-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
Bacterial growth in multicellular communities, or biofilms, offers many potential advantages over single-cell growth, including resistance to antimicrobial factors. Here we describe the interaction between the biofilm-promoting components curli fimbriae and cellulose of uropathogenic E. coli and the endogenous antimicrobial defense in the urinary tract. We also demonstrate the impact of this interplay on the pathogenesis of urinary tract infections. Our results suggest that curli and cellulose exhibit differential and complementary functions. Both of these biofilm components were expressed by a high proportion of clinical E. coli isolates. Curli promoted adherence to epithelial cells and resistance against the human antimicrobial peptide LL-37, but also increased the induction of the proinflammatory cytokine IL-8. Cellulose production, on the other hand, reduced immune induction and hence delayed bacterial elimination from the kidneys. Interestingly, LL-37 inhibited curli formation by preventing the polymerization of the major curli subunit, CsgA. Thus, even relatively low concentrations of LL-37 inhibited curli-mediated biofilm formation in vitro. Taken together, our data demonstrate that biofilm components are involved in the pathogenesis of urinary tract infections by E. coli and can be a target of local immune defense mechanisms.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1001010
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001010Summary
Bacterial growth in multicellular communities, or biofilms, offers many potential advantages over single-cell growth, including resistance to antimicrobial factors. Here we describe the interaction between the biofilm-promoting components curli fimbriae and cellulose of uropathogenic E. coli and the endogenous antimicrobial defense in the urinary tract. We also demonstrate the impact of this interplay on the pathogenesis of urinary tract infections. Our results suggest that curli and cellulose exhibit differential and complementary functions. Both of these biofilm components were expressed by a high proportion of clinical E. coli isolates. Curli promoted adherence to epithelial cells and resistance against the human antimicrobial peptide LL-37, but also increased the induction of the proinflammatory cytokine IL-8. Cellulose production, on the other hand, reduced immune induction and hence delayed bacterial elimination from the kidneys. Interestingly, LL-37 inhibited curli formation by preventing the polymerization of the major curli subunit, CsgA. Thus, even relatively low concentrations of LL-37 inhibited curli-mediated biofilm formation in vitro. Taken together, our data demonstrate that biofilm components are involved in the pathogenesis of urinary tract infections by E. coli and can be a target of local immune defense mechanisms.
Introduction
It has been recognized that bacteria in their natural milieu seldom grow as non-differentiated, single cell organisms. Instead, they form multicellular communities, biofilms, showing coordinated behavior [1]. Classically, biofilm formation includes surface adherence, cell-cell interactions, and production of extracellular matrix [2]. The extracellular matrix contributes to the development of higher-ordered three-dimensional structures that offer advantages to the bacteria, such as increased resistance to antimicrobial substances, mechanical forces and to nutrient depletion [3]–[5]. During urinary tract infections (UTI), the role of bacterial biofilms has previously been established in the presence of indwelling catheters [6]. However, uropathogenic E. coli also forms biofilm-like structures on and inside host cells in the absence of a foreign body [7]–[9], and the ability to form biofilms has been related to persistence of bacteria in the urinary tract [10].
Curli belong to a class of fibers known as amyloids [11] and are involved in adhesion to surfaces, cell aggregation and, finally, biofilm development. Functionally and genetically, curli are linked to cellulose [12], another extracellular matrix component of biofilms formed by bacteria from the family Enterobacteriaceae. Bacterial cellulose has mostly been investigated in soil bacteria of the family Rhizobiaceae, where this polysaccharide is required for the firm adherence and aggregation of bacteria at the root hair tip of plants [13]. Although the production of cellulose is common among many bacterial species, its biological function and role during infection is not entirely clear. When cellulose is expressed together with curli, the two substances produce a highly inert, hydrophobic extracellular matrix around the bacteria [14]. Biofilms built from curli and cellulose have widely been investigated on abiotic surfaces [15], [16] and in commensal intestinal E. coli isolates [17], [18]. Less information is available about the role of curli and cellulose during E. coli infection of the urinary tract [10], [19].
Recently, we demonstrated that epithelial cells of the urinary tract up-regulate the production of the human antimicrobial peptide LL-37 upon infection with uropathogenic E. coli [20]. Thus, the cathelicidin LL-37 plays an important role in the protection against infections of the urinary tract. The proform of LL-37, hCAP-18, is mainly produced by epithelial cells and neutrophils [21], [22]. After processing [23], the active LL-37 peptide is released and exhibits its bactericidal activity by interaction with the bacterial cell membrane [24].
In the current project, we sought to study the presence of curli and cellulose in E. coli isolated from uncomplicated community-acquired UTI and their impact on early UTI pathogenesis. In addition, we here investigate the influence of LL-37 on curli-mediated biofilm formation in E. coli. We suggest that curli and cellulose protect the bacterium from immune defense mechanisms and in addition modulate the immune response of the host. We furthermore demonstrate an interaction of curli and LL-37, especially that LL-37 inhibits the polymerization of CsgA, the major subunit of curli.
Results
Uropathogenic E. coli isolates have higher adhesion capacity and produce more biofilm than commensal E. coli
A total of 99 E. coli isolates were collected from urine of patients with UTI and 77 isolates were obtained from fecal samples of healthy individuals. Each isolate was assessed for biofilm formation using a standard microtiter assay (see Materials and Methods). On average, uropathogenic bacteria adhered significantly better and formed more biofilm as compared to fecal isolates (P<0.0001, Figure 1A).
Fig. 1. Biofilm expression by uropathogenic and fecal E. coli isolates. 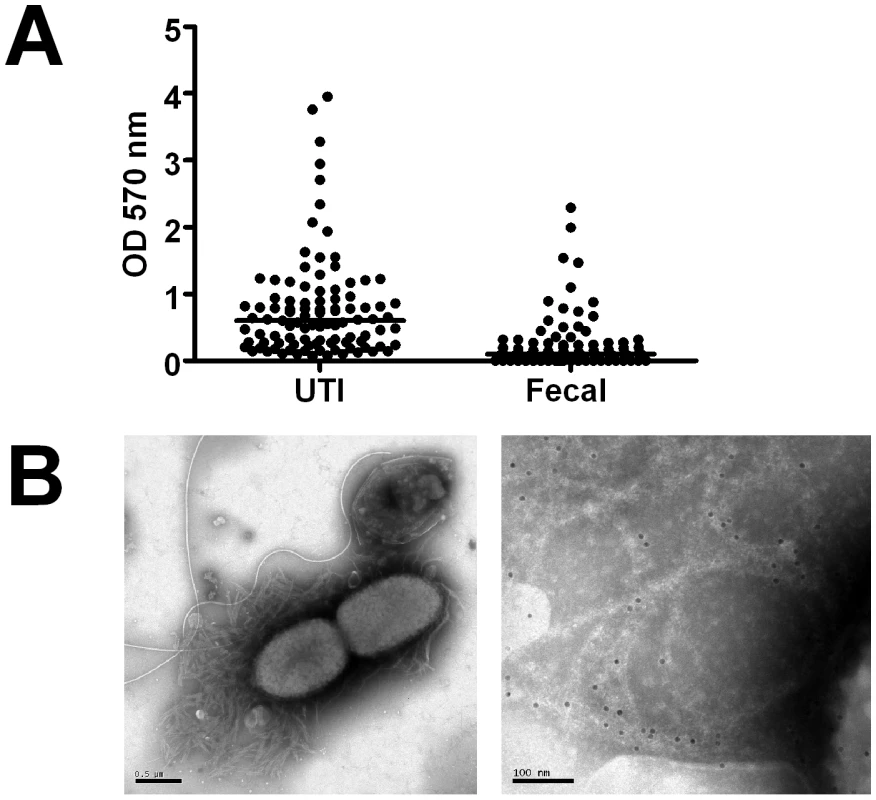
(A) Adhesion capacity and thickness of biofilm produced by E. coli isolates collected from urine of patients with urinary tract infections (UTI, n = 99) and from fecal samples of healthy individuals (Fecal, n = 77) was measured. Individual values and medians are presented, depicted as optical density (OD) at 570 nm after dissolution of crystal violet. The difference is significant (P<0.0001, Mann-Whitney U test). (B) Isolates from urine samples were investigated by electron microscopy. The left image shows an overview, the right image is a magnification showing immunogold-labelled curli. The scale bars show 0.5 µm (left) and 100 nm (right), respectively. Uropathogenic and commensal E. coli isolates produce extracellular matrixes with similar composition
Curli and cellulose production by all isolates was monitored on Congo red and Calcofluor containing plates. To better mimic the host environment, we chose to analyze bacteria grown at 37°C. Based on the uptake of Congo red and fluorescence after the exposure of Calcofluor plates to UV light, we could identify that approximately half of the uropathogenic and commensal E. coli isolates expressed curli (54% and 45%, respectively), 30% of the uropathogens and 16% of the commensals expressed curli and cellulose together. This morphotype was significantly associated to uropathogenic E. coli (P = 0.032). The expression of cellulose alone was rarely detected in either collection (5% and 10%, respectively). Nearly all isolates were positive for expression of type 1 fimbriae, irrespective of their origin (99% of the uropathogenic and 92% of the fecal isolates).
Curli are expressed in isolates from fresh urine samples
To confirm the expression of curli in vivo, we collected fresh urine samples from patients with community-acquired E. coli UTI. Bacteria were analyzed directly from the urine by dot blot analysis and electron microscopy. Ten of seventeen investigated clinical E. coli isolates (59%) reacted with antibodies towards CsgA. This was in line with their Congo red/Calcofluor phenotype and the overall prevalence of curli in uropathogenic E. coli investigated here (54%). Bundles of curli expressed during UTI were visualized by electron microscopy and their identity was confirmed by gold-labeled antibodies (Figure 1B).
Expression of curli increases the propensity of E. coli to cause infection
The relevance of curli and cellulose expression on bacterial adhesion and immune induction in target cells was investigated by the interaction of bacteria with human cells in vitro (Figure 2). Bladder (UROtsa, T24) and renal (A498) epithelial cells were infected with the uropathogenic E. coli strain No. 12, producing curli and cellulose; and its isogenic mutants lacking curli and/or cellulose. The wild-type strain and its mutants also expressed type 1 fimbriae to similar extent. The total number of bacteria after 30 min of cell infection was determined. Curli expression resulted in an increased number of cell-associated bacteria in the presence or absence of cellulose (P<0.0001, Figure 2A). Likewise, levels of IL-8 were significantly higher in supernatants of cells infected with curliated E. coli compared to those induced by the respective non-curliated strain (P = 0.001 and P<0.0001 for cellulose-expressing and lacking strains, respectively, Figure 2B). Cellulose, on the other hand, reduced the ability of bacteria to adhere (P<0.0001 and P = 0.001 in the presence and absence of curli, respectively, Figure 2A). In curliated bacteria, cellulose expression significantly reduced the induction of IL-8 (P = 0.001, Figure 2B). The role of curli and cellulose on adherence and IL-8 induction was confirmed by complementation of the curli and cellulose-deficient mutants which restored the wild-type phenotype (Figure 2C+D).
Fig. 2. Adhesion and immune induction by E. coli expressing or lacking curli and/or cellulose. 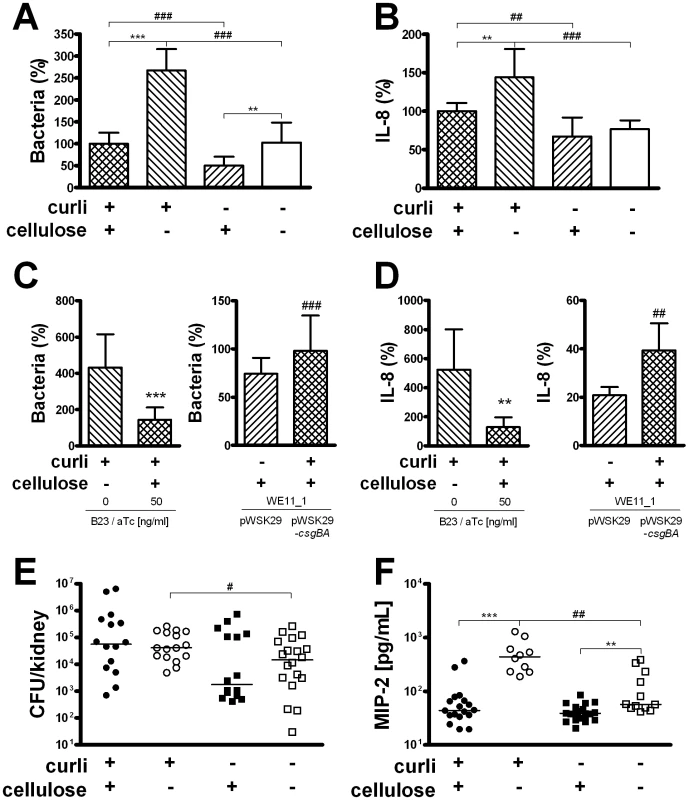
(A) Adhesion to renal epithelial cells A498 was measured after 30 min. Curliated strains adhered significantly better to renal epithelial cells than strains lacking curli, independent of the expression of cellulose (### P<0.0001, t-test). Cellulose expression decreased the number of cell-associated bacteria in curliated (*** P<0.0001, t-test) and non-curliated strains (** P = 0.001, t-test). Results from three independent experiments in quadruplicates are shown as mean and standard deviation. Similar results were obtained for bladder epithelial cells (data not shown). (B) Induction of IL-8 was measured in culture supernatants of renal epithelial cells A498 stimulated with E. coli for 24 h. Curliated bacteria induced a significantly stronger IL-8 response than the mutants lacking curli in the presence (## P = 0.001, t-test) and absence (### P<0.0001, t-test) of cellulose. In curliated bacteria, the expression of cellulose reduced IL-8 induction (** P = 0.001, t-test). Results from three independent experiments in quadruplicate are shown as mean and standard deviation. Similar results were obtained for bladder epithelial cells (data not shown). (C+D) The phenotype of E. coli No. 12 could be restored by complementation of its mutants. Cellulose expression in strain B23 is inducible by aTc (left panels) and reduces adherence and IL-8 induction (*** P<0.0001 and ** P = 0.007, respectively, t-test). The curli subunits CsgA and CsgB are expressed from pWSK29-csgBA in strain WE11_1 (right panels) and increases adherence and IL-8 induction compared to WE11_1 carrying the vector pWSK29 only (*P = 0.048 and ** P = 0.003, respectively, t-test). Results in A498 cells are shown as mean and standard deviation. Data from three experiments in quadruplicate for adherence and from two experiments in triplicate for IL-8 induction are presented. (E) Mice were infected with the isogenic E. coli strains for 1 h. The curliated mutant were isolated from kidneys in significantly higher numbers than the double knockout (# P = 0.026, Mann-Whitney U test). Individual values from n = 8–10 mice/group and medians are shown. (F) Levels of MIP-2 were measured in kidney tissue of infected mice after 16 h. In the absence of cellulose, the curliated mutant strain induced higher levels of MIP-2 compared to the non-curliated strain (## P = 0.001, Mann-Whitney U test). Expression of cellulose reduced the induction of MIP-2 in the presence (*** P<0.0001, Mann-Whitney U test) and absence of curli (** P = 0.001, Mann-Whitney U test). Individual values from n = 5–10 mice/group and medians are shown. To confirm the role of curli and cellulose during the initial infection steps, mice were infected with the isogenic E. coli strains. After 1 h of infection, the expression of curli increased the number of bacteria significantly only in the absence of cellulose (P = 0.026, Figure 2E), whereas the comparison between the wild-type strain and the curli-lacking mutant was not significant. However, comparing the pair of curliated strains with the pair of non-curliated mutants, the effect of curli on adherence in vitro was supported (P = 0.007). Similar to the cell culture experiments, the expression of curli alone increased the induction of MIP-2 (P = 0.001, Figure 2F). Interestingly, the inhibitory effect of cellulose was even more pronounced in vivo, and was also observed in the absence of curli (P<0.0001 and P = 0.001 in the presence and absence of curli, respectively, Figure 2F). Moreover, none of the strains expressing cellulose induced MIP-2 levels significantly higher than levels in control mice inoculated with sterile PBS (32–52 pg/ml).
Bacterial cellulose influences neutrophil recruitment and elimination of E. coli from the kidney
In the initial stages of UTI, curli promoted colonization (Figure 2A+E). We further investigated the later course of UTI. Mice were infected with isogenic strains expressing curli and/or cellulose, and kidneys were analyzed 48 h post infection (p.i.). MIP-2 is the major neutrophil chemoattractant in the urinary tract [25]. Corresponding to immune induction (Figure 2F), the curliated mutant was more efficiently eliminated after 48 h p.i. than the wild-type strain with cellulose (P = 0.011, Figure 3A).
Fig. 3. Cellulose delays bacterial elimination in vivo. 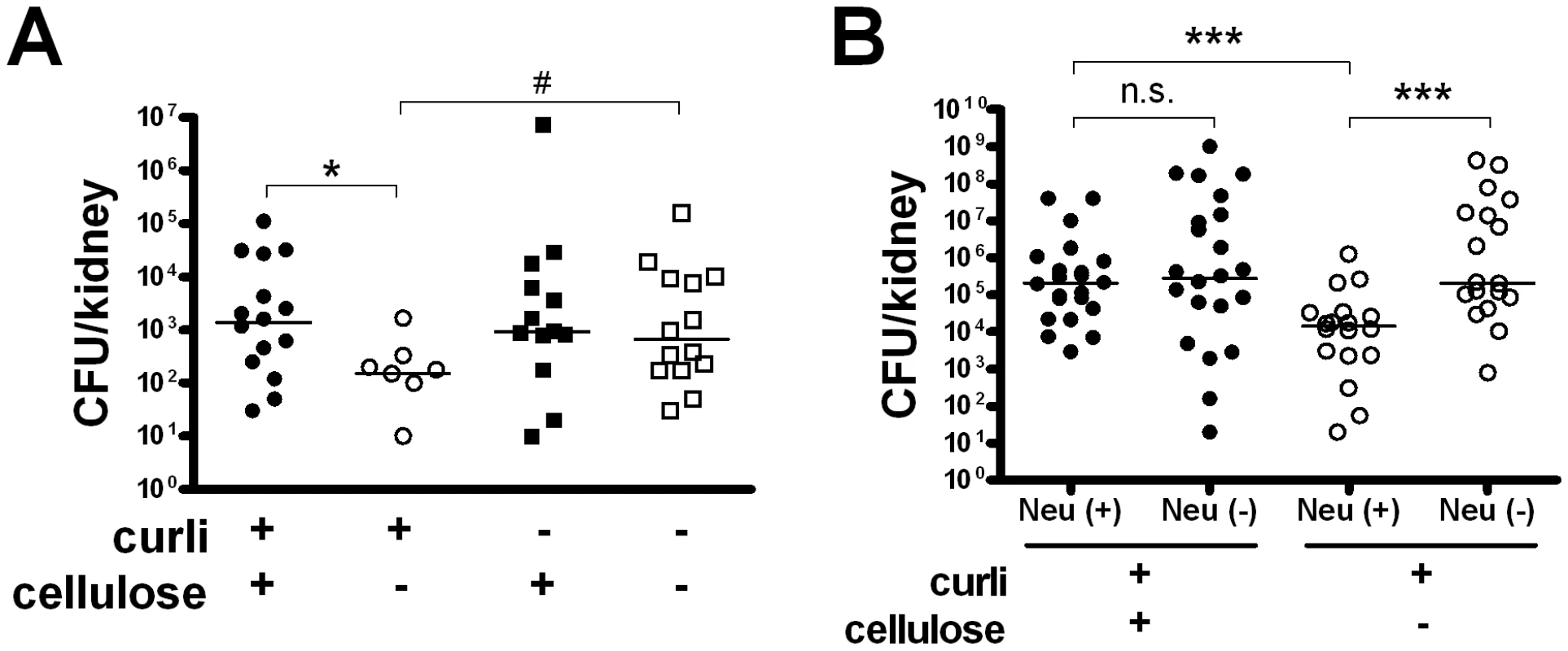
(A) The expression of cellulose in curliated strains increased the number of bacteria in kidneys determined at 48 h p.i. (* P = 0.011, Mann-Whitney U test). The expression of curli in the absence of cellulose, on the other hand, mediated a more rapid elimination (# P = 0.011, Mann-Whitney U test). Individual values from n = 5–8 mice/group and medians are shown. (B) Cellulose expression reduces bacterial clearance by neutrophils. Control mice (Neu +) and mice with induced neutropenia (Neu −) were infected with curliated E. coli strains, and the bacterial load in the kidneys was determined 48 h p.i. A difference between bacteria with or without cellulose was only seen in the presence of neutrophils (P = 0.001, Mann-Whitney U test). Individual values from n = 9–12 mice/group and medians are shown. To further investigate the role of cellulose in this process, we induced neutropenia in mice prior to infection. Neutrophil-depleted and control mice were infected with curliated bacteria with or without cellulose. Clearance by neutrophils was more efficient for bacteria lacking cellulose (Figure 3B). In neutrophil-depleted mice, the number of cellulose-deficient bacteria after 48 h was as high as those of the wild-type strain.
Curli increase resistance to the antimicrobial peptide LL-37
To understand the mechanism underlying the more efficient infection by curliated bacteria, we specifically investigated the antimicrobial activity of bladder and renal epithelial cells on adhered bacteria. For this purpose, bacteria were coincubated with cells for 30 min and adherent bacteria were then subjected to a staining procedure allowing the discrimination between live and dead bacteria. Dependent on the expression of curli and cellulose, 19% to 67% of cell-associated bacteria were killed. Curli but also cellulose protected bacteria from antimicrobial activities of the cells (Figure 4A, P<0.001 for curli and P = 0.024 and P = 0.003 for cellulose, respectively), most efficiently when both structures were expressed together.
Fig. 4. Curli increase the resistance to the antimicrobial peptide LL-37. 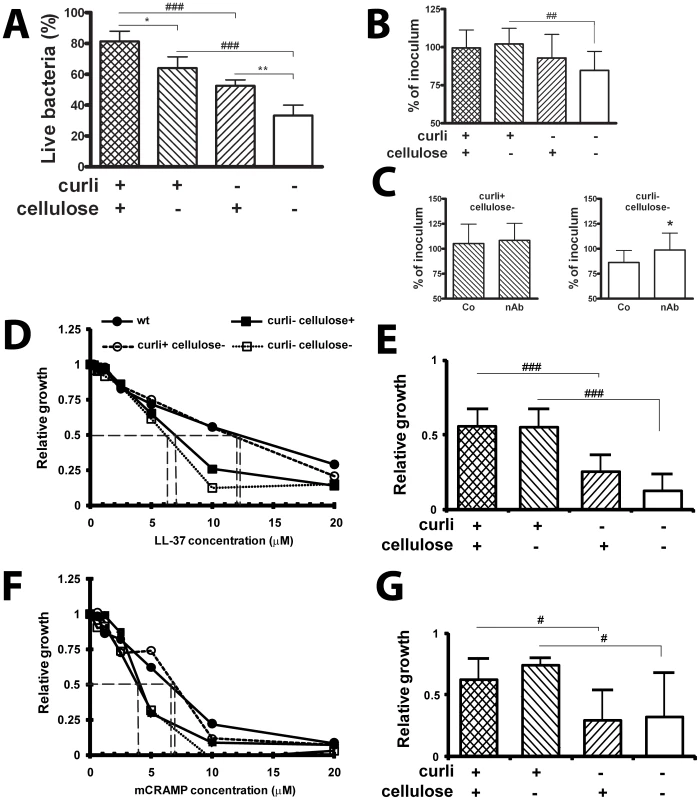
(A) Bladder epithelial cells T24 were infected with bacteria for 30 min and adherent bacteria were subjected to LIVE/DEAD staining. Curli and cellulose expression enhanced bacterial resistance to antimicrobial properties (### P<0.001 for curliated strains versus non-curliated strains, * P = 0.023 and ** P = 0.003 for cellulose expressing strains with or without curli, t-test). Combined data from four experiments are shown. (B+C) Bacteria were exposed to conditioned medium of bladder epithelial cells T24 stimulated with phenylbutyrate to enhance LL-37 production. Curli expression enhanced bacterial survival over 30 min (## P = 0.006, t-test) (B). Results from three experiments in triplicate are shown. Conditioned medium was incubated with neutralizing anti-LL-37-antibodies (nAb) or isotype control antibodies (Co) prior to bacterial inoculation. Neutralizing of LL-37 had no effect on viability of the curliated strain (left) but enhanced viability of the double knockout (right, * P = 0.047, t-test) (C). Results from four experiments in triplicate are shown. (D–G) The susceptibility to LL-37 and mCRAMP of E. coli strains expressing or lacking curli or cellulose was tested by the broth dilution method. The expression of curli increased the resistance to both LL-37 (D+E) and mCRAMP (F+G). A significant difference of bacterial growth was observed at 10 µM LL-37 between curliated and non-curliated strains (### P<0.001, t-test). The curliated strains were also significantly more resistant to 5 µM mCRAMP than bacteria not producing curli (# P<0.05, t-test). An increased resistance to both cathelicidins was not observed for cellulose. Mean and standard deviation from data of two separate experiments in triplicates are shown. The IC50 is indicated by a broken line. Bladder and renal epithelial cells are known to produce cathelicidins in response to E. coli infection, in particular LL-37 in humans and mCRAMP in mice, respectively [20]. To relate the observed antimicrobial activity of uroepithelial cells to this peptide, bacteria were exposed to conditioned medium from cells stimulated with phenylbutyrate to enhance LL-37 production [26]. After 30 min of incubation, the number of curli-producing bacteria was almost unchanged (99% and 102% of the inoculated concentration), whereas bacteria lacking curli were reduced to 93% and 85% in the presence or absence of cellulose, respectively (Figure 4B). The most pronounced difference was observed due to curli in the absence of cellulose (102% versus 85%, P = 0.006). Hence, we chose these two mutant strains for neutralizing experiments. Prior to inoculation, the activity of LL-37 in the culture medium was inhibited by neutralizing antibodies. While the number of viable curliated bacteria did not differ after 30 min (Figure 4C, left), the number of bacteria lacking curli was significantly higher in the presence of LL-37-specific antibodies compared to the samples treated with an irrelevant isotype control antibody (Figure 4C, right, P = 0.047).
We further investigated the influence of curli expression on bacterial sensitivity to LL-37 and mCRAMP more specifically by a broth dilution method. When bacteria were initially grown in biofilm, the concentration of LL-37 at which bacterial growth was inhibited to 50% (IC50) was 12 µM for the curliated strains. However, the IC50 for the non-curliated strains was only 6–7 µM (Figure 4D). At 10 µM LL-37, the relative growth of the curliated strains was significantly higher than growth of the non-curliated strains (P = 0.001 and P<0.001 in the presence and absence of cellulose, respectively, Figure 4E). Similar results were obtained for the mouse cathelicidin mCRAMP (Figure 4F+G). The IC50 value was higher for the curliated strains than for the non-curliated strains (7 versus 4 µM, Figure 4F). At 5 µM mCRAMP, the relative growth differed significantly between the curliated and the non-curliated strains (P = 0.034 and P = 0.037 in the presence and absence of cellulose, respectively, Figure 4G). The same bacteria were then pre-grown planktonically, where curli expression is suppressed. When grown under such conditions, no significant difference in the resistance against both LL-37 and mCRAMP was observed between the strains (data not shown). These data indicate that curli is a biofilm component that counteracts the bactericidal effect of cathelicidins and may contribute to the increased resistance of E. coli growing in biofilm. In contrast to curli, cellulose did not influence the IC50 of cathelicidins (Figure 4D–G).
LL-37 binds to curli fimbriae
In order to elucidate one possible mechanism that could influence the increased resistance of curliated bacteria against LL-37, the binding of LL-37 to wild-type curli and recombinant CsgA was assessed. A precipitation assay showed a pronounced decrease of LL-37 in supernatants from samples containing wild-type curli or polymerized CsgA (Figure 5A). Further, LL-37 binding to both monomeric and polymeric CsgA was demonstrated by surface plasmon resonance (Figure 5B). By comparing the response during loading of the peptide in a time frame of 0–180 s, the sensogram of LL-37 demonstrated a higher association with CsgA than the control peptides, i.e. scrambled LL-37 (sLL-37) and the vasoactive intestinal peptide (VIP) [27]. Furthermore, the binding curves reveal that the control peptides had faster dissociation rates than LL-37, indicating a weaker binding to CsgA. This was especially pronounced for the binding to polymeric CsgA. Determination of binding constants was precluded, since LL-37 and in particular CsgA forms oligomers and polymers, respectively, and thus generate several different assemblies.
Fig. 5. LL-37 binds to recombinant polymerized CsgA and isolated wild-type curli. 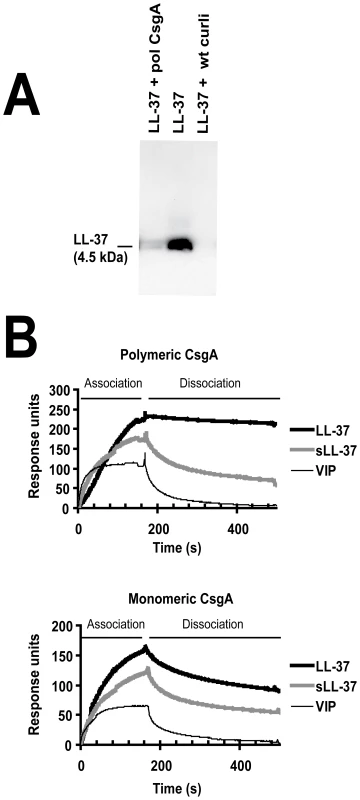
(A) Western blot analysis of supernatants after precipitation of LL-37 with curli. By adding polymeric CsgA (pol CsgA) or wild-type curli (wt curli) to a solution of 0.1 µM LL-37, the levels of LL-37 decreased in the supernatants after centrifugation. (B) Surface plasmon resonance. LL-37 exhibits a stronger association and lower dissociation rates to both polymeric (upper panel) and monomeric CsgA (lower panel) compared to the control peptides sLL-37 and VIP. LL-37 prevents adherence and biofilm formation in vitro
At concentrations below the IC50 for bacterial growth, LL-37 inhibited curli-mediated biofilm formation with a reduction of more than 80% at 2.5 µM for both the wild-type (data not shown) and the cellulose-negative E. coli strain (Figure 6). To investigate the specificity for the inhibitory capacity, sLL-37 and VIP were analyzed in the same assay. Our results showed that the same concentration of these peptides reduced biofilm formation by only 10%, which is a significantly lower reduction than the effect of LL-37 (P = 0.001, Figure 6B). This indicates a sequence-specific inhibition of curli-mediated biofilm by LL-37.
Fig. 6. LL-37 prevents formation of biofilm by E. coli in vitro. 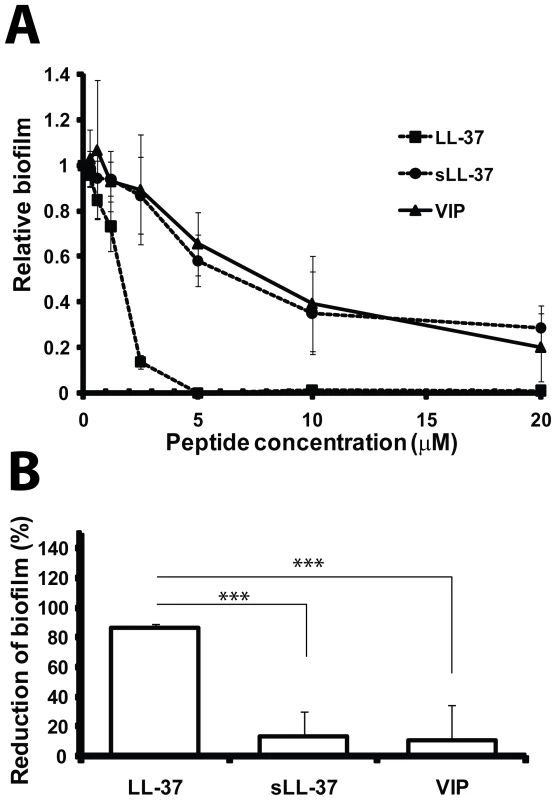
(A) Different concentrations of LL-37 and the control peptides sLL-37 and VIP were added to the curli-expressing mutant. (B) At 2.5 µM, LL-37 caused more than 80% reduction of biofilm production, whereas the same concentration of the control peptides gave a reduction of only ∼10%. Mean and standard deviation from data of two separate experiments in triplicates are shown. The difference between LL-37 versus sLL-37 or VIP at 2.5 µM was statistically significant (*** P = 0.001, t-test). Similar results were obtained for the wild-type strain expressing both curli and cellulose (data not shown). LL-37 inhibits the polymerization of CsgA
To explain a possible cause for the inhibition of biofilm formation by LL-37, the effect of LL-37 on curli formation was investigated. For this purpose, we utilized monomeric recombinant CsgA, the major subunit of curli, which spontaneously polymerizes [28]. CsgA polymerization was monitored with thioflavin T (ThT), a fluorescent dye that binds to polymerized, but not to monomeric CsgA. Our results demonstrated that CsgA polymerization started immediately after incubation at 37°C and reached a stationary phase after approximately 300 min (red line in Figure 7A). After prolonged incubation, the fluorescence declined, most likely due to degradation of ThT and/or precipitation of fibers [28]. The polymerization was inhibited by LL-37 in a dose-dependent manner, and at a molar ratio of 1∶1 (CsgA∶LL-37) fiber formation was completely inhibited (Figure 7A, left panel). The control peptides sLL-37 and VIP had a less pronounced effect on CsgA polymerization than LL-37 (Figure 7A, right panel).
Fig. 7. LL-37 inhibits CsgA polymerization. 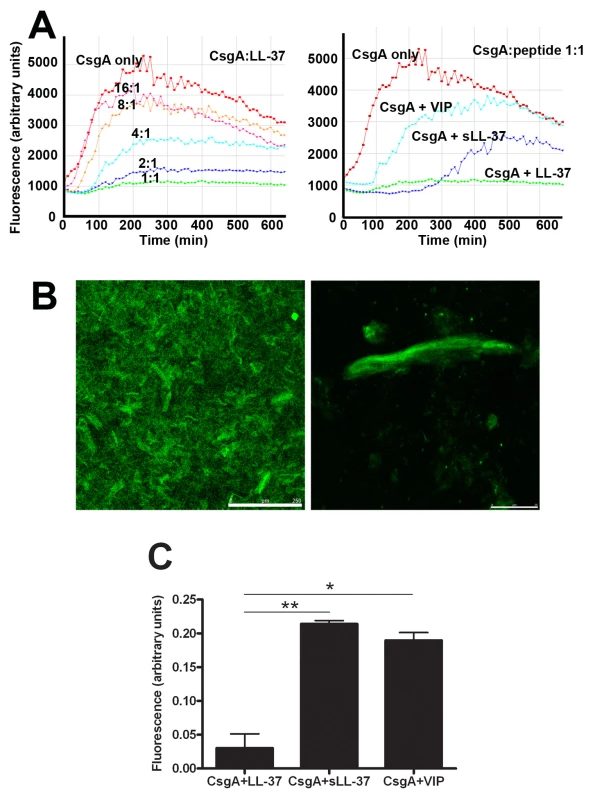
(A) Monomeric CsgA was incubated without or with different concentrations of LL-37 (left). The CsgA monomers formed fibers that could be detected by the fiber-specific fluorescent dye Thioflavin (ThT). When bound to fibers, ThT gave rise to fluorescence that was detected by a Tecan plate reader. The fiber formation was inhibited by LL-37 in a dose-dependent manner and was completely inhibited at a molar ratio of 1∶1 (CsgA∶LL-37). As control peptides, VIP and sLL-37 were included (right). (B+C) Confocal images of polymerized CsgA stained with ThT. (B) The left image shows an overview, the right image a magnification of an aggregate of fibers. Scale bars are 250 µm (left) and 25 µm (right), respectively. (C) Fluorescent signals from CsgA incubated with LL-37, sLL-37, or VIP were quantified. Values were corrected for the contribution of the peptides themselves and are expressed in relation to the signal from CsgA alone. The inhibitory effect of LL-37 is significantly stronger than the effect of sLL-37 (** P = 0.007) or VIP (* P = 0.011). Combined results from two experiments are presented. Similar results could be achieved using confocal microscopy. After 20 h incubation of CsgA alone, we could clearly detect fiber structures stained with ThT (Figure 7B) or Congo red (data not shown). In line with the results described above, these fibers were not detected when LL-37 was present in a molar ratio of 1∶1, giving a fluorescence of only 0.05 arbitrary units compared to CsgA alone (1 arbitrary unit, Figure 7C). The control peptides sLL-37 and VIP reduced the fluorescence intensity to approximately 0.2 arbitrary units, suggesting a lower inhibitory capacity than LL-37. Thus, inhibition of CsgA fiber formation was evidently stronger for LL-37 (P = 0.007 and P = 0.011 versus sLL-37 and VIP, respectively, Figure 7C).
The structure and the levels of CsgA monomers remain stable in the presence of LL-37
To confirm the inhibition of CsgA polymerization we sought to analyse the stability of the CsgA monomer in the absence and presence of LL-37. Freshly purified, monomeric CsgA (10 µM) was incubated for 20 h at 37°C without or with different concentrations of LL-37 and was subsequently separated by SDS-PAGE. After staining with Coomassie Blue, bands corresponding to LL-37 and/or CsgA in monomeric, dimeric or tetrameric form were visualized. When CsgA was incubated alone, monomers were not visible although sometimes dimers and/or tetramers could be observed. This finding indicates spontaneous formation of CsgA oligomers and/or larger polymers that can not migrate into the gel due to their size. However, in the presence of LL-37, a band migrating at 15 kDa, the predicted size of monomeric CsgA, could be observed (Figure 8A). This was already seen at a molar ratio of 16∶1 (CsgA∶LL-37). To exclude degradation of CsgA as a possible explanation for the lack of the gel band, polymerized CsgA was treated with 90% formic acid, dissolving polymeric CsgA into monomers. After this treatment, a band corresponding to monomeric CsgA was detectable in the gel (Figure 8A).
Fig. 8. The monomeric form of CsgA remains stable in the presence of LL-37. 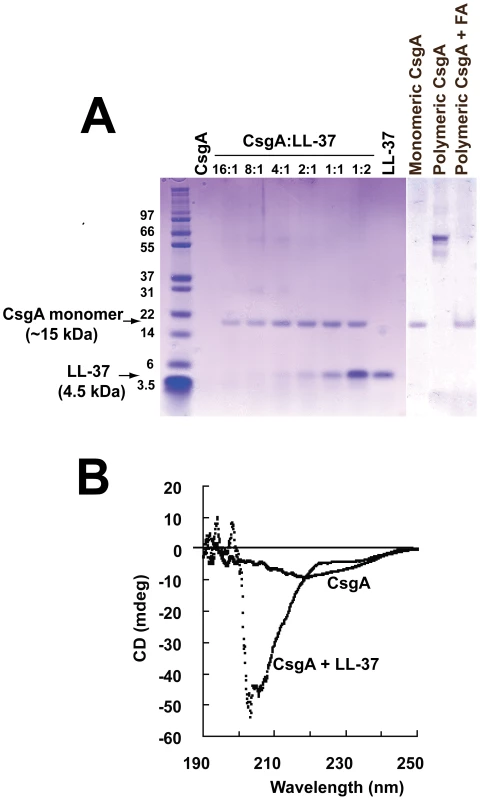
(A) Monomeric CsgA was incubated for 20 h at 37°C without or with LL-37. When CsgA is incubated together with LL-37, the CsgA monomer is visible after SDS-PAGE, whereas the monomer is not detected in the absence of LL-37. When polymeric CsgA is treated with formic acid (FA), the CsgA monomer is detectable, excluding degradation of CsgA. The bands of ∼30 and ∼60 kDa are most likely the dimer and tetramer of CsgA, respectively. (B) The stability of the monomeric form of CsgA in the presence of LL-37 was also confirmed with CD spectroscopy after 60 h incubation. The CD spectrum reveals that CsgA alone exhibits a fiber-like structure with weak beta-sheet conformation and a decreased solubility. In contrast, CsgA incubated together with LL-37 displays an unstructured, random coil structure. The spectrum of LL-37 alone was subtracted from the spectrum of CsgA + LL-37. The impact of LL-37 on the structure of curli was investigated with circular dichroism (CD) spectroscopy. In line with previous findings, polymeric CsgA exhibited a beta-sheet conformation with a minimum around 218–220 nm (Figure 8B) [28]. Furthermore, the low signal amplitude indicates a decreased solubility due to polymerization (Figure 8B). In contrast, CsgA together with LL-37 displayed a random coil structure as has been described for monomeric CsgA [28]. This result indicates that LL-37 is able to stabilize CsgA in an unstructured form.
Discussion
In the present study, we show that the majority of uropathogenic E. coli from uncomplicated community-acquired UTI adheres stronger and produces more biofilm compared to commensal bacteria. Two major extracellular components in E. coli biofilm are curli and cellulose. We here sought to explore their role in the course of UTI and their interaction with the human antimicrobial peptide LL-37. During early stages of UTI, curli promote colonization and immune induction. Cellulose in contrast reduces MIP-2 induction, followed by impaired bacterial eradication by neutrophils. The antimicrobial peptide LL-37 produced by uroepithelial cells and neutrophils in the urinary tract interacts with curli-mediated biofilms. Curli bind LL-37, and thus protects the bacterial cell against the bactericidal activity of LL-37. On the other hand, by binding to CsgA monomers and likely also shorter oligomers, LL-37 inhibits CsgA polymerization and curli formation.
We here for the first time provide evidence that curli are present on E. coli in fresh urine of infected patients that are not catheterized (Figure 1B). The expression of curli or cellulose was equally common among E. coli isolates from UTI and commensal fecal isolates. However, the combined expression of curli and cellulose was the most common phenotype among uropathogenic isolates whereas most of the fecal isolates expressed only curli.
In the pathogenesis of infection, curli fimbriae have previously been implicated in the attachment and invasion of host cells, interaction with host proteins and activation of the immune system [11], [29], [30]. Cytokine induction by Salmonella has been associated with binding of CsgA to toll-like receptor 2 [31], which is expressed on bladder and renal epithelial cells [32]. In our clinical samples, urinary IL-8 levels did not correspond to curli expression, and did not differ between isolates expressing different biofilm morphotypes (data not shown). However, virtually all tested clinical UTI isolates expressed type 1 fimbriae. Since type 1 fimbriae and other bacterial factors such as lipopolysaccharides are potent inducers of IL-8, the impact of curli on IL-8 induction was possibly masked [32], [33]. In addition, it can not be ruled out that the lag time between onset of symptoms and the first visit to the hospital, when urine samples were obtained, also influenced the results.
However, we did observe a clear correlation between curli expression and IL-8 induction in bladder and renal epithelial cells (Figure 2B) as well as MIP-2 in mice infected with the isogenic strains (Figure 2F). Interestingly, curli-dependent IL-8 induction was also observed in A498 kidney cells, which have been found to lack toll-like receptor 2 [32], [34]. It has been reported for this cell line, that IL-8 induction is probably increased by type 1 fimbriae-mediated attachment [32], [35]. We speculate that adherence enhanced by curli could similarly increase the immune induction in A498 cells in our experiments. Another explanation would be that there is an alternative route not yet identified mediating the immune response.
Recruitment of neutrophils is mediated by IL-8 and MIP-2 in humans and mice, respectively [33], [36]. The crucial function of MIP-2 and neutrophils in the defense of the urinary tract [25] is illustrated here by less efficient elimination of the curliated, highly immunogenic cellulose mutant strain in neutrophil-depleted mice (Figure 3B). In contrast, clearance of the wild-type strain expressing cellulose is not significantly influenced by the lack of neutrophils (Figure 3B), consistent with low levels of MIP-2 detected in wild-type infected mice (Figure 2F). It is well known that in wound healing bacterial cellulose itself does not induce inflammation [37]. The role of bacterial cellulose in the pathogenesis of infections, however, has previously not been established. Our results suggest a protective role against the immune system. Cellulose might mask bacterial surface structures, hence avoiding immune recognition and cytokine induction, or alternatively, actively decrease the immune response. In the cell culture model, we see a significant reduction of IL-8 after infection with the wild-type strain compared to the mutant expressing only curli, whereas there is no reduction due to cellulose in the absence of curli. It is reasonable to believe that cellulose might be able to cover the relatively short curli fibers but not longer structures such as type 1 fimbriae, which are expressed by all four strains utilized in this study. In the mouse model, we see a reduced MIP-2 induction in the presence of cellulose also in the absence of curli (Figure 2F). Moreover, the inhibitory effect of cellulose on MIP-2 induction appears to be stronger. Thus, the production of cellulose might be an efficient protection for bacteria not only against environmental conditions but also against immune defense mechanisms in vivo.
We have recently shown that LL-37 plays a crucial role in urinary tract innate immune defense [20]. Here we demonstrate increased resistance of curliated bacteria towards the antimicrobial properties of uroepithelial cells (Figure 4A–C), which is at least partly based on increased resistance against LL-37 (Figure 4C–E). We confirmed the relevance of this observation for the mouse infection model by investigating the susceptibility of wild-type and mutant E. coli against mCRAMP, the murine LL-37 ortholog. Our results revealed that the curliated strains are more resistant also against the mouse cathelicidin (Figure 4F+G), indicating a similar interaction with curli as demonstrated for LL-37.
Curli fibers are mainly composed of polymerized CsgA. By precipitation of LL-37 in the presence of wild-type curli or recombinant polymeric CsgA, we demonstrate binding between the peptide and the protein (Figure 5A). Antimicrobial peptides including LL-37 kill their target cells by a peptide-bacterial membrane interaction that leads to lysis of the bacterium [24]. Our finding suggests that LL-37 might be trapped in the net of curli covering the bacterial surface. This prevents LL-37 from reaching the bacterial membrane and lysing the cell. In contrast, bacteria without curli lack this protection and are more easily killed during adherence and invasion into the uroepithelium. A similar protective role against cationic antimicrobial peptides has been observed for the biofilm components alginate in Pseudomonas aeruginosa [38] and the polysaccharide intercellular adhesion (PIA) in Staphylococcus epidermidis [39]. We also observed partial protection mediated by cellulose, the polysaccharide component in E. coli biofilm (Figure 4A+B). However, this effect was not as pronounced as the protection by curli and could not be related to LL-37, since cellulose production did not influence bacterial susceptibility to the cathelicidins LL-37 or mCRAMP in vitro (Figure 4D–G).
Interestingly, we demonstrate that LL-37 inhibits the formation of curli-promoted biofilm formation in vitro (Figure 6). We also show that LL-37 prevents CsgA polymerization (Figures 7+8), and speculate that LL-37 inhibits biofilm establishment by direct interference with CsgA assembly. The binding of LL-37 to both monomeric and polymeric CsgA might block reactive surfaces that are crucial for the CsgA-CsgA interaction [40]. There are five segments in the CsgA amino acid sequence that are conserved and share similarity to each other. They are characterized by conserved Ser, Gln and Asn residues [11], [40], but these repeats also contain acidic residues that may contribute to an electrostatic interaction with the cationic peptide LL-37. Based on the general structures of amyloids [41], each of these repeats is predicted to form a strand-loop-strand motif in a strong hydrogen bonding network [11], which might be prohibited by the binding of LL-37. Considering the function of curli during infection, the prevention of curli generation would provide an effective host defense mechanism. Our in vivo results demonstrate, despite the initial advantage of curliated bacteria, that they are eradicated more efficiently at later stages of infection. The expression of many virulence factors is highly regulated by environmental conditions, and this has also been shown for curli. Curli are maximally expressed in stationary phase and participate in the initial stage of biofilm, i.e. irreversible attachment, whereas expression might be down-regulated during biofilm maturation correlating to later stages of infection [11], [42]. Reduced curli expression by bacteria colonizing the kidney makes them more vulnerable towards LL-37. In addition to increased bacterial sensitivity, incoming neutrophils release high amounts of LL-37 that contributes to the antibacterial defense. In growing bacteria, the generation of new curli fibers might be inhibited by LL-37, reducing both protection and ability to colonize the host tissue. Remarkably, biofilm-inhibitory concentrations of LL-37 were much lower than bactericidal concentrations and within a range which can be present in vivo [20], [43]. In contrast, subinhibitory concentrations of exogenous antimicrobial drugs, e.g. aminoglycosides, seem to stimulate bacteria to produce biofilm [44]. Moreover, bacteria grown in biofilm are less susceptible to most exogenous antimicrobial agents [45].
Biofilm inhibition by antimicrobial polypeptides has previously been described for Pseudomonas aeruginosa. Both LL-37 [46] and lactoferrin [47] increased bacterial surface motility mediated by type IV pili. A direct interaction with biofilm components was not investigated in these studies. The effect was rather related to an influence of LL-37 on the bacterial gene expression profile [46] or an influence of lactoferrin on free iron [47]. These and our findings stress an important anti-biofilm role of antimicrobial polypeptides in host defense. It is likely that the anti-biofilm activity is a general strategy for these host defense molecules to keep potential pathogenic bacteria more vulnerable to killing in various tissues, including the urinary tract.
In conclusion, we demonstrate that uropathogenic E. coli by expressing curli are able to modulate the immune response and display increased virulence. Cellulose, on the other hand, may reduce adherence and immunogenicity by masking bacterial surface structures, thereby evading the immune system. We also show that defense mechanisms in the urinary tract interfere with these biofilm components; curli protect the bacteria from being killed by LL-37, in contrast LL-37 is inhibiting the formation of curli fibers. This inhibition might be an important host defense mechanism in the protection against UTI.
Materials and Methods
Patients
The studies have been approved by the ethics committee of the Karolinska University Hospital, and written informed consent has been obtained from the patients and parents of the children, respectively, in accordance with the ethics permission.
The clinical study included 98 patients with UTI; 36 children [20] and 62 adult women [48]. One woman suffered from two episodes of UTI with different E. coli isolates. The diagnostic criterion of acute UTI was the presence of ≥105 CFU of E. coli per ml of freshly voided urine. Except for bacteriuria, the diagnostic criteria of acute pyelonephritis were: body temperature ≥38°C and laboratory signs of systemic inflammation, either C-reactive protein ≥20 mg/liter or erythrocyte sedimentation rate ≥20 mm/h, respectively. In addition, fecal commensal E. coli isolates were collected from 77 adults in connection with routine outpatient health examination. None of them had a history of symptomatic UTI or recent gastrointestinal disease, and their urine did not yield E. coli on cultivation [48].
Human cell lines
Two human bladder epithelial cell lines and one human renal epithelial cell line were utilized. Virus-immortalized bladder epithelial cells UROtsa were kindly provided by Prof. Scott Garrett, Department of Pathology, University of North Dakota and cultured as described previously [20], [49]. Bladder epithelial cells T24 (HTB-4; American Type Culture Collection (ATCC), Rockville, MD, USA) were cultured in McCoy's 5A medium containing glutamine (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen). Human renal epithelial cells A498 (HTB-44; ATCC) were cultured as described before [20].
Bacteria for in vitro and in vivo experiments
For further investigation, E. coli isolate No. 12 from a child with pyelonephritis was chosen. This was a typical isolate expressing both curli and cellulose as well as type 1 fimbriae and yielding an approximately median level of biofilm as measured on microtiter plates. One-step knockout of bcsA and csgBA was carried out according to the protocol of Datsenko and Wanner with modifications [17], [50]. The following mutants were constructed using oligos listed in Table 1; WE1 bcsA::Cm, deficient in cellulose production; WE11 csgBA::Cm, deficient in curli production; and WE16 csgBA bscA::Cm, deficient in both cellulose and curli production. Expression of curli and cellulose in strain No. 12 was confirmed by these knockouts. Production of type 1 fimbriae under the culture conditions used for experiments was confirmed by yeast agglutination (see below).
Tab. 1. Oligos and plasmids used for mutant construction and complementation. 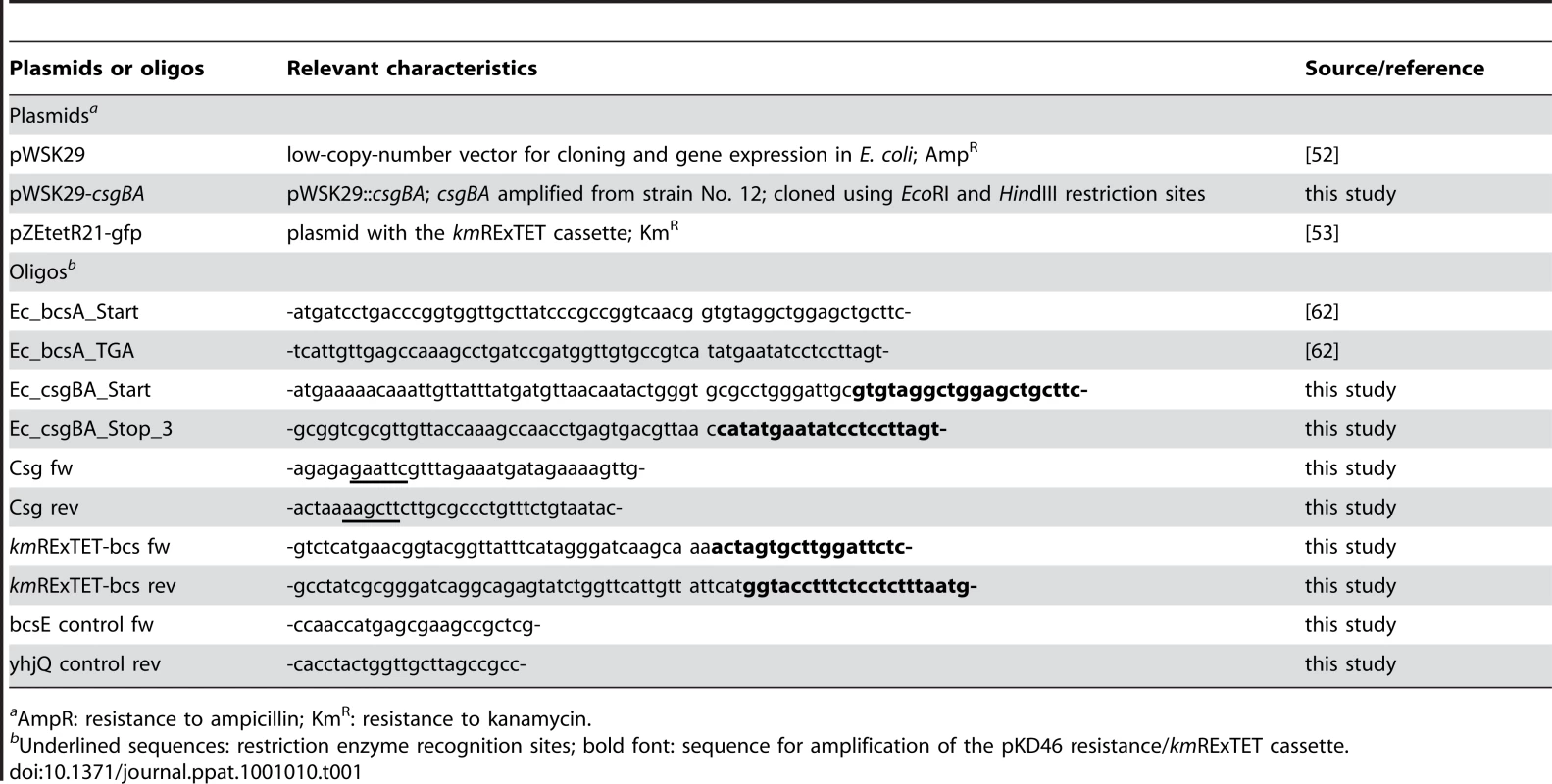
AmpR: resistance to ampicillin; KmR: resistance to kanamycin. For relevant control experiments, complementation equivalents for the mutants WE1 and WE11 were constructed. The complementation of strain WE11 was achieved as follows: First, the chloramphenicol cassette of the curli-deficient strain WE11 csgBA::Cm was removed by Flp-catalyzed excision as described elsewhere [51]; resulting in the Cm-sensitive, curli synthesis-deficient strain WE11_1. The removal was confirmed by PCR using oligos Csg fw and Csg rev (Table 1). The DNA region comprising the csgBA operon was amplified from strain No. 12 using the above mentioned oligos and cloned into vector pWSK29 [52]. Finally, the obtained plasmid pWSK29-csgBA was transferred into strain WE11_1. The ability of strain WE11_1 containing pWSK29-csgBA to produce curli was demonstrated by morphotype assessment on Congo red plates.
The complementation of the cellulose production-deficient strain WE1 proved to be more complex, since plasmid-based complementation approaches failed. Therefore, strain B23 was constructed which originates from wild-type strain No. 12 and carries an inducible promoter upstream of the bcs operon. In short, the previously described kmRExTET cassette [53] which contains the anhydrotetracycline (aTc) inducible tetA promoter was amplified using oligos kmRExTET-bcs fw and kmRExTET-bcs rev (Table 1) and inserted upstream of the bcs operon using the protocol of Datsenko and Wanner with modifications [50]. Insertion of the kmRExTET cassette was confirmed by PCR using oligos bcsE control fw and yhjQ control rev (Table 1). In the resulting strain B23, cellulose production became an aTc-dependent occurrence due to the insertion of the kmRExTET cassette as previously communicated [54]. In the absence of aTc, the morphotype of strain B23 is consistent to the morphotype of strain WE1 bcsA::Cm. In presence of aTc, cellulose production in strain B23 is restored to comparable levels than in the wild-type strain No. 12 as judge on Congo red plates. Thus strain B23 grown under inducing conditions can be used as a complemented equivalent.
Biofilm assays
Microtiter plate method to measure bacterial adhesion and thickness of biofilm
To measure the ability of the bacteria to adhere and to form biofilm a crystal violet assay in polystyrene microtiter plates (Costar, Corning, NY, USA) was performed [17]. Bacteria were grown in Luria-Bertani (LB) broth without salt for 24 h at 37°C without shaking. Biofilm was then stained with crystal violet (3%). The dye was solubilized with ethanol (95%) and the optical density was measured at 570 nm.
Morphotype analysis on Congo red and Calcofluor plates
Bacteria were grown at 37°C for 24 h on Congo red and Calcofluor plates and analyzed as described previously [17]. The mutants generated from strain No. 12 served as controls for the analysis of clinical isolates.
Expression of type 1 fimbriae
Expression of type 1 fimbriae was tested by mannose-sensitive agglutination of yeast cells. Bacteria were grown in LB broth without shaking to induce expression of type 1 fimbriae, centrifuged and suspended in PBS (approximately 1010 CFU/ml). Bacteria were mixed 1∶1 with a suspension of Baker's yeast (Saccharomyces cerevisiae, 3% in PBS) and inspected for agglutination. Specificity of the reaction was tested by the inhibitory effect of mannose (5% in PBS). Bacteria were subcultured in broth up to three times before considered negative for expression of type 1 fimbriae. To quantify fimbrial expression in the isogenic strains, the bacterial suspension was subjected to serial two-fold dilution and mixed with an equal volume of yeast suspension. Agglutination was monitored and the optical density of the highest dilution giving a positive result was recorded. Specificity of agglutination was confirmed by mannose sensitivity.
Cell experiments
All assays were performed using both bladder (UROtsa, T24) and renal epithelial (A498) cells grown on 24-well plates (Costar). Experiments were performed in quadruplicates and repeated at least three times independently. Wild-type and mutant E. coli strains were cultured for 24 h at 37°C on LB agar plates without salt to promote the formation of biofilm. Medium was supplemented with ampicillin (100 µg/ml) or aTc (50 ng/ml) if appropriate. Colonies were scraped off and suspended in PBS. Bacterial cell clusters were then removed by centrifugation at 150×g for 10 min. The number of bacteria was determined spectrophotometrically at 600 nm and confirmed by viable count on blood agar plates after serial dilutions in PBS.
Cell infection
The experiments were performed in serum-free medium supplemented with gentamicin (40 µg/ml). Confluent layers of cells were infected with 106 CFU/ml of E. coli. Cells incubated with medium only served as controls. After incubation at 37°C in 5% CO2 and 80% humidity for 24 h, medium was aspirated, centrifuged at 350×g for 10 min and stored at −20°C prior to ELISA analysis. The viability of cells during the experiments was confirmed using the Trypan blue method.
Adhesion assay
To evaluate the ability of bacteria to adhere to epithelial cells, 106 CFU/ml of E. coli was added to cell culture wells in serum - and antibiotic-free medium and incubated at 37°C. After 10 or 30 min, cells were washed three times with PBS (37°C) to remove non-adherent bacteria. To collect cell-associated bacteria, ice-cold PBS with 1% Triton X-100 was added. Lysates were plated on blood agar plates after serial dilution in PBS and bacterial numbers were counted after over-night incubation at 37°C.
Epithelial cell antimicrobial assays
To access the inhibitory activity of epithelial cells on E. coli growth and viability two experimental settings were employed.
Antimicrobial activity on adherent bacteria
The viability of cell-adherent bacteria expressing or lacking curli and/or cellulose was investigated by LIVE/DEAD staining. For this purpose, E. coli were cultured as described above and 107 CFU/ml in serum - and antibiotic-free medium were added to confluent cell layers grown on sterile glass cover slips. After 30 min of incubation at 37°C, non-adherent bacteria were removed by washing three times with PBS. In order to include intracellular bacteria in the staining process, cells were permeabilized with saponin (0.2% in PBS, Sigma-Aldrich, St. Louis, MO, USA) for 5 min before addition of the two-component LIVE/DEAD BacLight stain (Invitrogen) diluted in 0.2% saponin. After 5 min, cells were washed with PBS and lightly fixed in freshly diluted 0.1% paraformaldehyde (PFA) for 15 min. At this concentration, PFA did not affect the fluorescence intensity of any of the dye components. Cover slips were mounted in ProLong Gold antifade mounting medium (Invitrogen) and immediately examined in a Leica TCS SP5 confocal microscope. To ensure correct discrimination between live and dead bacteria, stained green and red, respectively, microscope filter acquisition settings were adjusted using preparations of live and/or dead bacteria. Staining of the cell nucleus with both the cell permeable green dye and the cell impermeable red dye served as a positive control for cell permeabilization. Red or green bacteria were manually counted in microscope images of two separate preparations of three or four independent experiments.
Antimicrobial activity of conditioned medium
In order to investigate secreted antimicrobial components and to specify the active compound, bacteria were incubated in conditioned medium. To especially investigate the influence of LL-37 on bacterial viability in relation to curli and cellulose expression, production of LL-37 was stimulated with phenylbutyrate prior to medium collection [26]. Cells were grown in complete medium to reach ∼80% confluence and medium was then exchanged to serum-free medium supplemented with 4 mM 4-phenylbutyrate (Tocris Bioscience, Bristol, UK). After additional 48 h, medium was collected and cells were removed by centrifugation at 300×g for 10 min. Bacteria grown as described above and suspended in PBS were added to conditioned medium at a final concentration of 104 CFU/ml. Aliquots of 150 µL were transferred to wells of a polypropylene microtiterplate (Costar) and incubated at 37°C for 30 min with shaking. Thereafter, the number of live bacteria in the conditioned medium was determined by viable count and expressed in relation to the concentration in the inoculum.
To relate the antimicrobial effect to LL-37, monoclonal mouse anti-LL-37 antibodies [55] were added to the conditioned medium at a concentration of 1 µg/ml and incubated for 30 min at 37°C prior to inoculation of bacteria. For control purposes, supernatants were equally treated with mouse IgG1κ isotype control antibodies (BD Biosciences, San Diego, CA, USA). The antimicrobial activity was determined as described above.
Measurement of IL-8 and MIP-2 levels
Before ELISA analysis, urine was centrifuged at 350×g for 10 min to remove cells and larger particles. ELISA kits for human IL-8 or mouse MIP-2 were obtained from R&D systems (Abingdon, UK). IL-8 or MIP-2 levels were determined according to the manufacturer's instructions. The lower detection limit for IL-8 and MIP-2 was 31.3 pg/ml and 15.6 pg/ml, respectively. The urinary levels of creatinine were analyzed colorimetrically, and the levels of IL-8 were expressed as IL-8/creatinine ratios.
Electron microscopy of E. coli in fresh urine
Urine was collected from patients with E. coli UTI and without having a catheter. A drop of urine was incubated on carbon/Formvar-coated 400-mesh copper grids (GilderGrids, Lincolnshire, UK) for one minute. Immunostaining was performed as described previously with minor modification [56]. Briefly, grids were blocked with 1% BSA/PBS for 5 min, then incubated with anti-CsgA [28] (1∶200 in 0.1% BSA/PBS) for 60 min at 37°C, followed by incubation with anti-rabbit IgG-10-nm gold antibodies (1∶15 in 0.1% BSA/PBS; Sigma-Aldrich) for 30 min at 37°C. Grids were stained with 2% tungstophosphoric acid (Merck, Darmstadt, Germany) at pH 6. Analysis were performed using a FEI Tecnai Spirit electron microscope (Eindhoven, The Netherlands) at 80 kV accelerating voltage.
Dot blot analysis
Urine was centrifuged at 300×g for 10 min to remove cells and larger particles. Bacteria from the supernatant were collected by centrifugation at 3500×g for 10 min. A 2-µL aliquot of the pellet suspended in a minimal volume of PBS was transferred to a nitrocellulose membrane (Invitrogen) and air dried for 15 minutes. Immunostaining was performed as described previously [56]. Briefly, membranes were blocked with 1% milk/1% BSA/PBS for 2 h at room temperature, incubated with anti-CsgA [28] (1∶5000 in 1% milk/1% BSA/PBS) for 1 h, followed by incubation with anti-rabbit horse-radish-peroxidase conjugated antibodies (1∶3000 in 1% milk/1% BSA/PBS; Bio-Rad Laboratories, Hercules, CA, USA) for 1 h.
Animal experiments
Mouse experiments were approved by the Northern Stockholm Animal Ethics Committee and experiments were carried out according to FELASA guidelines and in compliance with the Committee's requirements.
Bacterial infection
Female NMRI mice of 8–10 weeks age were caged in groups of 3–5 animals in standard cages. After water deprivation for 4 h, mice were anaesthetized with isoflurane (Forene™; Abbott Scandinavia, Solna, Sweden) and infected transurethrally with 50 µl of a bacterial suspension of 109 CFU/ml, using a soft sterile polyethylene catheter (outer diameter 0.61 mm, inner diameter 0.28 mm; Clay Adams, Becton Dickinson, Franklin Lakes, NJ, USA) with lubricant. Sterile PBS was used for control mice. After 1, 16 or 48 h, mice were sacrificed by cervical dislocation and bladders and kidneys were aseptically removed. Bladders taken 1 h p.i. were cut open and washed in ice-cold PBS three times to remove non-adherent bacteria. The organs were homogenized in 1 ml PBS and serial dilutions of the homogenate were plated on blood agar plates for viable count. For measurement of MIP-2, homogenized samples were centrifuged at 350×g for 10 min and the supernatants were stored at −20°C prior to ELISA analysis. A total of 126 animals including controls were infected. Mice without bacterial growth in any of the organs were regarded as non-infected and therefore excluded from further analysis; only MIP-2 values from infected organs were used for statistical analysis.
Neutrophil depletion
To induce neutropenia, 100 µg of RB6-8C5 monoclonal antibody (R&D systems) was administered intravenously 24 h before infection [20]. Control animals received an equal volume of sterile saline. Neutropenia at the time of infection and at the end of the experiment was confirmed on Giemsa-stained blood smears.
Bacterial sensitivity to LL-37 and mCRAMP
The susceptibility of wild-type and mutant E. coli strains to synthetic LL-37 and mCRAMP (Innovagen AB, Lund, Sweden) was determined using a broth microdilution method. Briefly, bacteria were grown overnight at 37°C on LB plates with or without salt, inhibiting or promoting the formation of biofilm, respectively. Then, bacteria were suspended in PBS and diluted in LB broth without salt in a concentration of 105 CFU/ml. The bacterial concentration was verified by viable count after serial dilutions in PBS. Bacteria (90 µl suspension) were grown in 96-well plates in the presence of 10 µl aqueous solution of synthetic LL-37 or mCRAMP in final concentrations ranging from 0.6 µM to 20 µM in 2-fold dilutions. After 20 h, bacterial viability was measured colorimetrically by reduction of Alamar blue (BioSource International, Camarillo, CA, USA) for 1 h at 37°C [57]. The IC50 was determined as the peptide concentration that gave 50% reduction of the absorbance at 570 nm relative to bacteria grown without peptide.
Effect of LL-37 on formation of biofilm
Plates were filled with 90 µL bacterial culture of planktonic cells and 10 µL of aqueous solution of LL-37 in the same concentrations as in the susceptibility assay. As control peptides, sLL-37 (Innovagen AB) and VIP [27] with similar structural properties as LL-37 were utilized. The plates were incubated without shaking at 37°C for 20 h. After incubation, the amount of biofilm formed was determined as described above.
Purification of recombinant CsgA-His6
CsgA-His6 and CsgG, a lipoprotein that is required for CsgA secretion [58], were overexpressed in LSR12 (C600::csg). The purification procedure of recombinant CsgA-His6 was performed as previously described with some modifications [59]. The filtrate was incubated with nickel-nitrilotriacetic acid (Ni-NTA) agarose (Invitrogen) for 1 h at 4°C with shaking, centrifuged at 200×g for 5 min and transferred to a polyprep chromatography column (Bio-Rad, Hercules, CA, USA). To confirm protein identity of CsgA-His6, the isolated protein was prepared and subjected to SDS-PAGE as described below, and thereafter the protein was transferred from the gel to a polyvinylidene fluoride (PVDF) membrane (Invitrogen) at 160 mA for 60 min. The band of 15 kDa, corresponding to the molecular weight of CsgA-His6, was excised from the membrane stained with Coomassie Blue and analysed with N-terminal sequence analysis as has been described [60]. Since it has previously been shown that CsgA-His6 has the same polymerizing properties as wild-type CsgA [59], we refer to CsgA-His6 as CsgA.
Isolation of wild-type curli
The cellulose-deficient isogenic mutant of E. coli No. 12 was used to purify wild-type curli, as has been described [17]. Colonies were harvested and suspended in 0.05 M Tris-buffer by using an omnimixer. Bacterial cell debris was discarded by centrifugation at 8000×g for 15 min and curli protein was precipitated by adding 0.1 M MgCl2 and 0.15 M NaCl. The aggregated curli were centrifuged at 16000×g for 15 min, and the pellet was dissolved in 10 mM TRIS, 1 mM EDTA, pH 7.5 with 2% 3-((3-cholamidopropyl)dimethylammonium)-1-propanesulfonate (CHAPS). After incubation for 45 min at 95°C, the solution was centrifuged at 20000×g for 10 min. The pellet containing curli was washed three times with water. Finally, curli were suspended in PBS and used for binding studies with LL-37.
Binding of LL-37 to CsgA
Precipitation of LL-37 with curli
LL-37 (0.1 µM in PBS) was mixed with 5 µM wild-type curli, or 5 µM recombinant polymerized CsgA. As control, the same concentration of LL-37 without curli or CsgA was utilized. The samples were incubated for 1 h at 37°C with shaking and centrifuged for 30 min at 10000×g. An aliquot of the supernatants was analyzed for the presence of LL-37 with Western blot.
Surface plasmon resonance
Binding analysis of LL-37 to both monomeric and polymeric CsgA was performed with surface plasmon resonance (Biacore 3000 instrument; Biacore AB, Uppsala, Sweden). Both monomeric and polymeric CsgA was immobilized on a CM5 sensor chip (Biacore AB) surface by amine coupling. The chip surface was normalized in 70% glycerol and lanes on the chip were activated with injection of 35 µL 0.05 M N-hydroxysuccinimide (NHS)/0.2 M N-ethyl-N′-[3-dimethylamino) propyl]carbodiimide (EDC). Polymeric or monomeric CsgA (1 µM in 10 mM sodium acetate, pH 4.5) was then immobilized on the chip to 7000 response units and 2000 response units, respectively. After immobilization, the lanes were subjected to 60 µL ethanolamine (1 M, pH 8.5) to deactivate remaining activated carboxylic groups. One lane, activated and deactivated, was used as negative control (without addition of CsgA). Standard Biacore HBS-EP (Biacore AB) was utilized as running buffer, and 0.1 µM LL-37, sLL-37 or VIP were injected at 20 µl/min for 3 min. The surface was regenerated after each cycle with 100 mM HCl.
SDS-PAGE
Samples were prepared in LDS (lithium dodecyl sulphate) sample buffer (4∶1) and incubated at 70°C for 10 min. Electrophoresis was performed using 4–12% Bis-tris NU-PAGE gels (Invitrogen) at 200 V for 35 min. Gels were stained with Coomassie Blue for 1 h and destained over night in 90% water, 8% methanol and 2% acetic acid (vol/vol/vol).
Western blot analysis
Sample preparation and SDS-PAGE was carried out as described above and Western blot was performed as previously described [20]. The antibodies used were monoclonal mouse anti-LL-37 (0.6 µg/ml in 5% fat-free milk/PBS) [55] and horse radish peroxidase-conjugated anti-mouse IgG (diluted 1∶5000).
CD spectroscopy
Samples containing 40 µM CsgA in 50 mM potassium phosphate buffer (KPi) and 0.02% NaN3, pH 7.2, with and without 10 µM LL-37 were incubated for 60 h at 37°C. The samples were then assayed with a Jasco J-810 spectropolarimeter from 190 to 250 nm in a quartz cell with a 1-mm path length at 20°C. The spectrum of buffer alone and LL-37 in buffer was subtracted from the spectra for CsgA alone and for CsgA together with LL-37, respectively.
Thioflavin T assays
Tecan plate reader
Purified recombinant monomeric CsgA (10 µM) with or without different concentrations of the peptides LL-37, sLL-37 or VIP was mixed with 20 µM of the fiber-specific fluorescent probe ThT (Sigma-Aldrich). Fluorescence was measured with a Tecan infinite M200 reader (Tecan Nordic AB, Täby, Sweden). The excitation and emission wavelength was 430 and 490 nm, respectively. Measurements were conducted at 37°C every 10 min after shaking the sample for five seconds. Background fluorescence of the peptides themselves was subtracted.
Confocal microscopy
Recombinant monomeric CsgA was mixed with equimolar LL-37, sLL-37 or VIP and 20 µM ThT, and incubated over night at 37°C, washed twice, and suspended in 10 mM Kpi, pH 7.2 before mounting in ProLong Gold antifade mounting medium (Invitrogen). Images were acquired on a Leica TCS SP5 confocal microscope using a 20× objective. Fluorescence intensity was quantified using ImageJ software [61]. The difference in fluorescence intensity of CsgA with and without peptides was calculated and corrected for the contribution of fluorescence from the peptides themselves.
Data analysis
Data were compared with student's t-test, Mann-Whitney U test or Fisher's exact test as appropriate. P values of less than 0.05 were considered as statistically significant.
Zdroje
1. RobertsRC
MohrCD
ShapiroL
1996 Developmental programs in bacteria. Curr Top Dev Biol 34 207 257
2. CostertonJW
LewandowskiZ
CaldwellDE
KorberDR
Lappin-ScottHM
1995 Microbial biofilms. Annu Rev Microbiol 49 711 745
3. FolkessonA
HaagensenJAJ
ZampaloniC
SternbergC
MolinS
2008 Biofilm induced tolerance towards antimicrobial peptides. PLoS ONE 3 e1891
4. Hall-StoodleyL
CostertonJW
StoodleyP
2004 Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Micro 2 95 108
5. ShapiroJA
1998 Thinking about bacterial populations as multicellular organisms. Ann Rev Microbiol 52 81 104
6. TrautnerBW
DarouicheRO
2004 Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control 32 177 183
7. AndersonGG
PalermoJJ
SchillingJD
RothR
HeuserJ
2003 Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301 105 107
8. JusticeSS
HungC
TheriotJA
FletcherDA
AndersonGG
2004 Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci USA 101 1333 1338
9. AndersonGG
DodsonKW
HootonTM
HultgrenSJ
2004 Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol 12 424 430
10. SotoSM
SmithsonA
HorcajadaJP
MartinezJA
MensaJP
2006 Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin Microbiol Infect 12 1034 1036
11. BarnhartMM
ChapmanMR
2006 Curli Biogenesis and Function. Annu Rev Microbiol 60 131 147
12. ZogajX
NimtzM
RohdeM
BokranzW
RömlingU
2001 The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39 1452 1463
13. RömlingU
2002 Molecular biology of cellulose production in bacteria. Res Microbiol 153 205 212
14. RömlingU
2005 Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell Mol Life Sci 62 1234 1246
15. GualdiL
TagliabueL
BertagnoliS
IeranoT
De CastroC
2008 Cellulose modulates biofilm formation by counteracting curli-mediated colonization of solid surfaces in Escherichia coli. Microbiology 154 2017 2024
16. UhlichGA
CookePH
SolomonEB
2006 Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Appl Environ Microbiol 72 2564 2572
17. BokranzW
WangX
TschäpeH
RömlingU
2005 Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J Med Microbiol 54 1171 1182
18. WangX
RochonM
LamprokostopoulouA
LünsdorfH
NimtzM
2006 Impact of biofilm matrix components on interaction of commensal Escherichia coli with the gastrointestinal cell line HT-29. Cell Mol Life Sci 63 2352 2363
19. KikuchiT
MizunoeY
TakadeA
NaitoS
YoshidaS-i
2005 Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol Immunol 49 875 848
20. ChromekM
SlamováZ
BergmanP
KovácsL
PodrackáLu
2006 The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12 636 641
21. GudmundssonGH
AgerberthB
OdebergJ
BergmanT
OlssonB
1996 The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 238 325 332
22. Frohm NilssonM
SandstedtB
SørensenO
WeberG
BorregaardN
1999 The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with Interleukin-6. Infect Immun 67 2561 2566
23. SørensenOE
FollinP
JohnsenAH
CalafatJ
TjabringaGS
2001 Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97 3951 3959
24. OrenZ
LermanJC
GudmundssonGH
AgerberthB
ShaiY
1999 Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J 341 501 513
25. HangL
HaraokaM
AgaceWW
LefflerH
BurdickM
1999 Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol 162 3037 3044
26. SteinmannJ
HalldórssonS
AgerberthB
GudmundssonGH
2009 Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother 53 5127 5133
27. SaidS
MuttV
1970 Polypeptide with broad biological activity: isolation from small intestine. Science 169 1217 1218
28. WangX
SmithDR
JonesJW
ChapmanMR
2007 In vitro polymerization of a functional Escherichia coli amyloid protein. J Biol Chem 282 3713 3719
29. BianZ
BraunerA
LiY
NormarkS
2000 Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis 181 602 612
30. GophnaU
BarlevM
SeijffersR
OelschlagerTA
HackerJ
2001 Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect Immun 69 2659 2665
31. TükelÇ
RaffatelluM
HumphriesAD
WilsonRP
Andrews-PolymenisHL
2005 CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol 58 289 304
32. BäckhedF
SöderhällM
EkmanP
NormarkS
Richter-DahlforsA
2001 Induction of innate immune responses by Escherichia coli and purified lipopolysaccharide correlate with organ - and cell-specific expression of Toll-like receptors within the human urinary tract. Cell Microbiol 3 153 158
33. AgaceWW
1996 The role of the epithelial cell in Escherichia coli induced neutrophil migration into the urinary tract. Eur Respir J 9 1713 1728
34. FrendéusB
WachtlerC
HedlundM
FischerH
SamuelssonP
2001 Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol Microbiol 40 37 51
35. HedlundM
FrendéusB
WachtlerC
HangL
FischerH
2001 Type 1 fimbriae deliver an LPS - and TLR4-dependent activation signal to CD14-negative cells. Mol Microbiol 39 542 552
36. GodalyG
ProudfootAE
OffordRE
SvanborgC
AgaceWW
1997 Role of epithelial interleukin-8 (IL-8) and neutrophil IL-8 receptor A in Escherichia coli-induced transuroepithelial neutrophil migration. Infect Immun 65 3451 3456
37. CzajaW
KrystynowiczA
BieleckiS
BrownJRM
2006 Microbial cellulose–the natural power to heal wounds. Biomaterials 27 145 151
38. ChanC
BurrowsLL
DeberCM
2005 Alginate as an auxiliary bacterial membrane: binding of membrane-active peptides by polysaccharides. J Pep Res 65 343 351
39. VuongC
VoyichJM
FischerER
BraughtonKR
WhitneyAR
2004 Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6 269 275
40. CollinsonSK
ParkerJMR
HodgesRS
KayWW
1999 Structural predictions of AgfA, the insoluble fimbrial subunit of Salmonella thin aggregative fimbriae. J Mol Biol 290 741 756
41. MakinOS
SerpellLC
2005 Structures for amyloid fibrils. FEBS J 272 5950 5961
42. PrüßBM
BesemannC
DentonA
WolfeAJ
2006 A complex transcription network controls the early stages of biofilm development by Escherichia coli. J Bacteriol 188 3731 3739
43. OngPY
OhtakeT
BrandtC
StricklandI
BoguniewiczM
2002 Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347 1151 1160
44. HoffmanLR
D'ArgenioDA
MacCossMJ
ZhangZ
JonesRA
2005 Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436 1171 1175
45. FuxCA
CostertonJW
StewartPS
StoodleyP
2005 Survival strategies of infectious biofilms. Trends Microbiol 13 34 40
46. OverhageJ
CampisanoA
BainsM
TorfsECW
RehmBHA
2008 Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun 76 4176 4182
47. SinghPK
ParsekMR
GreenbergEP
WelshMJ
2002 A component of innate immunity prevents bacterial biofilm development. Nature 417 552 555
48. BraunerA
KatouliM
TullusK
JacobsonS
1990 Production of cytotoxic necrotizing factor, verocytotoxin and haemolysin by pyelonephritogenic Escherichia coli. Eur J Clin Microbiol Infect Dis 9 762 767
49. RossiMR
MastersJR
ParkS
ToddJH
GarrettSH
2001 The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect 109 801 808
50. DatsenkoKA
WannerBL
2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97 6640 6645
51. CherepanovPP
WackernagelW
1995 Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158 9 14
52. WangRF
KushnerSR
1991 Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100 195 199
53. Da ReS
Le QuereB
GhigoJ-M
BeloinC
2007 Tight modulation of Escherichia coli bacterial biofilm formation through controlled expression of adhesion factors. Appl Environ Microbiol 73 3391 3403
54. Le QuéréB
GhigoJ-M
2009 BcsQ is an essential component of the Escherichia coli cellulose biosynthesis apparatus that localizes at the bacterial cell pole. Mol Microbiol 72 724 740
55. YoshioH
TollinM
GudmundssonGH
LagercrantzH
JornvallH
2003 Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res 53 211 216
56. EpsteinEA
ReizianMA
ChapmanMR
2009 Spatial clustering of the curlin secretion lipoprotein requires curli fiber assembly. J Bacteriol 191 608 615
57. PettitRK
WeberCA
KeanMJ
HoffmannH
PettitGR
2005 Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother 49 2612 2617
58. RobinsonLS
AshmanEM
HultgrenSJ
ChapmanMR
2006 Secretion of curli fibre subunits is mediated by the outer membrane-localized CsgG protein. Mol Microbiol 59 870 881
59. ChapmanMR
RobinsonLS
PinknerJS
RothR
HeuserJ
2002 Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295 851 855
60. TollinM
BergssonG
Kai-LarsenY
LengqvistJ
SjövallJ
2005 Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci 62 2390 2399
61. AbramoffMD
MagelhaesPJ
RamSJ
2004 Image processing with ImageJ. Biophotonics International 11 36 42
62. MonteiroC
SaxenaI
WangX
KaderA
BokranzW
2009 Characterization of cellulose production in Escherichia coli Nissle 1917 and its biological consequences. Environ Microbiol 11 1105 1116
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



