-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaComparative Phenotypic Analysis of the Major Fungal Pathogens and
Candida species are among the most common causes of fungal infection worldwide. Infections can be both community-based and hospital-acquired, and are particularly associated with immunocompromised individuals. Candida albicans is the most commonly isolated species and is the best studied. However, other species are becoming of increasing concern. Candida parapsilosis causes outbreaks of infection in neonatal wards, and is one of the few Candida species that is transferred from the hands of healthcare workers. C. parapsilosis, like C. albicans, grows as biofilms (cell communities) on the surfaces of indwelling medical devices like feeding tubes. We describe here the construction of a set of tools that allow us to characterize the virulence properties of C. parapsilosis, and in particular its ability to grow as biofilms. We find that some of the regulatory mechanisms are shared with C. albicans, but others are unique to each species. Our tools, based on selectively deleting regulatory genes, will provide a major resource to the fungal research community.
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004365
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004365Summary
Candida species are among the most common causes of fungal infection worldwide. Infections can be both community-based and hospital-acquired, and are particularly associated with immunocompromised individuals. Candida albicans is the most commonly isolated species and is the best studied. However, other species are becoming of increasing concern. Candida parapsilosis causes outbreaks of infection in neonatal wards, and is one of the few Candida species that is transferred from the hands of healthcare workers. C. parapsilosis, like C. albicans, grows as biofilms (cell communities) on the surfaces of indwelling medical devices like feeding tubes. We describe here the construction of a set of tools that allow us to characterize the virulence properties of C. parapsilosis, and in particular its ability to grow as biofilms. We find that some of the regulatory mechanisms are shared with C. albicans, but others are unique to each species. Our tools, based on selectively deleting regulatory genes, will provide a major resource to the fungal research community.
Introduction
More than 300 Candida species have been described to date [1]. Although all Candida species are Ascomycetes (belonging to the Saccharomycetales), they are paraphyletic, and do not share a recent common ancestor [2]. As a result, they have few shared characteristics. The term “Candida” suggests that they are asexual species, but sexual or cryptic sexual cycles are increasingly being identified [3], [4], [5], [6]. Most well studied Candida species belong to the monophyletic CTG clade, where the codon CTG is translated as serine rather than leucine [2], [7]. These include the major human fungal pathogens Candida albicans, Candida parapsilosis and Candida tropicalis [8], [9]. Whereas C. albicans is still the most common cause of candidiasis, C. parapsilosis and the non-CTG clade species Candida glabrata are increasing in frequency [8], [9], [10]. Properties of C. albicans associated with the ability to cause disease have been well characterized, and include growth in yeast and hyphal forms, epigenetic switching from white to opaque cells, secretion of hydrolases, and adhesion and biofilm development (reviewed in [11]). While some of these properties are likely to be shared with other CTG-clade species, many are species or lineage specific. For example, only C. albicans and its close relative Candida dubliniensis can grow in truly hyphal forms, and white-opaque switching and the associated parasexual cycle have only been described in C. albicans, C. dubliniensis and C. tropicalis [12], [13], [14]. C. parapsilosis, a major cause of infection in premature neonates [15], does not appear to have a sexual cycle [16], [17] and does not undergo white-opaque switching [18]. Unlike other Candida species, C. parapsilosis is often isolated from the hands of health care workers and has been responsible for causing outbreaks of infection [19], [20], [21], [22], [23], [24]. C. parapsilosis is responsible for approximately 20% of Candida infections particularly in infants less than 1 year old [25], [26].
One of the major factors of Candida species associated with pathogenicity is their ability to grow as biofilms on implanted medical devices [27]. Biofilms are composed of communities of microorganisms associated with a surface and embedded in an extracellular matrix, and are believed to be the major growth form of microorganisms in nature [28]. Biofilms are extremely refractory to antimicrobial therapy and treatment usually involves removal of the infected device. Biofilm formation in C. albicans has been well characterized and occurs in several stages (reviewed in [29], [30]). The first step involves yeast cells adhering to a substrate surface. This is followed by a period of cellular growth, or biofilm initiation. During the maturation stage, hyphae are produced and cells become encased in an extracellular matrix (ECM). The final stage is dispersal, when yeast cells break away from the biofilm structure and disseminate around the body to seed new sites of infection [31], [32].
Although many Candida species form biofilms, the structures are highly variable [28], [33]. C. albicans biofilms consist of a compact basal layer of yeast cells and a thicker less compact hyphal layer all surrounded by an ECM composed mainly of carbohydrate [34]. In contrast C. parapsilosis does not make true hyphae, and biofilms are composed of yeast and pseudohyphal cells only [27], [35], [36]. The ability of C. parapsilosis to produce biofilm is also highly strain dependent [28], [33].
Many of the key regulators of biofilm formation in C. albicans have been identified (reviewed in [37]). Hyphal formation is a pivotal step, and mutants blocked in filamentation are often impaired in biofilm development [38]. Nobile et al [39] identified a network of six transcription factors (BCR1, EFG1, TEC1, ROB1, NDT80 and BRG1) that play a major role in regulating C. albicans biofilm growth. In addition, Finkel et al [40] identified 30 transcription factors required for adhesion, some of which (such as Bcr1) are also necessary for mature biofilm development. We have previously shown that C. parapsilosis orthologs of BCR1 and EFG1 are required for biofilm formation in this species [18], [35]. However, even though the function of the transcription factors is at least partially conserved, many of the gene targets are different, and some conserved targets of Bcr1 have different functions [41]. For example the CFEM family of cell wall proteins is required for biofilm development in C. albicans, but not in C. parapsilosis [41].
We report here the construction of the first large-scale gene deletion collection in C. parapsilosis, targeting 100 genes representing transcription factors, protein kinases and species-specific genes. We carry out a detailed comparison of C. parapsilosis and C. albicans phenotypes, particularly in relation to biofilm development. We find that overall, the molecular function of orthologous genes is generally conserved between the two species. However, there are also important differences. BRG1 and TEC1, transcription factors required for biofilm development in C. albicans, do not have the same role in C. parapsilosis. CZF1, UME6, CPH2 and GZF3 are regulators of biofilm development in C. parapsilosis only.
Results
Construction of a gene knockout collection in Candida parapsilosis
Most of the available gene disruption collections in C. albicans target transcription factors or protein kinases [42], [43], [44], [45]. We therefore selected similar genes from C. parapsilosis (Figure 1, Tables S1, S2). In total, we chose genes encoding 73 transcription factors, 16 protein kinases, 1 putative RNA-binding protein, 1 putative tRNA-methyl transferase, 6 genes that are apparently unique to C. parapsilosis, and members of the CFEM family of transmembrane proteins [41], [46], [47]. We selectively deleted entire open reading frames rather than generating random insertions, to facilitate downstream analysis. The C. parapsilosis genome is diploid and therefore requires two rounds of gene disruption to create a homozygous mutant. Gene disruptions in C. parapsilosis have previously been carried out using a recyclable SAT1 (nourseothricin resistance) cassette [35], [36]. Although this approach is successful, it is slow and very inefficient. Instead, we adapted a fusion PCR method previously developed for gene deletion in C. albicans, in which each allele is replaced with a heterologous selectable marker [45] (Figure 1A, Figure S1).
Fig. 1. Phenotype screening of C. parapsilosis gene knockout strains. 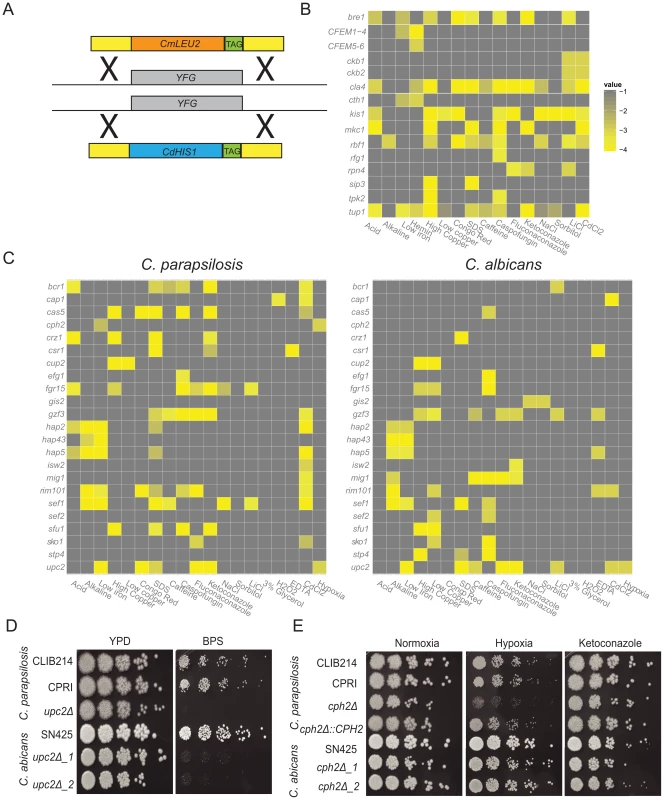
(A) Both alleles of 100 C. parapsilosis genes (YFG) were deleted by replacement with C. maltosa LEU2 and C. dubliniensis HIS1 by homologous recombination. Unique barcode sequences (TAG) were included in each deletion. Two independent replicates of each strain were constructed. (B,C) Each deletion strain was grown overnight in a 96 well plate in YPD media, diluted 1∶100 into fresh YPD and replica plated to test media using a 48-pin replicator. Each plate contained the control strains CLIB214 and CPRI in duplicate. Growth of each deletion strain was scored in comparison to the control strains. Strains that were scored in C. parapsilosis only are shown in (B), and strains with a detectable phenotype in both C. albicans and C. parapsilosis are shown in (C). Only scores <−2.5 are shown, and isolates with no growth defect on the selected media are not included. The full data set is available in Table S2. (D) Deleting UPC2 in both C. parapsilosis and C. albicans results in reduced growth in low iron conditions (YPD, and YPD+0.15 mM BPS). (E) Cells were grown overnight in YPD, diluted in PBS and plated on YPD containing 0.005 µg ml−1 ketoconazole where indicated, and incubated at 30°C in 21% or 1% O2 for 2 days. Deleting CPH2 in C. parapsilosis results in a growth defect under hypoxic (1% oxygen) conditions, but there is no effect on the equivalent deletion of C. albicans. Growth is restored by re-introducing one allele of CpCPH2. There is no effect on ketoconazole sensitivity in either species. Deletions were constructed in the C. parapsilosis type strain, CLIB214. Auxotrophic mutations in LEU2 and HIS1 were first generated using a SAT1 cassette, yielding strain CPL2H1 (see methods) (Figure S1, Table S1) [35]. Candidate genes were then deleted by replacing one allele with HIS1 from C. dubliniensis, and one with LEU2 from C. maltosa (Figure 1A, Figure S1). All mutant strains were confirmed by PCR using primers inside CdHIS1 or CmLEU2 and a primer outside of the integration sites at both the 5′ and 3′ end of the gene. Deletion of the target open reading frame was also confirmed using PCR. A control strain (CPRI) was created by integrating CdHIS1 and CmLEU2 at the site of the original HIS1 alleles (Figure S1). Both CLIB214 and CPRI strains were used as controls for the majority of experiments.
Two independent homozygous deletion mutants were generated for each targeted gene, which increases the probability that an observed phenotype in both strains is a result of the gene deletion, and not from a secondary effect. A unique 20 base pair sequence tag (barcode) was also incorporated into each mutant strain, which will facilitate future competition experiments. In total, 200 barcoded deletion strains were constructed (Table S1).
Phenotypic screen of deletion collection
Growth of the deletion collection was determined in 42 different conditions, designed to identify nutritional, cell wall, osmotic and oxidative stress phenotypes, and response to antifungal drugs (Table S2). Some strains had severe growth defects, and were not included in the phenotype analysis (Table S2). For the remainder, each independent strain was grown in YPD in 96 well plates (two replicates per candidate gene), and then replica-plated using a 48-pin replicator to selective media. YPD was used as the base media except where the effect of different carbon sources was tested. Several drugs and chemicals were tested at a range of concentrations (Table S2). Growth was scored using a simple scoring system (−4 to +1, where 0 is the same as the control strains). The scores were averaged between sister strains and replicate screens and were converted to heatmaps (Figure 1B,C). Of the 73 transcription factor deletions constructed in C. parapsilosis, 64 orthologous deletions were available from a large scale screen in C. albicans [43], and another (UME6) was obtained from Banerjee et al [48]. Growth of the C. albicans strains was monitored under similar conditions as for C. parapsilosis except that lower concentrations of ketoconazole, fluconazole and caspofungin and higher concentrations of CdCl2 were used, and utilization of heme was not tested (Table S2). Nine transcription factor deletions are available in C. parapsilosis only. The phenotypes of these, and of the C. parapsilosis protein kinase deletions, are described in the supporting information (Text S1, Table S2).
Figure 1C shows a comparison of the phenotypic profiles of orthologous deletions in the two species. Thirty-five gene deletions are not shown because they have no, or little, effect on growth in any condition tested for either species (Table S2). Changes in colony morphology were not recorded. Another four strains (deletions of ACE2, NRG1, SSN6 and TUP1) were removed because the phenotypes are difficult to score in C. albicans or in both species, mostly because they significantly affect filamentation. Deleting RBF1 and RPN4 in C. albicans and NDT80 in C. parapsilosis results in dramatic reduction in growth; the NDT80 deletion is therefore not shown, and the RBF1 and RPN4 deletions are included with the C. parapsilosis-only data (Figure 1B). Many phenotypes previously described in C. albicans are shared in C. parapsilosis. These include the role of CAP1 in the oxidative stress response (sensitivity to cadmium chloride [49]), enhanced sensitivity of CSR1 deletion to metal chelators (EDTA; [43]) and the role of HAP2, HAP5, HAP43 and SEF1 in regulating the response to iron (deletions have reduced growth to low iron, resulting from addition of the iron chelator BPS [50], [51]), of RIM101 as a regulator of the response to alkaline conditions [42], and UPC2, which determines sensitivity to azole drugs [52], [53]. The function of many of these regulators is conserved across a wide evolutionary distance, at least since the common ancestor with S. cerevisiae [54], [55], [56], [57], [58], [59], [60]. Regulation of the iron and copper response is similar in both species though there are some subtle differences (Text S1).
Some phenotypes conserved between the two species have not previously been reported. These include the sensitivity of the upc2 deletions to the presence of the iron chelator, BPS (Figure 1D). Upc2 is also required for growth on xylose as the main carbon source in both C. albicans and C. parapsilosis (Table S2). However, despite the overall similarity, there are also significant differences between the two species. Several deletion strains (e.g. BCR1, CPH2, GIS2, ISW2, MIG1, SEF2, STP4) have no shared phenotypes. Some gene deletions have pleiotropic effects in C. parapsilosis; for example, deleting SEF1 results in reduced growth in many conditions, whereas the equivalent deletion in C. albicans results in much fewer phenotypes (Table S2). One of the most obvious differences between C. albicans and C. parapsilosis occurs during growth in low oxygen (hypoxic) conditions (Table S2, Figure 1C,E). Deleting UPC2 confers sensitivity to hypoxia in both, as previously reported [43], [53], [61], [62]. No other C. albicans gene deletion tested affects hypoxic growth. However, in C. parapsilosis, deleting CPH2 also reduces growth in hypoxia, and growth is restored when the wildtype gene is reintroduced (Figure 1E). In addition, we have previously shown that expression of CpCPH2 is increased during growth in hypoxia [63]. However, deleting CpCPH2 does not affect sensitivity to azole drugs (Figure 1E).
We have previously shown that in C. parapsilosis, similar to C. albicans, members of the CFEM family are important for heme utilization [41], [64], [65]. Whereas C. albicans has five family members, in C. parapsilosis the family has expanded to seven (CFEM1-7). Here, we deleted CFEM1-CFEM4 together, CFEM5 and CFEM6 together, and CFEM7 alone (Table S2). Similar to previous reports, strains missing CFEM1-CFEM4 or CFEM5-CFEM6 are unable to use heme as a sole source of iron (Figure 1B, Table S2, [41]). However, deleting CFEM7 alone had no effect (Table S2). We also find that deleting CTH1 (CPAR2_407950) renders cells sensitive to iron, and unable to use hemin as a sole source of iron (Figure 1B). Expression of CpCTH1 is induced in low iron conditions [41]. CpCTH1 is an ortholog of both members of the CTH1/CTH2 gene pair in S. cerevisiae, proteins that bind to RNA transcripts from iron metabolic genes, targeting them for degradation [66], [67]. Our results suggest that CTH1 forms part of the iron regulatory pathway in C. parapsilosis.
Identification of regulators of biofilm development
The effect of the C. parapsilosis gene deletions on biofilm development on polystyrene surfaces was determined visually using crystal violet staining, and by measurement of biomass (dry weight). Several deletion strains (ADA2, MSS2, VPS34, NDT80 and YCK2) exhibited growth defects on YPD and were not included in the biofilm screen. Of the 95 unique deletions tested, eight had obvious visual defects and significantly reduced dry weight formation, including seven transcription factors (EFG1, CZF1, GZF3, UME6, CPH2, BCR1 and ACE2) and one protein kinase (MKC1) (Figure 2A,B). Some other deletion strains had minor effects on biofilm growth, but only these eight had significant and reproducible reductions in biofilm mass. Reintroducing the intact genes restored biofilm growth (Figure 2C; restoring BCR1 and EFG1 have been described previously [18], [35]). These mutants displayed no significant defects in growth in liquid culture in biofilm media, except that the ACE2 deletion has a cell-separation defect [68].
Fig. 2. Identification of biofilm regulators in C. parapsilosis. 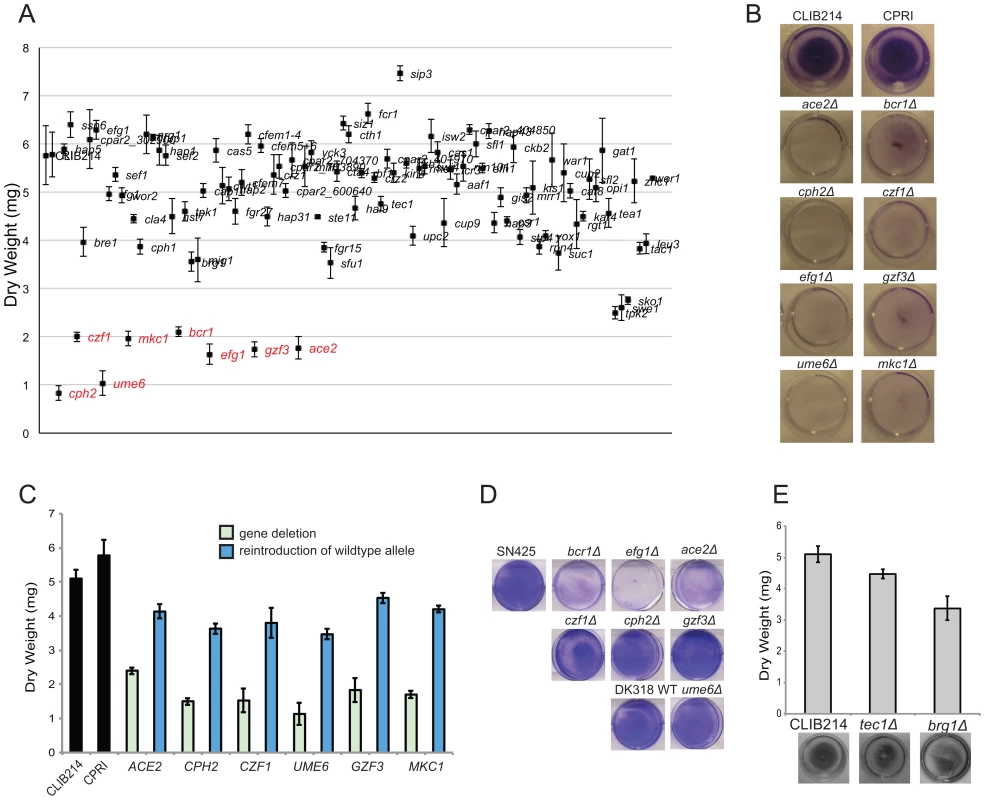
(A) 95 C. parapsilosis knockout strains (Table S2) and two control strains were grown under standard biofilm conditions in 6 well plates. Two wells were combined for each strain, and the dry weight of the biofilm was determined. The standard deviation from three independent measurements is shown. Strains with statistically significant reductions in dry weight are indicated in red (p<0.0005). (B) The indicated C. parapsilosis strains were grown under standard biofilm conditions in 24 well plates, and the biofilm was stained with crystal violet. (C) A wild type copy of each gene was re-introduced into the relevant C. parapsilosis mutant strain as indicated, and the biofilm biomass was measured. Re-introduction of one allele restored biofilm to levels similar to the control strains, CLIB214 and CPRI. (D) C. albicans strains with the indicated gene deletions were grown in 6 well plates in Spider media for 48 h, and stained with crystal violet. The relevant C. albicans wild type strains were included for comparison. Deleting BCR1, EFG1 and ACE2 dramatically reduces biofilm formation, but deleting CPH2, CZF1, GZF3, or UME6 has little effect. (E) Biofilms from C. parapsilosis strains carrying deletions of TEC1 and BRG1 were grown in 24 well plates in SD media with 50 mM glucose and were stained with crystal violet (bottom) or dry weights were measured (top). The gene deletions have no significant effect on biofilm formation. A recent study identified a network of six transcription factors (BCR1, EFG1, TEC1, NDT80, BRG1 and ROB1) that regulate biofilm formation in C. albicans [39]. In a separate study, ACE2 was shown to be required for adhesion and subsequent biofilm development in the same species [40]. To determine the overlap between the networks regulating biofilm growth in C. albicans and C. parapsilosis, we directly compared the effect of deleting the orthologous transcription factors in the two species (Figure 2D,E). Both species were grown in conditions that maximize biofilm development [35], [38], [41]. We confirmed that deleting BCR1, EFG1 and ACE2 reduces biofilm development in C. albicans, similar to C. parapsilosis (Figure 2D), whereas deleting CZF1, GZF3, UME6, or CPH2 has little effect on C. albicans biofilm growth in our assay (Figure 2D).
From the remaining genes in the C. albicans biofilm regulatory network, orthologs of TEC1, NDT80, and BRG1 were not identified in our large-scale screen of biofilm-defective mutants in C. parapsilosis (Figure 2A). We confirmed that deleting TEC1 or BRG1 did not dramatically reduce C. parapsilosis biofilm formation in follow-up tests (Figure 2E). However, we could not determine the role of the NDT80 ortholog, because unlike in C. albicans, deleting NDT80 in C. parapsilosis results in a significant growth defect. There is no ortholog of the final member of the C. albicans network, ROB1, in the C. parapsilosis genome [39], [47].
Structure of C. parapsilosis biofilms
We used confocal laser scanning microscopy (CLSM) to visualize the morphology and structure of C. parapsilosis biofilms growing on the surface of Thermanox slides and stained with concanavalin A [63] (Figure 3). The control strains produce a compact biofilm consisting of layers of yeast and pseudohyphal cells. The biofilm layer is thinner than previously reported (10–20 µm, [35]), but it is reproducible.
Fig. 3. Deleting regulators alters biofilm structure of C. parapsilosis. 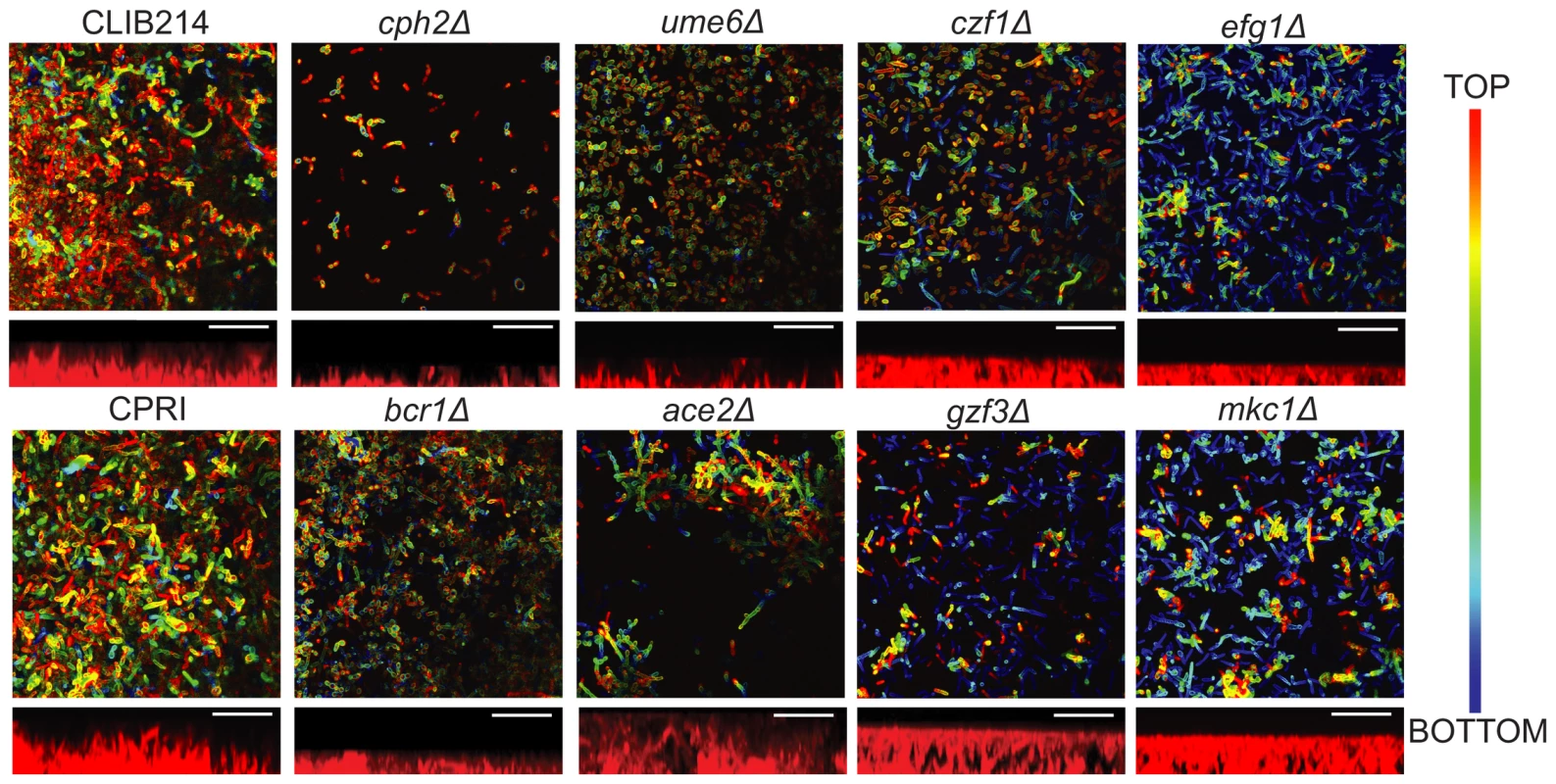
Biofilm structure was determined using confocal microscopy. Biofilms from the indicated strains were grown on Thermanox slides in SD media with 50 mM glucose, stained with conA and visualized using a Zeiss LSM510 confocal scanning microscope. False-color images are shown. Side view images showing the approximate thickness of the biofilm were created using Fiji. Images were restricted on the vertical scale to prevent aberrant measurements from small regions of the biofilm. Scale bars (20 µm) are shown. CLIB214 (11 µm), CPRI (15 µm), cph2Δ (4 µm), ume6Δ (6 µm), czf1Δ (10 µm), efg1Δ (6 µm), bcr1Δ (5 µm), ace2Δ (20 µm), gzf3Δ (12 µm), and mkc1Δ (11 µm). Deleting CPH2, UME6, EFG1 and BCR1 in C. parapsilosis results in biofilms with very few layers that are consistently thinner than those produced by the control strains. Biofilms generated by CPH2, UME6 and CZF1 deletions are mainly composed of yeast cells, with few pseudohyphal cells present, whereas the BCR1 mutant produces a thin biofilm of both yeast and pseudohyphal cells. The ACE2 deletion generates patchy biofilm, consisting of clumps of pseudohyphal cells across the surface of the slide, probably due to the cell separation defect caused by this mutation [68]. Biofilms produced by the GZF3 and MKC1 deletion strains have both yeast and pseudohyphal cells present in several layers; however the biofilm produced is not as compact as the wild type (Figure 3). We have previously shown that deleting EFG1 increases morphological switching between “wrinkled” and “smooth” colonies, both of which have reduced biofilm development [18]. We did not differentiate between different colony morphologies in the assay presented here. However, CPH2, UME6 and CZF1 deletion strains all have reduced colony wrinkling compared to wildtype (not shown).
Characterization of biofilm defective mutants in vivo
Biofilm development in vivo is substantially different from the in vitro models used here. In particular, biofilms formed in catheters undergo stress from blood flow. We therefore used an established rat central venous catheter (CVC) model of infection [69] to test the effect of deleting regulatory genes in C. parapsilosis (Figure 4). We compared the deletion constructs to the control CPRI strain, because biofilm development by the wildtype strain was variable in this model. C. parapsilosis biofilms in vivo consist of yeast cells, matrix and host cells. We have previously shown that deleting BCR1 greatly reduces biofilm formation in vivo, whereas deleting EFG1 has a more minor effect [18], [41]. Here we show that deleting UME6 and CZF1 also greatly reduce biofilm formation (Figure 4). The biofilm from the UME6 deletion consists of a single layer of yeast cells with little obvious matrix, whereas the CZF1 deletion produces little obvious biofilm. Deleting ACE2 results in a clumpy biofilm. In contrast to the in vitro assay, deleting CPH2, GZF3 and MKC1 has little effect on biofilm growth in vivo.
Fig. 4. Deletion of regulators disrupts biofilm formation in vivo. 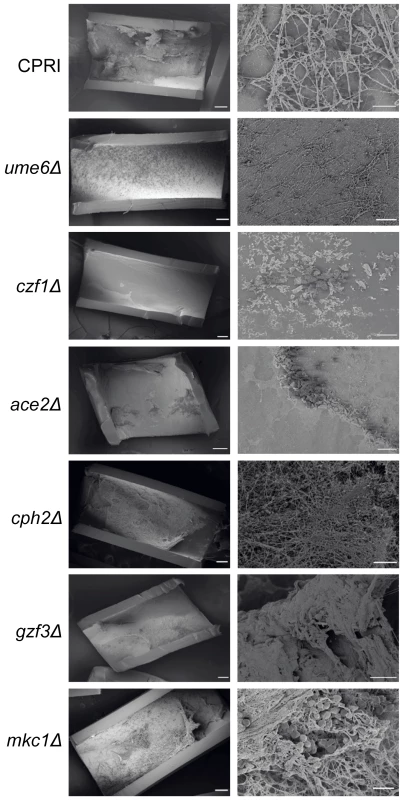
The indicated C. parapsilosis strains were inoculated into catheters in the rat CVC model, and biofilms were visualized by scanning electron microscopy (SEM) after 24 hours. Deleting UME6, CZF1 and ACE2 reduces biofilm formation in comparison to the control strain, CPRI, whereas deleting CPH2, GZF3 and MKC1 has little effect. BCR1 and EFG1 deletions were assayed in this model previously [18], [41]. Scale bars correspond to 200 µm and 20 µm. Transcriptional profile of C. parapsilosis biofilms
We previously used microarray analysis designed from a partial genome sequence to determine the transcriptional profile of C. parapsilosis biofilms growing under flow conditions in a fermenter [63]. In general, we found that the transcriptional response of biofilms is similar to that of cells growing in hypoxic conditions, and is associated with upregulation of fatty acid metabolism genes. Here, we used RNA-seq analysis to characterize the transcription profile of cells growing in static conditions, the same as those used to identify biofilm regulators. Compared to planktonic conditions, 777 genes are up-regulated and 662 genes are down-regulated in biofilms (log2FC+/ − 1.5, adjusted p-value <0.001) (Table S3). Upregulated genes are enriched for processes associated with lipid/fatty acid oxidation and transmembrane transport, whereas downregulated genes are enriched in translation, macromolecule and amino acid biosynthetic processes and cellular component assembly (Table S4).
We used Gene Set Enrichment Analysis (GSEA) to identify gene categories that are over-represented in the biofilm transcriptome. GSEA allows the identification of statistically significant overlaps between a ranked gene list (e.g. the C. parapsilosis biofilm transcriptome), and gene lists (or gene sets) identified in other experimental analyses. We used a collection of 8,852 gene sets derived from microarray and ChIP (chromatin immunoprecitation) experiments from C. albicans and from protein-protein interactions from S. cerevisiae that were collected and described by Sellam et al [70]. We extracted a set of C. albicans orthologs of our ranked list of genes differentially expressed in C. parapsilosis biofilms, and looked for similarities between this gene list and the gene sets described by Sellam et al [70]. The network of similar gene sets was visualized using Cytoscape [71] (Figure 5, Figure S2). In these figures, nodes represent gene sets, and edges connect nodes sharing a significant number of genes. Clustering algorithms in Cytoscape group highly interconnected and similar gene sets together. We have colored nodes that included genes upregulated in C. parapsilosis biofilms in red, and those that include downregulated genes in blue. Figure 5 shows that upregulated genes share similarities with gene sets associated with transport (including carboxylic acid and drug transport) in C. albicans. There is also a significant overlap with the C. albicans biofilm regulatory network described by Nobile et al [39]. The transport and biofilm networks are connected via Ndt80 (Figure 5). Downregulated genes are enriched for processes associated with translation and the ribosome.
Fig. 5. Gene Set Enrichment Analysis of the C. parapsilosis biofilm transcriptome. 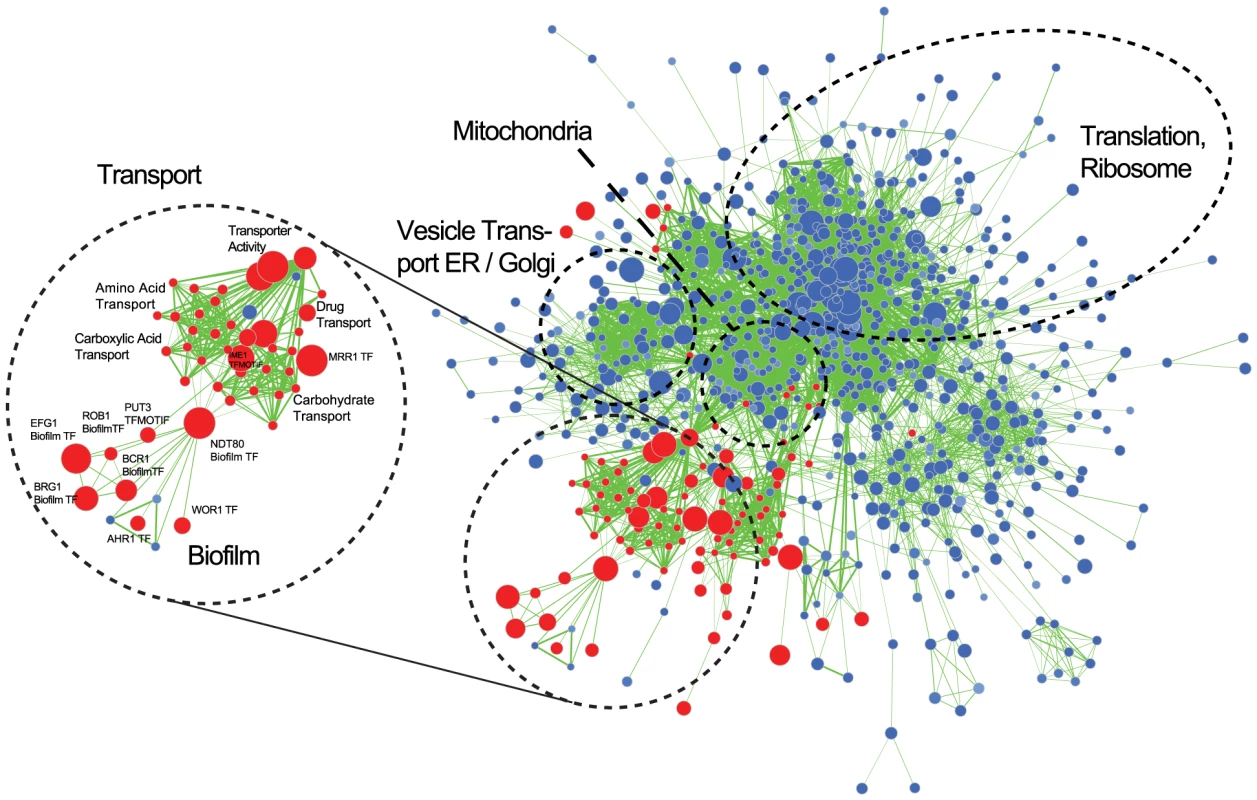
Visualization of gene sets enriched in the C. parapsilosis wild type biofilm transcriptome compared to C. parapsilosis planktonic transcriptome. Gene sets are obtained from C. albicans and S. cerevisiae data [70]. C. albicans gene sets enriched in genes up-regulated in C. parapsilosis biofilms are shown in red, and gene sets enriched in down-regulated genes are in blue. The size of the nodes represents the number of genes in the gene sets in C. albicans, and the edges represent an overlap of genes between gene sets. Sub-networks of interest are highlighted in black circles. “TF” indicates dataset from C. albicans transcription factor deletions, and “Biofilm” indicates the C. albicans biofilm regulatory network form Nobile at al [39]. A larger version of the figure is available as Figure S2, and the Cytoscape data is provided as Dataset S1. Comparison to C. albicans biofilm transcriptome
The similarity between the C. parapsilosis biofilm transcriptome and the C. albicans biofilm regulatory network prompted us to directly compare the biofilm transcriptional profiles of the two species, using the C. albicans data from Nobile et al [39] (Figure 6A). In C. albicans 785 genes are up-regulated and 300 genes down-regulated in biofilm compared to planktonic cells. There are 192 genes upregulated in biofilms in both species (Figure 6A). These are enriched in oxidoreductases and in pathways associated with carboxylic acid metabolism. Interestingly, genes upregulated in C. albicans only (and not in C. parapsilosis) are enriched in processes associated with biofilm formation and adhesion. Many are transcription factors, some of which we have shown have no role in biofilm development in C. parapsilosis (BRG1, WAR1 [40], CRZ2 [40] and ZNC1 [40]), and others that we have not tested, but which are not differentially expressed in biofilms e.g. GCN4 [72]. Genes upregulated only in C. parapsilosis are enriched in pathways associated with transmembrane and drug transport. This category includes a large number of genes with no annotation, suggesting that their function may be specific to C. parapsilosis.
Fig. 6. Transcriptional profiling of C. parapsilosis biofilms. 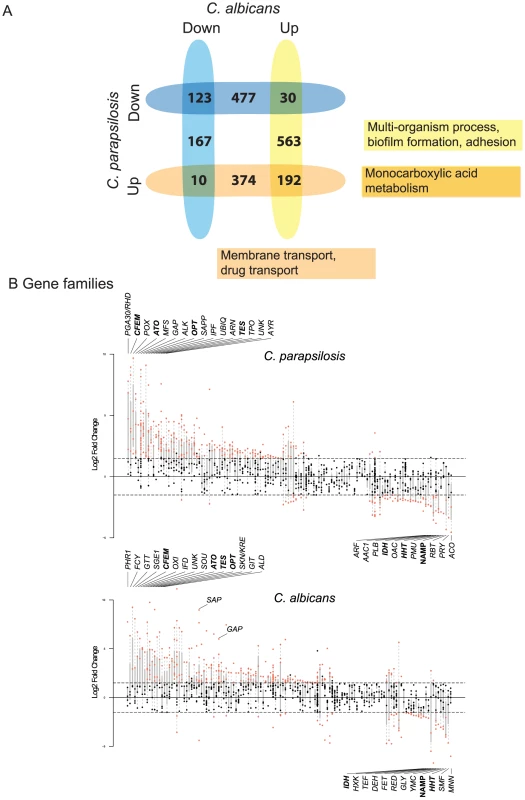
Comparison of genes differentially expressed in C. parapsilosis biofilms with C. albicans biofilms (from [39]). The intersections show genes that are differentially regulated in biofilms in both species, and the remaining numbers indicate genes that differentially regulated in one species only. Up (blue) and down (yellow) regulated genes are shown separately. The most common GO processes in upregulated genes are indicated. Gene families enriched in differentially expressed genes in biofilms in C. parapsilosis (top) and C. albicans (bottom). Each vertical line represents a gene family. Families are ordered with respect to mean expression from highest (left) to lowest (right). Each dot represents an individual gene; those that are differentially expressed are highlighted in red. The families with the highest and lowest expression are named, as are others discussed in the text. Families highlighted in bold text are highly differentially expressed in biofilms in both species. One of the difficulties comparing gene expression profiles between species is the reliance on the identification of orthologs present in both. This may underestimate the importance of genes that are found in one species only. In addition, it is very difficult to evaluate the roles of gene families that have different numbers or members in different Candida species [73]. We therefore categorized the gene families in C. albicans and C. parapsilosis using Markov Clustering (MCL; [74]) and looked for evidence of family enrichment among differentially expressed genes in biofilms (again using the data from [39] for C. albicans). Figure 6B shows the gene families ordered by average gene expression for both species (from highest to lowest expression; full data in Table S5).
The CFEM family, the ATO family of putative ammonia transporters [75], the OPT family of oligopeptide transporters [76] and the TES family of acyl CoA-thioesterases are among the most enriched among upregulated genes in both species (Figure 6, Table S5). General amino acid permeases (GAP family) and secreted aspartyl proteases (SAP family) are also among the most highly expressed in C. parapsilosis, and are enriched among up-regulated genes in C. albicans (Figure 6B). The most downregulated genes enriched in both species include a family involved in Nucleic Acid Metabolic Processes (NAMP) (including TUP1, a major hyphal regulator in C. albicans that is required for biofilm growth [77]) and histone genes (HHT family). The PGA30/RHD3 family has the highest average expression in C. parapsilosis; these encode GPI-anchored proteins predicted to be localized to the cell wall [78].
Identification of targets of biofilm regulators
To identify the genes that are regulated in C. parapsilosis biofilms, we used RNA-seq to compare the transcriptional profiles of biofilms from strains deleted for EFG1, CZF1, UME6, CPH2, BCR1 and ACE2 to biofilms from wildtype strains (Figure 7). We were unable to isolate RNA of sufficient quality from strains deleted for GZF3 or MKC1, suggesting that there may be changes in the extracellular matrix or cell wall that we cannot directly observe. Only seven genes are downregulated in biofilms of all deletion strains and upregulated in the wildtype (ARO10, ATO1, PUT4, STE18, HGT17, CPAR2_803700, CPAR2_805760). We have not yet characterized the roles of these genes in biofilm development.
Fig. 7. Comparison of transcription profiles of biofilm regulators. 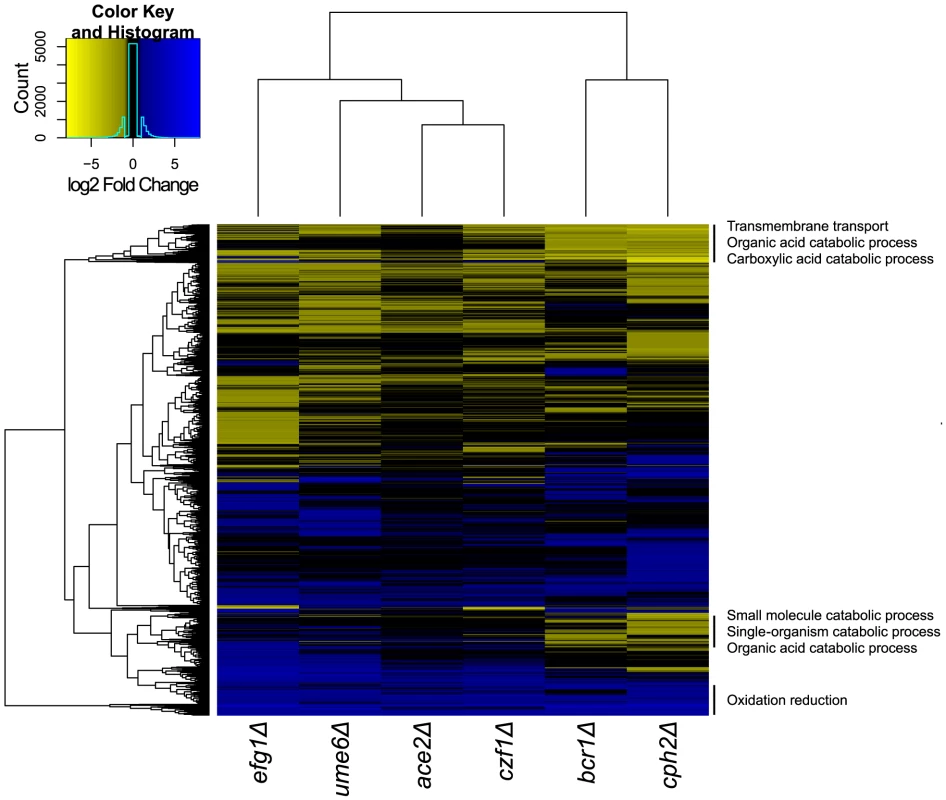
Gene expression profiles from the indicated C. parapsilosis strains were determined using RNA-seq of three independent replicates and compared to the profile of wildtype biofilms. Similar expression patterns were clustered using Bioconductor [114]. The major GO patterns associated with specific clusters are shown. Processes associated with transmembrane transport, organic acid catabolism and carboxylic acid metabolism are upregulated in most of the deletion biofilms, compared to wildtype (Figure 7). The transcriptional profiles of biofilms from the BCR1 and CPH2 deletion cluster close together (Figure 7), suggesting that these transcription factors regulate a core set of genes. We also used GSEA to compare the gene sets regulated by some of the transcription factors with the biofilm network described in Figure 5. Figure 8 shows that there is a high degree of overlap between the gene sets enriched in the BCR1 and CPH2 deletion and in the wildtype biofilm/planktonic comparison. Gene sets associated with carboxylic acid transport and with carbohydrate transport are downregulated in both. The targets of Efg1, a known biofilm regulator in C. parapsilosis [18] have the smallest overlap with the biofilm network (Figure 8C). Again, the overlap includes gene sets associated with carboxylic acid transport.
Fig. 8. GSEA analysis of biofilms formed by C. parapsilosis deletion strains. 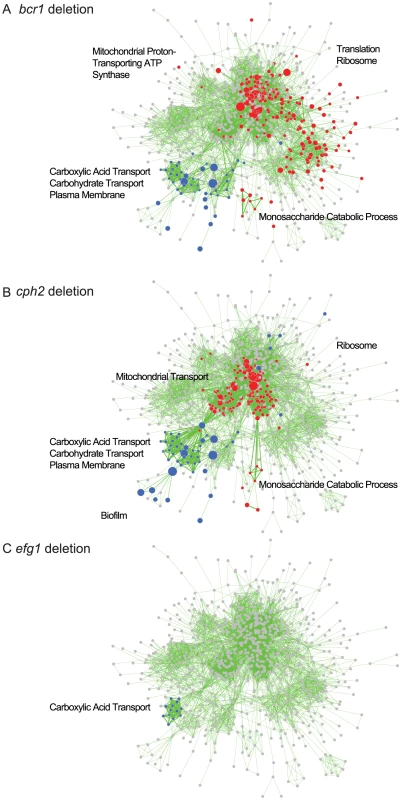
The panels show enriched genesets from GSEAPreRanked analysis of biofilms from (A) bcr1, (B) cph2 and (C) efg1 deletion strains overlayed on the transcriptional profile of C. parapsilosis biofilms compared to planktonic cells from Figure 5. Gene sets enriched in upregulated genes in the deletion biofilms compared to wildtype biofilms are shown in red, and gene sets enriched in downregulated genes are shown in blue. Gene sets that are not changed in the deletion strains are shown in gray. Enrichment is visualized using Cytoscape, as described in Figure 5. A larger version of the figure is available as Figure S3, and the Cytoscape data is provided as Dataset S1. Discussion
Conservation of phenotypes between C. albicans and C. parapsilosis
The phenotype screen revealed a high conservation in the regulation of different biological processes between C. albicans and C. parapsilosis. Overall, 15 of the 23 transcription factors in Figure 1C share at least one major phenotype in C. albicans and C. parapsilosis. Some shared phenotypes, such as role of UPC2 in the response to low iron conditions, have not previously been highlighted. However, Homann et al [43] noticed that deleting UPC2 in C. albicans resulted in the accumulation of a pink color after several days of growth on BPS, possibly signaling formation of a ferrous-BPS complex. Deleting UPC2 also results in a growth defect of Yarrowia lipolytica in low iron conditions [79]. It is therefore likely that this transcription factor is involved in regulating the response to iron in several fungal species.
Despite the overall similarity observed in the phenotypic screen there are some significant differences, including the role of CPH2 in the hypoxic response of C. parapsilosis. In C. albicans, CPH2 is a regulator of hyphal growth with no known hypoxic role [80], [81]. It has a basic helix-loop-helix (bHLH) DNA-binding domain, and is probably a remnant of the Sterol Regulatory Binding Proteins (SREBPs) that regulate sterol synthesis and the hypoxic response in filamentous fungi and in Schizosaccharomyces pombe; species that have no recognizable Upc2 [79], [82], [83]. Most species in the Saccharomycotina (including Saccharomyces and Candida species) have lost domains from their SREBP homologs, and the remaining remnants have no known role in regulating sterol synthesis, or in the hypoxic response [79], [83]. Y. lipolytica, an outgroup of the Saccharomycotina, is a notable exception in that it retains a full length SREBP, and has gained a Upc2 ortholog [79]. However, even in this species Upc2 is the main regulator of sterol synthesis, although SREBP has retained some role in regulating the hypoxic response, particularly in the control of filamentation [79]. In C. parapsilosis, deleting CPH2 does not affect sensitivity to ketoconazole (Figure 1E), indicating that its role in hypoxia is unlikely to be related to regulating expression of ergosterol genes. Elucidating the role of CPH2 in the hypoxic response of C. parapsilosis will require substantial further investigation. However it appears that Cph2 and SREBPs have retained a previously unsuspected role in regulating the hypoxic response in the Saccharomycotina.
Regulation of biofilm development
We identified seven transcription factors and one protein kinase that are important regulators of C. parapsilosis biofilm development in vitro (Figure 2). Deleting CPH2, GZF3 and the protein kinase MKC1 has no effect on biofilm development in the in vivo rat catheter model. This suggests that biofilm development may be context dependent. A similar phenomenon has been described in C. albicans; for example, CaBRG1 is required for biofilm formation in vitro but not in vivo [39], and deleting CaRHR2 reduces biofilm formation in the central venous catheter model but not in an oral pharyngeal model [84]. However, we note that in C. parapsilosis the GZF3 and MKC1 deletions strains have minor effects on the structure of biofilms on Thermanox slides (Figure 3), suggesting that their role as biofilm regulators may be restricted to growth in specific conditions.
Two of the C. parapsilosis biofilm regulators (BCR1 and EFG1) are conserved with the well-characterized C. albicans biofilm circuit [39], and a third (ACE2) is also likely to regulate biofilm development in both species (Figure 9, [40], [68]). Five (CPH2, UME6, CZF1, GZF3 and MKC1) appear to be unique to C. parapsilosis. We previously showed that the role of Bcr1 as a regulator of biofilm development in C. parapsilosis shares some similarities with that of its ortholog in C. albicans [85]. However, there are significant differences in the two species. For example, some of the conserved targets (e.g. the CFEM family) are required for biofilm development in C. albicans and not in C. parapsilosis [41]. The role of Bcr1 is also strain dependent. In C. parapsilosis, strains which make relatively low levels of biofilm (like CLIB214, the isolate used throughout this study) are Bcr1-dependent, while those that generate high levels of biofilm are not [86]. In C. albicans, “sexual” or “pheromone-stimulated” biofilms are made by cells that are homozygous at the mating MTL locus, and they are distinguished from the more general pathogenic biofilms. There is some evidence that both kinds of biofilms require Bcr1 [87], whereas other studies suggest that Bcr1-dependent expression of CFEM genes is required for drug resistance in pathogenic biofilms only [88], [89]. “Sexual” biofilms have not been described in C. parapsilosis, where most (and possibly all) isolates are of a single mating type [17], [90]. There are therefore aspects of the roles of Bcr1 in both species than remain to be elucidated.
Fig. 9. Comparison of the C. parapsilosis biofilm network with C. albicans. 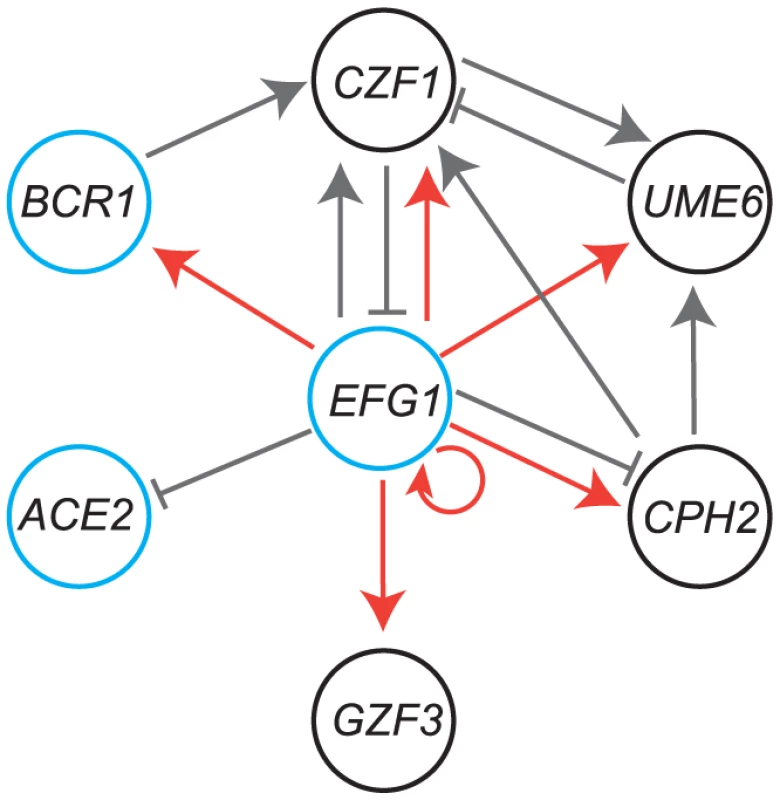
Genes in black circles are major regulators of biofilm development in C. parapsilosis only, whereas those in blue circles have a conserved role in C. albicans. Gray lines indicate regulation of expression from RNA-seq data; arrowheads show activation, and solid lines show repression. Red lines show direct binding of Efg1 to promoters of genes in planktonic growth, from Connolly et al [18]. Like Bcr1, Efg1 also regulates biofilm development in both C. albicans and C. parapsilosis [18], [39], [87], [91], [92]. In C. albicans Efg1 plays a central role in networks regulating high frequency epigenetic switching from white-to-opaque cells [93], and in filamentation of both cell types [12]. In addition, it is required for virulence [94] and for drug resistance [95]. In C. parapsilosis, Efg1 also regulates a high frequency switching system [18]. In both species, Efg1 directly bind to the promoters of a large number of transcription factors [18], [39]. Many of the promoters are exceptionally long, suggesting that they are regulated by several transcription factors [18], [39]. It is therefore likely that Efg1 is a member of several regulatory circuits in both species.
Although Ace2 was not identified as a component of the C. albicans biofilm regulatory circuit by Nobile et al [39], we and others have shown that it is required for biofilm development in this species [40], [68], which we confirmed in the current assay (Figure 2D). Ace2 regulates expression of genes during late M/early G1 phase of the cell cycle in S. cerevisiae and C. albicans [68], [96], [97]. The transcription factor is part of the RAM network (Regulation of Ace2 and Morphogenesis) that controls exit from mitosis in many fungi (reviewed in [98]). Ace2 regulates adherence of C. albicans, leading to sparse biofilms, and is target of Snf5, a chromatin regulator [40]. Deleting CpACE2 results in a cell separation defect, and expression of many targets shared with CaACE2 is reduced (e.g. CHT1, CHT3, SCW11) (Table S3). The function of Ace2 in regulating biofilm development in Candida species is therefore likely to be conserved.
Deleting the transcription factors UME6, CPH2, CZF1 and GZF3 reduces biofilm development in C. parapsilosis only (Figure 2A,D). We did not test the effect of deleting the MKC1 kinase ortholog in C. albicans. It remains possible that the orthologous transcription factors may play a more minor role in biofilm growth of C. albicans. For example, overexpressing UME6 enhances biofilm development in both species [48], [99]. However, we detected very little reduction in C. albicans biofilms formed by ume6 deletion strains (Figure 2D, and two deletions in a different genetic background that are not shown), and it is clear that the phenotype of the UME6 deletion in C. parapsilosis is considerably more severe. In S. cerevisiae, UME6 is an activator of gene expression during early meiosis, whereas in C. albicans, the ortholog regulates filamentation. Expression of UME6 is also increased during pseudohyphal growth in C. parapsilosis [99].
The CPH2 regulon in C. albicans is not known, but an early microarray study suggests that CaCPH2 regulates expression of filamentation genes, many of which are also targets of Efg1 [100]. Other regulators of filamentation, such as NRG1, also control biofilm formation in both C. albicans and C. parapsilosis [48]. It is likely that filamentation is more important for C. albicans biofilms; although both species can grow as filaments, only C. albicans make true hyphae, and many mutants locked in the yeast phase make poor biofilms [101], [102]. Ume6 and Cph2 may therefore have functions separate to filamentation that are important for biofilm formation in C. parapsilosis.
Although deleting CZF1 does not reduce mature biofilm development in C. albicans, the transcription factor is required for early stage adherence [40]. The functional targets are not known [40]. In C. parapsilosis, CZF1 regulates expression of transporters (Table S3); their role in biofilm development remains to be elucidated. GZF3 encodes a GATA-type transcription factor that is induced during oxidative stress in C. albicans [103]. Expression is induced in biofilms, but there is no evidence that it plays a major role in C. albicans biofilm development [39].
Elucidation of the biofilm transcriptome
We used RNA-seq and network analysis to characterize the transcriptome of C. parapsilosis biofilms. Gene sets enriched in genes downregulated in biofilms are associated with translation and with ribosome function, which probably reflects reduced growth in biofilms compared to planktonic cells (Figure 5). Genes up-regulated in C. parapsilosis biofilms are enriched for pathways associated with transport (including carboxylic acid transport), and are also enriched in gene sets that are upregulated in C. albicans biofilms [39]. A more direct comparison of the C. parapsilosis and C. albicans biofilm transcriptomes revealed that similarities between the species center on metabolic changes, and in particular monocarboxylic acid metabolism (Figure 6).
Some of the shared response may be related to the fact that biofilms are hypoxic environments [32], [63]. For example, 43 genes that are up-regulated in C. parapsilosis and C. albicans [39] biofilms and in both species in hypoxic conditions [61], [104] are enriched for processes associated with carbohydrate and lipid metabolism, which may reflect changes occurring during adaptation to hypoxic environments. Other metabolic features, such as glycerol metabolism, are also increasingly being recognized as potential virulence factors [105]. Expression of RHR2, glycerol-3-phosphatase is important for development of C. albicans biofilms, and it is suggested that glycerol levels directly regulate expression of adhesins [105]. RHR2 expression is also increased in C. parapsilosis biofilms (Table S3).
Analysis of gene family enrichment was also used to compare the biofilm transcriptional response of these two species. The ATO, TES, OPT and CFEM families are enriched in both C. parapsilosis and C. albicans biofilms (Figure 6B). Although the roles of the ATO and TES families in biofilm development of C. albicans has not been elucidated, increased oligopeptide transfer (OPT) has been associated with early biofilm stages [106]. Some members of the CFEM family are regulated by Bcr1 in both C. albicans and C. parapsilosis [41], [44], [107]. Surprisingly, although CFEM genes are required for biofilm development in C. albicans [108] they do not have a similar role in C. parapsilosis [41]. We further confirmed this here by deleting the CFEM genes in C. parapsilosis in clusters (CFEM1-4 together, CFEM5-6 together and CFEM7). None of the deletion strains were defective in biofilm formation (Table S2). However, the conservation of expression of the four gene families in C. albicans and C. parapsilosis suggest that they may be suitable targets for subsequent study of core biofilm components.
Somewhat surprisingly, processes associated with adhesion and those classified as being involved in biofilm formation by the Candida Genome Database [109] are enriched in C. albicans biofilms and not in C. parapsilosis biofilms (Figure 6A). These may be because biofilm development in C. albicans is strongly correlated with the switch from yeast to hyphal growth [29]. One of the major differences between C. parapsilosis and C. albicans is that C. parapsilosis does not make true hyphae. Biofilms produced by C. albicans are composed of a mixture of yeast, pseudohyphal and hyphal cells, with the yeast cells forming the basal layer and an upper layer of hyphal cells [29]. In contrast C. parapsilosis biofilm contains only yeast and pseudohyphal cells in compact layers [28].
Identification of the C. parapsilosis biofilm circuit
We used RNA-seq to identify the targets of the major C. parapsilosis transcriptional regulators during biofilm growth. The Bcr1 and Cph2 regulons are similar (Figure 7) and they have a high degree of overlap with genes enriched in the biofilm transcriptome (Figure 8). This suggests that Cph2 and Bcr1 are major biofilm regulators in C. parapsilosis. Both transcription factors regulate expression of genes involved in transport of carboxylic and other organic acids, and in glycolysis and monosaccharide metabolism. We observed that carboxylic acid transport or metabolism gene sets are enriched in the genes differentially regulated in many of the deletion strains (Figure 7). Even Efg1, which otherwise has little overlap with the C. parapsilosis biofilm network, regulates carboxylic acid transport (Figure 8C). In addition, genes that are differentially regulated in both C. albicans and C. parapsilosis biofilms are enriched in processes associated with carboxylic acid metabolism (Figure 6A). This supports our hypothesis that regulation of metabolism is important for biofilm production in both species. The shared role of Bcr1 as a biofilm regulator in the two species may therefore be related to regulation of carboxylic acid metabolism. In C. parapsilosis biofilms, Cph2 regulates expression of transporters and oxidoreductases, including the highly expressed ATO (Ammonia Transporter Outward) family. The cph2 GSEA network is enriched for gene sets associated with C. albicans biofilms (Figure 8B), suggesting that CpCPH2 may perform some of the role of the C. albicans biofilm regulators.
We exploited the RNA-seq data to look for evidence that the transcriptional regulators we identified work together in a network (Figure 9). We also included ChIP-seq data for Efg1 from planktonic cells [18]. We placed Efg1 at the center of the network to simplify the model, because it has many connections with the other regulators. Efg1 is also known to have many roles in both species [18], [95], [110], [111], [112], [113]. Several of the transcription factors regulate expression of other members of the putative network; some are activators (for example deleting EFG1 reduces expression of CZF1) and some as repressors (deleting CZF1 increases expression of EFG1). Some are linked to the putative network by only one connection (ACE2 and GZF3). The role of ACE2 in biofilm development is likely to be associated with its function in regulating cell separation [68], [97], and it may be more important for adherence rather than for mature biofilm growth [40].
It is unlikely that we have identified all the biofilm regulators in C. parapsilosis. For example, our transcription factor deletion collection is not complete. In addition, we have not obtained transcriptional profiling data for the GZF3 deletion, and we have not identified the direct targets of the transcription factors (for example using ChIP-seq). We were also unable to determine the role of NDT80, which our GSEA analysis suggests may be involved (Figure 5). Overall, our analysis suggests that there is some degree of conservation of biofilm networks in C. albicans and C. parapsilosis. This is not surprising, considering the two species are closely related [2]. However, there are also significant differences, with four transcription factors specific to C. parapsilosis, and three unique to C. albicans. In future work, careful characterization the C. parapsilosis biofilm regulon will help us to elucidate the evolution of the networks in these species.
Materials and Methods
Strains and growth conditions
Candida parapsilosis strains (Table S1) were grown in YPD medium (1% yeast extract, 2% peptone, 2% glucose) at 30°C. For colony selection 2% agar was added. To select for transformants, nourseothricin (Werner Bioagents Jena, Germany) was added to YPD agar at a final concentration of 200 µg ml−1. Transformants containing the LEU2 and HIS1 markers were selected on synthetic complete (SC; 0.19% yeast nitrogen base without amino acids and ammonium sulphate, 0.5% ammonium sulphate, 2% dextrose, 0.075% mixture of amino acids, 2% agar) media without leucine or histidine. For biofilm formation, C. parapsilosis was grown in synthetic defined (SD) medium (0.67% yeast nitrogen base) containing 50 mM glucose. C. albicans was grown in Spider media (1% nutrient broth, 1% mannitol, 0.2% potassium phosphate). The media used for phenotype screening is shown in Table S2. All deletion strains were grown in 96 well plates in YPD media at 30°C overnight. The cultures were then diluted 1∶100 into a new 96 well plate containing fresh YPD media. The strains were then pinned onto agar plates using a 48 pin bolt replicator. Plates were incubated at 30°C and photographed after 2 and 3 days of growth. Each knockout was scored on growth in comparison to the control strains (CLIB214 and CPRI) on the same media, where −4 indicates a severe growth defect, −3, −2, and −1 indicate strong, moderate and marginal growth defects, 0 is similar to the control strains, and +1 is stronger growth than control strains. Scores were assigned only where the two independent replicates had the same behavior. Screens were repeated at least twice. Growth on different chemical concentrations (e.g. CuCl2, ketoconazole) were combined to give a single score (see Table S2). Deletion strains with interesting phenotypes were further validated by plating exact numbers of cells in decreasing concentration on test media. Control strains were included in each plate. Scores were converted to a Heatmap using Bioconductor [114].
Construction of deletion strains in C. parapsilosis
To delete LEU2 (CPAR2_805510), approximately 500 bp upstream and downstream of the open reading frame was amplified using the primer pairs CpLEU2KO1/CpLEU2KO2 and CpLEU2KO3/CpLEU2KO4 respectively (Table S6). Primers KO1 and KO2 contain recognition sites for KpnI and ApaI respectively and KO3 and KO4 for SacII and SacI. The PCR products were purified using a Qiagen PCR purification kit and ligated at either end of a SAT1 flipper cassette in pCD8 [115], generating plasmid pCpLEU2. The entire cassette plus flanking regions were excised by digestion with KpnI and SacI, gel purified and transformed into C. parapsilosis CLIB214. Integration of the cassette at LEU2 was confirmed by PCR using a primer 5′ to LEU2 (CpLEU2KO5) and a primer from inside the cassette (BUT237). Intact LEU2 alleles were identified by PCR with CpLEU2KO5 and CpLEU2KO6. Primers CpLEU2KO1 and CpLEU2KO4 were used to verify recycling of the cassette and deletion of the LEU2 gene. The same cassette was used to delete both LEU2 alleles (Figure S1). A similar method was used to delete the HIS1 gene (CPAR2_100200) using primers CpHIS1KO1 and CpHIS1KO2 to amplify an upstream region and primers CpHIS1KO3 and CpHIS1KO4 to amplify a downstream region. Integration of the cassette was confirmed by PCR using a primer 5′ to the gene CpHIS1KO5 and BUT237. To confirm the presence of an intact allele the primer pair CpHIS1KO5 and CpHIS1KO6 were used and primer pair CpHIS1KO1 and CpHIS1KO4 were used to verify recycling of the cassette and deletion of the HIS1 (Figure S1). The leu2−/his1− strain (CPL2H1) was used as a background for all other deletion constructs.
Target genes were deleted in strain CPL2H1 using a fusion PCR method described in Noble et al [45]. Target genes are listed in Tables S1 and S2, and the primers used are listed in Table S6. Approximately 500 bp upstream and downstream of the target gene was amplified using Phusion Taq (New England BioLabs) with primer pairs 1/3 and primers 4/6. The annotated CZF1 open reading frame was corrected (and elongated) by re-sequencing. The selectable markers, C. dubliniensis HIS1 and C. maltosa LEU2 genes were amplified using primers 2 (universal primer) and primer 5 from the plasmids pSN52 and pSN40 respectively. All PCR products were purified using a Qiagen PCR purification kit. The 5′ tails of primers 2 and 3 have complementary regions, as do primers 4 and 5. The 5′ PCR product, the 3′ PCR product and one of the selectable marker products were fused by PCR using primers 1 and 6. The resulting disruption cassette was transformed into the background strain (Figure 1, Figure S1). The first allele was always deleted using the CmLEU2 selectable marker and the second allele using the CdHIS1 gene.
Correct integration of the marker gene at the target locus was confirmed by PCR of both ends of the deletion construct; the 5′ region was confirmed using primers 5′check and either LEUcheck1/HIScheck1, and the 3′ region using primers 3′ check and either LEUcheck2/HIScheck2. Loss of the open reading frames was also confirmed using the appropriate ORF primers (Table S6).
To create the control reintegration strain, CPRI, upstream and downstream regions of the deleted HIS1 gene were amplified using primers CpHIS1KO1/CpHIS-3 and CpHIS-4/CpHISKO7. The Candida dulbiniensis HIS1 and Candida maltosa LEU2 genes were amplified from the appropriate plasmids using primers 2 (universal primer) and CpHIS-5. The resulting PCR product were fused using primers CpHIS1KO1 and CpHIS1KO7 and transformed into the background strain and in turn re-introducing the C. dulbiniensis HIS1 and C. maltosa LEU2 genes at the original site of the C. parapsilosis HIS1 alleles. Correct integration of the marker genes were confirmed by PCR using the primer CpHIS1KO5 and either LEUcheck1 or HIScheck1.
The cph2, czf1, gzf3, ace2, mkc1 and ume6 mutant strains were complemented by introducing one copy of the gene at its original site on the genome [87]. Briefly the entire ORF and promoter were amplified using the primers listed in Table S6. The resulting PCR products were digested using the enzyme sites incorporated into each primer and cloned into pSFS2a. The plasmid was then linearized within the promoter region using the relevant enzyme (MKC1 and UME6 (HpaI), CPH2 and CZF1 (MluI), GZF3 (BsmBI), ACE2 (BsiWI)) and transformed into the appropriate background mutant strain. Transformants were selected on nourseothricin plates and correct integration of the plasmid was determined by PCR and using one primer upstream of the promoter and one inside the ORF (Table S6).
Transformation of C. parapsilosis
Strains were transformed by electroporation as described previously with some modifications [115]. After electroporation, 950 µl of fresh YPD was added immediately and the mixture incubated at 30°C for 3–4 h. Following incubation, cells were pelleted, washed once in 1 ml of water and resuspended in 300 µl of water. 100 µl was plated onto YPD plates supplemented with nourseothricin at a concentration of 200 µg ml−1. Transformants were obtained following 48 h of incubation at 30°C.
The SAT1 cassette was recycled by growing overnight in YPM (1% yeast extract, 2% peptone and 2% maltose). 100 cells were plated onto YPD plates containing 10 µg ml−1 of nourseothricin and incubated overnight at 30°C. Following incubation a mixture of large and small colonies were visible on the plate. Small colonies were restreaked onto fresh YPD agar plates and checked for nourseothricin sensitivity. The second allele was deleted using the same protocol.
Most deletion strains were constructed using chemical transformation. An overnight culture was diluted to an A600 of 0.2 in 30 ml of YPD broth. This was grown at 30°C to an A600 of 1. The culture was centrifuged at 4000 g for 5 min and the pellet resuspended in 3 ml of ice-cold water. The re-suspended pellet was centrifuged again as above and the pellet re-suspended in 200 µl of ice-cold TE-LiOAC (0.1M lithium acetate, 10 mM Tris and 1 mM EDTA). A transformation mix was set up that contained 10 µl of boiled and cooled salmon sperm DNA (10 mg ml−1), 20–30 µl of fusion PCR product and 100 µl of competent cells. This was incubated at 30°C for 30 min followed by addition of 700 µl of PLATE (0.1M lithium acetate, 10 mM Tris, 1 mM EDTA and 40% PEG 3350). Samples were incubated overnight at 30°C. Cells were heat shocked at 44°C for 15 min, centrifuged, and washed with 1 ml of YPD. The cells were centrifuged again and finally re-suspended in 100 µl of YPD followed by incubation at 30°C for 2 h. Cultures were then spread on the appropriate drop-out agar plates and incubated for 2–3 days at 30°C.
Biofilm assays
Strains were tested for biofilm development crystal violet staining and direct observation using Nunclon Delta 24-well polystyrene plates as follows. C. parapsilosis trains were grown overnight at 30°C and washed twice in phosphate buffered saline (PBS), diluted to an A600 of 1 in SD media with 50 mM glucose, and 1 ml was added to each well. The cultures were incubated for 2 h at 37°C at 50 rpm. Wells were then washed once with 1 ml of PBS to remove non-adherent cells. 1 ml of fresh SD 50 mM glucose media was then added to each well. Plates were then incubated for 48 h at 37°C at 50 rpm. The supernatants were removed, and each well was washed twice with 1 ml of PBS. Plates were allowed to dry overnight at room temperature. Biofilms were stained with 500 µl 0.4% crystal violet for 10 min. The dye was removed and wells were washed with PBS. Plates were allowed to dry and biofilm was photographed. For C. albicans biofilms, 6 well plates were pre-treated with 10% fetal bovine serum overnight which was removed by washing with 1 ml of PBS. C. albicans strains from overnight cultures were washed twice with PBS, diluted to a starting A600 of 0.5 in Spider media and 5 ml was added to the pre-treated wells. The plates were incubated for 90 min at 37°C at 100 rpm. Each well was then washed once with PBS and fresh spider media was added followed by incubation for 48 h at 37°C at 100 rpm. The supernatants were removed and each well was washed twice with 1 ml of PBS.
For dry mass measurements, C. parapsilosis biofilms were formed in Nunclon Delta 6 well plates. Assays were set up as for the 24-well plates, except that 5 ml of media was used in each well. After the final wash, 1 ml of PBS was added to each well and adherent biofilms were scrapped from the bottom of the wells. The contents of two wells were vacuum filtered over a pre-weighed 0.8 µm nitrocellulose filter (Millipore). The filters were dried overnight in a warm room and weighed the following day. The average total biomass of each strain was calculated for 3 independent samples by subtracting the initial weight of the filter from the final weight. Statistical significance was calculated using the students two tailed paired t-test, and only those with a P-value <0.005 were retained.
Confocal microscopy
C. parapsilosis biofilms were grown on Thermanox (Nunc) slides in in 6 well plates. Briefly, overnight cultures were washed twice in PBS and diluted to an A600 of 1 in SD media with 50 mM glucose. 5 ml was added to each well, and incubated for 2 h at 37°C at 50 rpm. The slides were gently removed and placed into a fresh well containing 5 ml of PBS, and gently placed into a well containing fresh SD/50 mM glucose media and incubated for 48 h at 37°C at 50 rpm. The slides were removed and washed as above, and then stained with 25 µg ml−1 concanavalin A (conA)-Alexa Fluor 594 conjugate (C-11253; Bio-science) for 45 min at 37°C. The liquid was removed from each well, and the Thermonox slides were flipped and placed on a 35-mm-diameter glass-bottomed petri dish (MatTek Corp., Ashland, MA). The biofilms were observed with a Zeiss LSM510 confocal scanning microscope with a ×40-magnification oil objective. A HeNe1 laser was used to excite at a 543-nm wavelength. All images were captured and analyzed using a Zeiss LSM Image Browser and Fiji.
RNA-seq analysis
Biofilms for RNA-seq analysis were grown in SD media with 50 mM glucose at 37°C in Nunc 6 well plates. The biofilm was scraped from the bottom of each well and combined for RNA isolation. The number of wells combined depended on the amount of biofilm produced and the amount of biomass needed to obtain sufficient RNA for library preparation (6 wells for CLIB214 and 6–10 wells for the deletion strains). RNA was isolated using an Ambion Ribopure-Yeast RNA kit. To isolate RNA for planktonic cells, strains were grown to an A600 of 1 in SD media with 50 mM glucose at 37°C.
Strand specific RNA-seq library preparation and sequencing was carried out by BGI (www.genomics.cn, Hong-Kong). Paired-end reads (Illumina HiSeq 2000, 2×90 bp, 2 GB clean data) were obtained from three biological replicates from wild type (Candida parapsilosis CLIB214) in planktonic and biofilm conditions, and from ace2, cph2, efg1, czf1, ume6 and bcr1 deletion strains in biofilms. Two replicates were obtained form one deletion construct, and the third from the independent replicate strain. Samples were aligned to the genome [61] using TopHat2 [116]. HTSeq [27] was used to count mapped reads per gene. Differentially expressed genes were identified using DESeq2 [117] with an adjusted p-value threshold of 0.001 and a log2 fold change threshold of −1.5 and 1.5. Default parameters in DESeq2 were used, except that Cook distance filtering was turned off. Significantly differentially expressed genes were clustered using hierarchical clustering in R [114]. C. albicans orthologs were obtained from the Candida Gene Order Browser (CGOB) [47]. The GO term finder from Candida Genome Database [109] was used to carry out Gene Ontology analyses. All RNA-seq data is available from GEO accession number GSE57451.
Gene family analysis
Gene families in C. parapsilosis and C. albicans were identified using Blast similarity searches [118] and the MCL algorithm [74]. In both species an inflation factor of 2.1 was used with MCL to robustly identify similar clusters. The average log2 fold change of significantly differentially expressed genes in each cluster was calculated, and ranked from high to low (Figure 8, Table S3). For C. albicans biofilms, gene expression levels were reported from a mixture of RNA-seq and microarray analysis [39]. We used significant RNA-seq values were available, and values from microarrays if the equivalent RNA-seq data was not significant. P-values for microarrays were not available. Significant genes were defined as log2 fold change greater than 1.5 or less than −1.5, and with an adjusted p-value less than 0.001 (for RNA-seq only). Plots were generated in R and analyzed manually.
Gene Set Enrichment Analysis
The pre-ranked Gene Set Enrichment Analysis tool (GSEAPreRanked, see http://www.broadinstitute.org/gsea/) was used to determine gene sets that are enriched in differentially expressed genes from the RNA-seq data. Only C. parapsilosis genes with orthologs in C. albicans were included, with gene sets provided by Dr. Andre Nantel (http://www.candidagenome.org/download/community/GSEA_Nantel_2012/) [70]. Genes were ranked from highest to lowest by log2 fold change. GSEAPreRanked was used with the default options, excluding gene sets with less than 5 or more than 1000 genes, resulting in the analysis of 5249 gene sets in total. Significant results were defined as p-value lower than 0.005 and an FDR Q-value lower than 0.1. GSEAPreRanked results were further analyzed using Cytoscape (version 2.8.2) and the EnrichmentMap plugin (version 1.2, [71]) using an overlap coefficient cutoff of 0.5 and default settings. The size of the nodes correlates to the number of genes in the gene sets from Sellam et al [70], which are based on C. albicans. We use a background of C. parapsilosis orthologs for the GSEA, but the networks generated in Cytoscape reflect the original gene sets from Sellam et al [70].
Supporting Information
Zdroje
1. Lachance M-A, Boekhout T, Scorzetti G, Fell JW, Kurtzmann CP (2011) Candida Berkhout (1923). In: Kurtzman CP, Fell JW, Boekhout T, editors. The Yeasts, a Taxonomic Study. 5th ed. Amsterdam: Elsevier. pp. 987–1278.
2. FitzpatrickDA, LogueME, StajichJE, ButlerG (2006) A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol Biol 6 : 99.
3. WongS, FaresMA, ZimmermannW, ButlerG, WolfeKH (2003) Evidence from comparative genomics for a complete sexual cycle in the ‘asexual’ pathogenic yeast Candida glabrata. Genome Biol 4: R10.
4. SeervaiRN, JonesSKJr, HirakawaMP, PormanAM, BennettRJ (2013) Parasexuality and ploidy change in Candida tropicalis. Eukaryot Cell 12 : 1629–1640.
5. HickmanMA, ZengG, ForcheA, HirakawaMP, AbbeyD, et al. (2013) The ‘obligate diploid’ Candida albicans forms mating-competent haploids. Nature 494 : 55–59.
6. SherwoodRK, ScadutoCM, TorresSE, BennettRJ (2014) Convergent evolution of a fused sexual cycle promotes the haploid lifestyle. Nature 506 : 387–390.
7. SantosMA, GomesAC, SantosMC, CarretoLC, MouraGR (2011) The genetic code of the fungal CTG clade. C R Biol 334 : 607–611.
8. Lass-FlorlC (2009) The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 52 : 197–205.
9. PfallerMA, DiekemaDJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20 : 133–163.
10. PfallerMA, CastanheiraM, MesserSA, MoetGJ, JonesRN (2010) Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009). Diagn Microbiol Infect Dis 68 : 278–283.
11. MayerFL, WilsonD, HubeB (2013) Candida albicans pathogenicity mechanisms. Virulence 4 : 119–128.
12. SiH, HerndayAD, HirakawaMP, JohnsonAD, BennettRJ (2013) Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog 9: e1003210.
13. PormanAM, HirakawaMP, JonesSK, WangN, BennettRJ (2013) MTL-independent phenotypic switching in Candida tropicalis and a dual role for Wor1 in regulating switching and filamentation. PLoS Genet 9: e1003369.
14. XieJ, DuH, GuanG, TongY, KourkoumpetisTK, et al. (2012) N-Acetylglucosamine induces White-to-Opaque switching and mating in Candida tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot Cell 11 : 773–782.
15. PammiM, HollandL, ButlerG, GacserA, BlissJM (2013) Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J 32: e206–216.
16. ButlerG (2010) Fungal sex and pathogenesis. Clin Microbiol Rev 23 : 140–159.
17. SaiS, HollandL, McGeeCF, LynchDB, ButlerG (2011) Evolution of mating within the Candida parapsilosis species group. Eukaryot Cell 10 : 578–587.
18. ConnollyLA, RiccombeniA, GrozerZ, HollandLM, LynchDB, et al. (2013) The APSES transcription factor Efg1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol Microbiol 90 : 36–53.
19. BarchiesiF, CaggianoG, Falconi Di FrancescoL, MontagnaMT, BarbutiS, et al. (2004) Outbreak of fungemia due to Candida parapsilosis in a pediatric oncology unit. Diagn Microbiol Infect Dis 49 : 269–271.
20. ClarkTA, SlavinskiSA, MorganJ, LottT, Arthington-SkaggsBA, et al. (2004) Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J Clin Microbiol 42 : 4468–4472.
21. DiazGranadosCA, MartinezA, DeazaC, ValderramaS (2008) An outbreak of Candida spp. bloodstream infection in a tertiary care center in Bogota, Colombia. Braz J Infect Dis 12 : 390–394.
22. DizbayM, KalkanciA, SezerBE, AktasF, AydoganS, et al. (2008) Molecular investigation of a fungemia outbreak due to Candida parapsilosis in an intensive care unit. Braz J Infect Dis 12 : 395–399.
23. LevinAS, CostaSF, MussiNS, BassoM, SintoSI, et al. (1998) Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn Microbiol Infect Dis 30 : 243–249.
24. van AsbeckEC, HuangYC, MarkhamAN, ClemonsKV, StevensDA (2007) Candida parapsilosis fungemia in neonates: genotyping results suggest healthcare workers hands as source, and review of published studies. Mycopathologia 164 : 287–293.
25. AlmiranteB, RodriguezD, Cuenca-EstrellaM, AlmelaM, SanchezF, et al. (2006) Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 44 : 1681–1685.
26. ClerihewL, LamagniTL, BrocklehurstP, McGuireW (2007) Candida parapsilosis infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 92: F127–129.
27. KuhnDM, ChandraJ, MukherjeePK, GhannoumMA (2002) Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun 70 : 878–888.
28. SilvaS, HenriquesM, MartinsA, OliveiraR, WilliamsD, et al. (2009) Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol 47 : 681–689.
29. BlankenshipJR, MitchellAP (2006) How to build a biofilm: a fungal perspective. Curr Opin Microbiol 9 : 588–594.
30. SeneviratneCJ, JinL, SamaranayakeLP (2008) Biofilm lifestyle of Candida: a mini review. Oral Dis 14 : 582–590.
31. RamageG, MowatE, JonesB, WilliamsC, Lopez-RibotJ (2009) Our current understanding of fungal biofilms. Crit Rev Microbiol 35 : 340–355.
32. SellamA, Al-NiemiT, McInnerneyK, BrumfieldS, NantelA, et al. (2009) A Candida albicans early stage biofilm detachment event in rich medium. BMC Microbiol 9 : 25.
33. PannanusornS, FernandezV, RomlingU (2013) Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses 56 : 264–272.
34. KuhnDM, ChandraJ, MukherjeePK, GhannoumMA (2002) Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun 70 : 878–888.
35. DingC, ButlerG (2007) Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot Cell 6 : 1310–1319.
36. GacserA, TrofaD, SchaferW, NosanchukJD (2007) Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest 117 : 3049–3058.
37. FinkelJS, MitchellAP (2011) Genetic control of Candida albicans biofilm development. Nature Rev Microbiol 9 : 109–118.
38. NobileCJ, MitchellAP (2006) Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 8 : 1382–1391.
39. NobileCJ, FoxEP, NettJE, SorrellsTR, MitrovichQM, et al. (2012) A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 148 : 126–138.
40. FinkelJS, XuW, HuangD, HillEM, DesaiJV, et al. (2012) Portrait of Candida albicans adherence regulators. PLoS Pathog 8: e1002525.
41. DingC, VidanesGM, MaguireSL, GuidaA, SynnottJM, et al. (2011) Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS ONE 6: e28151.
42. DavisDA, BrunoVM, LozaL, FillerSG, MitchellAP (2002) Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162 : 1573–1581.
43. HomannOR, DeaJ, NobleSM, JohnsonAD (2009) A phenotypic profile of the Candida albicans regulatory network. PLoS Genet 5: e1000783.
44. NobileCJ, MitchellAP (2005) Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol 15 : 1150–1155.
45. NobleSM, JohnsonAD (2005) Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell 4 : 298–309.
46. FitzpatrickDA, O'GaoraP, ByrneKP, ButlerG (2010) Analysis of gene evolution and metabolic pathways using the Candida Gene Order Browser. BMC Genomics 11 : 290.
47. MaguireSL, OheigeartaighSS, ByrneKP, SchroderMS, O'GaoraP, et al. (2013) Comparative genome analysis and gene finding in Candida species using CGOB. Mol Biol Evol 30 : 1281–1291.
48. BanerjeeM, ThompsonDS, LazzellA, CarlislePL, PierceC, et al. (2008) UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell 19 : 1354–1365.
49. Alonso-MongeR, Navarro-GarciaF, RomanE, NegredoAI, EismanB, et al. (2003) The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot Cell 2 : 351–361.
50. BaekYU, LiM, DavisDA (2008) Candida albicans ferric reductases are differentially regulated in response to distinct forms of iron limitation by the Rim101 and CBF transcription factors. Eukaryot Cell 7 : 1168–1179.
51. ChenC, PandeK, FrenchSD, TuchBB, NobleSM (2011) An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10 : 118–135.
52. HootSJ, OliverBG, WhiteTC (2008) Candida albicans UPC2 is transcriptionally induced in response to antifungal drugs and anaerobicity through Upc2p-dependent and -independent mechanisms. Microbiology 154 : 2748–2756.
53. SynnottJM, GuidaA, Mulhern-HaugheyS, HigginsDG, ButlerG (2010) Regulation of the hypoxic response in Candida albicans. Eukaryot Cell 9 : 1734–1746.
54. DrakulicT, TempleMD, GuidoR, JarolimS, BreitenbachM, et al. (2005) Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species, and ageing in Saccharomyces cerevisiae. FEMS Yeast Res 5 : 1215–1228.
55. ZhaoH, EideDJ (1997) Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae. Mol Cell Biol 17 : 5044–5052.
56. ThieleDJ (1988) ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol 8 : 2745–2752.
57. OlesenJT, GuarenteL (1990) The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev 4 : 1714–1729.
58. LambTM, MitchellAP (2003) The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol Cell Biol 23 : 677–686.
59. VikA, RineJ (2001) Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol 21 : 6395–6405.
60. ChenC, PandeK, FrenchSD, TuchBB, NobleSM (2011) An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe 10 : 118–135.
61. GuidaA, LindstadtC, MaguireSL, DingC, HigginsDG, et al. (2011) Using RNA-seq to determine the transcriptional landscape and the hypoxic response of the pathogenic yeast Candida parapsilosis. BMC Genomics 12 : 628.
62. MacPhersonS, AkacheB, WeberS, De DekenX, RaymondM, et al. (2005) Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob Agents Chemother 49 : 1745–1752.
63. RossignolT, DingC, GuidaA, d'EnfertC, HigginsDG, et al. (2009) Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot Cell 8 : 550–559.
64. WeissmanZ, KornitzerD (2004) A family of Candida cell surface haem-binding proteins involved in haemin and haemoglobin-iron utilization. Mol Microbiol 53 : 1209–1220.
65. WeissmanZ, ShemerR, ConibearE, KornitzerD (2008) An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans. Mol Microbiol 69 : 201–217.
66. PuigS, AskelandE, ThieleDJ (2005) Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120 : 99–110.
67. PuigS, VergaraSV, ThieleDJ (2008) Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab 7 : 555–564.
68. KellyMT, MacCallumDM, ClancySD, OddsFC, BrownAJ, et al. (2004) The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol Microbiol 53 : 969–983.
69. AndesD, NettJ, OschelP, AlbrechtR, MarchilloK, et al. (2004) Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect Immun 72 : 6023–6031.
70. SellamA, van Het HoogM, TebbjiF, BeaurepaireC, WhitewayM, et al. (2014) Modeling the transcriptional regulatory network that controls the early hypoxic response in Candida albicans. Eukaryot Cell 13 : 675–90 doi: 10.1128/EC.00292-13
71. MericoD, IsserlinR, StuekerO, EmiliA, BaderGD (2010) Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS ONE 5: e13984.
72. Garcia-SanchezS, AubertS, IraquiI, JanbonG, GhigoJM, et al. (2004) Candidaalbicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot Cell 3 : 536–545.
73. Butler G, Lorenz M, Gow NAR (2012) Evolution and genomics of the pathogenic Candida species complex. In: Sibley DL, Howlett BK, Heitman J, editors. Evolution of virulence in eukaryotic microbes: John Wiley & Sons, Inc. pp. 404–421.
74. EnrightAJ, Van DongenS, OuzounisCA (2002) An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res 30 : 1575–1584.
75. PalkovaZ, DevauxF, IcicovaM, MinarikovaL, Le CromS, et al. (2002) Ammonia pulses and metabolic oscillations guide yeast colony development. Mol Biol Cell 13 : 3901–3914.
76. LubkowitzMA, HauserL, BreslavM, NaiderF, BeckerJM (1997) An oligopeptide transport gene from Candida albicans. Microbiology 143 : 387–396.
77. CaoYY, CaoYB, XuZ, YingK, LiY, et al. (2005) cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob Agents Chemother 49 : 584–589.
78. De GrootPW, HellingwerfKJ, KlisFM (2003) Genome-wide identification of fungal GPI proteins. Yeast 20 : 781–796.
79. MaguireSL, WangC, HollandLM, BrunelF, NeuvegliseC, et al. (2014) Zinc finger transcription factors displaced SREBP proteins as the major sterol regulators during Saccharomycotina evolution. PLoS Genet 10: e1004076.
80. LaneS, BirseC, ZhouS, MatsonR, LiuH (2001) DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J Biol Chem 276 : 48988–48996.
81. LaneS, ZhouS, PanT, DaiQ, LiuH (2001) The basic helix-loop-helix transcription factor Cph2 regulates hyphal development in Candida albicans partly via TEC1. Mol Cell Biol 21 : 6418–6428.
82. BienCM, EspenshadePJ (2010) Sterol regulatory element binding proteins in fungi: hypoxic transcription factors linked to pathogenesis. Eukaryot Cell 9 : 352–359.
83. ButlerG (2013) Hypoxia and gene expression in eukaryotic microbes. Ann Rev Microbiol 67 : 291–312.
84. Di RienziSC, CollingwoodD, RaghuramaMK, BrewerBJ (2009) Fragile genomic sites are associated with origins of replication. Gen Biol Evol 2009 : 350–363.
85. NelissenB, De WachterR, GoffeauA (1997) Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol Rev 21 : 113–134.
86. PannanusornS, Ramirez-ZavalaB, LunsdorfH, AgerberthB, MorschhauserJ, et al. (2014) Characterization of biofilm formation and the role of BCR1 in clinical isolates of Candida parapsilosis. Eukaryot Cell 13 : 438–451.
87. LinCH, KabrawalaS, FoxEP, NobileCJ, JohnsonAD, et al. (2013) Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. PLoS Pathog 9: e1003305.
88. SrikanthaT, DanielsKJ, PujolC, KimE, SollDR (2013) Identification of genes upregulated by the transcription factor Bcr1 that are involved in impermeability, impenetrability, and drug resistance of Candida albicans a/alpha biofilms. Eukaryot Cell 12 : 875–888.
89. YiS, SahniN, DanielsKJ, LuKL, SrikanthaT, et al. (2011) Alternative Mating Type Configurations (a/alpha versus a/a or alpha/alpha) of Candida albicans Result in Alternative Biofilms Regulated by Different Pathways. PLoS Biol 9: e1001117.
90. LogueME, WongS, WolfeKH, ButlerG (2005) A genome sequence survey shows that the pathogenic yeast Candida parapsilosis has a defective MTLa1 allele at its mating type locus. Eukaryot Cell 4 : 1009–1017.
91. RamageG, SavilleSP, ThomasDP, Lopez-RibotJL (2005) Candida biofilms: an update. Eukaryot Cell 4 : 633–638.
92. StichternothC, ErnstJF (2009) Hypoxic adaptation by Efg1 regulates biofilm formation of Candida albicans. Appl Environ Microbiol 3663–3672.
93. ZordanRE, MillerMG, GalgoczyDJ, TuchBB, JohnsonAD (2007) Interlocking transcriptional feedback loops control White-Opaque switching in Candida albicans. PLoS Biol 5: e256.
94. LoHJ, KohlerJR, DiDomenicoB, LoebenbergD, CacciapuotiA, et al. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90 : 939–949.
95. BinkA, GovaertG, VandenboschD, KucharikovaS, CoenyeT, et al. (2012) Transcription factor Efg1 contributes to the tolerance of Candida albicans biofilms against antifungal agents in vitro and in vivo. J Med Microbiol 61 : 813–819.
96. DohrmannPR, ButlerG, TamaiK, DorlandS, GreeneJR, et al. (1992) Parallel pathways of gene regulation: homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev 6 : 93–104.
97. DoolinMT, JohnsonAL, JohnstonLH, ButlerG (2001) Overlapping and distinct roles of the duplicated yeast transcription factors Ace2p and Swi5p. Mol Microbiol 40 : 422–432.
98. SaputoS, Chabrier-RoselloY, LucaFC, KumarA, KrysanDJ (2012) The RAM network in pathogenic fungi. Eukaryot Cell 11 : 708–717.
99. LackeyE, VipulanandanG, ChildersDS, KadoshD (2013) Comparative evolution of morphological regulatory functions in Candida species. Eukaryot Cell 12 : 1356–1368.
100. LaneS, BirseC, ZhouS, MatsonR, LiuH (2001) DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J Biol Chem 276 : 48988–48996.
101. ThompsonDS, CarlislePL, KadoshD (2011) Coevolution of morphology and virulence in Candida species. Eukaryot Cell 10 : 1173–1182.
102. RenD, BedzykLA, ThomasSM, YeRW, WoodTK (2004) Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol 64 : 515–524.
103. WangY, CaoYY, JiaXM, CaoYB, GaoPH, et al. (2006) Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic Biol Med 40 : 1201–1209.
104. SetiadiER, DoedtT, CottierF, NoffzC, ErnstJF (2006) Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J Mol Biol 361 : 399–411.
105. DesaiJV, BrunoVM, GangulyS, StamperRJ, MitchellKF, et al. (2013) Regulatory role of glycerol in Candida albicans biofilm formation. MBio 4: e00637–00612.
106. YeaterKM, ChandraJ, ChengG, MukherjeePK, ZhaoX, et al. (2007) Temporal analysis of Candida albicans gene expression during biofilm development. Microbiology 153 : 2373–2385.
107. NobileCJ, AndesDR, NettJE, SmithFJ, YueF, et al. (2006) Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog 2: e63.
108. PerezA, PedrosB, MurguiA, CasanovaM, Lopez-RibotJL, et al. (2006) Biofilm formation by Candida albicans mutants for genes coding fungal proteins exhibiting the eight-cysteine-containing CFEM domain. FEMS Yeast Res 6 : 1074–1084.
109. InglisDO, ArnaudMB, BinkleyJ, ShahP, SkrzypekMS, et al. (2012) The Candida genome database incorporates multiple Candida species: multispecies search and analysis tools with curated gene and protein information for Candida albicans and Candida glabrata. Nucleic Acids Res 40: D667–674.
110. BraunBR, JohnsonAD (2000) TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155 : 57–67.
111. DoedtT, KrishnamurthyS, BockmuhlDP, TebarthB, StempelC, et al. (2004) APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol Biol Cell 15 : 3167–3180.
112. LiuH (2001) Transcriptional control of dimorphism in Candida albicans. Curr Opin Microbiol 4 : 728–735.
113. SohnK, UrbanC, BrunnerH, RuppS (2003) EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol Microbiol 47 : 89–102.
114. GentlemanRC, CareyVJ, BatesDM, BolstadB, DettlingM, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80.
115. DingC, ButlerG (2007) Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot Cell 6 : 1310–1319.
116. KimD, PerteaG, TrapnellC, PimentelH, KelleyR, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36.
117. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
118. AltschulSF, GishW, MillerW, MyersEW, LipmanDJ (1990) Basic local alignment search tool. J Mol Biol 215 : 403–410.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



