-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
Bats are reservoirs for a wide range of zoonotic agents including lyssa-, henipah-, SARS-like corona-, Marburg-, Ebola-, and astroviruses. In an effort to survey for the presence of other infectious agents, known and unknown, we screened sera from 16 Pteropus giganteus bats from Faridpur, Bangladesh, using high-throughput pyrosequencing. Sequence analyses indicated the presence of a previously undescribed virus that has approximately 50% identity at the amino acid level to GB virus A and C (GBV-A and -C). Viral nucleic acid was present in 5 of 98 sera (5%) from a single colony of free-ranging bats. Infection was not associated with evidence of hepatitis or hepatic dysfunction. Phylogenetic analysis indicates that this first GBV-like flavivirus reported in bats constitutes a distinct species within the Flaviviridae family and is ancestral to the GBV-A and -C virus clades.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000972
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000972Summary
Bats are reservoirs for a wide range of zoonotic agents including lyssa-, henipah-, SARS-like corona-, Marburg-, Ebola-, and astroviruses. In an effort to survey for the presence of other infectious agents, known and unknown, we screened sera from 16 Pteropus giganteus bats from Faridpur, Bangladesh, using high-throughput pyrosequencing. Sequence analyses indicated the presence of a previously undescribed virus that has approximately 50% identity at the amino acid level to GB virus A and C (GBV-A and -C). Viral nucleic acid was present in 5 of 98 sera (5%) from a single colony of free-ranging bats. Infection was not associated with evidence of hepatitis or hepatic dysfunction. Phylogenetic analysis indicates that this first GBV-like flavivirus reported in bats constitutes a distinct species within the Flaviviridae family and is ancestral to the GBV-A and -C virus clades.
Introduction
Bats (order Chiroptera), after rodents, comprise the most diverse group of mammals with more than 1,100 species. They are present on six continents, often have substantial habitat overlap with humans [1] and harbor several zoonotic viruses causing significant human morbidity and mortality, including Ebola - and Marburgvirus, Nipah virus (NiV), and SARS-like coronaviruses [2]–[5]. Proximity of bats to human populations may facilitate the zoonotic transmission of viruses either through direct contact, via amplifying domestic animal hosts, or through food-borne routes [6]–[8].
The current study was set up as part of a viral discovery effort to target key wildlife reservoirs in emerging disease hotspots. Bangladesh is a ‘hotspot’ for emerging zoonotic diseases [9], with a relatively high diversity of wildlife that likely harbors new zoonotic pathogens, one of the densest human populations on the planet, and a high level of connectivity between people, domestic animals and wildlife. In Bangladesh and India, frugivorous Pteropus giganteus bats have been identified as a reservoir for NiV [10], [11], which has been recognized as the cause of several outbreaks of encephalitis [12]–[14]. Pteropus giganteus bats are common throughout the Indian subcontinent, living in close association with humans and feeding on cultivated fruit [14]. NiV transmission from bats to humans has been linked with the harvest and consumption of raw date palm sap, which becomes contaminated with bat feces, urine or saliva overnight when bats such as P. giganteus come to feed from the collecting pots [14], [15]. Date palm sap or other foods eaten by both bats and people, may also serve as a vehicle for transmission of other bat-borne agents.
Several zoonotic flaviviruses, including Japanese encephalitis virus, West Nile virus, and Kyasanur forest virus have been identified in bats; however, to date, GB viruses have not [1]. GB viruses A and C (GBV-A and -C) represent two recently identified species that are currently unassigned members of the family Flaviviridae [16]. GBV-A viruses have been described in New World primates and are not known to infect humans [17]–[19], while GBV-C (also known as Hepatitis G virus (HGV)) have frequently been isolated from humans in many regions of the World, including India and Bangladesh [19]–[23], and from wild chimpanzees (Pan troglodytes) in Africa [24], [25]. Here we describe discovery of a virus in the serum of healthy bats in Bangladesh, tentatively named GB virus D (GBV-D), that is distantly related to GBV-A and -C and represents a new member of the family Flaviviridae.
Materials and Methods
Ethics statement
Every effort was made to minimize bat stress and avoid injury during capture, restraint, and sampling procedures. This study was conducted following Wildlife Trust institutional guidelines under IACUC approval G2907 issued by Tufts New England Medical Center, Boston, Massachusetts.
Bat sample collection
As part of a longitudinal surveillance study of Nipah virus in bats, 98 free-ranging P. giganteus bats were caught from a colony of approximately 1800 individuals in the Faridpur district of Bangladesh in December 2007 (Figure 1). Each bat was anesthetized using isoflurane gas; morphometric measurements (weight, forearm length, head length, and body condition) were taken and bats were aged [10]. Each bat was marked for future identification using an RFID microchip (AVID corp, www.avidid.com) implanted subcutaneously between the scapulae. Three mL of blood were collected and placed into serum separator tubes (vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA). Serum was allowed to separate overnight at 4°C then drawn off without centrifugation and immediately frozen using a liquid nitrogen dry shipper. To inactivate potentially infectious agents, serum samples were heat-treated at 56°C for 30 min and then stored at −70°C. For RNA extraction, 250 µL of serum was added to 750 µL Tri-Reagent LS (Molecular Research Center, Cincinnati, OH, USA). Saliva was collected from the bat's throat using a sterile cotton swab. Urine was collected either by catching urine in a 1.0 mL sterile cryovial while the bat was urinating, or by urethral swab. Urine and saliva swabs were immediately placed into 1 mL Tri-Reagent LS and frozen in liquid nitrogen.
Fig. 1. Map showing the location of the bat colony in Faridpur district, Bangladesh from which GBV-D was identified. 
Unbiased high-throughput pyrosequencing (UHTS)
Total RNA from serum was extracted for UHTS analysis to screen for the presence of microorganisms. Five microliters of total RNA from each bat were combined into 4 pools: 4 pregnant bats; 4 non-pregnant female bats, and 2 pools of 4 adult male bats, respectively. Reverse transcription (RT) was performed on DNase I-treated (DNA-free, Ambion Inc., Austin, TX, USA) RNA pools to generate cDNA using Superscript II RT (Invitrogen, Carlsbad, CA, USA) and random octamers linked to a defined arbitrary, 17-mer primer sequence tail (MWG, Huntsville, AL, USA) [26]. After RNase H treatment cDNA was amplified by the polymerase chain reaction (PCR), applying a 9∶1 mixture of the defined 17-mer primer sequence and the random octamer-linked 17-mer primer sequence, respectively [27]. Products of >70 base pairs (bp) were selected by column purification (MinElute, Qiagen, Hilden, Germany) and ligated to specific linkers for sequencing on the 454 Genome Sequencer FLX (454 Life Sciences, Branford, CT, USA) without DNA fragmentation [28], [29]. Sequences were analyzed using software applications implemented at the GreenePortal website (http://tako.cpmc.columbia.edu/Tools/).
Genome sequencing
Multiple forward and reverse primers for RT-PCR (available upon request) were designed using the sequences obtained by UHTS in order to fill gaps between fragments. Amplifications were performed with Bio-X-act (Bioline, London, UK) according to manufacturer's protocols. Products were size fractionated by electrophoresis and directly sequenced in both directions with ABI PRISM Big Dye Terminator 1.1 Cycle Sequencing kits (Perkin-Elmer Applied Biosystems, Foster City, CA, USA) at a commercial facility (Genewiz, South Plainfield, NJ, USA). Additional methods applied to obtain the genome sequence included touch-down PCR [30], 2-step walking PCR [31], and 3′ - and 5′ - RACE (Invitrogen).
Quantitative real-time PCR
A real time Taqman PCR assay was developed to screen bat samples for GBV-D. Reactions were performed in a 25 µL volume by using commercial Taqman Universal Master Mix (Applied Biosystems, Foster City, CA, USA). Primers and probe were designed to target a 60 nt region in the NS4A gene region: Fadi-forward, 5′ - gCAgCTgCgTgTgCCA; Fadi-reverse, 5′ - ACACCCATgATgTTACCACgAC; Fadi-probe, 5′ - FAM - AggACCCggTCgCTCCAgCA-T-BQX (TIB Molbiol, Adelphia, NJ, USA). Cycling conditions were: 50°C for 2 min, and 95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and 60°C for 1 min. Thermal cycling was performed in an ABI 7300 real-time PCR system (Applied Biosystems).
Serum chemistry
A liver function panel was conducted at the International Center for Diarrheal Disease Research (Dhaka, Bangaldesh) using non heat-treated bat sera (Automated Chemistry Analyzer AU 640, Olympus Corporation, Tokyo, Japan). The following parameters were analyzed: total protein, albumin, globulin, albumin∶globulin ratio, total cholesterol, total bilirubin , alkaline phosphatase, alanine transferase, aspartate aminotransferase, gamma glutamyltransferase , and lactate dehydrogenase.
Phylogenetic and sequence analyses
Sequence alignments were generated with ClustalW software [32] and phylogenetic relationships deduced using Geneious software [33]. Statistical significance was assessed by bootstrap re-sampling of 1000 pseudoreplicate data sets. Sequence relations were determined from p-distance matrices calculated with pairwise deletion for missing data and homogeneous patterns among lineages based on ClustalW alignments as implemented in MEGA software [34]. Sliding window similarity analysis was performed using SimPlot [35]. Potential signalase cleavage sites, glycosylation sites, and phosphorylation sites were analyzed using the respective prediction servers available at the Center for Biological Sequence Analysis (http://www.cbs.dtu.dk/services/).
Results
Identification of a GB-like agent from bats
Total RNA from the serum of healthy bats captured at a roost in the Faridpur district of Bangladesh was extracted for UHTS analysis. Extracts of 16 individual bats were combined into 4 pools consisting of 4 pregnant adult bats, 4 non-pregnant adult female bats, or 2×4 adult male bats. Each pool yielded between 1,400 and 2,000 assembled contigs or singlton reads (representing 50,000–75,000 reads ranging in size from 31–328 nt). Two reads of 238 and 215 nucleotides (nt) derived from the pregnant bat pool had distant homology to GBV-A sequences at the deduced amino acid (aa) level in the E2 and NS4A gene regions respectively (BLASTX); no homology was detected by searches at the nt level (BLASTN; local copy of the executables with standard settings except that the reward for a nucleotide match was set to 2 instead of 1). No viral sequences were detected in other pools at the nt or aa levels. Screening of the individual RNA preparations from the pregnant bat pool using primers derived from the UHTS reads confirmed the presence of the GBV-like sequence in the serum of bat 93. A quantitative real time PCR assay indicated a load of approximately 30 000 RNA copies in bat-93 serum extract, and identified an additional 4 positive bat sera from the original 98 samples (5/98; 5%), indicating serum loads ranging from 350 to 70,000 RNA copies per assay. These positive samples came from male bats that were not included in the initial UHTS pools. Extracts of saliva from the five positive bats indicated a load of approximately 200 RNA copies in bat 93; no signal was obtained with urine extracts from the five positive bats.
Genomic characterization of GBV-D
Near full-length genome sequence was generated from bat-93 and a second positive serum (bat 68), applying primers crossing gaps between UHTS reads as well as touch-down PCR [30], 2-step walking PCR [31], and 3′ - and 5′-RACE (Invitrogen) protocols. The two genome sequences were 96% identical at the nt level (GenBank Accession nos. GU566734 and GU566735), indicating two strains of the same virus. Comparison of deduced polyprotein sequence to other GBV and hepaciviruses indicated highest nt and aa sequence identities to GBV-A and -C (Table 1, Figure 2). The genomic sequence of the GBV-like virus identified in P. giganteus bats, tentatively named GBV-D, comprises 9,633 nt with 52 nt of potentially 5′-untranslated region (UTR), one continuous open reading frame (ORF) of 9318 nt (3106 aa) and 265 nt of 3′-UTR (Figure 3).
Fig. 2. Sliding window similarity analysis between GBV-D and other GBV and hepaciviruses (amino acid sequence; window, 160; step, 20). 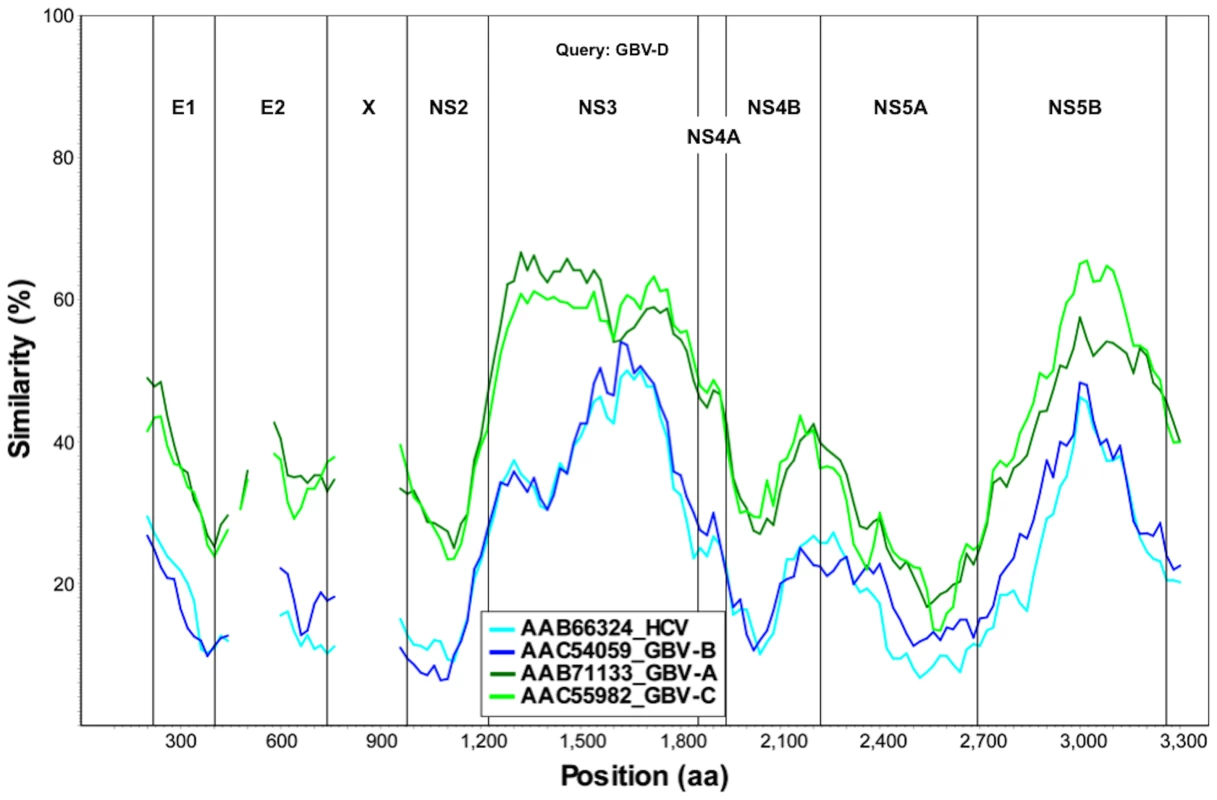
Fig. 3. Genomic organization of GBV-D, a novel flavivirus identified in the sera of frugivorous bats in Bangladesh. 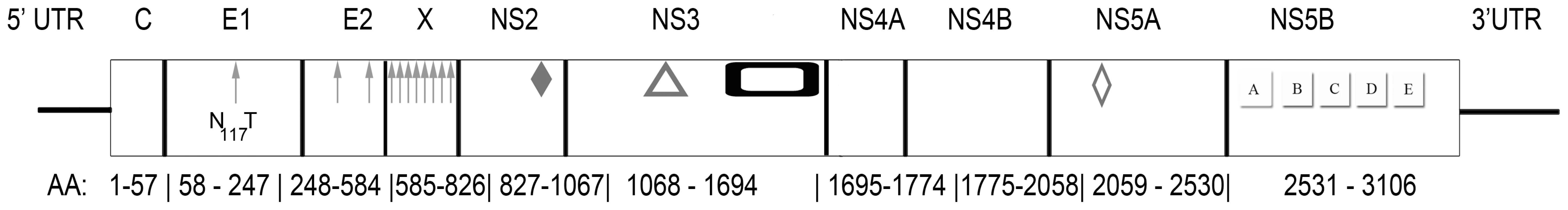
Arrows, glycosylation sites; solid diamond, active center sites H921, E1011, and C1032 in the autocatalytic NS2/NS3 endoprotease domain; triangle, catalytic triad H1123, D1147, S1204 of NS3 serine protease; rectangle, NS3 helicase and DEAD-like helicase motifs; open diamond, zinc finger motif; and NS5 polymarase motifs A (T2744VDAICFDSCIT), B (R2802ASGVLTTSSSNCISSFLKVSAAC), C (F2835LIHGDDVMII), D (L2876DTAQSCSA),and E (H2900YFLSTDFR) motifs. Tab. 1. Percent sequence similarity between GBV-D (bat-68), -A, -C, and hepaciviruses. 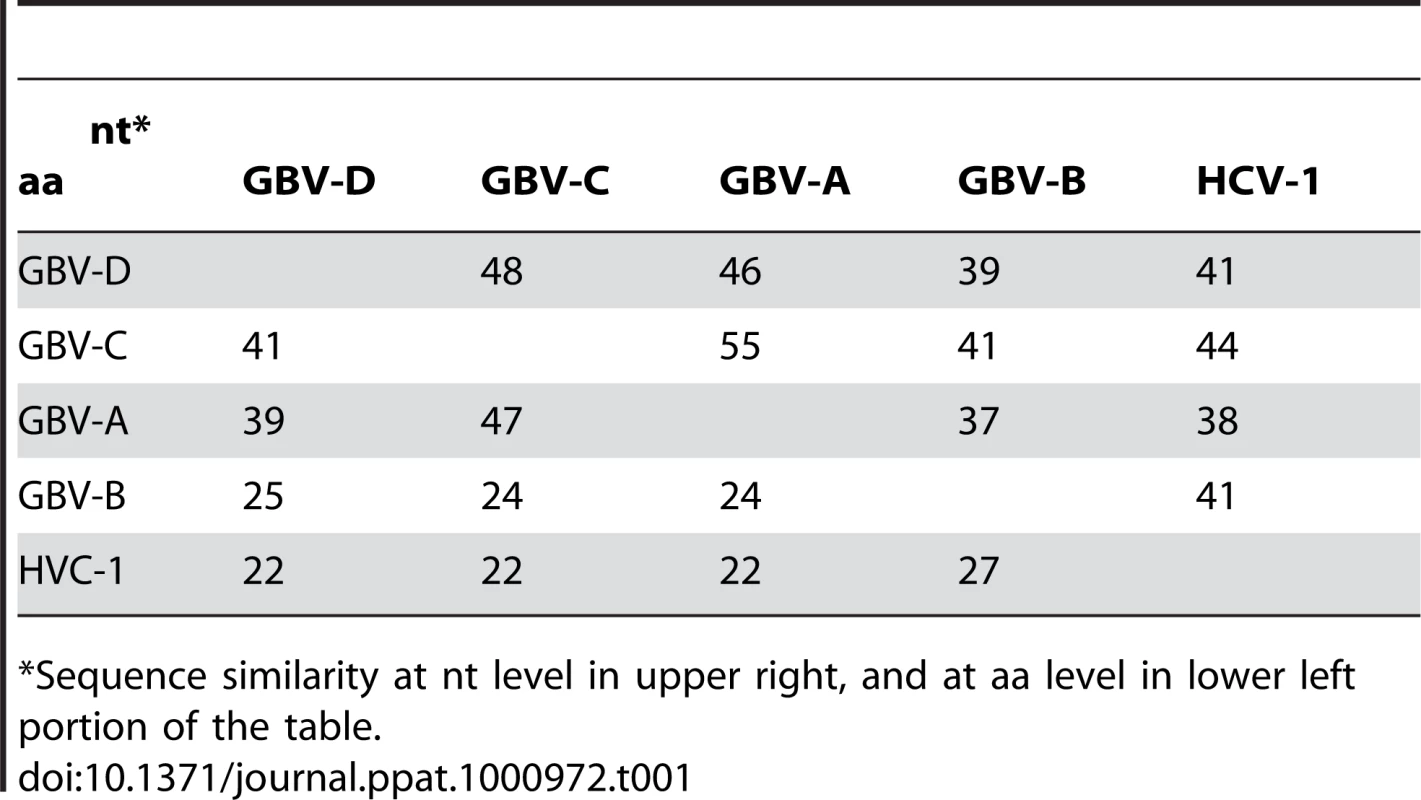
*Sequence similarity at nt level in upper right, and at aa level in lower left portion of the table. Mature structural proteins in GB viruses, as well as other flaviviruses, are the product of cleavage by host signal peptidase [36]. In GBV-D the first potential signal sequence cleavage site is present after a stretch of 57, largely basic aa (6 kDa, pI = 12), followed by sequence homologous to E1 (pfam 01539, http://pfam.sanger.ac.uk/) (Figure 3). The single glycosylation site N177IT present in that sequence is located in a position comparable to GBV-C, -A, -B and HCV glycosylation sites. Identification of the downstream E2 termini is less apparent as the next 580 aa contain multiple potential signal sequences and 10 potential glycosylation sites that indicate no homology to hepaciviral E2/NS1 (pfam 01560), until the sequence aligns with N-terminal NS2 motifs (pfam 01538) (Figure 2, Figure 3). However, despite similarity to pfam 01538 no signal sequence compatible with cleavage at A759/A was found; cleavage may occur at G826/R, which combined with potential signalase cleavage at A584/F may indicate the existence of a heavily glycosylated potential 26 kDa product instead of the p7 trans-membrane protein identified in HCV [37]–[39] or the 13 kDa variant described in GBV-B [40], [41]. Conserved C-terminal motifs of the autocatalytic NS2/NS3 endoprotease domain are compatible with NS2/NS3 cleavage at S1067/A and comparable to other GBV and HCV [42]. Figure 3 indicates potential cleavage sites for NS3 (peptidase S29, pfam 02907; DEAD box helicase, pfam 07652; helicase C, pfam 00271), NS4A (pfam 01006), NS4B (pfam 01001), NS5A (domain-1a zinc finger, pfam 08300; domain-1b, pfam 08301), and NS5B (pfam 00998).
Conserved aa motifs were recognized in NS proteins. RNA-dependent RNA polymerase (RdRp) motifs in RdRp block III that are conserved with respect to other GBV and hepaciviruses were identified in NS5B (Figure 3) [43]–[46]. Potential phosphorylation sites are present at multiple serine (9), threonine (14) and tyrosine (4) residues in NS5A, compatible with its possible function as a phosphorylation-regulated mediator of viral replication [47]. However, significant conservation of primary sequence is not obvious for phosphorylation sites, proline-rich, or interferon-sensitivity determining region motifs [48]–[50]. The C-terminal portion of NS3 has homology to conserved NTPase/helicase motifs [51]; the N-terminal portion includes conserved active triad residues H1123, D1147, S1204 of serine protease [52], the viral protease responsible for cleavage of mature non-structural proteins [53]. Likewise, the active triad H991, E1011, C1032 of the cis-acting protease activity in the C-terminal portion of NS2 is conserved with respect to other GBV and HCV [42]. The only other discernable motif identified was a well-conserved N75 C/D C motif at the N-terminus of E1 (Figure 3) [54].
Phylogenetic analysis
Phylogenetic analysis of GBV-D was performed in comparison to selected representatives of GBV-A, GBV-B, GBV-C and HCV. Analysis of NS5B aa sequence (Figure 4A) confirmed a closer relationship of GBV-D to GBV-A and -C than to GBV-B or HCV as also indicated by pairwise sequence comparisons (Table 1). The same relationships were also apparent when NS3, or the complete polyprotein sequence were analyzed (Figure 4B and C, respectively). All three trees show GBV-D consistently at the root of the GBV-A/-C viruses, indicating an independent phylogenetic clade compatible with a separate species distinct from the recently created genus Hepacivirus [16].
Fig. 4. Phylogenetic relationship of GBV-D to other GBV and hepaciviruses. 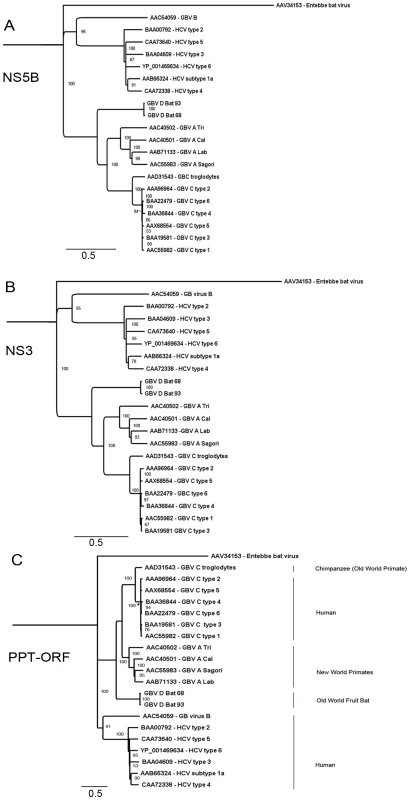
GBV-D amino acid sequences for A: NS5B, B: NS3, and C: the polyprotein (PPT) were analyzed in comparison to representative sequences of GBV-A, -B, -C and hepatitis C viruses. GenBank accession numbers for the respective sequences are indicated. Entebbe bat virus was used as an outgroup; distance in substitutions per site is indicated by scale bars; percent bootstrap support for values greater than 85% is indicated at respective nodes. Serum chemistries
A liver serum chemistry panel was conducted on sera from 15 bats, the five GBV-D infected and 10 non-infected animals. Standard assays to detect hepatitis and/or impaired liver function were performed [55]. Levels of total protein, alanine transferase, aspartate aminotransferase and total cholesterol were within published ranges reported for P. giganteus, except for bat 33 (infected) and bat 73 (uninfected), which had modest elevation in aspartate aminotransferase. Reference values for albumin, globulin, albumin∶globulin ratio, total bilirubin, alkaline phosphatase, gamma glutamyltransferase and lactate dehydrogenase are not available for P. giganteus, however, values were comparable to those reported for other Pteropus species [56]. Mean values did not significantly differ between infected and uninfected bats (Table 2).
Tab. 2. Liver function values from Pteropus giganteus bats. 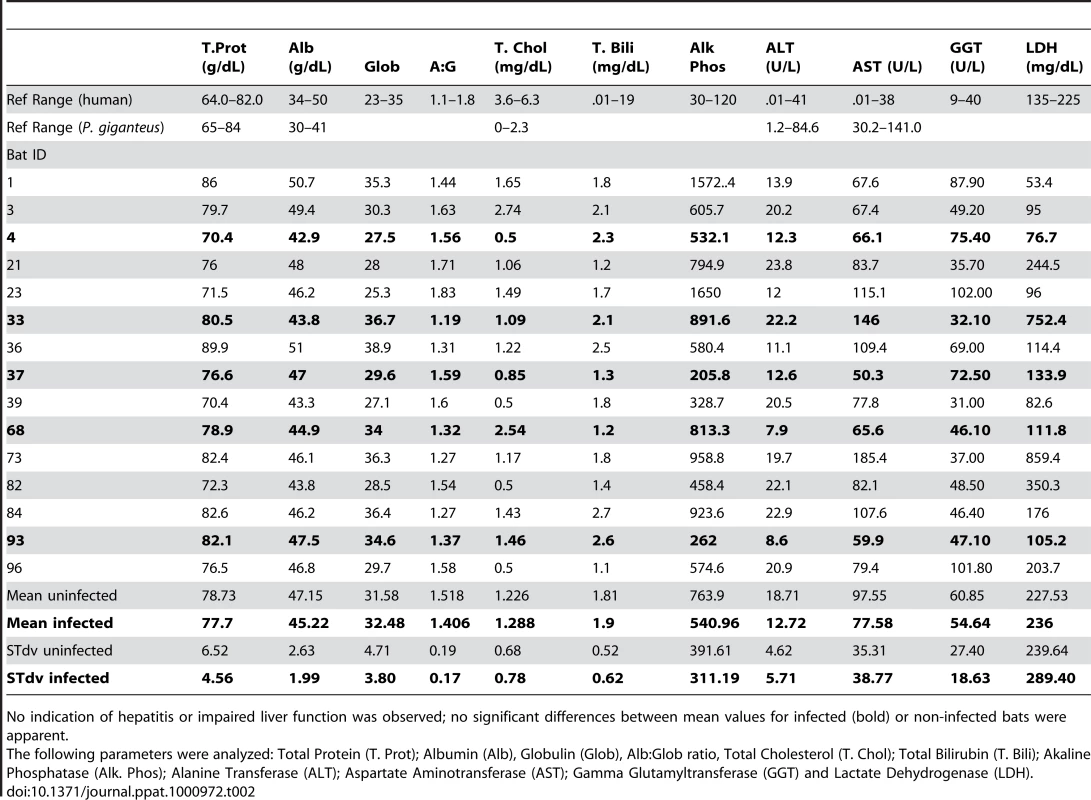
No indication of hepatitis or impaired liver function was observed; no significant differences between mean values for infected (bold) or non-infected bats were apparent. Discussion
Molecular analyses of sera from Pteropus giganteus bats from Faridpur, Bangladesh led to the identification of a 9,633 nt sequence consistent in genomic organization with known GBV and other species within the family Flaviviridae [16]. Whereas previous studies of bats have employed assays that test for known pathogens, ours is the first report of an unbiased molecular approach to pathogen discovery in this important reservoir of emerging infectious diseases. The modest yield of novel microbial sequences may reflect the choice of sample (e.g., serum vs feces, tissue or another specimen), competition between host and microbial template during unbiased amplification, or both. Efforts to address template competition are under way that include subtraction of host nucleic acids or the use of semi-random primers that do not amplify host sequences. Such efforts will likely enhance the sensitivity and throughput of unbiased sequencing technologies for pathogen discovery.
The discovery of this chiropteran flavivirus broadens both the taxonomical and geographical distribution of GB-like viruses. Three types of GB viruses have been described: GBV-A, -B and -C [18], [19], [24], [25], [54], [57]. GBV-B, which has never been found in humans and was only reported in captive tamarins after serial passage of the original human GB serum [58], is most closely related to HCV and was recently classified together with HCV into a new genus, Hepacivirus, within the family Flaviviridae [16]. GBV-A and -C remain unclassified members of the family. GBV-A have been isolated from several New World monkeys. Different genotypes appear to be associated with specific monkey species of the genera Saguinus, Callithrix (Callitrichidae family) and Aotus (Aotidae family), without any clinical signs associated with infection [24], [54], [57]. GBV-C have been isolated from humans with non-A-E hepatitis; however, its pathogenicity is unknown and the virus is widespread in the human population [21], [59]–[61]. Population studies showed that GB viruses are enzoonotic and species-specific within both Old and New World nonhuman primates as well as humans, and have likely co-evolved with their hosts over long periods of time [62]. Previously, the only GBV found in the Old world was GBV-C from chimpanzees (in Africa) and humans. Although GBV-C were found in humans, GB viruses have not been previously reported in primates or other animals on the Indian subcontinent.
GBV-C and -A are remarkable for a truncated or missing capsid (C) protein [18], [19]. Due to exhaustion of our samples we were unable to complete assessment of the 5′-terminal sequence; nonetheless, RACE experiments suggest that GBV-D likely codes for a short basic peptide, instead of a full-length C protein. The first methionine (M1) predicts a peptide of 57 aa (pI = 12); however, the more favorable Kozak context [63] of M3 indicates a 55 aa peptide. After signalase cleavage from the polyprotein precursor, this peptide may be functional, possibly influencing maturation of, or directly binding to, the E1 and/or E2 glycoproteins.
Phylogenetic analyses of NS5B, NS3 and complete polyprotein sequence place GBV-D at the root of the GBV-A and -C clades and are consistent with a model wherein GBV-D is ancestral to GBV-A and -C clades. Mixed relationships indicative of recombination events [64] were not evident (Figure 2, Figure 4). Both pteropid bats and chimpanzees are restricted to the Old World. While the range of chimpanzees (Africa) and P. giganteus (the Indian subcontinent) do not overlap, it is possible that other primate species in Bangladesh or India, such as macaques, or other fruit bats in Africa such as Eidelon spp., whose range overlaps that of chimpanzees, may carry related viruses. While GBV-A is only known from primates of the New World, an African origin has been suggested for GBV-C based on a 12-aa indel sequence in NS5A [65]. Although the NS5A sequence of GBV-D, similar to that of GBV-A, appears elongated in the indel region, compatible with their respective earlier phylogenetic branching compared to GBV-C, little sequence conservation is observed in that region.
The bats in this study, like primates infected with their associated GBV [66], all appeared to be healthy. The lack of chemical evidence of hepatic inflammation or dysfunction suggests that this virus may not target hepatic cells in bats. This is consistent with the behavior of GBV-A in its natural primate hosts [54]. In contrast, elevated alanine transferase levels and mild hepatitis are observed in experimental infections of macaques with GBV-C isolates from humans [67]. Five percent of the bats we studied were infected with one of at least two different strains of GBV-D, which suggests widespread viral circulation within this species. The observation that bats are asymptomatically infected with diverse strains that constitute a distinct phylogenetic clade is compatible with a co-evolutionary relationship between GBV and their hosts [57], [62], and supports the hypothesis that P. giganteus bats may be a natural reservoir for GBV-D. In one case we were able to detect GBV-D nucleic acid in saliva. This suggests a potential route for viral transmission via fighting or grooming behavior, or via food shared by bats.
Pteropus giganteus is a frugivorous bat species that carries NiV, a zoonotic paramyxovirus [10], [11]. This species lives in close association with humans in Bangladesh and bats have been observed drinking from (and urinating into) date palm sap collecting pots [14]. Human consumption of contaminated palm juice is proposed to be a major route of NiV transmission [68]. Although it is unclear whether infectious virus was present in bat saliva, the observation that saliva can contain GBV-D nucleic acids provides a biologically plausible mechanism for transmission from infected bats to other hosts. While it is currently unknown whether GBV-D virus occurs in humans, up to 20% of non-A-E hepatitis cases remain unexplained [19].
Zdroje
1. CalisherCH
ChildsJE
FieldHE
HolmesKV
SchountzT
2006 Bats: Important reservoir hosts of emerging viruses. Clin Microbiol Rev 19 531 538
2. TownerJS
PourrutX
AlbarinoCG
NkogueCN
BirdBH
2007 Marburg virus infection detected in a common African bat. PLoS ONE 2 e764, 761 765
3. EpsteinJH
FieldHE
LubyS
PulliamJRC
DaszakP
2006 Nipah virus: Impact, Origins, and Causes of Emergence. Curr Infect Dis Rep 8 59 65
4. LiW
ShiZ
YuM
RenW
SmithC
2005 Bats are natural reservoirs of SARS-like coronaviruses. Science 310 676 679
5. LeroyEM
KumulunguiB
PourrutX
RouquetP
HassaninA
2005 Fruit bats as reservoirs of Ebola virus. Nature 438 575 576
6. ChuaK
BelliniW
RotaP
HarcourtB
TaminA
2000 Nipah virus: A recently emergent deadly paramyxovirus. Science 288 1432 1435
7. SelveyL
WellsRM
McCormackJG
AnsfordAJ
MurrayPK
1995 Infection of humans and horses by a newly described morbillivirus. Med J Aust 162 642 645
8. McCollK
TordoN
Aguilar-SetienA
2000 Bat lyssavirus infections. Rev sci tech Off int Epiz 19 177 196
9. JonesKE
PatelNG
LevyMA
StoreygardA
BalkD
2008 Global trends in emerging infectious diseases. Nature 451 990 U994
10. EpsteinJH
PrakashV
SmithCS
DaszakP
McLaughlinAB
2008 Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerg Infect Dis 14 1309 1311
11. HsuVP
HossainMJ
ParasharUD
AliMM
KsiazekTG
2004 Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis 10 2082 2087
12. ChadhaMS
ComerJA
LoweL
RotaPA
RollinPE
2006 Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis 12 235 240
13. GurleyES
MontgomeryJM
HossainMJ
BellM
AzadAK
2007 Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis 13 1031 1037
14. LubySP
HossainMJ
GurleyES
AhmedBN
BanuS
2009 Recurrent Zoonotic Transmission of Nipah Virus into Humans, Bangladesh, 2001–2007. Emerg Infect Dis 15 1229 1235
15. LubyS
RahmanM
HossainMJ
BlumLS
HusainNM
2006 Foodborne Transmission of Nipah Virus, Bangladesh. Emerg Infect Dis 12
16. TheilHJ
2005 Family Flaviviridae.
FauquetClaudeM
MayoMA
ManiloffJ
DesselbergerU
BallLA
Virus Taxonomy: VIIIth Report of the International Committee on Taxonomy of Viruses. 2 ed San Diego Academic Press 979 996
17. ErkerJC
DesaiSM
LearyTP
ChalmersML
MontesCC
1998 Genomic analysis of two GB virus A variants isolated from captive monkeys. J Gen Virol 79 41 45
18. LearyTP
DesaiSM
ErkerJC
MushahwarIK
1997 The sequence and genomic organization of a GB virus A variant isolated from captive tamarins. J Gen Virol 78 2307 2313
19. LearyTP
MuerhoffAS
SimonsJN
PilotMatiasTJ
ErkerJC
1996 Sequence and genomic organization of GBV-C: A novel member of the flaviviridae associated with human non-A-E hepatitis. J Med Virol 48 60 67
20. LinnenJ
WagesJ
ZhangKeckZY
FryKE
KrawczynskiKZ
1996 Molecular cloning and disease association of hepatitis G virus: A transfusion-transmissible agent. Science 271 505 508
21. KaoJH
ChenPJ
ChenDS
1996 GBV-C in the aetiology of fulminant hepatitis. Lancet 347 120 120
22. KarP
MukhopadhyayS
GopalkrishnaV
DasBC
2000 Infection with hepatitis G-virus and viral hepatitis in India. Curr Sci 78 189 194
23. KondoY
MizokamiM
NakanoT
KatoT
OhbaK
1997 Genotype of GB virus C hepatitis G virus by molecular evolutionary analysis. Virus Res 52 221 230
24. AdamsNJ
PrescottLE
JarvisLM
LewisJCM
McClureMO
1998 Detection in chimpanzees of a novel flavivirus related to GB virus-C hepatitis G virus. J Gen Virol 79 1871 1877
25. BirkenmeyerLG
DesaiSM
MuerhoffAS
LearyTP
SimonsJN
1998 Isolation of a GB virus-related genome from a chimpanzee. J Med Virol 56 44 51
26. PalaciosG
QuanPL
JabadoOJ
ConlanS
HirschbergDL
2007 Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerg Infect Dis 13 73 81
27. QuanPL
PalaciosG
JabadoOJ
ConlanS
HirschbergDL
2007 Detection of respiratory viruses and subtype identification of influenza a viruses by GreeneChipResp oligonucleotide microarray. J Clin Microbiol 45 2359 2364
28. Cox-FosterDL
ConlanS
HolmesEC
PalaciosG
EvansJD
2007 A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318 283 287
29. MarguliesM
EgholmM
AltmanWE
AttiyaS
BaderJS
2005 Genome sequencing in microfabricated high-density picolitre reactors. Nature 437 376 380
30. KorbieDJ
MattickJS
2008 Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat Protoc 3 1452 1456
31. PilhoferM
BauerAP
SchrallhammerM
RichterL
LudwigW
2007 Characterization of bacterial operons consisting of two tubulins and a kinesin-like gene by the novel Two-Step Gene Walking method. Nucleic Acids Res 35 8
32. ChennaR
SugawaraH
KoikeT
LopezR
GibsonTJ
2003 Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31 3497 3500
33. DrummondAJAB
CheungM
HeledJ
KearseM
MoirR
Stones-HavasS
ThiererT
WilsonA
2009 Geneious v4.6, Available from http://www.geneious.com/
34. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol & Evol 24 1596 1599
35. LoleKS
BollingerRC
ParanjapeRS
GadkariD
KulkarniSS
1999 Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73 152 160
36. ChambersTJ
HahnCS
GallerR
RiceCM
1990 Flavivirus genome organization, expression, and replication. Ann Rev Microbiol 44 649 688
37. LinC
LindenbachBD
PragaiBM
McCourtDW
RiceCM
1994 Processing in the Hepatitis-C virus E2-N2 region - identification of P7 and 2 Distinct E2-specific products with different C-termini. J Virol 68 5063 5073
38. MizushimaH
HijikataM
AsabeSI
HirotaM
KimuraK
1994 2 Hepatitis-C virus glycoprotein E2 products with different C-termini. J Virol 68 6215 6222
39. GriffinS
ClarkeD
McCormickC
RowlandsD
HarrisM
2005 Signal peptide cleavage and internal targeting signals direct the hepatitis C virus p7 protein to distinct intracellular membranes. J Virol 79 15525 15536
40. GhibaudoD
CohenL
PeninF
MartinA
2004 Characterization of GB virus B polyprotein processing reveals the existence of a novel 13-kDa protein with partial homology to hepatitis C virus p7 protein. J Biol Chem 279 24965 24975
41. TakikawaS
EngleRE
EmersonSU
PurcellRH
St ClaireM
2006 Functional analyses of GB virus B p13 protein: Development of a recombinant GB virus B hepatitis virus with a p7 protein. PNAS 103 3345 3350
42. BelyaevAS
ChongS
NovikovA
KongpachithA
MasiarzFR
1998 Hepatitis G virus encodes protease activities which can effect processing of the virus putative nonstructural proteins. J Virol 72 868 872
43. FerronF
BussettaC
DutartreH
CanardB
2005 The modeled structure of the RNA dependent RNA polymerase of GBV-C Virus suggests a role for motif E in Flaviviridae RNA polymerases. Bmc Bioinformatics 6
44. KooninEV
1991 The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol 72 2197 2206
45. PochO
SauvagetI
DelarueM
TordoN
1989 Identification of 4 conserved motifs among the RNA-dependent polymerase encoding elements. Embo Journal 8 3867 3874
46. MullerR
PochO
DelarueM
BishopDHL
BouloyM
1994 Rift-Valley Fever virus L-segment - correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J Gen Virol 75 1345 1352
47. HuangY
StaschkeK
De FrancescoR
TanSL
2007 Phosphorylation of hepatitis C virus NS5A nonstructural protein: A new paradigm for phosphorylation-dependent viral RNA replication? Virology 364 1 9
48. NandaSK
HerionD
LiangTJ
2006 Src homology 3 domain of hepatitis C virus NS5A protein interacts with Bin1 and is important for apoptosis and infectivity.(vol 130, pg 794, 2006). Gastroenterology 131 687 687
49. TanSL
KatzeMG
2001 How hepatitis C virus counteracts the interferon response: The jury is still out on NS5A. Virology 284 1 12
50. TanSL
NakaoH
HeYP
VijaysriS
NeddermannP
1999 NS5A, a nonstructural protein of hepatitis C virus, binds growth factor receptor-bound protein 2 adaptor protein in a Src homology 3 domain/ligand-dependent manner and perturbs mitogenic signaling. PNAS 96 5533 5538
51. DumontS
ChengW
SerebrovV
BeranRK
TinocoI
2006 RNA translocation and unwinding mechanism of HCVNS3 helicase and its coordination by ATP. Nature 439 105 108
52. ScarselliE
UrbaniA
SbardellatiA
TomeiL
DeFrancescoR
1997 GB virus B and hepatitis C virus NS3 serine proteases share substrate specificity. J Virol 71 4985 4989
53. BartenschlagerR
1999 The NS3/4A proteinase of the hepatitis C virus: unravelling structure and function of an unusual enzyme and a prime target for antiviral therapy. J Viral Hepatitis 6 165 181
54. SchlauderGG
DawsonGJ
SimonsJN
PilotmatiasTJ
GutierrezRA
1995 Molecular and serologic analysis in the transmission of the GB hepatitis agents. J Med Virol 46 81 90
55. DufourDR
LottJA
NolteFS
GretchDR
KoffRS
2000 Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests; 2000. 2027 2049 Amer Assoc Clinical Chemistry
56. HeardDJ
De YoungJL
GoodyearB
EllisGA
1997 Comparative rectal bacterial flora of four species of flying fox (Pteropus sp.). J Zoo Wild Med 28 471 475
57. BukhJ
ApgarCL
1997 Five new or recently discovered (GBV-A) virus species are indigenous to New World monkeys and may constitute a separate genus of the Flaviviridae. Virology 229 429 436
58. DeinhardF
HolmesAW
CappsRB
PopperH
1967 Studies on transmission of human viral hepatitis to marmoset monkeys. 1. Transmission of disease serial passages and description of liver lesions. J Exp Med 125 673 &
59. AlterMJ
GallagherM
MorrisTT
MoyerLA
MeeksEL
1997 Acute non-A-E hepatitis in the United States and the role of hepatitis G virus infection. New Engl J Med 336 741 746
60. StapletonJT
2003 GB virus type C/hepatitis G virus. Seminars in Liver Disease 23 137 148
61. ShengWH
HungCC
WuRJ
WangJT
ChenPJ
2007 Clinical impact of GB virus C viremia on patients with HIV type 1 infection in the era of highly active antiretroviral therapy. Clin Infect Dis 44 584 590
62. CharrelRN
De MiccoP
de LamballerieX
1999 Phylogenetic analysis of GB viruses A and C: evidence for cospeciation between virus isolates and their primate hosts. J Gen Virol 80 2329 2335
63. KozakM
1999 Initiation of translation in prokaryotes and eukaryotes. Gene 234 187 208
64. WorobeyM
HolmesEC
2001 Homologous recombination in GB virus C/hepatitis G virus. Mol Biol & Evol 18 254 261
65. TanakaY
MizokamiM
OritoE
OhbaK
KatoT
1998 African origin of GB virus C hepatitis G virus. FEBS Lett 423 143 148
66. LearyTP
DesaiSM
YamaguchiJ
ChalmersML
SchaluderGG
1997 Species-specific variants of GB virus A in captive monkeys (vol 70, pg 9028, 1996). J Virol 71 8953 8953
67. ChengY
ZhangWZ
LiJ
LiBA
ZhaoJM
2000 Serological and histological findings in infection and transmission of GBV-C/HGV to macaques. J Med Virol 60 28 33
68. LubySP
GurleyES
HossainMJ
2009 Transmission of Human Infection with Nipah Virus. Clin Infect Dis 49 1743 1748
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



