-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA Damage Triggers Genetic Exchange in
Many organisms respond to DNA damage by inducing expression of DNA repair genes. We find that the human stomach pathogen Helicobacter pylori instead induces transcription and translation of natural competence genes, thus increasing transformation frequency. Transcription of a lysozyme-like protein that promotes DNA donation from intact cells is also induced. Exogenous DNA modulates the DNA damage response, as both recA and the ability to take up DNA are required for full induction of the response. This feedback loop is active during stomach colonization, indicating a role in the pathogenesis of the bacterium. As patients can be infected with multiple genetically distinct clones of H. pylori, DNA damage induced genetic exchange may facilitate spread of antibiotic resistance and selection of fitter variants through re-assortment of preexisting alleles in this important human pathogen.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1001026
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001026Summary
Many organisms respond to DNA damage by inducing expression of DNA repair genes. We find that the human stomach pathogen Helicobacter pylori instead induces transcription and translation of natural competence genes, thus increasing transformation frequency. Transcription of a lysozyme-like protein that promotes DNA donation from intact cells is also induced. Exogenous DNA modulates the DNA damage response, as both recA and the ability to take up DNA are required for full induction of the response. This feedback loop is active during stomach colonization, indicating a role in the pathogenesis of the bacterium. As patients can be infected with multiple genetically distinct clones of H. pylori, DNA damage induced genetic exchange may facilitate spread of antibiotic resistance and selection of fitter variants through re-assortment of preexisting alleles in this important human pathogen.
Introduction
The Gram-negative human stomach pathogen Helicobacter pylori occupies an exposed niche on the surface of the stomach epithelium and faces a chronic inflammatory response. Despite these challenges, H. pylori colonizes 50% of the world's population and chronic infection leads to gastritis, peptic ulcer disease, gastric cancer and gastric mucosal-associated lymphoid tissue lymphoma in a subset of infected patients [1]. Eradication requires a 7–14 day course of multiple antibiotics [2] and treatment failure due to chromosomally encoded antibiotic resistance is common [3].
Comparative genomic studies of H. pylori strains from diverse global populations revealed extensive genetic diversity in nucleotide sequence and gene content as well as a genome in linkage equilibrium [4], [5], [6], [7]. Allelic diversity is generated by mutation. Some isolates of H. pylori, including the one used in this study, have a mutation rate similar to E. coli [8], [9], whereas other studies have found a 10–700 fold higher mutation frequency in clinical isolates that may drive strain variation [10], [11]. Although infection of humans with a single strain is most common, mixed infection with distinct strains and genetic exchange between strains is observed [12], [13], [14], [15], [16], [17]. Most clinical isolates show natural competence for DNA uptake and recombination into the genome [18] which likely contributes to the recombination signatures observed in H. pylori genomes [4]. Exogenous DNA is transported into the cell by the Com type IV secretion system (T4SS), which is homologous to type IV systems found in Agrobacterium tumefaciens and other Gram-negative species [19]. These systems are known to transport DNA and proteins, but H. pylori is the only species known to use a T4SS for natural competence [20]. Unlike other organisms which carefully regulate competence, H. pylori competence is constitutive during logarithmic growth [21]. Thus, new alleles created by mutation can spread and re-assort by DNA exchange during mixed infection generating the heterogeneous populations observed in clinical isolates [4].
All organisms encode genetic programs for response to stressful conditions including DNA damage. In H. pylori, homologous recombination is required for resistance to antimicrobial agents that create DNA double strand breaks (DSBs) such as ciprofloxacin and colonization of the mouse stomach [22], [23]. The AddAB helicase-nuclease complex resects DSBs and loads RecA onto single strand DNA (ssDNA), which then mediates strand exchange, leading to homologous recombination and repair [23]. The requirement of RecA plus AddAB for efficient stomach colonization suggests that in the stomach H. pylori is either exposed to DNA damage that must be repaired or requires some other recombination-mediated event.
In some bacterial species, DNA damage induces a transcriptional program called the SOS response, which can include genes involved in DNA repair, cell cycle control and low-fidelity polymerases. The SOS response is triggered when RecA binds ssDNA, thus activating its co-protease activity towards LexA, a transcriptional repressor [24]. Expression of many other genes is changed by DNA damage in a LexA-independent manner and the genes expressed vary by species [25], [26]. Genome sequencing revealed that H. pylori lacks lexA, low-fidelity polymerases, and an obvious cell cycle repressor, suggesting that H. pylori lacks the SOS response [5], [27]. Various Gram-negative and Gram-positive organisms also lack lexA, including Campylobacter jejuni and Streptococcus pneumoniae, but only limited studies of their responses to DNA damage are available. In response to a short pulse of DNA damage, C. jejuni induces several genes including mfd, which encodes a transcription-coupling repair factor [28]. In S. pneumoniae, DNA damage and other stresses induce genetic competence [29]. The conservation of LexA-independent responses is unclear and the transcriptional response to DNA damage in H. pylori has not been described. Identification of the complete set of DNA damage responsive genes thus promised to provide insight into how this important pathogen responds to DNA damage and adapts to its environment. This work indicates that induction of competence is a major component of the H. pylori response to DNA damage and suggests a close relationship between DNA damage and genetic diversification during stomach colonization.
Results/Discussion
The H. pylori transcriptional response to DNA damage is distinct from SOS
To define critical pathways for an H. pylori DNA damage response, cDNA based microarrays [7] were used to measure transcriptional changes in cells undergoing DNA damage. Wild-type cells were exposed to the antibiotic ciprofloxacin, which binds DNA gyrase, causing DSBs [30], and compared to untreated wild-type cells. Using Significance Analysis of Microarrays (SAM, [31]), we observed significant induction of 127 genes and repression of 170 genes in ciprofloxacin-treated cells relative to untreated cells (1% false discovery rate (FDR)) (Table S1).
To further define the response to DNA damage, transcriptional changes were similarly measured in cells lacking addA, which is required for DSB repair by homologous recombination [22]. It is likely that the ΔaddA mutant accumulates unrepaired DNA damage, because cells lacking addA replicate 1.1 fold slower than wild - type cells, which translates to a 100-fold decrease in CFU after 20 generations (Figure S1A and [32]). In contrast, cells lacking DNA single strand break repair due to mutation of recR [32] replicated with the same efficiency as wild-type cells (Figure S1B). These data suggest that DSBs occur during growth in broth culture, and that therefore cells lacking DSB repair, including the ΔaddA mutant, undergo chronic DNA damage. Microarray analysis revealed that the ΔaddA mutant showed induction of 67 genes and repression of 167 genes, compared to wild-type cells during logarithmic growth using a 5% FDR (SAM) (Table S1). The ΔaddA mutant showed weaker transcriptional induction than ciprofloxacin, necessitating use of a higher FDR, possibly because ciprofloxacin treatment causes acute DNA damage whereas the ΔaddA mutant undergoes chronic damage. We also queried transcriptional changes in cells lacking single strand break repair (ΔrecR mutant), but observed no significant changes in gene expression (Table S2). These observations suggest that lack of DSB repair in the ΔaddA mutant causes transcriptional changes.
Comparison of the transcriptional profiles of DNA damage from ciprofloxacin treated cells and the ΔaddA mutant cells demonstrated a strong correlation (r2 = 0.9) between their induced and repressed gene sets (Figure 1A,B). Indeed, 41 induced genes (Figure 1B) and 41 repressed genes identified by SAM were common to both profiles and this overlap was statistically significant (p<0.001, χ2 test), demonstrating that these two DNA damaging conditions regulated a similar subset of genes. We focused on the 41 genes induced in both ciprofloxacin treated cells and the ΔaddA mutant (Figure 1B). No DNA repair genes, a hallmark of the SOS response, were induced in both conditions, but we were surprised to note several genes involved in natural competence for DNA transformation (explored further below). Our findings are similar to those in diverse species, which demonstrate that DNA repair genes are only one of many classes of genes regulated by DNA damage [25], [26]. Consistent with these prior studies, we found genes required for energy metabolism, membrane proteins, and fatty acid biosynthesis (Table S1) are regulated in response to DNA damage, although the contribution of these genes to survival in the face of DNA damage is not well understood in any species. Several cell division genes were also induced (minE, ftsK, fic); however there is no obvious homolog of the SOS-regulated cell division inhibitor sulA in H. pylori [33]. Interestingly, translation factors were also induced. Although induction of translation has not been observed as part of the DNA damage response in other bacterial species, we explore below its contribution to the DNA damage response. Finally, 30% of the induced genes are species-specific genes, which may function in cellular responses to DNA damage or have co-opted an existing regulatory pathway for their expression [34].
Fig. 1. DNA damage induces natural competence. 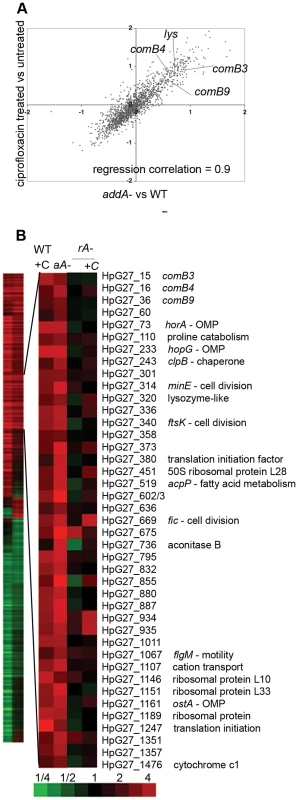
A. Ciprofloxacin exposure and addA mutation lead to similar transcriptional changes. A comparison of log2 ratios of gene expression changes measured by microarray for ciprofloxacin treatment (2.5 hours, 10× MIC) vs untreated and untreated ΔaddA mutant vs wild-type cells during logarithmic growth. Each condition is represented as mean values measured from multiple microarray experiments from three independent cultures. Regression correlation = 0.9. B. RecA is required for induction of DNA damage responsive genes. Heat map showing 471 genes with 1.6 fold change upon treatment with ciprofloxacin (column 1) or in the ΔaddA mutant (column 2). The enlarged panel shows genes with statistically significant induction in both ciprofloxacin-treated cells and the ΔaddA mutant, as determined by SAM (DNA damage responsive genes), with annotation from strain G27 [46]. Untreated wild-type cells compared to either wild-type cells treated with ciprofloxacin (WT+C), untreated ΔaddA mutant (aA−), untreated ΔrecA mutant (rA−) or the ΔrecA mutant treated with ciprofloxacin (+C). Bottom, scale bar for heat maps in fold change. OMP = outer membrane protein, genes with no annotation are left blank. RecA is required for the transcriptional response to DNA damage
RecA expression is often induced by DNA damage, thus increasing induction of SOS [25], [26], [35]. Although H. pylori seems to lack lexA, it seemed possible that RecA may be required for a transcriptional response to DNA damage. Thus we specifically queried the expression of RecA in response to DNA damage. Real-time quantitative PCR (qPCR) of either ciprofloxacin-treated wild-type cells or cells lacking addA revealed expression of recA was slightly repressed (Table 1). To test whether recA is required for the induction of DNA damage regulated genes, cDNA microarrays were used to measure transcriptional changes in cells lacking recA that were either untreated or treated with ciprofloxacin (Figure 1B). Only seven genes were induced in response to ciprofloxacin treatment in cells lacking recA and there was no overlap with the DNA damage responsive genes defined above. The absence of a transcriptional response in cells lacking recA suggests that RecA participates in sensing and transmission of the DNA damage signal, despite the absence of lexA in H. pylori.
Tab. 1. Transcription of the com T4SS is induced by DNA damage but recA expression is not. 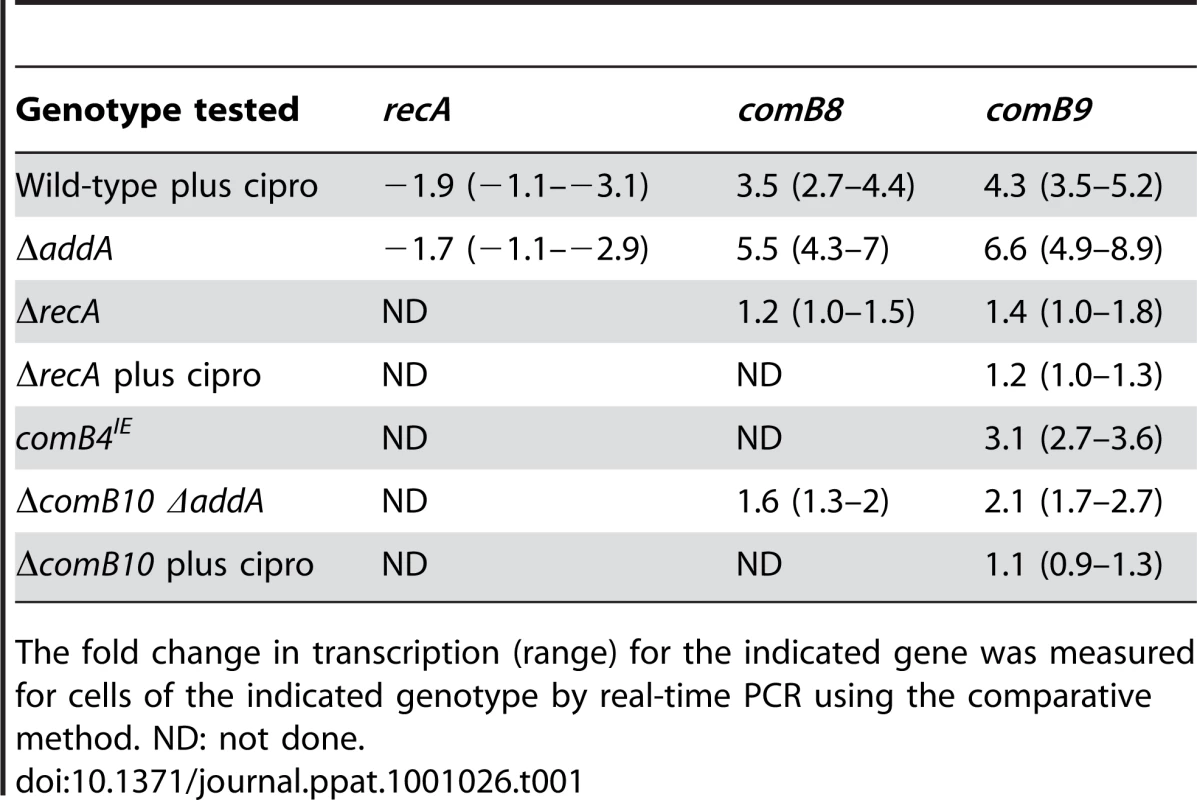
The fold change in transcription (range) for the indicated gene was measured for cells of the indicated genotype by real-time PCR using the comparative method. ND: not done. Natural competence is induced by DNA damage, but not other cellular stresses
Gene-set analysis of gene ontology (GO) terms was used to further identify pathways undergoing transcription changes in response to ciprofloxacin treatment. As genes in the same GO classes are both induced and repressed during the DNA damage response in diverse organisms [25], [26] a bipartite signal may be expected for some gene sets. Thus, a statistic was calculated for each GO term based on the absolute value of fold-induction (Materials and Methods). Genetic exchange was among the terms significantly enriched in wild-type cells treated with ciprofloxacin (Table S3). The genetic exchange category includes several genes that comprise the com T4SS and are essential for natural transformation [19], [36]. Moreover, com T4SS components comB3, comB4 and comB9, which reside in two separate operons, comB2-4 and comB6-10 [36], [37], are among the 41 genes significantly induced by DNA damage (Figure 1A,B). qPCR confirmed transcriptional induction of comB8 and comB9 in wild-type cells treated with ciprofloxacin but not in ΔrecA cells (Table 1).
Many organisms are competent only under certain environmental conditions, such as starvation [38]. In contrast, H. pylori is competent throughout logarithmic growth [21] and little is known about its regulation. Since expression of the com T4SS was DNA damage inducible, we tested whether natural transformation is increased by DNA damage. Wild-type cells were exposed to ciprofloxacin at increasing concentrations and the frequency of transformation with exogenously added genomic DNA harboring an antibiotic-resistance cassette was measured. Cells treated with the minimum inhibitory concentration of ciprofloxacin [39] had a 4–5 fold higher frequency of transformation than untreated cells (Figure 2A,B) and the frequency of transformation was easily saturated (Figure 2B). Further increasing the concentration of ciprofloxacin decreased transformation frequency, possibly due to higher levels of DNA damage (Figure 2A). No transformants were obtained after ciprofloxacin treatment of the ΔcomB10 mutant at any concentration, demonstrating that the ciprofloxacin-induced increase in natural transformation depends on activity of the com T4SS.
Fig. 2. DNA damage increases natural transformation. 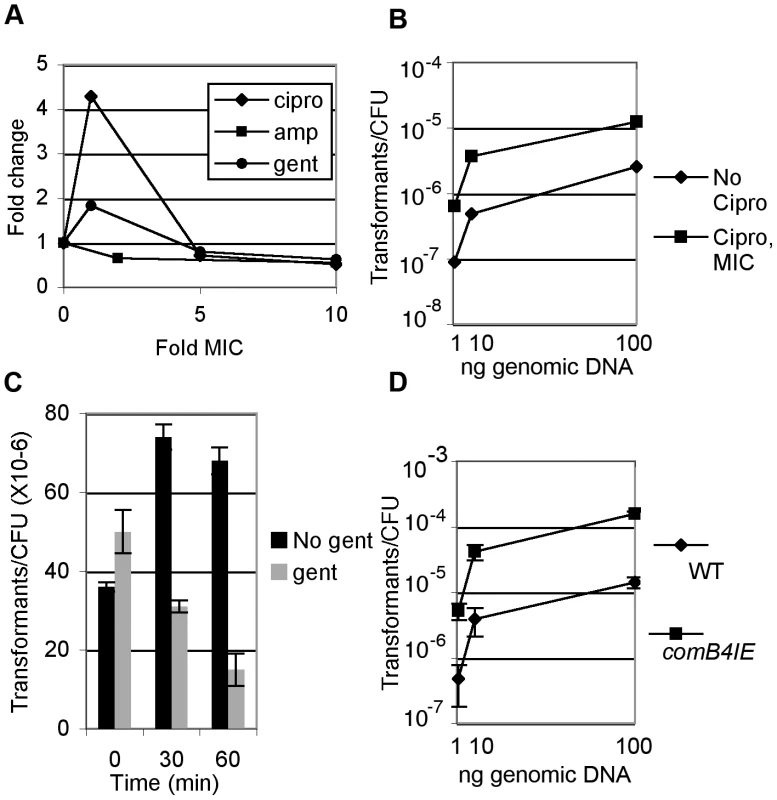
A. Wild-type cells were treated at or above the MIC of ciprofloxacin (cipro), ampicillin (amp) (0.016 ug/ml [50]) or gentamicin (1 ug/ml, data not shown) for 2.5 hours and transformed with 20ng genomic DNA harboring an antibiotic resistance cassette and the fraction of cells transformed per CFU was determined. B. Wild-type cells were treated with the minimum inhibitory concentration (MIC) of ciprofloxacin for 2.5 hours and the fraction of cells transformed by genomic DNA per CFU was determined. Wild-type transformation frequency ranges between 10−7 and 10−4, depending on DNA concentration and experiment and for each panel, error bars are the standard deviation of the mean with at least three replicates for each point and a representative from two experiments is shown. C. Wild-type cells were treated with 10× MIC gentamicin for the indicated time and the fraction of cells transformed by genomic DNA per CFU was determined either by plating directly to selective medium or by plating to non-selective medium to allow translation of the selective marker prior to selection. D. Increased expression of comB4 (comB4IE) increases natural transformation. The fraction of wild-type cells and comB4IE cells transformed by genomic DNA was determined. We also investigated whether other classes of antibiotics influence natural transformation. No increase in transformation was observed after treatment with ampicillin and there was little change in cells treated with gentamicin (Figure 2A). To further explore whether the slight increase in transformation observed with gentamicin at the minimal inhibitory concentration (MIC) resulted from weak induction of the DNA damage response, we queried the transcriptional response of H. pylori to gentamicin by microarray analysis. We observed induction of 80 genes (Table S4) and repression of 114 genes using a 1%FDR. Comparison of the gentamicin responsive genes to the DNA damage responsive genes showed an overlap of only 4 induced genes and 4 repressed genes, which was not statistically significant in either case (p = 0.2, χ2 test).
As translation factors are induced by DNA damage (Figure 1B), we determined whether ongoing translation is required for natural competence. Cells were pre-treated for various times with gentamicin at 10× MIC to fully block translation, then transformed with genomic DNA. Release of cells from gentamicin was required to recover expression of the antibiotic resistance cassette prior to selection (data not shown). One hour of pre-treatment with gentamicin caused a 5-fold reduction in transformation frequency (Figure 2C). Inhibition of transformation by gentamicin suggests that some component of the natural competence pathway must be continually synthesized and that transcriptional induction of translation by DNA damage is necessary for induction of natural competence. Taken together, these results show that induction of natural transformation is a specific response to DNA damaging agents probably mediated by increased transcription and translation of competence genes.
DNA uptake sustains expression of DNA damage responsive genes
As mentioned above, previous work had suggested that competence was constitutive in H. pylori. To explore whether a component of the natural transformation machinery is limiting for competence, we tested whether expression of a single com component might increase transformation. Merodiploids with increased expression of competence apparatus components, comB10 and dprA, a cytosolic protein required for competence, showed no increase in transformation frequency (data not shown); however two independent clones of the comB4 merodiploid (comB4IE for increased expression) were found to have a 5–8 fold increased transformation frequency (Figure 2D).
ComB4 is the only known ATPase of the Com T4SS and is thought to drive translocation of DNA through the competence machinery [19]. Increased competence in comB4IE cells could result from either increased activity of the ComB4 ATPase or increased expression of the whole com T4SS. qPCR of comB9 showed that its transcription was increased in comB4IE cells (Table 1) prompting us to perform microarray analysis of comB4IE cells. Although no genes were significantly repressed, there were 167 genes significantly induced (SAM 5% FDR) and 57 overlapped with the induced gene set for ciprofloxacin, which was statistically significant (p<0.001, χ2 test) (Table S5). In addition, SAM analysis demonstrated that 25 of the 41 genes regulated by DNA damage were induced, including com T4SS components comB3 (HpG27_15) and comB9 (HpG27_36) (Figure 3A) further suggesting activation of at least a subset of the DNA damage induced transcriptional program. The comB4IE cells showed similar sensitivity to DNA damaging agents and an equivalent mutation rate to wild-type cells (Table 2), suggesting these cell do not accumulate unrepaired DNA damage. Thus, increased expression of comB4 produces a similar effect as ciprofloxacin treatment and the ΔaddA mutant, although it does not appear to cause DNA damage.
Fig. 3. com T4SS control of the DNA damage response. 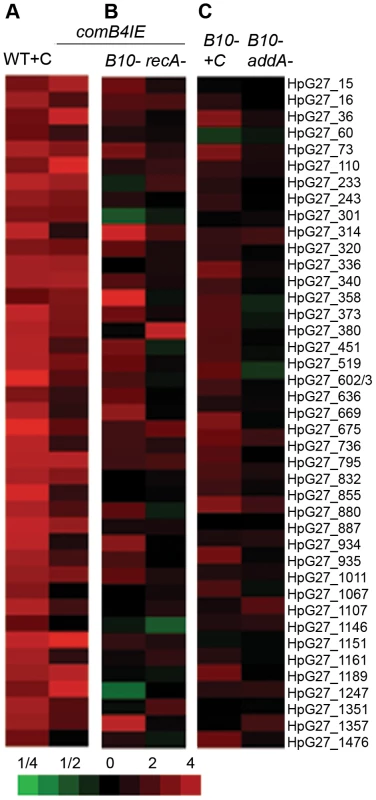
A. The comB4 merodiploid (comB4IE) induces expression of DNA damage responsive genes. Untreated wild-type cells are compared to either wild-type cells treated with ciprofloxacin (WT+C) or untreated comB4IE cells and the mean of fold change in RNA expression measured by microarray from three independent cultures is shown. Bottom, scale bar indicates fold change for heat maps in A,B,C. B. Increased expression of DNA damage responsive genes in comB4IE cells requires recA and the com T4SS. Untreated wild-type cells are compared to either comB4IE ΔcomB10 (comB4IE B10) cells, or comB4IE ΔrecA cells and data is represented as in Figure 1A. C. Full induction of DNA damage responsive genes by DNA damage requires the com T4SS. Untreated wild-type cells are compared to ciprofloxacin treated ΔcomB10 mutant cells (B10- +C) and untreated ΔaddA ΔcomB10 double mutant cells (B10- addA-). Data is represented as in Figure 1A. Tab. 2. Mutation rate and sensitivity to DNA damage are unchanged in cells expressing the DNA damage response. 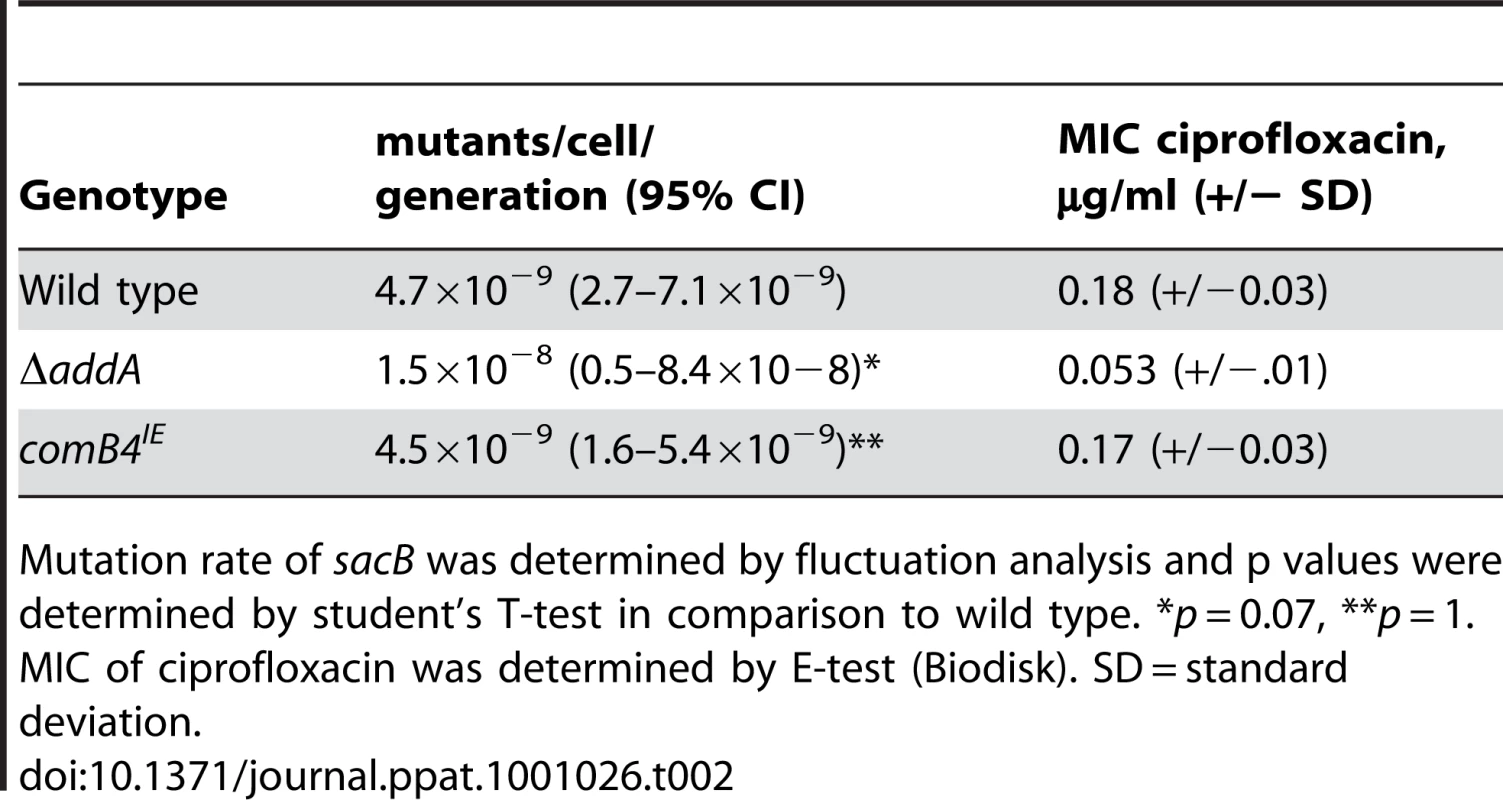
Mutation rate of sacB was determined by fluctuation analysis and p values were determined by student's T-test in comparison to wild type. *p = 0.07, **p = 1. MIC of ciprofloxacin was determined by E-test (Biodisk). SD = standard deviation. We next defined the requirements for transcriptional induction of DNA damage responsive genes in comB4IE. recA was required for induction (Figure 3B), suggesting that transcriptional induction of the DNA damage responsive genes in comB4IE occurs through a similar pathway as in ciprofloxacin treated cells. We hypothesized that increased DNA uptake in comB4IE cells is sensed by RecA and leads to transcriptional induction of the DNA damage responsive gene set. In support of this hypothesis, blocking DNA uptake by mutation of comB10 significantly decreases transcriptional induction of DNA damage responsive genes in the comB4IE cells (Figure 3B). Comparison of the comB4IE transcriptional profile with either the comB4IE ΔcomB10 mutant or the comB4IE ΔrecA mutant profile showed no statistically significant associations (χ2 test, p = 0.2). One possible explanation for these findings is that increased DNA uptake induces DNA damage responsive genes. Alternatively, a component of the natural competence machinery may act as a transcriptional regulator of the DNA damage responsive genes.
To further support the role for DNA uptake in stimulating the DNA damage response, we tested whether natural competence is required to stimulate transcription in cells undergoing DNA damage. In the ΔaddA ΔcomB10 double mutant and the ΔcomB10 mutant treated with ciprofloxacin, qPCR revealed that comB9 was not induced (Table 1). Microarray analysis revealed no transcriptional changes in the ΔcomB10 single mutant compared to wild-type cells (data not shown) and no induction of DNA damage responsive genes in the ΔaddA ΔcomB10 double mutant (Figure 3C). In the ΔcomB10 mutant treated with ciprofloxacin, only 4 of 41 DNA damage responsive genes were significantly induced (SAM 5% FDR), but close inspection of the microarray data indicated that the DNA damage response was weakly induced (e.g. HPG27_36, HPG27_73, Figure 3C), suggesting a role for natural competence in sustaining expression of DNA damage responsive genes.
A phage lysozyme-like gene contributes to DNA donation
Since no exogenous DNA was added to the transcriptional profiling experiments, cells in culture are the likely source of DNA taken up by the com T4SS [8]. The rate of genetic exchange between ΔcomB10 mutant (donor) and wild-type (recipient) cells was measured by fluctuation analysis. Genetic exchange of a single chromosomally encoded antibiotic resistance gene occurred at a rate of 2.4–5.8×10−10 exchanges/cell/generation, suggesting that cells are constantly exposed to free DNA liberated from other cells in the culture. Closer inspection of the genes regulated by DNA damage revealed an induced gene (lys, HPG27_320) that is homologous to phage T4 lysozyme and has demonstrated lysozyme activity [40]. We hypothesized that this protein lyses neighboring cells, thus liberating DNA for uptake during culture. To test this idea, we used stationary phase cells that were non-competent (the ΔcomB10 mutant) as donor cells so that genetic exchange is unidirectional. Logarithmically growing wild-type cells (recipient) showed a 12-fold higher transformation efficiency than the Δlys mutant recipient (Figure 4A), indicating that the lysozyme expressing cells could obtain more DNA from donor cells for transformation. Moreover, the Δlys mutant is transformed with purified genomic DNA at the same or higher frequency than wild-type cells (Figure 4B), indicating that Lys is not required for transformation. These results suggest that a DNA damage-induced lysozyme may target susceptible cells in culture and provide a source of DNA for uptake. DNA uptake then activates the DNA damage responsive genes in a positive feedback loop (Figure 5).
Fig. 4. Cells lacking lysozyme take up more genomic DNA than wild-type, but are less able to acquire DNA from donor cells. 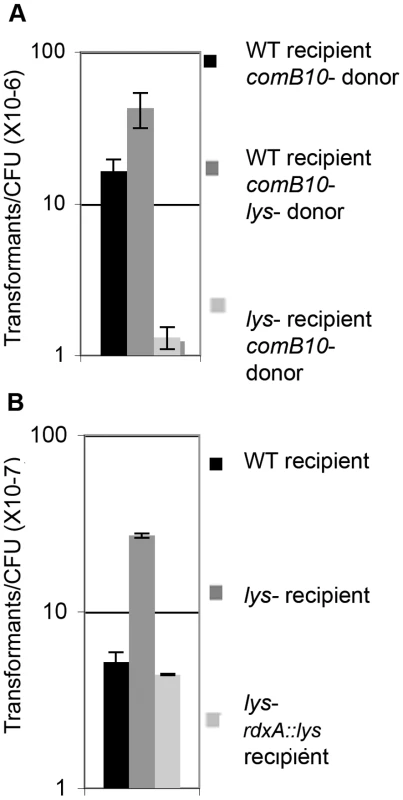
A. Log-phase recipient cells were mixed with stationary phase donor cells and the frequency of transformation was determined. B. The fraction of cells of the indicated genotype transformed by genomic DNA per CFU was determined using 10 ng genomic DNA. Error bars are the standard deviation of the mean with at least three replicates for each point and a representative of two independent experiments is shown. Fig. 5. Model for positive feedback of DNA on DNA damage responsive genes. 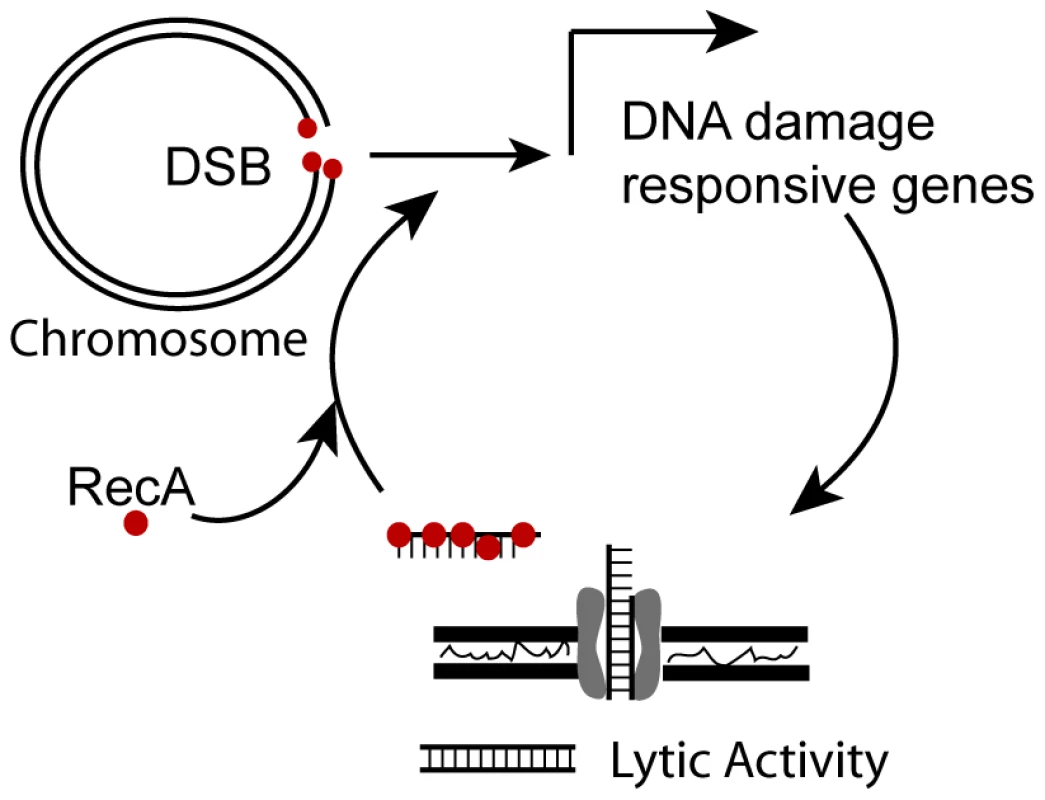
RecA binds DSBs and transcription of DNA damage responsive genes is induced that includes a lysozyme-like protein, which may be used to acquire DNA and the com T4SS, which transports DNA in the cell. Once in the cytosol, transforming DNA is bound by RecA and further induces transcription of DNA damage responsive genes. The H. pylori DNA damage response does not affect mutation rate
GO analysis gave no indication of induction of DNA repair functions by DNA damage (Table S3), but many of DNA damage responsive genes are not annotated and might have been missed. Since the ΔcomB10 mutant does not induce DNA damage responsive genes, we investigated whether the ΔcomB10 mutant is sensitive to DNA damaging agents, but sensitivity to ciprofloxacin and mutation rate were indistinguishable from wild-type cells (Table 2). In E. coli, mutation rates are increased by the SOS response through induction of error prone polymerases [24], therefore we determined the mutation rate for H. pylori cells that constitutively induce DNA damage responsive genes. The comB4IE cells have a mutation rate equivalent to wild-type cells, whereas the repair-defective ΔaddA cells have a rate that is only slightly elevated and is not statistically distinguishable from wild type (Table 2). Thus, even under stressful conditions this H. pylori strain maintains a low mutation rate, which further supports the hypothesis that H. pylori strain variation is driven by recombination among diverse strains [14], [15].
Natural competence can be detrimental during stomach colonization
Our results suggest that competence is a major output of the DNA damage response in H. pylori, but does not contribute to DNA repair or mutation. A mouse colonization assay [41] was used to further explore the relationship between DNA damage and competence during infection. The ΔcomB10 mutant showed equivalent colonization to wild-type cells in a competition assay in which differentially marked mutant and wild-type cells were introduced into the mouse stomach for one week (Figure 6A). In contrast, the ΔaddA mutant cells are compromised for infection in both competition and single strain infections [22]. Since the ΔaddA mutant can colonize, albeit with lower efficiency than wild-type cells, we tested whether the comB10 mutation would further compromise the ΔaddA mutant colonization by virtue of its requirement for activation of DNA damage responsive genes and competence. As shown in Figure 6A, the opposite result was observed: the ΔcomB10 ΔaddA double mutant showed enhanced colonization relative to the ΔaddA mutant, but not the ΔcomB10 mutant. We tested whether this effect is specific to cells lacking DSB repair. The ΔrecR mutant, which lacks single strand break repair, is defective for stomach colonization, but this defect is not suppressed by the loss of com T4SS activity (Figure 6B). The enhancement of growth by loss of com T4SS activity was not observed in broth culture. Although the ΔaddA mutant grows slower than wild-type cells in broth culture (Figure S1), the ΔaddA mutant and the ΔaddA ΔcomB10 double mutant grew at the same rate (Figure 6C). In total, our results suggest that neither competence nor DNA damage responsive genes contribute significantly to DNA repair during culture or initial stomach colonization. Furthermore, during colonization, the com T4SS exerts a fitness cost in the context of a DNA repair mutant. Thus, the observed transcriptional and translational control over natural competence may represent mechanisms to control a costly process during colonization.
Fig. 6. DNA damage responsive genes do not contribute to DNA repair during stomach colonization. 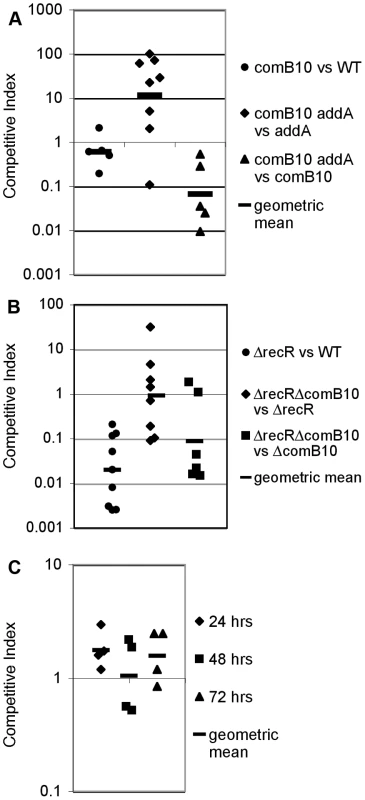
Each data point shows the competitive index of mutant cells vs wild-type cells or the indicated double mutant compared to either single mutant for a single mouse after one-week stomach colonization (A, B) or for a single well during co-culture in broth (C) and bars indicate the geometric mean. A. Competition between the ΔcomB10 mutant and wild-type cells shows no defect during stomach colonization; however stomach colonization of the ΔaddA mutant is improved by disruption of natural competence. B. Competition between the ΔrecR mutant and wild-type cells shows a strong defect during stomach colonization, but is unaffected by disruption of natural competence. C. Competence does not affect growth of the ΔaddA mutant in broth culture. The ΔaddA ΔcomB10 double mutant and the ΔaddA mutant were maintained in logarithmic growth for three days in broth culture by dilution. Conclusions
Our data reveal a connection between natural competence and the response to DNA damage in H. pylori. Similar to our observations in H. pylori, natural competence is induced by DNA damage and other stresses in the Gram-positive organism S. pneumoniae [29]. In contrast, S. pneumoniae regulation of competence and its molecular machinery for DNA uptake are completely different from H. pylori [19], [38], suggesting induction of competence in response to DNA damage is the product of convergent evolution. In H. pylori our data support a model (Figure 5) whereby DNA damage induces RecA-dependent expression of both a lysozyme-like protein, which stimulates donation of DNA from susceptible H. pylori, and the com T4SS, which increases the import of foreign DNA. Through a second RecA-dependent mechanism, DNA acquired via the com T4SS induces DNA damage responsive genes, thus amplifying the signal. A similar mechanism for signal amplification occurs in eukaryotic cells in which processing of DNA breaks creates single stranded DNA oligonucleotides that trigger the DNA damage checkpoint [42]. This newly described connection between the DNA damage response and DNA uptake suggests that natural competence contributes to persistence of H. pylori in its human host and explains its retention in most clinical isolates [18]. As patients are sometimes infected with more than one distinct strain [12], [13], [14], [15], up-regulation of natural competence may increase exchange of antibiotic resistance alleles and facilitate selection of fitter variants through re-assortment of pre-existing alleles. Our study suggests that H. pylori have co-opted signals of their harsh environment, namely DNA damage and extracellular DNA to induce genetic exchange within a heterogeneous population.
Materials and Methods
Ethics statement
All animal studies were done under practices and procedures of Animal Biosafety Level 2. The facility is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. The FHCRC Institutional Animal Care and Use Committee approved all activities.
Media and antibiotics
H. pylori strains were grown on solid horse blood agar (HB) plates containing 4% Columbia agar base (BD Bioscience), 5% defibrinated horse blood (HemoStat Laboratories), 0.2% β-cyclodextrin (Sigma), vancomycin (Sigma; 10 µg ml−1), cefsulodin (Sigma; 5 µg ml−1), polymyxin B (Sigma; 2.5 U ml−1), trimethoprim (Sigma; 5 µg ml−1) and amphotericin B (Sigma; 8 µg ml−1) at 37°C either under a microaerobic atmosphere generated using a CampyGen sachet (Oxoid) in a gas pack jar or in an incubator equilibrated with 14% CO2 and 86% air. For liquid culture, H. pylori was grown in Brucella broth (BD Biosciences) containing 10% fetal bovine serum (BB10; Hyclone) with shaking in a gas pack jar with a CampyGen sachet. For antibiotic resistance marker selection, bacterial media were additionally supplemented with kanamycin (50 µg ml−1), chloramphenicol (Cm; 15 µg ml−1) or metronidazole (Mtz; 36 µg ml−1). When culturing bacteria from mouse stomachs, Bacitracin (Bac; 200 µg ml−1) was added to eliminate contamination. For cDNA microarray and natural transformation assays BB10 medium was supplemented with ciprofloxacin, ampicillin, or gentamicin as indicated (Sigma).
Strains and plasmids
All H. pylori isogenic mutants were generated as described [23] in strain NSH57 [43]. Strains are listed in Table S6 and oligonucleotides in Table S7. All complementation constructs were generated and introduced into H. pylori as described [23].
Antibiotic resistance testing
H. pylori were grown overnight in liquid culture to optical density at 600 nm (OD600) 0.3 and 200 µL was plated on solid medium lacking all other antimicrobials, incubated for 30 minutes in a CO2 incubator. E-test strips (AB Biodisk) were then placed on the plates, which were further incubated for 2 days and read according to the manufacturers instructions.
RNA isolation and DNA microarray analysis
An overnight liquid culture was grown to (OD600) 0.8, then collected on 0.1 µm pore size filters (Whatman) and frozen in liquid nitrogen. RNA was extracted as described [44]. Approximately 10 µg RNA was reverse transcribed with Superscript II (Invitrogen), 1.5 mM each dATP, dCTP, dGTP, 0.75 mM each dTTP, 5-(3-aminoallyl)-2′-deoxyuridine-5′-triophosphate, (aa-dUTP) and random octamer primers (Fisher). To hydrolyze RNA, 100 mM EDTA, 200 mM NaOH was added and the mixture was heated to 65°C for 15 minutes. cDNA was purified over DNA Clean and Concentrator-5 (Zymoresearch), eluted with 50 mM sodium bicarbonate and coupled to Cy3 - (untreated sample) or Cy5 - (treated sample) mono NSH ester (Amersham) for one hour at room temperature. Treated and untreated samples were then mixed and unincorporated dye removed over a DNA Clean and Concentrator-5 (Zymoresearch), with samples eluted into 10 mM Tris-HCl and prepared for hybridization to custom DNA microarrays as described [45].
Microarray scanning and analysis were performed on a GenePix 4000B scanner (Axon) using GenePix Pro 6.0 software (Axon). Spots were filtered for slide abnormalities and signal from duplicate spots were averaged. These data were stored and processed in the Stanford Microarray database (http://smd.stanford.edu/). Values for genes found in strain G27 by comparative genomic hybridization [15], [46] were extracted from this set and filtered for a regression correlation >0.6 of the Log2 red/green normalized ratio (mean). These data sets were then either analyzed using SAM [31] or clustered using Cluster and visualized with Treeview (http://rana.lbl.gov/EisenSoftware.htm). To determine whether the overlap between arrays was significant, we used a chi-squared test comparing the induced genes in both conditions to the repressed or unchanged genes for each condition. Raw microarray data have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE19334 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19334).
Creation of a gene ontology for H. pylori and gene set analysis
Two gene ontologies were created for H. pylori. GO terms associated with H. pylori strain 26695 were downloaded from the Uni-prot database (http://www.uniprot.org/). We then hand-annotated genes that were not identified on this database using GO terms from Amigo (amigo.geneontology.org). H. pylori-specific and conserved hypothetical genes were identified using genolist.pasteur.fr/PyloriGene/ and GO terms were created for these categories. This procedure produced the generation0 GO list. In addition, the Bioconductor GO.db was used to identify the parent each of those GO terms, to provide a more general set of associations for each gene and creating the generation1 gene ontology.
Each array was normalized individually to adjust values for dye effects and background-corrected in the red (treated) and green (untreated) channel data. Array effects were normalized across 10 microarrays from ciprofloxacin treated cells, comB4IE cells and the ΔaddA mutant. Expression was calculated as the difference of the normalized log2 ratio of the red and green channels in each array. Finally, the two or four values for each probe on an array were averaged. We used the R package ‘limma’ to estimate treatment effects on expression.
A GSEA-style approach was used to assess differential expression for each of GO term with 10 or more associated probes [47]. A statistic was generated by summing the absolute value of the test t-statistics of probes associated with a GO term. A permutation test was used to estimate the p-value since the analytical function describing this distribution is not known.
qPCR
RNA was extracted as described above and reverse transcribed in a standard reaction with Superscript II (Invitrogen). qPCR was performed in a standard reaction using SYBR green on an ABI prism 7900HT sequence detection system (Applied Biosystems). Expression differences were calculated using the ΔΔCT method.
Quantification of natural transformation
Cells of the indicated genotype were grown overnight to approximately OD600 0.9 in shaking culture, then placed in 96 well plates (6×107 cells/well), and the indicated amount of genomic DNA harboring the aph3 gene, which confers kanamycin resistance, at a neutral locus [48] was added. After one hour, appropriate dilutions were plated to non-selective medium to determine the number of colony forming units. In addition, 50 µL was plated to non-selective plates, incubated for 24 hours to allow expression of the antibiotic resistance cassette, then replica plated to selective medium to determine the frequency of transformation. In some cases, cells were directly plated to selective medium, as described in the text.
To determine the frequency of transformation using stationary phase cells as donors, the recipient cells were grown overnight to log phase. Donor cells were grown to OD600 = 2 and then incubated for a further 16 hours. Donor and recipient cells were mixed 1∶1 for three hours, then plated to the appropriate selective medium to determine the frequency of transformation of the recipient cells to the donor genotype.
Competition in broth culture
Cells were grown overnight in liquid culture to mid-log phase, diluted to OD600 = 0.0015 for each clone and grown 24 hours in 96 well plates. After 24 and 48 hours, cells were diluted 450-fold into fresh medium and incubated for another 24 hours. At each time point, cells were titred for colony forming units (CFU) on selective medium and non-selective medium to determine the ratio of each clone in the mixture. The competitive index was determined by dividing the CFU ratio of the two clones at each time point by the starting ratio.
Mutation rate and rate of exchange between cells in culture
Mutation rate was measured in cells harboring a dual cassette consisting of aph3, which confers kanamycin resistance and sacB, which confers sensitivity to sucrose that was integrated at the omp27 locus (for wild-type and comB4IE cells) and at the rdxA locus (for wild-type and ΔaddA cells). Cells were grown overnight in liquid culture to mid-log phase, diluted to 10 cells/200µL in BB10 in 20 wells of a 96 well plate and incubated for 72 hours or 96 hours (for the ΔaddA mutant). The entire well was plated to medium containing sucrose and 4 wells were titred on non-selective medium to determine average cell number. The mutation rate was calculated using the maximum likelihood method. To determine the rate of exchange between cells in culture, cells were similarly diluted and then mixed together. Wild-type cells were marked with the aph3 gene at a neutral locus [48] and the DNA donor, ΔcomB10::cat, is resistant to chloramphenicol. The entire well was plated to medium containing kanamycin and chloramphenicol. The rate of exchange was calculated using the maximum likelihood method [49].
Mouse colonization
Female C57BL/6 mice 24–28 days old were obtained from Charles River Laboratories and certified free of endogenous Helicobacter infection by the vendor. The mice were housed in sterilized microisolator cages with irradiated PMI 5053 rodent chow, autoclaved corn cob bedding, and acidified, reverse-osmosis purified water provided ad libitum. All mouse colonization experiments were performed exactly as described [23].
Supporting Information
Zdroje
1. PeekRMJr
BlaserMJ
2002 Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2 28 37
2. CheyWD
WongBC
2007 American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102 1808 1825
3. GrahamDY
2009 Efficient identification and evaluation of effective Helicobacter pylori therapies. Clin Gastroenterol Hepatol 7 145 148
4. SuerbaumS
SmithJM
BapumiaK
MorelliG
SmithNH
1998 Free recombination within Helicobacter pylori. Proc Natl Acad Sci U S A 95 12619 12624
5. AlmRA
LingLS
MoirDT
KingBL
BrownED
1999 Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397 176 180
6. GressmannH
LinzB
GhaiR
PleissnerKP
SchlapbachR
2005 Gain and loss of multiple genes during the evolution of Helicobacter pylori. PLoS Genet 1 e43
7. SalamaN
GuilleminK
McDanielTK
SherlockG
TompkinsL
2000 A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc Natl Acad Sci U S A 97 14668 14673
8. BaltrusDA
GuilleminK
PhillipsPC
2008 Natural transformation increases the rate of adaptation in the human pathogen Helicobacter pylori. Evolution 62 39 49
9. LuriaSE
DelbruckM
1943 Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics 28 491 511
10. BjörkholmB
SjolundM
FalkPG
BergOG
EngstrandL
2001 Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc Natl Acad Sci U S A 98 14607 14612
11. KangJM
IovineNM
BlaserMJ
2006 A paradigm for direct stress-induced mutation in prokaryotes. FASEB J 20 2476 2485
12. TaylorNS
FoxJG
AkopyantsNS
BergDE
ThompsonN
1995 Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. J Clin Microbiol 33 918 923
13. BergDE
GilmanRH
Lelwala-GurugeJ
SrivastavaK
ValdezY
1997 Helicobacter pylori populations in Peruvian patients. Clin Infect Dis 25 996 1002
14. SchwarzS
MorelliG
KusecekB
ManicaA
BallouxF
2008 Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog 4 e1000180
15. SalamaNR
Gonzalez-ValenciaG
DeatherageB
Aviles-JimenezF
AthertonJC
2007 Genetic analysis of Helicobacter pylori strain populations colonizing the stomach at different times postinfection. J Bacteriol 189 3834 3845
16. TalaricoS
GoldBD
FeroJ
ThompsonDT
GuarnerJ
2009 Pediatric Helicobacter pylori isolates display distinct gene coding capacities and virulence gene marker profiles. J Clin Microbiol In Press
17. IsraelDA
SalamaN
KrishnaU
RiegerUM
AthertonJC
2001 Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc Natl Acad Sci U S A 98 14625 14630
18. YehYC
ChangKC
YangJC
FangCT
WangJT
2002 Association of metronidazole resistance and natural competence in Helicobacter pylori. Antimicrob Agents Chemother 46 1564 1567
19. HofreuterD
OdenbreitS
HaasR
2001 Natural transformation competence in Helicobacter pylori is mediated by the basic components of a type IV secretion system. Mol Microbiol 41 379 391
20. Alvarez-MartinezCE
ChristiePJ
2009 Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev 73 775 808
21. BaltrusDA
GuilleminK
2006 Multiple phases of competence occur during the Helicobacter pylori growth cycle. FEMS Microbiol Lett 255 148 155
22. AmundsenSK
FeroJ
HansenLM
CromieGA
SolnickJV
2008 Helicobacter pylori AddAB helicase-nuclease and RecA promote recombination-related DNA repair and survival during stomach colonization. Mol Microbiol 69 994 1007
23. AmundsenSK
FeroJ
SalamaNR
SmithGR
2009 Dual Nuclease and Helicase Activities of Helicobacter pylori AddAB Are Required for DNA Repair, Recombination, and Mouse Infectivity. J Biol Chem 284 16759 16766
24. GalhardoRS
HastingsPJ
RosenbergSM
2007 Mutation as a stress response and the regulation of evolvability. Crit Rev Biochem Mol Biol 42 399 435
25. CirzRT
JonesMB
GinglesNA
MinogueTD
JarrahiB
2007 Complete and SOS-mediated response of Staphylococcus aureus to the antibiotic ciprofloxacin. J Bacteriol 189 531 539
26. CirzRT
O'NeillBM
HammondJA
HeadSR
RomesbergFE
2006 Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin. J Bacteriol 188 7101 7110
27. TombJF
WhiteO
KerlavageAR
ClaytonRA
SuttonGG
1997 The complete genome sequence of the gastric pathogen Helicobacter pylori [see comments] [published erratum appears in Nature 1997 Sep 25;389(6649):412]. Nature 388 539 547
28. HanJ
SahinO
BartonYW
ZhangQ
2008 Key role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog 4 e1000083
29. PrudhommeM
AttaiechL
SanchezG
MartinB
ClaverysJP
2006 Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313 89 92
30. MooreRA
BecktholdB
WongS
KureishiA
BryanLE
1995 Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob Agents Chemother 39 107 111
31. TusherVG
TibshiraniR
ChuG
2001 Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98 5116 5121
32. MarsinS
MathieuA
KortulewskiT
GueroisR
RadicellaJP
2008 Unveiling novel RecO distant orthologues involved in homologous recombination. PLoS Genet 4 e1000146
33. MiyagishimaSY
WolkCP
OsteryoungKW
2005 Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol Microbiol 56 126 143
34. PerezJC
GroismanEA
2009 Evolution of transcriptional regulatory circuits in bacteria. Cell 138 233 244
35. da RochaRP
PaquolaAC
Marques MdoV
MenckCF
GalhardoRS
2008 Characterization of the SOS regulon of Caulobacter crescentus. J Bacteriol 190 1209 1218
36. KarnholzA
HoeflerC
OdenbreitS
FischerW
HofreuterD
2006 Functional and topological characterization of novel components of the comB DNA transformation competence system in Helicobacter pylori. J Bacteriol 188 882 893
37. SharmaCM
HoffmannS
DarfeuilleF
ReignierJ
FindeissS
The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464 250 5
38. ClaverysJP
PrudhommeM
MartinB
2006 Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu Rev Microbiol 60 451 475
39. KangJ
HuangS
BlaserMJ
2005 Structural and functional divergence of MutS2 from bacterial MutS1 and eukaryotic MSH4-MSH5 homologs. J Bacteriol 187 3528 3537
40. MarsichE
ZuccatoP
RizziS
VetereA
ToninE
2002 Helicobacter pylori expresses an autolytic enzyme: gene identification, cloning, and theoretical protein structure. J Bacteriol 184 6270 6279
41. SalamaNR
OttoG
TompkinsL
FalkowS
2001 Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun 69 730 736
42. JazayeriA
BalestriniA
GarnerE
HaberJE
CostanzoV
2008 Mre11-Rad50-Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J 27 1953 1962
43. BaldwinDN
ShepherdB
KraemerP
HallMK
SycuroLK
2007 Identification of Helicobacter pylori genes that contribute to stomach colonization. Infect Immun 75 1005 1016
44. MerrellDS
ThompsonLJ
KimCC
MitchellH
TompkinsLS
2003 Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun 71 6510 6525
45. ThompsonLJ
MerrellDS
NeilanBA
MitchellH
LeeA
2003 Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect Immun 71 2643 2655
46. BaltrusDA
AmievaMR
CovacciA
LoweTM
MerrellDS
2008 The Complete Genome Sequence of Helicobacter pylori strain G27. J Bacteriol
47. HahneF
HuberW
GentlemanR
FalconS
2008 Bioconductor Case Studies: Springer Verlag
48. LangfordML
ZabaletaJ
OchoaAC
TestermanTL
McGeeDJ
2006 In vitro and in vivo complementation of the Helicobacter pylori arginase mutant using an intergenic chromosomal site. Helicobacter 11 477 493
49. RoscheWA
FosterPL
2000 Determining mutation rates in bacterial populations. Methods 20 4 17
50. HachemCY
ClarridgeJE
ReddyR
FlammR
EvansDG
1996 Antimicrobial susceptibility testing of Helicobacter pylori. Comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn Microbiol Infect Dis 24 37 41
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



