-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSurvival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
Phlebotomine sand flies that transmit the protozoan parasite Leishmania differ greatly in their ability to support different parasite species or strains in the laboratory: while some show considerable selectivity, others are more permissive. In “selective” sand flies, Leishmania binding and survival in the fly midgut typically depends upon the abundant promastigote surface adhesin lipophosphoglycan (LPG), which exhibits species - and strain-specific modifications of the dominant phosphoglycan (PG) repeat units. For the “selective” fly Phlebotomus papatasi PpapJ, side chain galactosyl-modifications (scGal) of PG repeats play key roles in parasite binding. We probed the specificity and properties of this scGal-LPG PAMP (Pathogen Associated Molecular Pattern) through studies of natural isolates exhibiting a wide range of galactosylation patterns, and of a panel of isogenic L. major engineered to express similar scGal-LPG diversity by transfection of SCG-encoded β1,3-galactosyltransferases with different activities. Surprisingly, both ‘poly-scGal’ and ‘null-scGal’ lines survived poorly relative to PpapJ-sympatric L. major FV1 and other ‘mono-scGal’ lines. However, survival of all lines was equivalent in P. duboscqi, which naturally transmit L. major strains bearing ‘null-scGal’-LPG PAMPs. We then asked whether scGal-LPG-mediated interactions were sufficient for PpapJ midgut survival by engineering Leishmania donovani, which normally express unsubstituted LPG, to express a ‘PpapJ-optimal’ scGal-LPG PAMP. Unexpectedly, these “L. major FV1-cloaked” L. donovani-SCG lines remained unable to survive within PpapJ flies. These studies establish that midgut survival of L. major in PpapJ flies is exquisitely sensitive to the scGal-LPG PAMP, requiring a specific ‘mono-scGal’ pattern. However, failure of ‘mono-scGal’ L. donovani-SCG lines to survive in selective PpapJ flies suggests a requirement for an additional, as yet unidentified L. major-specific parasite factor(s). The interplay of the LPG PAMP and additional factor(s) with sand fly midgut receptors may determine whether a given sand fly host is “selective” or “permissive”, with important consequences to both disease transmission and the natural co-evolution of sand flies and Leishmania.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001185
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001185Summary
Phlebotomine sand flies that transmit the protozoan parasite Leishmania differ greatly in their ability to support different parasite species or strains in the laboratory: while some show considerable selectivity, others are more permissive. In “selective” sand flies, Leishmania binding and survival in the fly midgut typically depends upon the abundant promastigote surface adhesin lipophosphoglycan (LPG), which exhibits species - and strain-specific modifications of the dominant phosphoglycan (PG) repeat units. For the “selective” fly Phlebotomus papatasi PpapJ, side chain galactosyl-modifications (scGal) of PG repeats play key roles in parasite binding. We probed the specificity and properties of this scGal-LPG PAMP (Pathogen Associated Molecular Pattern) through studies of natural isolates exhibiting a wide range of galactosylation patterns, and of a panel of isogenic L. major engineered to express similar scGal-LPG diversity by transfection of SCG-encoded β1,3-galactosyltransferases with different activities. Surprisingly, both ‘poly-scGal’ and ‘null-scGal’ lines survived poorly relative to PpapJ-sympatric L. major FV1 and other ‘mono-scGal’ lines. However, survival of all lines was equivalent in P. duboscqi, which naturally transmit L. major strains bearing ‘null-scGal’-LPG PAMPs. We then asked whether scGal-LPG-mediated interactions were sufficient for PpapJ midgut survival by engineering Leishmania donovani, which normally express unsubstituted LPG, to express a ‘PpapJ-optimal’ scGal-LPG PAMP. Unexpectedly, these “L. major FV1-cloaked” L. donovani-SCG lines remained unable to survive within PpapJ flies. These studies establish that midgut survival of L. major in PpapJ flies is exquisitely sensitive to the scGal-LPG PAMP, requiring a specific ‘mono-scGal’ pattern. However, failure of ‘mono-scGal’ L. donovani-SCG lines to survive in selective PpapJ flies suggests a requirement for an additional, as yet unidentified L. major-specific parasite factor(s). The interplay of the LPG PAMP and additional factor(s) with sand fly midgut receptors may determine whether a given sand fly host is “selective” or “permissive”, with important consequences to both disease transmission and the natural co-evolution of sand flies and Leishmania.
Introduction
Leishmania are protozoan parasites that cause a spectrum of human diseases that range from self-healing cutaneous lesions to potentially fatal visceral forms. Leishmaniasis is re-emerging as a significant world health problem, with approximately 12 million people presently infected and 2 million new cases diagnosed each year (www.who.int/leishmaniasis/burden).
The world-wide distribution of different Leishmania is determined by the availability of transmission-competent sand fly vectors. When a sand fly bites an infected vertebrate host, Leishmania amastigotes residing within macrophages and other cell types are taken up in the blood meal which is surrounded by a midgut peritrophic matrix that lasts for several days. During this time amastigotes differentiate into motile, replicating promastigote forms which reside in the extracellular lumen of the sand fly alimentary tract (rev. in [1], [2], [3]). Barriers to Leishmania development in this compartment include the chitin-containing peritrophic matrix which completely encases the blood meal, and the many hydrolytic enzymes and anti-microbial molecules secreted into the gut lumen (rev. in [2], [3], [4], [5]). Eventually the remnants of the digested blood meal are excreted by the sand fly, and this is a crucial juncture for Leishmania promastigotes. In transmission-competent sand flies, parasites attach to the midgut epithelium and go on to establish a stable infection; in a transmission-refractory vector, unattached parasites are expelled when the sand fly defecates (rev. in [2], [3], [4]). As the sand fly prepares to feed again, promastigotes transition through several forms that culminate in infectious metacyclic parasites which express a modified surface that cannot bind to the midgut epithelium (rev. in [1], [3], [4], [6]). Thus, a key step in Leishmania transmission is stage-specific midgut attachment which allows Leishmania development to proceed.
Two distinct mechanisms for regulating Leishmania attachment to sand fly midgut epithelium have been identified to date (rev. in [1], [6], [7], [8]). One mechanism, utilized in “selective” Phlebotomus papatasi sand flies that support the complete development of only a single Leishmania species, involves a sand fly midgut epithelium receptor that binds the parasite lipophosphoglycan (LPG) adhesin. LPG is an abundant glycolipid that covers the entire surface, including the flagellum, of all Leishmania promastigote stages [9]. The basic LPG structure is highly conserved in all Leishmania species, consisting of a glycosyl-phosphatidyl-inositol lipid anchor to which is attached a long polymer of 10–30 phosphoglycan (PG) repeating units (6Galβ1,4-Manα1-PO4), and terminated by a small neutral oligosaccharide cap (rev. in [10], [11]). The PG repeating units are often modified by strain-, species-, and developmental stage-specific modifications that have been implicated in the midgut attachment and release of several Leishmania species in their respective natural vectors [12], [13], [14], [15]. A second, LPG-independent sand fly midgut binding mechanism was recently identified using LPG-deficient Leishmania and several “permissive” sand flies that support the development of a broad range of Leishmania species in the laboratory [2], [7], [8], [16]. While the precise binding modality is uncertain, the involvement of vector glycans has been suggested [8], [16]. Leishmania phosphoglycans and/or other LPG2-dependent molecule(s) are also required for parasite survival in “permissive” sand flies [8], [17]. Thus sugars, in the form of surface glycoconjugates, are key players in the productive interactions between Leishmania and sand flies that are necessary for disease transmission. This is in agreement with the general principle that important surface interactions between many microbes and their hosts involve complex glycoconjugates binding to receptors (rev. in [18]).
In this study we focused on the interactions between Leishmania major promastigotes and Phlebotomus papatasi sand flies. Phlebotomus papatasi is a “selective” vector which, despite its wide distribution in regions endemic for transmission of several Leishmania species, transmits only Leishmania major in nature and in the laboratory (rev. in [1], [2], [3], [4], [6]. In this vector, specificity is controlled by a stage-specific modification in the LPG adhesin [6], [12]. Midgut attachment is mediated by modified PG repeats bearing side chain β1,3 galactosyl residues (scGal), which form the ligand recognized by the midgut LPG receptor PpGalec identified in Jordan Valley strain P. papatasi (PpapJ) sand flies [19]. As L. major procyclics develop into infectious metacyclic forms, procyclic form LPG is shed and replaced by metacyclic form LPG, which has increased numbers of PG repeats and scGal residues masked by the addition of terminal arabinose “caps”; these modifications block binding to PpGalec receptors [19] and facilitate detachment from the midgut [19], [20]. Laboratory infections established the requirement for scGal-LPG in PpapJ midgut survival: L. major mutants or Leishmania species expressing scGal-deficient LPG, or lacking LPG entirely, could not establish stable PpapJ infections [12], [16], [21], [22], [23], [24] and bound poorly to isolated PpapJ midguts and recombinant PpGalec receptors in vitro [19].
Notably, geographically diverse L. major strains express very different LPG side chain galactosylation patterns [which we refer to hereafter as scGal-LPG PAMPs (Pathogen Associated Molecular Patterns)], showing a Southwest-to-Northeast cline across its range from ‘null-scGal’ to ‘poly-scGal’ LPG PAMPs ([23], [25], [26], [27]; Cardoso et al., in preparation}. For example, Senegalese strain SD procyclic LPG has such low levels of single βGal modifications that it is effectively unmodified [‘null-scGal’ LPG PAMP; [23] and this report}. In contrast, Israeli strain FV1 procyclic LPG is highly modified with primarily single βGal residues (‘mono-scGal’ LPG PAMP; [27]), and Central Asian strain LV39 clone 5 (LV39c5) procyclic LPG is highly modified by long polymers of up to 8 βGal residues (‘poly-scGal’ LPG PAMP; [28], [29]). Amongst these natural L. major strains, FV1 is sympatric with the “selective” P. papatasi PpapJ sand fly, while SD parasites are sympatric with P. duboscqi, a closely related sibling species of P. papatasi (rev. in [3]).
Previously, we hypothesized that different scGal-LPG PAMPs resulted from the combined activity of the seven telomeric SCG (Side Chain Galactose) gene family members, which encode PG-side chain-β1,3-galactosyltransferases. SCGs exhibit different activities, combining to varying extents ‘initiating’ activities able to attach the first βGal residue to the basic PG repeat, and ‘elongating’ activities able to add additional βGal residues to the initiated βGal side chain ([30], [31]; Dobson et al., in preparation). In this work, we made use of this suite of diverse SCG activities to engineer isogenic parasites bearing defined scGal-LPG PAMPs. Using both naturally-occurring L. major strains and SD-SCG transfectant lines, we show that a specific scGal-LPG PAMP is optimal for long-term parasite survival in selective PpapJ sand flies, which preferred highly substituted scGal-LPG PAMPs bearing mono-galactosyl chains, neither “too short” nor “too long”. The “PpapJ-optimal” scGal-LPG PAMP was not sufficient, however, to enhance survival of L. donovani-SCG transfectants in PpapJ sand flies. These findings lead us to propose a two-component model for long-term Leishmania survival in “selective” PpapJ sand flies: 1) a specific scGal-LPG PAMP recognized by PpGalec midgut receptors and 2) an as yet unidentified L. major species-specific factor(s).
Methods
Leishmania strains and transfections
Leishmania major strain Friedlin V1 (FV1) is a clonal derivative of the Friedlin line (MHOM/IL/80/Friedlin), L. major strain LV39 clone 5 (LV39c5) is a clonal derivative of the LV39 line (RHO/SU/59/P), L. major strain SD 75.1 (SD) is a clonal derivative of the NIH/SD line (MHOM/SN/74/SD), L. donovani Sudanese strain 1S-2D clone Ld4 (Ld) is a clonal derivative (MHOM/SD/00/1S-2D), and L. mexicana strain M379 is a clonal derivative (MYNC/BZ/62/M379). All wild type (WT) lines showed good infectivity in animal models and in their natural sand fly vectors [24], [32], [33], [34]. Cells were grown in complete M199 medium containing 10% heat-inactivated fetal bovine serum, penicillin (50 units/ml), streptomycin (50 µg/ml), HEPES pH 7.4 (40.5 mM), adenine (0.1 mM), biotin (0.0001%), biopterin (2 µg/ml), and hemin (0.0005%), at 25°C as described [35]. Procyclic promastigotes were harvested from logarithmically growing cultures.
Promastigotes were transfected by electroporation, using a low voltage [35] or high voltage [36] protocol. Clonal lines were obtained by plating on semisolid M199 media containing the appropriate selective drug concentration: 50 µg/ml hygromycin B (HYG), 20 µg/ml phleomycin (PHLEO), 15 µg/ml G418 (NEO), or 100 µg/ml nourseothricin (SAT).
Molecular constructs and transfectants
L. major strain FV1 was the source of all SCG genes used in this study. Episomal expression constructs used here include the cosmid vector cLHYG (strain B890; [37]), pXK(NEO)-SCG2 (B3900; [30]), SCG3 cosmid B3979 [30], and pXG(NEO)-LMSAP1 (B3092; [34]). The integrating SCG open reading frame (ORF) constructs pIR1SAT-SCG1 (B5097), pIR1SAT-SCG3 (B5101), pIR1SAT-SCG4 (B5103), and pIR1SAT-SCG5 (B5170) were created as follows: SCG ORFs liberated by BamHI digestion of appropriate pXG(PHLEO)-SCG ORF constructs (Dobson et al., in preparation) were ligated into the BglII expression site of pIR1SAT (B3541; [36]). Each pIR1SAT-SCG construct was digested with SwaI restriction enzyme, dephosphorylated with calf alkalline phosphatase, and gel-purified to yield linear SSU::IR1SAT-SCG ORF targeting fragments for integration into the ribosomal RNA small subunit (SSU) locus by homologous recombination during transfection [36]. Integrated SCG transfectants are referred to by the gene name and location, i.e. SD-SSU:SCG3 has the FV1 strain SCG3 ORF (SSU::IR1satSCG3) integrated into the SD ribosomal SSU locus.
We used three Ld-transfectant lines developed previously [30]: a ‘null-scGal’ LPG PAMP line devoid of any LPG side chain modification, transfected with the episomal cosmid vector cLHYG (Ld-vector); and two different lines exhibiting ‘mono-scGal’LPG PAMPs, one transfected with SCG3 cosmid B3979 (Ld-cSCG3), and a second transfected with the SCG2 ORF expression construct pXK-SCG2 (Ld-pSCG2).
Purification and analysis of LPG
LPG was prepared from exponentially-growing promastigotes as described [38]. To assess side chain modifications, phosphoglycan repeats were depolymerized using mild acid hydrolysis, dephosphorylated using E. coli alkaline phosphatase, covalently labeled with 1-aminopyrene-3,6,8-trisulfonate, and analyzed by Dionex HPLC chromatography [14] or capillary electrophoresis [39], comparing migration distances with oligomeric glucose standards.
Analysis of secreted acid phosphatase (SAP) levels
Procyclic promastigotes (5×105/ml) were grown in complete M199 medium to a final density of 1×107/ml. Culture supernatants were collected and centrifuged for 10 min at 2500 rpm in a Sorvall RT7000 centrifuge to remove cells and debris. 10 microliter samples of clarified culture supernatant were electrophoresed on a non-denaturing polyacrylamide gel and the gel then stained for SAP enzyme activity using α-naphthyl acid phosphate plus Fast Garnet GBC as described [40], [41]. SAP was quantitated using the AlphaImager version 5.5 gel documentation system spot densitometry program (Alpha Innotech, San Leandro, CA).
Sand fly infection and dissection
Sand fly colonies were reared at the Division of Entomology, Walter Reed Army Institute of Medical Research and at the Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, NIH. The following species were used in this study: Phlebotomus papatasi from colonies originating from the Jordan Valley (PpapJ), Phlebotomus duboscqi from colonies originating from Mali (PdubM), and Phlebotomus argentipes from colonies originating from India (PargIN).
Female 3 - to 5-day-old sand flies were fed through a chick skin membrane using a feeding device containing a mixture of heparin-treated mouse blood and logarithmic phase promastigotes, as described [24]. The concentration of promastigotes used varied depending on the experiment, from 1×106 to 20×106 parasites/ml. Blood-engorged sand flies were separated and maintained at 28 C with 30% sucrose (v/v). At various times after feeding, flies were anesthetized, their midguts dissected and homogenized, and the number of released midgut promastigotes counted using a hemocytometer as described [24].
Statistical analyses
Parasite numbers in the midguts of infected flies after blood meal excretion do not follow a Gaussian distribution. This is likely the result of flies within groups having either completely lost their infections or retained parasites that grow exponentially prior to the time of dissection. Therefore, data sets were compared using a nonparametric Mann Whitney test. Mann Whitney calculations were done using Prism 4 (Graphpad Software, Inc. San Diego, CA).
Results
Three natural L. major strains show varying patterns of LPG side chain galactosylation
LPGs from three geographically distinct L. major strain procyclic promastigotes were purified, subjected to mild acid hydrolysis and dephosphorylation, and isolated PG repeat structures assessed by capillary electrophoresis (Methods, Table S1). Side-chain galactosylation can be characterized by two parameters: the fraction of PG repeats that were modified, and the number of βGal residues attached. In these studies we found two general patterns of LPG side chain galactosylation: one in which little or no βGal was added; and a second in which 50–90% of the PG repeats were modified, with varying numbers of βGal residues. From these data we found it useful to calculate a single parameter for comparisons amongst lines, the “average scGal chain length”, obtained by multiplying the fraction of modified PG repeats times the average number of βGal residues added per modified repeat (Tables 1, S1).
Tab. 1. Effect of LPG galactosylation pattern on Leishmania survival in “selective” Phlebotomus papatasi PpapJ sand flies. 
The L. major (FV1, LV39c5, SD) and L. donovani (Ld) wild-type (“WT”) and transfectant lines used in experimental PpapJ laboratory infections are described in the text, with supporting data in Tables S1 to S3 and Methods. Senegalese strain SD LPG was mostly unmodified (0.02 avg. scGal chain length), consistent with prior studies using specific antisera and lectins suggesting that SD LPG was largely unmodified [23]. In contrast, Israeli strain FV1 LPG was extensively modified with predominantly single βGal residues (0.8 avg. scGal chain length). Central Asian strain LV39c5 LPG was also highly modified, but with longer polymers of up to 8 βGal residues (3.1 avg. scGal chain length). We refer to these three prototypic LPG galactosylation patterns as ‘null-scGal’, ‘mono-scGal’, and ‘poly-scGal’ LPG PAMPs (Pathogen-Associated Molecular Patterns), respectively. The results with FV1 and LV39c5 confirmed and extended previous studies [27], [28], [29], and were undertaken to guard against changes in LPG side chain composition occurring during laboratory propagation, as described previously [25], [42].
L. major survival in selective Phlebotomus papatasi PpapJ sand flies requires a specific scGal-LPG PAMP
PpapJ sand flies were fed on the indicated L. major-infective mouse blood and midgut infections were assessed 48 hr later, a time when parasites remain within the blood meal encased by the peritrophic membrane (Fig. 1A, “+ blood, d2”). At this time all three L. major strains showed high parasite numbers in most flies examined (>33,000 parasites/midgut), with the highest numbers observed in flies infected with the SD strain, likely reflecting the faster generation time of this strain. Thus differences in the scGal-LPG PAMPs did not affect the early survival and growth of L. major promastigotes, as expected since even LPG-deficient parasites survive normally in sand flies during this interval [16], [22], [24].
Fig. 1. Galactosylated LPG does not ensure survival of L. major promastigotes in Jordan Valley strain P. papatasi PpapJ sand flies. 
Female PpapJ sand flies were membrane fed on infective mouse blood containing the indicated L. major strain (LPG galactosylation pattern in parentheses) at concentrations of 4×106 (panel A) or 8×106 (panel B) per ml. At the indicated day (“d”) after feeding, midguts were dissected and the number of viable promastigotes determined by counting under a hemocytometer. “+ blood” denotes midguts that retained the blood meal, and “no blood” denotes midguts that had no detectable blood as a result of the digested blood meal having been expelled. Each symbol represents the number of parasites in a single sand fly midgut, and each bar represents the mean number of parasites for each group. The percentages of infected flies in each group are shown in italics. P values shown were calculated for the indicated pairs of infected flies. Results from two independent experiments (panels A,B) are shown. By day 5 post-feeding, the sand fly peritrophic matrix disintegrates and the remains of the digested blood meal are expelled. At this time there were clear differences amongst the L. major strains in their ability to survive (Fig. 1A, “no blood, d5”). In agreement with previous studies [22], [24], ‘mono-scGal’ FV1 persisted in most PpapJ flies at high levels (82% flies infected, 16200±16600 parasites/midgut). In contrast, ‘poly-scGal’ LV39c5 survived poorly (53% flies infected, 3860±4840 parasites/midgut; p<0.013), as did ‘null-scGal’ SD, with the exception of two strongly-infected outliers (38% flies infected, 6660±18600 parasites/midgut; p<0.005). The poor survival of ‘null-scGal’ SD was expected, as un-galactosylated LPG cannot bind to midgut PpGalec receptors [19], resulting in unattached parasites being excreted with the digested blood meal remnants [21], [24]. However, the poor PpapJ survival of ‘poly-scGal’ LV39c5 (Fig. 1A,B; [24]) suggested that a specific scGal-LPG PAMP, rather than simply the presence of galactosylated LPG, controls L. major promastigote survival in PpapJ midguts following blood meal expulsion.
Generation of isogenic L. major parasites bearing a range of scGal-LPG PAMPs
Since the three L. major strains studied here show an average nucleotide sequence divergence of 0.15% [43], comparable to that amongst many L. major strains, molecular differences other than scGal-LPG PAMPs were potentially responsible for the survival differences we observed in selective PpapJ sand fly infections. To generate different scGal-LPG PAMPs in an isogenic scGal-deficient LPG background, we introduced into the SD line a series of constructs expressing members of the previously characterized SCG family of telomeric phosphoglycan-side chain-(β1,3)galactosyltransferases (PG-scβGalTs) [30], [31] Critical to these studies is the fact that SCG-encoded PG-scβGalTs have different enzymatic properties mediating the addition of different numbers of scGal residues, ranging from 0 to 12 ([30], [31]; Dobson et al., in preparation). Thus, SD promastigotes were transfected with different SCG constructs, using either the episomal pXG-type vector which expresses passenger ORFs at moderate levels [37], episomal cosmids identified previously bearing SCG genes [30], or the integrating pIR1SAT vector which expresses passenger ORFs at high levels following integration into the ribosomal RNA small subunit (SSU) locus [36]. LPGs were purified from SD transfectants and LPG galactosylation patterns determined as described above (Methods); from these studies we chose a key set of SD-SCG lines exhibiting a range of scGal-LPG PAMPs (Tables 1, S1) briefly summarized here.
SD transfectants bearing an integrated catalytically inactive SCG5 ORF (SD-SSU:SCG5) synthesized scGal-deficient LPG indistinguishable from the parental WT SD line (‘null-scGal’ LPG PAMP; 0.02 avg. scGal chain length). Two SD transfectants expressed ‘mono-scGal’ LPG PAMPs: SD-cSCG3 (0.9 avg. scGal chain length), containing the episomal SCG3 cosmid B3979; and SD-SSU:SCG3 (1.3 avg. scGal chain length), containing an integrated SCG3 ORF (SSU::IR1SAT-SCG3). SD-SSU:SCG4 transfectants bearing an integrated SCG4 ORF (SSU::IR1SAT-SCG4) synthesized a ‘poly-scGal’ LPG PAMP (3.1 avg. scGal chain length). A novel ‘oligo-scGal’ LPG PAMP (1.9 avg. scGal chain length) was synthesized by SD-SSU:SCG1, which bears an integrated SCG1 ORF (SSU::IR1sat-SCG1). Together these SD-transfectant scGal-LPG PAMPs spanned the natural range of L. major LPG side chain variation as well as providing new LPG galactosylation patterns for study.
To confirm that SD transfectants had not experienced a general non-specific loss of “sand fly virulence” during their generation and propagation in the laboratory, we examined their survival in two independent infections involving Phlebotomus duboscqi PdubM sand flies originating from Mali (Fig. S1 A,B). P. duboscqi is a sibling species of P. papatasi, and PdubM flies are able to support the full development of WT SD in the laboratory [23]. Although L. major survival in P. duboscqi is LPG-dependent, it is not strongly affected by scGal-LPG PAMPs since various ‘null-scGal’, ‘mono-scGal’, or ‘poly-scGal’ L. major strains have been shown to survive expulsion of the digested blood meal [8], [17], [22], [44]. Female PdubM sand flies were allowed to feed on the indicated L. major-infective mouse blood containing ‘null-scGal’ (WT SD, SD-SSU:SCG5), ‘mono-scGal’ (WT FV1, SD-SSU:SCG3), ‘oligo-scGal’ (SD-SSU:SCG1), or ‘poly-scGal’ (SD-SSU:SCG4) promastigotes. As expected, all PdubM flies were successfully infected with high numbers of parasites at early time points (Fig. S1, “+ blood” panels). Following expulsion of the digested blood meal, PdubM flies infected with all L. major lines retained high numbers of midgut parasites (Fig. S1 “no blood” panels) and each line went on to establish fully mature infections in the PdubM anterior midgut by day 12 post-feeding (data not shown). These data argue against a general non-specific loss in the ability of SD-SCG transfectants to survive in the phlebotomine sand fly midgut environment.
Effect of scGal-LPG PAMPs on SD transfectant survival in “selective” PpapJ sand flies
PpapJ flies were allowed to feed on the indicated L. major-infective mouse blood containing SD transfectants expressing different scGal-LPG PAMPs. The results from two independent experiments are shown (Fig. 2A,B). At early times post-infection when the midgut blood meal was retained, all SD transfectants behaved similarly: 100% of PpapJ flies were infected with high numbers of promastigotes, similar to control ‘mono-scGal’ WT FV1 infections (Fig. 2A,B, “+ blood, d2”). However, we observed clear differences in PpapJ midgut survival amongst SD lines expressing different scGal-LPG PAMPs after the digested blood meal had passed out of the midgut (Fig. 2A,B, “no blood, d5”; Tables 1, S2).
Fig. 2. Survival of L. major SD-SCG transfectants in PpapJ sand flies is dependent on expression of specific scGal-LPG PAMPs. 
Female PpapJ flies were membrane fed on the indicated L. major-infective mouse blood and the number of viable parasites per midgut determined on the indicated day post-feeding as described in Fig. 1. SD-transfectant lines are described in the text, with additional data in Table S1. Infective mouse blood contained 5×106 (panel A) or 10×106 (panel B) parasites per ml. Results from two independent experiments (panels A,B) are shown. First, and as expected, SD-SSU:SCG5 transgenic parasites expressing a ‘null-scGal’ LPG PAMP survived poorly following blood meal excretion, with a 81–92% decrease in mean parasite numbers relative to control FV1-infected flies (p<0.0005), and 38–40% of PpapJ flies having lost their infections (Fig. 2A,B, “no blood, d5”).
Second, SD transfectants expressing ‘mono-scGal’ LPG PAMPs (SD-cSCG3, SD-SSU:SCG3) generally survived well post-blood meal expulsion (Fig. 2A,B, “no blood, d5”). Most flies remained infected, and mean SD-cSCG3 and SD-SSU:SCG3 parasite numbers (18600±4732, 18900±4900 and 18800±5100, 13200±4800 parasites/midgut, respectively) were not significantly different from control WT FV1 infections (13500±3500, 30000±4400 parasites/midgut). By contrast, ‘mono-scGal’ SD-cSCG3 survival was significantly enhanced relative to ‘null-scGal’ SD-SSU:SCG5 (2560±1720, p<0.0004 and 2420±600 parasites/midgut, p<0.003; Fig. 2A,B). Although ‘mono-scGal’ SD-SSU:SCG3 survival was also enhanced relative to ‘null-scGal’ SD-SSU:SCG5, this difference reached significance in only one experiment (p<0.0002 and p<0.264; Fig. 2A,B).
Third, SD-SSU:SCG1 transfectants expressing a novel ‘oligo-scGal’ LPG PAMP survived poorly. Only 37–55% of SD-SSU:SCG1 flies remained infected post-blood meal expulsion and parasite levels (4120±1940, 12500±6300 parasites/midgut) were significantly reduced relative to control WT FV1-infected flies (p<0.018 and p<0.06; Fig. 2A,B). In fact, ‘oligo-scGal’ SD-SSU:SCG1 survival was not significantly better than observed for ‘null-scGal’ SD-SSU:SCG5 (p<0.968 and p<0.954; Fig. 2A,B).
Lastly, and consistent with the results from natural isolates, SD-SSU:SCG4 transfectants expressing a ‘poly-scGal’ LPG PAMP survived poorly. Only 42–56% of SD-SSU:SCG4 flies remained infected and parasite levels (683±215, 2267±831 parasites/midgut) were significantly decreased, 92–95% relative to control WT FV1 infections (p<0.0007 and p<0.0001, Fig. 2A,B). Thus ‘poly-scGal’ SD-SSU:SCG4 PpapJ survival was not significantly better than ‘null-scGal’ SD-SSU:SCG5 parasites.
These findings are summarized in Fig. 3, showing the relationship between relative PpapJ survival post-blood meal expulsion and the average scGal chain length in purified procyclic promastigote LPG. Isogenic SD-SCG transfectants whose LPG closely approximates the ‘mono-scGal’ LPG PAMP of the WT FV1 line (i.e. SD-cSCG3, SD-SSU:SCG3) clearly survived well in PpapJ sand flies. In contrast, isogenic SD transfectants expressing either scGal-deficient LPG (‘null-scGal’ SD-SSU:SCG5) or LPG with longer side chain polymers (‘oligo-scGal’ SD-SSU:SCG1, ‘poly-scGal’ SD-SSU:SCG4) survived poorly in PpapJ flies, mirroring infection outcomes with naturally-occurring L. major strains SD or LV39c5 (‘null-scGal’ or ‘poly-scGal’ LPG PAMPs, respectively). Together, these data firmly implicate the scGal-LPG PAMP causally in controlling the ability of PpapJ flies to support L. major midgut survival post-blood meal expulsion.
Fig. 3. Relationship between L. major scGal-LPG PAMPs and PpapJ midgut survival post-blood meal expulsion. 
Relative survival of L. major promastigotes in infected PpapJ sand flies which have expelled their digested blood meal is plotted as a function of the average LPG scGal length, using data in Table 1. The average (±SEM) of two or more independent experiments is shown for FV1 (▪), LV39c5 (▴), SD-SSU:SCG5 (open pentagon), SD-cSCG3(⋄), SD-SSU:SCG3 (□), SD-SSU:SCG1 (▵), and SD-SSU:SCG4 (○). We include data here for SD (•) from a single experiment, which is consistent with previous studies [21], [23]. Are “PpapJ-optimal” scGal-LPG PAMPs sufficient to enhance PpapJ survival of a different Leishmania species?
The studies above established that a ‘mono-scGal’ LPG PAMP was necessary for L. major survival in selective PpapJ sand flies, following blood meal expulsion. We next asked whether this scGal-LPG PAMP would be sufficient, by examining its effect on the PpapJ survival of a different Leishmania species. We chose L. donovani Sudanese strain 1S-2D (Ld) since these parasites possess unmodified LPG (‘null-scGal’ LPG PAMP; [11], [45]) and have been shown to survive poorly in PpapJ sand flies [12], [24]. We used three Ld-transfectant lines developed previously [30]: a ‘null-scGal’ line devoid of any side chain sugars (Ld-vector, 0 avg. scGal length) and two different lines exhibiting ‘mono-scGal’ LPG PAMPs, Ld-cSCG3 and Ld-pSCG2 (0.7 and 1.1 avg. scGal chain length, respectively; Tables 1, S1).
When PpapJ sand flies were fed on L. donovani-infective mouse blood containing ‘null-scGal’ Ld-vector or ‘mono-scGal’ Ld-cSCG3 promastigotes, all flies were successfully infected with comparably high numbers of parasites when examined at a time when the midgut blood meal was present (Fig. 4A, PpapJ “+ blood, d3” panel). Thus, these parasites were able to survive well in the initial steps of sand fly infection. However, following expulsion of the blood meal at day 5 post-feeding, parasites from both of these lines were completely lost in >90% of PpapJ flies, and those flies retaining infections had very low levels of parasites (180 and 125 parasites/midgut respectively; Fig. 4A, PpapJ “no blood, d5”; Table S3). Thus, despite generation of the optimal highly substituted ‘mono-scGal’ LPG PAMP in the Ld-cSCG3 line, survival in PpapJ was not enhanced (Table 1). As a control, these Ld transfectants were fed to P. argentipes PargIN, a natural vector of Ld transmission originating from India [12], [24]. Previous studies have shown that midgut survival of both L. donovani and L. major in this “permissive” sand fly species is not strongly affected by LPG galactosylation patterns [8], [12], [24]. Due to the limited number of PargIN flies available for analysis, a single infection time point was analyzed comparing flies without blood meal remnants in the midgut on day 5 post-feeding. In contrast to the loss of midgut infections in PpapJ flies, both Ld-vector and Ld-cSCG3 promastigotes persisted and were maintained a moderate infection intensity in most PargIN flies after the digested blood meal was expelled (88% or 78% infected flies; 11263 or 6822 parasites/midgut; Fig. 4A PargIN “no blood, d5”, Table S3). These data argue against a general non-specific loss in the ability of these Ld transfectants to survive in the phlebotomine sand fly midgut environment.
Fig. 4. Expression of “PpapJ-optimal” scGal-LPG PAMPs in L. donovani-SCG transfectants does not improve survival in PpapJ sand flies. 
Female sand flies were membrane fed on the indicated Leishmania-infective mouse blood and the number of viable parasites per midgut determined on the indicated day post-feeding as described in Fig. 1. Ld transfectant lines are described in the text, with additional data in Table S1. In panel A, P. papatasi PpapJ (“PpapJ”) and P. argentipes PargIN (“PargIN”) flies fed on infective blood containing 10×106 parasites per ml. In separate experiments, P. papatasi PpapJ flies fed on infective blood containing 5×106 (panel B) or 20×106 (panel C) parasites per ml. In separate experiments PpapJ flies were fed on Leishmania-infective mouse blood containing ‘null-scGal’ Ld-vector, ‘mono-scGal’ Ld-pSCG2, or control ‘mono-scGal’ WT L. major FV1 promastigotes (Fig. 4B,C). As expected, most PpapJ flies were infected with high numbers of parasites prior to expulsion of the blood meal, although the numbers of Ld-vector and Ld-pSCG2 were significantly less than control WT L. major FV1 (Fig. 4B “+ blood, d3” panel). However, in PpapJ flies that had expelled their blood meal, neither Ld-vector nor Ld-pSCG2 survived (0% infected flies), whereas good survival was seen with the WT FV1 control (100% infected, 13200 parasites/midgut; Fig. 4B “no blood, d4” panel; Table S2). When PpapJ sand flies were infected with a 4-fold higher concentration of parasites to compensate for the diminished early growth of Ld transfectants compared to WT FV1, we again observed poor survival of both ‘mono-scGal’ Ld-pSCG2 and ‘null-scGal’ Ld-vector parasites after the midgut blood meal had been expelled (Fig. 4C “no blood, d5” panel), despite massive parasite loads in midguts that retained their blood meals at day 3 post-feeding (Fig. 4C “+ blood, d3” panel). Ld-vector and Ld-pSCG2 numbers were each significantly decreased relative to control WT FV1 (>90%, p<0.001), although a higher percentage of PpapJ flies remained infected (38% of Ld-vector, 52% of Ld-pSCG2, 100% of WT FV1; Fig. 4C “no blood, d5” panel). These results are consistent with early observations regarding the ability of high concentration of promastigotes in the artificial blood meal to overcome the natural resistance of P. papatasi to infection with L. donovani [12], [24], [46]. Together, these data suggest that while necessary for survival and transmission of L. major in “selective” PpapJ sand flies, the ‘mono-scGal’ LPG PAMP alone is not sufficient to rescue L. donovani-SCG promastigotes in PpapJ sand flies during the critical time of blood meal expulsion.
Competition with high levels of scGal-modified secreted acid phosphatase (SAP) is unlikely to account for poor PpapJ survival of ‘mono-scGal’ Ld-SCG promastigotes
Unlike L. major, L. donovani and most other Leishmania species secrete high levels of acid phosphatases (SAPs) covalently modified by PG repeats [47], [48], [49]. Since PG repeats attached to SAP bear the same covalent side chain modifications as LPG PG repeats [29], [41], [50], ‘mono-scGal’ SAP could potentially compete for Ld-cSCG3 and Ld-pSCG2 promastigote binding to PpapJ midgut PpGalec receptors, thereby accounting for their failure to survive expulsion of the digested blood meal. To test this hypothesis, we engineered L. major FV1 to express high levels of SAP (Methods). High levels of active SAP were detected in the culture medium of all FV1-SAP transfectant lines, more than 1100 times higher than SAP levels in WT FV1 or control FV1-vector transfectant culture media and thus comparable to SAP levels secreted by L. donovani and other Leishmania species promastigotes (Table S4). However, in two independent experiments involving infections of PpapJ flies with L. major-infective mouse blood, FV1-SAP1 over-expressors survived as well as control FV1-vector promastigotes, both prior to and after expulsion of the digested blood meal (Fig. 5A,B “+ blood, d3” and “no blood, d4” panels, respectively). These data argue that competition with ‘mono-scGal’ SAP is unlikely to account for the poor PpapJ survival of Ld-SCG transfectants expressing the ‘mono-scGal’ LPG PAMP preferred by this sand fly.
Fig. 5. L. major FV1 promastigote survival in PpapJ infections is unaffected by over-expression of secreted acid phosphatase (SAP). 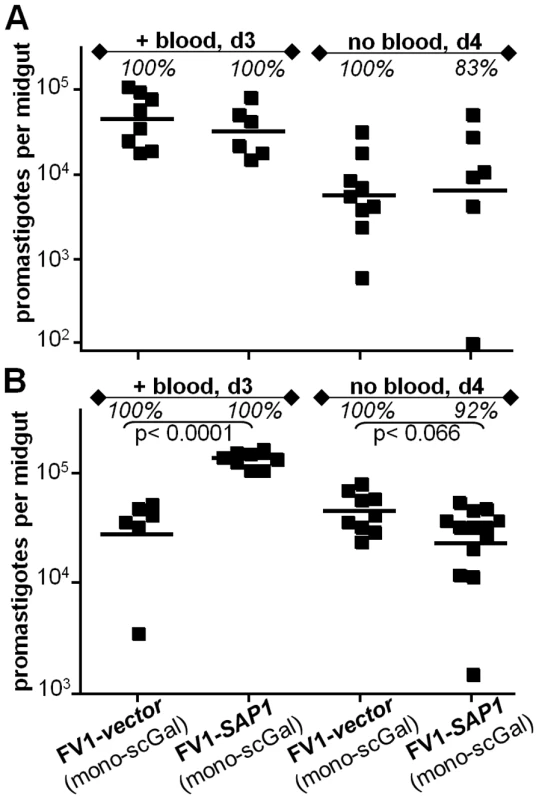
Female PpapJ flies were membrane fed on the indicated L. major-infective mouse blood (4×106 parasites per ml) and the number of viable parasites per midgut determined on the indicated day post-feeding as described in Fig. 1. FV1-transfectant lines are described in the text, with additional data in Table S4. High levels of active SAP were detected in the culture medium of all FV1-SAP transfectants, but not in WT FV1 or FV1-vector lines (Table S4). Results from two independent experiments (panels A,B) are shown. Discussion
In this report we have studied the ability of parasites bearing different LPG side chain galactosylation PAMPS to interact with a “selective” sand fly Leishmania host, Phlebotomus papatasi PpapJ originating from the Jordan Valley. Previous data have shown that galactosylated LPG plays a key role in mediating L. major midgut survival and binding in this sand fly species, and that binding was mediated by the PpapJ midgut LPG receptor PpGalec [12], [19], [21], [24]. We show here first that this scGal-LPG PAMP is more complex than originally proposed, as only parasites bearing short ‘mono-scGal’ LPG PAMPs survived expulsion of the digested blood meal in infected PpapJ sand flies. This was shown using both natural L. major isolates exhibiting a wide range of scGal-LPG PAMPs (Fig. 1) and isogenic derivatives of the normally scGal-deficient L. major strain SD engineered to express different scGal-LPG PAMPs via transfection of different SCG-encoded PG-side chain-(β1,3)galactosyltransferases (Fig. 2). Thus, like the fairy-tale character Goldilocks, PpapJ sand flies show an exquisite specificity for the “just right” ‘mono-scGal’ LPG PAMP, rejecting those scGal-LPG PAMPs that are either “too short” (‘null scGal’, <0.02 avg. scGal chain length) or “too long” (‘oligo-scGal’ and ‘poly-scGal’; ≥1.9 avg. scGal chain length).
Several molecular scenarios may account for the inability of L. major promastigotes expressing ‘oligo-scGal’ or ‘poly-scGal’ LPG to survive expulsion of the blood meal in PpapJ flies. Since all PpapJ-competent lines expressed LPG containing a high percentage of PG repeats bearing a single βGal residue (50–59%, Table S1), it seems likely that the low level of mono-galactosylated PG repeats in ‘oligo-scGal’ and ‘poly-scGal’ L. major lines (6–9%, Table S1) is not sufficient to mediate binding to PpGalec midgut receptors. An alternative, non-exclusive model considers interference by modified PG repeats decorated with long chains of poly-scGal residues, which could sterically interfere with the productive binding of the mono-galactosylated PG repeats. This latter model could be probed by testing parasites bearing LPG substitutions clustered differentially along the “backbone” of polymeric PG repeats; however, methods for engineering such parasites are not yet available.
Do scGal-LPG modifications control L. major “selectivity” in all Phlebotomus papatasi?
As noted earlier, many workers have grouped sand fly species according to their ability to support in experimental infections the survival (and, in some cases, experimental transmission) of a wide versus limited range of Leishmania species [7], [8], [12], [16], with the former group termed “permissive” sand flies and the latter termed “selective” or “restricted”. The availability of Leishmania mutants specifically defective in LPG (through the deletion of the gene encoding the LPG-specific galactofuranosyltransferase LPG1) has shown that in general, “selective” sand fly species show a strong role for LPG in midgut survival and binding, while the “permissive” sand fly species show little LPG dependency [7], [8], [12], [16], [17], [24]. Our panel of engineered and natural L. major, varying greatly in scGal-LPG modification, allowed us to compare the effects seen in a “selective” sand fly, P. papatasi PpapJ from the Jordan Valley, which showed a strong preference for ‘mono-scGal’ LPG PAMPs (Figs. 1–3).
Recently, we have completed studies of more than 15 L. major isolates that reveal a range in the extent of procyclic promastigote scGal-LPG modification, with a general cline proceeding from scGal-deficient ‘null-scGal’ LPG modification in West Africa to short chain ‘mono-scGal’ modification in the Middle East to long chain ‘poly-scGal’ modification in Central Asia (Cardoso et al., in preparation). Together with the findings presented here, the stage is now set for further explorations of the role of scGal-LPG PAMPs in L. major transmission in other natural settings. Since one natural P. papatasi sand fly vector in this geographic range showed differing abilities to support Leishmania growth which were dependent on scGal-LPG PAMPs (Figs. 1–3), it seems likely these may play an important role and perhaps even a driving force in the evolution of parasite/vector selectivity. For example, all Israeli L. major lines whose LPG has been characterized show ‘mono-scGal’ LPG PAMPs (V121 strain, avg. 1.1 scGal length; L580 strain, avg. 0.7 scGal length; calculated from data in [25], [27]) and correspondingly, the ability of a P. papatasi PpapJ colony established from wild caught flies from the Jordan Valley to support L. major midgut survival is strongly dependent on this scGal-LPG PAMP. In this respect it will be interesting to examine the properties of P. papatasi sand flies from Central Asia, including potential structural diversity in their PpGalec midgut LPG receptor, as L. major from this region typically elaborate a ‘poly-scGal’ LPG PAMP similar to that of LV39c5 (Cardoso et al., in preparation). Our work demonstrating a geographical origin-based specificity between PpapJ sand fly vector and L. major strains also complements the work of Elfari et al. [51] who demonstrated evidence for genetic and biological diversity in L. major strains that correlated with geographical origin and their ability to infect only sympatric animal reservoir hosts.
“PpapJ-optimal” scGal-LPG modifications are not sufficient to confer PpapJ midgut survival to L. donovani
While expression of appropriate scGal-LPG PAMPs is necessary for the survival of L. major in the PpapJ sand fly midgut, is it sufficient? We tested this by engineering the ‘mono-scGal’ LPG PAMP into a Sudanese strain of L. donovani which normally expresses a completely unmodified LPG coat [45]. We showed by biochemical analyses and agglutination tests (Table S1, [30], [31]) that the engineered scGal-LPG PAMPs in L. donovani-SCG transfectants were faithful replicas of L. major ‘mono-scGal’ LPG PAMPs synthesized by natural WT L. major FV1 and engineered SD-SCG3 transfectants, all of which exhibited robust long-term survival in PpapJ laboratory infections (Tables 1, S2). However, L. donovani-SCG lines bearing a ‘mono-scGal’ LPG surface coat remained unable to survive following expulsion of the blood meal in infected PpapJ flies (Fig. 4; Tables 1, S2, S3).
We then explored several possible mechanisms that could account for the failure of L. donovani bearing an L. major FV1 LPG “surface” to survive. First was the possibility that secretion of scGal-modified acid phosphatases (SAPs, [47], [48], [50]) competed for LPG-dependent midgut binding and parasite survival. While SAP-deficient L. donovani are not available, reconstruction experiments in L. major FV1 promastigotes expressing high levels of PG-modified SAPs (Fig. 5, Table S4) failed to reveal any alterations in PpapJ survival. Thus, competition by L. donovani scGal-SAP is unlikely to account for the failure of Ld-SCG promastigotes to survive in PpapJ midguts. A second reason was that the engineered ‘mono-scGal’ L. donovani were unable to withstand PpapJ midgut conditions, since early killing of L. donovani promastigotes in the P. papatasi midgut has been reported [52]. In fact, in comparison to the sympatric L. major FV1 strain, the L. donovani lines showed reduced growth in the early blood fed midgut (Fig. 4B), due either to their slower generation times, and/or their greater sensitivity to midgut digestive enzymes. Nevertheless, when the differences in the concentration of parasites present prior to blood meal excretion were overcome by initiating infection with a high dose inoculum, the L. donovani lines were still largely absent in flies that had passed their blood meals (Fig. 4C). Furthermore, L. donovani transfectants were able to survive within the midgut of P. argentipes PargIN sand flies (Fig. 4A). Importantly, survival in this sand fly species cannot be attributed simply to a more permissive midgut environment, as P. argentipes restricts survival of lpg2 - Ld lines which lack LPG and other PGs, evidence of a strongly hydrolytic midgut environment [8], [24]. These data argue that the inability of WT or engineered L. donovani lines to survive in PpapJ sand flies is not due to an inability to withstand the midgut environment, and the timing of the loss of infection is consistent with their failure to attach to the midgut.
Additional factors may be required to mediate Leishmania - sand fly midgut interactions
Thus, while specific scGal-LPG PAMPs are necessary for L. major persistence and midgut binding during expulsion of the blood meal in PpapJ flies, the inability of L. donovani expressing the appropriate L. major scGal-LPG PAMP to survive in the same fly strain suggests most simply that this interaction, while necessary, is not sufficient for midgut attachment. This in turn would argue that an additional parasite ligand(s) must be required, one shared in the closely related L. major strains but lacking in L. donovani, which diverged from L. major >80 million years ago [53]. In this model, generation of proper scGal-LPG PAMPs in L. major SD would be sufficient to promote survival, since L. major strains would retain this second L. major-specific interaction; but insufficient in L. donovani, where the second interaction was absent due to evolutionary divergence or loss. In contrast to Ld-SCG transfectants, which “inherited by transfection” only the scGal-LPG-dependent ligand, the enhanced P. papatasi survival of L. infantum - L. major hybrids observed by Volf et al. (relative to L. infantum; [54]) is thus predicted to result from the inheritance of both L. major-specific scGal-LPG-dependent and -independent ligands.
Whether this postulated second interaction is mediated through a second species-specific receptor for LPG, or an LPG-independent ligand such as the one proposed by Myskova et al. to control midgut binding of certain Leishmania species in “permissive” sand fly vectors [8], [16], is unknown. Perhaps the PpapJ ‘mono-scGal’ LPG midgut receptor PpGalec collaborates with a co-receptor, similar to the interactions of certain other pattern recognition receptors such as Toll-like receptors (TLR1/2/6) with each other or with other receptors (Dectin-1, CD14, TLR4; reviewed in [55], [56].
This putative species-specific co-receptor may be especially relevant to the interaction of L. major strains with P. duboscqi sand flies. This vector, while unable to support the survival of L. major lines completely deficient in LPG biosynthesis [8], [22], [44], is not sensitive to differences in L. major LPG galactosylation patterns (Fig. S1) and naturally transmits L. major strains in West Africa bearing effectively ‘null-scGal’ LPG. Nonetheless, P. duboscqi is a “selective” vector, permitting only the development of L. major in experimental infections ([8], Sacks et al., unpublished). These data suggest that the few interactions between predicted P. duboscqi PpGalec midgut LPG receptors [19] and the low number of mono-galactosylated PG repeats in WT SD LPG (2%, Table S1) is sufficient to mediate parasite attachment to the PdubM midgut epithelium, in concert with a second L. major-specific midgut binding interaction that is especially strong in this particular sand fly species. It is also possible that a scGal-independent ligand present on L. major LPG binds to the alternative receptor and is a sufficient interaction to maintain infection in the PdubM vector. When the factor(s) controlling parasite LPG-independent binding and survival of Leishmania in “selective” and “permissive” sand fly species becomes known, it should be possible to test these hypotheses.
Supporting Information
Zdroje
1. BatesPA
2008 Leishmania sand fly interaction: progress and challenges. Curr Opin Microbiol 11 340 344
2. VolfP
HostomskaJ
RohousovaI
2008 Molecular Crosstalks in Leishmania-Sandfly-Host Relationships. Parasite 15 237 243
3. SacksDL
LawyerP
KamhawiS
2008 The biology of Leishmania – sand fly interactions.
MylerPJ
FaselN
Leishmania - After the Genome Norwish, UK Caister Academic Press 205 238
4. KamhawiS
2006 Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol 22 439 445
5. OliveiraF
JochimRC
ValenzuelaJG
KamhawiS
2009 Sand flies, Leishmania, and transcriptome-borne solutions. Parasitol Int 58 1 5
6. SacksDL
2001 Leishmania-sand fly interactions controlling species-specific vector competence. Cell Microbiol 3 189 196
7. VolfP
MyskovaJ
2007 Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol 23 91 92
8. SvarovskaA
AntTH
SeblovaV
JecnaL
BeverleySM
2010 Leishmania major glycosylation mutants require phosphoglycans (lpg2-) but not lipophosphoglycan (lpg1-) for survival in permissive sand fly vectors. PLoS Negl Trop Dis 4 e580
9. PimentaPF
SaraivaEM
SacksDL
1991 The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp Parasitol 72 191 204
10. IlgoutzSC
McConvilleMJ
2001 Function and assembly of the Leishmania surface coat. Int J Parasitol 31 899 908
11. TurcoSJ
DescoteauxA
1992 The lipophosphoglycan of Leishmania parasites. Ann Rev Micro 46 65 94
12. PimentaPF
SaraivaEM
RowtonE
ModiGB
GarrawayLA
1994 Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc Natl Acad Sci U S A 91 9155 9156
13. KamhawiS
ModiGB
PimentaPF
RowtonE
SacksDL
2000 The vectorial competence of Phlebotomus sergenti is specific for Leishmania tropica and is controlled by species-specific, lipophosphoglycan-mediated midgut attachment. Parasitology 121 Pt 1 25 33
14. MahoneyAB
SacksDL
SaraivaE
ModiG
TurcoSJ
1999 Intra-species and stage-specific polymorphisms in lipophosphoglycan structure control Leishmania donovani-sand fly interactions. Biochemistry 38 9813 9823
15. SoaresRP
BarronT
McCoy-SimandleK
SvobodovaM
WarburgA
2004 Leishmania tropica: intraspecific polymorphisms in lipophosphoglycan correlate with transmission by different Phlebotomus species. Exp Parasitol 107 105 114
16. MyskovaJ
SvobodovaM
BeverleySM
VolfP
2007 A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microbes Infect 9 317 324
17. SecundinoN
KimblinN
PetersNC
LawyerP
CapulAA
2010 Proteophosphoglycan confers resistance of Leishmania major to midgut digestive enzymes induced by blood feeding in vector sand flies. Cell Microbiol 12 906 918
18. LloydDH
ViacJ
WerlingD
RemeCA
GattoH
2007 Role of sugars in surface microbe-host interactions and immune reaction modulation. Vet Dermatol 18 197 204
19. KamhawiS
Ramalho-OrtigaoM
PhamVM
KumarS
LawyerPG
2004 A role for insect galectins in parasite survival. Cell 119 329 341
20. PimentaPF
TurcoSJ
McConvilleMJ
LawyerPG
PerkinsPV
1992 Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science 256 1812 1815
21. ButcherBA
TurcoSJ
HiltyBA
PimentaPF
PanunzioM
1996 Deficiency in β1,3-galactosyltransferase of a Leishmania major lipophosphoglycan mutant adversely influences the Leishmania-sand fly interaction. J Biol Chem 271 20573 20579
22. CihakovaJ
VolfP
1997 Development of different Leishmania major strains in the vector sandflies Phlebotomus papatasi and P. duboscqi. Ann Trop Med Parasitol 91 267 279
23. JoshiPB
SacksDL
ModiG
McMasterWR
1998 Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (GP63). Mol Microbiol 27 519 530
24. SacksDL
ModiG
RowtonE
SpäthG
EpsteinL
2000 The role of phosphoglycans in Leishmania-sand fly interactions. Proc Natl Acad Sci U S A 97 406 411
25. McConvilleMJ
SchnurLF
JaffeC
SchneiderP
1995 Structure of Leishmania lipophosphoglycan: inter - and intra-specific polymorphism in Old World species. Biochem J 310 807 818
26. McConvilleMJ
Thomas-OatesJE
FergusonMA
HomansSW
1990 Structure of the lipophosphoglycan from Leishmania major. J Biol Chem 265 19611 19623
27. McConvilleMJ
TurcoSJ
FergusonMA
SacksDL
1992 Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. Embo J 11 3593 3600
28. DobsonDE
MengelingBJ
CilmiS
HickersonS
TurcoSJ
2003 Identification of genes encoding arabinosyltransferases (SCA) mediating developmental modifications of lipophosphoglycan (LPG) required for sand fly transmission of Leishmania major. J Biol Chem 278 28840 28848
29. CapulAA
BarronT
DobsonDE
TurcoSJ
BeverleySM
2007 Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J Biol Chem 282 14006 14017
30. DobsonDE
ScholtesLD
ValdezKE
SullivanDR
MengelingBJ
2003 Functional identification of galactosyltransferases (SCGs) required for species-specific modifications of the lipophosphoglycan adhesin controlling Leishmania major-sand fly interactions. J Biol Chem 278 15523 15531
31. DobsonDE
ScholtesLD
MylerPJ
TurcoSJ
BeverleySM
2006 Genomic organization and expression of the expanded SCG/L/R gene family of Leishmania major: internal clusters and telomeric localization of SCGs mediating species-specific LPG modifications. Mol Biochem Parasitol 146 231 241
32. AndersonCF
MendezS
SacksDL
2005 Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol 174 2934 2941
33. KimblinN
PetersN
DebrabantA
SecundinoN
EgenJ
2008 Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci U S A 105 10125 10130
34. SpathGF
EpsteinL
LeaderB
SingerSM
AvilaHA
2000 Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A 97 9258 9263
35. KaplerGM
CoburnCM
BeverleySM
1990 Stable transfection of the human parasite Leishmania delineates a 30 kb region sufficient for extra-chromosomal replication and expression. Mol Cell Biol 10 1084 1094
36. RobinsonKA
BeverleySM
2003 Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol Biochem Parasitol 128 217 228
37. HaDS
SchwarzJK
TurcoSJ
BeverleySM
1996 Use of the Green Fluorescent Protein as a marker in transfected Leishmania. Molec Biochem Parasitol 77 57 64
38. OrlandiPAJr
TurcoSJ
1987 Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem 262 10384 10391
39. BarronTL
TurcoSJ
2006 Quantitation of Leishmania lipophosphoglycan repeat units by capillary electrophoresis. Biochim Biophys Acta 1760 710 714
40. KatakuraK
KobayashiA
1988 Acid phosphatase activity of virulent and avirulent clones of Leishmania donovani promastigotes. Infect Immun 56 2856 2860
41. ZuffereyR
AllenS
BarronT
SullivanDR
DennyPW
2003 Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem 278 44708 44718
42. da SilvaR
SacksDL
1987 Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infect Immun 55 2802 2806
43. AkopyantsNS
KimblinN
SecundinoN
PatrickR
PetersN
2009 Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science 324 265 268
44. BoulangerN
LowenbergerC
VolfP
UrsicR
SigutovaL
2004 Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect Immun 72 7140 7146
45. SacksDL
PimentaPF
McConvilleMJ
SchneiderP
TurcoSJ
1995 Stage-specific binding of Leishmania donovani to the sand fly vector midgut is regulated by conformational changes in the abundant surface lipophosphoglycan. J Exp Med 181 685 697
46. Adler SaTO
1927 The behaviour of cultures of Leishmania sp. in Phlebotomus papatasi. Annals of Tropical Medicine and Parasitology 21 111 134
47. LippertDN
DwyerDW
LiF
OlafsonRW
1999 Phosphoglycosylation of a secreted acid phosphatase from Leishmania donovani. Glycobiology 9 627 636
48. ShakarianAM
DwyerDM
2000 Structurally conserved soluble acid phosphatases are synthesized and released by Leishmania major promastigotes. Exp Parasitol 95 79 84
49. ShakarianAM
JoshiMB
YamageM
EllisSL
DebrabantA
2003 Members of a unique histidine acid phosphatase family are conserved amongst a group of primitive eukaryotic human pathogens. Mol Cell Biochem 245 31 41
50. WieseM
IlgT
LottspeichF
OverathP
1995 Ser/Thr-rich repetitive motifs as targets for phosphoglycan modifications in Leishmania mexicana secreted acid phosphatase. Embo J 14 1067 1074
51. ElfariM
SchnurLF
StrelkovaMV
EisenbergerCL
JacobsonRL
2005 Genetic and biological diversity among populations of Leishmania major from Central Asia, the Middle East and Africa. Microbes Infect 7 93 103
52. SchleinY
JacobsonRL
1998 Resistance of Phlebotomus papatasi to infection with Leishmania donovani is modulated by components of the infective bloodmeal. Parasitology 117 467 473
53. TuonFF
NetoVA
AmatoVS
2008 Leishmania: origin, evolution and future since the Precambrian. FEMS Immunol Med Microbiol 54 158 166
54. VolfP
BenkovaI
MyskovaJ
SadlovaJ
CampinoL
2007 Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int J Parasitol 37 589 593
55. KawaiT
AkiraS
2010 The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11 373 384
56. LeeMS
KimYJ
2007 Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem 76 447 480
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



