-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPotentiation of Epithelial Innate Host Responses by Intercellular Communication
The epithelium efficiently attracts immune cells upon infection despite the low number of pathogenic microbes and moderate levels of secreted chemokines per cell. Here we examined whether horizontal intercellular communication between cells may contribute to a coordinated response of the epithelium. Listeria monocytogenes infection, transfection, and microinjection of individual cells within a polarized intestinal epithelial cell layer were performed and activation was determined at the single cell level by fluorescence microscopy and flow cytometry. Surprisingly, chemokine production after L. monocytogenes infection was primarily observed in non-infected epithelial cells despite invasion-dependent cell activation. Whereas horizontal communication was independent of gap junction formation, cytokine secretion, ion fluxes, or nitric oxide synthesis, NADPH oxidase (Nox) 4-dependent oxygen radical formation was required and sufficient to induce indirect epithelial cell activation. This is the first report to describe epithelial cell-cell communication in response to innate immune activation. Epithelial communication facilitates a coordinated infectious host defence at the very early stage of microbial infection.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001194
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001194Summary
The epithelium efficiently attracts immune cells upon infection despite the low number of pathogenic microbes and moderate levels of secreted chemokines per cell. Here we examined whether horizontal intercellular communication between cells may contribute to a coordinated response of the epithelium. Listeria monocytogenes infection, transfection, and microinjection of individual cells within a polarized intestinal epithelial cell layer were performed and activation was determined at the single cell level by fluorescence microscopy and flow cytometry. Surprisingly, chemokine production after L. monocytogenes infection was primarily observed in non-infected epithelial cells despite invasion-dependent cell activation. Whereas horizontal communication was independent of gap junction formation, cytokine secretion, ion fluxes, or nitric oxide synthesis, NADPH oxidase (Nox) 4-dependent oxygen radical formation was required and sufficient to induce indirect epithelial cell activation. This is the first report to describe epithelial cell-cell communication in response to innate immune activation. Epithelial communication facilitates a coordinated infectious host defence at the very early stage of microbial infection.
Introduction
Intestinal epithelial cells line the enteric mucosal surface and provide a physical barrier to maintain the integrity of this vulnerable body surface and prevent invasive infection by luminal microorganisms. Like professional immune cells, intestinal epithelial cells express receptors of the innate immune system such as Toll-like receptors (TLR) or nuclear oligomerization domain (NOD)-like receptors (NLR) [1], [2]. Recognition of microbial structures leads to epithelial production of antimicrobial effector molecules and proinflammatory chemoattractive mediators. Thus, it facilitates an active role in the initiation of the mucosal host response [3], [4], [5]. The recruitment of professional immune cells to the site of infection occurs within hours and provides a highly efficient dynamic mechanism of the epithelial host defence. It remains unclear, however, how low number of pathogenic microorganisms as well as the limited spectrum and only moderate amount of chemokine secretion per epithelial cell facilitates stimulation of an effective host defence. We therefore hypothesized that a horizontal intercellular communication between intestinal epithelial cells might help to induce a coordinated epithelial response towards infectious challenge and thereby to amplify the epithelial innate host defence.
Listeria monocytogenes is an important human pathogen that causes meningitis, sepsis, and abortion in susceptible individuals. It is acquired with food such as unpasteurized milk and cheese and enters the body following penetration through the intestinal epithelial barrier. The microbial pathogenesis and the bacteria-host cell interaction of this facultative intracellular bacterium has been studied for many years [6]. L. monocytogenes induces its own internalization and subsequently lyses the endosomal membrane of its host cell by the secretion of listeriolysin O (LLO) and phospholipases, thus gaining access to the cytosolic space. Here, Listeria upregulates polar expression of ActA that recruits and polymerizes host actin filaments resulting in propulsive locomotion. Together with LLO and the phospholipases this allows to enter neighbouring cells and to spread within the epithelial cell layer. Importantly, recognition of Listeria by the epithelial innate immune system only occurs after internalization and lysis of the endosomal membrane through cytosolic innate immune receptors [7], [8], [9], [10]. Since infection of individual cells can be traced using reporter gene technology, L. monocytogenes provides an excellent model to study cellular responses in respect to immune recognition at the single cell level.
In the present study, we analyzed innate immune recognition and epithelial responses at the single cell level using the model of Listeria infection of polarized intestinal epithelial cells in addition to transfection and microinjection. We present the surprising finding that non-infected epithelial cells were the main source of chemokine secretion in response to bacterial challenge. We identify oxygen radical species produced by NADPH oxidase (Nox) 4 in response to cytosolic bacteria to facilitate horizontal intercellular communication and chemokine production by non-infected cells. These results provide the first experimental evidence for a yet unknown mechanism of intercellular communication between epithelial cells in response to innate immune stimulation and thus significantly broaden our understanding of mucosal innate host defence.
Results
Invasion-dependent recognition but indirect epithelial cell activation after Listeria infection
Infection of a confluent monolayer of intestinal epithelial m-ICcl2 cells with wild-type (wt) L. monocytogenes induced rapid cellular activation illustrated by secretion of the proinflammatory chemokine Cxcl-2 (Fig. 1A). Strong epithelial activation was only observed using wt Listeria able to reach the cytosolic space (Fig. 1B) facilitating recognition by cytoplasmic innate immune receptor molecules (Fig. 1C) [7], [8], [9], [10]. Bacterial mutants unable to lyse the endosomal membrane such as isogenic hly or hly/plcA/plcB triple mutants as well as heat inactivated bacteria exhibited a significantly reduced or even absent epithelial activation (Fig. 1B and D). Of note, lack of hly or hly, plcA, and plcB expression did not affect bacterial invasion or intracellular viability (Fig. S1A). Endosomal lysis-dependent stimulation of L. monocytogenes infected epithelial cells was also observed using flow cytometry. A time-dependent increase of the number of Cxcl-2+ and Cxcl-5+ epithelial cells was detected after infection with wt Listeria (Fig. 1E). In contrast, a strongly reduced number of epithelial cells stained positive for Cxcl-2 after infection with hly mutant Listeria (Fig. 1F). In accordance with the published literature, internalization-dependent activation was observed in epithelial cells, but not in macrophages (Fig. S1B). These results suggested that activation of epithelial cells occurred primarily in directly Listeria-infected cells.
Fig. 1. Listeria-induced activation of intestinal epithelial cells is largely dependent on invasion and endosomal lysis. 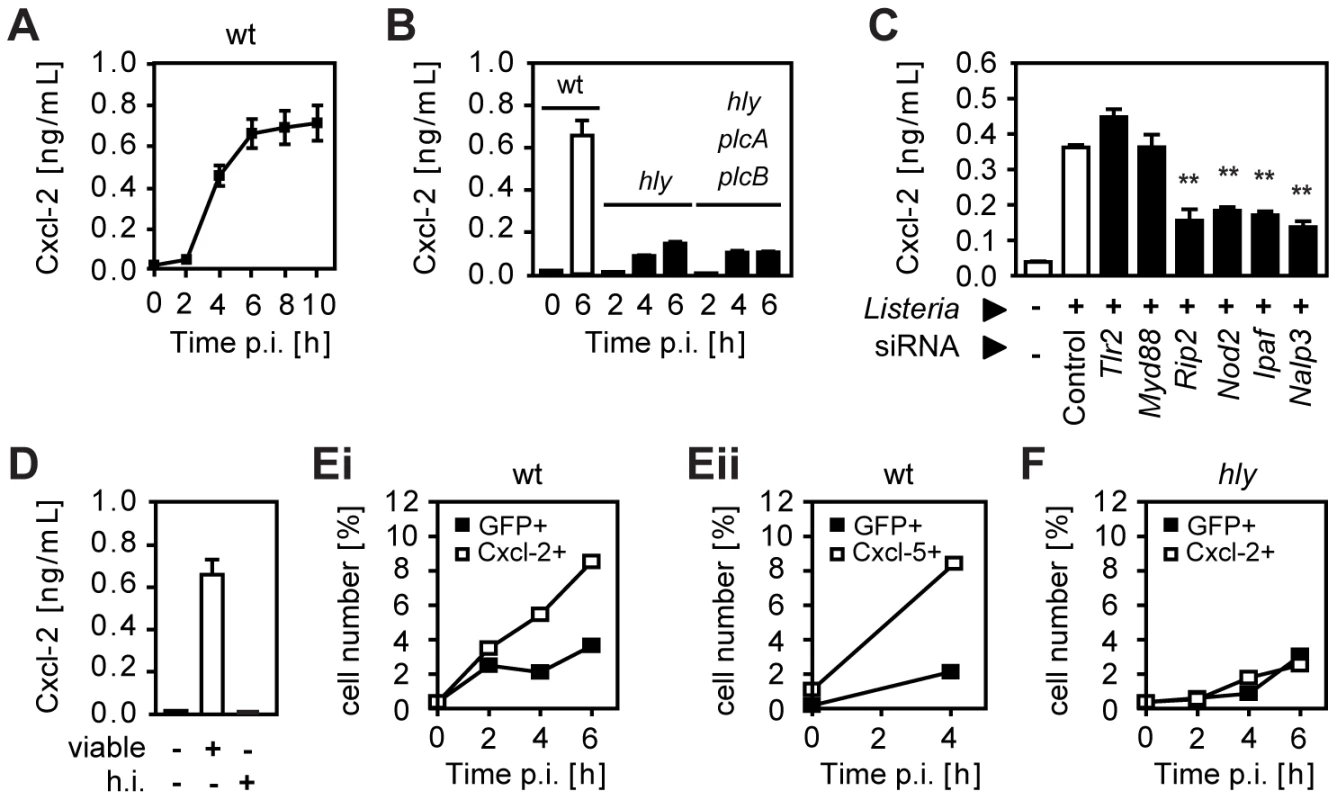
(A) m-ICcl2 cells were infected with wild-type (wt) Listeria monocytogenes. Cxcl-2 was determined after the indicated time in cell culture supernatant by ELISA. (B) m-ICcl2 cells were infected with wt, hly mutant or hly/plcA/plcB triple mutant L. monocytogenes. Cxcl-2 was determined after the indicated time in cell culture supernatant by ELISA. (C) m-ICcl2 cells were treated with small interfering RNA (siRNA) control or siRNA Tlr2, MyD88, Rip2, Nod2, Ipaf or Nalp3 and infected with actA mutant L. monocytogenes. Cxcl-2 was determined 4 h after infection in cell culture supernatant by ELISA. **, p<0.01. (D) m-ICcl2 cells were infected with viable or heat inactivated (h.i.) wt L. monocytogenes. Cxcl-2 was determined 6 h after infection in cell culture supernatant by ELISA. (E) m-ICcl2 cells were infected with wt L. monocytogenes expressing green fluorescence protein (GFP) under control of the actA promoter (PactA-gfp). The number of GFP+ (Listeria-infected, black square) or immunolabelled (Ei) Cxcl-2+ or (Eii) Cxcl-5+ (white square) cells was determined after the indicated time by flow cytometry. (F) m-ICcl2 cells were infected with hly mutant PactA-gfp L. monocytogenes. The number of GFP+ (Listeria-infected, black square) or immunolabelled Cxcl-2+ (white square) cells was determined after the indicated time by flow cytometry. All experiments were performed at a multiplicity of infection of 100∶1. Results are representative for three independent experiments and are presented as mean ± SD (ELISA) or show one representative experiment. To monitor Listeria infection and cellular activation simultaneously at the single cell level, bacteria transformed with a vector expressing green fluorescence protein (GFP) either under control of the inducible actA promoter [11] or the constitutive sod promoter [12] were used for subsequent experiments (for details see Table 1). Surprisingly, flow cytometry revealed that the vast majority of Cxcl-2+ epithelial cells (95%) were Listeria-negative. In addition, only a minor fraction of GFP-positive, Listeria-infected epithelial cells exhibited MIP-2 synthesis (Fig. 2A). Similar results were obtained using biotinylated Listeria (Fig. S2A). These results were confirmed by immunohistological staining. Cxcl-2 and Cxcl-5 synthesis was not restricted to GFP+ Listeria-infected cells but, in fact, predominantly detected in neighbouring non-infected epithelial cells (Fig. 2B and Fig S2B). Also flow cytometric cell sorting and quantitative RT-PCR analysis strongly supported this unexpected result. A marked upregulation of Cxcl-2 and Cxcl-5 mRNA expression was detected in GFPlow expressing (Listeria-negative, Fig. S2C) epithelial cells despite the absence of detectable Listeria DNA (Fig. 2C). Thus, although epithelial activation requires lysis of the endosomal membrane and contact with cytosolic innate immune receptors, a transcriptional cellular response was mainly observed in non-infected cells.
Fig. 2. Analysis of bacterial infection and epithelial activation at the single cell level. 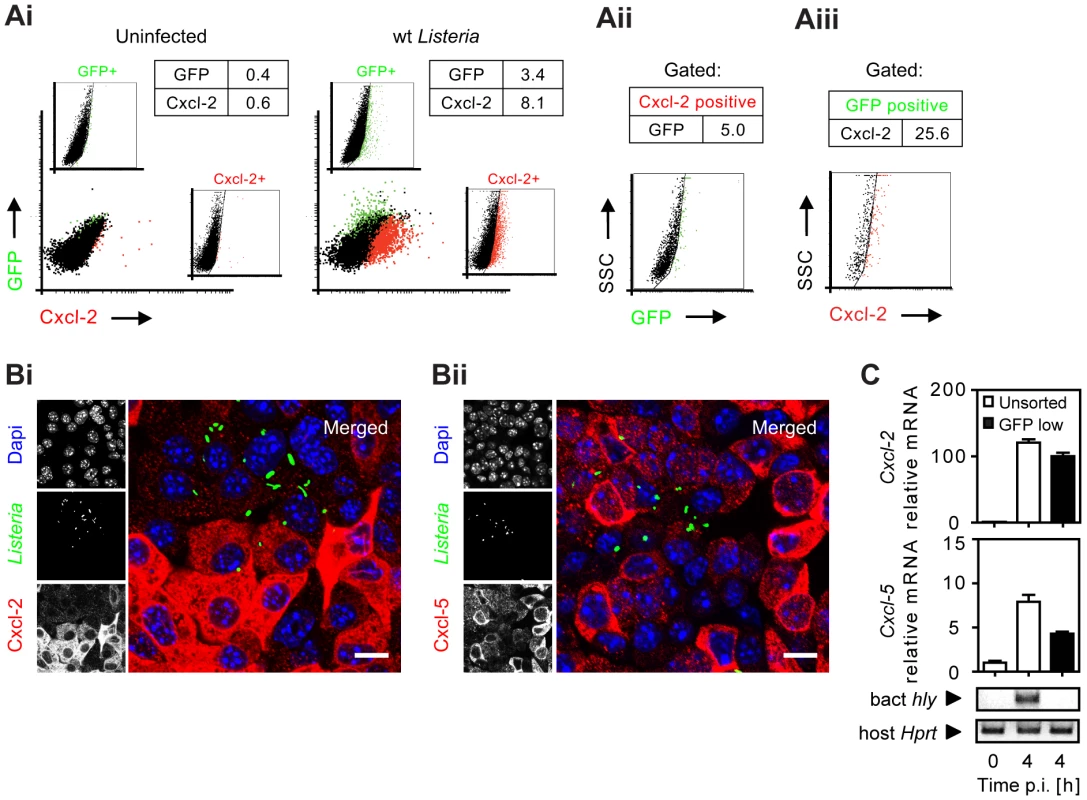
(A) m-ICcl2 cells were left uninfected (Ai, left) or infected with wt PactA-gfp L. monocytogenes (Ai, right). The number [%] of GFP+ (Listeria-infected, green) or immunolabelled Cxcl-2+ (red) cells was visualized 4 h after infection by flow cytometry. Single channel analysis (GFP: FL-1; Cxcl-2: FL-4) was depicted on the side of the axis. (Aii) The number of GFP+ (Listeria-infected) cells among activated, Cxcl-2+ cells and (Aiii) the proportion of Cxcl-2+ cells among GFP+ (Listeria-infected) cells was demonstrated gating on the respective population. (B) m-ICcl2 cells were infected with wt PactA-gfp L. monocytogenes. Intracellular Listeria (GFP+, green) and immunolabelled (Bi) Cxcl-2 (red) or (Bii) Cxcl-5 (red) was visualized 4 h after infection by fluorescence microscopy. Magnification ×400, counterstaining with Dapi (blue). Scale bar, 5 µm. (C) m-ICcl2 cells were infected with actA mutant L. monocytogenes expressing constitutively GFP under control of the sod promoter (Psod-gfp). 4 h after infection the GFPlow expressing (Listeria-negative) cell fraction was sorted by flow cytometry (see Fig. S2C) and analysed for Cxcl-2 (upper) or Cxcl-5 (lower) mRNA expression by RT-PCR. The absence of detectable Listeria in the sorted GFPlow cell fraction was demonstrated by PCR amplification of the bacterial hly gene (sensitivity limit: 103–104 genome copies). All experiments were performed at a multiplicity of infection of 100∶1. Results are representative for three independent experiments and are presented as mean ± SD (RT-PCR) or show one representative experiment. Tab. 1. Listeria strains used in this study. 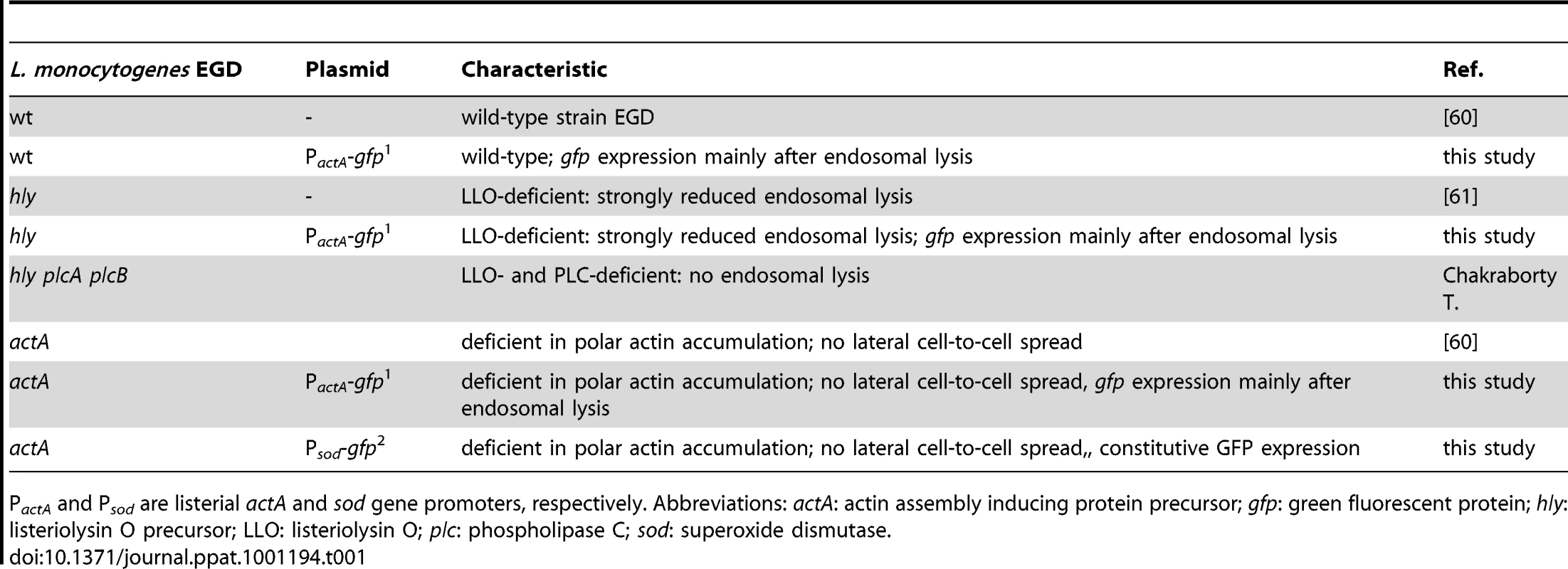
PactA and Psod are listerial actA and sod gene promoters, respectively. Abbreviations: actA: actin assembly inducing protein precursor; gfp: green fluorescent protein; hly: listeriolysin O precursor; LLO: listeriolysin O; plc: phospholipase C; sod: superoxide dismutase. Cell-to-cell spread, attachment-induced activation, or listeriolysin are not responsible for indirect cell activation
Several mechanisms might account for the observed activation of Listeria-negative epithelial cells. Activated epithelial cells might only appear to be Listeria-negative due to secondary bacterial escape facilitated by propulsion through ActA-induced actin polymerization in the cytosol and subsequent invasion of the neighbouring cell. Cells primarily infected but secondarily left by lateral spread might thereby appear Listeria-negative but in fact would have been previously in contact with cytosolic bacteria (and thus were, in fact, directly activated). To avoid lateral cell-to-cell spread and restrict intraepithelial bacteria to apically infected cells, a Listeria actA mutant strain was employed. ActA-deficient Listeria exhibited a moderately reduced epithelial invasion (Fig. S3A), a lower percentage of infected epithelial cells, and an enhanced number of bacteria per cell (Fig. 3A and 3B). Nevertheless, high numbers of Cxcl-2 producing epithelial cells (Fig. 3B) and a strong chemokine secretion (Fig. 3C) was observed. Also, the number of activated, Cxcl-2 producing epithelial cells remained significantly higher than the number of Listeria-infected cells reaching approximately 10-fold excess of Cxcl-2+ cells (Fig. 3B and Fig. S3B). Thus, indirect activation of epithelial cells was not due to escape from previously infected cells by ActA-driven secondary lateral spread.
Fig. 3. Epithelial activation is not due to bacterial cell-to-cell spread, extracellular attachment, or secreted listeriolysin. 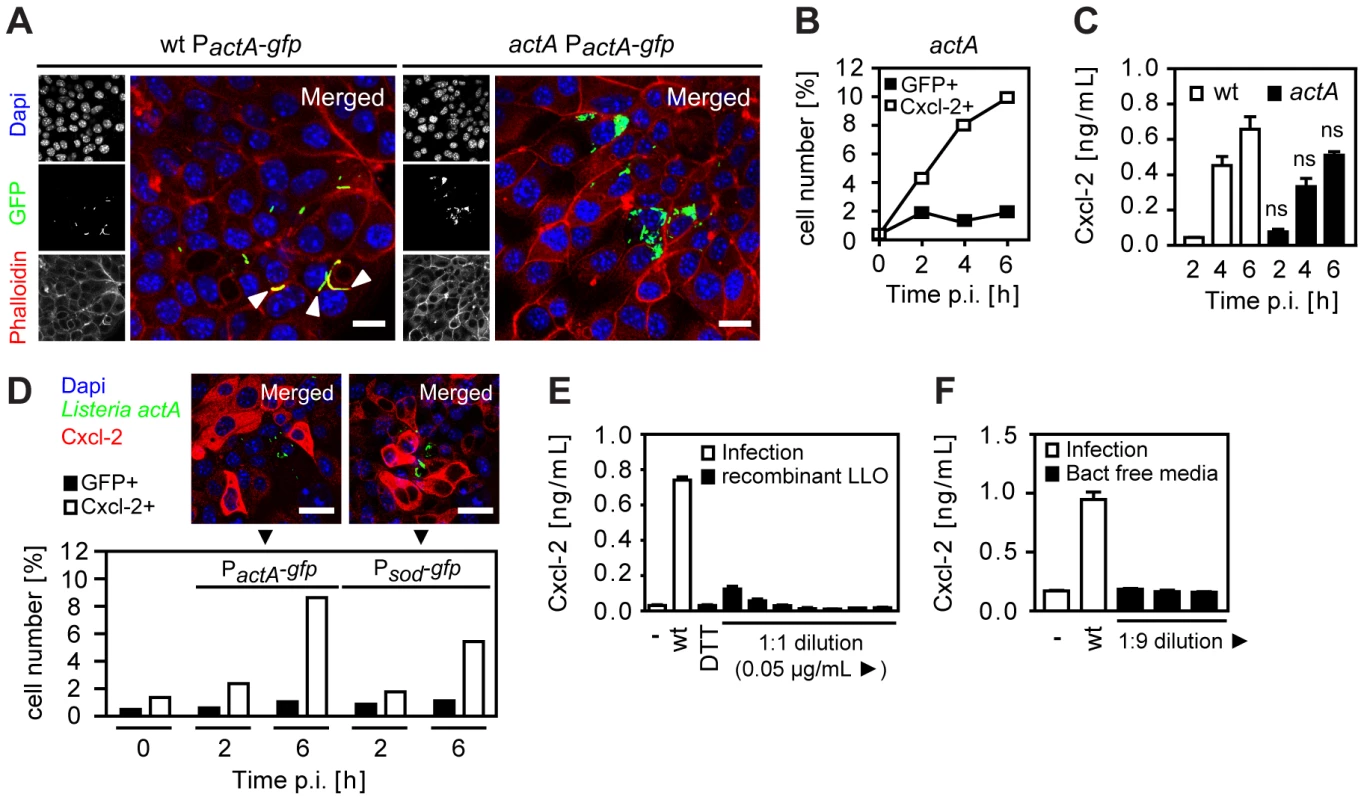
(A) m-ICcl2 cells were infected with wt (left) or actA mutant (right) PactA-gfp Listeria monocytogenes. Intracellular Listeria (GFP+, green) was visualized 6 h after infection by fluorescence microscopy. Magnification ×400, counterstaining with phalloidin (red) and Dapi (blue). White arrows indicate the actin tail assembly by wt Listeria. Scale bar, 5 µm. (B) m-ICcl2 cells were infected with actA mutant PactA-gfp L. monocytogenes. The number of GFP+ (Listeria-infected, black square) or immunolabelled Cxcl-2+ (white square) cells was determined after the indicated time by flow cytometry. (C) m-ICcl2 cells were infected with wt (white) or actA mutant (black) L. monocytogenes. Cxcl-2 was determined after the indicated time in cell culture supernatant by ELISA; ns, not significant (mutant versus wt for the indicated time points). (D) m-ICcl2 cells were infected with actA mutant PactA-gfp or Psod-gfp L. monocytogenes. The number of GFP+ (Listeria-infected, black) or immunolabelled Cxcl-2+ (white) cells was determined after the indicated time by flow cytometry (lower panel). Additionally, intracellular Listeria (GFP+, green) and immunolabelled Cxcl-2 (red) was visualized 4 h after infection by fluorescence microscopy (upper panel). Magnification ×400, counterstaining with Dapi (blue). Scale bar, 5 µm. (E) m-ICcl2 cells were infected with wt L. monocytogenes or exposed to recombinant listeriolysin at lytic to sublytic concentrations or to the solvent control (DTT). Cxcl-2 was determined 4 h after infection in cell culture supernatant by ELISA. (F) m-ICcl2 cells were infected with wt L. monocytogenes or exposed to undiluted or diluted filtered cultures (bact free media, normalised for multiplicity of infection of 100∶1) of wt L. monocytogenes grown in m-ICcl2 cell culture media. Cxcl-2 was determined 4 h after infection in cell culture supernatant by ELISA. All infection experiments were performed at a multiplicity of infection of 100∶1. Results are representative for three independent experiments and are presented as mean ± SD (ELISA) or show one representative experiment. Epithelial cell stimulation could also be induced by bacteria either attached to the plasma membrane or remaining intraendosomal and membrane enclosed. To exclude a significant role of attached or intraendosomal Listeria, Cxcl-2+ and GFP+ epithelial cells were quantified after infection with Listeria expressing GFP either constitutively under control of the superoxide dismutase promoter (Psod-gfp) or inducible under the control of the actA promoter (PactA-gfp) (Table 1). Whereas Psod-gfp carrying Listeria exhibited strong reporter expression after growth in bacterial culture medium, only a moderate fluorescence was detected in PactA-gfp –positive bacteria (Fig. S3C). In contrast, strong GFP expression was noted in PactA-gfp Listeria isolated from infected epithelial cells (Fig. S3D). Flow cytometric detection of infected epithelial cells was observed after wt, but not hly mutant PactA-gfp Listeria illustrating the endosomal lysis-dependent induction of the actA promoter-driven GFP reporter gene expression (Fig. S3E). Infection with Psod-gfp or PactA-gfp carrying wt Listeria resulted in a significant number of Listeria-infected epithelial cells. Importantly, a higher number of Cxcl-2+ activated cells as compared to Listeria-infected cells was observed by flow cytometry after infection with both reporter constructs and epithelial Cxcl-2 synthesis was similarly noted in Listeria-negative epithelial cells (Fig. 3D). These results suggest that indirect epithelial activation was not a result of attached or intraendosomal bacteria [13].
Finally, the activation of Listeria-negative epithelial cells might be due to the stimulatory effect of secreted bacterial molecules, such as the cytolytic listeriolysin O (LLO) [14], [15]. Therefore, the membrane damaging as well as the stimulatory effect of recombinant listeriolysin (rLLO) on red blood cells (RBC) and epithelial m-ICcl2 cells was analysed. High concentrations of rLLO induced significant hemoglobin and detectable lactate dehydrogenase (LDH) release by RBCs and epithelial m-ICcl2 cells, respectively (Fig. S3F and Fig. S3G). Quantitation of epithelial cell activation in response to rLLO, however, revealed an only minor response as compared to epithelial Cxcl-2 secretion after viable wt L. monocytogenes infection (Fig. 3E). Similarly, no significant Cxcl-2 secretion by epithelial cells was noted in response to bacteria-free culture supernatant derived from Listeria cultures with bacterial counts precisely corresponding to the infection model described above (Fig. 3F). Yet, culture supernatants derived from wild-type or ActA-deficient bacteria exhibited significant hemolytic activity, in contrast to supernatant from hly-deficient Listeria (Fig. S3H). No significant membrane damage was noted after infection of intestinal epithelial cells with wt, actA, or hly mutant Listeria (Fig. S3I). Although a supportive effect of released bacterial factors cannot be excluded, these results suggest that bacterial mediators do not play a major role in the observed indirect epithelial activation. Thus, neither basolateral cell-to-cell spread nor membrane attachment, or the secretion of LLO in the cell culture supernatant appear to be responsible for indirect epithelial cell activation after L. monocytogenes infection. This suggests the presence of a previously unrecognized mechanism of epithelial intercellular communication in response to bacterial infection.
Indirect epithelial activation is not induced by epithelial transcriptional activation per se
To examine whether indirect epithelial stimulation by horizontal cell-to-cell communication might be a general effect of transcriptional activation of intestinal epithelial cells, a bicistronic expression vector encoding the NF-κB subunit RelA/p65 together with GFP under the control of a constitutive cytomegalovirus (CMV) promoter was employed (Fig. S4A). Transient overexpression of RelA/p65 alone or bicistronic expression of RelA/p65 and GFP readily induced epithelial activation as illustrated by NF-κB reporter gene upregulation (Fig. S4B) and enhanced chemokine secretion (Fig. S4C). Although RelA/p65-mediated Cxcl-2 production exhibited a slower kinetic as compared to following Listeria infection, a significant number of Cxcl-2+ cells was detected. Of note, RelA/p65-mediated cellular activation was restricted to GFP+, i.e. directly activated epithelial cells (Fig. S4D). Cxcl-2 production by GFP+ cells increased strongly (0.1 versus 2.4%), whereas the number of Cxcl-2+ cells in the GFP- population remained virtually unchanged (0.8% versus 1.2%). In addition, the number of Cxcl-2+ epithelial cells did not exceed the number of transfected GFP+ cells at any time (Fig. S4E). Thus, epithelial activation per se does not induce indirect cell activation by horizontal intercellular communication. Indirect epithelial activation appears rather to be induced by innate immune signal transduction upstream of transcription factor activation.
Analysis of cytokine secretion, ion channel stimulation, and gap junction activity in horizontal cell-cell communication
Next we investigated the mechanism underlying horizontal cell-to-cell communication and coordinated epithelial chemokine upregulation in response to Listeria infection. Functional gap junctional transport was examined by microinjection of transferable Lucifer Yellow together with non-transferable high molecular weight dextran. Fluorescence imaging visualized transport of Lucifer Yellow from the microinjected cell to the surrounding neighbouring cells. Addition of inhibitors of gap junctional transport, effectively reduced lateral diffusion of Lucifer Yellow after microinjection (Fig. 4A and B). Inhibition of gap junctional intercellular communication, however, did not decrease the number of activated epithelial cells after Listeria infection as illustrated by the unaltered high ratio of activated (Cxcl-2+) to infected (GFP+) epithelial cells measured by flow cytometry (Fig. 4C). Although these results do not completely rule out transfer of very small signaling molecules by gap junctional transport channels, they do not support a major role in the process of horizontal communication.
Fig. 4. Inhibition of gap junctional transport, cytokine and prostaglandin secretion, or ion fluxes does not affect epithelial intercellular communication. 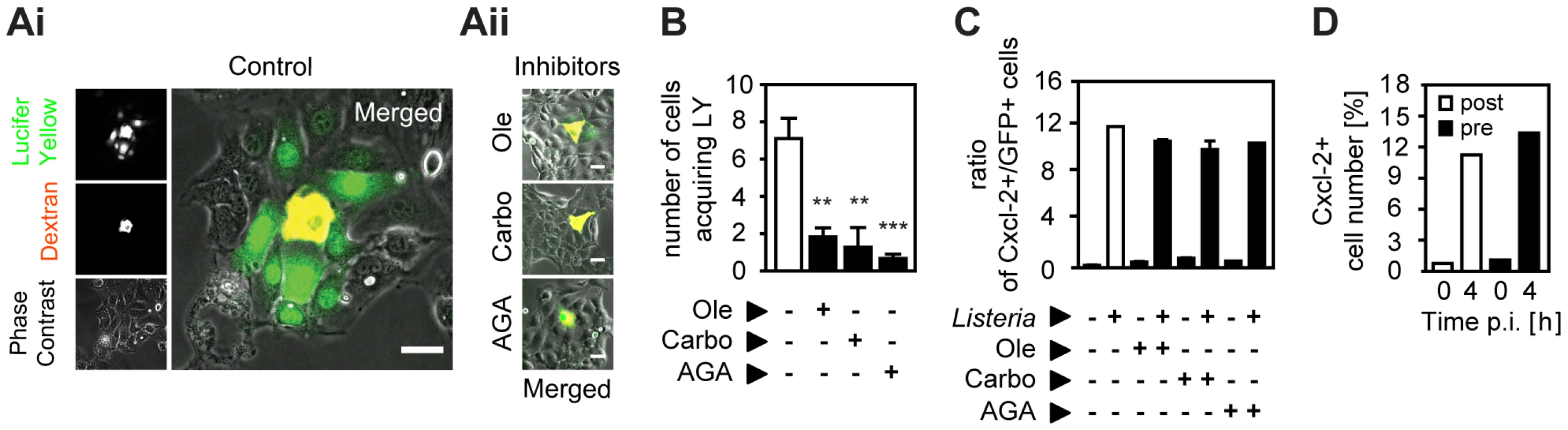
(A) m-ICcl2 cells were microinjected with a mixture of Texas Red-conjugated dextran (red) and the gap junction permissible Lucifer Yellow in the absence (Ai, Control) or presence (Aii) of the gap junction inhibitor oleamide (Ole, 0.1 mM), carbonoxolone (Carbo, 0.01 mM), or α-glycerrhetinic acid (AGA, 0.1 mM). The cellular spread of Lucifer Yellow was visualized 5 min after microinjection by live imaging fluorescence microscopy. Magnification ×400. Phase contrast images were added to visualize single cells. Scale bar, 5 µm. (B) Number of cells acquiring Lucifer Yellow (LY) by gap junction mediated transport after microinjection in the absence or presence of gap junction inhibitors. A minimum of 10 microinjected cells were analysed per experiment; **, p<0.01, ***, p<0.005. (C) m-ICcl2 cells were infected with actA mutant PactA-gfp L. monocytogenes. The ratio of immunolabelled Cxcl-2+ cells to GFP+ (Listeria-infected) cells was determined 4 h after infection in the absence or presence of gap junction inhibitors by flow cytometry. (D) m-ICcl2 cells were infected with actA mutant Psod-gfp L. monocytogenes. The number of immunolabelled Cxcl-2+ cells was determined after the indicated time by flow cytometry. Brefeldin A was added 60 min after (post, white bars) or 30 min prior to (pre, black bars) infection. All infection experiments were performed at a multiplicity of infection of 100∶1. Results are representative for three independent experiments and are presented as mean ± SD (microinjection) or show one representative experiment. Similarly, the potential role of a secreted protein messenger was examined. Intestinal epithelial m-ICcl2 cells were exposed to brefeldin A (BFA), an effective inhibitor of the secretion of newly synthesized proteins (Fig. S4F), prior and after infection with actA mutant L. monocytogenes. The number of Listeria-induced Cxcl-2+ cells, however, was not altered irrespective whether BFA was administered 30 min prior or 60 min after infection (Fig. 4D). Second, cell culture medium was obtained 10, 20, 30, 40, or 60 min after Listeria infection, centrifuged to remove bacteria, and immediately transferred to naïve uninfected epithelial cells. Yet no epithelial activation was observed after exposure to conditioned culture supernatant despite significant Cxcl-2 synthesis detected in the Listeria infected cell population (Fig. S4G). Of note, factors released by Listeria-infected cells might be unstable or immediately bound to neighbouring cells preventing their efficient release in the conditioned cell culture supernatant.
Finally, widely used pharmacological inhibitors of prostaglandin synthesis and known intestinal epithelial ion channels were employed. Indomethacin, an inhibitor of cyclooxygenase isoenzymes (COX1, COX2) involved in prostaglandin synthesis, thapsigargin, an inhibitor of the endoplasmatic Ca2+ATPase, CFTR II, a selective apical Cl− ion channel inhibitor, and bumetanide, an inhibitor of a basolateral epithelial Na+K+Cl− cotransporter had no significant influence on the number of activated epithelial cells after Listeria infection illustrated as ratio of activated (Cxcl-2+) to infected (GFP+) cells (Fig. S4H). These results do not identify a significant role of gap junctional transport, secreted protein or prostaglandin mediators, or ion fluxes in the observed indirect activation of epithelial cells after L. monocytogenes infection.
Listeria-induced horizontal epithelial communication depends on oxygen radical synthesis
Since unstable and highly reactive host-derived factors were not excluded by the previous experiments, a possible involvement of oxygen or nitrogen radicals in horizontal epithelial cell-cell communication was subsequently evaluated. Expression of members of two enzyme families, NADPH oxidases and nitric oxide synthase (NOS), has been described in epithelial cells [16]. Indeed, addition of the NADPH oxidase inhibitor diphenylene iodonium (DPI) resulted in a significant reduction of Listeria-induced epithelial activation (Fig. 5A). DPI did not reduce Listeria survival in epithelial cells (Fig. S5B) and had no effect on LPS or PMA-induced epithelial activation (Fig. S5A). In contrast to DPI, the NOS inhibitor N (G)-nitro-L - arginine methyl ester (L-NAME) did not influence the number of activated epithelial cells (Fig. 5B). In accordance with an inhibitory effect of DPI, synthesis of reactive oxygen intermediates (ROI) after Listeria infection was observed (Fig. 5C). ROI was detected in focal areas of confluent epithelial cells surrounding Listeria-positive, infected cells in accordance with local production and lateral spread of ROI as early as 10 min after infection (Fig. 5D).
Fig. 5. Epithelial intercellular communication is dependent on Listeria-induced oxygen radical synthesis. 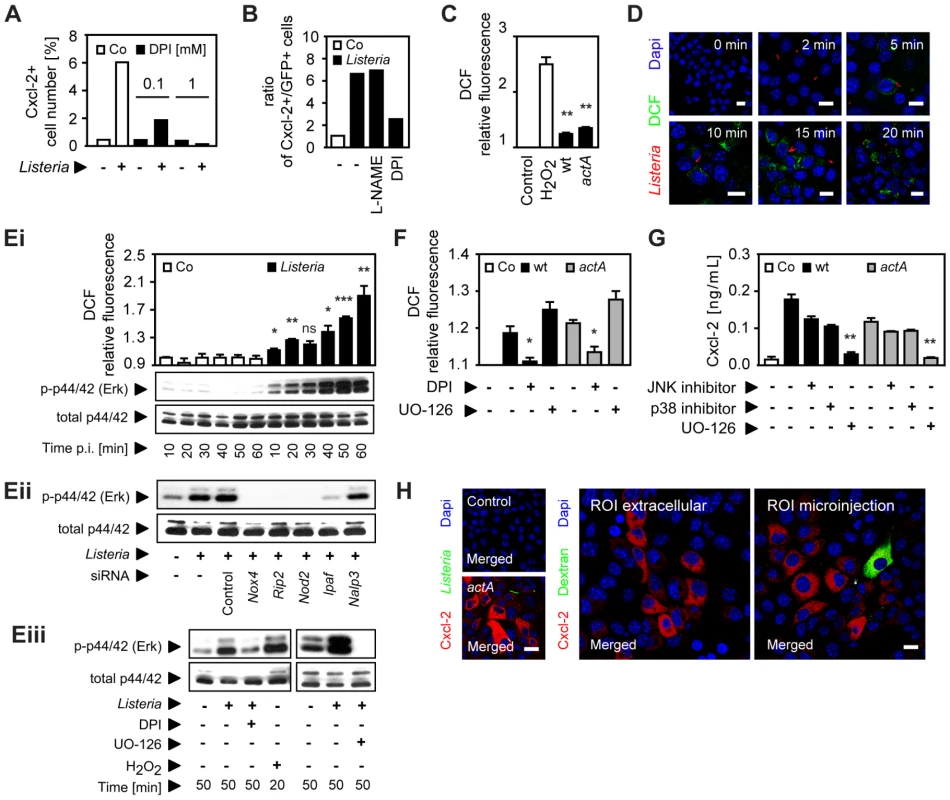
(A) m-ICcl2 cells were infected with actA mutant PactA-gfp Listeria monocytogenes. The number of immunolabelled Cxcl-2+ cells was determined 4 h after infection in the absence or presence of diphenylene iodonium (DPI, mM) by flow cytometry. (B) m-ICcl2 cells were infected with actA mutant PactA-gfp L. monocytogenes. The ratio of immunolabelled Cxcl-2+ cells to GFP+ (Listeria-infected) cells was determined 4 h after infection in the absence or presence of N (G)-nitro-L- arginine methyl ester (L-NAME) or DPI (each at 0.1 mM) by flow cytometry. (C) m-ICcl2 cells were loaded with DCF-DA and infected with wt or actA mutant L. monocytogenes. DCF relative fluorescence, reflecting reactive oxygen intermediates (ROI) production was quantified 20 min after infection by fluorescence spectroscopy. H2O2 (1 mM) was used as positive control. (D) m-ICcl2 cells were loaded with DCF-DA and infected with immunolabelled wt L. monocytogenes (red). DCF fluorescence, reflecting ROI production was visualized at the indicated time after infection by fluorescence microscopy. Magnification ×400, counterstaining with Dapi (blue). Scale bar, 5 µm. (Ei) m-ICcl2 cells were loaded with DCF-DA and infected with wt L. monocytogenes. DCF relative fluorescence, reflecting ROI production (upper panel) or p44/42 (Erk) phosphorylation (lower panel) was determined at the indicated time after infection by fluorescence spectroscopy or by immunoblotting, respectively. (Eii) m-ICcl2 cells were treated with small interfering RNA (siRNA) control or siRNA Nox4, Rip2, Nod2, Ipaf or Nalp3 and infected with wt L. monocytogenes. p44/42 phosphorylation was determined 50 min after infection by immunoblotting. (Eiii) m-ICcl2 cells were infected with wt L. monocytogenes in the absence or presence of DPI (0.1 mM) or exposed to H2O2 (1 mM) [left], or alternatively in the absence or presence of UO-126 (10 µM) [right]. p44/42 phosphorylation was determined after the indicated time by immunoblotting. (F) m-ICcl2 cells were loaded with DCF-DA and infected with wt (black square) or actA mutant (gray square) L. monocytogenes. DCF relative fluorescence, reflecting ROI production was quantified 20 min after infection in the absence or presence of DPI (0.1 mM) or UO-126 (10 µM) by fluorescence spectroscopy. (G) m-ICcl2 cells were infected with wt (black square) or actA mutant (gray square) L. monocytogenes. Cxcl-2 was determined 4 h after infection in the absence or presence of the indicated inhibitors in cell culture supernatant by ELISA. Used inhibitors: JNK inhibitor (1 µg/mL), p38 inhibitor (2 µg/mL), UO-126 (10 µM). (H) m-ICcl2 cells were left untreated (left, upper), infected with actA mutant PactA-gfp L. monocytogenes (GFP+, green, left, lower), or exposed to cumene hydroperoxide (0.5 mM) in cell culture medium (ROI extracellular, middle panel), or microinjected with cumene hydroperoxide (ROI microinjection, 0.5 mM, left panel). Immunolabelled Cxcl-2 (red) was visualized 4 h after infection or stimulation by fluorescence microscopy. The microinjected cell was loaded with Texas Red-conjugated dextran (green). Magnification ×400, counterstaining with Dapi (blue). Scale bar, 5 µm. All experiments were performed at a multiplicity of infection of 100∶1. Results are representative for three independent experiments and are presented as mean ± SD (ROI, ELISA) or show one representative experiment. *, p<0.05, **, p<0.01, ***, p<0.005, ns, not significant. Innate immune receptor stimulation by Listeria infection of epithelial cells resulted in rapid activation of the mitogen-activated protein (MAP) kinase Erk in a ROI-dependent manner (Fig. 5E). Whereas impairment of the MAP kinase Erk had no significant effect on ROI production (Fig. 5F), Listeria-induced Cxcl-2 synthesis by intestinal epithelial cells was completely abrogated by Erk inhibition and partially also dependent on the MAP kinases p38 and JNK (Fig. 5G). Of note, Erk inhibition did not affect bacterial invasion and the viability of intracellular Listeria (Fig. S5B). Finally, exposure of epithelial cells to cumene hydroperoxide, a ROI liberating organic agent within the cell culture medium or by microinjection induced Cxcl-2 synthesis in neighbouring cells similar to L. monocytogenes infection (Fig. 5H). Thus, Listeria-infection induces significant epithelial ROI synthesis, which in turn mediates MAP kinase Erk activation and downstream Cxcl-2 production.
Oxygen radical synthesis is performed by an oligomeric protein complex involving a cell type-specific NADPH-oxidase (Nox) protein. Only significant expression of the Nox4 isoform was detected in primary small intestinal epithelial cells (Fig. 6A). Nox4 synthesis was restricted to intestinal epithelial cells as demonstrated by immunostaining with a paranuclear expression pattern in accordance with a previous report (Fig. 6B) [16]. Importantly, downregulation of Nox4 expression in epithelial cells by siRNA interference significantly reduced Cxcl-2 secretion (Fig. 6C) and ROI production upon Listeria-infection (Fig. 6D). In contrast, downregulation of Nox4 expression did not alter LPS - or PMA-induced chemokine secretion (Fig. S6). Thus, ROI production by Nox4 appears to be both necessary and sufficient to induce horizontal cell-cell communication in intestinal epithelial cells leading to chemokine secretion in neighbouring cells in response to Listeria infection.
Fig. 6. Activation of the NADPH oxidase (Nox) 4 mediates indirect epithelial activation. 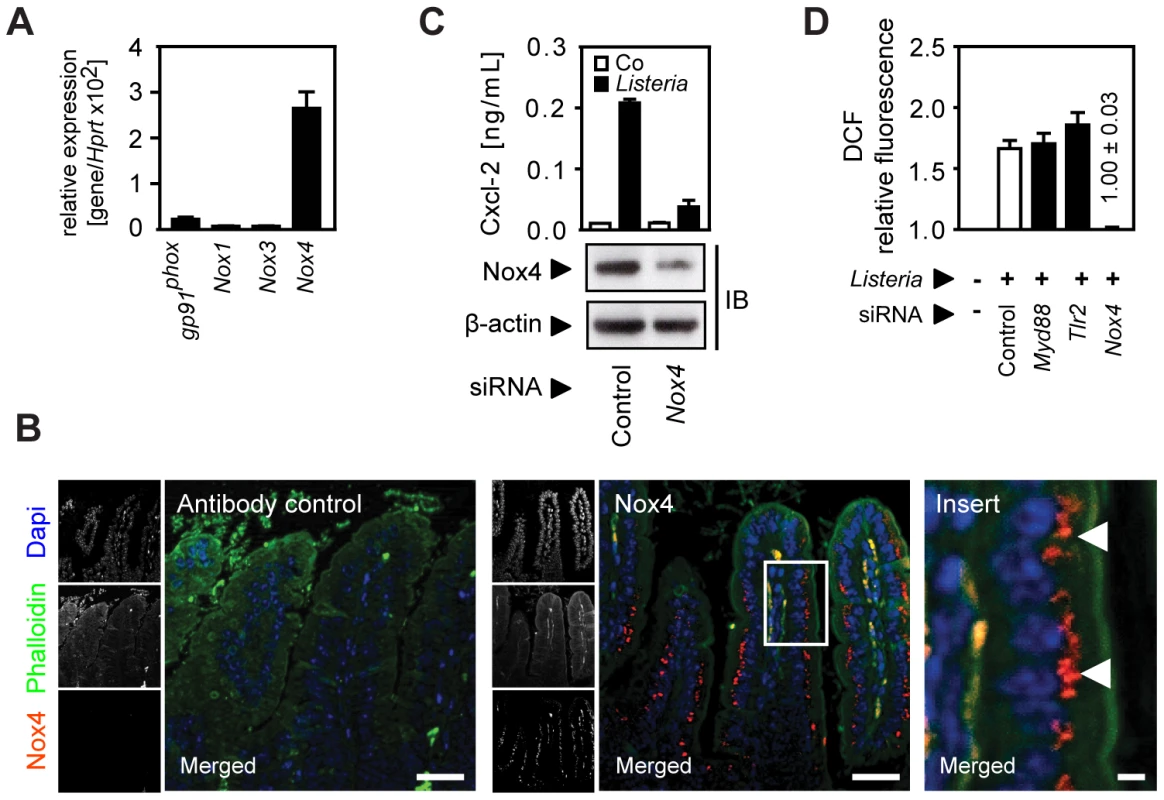
(A) Expression of gp91phox (Nox2), Nox1, 3, and 4 in isolated highly pure (>98% E-cadherin+, CD45−) primary intestinal epithelial cells. (B) Immunostaining for Nox4 expression in sections of C57BL/6 mouse small intestinal tissue. Magnification ×200; counterstaining with phalloidin (green) and Dapi (blue). Scale bar, 50 µm. For demonstration of the subcellular localisation of Nox4 (red), the insert (white frame) was enlarged ×630 (right panel). White arrows highlight the subcellular localization of Nox4. Scale bar, 5 µm. (C) m-ICcl2 cells were treated with small interfering RNA (siRNA) control or siRNA Nox4 and left uninfected (white) or were infected (black) with actA mutant L. monocytogenes. Cxcl-2 was determined 4 h after infection in cell culture supernatant by ELISA (upper panel). Nox4 downregulation was verified by immunoblotting (lower panel). (D) m-ICcl2 cells were treated with small interfering RNA (siRNA) control or siRNA MyD88, Tlr2, or Nox4, loaded with DCF-DA and infected with actA mutant L. monocytogenes. DCF relative fluorescence, reflecting ROI production, was quantified 20 min after infection by fluorescence spectroscopy. All infection experiments were performed at a multiplicity of infection of 100∶1. Results are representative for three independent experiments and are presented as mean ± SD (ELISA, ROI) or show one representative experiment. Discussion
Communication between individual cells is a fundamental feature of multicellular organisms. For instance, it mediates a coordinated reaction of muscle cell contraction, and allows neuronal signal transmission or endocrinological regulatory circuits. Cell-cell communication is also characteristic for the complex regulatory networks of the adaptive immune system. Cytokines bridge anatomical distances to coordinate and amplify the host response against pathogens. In the present study we investigated whether cell-cell communication between neighbouring cells might also contribute to innate immune activation within a confluent epithelial cell layer to coordinate the antimicrobial host defence at an early stage of the infection. Although inhibition of the overall epithelial responses by interference with the production of soluble mediators and gap junction integrity had previously been noted, the process of immune stimulation and cellular response upon bacterial infection has not been studied at the single cell level [17], [18], [19], [20], [21]. The present study therefore represents the first report to demonstrate epithelial horizontal cell-cell communication upon bacterial innate immune stimulation.
For three reasons, Listeria infection of confluent intestinal epithelial cells represents an ideal model to study epithelial cell-cell communication downstream of innate immune stimulation. First, similar to other pathogenic bacteria Listeria monocytogenes escapes from the endosomal vacuole and proliferates within the host cell cytosol [6]. Endosomal escape is associated with a dramatic change in bacterial gene expression. Although expressed at low levels also during in vitro culture, a very strong upregulation of the actin polymerizing protein ActA provides an excellent reporter for detection of cytosolic entry [22]. Second, bacteria lacking hly, plcA, or plcB mediating endosomal lysis only induce an only minor activation which might result from intraendosomal recognition or a so far unidentified minor mechanism of endosomal escape. Thus, in contrast to macrophages that recognize Listeria also at the plasma membrane, epithelial cell stimulation is mainly observed when bacteria reach the cytosol, facilitating contact with cytosolic innate immune receptors such as Ipaf, Nalp3, and Nod2 [9], [10]. This finding excludes innate immune recognition and receptor-mediated initiation of signal transduction in non-infected, Listeria-negative cells. Third, one amino acid exchange between the mouse and human E-cadherin causes a strongly reduced infection rate in murine epithelial cells [23], leaving most cells of a confluent cell layer uninfected and accessible to the analysis of indirect cellular activation. Using reporter gene technology, intracellular chemokine staining and flow cytometric analysis, we were able to demonstrate that the chemokine secretion in response to Listeria infection is mainly derived from uninfected, indirectly activated epithelial cells. Of note, the commonly used quantification of cytokine secretion in the cell culture supernatant or immunoblotting of total cell lysate proteins would not have disclosed this surprising finding.
Epithelial stimulation on the transcriptional level by p65/RelA overexpression did not result in detectable indirect cell activation. Several possible mechanisms of horizontal cell-cell communication downstream of innate immune receptor signaling were therefore considered. In response to microbial stimulation, epithelial cells produce chemokines, prostaglandins, and cause local alterations of ion concentrations by regulating transmembrane ion channel activity. Also, gap junctional intercellular communication (GJIC) represents a direct cytosolic connection and might be used to forward the information of innate immune recognition within the epithelial cell layer [17], [21]. Ca2+ fluxes via intercellular gap junctions have been shown to promote lung epithelial chemokine secretion [18] and intact gap junction formation has also been linked to innate immune stimulation and maintenance of the epithelial barrier [19]. On the other hand, connexin-26 hemichannel-mediated Ca2+ signaling has also been proposed to promote bacterial invasion and lateral spread [24]. Yet, neither protein secretion, nor ion channel activity or gap junction formation appeared to be involved in Listeria-induced indirect epithelial cell activation.
Instead, our results indicate an important role of reactive oxygen intermediates (ROI) in horizontal epithelial cell-cell communication. ROI represent reduction products of molecular oxygen such as the radical superoxide (•O2−) and hydroxyl (•OH), and the non-radical hydrogen-peroxide (H2O2). ROI production by professional phagocytes during oxidative burst provides significant bactericidal activity but synthesis is also observed in non-phagocytic cells [25]. ROI at subtoxic doses has been recognized as an important intracellular signal transducing molecule during the recent years [26], [27], [28], [29]. In accordance with our results ROI-induced activation of MAP kinase activity has been reported [30], [31], [32], [33]. In addition, an involvement of ROI in the cellular signaling leading to NF-κB activation [34], apoptosis [31], [35], epidermal growth factor receptor signaling [36], regulation of cellular proliferation [37], and antimicrobial peptide production [38], [39] has been described. ROI was also shown to prime Drosophila melanogaster hematogenic progenitor cells for differentiation [40] and to play an important role in the fruit fly's intestinal immunity [41]. Whereas the half-life of oxygen radical hydroxyl (•OH) is extremely short (10−9s) and the superoxide •O2− is membrane impermeable [40], H2O2 is able to diffuse to neighbouring cells and induce cellular activation. Indeed, a tissue gradient of H2O2 was shown to induce rapid recruitment of leukocytes into the wound margin following endothelial hypoxia [42], [43].
NADPH oxidase activation has previously been linked to innate immune mediated antimicrobial killing [25] as well as receptor signal transduction [44], [45], [46], [47], [48], [49], [50], [51]. Here we for the first time report Nox4 expression by intestinal epithelial cells and demonstrate Nox4-mediated ROI production in response to bacterial infection. Although enhanced Nox4 mRNA expression was shown to result in increased ROI production [52], the initiation of Nox4-dependent ROI production upon Listeria infection was noted as early as 5–10 minutes after bacterial challenge. This excludes a significant role of transcriptional regulation of Nox4 in our model. Whereas the prototypical NADPH oxidase of phagocytes, gp91phox (Nox2), requires cytosolic proteins such as p47phox to form a functional NADPH oxidase complex, Nox4 functions independent of cytosolic accessory proteins. Interestingly, Nox expression has previously been linked to innate immune receptor signaling: Nox4 activation was shown to be involved in TLR4-mediated NF-κB activation in human epithelial kidney cells and monocytes [47], [53]. In contrast, our results revealed activation of intestinal epithelial cells by Listeria infection in a Nod2-, Ipaf-, and Nalp3-dependent fashion which was followed by ROI production and subsequent MAP kinase signaling. Our data are therefore in accordance with previous reports on MAP kinase activation after Nox4-mediated ROI production [54], [55]. A future analysis of the local paracellular concentration of the different species of oxygen radicals might help to improve our understanding of the regulatory role of Nox4-mediated ROI production for epithelial cell-cell communication. Interestingly, reduced chemokine synthesis was noted in directly infected, Listeria-positive cells. These cells were also impaired to respond to secondary innate immune stimulation illustrating the immune evasive behaviour of L. monocytogenes (data not shown). Although the underlying mechanism is currently not resolved, high concentrations of ROI were previously associated with reduced susceptibility to immunostimulatory agents [56]. Yet other bacterial or host factors such as antioxidant enzymes might reduce local ROI concentrations and interfere with cellular activation and chemokine production in infected epithelial cells.
In conclusion, our data for the first time analyzed intestinal epithelial activation in response to bacterial infection at a single cell level. We could detect Nox4 expression by intestinal epithelial cells which facilitated rapid ROI production upon infection and paracrine activation of neighbouring cells (Fig. 7). Our findings thus identify horizontal cell-cell communication to allow a coordinated innate immune activation of the intestinal epithelium. The present work significantly broadens our knowledge on the complex processes that underlie mucosal innate immune stimulation and illustrates the specific role of epithelial cells for an efficient activation of the antimicrobial host defence.
Fig. 7. Model of horizontal intercellular communication between intestinal epithelial cells. 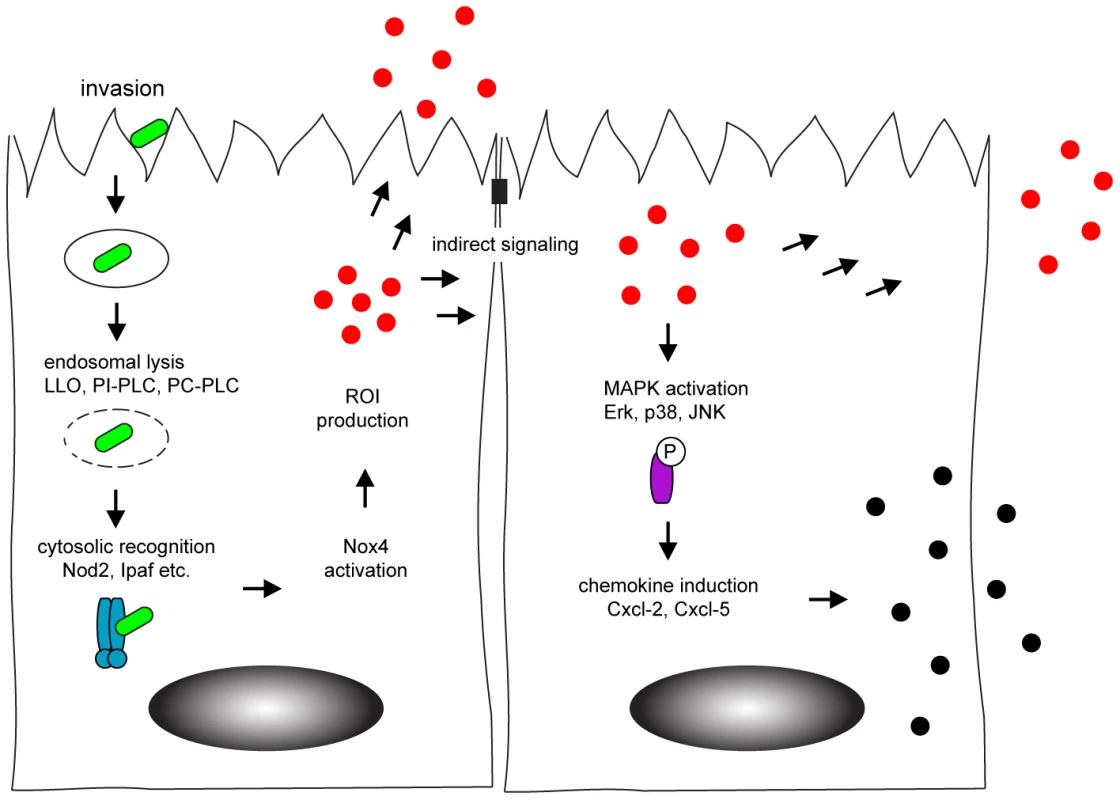
Following bacteria-induced internalization, Listeria monocytogenes (green) escapes from the endosome by listeriolysin (LLO) and phospholipase-mediated rupture. This facilitates recognition by cytosolic innate immune receptors (blue) that activate the NADPH oxidase (Nox) 4 and induce the production of the reactive oxygen intermediates (red). Reactive oxygen intermediates contribute to the activation of uninfected, neighbouring cells. Indirect stimulation subsequently initiates the MAPK-dependent secretion of chemokines (black) to attract professional immune cells to the site of infection. Materials and Methods
Antibodies and reagents
Intracellular Cxcl-2 (MIP-2) and Cxcl-5 was detected using rabbit antibodies from Nordic Biosite (Täby, Sweden). The rabbit polyclonal anti-actin antiserum was from Sigma-Aldrich (Taufenkirchen, Germany). The rabbit-anti-mouse Nox4 antiserum was obtained by immunization with recombinant peptide. The rabbit anti-p-p44/42 (phospho-Erk) and the mouse anti-p44/42 (total-Erk) was from Cell Signaling Technology (Beverly, MA, USA). The rabbit anti-Listeria antibody was from Dunn Labortechnik GmbH (Asbach, Germany). Cy5-, Cy3-, HRPO-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA, USA) and the Alexa Fluor (AF) 488-, and AF 555-conjugated donkey anti-rabbit IgG (H+L) was from Invitrogen (Molecular Probes). The MFP590 - and MFP488-labelled phalloidin were purchased from MoBiTec GmbH (Goettingen, Germany). The Sulfo NHS-LC-Biotin was obtained from Pierce, Thermo Scientific (Rockford, IL, USA). Escherichia coli K12 D31m4 LPS was ordered from List Biological Laboratories (Campbell, CA, USA). Recombinant LLO (rLLO) was expressed and purified exactly as described before [57]. rLLO was applied to cells in a serial dilution with 0.05 µg/mL as highest concentration. Cxcl-2 was quantified using an ELISA from Nordic Biosite or R&D Systems (Quantikine, R&D Systems GmbH, Wiesbaden, Germany). The NF-κB reporter construct pBIIX-luciferase carrying two copies (2× NF-κB) of the κB sequences from the Igκ enhancer was provided by S. Ghosh (Yale University Medical School, New Haven, CT, USA). Luciferase activity was quantified with luciferin substrate (PJK GmbH, Kleinblittersdorf, Germany). The bicistronic RelA/p65 expression plasmid was cloned by removing the nef gene from a pCG-nef-IRES-GFP expression plasmid (provided by J. Muench, Institute of Virology, University Clinic of Ulm, Germany) by digestion with the restriction enzymes XbaI and MluI (Fermentas, St Leon-Rot, Germany) and replacing it in frame with the p65 encoding gene amplified from a p65 expression plasmid (obtained from by U. Pahl, University Clinic, Freiburg, Germany) using the forward: 5′-ACC TCT AGA CCA TGG ACG ATC TGT TTC C-3′ and reverse: 5′-ACG ACG CGT GCA CCT TAG GAG CTG ATC TGA-3′ primers and digested with XbaI/MluI prior to ligation. Plasmid DNA for transfection was prepared using the endotoxin-free plasmid kit from Qiagen (Hilden, Germany). Targeted siRNA probes (Tlr2, Rip2, Nox4, Card12, Cias1, control siRNA,) were from Qiagen (Hilden, Germany), the Card15 siRNA was from Santa Cruz (Heidelberg, Germany). Lipofectamin 2000 (Invitrogen, Carlsbad, CA, USA) and INTERFERin (Polyplus Transfection, New York, NY, USA) were used for plasmid and siRNA transfection, respectively. The pharmacological inhibitors and radical donors oleamide, carbonoxolone, α-glycerrhetinic acid, brefeldin A, thapsigargin, CFTR inhibitor II (CFTR II.), indomethacin, bumetanide, N-(G)-nitro-L - arginine methyl ester (L-NAME), UO-126, hydrogen peroxide (H2O2) and cumene hydroperoxide were purchased from Sigma Aldrich. The p38 inhibitor 3-O-Acetyl-beta-boswellic acid and the L-stereoisomer JNK inhibitor 1 were from Enzo Life Sciences (Lörrach, Germany), and diphenylene iodonium (DPI) from Cayman Chemical (Hamburg, Germany). Defibrinated sheep red blood cells (SRBC) were purchased from Oxoid (Basingstoke, UK). The LDH Cytotoxicity Assay Kit was from Cayman Chemical (Hamburg, Germany). Colorimetric (ELISA, LDH), luminescent (luciferase) and fluorescent (ROI) measurements were carried out using a Victor3 Multilabel Plate Reader (Perkin Elmer, Waltham, MA, USA). Cell culture reagents were purchased from Invitrogen. All other reagents were obtained from Sigma Aldrich (Taufkirchen, Germany) if not stated otherwise.
Cell culture and stimulation assays
The m-ICcl2 small intestinal epithelial cell line has previously been described [58]. Cells were cultured in a modified, hormonally defined medium with DMEM and F12 (vol 1∶1) supplemented with 5% FCS, 2% glucose, 20 mM Hepes, 2 mM glutamin, 5 µg/mL insulin, 50 nM dexamethasone, 60 nM sodium selenite, 10 ng/mL epithelial growth factor, 5 µg/mL transferrin, and 1 nM 3,3′,5-triiodo-L-thyronine sodium salt. Cell passages 42–70 were used. Cells were grown at 37°C in a 5% CO2 atmosphere on collagen-coated cell culture plates or chambers to reach a polarized, confluent monolayer. Rat tail collagen was ordered from Institut Jacques Boy (Reims, France). Specific targeted or control siRNA was transfected at a final concentration of 10 nM 36 hours prior to functional analysis. Stimulation with lipopolysaccharide (LPS) was performed at a final concentration of 10 ng/mL.
Bacterial strains and epithelial infection
Listeria monocytogenes EGD wild-type (wt), actA, hly deletion mutant strains and the hly/plcA/plcB triple mutant strain are described in Table 1. Fluorescent bacteria were generated by transformation [59] with GFP expression vectors under the control of the actA or sod promoter (PactA-gfp, Psod-gfp; Table 1) Bacteria were routinely grown in Brain Heart Infusion (BHI) broth, supplemented with antibiotics when required. Overnight cultures were diluted 1∶50, grown to middle logaritmic phase (OD600) with mild agitation at 37°C, washed, and added in cell culture medium at the multiplicity of infection (m.o.i.) of 100∶1 (if not stated otherwise) followed by centrifugation (1500 rpm, 5 min, 4°C). 60 minutes after addition of bacteria, epithelial monolayers were washed three times with PBS, and fresh medium containing 50 µg/mL gentamycin was added to the culture medium to restrict extracellular bacterial growth. Unless indicated otherwise, infections were completed after 4 h post infection. To quantify bacterial invasion, co-culture of 20, 40 or 60 min was followed by 1 h incubation in fresh cell culture medium supplemented with 50 µg/mL gentamycin. For 4 h and 6 h infection, gentamycin was supplemented 60 minutes after addition of bacteria, and incubation was carried out for additional 3 h or 5 h. After washing, cells were lysed in 0.1% Triton/H2O and the number of intracellular bacteria was determined (CFU) by serial dilution and plating. Bacteria free conditioned medium were prepared by centrifugation or filtering of cell culture medium, and immediately applied on naïve, uninfected m-ICcl2 cells. The rabbit anti-Listeria antibody was used for immunolabelling of bacteria (1∶500). For alternative intracellular detection of Listeria, bacteria were biotinylated prior to infection according to the manufacturers protocol. Pharmacological inhibitors were added 30 min prior to infection if not stated otherwise.
Immunofluorescence microscopy and flow cytometric analysis
For intracellular Cxcl-2 or Cxcl-5 visualization, brefeldin A (0.5 µg/mL) was added to the cell culture medium 1 h after stimulation. Cells were fixed in 3% PFA and incubated with anti-Cxcl-2 or Cxcl-5 antiserum (1∶100). Nox4 was detected in formalin-fixed sections of mouse small intestine by incubation with a rabbit anti-Nox4 antiserum (1∶100) for 1 h at room temperature, followed after washing by a TR-conjugated secondary antibody. Cells were mounted in Vectashield Mounting Medium with Dapi (Vector Laboratories, Eching, Germany) and visualized using a Leica DM IRB Inverted Research Microscope with a TCS SP2 AOBS scan head (Leica Microsystems GmbH, Wetzlar, Germany). For fluorescent detection, immunolabelled Listeria was additionally stained with AF 555-conjugated secondary antibody prior to infection, or biotinylated bacteria were labelled by streptavidin-conjugated Cy3. For flow cytometry cells were trypsinized and fixed in Cytofix (BD Biosciences). Cxcl-2 or Cxcl-5 was stained following permeabilization in 0.5% saponin/1% FCS/PBS buffer. Analysis was performed on a FACS Calibur apparatus (BD Biosciences). The data acquisition on GFP+ (recorded in channel Fl-1) and Cxcl-2+ or Cxcl-5+ cells (Cy5-conjugated, Fl-4) was restricted to a total number of 10.000 events. The data acquisition on GFP+ (recorded in channel Fl-1) bacteria was restricted to a total number of 100.000 events. Flow cytometry cell sorting was performed using a MoFlo (XDP Upgrade, Beckman-Coulter) at the Cell Sorting Facility, Medical School, Hanover.
Immunoblotting
Cell were lysed in 3∶1 WB/SB vol/vol (WB: 50 mM Tris, pH 7.4, 120 mM NaCl; SB: 250 mM Tris, pH 6.5, 8% SDS, 40% glycerol; supplemented with a proteinase inhibitor cocktail [Complete Mini, Roche Diagnostics]). Samples were sonified and the protein concentration was determined (DC Protein Assay; Bio-Rad Laboratories). Protein was separated on 11% acrylamide gels and blotted on nitrocellulose. Membranes were incubated overnight at 4°C with the primary antibody. Detection was performed using peroxidase-labelled goat anti–rabbit or goat anti-mouse secondary antibodies in combination with the ECL kit (GE Healthcare). Before restaining, membranes were stripped for 45 min at 50°C in 62.5 mM Tris HCl, pH 6.7, 100 mM ß-mercaptoethanol and 2% SDS, followed by three 15-min washing steps.
PCR and quantitative RT-PCR analysis
Cells were divided after cell sorting. DNA extraction was performed following incubation in lysosyme (10 mg/mL), proteinase K (10 mg/mL), and 5% SDS using TRIzol (Invitrogen) according to the manufacturer's instruction. DNA was washed in sodium citrate (0.1 mM) and precipitated in 75% ethanol. Listeria genomic DNA was detected by PCR (Taq DNA polymerase from Invitrogen) using primers specific for the listerial hly gene (forward: 5′-ATG TAA ACT TCG GCG CAA CT-3′, reverse: 5′-TCG TGT GTG TTA AGC GGT TT-3′, annealing 57°C, cycles 35). A fragment encoding eukaryotic hypoxanthine phosphoribosyltransferase (Hprt) was amplified using oligonucleotides 5′-TGC TGA CCT GCT GGA TTA CA-3′ and 5′-GCT TAA CCA GGG AAA GCA AA-3′ (annealing temperature 59°C, cycles 32) as control. Amplification products were analysed on a 2% agarose gel and visualized with SYBR Safe (Invitrogen). Total RNA was extracted using the RNeasy Protect Cell Mini Kit (Qiagen) and first-strand cDNAs was synthesized using oligo-dT primers. Real-time PCR was prepared with absolute QPCR ROX mix (Thermoscientific), sample cDNA, intron-spanning forward and reverse primers, as well as the 6-carboxy-fluorescein-conjugated target probe provided in the commercial TaqMan gene expression assay for murine Hprt1 and Cxcl-2 or Cxcl-5 (Applied Biosystems). Analysis were performed using an ABI PRISM Sequence Detection System 7000 (Applied Biosystems). Samples were normalized to the endogenous control. Results were calculated by use of the Δ2-CT method and are presented as fold induction of target gene transcripts in stimulated relative to unstimulated controls.
Reactive oxygen radical detection
The fluorogenic probe 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (DCF-DA, Invitrogen) was used for reactive oxygen intermediates (ROI) visualization. Prior to stimulation, cells were incubated with DCF-DA (10 µg/mL) for 30 min at 37°C. After stimulation or infection for 20 min cells were rapidly rinsed with PBS, fixed in 3% PFA, washed twice with PBS and analyzed by fluorescence microscopy. For quantitative analysis of oxygen radical production, cells were rinsed with PBS to remove the free probe, and lysed in 200µl of 1% Triton/H2O. The lysate was transferred into microcentrifuge tubes, sedimented at 8,000×g for 5 min at 4°C, and 100 µl aliquots were dispensed in 96-well plates in triplicate. The index of oxidation (DCF) was calculated as the ratio of fluorescence intensity as compared to an untreated control.
Microinjection
Cells were grown in collagen-coated 8-well chamber slides (Nunc, Rochester, NY) continuously bathed in cell culture medium. The 70 kDa high molecular weight gap junction impermeant fluorescent compound Texas Red Dextran (Molecular Probes, 10 mg/mL) was mixed with either the <1 kDa low molecular weight Lucifer Yellow (Molecular Probes, 10 mg/mL) or 0.5 mM cumene hydroperoxide in injection buffer (25 mM HEPES, 125 mM K-acetate, 5 mM Mg-acetate, pH 7.1). Fluorescent mixtures were loaded into individual Femtotips II injection capillars (Eppendorf, Hamburg, Germany). Cells were transferred to a LSM 510 META laser scanning confocal microscope equipped with an inverted Axiovert 200M stand (Carl Zeiss, Germany) and single cell microinjection was performed by using an InjectMan NI2/Femtojet injector system at pi: 180 hPa, ti: 0.2s, pc: 25 hPa. A minimum of 10 microinjected cells were analyzed per experiment. To study gap junctional intercellular communication, cells were analysed by live imaging microscopy after 5 min incubation. For ROI donor cumene hydroperoxide stimulation, cells were incubated 1 h, washed, and incubated in prewarmed fresh cell culture medium for an additional 3 h in the presence of 0.5 µg/mL brefeldin A. Cells were fixed in 3% PFA and further analyzed by intracellular chemokine staining and fluorescence microscopy.
Statistical analysis
All experiments were performed at least three times and results are given as the mean ± standard deviation (SD) of one representative experiment. Statistical analyses were performed using the Student's t test. A p value<0.05 (*) or <0.01 (**) was considered significant.
Supporting Information
Zdroje
1. LotzM
MenardS
HornefM
2007 Innate immune recognition on the intestinal mucosa. Int J Med Microbiol 297 379 392
2. LeeJ
MoJH
ShenC
RuckerAN
RazE
2007 Toll-like receptor signaling in intestinal epithelial cells contributes to colonic homoeostasis. Curr Opin Gastroenterol 23 27 31
3. KobayashiKS
ChamaillardM
OguraY
HenegariuO
InoharaN
2005 Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307 731 734
4. SchillingJD
MartinSM
HungCS
LorenzRG
HultgrenSJ
2003 Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 100 4203 4208
5. AndoneguiG
BonderCS
GreenF
MullalySC
ZbytnuikL
2003 Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest 111 1011 1020
6. FreitagNE
PortGC
MinerMD
2009 Listeria monocytogenes - from saprophyte to intracellular pathogen. Nat Rev Microbiol 7 623 628
7. McCaffreyRL
FawcettP
O'RiordanM
LeeKD
HavellEA
2004 A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci U S A 101 11386 11391
8. LeberJH
CrimminsGT
RaghavanS
Meyer-MorseNP
CoxJS
2008 Distinct TLR - and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog 4 e6
9. WarrenSE
MaoDP
RodriguezAE
MiaoEA
AderemA
2008 Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol 180 7558 7564
10. CorrSC
O'NeillLA
2009 Listeria monocytogenes infection in the face of innate immunity. Cell Microbiol
11. DietrichG
BubertA
GentschevI
SokolovicZ
SimmA
1998 Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol 16 181 185
12. KohlerR
BubertA
GoebelW
SteinertM
HackerJ
2000 Expression and use of the green fluorescent protein as a reporter system in Legionella pneumophila. Mol Gen Genet 262 1060 1069
13. HerskovitsAA
AuerbuchV
PortnoyDA
2007 Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog 3 e51
14. GekaraNO
WestphalK
MaB
RohdeM
GroebeL
2007 The multiple mechanisms of Ca2+ signalling by listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Cell Microbiol 9 2008 2021
15. TsuchiyaK
KawamuraI
TakahashiA
NomuraT
KohdaC
2005 Listeriolysin O-induced membrane permeation mediates persistent interleukin-6 production in Caco-2 cells during Listeria monocytogenes infection in vitro. Infect Immun 73 3869 3877
16. ChenK
KirberMT
XiaoH
YangY
KeaneyJFJr
2008 Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181 1129 1139
17. LeaphartCL
QureshiF
CetinS
LiJ
DubowskiT
2007 Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 132 2395 2411
18. MartinFJ
PrinceAS
2008 TLR2 regulates gap junction intercellular communication in airway cells. J Immunol 180 4986 4993
19. EyB
EykingA
GerkenG
PodolskyDK
CarioE
2009 TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem 284 22332 22343
20. WuF
TymlK
WilsonJX
2008 iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J Cell Physiol 217 207 214
21. PatelSJ
KingKR
CasaliM
YarmushML
2009 DNA-triggered innate immune responses are propagated by gap junction communication. Proc Natl Acad Sci U S A 106 12867 12872
22. Shetron-RamaLM
MarquisH
BouwerHG
FreitagNE
2002 Intracellular induction of Listeria monocytogenes actA expression. Infect Immun 70 1087 1096
23. LecuitM
DramsiS
GottardiC
Fedor-ChaikenM
GumbinerB
1999 A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. Embo J 18 3956 3963
24. Tran Van NhieuG
ClairC
BruzzoneR
MesnilM
SansonettiP
2003 Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol 5 720 726
25. SegalAW
2005 How neutrophils kill microbes. Annu Rev Immunol 23 197 223
26. SundaresanM
YuZX
FerransVJ
IraniK
FinkelT
1995 Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270 296 299
27. ThannickalVJ
FanburgBL
2000 Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279 L1005 1028
28. KimuraH
SawadaT
OshimaS
KozawaK
IshiokaT
2005 Toxicity and roles of reactive oxygen species. Curr Drug Targets Inflamm Allergy 4 489 495
29. AllenRG
TresiniM
2000 Oxidative stress and gene regulation. Free Radic Biol Med 28 463 499
30. ThomasSR
ChenK
KeaneyJFJr
2002 Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem 277 6017 6024
31. El-NajjarN
ChatilaM
MoukademH
VuorelaH
OckerM
2009 Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis
32. SongHJ
KimJS
LeeMJ
NamYS
SohnUD
2007 Reactive oxygen species mediate ET-1-induced activation of ERK1/2 signaling in cultured feline esophageal smooth muscle cells. Arch Pharm Res 30 1080 1087
33. McCubreyJA
LahairMM
FranklinRA
2006 Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 8 1775 1789
34. BrarSS
KennedyTP
SturrockAB
HuecksteadtTP
QuinnMT
2002 NADPH oxidase promotes NF-kappaB activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 282 L782 795
35. JinS
RayRM
JohnsonLR
2008 TNF-alpha/cycloheximide-induced apoptosis in intestinal epithelial cells requires Rac1-regulated reactive oxygen species. Am J Physiol Gastrointest Liver Physiol 294 G928 937
36. AmmendolaR
RuocchioMR
ChiricoG
RussoL
De FeliceC
2002 Inhibition of NADH/NADPH oxidase affects signal transduction by growth factor receptors in normal fibroblasts. Arch Biochem Biophys 397 253 257
37. RichardsSM
ClarkEA
2009 BCR-induced superoxide negatively regulates B-cell proliferation and T-cell-independent type 2 Ab responses. Eur J Immunol 39 3395 3403
38. Mendez-SamperioP
PerezA
TorresL
2009 Role of reactive oxygen species (ROS) in Mycobacterium bovis bacillus Calmette Guerin-mediated up-regulation of the human cathelicidin LL-37 in A549 cells. Microb Pathog 47 252 257
39. YangCS
ShinDM
KimKH
LeeZW
LeeCH
2009 NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol 182 3696 3705
40. Owusu-AnsahE
BanerjeeU
2009 Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461 537 541
41. RyuJH
HaEM
OhCT
SeolJH
BreyPT
2006 An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. Embo J 25 3693 3701
42. NiethammerP
GrabherC
LookAT
MitchisonTJ
2009 A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459 996 999
43. MillarTM
PhanV
TibblesLA
2007 ROS generation in endothelial hypoxia and reoxygenation stimulates MAP kinase signaling and kinase-dependent neutrophil recruitment. Free Radic Biol Med 42 1165 1177
44. Ogier-DenisE
MkaddemSB
VandewalleA
2008 NOX enzymes and Toll-like receptor signaling. Semin Immunopathol 30 291 300
45. MatsuzawaA
SaegusaK
NoguchiT
SadamitsuC
NishitohH
2005 ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6 587 592
46. LambethJD
2004 NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4 181 189
47. ParkHS
JungHY
ParkEY
KimJ
LeeWJ
2004 Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol 173 3589 3593
48. PicardC
PuelA
BonnetM
KuCL
BustamanteJ
2003 Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299 2076 2079
49. LipinskiS
TillA
SinaC
ArltA
GrasbergerH
2009 DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 122 3522 3530
50. TattoliI
CarneiroLA
JehannoM
MagalhaesJG
ShuY
2008 NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep 9 293 300
51. DostertC
PetrilliV
Van BruggenR
SteeleC
MossmanBT
2008 Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320 674 677
52. SerranderL
CartierL
BedardK
BanfiB
LardyB
2007 NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406 105 114
53. ParkHS
ChunJN
JungHY
ChoiC
BaeYS
2006 Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 72 447 455
54. LiJ
StouffsM
SerranderL
BanfiB
BettiolE
2006 The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17 3978 3988
55. GoettschC
GoettschW
MullerG
SeebachJ
SchnittlerHJ
2009 Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem Biophys Res Commun
56. KumarA
WuH
Collier-HyamsLS
HansenJM
LiT
2007 Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. Embo J 26 4457 4466
57. DarjiA
ChakrabortyT
NiebuhrK
TsonisN
WehlandJ
1995 Hyperexpression of listeriolysin in the nonpathogenic species Listeria innocua and high yield purification. J Biotechnol 43 205 212
58. BensM
BogdanovaA
CluzeaudF
MiquerolL
KerneisS
1996 Transimmortalized mouse intestinal cells (m-ICc12) that maintain a crypt phenotype. Am J Physiol 270 C1666 1674
59. ParkSF
StewartGS
1990 High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94 129 132
60. HaufN
GoebelW
FiedlerF
SokolovicZ
KuhnM
1997 Listeria monocytogenes infection of P388D1 macrophages results in a biphasic NF-kappaB (RelA/p50) activation induced by lipoteichoic acid and bacterial phospholipases and mediated by IkappaBalpha and IkappaBbeta degradation. Proc Natl Acad Sci U S A 94 9394 9399
61. SlaghuisJ
GoetzM
EngelbrechtF
GoebelW
2004 Inefficient replication of Listeria innocua in the cytosol of mammalian cells. J Infect Dis 189 393 401
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



