-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
Dendritic cells (DCs) contribute to human immunodeficiency virus type 1 (HIV-1) transmission and dissemination by capturing and transporting infectious virus from the mucosa to draining lymph nodes, and transferring these virus particles to CD4+ T cells with high efficiency. Toll-like receptor (TLR)-induced maturation of DCs enhances their ability to mediate trans-infection of T cells and their ability to migrate from the site of infection. Because TLR-induced maturation can be inhibited by nuclear receptor (NR) signaling, we hypothesized that ligand-activated NRs could repress DC-mediated HIV-1 transmission and dissemination. Here, we show that ligands for peroxisome proliferator-activated receptor gamma (PPARγ) and liver X receptor (LXR) prevented proinflammatory cytokine production by DCs and inhibited DC migration in response to the chemokine CCL21 by preventing the TLR-induced upregulation of CCR7. Importantly, PPARγ and LXR signaling inhibited both immature and mature DC-mediated trans-infection by preventing the capture of HIV-1 by DCs independent of the viral envelope glycoprotein. PPARγ and LXR signaling induced cholesterol efflux from DCs and led to a decrease in DC-associated cholesterol, which has previously been shown to be required for DC capture of HIV-1. Finally, both cholesterol repletion and the targeted knockdown of the cholesterol transport protein ATP-binding cassette A1 (ABCA1) restored the ability of NR ligand treated cells to capture HIV-1 and transfer it to T cells. Our results suggest that PPARγ and LXR signaling up-regulate ABCA1-mediated cholesterol efflux from DCs and that this accounts for the decreased ability of DCs to capture HIV-1. The ability of NR ligands to repress DC mediated trans-infection, inflammation, and DC migration underscores their potential therapeutic value in inhibiting HIV-1 mucosal transmission.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000981
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000981Summary
Dendritic cells (DCs) contribute to human immunodeficiency virus type 1 (HIV-1) transmission and dissemination by capturing and transporting infectious virus from the mucosa to draining lymph nodes, and transferring these virus particles to CD4+ T cells with high efficiency. Toll-like receptor (TLR)-induced maturation of DCs enhances their ability to mediate trans-infection of T cells and their ability to migrate from the site of infection. Because TLR-induced maturation can be inhibited by nuclear receptor (NR) signaling, we hypothesized that ligand-activated NRs could repress DC-mediated HIV-1 transmission and dissemination. Here, we show that ligands for peroxisome proliferator-activated receptor gamma (PPARγ) and liver X receptor (LXR) prevented proinflammatory cytokine production by DCs and inhibited DC migration in response to the chemokine CCL21 by preventing the TLR-induced upregulation of CCR7. Importantly, PPARγ and LXR signaling inhibited both immature and mature DC-mediated trans-infection by preventing the capture of HIV-1 by DCs independent of the viral envelope glycoprotein. PPARγ and LXR signaling induced cholesterol efflux from DCs and led to a decrease in DC-associated cholesterol, which has previously been shown to be required for DC capture of HIV-1. Finally, both cholesterol repletion and the targeted knockdown of the cholesterol transport protein ATP-binding cassette A1 (ABCA1) restored the ability of NR ligand treated cells to capture HIV-1 and transfer it to T cells. Our results suggest that PPARγ and LXR signaling up-regulate ABCA1-mediated cholesterol efflux from DCs and that this accounts for the decreased ability of DCs to capture HIV-1. The ability of NR ligands to repress DC mediated trans-infection, inflammation, and DC migration underscores their potential therapeutic value in inhibiting HIV-1 mucosal transmission.
Introduction
Worldwide, heterosexual transmission accounts for most new HIV-1 infections, with a majority of these occurring in developing countries [1], [2]. Clearly, controlling heterosexual transmission of HIV-1 would be a significant step toward reducing this global pandemic. To achieve this goal, it will be important to delineate the cellular and molecular events that promote or restrict virus transmission and dissemination.
Immune cells within the vaginal, cervical, or rectal mucosa are thought to be the primary targets of infection in the sexual transmission of HIV-1 [1], [3], [4]. These target cells include sub-epithelial CD4+ T lymphocytes, intra-epithelial Langerhans cells, macrophages, submucosal plasmacytoid DCs (pDCs), and myeloid (or conventional) DCs (mDCs) located within the lamina propria [4], [5], [6], [7], [8], [9], [10], [11]. DCs, in particular, play a central role in HIV-1 transmission. DCs are thought to capture cell-free HIV-1 particles from the intralumenal space or from the mucosa after transcytosis across or leakage of HIV-1 particles through the epithelial barrier or by contacting HIV-1-infected cells introduced into the mucosa through abrasions or ulcerative lesions [6], [12], [13]. In addition, studies examining vaginal transmission of SIVmac in a rhesus macaque model of AIDS have implicated DCs in virus dissemination from the mucosa to draining lymph nodes [6], [14]. Moreover, DCs are the predominant infected migratory cell type harboring HIV-1 from virus exposed cervical tissue explants [15] supporting the idea that they are involved in virus dissemination. Upon capture, DCs can deliver infectious HIV-1particles to draining lymph nodes that contain large numbers of CD4+ T cells [16], [17]. The close contact between virus-laden DCs and CD4+ T cells facilitates cell-to-cell transmission and viral spread [18], [19]. In addition to their roles in virus transmission and dissemination, DCs can produce proinflammatory cytokines that create a microenvironment that favors virus replication [20], [21], [22]. Recent reports have demonstrated that DCs matured by exposure to pathogens encoding Toll-like receptor (TLR) ligands or to proinflammatory cytokines are capable of enhanced HIV-1 trans-infection [23], [24], [25] and chemokine-directed migration [26], [27], suggesting that agents capable of preventing inflammation and DC maturation may be able to limit HIV-1 transmission and dissemination.
NRs are a superfamily of ligand-activated transcription factors that includes classic hormone receptors, as well as the so-called orphan receptors and adopted orphan receptors whose natural ligands are either unknown or recently discovered [28], [29]. Included in these latter two families are peroxisome-proliferator activated receptors (PPAR) and liver X receptors (LXR). Ligand-activated PPARγ and LXR are bifunctional modulators of gene expression, capable of either activating or repressing transcription in a promoter-specific manner. Importantly, PPARγ and LXR are potent inhibitors of inflammation and are capable of repressing cytokine and chemokine production by Toll-like receptor (TLR)-activated macrophages and DCs through trans-repression mechanisms involving the failure to clear co-repressor complexes from promoters or through direct antagonism of transcription factors such as the p65 subunit of NF-κB, AP-1, STATs, and IRF3 [30], [31], [32], [33], [34], [35], [36], [37], [38]. The effects of PPARγ and LXR on TLR signaling are complex and a number of studies have demonstrated that each NR inhibits different subsets of inflammatory genes [32], [34]. For example, LXR signaling represses TLR4-induced expression of iNOS, COX-2, and IL-6 in murine macrophages, while PPARγ signaling represses IL-1β, GCSF, MCP-1, MCP-3, and MIP-1α expression [32]. Here, we show that PPARγ and LXR signaling acutely prevents TLR-activated expression of the proinflammatory cytokines TNF-α, IL-6, and IL-8, which have been implicated as co-factors for enhanced mucosal transmission of HIV-1. Moreover, PPARγ and LXR signaling inhibit the expression of the chemokine receptor CCR7, thereby preventing DC chemotaxis in response to gradients of CCL21, a process thought to be involved in DC migration from mucosal surfaces to draining lymph nodes.
As opposed to their inhibitory effects on inflammatory gene expression, ligand-activated PPARγ and LXR induce expression of genes involved in lipid and cholesterol metabolism, as well as cholesterol transport, including ABCA1 and ABCG1 [29], [39], [40], [41]. Importantly, many studies have demonstrated that cholesterol plays an essential role in HIV-1 biology. Cholesterol must be present in both the target cell membranes and HIV-1 particles for efficient virus binding and fusion [42], [43], [44], [45], [46]. In addition, nascent HIV-1 particles bud through cholesterol-rich lipid rafts [47], [48] and infectious particles enter target cells through cholesterol-rich lipid rafts [42], [49], [50]. Finally, studies using the cholesterol chelator, methyl-β-cyclodextrin, demonstrated that cholesterol is required for DC binding of virus particles [51]. Interestingly, PPARs and LXR are expressed at high levels in HIV-1 target cells such as macrophages and DCs [28], [29]. Therefore, we hypothesized that PPARγ and LXR-mediated changes in cholesterol metabolism and trafficking might contribute to their ability to inhibit the transmission of HIV-1 from DCs to T cells. Our results demonstrate that PPARγ and LXR signaling inhibit the capture of HIV-1 by DCs, and its subsequent transfer to CD4+ T cells. These effects are due to up-regulation of ABCA1-dependent cholesterol efflux, a mechanism distinct from the effects of PPARγ and LXR signaling on DC migration and proinflammatory cytokine production. Collectively, our data suggest that the bifunctional activities of ligand activated PPARγ and LXR can be exploited to inhibit multiple distinct steps in HIV-1 mucosal transmission and dissemination.
Results
Treatment with PPARγ and LXR ligands prevents the maturation of MDDCs
TLR signaling induced by sexually transmitted pathogens is thought to enhance HIV-1 mucosal transmission in part by promoting local inflammation. Inflammation not only activates HIV-1 target cells but, importantly, it also induces DC maturation and the subsequent migration of HIV-1-carrying DCs to local lymph nodes where they can contribute to virus dissemination [16], [17]. We were therefore interested in determining whether the anti-inflammatory activities of ligand-activated PPARγ and LXR [34], [52], [53] could be exploited to limit DC functions involved in HIV-1 transmission and pathogenesis. To examine the effects of PPARγ and LXR signaling on DC maturation, human monocyte-derived DCs (MDDCs) were treated with E. coli K12 LPS, a TLR4 ligand, in the presence or absence of ligands for PPARγ and LXR. As expected, LPS treatment upregulated the expression of surface markers associated with maturation, such as HLA-DR, CD80, CD86, and CD83, downregulated the expression of surface markers associated with an immature phenotype, such as the C-type lectin DC-SIGN, but had no effect on the expression of the pan-DC marker CD11c (Figure 1A and data not shown). Notably, treatment of MDDCs with the PPARγ ligand ciglitazone or the LXR ligand TO-901317 inhibited LPS-dependent upregulation of cell-surface expression of HLA-DR, CD80, and CD86 (Figure 1A). Similarly, we found that ciglitazone or TO-901317 treatment inhibited human MDDC maturation in response to the TLR2 ligand PAM3CSK4 (data not shown).
Fig. 1. PPARγ and LXR ligand treatment prevents MDDC maturation and pro-inflammatory cytokine production. 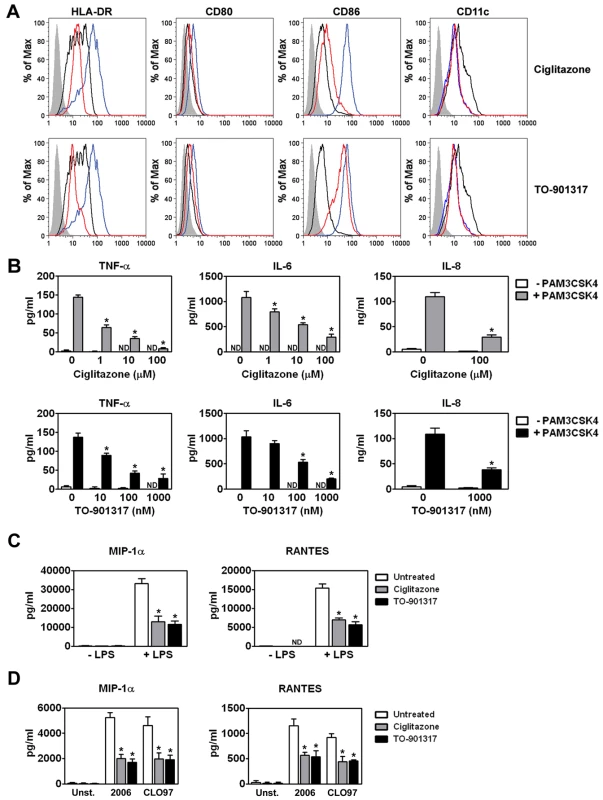
(A) MDDCs were treated with LPS for 48 hours in the presence or absence of the PPARγ ligand ciglitazone (100 µM; upper panel) or the LXR ligand TO-901317 (1 µM; lower panel). NR ligands were added one hour prior to TLR ligand addition. MDDCs were then stained for surface expression of HLA-DR, CD80, CD86, and CD11c and analyzed by flow cytometry. Shaded histogram, isotype control. Black line, iMDDCs. Blue line, LPS-treated MDDCs. Red line, LPS and nuclear receptor ligand-treated MDDCs. (B) MDDCs were treated with PAM3CSK4 in the presence or absence of 100 µM ciglitazone (upper panels) or 1 µM TO-901317 (lower panels). Cell-free supernatants were analyzed for TNF-α, IL-6, and IL-8 by ELISA. (n = 3) (C) MDDCs were treated with 100 ng/ml LPS in the presence or absence of 100 µM ciglitazone or 1 µM TO-901317. Cell-free supernatants were analyzed for MIP-1α and RANTES. (n = 3) (D) pDCs were treated with 5 µM CpG ODN 2006 or 1 µg/ml CLO97 in the presence or absence of 100 µM ciglitazone or 1 µM TO-901317. Cell-free supernatants were analyzed for MIP-1α and RANTES. (n = 4). * p<0.001 compared to TLR ligand-treated controls; ND, not detected. We next examined the effects of ciglitazone and TO-901317 treatment on TLR-induced proinflammatory cytokine and chemokine production. We found that treatment with these PPARγ and LXR ligands prevented the release of proinflammatory cytokines and chemokines such as TNF-α, IL-6, and IL-8 by PAM3CSK4-activated MDDCs (Figure 1B). In addition, PPARγ and LXR treatment also prevented the release of the chemokines MIP-1α and RANTES, which are important for the recruitment of CD4+ T cells to sites of infection, both from MDDC in response to the TLR4 ligand LPS (Figure 1C) and from plasmacytoid DCs (pDCs) in response to the TLR7 ligand CLO97 and the TLR9 ligand CpG ODN 2006 (Figure 1D). Importantly, PPARγ and LXR signaling inhibited TLR-induced proinflammatory cytokine and chemokine production coincident with TLR ligation (data not shown), suggesting that NR-mediated inhibition most likely acts through a trans-repression mechanism [34]. The concentrations of the PPARγ ligand ciglitazone and the LXR ligand TO-901317 necessary to see a reduction in DC maturation and the production of pro-inflammatory cytokines and chemokines did not affect MDDC viability as measured by LDH release or mitochondrial activity (Figure S1 and data not shown).
NR signaling prevents MDDC migration in response to CCL21
In addition to transmitting HIV-1 to T cells with high efficiency, DCs can also contribute to HIV-1 pathogenesis by binding virus and then migrating from mucosal sites of infection to regional lymph nodes. In this way, DCs can contribute to viral dissemination. Studies have shown that mature DCs have a greater migratory capacity than immature DCs [26], [27]. This led us to examine whether NR signaling would also inhibit MDDC migration through a 5 µm pore size Transwell insert in response to the chemokine CCL21, which has been shown to be important for DC migration in vivo [27]. We found that LPS-matured MDDCs (mMDDCs) migrated in response to a CCL21 gradient and that co-treatment with PPARγ or LXR ligands repressed this migration approximately 2-fold (Figure 2A). In contrast, immature MDDCs (iMDDCs) migrated quite poorly in response to CCL21 and, consequently, NR ligand treatment had a limited effect. Expression of CCR7, a receptor for CCL21, is upregulated in DCs in response to TLR engagement [26], [54]. Notably, treatment with PPARγ and LXR ligands prevented the LPS-induced upregulation of CCR7 (Figure 2B), which may partly explain why NR ligand-treated MDDCs migrate poorly in response to CCL21. Together, these data suggest that PPARγ and LXR signaling inhibit DC migration by preventing TLR-induced DC maturation.
Fig. 2. PPARγ and LXR ligand treatment prevents MDDC migration in response to CCL21. 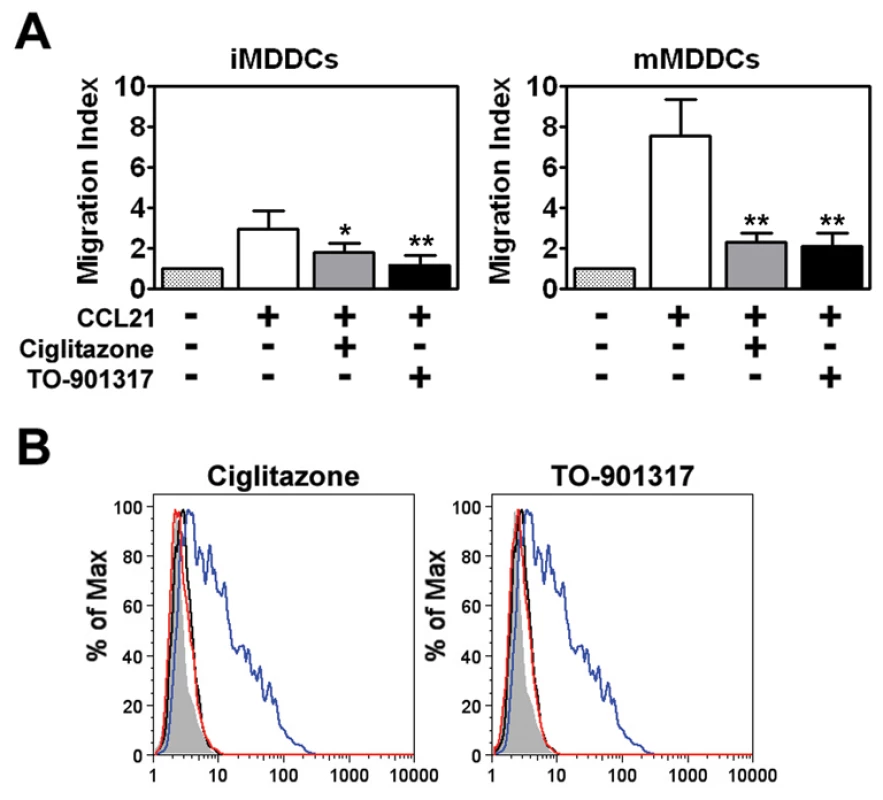
(A) Immature (left panel) or mature (right panel) MDDCs were seeded in the upper chamber of a Transwell insert and allowed to migrate in response to medium or a CCL21 gradient in the lower chamber. Migration indexes were determined after four hours. On average, approximately 14% of immature MDDCs (0.34×105 cells) and 40% of LPS-matured MDDCs (1.08×105 cells) migrated through the Transwell insert in response to CCL21. (n = 3) * p<0.005, ** p<0.001 compared to CCL21-treated controls (B) MDDCs were treated with LPS for 48 hours in the presence or absence of 100 µM ciglitazone or 1 µM TO-901317 and analyzed for CCR7 expression by flow cytometry. Shaded histogram, isotype control. Black line, iMDDCs. Blue line, LPS-treated MDDCs. Red line, LPS and nuclear receptor ligand-treated MDDCs. NR ligands inhibit MDDC-mediated trans-infection of HIV-1 to T cells
DCs are thought to play a critical role in virus dissemination by capturing HIV-1 and transferring it to T cells [5], [24], [55]. We therefore examined whether NR ligands could modulate DC-mediated HIV-1 trans-infection. iMDDCs were treated with ciglitazone or TO-901317 for 48 hours, extensively washed, and then incubated for four hours with either a single-round replication-competent HIV-1 reporter virus packaged with an R5-tropic envelope or with wild-type HIV-1. Following incubation with the virus, MDDCs were washed extensively to remove unbound virus and then cultured directly with autologous T cells or in the upper well of a Transwell insert separated from the T cells by a 0.4 µm membrane. Although HIV-1 replicated very poorly in immature MDDCs (Figure 3), we found that DCs were able to mediate T cell infection when directly cultured with the T cells or when separated from them by the Transwell insert (Figure 3), suggesting that a portion of the MDDC-mediated trans-infection is mediated by either exosome-associated HIV-1 [56] or virus shed from the surface of MDDCs [57].
Fig. 3. MDDCs are poorly infected with HIV-1 but can mediate trans-infection of T cells. 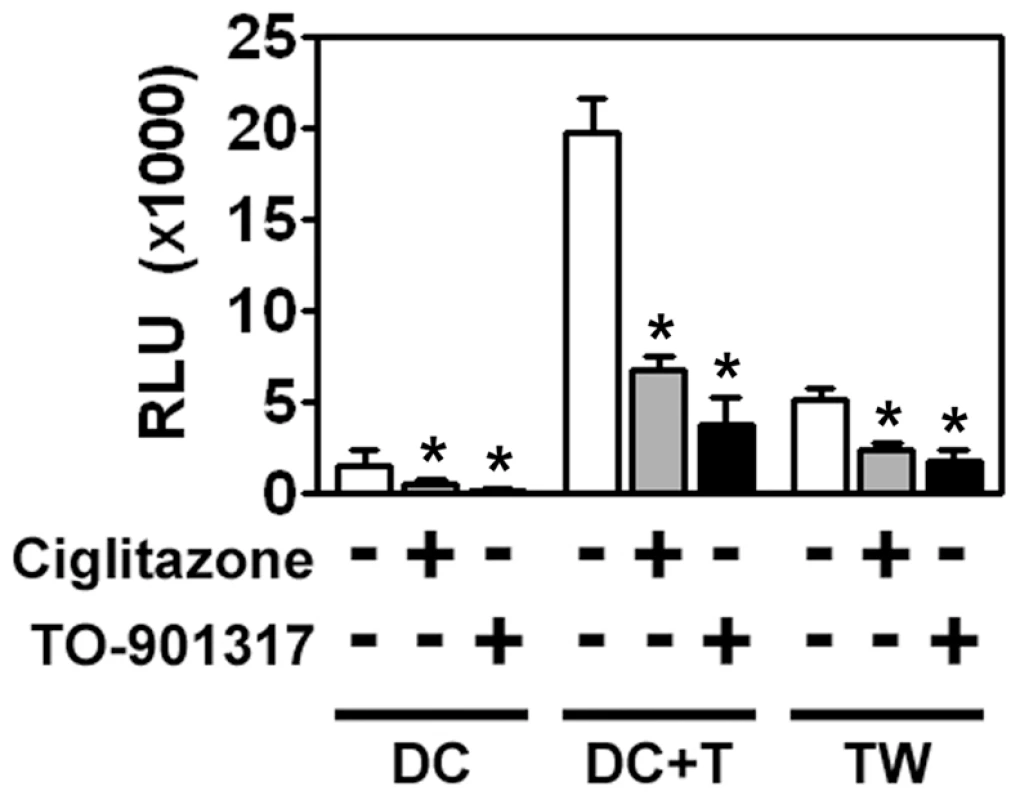
iMDDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317, washed extensively, and then incubated with a single round replication-competent HIV-1 reporter virus encoding luciferase and pseudotyped with R5-tropic envelope glycoproteins. After four hours, unbound virus was removed by extensive washing and the MDDCs were incubated directly with PM1 T cells or separated from the PM1 T cells by a Transwell insert with a 0.4 µm membrane for 48 hours. The cells were then harvested, lysed, and assayed for luciferase activity. (n = 4) DC, MDDCs cultured in the absence of T cells. DC + T, MDDCs cultured directly with T cells. TW, MDDCs separated from T cells by a Transwell insert. * p<0.001. Most importantly, we found that PPARγ and LXR ligands inhibited trans-infection up to 5-fold underscoring their potential to limit HIV-1 transmission (Figure 3). NR signaling inhibits trans-infection of T cells by both single-round replication competent virus (Figure 3) and wild-type replication competent virus (Figure 4A), suggesting that the majority of virus transferred to T cells is due to virus captured by the DC and not due to newly synthesized virus. Because mature DCs capture and transfer HIV-1 to T cells with higher efficiency than immature DCs [23], [24], [25], we next determined whether PPARγ or LXR ligands could inhibit trans-infection mediated by LPS - or PAM3CSK4-matured MDDCs. PPARγ and LXR signaling repressed trans-infection of autologous primary T cells mediated by both immature, LPS-matured MDDCs (Figure 4A), and PAM3CSK4-matured MDDCs (Figure 4B), suggesting that the repression is independent of MDDC maturation. To confirm NR-dependent maturation-independent repression of DC-mediated HIV-1 trans-infection, we matured MDDCs with LPS for two days prior to treatment with NR ligands and then assayed for HIV-1 transfer. As seen in figure 4C, the ability of mature MDDCs to transfer virus was impaired when treated with PPARγ and LXR ligands. In addition, we found that PPARγ and LXR ligand treatment of MDDCs prevented trans-infection over a wide range of input virus (Figure 4D). Of note, NR-ligand treatment inhibited immature and mature MDDC-mediated trans-infection of both R5 - and X4-tropic envelope glycoprotein-pseudotyped single-round replication competent reporter viruses and replication-competent R5 - and X4 - tropic wild-type HIV-1 (data not shown). Together these data suggest that, unlike PPARγ - and LXR-mediated inhibition of migration, the inhibition of trans-infection is independent of the maturation state of the DC. Importantly, MDDC-mediated trans-infection is also inhibited by rosiglitazone (Figure 4E), a PPARγ agonist that is currently licensed for the systemic treatment of type II diabetes.
Fig. 4. NR ligand treatment prevents HIV-1 trans-infection. 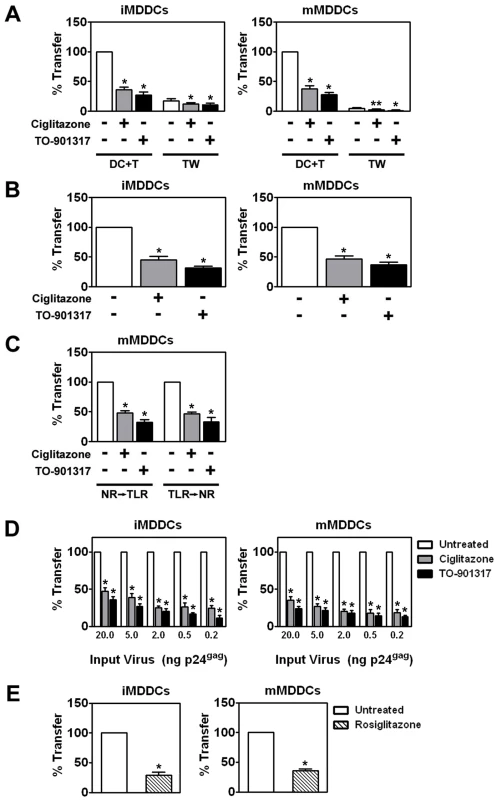
(A) Immature (left panel) and LPS-matured (right panel) MDDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317 (NR ligands were added one hour prior to TLR ligand addition), incubated with replication competent HIV-1ADA, washed, and cultured directly with autologous T cells (DC + T) or separated from the T cells by a Transwell insert (TW) for a period of 48 hours. Virus transfer and replication was measured by p24gag ELISA. DC + T, MDDCs cultured directly with T cells. TW, MDDCs separated from T cells by a Transwell insert. Mean transfer/replication values: 9.18 ng p24gag for untreated iMDDCs cultured directly with T cells; and 85.5 ng p24gag for untreated mMDDCs cultured directly with T cells. (n = 5) * (B) Immature (left panel) and PAM3CSK4-matured (right panel) MDDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317, washed extensively, and then incubated with HIV-1ADA. After four hours, unbound virus was removed by extensive washing and the MDDCs were incubated directly with autologous primary T for 48 hours. The cells were then harvested, lysed, and assayed for virus transfer by p24gag ELISA. (n = 3) (C) MDDCs were treated with LPS and either 100 µM ciglitazone or 1 µM TO-901317 for 48 hours (NR → TLR) or with LPS for 48 hours prior to treatment with NR ligands for an additional 48 hours (TLR → NR). MDDCs were incubated with HIV-1ADA, washed, and cultured directly with autologous T cells. Virus transfer was measured by p24gag ELISA. (n = 3) (D) Immature (left panel) and LPS-matured (right panel) MDDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317 and incubated with increasing amounts of HIV-1ADA, washed extensively, and then cultured with autologous T cells. Virus transfer was measured by p24gag ELISA. (n = 3) (E) Immature (left panel) and LPS-matured (right panel) MDDCs were treated for 48 hours with 1 µM rosiglitazone (hatched bars), incubated with HIV-1ADA, washed, and cultured directly with autologous T cells. Virus transfer was measured by p24gag ELISA. (n = 3) * p<0.001, ** p<0.005 compared to untreated controls. PPARγ and LXR ligands inhibit trans-infection at least in part by blocking HIV-1 capture by MDDCs
Next, we wanted to examine the mechanism accounting for the inhibition of trans-infection. We began by examining the effects of PPARγ or LXR ligand treatment on HIV-1 binding to MDDCs. Ciglitazone and TO-901317 treatment led to a 2 to 5-fold decrease in the amount of HIV-1 associated with MDDCs as measured by an ELISA for the HIV-1 p24 capsid protein (Figure 5A). Another PPARγ ligand, rosiglitazone, was also tested and had a comparable effect on HIV-1 capture (Figure 5B). Treatment with these NR ligands also inhibited the capture of HIV-1 by DCs at 4°C, suggesting that NR ligand treatment prevents DC binding of HIV-1 (Figure S2). In addition, we found that PPARγ and LXR ligand treatment of MDDCs prevented capture over a wide range of input virus (Figure 5C). Although NR signaling can repress inflammatory gene expression by a trans-repression mechanism [30], [31], [32], [33], [34], [36], [37], [38], [52], it likely decreases HIV-1 capture through a different mechanism. MDDCs must be treated with PPARγ and LXR ligands for at least 12 hours in order to observe inhibition of virus capture (Figure 5D), suggesting that changes in cellular gene expression are required for the observed effect. Though the amount of virus captured by MDDCs upon NR ligand treatment was reduced, the relative amount of virus particles internalized was similar (Figure 5E) suggesting that reduced ability of MDDCs to capture HIV-1 particles upon NR ligand treatment was not due to gross reduction in cellular endocytic function. To confirm that NR ligand treatment does not alter the ability of MDDCs to internalize particles, we examined their effects on the ability of MDDCs to macropinocytose FITC-labeled dextran. NR ligand treatment had no effect on FITC-dextran internalization by immature or mature MDDCs (Figure S3 and data not shown).
Fig. 5. NR ligand treatment prevents HIV-1 trans-infection by blocking the capture of HIV-1 particles by MDDCs. 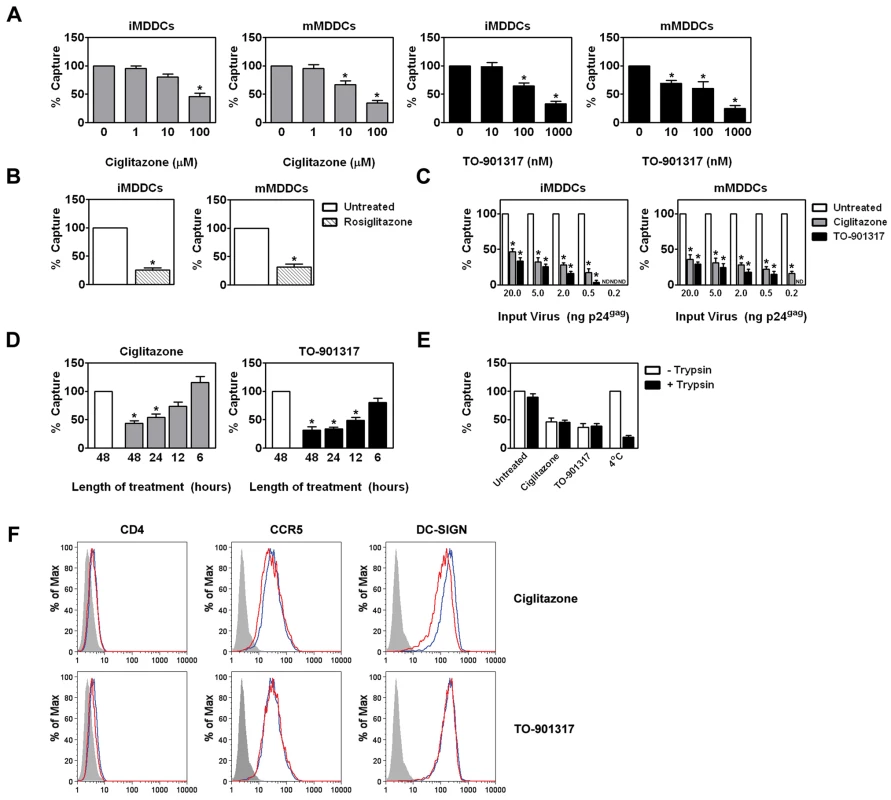
(A) Immature (left panel) and mature (right panel) MDDCs were treated with increasing concentrations of ciglitazone or TO-901317 for 48 hours. The MDDCs were then incubated with 5 ng p24gag equivalent of HIV-1ADA and virus capture was measured by p24gag ELISA. Mean capture values: 622.93 pg p24gag for untreated iMDDCs; and 1.80 ng p24gag for untreated mMDDCs. (n = 4) (B) Immature (left panel) and mature (right panel) MDDCs were treated with 1 µM rosiglitazone (hatched bars) for 48 hours and analyzed as in (A). (n = 3) (C) Immature (left panel) and LPS-matured (right panel) MDDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317 and incubated with increasing amounts of HIV-1ADA, washed extensively, and lysed. Virus capture was measured by p24gag ELISA. (n = 3) (D) iMDDCs were treated with 100 µM ciglitazone or 1 µM TO-901317 for various times, incubated with HIV-1ADA, and assayed for virus capture by p24gag ELISA. (n = 3) (E) Immature MDDCs were treated with ciglitazone (100 µM) or TO-901317 (1 µM) for 48 hours, incubated with HIV-1ADA for four hours at 37°C, and then washed to remove unbound virus. Some cells were incubated with virus at 4°C (as indicated). The cells were then treated with 0.5% trypsin for 5 minutes at 37°C to degrade surface-bound virus particles, washed twice in culture medium, and lysed as above. Virus capture was measured by p24gag ELISA. (n = 3) (F) iMDDCs were treated with either 100 µM ciglitazone or 1 µM TO-901317 for 48 hours and cell-surface expression of CD4, CCR5, and DC-SIGN was measured by flow cytometry. Shaded histogram, isotype control. Blue line, iMDDCs. Red line, nuclear receptor ligand-treated iMDDCs. * p<0.001 compared to untreated controls, ND, not detected. NR ligand treatment does not alter the expression of HIV-1 attachment factors
Our data suggest that changes in cellular gene expression are necessary for the observed decrease in HIV-1 capture by MDDCs. We therefore considered the possibility that PPARγ and LXR ligand treatment altered the expression of known HIV-1 attachment factors expressed on the surface of immature MDDCs. However, we found that NR ligand treatment did not alter the expression of CD4, CCR5, or DC-SIGN (Figure 5F), which have been implicated in DC capture of HIV-1 [5], [58]. Despite these findings, we cannot rule out whether NR signaling alters the expression of other factors implicated in HIV-1 attachment such as other C-type lectins [59], [60], [61], [62], heparan sulfate proteoglycans [63], [64], [65], or GSLs [66], [67], [68], [69], [70].
NR ligand treatment prevents HIV-1 capture and transfer by myeloid DCs
Although MDDCs are a faithful representation of myeloid or conventional DCs (mDCs) with respect to their interactions with HIV-1 [25], we decided to utilize mDCs freshly isolated from the peripheral blood of healthy volunteers. We found that PPARγ and LXR signaling inhibited the ability of immature and LPS-matured mDCs to capture HIV-1ADA and transfer it to autologous T cells (Figure 6) in a manner consistent with results obtained using MDDCs.
Fig. 6. PPARγ and LXR ligand treatment inhibit myeloid DC-mediated HIV-1 capture and trans-infection. 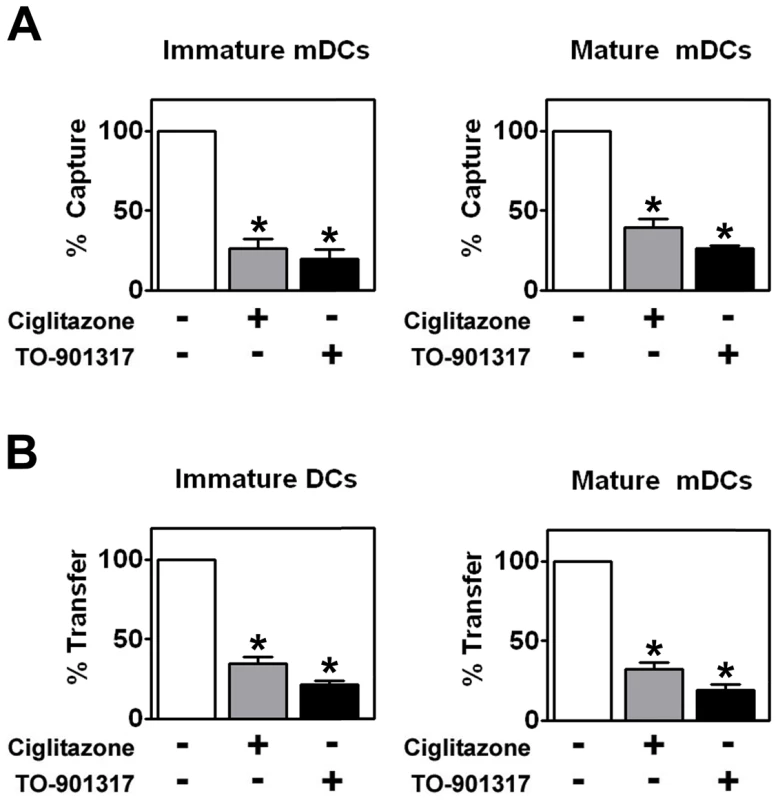
(A) Immature (left panel) and mature (right panel) mDCs were treated with 100 µM ciglitazone or 1 µM TO-901317 for 48 hours. The mDCs were then incubated with HIV-1ADA and virus capture was measured by p24gag ELISA. (n = 4) (B) Immature (left panel) and LPS-matured (right panel) mDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317, incubated with HIV-1ADA, washed, and cultured directly with autologous T cells. Virus transfer was measured by p24gag ELISA. (n = 4) * p<0.001 compared to untreated controls. PPARγ and LXR signaling does not prevent virological synapse formation
Because direct DC-T cell contact is required for efficient virus transfer [24], [57] (and Figure 3), we wanted to determine whether NR ligand treatment interfered with the ability of MDDCs to form conjugates with T cells. Using a FACS-based conjugate formation assay [71], we determined that NR ligand-treated MDDCs were able to form conjugates with primary autologous T cells in a manner similar to untreated MDDCs (Figure 7A). Because NR ligand treatment did not alter the ability of DCs to form conjugates with T cells, we next wanted to examine whether such treatment prevented the formation of functional virological synapses between DCs and T cells. Confocal microscopy data suggest that PPARγ and LXR ligand-treated DCs are capable of forming virological synapses, as indicated by co-localization of virus and the tetraspanin CD81 at the site of DC-T cell contact (Figure 7B). However, number of virus particles localized at the virological synapse is decreased in NR ligand-treated cells. Taken together, our data suggest that NR signaling impairs the ability of MDDCs to transfer virus to T cells by inhibiting the capture of HIV-1 by MDDCs.
Fig. 7. PPARγ and LXR ligand treatment do not prevent DC-T cell conjugate or virological synapse formation. 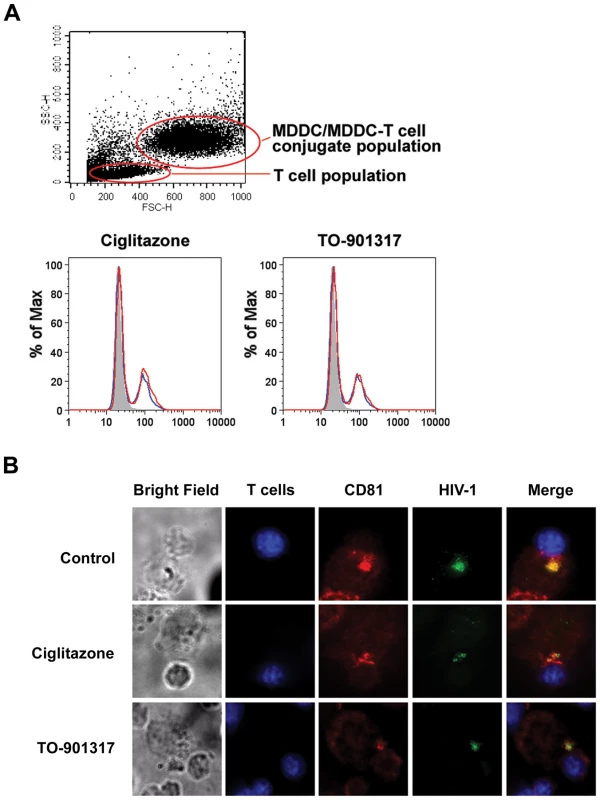
(A) Primary autologous CD4+ T cells were labeled with the cytoplasmic dye CMTMR for 30 minutes at 37°C, washed three times with PBS to remove excess dye, and incubated overnight at 37°C. Following labeling, 5×105 T cells were incubated with 2.5×105 unlabeled iMDDCs for four hours at 37°C. The conjugates were then fixed in 2% paraformaldehyde and conjugate formation was assessed by flow cytometry with gating on the MDDC population. Shaded histogram, unlabeled MDDCs without T cells. Blue line, untreated MDDCs with T cells. Red line, nuclear receptor ligand-treated MDDCs with T cells. (B) Primary autologous CD4+ T cells were labeled with the cytoplasmic dye CMCA (CellTracker Blue, Molecular Probes) for 30 minutes at 37°C, washed three times with PBS to remove excess dye, and incubated overnight at 37°C. 2.5×105 unlabeled LPS-matured MDDCs were incubated with 100 ng HIV-1NL4-3 virions packaged with Vpr-EGFP for four hours at 37°C, washed four times with PBS, and incubated with 5×105 CMCA-labeled autologous T cells for four additional hours. The cells were fixed in 1% paraformaldehyde, stained with anti-CD81-PE, and analyzed by deconvolution microscopy. PPARγ and LXR ligands inhibit HIV-1 capture by MDDCs in an envelope glycoprotein-independent manner
Recent studies have demonstrated that DCs can bind to infectious HIV-1 and envelope-deficient virus-like particles (VLPs) in a GSL-dependent, viral envelope glycoprotein-independent manner [72], [73]. We therefore wanted to assess whether triggering PPARγ and LXR signaling could alter the ability of MDDCs to bind virus independently of the envelope glycoprotein gp120. We found that PPARγ and LXR ligand treatment led to a 2 to 5-fold decrease in the amount of envelope glycoprotein (Env)-deficient HIV-1 particles captured by both immature and mature MDDCs (Figure 8A), suggesting that GSL-based virus-DC interactions may be targeted by NR signaling. To demonstrate that this interaction is truly envelope glycoprotein-independent, we also examined the effects of PPARγ and LXR signaling on the ability of DCs to capture HIV-1 particles pseudotyped with the glycoproteins of vesicular stomatitis virus (VSV), Ebola virus (EboV), and Marburg virus (MarV). As shown in figure 8B, treatment with PPARγ and LXR ligands inhibited the ability of DCs to capture EboV or MarV glycoprotein-pseudotyped HIV-1 particles, whereas the treatment had no effect on the ability of DCs to capture VSV-G-pseudotyped particles. Since, like HIV-1, both EboV and MarV glycoproteins are known to require cholesterol for infection [74], [75], whereas VSV-G does not [43], [75], [76], this suggested that PPARγ and LXR might be exerting their effects through the regulation of cellular cholesterol.
Fig. 8. PPARγ and LXR ligand treatment prevents envelope glycoprotein-independent capture of virus by MDDCs. 
(A) Immature (left panel) and mature (right panel) MDDCs were treated with 100 µM ciglitazone or 1 µM TO-901317 and incubated with a single round replication-competent HIV-1 reporter virus encoding luciferase that was either pseudotyped with R5-tropic envelope glycoproteins (R5) or that lacked envelope glycoproteins (ΔEnv). Virus capture was measured by p24gag ELISA. (n = 3) (B) Immature MDDCs were treated with100 µM ciglitazone or 1 µM TO-901317 and incubated with a single round replication-competent HIV-1 reporter virus encoding luciferase that was packaged with envelope glycoproteins from VSV (VSV-G), Ebola virus Zaire (EboV-Z), Ebola virus Sudan (EboV-S), or Marburg virus (MarV). Virus capture was measured by p24gag ELISA. Mean capture values for untreated iMDDCs: 1.13 ng VSV-G-pseudotyped HIV-1; 251.83 pg EBOV-Z-pseudotyped HIV-1; 324.10 pg EBOV-S-pseudotyped HIV-1; and 415.07 pg MARV-pseudotyped HIV-1. (n = 3) * p<0.001 compared to untreated controls. PPARγ and LXR ligand inhibits HIV-1 capture by MDDCs in a cholesterol-dependent manner
Previous studies have shown that DC capture of HIV-1 is dependent upon the cholesterol content of the cell membrane [51]. Since both PPARγ and LXR are known to modulate genes involved in cholesterol metabolism and transport [29], [39], [40], [77], we were interested in determining whether ciglitazone or TO-901317 affected the cholesterol content of MDDCs. Treatment with PPARγ and LXR ligands increased cholesterol efflux from immature MDDCs approximately 2 to 3-fold (Figure 9A) and led to a concomitant 2-fold decrease in the amount of cholesterol in immature MDDCs (Figure 9B). We next wanted to see if cholesterol depletion resulting from PPARγ and LXR ligand treatment was responsible for the decreased ability of MDDCs to capture and transfer HIV-1. In order to do this, we replenished membrane cholesterol in PPARγ and LXR ligand-treated MDDCs using cholesterol-saturated methyl-β-cyclodextrin and assayed for HIV-1 capture and transfer to T cells. Cholesterol repletion of NR ligand-treated MDDCs with cholesterol-saturated methyl-β-cyclodextrin restored cholesterol content (Figure 9B) and, importantly, fully restored the ability of both immature and mature MDDCs to capture HIV-1 (Figure 9C) and transfer it to CD4+ T cells (Figure 9D).
Fig. 9. PPARγ and LXR signaling inhibit HIV-1 capture and transfer by MDDCs via ABCA1-dependent cholesterol efflux. 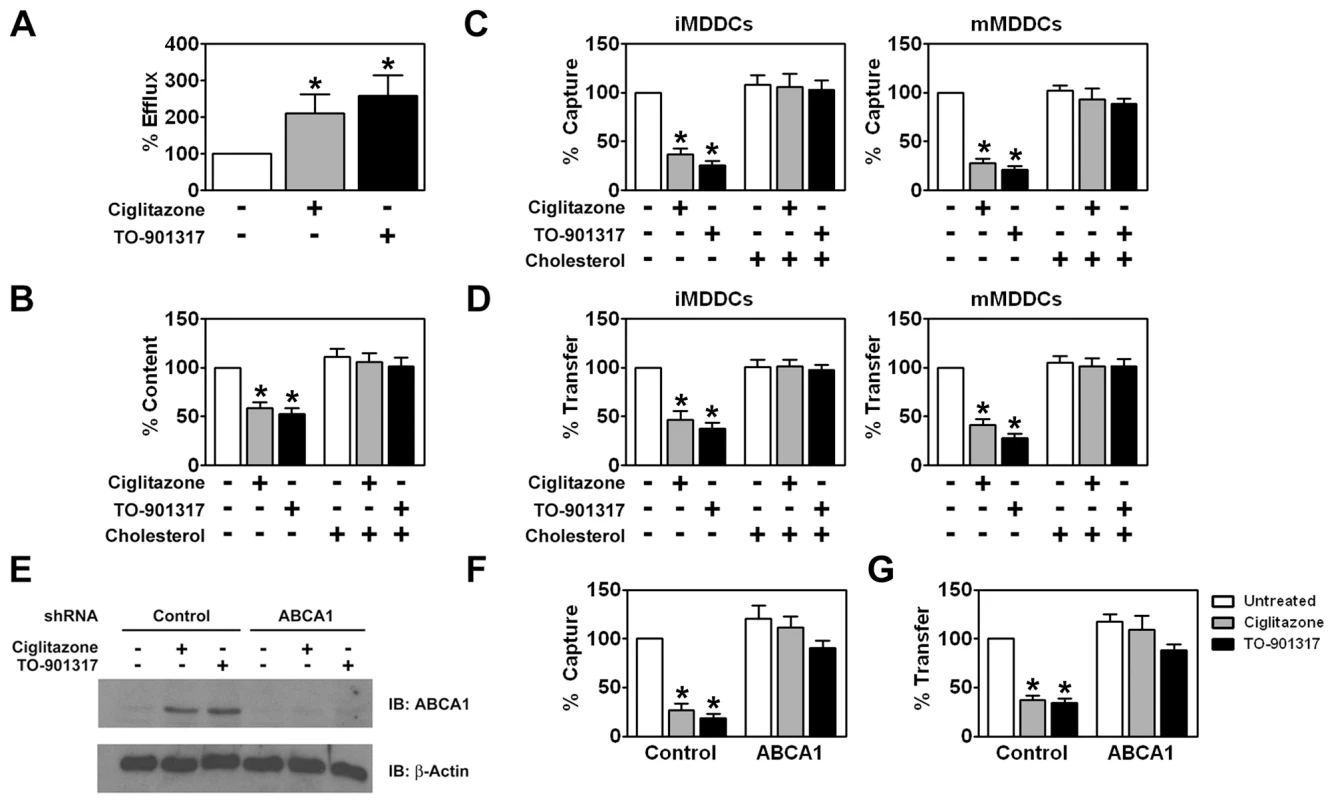
(A) iMDDCs were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317 and cholesterol efflux into the culture supernatant was measured. (n = 3) (B) iMDDCs were treated for 48 hours with 100 µM ciglitazone and 1 µM TO-901317. The cells were then lysed and cholesterol content of the cell lysates was measured. (n = 3) (C-D) Immature (left panel) and mature (right panel) MDDCs were treated with 100 µM ciglitazone or 1 µM TO-901317 for 48 hours. NR ligand-treated cells were then incubated with 300 µM cholesterol in the form of cholesterol-saturated methyl-β-cyclodextrin. Following cholesterol repletion, the cells were used in capture (C) and transfer assays (D) as described in the legend of figure 2. (n = 3) (E) iMDDCs transfected with either control shRNA or shRNA directed against ABCA1 were treated for 48 hours with 100 µM ciglitazone or 1 µM TO-901317. Levels of ABCA1 and β-actin were measured by immunoblot using an anti-ABCA1 and anti-β-actin antibodies, respectively. (n = 3) (F) iMDDCs transfected with either control shRNA or shRNA directed against ABCA1 were treated for 24 hours with 100 µM ciglitazone (gray bars) or 1 µM TO-901317 (black bars). The MDDCs were then incubated with HIV-1ADA and virus capture was measured by p24gag ELISA. (n = 3) (G) iMDDCs transfected with either control shRNA or shRNA directed against ABCA1 were treated for 24 hours with 100 µM ciglitazone (gray bars) or 1 µM TO-901317 (black bars). The MDDCs were then incubated with HIV-1ADA, washed extensively, and then incubated with autologous T cells. Virus transfer was measured by p24gag ELISA. Transfer assays with shRNA-treated DCs were performed using cells from two independent donors. * p<0.001 compared to untreated controls. PPARγ and LXR signaling upregulate expression of ATP-binding cassette protein A1 (ABCA1) that facilitates the apoA1-dependent efflux of cholesterol from cells [39], [41], [77]. We therefore examined whether treatment of DCs with ciglitazone or TO-901317 affected ABCA1 expression. We found by western blot analysis that both NR ligands increased ABCA1 expression (Figure 9E). Importantly, targeted knockdown of ABCA1 using shRNA abrogated the effect of PPARγ and LXR ligand treatment on cholesterol efflux (data not shown), HIV-1 capture by DCs (Figure 9F), and HIV-1 transfer to T cells (Figure 9G). These findings suggest that ligand-activated PPARγ and LXR mediate their effects through the depletion of cholesterol from the DC plasma membrane via the up-regulation of the ABCA1 cholesterol transport protein. It will be interesting to determine whether HIV-1 particles interact directly with cholesterol in the plasma membrane of DCs or with factors that localize to cholesterol-rich lipid rafts.
Discussion
Sexual transmission of HIV-1 is enhanced by inflammatory and ulcerative co-infections with STI pathogens that cause diseases such as genital herpes, gonorrhea, syphilis, Chlamydia, bacterial vaginosis, and fungal infections [78], [79], [80], [81], [82], [83]. This enhanced susceptibility to infection may be due to a number of factors, including disruption of epithelial integrity [6], [11], [14], [84], [85], [86], [87], recruitment of HIV-1 target cells such as Langerhans cells, DCs, macrophages, and T lymphocytes to sites of inflammation [8], and activation of HIV-1 expression by pro-inflammatory cytokines or microbial components [21], [22], [88], [89], [90], [91], [92]. It is likely that STI pathogens enhance these latter two processes, at least in part, through engagement of the TLR family of innate immune receptors. Clearly, prophylactic methods that inhibit infection of the genital or rectal mucosa would significantly limit the global spread of HIV. To this end, considerable efforts have been directed toward the development of microbicides that interfere with virus integrity or with key steps in virus replication. However, to date, little attention has been paid to targeting cellular pathways involved in active suppression of inflammation and its effects on mucosal HIV-1 infection and virus dissemination. With this in mind, we have focused our efforts on the nuclear receptor family of transcription factors that have recently been shown to be potent inhibitors of TLR-induced inflammation [30], [31], [32], [33], [34], [36], [37], [38]. Here, we demonstrate that PPARγ and LXR signaling inhibit several aspects of DC biology that are important for HIV-1 mucosal transmission. These include TLR-induced pro-inflammatory cytokine expression, DC migration in response to the chemokine, CCL21, and, importantly, DC-mediated capture of infectious virus particles and trans-infection of CD4+ T cells. Our findings highlight the therapeutic potential of PPARγ and LXR ligands as topical treatments that could be used in conjunction with conventional microbicides to limit mucosal transmission of HIV-1.
DC-mediated trans-infection of T cells is thought to play a critical role in the mucosal transmission of HIV-1. Studies suggest that DCs can mediate trans-infection either by internalizing infectious virions into a protected tetraspanin-rich intracellular compartment, or deep membrane invaginations contiguous with the cell surface, and releasing them for the subsequent infection of T cells [5], [56], [73], [93], [94], [95] or by retaining virions at the cell surface and transferring them to T cells [57], [95]. Regardless of the mechanism, maturation of DCs with ligands for TLRs such as TLR4 and TLR2/TLR1 increases DC-mediated HIV-1 capture and trans-infection of T cells. DC maturation also contributes to HIV-1 mucosal transmission in a number of other ways. Mature DCs create a pro-inflammatory environment that favors virus replication [20], [88], [96], [97], [98] and leads to disruption of the mucosal integrity [83], [99]. Mature DCs may also contribute to virus dissemination by virtue of their enhanced ability to traffic to regional lymph nodes in response to chemokine gradients and, once there, transfer virus to resident CD4+ T cells. Here we show that PPARγ or LXR ligand treatment can prevent DC maturation as measured by the expression of cell surface markers such as HLA-DR, CD80, and CD86 (Figure 1A). Importantly, treatment with PPARγ or LXR ligands also potently inhibit expression of maturation-associated pro-inflammatory cytokines (Figure 1B), such as TNF-α and IL-6 and the pro-inflammatory chemokine, IL-8, that have been shown to augment HIV-1 replication in infected cells and to increase HIV-1 transmission to T cells [21], [22], [91], [100]. Moreover, we demonstrate that PPARγ and LXR signaling can interfere with the migration of DCs in response to a CCL21 chemokine gradient (Figure 2A). This appears to be due to the effects of PPARγ and LXR signaling on the expression of CCR7 (Figure 2B), one of the receptors for CCL21. CCR7 is up-regulated upon DC maturation and has been shown to be important for the migration of DCs from the mucosa to regional lymph nodes in vivo [54]. By preventing DC migration in response to CCL21, PPARγ and LXR ligands may help to block the dissemination of DC-associated virus from mucosal sites of infection to regional lymph nodes.
Recent studies demonstrated that activation/maturation of DCs through TLR4 or TLR2/TLR1 enhances HIV-1 transmission to target cells via increased HIV-1 capture [23], [24], [25], [92] and Figure 4 and 5). Here, we demonstrate that activating PPARγ or LXR signaling pathways in DCs decreases the ability of both immature and TLR-matured DCs to capture and transfer HIV-1 to T cells (Figure 3, 4A and 5A). Furthermore, NR signaling can inhibit HIV-1 transfer by previously matured DCs (Figure 4C) These results suggest that PPARγ and LXR signaling alter other pathways involved with HIV-1 trans-infection that are independent of the maturation state of the DC (Figure 4C), however we cannot rule out the possibility that the prevention of DC maturation may contribute to the NR-mediated decrease in HIV-1 capture and transfer. Many studies have demonstrated a role for PPARγ and LXR signaling in cholesterol metabolism and transport [29], [39], [40]. For example, both signaling pathways stimulate the expression of ABCA1 and ABCG1, which have been implicated in apolipoprotein A1 (ApoA1) - and high density lipoprotein (HDL)-mediated cholesterol efflux, respectively [39]. Given the importance of cholesterol for a number of aspects of HIV-1 biology, including virus binding and infection [42], [43], [44], [45], [47], [48], [49], [50], [51], [76], we hypothesized that PPARγ and LXR signaling was altering the cholesterol content of DC membranes, thereby rendering them incapable of efficiently binding HIV-1 particles. Previous studies have demonstrated that treatment with cholesterol depleting drugs, such as methyl-β-cyclodextrin, or with cholesterol synthesis inhibitors, such as HMGCoA-reductase inhibitors (statins), alters the ability of cells, including DCs, to bind HIV-1 and renders them refractory to HIV-1 infection [42], [43], [45], [49], [50], [51], [101]. Here, we show that cholesterol repletion of PPARγ and LXR ligand-treated DCs reverses the effects of the NR ligands on virus capture and transfer (Figure 9C and 9D), confirming that PPARγ and LXR are mediating their effects through membrane cholesterol. In addition, targeted shRNA knock-down of ABCA1 abrogates the effects of PPARγ and LXR signaling on HIV-1 capture and transfer (Figure9F and 9G). A recent study suggests that LXR-dependent cholesterol efflux in macrophages is mediated entirely through ABCA1, with little to no contribution from ABCG1 [102]. We cannot, however, formally exclude a contribution from ABCG1-dependent cholesterol efflux to the effects we report here. Our data show that PPARγ and LXR signaling decrease cellular cholesterol content, which may in turn deplete cholesterol from membrane lipid rafts. It will be interesting to determine whether treatment of DCs with PPARγ and LXR ligands disrupts lipid rafts and whether this accounts for the decreased ability or NR-treated DCs to capture and transfer HIV-1.
We found that PPARγ and LXR ligand treatments do not alter the levels of a number of known virus attachment factors expressed on DCs including CD4, CCR5, and DC-SIGN (Figure 5F). Moreover, PPARγ and LXR signaling prevents the capture of Env-deficient HIV-1 virus like particles (Figure 8A), suggesting that virus envelope glycoprotein/receptor interactions are not involved in the observed effect. That the effects of PPARγ and LXR signaling on HIV-1 capture are virus envelope glycoprotein-independent is supported by our finding that treatment of DCs with ciglitazone or TO-901317 prevents the capture of viral particles pseudotyped with the envelope glycoproteins of Ebola virus and Marburg virus (Figure 8B). Interestingly, these two viruses are known to require cholesterol for infection [74], [75]. In contrast, treatment with the NR ligands had no effect on the ability of DCs to capture virus particles pseudotyped with the envelope glycoprotein of VSV. Previous studies have demonstrated that VSV-G-pseudotyped HIV-1 particles are efficiently captured by cells depleted of cholesterol using methyl-β-cyclodextrin [43], [75], [76]. These data further support the hypothesis that PPARγ and LXR signaling alter the membrane cholesterol content of DCs, rendering them incapable of efficiently capturing HIV-1 particles.
Although NR ligand treatment limits the expression of immune-activating cytokines and co-stimulatory molecules that are up-regulated as DCs mature, we found that it does not alter the ability of DCs to form conjugates with T cells. The number of DC-T cell conjugates formed with PPARγ and LXR ligand-treated DCs was comparable to that of control untreated DCs (Figure 7A). It will be interesting to determine whether these conjugates represent functional immunological synapses between DCs and T cells. It is worth noting that DC-to-T cell transfer of HIV-1 most likely occurs through the formation of virological synapses [103], [104], [105], [106], [107], [108]. We found that NR ligand treatment does not prevent the formation of virological synapses between DCs and T cells as assessed by confocal microscopy, although ligand treatment does seem to decrease the amount of virus concentrated at the virological synapse (Figure 7B).
Beyond demonstrating the ability of PPARγ and LXR signaling pathways to prevent DC capture and transfer of virus, our results provide support for a number of observations regarding the interactions between DCs and HIV-1. First, we demonstrate that immature DCs can transfer single round replication competent virus to T cells through a Transwell insert that prevents direct contact between the two cell types (Figure 3). Although direct cell-cell contact is required for efficient virus transfer, our data suggest that approximately 20% of infectious virus can be transferred by immature DCs via exosomes or shedding from the cell surface. In contrast, although mature DCs bind approximately 10-fold more virus, less than 10% of transfer is mediated through cell-surface bound viral particles (Figure 4A). Second, our data suggest that a large percentage of virions captured by DCs is internalized or otherwise protected from proteases (Figure 5E). Previous studies have demonstrated that DCs internalize HIV-1, resulting in either degradation of virus particles [56], [65], [109], establishment of productive infection [110], [111], [112], or sequestration into protected intracellular compartments [56], [73], [94], [95]. Although PPARγ and LXR signaling alters the amount of virus captured by DCs, it does not seem to alter the percentage of captured virus that is internalized by DCs (Figure 5E). This is not surprising, since PPARγ and LXR ligand treatment does not alter the endocytic capacity of DCs, as measured by the internalization of FITC-dextran (Figure S2). Finally, our data confirm that DCs can bind virus particles in a gp120-independent manner (Figure 8A). Recent reports demonstrate that host cell-derived GSLs incorporated into the budding virus particle play a critical role in mediating HIV-1 capture by immature and mature DCs in a gp120-independent manner [72], [73]. Taken together with current and previous findings that cholesterol depletion from DC membranes prevents HIV-1 binding [51] (and Figure 9C), these data argue for the presence of a yet-to-be-identified GSL-recognizing attachment factor(s) within lipid raft-like membrane microdomains at the surface of DCs whose function is compromised upon NR ligand treatment.
NR signaling may have beneficial effects on the prevention of HIV-1 transmission beyond the effects on pro-inflammatory cytokine production, migration, and virus capture and transfer. STIs, through engagement of TLRs, and STI/TLR-induced inflammation, can directly activate HIV-1 replication in infected cells. Our data suggest that both PPARγ and LXR ligands repress HIV-1 replication in DCs (Figure 3), although the levels of replication in this cell type are quite low. This finding is consistent with previous studies that have shown that PPARγ ligands repress HIV-1 expression in infected monocytes and macrophages [113], [114], [115]. Recent findings from our laboratory suggest that NR-mediated repression of HIV-1 replication is due to trans-repression (T. Hanley and G. Viglianti, manuscript in preparation), as is thought to be the case for NR-mediated repression of pro-inflammatory cytokine production [30], [31], [32], [33], [34], [35], [36], [37], [38]. Although our data suggest that the majority of virus transferred to T cells is due to virus captured by DCs, and not due to virus newly synthesized in infected DCs, NR-mediated inhibition of HIV-1 replication may contribute to the inhibition of trans-infection that we report here. By preventing HIV-1 replication, in addition to DC migration, pro-inflammatory cytokine and chemokine production, and trans-infection, PPARγ and LXR ligands may block the dissemination of DC-associated virus from the local site of infection to regional lymph nodes.
In the absence of an effective vaccine for HIV-1, the development of topical microbicides that block the early steps of HIV-1 infection and transmission may represent the best option for containing the spread of this global pandemic. To date, there has been limited success with antiviral microbicides. In order to ensure success with future microbicide development, a much greater understanding of the mechanisms involved in the very early stages of mucosal infection and transmission of HIV-1, and the role of DCs in HIV-1 pathogenesis, in particular, are required. Our results contribute to a better delineation of the mechanisms underlying the HIV-1 trans-infection activity of DCs, while having implications for the development of new anti-HIV microbicide strategies. PPARγ and LXR ligands are small lipophilic molecules that readily diffuse across cell membranes and might be amenable to topical formulations. Two PPARγ agonists, rosiglitazone and pioglitazone, are currently approved for the systemic treatment of type II diabetes. A limitation of the present study is that we have not yet examined the effects of NR signaling on HIV-1 transmission in the context of a complex tissue model or an animal model. Despite this limitation, the anti-inflammatory and anti-HIV-1 activities of PPARγ and LXR provide a solid rationale for considering them as drug targets that can act synergistically with conventional anti-viral microbicides that target other aspects of mucosal transmission including virion structure, virus binding/entry, or reverse transcription.
Materials and Methods
Ethics statement
This research has been determined to be exempt by the Institutional Review Board of the Boston University Medical Center since it does not meet the definition of human subjects research.
Cell isolation and culture
Primary human CD14+ monocytes were isolated from the peripheral blood mononuclear cells (PBMCs) of healthy donors using anti-CD14 magnetic beads (Miltenyi Biotec) per the manufacturer's instructions. CD14+ monocytes (1.5×106 cells/ml) were cultured in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.29 mg/ml L-glutamine, 1000 U/ml IL-4 (PeproTech), and 1400 U/ml GM-CSF (PeproTech) for 6-8 days at the end of which the cells acquired an immature dendritic cell phenotype as assessed by flow cytometry (CD11c+, DC-SIGN+, HLA-DRlo, CD80−, CD86−). Cells were given fresh medium supplemented with IL-4 and GM-CSF every 2 days. Mature dendritic cells were obtained following 48 hour exposure to 100 ng/ml ultra-pure E. coli K12 LPS or 100 ng/ml PAM3CSK4. Primary human myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) were isolated from monocyte - and B cell-depleted PBMCs using anti-CD11c and anti-BDCA4 magnetic beads (Miltenyi Biotec) per the manufacturer's instructions. mDCs were cultured in RPMI 1640 with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.29 mg/ml L-glutamine, 1000 U/ml IL-4, and 1400 U/ml GM-CSF. pDCs were cultured in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.29 mg/ml L-glutamine, and 10 ng/ml IL-3 (PeproTech). Primary human CD4+ T cells were isolated from CD14-depleted peripheral blood mononuclear cells using anti-CD4 magnetic beads (Miltenyi Biotec) per the manufacturer's instructions. CD4+ T cells (2×106 cells/ml) were cultured in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.29 mg/ml L-glutamine, 50 U/ml IL-2 (R&D Systems), and 5 µg/ml PHA-P (Sigma) for 6-8 days at the end of which the cells acquired a memory T cell phenotype as assessed by flow cytometry (CD3+, CD4+, CD45RO+, CD45RA–). 293T cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.29 mg/ml L-glutamine. MAGI-CCR5 cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.29 mg/ml L-glutamine, 500 µg/ml G418, 1 µg/ml puromycin, and 0.1 µg/ml hygromycin B. PM1 cells were cultured in RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.29 mg/ml L-glutamine.
Nuclear receptor and Toll-like receptor ligands
The LXR ligand TO-901317 was purchased from Calbiochem. The PPARγ ligands ciglitazone and rosiglitazone were purchased from Cayman Chemicals. The ligands were reconstituted in DMSO. The TLR2 ligand PAM3CSK4, the TLR4 ligand E. coli K12 LPS, the TLR7 ligand CLO97, and the TLR9 ligand CpG ODN 2006 were purchased from Invivogen. Unless otherwise noted, DCs were treated with PPARγ and LXR ligands for 24–48 hours, beginning one hour prior to treatment with TLR ligands.
Virus production
Replication competent R5-tropic HIV-1ADA and X4-tropic HIV-1NL4-3 were generated by infection of PM1 cells. Single-round replication-competent HIV-1-based reporter viruses were generated by packaging a luciferase expressing reporter virus, BruΔEnvLuc2, with the envelope glycoproteins from CCR5-tropic HIV-1(Ada-M), CXCR4-tropic HIV-1(HXB2), VSV (VSV-G), Ebola virus Zaire (EboV-Z), Ebola virus Sudan (EboV-S), or Marburg virus (MarV). EGFP-labeled virus particles were generated by co-transfection of the pro-viral clone HIV-1NL4-3 with an expression vector encoding a Vpr-EGFP fusion protein. Virus stocks were generated by transfecting HEK293T cells using the calcium phosphate method. All viruses were titered on MAGI-CCR5 cells and p24gag content was determined by ELISA.
Transfer assays
To assess DC-mediated transfer of HIV-1 to T cells, DCs were incubated with Ada-M - or HXB2-pseudotyped HIV-luciferase reporter virus at an MOI = 0.1 (37.8–40.4 ng p24gag) for four hours at 37°C. Cells were washed five times with PBS to remove unbound virus, seeded in 96-well plates (2.5×105 cells/well), and then cultured with either PM1 T cells (5×105 cells/well) or autologous primary CD4+ T cells (5×105 cells/well) for 48 hours. In some instances, the DCs were seeded in 24-well plates separated from the T cells by a Transwell insert (Corning) with a 0.4 µm pore size. As controls, virus-exposed DCs and virus-exposed T cells were cultured alone for 48 hours. After 48 hours, the cells were harvested, washed two times with PBS, and lysed in PBS/0.02% Triton X-100. Protein levels in cell lysates were determined using a modified Lowry protein assay (BioRad) and luciferase activity was measured using luciferase reagent (Promega) and a MSII luminometer (Molecular Devices). In some experiments, replication competent R5-tropic HIV-1ADA or X4-tropic HIV-1NL4-3 (5 ng p24gag) were used in place of packaged reporter virus and transfer was measured by p24gag ELISA.
Capture assays
DCs (2.5×105 cells/well) were incubated with replication competent R5-tropic HIV-1ADA or X4-tropic HIV-1NL4-3 (5 ng p24gag) for three to four hours at 37°C. Cells were washed four to five times with PBS to remove unbound virus, and lysed in PBS/10%FBS/0.5% Triton X-100. In some experiments, virus-exposed DCs were incubated with 0.5% trypsin for 5 minutes at 37°C to degrade surface-bound virus particles, washed twice in culture medium, and then lysed as above. An ELISA was used to determine the amount of p24gag protein associated with the cells. In some experiments, Ada-M-, HXB2-, VSV-G, EboV-, or MarV-packaged HIV-luciferase reporter virus (5 ng p24gag) or an equal amount of reporter virus lacking envelope glycoproteins (ΔEnv) was used.
DC-T cell conjugate formation assays
Primary CD4+ T cells were labeled with the cytoplasmic dye CMTMR (CellTracker Orange, Molecular Probes) for 30 minutes at 37°C and then washed three times with PBS to remove excess dye. Cells were then incubated for 16 hours at 37°C and washed twice with PBS prior to use in conjugate formation assays. Following labeling, 5×105 T cells were incubated with 2.5×105 unlabeled iMDDCs for four hours at 37°C in a total volume of 200 µl. The conjugates were then fixed by gently adding an equal volume of 4% paraformaldehyde. Samples were run immediately through a flow cytometer. Conjugate formation was assessed by fluorescence associated with the MDDC population.
Virological synapse formation assays
Primary CD4+ T cells were labeled with the cytoplasmic dye CMCA (CellTracker Blue, Molecular Probes) for 30 minutes at 37°C and then washed three times with PBS to remove excess dye. T cells were then incubated for 16 hours at 37°C and washed twice with PBS prior to use in virological synapse formation assays. 2.5×105 unlabeled mMDDCs were incubated with 100 ng HIV-1NL4-3 virions packaged with Vpr-EGFP for four hours at 37°C, washed four times with PBS, and incubated with 5×105 CMCA-labeled autologous T cells for four additional hours. The cells were fixed in 1% paraformaldehyde, stained with anti-CD81-PE (BD Pharmingen). Z-stacks were captured on the Nikon deconvolution wide-field Epifluorescence Scope at 100×. Using ImageJ software, the images were deconvolved and the fluorescence was summed.
Cholesterol repletion assays
Cholesterol-saturated methyl-β-cyclodextrin was prepared as previously described [116]. Briefly, cholesterol powder was added to 240 mM methyl-β-cyclodextrin solution at 1.16 mg/ml, agitated overnight, and filter sterilized using a 0.22-µm filter. To replete cholesterol, MDDCs were incubated with cholesterol-saturated methyl-β-cyclodextrin at a concentration of 300 µM cholesterol for 30 minutes at 37°C and then washed five times with PBS before being used in virus capture and transfer studies.
Transwell migration assays
MDDCs (2.5×105 cells) were seeded above a Transwell insert with a 5 µm pore size and allowed to migrate through the insert in response to medium or CCL21 (PeproTech). Cells above and below the Transwell insert were fixed in 2% paraformaldehyde and counted in a hemocytometer to determine the relative migratory capacity of the MDDCs. Migration index was calculated by dividing the number of experimental cells that migrated in response to CCL21 by the number of untreated cells that migrated in response to media alone.
Cholesterol efflux assays
Cholesterol efflux into cell-free culture supernatants and cholesterol content of lysed MDDCs were measured using the AmplexRed cholesterol assay kit per the manufacturer's instructions (Invitrogen).
shRNA knock-down of ABCA1
MDDCs were transfected with plasmids that encoded either a mixture of three to five shRNAs directed against ABCA1 or a mixture of control shRNAs (Santa Cruz Biotechnology) and a puromycin-resistance gene using Oligofectamine (Invitrogen) per the manufacturer's instructions. Transfected cells were selected by culture in the presence of puromycin for 48 hours and then used for cholesterol efflux assays, used for HIV-1 capture assays, or lysed for immunoblot analysis to measure ABCA1 expression.
Flow cytometry
MDDC phenotypes were assessed using antibodies against HLA-DR, CD80, CD86, DC-SIGN, CD11c, CD4, CCR5, CXCR4, and CCR7. Primary CD4+ T cell phenotypes were assessed using antibodies to CD3, CD4, CD8, CD45RO, CD45RA, CCR5, and CXCR4. Flow cytometric data was acquired using a Becton-Dickenson FACScan II and data was analyzed using FlowJo software.
Cytokine and chemokine release assays
MDDCs (2.5×105 cells/well) or pDCs (1×105 cells/well) were treated with PAM3CSK4 (100 ng/ml), LPS (100 ng/ml), CLO97 (1 µg/ml), or CpG ODN 2006 (5 µM) for 24 hours in the presence or absence of nuclear receptor ligands as described in the legend to figure 1. Cell-free culture supernatants were collected and analyzed for TNF-α (eBioscience), IL-6 (eBioscience), IL-8 (BioLegend), MIP-1α (PeproTech), and RANTES (PeproTech) release by commercially-available ELISA following the manufacturer's instructions.
Cell viability assays
MDDC cell viability was assessed by trypan blue dye exclusion, MTT cytotoxicity assay, and LDH release using a commercial kit (Promega) per the manufacturer's instructions.
Statistical analysis
Untreated control and ligand-treated experimental samples were compared using a two-tailed t-test. Experiments were performed in duplicate (mDCs and pDCs) or triplicate (MDDCs) using cells from a minimum of three different donors as indicated in the figure legends (n). Data are presented as the mean ± standard deviation of pooled data from at least three donors.
Accession numbers
PPARγ Swiss-Prot # P37231; LXRα Swiss-Prot # Q13133; LXRβ Swiss-Prot # P55055; CCR7 Swiss-Prot # P32248; CCL21 Swiss-Prot # O00585;TLR1 Swiss-Prot # Q15399; TLR2 Swiss-Prot # O60603; TLR4 Swiss-Prot # O00206; TLR7 Swiss-Prot # Q9NYK1; TLR9 Swiss-Prot # C3W5P5; ABCA1 Swiss-Prot # O95477; ABCG1 Swiss-Prot # P45844.
Supporting Information
Zdroje
1. ShattockRJ
MooreJP
2003 Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 1 25 34
2. GouwsE
WhitePJ
StoverJ
BrownT
2006 Short term estimates of adult HIV incidence by mode of transmission: Kenya and Thailand as examples. Sex Transm Infect 82 Suppl 3 iii51 55
3. PopeM
HaaseAT
2003 Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med 9 847 852
4. MillerCJ
LiQ
AbelK
KimEY
MaZM
2005 Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol 79 9217 9227
5. GeijtenbeekTB
KwonDS
TorensmaR
van VlietSJ
van DuijnhovenGC
2000 DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100 587 597
6. HuJ
GardnerMB
MillerCJ
2000 Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol 74 6087 6095
7. FongL
MengozziM
AbbeyNW
HerndierBG
EnglemanEG
2002 Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J Virol 76 11033 11041
8. GuptaP
CollinsKB
RatnerD
WatkinsS
NausGJ
2002 Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol 76 9868 9876
9. JamesonB
BaribaudF
PohlmannS
GhavimiD
MortariF
2002 Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol 76 1866 1875
10. PudneyJ
QuayleAJ
AndersonDJ
2005 Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 73 1253 1263
11. HladikF
SakchalathornP
BallweberL
LentzG
FialkowM
2007 Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26 257 270
12. BomselM
1997 Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 3 42 47
13. BobardtMD
ChatterjiU
SelvarajahS
Van der SchuerenB
DavidG
2007 Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol 81 395 405
14. SpiraAI
MarxPA
PattersonBK
MahoneyJ
KoupRA
1996 Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 183 215 225
15. HuQ
FrankI
WilliamsV
SantosJJ
WattsP
2004 Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med 199 1065 1075
16. BanchereauJ
SteinmanRM
1998 Dendritic cells and the control of immunity. Nature 392 245 252
17. SteinmanRM
Granelli-PipernoA
PopeM
TrumpfhellerC
IgnatiusR
2003 The interaction of immunodeficiency viruses with dendritic cells. Curr Top Microbiol Immunol 276 1 30
18. CameronPU
FreudenthalPS
BarkerJM
GezelterS
InabaK
1992 Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257 383 387
19. PopeM
BetjesMG
RomaniN
HirmandH
CameronPU
1994 Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78 389 398
20. KadowakiN
HoS
AntonenkoS
MalefytRW
KasteleinRA
2001 Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194 863 869
21. PoliG
KinterA
JustementJS
KehrlJH
BresslerP
1990 Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A 87 782 785
22. PoliG
BresslerP
KinterA
DuhE
TimmerWC
1990 Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med 172 151 158
23. DongC
JanasAM
WangJH
OlsonWJ
WuL
2007 Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis - and trans-infection. J Virol 81 11352 11362
24. WangJH
JanasAM
OlsonWJ
WuL
2007 Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol 81 8933 8943
25. Izquierdo-UserosN
BlancoJ
ErkiziaI
Fernandez-FiguerasMT
BorrasFE
2007 Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol 81 7559 7570
26. HarmanAN
WilkinsonJ
ByeCR
BosnjakL
SternJL
2006 HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J Immunol 177 7103 7113
27. ShiGX
HarrisonK
HanSB
MoratzC
KehrlJH
2004 Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling. J Immunol 172 5175 5184
28. GlassCK
OgawaS
2006 Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6 44 55
29. CastrilloA
TontonozP
2004 Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol 20 455 480
30. JiangC
TingAT
SeedB
1998 PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391 82 86
31. RicoteM
LiAC
WillsonTM
KellyCJ
GlassCK
1998 The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391 79 82
32. JosephSB
CastrilloA
LaffitteBA
MangelsdorfDJ
TontonozP
2003 Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9 213 219
33. CastrilloA
JosephSB
VaidyaSA
HaberlandM
FogelmanAM
2003 Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell 12 805 816
34. OgawaS
LozachJ
BennerC
PascualG
TangiralaRK
2005 Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122 707 721
35. WalcherD
KummelA
KehrleB
BachH
GrubM
2006 LXR activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol 26 1022 1028
36. AppelS
MirakajV
BringmannA
WeckMM
GrunebachF
2005 PPAR-gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood 106 3888 3894
37. PirainoG
CookJA
O'ConnorM
HakePW
BurroughsTJ
2006 Synergistic effect of peroxisome proliferator activated receptor-gamma and liver X receptor-alpha in the regulation of inflammation in macrophages. Shock 26 146 153
38. GhislettiS
HuangW
OgawaS
PascualG
LinME
2007 Parallel SUMOylation-dependent pathways mediate gene - and signal-specific transrepression by LXRs and PPARgamma. Mol Cell 25 57 70
39. ChawlaA
BoisvertWA
LeeCH
LaffitteBA
BarakY
2001 A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 7 161 171
40. AkiyamaTE
SakaiS
LambertG
NicolCJ
MatsusueK
2002 Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol 22 2607 2619
41. VenkateswaranA
LaffitteBA
JosephSB
MakPA
WilpitzDC
2000 Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A 97 12097 12102
42. LiaoZ
CimakaskyLM
HamptonR
NguyenDH
HildrethJE
2001 Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res Hum Retroviruses 17 1009 1019
43. GuyaderM
KiyokawaE
AbramiL
TurelliP
TronoD
2002 Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J Virol 76 10356 10364
44. GrahamDR
ChertovaE
HilburnJM
ArthurLO
HildrethJE
2003 Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J Virol 77 8237 8248
45. LiaoZ
GrahamDR
HildrethJE
2003 Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res Hum Retroviruses 19 675 687
46. PopikW
AlceTM
2004 CD4 receptor localized to non-raft membrane microdomains supports HIV-1 entry. Identification of a novel raft localization marker in CD4. J Biol Chem 279 704 712
47. NguyenDH
HildrethJE
2000 Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol 74 3264 3272
48. OnoA
FreedEO
2001 Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A 98 13925 13930
49. ManesS
del RealG
LacalleRA
LucasP
Gomez-MoutonC
2000 Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep 1 190 196
50. CarterGC
BernstoneL
SanganiD
BeeJW
HarderT
2009 HIV entry in macrophages is dependent on intact lipid rafts. Virology 386 192 202
51. GummuluruS
RogelM
StamatatosL
EmermanM
2003 Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J Virol 77 12865 12874
52. GossetP
CharbonnierAS
DeleriveP
FontaineJ
StaelsB
2001 Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol 31 2857 2865
53. StrausDS
GlassCK
2007 Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28 551 558
54. SallustoF
SchaerliP
LoetscherP
SchanielC
LenigD
1998 Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 28 2760 2769
55. BaribaudF
DomsRW
PohlmannS
2002 The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin Ther Targets 6 423 431
56. WileyRD
GummuluruS
2006 Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A 103 738 743
57. CavroisM
NeidlemanJ
KreisbergJF
GreeneWC
2007 In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog 3 e4
58. TurvilleSG
ArthosJ
DonaldKM
LynchG
NaifH
2001 HIV gp120 receptors on human dendritic cells. Blood 98 2482 2488
59. BashirovaAA
GeijtenbeekTB
van DuijnhovenGC
van VlietSJ
EileringJB
2001 A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J Exp Med 193 671 678
60. PohlmannS
SoilleuxEJ
BaribaudF
LeslieGJ
MorrisLS
2001 DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci U S A 98 2670 2675
61. NguyenDG
HildrethJE
2003 Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur J Immunol 33 483 493
62. SabadoRL
BabcockE
KavanaghDG
TjomslandV
WalkerBD
2007 Pathways utilized by dendritic cells for binding, uptake, processing and presentation of antigens derived from HIV-1. Eur J Immunol 37 1752 1763
63. PatelM
YanagishitaM
RoderiquezG
Bou-HabibDC
OraveczT
1993 Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses 9 167 174
64. SaphireAC
BobardtMD
ZhangZ
DavidG
GallayPA
2001 Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol 75 9187 9200
65. de WitteL
BobardtM
ChatterjiU
DegeestG
DavidG
2007 Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci U S A 104 19464 19469
66. BhatS
SpitalnikSL
Gonzalez-ScaranoF
SilberbergDH
1991 Galactosyl ceramide or a derivative is an essential component of the neural receptor for human immunodeficiency virus type 1 envelope glycoprotein gp120. Proc Natl Acad Sci U S A 88 7131 7134
67. HammacheD
PieroniG
YahiN
DelezayO
KochN
1998 Specific interaction of HIV-1 and HIV-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside GM3. J Biol Chem 273 7967 7971
68. HugP
LinHM
KorteT
XiaoX
DimitrovDS
2000 Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J Virol 74 6377 6385
69. PuriA
HugP
JerniganK
BarchiJ
KimHY
1998 The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc Natl Acad Sci U S A 95 14435 14440
70. NehetePN
VelaEM
HossainMM
SarkarAK
YahiN
2002 A post-CD4-binding step involving interaction of the V3 region of viral gp120 with host cell surface glycosphingolipids is common to entry and infection by diverse HIV-1 strains. Antiviral Res 56 233 251
71. PuigdomenechI
MassanellaM
Izquierdo-UserosN
Ruiz-HernandezR
CurriuM
2008 HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology 5 32
72. HatchSC
ArcherJ
GummuluruS
2009 Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV-1) particles is a crucial determinant for dendritic cell-mediated HIV-1 trans-infection. J Virol 83 3496 3506
73. Izquierdo-UserosN
Naranjo-GomezM
ArcherJ
HatchSC
ErkiziaI
2009 Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 113 2732 2741
74. BavariS
BosioCM
WiegandE
RuthelG
WillAB
2002 Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J Exp Med 195 593 602
75. YonezawaA
CavroisM
GreeneWC
2005 Studies of ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol 79 918 926
76. PopikW
AlceTM
AuWC
2002 Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J Virol 76 4709 4722
77. ChinettiG
LestavelS
BocherV
RemaleyAT
NeveB
2001 PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 7 53 58
78. WasserheitJN
1992 Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 19 61 77
79. RoyceRA
SenaA
CatesWJr
CohenMS
1997 Sexual transmission of HIV. N Engl J Med 336 1072 1078
80. FlemingDT
WasserheitJN
1999 From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 75 3 17
81. HesterRA
KennedySB
2003 Candida infection as a risk factor for HIV transmission. J Womens Health (Larchmt) 12 487 494
82. CelumC
LevineR
WeaverM
WaldA
2004 Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ 82 447 453
83. GalvinSR
CohenMS
2004 The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2 33 42
84. HaaseAT
2005 Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol 5 783 792
85. WuL
2008 Biology of HIV Mucosal Transmission. Curr Opin HIV AIDS 3 534 540
86. BouschbacherM
BomselM
VerroneseE
GoffloS
GanorY
2008 Early events in HIV transmission through a human reconstructed vaginal mucosa. Aids 22 1257 1266
87. HladikF
McElrathMJ
2008 Setting the stage: host invasion by HIV. Nat Rev Immunol 8 447 457
88. LoreK
SonnerborgA
OlssonJ
PattersonBK
FehnigerTE
1999 HIV-1 exposed dendritic cells show increased pro-inflammatory cytokine production but reduced IL-1ra following lipopolysaccharide stimulation. Aids 13 2013 2021
89. EquilsO
SchitoML
KarahashiH
MadakZ
YaraliA
2003 Toll-like receptor 2 (TLR2) and TLR9 signaling results in HIV-long terminal repeat trans-activation and HIV replication in HIV-1 transgenic mouse spleen cells: implications of simultaneous activation of TLRs on HIV replication. J Immunol 170 5159 5164
90. ZhangJ
LiG
BaficaA
PantelicM
ZhangP
2005 Neisseria gonorrhoeae enhances infection of dendritic cells by HIV type 1. J Immunol 174 7995 8002
91. de JongMA
de WitteL
OudhoffMJ
GringhuisSI
GallayP
2008 TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. J Clin Invest 118 3440 3452
92. ThibaultS
FromentinR
TardifMR
TremblayMJ
2009 TLR2 and TLR4 triggering exerts contrasting effects with regard to HIV-1 infection of human dendritic cells and subsequent virus transfer to CD4+ T cells. Retrovirology 6 42
93. TurvilleSG
SantosJJ
FrankI
CameronPU
WilkinsonJ
2004 Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103 2170 2179
94. GarciaE
PionM
Pelchen-MatthewsA
CollinsonL
ArrighiJF
2005 HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6 488 501
95. YuHJ
ReuterMA
McDonaldD
2008 HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog 4 e1000134
96. ChunTW
EngelD
MizellSB
EhlerLA
FauciAS
1998 Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med 188 83 91
97. McGowanI
ElliottJ
FuerstM
TaingP
BoscardinJ
2004 Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr 37 1228 1236
98. NarimatsuR
WoldayD
PattersonBK
2005 IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res Hum Retroviruses 21 228 233
99. CarrenoMP
KrieffC
IrinopoulouT
KazatchkineMD
BelecL
2002 Enhanced transcytosis of R5-tropic human immunodeficiency virus across tight monolayer of polarized human endometrial cells under pro-inflammatory conditions. Cytokine 20 289 294
100. LaneBR
LoreK
BockPJ
AnderssonJ
CoffeyMJ
2001 Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J Virol 75 8195 8202
101. del RealG
Jimenez-BarandaS
MiraE
LacalleRA
LucasP
2004 Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med 200 541 547
102. LarredeS
QuinnCM
JessupW
FrisdalE
OlivierM
2009 Stimulation of Cholesterol Efflux by LXR Agonists in Cholesterol-Loaded Human Macrophages Is ABCA1-Dependent but ABCG1-Independent. Arterioscler Thromb Vasc Biol
103. GrootF
WelschS
SattentauQJ
2008 Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111 4660 4663
104. GoussetK
AblanSD
CorenLV
OnoA
SoheilianF
2008 Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog 4 e1000015
105. JollyC
SattentauQJ
2005 Human immunodeficiency virus type 1 virological synapse formation in T cells requires lipid raft integrity. J Virol 79 12088 12094
106. McDonaldD
WuL
BohksSM
KewalRamaniVN
UnutmazD
2003 Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300 1295 1297
107. RudnickaD
FeldmannJ
PorrotF
WietgrefeS
GuadagniniS
2009 Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol 83 6234 6246
108. ChenP
HubnerW
SpinelliMA
ChenBK
2007 Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol 81 12582 12595
109. MorisA
PajotA
BlanchetF
Guivel-BenhassineF
SalcedoM
2006 Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 108 1643 1651
110. Granelli-PipernoA
ShimeliovichI
PackM
TrumpfhellerC
SteinmanRM
2006 HIV-1 selectively infects a subset of nonmaturing BDCA1-positive dendritic cells in human blood. J Immunol 176 991 998
111. BlauveltA
AsadaH
SavilleMW
Klaus-KovtunV
AltmanDJ
1997 Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J Clin Invest 100 2043 2053
112. Granelli-PipernoA
DelgadoE
FinkelV
PaxtonW
SteinmanRM
1998 Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M - and T-tropic virus to T cells. J Virol 72 2733 2737
113. SkolnikPR
RabbiMF
MathysJM
GreenbergAS
2002 Stimulation of peroxisome proliferator-activated receptors alpha and gamma blocks HIV-1 replication and TNFalpha production in acutely infected primary blood cells, chronically infected U1 cells, and alveolar macrophages from HIV-infected subjects. J Acquir Immune Defic Syndr 31 1 10
114. HayesMM
LaneBR
KingSR
MarkovitzDM
CoffeyMJ
2002 Peroxisome proliferator-activated receptor gamma agonists inhibit HIV-1 replication in macrophages by transcriptional and post-transcriptional effects. J Biol Chem 277 16913 16919
115. PotulaR
RamirezSH
KnipeB
LeibhartJ
SchallK
2008 Peroxisome proliferator-activated receptor-gamma activation suppresses HIV-1 replication in an animal model of encephalitis. Aids 22 1539 1549
116. NguyenDH
TaubD
2002 CXCR4 function requires membrane cholesterol: implications for HIV infection. J Immunol 168 4121 4126
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



