-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
Severe short stature in humans has many causes including defects in skeletal and hormonal growth regulation. Primordial dwarfisms are congenital growth defects that involve mutations in genes for DNA repair, DNA replication, splicing of U12 introns, and centrosome dynamics. We discovered that the spontaneous, dwarf mouse mutant chagun is caused by loss-of-function of the gene Poc1a, which encodes protein of the centriole 1A. Mutants exhibit disproportionate dwarfism, and bones formed by endochondral and intramembranous ossification processes are affected. The epiphyseal growth plates of their long bones are disorganized. The chagun males are infertile due to Sertoli cell dysfunction and the failure of germ cells to complete meiosis. The chagun mouse is a model for human dwarfism and provides insight into the mechanism whereby this centriolar protein affects bone growth and spermatogenesis.
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005569
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005569Summary
Severe short stature in humans has many causes including defects in skeletal and hormonal growth regulation. Primordial dwarfisms are congenital growth defects that involve mutations in genes for DNA repair, DNA replication, splicing of U12 introns, and centrosome dynamics. We discovered that the spontaneous, dwarf mouse mutant chagun is caused by loss-of-function of the gene Poc1a, which encodes protein of the centriole 1A. Mutants exhibit disproportionate dwarfism, and bones formed by endochondral and intramembranous ossification processes are affected. The epiphyseal growth plates of their long bones are disorganized. The chagun males are infertile due to Sertoli cell dysfunction and the failure of germ cells to complete meiosis. The chagun mouse is a model for human dwarfism and provides insight into the mechanism whereby this centriolar protein affects bone growth and spermatogenesis.
Introduction
Normal adult stature in humans is achieved primarily through regulation of long bone growth, which occurs through endochondral ossification [1,2]. This process begins with the differentiation of mesenchymal stem cells into chondrocytes in regions of the body where skeletal elements will eventually reside. Hypertrophic differentiation of these chondrocytes directs the vascularization of the forming element, allowing osteoblasts to enter and commence mineralization of the cartilage-based template. Pools of cells at the epiphyses of the long bones retain their cartilage identity as a means to secure the progressive addition of bone matrix throughout the period of skeletal growth. These structures are the epiphyseal growth plates. Tight control of chondrocyte proliferation and terminal hypertrophic differentiation allows new bone tissue to replace terminally differentiated chondrocytes in a spatially and temporally regulated manner, ensuring the proper growth of the skeletal elements and the individual overall. Disruption of these processes can lead to skeletal dysplasias.
The growth plate maintains a highly ordered architecture to carry out its function and is divided into three distinct zones: the resting, the proliferative, and the hypertrophic zone [1,2]. Perturbation of growth plate organization can result in profound growth defects in mice and humans [3–5]. Resting chondrocytes have a low rate of cell proliferation, and the plane of cell division is not controlled. In contrast, rapidly dividing chondrocytes in the proliferative zone undergo directed cytokinesis orthogonal to the main direction of bone growth, and then intercalate to form distinctive columns of disc-shaped chondrocytes [3,6]. Major orchestrators of growth plate architecture include the primary cilium [3], the WNT-planar cell polarity [6], TGFβ, BMP, and hedgehog signaling pathways [4].
Primordial dwarfisms are a subset of growth insufficiency disorders that are classified as forms of skeletal dysplasia [7,8]. Primordial dwarfism typically involves a proportionate reduction in longitudinal growth that commences early in fetal life [7]. Primordial dwarfisms result in profound reductions in height, and many are associated with head and/or facial dysmorphisms [1]. The primordial dwarfisms include Seckel Syndrome, Microcephalic Osteodysplastic Primordial Dwarfism I-III (MOPD I-III), and Meier-Gorlin Syndrome (MGS) [7]. These disorders interfere with processes that are intimately connected to the cell cycle rather than an endocrine disturbance. Common pathways that are disrupted in primordial dwarfism patients include: DNA repair [9–12], DNA replication [13–16], centrosome dynamics [12–15,17–23], and splicing of minor, or U12 introns, in mRNA [24,25].
We report discovery of the gene responsible for autosomal recessive skeletal dysplasia and male infertility in chagun mice [26]. The mutation is a LINE-1 mediated insertion of a processed Cenpw cDNA into Poc1a, which encodes protein of the centriole 1A. This disruption causes exon skipping, and the mutant protein lacks a highly conserved WD-40 domain necessary for normal spindle pole and cilia formation. POC1A is necessary for normal growth plate architecture, craniofacial development, and spermatogenesis. The mechanism underlying the growth defect is cell death and failure to regulate polarized cell division and intercalation at the growth plate. This animal model clarifies the molecular basis for the clinical features in human patients with POC1A mutations and predicts that male patients will be azoospermic [21,22].
Results
chagun causes disproportionate growth of the skull and other skeletal elements
The chagun mutation arose on the DBA/2J strain and was mapped to a 6 Mb region of Chr 9 using recombinant progeny from an F1 x F1 intercross with CAST/EiJ [26]. Initial studies revealed an autosomal recessive disproportionate growth disorder, likely caused by disorganization of the proliferative zone of the growth plate. We used dermestid beetle preparations to characterize the mutant bones (Fig 1). The chagun mutant skull has a slightly domed and disproportionate shape. The skull and mandible are foreshortened along the length, but the widths are similar to wild type. Incisor and molar teeth appear normal.
Fig. 1. Abnormal skull and long bone morphology in chagun mutants. 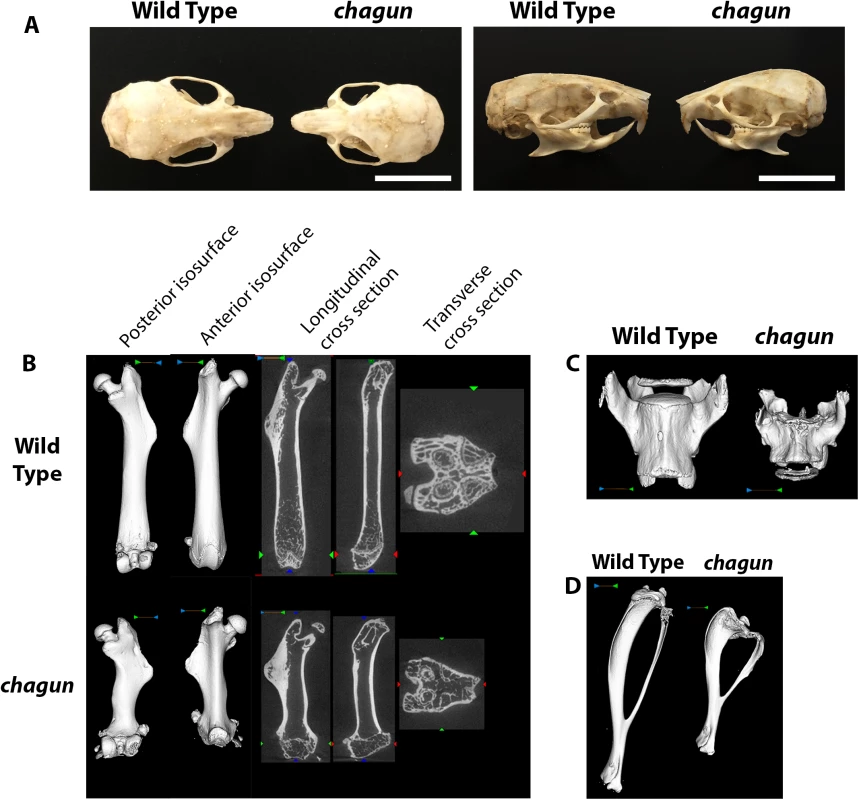
A. Skulls of wild type and chagun mice were stripped of soft tissues by incubation in a dermastid beetle colony. Scale bar = 1cm. B-D. Micro-CT scans and volume reconstructions of the femur (B), lumbar vertebrae (C), and tibia (D) in 4-month-old male wild type and chagun mice. We used micro-CT to assess bone architecture and mineralization (Fig 1). Representative cross sections and 3D volumetric renderings of 4-month-old male mice were selected based on medial bone mineral content and are shown with matched display settings. We found no differences in cortical mineralization, but the trabecular bone was thinner in both vertebrae and metaphyseal long bones of the mutants. The chagun femora are obviously short, with undulating cortical bone and lateral third trochanter irregularities. Disorganization at the distal femoral growth plate is apparent, likely leading to the noticeable lack of trabecular bone in the metaphysis. Metaphyseal periosteal shaping appears to be significantly impacted by the mutation, with abnormal anterior-posterior and medial-lateral shaping. Similar findings were observed in the tibia, with decreased tibial length, proximal tibial growth plate abnormalities, and excessive proximal tibial and mid-fibular flaring. Skeletal shortening extends to the axial skeleton, with pronounced loss of height in lumbar vertebrae.
The chagun growth defect is caused by a mutation in Poc1a
Genome-wide exome capture and high throughput sequencing of a chagun genomic DNA sample was conducted (http://www.broadinstitute.org/mouse-mutant-resequencing). No obvious deleterious mutations were uncovered in the mapped critical region using this approach. There were some synonymous changes and a single missense mutation that encoded an amino acid present in normal individuals of other species. Some variants were detected in intronic regions captured with the exons, but none appeared likely to disrupt splicing or create ectopic splice donors or acceptors (S1 Table).
We extended our search using regional DNA capture to enrich for all non-repetitive genomic DNA in the most broadly defined 8.5 Mb chagun critical region (D9Mit183-D9Mit212). We captured genomic DNA from an obligate carrier (cha/+) and a known mutant (cha/cha), and obtained high throughput sequence with at least 20X coverage for approximately 90% of the bases sequenced from both samples. Manual sequence curation was carried out to detect insertions, deletions, inversions, etc. An insertion of an L1Md-GF-5 end non-LTR transposable element [27] was detected in an intron of the gene that encodes collagen type VI, alpha 6 (Col6a6). This element is not reported in 16 mouse reference genomes (http://www.sanger.ac.uk/resources/mouse/genomes/) [28]. PCR amplification and Sanger sequencing of this region in DNA samples from affected, unaffected, and DBA/2J mice indicates that it is present in all three samples. Thus, this transposable element does not cause the mutant phenotype. The difficulty inherent in sequencing and mapping repetitive elements likely led to omission of this element from the reference genomes.
Manual sequence curation revealed a steep decrease in sequence coverage near exon 8 of Poc1a, which encodes protein of the centriole 1A (S1 Fig). This is more pronounced in the mutant than the heterozygote. In addition, many captured fragments from this region have mismatched paired end sequencing reads, where one read maps to chromosome 9 and the other to chromosomes 5, 10, or 12. PCR amplification of Poc1a exon 8 and flanking intronic regions produced a product that is ~500 bp larger in genomic DNA from chagun mice than in unaffected animals (Fig 2). Sanger sequencing of the genomic amplification products indicated that exon 8 is disrupted by the insertion of a nearly full-length transcript of centromere protein W (Cenpw) including a 61 nucleotide poly-A tail that is 24 bp downstream from the common polyadenylation sequence AUUAAA (S1 Fig). The total size of the insertion is 495 bp, and it includes a 5’ untranslated region (UTR) lacking only seven bp relative to the published transcription start site, all three exons, and a 3’UTR. This insertion is unique to chagun; it is not present in any of the 16 additional strains analyzed. The bona fide Cenpw gene is located on mouse chromosome 10, and processed Cenpw pseudogenes exist on chromosomes 5 and 12. The sequence of the Cenpw insert in Poc1a shares 100% identity with the exons of Cenpw on chromosome 10, and 90% and 84% identity with the pseudogenes on chromosomes 12 and 5, respectively. Thus, the inserted sequences of Cenpw in exon 8 of Poc1a are derived from a processed transcript of the bona fide Cenpw gene.
Fig. 2. Insertion of a processed cDNA into exon 8 of Poc1a underlies the chagun phenotype. 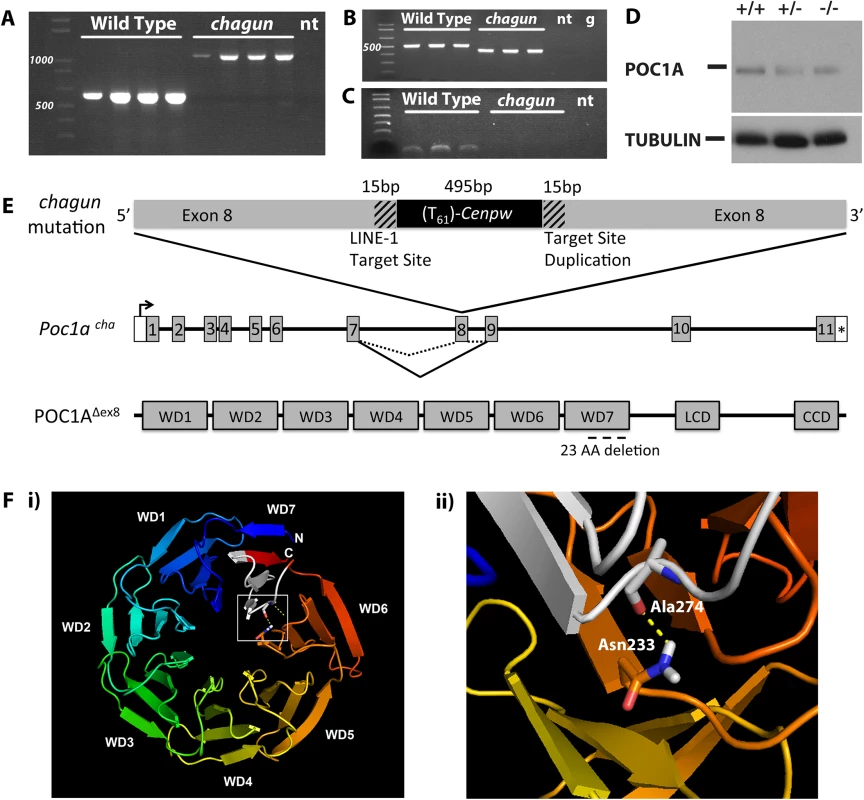
A. Normal and chagun mutant genomic DNA samples (N = 4 mice per genotype) were amplified with primers flanking Poc1a exon 8. The products were separated by gel electrophoresis and stained with ethidium bromide. A 1,100 bp product was produced from the chagun genomic DNA samples and the predicted product 613 bp in wild-type genomic DNA. B. Poc1a transcripts were analyzed by RT-PCR using RNA from tibia (N = 3 per genotype) and primers specific to exons 5 and 10. A 526 bp product was detected in wild types, but a ~450 bp product was detected in chagun mutants. C. Poc1a transcripts in tibia were analyzed using primers in exons 7 and 8. The expected size product was detected in wild types but no amplification products were detected in chagun mutants. D. Protein extracts from P3 tibiae of wild type (+/+), heterozygote (+/-) and chagun mutants (-/-) were run on a 12% SDS-PAGE gel, transferred to a membrane, and probed with primary antibodies specific for POC1A and tubulin. Both images are exposures taken from the same blot. E. Schematic diagram of the processed Cenpw cDNA insertion into Poc1a, the effects on mRNA splicing, and the predicted protein. A portion of the Poc1a coding sequence is duplicated (hatched regions) at the 3’ end of the Cenpw insertion (black) within exon 8. The insertion results in skipping exon 8, and the predicted protein lacks a portion of the last WD-40 repeat domain. F. Molecular modeling of WD40 domains and amino acid interactions. i) Molecular model of the WD domains of mouse POC1A. The N- and C-termini of POC1A, which do not form part of the WD domains, have not been modeled. The chagun mutation is indicated in white within WD7. ii) A predicted hotspot of amino acid interactions, Asn233, forms a hydrogen bond with Ala274, which resides within WD7 and the chagun deletion. LCD: Low complexity domain, CCD: Coiled-coil domain, no template (nt), Genomic DNA (g). The insertion does not delete any DNA from Poc1a exon 8. The exon is interrupted by the insertion of the Cenpw transcript in reverse orientation relative to Poc1a. The point of insertion is flanked by short (15 bp) stretches of identical Poc1a sequence (5’-CACCGTTGCCTTTTC-3’). This arrangement is consistent with target-site duplications characteristic of LINE-1 retrotransposon-mediated insertions (Reviewed in [29]), and with the consensus LINE-1 endonuclease ORF2 insertion site sequence (5’ TTTTC|A), just before the insertion of the Cenpw transcript [30–33].
The processed Cenpw cDNA insertion causes exon skipping but retains the Poc1a reading frame
RNA extracted from tibiae was prepared for RT-PCR analysis of Poc1a transcripts. The chagun cDNA produced a smaller amplification product than that detected in normal tibia (Fig 2). Sanger sequencing of the mutant RT-PCR product revealed that exon 8 of Poc1a is skipped precisely. No splicing into exon 8 was detected in chagun mutants (Fig 2). Quantitative RT-PCR was carried out using probes designed to amplify exons 2–3 at the 5’ end of the Poc1a transcript and exon 11–12 at the 3’ end of the transcript. The same level of transcripts were detected in RNA from wild type and chagun mutant tibiae using probes for both the 5’ or 3’ ends of the transcript (S1 Fig). This suggests that exon 8 is cleanly skipped and that the mutant transcript is similar in stability to the wild type.
Skipping exon 8 preserves the reading frame of the Poc1a transcript. The mutant transcript is predicted to produce a POC1A protein that lacks 23 amino acids within the seventh and final 40 amino acid WD40 repeat (Fig 2). Western blots were carried out to detect POC1A protein in tibiae using a polyclonal antibody generated against full-length human POC1A, which is highly conserved (89% identity, UniProt) between human and mouse. A single band of similar intensity was detected in wild type and mutant tibia (Fig 2). Pan-tubulin immunoreactivity was used to normalize protein preparations. The molecular structure of mouse POC1A was modeled using the WD40 structure predictor algorithm [34]. Molecular modeling suggests that the deletion would destabilize the seven bladed propeller structure characteristic of WD40 repeat domain proteins because the first and seventh WD40 repeats normally interlock to form the propeller, and the mutant protein lacks 23 of 40 amino acids that would comprise the seventh propeller. Additionally, the WDSP algorithm predicts certain amino acids to be hotspots for protein-protein interactions based on their biochemical properties and their location on the top face of the propeller structure [35]. There is a predicted hotspot in WD6, Asn233, which normally forms hydrogen bonds with Ala274, a residue that is deleted in chagun mutants. The loss of this interaction likely disrupts the protein-protein interactions of Asn233.
The chagun mutant is rescued by a Poc1a BAC transgene and phenocopied by Poc1a null mice
To validate the causal role of the mutation in Poc1a in the chagun phenotype, we undertook a BAC transgenic rescue experiment [36]. The selected BAC clone RP24-384G5 contains four genes in addition to Poc1a: Twf2, Tlr9, Alas1, and Dusp7. These genes do not cause problems when overexpressed, nor do their known loss-of-function phenotypes mimic any aspect of chagun mutants (S2 Table). Two of three independent BAC transgene insertion sites in founder mice provided completely penetrant correction of the chagun phenotype and caused no abnormalities in control transgene-positive animals. Transgenic chagun animals exhibit normal growth and testicular development (Fig 3). Body weight and length, as well as testicular weight, appearance, and histology were normal. This evidence supports our assertion that loss of Poc1a function due to the exon 8 insertion causes the chagun mutant phenotype.
Fig. 3. A Poc1a BAC transgene rescues chagun, and a null allele phenocopies chagun. 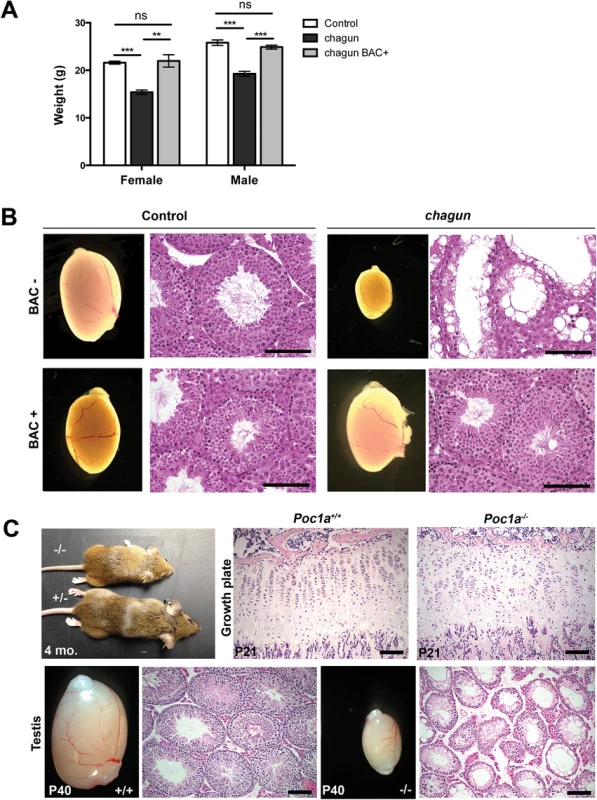
A. Female and male mice of the indicated genotypes were weighed at 6 wks of age. N = 5-19/group. B. Adult male animals of the indicated genotypes were analyzed for testis morphology (0.8X objective) and histology. Testis sections were stained with hematoxylin and eosin. Scale bar = 100 μm. C. Bones were collected from Poc1a+/+ and Poc1a-/- animals at P21 and testes from P40 animals. Samples were fixed, sectioned and stained with hematoxylin and eosin. Scale bar = 100 μm. We obtained embryonic stem (ES) cells from the knockout mouse project in which exons 3–6 of the Poc1a locus were deleted and replaced by a gene trap internal ribosome entry site (IRES) LacZ and a neomycin resistance expression cassette (S2 Fig). We used these ES cells to generate mice with a null allele of Poc1a [37]. Heterozygous carriers for the LacZ knock-in were normal, and mice homozygous for this null allele phenocopied the growth insufficiency and hypogonadism of chagun mutants (Fig 3).
POC1A is normally expressed in tibial growth plates and seminiferous tubules of wild type mice
We performed immunohistochemistry (IHC) using an antibody raised against a 50 amino acid peptide that contains the entire seventh WD40-repeat domain of POC1A. We detected POC1A in the proliferative zone of two-week old wild type (postnatal day 14; P14) tibial growth plates (Fig 4). The discoid chondrocytes in this zone have robust POC1A staining in the cytoplasm. Twenty-one amino acids of the peptide used to raise antibodies are intact in Poc1acha/cha mutants, and staining is observed in Poc1cha/cha mutant tibia. POC1A is also expressed in seminiferous tubules of the testis, and the immunostaining varies depending on stage of the seminiferous cycle. In wild type mice, POC1A protein is detectable in the cytoplasm of Sertoli cells and as single puncta in spermatozoa and spermatids, which likely represents labeling the centrosome of the spermatids as they begin to form flagella [38–40]. Little POC1A staining is observed in the testes of Poc1cha/cha mutants, suggesting that the mutant protein may be unstable or not retained in this tissue. Poc1a knockout mouse testes do not stain with this antibody, confirming the specificity of the antibody for POC1A (Fig 4 and S3 Fig). The expression of POC1A in wild type tibia is consistent with the observed defects in mutant chondrocyte proliferation and expression in wild type male germ cells and Sertoli cells suggests that one or both cell types could be involved in the infertility of male mutants.
Fig. 4. POC1A is expressed in the growth plate and seminiferous tubules. 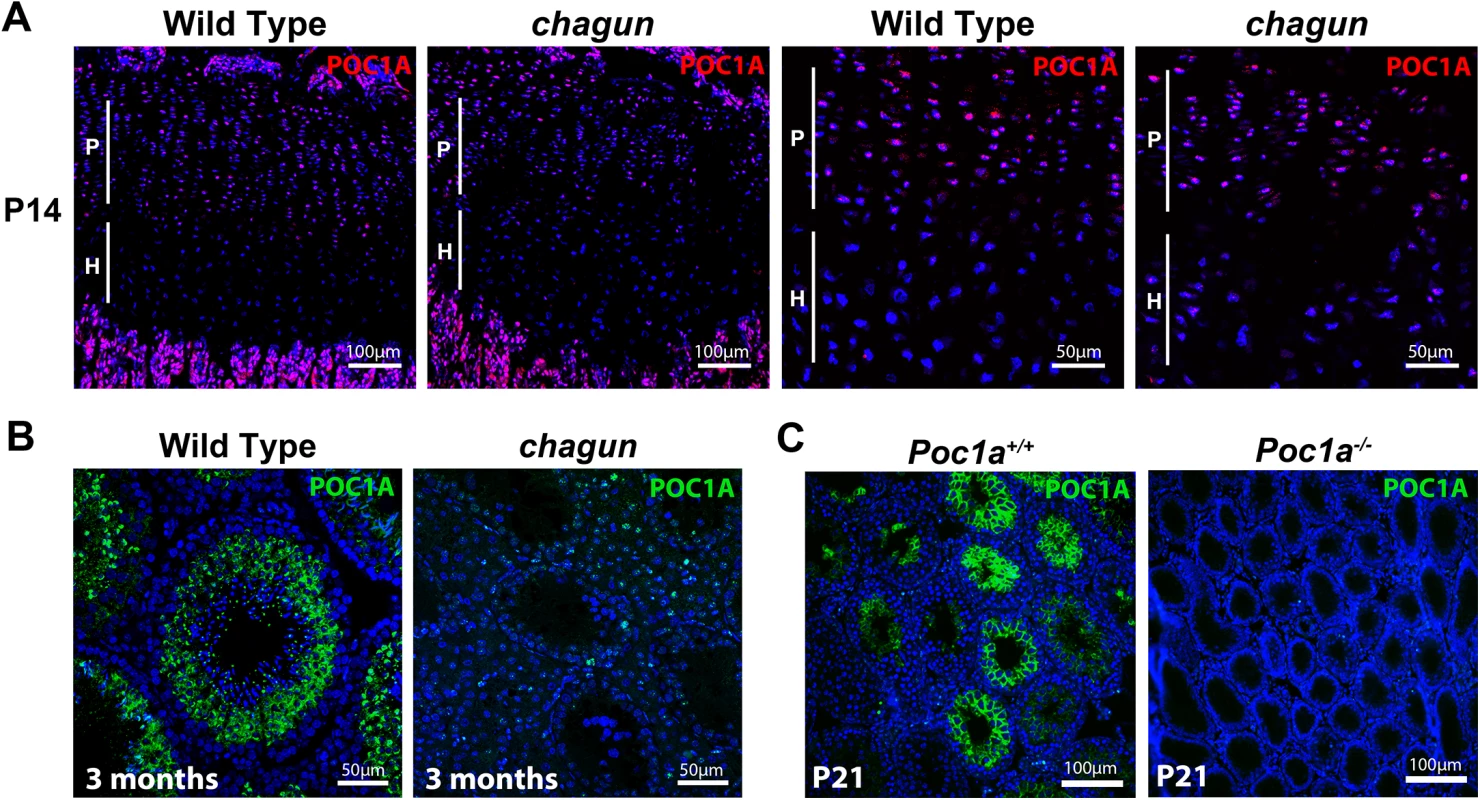
A. POC1A immunostaining of sections from postnatal day 14 (P14) wild type and Poc1acha/cha mutant tibial growth plates reveals strongest expression of POC1A (magenta) in the proliferative zone. B. POC1A immunostaining was carried out on testis sections from 3 mo old wild type and Poc1acha/cha mutants. C. POC1A staining was also performed on testis sections from P21 wild type and Poc1a knockout mice. Scale bars as indicated. Poc1acha/cha causes defects in the primary cilium and mitotic spindle in mouse embryonic fibroblasts
The centrosome is the microtubule-organizing center of the cell, and it is responsible for formation of the primary cilium and the mitotic spindle, and organization of the Golgi apparatus [41,42]. The centrosome is comprised of a mother and daughter centriole, and POC1A is a centriolar protein. Therefore, we hypothesized that the Poc1acha/cha mutation could affect any or all of these centrosome-dependent organelles. To test this prediction, mouse embryonic fibroblasts (MEFs) were isolated independently from three wild type and three mutant embryos and cultured for 24 hours on gelatin-coated coverslips. MEFs were either supplemented with serum to promote cell division and formation of mitotic spindles or serum-starved to facilitate visualization of primary cilia. After the culture period, cells were fixed and immunocytochemistry was conducted.
Immunostaining with an antibody against acetylated tubulin, which labels the mitotic spindle and the primary cilium, revealed the effects of the mutation on these two cellular structures. MEFS cultured with serum revealed dividing wild type cells with equal bipolar spindles, as expected (Fig 5). The dividing Poc1cha/cha cells, however, frequently displayed evidence of centrosome amplification, with three or four spindle poles present in a single cell. Centrosome amplification likely results in aneuploidy and cell death. Immunostaining with a GM130 antibody revealed the presence of Golgi near the nuclei of wild type and Poc1acha/cha mutant MEFs. No obvious differences in Golgi organization were noted. The majority of wild type MEFs cultured without serum had a primary cilium detected by acetylated tubulin immunostaining. Significantly more MEFs from wild type embryos had detectable cilia than Poc1acha/cha mutants, 87% vs. 28% respectively. The lengths of the measureable cilia were similar in wild types and mutants, 3.06 μm vs. 2.67 μm, respectively. Loss of Poc1a function leads to defects in centrosome-mediated processes, including formation of a bipolar spindle and formation or maintenance of the primary cilium in MEFS.
Fig. 5. MEFs isolated from Poc1acha/cha embryos exhibit aberrant mitotic spindles and primary cilia. 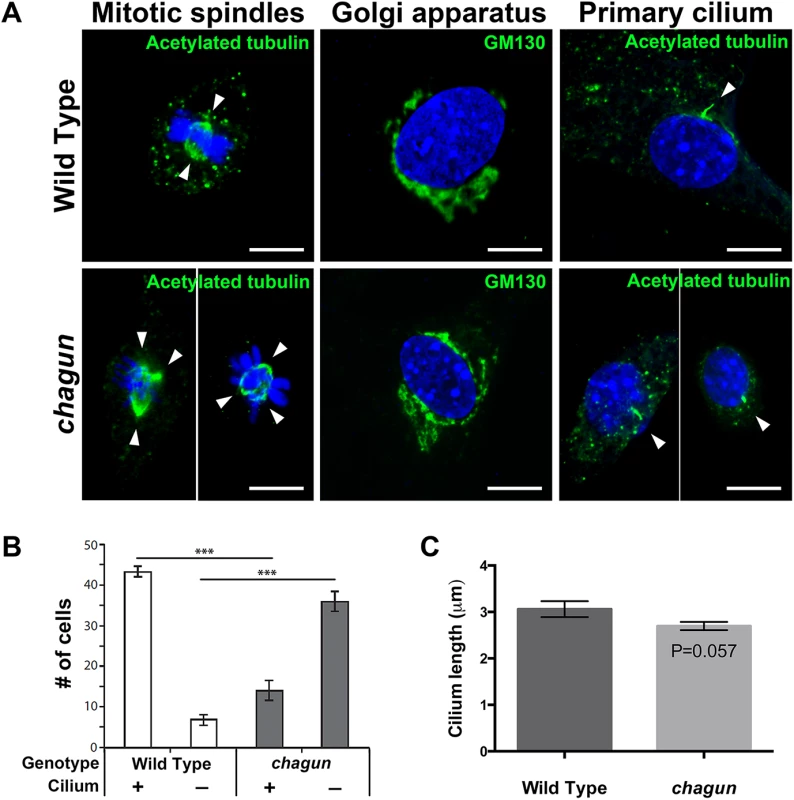
A. Confocal microscopy images from MEFs of the indicated genotypes incubated with primary antibodies for anti-acetylated tubulin (green) to visualize mitotic spindles (left panels), GM130 to identify Golgi (middle panels) and primary cilia (right panels). All are counterstained with DAPI to visualize nuclei. White arrowheads indicate spindle poles. Scale bar = 10 μm. B. Quantification of the number of anti-acetylated tubulin-stained MEFs of the indicated genotypes with a primary cilium that looks normal in appearance. Using confocal microscopy, 50 cells were counted per slide at 2 slides per sample, and 3 samples per genotype. The mean +/- standard error of the mean are displayed, and data was analyzed by Two-Way ANOVA and Tukey’s Post-Hoc Test were used to analyze statistical significance. The asterisks indicate p < 0.001. C. Quantification of cilium length from a total of 93 Poc1acha/cha MEFs and 113 wild type MEFs, from 3 lines of each genotype. The mean +/- standard error of the mean are displayed, and the data was analyzed by a generalized estimating equation. The Poc1acha/cha proliferative zone becomes progressively disorganized with age, and displays perturbed chondrocyte polarity and increased cell death
The growth plates of vertebrae and long bones of Poc1acha/cha mice are obviously disorganized [26]. Histology of tibiae from the neonatal period to P21 indicates that the growth plate becomes progressively more disorganized with age. At P0, the mutant growth plates look normal (Fig 6), but by P15, the coin stack structure of the chondrocytes in the proliferative zone begins to deteriorate in mutants, and some mutant chondrocytes have a rounded, rather than flat, appearance. By P21, Poc1acha/cha tibial growth plates are even more severely disorganized (S4 Fig)
Fig. 6. The Poc1acha/cha long bone growth plates become disorganized. 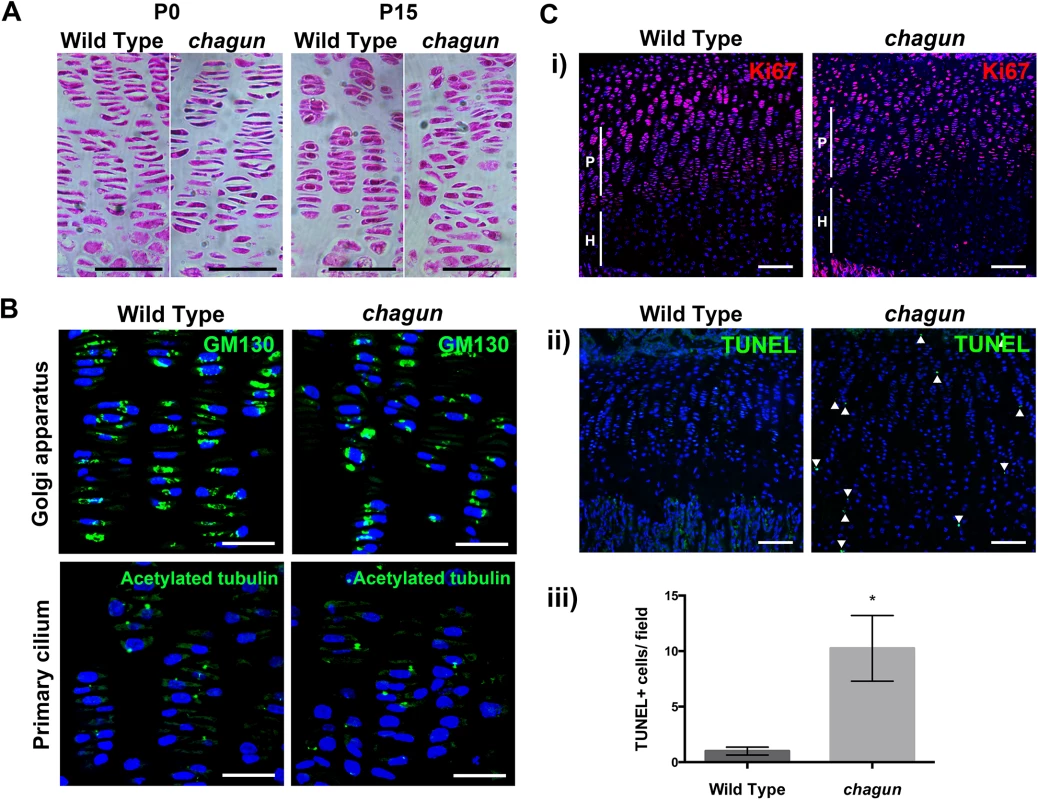
A. Standard hematoxylin and eosin histological staining was carried out on paraffin sections from P0 and P15 tibial growth plates. Scale bar = 50 μm. B. Immunohistochemistry using a primary antibody against the Golgi marker GM130 or acetylated tubulin counterstained with DAPI in wild type and mutant P15 tibia sections. Scale bar = 25 μm. C. Immunohistochemistry for primary antibodies against the proliferation marker Ki67 (i) or cell death marker TUNEL (ii), with quantification of the number of TUNEL-positive cells per field of view in wild type and chagun tibia (iii). Scale bar = 100 μm. The subcellular position of the Golgi within the columnar chondrocytes of the proliferative zone can provide information about the polarity of the cell [3,43]. The Golgi is typically located to the right or left of the nucleus in chondrocytes, towards the edge of the coin stack column. To test whether the Golgi are positioned normally in Poc1acha/cha chondrocytes, immunohistochemistry was conducted on sections of the tibial growth plate utilizing primary antibodies against the Golgi marker GM130. The lateral subcellular positioning of the Golgi is evident in both wild type and mutant cells in the proliferative zones (Fig 6). The mutants have more nuclei that are more rounded, and the mutant cells appear mal-rotated relative to the cellular column. This indicates that the chondrocytes in Poc1acha/cha mice exhibit some polarized Golgi localization, but fail to maintain cell shape or ability to re-align after cell division.
The primary cilia of chondrocytes in the columns of the proliferative zone are oriented in the direction of the column—pointing toward the resting or hypertrophic zone [3,44,45]. We conducted immunohistochemistry of tibia using a primary antibody against acetylated tubulin on sections from P15 wild type and Poc1acha/cha mutant mice to test whether primary cilia were properly oriented in the mutant growth plate. Wild type growth plates displayed normal orientation of the primary cilia within their columns. Primary cilia were detected in the Poc1acha/cha growth plate, and some of them are oriented properly (Fig 6).
Formation of multipolar spindles could lead to premature chondrocyte cell death. Ki67 staining is similar in Poc1acha/cha mice, being largely expressed to the proliferative zone. TUNEL staining of P15 tibial sections demonstrated a substantial increase (10.3 fold, p = 0.002) in TUNEL-positive nuclei in the Poc1acha/cha growth plate (Fig 6). Together, these data indicate that the proliferating chondrocytes in Poc1acha/cha mice fail to maintain their cell shape, fail to intercalate properly after cell division, and undergo a significantly higher rate of cell death.
Germ cell maturation arrest during postnatal life in Poc1acha/cha males leads to infertility
Poc1acha/cha males are infertile. In adult mutant males, the latest stage of spermatogenesis that is detected is the pachytene stage, and hypogonadism ensues with age [26]. However, the time of initial germ cell disruption during postnatal development was not previously determined. To address this, we conducted histological analysis of the testis in normal and Poc1acha/cha mutants from birth to adulthood. Mutants were identified by Poc1a exon 8 genotyping and periodic acid-Schiff (PAS) histological staining was conducted on cross-sections of testes from males at postnatal day 1 (P1), P7, P14, P21, and 12 weeks (Fig 7). Immediately after birth (P1), the number of germ cell and Sertoli cell nuclei per tubule and the diameter of seminiferous cords in testis cross-sections from Poc1acha/cha males were indistinguishable from wild type counterparts, indicating that during embryonic development the proliferation and migration of germ cells to the genital ridge to form testis cords occurs normally. At P7, seminiferous cords from Poc1acha/cha males are still morphologically similar to wild type; however, the number of γH2AX positive cells is reduced in mutant males, indicating loss of spermatocytes at the earliest stages of meiosis (S5 Fig). By P14 the morphology of seminiferous tubule cross-sections from Poc1acha/cha mutants are dramatically different from controls. Progression of germ cell maturation to the pachytene spermatocyte stage is clearly evident in tubules of wild-type mice at P14. In contrast, only a few spermatocytes are detected in the mutant tubules. At P21, germ cell maturation is still arrested in testes of Poc1acha/cha mutants, and most of the germline appears to be lost in some of the tubules. The testis of sexually mature 12 week old wild type mice contain all stages of spermatogenesis, including undifferentiated and differentiating spermatogonia, primary and secondary spermatocytes, and round and elongating spermatids. In contrast, very few germ cells are present in testis cross-sections from 12 wk Poc1acha/cha mutants. Although some tubules contain a few leptotene or pachytene spermatocytes, most tubules appear to be devoid of all differentiating germ cells and contain spermatogonia only. None of the tubules in Poc1acha/cha adult mice contain secondary spermatocytes or post-meiotic round/elongating spermatids. Taken together, these observations demonstrate that the Poc1acha/cha testis phenotype is caused by a postnatal defect in spermatogenesis beginning at the pre-leptotene stage of meiosis, rather than impairment of testis cord formation during embryogenesis.
Fig. 7. Poc1acha/cha males exhibit progressive germ cell loss. 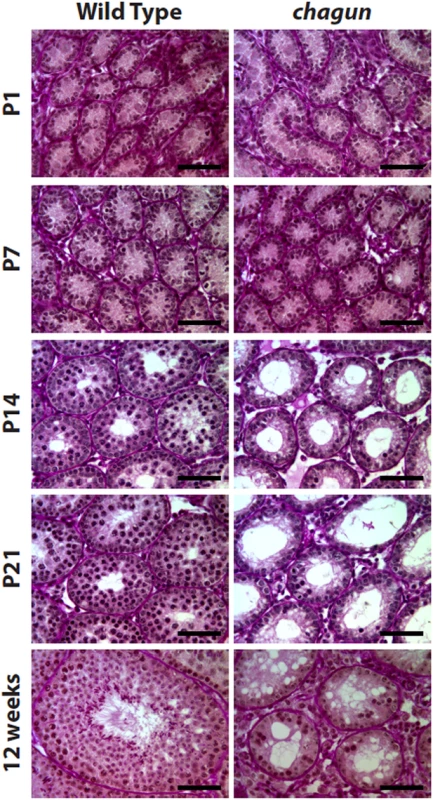
Paraffin sections of testes tissues collected from wild type and Poc1acha/cha mice at the indicated ages were stained with Periodic acid-Schiff reagent. At P1 and P7 Poc1acha/cha tubules resemble wild type seminiferous tubules, but from P14 through 12 wks, the Poc1acha/cha tubules are obviously abnormal. (N = 3 animals of each genotype at each age, scale bar = 50 μm). Spermatogonial stem cell transplant reveals defects in mutant Sertoli cells and germ cells
Sertoli cells function to nourish the germ cells, phagocytose cytoplasmic remnants during spermatogenesis, and promote the translocation of the developing germ cells from the base of the tubule to the lumen. Impaired spermatogenesis could result from defects in Sertoli cells, germ cells, or both. To assess the relative contributions of Sertoli cells and germ cells, we used immunohistochemistry to detect these cell types, with primary antibodies against SOX9, PLZF (promyelocytic leukemia zinc finger protein) and c-KIT. SOX9 is a marker of Sertoli cells. PLZF marks the undifferentiated spermatogonial population that consists of spermatogonial stem cells (SSCs) and progenitor spermatogonia that are primed to undergo differentiation [46,47]. The expression of c-KIT indicates that spermatogonia have initiated differentiation [48]. At P14 the number of Sertoli cell nuclei per tubule cross-section is not different in wild type and Poc1acha/cha males (WT = 23.3±2.3 cells/tubule vs Poc1acha/cha = 26.6±4.0 cells/tubule, p = 0.52). At 3 months of age, the number of Sertoli cells per tubule is also not diminished in Poc1acha/cha testis. The apparent increase in Sertoli cell number per tubule in mutants (Fig 8A) is likely due to reduced tubule size and defective spermatogenesis in mutants that arbitrarily causes a greater concentration of Sertoli cells per mm of tubule length. The number of PLZF+ cells per testis section does not differ in wild type and mutant testes at 3 mo (Fig 8A), or at earlier ages (S6 Fig). The quantitation of undifferentiated spermatogonia (i.e. PLZF+ cells) in wild type vs Poc1acha/cha testis at each age was as follows: P7 : 8.1 ±0.4 vs 4.8±1.2, p = 0.07. P14 : 13.4±1.6 vs. 9.5±0.2, p = 0.07. P30 : 5.9±0.9 vs 9.5±1.4, p = 0.1. The presence of c-KIT+ spermatogonia indicates that spermatogonial differentiation occurs in both wild type and Poc1acha/cha testes (Fig 8).
Fig. 8. Spermatogonial stem cell (SSCs) transplants reveal Sertoli cell and germ cell defects in Poc1acha/cha mice. 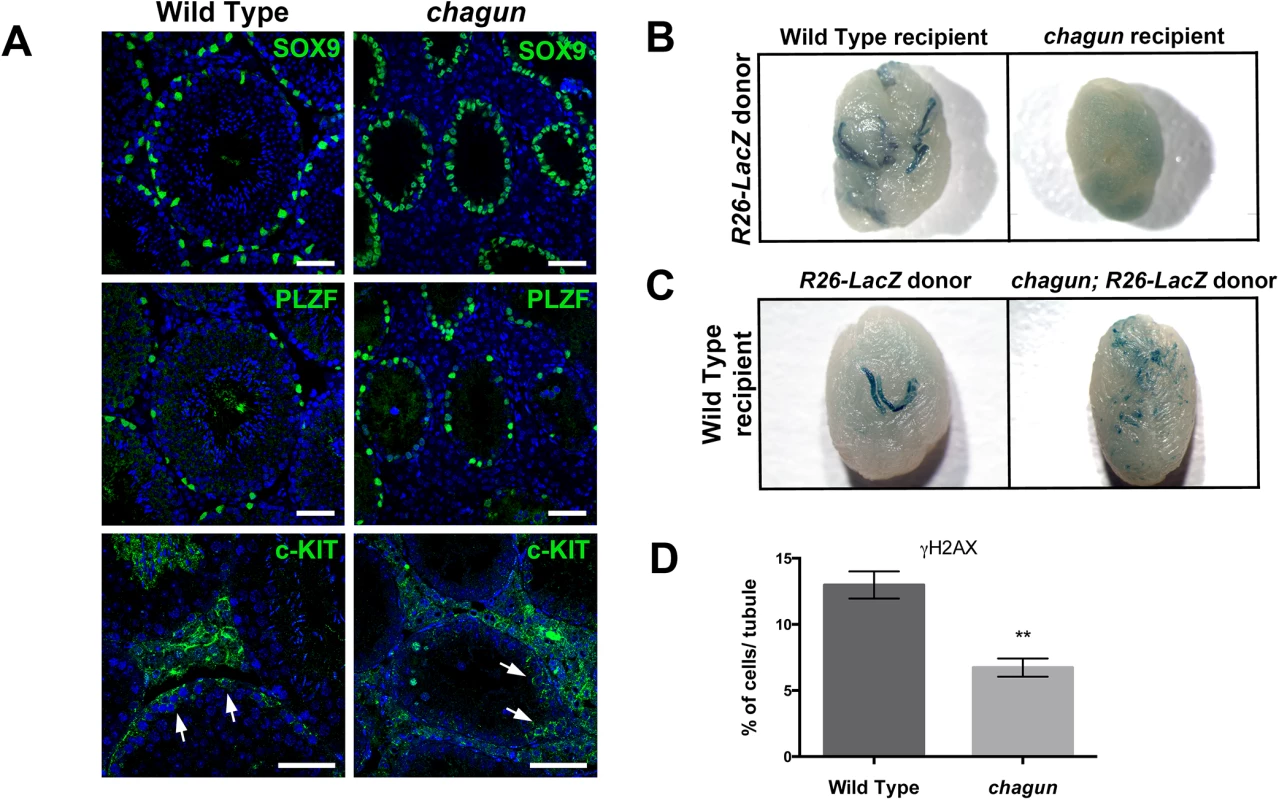
A. Testis sections from 3 mo wild type and Poc1acha/cha mice were immunostained for SOX9, a Sertoli cell marker, and PLZF, an undifferentiated spermatogonial marker, and counterstained with DAPI to visualize nuclei (N = 3 animals of each genotype, scale bar = 50 μm). B. Testes from busulfan treated wild-type recipient or Poc1acha/cha mutant recipients ~3 months after transplantation with wild-type LacZ labeled SSCs. The testes (N = 8) of recipient mice (N = 4) were transplanted for each genotype. C. Testes from busulfan-treated wild-type nude recipient mice ~3 months after transplantation with wild type LacZ-labeled SSCs or Poc1acha/cha SSCs labeled with LacZ. For both B and C, blue stained areas in recipient testes represent colonies of donor-derived spermatogenesis generated from engrafted SSCs. D. Quantification of γH2AX-positive cells in seminiferous tubules of 3 wild type and 3 Poc1acha/cha testes at P7. The histone marker γH2AX is rapidly phosphorylated in response to double strand breaks, and it recruits DNA damage response factors. It is prominently expressed in all intermediate and B spermatogonia and in pre-leptotene to zygotene spermatocytes. While the proportion of tubules in Poc1acha/cha testes with γH2AX staining is similar to wild types at P7 (61%±14% vs 65%±8%, not significant), there is a major reduction (1.9 fold) in the number of γH2AX-positive cells within these tubules in the Poc1acha/cha mutant testes: 12.98% vs 6.73%, respectively (Fig 8, Panel D). The defect is much more severe at P14 (S5 Fig). This suggests a loss of germ cells early in meiosis at the pre-leptotene and zygotene stages, resulting in some spermatogonia at the pachytene stage and none at later stages. Condensation of chromosomes and formation of synaptonemal complexes takes place at these early meiotic stages.
Next, we utilized spermatogonial stem cell (SSC) transplantation to determine whether the spermatogenesis defect was attributable to poor Sertoli cell function, intrinsic defects of the germ cells, or both [49]. In the first set of experiments, we transplanted Rosa-LacZ marked wild type spermatogonia into the seminiferous tubules of wild type recipients pre-treated with busulfan to eliminate the endogenous germline, or alternatively into adult Poc1acha/cha mutant males with impaired spermatogenesis (N = 4 recipients and 8 testes per genotype). Approximately 3 months after transplantation, X-gal staining revealed that the wild type donor SSCs generated colonies of donor-derived spermatogenesis in wild type recipient tubules, as expected. No colonization was detected in any of the Poc1a mutant testes (Fig 8B). The failure of Poc1a mutant testes to support the engraftment of wild-type SSCs is not attributable to the presence of residual mutant SSCs because donor SSCs can colonize normal testes with normal spermatogenesis occurring, albeit at a much lower rate [50]. These results are consistent with a Sertoli cell defect in the Poc1a mutants that could impair the homing ability of transplanted wild-type SSCs and/or re-establishment of colonies of continual spermatogenesis. Impaired homing seems unlikely to be the major cause because a primary spermatogonial population is established during neonatal development in Poc1acha/cha mutant testes. Thus, migration of germ cells from the lumen of the cords to the basement membrane during postnatal development occurs normally in Poc1acha/cha males.
For the second set of experiments, we crossed the Rosa-LacZ marker into the Poc1a cha/+ stock to produce Poc1acha/cha males with LacZ marked germ cells. The marked, mutant spermatogonia were transferred into the seminiferous tubules of busulfan-treated wild type recipient nude mice. As a control, Rosa-LacZ marked wild-type spermatogonia were transferred to identical recipients at the same time. As expected, the wild-type SSCs generated robust colonies of spermatogenesis with uniform blue staining in testes of all recipients (N = 4 recipient mice and 8 testes). In contrast, only small patches of spermatogenesis with non-uniform blue staining were generated from Poc1acha/cha mutant SSCs, consistent with blunted expansion of colonizing SSCs and arrested spermatogenesis. Transplantation of mutant cells did not generate densely stained colonies of donor-derived spermatogenesis in any recipient transplanted testes (N = 4 recipients and 8 testes). Taken together, these findings suggest that the SSCs of Poc1acha/cha mutants are capable of initiating a colonization of a wild type microenvironment, but that intrinsic defects in the germ cells cause arrested spermatogenesis, even with wild type Sertoli cell support. These results are consistent with the idea that POC1A expression in both germ cells and Sertoli cells is important for normal testicular function.
Discussion
The chagun mutation is a LINE-1 retrotransposon-mediated insertion of a processed Cenpw transcript in exon 8 of Poc1a
We report the discovery of the genetic basis of skeletal dysplasia and spermatogenic failure in chagun mutants. Insertion of a processed Cenpw transcript into exon 8 of the gene that encodes protein of the centriole 1A (Poc1a) causes elimination of a portion of one of the seven highly conserved WD40-repeat domains and abrogates function. We conclude this based on: 1) identification of an exonic insertion that causes skipping of exon 8 in Poc1a mRNA transcripts, 2) co-segregation of this Poc1a mutation with the chagun mutant phenotype, 3) the lack of unique insertions, deletions, or coding region mutations in other genes within the chagun critical interval, 4) successful BAC transgenic rescue of the mutant phenotype, 5) recapitulation of the mutant phenotype with a null, lacZ knock-in allele, and 6) expression of Poc1a in the affected tissues. Taken together, this provides compelling evidence that the insertion in Poc1a causes the chagun phenotype.
Failure to detect the insertion by exome sequencing is probably attributable to the fact that all of the exon 8 genomic DNA sequence is still present in the mutants. The insertion of the Cenpw processed transcript increases the size of exon 8 from 69 bp to 564 bp, and increased exon size can cause skipping [51]. Skipping of exon 8 maintains the Poc1a open reading frame, and the predicted POC1A mutant protein lacks 23 amino acids in the most C-terminal, highly conserved WD40 repeat domain. POC1A has seven WD40 repeats that are expected to form a seven bladed, circular beta propeller structure. The lesion in the 7th repeat likely causes a failure of the blades to interlock. The mutant POC1A protein is readily detectable in tibia but not testis of Poc1acha/cha mice, suggesting tissue specific effects on protein stability.
The Cenpw cDNA insertion appears to be a LINE-1 mediated event because it is flanked by a 15 bp target site duplication. In addition, the sequence 5’ TTTC|A in wild type exon 8 matches the ORF2 consensus (reviewed in [29]). Cleavage between the C and A at these sites permits target-primed reverse transcription by ORF2 reverse transcriptase [30,52,53], resulting in insertion of the transcript into exon 8 of Poc1a by the LINE-1 encoded proteins [54,55]. We are aware of only two other examples of LINE-1-mediated mutagenesis that involve non-LINE1 transcripts [56–58]. An insertion of a retrogene into an intron of FGF4 causes short stature in 19 dog breeds, and early dog breeders apparently selected for it. The insertion causes atypical expression of FGF4 in chondrocytes, rather than loss of function that we observed in Poc1acha/cha mice. Chronic granulomatous disease, an immunodeficiency disorder in humans, is caused by a LINE-1 mediated insertion of a partially processed transcript into an intron of the CYBB gene, which encodes a cytochrome that is essential for phagocytosis by leukocytes. The insertion causes loss of function; it disrupts splicing, resulting in incorporation of a novel exon and premature termination. Processed pseudogenes have also been detected in cancer genomes [59]. The difficulty in identifying the chagun mutation by exome sequencing suggests that the paucity of examples of LINE-1 mediated mutagenesis could, in part, due to alignment issues with the programs used to analyze exome sequencing data, resulting in ascertainment bias.
The Poc1acha/cha mutant models a human skeletal dysplasia syndrome
Two mutations in POC1A (p.Arg81X and p.Leu171Pro) were recently reported in separate consanguineous pedigrees with severe short stature and craniofacial abnormalities [21,22]. Sarig, Sprecher, and colleagues labeled the syndrome a primordial dwarfism called SOFT Syndrome (short stature, onychodysplasia, facial dysmorphism, and hypotrichosis) [21]. Shaheen, Alkuraya and colleagues reported the p.Leu171Pro mutation, and they noted global developmental delay including cognitive impairment in some affected individuals, but they did not observe hair or nail defects [22]. We observed no fur or nail abnormalities in the chagun mutants or ES derived Poc1a null mutants. There was no discussion of hypogonadism or fertility of the human subjects in either report. The POC1A mutation p.Arg81X permits significant read through translation, which left open the possibility that it is a hypomorphic allele. The growth insufficiency, however, is severe in patients with either mutation. Mice homozygous for the ES-derived null allele of Poc1a have the same growth defect, skeletal dysplasia, and testicular features as Poc1acha/cha mice. This indicates that Poc1acha is a loss of function allele. The variable secondary features in the two families could be due to functional differences between the two alleles, contributions from mutations in other genes, or genetic modifiers. Both mutant mice exhibit severe growth abnormalities and craniofacial dysmorphism, and provide excellent models for the major features of the human syndrome.
Loss of Poc1a function leads to defects in spindle poles and primary cilia in embryonic fibroblasts
In metazoans, the centrosome regulates organization of microtubules and progression of the cell cycle [60]. Two centrioles, cylindrical, tubulin-rich structures that, together with additional peri-centriolar material, form a single centrosome in each cell. The centrosome is typically located centrally near the nucleus of the cell, but it moves to the leading edge of polarized, migrating cells. At the G1 to S transition, the centrosome begins to duplicate, and after the G2 to M transition, the mother and daughter centrosomes migrate to opposite poles of the cell and form the mitotic spindles. The centrosomes are not strictly required for spindle formation, but they are believed to enhance its efficacy and ensure the fidelity of cell division. While cells are in the quiescent state the centrosome migrates to the cell surface and forms the basal body of the primary cilium. The centrosome and Golgi apparatus are juxtaposed during interphase and are thought to interact functionally for directional protein transport.
Proteome analysis in Chlamydomonas and Tetrahymena identified eighteen different proteins of the centriole, including Poc1, which is a core component of the centriole and basal body in all organisms with motile cilia [61]. Vertebrates have two genes, Poc1a and Poc1b, which are broadly expressed, exhibit 49% amino acid identity, and have overlapping function in cell culture [62]. Embryonic fibroblasts from Poc1acha/cha embryos exhibit a number of anomalies in centrosome-mediated processes. The majority of dividing mutant fibroblasts form aberrant mitotic spindles, and the primary cilia are infrequent and abnormally shaped. We did not notice any obvious defects in the organization of the Golgi. We observed binuclear Poc1acha/cha MEF cells, which are likely indicative of failed cytokinesis, similar to observations of Poc1 dysfunction in Tetrahymena [63]. Skin fibroblasts from human patients with POC1A dysfunction also had abnormalities in mitotic spindle polarity and the formation and length of the primary cilium [21], and although centrosome ultrastructure was normal, centrosome number increased and Golgi trafficking was impaired [22]. Knock down of Poc1a in cells leads to an inability to recruit markers of a mature centrosome [62]. Taken together, these data suggest that although POC1A deficient fibroblasts can form normal looking centriolar structures, the recruitment of proteins to the centriole is defective, causing abnormal centrosome function and increased number of centrosomes per cell. This leads to multipolar spindles and failed cytokinesis. The centrosome abnormalities also lead to defects in cilia formation and/or maintenance. Poc1acha/cha chondrocytes likely have defects in centrosomes, spindle poles and cytokinesis similar to those observed in Poc1acha/cha embryonic fibroblasts and POC1A patient fibroblasts [21,22].
Poc1a is required for the morphology, alignment and survival of proliferating chondrocytes
Cell division in the growth plate is an intriguing process in which chondrocytes undergo polarized cell division orthogonal to the main direction of bone growth and spread back over one another to form columnar structures comprised of disc-shaped chondrocytes [6,64]. The growth plates of newborn Poc1acha/cha mutants have normal organization into zones and normal morphology of proliferating chondrocytes, but by puberty the proliferating chondrocytes are losing the typical coin-stack structure, and their shape is abnormally rounded rather than flat. As the proliferating cells become more disorganized, apoptosis is increased, and the final size of the proliferative zone is reduced. Although mutant chondrocytes can produce cilia and exhibit polarization of the Golgi, the function of the centrosomes and cilia are likely impaired. This view is supported by the similar chondrocyte disorganization and abnormally round cells in mice with disruption of Kif3a, a kinesin II motor complex protein required for intraflagellar transport and cilia formation [3]. Cilia defects are known to affect the Wnt/Planar cell polarity pathway, which is important for cellular shape, migration and organization [42,65,66]. The primary cilium is also important in the cellular response to mechanical cues that govern growth plate structure. For example, disruptions of the osteoblast primary cilium affect mechanoresponsiveness, mesenchymal cell differentiation [67], and the response to mechanical loading [68,69]. Thus, the centrosomal defects in Poc1a mutants could account for abnormal microtubule organization affecting chondrocyte morphology, aneuploidy leading to increased cell death, and poor cilia function influencing cell alignment at the growth plate.
Poc1a is required for both Sertoli cell function and germ cell differentiation
Testosterone levels, seminal vesicle development, and Leydig cell structure are all normal in Poc1acha/cha males [26], suggesting normal function of the hypothalamic-pituitary-gonadal axis. The germline is established normally in Poc1acha/cha males during embryogenesis and early neonatal life, as indicated by normal testicular morphology and PLZF-positive spermatogonia. Hypogonadism and infertility arise later in postnatal development when spermatogonia normally undergo a transition from mitotic to meiotic divisions. Defects in germ cells and Sertoli cells contribute to the infertility. Mutant SSCs transplanted into wild type hosts are capable of initial colonization, but they do not establish colonies typical of normal spermatogenesis. There are examples of spermatocytes with chromosome mispairing or DNA repair defects that are arrested at the pachytene checkpoint and undergo apoptosis [70]. In the current study, we extended the assessment of spermatogenic defects and discovered a reduction of meiotic cells at P7 in Poc1acha/cha males compared to wild type counterparts, which is when pre-leptotene spermatocytes first arise in the male germ cell lineage. Also, we found that the number of spermatocytes is greatly reduced in Poc1acha/cha males at P14 when pachytene stage cells first arise. Together, these findings indicate that defects in germ cell maturation begin to arise at the earliest stages of meiosis but become more pronounced as meiotic progression ensues, eventually leading to elimination of a majority of the population. We predict that Poc1a mutant spermatocytes are aneuploid, which triggers apoptosis at the pachytene checkpoint. Also, our findings suggest that Poc1acha/cha Sertoli cells do not support the engraftment of wild type SSCs or development into mature sperm, possibly due to defects in the microtubule organizing center and/or failure to secrete factor(s) essential for spermatogonial survival and/or germ cell maturation.
The arrangement of microtubules in Sertoli cells is unique and highly specific to this cell type [71]. Defective centrosomes lacking POC1A could disrupt microtubule organization, leading to defective SSC niches [72] and/or an inability to support cells at later stages of spermatogenesis. The inability of wild-type SSCs to colonize Poc1acha/cha testes suggests that the mutation disrupts the ability of the testis microenvironment to support engraftment of normal SSCs in the niche at the basement membrane. Compromised trafficking of components required for the germ cells, or even the ability of the Sertoli cells to support the development of spermatocytes and spermatids could contribute to impaired spermatogenesis in Poc1acha/cha mutant testes. Immature or multiple centrosomes could impair the ability of Sertoli cells to support differentiation and development of spermatozoa from SSCs.
POC1A and POC1B have unique and overlapping functions
Poc1a and Poc1b are both expressed in testis and bone (Fig 4 and S7 Fig), and have functional overlap in cell culture [62,63]. Mutations in human POC1B, however, cause a very different disorder. Homozygotes for the p.Arg106Pro mutation in POC1B have severe, syndromic retinal ciliopathy with defects in kidney and cerebellar function [73]. Individuals with p.Gln67del mutations also have recessive, non-syndromic cone rod dystrophy [74]. The basis for these tissue-specific effects is not clear.
Summary
A loss of function of Poc1a causes skeletal dysplasia and male infertility in chagun mice. Insertion of a processed cDNA causes exon skipping and predicted deletion of 23 highly conserved amino acids necessary for the structural integrity of the protein. POC1A is expressed in the growth plate of long bones and the seminiferous tubules of the testis, the tissues with the most obvious cellular phenotypes. Several processes contribute to the growth defect. First, proliferating chondrocytes undergo enhanced cell death, likely due to multipolar spindle formation. Second, rapidly proliferating chondrocytes fail to maintain the characteristic flattened cell shape and fail to intercalate into a cellular column after cell division, consistent with defective cilia function. Male infertility is a consequence of the inability of Sertoli cells to support germ cell development, as well as germ cell defects in spermatogenesis. The Poc1acha/cha mouse has provided information on the molecular mechanism underlying clinical features of human patients, and highlights male fertility as a potential area of interest for clinicians examining adult male patients with mutations in Poc1a. The Poc1acha/cha and Poc1atm1(KOMP)Mpb mice are valuable tools for studying the molecular mechanisms of dwarfisms and for testing therapeutic interventions.
Materials and Methods
Mice
The chagun mutation arose spontaneously on the DBA/2J strain in Dr. Linda D. Siracusa’s laboratory at Thomas Jefferson University (Philadelphia, Pennsylvania). The mice were transferred to the University of Michigan (Ann Arbor, Michigan). Recently they have been maintained on a hybrid background consisting of C57BL/6J and DBA/2J background strains, and also outcrossed to the FVB/NJ strain for routine maintenance.
The BAC RP24-384G5 was purchased from Children’s Hospital of Oakland Research Institute (CHORI). Transgenic mice carrying this BAC were generated by the University of Michigan Transgenic Animal Model Core [36].
Embryonic stem cells containing the Poc1a tm1(KOMP)Mbp mutation, CSD45930, were purchased from the KOMP (University of California, Davis, CA). These stem cells, JM8A3.N1, were derived from the C57BL/6N-Atm1Brd strain. Two targeted clones were used: DEPD00572_5_B12 (Poc1a_B12), and DEPD00572_5_C09 (Poc1a_C9). These stem cells were injected into blastocysts and transferred to pseudopregnant surrogate mothers by the University of Michigan Transgenic Animal Model Core. Chimeras were mated to C57BL/6J females to obtain germline transmission.
All mice were housed in a specific pathogen free facility with 12-h light, 12-h dark cycle in ventilated cages with unlimited access to tap water and Purina 5020 chow. All procedures using mice were approved by the University of Michigan Committee on Use and Care of Animals (UCUCA), and the Washington State University Institutional Animal Care and Use Committee (IACUC), and all experiments were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guidelines of the Care and Use of Experimental Animals. Euthanasia was conducted by CO2 inhalation, except for newborn mice that are unresponsive to this method. Decapitation was used for those animals. Surgery was conducted with approved anesthetics. Experienced veterinary care was provided.
Genotyping protocols
Two protocols were used to genotype for chagun. Before the chagun mutation was uncovered, the genotype at cha was inferred based on polymorphic flanking markers. Primers were designed to amplify regions of genomic DNA flanking the chagun critical interval that contained informative SNPs. These SNPs differ between the mutant (DBA/2J) and the wild-type (FVB/NJ) backgrounds: rs30174769, rs29637716. These amplification products were digested with Bsr1 endonuclease and run on a 2% agarose/tris-boric acid-EDTA gel to visualize the different bands that segregated differentially between the two background strains. After the mutation was uncovered, mutants, heterozygous, and wild-type animals were distinguished using primers designed to detect the presence of the insertion from flanking sequence (two potential products; a small wild-type allele, and the larger chagun allele with the insertion).
Animals from the BAC transgenic rescue experiment were genotyped using the polymorphic SNP markers, and at least two additional sets of primers that amplify products specific to the BAC backbone. All primers anneal optimally at 60°C. The forward (Fwd) and reverse (Rev) primer sequences are:
rs30174769 Fwd: 5’-TGAGCGCCAACACAGTAAAAA-3’
rs30174769 Rev: 5’-GTGGGAGCAAGAGACAAAGAGAT-3’
rs29637716 Fwd: 5’-GCGGGGTGCCTGGGTCTCC-3’
rs29637716 Rev: 5’-TCATACAGCACACGCACTTACACA-3’
T7 Fwd: CGGGGCACATTTCATTACCTCTTT
T7 Rev: GCTCGGCATGCACAACTGATTA
SACBII Fwd: TTGACGGAGACGGAAAAACATA
SACBII Rev: ACCGCGTGAATCAGTGAACAAGTA
WT and chagun allele Fwd: 5’-TGTCCCACTGCCACTGCCACTCA-3’
WT and chagun allele Rev: 5’-GGAAGACTCGCCCCACAGGACTCA-3’
Animals were genotyped for the presence of the Poc1a tm1(KOMP)Mbp mutation by PCR with primers for LacZ and primers in Poc1a exons 6 and 7, which are not present in the Poc1a tm1(KOMP)Mbp mutant allele. Primers for LacZ amplification: Fwd: ATCCTCTGCATGGTCAGGTC Rev: CGTGGCCTGATTCATTCC. Amplification was at 94C for 3 min followed by 35 cycles of 94C 30sec, 58C 30 sec, 72C 30sec, followed by 72C 10min. Primers for detection of wt Poc1a: Fwd: TCTGCTTTGCGGTGTACGAA Rev: TTGGGTAGGGTGGGGTACAT. The conditions for this PCR are 92C for 2 min followed by 30 cycles of 92C 10sec, 57C 30sec, 72C 30sec.
Targeted genomic DNA capture
Two different capture approaches were taken. The Broad Institute conducted genome-wide exome capture and sequencing (Mutant Mouse Resequencing Project, The Broad Institute of MIT and Harvard, Cambridge, MA). Regional targeted enrichment was performed using custom capture probes targeting non-repetitive sequences within the chagun critical interval, and targeted enrichment libraries were generated (Roche/Nimblegen, Madison, Wisconsin). Genomic DNA samples from both a heterozygote (known carrier) and a chagun mutant were used to generate these targeted enrichment libraries, which were indexed separately to allow for multiplexed high-throughput sequencing (IlluminaHiSeq) by the University of Michigan DNA Sequencing Core. DNA Sequence reads were aligned to the B6 reference genome (mm9) using BWA [6]. Custom perl scripts were used to identify the location of potential insertional elements, using the paired-end read mapping data, and these were manually reviewed using Broad Institute’s Integrative Genomics Viewer to visualize and evaluate the DNA sequence data [28].
PCR amplification of genomic DNA
The region around Poc1a exon 8 was amplified by PCR of genomic DNA using the following primer sequences: Fwd: 5’-TGTCCCACTGCCACTGCCACTCA-3’, and Rev: 5’-GGAAGACTCGCCCCACAGGACTCA-3’. Optimal annealing temperature: 60C. PCR was conducted with the GoTaq DNA Polymerase and buffers provided by the distributor (Promega). Sanger sequencing was completed by the University of Michigan DNA Sequencing Core.
Extraction of RNA and generation of cDNA via reverse transcription
Extraction of total RNA was completed with the RNAqueous 4-PCR Kit (Ambion) according to the manufacturer’s instructions. The total RNA was utilized to generate cDNA with Oligo dT Primers (Invitrogen/Life Technologies) and Superscript II Reverse Transcriptase (Invitrogen/Life Technologies) according to the manufacturer’s instructions. The cDNA was used as a template in standard PCR reactions using GoTaq DNA Polymerase (Promega) and the following primers to amplify two regions in the Poc1a cDNA:
Exon 5–10 Fwd: 5’-CAAGACCAGCCGGGAATGTATC-3’
Exon 5–10 Rev: CTGGGGCTCGCCTTGAACTGACTC-3’
Optimal annealing temperature: 60°C.
Exon 7–8 Fwd: 5’ - CCATCGGGAAACTACCTCATCAC-3’
Exon 7–8 Rev: 5’-AAATACTCCCCCGTTCTTG-3’
Optimal annealing temperature: 55°C. Sequencing was completed by the University of Michigan DNA Sequencing Core.
Real-time quantitative PCR
TaqMan Universal PCR Master Mix (Applied Biosystems/Life Technologies) was used according to the manufacturer’s instructions. The following TaqMan probes were included to test expression levels of Poc1a: Exon 2–3 Assay ID: Mm01235877_m1. Exon 10–11 Assay ID: Mm01235875_m1. Reactions were loaded into MicroAmp Optical 96-well reaction plates (Applied Biosystems/Life Technologies) and run using an Applied Biosystems 7500 Real-Time PCR System.
POC1A western blot analysis
Isolation of protein from postnatal day 3 tibiae and western blot analyses were carried out as previously described [75]. The blot was incubated with a 1 : 500 dilution of a mouse anti-human POC1A primary antibody (Abcam, ab67698) overnight at 4°C. The blot was incubated with a 1 : 5000 dilution of a goat anti-mouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, Inc. #115-035-003) for one hour at room temperature. The blot was stripped and incubated with a 1 : 5000 dilution of a rat anti-yeast tubulin antibody (Abcam, ab6160) overnight at 4°C. The blot was incubated with a 1 : 10,000 dilution of a goat anti-Rat IgG secondary antibody (Jackson ImmunoResearch Laboratories, # 112-035-102) for one hour at room temperature. All antibodies were diluted in a blocking solution made up of 1% weight:volume Bovine Serum Albumin;Tris-buffered saline with Tween-20.
Bone preparations and analysis
Adult skulls and were completely stripped of flesh by incubation in a dermastid beetle colony (http://www.lsa.umich.edu/ummz/mammals/dermestarium/default.asp.) Briefly, mice were euthanized, and bony parts were trimmed of excess flesh. These specimens were fed to the beetles. After the remaining soft tissues were removed, the bones were frozen at ~-20 C for 72 hrs, and the dead beetles removed.
To assess shape and mineralization of bones, mice were euthanized, bones were dissected and skin and flesh trimmed. Specimens were imaged in water using a cone beam microCT system (eXplore Locus SP; E Healthcare Pre-Clinical Imaging, London, ON, Canada). Scan parameters were 0.5 degree rotational increment, 4 frames averaged, 80 kVp/80 μA X-ray, and 0.508 mm Al filter plus beam flattener to reduce beam hardening artifacts [76]. Volumes were reconstructed at 18 μm isotropic voxel size and calibrated for grayscale value by a manufacturer-provided phantom of air, water, and hydroxyapatite-mimicking material.
Bone and testis histology
Tibiae were dissected from 15 day old animals, fixed overnight in 4% paraformaldehyde (PFA), rinsed in PBS, and decalcified in 14% EDTA solution (weight:volume) for approximately 7 days, changing the solution each day. Testes were removed from mice of the listed ages and fixed overnight in Bouin’s Fixative Solution (Sigma). Both the testes and tibiae were then dehydrated through an ethanol series and embedded in paraffin. Sections were stained with hematoxylin and eosin (tibiae) or periodic acid-Schiff’s reagent (testes) according to standard protocols.
Immunohistochemistry
Immunohistochemistry was conducted after removing paraffin from the samples by incubation of slides in xylenes and rehydration in an ethanol series to 1X PBS. Afterward, the testis sections were boiled in a 100 mM solution of citric acid (pH 6.0) for 10 minutes to expose epitopes, and cooled. Tibia sections were fixed for 10 minutes in 4% PFA, washed in PBS, and were either incubated with proteinase K in a buffer containing 50 mM Tris base, 1 mM EDTA and 0.5% Triton-X100 (acetylated tubulin), or placed in citric acid (pH 6.0) heated to 65°C for 1 hour (GM130, POC1A). Both testis and tibia sections were incubated for 20 minutes in solution of 3% hydrogen peroxide diluted in methanol to quench endogenous peroxidase activity. The slides were blocked in a solution included in the Tyramide Signal Amplification (TSA) Kit (Perkin-Elmer #SAT701001EA) or Mouse-On-Mouse (M.O.M) Blocking Reagent (Vector #BMK-2202) for one hour at room temperature. This was followed by an overnight incubation with primary antibodies in either TSA Block or Vector Mouse-On-Mouse Diluant at 4C. The rabbit anti-rat POC1A antibody was raised against rat POC1A aa 242–291, which is homologous to mouse POC1A aa 280–329. The mouse aa 269–308 comprise the WD7 repeat. The primary antibodies include: rabbit anti-rat POC1A (Abcam, ab135361, diluted 1 : 100), rabbit anti-human PLZF (Santa Cruz Biotechnology Inc., sc-22839 diluted 1 : 50), rabbit anti-SOX9 (Millipore, # AB5535, diluted 1 : 200), mouse anti-acetylated tubulin (Sigma #T6793, diluted 1 : 500), mouse anti-GM130 (BD Biosciences #610822, diluted 1 : 200), rabbit anti-KI67 (Novocastra NCL-Ki67p, diluted 1 : 250), rabbit anti-γH2AX (Ser139) (20E3) (Cell Signaling Technology #9718, diluted 1 : 500), and rabbit anti-c-KIT antibody (Cell Signaling Technology, # 3074S, diluted 1 : 400). Sections were washed in PBS, and incubated with the following secondary antibodies at room temperature: goat anti-rabbit biotin-conjugated secondary (Jackson ImmunoResearch Laboratories Inc., #111-067-003, POC1A, PLZF, SOX9, Ki67, γH2AX, and c-KIT for 1 hour), or the biotinylated anti-mouse secondary included in the M.O.M Kit from Vector Laboratories according to the manufacturer’s instructions. The subsequent steps were carried out according to the instructions provided in the TSA Fluorescein Tyramide Kit (TSA-FITC Kit) by Perkin-Elmer. Sections were counterstained with DAPI to reveal nuclei, cover slipped and photographed with a Leica Leitz DMRB/E compound microscope. Quantitation was done on three animals per genotype and age on 5–10 sections per individual and presented ± std. dev.
MEF analysis
Mouse embryonic fibroblasts (MEFs) were isolated from individual embryos at embryonic day 13.5 by the University of Michigan Transgenic Animal Model Core, and stocks were genotyped for Poc1acha/cha and frozen. MEFs were thawed, grown on gelatin-coated coverslips for two days, then serum treated or serum starved for 24 hours and fixed for 20 minutes in 4% paraformaldehyde at room temperature. The coverslips were washed in PBS, permeabilized in 0.1% SDS for 10 minutes at room temperature, and were incubated with the same primary antibodies for acetylated tubulin and GM130 listed above. The coverslips treated with acetylated tubulin primary antibody were then incubated in an Alexa 488 conjugated anti - mouse secondary (Life Technologies, # A-21141). The coverslips incubated with the GM130 primary were incubated with the M.O.M. anti-mouse Biotinylated IgG included in the M.O.M. Kit (Vector Laboratories). The GM130-incubated coverslips were then treated in the same way as the tissue sections according to the TSA-FITC Kit (Perkin-Elmer). All coverslips were counterstained with DAPI and photographed using an Olympus FluoView Laser Scanning Confocal Microscope (Microscopy and Image Analysis Laboratory, University of Michigan, Ann Arbor, MI).
Apoptosis analysis by TUNEL staining
Sections through P15 tibiae were de-waxed in xylene, rehydrated through an ethanol series to 1 X PBS, and then pretreated by incubating the slides in citric acid (pH 6.0) at 65°C for 1 hour. Slides were washed in PBS and treated according to the manufacturer’s instructions thereafter (Roche In Situ Cell Death Detection Kit, Fluorescein, #11684795910). Quantification was done on three separate sections from two different mice of each genotype and presented as average per section ± standard error. The two-tailed T test was applied to assess significance.
Spermatogonial transplantation assays
Rosa-Lac Z mice (Jackson Laboratories, Bar Harbor, ME, #002073) were crossed to Poc1acha/cha homozygous females to generate reporter positive heterozygous mice for intercrossing and generation of reporter positive wild type and mutant males for the experiments requiring labeled mutant germ cells. Spermatogonial stem cell transplantation was performed as described previously [77]. Briefly, single cell suspensions from donor testes were generated by two-step enzymatic digestion and spermatogonia were enriched by selection with a 30% continuous Percoll gradient. Adult wild type recipient mice were prepared by busulfan treatment (60 mg/kg of body weight) at least 6 weeks before transplantation to deplete endogenous germ cells as described previously [77]. Adult Poc1a mutant recipient mice were not prepared with busulfan treatment because the endogenous germline was already depleted. For experiments involving Poc1a mutant and wild type counterparts as SSC donors, NCr nude mice (Taconic) were used as recipients to avoid immunological incompatibility. For all transplantations, donor cells were re-suspended in mouse serum-free injection media [78] at 1X106 cells/ml and approximately 10 μl of cell suspension was microinjected into the seminiferous tubules of each recipient testis. The recipient testes were examined for donor-derived colonies of spermatogenesis via X-Gal staining ~3 months after transplantation.
Protein structure prediction
The molecular structure of mouse POC1A was predicted using the WD40 structure predictor algorithm (WDSP) [34] and models generated using PyMOL (The PyMOL Molecular Graphics System, Version 1.7.2.2 Schrödinger, LLC).
Supporting Information
Zdroje
1. Krakow D, Rimoin DL (2010) The skeletal dysplasias. Genet Med 12 : 327–341. doi: 10.1097/GIM.0b013e3181daae9b 20556869
2. Karsenty G, Kronenberg HM, Settembre C (2009) Genetic control of bone formation. Annu Rev Cell Dev Biol 25 : 629–648. doi: 10.1146/annurev.cellbio.042308.113308 19575648
3. Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R (2007) Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol 305 : 202–216. 17359961
4. Zhang J, Tan X, Li W, Wang Y, Wang J, et al. (2005) Smad4 is required for the normal organization of the cartilage growth plate. Dev Biol 284 : 311–322. 16023633
5. Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, et al. (2011) NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet 88 : 106–114. doi: 10.1016/j.ajhg.2010.12.004 21211617
6. Li Y, Dudley AT (2009) Noncanonical frizzled signaling regulates cell polarity of growth plate chondrocytes. Development 136 : 1083–1092. doi: 10.1242/dev.023820 19224985
7. Klingseisen A, Jackson AP (2011) Mechanisms and pathways of growth failure in primordial dwarfism. Genes Dev 25 : 2011–2024. doi: 10.1101/gad.169037 21979914
8. Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, et al. (2011) Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A 155A: 943–968. doi: 10.1002/ajmg.a.33909 21438135
9. O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33 : 497–501. 12640452
10. Ogi T, Walker S, Stiff T, Hobson E, Limsirichaikul S, et al. (2012) Identification of the first ATRIP-deficient patient and novel mutations in ATR define a clinical spectrum for ATR-ATRIP Seckel Syndrome. PLoS Genet 8: e1002945. doi: 10.1371/journal.pgen.1002945 23144622
11. Qvist P, Huertas P, Jimeno S, Nyegaard M, Hassan MJ, et al. (2011) CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS Genet 7: e1002310. doi: 10.1371/journal.pgen.1002310 21998596
12. Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, et al. (2011) CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet 43 : 23–26. doi: 10.1038/ng.725 21131973
13. Bicknell LS, Bongers EM, Leitch A, Brown S, Schoots J, et al. (2011) Mutations in the pre-replication complex cause Meier-Gorlin syndrome. Nat Genet 43 : 356–359. doi: 10.1038/ng.775 21358632
14. Bicknell LS, Walker S, Klingseisen A, Stiff T, Leitch A, et al. (2011) Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat Genet 43 : 350–355. doi: 10.1038/ng.776 21358633
15. de Munnik SA, Bicknell LS, Aftimos S, Al-Aama JY, van Bever Y, et al. (2012) Meier-Gorlin syndrome genotype-phenotype studies: 35 individuals with pre-replication complex gene mutations and 10 without molecular diagnosis. Eur J Hum Genet 20 : 598–606. doi: 10.1038/ejhg.2011.269 22333897
16. Guernsey DL, Matsuoka M, Jiang H, Evans S, Macgillivray C, et al. (2011) Mutations in origin recognition complex gene ORC4 cause Meier-Gorlin syndrome. Nat Genet 43 : 360–364. doi: 10.1038/ng.777 21358631
17. Al-Dosari MS, Shaheen R, Colak D, Alkuraya FS (2010) Novel CENPJ mutation causes Seckel syndrome. J Med Genet 47 : 411–414. doi: 10.1136/jmg.2009.076646 20522431
18. Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, et al. (2008) Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet 40 : 232–236. 18157127
19. Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, et al. (2008) Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 319 : 816–819. doi: 10.1126/science.1151174 18174396
20. Dauber A, Lafranchi SH, Maliga Z, Lui JC, Moon JE, et al. (2012) Novel microcephalic primordial dwarfism disorder associated with variants in the centrosomal protein ninein. J Clin Endocrinol Metab 97: E2140–2151. doi: 10.1210/jc.2012-2150 22933543
21. Sarig O, Nahum S, Rapaport D, Ishida-Yamamoto A, Fuchs-Telem D, et al. (2012) Short stature, onychodysplasia, facial dysmorphism, and hypotrichosis syndrome is caused by a POC1A mutation. Am J Hum Genet 91 : 337–342. doi: 10.1016/j.ajhg.2012.06.003 22840363
22. Shaheen R, Faqeih E, Shamseldin HE, Noche RR, Sunker A, et al. (2012) POC1A truncation mutation causes a ciliopathy in humans characterized by primordial dwarfism. Am J Hum Genet 91 : 330–336. doi: 10.1016/j.ajhg.2012.05.025 22840364
23. Stiff T, Alagoz M, Alcantara D, Outwin E, Brunner HG, et al. (2013) Deficiency in origin licensing proteins impairs cilia formation: implications for the aetiology of Meier-Gorlin syndrome. PLoS Genet 9: e1003360. doi: 10.1371/journal.pgen.1003360 23516378
24. He H, Liyanarachchi S, Akagi K, Nagy R, Li J, et al. (2011) Mutations in U4atac snRNA, a component of the minor spliceosome, in the developmental disorder MOPD I. Science 332 : 238–240. doi: 10.1126/science.1200587 21474760
25. Edery P, Marcaillou C, Sahbatou M, Labalme A, Chastang J, et al. (2011) Association of TALS developmental disorder with defect in minor splicing component U4atac snRNA. Science 332 : 240–243. doi: 10.1126/science.1202205 21474761
26. Cha KB, Karolyi IJ, Hunt A, Wenglikowski AM, Wilkinson JE, et al. (2004) Skeletal dysplasia and male infertility locus on mouse chromosome 9. Genomics 83 : 951–960. 15177549
27. Goodier JL, Ostertag EM, Du K, Kazazian HH Jr. (2001) A novel active L1 retrotransposon subfamily in the mouse. Genome Res 11 : 1677–1685. 11591644
28. Nellaker C, Keane TM, Yalcin B, Wong K, Agam A, et al. (2012) The genomic landscape shaped by selection on transposable elements across 18 mouse strains. Genome Biol 13: R45. doi: 10.1186/gb-2012-13-6-r45 22703977
29. Beck CR, Garcia-Perez JL, Badge RM, Moran JV (2011) LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet 12 : 187–215. doi: 10.1146/annurev-genom-082509-141802 21801021
30. Feng Q, Moran JV, Kazazian HH Jr., Boeke JD (1996) Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell 87 : 905–916. 8945517
31. Rondeau G, Moreau I, Bezieau S, Petit JL, Heilig R, et al. (2001) Comprehensive analysis of a large genomic sequence at the putative B-cell chronic lymphocytic leukaemia (B-CLL) tumour suppresser gene locus. Mutat Res 458 : 55–70. 11691637
32. Morrish TA, Gilbert N, Myers JS, Vincent BJ, Stamato TD, et al. (2002) DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet 31 : 159–165. 12006980
33. Jurka J (1997) Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc Natl Acad Sci U S A 94 : 1872–1877. 9050872
34. Wang Y, Jiang F, Zhuo Z, Wu XH, Wu YD (2013) A method for WD40 repeat detection and secondary structure prediction. PLoS One 8: e65705. doi: 10.1371/journal.pone.0065705 23776530
35. Wu XH, Wang Y, Zhuo Z, Jiang F, Wu YD (2012) Identifying the hotspots on the top faces of WD40-repeat proteins from their primary sequences by beta-bulges and DHSW tetrads. PLoS One 7: e43005. doi: 10.1371/journal.pone.0043005 22916195
36. Van Keuren ML, Gavrilina GB, Filipiak WE, Zeidler MG, Saunders TL (2009) Generating transgenic mice from bacterial artificial chromosomes: transgenesis efficiency, integration and expression outcomes. Transgenic Res 18 : 769–785. doi: 10.1007/s11248-009-9271-2 19396621
37. Behringer R, Gertsenstein M, Nagy KV, Nagy A (2014) Manipulating the Mouse Embryo. A laboratory manual. NY: Cold Spring Harbor Press.
38. Manandhar G, Simerly C, Salisbury JL, Schatten G (1999) Centriole and centrin degeneration during mouse spermiogenesis. Cell Motil Cytoskeleton 43 : 137–144. 10379838
39. Avasthi P, Scheel JF, Ying G, Frederick JM, Baehr W, et al. (2013) Germline deletion of Cetn1 causes infertility in male mice. J Cell Sci 126 : 3204–3213. doi: 10.1242/jcs.128587 23641067
40. Escalier D (2002) Genetic approach to male meiotic division deficiency: the human macronuclear spermatozoa. Mol Hum Reprod 8 : 1–7. 11756563
41. Gonczy P (2012) Towards a molecular architecture of centriole assembly. Nat Rev Mol Cell Biol 13 : 425–435. doi: 10.1038/nrm3373 22691849
42. May-Simera HL, Kelley MW (2012) Cilia, Wnt signaling, and the cytoskeleton. Cilia 1 : 7. doi: 10.1186/2046-2530-1-7 23351924
43. Kuss P, Kraft K, Stumm J, Ibrahim D, Vallecillo-Garcia P, et al. (2014) Regulation of cell polarity in the cartilage growth plate and perichondrium of metacarpal elements by HOXD13 and WNT5A. Dev Biol 385 : 83–93. doi: 10.1016/j.ydbio.2013.10.013 24161848
44. Ascenzi MG, Blanco C, Drayer I, Kim H, Wilson R, et al. (2011) Effect of localization, length and orientation of chondrocytic primary cilium on murine growth plate organization. J Theor Biol 285 : 147–155. doi: 10.1016/j.jtbi.2011.06.016 21723296
45. de Andrea CE, Wiweger M, Prins F, Bovee JV, Romeo S, et al. (2010) Primary cilia organization reflects polarity in the growth plate and implies loss of polarity and mosaicism in osteochondroma. Lab Invest 90 : 1091–1101. doi: 10.1038/labinvest.2010.81 20421870
46. Jakob S, Lovell-Badge R (2011) Sex determination and the control of Sox9 expression in mammals. FEBS J 278 : 1002–1009. doi: 10.1111/j.1742-4658.2011.08029.x 21281448
47. Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, et al. (2004) Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet 36 : 653–659. 15156143
48. Yang QE, Racicot KE, Kaucher AV, Oatley MJ, Oatley JM (2013) MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development 140 : 280–290. doi: 10.1242/dev.087403 23221369
49. Oatley JM, Brinster RL (2008) Regulation of spermatogonial stem cell self-renewal in mammals. Annu Rev Cell Dev Biol 24 : 263–286. doi: 10.1146/annurev.cellbio.24.110707.175355 18588486
50. Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, et al. (2003) Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod 69 : 412–420. 12672656
51. Hertel KJ (2008) Combinatorial control of exon recognition. J Biol Chem 283 : 1211–1215. 18024426
52. Luan DD, Korman MH, Jakubczak JL, Eickbush TH (1993) Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell 72 : 595–605. 7679954
53. Cost GJ, Feng Q, Jacquier A, Boeke JD (2002) Human L1 element target-primed reverse transcription in vitro. EMBO J 21 : 5899–5910. 12411507
54. Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, et al. (2001) Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol 21 : 1429–1439. 11158327
55. Esnault C, Maestre J, Heidmann T (2000) Human LINE retrotransposons generate processed pseudogenes. Nat Genet 24 : 363–367. 10742098
56. Garcia-Perez JL, Doucet AJ, Bucheton A, Moran JV, Gilbert N (2007) Distinct mechanisms for trans-mediated mobilization of cellular RNAs by the LINE-1 reverse transcriptase. Genome Res 17 : 602–611. 17416749
57. Parker HG, VonHoldt BM, Quignon P, Margulies EH, Shao S, et al. (2009) An expressed fgf4 retrogene is associated with breed-defining chondrodysplasia in domestic dogs. Science 325 : 995–998. doi: 10.1126/science.1173275 19608863
58. de Boer M, van Leeuwen K, Geissler J, Weemaes CM, van den Berg TK, et al. (2014) Primary Immunodeficiency Caused by an Exonized Retroposed Gene Copy Inserted in the CYBB Gene. Hum Mutat 35 : 486–496. doi: 10.1002/humu.22519 24478191
59. Cooke SL, Shlien A, Marshall J, Pipinikas CP, Martincorena I, et al. (2014) Processed pseudogenes acquired somatically during cancer development. Nat Commun 5 : 3644. doi: 10.1038/ncomms4644 24714652
60. Vitre BD, Cleveland DW (2012) Centrosomes, chromosome instability (CIN) and aneuploidy. Curr Opin Cell Biol 24 : 809–815. doi: 10.1016/j.ceb.2012.10.006 23127609
61. Keller LC, Geimer S, Romijn E, Yates J 3rd, Zamora I, et al. (2009) Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol Biol Cell 20 : 1150–1166. doi: 10.1091/mbc.E08-06-0619 19109428
62. Venoux M, Tait X, Hames RS, Straatman KR, Woodland HR, et al. (2013) Poc1A and Poc1B act together in human cells to ensure centriole integrity. J Cell Sci 126 : 163–175. doi: 10.1242/jcs.111203 23015594
63. Pearson CG, Osborn DP, Giddings TH Jr., Beales PL, Winey M (2009) Basal body stability and ciliogenesis requires the conserved component Poc1. J Cell Biol 187 : 905–920. doi: 10.1083/jcb.200908019 20008567
64. Romereim SM, Conoan NH, Chen B, Dudley AT (2014) A dynamic cell adhesion surface regulates tissue architecture in growth plate cartilage. Development 141 : 2085–2095. doi: 10.1242/dev.105452 24764078
65. Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19: R526–535. doi: 10.1016/j.cub.2009.05.025 19602418
66. Singla V, Reiter JF (2006) The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313 : 629–633. 16888132
67. Tummala P, Arnsdorf EJ, Jacobs CR (2010) The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cell Mol Bioeng 3 : 207–212. 20823950
68. Rais Y, Reich A, Simsa-Maziel S, Moshe M, Idelevich A, et al. (2015) The growth plate's response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell Mol Life Sci 72 : 597–615. doi: 10.1007/s00018-014-1690-4 25084815
69. Shao YY, Wang L, Welter JF, Ballock RT (2012) Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50 : 79–84. doi: 10.1016/j.bone.2011.08.033 21930256
70. de Rooij DG, de Boer P (2003) Specific arrests of spermatogenesis in genetically modified and mutant mice. Cytogenet Genome Res 103 : 267–276. 15051947
71. Vogl AW, Vaid KS, Guttman JA (2008) The Sertoli cell cytoskeleton. Adv Exp Med Biol 636 : 186–211. doi: 10.1007/978-0-387-09597-4_11 19856169
72. Oatley JM, Brinster RL (2012) The germline stem cell niche unit in mammalian testes. Physiol Rev 92 : 577–595. doi: 10.1152/physrev.00025.2011 22535892
73. Beck BB, Phillips JB, Bartram MP, Wegner J, Thoenes M, et al. (2014) Mutation of POC1B in a severe syndromic retinal ciliopathy. Hum Mutat 35 : 1153–1162. doi: 10.1002/humu.22618 25044745
74. Roosing S, Lamers IJ, de Vrieze E, van den Born LI, Lambertus S, et al. (2014) Disruption of the basal body protein POC1B results in autosomal-recessive cone-rod dystrophy. Am J Hum Genet 95 : 131–142. doi: 10.1016/j.ajhg.2014.06.012 25018096
75. Geister KA, Brinkmeier ML, Hsieh M, Faust SM, Karolyi IJ, et al. (2013) A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for acromesomelic dysplasia, Maroteaux type. Hum Mol Genet 22 : 345–357. doi: 10.1093/hmg/dds432 23065701
76. Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA (2009) Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone 45 : 1104–1116. doi: 10.1016/j.bone.2009.07.078 19651256
77. Oatley JM, Brinster RL (2006) Spermatogonial stem cells. Methods Enzymol 419 : 259–282. 17141059
78. Kubota H, Avarbock MR, Brinster RL (2004) Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 101 : 16489–16494. 15520394
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



