-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGene-Regulatory Logic to Induce and Maintain a Developmental Compartment
article has not abstract
Published in the journal: . PLoS Genet 11(10): e32767. doi:10.1371/journal.pgen.1005543
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1005543Summary
article has not abstract
Forty-four years ago, Antonio García-Bellido utilized, for the first time in biology, the clonal analysis technique to characterize the parameters of proliferation and growth of a developing organ—in this case, the highly proliferative wing primordium of Drosophila [1]. A couple of years later, using the same technique, García-Bellido and two of his PhD students, Ginés Morata and Pedro Ripoll, reported the existence of adjacent cell populations that do not mix, which he called compartments [2]. Developmental compartmentalization of growing organs was subsequently demonstrated in all ectodermal derivatives of the fruit fly, as well as in vertebrate limbs and in the central nervous system. One of the most remarkable features of compartments is that their boundaries also act as organizing centers. Compartments are defined by the restricted expression and activity of the so-called selector genes. The homeodomain-encoding gene engrailed was rapidly identified as the one specifying the posterior compartment of all ectodermal derivatives of the fruit fly [3,4]. It was not until the early 1990s that the LIM-homeodomain-encoding gene apterous (ap) was shown to specify the dorsal compartment in the Drosophila wing [5]. These selector genes share three distinct activities: specification of compartment identity, localization of an organizing center at the compartment boundary, and establishment of the lineage restriction border. The ap selector gene rapidly became a paradigm in the identification of the signaling molecules, cell adhesion proteins, and transcription factors that mediate the construction of compartments and organizing centers [6–9]. However, how ap expression is maintained in order to fulfill its functions in all the cells of the dorsal compartment during the five days of growth and proliferation has remained largely unknown. In this issue of PLOS Genetics, Carlos Estella and colleagues [10] unravel the molecular mechanisms underlying the initiation and maintenance of ap expression in the Drosophila wing primordium. A combination of enhancers, autoregulatory and feed-forward mechanisms, and epigenetics appears to be at play.
Perhaps the best way of identifying the noncoding regions involved in the regulation of expression of a gene of interest is through classical genetics and phenotypic characterization. Indeed, this is the approach taken by Estella and colleagues in their analysis of the ap locus. A series of endogenous deletions were performed to identify the regulatory sequences required to drive ap expression and give rise to normal-looking adult wings. By doing so, the authors functionally identified a proximal region harboring a Polycomb Response Element (PRE) and a distal fragment containing a previously identified enhancer, whose deletions severely compromised ap expression and wing development. The authors next undertook a systematic in situ dissection of the ap cis-regulatory domain by bringing back sub-fragments of a previously deleted region and analyzing their capacity to rescue the wing phenotype. When combined, the PRE and two enhancers located in a distal fragment were sufficient to rescue both ap expression and wing formation.
So far so good, but what is the regulatory logic that combines the input of two distinct enhancers and a PRE to induce the expression and maintenance of ap expression in all dorsal cells throughout development? The authors again approached this issue in a clever manner. They constructed a battery of lacZ reporter lines to decipher how these enhancers were expressed in the wing, to identify the transcription factor involved in their regulation, and to analyze the contribution of the PRE to this process. As expected, the two functional enhancers located in a distal fragment and identified by the in situ rescue experiment drove expression of the lacZ reporter in dorsal cells of the wing primordium. The isolated ap-E enhancer is initially expressed in all dorsal cells and responds to the early activity of the Epidermal Growth Factor (EGF)-Receptor ligand Vein, as does the whole ap locus [11]. Interestingly, its initial expression coincides in time with the onset of ap expression and the initiation of the organizing activities of the compartment boundary through the activation of the Notch receptor. Later in development, expression of the ap-E enhancer fades away from the dorsal compartment of the wing primordium, and the ap-DV enhancer starts to be expressed in exactly the same region as a consequence of the activity of the Apterous and Vestigial proteins. It is interesting to note that Vestigial is a target of Notch [12]. Thus, both an Apterous-dependent autoregulatory mechanism and a feed-forward mechanism, through the activity of Notch and Vestigial, drive the expression of the ap-DV enhancer. The authors observed that these two enhancers were not able—either alone or in combination—to completely reproduce the ap expression pattern. Thus, additional elements appear to be at work. Following the logic of the in situ rescue experiments, the combination of the ap-E and ap-DV enhancers, together with the ap-PRE, gave rise to robust expression of the lacZ reporter in all dorsal cells throughout wing development. Interestingly, the ability of the ap-PRE to drive robust expression was shown to rely on the activity of the Trithorax group of proteins.
Taken together, the results from Estella and colleagues reveal a three-step molecular mechanism to initiate and maintain ap expression in all dorsal cells throughout development (Fig 1). Whereas the onset of ap expression relies on the restricted expression of the EGF receptor ligand Vein and on the activity of the EGF-Receptor responsive transcription factor Pointed-P1 in the presumptive dorsal compartment, the maintenance of ap expression during the subsequent stages of wing development is led by an Apterous-dependent autoregulatory loop, a Vestigial-mediated feed-forward mechanism, and the activity of the PRE. This initiation and maintenance mechanism has remarkable commonalities with the logic followed by other developmental genes whose expression has to be precisely initiated but also robustly maintained throughout the dramatic increase in tissue size and cell number that occurs in the primordia of the adult fly [13,14]. Forty-two years after the discovery of compartments, the Drosophila wing is still unraveling common principles of development.
Fig. 1. Illustration of the apterous locus with its regulatory regions involved in the control of its expression in the Drosophila wing. 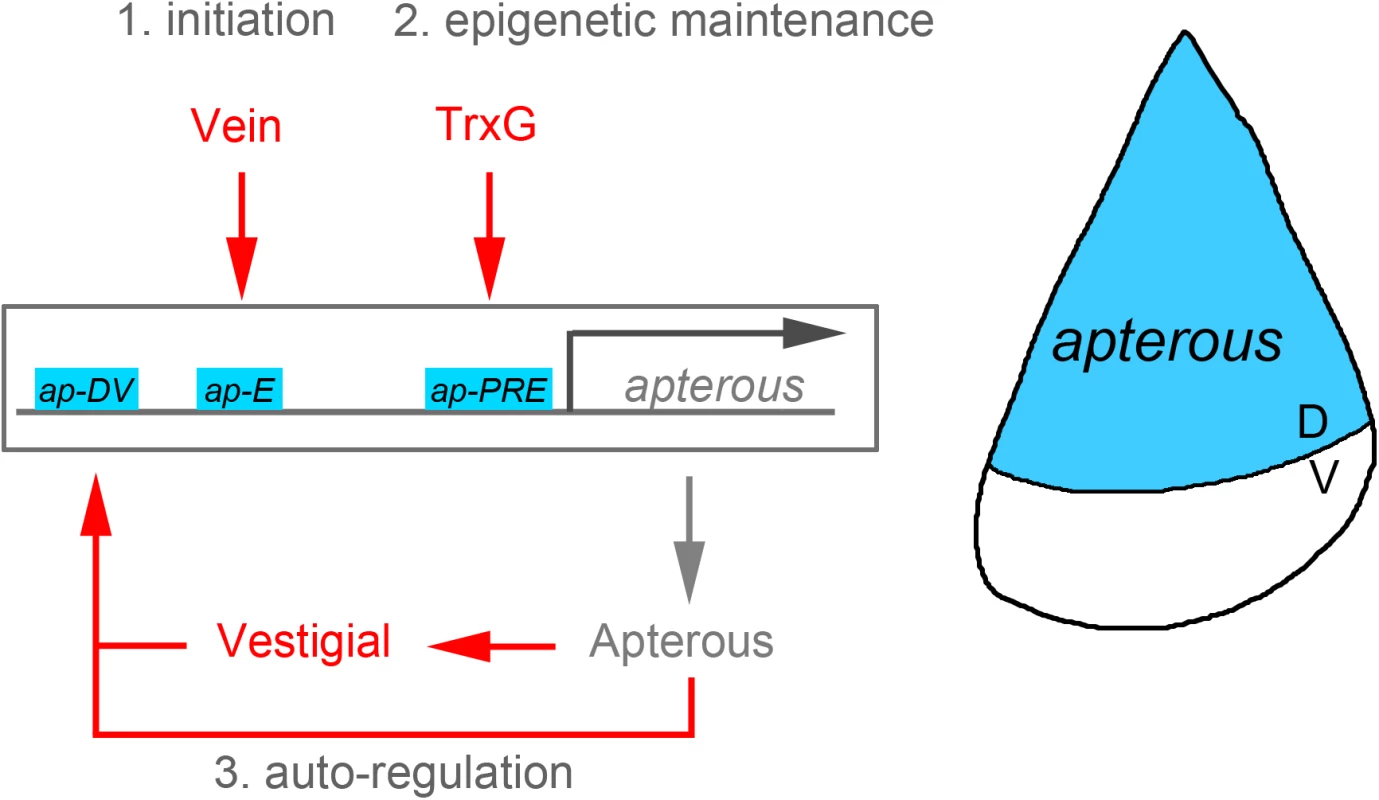
Whereas the onset of apterous expression in the dorsal compartment of the early wing primordium relies on the activity of the EGF receptor ligand Vein, the maintenance of its expression during subsequent developmental stages depends on both autoregulatory and epigenetic mechanisms.
Zdroje
1. García-Bellido A, Merriam JR (1971) Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev Biol 24 : 61–87. 5001010
2. Garcia-Bellido A, Ripoll P, Morata G (1973) Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol 245 : 251–253. 4518369
3. Garcia-Bellido A, Santamaria P (1972) Developmental analysis of the wing disc in the mutant engrailed of Drosophila melanogaster. Genetics 72 : 87–104. 4627463
4. Morata G, Lawrence PA (1975) Control of compartment development by the engrailed gene in Drosophila. Nature 255 : 614–617. 1134551
5. Diaz-Benjumea FJ, Cohen SM (1993) Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell 75 : 741–752. 8242746
6. Irvine K, Wieschaus E (1994) fringe, a boundary specific signalling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell 79 : 595–606. 7954826
7. Milan M, Weihe U, Perez L, Cohen SM (2001) The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell 106 : 785–794. 11572783
8. Milan M, Weihe U, Tiong S, Bender W, Cohen SM (2001) msh specifies dorsal cell fate in the Drosophila wing. Development 128 : 3263–3268. 11546743
9. Diaz-Benjumea FJ, Cohen SM (1995) Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development 121 : 4215–4225. 8575321
10. Bieli D, Kanca O, Requena D, Hamaratoglu F, Gohl D, Schedl P, et al. (2015) Establishment of a developmental compartment requires interactions between three synergistic cis-regulatory modules. PLoS Genet 11(9) e1005376 doi: 10.1371/journal.pgen.1005376
11. Wang SH, Simcox A, Campbell G (2000) Dual role for Drosophila epidermal growth factor receptor signaling in early wing disc development. Genes Dev 14 : 2271–2276. 10995384
12. Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, et al. (1996) Integration of positional signals and regulation of wing formation by Drosophila vestigial gene. Nature 382 : 133–138. 8700202
13. Estella C, McKay DJ, Mann RS (2008) Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev Cell 14 : 86–96. doi: 10.1016/j.devcel.2007.11.002 18194655
14. Pérez L, Barrio L, Cano D, Fiuza U, Muzzopappa M, et al. (2011) Enhancer-PRE communication contributes to expanding the domains of gene expression in proliferating primordia. Development 138 : 3135–3145. doi: 10.1242/dev.065599 21715425
Štítky
Genetika Reprodukční medicína
Článek Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation DevelopmentČlánek A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor PelotaČlánek A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in OvulationČlánek Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAsČlánek FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2Článek Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion inČlánek The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly SiteČlánek Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem CellsČlánek A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal ErythropoiesisČlánek Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation inČlánek Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in OocytesČlánek MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 10
-
Všechny články tohoto čísla
- Gene-Regulatory Logic to Induce and Maintain a Developmental Compartment
- A Decad(e) of Reasons to Contribute to a PLOS Community-Run Journal
- DNA Methylation Landscapes of Human Fetal Development
- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- Evidence of Selection against Complex Mitotic-Origin Aneuploidy during Preimplantation Development
- Transcriptional Derepression Uncovers Cryptic Higher-Order Genetic Interactions
- Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation
- Virus Satellites Drive Viral Evolution and Ecology
- A Novel Route Controlling Begomovirus Resistance by the Messenger RNA Surveillance Factor Pelota
- Sequence to Medical Phenotypes: A Framework for Interpretation of Human Whole Genome DNA Sequence Data
- Your Data to Explore: An Interview with Anne Wojcicki
- Modulation of Ambient Temperature-Dependent Flowering in by Natural Variation of
- The Ciliopathy Protein CC2D2A Associates with NINL and Functions in RAB8-MICAL3-Regulated Vesicle Trafficking
- PPP2R5C Couples Hepatic Glucose and Lipid Homeostasis
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
- Intermediate Levels of CodY Activity Are Required for Derepression of the Branched-Chain Amino Acid Permease, BraB
- "Missing" G x E Variation Controls Flowering Time in
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Type IV Collagen Controls the Axogenesis of Cerebellar Granule Cells by Regulating Basement Membrane Integrity in Zebrafish
- Loss of a Conserved tRNA Anticodon Modification Perturbs Plant Immunity
- Genome-Wide Association Analysis of Adaptation Using Environmentally Predicted Traits
- Oriented Cell Division in the . Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1
- Disproportionate Contributions of Select Genomic Compartments and Cell Types to Genetic Risk for Coronary Artery Disease
- A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Ovulation
- The RNAPII-CTD Maintains Genome Integrity through Inhibition of Retrotransposon Gene Expression and Transposition
- Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs
- Allelic Variation of Cytochrome P450s Drives Resistance to Bednet Insecticides in a Major Malaria Vector
- SCARN a Novel Class of SCAR Protein That Is Required for Root-Hair Infection during Legume Nodulation
- IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in
- NINL and DZANK1 Co-function in Vesicle Transport and Are Essential for Photoreceptor Development in Zebrafish
- Decay-Initiating Endoribonucleolytic Cleavage by RNase Y Is Kept under Tight Control via Sequence Preference and Sub-cellular Localisation
- Large-Scale Analysis of Kinase Signaling in Yeast Pseudohyphal Development Identifies Regulation of Ribonucleoprotein Granules
- FANCI Regulates Recruitment of the FA Core Complex at Sites of DNA Damage Independently of FANCD2
- LINE-1 Mediated Insertion into (Protein of Centriole 1 A) Causes Growth Insufficiency and Male Infertility in Mice
- Hsp90-Associated Immunophilin Homolog Cpr7 Is Required for the Mitotic Stability of [URE3] Prion in
- Genome-Scale Mapping of σ Reveals Widespread, Conserved Intragenic Binding
- Uncovering Hidden Layers of Cell Cycle Regulation through Integrative Multi-omic Analysis
- Functional Diversification of Motor Neuron-specific Enhancers during Evolution
- The GTP- and Phospholipid-Binding Protein TTD14 Regulates Trafficking of the TRPL Ion Channel in Photoreceptor Cells
- The Gyc76C Receptor Guanylyl Cyclase and the Foraging cGMP-Dependent Kinase Regulate Extracellular Matrix Organization and BMP Signaling in the Developing Wing of
- The Ty1 Retrotransposon Restriction Factor p22 Targets Gag
- Functional Impact and Evolution of a Novel Human Polymorphic Inversion That Disrupts a Gene and Creates a Fusion Transcript
- The Dedicated Chaperone Acl4 Escorts Ribosomal Protein Rpl4 to Its Nuclear Pre-60S Assembly Site
- The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study
- Parent-of-Origin Effects of the Gene on Adiposity in Young Adults
- Chromatin-Remodelling Complex NURF Is Essential for Differentiation of Adult Melanocyte Stem Cells
- Retinoic Acid Receptors Control Spermatogonia Cell-Fate and Induce Expression of the SALL4A Transcription Factor
- A Systems Approach Identifies Essential FOXO3 Functions at Key Steps of Terminal Erythropoiesis
- Protein O-Glucosyltransferase 1 (POGLUT1) Promotes Mouse Gastrulation through Modification of the Apical Polarity Protein CRUMBS2
- KIF7 Controls the Proliferation of Cells of the Respiratory Airway through Distinct Microtubule Dependent Mechanisms
- Integration of Posttranscriptional Gene Networks into Metabolic Adaptation and Biofilm Maturation in
- Lateral and End-On Kinetochore Attachments Are Coordinated to Achieve Bi-orientation in Oocytes
- Protein Homeostasis Imposes a Barrier on Functional Integration of Horizontally Transferred Genes in Bacteria
- A New Method for Detecting Associations with Rare Copy-Number Variants
- Histone H2AFX Links Meiotic Chromosome Asynapsis to Prophase I Oocyte Loss in Mammals
- The Genomic Aftermath of Hybridization in the Opportunistic Pathogen
- A Role for the Chaperone Complex BAG3-HSPB8 in Actin Dynamics, Spindle Orientation and Proper Chromosome Segregation during Mitosis
- Establishment of a Developmental Compartment Requires Interactions between Three Synergistic -regulatory Modules
- Regulation of Spore Formation by the SpoIIQ and SpoIIIA Proteins
- Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes
- Alkaline Ceramidase 3 Deficiency Results in Purkinje Cell Degeneration and Cerebellar Ataxia Due to Dyshomeostasis of Sphingolipids in the Brain
- ACLY and ACC1 Regulate Hypoxia-Induced Apoptosis by Modulating ETV4 via α-ketoglutarate
- Quantitative Differences in Nuclear β-catenin and TCF Pattern Embryonic Cells in .
- HENMT1 and piRNA Stability Are Required for Adult Male Germ Cell Transposon Repression and to Define the Spermatogenic Program in the Mouse
- Axon Regeneration Is Regulated by Ets–C/EBP Transcription Complexes Generated by Activation of the cAMP/Ca Signaling Pathways
- A Phenomic Scan of the Norfolk Island Genetic Isolate Identifies a Major Pleiotropic Effect Locus Associated with Metabolic and Renal Disorder Markers
- The Roles of CDF2 in Transcriptional and Posttranscriptional Regulation of Primary MicroRNAs
- A Genetic Cascade of Modulates Nucleolar Size and rRNA Pool in
- Inter-population Differences in Retrogene Loss and Expression in Humans
- Cationic Peptides Facilitate Iron-induced Mutagenesis in Bacteria
- EP4 Receptor–Associated Protein in Macrophages Ameliorates Colitis and Colitis-Associated Tumorigenesis
- Fungal Infection Induces Sex-Specific Transcriptional Changes and Alters Sexual Dimorphism in the Dioecious Plant
- FLCN and AMPK Confer Resistance to Hyperosmotic Stress via Remodeling of Glycogen Stores
- MET18 Connects the Cytosolic Iron-Sulfur Cluster Assembly Pathway to Active DNA Demethylation in
- Sex Bias and Maternal Contribution to Gene Expression Divergence in Blastoderm Embryos
- Transcriptional and Linkage Analyses Identify Loci that Mediate the Differential Macrophage Response to Inflammatory Stimuli and Infection
- Mre11 and Blm-Dependent Formation of ALT-Like Telomeres in Ku-Deficient
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- Identification of a Single Strand Origin of Replication in the Integrative and Conjugative Element ICE of
- The Type VI Secretion TssEFGK-VgrG Phage-Like Baseplate Is Recruited to the TssJLM Membrane Complex via Multiple Contacts and Serves As Assembly Platform for Tail Tube/Sheath Polymerization
- The Dynamic Genome and Transcriptome of the Human Fungal Pathogen and Close Relative
- Secondary Structure across the Bacterial Transcriptome Reveals Versatile Roles in mRNA Regulation and Function
- ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Regeneration
- Pelle Modulates dFoxO-Mediated Cell Death in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Single Strand Annealing Plays a Major Role in RecA-Independent Recombination between Repeated Sequences in the Radioresistant Bacterium
- The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals
- Genome Wide Identification of SARS-CoV Susceptibility Loci Using the Collaborative Cross
- DCA1 Acts as a Transcriptional Co-activator of DST and Contributes to Drought and Salt Tolerance in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



