-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHost Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
article has not abstract
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004273
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1004273Summary
article has not abstract
Introduction
Apicomplexans form a large phylum of parasitic protists, some of which cause severe diseases in humans. Most notorious is Plasmodium, the agent of malaria, which kills around a million people each year, mostly young children in Africa. Most successful is Toxoplasma, which parasitizes nearly a third of the human population, making those people at risk of life-threatening complications, primarily encephalitis or pneumonia, in case of immunosuppression. Other apicomplexans of human importance include Cryptosporidium, Isospora, and Sarcocystis, which are opportunistic pathogens that cause severe diarrhea often associated with AIDS. Several apicomplexan parasites cause heavy losses in livestock, particularly Theileria and Babesia in cattle and Eimeria in poultry.
Most apicomplexans are obligate intracellular parasites. Their extracellular stages, called zoites, display several conserved features: they are elongated and polarized cells, their shape is maintained by a set of microtubules running longitudinally, and their anterior pole contains secretory vesicles, called micronemes and rhoptries, which secrete their content at the anterior tip of the parasite. Most zoites also share two unique properties among eukaryotic cells. They move on substrate by a gliding type of motility, i.e., without overt deformation of the cell shape, at speeds of several microns per second. They also typically invade host cells by forming a ring-like junction with the host cell membrane. Zoites slide through the junction into an invagination of the host cell surface that becomes the parasitophorous vacuole (PV) after pinching off from the host cell plasma membrane, in a process that takes less than a minute. Once inside the PV niche, the zoite can multiply into multiple new zoites that eventually egress the infected cell to infect new host cells.
Much work has been performed since the late 1970s to understand the cellular and molecular bases of host cell invasion by apicomplexans, using various zoites as models. The overall invasion process encompasses several steps, including loose followed by intimate attachment, reorientation relative to the host cell surface, and organelle discharge with junction formation. The ultimate step, sliding through the junction inside the PV and called here internalization, is commonly viewed as powered by the zoite submembrane actin-myosin motor. The junction is thought to act as a traction point for the motor, to bridge the cortical cytoskeletons in the two cells, and to be made of parasite proteins conserved in the apicomplexan phylum. In this review, we confront these established notions with genetic data recently obtained in Plasmodium and Toxoplasma parasites.
The Junction: From “Moving” to Stationary
The first observation of a junction between an apicomplexan zoite and its host cell was made using Plasmodium merozoites and their target cells, erythrocytes [1]. Electron microscopy showed that the merozoite, after initial random binding, reorients so that its apical tip faces the erythrocyte surface, and then induces a circumferential zone of close apposition of the zoite and erythrocyte membranes over ∼250 nm and the thickening of the inner leaflet of the erythrocyte membrane [1]. This junctional area was described as “actively moving down the body of the merozoite,” since the poorly motile merozoite was not thought to be capable of actively moving inside the cell, and was thus termed “moving junction” [1].
Studies in the 1980s focused on the highly motile Eimeria sporozoites. They showed that several activities at the zoite surface were dependent on parasite actin, including the posterior translocation (capping) of various surface ligands and beads [2]. Videomicroscopic studies revealed that host cell invasion by Eimeria sporozoites was continuous with extracellular gliding [3]. This led to the proposal that the zoite actin-based system would power both gliding motility and host cell invasion by capping substrate-binding ligands or the junction, respectively, which implied that the zoite actively moved inside the host cell [3], [4]. After myosins were identified in Toxoplasma [5] and in Plasmodium [6], it was assumed that an actin-myosin motor powered the zoite motile processes.
The role of the host cell during zoite invasion has been studied mainly with Toxoplasma tachyzoites, which can be made to invade host cells at high frequency and synchronicity. The host cell was initially described as displaying no detectable actin reorganization and playing no active role during tachyzoite invasion [7], [8]. More recent work found that Toxoplasma tachyzoites induced, specifically at the junction, host actin polymerization and recruitment of the Arp2/3 complex, an actin-nucleating factor, which is important for tachyzoite entry [9]. Videomicroscopic studies showed a stationary ring of host F-actin at the parasite constriction, in agreement with the junction acting as an anchor for zoite traction inside the cell. In addition to de novo actin polymerization at the junction, tachyzoite invasion also requires disorganization of the host cortical actin meshwork. This activity is in part dependent on Toxofilin [10], a Toxoplasma protein that sequesters actin monomers in vitro [11] and promotes actin turnover at the leading edge of the cell [10]. Localized actin disassembly might thus release G-actin necessary to feed actin reassembly at the junction, regulated by recruited Arp2/3 complex, to anchor the junction to the host cortical cytoskeleton.
The Parasite Motor
The motor is located in the space (∼20 nm) between the zoite plasma membrane and the inner membrane complex (IMC), a continuous layer of flattened vesicles apposed onto the microtubule structure typical of alveolates (Figure 1A) [12]. The development in the 1990s of gene targeting techniques in Toxoplasma and Plasmodium has allowed the identification of some of the main players of the apicomplexan motor. The first proposed motor-substrate link was the thrombospondin-related anonymous protein (TRAP), a transmembrane protein of Plasmodium sporozoites conserved in the phylum that was found to be essential for sporozoite gliding [13]. The first (and, to date, only) myosin shown to operate during apicomplexan gliding is MyoA, a single-headed unconventional myosin of the apicomplexan-specific XIV class, which was shown to be crucial for gliding of Toxoplasma tachyzoites [14]. The orientation of the motor was determined by two main findings: the association of the cytoplasmic tails of Plasmodium TRAP and its MIC2 ortholog in Toxoplasma with actin [15] and the localization of the MyoA light chain (also called myosin A tail domain interacting protein, MTIP) in the IMC [16].
Fig. 1. Molecular models of apicomplexan gliding and invasion. 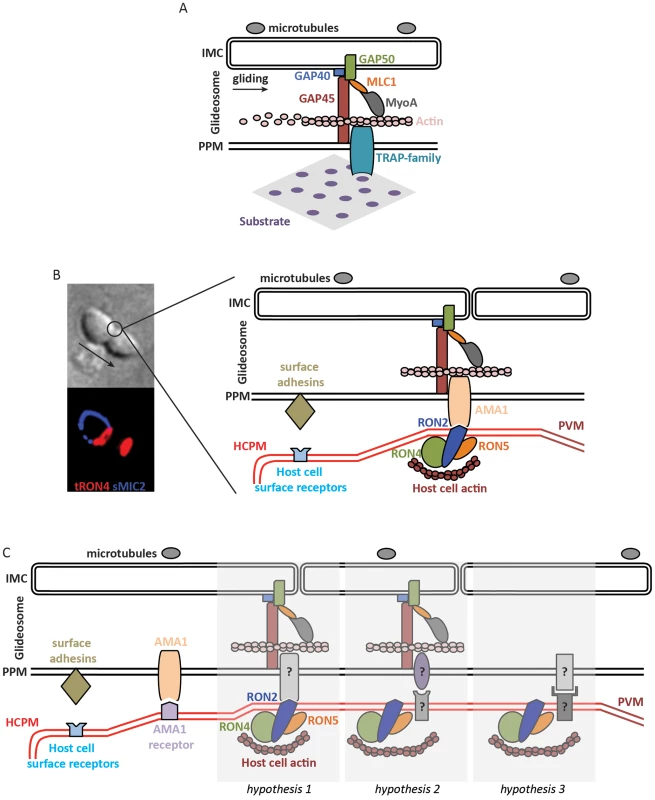
A. The parasite motor (glideosome) is located in the space between the parasite plasma membrane (PPM) and the inner membrane complex (IMC) apposed to the microtubules. Gliding motility is mediated by the binding of the ectodomain of transmembrane TRAP-family proteins to a solid substrate, while the cytoplasmic tail of the protein is linked to the parasite motor. The integrity of the glideosome is maintained by the gliding-associated protein 45 (GAP45), which is anchored to the PPM at one end and to the IMC, via GAPs 40 and 50, at the other end. The link between the GAPs, and ultimately the IMC, to actin is provided by Myosin A (MyoA) and the MyoA Light Chain 1 (MLC1). The movement of the cell is the consequence of the capping, by myosin-actin activity, of the TRAP-family protein. B. The model of invasion seen as the junction structured by the AMA1-RON complex. The figure on the left shows a Toxoplasma tachyzoite invading a host cell. The arrow indicates the direction of movement. Immunostaining of surface MIC2 (sMIC2) stains the part of the zoite cell still extracellular (blue), while the rest of the cell, already internalized, is not stained. Immunostaining of total RON4 (tRON4, red) marks the junction as a ring at the point of constriction, indicated by the circle, and the rhoptries at the apical pole of the zoite cell. After a first step of adhesion to the host cell plasma membrane (HCPM) mediated by parasite surface adhesins and host cell surface receptors, the binding of the transmembrane protein AMA1 to RON2, inserted at the host cell membrane and complexed with RONs 4 and 5, forms the junction. The link to the parasite motor is as in (A), while host actin recruited at the junction provides the link to the host cell cytoskeleton. The movement of the zoite towards the interior of the newly formed parasitophorous vacuole membrane (PVM) is thus a consequence of the capping of AMA1, which would be anchored at the junction by binding to RON2. C. Models of zoite invasion in which the functions of AMA1 and RONs are dissociated. Color codes and acronyms are as in (A) and (B). After a first step of adhesion mediated by parasite surface adhesins and host cell surface receptors, AMA1 binding to a host cell receptor provides a strong attachment between the zoite and host cell membranes, possibly leading to reorientation of the zoite to allow junction formation. Three different hypotheses could then explain junction formation: 1. A still-unknown transmembrane parasite protein binds to the motor and to RON2, taking the place previously assigned to AMA1. 2. Unknown proteins structure the junction and connect the parasite motor to the host cell cortical actin, in which case the role of the RONs at the junction is not structural. 3. Unknown proteins structure the junction without a role of the parasite motor during invasion. Actin polymerization in apicomplexans has been studied in some detail. In these parasites, which contain a limited repertoire of actin-binding factors [17], actin forms inherently short and unstable filaments [18], and normal zoite gliding requires rapid actin dynamics [19]. In the absence of Arp2/3 complex in these parasites, F-actin nucleation is promoted by formins [20], [21], which were detected as an apical ring at the junction, like actin itself [22], in the invading Plasmodium merozoite [20]. Actin depolymerization is controlled by actin-depolymerizing factor (ADF) [23] while profilin sequesters monomeric actin [24], [25]. How actin filaments are connected to the cytoplasmic tails of the TRAP/MIC2 proteins remains unclear. The link was originally thought to occur via aldolase [15], a glycolytic enzyme that binds the cytoplasmic tails of the TRAP/MIC2 proteins and is known to bundle actin filaments in mammalian cells. However, genetic data have now clearly shown that aldolase does not play such a mechanical, bridging role during gliding motility and invasion [26], [27] but is important for providing energy via its glycolytic activity [28]. Finally, structural components of the motor complex have also been identified, primarily in Toxoplasma, called gliding-associated protein 45 (GAP45) [29], [30], GAP50 [29], and GAP40 [31]. Although the individual contributions of the GAP proteins during gliding motility and invasion remain uncertain, they are thought to maintain the cohesion and integrity of the pellicle during zoite gliding and invasion, especially via GAP45 that spans the entire space between the plasma membrane and the IMC and anchors the motor complex at the IMC (Figure 1A) [31], [32].
The motor is still typically viewed as linear, i.e., as “linear arrangements of transmembrane proteins transducing the force generated by the actin-myosin motor and posteriorly capped,” as originally proposed by King [2]. However, recent studies using biophysical approaches to measure the force Plasmodium sporozoites exert on the surface during gliding indicate that zoite movement is not continuous, as predicted by the linear motor, but follows a stick-and-slip pattern [33]. This involves, in addition to backward capping of adhesion proteins, the formation/disengagement of adhesion sites at the front and rear ends of the zoite. Interestingly, TRAP appears to have a key role in the release of adhesion sites, not in retrograde capping (in stick but not in slip), while actin is important for both processes. Moreover, sporozoites lacking the motor-binding TRAP-like protein (TLP), which glide less efficiently by more frequently detaching from the substrate, were complemented by the addition of actin stabilizing drugs [34]. These data illustrate the complex bases of apicomplexan gliding, which may be more akin to crawling of mammalian cells than previously anticipated.
What Is in the Junction?
Zoite-specific proteins?
Numerous proteins in Plasmodium merozoites have been described as being released from apical organelles, important for invasion and found at the junction. This is the case of several members of the micronemal erythrocyte binding like (EBL) family of proteins, such as Plasmodium falciparum erythrocyte-binding antigen 175 (EBA175) [35] and P. knowlesi Duffy binding protein (DBP) [36]. This is also the case of several rhoptry proteins, including the P. falciparum reticulocyte-binding like (RBL) homologue PfRh1 [37], PfRh2a [38], and PfRh5 [39], and of the rhoptry-associated leucine zipper-like protein 1 (RALP1) [40]. However, function is difficult to assess in the Plasmodium merozoite, typically relying on antibody inhibition and negative transfection experiments. In Toxoplasma, one member of the micronemal MIC family of proteins, MIC8, was shown to be specifically crucial for junction formation [41]. Importantly, none of these proteins is conserved across the phylum and most are stage-specific. If these proteins are indeed part of the junction, the latter might then be at least in part zoite-specific. If instead the junction is composed of a molecular core conserved across apicomplexans, then these stage-specific proteins may constitute adaptations to particular zoite-host cell combinations.
Motor-binding proteins?
Since the junction is viewed as a traction point for the motor, other candidates for junction components were the transmembrane proteins involved in gliding motility, i.e., the TRAP family of proteins including TRAP, TLP, and TRAP-related protein (TREP) in the Plasmodium sporozoite and MIC2 in the Toxoplasma tachyzoite [42]. However, there is no evidence that any of these proteins participates specifically at the junction during host cell invasion. In the Toxoplasma tachyzoite, inactivation of MIC2 impairs but does not preclude motility or invasion [43] and MIC2 is not specifically enriched at the junction during tachyzoite internalization. The hypothesis that TRAP/MIC2 might play a role as junction components was also favored by the presence in their extracellular domains of one or more A domains of von Willebrand factor, which are homologous to integrin I domains and thus potential ligands of host cell surface receptors [44]. However, the A domains of the TRAP family member CS and TRAP-related protein (CTRP), expressed by the Plasmodium motile ookinete stage that does not invade host cells, were shown to be important for gliding motility [45]. Together, these data favor the view that the TRAP family of proteins is involved in gliding motility, but not specifically for host cell entry.
AMA1-RON complexes?
In 2005, two papers identified a set of parasite proteins in Toxoplasma tachyzoites, called rhoptry neck proteins (RON), which specifically marked the constriction around invading zoites in a ring-like manner (Figure 1B) [46], [47]. Later, a ring-like staining of RON proteins was also shown in invading Plasmodium merozoites [48]. It was additionally found that in tachyzoite extracts, several RON proteins formed a complex with apical membrane antigen 1 (AMA1) [46], a transmembrane protein first identified in Plasmodium merozoites [49] and a leading malaria vaccine candidate. In humans, evidence suggests that AMA1 is an important target of naturally acquired protective antibodies preventing merozoite invasion of erythrocytes [50]. AMA1 vaccines have demonstrated protective efficacy in rodent and simian models against blood-stage challenge with the homologous strain [51], although human vaccine trials using AMA1 have shown poor efficacy so far [52].
Both AMA1 and RON proteins are conserved in the apicomplexan phylum and the AMA1-RON complex has now been detected in extracts from Toxoplasma tachyzoites and sporozoites [53], Plasmodium merozoites of various species [54], and Neospora [55]. Several independent lines of evidence have first favored the view that the AMA1-RON complex might constitute the building block of the junction. (i) The ectodomain of AMA1 binds RON2 [56], [57], which inserts into the host cell membrane [58] and is thought to bind to the cell cortical cytoskeleton via other RON proteins, a view recently strengthened by the observation that Toxoplasma RON4 may bind host cell tubulin [59]. (ii) The crystal structure of the AMA1–RON2 interaction in Toxoplasma [60] and Plasmodium [61] reveals a conserved RON2 loop that inserts deep into a hydrophobic groove in AMA1, suggesting that it might withstand mechanical forces and act as the traction point for the zoite motor. (iii) Antibodies or peptides that inhibit the AMA1–RON2 interaction reduce host cell invasion by Toxoplasma tachyzoites and Plasmodium merozoites [62]–[65]. These results clearly pointed to the view that cross-membrane AMA1-RON2 complexes shaped the junction for zoite internalization [66], [67] (Figure 1B). Consequently, the conserved AMA1–RON2 interaction has sparked much interest as a broad target for intervention against apicomplexan parasites [68], [69].
AMA1-Dependent Attachment and AMA1-Independent Internalization
The development of conditional mutagenesis techniques in Toxoplasma [14], [70] and Plasmodium [71] has allowed the addressing of the functions of AMA1 and the RON complex (Table 1). All attempts to directly inactivate either RON4 or RON2 in Toxoplasma and Plasmodium have failed so far. P. berghei RON4 conditional sporozoites, obtained by Flp/FRT-mediated recombination, are unable to invade hepatocytes [72]. P. berghei RON2 conditional sporozoites, obtained by a promoter swap strategy, are unable to invade mosquito salivary gland cells [73]. Toxoplasma gondii RON5 and RON2 conditional tachyzoites, generated with a Tet-repressible promoter, are drastically impaired in invasion [74], [75]. Therefore, the RON proteins appear to play crucial roles during host cell invasion by all zoites.
Tab. 1. Mutants of interest in studies on host cell invasion by apicomplexans. 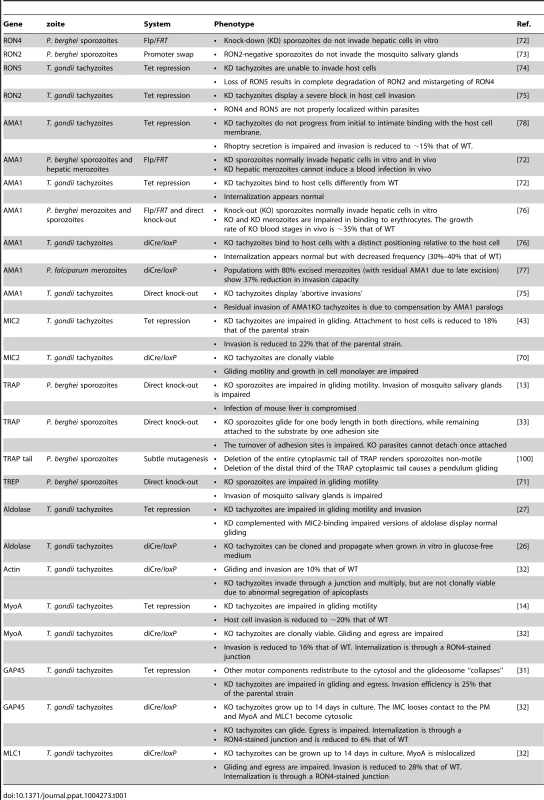
In contrast, all AMA1 knock-down (AMA1KD) or knock-out (AMA1KO) zoites constructed are still invasive. AMA1KD P. berghei sporozoites invade hepatocytes 3-fold better than the wild type (WT) in vitro and in vivo [72], and AMA1KO Toxoplasma tachyzoites and P. berghei merozoites are internalized into host cells indistinguishably from the WT, i.e. systematically form normal RON rings and are internalized at normal speed [72], [76]. Nonetheless, AMA1KO Toxoplasma tachyzoites and P. berghei merozoites display reduced invasion efficiency [72], [76], [77], along with a major impairment in host cell attachment [72], [76]. A recent report suggested that the adhesion defect of AMA1KO tachyzoites might be secondary to a failure to form a normal junction leading to parasite detachment from the cell, based on immunofluorescence (IF) assays of rhoptry secretion [75]. However, what causes rhoptry secretion is still unknown and no direct evidence was provided that AMA1KO tachyzoites formed an abnormal junction before detaching. AMA1KO tachyzoites observed by real-time imaging did not display abortive invasions and their attachment defect (upright instead of flattened positioning relative to the cell surface) concerned the entire population, irrespective of invasion [72], [76]. In Plasmodium, AMA1KO merozoites were 15-fold less adhesive to erythrocytes than controls after only 3 minutes incubation, which cannot be accounted for by failed invasions given that merozoite invasion efficiency is less than 5% [76]. A primary role of AMA1 in zoite binding to host cells is also in agreement with earlier work showing that AMA1 mediates attachment of Toxoplasma tachyzoites [78] and Plasmodium merozoites [79] to their respective host cells, and that Plasmodium AMA1 binds erythrocytes [80]–[82] and to the erythrocyte membrane receptor Kx [83].
Recent work revealed that the Toxoplasma genome encodes paralogs of AMA1 and RON2 in specific combinations. In the tachyzoite, RON2 interacts with AMA1 or AMA2 and RON2L1 with AMA4 [75], while in the sporozoite RON2L2 interacts with AMA3 in a manner mutually exclusive with tachyzoite paralogs [84]. Therefore, the hypothesis was raised that AMA1 paralogs might account for the residual invasive capacity of AMA1KO tachyzoites [75], [84]. In agreement with this, the latter were found, during parasite selection, to up-regulate AMA2 as well as the RON2L1–AMA4 pair [75]. However, evidence that these paralogs act at and/or structure the junction is lacking, and the weaker affinity of RON2 for AMA2 compared with AMA1 [75] is at odds with the fully efficient internalization of AMA1KO [76] (supposedly mediated by AMA2). In Plasmodium, the compensation theory appears particularly unlikely. The AMA1KD Flp/FRT sporozoites undergo AMA1 excision after parasite selection and yet are 100% invasive [76]. Additionally, Plasmodium expresses no AMA1 or RON2 paralog. The protein most closely related to AMA1 is the trans-membrane protein MAEBL, with which it shares the presence of a cysteine-rich domain but differs by an unrelated cytoplasmic tail and the absence of RON2-binding ability. MAEBL was shown by gene targeting in both P. berghei [85] and P. falciparum [86] sporozoites to function as a stage-specific adhesion; it mediates oocyst sporozoite binding to the mosquito salivary glands, but not internalization into hepatocytes. This further suggests that AMA1, its paralogs in Toxoplasma, and MAEBL in Plasmodium form a family of stage-specific, host cell–binding proteins.
Current Hypotheses
If AMA1 primarily mediates zoite intimate binding to host cell surfaces, irrespective of RON2 interaction and junction assembly, what could be the role of the conserved and therefore important AMA1–RON2 interaction? AMA1 might still bind to RON2, possibly to help further stabilize the zoite prior to internalization, although direct evidence for such a step is still lacking. Alternatively, AMA1–RON2 interactions might serve to process AMA1 at the junction during internalization of the AMA1-covered zoite. For example, interaction with RON2 might serve to disengage the AMA1–host cell receptor interaction and help the zoite slide free inside the PV, separated from the vacuole membrane. In agreement with this, RON2 binding induces conformational changes in AMA1 [60], which might impact AMA1 processing by the substilisin-like protease SUB2 [87] or intramembrane rhomboids [88]. Likewise, RON2L2 binding alters AMA3, including allosterically in its membrane-proximal domain [84]. Such AMA processing function of the AMA–RON2 interactions would be dispensable for internalization and yet block invasion if perturbed, reconciling the inhibition and genetic data.
The contribution of the apparently essential RON proteins is also unclear. They might be structural components of the junction, by linking it to the host cell cytoskeleton (Figure 1C, hypothesis 1), or might not be part of the force-transducing link (Figure 1C, hypothesis 2). Perhaps favoring the latter, the RON proteins are present in apicomplexans that are not known to form a junction, like Theileria [89], which raises the possibility that another zoite–host cell interaction might structure the junction, possibly involving host cell receptor(s). In any hypothesis, the junction constitutes a traction point for zoite internalization into the host cell.
Motor-Independent Entry
The Toxoplasma tachyzoite is ideal to study zoite invasion, not just due to the frequency of observable invasion events but also the genetic tractability of the parasite. The use of a transcriptional regulation system based on artificial Tet-transactivators (TATi) allowed the generation of knock-down mutants and the functional dissection of individual components of the motor (Table 1). As already said, knocking down MIC2 [43] or MyoA [14] does not result in a complete block in host cell invasion. Even knocking down GAP45, while leading to the detachment of the IMC from the plasma membrane (PM) and the release of the motor complex in the cytosol, does not abolish host cell entry [31]. In contrast, as mentioned above, a knock-down for MIC8 does not affect gliding motility but completely blocks host cell invasion due to an inability to form a junction [41]. These partial phenotypes were typically explained by the leakiness of the Tet-inducible system, but were also a hint that the motor might not be essential for host cell invasion.
The current adaptation of a conditional recombination system based on dimerizable Cre has allowed the construction of a series of tachyzoite mutants completely lacking individual components of the motor complex, including MyoA, GAP45, MLC1, and Act1 [32], [70]. All of these mutants are affected in invasion efficiency but retain some invasive capacity (Table 1). Strikingly, tachyzoites devoid of MyoA, MLC1, or Act1, which is a single copy gene in Toxoplasma, can invade host cells through a junction [32], demonstrating that at least junction formation is independent of connection to the motor. Moreover, GAP45KO tachyzoites, in which the IMC detaches from the parasite membrane and MyoA and MLC1 become cytosolic, remain motile and also invade [32]. These genetic data suggest that tachyzoites can move and enter host cells without a functional motor. However, whether this motor-independent entry pathway is the normal pathway used by the WT, or an alternative pathway discernable only when the motor is not functional, remains to be seen.
How could tachyzoites with a deficient actin-myosin motor invade host cells? Until recently, actin polymerization and actin-myosin contraction were thought to underlie force generation during movement. However, this view is currently being challenged by new models, in which hydrodynamic forces generate changes in cell shape during motility [90]. Indeed, there is mounting evidence that osmotic pressure and hydrodynamic fluids are critical for motility of amoeboid cells, while the actin-myosin system is critical for the formation and release of attachment sites and associated traction forces [91]. A recent report demonstrates the poroelastic nature of the cytosol [92], where force can be generated by differences in hydrodynamic pressure that can be higher in one part of a cell than another, leading to tension. This pressure can be generated by actin-myosin activity or by the localized activation of osmogenic ion transporters in the plasma membrane [90]. In agreement with such a model, Na+/H+ antiporters have been implicated in invasion and egress of host cells by Toxoplasma tachyzoites [93]–[95], and monovalent ion concentrations have been involved in gliding motility and host cell invasion efficiencies in Toxoplasma [96], [97]. Based on this, a gelsolation model for gliding motility and zoite internalization, in which the acto-myosin system of the parasite is required as a clutch for force transmission but not for the generation of the force itself, has recently been proposed [32].
Another possibility that cannot be excluded is that the force for parasite internalization might originate from the host cell. Theileria sporozoites and merozoites, which lack an IMC and subpellicular microtubules and are not motile, invade host cells in any orientation, without a junction, and by a mechanism of circumferential zippering of parasite and host cell membranes [89]. Others, like Cryptosporidium sporozoites, are motile but rest on the host cell surface and induce the formation of host cell membrane folds that progressively encapsulate the “epicellular parasite” inside the PV [98]. Interestingly, these membrane protrusions recruit a host cell Na+/glucose cotransporter and aquaporin 1, which generate localized water influx and are required for parasite invasion [99].
If zoite internalization is powered by the host cell or by hydrodynamic forces, then the junction would no longer be connected to the parasite motor. In this case, the junction might serve as a membrane “seal,” possibly regulating protein processing upon entry into the PV, facilitating membrane dynamics, fluidity and curvature, and/or ensuring correct formation of the PV (Figure 1C, hypothesis 3).
Conclusions
New mutagenesis data question the current view that apicomplexan zoites invade host cells by a unique pathway involving their motor and AMA1–RON2 interactions as traction points at the junction. AMA1 appears not to be involved in junction function and the RON proteins are the only parasite proteins known to functionally associate with the junction. Whether the RON complex transmits force at the junction remains uncertain, though the evidence of a link between the RON proteins and the host cell cytoskeleton points to this direction. The conservation of the RON proteins in apicomplexans and of their essential role in invasion suggests a conserved molecular kit for junction formation. Still, there is no definitive evidence for such a conserved apicomplexan junction core, and at least part of its structure might be stage-specific. Whether the zoite provides all pieces of the junction, or whether the host cell also provides receptors, possibly located in specific microdomains, is also unclear.
Moreover, it now appears that the force required for gliding motility and host cell entry might, at least in Toxoplasma, be generated in a motor-independent manner. Whether this holds true for other apicomplexan genera remains to be seen. In any event, apicomplexan invasion of host cells appears more complex than previously thought. Dissecting the process and its possible versatility will require establishing novel experimental approaches, including biophysical, and investigating unconventional force-generation means in zoites, as well as in host cells, that might facilitate, or even replace, the activity of the parasite motor.
Zdroje
1. AikawaM, MillerLH, JohnsonJ, RabbegeJ (1978) Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 77 : 72–82.
2. KingCA (1988) Cell motility of sporozoan protozoa. Parasitol Today 4 : 315–319.
3. RussellDG, SindenRE (1981) The role of the cytoskeleton in the motility of coccidian sporozoites. J Cell Sci 50 : 345–359.
4. RussellDG (1983) Host cell invasion by Apicomplexa: an expression of the parasite's contractile system? Parasitology 87 (Pt 2): 199–209.
5. SchwartzmanJD, PfefferkornER (1983) Immunofluorescent localization of myosin at the anterior pole of the coccidian, Toxoplasma gondii. J Protozool 30 : 657–661.
6. PinderJC, FowlerRE, DluzewskiAR, BannisterLH, LavinFM, et al. (1998) Actomyosin motor in the merozoite of the malaria parasite, Plasmodium falciparum: implications for red cell invasion. J Cell Sci 111 (Pt 13): 1831–1839.
7. DobrowolskiJM, SibleyLD (1996) Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84 : 933–939.
8. MorisakiJH, HeuserJE, SibleyLD (1995) Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci 108 (Pt 6): 2457–2464.
9. GonzalezV, CombeA, DavidV, MalmquistNA, DelormeV, et al. (2009) Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe 5 : 259–272.
10. Delorme-WalkerV, AbrivardM, LagalV, AndersonK, PerazziA, et al. (2012) Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci 125 : 4333–4342.
11. DelormeV, CaylaX, FaureG, GarciaA, TardieuxI (2003) Actin dynamics is controlled by a casein kinase II and phosphatase 2C interplay on Toxoplasma gondii Toxofilin. Mol Biol Cell 14 : 1900–1912.
12. MorrissetteNS, MurrayJM, RoosDS (1997) Subpellicular microtubules associate with an intramembranous particle lattice in the protozoan parasite Toxoplasma gondii. J Cell Sci 110 (Pt 1): 35–42.
13. SultanAA, ThathyV, FrevertU, RobsonKJ, CrisantiA, et al. (1997) TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell 90 : 511–522.
14. MeissnerM, SchluterD, SoldatiD (2002) Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298 : 837–840.
15. JewettTJ, SibleyLD (2003) Aldolase forms a bridge between cell surface adhesins and the actin cytoskeleton in apicomplexan parasites. Mol Cell 11 : 885–894.
16. BergmanLW, KaiserK, FujiokaH, CoppensI, DalyTM, et al. (2003) Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J Cell Sci 116 : 39–49.
17. SchulerH, MatuschewskiK (2006) Regulation of apicomplexan microfilament dynamics by a minimal set of actin-binding proteins. Traffic 7 : 1433–1439.
18. SkillmanKM, DiraviyamK, KhanA, TangK, SeptD, et al. (2011) Evolutionarily divergent, unstable filamentous actin is essential for gliding motility in apicomplexan parasites. PLoS Pathog 7: e1002280.
19. MehtaS, SibleyLD (2011) Actin depolymerizing factor controls actin turnover and gliding motility in Toxoplasma gondii. Mol Biol Cell 22 : 1290–1299.
20. BaumJ, TonkinCJ, PaulAS, RugM, SmithBJ, et al. (2008) A malaria parasite formin regulates actin polymerization and localizes to the parasite-erythrocyte moving junction during invasion. Cell Host Microbe 3 : 188–198.
21. DaherW, PlattnerF, CarlierMF, Soldati-FavreD (2010) Concerted action of two formins in gliding motility and host cell invasion by Toxoplasma gondii. PLoS Pathog 6: e1001132.
22. AngrisanoF, RiglarDT, SturmA, VolzJC, DelvesMJ, et al. (2012) Spatial localisation of actin filaments across developmental stages of the malaria parasite. PLoS ONE 7: e32188.
23. MehtaS, SibleyLD (2010) Toxoplasma gondii actin depolymerizing factor acts primarily to sequester G-actin. J Biol Chem 285 : 6835–6847.
24. SkillmanKM, DaherW, MaCI, Soldati-FavreD, SibleyLD (2012) Toxoplasma gondii profilin acts primarily to sequester G-actin while formins efficiently nucleate actin filament formation in vitro. Biochemistry 51 : 2486–2495.
25. PlattnerF, YarovinskyF, RomeroS, DidryD, CarlierMF, et al. (2008) Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe 3 : 77–87.
26. ShenB, SibleyLD (2014) Toxoplasma aldolase is required for metabolism but dispensable for host-cell invasion. Proc Natl Acad Sci U S A 111 : 3567–3572.
27. StarnesGL, CoinconM, SyguschJ, SibleyLD (2009) Aldolase is essential for energy production and bridging adhesin-actin cytoskeletal interactions during parasite invasion of host cells. Cell Host Microbe 5 : 353–364.
28. PomelS, LukFC, BeckersCJ (2008) Host cell egress and invasion induce marked relocations of glycolytic enzymes in Toxoplasma gondii tachyzoites. PLoS Pathog 4: e1000188.
29. GaskinsE, GilkS, DeVoreN, MannT, WardG, et al. (2004) Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J Cell Biol 165 : 383–393.
30. GilkSD, GaskinsE, WardGE, BeckersCJ (2009) GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot Cell 8 : 190–196.
31. FrenalK, PolonaisV, MarqJB, StratmannR, LimenitakisJ, et al. (2010) Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe 8 : 343–357.
32. EgarterS, AndenmattenN, JacksonAJ, WhitelawJA, PallG, et al. (2014) The Toxoplasma Acto-MyoA Motor Complex Is Important but Not Essential for Gliding Motility and Host Cell Invasion. PLoS ONE 9: e91819.
33. MunterS, SabassB, Selhuber-UnkelC, KudryashevM, HeggeS, et al. (2009) Plasmodium sporozoite motility is modulated by the turnover of discrete adhesion sites. Cell Host Microbe 6 : 551–562.
34. HellmannJK, PerschmannN, SpatzJP, FrischknechtF (2013) Tunable substrates unveil chemical complementation of a genetic cell migration defect. Adv Healthc Mater 2 : 1162–1169.
35. GilbergerTW, ThompsonJK, ReedMB, GoodRT, CowmanAF (2003) The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking. J Cell Biol 162 : 317–327.
36. SinghAP, OzwaraH, KockenCH, PuriSK, ThomasAW, et al. (2005) Targeted deletion of Plasmodium knowlesi Duffy binding protein confirms its role in junction formation during invasion. Mol Microbiol 55 : 1925–1934.
37. GunalanK, GaoX, YapSS, HuangX, PreiserPR (2013) The role of the reticulocyte-binding-like protein homologues of Plasmodium in erythrocyte sensing and invasion. Cell Microbiol 15 : 35–44.
38. GunalanK, GaoX, LiewKJ, PreiserPR (2011) Differences in erythrocyte receptor specificity of different parts of the Plasmodium falciparum reticulocyte binding protein homologue 2a. Infect Immun 79 : 3421–3430.
39. BaumJ, ChenL, HealerJ, LopatickiS, BoyleM, et al. (2009) Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int J Parasitol 39 : 371–380.
40. ItoD, HasegawaT, MiuraK, YamasakiT, ArumugamTU, et al. (2013) RALP1 is a rhoptry neck erythrocyte-binding protein of Plasmodium falciparum merozoites and a potential blood-stage vaccine candidate antigen. Infect Immun 81 : 4290–4298.
41. KesslerH, Herm-GotzA, HeggeS, RauchM, Soldati-FavreD, et al. (2008) Microneme protein 8—a new essential invasion factor in Toxoplasma gondii. J Cell Sci 121 : 947–956.
42. MorahanBJ, WangL, CoppelRL (2009) No TRAP, no invasion. Trends in Parasitology 25 : 77–84.
43. HuynhMH, CarruthersVB (2006) Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog 2: e84.
44. SongG, KoksalAC, LuC, SpringerTA (2012) Shape change in the receptor for gliding motility in Plasmodium sporozoites. Proc Natl Acad Sci U S A 109 : 21420–21425.
45. RamakrishnanC, DessensJT, ArmsonR, PintoSB, TalmanAM, et al. (2011) Vital functions of the malarial ookinete protein, CTRP, reside in the A domains. Int J Parasitol 41 : 1029–1039.
46. AlexanderDL, MitalJ, WardGE, BradleyP, BoothroydJC (2005) Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog 1: e17.
47. LebrunM, MichelinA, El HajjH, PoncetJ, BradleyPJ, et al. (2005) The rhoptry neck protein RON4 re-localizes at the moving junction during Toxoplasma gondii invasion. Cell Microbiol 7 : 1823–1833.
48. RiglarDT, RichardD, WilsonDW, BoyleMJ, DekiwadiaC, et al. (2011) Super-resolution dissection of coordinated events during malaria parasite invasion of the human erythrocyte. Cell Host Microbe 9 : 9–20.
49. DeansJA, AldersonT, ThomasAW, MitchellGH, LennoxES, et al. (1982) Rat monoclonal antibodies which inhibit the in vitro multiplication of Plasmodium knowlesi. Clin Exp Immunol 49 : 297–309.
50. ThomasAW, TrapeJF, RogierC, GoncalvesA, RosarioVE, et al. (1994) High prevalence of natural antibodies against Plasmodium falciparum 83-kilodalton apical membrane antigen (PF83/AMA-1) as detected by capture-enzyme-linked immunosorbent assay using full-length baculovirus recombinant PF83/AMA-1. Am J Trop Med Hyg 51 : 730–740.
51. RemarqueEJ, FaberBW, KockenCH, ThomasAW (2008) Apical membrane antigen 1: a malaria vaccine candidate in review. Trends Parasitol 24 : 74–84.
52. LaurensMB, TheraMA, CoulibalyD, OuattaraA, KoneAK, et al. (2013) Extended safety, immunogenicity and efficacy of a blood-stage malaria vaccine in malian children: 24-month follow-up of a randomized, double-blinded phase 2 trial. PLoS ONE 8: e79323.
53. PoukchanskiA, FritzHM, TonkinML, TreeckM, BoulangerMJ, et al. (2013) Toxoplasma gondii sporozoites invade host cells using two novel paralogues of RON2 and AMA1. PLoS ONE 8: e70637.
54. NarumDL, NguyenV, ZhangY, GlenJ, ShimpRL, et al. (2008) Identification and characterization of the Plasmodium yoelii PyP140/RON4 protein, an orthologue of Toxoplasma gondii RON4, whose cysteine-rich domain does not protect against lethal parasite challenge infection. Infect Immun 76 : 4876–4882.
55. StraubKW, ChengSJ, SohnCS, BradleyPJ (2009) Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell Microbiol 11 : 590–603.
56. LamarqueM, BesteiroS, PapoinJ, RoquesM, Vulliez-Le NormandB, et al. (2011) The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog 7: e1001276.
57. TylerJS, BoothroydJC (2011) The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathog 7: e1001282.
58. BesteiroS, MichelinA, PoncetJ, DubremetzJF, LebrunM (2009) Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5: e1000309.
59. TakemaeH, SugiT, KobayashiK, GongH, IshiwaA, et al. (2013) Characterization of the interaction between Toxoplasma gondii rhoptry neck protein 4 and host cellular beta-tubulin. Sci Rep 3 : 3199.
60. TonkinML, RoquesM, LamarqueMH, PugniereM, DouguetD, et al. (2011) Host cell invasion by apicomplexan parasites: insights from the co-structure of AMA1 with a RON2 peptide. Science 333 : 463–467.
61. Vulliez-Le NormandB, TonkinML, LamarqueMH, LangerS, HoosS, et al. (2012) Structural and functional insights into the malaria parasite moving junction complex. PLoS Pathog 8: e1002755.
62. CollinsCR, Withers-MartinezC, HackettF, BlackmanMJ (2009) An inhibitory antibody blocks interactions between components of the malarial invasion machinery. PLoS Pathog 5: e1000273.
63. RichardD, MacRaildCA, RiglarDT, ChanJA, FoleyM, et al. (2010) Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem 285 : 14815–14822.
64. SrinivasanP, BeattyWL, DioufA, HerreraR, AmbroggioX, et al. (2011) Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci U S A 108 : 13275–13280.
65. SrinivasanP, YasgarA, LuciDK, BeattyWL, HuX, et al. (2013) Disrupting malaria parasite AMA1-RON2 interaction with a small molecule prevents erythrocyte invasion. Nat Commun 4 : 2261.
66. BaumJ, CowmanAF (2011) Biochemistry. Revealing a parasite's invasive trick. Science 333 : 410–411.
67. ShenB, SibleyLD (2012) The moving junction, a key portal to host cell invasion by apicomplexan parasites. Curr Opin Microbiol 15 : 449–455.
68. MacraildCA, AndersRF, FoleyM, NortonRS (2011) Apical membrane antigen 1 as an anti-malarial drug target. Curr Top Med Chem 11 : 2039–2047.
69. MillerLH, AckermanHC, SuXZ, WellemsTE (2013) Malaria biology and disease pathogenesis: insights for new treatments. Nat Med 19 : 156–167.
70. AndenmattenN, EgarterS, JacksonAJ, JullienN, HermanJP, et al. (2013) Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nat Methods 10 : 125–127.
71. CombeA, GiovanniniD, CarvalhoTG, SpathS, BoissonB, et al. (2009) Clonal conditional mutagenesis in malaria parasites. Cell Host Microbe 5 : 386–396.
72. GiovanniniD, SpathS, LacroixC, PerazziA, BargieriD, et al. (2011) Independent roles of apical membrane antigen 1 and rhoptry neck proteins during host cell invasion by apicomplexa. Cell Host Microbe 10 : 591–602.
73. Murata E, Tokunaga N, Tachibana M, Tsuboi T, Torii M, et al. (2012) The investigation of the mechanism how malaria sporozoites invade salivary glands. Molecular Approaches to Malaria Meeting 2012. Abstract Book. Lorne, Australia.
74. BeckJR, ChenAL, kimEW, BradleyPJ (2014) RON5 is critical for organization and function of the Toxoplasma moving junction complex. PLoS Pathog 10: e1004025.
75. LamarqueMH, RoquesM, Kong-HapM, TonkinML, RugarabamuG, et al. (2014) Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat Commun 5 : 4098.
76. BargieriDY, AndenmattenN, LagalV, ThibergeS, WhitelawJA, et al. (2013) Apical membrane antigen 1 mediates apicomplexan parasite attachment but is dispensable for host cell invasion. Nat Commun 4 : 2552.
77. YapA, AzevedoMF, GilsonPR, WeissGE, O'NeillMT, et al. (2014) Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell Microbiol 16 : 642–656.
78. MitalJ, MeissnerM, SoldatiD, WardGE (2005) Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell 16 : 4341–4349.
79. MitchellGH, ThomasAW, MargosG, DluzewskiAR, BannisterLH (2004) Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun 72 : 154–158.
80. FraserTS, KappeSH, NarumDL, VanBuskirkKM, AdamsJH (2001) Erythrocyte-binding activity of Plasmodium yoelii apical membrane antigen-1 expressed on the surface of transfected COS-7 cells. Mol Biochem Parasitol 117 : 49–59.
81. UrquizaM, SuarezJE, CardenasC, LopezR, PuentesA, et al. (2000) Plasmodium falciparum AMA-1 erythrocyte binding peptides implicate AMA-1 as erythrocyte binding protein. Vaccine 19 : 508–513.
82. ValbuenaJ, RodriguezL, VeraR, PuentesA, CurtidorH, et al. (2006) Synthetic peptides from Plasmodium falciparum apical membrane antigen 1 (AMA-1) specifically interacting with human hepatocytes. Biochimie 88 : 1447–1455.
83. KatoK, MayerDC, SinghS, ReidM, MillerLH (2005) Domain III of Plasmodium falciparum apical membrane antigen 1 binds to the erythrocyte membrane protein Kx. Proc Natl Acad Sci U S A 102 : 5552–5557.
84. PoukchanskiA, FritzHM, TonkinML, TreeckM, BoulangerMJ, et al. (2013) Toxoplasma gondii sporozoites invade host cells using two novel paralogues of RON2 and AMA1. PLoS ONE 8: e70637.
85. KariuT, YudaM, YanoK, ChinzeiY (2002) MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med 195 : 1317–1323.
86. SaenzFE, BaluB, SmithJ, MendoncaSR, AdamsJH (2008) The transmembrane isoform of Plasmodium falciparum MAEBL is essential for the invasion of Anopheles salivary glands. PLoS ONE 3: e2287.
87. OlivieriA, CollinsCR, HackettF, Withers-MartinezC, MarshallJ, et al. (2011) Juxtamembrane shedding of Plasmodium falciparum AMA1 is sequence independent and essential, and helps evade invasion-inhibitory antibodies. PLoS Pathog 7: e1002448.
88. ParussiniF, TangQ, MoinSM, MitalJ, UrbanS, et al. (2012) Intramembrane proteolysis of Toxoplasma apical membrane antigen 1 facilitates host-cell invasion but is dispensable for replication. Proc Natl Acad Sci U S A 109 : 7463–7468.
89. ShawMK (2003) Cell invasion by Theileria sporozoites. Trends Parasitol 19 : 2–6.
90. MitchisonTJ, CharrasGT, MahadevanL (2008) Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin Cell Dev Biol 19 : 215–223.
91. KerenK, YamPT, KinkhabwalaA, MogilnerA, TheriotJA (2009) Intracellular fluid flow in rapidly moving cells. Nat Cell Biol 11 : 1219–1224.
92. MoeendarbaryE, ValonL, FritzscheM, HarrisAR, MouldingDA, et al. (2013) The cytoplasm of living cells behaves as a poroelastic material. Nat Mater 12 : 253–261.
93. FranciaME, WicherS, PaceDA, SullivanJ, MorenoSN, et al. (2011) A Toxoplasma gondii protein with homology to intracellular type Na(+)/H(+) exchangers is important for osmoregulation and invasion. Exp Cell Res 317 : 1382–1396.
94. KarasovAO, BoothroydJC, ArrizabalagaG (2005) Identification and disruption of a rhoptry-localized homologue of sodium hydrogen exchangers in Toxoplasma gondii. Int J Parasitol 35 : 285–291.
95. ArrizabalagaG, RuizF, MorenoS, BoothroydJC (2004) Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. J Cell Biol 165 : 653–662.
96. EndoT, TokudaH, YagitaK, KoyamaT (1987) Effects of extracellular potassium on acid release and motility initiation in Toxoplasma gondii. J Protozool 34 : 291–295.
97. EndoT, YagitaK (1990) Effect of extracellular ions on motility and cell entry in Toxoplasma gondii. J Protozool 37 : 133–138.
98. ValigurovaA, JirkuM, KoudelaB, GelnarM, ModryD, et al. (2008) Cryptosporidia: epicellular parasites embraced by the host cell membrane. Int J Parasitol 38 : 913–922.
99. ChenXM, O'HaraSP, HuangBQ, SplinterPL, NelsonJB, et al. (2005) Localized glucose and water influx facilitates Cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc Natl Acad Sci U S A 102 : 6338–6343.
100. KappeS, BrudererT, GanttS, FujiokaH, NussenzweigV, et al. (1999) Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J Cell Biol 147 : 937–943.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



