-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIntergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
Many species of arthropod are infected by deleterious inherited micro-organisms. Typically these micro-organisms are inherited maternally. Consequently, some, particularly bacteria of the genus Wolbachia, employ a variety of strategies that favour female over male hosts. These strategies include feminisation, induction of parthenogenesis and male-killing. These strategies result in female biased sex ratios in host populations, which lead to selection for host factors that promote male production. In addition, the intra-genomic conflict produced by the difference in transmission of these cytoplasmic endosymbionts and nuclear factors will impose a pressure favouring nuclear factors that suppress the effects of the symbiont. During investigations of the diversity of male-killing bacteria in ladybirds (Coccinellidae), unexpected patterns of vertical transmission of a newly discovered male-killing taxon were observed in the ladybird Cheilomenes sexmaculata. Initial analysis suggested that the expression of the bacterial male-killing trait varies according to the male(s) a female has mated with. By swapping males between females, a male influence on the expression of the male-killing trait was confirmed. Experiments were then performed to determine the nature of the interaction. These studies showed that a single dominant allele, which rescues male progeny of infected females from the pathological effect of the male-killer, exists in this species. The gene shows typical Mendelian autosomal inheritance and is expressed irrespective of the parent from which it is inherited. Presence of the rescue gene in either parent does not significantly affect the inheritance of the symbiont. We conclude that C. sexmaculata is host to a male-killing γ-proteobacterium. Further, this beetle is polymorphic for a nuclear gene, the dominant allele of which rescues infected males from the pathogenic effects of the male-killing agent. These findings represent the first reported case of a nuclear suppressor of male-killing in a ladybird. They are considered in regard to sex ratio and intra-genomic conflict theories, and models of the evolutionary dynamics and distribution of inherited symbionts.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000987
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000987Summary
Many species of arthropod are infected by deleterious inherited micro-organisms. Typically these micro-organisms are inherited maternally. Consequently, some, particularly bacteria of the genus Wolbachia, employ a variety of strategies that favour female over male hosts. These strategies include feminisation, induction of parthenogenesis and male-killing. These strategies result in female biased sex ratios in host populations, which lead to selection for host factors that promote male production. In addition, the intra-genomic conflict produced by the difference in transmission of these cytoplasmic endosymbionts and nuclear factors will impose a pressure favouring nuclear factors that suppress the effects of the symbiont. During investigations of the diversity of male-killing bacteria in ladybirds (Coccinellidae), unexpected patterns of vertical transmission of a newly discovered male-killing taxon were observed in the ladybird Cheilomenes sexmaculata. Initial analysis suggested that the expression of the bacterial male-killing trait varies according to the male(s) a female has mated with. By swapping males between females, a male influence on the expression of the male-killing trait was confirmed. Experiments were then performed to determine the nature of the interaction. These studies showed that a single dominant allele, which rescues male progeny of infected females from the pathological effect of the male-killer, exists in this species. The gene shows typical Mendelian autosomal inheritance and is expressed irrespective of the parent from which it is inherited. Presence of the rescue gene in either parent does not significantly affect the inheritance of the symbiont. We conclude that C. sexmaculata is host to a male-killing γ-proteobacterium. Further, this beetle is polymorphic for a nuclear gene, the dominant allele of which rescues infected males from the pathogenic effects of the male-killing agent. These findings represent the first reported case of a nuclear suppressor of male-killing in a ladybird. They are considered in regard to sex ratio and intra-genomic conflict theories, and models of the evolutionary dynamics and distribution of inherited symbionts.
Introduction
Cytoplasmic sex ratio distorters have been reported from many invertebrates [1]. One group of distorters comprises a diverse array of bacteria which distort the secondary sex ratio of their hosts towards females by killing male hosts early in embryogenesis [2], [3]. Infected females gain an advantage over uninfected females via inbreeding avoidance, resource reallocation or reduction in sibling competition. Theory predicts that, within populations biased towards females as a result of the action of maternally inherited cytoplasmic sex ratio distorters with incomplete vertical transmission, selection will favour autosomal sex ratio compensation [4], [5]. This could occur by distortion of the primary sex ratio or by distortion of the secondary sex ratio towards males, if loss of female offspring is compensated for by increased fitness of male progeny. No such case has been demonstrated in a diploid harbouring a male-killer (but see data of male-biased families in [6], [7]. In addition, selection may favour the evolution of autosomal genes that reduce the vertical transmission or the phenotypic effects of sex ratio distorting bacteria [8]. Autosomal genes that suppress the sex ratio phenotype are known for both cytoplasmic male sterility in plants [9] and sex chromosome meiotic drive in dipterans [10]. They are also suspected in the woodlouse Porcellinoides pruinosus which hosts a feminising Wolbachia [11]. A nuclear suppressor of male-killing could kill the male-killer, or reduce its vertical transmission, or prevent the symbiont from killing males, either by blocking male host recognition, or by blocking the killing act. Such a suppressor has been reported in Drosophila prosaltans where it is suggested there is a recessive allele that prevents transmission of the male-killer [12]. A suppressor conferring resistance has been demonstrated the butterfly Hypolimnas bolina infected with the male-killing Wolbachia strain wBol1, where infected Southeast Asian H. bolina produce a 1∶1 sex ratio [13]. It has been suggested that the widespread occurrence of males testing positive for known male-killers found via PCR screening of samples of 21 ladybird species, could be indicative of nuclear suppression [14]. Suppressors at fixation might also explain the findings in Drosophila recens and Ephestia cautella, where the Wolbachia strains they harbour cause male-killing when transferred to con-generic host species, although not in the original species [15], [16].
Cheilomenes sexmaculata harbours a male-killer [17]. This male-killer is transovarially transmitted, is horizontally transferable in haemolymph by microinjection and curable by both high temperature and tetracycline treatment [17], [18]. The causative agent associated with male-killing has previously been reported to be similar to that causing male-killing in Harmonia axyridis [18], a Spiroplasma [19].
Results
Phenotypic assessments, based on egg hatch rates and progeny sex ratio, of a small sample from Tokyo showed that two of 15 matrilines had traits consistent with male-killing, with less than 50% of eggs hatching and female-biased progeny sex ratios (family Mk1 - 7 males, 73 females; family Mk2 - 2 males, 14 females) prior to antibiotic (tetracycline) treatment. Antibiotic treatment effected a cure of the trait, egg hatch rates and the proportion of males both increasing after treatment (Mk1 - 71 males, 76 females; Mk2 - 87 males, 93 females).
Identity of the male-killer was established by PCR amplification of the 16S rDNA gene using general eubacterial primers 27f and 1495r [20], cloning [21] and sequencing the gene. Briefly, genomic DNA was extracted [19] from females producing female biased sex ratios from both male-killer (m-k) lines (parental females, F1 and F2 progeny), from F1 eggs of both m-k lines, from F1 and F2 females from a normal (N) sex ratio line (N12) that never produced a biased sex ratio (five generations, 37 families), and from F1 and F2 progeny from antibiotic treated females from both m-k lines. The m-k line females from all three generations, the m-k eggs, but not the N line or progeny from antibiotic treated females, generated a 16S rDNA PCR product, which was then cloned and sequenced (1517 bp). A majority rule consensus sequence was generated for the bacterium to account for PCR errors (total 22 sequences, of a total of 26 sequences generated at this stage, 3 contaminant Streptococcus lactis and one pGEM non-transformant also sequenced). The sequence produced was submitted to EMBL (accession number AJ272038). This was compared with sequences in the EMBL, Genbank, DDBJ and PDB databases using BLASTN [22]. The sequence was identified as a γ-proteobacterium with closest similarity to a variety of secondary symbionts, of aphids (97-98% identity), in particular Hamiltonella defensa (98% identity), of whiteflies, particularly of Bemisia tabaci (97% identity) and to a number of bacteria of the genus Yersinia. To investigate these relationships further, a phylogeny was produced using 16S rDNA sequences from 25 of the closest matches from the BLASTN search (Figure 1). It is interesting to note that the male-killer clearly falls within the clade of aphid secondary symbionts and is distinct from both the whitefly symbiont clade and the Yersinia clade. The tree is not particularly informative regarding relationships within the aphid symbiont clade, with almost all of the bootstrap values being below 500. However, it is certainly suggestive of horizontal transfer of symbionts both between aphid species, as has been reported [23], [24], [25] and between prey and predator species as has been suggested for other coccinellid male-killers [26], [27].
Fig. 1. 16S rDNA phylogenetic tree indicating the position of the C. sexmaculata male-killer amongst closely related bacteria. 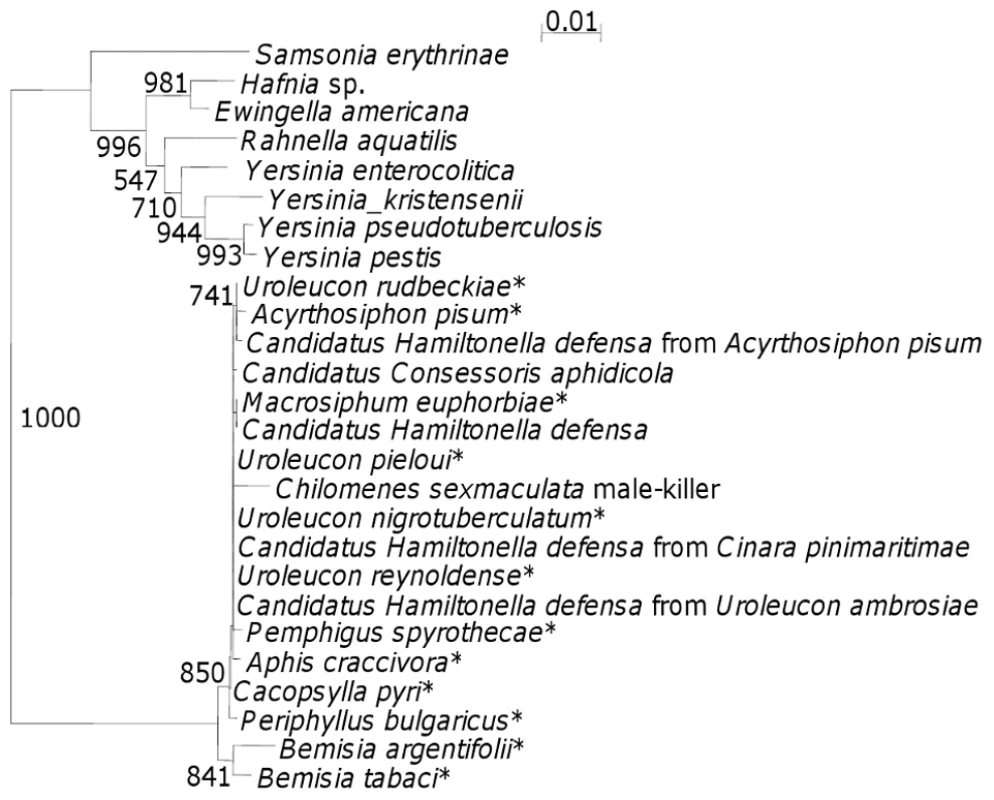
The numbers indicate bootstrap values (values within the clade containing the C. sexmaculata male-killer are low (most <500) and are not shown). * indicates secondary symbiont host name. The 16S rDNA phylogeny highlights one further interesting feature of this male-killer, as it supports the suggestion of horizontal transfer of symbionts with a change in phenotype between the alternative hosts. Secondary symbionts are known to confer protection to their hosts against natural enemies [25 for review], but natural enemy protection co-occurring with reproductive parasitism in a clade has to date only been established for Wolbachia [28].
This finding represents the second instance of a γ-proteobacterium causing male-killing [29], and the first recorded instance in a coccinellid. To confirm the absence of other known male-killers, in particular Spiroplasma, but also Rickettsia, Wolbachia and Flavobacteria, specific PCR assays were carried out on individuals from both male-killer lines, from the parental and F1 generations. In all cases these tests proved negative [27]. The male-killer in this sample of C. sexmaculata is thus not the same as that identified from H. axyridis [19], as has previously been assumed [18].
Crosses designed to test the inheritance of the trait, involving F1 females from the two putative male-killer lines (Mk1 and Mk2), with males from normal sex ratio matrilines produced unexpected progeny sex ratios (Table 1). All three crosses using males from one line (N5) produced significantly female-biased sex ratios, one (Mk1.1) being almost all female, the others (Mk1.4 and Mk2.3) giving approximately 2∶1 ratios of females to males. The remaining seven crosses, involving males from two other lines (N1 and N8) produced normal (approximately 1∶1) sex ratios.
Tab. 1. Outcome of crosses designed to test the inheritance of the m-k trait in two matrilines of C. sexmaculata. 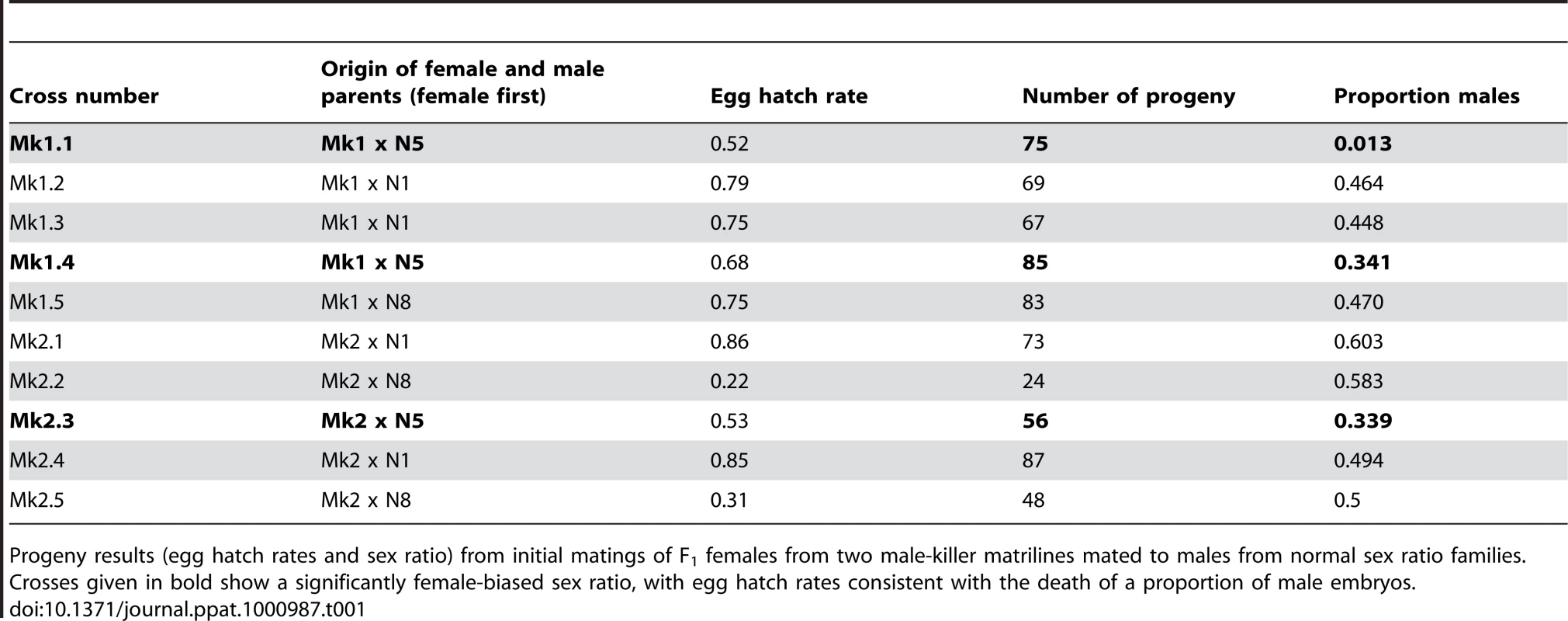
Progeny results (egg hatch rates and sex ratio) from initial matings of F1 females from two male-killer matrilines mated to males from normal sex ratio families. These data suggest either an exceptionally low and variable vertical transmission of the sex biasing trait, or paternal influence on progeny sex ratios.
To test the latter possibility, male parents were transferred between some of the crosses, the male (N5) from Mk1.1 being mated multiply to the female from Mk1.2 and once to the female from Mk1.5, and the male (N1) from Mk1.3 being mated to the Mk1.1 female. In all cases the progeny sex ratios changed following these additional matings (Figure 2), that of Mk1.1 rising from 0.013 to 0.355, with those of Mk1.2 and Mk1.5 becoming significantly female-biased. The Mk1.2 female, mated multiply to the second male produced a strong female bias from three days after introduction of the new male and for the remainder of her life. However, the Mk1.5 female, mated just once to a second male, again after a lag (four days) produced a strong and significant female bias (14 male∶42 female) over four days, before her progeny sex ratio reverted towards normality. This reversion suggests that sperm from the singly mated second male was used for a block of time before utilisation reverted to sperm from the multiply mated first male.
Fig. 2. The effect of changing the male on progeny sex ratios produced by C. sexmaculata females. 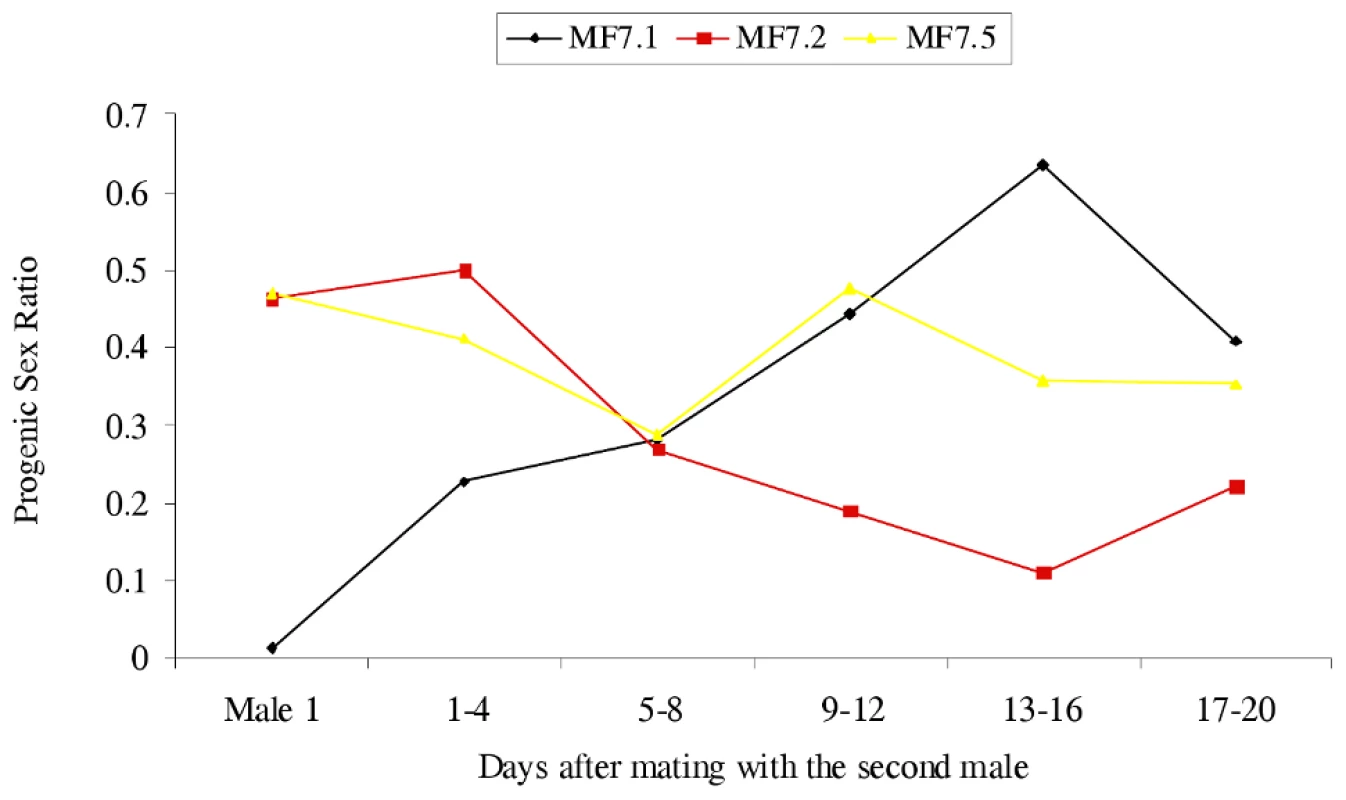
Progeny sex ratios of three F1 females, from a female biased matriline (Mk1) each mated twice. Male 1 indicates progeny from the first mating (see Table 1). Sex ratios after mating with the second male are given in four-day blocks. See Table S1 for data upon which the Figure is based. Extended replication of this mate swapping procedure using both male-killing matrilines showed that changes in the sex ratio following change of male were reversible if males were changed back (data available on request). These data suggest the presence of a factor, acting through sperm or some other element in the ejaculate, that inhibits the male-killing action of the bacterium. The initial data could be explained by a unifactorial dominant nuclear gene.
Four questions were addressed to test this hypothesis and detail the nature of the suppressor system.
-
Is the suppressor inherited in a Mendelian fashion?
-
Is expression of the suppressor affected by the sex of the parent from which it is inherited?
-
Does the action of the suppressor affect the inheritance of the male-killer in female hosts?
-
Do ‘rescued’ males carry the male-killer?
To address i) and ii) on the basis of the initial hypothesis, expected progeny sex ratios from monogamous crosses involving individuals of alternative genotypes with respect to both the male-killer and the suppressor locus (alleles: suppressor = res+, non suppressor = res−) were calculated (Table 2).
Tab. 2. Results of single pair crosses of individuals of inferred male-killer status and suppressor gene genotype. 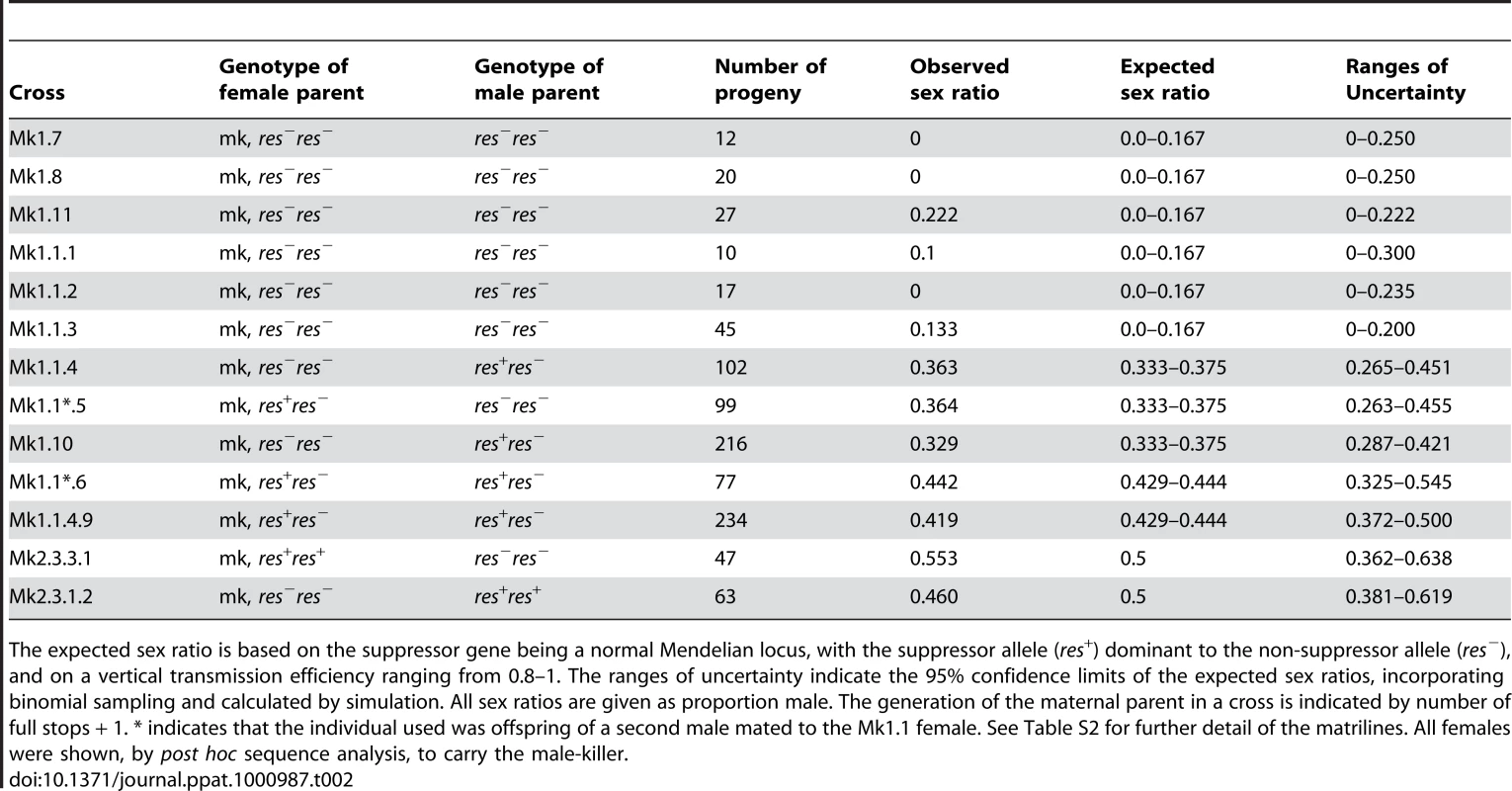
The expected sex ratio is based on the suppressor gene being a normal Mendelian locus, with the suppressor allele (res+) dominant to the non-suppressor allele (res−), and on a vertical transmission efficiency ranging from 0.8–1. The ranges of uncertainty indicate the 95% confidence limits of the expected sex ratios, incorporating binomial sampling and calculated by simulation. All sex ratios are given as proportion male. The generation of the maternal parent in a cross is indicated by number of full stops + 1. * indicates that the individual used was offspring of a second male mated to the Mk1.1 female. See Table S2 for further detail of the matrilines. All females were shown, by post hoc sequence analysis, to carry the male-killer. Crosses involving individuals of inferred suppressor genotype, the females being F1, F2 or F3 from male-killer matrilines, produced progeny sex ratios consistent with expectation on the basis of Mendelian inheritance (Table 2). The results indicate that the expression of the suppressor is not affected by the sex of the parent from which the suppressor is inherited. For example, cross Mk1.1.4.9, in which both parents were heterozygous for the suppressor gave a progeny sex ratio of 0.419 (n = 234), close to expectation and significantly different from both the maximum expected sex ratio if only paternally derived suppressors are expressed (χ21 = 4.869, p<.05), and from a 1∶1 sex ratio (χ21 = 6.171, p<0.05).
Mk1 and Mk2 were maintained for five generations, 118 families (minimum number of progeny = 10; mean number of progeny = 62.7) being reared. In 51 of these the genotype with respect to the rescue gene locus was inferred for both parents prior to progeny being obtained. In 46 families, results were consistent with the theorised autosomal (or pseudoautosomal) nature of the locus. The locus is not sex-linked. In the remaining five, 16S rDNA sequence analysis, performed post hoc, showed that the female parent lacked the male-killer. This proportion of revertants is roughly consistent with the estimated vertical transmission efficiency, a, of the male-killer seen in Mk1 (a = 0.89) and Mk2 (a = 0.83).
To address question iii), two female F2 progeny from predominantly female families of both the Mk1 and Mk2 matrilines were crossed to homozygous res+ males. The progeny sex ratio in each of these families was close to 1∶1. res+res− female offspring from these crosses were mated to res−res− males. Of eight crosses, one produced a normal sex ratio, the remainder producing ratios approximating to 1 male: 2 female (data not shown). Given the vertical transmission efficiency of this male-killer, these results show that the male-killer is inherited through females, even when its pathological effect on males has been suppressed. Verification was obtained by sequencing the 16S rDNA PCR product from parents and six progeny (3 male, 3 female) of two of the families that produced a 1 male: 2 female sex ratio. Female parents, all male progeny and five of the six female progeny were shown to bear the male-killer.
Verification that the suppressor acts as a rescue gene was obtained by demonstrating that adult males from suppressed male-killer females produced 16S rDNA PCR product with the same sequence as the γ-proteobacterium identified as the male-killer (question iv)). Two male progeny from two m-k families, one of which produced a 1∶2 sex ratio and one which produced a normal sex ratio (Mk1.4 and Mk2.5, respectively) were submitted to the same PCR cloning and sequencing protocol as used for the initial identification of the male-killer. In each case the γ-proteobacterium was shown to be present. The male-killer is thus present but not expressed in ‘rescued’ males.
Crosses of such males, inferred to be res+res− and to carry the male-killer, to res−res− females lacking the male-killer produced normal progeny sex ratios. The male-killer is thus not inherited from males. Taken together, these results suggest that the endosymbiont is not killed by the suppressor. Rather the suppressor acts as a rescue gene for males.
Discussion
The discovery of a nuclear gene that rescues males from the pathological effects of a maternally inherited bacterium that otherwise kills males, to the benefit of the males' female siblings that carry and vertically transmit the bacterium, is in accord with theories of sex ratio [30], [31] and intra-genomic conflict [4], [5]. Selection favouring a suppressor gene will be a direct consequence of sex ratio distortion. Autosomal genes that act against sex ratio distorters have been recorded in isopods infected with feminising Wolbachia and in a butterfly infected with male-killing Wolbachia. In Armadillium vulgare, the main effect of such genes is to reduce bacterial transmission to progeny [32]. In contrast, in P. pruinosus, autosomal genes are conjectured to prevent the feminising effect of Wolbachia [11]. This is analogous to the situation in C. sexmaculata and H. bolina [13] where observations are compatible with a single, dominant autosomal locus suppressing the male-killing effect of the bacteria.
Most models considering the evolutionary interactions between sex ratio distorting symbionts and suppressors are based on the assumption that the suppressor will kill the symbiont or reduce its vertical transmission [33], [34]. The dynamics of a male rescue gene may be quite different, and will depend on its cost, if any, in the absence of the male-killer. A male-killer in the presence of a male rescue gene should be selected against, due to the cost on hosts of carrying the male-killer and the lack of fitness advantages to infected females resulting from male death [3]. Such a male-killer then has several alternative fates. It may be selected to extinction. It may become polymorphic, male killer prevalence being determined by its transmission dynamics and fitness compensation and the costs to hosts of both it and the rescue gene. It may circumvent the rescue gene by evolving a different mechanism to kill males (cf. the double feminising effect of Wolbachia in A. vulgare [35]). Finally, it may reduce its cost on hosts, becoming costless (persistence would require vertical transmission close to 1) or even beneficial - cytoplasmic male-killers are an exquisite testing ground for theories of virulence [3].
It is interesting to compare the situation in C. sexmaculata with that in H. bolina. In the latter, the suppressor has recently and rapidly spread to fixation in Southeast Asian populations, but is absent from Polynesian populations [13]. A recent model [36] examines the dynamics of such systems with reference to host suppressors of male-killing and Wolbachia that are also able to induce cytoplasmic incompatibility (CI). Here the model predicts that (in the absence of CI, and so pertinent to this study) the maximum cost of a dominant suppressor of male-killing that allows invasion of a host population will increase as the male-killer prevalence increases. The model also predicts (in the absence of CI) that a costly suppressor that does invade will become polymorphic and the frequency of the male-killer will be reduced. Further, the authors suggest that polymorphic suppressors of the male-killing action of non-Wolbachia male-killers (not known to induce CI) should be more common than of Wolbachia male-killers. Our findings fit well with this model.
In contrast to the male-killer in C. sexmaculata, the male-killing Wolbachia in H. bolina may also cause CI and where this occurs the model demonstrates that the dynamics of the system are altered, with corresponding changes in the rate of spread, fixation and frequency of infection that depend on the level of CI, the cost of the suppressor, the transmission efficiency and the initial male-killer frequency. Suppressor spread may be inhibited, where there is CI, but where a suppressor does spread it is predicted to lead to fixation of both itself and the infection, giving an appearance of a population exhibiting only CI [36].
The Fuchu population of C. sexmaculata studied here is polymorphic for the rescue gene. Further investigation of whether this is a balanced or transient polymorphism, and determination of whether the rescue gene imposes a cost on bearers, would provide valuable insight into the dynamics of the spread of suppressors, as well as the generality of the findings in H. bolina. Establishment of the frequencies of the rescue gene and male-killer in different populations of C. sexmaculata would be valuable. Further, molecular investigation of coccinellids with ecological traits making them liable to male-killer invasion, but in which searches for male-killers using phenotypic assays have proved negative, may reveal presence of beneficial symbionts that are a peaceful resolution of an evolutionary arms race between a male-killer and a suppressor system.
Methods
Nomenclature
Lines were designated either Male-killer – Mk, or Normal – N, to reflect the phenotypic status of the P1 females in the original sample. The lines were then numbered sequentially within the two categories, hence Mk1 and Mk2 were the two male-killing lines and N1-N13 the 13 normal lines. Subsequent generations show the parental name followed by a ‘.’ to indicate a new generation, and then a number e.g. Mk1.3 is the third cross generated from F1 female progeny of Mk1 and Mk1.1.3 is the third cross generated from an F2 female, progeny of Mk1.1.
These numbers simply reflect P1 phenotype and indicate the matriline. They do not reflect rescue gene status and hence do not necessarily indicate F1 (or subsequent generation) phenotype. Rescue gene allelic status is indicated by res+ and res- for suppressor and non-suppressor, respectively.
Production of suppressor free males
For some tests, males known to lack the male-killer suppressor were necessary. These were obtained by tetracycline treatment of singly mated, male-killer bearing females (for both Mk1 and Mk2). Females were mated once and male-killer status confirmed. Females showing characteristic half hatch rates were treated with antibiotic. Where these initial clutches produced only female progeny, and assuming, as was subsequently shown, that res+ was expressed when inherited from the female parent, males produced in the later clutches of these crosses would be homozygous res−.
Molecular detection of the male-killer
Different categories of ladybird were assessed for the presence of a bacterial male-killer by performing PCR using general eubacterial primers that amplify the 16S rRNA gene (primer pair 27f, 1495r) [20]. The PCR was carried out using Expand High Fidelity PCR System (Boehringer Mannheim). The product was purified using Microcon Microconcentrators (Amicon Ltd.) and ligated into pGEM T-vector (Promega). The resulting plasmids were transformed into E. coli DH5α as described by Hurst et al. [21]. Plasmids containing insert DNA were purified using Wizard Minipreps DNA purification system (Promega). Inserts were sequenced using the ABI PRISM BigDye Terminator cycle-sequencing ready-reaction kit (Perkin Elmer) and visualised on an ABI 377 automated sequencer. Primers pUC/M13 forward and reverse, 27f and 1495r and internal primers [37] were used to sequence both strands of the whole unit.
Phylogenetic analysis
The 16SrDNA sequence generated above was aligned with 16S rDNA sequences from 25 different BLASTN matches with high alignment scores, which were downloaded from the nr database. The accession numbers of the 25 sequences used were: AY296733, CP001277, AF293622, EU348313, AY264676, AF293626, AF293616, AY692361, AY264675, AY136161, AY136136, AY136164, AY136162, AY136163, AY136145, AY136156, AY136148, EU178101, AB273745, AM403659, FM955884, AL590842, CP001048, NR028786, U90757. Sequences were aligned with ClustalW2 [38], minor manual adjustments were made using Seaview [39], and a neighbour-joining tree was generated excluding sites with a gap in any sequence, using Kimura's 2 parameter correction, with 1000 bootstrapped replicates, implemented in ClustalW2 [38]. The tree was displayed using NJPlot [40].
Calculation of expected progeny ratios
Under a model where the suppressor is a single gene, with alleles res+ and res−, where res+ is a dominant rescue allele and where the vertical transmission efficiency of the male-killer is between 0.8–1.0, the expected progeny ratios from different crosses can be calculated as follows:
In all cases female progeny will survive, whether infected or not; what varies is the proportion of males that inherit the infection and further the proportion of those inheriting the infection which carry a res+ allele and so (under this model) survive. If the proportion of progeny inheriting the male-killer varies between 0.8 and 1.0 a maximum of 20% of the progeny (10% male and 10% female) will lack the male-killer.
In a cross where both parents are free from the suppressor (res− res− x res− res−) and the parental female is infected with the male-killer, none of the progeny will inherit a suppressor and hence all the male progeny that receive the male-killer will die. This will be between 80 and 100% of the males, i.e. if vertical transmission is 1, 0 males will survive, if the vertical transmission is 0.8, 20% of the progeny will not inherit the male-killer, 10% of these are male, and will now survive, increasing the sex ratio to 0.1/0.6 = 0.167.
Similarly if the parents are res− res+ x res− res− half the progeny will inherit a suppressor allele. If vertical transmission is 100% one quarter of the progeny will die (males with no suppressor), and one third, 0.333, of the remaining progeny will be male. If vertical transmission is 80% this would mean of the 20% of the progeny that fail to inherit the male-killer, 10% will be male (as above), of which half will be res− and so will now survive, increasing the proportion male to 0.3/0.8 = 0.375.
If the parents are res+ res− x res+ res− three-quarters of the progeny will inherit a suppressor so 3/7 or 0.429 of the surviving progeny will be male assuming all inherit the male-killer. If vertical transmission is 80%, again 10% of the progeny that fail to inherit the male killer will be male. Now only a quarter of males are res−, hence another 2.5% will survive, making the proportion male 0.4/0.9 = 0.444.
Finally, if one parent is homozygous res+ then all progeny inherit a copy of the suppressor, all will survive and the proportion male will be 0.5, regardless of the vertical transmission efficiency.
These calculations assume that in the absence of the male-killer the sex ratio would be 1∶1, that the male-killer is inherited equally by males and females and that it always kills males unless there is a suppressor present.
Simulations
Simulations were carried out to estimate the 95% confidence limits of the expected sex ratios. This can be illustrated using Mk 1.7. Here the range of possible sex ratios is from 0–0.167, and the sample size is 12. First, a random number is chosen, and on the basis of this, a sex ratio is randomly chosen from an even distribution from 0 to 0.167. Then, using this sex ratio, a binomial sample of 12 individuals is created, of which between 0 and (theoretically) 12 will be male. This process was repeated 100,000 times, to produce a distribution of the numbers of males seen in samples of 12. In this case the distribution is:
0 males: 41.8%
1 male: 30.7%
2 males: 17.2%
3 males: 7.3%
4 males: 2.3%
5 males: 0.5%
6 males: 0.1%
From these values it is concluded that any number of males in the data above 3 would give significant evidence against the hypothesis, since this has a chance of happening that is below 5%, and thus the 95% confidence limits run from zero to three males from 12, or from 0–0.25 as the sex ratio. Corresponding calculations were carried out for each cross listed in Table 2.
Accession number mentioned in text
EMBL: AJ272038
Supporting Information
Zdroje
1. WerrenJH
O'NeillSL
1997 The evolution of heritable symbionts.
O'NeillSL
HoffmannAA
WerrenJH
Influential Passengers Oxford Oxford University Press 1 41
2. MajerusMEN
HurstGDD
1997 Ladybirds as a model system for the study of male-killing symbionts. Entomophaga 42 13 20
3. HurstGDD
HurstLD
MajerusMEN
1997 Cytoplasmic sex-ratio distorters.
O'NeillSL
HoffmannAA
WerrenJH
Influential Passengers Oxford Oxford University Press 125 154
4. WerrenJH
1987 The coevolution of autosomal and cytoplasmic sex ratio factors. J Theor Biol 124 317 334
5. HurstLD
1992 Intragenomic conflict as an evolutionary force. P Roy Soc Lond B Bio 248 91 99
6. OwenDF
ChanterDO
1969 Population biology of tropical African butterflies. Sex ratio and genetic variation in Acraea encedon. J Zool 157 345 374
7. MajerusMEN
MajerusTMO
2000 Female-biased sex ratio due to male-killing in the Japanese ladybird Coccinula sinensis. Ecol Entomol 25 234 238
8. UyenoyamaMK
FeldmanMW
1978 The genetics of sex ratio distortion by cytoplasmic infection under maternal and contagious transmission: an epidemiological study. Theor Popul Biol 14 471 497
9. FrankSA
1989 The evolutionary dynamics of cytoplasmic male sterility. Am Nat 133 345 376
10. MerçotH
LlorenteB
JacquesM
AtlanA
Montchamp-MoreauC
1995 Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics 141 1015 1023
11. JuchaultP
FrelonM
BouchonD
RigaudT
1994 New evidence for feminizing bacteria in terrestrial isopods: evolutionary implications. C R Acad Sci Paris, Série III 317 225 230
12. CavalcantiAGL
FalcaoDN
CastroLE
1957 “Sex-ratio” in Drosophila prosaltans - a character due to interaction between nuclear genes and cytoplasmic factors. Am Nat 91 327 329
13. HornettEA
CharlatS
DuplouyAMR
DaviesN
RoderickGK
2006 Evolution of Male-Killer Suppression in a Natural Population. PLoS Biol 4 e283
14. WeinertLA
TinsleyMC
TemperleyM
JigginsFM
2007 Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biol Lett 3 678 681
15. JaenikeJ
2007 Spontaneous emergence of a new Wolbachia phenotype. Evolution 61 2244 2252
16. SasakiT
KuboT
IshikawaH
2002 Interspecific transfer of Wolbachia between two lepidopteran insects expressing cytoplasmic incompatibility: a Wolbachia variant naturally infecting Cadra cautella causes male killing in Ephestia kuehniella. Genetics 162 1313 1319
17. NiijimaK
NakajimaK
1981 Abnormal sex ratio in Menochilus sexmaculatus (Fabricius). B Fac Agr Tamagawa U 21 59 67
18. GotohT
NiijimaK
1986 Characteristics and agent(s) of abnormal sex ratio (SR) in two aphidophagous coccinellid species.
HodekI
Ecology of the Aphidophaga Prague Academia 545 550
19. MajerusTMO
SchulenburgJHGvd
MajerusMEN
HurstGDD
1999 Molecular identification of a male-killing agent in the ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Insect Mol Biol 8 551 555
20. WeisbergWG
BarnsSM
PelletierDA
LaneDJ
1991 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173 697 703
21. HurstGDD
JigginsFM
SchulenbergJHGvd
BertrandD
WestSA
1999 Male-killing Wolbachia in two species of insect. P Roy Soc Lond B Bio 266 735 740
22. ZhangZ
SchwartzS
WagnerL
MillerW
2000 A greedy algorithm for aligning DNA sequences. J Comput Biol 7 203 14
23. SandstromJP
RussellJA
WhiteJP
MoranNA
2001 Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol 10 217 228
24. RussellJA
LatorreA
Sabater-MunozB
MoyaA
MoranNA
2003 Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol 12 1061 1075
25. OliverKM
DegnanPH
BurkeGR
MoranNA
Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55 247 66
26. MajerusTMO
MajerusMEN
2010 Discovery and identification of a male-killing agent in the Japanese ladybird Propylea japonica (Coleoptera: Coccinellidae). BMC Evol Biol 10 37
27. MajerusTMO
2001 The Evolutionary Genetics of Male killing in the Coccinellidae. PhD thesis. University of Cambridge, Department of Genetics
28. HedgesLM
BrownlieJC
O'NeillSL
JohnsonKN
2008 Wolbachia and virus protection in insects. Science 322 702
29. GhernaRL
WerrenJH
WeisburgW
CoteR
WoeseCR
1991 Arsenophonus nasoniae, genus novel, species novel, causative agent of Son Killer trait in the parasitic wasp, Nasonia vitripennis. Int J Syst Bacteriol 41 563 565
30. FisherRA
1930 The Genetical Theory of Natural Selection. Oxford Oxford University Press 318
31. HamiltonWD
1967 Extraordinary sex ratios. Science 156 477 488
32. RigaudT
JuchaultP
1992 Genetic control of the vertical transmission of a cytoplasmic sex factor in Armadillium vulgare atr. (Crustacea, Oniscidea). Heredity 68 47 52
33. StouthamerR
1997 Wolbachia-induced parthenogenesis.
O'NeillSL
HoffmannAA
WerrenJH
Influential Passengers Oxford Oxford University Press 102 124
34. RandersonJP
SmithGC
HurstLD
2000 The evolutionary dynamics of male-killers and their hosts. Heredity 84 152 160
35. RigaudT
1997 Inherited microorganisms and sex determination of arthropod hosts.
O'NeillSL
HoffmannAA
WerrenJH
Influential Passengers Oxford Oxford University Press 81 101
36. HornettEA
EngelstädterJ
HurstGD
2010 Hidden cytoplasmic incompatibility alters the dynamics of male-killer/host interactions. J Evolution Biol 23 479 487
37. LaneDJ
PaceB
OlsenGJ
StahlDA
SoginML
1985 Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. P Natl Acad Sci U S A 82 6955 6959
38. LarkinMA
BlackshieldsG
BrownNP
ChennaR
McGettiganPA
2007 ClustalW and ClustalX version 2. Bioinformatics 23 2947 2948
39. GouyM
GuindonS
GascuelO
2010 SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27 221 224
40. PerrièreG
GouyM
1996 WWW-Query: An on-line retrieval system for biological sequence banks. Biochimie 78 364 369
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



