-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStructural Insights into Viral Determinants of Nematode Mediated
Transmission
Many animal and plant viruses rely on vectors for their transmission from host to
host. Grapevine fanleaf virus (GFLV), a picorna-like virus from
plants, is transmitted specifically by the ectoparasitic nematode
Xiphinema index. The icosahedral capsid of GFLV, which
consists of 60 identical coat protein subunits (CP), carries the determinants of
this specificity. Here, we provide novel insight into GFLV transmission by
nematodes through a comparative structural and functional analysis of two GFLV
variants. We isolated a mutant GFLV strain (GFLV-TD) poorly transmissible by
nematodes, and showed that the transmission defect is due to a glycine to
aspartate mutation at position 297 (Gly297Asp) in the CP. We next determined the
crystal structures of the wild-type GFLV strain F13 at 3.0 Å and of
GFLV-TD at 2.7 Å resolution. The Gly297Asp mutation mapped to an exposed
loop at the outer surface of the capsid and did not affect the conformation of
the assembled capsid, nor of individual CP molecules. The loop is part of a
positively charged pocket that includes a previously identified determinant of
transmission. We propose that this pocket is a ligand-binding site with
essential function in GFLV transmission by X. index. Our data
suggest that perturbation of the electrostatic landscape of this pocket affects
the interaction of the virion with specific receptors of the nematode's
feeding apparatus, and thereby severely diminishes its transmission efficiency.
These data provide a first structural insight into the interactions between a
plant virus and a nematode vector.
Published in the journal: . PLoS Pathog 7(5): e32767. doi:10.1371/journal.ppat.1002034
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002034Summary
Many animal and plant viruses rely on vectors for their transmission from host to
host. Grapevine fanleaf virus (GFLV), a picorna-like virus from
plants, is transmitted specifically by the ectoparasitic nematode
Xiphinema index. The icosahedral capsid of GFLV, which
consists of 60 identical coat protein subunits (CP), carries the determinants of
this specificity. Here, we provide novel insight into GFLV transmission by
nematodes through a comparative structural and functional analysis of two GFLV
variants. We isolated a mutant GFLV strain (GFLV-TD) poorly transmissible by
nematodes, and showed that the transmission defect is due to a glycine to
aspartate mutation at position 297 (Gly297Asp) in the CP. We next determined the
crystal structures of the wild-type GFLV strain F13 at 3.0 Å and of
GFLV-TD at 2.7 Å resolution. The Gly297Asp mutation mapped to an exposed
loop at the outer surface of the capsid and did not affect the conformation of
the assembled capsid, nor of individual CP molecules. The loop is part of a
positively charged pocket that includes a previously identified determinant of
transmission. We propose that this pocket is a ligand-binding site with
essential function in GFLV transmission by X. index. Our data
suggest that perturbation of the electrostatic landscape of this pocket affects
the interaction of the virion with specific receptors of the nematode's
feeding apparatus, and thereby severely diminishes its transmission efficiency.
These data provide a first structural insight into the interactions between a
plant virus and a nematode vector.Introduction
Efficient transmission from host to host by vectors is an important biological feature shared by many animal and plant viruses. Arthropods transmit many viruses to mammals and plants. Examples include highly pathogenic viruses such as Rift Valley fever virus, Dengue virus or Chikungunya virus, primarily transmitted to animals and humans by Aedes spp. mosquitoes [1], [2], Tick-borne encephalitis virus transmitted by ticks [3] or Sharka/plum pox virus disease affecting stone fruits and vectored by aphids. In animals, transmission by vectors is limited to some genera such as Alphavirus Flavivirus, Rhabdovirus or Reoviridae and requires a replication cycle in the vector [4]. In contrast, nearly all plant viruses depend on vectors for their transmission. Non-enveloped viruses - the vast the majority of all plant viruses - are generally specifically acquired by their vectors, but do not replicate in them [5], [6], [7], [8].
Over the years, virus transmission has gradually been recognized as a specific process but the molecular mechanisms governing the recognition between a virus and its vector are far from being unraveled. Comparative studies of transmissible and non-transmissible plant virus isolates have led to the identification of determinants in capsid proteins (CP) [9], [10], [11], [12]. In addition to the CP, some viruses require additional viral proteins referred to as helper components for their transmission by vectors (HC) [7], [8], [13]. HCs are viral proteins capable of engaging interactions with the viral CP and putative receptor molecules from the vector. Thus, they act as bridging molecules.
Various motifs in CPs or HCs required for transmission are described for a broad range of plant viruses, in particular members of the genera Potyvirus, Caulimovirus and Cucumovirus vectored by aphids. For example, the rod shaped potyviruses have DAG and PTK motifs in their CP and HC-pro, respectively [14], [15], [16]. In contrast, in the icosahedral Cucumber mosaic virus (CMV), the CP is the sole viral determinant of transmission [17]. There, the CP that folds into ß-barrel domains exposes a conserved and negatively charged βH-βI loop exposed at the surface of the virion to establish electrostatic interactions with components inside the aphid's mouthparts [18], [19]. In Cauliflower mosaic virus (CaMV), transmission necessitates two HC proteins named P2 and P3 in addition to the CP. Together these proteins form a transmissible viral complex whose assembly depends on interactions between coiled-coil domains [20], [21], [22] and components of the host plants [23]. This complex is thought to be specifically retained in the acrostyle, a specialized anatomical structure in the aphid stylet where virus receptor proteins accumulate [24], [25].
Less is known about the transmission by ectoparasitic nematodes of soil-borne viruses belonging to the genera Nepovirus and Tobravirus. In the rod-shaped tobraviruses, the partly unstructured C-terminal tail of the CP is necessary but not sufficient to promote transmission and other viral proteins may act as HC [26], [27], [28]. In nepoviruses, the CP that assembles into icosahedral particles is the sole viral determinant involved in transmission specificity, as shown for Grapevine fanleaf virus (GFLV) and Arabis mosaic virus (ArMV) which are transmitted by two different species of Xiphinema nematodes, X. index and X. diversicaudatum, respectively [29], [30]. Recently, a 3D homology model of GFLV based on the crystal structure of Tobacco ringspot virus (TRSV) [31], revealed the existence of a stretch of 11 amino acids within the BC loop of the B-domain that differs between GFLV and ArMV. The transmission of GFLV by X. index was abolished when this sequence was replaced by the corresponding region from ArMV. Hence, this loop has all the properties of a determinant for GFLV transmission [32].
The general feature that emerges from all these analyses is that transmission of non-circulative plant viruses involves well-defined and precise interactions between viral and vector molecules. In this respect, parallels can be established with virus-receptor interactions used by animal viruses to enter host cells [33]. However, our current knowledge of the vector-assisted transmission of animal or plant viruses lags far behind that of animal virus-receptor interaction whose details are known in some cases up to the atomic resolution. In the coming years the challenge will be to characterize the key molecules of the vectors engaged in transmission and to gain high-resolution structural insights into their interactions with the cognate viruses.
To understand the molecular details controlling virus-vector interactions, we have use the model pathosystem GFLV - X. index. Here, we have identified a GFLV variant (GFLV-TD) poorly transmitted by X. index that differs from its parent strain (GFLV-F13) by a single Gly297Asp mutation. Using X-Ray crystallography in combination with cryo-electron microscopy 3D reconstruction, we solved the crystal structures of GFLV-TD and GFLV-F13 at 2.7 Å and 3.0 Å resolution, respectively. These 3D structures highlighted the dramatic effect of a single amino acid substitution in GFLV transmission and helped identify a pocket at the virus surface with predicted function in the specific recognition of GFLV by X. index. Altogether, the presented results give a first structural insight into the molecular mechanism needed for the specific binding of a plant virus to its nematode vector.
Results
Identification and characterization of a GFLV variant defective in nematode transmission
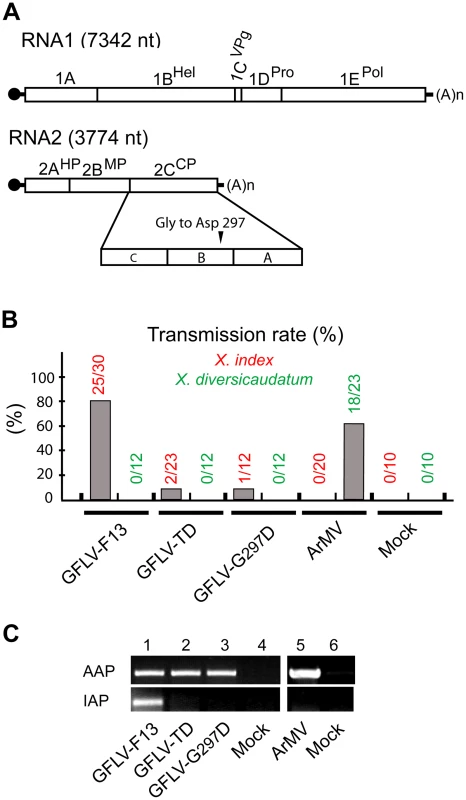
GFLV strain F13 (GFLV-F13) was first isolated from an infected grapevine in southern France in 1964 [34]. In agreement with its classification in the Nepovirus genus, it contains a bipartite, linear, single stranded positive sense RNA genome. RNA1 plays an essential role in replication and RNA2 is necessary for movement and encapsidation (Figure 1A). Ever since its isolation, GFLV-F13 was propagated by mechanical inoculation of the systemic herbaceous host Chenopodium quinoa. After four decades of successive passages onto C. quinoa, the nematode transmission of varied GFLV-F13 inocula was assessed. This led to the identification of a variant poorly transmitted by X. index named GFLV-TD (Figure 1B). Beside the defect in transmissibility, GFLV-TD was indistinguishable from its wild-type parental strain GFLV-F13 in terms of symptom development on C. quinoa, reactivity to GFLV antibodies in DAS-ELISA and virus purification yields (data no shown). Similarly, in transmission assays (Figure S1), no difference in the ability of X. index to ingest GFLV-F13 and GFLV-TD was detected by RT-PCR after a monthly acquisition access period (AAP) (Figure 1C, top panel). However, at the end of the inoculation access period (IAP), GFLV-TD was not detectable by RT-PCR in X. index (Figure 1C, bottom panel), suggesting that it is poorly or not retained by nematodes. These results were consistent with the transmission deficiency of GFLV-TD likely due to the paucity or incapacity of the virus to be retained by the vector at specific sites within its feeding apparatus.
Since the CP is the sole determinant required for GFLV transmission [29], [30], the GFLV-TD CP coding sequence was characterized by IC-RT-PCR and sequencing to identify potential amino acid mutations. A single Gly to Asp mutation at position 297 was found. To assess whether this mutation explained the deficiency in nematode transmission of GFLV-TD, it was introduced into the GFLV-F13 RNA2-encoded CP gene by site-directed mutagenesis of the corresponding cDNA infectious clone [35]. Similar to the natural GFLV-TD variant, the site-directed mutant, named GFLV-G297D, was poorly transmitted by X. index (Figure 1B). In addition, GFLV-G297D was not retained by the vector after the IAP, therefore mimicking GFLV-TD (Figure 1C). These results confirm the critical role of Gly297 in GFLV transmission efficiency.
GFLV-F13 and GFLV-TD structures
To determine their atomic structures, GFLV-TD and GFLV-F13 virions were crystallized as described [36]. Two crystal forms were obtained and analyzed (Table 1). The asymmetric unit of the GFLV-TD crystal (PDBid 2Y26) contains 20 CP subunits and that of GFLV-F13 contains 60 subunits, i.e. the entire virion. The structure of GFLV-TD was solved by molecular replacement using a cryo-electron microscopy model at 16.5 Å resolution (Figure S2) followed by solvent flattening, non crystallographic symmetry (NCS) averaging and refinement at 2.7 Å (Table 1). The complete GFLV-TD particle was generated by symmetry operations and used as a model to solve the structure of GFLV-F13 (PDBid 2Y7T, 2Y7U, 2Y7V) by molecular replacement at 3.0 Å (Table 1).
Tab. 1. Crystallographic analysis of GFLV particles. 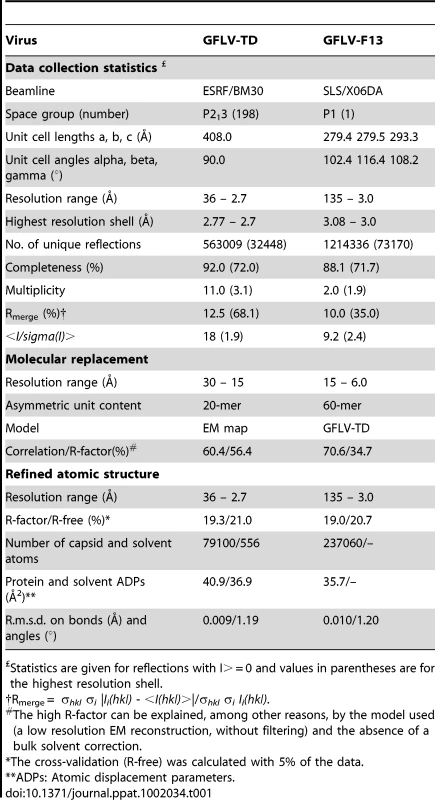
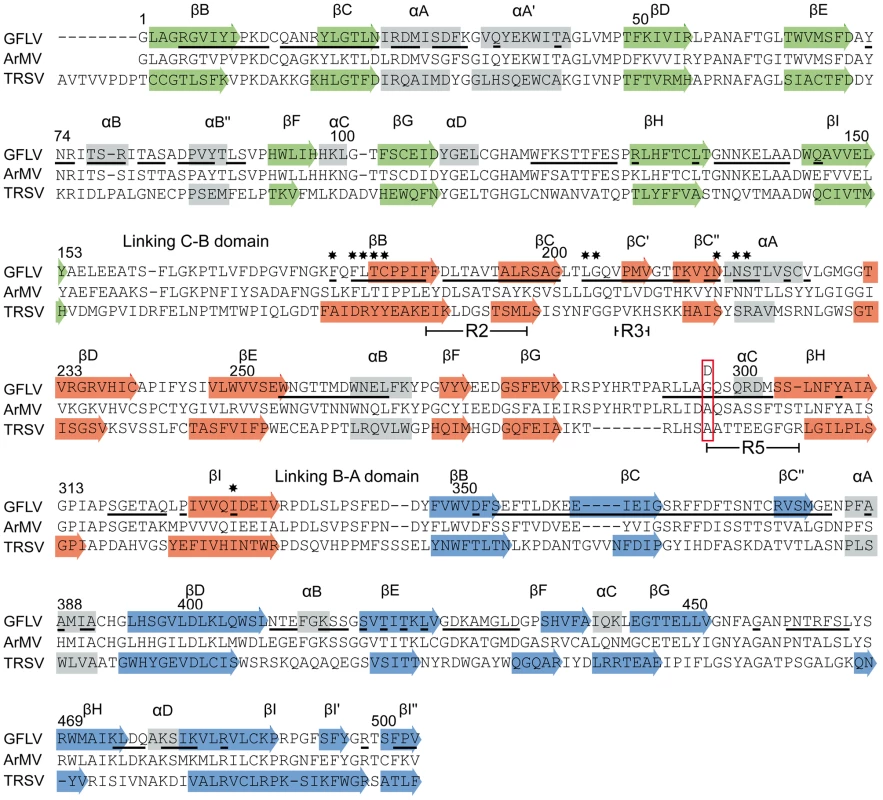
**ADPs: Atomic displacement parameters. In both cases, the icosahedral GFLV capsid is formed by 60 copies of the CP arranged according to a pseudo T = 3 symmetry (Figure 2A). The CP folds into three jelly-roll β sandwiches. To follow the TRSV nomenclature, the three jelly-roll domains were named C, B, and A from the N - to C - termini, respectively. Two linking peptides connect the C-B and B-A domains (Figure 2B). The B and C domains clustered at the 3-fold axis. Five A-domains organized around the 5-fold axis form a protrusion at the capsid's surface (Figure 2A). The particle outer radius seen down the 5-fold, 3-fold and 2-fold axes is 155 Å, 141 Å and 130 Å, respectively (Figure 2C). The A-domain deviates most from the β sandwich fold of the other domains with an extensive insertion between the βC and the βD strands that comprises one additional strand (Figure 3). This is in contrast with the capsid structures of closely related comoviruses where two strands are added at this position [37]. Along each 5-fold axis, i.e. the summit of the pentamers, a channel with an inner diameter of 7.1 Å contains an additional electron density that may be attributed to an ion (Figure 2D). However, the distance to the neighboring Lys atoms is incompatible with direct hydrogen or ionic bonding (Figure 2D), and suggests, in agreement with the presence of surrounding density peaks, that the ion is linked via intermediate water molecules.
Fig. 2. Crystal structures of GFLV-F13 and GFLV-TD. 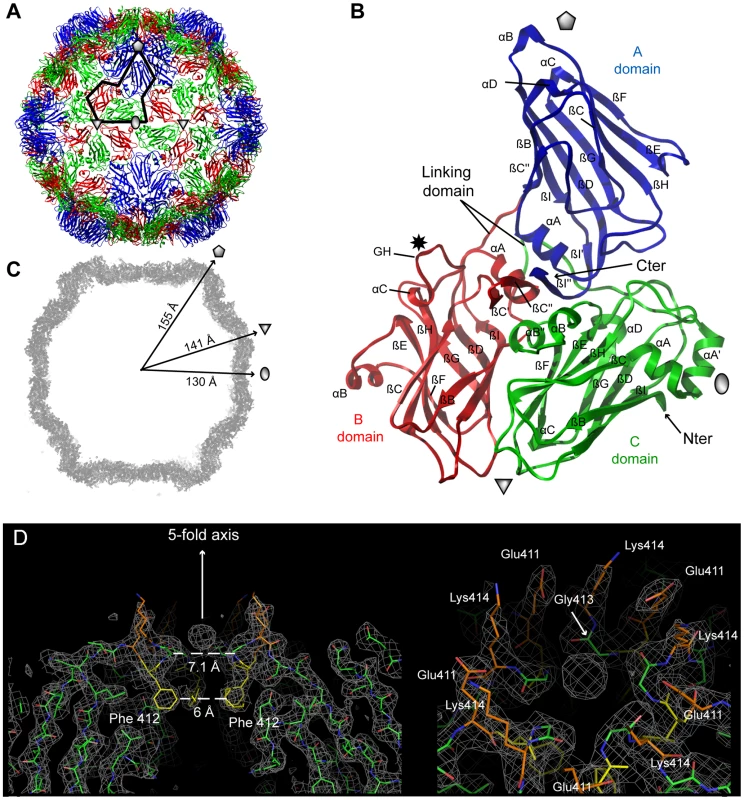
The structural variability of CP subunits within a capsid was very low. The average root-mean-square distances (r.m.s.d.) of pair-wise CP superposition were 0.07±0.01 Å and 0.09±0.02 Å for GFLV-TD (20 CPs) and for GFLV-F13 (60 CPs), respectively (Table S1). The superposition of the GFLV-F13 asymmetric unit (20 CPs) onto one third of the GFLV-TD caspid as rigid blocks, led to an r.m.s.d. of 0.4 Å for 10080 Cα positions. Higher deviations were found locally with a maximum distance of 1.9 Å at crystal packing contacts. At the level of individual CPs, the two viruses were very similar with an average r.m.s.d. of 0.13±0.01 Å over 504 Cα atoms (Table S1, Figure S3A). Overall we could not find any significant conformational change, neither between the two variants, nor inside their respective capsid.
Comparison of GFLV and TRSV structures
GFLV and TRSV are both transmitted by Xiphinema nematodes [38], [39]. As mentioned above, a 3D model of GFLV based on the crystal structure of TRSV helped identify a region at the virion's surface with function in nematode transmission [32]. As expected from CP sequence homology, the CP of GFLV and TRSV display similar 3D architectures with a good superimposition of the CP folds (Figure S3B). Both virions have about the same outer dimensions but those of TRSV are slightly smaller than those of GFLV. The greatest capsid radius of TRSV measured down the 5-fold, 3-fold and 2-fold symmetry axes is 155, 137 and 123 Å [31]. Overall contacts between the CP subunits of GFLV are the same as those described for TRSV [31]. Subunit interfaces on the 2-fold and 3-fold axes involve the αA' helix in the C domain and the βHI and βBC loops in the B and C domains, respectively (Figure S4). The three jelly-roll domains of the GFLV and TRSV CPs are nearly identical, except for the presence of extra α helices and two supplementary β sheets in the GFLV structure (Figure 3). The independent superimposition of the C, B and A domains showed the A is the most divergent and C domains the most conserved (Table S1). The most striking difference between TRSV and GFLV is the GH loop located at the outer surface of the B domain. In GFLV this loop is longer and much more prominent than in TRSV (Figure S3B). Also, the N-terminal tail facing the interior of the capsid in TRSV is absent in GFLV (Figure S3B). This tail accounts almost exclusively for the size differences between the two CPs (504 residues in GFLV vs 513 in TRSV).
Functional role of residue 297 in transmission
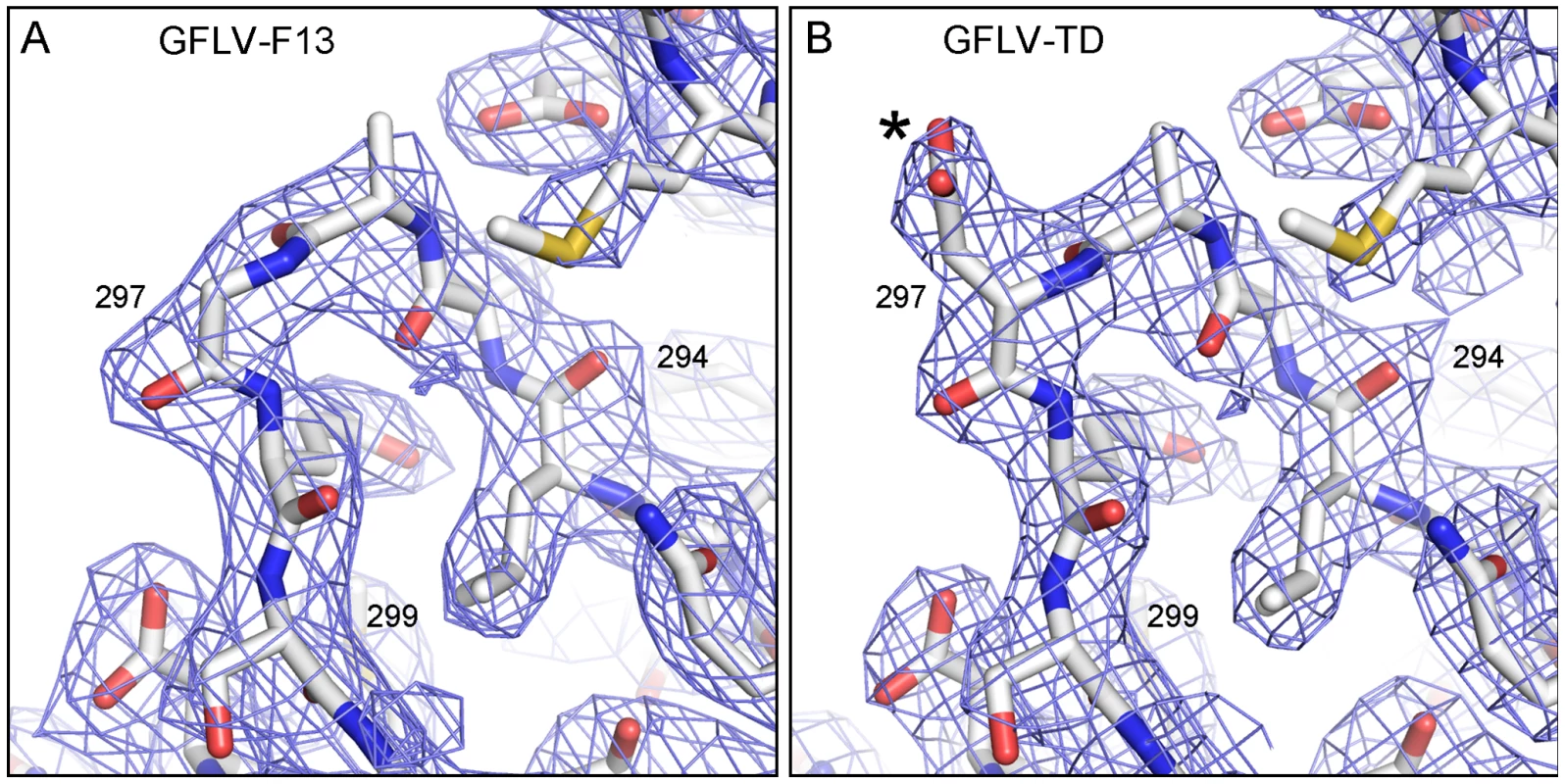
We previously hypothesized that residues important for transmission are exposed at the virion outer surface [32]. According to the GFLV crystal structures, 381 out of 504 CP residues are accessible to the solvent and 208 of them are located at the surface of the virion (underlined in Figure 3). Remarkably, among those, residue 297 lies in the most exposed part of the GH loop in the B-domain and is highly accessible to the solvent (Figure 2B, Figure S5). Sequence information and experimental electron density unambiguously helped identify an Asp side chain at this position in GFLV-TD (Figure 4). The conformation of the GH loops in the structures from GFLV-F13 and GFLV-TD is nearly identical with a maximum distance of 0.18 Å between equivalent atoms (Figure 4) and therefore, cannot account for the loss of GFLV-TD transmission. Consequently, in the absence of major differences between both structures, the addition of a negatively charged side chain per CP resulting from Gly297Asp substitution is presumably responsible for the loss of transmissibility by the nematode.
As mentioned above, a stretch of 11 residues within the CP named region 2 (R2) is essential for GFLV transmission by X. index [32]. Knowing that CP amino acid 297 also affects transmission efficiency and that Gly297 and R2 are relatively close together (Figure S5), we investigated whether both could act synergistically. To address this issue, GFLV amino acid residues in both locations were exchanged by their ArMV counterparts. The single substitution Gly297Ala generated a recombinant named GFLV-G297A and the dual substitution of R2 and Gly297 generated a recombinant named GFLV-R2G297A. Transmission assays showed that GFLV-G297A was transmitted by X. index but not by X. diversicaudatum (Figure 5). In contrast, GFLV-R2G297A was no longer transmitted by either nematode species (Figure 5), although virions were ingested by nematodes during AAP (Figure S6). These results indicate that Gly297 can be substituted by Ala but not by Asp without effect on transmission by X. index. Moreover, the simultaneous substitution of Gly297 and R2 by ArMV sequences is not sufficient to confer transmission by X. diversicaudatum, suggesting that additional residues may be involved.
Fig. 5. Nematode transmission of GFLV CP mutants. 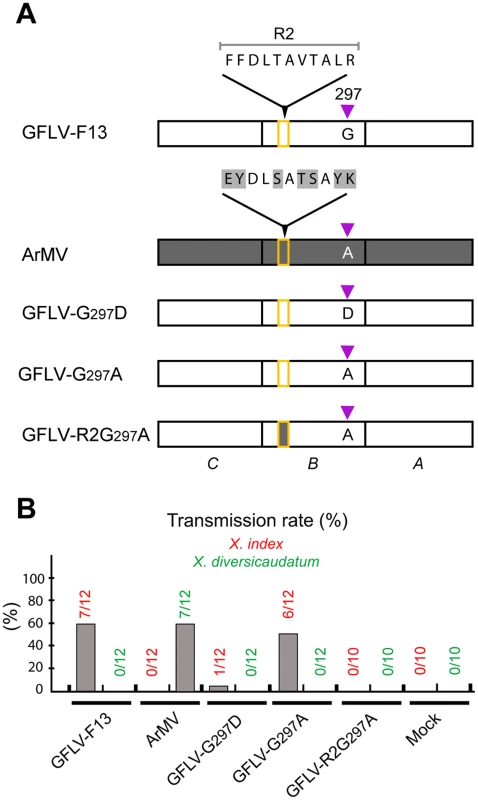
Identification of a putative ligand-binding pocket
The GFLV structure was inspected in the proximity of the residue Gly297 and of the region R2 to identify additional residues that may act as transmission determinants. Gly297 and R2 are located at the edge of a positively charged pocket within the B-domain, whereas most of the GFLV outer surface is negatively charged (Figure 6A). The walls of this pocket are formed essentially by the GH, BC and C′C″ loops encompassing Gly297, R2 and the previously defined region R3 [32], respectively (Figure 6B). The base of the pocket (Figure 6B, purple residues) is formed by at least 11 residues deeply embedded in the capsid shell but still accessible to the solvent (Figure 3, stars). In the crystal structures of GFLV-F13 and GFLV-TD, the residues of the GH, BC and C′C″ loops are well exposed at the outer surface of the capsid (Figure 3, Figure S5). This includes the residues Phe188+189, Thr192+195 and Leu197 from R2 which are different between GFLV and ArMV and may participate in the specific binding of GFLV to X. index (Figure 3 and [32]). Altogether, our data suggest that a positively charged pocket located within the GFLV CP B-domain between the 3-fold and 5-fold axes may constitute a ligand recognition site.
Fig. 6. Putative ligand-binding pocket. 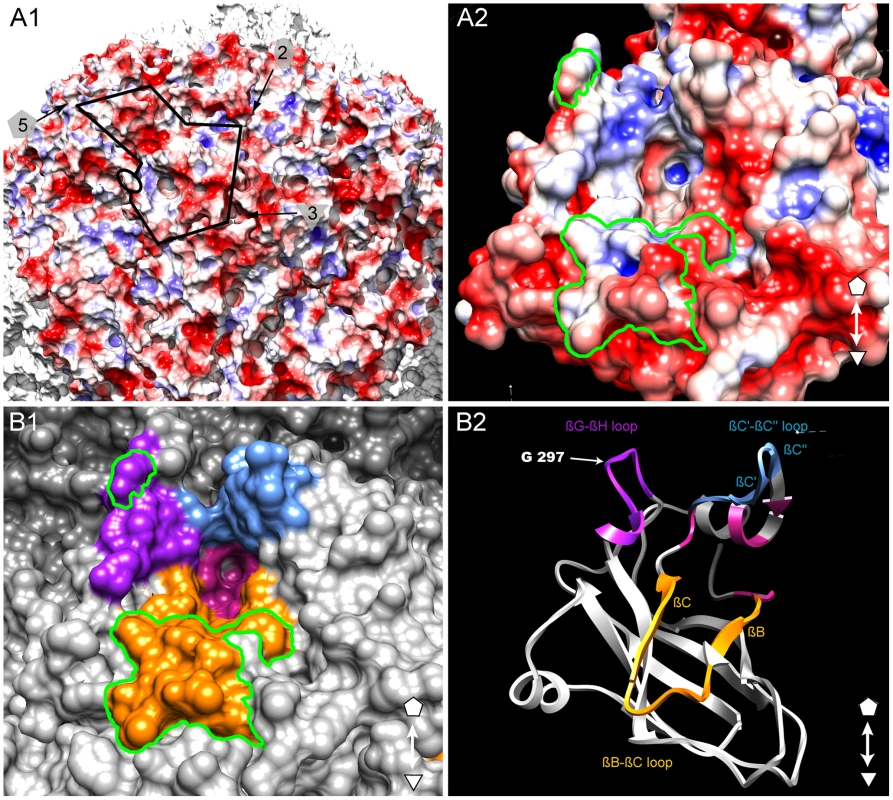
Discussion
GFLV-TD is a natural variant of GFLV-F13 that emerged spontaneously in the greenhouse after multiple mechanical passages in C. quinoa plants over time. Loss of virus transmission is not uncommon under such experimental conditions [12], [40], [41], [42], [43]. However, to our knowledge this is the first isolation and characterization of a spontaneously occurring transmission-deficient nepovirus. In the case of GFLV-TD, CP sequencing revealed that a single Gly297Asp mutation had occurred. A reverse genetics approach confirmed the involvement of CP residue 297 in the transmission deficiency of GFLV-TD by X. index. In addition, the defect in transmission was correlated with a lack of virus retention by X. index, although virus acquisition by nematodes was not affected. Therefore, Gly297 is a bona fide determinant of GFLV transmission efficiency.
The high-resolution structure of GFLV reveals an overall organization well in agreement with its classification in the order Picornavirales within the picorna-like super family [44], [45]. The GFLV capsid consists of 60 subunits, each containing three distantly related jellyroll domains that may have arisen by triplication of a single copy present in some ancestor virus and subsequent divergent evolution [31], [46]. The high degree of similarity of the 3D structures of GFLV and TRSV (Figure S3, Table S1) is in agreement with their classification in the same genus [47]. Yet, the superposition of their capsid proteins is not perfect. This is mainly due to small differences in the orientation of subunits within particles and the length of surface loops, e.g. GH loop in the B-domain. These differences certainly explain why classical molecular replacement using homology models was unsuccessful. Indeed, our initial 3D model of GFLV [32] resembled more TSRV from which it was derived than the actual crystal structure (Figure S3C). In contrast, the 16.5 Å cryoEM map of GFLV (Figure S2) rapidly led to an unambiguous solution. Overall, the resulting structures of GFLV-F13 and GFLV-TD have identical architectures although they were determined in different crystalline packings [36]. These findings indicate that particles are quite rigid and, more importantly, that the differential ability to be transmitted is not due to a conformational modification but rather to an alteration of the physical-chemical properties of their outer surface.
Single point mutations detrimental to virus transmission often affect highly conserved residues. For instance, single mutations in the conserved HI loop of CMV either reduce or abolish aphid transmission [18]. Also, single mutations in the conserved PTK motif of ZYMV HC-Pro [14] or in the DAG motif of TYMV CP [48] hinder aphid transmission of potyviruses. In GFLV, Gly297 is a highly conserved amino acid of the GH loop and our structure shows that it is very accessible to the solvent. Out of the 238 GFLV CP sequences available to date in GenBank, only three allelic variants exist at this position: Ser297 (accession number 38604190), Asn297 (accession number 86450421) and Asp297 (reported for GFLV isolate CACSB5 from California with accession number 299118269 [49] and this work). The transmissibility of the Ser297 and Asn297 allelic variants and CACSB5 isolate is unknown. Here we show that the Gly297Asp strongly affects transmission. We also found that the Gly297Ala single mutant (GFLV-G297A) is still transmitted by X. index, although Ala is the most frequent residue at position 297 in the CP of ArMV strains. Altogether, this indicates that the nature of the side chain of residue occupying the position 297 is decisive for vector recognition.
Since the same structure is observed in GFLV-TD and GFLV-F13, a conformational effect of the Gly297Asp mutation cannot account for the deficiency in transmission of GFLV-TD. However, the Asp297 side chain could create a steric hindrance and thereby interfere with proper recognition of a ligand within the nematode feeding apparatus. A more likely scenario is that Asp297 perturbs the electrostatic potential at the surface of the virions and their solvation shell via the addition of 60 negative charges in GFLV-TD. A striking consequence of this alteration is a 2.5-fold increase of the solubility of GFLV-TD with respect to that of wild-type GFLV-F13. Another one is the different crystal packing [36]. In the same way, alteration of the electrostatic potential may also impair the binding and retention of GFLV inside the nematode feeding apparatus, thereby reducing its transmissibility. Future work will clarify which hypothesis, electrostatic potential or steric hindrance, contributes most to the loss of transmission of GFLV-TD.
Our results show that Gly297 and region R2 are transmission determinants but they cannot alone explain the strict transmission specificity between GFLV and X. index. Thus, these residues may be part of an ensemble of surface residues with ligand binding properties. In view of our structural data, it appears that they are located at the edge of a pocket near the 3 fold axis whose walls are formed essentially by the GH, BC and C′C″ loops within the B-domain. This pocket is remarkable in several respects. First, it is positively charged whereas most of the GFLV outer surface is negatively charged (Figure 5A). Second, all three loops contain residues that are protruding from the capsid outer surface (Figure 3, Figure S5) and are therefore likely to be recognized by compounds of the nematode feeding apparatus. Finally, these three loops were previously identified for their possible involvement in nematode transmission and the function of region R2 encompassing the BC loop was experimentally proven [32]. For all these reasons, we suggest that this pocket may constitute a ligand recognition site with critical function in GFLV transmission by X. index. We also note that its topology resembles the receptor-binding site of other picorna-like viruses, in particular the heparin binding site of Foot-and-mouth disease virus (FMDV) where the pocket occupies a similar position within the icosahedral asymmetric unit (Figure S7) and contains important polar and positively charged residues with ligand binding properties [50], [51]. Whether the occurrence of negatively charged residues in the pocket is detrimental for GFLV transmission by its vector needs to be confirmed. Indeed, so far only two mutants, namely Phe188Glu (i.e. the first residue of R2, [32]) and Gly297Asp (described as GFLV-TD in this work) have been identified in which an alteration of the net electrostatic charge inside the putative ligand-binding pocket was correlated to a defect in virus transmission.
This work provides a new framework for further analyses aiming at precisely defining the structure and charge properties of the binding pocket and of its importance for GFLV transmission by nematodes. The knowledge of the underlying molecular mechanisms is a prerequisite for the identification of a ligand within the nematode feeding apparatus and the subsequent development of novel strategies to control virus propagation in vineyards.
In conclusion, effective virus transmission from host to host relies on a specific interaction with a vector. Here, we have identified structural features involved in such interaction on the surface of a 30 nm icosahedral nepovirus. We showed that a single mutation (Gly297Asp) in the GH loop within the CP B domain is sufficient to diminish GFLV transmission by its ectoparasitic nematode vector X. index. In the absence of any detectable difference in the resolved 3D structures of the wild-type virus and a transmission deficient mutant, we come to the conclusion that the introduction of a negative charge at a precise position in each of the 60 protein subunits of the capsid is sufficient to diminish virus retention inside the nematode's feeding apparatus and thereby hinder virus transmission. We have also delimited a positively charged pocket formed at the surface of the protein capsid which may constitute a binding site for the vector. These findings open new perspectives for the mapping of the ligand recognition site on the virus and the identification of a viral receptor or ligand in the nematode. Providing deeper insights into virus-vector interactions at the atomic level will help understand the origin of the specificity of virus-vector interactions and facilitate the implementation of new strategies to break the viral cycle.
Materials and Methods
Virus strains and plant infection with viral transcripts
GFLV and ArMV strains were isolated from naturally infected grapevines and propagated in the systemic host C. quinoa. Full-length cDNA clones of GFLV-F13 RNA1 and RNA2 are available [35]. They were used to produce RNA molecules by in vitro transcription as described previously [52]. Transcripts of either wild-type GFLV RNA1 and RNA2 or GFLV RNA1 and mutated RNA2 were mechanically inoculated to C. quinoa [35]. Virus infection was assessed in uninoculated apical leaves of C. quinoa plants 2 to 3 weeks post-inoculation by double-antibody sandwich (DAS)-enzyme-linked immunosorbent assay (ELISA) with specific γ-globulins to GFLV and ArMV. Samples were considered positive if their optical density (OD405nm) readings were at least three times those of healthy controls after 120 min of substrate hydrolysis.
GFLV purification and crystallization
Viral particles were purified mainly as described in [53] with one additional 60 to 10% (m/v) sucrose gradient that was performed at 210,000× g in SW41 rotor (Beckman) for 2.5 h. Purified virions were resuspended in sterile water and filtered through a 0.22 µm pore-size Ultrafree-MC membrane (Millex) prior to storage at 4°C. Crystallization by vapor diffusion at 20°C in sub-microliter sitting drops and structural analyses were performed as described [36].
Mutagenesis of GFLV RNA2
Plasmid pVecAcc65I2ABC, carrying a full-length cDNA copy of GFLV RNA2 was used as template for the production of chimeric CP genes harboring a mutated amino acid in position 297 by PCR site directed mutagenesis overlap extension mutagenesis [32]. Plasmid pVecAcc65I2ABCG2 is a derivative of pVecAcc65I2ABC carrying the CP region R2 in position nts 2,609–2,640 (nucleotide positions are given according to the GFLV-F13 RNA2 sequence, GenBank accession no. NC_003623) [32]. Residue 297 (corresponding to codon nts 2,936–2,938) was mutated into an aspartic acid, using pVecAcc65I2ABC as template, the mutagenic primer pair mutDF/mutDR and the external primer pair 18/36 (Table S2). Mutagenic PCR-amplified products were digested with Acc65I (nts 2,678–2683) and BglII (nts 3,055–3,060) and cloned into the corresponding sites in pVecAcc65I2ABC to yield pVecAcc65I2ABCG297D. Residue 297 was mutated into Alanine with the mutagenic primers mutAF/mutAR and the external primers 18/36 (Table S2); PCR-amplified products were digested with Acc65I and BglII, and cloned into the corresponding sites in pVecAcc65I2ABC and pVecAcc65I2ABCG2 to yield pVecAcc65I2ABCG297A and [54] pVecAcc65I2ABCG2-G297A, respectively. Each PCR reaction was carried out as described [32]. For simplicity, transcripts and mutant viruses derived from these constructs were referred to as GFLV-G297D (plasmid pVecAcc65I2ABCG297D), GFLV-G297A (plasmid pVecAcc65I2ABCG297A), and GFLV-R2G297A (plasmid pVecAcc65I2ABCG2-G297A). The integrity of all GFLV RNA2 clones was verified by DNA sequencing.
Nematode transmission tests and detection of GFLV and ArMV in nematodes
Nematode transmission assays were performed in two steps of 4 weeks each, the acquisition access period and the inoculation access period [30]. C. quinoa and Nicotiana benthamiana were used as source and bait plants for transmission assays with X. diversicaudatum and X index, respectively. Transmission tests were performed using 200 nematodes per plant. The presence of GFLV and ArMV was verified in total RNA extracts from nematodes by reverse-transcription (RT)-polymerase-chain reaction (PCR) as described [30].
Characterization of GFLV RNA2 progeny
The progeny of GFLV RNA2 CP sequence was characterized in infected plants by immuno-capture (IC)-RT-PCR and sequencing as described in [29], except that two cDNA fragments were amplified with primer pairs 397/227 and 115/18 (see Table S2). Sequences were analyzed with ContigExpress (Vector NTI Software, InforMax).
Cryo-electron microscopy 3D reconstruction
Purified GFLV particles were applied to a quantifoil R 2/2 carbon grid (Quantifoil Micro Tools GmbH, Germany), blotted by filter paper, and flash-frozen in liquid ethane to make a vitreous-ice embedded sample. Electron micrographs were recorded under low-dose conditions at liquid-N2 temperature with a JEOL 2010 operating at 200 kV microscope. Micrographs collected at X 50,000 magnification with a defocus range of 1.3–2.5 µm were digitized on a Nikon Coolscan 9000 ED with a step size of 10 µm. The images were coarsened by a factor of 2, resulting in a pixel size corresponding to 4 Å at the specimen level. The semi-automatic X3D program (J.F. Conway) was used for picking particles. The defocus value was estimated for each micrograph using CTFFIND3 [55], and phases flipped using CTFMIX [56]. Particle origins and orientations were determined and refined using the model-based orientation determination method [57]. The GFLV reconstruction was determined using as starting model the 3D reconstruction of TRSV filtered at 40 Å resolution. The density map was calculated by Fourier-Bessel formalism as described [57], and implemented in the EM3DR program. Resolution was estimated using the Fourier shell correlation (FSC) criterion with a cutting level of 0.5 [58]. The final density map computed at 16.5 Å resolution includes 2,424 particles extracted from 8 micrographs.
X-ray structure determination and analysis
X-ray diffraction data from GFLV-F13 and GFLV-TD were collected on crystal-cooled samples (Table 1) at FIP-BM30 beamline (ESRF, Grenoble, France) and at X06DA beamline (SLS, Villingen, Switzerland). They were reduced using the XDS package [59].
Diffraction data were phased by molecular replacement using AMoRe [60] followed by non-crystallographic symmetry (NCS) averaging and solvent flattening using RAVE [61], [62]. Attempts to phase data using TRSV-based homology models generated by Modeller [63] were not successful. In contrast, the 3D EM reconstruction led to a clear molecular replacement solution with cubic data in the 30-15 Å resolution range. The orientations of viral particles within the cubic crystal were identified by inspection of the self-rotation function calculated at the highest resolution available (4.5 Å for GFLV-F13 and 2.85 Å for GFLV-TD). Self-rotations corresponding to four differently oriented icosahedral particles were found. Calculation of the translation-function using the correctly oriented 3D EM model showed that four icosahedral particles were present in the unit cell, each sharing one of its 3-fold axis with the crystal. The molecular replacement solutions defined the molecular boundaries (masks) of the particles within the cubic crystals. Based on the icosahedral symmetry of the 3D EM model, the rigid-body operators relating equivalent regions within the molecular boundaries were defined (20 NCS x 3 crystallographic transformations). An iterative procedure of phase extension from 16.5 Å to the maximum available resolution was then carried out by using density modification techniques, including NCS map averaging, solvent flattening and intermediate steps where the molecular masks and the NCS operators were refined.
The incorporation of high-resolution data finally converged to an experimental map at 2.85 Å which allowed the rapid rebuilding of GFLV subunit from homology models. The atomic model of GFLV-TD was refined with PHENIX [64] with cubic data reprocessed at 2.7 Å resolution. NCS constrains were applied to the ensemble of monomers in the asymmetric unit except three regions which changed conformation due to packing contacts (Tyr 9, loops 15–19 and 259–265). Water molecules were added after convergence of capsid refinement. Strong peaks in the difference map were examined in Coot [65] to identify 28 solvent molecules around one monomer A. They were then transferred by symmetry to subunits B-T and a total of 556 solvent sites were assigned in the final model. Strong density peaks were also observed on the 5-fold axes of the capsid indicating the presence of a large ion, possibly a phosphate. A ring of solvent molecules bridging the ion to the CP monomers was clearly seen in 2 out of 4 pentamers of the cubic asymmetric unit. However, this ion could not be explicitly identify (no exploitable anomalous signal) and was not included in the model. The structure of the GFLV-F13 particle was solved by MR using the GFLV-TD model and was refined at 3 Å resolution. No solvent molecule was included, since it was not possible at this resolution to describe a common hydration pattern for the 60 viral subunits in the asymmetric unit. The stereochemical quality (Table 1) of final models was assessed with Coot and Procheck [65] and all residues were in the allowed regions of the Ramachandran plot. The totality of the CP amino acids (504 residues per subunit) was observed in both GFLV-F13 and -TD GFLV structures. Atomic coordinates have been deposited in the Protein Databank (GFLV-TD: pdb ID 2Y26; GFLV-F13 : 2Y7T, 2Y7U, 2Y7V).
GFLV structures were compared with lsqman [61]. R.m.s.d. on Cα positions were calculated for each pairwise superimposition of CPs observed in the cubic (GFLV-TD) and in the monoclinic (GFLV-F13) asymmetric units. Average r.m.s.d. were derived from the former analysis and are reported in Table S1, as well as the r.m.s.d of GFLV CP vs TRSV CP and GFLV CP model based on TRSV.[61]. Solvent accessible surface was calculated with a probe radius of 1.4 Å, with the program MSMS [66]. The analysis of the surface potential was performed with APBS [57], [67]. Figures were prepared using PyMol (http://www.pymol.org/) and Chimera [67].
Supporting Information
Zdroje
1. HalsteadSB
2008
Dengue virus-mosquito interactions.
Annu Rev Entomol
53
273
291
2. KyleJLHarrisE
2008
Global Spread and Persistence of Dengue.
Annu Rev Microbiol
62
71
92
3. CharrelRNAttouiHButenkoAMCleggJCDeubelV
2004
Tick-borne virus diseases of human interest in
Europe.
Clin Microbiol Infect
10
1040
1055
4. WeaverSCReisenWK
2010
Present and future arboviral threats.
Antiviral Res
85
328
345
5. Andret-LinkPFuchsM
2005
Transmission specificity of plant viruses by
vectors.
J Plant Pathol
87
153
165
6. HogenhoutSAAmmarEDWhitfieldAERedinbaughMG
2008
Insect vector interactions with persistently transmitted
viruses.
Annu Rev Phytopathol
46
327
359
7. NgJCKFalkBW
2006
Virus-vector interactions mediating nonpersistent and
semipersistent transmission of plant viruses.
Annu Rev Phytopathol
44
183
212
8. BlancS
2007
Virus transmission - Getting out and in.
Plant Cell Monogr
7
1
28
9. AtreyaCDRaccahBPironeTP
1990
A point mutation in the coat protein abolishes aphid
transmissibility of a potyvirus.
Virology
178
161
165
10. Lopez-MoyaJJWangRYPironeTP
1999
Context of the coat protein DAG motif affects potyvirus
transmissibility by aphids.
J Gen Virol
80
3281
3288
11. PerryKLZhangLPalukaitisP
1998
Amino acid changes in the coat protein of cucumber mosaic virus
differentially affect transmission by the aphids Myzus
persicae and Aphis gossypii.
Virology
242
204
210
12. KakaniKSgroJYRochonD
2001
Identification of specific cucumber necrosis virus coat protein
amino acids affecting fungus transmission and zoospore
attachment.
J Virol
75
5576
5583
13. SyllerJ
2006
The roles and mechanisms of helper component proteins encoded by
potyviruses and caulimoviruses.
Physiol Mol Plant Pathol
67
119
130
14. PengYHKadouryDGal-OnAHuetHWangY
1998
Mutations in the HC-Pro gene of zucchini yellow mosaic potyvirus:
effects on aphid transmission and binding to purified
virions.
J Gen Virol
79
897
904
15. BlancSAmmarEDGarcia-LampasonaSDoljaVVLlaveC
1998
Mutations in the potyvirus helper component protein: effects on
interactions with virions and aphid stylets.
J Gen Virol
79
3119
3122
16. HuetHGal-OnAMeirELecoqHRaccahB
1994
Mutations in the helper component protease gene of zucchini
yellow mosaic virus affect its ability to mediate aphid
transmissibility.
J Gen Virol
75
1407
1414
17. ChenBFranckiRI
1990
Cucumovirus transmission by the aphid Myzus
persicae is determined solely by the viral coat
protein.
J Gen Virol
71
939
944
18. LiuSHeXParkGJosefssonCPerryKL
2002
A Conserved Capsid Protein Surface Domain of Cucumber Mosaic
Virus Is Essential for Efficient Aphid Vector Transmission.
J Virol
76
9756
9762
19. NgJCJosefssonCClarkAJFranzAWPerryKL
2005
Virion stability and aphid vector transmissibility of Cucumber
mosaic virus mutants.
Virology
332
397
405
20. LehVJacquotEGeldreichAHaasMBlancS
2001
Interaction between the open reading frame III product and the
coat protein is required for transmission of cauliflower mosaic virus by
aphids.
J Virol
75
100
106
21. HohFUzestMDruckerMPlisson-ChastangCBronP
2010
Structural insights into the molecular mechanisms of Cauliflower
mosaic virus transmission by its insect vector.
J Virol
4706
4713
22. LehVJacquotEGeldreichAHermannTLeclercD
1999
Aphid transmission of cauliflower mosaic virus requires the viral
PIII protein.
EMBO J
18
7077
7085
23. MartinièreAGarganiDUzestMLautredouNBlancS
2009
A role for plant microtubules in the formation of
transmission-specific inclusion bodies of Cauliflower mosaic
virus.
Plant J
58
135
146
24. UzestMGarganiDDruckerMHebrardEGarzoE
2007
A protein key to plant virus transmission at the tip of the
insect vector stylet.
Proc Natl Acad Sci USA
104
17959
17964
25. UzestMGarganiDDombrovskyACazevieilleCCotD
2010
The “acrostyle”: A newly described anatomical
structure in aphid stylets.
Arthropod Struct Dev
39
221
229
26. HernandezCVisserPBBrownDJBolJF
1997
Transmission of tobacco rattle virus isolate PpK20 by its
nematode vector requires one of the two non-structural genes in the viral
RNA 2.
J Gen Virol
78
465
467
27. MacFarlaneSAWallisCVBrownDJ
1996
Multiple virus genes involved in the nematode transmission of pea
early browning virus.
Virology
219
417
422
28. VassilakosNVelliosEKBrownECBrownDJMacFarlaneSA
2001
Tobravirus 2b protein acts in trans to
facilitate transmission by nematodes.
Virology
279
478
487
29. Andret-LinkPSchmitt-KeichingerCDemangeatGKomarVFuchsM
2004
The specific transmission of Grapevine fanleaf virus by its
nematode vector Xiphinema index is solely determined by the viral coat
protein.
Virology
320
12
22
30. MarmonierASchellenbergerPEsmenjaudDSchmitt-KeichingerCRitzenthalerC
2010
The coat protein determines the specificity of virus transmission
by Xiphinema diversicaudatum.
J Plant Pathol
92
275
279
31. ChandrasekarVJohnsonJE
1998
The structure of tobacco ringspot virus: a link in the evolution
of icosahedral capsids in the picornavirus superfamily.
Structure
6
157
171
32. SchellenbergerPAndret-LinkPSchmitt-KeichingerCBergdollMMarmonierA
2010
A stretch of 11 amino acids in the ßB-ßC loop of the
coat protein of grapevine fanleaf virus is essential for transmission by the
nematode Xiphinema index.
J Virol
84
7924
7933
33. MarshMHeleniusA
2006
Virus Entry: Open Sesame.
Cell
124
729
740
34. VuittenezMMunckMCKuszalaJ
1964
Souches de virus à haute aggressivité
isolées de vignes atteintes de dégenerescence
infectieuse.
Etudes Virol Appliquée
5
69
78
35. ViryMSerghiniMAHansFRitzenthalerCPinckM
1993
Biologically active transcripts from cloned cDNA of genomic
Grapevine fanleaf nepovirus RNAs.
J Gen Virol
74
169
174
36. SchellenbergerPDemangeatGLemaireORitzenthalerCBergdollM
2011
Strategies for the crystallization of viruses: Using phase
diagrams and gels to produced 3D crystals of Grapevine fanleaf
virus.
J Struct Biol
174
344
351
37. LinTJohnsonJE
2003
Structures of Picorna-Like Plant Viruses: Implications and
Applications.
Adv Virus Research
62
167
239
38. McGuireJM
1964
Efficiency of Xiphinema americanum as a vector of tobacco
ringspot virus.
Phytopathol
54
799
801
39. MartelliGPTaylorCE
1990
Distribution of viruses and their nematode
vectors.
HarrisK
Advances in Disease Vector Research
New York
Springer-Verlag
151
189
40. GeraALoebensteinGRaccahB
1979
Protein coats of two strains of Cucumber mosaic virus affect
transmission by Aphis gossypii.
Phytopathology
69
396
399
41. SimonsJN
1976
Aphid Transmission of a nonaphid-transmissible strain of Tobacco
etch Virus.
Phytopathology
66
652
654
42. RobbinsMAReadeRDRochonDM
1997
A cucumber necrosis virus variant deficient in fungal
transmissibility contains an altered coat protein shell
domain.
Virology
234
138
146
43. MorenoAHebrardEUzestMBlancSFereresA
2005
A single amino acid position in the helper component of
cauliflower mosaic virus can change the spectrum of transmitting vector
species.
J Virol
79
13587
13593
44. Le GallOChristianPFauquetCMKingAMKnowlesNJ
2008
Picornavirales, a proposed order of positive-sense
single-stranded RNA viruses with a pseudo-T = 3 virion
architecture.
Arch Virol
153
715
727
45. KooninEVWolfYINagasakiKDoljaVV
2008
The Big Bang of picorna-like virus evolution antedates the
radiation of eukaryotic supergroups.
Nat Rev Micro
6
925
939
46. Chen StauffacherZCLiYSchmidtTBomuWKamerG
1989
Protein-RNA interactions in an icosahedral virus at 3.0 A
resolution.
Science
245
154
159
47. SanfaçonH
2008
Nepovirus.
MahyBWJvan RegenmortelMHV
Encyclopedia of Virology
Oxford
Elsevier
405
413
48. AtreyaPLAtreyaCDPironeTP
1991
Amino acid substitutions in the coat protein result in loss of
insect transmissibility of a plant virus.
Proc Natl Acad Sci U S A
88
7887
7891
49. OliverJEVigneEFuchsM
2010
Genetic structure and molecular variability of Grapevine fanleaf
virus populations.
Virus Res
152
30
40
50. FryEELeaSMJacksonTNewmanJWIEllardFM
1999
The structure and function of a foot-and-mouth disease
virus-oligosaccharide receptor complex.
EMBO J
18
543
554
51. FryEENewmanJWICurrySNajjamSJacksonT
2005
Structure of Foot-and-mouth disease virus serotype A1061 alone
and complexed with oligosaccharide receptor: receptor conservation in the
face of antigenic variation.
J Gen Virol
86
1909
1920
52. BelinCSchmittCGaireFWalterBDemangeatG
1999
The nine C-terminal residues of the Grapevine fanleaf
nepovirus movement protein are critical for systemic virus
spread.
J Gen Virol
80
1347
1356
53. PinckLFuchsMPinckMRavelonandroMWalterB
1988
A satellite RNA in Grapevine fanleaf virus
strain F13.
J Gen Virol
69
233
239
54. RitzenthalerCNebenfuhrAMovafeghiAStussi-GaraudCBehniaL
2002
Reevaluation of the effects of brefeldin A on plant cells using
tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent
protein and COPI antisera.
Plant Cell
14
237
261
55. MindellJAGrigorieffN
2003
Accurate determination of local defocus and specimen tilt in
electron microscopy.
J Struct Biol
142
334
347
56. ConwayJStevenA
1999
Methods for reconstructing density maps of “single”
particles from cryoelectron micrographs to subnanometer
resolution.
J Struct Biol
128
106
118
57. BakerTSChengRH
1996
A model-based approach for determining orientations of biological
macromolecules imaged by cryoelectron microscopy.
J Struct Biol
116
120
130
58. van HeelMHarauzG
1986
Resolution criteria for three dimensional
reconstruction.
Optik (Jena)
73
119
122
59. KabschW
2001
XDS in International Tables for Crystallography.
RossmannMGArnoldE
Crystallography of Biological Macromolecules: Dordrecht: Kluwer Academic
Publishers
60. NavazaJ
1994
AMoRe: an automated package for molecular
replacement.
Acta Cryst
50
157
163
61. KleywegtGJ
1996
Use of non-crystallographic symmetry in protein structure
refinement.
Acta Crystallogr D Biol Crystallogr
52
842
857
62. KleywegtGJJonesTA
1999
Software for handling macromolecular envelopes.
Acta Cryst D
55
941
944
63. FiserASaliA
2003
Modeller: generation and refinement of homology-based protein
structure models.
Meth Enzymol
374
461
491
64. AdamsPDGrosse-KunstleveRWHungLWIoergerTRMcCoyAJ
2002
PHENIX: building new software for automated crystallographic
structure determination.
Acta Crystallogr D Biol Crystallogr
58
1948
1954
65. EmsleyPCowtanK
2004
Coot: model-building tools for molecular
graphics.
Acta Crystallogr D Biol Crystallogr
60
2126
2132
66. SannerMFOlsonAJSpehnerJC
1996
Reduced surface: an efficient way to compute molecular
surfaces.
Biopolymers
38
305
320
67. PettersenEFGoddardTDHuangCCCouchGSGreenblattDM
2004
UCSF Chimera - A visualization system for exploratory research
and analysis.
J Comput Chem
25
1605
1612
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus SpeciesČlánek SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion ReleaseČlánek Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T CellsČlánek A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the CytosolČlánek Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- Infections Are Virulent and Inhibit the Human Malaria Parasite in
- A Gamma Interferon Independent Mechanism of CD4 T Cell Mediated Control of Infection
- MDA5 and TLR3 Initiate Pro-Inflammatory Signaling Pathways Leading to Rhinovirus-Induced Airways Inflammation and Hyperresponsiveness
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- An E2F1-Mediated DNA Damage Response Contributes to the Replication of Human Cytomegalovirus
- Quantitative Subcellular Proteome and Secretome Profiling of Influenza A Virus-Infected Human Primary Macrophages
- Distribution of the Phenotypic Effects of Random Homologous Recombination between Two Virus Species
- Inhibition of Both HIV-1 Reverse Transcription and Gene Expression by a Cyclic Peptide that Binds the Tat-Transactivating Response Element (TAR) RNA
- A Viral Satellite RNA Induces Yellow Symptoms on Tobacco by Targeting a Gene Involved in Chlorophyll Biosynthesis using the RNA Silencing Machinery
- Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Investigating the Host Binding Signature on the PfEMP1 Protein Family
- Human Neutrophil Clearance of Bacterial Pathogens Triggers Anti-Microbial γδ T Cell Responses in Early Infection
- Septation of Infectious Hyphae Is Critical for Appressoria Formation and Virulence in the Smut Fungus
- A Family of Helminth Molecules that Modulate Innate Cell Responses via Molecular Mimicry of Host Antimicrobial Peptides
- Phospholipids Trigger Capsular Enlargement during Interactions with Amoebae and Macrophages
- CTL Escape Mediated by Proteasomal Destruction of an HIV-1 Cryptic Epitope
- Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens
- Extensive Genome-Wide Variability of Human Cytomegalovirus in Congenitally Infected Infants
- The Antiviral Efficacy of HIV-Specific CD8 T-Cells to a Conserved Epitope Is Heavily Dependent on the Infecting HIV-1 Isolate
- Epstein-Barr Virus Infection of Polarized Epithelial Cells via the Basolateral Surface by Memory B Cell-Mediated Transfer Infection
- Reactive Oxygen Species Hydrogen Peroxide Mediates Kaposi's Sarcoma-Associated Herpesvirus Reactivation from Latency
- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- The Dot/Icm System Delivers a Unique Repertoire of Type IV Effectors into Host Cells and Is Required for Intracellular Replication
- AAV Exploits Subcellular Stress Associated with Inflammation, Endoplasmic Reticulum Expansion, and Misfolded Proteins in Models of Cystic Fibrosis
- Suboptimal Activation of Antigen-Specific CD4 Effector Cells Enables Persistence of In Vivo
- SIV Nef Proteins Recruit the AP-2 Complex to Antagonize Tetherin and Facilitate Virion Release
- Dual Function of the NK Cell Receptor 2B4 (CD244) in the Regulation of HCV-Specific CD8+ T Cells
- Transition of Sporozoites into Liver Stage-Like Forms Is Regulated by the RNA Binding Protein Pumilio
- A Large and Intact Viral Particle Penetrates the Endoplasmic Reticulum Membrane to Reach the Cytosol
- Transcriptome Analysis of in Human Whole Blood and Mutagenesis Studies Identify Virulence Factors Involved in Blood Survival
- Interleukin-13 Promotes Susceptibility to Chlamydial Infection of the Respiratory and Genital Tracts
- Structural Insights into Viral Determinants of Nematode Mediated Transmission
- Protective Efficacy of Serially Up-Ranked Subdominant CD8 T Cell Epitopes against Virus Challenges
- Viral CTL Escape Mutants Are Generated in Lymph Nodes and Subsequently Become Fixed in Plasma and Rectal Mucosa during Acute SIV Infection of Macaques
- Taking Some of the Mystery out of Host∶Virus Interactions
- Viral Small Interfering RNAs Target Host Genes to Mediate Disease Symptoms in Plants
- : An Emerging Cause of Sexually Transmitted Disease in Women
- Mitochondrial Ubiquitin Ligase MARCH5 Promotes TLR7 Signaling by Attenuating TANK Action
- The Hexamer Structure of the Rift Valley Fever Virus Nucleoprotein Suggests a Mechanism for its Assembly into Ribonucleoprotein Complexes
- Acquisition of Human-Type Receptor Binding Specificity by New H5N1 Influenza Virus Sublineages during Their Emergence in Birds in Egypt
- Stromal Down-Regulation of Macrophage CD4/CCR5 Expression and NF-κB Activation Mediates HIV-1 Non-Permissiveness in Intestinal Macrophages
- A Component of the Xanthomonadaceae Type IV Secretion System Combines a VirB7 Motif with a N0 Domain Found in Outer Membrane Transport Proteins
- Perturbs Iron Trafficking in the Epithelium to Grow on the Cell Surface
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Crystal Structure and Functional Analysis of the SARS-Coronavirus RNA Cap 2′-O-Methyltransferase nsp10/nsp16 Complex
- Lymphoadenopathy during Lyme Borreliosis Is Caused by Spirochete Migration-Induced Specific B Cell Activation
- The OXI1 Kinase Pathway Mediates -Induced Growth Promotion in Arabidopsis
- : An Emerging Cause of Sexually Transmitted Disease in Women
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání