-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
Occurrence of intraspecific variation in parasite virulence, a prerequisite for coevolution of hosts and parasites, has largely been reported. However, surprisingly little is known of the molecular bases of this variation in eukaryotic parasites, with the exception of the antigenic variation used by immune-evading parasites of mammals. The present work aims to address this question in immune suppressive eukaryotic parasites. In Leptopilina boulardi, a parasitic wasp of Drosophila melanogaster, well-defined virulent and avirulent strains have been characterized. The success of virulent females is due to a major immune suppressive factor, LbGAP, a RacGAP protein present in the venom and injected into the host at oviposition. Here, we show that an homologous protein, named LbGAPy, is present in the venom of the avirulent strain. We then question whether the difference in virulence between strains originates from qualitative or quantitative differences in LbGAP and LbGAPy proteins. Results show that the recombinant LbGAPy protein has an in vitro GAP activity equivalent to that of recombinant LbGAP and similarly targets Drosophila Rac1 and Rac2 GTPases. In contrast, a much higher level of both mRNA and protein is found in venom-producing tissues of virulent parasitoids. The F1 offspring between virulent and avirulent strains show an intermediate level of LbGAP in their venom but a full success of parasitism. Interestingly, they express almost exclusively the virulent LbGAP allele in venom-producing tissues. Altogether, our results demonstrate that the major virulence factor in the wasp L. boulardi differs only quantitatively between virulent and avirulent strains, and suggest the existence of a threshold effect of this molecule on parasitoid virulence. We propose that regulation of gene expression might be a major mechanism at the origin of intraspecific variation of virulence in immune suppressive eukaryotic parasites. Understanding this variation would improve our knowledge of the mechanisms of transcriptional evolution currently under active investigation.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001206
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001206Summary
Occurrence of intraspecific variation in parasite virulence, a prerequisite for coevolution of hosts and parasites, has largely been reported. However, surprisingly little is known of the molecular bases of this variation in eukaryotic parasites, with the exception of the antigenic variation used by immune-evading parasites of mammals. The present work aims to address this question in immune suppressive eukaryotic parasites. In Leptopilina boulardi, a parasitic wasp of Drosophila melanogaster, well-defined virulent and avirulent strains have been characterized. The success of virulent females is due to a major immune suppressive factor, LbGAP, a RacGAP protein present in the venom and injected into the host at oviposition. Here, we show that an homologous protein, named LbGAPy, is present in the venom of the avirulent strain. We then question whether the difference in virulence between strains originates from qualitative or quantitative differences in LbGAP and LbGAPy proteins. Results show that the recombinant LbGAPy protein has an in vitro GAP activity equivalent to that of recombinant LbGAP and similarly targets Drosophila Rac1 and Rac2 GTPases. In contrast, a much higher level of both mRNA and protein is found in venom-producing tissues of virulent parasitoids. The F1 offspring between virulent and avirulent strains show an intermediate level of LbGAP in their venom but a full success of parasitism. Interestingly, they express almost exclusively the virulent LbGAP allele in venom-producing tissues. Altogether, our results demonstrate that the major virulence factor in the wasp L. boulardi differs only quantitatively between virulent and avirulent strains, and suggest the existence of a threshold effect of this molecule on parasitoid virulence. We propose that regulation of gene expression might be a major mechanism at the origin of intraspecific variation of virulence in immune suppressive eukaryotic parasites. Understanding this variation would improve our knowledge of the mechanisms of transcriptional evolution currently under active investigation.
Introduction
Models of host-parasite coevolution all assume occurrence of genetic variation for host resistance and parasite virulence, such as well-described for plant-pathogen interactions [1] and in a few host-parasite models [2][3]. Advances in understanding coevolutionary interactions thus require unraveling the molecular bases of host resistance and parasite virulence, and then acquiring data on how polymorphism in genes controlling these traits affect parasitism success in the field [4]. This will enable direct observation, rather than inference, of the host-parasite coevolutionary dynamics.
One largely unaddressed question in eukaryotic parasites is the basis of intraspecific variation of virulence. Regarding immune aspects, the only described mechanism is, to our knowledge, the antigenic variation used by fungal and protozoan parasites to hide themselves from the mammalian immune system [5]–[10]. Whether changes associated with virulence variation in immune suppressive parasites are qualitative and/or quantitative and what is their nature, still remain to be assessed.
The biological models of interacting species that satisfy all the requirements to study coevolutionary processes, from molecular tools to population polymorphisms, are scarce and Drosophila-parasitoid wasps interactions are undoubtedly among the best of them [11]. Variation in both Drosophila resistance and parasitoid virulence is observed in the field [12]–[14], and some major genes involved in these traits have been characterized [15]–[18]. Based on these data, we address here the question of the origin of intraspecific variation of virulence of Drosophila parasitoids.
Endoparasitoid wasps develop inside the body of their arthropod host, which will die as a result of the interaction [19],[20]. To face the immune defense of the host, they have evolved original strategies ranging from displaying surface features that prevent their recognition to altering components of the host immune system using venom proteins, virus-like particles or wasp-specific viruses, polydnaviruses [21],[22]. However, occurrence of virulence variation together with molecular identification and characterization of the factors involved in immune suppression has only been reported in the wasp Leptopilina boulardi [15],[16],[23],[24].
Drosophila melanogaster immune response to a parasitoid consists in the formation of a multicellular melanized capsule around the wasp egg that results in the death of the parasitoid [25]. Plasmatocytes first attach to the parasitoid egg and spread around it, then lamellocytes adhere to them to form multiple cell layers [26],[27].
Two types of L. boulardi wasps have been described based on their virulence properties against Drosophila hosts. The ISm isofemale line, highly virulent against D. melanogaster (and so-called “virulent” line) is representative of Mediterranean L. boulardi wasps both in its virulence properties and venom protein profile [23],[28]. It produces in its venom a RacGAP domain-containing protein, named LbGAP, whose injection in D. melanogaster larvae mimics the egg protection provided by parasitism [23],[24],[29]. LbGAP has a RacGAP activity and induces changes in the morphology of D. melanogaster lamellocytes [23],[24]. It specifically interacts with and inactivates two Drosophila Rho GTPases, Rac1 and Rac2 [15], both required for successful encapsulation of Leptopilina eggs [27],[30]. Interestingly, ISm wasps are not virulent against the host species D. yakuba, and, accordingly, host lamellocytes remain unchanged in presence of ISm venom [31].
By contrast, the success of the ISy isofemale line of L. boulardi depends on the host phenotype and it was then called “avirulent” [12],[13]. This line originates from Congo, where variation of parasitism success toward D. melanogaster was observed, due to occurrence of a polymorphism in the L. boulardi virulence phenotype [13],[28]. This polymorphism is likely due to the fact that several Drosophila host species are available in tropical Africa that can successfully be parasitized, and that virulence toward D. melanogaster may be costly for the parasitoid [13],[28]. Using the avirulent ISy line, resistant and susceptible D. melanogaster reference strains were obtained from a sympatric Congolese D. melanogaster population [32]. However, resistance to ISy parasitoids is not restricted to Congo but is present at high frequencies in tropical as well as Mediterranean host populations [13].
ISy parasitoids are able to suppress the immune defenses of susceptible larvae of D. melanogaster as well as of other host species such as D. yakuba [12],[16]. However, their parasitism success is not associated with an alteration of the host lamellocyte shape [24],[31]. Accordingly, at the protein level, no major band of the size of LbGAP was observed in electrophoretic analysis of ISy venom producing tissues [23]. Taken together, all these data point to LbGAP as a major virulence factor in Mediterranean L. boulardi parasitoids, involved in variation of parasitism success against D. melanogaster. In ISy tropical parasitoids, the venom contains a serpin that alters melanisation in the hemolymph of D. yakuba larvae [16], and might be responsible for the delayed encapsulation induced by injection of total venom [31]. The way ISy females counteract the immune defenses of susceptible D. melanogaster flies is yet totally unknown.
In this study, we first confirm that LbGAP is required for virulence against resistant D. melanogaster hosts. We demonstrate that both the virulent and avirulent parasitoid lines produce a RacGAP protein in their venom, and we report the sequence of the RacGAP gene homologous to LbGAP from the avirulent line (LbGAPy). We then compare the activity of LbGAP and LbGAPy proteins, their level of interaction with their Rac targets and their localization in the lamellocytes of parasitized hosts. Finally, we present quantitative data on LbGAP and LbGAPy at the mRNA and protein levels on virulent, avirulent, and F1 parasitoids. Results show that differences between virulent and avirulent parasitoids regarding the RacGAP toxin are only quantitative and very likely due to variation in cis-regulation of gene expression.
Results
LbGAP is necessary for L. boulardi virulence
Previous work had shown that LbGAP is sufficient for successful parasitism of resistant D. melanogaster hosts by virulent parasitoid females. In order to determine whether this factor is also necessary for virulence, we performed experiments of injection of ISm venom in resistant D. melanogaster larvae, which is known to protect avirulent ISy eggs from encapsulation [24],[29]. In the present work, ISm venom was incubated before injection either with a specific polyclonal antibody against LbGAP or with the preimmune serum as a control, and larvae were then submitted to parasitism by ISy females. Venom incubated with the preimmune serum conferred active protection to avirulent eggs, with only 18.6% of encapsulation (Figure 1A). By contrast, incubation of ISm venom with the antibody against LbGAP led to 75.9% of avirulent eggs being encapsulated (Chi2 = 38.43 ; ddl = 1; p<0.001). A second experiment was performed in which the LbGAP antibody or the preimmune serum were injected alone into resistant host larvae that were subsequently parasitized by virulent ISm parasitoids. The encapsulation rates were 0% with the preimmune serum and 34.8% following injection of the LbGAP antibody, respectively (Chi2 = 24.49 ; ddl = 1 ; p<0.001; Figure 1B). These results demonstrate that LbGAP is a venom protein needed for L. boulardi virulence against resistant D. melanogaster flies.
Fig. 1. The RacGAP protein is necessary for virulence of ISm females. 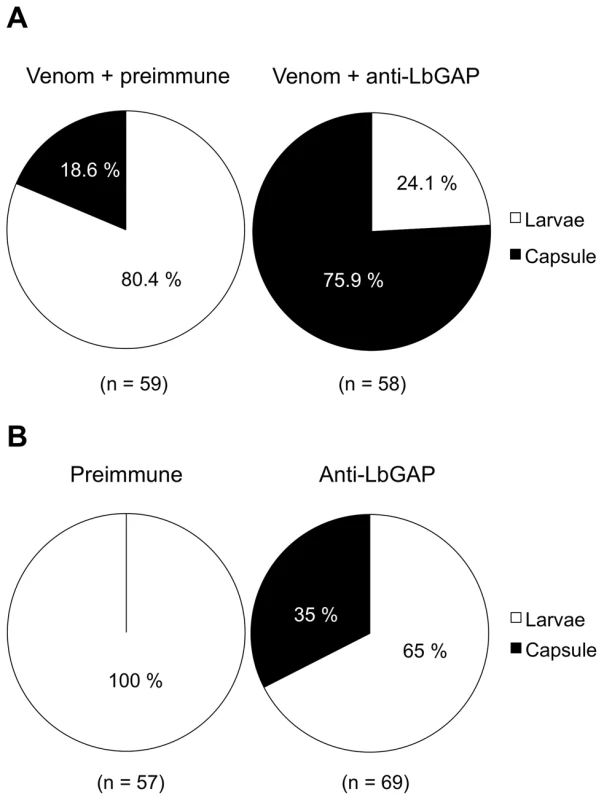
(A) D. melanogaster resistant L2 larvae were injected with ISm venom incubated either with the pre-immune serum as a control or a specific polyclonal antibody against LbGAP, then parasitized with the ISy avirulent line. The encapsulation rate was recorded after 48 h. In brackets are the numbers of injected larvae. (B) D. melanogaster resistant L2 larvae were injected with the pre-immune serum as a control or the specific antibody against LbGAP, and then parasitized with the ISm virulent line. The encapsulation rate was recorded after 48 h. In brackets are the numbers of injected larvae. A LbGAP homolog in the ISy L. boulardi line
Whether an homolog of LbGAP is expressed in ISy parasitoids was questioned by performing PCR experiments on cDNAs from ISy venom-producing tissues, using primers designed from the sequence of LbGAP [23]. A 914 bp amplicon was obtained whose sequence contains a 861 bp ORF (GenBank accession number GU300066) encoding a predicted protein of 286 amino acids that was named LbGAPy (Figure 2A). Like LbGAP, this protein starts with a N-terminal signal peptide of 20 amino acids allowing its extracellular export, and it contains a RhoGAP domain. The nucleotide sequences of LbGAP and LbGAPy are 95.2% identical, while LbGAP and LbGAPy proteins share 89.5% identity and 94.8% similarity (Figure 2B). LbGAPy contains the four amino acid residues Arg74, Lys111, Arg115 and Ser190, described to be involved in LbGAP interaction with Rac GTPases [15]. Arg74 is conserved in all GAP proteins and forms an arginine-finger that stabilizes the GTPase invariant glutamine residue 61 or 63 to facilitate the catalysis of GTP to GDP [33]. The main difference between LbGAPy and LbGAP sequences is located at the C-terminal end of the protein outside the RhoGAP domain (Figure 2B).
Fig. 2. Sequence analysis of LbGAPy, the LbGAP homolog from the ISy L. boulardi line. 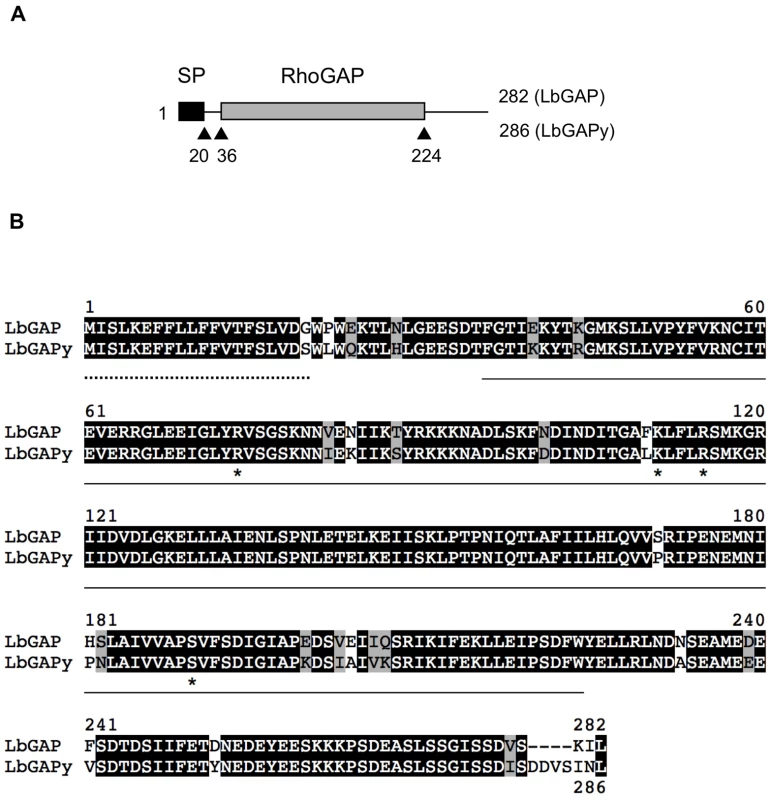
(A) Schematic representation of LbGAP and LbGAPy amino acid sequences. The signal peptide (SP) and the RhoGAP domain are shown as black and gray rectangles, respectively. (B) Sequence alignment of LbGAP and LbGAPy amino acid sequences. Residues identical or similar are highlighted in black and grey, respectively. The signal peptide is indicated by a dotted line. The RhoGAP domain is underlined. Stars identify LbGAP residues involved in the interaction with Rac GTPases. RacGAP activity of LbGAPy
LbGAP was previously shown to display a GAP activity with a strong preference for Rac-GTPases [15]. In order to determine if LbGAPy may have a similar GAP function in host cells, we carried out in vitro GAP assays using the LbGAPy protein produced in E. coli. Experiments were performed with human RhoA, Rac1 and Cdc42 Rho-GTPases. Human Ras, belonging to the Ras-GTPase family, was included as a negative control while LbGAP as well as the GAP domain from human p50 RhoGAP (which stimulates GTPase activities of RhoA, Rac1 and Cdc42 in vitro), were used as positive controls. Other negative controls consisted in the omission of either small G-protein or GAP protein. Similarly to LbGAP, LbGAPy significantly increased the GTPase activity of human Rac1 and Cdc42 but not of RhoA and Ras (F = 109.2; df = 11; p<0.001; Figure 3A). As for LbGAP, the GAP activity towards Rac1 was four times higher than towards Cdc42, suggesting that Rac-GTPases are the preferred substrates of LbGAPy as well.
Fig. 3. LbGAPy displays a GAP activity in vitro and interacts with D. melanogaster Rac1 and Rac2. 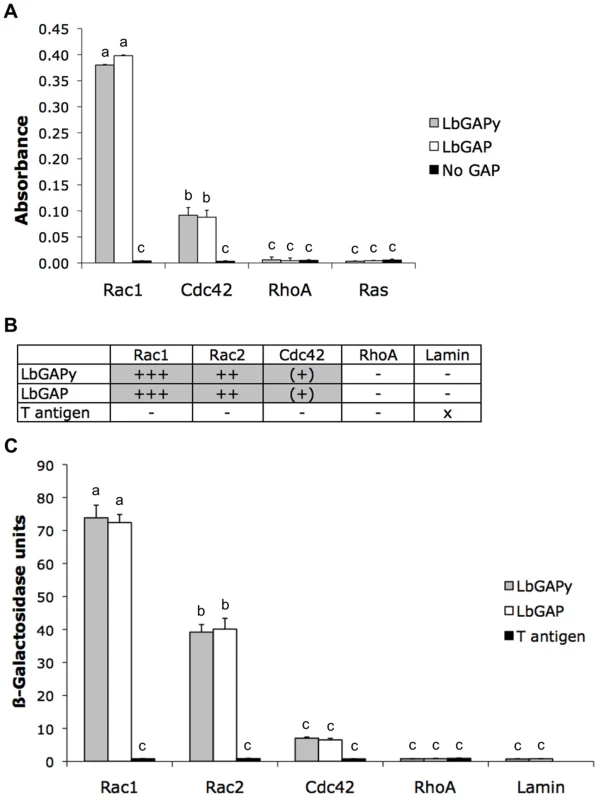
(A) Absorbance at 650 nm, which is correlated with the amount of Pi released from GTP-bound human Rac1, Cdc42, RhoA or Ras, was measured in the presence of LbGAPy or LbGAP or in the absence of any GAP. Bars: GAP activity in the presence of LbGAPy (grey bars), in the presence of LbGAP (open bars), in the absence of GAP (black bars). For each value, error bars represent the standard error of three measurements. (B) Results based on growth on selective medium lacking histidine and qualitative ß-galactosidase overlay assays. x : non-tested; − : no interaction; (+) : very weak interaction; ++ : mean interaction, ++++ : strong interaction. (C) Interactions with Rac1G12V, Rac2G12V, Cdc42G12V, RhoAG14V and Lamin (negative control) assayed by measuring beta-galactosidase activity in total protein extracts. Grey bars : beta-galactosidase activity in the presence of LbGAPy. Open bars : beta-galactosidase activity in the presence of LbGAP. Black bars : beta-galactosidase activity in the presence of T antigen (negative control). For each value, error bars represent the standard error of three measurements. Physical interaction of LbGAPy with Rac1 and Rac2 GTPases
As LbGAP is known to specifically interact with Drosophila Rac1 and Rac2 [15], we questioned whether LbGAPy similarly targets these Rac-GTPases by performing yeast two-hybrid analyses. In order to stabilize interactions, we used the G12V mutated forms of Drosophila Rac1, Rac2 and Cdc42 GTPases and the G14V mutated form of Drosophila RhoA. Each of these mutants is deficient in GTPase activity and therefore constitutively blocked in the GTP-bound active conformation. Fusions of the GAL4 activation domain with LbGAP were expressed in yeast together with fusions of the LexA-DNA binding domain either with Rac1G12V, Rac2G12V, Cdc42G12V or RhoAG14V. Direct in vivo interaction of LbGAPy with small GTPases was measured as the ability of transformed yeast to activate the transcription of HIS3 and lacZ reporter genes, both under the control of the LexA-binding sequences. Yeast growth on a selective medium lacking histidine revealed that, similarly to LbGAP, LbGAPy interacts with Rac1G12V and Rac2G12V but only weakly with Cdc42G12V, and has no interaction with RhoAG14V (Figure 3B).
The strength and specificity of the interaction between LbGAPy and Rac GTPases was then estimated by titration of ß-galactosidase activity (Figure 3C). Substantial activity was seen using coexpression of GAL4AD-LbGAPy and either LexABD-Rac1G12V or LexABD-Rac2G12V but not in combination with non-specific sequences. The beta-galactosidase activity resulting from the interaction between LbGAPy and Rac GTPases was similar to that obtained using LbGAP and the same Rac GTPases (F = 92.2; df = 13; p<0.001; Figure 3C), thus demonstrating that this interaction is as strong and specific as the one demonstrated with LbGAP.
Differences in LbGAP and LbGAPy amounts in host target cells
We previously showed that LbGAP enters plasmatocytes and lamellocytes in ISm-parasitized D. melanogaster larvae and that morphological changes in lamellocytes are correlated with the intracellular quantity of LbGAP [15]. Using the polyclonal antibody raised against LbGAP that equally recognizes LbGAPy (see below), immunolocalization experiments were performed on hemolymph from D. melanogaster larvae 48 hours following parasitization by either ISm or ISy females. As expected, the majority of lamellocytes from ISm-parasitized larvae had a modified morphology and contained LbGAP (red intracytoplasmic fluorescent dots; Figure 4A and 4B). In contrast, lamellocytes from ISy-parasitized larvae remained largely unmodified and very few contained LbGAPy dots (Figure 4C and 4D). Among these, the number of dots was usually less than five whereas many lamellocytes from ISm-parasitized larvae contained more than 30 dots.
Fig. 4. LbGAP and LbGAPy are detected in lamellocytes of parasitized D. melanogaster larvae. 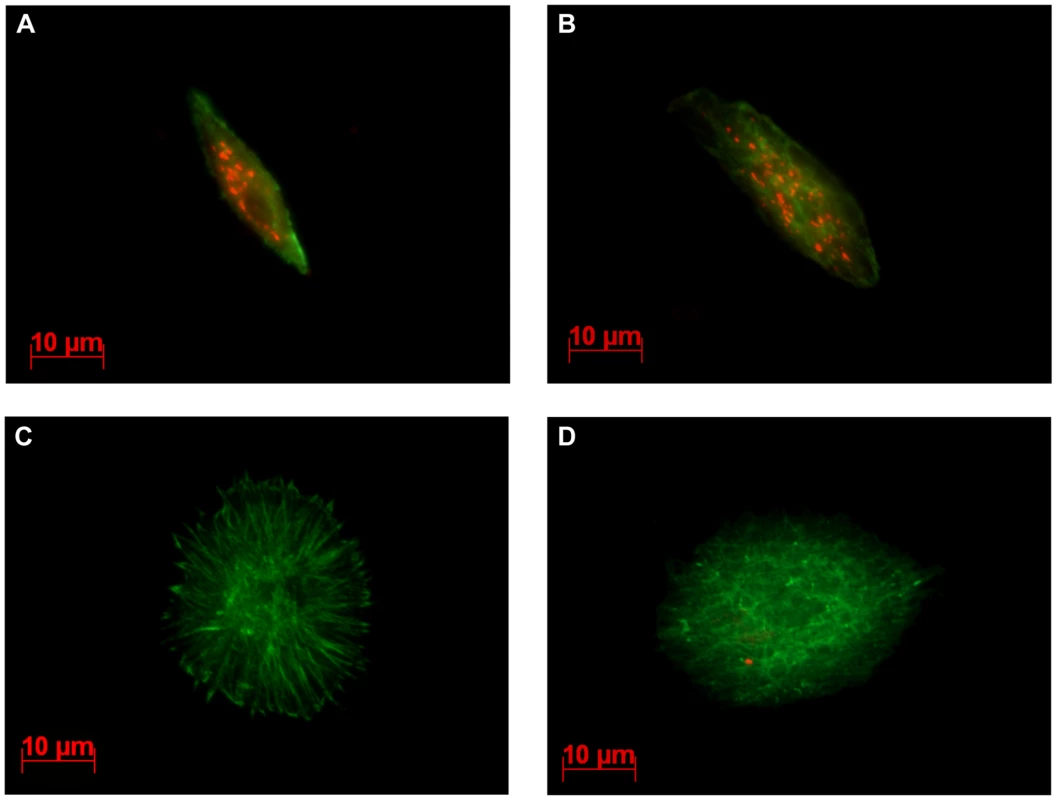
Immunocytochemical detection of LbGAP was performed on hemolymph collected from third-instar Drosophila larvae, 15hrs following parasitism. The LbGAP toxin is visualized as red spots while actin is detected by green labeled phalloidin. In larvae parasitized with ISm (A and B), most lamellocytes contain numerous LbGAP spots in their cytoplasm and their morphology is modified. In contrast, lamellocytes from larvae parasitized with ISy parasitoids are generally unchanged and without LbGAPy spots (C), a few of them containing a small number of LbGAPy spots (D). Different levels of LbGAP and LbGAPy expression in venom-producing tissues and female residual bodies
qRT-PCR experiments were performed on ISm and ISy female samples of the same age to quantify differences in expression levels of LbGAP and LbGAPy genes between venom-producing tissues and the rest of the bodies, and to compare expression levels of LbGAP and LbGAPy in their respective parasitoid line. A 2200-fold higher expression was observed for LbGAP in ISm venom-producing tissues as compared to the rest of the female body (Figure 5A; Student t-test : t = −19.1, df = 12, p<0.001). Expression of LbGAPy was also higher in ISy venom producing tissues than in the rest of the body, but only with a 270-fold higher expression level (Figure 5A; Student t-test: t = −16.6, df = 10, p<0.001). When comparing parasitoid strains, LbGAPy was approximately 30 times less expressed in ISy venom-producing tissues than LbGAP in ISm venom-producing tissues (Figure 5B; Student t-test: t = −9.4, df = 15, p<0.001).
Fig. 5. LbGAP expression in ISm females is higher than LbGAPy expression in ISy females. 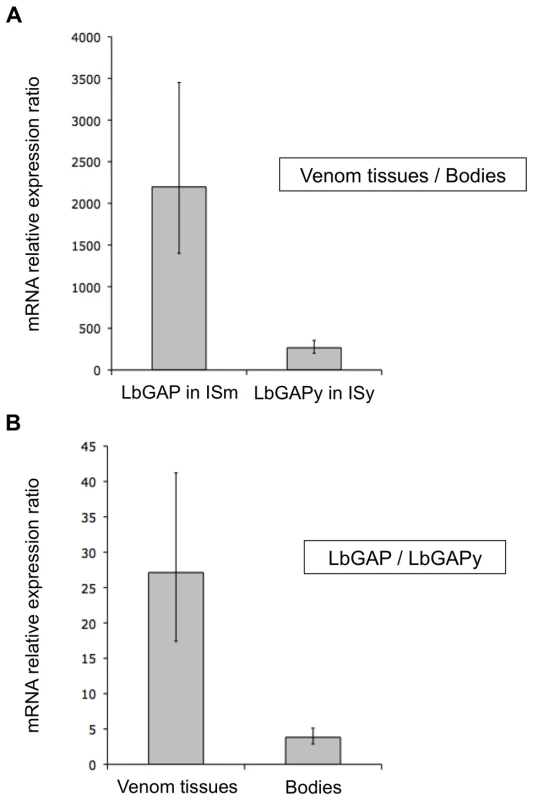
(A) Relative expression of LbGAP (ISm line) and LbGAPy (ISy line) in venom-producing tissues compared to the rest of the body without venom-producing tissues. (B) Ratio of relative expression of LbGAP (ISm line) compared to LbGAPy (ISy line) in venom-producing tissues and in the rest of the body without venom-producing tissues. For each value, error bars represent the standard error of three measurements. No significant difference in LbGAP and LbGAPy gene copy number
To determine whether the difference in the number of LbGAP and LbGAPy transcripts results from a difference in the number of gene copy, qPCR experiments were performed on genomic DNA from the venom-producing tissues and from the rest of the female bodies of ISm and ISy parasitoids, respectively. No significant differences were found between the copy number of the LbGAP gene in ISm venom producing tissues and ISm residual female bodies or of the LbGAPy gene in ISy venom producing tissues and ISy residual bodies (df = 3, F = 1.4651, p = 0.2953).
Differences in LbGAP and LbGAPy protein amounts in venom producing tissues
In previous Western blot experiments using a specific polyclonal antibody against the recombinant LbGAP protein, no signal was observed in ISy venom-producing tissues, possibly because the technique employed was not sensitive enough or because the antibody does not recognize LbGAPy [23]. These hypotheses were tested by producing both LbGAP and LbGAPy as GST-fusion proteins in Escherichia coli and using them to perform dot blot experiments on serial dilutions starting from the same quantity of these proteins. Our results show that the antibody recognizes specifically the LbGAP and LbGAPy recombinant proteins with the same efficiency (Figure 6A).
Fig. 6. LbGAP amount in ISm females is higher than LbGAPy amount in ISy females. 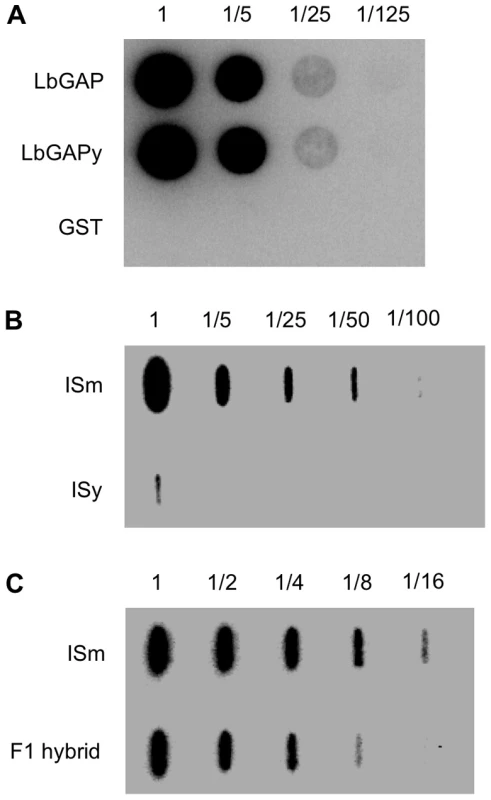
(A) Dot blot experiments on serial dilutions of the recombinant proteins GST-LbGAP and GST-LbGAPy using a LbGAP-specific polyclonal antibody. GST-tag alone was used as a control. (B) Slot blot on serial dilution of protein extract starting from twenty ISm and twenty ISy venom apparatus. (C) Slot blot on serial dilution of protein extract starting from five ISm and five F1 hybrid venom apparatus. We then performed dot blot experiments with serial dilutions of total protein extracts from 20 ISm and 20 ISy venom-producing tissues (which represent the same amount of protein), using the anti-LbGAP polyclonal antibody. LbGAP was easily detected in ISm sample dilutions while LbGAPy could only be detected in the undiluted ISy extracts. The quantity of LbGAPy was then estimated to be 60 times less than that of LbGAP in ISm venom tissues (Figure 6B).
A threshold effect of LbGAP quantity on virulence?
In order to further investigate the role of LbGAP quantity in ISm virulence, we performed crossing experiments between ISm females and ISy males and we assessed the virulence level of the F1 offspring, as well as the LbGAP/LbGAPy quantity in F1 venom-producing tissues, using dot-blot experiments. The amount of the LbGAP/LbGAPy proteins in F1 venom-producing tissues was approximately half the amount of LbGAP in ISm (Figure 6C). In parasitism experiments with the resistant strain of D. melanogaster in which ISy parasitoids are highly encapsulated (virulence level 5.7%), virulence of F1 hybrids (virulence level 98%) did not significantly differ from virulence of ISm parasitoids (virulence level 100% ; p<0.001).
Variation of expression of LbGAP and LbGAPy is likely under the control of cis-acting elements
To determine whether the variation of expression of LbGAP and LbGAPy is under the control of cis - or trans-acting elements, we performed PCR experiments on cDNA obtained from venom-producing tissues of ISm, ISy and F1 females. Two sets of primers were designed that respectively amplify LbGAP and LbGAPy and their specificity was tested using genomic DNA extracted from total bodies: a 670 bp and a 684 bp PCR product were amplified from ISm and ISy females, respectively, while both fragments were amplified from F1 individuals. Using ISm and F1 cDNAs and the LbGAP-specific primers, an intense band corresponding to a 252 bp amplicon was obtained. By contrast, only a faint band of 265 bp was observed using the LbGAPy-specific primers and cDNA from ISy or F1 individuals (Figure 7). Control of genomic DNA and cDNA quantities were performed using the internal transcribed spacer 2 (ITS2) gene.
Fig. 7. Variation of expression of LbGAP and LbGAPy. 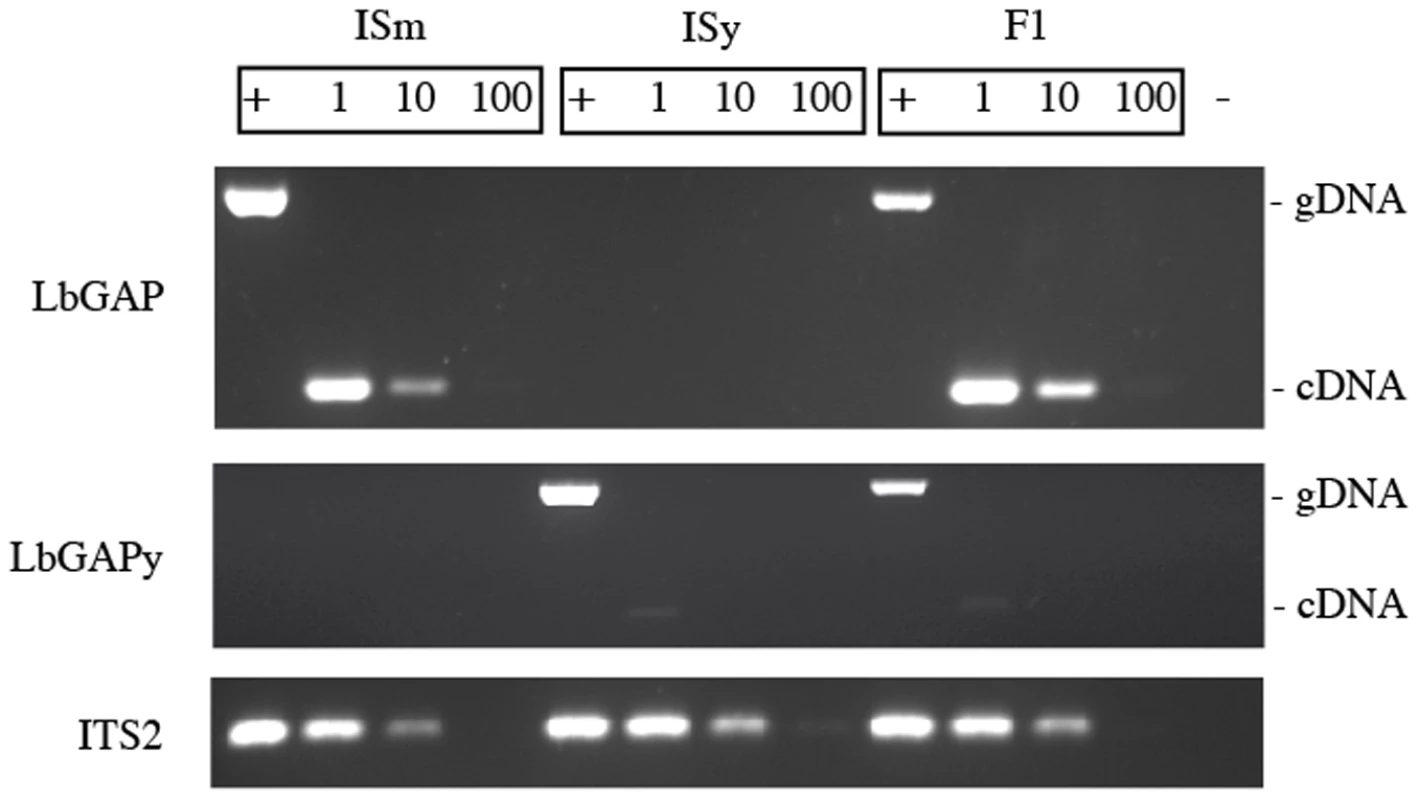
PCR experiments were performed on genomic DNA (+) to assess the specificity of the LbGAP- and LbGAPy-specific primers, and on serial dilutions of cDNA templates (1, 1/10, 1/100) to determine whether the variation of expression of LbGAP and LbGAPy is under the control of cis- or trans-acting elements. The ITS2 ribosomal sequence was used as control to assess the quantity of the DNA and RNA samples. (−) Negative control. Overall, these results show that F1 individuals overexpress the LbGAP and not the LbGAPy allele in venom tissues. This strongly suggests that variation of expression of LbGAP and LbGAPy is under the control of cis - rather than trans-acting elements.
Discussion
Intraspecific variation in virulence occurs in several eukaryotic parasite species [31],[34]–[36], but it has only been explained in some mammalian parasites such as Toxoplasma gondii, Trypanosoma brucei or Plasmodium falciparum. In these species, antigenic variation occurs, mediated by the differential expression of surface molecules [5]–[10]. In parasitoid wasps, intraspecific variation in virulence has been reported in three species, Asobara tabida, Cotesia sesamiae and L. boulardi. In A. tabida, it is associated with a difference in immunoevasion capacities [35] in relation with the degree of embedment of the parasitoid egg in host tissues [37],[38]. However, nothing is known of the mechanism by which A. tabida eggs adhere to host tissues. Parasitism success of C. sesamiae in the host Busseola fusca relies on the suppression of host immune defenses by polydnaviruses (PDVs) injected with the egg. Differences in the sequence of one PDV gene (CrV1) and in its level of expression in the host exist between virulent and avirulent parasitoids [34],[39] but CrV1 role in virulence of C. sesamiae has not been demonstrated.
In L. boulardi, two virulence factors have been characterized and extensively studied in the venom, one in each of two lines, ISm and ISy, that display opposite virulence properties towards Drosophila host species [15],[16],[23],[31],[40]. L. boulardi then certainly provides the best parasitoid model to address the issue of the molecular bases of variation in virulence of an immune suppressive parasite.
The LbGAP protein appears as a major band in venom protein electrophoretic patterns of all strains virulent against D. melanogaster analyzed to date, but it was not observed in the avirulent ISy line [23]. The demonstration that LbGAP represents a major toxin, sufficient for parasitoid virulence toward this host species, comes from experimental evidence that the proteins eluted from this band, when injected into host larvae, conferred the same protection to avirulent parasitoid eggs as injection of total ISm venom [23],[24],[29]. Here, we show that this protection is abolished if the venom is previously incubated with an anti-LbGAP antibody. Also, injection of the antibody into host larvae before parasitism significantly decreases the success of ISm parasitoids. LbGAP is thus the main factor responsible for protection of L. boulardi eggs in resistant D. melanogaster hosts, and it is necessary for parasitoid virulence. This might explain why virulence is reported to be controlled by a single chromosomal factor despite the presence of several proteins in the venom [41]. Variation in LbGAP is then likely responsible for most of the variation of virulence between L. boulardi strains.
Here, we show that a gene homologous to LbGAP (LbGAPy) is expressed in venom-producing tissues of the avirulent parasitoid line and that the protein is present in the venom. The previous extensive characterization of LbGAP [15],[23],[24] thus provided a unique opportunity to assess whether variation of virulence is due to quantitative differences in this toxin or to qualitative changes that would impair binding to its targets or reduce its activity.
LbGAP and LbGAPy deduced amino acid sequences are 89% identical, and both contain a signal peptide and a conserved GAP domain. The recombinant proteins have a similar level of GAP activity and they interact with the same host targets, in agreement with the conservation of critical interacting amino acid residues [15]. Altogether, no qualitative difference was observed between LbGAP and LbGAPy toxins in our functional assays that could explain variation in virulence between virulent and avirulent parasitoids. Occurrence of in vivo differences in protein binding or activity in fly hemocytes cannot be ruled out but is very unlikely.
We previously showed that LbGAP is present in high amounts inside lamellocytes of ISm parasitized hosts [15]. Following parasitism by ISy, LbGAPy could also be detected inside host lamellocytes, but in a much lower number of cells and, when present, in a much lower quantity compared to LbGAP. Moreover, the morphology of LbGAPy-containing lamellocytes remained unchanged, in agreement with the previous observation that LbGAP quantity in a cell correlates with the degree of shape alteration [15]. LbGAP and LbGAPy are then both able to “enter” host hemocytes but the quantity of LbGAP in ISm venom is 60-fold higher than that of LbGAPy in ISy venom. This difference is sufficient to explain the difference in the amount of the two toxins inside host lamellocytes. A different rate of entry between LbGAP and LbGAPy in host cells cannot be ruled out but it would not be detected given the low quantity of LbGAPy in ISy venom. Such a high difference in quantity is probably responsible for the absence of detection of LbGAPy in ISy venom in a previous study [23]. Interestingly, the toxin amount in venom was twice lower in F1 hybrids than in ISm parasitoids while F1 hybrids were as virulent as ISm on D. melanogaster resistant flies. This also supports the idea that a minimal quantity of LbGAP is necessary for L. boulardi success in resistant D. melanogaster, and suggests the possible existence of a threshold effect on LbGAP/LbGAPy quantity in the virulence phenotype.
The production of high amounts of LbGAP is probably under strong selection in Mediterranean areas where resistant D. melanogaster are often encountered as hosts [13]. The selection in tropical Africa would be relaxed due to the occurrence of alternative host species and to a possible cost of LbGAP overproduction. The reason why no resistance to virulent parasitoids has been described yet in D. melanogaster, while resistance to avirulent parasitoids is found at high frequencies, might be that resistance to injection of high amounts of LbGAP is difficult to evolve. Rac GTPases, the targets of LbGAP, are highly conserved proteins due to their key role in cell functions and target modification is unlikely to evolve. Some removal of LbGAP from the host hemolymph is performed via phagocytosis by host plasmatocytes [15]. However, the high quantity of the LbGAP injected, together with the fact that the toxin quickly enters host lamellocytes, may encompass phagocytosis capacities. Evolution of resistance would thus require evolution in the potential of detoxification by host hemocyte cells or of degradation of the toxin, for instance via host proteases. A connected question is the reason why LbGAP is not efficient on D. yakuba lamellocytes in spite of the total conservation of its Rac targets between the two host species. Answers might involve differences in their intrinsic potential of degradation of foreign proteins, the higher number of hemocytes cells recorded in D. yakuba [42], or differences in lamellocytes that would influence the capacity of “entry” of LbGAP.
Differences in the RacGAP protein amounts in venom of the two parasitoid lines were correlated with differences in the amount of LbGAP/LbGAPy mRNA in venom-producing tissues, while we found no difference in gene copy number using genomic DNA from venom tissues or residual bodies. This allowed us to conclude that variation in the RacGAP toxin between virulent and avirulent strains is mainly quantitative. It likely results from differences in regulation of gene transcription in venom-producing tissues, even if the hypothesis of a difference in LbGAP and LbGAPy mRNA stability cannot be totally ruled out.
One of the characteristics of most parasitoid venom proteins is their high amount in venom compared to other tissues, which often correlates with a high level of expression of their coding genes [16],[43]–[50]. Here, we found a much higher mRNA level of the RacGAP toxin in venom-producing tissues of the two parasitoid lines than in the rest of the body. Such a tissue-specific change of expression is one of the traits likely selected in the process of re-use of a protein as a virulence factor, because such factors need to be delivered into the host via injection of venom at each oviposition event [51]. Transcription of the LbGAP/LbGAPy gene is thus regulated both in a tissue-specific manner and differently between virulent and avirulent strains.
Changes in gene regulation are now believed to play a prominent role in evolution of biological diversity. Regulatory factors that control gene expression are mainly transcription factors that bind to cis-regulatory elements in the upstream sequences of the gene, and microRNAs (miRNA) [52]. Specific gene expression in parasitoid venom-producing tissues might be driven by the availability of transcription factors that are tissue-specific [53]. Differences in the binding level of transcription factors to cis-regulating sequences might explain the different level of expression between parasitoid lines. They could originate either from evolutionary changes in these sequences, as recently reported at the inter-specific level [54] or from variation in accessibility of cis-regulating sequences [55]. In L. boulardi F1 individuals between ISm and ISy strains, it is the LbGAP allele that is overexpressed in the venom, the transcription of the LbGAPy allele remaining very low. This result supports the hypothesis of variation in cis-regulation of LbGAP expression. Full characterization of the mechanisms involved in regulation of LbGAP transcription will involve cloning and comparing upstream gene sequences and promoter regions between parasitoid strains, characterizing binding of transcription factors, and if necessary analyzing the miRNA expressed in venom protein-secreting cells since changes in miRNA-mediated gene regulation [52] can allow a quick and reversible diversification of the gene expression program. This would provide insights in understanding the mechanisms of transcriptional evolution, currently under active investigation, at the intra-species level.
The regulation of transcription of a venom factor reported here is the first described mechanism at the origin of intraspecific variation in immune suppressive properties of a parasite. An open area of research is now to define how common is this mechanism and whether its occurrence is linked to the nature of the virulence factors, is in relation with the taxonomy, or might be parasitoid specific. Parasitoids are major auxiliaries in the control of insect pests and their host range and specificity are widely discussed in the literature [56],[57]. Estimations of the potential for evolution of virulence molecules and acquisition of new virulence factors in a parasitoid species are essential information to understand and improve the results of biological control assays.
Materials and Methods
Biological material
The origins of the L. boulardi ISy (Gif stock number 486) and ISm (Gif stock number 431) isofemale lines have been previously described [41]. Briefly, ISy derives from a single female originating from Brazzaville (Congo) while ISm derives from a single female collected in Nasrallah (Tunisia). ISm females are highly virulent against D. melanogaster while parasitism success of ISy females depends on the resistant/susceptible genotype of the host [12]. L. boulardi F1 hybrid females were obtained from crosses between ISm females and ISy males. Both ISm and ISy lines, as well as F1 hybrids, were reared on a susceptible D. melanogaster strain (Gif stock, number 1333), at 25°C. After emergence, adults of both lines were kept at 18°C on agar medium with honey.
Parasitism experiments
For parasitism experiments, the D. melanogaster YR strain (Gif stock, number 1088), resistant to L. boulardi ISy parasitoids, was used as host [32]. In each experiment, 30 second-instar host larvae (L2) were parasitized during 4 hours by one parasitoid female. The encapsulation ability was estimated 48 hours later by counting the number of encapsulated eggs after dissection of late third-instar larvae. Virulence was expressed as the ratio of non-encapsulated parasitoid eggs to the number of mono-parasitized hosts.
Injection experiments
The first injection experiment was performed using freshly collected venom from ISm parasitoid females. The venom apparatus of 20 individuals were carefully removed and placed in 20 µl of Ringer's saline solution. The sample was homogenized manually in an Eppendorf tube and the extract was centrifuged at 500×g, 4°C for 5 min to eliminate the cellular debris. 10 µl of the supernatant were then incubated during one hour at 4°C either with the preimmune serum or the specific anti-LbGAP polyclonal antibody, both diluted 1∶10. Finally, 20 nl of the incubated venom were injected in L2 D. melanogaster YR larvae using a Nanoject II injector (Drummond Scientific Company, Broomall, PA). Approximately 180 larvae were injected then parasitized by ISy parasitoid females as described above but during a two hours period.
In the second injection experiment, 20 nl of the preimmune serum or of the specific anti-LbGAP polyclonal antibody, both diluted 1∶10, were injected in approximately 180 L2 D. melanogaster YR larvae which were then parasitized by ISm females as described above.
Cloning and sequence analysis
To obtain the sequence of the LbGAPy cDNA, total RNA was extracted from L. boulardi ISy venom producing tissues using the TRIzol reagent (Invitrogen). RT-PCR was then performed using two specific primers designed from the cDNA sequence of LbGAP [23], 5′-CATAATTTTCAAATCTTCAACTTTTTTAGA-3′ and 5′-TTAGTCTCTGCACTTTTTCTCA TTTGATGT-3′. The amplified fragments were cloned into the pCR2.1-TOPO vector (Invitrogen) and sequenced.
Pairwise sequence comparisons were performed using the EMBOSS program Needle at EMBL-EBI (http://www.ebi.ac.uk/emboss/align/). The search for domains was performed using CDD (Conserved Domain Database) at NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) and InterProScan at EMBL-EBI (http://www.ebi.ac.uk/Tools/InterProScan/). Occurrence and position of the signal peptide cleavage site were predicted using SignalP at CBS (http://www.cbs.dtu.dk/services/SignalP/) and Phobius at SBC (http://phobius.sbc.su.se/).
In vitro GAP assays
In vitro GAP assays with recombinant GST-LbGAP and GST-LbGAPy proteins were performed in triplicates using the RhoGAP Assay Biochem Kit from Cytoskeleton Inc. P values were generated by ANOVA followed by pairwise comparisons using pairwise t tests performed with the R software package (http://www.r-project.org/).
Yeast two-hybrid analysis
The LbGAPy cDNA was inserted into the pGADT7 vector by homologous recombination in yeast strain JD53 (MATa, his3–200, leu2–3112, lys2–801, trp1–63, ura3–52). Interactions between LbGAP and mutated forms of RhoA, Rac1, Rac2 and Cdc42 GTPases were then examined individually by mating as previously described [15]. The plasmids expressing GTPase proteins were tested against the pGADT7 empty vector and the pGADT7-T control vector that encodes a fusion between the GAL4 activation domain and SV40 large T-antigen. Reciprocally, the plasmid producing LbGAPy was tested against the pLex-Lamin control vector. Interactions between LbGAP and Rac1 and Rac2 GTPases were used as positive controls [15]. Interactions were first tested by spotting five-fold serial dilutions of cells on minimal medium lacking histidine and supplemented with 3-amino-triazole at 0.5 mM to reduce the number of false positives. Quantification of ß-galactosidase activity in liquid assays was then performed according to the Yeast Protocols Handbook PT3024-1 (Clontech Laboratories, Inc.) except that yeast cells were lysed using glass beads (Sigma). P values were generated by ANOVA followed by pairwise comparisons using pairwise t tests performed with the R software package.
Production of recombinant proteins
GST-LbGAP was produced using a previously obtained construct [23] corresponding to the full-length LbGAP cDNA (without the signal peptide) cloned into the pGEX-5X-1 vector (GE Healthcare). For production of GST-LbGAPy, a cDNA fragment corresponding to the mature LbGAPy protein was amplified by RT-PCR from total RNA from ISy venom-producing tissues. The amplified fragment was cloned into the pGEX-4T-2 vector (GE Healthcare) using EcoRI and XhoI restriction sites. Competent BL21 Escherichia coli cells were subsequently transformed with the recombinant plasmids. The production and purification of the GST-LbGAP and GST-LbGAPy fusion proteins and GST alone were performed according to the GST Gene Fusion System Handbook (GE Healthcare).
Immunocytochemistry
Immunocytochemical experiments were performed as previously described [15], using a rabbit anti-LbGAP polyclonal antibody [23] and Phalloidin-X5-FluoProbe 505 (Interchim) to visualize F-actin.
Quantitative real-time RT-PCR
Total RNA was isolated either from dissected venom apparatus or from the rest of the female bodies (without venom-producing tissues) using the TRIzol reagent (Invitrogen), and reverse-transcribed using the iScript cDNA Synthesis Kit (BioRad). qPCR reactions were then carried out on an Opticon monitor 2 (BioRad) using the Absolute qPCR SYBR MasterMix Plus for SYBR Green I No ROX (Eurogentec) and the specific primers 5′-TGAAAGGGCGAATAATTGATG-3′ and 5′-TTTGGTGGAAGTTTGGAA-3′ for LbGAP and LbGAPy, respectively. PCR conditions were as follows: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 60°C for 30 s and 68°C for 30 s. Each reaction was performed in triplicate and the mean of three independent biological replicates (venom-producing tissues) or two independent biological replicates (rest of the bodies) was calculated. All data were normalized using the ITS2 (Internal Transcribed Spacer 2) ribosomal sequence as a control and results were analyzed using the ΔCt method. P values were generated by Student's t test with the R software package.
Quantitative real-time PCR
Genomic DNA was isolated either from dissected venom apparatus or from the rest of female bodies (without venom apparatus) using the DNeasy Blood & Tissue Kit (QIAGEN). qPCR reactions were then carried out as described above. Each reaction was performed in triplicate and the mean of three independent biological replicates was calculated. All data were normalized using the ITS2 ribosomal sequence as a control and analyzed using qBase software (http://medgen.ugent.be/qbase/). P values were generated by ANOVA followed by pairwise comparisons using pairwise t tests performed with the R software package.
PCR and RT-PCR experiments with primers specific to LbGAP and LbGAPy
Genomic DNA isolation, total RNA isolation, and reverse transcription were performed as described above. The specific primer pairs were 5′-CTCCTGAAGACAGTGTAGAAATTATTC-3′ and 5′-GAATTTTTGAAACATCACTCGAAATA-3′ for LbGAP and 5′-GCTCCT AAAGACAGTATAGCAATTGTTA-3′ and 5′-AGATTAATTGAAACATCATCCGAAAT-3′ for LbGAPy. PCR was performed using GoTaq DNA Polymerase (Promega) as follows: 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 60°C for 30 s and 72°C for 45 s. Serial 1∶10 dilutions were used for cDNA templates. Amplification products were analyzed on a 2% ethidium bromide-stained agarose gel.
Blotting experiments
For dot blot experiments with recombinant GST-LbGAP and GST-LbGAPy proteins, serial 1∶5 dilutions, starting from 10 ng of recombinant protein, were blotted onto a nitrocellulose membrane. GST alone was used as a negative control. Non-specific binding sites were blocked by overnight incubation at 4°C with TBST-2% milk buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.3% Tween 20). The membrane was probed 1 h with the LbGAP antiserum used at a 1∶5000 dilution. After three washes with TBST buffer, specifically bound antibodies were detected using anti-rabbit IgG horseradish peroxidase conjugate (Sigma) used at a 1∶15000 dilution for a 1 h incubation period. The membrane was revealed using the chemiluminescent Immobilon Western substrate (Millipore). Relative spot intensities were digitalized and quantified using GeneSnap and GeneTools softwares (Syngene).
For slot blot experiments comparing ISm and ISy extracts, 20 female venom apparatus were dissected in 20 µl of Ringer's solution and centrifuged for 2 min at 500×g. The supernatant was then diluted in 200 µl of denaturation solution (10 mM Tris-HCl pH 8, 100 mM NaPO4, 8 M Urea) and blotted onto a nitrocellulose membrane. For slot blot experiments comparing ISm and F1 hybrid extracts, only 5 female venom apparatus were used for each strain. The quantity of protein in the samples was determined using the Coo Protein Assay (Biorad) and found to be equivalent between the different parasitoid strains.
Accession numbers
The GenBank accession number for the nucleotide sequence of LbGAPy is GU300066.
Zdroje
1. FrankSA
1992 Models of plant-pathogen coevolution. Trends Genet 8 213 219
2. EbertD
2008 Host–parasite coevolution: Insights from the Daphnia–parasite model system. Curr Opin Microbiol 11 290 301
3. DubuffetA
ColinetD
AnselmeC
DupasS
CartonY
2009 Variation of Leptopilina boulardi success in Drosophila hosts: what is inside the black box ? Adv Parasitol 70 147 188
4. AllenDE
LittleTJ
2009 Exploring the molecular landscape of host-parasite coevolution. Cold Spring Harb Symp Quant Biol 74 169 176
5. CrabbBS
CowmanAF
2002 Plasmodium falciparum virulence determinants unveiled. Genome Biol 3 11 reviews1031
6. ChookajornT
PonsuwannaP
CuiL
2008 Mutually exclusive var gene expression in the malaria parasite: multiple layers of regulation. Trends Parasitol 24 455 61
7. DelibasSB
ErtabaklarH
ErtugS
2006 Evaluation of antigenic variations between two virulent toxoplasma strains. J Med Microbiol 55 1333 1335
8. HutchinsonOC
PicozziK
JonesNG
MottH
SharmaR
2007 Variant Surface Glycoprotein gene repertoires in Trypanosoma brucei have diverged to become strain-specific. BMC Genomics 8 234
9. PaysE
VanhammeL
Pérez-MorgaD
2004 Antigenic variation in Trypanosoma brucei: facts, challenges and mysteries. Curr Opin Microbiol 7 369 374
10. ScherfA
Lopez-RubioJJ
RiviereL
2008 Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol 62 445 70
11. DupasS
DubuffetA
CartonY
PoiriéM
2009 Local, geographic and phylogenetic scales of coevolution in Drosophila-parasitoid interactions. Adv Parasitol 70 281 295
12. DubuffetA
DupasS
FreyF
DrezenJ-M
PoiriéM
2007 Genetic interactions between the parasitoid wasp Leptopilina boulardi and its Drosophila hosts. Heredity 98 21 27
13. DupasS
CartonY
PoiriéM
2003 The genetic dimension of the coevolution of virulence resistance in Drosophila-parasitoid wasps relationships. Heredity 90 84 89
14. KraaijeveldAR
GodfrayHC
1999 Geographic patterns in the evolution of resistance and virulence in Drosophila and its parasitoids. Am Nat 153 S61 S74
15. ColinetD
SchmitzA
DepoixD
CrochardD
PoiriéM
2007 Convergent use of RhoGAP proteins by eukaryotic parasites and bacterial pathogens. PLoS Pathog 3 e203
16. ColinetD
DubuffetA
CazesD
MoreauS
DrezenJ
2009 A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev Comp Immunol 33 681 689
17. HitaMT
PoiriéM
LeblancN
LemeunierF
LutcherF
1999 Genetic localization of a Drosophila melanogaster resistance gene to a parasitoid wasp and physical mapping of the region. Genome Res 9 471 81
18. HitaM
EspagneE
LemeunierF
PascualL
CartonY
2006 Mapping candidate genes for Drosophila melanogaster resistance to the parasitoid wasp Leptopilina boulardi. Genet Res 88 81 91
19. GodfrayHJC
1994 Parasitoids: Behavioral and Evolutionary Ecology Princeton University Press 488
20. QuickeDLJ
1997 Parasitic wasps London Cambridge University Press edition 492
21. MoreauSJM
GuillotS
2005 Advances and prospects on biosynthesis, structures and functions of venom proteins from parasitic wasps. Insect Biochem Mol Biol 35 1209 1223
22. PennacchioF
StrandMR
2006 Evolution of developmental strategies in parasitic Hymenoptera. Annu Rev Entomol 51 233 258
23. LabrosseC
StasiakK
LesobreJ
GrangeiaA
HuguetE
2005 A RhoGAP protein as a main immune suppressive factor in the Leptopilina boulardi (Hymenoptera, Figitidae)-Drosophila melanogaster interaction. Insect Biochem Mol Biol 35 93 103
24. LabrosseC
EslinP
DouryG
DrezenJM
PoiriéM
2005 Haemocyte changes in D. melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: a Rho-GAP protein as an important factor. J Insect Physiol 51 161 170
25. CartonY
PoiriéM
NappiAJ
2008 Insect immune resistance to parasitoids. Insect Science 15 67 87
26. RussoJ
DupasS
FreyF
CartonY
BrehelinM
1996 Insect immunity: early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology 112 135 142
27. WilliamsMJ
AndoI
HultmarkD
2005 Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes Cells 10 813 823
28. DupasS
BoscaroM
1999 Geographic variation and evolution of immunosuppressive genes in a Drosophila parasitoid. Ecography 22 284 291
29. LabrosseC
CartonY
DubuffetA
DrezenJM
PoiriéM
2003 Active suppression of D. melanogaster immune response by long gland products of the parasitic wasp Leptopilina boulardi. J Insect Physiol 49 513 522
30. WilliamsMJ
WiklundML
WikmanS
HultmarkD
2006 Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci 119 2015 2024
31. DubuffetA
DouryG
LabrousseC
DrezenJ-M
CartonY
2008 Variation of success of Leptopilina boulardi in Drosophila yakuba: the mechanisms explored. Dev Comp Immunol 32 597 602
32. CartonY
FreyF
NappiA
1992 Genetic determinism of the cellular immune reaction in Drosophila melanogaster. Heredity 69 393 399
33. RittingerK
WalkerPA
EcclestonJF
NurmahomedK
OwenD
1997 Crystal structure of a small G protein in complex with the GTPase-activating protein RhoGAP. Nature 389 758 762
34. GitauCW
Gundersen-RindalD
PedroniM
MbugiJP
DupasS
2007 Differential expression of the CrV1 haemocyte inactivation-associated polydnavirus gene in the African maize stem borer Busseola fusca (Fuller) parasitized by two biotypes of the endoparasitoid Cotesia sesamiae (Cameron). J Insect Physiol 53 676 684
35. KraaijeveldAR
van AlphenJ
1994 Geographical variation in resistance of the parasitoid Asobara tabida against encapsulation by Drosophila melanogaster larvae: the mechanisms explored. Physiol Entomol 19 9 14
36. Ramos-MartinezE
Olivos-GarciaA
SaavedraE
NequizM
SanchezEC
2009 Entamoeba histolytica: oxygen resistance and virulence. Int J Parasitol 39 693 702
37. MonconduitH
PrévostG
1994 Avoidance of encapsulation by Asobara tabida, a larval parasitoid of Drosophila species. Norw J Agric Sci 16 9 14
38. EslinP
GiordanengoP
FourdrainY
PrévostG
1996 Avoidance of encapsulation in the absence of VLP by a braconid parasitoid of Drosophila larvae: an ultrastructural study. Can J Zool 74 2193 2198
39. DupasS
GitauCW
BrancaA
Le RuBP
SilvainJF
2008 Evolution of a polydnavirus gene in relation to parasitoid-host species immune resistance. J Hered 99 491 499
40. RussoJ
BrehelinM
CartonY
2001 Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi. J Insect Physiol 47 167 172
41. DupasS
FreyF
CartonY
1998 A single parasitoid segregating factor controls immune suppression in Drosophila. J Hered 89 306 311
42. DupasS
MorandS
EslinP
2004 Evolution of hemocyte concentration in the melanogaster subgroup species. C R Biol 327 139 47
43. AsgariS
ReinekeA
BeckM
SchmidtO
2002 Isolation and characterization of a neprilysin-like protein from Venturia canescens virus-like particles. Insect Mol Biol 11 477 485
44. CrawfordAM
BrauningR
SmolenskiG
FergusonC
BartonD
2008 The constituents of Microctonus sp. parasitoid venoms. Insect Mol Biol 17 313 324
45. de GraafDC
AertsM
BrunainM
DesjardinsCA
JacobsFJ
2010 Insights into the venom composition of the ectoparasitoid wasp Nasonia vitripennis from bioinformatic and proteomic studies. Insect Mol Biol 19 11 26
46. ParkinsonN
SmithI
WeaverR
EdwardsJP
2001 A new form of arthropod phenoloxidase is abundant in venom of the parasitoid wasp Pimpla hypochondriaca. Insect Biochem Mol Biol 31 57 63
47. PriceD
BellH
HinchliffeG
FitchesE
WeaverR
2009 A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae). Insect Mol Biol 18 195 202
48. VinchonS
MoreauSJM
DrezenJM
PrévostG
CherquiA
2010 Molecular and biochemical analysis of an aspartylglucosaminidase from the venom of the parasitoid wasp Asobara tabida (Hymenoptera: Braconidae). Insect Biochem Mol Biol 40 38 48
49. ZhangG
SchmidtO
AsgariS
2006 A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev Comp Immunol 30 756 764
50. ZhuJY
YeGY
HuC
2008 Molecular cloning and characterization of acid phosphatase in venom of the endoparasitoid wasp Pteromalus puparum (Hymenoptera: Pteromalidae). Toxicon 51 1391 1399
51. PoiriéM
CartonY
DubuffetA
2009 Virulence strategies in parasitoid Hymenoptera as an example of adaptive diversity. C R Biol 332 311 320
52. HobertO
2008 Gene regulation by transcription factors and microRNAs. Science 319 1785 1786
53. WilsonMD
OdomDT
2009 Evolution of transcriptional control in mammals. Curr Opin Genet Dev 19 579 585
54. WittkoppP
2010 Variable transcription factor binding: A mechanism of evolutionary change. PLoS Biol 8 e1000342
55. BradleyRK
LiX-Y
TrapnellC
DavidsonS
PachterL
2010 Binding site turnover produces pervasive quantitative changes in transcription factor binding between closely related Drosophila species. PLoS Biol 8 e1000343
56. CoryJS
MyersJH
2000 Direct and indirect ecological effects of biological control. Trends Ecol Evol 15 137 139
57. LoudaSM
PembertonRW
JohnsonMT
FollettPA
2003 Non target effects-the Achilles' heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu Rev Entomol 48 365 396
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



