-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
Complement forms an important arm of innate immunity against invasive meningococcal infections. Binding of the alternative complement pathway inhibitor factor H (fH) to fH-binding protein (fHbp) is one mechanism meningococci employ to limit complement activation on the bacterial surface. fHbp is a leading vaccine candidate against group B Neisseria meningitidis. Novel mechanisms that meningococci employ to bind fH could undermine the efficacy of fHbp-based vaccines. We observed that fHbp deletion mutants of some meningococcal strains showed residual fH binding suggesting the presence of a second receptor for fH. Ligand overlay immunoblotting using membrane fractions from one such strain showed that fH bound to a ∼17 kD protein, identified by MALDI-TOF analysis as Neisserial surface protein A (NspA), a meningococcal vaccine candidate whose function has not been defined. Deleting nspA, in the background of fHbp deletion mutants, abrogated fH binding and mAbs against NspA blocked fH binding, confirming NspA as a fH binding molecule on intact bacteria. NspA expression levels vary among strains and expression correlated with the level of fH binding; over-expressing NspA enhanced fH binding to bacteria. Progressive truncation of the heptose (Hep) I chain of lipooligosaccharide (LOS), or sialylation of lacto-N-neotetraose LOS both increased fH binding to NspA-expressing meningococci, while expression of capsule reduced fH binding to the strains tested. Similar to fHbp, binding of NspA to fH was human-specific and occurred through fH domains 6–7. Consistent with its ability to bind fH, deleting NspA increased C3 deposition and resulted in increased complement-dependent killing. Collectively, these data identify a key complement evasion mechanism with important implications for ongoing efforts to develop meningococcal vaccines that employ fHbp as one of its components.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1001027
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001027Summary
Complement forms an important arm of innate immunity against invasive meningococcal infections. Binding of the alternative complement pathway inhibitor factor H (fH) to fH-binding protein (fHbp) is one mechanism meningococci employ to limit complement activation on the bacterial surface. fHbp is a leading vaccine candidate against group B Neisseria meningitidis. Novel mechanisms that meningococci employ to bind fH could undermine the efficacy of fHbp-based vaccines. We observed that fHbp deletion mutants of some meningococcal strains showed residual fH binding suggesting the presence of a second receptor for fH. Ligand overlay immunoblotting using membrane fractions from one such strain showed that fH bound to a ∼17 kD protein, identified by MALDI-TOF analysis as Neisserial surface protein A (NspA), a meningococcal vaccine candidate whose function has not been defined. Deleting nspA, in the background of fHbp deletion mutants, abrogated fH binding and mAbs against NspA blocked fH binding, confirming NspA as a fH binding molecule on intact bacteria. NspA expression levels vary among strains and expression correlated with the level of fH binding; over-expressing NspA enhanced fH binding to bacteria. Progressive truncation of the heptose (Hep) I chain of lipooligosaccharide (LOS), or sialylation of lacto-N-neotetraose LOS both increased fH binding to NspA-expressing meningococci, while expression of capsule reduced fH binding to the strains tested. Similar to fHbp, binding of NspA to fH was human-specific and occurred through fH domains 6–7. Consistent with its ability to bind fH, deleting NspA increased C3 deposition and resulted in increased complement-dependent killing. Collectively, these data identify a key complement evasion mechanism with important implications for ongoing efforts to develop meningococcal vaccines that employ fHbp as one of its components.
Introduction
The complement system forms an important arm of innate immune defenses against Neisseria meningitidis. The presence of antibody-dependent complement-mediated serum bactericidal activity predicts protection against invasive disease [1]. Individuals deficient in components of the alternative or terminal complement pathways are predisposed to recurrent episodes of meningococcal infection [2], [3], [4], [5]. In order to survive in its human host, the meningococcus must evade killing by complement (either direct lysis by the terminal pathway or complement-dependent opsonophagocytosis).
Capsular polysaccharide expression is probably the most important determinant of meningococcal virulence. Expression of capsular polysaccharide renders the organism more serum resistant [6], [7], although the molecular basis for capsule-mediated serum resistance remains undefined. In addition, scavenging host complement inhibitors by meningococcal membrane proteins constitutes an important mechanism of subverting complement attack. Opc has recently been shown to bind to vitronectin [8] and contribute to serum resistance [9]. Porin (Por) A (PorA) binds to C4b-binding protein, although binding is best observed under hypo-osmolar conditions [10]. The molecule that has received much attention in recent literature is factor H-binding protein (fHbp; also known as LP2086 [11] or Genome-derived Neisserial Antigen (GNA) 1870 [12]) that binds to the alternative pathway inhibitor, factor H (fH) [13], [14]. FH acts as a cofactor in the factor I-mediated cleavage of C3b to the hemolytically inactive molecule iC3b [15], prevents the association of factor B with C3b thereby retarding the formation of the alternative pathway C3 convertase (C3b,Bb) and irreversibly dissociates the alternative pathway C3 convertase once it is formed [16], [17]. Based on its amino acid sequence, fHbp has been classified into 3 variants [12], or into 2 subfamilies [11], or more recently, into seven modular groups [18], [19]. Despite the fairly extensive fHbp sequence variation among strains, representative strains from each variant family bind to fH [13]. The co-crystal structure of variant 1 (subfamily B) fHbp with a fragment of fH revealed an extensive interaction surface of ∼2,860 Å2 [14]. fHbp is currently being evaluated as protein vaccine candidate against group B meningococcal disease and has shown promise in Phase III clinical trials [20].
In light of the use of fHbp as a vaccine, it is important to define alternative means of complement evasion that the meningococcus may employ, in particular scavenging fH. fHbp expression levels vary markedly across strains. Additional mechanisms to bind to host fH could undermine the efficacy of fHbp-based vaccines. In this report we have characterized Neisserial surface protein A (NspA) as a second acceptor molecule for fH on meningococci and have established its role in enhancing meningococcal serum resistance. It is noteworthy that NspA has received attention as a possible group B meningococcal vaccine; identification of a novel function for this protein highlights the potential utility of microbial fH binding molecules as vaccine antigens.
Results
fH binds to fHbp deletion mutants: evidence for an alternate fH ligand on meningococci
Human fH binds to the meningococcal surface molecule, fHbp and meningococcal strains, such as H44/76, do not show any detectable binding of fH by flow cytometry following deletion of fHbp ([13] and Figure 1A). However, we observed a small but reproducible, albeit statistically insignificant, binding of fH to fHbp mutants of some meningococcal strains, such as A2594, BZ198 and Z2087 by flow cytometry (grey shaded histograms, Figure 1A) relative to control histograms (Figure 1A, histograms depicted by broken lines). The amount of fH that bound to the fHbp deletion mutants of these strains was reduced compared to their wild-type fHbp expressing parents (histograms depicted by solid lines, Figure 1A). By contrast, fH binding to the fHbp deletion mutant of strain H44/76 was below the level of detection by FACS. These data indicate that some strains of meningococci may express a second molecule that binds to human fH.
Fig. 1. FH binding to fHbp mutants of select N. meningitidis strains and their isogenic capsule deficient mutants. 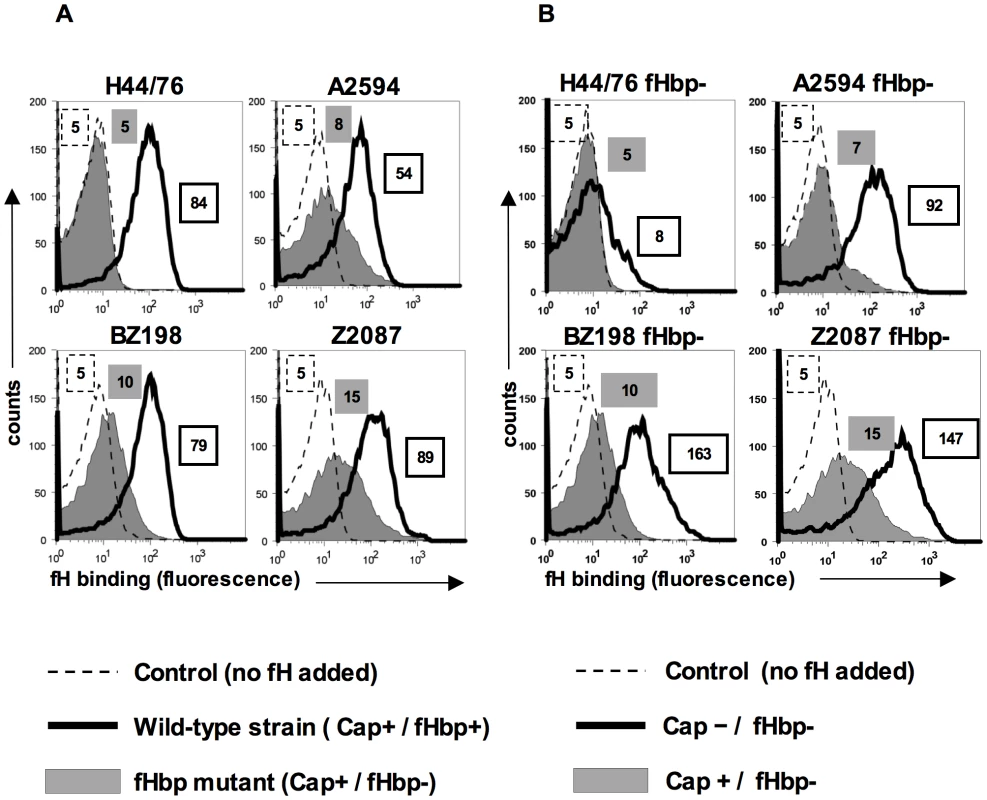
A. fH binding to wild-type meningococcal strains H44/76, A2594, BZ198 and Z2087 and their fHbp deletion mutants was examined by flow cytometry. Bacteria were incubated with purified human fH at a concentration of 20 µg/ml and bound fH was detected with polyclonal sheep anti-human fH. Representative controls with the wild-type strains where fH was omitted from the reaction mixture are shown by the broken line. The x-axis represents fluorescence on a log10 scale and the y axis is the number of events. Median fluorescence is indicated to the right of each histogram. B. Capsule expression hinders fH binding to fHbp deletion meningococcal mutants. fHbp deletion mutants of encapsulated strains H44/76, A2594, BZ198 and Z2087 and their isogenic unencapsulated mutants were incubated with fH at a concentration of 20 µg/ml and bacteria-bound fH was detected with sheep anti-human fH. fH binding to the encapsulated (Cap+) strains is shown by the shaded histogram and binding to the isogenic unencapsulated (Cap−) mutant is shown by the solid line. Representative controls with the wild-type strains where fH was omitted from the reaction mixture are shown by the broken line. The x-axis represents fluorescence on a log10 scale and the y axis the number of events. Median fluorescence is indicated to the right of each histogram. Expression of capsular polysaccharide decreases binding of fH to fHbp-negative meningococci
Capsule expression in N. meningitidis is subject to phase variation [21]. Down-regulation of capsule expression occurs during certain stages of pathogenesis, for example, while traversing the epithelial barrier [22]. Further, constitutively unencapsulated strains are commonly found as carriage isolates [23], [24], [25] and may contribute to the development of naturally acquired immunity. We have previously demonstrated that expression of capsule in group B meningococcal strain H44/76 reduces binding of the complement regulatory binding protein, C4b-binding protein (C4BP) by about 50% [10]. To determine if the expression of capsular polysaccharide similarly affects binding of fH to meningococci that lack fHbp expression, we assessed binding of fH to meningococcal strains in which capsule production had been abrogated. Deleting capsule from the fHbp mutants of strains A2594, Z2087, BZ198 and H44/76 revealed that isogenic capsule negative (Cap−) fHbp mutants bound more fH than their corresponding capsule expressing (Cap+) counterparts (Figure 1B). Consistent with previous observations [13], [26], deletion of capsule from meningococcal strains that expressed fHbp did not significantly alter binding of fH to meningococci (data not shown). While the fHbp mutants of A2594, Z2087 and BZ198 showed a marked increase in fH binding with loss of capsule, only a minimal increase in fH binding was seen in unencapsulated (Cap−) fHbp mutant of H44/76, suggesting that the second acceptor for fH was expressed in variable amounts across strains. These data show that meningococcal strains possess a molecule distinct from fHbp that serves as a ligand for human fH and that binding of fH to this molecule is inhibited, to some extent, by capsule expression.
LOS HepI chain length is inversely proportional to fH binding to fHbp-negative meningococci
LPS length can affect binding of complement inhibitors such as fH to bacteria [27]. In Neisseria, many of the genes involved in synthesis of lipooligosaccharide (LOS) are subject to reversible phase variation and a consequence is that the length of glycan extensions from HepI varies [28]. Previous work in our laboratory has shown that altering the length of glycan extensions from HepI affects binding of the complement inhibitor, C4BP, to gonococci [29]. To determine if HepI glycan extensions similarly affect binding of fH to its second ligand on meningococci, we studied the effects of truncating the glycan residues from HepI on fH binding to meningococci that lack fHbp. Wildtype strain A2594 expresses a lacto-N-neotetraose (LNT) extension from HepI and the wildtype LOS is not modified with sialic acid (LNT LOS sia-/Figure 2A, blue). Mutants that express a lactose extending from heptose I (lgtA mutants/L8 LOS/Figure 2A, green) or no saccharides off HepI (lgtF mutants/unsubstituted HepI LOS/Figure 2A, red) were created in the background of strains A2594 encapsulated (Cap+) and A2594 Cap−. As seen in Figure 2B, truncation of the HepI chain of LOS results in a progressive increase of fH binding to both Cap+ (left panel) and Cap − (right panel) meningococci. For a given LOS phenotype, the Cap+ mutant bound less fH than the corresponding Cap − mutant, confirming the observation above (Figure 1B) that capsule expression negatively impacted fH binding to the second receptor. In both Cap+ and Cap − backgrounds, the trend of increasing fH binding as the length of LOS HepI chain length decreases was statistically significant (Supplementary Table S1; p-value for trend test = 0.007). The inhibitory influence of capsule on fH binding to the second meningococcal fH receptor decreased as HepI LOS chain length decreased. For example, the differences in fH binding between the Cap+ and Cap − isogenic mutants were least apparent when the HepI of LOS was unsubstituted (red graphs in Figure 2B).
Fig. 2. Inverse relationship between fH binding to fHbp mutants and length of HepI glycans extensions. 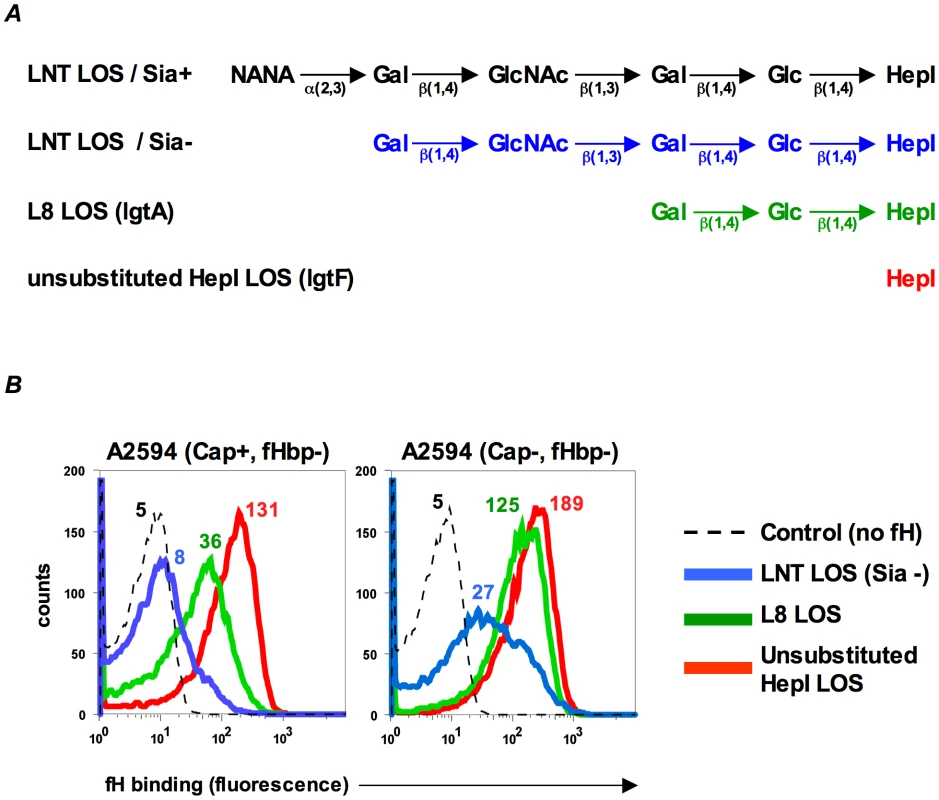
A. Schematic depicting the LOS HepI glycan extensions of the strains used in this study. B. Binding of fH (10 µg/ml) increases as LOS HepI chain length decreases. fH binding to Cap+ (left panel) and Cap− (right panel) isogenic mutants of A2594 that express either unsialylated LNT LOS (blue graphs), L8 LOS (green graphs) or unsubstituted HepI LOS (red graphs) was measured by flow cytometry. Numbers represent median fluorescence of fH binding from a single representative experiment; color corresponds to the color of the graph. Median fluorescence from three independent experiments was used to perform a Cuzick's nonparametric test for trend across ordered groups. The trend of fH binding increasing as HepI chain length decreases is statistically significant (p-value for trend test p = 0.007). The control (dashed histogram) represents a reaction mixture in which fH was excluded; controls with all mutants yielded similar results and a representative control obtained using the LNT LOS-expressing strain is shown. Axes are as described for Figure 1A. LOS sialylation enhances fH binding to fHbp-negative meningococci
In N. gonorrhoeae the modification of LNT LOS with sialic acid dramatically enhances the binding of fH [30], probably by increasing the access of fH to porin [31]. However, LOS sialylation has not been reported to enhance binding of fH to meningococci [26], [31]. Meningococcal strains that belong to groups B, C, W-135 and Y can endogenously sialylate their LNT LOS [32]. Group A strains do not have the capacity to synthesize 5′-cytidinemonophospho-N-acetylneuraminic acid (CMP-NANA; the donor molecule for sialic acid) and thus cannot endogenously sialylate their LNT LOS [33], [34]. However, they can scavenge CMP-NANA from the host to sialylate their LNT LOS. To determine if LOS sialylation affects binding of fH to fHbp negative meningococci we analyzed fH binding to fHbp − and Cap − mutants of group A strains A2594 and Z2087. The use of group A strains, that cannot endogenously sialylate their LOS, permitted us to study the effects of increasing amounts of LOS sialylation on fH binding by varying the amount of CMP-NANA added to growth media. As seen in Figure 3A, growth of both strains in CMP-NANA-containing media increased fH binding. Z2087 Cap − fHbp − was then grown in increasing amounts of CMP-NANA (Figure 3B); fH binding increased as CMP-NANA concentrations in the growth media were increased and maximal fH binding was achieved at 5 µg/ml of CMP-NANA. Taken together, the data presented thus far indicate that binding of fH to the putative meningococcal second fH receptor is enhanced by truncation of HepI glycan extensions, sialylation of LNT LOS or loss of capsule expression.
Fig. 3. Sialylation of lacto-N-neotetraose (LNT) LOS enhances binding of fH to fHbp-negative meningococci. 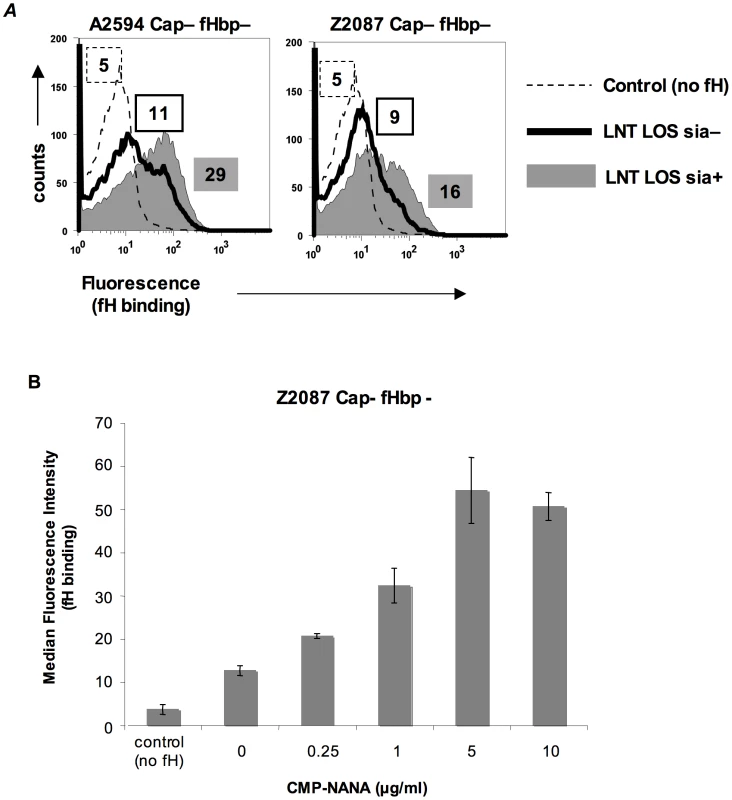
A. fH (10 µg/ml) binding to fHbp deletion mutants of unencapsulated (Cap−) derivatives of strains A2594 (upper panel) and Z2087 (lower panel) grown either with (sia+) or without (sia−) CMP-NANA (5 µg/ml) added to the growth media was measured by flow cytometry using anti-fH mAb 90×. Binding to sialylated (sia+) strains is shown by the shaded graphs and binding to strains without LOS sialic acid (sia−) by the solid line. Controls (no fH added) are shown by the dashed line. Numbers represent the median fluorescence of the corresponding histogram. B. fH binding to fHbp− LNT-bearing meningococci is dose dependent and saturatable. Z2087 mynB fHbp (Cap−, fHbp−, LNT LOS) was grown in media containing increasing concentrations of CMP-NANA ranging from 0 to 10 µg/ml. Bacteria were incubated with fH (20 µg/ml) and fH bound to bacteria was detected by flow cytometry using mAb 90×. The average median fluorescence from 3 independent experiments is plotted. Error bars represent standard deviations. The increases in fH binding were statistically significant (p-value<0.05) for all CMP-NANA concentrations prior to saturation. Controls and axes are as described in Figure 3A. Identification of NspA as the ligand for fH on fHbp-negative meningococci
Our studies indicate the presence of a second meningococcal receptor for human fH, distinct from the previously described fHbp. A Far Western ligand immuno-blotting assay was used to identify the putative second receptor molecule(s) present on fHbp deletion mutants of N. meningitidis. Membrane proteins prepared from strains A2594 (binds fH when its fHbp is deleted) and H44/76 (fHbp deletion mutant does not bind detectable amounts of fH by FACS) were separated on a 4–12% Bis-Tris gel and transferred to a PVDF membrane. Proteins that bound to fH were identified by probing the membrane with purified human fH and detecting bound fH with an anti-fH Ab (Figure 4A, right). An fHbp deletion mutant of each strain was used as a control. We focused on fH-reactive bands that were present in A2594 and A2594 fHbp−, but were either absent or expressed in reduced amounts on H44/76 and H44/76 fHbp−. A prominent fH-binding band of ∼17 kD was apparent in A2594 and A2594 fHbp−. This band was detected, but with lower intensity, in H44/76 and its fHbp deletion mutant (Figure 4A, right). This ∼17 kD band was not considered in our previous study where strain H44/76 was employed to identify fHbp as a fH binding molecule [13] because strain H44/76 expresses very low levels of this protein; we focused on the more prominent 29 kD fH-reactive band (fHbp) that was subsequently validated as the fH ligand on intact bacteria. A Coomassie blue stained gel showing the total membrane protein profile of each strain is shown for reference (Figure 4A, left).
Fig. 4. Identification of NspA as a meningococcal ligand for fH. 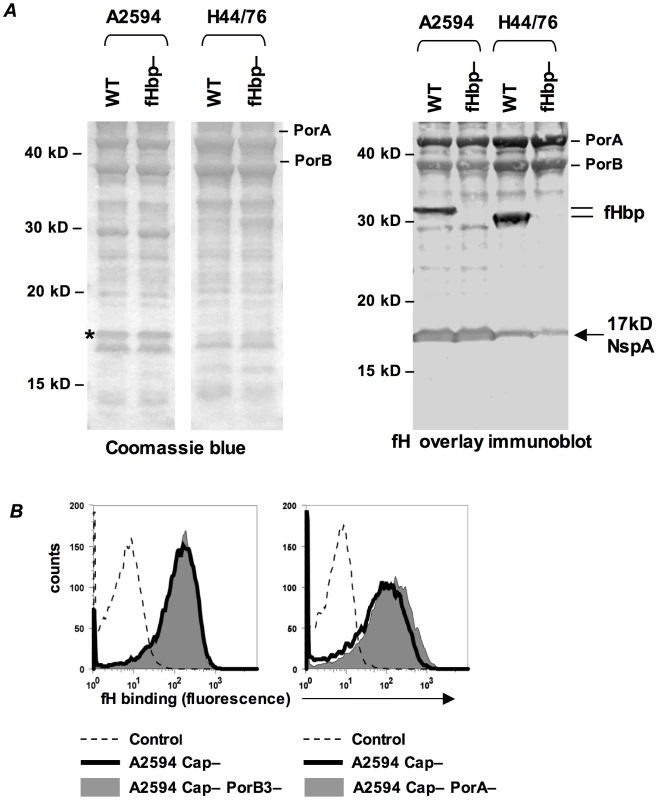
A. Membrane extracts from strains A2594 and H44/76 and their fHbp deletion mutants (fHbp−) were separated on a 4–12% Bis-Tris gel, transferred to a PVDF membrane by western blotting and probed with pure human fH (1 µg/ml). Bound fH was detected with sheep polyclonal anti-human fH. The locations of PorA, PorB and fHbp are indicated. A ∼17 kD molecule that also bound fH is indicated by the arrow. This protein was identified by MALDI-TOF analysis of the co-migrating band on a Coomassie blue stained gel (indicated by the asterisk) as NspA (see text). B. PorA and PorB are not ligands for fH on intact meningococci. porA and porB3 were deleted from the background of A2594 Cap− (right panel) and A2594 Cap− (left panel), respectively, and fH (10 µg/ml) binding was measured by flow cytometry using anti-fH mAb 90× as the detecting Ab. The shaded graph represents fH binding to the porin deletion mutants and the solid line represents fH binding to the parent strain. Controls and axes are as described in Figure 1A. To determine the identity of the ∼17 kD fH binding molecule, the region corresponding to the location of the ∼17 kD band was excised from a parallel Coomassie stained gel (indicated by the asterisk, Coomassie blue stained gel, Figure 4A) and this sample was subject to in-gel trypsin digestion and MALDI-TOF analysis followed by peptide mass fingerprinting that was compared with the Neisseria proteome. The protein band was defined as Neisserial surface protein A (NspA) using the Peptide Mass Fingerprint program for MS data and the MS/MS Ion Search program for CID data. The peptide ions covered 43% of the total protein sequence and no other statistically significant matches were identified. The data suggest that NspA could bind to human fH.
One caveat of a Far Western assay is that proteins presented in non-native conformations may interact in artificial ways with the ligand, in this case fH, and lead to the detection of “false positive” interactions. The data presented below indicate that NspA is likely the only additional fH ligand present in these strains and additional fH reactive bands present on the Far Western blot (Figure 4A, right) were not analyzed by MALDI-TOF.
Consistent with our previous observations [13], PorA and PorB also bound to fH on the western blot (Figure 4A, right). Purified H44/76 PorB3 binds to human fH by ELISA [35], but neither PorB3 nor PorA bind to fH in the context of intact H44/76 bacteria [13], [26], and we therefore did not anticipate these meningococcal porins to serve as ligands for fH on whole bacteria. The putative surface exposed loops of PorA and PorB show considerable variation across strains [36], [37], [38], [39] and thus it remained possible that the porin molecule(s) of A2594, but not H44/76, served as a ligand for fH. Deleting PorA or PorB3 from the background of A2594 Cap − did not diminish fH binding compared to the respective isogenic Por sufficient parent strains (right and left graphs of Figure 4B, respectively). This suggests that neither Por molecule contributed to fH binding to intact A2594 organisms and that the interaction of these proteins with fH in the Far Western assay is a “false positive”.
NspA shares structural similarities with the Neisserial opacity proteins (Opa) and we sought to determine if Opa might also bind fH. fH binding to an unencapsulated Z2087 strain that expressed Opa and its isogenic Opa negative mutant was indistinguishable (Supplementary data Figure S1), indicating that the Opa proteins were not ligands for fH.
NspA is a ligand for fH on intact meningococci
Several lines of evidence were used to independently verify that NspA is a ligand for fH on live, intact meningococci.
i) Deleting NspA abrogates fH binding to intact fHbp-negative bacteria
To determine the role of NspA in binding to human fH on intact fHbp-negative meningococci we constructed nspA deletion mutations in strains BZ198 and A2594. The BZ198 mutants were constructed in a Cap+ strain that expressed either sialylated LNT LOS (BZ198 is able to endogenously sialylate its LOS) or L8 LOS. The A2594 mutants were constructed in a Cap − strain expressing L8 LOS, to maximize binding of fH. All mutations were constructed in both fHbp+ and fHbp − backgrounds to compare the relative contribution of each of these ligands to fH binding. The expression of fHbp and NspA was verified in all strains by western blot using polyclonal anti-variant 1,2 and 3 fHbp antiserum and anti-NspA mAb Me-7, respectively (Figure 5A). Binding of fH to the nspA deletion mutants of BZ198 and A2594 was measured by flow cytometry and, as seen in Figure 5B, deleting nspA uniformly decreased fH binding to all strains. No residual fH binding to any of the strains was detected when both fHbp and NspA were deleted. These data, taken together with the direct binding of fH to NspA observed in the Far Western assay, confirmed that NspA served as a ligand for fH on intact bacteria. In addition, these data suggest that the increased fH binding observed in strains that express truncated (L8) LOS also occurred to NspA (i.e., no additional fH ligands were exposed).
Fig. 5. Deleting NspA decreases binding of fH to N. meningitidis. 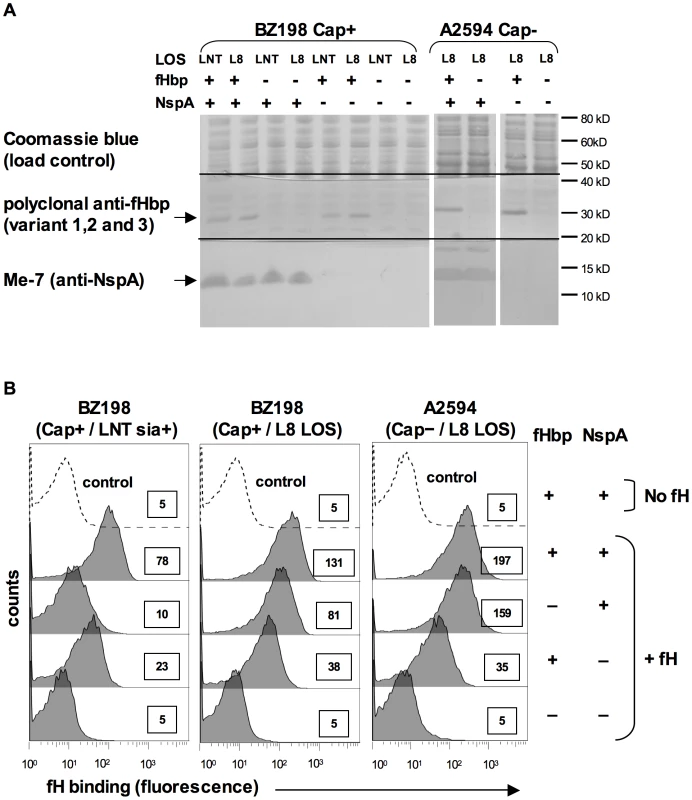
A. Expression of fHbp and NspA in BZ198 and A2594 derivatives as determined by western blotting of whole cell lysates followed by detection with polyclonal anti-fHbp (variant 1,2 and 3) or anti-NspA mAb Me-7 as indicated. After transfer, proteins migrating above ∼50 kD were stained with Coomassie blue and served as a loading control, proteins migrating between ∼20 kD and 40 kD were probed to detect fHbp and proteins migrating below 20 kD were probed to detect NspA. NspA migrates with an apparent molecular mass of approximately 15 kD when 4–12% Bis-Tris gels are used with MES running buffer. Also, of note, NspA is a heat-modifiable protein and the second larger anti-NspA-reactive band seen in some lanes is the result if incomplete heat denaturation. B. Strains BZ198 Cap+/LNT sia+, BZ198 Cap+/L8 LOS and A2594 Cap−/L8 LOS, and their fHbp, nspA or fHbp nspA double mutants were examined for their ability to bind to fH (20 µg/ml) by flow cytometry. The boxed numbers accompanying each histogram represents the median fluorescence of fH binding of the entire bacterial population. Controls (shown by the broken lines) represent fluorescence where fH was omitted from the reaction mixture; all strains yielded similar background binding and, for simplicity, only tracings obtained with the parent strains have been shown. Axes are as described for Figure 1A. LNT LOS sialylation enhanced binding of fH to fHbp-negative meningococci (Figure 3). To determine if the enhanced fH binding observed in sialylated fHbp-negative meningococci was dependent on NspA expression, we examined fH binding to an fHbp nspA double mutant. A2594 Cap − fHbp − NspA − with LNT LOS was grown in media containing CMP-NANA and, despite LNT LOS sialylation, there was no increase in fH binding noted by flow cytometry (data not shown). This upholds that the increased fH binding seen when the LNT LOS of the group A fHbp deletion mutants was sialylated (Figure 3A) was not because of fH binding to LOS sialic acid directly, but that the enhanced binding was dependent on the concomitant expression of NspA.
ii) Recombinant NspA expressed in E. coli binds to fH and binding of fH to NspA can be inhibited by an anti-NspA mAb
Microvesicles prepared from an E. coli strain expressing recombinant NspA [40] were used to demonstrate direct binding of fH to NspA by ELISA (Figure 6A). Dose-dependent binding of fH to NspA-containing vesicles was observed. In contrast, no binding of fH was observed to vesicles prepared from an E. coli strain harboring the plasmid without nspA at any of the fH concentrations tested. The specificity of fH binding to NspA containing vesicles was further validated by the ability of the anti-NspA mAb 14C7 to block binding of fH to vesicles containing NspA (Figure 6A).
Fig. 6. Recombinant NspA expressed in E. coli vesicles binds to fH. 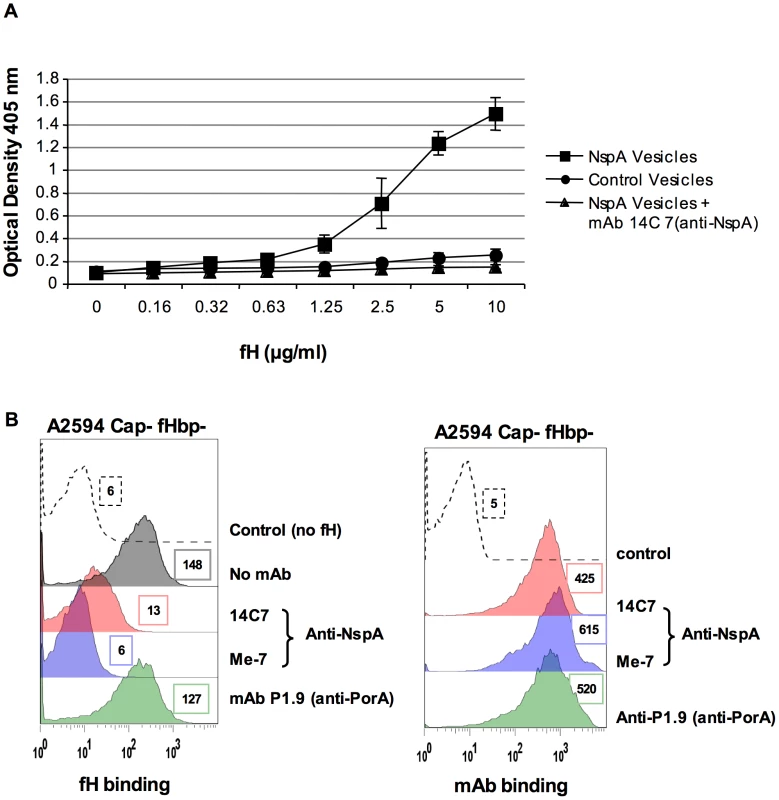
A. Binding of fH to microvesicles prepared from an E. coli strain expressing recombinant NspA (squares) or to vesicles prepared from the same E. coli strain transformed with the plasmid without the nspA gene (circles) was measured by ELISA. Binding of fH to vesicles harboring recombinant NspA was blocked by the anti-NspA mAb 14C7 (triangles). Each data point represents the arithmetic mean of the OD405nm reading from three independent experiments and error bars represent the standard deviation. B. Binding of fH to A2594 Cap− fHbp− LNT LOS sia− was measured in the presence of anti-NspA mAb Me-7 (purple shaded histogram), anti-NspA mAb 14C7 (pink shaded histogram) or anti-PorA mAb P1.9 (green shaded histogram); all mAbs were used at a concentration of 30 µg/ml and fH binding was detected using sheep anti-human fH. The control (histogram depicted by a dashed line) represents bacteria incubated with mAb Me-7, followed by addition of anti-fH and anti-sheep IgG FITC. A control where fH binding was measured in the absence of any added mAb is shown by the grey shaded histogram in the left panel. Surface binding of each mAb (shading as described above) to A2594 Cap− fHbp− LNT LOS sia− is also shown. The control (dashed line) represents bacteria incubated with anti-mouse IgG FITC. Median fluorescence is indicated to the right of each histogram. Additional evidence that NspA was an acceptor molecule for fH on the meningococcal surface was provided by the ability of the anti-NspA mAbs Me-7 and 14C7 to block binding of fH to strain A2594 Cap − fHbp − (Figure 6B). A control mAb (mAb P1.9) directed against the class I outer membrane protein porin A (PorA) did not affect fH binding to bacteria. Similar surface binding of the mAbs used, in the absence of fH, is shown in Figure 6B. Binding of fH in the absence of any added mAb was similar to that seen with mAb P1.9 (shaded grey histogram, left panel, Figure 6B).
iii) Increasing NspA expression enhances fH binding
NspA expression levels vary among meningococcal strains [41] and it appeared that the amount of fH binding mirrored expression levels. Consistent with the high levels of fH binding seen to their fHbp deletion mutants, strains BZ198, A2594 and Z2087 were also high NspA expressers as seen on western blot analysis of whole bacterial lysates (Figure 7A). Of the strains tested, H44/76 expressed the lowest levels of NspA, while C2120, W171 and Y2220 all expressed intermediate levels of NspA (Figure 7A). The results obtained by western blotting were confirmed by flow cytometry using anti-NspA mAb Me-7 (data not shown). Expression level of fHbp also varies among meningococcal strains [12] and fHbp expression in several strains is shown (Figure 7B). As noted previously, H44/76 is a high fHbp-expressing strain [13], [42] while fHbp expression in Y2220 barely exceeds the level of detection. The expression of fHbp and NspA did not vary within a strain in response to the genetic manipulations described herein (Supplementary Figure S2).
Fig. 7. fH binding increases with increasing NspA expression. 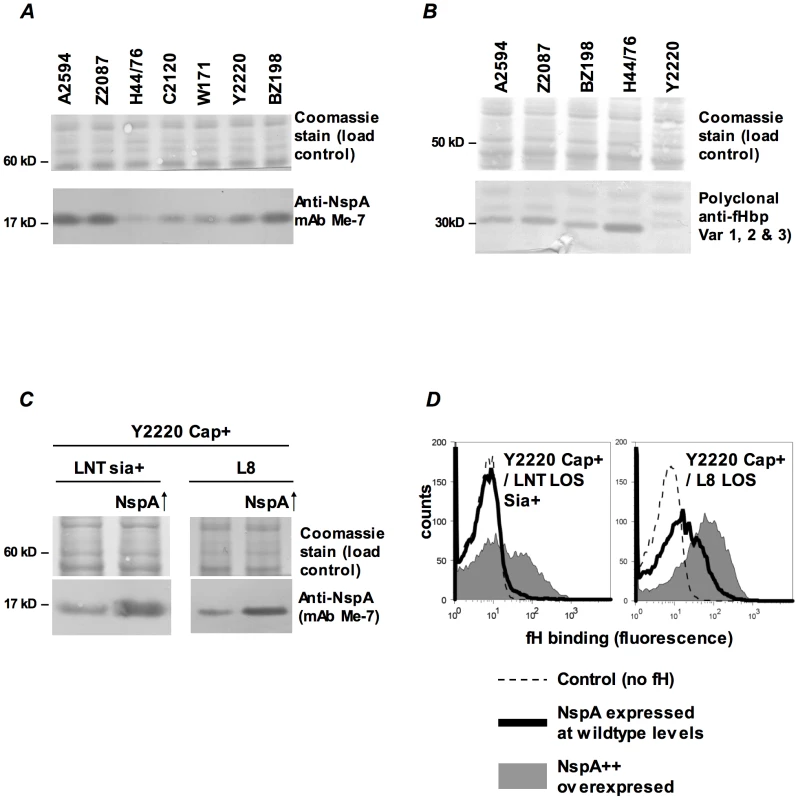
A. Comparison of NspA expression in Cap− derivatives of strains A2594, Z2087, H44/76, C2120, W171, Y2220 and BZ198 by western blotting of whole cell lysates followed by detection with anti-NspA mAb Me-7. After transfer, proteins migrating above 50 kD were stained with Coomassie blue and served as a loading control. B. Comparison of fHbp expression in strains A2594, Z2087, H44/76, BZ198 and Y2220 by western blotting of whole cell lysates followed by detection with polyclonal anti-variant 1, 2 and 3 fHbp. After transfer, proteins migrating above 40 kD were stained with Coomassie blue and served as a loading control. C. Overexpression of NspA. The porA promoter was used to increase NspA expression in the backgrounds of Y2220 Cap+/LNT sia+ and Y2220 Cap+/L8 LOS. Bacterial lysates were subject to western blotting and probed with mAb Me-7. Similar loading of parent and mutant strains was confirmed by Coomassie staining as described in A. D. Overexpression of NspA enhances fH binding to Y2220 Cap+/LNT sia+ and Y2220 Cap+/L8 LOS. fH binding to the parent strain, expressing wildtype levels of NspA, is shown by the solid line and binding to the NspA overexpressing isogenic mutant is shown by the grey shaded histogram. A representative control (no added fH) is shown by the dashed line. Axes are as described in Figure 1A. Fig. 8. Binding of fH/Fc fusion proteins to N. meningitidis fHbp and NspA mutants of A2594. 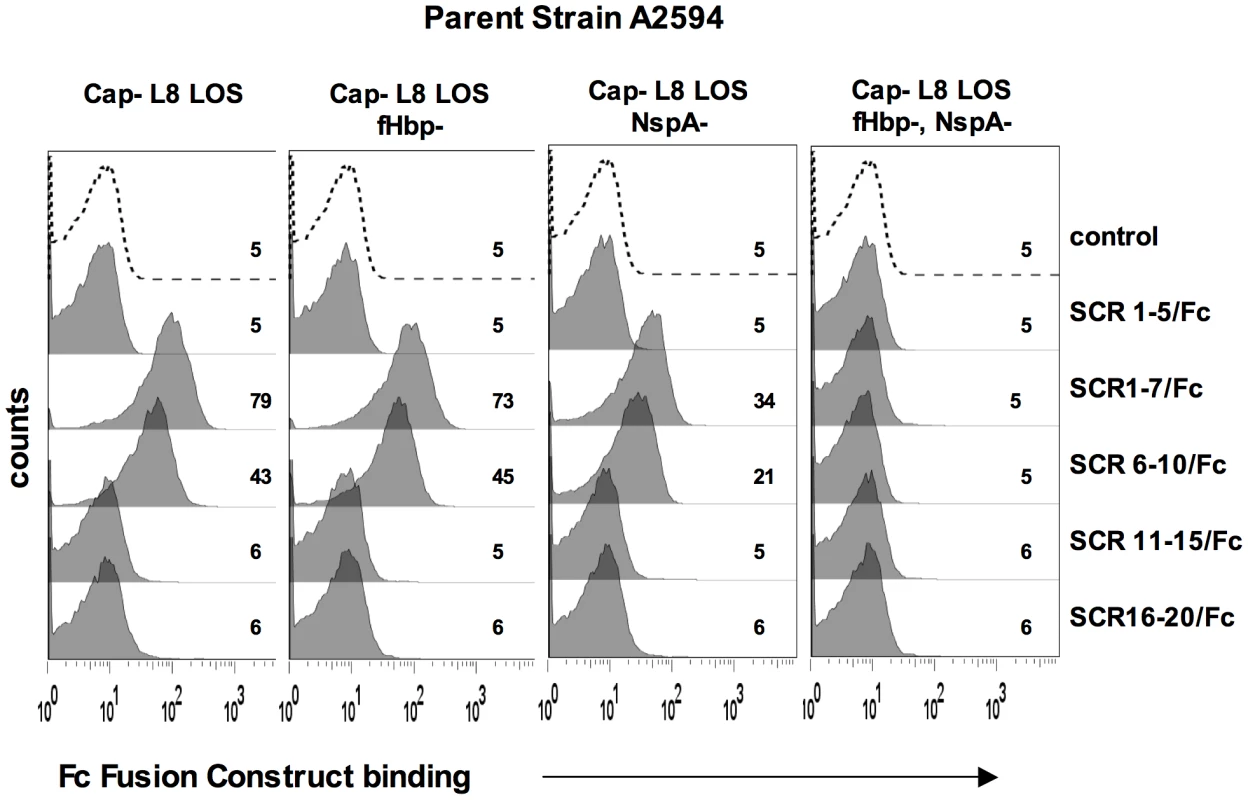
Binding of fH/Fc fusion constructs to A2594 Cap− L8 LOS and to its fHbp−, NspA− and fHbp− NspA− double mutants was assessed by flow cytometry using anti-mouse IgG FITC to disclosure the bound constructs. Strains that expressed either fHbp, NspA or both bound only to fH/Fc fusion constructs that contained SCR 6 and SCR 7 (SCR 1–7/Fc and SCR 6–10/Fc) of fH. In all graphs, the x-axis represents fluorescence on a log10 scale and the y-axis represents the number of events. No fusion protein is present in the control tube. One representative experiment of at least three independent experiments is shown. We hypothesized that increasing NspA expression would enhance fH binding. NspA expression was upregulated by placing nspA under control of the porA promoter in the background of wild-type strain Y2220 expressing sialylated LNT LOS (Cap+/LNT LOS sia+) and Y2220 Cap+/L8 LOS (Y2220 lgtA::kan). Strain Y2220 was chosen because it expresses very low levels of fHbp [13]. Thus, the effects of NspA over-expression on fH binding could be studied with minimal confounding background fH binding to fHbp. Enhanced NspA expression by the porA promoter was confirmed by western blotting (Figure 7C). Increased NspA expression was associated with increased fH binding (Figure 7D). Again, the strain that expressed the shorter (L8) LOS bound more fH that the isogenic mutant with LNT LOS.
Meningococcal strains such as, H44/76, C2120, W171 and Y2220 express low or intermediate levels of NspA, but do not bind fH when fHbp is deleted (data with H44/76 shown in Figure 1A and reference [13], Y2220 shown by solid black histogram in left panel of Figure 7D; data with C2120 Cap+ and W171 Cap+ are not shown). The Cap − mutants of these strains also show minimal fH binding when they express LNT LOS and when fHbp is deleted (Supplementary Figure S3). We speculated that further truncation of LOS in these strains would disclose fH binding. In accordance with this hypothesis, truncating LOS (lgtF mutants; HepI unsubstituted) resulted in increased fH binding (Supplementary Figure S3).
NspA mediates binding of fH to meningococci through interactions with fH short consensus repeats (SCRs) 6 and/or 7
FH comprises 20 short consensus repeat (SCR) domains arranged as a single chain [43]. Recently, the cocrystal complex of variant 1 fHbp with fH SCRs 6–7 showed an extensive area of interaction of fHbp with fH SCR 6 and minor points of contact with SCR 7 [14]. Site-directed mutagenesis studies also localized the fHbp binding domain in fH to SCR 6 [44]. To determine the fH SCRs involved in binding to NspA, we utilized fusion proteins that contain contiguous fH SCRs fused at their C-terminus to the Fc portion of IgG2a [44], [45]. The Fc fragment served as a ‘tag’ for symmetric detection of all fusion proteins. The ability of five fH/Fc fusion constructs (SCR 1–5/Fc, SCR 1–7/Fc, SCR 6–10/Fc, SCR 11–15/Fc and SCR 16–20/Fc) to bind to meningococcal strain A2594 Cap − L8 LOS and its isogenic fHbp−, NspA − and fHbp − NspA − double negative mutants, was examined by flow cytometry. Only those fH/Fc proteins that contained SCRs 6 and 7 (SCR 1–7/Fc, and SCR 6–10/Fc) bound to the NspA expressing strains that lacked fHbp. This result indicates that like fHbp, NspA binds to SCR 6 and/or 7. As expected, the SCR 6/7 containing constructs bound to fHbp expressing strains while none of the fH SCR/Fc constructs bound to mutants lacking both fHbp and NspA.
Factor H-like molecule 1 (FHL-1) comprises fH SCRs 1–7 plus four unique additional C-terminal amino acids (SFTL) [46]. FHL-1 also bound to Cap − fHbp − A2594 (Supplementary Figure S4), supporting the conclusion that SCRs 6 and/or 7 play a role in binding of fH to NspA. This finding is consistent with the NspA binding site residing in fH SCRs 6 and/or 7.
Together, these data suggest that SCR 6 and/or SCR 7 are important for binding of fH to NspA. Although less likely, these data do not unequivocally exclude a role for SCRs 8, 9 and 10 in binding of fH to NspA; studies to precisely localize the NspA binding region in fH are underway.
Species-specificity of fH binding to N. meningitidis expressing NspA
N. meningitidis and N. gonorrhoeae are exclusively human pathogens and the ability of these pathogens to evade complement-mediated killing in a species-specific fashion may contribute to the narrow host range of infection [45], [47], [48]. We have shown previously that gonococci bind specifically to human C4BP (and in some instances, chimpanzee C4BP) [48] and human fH [45]. Likewise, binding of fH to meningococcal fHbp is specific for human fH [47]. To determine if fH binding to NspA is also species specific we examined binding of fH from different primate species to N. meningitidis strain A2594 Cap − L8 LOS and its isogenic fHbp−, NspA − and fHbp − NspA − double-negative mutants by Western blotting (Figure 9A). Strains were incubated with 10% heat-inactivated human or primate sera to assess direct binding of fH to bacteria. Heat inactivation destroys heat labile complement components while leaving fH intact; inactivation of complement is necessary to prevent detection of complement C3b-mediated binding of fH to meningococci. Bound fH was detected by Western blot using polyclonal goat anti - human fH Abs. This Ab reacts with fH in the primate sera tested (Figure 9B) and as previously reported, detection of rhesus fH was slightly weaker [45]. Human fH bound well to all strains that expressed NspA (Figure 9A), but only weakly to strains that expressed fHbp but lacked NspA, which is consistent with the fH binding data present in Figure 6B. Very weak binding of chimpanzee fH to strains expressing NspA was also noted (Figure 9A). None of the strains tested bound rhesus fH when incubated with heat-inactivated rhesus sera (Figure 9A). The fHbp − NspA − strain showed barely detectable binding to human fH, and as expected, did not bind fH from the other primate species tested (Figure 9A).
Fig. 9. Meningococcal NspA binds selectively to human fH. 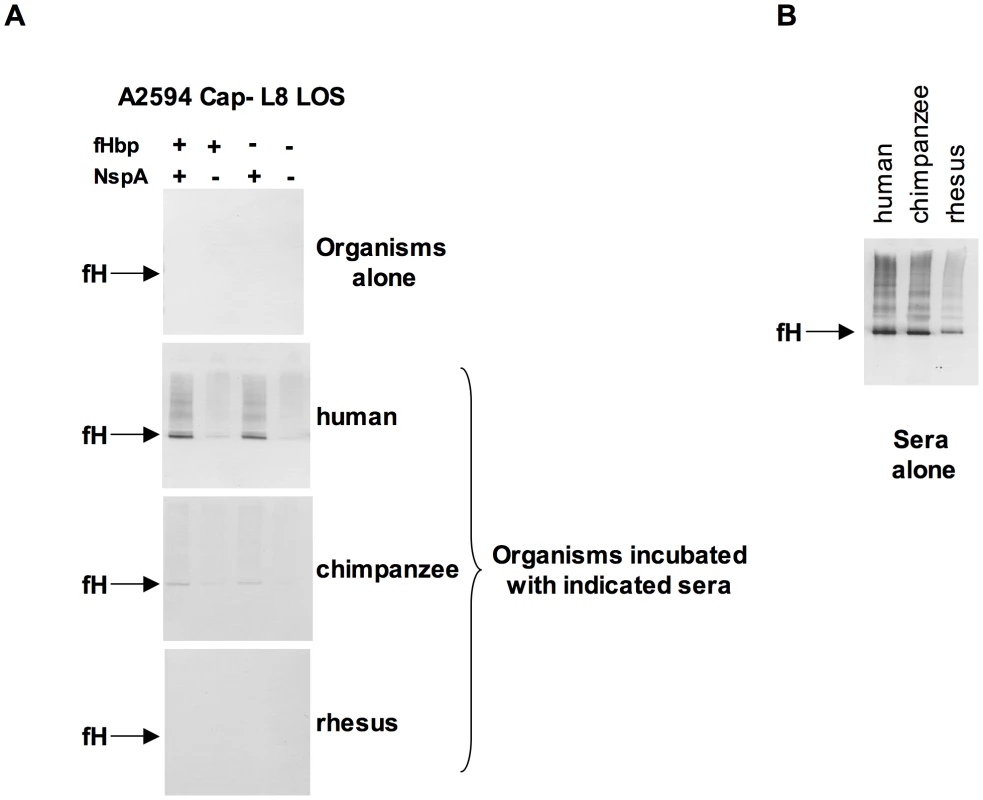
A. Unencapsulated N. meningitidis A2594 expressing L8 LOS and its fHbp−, NspA−, and fHbp−NspA− isogenic mutants were incubated with 10% (v/v) heat-inactivated human, chimpanzee and rhesus sera and Western blots were performed using polyclonal goat anti- human fH. B. Human, chimpanzee and rhesus serum controls at a dilution of 1/200 (v/v) were performed to ascertain that the goat polyclonal anti-human fH Abs recognized all primate fH tested. NspA expression enhances serum resistance and inhibits C3 deposition
fH functions to down-regulate the alternative pathway of complement and bacteria that bind to fH would be expected to be more resistant to the bactericidal action of serum than those that do not bind to fH. To determine the relative roles of fHbp and NspA in serum resistance we examined strains BZ198 Cap+ and A2594 Cap − each expressing L8 LOS and their isogenic mutants that lacked either fHbp or NspA or both for their ability to resist killing by normal human serum. The concentration of serum used was determined based on the survival of each parent strain in serum (Supplementary Figure S5). Loss of NspA expression in both instances resulted in greater sensitivity to complement-dependent killing (Figure 10A). It is noteworthy that in these high NspA-expressing strains, deleting fHbp did not negatively impact survival. Deleting fHbp from the high fHbp-expressing strain H44/76, which expresses low levels of NspA, results in decreased serum resistance [13], [42].
Fig. 10. NspA expression enhances resistance of meningococci to complement-dependent killing and limits C3 deposition on bacteria. 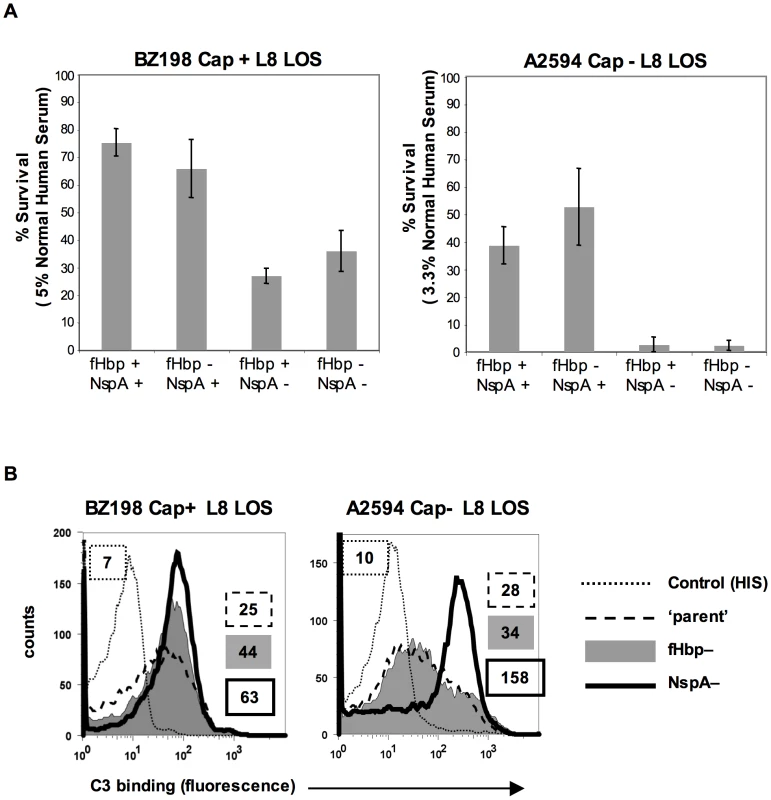
A. Strains BZ198 Cap+/L8 LOS and A2594 Cap−/L8 LOS and their isogenic mutant derivatives that lacked fHbp, NspA, or both fHbp and NspA were tested for their ability to resist killing by normal human serum in a serum bactericidal assay. The y-axis represents percent survival. Error bars indicate standard deviation calculated from 3 independent experiments. In all cases the decreased survival observed in strains that lack NspA was statistically significant (P<0.02 by a t-test) compared to the parent strain expressing NspA and fHbp B. C3 deposition on strains BZ198 Cap+/L8 LOS and A2594 Cap−/L8 LOS and their isogenic mutants that lacked either fHbp or NspA expression. The BZ198 Cap+ mutants were incubated with 40% NHS while the A2594 Cap− mutants were incubated with 20% NHS. C3 deposition on bacteria was detected by flow cytometry. Axes are as described for Figure 1A. Data with the double fHbp−/NspA− mutant was similar to the NspA− mutant and has been omitted for ease of visualization. fH limits C3 deposition by virtue of its ability to act as a cofactor in the factor I-mediated cleavage of C3b [15] and irreversibly dissociate alternative pathway C3 convertases (decay-accelerating activity) [16], [17]. As expected, mutant strains that lacked NspA bound more C3 than their NspA-sufficient ‘parent’ strains. The median fluorescence of C3 binding was ∼5-fold more with A2594 Cap−/L8 LOS/NspA − and ∼2.5-fold more with BZ198 Cap+/L8 LOS compared to their respective isogenic parent strains (Figure 10B). fH binding (Figure 5B) mirrored survival of bacteria in serum (Figure 10A) confirming that complement regulation by NspA occurred at the level of C3 deposition. Similarly, C3 deposition on BZ198 Cap+/LNT sia+/NspA − was ∼1.5-fold higher than on BZ198 Cap+/LNT sia+ (data not shown).
Complementation of fHbp − NspA − double mutants with NspA restores binding of fH and serum resistance to meningococci
Meningococcal strain A2594 Cap − L8 LOS lacking both fHbp and NspA was complemented, in trans, with NspAA2594 to verify that the loss in fH binding and concomitant decrease in serum resistance were not due to secondary changes. As expected, complementation with NspAA2594 resulted in expression of NspA as judged by both western blot (data not shown) and flow cytometry (Figure 11A). Restoration of NspA expression also restored the ability of fHbp − NspA − double mutants to bind fH (Figure 11A). The ability of the complemented strains to resist killing by NHS was assessed in a serum bactericidal assay. As shown above (Figure 10A), A2594 Cap − L8 LOS lacking both fHbp and NspA was more sensitive to serum killing than the parent strain expressing both of these proteins. Complementation with NspA, alone, restored serum resistance to the mutant strain lacking both fH ligands, albeit to levels less than that of the parent strain (Figure 11B). All three strains were completely (100%) killed in 6.6% NHS (data not shown). Overall, these data indicate that the lack of fH binding and decreased serum resistance observed in strains lacking NspA is because of lack of NspA expression and not the result of secondary changes in these isogenic strains.
Fig. 11. Complementation of fHbp NspA double mutants with NspA restores fH binding and serum resistance. 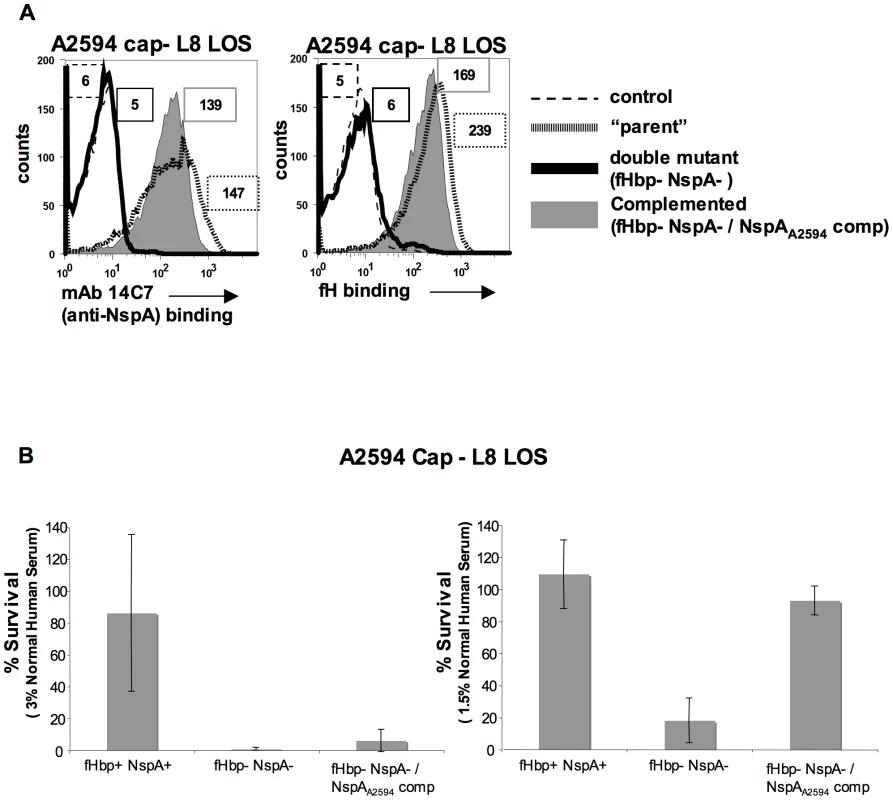
A. Meningococcal strain A2594 Cap−/L8 LOS (“parent”), its fHbp nspA double mutant (fHbp− NspA−) and its fHbp nspA double mutant complemented with NspA (fHbp− NspA−/NspAA2594 comp) were examined for their ability to bind to MAb 14C7 (anti-NspA) and to fH (20 µg/ml) by flow cytometry. The boxed numbers accompanying each histogram represents the median fluorescence of 14C7 or fH binding to the entire bacterial population. Controls (shown by the broken lines) represent fluorescence where either mAb 14C7 or fH or was omitted from the reaction mixture. Axes are as described for Figure 1A. B. Strain A2594 Cap−/L8 LOS (fHbp+ NspA+), its fHbp nspA double mutant (fHbp− NspA−) and its fHbp nspA double mutant complemented with NspA (fHbp− NspA−/NspAA2594 comp) were tested for their ability to resist killing by NHS at concentrations of 3% (left graph) or 1.5% (right graph) in a serum bactericidal assay. The y-axis represents percent survival. Error bars indicate standard deviation calculated from 3 independent experiments. With 1.5% NHS the decreased survival observed in strains that lack NspA was statistically significant (P<0.02 by a t-test) compared to the parent strain expressing both NspA and fHbp. Discussion
Several pathogens, including bacteria, fungi, parasites and viruses bind to fH, which inhibits complement activation on their surface (reviewed in [49], [50]). This work has characterized NspA as a ligand for human fH and has shown that NspA plays a role in conferring serum resistance to meningococci even in the absence of expression of the previously characterized fH-binding meningococcal molecule, fHbp. NspA interacts with fH SCRs 6 and/or 7 and like fHbp, preferentially binds to human fH.
It is interesting that all naturally occurring meningococcal strains reported thus far express both fHbp [12], [51] and NspA [52], suggesting an important role for these proteins in meningococcal pathogenesis. Prior to this study the function of NspA had not been defined. The factors that influence fH binding to NspA on intact bacteria have been characterized in this study, which provides insights into the pathophysiological conditions or niches where NspA-mediated fH binding may assume an important role. Meningococcal strains that are isolated from the nasopharynx are often unencapsulated and/or express L8 LOS [53]; high binding of fH to NspA under these conditions could point to a key role for NspA in survival of meningococci during nasopharyngeal colonization (a prerequisite of invasive disease) and in survival of carrier strains. The positive effects of NspA on bacterial survival are also seen in encapsulated strains that are high NspA expressers such as BZ198 when they express L8 LOS (Figure 10A). Meningococcal isolates often express more than one LOS species because many of the genes involved in LOS biosynthesis, including lgtA, are phase variable [28]. Thus, for example, a strain could express a combination of LNT and L8 LOS species [54], [55]. It is not clear how LOS sialylation, which represents elongation beyond the LNT structure, enhances fH binding to NspA. One possibility is that LOS and NspA lie in close proximity and expression of the unsialylated LNT hinders fH from binding to NspA; sialylation may alter the conformation of LOS thereby better exposing the fH binding region of NspA. Another possibility is that LOS sialic acid itself may act as part of the docking site for fH. Nevertheless, LOS sialylation is not essential for fH binding to NspA on intact organisms. Sialylation of N. gonorrhoeae LNT LOS also enhances fH binding, but the interaction in that instance requires the concomitant presence of the gonococcal PorB molecule [56]. LPS glycan extensions can negatively impact binding of complement inhibitors to gram-negative bacteria. As an example, expression of O-antigenic repeats on the LPS of Y. enterocolitica can block binding of fH to the Ail protein [27]. Neisseriae lack O-antigenic repeats, yet subtle changes in the core LOS structure can have profound impacts on the binding of complement inhibitors and serum resistance. The presence of the proximal Glc off HepI appears to be necessary for optimal C4b-binding protein (C4BP) binding to porin (Por) B.1B (Por1B)-expressing gonococci [29].
NspA forms an eight-stranded anti-parallel β-barrel and has four putative surface exposed loops. A conformational epitope that includes NspA loop 3 appears to be important for binding of mAbs Me-7 [57] and 14C7 [40] both of which inhibit fH binding to NspA on meningococci. It is therefore possible that NspA loop 3 plays a role in the interaction with fH, although steric hindrance by the surface-bound mAb could account for the ability of the mAbs to block fH binding. It would be of interest to determine whether NspA plays a role in fH binding to gonococci because of the extensive (∼95%) sequence similarity between gonococcal and meningococcal NspA.
Sera from humans may contain naturally-occurring antibodies that are directed against LNT-expressing LOS and is bactericidal against group B meningococci [58]. Phase variation of lgtA that results in L8 LOS expression could subvert killing by these naturally occurring anti-LNT antibodies. However, truncation of the HepI chain of LOS could have a negative effect on serum resistance because of increased accessibility of the 3-phosphoethanolamine (PEA) residue on HepII to C4b [59]; C4b amide-linked to PEA can lead to downstream complement activation that may result in bacterial killing. By virtue of enhanced fH binding, high NspA expressers may be able to dampen excessive complement activation that is initiated by C4b when LOS is truncated.
It is noteworthy that loss of NspA (leaving fHbp intact) from a high NspA expressing strain such as A2594 (intermediate fHbp expression levels) resulted in increased C3 deposition on bacteria, while loss of fHbp (leaving NspA intact) in that strain did not enhance C3 deposition (Figure 10B). Strain BZ198 also expresses high levels of NspA and intermediate levels of fHbp; loss of NspA resulted in greater enhancement of C3 deposition relative to that seen when fHbp was deleted (Figure 10B). We have shown previously that loss of fHbp in high-fHbp expressing strains such as H44/76 (expresses low levels of NspA) also increases C3 deposition [13], [42]. The relative abilities of the two ligands to regulate C3 deposition on different strains may reflect heterogeneity in their expression levels. In addition, variables such as the amount of capsule expression and the diversity of HepI LOS extensions could affect the amount of fH binding to NspA and thereby its ability to regulate C3 deposition. The relative roles of fHbp and NspA in regulating complement activation in the context of expression of the different capsular groups and varying LOS structures is a complex subject that merits further study. However, it is evident from the current study and from previous work [13], [42], [60], [61] that both molecules contribute to the ability of meningococci to resist killing by normal human serum.
fHbp has shown considerable promise as a vaccine candidate [20]. A vaccine that has fHbp as a component could lead to selection of meningococcal strains that either do not express, or express very low amounts of fHbp. Under such circumstances, high NspA expressers may have a survival advantage. Our data suggest that including NspA as part of a vaccine strategy that targets fH-binding proteins on N. meningitidis could, in theory, overcome this potential obstacle. Indeed, NspA has been intensively investigated as a vaccine candidate against group B meningococci [62], [63]. Both mAbs against NspA (including Me-7 and 14C7) and polyclonal Abs against NspA (raised by immunization of mice with meningococcal outer membrane vesicles that contained native NspA) were bactericidal and protected against experimental murine infection [52], [64]. Although recombinant NspA expressed in E. coli and purified from inclusion bodies elicited a good antibody response in humans, these antibodies were not bactericidal [62]. Recombinant NspA does not have the same conformation as NspA present in the meningococcal outer membrane [40], [64], suggesting that protective antibodies may be directed against conformational epitopes. Another intriguing possibility, in light of our observations that binding of fH to NspA is restricted to humans, is that human fH may bind to NspA in the vaccine formulation, which could have attenuated the antibody response to surface-exposed (and fH binding) NspA epitopes that otherwise would have elicited a more productive bactericidal antibody response; this possibility has been raised previously with regard to the use of fH binding proteins as vaccines [14], [65].
In summary, we have identified an important complement-evasion function for NspA, an antigen that has been studied for its potential as a group B meningococcal vaccine candidate. In addition to the implications for fHbp-based vaccines that are currently being developed, these findings set the stage for further studies to characterize NspA-fH interactions that could boost efforts to develop better meningococcal vaccines.
Materials and Methods
Ethics statement
This study was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical School. All subjects who donated blood for this study provided written informed consent.
Bacterial strains, mutagenesis and bacterial growth conditions
The relevant phenotypes of the mutants created in N. meningitidis are listed in Table 1. The characteristics meningococcal strains used in this study are listed in Supplementary Table S2. Bacteria were routinely grown on chocolate agar plates supplemented with IsoVitaleX equivalent at 37°C in an atmosphere enriched with 5% CO2. GC plates supplemented with IsoVitaleX equivalent were used for antibiotic selection. Antibiotics where used at the following concentrations when indicated; 100 µg/ml kanamycin, 7 µg/ml chloramphenicol, 5 µg/ml erythromycin, 50 µg/ml spectinomycin and 5 µg/ml tetracycline. Escherichia coli strains (Invitrogen, Carlsbad, CA) were routinely cultured in Luria Bertani (LB) broth or on LB agar. Antibiotic were used as needed at the following concentrations: 50 µg/ml kanamycin, 150 µg/ml ampicillin, 50 µg/ml chloramphenicol, 400 µg/ml erythromycin, 100 µg/ml spectinomycin and 12.5 µg/ml tetracycline.
Tab. 1. Description of genotype and phenotype of mutations used in this study. 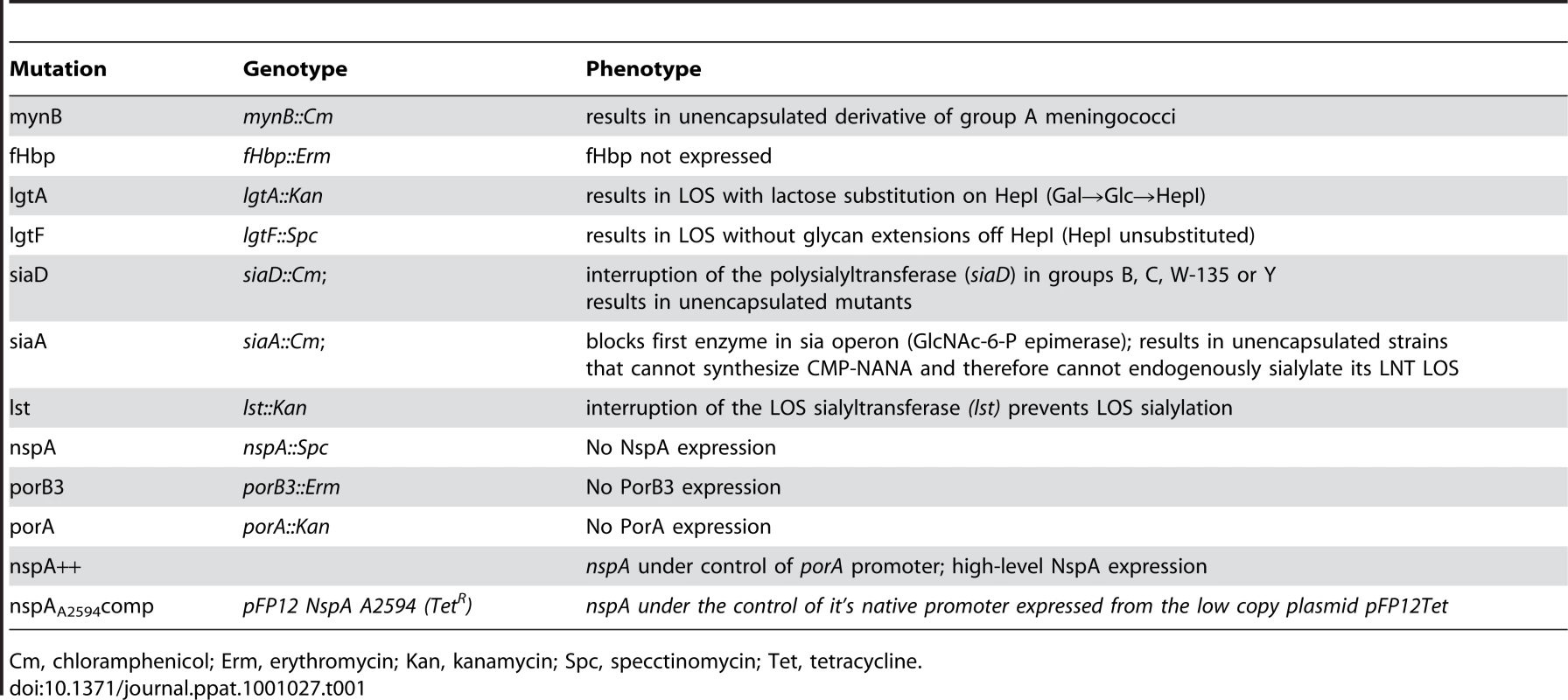
Cm, chloramphenicol; Erm, erythromycin; Kan, kanamycin; Spc, specctinomycin; Tet, tetracycline. Construction of lgtA [59], lgtF [59], mynB [56], siaD [59], lst [66] and fHbp [13] deletion mutants have all been described previously. Loss of capsule expression was verified by either colony hybridization or flow cytometry with the appropriate serogroup specific anti-capsule Ab. Anti-group A mAb JW-A-1 (IgG2a), anti-group C mAb KS-C-1 (IgG3), anti-group W-135 mAb JW-W1b (IgG2b) and anti-group Y mAb JW-Y2a (IgM) were provided by Dr. Dan M. Granoff (Childrens Hospital Oakland research Institute, Oakland, CA), while anti-group B mAb 2-2-B (IgM) was obtained from the National Institute for Biological Standards and Control (Potters Bar, Hertfordshire, U.K). In addition, inactivation of siaD (mynB in the case of group A) was verified by PCR. For serogroups A, C, Y and W-135 the confirmatory PCR was the amplification of a fragment corresponding to the predicted size of siaD (or mynB for group A) plus the resistance marker (∼2.5kb for serogroup A using primers NT2 5′-ATGATGGTAATGGGAAAAGAGT-3′ and NT4 5′-ATACTTAATAACAGAAAATGGCG-3′; ∼2.9 kb for serogroup C using primers BF 5′-AGCGTCAACGAATATGAAACATTAT-3′ and CR 5′-CTGCTTAACTTTATTAAGGGCATTG-3′ and ∼2.8 kb from serogroups W-135 and Y strains using primers W1618 5′-ATTCCCCATGAACTACATCAGAATA-3′ and W2766 5′-TAATGCAAACTCAATTGCAAAACTA-3′) coupled with the absence of a wildtype gene. In serogroup B strains siaD is inactivated with the 8.9 kb Tn1725 and a lack of amplification of the wild type siaD, in the presence of a positive control reaction, was used to demonstrate the lack of the wild type siaD. All Cap − serogroup B strains were verified by lack of reactivity to the anti-group B capsule mAb 2-2-B. LOS structure was verified in all strains and mutants by silver staining of protease K digested bacterial lysates that had been separated on 12% Bis-Tris gels (Invitrogen, Carlsbad, CA) using MES buffer (Invitrogen, Carlsbad, CA) as described previously [67]. In addition insertions in lgtA or lgtE were verified by PCR.
Mutant derivatives of strain A2594 that lacked PorA or PorB3 expression were constructed using DNA extracted from PorA and PorB3 deletion mutants in strain H44/76 (porA::kan and porB3::erm, respectively) that were provided by Dr. Peter Van der Ley (Laboratory of Vaccine Research, Netherlands Vaccine Institute, Bilthoven, The Netherlands).
NspA deletion mutants were constructed as follows. A 1.3 kb fragment of DNA containing nspA was amplified from N. meningitidis strain A2594 using the primers nspA_F114 (5′-CTCTTTAGGTTCTGCCAAAGGCTTC-3′) and nspA_R1122 (5′-ATGTTGTGAAGTGGGAAAGTGTTGC-3′) and the amplicon was cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, CA). The resulting plasmid was digested with HincII, deleting an internal 130 bp fragment of nspA, and ligated to a blunt spectinomycin resistance cassette containing aadA. Linearized plasmid DNA was used to transform N. meningitidis strains as previously described [68]. PCR was used to confirm the nspA::spc genotype and Western blot using anti-NspA mAb Me-7 were performed to demonstrated loss of NspA.
A derivative of the E. coli-Neisseria shuttle vector pFP12 was used to complement the nspA::spc mutations in trans. A 1,131 bp fragment, containing nspA with its native promoter and terminator was amplified from chromosomal DNA prepared from strain A2594 using the primers NspA-R1213 StuI 5′-GACAGGCCTGTTTTGGACATTTCGGATTCCTC-3′ and NspA-F102 SphI 5′-GACGCATGCCACTATATAAGCGCAAACAAATCG-3′. The amplified DNA was digested with StuI and SphI and cloned into identically digested pFP12-GNA1870 [69]. The resulting construct was then digested with ScaI to allow for the insertion of a blunt (BsrBI) TetM cassette. The resulting plasmid construct, pFP12 NspAA2594Tet, was confirmed by DNA sequencing and by Western blot analysis of E. coli cell lysates using ME-7. A2594 Cap − L8 (CmR, KanR) and its fHbp − (ermR) and NspA − (spcR) and fHbp − NspA − double mutants were transformed with pFP12 NspAA2594Tet as described above. Tetracycline resistant transformants were screened by PCR and Western blot with Me-7. In addition the DNA sequence of the complementing nspA was verified.
Expression of NspA was up-regulated in some strains that naturally express low levels of NspA by replacing the nspA promoter with the promoter of porA. In brief, an approximately 200 bp fragment of DNA containing the promoter region of porA was amplified by PCR from genomic DNA isolated from group B strain M986 using the following primers: 5′-CTCATCGATGGGCAAACACCCGATACG-3′ (introducing site ClaI) and 5′-CTCACGCGTGAGGTCTGCGCTTGAATTGTG-3′ (introducing site MluI). This fragment was ligated into a 700 bp region upstream of nspA amplified by PCR from Z1092 genomic DNA using primers 5′-CATAAGCTTCGTAGCGGTATCCGGCTGC-3′ and 5′-CGCTGCCGAAGATTTGCCGGCAAATCTTCGGCAGCG-3′. This, in turn, was ligated into the EcoRI and HindIII sites of the cloning vector pGEM3zf(-) (Promega Corporation, Madison, Wisconsin). An erythromycin resistance cassette, ermB, was inserted upstream of the porA promoter within the fragment upstream of nspA. N. meningitidis strain Z1092 was transformed by adding plasmid DNA in 10 mM MgCl2 to colonies of Z1092 and incubating at 37°C enriched with 5% CO2 for 5 hours prior to plating onto chocolate agar with 5 µg/ml or erythromycin. The level of NspA expression in erythromycin-resistant colonies was analyzed by SDS-PAGE and Western blotting using murine antisera raised against His-tagged-NspA. All analyzed transformants expressed approximately 5-times the level of NspA when compared with the wild-type strain (data not shown). DNA extracted from Z1092 overexpressing NspA was used to transform strain Y2220 and Y2220/L8 LOS; colonies resistant to erythromycin were screened for increased NspA production compared to the parent strain by Western blotting.
E. coli BL21 (DE3) pGMS 1.0 harboring a functional copy of nspA and the preparation of microvesicles that contain NspA have been described previously [40].
For simplicity, the capsule and LOS phenotype of each mutant has been designated as follows: encapsulated strains, Cap+; unencapsulated mutants, Cap−; sialylated lacto-N-neotetraose LOS, LNT sia+; unsialylated lacto-N-neotetraose LOS, LNT; LOS with lactose extension off HepI (lgtA mutants), L8 LOS and LOS with no HepI saccharide extensions (lgtF mutants), HepI unsubstituted.
Sera
Serum collected from a healthy human volunteer without a history of meningococcal disease and who had not received any meningococcal vaccines (normal human serum; NHS) was aliquoted and stored at −80°C till used. Hemolytic activity of the serum was confirmed using the Total Haemolytic Complement assay (Binding Site, Birmingham, U.K). Chimpanzee, baboon and rhesus sera were purchased from Bioreclamation (Bioreclamation, Hicksville, NY). Complement activity in the sera was destroyed by heating at 56°C for 30 minutes. The serum used did not contain any fHbp - or NspA-specific antibodies as revealed by western blots of whole bacterial lysates that were probed with serum.
Flow cytometry
Flow cytometry to detect bound fH was performed as described previously [44]. Briefly, bacteria grown overnight on chocolate agar plates were washed with Hanks Balanced Salt Solution (HBSS) containing 1mM Ca2+ and 1 mM Mg2+ (HBSS++) and suspended to a final concentration of 3×108 cells/ml; 108 organisms were centrifuged and incubated with fH purified from human plasma (Complement Technology, Inc.; concentration specified for each experiment). Bound fH was detected using either affinity-isolated sheep anti-human fH (Lifespan Biosciences) or an anti-fH mAb (Quidel, catalog no. A254 (mAb 90×)), as available. While the polyclonal antibody provided higher sensitivity, relative differences in fH binding among strains using the two reagents were similar. FITC conjugated anti-sheep IgG or anti-mouse IgG (Sigma) were used as secondary antibodies. All reaction mixtures were carried out in HBSS++/1% BSA in a final volume of 50 µl. Flow cytometry was performed using a FACSCalibur instrument (Becton Dickinson) and data analysis was performed using the FlowJo data analysis software package (www.TreeStar.com).
FH / murine Fc fusion constructs that contain contiguous fH SCR domains (SCRs 1–5, 1–7, 6–10, 11–15, or 16–20) fused to the N-terminus of the Fc fragment of murine IgG2a (fH/Fc fusion proteins) have been described in detail previously [45]. To detect binding of recombinant fH/Fc fusion proteins, bacteria were incubated with concentrated tissue culture supernatant containing 0.5 µg of recombinant fH/Fc protein (as determined by ELISA) in a final reaction volume of 100 µl for 30 min at 37°C. After washing, FITC-labeled goat anti-mouse IgG (Sigma-Aldrich) diluted 1∶100 in 1% BSA/HBSS++ was used to detect bacteria-bound fH/Fc fusion proteins.
Recombinant Factor H-like protein-1 (FHL-1) was generated as previously described [44]. Following incubation of bacteria with 0.5 µg purified FHL-1, bound FHL-1 was detected using monoclonal (mAb) 90× (specific for SCR1; detects both full-length fH and FHL-1) and FITC-conjugated anti-mouse IgG (Sigma) as previously described [44].
In some experiments, mAbs against NspA (mAb Me-7; IgG2a [57] and 14C7 [40]) were used to block fH binding to intact bacteria; mAb P1.9 against outer membrane protein PorA of strain A2594 (National Institute of Biological Standards and Control) was used as a control. Bacteria were incubated with tissue culture supernatants containing mAbs Me-7 or P1.9 (the concentration of mAb in supernatants was estimated by western blotting where serial dilutions of the supernatants were compared against purified mouse IgG standards of the same subclass) or purified mAb 14C7 for 15 min at 37°C followed by addition of purified fH. The reaction mixture was incubated for an additional 15 min and bound fH was detected using sheep anti-human fH as described above.
C3 deposition on bacteria that were incubated with normal human serum (concentration specified for each experiment) was measured using FITC-conjugated sheep anti-human C3 (Biodesign/Meridian Life Science, Inc.) as described previously [13].
Bacterial membrane preparations
Membranes were prepared from N. meningitidis strains A2594 and H44/76 and their fHbp deletion mutants as previously described [13], [70]. Briefly, bacteria harvested from five plates after overnight culture on chocolate agar were suspended in normal saline. Bacteria were washed, suspended in 5 ml of PBS containing 10 mM EDTA, and incubated at 60°C for 30 min. The bacterial suspensions were sheared by sequential passage through progressively smaller-gauge needles (18 - through 25-gauge). The resultant suspension was centrifuged at 5000×g for 10 min at 4°C to separate any intact cells and debris. The supernatant was collected and ultracentrifuged at 80,000×g for 90 min at 4°C to yield a pellet that was enriched in outer membranes.
Western blotting
Far Western blotting was used to assess fH binding to membrane preparations as described previously [13]. Membrane proteins were separated on a 4–12% Bis-Tris gel (Invitrogen Life Technologies) using MOPS running buffer. Proteins were transferred to polyvinylidene difluoride membranes (Millipore) and blocked with PBS-1% dry milk for 30 min at room temperature. Blocked membranes were incubated overnight at 4°C with fH (1 µg/ml in PBS-0.05% Tween 20). fH-binding proteins were detected using affinity-isolated sheep anti-human fH (1 µg/ml in PBS-0.05% Tween 20) and disclosed using anti-sheep IgG-alkaline phosphatase.
Western blotting was also used to assess NspA and fHbp expression. To detect NspA, membranes were probed with tissue culture supernatants that contained anti-NspA mAb Me-7 followed by anti-mouse IgG alkaline phosphatase. To detect fHbp (variant 1, 2 and 3), membranes were probed with rabbit polyclonal anti-serum that recognized variants 1, 2 and 3 fHbp diluted 1∶1000 in TBS 0.02% Tween 20 followed by anti-rabbit IgG alkaline phosphatase. To ensure equal loading across lanes, membranes were incised horizontally at the level of the ∼40–50 kD marker prior to the blocking step and stained with Coomassie blue (Imperial Protein Stain kit, Pierce).
Binding of human, chimpanzee and rhesus macaque fH to neisserial strains was measured by Western blotting as described previously [45]. Briefly, 108 bacteria were suspended in HBSS2+ and incubated for 30 min at 37°C with 10% (v/v) heat-inactivated NHS or heat-inactivated chimpanzee or rhesus serum in a final reaction volume of 100 µl. Bacteria were washed three times in HBSS2+, the bacterial pellets were lysed with lithium dodecyl sulfate sample buffer (Invitrogen, Carlsbad CA) and liberated fH was detected after electrophoresis and transfer to PVDF using goat polyclonal anti-human fH (Bethyl Laboratories, Montgomery, TX) that also recognizes chimpanzee and rhesus fH [45], followed by alkaline phosphatase-conjugated anti-goat IgG (Sigma). Human and the non-human primate sera alone served as positive controls.
MALDI-TOF analysis
Outer membrane proteins were separated by electrophoresis as described above and stained with colloidal Coomassie brilliant blue (Sigma-Aldrich). The band corresponding to the ∼17 kDa band that bound fH was excised, washed extensively and then digested “in gel” with trypsin as described elsewhere [71]. Digested peptides were further purified via micro Zip Tipping. Briefly, samples dried down to a 10 µl volume were acidified with 1–2 µl of 1% TFA and then loaded on a Zip Tipμ-C18 (Millipore, Corp) that had been pre-equilibrated with 0.1% TFA. After washing with twice with 10 µl aliquots of 0.1%, TFA samples were deposited directly onto the MALDI sample target using 1 µl of Matrix solution (15 mg/ml of 2,5-dihydroxybenzoic Acid (MassPrep DHB, Waters Corp.) in 50∶50 acetonitrile: 0.1% TFA). Samples were allowed to air dry prior to insertion into the mass spectrometer. Analysis was performed on a Kratos Axima QIT (Shimadzu Instruments) matrix-assisted-laser desorption/ionization (MALDI) mass spectrometer. Peptides were analyzed in positive ion mode in mid-mass range (700–3000 Da). The instrument was externally calibrated with Angiotensin II (1046.54 Da), P14R (1533.86 Da) and ACTH (18–39) (2465.20 Da). Precursors were selected based on signal intensity at a mass resolution width of 250 for CID fragmentation using Argon as the collision gas. Database searches were performed in house with Mascot (Matrix Sciences, Ltd.) using the Peptide Mass Fingerprint program for MS data and the MS/MS Ion Search program for CID data. All identifications were confirmed or established with CID (MS/MS) data.
Serum bactericidal assays
Susceptibility of meningococci to complement mediated killing was determined using a serum bactericidal assay as described previously [44], [72]. The optimal concentration of serum was determined empirically for each strain (Supplementary Figure S5). Bacteria from an overnight culture on chocolate agar plates were inoculated onto fresh chocolate agar and allowed to grow for ∼6 h at 37°C in 5% CO2. Normal human serum was obtained from a healthy human volunteer and stored at −70°C till used in bactericidal assays. Briefly, 2000 CFUs of meningococci were incubated with serum (concentrations specified for each experiment) in a final reaction volume of 150 µl. Aliquots of 25 µl were plated in duplicate at the start of the assay (t0) and after incubating the reaction mixture at 37°C for 30 min (t30). Survival was calculated as the number of viable colonies at t30 relative to baseline colony counts at t0. Each experiment was repeated at least three times.
ELISA to detect binding of fH to microvesicles containing NspA
E. coli BL21(DE3) (Invitrogen, Carlsbad, CA) harboring recombinant NspA on plasmid pGMS 1.0 and E. coli BL21(DE3) transformed with pBluescript II SK+ (Stratagene, La Jolla, CA) were used to prepare microvesicles as previously described [40]. An ELISA was used to detect fH binding to NspA containing vesicles. Microtiter wells were coated with either NspA-producing vesicles or with control vesicles each at a concentration of 10 µg/ml in PBS overnight at 22°C. Nonspecific biding sites were blocked with PBS/2.5% BSA for 2 h at 37°C. To demonstrate the ability of anti-NspA mAb 14C7 to block fH binding to NspA-containing vesicles, select wells were incubated with mAb 14C7 (10 µg/ml) in PBS/0.05% Tween 20 for 1 h at 37°C; the remaining wells were incubated with PBS/Tween alone. fH (concentrations ranging from 0 to 10 µg/ml) was then added to wells for 1 h at 37°C, and bound fH was detected using polyclonal sheep anti-human fH followed by anti-sheep IgG conjugated with alkaline phosphatase, each for 1 h at 37°C.
Statistical methods
Cuzick's nonparametric test for trend across ordered groups [73] was used to determine if there was a trend between the binding of fH and the length of glycan extensions from the HepI chain of LOS. Median fluorescence values from three independent experiments were used in the analysis. Strains expressing LNT LOS, L8 LOS and unsubstituted LOS were ordered decreasingly by the length of the HepI glycans extensions and scored as 5, 3 and 1, respectively. The measurement of binding was divided by the value of the control for normalizing. The analysis was done separately for strains A2594 Cap+ and A2594 Cap−. The results showed a statistically significant trend between fH binding and decreasing length of HepI glycan extensions for both Cap+ and Cap − strains (Table S2; results are the same for A2594 Cap+ and A2594 Cap−, p = 0.007).
For bactericidal assays the average survival was calculated from at least three independent experiments and error bars represent the standard deviation. A t-test was used to determine significance.
Supporting Information
Zdroje
1. GoldschneiderI
GotschlichEC
ArtensteinMS
1969 Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 129 1307 1326
2. FigueroaJ
AndreoniJ
DensenP
1993 Complement deficiency states and meningococcal disease. Immunol Res 12 295 311
3. FigueroaJE
DensenP
1991 Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 4 359 395
4. FijenCA
KuijperEJ
te BulteMT
DahaMR
DankertJ
1999 Assessment of complement deficiency in patients with meningococcal disease in The Netherlands. Clin Infect Dis 28 98 105
5. RossSC
DensenP
1984 Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 63 243 273
6. JarvisGA
VedrosNA
1987 Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun 55 174 180
7. UriaMJ
ZhangQ
LiY
ChanA
ExleyRM
2008 A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J Exp Med 205 1423 1434
8. SaECC
GriffithsNJ
VirjiM
2010 Neisseria meningitidis Opc invasin binds to the sulphated tyrosines of activated vitronectin to attach to and invade human brain endothelial cells. PLoS Pathog 6 e1000911
9. VirjiM
GriffithsNJ
Binding of Opc to vitronectin contributes to increased serum resistance of Neisseria meningitidis isolates.
van AlphenL
van der LeyP
van den DobbelsteenG
2008; Rotterdam, The Netherlands 166 167
10. JarvaH
RamS
VogelU
BlomAM
MeriS
2005 Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol 174 6299 6307
11. FletcherLD
BernfieldL
BarniakV
FarleyJE
HowellA
2004 Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 72 2088 2100
12. MasignaniV
ComanducciM
GiulianiMM
BambiniS
Adu-BobieJ
2003 Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med 197 789 799
13. MadicoG
WelschJA
LewisLA
McNaughtonA
PerlmanDH
2006 The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 177 501 510
14. SchneiderMC
ProsserBE
CaesarJJ
KugelbergE
LiS
2009 Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature
15. PangburnMK
SchreiberRD
Muller-EberhardHJ
1977 Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med 146 257 270
16. WeilerJM
DahaMR
AustenKF
FearonDT
1976 Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A 73 3268 3272
17. WhaleyK
RuddyS
1976 Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med 144 1147 1163
18. BeerninkPT
GranoffDM
2009 The modular architecture of meningococcal factor H-binding protein. Microbiology 155 2873 2883
19. PajonR
BeerninkPT
HarrisonLH
GranoffDM
2009 Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine
20. GranoffDM
2010 Review of group B meningococcal vaccines. Clin Infect Dis In Press
21. HammerschmidtS
MullerA
SillmannH
MuhlenhoffM
BorrowR
1996 Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol 20 1211 1220
22. DeghmaneAE
GiorginiD
LarribeM
AlonsoJM
TahaMK
2002 Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol Microbiol 43 1555 1564
23. ClausH
MaidenMC
MaagR
FroschM
VogelU
2002 Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology 148 1813 1819
24. ClausH
MaidenMC
WilsonDJ
McCarthyND
JolleyKA
2005 Genetic analysis of meningococci carried by children and young adults. J Infect Dis 191 1263 1271
25. YazdankhahSP
CaugantDA
2004 Neisseria meningitidis: an overview of the carriage state. J Med Microbiol 53 821 832
26. SchneiderMC
ExleyRM
ChanH
FeaversI
KangYH
2006 Functional significance of factor H binding to Neisseria meningitidis. J Immunol 176 7566 7575
27. Biedzka-SarekM
JarvaH
HyytiainenH
MeriS
SkurnikM
2008 Characterization of complement factor H binding to Yersinia enterocolitica serotype O:3. Infect Immun 76 4100 4109
28. BerringtonAW
TanYC
SrikhantaY
KuipersB
van der LeyP
2002 Phase variation in meningococcal lipooligosaccharide biosynthesis genes. FEMS Immunol Med Microbiol 34 267 275
29. RamS
NgampasutadolJ
CoxAD
BlomAM
LewisLA
2007 Heptose I glycan substitutions on Neisseria gonorrhoeae lipooligosaccharide influence C4b-binding protein binding and serum resistance. Infect Immun
30. RamS
SharmaAK
SimpsonSD
GulatiS
McQuillenDP
1998 A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med 187 743 752
31. MadicoG
NgampasutadolJ
GulatiS
VogelU
RicePA
2007 Factor H Binding and Function in Sialylated Pathogenic Neisseriae is Influenced by Gonococcal, but Not Meningococcal, Porin. J Immunol 178 4489 4497
32. MandrellRE
KimJJ
JohnCM
GibsonBW
SugaiJV
1991 Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol 173 2823 2832
33. KoganG
UhrinD
BrissonJR
JenningsHJ
1997 Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr Res 298 191 199
34. TsangRS
LawDK
TsaiC
NgL
2001 Detection of the lst gene in different serogroups and LOS immunotypes of Neisseria meningitidis. FEMS Microbiol Lett 199 203 206
35. EstabrookMM
JackDL
KleinNJ
JarvisGA
2004 Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J Immunol 172 3784 3792
36. DerrickJP
UrwinR
SukerJ
FeaversIM
MaidenMC
1999 Structural and evolutionary inference from molecular variation in Neisseria porins. Infect Immun 67 2406 2413
37. FeaversIM
SukerJ
McKennaAJ
HeathAB
MaidenMC
1992 Molecular analysis of the serotyping antigens of Neisseria meningitidis. Infect Immun 60 3620 3629
38. SukerJ
FeaversIM
AchtmanM
MorelliG
WangJF
1994 The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol Microbiol 12 253 265
39. UrwinR
FoxAJ
MusilekM
KrizP
MaidenMC
1998 Heterogeneity of the PorB protein in serotype 22 Neisseria meningitidis. J Clin Microbiol 36 3680 3682
40. HouVC
MoeGR
RaadZ
WuorimaaT
GranoffDM
2003 Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect Immun 71 6844 6849
41. MoeGR
TanS
GranoffDM
1999 Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect Immun 67 5664 5675
42. WelschJA
RamS
KoeberlingO
GranoffDM
2008 Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis 197 1053 1061
43. RipocheJ
DayAJ
HarrisTJ
SimRB
1988 The complete amino acid sequence of human complement factor H. Biochem J 249 593 602
44. ShaughnessyJ
LewisLA
JarvaH
RamS
2009 Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect Immun 77 2094 2103
45. NgampasutadolJ
RamS
GulatiS
AgarwalS
LiC
2008 Human Factor H Interacts Selectively with Neisseria gonorrhoeae and Results in Species-Specific Complement Evasion. J Immunol 180 3426 3435
46. FrieseMA
HellwageJ
JokirantaTS
MeriS
PeterHH
1999 FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol 36 809 818
47. GranoffDM
WelschJA
RamS
2009 Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun 77 764 769
48. NgampasutadolJ
RamS
BlomAM
JarvaH
JerseAE
2005 Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection. Proc Natl Acad Sci U S A 102 17142 17147
49. BlomAM
HallstromT
RiesbeckK
2009 Complement evasion strategies of pathogens-acquisition of inhibitors and beyond. Mol Immunol 46 2808 2817
50. KraiczyP
WurznerR
2006 Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol Immunol 43 31 44
51. MurphyE
AndrewL
LeeKL
DiltsDA
NunezL
2009 Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 200 379 389
52. MartinD
CadieuxN
HamelJ
BrodeurBR
1997 Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J Exp Med 185 1173 1183
53. JonesDM
BorrowR
FoxAJ
GrayS
CartwrightKA
1992 The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog 13 219 224
54. MackinnonFG
BorrowR
GorringeAR
FoxAJ
JonesDM
1993 Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microb Pathog 15 359 366
55. McLeod GriffissJ
BrandtBL
SaundersNB
ZollingerW
2000 Structural relationships and sialylation among meningococcal L1, L8, and L3,7 lipooligosaccharide serotypes. J Biol Chem 275 9716 9724
56. MadicoG
RamS
GetzlaffS
PrasadA
GulatiS
Sialylation of lacto-N-tetraose lipooligosaccharide in gonococci, but not meningococci, results in enhanced factor H binding: the modulatory role of gonococcal porin; 2004 September 5–September 10, 2004; Milwaukee, Wisconsin 230
57. CadieuxN
PlanteM
RiouxCR
HamelJ
BrodeurBR
1999 Bactericidal and cross-protective activities of a monoclonal antibody directed against Neisseria meningitidis NspA outer membrane protein. Infect Immun 67 4955 4959
58. EstabrookMM
JarvisGA
McLeod GriffissJ
2007 Affinity-purified human immunoglobulin G that binds a lacto-N-neotetraose-dependent lipooligosaccharide structure is bactericidal for serogroup B Neisseria meningitidis. Infect Immun 75 1025 1033
59. RamS
CoxAD
WrightJC
VogelU
GetzlaffS
2003 Neisserial lipooligosaccharide is a target for complement component C4b: Inner core phosphoethanolamine residues define C4b linkage specificity. J Biol Chem 278 50853 50862
60. SeibKL
OrienteF
Adu-BobieJ
MontanariP
FerliccaF
2010 Influence of serogroup B meningococcal vaccine antigens on growth and survival of the meningococcus in vitro and in ex vivo and in vivo models of infection. Vaccine
61. SeibKL
SerrutoD
OrienteF
DelanyI
Adu-BobieJ
2009 Factor H-binding protein is important for meningococcal survival in human whole blood and serum and in the presence of the antimicrobial peptide LL-37. Infect Immun 77 292 299
62. HalperinSA
LangleyJM
SmithB
WunderliP
KaufmanL
2007 Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine 25 450 457
63. MartinD
BrodeurBR
HamelJ
CoutureF
de AlwisU
2000 Candidate Neisseria meningitidis NspA vaccine. J Biotechnol 83 27 31
64. MoeGR
Zuno-MitchellP
HammondSN
GranoffDM
2002 Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect Immun 70 6021 6031
65. MeriS
JordensM
JarvaH
2008 Microbial complement inhibitors as vaccines. Vaccine 26 Suppl 8 I113 117
66. VogelU
ClausH
HeinzeG
FroschM
1999 Role of lipopolysaccharide sialylation in serum resistance of serogroup B and C meningococcal disease isolates. Infect Immun 67 954 957
67. GulatiS
CoxA
LewisLA
MichaelFS
LiJ
2005 Enhanced factor H binding to sialylated Gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in Gonococci. Infect Immun 73 7390 7397
68. LewisLA
GipsonM
HartmanK
OwnbeyT
VaughnJ
1999 Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol Microbiol 32 977 989
69. HouVC
KoeberlingO
WelschJA
GranoffDM
2005 Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J Infect Dis 192 580 590
70. GnehmHE
PeltonSI
GulatiS
RicePA
1985 Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Invest 75 1645 1658
71. LahmHW
LangenH
2000 Mass spectrometry: a tool for the identification of proteins separated by gels. Electrophoresis 21 2105 2114
72. McQuillenDP
GulatiS
RicePA
1994 Complement-mediated bacterial killing assays. Methods Enzymol 236 137 147
73. CuzickJ
1985 A Wilcoxon-type test for trend. Stat Med 4 87 90
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



