-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIntegration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
Adeno-associated virus type 2 (AAV) is known to establish latency by preferential integration in human chromosome 19q13.42. The AAV non-structural protein Rep appears to target a site called AAVS1 by simultaneously binding to Rep-binding sites (RBS) present on the AAV genome and within AAVS1. In the absence of Rep, as is the case with AAV vectors, chromosomal integration is rare and random. For a genome-wide survey of wildtype AAV integration a linker-selection-mediated (LSM)-PCR strategy was designed to retrieve AAV-chromosomal junctions. DNA sequence determination revealed wildtype AAV integration sites scattered over the entire human genome. The bioinformatic analysis of these integration sites compared to those of rep-deficient AAV vectors revealed a highly significant overrepresentation of integration events near to consensus RBS. Integration hotspots included AAVS1 with 10% of total events. Novel hotspots near consensus RBS were identified on chromosome 5p13.3 denoted AAVS2 and on chromsome 3p24.3 denoted AAVS3. AAVS2 displayed seven independent junctions clustered within only 14 bp of a consensus RBS which proved to bind Rep in vitro similar to the RBS in AAVS3. Expression of Rep in the presence of rep-deficient AAV vectors shifted targeting preferences from random integration back to the neighbourhood of consensus RBS at hotspots and numerous additional sites in the human genome. In summary, targeted AAV integration is not as specific for AAVS1 as previously assumed. Rather, Rep targets AAV to integrate into open chromatin regions in the reach of various, consensus RBS homologues in the human genome.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000985
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000985Summary
Adeno-associated virus type 2 (AAV) is known to establish latency by preferential integration in human chromosome 19q13.42. The AAV non-structural protein Rep appears to target a site called AAVS1 by simultaneously binding to Rep-binding sites (RBS) present on the AAV genome and within AAVS1. In the absence of Rep, as is the case with AAV vectors, chromosomal integration is rare and random. For a genome-wide survey of wildtype AAV integration a linker-selection-mediated (LSM)-PCR strategy was designed to retrieve AAV-chromosomal junctions. DNA sequence determination revealed wildtype AAV integration sites scattered over the entire human genome. The bioinformatic analysis of these integration sites compared to those of rep-deficient AAV vectors revealed a highly significant overrepresentation of integration events near to consensus RBS. Integration hotspots included AAVS1 with 10% of total events. Novel hotspots near consensus RBS were identified on chromosome 5p13.3 denoted AAVS2 and on chromsome 3p24.3 denoted AAVS3. AAVS2 displayed seven independent junctions clustered within only 14 bp of a consensus RBS which proved to bind Rep in vitro similar to the RBS in AAVS3. Expression of Rep in the presence of rep-deficient AAV vectors shifted targeting preferences from random integration back to the neighbourhood of consensus RBS at hotspots and numerous additional sites in the human genome. In summary, targeted AAV integration is not as specific for AAVS1 as previously assumed. Rather, Rep targets AAV to integrate into open chromatin regions in the reach of various, consensus RBS homologues in the human genome.
Introduction
The family of adeno-associated virus (AAV) represents defective, helper-dependent viruses that need to establish latency to ensure persistence in their primate hosts [1]. Upon natural infections in humans AAV genomes were shown to persist mainly as episomes and integrated AAV genomes were rarely detected [2]. The molecular mechanisms leading to integration have only been characterized for AAV type 2 that prefers integration near a site on human chromosome 19q13.42, called AAVS1 [3]. The specificity of AAV integration is mediated by the large regulatory AAV proteins, Rep78/68 [4]. During productive AAV replication in the presence of either adeno - or herpesvirus as a helper virus, Rep78/68 is required for AAV gene expression and DNA replication. The AAV origins of DNA replication reside in the 145 bp inverted terminal repeats (ITRs) that flank the 4.7 kb single-stranded AAV genome. Rep78 and/or Rep68 are expressed from the AAV p5 promoter and were shown to bind to the Rep-binding site (RBS) within the AAV-ITRs [5]. Rep unwinds the DNA and introduces a single-strand nick at the adjacent terminal resolution site (trs) [6]. The AAV-ITRs also serve as cis elements for chromosomal integration [4]. A RBS homologue present in the AAV p5 promoter was shown to mediate AAV integration in the absence of the ITRs [7]. DNA sequences homologous to the RBS and a nearby trs element were also found in AAVS1 [8], [9] and, in vitro, ternary complex formation of Rep68 with the AAV-ITR and AAVS1 was shown [10]. A 33 bp sequence of AAVS1 spanning the RBS and the trs element was sufficient to mediate AAV integration in vivo [4], [11]. AAV integrated at variable distances from the RBS in AAVS1 and sequence rearrangements were frequently found at AAV-chromosome junctions [8], [9], [12], [13], [14], [15]. Quantitative real-time PCR analysis of AAVS1-specific AAV-2 integration within hours after AAV-2 infection and at increasing MOIs showed that 10 to 20% of infected cells displayed AAV integration within a 4 kb region of AAVS1 on chromosome 19q13.42 [16], [17]. In AAV-infected and subsequently selected cell clones up to 80% of AAVS1-specific integration had been described before [18].
Although AAV has not been associated with disease in humans, it is well established that AAV Rep78/68 induces DNA damage, cell cycle arrest [19] and apoptosis [20]. In addition, AAV Rep interferes with helper adenovirus - [21] herpes simplex virus replication [22]. AAV holds much promise as a vector for gene therapy. As a rule, recombinant AAV vectors persist as non-integrated, nuclear episomes. AAV vectors lack the integration promoting rep gene and therefore only occasionally integrate into the host cell genome. The preferred integration of wildtype AAV-2 in chromosome 19q13.42 is unique and is commonly viewed as a specifically evolved virus-encoded targeting mechanism. Multiple attempts were published that aim to exploit Rep-mediated targeting specificity for chromosome 19q13.42 for the specific integration of gene therapy vectors [23], [24], [25], [26], [27], [28]. Yet chromosome 19q13.42 is not the only target region. The presence of alternative integration sites has long been postulated and in silico analysis detected numerous consensus Rep-binding sites in the human genome. Many of these bound Rep in vitro [29] but their in vivo accessibility for AAV integration has not been explored so far. From an evolutionary standpoint the assumption that AAV latency is ensured by more than one target site or mechanism appeared reasonable.
This study was designed to close the knowledge gap between AAVS1-specific and assumedly non-AAVS1-specific wildtype AAV integration and to compare the identified genomic sites to those preferred upon AAV vector transduction. An open survey of chromosomal integration preferences for wildtype AAV-2 was conducted and complemented by the bioinformatic analysis of genomic motifs and patterns in the genomic regions surrounding the integration loci.
Results
General strategy of LSM-PCR
The genomic structure of latent AAV in infected cells is highly variable. Wildtype AAV-2 was shown to integrate into the host cell genome, as well as persist as extrachromosomal, nuclear episomes [2], [30]. In either case multicopy, concatemeric structures predominate and often lead to unpredictable rearrangements involving the 145 bp inverted terminal repeats (ITRs). Therefore the retrieval of AAV-chromosome junctions suffers from the inherent problem of inefficient PCR reads through the hairpin ITR into the adjacent chromosomal sequences. This leads to a predominance of rearranged AAV genomes lacking chromosomal junctions in previous PCR-based studies [31], [32], [33]. Furthermore, previously cloned junctions often displayed unknown intervening sequences of varying lengths between AAV and the identified chromosomal sequence [12], [15], [16], [27], [34], [35], [36]. Therefore, unambiguous assignment of the AAV-derived and chromosome-derived parts of junctions requires sufficient DNA sequence lengths.
Several methods to identify virus-chromosome junctions have been developed to study retrovirus integration, where generally a single proviral copy per chromosomal site is found [37], [38]. The ultimate structure of the integrated long terminal repeat (LTR) is generally predictable in a way that allows an integration-specific PCR design. Linear amplification mediated (LAM)-PCR was initially designed to retrieve rare retroviral vector integration sites from small, clinical sample sizes [38]. We established a LAM-PCR with AAV primers in the “D” element of the AAV-ITR, the innermost and sole ITR region without internal inverse repetitions (Figure 1A). Unfortunately, pure AAV sequences with rearranged ITRs predominated, AAV-chromosome junctions were rare and the chromosomal DNA part often too short for unambiguous assignment to a unique genomic site. We then tested ligation-mediated (LM)-PCR that had been employed for broad surveys of lentivirus (HIV) or γ-retrovirus (MLV) integrations [39], [40], [41]. LM-PCR relies on a first LTR-specific primer. A linker is ligated to the first PCR strand that typically ends at the chosen restriction site within the unknown chromosomal sequence. A primer complementary to this linker ensures second strand synthesis and retrovirus-chromosome junctions are amplified by using a combination of retrovirus LTR-specific and linker-specific primer sets.
Fig. 1. Linker-selection-mediated (LSM) PCR for cloning of chromosomal AAV integration sites. 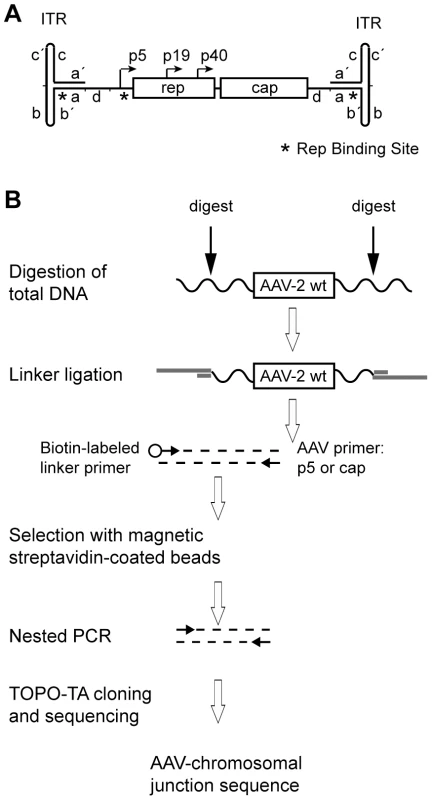
(A) Genome structure of AAV-2 with the rep and cap genes and their promoters flanked by inverted terminal repeats (ITRs) at either end of the ssDNA genome. The hairpin-structured ITRs contain internal repeat elements and complements thereof, represented by small letters. The positions of the Rep-binding sites (RBS) are represented by asterisks. (B) Schematic representation of the LSM-PCR strategy for amplification of AAV-chromosomal junction fragments. Wavy lines indicate chromosomal DNA. Linkers are displayed by thick, grey lines, AAV-specific primers by small, horizontal arrows. For restriction enzyme digestion indicated by vertical arrows either one of the following enzymes were used: PvuII or EcoRV (non-cut for AAV-2) or DraI (single-cut in AAV-2). For this study a variation of LM-PCR, named linker-selection-mediated (LSM)-PCR was developed which enriched for bona fide AAV-chromosome fusion sequences. The genomic DNA of AAV-infected cells was cleaved with restriction enzymes that lead to sufficiently sized DNA segments to allow unambiguous genomic assignment of the chromosomal junction (Figure 1B). DNA sequences were amplified with one primer for a unique AAV-sequence, either of the p5 promoter or of the cap gene. The other primer binds to the linker DNA attached to the unknown chromosomal site. The structure of the linkers forces the PCR to initiate within the AAV genome, thereby suppressing amplification of chromosomal DNAs lacking integrated AAV. The use of non-cut enzymes for AAV-2 DNA helped to circumvent the problem of ligating linkers to episomal, non-integrated AAV DNA sequences. To further enrich for AAV-chromosome junctions a biotin tag was attached to the 5′-end of the linker primer. Thus, chromosome-derived PCR products could be enriched by streptavidin-mediated magnetic bead selection. This lead to PCR products selected for both, the presence of AAV and of an unknown chromosomal DNA sequence.
AAV-2 integration sites
Using LSM-PCR a total of 1700 cloned PCR fragments were screened for DNA inserts of a minimal fragment size (>500 bp) to insure unambiguous detection of AAV-chromosome junctions. Out of 350 DNA sequence runs a total of 129 unique junction sites could be assigned to the human genome. Of these, 109 fulfilled the criteria outlined in the methods for unambiguous assignment of a single chromosomal site. Junctions were retrieved with non-cut enzymes for AAV-2, PvuII or EcoRV or with DraI, which cuts once in AAV-2 DNA outside of the region covered by the PCR. In addition, 43 wildtype AAV-2 infected Hela-derived single cell clones were generated of which eight harboured AAV-chromosome junctions that fulfilled the criteria outlined in the methods.
DNA sequence analysis revealed that AAV-2 wildtype integration sites were scattered over the entire human genome. The chromosomal distribution pattern is displayed in Figure 2A. Over one third of AAV integration sites were clustered at hotspots on chr. 19q13.42, on chr. 5p13.3 and on chr. 3p24.3 (Figure 2B–D). Infection with AAV in the absence of a helper virus leads to transient, low Rep expression. Many previous AAV integration studies used plasmid transfections of wildtype or vector AAV constructs often in combination with a high-level Rep expression construct. To evaluate whether high Rep expression influenced the target site preference of AAV, the sequence data of previously published transfection-based AAV integration sites [42] were reevaluated with the more stringent criteria outlined in the method. Of 157 DNA sequences retrieved after cotransfection of a rep-expression construct and an AAV vector plasmid 47 junction sequences fulfilled our criteria for unambiguous assignment of AAV to a unique chromosomal site (Table 1).
Fig. 2. Chromosomal distribution of AAV-2 integration sites. 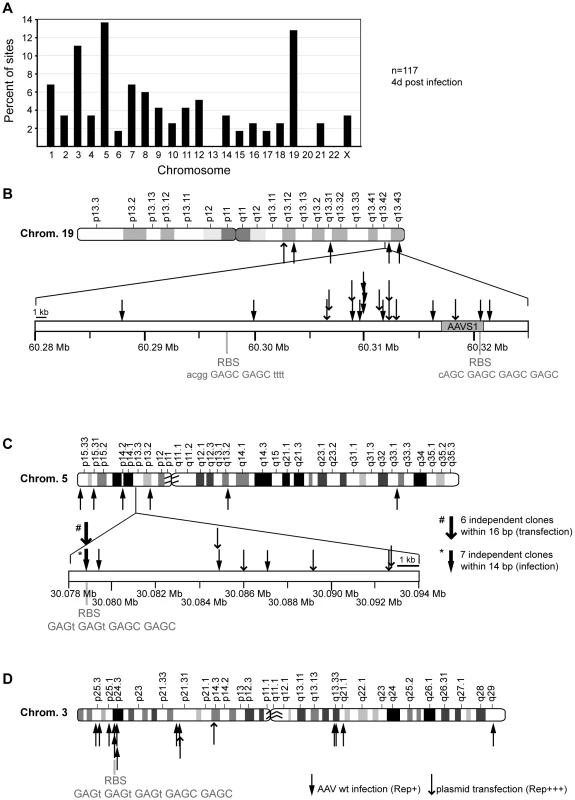
DNA of AAV-2 wildtype infected Hela cells was analyzed for viral integration by the LSM-PCR-method. DNA sequence data from 117 cloned AAV-chromosome junctions were assigned to unique loci. (A) Distributions of junctions on individual chromosomes are shown as percentage of the total 117. (B–D) AAV integration sites drawn to scale for chromosome 19 (B), 5 (C), and 3 (D). Shown are the chromosome ideograms and enlarged bands of hotspots found on chr. 19q13.42 (AAVS1) and chr. 5p13.3 (AAVS2). Solid arrows represent sites detected upon wildtype AAV-2 infection. Open arrows represent junctions stemming from cotransfection of AAV vector- and Rep-expression plasmids. Tab. 1. Summary of data sets analysed in this study. 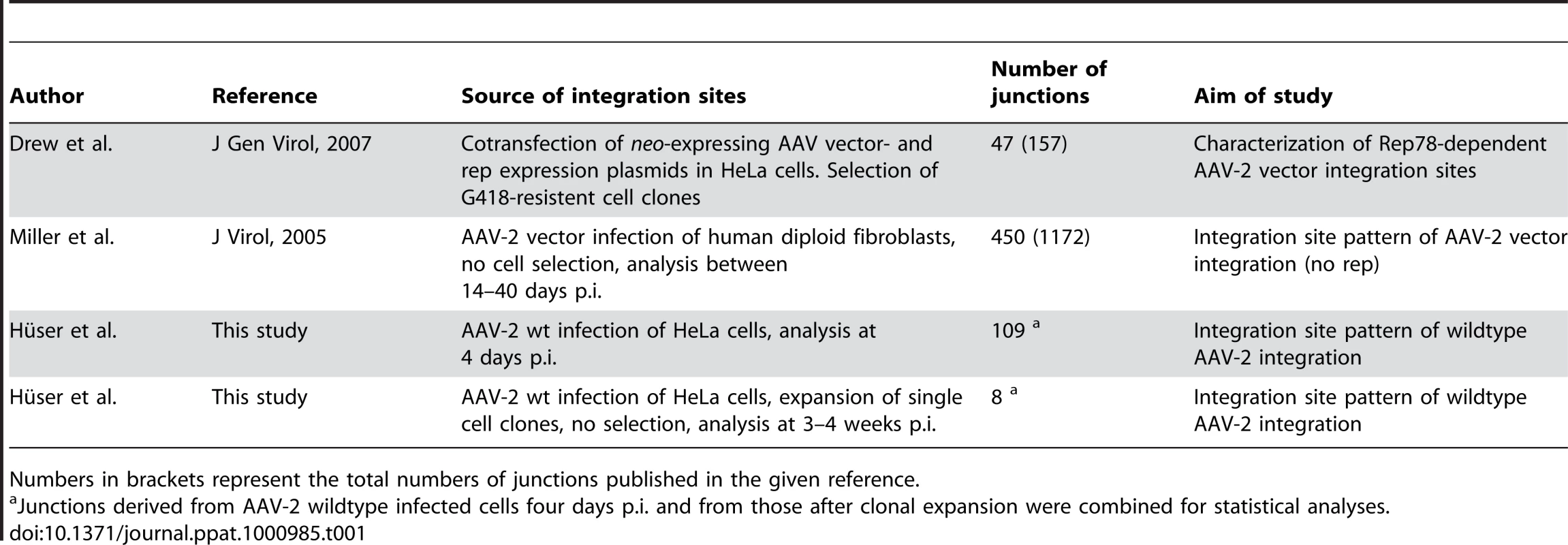
Numbers in brackets represent the total numbers of junctions published in the given reference. Integration hotspots
For AAV wildtype 10% of all retrieved junctions were detected at the hotspot on chr. 19q13.42 spread over a total of 33 kb around AAVS1 (Figure 2B). Only one out of twelve chr. 19q13.42-specific AAV junctions was located within the 4 kb region of AAVS1, where a consensus Rep-binding site and an adjacent trs site had been defined [4] The reevaluated distribution pattern of junctions generated by transfection of AAV vector - and Rep expression plasmids [42] was similar (Figure 2B). Latently AAV-infected Detroit 6 cells [43], [44] were analyzed as control. Using cap-specific primers the junction was detected within AAVS1 at nucleotide position 60,319,992. A second hotspot named AAVS2 was detected on the small arm of chr. 5p13.3 within an intergenic region, where ten independent integration sites were detected within 8 kb (Figure 2C). In seven of these junctions clustered within 14 bp AAV had integrated directly into a consensus Rep binding site. The reanalyzed chromosomal integrations from AAV plasmid transfection [42] displayed a similar pattern with six integrations within 16 bp of the consensus RBS (Figure 2C). The third hotspot named AAVS3 was found on chr. 3p24.3 (Figure 2D). Out of 13 sites detected on chr. 3, three integrations were clustered in a 8 kb region where a consensus Rep binding site GAGT GAGT GAGT GAGC GAGC was detected on the complement strand (Figure 2D).
Rep-binding affinity for RBS consensus sites in AAVS1,-S2, and S3
To evaluate the binding affinities of Rep to the consensus RBS of the hotspots on chr.5 and chr. 3 compared to the RBS of chr. 19 or within the AAV genome, double-stranded oligonucleotides spanning the respective RBS regions (Figure 3) were submitted to mobility shift assays (EMSA) with increasing amounts of purified MBP-Rep78. Since it was previously shown that GAGG repeats are deficient in binding to Rep [10], [45], a mutated oligo derived from the RBS of AAVS2 displaying GAGG GAGG GAGC GAGG was used as a control. As an additional control, a random oligonucleotide of similar length was used. As shown in figure 4, the RBS of AAVS3 contained five instead of four GAGY repeats and bound Rep with a two-fold higher affinity than the oligonucleotide spanning the AAVS1 RBS and trs (Figure 4B). The RBS of AAVS2 showed 76% of the Rep-binding affinity of the AAVS1 sequence (Figure 4C). In contrast, the relative binding affinity normalized to the AAVS1 sequence dropped to 13% with the mutated AAVS2 oligonucleotide, which was in the range of the random oligonucleotide (Figure 4C). These findings confirm the importance of the GAGY repeats in Rep binding. As expected, Rep78 displayed the highest affinities for oligonucleotides spanning the A-stem of the AAV-ITR or the AAV p5–promoter (Figure 4A, 4D). In summary, the newly discovered hotspots for AAV integration, AAVS2 on chr. 5 and AAVS3 on chr. 3 display RBS similarly proficient for Rep-binding as AAVS1.
Fig. 3. Sequence alignment of Rep-binding sites. 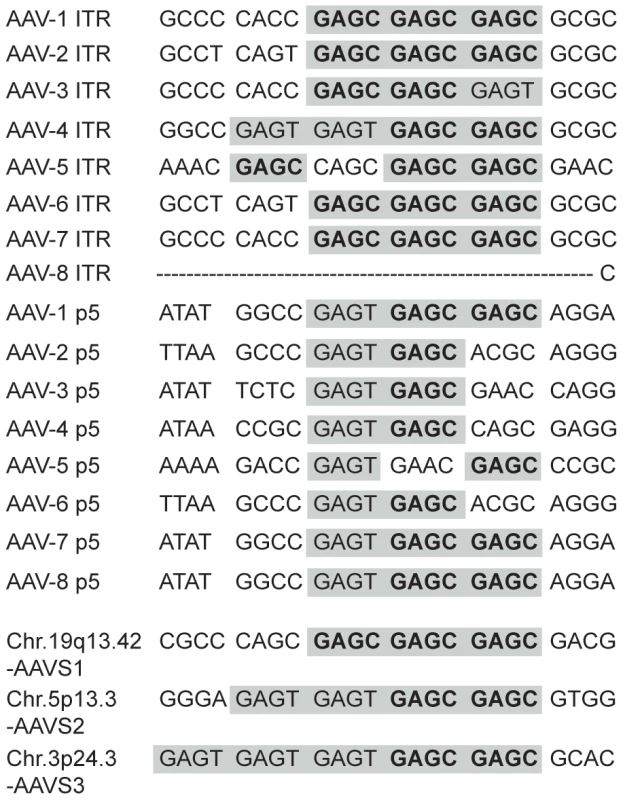
RBS elements present in the ITR and the p5 promoter of all known AAV serotypes are aligned and related to consensus RBS sites present at chromosomal integration hotspots. Rep binding element GAGC is displayed in bold letters. Both GAGC and GAGT elements are highlighted in grey. Fig. 4. Binding of MBP-Rep78 to Rep-binding sites of AAV-2 and of chromosomal integration hotspots. 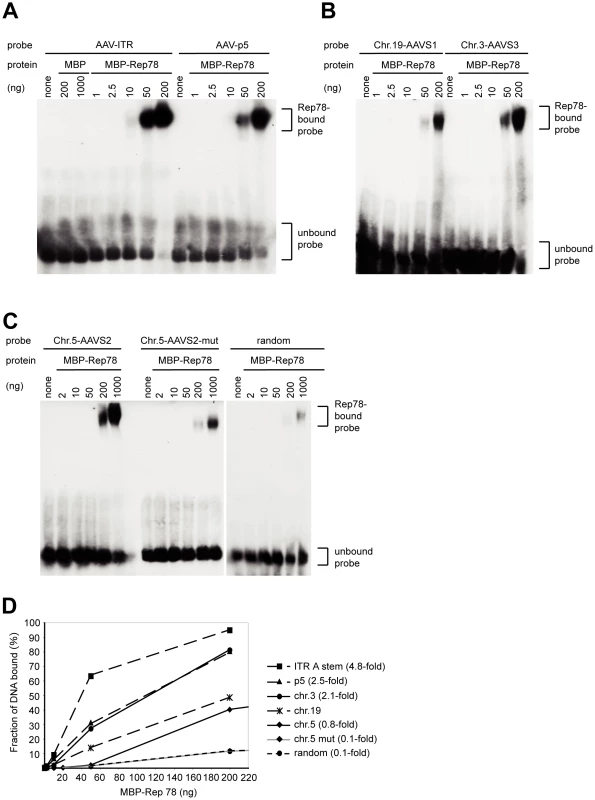
(A) to (C) Electrophoretic mobility shift assays (EMSA) were performed with 32P-labeled double-stranded oligonucleotides in the presence of increasing amounts of affinity-purified MBP-Rep78 as indicated above the autoradiograms. (D) Quantitative determination of the bound fractions of the different RBS and control oligonucleotide probes as a function of the amount of MBP-Rep78 protein in the binding reaction. EMSA gels shown in (A) to (C) were subjected to phosphorimager analysis to determine the relative amount of unbound and bound 32P-labeled oligonucleotides. The relative binding affinity was calculated as follows: The highest amount of Rep used in this assay (1000 ng) bound 31% of the random oligonucleotide. The amount of Rep that bound the same fraction of the other oligonucleotides was determined and normalized to the binding of the chr. 19 (AAVS1) oligonucleotide. Genomic features
To evaluate whether AAV-2 wildtype prefers specific motifs or genomic features for chromosomal integration the detected chromosomal junctions were compared to integration sites described for infection of human cells with a rep-deleted AAV-2 based vector [46]. The published DNA sequence files were reanalyzed using the criteria as outlined in the methods. This led to 450 junctions that could be included as an AAV vector-specific data set (Table 1). The preference for integration next to selected genomic features was analyzed for rep-positive AAV wildtype and for rep-deficient AAV vectors (Table 2). The data showed that the integration frequency of AAV wildtype in genes was higher than expected by chance (Table 2). The frequency was comparable to that of rep-deficient AAV vectors, thus confirming the findings by Miller et al. [46].
Tab. 2. Genomic features and chromatin state associated with AAV-2 wildtype- and AAV vector-derived integration sites. 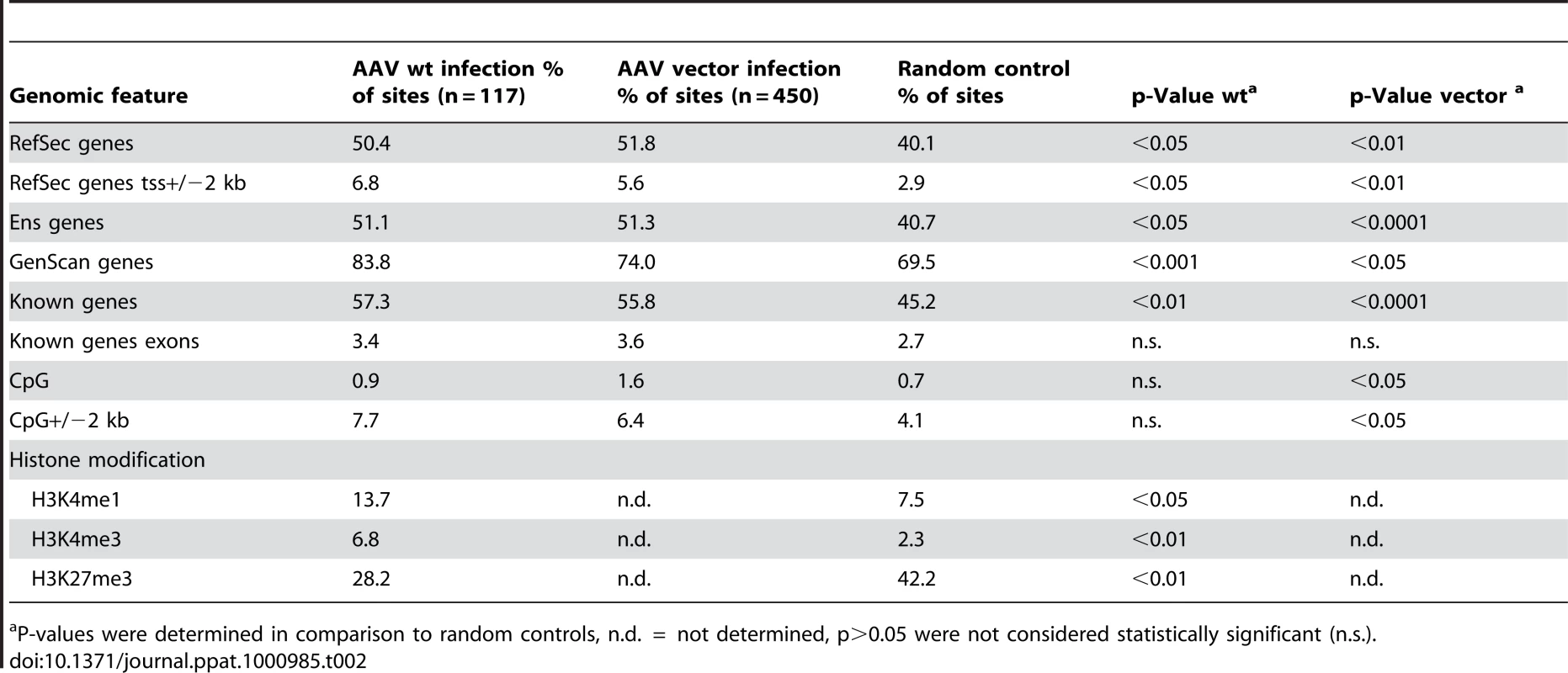
P-values were determined in comparison to random controls, n.d. = not determined, p>0.05 were not considered statistically significant (n.s.). Chromatin state at AAV integration sites
To analyze the effect of epigenetic modifications on AAV integration the association of integration sites with histone modifications as markers for open or closed chromatin were assessed by chromatin immunoprecipitation sequencing (ChIP-Seq) analysis as outlined in the methods. Trimethylated lysine 27 of histone 3 (H3K27me3) is correlated with gene repression (closed chromatin) [47], while methylation of lysine 4 in H3K4me3 and H3K4me1 is indicative of promoter or enhancer regions (open chromatin) [48]. As shown in table 2 the association of AAV wildtype with open chromatin regions is significantly higher than expected from random controls. Conversely, the respective association with closed chromatin is significantly reduced. In summary, AAV wildtype prefers integration into open chromatin whereas closed chromatin was avoided.
Bioinformatic analysis of the AAV integration sites
A series of publications have shown that fused combinations of two to four GAGC motifs bind to Rep78/68 of AAV-2 [4], [49], [50], [51], [52], [53]. Moreover, in vitro ternary complex formation of Rep68 with the AAV-2 ITR and AAVS1 of chr. 19q13.42 [10] led to the concept of Rep acting as an adapter that targets AAV to the human genome. Although only AAV-2 has been analyzed for chromosomal integration so far, all known AAV serotypes displayed various combinations of GAGC and/or GAGT motifs in the ITR and the p5 promoter. An alignment of these AAV elements to the integration hotspots AAVS1, AAVS2 and AAVS3 is displayed in Figure 3.
Based on these data we hypothesized that AAV-2 wildtype, due to the presence of Rep, prefers integration at chromosomal sites in closer proximity to consensus Rep binding sites than would be expected from control sites. The hypothesis was tested with the three sets of junctions derived from: 1. Infection with AAV-2 wildtype, 2. Cotransfection of plasmids coding for an AAV vector and a constitutive Rep-expression cassette, and 3. Infection with Rep-deficient AAV vectors (Table 1). The distances between any one integration site and its nearest Rep-binding site were determined in the human genome and compared to similarly determined distances of individual control sites to the nearest Rep-binding sites. Calculations were repeated using various combinations of RBS as displayed in Figure 5.
Fig. 5. Statistical analysis of distances of integration sites from human Rep-binding motifs. 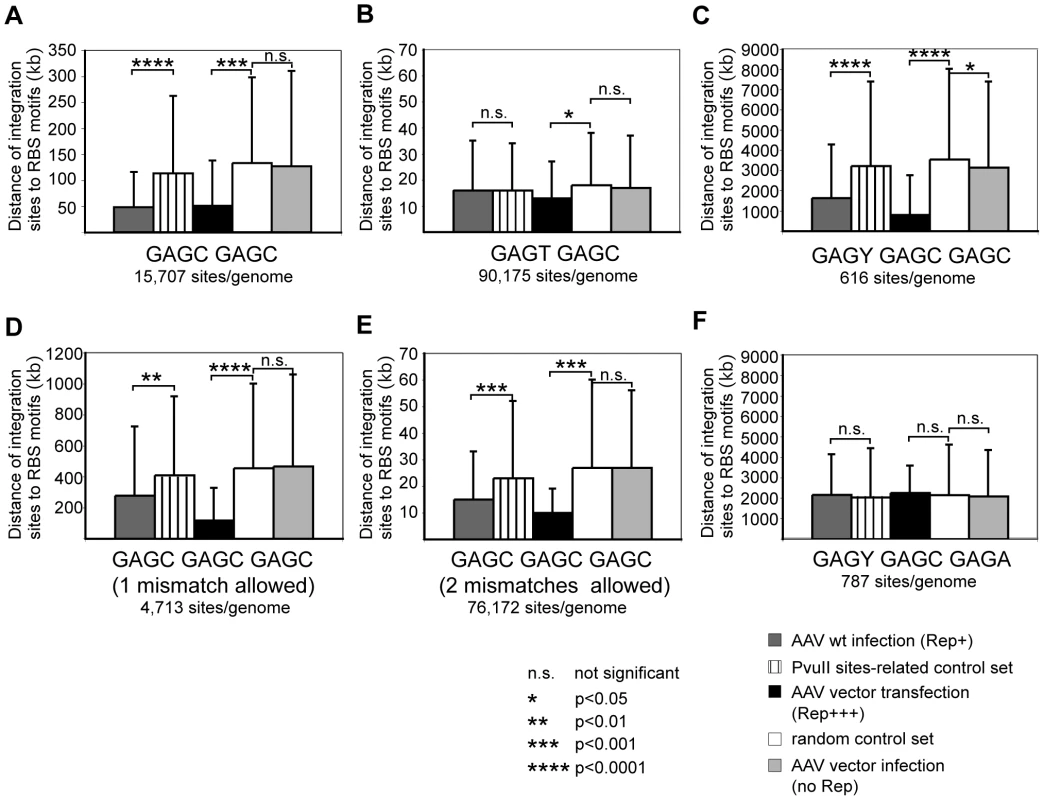
AAV integration sites of different data sets were analyzed for the proximity to the next Rep binding sites. Calculations were performed with the following putative Rep binding sites: GAGC GAGC (A); GAGT GAGC (B); GAGY GAGC GAGC (C); GAGC GAGC GAGC, one mismatch (D), or two mismatches allowed (E), and GAGY GAGC GAGA (F). Average distances of integration sites from putative Rep binding sites are displayed as mean +/− S.D. The levels of significance are marked by asterisks. P-values >0.05 were not considered statistically significant. P-values <0.01 were considered highly significant. For the analysis of wildtype AAV integration data a PvuII-related control set was used in addition to the random control set. The choice of randomly generated genomic control sites was considered optimal for comparative analysis of the three sets of data. Yet, a concern was the choice of restriction endonucleases for the identification of the wildtype AAV-2 integration sites by LSM-PCR. To control a bias introduced by a conceivable non-random genomic distribution of the restriction sites, the average distance of PvuII, EcoRV, or DraI-generated restriction sites to putative Rep-binding sites was compared to the average distances of random sites to Rep-binding sites. PvuII restriction sites were found to be closer to Rep-binding sites than random control sites (Figure S1). This was assumedly due to the high G+C content of the PvuII recognition sequence and of the consensus Rep-binding sites. Both EcoRV and DraI sites were found further apart from Rep-binding sites in accordance with their high A+T content (Figure S1). To circumvent any bias arising from the use of PvuII, the data set for AAV wildtype infection was calculated against the data set of random control sites as well as against the data sets for the restriction site–related controls. Since not more than two thirds of sites were generated with PvuII, the PvuII-related control sites would at most underestimate the association to Rep-binding sites and was therefore used as the most stringent control set. In addition all calculations were also performed with the set of random controls leading to similar findings (Figure S2).
The bioinformatic calculations with GAGC GAGC as a minimal Rep-binding site strikingly confirmed our hypothesis that integration of wildtype AAV takes place close to Rep-binding sites with very high significance (p <0.0001). A comparable effect was seen with the data set for AAV vectors in the presence of Rep (p<0.001). Most importantly, the set of integration sites for AAV vectors in the absence of Rep did not show any difference of integration site preference compared to random control sites (Figure 5A). With a frequency of 15,707 sites per human genome the Rep binding motif GAGC GAGC occurs sufficiently frequent to lead to a mean distance of around 50 kb to the next AAV integration site in the presence of Rep. In the absence of Rep the mean distance to AAV (vector) integration sites rises to around 130 kb (Figure 5A). To ensure that the presence of repetitive DNA in the random controls did not lead to a bias in the analysis, an independent control calculation was performed for AAV wt data using AAV vector infection data as background. The high significance level was maintained (data not shown). The significance of the Rep-associated preferential integration near GAGC GAGC sequences was further underlined by the results of similar calculations for the putative Rep-binding motif GAGT GAGC, where no such association was found. Only in the presence of presumably large amounts of Rep (AAV vector transfection, Rep+++) a small effect was seen (Figure 5B). Obviously the GAGT GAGC motif is not sufficient to attract Rep and the AAV genome for integration. When an additional GAGC repeat is added (GAGY GAGC GAGC) the integration preferences of AAV wildtype and Rep-expressing AAV vectors shifted to closer proximity to Rep-binding sites (p<0.0001). This is especially surprising since only 616 sites per human genome are found for GAGY GAGC GAGC (Figure 5C). To allow more potential Rep-binding site permutations, calculations were repeated with the consensus GAGC GAGC GAGC with one or two random mismatches. This led to a significantly decreased mean distance to AAV junctions in spite of the fact that up to 100-fold more genomic hits were found for the motifs (Figure 5D; E). A single nucleotide exchange in the GAGY GAGC GAGC motif (Figure 5F, GAGY GAGC GAGA) on the other hand led to a complete loss of association to AAV integration sites. This is surprising in view of the reported in vitro binding of Rep to this motif [45] and supports the assumption that the C at the 3′ end of the Rep binding motif is relevant for Rep-binding in vivo. Motifs GCCC GAGT GAGC and GAGT GAGC ACGC are part of the RBS in the viral p5 promoter. The individual motifs are found at very low frequency (n = 85, or n = 82, respectively) in the human genome, so that either no RBS was found in the same contig or the distance to the next RBS was more than several thousands kb. For these reasons we did not proceed with calculations for these motifs. To further exclude the possibility that the calculated associations with Rep binding sites were predominantly based on sequences assigned to the hotspots AAVS1 and AAVS2, the significance of the associations was re-evaluated with data sets omitted for the hotspot sequences (Table 3). The robustness of the data becomes evident by the fact that the highly significant association of AAV junctions to motifs GAGC GAGC and GAGY GAGC GAGC is maintained. In summary, AAV prefers integration sites in the vicinity of consensus Rep-binding elements, most prominently on chr. 19q13.42 (AAVS1), chr. 5p13.3 (AAVS2), and chr. 3p24.3 (AAVS3). But even in the absence of hotspots AAV still shows a highly significant integration preference for Rep-binding motifs at numerous additional sites in the human genome.
Tab. 3. Neighbourhood analysis of wildtype AAV-2 integration sites and RBS motifs found outside of the hotspots on chr. 19q13.42 (AAVS1) and on chr. 5p13.3 (AAVS2). 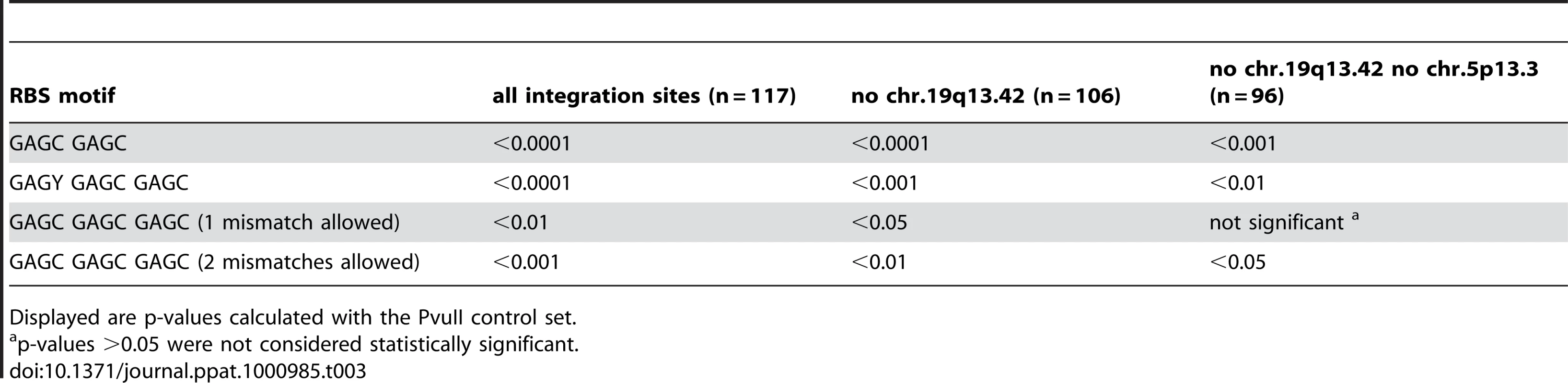
Displayed are p-values calculated with the PvuII control set. Discussion
This study represents the first genome-wide survey of wildtype AAV-2 integration in the human genome combined with a thorough bioinformatic analysis of the surrounding genome. We show here that wildtype AAV-2 infection leads to preferential integration in the vicinity of consensus Rep-binding sites (RBS) at defined hotspots as well as at numerous additional genomic sites. In contrast, AAV-2 vectors in the absence of Rep-expression integrate without discernable preference for consensus Rep-binding sites.
Hotspots of AAV integration
At the hotspot on chr. 19q13.42, up to 10% of all AAV junctions were scattered over a region of 33 kb, mostly in centromeric direction with regard to the previously defined core 4 kb AAVS1 site. AAV vectors in the absence of Rep expression do not show any preference for chr. 19q13.42 [46]. The here identified, novel hotspot AAVS2 on chr. 5p13.3 displayed roughly 8% of all junctions retrieved from wildtype AAV-2 infection and 23% of those retrieved from cotransfection of AAV vectors in the presence of Rep distributed over a region of 14 kb. A cluster of 13 independent junctions was found within 14 bp of the AAVS2 RBS that was shown to be similarly proficient in binding to Rep in vitro as is the RBS of AAVS1 (Figure 4). The high in vivo integration numbers may in part be due to the choice of HeLa as target cells. These are hypertriploid with up to 12 copies of the p-arm of chr. 5 [54]. The extra gain of integrations within the described 8 kb region is however unique for the AAVS2 site and not accompanied by a parallel increase of integrations at additional sites on the overrepresented p-arm of chr. 5, where 201 additional GAGC GAGC repeats and three additional GAGY GAGC GAGC repeats were counted. The only fourfold tetranucleotide repeat on the chr.5 p-arm is found in AAVS2 (GAGT GAGT GAGC GAGC; Figure 2C). In addition, junctions of rep-deficient AAV vector were reported to be underrepresented on chr. 5 [46].
A major difference between the hotspots on chr. 5 and chr. 19 concerns the presence of genes. The junctions identified on chr. 19 span the region of the transcribed gene for protein phosphatase 1, regulatory subunit 12C (PPP1R12C). The 8 kb AAVS2 sequence identified on chr. 5p13.3 represents an intergenic region to the best of current knowledge. It is well known that Rep expression leads to extensive rearrangements of AAVS1 [18], [55], [56]. Apparently, PPP1R12C is essential, since the majority of latently infected cell lines display gene duplications [57] and simultaneous AAV integrations in both alleles have never been reported. A currently unresolved question concerns the presence of a terminal resolution site (trs) next to the RBS of AAVS2 and AAVS3. In AAVS1 the spatial configuration of RBS and trs resembles that of the AAV-ITR. The trs element lies next to the RBS and serves as a nicking site for Rep [4]. In AAVS2 and AAVS3 the nearest perfect trs elements (5′-GTTGG-3′) are 400 and 500 bp away from the RBS, which represents the mean statistical occurrence for this motif. Unfortunately, the consensus nucleotide requirements for a functional trs element are not defined well enough to conduct a meaningful bioinformatic search. Therefore, the presence of nicking sites next to the RBS in AAVS2 or AAVS3 remains open at present.
Target site choice for AAV integration
Besides the identified integration hotspots numerous additional chromosomal junction sites were found for integrated wildtype AAV-2, scattered over the human genome. From the bioinformatic calculations it appeared that the perfect tetranucleotide repeat GAGC GAGC represented the minimal requirement for Rep-dependent targeted integration, and GAGY GAGC GAGC represents the optimized in vivo target sequence for wildtype AAV-2. Hotspots AAVS1, AAVS2, and AAVS3 display this core sequence fused to additional imperfect GAGY repeats. Other AAV serotypes display RBS sequences with similar numbers of GAGC and/or GAGT repeats, extended by additional imperfect repeats. AAV5 Rep co-crystallised with the hairpin-structured AAV5-ITR revealed that five Rep monomers bind to five consensus tetranucleotide repeats of the RBS, each of which was contacted by two Rep monomers from opposite faces of the DNA [58]. AAV2-Rep78/68 was shown to simultaneously bind to the RBS of the AAV-2 ITR and to that of AAVS1 [10]. Although it is currently unknown whether other AAV serotypes integrate at all, this is highly likely in view of the ability of both AAV-2 Rep and the relatively distant AAV-5 Rep to multimerize and simultaneously bind to clustered GAGY repeats.
In the initial descriptions of AAVS1, site-specific nicking of the trs by Rep bound to the adjacent RBS was viewed as preferred entry site for AAV recombination [4]. Meanwhile the majority of AAV integrations on chr. 19q13.42 were found many kb away from the RBS-trs combination, and neither AAVS2 or AAVS3 display obvious trs homologues next to the RBS. Therefore alternative explanations for RBS-dependent AAV integration should be considered. The potential use of preexisting chromosomal breakage sites recalls a mechanism already proposed for the integration of rep-deficient AAV vectors [34], [59]. Alternative integration concepts include the use of imperfect trs elements for nicking as shown in vitro [4], [60], [61], or the ability of Rep78 to induce DNA damage in vivo by single-strand nicking of cellular chromatin [19]. It is conceivable that the introduction of single-strand nicks occurs anywhere in accessible chromatin, even if the nicking site is hundreds or thousands of bp apart from the RBS on an extended DNA strand. HMGB1, an ubiquitous architectural protein that serves as key component of the chromatin remodelling complex may be of help [62]. Its long-known in vivo interaction with Rep [63] may help remodel the chromatin to make it accessible for nicking by Rep. Rep was also shown to contact other key players of the nucleosome remodelling complex as components of the transcription - or DNA replication machinery [64], [65], [66]. Any of these mechanisms can be exploited to open the chromatin for AAV integration. In summary, Rep with its combined DNA-binding and endonuclease activity appears to serve as a relatively imprecise targeting tool for AAV integration preferably in open chromatin regions in the reach of consensus Rep-binding sites prevalent in the human genome.
Implications for Rep-dependent targeting of AAV vector integration
The early finding that Rep would mediate site-specific AAV integration on chr. 19q13.42 had immediate implications for gene therapy. A variety of concepts were devised to incorporate Rep as an adapter to target AAV-ITR flanked transgenes to a specific site [26], [27], [28], [57], [67]. In the majority of cases appropriate cell selection or PCR for AAVS1 led to cells displaying the desired integration. The reported high frequencies of integration into AAVS1 are difficult to reconcile with our findings, unless the level of Rep expression is considered to have an impact on target site choice. Upon AAV infection Rep is only moderately expressed due to autoregulation of the AAV p5 promoter. Rep-dependent AAV vector transductions typically use strong heterologous promoters that lead to high and sustained Rep expression levels. Increasing Rep levels may increase the overall probability for integration anywhere in the genome, including at hotspots. Under these conditions AAVS1-specific integration will be detected more readily. This appears however to come at the price of genomic rearrangements in reach of alternative Rep-binding sites. Therefore, it is plausible that in the absence of any selection AAV integration into AAVS1 is typically unstable and difficult to detect.
In summary, Rep expression increases the probability for integration next to one of several genomic hotspots. However, the net genotoxic effect is unpredictable both with respect to the integrity of the AAV integration locus itself and with respect to the numerous additional sites where Rep binds and initiates chromosomal damage. Therefore, the current concept of a relatively precise site-specific targeting of AAV should be extended to a concept of a relative preference for accessible chromatin regions in the neighbourhood of any of the numerous consensus Rep-binding sites. More recent approaches for site-specific vector targeting try to exploit DNA sequence-specific zinc-finger nucleases to target a genomic sequence of wish [68]. Although zinc-finger nucleases are not free from off-target genotoxicity, at least the genomic targeting site for the transgene can be more precisely defined, a goal that appears to be inherently unachievable using Rep as an adapter molecule.
Materials and Methods
Cells
Detroit 6 cells harbouring latent AAV-2 genomes and HeLa cells were grown in Dulbecco's modified Eagles's medium (Gibco) supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 µg/ml).
AAV infection
Viral stocks of wildtype AAV-2 with infectious titers of 5×109 i.u./ml were prepared on HeLa cells as described before [16]. For the analysis of AAV integration sites 1.7×106 HeLa cells were seeded overnight on 10 cm diameter dishes and infected with AAV-2 at a MOI of 500. Cells were harvested at 96 hours post infection (p.i.) for the extraction of genomic DNA. The period of cell growth after infection was minimized to reduce the chances of selection of particular integration sites during cell proliferation. Alternatively, AAV-infected HeLa cells were seeded to microtiter plates at a dilution of 60 cells per plate and grown up as single-cell clones without drug selection.
Plasmids
Plasmid pTAV2-0 covers the AAV-2 wildtype genome (GenBank accession number AF043303), pRVK the 4 kb fragment of the AAVS1 locus on chromosome 19 (GenBank accession number S51329), and pAAVS1-TR covers an AAV-ITR/AAVS1 junction [16]. Plasmid pMBP-Rep78 encoding Rep78 fused to maltose-binding protein (MBP) was described before [69].
Production and purification of MBP-Rep78 fusion protein
MBP-Rep78 encoding Rep78 fused to maltose-binding protein was expressed und purified essentially as described [69]. Briefly, E.coli strain BL21 transformed with pMBP-Rep78 was grown at 30°C to an OD600 nm of 0.6 to 0.8. Production of MBP-Rep78 was induced with 0.3 mM IPTG for 3 h at 30°C. Cells were harvested by centrifugation and lysed by sonication for 2 min (30% duty cycle) in lysis buffer of 50 mM phosphate pH 7.8, 300 mM NaCl, 1% (v/v) Triton X-100, 0.1 mM PMSF. Cell debris was removed by centrifugation at 6500×g for 20 min at 4°C. The supernatant was adsorbed to amylose resin (New England Biolabs) in a batch process and the resin was washed extensively (5 washes with about 100 volumes of the resin) with lysis buffer. The adsorbed proteins were eluted with lysis buffer containing 10 mM maltose and analyzed for purity by SDS-polyacrylamide gel electrophoresis.
Electophoretic mobility shift assays (EMSA)
Binding of MPB-Rep78 fusion protein to 32P - labeled double-stranded oligonucleotide probes was detected by altered mobility of the probes in nondenaturating polyacrylamide gels essentially as described previously [70]. Briefly, oligonucleotides of 46–49 nt length were end-labeled with T4 polynucleotide kinase and annealed. EMSA reactions were performed for 20 min at 20°C as follows: 0.015 pmol of labeled DNA substrate was incubated with the indicated amounts of MBP or MBP-Rep78 in a binding buffer containing 25 mM HEPES-KOH (pH 7.8), 10 mM MgCl2, 40 mM NaCl, 1 mM DTT, 2% glycerol, 12.5 µg/ml BSA, 0,01% Nonidet P40 and 5 µg/ml salmon sperm DNA. The following oligonucleotides were used:
AAV-ITR (nucleotide position 85–133): GCCTCAGTGAGCGAGCGAGCGCGCAGAGAGGGAGTGGCCAACTCCATCA;
AAV-ITR complementary strand: TGATGGAGTTGGCCACTCCCTCTCTGCGCGCTCGCTCGCTCACTGAGGC
Chr. 19q13.42 (AAVS1): TGGCGGCGGTTGGGGCTCGGCGCTCGCTCGCTCGCTGGGCGGGCGGGC
Chr19 (AAVS1) complementary strand: GCCCGCCCGCCCAGCGAGCGAGCGAGCGCCGAGCCCCAACCGCCGCCA
Chr. 5p13.3 (AAVS2): AGCTGGACCCCACGCTCGCTCACTCACTCTCCCCTCACCGCTTTGT
Chr. 5 (AAVS2) complementary strand: ACAAAGCGGTGAGGGGAGAGTGAGTGAGCGAGCGTGGGGTCCAGCT
Chr. 3p24.3 (AAVS3) GCTTCCCAAGGGGAATGAATGTGCGCTCGCTCACTCACTCACTCCTCAC
Chr.3 (AAVS3) complementary strand: GTGAGGAGTGAGTGAGTGAGCGAGCGCACATTCATTCCCCTTGGGAAGC
Chr. 5MUT (AAVS2 mutated): AGCTGGACCCCACCCTCGCTCCCTCCCTCTCCCCTCACCGCTTTGT
Chr.5MUT (AAVS2 mutated), complementary strand: ACAAAGCGGTGAGGGGAGAGGGAGGGAGCGAGGGTGGGGTCCAGCT
AAV p5 (nucleotide position 245–292): TCACGCTGGGTATTTAAGCCCGAGTGAGCACGCAGGGTCTCCATTTTG
AAV p5 complementary strand: CAAAATGGAGACCCTGCGTGCTCACTCGGGCTTAAATACCCAGCGTGA
random control: CAGAGCAGCAGCACAGACGCTAGCAGATCTCCTGCGACCGGAGATGTG
random control, complementary strand: CACATCTCCGGTCGCAGGAGATCTGCTAGCGTCTGTGCTGCTGCTCTG
Preparation of genomic DNA
Total genomic DNA was extracted by SDS/proteinase K digestion followed by repeated phenol/chloroform extractions and ethanol precipitation, as described before [71]. High molecular weight DNA (2 µg) was digested with restriction enzymes that lead to a mean genomic fragment size of around 4 kb and produce blunt-ends ready for linker/adapter ligation. Non-cut enzymes for AAV-2 DNA were preferred, PvuII, EcoRV. Additional junctions were retrieved with DraI (one cut in AAV-2 wildtype DNA). Digested genomic DNA was purified by repeated phenol-chloroform extractions and precipitated with ethanol.
Linker-Selection-Mediated (LSM) PCR
A linker-based strategy described in [39], [40] and outlined in more detail in the manual of the GenomeWalker kit (Clontech) was modified as outlined in Figure 1B. The following oligos were used for linker construction: “Linkerlong” (5′GTA ATA CGA CTC ACT ATA CGG CAC GCG TGG TCG ACG GCC CGG GCT GGT 3′) and “linkershort” (5′ACC AGC CC 3′modifikation: 2′,3′-dideoxyC). Equal amounts of “linkerlong” and phosphorylated “linkershort” (100pmol each) were annealed and ligated to restriction enzyme-digested genomic DNA.
PCR-primers: The linker-primers were “P linker outside” with biotin attached to its 5′ end (5′-GTA ATA CGA CTC ACT ATA CGG C; Tm = 58.4°C) and “P linker nested” (5′-ACT ATA CGG CAC GCG TGG T; Tm = 58.8°C). Two AAV-2-specific primer sets were used. The first primer set covered the AAV p5 promoter: “AAV2p5” (5′-TCA AAA TGG AGA CCC TGC GTG CTC A; Tm = 64.6°C, AAV-2, nt 293–269), primer “AAV2p5 nested” (5′-TAA ATA CCC AGC GTG ACC ACA TGG TG; Tm = 64.8°C, AAV-2, nt 260–235). The other primer set is located in the cap gene region, as described before [2]: “CAPgsp1” (5′-GTC TGT TAA TGT GGA CTT TAC TGT GGA CAC; Tm = 65.4°C, AAV-2, nt 4320–4349) and “CAPgsp2” 5′-GTG TAT TCA GAG CCT CGC CCC AT; Tm = 64.2°C, AAV-2 nt 4357–4379).
The PCR reaction contained 0.2 mM dNTPs, linker primer and AAV specific primer (0.25 µM, each), 2.5 U proofreading hot-start polymerase (Herculase) in reaction buffer, as provided by the supplier (Stratagene). Of the preceding linker-ligation reaction 1–5 µl was added to a final volume of 50 µl. PCR conditions were as follows: 3 min at 98°C, followed by 10 cycles of 40 sec at 98°C, 30 sec at 65°C, and 4 min at 72°C, followed by 20 cycles of 40 sec at 98°C, 30 sec at 65°C, and 4 min + 10 sec per cycle at 72°C, terminated by an extension period of 10 min at 72°C. Biotin-labelled PCR products were further enriched on streptavidin-labelled Dynabeads M-280, as outlined by the supplier (Invitrogen). Subsequent nested PCR used conditions identical to the first round but with pairs of the nested PCR primers, as outlined above. Finally, to add overhangs of multiple As, PCR products were incubated with 1 U Taq polymerase (New England Biolabs).
Analysis of LSM PCR products
Products of LSM-PCR reactions were separated on agarose gels. To ensure sufficient chromosomal fragment lengths, PCR bands of a calculated minimal length (>500 bp) were excised and purified by the QIAEX II Gel extraction kit (Qiagen, Hilden, Germany). TOPO-TA cloning was performed as described [72]. Colonies were PCR-screened with the M13 forward (-20) and reverse primer pair (0.4 µM, each) with 0.2 mM dNTP, 2 U Taq polymerase (New England Biolabs) at the following conditions: 10 min at 94°C, followed by 30 cycles of 30 sec each at 94°C, 52°C, and 72°C, followed by 10 min at 72°C. Column-purified PCR products were submitted to DNA sequencing using the primer provided by the TOPO-TA cloning kit. DNA sequences were run on a CEQ2000 genetic analysis system (Beckman) using the CEQ Dye Terminator Cycle Sequencing Quick start kit (Beckman) and the run method LFR-a. Cycling conditions were as follows: 1 min at 96°C, followed by 30 cycles 20 sec at 96°C, 20 sec at 50°C and 4 min at 60°C.
Integration site determination
The genomic positions of AAV integration sites in the human genome (assembly from March 2006, hg18) were determined using the BLAT tool from the UCSC Genome Browser web site (http://genome.ucsc.edu/cgi-bin/hgBlat) [73]. A match was defined as a BLAT search result fulfilling all of the following criteria:
-
A human chromosome-derived part of the DNA sequence is at least 100 bp in length and of 98% or higher homology to the database.
-
A shorter chromosomal match is acceptable if it displays a minimum of 25 bp of a contiguous DNA sequence match.
-
A part of the sequence allows assignment of AAV.
-
In the case of unassigned base pairs between the AAV and the human part of the sequence, this sequence is no longer than 20 bp.
-
Sequences matching to multiple chromosomal regions (i.e. repeat regions) were discarded in view of the inability to unambiguously assign the surrounding genome for subsequent bioinformatic analysis (see below).
-
Duplicate AAV-chromosomal fusion sequences (identical viral and identical human DNA sequences) were counted only once.
In addition to the LSM-PCR derived sequences, the original DNA sequence files of 157 chromosomal junctions [42] kindly provided by Dr. G.W. Both, North Ride, Australia were reanalyzed applying the above inclusion criteria. This led to 47 DNA sequences suitable for our analysis (Table 1). In their study, HeLa cells had been cotransfected with plasmids for constitutive RSV-promoter-driven Rep78 expression and for recombinant AAV vectors expressing a SV40-promoter-driven neomycin gene [42]. Furthermore, 1100 DNA sequences from a published analysis of rep-deficient AAV vector integration sites in diploid human cells [46] were reanalyzed. Since the PCR methods employed in our study and in the one by Drew et al. [42] cannot detect the matching left and right junction sites generated by one AAV integration event, only one chromosomal junction was analyzed per rescued provirus. The original DNA sequence files (DU711025.1 to DU709854.1) of Miller et al. [46] were downloaded from the Genome Survey Sequences (GSS) Database of NCBI (http://www.ncbi.nlm.nih.gov/sites/entrez?db=nucgss) and reanalyzed using the UCSC March 2006 human genome build. The analysis led to a total of 450 junction sequences that fulfilled all of the above inclusion criteria for bioinformatic comparisons. For the subsequent data analysis we implemented software in C++ using the software library SeqAn [74] and several Python scripts.
Determination of distances of integration sites to putative Rep binding sites
For different Rep binding motifs, we computed the average distance of virus integration sites to the closest occurrences of Rep binding motifs within the genome. We supposed that the observed integration events were independent from each other and the sample size was high enough for assuming the distance to be normally distributed. To assess whether these distances differ significantly from expectation, several background models were generated:
-
For the background model “random”, we assumed that the probabilities for the observation of virus integrations were equally distributed among all conceivable positions in the genome. A program was implemented that computed the exact mean and standard deviation of this background distribution.
-
Since the integration site analysis required a suitable restriction enzyme site in the neighbourhood of the integrated virus three background-models for the restriction enzymes DraI, EcoRV or PvuII were generated. These models served as a corrective tool for an eventual bias of a non-uniform distribution of the respective restriction enzyme sites in the genome. For each AAV integration site observed, the distance to the closest restriction site was determined individually. Then, 1000 control sites per integration site were generated that displayed the same distance to randomly chosen restriction sites.
The generation of both, the data analysis and the background model was confined to those genomic contigs that contained at least one Rep binding motif, since otherwise the distance to the “closest Rep binding motif” would not be defined. A given set of AAV integration sites was considered to be significantly closer to Rep binding motifs than expected by chance, if the significance was calculated for all relevant background models. Data sets of AAV vectors were analyzed with the “random” background model. We applied a Z-test for determining statistical significances for the distances of integration sites to Rep binding sites. For comparing integration sites from AAV wildtype infection sites against those from rep-deficient AAV vector infection we applied the Student's t-test.
Presence of genomic features
AAV integration sites were examined for the occurrence of various genomic features using tables available in the UCSC database. For the determination of significant divergences from expectations, we compared the actual integration sites with a set of 100,000 randomly chosen control sites in the human genome using a two-tailed binomial test.
Analysis of chromatin state
Chromatin immunoprecipitation sequencing (ChIP-Seq) data were used to define the state of histone modifications in genomic regions of AAV integration. H3K27me3 domains determined by Cuddapah et al. were used as markers for closed chromatin (http://www.wip.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc=GSM325898) [75]. Regions enriched for H3K4 methylation (open chromatin) were determined as follows: The raw ChIP-Seq reads by Robertson et al. [76] (http://www.bcgsc.ca/data/histone-modification) were mapped to the human genome using Bowtie [77], and peaks were called using MACS [78]. H3K4me1/3 domains are then defined as 5 kb windows around the centers of the peaks.
Supporting Information
Zdroje
1. MuzyczkaN
BernsKI
2001
Parvoviridae: The viruses and their replication.
KnipeDM
HowleyPM
Fields Virology
Philadelphia
Lippincott
2327
2359
2. SchneppBC
JensenRL
ChenCL
JohnsonPR
ClarkKR
2005
Characterization of adeno-associated virus genomes isolated from human tissues.
J Virol
79
14793
14803
3. KotinRM
SiniscalcoM
SamulskiRJ
ZhuXD
HunterL
1990
Site-specific integration by adeno-associated virus.
Proc Natl Acad Sci U S A
87
2211
2215
4. LindenRM
WinocourE
BernsKI
1996
The recombination signals for adeno-associated virus site-specific integration.
Proc Natl Acad Sci U S A
93
7966
7972
5. SnyderRO
ImD-S
NiT
XiaoX
SamulskiRJ
1993
Features of the adeno-associated virus origin involved in substrate recognition by the viral Rep protein.
J Virol
67
6096
6104
6. ImD-S
MuzyczkaN
1990
The AAV origin-binding protein Rep68 is an ATP-dependent site-specific endonuclease with helicase activity.
Cell
61
447
457
7. PhilpottNJ
GomosJ
BernsKI
Falck-PedersenE
2002
A p5 integration efficiency element mediates Rep-dependent integration into AAVS1 at chromosome 19.
Proc Natl Acad Sci U S A
99
12381
12385
8. SamulskiRJ
ZhuX
XiaoX
BrookJD
HousmanDE
1991
Targeted integration of adeno-associated virus (AAV) into human chromosome 19 [published erratum appears in EMBO J 1992 Mar;11(3):1228].
EMBO J
10
3941
3950
9. KotinRM
LindenRM
BernsKI
1992
Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination.
Embo J
11
5071
5078
10. WeitzmanMD
KyöstiöSRM
KotinRM
OwensRA
1994
Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA.
Proc Natl Acad Sci U S A
91
5808
5812
11. MenesesP
BernsKI
WinocourE
2000
DNA sequence motifs which direct adeno-associated virus site-specific integration in a model system.
J Virol
74
6213
6216
12. YangCC
XiaoX
ZhuX
AnsardiDC
EpsteinND
1997
Cellular recombination pathways and viral terminal repeat hairpin structures are sufficient for adeno-associated virus integration in vivo and in vitro.
J Virol
71
9231
9247
13. TsunodaH
HayakawaT
SakuragawaN
KoyamaH
2000
Site-specific integration of adeno-associated virus-based plasmid vectors in lipofected HeLa cells.
Virology
268
391
401
14. PalomboF
MonciottiA
RecchiaA
CorteseR
CilibertoG
1998
Site-specific integration in mammalian cells mediated by a new hybrid baculovirus-adeno-associated virus vector.
J Virol
72
5025
5034
15. PieroniL
FipaldiniC
MonciottiA
CiminiD
SguraA
1998
Targeted integration of adeno-associated virus-derived plasmids in transfected human cells.
Virology
249
249
259
16. HüserD
WegerS
HeilbronnR
2002
Kinetics and frequency of adeno-associated virus site-specific integration into human chromosome 19 monitored by quantitative real-time PCR.
J Virol
76
7554
7559
17. HüserD
HeilbronnR
2003
Adeno-associated virus integrates site-specifically into human chromosome 19 in either orientation and with equal kinetics and frequency.
J Gen Virol
84
133
137
18. McCartyDM
YoungSMJr
SamulskiRJ
2004
Integration of adeno-associated virus (AAV) and recombinant AAV vectors.
Annu Rev Genet
38
819
845
19. BerthetC
RajK
SaudanP
BeardP
2005
How adeno-associated virus Rep78 protein arrests cells completely in S phase.
Proc Natl Acad Sci U S A
102
13634
13639
20. SchmidtM
AfioneS
KotinRM
2000
Adeno-associated virus type 2 Rep78 induces apoptosis through caspase activation independently of p53.
J Virol
74
9441
9450
21. Di PasqualeG
ChioriniJA
2003
PKA/PrKX activity is a modulator of AAV/adenovirus interaction.
Embo J
22
1716
1724
22. HeilbronnR
BürkleA
StephanS
zur HausenH
1990
The adeno-associated virus rep gene suppresses herpes simplex virus-induced DNA-amplification.
J Virol
64
3012
3018
23. CortesML
OehmigA
SaydamO
SanfordJD
PerryKF
2008
Targeted integration of functional human ATM cDNA into genome mediated by HSV/AAV hybrid amplicon vector.
Mol Ther
16
81
88
24. ZhangC
CortezNG
BernsKI
2007
Characterization of a bipartite recombinant adeno-associated viral vector for site-specific integration.
Hum Gene Ther
18
787
797
25. WangH
LieberA
2006
A helper-dependent capsid-modified adenovirus vector expressing adeno-associated virus rep78 mediates site-specific integration of a 27-kilobase transgene cassette.
J Virol
80
11699
11709
26. HowdenSE
VoullaireL
WardanH
WilliamsonR
VadolasJ
2008
Site-specific, Rep-mediated integration of the intact beta-globin locus in the human erythroleukaemic cell line K562.
Gene Ther
15
1372
1383
27. RecchiaA
ParksRJ
LamartinaS
ToniattiC
PieroniL
1999
Site-specific integration mediated by a hybrid adenovirus/adeno - associated virus vector.
Proc Natl Acad Sci U S A
96
2615
2620
28. RecchiaA
PeraniL
SartoriD
OlgiatiC
MavilioF
2004
Site-specific integration of functional transgenes into the human genome by adeno/AAV hybrid vectors.
Mol Ther
10
660
670
29. WonderlingRS
OwensRA
1997
Binding sites for adeno-associated virus Rep proteins within the human genome.
J Virol
71
2528
2534
30. SchneppBC
JensenRL
ClarkKR
JohnsonPR
2009
Infectious molecular clones of adeno-associated virus isolated directly from human tissues.
J Virol
83
1456
1464
31. Penaud-BudlooM
Le GuinerC
NowrouziA
ToromanoffA
CherelY
2008
Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle.
J Virol
82
7875
7885
32. NakaiH
IwakiY
KayMA
CoutoLB
1999
Isolation of recombinant adeno-associated virus vector-cellular DNA junctions from mouse liver.
J Virol
73
5438
5447
33. Vincent-LacazeN
SnyderRO
GluzmanR
BohlD
LagardeC
1999
Structure of adeno-associated virus vector DNA following transduction of the skeletal muscle.
J Virol
73
1949
1955
34. MillerDG
RutledgeEA
RussellDW
2002
Chromosomal effects of adeno-associated virus vector integration.
Nat Genet
30
147
148
35. DyallJ
SzaboP
BernsKI
1999
Adeno-associated virus (AAV) site-specific integration: formation of AAV-AAVS1 junctions in an in vitro system.
Proc Natl Acad Sci U S A
96
12849
12854
36. RizzutoG
GorgoniB
CappellettiM
LazzaroD
GloaguenI
1999
Development of animal models for adeno-associated virus site-specific integration.
J Virol
73
2517
2526
37. BushmanF
LewinskiM
CiuffiA
BarrS
LeipzigJ
2005
Genome-wide analysis of retroviral DNA integration.
Nat Rev Microbiol
3
848
858
38. SchmidtM
SchwarzwaelderK
BartholomaeC
ZaouiK
BallC
2007
High-resolution insertion-site analysis by linear amplification-mediated PCR (LAM-PCR).
Nat Methods
4
1051
1057
39. WuX
LiY
CriseB
BurgessSM
2003
Transcription start regions in the human genome are favored targets for MLV integration.
Science
300
1749
1751
40. SchroderAR
ShinnP
ChenH
BerryC
EckerJR
2002
HIV-1 integration in the human genome favors active genes and local hotspots.
Cell
110
521
529
41. MeekingsKN
LeipzigJ
BushmanFD
TaylorGP
BanghamCR
2008
HTLV-1 integration into transcriptionally active genomic regions is associated with proviral expression and with HAM/TSP.
PLoS Pathog
4
e1000027
42. DrewHR
LockettLJ
BothGW
2007
Increased complexity of wild-type adeno-associated virus-chromosomal junctions as determined by analysis of unselected cellular genomes.
J Gen Virol
88
1722
1732
43. CheungAKM
HogganMD
HauswirthWW
BernsKI
1980
Integration of the adeno-associated virus genome into cellular DNA in latently infected Human Detroit 6 cells.
J Virol
33
739
748
44. KotinRM
BernsKI
1989
Organization of adeno-associated virus DNA in latently infected Detroit 6 cells.
Virology
170
460
467
45. ChioriniJA
YangL
SaferB
KotinRM
1995
Determination of adeno-associated virus Rep68 and Rep78 binging sites by random sequence oligonucleotide selection.
J Virol
69
7334
7338
46. MillerDG
TrobridgeGD
PetekLM
JacobsMA
KaulR
2005
Large-scale analysis of adeno-associated virus vector integration sites in normal human cells.
J Virol
79
11434
11442
47. BarskiA
CuddapahS
CuiK
RohTY
SchonesDE
2007
High-resolution profiling of histone methylations in the human genome.
Cell
129
823
837
48. HeintzmanND
HonGC
HawkinsRD
KheradpourP
StarkA
2009
Histone modifications at human enhancers reflect global cell-type-specific gene expression.
Nature
459
108
112
49. ChioriniJA
WienerSM
OwensRA
KyöstiöSRM
KotinRM
1994
Sequence requirements for stable binding and function of Rep68 on the adeno-associated virus type 2 inverted terminal repeats.
J Virol
68
7448
7457
50. ImD-S
MuzyczkaN
1989
Factors that bind to adeno-associated virus terminal repeats.
J Virol
63
3095
3104
51. McCartyDM
RyanJH
ZolotukhinS
ZhouX
MuzyczkaN
1994
Interaction of the adeno-associated virus Rep protein with a sequence within the A palindrome of the viral terminal repeat.
J Virol
68
4998
5006
52. RyanJH
ZolotukhinS
MuzyczkaN
1996
Sequence requirements for binding of Rep68 to the adeno-associated virus terminal repeats.
J Virol
70
1542
1553
53. OwensRA
WeitzmanMD
KyöstiöSRM
CarterBJ
1993
Identification of a DNA-binding domain in the amino terminus of adeno-associated virus rep proteins.
J Virol
67
997
1005
54. MacvilleM
SchrockE
Padilla-NashH
KeckC
GhadimiBM
1999
Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping.
Cancer Res
59
141
150
55. YoungSMJr
SamulskiRJ
2001
Adeno-associated virus (AAV) site-specific recombination does not require a Rep-dependent origin of replication within the AAV terminal repeat.
Proc Natl Acad Sci U S A
98
13525
13530
56. HamiltonH
GomosJ
BernsKI
Falck-PedersenE
2004
Adeno-associated virus site-specific integration and AAVS1 disruption.
J Virol
78
7874
7882
57. HenckaertsE
DutheilN
ZeltnerN
KattmanS
KohlbrennerE
2009
Site-specific integration of adeno-associated virus involves partial duplication of the target locus.
Proc Natl Acad Sci U S A
106
7571
7576
58. HickmanAB
RonningDR
PerezZN
KotinRM
DydaF
2004
The nuclease domain of adeno-associated virus rep coordinates replication initiation using two distinct DNA recognition interfaces.
Mol Cell
13
403
414
59. RussellDW
2003
AAV loves an active genome.
Nat Genet
34
241
242
60. BristerJR
MuzyczkaN
1999
Rep-mediated nicking of the adeno-associated virus origin requires two biochemical activities, DNA helicase activity and transesterification.
J Virol
73
9325
9336
61. JangMY
YarboroughOH3rd
ConyersGB
McPhieP
OwensRA
2005
Stable secondary structure near the nicking site for adeno-associated virus type 2 Rep proteins on human chromosome 19.
J Virol
79
3544
3556
62. BianchiME
AgrestiA
2005
HMG proteins: dynamic players in gene regulation and differentiation.
Curr Opin Genet Dev
15
496
506
63. CostelloE
SaudanP
WinocourE
PizerL
BeardP
1997
High mobility group chromosomal protein 1 binds to the adeno-associated virus replication protein (Rep) and promotes Rep-mediated site-specific cleavage of DNA, ATPase activity and transcriptional repression.
Embo J
16
5943
5954
64. HermonatPL
SantinAD
BatchuRB
ZhanD
1998
The adeno-associated virus Rep78 major regulatory protein binds the cellular TATA-binding protein in vitro and in vivo.
Virology
245
120
127
65. WegerS
WendlandM
KleinschmidtJ
HeilbronnR
1999
The adeno-associated virus type 2 regulatory proteins Rep78/Rep68 interact with the transcriptional coactivator PC4.
J Virol
73
260
269
66. NashK
ChenW
SalganikM
MuzyczkaN
2009
Identification of cellular proteins that interact with the adeno-associated virus rep protein.
J Virol
83
454
469
67. GoncalvesMA
van NieropGP
TijssenMR
LefesvreP
Knaan-ShanzerS
2005
Transfer of the full-length dystrophin-coding sequence into muscle cells by a dual high-capacity hybrid viral vector with site-specific integration ability.
J Virol
79
3146
3162
68. CathomenT
JoungJK
2008
Zinc-finger nucleases: the next generation emerges.
Mol Ther
16
1200
1207
69. ChioriniJA
WeitzmanMD
OwensRA
UrcelayE
SaferB
1994
Biologically active rep proteins of adeno-associated virus type 2 produces as fusion proteins in Escherichia coli.
J Virol
68
797
804
70. CathomenT
ColleteD
WeitzmanMD
2000
A chimeric protein containing the N terminus of the adeno-associated virus rep protein recognizes its target site in an In vivo assay.
J Virol
74
2372
2382
71. HeilbronnR
zur HausenH
1989
A subset of herpes simplex replication genes induces DNA amplification within the host cell genome.
J Virol
63
3683
3692
72. HüserD
WegerS
HeilbronnR
2003
Packaging of human chromosome 19-specific adeno-associated virus (AAV) integration sites in AAV virions during AAV wild-type and recombinant AAV vector production.
J Virol
77
4881
4887
73. KentWJ
2002
BLAT–the BLAST-like alignment tool.
Genome Res
12
656
664
74. DöringA
WeeseD
RauschT
ReinertK
2008
SeqAn An efficient, generic C++ library for sequence analysis.
BMC Bioinformatics
9
11
75. CuddapahS
JothiR
SchonesDE
RohTY
CuiK
2009
Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains.
Genome Res
19
24
32
76. RobertsonAG
BilenkyM
TamA
ZhaoY
ZengT
2008
Genome-wide relationship between histone H3 lysine 4 mono - and tri-methylation and transcription factor binding.
Genome Res
18
1906
1917
77. LangmeadB
TrapnellC
PopM
SalzbergSL
2009
Ultrafast and memory-efficient alignment of short DNA sequences to the human genome.
Genome Biol
10
R25
78. ZhangY
LiuT
MeyerCA
EeckhouteJ
JohnsonDS
2008
Model-based analysis of ChIP-Seq (MACS).
Genome Biol
9
R137
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



