-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUbiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
Paramyxoviruses are known to replicate in the cytoplasm and bud from the plasma membrane. Matrix is the major structural protein in paramyxoviruses that mediates viral assembly and budding. Curiously, the matrix proteins of a few paramyxoviruses have been found in the nucleus, although the biological function associated with this nuclear localization remains obscure. We report here that the nuclear-cytoplasmic trafficking of the Nipah virus matrix (NiV-M) protein and associated post-translational modification play a critical role in matrix-mediated virus budding. Nipah virus (NiV) is a highly pathogenic emerging paramyxovirus that causes fatal encephalitis in humans, and is classified as a Biosafety Level 4 (BSL4) pathogen. During live NiV infection, NiV-M was first detected in the nucleus at early stages of infection before subsequent localization to the cytoplasm and the plasma membrane. Mutations in the putative bipartite nuclear localization signal (NLS) and the leucine-rich nuclear export signal (NES) found in NiV-M impaired its nuclear-cytoplasmic trafficking and also abolished NiV-M budding. A highly conserved lysine residue in the NLS served dual functions: its positive charge was important for mediating nuclear import, and it was also a potential site for monoubiquitination which regulates nuclear export of the protein. Concordantly, overexpression of ubiquitin enhanced NiV-M budding whereas depletion of free ubiquitin in the cell (via proteasome inhibitors) resulted in nuclear retention of NiV-M and blocked viral budding. Live Nipah virus budding was exquisitely sensitive to proteasome inhibitors: bortezomib, an FDA-approved proteasome inhibitor for treating multiple myeloma, reduced viral titers with an IC50 of 2.7 nM, which is 100-fold less than the peak plasma concentration that can be achieved in humans. This opens up the possibility of using an “off-the-shelf” therapeutic against acute NiV infection.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001186
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001186Summary
Paramyxoviruses are known to replicate in the cytoplasm and bud from the plasma membrane. Matrix is the major structural protein in paramyxoviruses that mediates viral assembly and budding. Curiously, the matrix proteins of a few paramyxoviruses have been found in the nucleus, although the biological function associated with this nuclear localization remains obscure. We report here that the nuclear-cytoplasmic trafficking of the Nipah virus matrix (NiV-M) protein and associated post-translational modification play a critical role in matrix-mediated virus budding. Nipah virus (NiV) is a highly pathogenic emerging paramyxovirus that causes fatal encephalitis in humans, and is classified as a Biosafety Level 4 (BSL4) pathogen. During live NiV infection, NiV-M was first detected in the nucleus at early stages of infection before subsequent localization to the cytoplasm and the plasma membrane. Mutations in the putative bipartite nuclear localization signal (NLS) and the leucine-rich nuclear export signal (NES) found in NiV-M impaired its nuclear-cytoplasmic trafficking and also abolished NiV-M budding. A highly conserved lysine residue in the NLS served dual functions: its positive charge was important for mediating nuclear import, and it was also a potential site for monoubiquitination which regulates nuclear export of the protein. Concordantly, overexpression of ubiquitin enhanced NiV-M budding whereas depletion of free ubiquitin in the cell (via proteasome inhibitors) resulted in nuclear retention of NiV-M and blocked viral budding. Live Nipah virus budding was exquisitely sensitive to proteasome inhibitors: bortezomib, an FDA-approved proteasome inhibitor for treating multiple myeloma, reduced viral titers with an IC50 of 2.7 nM, which is 100-fold less than the peak plasma concentration that can be achieved in humans. This opens up the possibility of using an “off-the-shelf” therapeutic against acute NiV infection.
Introduction
Nipah virus (NiV) is a highly pathogenic paramyxovirus that has recently emerged from fruit bats to cause fatal diseases in humans [1], [2], [3]. It was first identified as the etiologic agent responsible for an outbreak of severe encephalitis in Malaysia and Singapore that began in 1998 and continued into 1999 with a case-fatality rate of 40% [3]. In the initial cases of NiV infection, the virus is thought to have transmitted from pigs to humans, although it is able to infect a broad spectrum of animal hosts under natural and experimental conditions [1], [4]. Later outbreaks of NiV encephalitis in Bangladesh were associated with an increased mortality rate (up to 75%), and there has been evidence for direct human-to-human transmission [5]. The high virulence of the viruses and the absence of effective therapeutic modalities and vaccines have led to the classification of NiV and the closely-related Hendra virus (HeV) as Biosafety Level 4 (BSL4) pathogens [1]. Indeed, recent outbreaks of Hendra virus in Queensland, Australia (Aug-Sep 2009) have killed 3 horses and one veterinarian, and led to the quarantine of affected horse farms and potentially infected individuals [6]. Thus, NiV and HeV infections pose an ongoing threat to both agriculture and public health.
NiV and HeV comprise a new genus Henipavirus within the family Paramyxoviridae. This is a family of viruses with negative-stranded RNA genomes and lipid envelopes derived from the host cell membrane. The genome contains six principle genes: nucleocapsid (N), phosphoprotein (P), polymerase (L), matrix (M), fusion (F) and attachment (HN, H or G) proteins [7]. Paramyxoviruses are known to replicate in the cytoplasm, and progeny virions are released from the plasma membrane of the host cell. Viral assembly and budding are orchestrated by the matrix protein (M), a major structural protein underlying the viral envelope [7], [8], [9]. Previous studies have shown that when expressed alone in the cell, NiV-M in itself carries sufficient information for the spontaneous formation and release of viral-like particles (VLPs) in the absence of other viral components [10], [11], [12]. However, despite the identification of the YMYL motif in NiV-M as a potential late-domain [10] and the YPLGVG motif as another requirement for budding [12], the intracellular trafficking and budding pathways of NiV-M remain poorly defined. In our attempt to characterize the trafficking pathway of NiV-M, we found, quite unexpectedly, that it translocates to the nucleus at early stages of infection before localizing to the plasma membrane, suggesting a previously unappreciated role for the nuclear-cytoplasmic trafficking of the Nipah matrix protein in the viral life cycle.
Though paramyxoviruses replicate in the cytoplasm, nuclear localization of viral accessory proteins has been described before. For example, the W protein of NiV inhibits host interferon response by sequestering STAT1 in the nucleus [13], [14], [15], and a fraction of the V protein of human parainfluenza virus type 2 can be found in the nucleus [16]. The nuclear localization of viral structural proteins, however, is less expected. Within Paramyxoviridae, the matrix protein has been reported to localize to the nuclear compartment in three cases so far: Sendai virus (SeV) [17], Newcastle disease virus (NDV) [18], [19], [20] and human respiratory syncytial virus (HRSV) [21], [22], [23]. In SeV and NDV, although the nuclear localization of M was clearly described, the biological function of this nuclear localization remains undefined. Faaberg et al examined more than 10 strains of NDV and found that the degree of M nuclear localization appears unrelated to virulence per se [24]. In the case of HRSV, Ghildyal et al showed that nuclear extract from HRSV-infected cells supports in vitro transcription less efficiently compared to mock-infected cells, but it has yet to be demonstrated that this inhibition is directly attributable to M [23]. A recent study on HRSV showed that Crm1-dependent nuclear export of the matrix protein is important for viral assembly and budding, suggesting that nuclear trafficking of M is somehow involved in effectuating proper viral budding [21]. However, it remains unclear why M budding needs a nuclear transit phase or whether M's nuclear localization has additional biological functions.
For proteins larger than 40 kD, efficient transport across the nuclear membrane is mediated by specific import and export signals [25], [26]. There are two types of nuclear localization signals (NLSs) that have been well characterized. A monopartite NLS consists of one single cluster of positively charged amino acid residues such as lysine (K) or arginine (R), whereas a bipartite NLS contains two stretches of K/R residues conforming to the consensus (K/R)(K/R)-X10–12-(K/R)(K/R) (where X stands for any amino acid residue) [27], [28], [29]. Nuclear export signals (NESs) are less well-defined although leucine/isoleucine-rich stretches have been identified as NESs. The most well-defined nuclear export pathway involves the chromosomal region maintenance protein 1 (CRM-1), which recognizes these leucine/isoleucine-rich NESs [30], [31]. Additionally, post-translational modifications such as ubiquitination and SUMOylation have also been shown to regulate the nuclear-cytoplasmic trafficking of cellular proteins including p53, NF-kB, PTEN, and NEMO [32], [33], [34], [35], [36], although their involvement in viral protein trafficking is less known. Nevertheless, many viruses have evolved to co-opt the cellular ubiquitin/proteasome system as a means of manipulating the host cell cycle, evading the immune system as well as egressing from the infected cell [37], [38].
Here, we find in one viral structural protein, the use and convergence of three well known cellular pathways for nuclear-cytoplasmic trafficking. We report that proper nuclear-cytoplasmic trafficking of NiV-M is essential for viral budding, and that NiV-M's nuclear-cytoplasmic trafficking is regulated by a putative bipartite NLS, a leucine-rich NES, as well as potential ubiquitination on a conserved lysine residue located in the bipartite NLS itself. Not only does this lysine play key roles in both nuclear import and export, it is also indispensable for the plasma membrane targeting of NiV-M and its subsequent incorporation into virions. Live Nipah virus budding is exquisitely sensitive to ubiquitin depletion, which leads to the nuclear retention of NiV-M. Our results suggest the clinical use of FDA-approved proteasome inhibitors such as bortezomib (Velcade) as a potential “off-the-shelf” therapeutic against acute NiV infection.
Results
Nipah virus matrix protein transits through the nuclear compartment before it localizes to the plasma membrane
In order to examine the subcellular localization of Nipah virus matrix protein (NiV-M) during the natural course of viral infection, NiV-infected cells were fixed at different time points post-infection and processed for analysis by confocal microscopy. The polyclonal anti-NiV-M antibody used in these experiments was raised by immunizing rabbits with a peptide corresponding to amino acids 29–49 of NiV-M. We verified that this affinity purified antibody was highly specific to NiV-M and had very low background staining in M non-expressing cells (Fig. S1).
At early time points (between 8 and 16 hrs) post-infection, many cells had M protein primarily concentrated in the nuclei and fluorescence followed a discrete punctuate staining pattern (Figs. 1A to C). At later time points (20 to 24 hrs), NiV-M protein was distributed diffusely in both the cytoplasm and nucleus of infected cells (Figs. 1D and E). At the latest time-point examined (24 hrs), when syncytia have begun to form, NiV-M was more clearly localized to patches on the plasma membrane and filamentous membrane extensions.
Fig. 1. Nuclear-cytoplasmic trafficking of Nipah virus matrix protein (NiV-M) during live viral infection. 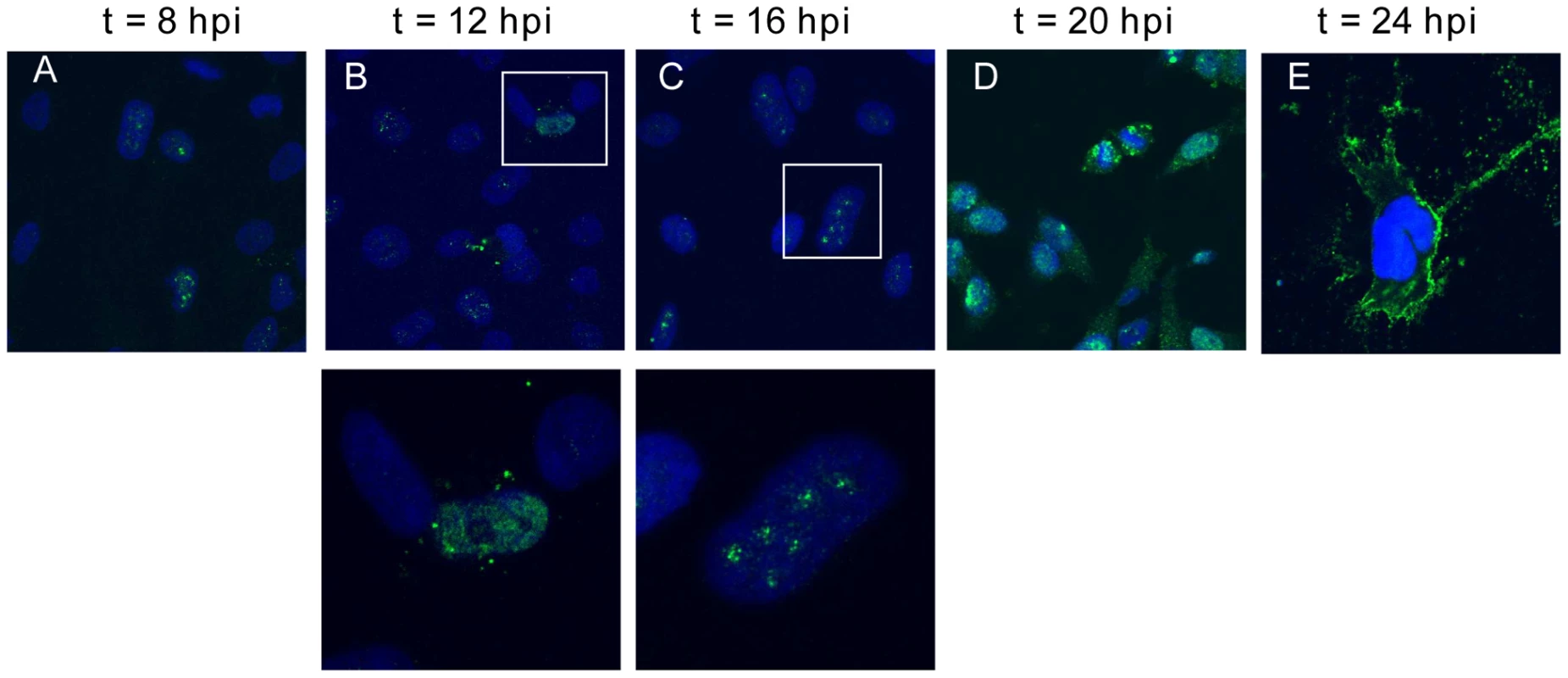
HeLa cells plated on poly-lysine-coated glass coverslips were incubated with Nipah virus Malaysia strain for 1 hr at 37°C and then fresh growth medium for up to 24 hrs. At (A) 8, (B) 12, (C) 16, (D) 20, and (E) 24 hpi, cells were fixed with 10% formalin, stained with rabbit anti-M polyclonal antibody and imaged on a confocal fluorescent microscope (63× magnification). DAPI was used for visualization of the nuclei. Insets in (B) and (C) indicate nuclear localization of M in infected cells. Experiments were performed under BSL4 conditions. To facilitate further biochemical characterizations and mutagenesis studies of NiV-M, we generated an N-terminally triple FLAG-tagged NiV-M expression construct (3XFLAG-M). This tagged protein, when expressed alone in HeLa cells, exhibited similar localization patterns as those seen during the natural course of viral infection. It concentrated in the nuclear compartment before the cytoplasmic staining became prominent (Fig. S2). A GFP-M fusion protein also behaved in a similar manner (data not shown).
NiV-M possesses a putative bipartite nuclear localization signal (NLS) and a leucine-rich nuclear export signal (NES)
The calculated molecular weight of NiV-M is 39 kD, which is around the upper limit for free diffusion across the nuclear envelope. Efficient nuclear import and export of proteins >20–40 kD usually require nuclear localization signals (NLSs) and nuclear export signals (NESs), respectively [26]. Sequence analysis of NiV-M revealed the presence of one cluster of positively charged amino acids analogous to known monopartite NLSs as well as a potential bipartite NLS consisting of two short stretches of lysines/arginines separated by ten other amino acid residues (Table 1). There are also two leucine/isoleucine-rich stretches in NiV-M that conform to the consensus for NESs (Table 2).
Tab. 1. Alignment of Nipah matrix sequence with known NLSs. 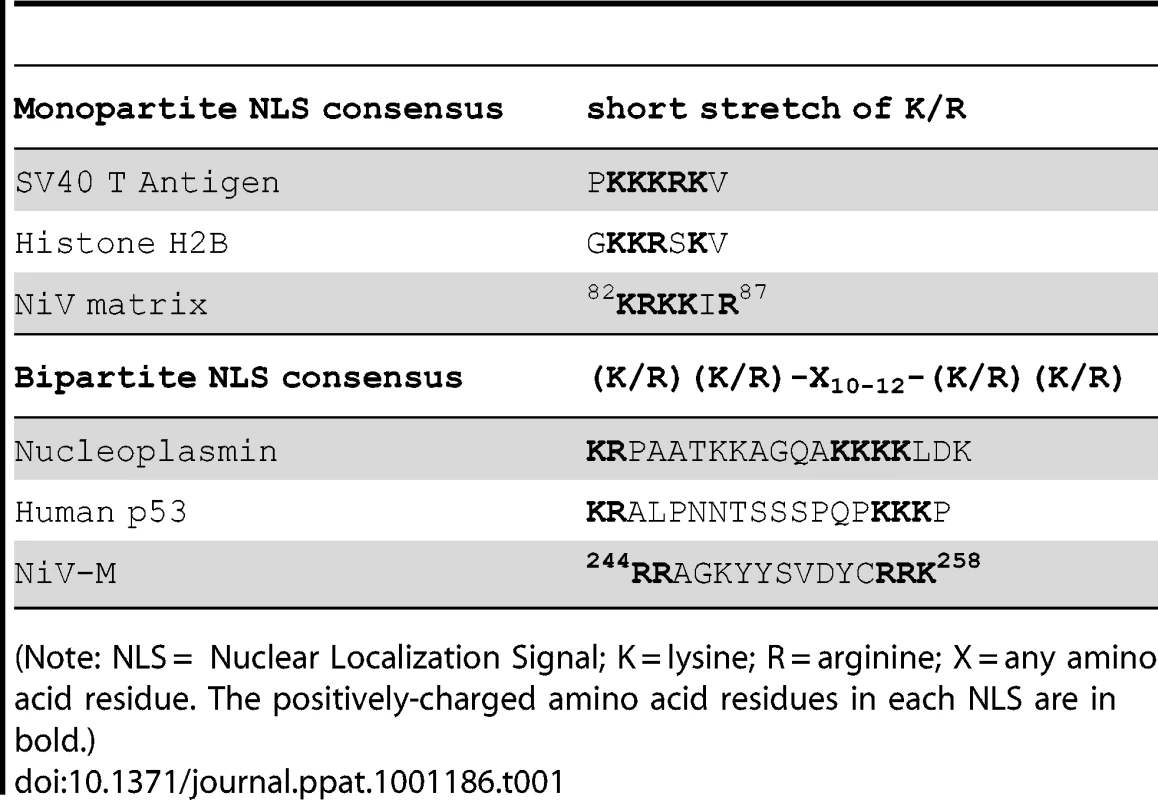
(Note: NLS = Nuclear Localization Signal; K = lysine; R = arginine; X = any amino acid residue. The positively-charged amino acid residues in each NLS are in bold.) Tab. 2. Alignment of Nipah matrix sequence with known NESs. 
(Note: NES = Nuclear Export Signal; L = Leucine; I = Isoleucine; X = any amino acid residue. The key L/I residues in each NES are in bold.) To test whether the NLSs are functional, we performed alanine substitution of key lysine/arginine residues. Subcellular localization of these mutants was examined by immunofluorescence microscopy (Fig. 2A), and quantification of the cytoplasmic/nuclear fluorescence intensity ratio was performed as described in Materials and Methods. The monopartite NLS mutant did not give a very obvious phenotype compared to the wild-type matrix protein and showed large cell-to-cell variations. This mutant was therefore excluded from further analysis. Mutating the first part of the bipartite signal (Mbp1) led to a mild nuclear exclusion phenotype, whereas mutating the second part (Mbp2) had a more apparent effect. When both parts of the bipartite NLS were mutated (Mbp1/2), nuclear import was most obviously impaired (Fig. 2A). These visual differences were confirmed by the quantification of the cytoplasmic/nuclear fluorescence intensity (C∶N) ratios shown in Fig. 2C. Note that Mbp1, Mbp2, and Mbp1/2 had statistically significant increases in C∶N ratios compared to Mwt, indicating increased cytoplasmic retention relative to nuclear import. Interestingly, we also noticed that while Mwt localized to punctuate structures in the cytoplasm as well as patches on the plasma membrane, the bi-partite NLS mutants, especially Mbp2 and Mbp1/2, exhibited more diffused localization patterns.
Fig. 2. Mutagenesis studies of potential nuclear localization signals (NLSs) and nuclear export signals (NESs) in NiV-M. 
Positively charged amino acid residues in the predicted monopartite and bipartite NLSs (A) or key leucine/isoleucine residues in the potential NESs (B) were mutated to alanines using site-directed mutagenesis. HeLa cells expressing the indicated proteins were stained with an anti-FLAG monoclonal antibody as well as DAPI. Representative fields are shown in (A) and (B), and (C) shows the quantification of cytoplasmic/nuclear fluorescence intensity (C:N) ratios for ∼10–50 individual cells analyzed for each mutant as described in Materials and Methods. Compared to Mwt, statistically significant increases in C:N ratios were observed for Mbp1 (p<0.01), Mbp2 (p<0.0001) and Mbp1/2 (p<0.0001) (unpaired t-test). Similarly, the key leucine/isoleucine residues in the potential NESs were mutated to alanines individually. All the NES mutants demonstrated nuclear retention phenotypes (Figs. 2B and C). However, previous studies have shown that deletion of the YMYL motif or the YPLGVG motif, originally thought to be late domain motifs, and neither of which conforms to a classical nuclear export sequence, also resulted in the nuclear retention of NiV-M [10], [12]. To test whether the two putative NESs in NiV-M are functional in the context of a heterologous protein, we adopted an experimental system similar to that developed by Henderson et al [39]. A fluorescent protein mCherry was fused to the C-terminus of the HIV Rev protein. This fusion protein localized to both the nucleus and the cytoplasm (Fig. 3A, panel a). When the endogenous NES in Rev was mutated (RevΔNES), the resulting fusion protein was restricted to the nuclear compartment (Fig. 3A, panel b), whereas the insertion of a short peptide corresponding to the first putative NES of NiV-M (amino acids 106–117) between RevΔNES and mCherry partially restored nuclear export (Fig. 3A, panel c). Insertion of a peptide corresponding to the second putative NES of NiV-M (amino acids 264–280) did not result in significant nuclear export of the fusion protein (data not shown). As a control, the endogenous Rev NES was inserted in the place of NiV-M NES, which led to significant nuclear export (Fig. 3A, panel d) as reported previously by other groups [39], [40]. Fig. 3B provides a semi-quantitative representation of the results in Fig. 3A by counting the relative distribution of the Rev-mCherry fusion proteins in the nucleus vs. cytoplasm of 100 transfected cells. These experiments were done in the presence of 5 µg/ml actinomycin D, which reduces the strength of the endogenous Rev NLS and therefore allows for the detection of relatively weak NESs in this reporter construct [39], [41], [42].
Fig. 3. NiV-M NES partially restores nuclear export to an NES-defective HIV Rev. 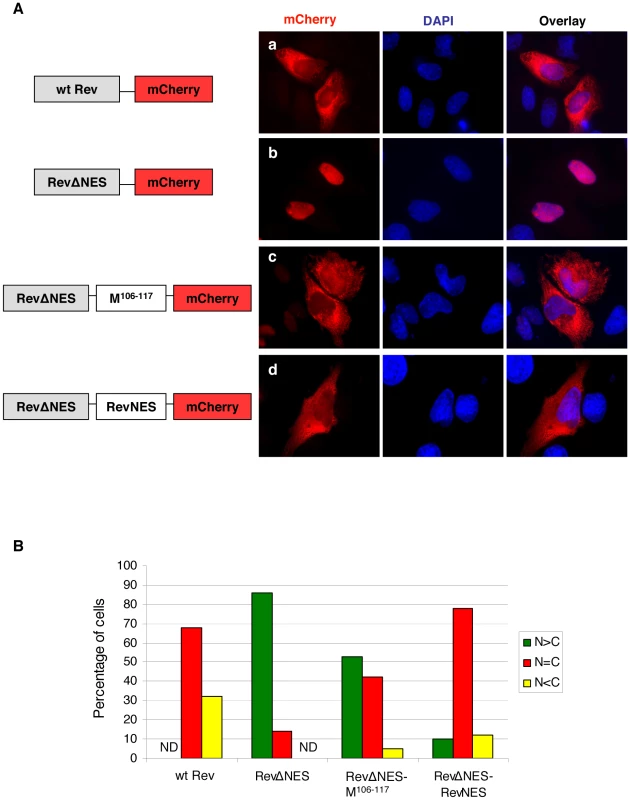
(A) HeLa cells were transiently transfected with plasmids encoding Rev-mCherry (panel a), RevΔNES-mCherry (panel b), RevΔNES-M106–117-mCherry (panel c), or RevΔNES-RevNES-mCherry (panel d). 24 hrs post transfection, cells were treated with 5 µg/ml actinomycin D for 4 hrs before fixation. Cells were stained with DAPI for visualization of the nuclei and imaged on a fluorescent microscope under 60× magnification. Representative images are shown in (A), and (B) shows the quantification of the percentage of cells with the fusion protein localized to only the nucleus (N>C), both the nucleus and the cytoplasm (N = C), or only the cytoplasm (N<C). For each mutant, at least 100 cells were counted. Both M106–117 and the endogenous NES from Rev were able to restore nuclear export to the RevΔNES-mCherry fusion protein. Our results so far show that NiV-M harbors a putative bi-partite NLS and two leucine/isoleucine-rich stretches that are important for nuclear export as suggested by mutagenesis studies. However, only the first leucine/isoleucine rich motif acts as a bona fide nuclear export signal in the context of a heterologous protein. These nuclear import/export phenotypes were recapitulated when we examined the localization of GFP-fused Mwt and NLS/NES mutants (Fig. S3).
Nuclear localization of NiV-M correlates with budding
The most important known function of viral matrix proteins is to mediate viral assembly and budding [7], [9]. Indeed, NiV-M, when expressed by itself in the cell, is able to form viral-like particles (VLPs) that spontaneously bud into the supernatant [10], [11], [12]. We confirmed that both 3XFLAG-tagged M and GFP-M were functional in a VLP budding assay (Figs. S4 and S5), although 3XFLAG-tagged M seemed to bud at reduced levels compared to the untagged M, especially at lower concentrations of transfected DNA. However, at concentrations we normally use for the VLP budding assay (1–2 µg of DNA), the budding index of 3XFLAG-M was not dramatically lower than untagged M. Since NiV-M was first localized to the nucleus before re-localizing to patches on the plasma membrane (Fig. 1), we sought to determine whether the nuclear-cytoplasmic trafficking of M is important for its ability to bud. We first examined the VLP budding of the NLS mutants (Fig. 4A) and found that, interestingly, the nuclear localization of M correlates with its ability to bud. Mbp1, which had a mild nuclear exclusion phenotype, formed VLPs at a moderately reduced level compared to wild-type M, whereas Mbp2 and Mbp1/2, which were more deficient in nuclear import, were also more severely impaired in their abilities to bud. Fig. 4B shows that all the NES mutants were also deficient in budding, presumably due to their nuclear retention and consequentially their inability to reach the plasma membrane where budding occurs. The budding phenotype of the NLS and NES mutants were quantified by determining their budding index as described in Materials and Methods and shown in Fig. 4C and 4D, respectively.
Fig. 4. Correlation between the nuclear localization of NiV-M and VLP budding. 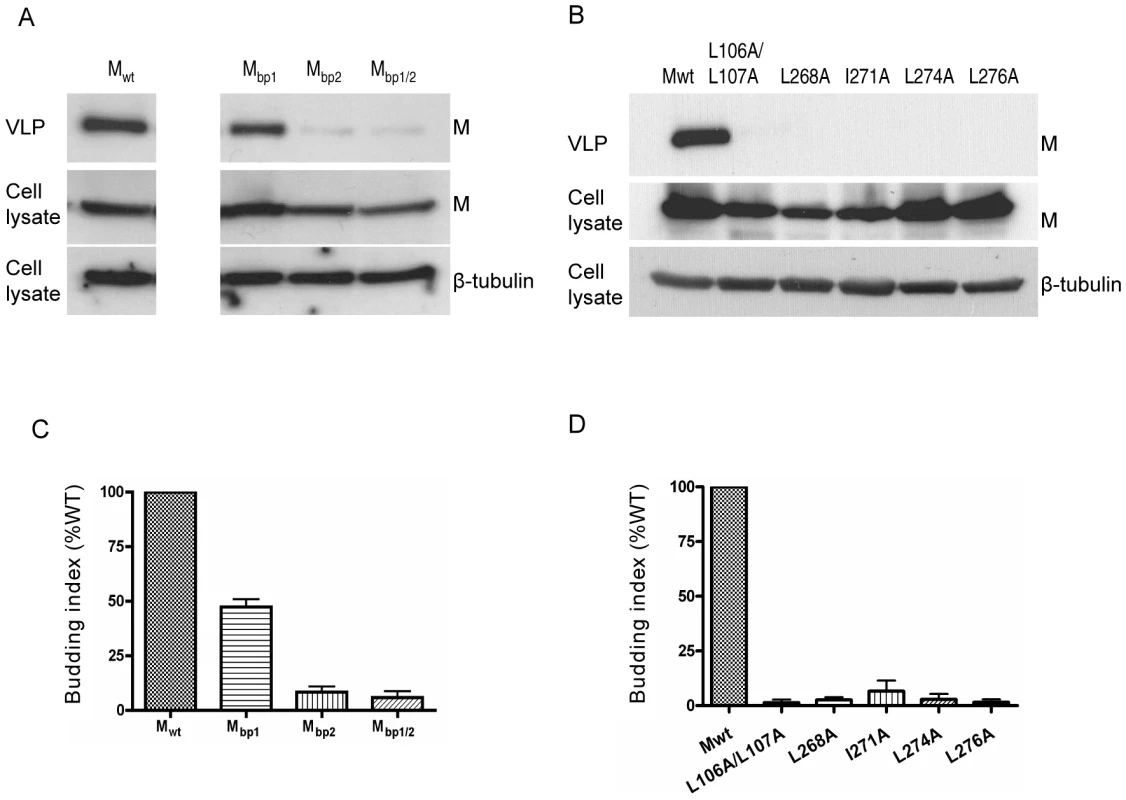
Viral-like particles were harvested from culture supernatants of cells expressing wild-type NiV-M, NLS mutants (A) or NES mutants (B) at 24 hpt as described in Materials and Methods. VLPs and the corresponding cell lysates were immunoblotted with an anti-FLAG antibody. The cell lysate blots were then stripped and re-probed with an anti-β-tubulin antibody as loading control. Representative results are shown in (A) and (B). (C) and (D) show the quantification of the budding index for the indicated wild-type and mutant NiV-M proteins as described in Materials and Methods. Error bars were calculated from three independent experiments. M mutants that were deficient in either nuclear import or export were also deficient in budding. The budding defect exhibited by the NLS and NES mutants is likely due to their nuclear import or export phenotypes rather than the disruption of their conformational integrity, as the budding defective mutants can associate and oligomerize with wild-type M (Fig. S6) and be rescued into VLPs by wild-type M (Fig. S7). Thus far, our data suggest that nuclear-cytoplasmic trafficking contributes to the eventual ability of NiV-M to bud.
A conserved lysine residue plays dual roles in regulating M nuclear-cytoplasmic trafficking
Functional NLSs have been described in the matrix protein of two other paramyxoviruses, namely human respiratory syncytial virus and Newcastle disease virus [18], [22]. Our finding that NiV-M possesses a putative NLS spurred us to look at the matrix proteins of other paramyxoviruses to determine the degree to which this motif might be conserved.
We aligned the matrix protein sequences of twelve viruses from different genera within the family Paramyxoviridae. Interestingly, in the same region where we identified the bipartite NLS in NiV-M, all twelve viruses had clusters of positively charged amino acids that could potentially function as bipartite NLSs (Fig. 5A). Specifically, the lysine residue in the second part of the bipartite NLS (K258 in NiV-M) was absolutely conserved, suggesting that the lysine itself, and not just the positive charge, might serve important functions. We therefore mutated K258 in NiV-M to an alanine versus an arginine.
Fig. 5. Dual functions of critical residue K258 in regulating NiV-M nuclear-cytoplasmic trafficking. 
(A) The matrix protein sequences of twelve viruses from different genera within the family Paramyxoviridae were aligned using CLUSTAL W (version 1.83). Positively charged amino acid residues that conform to the consensus for bipartite NLSs are colored green. The red arrow points to the lysine residue conserved among all twelve viruses. (B) K258 in NiV-M was mutated to alanine or arginine using site-directed mutagenesis. As control, K263, a non-conserved lysine in the vicinity of K258, was also mutated to arginine. HeLa cells transfected with the indicated constructs were stained with mouse anti-FLAG antibody and DAPI. K258A was excluded from the nucleus, whereas K258R was concentrated in the nucleus. The localization of K263R was similar to wild-type M. Quantification of cytoplasmic/nuclear fluorescence intensity ratios is shown in (C). As expected, the loss of positive charge impaired the function of the NLS. The K258A mutant was largely excluded from the nucleus, confirming that K258 was a critical part of the bipartite NLS (Fig. 5B). However, the K258R mutant, which retains the positive charge and should therefore have a localization pattern similar to wild-type M, gave a phenotype exactly the opposite of K258A. K258R was retained in the nucleus. This phenomenon was specific to K258, as mutating a nearby non-conserved lysine residue (K263) to arginine did not give an obvious nuclear retention phenotype (Fig. 5B). As in Fig. 2C, the C∶N ratios determined for each of these mutants confirmed the visual phenotypes observed (Fig. 5C). Thus, compared to Mwt, the nuclear-excluded K258A mutant had C∶N ratios significantly greater than 1 while the nuclear retained K258R mutants had C∶N ratios significantly less than 1 (p<0.0001 for both comparisons, unpaired t-test). This confirms the importance of the lysine residue itself at position 258 and suggests that potential modification(s) on K258 might be important for nuclear export. When K258 was mutated to arginine, the positive charge still allowed for nuclear import. However, arginines cannot be modified the same way that lysines are. Therefore, the K to R mutation could potentially compromise functions associated with the modified K258.
Ubiquitination regulates NiV-M nuclear-cytoplasmic trafficking
Lysine residues could be modified in different ways including ubiquitination, SUMOylation and acetylation. Interestingly, ubiquitination has previously been shown to be involved in the nuclear-cytoplasmic trafficking of cellular proteins such as NF-kB and p53 [35]. Specifically, monoubiquitination on the C-terminus of p53 regulates its nuclear export [32], [33]. Thus, we asked if NiV-M might exploit similar pathways for its nuclear-cytoplasmic trafficking behavior.
To test whether ubiquitin was involved, we took advantage of a well-characterized proteasome inhibitor, MG132. MG132 blocks proteasome-dependent degradation of poly-ubiquitinated proteins, thus depleting the cellular pool of free ubiquitin for new conjugations. It has previously been shown to inhibit retroviral budding, presumably because ubiquitin is required for a late step during viral assembly and egress [43], [44], [45], [46].
MG132 treatment resulted in the nuclear retention of GFP-M fusion protein (Fig. 6A, panel b) reminiscent of the phenotype of the K258R mutant (Fig. 5B). Similar results were obtained when another proteasome inhibitor, bortezomib, was used (Fig. S8). Overexpressing an HA-tagged ubiquitin (HA-Ub) in the cells was able to reverse the effect of MG132 (Fig. 6A, panel c), confirming that MG132's effect on the nuclear retention of NiV-M was indeed due to its effect on depleting the cellular pool of free ubiquitin, and suggesting that the ubiquitination of M is important for its nuclear export. As a specificity control, GFP-Mbp1/2, which is impaired in nuclear import (Fig. S3), did not accumulate in the nucleus upon MG132 treatment (Fig. 6A, panel d).
Fig. 6. Ubiquitination regulates NiV-M nuclear export. 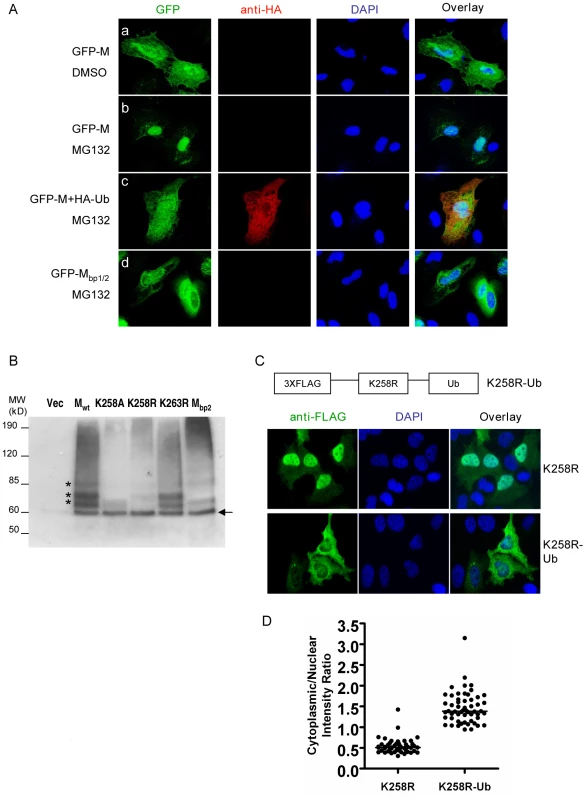
(A) Ubiquitin depletion by MG132 treatment inhibits M nuclear export. HeLa cells were transfected with GFP-M alone (panels a and b), GFP-M plus HA-Ub (panel c) or GFP-Mbp1/2 (panel d). 24 hpt, cells were treated with 50 µM MG132 or DMSO as indicated, fixed 6 hrs later with 2% paraformaldehyde, stained with DAPI as well as a mouse anti-HA antibody followed by Alexa594-conjugated goat-anti-mouse secondary antibody to identify cells expressing HA-Ub, and imaged on a fluorescent microscope. Representative images are shown. (B) Ubiquitination patterns of wild-type M and the indicated mutants. HEK293T cells were co-transfected with HA-Ub (in which all the lysines were mutated to arginines to specifically look at monoubiquitination) and the indicated 3XFLAG-tagged M mutants or empty vector as control. M was immunoprecipitated as described in Materials and Methods and the ubiquitinated species were detected by immunoblotting using an anti-HA antibody. The banding patterns of K258A, K258R and Mbp2 were different from Mwt, whereas K263R was similar to Mwt. (C) Mimicking monoubiquitination restores nuclear export to K258R. One copy of ubiquitin was fused in frame to the C-terminus of 3XFLAG-K258R, and HeLa cells expressing K258R or K258R-Ub were stained with an anti-FLAG antibody. Quantification of the cytoplasmic/nuclear fluorescence intensity ratio for each mutant is shown in (D). There is significant difference between the localization patterns of K258R and K258R-Ub (p<0.0001, unpaired t test). To biochemically detect the ubiquitinated NiV-M, we co-transfected triple-FLAG tagged Mwt or the indicated mutants with HA-Ub. Since polyubiquitination is usually associated with proteasome-dependent protein degradation, whereas monoubiquitination serves regulatory functions, we wanted to specifically look at the monoubiquitinated M species by using a mutated version of ubiquitin in which all the lysine residues are changed to arginines [33]. We immunoprecipitated M with an anti-FLAG antibody and detected the ubiquitinated species with an anti-HA antibody (Fig. 6B). For Mwt, there were at least four distinct bands at ∼8 kD intervals starting from 60 kD which represents the first monoubiquintinated band above the size of unconjugated 3XFLAG-M (Fig. 6B, arrow). These bands likely represent M monoubiquitinated on four different lysine residues. When the same experiment was performed using K258A or K258R mutant, the banding patterns were different from Mwt. The bottom band (indicated by the arrow) was the same for Mwt as well as the mutants, likely indicating monoubiquitination on a lysine residue other than K258. The three bands above it, however, were significantly reduced in the mutants compared to the wild-type. These bands likely represent ubiquitinated K258 as well as other lysines whose ubiquitination depends on K258. It was not totally unexpected that the banding patterns of K258A and K258R were not exactly identical. One of them was in the cytoplasm while the other in the nucleus, where distinct ubiquitination machineries might account for the difference. As controls, K263R had the same banding pattern as wild-type, whereas Mbp2, in which all three basic amino acid residues in the second part of the bipartite NLS including K258 were simultaneously mutated to alanines, showed a banding pattern similar to K258A.
We therefore hypothesized that ubiquitination on K258 in the nucleus might be necessary for the subsequent nuclear export of NiV-M. The altered subcellular localization of the K258R mutant was likely due to its lack of ubiquitination. To test this hypothesis, we constructed a fusion protein with one copy of ubiquitin fused in-frame to the C-terminus of K258R to mimic monoubiquitination [47], [48], [49], [50]. It has previously been shown that fusion to ubiquitin induces the nuclear export of p53 but had no effect on Max, a nuclear protein known not to be regulated by monoubiquitination [33]. Fusion to ubiquitin was able to restore nuclear export to K258R. While K258R was largely retained in the nucleus, the K258R-Ub fusion protein was clearly more cytoplasmic although in some cells, it was evenly distributed between the nucleus and cytoplasm (Fig. 6C). Fig. 6D quantifies the nuclear∶cytoplasmic ratios of K258R and K258R-Ub in ∼40–50 cells and confirms the visual phenotypes seen in Fig. 6C.
K258 is important for the membrane association and budding of NiV-M
Since K258 plays key roles in regulating NiV-M trafficking, we asked whether it might also affect the ability of M to bud. We therefore purified viral-like particles (VLPs) from the culture supernatants of HEK293T cells expressing Mwt, K258A, K258R, or K263R as control. Both K258A and K258R were deficient in budding, whereas K263R budded at similar levels compared to Mwt (Figs. 7A and B). K258R was defective in budding presumably because it was trapped in the nucleus and therefore not able to reach the plasma membrane where budding normally occurs, but it was less intuitively obvious why K258A, which was localized to the cytoplasm, was also budding-deficient.
Fig. 7. K258 is critical for NiV-M membrane association and budding. 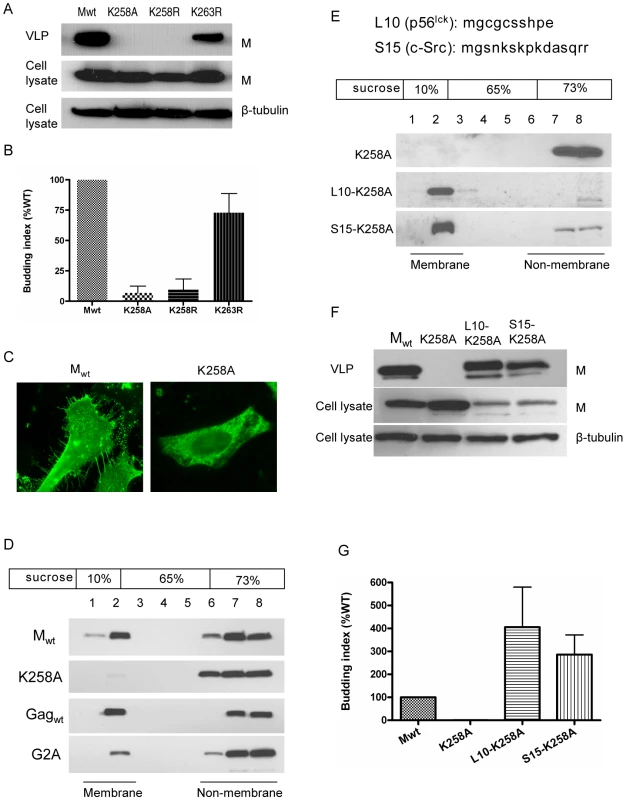
(A) NiV-M K258 mutants are deficient in VLP budding. VLP and cell lysate samples were prepared from cells expressing wild-type M, K258A, K258R or K263R at 24 hpt as described in Materials and Methods. Immunoblotting was performed using an anti-FLAG monoclonal antibody, then the cell lysate blot was stripped and re-probed with an anti-β-tubulin antibody as loading control. Both K258A and K258R were expressed in the cells at similar levels compared to wild-type M, but they were absent from the VLPs. The experiment was repeated three times and representative results are shown. (B) Quantification of the budding index for the wild-type and mutant NiV-M proteins shown in (A). (C) Wild-type M localized to membrane patches and fine filopodia extensions while the K258A mutant did not. (D) K258A is deficient in membrane association. HEK293T cells expressing wild-type NiV-M, K258A, wild-type HIV Gag, or a myristoylation mutant of HIV Gag (G2A) were harvested at 24 hpt. Cell homogenates were loaded at the bottom of a 10–73% discontinuous sucrose gradient and ultracentrifuged for 16 hrs at 100,000×g. Eight fractions were collected from the top, and proteins were extracted using methanol/chloroform prior to immunoblotting with anti-NiV-M (in the case of Mwt and K258A) or anti-myc (Gagwt and G2A) antibodies. Membrane-associated proteins were collected at the interface between 10% and 65% sucrose as “fraction 2” as described previously [71]. (E) Fusion to L10 or S15, the membrane targeting N-terminal peptide sequence from p56lck and c-Src, respectively, restores membrane association to the K258A mutant. Membrane flotation centrifugation was performed as in (D). (F) Rescue of K258A budding by L10 and S15. VLP and cell lysate samples were prepared from HEK293T cells expressing the indicated constructs and examined by immunoblotting using a rabbit anti-NiV-M antibody. The cell lysate blot was also probed with an anti-β-tubulin antibody as loading control. The experiment was repeated three times. Representative blots are shown in (F), and the quantification of the budding indices is shown in (G). A closer look at the microscopic images revealed that while Mwt localized to patches on the membrane as well as filopodia-like membrane extensions, K258A exhibited a more diffused cytoplasmic localization pattern indicative of a defect in membrane association (Fig. 7C). This was confirmed by a membrane flotation assay (Fig. 7D). While Mwt was distributed in both the membrane and non-membrane fractions, the K258A mutant was found almost exclusively in the non-membrane fractions. As controls, wild-type HIV Gag (Gagwt) and a myristoylation site mutant G2A were subjected to the same treatment. Gagwt was in both the membrane and non-membrane fractions, but G2A, which lacks membrane association, was found mainly in the non-membrane fractions as described previously [51], [52].
To confirm that the budding defect of K258A was due to its lack of membrane association rather than a conformational defect that prevented its incorporation into the virions, we tried to rescue the budding of K258A by fusing membrane targeting signals to its N-terminus. L10 is the minimal signal required for targeting p56lck to the lipid rafts, whereas S15 from c-Src targets proteins to non-raft membrane compartments [53], [54]. Both L10 and S15 were able to rescue the membrane association of K258A, as indicated by the presence of these fusion proteins in the membrane fractions as determined in our membrane flotation assay (Fig. 7E). Fusion to L10 and S15 also restored VLP budding to the K258A mutant (Figs. 7F and G). Indeed, a greater fraction of both L10-K258A and S15-K258A appeared to be in the membrane fractions which correlated with their increased budding index.
Proteasome inhibitors block Nipah virus budding
The proteasome inhibitor MG132, which we have previously shown to inhibit NiV-M nuclear export (Fig. 6A), reduced M VLP budding in a dose-dependent manner (Fig. 8A). This inhibition was not due to potential cytotoxic effect of MG132, as both endogenous (β-tubulin) and exogenous protein (NiV-M) expression levels in the cell lysates were very similar between MG132-treated and untreated samples. The budding inhibition was also seen when a different proteasome inhibitor, bortezomib, was used (Fig. S9). Moreover, the budding inhibition by MG132 could be reversed by overexpressing HA-Ub in the cells (Fig. 8A), confirming that the inhibition was indeed due to the depletion of cellular free ubiquitin. Similar phenotypes were also observed when a different cell line, HeLa, was used instead of HEK293T (Fig. S10), suggesting that this phenomenon is not a cell-type specific effect.
Fig. 8. MG132 and bortezomib inhibit NiV-M nuclear export during live viral infection and reduce viral titers. 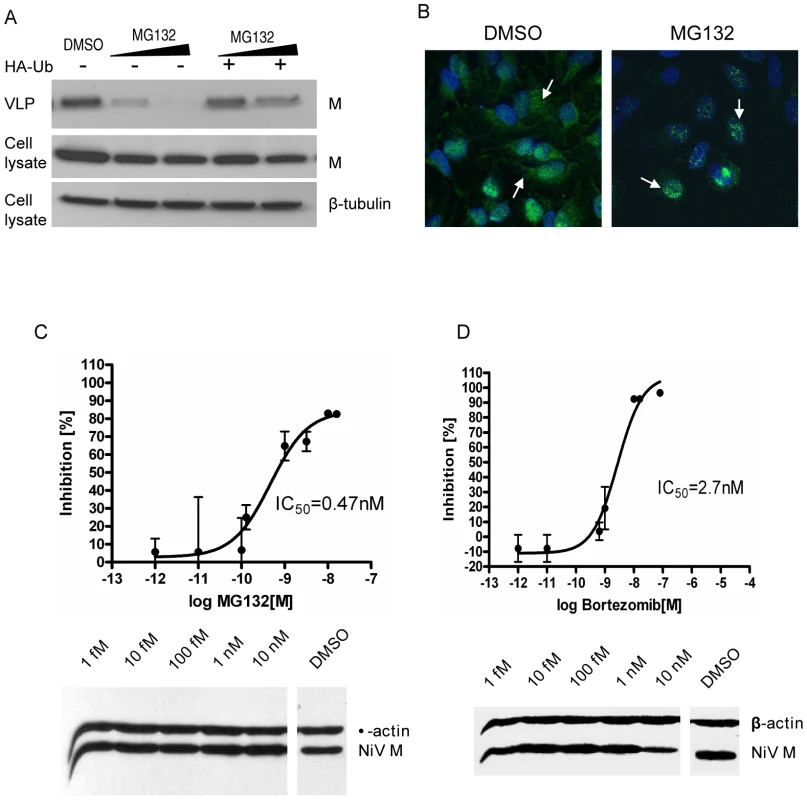
(A) NiV-M VLP budding in the presence of MG132. HEK293T cells expressing 3XFLAG-M (left three lanes) or 3XFLAG-M plus HA-Ub (right two lanes) were incubated with DMSO, 10 µM or 50 µM MG132 for 12 hrs, and VLPs produced during this period were harvested as described in Materials and Methods. VLPs and cell lysates were immunoblotted with an anti-FLAG antibody, then the cell lysate blot was stripped and re-probed with an anti-β-tubulin antibody as loading control. (B) MG132 altered M localization during live viral infection. HeLa cells infected with Nipah virus Malaysia strain were incubated with 50 µM MG132 or DMSO for 8 hrs starting from 15 hpi. Cells were then stained with an anti-M antibody and imaged on a confocal microscope. MG132 restricted M localization to the nuclear compartment. (C) and (D) Dose-response curves of Nipah viral titers in the presence of MG132 (C) or bortezomib (D). HeLa cells were incubated with NiV for 1 hr at 37°C and then fresh growth medium. 15 hpi, serial dilutions of MG132 or bortezomib were added, yielding final concentrations ranging from 10 nM to 1 fM. Considering the short half-life of bortezomib (9–15 hrs), it was re-added 12 hrs later. Supernatants were collected at 40 hpi and viral titers were determined by plaque assay. To calculate the 50% inhibitory concentration (IC50), the resulting data were fit to the sigmoidal dose-response curve (GraphPad Prism software version 4.00) using the equation: % inhibition = minimal inhibition + (maximal inhibition-minimal inhibition)/(1+10∧(LogIC50-Log drug concentration)). Results shown are from two independent experiments with triplicates for each data point. The infected cells were harvested, and the expression of cellular (β-actin) and viral (matrix) proteins was examined by immunoblotting. Next, we wanted to confirm the effect of proteasome inhibition on M localization and activity in the context of a live viral infection. MG132 altered M localization during live Nipah virus infection (Fig. 8B), restricting M to the nucleus similar to what we have seen using the transfection system. It also reduced viral titers in a dose-dependent manner, with an IC50 of 0.47 nM (Fig. 8C). Under our experimental conditions, at all concentrations tested here, MG132 did not seem to be toxic to the cells as indicated by the cytotoxicity assay (Fig. S11), and the expression levels of cellular (β-actin) and viral (matrix) proteins were very similar between MG132-treated and DMSO-treated cells (Fig. 8C, lower panel). However, MG132 has limited in vivo utility due to its configurational instability [55]. Therefore, we tested another proteasome inhibitor, bortezomib, which is an FDA-approved drug for treating multiple myeloma [56]. A dose response curve for bortezomib indicates that 50% inhibition of viral infection was achieved at 2.7 nM (Fig. 8D), which is 100-fold less than the peak plasma concentration (200–300 nM) that can be reached in humans [57], [58].
Discussion
Most negative-stranded RNA viruses, including paramyxoviruses such as Nipah virus (NiV), are known to replicate in the cytoplasm [7]. Quite unexpectedly, we found that the matrix protein of NiV transits through the nuclear compartment before reaching the plasma membrane both during live viral infection and when expressed alone in the cells.
Within the Paramyxoviridae, nuclear localization of matrix proteins has previously been described for Newcastle disease virus, Sendai virus and human respiratory syncytial virus [17], [20], [23]. However, to our knowledge, this phenomenon has not been directly associated with any biological functions. Our results suggest that the nuclear translocation of NiV-M is important for viral budding, as all the nuclear-excluded mutants are deficient in VLP formation (Figs. 4A, 4C, 7A and 7B). A straightforward explanation would be that post-translational modification(s) occurs in the nucleus which allows NiV-M to interact with the budding machinery once it is exported into the cytoplasm. Indeed, our data support the hypothesis that ubiquitination might be a key regulator of NiV-M intracellular trafficking and function.
The 76-amino-acid protein modifier ubiquitin is involved in the activity of many cellular as well as viral proteins [38], [59], [60]. Previous studies have shown that the ubiquitin-proteasome machinery is present in both the cytoplasmic and nuclear compartments of the cell [33], [61], [62], and a role for ubiquitin in protein nuclear/cytoplasmic trafficking has been demonstrated in the cases of cellular proteins including p53, PTEN and NF-kB [32], [33], [34], [35], [36]. We found that ubiquitination is important for NiV-M nuclear export as well as budding. Mutation of the putative ubiquitination site K258 altered M subcellular localization (Fig. 5B) and abrogated budding (Fig. 7A). These phenotypes were recapitulated when ubiquitin was depleted from the cells (Fig. 6A and Fig. 8). The involvement of ubiquitin in NiV-M budding is also reflected in our observation that overexpression of ubiquitin in the cells enhances M budding (Fig. S12). This effect is likely due to the ubiquitination of M per se instead of stimulating the cellular budding machinery, as the enhancement was not observed when a ubiquitination-site mutant was used.
Ubiquitin has previously been shown to be required for a late step during retroviral budding, namely the fission of virions from the cellular membrane, a process that involves the interaction between the late-domain in the Gag proteins and the cellular ESCRT complexes [37], [43], [63]. In the case of NiV-M, however, the dependence on ubiquitin seems to be via a different mechanism. Our finding that the potential ubiquitination-site mutant K258A was not membrane-associated seems to suggest a role for ubiquitin in targeting M to the plasma membrane. Plasma membrane targeting is usually mediated by N-terminal acylations such as the myristoylation of HIV Gag and the palmitoylation of Synaptosomal-associated protein of 25 kDa (SNAP-25) [64], [65]. Our sequence analysis of NiV-M did not reveal the presence of such signals, and membrane targeting is unlikely attributed solely to the interaction between M and viral transmembrane glycoproteins such as F and G, as M was able to reach the plasma membrane when expressed alone. It is possible that the ubiquitination of NiV-M might contribute to its recognition by a cellular factor that transports it to the plasma membrane. Fusing a copy of ubiquitin to the C-terminus of the K258R mutant to mimic monoubiquitination restored nuclear export (Fig. 6C), but this fusion protein was still not membrane-associated and failed to bud (data not shown), suggesting that the requirement for ubiquitin in the case of membrane targeting might be context-dependent. However, we cannot rule out the possibility that modifications other than ubiquitination might be involved.
Additionally, we found that membrane targeting positively correlates with budding. L10 and S15 peptides seem to be more potent membrane-targeting signals compared to the endogenous signal in NiV-M, as L10-K258A and S15-K258A were found predominantly in the membrane fractions whereas Mwt was present in both membrane and non-membrane fractions (Figs. 7D and F ). The more efficient membrane targeting conferred by L10 and S15 translated to higher levels of budding (Figs. 7G and H).
The cholesterol - and sphingolipid-rich membrane microdomains, or lipid rafts, have been implicated in the budding of some enveloped viruses including a few paramyxoviruses [66], [67], [68]. The fact that both L10 and S15, which target proteins to lipid raft and non-raft compartments, respectively, were equally capable of restoring NiV-M K258A budding seems to suggest that the budding of NiV-M does not require localization to the lipid rafts. This is also consistent with our observation that NiV-M localized to, but did not concentrate in lipid raft fractions (our unpublished observation). However, no conclusions can be drawn at this point as to where budding occurs during live viral infection.
NiV-M possesses two leucine/isoleucine-rich stretches, both of which are important for nuclear export, as mutating either one resulted in nuclear retention phenotypes (Figs. 2B and C). However, only one of them (amino acids 106–117) is functional in directing the nuclear export of a heterologous protein (Fig. 3). Moreover, the presence of a functional NES seems to be necessary but not sufficient for the nuclear export of NiV-M. This is demonstrated by the nuclear-retained K258R mutant, which still failed to be exported despite the presence of an intact NES. It seems that ubiquitination on K258, in addition to the NES, is required for efficient nuclear export. This is consistent with the hypothesis that ubiquitination changes the conformation of the protein, exposing the NES that is otherwise not accessible to cellular exportins [35], [49]. Alternatively, it is possible that K258 needs to be ubiquitinated for recognition by a yet-to-be-identified cellular ubiquitin-binding protein that forms an indispensable part of the nuclear export machinery. Previous studies have indicated that deleting the YMYL and YPLGVG motifs in NiV-M also results in nuclear retention [10], [12]. Since those two motifs are in proximity to the NES we identified in NiV-M, it is possible that the altered localization is due to NES masking induced by conformational changes resulting from the deletions.
Nipah virus causes fatal encephalitis in humans with high mortality rates, and there are currently no vaccines or effective therapeutics. We report here that proteasome inhibitors including MG132 and bortezomib potently reduce viral titers during live NiV infection. Bortezomib (marketed as Velcade) is an FDA-approved drug for treating multiple myeloma and mantle cell lymphoma. It is usually given to patients at a dose of 1.3 mg/m2 twice a week, and the mean maximum plasma concentration of the drug reaches 200–300 nM [57], [58]. Our inhibition curve (Fig. 8D) indicates that the IC50 of bortezomib is 2.7 nM, well below the clinically achievable plasma concentration, suggesting that it could potentially be used as an anti-viral against acute NiV infection.
Although the nuclear localization of paramyxoviral matrix proteins has been known for quite some time [17], [19], [20], [23], the biological function of this intracellular trafficking behavior remains enigmatic. Here, we provide evidence that, at least for Nipah virus matrix, nuclear transit and possible post-translational modification play critical roles in subsequent matrix-mediated viral budding. The Nipah matrix protein also illustrates the remarkably efficient use of multiple cellular trafficking machineries: that a single lysine residue in the putative bipartite NLS can serve as both a signal for nuclear import and a regulator for subsequent nuclear export. Also, the fact that the homologous lysine residue (K258 in NiV-M) in the bipartite NLS is highly conserved in all 5 genera of Paramyxoviridae suggests that the mechanisms described for NiV-M budding may extend to other paramyxoviruses. Finally, our findings suggest the potential use of bortezomib (Velcade) as treatment for acute henipavirus infections, or even prophylaxis in the case of high-risk exposure (such as veterinarians treating symptomatic horses in the Australian Hendra virus outbreaks). Although Velcade is an FDA-approved drug, it is not completely innocuous. However, it has the benefit of well-documented pharmacokinetic and toxicity profiles.
Materials and Methods
Cells and virus
VeroE6, HeLa and HEK293T cells were grown in Dulbecco's modified eagle medium (DMEM, Gibco) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 1% sodium pyruvate. The Nipah virus (NiV) strain Malaysia (kindly provided by the Special Pathogens Branch, CDC, Atlanta) was propagated in VeroE6 cells. Stock virus was harvested 48 hours post infection (hpi) and virus titer was calculated using the Reed–Muench method [69].
For infection, HeLa cells were incubated with NiV for 1 hr at 37°C, and then fresh medium containing 2% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin was added. For generating the dose-response inhibition curve in Figure 8, serial dilutions of MG132 or bortezomib were added at 15 hpi, yielding final concentrations ranging from 10 nM to 1fM. Considering the short half-life of bortezomib (9–15 hrs), it was re-added 12 hrs later. Supernatants were collected at 40 hpi and viral titers were determined by plaque assay.
For plaque assay, confluent monolayers of Vero cells (seeded in 12-well plates) were infected with 100 µl of serial tenfold dilutions of virus-containing cell supernatant. After 1 hr incubation at 37°C and 5% CO2, the inocula were removed and wells overlaid with a mixture of one part 1.0% methylcellulose and one part 2xMEM (Gibco, Invitrogen) supplemented with 2% FBS and 2% penicillin/streptomycin. The plates were incubated at 37°C and 5% CO2 for 3 days and then stained with 0.25% crystal violet in 10% buffered formalin. Plates were then washed and the plaques enumerated. All work with live virus was carried out under Biosafety Level 4 (BSL4) conditions in the Robert E. Shope BSL4 Laboratory, UTMB.
Plasmids and reagents
The open reading frame encoding Nipah virus matrix protein was codon-optimized and synthesized by Geneart Inc. (Regensburg, Germany) to facilitate expression in mammalian cells. NiV-M sequence was then amplified by PCR and inserted between the HindIII and XhoI sites in the pCMV-3Tag-1 vector (Stratagene) to generate 3XFLAG-M. 3XFLAG-tagged M mutants including Mmono, Mbp1, Mbp2, Mbp1/2, L106A/L107A, L268A, I271A, L274A, L276A, K258A, K258R, R256A/R257A and K263R were generated by QuikChange site-directed mutagenesis (Stratagene). GFP sequence was fused in-frame to the N-terminus of NiV-M by overlapping PCR to generate GFP-M, and untagged NiV-M and mutants were constructed by PCR amplification and insertion into the pcDNA3.1(+) vector (Invitrogen). HA-ubiquitin constructs (wild-type and a mutant in which all the lysines were mutated to arginines) have been described previously [70] and were purchased from Addgene (Addgene plasmids 17608 and 17603). K258R-Ub was generated by fusing one copy of ubiquitin in-frame to the C-terminus of 3XFLAG-K258R. L10 and S15 sequences were fused to the N-terminus of the untagged K258A mutant by PCR using primers 5′-agaagcttgccaccatgggctgtgtctgcagctcaaaccctgaagagcccgacatcaag, 5′ - ataagcttgccaccatgggtagcaacaagagcaagcccaaggatgccagccagcggcgcgagcccgacatcaag, and 5′ - gtcagcctcgagtcatcagcccttcagg.
Rev-mCherry was constructed by fusing the coding sequence of mCherry at the C-terminus of HIV Rev via a GGS linker followed by a KpnI restriction enzyme site. Amino acids L78 and L81 in Rev were mutated to alanines via site-directed mutagenesis to derive RevΔNES-mCherry. RevΔNES-M106–117-mCherry, RevΔNES-M264–280-mCherry and RevΔNES-RevNES-mCherry were constructed by inserting sequences corresponding to each NES between the GGS linker and the KpnI site. All the sequences were verified by DNA sequencing. Cell transfection was performed using BioT transfection reagent per manufacturer's instructions. MG132 was purchased from Calbiochem as a 10mM stock solution in DMSO, bortezomib (Velcade) was purchased from ChemieTek, and actinomycin D was purchased from Sigma.
Antibodies
Rabbit anti-NiV-M polyclonal antibodies were raised by immunizing rabbits with a purified peptide corresponding to amino acids 29–49 of NiV matrix protein (21st Century Biochemicals Inc.). Mouse anti-FLAG monoclonal antibody clone M2 was purchased from Stratagene. Mouse anti-HA and anti-myc (9E10) monoclonal antibodies were obtained from Covance and the Developmental Studies Hybridoma Bank at University of Iowa, respectively. Mouse anti-β-tubulin antibody was purchased from Sigma.
Production of viral-like particles (VLPs) and quantification of the budding index
HEK293T cells were transfected with NiV-M or M mutants expression constructs. 24 hpt, culture supernatants were collected and centrifuged at 2000 rpm for 5 min to get rid of contaminating cells. The cleared supernatants were then ultracentrifuged at 30,600 rpm on an AH-650 rotor (Thermo Scientific) for 2 hrs through a 20% (w/v) sucrose cushion. VLPs pelleted at the bottom of the tubes were resuspended in lysis buffer and subjected to immunoblotting. The intensities of the bands were quantified by densitometry with a VersaDoc Imaging System (Bio-Rad), and budding index was defined as the amount of M in the VLPs divided by the amount in the cell lysate and presented as % of wt M which is set as 100%.
Immunoprecipitation and immunoblotting
HEK293T cells expressing NiV-M or M mutants were harvested in a lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 0.25% (w/w) sodium deoxycholate, 5 mM N-ethylmaleimide (NEM) and protease inhibitors cocktail (Roche). Cell lysate was clarified by centrifugation at 21,000×g for 5 min before incubation overnight with mouse anti-FLAG antibody crosslinked to protein G-conjugated agarose beads (Pierce) with 10 mg/ml dimethyl pimelimidate (DMP). Beads were then extensively washed, and the bound proteins were eluted by boiling for 10 min in SDS protein loading buffer. Proteins were separated by SDS-PAGE, transferred to PVDF membranes and analyzed using appropriate antibodies.
Immunofluorescence microscopy and image analysis
For the live viral infection experiments, cells infected with NiV were fixed with 10% formalin for 24 hrs and removed from the BSL4. Cells were then permeabilized with 0.2% Triton X-100 in phosphate-buffered saline (PBS) for 5 min at room temperature (RT), washed with PBS and then incubated with rabbit polyclonal anti-NiV-M antibody. After extensive washing with PBS, cells were incubated with Alexa488-conjugated goat anti-rabbit secondary antibody (Invitrogen Molecular Probes) for 30 min at RT. Cells were then counterstained with DAPI. The slides were imaged on a Zeiss LSM 510 confocal microscope in the UTMB optical imaging core. For the cell transfection experiments, HeLa cells transfected with the indicated constructs were fixed with 2% paraformaldehyde, permeabilized with 0.2% Triton in PBS and stained with mouse anti-FLAG antibody followed by Alexa488 or 594-conjugated goat anti-mouse secondary antibodies. Cells were imaged on a Nikon Eclipse TE300 fluorescent microscope with MetaMorph software (Molecular Devices).
Image analysis was performed with MetaXpress software from Molecular Devices using the Enhanced Translocation module. The algorithm identifies nuclei as compartments using DAPI stain. The nuclear region was defined as the central region 20 pixels inset from the nuclear/cytoplasm boundary. The cytoplasmic region was defined as a disc beginning at the nuclear/cytoplasmic boundary and extending 5 pixels into the cytoplasm. Cells were manually included or excluded by inspection to insure that all cells included in the final scoring had the cytoplasm and nuclear regions correctly defined. A minimum cutoff intensity level was applied to ensure NiV matrix expression was sufficient. This was to exclude aberrant cell morphology and non-transfected cells. The statistic evaluated was the ratio of the average cytoplasmic region intensity to the average nuclear region intensity for each cell. Since the cytoplasmic/nuclear fluorescent intensity (C∶N) ratio for wild-type M is close to 1, C∶N ratios greater than 1 implies increased cytoplasmic retention whereas C∶N ratios less than 1 indicates increased nuclear retention. Between 10-50 cells were counted for Mwt and all mutants analyzed.
Membrane flotation centrifugation
Membrane flotation centrifugation was performed as described previously [71]. Briefly, transfected HEK293T cells were Dounce-homogenized in cold TNE buffer containing 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 0.1% 2-mercaptoethanol and protease inhibitors cocktail (Roche). Cell homogenates were clarified at 3,000 rpm for 30 min at 4°C to remove cell debris and nuclei. The cleared cell homogenate was mixed with 85% (w/v) sucrose solution to obtain 73% final concentration and loaded at the bottom of a 5 ml ultracentrifuge tube. The sample was then layered with 3 ml 65% and 0.8 ml 10% sucrose solutions and centrifuged at 100,000×g for 16 hrs at 4°C. Eight fractions (0.6 ml/fraction) were collected from the top and proteins were extracted with methanol/chloroform. Membrane-associated materials were harvested at the interface between 10% and 65% sucrose as “fraction 2”.
Supporting Information
Zdroje
1. EatonBT
BroderCC
MiddletonD
WangLF
2006 Hendra and Nipah viruses: different and dangerous. Nat Rev Microbiol 4 23 35
2. FieldH
YoungP
YobJM
MillsJ
HallL
2001 The natural history of Hendra and Nipah viruses. Microbes Infect 3 307 314
3. ChuaKB
BelliniWJ
RotaPA
HarcourtBH
TaminA
2000 Nipah virus: a recently emergent deadly paramyxovirus. Science 288 1432 1435
4. WeingartlHM
BerhaneY
CzubM
2009 Animal models of henipavirus infection: a review. Vet J 181 211 220
5. HsuVP
HossainMJ
ParasharUD
AliMM
KsiazekTG
2004 Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis 10 2082 2087
6. 2009 ProMED-mail PRO/AH/EDR> Hendra virus, human, equine - Australia (04): (QL) fatal. 20090903.3098
7. LambRA
ParksGD
2006 Paramyxoviridae: The Viruses and Their Replication.
KnipeDM
HowleyPM
Fields Virology. Fifth ed Philadelphia Lippincott, Williams and Wilkins 1449 1496
8. TakimotoT
PortnerA
2004 Molecular mechanism of paramyxovirus budding. Virus Res 106 133 145
9. GaroffH
HewsonR
OpsteltenDJ
1998 Virus maturation by budding. Microbiol Mol Biol Rev 62 1171 1190
10. CiancanelliMJ
BaslerCF
2006 Mutation of YMYL in the Nipah virus matrix protein abrogates budding and alters subcellular localization. J Virol 80 12070 12078
11. PatchJR
CrameriG
WangLF
EatonBT
BroderCC
2007 Quantitative analysis of Nipah virus proteins released as virus-like particles reveals central role for the matrix protein. Virol J 4 1
12. PatchJR
HanZ
McCarthySE
YanL
WangLF
2008 The YPLGVG sequence of the Nipah virus matrix protein is required for budding. Virol J 5 137
13. ShawML
CardenasWB
ZamarinD
PaleseP
BaslerCF
2005 Nuclear localization of the Nipah virus W protein allows for inhibition of both virus - and toll-like receptor 3-triggered signaling pathways. J Virol 79 6078 6088
14. ShawML
Garcia-SastreA
PaleseP
BaslerCF
2004 Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J Virol 78 5633 5641
15. CiancanelliMJ
VolchkovaVA
ShawML
VolchkovVE
BaslerCF
2009 Nipah virus sequesters inactive STAT1 in the nucleus via a P gene-encoded mechanism. J Virol 83 7828 7841
16. WatanabeN
KawanoM
TsurudomeM
KusagawaS
NishioM
1996 Identification of the sequences responsible for nuclear targeting of the V protein of human parainfluenza virus type 2. J Gen Virol 77 Pt 2 327 338
17. YoshidaT
Nagai Y'YoshiiS
MaenoK
MatsumotoT
1976 Membrane (M) protein of HVJ (Sendai virus): its role in virus assembly. Virology 71 143 161
18. ColemanNA
PeeplesME
1993 The matrix protein of Newcastle disease virus localizes to the nucleus via a bipartite nuclear localization signal. Virology 195 596 607
19. PeeplesME
WangC
GuptaKC
ColemanN
1992 Nuclear entry and nucleolar localization of the Newcastle disease virus (NDV) matrix protein occur early in infection and do not require other NDV proteins. J Virol 66 3263 3269
20. PeeplesME
1988 Differential detergent treatment allows immunofluorescent localization of the Newcastle disease virus matrix protein within the nucleus of infected cells. Virology 162 255 259
21. GhildyalR
HoA
DiasM
SoegiyonoL
BardinPG
2009 The respiratory syncytial virus matrix protein possesses a Crm1-mediated nuclear export mechanism. J Virol 83 5353 5362
22. GhildyalR
HoA
WagstaffKM
DiasMM
BartonCL
2005 Nuclear import of the respiratory syncytial virus matrix protein is mediated by importin beta1 independent of importin alpha. Biochemistry 44 12887 12895
23. GhildyalR
Baulch-BrownC
MillsJ
MeangerJ
2003 The matrix protein of Human respiratory syncytial virus localises to the nucleus of infected cells and inhibits transcription. Arch Virol 148 1419 1429
24. FaabergKS
PeeplesME
1988 Strain variation and nuclear association of Newcastle disease virus matrix protein. J Virol 62 586 593
25. KanwalC
LiH
LimCS
2002 Model system to study classical nuclear export signals. AAPS PharmSci 4 E18
26. TerryLJ
ShowsEB
WenteSR
2007 Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 318 1412 1416
27. DingwallC
LaskeyRA
1991 Nuclear targeting sequences–a consensus? Trends Biochem Sci 16 478 481
28. EfthymiadisA
ShaoH
HubnerS
JansDA
1997 Kinetic characterization of the human retinoblastoma protein bipartite nuclear localization sequence (NLS) in vivo and in vitro. A comparison with the SV40 large T-antigen NLS. J Biol Chem 272 22134 22139
29. SchlenstedtG
1996 Protein import into the nucleus. FEBS Lett 389 75 79
30. FukudaM
AsanoS
NakamuraT
AdachiM
YoshidaM
1997 CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 390 308 311
31. KauTR
WayJC
SilverPA
2004 Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer 4 106 117
32. LohrumMA
WoodsDB
LudwigRL
BalintE
VousdenKH
2001 C-terminal ubiquitination of p53 contributes to nuclear export. Mol Cell Biol 21 8521 8532
33. LiM
BrooksCL
Wu-BaerF
ChenD
BaerR
2003 Mono - versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302 1972 1975
34. TrotmanLC
WangX
AlimontiA
ChenZ
Teruya-FeldsteinJ
2007 Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128 141 156
35. ShcherbikN
HainesDS
2004 Ub on the move. J Cell Biochem 93 11 19
36. HuangTT
Wuerzberger-DavisSM
WuZH
MiyamotoS
2003 Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell 115 565 576
37. RandowF
LehnerPJ
2009 Viral avoidance and exploitation of the ubiquitin system. Nat Cell Biol 11 527 534
38. IsaacsonMK
PloeghHL
2009 Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe 5 559 570
39. HendersonBR
EleftheriouA
2000 A comparison of the activity, sequence specificity, and CRM1-dependence of different nuclear export signals. Exp Cell Res 256 213 224
40. PankivS
LamarkT
BruunJA
OvervatnA
BjorkoyG
2010 Nucleocytoplasmic shuttling of p62/SQSTM1 and its role in recruitment of nuclear polyubiquitinated proteins to promyelocytic leukemia bodies. J Biol Chem 285 5941 5953
41. LiSY
DavidsonPJ
LinNY
PattersonRJ
WangJL
2006 Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export. Glycobiology 16 612 622
42. RodriguezJJ
CruzCD
HorvathCM
2004 Identification of the nuclear export signal and STAT-binding domains of the Nipah virus V protein reveals mechanisms underlying interferon evasion. J Virol 78 5358 5367
43. PatnaikA
ChauV
WillsJW
2000 Ubiquitin is part of the retrovirus budding machinery. Proc Natl Acad Sci U S A 97 13069 13074
44. SchubertU
OttDE
ChertovaEN
WelkerR
TessmerU
2000 Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci U S A 97 13057 13062
45. VogtVM
2000 Ubiquitin in retrovirus assembly: actor or bystander? Proc Natl Acad Sci U S A 97 12945 12947
46. MoritaE
SundquistWI
2004 Retrovirus budding. Annu Rev Cell Dev Biol 20 395 425
47. HoellerD
CrosettoN
BlagoevB
RaiborgC
TikkanenR
2006 Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol 8 163 169
48. QianSB
OttDE
SchubertU
BenninkJR
YewdellJW
2002 Fusion proteins with COOH-terminal ubiquitin are stable and maintain dual functionality in vivo. J Biol Chem 277 38818 38826
49. CarterS
BischofO
DejeanA
VousdenKH
2007 C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol 9 428 435
50. LeeJC
WangGX
SchicklingO
PeterME
2005 Fusing DEDD with ubiquitin changes its intracellular localization and apoptotic potential. Apoptosis 10 1483 1495
51. SpearmanP
WangJJ
Vander HeydenN
RatnerL
1994 Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J Virol 68 3232 3242
52. Hermida-MatsumotoL
ReshMD
2000 Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J Virol 74 8670 8679
53. RodgersW
2002 Making membranes green: construction and characterization of GFP-fusion proteins targeted to discrete plasma membrane domains. Biotechniques 32 1044 1046, 1048, 1050–1041
54. Shenoy-ScariaAM
GauenLK
KwongJ
ShawAS
LublinDM
1993 Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol 13 6385 6392
55. ElliottPJ
ZollnerTM
BoehnckeWH
2003 Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med 81 235 245
56. Sanchez-SerranoI
2006 Success in translational research: lessons from the development of bortezomib. Nat Rev Drug Discov 5 107 114
57. PapandreouCN
DalianiDD
NixD
YangH
MaddenT
2004 Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol 22 2108 2121
58. OgawaY
TobinaiK
OguraM
AndoK
TsuchiyaT
2008 Phase I and II pharmacokinetic and pharmacodynamic study of the proteasome inhibitor bortezomib in Japanese patients with relapsed or refractory multiple myeloma. Cancer Sci 99 140 144
59. HickeL
2001 Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2 195 201
60. JohnsonES
2002 Ubiquitin branches out. Nat Cell Biol 4 E295 298
61. TanakaT
SorianoMA
GrusbyMJ
2005 SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity 22 729 736
62. NatoliG
ChioccaS
2008 Nuclear ubiquitin ligases, NF-kappaB degradation, and the control of inflammation. Sci Signal 1 pe1
63. Martin-SerranoJ
2007 The role of ubiquitin in retroviral egress. Traffic 8 1297 1303
64. SaadJS
MillerJ
TaiJ
KimA
GhanamRH
2006 Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A 103 11364 11369
65. GonzaloS
LinderME
1998 SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol Biol Cell 9 585 597
66. ManieSN
de BreyneS
VincentS
GerlierD
2000 Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J Virol 74 305 311
67. BrownG
RixonHW
SugrueRJ
2002 Respiratory syncytial virus assembly occurs in GM1-rich regions of the host-cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin-1. J Gen Virol 83 1841 1850
68. AliA
NayakDP
2000 Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology 276 289 303
69. ReedLJ
MuenchH
1938 A simple method of estimating fifty percent endpoints. The American Journal of Hygiene 493 397
70. LimKL
ChewKC
TanJM
WangC
ChungKK
2005 Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25 2002 2009
71. GuoX
RoldanA
HuJ
WainbergMA
LiangC
2005 Mutation of the SP1 sequence impairs both multimerization and membrane-binding activities of human immunodeficiency virus type 1 Gag. J Virol 79 1803 1812
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



