-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvolution of Plastic Transmission Strategies in Avian Malaria
Seasonal fluctuations in the environment affect dramatically the abundance of insect species. These fluctuations have important consequences for the transmission of vector-borne diseases. Here we contend that malaria parasites may have evolved plastic transmission strategies as an adaptation to the fluctuations in mosquito densities. First, our theoretical analysis identifies the conditions for the evolution of such plastic transmission strategies. Second, we show that in avian malaria Plasmodium parasites have the ability to increase transmission after being bitten by uninfected Culex mosquitoes. This demonstrates the ability of Plasmodium parasites to adopt plastic transmission strategies and challenges our understanding of malaria epidemiology.
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004308
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004308Summary
Seasonal fluctuations in the environment affect dramatically the abundance of insect species. These fluctuations have important consequences for the transmission of vector-borne diseases. Here we contend that malaria parasites may have evolved plastic transmission strategies as an adaptation to the fluctuations in mosquito densities. First, our theoretical analysis identifies the conditions for the evolution of such plastic transmission strategies. Second, we show that in avian malaria Plasmodium parasites have the ability to increase transmission after being bitten by uninfected Culex mosquitoes. This demonstrates the ability of Plasmodium parasites to adopt plastic transmission strategies and challenges our understanding of malaria epidemiology.
Introduction
All organisms experience some level of temporal variation in the quality of their environment. In response to these variations, many species have evolved specific strategies that allow them to limit or shut down growth and development until the conditions improve [1]. The best reported examples are dormancy in plants and diapause in insects, but similar strategies have also evolved in microbes. Bacteria can survive adverse conditions (e.g. desiccation, antibiotics) by entering a state of reduced metabolic activity called persistence [2], [3]. Several viruses (e.g. lambdoid phages, herpesviruses) have evolved the ability to integrate their host genome and enter a latent phase during which within-host replication is shut down, the infection is asymptomatic and transmission is very limited [4], [5]. Hence, the evolution of latent life cycle in pathogens may be viewed as an adaptation to temporal variations of the availability of susceptible hosts.
For vector-borne pathogens the abundance of vectors is a key parameter determining the quality of their environment. Vector density may vary in space due to intrinsic heterogeneities of their habitat (e.g. temperature, hygrometry). In malaria, for instance, spatial variation in mosquito abundance has a direct impact on the geographic distribution of prevalence [6]–[8]. Vector abundance may also vary widely through time [9]. Although inter-tropical regions are characterized by a relatively constant density of vectors, regions from higher latitudes experience a broad range of climatic seasonality, and very far from the equator mosquitoes are present for only a fraction of the year [10]–[12]. From the parasite's perspective, such temporal variation in vector density is analogous to the temporal variations in habitat quality experienced by other organisms. How have malaria parasites adapted to these temporal fluctuations in vector density?
Malaria is caused by Plasmodium spp., a prevalent vector-borne pathogen which is found infecting many vertebrate hosts, including humans, reptiles and birds. Plasmodium infections within the vertebrate host are characterized by drastic temporal changes in blood parasitaemia. After an initial acute phase, generally characterized by a very high number of parasites in the blood, the infection usually reaches a chronic phase where the parasitaemia stabilizes at low levels. During the chronic phase, however, blood parasites may go through short, intense, bouts of asexual replication during which parasitaemia increases temporarily. Little is known about the causes of such recrudescences but one potential trigger may be a weakening of the host's immunity [13]. In some, but not all, Plasmodium species the infection may entirely disappear from the blood stream, hiding in other host cells in the form of (dormant) exoerythrocytic stages. After a period of latency that can last months or even years, parasites may reappear in the blood stream. These relapses are due to the differentiation of dormant parasite stages into new erythrocytic stages. The dormant stages of Plasmodium were first described in birds [14], [15] and, later, in humans [16], [17] and reptiles [18], [19]. Relapses and recrudescences have been puzzling researchers ever since the first clinical symptoms were described in P. vivax-infected humans in the late 19th century [20], [21]. Why do some malaria species (e.g. P. falciparum) completely lack the ability to produce dormant stages in the vertebrate host? What are the ultimate causes of the production of recrudescences and relapses? Is this diversity of life cycles due to the temporal variation in vector density?
The ability to produce recrudescences and relapses may be a genetically fixed parasite strategy that has evolved as a way to match the dynamics of vector populations. Populations exposed to different fluctuations of vector density may thus evolve different strategies. In human malaria, the relapsing periodicity of different lineages of P. vivax supports this prediction [12], [22]. Indeed, lineages exhibiting frequent relapses have been sampled in Asia where the vector is present throughout the year. In contrast, long latency has been observed in lineages sampled in temperate zones where the mosquito vector is only present for a few months. In avian malaria, similarly, the differences in the within-host dynamics of Leucocytozoon spp. and Haemoproteus mansoni may have evolved to match the temporal fluctuations of their respective vector species (simuliid flies and Culicoides, respectively) [23].
Another explanation for these patterns may involve adaptive phenotypic plasticity. Phenotypic plasticity is the ability for a single genotype to exhibit different phenotypes in different environments [24], [25]. This contrasts with the above hypothesis (fixed strategy) where different relapsing strategies are associated with different genotypes. The ability to adopt a plastic exploitation strategy requires the ability to detect a change of the environment (i.e. cues) and the acquisition of such a sensing mechanism may be associated with direct fitness costs [24], [25]. In spite of these costs, phenotypic plasticity is often viewed as an adaptation to variable environments [24], [25]. Many pathogens have indeed evolved an unparalleled level of phenotypic plasticity in their life history traits to cope with the temporal variability of their habitat [26]–[28]. In Plasmodium, plasticity has been shown to be a response to various stressful conditions such as drug treatment and the presence of competitors [29], [30]. Some experimental evidence suggests that relapses may also be a plastic trait. P. vivax relapses are often observed in the spring and summer months irrespective of when the patients got the original infection [31], which suggests that the parasite may react to a change in the physiological state of the host or the environment. Relapses have also been observed in avian malaria, which has triggered several experimental studies to pinpoint the underlying environmental cues [32]. Some authors have proposed that spring relapses may result from increasing photoperiod and/or stress-induced hormonal changes [33]–[36]. Parasites may indirectly benefit from using hormonal and photoperiod cues because they often coincide with (or even anticipate) the appearance of vectors in temperate populations. Such indirect cues are, however, imperfect because vector abundance may be influenced by other, non-seasonal, factors. A more efficient strategy would be to react to direct cues such as mosquito bites which unambiguously indicate the presence of vectors [10], [31], [37]. Although there is some correlational evidence supporting this hypothesis, largely coming from longitudinal cohort studies [10], [37], [38], direct experimental evidence for this hypothesis is scarce and somewhat contradictory. In rodent malaria P.chabaudi, mice exposed to probing by Anopheles stephensi mosquitoes had higher and earlier parasite growth and gametocytaemia than control unexposed mice [39]. In contrast, however, Shutler et al. [40] found no evidence of facultative alteration in the timing or in the level of P. chabaudi or P. vinckei parasitaemia and gametocytogenesis as a consequence of mosquito probing. Rodent malaria is a laboratory model and, as such, may, not be the best system to test this hypothesis because An. stephensi is not the natural vector of rodent malaria [41]. In addition the parasites have been originally sampled from the tropical lowlands of the Congo Basin [42] an area where malaria transmission is high throughout the year [43] and thus the selective pressure for the evolution of plasticity in response to vector availability is expected to be weak. Finally, both rodent malaria experiments [39], [40] were carried out during the initial (acute) phase of the infection, i.e. when parasitaemia is already high (so no need to increase it further) and the infection recent (so the mosquitoes are probably still around). We contend that it is mainly in old (chronic state) infections that the parasite may accrue the greatest benefits from a plastic response to the bites of its vector. Finally, both of these studies used gametocyte density (the blood stages of Plasmodium that are transmissible to the vector) as a proxy for transmission but neither followed transmission all the way to the mosquito stage.
Here, we first present a theoretical model that studies the evolution of parasite transmission in a variable environment. This model explores the effects of the seasonality of mosquito dynamics on the evolution of virulence and transmission strategies. In particular it clarifies the selective pressures acting on the evolution of temporally variable transmission strategies and identifies the conditions driving the evolution of costly plastic transmission strategies triggered by the exposure to mosquito bites. Then, we carry out an experiment to test the following two hypotheses: (1) Plasmodium parasites plastically react to the biting of uninfected vectors by enhancing their within-host replication, and (2) this effect yields higher rates of transmission to the mosquito vector. For this purpose, we studied the interaction between Plasmodium relictum (the aetiological agent of the most prevalent form of avian malaria which is commonly found infecting Passeriform birds in Europe) and its natural vector, the mosquito Culex pipiens. P. relictum is a very convenient malaria parasite to address this issue because it is known to have a long chronic phase marked by sudden events of recrudescences and relapses [44]. Strictly speaking, relapses originate from the division and differentiation of dormant stages (called phanerozoites) that infect the endothelial cells of different organs such as the spleen and liver, while recrudescences originate from an increased replication of the blood stages [44]. In practice, however, it is very difficult to distinguish between recrudescences and true relapses and in the following we will use the term relapse to encompass both cases. We investigate whether bites of uninfected Cx. pipiens mosquitoes trigger parasite relapses in the blood of domestic canaries (Serinus canaria) chronically infected by P. relictum (lineage SGS1), as well as the concomitant effects on transmission in terms of mosquito infectivity (see Box 1 and Fig. 1).
Fig. 1. (A) Overview of the experiment with the 3 exposure sessions (grey areas). 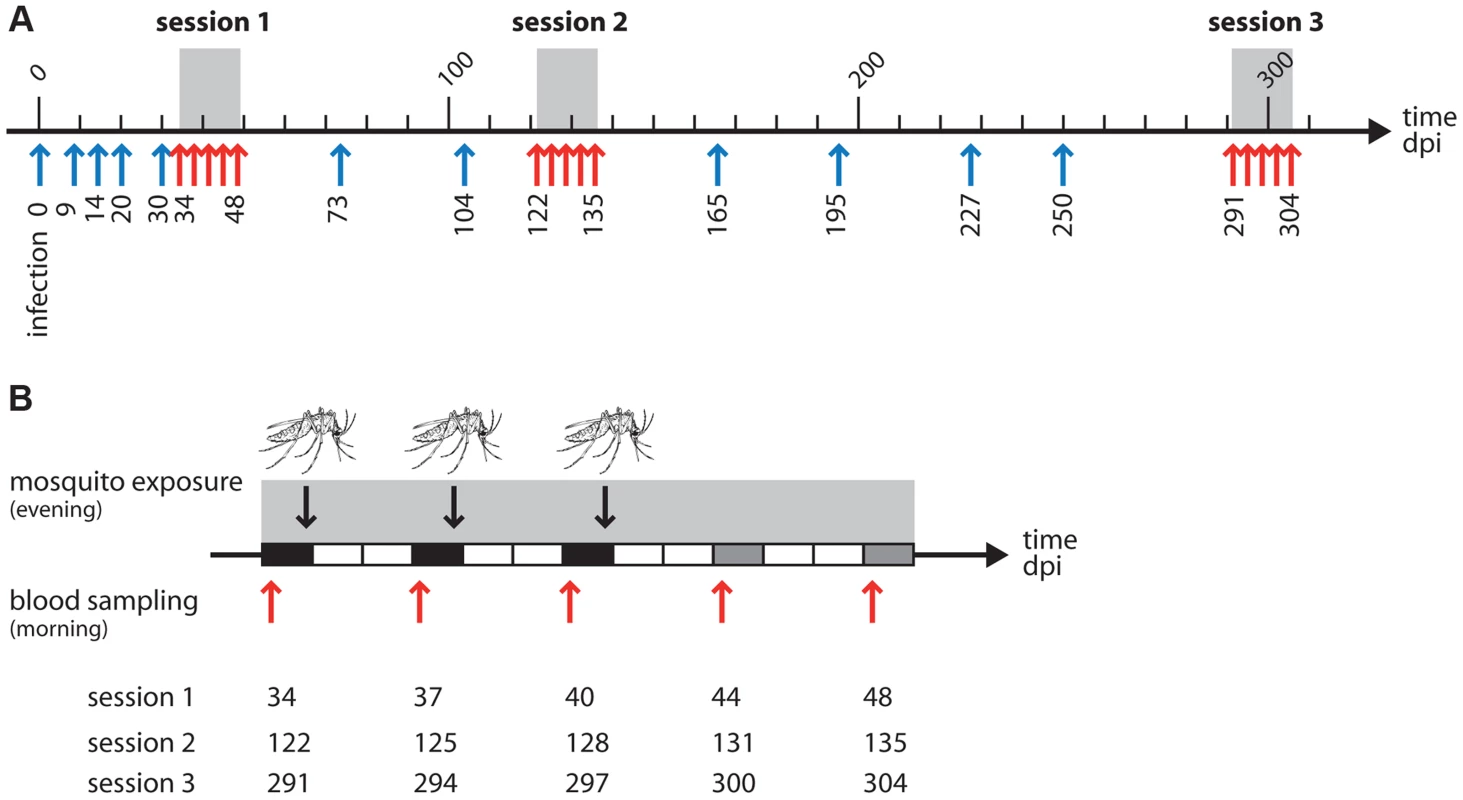
Arrows indicate the times at which blood samples were taken from the birds: red arrows for the 5 samples taken in and around the time of the mosquito exposure, blue arrows for the regular monitoring of parasitaemia before and between exposures. One unit = 10 days. (B) Zoom on an exposure session: each rectangle represents a day. Black rectangles: blood sample in the morning, mosquito exposure in the evening. Grey rectangles: blood sample in the morning, no mosquito exposure. White rectangles: no mosquito exposure, no blood sampling. Red arrows and figures underneath indicate dates where blood sampling took place in each of the 3 sessions. The mosquito drawing indicates a mosquito exposure. Box 1. Experimental design
Twenty birds experimentally inoculated with avian malaria parasite Plasmodium relictum were followed for over 300 days post-infection (dpi) to monitor the variation in blood parasitaemia. Birds were either exposed or unexposed (control) to mosquito bites. Exposure to mosquito bites took place in 3 consecutive “exposure sessions” (grey area in figure 1). Each session consisted of 3 “exposure days” separated by 3-day intervals: days 34, 37 and 40 dpi for the first session, days 122, 125 and 128 dpi for the second session, and days 291, 294 and 297 dpi for the third session. In exposure days, each bird in the “exposed” treatment was placed in a cage with a batch of 50 uninfected female mosquitoes for 2 hours; “control” birds were placed in identical conditions but without mosquitoes. Two different response variables were subsequently obtained:
-
To estimate changes in blood parasitaemia due to mosquito exposure, blood samples were taken from all (“exposed” and “control”) birds in the morning preceding the exposure as well as 3–4 days and 7–8 days afterwards (days 44 and 48 dpi, days 131 and 135 dpi and days 300 and 304 dpi for exposure sessions one, two and three, respectively). These are indicated by red arrows in Fig. 1. Bird parasitaemia was measured by qPCR on blood samples (see materials and methods for details). Regular monitoring of parasitaemia took place at several other time points before and between each of the exposure sessions (indicated by blue arrows in Fig. 1).
-
To estimate the effect of exposure on the prevalence and intensity of mosquito infections, after each exposure blood-fed mosquitoes from all the batches were maintained under standard laboratory conditions for 7 days. Fifteen haphazardly chosen mosquitoes were dissected to check for the presence (prevalence) and number (intensity) of oocysts in the midgut (see materials and methods).
Results
Theory: Evolution of plastic transmission strategies
To model the evolution of plastic transmission strategies we first need to model the epidemiological dynamics of malaria. For the sake of simplicity the vertebrate host population is assumed to be constant and equal to N = S(t)+I(t), where S(t) and I(t) are the densities of uninfected and infected hosts, respectively. In contrast, the density of the vector population may change through time. This may be particularly relevant in temperate environments where the mosquitoes do not reproduce in winter. In other words, the influx θ(t) of uninfected mosquitoes is assumed to change throughout the year (i.e. θ(t) fluctuates with a period T). As a consequence, the densities V(t) and VI(t) of uninfected and infected vectors also fluctuate through time. The following set of differential equations describes the temporal dynamics of the different types of hosts (the dot notation indicates differential over time):
Where d is the natural mortality rate of the vertebrate host and α is the virulence of malaria (the extra mortality induced by the infection); m and mI are the mortality rates of uninfected and infected vectors, respectively; β1 is the transmission rate from the vertebrate host to the vector; β2 is the transmission rate from the vector to the vertebrate host. Figure 2 presents a typical epidemiological dynamics in a seasonal environment.Fig. 2. Epidemiological dynamics of a vector-borne disease in a seasonal environment. 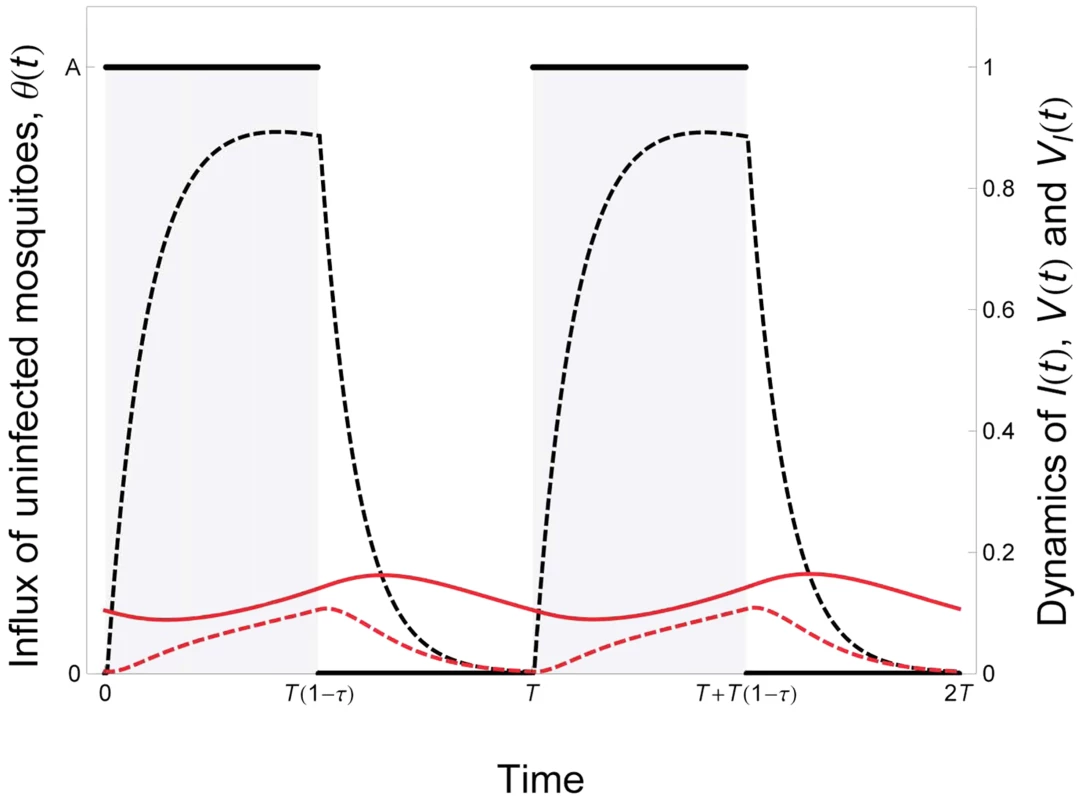
We consider here that the influx of mosquitoes, θ(t), is a periodic square wave (see equation (5) in the main text). The parameter T measures the duration of the period and the parameter τ measures seasonality: the fraction of time where the environment is not suitable for vector reproduction.The epidemiologic dynamics converges to a periodic equilibrium characterised by fluctuations of the uninfected and infected vector the densities: V(t) and VI(t) (black and red dashed lines, respectively). We also plot the dynamics of the density of infected hosts: I(t)/10 (red line). Parameter values: , , , . See default values in the Text S1 for other parameters. What are the consequences of the temporal variation in the availability of vectors on the evolution of malaria? More specifically, what is the effect of the shape of the function θ(t) on the evolution of the parasite? To study this question one can consider the fate of a mutant malaria strategy M that would alter its life history strategy in the vertebrate host. The replication in the vertebrate host is assumed to be governed by two traits of the parasite. The first trait, εF, governs the allocation to a fixed exploitation strategy that yields a within-host growth rate that does not vary with time. In contrast, the second trait, εP, governs allocation to a plastic exploitation strategy that may vary with time. More specifically we consider that when the parasite adopts this plastic trait within-host growth rate depends on the density of vectors in the population. In other words this plastic trait allows within-host growth rate to be conditional on the presence of vectors. Because within-host growth rate is assumed to affect virulence in the vertebrate host this yields:
As in classical models of virulence evolution [45], [46] more replication is costly because it may induce the death of the vertebrate host but it allows the parasite to transmit more efficiently. The parameters a and b govern the specific shape of the virulence-transmission trade-off (see equation (3) below). In addition we assume that the adoption of a plastic exploitation strategy requires the ability to acquire information regarding the availability of the vectors. The parameter c, therefore measures the fitness cost associated with a higher investment in the mechanisms allowing such plasticity. Only the transmission rate, β1, from the vertebrate host to the vector is assumed to be affected by the parasite exploitation strategy (i.e. β2 is assumed to be constant) which yields:
Note that in this model virulence and transmission vary in time only if the parasite allocates some resources in the development of a plastic trait (i.e. εP>0).Integrating the change in frequency of the mutant parasite genotype M over one period of the fluctuation allows deriving a condition for the invasion of the mutant (see Text S1):
where , , and .The first term in the above equation for sM is the classical benefit associated with higher investment in transmission. If the mutant invests more than the resident in transmission (i.e. Δβ1>0) the fitness increase depends on , which measures the availability of both uninfected hosts and vectors over the period of the fluctuation of the environment. The second term in sM is the classical cost of virulence if the mutant exploits the host more aggressively than the resident (i.e. Δα>0). The final term in sM measures the potential benefit associated with plastic transmission strategies. This term depends on the covariance between the availability of uninfected vectors, the availability of uninfected vertebrate hosts and the investment in transmission from vertebrate to mosquito hosts. The mutant may gain a fitness advantage if its conditional transmission rate can better track the fluctuations of the density of uninfected hosts. In other words this final term indicates that in a fluctuating environment it is adaptive to invest on transmission whenever uninfected hosts and mosquitoes reach high densities simultaneously. We can use this analysis to look at different evolutionary scenarios.
Without plasticity
For the sake of simplicity let us focus first on the influence of seasonality on the evolution of the fixed exploitation strategy in the absence of plasticity. In this case εP = 0 and neither virulence nor transmission vary in time. Consequently the final term in sM drops and evolution is driven by the balance between the benefit of transmission and the cost of virulence. In our model the cost of virulence is not affected by the epidemiological dynamics (infected hosts die because of the infection irrespective of the presence of the vectors). In contrast, the benefit of transmission is weighted by the quantity which measures the overall opportunity of contacts between uninfected hosts and vectors. Fluctuations in θ over time may not necessarily affect the quantity and in these situations seasonality has no impact on virulence evolution (Text S1). Yet, contrasting situations with or without wintering season dramatically affects the average influx of vectors and consequently the quantity . To study the effect of seasonality we model the fluctuations of the influx of uninfected mosquitoes as a periodic square wave (Fig. 2):
Where A is the influx of uninfected mosquitoes when the environment is favorable for mosquito reproduction, T is the period of the fluctuations, τ is the fraction of time unsuitable for mosquito reproduction and H(x) is the discontinuous unit step function taking the value 0 (when x<0) or 1 (when x>0). In Figure 3 we show that when seasonality increases the quantity drops, the benefit of transmission is reduced and the parasite evolves toward lower virulence and lower transmission rates. In other words our model predicts that, away from the tropics, malaria population will experience more seasonal environments and the investment in the fixed level of host exploitation drops to avoid the cost of virulence when no vectors are around. This should yield lower levels of transmission and virulence in higher latitudes.Fig. 3. Evolution of allocation to fixed pathogen strategy, εF, as a function of seasonality, τ. 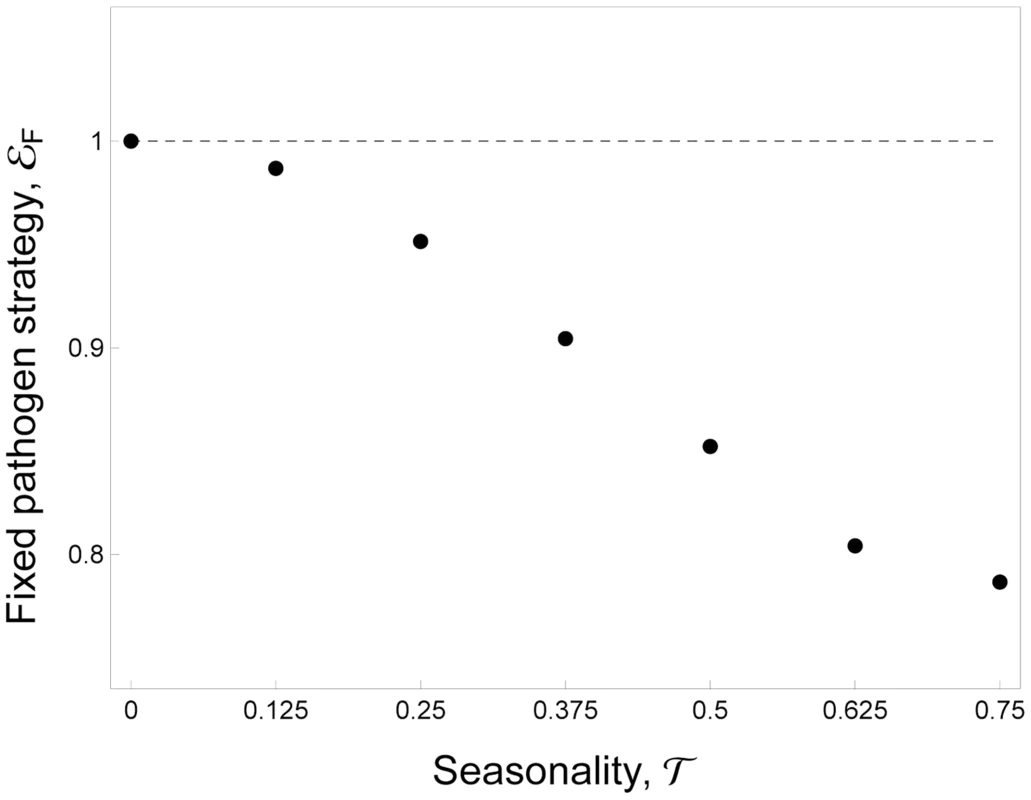
A higher investment in εF indicates that the pathogen invests more in transmission (and virulence). Parameter values: εP = 0. See default values in the Text S1 for the other parameters. With plasticity
Our model can also be used to understand the conditions leading to the evolution of plastic transmission strategies. In this case we allow both fixed and plastic exploitation strategies (i.e. εF and εP) to evolve freely. Figure 4 shows that when the vectors are present throughout the year the pathogen does not invest in a costly plastic strategy. In this case, the parasite population evolves toward the fixed level of host exploitation studied in the previous scenario and resulting from the balance between the benefit and the cost of parasite virulence. When the environment becomes more seasonal (i.e. τ>0) the allocation to a fixed exploitation strategy drops and the pathogen invests in the costly plastic strategy (Fig. 4). The plastic strategy allows allocating resources to host exploitation only when vectors are around. Hence this relatively simple model confirms that a costly plastic transmission strategy that depends on the availability of vectors in the environment can outcompete a constant exploitation strategy. Note that for intermediate levels of seasonality the evolutionarily stable exploitation strategy is a mixture between a constant and a plastic strategy.
Fig. 4. Joint evolution of (A) the plastic pathogen strategy, εP and of (B) the fixed pathogen strategy, εF for different values of seasonality, τ, and for different costs of plasticity, c. 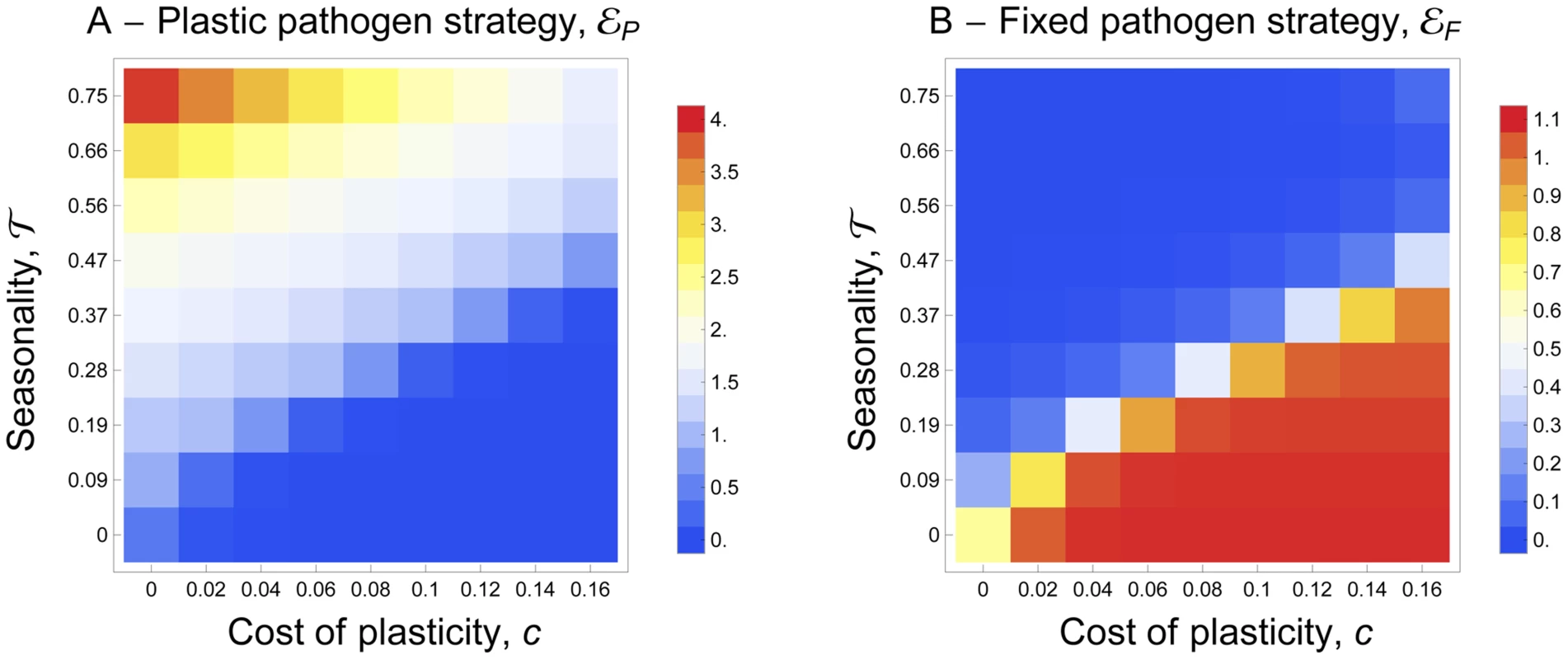
The color shading indicates the value of the pathogen strategies and the warmer color indicates higher values. A higher investment in εP indicates that the pathogen invests more into the mechanisms that allow it to react to the presence of mosquitoes. A higher investment in εF indicates that the pathogen invests more into transmission (and virulence). For both strategies the lower value (blue) is 0. The maximal value (red) of εP is 4 and the maximal value (red) of εF is 1.1. See default values in the Text S1 for the other parameters. Experiment with avian malaria
The experimental design is presented in Box 1. In brief, we followed 20 experimentally infected birds over 300 days post infection and monitored within-host parasitaemia and transmission to vectors. Birds were assigned to two treatments: “exposed” or “control” (unexposed) to uninfected mosquito bites during 3 sessions (starting 34, 122 and 291 days post infection, see Fig. 1A). During each session the exposed birds were bitten by a batch of 50 female mosquitoes every 3 days (see Fig. 1B).
Parasitaemia
The parasitaemia initially followed a bell-shape function typical of acute Plasmodium infections: peaking at day 14 pi and decreasing thereafter (Fig. 5). The infection subsequently entered a long-lasting chronic state, which was characterized by a low (but detectable) blood parasitaemia over several months (Fig. 5).
Fig. 5. Dynamics of blood parasitaemia (Log(RQ+1), mean ± s.e.) of Plasmodium relictum (lineage SGS1) in birds that were either unexposed (open circles, dashed line) or exposed to mosquito bites (filled circles, solid line). 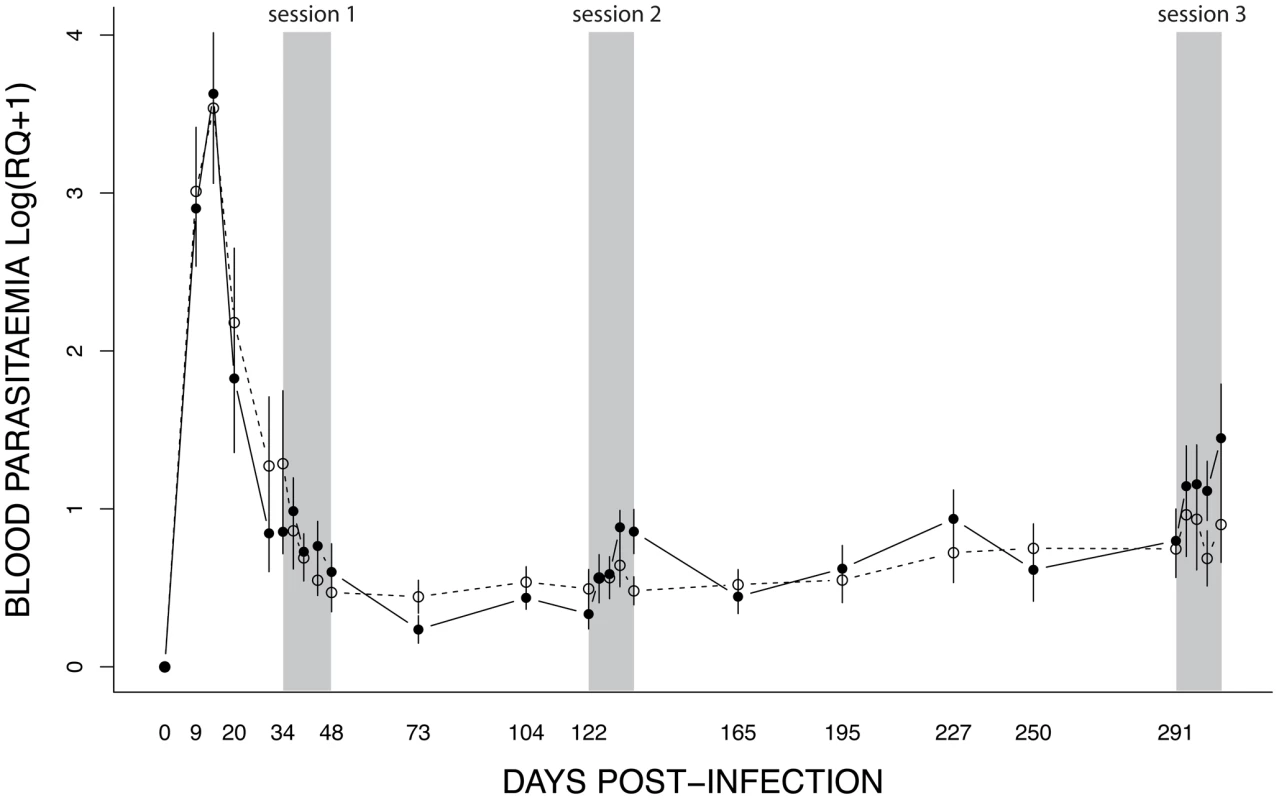
Mosquito exposure (refer to Materials & Methods for details) took place in three consecutive sessions (grey areas): 34–48 dpi, 122–135 dpi and 291–304 dpi. The effect of mosquito exposure on blood parasitaemia was analysed separately for each of the 3 exposure sessions. In the first (34–48 dpi) session, parasitaemia was still decreasing after the initial (acute) phase (time effect: χ21 = 11.14, P = 0.0008) but this decrease was independent of whether the birds had been exposed to mosquitoes or not (exposure effect: χ21 = 0.007, P = 0.9345) (Fig. 6A). In the second (122–135 dpi) session, however, bird parasitaemia differed between the exposed and the unexposed (control) birds. Whereas in the control birds the total number of parasites remained roughly constant with time, parasitaemia in the exposed group increased significantly with time (exposure*time: χ21 = 9.18, P = 0.0024, Fig. 6B). In the third (291–304 dpi) session, parasitaemia showed a similar trend towards a higher parasitaemia in exposed mosquitoes (Fig. 6C), but this trend was not statistically significant (exposure: χ21 = 0.40, P = 0.5270; time: χ21 = 18.87, P = 0.0003). At this time point, however, several birds had died, which reduced the statistical replication and limited the statistical power of the test (n = 6 birds alive on day 291, n = 3 and n = 4 on day 304 for exposed and control birds, respectively) (see Table S1 for group sample sizes).
Fig. 6. Details of the dynamics of blood parasitaemia (Log(RQ+1), mean ± s.e.) for the 3 exposure sessions (see Fig. 4): (A) session 1 (34–48 dpi), (B) session 2 (122–135 dpi) and (C) session 3 (291–304 dpi). 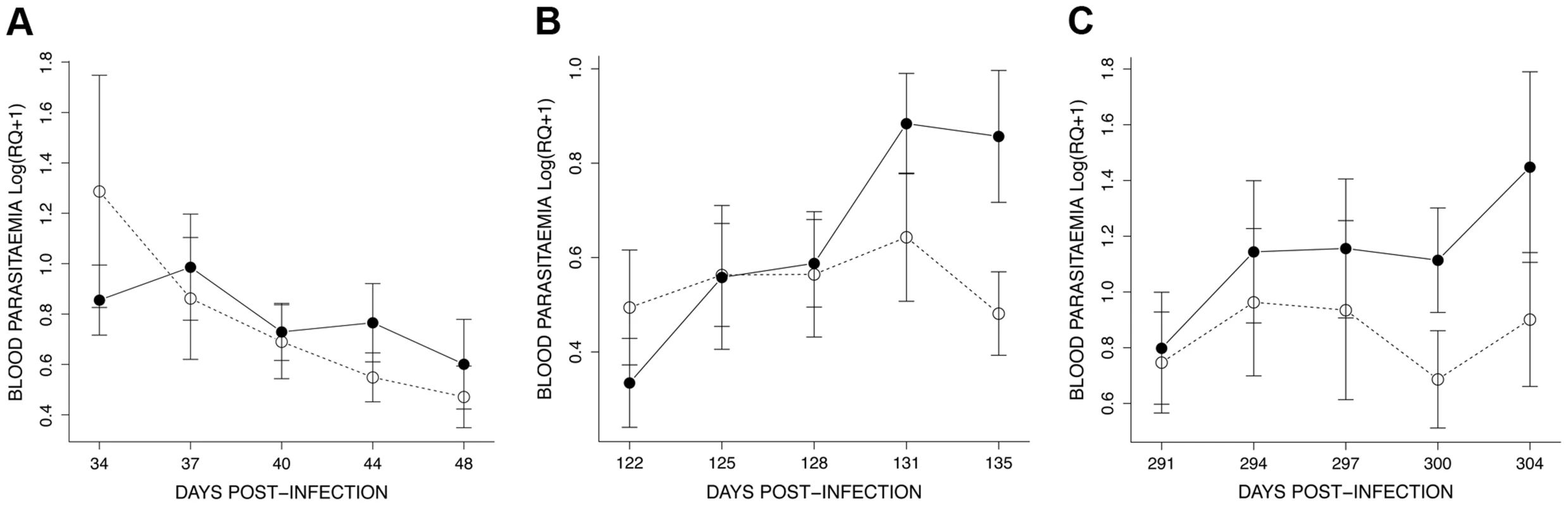
Unexposed (open circles, dashed line) or exposed to mosquito bites (filled circles, solid line). It is worth noting that the effect of exposure to mosquito bites seems to be short-lived. Indeed, one month after the second exposure session (165 dpi) we did not detect any difference in parasitaemia between the exposed and the control birds (χ21 = 0.03, P = 0.5932, see Fig. 5).
Mosquito infection
In the first exposure session, infection prevalence (proportion of mosquitoes containing at least 1 oocyst) was extremely high among the first batch of mosquitoes (Table S2) but decreased in subsequent batches (contrast 34+37vs. 40 dpi, χ21 = 51.71, P<0.0001; Fig. 7A). This effect is linked to the decrease in overall parasitaemia (χ21 = 48.45, P<0.0001) and is likely due to the fact that the first session occurred at the end of the acute phase and before the start of the chronic phase (see Fig. 5). In contrast, in both the second and third exposure sessions infection prevalence increased significantly in successive mosquito batches (second exposure session: contrast 122 vs. 125+128 dpi, χ21 = 66.34, P<0.0001; third exposure session: χ22 = 25.99, P<0.0001, here all time points differed from each other, Fig. 7B and B).
Fig. 7. Boxplot of the proportion of infected mosquitoes among 15 haphazardly chosen blood fed individuals on each bird (harbouring at least 1 oocyst in the midgut) for the 3 exposure sessions (see Fig. 4): (A) session 1 (34–40 dpi), (B) session 2 (122–128 dpi) and (C) session 3 (291–297 dpi). 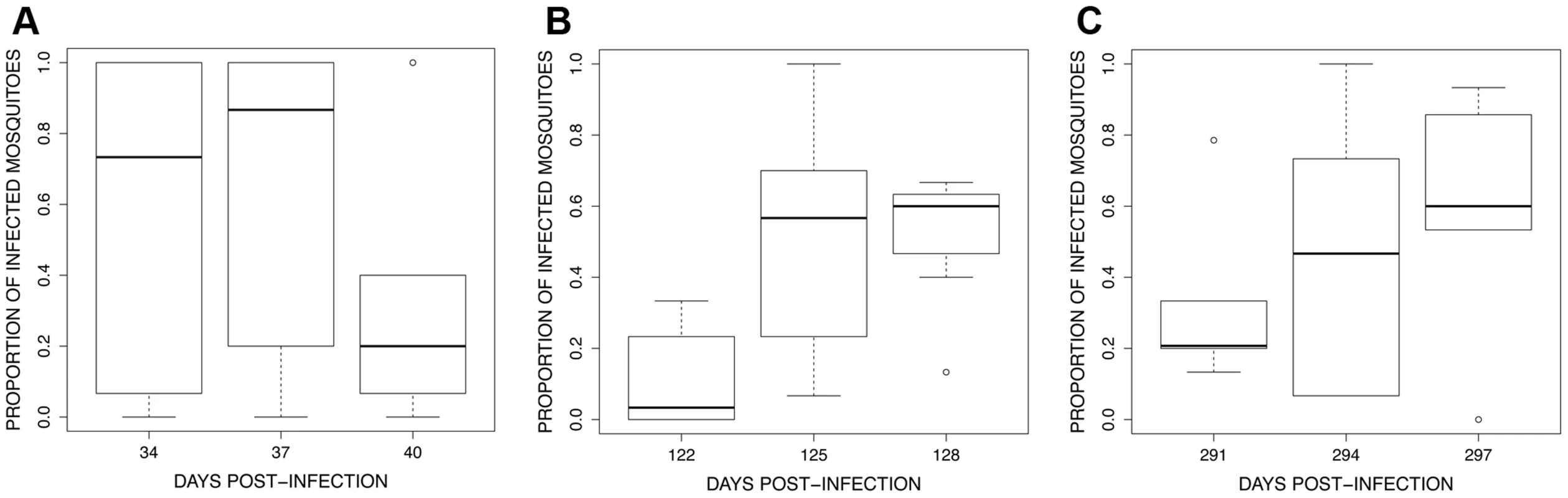
The figure shows the median proportion of infected mosquitoes (horizontal black bars). The white boxes below and above the median indicate the first and third quartiles respectively. Dashed lines delimit 1.5 times the inter-quartile range on both side of the box, above which individual counts are considered outliers and marked as dots. The analysis of oocyst burden only included mosquitoes having one or more oocysts in the midgut. Oocyst burden showed a consistent pattern across the three exposure sessions: the number of oocysts increased significantly between the first and second mosquito batches but decreased thereafter (batch time effect: χ22 = 1147, P<0.0001, χ22 = 546.17, P<0.0001 and χ22 = 389.84, P<0.0001 for the first, second and third exposure sessions respectively; all contrast analyses were significant; Fig. 8).
Fig. 8. Boxplot of the number of oocysts per midgut among 15 haphazardly chosen blood fed individuals on each bird (only includes mosquitoes harbouring ≥1 oocysts) for the 3 exposure sessions (see <em class="ref">Fig. 4</em>): (A) session 1 (34–40 dpi), (B) session 2 (122–128 dpi) and (C) session 3 (291–297 dpi). 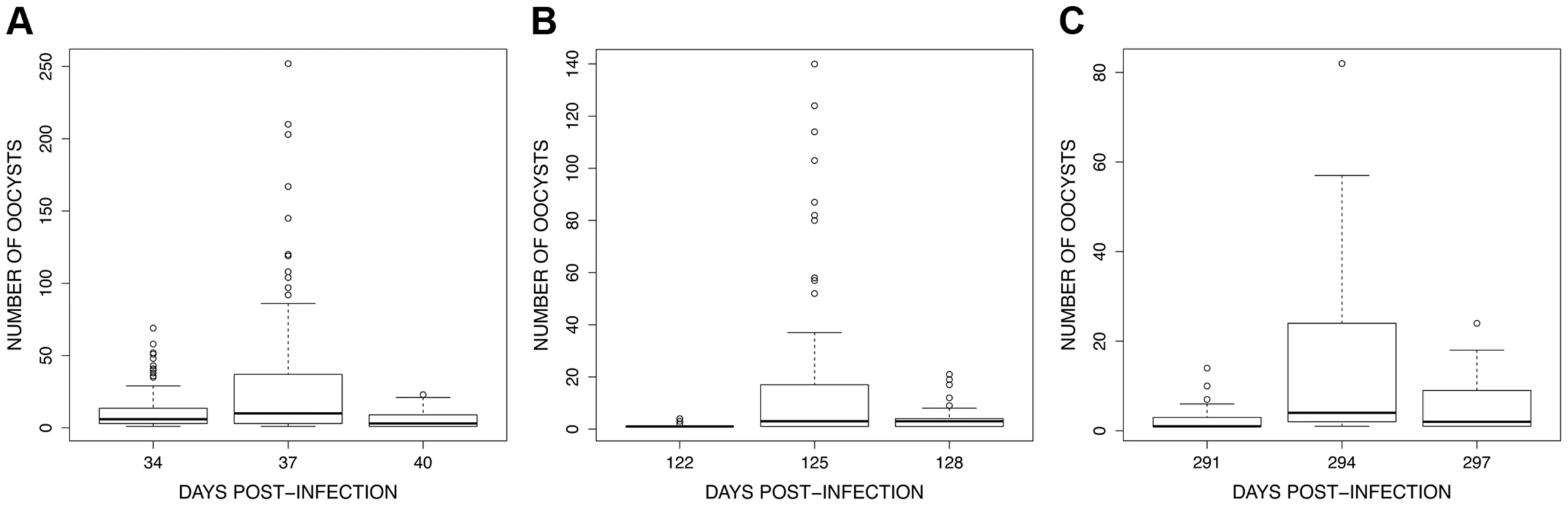
To verify that the control birds were still infective to mosquitoes, they were exposed to mosquitoes on day 307 pi. Only three birds survived to this point and all three were infective to mosquitoes (Table S2).The infection rate of mosquitoes biting the control birds was compared to the infection rate of the last batch of mosquitoes biting the exposed birds (297 dpi). As expected from the above results, the infection rate of mosquitoes biting a bird for the first time was significantly lower than the infection rate of mosquitoes biting a bird which has been recently bitten by two batches of mosquitoes in previous days (infection rate of mosquitoes biting the control birds: 0.36±0.12, exposed birds 0.58±0.10; χ21 = 5.76, P = 0.0164).
Discussion
Plasticity has evolved as an adaptation to the variability of the environment in many organisms [25], [47], including pathogens [27], [28], [48], [49]. Here we contend that the evolution of fixed or plastic dormancy strategies in Plasmodium may be an adaptation to the seasonal fluctuations of vector densities. We explore this hypothesis with a theoretical model and test experimentally some of our predictions in avian malaria.
Theory
How do malaria parasites adapt to the density fluctuations of their insect vectors? To answer this question we started by studying the evolution of transmission strategies using a classical epidemiological model for a vector-borne pathogen. This theoretical approach helps clarify the multiple effects of temporal fluctuations of vector populations. We first considered the evolution of a fixed allocation to virulence and transmission. Our analysis shows that the effect of the temporal variation is driven by its effect on the average density of susceptible hosts and vectors over one period of the fluctuation. In particular we show that in more seasonal environments (e.g. higher latitudes), where the vectors can pullulate only for a few months, lower levels of virulence and transmission should be selected. This is because, in our model, seasonality reduces the average number of vectors. In the absence of the vector, investing in transmission becomes maladaptive because within-host reproduction is associated with higher virulence and host death. This result is very similar to the effect of periodic host absence on the evolution of phytopathogens when there is a trade-off between pathogen transmission and pathogen survival [50]. In addition, our predictions agree with recent models studying the effect of seasonality on virulence evolution [51], in that if the fluctuations of vector density do not affect the mean density of susceptible vectors over time, we expect no evolutionary consequences. Interestingly, our prediction on the effect of seasonality (Fig. 3) is consistent with the geographical distribution of relapsing strategies in P. vivax [22]. P. vivax genotypes sampled near the equator (where seasonality is minimal) invest in higher transmission strategies (higher rates of relapse) than P. vivax genotypes sampled in higher latitudes. In other words, in P. vivax malaria latitude is a very good predictor of the rate of relapses (Fig. 9).
Fig. 9. Effect of latitude on the relapsing rate of Plasmodium vivax. The data was obtained from the supplementary Table S1 of Battle et al. [22]. Each dot represents a parasite strain originating from different locations. ![Effect of latitude on the relapsing rate of <i>Plasmodium vivax</i>. The data was obtained from the supplementary <em class="ref">Table S1</em> of Battle <i>et al.</i> <em class="ref">[22]</em>. Each dot represents a parasite strain originating from different locations.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/feafdde0272555c755db40af94cd5a24.png)
The latitude of origin has a significant effect on the observed time to first relapse (R2 = 0.4966, F1,232 = 228.9, P<0.0001). In a second step of the analysis we allowed plastic transmission strategies to evolve. In particular, we assumed that the malaria pathogens have the ability to sense the density of vectors through exposure to mosquito bites. We derived the condition promoting the evolution of such plastic behaviours when investment in this strategy is associated to a direct fitness cost on transmission. Koelle et al. [52] derived a similar result in a model of pathogen adaptation to seasonal fluctuations but without highlighting the force driving adaptive plasticity. Kumo and Sasaki [53] showed that the sensitivity to seasonality in a directly transmitted pathogen is driven by the correlation between the seasonal variation in transmission rate and the density of susceptible hosts. In our model the sensitivity to seasonality is governed by the fluctuation of mosquito density and plasticity. Similarly we show that what selects for plasticity is the covariance between transmission and the availability of hosts (both the vertebrate hosts and the vectors). In other words, plasticity evolves when mosquito bites provide accurate information on the availability of susceptible hosts. Cohen [54] obtained very similar results on the evolution of conditional dormancy strategies in randomly varying environments. The evolution of conditional dormancy depends on the correlation between the cue and the quality of the environment for individuals leaving the dormant state [54] (see also [55], [56]). In our model the correlation between the cue (mosquito bites) and the abundance of susceptible hosts depends on seasonality: as expected, plasticity evolves more readily when mosquitoes are only around for a short period of time.
Experiment
Have malaria parasites evolved the ability to respond plastically to mosquito bites on its vertebrate host? Previous work on acute rodent malaria infections has produced somewhat contrasting results [39], [40]. These earlier studies had in common that (i) they used an unnatural mosquito-Plasmodium combination, (ii) they were carried out using parasites collected in a high-transmission tropical environment several decades ago and (iii) were carried out when the infection is already at its highest level within the vertebrate host. Here we use a natural mosquito-Plasmodium combination to test the effect of mosquito bites on parasite transmission during the chronic phase of the infection. We used a P. relictum lineage (SGS1) which had been sampled from wild house sparrows in 2009 in a high latitude habitat (Dijon, France) where the environment is characterized by marked seasonal patterns, including variations in mosquito prevalence [57]. In addition, rather than inferring parasite transmissibility solely from the host's parasitaemia, we also quantified the number of parasites that made it all the way to the gut (oocyst) stages of the biting mosquitoes. Our experiment confirmed our two main predictions. First, P. relictum SGS1 reacts to mosquito bites by increasing its overall parasitaemia in the blood. As expected, this effect was not present during the acute infection (first exposure session) because transmission is always high at this stage, but became apparent during the chronic stage of the infection (second and third exposure sessions). Second, this increased parasitaemia resulted in higher probability of infection to mosquitoes and thus in higher transmission rates. The results were consistent at the chronic stage of the infection (exposure sessions 2 and 3): there was a significant increase in mosquito infection rate after exposure to mosquito bites.
Blood stage malaria infections comprise both asexual (replicating) and sexual (transmissible) stages. However, the molecular tools used to quantify overall parasitaemia in this study did not allow us to distinguish between these two parasite life stages. In other malaria systems the conversion rate between the asexual and the sexual (gametocyte) stages, and the resulting sex ratio of the gametocytes may be highly plastic [30], [58], so that overall parasitaemia may not necessarily be a good predictor of gametocyte density and/or transmission. Although nothing is yet known about the conversion rates or sex allocation strategies in P. relictum, in our experiment the increase in parasitaemia was accompanied by a significant increase in the number of infected mosquitoes, suggesting a concomitant increase in gametocyte density in birds exposed to mosquito bites. However, to directly test this hypothesis, we compared the gametocytaemia of exposed and unexposed birds by counting the visible gametocytes in the thick blood smears taken after the exposure (see Text S2). Contrary to expectations, however, we found no clear and consistent evidence that mosquito bites result in higher gametocytaemia. One potential explanation of this lack of consistency is an error in our estimate of gametocytaemia. The application of molecular techniques for the quantification of gametocytes has indeed called into question the use of microscopic methods to quantify Plasmodium gametocytes [59]. In particular, these studies have shown that submicroscopic gametocyte densities are common [60], [61] and can readily infect mosquitoes [62]. Unfortunately these molecular tools are currently only available for P. falciparum and P. chabaudi, and no equivalent tools exist to estimate gametocytaemia in P. relictum.
A potential caveat of these results is that all the mosquitoes used in the same exposure session emerged roughly at the same date. As a consequence, females from the second and third mosquito batches were 3 and 6 days older (respectively) than mosquitoes used in the first batch. To control for a potential confounding effect of female age on transmission we therefore carried out another experiment using females of identical age (7 days old) at each exposure session. This experiment was carried out using laboratory (SLAB strain) mosquitoes and although the oocyst infection intensities were overall lower, we obtained qualitatively similar effects as in the main experiment (see Text S3). In addition, earlier studies have found either that age has little effect on mosquito vector competence [63] or that older mosquitoes have a lower prevalence and intensity of infection than their younger counterparts [64]. Hence, the increase in oocyst prevalence and intensity observed in consecutive exposure sessions in our main experiment cannot be explained by differences in the age of the mosquitoes used.
Mechanisms
The proximal mechanism governing this form of plasticity remains to be investigated. How do parasites in the blood or in tissues perceive mosquito bites? A plethora of substances and molecules present in the salivary fluid are injected when mosquitoes probe and feed [65]. The primary role of these molecules is to combat host homeostasis and to regulate inflammation at the biting site to facilitate blood uptake. Vector salivary lysates have been shown to stimulate within-host growth of Leishmania parasites [66] and may also trigger plastic life-history strategies in Plasmodium. In addition, host anaemia, erythropoeisis, and asexual density have all been shown to be associated with the onset of gametocytogenesis in rodent malaria [67]–[70]. Shutler et al. [40] suggested that blood feeding mosquitoes may cause host anaemia thereby triggering gametocytogenesis in P. chabaudi. Our data, however, do not support this hypothesis, because birds exposed to mosquitoes had similar or even higher haematocrit than control birds (see Text S3). In addition, previous findings obtained using P. gallinaceum have shown that in this avian malaria parasite, parasitaemia and gametocytaemia are not affected by host anaemia [71]. The study of the mechanisms governing plastic transmission strategies in avian malaria is hampered by the lack of available molecular tools to quantify and sex gametocytes (e.g. [72] for rodent malaria). Mechanistic studies can reveal fascinating pathogen strategies. For instance, a recent study on the Cauliflower mosaic virus (CaMV) has shown that when aphids feed on the infected plants the virus reacts instantly (and reversibly) to maximize its transmission. For this purpose it modifies the distribution of a specialised set of proteins which are essential for virus transmission [73]. In the absence of the vector, these proteins, which are toxic for the plant, are neatly packed away inside specialised structures called “transmission bodies”. This study not only represents an excellent example of the ability of some vector-borne pathogens to adopt plastic transmission strategies but it also demonstrates the sophisticated molecular and cellular mechanisms that may be involved.
Perspectives
Theory
The theoretical framework we developed could be used to consider other forms of fluctuations of the environment. For instance, at a short time scale, most malaria parasites show a periodicity in replication burst and allocation to parasite transmissible stages. The synchronicity between this periodicity and the fluctuation of vector density is very important for transmission success [74] and Hawking postulated that the periodicity of malaria may have evolved as a way to maximize the availability of mature gametocytes when mosquitoes feed [75]. Although this hypothesis remains controversial [76], [77] our approach could help identify the conditions that may promote the evolution of cell cycle coordination in malaria as a response to daily fluctuations of vector availability.
Our theoretical framework could be readily extended to consider the evolution of other cues triggering higher transmission rates. In the field, co-infections of different Plasmodium strains or species are the norm rather than the exception [78], [79]. The relapsing strategy of malaria may depend on the presence of other competing pathogens within the host [80]. It would be particularly interesting to investigate the effect of coinfections on the activation of relapses [81], [82].
Experimental
Our model predicts that seasonality could have a huge impact on the evolution of pathogens (see Fig. 4). Figure 9 shows data in P. vivax supporting our prediction on the evolution of fixed pathogen strategies. Testing our predictions regarding the effect of seasonality on the evolution of plasticity may, however, be more challenging. The well recorded worldwide distribution of avian malaria (MalAvi Database, [83]), however, provides an unparalleled opportunity to test this hypothesis. We predict that tropical lineages should exhibit lower potential for plasticity and lower response to external stimuli (such as mosquito bites) than temperate lineages.
It is important to investigate further the role of the quantity and the quality of the mosquitoes on the reactivation of dormant parasites. Quantity-wise, the threshold number of mosquitoes required for the reactivation to take place should be established. Billingsley et al. [39] reported an effect of the amount of mosquitoes biting on gametocytaemia of rodent malaria. Quality-wise, it would be interesting to study if plasticity is specific to particular vector species. Indeed, we may expect Plasmodium to respond only to the bites of mosquitoes species that serve as competent vectors of the disease [66]. Alternatively, a general response to the bites of non-vector species may be indicative of an evolutionary constraint (the inability of the parasite to distinguish between vector and non-vector signals), or of a temporal correlation in the abundance of vector and non-vector species in a given area.
Conclusions
We identified the conditions that promote the evolution of plastic transmission strategies in a fluctuating environment. In line with our theoretical predictions, we show that P. relictum has the ability to boost its own transmission during the chronic phase of the vertebrate infection after being exposed to mosquito bites. Whether this ability extends to other Plasmodium species and in particular to human malaria remains to be investigated. In P. vivax the data presented in Figure 9 indicates a strong effect of latitude (i.e. seasonality) on relapses and transmission. The role of plastic transmission strategies on this pattern is unclear but it deserves further investigation. This may help define better control strategies, with more specific recommendations on both spatial and temporal implementations of targeted interventions against malaria hotspots [84]. The study of plastic transmission strategies may also be relevant to many other pathogens that are known to alternate between acute and dormant phases such as varicella zoster virus [85] Herpes Simplex virus [86], Mycobacterium tuberculosis [87] and HIV [88]. Such dormant parasites pose considerable therapeutic challenges and much would be gained from understanding the cues underlying the switch between dormant and acute stages in these pathogens [89]–[91]. In conclusion, a better understanding of the ecological determinants as well as the evolutionary forces governing parasite relapses is not only of academic interest: it is also urgently needed to improve the efficacy of public health strategies.
Materials and Methods
Ethics statement
Animal experiments were carried out in strict accordance with the National Charter on the Ethics of Animal Experimentation of the French Government, and all efforts were made to minimise suffering. Experiments were approved by the Ethical Committee for Animal Experimentation established by the authors' institution (CNRS) under the auspices of the French Ministry of Education and Research (permit number CEEA - LR-1051).
Malaria parasites and mosquitoes
Plasmodium relictum (lineage SGS1) is a generalist parasite and the most prevalent form of avian malaria in Europe, infecting over 30 birds species in the order Passeriformes [44], [83]. Our strain was originally isolated from wild house sparrows caught in the region of Dijon (France) and maintained in the laboratory via passages to naïve canaries either by intraperitoneal injection or through the bite of infected Culex pipiens mosquitoes.
Experiments were conducted with wild Cx. pipiens pipiens mosquitoes. Cx. pipiens is the natural vector of P. relictum in the wild [44], [92], [93]. Thousands of L3 and L4 larvae were collected from a single sewage treatment lagoon in the village of Triadou (20 km north Montpellier, France) using a hand net and reared till adulthood under standard laboratory conditions [94]. We used females 7, 10 and 13 days after emergence that had had no prior access to blood, had been maintained on glucose solution (10%) since their emergence, and had been starved (but provided with water) for 6 h before the experiment.
Experimental design
Experiments were carried out using (1-year old) domestic canaries (Serinus canaria). Prior to the experiments, a small amount (ca. 15–25 µL) of blood was collected from the brachial vein of each of the birds and used for molecular sexing [95], as well as to verify that they were free from any previous haemosporidian infections [96]. Twenty birds were experimentally inoculated on the 3rd July 2010 (day 0, see Box 1 and Fig. 1) by means of an intraperitoneal injection of ca. 50–100 µL of an infected blood pool. The blood pool was constituted of a mixture of blood from 8 infected canaries that had been inoculated with the parasite 12 days previously following standard laboratory procedures [97]. Note that unlike what happens in some Plasmodium parasites such as P. vivax, the artificial infection with P. relictum via the inoculation of infected blood containing merozoites does not prevent the formation of exoerythrocytic stages [44], [98]. One bird failed to get infected and the remaining infected birds were assigned to two treatments: “exposed” (n = 10) or “unexposed” (control, n = 9) to mosquito bites. This assignment was made by balancing the gender of birds and the magnitude in the peak parasitaemia during the acute phase between the two treatments. This experimental design thus allowed mosquitoes to both probe and blood feed on the birds, and in this respect it contrasts with previous designs where only probing was allowed [39], [40].
Exposure to mosquito bites took place in August 2010 (first exposure session), and repeated in November 2010 and April 2011 (second and third exposure sessions). Each of these exposure sessions consisted of 3 “exposure days” separated by 3 day intervals: days 34, 37 and 40 post-infection (dpi) for the first exposure session, days 122, 125 and 128 dpi for the second exposure session, and days 291, 294 and 297 dpi for the third exposure session (Box 1 and Fig. 1A). In the morning of each exposure day, a small (ca. 15–25 µL) amount of blood was taken from the brachial vein of all (“exposed” and “control”) birds to quantify parasitaemia (see below). In the evening, birds allocated to the “exposed” treatment were placed inside a cage (dimensions L40×W30×H30 cm) with a batch of 50 uninfected female mosquitoes for 2 hours (8–10pm). Around 30 females blood fed on the birds during this time (see Table S3) which is close to available estimations in the wild [99]. Tables S2 and S3 provide the full details of the number of replications (number of blood fed mosquitoes, number of mosquitoes dissected) for each exposure session. To minimize host defensive behaviours that may alter the mosquito biting process during the assay, we immobilized birds in a specially designed PVC tube that rendered their legs accessible to the mosquitoes while protecting the rest of the body from the bites [97]. “Control” birds were placed in identical conditions but without mosquitoes. Immediately after each exposure, blood-fed mosquitoes from each cage (n = 10) and time point (3 exposure sessions, 3 days per session) were collected, isolated in a new cage, and maintained under standard laboratory conditions for 7 days. Fifteen haphazardly chosen mosquitoes were dissected to check for the presence (prevalence) and number (intensity) of oocysts in the midgut [94].
In each exposure session, two further blood samples were taken from all experimental birds, 3–4 days and 7–8 days after the last exposure day (days 44 and 48 dpi, days 131 and 135 dpi and days 300 and 304 dpi, for the first to third exposure sessions, Fig. 1B). For each exposure session we therefore obtained 5 different blood samples (red arrows in Fig. 1A and 1B). These blood samples were used to quantify total parasitaemia using previously published qPCR procedures [97] and gametocytaemia by microscopic examination (see Text S2). In addition, blood samples were taken at regular intervals throughout the experiment to monitor parasitaemia before and between the exposure sessions (blue arrows in Fig. 1A).
Statistical analyses
The statistical analyses were run using the R software (v. 2.14.0). Analyses were carried out separately for each exposure session. Variation in parasitaemia (log-transformed (RQ+1)) was analyzed using linear mixed-effect models (lme function, nlme package) with bird as a random effect to account for the repeated sampling of individual hosts. A generalized linear mixed-effect models GLMM (glmer function, lme4 package, binomial distribution) was carried out to study variation in gametocytaemia (proportion of gametocytes). Bird and time were included as random and fixed factors, respectively.
Variation in the infection prevalence (proportion of individuals harbouring at least 1 oocyst) and the oocystaemia (number of oocysts, only for infected mosquitoes) was analysed using GLMMs (glmer function, lme4 package, with binomial and Poisson distributions, respectively). Bird and time (i.e. time between the 5 different blood samples, see red arrows in Fig. 1b) were included as random and fixed factors, respectively. Here, time was considered as a factorial explanatory variable.
When appropriate, a posteriori contrasts were carried out by aggregating factor levels that did not significantly differ from each other and by testing the fit of the simplified model [100]. The significance of explanatory variables was established by comparing the change in deviance with and without the term to a χ2 distribution. Degrees of freedom correspond to the difference in the number of terms in the model.
Supporting Information
Zdroje
1. MeyersLA, BullJJ (2002) Fighting change with change: adaptive variation in an uncertain world. Trends Ecol Evol 17 : 551–557.
2. MasseyRC, BucklingA, PeacockSJ (2001) Phenotypic switching of antibiotic resistance circumvents permanent costs in Staphylococcus aureus. Curr Biol 11 : 1810–1814.
3. BalabanNQ, MerrinJ, ChaitR, KowalikL, LeiblerS (2004) Bacterial persistence as a phenotypic switch. Science 305 : 1622–1625.
4. StewartFM, LevinBR (1984) The population biology of bacterial viruses: why be temperate. Theor Pop Biol 26 : 93–117.
5. StumpfMPH, LaidlawZ, JansenVAA (2002) Herpes viruses hedge their bets. Proc Natl Acad Sci USA 99 : 15234–15237.
6. TrapeJF, Lefebvre-ZanteE, LegrosF, NdiayeG, BouganaliH, et al. (1992) Vector density gradients and the epidemiology of urban malaria in Dakar, Senegal. Am J Trop Med Hyg 47 : 181–189.
7. RogersDJ, RandolphSE, SnowRW, HaySI (2002) Satellite imagery in the study and forecast of malaria. Nature 415 : 710–715.
8. MbogoCM, MwangangiJM, NzovuJ, GuW, YanG, et al. (2003) Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg 68 : 734–742.
9. OesterholtMJAM, BousemaJT, MwerindeOK, HarrisC, LushinoP, et al. (2006) Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malaria J 5 : 98.
10. PaulREL, DialloM, BreyPT (2004) Mosquitoes and transmission of malaria parasites - not just vectors. Malaria J 3: e39.
11. PonçonN, TotyC, L'AMBERTG, Le GoffG, BrenguesC, et al. (2007) Population dynamics of pest mosquitoes and potential malaria and West Nile virus vectors in relation to climatic factors and human activities in the Camargue, France. Med Vet Entomol 21 : 350–357.
12. WhiteNJ (2011) Determinants of relapse periodicity in Plasmodium vivax malaria. Malaria J 10 : 297.
13. McLeanSA, PersonCD, PhillipsRS (1982) Plasmodium chabaudi: relationship between the occurence of recrudescenct parasitaemias in mice and the effective levels of acquired immunity. Exp Parasitol 54 : 213–221.
14. HuffCG, BloomW (1935) A malarial parasite infecting all blood and blood-forming cells of birds. J Infect Dis 57 : 315–336.
15. JamesSP, TateP (1937) New knowledge of the life-cycle of malaria parasites. Nature 139 : 545.
16. ShorttHE, GarnhamPCC, CovellG, ShutePG (1948) Pre-erythrocytic stage of human malaria, Plasmodium vivax. Brit Med J 1 : 547.
17. CogswellFB (1992) The hypnozoite and relapse in primate malaria. Clin Microbiol Rev 5 : 26–35.
18. ThompsonPE, HuffCG (1944) A saurian malarial parasite, Plasmodium mexicanum, N. Sp., with both elongatum and gallinaceum-types of exoerythrocytic stages. J Infect Dis 74 : 48–67.
19. TelfordSRJr (1989) Discovery of the pre-erythrocytic stages of a saurian malaria parasite, hypnozoites, and a possible mechanism for the maintenance of chronic infections throughout the life of the host. Int J Parasitol 19 : 597–616.
20. Thayer WLotmf, p. 326. (1897) Lectures on the malarial fevers. New York: D. Appleton & Co.
21. CoatneyGR (1976) Relapse in malaria: an enigma. J Parasitol 62 : 2–9.
22. BattleKE, KarhunenMS, BhattS, GethingPW, HowesRE, et al. (2014) Geographical variation in Plasmodium vivax relapse. Malaria J 13 : 144.
23. AllanRA, MahrtJL (1989) Influence of transmission period on primary and relapse patterns of infection of Leucocytozoon spp. and Haemoproteus mansoni. Am Midl Nat 121 : 341–349.
24. PigliucciM (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20 : 481–486.
25. ScheinerSM (1993) Genetics and evolution of phenotypic plasticity. Annu Rev Ecol Syst 24 : 35–68.
26. ReeceSE, RamiroRS, NusseyDH (2009) Plastic parasites: sophisticated strategies for survival and reproduction? Evol Appl 2 : 11–23.
27. BabayanSA, ReadAF, LawrenceRA, BainO, AllenJE (2010) Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy. PLoS Biol 8: e1000525.
28. LeggettHC, BenmayorR, HodgsonDJ, BucklingA (2013) Experimental Evolution of Adaptive Phenotypic Plasticity in a Parasite. Curr Biol 23 : 139–142.
29. ReeceSE, AliE, SchneiderP, BabikerHA (2010) Stress, drugs and the evolution of reproductive restraint in malaria parasites. Proc R Soc B Biol Sci 277 : 3123–3129.
30. PollittLC, MideoN, DrewDR, SchneiderP, ColegraveN, et al. (2011) Competition and the evolution of reproductive restraint in malaria parasites. Am Nat 177 : 358–367.
31. HuldenL, HuldenL (2011) Activation of the hypnozoite: a part of Plasmodium vivax life cycle and survival. Malaria J 10 : 90.
32. ManwellRD (1929) Relapse in bird malaria. Am J Epidemiol 9 : 308–345.
33. AppelgateJE (1970) Population changes in latent avian malaria infections associated with season and corticosterone treatment. J Parasitol 56 : 439–443.
34. AppelgateJE, BeaudoinRL (1970) Mechanisms of spring relapse in avian malaria: effects of gonodropin and corticosterone. J Wildlife Dis 6 : 443–447.
35. PearsonRD (2002) Is prolactin responsible for avian, saurian, and mammalian relapse and periodicity of fever in malarial infections? Can J Zool 80 : 1313–1315.
36. ValkiunasG, BairleinF, IezhovaTA, DolnikOV (2004) Factors affecting the relapse of Haemoproteus belopolskyi infections and the parasitaemia of Trypanosoma spp. in a naturally infected European songbird, the blackcap, Sylvia atricapilla. Parasitol Res 93 : 218–222.
37. HuldenL, HuldenL, HeliovaaraK (2008) Natural relapses in vivax malaria induced by Anopheles mosquitoes. Malaria J 7 : 64.
38. LawalyR, KonateL, MarramaL, DiaI, DialloD, et al. (2012) Impact of mosquito bites on asexual parasite density and gametocyte prevalence in asymptomatic chronic Plasmodium falciparum infections and correlation with IgE and IgG titers. Infect Immun 80 : 2240–2246.
39. BillingsleyPF, SnookLS, JohnstonVJ (2005) Malaria parasite growth is stimulated by mosquito probing. Biol Lett 1 : 185–189.
40. ShutlerD, ReeceSE, MullieA, BillingsleyPF, ReadAF (2005) Rodent malaria parasites Plasmodium chabaudi and P. vinckei do not increase their rates of gametocytogenesis in response to mosquito probing. Proc R Soc B Biol Sci 272 : 2397–2402.
41. Killick-Kendrick R (1978) Taxonomy, zoology and evolution. In: Killick-Kendrick R, Peters W, editors. Rodent Malaria. London: Academic Press. pp. 1–52.
42. LandauI, ChabaudA (1994) Plasmodium species infecting Thamnomys rutilans: a zoological study. Adv Parasitol 33 : 50–90.
43. Roca-FeltrerA, SchellenbergJR, SmithL, CarneiroI (2009) A simple method for defining malaria seasonality. Malaria J 8 : 276.
44. Valkiūnas G (2005) Avian Malaria Parasites and Other Haemosporidia. Boca Raton, FL., USA: CRC Press.
45. FrankSA (1996) Models of parasite virulence. Q Rev Biol 71 : 37–78.
46. AlizonSA, HurfordA, MideoN, van BaalenM (2009) Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 22 : 245–259.
47. BeldadeP, MateusARA, KellerRA (2011) Evolution and molecular mechanisms of adaptive developmental plasticity. Mol Ecol 20 : 1347–1363.
48. DuneauD, EbertD (2012) Host sexual dimorphism and parasite adaptation. PLoS Biol 10: e1001271.
49. MideoN, ReeceSE (2012) Plasticity in parasite phenotypes: evolutionary and ecological implications for disease. Future Microbiol 7 : 17–24.
50. Van Den BergF, BacaërN, MetzJAJ, LannouC, Van Den BoschF (2011) Periodic host absence can select for higher or lower parasite transmission rates. Evol Ecol 25 : 121–137.
51. DonnellyR, BestA, WhiteA, BootsM (2013) Seasonality selects for more acutely virulent parasites when virulence is density dependent. Proceedings of the Royal Society B: Biological Sciences, 280(1751). Proc R Soc B Biol Sci 280 : 20122464.
52. KoelleK, PascualM, YunusM (2005) Pathogen adaptation to seasonal forcing and climate change. Proc R Soc B Biol Sci 272 : 971–977.
53. KamoM, SasakiA (2005) Evolution toward multi-year periodicity in epidemics. Ecol Lett 8 : 378–385.
54. CohenD (1967) Optimizing reproduction in a randomly varying environment when a correlation may exist between the conditions at the time a choice has to be made and the subsequent outcome. J Theor Biol 16 : 1–14.
55. GavriletsS, ScheinerSM (1993) The genetics of phenotypic plasticity. 5. Evolution of reaction norm shape. J Evol Biol 6 : 31–48.
56. LandeR (2009) Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J Evol Biol 22 : 1435–1446.
57. Cellier-HolzemE, Esparza-SalasR, GarnierS, SorciG (2010) Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int J Parasitol 40 : 1447–1453.
58. ReeceSE, DrewDR, GardnerA (2008) Sex ratio adjustment and kin discrimination in malaria parasites. Nature 453 : 609–614.
59. BabikerHA, SchneiderP, ReeceSE (2008) Gametocytes: insights gained during a decade of molecular monitoring. Trends Parasitol 24 : 525–530.
60. Abdel-WahabA, Abdel-MuhsinAMA, AliE, SuleimanS, AhmedS, et al. (2002) Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. J Infect Dis 185 : 1838–1842.
61. SchneiderP, SchooneG, SchalligH, VerhageD, TelgtD, et al. (2004) Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol 137 : 35–41.
62. SchneiderP, BousemaJT, GouagnaLC, OtienoS, Van de Vegte-BolmerM, et al. (2007) Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg 76 : 470–474.
63. OkechBA, GouagnaLC, KabiruEW, BeierJC, YanGY, et al. (2004) Influence of age and previous diet of Anopheles gambiae on the infectivity of natural Plasmodium falciparum gametocytes from human volunteers. J Insect Sci 4 : 33.
64. TerzianLA, StahlerN, IrreverreF (1956) The effects of aging, and the modifications of these effects, on the immunity of mosquitoes to malarial infection. J Immunol 76 : 308–313.
65. FontaineA, DioufI, BakkaliN, MisseD, PagesF, et al. (2011) Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasites Vectors 4 : 187.
66. TitusRG, RibeiroJM (1988) Salivary gland lysates from the sand fly Lutzomyialongipalpis enhance Leishmania infectivity. Science 239 : 1306–1308.
67. CameronA, ReeceSE, DrewDR, HaydonDT, YatesAJ (2013) Plasticity in transmission strategies of the malaria parasite, Plasmodium chabaudi: environmental and genetic effects. Evol Appl 6 : 365–376.
68. CarterLM, KafsackBFC, LlinasM, MideoN, PollittLC, et al. (2013) Stress and sex in malaria parasites: Why does commitment vary? Evol Med Publ Heath 2013 : 135–147.
69. GautretP, CoquelinF, ChabaudAG, LandauI (1997) The production of gametocytes by rodent Plasmodium species in mice during phenylhydrazine induced reticulocytosis. Acta Parasitol 42 : 65–67.
70. ReeceSE, DuncanAB, WestSA, ReadAF (2005) Host cell preference and variable transmission strategies in malaria parasites. Proc R Soc B Biol Sci 272 : 511–517.
71. PaulREL, CoulsonTN, RaibaudA, BreyPT (2000) Sex determination in malaria parasites. Science 287 : 128–131.
72. DrewDR, ReeceSE (2007) Development of reverse-transcription PCR techniques to analyse the density and sex ratio of gametocytes in genetically diverse Plasmodium chabaudi infections. Mol Biochem Parasitol 156 : 199–209.
73. MartiniereA, BakA, MaciaJL, LautredouN, GarganiD, et al. (2013) A virus responds instantly to the presence of the vector on the host and forms transmission morphs. eLife 2: e00183.
74. O'DonnellAJ, SchneiderP, McWattersHG, ReeceSE (2011) Fitness costs of disrupting circadian rhythms in malaria parasites. Proc R Soc B Biol Sci 278 : 2429–2436.
75. HawkingF, WormsMJ, GammageK, GoddardPA (1966) The biological purpose of the blood-cycle of the malaria parasite Plasmodium cynomolgi. Lancet 288 : 422–424.
76. MideoN, ReeceSE, SmithAL, MetcalfCJE (2013) The Cinderella syndrome: why do malaria-infected cells burst at midnight? Trends Parasitol 29 : 10–16.
77. GreischarMA, ReadAF, BjørnstadON (2014) Synchrony in malaria infections: How intensifying within-host competition can be adaptive. Am Nat 183: E36–E49.
78. MayxayM, PukrittayakameeS, NewtonPN, WhiteNJ (2004) Mixed-species malaria infections in humans. Trends Parasitol 20 : 233–240.
79. ValkiūnasG, BenschS, IezhovaTA, KrižanauskienėA, HellgrenO, et al. (2006) Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92 : 418–422.
80. ShanksGD, WhiteNJ (2013) The activation of vivax malaria hypnozoites by infectious diseases. Lancet Infect Dis 13 : 900–906.
81. ChenNH, AuliffA, RieckmannK, GattonM, ChengQ (2007) Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis 195 : 934–941.
82. ImwongM, SnounouG, PukrittayakameeS, TanomsingN, KimJR, et al. (2007) Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis 195 : 927–933.
83. BenschS, HellgrenO, Perez-TrisJ (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9 : 1353–1358.
84. BousemaT, GriffinJT, SauerweinRW, SmithDL, ChurcherTS, et al. (2012) Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med 9: e1001165.
85. GildenD, MahalingamR, NagelMA, PugazhenthiS, CohrsRJ (2011) Review: the neurobiology of varicella zoster virus infection. Neuropath Appl Neurobiol 37 : 441–463.
86. WilsonAC, MohrI (2012) A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 20 : 604–611.
87. CardonaPJ, Ruiz-ManzanoJ (2004) On the nature of Mycobacterium tuberculosis-latent bacilli. Eur Respir J 24 : 1044–1051.
88. MarcelloA (2006) Latency: the hidden HIV-1 challenge. Retrovirology 3 : 7.
89. PerngGC, JonesC (2010) Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. Interdisc Persp Infect Dis vol 2010 : 262415.
90. LiebermanPM (2013) Keeping it quiet: chromatin control of gammaherpesvirus latency. Nature Rev Microbiol 11 : 863–875.
91. SpinaCA, AndersonJ, ArchinNM, BosqueA, ChanJ, et al. (2013) An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 9: e1003834.
92. KimuraM, DarbroJM, HarringtonLC (2010) Avian malaria parasites share congeneric mosquito vectors. J Parasitol 96 : 144–151.
93. LalubinF, DelédevantA, GlaizotO, ChristeP (2013) Temporal changes in mosquito abundance (Culex pipiens), avian malaria prevalence and lineage composition. Parasites Vectors 6 : 307.
94. VézilierJ, NicotA, GandonS, RiveroA (2010) Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malaria J 9 : 379.
95. GriffithsR, DoubleMC, OrrK, DawsonRJG (1998) A DNA test to sex most birds. Mol Ecol 7 : 1071–1075.
96. WaldenströmJ, BenschS, HasselquistD, OstmanO (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90 : 191–194.
97. CornetS, NicotA, RiveroA, GandonS (2013) Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett 16 : 323–329.
98. HuffCG (1947) Life cycle of malarial parasites. Annual Reviews in Microbiology 1 : 43–60.
99. GriffingSM, KilpatrickAM, ClarkL, MarraPP (2007) Mosquito landing rates on nesting American robins (Turdus migratorius). Vector-Borne Zoo Dis 7 : 437–443.
100. Crawley MJ (2007) The R Book. England: John Wiley & Sons, Ltd.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání








