suggest that P. falciparum infection might also be positively modulated by elastase, and counteracted by Agaphelin. We have also verified that Agaphelin does not promote bleeding, according to the tail transection method. It has become clear that the relative contributions of the intrinsic and extrinsic pathways in thrombosis and hemostasis are different. For instance, targeting components of the contact pathway has emerged as an alternative to prevent thrombosis without increase in bleeding [64]. Because neutrophils play a major role in thrombosis through NETs formation—which is particularly relevant in activation of the contact pathway [18], [64]—our results support the notion that targeting neutrophil functions, and likely other components of the innate immune response, is an alternative strategy to prevent pathologic inflammation and thrombosis [5], [6], [16]–[21]. However, the relative contribution of blockade of neutrophils, platelets, or coagulation by Agaphelin in the inhibition of thrombus formation remains to be determined. With respect to the dose, Agaphelin at 1 mg/kg equals 20 µg per animal (antithrombotic effect) and may reach a maximum theoretical plasma concentration of 1–2 µM which is at least 50 fold above the KD for elastase (assuming no losses and 1.5-mL volemia). Consistent with our findings, Elafin, a potent elastase inhibitor purified from bronchial secretions, blocks inflammation in several in vivo models and is under clinical trial to demonstrate whether it can attenuate myocardial infarction, ischemia-reperfusion injury in patients undergoing coronary bypass surgery [14]. Accordingly, Agaphelin can potentially be used as a drug or as prototype to develop inhibitors of elastase in several pathologic conditions [6], [16]–[21], [29].
On the vector side, Agaphelin is the first salivary antihemostatic to be upregulated by P. falciparum infection. In addition, it displays a novel anti-thrombotic mechanism which is mediated by inhibition of neutrophil-associated elastase. Accordingly, Agaphelin may contribute to the repertoire of salivary anti-hemostatics from An. gambiae, which includes Anophelin (anticoagulant) [65], apyrase (platelet inhibitor) [2], [3], antigen-5 members (antioxidant) [66], and peroxidase (vasodilator) [67]. SGs are also critical in allowing sporozoites to become infective [68] and might also facilitate Plasmodium sp. survival in the skin by blocking the effects of neutrophil-derived elastase and proteinase-3. In fact, human neutrophil elastase is known to degrade the major circumsporozoite protein of the infective stage of P. vivax and to interfere with cytoadherance on erythrocytes infected with P. falciparum [69]. Therefore, blockage of elastase by Agaphelin could potentially protect the sporozoite during its residency period and transit in the skin. Testing the effect of gene silencing of Agaphelin through siRNA or transgenic approach may potentially determine its role in parasite transmission to the host. Nevertheless, our data suggest that P. falciparum induces expression of genes in the invertebrate host that can be used to manipulate the vertebrate host's immune and hemostatic environment, potentially for the parasite's benefit. These results suggest that the parasite changes the local SG milieu to generate a more favorable environment for its development, which could also contribute to increase its infectivity. Upregulation of genes involved in stress response and antimicrobial response also indicates that the presence of the parasite on SGs is not free of costs for the mosquito. Altogether, our results suggest that a notable interplay exist in parasite-vector-host interactions.
Materials and Methods
Sporozoite preparation
An. gambiae mosquitoes were infected with P. falciparum (3D7) by allowing mosquitoes to feed on 14 day-old gametocyte cultures prepared as previously described [70]. Infections in the midgut were assessed by dissecting mosquitoes 9 days post infection and staining midguts with 0.1% mercurochrome solution in water. Infected mosquitoes and uninfected controls were used to isolate midguts at day 9 post infection and SGs at day 20. Dissected tissues were immediately placed on RNAlater solution (Ambion Inc. Austin, TX, USA) and stored at −80°C until further processing. As a technical note, the laboratory infection of A. gambiae with P. falciparum gametocyte cultures can achieve infection loads of oocysts in the midguts that are higher than those from natural infections that lead to <5 oocysts in the midgut. It is therefore expected that the infection load of the salivary gland with sporozoites would be higher in the case of laboratory infections (9,000–68,000) [71] versus field infections. Nevertheless, there is high variability in the number of sporozoites in salivary glands of field infected Anopheles. For example, it was found in Kenya that salivary glands of naturally infected A. gambiae ranged contained from 125 to 79,875 sporozoites. About half of the mosquitos had sporozoite loads higher than 1,000 [72]. This indicates that the laboratory infections of An. gambiae with P. falciparum can achieve in general high loads of sporozoites in the salivary glands but are still within levels that can be found in nature.
RNA extraction and T7 RNA amplification
Three pools—each containing whole organs from approximately 20 mosquitos—were used for total RNA extraction using Trizol reagent (Invitrogen, San Diego, CA, USA). For infected organs, we extracted the mosquito and parasite RNA simultaneously. RNA was amplified and labeled using two rounds of linear amplification according to the manufacturer's protocol (GeneChip® two-cycle cDNA synthesis kit; Affymetrix, Santa Clara, CA, USA). RNA quality and purity before and after amplification were assessed by high-resolution electrophoresis using the Agilent 2100 Bioanalyser system (Agilent Technologies, Inc., Santa Clara, CA, USA).
Labeling and hybridization
Labeled targets from the samples were combined with 2× hybridization buffer, 3 nM B2 control oligo (Affy 900457), and 20× spike (Affy 51214) in DMSO and nuclease-free water, making a final volume of 200 µl for the 48 individual hybridizations to commercial Affymetrix GeneChip Plasmodium/Anopheles containing both the P. falciparum and An. gambiae genomes (Cat# 900511; Affymetrix). The hybridization cocktail—including the components listed above—was transferred into the chip and hybridized at a constant temperature of 45°C for approximately 48 h using the approved Affymetrix 640 oven, 500 k.
Fluidics and scanning
Each chip was filled with 200 µl of wash buffer A, then processed on the fluidics station 450 using a stain mixture of 2× MES stain buffer, 50 mg/ml BSA, 1 mg/ml of streptavidin phycoerythrin, and water to make up 600 µl total volume for each stain and a holding buffer added to make up 600 µl total volume for storage and scanning. Upon completion of the fluidics process, each chip was scanned using the Affymetrix 7Gplus GeneChip scanner to create the image files (dat files).
Data sharing
All data are MIAME compliant and were deposited on the Gene Expression Omnibus database (GSM465895, GSM465898, GSM465905, GSM465901, GSM465904, GSM465896) [73].
Quality analysis
GeneChip Operating Software (GCOS v 1.4) was used to convert the image files to cell-intensity data (cel files). All cel files—representing individual samples—were normalized using the scaling method within GCOS and a scaled target of 500 using a P. falciparum filter to produce the analyzed files (chp files) along with the report files (RPT) and a pivot table for export into other software. A report was generated from the chp files (along with selected raw data) summarizing various quality and statistical aspects from the chips. From the raw data, the expected signal gradient was produced from the Affymetrix 20× spike in hybridization controls with creX, bioD, bioC, and bioB at 100, 25, 5, and 1.5 pM (supplemental material).
Data analysis and annotation
The pivot table from all samples, mentioned above, was created including calls, call P-value, and signal intensity for each gene. Genes with p values <0.05 and fold change >2 were considered as being significant. The table was imported into GeneSpring GX 7.3 and normalized to a constant value of 500. A hierarchical clustering (condition tree) using a Pearson correlation similarity measure with average linkage was used to produce the dendrogram indicating the cluster from these biological samples. We electronically annotated the entire P. falciparum genome using the default settings of Blast2GO [57] and by searching further information on Anoxcel [58] and Biomart [59]. We calculated the correlation between the Affy microarray data and the nanostring data with the Pearson's product moment correlation statistic using the R language [60].
Nanostring data
Total amplified RNA extracted from midguts and SGs was used to determine mRNA abundance by nanostring technology. In brief, amplified RNA from each sample was allowed to hybridize with the capture and reporter probe and incubated overnight at 65°C according to the nCounter gene expression assay manual (NanoString Technologies, Seattle, WA, USA). After the washes, purified Target/Probe complexes were eluted off and immobilized in the cartridge for data collection carried out in the nCounter digital analyzer. Data were normalized according to the guidelines provided by NanoString Technologies using the nCounter RCC collector worksheet and analyzed as described above.
Synthesis of Agaphelin
The peptide was synthesized using an automated peptide synthesizer, model 433A (Applied Biosystems, Fullerton, CA, USA) with Fmoc strategy and HBTU/DIPEA as the coupling reagent. Novabiochem Fmoc-Phe-Wang-LL resin (0.20 mmol) (EMD Millipore, division of Merck KGaA, Darmstadt, Germany) was used as the solid phase. The side-chain protecting groups used in synthesis were Trt for Asn, Cys, Gln, and His; OtBu for Glu and Asp; Pbf for Arg; and tBu for Ser, Thr, and Tyr. The coupling reaction time was 1 h, and 4-methylpiperidine (20%)/N-methylpyrrolidone was used to remove the Fmoc group at every step. Peptide resin was washed with N-methylpyrrolidone and dichloromethane and dried in vacuo to yield the protected peptide-resin. The peptidyl resin was treated with a cleavage mixture of trifluoroacetic acid/thioanisole/1,2-ethanedithiol/triisopropylsilane (90∶5∶3∶2, v/v/v/v; 40 ml) for 2.5 h to remove protecting groups and peptide from the resin. After filtration of the exhausted resin, the solvent was concentrated in vacuo and the residue was triturated with methyl t-butyl ether. The solid peptide was filtered off, washed with methyl t-butyl ether, and vacuum dried. The crude peptide was purified by preparative reversed-phase high-performance liquid chromatography (HPLC), and purity grade was checked by analytical HPLC analyses and mass spectrometry using a matrix-assisted laser desorption ionization time-of-flight mass spectrometer Axima CFR+ (Shimadzu Scientific Instruments. Columbia, MD, USA). Pure fractions were combined, frozen, and lyophilized to afford linear (unfolded) peptide. A second Kazal-type inhibitor from Triatoma infestans (gi149898841) was synthesized and refolded as above. Peptide was 94.33% pure with molecular mass 5705.5 da.
Folding of Agaphelin
Linear, purified peptide Agaphelin (1–58) was dissolved in 6 M guanidin•HCl/200 mM PBS at a concentration of 2 mmol, and the solution was introduced by a Harvard Apparatus “Elite 11” through a syringe, to a 30 times larger volume of the stirred solution of degassed, 50 mM Tris/1 mM EDTA, pH∼8, containing reduced glutathione and oxidized glutathione at concentrations of 1.6 mM and 0.2 mM, respectively. The progress of folding was monitored by HPLC using a gradient of acetonitrile/water with UV monitoring at 215 nm. After 3 h, the reaction mixture was acidified with 2% trifluoroacetic acid to pH 5. The folded peptide was isolated by preparative reverse-phase HPLC and its purity checked by HPLC analyses and mass spectrometry using a matrix-assisted laser desorption ionization time-of-flight mass spectrometer Axima CFR+ (Shimadzu Scientific Instruments). Pure fractions were combined, frozen, and lyophilized to afford pure, folded peptide. Agaphelin molecular mass is 6273 Da (58 amino acids, mature form) with an estimated pI 5.09. Extinction coefficient at 280 nm is 3355; A280 nm/cm 0.1% (1 mg/ml), 0.535. Agaphelin was diluted in PBS (1–1.5 mM) and frozen at −80°C.
Clustal alignment and phylogenetic tree
A sequence similarity search to Agaphelin (gi 118789673) was performed using protein PSI-BLAST against Arthropoda organisms with a threshold of 0.005. Other Kazal domain-containing proteins from Triatoma infestans (gi| 149898841, gi| 149898876), Triatoma brasiliensis (gi| 116267193), Tabanus yao (gi| 161897822, gi| 241914365, gi| 241914367), Hybomitra bimaculata (gi| 94730670, vasotab), Dipetalogaster maxima (gi| 4033530, dipetalogastin), Rhodnius prolixus (gi| 730939, rhodniin), Hirudo medicinalis (gi| 124043, bdellin B-3), and Hirudo nipponia (gi| 14279682, bdellin-KL) were also added. Sequences from the nonreduntant (NR) protein database of the National Center for Biotechnology Information (NCBI) are represented by six letters followed by the NCBI gi| accession number. The six letters derive from the first three letters of the genus and the first three letters from the species name. Protein sequences were aligned by the ClustalX software program [74]. The phylogenetic tree was performed with the Mega package [75] after 10,000 bootstraps with the neighbor joining algorithm using the poisson model and pairwise deletion.
Structural modeling and alignment
The sequence of the Agaphelin protein (AGAP007907-PA) was downloaded from Vectorbase [76]. The presence and location of a signal peptide was predicted using a SignalP 4.1 Server [77]. The signal peptide was removed from the protein sequence, and the structure was homology modeled using Swiss-Model Automated Mode [78], Phyre2 [79], and I-Tasser [80], [81]. The structures were then aligned to its best template (T. infestans Infestin 4, PDBID: 2ERW) and to each other using the multiple alignment of protein structures and consensus identification server [82]. Secondary structure prediction was done for each individual model using DSSP, and the percent composition of each secondary structure motif was calculated for the three models [83]. The aligned structures of Agaphelin and Infestin 4 were further processed using the PyMOL molecular graphics system (v. 1.4.1; Schrödinger, LLC, San Diego, CA, USA).
Analytical SEC-MALS HPLC
The solution state of the peptide was analyzed using analytical size-exclusion chromatography with on-line multi-angle light scattering (SEC-MALS). Chromatography was performed on an Alliance HPLC system (Waters Corporation, Milford, MA, USA) connected in series to a multi-angle Dawn Heleos light-scattering detector and a quasi-electric light scatter detector (Wyatt Technology, Santa Barbara CA, USA).
Protein separation was done on a TSKgel G2000SW×l column (Tosoh Bioscience, King of Prussia, PA, USA). The column was equilibrated in mobile phase consisting of 1.04 mM KH2PO4, 2.97 mM Na2P04•7 H20, 308 mM NaCl, 0.02% azide, pH 7.4, and the samples were run using an isocratic elution at 0.5 ml/min. A gel filtration standard (Bio-Rad, Hercules, CA, USA) was run for size comparison as well as a 125-µg injection of BSA for configuration of the MALS data. The HPLC data was analyzed with Empower software and the light scattering data with Astra software.
Circular dichroism
The circular dichroism spectrum (CDS) of the peptide was recorded on a Jasco J-815 CD spectropolarimeter over the wavelength range of 260–190 nm. Data were collected using a slit bandwidth of 1.0 nm and a signal averaging time of 1.0 second in a 1-mm quartz cuvette. Raw data measured in millidegrees were converted into ellipticity (degrees cm2 dmol−2). CDS was deconvoluted using the DichroWeb interface (http://dichroweb.cryst.bbk.ac.uk) [84]. Results having a normalized root mean square of ≤0.3 were used to calculate secondary structure composition.
Serine protease inhibition screen
The screen was performed as previously described [58], [85]. Except for the enzyme concentrations, all the rest was kept constant. The following assay concentrations were used. Thrombin (0.01 nM), α-chymotrypsin (0.03 nM), plasmin (0.8 nM), and chymase (0.45 nM) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Human skin β-tryptase (0.01 nM) was purchased from Promega (Madison, WI, USA), FXa (0.33 nM) from EMD Biosciences, Inc. (San Diego, CA, USA), FXIIa (0.1 nM) from Haematologic Technologies Inc. (Essex Junction, VT, USA), kallikrein (0.04 nM) from Fitzgerald Industries International (Concord, MA, USA), elastase (0.06 nM) from Elastin Products Company, Inc. (Owensville, MO, USA), and Cathepsin G (5.3 nM), FXIa (0.06 nM), urokinase plasminogen activator (uPA; 0.25 nM), and tissue plasminogen activator (t-PA; 0.02 nM) were purchased from Molecular Innovations (Southfield, MI, USA). Matriptase (0.03 nM) was obtained from R&D Systems (Minneapolis, MN, USA), proteinase 3 (11 nM) from Merck-Millipore (Billerica, MA, USA), and sequencing-grade trypsin (0.1 nM) was purchased from Roche Molecular Biochemicals (Indianapolis, IN, USA).
aPTT and PT
aPTT and PT were evaluated on a STart 4 stago coagulometer (Diagnostica Stago, Parsippany, NJ, USA). Freeze-dried, citrated, normal human plasma was resuspended in ultra-pure water. For the aPTT, plasma (50 µl) was incubated with 7 µl Agaphelin or PBS (control) in appropriate cuvettes and placed in the coagulometer for 2 min at 37°C. Then, 50 µl of pre-warmed aPTT reagent (STA PTT; Diagnostica Stago, Asnières sur Seine, France) was added and incubated for an additional 2 min. CaCl2 (50 µl at 25 mM) was added to start reactions. For PT, plasma (50 µl) was incubated with Agaphelin or PBS (control) and placed in the coagulometer for 2 min at 37°C. Then, 100 µl of the PT reagent (NEOplastine CI plus; Diagnostica Stago) was added. Time for clot formation was recorded in duplicate.
Surface plasmon resonance (SPR)
SPR instrumentation (XPR36), GLC sensor chip, and amine coupling reagents containing N-hydroxysulfosuccinimide (sulfo-NHS), N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDAC), and ethanolamine HCl were obtained from Bio-Rad. Synthetic refolded Agaphelin was diluted to a concentration of 100 µg/ml in NaOAc buffer (10 mM, pH 4.5) and coupled to the surface of a GLC chip using the manufacture's amine-coupling chemistry as described in the XPR36 system manual. Briefly, the surface of the sensor chip was first activated with EDC/NHS, followed by addition of the peptide. The surface was blocked using ethanolamine. Employing these conditions, surfaces containing densities of 408.27 resonance units of peptide were generated. Surface regeneration was done using 10 mM HCl. Sensograms were recorded and normalized to a baseline of 0 resonance units. Equivalent volumes of elastase were also injected over a mock, no-protein, blocked surface to serve as a blank sensogram for subtraction of bulk refractive index background. Sensograms were analyzed for fitting using XPR36 software (Bio-Rad). A Langmuir single-site binding model (A+B = AB) was used for analysis of interaction of the Kazal peptide and elastase surface.
Chemotaxis assay
HL-60 cells (human promyelocytic cells) (American Type Culture Collection, Manassas, VA, USA) were maintained in an undifferentiated state in RPMI 1640 media containing 10% fetal bovine serum and 25 mM Hepes at 37°C in a humidified 5% CO2 atmosphere. HL-60 cells differentiated in culture medium containing 1.3% DMSO for 5 days before experiments. The EZ-TAXIScan chamber (Effector Cell Institute, Tokyo, Japan) was assembled as described by the manufacturer. HL-60 cells were incubated for 1 h with Agaphelin at 37°C. Cell migration was recorded every 15 s for 30 min at 37°C in a humidified environmental chamber. Coverslips and chips used in the chamber were coated with 1% BSA at room temperature (RT) for 1 h. All glass coverslips were ultrasonicated and washed before use. Cell migration analysis was conducted with DIAS software.
Platelet aggregation
Platelet-rich plasma was obtained by plateletpheresis from medication-free platelet donors at the Department of Transfusion Medicine (NIH blood bank). Washing of platelets and platelet aggregation were performed in a Lumi-aggregometer (Chronol-Log, Haverstown, MD, USA) as described [86], [87].
Vasodilation
Contraction of rat aortic ring preparations by U 46619 was measured isometrically and recorded with transducers from Harvard Apparatus Inc. (Holliston, MA, USA) as reported [88]. A modified Tyrode solution was prepared with the addition of 5 mM Hepes; pH was adjusted to 7.4, and the solution was oxygenated by continuous bubbling of air throughout the assays. In the first assay, aortic rings were suspended in a 0.5-ml bath kept at 36°C; they were pre-constricted by 100 nM U-46619 before addition of Agaphelin to give final concentrations of 1 µM, or SG homogenates of R. prolixus (0.04 of one pair of glands/ml, with an approximate final concentration of nitrophorins of 1 µM as positive control) [89]. Additions to the bath were never greater than 5% of the volume of the bath.
TFPI cleavage
In a PCR tube, Agaphelin at different concentrations (0.1 µM, and 1 µM) was incubated with 1 µg of TFPI (R&D Systems), in a final volume of 20 µl in PBS, followed by addition of 1 µl PBS (control) or human neutrophil elastase (Molecular Innovations; final concentration: 0.8 µg/ml). After 2 h at RT, reactions were stopped by addition of Laemmli buffer (supplemented with dithiothreitol) and boiled for 5 min. Proteins were separated by 4% to 12% NuPAGE (MES buffer); gels were stained with Coomassie Blue R-250 and destained with 40% methanol. See Blue molecular weight (mol. wt.) markers were used (Invitrogen).
Neutrophils isolation
Whole blood (5 mL collected in 0.5 mL of 3.2% sodium citrate) from healthy donors was diluted in an equal volume of PBS, layered over 5 mL of HISTOPAQUE solution (10771, Sigma Aldrich), and centrifuged for 40 min, at 400×g, at room temperature. The lower interphase having granulocytes was collected and transferred to a 15-ml Falcon tube and resuspended in 10 ml ammonium chloride lysis buffer (1.7 M NH4Cl, 0.1 M KCO3, 1 mM EDTA) to lyse red blood cells. Lysis was carried out twice followed by centrifugation for 10 min at 400×g. Neutrophils were washed with PBS and resuspended at 1×106 cells/mL in high glucose DMEM (GIBCO). Neutrophils were kept on ice.
Neutrophil-induced coagulation
Purified neutrophils (500 µl, 1×105/ml, in RPMI without serum) were incubated with RPMI, Agaphelin (1 µM) or DNAse (Dornase alfa, Genentech Inc. 4 µg/ml) at 37°C for 1 hr, prior stimulation with RPMI (negative control), or 50 nM PMA (at 37°C for 3 h). Then 50 µl of this suspension was added to 50 µl of fresh plasma (collected in citrate) in a coagulometer cuvette (KC4 Delta Coagulometer, Tcoag Ireland Limited, Wicklow, Ireland). Reactions were started by addition of 12.5 mM CaCl2 (final concentrations).
NETs formation
5×104 neutrophils (in 100 µl DMEM) treated with PBS or with Agaphelin (1 µM) were seeded onto 13 mm cover-slips (Glasscyto) and incubated for 1 h, at 37°C, prior to stimulation with Phorbol 12-myristate 13-acetate (PMA), 5 nM (Sigma Chemical Co) for 3 h in DMEM. Cells were fixed with 500 µl of 4% paraformaldehyde (final concentrations) for 10 min, washed 3 times with PBS and incubated for 10 min with blocking solution (PBS, 10%FBS, 5 mg/ml BSA). Samples were incubated with goat polyclonal anti-human histone H3 antibody at 1∶50 (Abcam, 5103) diluted in blocking solution. Samples were washed 3 times with blocking solution followed by 2 h incubation with rabbit anti-goat IgG labeled with Alexa 488 at 1∶500 (Molecular Probes) diluted in blocking buffer and Hoechst 33342 at 1∶1000 diluted in blocking buffer. NETs were visualized under a confocal microscope (Leica, Confocal Microscope LEICA DMI4000 TCS SPE, 20×). Images analyses were performed using Image J software (NIH). NETs were quantified according to Farley et al [56]. Briefly, NETs were identified on digitalized images as Hoechst-positive fibrils emanating from cells with an overall length at least twice as long as the cell diameter and were counted for at least two fields of view per variable. Results were expressed as the percentage of NETs/total number of granulocytes.
Paw edema
Female C57BL/6 mice weighing 20–30 g (6–8 weeks old) were used. Experimental protocol reference number 276 was approved by the IACUC/Ethical Committee of the Universidade Federal do Triângulo Mineiro. The carrageenan-induced hind paw inflammation model was used to investigate the anti-inflammatory role of Agaphelin. Prior to each injection, the basal footpad thickness of each mouse was recorded using a caliper (Mitutoyo America Corp., Aurora, IL, USA). Subsequently, 40 µl of carrageenan (2% in saline) was administered by intraplantar injection in each posterior footpad in the absence or presence of two different concentrations of LPS-free Agaphelin. As control, groups of mice received the same volume of saline (vehicle) in the presence of Agaphelin only. As an index of edema formation, paw thickness was measured at 4 and 24 h post injection. Each data point is the mean±SD of four paws.
Tissue myeloperoxidase (MPO) assay
To measure the presence of neutrophils in the paw, activity of MPO in paw tissues was measured as previously described [90]. Briefly, paw tissues from the different groups were homogenized with an electrical tissue homogenizer (30,000 rpm) in 0.5 mL ice-cold buffer (0.1 M NaCl, 0.08 M NaPO4, 0.015 M EDTA), pH 4.7, and centrifuged at 3000 rpm for 15 min at 4°C. The pellet was subjected to hypotonic lysis with 200 µl of 0.2% NaCl solution (0.1 M) for 30 s followed by addition of an equal volume of a solution containing 0.2% NaCl). After centrifugation at 3000 rpm for 15 min, the pellet was resuspended in 0.5 mL of 0.05 M NaPO4 buffer, pH 5.4, containing 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich, EUA) and re-homogenized. The homogenate was frozen and thawed three times and then centrifuged again at 12 000 rpm for 15 min at 4°C. MPO activity in the re-suspended pellet was assayed by measuring in a spectrophotometer the change in absorbance at 450 nm using 25 µL of tetramethylbenzidine 1.6 mM (BD pharmigen) and hydrogen peroxide (0.5 mM). A unit of MPO activity was defined as that converting 1 µmol of hydrogen peroxide to water in 1 min at 22°C.
FeCl3-induced artery thrombosis
BALB/c mice were anesthetized with intramuscular xylazine (16 mg/kg) followed by ketamine (100 mg/kg). Experimental protocol number IBQM081-05/16 was approved by IACUC/Ethical Committee of the Universidade Federal do Rio de Janeiro. The right common carotid artery was isolated through a midline cervical incision, and blood flow was continuously monitored using a 0.5-VB Doppler flow probe coupled to a TS420 flow meter (Transonic Systems, Ithaca, NY, USA) as described [58]. Fifteen min before induction of thrombosis, animals were injected in the tail vein with 50 µl Agaphelin (1 mg/kg) or vehicle (PBS). Thrombus formation was induced by applying a piece of filter paper (1×2 mm) saturated with 7.5% FeCl3 solution on the adventitial surface of the artery for 3 min. After exposure, the filter paper was removed and the vessel was washed with sterile normal saline. Carotid blood flow was continuously monitored for 60 min or until complete occlusion (0 flow for at least 10 s) occurred. ANOVA using Tukey as a multiple comparison post-test was used. P≤0.05 was considered statistically significant.
Tail bleeding assay
Mice were anesthetized with intramuscular xylazin (16 mg/kg) followed by ketamine (100 mg/kg) and injected intravenously with PBS or Agaphelin (1.0 mg/kg) in a 100 µl volume. After 15 min, the distal 2-mm segment of the tail was removed and immediately immersed in 40 ml distilled water warmed to 37°C. Samples were properly homogenized and absorbance determined at 540 nm to estimate hemoglobin content. No animal was allowed to bleed for more than 30 min.
Bacterial strains
Bacterial strains were obtained either from American Type Culture Collection or Eurofins (Chantilly, VA, USA). They were maintained at −80°C in nutrient broth, brain-heart infusion broth, or tryptic soy broth, all with a final concentration of 20% glycerol (v/v). For the experiment, strains were thawed and streaked onto agar media plates for single colonies (1.5% w/v agar); colonies were then picked for liquid culture. Liquid cultures were grown on cation adjusted MHB. Minimum inhibitory concentration inocula were prepared by diluting cultures to achieve a cell density of 1×106 cells/ml.
Minimum inhibitory concentration (MIC)
Broth microdilution MIC testing was performed by Emeryville Pharmaceutical Services (Emeryville, CA, USA) using the procedure described by NCCLS publication M7-A6. The highest concentration of Agaphelin peptide tested was 512 µg/ml, and the recorded MIC values were the lowest concentration of the test article that completely inhibited growth, which was checked visually after 24 h of incubation. Each strain was tested against ciprofloxacin (1024-0 ng/ml) for quality control.
Minimum bactericidal concentration (MBC)
Agar dilution plate count MBC was performed by Emeryville Pharmaceutical Services. MBC values were determined using the procedure described in NCCLS publication M26-A. The highest concentration of peptide tested was 512 µg/ml, and the MBC values recorded were the lowest concentration of each test article in which the plate count was at least 3 standard deviations below that representing 0.1% of the final inoculum count.
Ethics statement
All treatments were performed and conducted in accordance with the NIH guidelines for the welfare of experimental animals and approved by the Ethical Committee of the Federal University of Triângulo Mineiro (Number#276) and Federal University of Rio de Janeiro (IBQM081-05/16).
Statistical analysis
Results are expressed as means ± SE. Statistical differences among the groups were analyzed by t-test or ANOVA using a multiple comparison post-test. Significance was set at p≤0.05 (GraphPad Prism Software, San Diego, CA, USA).
Zdroje
1. ChoumetV, AttoutT, ChartierL, KhunH, SautereauJ, et al. (2012) Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS One 7: e50464.
2. RibeiroJM, FrancischettiIM (2003) Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol 48 : 73–88.
3. FrancischettiIM (2010) Platelet aggregation inhibitors from hematophagous animals. Toxicon 56 : 1130–1144.
4. PhillipsonM, KubesP (2011) The neutrophil in vascular inflammation. Nat Med 17 : 1381–1390.
5. SchulzC, EngelmannB, MassbergS (2013) Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost 11 Suppl 1 : 233–241.
6. CarboneF, NencioniA, MachF, VuilleumierN, MontecuccoF (2013) Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost 110 : 501–514.
7. MartinodK, WagnerDD (2014) Thrombosis: tangled up in NETs. Blood 123 : 2768–2776.
8. YippBG, KubesP (2013) NETosis: how vital is it? Blood 122 : 2784–2794.
9. AbeH, OkajimaK, OkabeH, TakatsukiK, BinderBR (1994) Granulocyte proteases and hydrogen peroxide synergistically inactivate thrombomodulin of endothelial cells in vitro. J Lab Clin Med 123 : 874–881.
10. HiguchiDA, WunTC, LikertKM, BrozeGJJr (1992) The effect of leukocyte elastase on tissue factor pathway inhibitor. Blood 79 : 1712–1719.
11. BrozeGJJr, GirardTJ (2012) Tissue factor pathway inhibitor: structure-function. Front Biosci 17 : 262–280.
12. MassbergS, GrahlL, von BruehlML, ManukyanD, PfeilerS, et al. (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 16 : 887–896.
13. RufW, RuggeriZM (2010) Neutrophils release brakes of coagulation. Nat Med 16 : 851–852.
14. AlamSR, NewbyDE, HenriksenPA (2012) Role of the endogenous elastase inhibitor, elafin, in cardiovascular injury: from epithelium to endothelium. Biochem Pharmacol 83 : 695–704.
15. PhamCT (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6 : 541–550.
16. HirahashiJ, MekalaD, Van ZiffleJ, XiaoL, SaffaripourS, et al. (2006) Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity 25 : 271–283.
17. SchofieldZV, WoodruffTM, HalaiR, WuMC, CooperMA (2013) Neutrophils-a key component of ischemia-reperfusion injury. Shock 40 : 463–470.
18. von BruhlML, StarkK, SteinhartA, ChandraratneS, KonradI, et al. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209 : 819–835.
19. FuchsTA, BrillA, DuerschmiedD, SchatzbergD, MonestierM, et al. (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107 : 15880–15885.
20. JinR, YangG, LiG (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87 : 779–789.
21. SunZ, YangP (2004) Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol 5 : 182–190.
22. HenriksenPA (2014) The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr Opin Hematol 21 : 23–28.
23. LovatoDV, Nicolau de CamposIT, AminoR, TanakaAS (2006) The full-length cDNA of anticoagulant protein infestin revealed a novel releasable Kazal domain, a neutrophil elastase inhibitor lacking anticoagulant activity. Biochimie 88 : 673–681.
24. WikelS (2013) Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol 4 : 337.
25. NuttallPA, LabudaM (2004) Tick-host interactions: saliva-activated transmission. Parasitology 129 Suppl: S177–189.
26. ChampagneDE (2005) Antihemostatic molecules from saliva of blood-feeding arthropods. Pathophysiol Haemost Thromb 34 : 221–227.
27. Sa-Nunes A, Oliveira CJ (2010) Sialogenins and Immunomodulators Derived from Blood Feeding Parasites. Toxins and Hemostasis From bench to bedside Editors R M Kini, K J Clemetson, FS Markland, MA McLane, T Morita Springer, NY (2010): : 131–152.
28. MillerLH, AckermanHC, SuXZ, WellemsTE (2013) Malaria biology and disease pathogenesis: insights for new treatments. Nat Med 19 : 156–167.
29. AveryJW, SmithGM, OwinoSO, SarrD, NagyT, et al. (2012) Maternal malaria induces a procoagulant and antifibrinolytic state that is embryotoxic but responsive to anticoagulant therapy. PLoS One 7: e31090.
30. BakerDA, NolanT, FischerB, PinderA, CrisantiA, et al. (2011) A comprehensive gene expression atlas of sex - and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics 12 : 296.
31. LuSM, LuW, QasimMA, AndersonS, ApostolI, et al. (2001) Predicting the reactivity of proteins from their sequence alone: Kazal family of protein inhibitors of serine proteinases. Proc Natl Acad Sci U S A 98 : 1410–1415.
32. TakacP, NunnMA, MeszarosJ, PechanovaO, VrbjarN, et al. (2006) Vasotab, a vasoactive peptide from horse fly Hybomitra bimaculata (Diptera, Tabanidae) salivary glands. J Exp Biol 209 : 343–352.
33. KrowarschD, CierpickiT, JelenF, OtlewskiJ (2003) Canonical protein inhibitors of serine proteases. Cell Mol Life Sci 60 : 2427–2444.
34. RimphanitchayakitV, TassanakajonA (2010) Structure and function of invertebrate Kazal-type serine proteinase inhibitors. Dev Comp Immunol 34 : 377–386.
35. CerRZ, MudunuriU, StephensR, LebedaFJ (2009) IC50-to-Ki: a web-based tool for converting IC50 to Ki values for inhibitors of enzyme activity and ligand binding. Nucleic Acids Res 37: W441–445.
36. KanegasakiS, NomuraY, NittaN, AkiyamaS, TamataniT, et al. (2003) A novel optical assay system for the quantitative measurement of chemotaxis. J Immunol Methods 282 : 1–11.
37. Si-TaharM, PidardD, BalloyV, MoniatteM, KiefferN, et al. (1997) Human neutrophil elastase proteolytically activates the platelet integrin alphaIIbbeta3 through cleavage of the carboxyl terminus of the alphaIIb subunit heavy chain. Involvement in the potentiation of platelet aggregation. J Biol Chem 272 : 11636–11647.
38. PapayannopoulosV, MetzlerKD, HakkimA, ZychlinskyA (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191 : 677–691.
39. MaD, MizuriniDM, AssumpcaoTC, LiY, QiY, et al. (2013) Desmolaris, a novel factor XIa anticoagulant from the salivary gland of the vampire bat (Desmodus rotundus) inhibits inflammation and thrombosis in vivo. Blood 122 : 4094–4106.
40. OwensAP3rd, LuY, WhinnaHC, GachetC, FayWP, et al. (2011) Towards a standardization of the murine ferric chloride-induced carotid arterial thrombosis model. J Thromb Haemost 9 : 1862–1863.
41. EcklyA, HechlerB, FreundM, ZerrM, CazenaveJP, et al. (2011) Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost 9 : 779–789.
42. MikolajczakSA, Silva-RiveraH, PengX, TarunAS, CamargoN, et al. (2008) Distinct malaria parasite sporozoites reveal transcriptional changes that cause differential tissue infection competence in the mosquito vector and mammalian host. Mol Cell Biol 28 : 6196–6207.
43. Rosinski-ChupinI, ChertempsT, BoissonB, PerrotS, BischoffE, et al. (2007) Serial Analysis of Gene Expression in Plasmodium berghei salivary gland sporozoites. BMC Genomics 8 : 466.
44. KaiserK, MatuschewskiK, CamargoN, RossJ, KappeSH (2004) Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol 51 : 1221–1232.
45. van HoefV, BreugelmansB, SpitJ, SimonetG, ZelsS, et al. (2013) Phylogenetic distribution of protease inhibitors of the Kazal-family within the Arthropoda. Peptides 41 : 59–65.
46. LiXC, WangXW, WangZH, ZhaoXF, WangJX (2009) A three-domain Kazal-type serine proteinase inhibitor exhibiting domain inhibitory and bacteriostatic activities from freshwater crayfish Procambarus clarkii. Dev Comp Immunol 33 : 1229–1238.
47. OwenCA, CampbellMA, SannesPL, BoukedesSS, CampbellEJ (1995) Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol 131 : 775–789.
48. YoungRE, ThompsonRD, LarbiKY, LaM, RobertsCE, et al. (2004) Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. J Immunol 172 : 4493–4502.
49. AoshibaK, NagaiA, TakizawaT (1991) Effects of proteinase inhibitors on polymorphonuclear neutrophil polarization. Tohoku J Exp Med 165 : 165–170.
50. WoodmanRC, ReinhardtPH, KanwarS, JohnstonFL, KubesP (1993) Effects of human neutrophil elastase (HNE) on neutrophil function in vitro and in inflamed microvessels. Blood 82 : 2188–2195.
51. ReevesEP, BanvilleN, RyanDM, O'ReillyN, BerginDA, et al. (2013) Intracellular secretory leukoprotease inhibitor modulates inositol 1,4,5-triphosphate generation and exerts an anti-inflammatory effect on neutrophils of individuals with cystic fibrosis and chronic obstructive pulmonary disease. Biomed Res Int 2013 : 560141.
52. AdkisonAM, RaptisSZ, KelleyDG, PhamCT (2002) Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest 109 : 363–371.
53. JenneCN, UrrutiaR, KubesP (2013) Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol 35 : 254–261.
54. PilsczekFH, SalinaD, PoonKK, FaheyC, YippBG, et al. (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185 : 7413–7425.
55. BraianC, HogeaV, StendahlO (2013) Mycobacterium tuberculosis - induced neutrophil extracellular traps activate human macrophages. J Innate Immun 5 : 591–602.
56. FarleyK, StolleyJM, ZhaoP, CooleyJ, Remold-O'DonnellE (2012) A serpinB1 regulatory mechanism is essential for restricting neutrophil extracellular trap generation. J Immunol 189 : 4574–4581.
57. TaggartCC, CryanSA, WeldonS, GibbonsA, GreeneCM, et al. (2005) Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J Exp Med 202 : 1659–1668.
58. CollinN, AssumpcaoTC, MizuriniDM, GilmoreDC, Dutra-OliveiraA, et al. (2012) Lufaxin, a novel factor Xa inhibitor from the salivary gland of the sand fly Lutzomyia longipalpis blocks protease-activated receptor 2 activation and inhibits inflammation and thrombosis in vivo. Arterioscler Thromb Vasc Biol 32 : 2185–2198.
59. MizuriniDM, FrancischettiIM, AndersenJF, MonteiroRQ (2010) Nitrophorin 2, a factor IX(a)-directed anticoagulant, inhibits arterial thrombosis without impairing haemostasis. Thromb Haemost 104 : 1116–1123.
60. NazarethRA, TomazLS, Ortiz-CostaS, AtellaGC, RibeiroJM, et al. (2006) Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thromb Haemost 96 : 7–13.
61. FurieB, FurieBC (2008) Mechanisms of thrombus formation. N Engl J Med 359 : 938–949.
62. GleissnerCA, von HundelshausenP, LeyK (2008) Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol 28 : 1920–1927.
63. WatsonSP (2009) Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des 15 : 1358–1372.
64. RenneT, SchmaierAH, NickelKF, BlombackM, MaasC (2012) In vivo roles of factor XII. Blood 120 : 4296–4303.
65. FrancischettiIM, ValenzuelaJG, RibeiroJM (1999) Anophelin: kinetics and mechanism of thrombin inhibition. Biochemistry 38 : 16678–16685.
66. AssumpcaoTC, MaD, SchwarzA, ReiterK, SantanaJM, et al. (2013) Salivary Antigen-5/CAP Family Members Are Cu2+-dependent Antioxidant Enzymes That Scavenge OFormula and Inhibit Collagen-induced Platelet Aggregation and Neutrophil Oxidative Burst. J Biol Chem 288 : 14341–14361.
67. RibeiroJM, ValenzuelaJG (1999) Purification and cloning of the salivary peroxidase/catechol oxidase of the mosquito Anopheles albimanus. J Exp Biol 202 : 809–816.
68. KappeSH, KaiserK, MatuschewskiK (2003) The Plasmodium sporozoite journey: a rite of passage. Trends Parasitol 19 : 135–143.
69. JanoffA, RothWJ, SinhaS, BarnwellJW (1988) Degradation of plasmodial antigens by human neutrophil elastase. J Immunol 141 : 1332–1340.
70. IfedibaT, VanderbergJP (1981) Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294 : 364–366.
71. PonnuduraiT, LensenAH, Van GemertGJ, BensinkMP, BolmerM, et al. (1989) Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98 Pt 2 : 165–173.
72. KabiruEW, MbogoCM, MuiruriSK, OumaJH, GithureJI, et al. (1997) Sporozoite loads of naturally infected Anopheles in Kilifi District, Kenya. J Am Mosq Control Assoc 13 : 259–262.
73. EdgarR, DomrachevM, LashAE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research 30 : 207–210.
74. ThompsonJD, GibsonTJ, PlewniakF, JeanmouginF, HigginsDG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25 : 4876–4882.
75. KumarS, TamuraK, NeiM (2004) MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5 : 150–163.
76. MegyK, EmrichSJ, LawsonD, CampbellD, DialynasE, et al. (2012) VectorBase: improvements to a bioinformatics resource for invertebrate vector genomics. Nucleic Acids Res 40: D729–734.
77. PetersenTN, BrunakS, von HeijneG, NielsenH (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8 : 785–786.
78. BordoliL, SchwedeT (2012) Automated protein structure modeling with SWISS-MODEL Workspace and the Protein Model Portal. Methods Mol Biol 857 : 107–136.
79. KelleyLA, SternbergMJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4 : 363–371.
80. ZhangY (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9 : 40.
81. RoyA, KucukuralA, ZhangY (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5 : 725–738.
82. IlinkinI, YeJ, JanardanR (2010) Multiple structure alignment and consensus identification for proteins. BMC Bioinformatics 11 : 71.
83. KabschW, SanderC (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22 : 2577–2637.
84. WhitmoreL, WallaceBA (2008) Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89 : 392–400.
85. ChmelarJ, OliveiraCJ, RezacovaP, FrancischettiIM, KovarovaZ, et al. (2011) A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 117 : 736–744.
86. MaD, AssumpcaoTC, LiY, AndersenJF, RibeiroJ, et al. (2012) Triplatin, a platelet aggregation inhibitor from the salivary gland of the triatomine vector of Chagas disease, binds to TXA(2) but does not interact with glycoprotein PVI. Thromb Haemost 107 : 111–123.
87. FrancischettiIM, GhazalehFA, ReisRA, CarliniCR, GuimaraesJA (1998) Convulxin induces platelet activation by a tyrosine-kinase-dependent pathway and stimulates tyrosine phosphorylation of platelet proteins, including PLC gamma 2, independently of integrin alpha IIb beta 3. Arch Biochem Biophys 353 : 239–250.
88. AssumpcaoTC, AlvarengaPH, RibeiroJM, AndersenJF, FrancischettiIM (2010) Dipetalodipin, a novel multifunctional salivary lipocalin that inhibits platelet aggregation, vasoconstriction, and angiogenesis through unique binding specificity for TXA2, PGF2alpha, and 15(S)-HETE. J Biol Chem 285 : 39001–39012.
89. WeichselA, AndersenJF, ChampagneDE, WalkerFA, MontfortWR (1998) Crystal structures of a nitric oxide transport protein from a blood-sucking insect. Nat Struct Biol 5 : 304–309.
90. GarletGP, CardosoCR, CampanelliAP, FerreiraBR, Avila-CamposMJ, et al. (2007) The dual role of p55 tumour necrosis factor-alpha receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin Exp Immunol 147 : 128–138.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



